Contents
Familial dysbetalipoproteinemia
Familial dysbetalipoproteinemia also called dysbetalipoproteinemia, hyperlipoproteinemia type 3, remnant hyperlipidemia, dysbetalipoproteinemia or “broad beta disease” is a inherited lipid disorder that causes your body to breakdown (metabolize) fats (lipids) incorrectly resulting in accumulation of triglyceride- and cholesterol-rich lipoproteins (remnant particles) in plasma 1, 2, 3, 4, 5, 6, 7, 8. Familial dysbetalipoproteinemia is usually caused by a recessively inherited mutation in the Apolipoproteins E (APOE) gene (e2e2), but variants with dominant inheritance have also been described 9. The APOE gene provides instructions for making a protein called apolipoprotein E. The apolipoprotein E (APOE) combines with fats (lipids) in your body to form molecules called lipoproteins. Lipoproteins are responsible for packaging cholesterol and other fats and carrying them through the bloodstream. Maintaining normal levels of cholesterol is essential for the prevention of disorders that affect the heart and blood vessels (cardiovascular diseases), including heart attack and stroke. Because of the mutation in the APOE gene, people with familial dysbetalipoproteinemia develop the buildup of lipids (fats) in their body (hyperlipidemia) and can lead to the development of multiple small, yellow skin growths called xanthomas. Xanthomas develop primarily due to fat (lipid) leakage from the blood vessels into surrounding tissues, where macrophages engulf these lipids. Accumulation of lipids in the macrophages leads to formation of “foamy macrophages” 10. Affected individuals may also develop the buildup of fatty materials and scar-like tissue in the lining of their arteries called atherosclerosis blocking blood flow and potentially leading to heart attack or stroke. Without treatment, individuals with familial dysbetalipoproteinemia are 5-10 times more likely to develop premature cardiovascular disease 11, 12.
Familial dysbetalipoproteinemia is estimated to affect approximately 1 in 5,000 to 1 in 10,000 people in the general population. It was shown that the prevalence of familial dysbetalipoproteinemia was 0.7% (one in 143) in the population of Utah, USA, and 2.7% (one in 37) among patients with premature coronary artery disease 13. In a more recent study, the prevalence of familial dysbetalipoproteinemia among US adults in two population-based samples was 0.2 to 0.8% 14. This estimate is based on analyzing plasma lipoproteins by ultracentrifugation. Recent data suggest that the real prevalence of familial dysbetalipoproteinemia may be underestimated 15, 16.
Familial dysbetalipoproteinemia affects males more often than females. The symptoms of familial dysbetalipoproteinemia may vary from person to person. Some individuals may not show any symptoms (asymptomatic). Symptoms of familial dysbetalipoproteinemia often do not appear unless additional conditions are present such as diabetes, obesity or underactive thyroid (hypothyroidism) 17.
The most common feature associated with familial dysbetalipoproteinemia is the development of xanthomas, which are deposits of fatty materials (lipids) in the skin and appear as multiple yellow bumps (papules) on or just beneath the skin. Xanthomas may form on different parts of the body including the hands, elbows, knees, knuckles, arms, legs, and buttocks. Although very rare, finding an xanthomas on the palms of the hands, a condition called xanthoma striata palmaris, on physical examination of the patient, is considered pathognomonic of familial dysbetalipoproteinemia and has not been reported in any other disorder 18, 19, 20, 21, 22, 23. Xanthomas can also develop within the tendons of the rear lower legs (Achilles tendon) and occasionally on the fingers. Some affected individuals may have fatty deposits within the corneas of the eyes (arcus lidus corneae). Of the 10-15% of people who develop symptoms, this most often happens in early adulthood. Most people begin to experience symptoms in early adulthood, although some individuals have symptoms beginning in childhood or late adulthood. Women are rarely affected until after menopause.
The risk for developing coronary artery disease is 5-10 times higher for an individual with familial dysbetalipoproteinemia compared to the general population. Individuals with familial dysbetalipoproteinemia may develop thickening and blockage of various blood vessels (atherosclerosis) due to the buildup of fatty material (lipids). Atherosclerosis may result in coronary heart disease or peripheral vascular disease. Coronary heart disease results from blockage of the blood supply to the heart potentially resulting in chest pain (angina) and heart attack. Peripheral artery disease is a general term for disease of the blood vessels outside of the heart and brain. It results from blockage of the blood flow to various organs and the extremities. Decreased blood flow to your legs may result in cramping and cause a limp (claudication). Some individuals may have an abnormally enlarged liver or spleen (hepatosplenomegaly).
Individuals with familial dysbetalipoproteinemia may eventually develop inflammation of the pancreas (pancreatitis). Chronic pancreatitis may result in back pain, diarrhea, yellow-colored skin (jaundice), and potentially the development of diabetes. Pancreatitis can also lead to the development of pancreatic cancer.
Familial dysbetalipoproteinemia diagnosis can be made based upon a thorough clinical evaluation, a detailed patient and family history and identification of characteristic findings such as xanthomas. Arterial imaging and a cardiac stress test can identify signs of silent atherosclerosis in young adults.
A blood test called a lipid panel or lipid profile can measure your cholesterol levels typically reports:
- Low-density lipoprotein (LDL) cholesterol is sometimes called “bad” cholesterol because a high LDL level leads to the buildup of plaque in your arteries.
- High-density lipoprotein (HDL) cholesterol is sometimes called “good” cholesterol because it helps your body get rid of cholesterol. HDL carries cholesterol from other parts of your body back to your liver. Your liver then removes the cholesterol from your body.
- Total cholesterol = HDL + LDL + 20% triglycerides. “Total cholesterol” is the total amount of cholesterol that’s circulating in your blood.
- Very low-density lipoprotein (VLDL) cholesterol is sometimes called “bad” cholesterol because it too contributes to the buildup of plaque in your arteries. But VLDL and LDL are different; VLDL mainly carries triglycerides and LDL mainly carries cholesterol.
- Triglycerides. Triglycerides are the most common type of fat in your body. Triglycerides come from foods, especially butter, oils, and other fats you eat. Triglycerides also come from extra calories your body does not need right away. Unused calories (energy you don’t need right away) are stored as triglycerides in fat cells, thus triglycerides is a major source of energy for your body. When your body needs energy, it releases the triglycerides. In between meals, triglycerides are released from fat tissue to be used as an energy source for your body. Most triglycerides are carried in the blood by lipoproteins called very low-density lipoproteins (VLDL).
In familial dysbetalipoproteinemia the lipid profile showed mixed hyperlipidemia with high total cholesterol, elevated LDL cholesterol and elevated triglyceride levels, although familial dysbetalipoproteinemia can also present as isolated hypertriglyceridemia or hypercholesterolemia 24, 25. Furthermore, an increased ratio of very low-density lipoproteins (VLDL) to plasma triglycerides is also suggestive of familial dysbetalipoproteinemia.
A test known as electrophoresis may be used to show abnormal lipoproteins 8. Electrophoresis is a laboratory test that measures protein levels in the blood or urine by using an electric current to separate proteins by molecular size.
Genetic testing with Next Generation Sequencing of the APOE gene can confirm diagnosis of familial dysbetalipoproteinemia. Many laboratories can perform APOE genotyping for the common isoforms in the APOE gene (e2, e3, or e4). If genetic testing identifies two e2 versions of the APOE gene in an individual who is experiencing symptoms (xanthomas, high cholesterol and triglycerides), then a diagnosis of familial dysbetalipoproteinemia can be made.
The most important treatment for familial dysbetalipoproteinemia is a heart-healthy lifestyle, which includes:
- A heart-healthy diet, which limits the amount of saturated and trans fats that you eat. It encourages you to choose a variety of nutritious foods, including fruits, vegetables, whole grains, and lean meats. Healthy-eating plans include the Dietary Approaches to Stop Hypertension (DASH) diet and the Mediterranean diet, emphasize eating vegetables, fruits, high-fiber whole grains and lean protein. Healthy-eating plans tend to recommend limiting sugar-sweetened beverages, alcohol, salt, sugar and fat, especially saturated fat and trans fat.
- Limiting saturated fats found in fatty cuts of meats, dairy products, and desserts
- Eating whole grains, fruits, and vegetables rather than refined carbohydrates such as sweets and other high-sugar foods
- Eating a variety of nuts
- Preparing foods with little or no salt
- Getting regular physical activity. Studies have shown that physical activity can lower LDL “bad” cholesterol and triglycerides and raise your “good” HDL cholesterol. For example, resistance training among postmenopausal women may decrease total cholesterol, LDL cholesterol, and triglycerides. Aim for at least 30 minutes of exercise on most days. It’s recommended that you do at least 150 minutes of moderate intensity exercise per week. Moderate exercise is when you feel warm and comfortably breathless like when walking or pushing a lawn mower. Intense exercise is when you breathe hard and fast like when running, swimming or cycling. The recommended types of exercise for improving heart health are:
- Aerobic exercise – when you’re moving your body in a way that makes you warm and slightly out of breath like when walking, cycling, doing housework or gardening. Over time, this type of exercise helps your heart and circulatory system to work better by helping to lower your blood pressure and resting heart rate, improving cholesterol levels and helping you maintain a healthy weight
- Balance and flexibility exercise – exercise like yoga, tai chi and Pilates where we hold our bodies in less stable positions. These exercises make sure our muscles don’t get too tight and keep us flexible, helping avoid pain or injury and reduce the risk of having falls
- Resistance exercise – resistance training like lifting weights or using resistance bands and cables to strengthen your muscles. The stronger your muscles are, the harder they can work which takes the strain off your heart making it easier to do everyday tasks. Check in with your doctor before you start any resistance training as it may not be suitable for some people with heart conditions.
- Aiming for a healthy weight. Research has shown that adults with overweight and obesity can lower “bad” LDL cholesterol and raise “good” HDL cholesterol by losing only 3% to 5% of their weight. Losing 7% of your body weight can reduce insulin resistance and blood pressure and decrease your risk of diabetes. Mateo-Gallego et al. 26 showed that a weight loss of 5% of total weight in overweight adults with familial combined hyperlipidemia significantly reduces triglyceride and non-HDL cholesterol levels at 3 and 6 months. This justifies the role of weight loss in overweight patients with familial combined hyperlipidemia to complement lipid-lowering therapy in familial combined hyperlipidemia 27. In fact, any amount of weight loss is beneficial. It’s also important to maintain your weight loss. If you’re struggling with losing weight and keeping it off, talk to your doctor about what options might be available to help you, such as medications or weight-loss surgery.
- Managing stress. Research has shown that chronic stress can sometimes increase LDL cholesterol levels and decrease HDL cholesterol levels. Physical activity, meditation, yoga and other programs can help you handle stress and improve your emotional and physical health.
- Quitting smoking. If you smoke, quit. Smoking can raise your risk of heart disease and heart attack and worsen other heart disease risk factors. Talk with your doctor about programs and products that can help you quit smoking. Also, try to avoid secondhand smoke.
- Getting enough good quality sleep. Getting 7 to 9 hours of sleep a day lowers your risk for high “bad” cholesterol (LDL) and total cholesterol.
- Limiting alcohol. Visit the National Institute on Alcohol Abuse and Alcoholism for resources on support and treatment to stop drinking.
If making lifestyle changes is not enough, you may need to take medicines. Your doctor might suggest medications to help control your blood pressure, cholesterol and blood sugar levels. Drugs that have shown to be effective for reducing lipid levels include statin, fibrates, and nicotinic acid 28. Bile-acid-binding resins, such as cholestyramine (Prevalite), colesevelam (Welchol) and colestipol (Colestid) are not effective for the treatment of familial dysbetalipoproteinemia; they may actually raise blood levels of beta-lipoproteins.
- Statins. Statins are the most common medicine used to treat high blood cholesterol. Statins reduce the amount of cholesterol made in your liver. Studies have shown that statins lower the risk of heart attack and stroke in people with high LDL cholesterol. Statins usually don’t cause side effects, but they may raise the risk of diabetes. However, this mainly happens in people already at high risk of diabetes, such as those who have prediabetes, overweight or obesity, or metabolic syndrome. Statins may also cause abnormal results on liver enzymes tests, but actual liver damage is extremely rare. Other rare side effects include muscle damage and cognitive impairment.
- Cholesterol absorption inhibitors. Your small intestine absorbs the cholesterol from your diet and releases it into your bloodstream. The drug ezetimibe (Zetia) helps reduce blood cholesterol by limiting the absorption of dietary cholesterol. Ezetimibe can be used with a statin drug.
- Bempedoic acid. This newer drug works in much the same way as statins but is less likely to cause muscle pain. Adding bempedoic acid (Nexletol) to a maximum statin dosage can help lower LDL significantly. A combination pill containing both bempedoic acid and ezetimibe (Nexlizet) also is available.
- PCSK9 inhibitors are injected under the skin every few weeks and are expensive. Your liver makes the protein, PCSK9. PCSK9 destroys parts of cells in the liver that allow LDL cholesterol to be absorbed. By stopping the PCSK9 protein, these inhibitors can reduce LDL cholesterol levels. These drugs can help the liver absorb more LDL cholesterol, which lowers the amount of cholesterol circulating in your blood. PCSK9 inhibitors have a strong effect on the lipid profile primarily reducing LDL-cholesterol (-50 to -60%) but also reducing lipoprotein-a (Lp[a]) (-20 to -30%). PCSK9 inhibitors have little effect on triglyceride levels (-12 to -17%) 29, 30. Alirocumab (Praluent) and evolocumab (Repatha) might be used for people who have a genetic condition that causes very high levels of LDL or in people with a history of coronary disease who have intolerance to statins or other cholesterol medications 31, 32. Your cardiologist may prescribe a PCSK9 inhibitor in patients with familial dysbetalipoproteinemia resistant or intolerant to statin and/or fibrate therapy 33. In 2021, the United States Food and Drug Administration (FDA) approved the PCSK9 inhibitor, inclisiran (Leqvio) joining the already approved alirocumab (Praluent), for patients with familial hypercholesterolemia 34. The most common side effects are itching, pain, or swelling at the place where you injected it.
The treatment target in familial dysbetalipoproteinemia is non-HDL cholesterol instead of LDL-cholesterol (LDL-C) 35 because familial dysbetalipoproteinemia patients usually have low to normal LDL-cholesterol levels and normal HDL-cholesterol concentration 36. LDL-cholesterol is low because the conversion of very low-density lipoprotein (VLDL) to LDL-cholesterol is decreased 37. Studies in familial dysbetalipoproteinemia patients that compared statin to fibrate therapy found that statins lower LDL-cholesterol (LDL-C) levels and, to a lesser degree, triglycerides (TG), but do not increase HDL cholesterol, while fibrates improve triglycerides (TG) and HDL cholesterol, but not LDL-cholesterol (LDL-C) 38, 39, 40. Both statins and fibrates reduce apoB, although statins reduce apoB more effectively (33% vs. 17%) 40. Adding a fibrate to statin therapy in familial dysbetalipoproteinemia patients resulted in improved fasting levels of total cholesterol (TC), triglycerides (TG), and HDL cholesterol compared with statin monotherapy, but these changes were nonsignificant 41, 42, which was probably due to insufficient power because fibrate was only added in patients who did not sufficiently respond to statin monotherapy. The European Society of Cardiology and European Atherosclerosis Society Guidelines guidelines mentioned that in familial dysbetalipoproteinemia “most cases respond well to treatment with a statin or, if dominated by high triglycerides (TG), a fibrate” and that “often a combination of a statin and a fibrate may be needed” 43.
The recommended treatment in guidelines for familial dysbetalipoproteinemia is statin-fibrate combination therapy because statins increase hepatic LDL uptake, reduce VLDL production, and decrease cardiovascular disease risk, while fibrates reduce triglycerides (TG) 44, 43. However, in clinical practice only 10% of the familial dysbetalipoproteinemia patients are treated with statin/fibrate combination 35, which is probably due to an ongoing debate about the efficacy of fibrate therapy in reducing clinical endpoints in patients with type 2 diabetes 45 and the risk of adverse events, such as myalgia and rhabdomyolysis during statin/fibrate combination therapy 46. Furthermore, clinical trial evidence to substantiate treatment decisions in familial dysbetalipoproteinemia is scarce. Two clinical trials that included a total of 31 familial dysbetalipoproteinemia patients showed that statin/fibrate combination therapy decreased fasting levels of total cholesterol, triglycerides, and VLDL-cholesterol (VLDL-C) in 12 patients that remained hypercholesterolemic on monotherapy with either fibrate or statin 41, 42. With regard to after meal lipids, a study that evaluated the effect of statins on after meal fat clearance in five familial dysbetalipoproteinemia patients showed significant reductions in both VLDL synthesis and absolute cholesterol absorption compared with no statin treatment, but no improvement in the delayed after meal fat clearance 47.
Xanthomas can sometimes be removed surgically. Cardiovascular disease is treated according to the symptoms that present. Because estrogen improves the clearance of specific lipids associated with familial dysbetalipoproteinemia from the bloodstream, estrogen therapy may help some postmenopausal women with this disorder.
Genetic counseling is recommended for people with familial dysbetalipoproteinemia and their families. Other treatment is symptomatic and supportive.
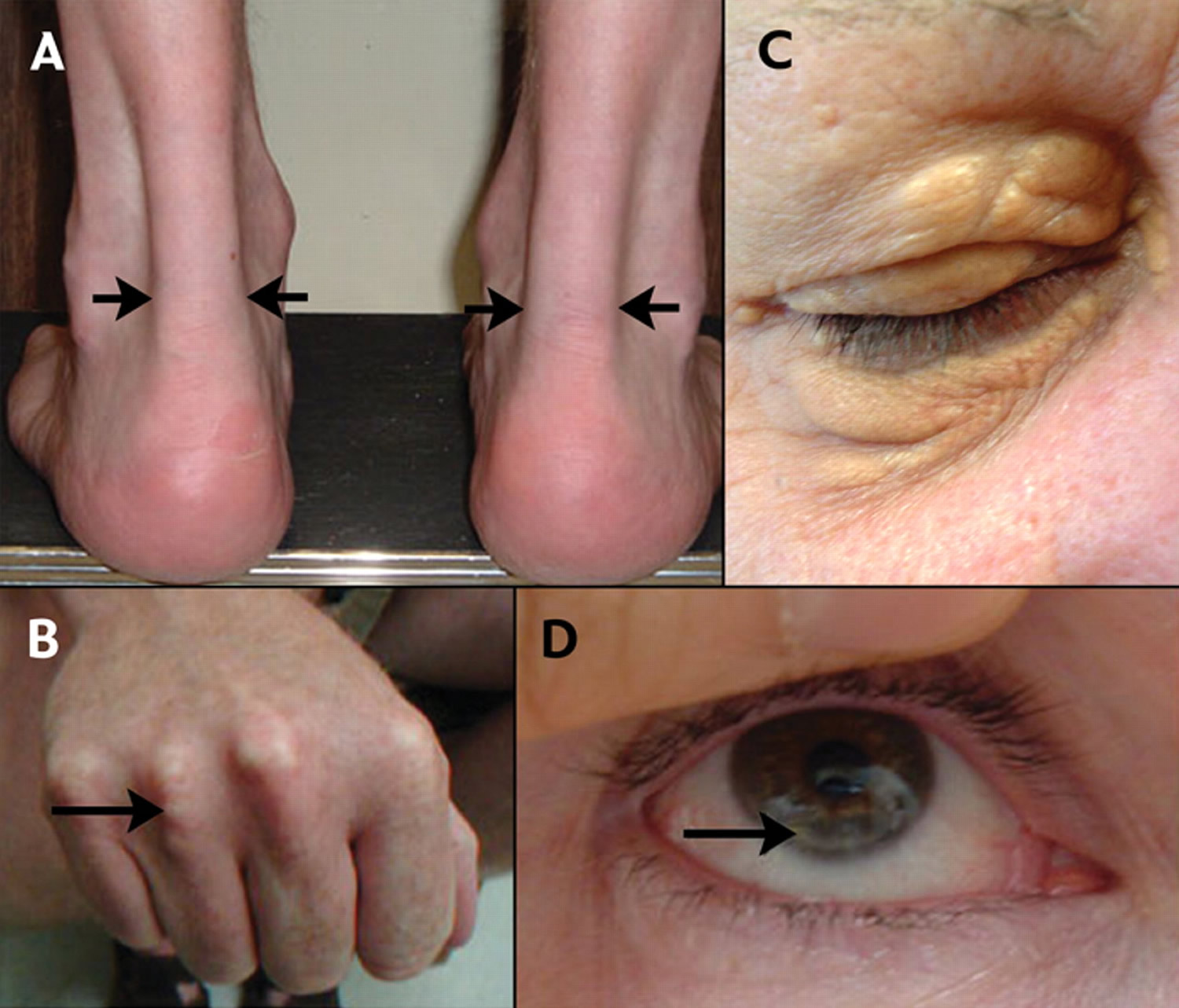
Figure 1. Xanthomas in familial hypercholesterolemia
Footnotes: Xanthomas are lesions characterized by accumulations of lipid-laden macrophages. Xanthomas are commonly associated with all types of hyperlipidemia. (A) Lateral borders of thickened Achilles’ tendons are shown with arrows due to cutaneous deposits of cholesterol, called xanthoma, in the Achilles tendon. (B) Tendinous xanthomas can also occur in the extensor tendons of the hands (shown), feet, elbows and knees. (C) Xanthelasmas are cholesterol deposits in the eyelids. (D) Arcus cornealis (corneal arcus) is a greyish-white ring of cholesterol infiltration around the corneal rim (arrow). A corneal arcus before the age of 45 is also a significant indicator to strongly suspect familial hypercholesterolemia.
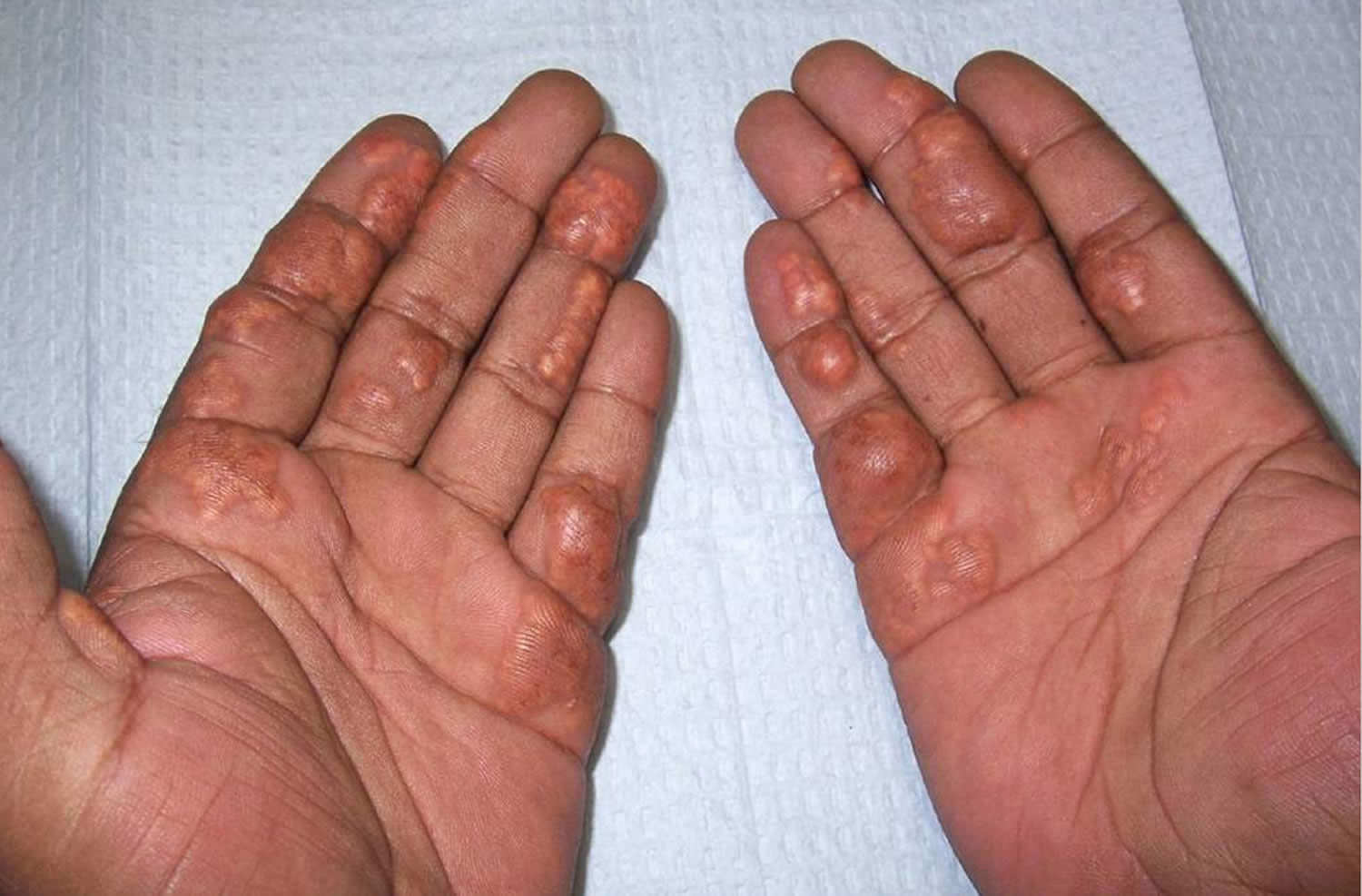
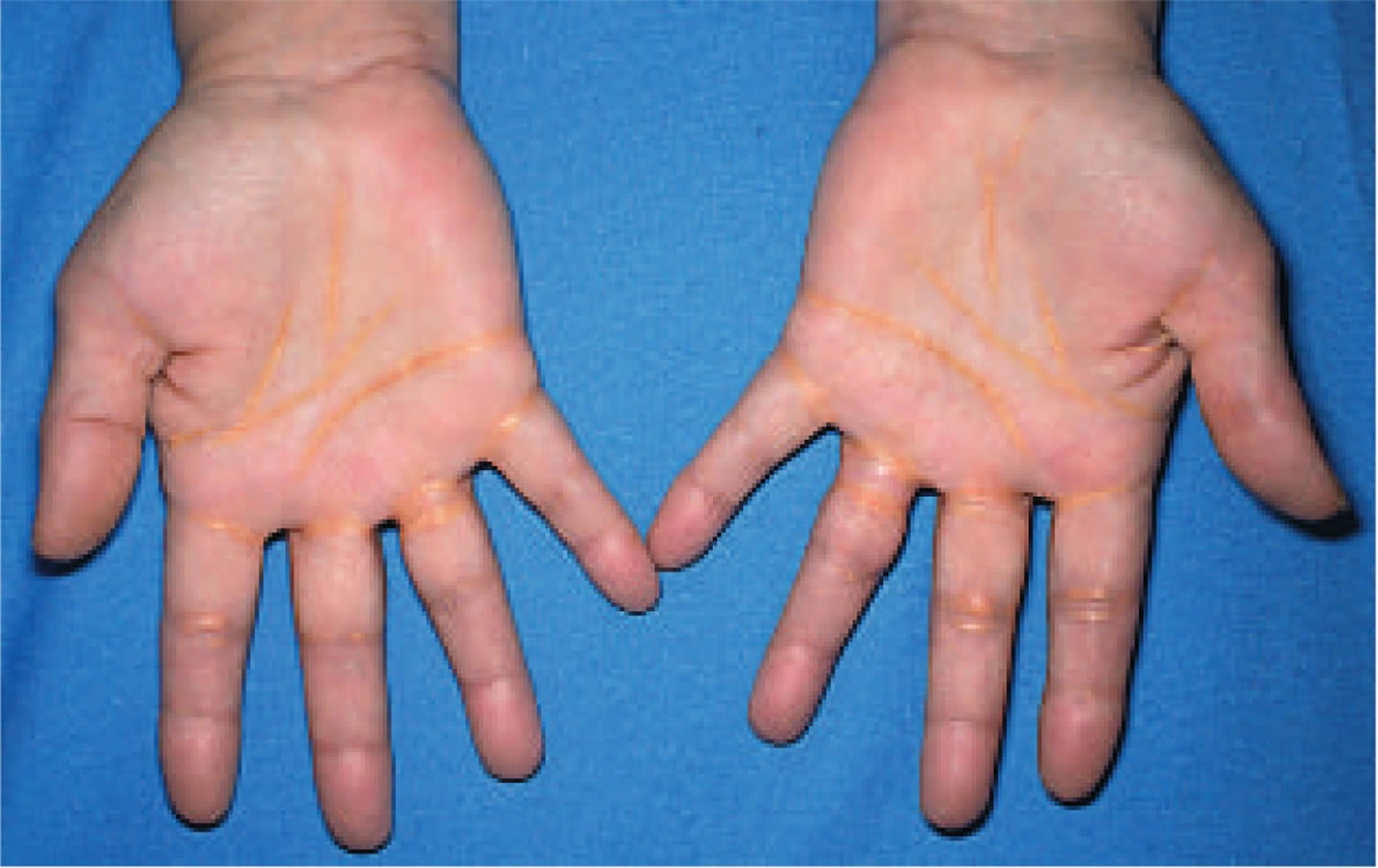
Figure 2. Palmar xanthoma (palmar crease xanthoma)
Footnotes: Palmar xanthomas commonly referred to as xanthoma striatum palmare typically appear as orange-yellow plaques along the plamar creases. These can also be present along the wrist creases.
[Source 18 ]Figure 3. Palmar xanthoma in familial dysbetalipoproteinemia
[Source 23 ]Familial dysbetalipoproteinemia causes
Familial dysbetalipoproteinemia is a genetic condition caused by changes in the Apolipoproteins E (APOE) gene 9. The APOE gene provides instructions for making a protein called apolipoprotein E. This protein combines with fats (lipids) in the body to form molecules called lipoproteins. Lipoproteins are responsible for packaging cholesterol and other fats, carrying them through the bloodstream, and helping clear them from the bloodstream.
There are different versions (alleles) of the APOE gene. The major versions are called e2, e3, and e4 48. Every person has two copies of the APOE gene in some combination of these different versions. As a result, there are three homozygous (e2e2, e3e3, and e4e4) and three heterozygous (e2e3, e3e4, and e2e4) haplotypes 48. The most common version is e3, which is found in more than half of the general population. Most familial dysbetalipoproteinemia patients are homozygous for apolipoprotein e2 (two copies of the APO e2 [e2e2 genotype]), which is associated with decreased binding of apolipoprotein E (APOE) to the LDL receptor 1. ApoE2 has the lowest affinity to the LDL-receptor of all apoE isoforms and clears dietary fats from the body at a slower rate than Apo e3 49. This is why in APO e2 (e2e2 genotype) patients triglyceride- and cholesterol-rich lipoproteins (remnant particles) accumulate in their plasma. Also, the APOE gene is associated with Alzheimer’s disease. However, individuals with two copies of the APOE e2 (e2e2) variant have a low risk to develop Alzheimer’s disease.
The presence of two APOE e2 (e2e2) genes by itself usually does not result in the development of symptoms. In fact, only about 10-15 percent of individuals with two copies of the APOE e2 (e2e2) genetic variant develop outward symptoms of familial dysbetalipoproteinemia. Researchers believe that additional genetic, environmental, or hormonal factors play a role in the development of familial dysbetalipoproteinemia. These factors may include the presence of other disorders (e.g., hypothyroidism, insulin resistance, diabetes), obesity, or age 50, 51, 52, 53, 54. If this secondary factor can be eliminated, lipid profiles may become “normal” again 33. In women, low estrogen levels may contribute to the development of symptoms, which is why familial dysbetalipoproteinemia occurs in women after menopause or in pregnancy 50, 55, 56. Of people with homozygous (two copies of the APOE e2 [e2e2]) genotype, 15% develops familial dysbetalipoproteinemia, which is associated with secondary risk factors such as obesity and insulin resistance, inhibition or even degradation of the heparan sulfate proteoglycan receptor can occur, leading to the accumulation of remnant lipoproteins and the development of familial dysbetalipoproteinemia 50.
It was also shown that around 10% of patients could have the autosomal dominant familial dysbetalipoproteinemia associated with a single copy of the defective APOE allele. Approximately 30 APOE variants associated with the autosomal dominant familial dysbetalipoproteinemia have been reported 57, 58, 59. However, there are insufficient data regarding the relationship between the described APOE variants and familial dysbetalipoproteinemia 59.
Familial dysbetalipoproteinemia inheritance pattern
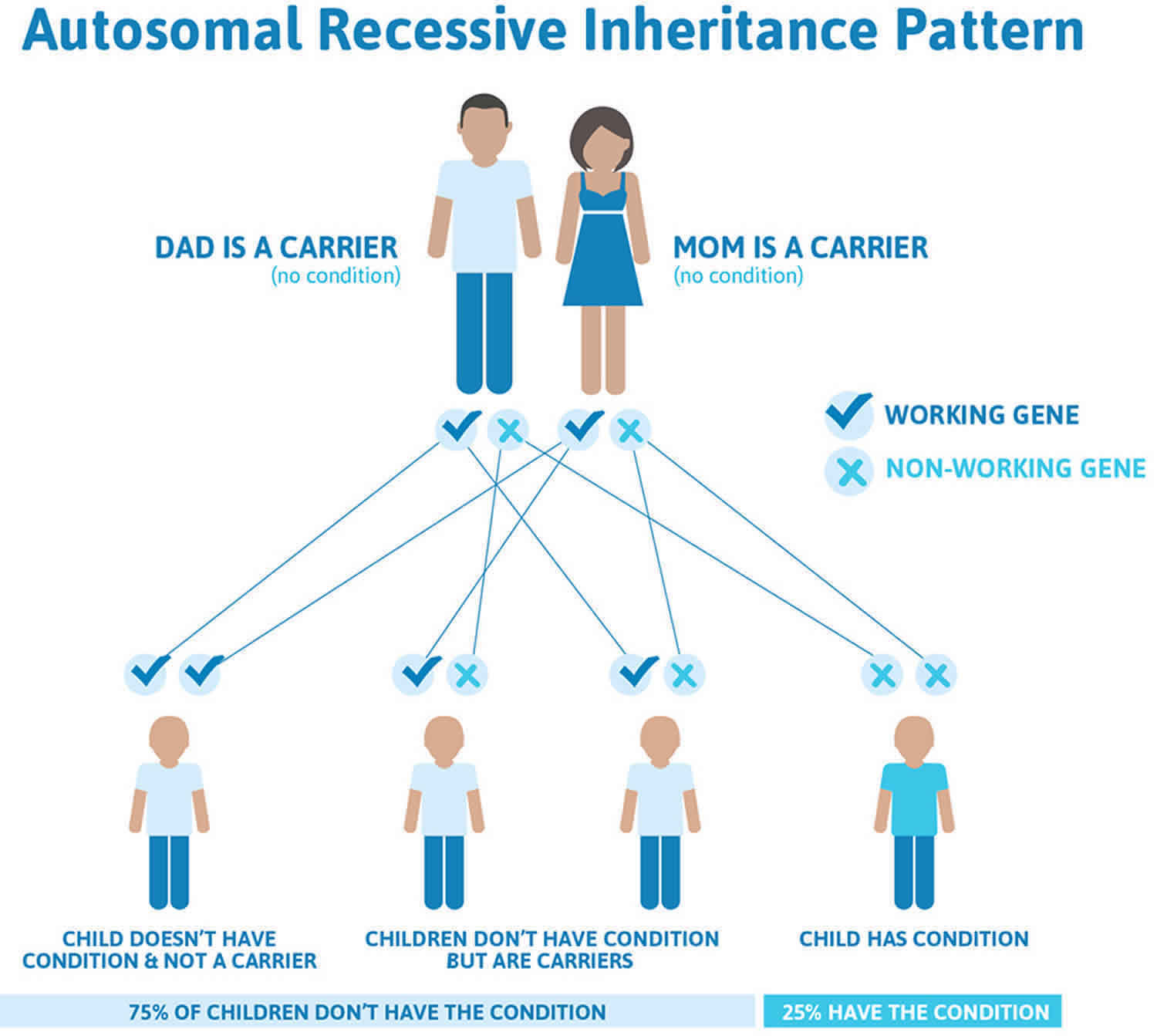
Familial dysbetalipoproteinemia is most often (more than 90% of cases) inherited in an autosomal recessive pattern 60. Most genetic diseases are determined by the status of the two copies of a gene, one received from the father and one from the mother. Recessive genetic disorders occur when an individual inherits two copies of an abnormal gene for the same trait, one from each parent. If an individual inherits one normal gene and one gene for the disease, the person will be a carrier for the disease but usually will not show symptoms. The risk for two carrier parents to both pass the altered gene and have an affected child is 25% with each pregnancy. The risk to have a child who is a carrier like the parents is 50% with each pregnancy. The chance for a child to receive normal genes from both parents is 25%. The risk is the same for males and females.
Parents who are close blood relatives (consanguineous) have a higher chance than unrelated parents to both carry the same abnormal gene, which increases the risk to have children with a recessive genetic disorder.
About 10% of familial dysbetalipoproteinemia is caused by versions of the APOE gene that are inherited in an autosomal dominant pattern. Dominant genetic disorders occur when only a single copy of an abnormal gene is necessary to cause a particular disease. The abnormal gene can be inherited from either parent. The risk of passing the abnormal gene from an affected parent to an offspring is 50% for each pregnancy. The risk is the same for males and females.
In some individuals, familial dysbetalipoproteinemia is due to a spontaneous (de novo) genetic mutation that occurs in the egg or sperm cell. In such situations, familial dysbetalipoproteinemia is not inherited from the parents.
Individuals with the dominant forms of familial dysbetalipoproteinemia may experience symptoms from birth. Additional genetic, environmental and hormonal factors may determine the severity of the disorder.
Figure 4. Familial dysbetalipoproteinemia an autosomal recessive pattern
Familial dysbetalipoproteinemia prevention
Familial dysbetalipoproteinemia is caused by the APOE gene mutation that’s passed down from one or both parents. People who have familial dysbetalipoproteinemia are born with it. This change prevents the body from ridding itself of the type of cholesterol that can build up in the arteries and cause premature heart disease. People who have familial dysbetalipoproteinemia have a higher risk of heart disease and death at a younger age.
Healthy-lifestyle habits also can help reduce your risk of heart disease, and some may lower your cholesterol:
- Lose extra pounds. Losing weight can help lower cholesterol.
- Eat a heart-healthy diet. Focus on plant-based foods, including fruits, vegetables and whole grains. Limit saturated fats and trans fats (such as red meat and most dairy products). Choose healthier fats. This includes lean meats, avocados, nuts, and low-fat dairy items. Avoid foods that contain trans fat (such as fried and packaged foods). Look for foods that are rich in omega-3 fatty acids. These foods include salmon, herring, walnuts, and almonds. Some egg brands contain omega-3.
- Exercise regularly. Aim for at least 30 minutes of moderate-intensity exercise five times a week.
- Don’t smoke. If you smoke, find a way to quit.
Familial dysbetalipoproteinemia symptoms
The symptoms of familial dysbetalipoproteinemia may vary from person to person. Some individuals may not show any symptoms (asymptomatic). Symptoms of familial dysbetalipoproteinemia often do not appear unless additional conditions are present such as diabetes, obesity or underactive thyroid (hypothyroidism) 17. The most common feature associated with familial dysbetalipoproteinemia is the development of xanthomas, which are deposits of fatty materials (lipids) in the skin and appear as multiple yellow bumps (papules) on or just beneath the skin. Xanthomas may form on different parts of the body including the hands, elbows, knees, knuckles, arms, legs, and buttocks. Although very rare, finding an xanthomas on the palms of the hands, a condition called xanthoma striata palmaris, on physical examination of the patient, is considered pathognomonic of familial dysbetalipoproteinemia and has not been reported in any other disorder 18, 19, 20, 21, 22, 23. Xanthomas can also develop within the tendons of the rear lower legs (Achilles tendon) and occasionally on the fingers. Some affected individuals may have fatty deposits within the corneas of the eyes (arcus lidus corneae). Of the 10-15% of people who develop symptoms, this most often happens in early adulthood. Most people begin to experience symptoms in early adulthood, although some individuals have symptoms beginning in childhood or late adulthood. Women are rarely affected until after menopause.
The risk for developing coronary artery disease is 5-10 times higher for an individual with familial dysbetalipoproteinemia compared to the general population. Individuals with familial dysbetalipoproteinemia may develop thickening and blockage of various blood vessels (atherosclerosis) due to the buildup of fatty material (lipids). Atherosclerosis may result in coronary heart disease or peripheral vascular disease. Coronary heart disease results from blockage of the blood supply to the heart potentially resulting in chest pain (angina) and heart attack. Peripheral artery disease is a general term for disease of the blood vessels outside of the heart and brain. It results from blockage of the blood flow to various organs and the extremities. Decreased blood flow to your legs may result in cramping and cause a limp (claudication). Some individuals may have an abnormally enlarged liver or spleen (hepatosplenomegaly).
Individuals with familial dysbetalipoproteinemia may eventually develop inflammation of the pancreas (pancreatitis). Chronic pancreatitis may result in back pain, diarrhea, yellow-colored skin (jaundice), and potentially the development of diabetes. Pancreatitis can also lead to the development of pancreatic cancer.
Familial dysbetalipoproteinemia diagnosis
There are usually no signs or symptoms that you have familial dysbetalipoproteinemia. Familial dysbetalipoproteinemia diagnosis can be made based upon a thorough clinical evaluation, a detailed patient and family history and identification of characteristic findings such as xanthomas. Arterial imaging and a cardiac stress test can identify signs of silent atherosclerosis in young adults.
A blood test called a lipid panel or lipid profile can measure your cholesterol levels typically reports:
- Low-density lipoprotein (LDL) cholesterol is sometimes called “bad” cholesterol because a high LDL level leads to the buildup of plaque in your arteries.
- High-density lipoprotein (HDL) cholesterol is sometimes called “good” cholesterol because it helps your body get rid of cholesterol. HDL carries cholesterol from other parts of your body back to your liver. Your liver then removes the cholesterol from your body.
- Total cholesterol = HDL + LDL + 20% triglycerides. “Total cholesterol” is the total amount of cholesterol that’s circulating in your blood.
- Very low-density lipoprotein (VLDL) cholesterol is sometimes called “bad” cholesterol because it too contributes to the buildup of plaque in your arteries. But VLDL and LDL are different; VLDL mainly carries triglycerides and LDL mainly carries cholesterol.
- Triglycerides. Triglycerides are the most common type of fat in your body. Triglycerides come from foods, especially butter, oils, and other fats you eat. Triglycerides also come from extra calories your body does not need right away. Unused calories (energy you don’t need right away) are stored as triglycerides in fat cells, thus triglycerides is a major source of energy for your body. When your body needs energy, it releases the triglycerides. In between meals, triglycerides are released from fat tissue to be used as an energy source for your body. Most triglycerides are carried in the blood by lipoproteins called very low-density lipoproteins (VLDL).
In familial dysbetalipoproteinemia the lipid profile showed mixed hyperlipidemia with high total cholesterol, elevated LDL cholesterol and elevated triglyceride levels, although familial dysbetalipoproteinemia can also present as isolated hypertriglyceridemia or hypercholesterolemia 24, 25. Furthermore, an increased ratio of very low-density lipoproteins (VLDL) to plasma triglycerides is also suggestive of familial dysbetalipoproteinemia.
A test known as electrophoresis may be used to show abnormal lipoproteins 8. Electrophoresis is a laboratory test that measures protein levels in the blood or urine by using an electric current to separate proteins by molecular size.
Genetic testing with Next Generation Sequencing of the APOE gene can confirm diagnosis of familial dysbetalipoproteinemia. Many laboratories can perform APOE genotyping for the common isoforms in the APOE gene (e2, e3, or e4). If genetic testing identifies two e2 versions of the APOE gene in an individual who is experiencing symptoms (xanthomas, high cholesterol and triglycerides), then a diagnosis of familial dysbetalipoproteinemia can be made.
Familial dysbetalipoproteinemia treatment
Most individuals with familial dysbetalipoproteinemia respond well to dietary therapy that consists of a diet that is low in cholesterol and saturated fat. The reduction of the intake of dietary cholesterol and other fats generally prevents xanthomas and high lipid levels in your blood (hyperlipidemia). Exercise in addition to dietary therapy may help lower lipid levels.
The most important treatment for familial dysbetalipoproteinemia is a heart-healthy lifestyle, which includes:
- A heart-healthy diet, which limits the amount of saturated and trans fats that you eat. It encourages you to choose a variety of nutritious foods, including fruits, vegetables, whole grains, and lean meats. Healthy-eating plans include the Dietary Approaches to Stop Hypertension (DASH) diet and the Mediterranean diet, emphasize eating vegetables, fruits, high-fiber whole grains and lean protein. Healthy-eating plans tend to recommend limiting sugar-sweetened beverages, alcohol, salt, sugar and fat, especially saturated fat and trans fat.
- Limiting saturated fats found in fatty cuts of meats, dairy products, and desserts
- Eating whole grains, fruits, and vegetables rather than refined carbohydrates such as sweets and other high-sugar foods
- Eating a variety of nuts
- Preparing foods with little or no salt
- Getting regular physical activity. Studies have shown that physical activity can lower LDL “bad” cholesterol and triglycerides and raise your “good” HDL cholesterol. For example, resistance training among postmenopausal women may decrease total cholesterol, LDL cholesterol, and triglycerides. Aim for at least 30 minutes of exercise on most days. It’s recommended that you do at least 150 minutes of moderate intensity exercise per week. Moderate exercise is when you feel warm and comfortably breathless like when walking or pushing a lawn mower. Intense exercise is when you breathe hard and fast like when running, swimming or cycling. The recommended types of exercise for improving heart health are:
- Aerobic exercise – when you’re moving your body in a way that makes you warm and slightly out of breath like when walking, cycling, doing housework or gardening. Over time, this type of exercise helps your heart and circulatory system to work better by helping to lower your blood pressure and resting heart rate, improving cholesterol levels and helping you maintain a healthy weight
- Balance and flexibility exercise – exercise like yoga, tai chi and Pilates where we hold our bodies in less stable positions. These exercises make sure our muscles don’t get too tight and keep us flexible, helping avoid pain or injury and reduce the risk of having falls
- Resistance exercise – resistance training like lifting weights or using resistance bands and cables to strengthen your muscles. The stronger your muscles are, the harder they can work which takes the strain off your heart making it easier to do everyday tasks. Check in with your doctor before you start any resistance training as it may not be suitable for some people with heart conditions.
- Aiming for a healthy weight. Research has shown that adults with overweight and obesity can lower “bad” LDL cholesterol and raise “good” HDL cholesterol by losing only 3% to 5% of their weight. Losing 7% of your body weight can reduce insulin resistance and blood pressure and decrease your risk of diabetes. Mateo-Gallego et al. 26 showed that a weight loss of 5% of total weight in overweight adults with familial combined hyperlipidemia significantly reduces triglyceride and non-HDL cholesterol levels at 3 and 6 months. This justifies the role of weight loss in overweight patients with familial combined hyperlipidemia to complement lipid-lowering therapy in familial combined hyperlipidemia 27. In fact, any amount of weight loss is beneficial. It’s also important to maintain your weight loss. If you’re struggling with losing weight and keeping it off, talk to your doctor about what options might be available to help you, such as medications or weight-loss surgery.
- Managing stress. Research has shown that chronic stress can sometimes increase LDL cholesterol levels and decrease HDL cholesterol levels. Physical activity, meditation, yoga and other programs can help you handle stress and improve your emotional and physical health.
- Quitting smoking. If you smoke, quit. Smoking can raise your risk of heart disease and heart attack and worsen other heart disease risk factors. Talk with your doctor about programs and products that can help you quit smoking. Also, try to avoid secondhand smoke.
- Getting enough good quality sleep. Getting 7 to 9 hours of sleep a day lowers your risk for high “bad” cholesterol (LDL) and total cholesterol.
- Limiting alcohol. Visit the National Institute on Alcohol Abuse and Alcoholism for resources on support and treatment to stop drinking.
If making lifestyle changes is not enough, you may need to take medicines. Your doctor might suggest medications to help control your blood pressure, cholesterol and blood sugar levels. Drugs that have shown to be effective for reducing lipid levels include statin, fibrates, and nicotinic acid 28. Bile-acid-binding resins, such as cholestyramine (Prevalite), colesevelam (Welchol) and colestipol (Colestid) are not effective for the treatment of familial dysbetalipoproteinemia; they may actually raise blood levels of beta-lipoproteins.
- Statins. Statins are the most common medicine used to treat high blood cholesterol. Statins reduce the amount of cholesterol made in your liver. Studies have shown that statins lower the risk of heart attack and stroke in people with high LDL cholesterol. Statins usually don’t cause side effects, but they may raise the risk of diabetes. However, this mainly happens in people already at high risk of diabetes, such as those who have prediabetes, overweight or obesity, or metabolic syndrome. Statins may also cause abnormal results on liver enzymes tests, but actual liver damage is extremely rare. Other rare side effects include muscle damage and cognitive impairment.
- Cholesterol absorption inhibitors. Your small intestine absorbs the cholesterol from your diet and releases it into your bloodstream. The drug ezetimibe (Zetia) helps reduce blood cholesterol by limiting the absorption of dietary cholesterol. Ezetimibe can be used with a statin drug.
- Bempedoic acid. This newer drug works in much the same way as statins but is less likely to cause muscle pain. Adding bempedoic acid (Nexletol) to a maximum statin dosage can help lower LDL significantly. A combination pill containing both bempedoic acid and ezetimibe (Nexlizet) also is available.
- PCSK9 inhibitors are injected under the skin every few weeks and are expensive. Your liver makes the protein, PCSK9. PCSK9 destroys parts of cells in the liver that allow LDL cholesterol to be absorbed. By stopping the PCSK9 protein, these inhibitors can reduce LDL cholesterol levels. These drugs can help the liver absorb more LDL cholesterol, which lowers the amount of cholesterol circulating in your blood. PCSK9 inhibitors have a strong effect on the lipid profile primarily reducing LDL-cholesterol (-50 to -60%) but also reducing lipoprotein-a (Lp[a]) (-20 to -30%). PCSK9 inhibitors have little effect on triglyceride levels (-12 to -17%) 29, 30. Alirocumab (Praluent) and evolocumab (Repatha) might be used for people who have a genetic condition that causes very high levels of LDL or in people with a history of coronary disease who have intolerance to statins or other cholesterol medications 31, 32. Your cardiologist may prescribe a PCSK9 inhibitor in patients with familial dysbetalipoproteinemia resistant or intolerant to statin and/or fibrate therapy 33. In 2021, the United States Food and Drug Administration (FDA) approved the PCSK9 inhibitor, inclisiran (Leqvio) joining the already approved alirocumab (Praluent), for patients with familial hypercholesterolemia 34. The most common side effects are itching, pain, or swelling at the place where you injected it.
The treatment target in familial dysbetalipoproteinemia is non-HDL cholesterol instead of LDL-cholesterol (LDL-C) 35 because familial dysbetalipoproteinemia patients usually have low to normal LDL-cholesterol levels and normal HDL-cholesterol concentration 36. LDL-cholesterol is low because the conversion of very low-density lipoprotein (VLDL) to LDL-cholesterol is decreased 37. Studies in familial dysbetalipoproteinemia patients that compared statin to fibrate therapy found that statins lower LDL-cholesterol (LDL-C) levels and, to a lesser degree, triglycerides (TG), but do not increase HDL cholesterol, while fibrates improve triglycerides (TG) and HDL cholesterol, but not LDL-cholesterol (LDL-C) 38, 39, 40. Both statins and fibrates reduce apoB, although statins reduce apoB more effectively (33% vs. 17%) 40. Adding a fibrate to statin therapy in familial dysbetalipoproteinemia patients resulted in improved fasting levels of total cholesterol (TC), triglycerides (TG), and HDL cholesterol compared with statin monotherapy, but these changes were nonsignificant 41, 42, which was probably due to insufficient power because fibrate was only added in patients who did not sufficiently respond to statin monotherapy. The European Society of Cardiology and European Atherosclerosis Society Guidelines guidelines mentioned that in familial dysbetalipoproteinemia “most cases respond well to treatment with a statin or, if dominated by high triglycerides (TG), a fibrate” and that “often a combination of a statin and a fibrate may be needed” 43.
The recommended treatment in guidelines for familial dysbetalipoproteinemia is statin-fibrate combination therapy because statins increase hepatic LDL uptake, reduce VLDL production, and decrease cardiovascular disease risk, while fibrates reduce triglycerides (TG) 44, 43. However, in clinical practice only 10% of the familial dysbetalipoproteinemia patients are treated with statin/fibrate combination 35, which is probably due to an ongoing debate about the efficacy of fibrate therapy in reducing clinical endpoints in patients with type 2 diabetes 45 and the risk of adverse events, such as myalgia and rhabdomyolysis during statin/fibrate combination therapy 46. Furthermore, clinical trial evidence to substantiate treatment decisions in familial dysbetalipoproteinemia is scarce. Two clinical trials that included a total of 31 familial dysbetalipoproteinemia patients showed that statin/fibrate combination therapy decreased fasting levels of total cholesterol, triglycerides, and VLDL-cholesterol (VLDL-C) in 12 patients that remained hypercholesterolemic on monotherapy with either fibrate or statin 41, 42. With regard to after meal lipids, a study that evaluated the effect of statins on after meal fat clearance in five familial dysbetalipoproteinemia patients showed significant reductions in both VLDL synthesis and absolute cholesterol absorption compared with no statin treatment, but no improvement in the delayed after meal fat clearance 47.
Xanthomas can sometimes be removed surgically. Cardiovascular disease is treated according to the symptoms that present. Because estrogen improves the clearance of specific lipids associated with familial dysbetalipoproteinemia from the bloodstream, estrogen therapy may help some postmenopausal women with this disorder.
Genetic counseling is recommended for people with familial dysbetalipoproteinemia and their families. Other treatment is symptomatic and supportive.
- Koopal C, Marais AD, Visseren FL. Familial dysbetalipoproteinemia: an underdiagnosed lipid disorder. Curr Opin Endocrinol Diabetes Obes. 2017 Apr;24(2):133-139. doi: 10.1097/MED.0000000000000316[↩][↩]
- Marais D. Dysbetalipoproteinemia: an extreme disorder of remnant metabolism. Curr Opin Lipidol 2015; 26: 292–297. doi: 10.1097/MOL.0000000000000192[↩]
- Blum C.B. Type III hyperlipoproteinemia: Still worth considering? Prog. Cardiovasc. Dis. 2016;59:119–124. doi: 10.1016/j.pcad.2016.07.007[↩]
- Marais A.D., Solomon G.A., Blom D.J. Dysbetalipoproteinaemia: A mixed hyperlipidaemia of remnant lipoproteins due to mutations in apolipoprotein E. Crit. Rev. Clin. Lab. Sci. 2014;51:46–62. doi: 10.3109/10408363.2013.870526[↩]
- de Beer F, Stalenhoef AF, Hoogerbrugge N, Kastelein JJ, Gevers Leuven JA, van Duijn CM, Havekes LM, Smelt AH. Expression of type III hyperlipoproteinemia in apolipoprotein E2 (Arg158 –> Cys) homozygotes is associated with hyperinsulinemia. Arterioscler Thromb Vasc Biol. 2002 Feb 1;22(2):294-9. doi: 10.1161/hq0202.102919[↩]
- Heidemann BE, Wolters FJ, Kavousi M, Gruppen EG, Dullaart RP, Marais AD, Visseren FL, Koopal C. Adiposity and the development of dyslipidemia in APOE ε2 homozygous subjects: A longitudinal analysis in two population-based cohorts. Atherosclerosis. 2021 May;325:57-62. doi: 10.1016/j.atherosclerosis.2021.04.001[↩]
- Marais D. Dysbetalipoproteinemia: an extreme disorder of remnant metabolism. Curr Opin Lipidol. 2015 Aug;26(4):292-7. doi: 10.1097/MOL.0000000000000192[↩]
- Fredrickson DS, Morganroth J, Levy RI. Type III hyperlipoproteinemia: an analysis of two contemporary definitions. Ann Intern Med. 1975 Feb;82(2):150-7. doi: 10.7326/0003-4819-82-2-150[↩][↩][↩]
- Weintraub MS, Eisenberg S, Breslow JL. Dietary fat clearance in normal subjects is regulated by genetic variation in apolipoprotein E. J Clin Invest 1987; 80: 1571–1577. doi: 10.1172/JCI113243[↩][↩]
- Manchanda Y, Sharma VK. Intertriginous xanthomas: a marker of homozygous type IIa hyperlipoproteinemia. Int J Dermatol. 2004;43(9):676–7. doi: 10.1111/j.1365-4632.2004.01931.x[↩]
- Devaraj S, Vega G, Lange R et al. Remnant-like particle cholesterol levels in patients with dysbetalipoproteinemia or coronary artery disease. Am J Med 1998; 104: 445–450. doi: 10.1016/s0002-9343(98)00089-8[↩]
- Varbo A, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013; 128: 1298–1309. doi: 10.1161/CIRCULATIONAHA.113.003008[↩]
- Hopkins P.N., Nanjee M.N., Wu L.L., McGinty M.G., Brinton E.A., Hunt S.C., Anderson J.L. Altered composition of triglyceride-rich lipoproteins and coronary artery disease in a large case–control study. Atherosclerosis. 2009;207:559–566. doi: 10.1016/j.atherosclerosis.2009.05.016[↩]
- Pallazola V.A., Sathiyakumar V., Park J., Vakil R.M., Toth P.P., Lazo-Elizondo M., Brown E., Quispe R., Guallar E., Banach M., et al. Modern prevalence of dysbetalipoproteinemia (Fredrickson-Levy-Lees type III hyperlipoproteinemia) Arch. Med. Sci. 2019;16:993–1003. doi: 10.5114/aoms.2019.86972[↩]
- Hopkins P.N., Brinton E.A., Nanjee M.N. Hyperlipoproteinemia type 3: The forgotten phenotype. Curr. Atheroscler. Rep. 2014;16:440. doi: 10.1007/s11883-014-0440-2[↩]
- Boot C.S., Luvai A., Neely R.D.G. The clinical and laboratory investigation of dysbetalipoproteinemia. Crit. Rev. Clin. Lab. Sci. 2020;57:458–469. doi: 10.1080/10408363.2020.1745142[↩]
- Feussner G, Ziegler R. Expression of type III hyperlipoproteinaemia in a subject with secondary hypothyroidism bearing the apolipoprotein E2/2 phenotype. J Intern Med. 1991 Aug;230(2):183-6. doi: 10.1111/j.1365-2796.1991.tb00428.x[↩][↩]
- Nagarajan DV, Boreham PA, Parfitt VJ. Palmar striated xanthomas. Postgrad Med J. 2003 Dec;79(938):690. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1742886/pdf/v079p00690.pdf[↩][↩][↩]
- Palmar xanthoma: Mirror to pernicious underlying condition. The Gulf Journal of Dermatology and Venereology Volume 24, No.2, October 2017. http://www.gulfdermajournal.net/pdf/2017-10/6.pdf[↩][↩]
- Rothschild M, Duhon G, Riaz R, Jetty V, Goldenberg N, Glueck CJ, Wang P. Pathognomonic Palmar Crease Xanthomas of Apolipoprotein E2 Homozygosity-Familial Dysbetalipoproteinemia. JAMA Dermatol. 2016 Nov 1;152(11):1275-1276. doi: 10.1001/jamadermatol.2016.2223[↩][↩]
- Blom DJ, Byrnes P, Jones S, Marais AD. Dysbetalipoproteinaemia—clinical and pathophysiological features. S Afr Med J. 2002;92(11):892-897.[↩][↩]
- Morganroth J, Levy RI, Fredrickson DS. The biochemical, clinical, and genetic features of type III hyperlipoproteinemia. Ann Intern Med. 1975 Feb;82(2):158-74. doi: 10.7326/0003-4819-82-2-158[↩][↩]
- Sharma D, Thirkannad S. Palmar xanthoma-an indicator of a more sinister problem. Hand (N Y). 2010 Jun;5(2):210-2. doi: 10.1007/s11552-009-9206-7[↩][↩][↩]
- Heidemann BE, Koopal C, Baass A, Defesche JC, Zuurbier L, Mulder MT, Roeters van Lennep JE, Riksen NP, Boot C, Marais AD, Visseren FLJ. Establishing the relationship between familial dysbetalipoproteinemia and genetic variants in the APOE gene. Clin Genet. 2022 Oct;102(4):253-261. doi: 10.1111/cge.14185[↩][↩]
- Marais D. Dysbetalipoproteinemia: an extreme disorder of remnant metabolism. Curr Opin Lipidol 2015; 26: 292–297. doi: 10.1097/MOL.000000000000019[↩][↩]
- Mateo-Gallego R, Perez-Calahorra S, Cofán M, Baila-Rueda L, Cenarro A, Ros E, Puzo J, Civeira F. Serum lipid responses to weight loss differ between overweight adults with familial hypercholesterolemia and those with familial combined hyperlipidemia. J Nutr. 2014 Aug;144(8):1219-26. doi: 10.3945/jn.114.191775[↩][↩]
- Martin SS, Abd TT, Jones SR, Michos ED, Blumenthal RS, Blaha MJ. 2013 ACC/AHA cholesterol treatment guideline: what was done well and what could be done better. J Am Coll Cardiol. 2014 Jun 24;63(24):2674-8. doi: 10.1016/j.jacc.2014.02.578[↩][↩]
- François Mach, Colin Baigent, Alberico L Catapano, et al. ESC Scientific Document Group, 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS), European Heart Journal, Volume 41, Issue 1, 1 January 2020, Pages 111–188, https://doi.org/10.1093/eurheartj/ehz455[↩][↩]
- Robinson JG, Farnier M, Krempf M et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1489–1499. doi: 10.1056/NEJMoa1501031[↩][↩]
- Sabatine MS, Giugliano RP, Wiviott SD et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1500–1509. doi: 10.1056/NEJMoa1500858[↩][↩]
- Sabatine MS, Giugliano RP, Keech AC et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017; 376: 1713–1722. doi: 10.1056/NEJMoa1615664[↩][↩]
- Schwartz GG, Steg PG, Szarek M et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med 2018; 379: 2097–2107. doi: 10.1056/NEJMoa1801174[↩][↩]
- Waldmann E, Wu L, Busygina K, Altenhofer J, Henze K, Folwaczny A, Parhofer KG. Effect of PCSK9 inhibition with evolocumab on lipoprotein subfractions in familial dysbetalipoproteinemia (type III hyperlipidemia). PLoS One. 2022 Mar 23;17(3):e0265838. doi: 10.1371/journal.pone.0265838[↩][↩][↩]
- FDA approves add-on therapy to lower cholesterol among certain high-risk adults. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-add-therapy-lower-cholesterol-among-certain-high-risk-adults[↩][↩]
- Koopal C, Retterstøl K, Sjouke B, Hovingh GK, Ros E, de Graaf J, Dullaart RP, Bertolini S, Visseren FL. Vascular risk factors, vascular disease, lipids and lipid targets in patients with familial dysbetalipoproteinemia: a European cross-sectional study. Atherosclerosis. 2015 May;240(1):90-7. doi: 10.1016/j.atherosclerosis.2015.02.046[↩][↩][↩][↩]
- Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, Keavney B, Collins R, Wiman B, de Faire U, Danesh J. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007 Sep 19;298(11):1300-11. doi: 10.1001/jama.298.11.1300[↩][↩]
- Mahley RW, Huang Y, Rall SC Jr. Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J Lipid Res. 1999 Nov;40(11):1933-49.[↩][↩]
- Zhao SP, Smelt AH, Van den Maagdenberg AM, Van Tol A, Vroom TF, Gevers Leuven JA, Frants RR, Havekes LM, Van der Laarse A, Van ‘t Hooft FM. Plasma lipoproteins in familial dysbetalipoproteinemia associated with apolipoproteins E2(Arg158–>Cys), E3-Leiden, and E2(Lys146–>Gln), and effects of treatment with simvastatin. Arterioscler Thromb. 1994 Nov;14(11):1705-16. doi: 10.1161/01.atv.14.11.1705[↩][↩]
- Zhao SP, Smelt AH, Leuven JA, Vroom TF, van der Laarse A, van ‘t Hooft FM. Changes of lipoprotein profile in familial dysbetalipoproteinemia with gemfibrozil. Am J Med. 1994 Jan;96(1):49-56. doi: 10.1016/0002-9343(94)90115-5[↩][↩]
- Kawashiri MA, Kobayashi J, Nohara A, Noguchi T, Tada H, Nakanishi C, Inazu A, Mabuchi H, Yamagishi M. Impact of bezafibrate and atorvastatin on lipoprotein subclass in patients with type III hyperlipoproteinemia: result from a crossover study. Clin Chim Acta. 2011 May 12;412(11-12):1068-75. doi: 10.1016/j.cca.2011.02.026[↩][↩][↩][↩]
- Feussner G, Eichinger M, Ziegler R. The influence of simvastatin alone or in combination with gemfibrozil on plasma lipids and lipoproteins in patients with type III hyperlipoproteinemia. Clin Investig. 1992 Nov;70(11):1027-35. doi: 10.1007/BF00180314[↩][↩][↩][↩]
- Illingworth DR, O’Malley JP. The hypolipidemic effects of lovastatin and clofibrate alone and in combination in patients with type III hyperlipoproteinemia. Metabolism. 1990 Apr;39(4):403-9. doi: 10.1016/0026-0495(90)90256-c[↩][↩][↩][↩]
- Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT; ESC Scientific Document Group. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016 Oct 14;37(39):2999-3058. doi: 10.1093/eurheartj/ehw272[↩][↩][↩][↩]
- Guyton JR. Treatment of type III hyperlipoproteinemia. Am Heart J. 1999 Jul;138(1 Pt 1):17-8. doi: 10.1016/s0002-8703(99)70239-5[↩][↩]
- ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010 Apr 29;362(17):1563-74. doi: 10.1056/NEJMoa1001282. Epub 2010 Mar 14. Erratum in: N Engl J Med. 2010 May 6;362(18):1748.[↩][↩]
- Amend KL, Landon J, Thyagarajan V, Niemcryk S, McAfee A. Incidence of hospitalized rhabdomyolysis with statin and fibrate use in an insured US population. Ann Pharmacother. 2011 Oct;45(10):1230-9. doi: 10.1345/aph.1Q110[↩][↩]
- Gylling H, Relas H, Miettinen TA. Postprandial vitamin A and squalene clearances and cholesterol synthesis off and on lovastatin treatment in type III hyperlipoproteinemia. Atherosclerosis. 1995 May;115(1):17-26. doi: 10.1016/0021-9150(94)05495-5[↩][↩]
- Blokhina AV, Ershova AI, Kiseleva AV, Sotnikova EA, Zharikova AA, Zaicenoka M, Vyatkin YV, Ramensky VE, Kutsenko VA, Shalnova SA, Meshkov AN, Drapkina OM. Applicability of Diagnostic Criteria and High Prevalence of Familial Dysbetalipoproteinemia in Russia: A Pilot Study. Int J Mol Sci. 2023 Aug 24;24(17):13159. doi: 10.3390/ijms241713159[↩][↩]
- Phillips MC. Apolipoprotein E isoforms and lipoprotein metabolism. IUBMB Life 2014; 66: 616–623. doi: 10.1002/iub.1314[↩]
- Smelt A.H.M., De Beer F. Apolipoprotein E and familial dysbetalipoproteinemia: Clinical, biochemical, and genetic aspects. Semin. Vasc. Med. 2004;4:249–257. doi: 10.1055/s-2004-861492[↩][↩][↩]
- De Beer F., Stalenhoef A.F.H., Hoogerbrugge N., Kastelein J.J.P., Gevers Leuven J.A., van Duijn C.M., Havekes L.M., Smelt A.H. Expression of type III hyperlipoproteinemia in apolipoprotein E2 (Arg158 → Cys) homozygotes is associated with hyperinsulinemia. Arterioscler. Thromb. Vasc. Biol. 2002;22:294–299. doi: 10.1161/hq0202.102919[↩]
- Heidemann B.E., Wolters F.J., Kavousi M., Gruppen E.G., Dullaart R.P., Marais A.D., Visseren F.L., Koopal C. Adiposity and the development of dyslipidemia in APOE ε2 homozygous subjects: A longitudinal analysis in two population-based cohorts. Atherosclerosis. 2021;325:57–62. doi: 10.1016/j.atherosclerosis.2021.04.001[↩]
- Corsetti J.P., Love T.M., Sparks C.E., Bakker S.J.L., Dullaart R.P.F. Insulin resistance involvement in prevalence of familial dysbetalipoproteinemia in ε2ε2 subjects by Bayesian network modeling. Clin. Biochem. 2018;59:31–36. doi: 10.1016/j.clinbiochem.2018.06.009[↩]
- Koopal C., Retterstøl K., Sjouke B., Hovingh G.K., Ros E., de Graaf J., Dullaart R.P., Bertolini S., Visseren F.L. Vascular risk factors, vascular disease, lipids and lipid targets in patients with familial dysbetalipoproteinemia: A European cross-sectional study. Atherosclerosis. 2015;240:90–97. doi: 10.1016/j.atherosclerosis.2015.02.046[↩]
- Basaran A. Pregnancy-induced hyperlipoproteinemia: Review of the literature. Reprod. Sci. 2009;16:431–437. doi: 10.1177/1933719108330569[↩]
- Chuang T.Y., Chao C.L., Lin B.J., Lu S.C. Gestational hyperlipidemic pancreatitis caused by type III hyperlipoproteinemia with apolipoprotein E2/E2 homozygote. Pancreas. 2009;38:716–717. doi: 10.1097/MPA.0b013e3181ac6dc1[↩]
- Koopal C., Marais A.D., Westerink J., Visseren F.L.J. Autosomal dominant familial dysbetalipoproteinemia: A pathophysiological framework and practical approach to diagnosis and therapy. J. Clin. Lipidol. 2017;11:12–23. doi: 10.1016/j.jacl.2016.10.001[↩]
- Abou K.Y., Rabes J.P., Boileau C., Varret M. APOE gene variants in primary dyslipidemia. Atherosclerosis. 2021;328:11–22. doi: 10.1016/j.atherosclerosis.2021.05.007[↩]
- Heidemann B.E., Koopal C., Baass A., Defesche J.C., Zuurbier L., Mulder M.T., Roeters van Lennep J.E., Riksen N.P., Boot C., Marais A.D., et al. Establishing the relationship between familial dysbetalipoproteinemia and genetic variants in the APOE gene. Clin. Genet. 2022;102:253–261. doi: 10.1111/cge.14185[↩][↩]
- Koopal C., Marais A.D., Visseren F.L.J. Familial dysbetalipoproteinemia: An underdiagnosed lipid disorder. Curr. Opin. Endocrinol. Diabetes Obes. 2017;24:133–139. doi: 10.1097/MED.0000000000000316[↩]