What is formaldehyde
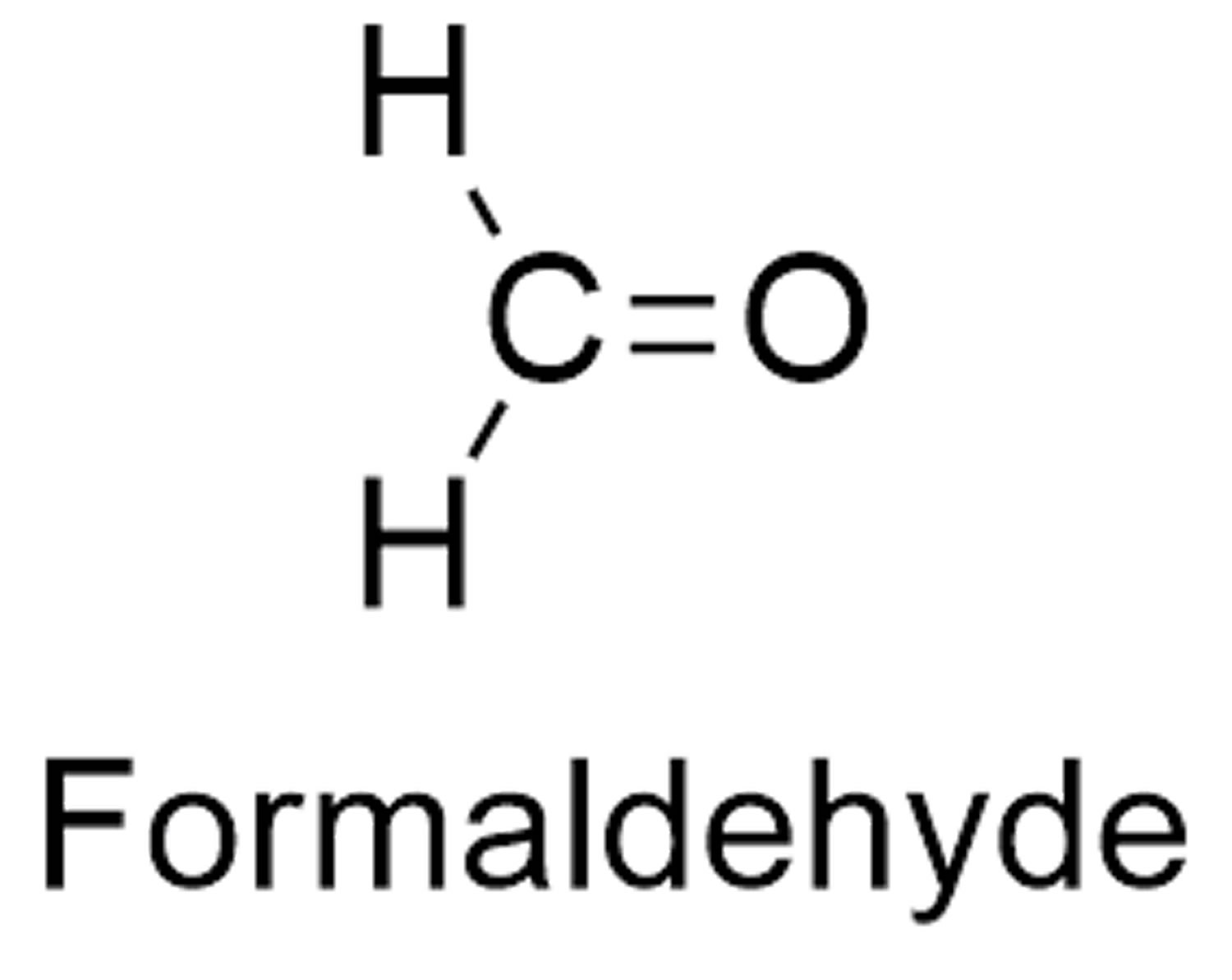
Formaldehyde (CH2O) is also known as methanal, methylene oxide, oxymethylene, methylaldehyde, oxomethane, and formic aldehyde. At room temperature, formaldehyde is a colorless gas with a pungent, irritating and pungent odor, highly flammable gas that is sold commercially as 30–50% (by weight) aqueous solutions 1. Formaldehyde (CH2O) is highly reactive, readily undergoes polymerization, is highly flammable, and can form explosive mixtures in air. Formaldehyde (CH2O) decomposes at temperatures above 150 °C. Formaldehyde is readily soluble in water, alcohols, and other polar solvents. In aqueous solutions, formaldehyde hydrates and polymerizes and can exist as methylene glycol, polyoxymethylene, and hemiformals. Solutions with high concentrations (>30%) of formaldehyde become turbid as the polymer precipitates. As a reactive aldehyde, formaldehyde can undergo a number of self-association reactions, and it can associate with water to form a variety of chemical species with properties different from those of the pure monomolecular substance. These associations tend to be most prevalent at high concentrations of formaldehyde; hence, data on properties at high concentrations are not relevant to dilute conditions.
Formaldehyde is produced worldwide on a large scale. Formaldehyde is produced worldwide on a large scale by catalytic, vapour-phase oxidation of methanol. Formaldehyde is used mainly in the production of resins that are used as adhesives and binders for wood products, pulp, paper, glasswool and rockwool. Formaldehyde is also used extensively in the production of plastics and coatings, in textile finishing and in the manufacture of industrial chemicals. It is used as a disinfectant and preservative (formalin) in many applications.
When formaldehyde is released to or formed in air, most of it degrades, and a very small amount moves into water. When formaldehyde is released into water, it does not move into other media but is broken down. Formaldehyde does not persist in the environment, but its continuous release and formation result in long-term exposure near sources of release and formation.
- The International Agency for Research on Cancer 2, 3 has classified formaldehyde is carcinogenic to humans (Group 1).
- Based on the same evidence, the U.S. National Toxicology Program 13th Report on Carcinogens determined that formaldehyde is known to be a human carcinogen 4. In an earlier assessment, the U.S. EPA IRIS program determined that formaldehyde was a probable human carcinogen, based on limited evidence for cancer in formaldehyde-exposed workers and evidence of nasal tumors in laboratory animals.
Formaldehyde exposure:
- Indoor air may contain formaldehyde from pressed wood products and smoke from cigarettes and poorly vented gas stoves, wood-burning stoves, or kerosene heaters. Workers in mortuaries, hospitals, medical laboratories, or other places that make or use formaldehyde or formaldehyde-containing products may be exposed to higher levels of formaldehyde than the general public. These workers may breathe in formaldehyde or have skin contact with formaldehyde.
- Irritation of the skin, eyes, nose and throat occurred in some individuals with short-term exposure to low concentrations of formaldehyde in air. Some people with asthma were especially sensitive to formaldehyde. Allergic skin reactions have been reported in some formaldehyde-exposed workers. Lightheadedness, dizziness, and incoordination also have been reported by some laboratory technicians exposed to formaldehyde in air. Direct contact with formaldehyde solutions has caused serious eye damage in some cases. Drinking large amounts of formaldehyde solutions can cause severe pain, vomiting, unconsciousness, and possible death. A number of studies of formaldehyde-exposed workers found evidence for increased risk of dying from myeloid leukemia or cancer of the nose or pharynx. Nasal tissue damage and nose tumors were found in laboratory animals who breathed in moderate concentrations of formaldehyde in air for 6 hours per day for most of their lives.
An air quality formaldehyde guideline of 0.1 mg/m³ has been derived based upon the development of nose and throat irritation in humans; this guidance value is to be used with a 30-min averaging time 5. A drinking-water guideline for formaldehyde of 900 µg/litre has been derived based on a no-observed-adverse-effect level (NOAEL) of 15 mg/kg body weight divided by an uncertainty factor of 100, and assuming 20% intake from water 6.
Pure formaldehyde is not available commercially but is sold as 30–50% (by weight) aqueous solutions. Formalin (37% formaldehyde) is the most common solution. Methanol or other substances are usually added to the solution as stabilizers to reduce the intrinsic polymerization of formaldehyde. In solid form, formaldehyde is marketed as trioxane [(CH2O)3] and its polymer paraformaldehyde, with 8–100 units of formaldehyde.
Formaldehyde is ubiquitously present in the environment as a result of natural sources (including forest fires) and from man-made sources, such as automotive and other fuel combustion and industrial on-site uses. The major source of atmospheric formaldehyde is the photochemical oxidation and incomplete combustion of hydrocarbons 7. Secondary formation also occurs, by the oxidation of natural and anthropogenic organic compounds present in air. Motor vehicles are the largest direct human source of formaldehyde in the environment of the source country. The highest concentrations measured in the environment occur near human activities; these are of prime concern for the exposure of humans and other biota. Releases from industrial processes are considerably less. Industrial uses of formaldehyde include the production of resins and fertilizers.
Formaldehyde natural sources
- Formaldehyde occurs naturally in the environment and is the product of many natural processes. It is released during biomass combustion, such as forest and brush fires (Howard, 1989; Reinhardt, 1991). In water, it is also formed by the irradiation of humic substances by sunlight 8.
- As a metabolic intermediate, formaldehyde is present at low levels in most living organisms 9. Formaldehyde is emitted by bacteria, algae, plankton, and vegetation10.
- Human body turnover of formaldehyde was estimated to be approximately 0.61-0.91 mg/kg body weight per minute and 878-1310 mg/kg body weight per day assuming a half life of 1-1.5 min. Compared with formaldehyde turnover and the background levels of formaldehyde from food sources (1.7-1.4 mg/kg body weight per day for a 60-70 kg person), including from dietary methanol, the relative contribution of external formaldehyde from consumption of animal products (milk, meat) from target animals exposed to formaldehyde-treated feed was negligible (<0.001 %). Oral exposure to formaldehyde from
aspartame involves metabolism to methanol and further oxidation to formaldehyde. At the current ADI (acceptable daily intake) of 40 mg/kg body weight per day for aspartame, formaldehyde would be approximately 4 mg/kg body weight per day and represent only 0.3-0.4 % of the endogenous turnover of formaldehyde. Acceptable daily intake (ADI) is a measure of the amount of a specific substance in food or drinking water that can be ingested on a daily basis over a lifetime without an appreciable health risk 11.
Formaldehyde man made sources
Man made sources of formaldehyde include direct sources such as fuel combustion, industrial on-site uses, and off-gassing from building materials and consumer products 12.
Although formaldehyde is not present in gasoline, it is a product of incomplete combustion and is released, as a result, from internal combustion engines. The amount generated depends primarily on the composition of the fuel, the type of engine, the emission control applied, the operating temperature, and the age and state of repair of the vehicle. Therefore, emission rates are variable 13.
Based on data for 1997 reported to the National Pollutant Release Inventory, on-road motor vehicles are the largest direct source of formaldehyde released into the Canadian environment. Data on releases from on-road vehicles were estimated by modelling, based on assumptions outlined in Environment Canada 14. The amount estimated by modelling to have been released in 1997 from on-road motor vehicles was 11,284 tonnes 15. While Environment Canada 15 did not distinguish between gasoline-powered and diesel-powered vehicles, it has been estimated, based on emissions data from these vehicles, that they account for about 40% and 60% of on-road automotive releases, respectively. Aircraft emitted an estimated 1730 tonnes, and the marine sector released about 1175 tonnes 15. It can be expected that the rates of release of formaldehyde from automotive sources have changed and will continue to change; many current and planned modifications to automotive emission control technology and gasoline quality would lead to decreases in the releases of formaldehyde and other volatile organic compounds 15.
Other man made combustion sources (covering a range of fuels from wood to plastics) include wood-burning stoves, fireplaces, furnaces, power plants, agricultural burns, waste incinerators, cigarette smoking, and the cooking of food 16. Cigarette smoking in Canada is estimated to produce less than 84 tonnes per year, based on estimated emission rates 17 and a consumption rate of approximately 50 billion cigarettes per year 18. Canadian coal-based electricity generating plants are estimated to emit 0.7–23 tonnes per year, based on US emission factors 19, the high heating value of fuel, and Canadian coal consumption in 1995. A gross estimate of formaldehyde emissions from municipal, hazardous, and biomedical waste in Canada is 10.6 tonnes per year, based on measured emission rates from one municipal incinerator in Ontario 13.
Industrial releases of formaldehyde can occur at any stage during the production, use, storage, transport, or disposal of products with residual formaldehyde. Formaldehyde has been detected in emissions from chemical manufacturing plants 13, pulp and paper mills, forestry product plants 13, tire and rubber plants (Environment Canada, 1997a), petroleum refining and coal processing plants 20, textile mills, automotive manufacturing plants, and the metal products industry 13.
In Canada, about 1424 tons were released into the environment from industrial sites in 1997, from which about 20 tons per annum were releases to surface waters by 4 sites, with reported releases to different media as follows: 1339.3 tonnes to air, 60.5 tonnes to deep-well injection, 19.4 tonnes to surface water, and 0 tonnes to soil. From 1979 to 1989, about 77 tonnes were spilled in Canada as a result of 35 reported incidents. Releases of formaldehyde to groundwater from embalming fluids in bodies buried in cemeteries are expected to be very small based on groundwater samples and the estimated loading rates of six cemeteries in Ontario 21. The US TRI gives industrial releases of formaldehyde for 1999 with about 6,000 tons per annum to air and about 175 tons per annum to surface waters 22. From the direct use of the substance as e.g. biocide it can be assumed that a very high amount is released into the environment. With an amount of 75,000 to 90,000 t/a worldwide this is a significant pollution source. It can be estimated that formaldehyde contained in consumer products, like cleaning agents is released completely into the wastewater. In addition, reported use of formaldehyde in fish farming and in animal husbandry may lead to a significant environmental exposure.
Formaldehyde has been detected in the off-gassing of formaldehyde products such as wood panels, latex paints, new carpets, textile products, and resins. While emission rates have been estimated for some of these sources, there are insufficient data for estimating total releases 23. In some countries, there have been regulatory and voluntary initiatives to control emissions from building materials and furnishings, since these are recognized as the major sources of elevated concentrations of formaldehyde in indoor air.
Formaldehyde and cancer in humans
The expert working group at the International Agency for Research on Cancer has determined that there is now sufficient evidence that formaldehyde causes nasopharyngeal cancer in humans, a rare cancer in developed countries 2. “Their conclusion that there is adequate data available from humans for an increased risk of a relatively rare form of cancer (nasopharyngeal cancer), and a supporting mechanism, demonstrates the value and strengths of the Monographs Programme,” 24. The International Agency for Research on Cancer working group also found limited evidence for cancer of the nasal cavity and paranasal sinuses and “strong but not sufficient evidence” for leukemia 24, 2. The finding for leukemia reflects the epidemiologists’ finding of strong evidence in human studies coupled with an inability to identify a mechanism for induction of leukemia, based on the data available at this time. The International Agency for Research on Cancer Working Group further stated that “It is
possible that formaldehyde itself can reach the bone marrow following inhalation, although the evidence is inconsistent” 2. Since that time (2006), Zhang et al. 25, reviewed potential pathways by which formaldehyde could act as a leukemogen. Three mechanisms were suggested:
- By damaging stem cells in the bone marrow directly, as most other leukemogens do;
- By damaging hematopoietic stem/progenitor cells circulating in the peripheral blood and
- By damaging the primitive pluri-potent stem cells present within the nasal turbinates and/or olfactory mucosa.
Endogenous formaldehyde turnover in humans
Formaldehyde is an important metabolic intermediate that is physiologically present in all cells at an intracellular concentration of around 12 mg/L (400 μM) 26. There are numerous sources of endogenous formaldehyde including the one carbon pool, amino acid metabolism (serine, glycine, methionine, and choline), methanol metabolism, lipid peroxidation, and P450 dependent demethylation (e.g. O-, N-, and S-methyl) 27. The metabolism of formaldehyde is rapid and catalyzed by glutathione-dependent formaldehyde dehydrogenase (which is also known as alcohol dehydrogenase 5, ADH5) and Sformyl-glutathione hydrolase to formic acid. Formic acid then enters the one-carbon pool where it can be incorporated as a methyl group into nucleic acids and proteins and is either excreted in the urine or oxidised to carbon dioxide and exhaled at a significantly slower rate than its formation from formaldehyde (formic acid halflife in plasma is between 1 and 6 hours) 27. ADH5 has been detected in all human tissues at all stages of development, from embryo through adult. The electrophilic nature of formaldehyde makes it reactive towards a variety of endogenous molecules, including glutathione, proteins, nucleic acids and folic acid 28. Ideally, an evaluation of the fate of formaldehyde in vivo requires a distinction between products which are normal cellular metabolites, those which are detoxification products and those which are formed chemically in localized tissues due to the reactivity of formaldehyde 27.
In order to estimate the synthesis and metabolism of formaldehyde in the human body, authors in the scientific literature have assumed that it is present in all aqueous body fluids because of its water solubility and have estimated its half life in humans as 1-1.5 min 29. Blood and intracellular steady state concentrations of formaldehyde have been estimated in humans to be around 2.6 mg/L (87 μM) and 12 mg/L (400 μM), respectively 29. Based on blood steady state concentrations and half life values in humans of 1-1.5 min, formaldehyde turnover was estimated to be approximately 0.61-0.91mg/kg body weight per minute corresponding to a daily turnover of 878-1310 mg/kg body weight per day 11.
Formaldehyde in food
Background levels in food products of formaldehyde are very variable and range from values around 0.1-0.3 mg/kg in milk to over 200 mg/kg in some fish species 11. Background levels of formaldehyde have been measured in milk, meat, plant material, mushrooms and fish.
- Formaldehyde is present in the milligram range with the lowest level measured in fresh milk (0.013 – < 1 mg/kg) 30.
- In pig tissues, background formaldehyde levels (n=3) has been measured with values of 11.8±0.17, 8.75±0.28, 6.24±0.12 mg/kg in liver, kidney and muscle, respectively 31.
- In meat, background levels of formaldehyde ranged from 2.5 mg/kg in sandwich paste from poultry, 2.9-4.6 mg/kg in cold meat cuts, ham from poultry, turkey, up to 10-20.7 mg/kg in sausages and up to 224-267 mg/kg in the outer layer of smoked ham 30.
- In fish, formaldehyde background levels show the highest values measured in food with extreme values 232-293 mg/kg in deep frozen hake, lowest values in haddock and mullet 1.47-4.87 mg/kg and average values in cod around 100 mg/kg 32. Formaldehyde develops postmortem in marine fish and crustaceans, from the enzymatic reduction of trimethylamine oxide to formaldehyde and dimethylamine 33. While formaldehyde may be formed during the ageing and deterioration of fish flesh, high levels do not accumulate in the fish tissues, due to subsequent conversion of the formaldehyde formed to other chemical compounds 34. However, formaldehyde accumulates during the frozen storage of some fish species, including cod, pollack, and haddock 33. Formaldehyde formed in fish reacts with protein and subsequently causes muscle toughness 35, which suggests that fish containing the highest levels of formaldehyde (e.g., 10–20 mg/kg) may not be considered palatable as a human food source.
- In plant material such as fruit vegetables, background formaldehyde levels (mg/kg) range from 6.3 and 6.8 in apples and carrots, 9.0 in fresh water melon, 9.5 in apricot to 13.3 in tomatoes, 16.3 in bananas, 19.5 in potatoes to 26.9, 31 and 35 in cauliflower, kohlrabi and large beetroot respectively 30.
Higher concentrations of formaldehyde (i.e., up to 800 mg/kg) have been reported in fruit and vegetable juices in Bulgaria 36; however, it is not clear if these elevated levels arise during processing. Formaldehyde is used in the sugar industry to inhibit bacterial growth during juice production 37. In a study conducted by Agriculture Canada, concentrations of formaldehyde were higher in sap from maple trees that had been implanted with paraformaldehyde to deter bacterial growth in tap holes 38. The resulting maple syrup contained concentrations up to 14 mg/kg, compared with less than 1 mg/kg in syrup from untreated trees.
In other processed foods, the highest concentrations (i.e., 267 mg/kg) have been reported in the outer layer of smoked ham 39) and in some varieties of Italian cheese, where formaldehyde is permitted for use under regulation as a bacteriostatic agent 40. Hexamethylenetetramine, a complex of formaldehyde and ammonia that decomposes slowly to its constituents under acid conditions, has been used as a food additive in fish products such as herring and caviar in the Scandinavian countries 41.
Concentrations of formaldehyde in a variety of alcoholic beverages ranged from 0.04 to 1.7 mg/litre in Japan 42 and from 0.02 to 3.8 mg/liter in Brazil (de Andrade et al., 1996). In earlier work conducted in Canada, Lawrence & Iyengar (1983) compared levels of formaldehyde in bottled and canned cola soft drinks (7.4–8.7 mg/kg) and beer (0.1–1.5 mg/kg) and concluded that there was no significant increase in the formaldehyde content of canned beverages due to the plastic inner coating of the metal containers. Concentrations of 3.4 and 4.5 mg/kg in brewed coffee and 10 and 16 mg/kg in instant coffee were reported in the USA 43. These concentrations reflect the levels in the beverages as consumed.
In a more recent study, the concentrations of formaldehyde in commercial 2% milk and in fresh milk from cows fed on a typical North American dairy total mixed diet were determined. Concentrations in the fresh milk (i.e., from Holstein cows, morning milking) ranged from 0.013 to 0.057 mg/kg, with a mean concentration (n = 18) of 0.027 mg/kg, while concentrations in processed milk (i.e., 2% milk fat, partly skimmed, pasteurized) ranged from 0.075 to 0.255 mg/kg, with a mean concentration (n = 12) of 0.164 mg/kg. The somewhat higher concentrations in the commercial 2% milk were attributed to processing technique, packaging, and storage, but these factors were not assessed further 44.
The degree to which formaldehyde in various foods is bioavailable following ingestion is not known.
From these figures, background levels in food products of formaldehyde are very variable and range from values below 1 mg/kg in milk to over 200 mg/kg in some fish species 32. Assuming a person would be eating one kilogram of food per day (including milk, fish, meat, ham, vegetables, fruit) and giving of the variability of background formaldehyde concentration in raw commodities and food products, it was assumed that oral exposure to formaldehyde in humans from dietary sources would not exceed 100 mg formaldehyde per day corresponding to 1.7 and 1.4 mg/kg body weight per day for 60 kg and 70 kg respectively. It should be noted that these exposure estimates do not include endogenously produced formaldehyde from dietary and endogenous sources of methanol. Table 1 summarizes the background levels of formaldehyde in a range of food commodities as described in literature.
Table 1. Background levels of formaldehyde in food
| Food Product | Formaldehyde content mg/kg |
| Meat and poultry | 5.7-20 mg/kg |
| Fish | 6.4-293 mg/kg |
| Milk and milk products | 0.01-0.80 mg/kg |
| Sugar and sweeteners | 0.75 mg/kg |
| Fruit and vegetables | 6-35 mg/kg |
| Coffee | 3.4-16 mg/kg |
| Alcohol beverages | 0.27-3.0 mg/kg |
Considering such wide variability in formaldehyde concentrations and assuming a person would be eating one kilogram of food per day, it was assumed that oral exposure to formaldehyde in humans would not exceed 100 mg formaldehyde per day, corresponding to 1.7 and 1.4 mg/kg body weight per day for 60 kg and 70 kg respectively. Carry over in animal tissues has been measured in a few limited tissue deposition studies, mostly in cows and the data showed a maximum increase in formaldehyde concentration between 0.1-0.2 mg/kg milk or meat. Such levels of formaldehyde resulting from the consumption of milk or meat from animals fed with formaldehyde-supplemented feed would represent 0.1-0.3 % of human oral exposure from background levels in food products and would be below 0.001 % considering the endogenous formaldehyde turnover. Such levels were considered negligible 11.
Based on the analysis of the European Food Safety Authority Scientific Panel on Food Additives and Nutrient Sources added to Food using actual usage data, methanol from aspartame was estimated to contribute to 0.5-9.7 % of the total daily exposure to endogenous and exogenous methanol. For formaldehyde, and assuming exposure to aspartame at the current Acceptable Daily Intake of 40 mg/kg body weight per day together with a 10 % conversion to methanol with further conversion to formaldehyde, a daily exposure of 4 mg/kg body weight per day is estimated. This exposure would only contribute to approximately 0.3-0.4 % of human oral exposure from background levels in food products and endogenous turnover.
Summary
- Formaldehyde is an essential metabolic intermediate present in all cells at an intracellular concentration of around 400 μM (12 mg/L) which is synthesised endogenously. Sources of endogenous formaldehyde include the one carbon pool, amino acid metabolism, methanol metabolism, lipid peroxidation, and P450 dependent demethylation.
- Formaldehyde metabolism is rapid and catalysed by glutathione-dependent formaldehyde dehydrogenase and S-formyl-glutathione hydrolase to formic acid. Formic acid then enters the one-carbon pool and is either excreted in the urine or oxidised to carbon dioxide and exhaled. The electrophilic nature of formaldehyde makes it reactive with a variety of endogenous molecules, including glutathione, proteins, nucleic acids, and folic acid.
- Formaldehyde concentration in the blood of mammals resulting from endogenous production is similar in different species with 2.2, 2.4 and 2.6 mg/L in the rat, monkey and humans respectively. Formaldehyde half life in humans is very short and has been estimated to be within 1-1.5 min associated with a volume of distribution approximately corresponding to total body water (49 L). Total content of formaldehyde in the human body has been estimated assuming its presence in all aqueous body fluids and a range of half-lives of 1 and 1.5 min, daily endogenous turn over of formaldehyde has been estimated to be around 878-1310 mg/kg body weight per day.
- Background levels in food products of formaldehyde are very variable and range from values below 1 mg/kg in milk to over 200 mg/kg in some fish species. Considering the wide variability of formaldehyde concentrations in food, daily exposure to formaldehyde from would not exceed 100 mg per kilogram of food and per person.
- Carry over in animal tissue or products of formaldehyde has been measured in a few limited tissue deposition studies mostly available in cows and showed a maximum increase in concentration between 0.1-0.2 mg/kg milk or meat (muscle). The levels of formaldehyde resulting from the consumption of milk or meat from animals fed with formaldehydesupplemented feed would be around 0.1 % of the background levels of formaldehyde in food products and below 0.001 % of daily endogenous turnover of formaldehyde. Such levels are considered negligible.
- Oral exposure to formaldehyde derived from aspartame-derived methanol at the current acceptable daily intake of aspartame would be 4 mg/kg body weight per day assuming a 10 % conversion. Such exposure only represents 0.3-0.4 % of the combined background level in food and the daily turnover of formaldehyde for an adult.
Formaldehyde exposure
The global production of formaldehyde in 1999 is estimated to be 5 – 6 million tons. Formaldehyde is mainly used as an intermediate in the chemical industry for the production of condensed resins for the wood, paper and textile processing industries and in the synthesis of methylene dianiline, diphenylmethane diisocyanate, hexamethylenetetraamine, trimethylol propane, neopentylglycol, pentaerythritol and acetylenic agents 7. Aqueous solutions of formaldehyde are employed as germicides, bactericides and fungicides 7. The use of formaldehyde as biocide and in other applications is estimated to be 1.5 % of the total production, i.e. 75,000 to 90,000 ton per annum related to the worldwide production amount.
Formaldehyde is also used as a preservative in a large number of consumer products, such cosmetics and household cleaning agents. Tobacco smoke as well as urea-formaldehyde foam insulation and formaldehyde-containing disinfectants are all important sources of formaldehyde exposure. Releases into the environment are likely to occur during production and processing as intermediate as well as from use of products containing the substance.
Since formaldehyde (also a product of intermediary metabolism) is water soluble, highly reactive with biological macromolecules, and rapidly metabolized, adverse effects resulting from exposure are observed primarily in those tissues or organs with which formaldehyde first comes into contact (i.e., the respiratory and aerodigestive tract, including oral and gastrointestinal mucosa, following inhalation or ingestion, respectively) 12.
Sensory irritation of the eyes and respiratory tract by formaldehyde has been observed consistently in clinical studies and epidemiological surveys in occupational and residential environments. 12 At concentrations higher than those generally associated with sensory irritation, formaldehyde may also contribute to the induction of generally small, reversible effects on lung function.
For the general population, dermal exposure to concentrations of formaldehyde, in solution, in the vicinity of 1–2% (10,000–20,000 mg/liter) is likely to cause skin irritation; however, in hypersensitive individuals, contact dermatitis can occur following exposure to formaldehyde at concentrations as low as 0.003% (30 mg/liter) 12. In North America, less than 10% of patients presenting with contact dermatitis may be immunologically hypersensitive to formaldehyde. Although it has been suggested in case reports for some individuals that formaldehyde-induced asthma was attributable to immunological mechanisms, no clear evidence has been identified. However, in studies with laboratory animals, formaldehyde has enhanced their sensitization to inhaled allergens 12.
Following inhalation in laboratory animals, formaldehyde causes degenerative non-neoplastic effects in mice and monkeys and nasal tumours in rats. In vitro, formaldehyde induced DNA–protein crosslinks, DNA single-strand breaks, chromosomal aberrations, sister chromatid exchange, and gene mutations in human and rodent cells. Formaldehyde administered by inhalation or gavage to rats in vivo induced chromosomal anomalies in lung cells and micronuclei in the gastrointestinal mucosa. The results of epidemiological studies in occupationally exposed populations are consistent with a pattern of weak positive responses for genotoxicity, with good evidence of an effect at site of contact (e.g., micronucleated buccal or nasal mucosal cells). Evidence for distal (i.e., systemic) effects is equivocal. Overall, based on studies in both animals and humans, formaldehyde is weakly genotoxic, with good evidence of an effect at site of contact, but less convincing evidence at distal sites. Epidemiological studies taken as a whole do not provide strong evidence for a causal association between formaldehyde exposure and human cancer, although the possibility of increased risk of respiratory cancers, particularly those of the upper respiratory tract, cannot be excluded on the basis of available data. Therefore, based primarily upon data derived from laboratory studies, the inhalation of formaldehyde under conditions that induce cytotoxicity and sustained regenerative proliferation is considered to present a carcinogenic hazard to humans.
Environmental toxicity data are available for a wide range of terrestrial and aquatic organisms. Based on the maximum concentrations measured in air, surface water, effluents, and groundwater in the sample exposure scenario from the source country and on the estimated no-effects values derived from experimental data for terrestrial and aquatic biota, formaldehyde is not likely to cause adverse effects on terrestrial or aquatic organisms.
Consumer products
Formaldehyde and formaldehyde derivatives are present in a wide variety of consumer products 45 to protect the products from spoilage by microbial contamination. Formaldehyde is used as a preservative in household cleaning agents, dishwashing liquids, fabric softeners, shoe care agents, car shampoos and waxes, carpet cleaning agents, etc. 46. Levels of formaldehyde in hand dishwashing liquids and liquid personal cleansing products available in Canada are less than 0.1% (w/w).
Formaldehyde has been used in the cosmetics industry in three principal areas: preservation of cosmetic products and raw materials against microbial contamination, certain cosmetic treatments such as hardening of fingernails, and plant and equipment sanitation 47. Formaldehyde is also used as an antimicrobial agent in hair preparations, lotions (e.g., suntan lotion and dry skin lotion), makeup, and mouthwashes and is also present in hand cream, bath products, mascara and eye makeup, cuticle softeners, nail creams, vaginal deodorants, and shaving cream 37.
Some preservatives are formaldehyde releasers. The release of formaldehyde upon their decomposition is dependent mainly on temperature and pH. Information on product categories and typical concentrations for chemical products containing formaldehyde and formaldehyde releasers was obtained from the Danish Product Register Data Base (PROBAS) by Flyvholm & Andersen (1993). Industrial and household cleaning agents, soaps, shampoos, paints/lacquers, and cutting fluids comprised the most frequent product categories for formaldehyde releasers. The three most frequently registered formaldehyde releasers were bromonitropropanediol, bromonitrodioxane, and chloroallylhexaminium chloride 48.
Formaldehyde is present in the smoke resulting from the combustion of tobacco products. Estimates of emission factors for formaldehyde (e.g., µg/cigarette) from mainstream and sidestream and environmental tobacco smoke have been determined by a number of different protocols for cigarettes in several countries.
A range of mainstream smoke emission factors from 73.8 to 283.8 µg/cigarette was reported for 26 US brands, which included non-filter, filter, and menthol cigarettes of various lengths 49. Differences in concentrations reflect differences in tobacco type and brand. More recent information is available from the British Columbia Ministry of Health from tests conducted on 11 brands of Canadian cigarettes. Mainstream smoke emission factors ranged from 8 to 50 µg/cigarette when tested under standard conditions.
Levels of formaldehyde are higher in sidestream smoke than in mainstream smoke. Guerin et al. 50 reported that popular commercial US cigarettes deliver approximately 1000–2000 µg formaldehyde/cigarette in their sidestream smoke. Schlitt & Knöppel 51 reported a mean (n = 5) formaldehyde content of 2360 µg/cigarette in the sidestream smoke from a single brand in Italy. Information from the British Columbia Ministry of Health from tests conducted on 11 brands of Canadian cigarettes indicates that emission factors from sidestream smoke ranged from 368 to 448 µg/cigarette.
Emission factors for toxic chemicals from environmental tobacco smoke, rather than from mainstream or sidestream smoke, have also been determined. This is in part due to concerns that emission factors for sidestream smoke may be too low for reactive chemicals such as formaldehyde, due to losses in the various apparati used to determine sidestream smoke emission factors. Daisey et al. 52 indicated that environmental tobacco smoke emission factors for formaldehyde from six US commercial cigarettes ranged from 958 to 1880 µg/cigarette, with a mean of 1310 ± 349 µg/cigarette. Data concerning emission factors for formaldehyde from environmental tobacco smoke produced by Canadian cigarettes were not identified.
Clothing and fabrics
Formaldehyde-releasing agents provide crease resistance, dimensional stability, and flame retardance for textiles and serve as binders in textile printing 53. Durable-press resins or permanent-press resins containing formaldehyde have been used on cotton and cotton/polyester blend fabrics since the mid-1920s to impart wrinkle resistance during wear and laundering. Hatch & Maibach (1995) identified nine major resins used. These differ in formaldehyde-releasing potential during wear and use.
Priha (1995) indicated that formaldehyde-based resins, such as UF resin, were once more commonly used for crease resistance treatment; more recently, however, better finishing agents with lower formaldehyde release have been developed. Totally formaldehyde-free crosslinking agents are now available, and some countries have legally limited the formaldehyde content of textile products. In 1990, the percentage of durable-press fabric manufactured in the USA finished with resins rated as having high formaldehyde release was 27%, about one-half the percentage in 1980, according to Hatch & Maibach 54. It has been reported that the average level contained by textiles made in the USA is approximately 100–200 µg free formaldehyde/g 55.
Piletta-Zanin et al. 56 studied the presence of formaldehyde in moist baby toilet tissues and tested 10 of the most frequently sold products in Switzerland. One product contained more than 100 µg/g, five products contained between 30 and 100 µg/g, and the remaining four products contained less than 30 µg formaldehyde/g.
Building materials
The emission of formaldehyde from building materials has long been recognized as a significant source of the elevated concentrations of formaldehyde frequently measured in indoor air. Historically, the most important indoor source among the many materials used in building and construction has been urea-formaldehyde foam insulation, which is produced by the aeration of a mixture of urea-formaldehyde resin and an aqueous surfactant solution containing a curing catalyst 57. Urea-formaldehyde foam insulation was banned from use in Canada in 1980 and in the USA in 1982, although the US ban was subsequently overturned.
Pressed wood products (i.e., particleboard, medium-density fibreboard, and hardwood plywood) are now considered the major sources of residential formaldehyde contamination 58. Pressed wood products are bonded with urea-formaldehyde resin; it is this adhesive portion that is responsible for the emission of formaldehyde into indoor air. The emission rate of formaldehyde is strongly influenced by the nature of the material. Generally, release of formaldehyde is highest from newly made wood products. Emissions then decrease over time, to very low rates, after a period of years 59.
Concentrations of formaldehyde in indoor air are primarily determined by such factors as source strength (i.e., mass of substance released per unit time or per unit area), loading factors (i.e., the ratio of the surface area of a source [e.g., a particleboard panel] to the volume of an enclosed area [e.g., a room] where the source is present), and the presence of source combinations 59. Emission rates for formaldehyde from pressed wood products determined by emission chamber testing in Canada 60 are now typically less than 0.3 mg/m2 per hour 61.
Formaldehyde release from pressed wood materials is greater in mobile homes than in conventional housing, as mobile homes typically have higher loading ratios (e.g., exceeding 1 m2/m³) of these materials. In addition, mobile homes can have minimal ventilation, are minimally insulated, and are often situated in exposed sites subject to temperature extremes 62.
The use of scavengers (e.g., urea) to chemically remove unreacted formaldehyde while the curing process is taking place has been investigated as a control measure. Other reactants could be used to chemically modify the formaldehyde to a non-toxic derivative or convert it to a non-volatile reaction product. There has also been work to effectively seal the resin and prevent the residual formaldehyde from escaping 63. Surface coatings and treatments (e.g., paper and vinyl decorative laminates) can significantly affect the potential for off-gassing and in some cases can result in an order of magnitude reduction in the emission rates for formaldehyde from pressed wood products 64. On the other hand, high emissions of formaldehyde during the curing of some commercially available conversion varnishes (also known as acid-catalyst varnishes) have been reported. An initial emission rate of 29 mg formaldehyde/m² per hour was determined for one product 65.
Emission rates for formaldehyde from carpets and carpet backings, vinyl floorings, and wall coverings in the source country (Canada) are now generally less than 0.1 mg/m² per hour 61.
Formaldehyde uses
Formaldehyde is used mostly to make resins used in building materials, coatings for paper and clothing fabrics, and synthetic fibers. Phenolic, urea, and melamine resins have wide uses as adhesives and binders in the wood-production, pulp-and-paper, and the synthetic vitreousfibre industries, in the production of plastics and coatings, and in textile finishing. Polyacetal resins are widely used in the production of plastics. Formaldehyde is also used extensively as an intermediate in the manufacture of industrial chemicals, such as 1,4-butanediol, 4,4′-methylenediphenyl diisocyanate, penta-erythritol, and hexamethylenetetramine. Formaldehyde is used directly in aqueous solution (known as formalin) as a disinfectant and preservative in many applications 66.
A second major use is as a starting chemical to make other chemicals. It is found in smoke from burning tobacco or fuels. Building materials with formaldehyde include certain insulation materials, glues, and pressed wood products like particle board, plywood, and fiberboard. Products containing urea and formaldehyde are used as slow-release nitrogen fertilizers in farming and gardening. Formaldehyde is also used as a preservative in mortuaries and medical laboratories, and as an antimicrobial agent and disinfectant for industrial processes and some household purposes.
Formaldehyde is used in the animal feed industry, where it is added to ruminant feeds to improve handling characteristics. The food mixture contains less than 1% formaldehyde, and animals may ingest as much as 0.25% formaldehyde in their diet 41. Formalin has been added as a preservative to skim milk fed to pigs in the United Kingdom 67 and to liquid whey (from the manufacture of cheddar and cottage cheeses) fed to calves and cows in Canada. Maximum concentrations in the milk of cows fed whey with the maximum level of formalin tested (i.e., 0.15%) were up to 10-fold greater (i.e., 0.22 mg/kg) than levels in milk from control cows fed whey without added formalin 68.
Formaldehyde toxicity
Extensive recent data are available for concentrations of formaldehyde in air at industrial, urban, suburban, rural, and remote locations in the source country (Canada). There are fewer but still considerable data on concentrations in indoor air, which are higher. Data on concentrations in water are more limited. Although formaldehyde is a natural component of a variety of foodstuffs, monitoring has generally been sporadic and source directed. Based on available data, the highest concentrations of formaldehyde occurring naturally in foods are in some fruits and marine fish. Formaldehyde may also be present in food due to its use as a bacteriostatic agent in production and its addition to animal feed to improve handling characteristics. Formaldehyde and formaldehyde derivatives are also present in a wide variety of consumer products to protect the products from spoilage by microbial contamination. The general population is also exposed during release from combustion (e.g., from cigarettes and cooking) and emission from some building materials, such as pressed wood products.
Formaldehyde is a highly reactive gas that is absorbed quickly at the point of contact and is also produced by endogenous metabolism 7. Formaldehyde is rapidly metabolized, such that exposure to high concentrations (up to 15 ppm in rats) does not result in increased blood concentrations. Repeated formaldehyde exposure caused toxic effects only in the tissues of direct contact after inhalation, oral or dermal exposure characterized by local cytotoxic destruction and subsequent repair of the damage 7. The typical locations of lesions in experimental animals were the nose after inhalation, the stomach after oral administration and the skin after dermal application. The nature of the lesions depended on the inherent abilities of the tissues involved to respond to the noxious event and on the local concentration of the substance. Atrophy and necrosis as well as hyper- and metaplasia of epithelia may occur. The most sensitive No Observed Adverse Effect Levels (NOAELs) for morphological lesions were between 1 and 2 ppm for inhalation exposure and about 260 mg/liter in drinking water 7.
Formaldehyde had acute effects in mammals: LD50 (lethal dose 50 is where 50% of the test subjects die) (rat, oral) 600 – 800 mg/kg body weight, LC50 (lethal concentration 50 is the lethal concentration required to kill 50% of the population) (rat, inhalation, 4 hour) 578 mg/m3 (480 ppm) 7. Inhalation of high concentrations ( > 120 mg/m³) of formaldehyde caused hypersalivation, acute dyspnea, vomiting, muscular spasms, convulsions and finally deaths. Histopathology examination showed respiratory tract irritation, bronchioalveolar constriction and lung oedema. Formaldehyde was irritating to the eyes, and aqueous solutions of formaldehyde (0.1% to 20%) were irritating to the skin of rabbits. Formaldehyde was sensitising in the guinea pig maximization test and the local lymph node assay with mice. On the other hand, specially designed studies (IgE tests, cytokine secretion profiles of lymph node cells) did not reveal evidence of respiratory sensitization in mice.
Formaldehyde is weakly genotoxic and was able to induce gene mutations and chromosomal aberrations in mammalian cells. DNA-protein crosslinks are a sensitive measure of DNA modification by formaldehyde. However, the genotoxic effects were limited to those cells, which are in direct contact with formaldehyde, and no effects could be observed in distant-site tissues. In conclusion, formaldehyde is a direct acting locally effective mutagen 7.
In humans, transient and reversible sensory irritation of the eyes and respiratory tract has been observed in clinical studies and epidemiological surveys 7. Odor threshold for most people ranges between 0.5 and 1 ppm (parts per million). PPM (parts per million) is a way of expressing very dilute concentrations of substances. Parts per million (ppm) means out of a million. Usually describes the concentration of something in water or soil. One ppm is equivalent to 1 milligram of something per liter of water (mg/l) or 1 milligram of something per kilogram soil (mg/kg). In general, eye irritation, the most sensitive endpoint, is associated with airborne concentrations beginning in the range of 0.3 to 0.5 ppm. Eye irritation does not become significant until about 1 ppm, and rapidly subsides. Moderate to severe eye, nose and throat irritation occurs at 2 to 3 ppm 7. Sensory irritation has also been reported at lower exposure levels, but is then difficult to distinguish from background. Most studies show no effect on lung function in either asthmatics or non-asthmatics. Formaldehyde causes skin irritation and has corrosive properties when ingested. In some individuals, contact dermatitis may occur at challenge concentrations as low as 30 ppm 7.
The majority of the general population is exposed to airborne concentrations of formaldehyde less than those associated with sensory irritation (i.e., 0.083 ppm [0.1 mg/m³]). However, in some indoor locations, concentrations may approach those associated with eye and respiratory tract sensory irritation in humans. Risks of cancer estimated on the basis of a biologically motivated case-specific model for calculated exposure of the general population to formaldehyde in air based on the sample exposure scenario for the source country (Canada) are exceedingly low. This model incorporates two-stage clonal growth modelling and is supported by dosimetry calculations from computational fluid dynamics modelling of formaldehyde flux in various regions of the nose and single-path modelling for the lower respiratory tract.
Chronic inhalation of concentrations of 10 ppm and higher led to clear increases in nasal tumor incidence in rats 7. Most of the nasal tumors were squamous cell carcinomas (SCCs). Marked non-neoplastic pathological lesions of the nasal epithelium accompanied them. No increased incidence of tumors was found in other organs after inhalation, and administration routes other than inhalation did not result in local or systemic tumor formation 7. The damage of nasal tissue played a crucial role in the tumor induction process, since nasal cancer was only found at concentrations inducing epithelial degeneration and increased cell proliferation. Thus the stimulation of cell proliferation seems to be an important prerequisite for tumor development. Although formaldehyde exhibits some genotoxic activity, the correlation between cytotoxicity, cell proliferation and the induction of nasal cancer in rats provides a convincing scientific basis for the cause of the carcinogenic response to be cytotoxicity driven. In contrast to that, no significant numbers of tumors were seen in mice and Syrian hamsters following chronic exposure to concentrations up to 14.3 or 30 ppm, respectively 7. These clear species differences appeared to be related, in part, to the local dosimetry and disposition of formaldehyde in nasal tissues. Species differences in nasal anatomy and respiratory physiology may have a profound effect on susceptibility to formaldehyde-induced nasal tumors.
In epidemiological studies in occupationally exposed human populations, there is limited evidence of a causal association between formaldehyde exposure and nasal tumors 7. Taking into account the extensive information on its mode of action, formaldehyde is not likely to be a potent carcinogen to humans under low exposure conditions 7.
There are no indications of a specific toxicity of formaldehyde to fetal development and no effects on reproductive organs were observed after chronic oral administration of formaldehyde to male and female rats. Amounts of formaldehyde which produce marked toxic effects at the portal of entry, do not lead to an appreciable systemic dose and thus do not produce systemic toxicity. This is consistent with formaldehyde’s high reactivity with many cellular nucleophiles and its rapid metabolic degradation.
Common sources of exposure include vehicle emissions, particle boards and similar building materials, carpets, paints and varnishes, foods and cooking, tobacco smoke, and the use of formaldehyde as a disinfectant. Levels of formaldehyde in outdoor air are generally low but higher levels can be found in the indoor air of homes.
Occupational exposure to formaldehyde occurs in a wide variety of occupations and industries: for example, it is estimated that more than one million workers are exposed to some degree across the European Union. Short-term exposures to high levels have been reported for embalmers, pathologists and paper workers. Lower levels have usually been encountered during the manufacture of man-made vitreous fibres, abrasives and rubber and in formaldehyde production industries. A very wide range of exposure levels has been observed in the production of resins and plastic products. The development of resins that release less formaldehyde and improved ventilation has resulted in decreased exposure levels in many industrial settings in recent decades.
- Formaldehyde http://www.inchem.org/documents/cicads/cicads/cicad40.htm [↩]
- FORMALDEHYDE. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100F-29.pdf[↩][↩][↩][↩]
- IARC CLASSIFIES FORMALDEHYDE AS CARCINOGENIC TO HUMANS. PRESS RELEASE N° 153; 15 June 2004 https://www.iarc.fr/en/media-centre/pr/2004/pr153.html[↩]
- Report on Carcinogens Background Document for Formaldehyde. January 22, 2010. https://ntp.niehs.nih.gov/ntp/roc/twelfth/2009/november/formaldehyde_bd_final.pdf[↩]
- WHO (2000) Air quality guidelines for Europe, 2nd ed. Copenhagen, World Health Organization, Regional Office for Europe (WHO Regional Publications, European Series, No. 91).[↩]
- IPCS (1996) Guidelines for drinking-water quality. Vol. 2. Health criteria and other supporting information. Geneva, World Health Organization, International Programme on Chemical Safety, 973 pp.[↩]
- Formaldehyde. http://www.inchem.org/documents/sids/sids/FORMALDEHYDE.pdf[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kieber RJ, Zhou X, Mopper K (1990) Formation of carbonyl compounds from UV-induced photodegradation of humic substances in natural waters: Fate of riverine carbon in the sea. Limnology and oceanography, 35(7):1503–1515.[↩]
- IARC (1995) Wood dust and formaldehyde. Lyon, International Agency for Research on Cancer, pp. 217–375 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 62).[↩]
- Nuccio J, Seaton PJ, Kieber RJ (1995) Biological production of formaldehyde in the marine environment. Limnology and oceanography, 40(3):521–527.[↩]
- Endogenous formaldehyde turnover in humans compared with exogenous contribution from food sources. European Food Safety Authority. EFSA Journal 2014;12(2):3550 https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2014.3550[↩][↩][↩][↩][↩]
- Formaldehyde http://www.inchem.org/documents/cicads/cicads/cicad40.htm[↩][↩][↩][↩][↩]
- Environment Canada (1999a) Canadian Environmental Protection Act — Priority Substances List — Supporting document for the environmental assessment of formaldehyde. Hull, Quebec, Environment Canada, Commercial Chemicals Evaluation Branch.[↩][↩][↩][↩][↩]
- Environment Canada (1996) Summary report — 1994. National Pollutant Release Inventory (NPRI). Hull, Quebec, Environment Canada, Pollution Data Branch.[↩]
- Environment Canada (1997b) Results of the CEPA Section 16 Notice to Industry respecting the second Priority Substances List and di(2-ethylhexyl) phthalate. Hull, Quebec, Environment Canada, Commercial Chemicals Evaluation Branch, Use Patterns Section.[↩][↩][↩][↩]
- Baker DC (1994) Projected emissions of hazardous air pollutants from a Shell coal gasification process-combined-cycle power plant. Fuel, 73(7):1082–1086.[↩]
- IPCS (1989) Formaldehyde. Geneva, World Health Organization, International Programme on Chemical Safety, 219 pp. Environmental Health Criteria 89.[↩]
- Health Canada (1997) Health Canada 1994 Survey on Smoking in Canada. Chronic diseases in Canada, 18(3):120–129.[↩]
- Sverdrup GM, Riggs KB, Kelly TJ, Barrett RE, Peltier RG, Cooper JA (1994) Toxic emissions from a cyclone burner boiler with an ESP and with the SNOX demonstration and from a pulverized coal burner boiler with an ESP/wet flue gas desulfurization system. Presented at the 87th Annual Meeting and Exhibition of the Air and Waste Management Association, Cincinnati, OH, 19–24 June 1994 (94-WA73.02).[↩]
- US EPA (1993) Motor vehicle-related air toxics study. Ann Arbor, MI, US Environmental Protection Agency, Office of Mobile Sources, Emission Planning and Strategies Division, April (EPA 420-R-93-005).[↩]
- Chan GS, Scafe M, Emami S (1992) Cemeteries and groundwater: An examination of the potential contamination of groundwater by preservatives containing formaldehyde. Toronto, Ontario, Ontario Ministry of the Environment, Water Resources Branch.[↩]
- TRI (1994) 1992 Toxics Release Inventory, public data release. Washington, DC, US Environmental Protection Agency, Office of Pollution Prevention and Toxics, p. 236.[↩]
- Environment Canada (1995) Technical briefing note on composite wood panels. Report prepared for Environmental Choice Program, Environment Canada, Ottawa, Ontario, 18 May 1995.[↩]
- IARC CLASSIFIES FORMALDEHYDE AS CARCINOGENIC TO HUMANS. PRESS RELEASE N° 153, 15 June 2004 https://www.iarc.fr/en/media-centre/pr/2004/pr153.html[↩][↩]
- Zhang LSCED, Steinmaus C, Eastmond DA et al. (2009). Formaldehyde exposure and leukemia: a new meta-analysis and potential mechanisms. Mutat Res, 681: 150–168. doi:10.1016/j.mrrev.2008.07.002 https://www.ncbi.nlm.nih.gov/pubmed/18674636[↩]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2013. Scientific Opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA Journal 2013;11(12):3496, 263 pp. doi:10.293/j.efsa.2013.3496[↩]
- Dhareshwar SS and Stella VJ, 2008. Your Prodrug Releases Formaldehyde: Should you be Concerned? No! Journal of Pharmaceutical Sciences, 97, 4184-4193.[↩][↩][↩]
- NTP (National Toxicology Program), 2011. Formaldehyde. Report on Carcinogens, Twelfth Edition.[↩]
- Sullivan JB and Krieger GR, 2001. Formaldehyde. In: Clinical Environmental Health Toxic Exposures. 2nd ed. Philadelphia: Lippincott Williams and Wilkins, 1006-1014.[↩][↩]
- Trezl L, Csiba A, Juhasz S, Szentgyorgyi M, Lombai G and Hullan L, 1997. Endogenous formaldehyde level of foods and its biological significance. Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 205, 300-304.[↩][↩][↩]
- Rétfalvi T, Németh ZI, Sarudi I and Albert L. 1998 Alteration of endogenous formaldehyde level following mercury accumulation in different pig tissues. Acta Biologica Hungarica, 49, 375-379[↩]
- Weng X, Chon CH, Jiang H and Li D, 2009. Rapid detection of formaldehyde concentration in food on a polydimethylsiloxane (PDMS) microfluidic chip. Food Chemistry, 114, 1079-1089.[↩][↩]
- Sotelo CG, Piñeiro C, Pérez-Martín RI (1995) Denaturation of fish protein during frozen storage: role of formaldehyde. Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 200:14–23.[↩][↩]
- Tsuda M, Frank N, Sato S, Sugimura T (1988) Marked increase in the urinary level of N-nitrosothioproline after ingestion of cod with vegetables. Cancer research, 48:4049–4052.[↩]
- Yasuhara A, Shibamoto T (1995) Quantitative analysis of volatile aldehydes formed from various kinds of fish flesh during heat treatment. Journal of agricultural and food chemistry, 43:94–97.[↩]
- Tashkov W (1996) Determination of formaldehyde in foods, biological media and technological materials by head space gas chromatography. Chromatographia, 43(11/12):625–627.[↩]
- ATSDR (1999) Toxicological profile for formaldehyde. Atlanta, GA, US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry.[↩][↩]
- Baraniak Z, Nagpal DS, Neidert E (1988) Gas chromatographic determination of formaldehyde in maple syrup as 2,4-dinitrophenylhydrazone derivative. Journal of the Association of Official Analytical Chemists, 71(4):740–741.[↩]
- Brunn W, Klostermeyer H (1984) [Detection and determination of formaldehyde in foods.] Lebensmittelchemie und Gerichtliche Chemie, 38:16–17 (in German[↩]
- Restani P, Restelli AR, Galli CL (1992) Formaldehyde and hexamethylenetetramine as food additives: chemical interactions and toxicology. Food additives and contaminants, 9(5):597–605.[↩]
- Scheuplein RJ (1985) Formaldehyde: The Food and Drug Administration’s perspective. In: Turoski V, ed. Formaldehyde — analytical chemistry and toxicology. Washington, DC, American Chemical Society, pp. 237–245. Advances in Chemistry Series 210.[↩][↩]
- Tsuchiya H, Ohtani S, Yamada K, Akagiri M, Takagi N, Sato M (1994) Determination of formaldehyde in reagents and beverages using flow injection. Analyst, 119:1413–1416.[↩]
- Hayashi T, Reece CA, Shibamoto T (1986) Gas chromatographic determination of formaldehyde in coffee via thiazolidine derivative. Journal of the Association of Official Analytical Chemists, 69(1):101–105[↩]
- Kaminski J, Atwal AS, Mahadevan S (1993) Determination of formaldehyde in fresh and retail milk by liquid column chromatography. Journal of the Association of Official Analytical Chemists international, 76(5):1010–1013.[↩]
- Preuss PW, Dailey RL, Lehman ES (1985) Exposure to formaldehyde. In: Turoski V, ed. Formaldehyde — analytical chemistry and toxicology. Washington, DC, American Chemical Society, pp. 247–259. Advances in Chemistry Series 210.[↩]
- IPCS (1989) Formaldehyde. Geneva, World Health Organization, International Programme on Chemical Safety, 219 pp. (Environmental Health Criteria 89).[↩]
- Jass HE (1985) History and status of formaldehyde in the cosmetics industry. In: Turoski V, ed. Formaldehyde — analytical chemistry and toxicology. Washington, DC, American Chemical Society, pp. 229–236 (Advances in Chemistry Series 210).[↩]
- Flyvholm M-A, Andersen P (1993) Identification of formaldehyde releasers and occurrence of formaldehyde and formaldehyde releasers in registered chemical products. American journal of industrial medicine, 24:533–552.[↩]
- Miyake T, Shibamoto T (1995) Quantitative analysis by gas chromatography of volatile carbonyl compounds in cigarette smoke. Journal of chromatography, A 693:376–381.[↩]
- Guerin MR, Jenkins RA, Tomkins BA (1992) The chemistry of environmental tobacco smoke: composition and measurement. Boca Raton, FL, Lewis Publishers.[↩]
- Schlitt H, Knöppel H (1989) Carbonyl compounds in mainstream and sidestream cigarette smoke. In: Bieva C, Courtois Y, Govaerts M, eds. Present and future of indoor air quality. Amsterdam, Excerpta Medica, pp. 197–206.[↩]
- Daisey JM, Mahanama KRR, Hodgson AT (1994) Toxic volatile organic compounds in environmental tobacco smoke: Emission factors for modelling exposures of California populations. Prepared for Research Division, Air Resources Board, California Environmental Protection Agency, Sacramento, CA, 109 pp. (Contract No. A133-186).[↩]
- Priha E (1995) Are textile formaldehyde regulations reasonable? Experiences from the Finnish textile and clothing industries. Regulatory toxicology and pharmacology, 22:243–249.[↩]
- Hatch KL, Maibach HI (1995) Textile dermatitis: an update (I). Resins, additives and fibers. Contact dermatitis, 32:319–326.[↩]
- Scheman AJ, Carrol PA, Brown KH, Osburn AH (1998) Formaldehyde-related textile allergy: an update. Contact dermatitis, 38:332–336.[↩]
- Piletta-Zanin PA, Pasche-Koo F, Auderset PC, Huggenberger D, Saurat J-H, Hauser C (1996) Detection of formaldehyde in ten brands of moist baby toilet tissue by the acetylacetone methods and high-performance liquid chromatography. Dermatology, 193:170.[↩]
- Meek ME, Atkinson A, Sitwell J (1985) Background paper on formaldehyde prepared for WHO working group on indoor air quality: radon and formaldehyde. Ottawa, Ontario, Health and Welfare Canada, Health Protection Branch, Bureau of Chemical Hazards, pp. 1–124.[↩]
- Etkin DS (1996) Volatile organic compounds in indoor environments. Arlington, MA, Cutter Information Corp., 426 pp.[↩]
- Godish T (1988) Residential formaldehyde contamination: sources and levels. Comments on toxicology, 2(3):115–134.[↩][↩]
- Piersol P (1995) Build Green and conventional materials off-gassing tests. Prepared by ORTECH Corporation for Canada Mortgage and Housing Corporation, 6 February 1995, 24 pp. (Report No. 94-G53-B0106).[↩]
- Health Canada (2000) Draft supporting documentation for PSL2 assessments. Human exposure assessment for formaldehyde. Ottawa, Ontario, Health Canada, Health Protection Branch, Priority Substances Section, January 2000.[↩][↩]
- Meyer B, Hermanns K (1985) Formaldehyde release from pressed wood products. In: Turoski V, ed. Formaldehyde — analytical chemistry and toxicology. Washington, DC, American Chemical Society, pp. 101–116 (Advances in Chemistry Series 210).[↩]
- Tabor RG (1988) Control of formaldehyde release from wood products adhesives. Comments on toxicology, 2(3):191–200.[↩]
- Kelly TJ, Smith DL, Satola J (1999) Emission rates of formaldehyde from materials and consumer products found in California homes. Environmental science and technology, 33(1):81–88.[↩]
- McCrillis RC, Howard EM, Guo Z, Krebs KA, Fortmann R, Lao H-C (1999) Characterization of curing emissions from conversion varnishes. Journal of the Air and Waste Management Association, 49:70–75.[↩]
- IARC (2006). Formaldehyde, 2-butoxyethanol and 1-tertbutoxypropan-2-ol. IARC Monogr Eval Carcinog Risks Hum, 88: 1–478. https://www.ncbi.nlm.nih.gov/pubmed/9559107[↩]
- Florence E, Milner DF (1981) Determination of free and loosely protein-bound formaldehyde in the tissues of pigs fed formalin-treated skim milk as a protein supplement. Journal of the science of food and agriculture, 32:288–292.[↩]
- Buckley KE, Fisher LJ, Mackay VG (1988) Levels of formaldehyde in milk, blood, and tissues of dairy cows and calves consuming formalin-treated whey. Journal of agricultural and food chemistry, 36:1146–1150.[↩]