Contents
What is trisodium phosphate
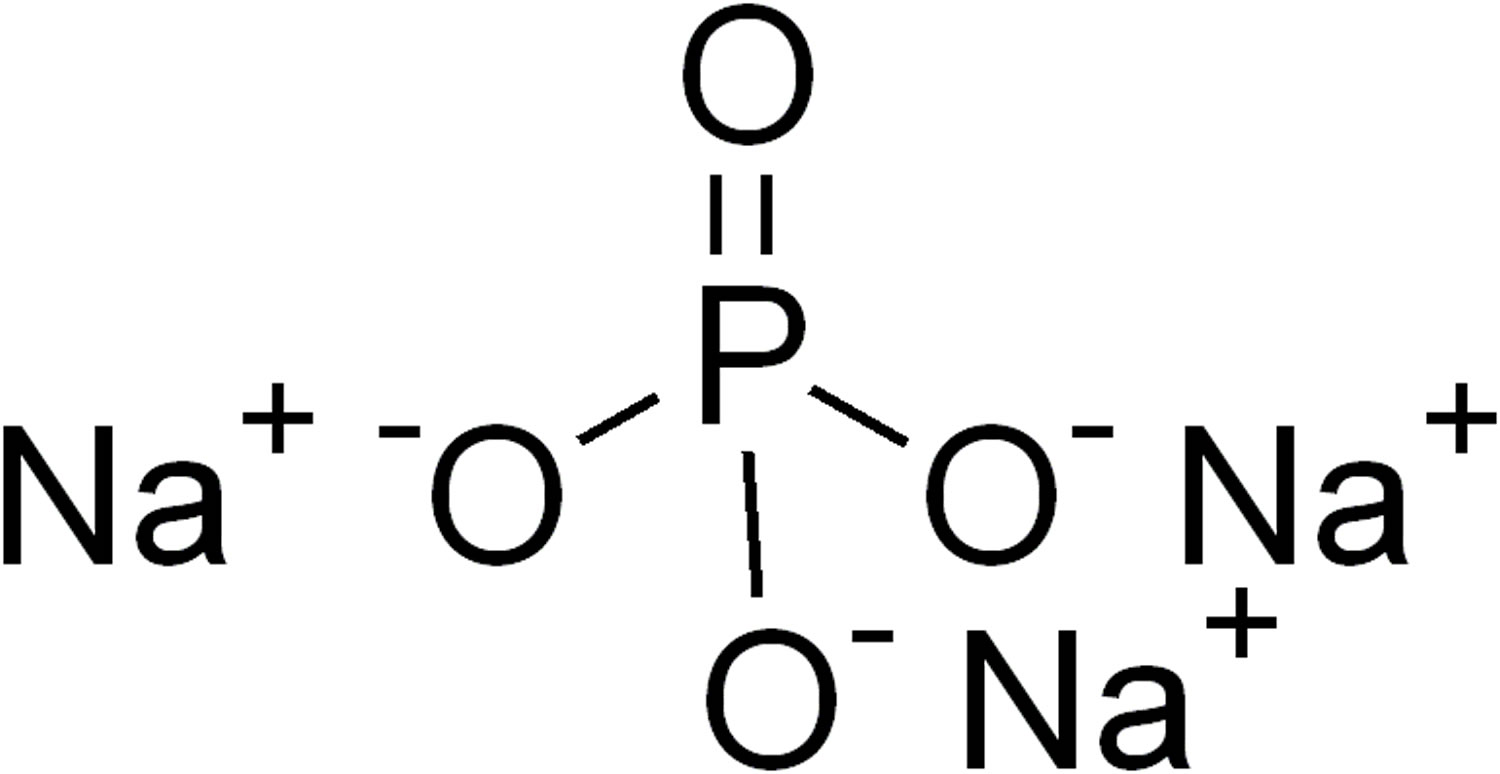
Trisodium phosphate Na3PO4 (trisodium orthophosphate or trisodium monophosphate or food additive E 339ii) is presently used in the USA to kill or reduce the number of bacteria, such as salmonella or campylobacter on poultry 1. Trisodium phosphate is a permitted food additive in Europe identified as E 339ii and authorized in several processed foods, including meat products 2. In the USA, sodium phosphates (mono-, di-, and tri-) are considered GRAS (generally recognized as safe) by the US Food and Drug Administration (FDA) as multipurpose ingredients in food (21 CFR 182.1778). This GRAS status recognition was issued through experience based on common use in food and considering that the substance was used in food prior to January 1, 1958 and has been approved by the US Department of Agriculture-Food Safety and Inspection Service at levels of 8–12% with a high pH value (pH 12) as an antimicrobial agent on raw chilled poultry carcasses that have been passed for wholesomeness. Poultry carcasses are either sprayed with or dipped in the trisodium phosphate solution for up to 15 seconds at a solution temperature of 13–17°C 3. Trisodium phosphate exerts a destructive effect on pathogens and a “detergent effect” that allows the removal of bacteria by the washing process 4. The mechanism of action of trisodium phosphate is based on its high alkalinity in solution (pH 12.1) that can disrupt cell membranes and remove fat films causing the cell to leak intracellular fluid. It can also act as a surfactant contributing to elimination of bacteria not yet strongly adhered to the surface of poultry skin 5. The lowest effective trisodium phosphate concentration for microbial control is 8%.
The mechanisms of the trisodium phosphate mode of action include:
- Surfactant properties,
- Destruction due to high pH (pH 12.1),
- Removal of loosely associated bacteria from the skin,
- Removal of carcass surface fat, invariably resulting in removal of bacteria attached to the fat, and
- Destruction of the bacterial cell wall 6.
- It is hypothesized that the increased wetting ability of hot water and trisodium phosphate physically remove bacteria in addition to killing them.
Trisodium phosphate is ionized in water generating Na+ and PO43- ions. These ions can be absorbed into the carcass but no further reactions are likely. The poultry carcass can be affected when exposed to the high alkalinity of the solutions. For instance, the action of endogenous poultry muscle enzymes or the water retention capacity could be altered during the post-treatment period of time. However, a study on broiler products reported no detectable effects of treatment on taste, texture or appearance 7. There would be no possibility of the formation of semicarbazide after treatment with trisodium phosphate.
Figure 1. Trisodium phosphate
The rapid dissociation of trisodium phosphate into its constituent ions and the relatively low chemical reactivity of Na+ and PO43- ions makes it very unlikely that significant levels of by-products would be produced after treatment of poultry carcasses 8.
At this moment, there is no indication that the use of a trisodium phosphate could support the spread of resistance to therapeutic antimicrobials by direct selection, although it may be possible by indirect selection. Despite a long history of use, there are currently no published data to conclude that the application of trisodium phosphate to remove microbial contamination of poultry carcasses at the proposed conditions of use will lead to the occurrence of acquired reduced susceptibility to these substances 9. Similarly, there are currently no published data to conclude that the application of trisodium phosphate to remove microbial contamination of poultry carcasses at the proposed conditions of use will lead to resistance to therapeutic antimicrobials 9.
What is trisodium phosphate used for?
Trisodium phosphate is typically used in poultry processing in the United States either as sprays or washes for on-line reprocessing, or added to the chiller water (chiller bath applications) to limit the potential for microbial cross-contamination. Treatment of poultry carcasses with trisodium phosphate was effective in reducing populations of food-borne pathogens including Salmonella, Campylobacter, Escherichia coli O157:H7, Listeria, Staphylococcus aureus as well as spoilage bacteria including Pseudomonas and Lactobacillus 10.
Trisodium phosphate, has been approved by regulatory agencies in the United States for use in poultry process water within a concentration range of 8 to 12%. The trisodium phosphate solution must be maintained at a temperature of 7.2 to 12.8 °C and applied by spraying or dipping carcasses for up to 15 seconds (9 CFR 424.21(c) and FDA regulation 21 CFR 182.1778). Since 1994, interim approval has been granted to use trisodium phosphate as a processing aid for the purpose of
reducing microorganisms when: (1) applied as a spray or dip to raw, unchilled carcasses for up to 15 seconds in an 8 to 12 % solution of trisodium phosphate maintained within a temperature range of 18.3 to 29.4 °C; and (2) applied as a spray or dip to raw, unchilled poultry giblets for up to 30 seconds with an 8 to 12% solution of trisodium phosphate, until rule making approving its use can be finalized. Carcass exposure time is controlled by line speed and length of the application cabinet. A typical application is approximately 15 seconds at full line speed. If the line is stopped for more than 5 minutes, carcasses in the application cabinet are segregated and condemned.
According to previous estimations by the Scientific Committee on Veterinary Measures relating to Public Health 11, the treatment of poultry carcasses with trisodium phosphate would incorporate 480 mg trisodium phosphate per kg carcass. Based on meat consumption data in European adults, potential daily exposure to trisodium phosphate for a 60 kg individual would be up to 1.21 mg/kg body weight at the mean and up to 2.08 and 2.80 mg/kg body weight at the 95th and 99th percentile of meat consumption, respectively.
A maximum tolerable daily intake of 70 mg/kg body weight for phosphates was established by the Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) 12. The Scientific Committee on Food 13 confirmed the maximum tolerable daily intake value of 70 mg/kg body weight estimated by the Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) for phosphates used as food additives. Both evaluations concluded that the main risk related to the ingestion of these additives was their potential effect on the calcium-phosphorus-magnesium balance of the body 8.
Based on meat consumption data in European adults, as an estimate of poultry consumption, potential dietary exposure to trisodium phosphate for a 60 kg individual would be up to 1.2 mg/kg body weight per day at the mean, reaching up to 2.1 and 2.8 mg/kg body weight at the 95th and 99th percentiles of meat consumption, respectively 8.
Treated poultry carcasses are only consumed after processing (cooking, frying, etc) and final concentrations of phosphate residues to which the consumer would actually be exposed are likely less than what has been estimated above. Dietary exposures would thus only be a fraction of maximum tolerable daily intake value (up to 4 %, 99th percentiles) and the European Food Safety Authority Panel considers that this exposure is of no safety concern 8.
On the basis of the available data, the European Food Safety Authority Panel considers that treatment of poultry carcasses with trisodium phosphate as described is of no safety concern 8. The European Food Safety Authority Panel considers that the rapid dissociation of trisodium phosphate into its constituent ions (Na+ and PO43- ions) and their relatively low chemical reactivity make it very unlikely that by-products of toxicological relevance are formed after this treatment. There is no possibility of formation of semicarbazide from the use of trisodium phosphate 8.
The European Food Safety Authority Panel notes that the initial health concerns about semicarbazide are no longer relevant 8. As set out in the European Food Safety Authority opinion on semicarbazide 14, new data showed that semicarbazide is not genotoxic in vivo.
Phosphorus
Phosphorus is most commonly found in nature in its pentavalent form in combination with oxygen, as phosphate (PO43-). Phosphorus (as phosphate) is an essential constituent of all known protoplasm and its content is quite uniform across most plant and animal tissues. Except for specialized cells with high ribonucleic acid content, and for nervous tissue with high myelin content, tissue phosphorus occurs at concentrations ranging approximately from 0.25 to 0.65 mmol (7.8 to 20.1 mg)/g protein 15. A practical consequence is that, as organisms consume other organisms lower in the food chain (whether animal or plant), they automatically obtain their phosphorus.
Phosphorus is a mineral that makes up 1% of a person’s total body weight. Phosphorus is the second most abundant mineral in your body. Phosphorus is present in every cell of your body. Most of the phosphorus in the body is found in the bones and teeth. Eighty-five percent of adult body phosphorus is in bone. The remaining 15 percent is distributed through the soft tissues 16.
Phosphorus makes up about 0.5 percent of the newborn infant body 17 and from 0.65 to 1.1 percent of the adult body 18. Total phosphorus concentration in whole blood is 13 mmol/liter (40 mg/dl), most of which is in the phospholipids of red blood cells and plasma lipoproteins. Approximately 1 mmol/liter (3.1 mg/dl) is present as inorganic phosphate (Pi). This inorganic phosphate component, while a tiny fraction of body phosphorus (< 0.1 percent), is of critical importance. In adults this component makes up about 15 mmol (465 mg) and is located mainly in the blood and extracellular fluid. It is into this inorganic phosphate compartment that phosphate is inserted upon absorption from the diet and resorption from bone and from this compartment that most urinary phosphorus and hydroxyapatite mineral phosphorus are derived. This compartment is also the primary source from which the cells of all tissues derive both structural and high-energy phosphate.
- The main function of phosphorus is in the formation of bones and teeth.
- Phosphorus plays an important role in how your body uses carbohydrates and fats. Phosphorus is also needed for the body to make protein for the growth, maintenance, and repair of cells and tissues. Phosphorus also helps the body make ATP, a molecule the body uses to store energy.
Structurally, phosphorus occurs as phospholipids, which are a major component of most biological membranes, and as nucleotides and nucleic acids. The functional roles include: (1) the buffering of acid or alkali excesses, hence helping to maintain normal pH; (2) the temporary storage and transfer of the energy derived from metabolic fuels; and (3) by phosphorylation, the activation of many catalytic proteins. Since phosphate is not irreversibly consumed in these processes and can be recycled indefinitely, the actual function of dietary phosphorus is first to support tissue growth (either during individual development or through pregnancy and lactation) and, second, to replace excretory and dermal losses. In both processes it is necessary to maintain a normal level of Pi in the extracellular fluid (ECF), which would otherwise be depleted of its phosphorus by growth and excretion.
Phosphorus works with the B vitamins. It also helps with the following:
- Kidney function
- Muscle contractions
- Normal heartbeat
- Nerve signaling
According to Institute of Medicine recommendations, the recommended dietary intakes of phosphorus are as follows:
- 0 to 6 months: 100 milligrams per day (mg/day)*
- 7 to 12 months: 275 mg/day*
- 1 to 3 years: 460 mg/day
- 4 to 8 years: 500 mg/day
- 9 to 18 years: 1,250 mg
- Adults: 700 mg/day
Pregnant or lactating women:
- Younger than 18: 1,250 mg/day
- Older than 18: 700 mg/day
*AI or Adequate Intake
Table 1. Usual mean daily phosphorus intake and dietary recommended intake for phosphorus by gender and age
| Dietary recommended intake | ||||
| Age | Usual phosphorus intake | EAR | UL | RDA |
| years | mg/day | mg/day | ||
| Men | ||||
| 1–3 | 1030 ± 26.3 | 380 | 3000 | 460 |
| 4–8 | 1145 ± 27.4 | 405 | 3000 | 500 |
| 9–13 | 1321 ± 35.4 | 1055 | 4000 | 1250 |
| 14–18 | 1681 ± 61.5 | 1055 | 4000 | 1250 |
| 19–30 | 1656 ± 53.4 | 580 | 4000 | 700 |
| 31–50 | 1727 ± 25.0 | 580 | 4000 | 700 |
| 51–70 | 1492 ± 30.0 | 580 | 4000 | 700 |
| ≥71 | 1270 ± 27.6 | 580 | 3000 | 700 |
| Women | ||||
| 1–3 | 1030 ± 26.3 | 380 | 3000 | 460 |
| 4–8 | 1145 ± 27.4 | 405 | 3000 | 500 |
| 9–13 | 1176 ± 57.5 | 1055 | 4000 | 1250 |
| 14–18 | 1067 ± 29.8 | 1055 | 4000 | 1250 |
| 19–30 | 1120 ± 40.8 | 580 | 4000 | 700 |
| 31–50 | 1197 ± 25.0 | 580 | 4000 | 700 |
| 51–70 | 1106 ± 34.0 | 580 | 4000 | 700 |
| ≥71 | 985 ± 28.8 | 580 | 3000 | 700 |
Footnotes: Usual daily phosphorus intake data from What We Eat in America, NHANES 2005–2006 (unpublished data from Alanna Moshfegh, U.S. Department of Agriculture) are expressed as means. The dietary recommended intake levels for phosphorus were established by the Institute of Medicine, Food, and Nutrition Board in 1997 19.
- Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
- Estimated Average Requirement (EAR): Average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals.
- Tolerable Upper Intake Level (UL): Maximum daily intake unlikely to cause adverse health effects.
Regulation of the Serum Inorganic Phosphate Concentration
Inorganic phosphate (Pi) levels are only loosely regulated. Normal inorganic phosphate (Pi) levels decline with age from infancy to maturity (Table 2). The most likely reason for the higher inorganic phosphate (Pi) in newborn infants than in older children and adults is the lower glomerular filtration rate (GFR) of infants. GFR is about 32 ml/min/1.73 m2 at about 1 week of age, and rises to 87 at 4 to 6 months 21. In the first months of life, plasma inorganic phosphate (Pi) concentration appears to be a reflection both of renal glomerular maturity and of the amount of dietary intake. Mean serum inorganic phosphate (Pi) appears to decline by about 0.3 mmol/liter (0.9 mg/ dl) across the second half of the first year of life 22. Human milk-fed, compared with formula-fed, infants have a slightly lower plasma inorganic phosphate (Pi) (2.07 versus 2.25 mmol/liter or 6.4 versus 7.0 mg/dl) which is simply a function of differences in intake 22; Caucasian compared with African American infants have a slightly higher plasma Pi irrespective of type of milk feeding 22.
Table 2. Normative Values for Serum Inorganic Phosphorus (mmol/liter) for Age
| Age (y) | Mean | 2.5 Percentile | 97.5 Percentile |
|---|---|---|---|
| 0–0.5 | 2.15 | 1.88 | 2.42 |
| 2 | 1.81 | 1.43 | 2.20 |
| 4 | 1.77 | 1.38 | 2.15 |
| 6 | 1.72 | 1.33 | 2.11 |
| 8 | 1.67 | 1.29 | 2.06 |
| 10 | 1.63 | 1.24 | 2.01 |
| 12 | 1.58 | 1.19 | 1.97 |
| 14 | 1.53 | 1.15 | 1.92 |
| 16 | 1.49 | 1.10 | 1.88 |
| 20 | 1.39 | 1.01 | 1.78 |
| Adult | 1.15 | 0.87 | 1.41 |
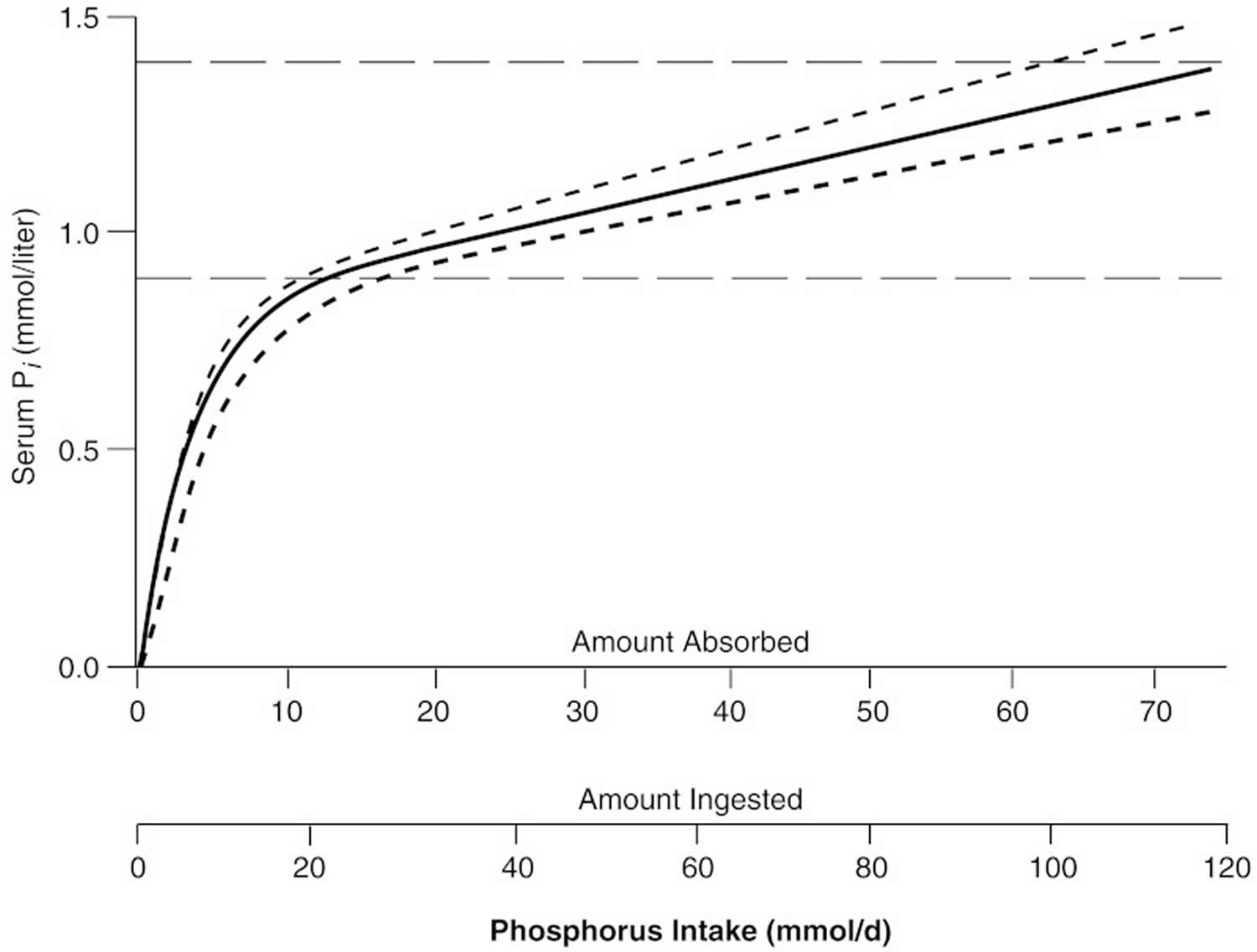
The general relationship between absorbed phosphorus intake and plasma inorganic phosphate (Pi) in adults is shown in Figure 2. The relationship shown in Figure 4 holds only in adult individuals with adequate renal function; that is, the slow rise of plasma inorganic phosphate (Pi) with rising phosphorus intake over most of the intake range applies only so long as excess absorbed phosphate can be spilled into the urine. However, in individuals with reduced renal function, phosphorus clearance remains essentially normal so long as GFR is at least 20 percent of mean adult normal values, largely because tubular reabsorption is reduced to match the reduction in filtered load. Below that level, excretion of absorbed phosphate requires higher and higher levels of plasma inorganic phosphate (Pi) to maintain a filtered load at least equal to the absorbed load. This is the reason for the hyperphosphatemia typically found in patients with end-stage renal disease.
Figure 2. Relation of serum inorganic phosphate (Pi) to absorbed intake in adults with normal renal function
Phosphorus Food Sources
Phosphates are found in foods as naturally occurring components of biological molecules and as food additives in the form of various phosphate salts. These salts are used in processed foods for nonnutrient functions, such as moisture retention, smoothness, and binding.
Phosphates occur naturally in the form of organic esters in many kinds of food, the main food sources are the protein food groups of meat and milk. A diet that includes the right amounts of calcium and protein will also provide enough phosphorus. Whole-grain breads and cereals contain more phosphorus than cereals and breads made from refined flour. However, the phosphorus is stored in a form that is not absorbed by humans. These phosphate esters are organically bound and only partially absorbed in the gastrointestinal tract. Fruits and vegetables contain only small amounts of phosphorus. Phosphates are important for human health since they are responsible for growth, maintenance and repair of tissues and cells of living organisms.
In infants, dietary intake of phosphorus spans a wide range, depending on whether the food is human milk, cow milk, adapted cow milk formula, or soy formula (see Table 4). Moreover, the phosphorus concentration of human milk declines with progressing lactation, especially between 4 and 25 weeks of lactation 23. By contrast, more of the variation in dietary intake of phosphorus in adults is due to differences in total food intake and less to differences in food composition. Phosphorus contents of adult diets average about 62 mg (2 mmol)/100 kcal in both sexes 24, and phosphorus:energy ratios exhibit a coefficient of variation of only about one-third that of total phosphorus intake. Nevertheless, individuals with high dairy product intakes will have diets with higher phosphorus density values, since the phosphorus density of cow milk is higher than that of most other foods in a typical diet. The same is true for diets high in colas and a few other soft drinks that use phosphoric acid as the acidulant. A 12-ounce serving of such beverages contains about 50 mg (< 2 mmol), which is only 5 percent of the typical intake of an adult woman. However, when consumed in a quantity of five or more servings per day, such beverages may contribute substantially to total phosphorus intake.
Table 3. Contribution of food categories to phosphorus intake and examples of phosphorus containing generally recognized as safe ingredients frequently used in processing foods in each category
| Food Category | % Contribution to Phosphorus Intake | Examples of Phosphorus Ingredients Used in Processing Foods in Each Category2 |
| Milk and dairy | 20.9 | Phosphoric acid, sodium phosphate, calcium phosphate, potassium tripolyphosphate |
| Mixed dishes: grain-based | 10.1 | Modified food starch, sodium acid pyrophosphate, disodium phosphate |
| Breads, rolls, and tortillas | 5.8 | Sodium aluminum phosphate, mono-calcium phosphate, sodium acid pyrophosphate |
| Quick breads, bread products, sweet bakery products | 5.2 | Sodium acid pyrophosphate, sodium aluminum phosphate, mono-calcium phosphate, dicalcium phosphate, calcium acid pyrophosphate |
| Poultry | 5.1 | Sodium tripolyphosphate, sodium tripoly/sodium hexa-meta-phosphate blends, sodium acid pyrophosphate, tetrasodium pyrophosphate |
| Pizza | 4.8 | Disodium phosphate, tricalcium phosphate, tetrasodium pyrophosphate, sodium acid pyrophosphate |
| Vegetables | 4.8 | Mono-calcium phosphate, sodium phosphate, disodium phosphate, sodium acid pyrophosphate, disodium hydrogen pyrophosphate |
| Mixed dishes: meat, poultry, seafood | 4.5 | Sodium tripolyphosphate, sodium acid pyrophosphate, tricalcium phosphate, trisodium phosphate |
| Cured meats and poultry | 4.4 | Sodium tripolyphosphate, tetrasodium pyrophosphate, sodium acid pyrophosphate |
| Meats | 4.2 | Potassium tripolyphosphate, tetrapotassium pyrophosphate, sodium hexa-meta-phosphate |
| Plant-based protein foods | 3.7 | Sodium hexa-meta-phosphate, sodium tripolyphosphate |
| Cereals | 3.2 | Disodium phosphate, tricalcium phosphate, trisodium phosphate |
| Eggs | 2.8 | Sodium hexa-meta-phosphate, tetrasodium pyrophosphate, mono-sodium phosphate |
| Seafood | 2.5 | Sodium acid pyrophosphate, potassium tripolyphosphate, tetrapotassium pyrophosphate, sodium tripolyphosphate |
| All other food categories | 18 | |
| Savory snacks, crackers, snack/meal bars | <2.5 | Calcium phosphate, sodium hexa-meta-phosphate, tricalcium phosphate |
| Other desserts | <2.5 | Calcium phosphate, modified corn starch, disodium phosphate, tetrasodium pyrophosphate |
| Candy (chocolate) | <2.5 | Lecithin |
| Sugar sweetened/diet beverages/alcoholic beverages | <2.5 | Phosphoric acid |
| 100% juice | <2.5 | Calcium phosphate |
| Fruits | <2.5 | Mono-calcium phosphate |
| Soups | <2.5 | Mono-potassium phosphate |
| Cooked grains | <2.5 | Disodium phosphate, tricalcium phosphate |
| Condiments/sauces | <2.5 | Phosphoric acid, disodium phosphate, modified food starch, sodium hexa-mono-phosphate |
| Fats and oils | <2.5 | None found |
Footnotes:
Unpublished data source: Alanna Moshfegh, U.S. Department of Agriculture, What We Eat in America, NHANES 2009–2010. The U.S. Food and Drug Administration considers the phosphate-containing ingredients shown in this table to be generally recognized as safe under conditions of their intended use in foods.
All of these phosphorus-containing ingredients were granted generally recognized as safe status between 1975 and 1980. Data source: Ingredients labels on products of processed foods currently in the marketplace.
[Source 20]Table 4. Average Phosphorus Content and Calcium:Phosphorus Molar Ratio of Various Infant Feedings
| Feeding Type | P (mmol/liter) | Ca:P Molar Ratio |
|---|---|---|
| Human milka | ||
| 1 week | 5.1 ± 0.9 | 1.3:1 |
| 4 weeks | 4.8 ± 0.8 | 1.4:1 |
| 16 weeks | 3.9 ± 0.5 | 1.5:1 |
| Cows’ milk formula | 12 | 1.0:1 |
| Soy formulab | 15 | 1.2:1 |
| Whole cows’ milk | 30 | 1.0:1 |
Footnotes:
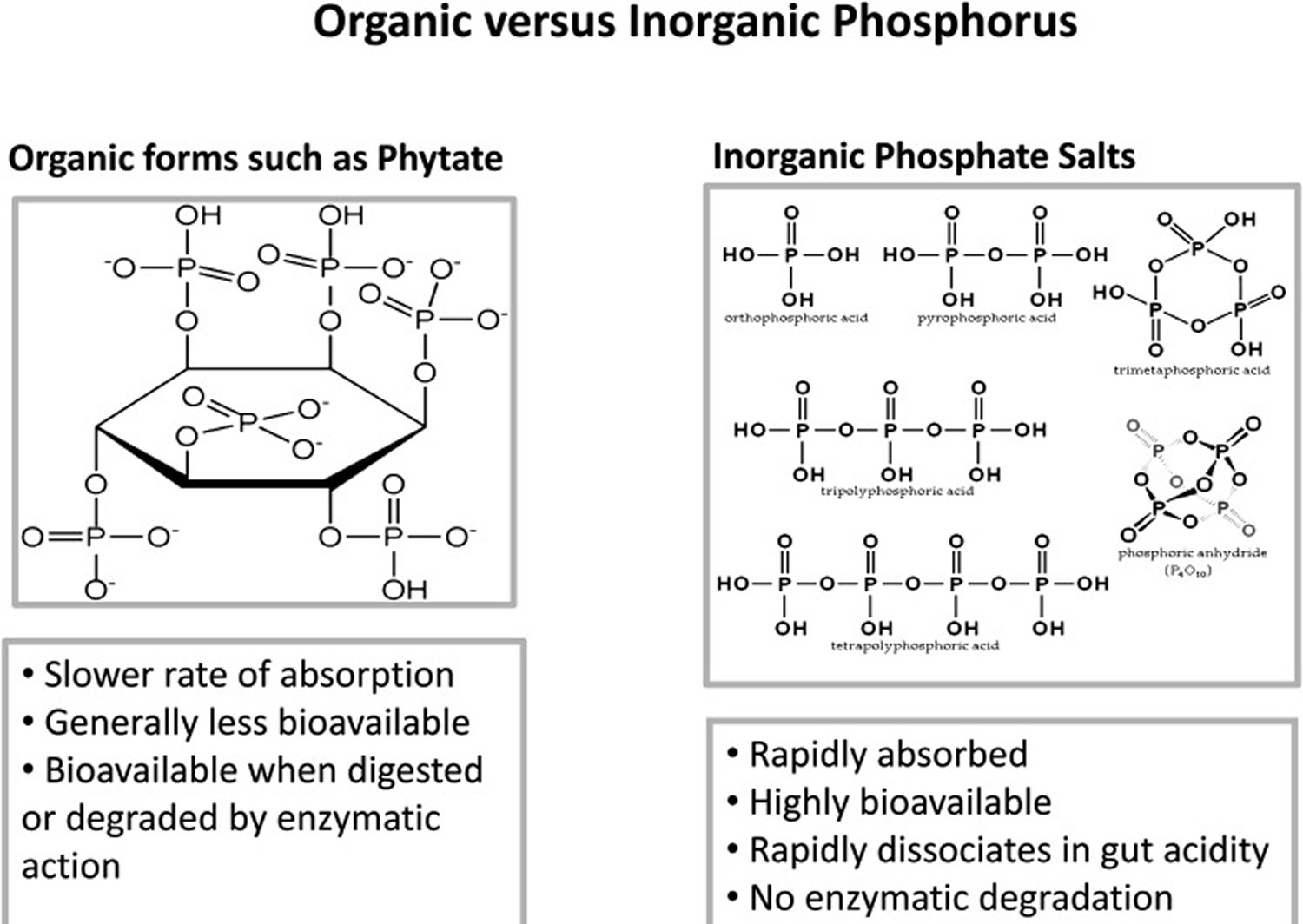
Food phosphorus is a mixture of inorganic and organic forms (see Figure 3). Intestinal phosphatases hydrolyze the organic forms contained in ingested protoplasm, and thus most phosphorus absorption occurs as inorganic phosphate. On a mixed diet, net absorption of total phosphorus in various reports ranges from 55 to 70 percent in adults 27 and from 65 to 90 percent in infants and children 28. There is no evidence that this absorption efficiency varies with dietary intake. In the data from both Stanbury 29 and Lemann 30, the intercept of the regression of adult fecal phosphorus on dietary phosphorus is not significantly different from zero, and the relationship is linear out to intakes of at least 3.1 g (100 mmol)/day. This means that there is no apparent adaptive mechanism that improves phosphorus absorption at low intakes. This is in sharp contrast to calcium, for which absorption efficiency increases as dietary intake decreases 31 and for which adaptive mechanisms exist that improve absorption still further at habitual low intakes 32.
A portion of phosphorus absorption is by way of a saturable, active transport facilitated by 1,25-dihydroxyvitamin D (1,25(OH)2D) 33. However, the fact that fractional phosphorus absorption is virtually constant across a broad range of intakes suggests that the bulk of phosphorus absorption occurs by passive, concentration-dependent processes. Also, even in the face of dangerous hyperphosphatemia (high blood phosphate), phosphorus continues to be absorbed from the diet at an efficiency only slightly lower than normal 34.
Phosphorus absorption is reduced by ingestion of aluminum-containing antacids and by pharmacologic doses of calcium carbonate. There is, however, no significant interference with phosphorus absorption by calcium at intakes within the typical adult range.
Excretion of endogenous phosphorus is mainly through the kidneys. Inorganic serum phosphate is filtered at the glomerulus and reabsorbed in the proximal tubule. The transport capacity of the proximal tubule for phosphorus is limited; it cannot exceed a certain number of mmol per unit time. This limit is called the tubular maximum for phosphate (TmP). Tubular maximum for phosphate (TmP) varies inversely with parathyroid hormone (PTH) concentration; parathyroid hormone (PTH) thereby adjusts renal clearance of inorganic phosphate (Pi). At filtered loads less than the tubular maximum for phosphate (TmP) (for example, at low plasma inorganic phosphate (Pi) values), most or all of the filtered load is reabsorbed, and thus plasma phosphate levels can be at least partially maintained. By contrast, at filtered loads above the tubular maximum for phosphate (TmP), urinary phosphorus is a linear function of plasma phosphate 30. In a healthy adult, urine phosphorus is essentially equal to absorbed diet phosphorus, less small amounts of phosphorus lost in shed cells of skin and intestinal mucosa.
This regulation of phosphorus excretion is apparent from early infancy. In infants, as in adults, the major site of regulation of phosphorus retention is at the kidney. In studies of infants receiving different calcium intakes 35, phosphorus retention did not differ even with high amounts of dietary calcium (calcium:phosphorus [Ca:P] molar ratios of 0:6, 1:1, or 1.4:1). Any reduction in absorption of phosphorus due to high amounts of dietary calcium were compensated for by parallel reductions in renal phosphorus excretion 35. The least renal excretory work to maintain normal phosphorus homeostasis would be achieved with human milk as the major source of minerals during the first year of life.
Figure 3. Two types of phosphorus in food supply, natural (organic forms) and added (inorganic forms)
[Source 20]What happens with too much phosphorus / phosphate intake?
As shown in Figure 2, serum inorganic phosphate (Pi) rises as total phosphorus intake increases. Excess phosphorus intake from any source is expressed as hyperphosphatemia, and essentially all the adverse effects of phosphorus excess are due to the elevated in inorganic phosphate (Pi) the ECF (extracellular fluid).
The principal effects that have been attributed to hyperphosphatemia are:
- Adjustments in the hormonal control system regulating the calcium economy,
- Ectopic (metastatic) calcification, particularly of the kidney,
- In some animal models, increased porosity of the skeleton, and
- A suggestion that high phosphorus intakes could reduce calcium absorption by complexing calcium in the chyme.
Concern about high phosphorus intake has been raised in recent years because of a probable population-level increase in phosphorus intake through such sources as cola beverages and food phosphate additives 15.
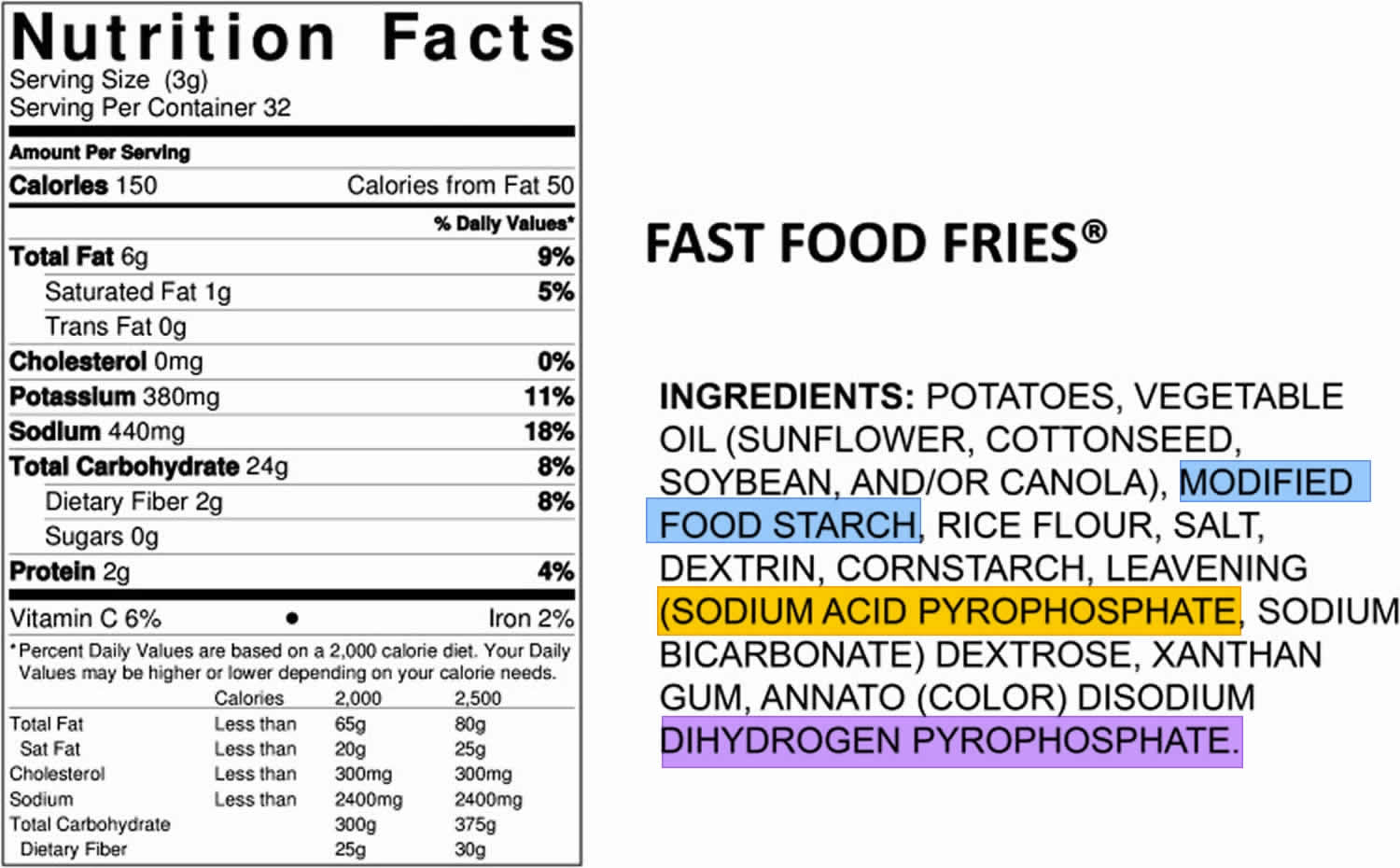
The phosphorus content of the U.S. food supply continues to increase as food manufacturers find new and effective ways to improve taste, speed of preparation, shelf life, or convenience of products through the addition of phosphate ingredients. This growing use of phosphate additives is captured in the nutrient database as foods are reanalyzed for their nutrient composition. An example of such a product can be seen in Figure 4, where the ingredients list shows 3 phosphate additives used to process fast food fries (modified food starch, sodium acid pyrophosphate, and disodium dihydrogen pyrophosphate). The growth in availability and intake of convenience and fast foods is contributing to the changing phosphorus content of the U.S. food supply and to increased intake of phosphorus by those individuals consuming more of these foods, essentially without their knowledge or understanding. For example, few consumers would consider french fries to be a source of phosphorus additives.
Figure 4. Food ingredients list showing 3 phosphate additives
Footnotes: The phosphorus content of the U.S. food supply continues to increase as food manufacturers find new and effective ways to improve taste, speed of preparation, shelf life, or convenience of products through the addition of phosphate ingredients. This growing use of phosphate additives is captured in the nutrient database as foods are reanalyzed for their nutrient composition. An example of such a product can be seen in Figure 1, where the ingredients list shows 3 phosphate additives used to process fast food fries (modified food starch, sodium acid pyrophosphate, and disodium dihydrogen pyrophosphate). The growth in availability and intake of convenience and fast foods is contributing to the changing phosphorus content of the U.S. food supply and to increased intake of phosphorus by those individuals consuming more of these foods, essentially without their knowledge or understanding. For example, few consumers would consider french fries to be a source of phosphorus additives.
[Source 20]It has been reported that high intakes of polyphosphates, such as are found in food additives, can interfere with absorption of iron, copper, and zinc36; however, described effects are small, and have not been consistent across studies 37. For this reason, as well as because trace mineral status may be low for many reasons, it was not considered feasible to use trace mineral status as an indicator of excess phosphorus intake. Nevertheless, given the trend toward increased use of phosphate additives in a variety of food products 38, it would be well to be alert to the possibility of some interference in individuals with marginal trace mineral status.
Unbalanced Phosphorus Intake Relative to Calcium Intake Affects Health
The typical dietary pattern of many Americans is high in phosphorus relative to calcium intake, and it is well established that the physiologic reaction to excess phosphorus intake is significantly influenced by its balance with calcium. Animal studies have shown that high dietary phosphorus relative to calcium can induce secondary hyperparathyroidism, bone resorption, lower peak bone mass, and fragile bones in young and old animals 39. Dietary guidelines recommend relative intakes of these minerals at 1:1 molar intake ratios or 1.5:1 mass intake ratios [calcium-to-phosphorus (Ca:P) ratio, both in milligrams] 39. In reality, the calcium-to-phosphorus (Ca:P) ratio in the typical U.S. diet is well below the recommended guidelines. For 25% of the U.S. population, these ratios are <0.6. In animal studies, mass intake ratios ≤0.5 have been shown to be detrimental to bone, even when calcium intake was considered adequate 39.
Pettifor et al. 40 noted the importance of balancing intake between these minerals in a chronic feeding study in young vitamin D–replete baboons. Baboons were fed 1 of 3 experimental diets, each with adequate phosphorus, but high, normal, or low calcium levels, containing 1:0.78, 1:2.2, and 1:7.7 calcium-to-phosphorus (Ca:P) mass ratios, respectively, and a fourth diet low in both minerals with a mass ratio of 1:2.3. By 16 months, baboons fed the low-calcium, normal-phosphorus diet (1:7.7) showed histologic evidence of hyperparathyroidism and bone loss, whereas baboons fed the low-calcium, low-phosphorus diet (1:2.3) showed only histologic features of osteomalacia (poorly calcified bone). These findings suggest that the balance in intake between these 2 minerals may have greater influence than the absolute level of phosphorus. This appears to be true for human populations as well, as shown in a cross-sectional study of young Finnish women 41. The calcium intake of the women was greater than the Recommended Dietary Allowance (RDA) in all but 1 quartile, whereas phosphorus intake was greater than twice the Recommended Dietary Allowance (RDA) in all quartiles. In the quartile with the lowest intake of calcium, the Ca:P intake ratio was 0.56 and the mean serum PTH concentration was significantly higher compared with the other quartiles, whose mass intake ratios were >0.7. Similar to animal models, high dietary phosphorus–induced, persistently elevated PTH has been shown to adversely affect peak bone mass and bone fragility with aging 39.
Recent clinical evidence from the Gambia stresses the importance of balance in the Ca:P intake ratio in children 42. Braithwaite et al. 42 showed that such an imbalance was present in Gambian children with active rickets who consumed natural food diets containing adequate dietary phosphorus but deficient in calcium (Ca:P molar ratio of 0.26–0.27). These children had higher fibroblast growth factor-23 [FGF-23] (65 vs. 54 RU/mL) than did local children without rickets consuming diets with higher molar intake ratios (0.32–0.49). In children, the skeletal effects of calcium deficiency may be exacerbated with greater imbalance in calcium and phosphorus intakes, even when phosphorus intake is low or moderate. In the Finnish study 41, adequate intake of calcium did not correct this imbalance when phosphorus intake was in great excess relative to calcium. Both natural and added sources of phosphorus appear to influence the Ca:P intake ratio. If either source of phosphorus is in excess relative to calcium, disordered homeostasis through an elevation in PTH and/or fibroblast growth factor-23 [FGF-23] can occur. These data suggest that the Ca:P intake ratio should be factored into analyses of the effect of high-phosphorus intake on chronic disease development.
Metastatic Calcification
The most serious, clearly harmful effect of hyperphosphatemia is calcification of nonskeletal tissues. This occurs when the calcium and phosphorus concentrations of ECF exceed the limits of solubility for secondary calcium phosphate (CaHPO4). This critical concentration is strongly dependent on amounts of other ions in the ECF, especially HCO3 – citrate, H+, and K+, and so cannot be unambiguously defined. However, tissue calcification virtually never occurs at ECF calcium × phosphorus ion products less than ∼4 (mmol/ liter)2 [∼1(mg/dl)2]. Although ECF in adults is normally less than half-saturated with respect to CaHPO4, elevation of plasma Pi, if extreme, can bring the ECF to the point of saturation. Although both calcium and phosphate are involved in such ectopic mineralization, ECF calcium levels are tightly regulated and are usually affected little by even large variations in calcium intake. By contrast, the sensitivity of ECF Pi to joint effects of diet and renal clearance means that an elevation in ECF Pi will usually be the cause of supersaturation. When calcification involves the kidney, renal function can deteriorate rapidly, renal phosphorus clearance drops, and ECF Pi rises yet further, leading to a rapid downhill spiral.
Under saturated conditions, susceptible tissue matrices will begin to accumulate CaHPO4 crystals, particularly if local pH rises above 7.4. Saturation of ECF with respect to calcium and phosphorus almost never occurs in individuals with normal renal function, mainly because urine phosphate excretion rises in direct proportion to dietary intake. As Figure 2 shows, the upper limit of the normal adult range for serum Pi typically occurs at absorbed intakes above 2.2 g (71 mmol)/day. At 62.5 percent absorption, that means ingested intakes above 3.4 g (110 mmol)/day. The 1994 CSFII data indicate that the reported intake at the ninety-fifth percentile was 2.5 g (81.7 mmol)/day in boys aged 14 through 18 years. Hyperphosphatemia from dietary causes becomes a problem mainly in patients with end-stage renal disease or in such conditions as vitamin D intoxication. When functioning kidney tissue mass is reduced to less than ∼20 percent of normal, the GFR becomes too low to clear typical absorbed loads of dietary phosphorus, and then even sharply reduced phosphorus diets may still be excessive as they lead to hyperphosphatemia.
Although metastatic calcification can occur in patients with endstage renal disease in whom ECF Pi levels are not adequately controlled, it is not known to occur from dietary sources alone in persons with adequate renal function. For that reason, calcification in previously normal kidneys produced by high phosphorus intakes has been studied mainly in rats and mice 43. Production of calcification has required very high phosphate loads over and above the animals’ already high basal phosphate intakes and in several reports has required partial reduction of renal tissue mass, as well.
Skeletal Porosity
Skeletal lesions associated with high phosphorus intakes have been described in rabbits 44 and bulls 45. As with kidney toxicity, the bony lesions required extremely large phosphate intakes (in rabbits, about 40-fold typical human intakes on a body weight basis, and in bulls, feeding of a ration designed to support milk production in cows). None of these situations has any evident direct relevance to human nutrition or to human dietary intake of phosphate. Krook et al. 45 also noted that bone loss develops in household pets and zoo animals fed human table scraps and meat. Despite acknowledging that such foods are poor in calcium, they attribute the bone loss to the high phosphorus content of such diets. Lacking evidence that phosphorus would produce this effect with diets adequate in calcium, this conclusion seems unwarranted. Finally, given the evidence cited above that high phosphorus intakes in humans do not lead to negative calcium balance or to increased bone resorption, it seems likely that the bone disease in other animals is more a consequence of low effective calcium intake than of high phosphorus intake per se.
Interference with Calcium Absorption
As noted, some concerns have been expressed that a high phosphorus intake could interfere with calcium nutrition by complexing calcium in the chyme and reducing its absorption 38. Given the relative absorption efficiencies of calcium and phosphorus, there would not be a stoichiometric excess of phosphorus relative to calcium in the chyme until the Ca:P intake ratio fell below 0.375:1. However, even this is a purely theoretical concern. In the studies of Spencer et al. 46, in which inorganic neutral phosphate was added to the diet, and of Heaney and Recker 47, who studied women on their habitual intakes of food phosphorus, even Ca:P ratios as low as 0.08:1 did not lower calcium absorption. Nevertheless, it must be noted that it is more difficult for the body to compensate for impaired calcium absorption at low dietary calcium intakes compared with higher intakes 48. As prior expert panels have noted 49, even the theoretical potential for interference with the calcium economy by high phosphorus intakes is effectively negated if calcium intake is adequate.
Conclusions
The evidence of increasing phosphorus intake is clear, with more compounds being added to the food supply and more foods consumed as processed or pre-prepared, and the risk of exceeding the current upper intake level is feasible for large segments of the population. Beyond that, there is accumulating evidence that both the high intakes and the poor balance of intake with other nutrients may place individuals at risk of kidney disease, bone loss, cardiovascular disease 50, 51 and other chronic health conditions 52. However, evidence linking these in the general population remains weak at this time 52.
- USDA (2002c) The use of trisodium phosphate as an antimicrobial agent in poultry processing in the United States. USDA-FSIS, Office of International Affairs. November 2002.[↩]
- EC (1995) Directive 95/2/EC of 20 february 1995 amended.[↩]
- Federal Register. Use of trisodium phosphate on raw, chilled poultry carcasses. Fed Regist. 1994;59:551–554.[↩]
- SCVPH (1998) Report on benefits and limitations of antimicrobial treatments for poultry carcasses, adopted on 30 October 1998.[↩]
- Capita R, Alonso-Calleja C, García-Fernández MC, Moreno B. (2002) Review: Trisodium phosphate (TSP) treatment for decontamination of poultry. Food Sci. Tech. Int. 8: 11-24.[↩]
- Keener, K. M., Bashor, M. P., Curtis, P. A., Sheldon, B. W. and Kathariou, S. 2004. Comprehensive review of Campylobacter and poultry processing. Comp. Rev. Food Sci. Food Saf. 3: 105-116.[↩]
- Hollender R, Bender FG, Jenkins RK, Black CL (1993) Consumer evaluation of chicken treated with a trisodium phosphate application during processing. Poultry Sci. 72: 755-759.[↩]
- Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Treatment of poultry carcasses with chlorine dioxide, acidified sodium chlorite, trisodium phosphate and peroxyacids. The EFSA Journal (2005) 297, p.16 of 27. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2006.297[↩][↩][↩][↩][↩][↩][↩]
- Assessment of the possible effect of the four antimicrobial treatment substances on the emergence of antimicrobial resistance – Scientific Opinion of the Panel on Biological Hazards. The EFSA Journal (2008) 659, 17-26. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2008.659[↩][↩]
- Capita R, Alonso-Calleja C, Garcia-Fernandez MC, Moreno B. Review: Trisodium phosphate (TSP) treatment for decontamination of poultry. Food Sci Technol Int. 2002;8:11–24.[↩]
- SCVPH (2003) Opinion on the evaluation of antimicrobial treatments for poultry carcasses, adopted on 14-15 April 2003.[↩]
- WHO (1982) Food additives series 17. Geneva.[↩]
- SCF (1991) Reports of the SCF, 25th series.[↩]
- EFSA (2005) Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to Semicarbazide in food. The EFSA Journal 219:1-36.[↩]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): National Academies Press (US); 1997. 5, Phosphorus. Available from: https://www.ncbi.nlm.nih.gov/books/NBK109813[↩][↩][↩][↩]
- Diem K. Documenta Geigy. Ardsley, NY: Geigy Pharmaceuticals; 1970.[↩]
- Fomon SJ, Nelson SE. Calcium, phosphorus, magnesium, and sulfur. In: Fomon SJ, editor. Nutrition of Normal Infants. St. Louis: Mosby-Year Book, Inc.; 1993. pp. 192–216.[↩]
- Aloia JF, Vaswani AN, Yeh JK, Ellis K, Cohn SH. Total body phosphorus in postmenospausal women. Miner Electrolyte Metab. 1984;10:73–76[↩]
- Institute of Medicine Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D and fluoride. Washington, DC: National Academies Press; 1997[↩]
- Calvo MS, Moshfegh AJ, Tucker KL. Assessing the Health Impact of Phosphorus in the Food Supply: Issues and Considerations. Advances in Nutrition. 2014;5(1):104-113. doi:10.3945/an.113.004861.[↩][↩][↩][↩]
- Brodehl J, Gellissen K, Weber HP. Postnatal development of tubular phosphate reabsorption. Clin Nephrol. 1982;17:163–171[↩]
- Specker BL, Lichtenstein P, Mimouni F, Gormley C, Tsang RC. Calcium-regulating hormones and minerals from birth to 18 months of age: A cross-sectional study. II. Effects of sex, race, age, season, and diet on serum minerals, parathyroid hormone, and calcitonin. Pediatrics. 1986;77:891–896.[↩][↩][↩]
- Atkinson SA, Alston-Mills BP, Lonnerdal B, Neville MC, Thompson MP. Major minerals and ionic constituents of human and bovine milk. In: Jensen RJ, editor. Handbook of Milk Composition. California: Academic Press; 1995. pp. 593–619.[↩]
- Carroll MD, Abraham S, Dresser CM. Dietary intake source data: United States, 1976–1980. Data from the National Health Survey, Vital and Health Statistics series 11. no. 231. Hyattsville, MD: National Center for Health Statistics, Public Health Service, U.S. Department of Health and Human Services; 1983. DHHS Publ. No. (PHS) 83–1681.[↩]
- Atkinson SA, Alston-Mills BP, Lonnerdal B, Neville MC, Thompson MP. Major minerals and ionic constituents of human and bovine milk. In: Jensen RJ, editor. Handbook of Milk Composition. California: Academic Press; 1995. pp. 593–619[↩]
- DeVizia B, Mansi A. Calcium and phosphorus metabolism in full-term infants. Monatsschr Kinderheilkd. 1992;140:S8–S12.[↩]
- Lemann J Jr. Calcium and phosphate metabolism: An overview in health and in calcium stone formers. In: Coe FL, Favus MJ, Pak CY, Parks JH, Preminger GM, editors. Kidney Stones: Medical and Surgical Management. Philadelphia, PA: Lippincott-Raven; 1996. pp. 259–288[↩]
- Ziegler EE, Fomon SJ. Lactose enhances mineral absorption in infancy. J Pediatr Gastroenterol Nutr. 1983;2:228–294.[↩]
- Stanbury SW. The phosphate ion in chronic renal failure. In: Hioco DJ, editor. Phosphate et Metabolisme Phosphocalcique. Paris: Sandoz Laboratories; 1971.[↩]
- Lemann J Jr. Calcium and phosphate metabolism: An overview in health and in calcium stone formers. In: Coe FL, Favus MJ, Pak CY, Parks JH, Preminger GM, editors. Kidney Stones: Medical and Surgical Management. Philadelphia, PA: Lippincott-Raven; 1996. pp. 259–288.[↩][↩]
- Heaney RP, Weaver CM, Fitzsimmons ML. Influence of calcium load on absorption fraction. J Bone Miner Res. 1990;5:1135–1138.[↩]
- Heaney RP, Recker RR, Stegman MR, Moy AJ. Calcium absorption in women: Relationships to calcium intake, estrogen status, and age. J Bone Miner Res. 1989;4:469–475.[↩]
- Chen TC, Castillo L, Korycka-Dahl M, DeLuca HF. Role of vitamin D metabolites in phosphate transport of rat intestine. J Nutr. 1974;104:1056–1060.[↩]
- Brickman AS, Coburn JW, Massry SG. 1,25 dihydroxy-vitamin D3 in normal man and patients with renal failure. Ann Intern Med. 1974;80:161–168.[↩]
- Moya M, Cortes E, Ballester MI, Vento M, Juste M. Short-term polycose substitution for lactose reduces calcium absorption in healthy term babies. J Pediatr Gastroenterol Nutr. 1992;14:57–61.[↩][↩]
- Bour NJS, Soullier BA, Zemel MB. Effect of level and form of phosphorus and level of calcium intake on zinc, iron and copper bioavailability in man. Nutr Res. 1984;4:371–379.[↩]
- Snedeker SM, Smith SA, Greger JL. Effect of dietary calcium and phosphorus levels on the utilization of iron, copper, and zinc by adult males. J Nutr. 1982;112:136–143.[↩]
- Calvo MS, Park YK. Changing phosphorus content of the U.S. diet: Potential for adverse effects on bone. J Nutr. 1996;126:1168S–1180S.[↩][↩]
- Calvo MS, Park YK. Changing phosphorus content of the US diet: potential for adverse effects on bone. J Nutr. 1996;126(suppl):1168S–80S[↩][↩][↩][↩]
- Pettifor JM, Marie PJ, Sly MR, du Bruyn DB, Ross F, Isdale JM, Dekerk W, Van der Walt WH. The effects of differing dietary calcium and phosphorus contents on mineral metabolism and bone histomorphometry in young vitamin D-replete baboons. Calcif Tissue Int. 1984;36:668–76[↩]
- Kemi VE, Kärkkäinen MUA, Rita HJ, Laaksonen MM, Outila TA, Lamberg-Allardt CJ. Low calcium:phosphorus ratio in habitual diets affects serum parathyroid hormone concentration and calcium metabolism in healthy women with adequate calcium intake. Br J Nutr. 2010;103:561–8[↩][↩]
- Braithwaite V, Jarjou LMA, Goldberg GR, Jones H, Pettifor JM, Prentice A. A follow-up study of Gambian children with rickets-like bone deformities and elevated plasma FGF-23: possible aetiological factors. Bone. 2012;50:218–25[↩][↩]
- NRC (National Research Council); Committee on Animal Nutrition; Board on Agriculture. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academy Press; 1995.[↩]
- Jowsey J, Balasubramaniam P. Effect of phosphate supplements on soft tissue calcification and bone turnover. Clin Sci. 1972;42:289–299[↩]
- Krook L, Whalen JP, Lesser GV, Berens DL. Experimental studies on osteoporosis. Methods Achiev Exp Pathol. 1975;7:72–108.[↩][↩]
- Spencer H, Kramer L, Osis D, Norris C. Effect of phosphorus on the absorption of calcium and on the calcium balance in man. J Nutr. 1978;108:447–457.[↩]
- Heaney RP, Recker RR. Effects of nitrogen, phosphorus, and caffeine on calcium balance in women. J Lab Clin Med. 1982;99:46–55[↩]
- Heaney RP. Vitamin D: Role in the calcium economy. In: Feldman D, Glorieux FH, Pike JW, editors. Vitamin D. San Diego, CA: Academic Press; 1997. pp. 485–497[↩]
- Chinn HI. Effects of dietary factors on skeletal integrity in adults: Calcium, phosphorus, vitamin D, and protein. 1981. Prepared for Bureau of Foods, Food and Drug Administration, U.S. Department of Health and Human Services, Washington, D.C.[↩]
- Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Am J Kidney Dis. 1998 Apr; 31(4):607-17. https://www.ncbi.nlm.nih.gov/pubmed/9531176/[↩]
- Serum phosphorus levels associate with coronary atherosclerosis in young adults. Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. J Am Soc Nephrol. 2009 Feb; 20(2):397-404. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2637043/[↩]
- Calvo MS, Moshfegh AJ, Tucker KL. Assessing the Health Impact of Phosphorus in the Food Supply: Issues and Considerations. Advances in Nutrition. 2014;5(1):104-113. doi:10.3945/an.113.004861. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3884091/[↩][↩]