Contents
What is ginkgo biloba
Ginkgo biloba also called Ginkgo, is one of the most ancient seed plant, often referred to as a “living fossil”, because Ginkgo species from as early as the Jurassic Period 170 million years ago have been identified 1. The sole surviving member of the Ginkgoaceae family, Ginkgo biloba, is a highly adaptable and hardy tree that is dioecious, having separate male and female plants. Some specimens are over 1,000 years old 2. Ginkgo trees begin reproducing after approximately 20 years of maturation, at which time the female produces fruit that is distinctly malodorous 2. The unique, bilobed leaves of Ginkgo biloba are fan-shaped and are similar in appearance to those of the maidenhair fern, giving rise to the common name “maidenhair tree” 2. This large tree may live over 1000 years and reach 40 m of height. Originally native to China, Ginkgo biloba is now cultivated worldwide. The attractive appearance of ginkgo trees and their robust nature have lead to widespread cultivation of Ginkgo biloba as an ornamental plant, expanding its range from its origins in China to every country in the temperate zone 3. In addition to its horticultural popularity, various parts of the Ginkgo biloba plant have been used for food or medicine. Ginkgo seeds are regularly consumed in Japan, Korea, and China, despite the fact that poisoning, manifesting as gastrointestinal distress, irritability, and tonic or clonic seizures, can result from overconsumption of seeds due to their high 4′-O-methylpyridoxine content 4.
Extract from Ginkgo biloba leaves has been used in traditional Chinese medicine for centuries to treat circulatory disorders, asthma, tinnitus, vertigo, and cognitive problems 5. Today, Ginkgo biloba extracts are one of the most commonly taken phytomedicines globally 6 and are often prescribed in Europe as a nootropic agent (cognitive enhancer) in old age and dementia 7. Ginkgo biloba extract contains mainly terpene trilactones, flavonol glycosides, biflavones, alkylphenols, phenolic acids, polyprenols and proanthocyanidins 8. The most prevalent of these groups are the flavonol glycosides (quercetin, catechin) 9. The terpenoids include ginkgolides and bilobalides, which represent unique components of Ginkgo biloba. Terpene trilactones, flavonol glycosides and proanthocyanidins are thought to be responsible for the pharmacological properties of Ginkgo biloba 5, whereas, ginkgolic acids are typically regarded as causing negative biological activities (e.g., cytotoxicity, mutagenicity) 10.
Ginkgotoxin, or 4′-O-methylpyridoxine, is also associated with toxicity, but is found primarily in Ginkgo biloba seeds and only in lesser amounts in the Ginkgo biloba leaves and extracts prepared from the leaves 11. In recent years, the use of fingerprinting to quantify key chemical constituents of plant-based formulations has been advocated as a quality control measure to ensure appropriate quantities of the biologically-active constituents and a lack of ginkgolic acids 8.
Figure 1. Ginkgo biloba
Ginkgo biloba is commonly available as a oral tablets, capsules or teas, but may also be available in liquid extracts. Ginkgo biloba extract is typically taken orally. Don’t eat raw or roasted Ginkgo biloba seeds, which can be poisonous.
Ginkgo extract is made from the leaves of the Ginkgo biloba tree. The Ginkgo biloba leaves contain a complex mixture of components. The exact formulation of Ginkgo biloba extract in products available to consumers in the U.S. can vary from manufacturer to manufacturer. Ginkgo is marketed in the U.S. as a dietary supplement and the FDA regulations for dietary supplements are different from regulations for prescription or over-the-counter drugs. Federal law does not require dietary supplements to go through the same standards of premarket testing for safety or efficacy as drugs intended to treat, cure, prevent, diagnose, or mitigate disease. In contrast, in Germany and France, Ginkgo biloba extract is regulated as a prescription drug and therefore, requires registration and adherence to specified content standards. The German Commission E (the German equivalent to the U.S. Food and Drug Administration [FDA]), which evaluated the efficacy and safety of herbals licensed for prescription in Germany, approved the monograph on Ginkgo biloba extract specifying contents of 22% to 27% flavone glycosides, 5% to 7% terpene lactones (2.8% to 3.4% ginkgolides A, B, C and 2.6% to 3.2% bilobalide), and not more than 5 ppm ginkgolic acids, due to their cytotoxic and allergenic potential 12. However, Ginkgo biloba products on the marketplace in the United States and elsewhere exhibit a wide range of component concentrations 13.
In the U.S., people take Ginkgo biloba for a wide variety of health reasons, mostly to improve brain function and memory. However, clinical trials designed to assess the efficacy of Ginkgo biloba extract have not produced consistent evidence of benefit.
On the basis of animal studies, several mechanisms have been proposed to explain the pharmacological properties of this plant: extract from Ginkgo biloba leaves inhibits platelet-activating factor 14 and enhances nitric oxide (NO) production in vessels, with subsequent effect on peripheral and cerebral blood flow 15. Ginkgo biloba extract is thought to modulate different neurotransmitter systems: it is a strong inhibitor of monoamine oxidase A (MOA) and synaptosomal uptake of DA (dopamine), 5-HT (serotonin) and norepinephrine 16. Additionally, Ginkgo biloba displays a free radical scavenger activity and has neuroprotective and antiapoptotic properties, such as inhibition of amyloid-β neurotoxicity and protection against hypoxic challenges and increased oxidative stress 17.
The effect of Ginkgo biloba has been studied in a variety of neuropsychiatric conditions. However, the general lack of evidence prevents drawing conclusions regarding Ginkgo biloba effectiveness in many neuropsychiatric conditions, such as autism, ADHD, addiction, generalized anxiety disorder, and tardive dyskinesia 9. Of all the psychiatric disorders reviewed, dementia has been the most extensively studied. A recent meta-analysis of eight studies in dementia showed that Ginkgo biloba differed significantly from placebo, providing beneficial effects both in cognition and activities of daily living. The results are consistent with another recent 2010 meta-analysis 18 on the effect of Ginkgo biloba on cognition. On the other hand, the review authors found a significant difference between Ginkgo biloba and placebo for activities of daily living in patients with dementia which were not significant in the aforementioned report 18.
Ginkgo biloba key facts
- There have been a lot of studies on the possible health effects and risks of people using Ginkgo biloba.
- There’s no conclusive evidence that Ginkgo biloba is helpful for any health condition.
- Ginkgo biloba doesn’t help prevent or slow dementia or cognitive decline, according to studies 19, 20, including the long-term Ginkgo Evaluation Memory Study, which enrolled more than 3,000 older adults, ages 75 or older, between 2000 and 2008 21. Half took ginkgo, half did not. All participants in the Ginkgo Evaluation of Memory (GEM) study took tests of their thinking abilities. The researchers found that 120 milligrams Ginkgo biloba taken twice daily was not effective in reducing the incidence of dementia, lessening cognitive decline, reducing blood pressure or hypertension, or reducing cardiovascular disease events 21.
- There’s no strong evidence that ginkgo helps with memory enhancement in healthy people 22, 23, blood pressure 24, intermittent claudication 25, tinnitus 26, age-related macular degeneration 27, the risk of having a heart attack or stroke 28 or with other conditions.
- Ongoing research is looking at whether a compound in Ginkgo biloba may help with diabetes.
Research on ginkgo use for specific conditions shows:
- Dementia. There isn’t enough evidence to support the use of ginkgo to prevent dementia or treat people with mild cognitive impairment.
- Claudication. A review of the research suggests that taking ginkgo has no significant benefits for people with this condition.
Ginkgo’s effect on memory enhancement has had conflicting results. While some evidence suggests that ginkgo extract might modestly improve memory in healthy adults, most studies indicate that ginkgo doesn’t improve memory, attention or brain function.
While ginkgo appears to be safe in moderate amounts, research doesn’t support use of the supplement to prevent or slow dementia or cognitive decline. Further research is needed to find out what role ginkgo might play in supporting brain function and treating other conditions.
Can Ginkgo biloba prevent memory loss and improve cognitive function?
Ginkgo biloba extract, derived from the leaves of the Ginkgo biloba tree, is often touted as a memory aid. But it appears unlikely that Ginkgo biloba extract can slow or prevent age-related memory problems, or memory loss associated with mild cognitive impairment or Alzheimer’s disease. Several small, early studies showed modest improvements in cognitive function for older adults with dementia. However, a number of larger studies haven’t confirmed that Ginkgo biloba extract prevents memory loss or slows the progression of cognitive decline or Alzheimer’s disease in older adults. In adults with normal cognition or mild cognitive impairment, Ginkgo biloba does not slow cognitive decline.
Although some studies have shown slight improvements in cognitive function for people taking Ginkgo biloba, most experts feel that Ginkgo biloba hasn’t lived up to its early promise and don’t recommend its use as a memory aid.
Should I stop or start taking Ginkgo biloba extract?
Consumers should be aware that studies have not consistently demonstrated that ginkgo improves brain function. The new National Toxicology Program 29 findings showing that both rats and mice develop cancer after long-term use should also be taken into consideration. Additionally, ginkgo has been shown to interact with other drugs, which can increase or decrease their effects. It is always important to give your health care provider a full picture of what you do to manage your health, including taking dietary supplements. This will help ensure coordinated and safe care.
What do scientists know about Ginkgo biloba safety?
- For many healthy adults, ginkgo appears to be safe when taken by mouth in moderate amounts.
- Side effects of ginkgo may include headache, stomach upset, and allergic skin reactions. If you’re older, have a known bleeding risk, or are pregnant you should be cautious about ginkgo possibly increasing your risk of bleeding.
- In a 2012 research study 29, rodents given ginkgo had an increased risk of developing liver and thyroid cancer at the end of the 2-year tests.
- Ginkgo may interact with some conventional medications, including anticoagulants (blood thinners), research reviews show.
- Eating fresh (raw) or roasted ginkgo seeds can be poisonous and have serious side effects.
Ginkgo biloba extract
The two mechanisms of action most often associated with the proclaimed health benefits of Ginkgo biloba extract are the antagonism of Platelet Activating Factor
(PAF) by the ginkgolides and the antioxidant action of the flavonoids 30. However, there are multiple mechanisms of action that are likely to impact disease pathways. Several mechanisms of action identified through in vitro or in vivo studies are hypothesized to play a role in the health promoting action of Ginkgo biloba extract. These include: antagonism of Platelet Activating Factor by the ginkgolides 31; antioxidant activity of the flavonoids attributed to scavenging of reactive oxygen species, chelation of metal ions, and increasing the concentration of superoxide dismutase and glutathione-S-transferase 32; antagonism of major inhibitory receptors of the central nervous system, glycine, and GABA receptors 33; modulation of neurotransmitter concentrations or receptor densities 34; reduction of nitric oxide release 34; inhibition of mitochondrial dysfunction 35; and modulation of P450 enzymes 36.
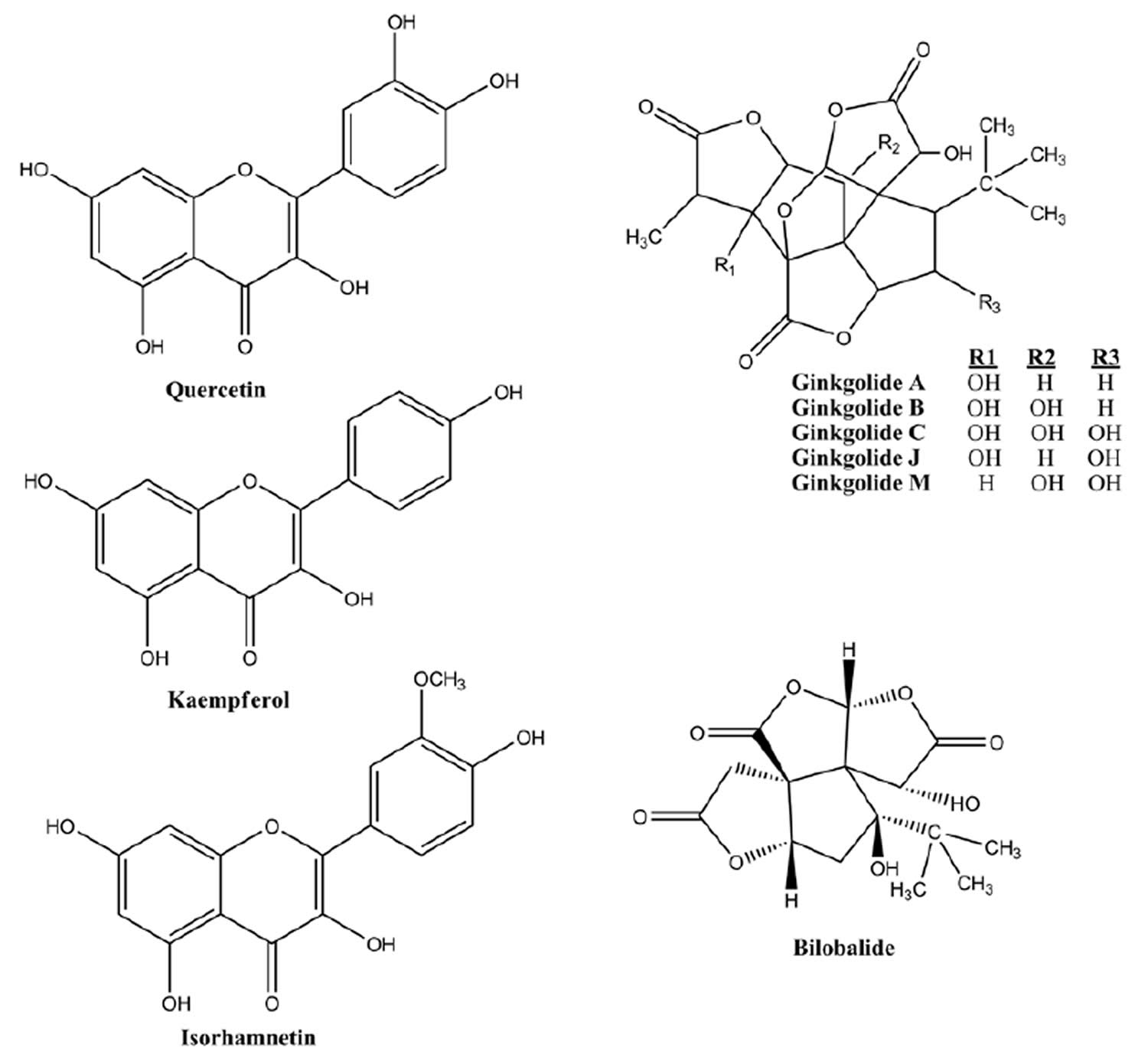
Figure 2. Ginkgo biloba extract bioactive compounds
Ginkgo biloba absorption, distribution, metabolism and excretion
Due to the complex nature of Ginkgo biloba extract with its many structurally diverse constituents, its precise pharmacokinetic profile remains undetermined. However, there have been many pharmacokinetics studies in humans and experimental animals that focus on key chemical classes such as the terpene trilactones
and select flavonol glycosides. In general, Ginkgo biloba extract is well absorbed in humans, rats, and rabbits after oral administration 37.
Ginkgo biloba uses
The therapeutic effect most frequently ascribed to Ginkgo biloba use in popular culture is improvement of memory and brain function, however, there are numerous other health benefits attributed to its use. In Germany, Ginkgo biloba is indicated for the treatment of intermittent claudication, decreased mental function (including forgetfulness, early dementia, and concentration problems), and tinnitus 38. Ginkgo biloba extract has been studied in humans as treatment for the neurological effects associated with Alzheimer’s disease, traumatic brain injury, stroke, dementia, normal aging, edema, tinnitus, and macular degeneration 39. Additionally, preclinical in vitro or experimental animal studies have explored the use of Ginkgo biloba for cardiovascular indications (thrombosis, embolism), anxiety/stress, sexual dysfunction, and cancer 39. Despite the popular use of Ginkgo biloba extract for numerous ailments, the largest clinical trial of Ginkgo biloba effects (Ginkgo Evaluation of Memory Study) failed to find an effect of Ginkgo biloba
on prevention of dementia 21, prevention of cognitive decline 40, reduction in cardiovascular disease-related events or mortality 41, or decreases in blood pressure or hypertension 42.
In the largest clinical trial of Ginkgo biloba to date, the Ginkgo Evaluation of Memory (GEM) study 21, the researchers looked at data on more than 3,000 people, ages 75 or older, between 2000 and 2008. Half took ginkgo, half did not. All participants in the Ginkgo Evaluation of Memory (GEM) study took tests of their thinking abilities. The researchers found that 120 milligrams Ginkgo biloba taken twice daily was not effective in reducing the incidence of dementia, lessening cognitive decline, reducing blood pressure or hypertension, or reducing cardiovascular disease events 21.
Ginkgo biloba dosage
Diamond et al. 43 reviewed the literature on the effectiveness of Ginkgo biloba extract and concluded
that positive effects on various endpoints were observed with dosages of 120 to 300 mg per day administered for a period of 3 to 12 weeks. Standardized Ginkgo biloba extract is typically taken in tablet or capsule form at doses in the range of 120 to 240 mg per day. In addition to the standardized extract, Ginkgo biloba is also available as a mother tincture (ethanol extraction of fresh leaves) and as dry leaves for use in tea 44.
Ginkgo biloba side effects
When used orally in moderate amounts, Ginkgo biloba appears to be safe for most healthy adults.
Ginkgo biloba can cause:
- Headache
- Dizziness
- Heart palpitations
- Upset stomach
- Constipation
- Allergic skin reactions
Don’t eat raw or roasted Ginkgo biloba seeds, which can be poisonous.
If you are epileptic or prone to seizures, avoid ginkgo. Large amounts of ginkgotoxin can cause seizures. Ginkgotoxin is found in Ginkgo biloba seeds and to a lesser extent, Ginkgo biloba leaves.
If you are older, have a bleeding disorder or are pregnant, don’t take Ginkgo biloba. The supplement might increase your risk of bleeding. If you’re planning to have surgery, stop taking Ginkgo biloba two weeks beforehand.
Ginkgo biloba might interfere with the management of diabetes. If you take ginkgo and have diabetes, closely monitor your blood sugar levels.
Bent et al. 45 assessed case reports and concluded a risk of spontaneous bleeding could be associated with Ginkgo biloba use. Kellermann and Kloft 46 found 21 reported cases of spontaneous bleeding associated with Ginkgo biloba extract through 2007, with one third of the cases involving concurrent use of antiplatelet or anticoagulant therapies. The hypothesized mechanism of toxicity is that antagonism of Platelet Activating Factor
(PAF) and collagen lead to inhibition of platelet aggregation 47. Kellermann and Kloft 46 conducted a systematic review and meta-analysis that did not find evidence of higher bleeding risk associated with Ginkgo biloba extract therapy. In isolated cases, ginkgo use was associated with increased blood pressure when combined with thiazide diuretics and coma when combined with trazodone 48. Seizures occurred in two previously well-controlled epileptics within 2 weeks of beginning Ginkgo biloba extract supplementation and resolved after stopping supplementation 49.
The ginkgolic acids present in the ginkgo plant are known to cause contact dermatitis in a manner similar to that elicited by urushiols in poison ivy with limited evidence of cross-reactivity between poison ivy and ginkgo 50. For this reason, in Germany, ginkgolic acids are restricted to less than 5 ppm in standardized preparations. Two cases of allergic skin reaction (diffuse mobilliform eruptions and acute generalized excanthematous pustulosis) following oral treatment with Ginkgo biloba products were reported, one in the United States 51 and one in Australia 52.
Some research has shown that rodents given Ginkgo biloba had an increased risk of developing liver and thyroid cancers
In 2012 the National Toxicology Program 29 looked at the long-term effects of Ginkgo biloba extract in mice and rats. Groups of 50 male and female rats received 100, 300, or 1,000 milligrams of Ginkgo biloba extract per kilogram of body weight, and groups of male or female mice received 200, 600, or 2,000 mg/kg each day. Similar groups of animals were given solutions of corn oil with no chemical added and served as the control groups. Ginkgo extract was given orally to the animals for up to 105 weeks. At the end of the two-year studies, tissues from more than 40 sites were examined for every animal. The National Toxicology Program study 29 found an increase in liver cancer in male and female mice, and in cancer of the thyroid gland in male and female rats and male mice.
What do the National Toxicology Program studies mean for humans?
The National Toxicology Program 29 rodent studies on Ginkgo biloba extract may be relevant to humans. However, these studies represent only the first step in determining if there is a human cancer risk from taking Ginkgo biloba extract as a dietary supplement. The next steps will include identification of components in the extract that may account for the cancer findings, along with the collection of additional information on human consumption of Ginkgo biloba extract.
Interactions with medicines
Possible interactions include:
- Alprazolam (Xanax). Taking Ginkgo biloba with this drug used to relieve symptoms of anxiety might reduce the drug’s effectiveness.
- Anticoagulants and anti-platelet drugs, herbs and supplements. These types of drugs, herbs and supplements reduce blood clotting. Taking Ginkgo biloba with them might increase your risk of bleeding.
- Anticonvulsants and seizure threshold lowering drugs, herbs and supplements. Large amounts of ginkgotoxin can cause seizures. Ginkgotoxin is found in ginkgo seeds and, to a lesser extent, ginkgo leaves. It’s possible that taking Ginkgo biloba could reduce the effectiveness of an anticonvulsant drug.
- Antidepressants. Taking Ginkgo biloba with certain antidepressants, such as fluoxetine (Prozac, Sarafem) and imipramine (Tofranil), might decrease their effectiveness.
- Certain statins. Taking ginkgo with simvastatin (Zocor) might reduce the drug’s effects. Ginkgo also appears to reduce the effects of atorvastatin (Lipitor).
- Diabetes drugs. Ginkgo biloba might alter your response to these drugs.
- Ibuprofen. It’s possible that combining Ginkgo biloba with ibuprofen (Advil, Motrin IB, others) might increase your risk of bleeding.
Toxicity Studies
Experimental animals
The LD50 (lethal dose 50 is where 50% of the test subjects die) of standardized Ginkgo biloba extract administered orally to mice was reported to be 7.73 g/kg
and the LD50 after intravenous administration was 1.1 g/kg for both rats and mice 53. There was no evidence of organ damage or impairment of hepatic or renal function when Ginkgo biloba extract was administered orally over 27 weeks to rats and mice at doses ranging from 100 to 1,600 mg/kg 53. Rats exposed to EGb 761®, which consists of 24% ginkgo-flavone glycosides and 6% terpenoids, at 4, 20, and 100 mg/kg per day for 2 years were reported to show no histopathological changes, however, details of this study are not available 54.
EGb 761®, originated by Dr Willmar Schwabe Pharmaceuticals 55 in the 1990s, is a well-defined mixture of active compounds extracted from Ginkgo biloba leaves according to a standardized procedure. EGb 761® contains approximately 24% flavone glycosides (primarily quercetin, kaempferol and isorhamnetin) and 6% terpene lactones (2.8% to 3.4% ginkgolides A, B and C, and 2.6% to 3.2% bilobalide). Ginkgolide B and bilobalide account for about 0.8% and 3% of the total extract, respectively. Other constituents include proanthocyanidins, glucose, rhamnose, organic acids, D-glucaric and ginkgolic acids. EGb 761 is applied in treating a wide range of disorders, such as hypertension, coronary heart disease, hyperlipidaemia, diabetes, asthma and cancer adjuvant therapy 56. There are many laboratory and trial reports focused on the applying of EGb in psychiatric medicine 57.
In a chronic study conducted in Fisher 344 rats with quercetin administered by dosed feed at concentrations of 0, 1,000, 10,000, or 40,000 ppm, there were no treatment-related effects on survival, clinical signs, organ weights, or hematological and clinical chemistry parameters 58. There were decreases in mean body weights and relative liver and kidney weights in the 40,000 ppm male and female rats.
- Zhou, Z., and Zheng, S. (2003). The missing link in Ginkgo evolution. Nature 423, 821-822.[↩]
- Mahadevan, S., and Park, Y. (2008). Multifaceted therapeutic benefits of Ginkgo biloba L.: Chemistry, efficacy, safety, and uses. J. Food Sci. 73, R14-R19.[↩][↩][↩]
- Del Tredici, P. (1991). Ginkgos and people: A thousand years of interaction. Arnoldia 51, 2-15.[↩]
- Kobayashi, D., Yoshimura, T., Johno, A., Sasaki, K., and Wada, K. (2011). Toxicity of 4′-O-methylpyridoxine-5′-glucoside in Ginkgo biloba seeds. Food Chem. 126, 1198-1202.[↩]
- Kleijnen J, Knipschild P. Ginkgo biloba. The Lancet. 1992;340(8828):1136–1139[↩][↩]
- Ernst E. The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John’s wort, ginseng, echinacea, saw palmetto, and kava. Annals of Internal Medicine. 2002;136(1):42–53[↩]
- Kennedy DO, Wightman EL. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Advances in Nutrition. 2011;2:32–50 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3042794/[↩]
- van Beek, T.A., and Montoro, P. (2009). Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 1216, 2002-2032.[↩][↩]
- Brondino N, De Silvestri A, Re S, et al. A Systematic Review and Meta-Analysis of Ginkgo biloba in Neuropsychiatric Disorders: From Ancient Tradition to Modern-Day Medicine. Evidence-based Complementary and Alternative Medicine : eCAM. 2013;2013:915691. doi:10.1155/2013/915691. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3679686/[↩][↩]
- Westendorf, J., and Regan, J. (2000). Induction of DNA strand-breaks in primary rat hepatocytes by ginkgolic acids. Pharmazie 55, 864-865.[↩]
- Arenz, A., Klein, M., Fiehe, K., Gross, J., Drewke, C., Hemscheidt, T., and Leistner, E. (1996). Occurrence of neurotoxic 4′-O-methylpyridoxine in Ginkgo biloba leaves, Ginkgo medications and Japanese Ginkgo food. Planta Med. 62, 548-551.[↩]
- Kressmann, S., Müller, W.E., and Blume, H.H. (2002). Pharmaceutical quality of different Ginkgo biloba brands. J. Pharm. Pharmacol. 54, 661-669.[↩]
- Gawron-Gzella, A., Marek, P., Chanaj, J., and Matlawska, I. (2010). Comparative analysis of pharmaceuticals and dietary supplements containing extracts from the leaves of Ginkgo biloba L. Acta Pol. Pharm. 67, 335-343.[↩]
- Koch E. Inhibition of platelet activating factor (PAF)-induced aggregation of human thrombocytes by ginkgolides: considerations on possible bleeding complications after oral intake of Ginkgo biloba extracts. Phytomedicine. 2005;12(1-2):10–16.[↩]
- Koltermann A, Hartkorn A, Koch E, Fürst R, Vollmar AM, Zahler S. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cellular and Molecular Life Sciences. 2007;64(13):1715–1722[↩]
- Yoshitake T, Yoshitake S, Kehr J. The Ginkgo biloba extract EGb 761 and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex: RESEARCH PAPER. British Journal of Pharmacology. 2010;159(3):659–668.[↩]
- Kampkötter A, Pielarski T, Rohrig R, et al. The Ginkgo biloba extract EGb761 reduces stress sensitivity, ROS accumulation and expression of catalase and glutathione S-transferase 4 in Caenorhabditis elegans. Pharmacological Research. 2007;55(2):139–147.[↩]
- Weinmann S, Roll S, Schwarzbach C, Vauth C, Willich SN. Effects of Ginkgo biloba in dementia: systematic review and meta-analysis. BMC Geriatrics. 2010;10, article 14 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2846949/[↩][↩]
- Long-term use of standardised ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. The Lancet Volume 11, ISSUE 10, P851-859, October 01, 2012. https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(12)70206-5/fulltext[↩]
- Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No.: CD003120. DOI: 10.1002/14651858.CD003120.pub3. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003120.pub3/full[↩]
- DeKosky, S.T., Williamson, J.D., Fitzpatrick, A.L., Ginkgo Evaluation of Memory (GEM) Study Investigators (2008). Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA 300, 2253-2262, 2730.[↩][↩][↩][↩][↩]
- Lee H, Birks J. Ginkgo biloba for cognitive improvement in healthy individuals (Protocol). Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No.: CD004671. DOI: 10.1002/14651858.CD004671. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD004671/full[↩]
- Birks J, Grimley Evans J, Van Dongen M. Ginkgo Biloba for Cognitive Impairment and Dementia (Cochrane Review). The Cochrane Library 2003, Issue 3.[↩]
- Brinkley TE, Lovato JF, Arnold AM, et al. Effect of Ginkgo biloba on blood pressure and incidence of hypertension in elderly men and women. American Journal of Hypertension. 2010;23(5):528-533. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2989407/[↩]
- Nicolaï SPA, Kruidenier LM, Bendermacher BLW, Prins MH, Stokmans RA, Broos PPHL, Teijink JAW. Ginkgo biloba for intermittent claudication. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No.: CD006888. DOI: 10.1002/14651858.CD006888.pub3. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD006888.pub3/full[↩]
- Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No.: CD003852. DOI: 10.1002/14651858.CD003852.pub3. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003852.pub3/full[↩]
- Evans JR. Ginkgo biloba extract for age-related macular degeneration. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No.: CD001775. DOI: 10.1002/14651858.CD001775.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD001775.pub2/full[↩]
- Zeng X, Liu M, Yang Y, Li Y, Asplund K. Ginkgo biloba for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No.: CD003691. DOI: 10.1002/14651858.CD003691.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003691.pub2/full[↩]
- NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF GINKGO BILOBA EXTRACT. https://ntp.niehs.nih.gov/ntp/about_ntp/trpanel/2012/february/drafttr578.pdf[↩][↩][↩][↩][↩]
- Smith, J.V., and Luo, Y. (2004). Studies on molecular mechanisms of Ginkgo biloba extract. Appl. Microbiol. Biotechnol. 64, 465-472[↩]
- Smith, P.F., Maclennan, K., and Darlington, C.L. (1996). The neuroprotective properties of the Ginkgo biloba leaf: A review of the possible relationship to platelet-activating factor (PAF). J. Ethnopharmacol. 50, 131-139.[↩]
- Shi, C., Liu, J., Wu, F., and Yew, D.T. (2010a). Ginkgo biloba extract in Alzheimer’s disease: From action mechanisms to medical practice. Int. J. Mol. Sci. 11, 107-123.[↩]
- Heads, J.A., Hawthorne, R.L., Lynagh, T., and Lynch, J.W. (2008). Structure-activity analysis of ginkgolide binding in the glycine receptor pore. J. Neurochem. 105, 1418-1427.[↩]
- Ahlemeyer, B., and Krieglstein, J. (2003). Neuroprotective effects of Ginkgo biloba extract. Cell. Mol. Life Sci. 60, 1779-1792.[↩][↩]
- Shi, C., Xiao, S., Liu, J., Guo, K., Wu, F., Yew, D.T., and Xu, J. (2010c). Ginkgo biloba extract EGb761 protects against aging-associated mitochondrial dysfunction in platelets and hippocampi of SAMP8 mice. Platelets 21, 373-379[↩]
- Rajaraman, G., Yang, G., Chen, J., and Chang, T.K. (2009). Modulation of CYP1B1 and CYP1A1 gene expression and activation of aryl hydrocarbon receptor by Ginkgo biloba extract in MCF-10A human mammary epithelial cells. Can. J. Physiol. Pharmacol. 87, 674-683.[↩]
- Li, C.L., and Wong, Y.Y. (1997). The bioavailability of ginkgolides in Ginkgo biloba extracts. Planta Med. 63, 563-565.[↩]
- van Beek, T.A., and Montoro, P. (2009). Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 1216, 2002-2032[↩]
- McKenna, D.J., Jones, K., and Hughes, K. (2001). Efficacy, safety, and use of ginkgo biloba in clinical and preclinical applications. Altern. Ther. Health Med. 75, 70-86, 88-90.[↩][↩]
- Snitz, B.E., O’Meara, E.S., Carlson, M.C., Arnold, A.M., Ives, D.G., Rapp, S.R., Saxton, J., Lopez, O.L., Dunn, L.O., Sink, K.M., DeKosky, S.T., and Ginkgo Evaluation of Memory (GEM) Study Investigators (2009). Ginkgo biloba for preventing cognitive decline in older adults: A randomized trial. JAMA 302, 2663-2670.[↩]
- Kuller, L.H., Ives, D.G., Fitzpatrick, A.L., Carlson, M.C., Mercado, C., Lopez, O.L., Burke, G.L., Furberg, C.D., DeKosky, S.T., and Ginkgo Evaluation of Memory Study Investigators (2010). Does Ginkgo biloba reduce the risk of cardiovascular events? Circ. Cardiovasc. Qual. Outcomes 3, 41-47.[↩]
- Brinkley, T.E., Lovato, J.F., Arnold, A.M., Furberg, C.D., Kuller, L.H., Burke, G.L., Nahin, R.L., Lopez, O.L., Yasar, S., and Williamson, J.D. (2010). Effect of Ginkgo biloba on blood pressure and incidence of hypertension in elderly men and women. Am. J. Hypertens. 23, 528-533[↩]
- Diamond, B.J., Shiflett, S.C., Feiwel, N., Matheis, R.J., Noskin, O., Richards, J.A., and Schoenberger, N.E. (2000). Ginkgo biloba extract: Mechanisms and clinical indications. Arch. Phys. Med. Rehabil. 81, 668-678.[↩]
- Ehrlich, S.D. (2010). Ginkgo biloba. University of Maryland Medical Center.[↩]
- Bent, S., Goldberg, H., Padula, A., and Avins, A. L. (2005). Spontaneous bleeding associated with Ginkgo biloba: A case report and systematic review of the literature. J. Gen. Intern. Med. 20, 657-661[↩]
- Kellermann, A.J. , and Kloft, C. (2011). Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? Pharmacotherapy 31, 490-502[↩][↩]
- Bent, S., Goldberg, H., Padula, A., and Avins, A. L. (2005). Spontaneous bleeding associated with Ginkgo biloba: A case report and systematic review of the literature. J. Gen. Intern. Med. 20, 657-661.[↩]
- Izzo, A.A., and Ernst, E. (2001). Interactions between herbal medicines and prescribed drugs: A systematic review. Drugs 61, 2163-2175.[↩]
- Granger, A.S. (2001). Ginkgo biloba precipitating epileptic seizures. Age Ageing 30, 523-525[↩]
- Lepoittevin, J.P., Benezra, C., and Asakawa, Y. (1989). Allergic contact dermatitis to Ginkgo bilobaL.: Relationship with urushiol. Arch. Dermatol. Res. 281, 227-230.[↩]
- Chiu, A.E., Lane, A.T., and Kimball, A.B. (2002). Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J. Am. Acad. Dermatol. 46, 145-146.[↩]
- Pennisi, R.S. (2006). Acute generalized exanthematous pustulosis induced by the herbal remedy Ginkgo biloba Med. J. Aust. 184, 583-584.[↩]
- Salvador, R.L. (1995). Herbal medicine – ginkgo. Can. Pharmacists J. 52, 39-41.[↩][↩]
- Woerdenbag, H.J., and De Smet, P.A.G.M. (2000). Adverse effects of and toxicity of Ginkgo extracts. In Ginkgo biloba (T.A. van Beek, Ed.), pp. 544-555. Harwood Academic Publishers, Amsterdam.[↩]
- Jaggy H, Koch E. Chemistry and biology of alkylphenols from Ginkgo biloba L. Pharmazie 1997;52(10):735-8.[↩]
- Clostre F. Ginkgo biloba extract (EGb 761). State of knowledge in the dawn of the year 2000. Annales Pharmaceutiques Françaises 1999;57(Suppl 1):8-88.[↩]
- Defeudis FV. Ginkgo Biloba Extract (EGb 761): Pharmacological Activity and Clinical Applications. Paris: Elsevier Press, 1991.[↩]
- Dunnick, J.K., and Hailey, J.R. (1992). Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Fundam. Appl. Toxicol. 19, 423-431.[↩]