Contents
- Gonadotropins
- Follicle stimulating hormone (FSH)
- Luteinizing hormone (LH)
- Human chorionic gonadotropin (hCG)
- Human chorionic gonadotropin function
- Human chorionic gonadotropin uses
- Human chorionic gonadotropin diagnostic use
- How is human chorionic gonadotropin used?
- What does human chorionic gonadotropin test result mean?
- How does the test that I do at home myself compare with the results of a test done in a lab?
- When is a blood human chorionic gonadotropin test ordered instead of a urine human chorionic gonadotropin?
- How many days after a miscarriage would it take for a urine pregnancy test to show a negative result?
- What is an ectopic pregnancy?
- Human chorionic gonadotropin as a Potential Biomarker for Preeclampsia
- Human chorionic gonadotropin as Serum Marker for Down’s Syndrome Screening
- Human chorionic gonadotropin as tumor marker
- Human Chorionic Gonadotropin (hCG) in Female Infertility Treatment
- Gonadotropin treatment
- Follicle stimulating hormone (FSH) therapeutic uses
- Luteinizing hormone (LH) use in Infertility and Assisted Reproductive Technology
- Gonadotropins use in Assisted Reproductive Technology
- Human chorionic gonadotropin (hCG) Therapeutic Uses in the Male
- Human chorionic gonadotropin (hCG) use in Assisted Reproductive Technology
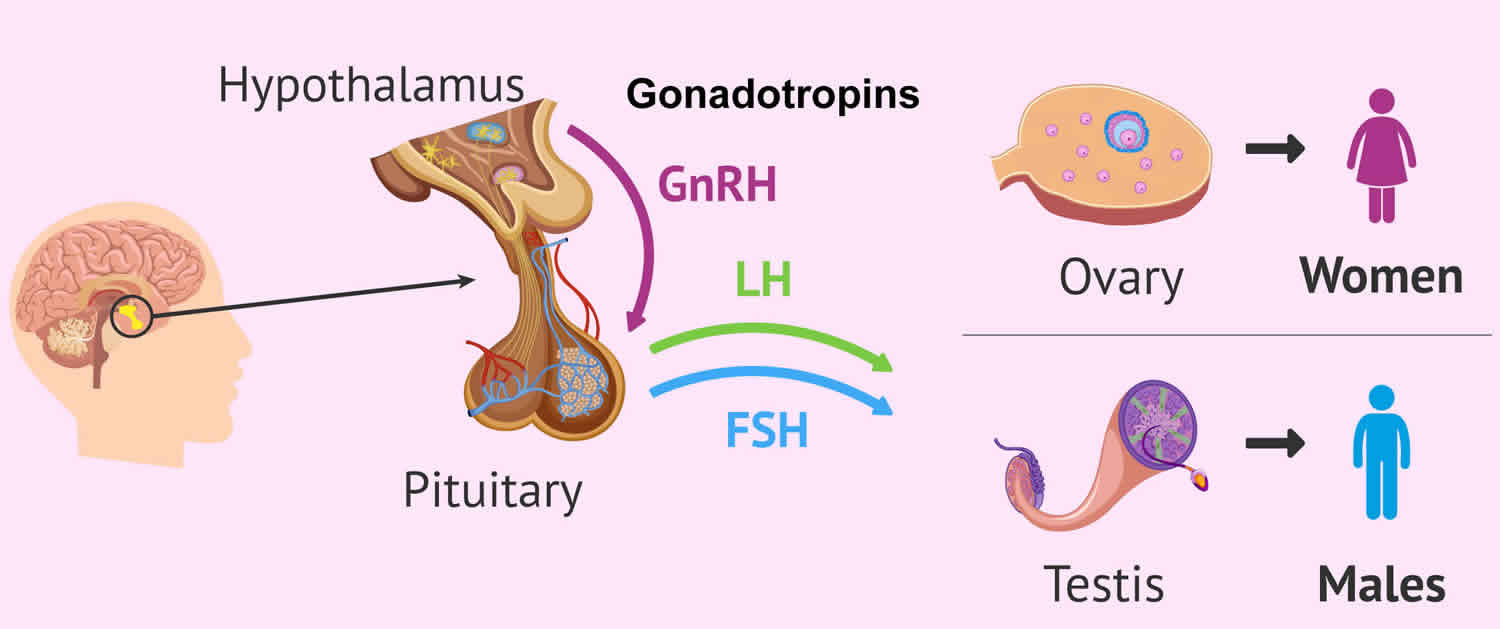
Gonadotropins
In the body, there are two types of gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), that are secreted from the anterior pituitary gland and that act on the gonads (i.e., the ovaries or testes) that are essential for normal sexual maturation and reproductive function in both males and females 1, 2, 3. Another type of gonadotropin found in women is human chorionic gonadotropin (hCG), which is produced by the placenta during pregnancy 3. The detection of human chorionic gonadotropin (hCG) forms the basis of pregnancy tests. Gonadotropins are glycoprotein hormones that regulate ovarian and testicular function and are essential for normal growth, sexual development and reproduction. In men, luteinizing hormone (LH) stimulates the Leydig cells in the testicles to make testosterone whilst the follicle-stimulating hormone (FSH) stimulates the development of sperm (spermatogenesis) by acting on special cells in the testes called Sertoli cells 4, 5, 6, 7. In women, gonadotropins (LH and FSH) cause the ovaries to make estrogen and progesterone. Gonadotrophs, cells that constitute about 10 percent of the pituitary gland, secrete two primary gonadotropins: luteinizing hormone (LH) and follicle-stimulating hormone (FSH) 2. Gonadotropin-releasing hormone (GnRH) causes the pituitary gland in the brain to make and secrete the hormones luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The amount and rate of secretion of these hormones vary widely at different ages and at different times during the menstrual cycle in women. Secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) is low in both males and females prior to puberty. Following puberty, more luteinizing hormone (LH) than follicle-stimulating hormone (FSH) is secreted. During the menstrual cycle there is a dramatic increase in the serum concentrations of both hormones at the time of ovulation, and the secretion of both hormones increases 10- to 15-fold in postmenopausal women.
The gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), are both glycoproteins made up of an alpha (α) and beta (β) subunit 8, 9. The alpha subunits are identical between the two hormones, but the beta subunit of each is different and gives each hormone its biological specificity 10, 11. The alpha (α) subunit of luteinizing hormone (LH) is made up of 92 amino acids, and the beta (β) subunit contains 120 amino acids 12, 11. The secretion of these hormones is regulated by the release of gonadotropin-releasing hormone (GnRH) secreted by the hypothalamus.
Luteinizing hormone (LH) plays an important role in sexual development and is produced by the pea-sized pituitary gland in the brain.
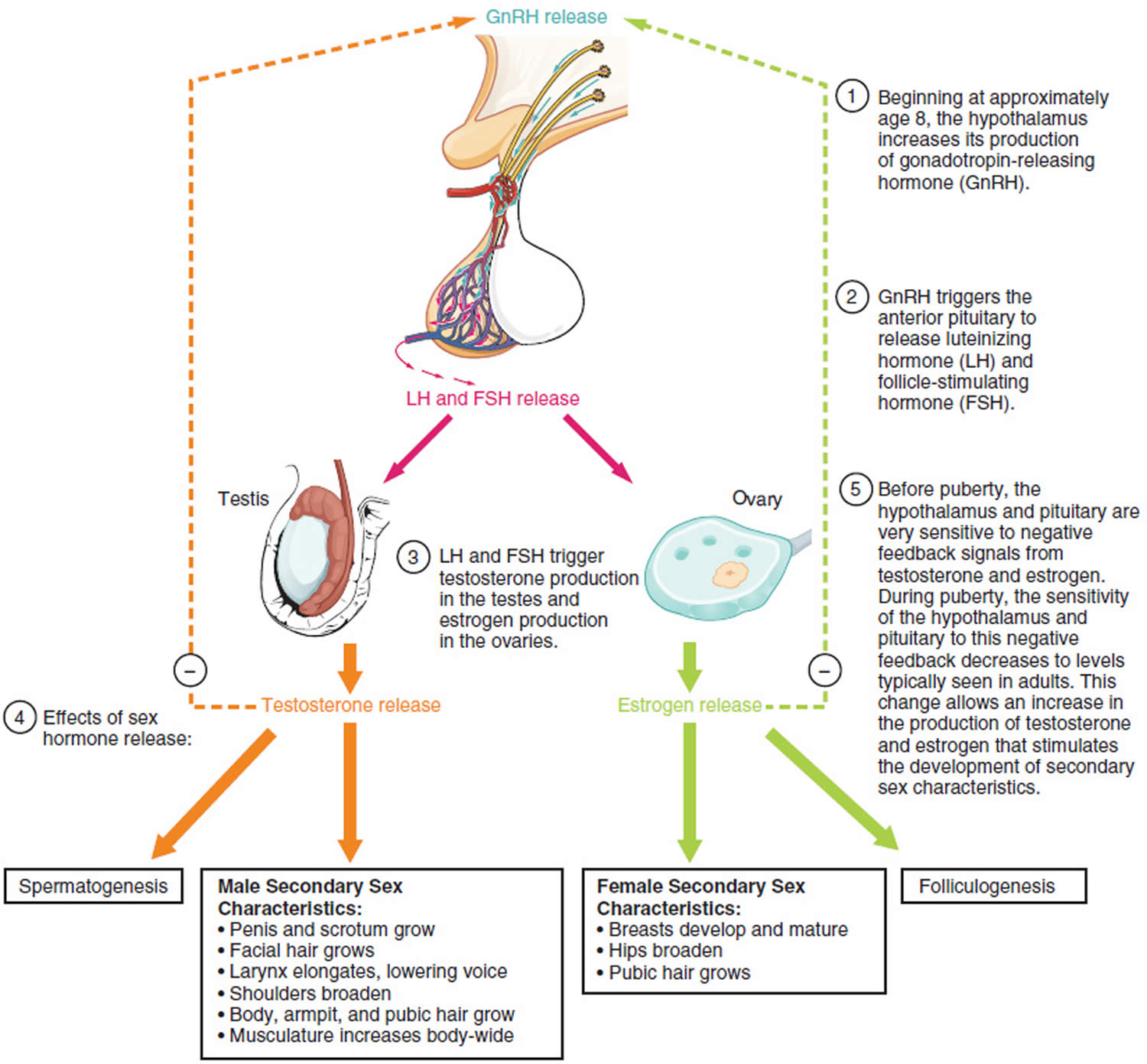
In children, luteinizing hormone (LH) levels are high right after birth, but then fall, remaining low until puberty approaches (usually between ages 10 and 14). At this time the hypothalamus, an almond-sized area of the brain that links the nervous system with the hormone-producing endocrine system, releases gonadotropin-releasing hormone (GnRH) that starts the changes of puberty. Gonadotropin-releasing hormone (GnRH) signals the pituitary gland to release two puberty hormones into the bloodstream: luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
Pulsatile secretion of gonadotropin-releasing hormone (GnRH) into the hypophyseal portal circulation represents the initial neuroendocrine step in the regulation of the hypothalamo-pituitary-gonadal axis (HPG axis) in both sexes. Thus, this specialized gonadotropin-releasing hormone (GnRH) neuronal network plays a commanding role in this biologic hierarchy and controls episodic gonadotropin secretion, modulates gonadal steroid feedback, and ultimately determines the initiation or suppression of pubertal development and fertility across the life cycle 13.
Under normal conditions, the gonadotropin-releasing hormone (GnRH) neuronal network undergoes a series of dynamic changes from fetal life to adulthood. The initiation of gonadotropin-releasing hormone (GnRH) secretion is initiated in early fetal life and remains active until the first several months of infancy (representing the “mini-puberty”), and then becomes remarkably dampened during the years of the childhood “quiescence” 14. At puberty, unknown biologic triggers re-ignite gonadotropin-releasing hormone (GnRH) secretion, resulting in full sexual maturation. Therefore, the controls of the reproductive axis are in dynamic flux, turning on and turning off in response to as-yet-unknown biologic signals at various points in the reproductive life cycle.
- In boys, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) work together to get the testes to begin producing testosterone, the hormone responsible for the physical changes of puberty and the production of sperm. Testosterone is the hormone that causes most of the changes in a boy’s body during puberty. Sperm cells must be produced for men to reproduce.
- In girls, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) prompt the ovaries to begin producing the hormone estrogen, which causes a girl’s body to mature and prepares her for menstruation. Estrogen, along with luteinizing hormone (LH) and follicle-stimulating hormone (FSH), causes a girl’s body to mature and prepares her for pregnancy.
- In men, follicle-stimulating hormone (FSH) stimulates the development of spermatozoa, in large part by acting on special cells in the testes called Sertoli cells. Luteinizing hormone (LH) stimulates the secretion of androgen (male) hormones by specialized cells in the testes called Leydig cells.
- In women, follicle-stimulating hormone (FSH) stimulates the synthesis of estrogens and the maturation of cells lining the spherical egg-containing structures known as Graafian follicles. In menstruating women, there is a preovulatory surge in serum follicle-stimulating hormone (FSH) and luteinizing hormone (LH) concentrations. The preovulatory surge of luteinizing hormone (LH) is essential for rupture of the Graafian follicle (ovulation), after which the egg enters the fallopian tube and travels to the uterus. The empty Graafian follicle becomes filled with progesterone-producing cells, transforming it into a corpus luteum. Luteinizing hormone (LH) stimulates the production of progesterone by the corpus luteum.
- Inhibin, a hormone secreted by the Graafian follicles of the ovaries and by the Sertoli cells of the testes, inhibits the secretion of follicle-stimulating hormone (FSH) from the pituitary gonadotrophs.
Patients with diseases involving the anterior pituitary gland often have gonadotropin deficiency. Thus, the disappearance of menstrual periods may be the first sign of a pituitary tumor or other pituitary disease in women. In men the most common symptoms of gonadotropin deficiency are loss of libido and erectile dysfunction. Isolated deficiencies of both luteinizing hormone (LH) and follicle-stimulating hormone (FSH) do occur but only rarely. In men isolated luteinizing hormone (LH) deficiency (“fertile eunuch”) is characterized by symptoms and signs of androgen deficiency; however, there is sufficient secretion of follicle-stimulating hormone (FSH) to permit the maturation of spermatozoa. Some pituitary tumors produce an excess of luteinizing hormone (LH) or follicle-stimulating hormone (FSH), whereas other pituitary tumors produce the hormonally inactive alpha chain subunit of the glycoprotein hormones.
Because luteinizing hormone (LH) and follicle-stimulating hormone (FSH) work so closely with each other, doctors often perform these tests together, as well as tests for testosterone (the major male sex hormone) and estradiol (a form of estrogen, the major female sex hormone). Taken together, the results can often provide a more complete picture of a person’s sexual maturation, and the well-being of the endocrine glands that produce these hormones.
Figure 1. Gonadotropins
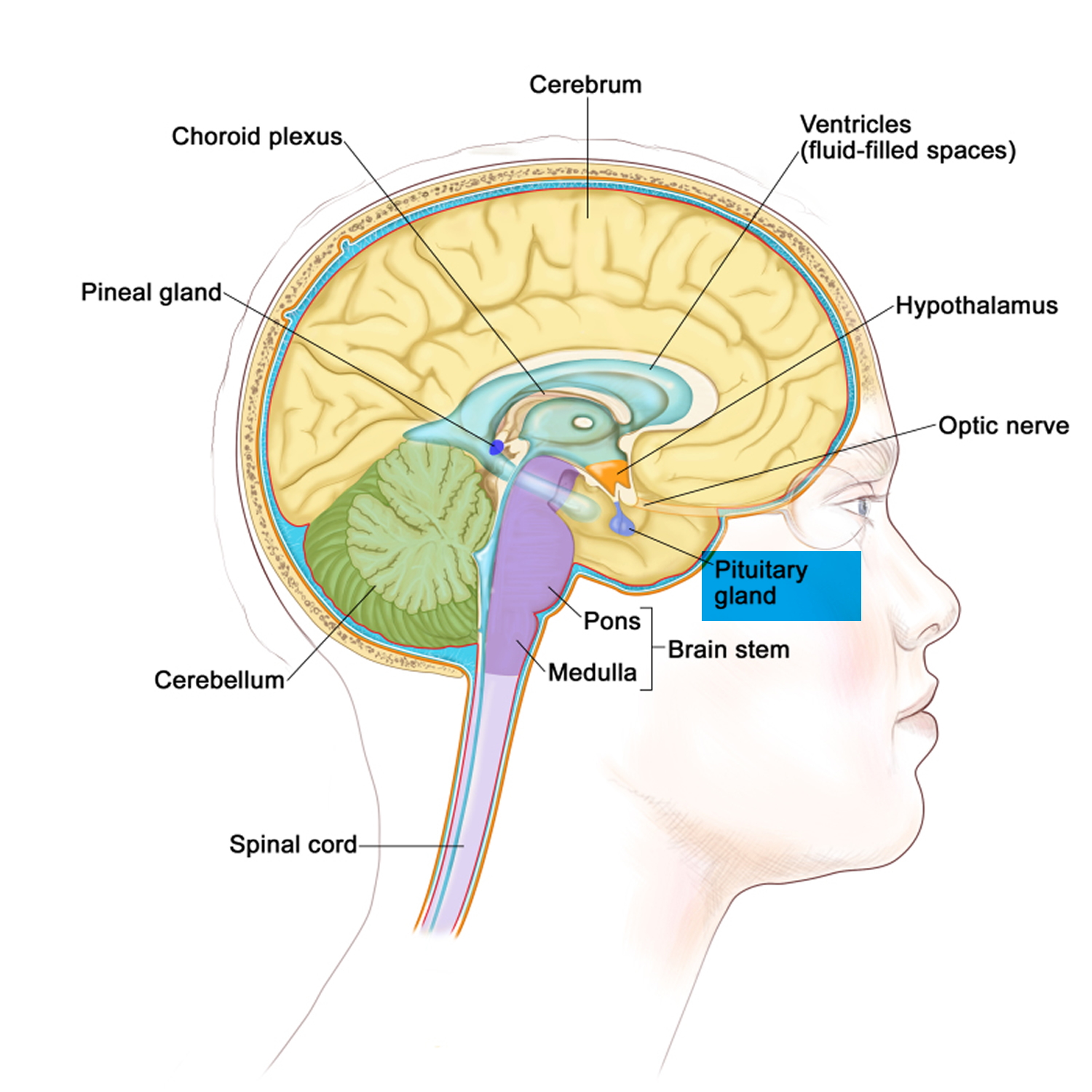
Figure 2. The pituitary gland location
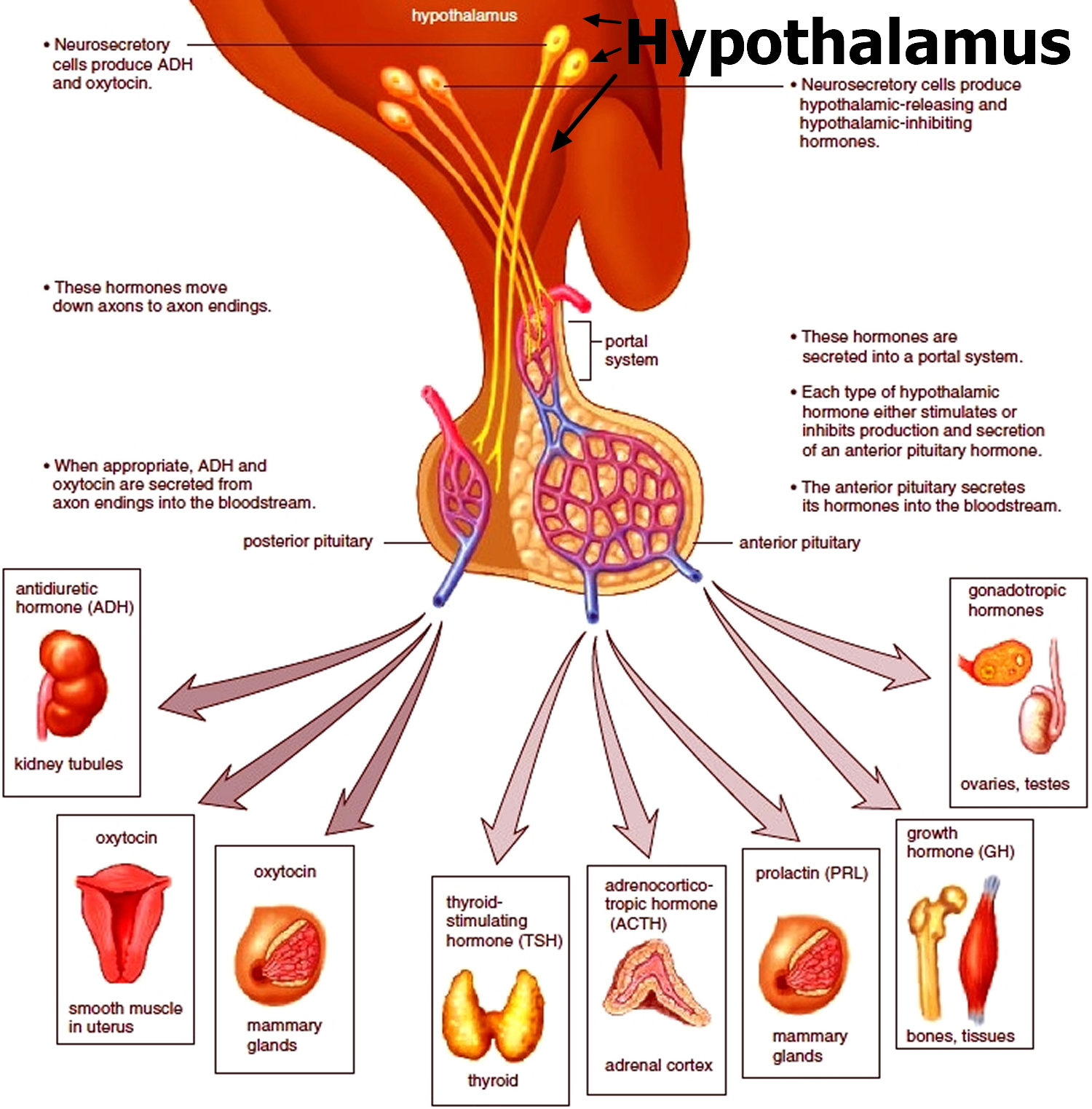
Figure 3. The hypothalamus and pituitary gland (anterior and posterior) endocrine pathways and target organs
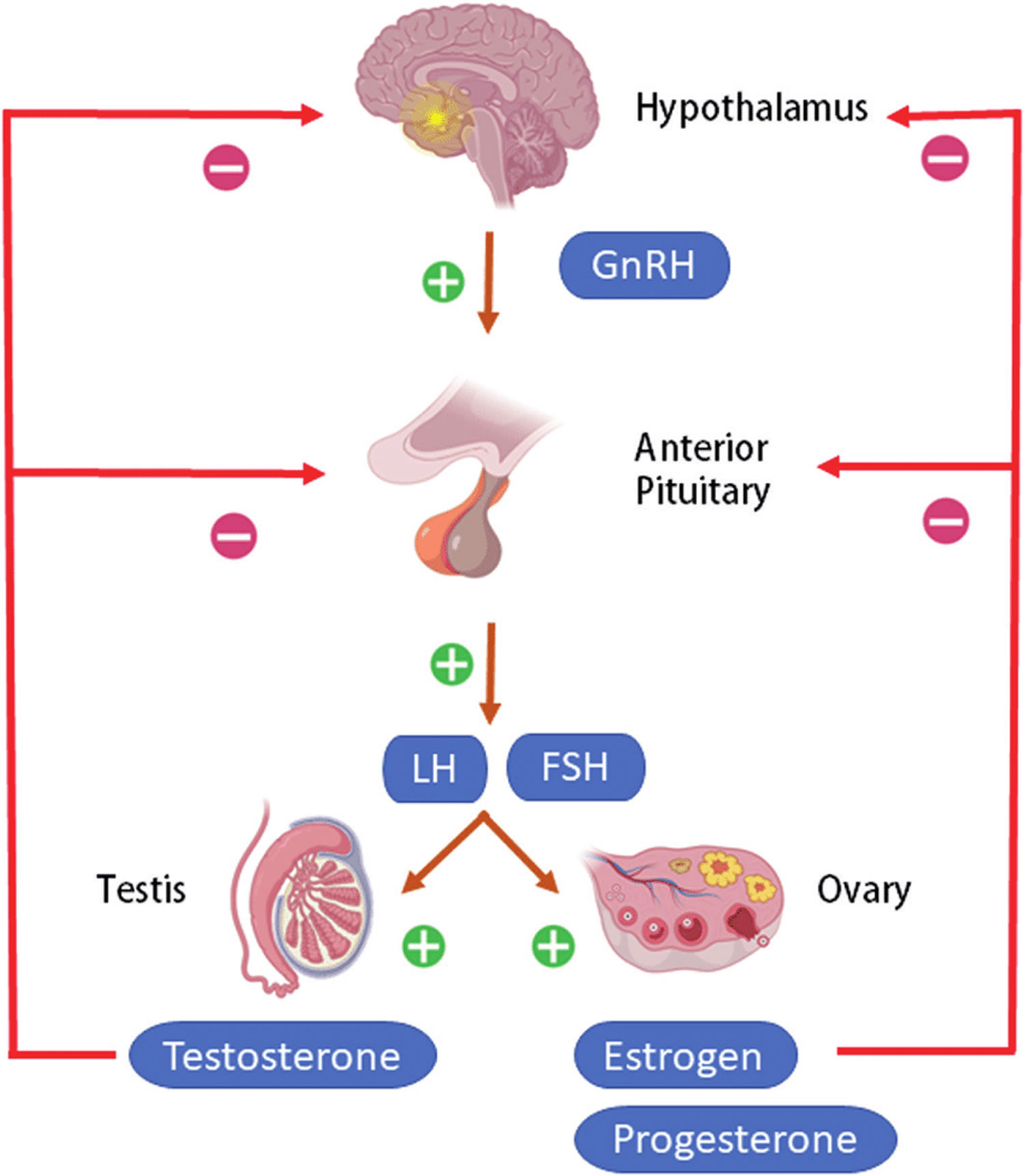
Figure 4. Hypothalamic–pituitary–gonadal axis
Footnotes: Hypothalamic–pituitary–gonadal (HPG) axis also known as the hypothalamic–pituitary–ovarian/testicular axis refers to the hypothalamus, pituitary gland, and gonadal glands as if these individual endocrine glands were a single entity. The hypothalamic control of reproduction is coordinated through the release of gonadotropin-releasing hormone (GnRH). Pulsatile secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus is required for both the initiation and maintenance of the hypothalamic–pituitary–ovarian/testicular axis in human. Pulsatile GnRH stimulates secretion of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), from the anterior pituitary gonadotropes. Gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in turn function at the gonads to stimulate the production of gametes and promote gonadal release of sex steroids [i.e., testosterone (T), estradiol (primarily 17β-estradiol, E2), and progesterone (P4)]. In females, ovarian follicles are stimulated by follicle-stimulating hormone (FSH) to grow and mature; luteinizing hormone (LH) stimulates ovulation and corpus luteum formation. In males, LH stimulates testicular Leydig cells to synthesize and secrete testosterone (T), which in turn maintains spermatogenesis in Sertoli cells through its paracrine action and exerts sexual and anabolic actions. While FSH acts on Sertoli cells to produce androgen-binding protein, which is critical for spermatogenesis initiation and ultimately augments sperm production. Along with guiding reproductive function in the peripheral tissues, these gonadal steroids [i.e., testosterone (T), estradiol (primarily 17β-estradiol, E2) and progesterone (P4)] secreted by ovaries and testis can inhibit gonadotropin-releasing hormone (GnRH)’s hypothalamic synthesis via a feedback loop, hence playing a vital role in regulating reproductive function. In both men and women, gonadal failure results in increased LH, because of loss of the negative feedback of estrogen at the hypothalamus and pituitary in women and from decreases in both androgen and estrogen feedback in men. In response to decreased levels of sex steroids as well as the loss of inhibin, FSH levels are also elevated following gonadal damage. Serum gonadotropin [luteinizing hormone (LH) and follicle-stimulating hormone (FSH)] and sex steroid values will differentiate between reproductive failure at the gonadal level or at the hypothalamic/pituitary level. Sex steroid levels will be low in both, but serum gonadotropin levels will be high in primary gonadal failure and low in those with hypothalamic or pituitary disease. The hallmark of primary gonadal failure from any cause is elevation of gonadotropin [luteinizing hormone (LH) and follicle-stimulating hormone (FSH)] levels, and this is the usual state in postpubertal patients receiving substantial doses of chemotherapy agents. Cranial radiation, on the other hand, may result in significant hypothalamic-pituitary dysfunction and secondary gonadal failure with low serum levels of gonadotropins.
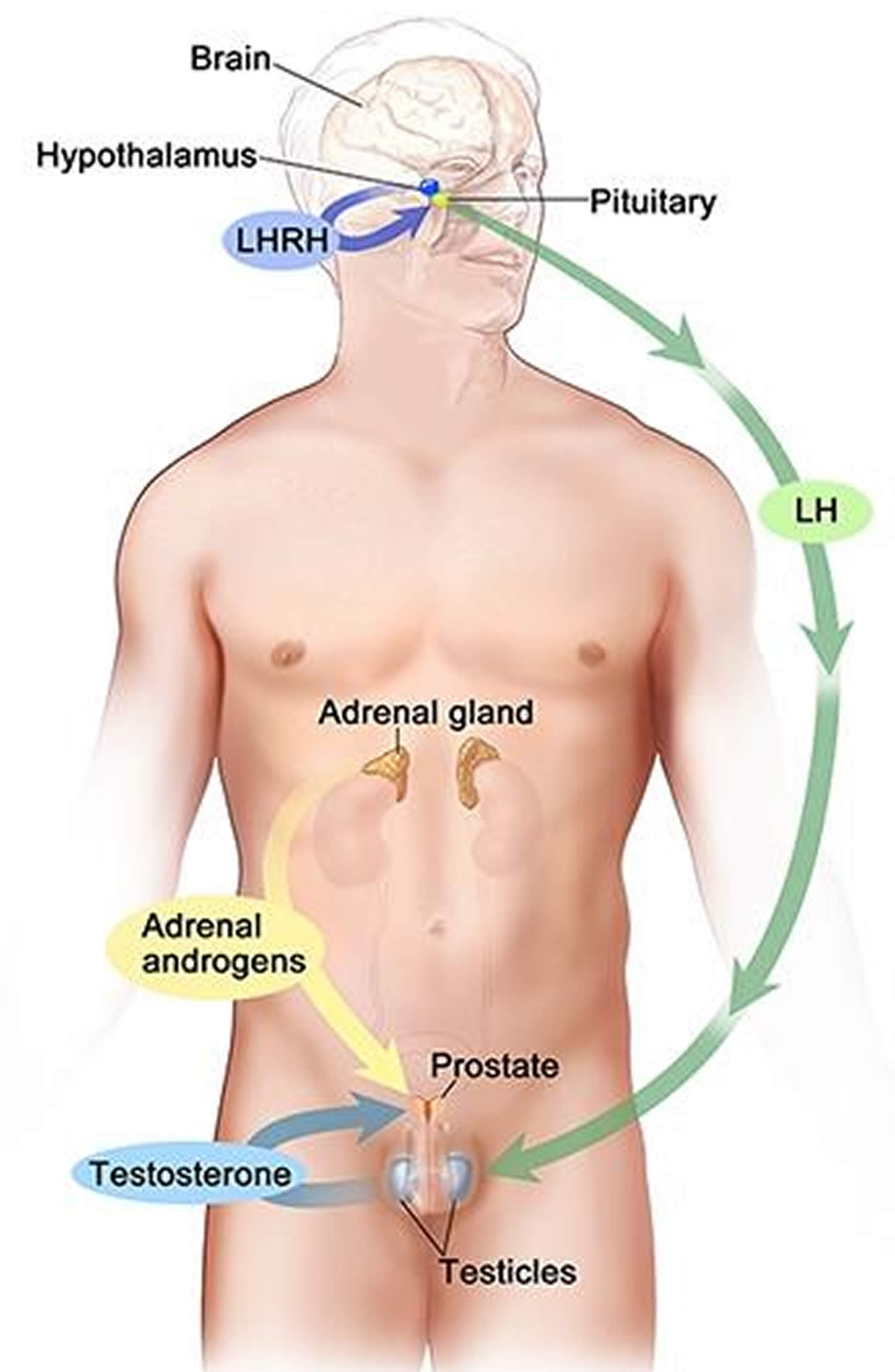
[Source 15 ]Figure 5. Testosterone production in men
Footnotes: Drawing shows that testosterone (androgen) production is regulated by luteinizing hormone (LH) and luteinizing hormone-releasing hormone (LHRH). The hypothalamus releases luteinizing hormone-releasing hormone (LHRH), which stimulates the release of LH (luteinizing hormone) from the pituitary gland. Luteinizing hormone (LH) acts on Leydig cells in the testes to produce the majority of testosterone in the body. Most of the remaining testosterone are produced by the adrenal glands. Testosterone are taken up by prostate cells, where they either bind to the androgen receptor (AR) directly or are converted to dihydrotestosterone (DHT), which has a greater binding affinity for the androgen receptor than testosterone.
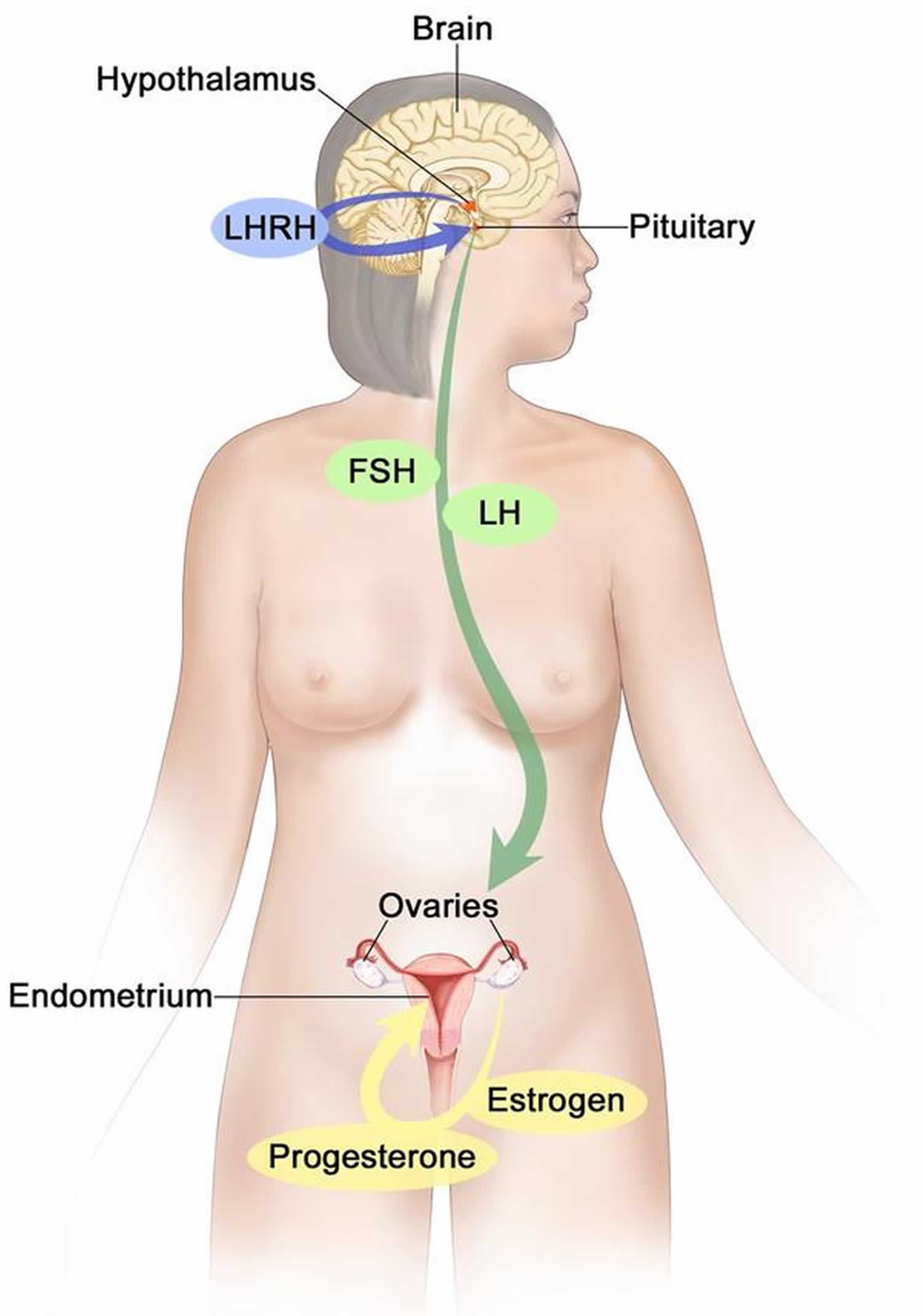
Figure 6. Estrogen and progesterone production in premenopausal women
Footnotes: Drawing shows that in premenopausal women, estrogen and progesterone production by the ovaries is regulated by luteinizing hormone (LH) and luteinizing hormone-releasing hormone (LHRH). The hypothalamus releases LHRH, which then causes the pituitary gland to make and secrete LH and follicle-stimulating hormone (FSH). LH and FSH cause the ovaries to make estrogen and progesterone, which act on the endometrium (inner lining of the uterus). When estrogen and progesterone production reaches a certain level during the menstrual cycle, these hormones act on the hypothalamus and pituitary to turn off production of LHRH, LH, and FSH. Estrogen is a steroid hormone that is responsible for the growth and regulation of the female reproductive system and secondary sex characteristics. Estrogen is produced by the granulosa cells of the developing follicle and exerts negative feedback on LH production in the early part of the menstrual cycle. However, once estrogen levels reach a critical level as oocytes mature within the ovary in preparation for ovulation, estrogen begins to exert positive feedback on LH production, leading to the LH surge through its effects on GnRH pulse frequency. Estrogen also has many other effects that are important for bone health and cardiovascular health in premenopausal patients 16.
Follicle stimulating hormone (FSH)
Follicle stimulating hormone (FSH) is a hormone produced by the anterior pituitary in response to gonadotropin-releasing hormone (GnRH) from the hypothalamus 17, 18. Follicle stimulating hormone (FSH) is a glycoprotein hormone with alpha (α) and beta (β) subunits. The beta subunit is unique to FSH, while the alpha subunit is the same as in thyroid stimulating hormone (TSH), human chorionic gonadotropin (hCG), and luteinizing hormone (LH) 19. Follicle stimulating hormone (FSH) regulates growth, sexual development and reproduction, including menstruation, follicular development and ovulation 1, 2.
FSH is regulated, at least in part, by gonadotropin-releasing hormone (GnRH) produced in the hypothalamus in response to multiple signals including circulating levels of sex hormones (i.e, estrogen, progesterone, and testosterone). The hypothalamus produces GnRH, and it is released into the hypophyseal portal circulation to act on G-protein-coupled receptors at gonadotropic cells of the anterior pituitary. Those gonadotropic cells produce FSH and luteinizing hormone (LH) and release them into the peripheral circulation. Gonadal sex hormones, estrogen, progesterone, and testosterone exert negative feedback, thus decreasing the secretion of FSH and LH 20.
Gonadotropin-releasing hormone (GnRH) release occurs in a pulsatile manner, with low pulse frequencies stimulating more FSH production and high pulse frequencies stimulating more luteinizing hormone (LH) production 18. Continuous use of gonadotropin-releasing hormone (GnRH) suppresses the release of FSH and LH from the anterior pituitary which inhibits ovulation and estrogen production in women. Clinically, GnRH agonists like leuprolide work via this mechanism 21. In women, negative feedback from estrogen levels inhibits FSH secretion 22. In men, levels of inhibin B, secreted by the Sertoli cells in response to FSH, inhibit FSH secretion via negative feedback 23.
- In females, FSH receptors are located in the Granulosa cells that surround developing ovarian follicles of the ovaries 24. FSH stimulates the growth and maturation of immature oocytes into mature (Graafian) secondary follicles before ovulation. FSH Receptors are G-protein coupled receptors and are found in the Granulosa cells and Granulosa cells initially produce the estrogen needed to maturate the developing dominant ovarian follicle. After 2 days of sustained elevation of estrogen levels, the LH surge causes luteinization of the Granulosa cells into LH receptive cells. This transition enables Granulosa cells to respond to LH levels and produce progesterone 16. The corpus luteum grows and secretes progesterone and some estrogen, which makes the endometrium more receptive to implantation. If fertilization does not occur, progesterone/estrogen levels fall, and the corpus luteum dies, forming the corpus albicans. These falling hormone levels stimulate FSH to begin recruiting follicles for the next cycle. If fertilization does occur, human chorionic gonadotropin (hCG) produced by the early placenta preserves the corpus luteum, maintaining progesterone levels until the placenta is able to make sufficient progesterone to support the pregnancy 25.
- In males, FSH receptors are found in the Sertoli cells of the testes 19. In men, FSH promotes the production (spermatogenesis) and function of sperm and androgen responsiveness in the testes.
Therefore, FSH is essential for sexual maturation and reproduction in both men and women. Partially and highly purified human menopausal urine derived FSH (Menotropins or human menopausal gonadotropin [hMG] or Menopur which also has LH activity); industrial production of therapeutic grade urinary FSH (urofollitropin, Bravelle) and recombinant DNA (rDNA)-derived human FSH (follitropin alpha, Follistim, Gonal F) are available and approved for use in treatment of infertility and hypogonadism 26. They are generally given by subcutaneous injection daily or several times weekly. The dose and appropriate regimen vary by indication. These agents should be used only by doctors with expertise in management of infertility and hypogonadism.
Follicle stimulating hormone (FSH) function
During fetal development, gonadotropin-releasing hormone (GnRH) producing neurons develop from the epithelium of the medial olfactory pit and then migrate to the hypothalamus 27. The anterior pituitary gland develops from Rathke’s pouch, a portion of the oral cavity 19. In the second and third trimesters of pregnancy, as well during the first 3 to 6 months of infancy, the pituitary gland secretes luteinizing hormone (LH) and follicle stimulating hormone (FSH) 28. LH and FSH levels peak mid-pregnancy as the first ovarian follicle or seminiferous tubule mature 27.
In males, follicle stimulating hormone (FSH) stimulates Sertoli cell proliferation, which is the most significant contributor to testicular volume in children 28. The Sertoli cells produce an anti-mullerian hormone (AMH), which causes the involution of the Mullerian ducts, preventing the formation of female internal genitalia 27.
During puberty, the hypothalamus secretes GnRH in a pulsatile manner, which stimulates the anterior pituitary to increase secretion of LH and FSH 27. In the male, follicle stimulating hormone (FSH) in combination with testosterone, which is under the control of luteinizing hormone (LH), is required for the initiation and maintenance of the quality and quantity of normal production of sperm (spermatogenesis) 29. In transgenic mice it appears FSH to be not essential for male fertility 30, spermatogenesis is not completely normal in the absence of FSH and, furthermore, the requirement for FSH is more critical in primates than in rats.
In the female follicle stimulating hormone (FSH) is necessary for the selection and growth of ovarian follicles and for the production of estrogens from androgen substrates. The gonadotrophic effects of FSH may be subserved by a number of intermediaries 31 that form part of the cellular and tissue response to FSH stimulation culminating in ovulation 32. Such cellular responses illustrate the complex nature of FSH since they indicate that FSH activity has many components, i.e., FSH is a growth factor or tropic hormone, a secretagogue, and a modulator of cellular development 33. It is generally thought that FSH exerts most of its intracellular actions via the cAMP-mediated signaling pathway, although FSH may also utilize other signal transduction pathways such as calcium ion 34.
The biological activity of FSH is the sum of a complex combination of processes: release from the pituitary, survival in the circulation, transport to the site of action (i.e., the gonad), binding to the receptor, and activation of signal transduction pathways.
The lack of the normal infancy peak of luteinizing hormone (LH) and follicle stimulating hormone (FSH) might identify and diagnose infants, especially males, who have hypogonadotropic hypogonadism.
Girls with monosomy Turner syndrome (45, XO) have an elevation of FSH up to 6 years old due to lack of negative feedback from nonfunctional ovaries, while girls with mosaicism (45, X/46, XX) have a much lower FSH elevation due to partial ovarian function 27.
FSH function in Females
- Estrogen production: FSH stimulates granulosa cells in the ovarian follicles to synthesize aromatase, which converts androgens produced by the thecal cells to estradiol.
- Follicular development and the menstrual cycle: During the follicular phase of the menstrual cycle, FSH stimulates the maturation of ovarian follicles. As a dominant follicle takes over and secretes estradiol and inhibin, FSH secretion is suppressed. When the dominant follicle produces enough estradiol to maintain levels of 200 to 300 pg/ml for 48 hours, the hypothalamus responds with a surge of GnRH which stimulates the secretion of gonadotropic hormones instead inhibiting them. FSH peaks at the same time as the LH surge that causes ovulation. FSH then remains low throughout the luteal phase, preventing the development of new follicles 19.
FSH function in Males
Follicle stimulating hormone (FSH), along with testosterone, is necessary for maintaining normal sperm count and function. Studies have shown that FSH deprivation not only lowers sperm count but also affects the quality of the remaining sperm 35.
Follicle stimulating hormone (FSH) diagnostic use
Elevated levels of follicle stimulating hormone (FSH) are associated with unresponsive gonads or hyperfunctioning pituitary adenomas. Low levels of FSH are associated with either hypothalamic or anterior pituitary dysfunction. The measurement of FSH in the blood is widely employed in the diagnosis of disorders of reproduction and development.
Clinical situations where follicle stimulating hormone (FSH) measurements are useful or are commonly requested 36:
- Anovulatory infertility (irregular and inconsistent menstrual blood flow [oligomenorrhea] or absence of monthly menstruation [amenorrhea]): To help determine whether cause is pituitary or gonadal in origin and to aid diagnosis of conditions such as polycystic ovary syndrome (PCOS)
- Suspected premature puberty: In addition to steroid hormone levels
- Delayed puberty: In addition to steroid hormone levels
- Azoospermia or severe low sperm count (oligospermia): To differentiate pituitary and gonadal causes
- Ovarian reserve: As a biological marker for the number of releasable oocytes; may be enhanced by measurements of inhibin and ovarian ultrasound to accurately stage the timing of the sample
- Menopausal status: A frequently requested test; FSH is not a good marker for timing of the menopause or of perimenopausal state
The primary use of follicle stimulating hormone (FSH) measurements is for assessment of gonadal function. Through classical endocrine feedback pathways, an elevated level of FSH indicates reduced gonadal function or gonadal failure, whereas a normal serum concentration of FSH suggests normal gonadal function (see Figures 4 to 6). A low serum FSH may indicate a problem at the level of the hypothalamus or pituitary.
A measurement of serum follicle stimulating hormone (FSH), with measurement of luteinizing hormone (LH) and either estradiol (E2) or testosterone (T), may be helpful in children with suspected premature puberty or in cases of delayed puberty, particularly as the application of sensitive assay methodologies permits detection of hormonal changes before clinical changes of puberty are observed 37. FSH measurement is indicated in men with azoospermia or severe oligospermia (low sperm count) to help determine the degree to which the problem is due to gonadal failure 38.
Ovarian reserve, or the total number of remaining oocytes within the ovary, declines with ovarian age, but this does not always equate with the age of the woman. A baseline measurement of serum FSH concentration, usually on day 3 of the menstrual cycle, is a fairly good predictor of ovarian reserve in women of reproductive years 39. A fluctuating baseline FSH level is indicative of compromised ovarian function. The picture is further enhanced if measurement of FSH is combined with serum estradiol and inhibin 40. In an irregular menstrual cycle it can be difficult to time collection of samples correctly, and therefore more than one sample may have to be taken, often in combination with an ultrasound scan of the ovaries, to help determine the stage in the cycle 41, 42, 43, 44. Measurement of FSH is also helpful in determining the presence of common disorders of reproduction such as polycystic ovary syndrome (PCOS), when classically the serum luteinizing hormone (LH) concentration is elevated, while FSH is usually normal 45. A single measurement of FSH is not predictive of the timing of menopause and is not usually recommended for this purpose, although it may be useful in developing a differential diagnosis to exclude other causes (endocarditis or pheochromocytoma for example) of symptoms such as hot flushes. Although various studies have been performed to characterize the perimenopausal status 46, 47, the practical use of FSH measurement is in the prediction of ovarian response to stimulation in the context of assisted reproduction.
In the United States FSH assays are used for investigations of menopausal status, diagnoses of infertility/amenorrhea, and infertility in men. Essentially, your doctor will wish to detect gross changes in FSH levels from the normal ranges associated with primary gonadal failure and hypogonadotrophic hypogonadism. The ratio of LH to FSH has been proposed as a good predictor of ovarian hyperstimulation syndrome 48. In such cases, particularly where a ratio of two measurements is made, it is important to maintain continuity of unitage between estimates derived from different assays over a period of time and thus from one standard preparation to the next.
FSH levels and Male infertility
If males present with small, firm testes and absence of sperm in the ejaculate (azoospermia) or low sperm count (oligospermia), elevated FSH levels can be used to differentiate Klinefelter syndrome from hypothalamic or pituitary insufficiency. If testicular size is normal and patients present with azoospermia or oligospermia, FSH levels can be used to determine whether the cause is a primary impairment of spermatogenesis or obstructive. In an obstructive cause of infertility, FSH levels remain normal, while a primary impairment of spermatogenesis will present with elevated FSH levels.
Several FSH preparations have been used to treat secondary hypogonadism in males. These preparations have been reasonably successful at inducing spermatogenesis and achieving paternity 35.
Polycystic ovary syndrome (PCOS)
With PCOS, many small sacs of fluid called cysts develop along the outer edge of the ovary. The small fluid-filled cysts contain immature eggs. These are called follicles. The follicles fail to regularly release eggs.
The symptoms of PCOS vary. A diagnosis of PCOS is made when you have at least two of these:
- Irregular periods. Having few menstrual periods or having periods that aren’t regular are common signs of PCOS. So is having periods that last for many days or longer than is typical for a period. For example, you might have fewer than nine periods a year. And those periods may occur more than 35 days apart. You may have trouble getting pregnant.
- Too much androgen (testosterone). High levels of the hormone androgen may result in excess facial and body hair. This is called hirsutism. Sometimes, severe acne and male-pattern baldness can happen, too.
- Polycystic ovaries. Your ovaries might be bigger. Many follicles containing immature eggs may develop around the edge of the ovary. The ovaries might not work the way they should.
PCOS signs and symptoms are typically more severe in people with obesity.
The exact cause of PCOS is unknown. Early diagnosis and treatment along with weight loss may lower the risk of long-term complications such as type 2 diabetes and heart disease.
In PCOS, the LH:FSH ratio is skewed due to persistently rapid GnRH pulses. These GnRH pulses lead to an increased LH: FSH ratio. This skewed ratio leads to the theca cells of the ovaries producing excess androgen while the granulosa cells do not produce enough aromatase to convert the androgens to estradiol 18.
There is no cure for PCOS with treatment focuses on managing the things that are concerning you. This could include infertility, hirsutism, acne or obesity. Specific treatment might involve lifestyle changes or medication. Birth control pills help women have normal periods, reduce male hormone levels, and clear acne. Treatments for infertility caused by PCOS may include medicines, surgery, and in vitro fertilization (IVF).
Hypogonadotropic Hypogonadism
Hypogonadotropic hypogonadism (HH) is a form of hypogonadism that is due to a problem with the pituitary gland or hypothalamus. Hypogonadism is a condition in which the male testes or the female ovaries produce little or no sex hormones.
Hypogonadotropic hypogonadism (HH) is caused by a lack of gonadotropin-releasing hormone (GnRH), follicle stimulating hormone (FSH) and luteinizing hormone (LH) that normally stimulate the ovaries or testes.
There are several causes of hypogonadotropic hypogonadism 50:
- Damage to the pituitary gland or hypothalamus from surgery, injury, tumor, infection, or radiation
- Genetic defects. Kallmann syndrome is an inherited form of hypogonadotropic hypogonadism. Some people with this condition also lose their sense of smell (anosmia).
- High doses or long-term use of opioid or steroid (glucocorticoid) medicines
- High prolactin level (a different hormone released by the pituitary)
- Severe stress
- Nutritional problems (both rapid weight gain or weight loss)
- Long-term (chronic) medical diseases, including chronic inflammation or infections
- Drug use, such as heroin or use or abuse of prescription opioid medicines
- Certain medical conditions, such as iron overload
Hypogonadotropic hypogonadism treatment depends on the source of the problem, but may involve:
- Injections of testosterone (in males)
- Slow-release testosterone skin patch (in males)
- Testosterone gels (in males)
- Estrogen and progesterone pills or skin patches (in females)
- Gonadotropin-releasing hormone (GnRH) injections
- Human chorionic gonadotropin (hCG) injections
Kallman syndrome
Kallmann syndrome also called congenital hypogonadotropic hypogonadism is a rare genetic disorder in humans that is defined by a delayed or absent of signs of puberty along with an absent or impaired sense of smell (anosmia) 51, 52. In Kallmann syndrome, the sense of smell is either diminished (hyposmia) or completely absent (anosmia). This feature distinguishes Kallmann syndrome from most other forms of hypogonadotropic hypogonadism such as normosmic idiopathic hypogonadotropic hypogonadism, which do not affect the sense of smell. Many people with Kallmann syndrome are not aware that they are unable to detect odors until the impairment is discovered through testing.
Kallmann syndrome can have a wide variety of additional signs and symptoms. These include a failure of one kidney to develop (unilateral renal agenesis), abnormalities of bones in the fingers or toes, a cleft lip with or without an opening in the roof of the mouth (a cleft palate), abnormal eye movements, hearing loss, and abnormalities of tooth development 51, 52. Some affected individuals have a feature called bimanual synkinesis, in which the movements of one hand are mirrored by the other hand. Bimanual synkinesis can make it difficult to do tasks that require the hands to move separately, such as playing a musical instrument.
Kallmann syndrome is caused by mutation (changes) in more than 20 genes 51, 52. Among the most common causes of Kallmann syndrome are mutations in the ANOS1, CHD7, FGF8, FGFR1, PROK2, or PROKR2 gene. In some cases, affected individuals have mutations in more than one of these genes. Additionally, researchers have identified mutations in other genes that may contribute to the development and features of Kallmann syndrome, but are unlikely to cause the disease on their own.
The genes associated with Kallmann syndrome play roles in the development of certain areas of the brain before birth. Although some of their specific functions are unclear, these genes appear to be involved in the formation and movement (migration) of a group of nerve cells that are specialized to process the sense of smell (olfactory neurons). These nerve cells originate in the developing nose and then migrate together to a structure in the front of the brain called the olfactory bulb, which is critical for the perception of odors. Studies suggest that the genes associated with Kallmann syndrome are also involved in the migration of neurons that produce a hormone called gonadotropin-releasing hormone (GnRH). Like olfactory neurons, GnRH-producing neurons migrate from the developing nose to the front of the brain. GnRH controls the production of several hormones that direct sexual development before birth and during puberty. These hormones are important for the normal function of the ovaries in women and testes in men.
Studies suggest that mutations in genes associated with Kallmann syndrome disrupt the migration of olfactory nerve cells and GnRH-producing nerve cells in the developing brain. If olfactory nerve cells do not extend to the olfactory bulb, a person’s sense of smell will be impaired or absent. Misplacement of GnRH-producing neurons in the brain prevents the production of other sex hormones, which interferes with normal sexual development and causes the characteristic features of hypogonadotropic hypogonadism. It is unclear how gene mutations lead to the other signs and symptoms that can occur in Kallmann syndrome. Because the features of this condition vary among individuals, additional genetic and environmental factors likely contribute to this disease.
Together, mutations in known genes account for about 30 percent of all cases of Kallmann syndrome. In cases without a mutation in one of the identified genes, the cause of the condition is unknown. Researchers are looking for additional genetic changes that can cause Kallmann syndrome.
Each of these genes have varied pattern of affecting families, i.e. inheritance (the way that the disorder passes from parents to offspring). All forms of Mendelian inheritance (autosomal dominant, autosomal recessive, and X-lined recessive) as well more complex oligogenic inheritance patterns are now recognized. Understanding the genetic basis of the disorder is crucial not only for genetic counseling for determine the risk of transmission to the next generation, but also for fostering new gene discovery as well as bench-to-bedside research.
Most of the time, people with Kallmann syndrome resulting from an ANOS1 gene mutation inherit the mutation from their mothers, who carry a single altered copy of the gene in each cell (and generally do not have any signs or symptoms of the condition). Other people have Kallmann syndrome as a result of a new mutation in the ANOS1 gene.
The diagnosis of Kallmann syndrome is based on the clinical evidence of arrested sexual maturation or hypogonadism and the incomplete sexual maturation by Tanner staging on physical examination. Apart from the physical examination, biochemical testing is also critical for diagnosis of Kallmann syndrome. As GnRH is not measurable, serum concentration of the gonadotropins (LH and FSH) and sex hormones are used for diagnosis.
Kallmann syndrome treatment involve hormone replacement therapies and this is usually tailored the clinical need of the patients. Typically, once the diagnosis is made, in both sexes, treatment is aimed at inducing puberty and maintaining normal hormonal levels. Subsequently, treatment may also be need for inducing fertility for achieving pregnancy.
Stress-Induced Hypogonadotropic Hypogonadism
When calorie intake falls short of energy expenditure, the physiological stress decreases hypothalamic gonadotropin-releasing hormone (GnRH) pulse frequency and amplitude, leading to low FSH and LH levels. This explains the anovulation and amenorrhea that can occur in female athletes and individuals with eating disorders due to lack of adequate caloric intake or excessive exercise 18, 19.
Primary Ovarian Insufficiency
In cases of amenorrhea with elevated levels of FSH, the problem lies in the ovaries. Premature ovarian failure occurs when ovarian failure and menopause occur before age 40. When this happens, FSH levels are elevated due to the lack of negative feedback from the ovaries. Although there may be multiple genetic causes, most cases are idiopathic.
Turner syndrome is the most common genetic disorder causing premature ovarian failure. Turner syndrome is a rare chromosomal disorder that affects only females. Turner syndrome is caused by the loss of an X chromosome where one of the X chromosomes (sex chromosomes) is missing (XO karyotype) or partially missing 53, 54, 55. The reason that this occurs is unknown and is believed to result from a random event (Turner Syndrome. https://rarediseases.org/rare-diseases/turner-syndrome/)). In some people, the chromosomal abnormality appears to arise spontaneously (de novo) due to an error in the division of a parent’s reproductive cells, either in the father’s sperm or the mother’s egg (Turner Syndrome. https://rarediseases.org/rare-diseases/turner-syndrome/)).
The genetic changes of Turner syndrome may be one of the following (Turner Syndrome. https://rarediseases.org/rare-diseases/turner-syndrome/)):
- Monosomy. The complete absence of an X chromosome generally occurs because of an error in the father’s sperm or in the mother’s egg. This results in every cell in the body having only one X chromosome.
- Mosaicism. In many people with Turner syndrome, only a certain percentage of cells may be affected. This is referred to as mosaicism. Specifically, some cells have the normal 46 chromosomes (one cell line) while other cells do not have the normal 46 chromosomes (second cell line). This second cell line may contain various abnormalities such as partial or complete loss of the X chromosome. In these cases, the loss of genetic material from the X chromosome usually occurs because of spontaneous errors very early during fetal development. Theoretically, individuals with Turner syndrome mosaicism may have fewer developmental problems because fewer cells are affected. However, this is difficult to predict. Further research is necessary to completely understand the complicated factors involved in the development of the various symptoms associated with Turner syndrome.
- X chromosome changes. Changed or missing parts of one of the X chromosomes can occur. Cells have one complete and one altered copy. This error can occur in the sperm or egg with all cells having one complete and one altered copy. Or the error can occur in cell division in early fetal development so that only some cells contain the changed or missing parts of one of the X chromosomes (mosaicism).
- Y chromosome material. In a small percentage of Turner syndrome cases, some cells have one copy of the X chromosome and other cells have one copy of the X chromosome and some Y chromosome material. These individuals develop biologically as female, but the presence of Y chromosome material increases the risk of developing a type of cancer called gonadoblastoma.
Turner syndrome can cause many different symptoms. The symptoms may be mild for some people. But for others, Turner syndrome can cause serious health problems. People with Turner syndrome may be born with heart and kidney defects. They usually don’t have typical sexual development and are infertile. They are also at risk for other health problems such as high blood pressure, type 2 diabetes, osteoporosis, and thyroid problems.
Some of the symptoms of Turner syndrome affect a person’s appearance. Most people with Turner syndrome are shorter than average. They may also have physical features such as:
- A neck that is short and has extra skin (a “webbed” neck)
- A low hairline in the back
- Low-set ears
- Swollen hands and feet
Girls with Turner syndrome will present with primary amenorrhea and underdeveloped ovaries (streak ovaries) and elevated FSH levels 56.
Turner syndrome may be diagnosed before birth (prenatally), during infancy or in early childhood. Occasionally, in females with mild signs and symptoms of Turner syndrome, the diagnosis is delayed until the teen or young adult years.
There is no cure for Turner syndrome, but there are treatments for some of the symptoms. Girls and women with Turner syndrome need ongoing medical care from a variety of specialists. Regular checkups and appropriate care can help most girls and women lead healthy, independent lives.
The primary treatments for nearly all girls and women with Turner syndrome include hormone therapies:
- Growth hormone (GH). Growth hormone therapy — usually given daily as an injection of recombinant human growth hormone — is typically recommended to increase height as much as possible at appropriate times during early childhood until the early teen years. Starting treatment early can improve height and bone growth.
- Estrogen replacement therapy (ERT). Most girls with Turner syndrome need to start estrogen and related hormone therapy in order to begin puberty. Often, estrogen therapy is started around age 11 or 12 years. Estrogen helps to promote breast development and improve the size (volume) of the uterus. Estrogen helps with bone mineralization, and when used with growth hormone, may also help with height. Estrogen replacement therapy usually continues throughout life, until the average age of menopause is reached.
Other treatments are tailored to address particular problems as needed. Assisted reproduction technologies can help some women with Turner syndrome get pregnant. Regular checkups have shown substantial improvements in the health and quality of life for girls and women with Turner syndrome.
Pituitary Adenomas
Pituitary adenomas can develop from any of the cell types in the pituitary. Pituitary adenomas derived from gonadotropic cells are most often nonfunctioning or function within normal hormone levels and are diagnosed due to symptoms from mass effect rather than hormone secretions. However, in sporadic, these tumors can secrete excess FSH and/or LH and can cause ovarian hyperstimulation 57.
GnRH Agonists and Antagonists medications
GnRH agonists initially stimulate secretion of LH and FSH, but when given continuously, suppress LH and FSH release. This results in ovarian suppression and decreased estrogen levels. GnRH antagonists can acutely suppress LH and FSH secretion 19.
Both GnRH agonists and antagonists have a role in the treatment of certain breast and prostate cancers, endometriosis, and uterine leiomyomas.
Assisted Reproduction Techniques (ART)
Techniques such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) help couples by using FSH to stimulate multiple follicles in the ovaries to harvest multiple eggs for fertilization. FSH is available as urinary FSH with or without LH or recombinant FSH 58. GnRH agonists or antagonists can be used during these cycles to prevent the LH surge and ovulation 19.
Luteinizing hormone (LH)
Luteinizing hormone (LH) is a hormone produced by the anterior pituitary in response to gonadotropin-releasing hormone (GnRH) from the hypothalamus 11, 18. Luteinizing hormone (LH) is essential for sexual development and reproduction in both men and women 1, 2. LH is regulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus which is sensitive to circulating levels of sex hormones (i.e., estrogen, progesterone, and testosterone). Gonadal sex hormones, estrogen, progesterone, and testosterone exert negative feedback, thus decreasing the secretion of LH 20.
LH interacts with receptors on ovarian follicles and promotes their maturation. In the middle of the menstrual cycle, a surge of LH triggers ovulation and production of progesterone by the corpus luteum that is necessary for the maturation of the uterine endometrium for implantation of the fertilized egg. In males, LH stimulates production of testosterone by the testes. Luteinizing hormone (LH) is used clinically in assisted reproduction techniques (ART) and in vitro fertilization (IVF) to stimulate ovarian follicle maturation. Both urinary derived (menotropin, Menopur, which also has FSH activity) and recombinant forms (lutropin alfa: Luveris) of human LH have been developed, but not all are available in the United States 26. LH is generally administered by subcutaneous injection in a cyclic and step-wise fashion. The dosages and regimens of administration vary by indication. These agents should be used only by health care workers with expertise in management of infertility and hypogonadism.
Luteinizing hormone (LH) function
Luteinizing hormone (LH) release is stimulated by gonadotropin-releasing hormone (GnRH) and inhibited by estrogen in females and testosterone in males. Luteinizing hormone (LH) has various functions, which differ between women and men. In both sexes, LH contributes to the maturation of primordial germ cells. In men, LH causes the testes’ Leydig cells to produce testosterone. In women, LH triggers the creation of steroid hormones from the ovaries 59. Additionally, LH helps regulate the length and order of the menstrual cycle in females by playing roles in ovulation and implantation of an egg in the uterus 60.
Luteinizing hormone (LH) function in Fetal Development
Luteinizing hormone (LH) and human chorionic gonadotropin (hCG) are 2 essential hormones in the development of both sexes. Their levels can be seen to fluctuate throughout development. In male fetuses, human chorionic gonadotropin (hCG) begins at a high level in the plasma and quickly decreases between weeks 10 and 20 of gestation and then slowly declines afterward 11. In contrast, LH secretion increases by week 10, peaks before week 20, and decreases gradually 11. Increased plasma levels of human chorionic gonadotropin (hCG) early on in gestation are a more significant contributor to testosterone production by Leydig cells than LH early in the development of a fetus 11. However, as LH levels rise, the regulation of testosterone formation changes to LH, which is driven by weeks 15 to 20 of gestation 11. This change in regulation can be exemplified by anencephalic male fetuses that are deficient in LH. In these fetuses, normal development of the male reproductive tract occurs while hCG levels are high initially. However, due to the lack of LH, the development of the external genitalia is impeded when hCG levels decrease around gestational weeks 15 to 20 61.
In female fetuses, the peak levels of LH are higher than in male fetuses; this has been thought to be due to negative feedback of higher testosterone levels on the hypothalamic-pituitary-gonadal axis in male fetuses. Female fetuses have a lower level of gonadal hormones during gestation because the development of the female reproductive tract is not dependent on circulating levels of LH or human chorionic gonadotropin (hCG). The developing ovary does not express LH/choriogonadotropin receptors until the 16th week of gestation, which is why there is minimal steroidogenesis in the ovary until after delivery of the fetus 61.
Luteinizing hormone (LH) function After Delivery
After delivery, regardless of sex, a sharp increase in luteinizing hormone (LH) levels is seen because the mother withdraws estrogen. After this temporary increase, LH levels begin to decline and stay at low basal levels until prepuberty starts in both sexes 61.
Luteinizing hormone (LH) function At Puberty
In the years leading up to puberty in both sexes, there is a slow increase in the secretion of luteinizing hormone (LH) at night. As puberty progresses, LH begins to be secreted less so in a nighttime pattern followed by a pulsatile pattern throughout the whole day. This increase in gonadotropin secretion helps to stimulate gonadal steroidogenesis, which is important for maturation 61.
Luteinizing hormone (LH) function in Males
In males, luteinizing hormone (LH) stimulates testosterone release by the Leydig cells of the testes.
Luteinizing hormone (LH) function in Females
In females, luteinizing hormone (LH) stimulates steroid hormone release from the ovaries, ovulation, and the release of progesterone after ovulation by the corpus luteum 62. Ovulation is made possible by the combined actions of the hypothalamus, pituitary, and ovary 60. The hypothalamus begins the ovulation process by releasing gonadotropin-releasing hormone (GnRH) in a pulsatile fashion. This pulsatile release causes the anterior pituitary to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which then act on the ovarian follicle. The ovarian follicle comprises 3 essential cells: theca, granulosa, and oocyte. Luteinizing hormone (LH) causes the theca cells to make androstenedione. Androstenedione then converts to estradiol via aromatase, which follicle-stimulating hormone (FSH) stimulates. Upon achieving a critical concentration of estradiol, the negative feedback on LH that normally occurs by estrogen is shut off, and it begins to have positive feedback on LH release, which causes an “LH surge”, which initiates ovulation. Once ovulation has occurred, the ovarian follicle becomes the corpus luteum. The corpus luteum secretes progesterone and is stimulated by LH or human chorionic gonadotropin (hCG) if a pregnancy occurs 19.
Progesterone is a steroid hormone that is responsible for preparing the endometrium for the uterine implantation of the fertilized egg and maintenance of pregnancy 60, 63. If a fertilized egg implants, the corpus luteum secretes progesterone in early pregnancy until the placenta develops and takes over progesterone production for the remainder of the pregnancy 63.
Luteinizing hormone (LH) and Testicular Dysfunction in Chronic Kidney Disease
Low libido, erectile dysfunction, and smaller testicular size are all signs of testicular dysfunction. All these signs can be present in end-stage kidney disease. Testosterone concentration in the plasma and how quickly testosterone production takes place are usually low in patients with chronic kidney disease. Spermatogenesis (sperm production) has been noted to be either lowered or completely absent as well 11. After kidney transplantation, the low libido, erectile dysfunction, and smaller testicular size changes can be reversed and return to normal 11. Studies have shown that this testicular dysfunction and altered testosterone concentration results from higher levels of luteinizing hormone (LH) in the plasma and lower amounts of secretory luteinizing hormone (LH) pulses seen in men with end-stage kidney disease when compared to healthy subjects or men who underwent a successful renal transplant. This is significant because LH’s pulsatile secretion is necessary for the testes’ gonadotropin receptors to function properly. Furthermore, sustained high levels of LH in the blood and testes can cause a loss of gonadotropin receptors in the testes 64.
Luteinizing hormone (LH) diagnostic use
Women use ovulation predictor kits to determine the exact time of ovulation while trying to get pregnant. These ovulation predictor kits quantify luteinizing hormone (LH) levels in the urine 63.
Human chorionic gonadotropin (hCG)
Human chorionic gonadotropin (hCG) is “the hormone of pregnancy” produced primarily by syncytiotrophoblastic cells of the placenta of a pregnant woman 65, 66, 67. Smaller amounts of human chorionic gonadotropin (hCG) are also produced in the pituitary gland, the liver, and the colon 68. Human chorionic gonadotropin (hCG) plays an important role in synchronizing fetal and endometrial developments. Early in pregnancy, the level of human chorionic gonadotropin (hCG) increases in the blood and is eliminated in the urine. A pregnancy test detects human chorionic gonadotropin (hCG) in the blood or urine and confirms or rules out pregnancy 67. Throughout pregnancy, human chorionic gonadotropin (hCG) is also a marker of placental function. Human chorionic gonadotropin (hCG) can also be used in medical diagnostics to detect some cancers.
In everyday clinical practice, human chorionic gonadotropin (hCG) is mainly used to diagnose pregnancy and to supervise first trimester adverse pregnancy outcomes. Abnormalities in the production and the circulating levels of hCG during specific periods of gestation have been associated with a large array of pregnancy complications, such as miscarriages 69, fetal chromosomal anomalies 70, pre-eclampsia 71, 72, disturbances in fetal growth and development 73 and gestational trophoblastic diseases 74. Trophoblastic cancers (hydatidiform mole, choriocarcinoma, and germ cell tumors) are associated with high serum levels of hCG-related molecules 65, 75.
Nevertheless, the persistence of low hCG concentrations in a non-pregnant woman is not always malignant and can even be benign 76, 77. In addition, very high concentrations of hCG have been shown to have deleterious effects on fetal tissues, notably on fetal gonadal steroidogenesis 78. To avoid this, the human fetal tissue macrophages are thought to eliminate excess hCG. Katabuchi et al. 79 have shown that hCG induces the formation of vacuoles in human monocytes and hypothesized that these vacuoles would be involved in the protection of fetal tissues.
Multiple factors influence hCG levels during pregnancy. Among them, endocrine disruptive chemicals (EDCs), particularly bisphenol A and para-nonylphenol, can modulate hCG production and cause fetal damage as well as long-lasting consequences in adult life 80.
Human chorionic gonadotropin (hCG) stimulates the corpus luteum to produce progesterone to maintain the pregnancy. Human chorionic gonadotropin (hCG), is crucially involved in processes such as implantation and placentation, two milestones of pregnancy whose successful progress is a prerequisite for adequate fetal growth. Moreover, hCG determines fetal fate by regulating maternal innate and adaptive immune responses allowing the acceptance of the foreign fetal antigens 81. As one of the first signals provided by the embryo to its mother, human chorionic gonadotropin (hCG) has the potential to regulate very early pregnancy-driven immune responses, allowing the establishment and preservation of fetal tolerance.
During the early weeks of pregnancy, human chorionic gonadotropin (hCG) is important in maintaining function of the corpus luteum. Circulating human chorionic gonadotropin (hCG) interacts with the luteinizing hormone receptors (LHRs) of the ovary, promoting the corpus luteum and its production of progesterone which is necessary to maintain pregnancy and support the growth of the fetus. Production of human chorionic gonadotropin (hCG) increases steadily during the first trimester (8-10 weeks) of a normal pregnancy, peaking around the 10th week after the last menstrual cycle. Levels then fall slowly during the remainder of the pregnancy. Human chorionic gonadotropin is no longer detectable within a few weeks after delivery.
When a pregnancy occurs outside of the uterus (ectopic pregnancy), the level of human chorionic gonadotropin (hCG) in the blood increases at a slower rate. When an ectopic pregnancy is suspected, measuring the level of human chorionic gonadotropin (hCG) in the blood (quantitative test) over time may be useful in helping to make a diagnosis of ectopic pregnancy.
Similarly, the human chorionic gonadotropin (hCG) blood level may be abnormal when the developing baby (fetus) has a chromosome defect such as Down syndrome. An human chorionic gonadotropin (hCG) test is used routinely in conjunction with a few other tests as part of screening for fetal abnormalities.
Injections of human chorionic gonadotropin (hCG) mimic the surge in luteinizing hormone (LH) that is necessary for ovulation and are used in the therapy of female infertility, in assisted reproduction techniques (ART). In clinical trials, hCG resulted in pregnancies in approximately 30% of women. Human chorionic gonadotropin (hCG) prepared from urine of pregnant women and was approved for use in the United States in 1967 as treatment of ovulatory dysfunction in women desiring pregnancy. Subsequently, recombinant forms of hCG have been developed and licensed for use. Currently, hCG is available as a powder or in solution generically and under trade names such as Novarel and Pregnyl. Recombinant hCG is available as Overle. The dose and regimen of hCG therapy varies by indication and it should be used only by physicians with expertise in the management of infertility and hypogonadism. Common side effects include headache, nausea, anorexia, and local injection reactions. Uncommon, but potentially severe adverse events include ovarian hyperstimulation syndrome.
Figure 7. Human chorionic gonadotropin (hCG)
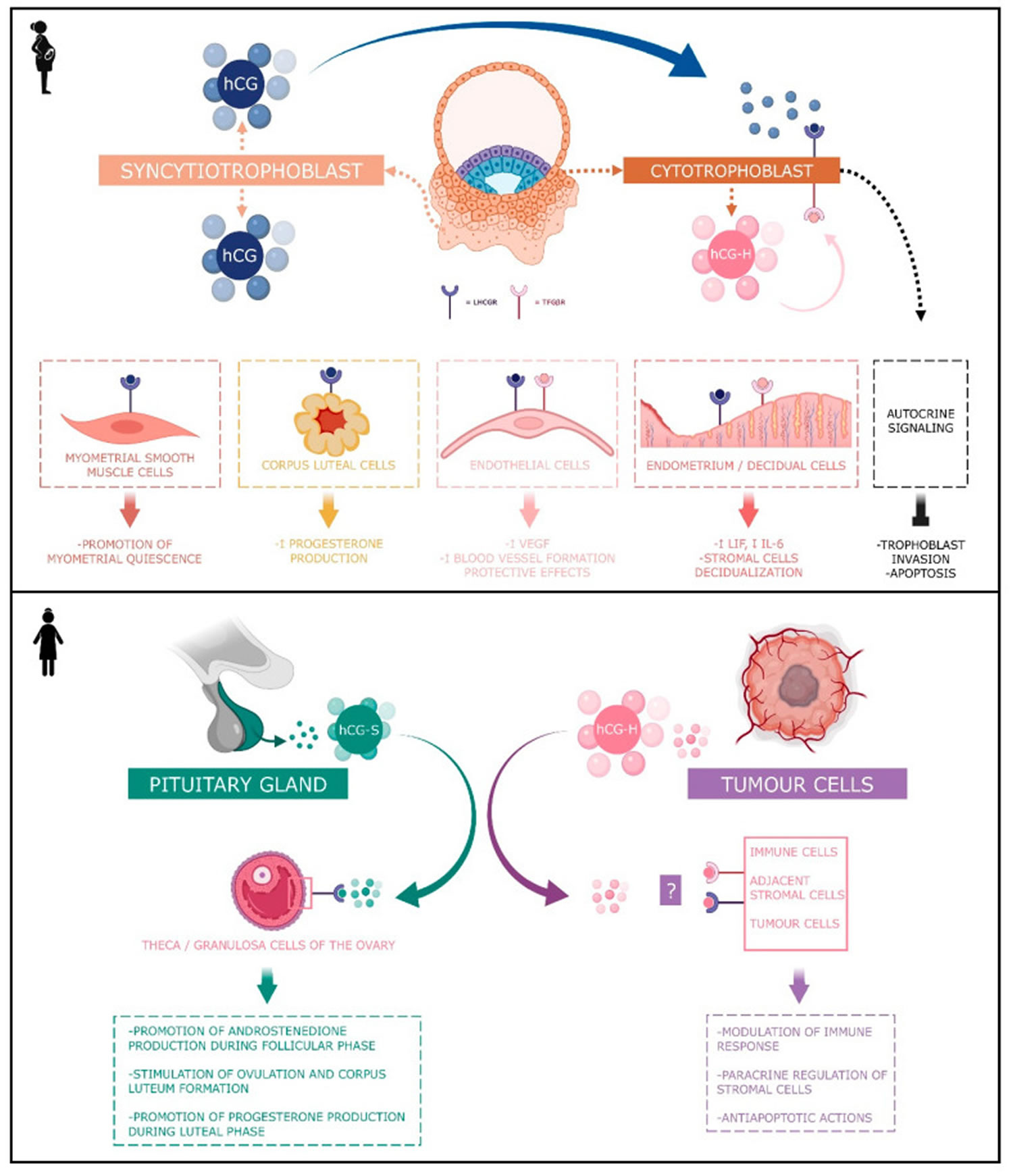
Figure 8. Human chorionic gonadotropin (hCG) actions during pregnancy and in non-pregnant woman
Footnotes: Human chorionic gonadotropin (hCG) has 4 major isoforms found in the serum and urine during pregnancy: classical hCG, hyperglycosylated hCG, free beta (β) subunit, and sulphated hCG 82. The classical form of hCG is schematized by a blue dot, the hyperglycosylated hCG by a pink dot and the sulphated form of hCG by a green dot. The blue receptor is the LH-hCG receptor (LHCGR) and the pink receptor is the transforming growth factor β receptor (TGFβR).
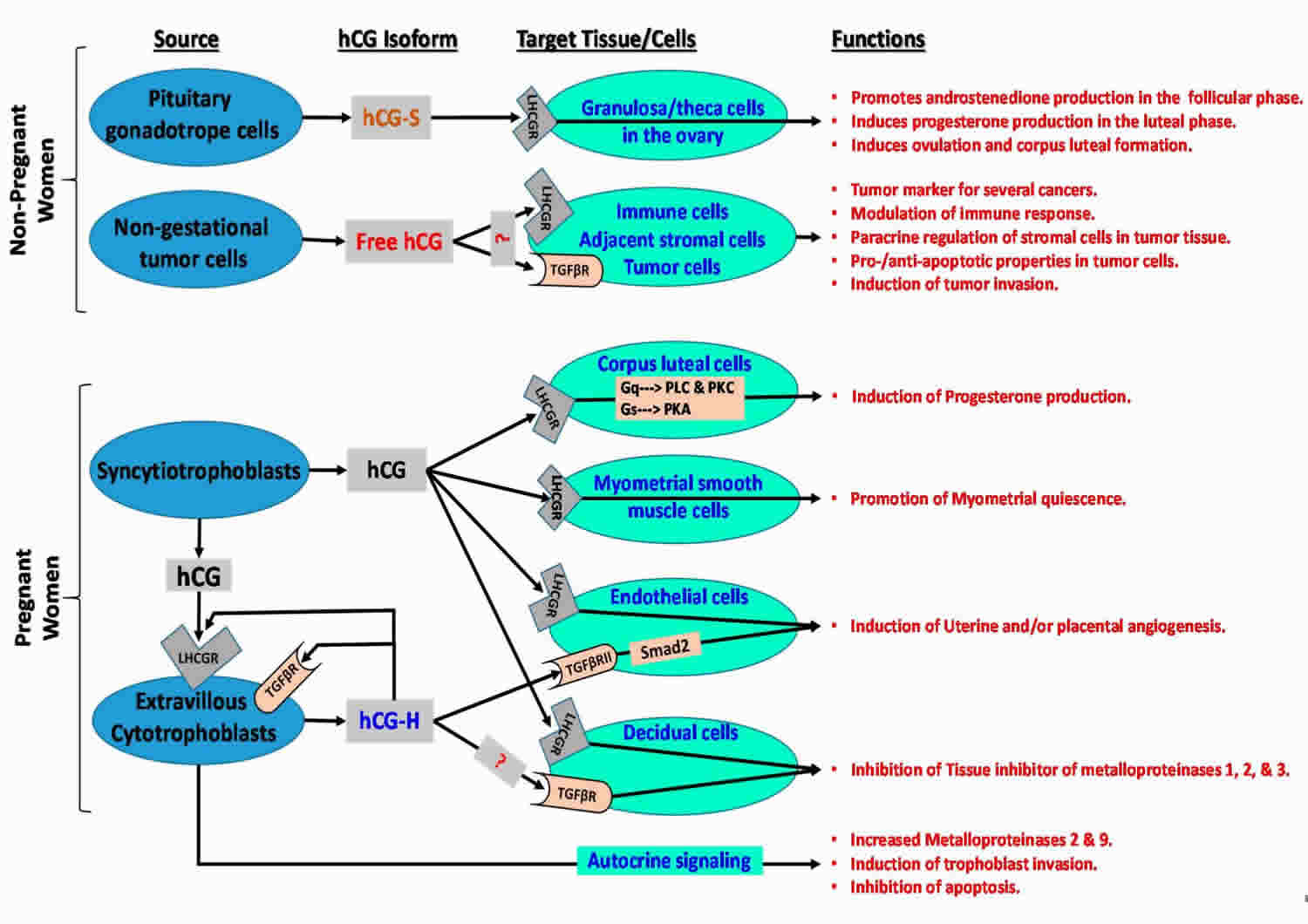
[Source 82 ]Figure 9. Human chorionic gonadotropin function
Footonotes: Human chorionic gonadotropin (hCG) isoforms function in non-pregnant and pregnant women.
Abbreviations: LHCGR = luteinizing hormone/choriogonadotropin receptor; TGFβR = transforming growth factor beta receptor; ?: hCG may bind to relevant receptor in target cells; Smad2 = similar to drosophila gene ‘mothers against decapentaplegic’ 2; Gq = heterotrimeric G protein subunit that activates phospholipase C (PLC)-associated protein kinase C (PKC); Gs = heterotrimeric G protein subunit that activates cAMP-dependent protein kinase A (PKA) signaling by activating adenylyl cyclase; hCH-S = sulfated hCG; hCG-H = hyperglycosylated hCG.
[Source 83 ]Human chorionic gonadotropin function
Human chorionic gonadotropin (hCG) has 4 major isoforms found in the serum and urine during pregnancy: classical hCG, hyperglycosylated hCG (H-hCG), free beta subunit (hCGβ), sulphated hCG, all of them with distinct biological functions 82, 84, 85, 86)):
- Classical human chorionic gonadotropin (hCG) is one of the first molecules secreted by the embryo 87. Its RNA is transcribed as early as the eight-cells stage 88 and the blastocyst produces the protein before implantation 89, 90. During the implantation, human chorionic gonadotropin (hCG) is mainly secreted by the syncytiotrophoblast and less by the cytotrophoblast 82. Human chorionic gonadotropin (hCG) can be detected in the maternal blood 10 days after ovulation. Its concentration reaches its top level around the 10th and 11th weeks of gestation 82. Afterwards, this level decreases and remains basal from the 12th week of gestation onwards until the end of the pregnancy. However, it remains significantly higher than in non-pregnant women (Betz D, Fane K. Human Chorionic Gonadotropin. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532950)), 91. By binding to its receptor called LH-hCG receptor (LHCGR), classical hCG acts on multiple types of cells: corpus luteum cells, myometrial smooth muscle cells, endothelial cells, and decidual cells. During the fifth and sixth days of embryogenesis, the blastocyst secretes hCG into the uterine cavity. This hormone binds to its LH-hCG receptor (LHCGR) on the deciduous surface. In response, the decidua prepares for implantation 92, 93, 94. Human chorionic gonadotropin (hCG) influences stromal cells by underpinning the decidualization and the prolactin secretion 95. D’Hauterive et al 93 have shown that the hCG–LHCGR complex also increases the secretion of leukemia inhibitory factor (LIF) and decreases the secretion of interleukine-6 (IL-6) by endometrial cells, factors affecting embryo implantation. This complex also promotes the differentiation of cytotrophoblasts into syncytiotrophoblasts 96. The hCG–LHCGR complex also regulates prostaglandin synthesis 97 and the formation of cAMP 98. Human chorionic gonadotropin (hCG) encourages trophoblast invasion and interstitial theca cell proliferation by over-modulating ERK and AKT signals 99, 100. Aside from this hCG-LHCGR complex, it has been shown that multiple hCG isoforms could stimulate trophoblastic invasion without regard to the LH-hCG receptor (LHCGR) 101.
- Hyperglycosylated hCG (H-hCG). Hyperglycosylated hCG β subunit has four oligosaccharide-linked instead of two in the classical form of the hCG β subunit 102. Hyperglycosylated hCG is massively produced during the first trimester of pregnancy, particularly by the extravillous cytotrophoblasts 103. Hyperglycosylated hCG represents the majority of the total hCG in the third week of gestation and 50% during the fourth week 82, 104. Then, it decreases rapidly until it completely disappears from the maternal blood circulation at the end of the first trimester 105. Hyperglycosylated hCG is useful for predicting pregnancy outcomes in women, with a first trimester suspicion of abortion. However, it is not considered as a better tool than the classical form of hCG 106. Hyperglycosylated hCG acts through autocrine instead of endocrine action. It decreases the apoptosis of trophoblast cells 107 and induces the implantation of the embryo 108 and trophoblastic invasion 109. Hyperglycosylated hCG is also massively secreted by choriocarcinomas and germ cell tumors 109, 110, 102. Its anti-apoptotic action would be achieved by its binding with the TGF-β receptor and independently of LH-hCG receptor (LHCGR). Hyperglycosylated hCG monitoring is useful in predicting Down’s syndrome 109, pre-eclampsia 111, and the therapeutic response to trophoblastic diseases, as well as in pregnancy predictions performed in in vitro fertilization (IVF) 112. The hyperglycosylated hCG (hCG-H) produced early in pregnancy and by various cancers contains more structurally-complex sialylated glycans than hyperglycosylated hCG (hCG-H) produced in mid and late pregnancy 113, 114, 115.
- Free beta subunit human chorionic gonadotropin (βhCG). Free beta subunit human chorionic gonadotropin (hCGβ) acts as an agonist of LH-hCG receptor (LHCGR) and an antagonist of the TGF-β receptor. High blood pressure during pregnancy (preeclampsia) could also be predicted by the abnormal rise in the circulating free β subunit of hCG (hCGβ). However, the association of beta subunit human chorionic gonadotropin (hCGβ) and inflammation, and oxidative stress in a pregnancy-caused hypertensive disorder, on the perinatal stage remains unclear. However, Wang et al. 116 demonstrated via a case–control study that the correlation of circulating free beta subunit human chorionic gonadotropin (hCGβ) levels with inflammatory and oxidative stress markers in patients with pregnancy-induced hypertension (preeclampsia) in perinatal stage was statistically significant. Like hyperglycosylated hCG (H-hCG), maternal serum free beta subunit human chorionic gonadotropin (hCGβ) is also used as a biomarker in first trimester screening for fetal Down’s syndrome 117. The free beta subunit human chorionic gonadotropin (hCGβ) also has a promotive action on cancer: germ cell malignancies, epithelial malignancies or carcinomas, adenocarcinomas, sarcomas, teratomas, blastomas, leukemias and lymphomas 118. For example, this action on the bladder carcinoma is exerted through the inhibition of apoptosis 119. According to Sirikunalai et al. 69, abnormally low (<0.5 MoM) or high (>2.0 MoM) free β subunit human chorionic gonadotropin (hCGβ) levels during gestation are generally associated with an increased risk of adverse pregnancy outcome (spontaneous abortion, preterm birth, low Apgar score, etc.).
- Sulphated human chorionic gonadotropin (hCG-S). Sulphated human chorionic gonadotropin (hCG-S) is produced by the pituitary gland in non-pregnant women and is secreted at the same time as LH during the cycle. Hence, its concentration ranges around one-fiftieth of the LH concentration 76, 120, 121, 122. While these levels are low, sulphated hCG is exactly 50 times more potent than LH 70 and acts the same way by stimulating androstenedione production during the follicular phase of the cycle as well as stimulating ovulation and corpus luteum formation. During the luteal phase, it may help stimulate progesterone production 76, 120, 121, 122. During the menstrual cycle, sulphated human chorionic gonadotropin (hCG-S) mediates several endocrine functions by inducing theca cell androstenedione production, corpus luteal progesterone production and by contributing to the process of ovulation. During pregnancy, syncytiotrophoblast derived hCG also induces corpus luteal cells to produce progesterone, whereas cytotrophoblast-derived hyperglycosylated hCG (hCG-H) appears to act as an autocrine and paracrine factor by activating the transforming growth factor-β receptor (TGFβR) mediated signaling 123, 124, 125, 126.
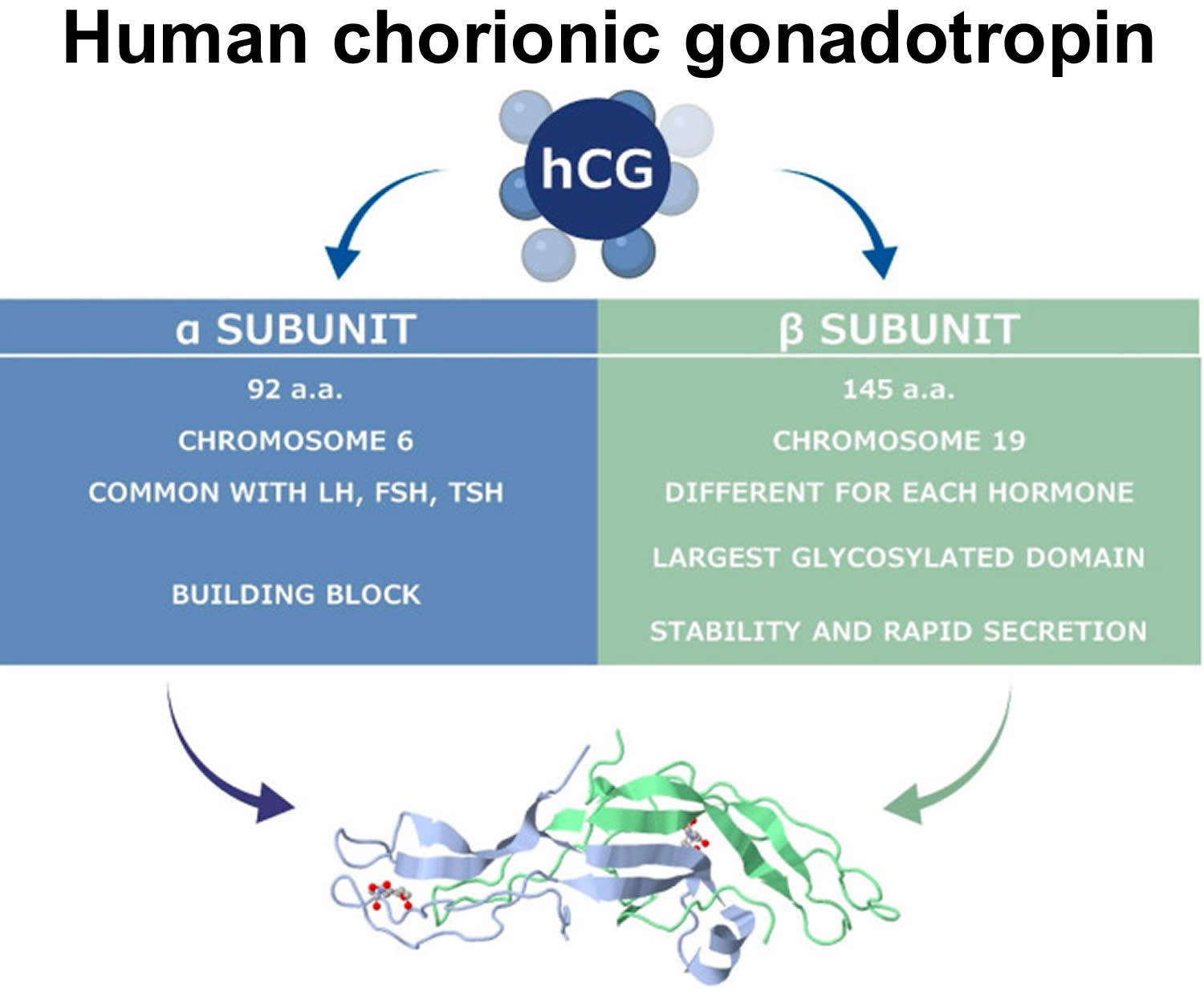
Human chorionic gonadotropin (hCG) is a glycoprotein composed of two subunits, the alpha (α) and beta (β) subunits 68. The alpha (α)-subunit of hCG is essentially identical to the alpha sub-units of the human pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as to the alpha sub-unit of human thyroid-stimulating hormone (TSH)113, 127, 128. The beta (β)-subunit of hCG, while structurally somewhat similar to the beta-subunit of luteinizing hormone (LH), differentiates hCG, hyperglycosylated hCG, and pituitary hCG from other molecules. Both human chorionic gonadotropin (hCG) and luteinizing hormone (LH) bind and function through a common luteinizing hormone/chorionic gonadotropin receptor (LHCGR) 128. The marked distinction between the human chorionic gonadotropin (hCG) and luteinizing hormone (LH) besides the absence of a beta subunit in LH is the difference in half-life. LH has a half-life of approximately 25 to 30 minutes 129, while human chorionic gonadotropin (hCG) has a much longer half-life at 24 to 37 hours 130, 131. This difference in half-life is critical to hCG’s function as a type of “super LH” during pregnancy to support the maintenance of an optimal intrauterine environment 132, 130, 133. As a result of human chorionic gonadotropin (hCG) structural homogeneity to luteinizing hormone (LH), hCG binds to luteinizing hormone/chorionic gonadotropin receptor (LHCGR) during the first 3–4 weeks of pregnancy, stimulating corpus luteal cells until the steroidogenic activity of the placenta produces sufficient progesterone to maintain pregnancy 134, 135, 136.
The hCG alpha (α)-subunit is encoded by a single CGA gene localized to chromosome 6q21.1-23 137, while the beta (β)-subunit is encoded by six non-allelic CGB1, 2, 3, 5, 7 and 8 genes localized to chromosome 19q13.3 138, 139. This gene cluster is widely accepted to have evolved as a result of duplication from the LHB gene 140. Expression of hCG genes is regulated by several hormones (corticosteroids, progesterone, GnRH), growth factors (placental growth hormone, leukemia inhibitory factor, vascular endothelial growth factor (VEGF)), cytokines (Interleukin (IL)-6, epidermal growth factors (EGF), tumor necrosis factor (TNF)-α), ligands of the nuclear receptor PPARγ and the homeobox gene (DLX3) 141, 142, 143, 144.
Human chorionic gonadotropin (hCG) is primarily metabolized by the liver with approximately 20% of circulating hCG excreted by the kidneys in the urine 145, 146. The beta-subunit is degraded in the kidney to make hCG β-core fragment (hCGβcf) which is measured by urine hCG tests 145, 147, 65. In early pregnancy, levels of beta-core fragment hCG (hCGβcf) in urine are low 113, while in the second trimester, approximately 80% of immunoreactive urinary hCG levels consists of hCGβcf 148.
The most well-known function of human chorionic gonadotropin (hCG) is the promotion of progesterone production during pregnancy. Human chorionic gonadotropin (hCG) stimulates ovarian corpus luteal cells to produce progesterone, therefore reinforcing the endometrial walls and preventing menstrual bleeding. This promotion of progesterone production is active in approximately 10% of the total length of the pregnancy or around 3 to 4 weeks following implantation. In a non-pregnant female, LH promotes progesterone production 128, 149, 150.
Studies done over recent years have shown hCG to be involved in a plethora of functions supporting the placenta, uterus, and fetus throughout pregnancy. These functions include the promotion of angiogenesis, immunosuppression, and blockage of phagocytosis of invading trophoblasts, promotion of growth and differentiation of fetal organs, and involvement in the adult brain and brainstem 151, 150.
Human chorionic gonadotropin (hCG) promotes the formation of new blood vessels (angiogenesis) and vasculogenesis through the upregulation of endocrine gland-derived vascular endothelial growth factor (EG-VEGF) 152. Uterine spinal arteries have hCG receptors that, when acted upon by hCG, undergo growth, and support the adequate blood supply and nutrition to the placenta. Human chorionic gonadotropin (hCG) also promotes the fusion of cytotrophoblast cells and their subsequent differentiation into syncytiotrophoblasts 151, 128.
Human chorionic gonadotropin (hCG) achieves many of its functions through the regulation of the expression of endocrine gland-derived vascular endothelial growth factor (EG-VEGF) and its receptors 152. The EG-VEGF receptors are G protein-coupled receptors (GPCRs), prokineticin 1 (PROKR1), and prokineticin 2. EG-VEGF is an angiogenic factor specific to endocrine tissues, including the placenta. EG-VEGF expression peaks around the same time as the peak of hCG concentration at approximately 8 to 11 weeks gestation 152. As an angiogenic factor, EG-VEGF expression increases in conditions of hypoxia. EG-VEGF and its receptors are regulators of both pathological and normal development of the fetus. EG-VEGF, PROKR1, and PROKR2 levels are significantly higher in fetal growth-restricted patients. Some have proposed that increases in EG-VEGF expression and its receptors brought on by increased levels of hCG are a form of compensation in fetal growth restriction 150, 153, 133, 149.
Maternal human chorionic gonadotropin (hCG) has implications in the development of fetal organs during development. There are hCG receptors in the fetal liver and kidney that are completely absent in adult organs. Human chorionic gonadotropin (hCG) has also been shown to support the growth and development of the umbilical cord 128, 133, 149.
Researchers have found hCG receptors in various areas of the adult female brain, including the hippocampus, hypothalamus, and brain stem. Speculation is that the presence of these receptors in the brain are involved in the pathophysiology of hyperemesis gravidarum. Other contributing factors may involve a combination of rising hormone levels overall, including estrogen, progesterone, and serum thyroxine, in addition to elevated hCG 128, 149, 154.
Human chorionic gonadotropin in Embryo Implantation and Placentation
Human chorionic gonadotropin (hCG) represents one of the first molecular messages send out by the pre-implanting embryo to regulate the implantation site and to ensure a timely initiation of the nidation process 155. Despite CGB gene expression was proven already in the 8-cell stage embryo 156, active secretion of human chorionic gonadotropin (hCG) starts at the blastocyst stage 157 and enables hCG detection in the maternal circulation 10 days after fertilization. Later on, human chorionic gonadotropin (hCG) is produced in high amounts by trophoblast cells 158 resulting in the highest hCG values between the 10th and 11th week of pregnancy. By the end of the first trimester, hCG levels decrease but remain elevated compared to non-pregnant individuals. A drop of hCG seems to be required for normal pregnancy progression 155. Blastocyst is an embryo that has formed a fluid-filled cavity and the cells have begun to form the early placenta and embryo, usually 5 days after ovulation or egg retrieval 159. A recent meta-analysis suggested that elevated hCG levels can be detected already at the end of the first trimester in women developing preterm preeclampsia (high blood pressure during pregnancy) 160 and hCG was suggested as a useful predictor for the development and severity of preeclampsia (high blood pressure during pregnancy) 161, 162.
Human chorionic gonadotropin angiogenic actions
Classical human chorionic gonadotropin (hCG) has angiogenic actions through the LHCGR (LH-hCG receptor) and achieves many of its functions through the regulation of the expression of endocrine gland-vascular endothelial growth factor (EG-VEGF) and its receptors 163, 164, 164, 66.
Human chorionic gonadotropin (hCG) increases blood vessel formation (angiogenesis) and the migration and maturation of pericytes in different in vitro and in vivo models. Through this action, the trophoblast can form plugs that prevent maternal blood from bleeding into the intervillous spaces during early pregnancy 66, 165, 166, 167, 168.
Human chorionic gonadotropin (hCG) also enhances the secretion of VEGF through the activation of NF-κB on angiogenesis during the luteal phase (Berndt S., D’Hauterive S.P., Blacher S., Pequeux C., Lorquet S., Munaut C., Applanat M., Hervé M.A., Lamandé N., Corvol P., et al. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J. 2006;20:2630–2632. doi: 10.1096/fj.06-5885fje)), 169, 170. In addition, hCG shields vascular endothelial cells against oxidative stress through the inhibition of apoptosis, activation of cell survival signaling, and mitochondrial function retention 171. Jing et al. 172 have shown that the decreased production of the β subunit human chorionic gonadotropin in early pregnant women could act on the expression of VEGF-MEK/ERK signal pathway by down-regulating it. It reduces angiogenesis and eventually leads to the abnormal angiogenesis of the villosities, a mechanism which may be an important factor of missed abortion 172.
As hyperglycosylated hCG subunit still presents a potent angiogenic effect but is acting regardless of LHCGR signaling pathways 140, 173. Gallardo et al. 174 have suggested that the striking overlapping of hCG and Heme oxygenase-1 (HO-1) functions in pregnancy could indicate that hCG hormonal effects are mediated by HO-1 activity, which may be affected by a HMOX1polymorphism in humans.
Human chorionic gonadotropin (hCG) and its hyperglycosylated isoform are accordingly considered pro-angiogenic molecules granting adequate fetal perfusion and fetal-maternal exchanges.
Human chorionic gonadotropin immunological actions
The immunomodulatory properties of human chorionic gonadotropin (hCG) are various and important for maternal tolerance of the embryo, an essential mechanism for the embryonic implantation and development 164, 175, 176. Immune cells situated in the uterine cavity play a key role in the embryo implantation 177, 178.
Several studies have supported the function of hCG in the prevention of fetoplacental tissue rejection through inhibitory immune-mediated mechanisms 179, 180. Some groups have shown that an anti-macrophage inhibitory factor is upregulated by human chorionic gonadotropin (hCG) activity during pregnancy, thus reducing macrophage activity at the uterine-placental interface 181, 182, 183 Other studies support a more proximate mechanism of action in which hCG directly suppresses immune actions taken against the fetus 151, 150, 184.
Human chorionic gonadotropin in Normal and Abnormal Pregnancies
Human chorionic gonadotropin (hCG) is a pregnancy supporting hormone and its clinical utility is primarily centered around its detection in early pregnancy, along with serial measurement during pregnancy and pregnancy-related complications. Early in pregnancy, the level of human chorionic gonadotropin (hCG) increases in the blood and is eliminated in the urine. A pregnancy test detects human chorionic gonadotropin (hCG) in the blood or urine and confirms or rules out pregnancy 67. Levels of hCG can vary widely between women with normal pregnancies. In viable pregnancies, a median hCG concentration of 126 IU/L is observed 12 days after embryo transfer, whereas levels below 76 IU/L are associated with early pregnancy loss 185. Typically, serum and urine concentrations of hCG rise exponentially in the first trimester of pregnancy, doubling about every 24 hours during the first 8 weeks 65. The peak is usually around 10 weeks of gestation and then levels decrease until about the 16th week of gestation where they remain fairly constant until term 186. Compared with an ongoing pregnancy, a failing pregnancy is generally associated with lower serum HCG levels, which gradually turns into a decrease 187. Patients who have hCG levels that plateau prior to 8 weeks or that fail to double commonly have a nonviable pregnancy, whether intra-uterine or extra-uterine 124, 188. Extra-uterine (ectopic) pregnancies usually have a rate-of-rise that is low without the typical doubling. However, given the large range of normal hCG levels and inconsistent rates-of-rise of this hormone, checking serum hCG levels is typically paired with ultrasound evaluation to improve sensitivity and specificity 189.
Human chorionic gonadotropin uses
Human chorionic gonadotropin diagnostic use
Human chorionic gonadotropin (hCG) is an important hormone in pregnancy, and its clinical utility is primarily centered around its detection in early pregnancy, along with serial measurement during pregnancy and pregnancy-related complications. Early in pregnancy, the level of human chorionic gonadotropin (hCG) increases in the blood and is eliminated in the urine. A pregnancy test detects human chorionic gonadotropin (hCG) in the blood or urine and confirms or rules out pregnancy 67. Levels of hCG can vary widely between women with normal pregnancies. Typically, serum and urine concentrations of hCG rise exponentially in the first trimester of pregnancy, doubling about every 24 hours during the first 8 weeks 65. The peak is usually around 10 weeks of gestation and then levels decrease until about the 16th week of gestation where they remain fairly constant until term 186. Patients who have hCG levels that plateau prior to 8 weeks or that fail to double commonly have a nonviable pregnancy, whether intra-uterine or extra-uterine. Extra-uterine (ectopic) pregnancies usually have a rate-of-rise that is low without the typical doubling. However, given the large range of normal hCG levels and inconsistent rates-of-rise of this hormone, checking serum levels is typically paired with ultrasound evaluation to improve sensitivity and specificity 189.
Certain cancers can also produce either human chorionic gonadotropin (hCG) or hCG-related hormone. Trophoblastic cancers (hydatidiform mole, choriocarcinoma, and germ cell tumors) are associated with high serum levels of hCG-related molecules 65, 75. Detection of hCG is also useful in the evaluation of trophoblastic disease, including complete and partial hydatidiform mole, postmolar tumor, gestational choriocarcinoma, testicular choriocarcinoma, and placental site trophoblastic disease. All of these entities produce hCG, varying levels of which are reported on commercial assays. A total hCG level of greater than 100,000 mIU/mL in early pregnancy, for example, is highly suggestive of a complete hydatidiform mole, although many normal pregnancies may reach this level at their peak around weeks 8 to 11 of gestation 190. Precise hCG measurements are important to assess the tumor mass, the successful treatment of trophoblastic cancer and to test for recurrence or persistence of trophoblastic disease 191.
The development of molar pregnancy correlates with fluxes in the levels of free beta-subunit of hCG. Return of hCG to zero following delivery or termination of pregnancy ranges from 7 to 60 days 192. Trending the fall of hCG levels can be important in termination of molar pregnancies and also following the termination of normal or ectopic pregnancies to be assured that the therapy has been successful. In a complete molar pregnancy, it is not uncommon to see large theca-lutein cysts as a result of increased stimulation of the ovaries by excess free beta-subunit hCG 193, 194, 195.
A molar pregnancy or hydatidiform mole, is a tumor arising from the trophoblast, which surrounds a blastocyst and subsequently develops into the chorion and amnion 194, 195. Hydatidiform mole may manifest as a complete or partial molar pregnancy. A complete hydatidiform mole is usually diploid with a 46 XX karyotype. There is trophoblastic hyperplasia producing a mass of multiple vesicles with little evidence of fetal and embryonic development. A partial hydatidiform mole is usually triploid due to dispermic fertilization or fertilization with an unreduced diploid sperm. In contrast to the complete mole, there is usually evidence of fetal development with an enlarged placenta 150, 194.
Patients with a history of prior molar pregnancy are at a 10-fold greater risk of a second hydatidiform pregnancy compared to the general population. The recommendation is that these women have their hCG levels monitored throughout pregnancy, as well as undergo evaluation by early ultrasonography 194, 195, 67.
Abnormal levels of hCG are associated with adverse pregnancy outcomes such as molar pregnancies and fetal growth restrictions. The intrauterine conditions are dependent upon placental function as the placenta is the main source of fetal nourishment. Suboptimal conditions due to an atrophic placenta may contribute to the risk of low birth weight. Several studies support the correlation between low birth weight and the risk of developing chronic conditions such as diabetes and hypertension later in life 196, 193, 194, 128.
Several clinical studies support the association of hCG concentration abnormalities with adverse fetal outcomes. This association varies with gestational age as hCG levels fluctuate throughout the pregnancy 194, 195, 193.
In the first trimester, low levels of hCG have correlated with spontaneous abortion and preeclampsia. Some studies have shown an association between low hCG concentrations (especially of the free beta-subunit of hCG) during the latter half of the first trimester and low birth weight due to attenuated fetal growth. Interestingly, some studies show that higher maternal hCG concentrations at the end of the first trimester are associated with fetal growth acceleration only in female-sex fetuses 196, 193.
In the second trimester, high levels of hCG have associations with gestational hypertension, spontaneous abortion, preeclampsia, fetal growth restriction (low birth weight), and pre-term delivery; this is in contrast to the association of low levels of hCG and low birth weight observed in the first trimester of pregnancy 193, 197.
How is human chorionic gonadotropin used?
Qualitative human chorionic gonadotropin (hCG) testing detects the presence of human chorionic gonadotropin (hCG) and is routinely used to screen for a pregnancy. This test may be performed by a laboratory, at a doctor’s office, or at home using a home pregnancy test kit. Methods will vary slightly but for most, a test strip is dipped into a collected cup of urine or exposed to a woman’s urine stream. A colored line (or other color change) appears within the time allotted per instructions, usually about 5 minutes. For accurate test results, it is important to carefully follow the test directions. (See the article on Home Testing: Avoiding Errors for more on this.) If the test is negative, it is often repeated several days later. Since human chorionic gonadotropin (hCG) rises rapidly, an initial negative test can turn positive within this time period.
Quantitative human chorionic gonadotropin (hCG) testing, often called beta human chorionic gonadotropin (β-hCG), measures the amount of human chorionic gonadotropin present in the blood. It may be used to confirm a pregnancy. It may also be used, along with a progesterone test, to help diagnose an ectopic pregnancy, to help diagnose and monitor a pregnancy that may be failing, and/or to monitor a woman after a miscarriage.
Human chorionic gonadotropin (hCG) blood measurements may also be used, along with a few other tests, as part of screening for fetal abnormalities.
Occasionally, an human chorionic gonadotropin test is used to screen for pregnancy if a woman is to undergo a medical treatment, be placed on certain drugs, or have other testing, such as x-rays, that might harm the developing baby. This is usually done to help confirm that the woman is not pregnant. It has become standard practice at most institutions to screen all female patients for pregnancy using a urine or blood human chorionic gonadotropin test before a medical intervention, such as an operation, that could potentially harm a fetus.
What does human chorionic gonadotropin test result mean?
- A negative human chorionic gonadotropin result means that it is unlikely that a woman is pregnant. However, tests performed too early in a pregnancy, before there is a significant human chorionic gonadotropin (hCG) level, may give false-negative results. The test may be repeated a few days later if there is a strong possibility of pregnancy.
- A positive human chorionic gonadotropin means that a woman is likely pregnant.
- However, blood or protein in the urine may cause false-positive pregnancy results. Urine human chorionic gonadotropin (hCG) tests may give a false-negative result if the urine is too diluted or if testing is done too soon in the pregnancy.
- Certain drugs such as diuretics and promethazine (an antihistamine) may cause false-negative urine results. Other drugs such as anti-convulsants, anti-parkinson drugs, hypnotics, and tranquilizers may cause false-positive results. The presence of protein in the urine (proteinuria), blood in the urine (hematuria), or excess pituitary gonadotropin may also cause a false positive.
- There are reports of false-positive blood human chorionic gonadotropin (hCG) results due to the presence of certain types of antibodies that some individuals produce or fragments of the human chorionic gonadotropin (hCG) molecule. Generally, if results are questionable, they may be confirmed by testing with a different method.
The blood level of human chorionic gonadotropin in a woman with an ectopic pregnancy usually rises at a slower rate than normal. Typically, human chorionic gonadotropin (hCG) levels double about every two days for the first four weeks of a normal pregnancy, then slow to every 31/2 days by six weeks. Those with failing pregnancies will also frequently have a longer doubling time early on or may even show falling human chorionic gonadotropin (hCG) concentrations during the doubling period. Human chorionic gonadotropin (hCG) concentrations will drop rapidly following a miscarriage. If human chorionic gonadotropin (hCG) does not fall to undetectable levels, it may indicate remaining human chorionic gonadotropin-producing tissue that will need to be removed (dilation and curettage – D&C).
How does the test that I do at home myself compare with the results of a test done in a lab?
Home pregnancy testing is very similar to qualitative urine human chorionic gonadotropin testing performed in the laboratory, but there are factors surrounding its use that are important to note.
- Home tests come with very specific directions that must be followed explicitly. If you are using a home test, follow the directions extremely carefully. There can be variability in sensitivity to detecting the presence of human chorionic gonadotropin with different brands of home pregnancy kits.
- Home tests are sometimes done too soon after the missed menstrual cycle to result in a positive test. It typically takes 10 days after a missed menstrual period before the presence of human chorionic gonadotropin can be detected by the urine test.
- All urine human chorionic gonadotropin tests should be done on a first morning urine sample, if possible. Urine becomes more dilute after ingestion of liquids (coffee, juice, water, etc.) and urine human chorionic gonadotropin concentrations may become too low to register as positive.
Generally, when used correctly, the home test should produce the same result as the urine human chorionic gonadotropin test done by your health practitioner. Blood testing for human chorionic gonadotropin is more sensitive than urine human chorionic gonadotropin testing, so sometimes a blood test will indicate pregnancy when the urine test is negative.
When is a blood human chorionic gonadotropin test ordered instead of a urine human chorionic gonadotropin?
Since human chorionic gonadotropin is not normally detected in the urine of a non-pregnant woman, a urine human chorionic gonadotropin is enough to confirm a pregnancy. This can also be done with a qualitative blood human chorionic gonadotropin test. Sometimes, however, it is important to know how much human chorionic gonadotropin is present to evaluate a suspected ectopic pregnancy or to monitor a woman following a miscarriage. In these circumstances, a health practitioner will order a quantitative blood human chorionic gonadotropin test.
How many days after a miscarriage would it take for a urine pregnancy test to show a negative result?
Urine human chorionic gonadotropin decreases at about the same rate as serum human chorionic gonadotropin, which can take anywhere from 9 to 35 days, with a median of 19 days. However, the timeframe for when an human chorionic gonadotropin result will be negative is dependent on what the human chorionic gonadotropin level was at the time of the miscarriage. Frequently, miscarriages are monitored with quantitative blood human chorionic gonadotropin testing. If the levels of human chorionic gonadotropin do not fall to undetectable levels, some human chorionic gonadotropin-producing tissue may remain and have to be removed.
What is an ectopic pregnancy?
An ectopic pregnancy occurs when the fertilized egg (ovum) implants somewhere other than in the uterus. This is a serious condition needing immediate treatment. Women with ectopic pregnancies often have abdominal pain and uterine bleeding. Usually, abnormally low levels of human chorionic gonadotropin are produced in ectopic pregnancies with slower-than-normal rates of increase.
Human chorionic gonadotropin as a Potential Biomarker for Preeclampsia
Pregnancies complicated by high blood pressure during pregnancy (preeclampsia) display an over-abundance of non-invasive syncytiotrophoblasts accompanied by inadequate cytotrophoblast invasion 198. Preeclampsia (high blood pressure during pregnancy) is frequently accompanied by low hyperglycosylated human chorionic gonadotrophin (hCG-H) serum levels during the first trimester of pregnancy (8–13 weeks) 199. A study by Kalkunte et al. 200 found higher hCG levels in serum from preeclamptic pregnancies at term compared with serum derived from normal pregnancies. The pathogenesis of preeclampsia is associated with altered glycosylation patterns and/or presence of sialyl Lewis antigens on hCG, which impairs the recruitment and/or expansion of tolerance-inducing immune cell types 71, 201. The combination of hyperglycosylated human chorionic gonadotrophin (hCG-H) together with pregnancy-associated plasma protein A (PAPP-A) levels, maternal mean arterial pressure and parity were shown to predict early onset preeclampsia 199. A meta-analysis of by Zhong et al. 202 evaluated first-trimester serum screening for 112,400 women in order to predict preeclampsia. Although the results showed no improvement in prediction, the detection rate of first trimester serum markers for early preeclampsia was observed to be better than that for late preeclampsia 199, 203, 204, 205.
Human chorionic gonadotropin as Serum Marker for Down’s Syndrome Screening
Measurements of free beta-human chorionic gonadotropin (hCGβ) levels in first trimester maternal serum proved to be extremely useful in screening for Down’s syndrome 83. Pregnancies complicated by Down’s syndrome are associated with elevated serum hCG and free beta-human chorionic gonadotropin (hCGβ) concentrations 83. Recommended screening for trisomy 21 (Down’s syndrome) includes a combination of maternal age, fetal nuchal translucency (NT) thickness, maternal serum hCGβ and PAPP-A at around 11–13 weeks gestation 206. However, for second trimester (15–22 weeks gestation) screening, hCG is combined with inhibin A, alpha-fetoprotein (AFP) and unconjugated estriol. To assess patient-specific risks for trisomy 21 or Down’s syndrome, a prior maternal age-related risk is multiplied by likelihood ratios determined from deviation of the measured markers from the respective expected levels 207. Furthermore, several markers of Down’s syndrome (β-core fragment hCG [hCGβcf], β human chorionic gonadotropin [hCGβ] and hyperglycosylated hCG [hCG-H]) can be detected in maternal urine 208. Among these, β-core fragment hCG (hCGβcf) is the major metabolic product of hCG in maternal urine with second trimester levels increasing in pregnancies complicated by Down’s syndrome 208. Urinary hyperglycosylated hCG (hCG-H) levels are also elevated in affected pregnancies although fewer cases have been tested for hCG-H compared to hCGβcf levels. Maternal serum hCG assays remain the preferred test for Down’s syndrome screening versus urine β-core fragment hCG (hCGβcf) or hyperglycosylated hCG (hCG-H) due to the wide standard deviation of the urine markers and the significant heterogeneity that exists among studies 208.
Human chorionic gonadotropin as tumor marker
Serum human chorionic gonadotropin (hCG) is also a tumor marker for certain types of testicular cancer (seminomas, nonseminomatous germ cell tumor and extragonadal tumors) in males and gestational trophoblastic disease (GTD) in females after pregnancy and germ cell tumors 209, 210, 211. Since hCG is not normally present in men or non-pregnant women, it is useful as a tumor marker. If the tumor or cancer is producing hCG, then the hCG test can be used to help detect and monitor tumor activity. If hCG is increased with these conditions, then the hCG test can be used as a diagnostic and monitoring tool. Tumor markers are substances, often proteins, that are produced by the cancer tissue itself or sometimes by the body in response to cancer growth. Because some of these substances can be detected in body samples such as blood, urine, and tissue, these markers may be used, along with other tests and procedures, to help detect and diagnose some types of cancer, predict and monitor a person’s response to certain treatments, and detect recurrence.
When testing hCG as a tumor marker, unlike detecting pregnancy, it is important to measure the intact (alpha + beta subunits) form of hCG. There may be some benefit to also measuring the beta-subunit of hCG (hCGβ) in certain tumors, and different labs will detect intact and beta human chorionic gonadotropin (hCGβ) differently. For this reason, it is very important to continue to use the same lab when monitoring testicular cancer, gestational trophoblastic disease or germ cell tumors.
Gestational trophoblastic disease (GTD) is a group of tumor types that develop in a woman’s uterus from the layer of cells surrounding an embryo that creates the placenta during a normal pregnancy (trophoblasts) and produces hCG 212. Gestational trophoblastic disease usually occurs at the beginning of pregnancy after an egg has been fertilized, but instead of supporting the growth of a fetus, the cells form abnormal tissue masses. This tissue is made of trophoblast cells and normally surrounds the fertilized egg in the uterus. Trophoblast cells help connect the fertilized egg to the wall of the uterus and form part of the placenta (the organ that passes nutrients from the mother to the fetus) 212. Sometimes there is a problem with the fertilized egg and trophoblast cells. Instead of a healthy fetus developing, a tumor forms. Until there are signs or symptoms of the tumor, the pregnancy will seem like a normal pregnancy. Most gestational trophoblastic disease is benign (not cancer) and does not spread, but some types become malignant (cancer) and spread to nearby tissues or distant parts of the body. According to the American Cancer Society, gestational trophoblastic disease occurs in about 1 in 1,000 pregnancies. It can also occur after a normal pregnancy or after a miscarriage or abortion.
The primary forms of gestational trophoblastic disease are 212:
- Hydatidiform mole also called a “molar pregnancy”, which may be complete (only tumor tissue) or a mixture of tumor and fetal tissue but does not develop into a viable baby; these are usually benign but must be surgically removed.
- Complete hydatidiform mole.
- Partial hydatidiform mole.
- Gestational Trophoblastic Neoplasia (GTN)
- Invasive mole – a hydatidiform mole that grows into the uterus wall; it must be surgically removed; however, the condition can persist if gestational trophoblastic disease tissue remains.
- Choriocarcinoma – a rare cancer that may develop from the gestational trophoblastic disease tumor tissue in about 2 to 7 per 100,000 pregnancies; these cancers can grow quickly and spread to other parts of the body.
- Placental-site trophoblastic tumors (PSTT; very rare). Placental site trophoblastic tumor arises at the site of placental attachment in the uterus. This tumor usually develops after a normal or aborted pregnancy but doesn’t often spread through the body.
- Epithelioid trophoblastic tumors (ETT; extremely rare). Epithelioid trophoblastic tumor is similar in nature to the choriocarcinoma but is now considered a separate disease. It may take many years after a pregnancy for this tumor to be detected and may have already spread to other parts of the body.
Testicular cancer is cancer that starts in the testicles. More than 90% of testicular cancers start in cells known as germ cells 213. These are the cells that make sperm. The main types of germ cell tumors in the testicles are seminomas and non-seminomas.
Seminomas is a slow-growing form of testicular cancer found in men in their 40s and 50s 213. Seminomas cancer is in the testes, but it can spread to the lymph nodes. Lymph node involvement is either treated with radiotherapy or chemotherapy. Seminomas are very sensitive to radiation therapy. Elevation of beta-human chorionic gonadotropin (hCGβ) is found in approximately 14% of patients with stage 1 pure seminomas before surgical procedure to remove one or both testicles (orchiectomy) and in about one-half of patients with metastatic seminomas 214, 215, 216. Stage 1 cancer has not spread beyond the testicle. Approximately 40% to 60% of men with nonseminomas have an elevated serum beta-hCG 217.
Non-seminomas, another type of testicular cancer, include embryonal carcinomas, teratomas, yolk sac carcinomas and choriocarcinomas 217, 213. Most nonseminomas are a mix of different types sometimes with seminoma cells too, but this doesn’t change the treatment of most non-seminoma cancers 213. Nonseminomas usually occur in men between their late teens and early 30s. For patients with nonseminomas, one of the most significant predictors of prognosis is the degree of tumor-marker elevation after the cancerous testicle has been removed 218. Alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (hCGβ), and lactase dehydrogenase (LDH) play an important role as serum tumor markers in the staging and monitoring of germ cell tumors and should be measured prior to removing the involved testicle 219. Elevated levels of serum tumor markers are often the earliest sign of relapse, making these markers useful for monitoring all stages of nonseminomas and metastatic seminomas. Tumors that appear to have a seminoma histology but are accompanied by an elevated serum level of alpha-fetoprotein (AFP) should be treated as nonseminomas because seminomas do not produce alpha-fetoprotein (AFP) 217.
- Embryonal carcinoma: These cells are found in about 40% of testicular tumors, but pure embryonal carcinomas occur only 3% to 4% of the time. When seen under a microscope, these tumors can look like tissues of very early embryos. This type of non-seminoma tends to grow rapidly and spread outside the testicle. Embryonal carcinoma can increase blood levels of a tumor marker protein called alpha-fetoprotein (AFP), as well as human chorionic gonadotropin (HCG).
- Choriocarcinoma: This is a very rare and fast-growing type of testicular cancer in adults. Pure choriocarcinoma is likely to spread rapidly to other parts of the body, including the lungs, bones, and brain. More often, choriocarcinoma cells are seen with other types of non-seminoma cells in a mixed germ cell tumor. These mixed tumors tend to have a somewhat better outlook than pure choriocarcinomas, although the presence of choriocarcinoma is always a worrisome finding. Choriocarcinoma increases blood levels of human chorionic gonadotropin (hCG).
- Teratoma: Teratomas are germ cell tumors with areas that, under a microscope, look like each of the 3 layers of a developing embryo: the endoderm (innermost layer), mesoderm (middle layer), and ectoderm (outer layer). Pure teratomas of the testicles are rare and do not increase alpha-fetoprotein (AFP) or human chorionic gonadotropin (hCG) levels. Most often, teratomas are seen as parts of mixed germ cell tumors.
An increased level of hCG is seen in some germ cell tumors (benign and cancerous).
Germ cell tumors and cancers occur primarily in the ovaries and testicles but can also rarely develop in other locations such as the chest.
- Germ cell tumors can occur in the egg-producing cells of the ovaries and are more often seen in younger women.
- Germ cell tumors can affect cells within the testicles that make sperm and account for more than 90% of testicular cancers.
Levels of hCG may also be elevated in other diseases such as liver, breast, lung, skin, and stomach cancers. Increased human chorionic gonadotropin (hCG) levels may also be seen in non-cancer conditions such as cirrhosis, duodenal ulcer, and inflammatory bowel disease.
Human Chorionic Gonadotropin (hCG) in Female Infertility Treatment
Human chorionic gonadotropin (hCG) is a pregnancy supporting hormone extracted from the urine of pregnant women and is an injectable hormone that is widely used as an ovulation trigger that helps with the final maturation of the eggs in the ovariain follicles and triggers the ovaries to release the mature eggs (ovulation) in women undergoing Assisted Reproductive Technology (ART) like intrauterine insemination (IUI), in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) 220. Human chorionic gonadotropin (hCG) also stimulates the corpus luteum to secrete progesterone to prepare the lining of the uterus for implantation of the fertilized egg.
Human chorionic gonadotropin (hCG) is structurally similar to the luteinizing hormone (LH) that is produced by a woman’s anterior pituitary gland 221. Human chorionic gonadotropin (hCG) acts on the ovary in a manner similar to a woman’s own luteinizing hormone (LH). Human chorionic gonadotropin (hCG), like LH, stimulates the final maturation of the eggs in the follicle. Human chorionic gonadotropin (hCG) also stimulates progesterone production from the ovary after egg retrieval. This progesterone is important to prepare the uterus for implantation of the embryo.
Human chorionic gonadotropin (hCG) and luteinizing hormone (LH) share the same receptor the LHCGR (LH-hCG receptor).
Intrauterine administration of synthetic or natural hCG around the time of embryo transfer in in vitro fertilization (IVF) is a novel approach that has been suggested to improve the outcomes of assisted reproduction treatment (ART) based on the fundamental role of hCG in embryo implantation and the early stages of pregnancy 128. The intervention involves intrauterine administration of hCG via an embryo transfer catheter. The hCG can be released at different points inside the uterine cavity (close to the internal cervical os, mid‐cavity, or near the fundus) within minutes, hours, or days before the actual embryo transfer. Human chorionic gonadotropin (hCG) sources for medical treatments include extraction from the urine of pregnant women (natural) or from cell cultures using recombinant DNA technology (rhCG). This 2018 Cochrane review found moderate quality evidence that women undergoing cleavage‐stage embryo transfer using an intrauterine (intracavity) administration of human chorionic gonadotropin (hCG) dose ≥ 500 IU have an improved live birth rate 222. There is insufficient evidence for intrauterine (intracavity) human chorionic gonadotropin (hCG) treatment for blastocyst transfer. There should be further trials with live birth as the primary outcome to identify the groups of women who would benefit the most from this intervention. There was no evidence that miscarriage was reduced following intrauterine (intracavity) human chorionic gonadotropin (hCG) administration, irrespective of embryo stage at transfer or dose of intrauterine (intracavity) human chorionic gonadotropin (hCG). Events were too few to allow conclusions to be drawn with regard to other complications 222.
Gonadotropin treatment
Highly purified and man-made (synthetic) gonadotropins have been developed and used in the treatment of hypogonadism and infertility. Synthetic forms of gonadotropin-releasing hormone (GnRH) have been used with the gonadotropins in assisted reproductive techniques (ART) and in vitro fertilization (IVF).
Follicle stimulating hormone (FSH) therapeutic uses
Therapeutic preparations of follicle stimulating hormone (FSH) are widely used in the treatment of infertility (Table 1). Partially and highly purified human menopausal urine derived FSH (Menotropins or human menopausal gonadotropin [hMG] or Menopur which also has LH activity); industrial production of therapeutic grade urinary FSH (urofollitropin, Bravelle) and recombinant DNA (rDNA)-derived human FSH (follitropin alpha, Follistim, Gonal F) are available and approved for use in treatment of infertility and hypogonadism 26. They are generally given by subcutaneous injection daily or several times weekly. The dose and appropriate regimen vary by indication. These agents should be used only by doctors with expertise in management of infertility and hypogonadism.
Therapeutic preparations of follicle stimulating hormone (FSH) use in assisted reproduction technology can be divided into 3 categories 36:
- Induction of ovulation when a single healthy oocyte is required.
- Induction of multiple ovulation or superovulation to maximize efficiency when assisted reproductive technologies are used that allow replacement of a fixed number of embryos.
- Stimulation of spermatogenesis.
Treatment of female infertility is a situation in which patients are otherwise generally healthy and common disorders of reproduction such as anovulatory infertility can be treated in a safe and effective way 223. However, there is a narrow dose-range for use of FSH between a threshold level required to stimulate growth of a follicle(s) and the maximal dose (ceiling dose) above which overstimulation can occur 224. Therefore there is a significant risk to health due to the iatrogenic induction of ovarian hyperstimulation syndrome or multiple pregnancies. Different physiological and clinical states can affect the levels of the threshold and ceiling for FSH treatment. Thus careful dose adjustment and monitoring of FSH levels and ovarian responses are required, particularly for women with polycystic ovary syndrome (PCOS) 225. This cannot be achieved without accurate and reproducible calibration of therapeutic products. However, the end point used for patient response to therapeutic preparations should also be carefully considered.
Table 1. Common causes of infertility and treatments that require follicle stimulating hormone (FSH)
| Cause | Treatment |
|---|---|
| Female infertility | |
| Ovulatory failure (oligo- or amenorrhea) | Ovulation induction |
| Primary ovarian failure | Superovulation followed by In vitro fertilization (IVF) using donated oocytes |
| Tubal or pelvic damage a | Superovulation and In vitro fertilization (IVF) |
| Endometriosis a | Superovulation and In vitro fertilization (IVF), gamete intra-fallopian tube transfer (GIFT), intra-uterine insemination (IUI) |
| Cervical mucus dysfunction or defects | Superovulation and intra-uterine insemination (IUI), gamete intra-fallopian tube transfer (GIFT), In vitro fertilization (IVF), zygote intra-fallopian tube transfer (ZIFT) |
| Antisperm antibodies | Superovulation and In vitro fertilization (IVF), intra-uterine insemination (IUI) |
| Idiopathic infertility | Superovulation and In vitro fertilization (IVF), gamete intra-fallopian tube transfer (GIFT), intra-uterine insemination (IUI) |
| Male infertility | |
| Sperm dysfunction | In vitro fertilization (IVF), intra-cytoplasmic sperm injection (ICSI) |
| Azoospermia | Stimulation of spermatogenesis with FSH if due to hypogonadotrophic hypogonadism or pituitary failure |
Footnote: a May be treated surgically in appropriate cases.
[Source 36 ]Luteinizing hormone (LH) use in Infertility and Assisted Reproductive Technology
Infertility is defined clinically as the inability to become clinically pregnant after at least 12 months of unprotected sexual intercourse. Female factors, male factors, or both can cause it. In women, it can be the result of ovulatory issues (ie, anovulation), obstructions of the fallopian tubes, and endometriosis. To become pregnant, many women undergo assisted reproductive technologies (ARTs), like intrauterine insemination (IUI) and in vitro fertilization (IVF) 226.
Proper development of a ovarian follicle and ovulation involves the combined effects of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and their bodily activities. This interplay between FSH and LH has also been shown to be important in assisted reproductive technology (ART). It has been found that low LH levels in the body can result in poor outcomes in Assisted Reproductive Technology (ART). Therefore, patients who have low endogenous LH, such as those with hypogonadotropic hypogonadism, can have an increase in the efficacy of ART with exogenous LH treatment 227. A study found that with supplementation of LH during the mid-follicular phase, there were better pregnancy results in women who had not responded optimally to conventional Assisted Reproductive Technology (ART). This outcome was thought to be due to the increased production of 17-beta-estradiol 60. Another study reported that fertilization, implantation, and clinical pregnancy rates were higher in patients who underwent Assisted Reproductive Technology (ART) that included recombinant human LH (rhLH) when compared to patients who underwent ART without recombinant human LH (rhLH). A lower apoptosis rate was also present in patients who underwent ART, which included recombinant human LH (rhLH) 12.
Although there are demonstrable benefits of LH supplementation during Assisted Reproductive Technology (ART), research also shows that LH levels can have unfavorable effects on ART 227. These adverse effects are thought to result from inhibition of Granulosa cell proliferation, atresia of immature ovarian follicles, and luteinization of preovulatory follicles before they would be under physiological conditions. Additionally, increased LH before ovulation has been shown to influence the conception and implantation of the embryo negatively 60.
Gonadotropins use in Assisted Reproductive Technology
To increase likelihood of pregnancy through assisted reproductive technology (ART), multiple eggs must be produced. This is accomplished through the administration of gonadotropins medications that directly stimulate the ovaries. Stimulation can be achieved with a variety of drug regimens. Gonadotropin medications come in several forms; Repronex® (menotropins for injection) and Menopur® (menotropins for injection) are a combination of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). They replace a woman’s own LH and FSH which are normally produced by the anterior pituitary gland. Bravelle®, Follistim® AQ Cartridge for use with Follistim Pen®, Follistim® AQ Vial, Gonal-F®, and Gonal-F® RFF Pen are preparations that contain only FSH. Follistim® AQ Cartridge for use with Follistim Pen®, Follistim® AQ Vial, Gonal-F®, and Gonal-F® RFF Pen are recombinant products which are made by genetically engineered cells. This process ensures uniform purity and potency. Because the dose of hormones that are used in ART is greater than what the body normally produces, the ovaries typically develop more than one oocyte as occurs in a natural cycle.
Gonadotropins act directly on the ovary to stimulate the growth of follicles (the structures in the ovaries which contain eggs). Granulosa cells within the follicles grow and develop which cause the follicles to enlarge and fill with follicular fluid. These developing follicles can be counted and measured using transvaginal ultrasound. As the follicles grow, they produce increasing amounts of estrogen, which can be measured with a laboratory blood test. Some physicians prefer one formulation or another. Your doctor can discuss this with you in more detail.
Gonadotropins Dosage and Monitoring
Gonadotropins are packaged in vials containing 37.5, 75 or 150 International Units (IU). Follistim AQ Pens and Gonal-F RFF Pens are packaged in pre-mixed injectable pens. Multi-dose vials of some medications are also available. Dosage may vary depending on the patient’s history. Patients will then have regularly scheduled transvaginal ultrasound examinations and serum estradiol tests. The dose of gonadotropins is then determined by the result of the ultrasound and estradiol tests. Most women require between seven to 10 days of gonadotropin therapy.
Bravelle®, and Repronex® are administered subcutaneously or by intramuscular injection, usually into the muscles of the buttocks. Gonal-F,® Follistim,® Follistim AQ Pens, and Gonal-F RFF Pens are administered subcutaneously, like an insulin or allergy shot.
Human chorionic gonadotropin (hCG) Therapeutic Uses in the Male
In the male human chorionic gonadotropin (hCG):
- Hypogonadotrophic hypogonadism.
- Delayed puberty associated with insufficient gonadotrophic pituitary function.
- Cryptorchism, not due to an anatomic obstruction.
- Preoperative preparation of ectopic testes.
- Infertility, in selected cases of deficient spermatogenesis.
Hypogonadism is impaired testicular function; this can occur due to a problem with the testes (primary hypogonadism or hypergonadotropic hypogonadism) or due to a problem with the hypothalamic-pituitary-gonadal axis (secondary hypogonadism or hypogonadotropic hypogonadism). These two entities can be distinguished by measuring serum LH and FSH concentrations 228. Primary hypogonadism or hypergonadotropic hypogonadism is characterized by a low serum testosterone level and oligo- or azoospermia in the presence of elevated serum LH and FSH concentrations 228. In contrast, secondary hypogonadism or hypogonadotropic hypogonadism is diagnosed in the setting of a low testosterone level and sperm count in association with low or inappropriately normal serum LH and FSH concentrations 228.
Men with secondary hypogonadism or hypogonadotropic hypogonadism have low levels of androgens in the plasma as well as a lack or delay of sexual maturity, which can cause symptoms such as a lack of libido, depression, increase in adipose tissue, and diminished erectile function 229.
Patients with secondary hypogonadism or hypogonadotropic hypogonadism usually have an issue with gonadotropin-releasing hormone (GnRH) signaling, which then causes a decrease in follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secretion. This decreased FSH and LH contributes to decreased androgen levels and reduced spermatogenesis. Studies have shown that giving these patients pulsatile GnRH or LH (or hCG) and FSH can help increase spermatogenesis and thus increase the sperm concentration in the ejaculate. Even then, most couples will need assisted reproductive technology (ART) to achieve pregnancy 230.
Testosterone therapy, whether by exogenous testosterone replacement or induction of endogenous testosterone production by human chorionic gonadotropin (hCG) is needed in all hypogonadotropic hypogonadism patients 228. Testosterone plays a number of important physiologic roles in the human and are required not only for virilization and normal sexual function, but also for maintenance of both muscle and bone mass, as well as normal mood and cognition.
While testosterone is the primary treatment modality used to induce and maintain secondary sexual characteristics and sexual function in men with hypogonadism, treatment with testosterone does not restore fertility. Therefore, in patients in whom fertility is the treatment goal, induction of gonadotropin secretion by pulsatile GnRH or exogenous gonadotropins is necessary. While hormone therapy is the mainstay of treatment for congenital hypogonadotropic hypogonadism; undescended testicle (cryptorchidism), if present, should be treated surgically with orchidopexy, ideally before the age of 2 years to improve fertility outcomes and reduce the risk of future testicular malignancy 231.
Exogenous Gonadotropin Therapy
The typical gonadotropin regimen for induction of spermatogenesis in men comprises human chorionic gonadotropin (hCG) in combination with follicle-stimulating hormone (FSH). Purified human chorionic gonadotropin (hCG) is an effective substitute for luteinizing hormone (LH) given the structural homology between these 2 hormones which act through the same receptor on Leydig cells 228. While a variety of FSH formulations are now available in different countries, there is little to choose between them in terms of therapeutic efficacy. Traditionally, FSH has been administered in the form of human menopausal gonadotropins (hMG) derived from the urine of postmenopausal women. Although human menopausal gonadotropins (hMG) has both FSH and LH activity, FSH activity predominates and LH activity is so low that combined administration with hCG is necessary to achieve fertility. Subsequently, highly purified urinary FSH preparations were developed, which have enhanced specific activity in comparison to hMG (10,000 IU/mg of protein vs 150 IU/mg of protein for hMG) 228. In the early 1990s, recombinant human follicle stimulating hormone (r-hFSH) formulations were developed, which have greater purity and specific activity than any of the urinary preparations and no intrinsic LH activity 232, 233. Recombinant human follicle stimulating hormone (r-hFSH) is produced in genetically engineered Chinese hamster ovary cells, in which the genes encoding the alpha and beta subunits have been introduced using recombinant DNA technology 232. Pharmacokinetic studies of r-hFSH indicate a half-life of 48 ± 5 hour and a dose-dependent increase in the serum level of FSH 232.
The subcutaneous route of administration is as effective as the intramuscular route for both gonadotropins and significantly increases patient compliance. Therapy is typically initiated with human chorionic gonadotropin (hCG) alone at a dose of 1,000 IU on alternate days and the dose titrated based on trough testosterone levels and testicular growth 234. Alternatively, recombinant human hCG can also be administered subcutaneously from a prefilled syringe. In some patients the dose of hCG can be decreased over time as testicular size increases. In the majority of patients with larger testes at baseline, spermatogenesis can be initiated with hCG alone most likely due to residual FSH secretion 235, 236. Once there is a plateau in the response to hCG which typically occurs at around 6 months, therapy with FSH (in one of the three forms described above) should be added at a dose of 75 IU on alternate days. If sperm output and testicular growth remain suboptimal the dose of FSH can be gradually increased to 150 U daily. Continuation of this combined regimen for 12-24 months induces testicular growth in almost all patients, spermatogenesis in approximately 80% and pregnancy rates in the range of 50% 237, 238, 239. In an Australian study of 75 men with hypogonadotropic hypogonadism treated with gonadotropins the median time for sperm to appear in the ejaculate was 7.1 months and for conception was just over 28 months 238. Similar data were reported in a compilation of clinical trial data from Asian, European, Australian and American patients 239. Factors predictive of better outcome include larger baseline testicular size and absence of cryptorchidism. The Australian study reported that prior androgen use is also a negative prognostic indicator of response 238. While the study investigators propose that gonadotropin therapy be considered to induce puberty based on their results, confirmation that such an approach is superior to the conventional practice of giving testosterone would require a large clinical trial the logistics of which would be challenging given the rarity of this patient population. Gynecomastia is the most common side effect of gonadotropin therapy and is due to human chorionic gonadotropin (hCG) stimulation of aromatase causing increased secretion of estradiol. This undesirable side effect can be prevented by using the lowest dose of human chorionic gonadotropin (hCG) capable of maintaining serum testosterone levels towards the lower end of the normal range.
In the majority of hypogonadotropic hypogonadism patients treated with gonadotropins sperm density remains below the normal range. However, failure to achieve a normal sperm density does not preclude fertility. Indeed, the median sperm concentrations reported at conception range from 5-8 million/mL 235, 238. While spermatogenesis can be initiated even in patients with very small testes 235, 240, a longer duration of therapy is typically required and it may take up to 24 months for spermatogenesis to be induced. Accordingly when discussing the issue of fertility with patients, experts recommend that they start treatment at least 6 to 12 months prior to the time at which fertility is desired. Once pregnancy is achieved, experts advise continuing therapy until at least the second trimester. If the couple plans to have another child in the near future, then hCG monotherapy should be continued. However, if a long interval is expected to elapse before the next pregnancy, it may be more convenient for the patient to resume testosterone therapy. Patients should also be offered the option of storing sperm for subsequent use in intrauterine insemination or intracytoplasmic sperm injection. In patients in whom the combination of hCG and FSH is required to induce spermatogenesis initially, treatment with hCG alone may be sufficient for subsequent pregnancies due to larger testicular size.
In patients with panhypopituitarism who fail to respond to gonadotropin therapy, the addition of recombinant growth hormone (rGH) therapy should be considered. It is thought that a direct effect of growth hormone on Leydig cells may play a role in the delayed puberty encountered commonly in patients with isolated growth hormone deficiency 241, 242. While small non-randomized studies suggest that recombinant growth hormone (rGH) may enhance the testosterone response to human chorionic gonadotropin (hCG) administration 241, larger, randomized studies are needed before a definitive decision about the benefit of recombinant growth hormone (rGH) in inducing spermatogenesis in men with hypopituitarism can be reached.
Pulsatile GnRH Therapy
The alternative to gonadotropin therapy is pulsatile administration of gonadotropin-releasing hormone (GnRH), which may be administered by a programmable, portable mini-infusion pump 243. While intravenous administration produces the most physiologic GnRH pulse contour and ensuing LH response 244, the subcutaneous route is clearly more practical for the long term treatment required to stimulate spermatogenesis. Based on the normative data 245, the frequency of GnRH administration that we employ is every 2 hours. The dose of gonadotropin-releasing hormone (GnRH) is titrated for each individual to ensure normalization of testosterone, LH and FSH and varies from 25 to 600 ng/kg per bolus. Patients on longterm therapy are monitored with serum testosterone and gonadotropin levels at monthly intervals. Once testicular volume reaches 6-8 mL, regular semen analyses are obtained. The majority of patients require treatment for 18-24 months to maximize testicular growth and achieve spermatogenesis, although the time taken to reach these endpoints tends to be shorter in those with a larger initial gonadal size. In an analysis of 76 men with idiopathic hypogonadotropic hypogonadism, pulsatile GnRH restored normal testosterone levels in 93% of cases and was successful in inducing spermatogenesis in 77% by 12 months and 82% by 24 months 246. As with gonadotropin therapy, testicular size is an important predictor of successful treatment while negative predictors include a history of cryptorchidism and a pre-treatment inhibin B level <60 pg/mL 246.
If pulsatile GnRH treatment fails, a mutation of GnRH receptor should be considered 247 and genetic testing arranged, if available. A second cause of failure of pulsatile GnRH treatment is the appearance of anti-GnRH antibodies, which typically occur in the setting of erratic compliance and are associated with a progressive decrease in T and gonadotropins levels.
Both exogenous gonadotropins and pulsatile GnRH are very effective in stimulating spermatogenesis. Most studies have no shown no advantage of either therapy in terms of testicular growth, onset of spermatogenesis, final sperm counts or pregnancy rates 248, 237. However, pulsatile GnRH therapy is not approved for induction of spermatogenesis by the Food and Drug Administration (FDA) in the United States, and its use is thus confined to specialist centers.
Human chorionic gonadotropin (hCG) use in Assisted Reproductive Technology
Human chorionic gonadotropin (hCG) is a hormone that supports the normal development of an egg in a woman’s ovary, and stimulates the release of the egg during ovulation. Human chorionic gonadotropin (hCG) is used to cause ovulation and to treat infertility in women, and to increase sperm count in men. Human chorionic gonadotropin (hCG) is also used in young boys when their testicles have not dropped down into the scrotum normally. This can be caused by a pituitary gland disorder. Although hCG can help you become pregnant, it should not be used during pregnancy. Tell your doctor right away if you become pregnant during treatment.
The action of human chorionic gonadotropin (hCG) is virtually identical to that of pituitary luteinizing hormone (LH), although human chorionic gonadotropin (hCG) appears to have a small degree of follicle stimulating hormone (FSH) activity as well. Human chorionic gonadotropin (hCG) stimulates production of gonadal steroid hormones by stimulating the interstitial cells (Leydig cells) of the testis to produce testosterone and the corpus luteum of the ovary to produce progesterone. Testosterone stimulation in the male leads to the development of secondary sex characteristics and may stimulate testicular descent when no anatomical impediment to descent is present. This descent is usually reversible when hCG is discontinued. During the normal menstrual cycle, luteinizing hormone (LH) participates with follicle stimulating hormone (FSH) in the development and maturation of the normal ovarian follicle, and the mid-cycle LH surge triggers ovulation. Human chorionic gonadotropin (hCG) can substitute for LH in this function. During a normal pregnancy, hCG secreted by the placenta maintains the corpus luteum after LH secretion decreases, supporting continued secretion of estrogen and progesterone and preventing menstruation.
Circulating human chorionic gonadotropin (hCG) interacts with the luteinizing hormone receptors (LHR) of the ovary, promoting the corpus luteum and its production of progesterone which is necessary to maintain pregnancy and support the growth of the fetus. Injections of hCG mimic the surge in LH that is necessary for ovulation and are used in the therapy of female infertility, in assisted reproduction techniques. In clinical trials, hCG resulted in pregnancies in approximately 30% of women. hCG prepared from urine of pregnant women and was approved for use in the United States in 1967 as treatment of ovulatory dysfunction in women desiring pregnancy. Subsequently, recombinant forms of hCG have been developed and licensed for use. Currently, hCG is available as a powder or in solution generically and under trade names such as Novarel and Pregnyl. Recombinant hCG is available as Overle. The dose and regimen of hCG therapy varies by indication and it should be used only by physicians with expertise in the management of infertility and hypogonadism. Common side effects include headache, nausea, anorexia, and local injection reactions. Uncommon, but potentially severe adverse events include ovarian hyperstimulation syndrome.
Human chorionic gonadotropin (hCG) Dosage and Administration
Human chorionic gonadotropin (hCG) medications are Profasi®, Ovidrel®, Novarel®,and Pregnyl® can be administered several different ways. The commonly administered dose is a single injection of 10,000 units. Once hCG is administered, ovulation usually occurs in approximately 36 to 40 hours. Therefore oocyte retrieval is routinely scheduled at 34-36 hours after hCG. This helps ensure maximal egg maturity, which is important for fertilization and embryo development. Occasionally, several doses of 2,500 units (usually every three days) are administered after egg retrieval to stimulate progesterone production. If your response to stimulation is particularly exuberant, the dose of hCG can be reduced to 5,000 units in an attempt to reduce the risk of ovarian hyperstimulation syndrome.
It typically takes 8-10 days for single injection of 10,000 units of hCG to be cleared from the blood stream. As hCG is the same hormone that is produced by a developing pregnancy, patients should not have a blood or urine pregnancy test sooner than 10 days following the hCG injection. If a pregnancy test is performed earlier, it may measure the hCG that was given by injection rather than measure hCG produced by a pregnancy.
- Marshall JC, Kelch RP. Gonadotropin-releasing hormone: role of pulsatile secretion in the regulation of reproduction. N Engl J Med. 1986 Dec 4;315(23):1459-68. doi: 10.1056/NEJM198612043152306[↩][↩][↩]
- Sadiq NM, Tadi P. Physiology, Pituitary Hormones. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557556[↩][↩][↩][↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Gonadotropins. [Updated 2018 Mar 26]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548856[↩][↩]
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22(6):764–786. doi: 10.1210/edrv.22.6.0446[↩]
- Griswold MD. Action of FSH on mammalial Sertoli cells. In: Russell LD, Griswold MD, editors. The Sertoli cell. Clearwater: Cache River Press; 1993. pp. 493–508.[↩]
- Zirkin BR, Awoniyi C, Griswold MD, Russell LD, Sharpe R. Is FSH required for adult spermatogenesis? J Androl. 1994 Jul-Aug;15(4):273-6.[↩]
- Simorangkir DR, Ramaswamy S, Marshall GR, Pohl CR, Plant TM. A selective monotropic elevation of FSH, but not that of LH, amplifies the proliferation and differentiation of spermatogonia in the adult rhesus monkey (Macacamulatta) Hum Reprod. 2009;24:1584–1595. doi: 10.1093/humrep/dep052[↩]
- Ciccone NA, Kaiser UB. The biology of gonadotroph regulation. Curr Opin Endocrinol Diabetes Obes. 2009 Aug;16(4):321-7. doi: 10.1097/MED.0b013e32832d88fb[↩]
- Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990 Feb;11(1):177-99. doi: 10.1210/edrv-11-1-177[↩]
- Goodman HM. Discovery of the luteinizing hormone of the anterior pituitary gland. Am J Physiol Endocrinol Metab. 2004 Nov;287(5):E818-9. doi: 10.1152/classicessays.00006.2004[↩]
- Nedresky D, Singh G. Physiology, Luteinizing Hormone. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539692[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ezcurra D, Humaidan P. A review of luteinising hormone and human chorionic gonadotropin when used in assisted reproductive technology. Reprod Biol Endocrinol. 2014 Oct 3;12:95. doi: 10.1186/1477-7827-12-95[↩][↩]
- Crowley WF Jr, Filicori M, Spratt DI, Santoro NF. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res. 1985;41:473–531.[↩]
- Waldhauser F, Weissenbacher G, Frisch H, Pollak A. Pulsatile secretion of gonadotropins in early infancy. Eur J Pediatr. 1981;137:71–4[↩]
- Gupta, Priya & Mahapatra, Archisman & Suman, Anjali & Singh, Rahul. (2021). Effect of Endocrine Disrupting Chemicals on HPG Axis: A Reproductive Endocrine Homeostasis. https://www.researchgate.net/publication/350037153_Effect_of_Endocrine_Disrupting_Chemicals_on_HPG_Axis_A_Reproductive_Endocrine_Homeostasis[↩]
- Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56(6):729-37. doi: 10.1507/endocrj.k09e-185[↩][↩]
- Orlowski M, Sarao MS. Physiology, Follicle Stimulating Hormone. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535442[↩]
- Stamatiades GA, Kaiser UB. Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol Cell Endocrinol. 2018 Mar 5;463:131-141. doi: 10.1016/j.mce.2017.10.015[↩][↩][↩][↩][↩]
- Barbieri RL. The endocrinology of the menstrual cycle. Methods Mol Biol. 2014;1154:145-69. doi: 10.1007/978-1-4939-0659-8_7[↩][↩][↩][↩][↩][↩][↩][↩]
- Clarke H, Dhillo WS, Jayasena CN. Comprehensive Review on Kisspeptin and Its Role in Reproductive Disorders. Endocrinol Metab (Seoul). 2015 Jun;30(2):124-41. doi: 10.3803/EnM.2015.30.2.124[↩][↩]
- Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018 Jan;218(1):68-74. doi: 10.1016/j.ajog.2017.05.045[↩]
- Shaw ND, Histed SN, Srouji SS, Yang J, Lee H, Hall JE. Estrogen negative feedback on gonadotropin secretion: evidence for a direct pituitary effect in women. J Clin Endocrinol Metab. 2010 Apr;95(4):1955-61. doi: 10.1210/jc.2009-2108[↩]
- Boepple PA, Hayes FJ, Dwyer AA, Raivio T, Lee H, Crowley WF Jr, Pitteloud N. Relative roles of inhibin B and sex steroids in the negative feedback regulation of follicle-stimulating hormone in men across the full spectrum of seminiferous epithelium function. J Clin Endocrinol Metab. 2008 May;93(5):1809-14. doi: 10.1210/jc.2007-2450[↩]
- Richards JS, Russell DL, Robker RL, Dajee M, Alliston TN. Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol. 1998 Oct 25;145(1-2):47-54. doi: 10.1016/s0303-7207(98)00168-3[↩]
- Reed BG, Carr BR. The Normal Menstrual Cycle and the Control of Ovulation. [Updated 2018 Aug 5]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279054[↩]
- Lunenfeld B, Lunenfeld E. Gonadotropic preparations–lessons learned. Fertil Steril. 1997 May;67(5):812-4. https://www.fertstert.org/article/S0015-0282(97)81389-1/pdf[↩][↩][↩]
- Lanciotti L, Cofini M, Leonardi A, Penta L, Esposito S. Up-To-Date Review About Minipuberty and Overview on Hypothalamic-Pituitary-Gonadal Axis Activation in Fetal and Neonatal Life. Front Endocrinol (Lausanne). 2018 Jul 23;9:410. doi: 10.3389/fendo.2018.00410[↩][↩][↩][↩][↩]
- Grinspon RP, Urrutia M, Rey RA. Male Central Hypogonadism in Paediatrics – the Relevance of Follicle-stimulating Hormone and Sertoli Cell Markers. Eur Endocrinol. 2018 Sep;14(2):67-71. doi: 10.17925/EE.2018.14.2.67[↩][↩]
- McLachlan RI, Wreford NG, O’Donnell L, de Kretser DM, Robertson DM. The endocrine regulation of spermatogenesis: independent roles for testosterone and FSH. J Endocrinol. 1996 Jan;148(1):1-9. doi: 10.1677/joe.0.1480001[↩]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997 Feb;15(2):201-4. doi: 10.1038/ng0297-201[↩]
- Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994 Dec;15(6):725-51. doi: 10.1210/edrv-15-6-725[↩]
- Christenson LK, Stouffer RL. Follicle-stimulating hormone and luteinizing hormone/chorionic gonadotropin stimulation of vascular endothelial growth factor production by macaque granulosa cells from pre- and periovulatory follicles. J Clin Endocrinol Metab. 1997 Jul;82(7):2135-42. doi: 10.1210/jcem.82.7.4169[↩]
- Li R, Phillips DM, Moore A, Mather JP. Follicle-stimulating hormone induces terminal differentiation in a predifferentiated rat granulosa cell line (ROG). Endocrinology. 1997 Jul;138(7):2648-57. doi: 10.1210/endo.138.7.5154[↩]
- Sharma OP, Flores JA, Leong DA, Veldhuis JD. Cellular basis for follicle-stimulating hormone-stimulated calcium signaling in single rat Sertoli cells: possible dissociation from effects of adenosine 3′,5′-monophosphate. Endocrinology. 1994 Apr;134(4):1915-23. doi: 10.1210/endo.134.4.8137759[↩]
- Nieschlag E, Simoni M, Gromoll J, Weinbauer GF. Role of FSH in the regulation of spermatogenesis: clinical aspects. Clin Endocrinol (Oxf). 1999 Aug;51(2):139-46. doi: 10.1046/j.1365-2265.1999.00846.x[↩][↩]
- Matthew P. Rose, Rose E. Gaines Das, Adam H. Balen, Definition and Measurement of Follicle Stimulating Hormone, Endocrine Reviews, Volume 21, Issue 1, 1 February 2000, Pages 5–22, https://doi.org/10.1210/edrv.21.1.0388[↩][↩][↩]
- Manasco PK, Umbach DM, Muly SM, Godwin DC, Negro-Vilar A, Culler MD, Underwood LE. Ontogeny of gonadotropin, testosterone, and inhibin secretion in normal boys through puberty based on overnight serial sampling. J Clin Endocrinol Metab. 1995 Jul;80(7):2046-52. doi: 10.1210/jcem.80.7.7608253[↩]
- Balen AH , Jacobs HS 1997 Male factor infertility. In: Balen AH, Jacobs HS (eds) Infertility in Practice. Churchill Livingstone, London, pp 213 –240[↩]
- Balen AH , Jacobs HS 1997 Assisted conception. In: Balen AH, Jacobs HS (eds) Infertility in Practice. Churchill Livingstone, London, pp 255 –286[↩]
- Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu HC, Jones GS, Rosenwaks Z. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988 Aug;50(2):298-307. doi: 10.1016/s0015-0282(16)60077-8[↩]
- Balen AH , Jacobs HS 1997 Investigating infertility. In: Balen AH, Jacobs HS (eds) Infertility in Practice. Churchill Livingstone, London, pp 39 –114[↩]
- Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989 Apr;51(4):651-4. doi: 10.1016/s0015-0282(16)60615-5[↩]
- Cameron IT, O’Shea FC, Rolland JM, Hughes EG, de Kretser DM, Healy DL. Occult ovarian failure: a syndrome of infertility, regular menses, and elevated follicle-stimulating hormone concentrations. J Clin Endocrinol Metab. 1988 Dec;67(6):1190-4. doi: 10.1210/jcem-67-6-1190[↩]
- Koskinen P, Penttilä TA, Anttila L, Erkkola R, Irjala K. Optimal use of hormone determinations in the biochemical diagnosis of the polycystic ovary syndrome. Fertil Steril. 1996 Mar;65(3):517-22. doi: 10.1016/s0015-0282(16)58146-1[↩]
- Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, Jacobs HS. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995 Aug;10(8):2107-11. doi: 10.1093/oxfordjournals.humrep.a136243[↩]
- Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996 Apr;81(4):1495-501. doi: 10.1210/jcem.81.4.8636357[↩]
- Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996 Mar;81(3):1038-45. doi: 10.1210/jcem.81.3.8772573[↩]
- Bódis J, Török A, Tinneberg HR. LH/FSH ratio as a predictor of ovarian hyperstimulation syndrome. Hum Reprod. 1997 Apr;12(4):869-70. doi: 10.1093/humrep/12.4.869[↩]
- Krishnan A, Muthusami S. Hormonal alterations in PCOS and its influence on bone metabolism. J Endocrinol. 2017 Feb;232(2):R99-R113. doi: 10.1530/JOE-16-0405[↩]
- Hypogonadotropic hypogonadism. https://medlineplus.gov/ency/article/000390.htm[↩]
- Kallmann Syndrome. https://rarediseases.org/rare-diseases/kallmann-syndrome[↩][↩][↩]
- Kallmann syndrome. https://medlineplus.gov/genetics/condition/kallmann-syndrome[↩][↩][↩]
- Turner syndrome. https://www.mayoclinic.org/diseases-conditions/turner-syndrome/symptoms-causes/syc-20360782[↩]
- Turner Syndrome. https://medlineplus.gov/turnersyndrome.html[↩]
- Turner Syndrome. https://rarediseases.org/rare-diseases/turner-syndrome/[↩]
- Pouresmaeili F, Fazeli Z. Premature ovarian failure: a critical condition in the reproductive potential with various genetic causes. Int J Fertil Steril. 2014 Apr;8(1):1-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3973172[↩]
- Kihara M, Sugita T, Nagai Y, Saeki N, Tatsuno I, Seki K. Ovarian hyperstimulation caused by gonadotroph cell adenoma: a case report and review of the literature. Gynecol Endocrinol. 2006 Feb;22(2):110-3. doi: 10.1080/09513590600581665[↩]
- Pouwer AW, Farquhar C, Kremer JA. Long-acting FSH versus daily FSH for women undergoing assisted reproduction. Cochrane Database Syst Rev. 2015 Jul 14;2015(7):CD009577. doi: 10.1002/14651858.CD009577.pub3[↩]
- Ilahi S, Ilahi TB. Anatomy, Adenohypophysis (Pars Anterior, Anterior Pituitary) [Updated 2022 Oct 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519039[↩]
- Kumar P, Sait SF. Luteinizing hormone and its dilemma in ovulation induction. J Hum Reprod Sci. 2011 Jan;4(1):2-7. doi: 10.4103/0974-1208.82351[↩][↩][↩][↩][↩]
- Choi J, Smitz J. Luteinizing hormone and human chorionic gonadotropin: distinguishing unique physiologic roles. Gynecol Endocrinol. 2014 Mar;30(3):174-81. doi: 10.3109/09513590.2013.859670[↩][↩][↩][↩]
- El Sayed SA, Fahmy MW, Schwartz J. Physiology, Pituitary Gland. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459247[↩]
- Holesh JE, Bass AN, Lord M. Physiology, Ovulation. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441996[↩][↩][↩]
- Rodger RS, Morrison L, Dewar JH, Wilkinson R, Ward MK, Kerr DN. Loss of pulsatile luteinising hormone secretion in men with chronic renal failure. Br Med J (Clin Res Ed). 1985 Dec 7;291(6509):1598-600. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1418441/pdf/bmjcred00477-0008.pdf[↩]
- Betz D, Fane K. Human Chorionic Gonadotropin. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532950[↩][↩][↩][↩][↩][↩]
- Ogino MH, Tadi P. Physiology, Chorionic Gonadotropin. [Updated 2022 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556118[↩][↩][↩]
- Cole LA, Laidler LL. Inherited human chorionic gonadotropin. J Reprod Med. 2010 Mar-Apr;55(3-4):99-102.[↩][↩][↩][↩][↩]
- Montagnana M, Trenti T, Aloe R, Cervellin G, Lippi G. Human chorionic gonadotropin in pregnancy diagnostics. Clin Chim Acta. 2011 Aug 17;412(17-18):1515-20. doi: 10.1016/j.cca.2011.05.025[↩][↩]
- Sirikunalai P., Wanapirak C., Sirichotiyakul S., Tongprasert F., Srisupundit K., Luewan S., Traisrisilp K., Tongsong T. Associations between maternal serum free beta human chorionic gonadotropin (β-hCG) levels and adverse pregnancy outcomes. J. Obstet. Gynaecol. 2016;36:178–182. doi: 10.3109/01443615.2015.1036400[↩][↩]
- Craig W.Y., Haddow J.E., Palomaki G.E., Roberson M. Major fetal abnormalities associated with positive screening tests for Smith-Lemli-Opitz syndrome (SLOS) Prenat. Diagn. 2007;27:409–414. doi: 10.1002/pd.1699[↩][↩]
- Norris W., Nevers T., Sharma S., Kalkunte S. Review: hCG, preeclampsia and regulatory T cells. Placenta. 2011;32:S182–S185. doi: 10.1016/j.placenta.2011.01.009[↩][↩]
- Barjaktarovic M., Korevaar T.I.M., Jaddoe V.W.V., de Rijke Y.B., Peeters R.P., Steegers E.A.P. Human chorionic gonadotropin and risk of pre-eclampsia: Prospective population-based cohort study. Ultrasound Obstet. Gynecol. 2019;54:477–483. doi: 10.1002/uog.20256[↩]
- Barjaktarovic M., Korevaar T.I., Jaddoe V.W., de Rijke Y.B., Visser T.J., Peeters R.P., Steegers E.A. Human chorionic gonadotropin (hCG) concentrations during the late first trimester are associated with fetal growth in a fetal sex-specific manner. Eur. J. Epidemiol. 2017;32:135–144. doi: 10.1007/s10654-016-0201-3[↩]
- Stevens F.T., Katzorke N., Tempfer C., Kreimer U., Bizjak G.I., Fleisch M.C., Fehm T.N. Gestational Trophoblastic Disorders: An Update in 2015. Geburtshilfe Und Frauenheilkd. 2015;75:1043–1050. doi: 10.1055/s-0035-1558054[↩]
- Cole LA, Butler S. Detection of hCG in trophoblastic disease. The USA hCG reference service experience. J Reprod Med. 2002 Jun;47(6):433-44.[↩][↩]
- Cole L.A. “Background” Human Chorionic Gonadotropin in Healthy, Nonpregnant Women. Clin. Chem. 2005;51:1765–1766. doi: 10.1373/clinchem.2005.056507[↩][↩][↩]
- De Backer B., Goffin F., Nisolle M., Minon J.M. Élévation faible d’hCG en dehors d’un contexte gravidique: À propos de deux cas et revue de la littérature [Persistent low hCG levels beyond pregnancy: Report of two cases and review of the literature] Ann. Biol. Clin. 2013;71:496–502. doi: 10.1684/abc.2013.0876[↩]
- Katabuchi H., Ohba T. Human chorionic villous macrophages as a fetal biological shield from maternal chorionic gonadotropin. Dev. Growth Differ. 2008;50:299–306. doi: 10.1111/j.1440-169X.2008.01030.x[↩]
- Yamaguchi M., Ohba T., Tashiro H., Yamada G., Katabuchi H. Human Chorionic Gonadotropin Induces Human Macrophages to Form Intracytoplasmic Vacuoles Mimicking Hofbauer Cells in Human Chorionic Villi. Cells Tissues Organs. 2013;197:127–135. doi: 10.1159/000342806[↩]
- Paulesu L., Rao C., Ietta F., Pietropolli A., Ticconi C. hCG and Its Disruption by Environmental Contaminants during Human Pregnancy. Int. J. Mol. Sci. 2018;19:914. doi: 10.3390/ijms19030914[↩]
- Human Chorionic Gonadotropin as a Pivotal Endocrine Immune Regulator Initiating and Preserving Fetal Tolerance. Int. J. Mol. Sci. 2017, 18(10), 2166; doi:10.3390/ijms18102166 http://www.mdpi.com/1422-0067/18/10/2166/htm[↩]
- d’Hauterive SP, Close R, Gridelet V, Mawet M, Nisolle M, Geenen V. Human Chorionic Gonadotropin and Early Embryogenesis: Review. Int J Mol Sci. 2022 Jan 26;23(3):1380. doi: 10.3390/ijms23031380[↩][↩][↩][↩][↩][↩][↩]
- Nwabuobi C, Arlier S, Schatz F, Guzeloglu-Kayisli O, Lockwood CJ, Kayisli UA. hCG: Biological Functions and Clinical Applications. Int J Mol Sci. 2017 Sep 22;18(10):2037. doi: 10.3390/ijms18102037[↩][↩][↩]
- Cole L.A. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod. Biol. Endocrinol. 2009;7:8. doi: 10.1186/1477-7827-7-8[↩]
- Cole LA. hCG, five independent molecules. Clin Chim Acta. (2012) 413:48–65. 10.1016/j.cca.2011.09.037[↩]
- ((Fournier T, Guibourdenche J, Evain-Brion D. Review: hCGs: different sources of production, different glycoforms and functions. Placenta. 2015 Apr;36 Suppl 1:S60-5. doi: 10.1016/j.placenta.2015.02.002[↩]
- Jarvela IY, Ruokonen A, Tekay A. Effect of rising hCG levels on the human corpus luteum during early pregnancy. Hum Reprod. (2008) 23:2775–81. 10.1093/humrep/den299[↩]
- Jurisicova A., Antenos M., Kapasi K., Meriano J., Casper R.F. Variability in the expression of trophectodermal markers β-human chorionic gonadotrophin, human leukocyte antigen-G and pregnancy specific β-1 glycoprotein by the human blastocyst. Hum. Reprod. 1999;14:1852–1858. doi: 10.1093/humrep/14.7.1852[↩]
- Bonduelle M.-L., Dodd R., Liebaers I., Van Steirteghem A., Williamson R., Akhurst R. Chorionic gonadotrophin-β mRNA, a trophoblast marker, is expressed in human 8-cell embryos derived from tripronucleate zygotes. Hum. Reprod. 1988;3:909–914. doi: 10.1093/oxfordjournals.humrep.a136808[↩]
- Lopata A., Hay D.L. The potential of early human embryos to form blastocysts, hatch from their zona and secrete HCG in culture. Hum. Reprod. 1989;4:87–94. doi: 10.1093/humrep/4.suppl_1.87[↩]
- Braunstein G.D., Rasor J., Danzer H., Adler D., Wade M.E. Serum human chorionic gonadotropin levels throughout normal pregnancy. Am. J. Obstet. Gynecol. 1976;126:678–681. doi: 10.1016/0002-9378(76)90518-4[↩]
- Ohlsson R., Larsson E., Nilsson O., Wahlström T., Sundström P. Blastocyst implantation precedes induction of insulin-like growth factor II gene expression in human trophoblasts. Development. 1989;106:555–559. doi: 10.1242/dev.106.3.555[↩]
- D’Hauterive S.P., Charlet-Renard C., Berndt S., Dubois M., Munaut C., Goffin F., Hagelstein M.-T., Noel A., Hazout A., Foidart J.-M., et al. Human chorionic gonadotropin and growth factors at the embryonic–endometrial interface control leukemia inhibitory factor (LIF) and interleukin 6 (IL-6) secretion by human endometrial epithelium. Hum. Reprod. 2004;19:2633–2643. doi: 10.1093/humrep/deh450[↩][↩]
- Srisuparp S., Strakova Z., Fazleabas A.T. The Role of Chorionic Gonadotropin (CG) in Blastocyst Implantation. Arch. Med. Res. 2001;32:627–634. doi: 10.1016/S0188-4409(01)00330-7[↩]
- Lobo S.C., Srisuparp S., Peng X., Fazleabas A.T. Uterine Receptivity in the Baboon: Modulation by Chorionic Gonadotropin. Semin. Reprod. Med. 2001;19:069–074. doi: 10.1055/s-2001-13913[↩]
- Shi Q.J., Lei Z.M., Rao C.V., Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology. 1993;132:1387–1395. doi: 10.1210/endo.132.3.7679981[↩]
- North R.A., Whitehead R., Larkins R.G. Stimulation by Human Chorionic Gonadotropin of Prostaglandin Synthesis by Early Human Placental Tissue. J. Clin. Endocrinol. Metab. 1991;73:60–70. doi: 10.1210/jcem-73-1-60[↩]
- Weedon-Fekjær M., Taskén K. Review: Spatiotemporal dynamics of hCG/cAMP signaling and regulation of placental function. Placenta. 2012;33:S87–S91. doi: 10.1016/j.placenta.2011.11.003[↩]
- Prast J., Saleh L., Husslein H., E Sonderegger S., Helmer H., Knöfler M. Human Chorionic Gonadotropin Stimulates Trophoblast Invasion through Extracellularly Regulated Kinase and AKT Signaling. Endocrinology. 2007;149:979–987. doi: 10.1210/en.2007-1282[↩]
- Palaniappan M., Menon K. Human Chorionic Gonadotropin Stimulates Theca-Interstitial Cell Proliferation and Cell Cycle Regulatory Proteins by a cAMP-Dependent Activation of AKT/mTORC1 Signaling Pathway. Mol. Endocrinol. 2010;24:1782–1793. doi: 10.1210/me.2010-0044[↩]
- Lee C.-L., Chiu C.N., Hautala L., Salo T., Yeung S.B.W., Stenman U.-H., Koistinen H. Human chorionic gonadotropin and its free β-subunit stimulate trophoblast invasion independent of LH/hCG receptor. Mol. Cell. Endocrinol. 2013;375:43–52. doi: 10.1016/j.mce.2013.05.009[↩]
- Cole LA, Butler SA. Hyperglycosylated human chorionic gonadotropin and human chorionic gonadotropin free beta-subunit: tumor markers and tumor promoters. J Reprod Med. 2008 Jul;53(7):499-512.[↩][↩]
- Sasaki Y, Ladner DG, Cole LA. Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. Fertil Steril. (2008) 89:1781–6. 10.1016/j.fertnstert.2007.03.010[↩]
- Cole LA. Hyperglycosylated hCG, a review. Placenta. (2010) 31:653–64. 10.1016/j.placenta.2010.06.005[↩]
- Guibourdenche J., Handschuh K., Tsatsaris V., Gerbaud P., Leguy M.C., Müller F., Brion D.E., Fournier T. Hyperglycosylated hCG Is a Marker of Early Human Trophoblast Invasion. J. Clin. Endocrinol. Metab. 2010;95:E240–E244. doi: 10.1210/jc.2010-0138[↩]
- Salas A., Gastón B., Barrenetxea J., Sendino T., Jurado M., Alcázar J.L. Predictive value of hyperglycosylated human chorionic gonadotropin for pregnancy outcomes in threatened abortion in first-trimester viable pregnancies. An. Sist. Sanit. Navar. 2021;44:23–31. doi: 10.23938/assn.0933[↩]
- Hamada A.L., Nakabayashi K., Sato A., Kiyoshi K., Takamatsu Y., Laoag-Fernandez J.B., Ohara N., Maruo T. Transfection of Antisense Chorionic Gonadotropin β Gene into Choriocarcinoma Cells Suppresses the Cell Proliferation and Induces Apoptosis. J. Clin. Endocrinol. Metab. 2005;90:4873–4879. doi: 10.1210/jc.2004-2458[↩]
- Sasaki Y., Ladner D.G., Cole L.A. Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. Fertil. Steril. 2008;89:1781–1786. doi: 10.1016/j.fertnstert.2007.03.010[↩]
- Cole L. Hyperglycosylated hCG. Placenta. 2007;28:977–986. doi: 10.1016/j.placenta.2007.01.011[↩][↩][↩]
- Cole L.A., Dai D., Butler S.A., Leslie K.K., Kohorn E.I. Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylated hCG. Gynecol. Oncol. 2006;102:145–150. doi: 10.1016/j.ygyno.2005.12.047[↩]
- Kovalevskaya G., Kakuma T., Schlatterer J., O’Connor J.F. Hyperglycosylated HCG expression in pregnancy: Cellular origin and clinical applications. Mol. Cell. Endocrinol. 2007;260–262:237–243. doi: 10.1016/j.mce.2006.02.021[↩]
- Bersinger N.A., Wunder D.M., Nicolas M., Birkhäuser M.H., Porquet D., Guibourdenche J. Serum Hyperglycosylated Human Chorionic Gonadotropin to Predict the Gestational Outcome in in vitro Fertilization/Intracytoplasmic Sperm Injection Pregnancies. Fetal Diagn. Ther. 2008;24:74–78. doi: 10.1159/000132412[↩]
- Stenman U.H., Tiitinen A., Alfthan H., Valmu L. The classification, functions and clinical use of different isoforms of HCG. Hum. Reprod. Update. 2006;12:769–784. doi: 10.1093/humupd/dml029[↩][↩][↩]
- Elliott M.M., Kardana A., Lustbader J.W., Cole L.A. Carbohydrate and peptide structure of the α- and β-subunits of human chorionic gonadotropin from normal and aberrant pregnancy and choriocarcinoma. Endocrine. 1997;7:15–32. doi: 10.1007/BF02778058[↩]
- Birken S., Yershova O., Myers R.V., Bernard M.P., Moyle W. Analysis of human choriogonadotropin core 2 o-glycan isoforms. Mol. Cell. Endocrinol. 2003;204:21–30. doi: 10.1016/S0303-7207(03)00153-9[↩]
- Wang R., Chen L., Wang X., Liu Y. Association between serum beta-human chorionic gonadotropin and inflammation, oxidative stress in pregnancy-induced hypertension. Microvasc. Res. 2021;135:104130. doi: 10.1016/j.mvr.2020.104130[↩]
- Ballantyne A., Rashid L., Pattenden R. Stability of maternal serum free β-hCG following whole blood sample transit: First trimester Down’s syndrome screening in Scotland. Ann. Clin. Biochem. 2022;59:87–91. doi: 10.1177/00045632211045250[↩]
- Cole L.A., Butler S. Hyperglycosylated hCG, hCGβ and Hyperglycosylated hCGβ: Interchangeable cancer promoters. Mol. Cell. Endocrinol. 2012;349:232–238. doi: 10.1016/j.mce.2011.10.029[↩]
- Butler S.A., Ikram M.S., Mathieu S., Iles R.K. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br. J. Cancer. 2000;82:1553–1556. doi: 10.1054/bjoc.2000.1177[↩]
- Cole L.A., Laidler L.L., Muller C.Y. USA hCG reference service, 10-year report. Clin. Biochem. 2010;43:1013–1022. doi: 10.1016/j.clinbiochem.2010.05.006[↩][↩]
- Birken S., Maydelman Y., Gawinowicz M.A., Pound A., Liu Y., Hartree A.S. Isolation and characterization of human pituitary chorionic gonadotropin. Endocrinology. 1996;137:1402–1411. doi: 10.1210/endo.137.4.8625917[↩][↩]
- Cole LA, Gutierrez JM. Production of human chorionic gonadotropin during the normal menstrual cycle. J Reprod Med. 2009 Apr;54(4):245-50.[↩][↩]
- Kovalevskaya G., Birken S., Kakuma T., Ozaki N., Sauer M., Lindheim S., Cohen M., Kelly A., Schlatterer J., O’Connor J.F. Differential expression of human chorionic gonadotropin (hCG) glycosylation isoforms in failing and continuing pregnancies: Preliminary characterization of the hyperglycosylated hCG epitope. J. Endocrinol. 2002;172:497–506. doi: 10.1677/joe.0.1720497[↩]
- Kovalevskaya G., Genbacev O., Fisher S.J., Caceres E., O’Connor J.F. Trophoblast origin of hCG isoforms: Cytotrophoblasts are the primary source of choriocarcinoma-like hCG. Mol. Cell. Endocrinol. 2002;194:147–155. doi: 10.1016/S0303-7207(02)00135-1[↩][↩]
- Cole L.A. hCG, five independent molecules. Clin. Chim. Acta Int. J. Clin. Chem. 2012;413:48–65. doi: 10.1016/j.cca.2011.09.037[↩]
- Berndt S., Blacher S., Munaut C., Detilleux J., Perrier d’Hauterive S., Huhtaniemi I., Evain-Brion D., Noel A., Fournier T., Foidart J.M. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-β receptor activation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013;27:1309–1321. doi: 10.1096/fj.12-213686[↩]
- Stenman U.H., Alfthan H. Determination of human chorionic gonadotropin. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:783–793. doi: 10.1016/j.beem.2013.10.005[↩]
- Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010 Aug 24;8:102. doi: 10.1186/1477-7827-8-102[↩][↩][↩][↩][↩][↩][↩][↩]
- Schalch DS, Parlow AF, Boon RC, Reichlin S. Measurement of human luteinizing hormone in plasma by radioimmunoassay. J Clin Invest. 1968 Mar;47(3):665-78. doi: 10.1172/JCI105762[↩]
- Faiman C, Ryan RJ, Zwirek SJ, Rubin ME. Serum FSH and HCG during human pregnancy and puerperium. J Clin Endocrinol Metab. 1968 Sep;28(9):1323-9. doi: 10.1210/jcem-28-9-1323[↩][↩]
- Rao CV. Differential properties of human chorionic gonadotrophin and human luteinizing hormone binding to plasma membranes of bovine corpora lutea. Acta Endocrinol (Copenh). 1979 Apr;90(4):696-710. doi: 10.1530/acta.0.0900696[↩]
- Cole LA, Dai D, Butler SA, Leslie KK, Kohorn EI. Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylated hCG. Gynecol Oncol. 2006 Aug;102(2):145-50. doi: 10.1016/j.ygyno.2005.12.047[↩]
- Strott CA, Yoshimi T, Ross GT, Lipsett MB. Ovarian physiology: relationship between plasma LH and steroidogenesis by the follicle and corpus luteum; effect of HCG. J Clin Endocrinol Metab. 1969 Sep;29(9):1157-67. doi: 10.1210/jcem-29-9-1157[↩][↩][↩]
- Cole L.A. hCG, the wonder of today’s science. Reprod. Biol. Endocrinol. 2012;10:24. doi: 10.1186/1477-7827-10-24[↩]
- Dufau M.L. The luteinizing hormone receptor. Ann. Rev. Physiol. 1998;60:461–496. doi: 10.1146/annurev.physiol.60.1.461[↩]
- Jameson JL, Hollenberg AN. Regulation of chorionic gonadotropin gene expression. Endocr Rev. 1993 Apr;14(2):203-21. doi: 10.1210/edrv-14-2-203[↩]
- Fiddes JC, Goodman HM. The gene encoding the common alpha subunit of the four human glycoprotein hormones. J Mol Appl Genet. 1981;1(1):3-18.[↩]
- Boorstein W.R., Vamvakopoulos N.C., Fiddes J.C. Human chorionic gonadotropin β-subunit is encoded by at least eight genes arranged in tandem and inverted pairs. Nature. 1982;300:419–422. doi: 10.1038/300419a0[↩]
- Rull K., Hallast P., Uuskula L., Jackson J., Punab M., Salumets A., Campbell R.K., Laan M. Fine-scale quantification of HCG bata gene transcription in human trophoblastic and non-malignant non-trophoblastic tissues. Mol. Hum. Reprod. 2008;14:23–31. doi: 10.1093/molehr/gam082[↩]
- Fournier T., Guibourdenche J., Evain-Brion D. Review: hCGs: Different sources of production, different glycoforms and functions. Placenta. 2015;36:S60–S65. doi: 10.1016/j.placenta.2015.02.002[↩][↩]
- Knofler M. What factors regulate HCG production in Down’s syndrome pregnancies? Regulation of HCG during normal gestation and in pregnancies affected by Down’s syndrome. Mol. Hum. Reprod. 1999;5:895–897. doi: 10.1093/molehr/5.10.895[↩]
- Handschuh K., Guibourdenche J., Cocquebert M., Tsatsaris V., Vidaud M., Evain-Brion D., Fournier T. Expression and regulation by PPARgamma of hCG α- and β-subunits: Comparison between villous and invasive extravillous trophoblastic cells. Placenta. 2009;30:1016–1022. doi: 10.1016/j.placenta.2009.09.006[↩]
- Handschuh K., Guibourdenche J., Tsatsaris V., Guesnon M., Laurendeau I., Evain-Brion D., Fournier T. Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-γ Endocrinology. 2007;148:5011–5019. doi: 10.1210/en.2007-0286[↩]
- Murthi P., Kalionis B., Cocquebert M., Rajaraman G., Chui A., Keogh R.J., Evain-Brion D., Fournier T. Homeobox genes and down-stream transcription factor PPARgamma in normal and pathological human placental development. Placenta. 2013;34:299–309. doi: 10.1016/j.placenta.2013.01.005[↩]
- Montagnana M., Trenti T., Aloe R., Cervellin G., Lippi G. Human chorionic gonadotropin in pregnancy diagnostics. Clin. Chim. Acta. 2011;412:1515–1520. doi: 10.1016/j.cca.2011.05.025[↩][↩]
- Nisula B.C., Blithe D.L., Akar A., Lefort G., Wehmann R.E. Metabolic fate of human choriogonadotropin. J. Steroid Biochem. 1989;33:733–737. doi: 10.1016/0022-4731(89)90485-8[↩]
- Wehmann R.E., Nisula B.C. Characterization of a discrete degradation product of the human chorionic gonadotropin β-subunit in humans. J. Clin. Endocrinol. Metab. 1980;51:101–105. doi: 10.1210/jcem-51-1-101[↩]
- Norman RJ, Menabawey M, Lowings C, Buck RH, Chard T. Relationship between blood and urine concentrations of intact human chorionic gonadotropin and its free subunits in early pregnancy. Obstet Gynecol. 1987 Apr;69(4):590-3.[↩]
- Toth P, Lukacs H, Gimes G, Sebestyen A, Pasztor N, Paulin F, Rao CV. Clinical importance of vascular LH/hCG receptors–a review. Reprod Biol. 2001 Nov;1(2):5-11.[↩][↩][↩][↩]
- Iles RK. Ectopic hCGbeta expression by epithelial cancer: malignant behaviour, metastasis and inhibition of tumor cell apoptosis. Mol Cell Endocrinol. 2007 Jan 2;260-262:264-70. doi: 10.1016/j.mce.2006.02.019[↩][↩][↩][↩][↩]
- Toth P, Li X, Rao CV, Lincoln SR, Sanfilippo JS, Spinnato JA 2nd, Yussman MA. Expression of functional human chorionic gonadotropin/human luteinizing hormone receptor gene in human uterine arteries. J Clin Endocrinol Metab. 1994 Jul;79(1):307-15. doi: 10.1210/jcem.79.1.8027246[↩][↩][↩]
- Berndt S, Blacher S, Perrier d’Hauterive S, Thiry M, Tsampalas M, Cruz A, Péqueux C, Lorquet S, Munaut C, Noël A, Foidart JM. Chorionic gonadotropin stimulation of angiogenesis and pericyte recruitment. J Clin Endocrinol Metab. 2009 Nov;94(11):4567-74. doi: 10.1210/jc.2009-0443[↩][↩][↩]
- Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br J Cancer. 2000 May;82(9):1553-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2363404/pdf/82-6691177a.pdf[↩]
- Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology. 1993 May;132(5):2262-70. doi: 10.1210/endo.132.5.8477671[↩]
- Schumacher A, Zenclussen AC. Human Chorionic Gonadotropin-Mediated Immune Responses That Facilitate Embryo Implantation and Placentation. Front Immunol. 2019 Dec 10;10:2896. doi: 10.3389/fimmu.2019.02896[↩][↩]
- Bonduelle ML, Dodd R, Liebaers I, Van Steirteghem A, Williamson R, Akhurst R. Chorionic gonadotrophin-beta mRNA, a trophoblast marker, is expressed in human 8-cell embryos derived from tripronucleate zygotes. Hum Reprod. (1988) 3:909–14. 10.1093/oxfordjournals.humrep.a136808[↩]
- Lopata A, Hay DL. The potential of early human embryos to form blastocysts, hatch from their zona and secrete HCG in culture. Hum Reprod. (1989) 4(8 Suppl.):87–94. 10.1093/humrep/4.suppl_1.87[↩]
- Jeschke U, Karsten U, Reimer T, Richter DU, Bergemann C, Briese V, et al.. Stimulation of hCG protein and mRNA in first trimester villous cytotrophoblast cells in vitro by glycodelin A. J Perinat Med. (2005) 33:212–8. 10.1515/JPM.2005.039[↩]
- Society for Assisted Reproductive Technology – Glossary and Terms. https://www.sart.org/patients/a-patients-guide-to-assisted-reproductive-technology/patient-resources/glossary/[↩]
- Than NG, Romero R, Tarca AL, Kekesi KA, Xu Y, Xu Z, et al.. Integrated systems biology approach identifies novel maternal and placental pathways of preeclampsia. Front Immunol. (2018) 9:1661. 10.3389/fimmu.2018.01661[↩]
- Panwar M, Kumari A, Hp A, Arora R, Singh V, Bansiwal R. Raised neutrophil lymphocyte ratio and serum beta hCG level in early second trimester of pregnancy as predictors for development and severity of preeclampsia. Drug Discov Ther. (2019) 13:34–7. 10.5582/ddt.2019.01006[↩]
- Jensen F, Wallukat G, Herse F, Budner O, El-Mousleh T, Costa SD, et al.. CD19+CD5+ cells as indicators of preeclampsia. Hypertension. (2012) 59:861–8. 10.1161/HYPERTENSIONAHA.111.188276[↩]
- Tsampalas M., Gridelet V., Berndt S., Foidart J.-M., Geenen V., D’Hauterive S.P. Human chorionic gonadotropin: A hormone with immunological and angiogenic properties. J. Reprod. Immunol. 2010;85:93–98. doi: 10.1016/j.jri.2009.11.008[↩]
- Polese B., Gridelet V., Araklioti E., Martens H., Perrier d’Hauterive S., Geenen V. The Endocrine Milieu and CD4 T-Lymphocyte Polarization during Pregnancy. Front. Endocrinol. 2014;5:106. doi: 10.3389/fendo.2014.00106[↩][↩][↩]
- Berndt S., D’Hauterive S.P., Blacher S., Pequeux C., Lorquet S., Munaut C., Applanat M., Hervé M.A., Lamandé N., Corvol P., et al. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J. 2006;20:2630–2632. doi: 10.1096/fj.06-5885fje[↩]
- Berndt S., Blacher S., D’Hauterive S.P., Thiry M., Tsampalas M., Cruz A., Péqueux C., Lorquet S., Munaut C., Noël A., et al. Chorionic Gonadotropin Stimulation of Angiogenesis and Pericyte Recruitment. J. Clin. Endocrinol. Metab. 2009;94:4567–4574. doi: 10.1210/jc.2009-0443[↩]
- Herr F., Baal N., Reisinger K., Lorenz A., McKinnon T., Preissner K., Zygmunt M. hCG in the Regulation of Placental Angiogenesis. Results of an In Vitro Study. Placenta. 2007;28:S85–S93. doi: 10.1016/j.placenta.2007.02.002[↩]
- Bourdiec A., Bédard D., Rao C.V., Akoum A. Human Chorionic Gonadotropin Regulates Endothelial Cell Responsiveness to Interleukin 1 and Amplifies the Cytokine-Mediated Effect on Cell Proliferation, Migration and the Release of Angiogenic Factors. Am. J. Reprod. Immunol. 2013;70:127–138. doi: 10.1111/aji.12080[↩]
- Reisinger K., Baal N., McKinnon T., Münstedt K., Zygmunt M. The gonadotropins: Tissue-specific angiogenic factors? Mol. Cell. Endocrinol. 2007;269:65–80. doi: 10.1016/j.mce.2006.11.015[↩]
- Zhang Z., Huang Y., Zhang J., Liu Z., Lin Q., Wang Z. Activation of NF-κB signaling pathway during HCG-induced VEGF expression in luteal cells. Cell Biol. Int. 2019;43:344–349. doi: 10.1002/cbin.11090[↩]
- Surico D., Farruggio S., Marotta P., Raina G., Mary D., Surico N., Vacca G., Grossini E. Human Chorionic Gonadotropin Protects Vascular Endothelial Cells from Oxidative Stress by Apoptosis Inhibition, Cell Survival Signalling Activation and Mitochondrial Function Protection. Cell. Physiol. Biochem. 2015;36:2108–2120. doi: 10.1159/000430178[↩]
- Jing G., Yao J., Dang Y., Liang W., Xie L., Chen J., Li Z. The role of β-HCG and VEGF-MEK/ERK signaling pathway in villi angiogenesis in patients with missed abortion. Placenta. 2021;103:16–23. doi: 10.1016/j.placenta.2020.10.005[↩][↩]
- Berndt S., Blacher S., Munaut C., Detilleux J., D’Hauterive S.P., Huhtaniemi I., Evain-Brion D., Noël A., Fournier T., Foidart J. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-β receptor activation. FASEB J. 2013;27:1309–1321. doi: 10.1096/fj.12-213686[↩]
- Gallardo V., González M., Toledo F., Sobrevia L. Role of heme oxygenase 1 and human chorionic gonadotropin in pregnancy associated diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020;1866:165522. doi: 10.1016/j.bbadis.2019.07.016[↩]
- Raghupathy R. Th 1-type immunity is incompatible with successful pregnancy. Immunol. Today. 1997;18:478–482. doi: 10.1016/S0167-5699(97)01127-4[↩]
- Schumacher A., Heinze K., Witte J., Poloski E., Linzke N., Woidacki K., Zenclussen A.C. Human Chorionic Gonadotropin as a Central Regulator of Pregnancy Immune Tolerance. J. Immunol. 2013;190:2650–2658. doi: 10.4049/jimmunol.1202698[↩]
- Lea R.G., Sandra O. Immunoendocrine aspects of endometrial function and implantation. Reproduction. 2007;134:389–404. doi: 10.1530/REP-07-0167[↩]
- Fujiwara H. Do circulating blood cells contribute to maternal tissue remodeling and embryo-maternal cross-talk around the implantation period? Mol. Hum. Reprod. 2009;15:335–343. doi: 10.1093/molehr/gap027[↩]
- Akoum A, Metz CN, Morin M. Marked increase in macrophage migration inhibitory factor synthesis and secretion in human endometrial cells in response to human chorionic gonadotropin hormone. J Clin Endocrinol Metab. 2005 May;90(5):2904-10. doi: 10.1210/jc.2004-1900[↩]
- Herrmann-Lavoie C, Rao CV, Akoum A. Chorionic gonadotropin down-regulates the expression of the decoy inhibitory interleukin 1 receptor type II in human endometrial epithelial cells. Endocrinology. 2007 Nov;148(11):5377-84. doi: 10.1210/en.2007-0368[↩]
- Matsuura T, Sugimura M, Iwaki T, Ohashi R, Kanayama N, Nishihira J. Anti-macrophage inhibitory factor antibody inhibits PMSG-hCG-induced follicular growth and ovulation in mice. J Assist Reprod Genet. 2002 Dec;19(12):591-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3455831/pdf/10815_2004_Article_450330.pdf[↩]
- Kamada M, Ino H, Naka O, Irahara M, Daitoh T, Mori K, Maeda N, Maegawa M, Hirano K, Aono T. Immunosuppressive 30-kDa protein in urine of pregnant women and patients with trophoblastic diseases. Eur J Obstet Gynecol Reprod Biol. 1993 Aug;50(3):219-25. doi: 10.1016/0028-2243(93)90204-p[↩]
- Majumdar S, Bapna BC, Mapa MK, Gupta AN, Devi PK, Subrahmanyam D. Pregnancy specific proteins: suppression of in vitro blastogenic response to mitogen by these proteins. Int J Fertil. 1982;27(2):66-9.[↩]
- CEDARD L, VARANGOT J, YANNOTTI S. [The metabolism of estrogens in human placentas artificially maintained in survival by perfusion in vitro]. C R Hebd Seances Acad Sci. 1962 Mar 5;254:1870-1. French.[↩]
- Poikkeus P., Hiilesmaa V., Tiitinen A. Serum HCG 12 days after embryo transfer in predicting pregnancy outcome. Hum. Reprod. 2002;17:1901–1905. doi: 10.1093/humrep/17.7.1901[↩]
- Cole LA. Immunoassay of human chorionic gonadotropin, its free subunits, and metabolites. Clin Chem. 1997 Dec;43(12):2233-43.[↩][↩]
- Korhonen J., Stenman U.H., Ylostalo P. Serum human chorionic gonadotropin dynamics during spontaneous resolution of ectopic pregnancy. Fertil. Steril. 1994;61:632–636. doi: 10.1016/S0015-0282(16)56638-2[↩]
- O’Connor J.F., Ellish N., Kakuma T., Schlatterer J., Kovalevskaya G. Differential urinary gonadotrophin profiles in early pregnancy and early pregnancy loss. Prenat. Diagn. 1998;18:1232–1240. doi: 10.1002/(SICI)1097-0223(199812)18:12<1232::AID-PD439>3.0.CO;2-Z[↩]
- Davies S, Byrn F, Cole LA. Human chorionic gonadotropin testing for early pregnancy viability and complications. Clin Lab Med. 2003 Jun;23(2):257-64, vii. doi: 10.1016/s0272-2712(03)00026-x[↩][↩]
- Menczer J, Modan M, Serr DM. Prospective follow-up of patients with hydatidiform mole. Obstet Gynecol. 1980 Mar;55(3):346-9. doi: 10.1097/00006250-198003000-00015[↩]
- Cole LA, Shahabi S, Butler SA, Mitchell H, Newlands ES, Behrman HR, Verrill HL. Utility of commonly used commercial human chorionic gonadotropin immunoassays in the diagnosis and management of trophoblastic diseases. Clin Chem. 2001 Feb;47(2):308-15.[↩]
- Butts SF, Guo W, Cary MS, Chung K, Takacs P, Sammel MD, Barnhart KT. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013 Aug;122(2 Pt 1):337-343. doi: 10.1097/AOG.0b013e31829c6ed6[↩]
- Barjaktarovic M, Korevaar TI, Jaddoe VW, de Rijke YB, Visser TJ, Peeters RP, Steegers EA. Human chorionic gonadotropin (hCG) concentrations during the late first trimester are associated with fetal growth in a fetal sex-specific manner. Eur J Epidemiol. 2017 Feb;32(2):135-144. doi: 10.1007/s10654-016-0201-3[↩][↩][↩][↩][↩]
- Cavaliere A, Ermito S, Dinatale A, Pedata R. Management of molar pregnancy. J Prenat Med. 2009 Jan;3(1):15-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3279094[↩][↩][↩][↩][↩][↩]
- Brouillet S, Murthi P, Hoffmann P, Salomon A, Sergent F, De Mazancourt P, Dakouane-Giudicelli M, Dieudonné MN, Rozenberg P, Vaiman D, Barbaux S, Benharouga M, Feige JJ, Alfaidy N. EG-VEGF controls placental growth and survival in normal and pathological pregnancies: case of fetal growth restriction (FGR). Cell Mol Life Sci. 2013 Feb;70(3):511-25. doi: 10.1007/s00018-012-1141-z[↩][↩][↩][↩]
- Visconti F, Quaresima P, Chiefari E, Caroleo P, Arcidiacono B, Puccio L, Mirabelli M, Foti DP, Di Carlo C, Vero R, Brunetti A. First Trimester Combined Test (FTCT) as a Predictor of Gestational Diabetes Mellitus. Int J Environ Res Public Health. 2019 Sep 28;16(19):3654. doi: 10.3390/ijerph16193654[↩][↩]
- Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br J Cancer. 2000 May;82(9):1553-6. doi: 10.1054/bjoc.2000.1177[↩]
- Huppertz B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension. 2008;51:970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607[↩]
- Keikkala E., Vuorela P., Laivuori H., Romppanen J., Heinonen S., Stenman U.H. First trimester hyperglycosylated human chorionic gonadotrophin in serum—A marker of early-onset preeclampsia. Placenta. 2013;34:1059–1065. doi: 10.1016/j.placenta.2013.08.006[↩][↩][↩]
- Kalkunte S., Navers T., Norris W., Banerjee P., Fazleabas A., Kuhn C., Jeschke U., Sharma S. Presence of non-functional hCG in preeclampsia and rescue of normal pregnancy by recombinant hCG. Placenta. 2010;31:A126.[↩]
- Kalkunte S., Boij R., Norris W., Friedman J., Lai Z., Kurtis J., Lim K.H., Padbury J.F., Matthiesen L., Sharma S. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am. J. Pathol. 2010;177:2387–2398. doi: 10.2353/ajpath.2010.100475[↩]
- Zhong Y., Zhu F., Ding Y. Serum screening in first trimester to predict pre-eclampsia, small for gestational age and preterm delivery: Systematic review and meta-analysis. BMC Pregnancy Childbirth. 2015;15:191. doi: 10.1186/s12884-015-0608-y[↩]
- Ong C.Y., Liao A.W., Spencer K., Munim S., Nicolaides K.H. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG Int. J. Obstet. Gynaecol. 2000;107:1265–1270. doi: 10.1111/j.1471-0528.2000.tb11618.x[↩]
- Smith G.C., Stenhouse E.J., Crossley J.A., Aitken D.A., Cameron A.D., Connor J.M. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J. Clin. Endocrinol. Metab. 2002;87:1762–1767. doi: 10.1210/jcem.87.4.8430[↩]
- Ranta J.K., Raatikainen K., Romppanen J., Pulkki K., Heinonen S. Decreased PAPP-A is associated with preeclampsia, premature delivery and small for gestational age infants but not with placental abruption. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;157:48–52. doi: 10.1016/j.ejogrb.2011.03.004[↩]
- Kagan K.O., Wright D., Baker A., Sahota D., Nicolaides K.H. Screening for trisomy 21 by maternal age, fetal nuchal translucency thickness, free β-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008;31:618–624. doi: 10.1002/uog.5331[↩]
- Wright D., Spencer K., Kagan K.K., Torring N., Petersen O.B., Christou A., Kallikas J., Nicolaides K.H. First-trimester combined screening for trisomy 21 at 7–14 weeks’ gestation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2010;36:404–411. doi: 10.1002/uog.7755[↩]
- Cuckle H. Biochemical screening for Down syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000;92:97–101. doi: 10.1016/S0301-2115(00)00431-0[↩][↩][↩]
- Stenman UH, Alfthan H, Hotakainen K. Human chorionic gonadotropin in cancer. Clin Biochem. 2004;37(7):549–61. 10.1016/j.clinbiochem.2004.05.008[↩]
- Bellet D, Lazar V, Bièche I, Paradis V, Giovangrandi Y, Paterlini P, Lidereau R, Bedossa P, Bidart JM, Vidaud M. Malignant transformation of nontrophoblastic cells is associated with the expression of chorionic gonadotropin beta genes normally transcribed in trophoblastic cells. Cancer Res. 1997 Feb 1;57(3):516-23.[↩]
- Marcillac I, Troalen F, Bidart JM, Ghillani P, Ribrag V, Escudier B, Malassagne B, Droz JP, Lhommé C, Rougier P, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992 Jul 15;52(14):3901-7.[↩]
- Gestational Trophoblastic Disease Treatment (PDQ®)–Patient Version. https://www.cancer.gov/types/gestational-trophoblastic/patient/gtd-treatment-pdq[↩][↩][↩]
- What Is Testicular Cancer? https://www.cancer.org/cancer/types/testicular-cancer/about/what-is-testicular-cancer.html[↩][↩][↩][↩]
- Gholam D, Fizazi K, Terrier-Lacombe MJ, Jan P, Culine S, Theodore C. Advanced seminoma–treatment results and prognostic factors for survival after first-line, cisplatin-based chemotherapy and for patients with recurrent disease: a single-institution experience in 145 patients. Cancer. 2003 Aug 15;98(4):745-52. doi: 10.1002/cncr.11574[↩]
- Oliver RT, Mason MD, Mead GM, von der Maase H, Rustin GJ, Joffe JK, de Wit R, Aass N, Graham JD, Coleman R, Kirk SJ, Stenning SP; MRC TE19 collaborators and the EORTC 30982 collaborators. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005 Jul 23-29;366(9482):293-300. doi: 10.1016/S0140-6736(05)66984-X[↩]
- Weissbach L, Bussar-Maatz R, Mann K. The value of tumor markers in testicular seminomas. Results of a prospective multicenter study. Eur Urol. 1997;32(1):16-22.[↩]
- Testicular Cancer Treatment (PDQ®)–Health Professional Version. https://www.cancer.gov/types/testicular/hp/testicular-treatment-pdq[↩][↩][↩]
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997 Feb;15(2):594-603. doi: 10.1200/JCO.1997.15.2.594[↩]
- Sturgeon CM, Duffy MJ, Stenman UH, et al. National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008 Dec;54(12):e11-79. doi: 10.1373/clinchem.2008.105601[↩]
- Loutradis D., Drakakis P., Vomvolaki E., Antsaklis A. Different ovarian stimulation protocols for women with diminished ovarian reserve. J. Assist. Reprod. Genet. 2007;24:597–611. doi: 10.1007/s10815-007-9181-2[↩]
- Cole L.A. Biological functions of hcg and hcg-related molecules. Reprod. Biol. Endocrinol. 2010;8:102–115. doi: 10.1186/1477-7827-8-102[↩]
- Craciunas L, Tsampras N, Raine-Fenning N, Coomarasamy A. Intrauterine administration of human chorionic gonadotropin (hCG) for subfertile women undergoing assisted reproduction. Cochrane Database Syst Rev. 2018 Oct 20;10(10):CD011537. doi: 10.1002/14651858.CD011537.pub3[↩][↩]
- Balen AH, Braat DD, West C, Patel A, Jacobs HS. Cumulative conception and live birth rates after the treatment of anovulatory infertility: safety and efficacy of ovulation induction in 200 patients. Hum Reprod. 1994 Aug;9(8):1563-70. doi: 10.1093/oxfordjournals.humrep.a138750[↩]
- Ben-Rafael Z, Levy T, Schoemaker J. Pharmacokinetics of follicle-stimulating hormone: clinical significance. Fertil Steril. 1995 Apr;63(4):689-700. doi: 10.1016/s0015-0282(16)57467-6[↩]
- White DM, Polson DW, Kiddy D, Sagle P, Watson H, Gilling-Smith C, Hamilton-Fairley D, Franks S. Induction of ovulation with low-dose gonadotropins in polycystic ovary syndrome: an analysis of 109 pregnancies in 225 women. J Clin Endocrinol Metab. 1996 Nov;81(11):3821-4. doi: 10.1210/jcem.81.11.8923819[↩]
- Nardelli AA, Stafinski T, Motan T, Klein K, Menon D. Assisted reproductive technologies (ARTs): evaluation of evidence to support public policy development. Reprod Health. 2014 Nov 7;11(1):76. doi: 10.1186/1742-4755-11-76[↩]
- Raju GA, Chavan R, Deenadayal M, Gunasheela D, Gutgutia R, Haripriya G, Govindarajan M, Patel NH, Patki AS. Luteinizing hormone and follicle stimulating hormone synergy: A review of role in controlled ovarian hyper-stimulation. J Hum Reprod Sci. 2013 Oct;6(4):227-34. doi: 10.4103/0974-1208.126285[↩][↩]
- Hayes F, Dwyer A, Pitteloud N. Hypogonadotropic Hypogonadism (HH) and Gonadotropin Therapy. [Updated 2013 Nov 25]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279078[↩][↩][↩][↩][↩][↩]
- Fraietta R, Zylberstejn DS, Esteves SC. Hypogonadotropic hypogonadism revisited. Clinics (Sao Paulo). 2013;68 Suppl 1(Suppl 1):81-8. doi: 10.6061/clinics/2013(sup01)09[↩]
- Clavijo RI, Hsiao W. Update on male reproductive endocrinology. Transl Androl Urol. 2018 Jul;7(Suppl 3):S367-S372. doi: 10.21037/tau.2018.03.25[↩]
- Canavese F, Mussa A, Manenti M, Cortese MG, Ferrero L, Tuli G, Macchieraldo R, Lala R. Sperm count of young men surgically treated for cryptorchidism in the first and second year of life: fertility is better in children treated at a younger age. Eur J Pediatr Surg. 2009 Dec;19(6):388-91. doi: 10.1055/s-0029-1241171[↩]
- Recombinant follicle stimulating hormone: development of the first biotechnology product for the treatment of infertility. Recombinant Human FSH Product Development Group. Hum Reprod Update. 1998 Nov-Dec;4(6):862-81. doi: 10.1093/humupd/4.6.862[↩][↩][↩]
- Mannaerts B, Fauser B, Lahlou N, Harlin J, Shoham Z, Bennink HC, Bouchard P. Serum hormone concentrations during treatment with multiple rising doses of recombinant follicle stimulating hormone (Puregon) in men with hypogonadotropic hypogonadism. Fertil Steril. 1996 Feb;65(2):406-10. doi: 10.1016/s0015-0282(16)58108-4[↩]
- King TF, Hayes FJ. Long-term outcome of idiopathic hypogonadotropic hypogonadism. Curr Opin Endocrinol Diabetes Obes. 2012 Jun;19(3):204-10. doi: 10.1097/MED.0b013e328353565b[↩]
- Burris AS, Rodbard HW, Winters SJ, Sherins RJ. Gonadotropin therapy in men with isolated hypogonadotropic hypogonadism: the response to human chorionic gonadotropin is predicted by initial testicular size. J Clin Endocrinol Metab. 1988 Jun;66(6):1144-51. doi: 10.1210/jcem-66-6-1144[↩][↩][↩]
- Vicari E, Mongioì A, Calogero AE, Moncada ML, Sidoti G, Polosa P, D’Agata R. Therapy with human chorionic gonadotrophin alone induces spermatogenesis in men with isolated hypogonadotrophic hypogonadism–long-term follow-up. Int J Androl. 1992 Aug;15(4):320-9. doi: 10.1111/j.1365-2605.1992.tb01131.x[↩]
- Büchter D, Behre HM, Kliesch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol. 1998 Sep;139(3):298-303. doi: 10.1530/eje.0.1390298[↩][↩]
- Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009 Mar;94(3):801-8. doi: 10.1210/jc.2008-1648[↩][↩][↩][↩]
- Warne DW, Decosterd G, Okada H, Yano Y, Koide N, Howles CM. A combined analysis of data to identify predictive factors for spermatogenesis in men with hypogonadotropic hypogonadism treated with recombinant human follicle-stimulating hormone and human chorionic gonadotropin. Fertil Steril. 2009 Aug;92(2):594-604. doi: 10.1016/j.fertnstert.2008.07.1720[↩][↩]
- Whitcomb RW, Crowley WF Jr. Clinical review 4: Diagnosis and treatment of isolated gonadotropin-releasing hormone deficiency in men. J Clin Endocrinol Metab. 1990 Jan;70(1):3-7. doi: 10.1210/jcem-70-1-3[↩]
- Kulin HE, Samojlik E, Santen R, Santner S. The effect of growth hormone on the Leydig cell response to chorionic gonadotrophin in boys with hypopituitarism. Clin Endocrinol (Oxf). 1981 Nov;15(5):463-72. doi: 10.1111/j.1365-2265.1981.tb00689.x[↩][↩]
- Chatelain PG, Sanchez P, Saez JM. Growth hormone and insulin-like growth factor I treatment increase testicular luteinizing hormone receptors and steroidogenic responsiveness of growth hormone deficient dwarf mice. Endocrinology. 1991 Apr;128(4):1857-62. doi: 10.1210/endo-128-4-1857[↩]
- Hoffman AR, Crowley WF Jr. Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982 Nov 11;307(20):1237-41. doi: 10.1056/NEJM198211113072003[↩]
- Spratt DI, Crowley WF Jr, Butler JP, Hoffman AR, Conn PM, Badger TM. Pituitary luteinizing hormone responses to intravenous and subcutaneous administration of gonadotropin-releasing hormone in men. J Clin Endocrinol Metab. 1985 Nov;61(5):890-5. doi: 10.1210/jcem-61-5-890[↩]
- Spratt DI, O’Dea LS, Schoenfeld D, Butler J, Rao PN, Crowley WF Jr. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH, and testosterone. Am J Physiol. 1988 May;254(5 Pt 1):E658-66. doi: 10.1152/ajpendo.1988.254.5.E658[↩]
- Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, Crowley WF Jr. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002 Sep;87(9):4128-36. doi: 10.1210/jc.2002-020518[↩][↩]
- Caron P, Chauvin S, Christin-Maitre S, Bennet A, Lahlou N, Counis R, Bouchard P, Kottler ML. Resistance of hypogonadic patients with mutated GnRH receptor genes to pulsatile GnRH administration. J Clin Endocrinol Metab. 1999 Mar;84(3):990-6. doi: 10.1210/jcem.84.3.5518[↩]
- Liu L, Banks SM, Barnes KM, Sherins RJ. Two-year comparison of testicular responses to pulsatile gonadotropin-releasing hormone and exogenous gonadotropins from the inception of therapy in men with isolated hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1988 Dec;67(6):1140-5. doi: 10.1210/jcem-67-6-1140[↩]