Contents
What is inositol
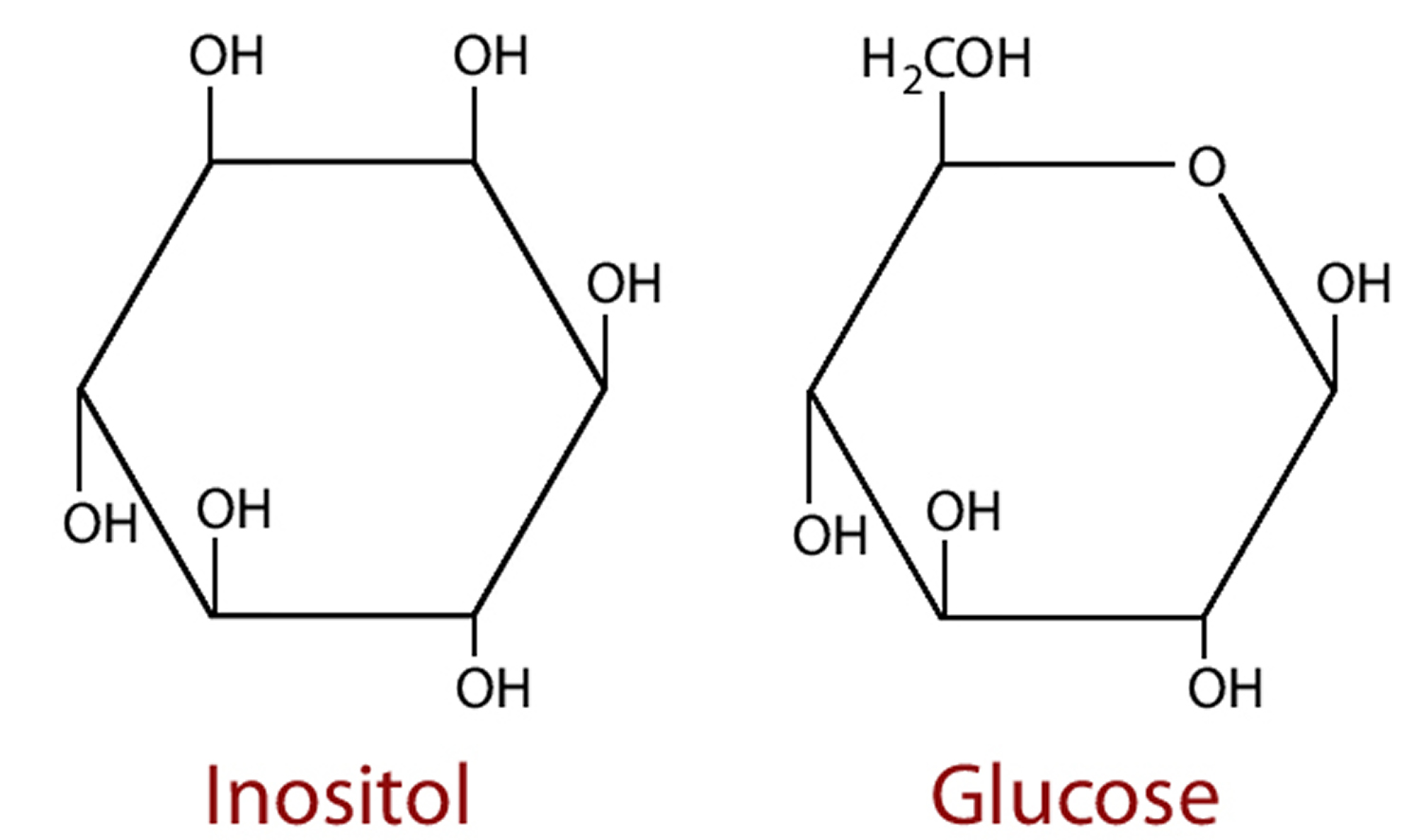
Inositol (1,2,3,4,5,6‐hexahydroxycyclohexane) is a naturally occurring sugar alcohol, an isomer of glucose, found in cell membrane phospholipids, plasma lipoproteins, and as the phosphate form in the cell nucleus with potential chemopreventive properties 1. Inositol is an essential nutrient required by human cells in culture for growth and survival 2. There are nine stereoisomers of inositol, of which myo‐, D‐chiro‐, scyllo‐, epi‐, muco‐, and neo‐inositol occur naturally, with the predominant form being myo‐inositol 3. It’s been further observed that the human body (namely liver and kidney) could produce up to 4 g/day on inositol 4. Inositol can be synthesized from glucose by means of conversion of glucose 6‐phosphate to inositol 1‐phosphate (Figure 2). Adults typically consume approximately 1 g of myo‐inositol per day which is present in a variety of foods including nuts, whole-grain cereals, plants, vegetables, citrus fruits, fruits and meats 5. Plants, particularly legumes, oil seeds, and grains, are particularly rich in myo‐inositol hexakisphosphate (IP6; phytic acid); this is mostly hydrolyzed to free inositol before absorption from the gut 6. Given that myo-inositol can be obtained through biosynthetic mechanisms as well as from dietary source, one would surmise that inositol deficiency is unlikely to happen. However, owing to its ion‐chelating properties, an excess of phytate from dietary sources could theoretically hinder absorption of cations, such as Ca2+, from the gut 7. Myo‐inositol in the nonphosphorylated form, typically available in vitamin supplements, does not have this property. Although less abundant, D‐chiro‐inositol is also obtained from dietary sources, principally in the methylated form 3‐O‐methyl‐D‐chiro‐inositol (pinitol) 8.
Inositol is not a prescription medication, but is widely available as a dietary supplement. Within the body, inositol is used for the production of inositol triphosphate (IP3) and diacylglycerol (DAG), important ‘second messengers’ allowing cell surface receptors for neurotransmitters, including serotonin (5 HT), to affect intracellular processes 9. As one of a number of intracellular phosphate compounds, inositol is involved in cell signaling and may stimulate tumor cell differentiation. Inositol has traditionally been considered to be a pseudo‐vitamin B (vitamin Bh or B8) although it has an uncertain status as a vitamin and a deficiency syndrome has not been identified in man 10. Inositol phospholipids are important in signal transduction. Scyllo-inositol (Scyllitol) has been investigated for the treatment of Alzheimer Disease.
Depletion of cellular content of inositol and of its isomers and phosphate derivatives, has been reported in both diabetic and cancerous tissues 11, while deregulation of myo-inositol metabolism has been found in a number of conditions (PCOS, metabolic syndrome), mechanistically and epidemiologically associated with high-glucose diet or altered glucose metabolism. Inositol deficit may first arise from low nutritional intake of phytate-rich foods, the principal alimentary source of myo-inositol 12. High glucose content inhibits myo-inositol uptake by cells, the gut, and the kidney. Moreover, through the activation of the polyol pathway, glucose extrudes myo-inositol from cells, thus “buffering” the increased osmolarity due to the augmented sorbitol levels. Biosynthesis of myo-inositol begins with G-6P enzymatic transformation into inositol-1-phosphate. However, high glucose levels indirectly inhibit myo-inositol biosynthesis, probably by increasing intracellular phosphatidic acid and consequently activating inositol hexakisphosphate kinase (IP6K1), the principal negative regulator of myo-inositol de novo synthesis. Ultimately, glucose increases catabolism and urinary loss of inositol through myo-inositol oxygenase (MIOX) activation and overexpression. To sum up: high glucose levels hinder inositol availability by increasing its degradation and by inhibiting both myo-inositol biosynthesis and absorption 12. These underappreciated mechanisms may likely account for acquired, metabolic deficiency in inositol bioavailability. How these effects could participate in the pathogenesis of different degenerative diseases still necessitates further studies.
While a few different hypotheses have been proposed to explain the protective role exerted by diets with high-fiber content 13, some early reports pointed out the presence of inositol(s) as causative protective agents. Such studies demonstrated that only fibers with high phytate content, such as cereals and legumes, show negative correlation with colon cancer, indicating that phytate, and not fiber, suppressed colon carcinogenesis 14. Moreover, it was showed that phytic acid exerts some effects originally attributed to fibers. Phytate increases the weights of the cecum and cecal digesta and reduces pH of cecal digesta 15. In addition, phytate improves the composition of cecal organic acids, microflora, and mucins, and decreases the levels of serum proinflammatory cytokines in rats fed a high-fat, mineral-sufficient diet 16.
Indeed, myo-Inositol and its phosphate derivatives (namely, inositol-hexaphosphate (IP6) also called phytate, or phytic acid) [inositol pentaphosphate (IP5), tetraphosphate (IP4), and triphosphate (IP3) are also called “phytates”] have been demonstrated to exert a plethora of valuable health effects—including anti-diabetic, anti-oxidant, anti-inflammatory, and anticancer effects 17. Hence, in recent decades many attempts have been conducted to deepen the understanding of inositol involvement in different physiological and pathological conditions, including fertility 18, regenerative processes 19, oogenesis 20 and sperm function 21, glucose 22 and fat metabolism 23, morphogenesis 24, neurological disorders 25, and respiratory function in newborn 26. For instance, in a very preliminary study, F344 rats were injected with azoxymethane to induce colon tumors. Colon cancer development was heralded by the appearance of aberrant crypts in colon epithelium. In the absence of surgical excision, these inflammatory-like lesions progressed towards full neoplastic transformation. Adding Inositol-hexaphosphate (IP6) to the drinking water significantly reduced the number and depth of the colonic crypts, as well as the incidence of colon tumors [83% in controls versus 25% in rats treated with inositol-hexaphosphate (phytate) 27. These preliminary results have been further strengthened by additional investigations, which evidenced that both inositol-hexaphosphate (phytate) and inositol play some appreciable anticancer effects in a number of in vitro and in vivo studies 28.
Inositol polyphosphates are important as second messengers in signal transduction, act as anti-oxidants, and mediate calcium regulation in membrane signaling. Nuclear inositol signaling may also play a role in DNA repair and chromatin remodeling 29. Due to these effects, inositol has been tested in a wide range of human clinical trials, including cancer prevention, autism, and psychiatric disorders. Pre-clinical studies show myo-inositol inhibits carcinogenesis by 40% to 50% in both the induction and post-initiation phase 30. In a Randomized phase IIb trial 31 of myo-Inositol in smokers with bronchial dysplasia were randomly assigned to receive oral placebo or myo-inositol, 9 g once/day for two weeks, and then twice/day for 6 months. Seventy four (n=38 myo-inositol and n=36 placebo) participants with a baseline and 6-month bronchoscopy were included in all efficacy analyses. The complete response and the progressive disease rates were 26.3% versus 13.9% and 47.4% versus 33.3%, respectively, in the myo-inositol and placebo arms. Compared with placebo, myo-inositol intervention significantly reduced IL-6 levels in bronchoalveolar lavage fluid over 6 months 31. Among those with a complete response in the myo-inositol arm, there was a significant decrease in a gene-expression signature reflective of phosphatidylinositol 3-kinase activation within the cytologically-normal bronchial airway epithelium. The heterogeneous response to myo-inositol suggests a targeted therapy approach based on molecular alterations is needed in future clinical trials to determine the efficacy of myo-inositol as a chemopreventive agent 31.
Lower than normal levels of inositol are found in the cerebrospinal fluid of people with depression 32. Post mortem studies have shown that levels of inositol in particular areas of the cerebral cortex of suicide victims, and people with bipolar disorder are also lowered 33. It has also been reported that the anti-manic effect of lithium treatment is associated with a reduction in inositol levels 34. These findings raised the possibility that increasing inositol levels in depression might be therapeutic. Treatment with inositol has been effective in so-called ‘animal models’ of depression 35. Inositol taken orally has been shown to increase inositol levels within the central nervous system in humans 36. However, based on a Cochrane Systematic Review 9, the currently available evidence does not indicate a clear benefit from the use of inositol in depression. It is currently unclear whether or not inositol is of benefit in the treatment of depression. Ongoing studies should reduce this uncertainty. People with depression and their clinicians may wish to await further evidence before using inositol in a widespread fashion.
Figure 1. Inositol (myo-inositol)
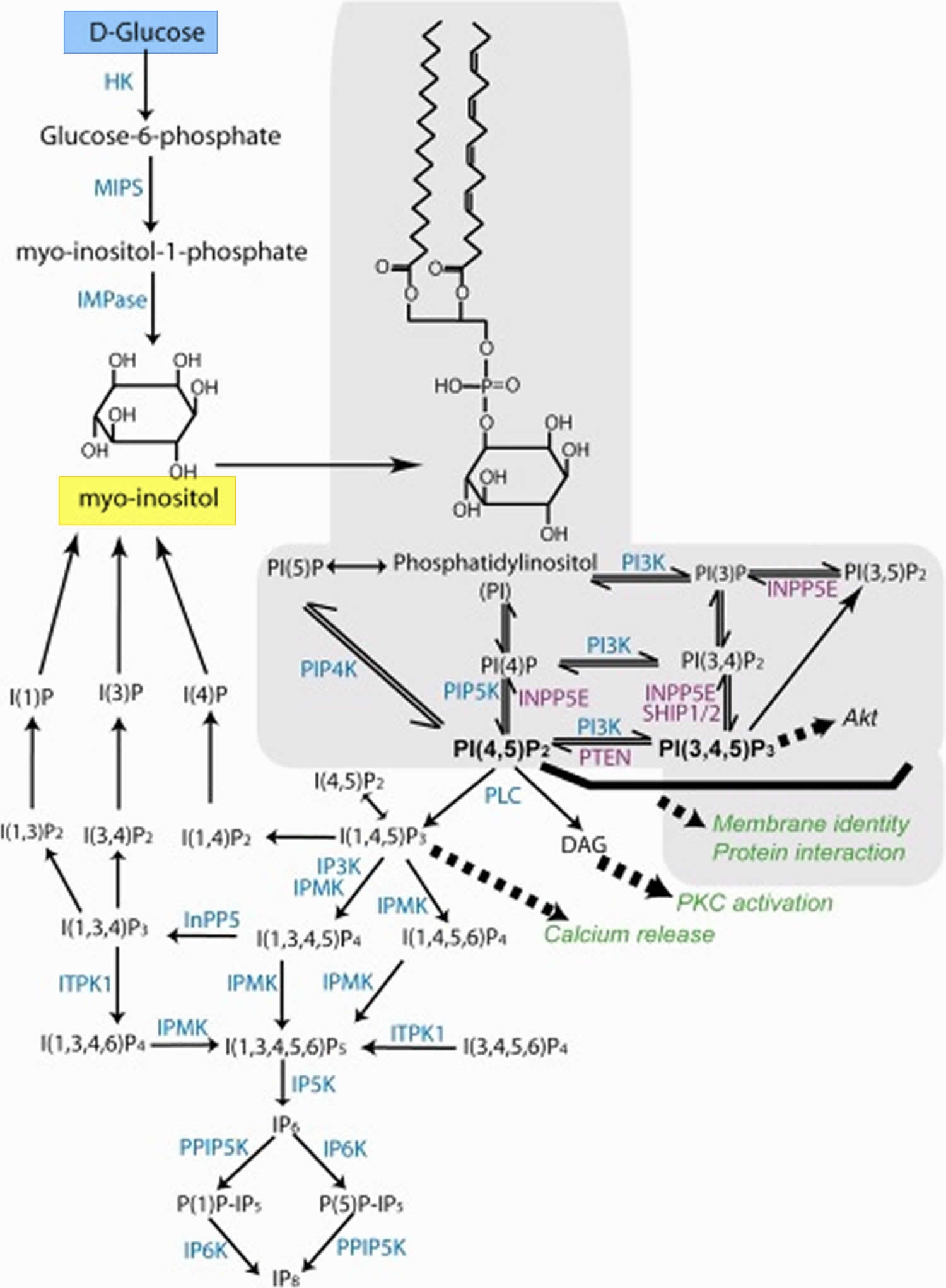
[Source 37]Figure 3. Myo-inositol synthesis
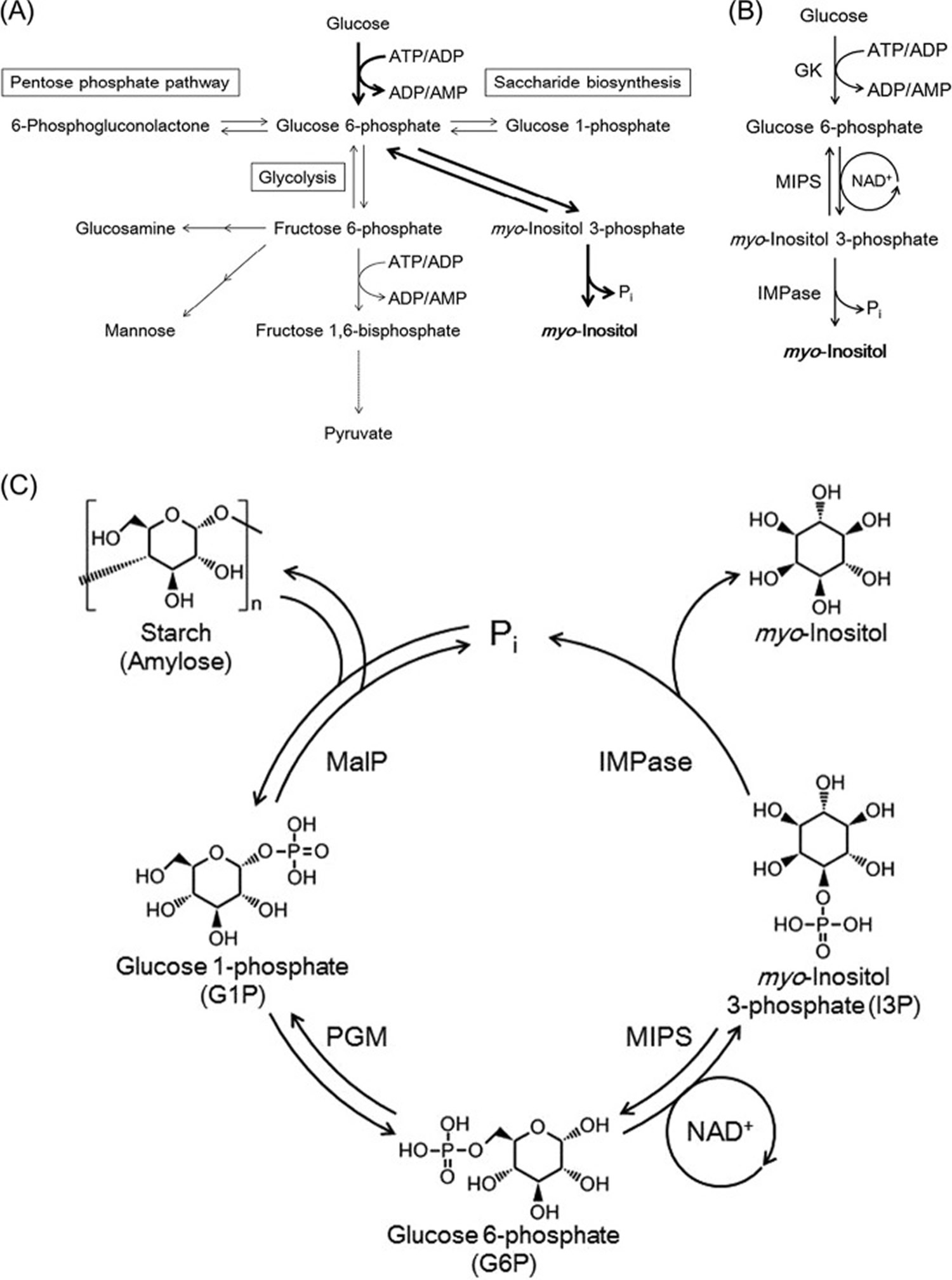 Footnotes: (A) Pathways related to glucose 6-phosphate metabolism. Enzyme reactions responsible for the conversion of glucose to myo-inositol are indicated with bold arrows. For simplicity, cofactors are not shown for some of the reactions. (B) The three enzyme reactions responsible for the conversion of glucose to myo-inositol. In the MIPS reaction, NAD+ is once reduced to NADH in the first half of the MIPS reaction but is regenerated in the second half of the reaction. (C) The designed pathway for the in vitro conversion of starch or amylose to myo-inositol. Abbreviations: GK, ATP- or ADP-dependent glucokinase; MIPS, myo-inositol-3-phosphate synthase; MalP: maltodextrin phosphorylase; PGM, phosphoglucomutase; IMPase, inositol monophosphatase.
Footnotes: (A) Pathways related to glucose 6-phosphate metabolism. Enzyme reactions responsible for the conversion of glucose to myo-inositol are indicated with bold arrows. For simplicity, cofactors are not shown for some of the reactions. (B) The three enzyme reactions responsible for the conversion of glucose to myo-inositol. In the MIPS reaction, NAD+ is once reduced to NADH in the first half of the MIPS reaction but is regenerated in the second half of the reaction. (C) The designed pathway for the in vitro conversion of starch or amylose to myo-inositol. Abbreviations: GK, ATP- or ADP-dependent glucokinase; MIPS, myo-inositol-3-phosphate synthase; MalP: maltodextrin phosphorylase; PGM, phosphoglucomutase; IMPase, inositol monophosphatase.
Inositol metabolism
Inositol is chiefly catabolized in the kidney, where the enzyme myo-inositol oxygenase specifically metabolizes myo-inositol through the glucuronate-xylulose pathway. Myo-inositol oxygenase is exclusively expressed in the renal cortical tubules and is markedly downregulated in acute kidney injury 39. Myo-inositol oxygenase catalyzes myo-inositol transformation into d-glucuronic acid, which is further metabolized into l-gulonate by aldehyde reductase. The latter is hence converted into xylulose and ribulose, which finally enter into the glycolytic pathway 40.
Nephrectomy in animals impairs myo-inositol degradation, while renal failure is associated with significant abnormalities in myo-inositol metabolism and both inositol plasma and urinary levels 41.
Furthermore, elevated inositol excretion (including both myo-inositol and d-Chiro-inositol) have been observed in diabetic animal models characterized by hyperglycemia and glycosuria, regardless of circulating insulin and body weight 42. Previous studies on animal models of diabetes documented an increased urinary loss involving only one isomer (myo-inositol or d-Chiro-inositol) 43, or both 42. Similarly, in humans, increased urinary myo-inositol has been consistently demonstrated in both type 2 diabetes 44, and type 1 diabetes 45. On the contrary, d-chiro-inositol has been alternatively found increased 46 or reduced 45. It is noticeable that, despite increased expression of the inositol transporter systems (SMT1/2), inositol reabsorption is significantly inhibited at the tubular level 47. Other pathophysiological processes should therefore explain increased urinary loss of inositol isomers in diabetic kidneys 48.
Increased urinary excretion significantly contributes to depleting inositol, and may represent an independent relevant cause of inositol deficiency during both renal failure and diabetes. However, renal depletion of myo-inositol persisted despite normalization of sorbitol levels in diabetic rats treated with an aldose reductase inhibitor, strongly suggesting that inositol depletion occurs independently of the polyol pathway 49.
Indeed, it has recently been observed that myo-inositol depletion happens even in insulin-resistant or hypertensive animals, without involvement of the inositol biosynthetic capability, while myo-inositol oxygenase activity steadily increases 50. Furthermore, myo-inositol oxygenase overexpression has been already observed in other models of animal diabetes. It is argued that, at least in diabetic, hyperglycemic animals, myo-inositol oxygenase overexpression may be fostered by xylose, an intermediate metabolite of the glucuronate-xylulose pathway (through a positive feedback), and further reinforced by xylulose-5-phosphate, which activates carbohydrate-responsive element binding protein 51. In turn, it is worth noting that a sustained activation of the glucuronate-xylulose pathway would be a significant source of oxidative stress 40, thus contributing to fibrosis and progressive impairment of renal function 52. Conversely, inhibition of myo-inositol oxygenase expression reduces renal (tubular) damage, improving renal function in diabetic animals. Myo-inositol oxygenase overexpression is modulated by glucose-induced transcription factors such as NFAT-5, ChREBP, Nrf-2, AP1, and cAMP-response element-binding protein. Post-translational modifications, chiefly controlled by PKA, PDK1 and PKC, activate myo-inositol oxygenase through phosphorylation of serine and threonine residues, mostly clustered in the N-terminal segment of the myo-inositol oxygenase enzyme 53. Aldehyde reductase is also strongly transcriptionally upregulated by high glucose ambience and oxidative stress stemming from the advanced glycation products 54. Thereby, high glucose levels enhance myo-inositol catabolism by overexpressing and post-translationally activating both myo-inositol oxygenase and aldehyde reductase. It is worth noting that the administration of antioxidants can strongly counteracts these effects, and improves the renal function 50.
These studies suggest a pivotal role of myo-inositol oxygenase in diabetes-associated renal damage. Moreover, as myo-inositol oxygenase overexpression is independent from the glucose-sorbitol pathway, that finding explain why by inhibiting aldose reductase (which catalyzes the main enzymatic step of that pathway) renal function could be only minimally improved in diabetic animals 49.
Diabetic nephropathy may directly arise from myo-inositol oxygenase deregulation, as suggested by studies on renal mitochondria from diabetic animals. Indeed, both in vivo and in vitro investigations revealed that myo-inositol oxygenase upregulation in the presence of high glucose levels induces mitochondrial fragmentation and depolarization, while inhibiting autophagic removal of damaged mitochondria through Pink1-dependent Mfn2–Parkin interaction 55. The net effect of these events would be disruption in the surveillance of mitochondrial quality control, accumulation of dysfunctional organelles, generation of reactive oxygen species, and increased apoptosis, leading to tubular injury.
These findings suggest that deregulation of glucose metabolism will trigger abnormalities in myo-inositol metabolism and can increase the production of inositol-dependent toxic metabolites, which ultimately will damage renal function and promote inositol urinary loss. Therefore, it is likely that during hyperglycemia, insulin resistance and/or hypertension, changes in kidney metabolism are directly responsible for increased inositol loss, including both myo-inositol and d-Chiro-inositol isomers 56.
What is inositol used for
Inositol and PCOS
There have been a few small clinical trials involving myo-inositol and d-chiro-inositol in PCOS (polycystic ovary syndrome) etiology and therapy. In tissue such as the liver both myo-inositol and d-chiro-inositol are involved in the insulin signaling, i.e. myo-inositol promotes glucose uptake and d-chiro-inositol glycogen synthesis. In reproductive tissue such as the ovary, myo-inositol regulates glucose uptake and follicle stimulating hormone (FSH) signaling, whereas d-chiro-inositol is devoted to the insulin-mediated androgen production. Unlike other tissues, ovary is not insulin resistant, indeed because the epimerase enzyme, which converts myo-inositol to d-chiro-inositol, is insulin dependent, the “d-chiro-inositol paradox” hypothesis suggests that in the ovary of PCOS women, an increased epimerase activity leads to a d-chiro-inositol overproduction and myo-inositol depletion 57. This imbalance could be the cause of the poor oocyte quality and the impairment in the FSH signaling. Owing to this situation, the focal point is the administration of both myo-inositol and d-chiro-inositol in a proper ratio for treating PCOS. Insulin resistance plays a pivotal role in PCOS. Insulin-sensitizer agents such as metformin and inositols have been shown to improve the endocrine and metabolic aspects of PCOS 58. This study compare the effects of metformin and inositols on the clinical and metabolic features of the women with PCOS. Fifty PCOS women with insulin resistance and/or hyperinsulinemia were randomized to treatment with metformin (1500 mg/day) or myo-inositol (4 g/day). Insulin resistance was defined as HOMA-insulin resistance >2.5, while hyperinsulinemia was defined as a value of area under the curve for insulin after a glucose load over the cutoff of our laboratory obtained in normal women. The women have been evaluated for insulin secretion, BMI, menstrual cycle length, acne and hirsutism, at baseline and after 6 months of therapy. The results obtained in both groups were similar 58. The insulin sensitivity improved in both treatment groups. The BMI significantly decreased and the menstrual cycle was normalized in about 50% of the women 58. No significant changes in acne and hirsutism were observed. The two insulin-sensitizers, metformin and myo-inositol, show to be useful in PCOS women in lowering BMI and ameliorating insulin sensitivity, and improving menstrual cycle without significant differences between the two treatments 58.
Inositol in preterm infants at risk for or having respiratory distress syndrome
In human infants with respiratory distress syndrome (RDS), a premature drop in serum inositol levels predicts a more severe course of the syndrome 59. Inositol supplementation increases the amount of saturated phosphatidylcholine in surfactant in newborns and produces a rise in serum inositol concentration 60. Inositol promotes maturation of the surfactant phospholipids phosphatidylcholine and phosphatidylinositol, and the synthesis of phosphatidylinositol in type II pneumocytes appears to be dependent on extracellular inositol concentrations 61. Compositional changes in fetal rat lung surfactant correlate with changes in plasma inositol levels, and supplementation increases phospholipid levels to normal in the deprived rat pup 62. In humans, free inositol levels in sera from preterm neonates are 2 to 20 times higher than are levels in maternal or adult sera (Bromberger 1986; Burton 1974; Lewin 1978). Studies in newborns suggest an endogenous synthesis of inositol during fetal life (Bromberger 1986; Pereira 1990). Human milk has a high concentration of inositol, with preterm milk being the richest source. Infants who are breast fed have higher serum inositol levels compared to those that are not breastfed at one to two weeks of life 63. These facts suggest a critical role for inositol in fetal and early neonatal life. Several studies have been published assessing serum inositol levels in the preterm human infant 63, 64 as well as the effects of inositol supplementation. As additional evidence has become available, another critical overview of the use of inositol supplementation that includes all known trials to date was warranted. Maintaining inositol concentrations similar to those occurring naturally in utero may reduce the rates of retinopathy of prematurity and bronchopulmonary dysplasia in preterm infants.
Inositol is effective in the management of preterm babies who have or are at a risk of infant respiratory distress syndrome 65. Inositol is administered intravenously as long as the infant is not on full oral feeds. When the infant progresses to full feeds inositol is given orally or via an oro-gastric tube. Inositol supplementation results in statistically significant and clinically important reductions in important short-term adverse neonatal outcomes. A large size multi-center randomized controlled trial is currently ongoing and the trial will likely confirm or refute the findings from this Cochrane systematic review.
Inositol and depression
Current scientific evidence does not support the use of inositol for the treatment of depression.
The current evidence for efficacy of inositol for depression consists of inadequate, small, randomized controlled trials and a single meta-analysis, as well as inclusion in a few systematic reviews along with other nutrient-based therapies for depressive disorders.
A 2014 meta-analysis 66 of seven randomized controlled trials (two bipolar studies, one bipolar and major depressive disorder study, two major depressive disorder studies, and two premenstrual dysphoric disorder studies) involving 242 participants found no significant treatment effect of inositol for depressed patients. However, inositol showed a trend of efficacy of depressive symptoms over placebo in patients with premenstrual dysphoric disorder.
A 2004 Cochrane review 9 of four double-blind, randomized controlled trials involving a total of 141 participants found no clear evidence of a therapeutic benefit.
Inositol dosage
For PCOS the myo-inositol dose used in a clinical trial was 4 g/day 58.
The myo-inositol through the diet has rarely been investigated, and researchers have to rely on indirect estimations, based on the consumption of phytate-rich aliments. Consuming mostly a vegetarian diet will give you mean daily inositol-hexaphosphate (IP6) intake of 1487 ± 791 mg/day) 67. Moreover, available data evidence shows huge differences among different countries, and an even more relevant variability among different studies performed in the same country. At a first glance, the daily myo-inositol intake does not exceed 500–700 mg/day for western countries, while higher consumption data have been recorded in Africa and Asia. Yet, in both cases, a wide variability has been recorded, probably reflecting hidden diversities (of societal, educational, nutritional origins) among the population under observation. For example, in a study conducted in India, huge differences have been tracked depending on the age and gender of subjects 68. Similar results have been reported among Egyptians 69 as well as in many other countries. Moreover, in Europe, contrary to what is generally assumed, inositol-hexaphosphate (phytate) intake is usually far below the supposed daily doses. For instance, in Italy, an early report has documented a broad range of 112–1367 mg phytic acid intake per day with an estimated mean value of 219 mg/day 70. A further study stated the mean phytic acid intake of the national Italian diet approximates 293 mg, defined as the average of typical diets from the north-west (288 mg), from the northeast (320 mg), and from the south of Italy (265 mg) 71.
Inositol side effects
There is a paucity of data on the safety and side effects of inositol. In a study on myo-inositol some paticipants reported syncope, dyspnea, dizziness, pain, arthralgia and gastrointestinal side effects 72. A 2014 meta-analysis of inositol for depression and anxiety disorders found that inositol marginally caused gastrointestinal upset compared with placebo. A 2011 European review on the safety of inositol had similar findings in that inositol induced gastrointestinal side effects such as nausea, flatus, and diarrhea.
- Inositol. https://pubchem.ncbi.nlm.nih.gov/compound/myo-inositol[↩]
- Eagle H, Oyama VI, Levy M, Freeman AE. Myo-inositol as an essential growth factor for normal and malignant human cells in tissue culture. The Journal of Biological Chemistry 1957;266(1):191-205.[↩]
- Leung KY, Mills K, Burren KA, et al. 2011. Quantitative analysis of myo‐inositol in urine, blood and nutritional supplements by high‐performance liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 879:2759–2763 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3632838/[↩]
- Involvement of inositol in reproduction. Beemster P, Groenen P, Steegers-Theunissen R. Nutr Rev. 2002 Mar; 60(3):80-7. https://www.ncbi.nlm.nih.gov/pubmed/11908744/[↩]
- Croze ML, Soulage CO. 2013. Potential role and therapeutic interests of myo‐inositol in metabolic diseases. Biochimie 95:1811–1827.[↩]
- Schlemmer U, Frolich W, Prieto RM, Grases F. 2009. Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res 53(Suppl 2):S330–S375.[↩]
- Wilson MS, Bulley SJ, Pisani F, et al. 2015. A novel method for the purification of inositol phosphates from biological samples reveals that no phytate is present in human plasma or urine. Open Biol 5:150014.[↩]
- Negishi O, Mun’im A, Negishi Y. 2015. Content of methylated inositols in familiar edible plants. J Agric Food Chem 63:2683–2688.[↩]
- Taylor MJ, Wilder H, Bhagwagar Z, Geddes J. Inositol for depressive disorders. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No.: CD004049. DOI: 10.1002/14651858.CD004049.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD004049.pub2/full[↩][↩][↩]
- Martindale, The Extra Pharmacopoeia, 30th ed, p1379[↩]
- Deja S., Dawiskiba T., Balcerzak W., Orczyk-Pawiłowicz M., Głód M., Pawełka D., Młynarz P. Follicular adenomas exhibit a unique metabolic profile. ¹H NMR studies of thyroid lesions. PLoS ONE. 2013;8:e84637 doi: 10.1371/journal.pone.0084637[↩]
- Dinicola S, Minini M, Unfer V, Verna R, Cucina A, Bizzarri M. Nutritional and Acquired Deficiencies in Inositol Bioavailability. Correlations with Metabolic Disorders. International Journal of Molecular Sciences. 2017;18(10):2187. doi:10.3390/ijms18102187 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5666868/[↩][↩]
- Cohen LA: Dietary fiber and breast cancer. Anticancer Res. 1999;19:3685–3688.[↩]
- Graf E., Eaton J.W. Dietary suppression of colonic cancer: Fiber or phytate? Cancer. 1985;56:717–718. doi: 10.1002/1097-0142(19850815)56:4<717::AID-CNCR2820560402>3.0.CO;2-4[↩]
- Nakahara N., Okazaki Y., Katayama T. Hypertrophic effect of dietary phytate on cecum in rats. Trace Nutr. Res. 2000;17:47–52.[↩]
- Okazaki Y., Katayama T. Dietary phytic acid modulates characteristics of the colonic luminal environment and reduces serum levels of proinflammatory cytokines in rats fed a high-fat diet. Nutr. Res. 2014;34:1085–1091. doi: 10.1016/j.nutres.2014.09.012[↩]
- Tantivejkul K., Vucenik I., Shamsuddin A.M. Inositol hexaphosphate (IP6) inhibits key events of cancer metastasis: I. In vitro studies of adhesion, migration and invasion of MDA-MB 231 human breast cancer cells. Anticancer Res. 2003;23:3671–3679. https://www.ncbi.nlm.nih.gov/pubmed/14666663[↩]
- Facchinetti F., Bizzarri M., Benvenga S., D’Anna R., Lanzone A., Soulage C., Di Renzo G.C., Hod M., Cavalli P., Chiu T.T., et al. Results from the international consensus conference on myo-inositol and d-chiro-inositol in obstetrics and gynecology: The link between metabolic syndrome and PCOS. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;195:72–76. doi: 10.1016/j.ejogrb.2015.09.024[↩]
- Tsui M.M., York J.D. Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling and nuclear processes. Adv. Enzyme Regul. 2010;50:324–337. doi: 10.1016/j.advenzreg.2009.12.002[↩]
- Chiu T.T., Rogers M.S., Law E.L., Briton-Jones C.M., Cheung L.P., Haines C.J. Follicular fluid and serum concentrations of myo-inositol in patients undergoing IVF: Relationship with oocyte quality. Hum. Reprod. 2002;17:1591–1596. doi: 10.1093/humrep/17.6.1591[↩]
- Condorelli R.A., La Vignera S., Di Bari F., Unfer V., Calogero A.E. Effects of myo-inositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 2011;15:129–134.[↩]
- Croze M.L., Soulage C.O. Potential role and therapeutic interests of myoinositol in metabolic diseases. Biochimie. 2013;95:1811–1827. doi: 10.1016/j.biochi.2013.05.011[↩]
- Santamaria A., Giordano D., Corrado F., Pintaudi B., Interdonato M.L., Vieste G.D., Benedetto A.D., D’Anna R. One-year effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome. Climacteric. 2012;15:490–495. doi: 10.3109/13697137.2011.631063[↩]
- Colazingari S., Fiorenza M.T., Carlomagno G., Najjar R., Bevilacqua A. Improvement of mouse embryo quality by myo-inositol supplementation of IVF media. J. Assist. Reprod. Genet. 2014;31:463–469. doi: 10.1007/s10815-014-0188-1[↩]
- Forlenza O.V., De-Paula V.J., Diniz B.S. Neuroprotective effects of lithium: Implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem. Neurosci. 2014;5:443–450. doi: 10.1021/cn5000309[↩]
- Hallman M., Epstein B.L. Role of myo-inositol in the synthesis of phosphatidylglycerol and phosphatidylinositol in the lung. Bioch. Biophys. Res. Comm. 1980;92:1151–1159. doi: 10.1016/0006-291X(80)90407-6[↩]
- Pretlow T.P., O’Riordan M.A., Somich G.A., Amini S.B., Pretlow T.G. Aberrant crypts correlate with tumor incidence in F344 rats treated with azoxymethane and phytate. Carcinogenesis. 1992;13:1509–1512. doi: 10.1093/carcin/13.9.1509[↩]
- Bizzarri M., Dinicola S., Bevilacqua A., Cucina A. Broad spectrum anti-cancer activity of myo-inositol and inositol hexakisphosphate. Int. J. Endocrinol. 2016;2016:5616807. doi: 10.1155/2016/5616807[↩]
- Signal transduction. Unexpected mediators of protein phosphorylation. York JD, Hunter T. Science. 2004 Dec 17; 306(5704):2053-5. https://www.ncbi.nlm.nih.gov/pubmed/15604398/[↩]
- Chemopreventive effects of myo-inositol and dexamethasone on benzo[a]pyrene and 4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanone-induced pulmonary carcinogenesis in female A/J mice. Wattenberg LW, Estensen RD. Cancer Res. 1996 Nov 15; 56(22):5132-5. http://cancerres.aacrjournals.org/content/56/22/5132.full-text.pdf[↩]
- Lam S, Mandrekar SJ, Gesthalter Y, et al. A Randomized Phase IIb Trial of myo-Inositol in Smokers with Bronchial Dysplasia. Cancer prevention research (Philadelphia, Pa). 2016;9(12):906-914. doi:10.1158/1940-6207.CAPR-15-0254 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5136333/[↩][↩][↩]
- Barkai AI, Dunner DL, Gross HA, Mayo P, Fieve RR. Reduced myo-inositol levels in cerebrospinal fluid from patients with affective disorder. Biological Psychiatry 1978;13(1):65-72.[↩]
- Shimon H, Agam G, Belmaker RH, Hyde TM, Kleinman JE. Reduced frontal cortex inositol levels in postmortem brain of suicide victims and patients with bipolar disorder. American Jornal of Psychiatry 1997;154(8):1148-50.[↩]
- Kofman O, Belmaker RH. Ziskind-Somerfeld Research Award 1993. Biochemical, behavioral, and clinical studies of the role of inositol in lithium treatment and depression. Biol Psychiatry 1993;34(12):839-52.[↩]
- Einat H, Belmaker RH. The effects of inositol treatment in animal models of psychiatric disorders. Journal of Affective Disorders 2001;62(1-2):113-21.[↩]
- Levine J, Rapaport A, Lev L, Bersudsky Y, Kofman O, Belmaker RH, et al. Inositol treatment raises CSF inositol levels. Brain Research 1993;627(1):168-70.[↩]
- Greene NDE, Leung K, Copp AJ. Inositol, neural tube closure and the prevention of neural tube defects. Lupo PJ, Zohn I, eds. Birth Defects Research. 2017;109(2):68-80. doi:10.1002/bdra.23533 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5353661/[↩]
- Fujisawa T, Fujinaga S, Atomi H. An In Vitro Enzyme System for the Production of myo-Inositol from Starch. Zhou N-Y, ed. Applied and Environmental Microbiology. 2017;83(16):e00550-17. doi:10.1128/AEM.00550-17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5541223/[↩]
- Yang Q., Dixit B., Wada J., Tian Y., Wallner E.I., Srivastva S.K., Kanwar Y.S. Identification of a renal-specific oxido-reductase in newborn diabetic mice. Proc. Natl. Acad. Sci. USA. 2000;97:9896–9901. doi: 10.1073/pnas.160266197[↩]
- Hankes L.V., Politzer W.M., Touster O., Anderson L. Myo-inositol catabolism in human pentosurics: The predominant role of the glucuronate-xylulosepentose phosphate pathway. Ann. N. Y. Acad. Sci. 1969;165:564–576[↩][↩]
- Pitkänen E. Changes in serum and urinary myo-inositol levels in chronic glomerulonephritis. Clin. Chim. Acta. 1976;71:461–468. doi: 10.1016/0009-8981(76)90097-8[↩]
- Kawa J.M., Przybylski R., Taylor C.G. Urinary chiro-inositol and myoinositol excretion is elevated in the diabetic db/db mouse and streptozotocin diabetic rat. Exp. Biol. Med. 2003;228:907–914. doi: 10.1177/153537020322800806[↩][↩]
- Portha B., Serradas P., Bailbé D., Suzuki K.-I., Goto Y., Giroix M.-H. Beta-cell insensitivity to glucose in the GK rat, a spontaneous nonobese model for type II diabetes. Diabetes. 1991;40:486–491. doi: 10.2337/diab.40.4.486[↩]
- Kunjara S., Wang D.Y., Greenbaum A.L., McLean P., Kurtz A., Rademacher T.W. Inositol phosphoglycans in diabetes and obesity: Urinary levels of IPG A-Type and IPG-P Type, and relationship to pathophysiological change. Mol. Genet. Metab. 1999;68:488–502. doi: 10.1006/mgme.1999.2936[↩]
- Ostlund R.E., Jr., McGill J.B., Herskowitz I., Kipnis D.M., Santiago J.V., Sherman W. D-chiro-inositol metabolism in diabetes mellitus. Proc. Natl. Acad. Sci. USA. 1993;90:9988–9992. doi: 10.1073/pnas.90.21.9988[↩][↩]
- Suzuki S., Kawasaki H., Satoh Y., Ohtomo M., Hirai M., Hirai A., Hirai S., Onoda M., Matsumoto M., Hinokio Y., et al. Urinary chiro-inositol excretion is an index marker of insulin sensitivity in Japanese Type II Diabetes. Diabetes Care. 1994;17:1465–1468. doi: 10.2337/diacare.17.12.1465[↩]
- Lahjouji K., Aouameur R., Bissonnette P., Coady M.J., Bichet D.G., Lapointe J.Y. Expression and functionality of the Na+/myo-inositol cotransporter SMIT2 in rabbit kidney. Biochim. Biophys. Acta. 2007;1768:1154–1159. doi: 10.1016/j.bbamem.2007.01.007[↩]
- Chang H.H., Choong B., Phillips A.R., Loomes K.M. The diabetic rat kidney mediates inosituria and selective urinary partitioning of d-chiroinositol. Exp. Biol. Med. 2015;240:8–14. doi: 10.1177/1535370214543064[↩]
- Raccah D., Coste T., Cameron N., Dufayet D., Vague P., Hohman T. Effect of the aldose reductase inhibitor tolrestat on nerve conduction velocity, Na/K ATPase activity, and polyols in red blood cells, sciatic nerve, kidney cortex, and kidney medulla of diabetic rats. J. Diabetes Compl. 1998;12:154–162. doi: 10.1016/S1056-8727(97)00093-7[↩][↩]
- Chang H.H., Chao H.N., Walker C.S., Choong S.Y., Phillips A., Loomes K.M. Renal depletion of myo-inositol is associated with its increased degradation in animal models of metabolic disease. Am. J. Physiol. Renal. Physiol. 2015;309:755–763. doi: 10.1152/ajprenal.00164.2015[↩][↩]
- Prabhu K.S., Arner R.J., Vunta H., Reddy C.C. Up-regulation of human myo-inositol oxygenase by hyperosmotic stress in renal proximal tubular epithelial cells. J. Biol. Chem. 2005;280:19895–19901. doi: 10.1074/jbc.M502621200[↩]
- Vallon V., Thomson S. Renal function in diabetic disease models: The tubular system in the pathophysiology of the diabetic kidney. Annu. Rev. Physiol. 2012;74:351–375. doi: 10.1146/annurev-physiol-020911-153333[↩]
- Nayak B., Kondeti V.K., Xie P., Lin S., Viswakarma N., Raparia K., Kanwar Y.S. Transcriptional and post-translational modulation of myo-inositol oxygenase by high glucose and related pathobiological stresses. J. Biol. Chem. 2011;286:27594–27611. doi: 10.1074/jbc.M110.217141[↩]
- Nakamura N., Obayashi H., Fujii M., Fukui M., Yoshimori K., Ogata M., Hasegawa G., Shigeta H., Kitagawa Y., Yoshikawa T., et al. Induction of aldose reductase in cultured human microvascular endothelial cells by advanced glycation end products. Free Radic. Biol. Med. 2000;29:17–25. doi: 10.1016/S0891-5849(00)00286-0[↩]
- Zhan M., Usman I.M., Sun L., Kanwar Y.S. Disruption of renal tubular mitochondrial quality control by myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol. 2015;26:1304–1321. doi: 10.1681/ASN.2014050457[↩]
- Dinicola S, Minini M, Unfer V, Verna R, Cucina A, Bizzarri M. Nutritional and Acquired Deficiencies in Inositol Bioavailability. Correlations with Metabolic Disorders. International Journal of Molecular Sciences. 2017;18(10):2187. doi:10.3390/ijms18102187. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5666868/[↩]
- Reflections on inositol(s) for PCOS therapy: steps toward success. Gynecological Endocrinology Volume 31, 2015 – Issue 7; 501-505. https://doi.org/10.3109/09513590.2015.1054802[↩]
- Comparison of two insulin sensitizers, metformin and myo-inositol, in women with polycystic ovary syndrome (PCOS). Gynecological Endocrinology Volume 33, 2017 – Issue 1, 39-42. https://doi.org/10.1080/09513590.2016.1236078[↩][↩][↩][↩][↩]
- Hallman M, Saugstad OD, Porreco RP, Epstein BL, Gluck L. Role of myo-inositol in regulation of surfactant phospholipids in the newborn. Early Human Devevelopment 1985;10(3-4):245-54.[↩]
- Hallman M, Arjomaa P, Hoppu K. Inositol supplementation in respiratory distress syndrome: Relationship between serum concentration, renal excretion, and lung effluent phospholipids. Journal of Pediatrics 1987;110(4):604-10.[↩]
- Hallman M. Effect of intracellular myo-inositol on the surfactant phospholipid synthesis in the fetal rabbit lung. Biochimica et Biophysica Acta 1984;795(1):67-78.[↩]
- Egberts J, Noort WA. Gestational age-dependent changes in plasma inositol levels and surfactant composition in the fetal rat. Pediatric Research 1986;20(1):24-7.[↩]
- Bromberger P, Hallman M. Myoinositol in small preterm infants: Relationship between intake and serum concentration. Journal of Pediatric Gastroenterology and Nutrition 1986;5(3):455-8.[↩][↩]
- Pereira GR, Baker L, Egler J, Corcoran L, Chiavacci R. Serum myoinositol concentrations in premature infants fed human milk, formula for infants, and parenteral nutrition. American Journal of Clinical Nutrition 1990;51(4):589-93.[↩]
- Howlett A, Ohlsson A, Plakkal N. Inositol in preterm infants at risk for or having respiratory distress syndrome. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No.: CD000366. DOI: 10.1002/14651858.CD000366.pub3. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD000366.pub3/full[↩]
- A meta‐analysis of inositol for depression and anxiety disorders. Human Psychopharmacology Volume 29, Issue 1, January 2014, pages 55-63. https://doi.org/10.1002/hup.2369[↩]
- Bindra G.S., Gibson R.S., Thompson L.U. [Phytate][calcium][zinc] ratios in asian immigrant lacto-ovo vegetarian diets and their relationship to zinc nutriture. Nutr. Res. 1986;6:475–483. doi: 10.1016/S0271-5317(86)80101-4[↩]
- Khokhar S., Fenwick G.R. Phytate content of Indian foods and intakes by vegetarian Indians of Hisar Region, Haryana State. J. Agric. Food Chem. 1994;42:2440–2444. doi: 10.1021/jf00047a014[↩]
- Murphy S.P., Calloway D.H., Beaton G.H. Schoolchildren have similar predicted prevalences of inadequate intakes as toddlers in village populations in Egypt, Kenya, and Mexico. Eur. J. Clin. Nutr. 1995;49:647–657[↩]
- Carnovale E., Lombardi-Boccia G., Lugaro E. Phytate and zinc content of italian diets. Hum. Nutr. Appl. Nutr. 1987;41:180–186[↩]
- Ruggeri S., De Santis N., Carnovale E. Intake and sources of phytic acid in Itanlian diets. In: Kozlowska H., Fornal J., Zdunczyk Z., editors. Bioactive Substances in Food of Plant Origin. Centre for Agrotechnology and Veterinary Sciences; Olsztyn, Poland: 1994. pp. 355–359.[↩]
- Lam S, Mandrekar SJ, Gesthalter Y, et al. A Randomized Phase IIb Trial of myo-Inositol in Smokers with Bronchial Dysplasia. Cancer prevention research (Philadelphia, Pa). 2016;9(12):906-914. doi:10.1158/1940-6207.CAPR-15-0254. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5136333/[↩]