Contents
What is licorice
Licorice (American) or liquorice (British) is the root of Glycyrrhiza glabra from which a sweet flavor can be extracted 1. Licorice is harvested from the plants’ roots and underground stems. The licorice plant is a herbaceous perennial legume native to southern Europe and parts of Asia, such as India. It is not botanically related to anise, star anise, or fennel, which are sources of similar flavoring compounds. Anise oil is often used instead of licorice root to flavor licorice candy.
In ancient Egypt, licorice was not eaten as strips or ropes of candy but was made into a sweet liquid drink 2. The extract of the plant called Glycyrrhiza is derived from the ancient Greek term ‘glykos’, meaning sweet, and ‘rhiza’, meaning root. Glycyrrhiza was indulged upon by many prophets and pharaohs.
Licorice is a popular sweetener found in many soft drinks, food products, snacks and herbal medicines. Most licorice is used as a flavoring agent for tobacco, particularly US blend cigarettes, to which licorice lends a natural sweetness and a distinctive flavour and makes it easier to inhale the smoke by creating bronchodilators, which open up the lungs 3. Licorice flavors are also used as candies or sweeteners, particularly in some European and Middle Eastern countries.
Centuries ago, licorice root was used in Greece, China, and Egypt for stomach inflammation and upper respiratory problems. Licorice is one of the most widely prescribed herbs in Chinese medicine. It is used to treat gastric ulcers when administered 20 to 30 minutes before meals through lining the stomach wall. The processed form of licorice (deglycyrrhizinated form of licorice or DGL) is not associated with adverse effects and can be used to treat peptic ulcer disease in combination with antacids (this combination has been marketed as Caved-S) 2. However, licorice is rarely used nowadays because of its side effects and the emergence of other more powerful classes of medications for treatment of peptic ulcers.
Today, people use licorice root as a dietary supplement for digestive problems, menopausal symptoms, cough, and bacterial and viral infections. Licorice supplements are available as capsules, tablets, and liquid extracts. People also use it as a shampoo.
Figure 1. Licorice root

Figure 2. Licorice plant

Benefits of licorice root
A number of studies of licorice root in people have been published, but not enough to support the use for any specific health condition.
- Glycyrrhizin—a compound found in licorice root—has been tested in a few clinical trials in hepatitis C patients, but there’s currently not enough evidence to determine if it’s helpful 4. Laboratory studies done in Japan (where an injectable glycyrrhizin compound is used in people with chronic hepatitis C who do not respond to conventional treatment) suggest that glycyrrhizin may have some effect against hepatitis C 5.
- Also in Japan, glycyrrhizin has been given intravenously for treatment of patients with chronic hepatitis B with improvement in liver functions and occasionally complete recovery. It was suggested that glycyrrhizin is able to suppress the secretion of both hepatitis B surface antigen and its intracellular transport 6, 7.
- There’s some evidence that topical licorice extract may improve skin rash symptoms, such as redness, swelling, and itching.
- In women, licorice has been used in conjunction with spironolactone in the treatment of polycystic ovary syndrome (PCOS) 8. This estrogenic activity of licorice has been well documented 9.
- Studies of licorice root extracts in people for cavities, mouth ulcers, and oral yeast infections have returned mixed results 10.
- Agarwal and colleagues demonstrated the beneficial effect of licorice gargle in reducing the risk of postoperative sore throat 11.
- Other beneficial effects of licorice include its role in bone metabolism which was described by Mattarello and colleagues 12 who demonstrated an increased parathyroid hormone and urinary calcium levels from baseline value in healthy women after 2 months of therapy. The postulated mechanism was the estrogen-like activity of isoflavans which are one of the constituents of licorice roots.
- Understanding the mechanism of action of licorice promoted its therapeutic benefit in several groups of patients. The binding of licorice to the MR explains its utility in patients with Addison’s disease. These effects are due to the affinity of glycyrrhetinic acid for mineralocorticoid receptor (see Figure 3 below) in addition to its plasma concentration being more than 5000 fold the concentration of aldosterone 13. Patients with postural hypotension caused by diabetic autonomic neuropathy have also shown improvement with licorice ingestion 14. Bernardi and colleagues studied the effects of prolonged ingestion of graded doses of licorice on serum potassium level in healthy volunteers 15. A significant fall in plasma potassium concentration from 4.3 to 3.5 mmol/liter was noticed, which occurred at the dose of 800 mg or more daily. An important clinical application for this was provided by Farese and colleagues, who demonstrated the use of licorice as an important tool to maintain predialysis potassium levels within a safe limit to decrease the risk of hyperkalemic (high blood potassium) arrhythmias in patients on chronic dialysis 16.
Licorice side effects and toxicity
After oral ingestion of licorice in humans, its main constituent, glycyrrhizic acid, is hydrolyzed to glycyrrhetic acid by intestinal bacteria possessing a specialized ß-glucuronidase. Glycyrrhetic acid is a 200–1000 times more potent inhibitor of 11-ß-hydroxysteroid dehydrogenase than glycyrrhizic acid; therefore, its pharmacokinetics is more relevant after oral administration.
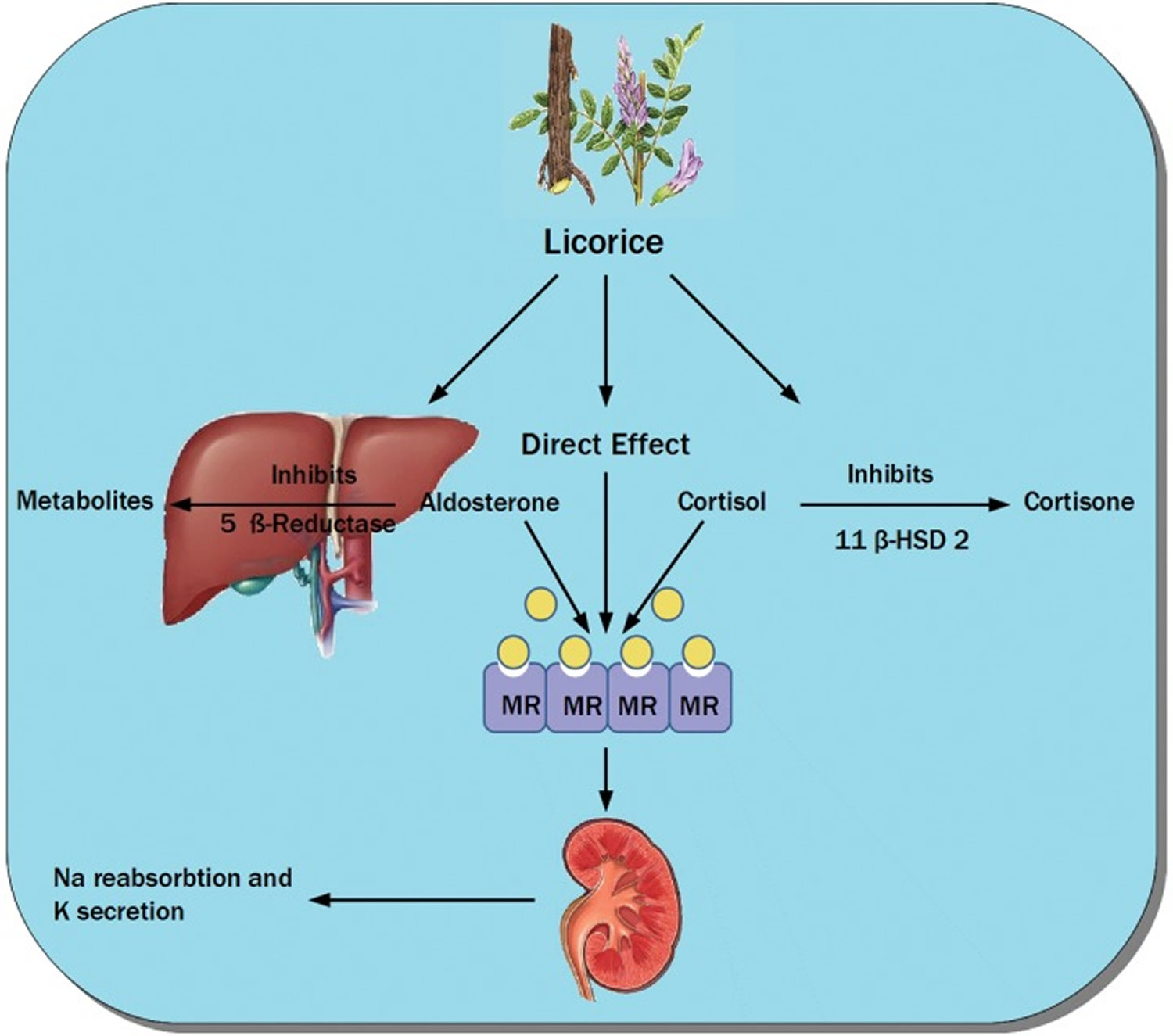
The active metabolites in licorice extract which are glycyrrhizic acid and glycyrrhetic acid can lead to a syndrome known as apparent mineralocorticoid excess 17. These side effects arise from the inhibition of the enzyme 11-ß-hydroxysteroid dehydrogenase and subsequent increase in the activity of cortisol. This effect is physiologically important because cortisol binds as avidly as aldosterone to the mineralocorticoid receptor 18. One form of this enzyme 11-ß-hydroxysteroid dehydrogenase type 2 (11-ß-HSD2) is mainly restricted in the kidneys to the aldosterone-sensitive sites in the collecting tubules. Licorice also has a mineralocorticoid-like activity not only by blocking 11-ß-HSD2 but also by directly binding to mineralocorticoid receptor 19.
Figure 3. Licorice mineralocortioid like activity
Note: Different mechanisms of action of licorice through inhibition of 11-ß-hydroxysteroid dehydrogenase type 2, 5 ß-reductase (which metabolizes aldosterone) and its direct action on the mineralocorticoid receptors causing sodium reabsorption and potassium secretion. MR= mineralocorticoid receptor; 11-ß-HSD 2 = 11-ß-hydroxysteroid dehydrogenase type 2.
[Source 2]Licorice main constituent, glycyrrhizic acid, mimics mineralocorticoids in its action (sodium reabsorbtion and potassium secretion) 2. The extent of metabolic and acid–base derangement can occasionally be severe enough to cause serious complications such as high blood pressure and low potassium levels, which could lead to heart and muscle problems 20.
Excessive consumption of licorice (more than 2 mg/kg/day of pure glycyrrhizinic acid, a licorice component) may result in adverse effects, and over consumption should be suspected clinically in patients presenting with otherwise unexplained hypokalemia (low blood potassium) and muscle weakness 2.
The complication associated with most fatalities is the arrhythmogenic effect of licorice mediated by hypokalemia and subsequent QT prolongation and possible torsade de pointes. The prognosis in the reported cases was poor, with six out of nine cases experiencing cardiac arrest 21, 22, 23, 24. Some reports described a picture of heart failure and acute pulmonary edema which mostly followed a licorice binge 25, 26. In these cases, recovery was the rule after implementing antifailure measures. A few cases presented with generalized edema which responded well to cessation of licorice and diuretic therapy 27, 28.
Several reports demonstrated the occurrence of ocular complications related to licorice ingestion 29, 30, 31. The underlying pathogenesis involves vasospasm of the optic nerve blood vessels leading to transient monocular or binocular visual loss/aberrations. All patients who experienced transient visual loss/aberrations had resolution of their visual symptoms; the aid of hyperbaric oxygen was required for one patient.
Table 1. Complications related to excess licorice intake
| Cardiovascular | Hypertension |
| Hypertensive encephalopathy | |
| Cardiac arrhythmias and death due to QT prolongation | |
| Heart failure and pulmonary edema | |
| Generalized edema | |
| Embolic ischemia | |
| Neurological | Hypokalemic myopathy Stroke |
| Rhabdomyolysis | |
| Carpal tunnel syndrome | |
| Licorice-induced myoclonus | |
| Occular deficits | |
| Electrolyte and renal abnormalities | Hypokalemia |
| Metabolic alkalosis | |
| Elevated CPK | |
| Acute tubular necrosis due to myoglobinuria | |
| Allergic reactions | Occupational asthma |
| Contact dermatitis | |
| Drug interaction | Inhibition of the P450 and CYP3A4 systems |
| Potentiation of the effect of warfarin therapy | |
| Digoxin toxicity due to licorice-induced hypokalemia |
CPK, creatine phosphokinase; CYP, cytochrome P450.
Some side effects are thought to be due to a chemical called glycyrrhizic acid. Licorice that has had this chemical removed (called DGL for deglycyrrhizinated licorice) may not have the same degree of side effects.
Taking licorice root containing glycyrrhizinic acid with medications that reduce potassium levels such as diuretics might be bad for your heart.
Factors that increase sensitivity to glycyrrhizin 2:
- Hypokalemia
- Prolonged gastrointestinal transit time
- Decreased 11-ß-hydroxysteroid dehydrogenase-2 activity
- Hypertension
- Anorexia nervosa
- Old age
- Female sex
Pregnant women should avoid using licorice root as a supplement or consuming large amounts of it as food.
- A Finnish study of mothers and their young children suggested that eating a lot of actual licorice root during pregnancy may harm a child’s developing brain, leading to reasoning and behavioral issues, such as attention problems, rule-breaking, and aggression 32.
Black Licorice Overdose
As it turns out, you really can overdose on candy—or, more precisely, black licorice 33.
Days before the biggest candy eating holiday of the year, the Food and Drug Administration (FDA) encourages moderation if you enjoy snacking on the old fashioned favorite.
So, if you’re getting your stash ready for Halloween, here’s some advice from FDA:
- If you’re 40 or older, eating 2 ounces of black licorice a day for at least two weeks could land you in the hospital with an irregular heart rhythm or arrhythmia.
FDA experts say black licorice contains the compound glycyrrhizin, which is the sweetening compound derived from licorice root. Glycyrrhizin can cause potassium levels in the body to fall. When that happens, some people experience abnormal heart rhythms, as well as high blood pressure, edema (swelling), lethargy, and congestive heart failure.
FDA’s Linda Katz, M.D., says last year the agency received a report of a black licorice aficionado who had a problem after eating the candy. And several medical journals have linked black licorice to health problems in people over 40, some of whom had a history of heart disease and/or high blood pressure.
FDA’s Linda Katz, M.D., says potassium levels are usually restored with no permanent health problems when consumption of black licorice stops.
Figure 4. Black licorice

If you have a fondness for black licorice, FDA is offering this advice:
- No matter what your age, don’t eat large amounts of black licorice at one time.
- If you have been eating a lot of black licorice and have an irregular heart rhythm or muscle weakness, stop eating it immediately and contact your healthcare provider.
- Black licorice can interact with some medications, herbs and dietary supplements. Consult a health care professional if you have questions about possible interactions with a drug or supplement you take.
How much licorice is too much ?
The main difficulty with licorice dosing lies in its availability in various forms such as candies, beverages, supplements and extracts that contain different amounts of the active components of licorice. In the United States, the manufacture of some dietary supplements, including licorice, is not closely regulated. Licorice and its derivatives, including ammoniated glycyrrhizin, are generally recognized as safe (GRAS) for use in foods by the US Food and Drug Administration (FDA) (21 CFR 184.1408). This chapter of regulations includes descriptions, specifications and maximum use levels (Table 2) for licorice and licorice derivatives. The FDA assumes that glycyrrhizin levels in foods do not pose a health hazard, provided that these foods are not consumed in excess.
Table 2. US Food and Drug Administration limitations for the use of licorice and its derivatives in foods
| Category of food | Maximum level in food (percent glycyrrhizin content of food) (as served) | Functional use |
|---|---|---|
| Baked foods, 170.3(n)(1) of this chapter | 0.05 | Flavor enhancer, 170.3(o)(11) of this chapter; flavoring agent, 170.3(o)(12) of this chapter. |
| Alcoholic beverages, 170.3(n)(2) of this chapter | 0.1 | Flavor enhancer, 170.3(o)(11) of this chapter; flavoring agent, 170.3(o)(12) of this chapter; surface-active agent, 170.3(o)(29) of this chapter. |
| Nonalcoholic beverages, 170.3(n)(3) of this chapter | 0.15 | Flavor enhancer, 170.3(o)(11) of this chapter; flavoring agent, 170.3(o)(12) of this chapter; surface-active agent, 170.3(o)(29) of this chapter. |
| Chewing gum, 170.3(n)(6) of this chapter | 1.1 | Flavor enhancer, 170.3(o)(11) of this chapter; flavoring agent, 170.3(n)(12) of this chapter. |
| Hard candy, 170.3(n)(25) of this chapter | 16.0 | Flavor enhancer, 170.3(o)(11) of this chapter; flavoring agent, 170.3(n)(12) of this chapter. |
| Herbs and seasonings, 170.3(n)(26) of this chapter | 0.15 | Flavor enhancer, 170.3(o)(11) of this chapter; flavoring agent, 170.3(n)(12) of this chapter. |
| Plant protein products, 170.3(n)(33) of this chapter | 0.15 | Flavor enhancer, 170.3(o)(11) of this chapter; flavoring agent, 170.3(n)(12) of this chapter. |
| Soft candy, 170.3(n)(38) of this chapter | 3.1 | Flavor enhancer, 170.3(o)(11) of this chapter; flavoring agent, 170.3(n)(12) of this chapter. |
| Vitamin or mineral dietary supplements | 0.5 | Flavor enhancer, 170.3(o)(11) of this chapter; flavoring agent, 170.3(n)(12) of this chapter. |
| All other foods except sugar substitutes, 170.3(n)(42) of this chapter. The ingredient is not permitted to be used as a nonnutritive sweetener in sugar substitutes | 0.1 | Flavor enhancer, 170.3(o)(11) of this chapter; flavoring agent, 170.3(n)(12) of this chapter. |
In 1991, the European Union proposed a provisional figure of 100 mg/day as the upper limit for ingestion of glycyrrhizin (approximately the amount found in 60–70 g licorice) 35. In April 2003, the Scientific Committee on Food confirmed an upper limit of 100 mg/day 36. This was based on data from human volunteer studies. The Dutch Nutrition Information Bureau advised against daily glycyrrhizin consumption in excess of 200 mg, assumed to correspond to 150 g of licorice confectionery 37.
Licorice fluid extracts contain approximately 10–20% glycyrrhizin; typical doses of 2–4 ml deliver 200–800 mg. A review concluded that about 2% of the regular consumers have a daily intake of glycyrrhizinic acid of over 100 mg/day 38. In 1994, Walker and Edwards demonstrated that a daily oral intake of 1–10 mg of glycyrrhizin, which corresponds to 1–5 g licorice, has been estimated to be a safe dose for most healthy adults 39. Two studies on healthy volunteers were published. In 1994, Bernardi and colleagues 15 administered daily doses of 108, 217, 380 and 814 mg glycyrrhizic acid, as ‘licorice pills’ for 4 weeks to four groups of 3 male and 3 female healthy volunteers. No observed-adverse-effect level (NOAEL) based on the study report was 217 mg/person/day. At higher dose levels, sodium retention and depression of plasma renin and aldosterone levels were observed. Female participants were slightly more sensitive to glycyrrhizinic acid than male participants. Two years later, in the double-blinded randomized placebo-controlled study by Bijlsma and colleagues 40, four groups of 10 healthy female volunteers received orally 0, 1, 2 or 4 mg of pure glycyrrhizinic acid/kg/day for 8 weeks. In this study the No observed-adverse-effect level (NOAEL) for glycyrrhizinic acid was 2 mg/kg/day. The European committee considered that the No observed-adverse-effect level (NOAEL) obtained in the study by Bijlsma and colleagues is more appropriate because this study comprised a larger group of volunteers (40 volunteers in contrast to 24 volunteers), a longer period of exposure (8 weeks in contrast to 4 weeks) and inclusion of a placebo control group.
- Liquorice. Wikipedia. https://en.wikipedia.org/wiki/Liquorice[↩]
- Omar HR, Komarova I, El-Ghonemi M, et al. Licorice abuse: time to send a warning message. Therapeutic Advances in Endocrinology and Metabolism. 2012;3(4):125-138. doi:10.1177/2042018812454322. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3498851/[↩][↩][↩][↩][↩][↩][↩]
- Boeken v. Phillip Morris Inc., 127 Cal. App. 4th 1640, 1673, 26 Cal. Rptr. 3d 638, 664 (2005).[↩]
- Dhiman RK, Chawla YK. Herbal medicines for liver diseases. Digestive Diseases and Sciences. 2005;50(10):1807-1812. https://www.ncbi.nlm.nih.gov/pubmed/16187178[↩]
- Matsumoto Y, Matsuura T, Aoyagi H, et al. Antiviral Activity of Glycyrrhizin against Hepatitis C Virus In Vitro. Polyak SJ, ed. PLoS ONE. 2013;8(7):e68992. doi:10.1371/journal.pone.0068992. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3715454/[↩]
- Therapeutic basis of glycyrrhizin on chronic hepatitis B. Sato H, Goto W, Yamamura J, Kurokawa M, Kageyama S, Takahara T, Watanabe A, Shiraki K. Antiviral Res. 1996 May; 30(2-3):171-7. https://www.ncbi.nlm.nih.gov/pubmed/8783808/[↩]
- Effects of glycyrrhizin on hepatitis B surface antigen: a biochemical and morphological study. Takahara T, Watanabe A, Shiraki K. J Hepatol. 1994 Oct; 21(4):601-9. https://www.ncbi.nlm.nih.gov/pubmed/7814808/[↩]
- Treatment of polycystic ovary syndrome with spironolactone plus licorice. Armanini D, Castello R, Scaroni C, Bonanni G, Faccini G, Pellati D, Bertoldo A, Fiore C, Moghetti P. Eur J Obstet Gynecol Reprod Biol. 2007 Mar; 131(1):61-7. https://www.ncbi.nlm.nih.gov/pubmed/17113210/[↩]
- Estrogen bioactivity in fo-ti and other herbs used for their estrogen-like effects as determined by a recombinant cell bioassay. Oerter Klein K, Janfaza M, Wong JA, Chang RJ. J Clin Endocrinol Metab. 2003 Sep; 88(9):4077-9. https://www.ncbi.nlm.nih.gov/pubmed/12970265/[↩]
- Messier C, Epifano F, Genovese S, et al. Licorice and its potential beneficial effects in common oro-dental diseases. Oral Diseases. 2012;18(1):32-39. https://www.ncbi.nlm.nih.gov/pubmed/21851508[↩]
- An evaluation of the efficacy of licorice gargle for attenuating postoperative sore throat: a prospective, randomized, single-blind study. Agarwal A, Gupta D, Yadav G, Goyal P, Singh PK, Singh U. Anesth Analg. 2009 Jul; 109(1):77-81. https://www.ncbi.nlm.nih.gov/pubmed/19535697/[↩]
- Effect of licorice on PTH levels in healthy women. Mattarello MJ, Benedini S, Fiore C, Camozzi V, Sartorato P, Luisetto G, Armanini D. Steroids. 2006 May; 71(5):403-8. https://www.ncbi.nlm.nih.gov/pubmed/16513152/[↩]
- Further studies on the mechanism of the mineralocorticoid action of licorice in humans. Armanini D, Lewicka S, Pratesi C, Scali M, Zennaro MC, Zovato S, Gottardo C, Simoncini M, Spigariol A, Zampollo V. J Endocrinol Invest. 1996 Oct; 19(9):624-9. https://www.ncbi.nlm.nih.gov/pubmed/8957748/[↩]
- Licorice ameliorates postural hypotension caused by diabetic autonomic neuropathy. Basso A, Dalla Paola L, Erle G, Boscaro M, Armanini D. Diabetes Care. 1994 Nov; 17(11):1356. https://www.ncbi.nlm.nih.gov/pubmed/7821181/[↩]
- Effects of prolonged ingestion of graded doses of licorice by healthy volunteers. Bernardi M, D’Intino PE, Trevisani F, Cantelli-Forti G, Raggi MA, Turchetto E, Gasbarrini G. Life Sci. 1994; 55(11):863-72. https://www.ncbi.nlm.nih.gov/pubmed/8072387/[↩][↩]
- Glycyrrhetinic acid food supplementation lowers serum potassium concentration in chronic hemodialysis patients. Farese S, Kruse A, Pasch A, Dick B, Frey BM, Uehlinger DE, Frey FJ. Kidney Int. 2009 Oct; 76(8):877-84. https://www.ncbi.nlm.nih.gov/pubmed/19641483/[↩]
- Mineralocorticoid activity of liquorice: 11-beta-hydroxysteroid dehydrogenase deficiency comes of age. Stewart PM, Wallace AM, Valentino R, Burt D, Shackleton CH, Edwards CR. Lancet. 1987 Oct 10; 2(8563):821-4. https://www.ncbi.nlm.nih.gov/pubmed/2889032/[↩]
- Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Funder JW, Pearce PT, Smith R, Smith AI. Science. 1988 Oct 28; 242(4878):583-5. https://www.ncbi.nlm.nih.gov/pubmed/2845584/[↩]
- Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22(phox) in human mononuclear leukocytes. Calò LA, Zaghetto F, Pagnin E, Davis PA, De Mozzi P, Sartorato P, Martire G, Fiore C, Armanini D. J Clin Endocrinol Metab. 2004 Apr; 89(4):1973-6. https://www.ncbi.nlm.nih.gov/pubmed/15070972/[↩]
- Jalili J, Askeroglu U, Alleyne B, et al. Herbal products that may contribute to hypertension. Plastic and Reconstructive Surgery. 2013;131(1):168-173. https://www.ncbi.nlm.nih.gov/pubmed/23271526[↩]
- [An unusual cause of cardiac arrest]. Campana A, Manzo M, Brigante M, Marrazzo N, Melchiorre G. Ital Heart J Suppl. 2003 Jun; 4(6):510-3. https://www.ncbi.nlm.nih.gov/pubmed/19400057/[↩]
- A sweet tooth as the root cause of cardiac arrest. Crean AM, Abdel-Rahman SE, Greenwood JP. Can J Cardiol. 2009 Oct; 25(10):e357-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2782510/[↩]
- Lethal licorice. Harris J. Aust Nurs J. 2000 Feb; 7(7):suppl 1-3. https://www.ncbi.nlm.nih.gov/pubmed/11894306/[↩]
- Torsades de Pointes induced by a combination of garenoxacin and disopyramide and other cytochrome P450, family 3, subfamily A polypeptide-4-influencing drugs during hypokalemia due to licorice. Miyamoto K, Kawai H, Aoyama R, Watanabe H, Suzuki K, Suga N, Kitagawa W, Miura N, Nishikawa K, Imai H. Clin Exp Nephrol. 2010 Apr; 14(2):164-7. https://www.ncbi.nlm.nih.gov/pubmed/19915794/[↩]
- Pulmonary edema following a licorice binge. Chamberlain JJ, Abolnik IZ. West J Med. 1997 Sep; 167(3):184-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1304525/pdf/westjmed00337-0056.pdf[↩]
- Echocardiographic findings of the heart resembling dilated cardiomyopathy during hypokalemic myopathy due to licorice-induced pseudoaldosteronism. Hasegawa J, Suyama Y, Kinugawa T, Morisawa T, Kishimoto Y. Cardiovasc Drugs Ther. 1998 Dec; 12(6):599-600. https://www.ncbi.nlm.nih.gov/pubmed/10410830/[↩]
- [Generalized edema caused by licorice: a new syndrome. Apropos of 3 cases]. Sailler L, Juchet H, Ollier S, Nicodème R, Arlet P. Rev Med Interne. 1993; 14(10):984. https://www.ncbi.nlm.nih.gov/pubmed/8009077/[↩]
- Liquorice-induced hypokalaemia and water retention in the absence of hypertension. Francini-Pesenti F, Puato M, Piccoli A, Brocadello F. Phytother Res. 2008 Apr; 22(4):563-5. https://www.ncbi.nlm.nih.gov/pubmed/18386259/[↩]
- Central retinal vein occlusion associated with liquorice ingestion. Hall RC, Clemett RS. Clin Exp Ophthalmol. 2004 Jun; 32(3):341. https://www.ncbi.nlm.nih.gov/pubmed/15180854/[↩]
- Ocular adverse effects associated with systemic medications : recognition and management. Santaella RM, Fraunfelder FW. Drugs. 2007; 67(1):75-93. https://www.ncbi.nlm.nih.gov/pubmed/17209665/[↩]
- Ocular side effects from herbal medicines and nutritional supplements. Fraunfelder FW. Am J Ophthalmol. 2004 Oct; 138(4):639-47. https://www.ncbi.nlm.nih.gov/pubmed/15488795/[↩]
- Räikkönen K, Pesonen A-K, Heinonen K, et al. Maternal licorice consumption and detrimental cognitive and psychiatric outcomes in children. American Journal of Epidemiology. 2009;170(9):1137-1146. https://www.ncbi.nlm.nih.gov/pubmed/19808634[↩]
- Black Licorice: Trick or Treat ? U.S. Food and Drug Administration. https://www.fda.gov/forconsumers/consumerupdates/ucm277152.htm[↩]
- CFR – Code of Federal Regulations Title 21. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=184.1408[↩]
- Too much of a good thing: a woman with hypertension and hypokalemia. Murphy SC, Agger S, Rainey PM. Clin Chem. 2009 Dec; 55(12):2093-6. http://clinchem.aaccjnls.org/content/55/12/2093.long[↩]
- Scientific Committee on Food (2003) Opinion of the Scientific Committee on Food on Glycyrrhizinic Acid and its Ammonium Salt. Brussels: European Commission Heath and Consumer Protection Directorate General.[↩]
- Fenwick G., Lutomski J., Nieman C. (1990) Liquorice, Glycyrrhiza glabra L. – composition, uses and analysis. Food Chem 38: 119–143.[↩]
- Maas P. (2000) Zoethout in levensmiddelen: onderzoek naar het glycyrrhizine gehalte van thee, kruidenmengsels, dranken en drop [Liquorice root in food stuffs: survey of the glycyrrhizin content of tea, herbal mixtures, alcoholic drinks and liquorice] (in Dutch). De Ware(n) Chemicus 30: 65–74.[↩]
- Licorice-induced hypertension and syndromes of apparent mineralocorticoid excess. Walker BR, Edwards CR. Endocrinol Metab Clin North Am. 1994 Jun; 23(2):359-77. https://www.ncbi.nlm.nih.gov/pubmed/8070427/[↩]
- Bijlsma J., Van Vloten P., Van Gelderen C., Mensinga T., Mout H., Elvers L., et al. (1996) Study into the effects of different dosages of glycyrrhizin in health female volunteers; in Dutch. RIVM report no. 348801004, RIVM, Bilthoven, The Netherlands.[↩]