Contents
- What is lactic acid
- Lactic acid build up in body and in muscles
- Excessive Muscle Activity
- Lactic acid test
- When is lactate test ordered?
- What does abnormal lactate test result mean?
- Is there anything I can do to decrease my lactate level?
- Why would a health practitioner choose to measure lactate in a blood sample from an artery rather than blood from a vein?
- Are there other ways to measure lactate than by sending a blood sample to the lab for testing?
- What is the lactate/pyruvate ratio and how is it used?
- Lactic acid uses
- Lactic acid risks
What is lactic acid
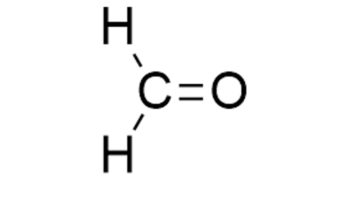
Lactic acid (C3H6O3) is a normal intermediate in the fermentation (oxidation, metabolism) of sugar. The concentrated form is used internally to prevent gastrointestinal fermentation. Lactic acid is a yellow to colorless crystal or liquid. Pure lactic acid is odorless. Other forms have a weak, unpleasant odor. Lactic acid has an acrid taste. Lactic acid mixes easily with water. Lactic acid is produced in most living organisms, its concentration increases in serum and muscle following exercise and is a component in blood and transfers into the body. Lactic acid is also found naturally in foods such as apples and other fruits, molasses, sour milk, wines, beer and plants. Lactic acid is also used as a food additive E 270, especially fermented foods like buttermilk, sourdoughs, beer and wine. Ultimately any absorbed lactic acid will be oxidized to give carbon dioxide and water.
Lactic acid (E 270) is a permitted food additive used in a variety of foods (e.g. nectars, jam, jellies, marmalades, mozzarella and whey cheese, fats of animal or vegetable origin for cooking and/or frying, canned and bottled fruits and vegetables, fresh pasta, beer) according to European Union Regulation (EC) No 1333/2008 on food additives 1. The Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) issued an opinion on lactic acid 2 allocating an acceptable daily intake (ADI) of ‘not limited’. In 1991, this acceptable daily intake (ADI) was supported by the Scientific Committee of Food 3.
Lactic acid is also a breakdown product of some chemicals present in the air reacting with light. Lactic acid is an important commercial chemical used to make some plasticizers, adhesives, pharmaceuticals and salts. Lactic acid is used in the leather tanning industry and as a solvent. Lactic acid is an ingredient in many household cleaning products and personal care products.
2% to 5% lactic acid solutions at temperatures of up to 55 °C applied either by spraying or misting is used for decontamination of beef carcasses, cuts and
trimmings.
Hyperlactatemia and lactic acidosis are among the most dangerous and life-threatening side effect that occurs during therapy with some nucleoside reverse transcriptase inhibitors, mainly didanosine and stavudine (d4T), also known as d-drugs. A prospective, follow-up study 4 aimed to examine the incidence rates and rate ratios of hyperlactatemia and lactic acidosis for each nucleoside reverse transcriptase inhibitor. Three hundred and ninety-six HIV-patients were included in final analysis comprising 783.8 person-years of follow-up. Between 1st January 2000 and 1st January 2008, 19 cases of hyperlactatemia and 15 cases of lactic acidosis were recorded 4. Between regimens with the significant impact for developing hyperlactatemia and lactic acidosis the lowest incidence rate was for didanosine (incidence rate=2.87 per 100 person-years and incidence rate=4.31 per 100 person-years, respectively), and the highest for didanosine+stavudine (incidence rate=10.17 per 100 person-years, and incidence rate=7.39 per 100 person-years, respectively). Compared to didanosine alone the rate ratio of hyperlactatemia was 2.67 for stavudine, and 4.06 for didanosine+stavudine. The rate ratio of lactic acidosis was 3.12 for stavudine, and 5.13 for didanosine+stavudine in comparison with didanosine alone. Other risk factors for hyperlactatemia were CD4 cell count less than 200 cells/cu mm and female sex. These results suggest that the use of stavudine alone or in combination with didanosine should not be used as first-line therapy, especially in patients with CD4 cell count less than 200 cells/cu mm and females if other treatment options are available.
What is normal lactic acid level in a human body?
The blood lactate concentration reflects a balance between production and uptake of lactate in tissues, and is normally between 0.5-1.8 mmol/L or 90 mg/L in a resting condition.
Elevated lactate is not clearly and universally defined but most studies use cut-offs between 2.0 and 2.5 mmol/L 5 whereas “high” lactate has been defined as a lactate level > 4 mmol/L in a number of studies 6.
“Lactic acidosis” is often used clinically to describe elevated lactate but should be reserved for cases where there is a corresponding acidosis (pH < 7.35) 7.
Lactic acid build up in body and in muscles
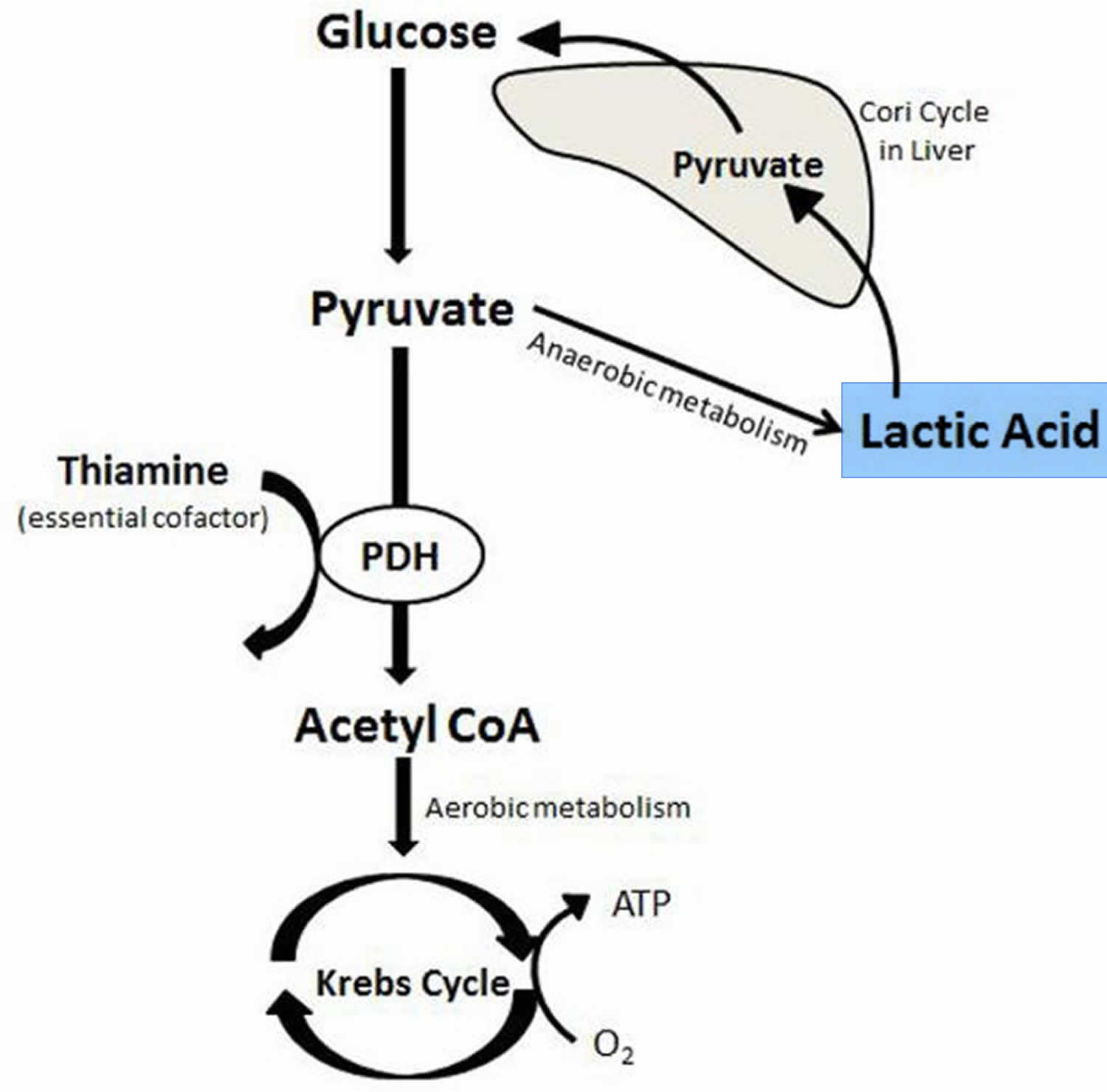
Lactate is produced by most tissues in the human body in carbohydrate and amino acid metabolism, with the highest level of production found in muscle 8. Lactate is also a natural component of very many foods, in particular fruits and fermented milk products. Under normal conditions, lactate is rapidly cleared by the liver with a small amount of additional clearance by the kidneys 9. In aerobic conditions, pyruvate is produced via glycolysis and then enters the Krebs cycle, largely bypassing the production of lactate. Under anaerobic conditions, lactate is an end product of glycolysis and feeds into the Cori cycle as a substrate for gluconeogenesis (see Figure 1). Lactate exists in two isomers: L-lactate and D-lactate. Current lactate measurements only include L-lactate (the primary isomer produced in humans). D-lactate is produced by bacteria in the human colon when they are exposed to large amounts of unabsorbed carbohydrates. In the setting of alteration in the intestinal flora and a high carbohydrate load (such as in short bowel syndrome) there will be an excess production of D-lactate, which can cross into the bloodstream and potentially cause neurologic symptoms.
Key points
- Elevated lactate can be caused by a number of conditions including shock, sepsis, cardiac arrest, trauma, seizure, ischemia, diabetic ketoacidosis, thiamine deficiency, malignancy, liver dysfunction, genetic disorders, toxins, and medications
- Elevated lactate has been associated with increased mortality in a number of diseases such as sepsis, trauma and cardiac arrest
- Decreased lactate clearance has been found to be associated with increased mortality in sepsis, post-cardiac arrest, trauma, burns and other conditions
- When approaching the patient with elevated lactate, the possibility of a multifactorial causes must be considered
- In spite of its imperfect sensitivity and specificity, the lactate blood test remains a clinically useful test that can alert a clinician to underlying hypoperfusion in need of immediate treatment or an etiology not readily apparent on initial evaluation
Figure 1. Lactic acid production and aerobic and anaerobic metabolism
Abbreviatons: ATP = Adenosine triphosphate; CoA = Coenzyme A; PDH = Pyruvate dehydrogenase
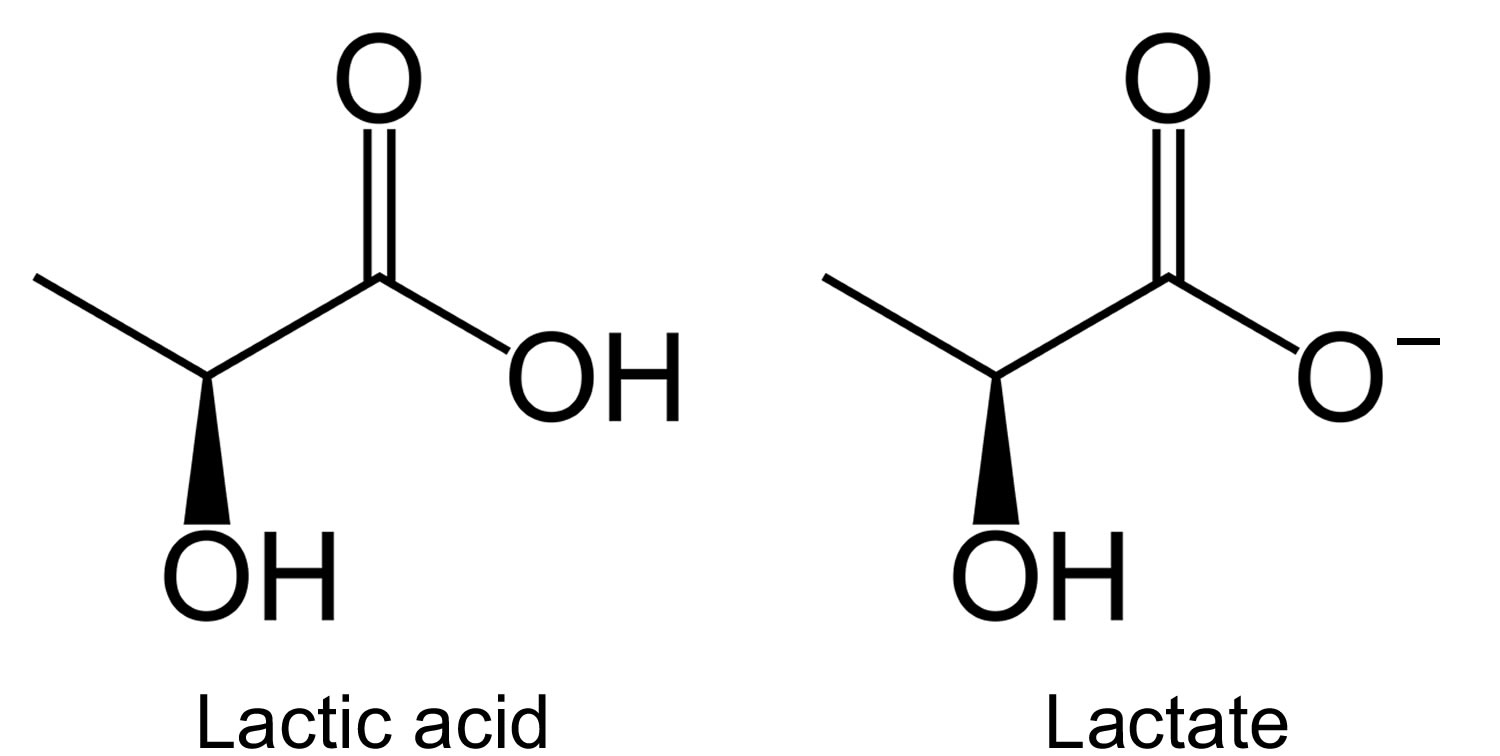
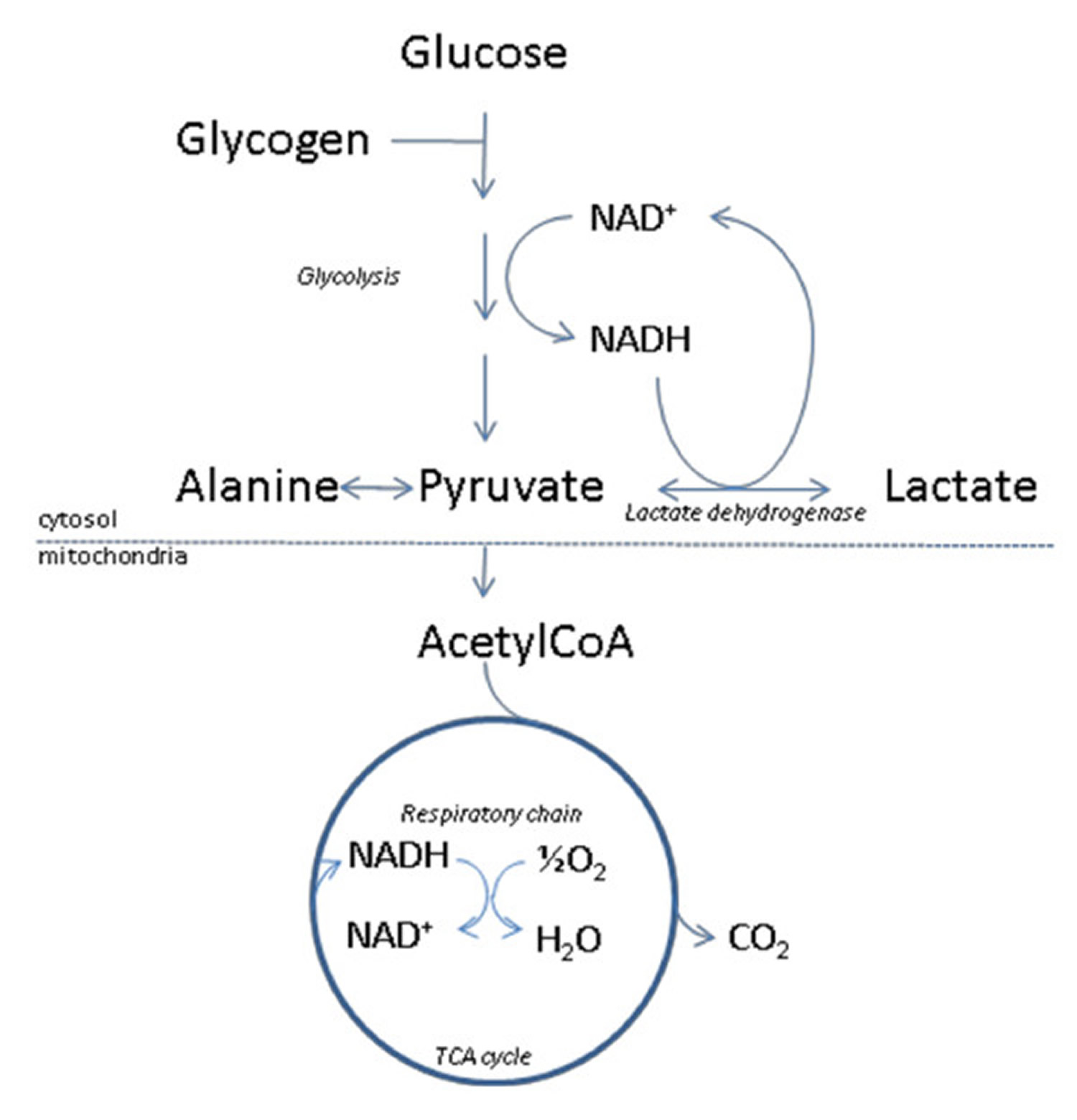
[Source 10]Lactate is one of the substances produced by cells as your body turns food into energy (cell metabolism). Lactate is formed by reduction of pyruvate, and is metabolized by oxidation to pyruvate in the reaction catalyzed by the cytosolic NAD-dependent lactate dehydrogenase (Figure 2). Depending on pH, lactate is sometimes present in the form of lactic acid. However, with the neutral pH maintained by the body, most of it will be present in the blood in the form of lactate.
Normally, the level of lactate in blood and cerebrospinal fluid (CSF) is low. Lactate is produced in excess by muscle cells, red blood cells, brain, and other tissues when there is insufficient oxygen at the cellular level or when the primary way of producing energy in the body’s cells is disrupted. Excess lactate can lead to lactic acidosis.
Lactic acid is mainly produced in muscle cells and red blood cells. Under conditions of heavy energy demand (and thus high oxygen need) skeletal muscles convert glucose into lactic acid to use for energy when oxygen levels are low (anaerobic), which is excreted from the muscle cells into the blood. In the liver this lactic acid is reduced to glucose.
The principal means of producing energy within cells occurs in the mitochondria, tiny power stations inside most cells of the body. The mitochondria use glucose and oxygen to produce ATP (adenosine triphosphate), the body’s primary source of energy. This is called aerobic energy production.
Whenever cellular oxygen levels decrease and/or the mitochondria are not functioning properly, the body must turn to less efficient energy production to metabolize glucose and produce ATP (adenosine triphosphate). This is called anaerobic energy production and the primary byproduct is lactic acid, which is processed (metabolized) by the liver.
Lactic acid can accumulate in the body and blood when it is produced faster than the liver can break it down.
Figure 2. Lactate metabolism
Footnote: Outline of lactate metabolism. With insufficient oxygen supply, pyruvate will be diverted to lactate, thereby assuring regeneration of NAD+ from NADH. This will enable glycolysis, and the accompanying ATP production to proceed.
The metabolic fate of pyruvate is mainly mitochondrial oxidation to carbon dioxide and water with accompanying energy production in the respiratory chain. The latter sequence of reactions are oxygen requiring, and with insufficient oxygen supply, or if pyruvate production for other reasons exceeds the capacity of oxidative metabolism, pyruvate will be diverted to lactate. This assures regeneration of NAD+ from NADH, which will enable glycolysis, and the accompanying ATP production to proceed. Due to the central role of the NAD-redox state for lactate production and metabolism, any metabolic condition giving rise to a steady-state increase in the cytosolic NADH/NAD+ ratio, will cause an increased net lactate production. This applies not only to conditions of hypoxia/anoxia in all tissues, but is also observed e.g. during extensive muscular work, and during alcohol metabolism by the liver. Lactate is released from tissues accompanied by a proton, and because lactic acid is fully dissociated at pH above approximately 6, excessive lactate production may thus give rise to lactic acidosis. The uptake of lactate from plasma takes place predominantly in liver and heart, where lactate will be used as an energy producing substrate or, in case of the liver, as a precursor for glucose formation.
[Source 11]Figure 3. Lactic acid and lactate chemical structure
There are a number of conditions that can cause high levels of lactate.
Excess lactate may indicate one or a combination of the following:
- Lack of oxygen (hypoxia)
- The presence of a condition that causes increased lactate production
- The presence of a condition that causes decreased clearance of lactate from the body
Times when your body’s oxygen level might drop include:
- During intense exercise
- When you have an infection or disease
Increased lactate levels may also be seen with thiamine (vitamin B1) deficiency. Thiamine serves as a co-factor for multiple cellular enzymes including pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, components essential to the tricarboxylic acid cycle and aerobic carbohydrate metabolism (see Figure 1). In the absence of thiamine, anaerobic metabolism predominates and lactate production increases 12. The development of elevated lactate in both serum and cerebrospinal fluid secondary to thiamine deficiency has been well described 13.
Table 1. Causes of elevated lactate
| Shock | Pharmacological agents* |
| Distributive | Linezolid |
| Cardiogenic | Nucleoside reverse transcriptase inhibitors |
| Hypovolemic | Metformin |
| Obstructive | Epinephrine |
| Post-cardiac arrest | Propofol |
| Regional tissue ischemia | Acetaminophen |
| Mesenteric ischemia | Beta2 agonists |
| Limb ischemia | Theophylline |
| Burns | |
| Trauma | Anaerobic muscle activity |
| Compartment syndrome | Seizures |
| Necrotizing soft tissue infections | Heavy exercise |
| Diabetic ketoacidosis | Excessive work of breathing |
| Drugs/toxins | Thiamine deficiency |
| Alcohols | Malignancy |
| Cocaine | Liver failure |
| Carbon monoxide | Mitochondrial disease |
| Cyanide |
Table 2. Common drugs and toxins associated with elevated lactate
| Drug/toxin | Risk factors | Proposed mechanism | Suggested treatment in addition to cessation of the offending agent |
|---|---|---|---|
| Metforminb | Congestive heart failure, kidney failure, liver failure or overdose | Inhibition of gluconeogenesis and mitochondrial impairment, inhibition of lactate elimination | Consider hemodialysis |
| Acetaminophen | Overdose | Impairment of the mitochondrial electron transport chain. Later hepatotoxicity and systemic effects. | Enteral activated charcoal and N-acetylcysteine. |
| NRTI | Female gender | Direct mitochondrial toxicity | No specific treatment |
| Linezolid | Possibly prolonged use in elderly patients | Direct mitochondrial toxicity | No specific treatment |
| Beta2-agonists | Not applicable | Beta2-adrenergic stimulation causing increased glycogenolysis, glycolysis and lipolysis. Free fatty acids released by lipolysis may inhibit PDH. | Depending on the clinical situation the beta2-agonist may/should be continued |
| Propofol | Prolonged high-dose use (Propofol Infusion Syndromed) | Impairment of the mitochondrial electron transport chain and fatty acid oxidation | Supportive treatment and potentially hemodialysis should be considered |
| Epinephrine | Not applicable | Likely due to beta2-adrenergic stimulation (see beta2-agonists) | Depending on the clinical situation epinephrine may be continued. |
| Theophylline | Overdose, though reported in standard doses | Increased levels of catecholamines (see beta2-agonists) | Enteral activated charcoal. Hemodialysis in severe cases. |
| Alcohols (ethanol, methanol, propylene glycol)b,c | Clinical relevance controversial and may be confounded by comorbidities (thiamine deficiency, seizures, sepsis, and other toxins) | Increased NADH levels due to ethanol metabolism may inhibit PDH and the utilization of lactate. Contributions from underlying comorbidities or possibly ketoacidosis may play a role | Identification and treatment of underlying disorders including administration of thiamine. |
| Cocaine | Not applicable | Beta2-adrenergic stimulation (see beta2-agonists). Vasoconstriction causing ischemia. | Supportive care and benzodiazepine |
| Carbon monoxide | Not applicable | Decreased oxygen-carrying capacity of the blood. | High-flow/hyperbaric oxygen. Consider co-exposure to cyanide |
| Cyanide | Not applicable | Noncompetitive inhibition of cytochrome c oxidase causing mitochondrial dysfunction and inability to utilize oxygen | Hydroxocobalamin or other cyanide antidote kit (Sodium nitrite, amyl nitrite, sodium thiosulfate). Consider co-exposure to carbon monoxide. |
Footnotes:
a: NRTI = Nucleoside reverse transcriptase inhibitor, PDH = Pyruvate dehydrogenase, NADH = Reduced nicotinamide adenine dinucleotide
b: See text for more details
c: Ethylene glycol may cause falsely elevated lactate levels
d: The Propofol Infusion Syndrome is characterized by cardiac failure, rhabdomyolysis, metabolic acidosis and renal failure.
When lactic acid production increases significantly, the affected person is said to have hyperlactatemia, which can then progress to lactic acidosis as more lactic acid accumulates. The body can often compensate for the effects of hyperlactatemia, but lactic acidosis can be severe enough to disrupt a person’s acid/base (pH) balance and cause symptoms such as muscular weakness, rapid breathing, nausea, vomiting, sweating, and even coma.
A test can be done to measure the amount of lactic acid in your blood. The lactate test measures the level of lactate in the blood at a given point in time or less commonly, in the cerebrospinal fluid (CSF). A normal lactate level indicates that a person does not have lactic acidosis, that there is sufficient oxygen at the cellular level, and/or that their signs and symptoms are not caused by lactic acidosis.
Excessive Muscle Activity
Lactate levels increase with heavy exercise, mainly due to anaerobic metabolism 15. Siegel and coworkers 16 found that lactate levels were elevated in 95% of collapsed marathon runners, with levels from 1.1 to 11.2 mmol/L.
Elevated lactate in the setting of acute severe asthma may be caused, at least in part, by excessive muscle work 17. Rabbat et al. 18 found that elevated lactate is common in acute severe asthma and that lactate increases in the first 6 hours after admission. They found no association with mortality or progression to respiratory failure. Beta agonists used in asthma treatment may also play a role due to excessive adrenergic stimulation (see Table 2) but the exact pathophysiology of elevated lactate in asthma warrants further research 19. Furthermore, excessive muscle work and respiratory muscle fatigue independent of the underlying etiology have been suggested to cause elevated lactate but further research is necessary to clarify this relationship 20.
Elevated lactate due to excessive muscle activity has also been associated with the use of restraints. A delirious or intoxicated patient may struggle against restraints and produce lactate due to muscle activity and tissue hypoxia. Sudden death has been reported in this population although whether that is a result of acidosis remains unknown. Proper sedation or alternative methods for restraint may be required for patient safety in this scenario 21.
Lactic acid test
The lactate test is primarily ordered to help determine if someone has lactic acidosis, a level of lactate that is high enough to disrupt a person’s acid-base (ph) balance.
Depending on pH, it is sometimes present in the form of lactic acid. However, with the neutral pH maintained by the body, most lactic acid will be present in the blood as lactate.
- Lactic acidosis is most commonly caused by an inadequate amount of oxygen in cells and tissues (hypoxia). If someone has a condition that may lead to a decreased amount of oxygen delivered to cells and tissues, such as shock or congestive heart failure, this test can be used to help detect and evaluate the severity of hypoxia and lactic acidosis. It may be ordered along with blood gases to evaluate a person’s acid/base balance and oxygenation.
- As lactic acidosis may also be caused by conditions unrelated to oxygen levels, this test may be used to evaluate someone who has a disease that can lead to increased lactate levels and who has signs and symptoms of acidosis. It may be ordered along with groups of tests, such as the comprehensive metabolic panel, basic metabolic panel or complete blood count (CBC), to determine if an underlying condition, such as liver or kidney disease, is causing lactic acidosis.
- The lactate test may also be used as part of an initial evaluation of someone who is suspected of having sepsis. Typically, if the person’s lactate level is above normal limits, treatment will be initiated without delay. If a person with sepsis can be diagnosed and treated promptly, their chances of recovery are significantly improved.
- Lactate levels may be ordered at intervals to help monitor hypoxia and response to treatment in a person being treated for an acute condition, such as sepsis, shock or heart attack, or a chronic condition, such as severe congestive heart failure.
A cerebrospinal fluid (CSF) lactate test may be ordered, along with a blood lactate test, to help distinguish between viral and bacterial meningitis.
When is lactate test ordered?
A lactate test may be ordered when someone has signs and symptoms of inadequate oxygen (hypoxia) such as:
- Shortness of breath
- Rapid breathing
- Paleness
- Sweating
- Nausea
- Muscle weakness
- Abdominal pain
- Coma
The test may be ordered when a person has signs and symptoms that a health practitioner suspects are related to sepsis, shock, heart attack, severe congestive heart failure, kidney failure, or uncontrolled diabetes.
The lactate test may be initially ordered with other tests to help evaluate a person’s condition. If lactate is significantly elevated, it may be ordered at intervals to monitor the condition.
CSF and blood lactate levels may be ordered when a person has signs and symptoms of meningitis, such as severe headaches, fever, delirium, and loss of consciousness.
What does abnormal lactate test result mean?
A high lactate level in the blood means that the disease or condition a person has is causing lactate to accumulate. In general, a greater increase in lactate means a greater severity of the condition. When associated with lack of oxygen, an increase in lactate can indicate that organs are not functioning properly.
However, the presence of excess lactate is not diagnostic. A health practitioner must consider a person’s medical history, physical examination, and the results of other diagnostic tests in order to determine the cause and to diagnose the underlying condition or disease.
A number of conditions can cause elevated lactate levels. They are separated into two groups according to the mechanism by which they cause lactic acidosis.
1) Type A lactic acidosis, the most common type, may be due to conditions that cause a person to be unable to breathe in enough oxygen (inadequate oxygen uptake in the lungs) and/or cause reduced blood flow, resulting in decreased transport of oxygen to the tissues (decreased tissue perfusion). Examples of type A conditions include:
- Shock from trauma or extreme blood loss (hypovolemia)
- Sepsis
- Heart attack
- Congestive heart failure
- Severe lung disease or respiratory failure
- Fluid accumulation in the lungs (Pulmonary edema)
- Very low level of red blood cells and/or low hemoglobin (severe anemia)
2) Type B lactic acidosis is not related to delivery of oxygen but reflects excess demand for oxygen or metabolic problems. Examples of type B causes include:
- Excessive lactate production
- Alcohols
- Ethanol
- Methanol
- Ethylene glycol
- Propylene glycol
- Impaired lactate use
- Liver disease
- Kidney disease
- Fructose metabolic defects
- Diabetes mellitus/Inadequately treated (uncontrolled) diabetes
- Leukemia
- AIDS
- Rare glycogen storage diseases (such as glucose-6-phosphatase deficiency)
- Use of certain drugs such as salicylates and metformin
- Exposure to toxins such as cyanide and methanol
- A variety of rare inherited metabolic and mitochondrial diseases that are forms of muscular dystrophy and affect normal ATP production (see the Related
- Content below for links to more information on these)
- Strenuous exercise, as with marathon runners
- Impaired oxygen use
- Disruption of mitochondrial oxidative phosphorylation
- Cyanide intoxication
- Carbon monoxide intoxication
- Linezolid
- Biguanides
- Nucleoside analog reverse transcription inhibitors
- Acetaminophen intoxication
- Acquired defects of the citric acid cycle or tricarboxylic acid cycle
- Thiamine deficiency
- Nutritional
- Cancer
- Alcoholism
- Gastrectomy
- Congenital defects of:
- Pyruvate transport
- Citric acid cycle or tricarboxylic acid cycle enzymes
- Pyruvate dehydrogenase complex
When someone is being treated for lactic acidosis or hypoxia, decreasing concentrations of lactate over time reflect a response to treatment.
When someone has signs and symptoms of meningitis, significantly increased cerebrospinal fluid lactate levels suggest bacterial meningitis while normal or slightly elevated levels are more likely to be due to viral meningitis.
Lactic acidosis is also associated with both inherited and acquired metabolic diseases. Lactic acid metabolism in the presence of altered gluconeogenesis, anaerobic glycolysis, and acid-base balance is a major factor in many disorders. Lactic acid can be formed only from pyruvic acid; therefore, disorders that increase pyruvate concentration, enhance lactic acid formation, or reduce lactic acid degradation cause lactic acidosis. Inborn metabolic errors that are accompanied by derangement of metabolic pathways of glucose, pyruvate, amino acids, and organic acids as well as toxic and systemic conditions that promote tissue hypoxia or mitochondrial injury result in lactic acidosis.
Is there anything I can do to decrease my lactate level?
Generally, no. However, if your elevated lactate level is due to an underlying condition that can be addressed, such as uncontrolled diabetes or a substance that can be avoided, such as ethanol, then you may be able to lower it. If you have been diagnosed with a condition, such as a metabolic disorder, following your prescribed treatment regimen should control your lactate level. If the increase is due to a temporary condition, such as shock or infection, then it will usually return to normal after the condition has been resolved.
Why would a health practitioner choose to measure lactate in a blood sample from an artery rather than blood from a vein?
Lactate measurements from arterial blood are thought to be more accurate and, because a tourniquet is not used, they are not generally affected by the collection process. A health practitioner may order an arterial lactate for these reasons or because a group of other tests called arterial blood gases (ABGs) are also being collected and the same sample can be used. When other arterial blood tests are not being ordered, a health practitioner may order a venous lactate because it provides an adequate evaluation of a person’s lactate level and because the collection process is not as uncomfortable.
Are there other ways to measure lactate than by sending a blood sample to the lab for testing?
Yes. Lactate may be measured using a small hand-held device much like a glucose meter at the point of care at a patient’s bedside, instead of in a laboratory. This type of monitoring is useful, for example, in emergency departments and intensive care units where rapid results are vital to the care of critically ill people. However, since the methods of measurement are different, the results from lactate point of care tests may not be comparable with those from tests performed in a laboratory.
What is the lactate/pyruvate ratio and how is it used?
A lactate/pyruvate ratio is a calculated result that may be used to differentiate between causes of lactic acidosis.
Pyruvate is a substance produced by and used by cells in the production of energy. The mitochondria within cells metabolize glucose in a series of steps to produce ATP, the body’s energy source. One of the steps involves pyruvate and the following step requires oxygen. When the oxygen level is low, pyruvate accumulates and is converted to lactate, resulting in an accumulation of lactate and lactic acidosis. An alternative cause is when there is impaired mitochondrial function and the pathway is interrupted, resulting in increased pyruvate and hence more lactate. The lactate/pyruvate ratio will be high in these cases.
However, there are certain congenital disorders (inborn errors of metabolism) in which pyruvate is not converted to lactate. One example is pyruvate dehydrogenase deficiency. In these cases, pyruvate will accumulate, the blood level will be high, and the lactate to pyruvate ratio will be low.
Lactic acid uses
Lactic acid has multiple uses in dyeing baths, as mordant in printing woolen goods, solvent for water-insoluble dyes. Lactic acid is also used for reducing chromates in mordanting wool, in manufacture of cheese, confectionery. Lactic acid is a component of babies’ milk formulas; acidulant in beverages; also used for acidulating worts in brewing. It is used in preparation of sodium lactate injections, and as ingredient of cosmetics, component of spermicidal jellies. Other uses of lactic acid: for removing Clostridium butyricum in manufacture of yeast; dehairing, plumping, and decalcifying hides, solvent for cellulose formate, flux for soft solder. Lactic acid is used to manufacture lactates which are used in food products, in medicine, and as solvents. Lactic acid is also a plasticizer, catalyst in the casting of phenolaldehyde resins.
Lactic acid risks
Eye, skin, nose, throat, and lung irritation may occur following exposure to lactic acid. Stomach ulcers have been observed from ingestion of high amounts of lactic acid. Death was reported from ingestion of a very high dose of lactic acid. In man, accidental intraduodenal administration of 100 mL 33% lactic acid was fatal within 12 hours 22. Diarrhea and weight loss were observed in healthy infants administered lactic acid as a dietary supplement. When 0.35% DL-lactic acid was administered to healthy babies from the tenth to the twentieth day of life, a threefold increase in the urinary excretion of the physiological L(+)-lactic acid and a twelve fold increase in the D(-)-lactic acid was observed 22. On withdrawing lactic acid from the diet the level of lactic acid excreted in the urine returned to normal. Since the racemic mixture used consisted of 80% of the L(+) and 20% of the D(-) forms it seems that the metabolism of the D(-) form by the young full-term baby is more difficult than the L(+) form. The increase in the urinary excretion of either form of lactic acid indicated that the young infant cannot utilize lactic acid at a rate which can keep up with 0.35% in the diet. A number of babies could not tolerate lactic acid 22. In such cases there was rapid loss of weight, frequent diarrhea, reduction of plasma bicarbonate and increased excretion of organic acids in the urine. All these effects were reversed on withdrawing lactic acid from the diet 22.
A skin test was performed using 49 a topic and 56 nonatopic patients to determine whether application of 2.5% lactic acid in water produces an urticarial reaction. Finn chambers containing 20 uL of test solution were fixed on the skin using porous tape for 20 min. Lactic acid produced no immediate reactions 23.
The ability of lactic acid to induce hyperkeratosis was evaluated 23. Lactic acid, 3 and 8%, pH 3, was applied to the outer aspect of the calf to induce scaling. When visible scaling and irritation occurred, the skin desquamation profile was altered. Control values were 5.7% for cell renewal and 1 for irritation, clinical scaling, desquamation amount, and desquame size. After 3 weeks of application of 3% lactic acid, the values increased to 27.8% for cell renewal, 1.9 for irritation, 1.5 for scaling and the desquamation amount, and 1.6 for desquame size. With 8% lactic acid, these values increased to 44.2% for cell renewal, 4.2 for irritation, 3.5 for scaling, 1.8 for desquamation amount, and 3.8 for desquame size.
Tumors were not induced following long-term exposure to lactic acid in laboratory animals. The potential for lactic acid to cause cancer in humans has not been assessed by the U.S. EPA IRIS program, the International Agency for Research on Cancer, or the U.S. National Toxicology Program 13th Report on Carcinogens.
Lactic acid effect on eye is similar to that of other acid of moderate strength, causing initial epithelial coagulation on cornea and conjunctiva, but having good prognosis if promptly washed off with water.
- Safety of lactic acid and calcium lactate when used as technological additives for all animal species. EFSA Journal 5 July 2017. https://doi.org/10.2903/j.efsa.2017.4938[↩]
- JECFA Joint FAO/WHO Expert Committee on Food Additives, 1974. Toxicological evaluation of some anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. Toxicological monographs: WHO Food Additives Series, no. 5.[↩]
- SCF (Scientific Committee for Food), 1991. Reports of the Scientific Committee for Food 25th series: First series of food additives of various technological functions (Opinion expressed on 18 May 1990). Directorate-General, Internal Market and Industrial Affairs https://ec.europa.eu/commission/index_en[↩]
- Dragovic G, Jevtovic D; Biomed Pharmacother 66 (4): 308-11, 2012 https://www.ncbi.nlm.nih.gov/pubmed/22658063[↩][↩]
- Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scandinavian journal of trauma, resuscitation and emergency medicine. 2011;19:74 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3292838/[↩]
- Callaway DW, Shapiro NI, Donnino MW, Baker C, Rosen CL. Serum lactate and base deficit as predictors of mortality in normotensive elderly blunt trauma patients. The Journal of trauma. 2009 Apr;66(4):1040–1044[↩]
- Definition of clinically relevant lactic acidosis in patients with internal diseases. Luft D, Deichsel G, Schmülling RM, Stein W, Eggstein M. Am J Clin Pathol. 1983 Oct; 80(4):484-9. https://www.ncbi.nlm.nih.gov/pubmed/6624712/[↩]
- Lactate kinetics in human tissues at rest and during exercise. van Hall G. Acta Physiol (Oxf). 2010 Aug; 199(4):499-508. https://www.ncbi.nlm.nih.gov/pubmed/20345411[↩]
- Contribution of liver and skeletal muscle to alanine and lactate metabolism in humans. Consoli A, Nurjhan N, Reilly JJ Jr, Bier DM, Gerich JE. Am J Physiol. 1990 Nov; 259(5 Pt 1):E677-84. https://www.ncbi.nlm.nih.gov/pubmed/2240206/[↩]
- Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate. Mayo Clinic proceedings. 2013;88(10):1127-1140. doi:10.1016/j.mayocp.2013.06.012. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3975915/[↩]
- Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine. 2011;19:74. doi:10.1186/1757-7241-19-74. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3292838/[↩]
- Butterworth RF. Thiamine deficiency-related brain dysfunction in chronic liver failure. Metab Brain Dis. 2009 Mar;24(1):189–196.[↩]
- Kountchev J, Bijuklic K, Bellmann R, Joannidis M. A patient with severe lactic acidosis and rapidly evolving multiple organ failure: a case of shoshin beri-beri. Intensive Care Med. 2005 Jul;31(7):1004.[↩]
- Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate. Mayo Clinic proceedings. 2013;88(10):1127-1140. doi:10.1016/j.mayocp.2013.06.012 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3975915/[↩][↩]
- Cerretelli P, Samaja M. Acid-base balance at exercise in normoxia and in chronic hypoxia. Revisiting the “lactate paradox” European journal of applied physiology. 2003 Nov;90(5–6):431–448.[↩]
- Siegel AJ, Januzzi J, Sluss P, et al. Cardiac biomarkers, electrolytes, and other analytes in collapsed marathon runners: implications for the evaluation of runners following competition. American journal of clinical pathology. 2008 Jun;129(6):948–951.[↩]
- Appel D, Rubenstein R, Schrager K, Williams MH., Jr. Lactic acidosis in severe asthma. The American journal of medicine. 1983 Oct;75(4):580–584.[↩]
- Rabbat A, Laaban JP, Boussairi A, Rochemaure J. Hyperlactatemia during acute severe asthma. Intensive Care Med. 1998 Apr;24(4):304–312.[↩]
- Prakash S, Mehta S. Lactic acidosis in asthma: report of two cases and review of the literature. Canadian respiratory journal : journal of the Canadian Thoracic Society. 2002 May-Jun;9(3):203–208.[↩]
- Roussos C. Respiratory muscle fatigue and ventilatory failure. Chest. 1990 Mar;97(3 Suppl):89S–96S.[↩]
- Alshayeb H, Showkat A, Wall BM. Lactic acidosis in restrained cocaine intoxicated patients. Tennessee medicine : journal of the Tennessee Medical Association. 2010 Nov-Dec;103(10):37–39.[↩]
- Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additives Ser 40 a,b,c: Lactic acid 1966 http://www.inchem.org/documents/jecfa/jecmono/40abcj44.htm[↩][↩][↩][↩]
- Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology, 17 (Suppl.1): 1-203, 1998[↩][↩]