Contents
- What is carbidopa and levodopa
- Before using levodopa precautions
- Carbidopa levodopa contraindications
- Carbidopa levodopa uses
- Carbidopa levodopa dosage
- Carbidopa levodopa side effects
- Levodopa side effects
- Carbidopa side effects
- Nervous system side effects
- Psychiatric side effects
- Cardiovascular side effects
- Gastrointestinal side effects
- Hematologic side effects
- Metabolic side effects
- Musculoskeletal side effects
- Respiratory side effects
- Dermatologic side effects
- Ocular side effects
- Genitourinary side effects
- Other side effects
- Hepatic side effects
- Renal side effects
- Carbidopa levodopa side effects long term
- Carbidopa levodopa overdose
What is carbidopa and levodopa
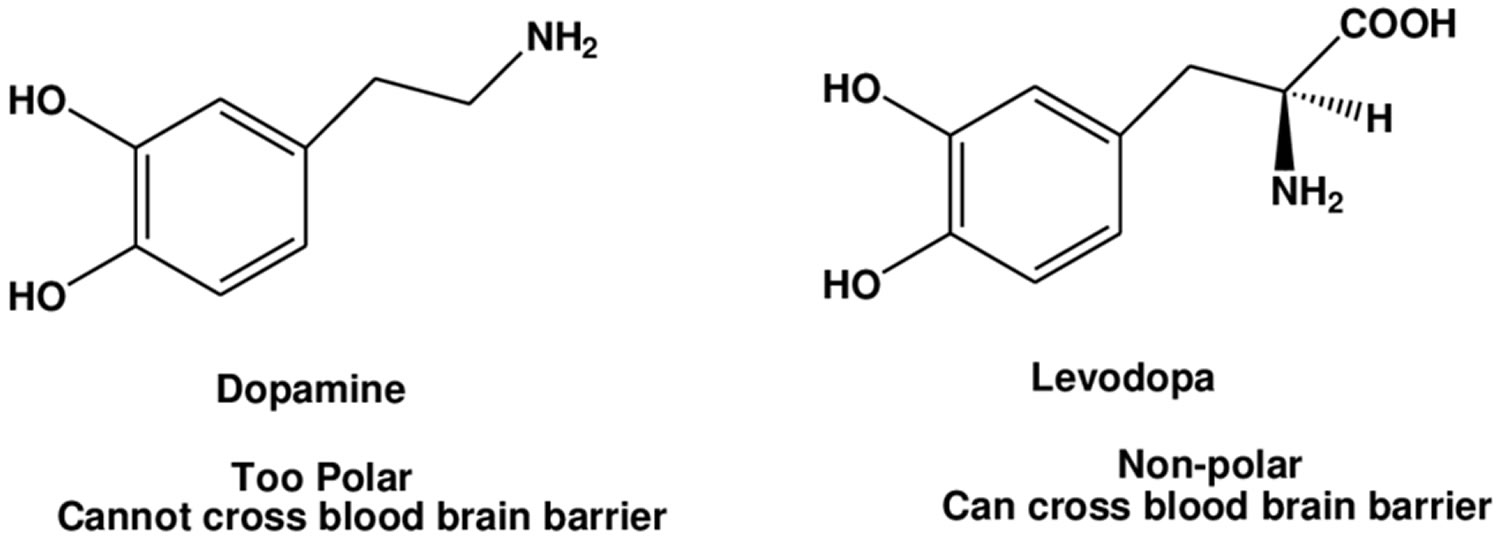
The combination of levodopa and carbidopa is used to treat the symptoms of Parkinson’s disease and Parkinson’s-like symptoms (parkinsonism) that may develop after encephalitis (swelling of the brain) or injury to the nervous system caused by carbon monoxide poisoning or manganese poisoning. Current evidence indicates that symptoms of Parkinson’s disease are related to depletion of dopamine in the corpus striatum. Administration of dopamine is ineffective in the treatment of Parkinson’s disease apparently because it does not cross the blood-brain barrier. However, levodopa, the metabolic precursor of dopamine (an aromatic amino acid precursor of dopamine), does cross the blood-brain barrier, and presumably is converted to dopamine in the brain. This is thought to be the mechanism whereby levodopa relieves symptoms of Parkinson’s disease. Levodopa is a prodrug that is converted to dopamine by DOPA decarboxylase and can cross the blood-brain barrier. Levodopa is the most commonly used treatment for Parkinson’s disease and levodopa is converted to dopamine in the brain. Carbidopa is a peripheral decarboxylase inhibitor that helps prevent the breakdown of levodopa before it can reach the brain and take effect. Carbidopa has little or no pharmacological activity when given alone in usual doses. Carbidopa inhibits the peripheral decarboxylation of levodopa to dopamine; and as carbidopa does not cross the blood-brain barrier, unlike levodopa, effective brain concentrations of dopamine are produced with lower doses of levodopa. At the same time, reduced peripheral formation of dopamine reduces peripheral side-effects, notably nausea and vomiting, and cardiac arrhythmias, although the dyskinesias and adverse mental effects associated with levodopa therapy tend to develop earlier. Carbidopa is a also used with levodopa to treat muscle symptoms similar to Parkinson’s disease that are caused by certain drugs such as chlorpromazine (Thorazine), fluphenazine (Prolixin), perphenazine (Trilafon), and others.
Note: Carbidopa has no antiparkinsonian effect when given alone. Carbidopa is indicated for use with carbidopa-levodopa or levodopa. Carbidopa does not decrease adverse reactions due to central effects of levodopa. When carbidopa is to be given to carbidopa-naive patients who are being treated with levodopa alone, the two drugs should be given at the same time. At least twelve hours should elapse between the last dose of levodopa and initiation of therapy with carbidopa and levodopa in combination. Start with no more than one-fifth (20%) to one-fourth (25%) of the previous daily dosage of levodopa when given without carbidopa. The addition of carbidopa with levodopa or carbidopa-levodopa reduces the peripheral effects (nausea, vomiting) due to decarboxylation of levodopa; however, carbidopa does not decrease the adverse reactions due to the central effects of levodopa. Because carbidopa permits more levodopa to reach the brain and more dopamine to be formed, certain adverse central nervous system (CNS) effects, e.g., dyskinesias (involuntary movements), may occur at lower dosages and sooner with levodopa in combination with carbidopa than with levodopa alone.
Parkinson’s disease is a progressive disorder of the nervous system marked by resting muscle tremors, muscle rigidity, decreased mobility, stooped posture, slow voluntary movements, and a mask-like facial expression. Parkinson’s disease affects over six million people worldwide, making it the most common neurodegenerative disease after Alzheimer’s disease 1. Parkinson’s disease can be very hard to live with because it severely restricts mobility and as a result makes daily activities increasingly difficult. Parkinson’s symptoms, including tremors (shaking), stiffness, and slowness of movement, are caused by a lack of dopamine, a natural substance usually found in the brain. There is no cure for Parkinson’s disease and its exact cause is not known, but there are effective treatments that can relieve the symptoms. Levodopa is in a class of medications called central nervous system agents. Although the optimal timing of the initiation of levodopa therapy is controversial, some investigators have suggested that early treatment of parkinsonism with levodopa delays disease progression and decreases mortality. Some patients require the combination of medicine, while others benefit from levodopa alone. By improving muscle control, levodopa allows more normal movements of the body. Levodopa works by being converted to dopamine in the brain. Carbidopa is in a class of medications called decarboxylase inhibitors. It works by preventing levodopa from being broken down before it reaches the brain. This allows for a lower dose of levodopa, which causes less nausea and vomiting.
Three main types of medication are commonly used for Parkinson’s disease:
- Levodopa
- Dopamine agonists. Dopamine agonists act as a substitute for dopamine in the brain and have a similar but milder effect compared with levodopa. They can often be given less frequently than levodopa. Sometimes dopamine agonists are taken at the same time as levodopa, as this allows lower doses of levodopa to be used.
- Monoamine oxidase-B inhibitors (e.g., selegiline and rasagiline). They block the effects of an enzyme or brain substance that breaks down dopamine (monoamine oxidase-B), increasing dopamine levels.
Your specialist can explain your medication options, including the risks associated with each medication, and discuss which may be best for you. Regular reviews will be required as the condition progresses and your needs change.
Catechol-O-methyltransferase (COMT) inhibitors are prescribed for people in later stages of Parkinson’s disease. They prevent levodopa from being broken down by the enzyme COMT.
Side effects of catechol-O-methyltransferase inhibitors include:
- nausea or vomiting
- diarrhoea
- abdominal pain
Carbidopa levodopa pharmacodynamics
When levodopa is administered orally it is rapidly decarboxylated to dopamine in extracerebral tissues so that only a small portion of a given dose is transported unchanged to the central nervous system. For this reason, large doses of levodopa are required for adequate therapeutic effect and these may often be accompanied by nausea and other adverse reactions, some of which are attributable to dopamine formed in extracerebral tissues.
The route of entry of levodopa is oral. Levodopa is rapidly absorbed from the small intestine by an active transport system for aromatic amino acids. Concentrations of drug in plasma usually peak between 0.5 and 2 hours after an oral dose. The rate of absorption of levodopa is greatly dependent upon the rate of gastric emptying, the pH of gastric juice and the length of time the drug is exposed to the degradative enzymes of the gastric mucosa and intestinal flora. Hyperacidity of gastric juice, and competition for absorption sites in the small intestine by amino acids each may interfere with the bioavailability of vodopa.
Distribution by route of exposure: Levodopa is widely distributed to most body tissues, but less to the central nervous system (CNS). Little unchanged drug reaches the cerebral circulation and probably less than 1% penetrates into the CNS.
Biological half-life by route of exposure: Levodopa has relatively short plasma half-life of 1 to 3 hours.
Levodopa metabolism: More than 95% of levodopa is decarboxylated by the widely distributed extracerebral aromatic l-amino acid decarboxylase. The drug is extensively decarboxylated in its first passage through the liver, which is rich in decarboxylase. A small amount is methylated to 3-0-methyldopa, which accumulates in the CNS due to its long half-life. Most is converted to dopamine, small amounts of which in turn are metabolized to norepinephrine and epinephrine (adrenaline). Biotransformation of dopamine proceeds rapidly to yield the principal excretion products, 3-4-dihydroxy-phenylacetic acid (DOPAC) and 3-methoxy-4-hydroxy-phenylacetic acid (homovanillic acid, HVA). At least 30 metabolites of levodopa have been identified. The evidence indicates that the metabolism of levodopa may be accelerated during prolonged therapy possibly due to enzyme induction.
Levodopa excretion: Metabolites of dopamine are rapidly excreted in the urine; 80% of a radioactively labelled dose is recovered within 24 hours. The principal metabolites 3-4-dihydroxy-phenylacetic acid and homovanillic acid account for up to 50% of the administered dose. Negligible amounts are found in the feces.
The incidence of levodopa-induced nausea and vomiting is less when levodopa is used with carbidopa than when levodopa is used without carbidopa. In many patients this reduction in nausea and vomiting will permit more rapid dosage titration.
Carbidopa inhibits decarboxylation of peripheral levodopa. Carbidopa has not been demonstrated to have any overt pharmacodynamic actions in the recommended doses. Carbidopa does not appear to cross the blood-brain barrier readily and does not affect the metabolism of levodopa within the central nervous system at doses of carbidopa that are recommended for maximum effective inhibition of peripheral decarboxylation of levodopa.
Carbidopa is combined with levodopa in carbidopa-levodopa or carbidopa-levodopa extended release tablets.
Since carbidopa’s decarboxylase-inhibiting activity is limited primarily to extracerebral tissues, administration of carbidopa with levodopa makes more levodopa available for transport to the brain. However, since levodopa and carbidopa compete with certain amino acids for transport across the gut wall, the absorption of levodopa and carbidopa may be impaired in some patients on a high protein diet.
Carbidopa levodopa pharmacokinetics
Levodopa is the metabolic precursor of dopamine, does cross the blood-brain barrier, and presumably is converted to dopamine in the basal ganglia. This is thought to be the mechanism whereby levodopa relieves symptoms of Parkinson’s disease, because it replaces depleted brain dopamine in these patients.
Carbidopa reduces the amount of levodopa required to produce a given response by about 75% and when administered with levodopa, increases both plasma levels and the plasma half-life of levodopa, and decreases plasma and urinary dopamine and homovanillic acid.
In clinical pharmacologic studies, simultaneous administration of separate tablets of carbidopa and levodopa produced greater urinary excretion of levodopa in proportion to the excretion of dopamine when compared to the two drugs administered at separate times.
Supplemental pyridoxine (vitamin B6) can be given to patients when they are receiving carbidopa and levodopa concomitantly or the fixed combination carbidopa-levodopa or carbidopa-levodopa extended release. Previous reports in the medical literature cautioned that high doses of vitamin B6 should not be taken by patients on levodopa therapy alone because exogenously administered pyridoxine would enhance the metabolism of levodopa to dopamine. The introduction of carbidopa to levodopa therapy, which inhibits the peripheral decarboxylation of levodopa to dopamine, counteracts the metabolic-enhancing effect of vitamin B6 (pyridoxine).
Before using levodopa precautions
In deciding to use levodopa, the risks of taking the medicine must be weighed against the good it will do. This is a decision you and your doctor will make.
Levodopa may cause some people to become dizzy, confused, or have blurred or double vision. Make sure you know how you react to levodopa before you drive, use machines, or do anything else that could be dangerous if you are not alert or not able to see well.
Dizziness, lightheadedness, or fainting may occur, especially when you get up from a lying or sitting position. Getting up slowly may help. If the problem continues or gets worse, check with your doctor.
For levodopa, the following should be considered:
Allergies
Tell your doctor if you have ever had any unusual or allergic reaction to levodopa or any other medicines. Also tell your health care professional if you have any other types of allergies, such as to foods, dyes, preservatives, or animals. For non-prescription products, read the label or package ingredients carefully.
Pediatric
Studies on levodopa have been done only in adult patients, and there is no specific information comparing use of levodopa or carbidopa in children with use in other age groups.
Geriatric
Elderly people are especially sensitive to the effects of levodopa. This may increase the chance of side effects during treatment.
Pregnancy
- Pregnancy Category C (all Trimesters): Animal studies have shown an adverse effect and there are no adequate studies in pregnant women OR no animal studies have been conducted and there are no adequate studies in pregnant women.
Breast Feeding
- Studies suggest that levodopa may alter milk production or composition. If an alternative to this medication is not prescribed, you should monitor the infant for side effects and adequate milk intake.
Patients with diabetes
Levodopa may cause test results for urine sugar or ketones to be wrong. Check with your doctor before depending on home tests using the paper-strip or tablet method.
Levodopa interactions with other medicines
When you are taking levodopa, it is especially important that your healthcare professional know if you are taking any of the medicines listed below. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Levodopa is always combined with carbidopa (a dopa decarboxylase inhibitor) to reduce peripheral side effects and enhance absorption.
Administration of levodopa with nonspecific inhibitors of monoamine oxidase inhibitor (MAO), such as phenelzine and tranylcypromine, markedly accentuates the actions of levodopa & may precipitate life-threatening hypertensive crisis & hyperpyrexia; nonspecific monoamine oxidase inhibitors always should be discontinued at least 14 days before levodopa is admin (note that this prohibition does not include the monoamine oxidase-B subtype-specific inhibitor selegiline, which, as discussed below, often is admin safely in combination with levodopa). Abrupt withdrawal of levodopa or other dopaminergic medications may precipitate the neuroleptic malignant syndrome more commonly observed after treatment with dopamine antagonists.
Antipsychotic drugs, such as phenothiazines, butyrophenones and reserpine can produce a parkinsonism-like syndrome, and since these drugs interfere with the therapeutic effects of levodopa, they are contraindicated. Therefore the phenothiazines should not be used to combat the emetic effect of levodopa. Nonspecific monoamine oxidase inhibitors interfere with inactivation of dopamine, norepinephrine and other catecholamines. Hence, they unpredictably exaggerate the central effects of levodopa and its catecholamine metabolites. Hypertensive crisis and hyperpyrexia are very real and dangerous sequelae of concurrent administration of two such drugs.
Using levodopa with any of the following medicines is not recommended. Your doctor may decide not to treat you with this medication or change some of the other medicines you take.
- Amisulpride
- Bromopride
- Clorgyline
- Furazolidone
- Iproniazid
- Isocarboxazid
- Linezolid
- Methylene Blue
- Moclobemide
- Nialamide
- Pargyline
- Phenelzine
- Procarbazine
- Sulpiride
- Toloxatone
- Tranylcypromine
Using levodopa with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Bupropion
- Isoniazid
- Macimorelin
- Metoclopramide
Using levodopa with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Fosphenytoin
- Indinavir
- Iron
- Kava
- Phenylalanine
- Phenytoin
- Spiramycin
- Tyrosine
For patients taking levodopa by itself:
- Pyridoxine (vitamin B6) has been found to reduce the effects of levodopa when levodopa is taken by itself. This does not happen with the combination of carbidopa and levodopa. If you are taking levodopa by itself, do not take vitamin products containing vitamin B6 during treatment, unless prescribed by your doctor.
- Large amounts of pyridoxine are also contained in some foods such as bananas, egg yolks, lima beans, meats, peanuts, and whole grain cereals. Check with your doctor about how much of these foods you may have in your diet while you are taking levodopa. Also, ask your health care professional for help when selecting vitamin products.
Interactions with Food/Tobacco/Alcohol
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Since protein may interfere with the body’s response to levodopa, high protein diets should be avoided. Intake of normal amounts of protein should be spaced equally throughout the day, or taken as directed by your doctor. Using alcohol or tobacco with certain medicines may also cause interactions to occur. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using levodopa with any of the following may cause an increased risk of certain side effects but may be unavoidable in some cases. If used together, your doctor may change the dose or how often you use levodopa, or give you special instructions about the use of food, alcohol, or tobacco.
Other Medical Problems
The presence of other medical problems may affect the use of levodopa. Make sure you tell your doctor if you have any other medical problems, especially:
- Diabetes mellitus (sugar diabetes)—The amount of insulin or antidiabetic medicine that you need to take may change
- Emphysema, asthma, bronchitis, or other chronic lung disease or
- Glaucoma or
- Heart or blood vessel disease or
- Hormone problems or
- Melanoma (a type of skin cancer) (or history of) or
- Mental illness—Levodopa may make the condition worse
- Kidney disease or
- Liver disease—Higher blood levels of levodopa may occur, increasing the chance of side effects
- Seizure disorders, such as epilepsy (history of)—The risk of seizures may be increased
- Stomach ulcer (history of)—The ulcer may occur again
Carbidopa-levodopa patient advice
It is important that carbidopa with levodopa be taken at regular intervals according to the schedule outlined by the health care provider. Caution patients not to change the prescribed dosage regimen and not to add any additional antiparkinson medications, including other carbidopa-levodopa preparations without first consulting a physician.
- Occasionally dark color may appear in saliva, urine, or sweat after ingestion of carbidopa.
- A change in diet to high-protein foods may delay the absorption of levodopa.
- Carbidopa-levodopa therapy may lead to sudden onset of sleep during daily activities or somnolence. Avoid driving or operating machinery if you experience these symptoms.
- As with levodopa alone, periodic evaluations of hepatic, hematopoietic, cardiovascular, and renal function are recommended during extended concomitant therapy with carbidopa and levodopa, or with carbidopa and carbidopa-levodopa, or any combination of these drugs.
Patients should be aware of a ‘wearing-off’ effect that may occur at the end of the dosing interval. Patients should to notify the prescriber if such response poses a problem to lifestyle.
Patients should be advised that occasionally dark color (red, brown, or black) may appear in saliva, urine, or sweat after ingestion of carbidopa and levodopa. Although the color appears to be clinically insignificant, garments may become discolored.
The patient should be advised that a change in diet to foods that are high in protein may delay the absorption of levodopa and may reduce the amount taken up in the circulation. Excessive acidity also delays stomach emptying thus delaying the absorption of levodopa. Iron salts (such as in multivitamin tablets) may also reduce the amount of levodopa available in the body. The above factors may reduce the clinical effectiveness of the carbidopa and levodopa therapy.
Alert patients to the possibility of sudden onset of sleep during daily activities, in some cases without awareness or warning signs, when they are taking dopaminergic agents, including levodopa. Advise patients to exercise caution while driving or operating machinery and that if they have experience somnolence and/or sudden sleep onset, they must refrain from these activities.
There have been reports of patients experiencing intense urges to gamble, increased sexual urges, and other intense urges, and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease, including carbidopa and levodopa. Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges, or other intense urges while taking carbidopa and levodopa. Physicians should consider dose reduction or stopping carbidopa and levodopa if a patient develops such urges while taking carbidopa with carbidopa/levodopa.
Patients taking carbidopa-levodopa products alone or with other dopaminergic drugs have reported suddenly falling asleep without prior warning of sleepiness while engaged in activities of daily living (includes operation of motor vehicles). Some of these episodes resulted in automobile accidents. Although many of these patients reported somnolence while on dopaminergic medications, some did perceive that they had no warning signs, such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some patients reported these events one year after the initiation of treatment.
Falling asleep while engaged in activities of daily living usually occurs in patients experiencing pre-existing somnolence, although some patients may not give such a history. For this reason, prescribers should continually reassess patients for drowsiness or sleepiness especially since some of the events occur after the start of treatment. Prescribers should be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities. Patients who have already experienced somnolence or an episode of sudden sleep onset should not participate in these activities during treatment with carbidopa when taking it with other carbidopa-levodopa products.
Before initiating treatment with carbidopa, advise patients about the potential to develop drowsiness and ask specifically about factors that may increase the risk for somnolence with carbidopa such as the use of concomitant sedating medications and the presence of sleep disorders. Consider discontinuing carbidopa in patients who report significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.). If treatment with carbidopa continues, patients should be advised not to drive and to avoid other potentially dangerous activities that might result in harm if the patients become somnolent. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
Impulse Control/Compulsive Behaviors
Postmarketing reports suggest that patients treated with anti-Parkinson medications can experience intense urges to gamble, increased sexual urges, intense urges to spend money uncontrollably, binge eating, and other intense urges. Patients may be unable to control these urges while taking one or more of the medications that are used for the treatment of Parkinson’s disease and that increase central dopaminergic tone, including carbidopa taken with levodopa and carbidopa. In some cases, although not all, these urges were reported to have stopped when the dose of anti-Parkinson medications was reduced or discontinued. Because patients may not recognize these behaviors as abnormal it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with carbidopa. Physicians should consider dose reduction or stopping carbidopa or levodopa if a patient develops such urges while taking carbidopa with carbidopa/levodopa.
Hallucinations/Psychotic-Like Behavior
Hallucinations and psychotic like behavior have been reported with dopaminergic medications. In general, hallucinations present shortly after the initiation of therapy and may be responsive to dose reduction in levodopa. Hallucinations may be accompanied by confusion and to a lesser extent sleep disorder (insomnia) and excessive dreaming. Carbidopa when taken with carbidopa-levodopa may have similar effects on thinking and behavior. This abnormal thinking and behavior may present with one or more symptoms, including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium.
Ordinarily, patients with a major psychotic disorder should not be treated with carbidopa and carbidopa-levodopa, because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson’s disease and may decrease the effectiveness of carbidopa.
Dyskinesia
Carbidopa may potentiate the dopaminergic side effects of levodopa and may cause or exacerbate preexisting dyskinesia.
Depression
Patients treated with carbidopa and carbidopa-levodopa should be observed carefully for the development of depression with concomitant suicidal tendencies.
Melanoma
Epidemiological studies have shown that patients with Parkinson’s disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the observed increased risk was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear.
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using carbidopa tablets for Parkinson’s disease.
Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
Laboratory Tests
Abnormalities in laboratory tests may include elevations of liver function tests such as alkaline phosphatase, SGOT (AST), SGPT (ALT), lactic dehydrogenase, and bilirubin. Abnormalities in blood urea nitrogen and positive Coombs test have also been reported. Commonly, levels of blood urea nitrogen, creatinine, and uric acid are lower during concomitant administration of Carbidopa and levodopa than with levodopa alone.
Levodopa and carbidopa-levodopa combination products may cause a false-positive reaction for urinary ketone bodies when a test tape is used for determination of ketonuria. This reaction will not be altered by boiling the urine specimen. False-negative tests may result with the use of glucose-oxidase methods of testing for glucosuria.
Hyperpyrexia and Confusion
Sporadic cases of a symptom complex resembling neuroleptic malignant syndrome (NMS) have been reported in association with dose reductions or withdrawal of certain antiparkinsonian agents such as levodopa, carbidopa-levodopa, or carbidopa-levodopa extended-release. Therefore, patients should be observed carefully when the dosage of levodopa or carbidopa-levodopa is reduced abruptly or discontinued, especially if the patient is receiving neuroleptics.
Neuroleptic malignant syndrome (NMS) is an uncommon but life-threatening syndrome characterized by fever or hyperthermia. Neurological findings, including muscle rigidity, involuntary movements, altered consciousness, mental status changes; other disturbances, such as autonomic dysfunction, tachycardia, tachypnea, sweating, hyper-or hypotension; laboratory findings, such as creatine phosphokinase elevation, leukocytosis, myoglobinuria, and increased serum myoglobin, have been reported.
The early diagnosis of this condition is important for the appropriate management of these patients. Considering neuroleptic malignant syndrome (NMS) as a possible diagnosis and ruling out other acute illnesses (e.g., pneumonia, systemic infection, etc.) is essential. This may be especially complex if the clinical presentation includes both serious medical illness and untreated or inadequately treated extrapyramidal signs and symptoms. Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system (CNS) pathology.
The management of neuroleptic malignant syndrome (NMS) should include: 1) intensive symptomatic treatment and medical monitoring and 2) treatment of any concomitant serious medical problems for which specific treatments are available. Dopamine agonists, such as bromocriptine, and muscle relaxants, such as dantrolene, are often used in the treatment of neuroleptic malignant syndrome (NMS); however, their effectiveness has not been demonstrated in controlled studies.
Carbidopa levodopa drug interactions
Symptomatic postural hypotension has occurred when carbidopa, given with levodopa or carbidopa-levodopa combination products, was added to the treatment of a patient receiving antihypertensive drugs. Therefore, when therapy with carbidopa, given with or without levodopa or carbidopa-levodopa combination products, is started, dosage adjustment of the antihypertensive drug may be required.
For patients receiving monoamine oxidase inhibitors (Type A or B). Concomitant therapy with selegiline or rasigiline and carbidopa and carbidopa-levodopa may be associated with severe orthostatic hypotension not attributable to carbidopa-levodopa alone.
There have been rare reports of adverse reactions, including hypertension and dyskinesia, resulting from the concomitant use of tricyclic antidepressants and carbidopa-levodopa preparations.
Dopamine D2 receptor antagonists (e.g., phenothiazines, butyrophenones, risperidone) and isoniazid may reduce the therapeutic effects of levodopa. In addition, the beneficial effects of levodopa in Parkinson’s disease have been reported to be reversed by phenytoin and papaverine. Patients taking these drugs with carbidopa and levodopa or carbidopa-levodopa combination products should be carefully observed for loss of therapeutic response.
Carbidopa and iron salts or multi vitamins containing iron salts should be co administered with caution. Iron salts can form chelates with levodopa and carbidopa and consequently reduce the bioavailability of carbidopa and levodopa.
Although metoclopramide may increase the bioavailability of levodopa by increasing gastric emptying, metoclopramide may also adversely affect disease control by its dopamine receptor antagonistic properties.
Droxidopa: Carbidopa may diminish the therapeutic effect of Droxidopa. Carbidopa may decrease serum concentrations of the active metabolite(s) of Droxidopa. Carbidopa may increase the serum concentration of Droxidopa. Monitor therapy
Spiramycin: May decrease the serum concentration of Carbidopa. And thus may decrease the effectiveness of levodopa. Monitor therapy
Test Interactions
False-positive reaction for urinary glucose with Clinitest®; false-negative reaction using Clinistix®; false-positive urine ketones with Acetest®, Ketostix®, Labstix®.
Carbidopa levodopa contraindications
Carbidopa is contraindicated in patients with known hypersensitivity to any component of this drug.
Levodopa is contraindicated for melanoma and glaucoma in patients with known hypersensitivity to the drug. Levodopa should be administered cautiously to patients with severe cardiovascular or pulmonary disease, asthma, renal, hepatic or endocrine disease. Care should be exercised in administering levodopa to patients with history of myocardial infarction who have residual atrial nodal or ventricular arrhythmias. If levodopa is necessary in this type of patient, it should be used in a facility with a coronary care unit or an intensive care unit.
Levodopa is contraindicated in patients with severe psychotic disorders, it should be used with caution in patients with psychiatric disturbance. This drug should not be used in pregnancy and should not be used in nursing mothers. The safety under the age of 12 has not been established.
Nonselective monoamine oxidase (MAO) inhibitors are contraindicated for use with levodopa or carbidopa-levodopa combination products with or without carbidopa. These inhibitors must be discontinued at least two weeks prior to initiating therapy with levodopa. Carbidopa-levodopa, or levodopa may be administered concomitantly with the manufacturer’s recommended dose of an MAO inhibitor with selectivity for MAO type B (e.g., selegiline HCl).
Levodopa or carbidopa-levodopa products, with or without carbidopa, are contraindicated in patients with narrow-angle glaucoma.
Carbidopa levodopa uses
Most people with Parkinson’s disease eventually need a medication called levodopa.
Levodopa is absorbed by the nerve cells in your brain and turned into the chemical dopamine, which is used to transmit messages between the parts of the brain and nerves that control movement.
Increasing the levels of dopamine using levodopa usually improves movement problems.
It is usually taken as a tablet or liquid, and is often combined with other medication, such as benserazide or carbidopa.
These medications stop the levodopa being broken down in the bloodstream before it has a chance to get to the brain.
They also reduce the side effects of levodopa, which include:
- feeling sick (nausea) or vomiting
- tiredness
- dizziness
If you’re prescribed levodopa, the initial dose is usually very small and will be gradually increased until it takes effect.
At first, levodopa can cause a dramatic improvement in the symptoms.
However, its effects can be less long-lasting over the following years – as more nerve cells in the brain are lost, there are fewer of them to absorb the medicine. This means the dose may need to be increased from time to time.
Long-term use of levodopa is also linked to problems such as uncontrollable, jerky muscle movements (dyskinesias) and “on-off” effects, where the person rapidly switches between being able to move (on) and being immobile (off).
Carbidopa levodopa is used in the treatment of idiopathic Parkinson disease (paralysis agitans), postencephalitic parkinsonism and symptomatic parkinsonism, which may follow injury to the nervous system by carbon monoxide and/or manganese intoxication.
Note: Administration of carbidopa allows use of a lower dosage of levodopa, more rapid titration, and a decrease in nausea and vomiting associated with levodopa; use with carbidopa/levodopa in patients requiring additional carbidopa; has no effect without levodopa.
Although the administration of carbidopa permits control of parkinsonism and Parkinson’s disease with much lower doses of levodopa, there is no conclusive evidence at present that this is beneficial other than in reducing nausea and vomiting, permitting more rapid titration, and providing a somewhat smoother response to levodopa.
Certain patients who responded poorly to levodopa alone have improved when carbidopa and levodopa were given concurrently. This was most likely due to decreased peripheral decarboxylation of levodopa rather than to a primary effect of carbidopa on the peripheral nervous system. Carbidopa has not been shown to enhance the intrinsic efficacy of levodopa.
In deciding whether to give carbidopa with carbidopa-levodopa or with levodopa to patients who have nausea and/or vomiting, the physician should be aware that, while many patients may be expected to improve, some may not. Since one cannot predict which patients are likely to improve, this can only be determined by a trial of therapy. It should be further noted that in controlled trials comparing carbidopa and levodopa with levodopa alone, about half the patients with nausea and/or vomiting on levodopa alone improved spontaneously despite being retained on the same dose of levodopa during the controlled portion of the trial.
Carbidopa is for use with carbidopa-levodopa in patients for whom the dosage of carbidopa-levodopa provides less than adequate daily dosage (usually 70 mg daily) of carbidopa.
Carbidopa is for use with levodopa in the occasional patient whose dosage requirement of carbidopa and levodopa necessitates separate titration of each medication.
Carbidopa is used with carbidopa-levodopa or with levodopa to permit the administration of lower doses of levodopa with reduced nausea and vomiting, more rapid dosage titration, and with a somewhat smoother response. However, patients with markedly irregular (“on-off”) responses to levodopa have not been shown to benefit from the addition of carbidopa.
Since carbidopa prevents the reversal of levodopa effects caused by pyridoxine (vitamin B6), supplemental pyridoxine (vitamin B6), can be given to patients when they are receiving carbidopa and levodopa concomitantly or as carbidopa-levodopa.
Carbidopa levodopa dosage
Take levodopa only as directed. Do not take more or less of it, and do not take it more often than your doctor ordered.
For patients taking carbidopa and levodopa extended-release tablets:
- Swallow the tablet whole without crushing or chewing, unless your doctor tells you not to. If your doctor tells you to, you may break the tablet in half.
Some people must take levodopa for several weeks or months before full benefit is received. Do not stop taking it even if you do not think it is working. Instead, check with your doctor.
Monitoring:
- Periodic evaluations of hepatic, hematopoietic, cardiovascular, and renal function.
- Psychiatric: Development of new or increased gambling urges, sexual urges, or other intense urges.
Levodopa dose
The dose of levodopa will be different for different patients. Follow your doctor’s orders or the directions on the label. The following information includes only the average doses of levodopa as a guide. If your levodopa dose is different, do NOT change it unless your doctor tells you to do so.
The amount of levodopa that you take depends on the strength of the levodopa. Also, the number of levodopa doses you take each day, the time allowed between doses, and the length of time you take the levodopa depend on the medical problem for which you are using the medicine.
For Parkinson’s disease
For oral levodopa dosage form (tablets):
- Adults and teenagers—At first, 250 milligrams (mg) two to four times a day. Your doctor may increase your dose if needed. However, the dose is usually not more than 8000 mg (8 grams) a day.
- Children up to 12 years of age—Use and dose must be determined by your doctor.
Missed dose
If you miss a dose of levodopa, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule. Do not double doses.
Levodopa and carbidopa combination
When carbidopa tablet is to be given to carbidopa-naive patients who are being treated with levodopa, the two drugs should be given at the same time, starting with no more than 20 to 25% of the previous daily dosage of levodopa when given without carbidopa tablets. At least twelve hours should elapse between the last dose of levodopa and initiation of therapy with carbidopa and levodopa.
Most patients respond to a 1:10 proportion of carbidopa and levodopa, provided the daily dosage of carbidopa is 70 mg or more a day. The maximum daily dosage of carbidopa should not exceed 200 mg, since clinical experience with larger dosages is limited. If the patient is taking carbidopa-levodopa, the amount of carbidopa in carbidopa-levodopa should be considered when calculating the total amount of carbidopa to be administered each day.
Patients receiving carbidopa-levodopa who require additional carbidopa
Some patients taking carbidopa-levodopa may not have adequate reduction in nausea and vomiting when the dosage of carbidopa is less than 70 mg a day, and the dosage of levodopa is less than 700 mg a day. When these patients are taking carbidopa-levodopa, 25 mg of carbidopa may be given with the first dose of carbidopa-levodopa each day. Additional doses of 12.5 mg or 25 mg may be given during the day with each dose of carbidopa-levodopa. Carbidopa may be given with any dose of carbidopa-levodopa as required for optimum therapeutic response. The maximum daily dosage of carbidopa, given as carbidopa tablets and as carbidopa-levodopa, should not exceed 200 mg.
Patients requiring individual titration of carbidopa and levodopa dosage
Although carbidopa-levodopa is the most frequently used of carbidopa and levodopa administration, there may be an occasional patient who requires individually titrated doses of these two drugs. In these patients, carbidopa should be initiated at a dosage of 25 mg three or four times a day. The two drugs should be given at the same time, starting with no more than one-fifth (20%) to one-fourth (25%) of the previous or recommended daily dosage of levodopa when given without carbidopa. In patients already receiving levodopa therapy, at least twelve hours should elapse between the last dose of levodopa and initiation of therapy with carbidopa and levodopa. A convenient way to initiate therapy in these patients is in the morning following a night when the patient has not taken levodopa for at least twelve hours. Health care providers who prescribe separate doses of carbidopa and levodopa should be thoroughly familiar with the directions for use of each drug.
Dosage adjustment
Dosage of carbidopa may be adjusted by adding or omitting one-half or one tablet a day. Because both therapeutic and adverse responses occur more rapidly with combined therapy than when only levodopa is given, patients should be monitored closely during the dose adjustment period. Specifically, involuntary movements will occur more rapidly when carbidopa and levodopa are given concomitantly than when levodopa is given without carbidopa. The occurrence of involuntary movements may require dosage reduction. Blepharospasm may be a useful early sign of excess dosage in some patients.
Current evidence indicates other standard antiparkinsonian drugs may be continued while carbidopa and levodopa are being administered. However, the dosage of such other standard antiparkinsonian drugs may require adjustment.
Interruption of therapy
Sporadic cases of hyperpyrexia and confusion have been associated with dose reductions and withdrawal of carbidopa-levodopa or carbidopa-levodopa Extended Release. Patients should be observed carefully if abrupt reduction or discontinuation of carbidopa-levodopa or carbidopa-levodopa Extended-Release is required, especially if the patient is receiving neuroleptics.
If general anesthesia is required, therapy may be continued as long as the patient is permitted to take fluids and medication by mouth. When therapy is interrupted temporarily, the patient should be observed for symptoms resembling neuroleptic malignant syndrome (NMS), and the usual daily dosage may be resumed as soon as the patient is able to take medication orally.
For oral levodopa and carbidopa tablet dosage form:
- Adults—At first, 1 tablet three or four times a day. Your doctor may need to change your dose, depending on how you respond to this combination medicine.
- Children and teenagers—Use and dose must be determined by your doctor.
For oral extended-release levodopa and carbidopa tablet dosage form:
- Adults—At first, 1 tablet two times a day. However, you may need to take more than this. Your doctor will decide the right dose for you, depending on your condition and the other medicines you may be taking for Parkinson’s disease.
- Children and teenagers—Use and dose must be determined by your doctor.
Usual Adult Dose for Parkinsonian Tremor
Patients on carbidopa-levodopa therapy requiring additional carbidopa:
- Initial dose: 25 mg orally with the first dose of carbidopa-levodopa each day
- Maintenance dose: Additional doses of 12.5 mg or 25 mg may be given with each dose of carbidopa-levodopa
- Maximum dose: 200 mg orally per day
Patients requiring individual titration of carbidopa and levodopa:
- Initial dose: 25 mg orally three to four times a day, given at the same time as levodopa
- Maximum dose: 200 mg orally per day
Comments:
- In patients already on levodopa, allow 12 hours between the last dose of levodopa and the initiation of therapy with carbidopa and levodopa.
- If the patient is taking carbidopa-levodopa, the amount of carbidopa in carbidopa-levodopa should be considered when calculating the total daily dose.
- Blepharospasm may be used as an early sign of excess dosage in some patients.
- Therapeutic and adverse responses occur more rapidly with concomitant carbidopa and levodopa compared with levodopa given alone.
- Patients should be carefully monitored for signs and symptoms of neuroleptic malignant syndrome if treatment is abruptly reduced or discontinued.
Dose adjustments
Dose reduction may be required if there is occurrence of involuntary movements.
Concomitant levodopa:
- Most patients respond to a 1:10 proportion of carbidopa and levodopa, provided the daily dosage of carbidopa is 70 mg or more per day.
- If levodopa and carbidopa are titrated individually, levodopa should be initiated at 20% to 25% of the previous or recommended daily dosage of levodopa when taken alone.
Renal dose adjustments
- Data not available
Liver dose adjustments
- Data not available
Dialysis
- Data not available.
Carbidopa levodopa side effects
Levodopa side effects
Along with its needed effects, a medicine may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Check with your doctor as soon as possible if any of the following side effects occur:
More common
- abnormal thinking: holding false beliefs that cannot be changed by fact
- agitation
- anxiety
- clenching or grinding of teeth
- clumsiness or unsteadiness
- confusion
- difficulty swallowing
- dizziness
- excessive watering of mouth
- false sense of well being
- feeling faint
- general feeling of discomfort or illness
- hallucinations (seeing, hearing, or feeling things that are not there)
- hand tremor, increased
- nausea or vomiting
- numbness
- unusual and uncontrolled movements of the body, including the face, tongue, arms, hands, head, and upper body
- unusual tiredness or weakness
Less common
- blurred vision
- difficult urination
- difficulty opening mouth
- dilated (large) pupils
- dizziness or lightheadedness when getting up from a lying or sitting position
- double vision
- fast, irregular, or pounding heartbeat
- hot flashes
- increased blinking or spasm of eyelids
- loss of bladder control
- mental depression
- other mood or mental changes
- skin rash
- unusual weight gain or loss
Rare
- back or leg pain
- bloody or black tarry stools
- chills
- convulsions (seizures)
- fever
- high blood pressure
- inability to move eyes
- loss of appetite
- pain, tenderness, or swelling of foot or leg
- pale skin
- prolonged, painful, inappropriate penile erection
- sore throat
- stomach pain
- swelling of face
- swelling of feet or lower legs
- vomiting of blood or material that looks like coffee grounds
Some side effects may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects. Check with your health care professional if any of the following side effects continue or are bothersome or if you have any questions about them:
More common
- abdominal pain
- dryness of mouth
- loss of appetite
- nightmares
- passing gas
Less common
- constipation
- diarrhea
- flushing of skin
- headache
- hiccups
- increased sweating
- muscle twitching
- trouble in sleeping
Levodopa may sometimes cause the urine, saliva, and sweat to be darker in color than usual. The urine may at first be reddish, then turn to nearly black after being exposed to air. Some bathroom cleaning products will produce a similar effect when in contact with urine containing levodopa. This is to be expected during treatment with levodopa. Also, levodopa may cause a bitter taste, or a burning sensation of the tongue.
Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional.
Call your doctor for medical advice about side effects.
Nervous system side effects
Nervous system side effects most frequently reported have included involuntary movements and mental status changes (in as many as 50% of treated patients on long-term therapy). The types of involuntary movements due to levodopa have been characterized as choreiform, dystonic and dyskinetic. Fluctuations in motor function occur frequently and often increase as the duration of therapy increases.
Choreiform movements due to levodopa therapy may occur in as many as 80% of patients treated for one year and frequently involve facial grimacing, exaggerated chewing, and twisting and protrusion of the tongue.
Several types of motor fluctuations may occur and result in “bradykinetic episodes”. Some motor fluctuations are related to the timing of levodopa dosage administration. For example, patients may experience “peak of the dose dyskinesia” and a wearing-off effect called “end of the dose akinesia”. The “wearing-off effect may result in early morning dystonia. Such motor fluctuations may be managed by increasing the frequency of dosage administration and decreasing the dose administered to achieve a smoother therapeutic effect.
Other motor fluctuations are not related to the timing of dose administration. Such fluctuations are characterized by sudden loss of levodopa effect which may last for minutes to hours and result in akinesia followed by a sudden return of levodopa effect. These “on-off” fluctuations may occur many times per day. “On-off” fluctuations may respond to more frequent dose administration.
Finally, akinesia paridoxica is a sudden episode of akinesia which occurs as patients begin to walk. Akinesia paridoxica frequently results in falls and often responds to levodopa dose reductions.
Other adverse nervous system effects due to levodopa include myoclonus, sleep disturbances (including insomnia, daytime somnolence, altered dreams and episodic nocturnal myoclonus), Meige’s syndrome (blepharospasm-oromandibular dystonia) and ocular dyskinesia. In addition, the orofacial movements induced by levodopa have occasionally been reported to cause severe dental erosion.
Some investigators have suggested that levodopa may cause brain dysfunction and may have negative effects on cognitive performance. Levodopa “drug holidays” have been proposed by some investigators as potentially beneficial (perhaps by causing dopamine receptor resensitization). However, the therapeutic value of these drug holidays is controversial.
Gastrointestinal side effects
Exacerbation of preexisting stomach ulcer disease with severe upper gastrointestinal bleeding has been reported.
Gastrointestinal side effects most commonly reported have included nausea and vomiting. Anorexia and gastrointestinal hemorrhage have been reported rarely.
Psychiatric side effects
Some authors have suggested that clozapine may be useful in the management of levodopa-induced psychotic symptoms.
Other investigators have suggested that levodopa may induce alterations in the noradrenergic systems of the CNS which may lead to panic attacks.
Psychiatric side effects have included hallucinations (particularly visual hallucinations), psychosis, confusion, anxiety, mania, hypomania, depression, rapid mood cycling, nightmares, and hypersexuality.
Neuroleptic malignant syndrome
Fever, altered consciousness, autonomic dysfunction and muscle rigidity are the hallmarks of the neuroleptic malignant syndrome. The neuroleptic malignant syndrome (NMS) is associated with a case fatality rate of about 20%. If withdrawal of dopaminergic therapy is suspected as the cause of NMS (neuroleptic malignant syndrome), dopaminergic therapy should be restarted. If a neuroleptic agent is suspected as the cause, the neuroleptic agent should be immediately discontinued. For patients with NMS (neuroleptic malignant syndrome) suspected to be due to neuroleptic therapy, consideration should be given to dantrolene (or bromocriptine) administration. Intensive monitoring and supportive care are indicated for all patients with NMS (neuroleptic malignant syndrome).
Sudden discontinuation or rapid tapering of levodopa therapy may result in acute worsening of parkinsonism or less frequently, in a syndrome resembling the neuroleptic malignant syndrome. Cases of psychologic levodopa addiction have also been reported rarely.
Cardiovascular side effects
Some authors have reported marked hemodynamic and clinical improvements in patients with congestive heart failure treated with oral levodopa. However, at least one author has reported marked hemodynamic deterioration following such treatment.
Cardiovascular side effects have included hypotension and syncope. Arrhythmias have also been reported rarely.
Dermatologic side effects
Dermatologic side effects have included a number of cases of malignant melanoma in patients taking levodopa for Parkinson’s Disease. Additionally, several cases of maculopapular skin rashes have been reported in patients taking levodopa-containing drugs.
Despite reports of melanoma occurring in levodopa-treated patients, some authors have suggested that a causal association is tenuous and other authors have suggested that levodopa may have an antitumor effect on melanoma. Nevertheless, the manufacturers of levodopa-containing drugs report that either the history of melanoma or the presence of suspicious skin lesions is a contraindication for the use of levodopa-containing drugs.
Immunologic side effects
Immunologic side effects have included rare reports of a lupus-like syndrome.
Hematologic side effects
Hematologic side effects reported rarely have included severe hemolytic and nonhemolytic anemias.
Respiratory side effects
Respiratory side effects have included dyskinesias (occasionally of life-threatening severity).
Liver side effects
Hepatic side effects have included rare cases of asterixis (without abnormalities of liver function tests). The manufacturer of levodopa-containing products reports that abnormal liver function tests may occur.
Endocrine side effects
Endocrine side effects have included elevated urinary vanillylmandelic acid levels which have occasionally led to confusion concerning the diagnosis of pheochromocytoma.
Kidney side effects
Renal side effects have included hypokalemia and hyponatremia. Chronic administration of levodopa may also slightly but significantly increase BUN without changes in the glomerular filtration rate.
Carbidopa side effects
Adverse reactions are associated with concomitant administration with levodopa.
Get emergency medical help if you have signs of an allergic reaction: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
The following side effects may occur when carbidopa is taken with levodopa.
Cardiovascular: Cardiac arrhythmia, chest pain, edema, flushing, hypertension, hypotension, myocardial infarction, orthostatic hypotension, palpitation, phlebitis, syncope
Central nervous system: Abnormal dreams, abnormal gait, agitation, anxiety, ataxia, confusion, decreased mental acuity, delusions, dementia, depression (with or without suicidal tendencies), disorientation, dizziness, drowsiness, euphoria, extrapyramidal reaction, falling, fatigue, glossopyrosis, hallucination, headache, Horner’s syndrome, impulse control disorder, insomnia, malaise, memory impairment, nervousness, neuroleptic malignant syndrome, nightmares, numbness, on-off phenomenon, paranoia, paresthesia, pathological gambling, peripheral neuropathy, psychosis, seizure (causal relationship not established), trismus
Dermatologic: Alopecia, bulla, diaphoresis, discoloration of sweat, skin rash
Endocrine and metabolic: Abnormal lactate dehydrogenase, glycosuria, hot flash, hyperglycemia, hypokalemia, increased libido (including hypersexuality), increased uric acid, weight changes
Gastrointestinal: Abdominal distress, abdominal pain, anorexia, bruxism, constipation, diarrhea, discoloration of saliva, duodenal ulcer, dysgeusia, dyspepsia, dysphagia, flatulence, gastrointestinal hemorrhage, heartburn, hiccups, nausea, sialorrhea, sore throat, vomiting, xerostomia
Genitourinary: Priapism, proteinuria, urinary frequency, urinary incontinence, urinary retention, urinary tract infection, urine discoloration
Hematologic and oncologic: Abnormal Coombs’ test, agranulocytosis, anemia, decreased hematocrit, decreased hemoglobin, hemolytic anemia, leukopenia, malignant melanoma, thrombocytopenia
Hepatic: Abnormal alanine aminotransferase, abnormal alkaline phosphatase, abnormal aspartate transaminase, abnormal bilirubin levels, abnormal lactate dehydrogenase
Hypersensitivity: Angioedema, hypersensitivity reaction (bulla, IgA vasculitis, pruritus, urticaria)
Neuromuscular and skeletal: Back pain, dyskinesia (including choreiform, dystonic, and other involuntary movements), leg pain, muscle cramps, muscle twitching, shoulder pain, tremor, weakness
Ophthalmic: Blepharospasm, blurred vision, diplopia, mydriasis, oculogyric crisis (may be associated with acute dystonic reactions)
Renal: Increased blood urea nitrogen, increased serum creatinine
Respiratory: Cough, dyspnea, hoarseness, upper respiratory tract infection
Call your doctor at once if you have:
- uncontrolled muscle movements in your face (chewing, lip smacking, frowning, tongue movement, blinking or eye movement);
- worsening of tremors (uncontrolled shaking);
- severe nausea, vomiting, or diarrhea;
- confusion, hallucinations, unusual changes in mood or behavior;
- depression or suicidal thoughts;
- seizure (convulsions); or
- severe nervous system reaction–very stiff (rigid) muscles, high fever, sweating, confusion, fast or uneven heartbeats, tremors, feeling like you might pass out.
Some people taking carbidopa with levodopa have fallen asleep during normal daytime activities such as working, talking, eating, or driving. Tell your doctor if you have any problems with daytime sleepiness or drowsiness.
You may have increased sexual urges, unusual urges to gamble, or other intense urges while taking this medicine. Talk with your doctor if this occurs.
You may notice that your sweat, urine, or saliva appears dark in color, such as red, brown, or black. This is not a harmful side effect, but it may cause staining of your clothes or bed sheets.
Common side effects may include:
- nausea, upset stomach;
- headache, dizziness;
- sleep problems (insomnia), dreaming more than usual;
- dry mouth, burning feeling in your tongue;
- weight changes; or
- abnormal liver function tests.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects.
Nervous system side effects
Symptoms related to neuroleptic malignant syndrome are characterized by fever or hyperthermia. Other findings include neurological symptoms such as muscle rigidity, involuntary movements, altered consciousness, mental status changes, other disturbances such as autonomic dysfunction, tachycardia, tachypnea, sweating, hyper- or hypotension, and laboratory findings such as elevated creatine phosphokinase, leukocytosis, myoglobinuria, and increased serum myoglobin.
A causal relationship with concomitant carbidopa and levodopa and the reported side effect of convulsions has not been established.
Frequency not reported: Bradykinetic episodes (“on-off” phenomenon), convulsions, dizziness, dyskinesias such as choreiform, dystonic and other involuntary movements, headache, neuroleptic malignant syndrome, oculogyric crises, paresthesia, peripheral neuropathy, somnolence, syncope, taste alterations
Psychiatric side effects
Frequency not reported: Agitation, bruxism, confusion, dementia, depression with or without development of suicidal tendencies, dream abnormalities (including nightmares), impulse control symptoms, increased libido (including hypersexuality), insomnia, pathological gambling, psychotic episodes (including delusions, hallucinations and paranoid ideation)
Psychiatric side effects were reported with concomitant administration of levodopa or carbidopa-levodopa.
Cardiovascular side effects
Frequency not reported: Cardiac irregularities, chest pain, hot flashes, hypertension, hypotension (including orthostatic hypotension), myocardial infarction, palpitation, phlebitis
Gastrointestinal side effects
Frequency not reported: Abdominal pain and distress, burning sensation of the tongue, constipation, dark saliva, development of duodenal ulcer, diarrhea, dry mouth, dyspepsia, dysphagia, flatulence, gastrointestinal bleeding, gastrointestinal pain, heartburn, hiccups, nausea, sialorrhea, vomiting
Hematologic side effects
Frequency not reported: Abnormal Coombs test, agranulocytosis, decreased hemoglobin and hematocrit, decreased white blood cell count, hemolytic and nonhemolytic anemia, leukopenia, thrombocytopenia
Metabolic side effects
Frequency not reported: Abnormal lactic dehydrogenase, anorexia, edema, elevated serum glucose, elevated serum potassium, weight gain, weight loss
Musculoskeletal side effects
Frequency not reported: Back pain, leg pain, muscle cramps, shoulder pain
Respiratory side effects
Frequency not reported: Bizarre breathing patterns, cough, dyspnea, pharyngeal pain, upper respiratory infection
Dermatologic side effects
Frequency not reported: Alopecia, angioedema, bullous lesions (including pemphigus-like reactions), dark sweat, flushing, Henoch-Schonlein purpura, increased sweating, malignant melanoma, pruritus, rash, urticaria
Ocular side effects
Frequency not reported: Blurred vision, dilated pupils, diplopia
Genitourinary side effects
Frequency not reported: Dark urine, priapism, urinary frequency, urinary incontinence, urinary retention, urinary tract infection
Other side effects
Frequency not reported: Asthenia, faintness, fatigue, hoarseness, malaise, sense of stimulation
Hepatic side effects
Frequency not reported: Abnormalities in alkaline phosphatase, SGOT (AST), SGPT (ALT), and bilirubin.
Renal side effects
Frequency not reported: Abnormal blood urea nitrogen (BUN), increased serum creatinine and uric acid, protein and glucose in the urine, white blood cells, bacteria and blood in the urine,
Carbidopa levodopa side effects long term
One of the complications of long‐term treatment of Parkinson’s disease with levodopa is the development of motor complications e.g. dyskinesia; a jerky, dance‐like movement of the body. Generally clinicians add on drugs (to the levodopa regimen) from one of the other three classes of anti‐Parkinsonian treatments available (e.g. dopamine agonists, catechol‐O‐methyl transferase inhibitors or monoamine oxidase type B inhibitors). However, despite clinical trials having shown that these drugs are beneficial compared to placebo, it remains unclear as to the best way to treat patients experiencing motor complications and in particular, whether one class of drug may be more effective than another.
Carbidopa levodopa overdose
Spasm or closing of eyelids are possible early sign of overdose. Nausea, vomiting, cardiac arrhythmias, involuntary movements of the body, including the face, tongue, arms, hand, head, and upper body; choreiform and other involuntary movements occur in 50% to 80% of patients. This effect is dose related.
Acute levodopa overdose was reported in a 61-yr old man following ingestion of 100 g of levodopa. He experienced hypertension initially, followed by hypotension for a few hrs, mild nausea, & severe anorexia that gradually resolved over 1 week. He had insomnia for about 1 week, confusion for 2 days and agitation for 3-4 days 2.
In the event of overdosage, general supportive measures should be employed, along with immediate gastric lavage. Intravenous fluids should be administered judiciously, and an adequate airway maintained. Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in respiratory arrest. Positive pressure ventilation techniques with a bag valve mask device may be beneficial. Monitor cardiac rhythm and treat arrhythmias as necessary. Electrocardiographic monitoring should be instituted and the patient carefully observed for the development of arrhythmias; if required, appropriate antiarrhythmic therapy should be given. The possibility that the patient may have taken other drugs as well as carbidopa should be taken into consideration. To date, no experience has been reported with dialysis; hence, its value in overdosage is not known. Pyridoxine is not effective in reversing the actions of carbidopa.
Based on studies in which high doses of levodopa and/or carbidopa were administered, a significant proportion of rats and mice given single oral doses of levodopa of approximately 1500-2000 mg/kg are expected to die (levodopa LD50 mouse oral 2363 mg/kg). A significant proportion of infant rats of both sexes are expected to die at a dose of 800 mg/kg. A significant proportion of rats are expected to die after treatment with similar doses of carbidopa. The addition of carbidopa in a 1:10 ratio with levodopa increases the dose at which a significant proportion of mice are expected to die to 3360 mg/kg.
- Schapira AHV. Parkinson’s disease. BMJ : British Medical Journal. 1999;318(7179):311-314. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1114782/[↩]
- Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn’s Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 879[↩]