Contents
What is macular hole
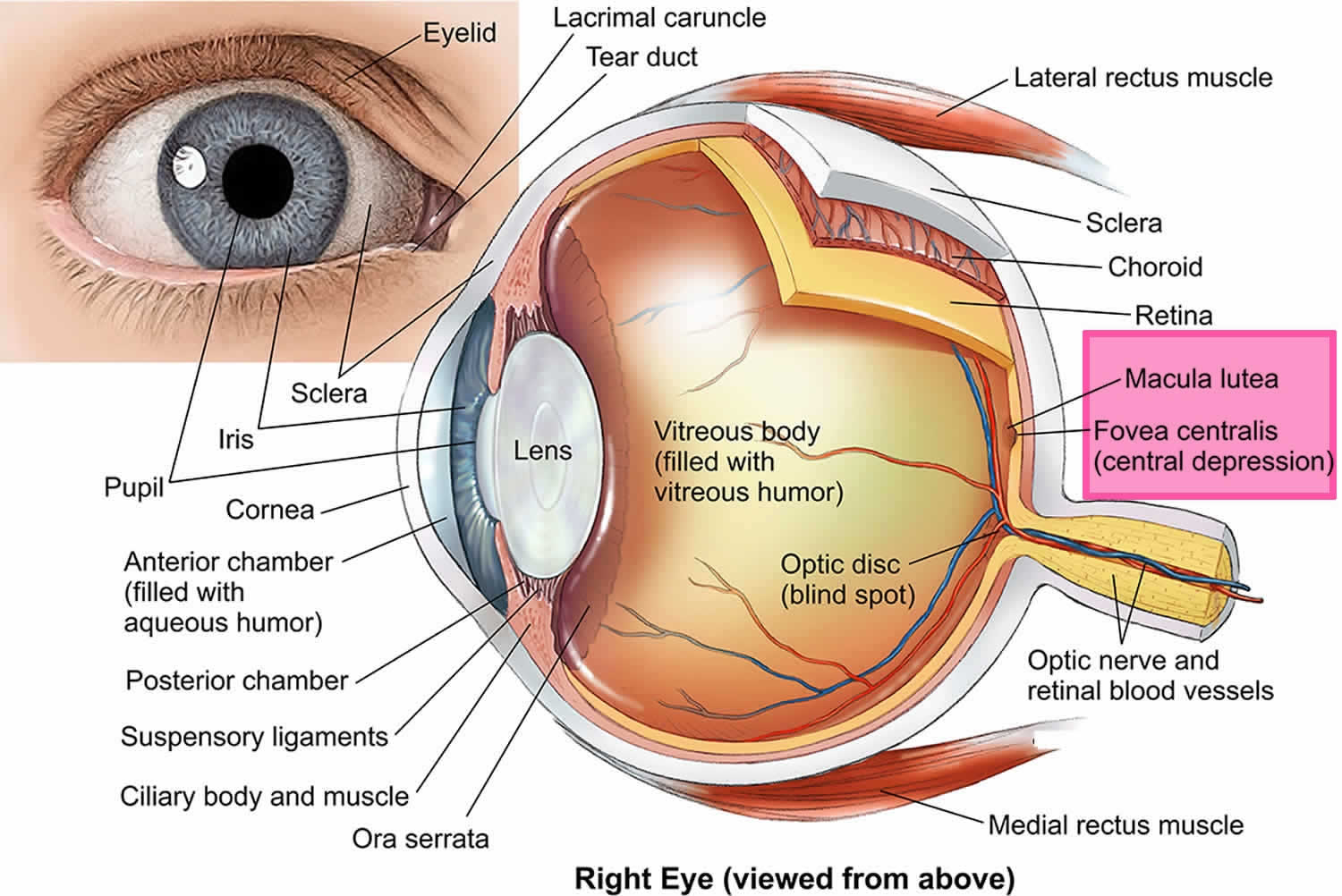
A macular hole is a small break in the macula, located in the center of your eye’s light-sensitive tissue called the fovea (the part of the eye that is responsible for sharp vision) and causes a small dark spot in the central vision, often preventing those with the condition from recognizing very small objects, and particularly from reading ordinary print. Macular holes can be seen in people with highly myopic eyes (who cannot see clearly in the distance) or following ocular trauma, but in the great majority of cases the cause is unknown (idiopathic).
The macula is responsible for your straight ahead vision, which provides the sharp, central vision you need for reading, driving, and seeing fine detail. While reading the print on this page, you are using your macula. The cornea and lens in the front of your eye focus the image of these words onto your macula. The macula converts the light from the image on this page into a neurological signal that is processed by your brain. A macular hole can cause blurred and distorted central vision. Macular holes are related to aging and usually occur in people over age 60.
Since the very center of the macular is the thinnest part of the retina, it is also relatively weak. Some patients have an abnormally tight attachment between the vitreous gel that fills the eye and the center of the macula. When the vitreous gel contracts with aging, or after an injury, some people can develop a macular hole. Interestingly, 90% of macular holes occur in females. The reason for the female predilection for macular holes is unknown.
Figure 1. Macular hole
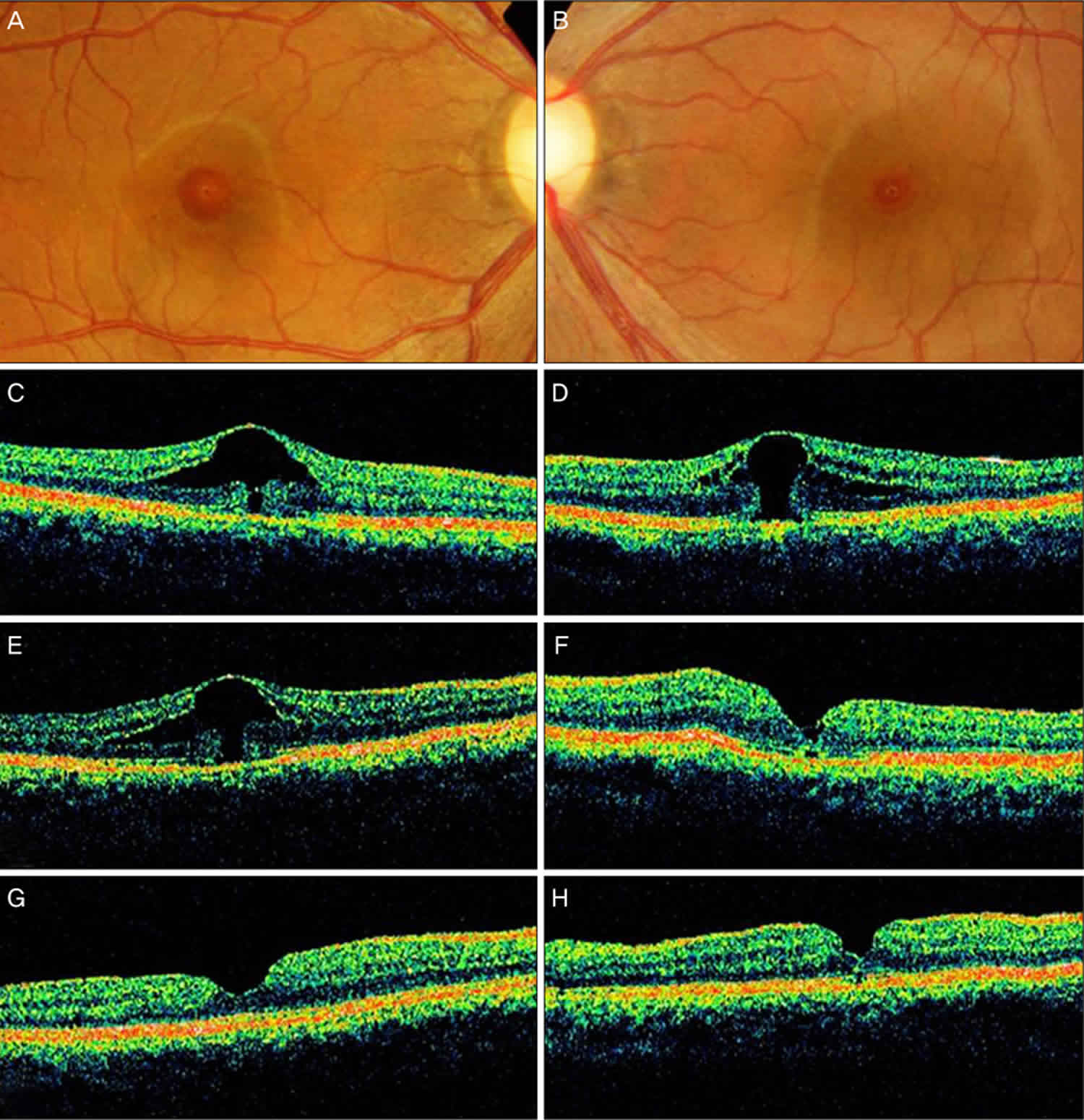
Footnote: A 36-year-old woman had complained of decreased vision for several months. Her past medical history included a radical mastectomy for breast carcinoma. After mastectomy, she had been started on toremifen, at a daily dose of 40 mg. Her visual acuity (VA) was 20/32 OD and 20/70 OS. No anterior segment abnormalities were detected. Fundus photograph and optical coherence tomographic images of both her eye. (A,C) At presentation, the image shows foveal cystic changes with outer retinal defect in the patient’s right eye. (B,D) At presentation, the image shows a stage 1b macular hole in the left eye. (E) The enlargement of outer foveal defect is noted in the right eye 4 months after initial presentation, with further deterioration of vision. (F) However, improved foveal contour and vision is noted after macular hole surgery in the left eye. (G,H) The closure of macular hole is achieved in both eyes after completing the surgery in both eyes.
The macular hole was successfully closed (Figure F). Fundus examination revealed aggravated cystic changes in the foveal center OD. Her VA was 20/125 OD and 20/32 OS at that time. An OCT scan of her right eye revealed an enlarged outer retinal defect (Figure E). Her right eye was also treated via pars plana vitrectomy, ILM peeling, and SF6 gas tamponade. She had discontinued her toremifen regimen in October 2005. In January 2006, a fundus examination and OCT scan revealed the successful closure of the macular hole (Figures G and H), and her visual acuity (VA) was improved to 20/30 OD and to 20/25 OS.
[Source 1 ]Figure 2. Human eye anatomy
No. Macular holes and age-related macular degeneration are two separate and distinct conditions, although the symptoms for each are similar. Both conditions are common in people 60 and over. An eye care professional will know the difference.
Macular hole stages
There are three stages to a macular hole:
- Foveal detachments (Stage I). Without treatment, about half of Stage I macular holes will progress.
- Partial-thickness holes (Stage II). Without treatment, about 70 percent of Stage II macular holes will progress.
- Full-thickness holes (Stage III).
The size of the hole and its location on the retina determine how much it will affect a person’s vision. When a Stage III macular hole develops, most central and detailed vision can be lost. If left untreated, a macular hole can lead to a detached retina, a sight-threatening condition that should receive immediate medical attention.
Macular hole causes
Most of the eye’s interior is filled with vitreous, a gel-like substance that fills about 80 percent of the eye and helps it maintain a round shape. The vitreous contains millions of fine fibers that are attached to the surface of the retina. As you age, the vitreous slowly shrinks and pulls away from the retinal surface. Natural fluids fill the area where the vitreous has contracted. This is normal. In most cases, there are no adverse effects. Some patients may experience a small increase in floaters, which are little “cobwebs” or specks that seem to float about in your field of vision.
However, if the vitreous is firmly attached to the retina when it pulls away, it can tear the retina and create a macular hole. Also, once the vitreous has pulled away from the surface of the retina, some of the fibers can remain on the retinal surface and can contract. This increases tension on the retina and can lead to a macular hole. In either case, the fluid that has replaced the shrunken vitreous can then seep through the hole onto the macula, blurring and distorting central vision.
Macular holes can also occur in other eye disorders, such as high myopia (nearsightedness), injury to the eye, retinal detachment, and, rarely, macular pucker.
If a macular hole exists in one eye, there is a 10-15 percent chance that a macular hole will develop in your other eye over your lifetime. Your doctor can discuss this with you.
Trauma
Of patients experiencing a contusion injury of the eye, 6% develop a macular hole following the trauma.
Trauma is also commonly associated with commotio retinae involving the macula, subretinal hemorrhage, and intraretinal hemorrhage.
Progressive high myopia (foveal schisis)
Patients with high myopia may develop foveal schisis and/or lamellar holes, which can progress to a full-thickness macular hole.
Of those patients in whom foveal schisis identified, 31% developed macular holes.
Tanaka et al 2 reviewed 24 eyes of 21 consecutive patients with lamellar holes and high myopia. Over a mean follow-up of 19.2 months, these lamellar macular holes remained very stable; only one eye progressed to a full-thickness macular hole.
Risk factors include axial eye length, macular chorioretinal atrophy, and vitreoretinal interface factors.
Preceding rhegmatogenous retinal detachment
Less than 1% of patients with a successfully repaired rhegmatogenous retinal detachment will present with a subsequent macular hole.
Vitreoretinal traction theory (idiopathic macular holes)
Vitreous syneresis results in shrinkage of cortical vitreous and traction on the fovea.
Macular hole symptoms
Patients with idiopathic macular holes present with a variety of symptoms. Macular holes often begin gradually. In the early stage of a macular hole, people may notice a slight distortion or blurriness in their straight-ahead vision or metamorphopsia. Straight lines or objects can begin to look bent or wavy. Reading and performing other routine tasks with the affected eye become difficult. Patients may characterize these symptoms as being mild and only apparent when reading or driving. Because the initial changes may be mild and gradual, it may be some time before the patient discovers that something is wrong with their vision. Macular holes may only be discovered when patients cover one eye and notice blurred vision and metamorphopsia in the opposite eye.
Rarely, some patients may describe the exact moment at which the hole developed, but more commonly, they describe the onset as slow and gradual if at all noticeable.
Later, a larger macular hole may produce a central defect, or scotoma, in the central vision of the patient.
Some patients may be asymptomatic, and the hole is diagnosed only on routine ophthalmologic examination.
Macular hole diagnosis
The visual acuity of the patient varies according to the size, location, and the stage of the macular hole. Patients with small, eccentric holes may retain excellent visual acuity in the range of 20/25 to 20/40. In addition, a macular hole that is not full thickness can have very good visual acuity in the range of 20/30 to 20/50. However, once a macular hole is well developed or full thickness, the usual range of visual acuity is from 20/80 to 20/400, averaging at 20/200.
Direct ophthalmoscopy
A full-thickness macular hole visualized with direct ophthalmoscopy is characterized by a well-defined round or oval lesion in the macula with yellow-white deposits at the base. These yellow dots probably represent lipofuscin-laden macrophages or nodular proliferations of the underlying pigment epithelium with associated eosinophilic material (see Figure 1 above).
Biomicroscopic (slit lamp) examination
With biomicroscopic (slit lamp) examination, a round excavation with well-defined borders interrupting the beam of the slit lamp can be observed.
In most patients, an overlying semitranslucent tissue, representing the pseudo-operculum, can be seen suspended over the hole. There is often a surrounding cuff of subretinal fluid (see Figure 1 above).
Cystic changes of the retina also may be evident at the margins of the hole. The retinal pigment epithelium is usually intact and normal in acute stages but may undergo chronic changes, such as atrophy and hyperplasia, with time.
Fine crinkling of the inner retinal surface caused by an epiretinal membrane may be present and sometimes may even distort the appearance of the hole.
Watzke-Allen and the laser aiming beam tests
The most useful diagnostic tests for ophthalmologists to distinguish full-thickness macular holes from other lesions are the Watzke-Allen and the laser aiming beam tests.
The Watzke-Allen test is performed at the slit lamp using a macular lens and placing a narrow vertical slit beam through the fovea. A positive test is elicited when patients detect a break in the bar of light that they perceive. This reaction is due to the fact that there is a lack of retinal material in the area of the hole, thus producing a central defect or scotoma. Narrowing or distortion of the bar of light is not diagnostic of full-thickness macular holes and should be interpreted with caution.
The laser aiming beam test also is performed similarly, but this time a small 50-µm spot size laser aiming beam is placed within the lesion. A positive test is obtained when the patient fails to detect the aiming beam when it is placed within the lesion but is able to detect it once it is placed onto normal retina.
In addition, some slit lamps are equipped with a setting to project a small test object, often a star, onto the fovea. Again, the patient is asked whether they perceive the test object.
Imaging Studies
Ocular coherence tomography (OCT)
Ocular coherence tomography (OCT) allows high-resolution cross-sectional imaging of the retina. OCT allows the physician to detect the presence of a macular hole as well as changes in the surrounding retina.
OCT can distinguish lamellar holes and cystic lesions of the macula from macular holes.
Also, the status of the vitreomacular interface can be evaluated. This allows the clinician to evaluate the earliest of the stages of a macular hole as well as evaluate for other vision-limiting conditions associated with macular holes, such as a surrounding cuff of subretinal fluid.
Fluorescein angiography
Fluorescein angiography (FA) may be a useful test in differentiating macular holes from masquerading lesions, such as cystoid macular edema (CME) and choroidal neovascularization (CNV).
Full-thickness stage 3 macular holes typically produce a window defect early in the angiogram and do not expand with time. The arteriovenous phase of the angiogram best demonstrates a granular hyperfluorescent window associated with the overlying pigment layer changes.
No leakage or accumulation of dye is observed as opposed to other lesions.
In cystoid macular edema (CME), a gradual accumulation of dye occurs in the cystoid spaces, eventually demonstrating a petaloid appearance late in the angiogram.
B-scan ultrasonography
B-scan ultrasonography may be helpful in elucidating the relationship of the macula to the vitreous; therefore, it may be helpful in staging macular hole but is not sensitive to distinguish a true macular hole from masquerading lesions.
Other Tests
Amsler grid abnormalities, although sensitive for macular lesions, are not specific for macular holes. Plotting of small central scotomas caused by full-thickness macular holes using the Amsler grid is difficult because of the poor fixation in the affected eye. However, bowing of the lines and micropsia frequently are appreciated. This could be attributable to the surrounding area of retinal edema and intraretinal cysts, which could be seen in macular holes as well as other lesions like choroidal neovascularization (CNV).
Microperimetry and multifocal electroretinography have also been used to evaluate patients with idiopathic macular holes. These studies show loss of retinal function corresponding to the macular hole with subsequent recovery of function following surgical repair of the hole.
Fundus autofluorescence results are abnormal in patients with full-thickness macular holes. There is a strong subfoveal autofluorescence signal in full-thickness macular holes that resolves upon anatomic hole closure 3.
Macular hole treatment
Case reports exist that describe the use of autologous plasmin for idiopathic and traumatic macular holes. Ongoing clinical trials are evaluating the role of plasmin as a means of “chemovitrectomy.” In these studies, case illustrations have demonstrated resolution of idiopathic macular holes following intravitreal injection of plasmin and no surgical intervention.
In October 2012, ocriplasmin (Jetrea) was approved by the US Food and Drug Administration (FDA) for the treatment of vitreomacular adhesion. Ocriplasmin is a recombinant proteolytic enzyme that underwent study by the MIVI-TRUST study group. This protease demonstrated activity against fibronectin and laminin. In a randomized, double-blind study, 652 eyes with vitreomacular adhesion were treated with an intravitreal injection of ocriplasmin. A secondary endpoint of the study was nonsurgical closure of a full-thickness macular hole (FTMH), which can result from vitreomacular adhesion. In the study, 40.6% of treated eyes experienced nonsurgical closure of the macular hole compared to 10.6% in the placebo group. FDA approval was based on this study. This injectable medication provides a nonsurgical means of treating macular holes 4.
A 2018 study 5 suggests slightly higher closure rates for full-thickness macular hole following ocriplasmin use. They described 6 patients with vitreomacular traction and a full-thickness macular hole. By 24 weeks’ of follow-up, four of the six full-thickness macular holes had closed. Other studies have confirmed a relatively low rate of macular hole closure even with release of vitreomacular traction despite careful selection of treatment candidates 6. One retrospective study suggests that only approximately 18% of patients with macular hole could be favorable candidates for ocriplasmin treatment 7.
There are concerns with the potential for retinal toxicity from the use of ocriplasmin. There is evidence of potential retinal toxicity associated with the use of ocriplasmin. Anatomic changes in the outer segments were seen in 7 of 17 patients reported in a series by Singh et al 8. Multiple case reports have also evaluated individual patients with ancillary studies such as electroretinography and perimetry 9. Fahim et al 10 suggest that the retinal dysfuction associated with ocriplasmin may be related to the enzymatic cleavage of intraretinal laminin. Subretinal deposits have also been reported following successful release of vitreomacular traction 11.
Macular hole surgery
Although some macular holes can seal themselves and require no treatment, surgery is necessary in many cases to help improve vision. Indications for consideration of the surgical management of macular holes are based on the presence of a full-thickness defect. Once this defect has developed, the potential for spontaneous resolution is low. Thus, surgical management is recommended with documentation of a stage 2 or higher, full-thickness macular hole. Stage 1 macular holes and lamellar holes are managed conservatively with observation at this time.
Pars plana vitrectomy has been used for more than a decade to treat full-thickness macular holes, which if left untreated cause a blind spot in central vision that only rarely improve naturally. Vitrectomy is a surgical technique involving the removal of the vitreous body (the clear gel that fills the eye). The surgeon inserts thin tubes called cannulas into the eyes through scleral (white part of the eye) incisions or incision of the eye wall to relieve traction exerted by the vitreous to the central retina and close the hole.
In this surgical procedure–called a vitrectomy–the vitreous gel is removed to prevent it from pulling on the retina and replaced with a bubble containing a mixture of air and gas. The bubble acts as an internal, temporary bandage that holds the edge of the macular hole in place as it heals. Surgery is performed under local anesthesia and often on an out-patient basis.
A prospective, randomized, and controlled series by the Vitrectomy for Treatment of Macular Hole Study Group for stage 2, 3, and 4 macular holes showed that vision was improved in surgically treated eyes compared with observed eyes. However, more frequent adverse effects were observed in the surgically treated eyes compared to the control eyes, with the most common adverse effects being macular retinal pigment epithelium changes and cataract development (cataractogenesis).
Some aspects of the macular hole surgery may vary, but the basic technique is the same. The anterior and middle vitreous is removed via a standard 3-port pars plana vitrectomy. Patients with macular hole frequently undergo vitrectomy using smaller gauge vitrectomy systems (ie, 27 gauge, 25 gauge, 23 gauge). Associated instruments have been developed for these smaller gauge, transconjunctival vitrectomy systems.
The critical step appears to be the removal of the perimacular traction. Factors contributing to this traction, such as the posterior hyaloid, the internal limiting membrane, and coexisting epimacular membranes, need to be addressed. The traction exerted by the posterior hyaloid on the macula should be relieved by either removing just the perimacular vitreous or combining it with the induction of a complete posterior vitreous detachment. Various surgical techniques have been described to accomplish this task, including the use of a soft-tipped silicon cannula or the vitrectomy cutter with the cutter disengaged. A “fish-strike sign” or bending of the silicon cannula has been described as a sign that the posterior hyaloid has been engaged. Then, it may be released from the underlying retina and removed with the vitrectomy cutter.
The removal of internal limiting membrane is considered to be a contributing factor in the success of macular hole surgeries. Its removal is also associated with a reduced risk of subsequent reopening of the macular hole 12. Internal limiting membrane peeling may be accomplished via a “rhexis” not unlike that of a capsulorrhexis in lens surgeries. Very fine forceps may be used to peel the internal limiting membrane from the underlying retina. Care should be taken not to include the deeper layers in the forceps’ grasp, which may further damage the surrounding retinal tissues. Currently, many surgeons use vital dyes such as indocyanine green or trypan blue to stain the internal limiting membrane, making it easier to visualize and manipulate. Triamcinolone acetonide is also used to assist with visualization of the internal limiting membrane for peeling.
Use of an inverted internal limiting membrane flap was first described in 2010 for repairing large macular holes 13. This technique involves reflecting the internal limiting membrane peeled from around the hole back over the hole so that it is covered with inverted internal limiting membrane tissue. Many authors 14, 15, 16 suspect that this technique would be beneficial for large macular holes and macular holes in patients with high myopia (ie, holes that have a low rate of closure with standard internal limiting membrane peeling techniques).
The Manchester Large Macular Hole Study 15 showed that the standard internal limiting membrane peeling was very effective for macular holes up to 650 microns. The closure rate decreased from more than 90% for holes smaller than 650 microns to only 76% for holes larger than 650 microns. The authors suggested using inverted internal limiting membrane flaps for these large holes. Rizzo et al 17 demonstrated a significant difference in hole closure rates for patients with axial eye lengths of more than 26mm (39% with internal limiting membrane peeling vs 88% with internal limiting membrane flap), as well as a less dramatic benefit for macular holes of more than 400 microns (79% with internal limiting membrane peeling vs 96% with internal limiting membrane flap).
Epiretinal membranes, if present, also should be removed. Techniques in completing this procedure vary from surgeon to surgeon.
After careful indirect ophthalmoscopic examination of the peripheral retina for tears, a total air-fluid exchange is performed to desiccate the vitreous cavity. A nonexpansile concentration of a long-acting gas is exchanged for air. Studies have shown that a longer period of internal tamponade equated to a higher success rate.
Sterile air and varying concentrations of either perfluoropropane or sulfur hexafluoride have been used based on surgeon preference for internal tamponade. The primary difference achieved using different gases is the duration of the gas bubble and, consequently, the amount of internal tamponade achieved within the first several days after surgery. Silicone oil has also been used as an internal tamponade for patients with difficulty positioning or altitude restrictions. However, the use of silicone oil necessitates a second subsequent surgery to remove the oil. Furthermore, the visual results are not comparable to the use of gas tamponade, possibly as a result of silicone oil toxicity at the level of the photoreceptors and retinal pigment epithelium layer (RPE).
Tafoya et al 18 showed, at 1 year, a final postoperative visual acuity difference of 20/96 (LogMAR 0.208) for silicone oil eyes versus 20/44 (LogMAR 0.453) for gas treated eyes. Lai et al 19 also showed the visual acuity advantage of gas tamponade with a smaller difference (20/70 vs 20/50). However, Lai et al also showed the rate of single operation closure being only 65% for silicone oil and 91% for gas tamponade 19. Thus, unless limited by patient circumstances, gas tamponade for macular hole repair is preferable to silicone oil tamponade.
Macular hole surgery recovery
Following surgery, patients must remain in a face-down position, normally for a day or two but sometimes for as long as two-to-three weeks. This position allows the bubble to press against the macula and be gradually reabsorbed by the eye, sealing the hole. As the bubble is reabsorbed, the vitreous cavity refills with natural eye fluids.
Maintaining a face-down position is crucial to the success of the macular hole surgery. Because this position can be difficult for many people, it is important to discuss this with your doctor before macular hole surgery.
If you cannot remain in a face-down position for the required period after macular hole surgery, vision recovery may not be successful. People who are unable to remain in a face-down position for this length of time may not be good candidates for a vitrectomy. However, there are a number of devices that can make the “face-down” recovery period easier on you. There are also some approaches that can decrease the amount of “face-down” time. Discuss these with your doctor.
Face-down positioning controversy
Historically, strict face-down positioning had been recommended for patients for up to 4 weeks, with consequent difficulties of compliance and patient quality of life during that period.
More evaluation placed into shorter periods of face-down positioning, though, traditionally, it has been believed that the shorter the period of face-down positioning, the lower the rate of successful hole closure. In 1997, Tournambe et al 20 described a pilot study of patients without face-down positioning. They reported a success rate with one surgery of 79% and suggested that pseudophakia was necessary for consideration of liberalization of positioning requirements.
The advent of internal limiting membrane peeling has encouraged a second look at minimal to no face-down positioning. Rubinstein et al 21 described a case series of 24 eyes of patients who underwent internal limiting membrane peeling and then did not position postoperatively. In this case series 21, 22 eyes were successfully closed and had visual improvement with both eyes that failed being stage 4 large holes.
Others have reported comparable, if not better, results in patients with only 1 day of positioning. Dhawahir-Scala et al 22 suggests that a critical factor is the size of the gas bubble on postoperative day 1 being greater than 70%. Tranos et al 23 showed, however, that there may be more rapid progression of cataract formation with less face-down positioning. Tranos et al 23 were among several authors who recommended combined phacovitrectomy for phakic patients to allow less stringent positioning requirements. Iezzi and Kapoor 24 reported a series of 68 eyes that underwent macular hole repair with broad internal limiting membrane peeling and SF6 tamponade without any face-down positioning. They reported a 100% rate of closure with a single procedure.
Alberti and Ia Cour 25 compared face-down positioning with nonsupine positioning and found equivalent macular hole closure rates and noninferiority of nonsupine positioning. However, they also identified that gas fill in nonsupine positioning correlated with macular hole closure. These authors 25 used perfluoropropane and internal limiting membrane peeling in their study.
A different study assessed face-down positioning and withholding face-down positioning, as well as using a shorter-acting gas such as sulfurhexafluoride 26. These authors determined that withholding face-down positioning (essentially nonsupine positioning) was noninferior to face-down positioning and that sulfurhexafluoride was noninferior to perfluoropropane. However, when they looked more carefully at their data, they identified risk factors for lowered macular hole closure success, including hole size larger than 400 microns, no internal limiting membrane peeling, older age of patient, and hole duration of greater than 9 months. They cautioned withholding face-down positioning based on their results.
Macular hole surgery risks
The most common risk following macular hole surgery is an increase in the rate of cataract development (cataractogenesis). In most patients, a cataract can progress rapidly, and often becomes severe enough to require removal. Other less common complications include infection and retinal detachment either during surgery or afterward, both of which can be immediately treated. Other surgical complications include iatrogenic retinal tears, enlargement of the macular hole, macular light toxicity and postoperative pressure elevation. Postoperative pressure elevation usually can be treated pharmacologically but may sometimes require an anterior chamber or vitreous tap.
For a few months after surgery, patients are not permitted to travel by air. Changes in air pressure may cause the bubble in the eye to expand, increasing pressure inside the eye.
Failure of hole closure/hole reopening
Histopathologic evaluation of specimens from patients with failed initial macular hole surgery demonstrated massive proliferation of cells and newly formed collagen associated with remaining internal limiting membrane. The residual internal limiting membrane and the associated collagen fibrils may become the source of persistent traction that prevents macular hole closure.
Retinal detachment/iatrogenic tears
The rate of postoperative retinal detachment is reported from 2-14%. Chung et al 27 identified that the induction of a posterior vitreous detachment during surgery was a key risk factor for the development of iatrogenic retinal breaks. They found an overall incidence of 14.6% (20 of 137 eyes) for retinal breaks. Only one retinal break (3.1%) was identified in a macular hole patient who did not require the induction of a posterior vitreous detachment. However, only 32 of 137 eyes undergoing vitrectomy for macular hole surgery did not require this step in the procedure, owing to the underlying pathophysiology of macular holes 27.
Visual field defects
Visual field defects have been noted following macular hole surgery.
They are related to dehydration of the nerve fiber layer.
The rate is reduced by shorter surgical times, lower air flow, and oblique placement of infusion cannulas caused by beveled incisions of smaller gauge vitrectomies.
Cataract formation
There is a small risk of hole reopening in the immediate postoperative period following cataract surgery.
Consideration of prophylaxis versus cystoid macular edema may reduce the risk of hole reopening after cataract surgery.
A retrospective case series by Bhatnagar et al 28 suggest that prior or simultaneous cataract extraction may carry a better long-term visual prognosis than cataract extraction following macular hole repair due to the risk of reopening of the hole following cataract surgery.
Macular hole surgery success rate
Vision improvement varies from patient to patient. People that have had a macular hole for less than six months have a better chance of recovering vision than those who have had one for a longer period. Discuss vision recovery with your doctor before your surgery. Vision recovery can continue for as long as three months after surgery.
Because complications, such as cataracts and retinal detachment, can follow treatment for macular holes, regular examinations are necessary.
Macular hole prognosis
In 1994, Wendel reported a series of 235 consecutive eyes undergoing repair of macular holes 29. In this series, 93% of patients were successfully managed with only a single operation; 60% patients experienced 4+ lines of visual improvement; and 84% patients experienced 2+ lines of improvement 29. Within this group, 58% of patients achieved 20/40 or better final visual acuity 29.
Multiple other studies cite similar success rates, though vision recovery may be protracted and also further delayed by onset of cataract formation. Use of internal limiting membrane (ILM) peeling may further increase the rate of single operation success, though it may potentially slow or affect final vision recovery. See Controversies surrounding the surgical repair of macular holes.
Optical coherence tomography (OCT) imaging preoperatively and postoperatively has provided additional prognostic data for visual recovery following macular hole surgery. Factors on optical coherence tomography (OCT) predictive of good visual acuity macular hole surgical outcome are as follows:
- Size of macular hole (minimum diameter < 311 µm)
- Traction on macular hole edges as defined by various parameters (eg, macular hole height)
- Development of a normal photoreceptor inner segment and outer segment junction, which can occur as early as 1 month postoperatively but typically by 6 months postoperatively.
While surgery for macular holes is considered elective, it is important for the patient to consider prognostically that there is potentially a risk for the fellow eye to develop a macular hole as well (12%).
- Estrogen Antagonist and Development of Macular Hole. Korean J Ophthalmol. 2010 Oct;24(5):306-309. English. https://doi.org/10.3341/kjo.2010.24.5.306[↩]
- Tanaka Y, Shimada N, Moriyama M, Hayashi K, Yoshida T, Tokoro T, et al. Natural history of lamellar macular holes in highly myopic eyes. Am J Ophthalmol. 2011 Jul. 152(1):96-99.e1[↩]
- Ciardella AP, Lee GC, Langton K, Sparrow J, Chang S. Autofluorescence as a novel approach to diagnosing macular holes. Am J Ophthalmol. 2004 May. 137 (5):956-9.[↩]
- Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012 Aug 16. 367(7):606-15.[↩]
- Muqit MMK, Hamilton R, Ho J, Tucker S, Buck H. Intravitreal ocriplasmin for the treatment of vitreomacular traction and macular hole- A study of efficacy and safety based on NICE guidance. PLoS One. 2018. 13 (5):e0197072.[↩]
- Feng HL, Roth DB, Hasan A, Fine HF, Wheatley HM, Prenner JL, et al. INTRAVITREAL OCRIPLASMIN IN CLINICAL PRACTICE: Predictors of Success, Visual Outcomes, and Complications. Retina. 2018 Jan. 38 (1):128-136.[↩]
- Chaudhary K, Mak MY, Gizicki R, Muni RH, Wong DT, Altomare F, et al. PROPORTION OF PATIENTS WITH MACULAR HOLE SURGERY WHO WOULD HAVE BEEN FAVORABLE OCRIPLASMIN CANDIDATES: A Retrospective Analysis. Retina. 2017 Jan. 37 (1):76-79.[↩]
- Singh RP, Li A, Bedi R, Srivastava S, Sears JE, Ehlers JP. Anatomical and visual outcomes following ocriplasmin treatment for symptomatic vitreomacular traction syndrome. Br J Ophthalmol. 2014 Mar. 98(3):356-60.[↩]
- Tibbetts MD, Reichel E, Witkin AJ. Vision Loss After Intravitreal Ocriplasmin: Correlation of Spectral-Domain Optical Coherence Tomography and Electroretinography. JAMA Ophthalmol. 2014 Feb 27.[↩]
- Fahim AT, Khan NW, Johnson MW. Acute Panretinal Structural and Functional Abnormalities After Intravitreous Ocriplasmin Injection. JAMA Ophthalmol. 2014 Feb 27.[↩]
- Chow N, Hong T, Chang A. Multimodal imaging of macular subretinal deposits following intravitreal ocriplasmin injection. Am J Ophthalmol Case Rep. 2018 Mar. 9:80-84.[↩]
- Rahimy E, McCannel CA. IMPACT OF INTERNAL LIMITING MEMBRANE PEELING ON MACULAR HOLE REOPENING: A Systematic Review and Meta-Analysis. Retina. 2016 Apr. 36 (4):679-87.[↩]
- Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010 Oct. 117 (10):2018-25.[↩]
- Narayanan R, Singh SR, Taylor S, Berrocal MH, Chhablani J, Tyagi M, et al. SURGICAL OUTCOMES AFTER INVERTED INTERNAL LIMITING MEMBRANE FLAP VERSUS CONVENTIONAL PEELING FOR VERY LARGE MACULAR HOLES. Retina. 2018 Apr 23.[↩]
- Ch’ng SW, Patton N, Ahmed M, Ivanova T, Baumann C, Charles S, et al. The Manchester Large Macular Hole Study: Is it time to reclassify large macular holes?. Am J Ophthalmol. 2018 Jul 30.[↩][↩]
- Kannan NB, Kohli P, Parida H, Adenuga OO, Ramasamy K. Comparative study of inverted internal limiting membrane (ILM) flap and ILM peeling technique in large macular holes: a randomized-control trial. BMC Ophthalmol. 2018 Jul 20. 18 (1):177.[↩]
- Rizzo S, Tartaro R, Barca F, Caporossi T, Bacherini D, Giansanti F. INTERNAL LIMITING MEMBRANE PEELING VERSUS INVERTED FLAP TECHNIQUE FOR TREATMENT OF FULL-THICKNESS MACULAR HOLES: A COMPARATIVE STUDY IN A LARGE SERIES OF PATIENTS. Retina. 2018 Sep. 38 Suppl 1:S73-S78.[↩]
- Tafoya ME, Lambert HM, Vu L, et al. Visual outcomes of silicone oil versus gas tamponade for macular hole surgery. Semin Ophthalmol. 2003 Sep. 18(3):127-31.[↩]
- Lai JC, Stinnett SS, McCuen BW. Comparison of silicone oil versus gas tamponade in the treatment of idiopathic full-thickness macular hole. Ophthalmology. 2003 Jun. 110(6):1170-4.[↩][↩]
- Macular hole surgery without face-down positioning. A pilot study. Retina. 1997;17(3):179-85. https://www.ncbi.nlm.nih.gov/pubmed/9196926[↩]
- Rubinstein A, Ang A, Patel CK. Vitrectomy without postoperative posturing for idiopathic macular holes. Clin Experiment Ophthalmol. 2007 Jul. 35(5):458-61.[↩][↩]
- Dhawahir-Scala FE, Maino A, Saha K et al. To posture or not to posture after macular hole surgery. Retina. 2008. 28:60-5.[↩]
- Tranos PG, Peter NM, Nath R, et al. Macular hole surgery without prone positioning. Eye. 2007 Jun. 21(6):802-6.[↩][↩]
- Iezzi R, Kapoor KG. No face-down positioning and broad internal limiting membrane peeling in the surgical repair of idiopathic macular holes. Ophthalmology. 2013 Oct. 120(10):1998-2003.[↩]
- Alberti M, la Cour M. NONSUPINE POSITIONING IN MACULAR HOLE SURGERY: A Noninferiority Randomized Clinical Trial. Retina. 2016 Apr 4.[↩][↩]
- Essex RW, Kingston ZS, Moreno-Betancur M, Shadbolt B, Hunyor AP, Campbell WG, et al. The Effect of Postoperative Face-Down Positioning and of Long- versus Short-Acting Gas in Macular Hole Surgery: Results of a Registry-Based Study. Ophthalmology. 2016 Feb 23.[↩]
- Chung SE, Kim KH, Kang SW. Retinal breaks associated with the induction of posterior vitreous detachment. Am J Ophthalmol. 2009 Jun. 147(6):1012-6.[↩][↩]
- Bhatnagar P, Kaiser PK, Smith SD, et al. Reopening of previously closed macular holes after cataract extraction. Am J Ophthalmol. 2007 Aug. 144(2):252-9.[↩]
- Wendel RT, Patel AC, Kelly NE. Chapter 120: Macular Hole Surgery. Guyer DR, Yannuzzi LA, Chang S, Shields JA, Green WR, eds. Retina-Vitreous-Macula. Philadelphia: WB Saunders Co; 1999. Vol 2: 1432-1448.[↩][↩][↩]