Contents
N-acetyl cysteine
N-acetylcysteine (NAC) also known as N-acetyl-cysteine or acetylcysteine, is a natural amino acid with powerful antioxidant with anti-inflammatory activity and a scavenger of reactive oxygen species (ROS) 1, 2. N-acetylcysteine (NAC) is a modified amino acid that is used as an antidote to treat acetaminophen (paracetamol) overdose to prevent acute liver injury and is almost 100% effective if given within 8 hours post-ingestion acetaminophen (paracetamol) 3, 4, 5, 6. People commonly use N-acetylcysteine (NAC) for cough in idiopathic pulmonary fibrosis and as a mucolytic agent in cystic fibrosis, chronic obstructive pulmonary disease (COPD), pneumonia, bronchitis, tracheobronchitis, tracheostomy patients, postoperative pulmonary complications, posttraumatic chest conditions and before diagnostic bronchoscopy to help with mucous plugging 3, 7, 8, 9, 10. N-acetylcysteine (NAC) is also used for flu, dry eye (keratoconjunctivitis sicca), contrast-induced nephropathy, acute liver failure, Alzheimer disease and many other conditions, but there is no good scientific evidence to support many of these uses 11, 12, 13, 14. There is also no good evidence to support using N-acetylcysteine (NAC) for COVID-19 7. Although many dietary supplement products contain N-acetylcysteine (NAC), the United States Food and Drug Administration (FDA) states that it’s illegal for dietary supplements to contain N-acetyl cysteine since it’s technically an approved drug. Prescription N-acetyl cysteine products are available under the guidance of a healthcare provider. N-acetylcysteine is found mostly in plants of the Allium species, especially in the onion (Allium cepa, 45 mg NAC/kg) 15, 16
N-acetylcysteine (NAC) has also been used as an antioxidant precursor to glutathione (GSH), a tripeptide made up of cysteine, glycine, and glutamic acid that is found in relatively high concentrations inside many cells 17. Therefore, the human body requires all three amino acids (i.e, cysteine, glycine, and glutamic acid) and adequate enzymatic function to make sufficient quantities of glutathione 18. The availability of the amino acid cysteine is known to be rate-limiting for the synthesis of glutathione, and it is well documented, both clinically and in animal studies, that cysteine supplementation most practically achieved by administration of N-acetylcysteine (NAC) can boost glutathione synthesis and levels 19, 10, yet a review of research data indicates the use of N-acetylcysteine (NAC) to boost glutathione synthesis and levels may be inconclusive or equivocal 20. Cysteine is a sulfur amino acid, which might imply that consuming cysteine-rich foods such as meat, fish, grains, dairy, soybean, and egg products, may also support glutathione synthesis 21, 22. A systematic review 23 that included twelve clinical trials utilizing N-acetylcysteine (NAC) supplementation with a specific focus on cognitive markers indicated that there may be some benefit to using N-acetylcysteine in certain populations; however, the studies were too variable in design and outcome to make any definitive conclusion 20.
Although N-acetylcysteine (NAC) is promising as a supplement to both boost glutathione levels and has the potential to reduce some of the issues related to oxidative stress 24, 25, the research up till now is inconclusive 26, 27, 28, 29. Furthermore, it is unclear if the effects of N-acetylcysteine (NAC) on oxidative stress are due to its antioxidant properties or due to increased glutathione synthesis 20. Moreover, it has been suggested that N-acetylcysteine (NAC) may work synergistically with other supplemental nutrients. For example, it has been postulated that glycine may be as important as cysteine when it comes to glutathione production, especially when concurrently supplemented with N-acetylcysteine 30. While further studies are required, it may be the better approach to supplement with both cysteine and glycine to see a boost in glutathione, especially among those who may not have adequate quantities of the amino acids or require higher levels of glutathione.
For example, in a small study 31 with eight healthy elderly adults and a control group of eight younger subjects, after measuring baseline glutathione synthesis in both groups, the older subjects were orally administered 0.81 mmol N-acetylcysteine per kg per day (around 132 mg/kg/day) and 1.33 mmol glycine/kg/day (roughly 100 mg/kg/day) for 14 days. Initially, the older subjects had 55.2% less glycine and 24.4% less cysteine in their red blood cells 31. They also had a 46.2% lower glutathione level than the controls 31. However, after N-acetylcysteine (NAC) supplementation, the glycine levels increased by 117.6% and the cysteine by 55.1%. Furthermore, they had a 94.6% higher glutathione concentration in their red blood cells, which also led to no statistical difference between the young controls and the elderly subjects in their glutathione levels 31. In addition, they experienced lower plasma oxidative stress and F2-isoprostanes 31. The researchers speculated that the typical reduction of glutathione in the elderly was due to a lower supply of glycine and cysteine, the precursors to glutathione synthesis, and that upon N-acetylcysteine (NAC) supplementation, they had the ability to stimulate synthesis and restore levels. Although impressive, it is important to recognize that this was a small study. It is worthwhile to note that there was also no translation to clinical benefit, as these subjects were healthy 20.
In another study 32 on five people with mild to moderate Parkinson’s disease and three controls, a high dose of N-acetylcysteine (3000 mg taken orally twice daily) for a period of four weeks led to an increase of cysteine levels and antioxidant measures, with no commensurate improvement in oxidative stress measurements (4-hydroxynonenal and malondialdehyde) nor did it increase the level of glutathione in the brain 32. Additionally, some of the participants experienced a worsening of Parkinson’s symptoms that were alleviated upon stopping the N-acetylcysteine supplementation 20.
Another study 33 looking at those with neurodegenerative disorders found that a single intravenous (IV) dose of N-acetylcysteine led to an increase of the blood reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio and levels of glutathione in the brain 33. Those who had the greatest percent change in reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio also had a greater percent change in their levels of glutathione in their brain. While the study was too small and of too short duration to make any conclusions about the role of N-acetylcysteine in these conditions, what is notable is that through intravenous (IV) administration of N-acetylcysteine, brain levels could be altered 20.
In a 12-week clinical trial with children (n = 31) with autism spectrum disorder administered 60 mg/kg/day in three doses (maximum dose of 4200 mg/day) found that although there was no significant impact on the social impairment associated with autism, there was a significant impact on boosting the glutathione levels in the children 34. Similar to other studies, more needs to be explored as to how N-acetylcysteine influences glutathione levels, factors that modify individual response to supplementation and how symptomatology interrelates. Glutathione-S-transferase (GST) polymorphisms may play a role in the efficacy of N-acetylcysteine 20. In one study 35 investigating the impact of N-acetylcysteine on noise-induced hearing loss in men (n = 53) taking 1200 mg per day for 14 days led to a significant reduction of noise-induced temporary threshold shift, or the amount of hearing loss after a period of heightened noise exposure in a work setting relative to baseline, or pre-shift levels. When the participants were grouped according to their glutathione-S-transferase (GST) genotypes, the researchers found that only those with the null genotypes experienced a significant effect from taking N-acetylcysteine 20.
N-acetylcysteine can cause side effects such as dry mouth, nausea, vomiting, and diarrhea. One of the properties of N-acetylcysteine is that it has an unpleasant smell and taste that some people find hard to tolerate. Vomiting after intravenous use has been reported in about 11% at doses of 150 mg/kg and one anaphylactic reaction has been reported 36. N-acetylcysteine has anticoagulant and platelet inhibiting properties and the use in patients with bleeding disorders or blood thinners may be relatively counterindicated 37. The use of N-acetylcysteine with patients on nitroglycerine should be cautioned, since it may cause hypotension 38. Other more rare side effects may include inflammation of the mucous membrane of the mouth (stomatitis), drowsiness, runny nose (rhinorrhea), and coughing up blood (hemoptysis) 39.
Table 1. N-acetylcysteine (NAC) potential mechanisms of action
| 1 | Action on glutathione | N-acetylcysteine restores glutathione (cysteine is rate limiting) as seen in cell and animal studies and clinically in acetaminophen overdose 40. |
| 2 | Stabilizes proteins/DNA | Protects proteins by crosslinking cysteine disulfide molecules 41. Various mechanisms of DNA repair/protection as seen in animal studies and human cell studies 42. |
| 3 | Scavenges free radicals | Scavenging property via the redox potential of thiols as demonstrated in cell culture 43. |
| 4 | Anti-inflammatory property | Reduces proinflammatory cytokines as seen in animal studies 44. |
| 5 | Antioxidant property | Reduces oxidative damage as seen in cell cultures 45. |
| 6 | Mucolytic property | Splits disulfide bonds in mucoproteins lowering viscosity demonstrated in purified mucus gels and tracheal explant systems and in vitro (in a pig tracheal pouch) models 46. |
| 7 | Mitochondrial resilience | Neurogenesis-inducing ability 47 reduces apoptosis of mitochondria as demonstrated in human dental pulp cells 48. |
| 8 | Metal chelation | Thiol groups provide binding sites for metals in animal studies 49. |
| 9 | Glutamate/dopamine homeostasis | Modulates glutamate and dopamine extensive studies in humans 12. |
| 10 | Antiviral properties | Immune modulation, anti-NF-KB properties, and other unexplored mechanisms observed in vitro and in vivo 50. |
| 11 | Vascular endothelial growth factor | Inhibition of vascular permeability as seen in human keratinocytes 51. |
| 12 | Adenosine triphosphate (ATP) and nitric oxide (NO) production | Increased ATP production in some cells like fibroblasts in vitro 52. Increased nitric oxide production as demonstrated in human studies 53. |
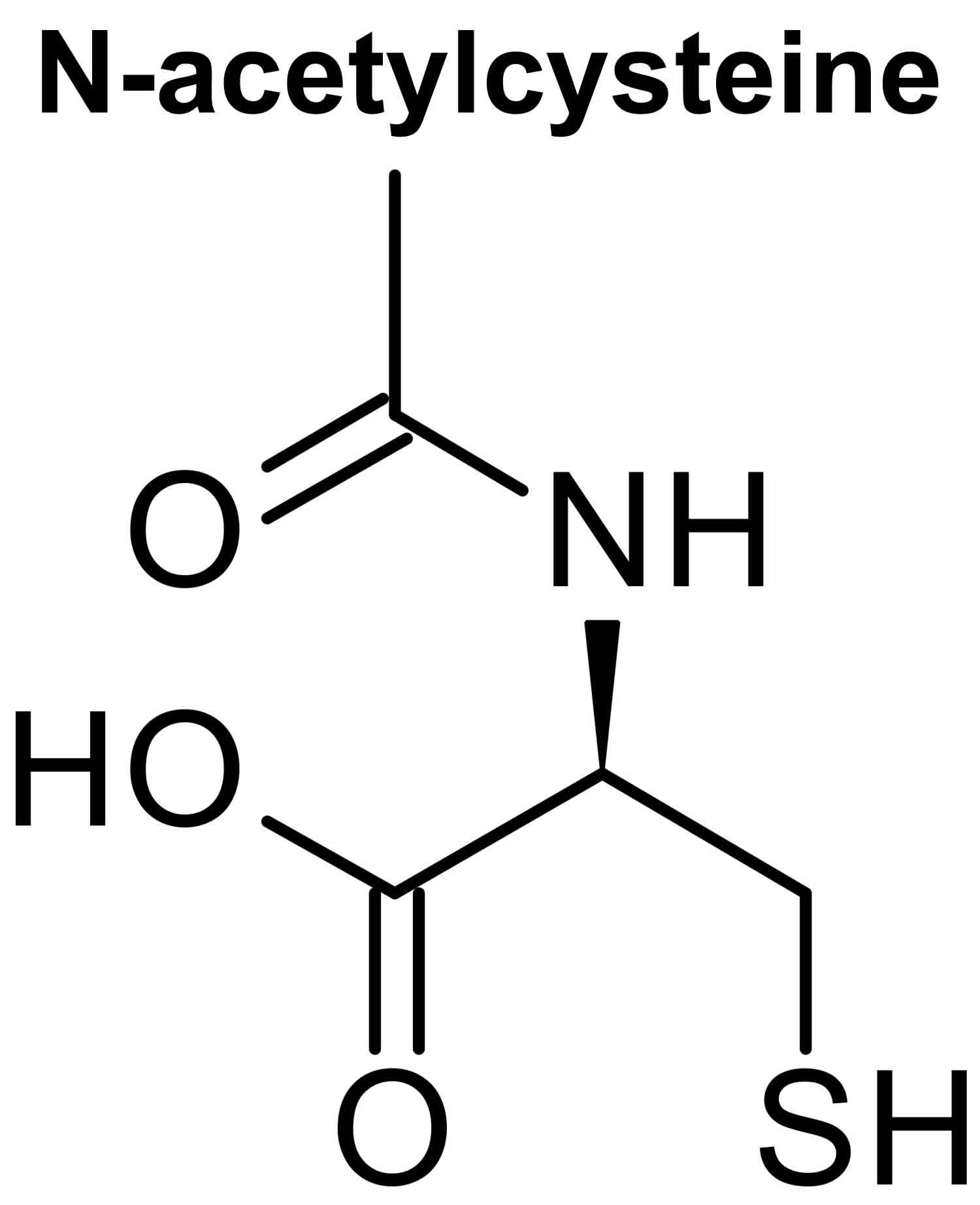
Figure 1. N-acetylcysteine structure
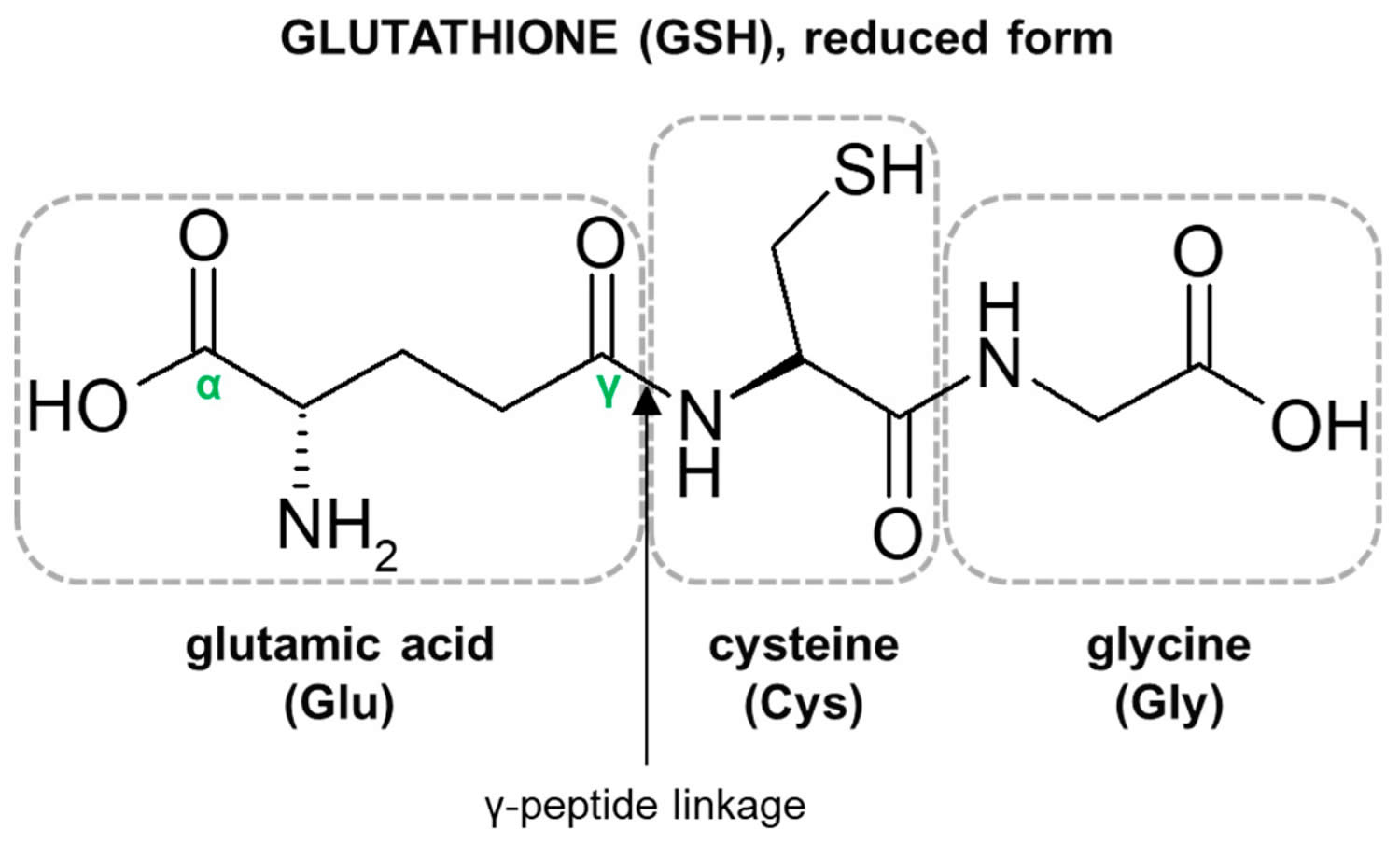
Figure 2. Glutathione structure
Footnote: Structure of the tripeptide glutathione (GSH) (reduced form). Glutamic acid (Glu) is linked in a gamma (γ) peptide linkage (via its gamma-carboxyl group) to cysteine (Cys), which in turn forms an alpah (α) peptide linkage with glycine (Gly).
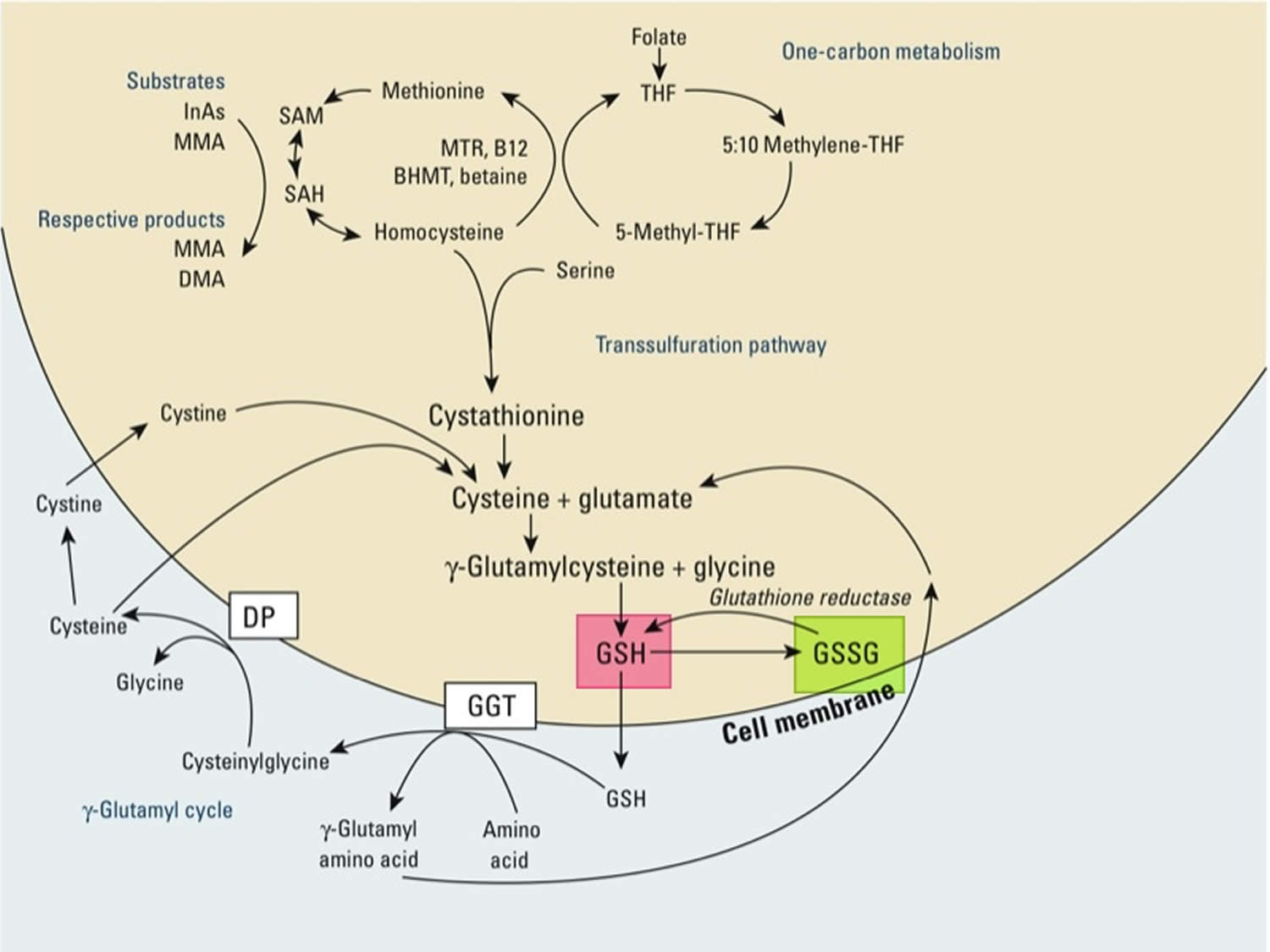
[Source 55 ]Figure 3. Synthesis and recycling of glutathione
Footnotes: One-carbon metabolism and glutathione (GSH) synthesis and metabolism. Folic acid is reduced to tetrahydrofolate (THF) and subsequently converted to 5-methyl THF. In a reaction catalyzed by methionine synthetase (MTR), the methyl group of 5‑methyl-THF can be transferred to homocysteine, generating methionine. Methionine is activated to form S‑adenosylmethionine (SAM), the universal methyl donor. The by-product of methylation reactions, S‑adenosylhomocysteine (SAH), is hydrolyzed to homocysteine. Homocysteine is either used to regenerate methionine or is directed to the transsulfuration pathway. Reduced glutathione (GSH) is a product of the transsulfuration pathway. Reduced glutathione (GSH) can serve as a continuous source of cysteine, which is extremely unstable, via the gamma‑glutamyl cycle. Reduced glutathione (GSH) is exported from the cell, and the enzyme gamma‑glutamyltransferase (GGT) transfers the gamma-glutamyl moiety of glutathione (GSH) to an amino acid, often cystine, producing cysteinylglycine and gamma-glutamyl amino acid. The gamma-glutamyl amino acid can be transported back into the cell and ultimately metabolized to glutamate. Cysteinylglycine is converted to cysteine and glycine by dipeptidase (DP). Cysteine is unstable extracellularly and can oxidize to cystine; both cysteine and cystine can be imported back into the cell for glutathione (GSH) production.
Abbreviations: B12 = vitamin B12; BHMT = betaine homocysteine methyltransferase; DMA = dimethylarsinic acid; InAs = inorganic acids; MMA = monomethylarsonic acid; GSSG = oxidized glutathione; GSH = reduced glutathione
[Source 56 ]N-acetylcysteine health benefits
Even though N-acetylcysteine has been used for more than 50 years, there are still many controversies surrounding it as a medicine as well as a dietary supplement 36, 57, 58, 59, 60, 61, 62, 63, 49, 64, 10.
Much of the research on N-acetylcysteine has used an inhaled, liquid form of this compound. The inhaled N-acetylcysteine, which is classified as a drug, not a dietary supplement, is approved by the U.S. Food and Drug Administration (FDA) as a mucolytic agent and for decreasing respiratory secretion viscosity 65. Products containing N-acetylcysteine are also sold as dietary supplements, however, the FDA indicated that N-acetylcysteine cannot lawfully be marketed as a dietary supplement.
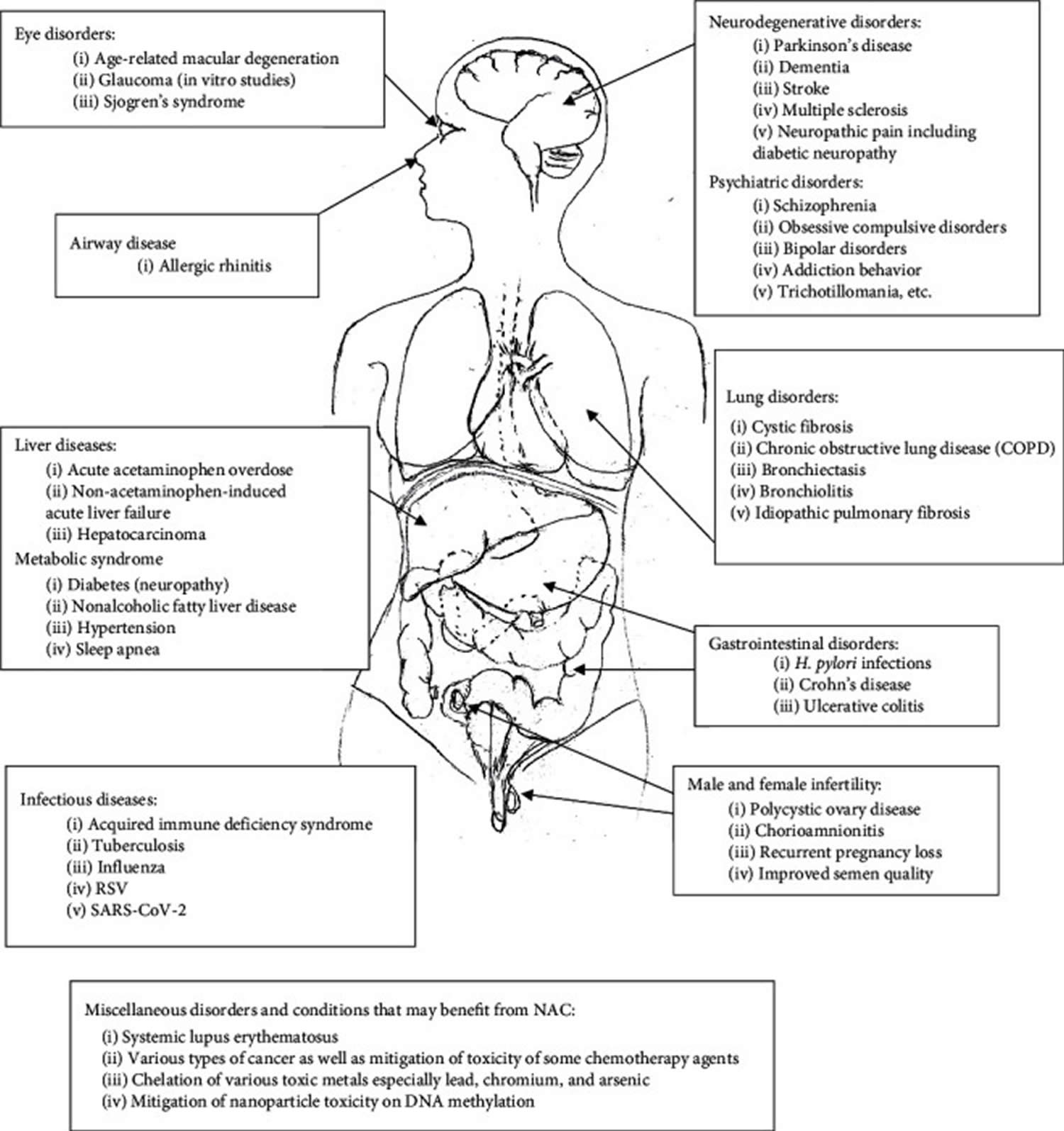
Figure 4. N-acetylcysteine health benefits
[Source 54 ]N-acetylcysteine is effective for:
- Acetaminophen (paracetamol) poisoning / acetaminophen overdosage 66, 67. Taking prescription N-acetyl cysteine by mouth or by IV reduces the death rate and prevents permanent harm caused by acetaminophen overdosage 68. Prescription products must be given by a healthcare provider. Optimal if given within 8 hours of acetaminophen ingestion; may be effective when given ≥24 hours after ingestion 69.
- Complete or partial lung collapse (atelectasis). Inhaling a prescription form of N-acetyl cysteine helps treat collapsed lungs caused by mucus blockage. Prescription products must be given by a healthcare provider.
- Mucolytic uses. N-acetylcysteine is used as an adjunctive treatment (add-on treatment used together with the primary treatment) for patients with abnormal, viscid, or inspissated mucous secretions associated with conditions such as acute and chronic bronchopulmonary disorders (e.g., pneumonia, bronchitis, emphysema, tracheobronchitis, chronic asthmatic bronchitis, tuberculosis, bronchiectasis, primary amyloidosis of the lung); atelectasis caused by mucus obstruction; pulmonary complications of cystic fibrosis 70; pulmonary complications of thoracic and cardiovascular surgery; and post-traumatic chest conditions.
- Cystic fibrosis. Cystic fibroses is a multiorgan genetic recessive disorder that affects 1/2500 births and is characterized by hyperviscoelastic sputum, neutrophilic inflammation, and infection. High-dose oral N-acetylcysteine was used safely in modulating inflammation, improving glutathione levels, and decreasing elastase activity 71. A recent study showed that lung function was maintained with oral N-acetylcysteine over 24 weeks, while there was deterioration on placebo; however, other markers of inflammation did not change 72. A 2013 Cochrane Review of nebulized N-acetylcysteine did not show significant benefit and nebulized N-acetylcysteine had a very bad taste and smell 73. Oral N-acetylcysteine studies were small and only showed minimal benefit 74. However, a recent study on nebulized N-acetylcysteine did show protection of lung function in children 75. Distal intestinal obstruction found in cystic fibroses patients may benefit from a nonsurgical alternative using N-acetylcysteine although further studies are warranted 76.
- Chronic obstructive lung disease (COPD) and chronic bronchitis. As early as 1985, it was reported that, in chronic obstructive lung disease (COPD), polymorphonuclear leukocyte oxidative damage was reduced with the use of N-acetylcysteine in a controlled in vitro cell model 77. More recently, a meta-analysis looked at chronic bronchitis and COPD treated with N-acetylcysteine, and there were significantly fewer exacerbations in the treated group than in the placebo group 78, 79. For those with documented airway obstruction, recommendations are to take 1200 mg/day, as a preventative 80. A quantitative systematic review using N-acetylcysteine in chronic bronchitis showed significant benefit in preventing exacerbation and the number needed to treat to achieve benefit was 5.8 81. Recommendations as add-on therapy for COPD and chronic bronchitis is considered a reasonable approach 82.
- Lung tests. Inhaling a prescription form of N-acetylcysteine is helpful to prepare people for diagnostic lung tests. Prescription products must be given by a healthcare provider.
- Care of people with a tube placed in their windpipe (tracheostomy care). Inhaling a prescription form of N-acetylcysteine helps prevent crusting in people with a tube in the windpipe. Prescription products must be given by a healthcare provider.
N-acetylcysteine possibly effective for:
- Age-related macular degeneration (AMD). The addition of N-acetylcysteine to cell cultures of retinal pigment epithelium resulted in significant reduction of oxidative damage in cell studies 83. N-acetylcysteine also upregulates reduced glutathione production and also reverses lipid peroxidation in these cells 84. This has been suggested as a novel new treatment for macular degeneration and clinical studies are warranted.
- Chest pain (angina). Taking N-acetyl cysteine by mouth or by IV seems to improve chest pain when used with the drug nitroglycerin. Taking N-acetyl cysteine by IV also seems to help prevent nitroglycerin tolerance, but it might increase the risk for headaches and low blood pressure. IV products can only be given by a healthcare provider.
- Autism. Taking N-acetyl cysteine by mouth might improve irritability in children and adolescents with autism. But it doesn’t seem to help other autism symptoms.
- Swelling (inflammation) of the main airways in the lung (bronchitis). Taking N-acetyl cysteine by mouth seems to reduce shortness of breath and coughing from chronic bronchitis. Also, taking N-acetyl cysteine by mouth for 3-36 months seems to prevent flare-ups.
- Bronchiectasis. There is in vitro evidence that biofilms are disrupted and prevented with use of N-acetylcysteine potentially reducing infection 85. A recent long-term randomized controlled trial study using N-acetylcysteine 600 mg bid resulted in fewer exacerbations, less 24-hour mucous production, and a significant improvement in quality of life 86.
- Bronchiolitis. Nebulized N-acetylcysteine used in children was found to be effective by reducing the clinical severity score within 3–5 days and resulted in earlier discharge 87. Further studies are in progress.
- Idiopathic pulmonary fibrosis. Idiopathic pulmonary fibrosis is a fatal lung disease with limited options and poor prognosis. In 2011, the use of three medications Azathioprine, Prednisone, and N-acetylcysteine in combination was halted because of increased hospitalizations and deaths 88. The evidence against the use of these three medications together was strong 88. Evidence has been mixed over the years but more recently a systematic review and meta-analyses have provided more clarity in the clinical use of N-acetylcysteine in pulmonary fibrosis showing benefit in improving oxygenation and reduced the decline in lung function; however, complications and mortality have remained similar 89.
- A lung disease that makes it harder to breathe (chronic obstructive pulmonary disease or COPD). Taking N-acetyl cysteine by mouth for at least 6 months seems to decrease flare-ups by about 40% in people with moderate to severe COPD. It seems to work best in people who are not already taking corticosteroids. In people with COPD who need to be hospitalized, taking N-acetyl cysteine in addition to regular treatment helps with recovery.
- Kidney damage caused by contrast dyes (contrast induced nephropathy). Taking N-acetyl cysteine by mouth, with or without other drugs, might help to prevent kidney problems caused by dyes used during some X-ray exams. But it only seems to help in people who already have poor kidney function.
- Non-acetaminophen-induced acute liver failure. The cause of non-acetaminophen-induced acute liver failure may include agents like viruses, drugs, toxins, herbal and traditional medications, and autoimmunity. Treatment includes removing the offending agent or specific treatments for the agent. N-acetylcysteine has been used in mushroom poisoning 90, herbicide (Paraquat) poisoning 91, chloroform poisoning 92, and protecting against polychlorinated biphenyls (PCB) induced steatosis 93 and other poisonings. N-acetyl cysteine has been used because of its antioxidant, anti-inflammatory, and vasodilating effects as seen in acetaminophen damage 94]. The use of N-acetylcysteine in non-acetaminophen-induced acute liver failure reduced mortality and average length of stay and improved survival 95, 96. A meta-analysis of prospective clinical trials reviewing N-acetylcysteine-treated and placebo-treated groups showed NAC to be safe and prolonged the survival of patients with native livers without transplantation but did not improve overall survival 97.
- High levels of homocysteine in the blood (hyperhomocysteinemia). Taking N-acetyl cysteine by mouth seems to reduce homocysteine levels, a possible risk factor for heart disease.
High levels of cholesterol or other fats (lipids) in the blood (hyperlipidemia). Taking N-acetyl cysteine by mouth seems to reduce levels of a blood fat called lipoprotein(a) in people with high levels of this blood fat. - Toxicity in people taking the cancer drug ifosfamide (Ifex). Taking N-acetyl cysteine by mouth seems to help prevent side effects of the cancer drug ifosfamide. But a drug called mesna seems to work better than N-acetyl cysteine.
- Crohn’s disease. Crohn’s disease is characterized by marked systemic oxidative stress even in those in clinical remission 98. In a double-blind randomized controlled trial (N = 168), the relapse rate of those on N-acetylcysteine 400 mg twice daily was significantly reduced compared to placebo while tapering off prednisolone 99.
- Ulcerative colitis. The use of antioxidant therapy in inflammatory bowel disease has been suggested in the past 100. In an randomized controlled trial, using 400 mg of N-acetylcysteine twice a day in ulcerative colitis patients who were on prednisolone taper, the relapse rate was significantly less in the treatment group. The endoscopic relapse rate, serum level of high-sensitivity C-reactive protein (hs-CRP), and fecal calprotectin level were all lower in the treatment group 101.
- Diabetic neuropathy, diabetic retinopathy, and diabetic nephropathy. N-acetylcysteine has been shown to benefit diabetic peripheral neuropathy in animals 102. Likewise, animal studies investigating diabetic retinopathy 103 and diabetic nephropathy 104 show promising results. Recently, a study in humans has shown significant benefit in painful peripheral neuropathy 105. Patients with painful neuropathy improved over 8 weeks with the use of N-acetylcysteine (600 mg bid) as adjuvant therapy to pregabalin. There was a >50% reduction in pain score compared to placebo.
- Flu (influenza). Taking N-acetyl cysteine by mouth seems to reduce flu symptoms. A double-blind randomized controlled trial in which 262 patients were given 600 mg of N-acetylcysteine or placebo for 6 months (during the winter) was conducted to determine the effect of long-term treatment on influenza 106. This study showed that only 25% of virus-infected patients in the N-acetylcysteine group were symptomatic compared to 79% of patients in the placebo group. There was a significant decrease in influenza-like episodes, severity, and length of time confined to bed and sharp reduction of both local and systemic symptoms in the N-acetylcysteine group. Replication of seasonal human influenza A viruses is inhibited by N-acetylcysteine along with inhibition of virus-induced proinflammatory responses 106.
- Kidney failure. Taking N-acetyl cysteine by mouth seems to help prevent problems such as heart attack and stroke in people with kidney failure.
- Heart attack. Giving N-acetyl cysteine by IV along with the drug nitroglycerin seems to help maintain heart function and reduce heart damage in people having a heart attack. Sometimes the drug streptokinase is also used along with N-acetyl cysteine and nitroglycerin. IV products can only be given by a healthcare provider.
- Liver cancer (hepatocarcinoma). Liver cancer, most commonly known as hepatocarcinoma, is a common cancer and treatment of this cancer with interferon-alpha 2A (IFN-α) has a relatively poor response rate of about 30%. N-acetylcysteine acts synergistically to improve the efficacy of the drug by decreasing tumor viability, increasing apoptosis, and decreasing expression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB). Inactivating the pathway of initiation, promotion, and progression of tumors by NF-kB can be done by using NAC as an adjuvant to IFN α 107.
- Male infertility. In idiopathic male infertility, an randomized controlled trial using N-acetylcysteine showed improvement of oxidative status along with semen quality (improved motility, viscosity, and volume) 108. The dosage was 600 mg twice daily. In another randomized controlled trial using selenium and N-acetylcysteine together, there was significant improvement in semen quality 109.
- Multiple sclerosis. A small randomized controlled trial study using intravenous N-acetylcysteine once weekly along with 500 mg twice a day for two months showed improvement in glucose metabolism in several areas of the brain as well as improved attention and cognition in self-reported scores in the treatment group 110. In a small study in progressive multiple sclerosis (MS), N-acetylcysteine at a dose of 1250 mg three times a day was well tolerated and resulted in sustained fatigue improvement 111. Glutathione levels are reduced in secondary progressive MS and N-acetylcysteine is able to improve this 112.
- Sjogren’s syndrome. Oral N-acetylcysteine used in a double-blind study has shown improvements in daytime thirst, ocular soreness, ocular irritability, and halitosis in Sjogren’s syndrome 113.
- Systemic lupus erythematosus. There is depletion of glutathione in patients with systemic lupus erythematosus as well as T-cell dysfunction. N-acetylcysteine replenishes glutathione and as an antioxidant in and of itself is able to inhibit mechanistic target of rapamycin (mTOR) in vitro 101. A double-blind randomized controlled trial pilot study using 2.4 gm of N-acetylcysteine daily safely and significantly improved lupus disease activity 114.
- Nonalcoholic fatty liver disease (NAFLD). There is evidence that N-acetylcysteine may block hepatic lipid accumulation and provide therapeutic benefit against metabolic complications found in nonalcoholic fatty liver disease (NAFLD). This is primarily due to the antioxidant effects and attenuation of lipid peroxidation 115. This is supported by most preclinical studies and a few clinical studies, and there is an urgent need for larger clinical studies. Nonalcoholic fatty liver disease (NAFLD) affects up to 25% of the population, a condition which may lead to significant pathology such as fibrosis of the liver.
- Parkinson’s disease. Dopamine may trigger apoptosis in neuronal cell cultures, which may initiate inappropriate loss of nigral cells in Parkinson’s disease. Thiols containing compounds like N-acetylcysteine are markedly protective by inhibiting dopamine-induced cell death in cell cultures 116. A clinical study using N-acetylcysteine both as a weekly intravenous infusion and 500 mg orally twice a day over three months significantly improved Parkinson symptoms and increased dopamine binding in the brain warranting further study 117.
- Polycystic ovary disease, chorioamnionitis, and recurrent pregnancy loss. Treatment with N-acetylcysteine may improve insulin sensitivity in women with polycystic ovary disease 118. A systematic review of randomized controlled trials using N-acetylcysteine as a supplement in polycystic ovary disease resulted in improved fertility, ovulation, and odds of having a live birth although these results were not as robust as studies using Metformin 119. The dosage in studies varied from 1200 mg/day to 1800 mg/day. Chorioamnionitis, a devastating infection with increased risk of cerebral palsy and other neurological sequalae, may benefit from intravenous N-acetylcysteine (both antenatally and postnatally) by reducing neuroinflammation 120. The use of N-acetylcysteine as an adjunct in recurrent pregnancy loss has been shown to improve the take-home-baby rate as compared to folic acid alone 121.
- Stroke. Acrolein-mediated damage after stroke has been implicated in the size of strokes in animal studies and N-acetylcysteine has been shown to reduce the size of the infarct 122. In a recent randomized double-blind placebo-controlled trial using N-acetylcysteine 4 grams four times a day for 72 hours at the onset (within 24 hours) of an ischemic stroke, the follow-up National Institute of Health Stroke Scale at 90 days resulted in a better outcome profile in both neurological deficit and disability 123.
N-acetylcysteine possibly ineffective for:
- Lou Gehrig’s disease (amyotrophic lateral sclerosis or ALS). Taking N-acetyl cysteine by IV doesn’t seem to improve ALS symptoms. IV products can only be given by a healthcare provider.
- Alzheimer’s disease. Alzheimer’s disease is the most common form of dementia among older people. Dementia is a brain disorder that seriously affects a person’s ability to carry out daily activities. Results of different studies showed that lipoic acid and N-acetylcysteine decreased levels of oxidative and apoptotic markers via protection of mitochondrial function 124, 125. Combination of both lipoic acid and N-acetylcysteine maximizes this protective effect suggesting that this may prevent mitochondrial decay associated with aging and age-related disorders such as Alzheimer’s disease.
- Dementia. Animal studies have shown significant promise improving cognitive function even though beta-amyloid pathology was unchanged 126. There is some evidence that N-acetylcysteine as an adjuvant may slow the progression of dementia; however, this effect was seen clinically in a nutraceutical containing several ingredients including N-acetylcysteine 127.
- Heart damage caused by certain cancer drugs (anthracycline cardiotoxicity). Taking N-acetyl cysteine by mouth doesn’t seem to prevent or treat heart damage caused by doxorubicin.
- A lung disease that affects newborns (bronchopulmonary dysplasia). Giving N-acetyl cysteine through a hole in the windpipe does not seem to prevent breathing problems in premature infants. This can only be given by a healthcare provider.
- Acute asthma. Studies at present do not support the use of N-acetylcysteine in acute asthma attacks because of lack of improvement of cough, wheezing, dyspnea, sputum expectoration, or night sleep 128. However, in animal models, steroid-resistant acute exacerbation of asthma did benefit from NAC 129. To date, there have been no long-term studies using N-acetylcysteine in prevention of recurrent asthma attacks by reducing inflammation and mucous plugging. NAC has been shown to reduce the allergen-induced nasal inflammatory cascade in allergic rhinitis in animal models 130. Topical application of N-acetylcysteine prior to ragweed exposure resulted in attenuation of the late phase allergic response mediated nasal symptoms 131.
- Cannabis use disorder. Taking N-acetyl cysteine by mouth doesn’t seem to improve depression or help reduce cannabis use in teens and adults with cannabis use disorder.
- Cystic fibrosis. Taking N-acetyl cysteine by mouth or inhaling it doesn’t seem to improve lung function in people with cystic fibrosis.
- Diabetes. Animal studies show that N-acetylcysteine may inhibit hepatic steatosis and development of glucose intolerance and improve lipid profiles 132, 133. In type 2 diabetic humans, there does not appear to be any benefit in improving glucose tolerance or ß-cell function with the addition of N-acetylcysteine in short-term trials (2 weeks) 134. Long-term trials are warranted.
- An inherited disorder marked by sensitivity to light (erythropoietic protoporphyria or EPP). Taking N-acetyl cysteine by mouth doesn’t seem to reduce light sensitivity in people with EPP.
- A digestive tract Helicobacter pylori (H. pylori) infection that can lead to ulcers. Taking N-acetyl cysteine by mouth along with usual treatments for H. pylori infection doesn’t help eliminate the infection any better than usual treatment 135.
- Swelling (inflammation) of the liver (hepatitis). Taking N-acetyl cysteine by mouth doesn’t seem to help treat viral hepatitis. It also doesn’t seem to improve response to interferon therapy in people with hepatitis C. But it might help prevent relapses in people with hepatitis C.
- HIV and AIDS. Taking N-acetyl cysteine by mouth doesn’t seem to improve immune function or reduce the amount of virus in the body in most people with HIV. In a double-blind placebo-controlled trial using 800 mg of N-acetylcysteine, there was a reduction in the decline of the CD4 count (number of a type of white blood cell) seen in the placebo group and tumor necrosis factor-alpha (TNF-alpha) levels were also reduced 136. Glutathione levels are improved with N-acetylcysteine, inhibiting actions of inflammatory cytokines and slowing cachexia and wasting 137.
- Low blood pressure (hypotension). Taking N-acetyl cysteine by mouth doesn’t seem to reduce the risk of kidney failure in people with long-term low blood pressure.
- Inability to become pregnant within a year of trying to conceive (infertility). Taking N-acetyl cysteine by mouth doesn’t seem to improve pregnancy rate or miscarriage rate in females with fertility problems.
- Liver transplant. Giving N-acetyl cysteine by IV during liver donor surgery doesn’t seem to prevent transplant rejection in liver transplant recipients. IV products can only be given by a healthcare provider.
- Swelling (inflammation) of the pancreas (pancreatitis). Taking N-acetyl cysteine by mouth doesn’t prevent pancreatitis in people having a certain procedure that can cause pancreas swelling. Also, taking N-acetyl cysteine by IV along with selenium and vitamin C doesn’t seem to improve pancreas function in people with serious pancreatitis. IV products can only be given by a healthcare provider.
- Recovery after surgery. Taking N-acetyl cysteine by mouth or by IV doesn’t seem to reduce the risk of heart attack, stroke, kidney injury, or death after heart surgery. IV products can only be given by a healthcare provider.
N-acetylcysteine likely ineffective for:
- Head and neck cancer. Taking N-acetyl cysteine by mouth doesn’t prevent new tumors or improve survival in people with head and neck cancer.
- Lung cancer. Taking N-acetyl cysteine by mouth doesn’t prevent new tumors or improve survival in people with lung cancer.
- Multiple organ failure. Giving N-acetyl cysteine by IV might increase the risk of death in people with multiple organ failure. IV products can only be given by a healthcare provider.
There is interest in using N-acetyl cysteine for a number of other purposes, but there isn’t enough reliable information to say whether it might be helpful.
Is N-acetylcysteine safe?
N-acetylcysteine is likely safe for most adults. N-acetylcysteine is an FDA-approved prescription drug.
When inhaled: N-acetylcysteine is likely safe for most adults, when used as a prescription medication. It can cause swelling in the mouth, runny nose, drowsiness, clamminess, and chest tightness.
Special precautions and warnings
Pregnancy
N-acetylcysteine is possibly safe when taken by mouth or inhaled during pregnancy. N-acetyl cysteine crosses the placenta, but there is no evidence that it harms the unborn child. But N-acetyl cysteine should only be used when medically needed.
Breastfeeding
There isn’t enough reliable information to know if N-acetylcysteine is safe to use during breast-feeding. Stay on the safe side and avoid use.
Children
N-acetyl cysteine is likely safe when taken by mouth in doses of 900-2700 mg daily for up to 12 weeks.
Allergy
Don’t use N-acetylcysteine if you are allergic to acetyl cysteine.
Asthma
N-acetylcysteine might cause bronchospasm in people with asthma if inhaled or taken by mouth. If you take N-acetyl cysteine and have asthma, you should be monitored by your healthcare provider.
Bleeding disorder
N-acetyl cysteine might slow blood clotting. N-acetyl cysteine might increase the risk of bruising and bleeding in people with bleeding disorders.
Surgery
N-acetylcysteine might slow blood clotting. This might increase the risk of bleeding during and after surgery. Stop taking N-acetyl cysteine at least 2 weeks before a scheduled surgery.
N-acetylcysteine interactions with medications and supplements
Major interactions
Do not take this combination
- Nitroglycerin. Nitroglycerin can dilate blood vessels and increase blood flow. Taking N-acetyl cysteine seems to increase the effects of nitroglycerin. This could increase the risk for side effects including headache, dizziness, and lightheadedness.
Moderate interactions
Be cautious with this combination.
- Activated charcoal. Activated charcoal is sometimes used to prevent poisoning in people who take too much acetaminophen (Tylenol) or other medications. Taking N-acetyl cysteine at the same time as activated charcoal might decrease how well it works for preventing poisoning.
- Chloroquine (Aralen). Chloroquine is a drug used to treat malaria. N-acetyl cysteine might reduce the effects of chloroquine against malaria.
- Medications for high blood pressure (antihypertensive drugs). N-acetyl cysteine might lower blood pressure. Taking N-acetyl cysteine along with medications that lower blood pressure might cause blood pressure to go too low. Monitor your blood pressure closely.
- Medications that slow blood clotting (anticoagulant / antiplatelet drugs). N-acetyl cysteine might slow blood clotting. Taking N-acetyl cysteine along with medications that also slow blood clotting might increase the risk of bruising and bleeding.
- Herbs and supplements that might lower blood pressure. N-acetyl cysteine might lower blood pressure. Taking it with other supplements that have the same effect might cause blood pressure to drop too much. Examples of supplements with this effect include andrographis, casein peptides, L-arginine, niacin, and stinging nettle.
- Herbs and supplements that might slow blood clotting. N-acetyl cysteine might slow blood clotting and increase the risk of bleeding. Taking it with other supplements with similar effects might increase the risk of bleeding in some people. Examples of supplements with this effect include garlic, ginger, ginkgo, nattokinase, and Panax ginseng.
N-acetylcysteine dosage
N-acetylcysteine dose varies significantly with various clinical studies and doses of 1200 mg daily or more are usually required to be clinically relevant 54. Studies in metabolic diseases show that 500 to 600 mg orally per day may be sufficient to reduce fatty liver disease. For Crohn’s disease and ulcerative colitis, doses of 800 mg per day seemed sufficient. Doses as high as 1250 mg orally three times a day have been used safely in multiple sclerosis (MS) and showed benefit in reducing fatigue. Doses of 8000 mg/day orally did not cause clinically significant reactions in HIV patients 138. Clearly dosing is still debated and much needs to be learned in this area.
- Liver impairment: In patients with severe liver disorders such as alcoholic liver cirrhosis or primary/secondary biliary cirrhosis (with Child-Pugh score of 5 to 7), the mean half-life is increased up to 80%. In comparison, there is a decrease in mean clearance by 30% when compared to a healthy adult. However, the existing medical literature does not provide evidence supporting a reduction in the dose of N-acetylcysteine for patients with hepatic impairment.
- Renal impairment: In individuals with chronic kidney disease, N-acetylcysteine is a safe intervention without significant adverse events 139.
- Pregnancy: No reports of fetal risk are apparent in pregnant women. Dosing for these patients can be initiated according to protocols similar to those used in the general population.
- Breastfeeding: Nursing mothers should pump and discard milk 30 hours after taking acetylcysteine to reduce infant exposure. NAC is minimally absorbed through inhalation; breastfeeding usually continues without extra precautions 140.
- Children: The mean half-life of N-acetylcysteine is elevated in newborns (11 hours) compared to adults (5.6 hours). According to the American Academy of Pediatrics, acetaminophen overdose remains the primary cause of hospitalizations in the United States due to poisoning in the pediatric population. Self-inflicted acetaminophen poisoning remains a particular problem, especially among adolescents 141, 142.
- Geriatric patients: To prevent fluid overload, adjust the volume of N-acetylcysteine injection for older patients or those who need fluid restriction. Fluid overload can lead to serious complications like hyponatremia and seizures.
- Drug-Drug Interaction: Nitroglycerin interacts moderately with N-acetylcysteine, as their coadministration may result in hypotension and nitroglycerin-induced headache 143. Carbamazepine when co-administered with N-acetylcysteine can result in a lower-than-desired blood concentration of carbamazepine.
Acetaminophen overdose and toxicity
Acetaminophen (paracetamol) is a pain medicine. Acetaminophen (paracetamol) overdose occurs when someone takes more than the recommended amount of this medicine. Acetaminophen (paracetamol) overdose is one of the most common poisonings. People often think that acetaminophen (paracetamol) is very safe. However, it can be deadly if taken in large doses. Adults should not take more than 3,000 mg of single-ingredient acetaminophen (paracetamol) a day. You should take less if you are 65 or more years old. Taking more, especially 7,000 mg or more, can lead to a severe acetaminophen (paracetamol) overdose problems. People with liver disease are more likely to develop serious complications of acetaminophen overdose. Overdose may be either acute (sudden or short-term) or chronic (long-term), depending on the acetaminophen doses taken, and symptoms may therefore vary. If you have liver or kidney disease, you should discuss the use of this drug with your doctor.
The decision to give N-acetylcysteine in acetaminophen (paracetamol) overdose depends on the likelihood of liver damage and toxicity in the patient 3. Assessment is obtained from a thorough history, physical examination, and serum acetaminophen (paracetamol) and liver transaminase concentrations 3.
A detailed history, including the quantity of acetaminophen (paracetamol) consumed, is necessary. The clinician must know whether consumption took place at once or over some time. History of co-ingestants like anticholinergic medications or opioids could cause delayed acetaminophen (paracetamol) absorption, and assessing the presence of risk factors, including malnutrition, alcoholism, or cirrhosis, have associations with decreased glutathione reserves.
Whether the acetaminophen (paracetamol) formulation is a regular or an extended-release (ER) preparation should be determined, as the extended-release (ER) formulation can cause a delayed peak serum concentration. The concurrent use of drugs that can induce CYP2E1 (for example, isoniazid and chronic alcohol consumption) should also be determined, as this increases the risk of hepatotoxicity.
The person with acetaminophen (paracetamol) overdose may receive 144:
- Activated charcoal
- Airway support, including oxygen, breathing tube through the mouth (intubation), and ventilator (breathing machine)
- Blood and urine tests
- Chest x-ray
- CT (computerized axial tomography) scan
- ECG (electrocardiogram, or heart tracing)
- Fluids through the vein (intravenous or IV)
- Laxative
- Medicines to treat symptoms, including an antidote, N-acetylcysteine (NAC), to counteract the effects of the drug
The Rumack-Mathew Nomogram is a useful tool to assess the risk of liver toxicity and the need to start N-acetylcysteine in single acute ingestion of acetaminophen (paracetamol) 145:
- If the time of ingestion of acetaminophen (paracetamol) is less than 4 hours, 4-hour levels of serum acetaminophen (paracetamol) are obtained and plotted on the nomogram. If the value is above the treatment line, starting N-acetylcysteine should be the course of action; if it is below, the risk of liver toxicity is virtually nonexistent.
- If the time of ingestion is between 4 and 24 hours and the time required to obtain serum acetaminophen (paracetamol) levels is less than 8 hours, one may wait for the acetaminophen (paracetamol) levels before deciding to start N-acetylcysteine. If the acetaminophen (paracetamol) levels reports are not obtainable until more than 8 hours, N-acetylcysteine can be started empirically and stopped if the levels are below the treatment line.
- If the dose is unclear or more than 24 hours have passed since ingestion, give the first dose of N-acetylcysteine and send acetaminophen (paracetamol) levels and transaminase levels. N-acetylcysteine can be continued if acetaminophen (paracetamol) levels are more than 10 mg/L or transaminases are elevated.
- In chronic ingestion, N-acetylcysteine therapy should be initiated if acetaminophen (paracetamol) levels are more than 20 mg/L or transaminases are elevated.
N-acetylcysteine may be given orally or IV with minimal differences in its effectiveness 146. The most common regimes are the 21-hour IV and 72-hour oral dosing protocols. N-acetylcysteine should be started in patients at risk of liver toxicity and continued if liver toxicity develops 3. N-acetylcysteine may be stopped following the completion of the protocol or upon resolution of liver toxicity, whichever occurs last 3. Both oral and IV routes of administration are equally efficacious in preventing and treating acetaminophen (paracetamol) toxicity 3. The IV route is preferable to the oral route in established liver failure and in patients who cannot tolerate oral N-acetylcysteine due to intractable vomiting or nausea 147.
The dosing schedule for the 21-hour IV N-acetylcysteine protocol 3:
- Loading dose: 150 mg/kg up to 15 g in 200 ml dextrose 5% water over 60 minutes.
- Second (maintenance) dose: 50 mg/kg up to a maximum of 5 g in 500 ml dextrose 5% water over 4 hours (12.5 mg/kg/hour).
- Third dose: 100 mg/kg up to 10 g in 1000 ml dextrose 5% water over 16 hours (6.25 mg/kg/hour).
The dosing schedule for the 72-hour oral N-acetylcysteine protocol is as follows 3:
- 140 mg/kg loading dose orally.
- After 4 hours of administering the loading dose, 70 mg/kg should be given every 4 hours for an additional 17 doses, for a total dose of 1330 mg/kg. The solution should be diluted to 5% and mixed with a soft drink or juice to enhance palatability.
- Any vomited doses should be re-administered.
N-acetylcysteine should be continued until acetaminophen (paracetamol) levels are undetectable, PT/INR is near normal, encephalopathy has resolved, transaminases are normal or are down-trending, and AST <1000 U/L 3. In the 21-hour IV protocol, the acetaminophen (paracetamol) levels and transaminase level testing should occur at 20 hours. The oral protocol requires checking at 24 hours 3. If acetaminophen (paracetamol) is undetectable and transaminase levels are normal, N-acetylcysteine can be discontinued at the end of the regime 3.
If the patient has a detectable acetaminophen (paracetamol) level or AST is still elevated, restarting N-acetylcysteine at 6.25 mg/kg per hour (for IV protocol) or 70 mg/kg every 4 hours (for oral protocol) is the proper course 3. This can be continued until the patient returns to normal mental status and INR is below 2.0 or if the patient obtains a liver transplant 3.
Lung diseases
According to the American College of Chest Physicians, for patients with moderate to severe chronic obstructive pulmonary disease (COPD) and a history of 2 or more exacerbations in the previous 2 years, oral N-acetylcysteine treatment is suggested to prevent acute exacerbations of COPD 148. If necessary, dilute the 20% solution and refrigerate any unused portion for 96 hours to avoid contamination 3. The 10% solution can be used directly without dilution 3. The recommendation is to administer a bronchodilator approximately 10 to 15 minutes before administering N-acetylcysteine via nebulization 3.
- Nebulization (facemask, mouthpiece): When using a face mask or mouthpiece for nebulization, the recommended dose for most patients is 3 mL to 5 mL (20% solution) or 6 mL to 10 mL (10% solution) 3 or 4 times a day.
- Nebulization (tent or croupette): In special circumstances where nebulization in a tent or roulette is required, adjust the dosage based on equipment and the patient’s needs. This method may involve large volumes of solution, occasionally up to 300 mL, during a single treatment period.
- Direct instillation: Administer 1 mL to 2 mL (10% to 20% solution) as frequently as every hour by direct instillation.
- Routine nursing care of tracheostomy: For patients with tracheostomy, administer 1 mL to 2 mL (10% to 20% solution) every 1 to 4 hours through instillation into the tracheostomy.
- Direct introduction in the bronchopulmonary tree: Introduce N-acetylcysteine directly into a specific segment of the bronchopulmonary tree by inserting a small plastic catheter into the trachea under local anesthesia and direct vision. Instill 2 mL to 5 mL of the 20% solution using a syringe connected to the catheter.
- Percutaneous intratracheal catheter: Using a percutaneous intratracheal catheter, administer 1 mL to 2 mL (20% solution) or 2 mL to 4 mL (10% solution) every 1 to 4 hours through a syringe attached to the catheter.
- Diagnostic bronchograms: N-acetylcysteine can enhance visibility and facilitate access to the underlying tissue. Before diagnostic bronchial studies, administer 2 or 3 doses of 1 mL to 2 mL (20% solution) or 2 mL to 4 mL (10% solution) through nebulization or intratracheal instillation 149.
N-acetylcysteine Treatment Monitoring
During IV N-acetylcysteine administration, patients require monitoring for manifestations of an anaphylactoid reaction (severe potentially life-threatening allergic reaction), in the case of acetaminophen toxicity, plasma or serum acetaminophen concentration post-loading dose and after the last maintenance dose is suggested to evaluate efficacy. As the pharmacokinetics of patients with severe liver impairment are altered, monitoring through liver function tests such as ALT, AST, bilirubin, and prothrombin time is necessary 150. Evaluation of kidney function should be performed by monitoring serum creatinine, blood urea nitrogen, blood glucose, and electrolytes before initiating the whole course of therapy. Patients with a medical history of asthma or bronchospasm administration of N-acetylcysteine require monitoring.
N-acetylcysteine contraindications
In patients with a tendency to develop fluid overload (eg, cardiomyopathy or congestive heart failure), the quantity of diluent fluid used in IV N-acetylcysteine must be appropriately titrated to prevent fluid overload 3. The total volume must be adjusted for patients requiring fluid restriction and those less than 40 kg. A pharmacist can perform this titration while preparing the N-acetylcysteine IV infusion 3. Monitor for acute flushing and erythema of the skin when given IV N-acetylcysteine; usually, they are associated with administration of the loading dose and resolve spontaneously after continued infusion. Monitor for serious anaphylactoid reactions (severe potentially life-threatening allergic reactions) for IV administration, and infusion may be stopped until the treatment of anaphylactoid symptoms has been initiated 151. N-acetylcysteine infusion should be used cautiously in patients with a history of bronchospasm or asthma.
IV N-acetylcysteine can cause rate-related anaphylactoid reactions (severe potentially life-threatening allergic reactions) in up to 18% of patients, which is not an issue with the oral route 152. Most anaphylactoid reactions are mild (6%) or moderate (10%), with severe reactions like bronchospasm and hypotension rare at 1% 3. Anaphylactoid reactions occur more commonly with lower acetaminophen levels than with higher acetaminophen levels 153. One possible explanation is that acetaminophen decreases the histamine release from mast cells and mononuclear cells proportionate to the dose ingested. Bronchospasm more commonly occurs in patients with pre-existing reactive airway diseases, like asthma. Bronchodilating agents are effective in treating these patients 3.
N-acetylcysteine side effects
N-acetylcysteine can cause side effects such as dry mouth, nausea, vomiting, diarrhea, flatus, and gastroesophageal reflux 3, 58, 10. One of the properties of N-acetylcysteine is that it has an unpleasant smell and taste that some people find hard to tolerate. Vomiting after intravenous use has been reported in about 11% at doses of 150 mg/kg and one anaphylactic reaction has been reported 36. N-acetylcysteine has anticoagulant and platelet inhibiting properties and the use in patients with bleeding disorders or blood thinners may be relatively counterindicated 37. The use of N-acetylcysteine with patients on nitroglycerine should be cautioned, since it may cause hypotension 38. Other more rare side effects may include inflammation of the mucous membrane of the mouth (stomatitis), drowsiness, runny nose (rhinorrhea), and coughing up blood (hemoptysis) 39.
IV N-acetylcysteine can cause rate-related anaphylactoid reactions (severe potentially life-threatening allergic reactions) in up to 18% of patients, which is not an issue with the oral route 152. Most anaphylactoid reactions are mild (6%) or moderate (10%), with severe reactions like bronchospasm and hypotension rare at 1% 3. Anaphylactoid reactions occur more commonly with lower acetaminophen levels than with higher acetaminophen levels 153. One possible explanation is that acetaminophen decreases the histamine release from mast cells and mononuclear cells proportionate to the dose ingested. Bronchospasm more commonly occurs in patients with pre-existing reactive airway diseases, like asthma. Bronchodilating agents are effective in treating these patients 3.
Get emergency medical help if you have signs of an allergic reaction to N-acetylcysteine:
- hives;
- difficult breathing;
- swelling of your face, lips, tongue, or throat.
When an anaphylactoid reaction occurs, N-acetylcysteine should be stopped immediately, and the patient should be treated with anti-histamine medication (eg, diphenhydramine) and IV fluid for hypotension 3. Vasopressors are not typically necessary. N-acetylcysteine therapy may restart at a slower rate after the resolution of the reaction 3. Oral N-acetylcysteine is the alternative approach if there is a persistent reaction 3. IV N-acetylcysteine can cause a spurious increase in INR, which normalizes when the infusion stops; it can also cause a false-positive result for urine ketones 154, 155. Oral N-acetylcysteine may cause vomiting in up to 33% of cases 156. In patients with preexistent gastrointestinal ulcers or varices, there may be concerns about inducing gastrointestinal bleeding with oral N-acetylcysteine.
N-acetylcysteine may cause serious side effects. See your doctor at once if you have:
- severe or ongoing vomiting;
- coughing up blood or vomit that looks like coffee grounds; or
- signs that the medicine may not be working–upper stomach pain, loss of appetite, dark urine, clay-colored stools, jaundice (yellowing of the skin or eyes).
Common side effects of N-acetylcysteine may include:
- nausea, vomiting, upset stomach;
- rash;
- fever.
This is not a complete list of side effects and others may occur. See your doctor for medical advice about side effects.
N-acetylcysteine overdose
Given N-acetylcysteine’s complicated regime, N-acetylcysteine has a high potential for iatrogenic errors, including overdose 157. The signs of N-acetylcysteine overdose are documented to include hemolysis, thrombocytopenia, metabolic acidosis, acute renal failure, and elevated serum bilirubin 3. Though the mild signs improve within a few days, severe signs may lead to fatal consequences. Massive accidental N-acetylcysteine administration of 100 mg/kg/hr had resulted in cerebral edema, seizures, uncal herniation, and permanent brain injury in a patient with an acetaminophen (paracetamol) overdose 158.
- Wang H-C, Chou H-C, Chen C-M. Molecular Mechanisms of Hyperoxia-Induced Neonatal Intestinal Injury. International Journal of Molecular Sciences. 2023; 24(5):4366. https://doi.org/10.3390/ijms24054366[↩]
- Radomska-Leśniewska D.M., Skopiński P. N-acetylcysteine as an anti-oxidant and anti-inflammatory drug and its some clinical applications. Cent. J. Immunol. 2012;37:57–66.[↩]
- Ershad M, Naji A, Patel P, et al. N-Acetylcysteine. [Updated 2024 Feb 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537183[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Acetylcysteine. [Updated 2016 Nov 7]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548401[↩]
- El-Maddawy ZK, El-Sayed YS. Comparative analysis of the protective effects of curcumin and N-acetyl cysteine against paracetamol-induced hepatic, renal, and testicular toxicity in Wistar rats. Environ Sci Pollut Res Int. 2018 Feb;25(4):3468-3479. doi: 10.1007/s11356-017-0750-3[↩]
- Scalley RD, Conner CS. Acetaminophen poisoning: a case report of the use of acetylcysteine. Am J Hosp Pharm. 1978 Aug;35(8):964-7.[↩]
- N-Acetyl Cysteine (NAC). https://medlineplus.gov/druginfo/natural/1018.html[↩][↩]
- Gracey M., Burke V., Anderson C. M. Treatment of abdominal pain in cystic fibrosis by oral administration of n-acetyl cysteine. Archives of Disease in Childhood. 1969;44(235):404–405. doi: 10.1136/adc.44.235.404[↩]
- Idiopathic Pulmonary Fibrosis. Center for Drug Evaluation and Research (CDER) and the U.S. Food and Drug Administration (FDA). https://www.fda.gov/media/91396/download[↩]
- Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin Biol Ther. 2008 Dec;8(12):1955-62. doi: 10.1517/14728220802517901[↩][↩][↩][↩]
- Adair JC, Knoefel JE, Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology. 2001 Oct 23;57(8):1515-7. doi: 10.1212/wnl.57.8.1515[↩]
- Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 2011 Mar;36(2):78-86. doi: 10.1503/jpn.100057[↩][↩]
- Javaherforooshzadeh F, Shaker Z, Rashidi M, Akhondzadeh R, Hayati F. The effect of N-acetyl cysteine injection on renal function after coronary artery bypass graft surgery: a randomized double blind clinical trial. J Cardiothorac Surg. 2021 Jun 5;16(1):161. doi: 10.1186/s13019-021-01550-7[↩]
- Fliser D., Laville M., Covic A., Fouque D., Vanholder R., Juillard L., Van Biesen W., Ad-hoc working group of ERBP A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol. Dial. Transplant. 2012;27(12):4263–4272. doi: 10.1093/ndt/gfs375[↩]
- Diniz Y.S., Rocha K.K.H.R., Souza G.A., Galhardi C.M., Ebaid G.M.X., Rodrigues H.G., Novelli Filho J.L.V.B., Cicogna A.C., Novelli E.L.B. Effects of N-acetylcysteine on sucrose-rich diet-induced hyperglycaemia, dyslipidemia and oxidative stress in rats. Eur. J. Pharmacol. 2006;543:151–157. doi: 10.1016/j.ejphar.2006.05.039[↩]
- Campos K.E., Diniz Y.S., Cataneo A.C., Faine L.A., Alves M.J.Q.F., Novelli E.L.B. Hypoglycaemic and antioxidant effects of onion, Allium cepa: Dietary onion addition, antioxidant activity and hypoglycaemic effects on diabetic rats. Int. J. Food Sci. Nutr. 2003;54:241–246. doi: 10.1080/09637480120092062[↩]
- Pizzorno J. Glutathione! Integr Med (Encinitas). 2014 Feb;13(1):8-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4684116[↩]
- Lu S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008[↩]
- Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. N-Acetylcysteine–a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol. 2007 Aug;7(4):355-9. doi: 10.1016/j.coph.2007.04.005[↩]
- Minich DM, Brown BI. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients. 2019 Sep 3;11(9):2073. doi: 10.3390/nu11092073[↩][↩][↩][↩][↩][↩][↩][↩]
- Jones D.P., Park Y., Gletsu-Miller N., Liang Y., Yu T., Accardi C.J., Ziegler T.R. Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition. 2011;27:199–205. doi: 10.1016/j.nut.2010.01.014[↩]
- Vasdev S., Singal P., Gill V. The antihypertensive effect of cysteine. The International Journal of Angiology: Official Publication of the International College of Angiology, Inc. 2009;18(1):7–21. doi: 10.1055/s-0031-1278316[↩]
- Skvarc D.R., Dean O.M., Byrne L.K., Gray L., Lane S., Lewis M., Fernanders B.S., Berk M., Marriott A. The effect of N-acetylcysteine (NAC) on human cognition—A systematic review. Neurosci. Biobehav. Rev. 2017;78:44–56. doi: 10.1016/j.neubiorev.2017.04.013[↩]
- Jannatifar R., Parivar K., Roodbari N.H., Nasr-Esfahani M.H. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod Biol. Endocrinol. 2019;17:24. doi: 10.1186/s12958-019-0468-9[↩]
- Zhang Q., Ju Y., Ma Y., Wang T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: A randomized controlled trial. Medicine. 2018;97:e13087. doi: 10.1097/MD.0000000000013087[↩]
- Rushworth G.F., Megson I.L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014;141:150–159. doi: 10.1016/j.pharmthera.2013.09.006[↩]
- Ellegaard P.K., Licht R.W., Nielsen R.E., Dean O.M., Berk M., Poulsen H.E., Mohebbi M., Nielsen C.T. The efficacy of adjunctive N-acetylcysteine in acute bipolar depression: A randomized placebo-controlled study. J. Affect. Disord. 2019;245:1043–1051. doi: 10.1016/j.jad.2018.10.083[↩]
- Berk M., Turner A., Malhi G.S., Ng C.H., Cotton S.M., Dodd S., Samuni Y., Tanious M., McAulay C., Dowling N., et al. A randomised controlled trial of a mitochondrial therapeutic target for bipolar depression: mitochondrial agents, N-acetylcysteine, and placebo. BMC Med. 2019;17:18. doi: 10.1186/s12916-019-1257-1[↩]
- Panizzutti B., Bortolasci C., Hasebe K., Kidnapillai S., Gray L., Walder K., Berk M., Mohebbi M., Dodd S., Gama C., et al. Mediator effects of parameters of inflammation and neurogenesis from a N-acetyl cysteine clinical-trial for bipolar depression. Acta Neuropsychiatr. 2018;30:334–341. doi: 10.1017/neu.2018.13[↩]
- McCarty MF, O’Keefe JH, DiNicolantonio JJ. Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection. Ochsner J. 2018 Spring;18(1):81-87. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5855430[↩]
- Sekhar R.V., Patel S.G., Guthikonda A.P., Reid M., Balasubramanyam A., Taffet G.E., Jahoor F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am. J. Clin. Nutr. 2011;94:847–853. doi: 10.3945/ajcn.110.003483[↩][↩][↩][↩][↩]
- Coles L.D., Tuite P.J., Öz G., Mishra U.R., Kartha R.V., Sullivan K.M., Cloyd J.C., Terpstra M. Repeated-dose oral N-Acetylcysteine in Parkinson’s disease: Pharmacokinetics and effect on brain glutathione and oxidative stress. J. Clin. Pharmacol. 2018;58:158–167. doi: 10.1002/jcph.1008[↩][↩]
- Holmay M.J., Terpstra M., Coles L.D., Mishra U., Ahlskog M., Oz G., Cloyd J.C., Tuite P.J. N-Acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin. Neuropharmacol. 2013;36:103–106. doi: 10.1097/WNF.0b013e31829ae713[↩][↩]
- Wink L.K., Adams R., Wang Z., Klaunig J.E., Plawecki M.H., Posey D.J., McDpugle C.J., Erickson C.A. A randomized placebo-controlled pilot study of N-acetylcysteine in youth with autism spectrum disorder. Mol. Autism. 2016;7:26. doi: 10.1186/s13229-016-0088-6[↩]
- Lin C.Y., Wu J.L., Shih T.S., Tsai P.J., Sun Y.M., Ma M.C., Guo Y.L. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res. 2010;269:42–47. doi: 10.1016/j.heares.2010.07.005[↩]
- Šalamon Š, Kramar B, Marolt TP, Poljšak B, Milisav I. Medical and Dietary Uses of N-Acetylcysteine. Antioxidants (Basel). 2019 Apr 28;8(5):111. doi: 10.3390/antiox8050111[↩][↩][↩]
- Niemi T. T. The effect of N-acetylcysteine on blood coagulation and platelet function in patients undergoing open repair of abdominal aortic aneurysm. Blood Coagulation and Fibrinolysis. 2006;17(1):29–34. doi: 10.1097/01.mbc.0000195922.26950.89[↩][↩]
- Ardissino D., Savonitto S., Merlini P. A., et al. Effect of transdermal nitroglycerin or N-acetylcysteine, or both, in the long-term treatment of unstable Angina pectoris. Journal of the American College of Cardiology. 1997;29(5):941–947. doi: 10.1016/s0735-1097(97)00005-3[↩][↩]
- Sansone RA, Sansone LA. Getting a Knack for NAC: N-Acetyl-Cysteine. Innov Clin Neurosci. 2011 Jan;8(1):10-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3036554/[↩][↩]
- Kerksick C., Willoughby D. The antioxidant role of glutathione and N-Acetyl-Cysteine supplements and exercise-induced oxidative stress. Journal of the International Society of Sports Nutrition. 2005;2(2):p. 38. doi: 10.1186/1550-2783-2-2-38[↩]
- Fass D., Thorpe C. Chemistry and enzymology of disulfide cross-linking in proteins. Chemical Review. 2018;118(3):1169–1198. doi: 10.1021/acs.chemrev.7b00123[↩]
- De Flora S., Izzotti A., D’Agostini F., Balansky R. Mechanisms of N-acetylcysteine in the prevention of DNA damage and cancer, with special reference to smoking-related end-points. Carcinogenesis. 2001;22(7):999–1013. doi: 10.1093/carcin/22.7.999[↩]
- Halasi M., Wang M., Chavan T. S., Gaponenko V., Hay N., Gartel A. L. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochemical Journal. 2013;454(2):201–208. doi: 10.1042/bj20130282[↩]
- Uraz S. N-acetylcysteine expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic acid-induced colitis in rats. Scandinavian Journal of Clinical and Laboratory Investigation. 2013;73(1):61–66. doi: 10.3109/00365513.2012.734859[↩]
- Zhitkovich A. N-Acetylcysteine: Antioxidant, aldehyde scavenger, and more. Chemical Research in Toxicology. 2019;32(7):1318–1319. doi: 10.1021/acs.chemrestox.9b00152[↩]
- Livingstone C. R., Andrews M. A., Jenkins S. M., Marriott C. Model systems for the evaluation of mucolytic drugs: acetylcysteine and S-carboxymethylcysteine. Journal of Pharmacy and Pharmacology. 1990;42(2):73–78. doi: 10.1111/j.2042-7158.1990.tb05357.x[↩]
- Fries G. R., Kapczinski F. N-acetylcysteine as a mitochondrial enhancer: a new class of psychoactive drugs? Brazilian Journal of Psychiatry. 2011;33:321–322. doi: 10.1590/s1516-44462011000400003[↩]
- Jiao Y. N-acetyl cysteine depletes reactive oxygen species and prevents dental monomer-induced intrinsic mitochondrial apoptosis in vitro in human dental pulp cells. PLOS ONE. 2016;11(1) doi: 10.1371/journal.pone.0147858.e0147858[↩]
- Rossignol D. A. The use of N-acetylcysteine as a chelator for metal toxicity. In: Frye R. E., Berk M., editors. The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine. Singapore: Springer Singapore; 2019. pp. 169–179.[↩][↩]
- Tomàs Casanova M. G. N-Acetylcysteine: An old drug with variable anti-influenza properties. Journal of Controversies in Biomedical Research. 2016;2(1):1–8. doi: 10.15586/jcbmr.2016.13[↩]
- Redondo P., Jimenez E., Perez A., García-Foncillas J. N-acetylcysteine downregulates vascular endothelial growth factor production by human keratinocytes in vitro. Archives of Dermatological Research. 2000;292(12):621–628. doi: 10.1007/s004030000187[↩]
- Douiev L., Soiferman D., Alban C., Saada A. The effects of ascorbate, N-acetylcysteine, and resveratrol on fibroblasts from patients with mitochondrial disorders. Journal of Clinical Medicine. 2016;6(1) doi: 10.3390/jcm6010001[↩]
- Martina V. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care. 2008;31(5):940–944. doi: 10.2337/dc07-2251[↩]
- Schwalfenberg GK. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J Nutr Metab. 2021 Jun 9;2021:9949453. doi: 10.1155/2021/9949453[↩][↩][↩]
- Potęga A. Glutathione-Mediated Conjugation of Anticancer Drugs: An Overview of Reaction Mechanisms and Biological Significance for Drug Detoxification and Bioactivation. Molecules. 2022 Aug 17;27(16):5252. doi: 10.3390/molecules27165252[↩]
- Hall MN, Niedzwiecki M, Liu X, Harper KN, Alam S, Slavkovich V, Ilievski V, Levy D, Siddique AB, Parvez F, Mey JL, van Geen A, Graziano J, Gamble MV. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ Health Perspect. 2013 Sep;121(9):1068-74. doi: 10.1289/ehp.1205727[↩]
- Raghu G, Berk M, Campochiaro PA, Jaeschke H, Marenzi G, Richeldi L, Wen FQ, Nicoletti F, Calverley PMA. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr Neuropharmacol. 2021;19(8):1202-1224. doi: 10.2174/1570159X19666201230144109[↩]
- Frye R.E., Andrus J.P., Lemley K.V., De Rosa S.C., Ghezzi P., Holmgren A., Jones D., Jahoor F., Kopke R., Cotgreave I., Bottiglieri T., Kaplowitz N., Nakamura H., Staal F., Ela S.W., Atkuri K.R., Tirouvanziam R., Heydari K., Sahaf B., Zolopa A., Mantovani J.J., Herzenberg L.A., Herzenberg L.A. In: The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine. Frye R.E., Berk M., editors. Singapore: Springer Nature; 2019. Pharmacology, Formulations, and Adverse Effects. p. 392.[↩][↩]
- Mokhtari V, Afsharian P, Shahhoseini M, Kalantar SM, Moini A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017 Apr-Jun;19(1):11-17. doi: 10.22074/cellj.2016.4872[↩]
- Deepmala, Kumar N., Berk M., et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neuroscience and Biobehavioral Reviews. 2015;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015[↩]
- Dludla P. V., Tiano L., Louw J., et al. The beneficial effects of N-acetyl cysteine (NAC) against obesity associated complications: a systematic review of pre-clinical studies. Pharmacological Research. 2019;146 doi: 10.1016/j.phrs.2019.104332.104332[↩]
- Fowdar K., Chen H., He Z., et al. The effect of N-acetylcysteine on exacerbations of chronic obstructive pulmonary disease: a meta-analysis and systematic review. Heart and Lung. 2017;46(2):120–128. doi: 10.1016/j.hrtlng.2016.12.004[↩]
- Devi N., Boya C. Bansal D. N-acetyl-cysteine as adjuvant therapy in female infertility: a systematic review and meta-analysis. Journal of Basic and Clinical Physiology and Pharmacology. 2020[↩]
- Shi Z., Puyo C. A. N-acetylcysteine to combat COVID-19: an evidence review. Therapeutics and Clinical Risk Management. 2020;16:1047–1055. doi: 10.2147/tcrm.s273700[↩]
- National Drug Code Directory. https://www.accessdata.fda.gov/scripts/cder/ndc/dsp_searchresult.cfm[↩]
- Prescott L.F., Illingworth R.N., Critchley J.A., Stewart M.J., Adam R.D., Proudfoot A.T. Intravenous N-acetylcystine: The treatment of choice for paracetamol poisoning. BMJ. 1979;2(6198):1097–1100. doi: 10.1136/bmj.2.6198.1097[↩]
- Prescott L.F., Park J., Ballantyne A., Adriaenssens P., Proudfoot A.T. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2(8035):432–434. doi: 10.1016/S0140-6736(77)90612-2[↩]
- Peterson R. G., Rumack B. H. N-acetylcysteine for acetaminophen overdosage (cont.) New England Journal of Medicine. 1977;296(9):p. 515. doi: 10.1056/nejm19770303296091[↩]
- Smilkstein M. J., Knapp G. L., Kulig K. W., Rumack B. H. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) New England Journal of Medicine. 1988;319(24):1557–1562. doi: 10.1056/nejm198812153192401[↩]
- Hurst GA, Shaw PB, LeMaistre CA. Laboratory and clinical evaluation of the mucolytic properties of acetylcysteine. Am Rev Respir Dis. 1967 Nov;96(5):962-70. doi: 10.1164/arrd.1967.96.5.962[↩]
- Tirouvanziam R., Conrad C. K., Bottiglieri T., et al. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(12):4628–4633. doi: 10.1073/pnas.0511304103[↩]
- Conrad C., Lamp J., Dunn C., et al. Long-term treatment with oral N-acetylcysteine: affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. Journal of Cystic Fibrosis. 2015;14(2):219–227. doi: 10.1016/j.jcf.2014.08.008[↩]
- Tam J, Nash EF, Ratjen F, Tullis E, Stephenson A. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst Rev. 2013 Jul 12;2013(7):CD007168. doi: 10.1002/14651858.CD007168.pub3[↩]
- Duijvestijn Y. C., Brand P. L. Systematic review of N-acetylcysteine in cystic fibrosis. Acta Paediatrica. 1999;88(1):38–41. doi: 10.1080/08035259950170574[↩]
- McNamara J., Zhang L., Demirel N. The effect of long term use of inhaled N-acetylcysteine on cystic fibrosis lung disease. C67. Suppurative Lung Diseases In Children. 2017A6142[↩]
- Schauble A. L., Bisaccia E. J., Lee G., Nazr S. Z. N-acetylcysteine for management of distal intestinal obstruction syndrome. The Journal of Pediatric Pharmacology and Therapeutics: JPPT: The Official Journal of PPAG. 2019;24(5):390–397. doi: 10.5863/1551-6776-24.5.390[↩]
- Simon LM, Suttorp N. Lung cell oxidant injury: decrease in oxidant mediated cytotoxicity by N-acetylcysteine. Eur J Respir Dis Suppl. 1985;139:132-5.[↩]
- Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001 Aug;46(8):798-825. https://goldcopd.org/gold-reports/[↩]
- Criner G.J., Bourbeau J., Diekemper R.L., Ouellette D.R., Goodridge D., Hernandez P., Curren K., Balter M.S., Bhutani M., Camp P.G., Celli B.R., Dechman G., Dransfield M.T., Fiel S.B., Foreman M.G., Hanania N.A., Ireland B.K., Marchetti N., Marciniuk D.D., Mularski R.A., Ornelas J., Road J.D., Stickland M.K. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147(4):894–942. doi: 10.1378/chest.14-1676[↩]
- Cazzola M., Calzatta L., Page C., et al. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. European Respiratory Review. 2015;24(137):451–461. doi: 10.1183/16000617.00002215[↩]
- Stey C., Steurer J., Bachmann S., Medici T. C., Tramèr M. R. The effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic review. European Respiratory Journal. 2000;16(2):253–262. doi: 10.1034/j.1399-3003.2000.16b12.x[↩]
- Sanguinetti C. M. N-acetylcysteine in COPD: why, how, and when? Multidisciplinary Respiratory Medicine. 2015;11:p. 8. doi: 10.1186/s40248-016-0039-2[↩]
- Terluk M. R., Ebeling M. C., Fisher C. R., et al. N-Acetyl-L-cysteine protects human retinal pigment epithelial cells from oxidative damage: implications for age-related macular degeneration. Oxidative Medicine and Cellular Longevity. 2019;2019:14. doi: 10.1155/2019/5174957.5174957[↩]
- Schimel A. M., Abraham L., Cox D., et al. N-acetylcysteine amide (NACA) prevents retinal degeneration by up-regulating reduced glutathione production and reversing lipid peroxidation. American Journal of Pathology. 2011;178(5):2032–2043. doi: 10.1016/j.ajpath.2011.01.036[↩]
- Blasi F., Page C., Cazzola M., et al. The effect of N-acetylcysteine on biofilms: implications for the treatment of respiratory tract infections. Respiratory Medicine. 2016;117:190–197. doi: 10.1016/j.rmed.2016.06.015[↩]
- Qi Q., Li C., Li Y., et al. Effect of N-acetylcysteine on exacerbations of bronchiectasis (BENE): a randomized controlled trial. Respiratory Research. 2019;20(1):p. 73. doi: 10.1186/s12931-019-1042-x[↩]
- Naz F., Raza A. B., Ijaz I., Kazi M. Y. Effectiveness of nebulized N-acetylcysteine solution in children with acute bronchiolitis. Journal of College of Physicians and Surgeons Pakistan. 2014;24(6):408–411. doi: 10.2014/JCPSP.408411[↩]
- Prednisone, Azathioprine and N-acetylcysteine for pulmonary fibrosis. New England Journal of Medicine. 2012;366(21):1968–1977. doi: 10.1056/nejmoa1113354[↩][↩]
- Feng F, Zhang J, Wang Z, Wu Q, Zhou X. Efficacy and safety of N-acetylcysteine therapy for idiopathic pulmonary fibrosis: An updated systematic review and meta-analysis. Exp Ther Med. 2019 Jul;18(1):802-816. doi: 10.3892/etm.2019.7579[↩]
- Smith M. R., Davis R. L. Mycetismus: a review. Gastroenterology Report (Oxf) 2016;4(2):107–112. doi: 10.1093/gastro/gov062[↩]
- Drault J. N., Baelen E., Mehdaoui H., Delord J. M., Flament F. Massive paraquat poisoning. Favorable course after treatment with n-acetylcysteine and early hemodialysis. Annales Françaises d’Anesthésie et de Réanimation. 1999;18(5):534–537. doi: 10.1016/s0750-7658(99)80127-0[↩]
- Dell’Aglio DM, Sutter ME, Schwartz MD, Koch DD, Algren DA, Morgan BW. Acute chloroform ingestion successfully treated with intravenously administered N-acetylcysteine. J Med Toxicol. 2010 Jun;6(2):143-6. doi: 10.1007/s13181-010-0071-0[↩]
- Lai I. K., Dhakal K., Gadupudi G. S., et al. N-acetylcysteine (NAC) diminishes the severity of PCB 126-induced fatty liver in male rodents. Toxicology. 2012;302(1):25–33. doi: 10.1016/j.tox.2012.07.007[↩]
- Harrison P. M., Wendon J. A., Gimson A. E. S. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. New England Journal of Medicine. 1991;324(26):1852–1857. doi: 10.1056/nejm199106273242604[↩]
- Nabi T., et al. Role of N-acetylcysteine treatment in non-acetaminophen-induced acute liver failure: a prospective study. Saudi Journal of Gastroenterology. 2017;23(3):169–175. doi: 10.4103/1319-3767.207711[↩]
- Mumtaz K., Nabi S., Rafiq N., Shah A. Role of N-acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatology International. 2009;3(4):563–570. doi: 10.1007/s12072-009-9151-0[↩]
- Hu J. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: a meta-analysis of prospective clinical trials. Clinics and Research in Hepatology and Gastroenterology. 2015;39(5):594–599. doi: 10.1016/j.clinre.2015.01.003[↩]
- Bourgonje A. R., Dijkstra G., Goor H. V., et al. Crohn’s disease in clinical remission is marked by systemic oxidative stress. Frontiers in Physiology. 2019;10(499) doi: 10.3389/fphys.2019.00499[↩]
- Masnadi Shirazi K, Sotoudeh S, Masnadi Shirazi A, Moaddab SY, Nourpanah Z, Nikniaz Z. Effect of N-acetylcysteine on remission maintenance in patients with ulcerative colitis: A randomized, double-blind controlled clinical trial. Clin Res Hepatol Gastroenterol. 2021 Jul;45(4):101532. doi: 10.1016/j.clinre.2020.08.010[↩]
- Moura F. A., Andrade K. Q. D., Santos J. C. F. D., Araújo O. R. P., Goulart M. O. F. Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biology. 2015;6:617–639. doi: 10.1016/j.redox.2015.10.006[↩]
- O’Loghlen A. N-acetyl-cysteine abolishes hydrogen peroxide-induced modification of eukaryotic initiation factor 4F activity via distinct signalling pathways. Cell Signal. 2006;18(1):21–31. doi: 10.1016/j.cellsig.2005.03.013[↩][↩]
- Sagara M. Inhibition of development of peripheral neuropathy in streptozotocin-induced diabetic rats with N-acetylcysteine. Diabetologia. 1996;39(3):263–269. doi: 10.1007/s001250050440[↩]
- Zhu Y., Zhang X.-L., Zhu B.-F., Ding Y.-N. Effect of antioxidant N-acetylcysteine on diabetic retinopathy and expression of VEGF and ICAM-1 from retinal blood vessels of diabetic rats. Molecular Biology Reports. 2012;39(4):3727–3735. doi: 10.1007/s11033-011-1148-9[↩]
- Nogueira G. B., Punaro G. R., Oliveira C. S., et al. N-acetylcysteine protects against diabetic nephropathy through control of oxidative and nitrosative stress by recovery of nitric oxide in rats. Nitric Oxide. 2018;78:22–31. doi: 10.1016/j.niox.2018.05.003[↩]
- Heidari N., Sajedi F., Mohammadi Y., Mirjalili M., Mehrpooya M. Ameliorative effects of N-acetylcysteine as adjunct therapy on symptoms of painful diabetic neuropathy. Journal of Pain Research. 2019;12:3147–3159. doi: 10.2147/jpr.s228255[↩]
- Geiler J., Doerr H.-W., Cinatl J., et al. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochemical Pharmacology. 2010;79(3):413–420. doi: 10.1016/j.bcp.2009.08.025[↩][↩]
- Kretzmann N. A., Chiela E., Matte U., Marroni N., Marroni C. A. N-acetylcysteine improves antitumoural response of Interferon alpha by NF-kB downregulation in liver cancer cells. Comparative Hepatology. 2012;11(1):p. 4. doi: 10.1186/1476-5926-11-4[↩]
- Ciftci H., Verit A., Savas M., Yeni E., Erel O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology. 2009;74(1):73–76. doi: 10.1016/j.urology.2009.02.034[↩]
- Safarinejad M. R., Safarinejad S. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study. J Urol. 2009;181(2):741–751. doi: 10.1016/j.juro.2008.10.015[↩]
- Monti D. A., Zabrecky G., Leist T. P., et al. N-acetyl cysteine administration is associated with increased cerebral glucose metabolism in patients with multiple sclerosis: an exploratory study. Frontiers in Neurology. 2020;11:p. 88. doi: 10.3389/fneur.2020.00088[↩]
- Krysko K. N-acetyl cysteine for fatigue in progressive multiple sclerosis: a pilot randomized double-blind placebo-controlled trial (P5.2-093) Neurology. 2019;92(15 Supplement):P5–093.[↩]
- Choi I. Y., Lee S.-P., Denney D. R., Lynch S. G. Lower levels of glutathione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3 T. Multiple Sclerosis. 2011;17(3):289–296. doi: 10.1177/1352458510384010[↩]
- Walters MT, Rubin CE, Keightley SJ, Ward CD, Cawley MI. A double-blind, cross-over, study of oral N-acetylcysteine in Sjögren’s syndrome. Scand J Rheumatol Suppl. 1986;61:253-8.[↩]
- Lai Z. W., Hanczko R., Bonilla E., Caza T. N. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis and Rheumatology. 2012;64(9):2937–2946. doi: 10.1002/art.34502[↩]
- Dludla P. V., Louw J., Tiano L., et al. N-acetyl cysteine targets hepatic lipid accumulation to curb oxidative stress and inflammation in NAFLD: a comprehensive analysis of the literature. Antioxidants (Basel) 2020;9(12) doi: 10.3390/antiox9121283[↩]
- Offen D., Ziv I., Sternin H., et al. Prevention of dopamine-induced cell death by thiol antioxidants: possible implications for treatment of Parkinson’s disease. Experimental Neurology. 1996;141(1):32–39. doi: 10.1006/exnr.1996.0136[↩]
- Monti D. A., Zabrecky G., Kremens D., et al. N-acetyl cysteine is associated with dopaminergic improvement in Parkinson’s disease. Clinical Pharmacology & Therapeutics. 2019;106(4):884–890. doi: 10.1002/cpt.1548[↩]
- Fulghesu A. M. N-acetyl-cysteine treatment improves insulin sensitivity in women with polycystic ovary syndrome. Fertility and Sterility. 2002;77(6):1128–1135. doi: 10.1016/s0015-0282(02)03133-3[↩]
- Thakker D., Raval A., Patel I., Walia R. N-acetylcysteine for polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled clinical trials. Obstetrics and Gynecology International. 2015;2015:13. doi: 10.1155/2015/817849.817849[↩]
- Jenkins D. D. Fetal and neonatal effects of N-acetylcysteine when used for neuroprotection in maternal chorioamnionitis. The Journal of Pediatrics. 2016;168:67–76.e6. doi: 10.1016/j.jpeds.2015.09.076[↩]
- Amin A. F., Shaaban O. M., Bediawy M. A. N-acetyl cysteine for treatment of recurrent unexplained pregnancy loss. Reproductive Biomedicine Online. 2008;17(5):722–726. doi: 10.1016/s1472-6483(10)60322-7[↩]
- Uemura T. Protective effects of brain infarction by N-acetylcysteine derivatives. Stroke. 2018;49(7):1727–1733. doi: 10.1161/strokeaha.118.021755[↩]
- Sabetghadam M., Abolfathi P., Mohammadi Y., Mehrpooya M. Evidence for a beneficial effect of oral N-acetylcysteine on functional outcomes and inflammatory biomarkers in patients with acute ischemic stroke. Neuropsychiatric Disease and Treatment. 2020;(16):1265–1278. doi: 10.2147/ndt.s241497[↩]
- Moreira PI, Harris PL, Zhu X, Santos MS, Oliveira CR, Smith MA, Perry G. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J Alzheimers Dis. 2007 Sep;12(2):195-206. doi: 10.3233/jad-2007-12210[↩]
- Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr. 2005 Dec 9;2(2):38-44. doi: 10.1186/1550-2783-2-2-38[↩]
- Joy T., Rao M. S., Madhyastha S., Pai K. Effect of N-acetyl cysteine on intracerebroventricular colchicine induced cognitive deficits, beta amyloid pathology, and glial cells. Neuroscience Journal. 2019;2019:13. doi: 10.1155/2019/7547382.7547382[↩]
- Hara Y. Evaluation of the neuroprotective potential of N-acetylcysteine for prevention and treatment of cognitive aging and dementia. Journal of Prevention of Alzheimer’Disease. 2017;4(3):201–206. doi: 10.14283/jpad.2017.22[↩]
- Aliyali M., Poorhasan Amiri A., Sharifpoor A., Zalli F. Effects of N-acetylcysteine on asthma exacerbation. Iranian Journal of Allergy, Asthma and Immunology. 2010;9(2):103–109. doi: 10.02/ijaai.103109[↩]
- Eftekhari P, Hajizadeh S, Raoufy MR, Masjedi MR, Yang M, Hansbro N, Li JJ, Foster PS. Preventive effect of N-acetylcysteine in a mouse model of steroid resistant acute exacerbation of asthma. EXCLI J. 2013 Mar 11;12:184-92. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4531779[↩]
- Guibas G. V., Spandou E., Meditskou S., Vyzantiadis T. A., Priftis K. N., Anogianakis G. N-acetylcysteine exerts therapeutic action in a rat model of allergic rhinitis. International Forum of Allergy and Rhinology. 2013;3(7):543–549. doi: 10.1002/alr.21145[↩]
- Lane C. J., Redding D., Gonzalez K. A., Cardenas V., Boldogh I., Sur S. Topical N-acetyl cysteine (NAC) reduces late phase nasal symptoms following ragweed challenge. Journal of Allergy and Clinical Immunology. 2009;123(2):p. S53. doi: 10.1016/j.jaci.2008.12.170[↩]
- Kaga A. K., Barbanera P. O., Carmo N. O. L. D., et al. Effect of N-acetylcysteine on dyslipidemia and carbohydrate metabolism in STZ-induced diabetic rats. International Journal of Vascular Medicine. 2018;2018:7. doi: 10.1155/2018/6428630.6428630[↩]
- Falach-Malik A, Rozenfeld H, Chetboun M, Rozenberg K, Elyasiyan U, Sampson SR, Rosenzweig T. N-Acetyl-L-Cysteine inhibits the development of glucose intolerance and hepatic steatosis in diabetes-prone mice. Am J Transl Res. 2016 Sep 15;8(9):3744-3756[↩]
- Szkudlinska M. A., von Frankenberg A. D., Utzschneider K. M. The antioxidant N-Acetylcysteine does not improve glucose tolerance or β-cell function in type 2 diabetes. Journal of Diabetes and its Complications. 2016;30(4):618–622. doi: 10.1016/j.jdiacomp.2016.02.003[↩]
- Fontes L. E. S., Martimbianco A. L. C., Zanin C., Riera R. N-acetylcysteine as an adjuvant therapy for Helicobacter pylori eradication. Cochrane Database Systematic Review. 2019;2 doi: 10.1002/14651858.CD012357.pub2.CD012357[↩]
- Akerlund B., Jarstrand C., Lindeke B., Sönnerborg A., Akerblad A. C., Rasool O. Effect of N-acetylcysteine(NAC) treatment on HIV-1 infection: a double-blind placebo-controlled trial. European Journal of Clinical Pharmacology. 1996;50(6):457–461. doi: 10.1007/s002280050140[↩]
- Roederer M., Ela S. W., Staal F. J., Herzenberg L. A. N-acetylcysteine: a new approach to anti-HIV therapy. AIDS Research and Human Retroviruses. 1992;8(2):209–217. doi: 10.1089/aid.1992.8.209[↩]
- De Rosa S. C. N-acetylcysteine replenishes glutathione in HIV infection. European Journal of Clinical Investigation. 2000;30(10):915–929. doi: 10.1046/j.1365-2362.2000.00736.x[↩]
- Ye M, Lin W, Zheng J, Lin S. N-acetylcysteine for chronic kidney disease: a systematic review and meta-analysis. Am J Transl Res. 2021 Apr 15;13(4):2472-2485[↩]
- Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-. Acetylcysteine. [Updated 2019 Feb 7]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500931[↩]
- Shadman KA, Edmonson MB, Coller RJ, Sklansky DJ, Nacht CL, Zhao Q, Kelly MM. US Hospital Stays in Children and Adolescents With Acetaminophen Poisoning. Hosp Pediatr. 2022 Feb 1;12(2):e60-e67. doi: 10.1542/hpeds.2021-005816[↩]
- Nadler A, Fein DM. Acetaminophen Poisoning. Pediatr Rev. 2018 Jun;39(6):316-318. doi: 10.1542/pir.2017-0093[↩]
- Iversen HK. N-acetylcysteine enhances nitroglycerin-induced headache and cranial arterial responses. Clin Pharmacol Ther. 1992 Aug;52(2):125-33. doi: 10.1038/clpt.1992.121[↩]
- Acetaminophen overdose. https://medlineplus.gov/ency/article/002598.htm[↩]
- Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012 Oct;28(4):499-516. doi: 10.1016/j.ccc.2012.07.006[↩]
- Green JL, Heard KJ, Reynolds KM, Albert D. Oral and Intravenous Acetylcysteine for Treatment of Acetaminophen Toxicity: A Systematic Review and Meta-analysis. West J Emerg Med. 2013 May;14(3):218-26. doi: 10.5811/westjem.2012.4.6885[↩]
- Keays R, Harrison PM, Wendon JA, Forbes A, Gove C, Alexander GJ, Williams R. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ. 1991 Oct 26;303(6809):1026-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1671790/pdf/bmj00150-0028.pdf[↩]
- Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015 Apr;147(4):894-942. doi: 10.1378/chest.14-1676[↩]
- Young C, McCormack S. N-Acetylcysteine Instillation During Bronchoscopy for Patients Requiring Non-Cystic Fibrosis-Related Mucus Secretion Clearance: A Review of Clinical Effectiveness and Guidelines [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019 Aug 2. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549208[↩]
- Levine M, O’Connor AD, Padilla-Jones A, Gerkin RD. Comparison of Prothrombin Time and Aspartate Aminotransferase in Predicting Hepatotoxicity After Acetaminophen Overdose. J Med Toxicol. 2016 Mar;12(1):100-6. doi: 10.1007/s13181-015-0504-x[↩]
- Yarema M, Chopra P, Sivilotti MLA, Johnson D, Nettel-Aguirre A, Bailey B, Victorino C, Gosselin S, Purssell R, Thompson M, Spyker D, Rumack B. Anaphylactoid Reactions to Intravenous N-Acetylcysteine during Treatment for Acetaminophen Poisoning. J Med Toxicol. 2018 Jun;14(2):120-127. doi: 10.1007/s13181-018-0653-9. Epub 2018 Feb 8. Erratum in: J Med Toxicol. 2018 Jun;14(2):173. doi: 10.1007/s13181-018-0657-5[↩]
- Kerr F, Dawson A, Whyte IM, Buckley N, Murray L, Graudins A, Chan B, Trudinger B. The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Ann Emerg Med. 2005 Apr;45(4):402-8. doi: 10.1016/j.annemergmed.2004.08.040[↩][↩]
- Waring WS, Stephen AF, Robinson OD, Dow MA, Pettie JM. Lower incidence of anaphylactoid reactions to N-acetylcysteine in patients with high acetaminophen concentrations after overdose. Clin Toxicol (Phila). 2008 Jul;46(6):496-500. doi: 10.1080/15563650701864760[↩][↩]
- Jepsen S, Hansen AB. The influence of N-acetylcysteine on the measurement of prothrombin time and activated partial thromboplastin time in healthy subjects. Scand J Clin Lab Invest. 1994 Nov;54(7):543-7. doi: 10.3109/00365519409088566[↩]
- Poon R, Hinberg I, Peterson RG. N-acetylcysteine causes false-positive ketone results with urinary dipsticks. Clin Chem. 1990 May;36(5):818-9.[↩]
- Yip L, Dart RC. A 20-hour treatment for acute acetaminophen overdose. N Engl J Med. 2003 Jun 12;348(24):2471-2. doi: 10.1056/NEJM200306123482422[↩]
- Hayes BD, Klein-Schwartz W, Doyon S. Frequency of medication errors with intravenous acetylcysteine for acetaminophen overdose. Ann Pharmacother. 2008 Jun;42(6):766-70. doi: 10.1345/aph.1K685[↩]
- Heard K, Schaeffer TH. Massive acetylcysteine overdose associated with cerebral edema and seizures. Clin Toxicol (Phila). 2011 Jun;49(5):423-5. doi: 10.3109/15563650.2011.583664[↩]