Contents
What is propylene glycol
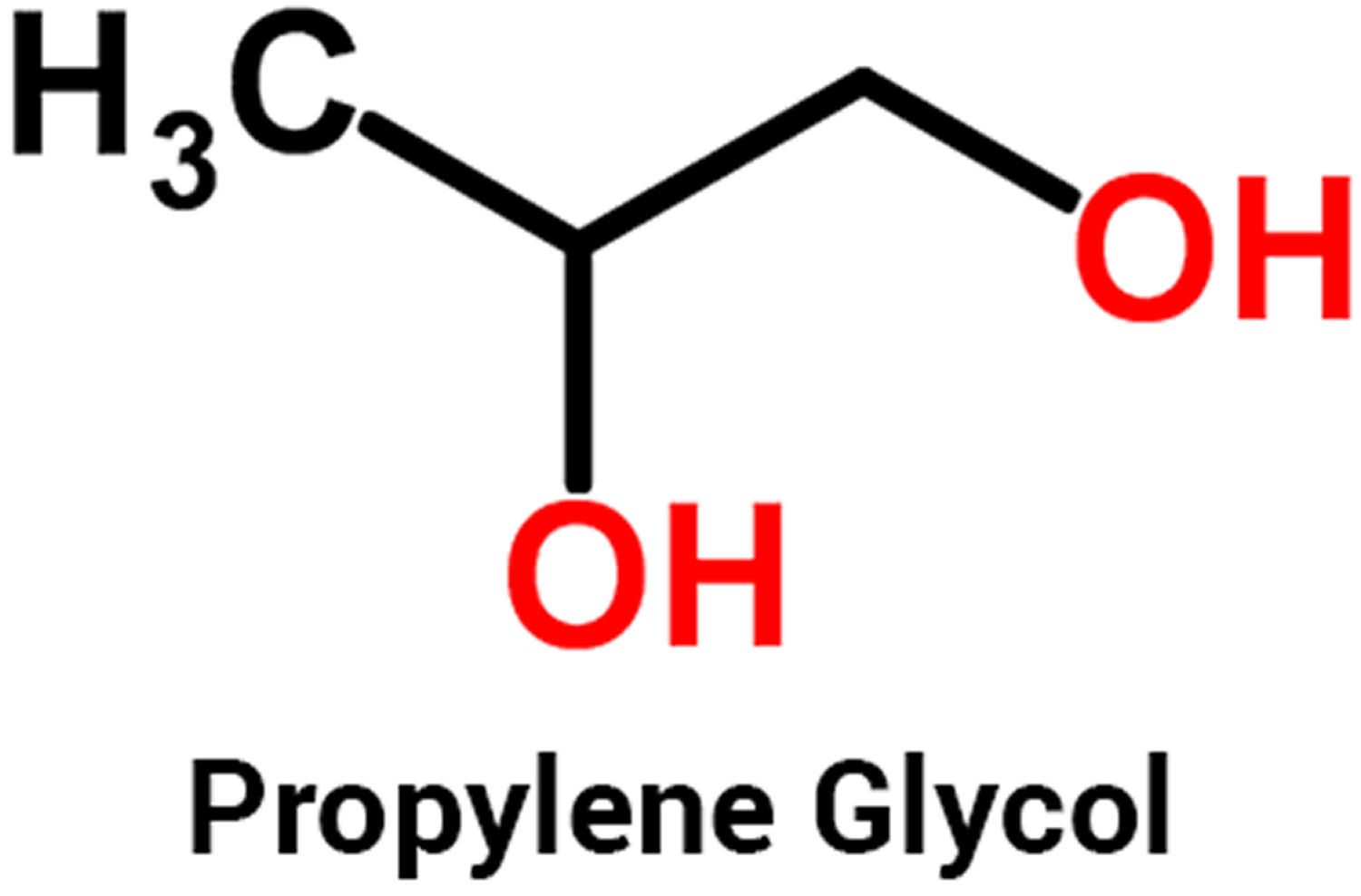
Propylene glycol (1,2-dihydroxypropane or 1,2-propanediol or methyl glycol or trimethyl glycol) is a water soluble alcohol and it is a clear liquid used in antifreeze and deicing solutions for cars, airplanes, and boats; to make polyester compounds; and as solvent in the paint and plastics industries. Propylene glycol is a clear, colorless, slightly syrupy liquids at room temperature. It may exist in air in the vapor form, although propylene glycol must be heated or briskly shaken to produce a vapor. Propylene glycol is practically odorless and tasteless. Propylene glycol is also used to create artificial smoke or fog used in fire-fighting training and in theatrical productions.
Propylene glycol is used in pharmaceuticals, as a drug vehicle (for example as a U.S. Food and Drug Administration (FDA)-approved solvent for intravenous diazepam) and preservative. Propylene glycol is also used in personal lubricants, in semi-moist pet food and as a humectant for tobacco. Propylene glycol is currently authorized as a food additive (E 1520). In the food industry propylene glycol (E 1520) is used as a solvent for food colors and flavors, as a humectant, preservative and emulsifier 1. Propylene glycol is used to absorb extra water and maintain moisture in certain medicines, cosmetics, or food products. The U.S. Food and Drug Administration (FDA) has classified propylene glycol as an additive that is “generally recognized as safe” for use in food, which means that it is acceptable for use in flavorings, drugs, and cosmetics, and as a direct food additive.
The Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) established an acceptable dietary intake of propylene glycol is 0 to 25 mg of propylene glycol for every kilogram (kg) of body weight for propylene glycol, which the European Food Safety Authority Panel on Contaminants in the Food Chain (CONTAM) endorses 2. The lowest oral LD50 (lethal dose 50 is a dose where 50% of the test subjects die) values range between 18 and 23.9 grams (5 different species) and the reported dermal LD50 is 20.8 grams 3. Propylene glycol is not genotoxic. Its use as previous cargo would not give rise to any concerns regarding possible irritancy or allergenicity. There are no reactions of concern with edible fats and oils, nor are any anticipated impurities likely to be present at levels of toxicological relevance.
Propylene glycol can enter your bloodstream if you breathe air containing mists or vapors from this compound. Propylene glycol can also enter your bloodstream through your skin if you come in direct contact with it and do not wash it off. If you eat products that contain propylene glycol, it may enter your bloodstream. Exposure of the general population to propylene glycol is likely since many foods, drugs, and cosmetics contain it.
Propylene glycol breaks down in the body in about 48 hours. However, studies of people and animals show that if you have repeated eye, skin, nasal, or oral exposures to propylene glycol for a short time, you may develop some irritation.
Propylene glycol increases the amount of acid in your body. However, large amounts of propylene glycol are needed to cause this effect.
Propylene glycol breaks down at the same rate as ethylene glycol, although it does not form harmful crystals when it breaks down. Frequent skin exposure to propylene glycol can sometimes irritate the skin.
Propylene glycol induces remarkably fewer adverse effects in both humans and animals than does ethylene glycol 4. Data describing either human or animal effects after exposure to propylene glycol were not as prevalent as those found for ethylene glycol. Human data came from case reports of clinical studies, adverse reactions to medical treatment, or accidental exposure. Animal data generally support those effects, or lack thereof, observed in humans.
Propylene glycol is essentially nonirritating to the skin and mildly irritating to the eyes. Numerous studies support that propylene glycol is not a skin sensitizer. Repeated exposures of rats to propylene glycol in drinking water or feed did not result in adverse effects at levels up to 10% in water (estimated at about 10 g/kg body weight/day) or 5% in feed (dosage reported as 2.5 g/kg body weight/day) for periods up to 2 years. In cats, two studies of at least 90 days duration show that a species-specific effect of increased Heinz bodies was observed (NOAEL = 80 mg/kg body weight/day; LOAEL = 443 mg/kg body weight/day), with other hematological effects (decrease in number of erythrocytes and erythrocyte survival) reported at higher doses (6-12% in diet, or 3.7-10.1 g/cat/day). No Observed Adverse Effect Level (NOAEL) is the highest dose at which there was not an observed toxic or adverse effect. The Lowest Observed Adverse Effect Level (LOAEL) is the lowest dose at which there was an observed toxic or adverse effect. Propylene glycol did not cause fetal or developmental toxicity in rats, mice, rabbits, or hamsters (NOAELs range from 1.2 to 1.6 g/kg body weight/day in four species). No reproductive effects were found when propylene glycol was administered at up to 5% in the drinking water (reported as 10.1 g/kg body weight/day) of mice. Propylene glycol was not a genetic toxicant as demonstrated by a battery of in vivo (micronucleus, dominant lethal, chromosome aberration) and in vitro (bacterial and mammalian cells and cultures) studies. No increase in tumors was found in all tissues examined when propylene glycol was administered in the diet of rats (2.5 g/kg body weight/day for 2 years), or applied to the skin of female rats (100% propylene glycol; total dose not reported; 14 months) or mice (mouse dose estimated at about 2 g/kg body weight/week; lifetime). These data support a lack of carcinogenicity for propylene glycol.
Propylene glycol allergy
There is evidence from clinical studies that propylene glycol is a weak irritant and skin sensitizer (challenge with 2 % solution or stronger), and may increase the reaction to some contact allergens (i.e. adjuvant-like effect), if there is co-exposure, for example when propylene glycol is used as a vehicle 5. Oral or IV administration of propylene glycol may exacerbate dermatitis in some individuals 6. Taking into account that the worst case residue levels of propylene glycol in the oils and fats is considered to be 100 mg/kg, and additionally that propylene glycol is only a weak or very weak irritant, allergen or adjuvant, the European Food Safety Authority Panel on Contaminants in the Food Chain (CONTAM) Panel considers that there would be no significant risk for adverse reactions due to irritancy or allergy from the use of propylene glycol.
Retrospective analysis of cross-sectional data compiled by the North American Contact Dermatitis Group 7 from 1996 to 2006 /was examined/. RESULTS: Of 23,359 patients, 810 (3.5%) had allergic patch-test reactions to 30% propylene glycol, 12.8% of the reactions were of definite clinical relevance (positive reaction to a personal product containing propylene glycol), 88.3% were considered to be currently relevant (definite, probable, or possible relevance), and 4.2% of reactions were occupation related, most commonly to mechanical and motor vehicle occupations. Common sources of propylene glycol were personal care products (creams, lotions, and cosmetics, 53.8%), topical corticosteroids (18.3%), and other topical medicaments (10.1%). In patients positive only to PG (n = 135), the face was most commonly affected (25.9%), followed by a scattered or generalized pattern (23.7%).
To characterize relevant allergens and irritants associated with food in patients referred to the North American Contact Dermatitis Group 8 for patch testing retrospective analysis of cross-sectional data from the North American Contact Dermatitis Group from 2001 to 2004 was performed. Of 10,061 patch-tested patients, 109 (1.1%) had a total of 122 reactions associated with food. Approximately two-thirds of patients (66%) were female, and one-third (36%) were atopic. The hands were the most common sites of dermatitis (36.7%). There were 78 currently relevant (definite, probable, or possible) allergic reactions to North American Contact Dermatitis Group standard series allergens with a food source; the most common allergen was nickel (48.7%), followed by Myroxilon pereirae (balsam of Peru) (20.6%) and propylene glycol (6.4%) 8.
Contact dermatitis has been reported from propylene glycol exposure in a wide variety of topical preparations and ingestion of propylene glycol in sensitized individuals has produced flares of dermatitis 9. Skin irritation resulting from topical exposure is manifest as erythematous reactions restricted to sites of exposure. The irritation potential is enhanced after prolonged dermal exposure, under dermal occlusion, and in combination with triethanolamine-stearate, a cosmetic emulsifier. The nature of the skin reaction of propylene glycol-sensitive patients has been a matter of controversy. In one study primary irritant reactions to the skin and type IV delayed hypersensitivity reactions were observed following oral ingestion or topical application of propylene glycol. However, in most cases, the skin reaction was due to a primary irritation, not to an allergic reaction 9.
Results from human patch testing show no sensitization potential of propylene glycol after semi-occlusive or occlusive epicutaneous application to the skin of volunteers (in excess of 300 subjects in total). These studies demonstrate that propylene glycol is not irritating to skin or eye, nor does it cause sensitization by skin contact 10.
Patch-test in humans, 15 uL 100% propylene glycol/test chamber for 48 hr. Results: not irritating 11.
In 6 human volunteers, pads containing /propylene glycol/ test substance were fixed to the forearm for 2 hr, observation time: 7 days. Results: not irritating 11.
Cream containing 12% propylene glycol was tested on 204 persons. Results: not sensitizing 12.
The irritant effects of propylene glycol was studied in humans (number of test subjects not indicated) using laser Doppler flowmetry 13. Propylene glycol was applied using three different methods: single open exposures to 1.0 mL propylene glycol on the ventral aspect of the thigh with and without occlusive patches for 5-15 min and repeated open exposure to 1.0 mL propylene glycol on the skin for 12 days. Blood flow at the test site was measured and used as an indication of irritation. propylene glycol, when administered under occlusive patches, caused weak erythema at the test site; the maximum reaction was measured 26 hr after exposure. Single open applications and repeated open exposure to propylene glycol did not cause any irritation reactions in this test 13.
A total of 866 patients with various dermatological conditions were patch tested (closed or covered patches) with 100% propylene glycol from April 1951 to April 1952 13. The patches were applied to clinically normal skin, and test sites were examined 48 hr after patch application. Positive reactions were observed in 138 (15.7%) patients. Reactions ranged from simple erythema (+) to erythema with induration and vesiculations (++++). 89 of the 138 patients with positive reactions suffered from dermatitis venenata. A seasonal fluctuation in the incidence of positive reactions was also noted. The incidence was at a minimum when the climate was hot and humid (July, August, and September 1951 in New York City) and significantly greater during the cooler and less humid seasons. Of the 84 patients involved with simultaneous testing with several samples of propylene glycol from different sources, positive reactions were observed in 15. There were no differences in patient responses to different brands of propylene glycol 23 of the 138 patients with positive reactions to 100% propylene glycol were patch tested with aqueous propylene glycol. There were only 5 positive responses to 10% propylene glycol, and the application of 2.5% propylene glycol in water to 3 of the 23 patients resulted in one positive reaction. Additionally, 16 of the patients with positive reactions to 100% propylene glycol were also tested by simple inunction of the test substance. There was no evidence of an inflammatory response to the the rubbing of propylene glycol into the skin either shortly after application or 48 hr later 13.
When 1,556 patients were patch tested with 100% propylene glycol, positive reactions were observed in 194 subjects 13. 4 patients had “true allergy” and the remainder had irritant reactions. Three groups of 42 patients with positive reactions to 100% propylene glycol were later tested with 3.2, 10, and 32 % propylene glycol, respectively, and the results were as follows: 3.2% propylene glycol (9 positive reactions), 10% propylene glycol (12 positive reactions), and 32% propylene glycol (20 positive reactions) 13.
Is propylene glycol safe?
The Scientific Committee on Food evaluated propylene glycol in 1996 and considered this substance as acceptable, noting that propylene glycol was a food additive with an ADI previously established by the Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) of 0-25 mg/kg body weight per day 14. In the 2003 Scientific Committee on Food evaluation of acceptable previous cargoes, propylene glycol was not further evaluated as it was already considered acceptable 15.
In 1993, the Scientific Committee on Food had also evaluated propylene glycol as a food additive, and concluded that “the uncertainty with regard to potential mutagenic effects at the germ cell level, the fact that most studies at the chromosomal level used limited protocols, that there is no in vitro assay for gene mutation in cultured mammalian cells as well as the absence of a carcinogenicity study in a second species leads the Committee to change the established full ADI into a temporary ADI of 25 mg/kg b.w.” 16. Propylene glycol is currently authorized as a food additive (E 1520).
The Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) at its 17th meeting in 1973 established an “Estimate of acceptable daily intake for man” of 0 to 25 mg/kg body weight for propylene glycol 17, following several previous evaluations, and specifications were also prepared 18. JECFA also evaluated propylene glycol as a flavoring substance in 2001. The evaluation was not finalised as further information was required whether propylene glycol is currently in use as a flavoring agent 19.
Propylene glycol was evaluated by the Scientific Committee on Food in 1978 as a substance intended for use in the manufacture of regenerated cellulose films 20. The Committee considered the substance toxicologically acceptable for the use intended on the basis of the Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) acceptable daily intake of 0-25 mg/kg body weight per day. In 1984, propylene glycol was evaluated by the Scientific Committee on Food as a substance intended for use in materials in contact with food and considered acceptable on the basis of the JECFA acceptable daily intake.
The US Agency for Toxic Substances and Disease Registry (ATSDR) has prepared a toxicological profile on propylene glycol 21. There was insufficient information to establish an oral reference value.
Absorption, distribution, metabolism and elimination
According to JECFA 17, propylene glycol is rapidly absorbed after oral administration and appears in the blood-stream. After a dose of 8 mL/kg body weight (equivalent to 8,284 mg/kg body weight) had been administered to dogs approximately 24 hours were required for complete elimination from the blood-stream. Conversion to lactic acid has been shown to be the normal metabolic pathway, via two biochemical pathways 19. Phosphorylated propylene glycol can be converted to acetolphosphate, lactaldehyde phosphate, lactyl phosphate, and then lactic acid. Non-phosphorylated propylene glycol is successively oxidized to lactaldehyde, methylgloyoxal, and lactic acid 19. High doses are likely to be excreted largely unchanged in the urine 19.
Acute toxicity
As reported by JECFA, acute toxicity studies on propylene glycol have been carried out in rats, mice, rabbits and guinea pigs, providing LD50 (lethal dose 50 where 50% of the test subjects die) values of greater than 19,000 mg/kg body weight in all these species 17.
Subacute, subchronic toxicity studies
Short-term oral toxicity studies of propylene glycol in rats, rabbits and dogs have shown no adverse effects at levels approximating 10 % in the diet 16. Hematological effects have been seen at high dose levels in some species, notably cats. Cats appear to be uniquely sensitive to hematological effects of ingested propylene glycol, manifest as an increase of Heinz bodies in circulating erythrocytes 22.
Genotoxicity
Propylene glycol has been extensively evaluated in a range of genetic toxicity test systems. The existing studies provide convincing evidence that it is not genotoxic 19. Chronic toxicity studies and carcinogenicity as cited by JECFA 19, in a study in which rats were given propylene glycol in the diet at a concentration of 2.45 % or 4.9 % (equivalent to 900 and 1,800 mg/kg body weight per day) for 2 years no treatment-related adverse effects were found on growth, and histological examination revealed no treatment-related effects 23. As cited by JECFA 19, in a study in which rats received propylene glycol in the diet at a concentration of 0, 310, 630, 1,300 or 2,500 mg/kg body weight per day for 2 years no treatment-related adverse effects on body weight gain, hematological, urinary, or clinical chemical end-points, or organ weights were found. The NOAEL was 1,300 mg/kg body weight per day 24. No Observed Adverse Effect Level (NOAEL) is the highest dose at which there was not an observed toxic or adverse effect. As cited by JECFA 19, in a study in which dogs received propylene glycol in the diet at a concentration of 0, 2000, or 5000 mg/kg body weight per day for 2 years increased erythrocyte destruction was found at 5,000 mg/kg body weight per day. No significant treatment-related effects on hematological, clinical chemical, or urinary end-points, or on gross or histological appearance were found 25.
Developmental and reproductive toxicity
In rats and mice, no adverse effects on reproductive performance were observed after oral treatment at doses as high as 10,000 mg/kg body weight per day during gestation of 1 generation or for multiple litters and 2 generations of mice 26 or inhalation exposure to 112 ppm for 18 months 27. Agency for Toxic Substances and Disease Registry (ATSDR) considered that further evaluation of the reproductive toxicity of propylene glycol was not necessary 22. As reported by JECFA, in a study to examine the potential of di(2-ethylhexyl)phthalate and its metabolites to cause testicular damage in rats after oral administration, a control group of six male Sprague Dawley rats were given propylene glycol orally at a dose of 2,000 mg/kg body weight per day for 5 days. Histopathological examination of testis, prostate and liver was done following
sacrifice on day 6. The testes of animals given propylene glycol were reported to contain occasional degenerated cells, most of which were in early meiotic prophase or undergoing meiotic division 28. In another study cited by JECFA 29, in which the effects of 15 chemicals, including propylene glycol, on differential ovarian follicle counts and reproductive performance were compared, propylene glycol was reported to have no effect on reproductive function 30.
How might I be exposed to propylene glycol?
- You can be exposed to propylene glycol by eating food products, using cosmetics, or taking medicine that contains it.
- If you work in an industry that uses propylene glycol or products containing propylene glycol, you could be exposed by breathing or touching these substances.
Propylene glycol has been approved for use at certain levels in food (food additive E 1520), cosmetics, and pharmaceutical products. If you eat food products, use cosmetics, or take medicines that contain it, you will be exposed to propylene glycol, but these amounts are not generally considered harmful. People who work in industries that use propylene glycol may be exposed by touching these products or inhaling mists from spraying them. These exposures tend to be at low levels, however. Propylene glycol is used to make artificial smoke and mists for fire safety training, theatrical performances, and rock concerts. These artificial smoke products may also be used by private citizens. These products are frequently used in enclosed spaces, where exposure may be more intense.
Is there a medical test to determine whether I have been exposed to propylene glycol?
Propylene glycol is generally considered to be a safe chemical, and is not routinely tested for, unless specific exposure, such as to a medicine or cosmetic, can be linked with symptoms.
Since propylene glycol breaks down very quickly in the body, it is very difficult to detect, even though symptoms may be present.
How likely is propylene glycol to cause cancer?
The Department of Health and Human Services, the International Agency for Research on Cancer (IARC), and the EPA have not classified propylene glycol for carcinogenicity. Animal studies have not shown propylene glycol to be carcinogen.
No increase in tumors was observed after twice weekly applications of propylene glycol to the skin of Swiss mice for 120 weeks, at doses up to 2 mg 31. Based on this information, its long history of use in consumer products, and structural activity considerations, it is extremely unlikely that exposure to levels of propylene glycol near hazardous waste sites would influence the incidence of cancer in the population living in the vicinity.
Propylene glycol in medicinal products for children
Clinically, the weak evidence and the reliance on predominantly non-comparative data and case reports, severely limit the robustness of any recommendation regarding safe propylene glycol exposure levels in children. A variety of relevant aspects are to be considered when considering a safe dose of propylene glycol. These include patient characteristics such as (developmental) age, weight, health status, concomitant medication, administration route, pattern and duration of use.
In pre-term infants where metabolism and secretion mechanisms are yet immature, accumulation of propylene glycol can occur more easily, thus leading to an increased potential toxicity 32. Propylene glycol is used as excipient for a variety of medicinal products frequently applied in an intensive care unit (ICU) setting, where the patient collective will arguably display a variety of severe health conditions that could accentuate but also mask any adverse effects due to propylene glycol. In this regard, studies considered for this review have been conducted in an ICU setting. Finally, the hardly quantifiable impact of co-medication and its excipients (e.g. mannitol) or sources of propylene glycol other than those investigated could potentially distort the clinical picture and the interpretation of data, accordingly.
The current regulatory recommendations concerning propylene glycol have been made for oral intake via food products (FAO/WHO) or focus on the alcohol effect of propylene glycol only (European Medicines Agency – EMA).
Attempts to define a safety threshold for propylene glycol administration have not been utterly conclusive so far. For children, it was concluded that “a median propylene glycol exposure of 34 mg/kg/24h seems well tolerated and does not affect normal postnatal maturational changes in renal, metabolic and hepatic function” 33. Determining a safe upper limit of propylene glycol exposure of 34 mg/kg/day might be a possible, rather conservative, approach. Importantly however, study specifics limit the generalizability of the proposed threshold: mainly (pre-term) infants in an ICU setting, short-term exposure (48h), use of historical controls, and assessment of selected endpoints only.
Based on the limited overall quality, non-clinical and clinical data available, the European Medicines Agency Committee considered that 32:
- No recommendation on a safe dose for propylene glycol can be made based on the current available data;
- Correlations between propylene glycol exposure, patient characteristics and reported adverse events are not established;
- While there is a trend of increasing safety concerns with propylene glycol doses in excess of several hundred mg /kg/day in infants, the limitations of the available data do not allow for a definite conclusion;
The European Medicines Agency Committee therefore concluded that well designed clinical trials investigating the safety of propylene glycol exposure and reflective of common clinical use in terms of duration and quantity are needed to allow a better understanding of propylene glycol safety in children. Additional information from non-clinical juvenile studies assessing the toxicity and toxicokinetics of propylene glycol following repeated administration in the relevant species and age groups may be considered useful to assess the safety risks (particularly central nervous system toxicity) inherent to the formulation.
Propylene glycol uses
Propylene glycol (1,2-dihydroxypropane or 1,2-propanediol or methyl glycol or trimethyl glycol) is a clear liquid used in antifreeze and deicing solutions for cars, airplanes, and boats; to make polyester compounds; and as solvent in the paint and plastics industries. Propylene glycol is also used to create artificial smoke or fog used in fire-fighting training and in theatrical productions.
Uses of propylene glycol, with percent of demand, are:
- Unsaturated polyester resins, 26 percent;
- Antifreeze and de-icing fluids, 22 percent;
- Food, drug and cosmetics uses, 18 percent;
- Liquid detergents, 11 percent;
- Functional fluids (inks, specialty anti-freeze, de-icing lubricants), 4 percent;
- Pet foods, 3 percent;
- Paints and coatings, 5 percent;
- Tobacco, 3 percent;
- Miscellaneous, including plasticizer use, 8 percent.
Propylene glycol is a synthetic liquid substance that absorbs water. Propylene glycol is used by the chemical, food, and pharmaceutical industries as an antifreeze when leakage might lead to contact with food. The Food and Drug Administration (FDA) has classified propylene glycol as an additive that is “generally recognized as safe” for use in food. It is used to absorb extra water and maintain moisture in certain medicines, cosmetics, or food products. It is a solvent for food colors and flavors, and in the paint and plastics industries.
Propylene glycol is used in pharmaceuticals, as a drug vehicle (for example as a U.S. Food and Drug Administration (FDA)-approved solvent for intravenous diazepam) and preservative. Propylene glycol is also used in personal lubricants, in semi-moist pet food and as a humectant for tobacco. Propylene glycol is currently authorized as a food additive (E 1520). In the food industry propylene glycol (E 1520) is used as a solvent for food colors and flavors, as a humectant, preservative and emulsifier 1. Propylene glycol is used to absorb extra water and maintain moisture in certain medicines, cosmetics, or food products. The U.S. Food and Drug Administration (FDA) has classified propylene glycol as an additive that is “generally recognized as safe” for use in food.
Propylene glycol toxicity
Below are symptoms of propylene glycol antifreeze poisoning in different parts of the body.
Airways and lungs
- Rapid breathing
- No breathing
Bladder and kidneys
- Blood in urine
- No urine output or decreased urine output
Eyes, Ears, Nose, and Throat
- Blurred vision
- Blindness
Heart and blood
- Rapid heartbeat
- Low blood pressure
Muscles and joints
- Leg cramps
Nervous system
- Coma
- Convulsions
- Dizziness
- Fatigue
- Headache
- Slurred speech
- Stupor (lack of alertness)
- Unconsciousness
- Unsteady walk
- Weakness
Skin
- Blue lips and fingernails
Stomach and gastrointestinal tract
- Nausea and vomiting
Home Care
Seek medical help right away. DO NOT make a person throw up unless poison control or a health care provider tells you to.
Use standard first aid and CPR for signs of shock or no heartbeat (cardiac arrest). Call your local poison control center or your local emergency services number for more help. You can call for any reason, 24 hours a day, 7 days a week.
Your local poison center can be reached directly by calling the national toll-free Poison Help hotline (1-800-222-1222) from anywhere in the United States. They will give you further instructions.
This is a free and confidential service. All local poison control centers in the United States use this national number. You should call if you have any questions about poisoning or poison prevention. It does NOT need to be an emergency.
What to Expect at the Emergency Room
Take the container with you to the hospital, if possible.
The healthcare provider will measure and monitor the person’s vital signs, including temperature, pulse, breathing rate, and blood pressure. The person may receive:
- Blood and urine tests
- Breathing support, including oxygen, tube through the mouth into the throat, and breathing machine
- Chest x-ray
- CT scan (advanced brain imaging)
- ECG (electrocardiogram or heart tracing)
- Intravenous fluids (through a vein)
- Medicines to reverse the effects of the poison
- Tube placed down the nose and into the stomach (sometimes)
Dialysis (kidney machine) treatment may be needed during recovery. This need may be permanent if kidney damage is severe.
Propylene glycol side effects
Non-asthmatic volunteers (n=27) were exposed to propylene glycol (propylene glycol) mist over 1 minute, during realistic training conditions 34. Geometric mean concentration of propylene glycol was 309 mg/m³ (range 176-851 mg/³), with the highest concentrations in the afternoon. The medical investigation was performed both before and after the exposure (within 15 minutes). It included an estimate of tear film stability break up time, nasal patency by acoustic rhinometry, dynamic spirometry, and a doctor’s administered questionnaire on symptoms. After exposure to propylene glycol mist for 1 minute tear film stability decreased, ocular and throat symptoms increased, forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) was slightly reduced, and self rated severity of dyspnea was slightly increased. No effect was found for nasal patency, vital capacity (VC), FVC, nasal symptoms, dermal symptoms, smell of solvent, or any systemic symptoms. Those exposed to the higher concentrations in the afternoon had a more pronounced increase of throat symptoms, and a more pronounced decrease of tear film stability. In four subjects who reported development of irritative cough during exposure to propylene glycol, FEV1 was decreased by 5%, but FEV1 was unchanged among those who did not develop a cough. Those who developed a cough also had an increased perception of mild dyspnea. Short exposure to propylene glycol mist from artificial smoke generators in discotheques, theaters, and aviation emergency training may cause acute ocular and upper airway irritation in non-asthmatic subjects. A few may also react with cough and slight airway obstruction.
Propylene glycol is estimated to be one-third as intoxicating as ethanol, with administration of large volumes being associated with adverse effects most commonly on the central nervous system, especially in neonates and children. Other adverse reactions reported, though generally isolated, include: ototoxicity; cardiovascular effects; seizures; and hyperosmolarity and lactic acidosis, both of which occur most frequently in patients with consumption of large quantities of propylene glycol or on administration to neonates, children under 4 years of age, pregnant women, and patients with hepatic or renal failure. Adverse effects may also occur in patients treated with disulfiram or metronidazole 35.
There are numerous reports of propylene glycol-induced serum hyperosmolarity following the topical administration of silver sulfadiazine. Systemic absorption of propylene glycol resulting in hyperosmolarity occurred with topical application of silver sulfadiazine cream in 15 burn patients with burns over more than 35% of their body surface area 36.
- Scientific Opinion on the evaluation of the substances currently on the list in the Annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part I of III. EFSA Journal 2011;9(12):2482. https://doi.org/10.2903/j.efsa.2011.2482[↩][↩]
- Scientific Opinion on the evaluation of the substances currently on the list in the Annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part I of III. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2011.2482[↩]
- Propylene Glycol. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=1122&tid=240[↩]
- PROPYLENE GLYCOL. Health Effects. https://www.atsdr.cdc.gov/toxprofiles/tp189-c2.pdf[↩]
- Andersen FA, ed. 1994. Final report on the safety assessment of Propylene Glycol and Polypropylene Glycols. Journal of the American College of Toxicology, 13, 437-491.[↩]
- Monograph on the Potential Human Reproductive and Developmental Effects of Propylene Glycol (March 2004) NIH Pub No. 04-4482 p.II-44 [↩]
- Positive patch-test reactions to propylene glycol: a retrospective cross-sectional analysis from the North American Contact Dermatitis Group, 1996 to 2006. Dermatitis. 2009 Jan-Feb;20(1):14-20. https://www.ncbi.nlm.nih.gov/pubmed/19321115[↩]
- Contact dermatitis associated with food: retrospective cross-sectional analysis of North American Contact Dermatitis Group data, 2001-2004. Dermatitis. 2008 Sep-Oct;19(5):252-60. https://www.ncbi.nlm.nih.gov/pubmed/18845115[↩][↩]
- Monograph on the Potential Human Reproductive and Developmental Effects of Propylene Glycol (March 2004) NIH Pub No. 04-4482 pp.II-28-29[↩][↩]
- Organization for Economic Cooperation and Development; Screening Information Data Set for 1,2-Dihydroxypropane (57-55-6) p.17, 2001[↩]
- Organization for Economic Cooperation and Development; Screening Information Data Set for 1,2-Dihydroxypropane (57-55-6) p.56, 2001[↩][↩]
- Organization for Economic Cooperation and Development; Screening Information Data Set for 1,2-Dihydroxypropane (57-55-6) p.60, 2001[↩]
- Cosmetic Ingredient Review Expert Panel; J Am Coll Toxicol 13 (6): 437-91; 1994[↩][↩][↩][↩][↩][↩]
- SCF (Scientific Committee on Food), 1997. Opinion on the potential risk to human health arising from the transport in ships’ tanks of oils and fats from substances proposed as acceptable previous cargoes (expressed on 20 September 1996). Annex VII to Document III/5693/96. DG III, European Commission, Brussels.[↩]
- SCF (Scientific Committee on Food), 2003. Updated opinion of the Scientific Committee on Food on the potential risk to human health arising from the transport in ships’ tanks of oils and fats from substances proposed as acceptable previous cargoes (expressed on 4 April 2003). Health and Consumer Protection Directorate-General, European Commission, Brussels.[↩]
- SCF (Scientific Committee on Food), 1996. Reports of the Scientific Committee for Food. Thirty-fifth Series. Opinions on the Scientific Committee for Food on: Propylene glycol. European Commission, Luxemburg.[↩][↩]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974. WHO Food Additives Series No. 5. Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. Seventeenth Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO, Geneva, Switzerland.[↩][↩][↩]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Propylene glycol. Specifications. Prepared at the 49th JECFA (1997).[↩]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2001. WHO Food Additives Series No 48. Safety evaluation of certain food additives and contaminants, Aliphatic acyclic diols, triols, and related substances. WHO, Geneva, Switzerland.[↩][↩][↩][↩][↩][↩][↩][↩]
- SCF (Scientific Committee on Food), 1978. Reports of the Scientific Committee for Food. Sixth series. Report of the Scientific Committee for Food on the positive list of substances to be authorized in the manufacture of regenerated cellulose films intended to come into contact with foodstuffs (Opinion expressed 28 September 1978). European Commission. Brussels-Luxemburg.[↩]
- Propylene Glycol. https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=240[↩]
- ATSRD (US Agency for Toxic Substances and Disease Registry), 1997. Toxicological profile for propylene glycol. September 1997.[↩][↩]
- Morris HJ, Nelson AA and Calvery HO, 1942. Journal of Pharmacology and Experimental Therapeutics, 74, 266. As cited in JECFA (2001).[↩]
- Gaunt IF, Carpanini FMB, Grasso P and Lansdown ABG, 1972. Long-term toxicity of propylene glycol in rats. Food and Cosmetics Toxicology, 10, 151-162. As cited by JECFA (2001).[↩]
- Weil CS, Woodside MD, Smyth MF and Carpenter CP, 1971. Results of feeding propylene glycol in the diet to dogs for two years. Food and Cosmetic Toxicology, 9, 479. As cited in JECFA (2001).[↩]
- Kavlock RJ, Short RD and Chemoff N, 1987. Further evaluation of and in vivo teratology screen. Teratogensis, Carcinogenisi and Mutagenesis, 7, 7-16. As cited by ATSDR (1997).[↩]
- Robertson OH, Loosli CG and Puck TT, 1947. Test for chronic toxicity of propylene glycol and triethylene glycol on monkeys and rats by vapor inhalation and oral administration. Journal of Pharmacology and Experimental Therapeutics, 91, 52-76. As Cited by ATSDR (1997).[↩]
- Sjoberg R, Bondesson U, Gray TJB and Ploen L, 1986. Effects of di-(2-ethylhexyl) phthalate and five of its metabolites on rat testis in vivo and in vitro. Acta Pharmacologica et Toxicologica, 58, 225-233.[↩]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2001. WHO Food Additives Series No 48. Safety evaluation of certain food additives and contaminants, Aliphatic acyclic diols, triols, and related substances. WHO, Geneva, Switzerland[↩]
- Bolon B, Bucci TJ, Warbritton AR, Chen JJ, Mattison DR and Heindel JJ, 1997. Differential Follicle Counts as a Screen for Chemically Induced Ovarian Toxicity in Mice: Results from Continuous Breeding Bioassays. Fundamental and Applied Toxicology, 39, 1-10. As cited by JECFA (2001).[↩]
- Stenback F, Shubik P. 1974. Lack of toxicity and carcinogenicity of some commonly used cutaneous agents. Toxicol Appl Pharmacol 30:7-13.[↩]
- Propylene glycol in medicinal products for children. European Medicines Agency Assessment Report. Article 5(3) of Regulation (EC) No 726/2004. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2014/03/WC500163989.pdf[↩][↩]
- Kulo et al., 2012. Biochemical tolerance during low dose propylene glycol exposure in neonates: A formulation-controlled evaluation[↩]
- Wieslander G et al; Occup Environ Med 58 (10): 649-55; 2001 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1740047/pdf/v058p00649.pdf[↩]
- Rowe, R.C., Sheskey, P.J., Quinn, M.E.; (Eds.), Handbook of Pharmaceutical Excipients 6th edition Pharmaceutical Press, London, England 2009, p. 593[↩]
- Goldfrank LR et al; Goldfrank’s Toxicologic Emergencies 7th Ed., McGraw-Hill, New York, N.Y. p.842; 2002[↩]