Contents
Respiratory acidosis
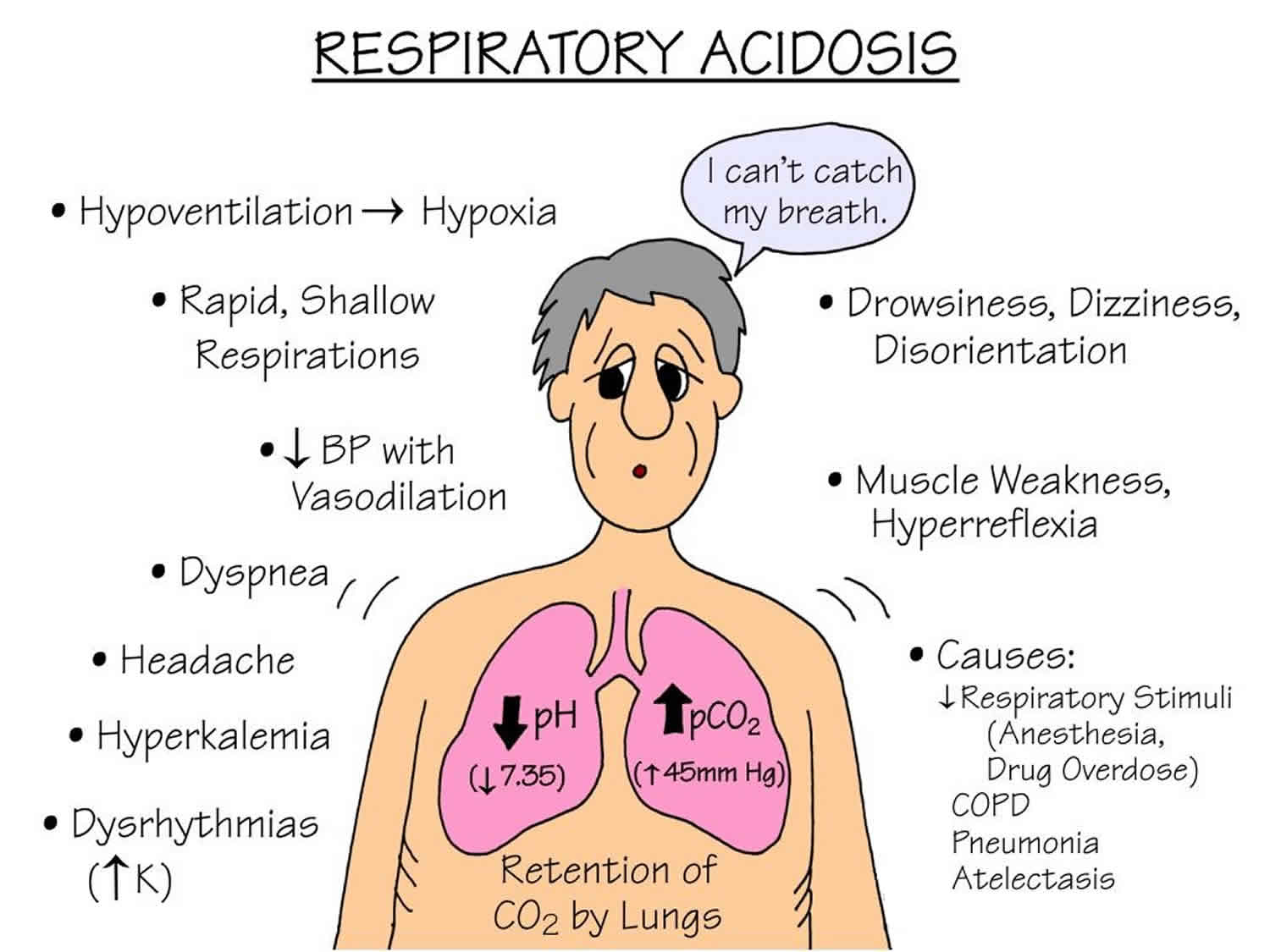
Respiratory acidosis is a condition of raised arterial partial pressure of carbon dioxide [PaCO2] and a reduced pH of less than 7.35 that develops when there is too much carbon dioxide (an acid) in the body. Other names for respiratory acidosis are hypercapnic acidosis or carbon dioxide acidosis. Respiratory acidosis is usually caused when the body is unable to remove enough carbon dioxide (CO2) through breathing, a failure of ventilation. Respiratory disease, such as bronchopneumonia, emphysema, asthma and chronic obstructive airways disease (COPD), may all be associated with hypoventilation sufficient to cause respiratory acidosis. In the presence of alveolar hypoventilation, 2 features commonly are seen are respiratory acidosis and hypercapnia. To compensate for the disturbance in the balance between carbon dioxide [CO2] (an acid) and bicarbonate [HCO3–] (a base), the kidneys begin to excrete more acid in the forms of hydrogen ions (H+) and ammonium (NH4) and reabsorb more base in the form of bicarbonate [HCO3–]. This compensation helps to normalize the pH.
Respiratory acidosis may cause slight elevations in ionized calcium and an extracellular shift of potassium. Acidosis decreases binding of calcium to albumin and tends to increase serum ionized calcium levels. However, the hyperkalemia is usually mild 1.
Some drugs (e.g. morphine and barbiturates) can also cause respiratory acidosis by depressing the respiratory center in the brain. Damage or trauma to the chest wall and the musculature involved in the mechanics of respiration may reduce ventilation rate. This explains the respiratory acidosis that can complicate the course of diseases such as poliomyelitis, Guillain-Barre syndrome and recovery from severe chest trauma.
Causes of respiratory acidosis include:
- Chest deformities, such as kyphosis and scoliosis
- Chest injuries
- Diseases affecting the nerves and muscles that signal the lungs to inflate or deflate
- Respiratory muscle/nerve disease (myasthenia gravis, botulism, muscular dystrophy, amyotrophic lateral sclerosis (ALS), Guillain-Barre syndrome)
- Long-term (chronic) lung disease
- Diseases of the airways such as asthma and chronic obstructive pulmonary disease (COPD)
- Diseases of the lung tissue (such as pulmonary fibrosis, which causes scarring and thickening of the lungs)
- Overuse of drugs that suppress breathing (including powerful pain medicines, such as narcotics, and sedative drugs or “downers,” such as benzodiazepines), often when combined with alcohol
- Severe obesity, which restricts how much the lungs can expand
- Obstructive sleep apnea.
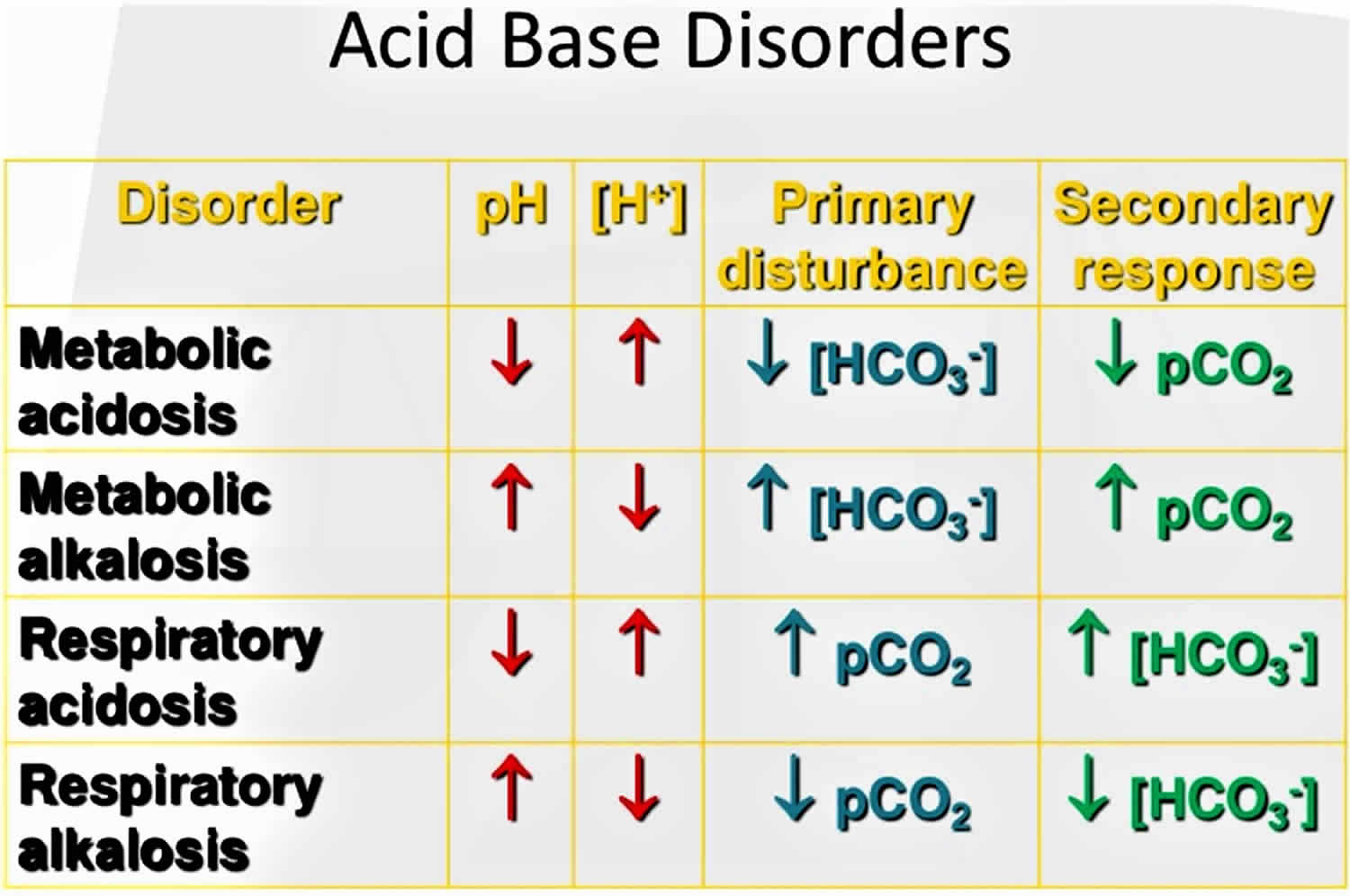
The primary disturbance seen in respiratory acidosis caused by the increased arterial partial pressure of carbon dioxide [PaCO2] is the decreased ratio of arterial bicarbonate [HCO3–] to arterial partial pressure of carbon dioxide [PaCO2], which leads to a lowering of the pH. In respiratory acidosis, the arterial blood gas (ABG) will show an elevated arterial partial pressure of carbon dioxide (PaCO2) (>45 mmHg), elevated bicarbonate [HCO3–] (>30 mmHg), and decreased pH (pH<7.35).
The respiratory acidosis can be further classified as acute or chronic based on the relative increase in bicarbonate [HCO3–] with respect to arterial partial pressure of carbon dioxide (PaCO2).
Acute respiratory acidosis is a condition in which carbon dioxide builds up very quickly because of failure of ventilation, before the kidneys can return the body to a state of balance. Acute respiratory acidosis may be due to cerebrovascular accidents (e.g., stroke), use of central nervous system (CNS) depressants such as opioids, or inability to use muscles of respiration because of disorders like myasthenia gravis, muscular dystrophy or Guillain-Barre Syndrome. Because of its acute nature, there is a slight compensation occurring minutes after the incidence. In cases of acute respiratory acidosis, bicarbonate [HCO3–] will have increased by 1 mEq/L for every 10 mmHg increase in arterial partial pressure of carbon dioxide (PaCO2) over a few minutes 2.
Chronic respiratory acidosis occurs over a long time. This leads to a stable situation, because the kidneys increase body chemicals, such as bicarbonate [HCO3–], that help restore the body’s acid-base balance. Chronic respiratory acidosis may be caused by chronic obstructive pulmonary disease (COPD) where there is a decreased responsiveness of the reflexes to states of hypoxia and hypercapnia. Other individuals who develop chronic respiratory acidosis may have fatigue of the diaphragm resulting from a muscular disorder. Chronic respiratory acidosis can also be seen in obesity hypoventilation syndrome, also known as Pickwickian syndrome, amyotrophic lateral sclerosis, and in patients with severe thoracic skeletal defects. In patients with chronic compensated respiratory disease and acidosis, an acute insult such as pneumonia or disease exacerbation can lead to ventilation/perfusion mismatch. In cases of chronic respiratory acidosis, bicarbonate [HCO3–] will have increased by 4 mEq/L for every 10 mmHg increase in arterial partial pressure of carbon dioxide (PaCO2) over a time course of 3 to 5 days 2. Some people with chronic respiratory acidosis get acute respiratory acidosis because an illness makes their condition worse. If the compensation does not occur in this pattern, a mixed respiratory-metabolic acidosis may be present.
In a patient who presents with unexplained respiratory acidosis, a drug screen may also be warranted. The effects of sedating drugs such as narcotics and benzodiazepines in depressing the central ventilatory drive and causing respiratory acidosis should be considered. These sedative drugs should be avoided, if possible, in patients with respiratory acidosis.
Once respiratory acidosis diagnosis has been made, the underlying cause of respiratory acidosis has to be treated. The hypercapnia (an elevation in the arterial carbon dioxide level) should be corrected gradually because rapid alkalization of the cerebrospinal fluid (CSF) may lead to seizures. Pharmacologic therapy can also be used to help improve ventilation. Bronchodilators like beta agonists, anticholinergic drugs, and methylxanthines can be used in treating patients with obstructive airway diseases. Naloxone can be used in patients who overdose on opioid use.
Figure 1. Acid-base disorders
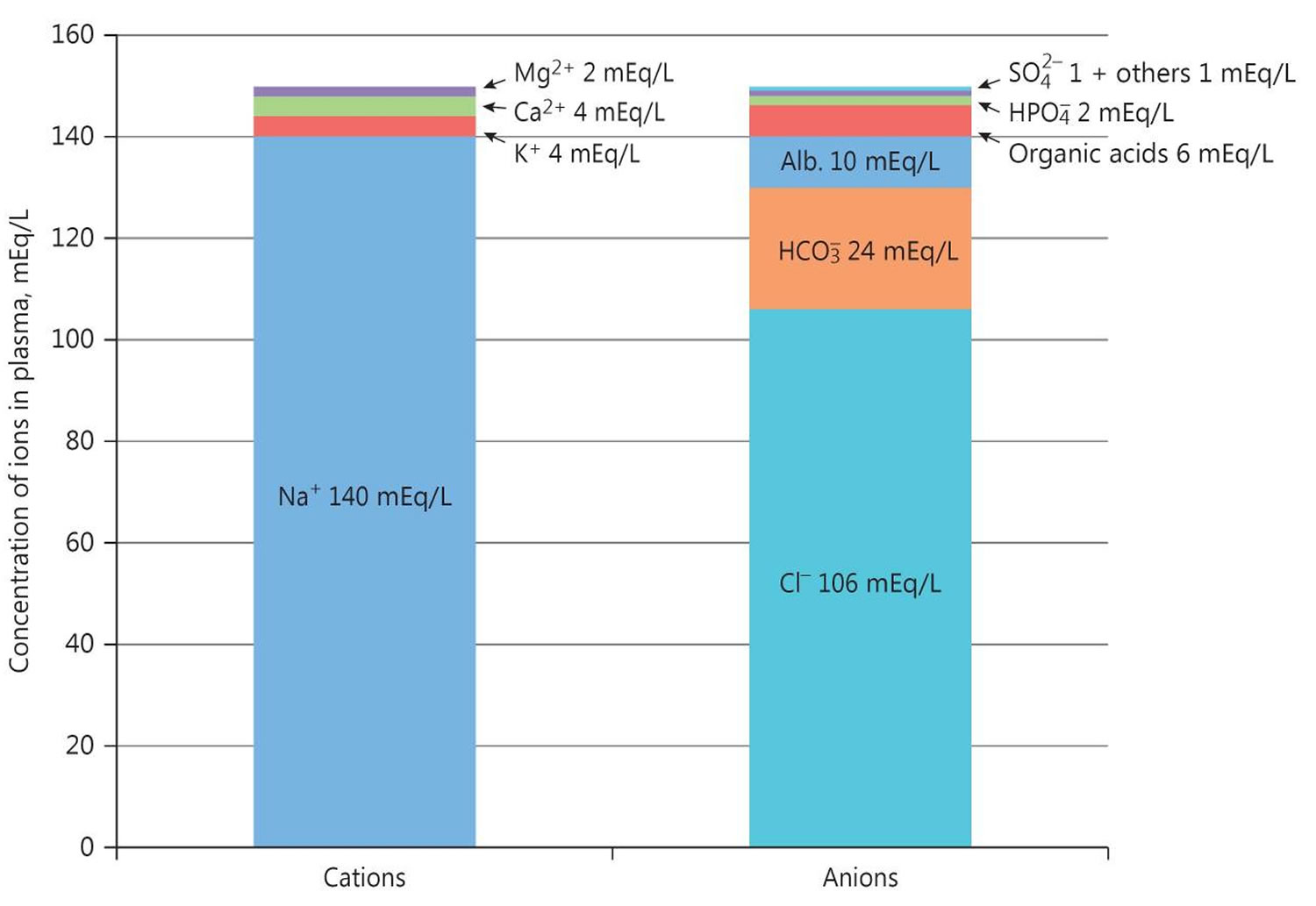
Figure 2. Normal anion gap levels
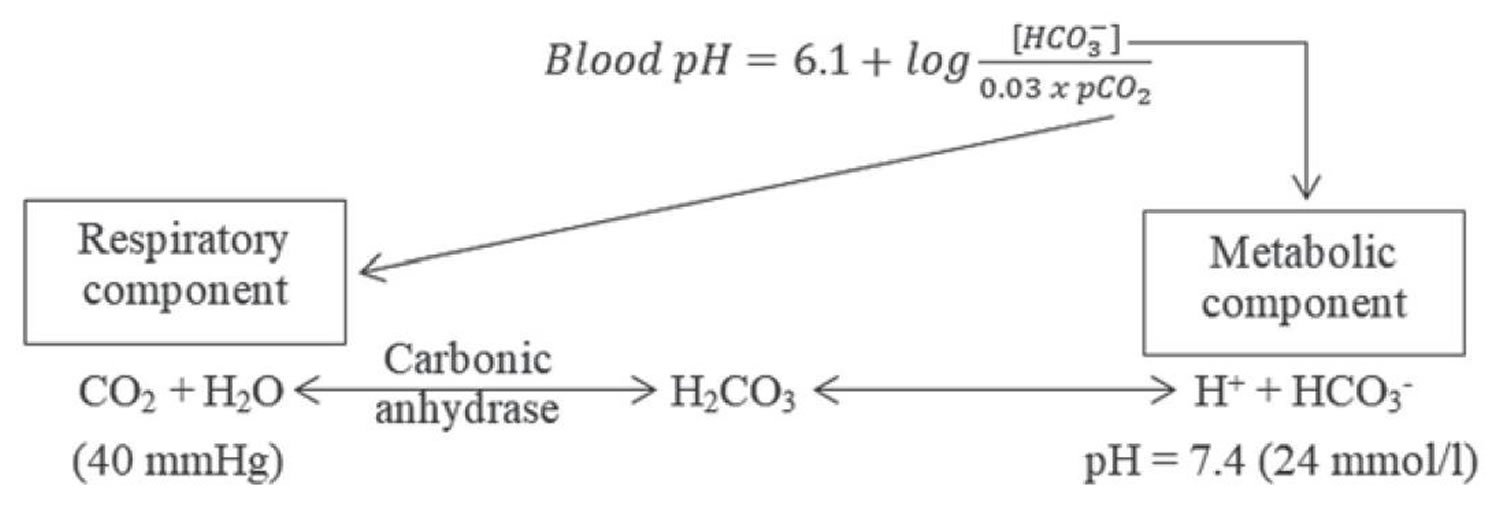
[Source 3 ]Figure 3. Henderson-Hasselbalch equation
[Source 4 ]Figure 4. Acid-base buffering system
Respiratory acidosis compensation
The primary ways the body deals with excessive acidity are through renal adaptations, respiration, and buffering with calcium from bone.
It is vital for life that pH does not waiver too far from normal, and the body will always attempt to return an abnormal pH towards normal when acid-base balance is disturbed. Compensation is the name given to this life-preserving process. To understand compensation, it is important to recall that pH is governed by the ratio bicarbonate [HCO3–] (a base)/arterial partial pressure of carbon dioxide (PaCO2) (an acid). So long as the ratio is normal, pH will be normal.
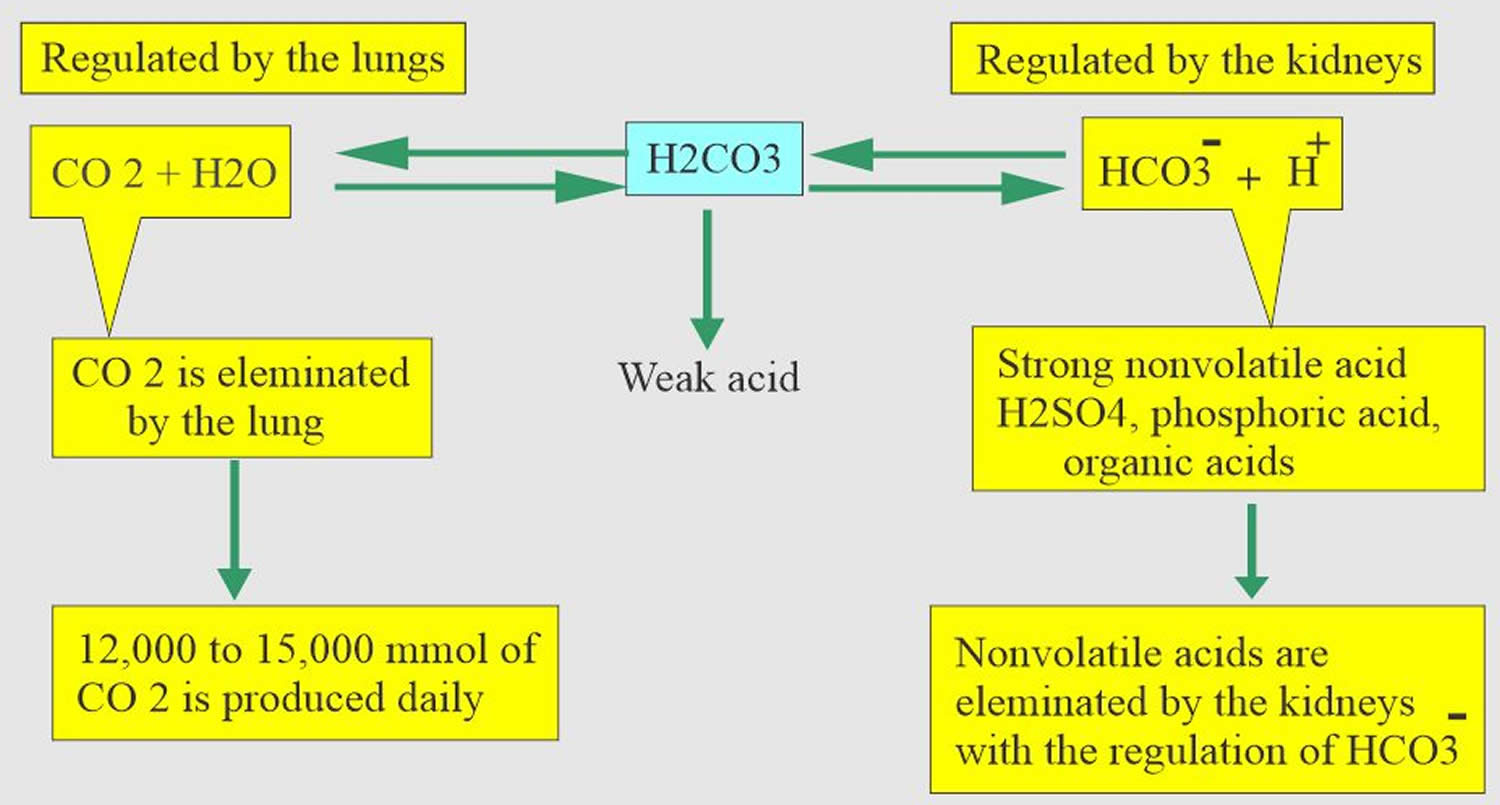
Normal body functions and metabolism generate large quantities of acids that must be neutralized and/or eliminated to maintain blood pH balance. Most of the acid is carbonic acid, which is created from carbon dioxide (CO2) and water (H2O). Carbon dioxide (CO2) is produced as the body uses glucose (sugar) or fat for energy. In its normal state, the body maintains carbon dioxide (arterial partial pressure of carbon dioxide [PaCO2]) in a well-controlled range from 38 to 42 mm Hg by balancing its production and elimination. Lesser quantities of lactic acid, ketoacids, and other organic acids are also produced.
According to the Henderson-Hasselbalch equation (Figure 3), maintaining physiological pH depends on arterial partial pressure of carbon dioxide (PaCO2), which in turn depends on alveolar ventilation (hypoventilation causes acidosis and hyperventilation causes alkalosis). The kidneys participate in maintaining the stable pH by reabsorption of bicarbonate (3,600 mmol of bicarbonate is filtrated in glomeruli during 24 hour) and excretion of hydrogen ions from nonvolatile acids (including sulfur and phosphate) as titratable acidity (0.3 mmol hydrogen ions/kg/day) and in the form of ammonium ion (0.7 mmol hydrogen ions/kg/day) 5, 6.
The lungs and kidneys are the major organs involved in regulating blood pH. And to compensate for the respiratory acidosis, your kidney increases retention of bicarbonate [HCO3–] and excretion of acid.
- The lungs flush acid out of the body by exhaling CO2. Raising and lowering the respiratory rate alters the amount of carbon dioxide (CO2) that is breathed out, and this can affect blood pH within minutes 7.
- The kidneys excrete acids in the urine, and they regulate the concentration of bicarbonate (HCO3–, a base) in blood. Acid-base changes due to increases or decreases in bicarbonate [HCO3–] concentration occur more slowly than changes in carbon dioxide (CO2), taking hours or days. Bicarbonate (HCO3–) reabsorption occurs in the kidneys in every part of the tubules. About 85–90% of the filtered bicarbonate is reabsorbed in the proximal tubules, 10% in the ascending arms of the Henle loop, 6% in the distal tubules, and 4% in the collecting tubules 5, 6.
Both of these processes are always at work, and they keep the blood pH in healthy people tightly controlled. The absolute quantities of acids or bases are less important than the balance between the two and its effect on blood pH.
Buffering systems that resist changes in pH also contribute to the regulation of acid and base concentrations. The main buffers in blood are hemoglobin (in red blood cells), plasma proteins, carbon dioxide (CO2), bicarbonate (HCO3–) and phosphates.
Carbon dioxide (CO2) plays a remarkable role in the human body mainly through pH regulation of the blood. The pH is the primary stimulus to initiate ventilation. In its normal state, the body maintains carbon dioxide (CO2) in a well-controlled range from 38 to 42 mm Hg by balancing its production and elimination. In a state of hypoventilation (breathing that is too shallow or too slow to meet the needs of the body), the body produces more carbon dioxide (CO2) than it can eliminate, causing a net retention of carbon dioxide (CO2). The increased carbon dioxide (CO2) is what leads to an increase in hydrogen ions (H+) and a slight increase in bicarbonate (HCO3–), as seen by a right shift in the following equilibrium reaction of carbon dioxide:
- Carbon dioxide (CO2) + water (H2O) -> H2CO3 (carbonic acid) -> HCO3– + H+
The buffer system created by carbon dioxide consists of the following three molecules in equilibrium: carbon dioxide (CO2), H2CO3 (carbonic acid), and bicarbonate (HCO3–). When hydrogen ions (H+) is high, bicarbonate (HCO3–) buffers the low pH. When hydroxide (OH–) is high, H2CO3 (carbonic acid) buffers the high pH. In respiratory acidosis, the slight increase in bicarbonate (HCO3–) serves as a buffer for the increase in hydrogen ions (H+), which helps minimize the drop in pH. The increase in hydrogen ions inevitably causes the decrease in pH, which is the mechanism behind respiratory acidosis.
Causes of respiratory acidosis
Causes of respiratory acidosis include:
- Chest deformities, such as kyphosis and scoliosis
- Chest injuries
- Diseases affecting the nerves and muscles that signal the lungs to inflate or deflate
- Respiratory muscle/nerve disease (myasthenia gravis, botulism, muscular dystrophy, amyotrophic lateral sclerosis (ALS), Guillain-Barre syndrome)
- Long-term (chronic) lung disease
- Diseases of the airways (such as asthma and COPD)
- Diseases of the lung tissue (such as pulmonary fibrosis, which causes scarring and thickening of the lungs)
- Overuse of drugs that suppress breathing (including powerful pain medicines, such as narcotics, and sedative drugs or “downers,” such as benzodiazepines), often when combined with alcohol
- Severe obesity, which restricts how much the lungs can expand
- Obstructive sleep apnea
- Lung-protective mechanical ventilation with permissive hypercapnia in the treatment of acute respiratory distress syndrome (ARDS); these patients typically are heavily sedated and may require paralytic agents
- Acute respiratory acidosis may develop rapidly during bronchoscopy-guided percutaneous dilation tracheostomy from a reduced minute ventilation (aimed at lung-protective ventilation) 8
- Hypermetabolic states such as sepsis, malignant hyperthermia, thyroid crisis, fever, and overfeeding can elevate carbon dioxide levels; similarly, administration of bicarbonate (HCO3–) to buffer acidosis or citrate-containing anticoagulants used in dialysis may lead to elevated arterial partial pressure of carbon dioxide (PaCO2) levels; these may result in the development of acute respiratory acidosis in the critically ill patient 9
Chronic respiratory acidosis occurs over a long time. This leads to a stable situation, because the kidneys increase body chemicals, such as bicarbonate, that help restore the body’s acid-base balance.
Acute respiratory acidosis is a condition in which carbon dioxide builds up very quickly, before the kidneys can return the body to a state of balance.
Some people with chronic respiratory acidosis get acute respiratory acidosis because an illness makes their condition worse.
Respiratory acidosis signs and symptoms
Respiratory acidosis symptoms can include:
- Confusion
- Fatigue
- Lethargy
- Shortness of breath
- Sleepiness
The clinical presentation of respiratory acidosis is usually a manifestation of its underlying cause. Signs and symptoms vary based on the length, severity, and progression of the disorder. Patients can present with dyspnea, anxiety, wheezing, and sleep disturbances. In some cases, patients may present with cyanosis due to hypoxemia. If the respiratory acidosis is severe and accompanied by prolonged hypoventilation, the patient may have additional symptoms such as altered mental status, myoclonus, and possibly even seizures. Respiratory acidosis leads to hypercapnia, which induces cerebral vasodilation. If severe enough, increased intracranial pressure and papilledema may ensue, increasing the risk of herniation and possibly even death. Cases of chronic respiratory acidosis may cause memory loss, impaired coordination, polycythemia, pulmonary hypertension, and heart failure. As the arterial partial pressure of carbon dioxide [PaCO2] increases, the anxiety may progress to delirium, and patients become progressively more confused, somnolent, and obtunded. This condition is sometimes referred to as carbon dioxide narcosis.
Persistence of apnea during sleep can lead to daytime hypersomnolence and headaches. In patients with an obvious source of respiratory acidosis, the offending agent needs to be removed or reversed.
Respiratory acidosis complications
Patients with chronic respiratory acidosis, by definition, have a component of alveolar hypoventilation. Partial arterial pressure of carbon dioxide (PaCO2) and bicarbonate levels are increased, and obligatory decreases in partial pressure of arterial oxygen (PaO2) also occur.
Complications are often related to the chronic hypoxemia, which can result in increased erythropoiesis (red blood cells formation) leading to secondary polycythemia.
Chronic hypoxia is a cause of pulmonary vasoconstriction. This physiologic response can, in the long term, lead to pulmonary hypertension, right ventricular failure, and cor pulmonale.
Hypopneas and apneas during sleep lead to impaired sleep quality and cerebral vasodilation, causing morning headaches, daytime fatigue, and somnolence.
High levels of carbon dioxide (CO2) can lead to confusion, often referred to as carbon dioxide narcosis. As a late complication of cerebral vasodilation, patients may have papilledema 10.
A study by Lun et al 11 indicated that in patients with acute exacerbation of COPD, those with either compensated or decompensated respiratory acidosis tend to have poorer lung function and a greater risk for future life-threatening events than do normocapnic patients.
A study by de Miguel-Díez et al 12 indicated that respiratory acidosis is one factor increasing the risk of rehospitalization for patients within 30 days of initial hospitalization for acute exacerbation of COPD and is also a risk factor for inhospital mortality in these readmitted patients. Other factors associated with rehospitalization and inhospital mortality included older age, malnutrition, nonobesity, and treatment with noninvasive ventilation 12.
Similarly, a study by Fazekas et al 13 indicated that in patients with COPD who survive a first episode of acute hypercapnic respiratory failure requiring noninvasive ventilation, severe respiratory acidosis predicts decreased long-term survival, as do chronic respiratory failure and lower body mass index (BMI).
In addition, a prospective study by Crisafulli et al 14 indicated that in patients who have been hospitalized for acute exacerbation of COPD, a modified Medical Research Council dyspnea score of 2 or greater and acute respiratory acidosis are independent risk factors, if present at admission, for a hospital stay of more than 7 days.
A study by Mochizuki et al 15 indicated that in intensive care unit (ICU) patients, mortality rates from acidemia differ by subtype. Of over 640,000 ICU patients, 57.8% were found to have acidemia. Metabolic, combined, and respiratory acidemia had prevalences of 42.9%, 30.3%, and 25.9%, respectively. Combined acidemia had the highest mortality rate (12.7%), followed by metabolic (11%) and respiratory (5.5%) acidemia. Hospital mortality in respiratory acidemia was best predicted by partial arterial pressure of carbon dioxide (PaCO2).
Respiratory acidosis diagnosis
Your healthcare provider will perform a physical examination and ask about your symptoms.
These tests can help diagnose acidosis. They can also determine whether the cause is a breathing problem (respiratory acidosis) or a metabolic problem (metabolic acidosis). Tests may include:
- Arterial blood gas (ABG)
- Basic metabolic panel, (a group of blood tests that measure your sodium and potassium levels, kidney function, and other chemicals and functions)
- A complete blood count (CBC) to evaluate for an infectious cause with elevated white blood count and fluid body status with hemoglobin and hematocrit values is useful.
- Thyroid-stimulating hormone (TSH) and a free T4 level should be considered in selected patients, since hypothyroidism may cause obesity, leading to obstructive sleep apnea (OSA) and sleep apnea–related hypoventilation.
Other tests that may be needed to determine the cause of the acidosis include:
- Pulmonary function test to measure breathing and how well the lungs are functioning
- Chest x-ray
- CT scanning and magnetic resonance imaging [MRI] of the brain should be considered if a central cause of hypoventilation and respiratory acidosis is suspected.
- Electromyography (EMG) and measurement of nerve conduction velocity are useful in diagnosing neuromuscular disorders (eg, myasthenia gravis, Guillain-Barré syndrome, and amyotrophic lateral sclerosis [ALS]), which can cause ventilatory muscle weakness.
- Measurement of transdiaphragmatic pressure is a useful diagnostic test for documenting respiratory muscle weakness. However, it is difficult to perform and is usually carried out only in specialized pulmonary function laboratories. The test is performed by placing an esophageal catheter with an esophageal balloon and a gastric balloon. The difference between the pressures measured at the 2 balloons is the transdiaphragmatic pressure. Patients with diaphragmatic dysfunction and paralysis have a decrease in maximal transdiaphragmatic pressure.
Physical examination
Physical examination findings in patients with respiratory acidosis are usually nonspecific and are related to the underlying illness or the cause of the respiratory acidosis.
Thoracic examination of patients with obstructive lung disease may demonstrate diffuse wheezing, hyperinflation (ie, barrel chest), decreased breath sounds, hyperresonance on percussion, and prolonged expiration. Rhonchi may also be heard.
Cyanosis may be noted if accompanying hypoxemia is present. Digital clubbing may indicate the presence of a chronic respiratory disease or other organ system disorders.
The patient’s mental status may be depressed if severe elevations of arterial partial pressure of carbon dioxide (PaCO2) are present. Patients may have asterixis, myoclonus, and seizures.
Papilledema may be found during the retinal examination. Conjunctival and superficial facial blood vessels may also be dilated.
A study by Zorrilla-Riveiro et al 16 of 212 patients indicated that in persons with dyspnea, nasal flaring is a sign of respiratory acidosis.
Respiratory acidosis lab values
In respiratory acidosis, the arterial blood gas (ABG) will show an elevated arterial partial pressure of carbon dioxide (PaCO2) (>45 mmHg), elevated bicarbonate [HCO3–] (>30 mmHg), and decreased pH (pH<7.35). The respiratory acidosis can be further classified as acute or chronic based on the relative increase in bicarbonate [HCO3–] with respect to arterial partial pressure of carbon dioxide (PaCO2). In cases of acute respiratory acidosis, bicarbonate [HCO3–] will have increased by 1 mEq/L for every 10 mmHg increase in arterial partial pressure of carbon dioxide (PaCO2) over a few minutes. In cases of chronic respiratory acidosis, bicarbonate [HCO3–] will have increased by 4 mEq/L for every 10 mmHg increase in arterial partial pressure of carbon dioxide (PaCO2) over a time course of 3 to 5 days. If the compensation does not occur in this pattern, a mixed respiratory-metabolic disorder may be present. In a patient who presents with unexplained respiratory acidosis, a drug screen may also be warranted.
The expected change in pH with respiratory acidosis can be estimated with the following equations:
- Acute respiratory acidosis: Change in pH = 0.008 × (40 – PaCO2)
- Chronic respiratory acidosis: Change in pH = 0.003 × (40 – PaCO2)
Arterial blood gas (ABG) analysis
Arterial blood gas (ABG) sampling, is a test often performed in an inpatient setting to assess the acid-base status of a patient. A needle is used to draw blood from an artery, often the radial artery, and the blood is analyzed to determine parameters such as the pH, arterial partial pressure of carbon dioxide (PaCO2), arterial partial pressure of oxygen (PaO2), bicarbonate (HCO3–), oxygen saturation (O2 Sat) and more. This allows the physician to understand the status of the patient better. ABGs are especially important in the critically ill. They are the main tool utilized in adjusting to the needs of a patient on a ventilator.
- Arterial partial pressure of carbon dioxide (PaCO2) as carbon dioxide tension, this measures the level of carbon dioxide in your blood.
- Arterial partial pressure of oxygen (PaO2) also known as oxygen tension, this measures how well oxygen is being transferred into your blood.
- Oxygen saturation (O2 Sat) is an assessment of the amount of oxygen in your blood that is based on measuring levels of hemoglobin. Hemoglobin is a protein found inside red blood cells that is responsible for carrying oxygen throughout the body.
- Bicarbonate (HCO3–) concentration: Bicarbonate (HCO3–) is an electrolyte, which is a type of mineral involved in managing your body’s acid-base balance. Most of the carbon dioxide (CO2) in your blood is stored in the form of bicarbonate, so this measurement helps reflect carbon dioxide (CO2) levels.
- Although not universal, some arterial blood gases tests include measurements of hemoglobin as well as altered forms of the hemoglobin protein. Examples of these potential additional measurements include:
- Methemoglobin: Methemoglobin is a form of hemoglobin that has been oxidized, changing its heme iron configuration from the ferrous (Fe2+) to the ferric (Fe3+) state. Unlike normal hemoglobin, methemoglobin does not bind oxygen and as a result cannot deliver oxygen to the tissues.
- Carboxyhemoglobin: Carboxyhemoglobin is a stable complex of carbon monoxide and hemoglobin that forms in red blood cells upon contact with carbon monoxide. This abnormal form of hemoglobin attaches to carbon monoxide and can interfere with oxygen’s ability to travel in the blood.
- Oxyhemoglobin: Oxyhemoglobin represents the fraction of oxygenated hemoglobin in relation to the total hemoglobin present, including non-oxygen-binding hemoglobins. In healthy individuals, oxyhemoglobin and oxygen saturation are approximately equal.
- Deoxyhemoglobin: This is the form of hemoglobin without oxygen in the blood.
The following are the most important Normal Values on an ABG:
- pH = 7.35 to 7.45
- Arterial partial pressure of carbon dioxide (PaCO2) = 35 to 45 mmHg
- Arterial partial pressure of oxygen (PaO2) = 75 to 100 mmHg
- Bicarbonate (HCO3–) = 22 to 26 mEq/L
- O2 Sat = greater than 95%
The ability to quickly and efficiently read an ABG is paramount to quality patient care.
- Look at the pH. Decide whether it is acidotic, alkalotic, or within the physiological range
- Arterial partial pressure of carbon dioxide (PaCO2) level determines respiratory contribution; a high level means the respiratory system is lowering the pH and vice versa.
- Bicarbonate (HCO3–) level denotes metabolic/kidney effect. An elevated bicarbonate (HCO3–) is raising the pH and vice versa.
- If the pH is acidotic, look for the number that corresponds with a lower pH. If it is a respiratory acidosis, the carbon dioxide (CO2) should be high. If the patient is compensating metabolically, the bicarbonate (HCO3–) should be high as well. A metabolic acidosis will be depicted with an bicarbonate (HCO3–) that is low.
- If the pH is alkalotic, again, determine which value is causing this. A respiratory alkalosis will mean the carbon dioxide (CO2) is low; a metabolic alkalosis should lend an bicarbonate (HCO3–) that is high. Compensation with either system will be reflected oppositely; for a respiratory alkalosis the metabolic response should be a low bicarbonate (HCO3–) and for metabolic alkalosis, the respiratory response should be a high carbon dioxide (CO2).
- If the pH level is in the physiological range but the arterial partial pressure of carbon dioxide (PaCO2) and/or bicarbonate (HCO3–) are not within normal limits, there is likely a mixed disorder. Also, compensation does not always occur; this is when clinical information becomes paramount.
- Sometimes it is difficult to ascertain whether a patient has a mixed disorder.
Other tests that are important to perform when analyzing the acid-base status of a patient include those that measure electrolyte levels and renal function. This helps the clinician gather information that can be used to determine the exact mechanism of the acid-base imbalance as well as the factors contributing to the disorders 2, 17.
Urinalysis
Urine pH is normally acidic, at less than 5.0. In acidemia, the urine normally becomes more acidic. If the urine pH is above 5.5 in the face of acidemia, this finding is consistent with a type 1 renal tubular acidosis (RTA). Alkaline urine is typical in salicylate toxicity.
Patients with ethylene glycol toxicity may present with calcium oxalate crystals, which appear needle shaped, in the urine.
Urine Anion Gap
Calculating the urine anion gap is helpful in evaluating some cases of non-anion gap metabolic acidosis. The major measured urinary cations are Na+ and K+, and the major measured urinary anion is Cl-:
- Urine anion gap = Urine Na + Urine K – Urine Cl
In the face of metabolic acidosis, the kidneys increase the amount of NH3 synthesized to buffer the excess H+ and NH4 Cl excretion increases. The increased unmeasured ammonium (NH4+) thus increases the measured anion Cl- in the urine, and the net effect is a negative anion gap, representing a normal response to systemic acidification. The finding of a positive urine anion gap in the face of non-anion gap metabolic acidosis points toward a renal acidification defect (eg, renal tubular acidosis) 18.
Ketone level
Elevations of ketones indicate diabetic, alcoholic, and starvation ketoacidosis 19.
The nitroprusside test is used to detect the presence of ketoacids in the blood and the urine. This test measures only acetoacetate and acetone; therefore, it may underestimate the degree of ketonemia and ketonuria, because it will not detect the presence of beta-hydroxybutyrate. This limitation of the test can be especially problematic in patients with ketoacidosis who cannot convert beta-hydroxybutyrate to acetoacetate because of severe shock or liver failure.
An assay for beta-hydroxybutyrate is unavailable in some hospitals. An indirect method to circumvent this problem is to add a few drops of hydrogen peroxide to a urine specimen. This enzymatically will convert beta-hydroxybutyrate into acetoacetate, which will be detected by the nitroprusside test.
Respiratory acidosis treatment
Once the diagnosis has been made, the underlying cause of respiratory acidosis has to be treated. The hypercapnia should be corrected gradually because rapid alkalization of the cerebrospinal fluid (CSF) may lead to seizures 2. Pharmacologic therapy can also be used to help improve ventilation. Bronchodilators like beta agonists, anticholinergic drugs, and methylxanthines can be used in treating patients with obstructive airway diseases. Naloxone can be used in patients who overdose on opioid use 20, 21.
Patients who are moribund, lethargic or confused need to be monitored in the intensive care unit (ICU). The criteria for admission to the intensive care unit (ICU) vary from institution to institution but may include patient confusion, lethargy, respiratory muscle fatigue, and a low pH (pH< 7.25).
Those who exhibit hypoventilation will need endotracheal intubation and mechanical ventilation. All patients who require tracheal intubation and mechanical ventilation must be admitted to the ICU. Most acute care facilities require that all patients being treated acutely with noninvasive positive-pressure ventilation (NIPPV) be admitted to the ICU.
The use of respiratory stimulants has not been shown to be effective in treating respiratory acidosis. Medroxyprogesterone has been used to stimulate the respiratory drive, but its benefits are questionable. Hypoxic patients, of course, need supplemental oxygen.
Oxygen therapy
Because many patients with hypercapnia are also hypoxemic, oxygen therapy may be indicated. Oxygen therapy is employed to prevent the sequelae of long-standing hypoxemia. Patients with COPD who meet the criteria for oxygen therapy have been shown to have decreased mortality when treated with continuous oxygen therapy. Oxygen therapy has also been shown to reduce pulmonary hypertension in some patients.
Oxygen therapy should be used with caution because it may worsen hypercapnia in some situations. For example, patients with COPD may experience exacerbation of hypercapnia during oxygen therapy. This observation is thought by many to be primarily a consequence of ventilation-perfusion mismatching, in opposition to the commonly accepted concept of a reduction in hypoxic ventilatory drive. The exact pathophysiology, however, remains controversial.
Hypercapnia is best avoided by titrating oxygen delivery to maintain oxygen saturation in the low 90% range and partial arterial pressure of oxygen (PaO2) in the range of 60-65 mm Hg.
Ventilatory support
Therapeutic measures that may be lifesaving in severe hypercapnia and respiratory acidosis include endotracheal intubation with mechanical ventilation and noninvasive positive pressure ventilation (NIPPV) techniques such as nasal continuous positive-pressure ventilation (NCPAP) and nasal bilevel ventilation. The latter techniques of NIPPV are preferred treatment for OHS and neuromuscular disorders, because they help to improve partial arterial pressure of oxygen (PaO2) and decrease the partial pressure of arterial carbon dioxide (PaCO2).
Noninvasive external negative-pressure ventilation devices are also available for the treatment of selected patients with chronic respiratory failure.
Rapid correction of the hypercapnia by the application of external noninvasive positive-pressure ventilation or invasive mechanical ventilation can result in alkalemia. Accordingly, these techniques should be used with caution.
A study comparing noninvasive techniques with invasive ventilation in myasthenic crisis found that patients who underwent noninvasive ventilation had better outcomes than patients who underwent invasive ventilation 22.
A 4-year retrospective study reported that noninvasive positive pressure ventilation (NIPPV) was highly beneficial in the treatment of COPD with hypercapnia (type 2) respiratory failure 23. NIPPV led to a decreased length of stay and a reduced cost of hospitalization.
Based on a literature review, Fielding-Singh et al 24 recommended that in refractory respiratory acidosis resulting from ARDS, patients be treated with “initial modest liberalization of tidal volumes, followed by neuromuscular blockade and prone positioning.”
A study by Nentwich et al 25 indicated that in patients with hypercapnia and concomitant renal failure, respiratory acidosis can be decreased and ventilation requirements reduced through the use of low-flow extracorporeal carbon dioxide (CO2) removal in combination with renal replacement therapy.
Investigational therapy
Extracorporeal carbon dioxide removal (ECCO2 R) is a newer technique for removing carbon dioxide via venovenous bypass without affecting oxygenation. Extracorporeal carbon dioxide removal (ECCO2 R) is being evaluated in the treatment of respiratory acidosis as a complication of the low tidal volume lung-protective ventilation with permissive hypercapnia. However, this technique has been associated with serious complications and requires more investigation 26.
- Respiratory Acidosis. https://emedicine.medscape.com/article/301574-overview#a7[↩]
- Patel S, Sharma S. Respiratory Acidosis. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482430[↩][↩][↩][↩]
- Berend K. Review of the Diagnostic Evaluation of Normal Anion Gap Metabolic Acidosis. Kidney Dis (Basel). 2017 Dec;3(4):149-159. doi: 10.1159/000479279[↩]
- Adamczak M, Surma S. Metabolic Acidosis in Patients with CKD: Epidemiology, Pathogenesis, and Treatment. Kidney Dis (Basel). 2021 Jun 4;7(6):452-467. doi: 10.1159/000516371[↩]
- Koeppen BM. The kidney and acid-base regulation. Adv Physiol Educ. 2009 Dec;33(4):275-81. doi: 10.1152/advan.00054.2009[↩][↩]
- Hamm LL, Nakhoul N, Hering-Smith KS. Acid-Base Homeostasis. Clin J Am Soc Nephrol. 2015 Dec 7;10(12):2232-42. doi: 10.2215/CJN.07400715[↩][↩]
- Brinkman JE, Sharma S. Respiratory Alkalosis. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482117[↩]
- Karagiannidis C, Merten ML, Heunks L, Strassmann SE, Schäfer S, Magnet F, Windisch W. Respiratory acidosis during bronchoscopy-guided percutaneous dilatational tracheostomy: impact of ventilator settings and endotracheal tube size. BMC Anesthesiol. 2019 Aug 9;19(1):147. doi: 10.1186/s12871-019-0824-5[↩]
- Kallet RH. A Comprehensive Review of Prone Position in ARDS. Respir Care. 2015 Nov;60(11):1660-87. doi: 10.4187/respcare.04271[↩]
- Pollock JM, Deibler AR, Whitlow CT, Tan H, Kraft RA, Burdette JH, Maldjian JA. Hypercapnia-induced cerebral hyperperfusion: an underrecognized clinical entity. AJNR Am J Neuroradiol. 2009 Feb;30(2):378-85. doi: 10.3174/ajnr.A1316[↩]
- Lun, C.-T., Tsui, M.S.N., Cheng, S.-L., Chan, V.L., Leung, W.-S., Cheung, A.P.S. and Chu, C.-M. (2016), AE-COPD and respiratory acidosis. Respirology, 21: 128-136. https://doi.org/10.1111/resp.12652[↩]
- de Miguel-Díez, J., Jiménez-García, R., Hernández-Barrera, V., Carrasco-Garrido, P., Puente Maestu, L., Ramírez García, L., and López de Andrés, A. (2016) Readmissions following an initial hospitalization by COPD exacerbation in Spain from 2006 to 2012. Respirology, 21: 489– 496. doi: 10.1111/resp.12705[↩][↩]
- Fazekas AS, Aboulghaith M, Kriz RC, Urban M, Breyer MK, Breyer-Kohansal R, Burghuber OC, Hartl S, Funk GC. Long-term outcomes after acute hypercapnic COPD exacerbation : First-ever episode of non-invasive ventilation. Wien Klin Wochenschr. 2018 Oct;130(19-20):561-568. doi: 10.1007/s00508-018-1364-6[↩]
- Crisafulli E, Ielpo A, Barbeta E, Ceccato A, Huerta A, Gabarrús A, Soler N, Chetta A, Torres A. Clinical variables predicting the risk of a hospital stay for longer than 7 days in patients with severe acute exacerbations of chronic obstructive pulmonary disease: a prospective study. Respir Res. 2018 Dec 27;19(1):261. doi: 10.1186/s12931-018-0951-4[↩]
- Mochizuki K, Fujii T, Paul E, Anstey M, Uchino S, Pilcher DV, Bellomo R. Acidemia subtypes in critically ill patients: An international cohort study. J Crit Care. 2021 Aug;64:10-17. doi: 10.1016/j.jcrc.2021.02.006[↩]
- Zorrilla-Riveiro JG, Arnau-Bartés A, Rafat-Sellarés R, García-Pérez D, Mas-Serra A, Fernández-Fernández R. Nasal flaring as a clinical sign of respiratory acidosis in patients with dyspnea. Am J Emerg Med. 2017 Apr;35(4):548-553. doi: 10.1016/j.ajem.2016.12.008[↩]
- Rajkumar P, Pluznick JL. Acid-base regulation in the renal proximal tubules: using novel pH sensors to maintain homeostasis. Am J Physiol Renal Physiol. 2018 Nov 1;315(5):F1187-F1190. doi: 10.1152/ajprenal.00185.2018[↩]
- Pereira PC, Miranda DM, Oliveira EA, Silva AC. Molecular pathophysiology of renal tubular acidosis. Curr Genomics. 2009 Mar;10(1):51-9. doi: 10.2174/138920209787581262[↩]
- Metabolic Acidosis Workup. https://emedicine.medscape.com/article/242975-workup#c12[↩]
- Cove ME, Federspiel WJ. Veno-venous extracorporeal CO2 removal for the treatment of severe respiratory acidosis. Crit Care. 2015 Apr 17;19(1):176. doi: 10.1186/s13054-015-0769-0[↩]
- Sharma S, Hashmi MF, Burns B. Alveolar Gas Equation. [Updated 2022 Aug 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482268[↩]
- Wu JY, Kuo PH, Fan PC, Wu HD, Shih FY, Yang PC. The role of non-invasive ventilation and factors predicting extubation outcome in myasthenic crisis. Neurocrit Care. 2009;10(1):35-42. doi: 10.1007/s12028-008-9139-y[↩]
- Zhang WB, Wang XY, Tian XY, Zhang H, Wang ZP, Gao YY. [Clinical value of noninvasive positive-pressure ventilation in chronic obstruction pulmonary disease combined with type II respiratory failure: a 4-year retrospective study]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008 Oct;20(10):601-3. Chinese.[↩]
- Fielding-Singh V, Matthay MA, Calfee CS. Beyond Low Tidal Volume Ventilation: Treatment Adjuncts for Severe Respiratory Failure in Acute Respiratory Distress Syndrome. Crit Care Med. 2018 Nov;46(11):1820-1831. doi: 10.1097/CCM.0000000000003406[↩]
- Nentwich J, Wichmann D, Kluge S, Lindau S, Mutlak H, John S. Low-flow CO2 removal in combination with renal replacement therapy effectively reduces ventilation requirements in hypercapnic patients: a pilot study. Ann Intensive Care. 2019 Jan 7;9(1):3. doi: 10.1186/s13613-019-0480-4[↩]
- Terragni PP, Birocco A, Faggiano C, Ranieri VM. Extracorporeal CO2 removal. Contrib Nephrol. 2010;165:185-196. doi: 10.1159/000313758[↩]