Contents
What is sleep disorder
A sleep disorder is a condition that prevents you from getting restful sleep and can cause daytime sleepiness and problems in functioning. Signs you may have a sleep disorder include persistent difficulty going to sleep or staying sleeping, irregular breathing or movement during sleep, and feeling sleepy during the day.
American adults average 6.9 hours of sleep each night, less than the seven to nine hours recommended by many sleep experts 1. Sleep disorders can lead to increased morbidity and higher health care costs. In controlled studies, volunteers who were restricted to four to five hours of sleep for a few days experienced worsening neurocognitive, behavioral, metabolic, and autonomic parameters 2. Several studies demonstrate that sleep deprivation leads to alterations in immune function with increased risk of infection, including pneumonia 3. Additionally, chronic sleep deprivation is associated with increased risk of diabetes mellitus, cardiovascular disease, cancer, and mortality 4, 5.
There are many different types of sleep disorders. They’re often grouped into categories that explain why they happen or how they affect you. Sleep disorders can also be grouped according to behaviors, problems with your natural sleep-wake cycles, breathing problems, difficulty sleeping or how sleepy you feel during the day.
Some common types of sleep disorders include:
- Insomnia, in which you have difficulty falling asleep or staying asleep throughout the night.
- Sleep apnea, in which you experience abnormal patterns in breathing while you are asleep. There are several types of sleep apnea.
- Restless legs syndrome (RLS), a type of sleep movement disorder. Restless legs syndrome, also called Willis-Ekbom disease, causes an uncomfortable sensation and an urge to move the legs while you try to fall asleep.
- Narcolepsy, a condition characterized by extreme sleepiness during the day and falling asleep suddenly during the day.
- Rapid eye movement (REM) sleep behavior disorder. Motor activity during sleep, acting out of dreams, polysomnography showing increased muscle tone.
There are many ways to help diagnose sleep disorders. Doctors can usually treat most sleep disorders effectively once they’re correctly diagnosed.
Patients with sleep disorders may be categorized into those who cannot sleep, those who will not sleep, those with excessive daytime sleepiness, and those with increased movements during sleep. Table 1 summarizes common sleep disorders.
Table 1. Summary of Common Sleep Disorders
| Disorder | Symptoms and signs | Most effective treatment |
|---|---|---|
Delayed sleep phase syndrome | Late sleep onset and wake-up time | Bright light therapy in the morning, low-dose melatonin in the evening |
Insomnia | Difficulty initiating or maintaining sleep, daytime sleepiness with inability to nap, daytime impairment (e.g., difficulty with memory, concentration, attention; worry about sleep; mood disturbance; irritability) | Cognitive behavior therapy, benzodiazepine receptor agonists |
Narcolepsy | Excessive daytime sleepiness, cataplexy, hallucinations upon falling asleep or awakening | Modafinil (Provigil) or stimulants, gamma hydroxybutyric acid (sodium oxybate [Xyrem]), selective serotonin reuptake inhibitors |
Obstructive sleep apnea | Snoring, witnessed apneas, gasping or choking, excessive daytime sleepiness | Continuous positive airway pressure |
Rapid eye movement sleep behavior disorder | Motor activity during sleep, acting out of dreams, polysomnography showing increased muscle tone | Clonazepam (Klonopin) or melatonin |
Restless legs syndrome | Uncomfortable sensation (e.g., “creepy crawly,” aching) in both legs, symptoms are worse in the evening, improve with movement such as walking or stretching | Dopaminergic agonists |
Sleep disorder test
Although many sleep disorders can be diagnosed using history alone, overnight polysomnography may be useful to assess for disorders such as obstructive sleep apnea (OSA). Polysomnography monitors brain wave activity (electroencephalogram), eye movements (electro-oculogram), muscle activity (electromyogram), heart rate and rhythm (electrocardiogram), and respiration (via nasal pressure transducer and oronasal thermistor, and oxygen saturation using pulse oximetry). Table 2 lists the most common indications for polysomnography.
Table 2. Common Indications for Polysomnography
Diagnosis of a sleep disorder | |
Narcolepsy | |
Parasomnias | |
Periodic limb movement disorder | |
Rapid eye movement sleep behavior disorder | |
Sleep-related breathing disorders, such as obstructive and central sleep apnea | |
Sleep-related seizure disorders | |
Evaluation of sleep-related symptoms | |
Sleep maintenance insomnia | |
Snoring | |
Unexplained daytime fatigue or sleepiness | |
Treatment of sleep-related breathing disorders (i.e., using positive airway pressure titration) | |
List of sleep disorders
Insomnia
Insomnia is difficulty going to sleep, or difficulty staying asleep. If you have insomnia, it is likely to not only affect your energy levels, but also your mood, health and quality of life. Most people need 7-9 hours sleep each night, so insomnia can lead to tiredness, poor concentration or mood changes. These can affect your ability to function, especially if it goes on for a long time. Insomnia may be experienced for no obvious reason, or it may be due to an underlying cause, such as stress, general health conditions, anxiety, depression, substance abuse or a sleep disorder. Learning good sleep habits and lifestyle changes can help you overcome insomnia. A doctor or health professional can give advice on effective strategies.
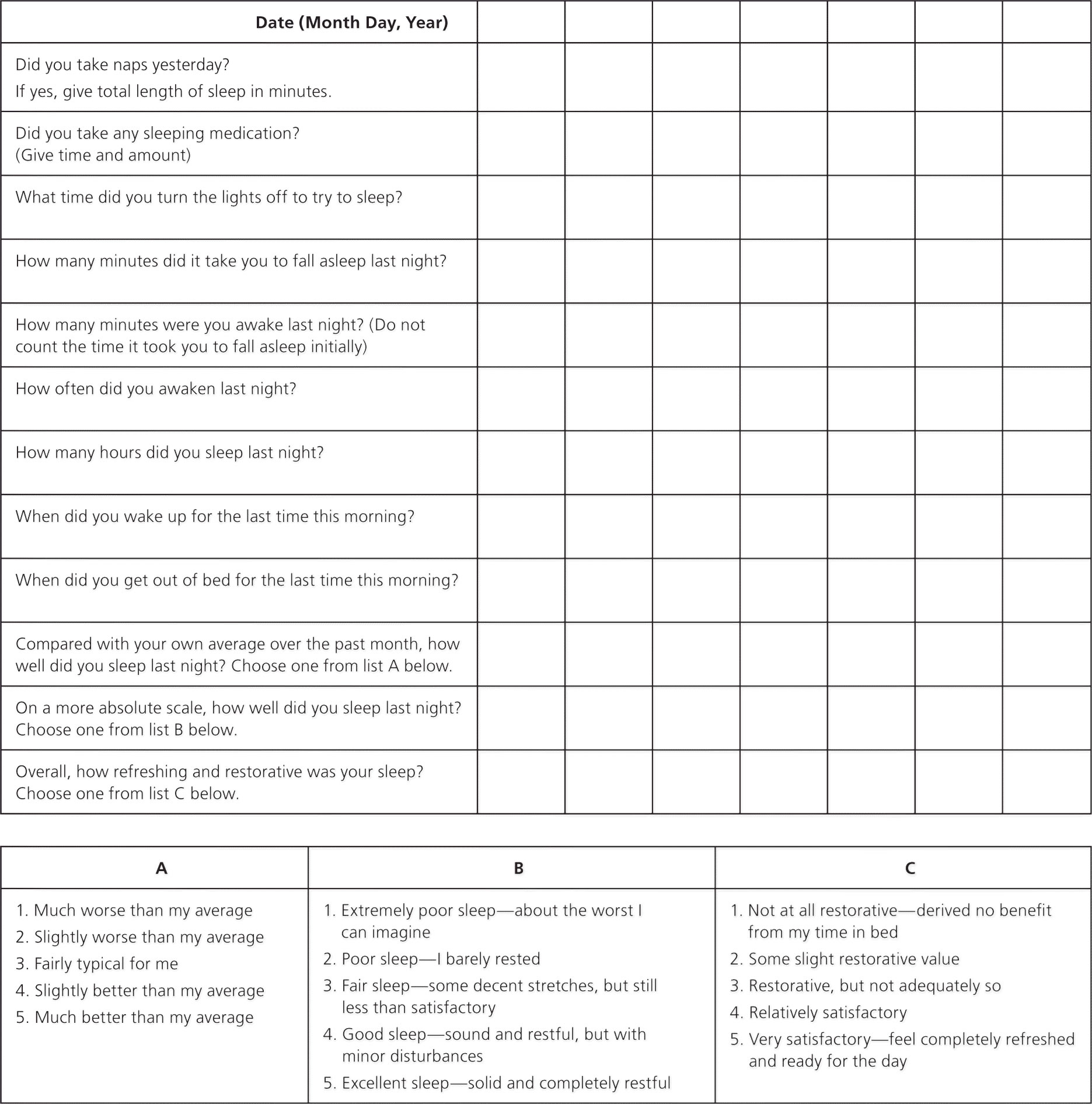
Approximately 10% of the U.S. population has had insomnia that occurred every night for at least two weeks 7; however, many do not discuss it with their physician. Insomnia is characterized by repeated difficulty with sleep initiation (the time it takes to fall asleep, normally less than 30 minutes; called sleep latency in sleep studies), duration (normally seven to nine hours per night for adults; also called sleep quantity), consolidation (sleep uninterrupted by arousals or awakenings), or quality that occurs despite adequate time and opportunity for sleep, resulting in daytime impairment 8. Daytime impairment may include fatigue; tiredness; difficulty with memory, concentration, and attention; worry about sleep; mood disturbances; or irritability. Insomnia is usually diagnosed with a patient history that includes evaluation for contributing psychiatric or medical conditions. Table 3 includes questions for patients that may be helpful in this assessment. Occasionally, a sleep log (Figure 1) or an actigraphy log (Figure 2) used for one to two weeks may be helpful. An actigraph is a device placed on the patient’s wrist during sleep to detect movements and sleep/wake patterns. The patient also records on this log, and the physician interprets the data based on the log and the actigraph. Overnight polysomnography is rarely needed, unless the history suggests concurrent sleep disorders or initial treatment is ineffective 9.
Figure 1. Sleep Log – to assess sleep behaviors over a period of time.
Figure 2. Sleep Actigraphy Log
Figure 3. Sleep Diary
Insomnia usually lasts for a short time, although it can go on for months or even years. Women and elderly people are more likely to suffer from it.
Short-term (acute) insomnia is often caused by a particular circumstance, such as a crisis at work or home. If your life is being affected you may seek professional help say from a doctor. To help you get by until things settle down, a doctor may assess that you are not developing something more serious like depression and suggest some techniques to improve sleep function. The use of any sleeping pills is only very short term and must be reviewed by your doctor regularly as they can be addictive.
If you have longer-term (chronic) insomnia doctors generally recommend making sure you have good sleep habits. They may also recommend non-drug treatments such as cognitive behavioral therapy, which is designed to help you change unhelpful thoughts and behaviors.
Cognitive behavior therapy (CBT) and hypnotic medications are treatment options for insomnia. Table 3 outlines the key points of cognitive behavior therapy (CBT) for insomnia. Primary care physicians can effectively administer CBT to treat chronic insomnia 10. Medication regimens approved by the U.S. Food and Drug Administration for insomnia are summarized in Table 4. Hypnotics may be associated with tolerance, dependence, and withdrawal symptoms, such as rebound insomnia, if a drug is abruptly stopped. However, studies show contradictory evidence for the development of tolerance and dependence 11. There are no comparative data on the effectiveness of short- or long-acting hypnotics for sleep onset or sleep maintenance insomnia, respectively.
Table 3. Cognitive Behavior Therapy for Insomnia
Cognitive therapy | |
Focuses on changing false beliefs and attitudes about sleep (e.g., everyone needs at least 8 hours of sleep for good health) | |
Sleep hygiene education | |
No pets in the bedroom | |
No caffeine consumption after 4 p.m. | |
Keep bedroom cool and conducive to sleep | |
No watching the bedroom clock | |
No nicotine use, especially in the evening | |
No exercising within 2 to 3 hours before bedtime | |
Sleep restriction | |
Time in bed can be reduced by estimating the actual total time that the patient is sleeping (e.g., if the patient is in bed for 8 hours but sleeps for 5.5 hours, time in bed could be reduced to 5.5 hours); time in bed usually should not be reduced to less than 5 hours | |
After sleep efficiency (ratio of time sleeping to time in bed) reaches 90%, the time in bed can be increased by 15 minutes every week | |
Stimulus control | |
Go to bed only when sleepy | |
Use the bedroom only for sleep and sex | |
Go to another room if unable to fall asleep within 15 to 20 minutes | |
Read or engage in other quiet activities and return to bed only when sleepy | |
Table 4. Medications for Insomnia
| Medication | Onset of action (minutes) | Half-life (hours) | Dose (mg) | Main indications | Common adverse effects | |
| Benzodiazepine receptor agonists | ||||||
| Eszopiclone (Lunesta) | 10 | 5 to 7 | 1 to 3 | Sleep maintenance insomnia | Metallic taste in the mouth, nausea and vomiting, dizziness, somnolence, rebound insomnia† | |
| Zaleplon (Sonata) | 30 | 1 | 5 to 20 | Sleep onset insomnia; can be given upon waking during night | Dizziness, headache, rebound insomnia† | |
| Zolpidem (Ambien) | 7 to 27 | 2 to 3 | 5 to 10 | Sleep onset insomnia | Somnolence, fatigue, drugged state, dizziness | |
| Occasionally sleep walking, talking, or eating; amnesia; increased fall risk in older persons; rebound insomnia† | ||||||
| Zolpidem CR (Ambien CR) | 30 | 3 to 4.5 | 6.25 to 12.5 | Sleep onset and maintenance insomnia | Similar to zolpidem | |
| Benzodiazepines | ||||||
| Estazolam | 120 | 10 to 24 | 0.5 to 2 | Sleep onset and maintenance insomnia | Somnolence, dizziness, ataxia, asthenia, rebound insomnia† | |
| Flurazepam | 15 to 45 | 40 to 114 | 15 to 30 | Sleep onset and maintenance insomnia | Taste disorder, somnolence, ataxia, dizziness, hangover, blurred vision, rarely leukopenia | |
| Quazepam (Doral) | 30 | 39 to 75 | 7.5 to 15 | Sleep onset and maintenance insomnia | Dyspepsia, xerostomia, dizziness, hangover, headache, fatigue | |
| Temazepam (Restoril) | 30 to 60 | 8 to 15 | 7.5 to 30 | Sleep onset and maintenance insomnia | Somnolence, blurred vision, hypotension, rebound insomnia† | |
| Triazolam (Halcion) | 15 to 30 | 2 to 5 | 0.125 to 0.25 | Sleep onset and maintenance insomnia | Somnolence, amnesia, ataxia, nausea and vomiting, dizziness, hepatotoxicity, rebound insomnia† | |
| Melatonin receptor agonist | ||||||
| Ramelteon (Rozerem) | 45 | 2 to 5 | 8 | Sleep onset insomnia | Dizziness, somnolence, fatigue, nausea, exacerbation of insomnia, hallucinations | |
NOTE: Medications approved by the U.S. Food and Drug Administration for the treatment of insomnia.
NA = not available.
†—If withdrawn suddenly after long-term use.
Although antihistamines, such as diphenhydramine (Benadryl), are widely used for insomnia, evidence on effectiveness and safety is very limited 12. In large clinical trials, melatonin had only minimal effect on initial sleep latency with little effect on total sleep time 13. Similarly, there are limited data on the effectiveness of sedating antidepressants, such as trazodone, for insomnia. However, they may be considered in patients with comorbid depression or if treatment with hypnotics fails 14.
A meta-analysis showed similar short-term effectiveness with hypnotics used alone and with CBT, whereas CBT alone resulted in greater reduction in sleep latency 15. Similarly, other studies have shown that CBT combined with pharmacotherapy is superior to pharmacotherapy alone, and improvement with CBT alone is maintained at 10 to 24 months of follow-up 16. In some studies, pharmacotherapy performed slightly or no better than placebo on various outcome measures 17.
Patients with insomnia who do not respond to medications and CBT should be referred to a sleep center for further testing and treatment.
Sleep Apnoea and Snoring
Sleep apnoea occurs when your breathing stops and starts repeatedly during sleep. It is caused by the temporary but repeated collapse of the airway at the back of the mouth. Common symptoms include loud snoring, waking up gasping and struggling to stay awake during the day.
Sleep apnoea has been linked to a range of health problems, including high blood pressure, heart disease, diabetes, and stroke. In many people, it can be treated using an oral appliance (like a mouthguard) that helps keep your airways open while you sleep. Other people will need nasal continuous positive airway pressure (CPAP) which pumps air under gentle pressure to help keep your airways open. Some people will benefit from surgery.
Causes of snoring include being overweight, drinking alcohol and smoking. Some allergies can also cause you to snore. Either way, if snoring is causing problems in your household, see your doctor.
Other sleep problems
Other sleep problems include:
- shift work disorder, which is sleepiness and/or insomnia associated with your work schedule.
- narcolepsy, which is repeatedly falling asleep during waking hours.
- restless legs syndrome, which is an irresistible urge to move the legs while trying to sleep.
Restless legs syndrome
Restless legs syndrome is a neurologic disorder that affects between 2.5% and 15% of the U.S. general population 18.
The condition is characterized by four essential features: (1) the intense urge to move the legs, usually accompanied or caused by uncomfortable sensations (e.g., “creepy crawly,” aching) in the legs; (2) symptoms that begin or worsen during periods of rest or inactivity; (3) symptoms that are partially or totally relieved by movements such as walking or stretching; and (4) symptoms that are worse or only occur in the evening or at night 8.
The diagnosis of restless legs syndrome is based on history findings. Because the condition can be a symptom of iron deficiency 19, a ferritin measurement may be warranted, even in the absence of anemia. A ferritin level less than 50 ng per mL (112 pmol per L) is associated with restless legs syndrome 20. Restless legs syndrome is familial in about 50% of patients, although it may be idiopathic or secondary to chronic renal failure, antidepressant use, pregnancy, or neuropathy. Most patients with restless legs syndrome have periodic limb movements during sleep, which are characterized by periodic episodes of repetitive, stereotypic movements that predominantly occur in the lower extremities. Patients’ bed partners may notice these movements.
Pharmacologic treatment of restless legs syndrome depends on the frequency of symptoms (Table 5). Dopaminergic agonists are first-line therapy for patients with nightly, persistent symptoms. Common adverse effects of dopaminergic agonists include insomnia, nasal congestion, swelling of the extremities, and daytime sleepiness. There have also been reports of increased tendency toward compulsive behaviors, such as gambling, in patients taking these medications. In patients with iron deficiency, iron supplementation may improve or resolve symptoms of restless legs syndrome.
Patients should be referred to a sleep clinic if symptoms of restless legs syndrome cannot be controlled or if augmentation symptoms (e.g., symptoms begin progressively earlier in the day or develop in the arms and trunk) occur despite appropriate treatment.
Table 5. Medications for Restless Legs Syndrome
| Symptoms | First choice | Alternative choice | ||
|---|---|---|---|---|
Occasional | Carbidopa/levodopa (Sinemet), 10 to 25 mg/100 mg at bedtime, as needed | Opiates
| ||
Frequent | Dopaminergic agonist
| Opiates
| ||
Nightly | Dopaminergic agonist
| Gabapentin (Neurontin) and pregabalin (Lyrica)
Opiates | ||
Painful | Gabapentin and pregabalin
Opiates | Dopaminergic agonists
| ||
[Source 6]
Narcolepsy
Narcolepsy is a chronic, debilitating condition with a prevalence of around 0.02% 21. Clinical features of narcolepsy usually begin in the teens or twenties, although diagnosis may be established years later. Onset after 50 years of age is unusual. The classic tetrad of narcolepsy is excessive sleepiness; cataplexy; hallucinations upon falling asleep (hypnagogic) and/or upon awakening (hypnopompic); and sleep paralysis (generalized, transient inability to move or speak during sleep-wake transitions).
Cataplexy is the sudden decrease or loss of voluntary muscle tone following an emotional trigger—usually laughter, but sometimes anger or surprise. It can manifest as jaw dropping, head nodding, arms dropping to the side, knees sagging, or the patient collapsing to the floor. These attacks may last from a few seconds to a few minutes, and the patient’s conscious awareness is preserved. The presence of cataplexy is highly specific for narcolepsy.
Referral to a sleep clinic is indicated if narcolepsy is suspected. The typical sequence of testing for suspected narcolepsy is one to two weeks of a sleep log or actigraphy to document sleep duration, followed by polysomnography to evaluate for other sleep disorders and document adequate sleep time, and concluding the next day with a multiple sleep latency test. The multiple sleep latency test is a daytime nap test to objectively assess for sleepiness and for onset of rapid eye movement (REM) sleep during naps. The combination of a mean sleep latency of less than eight minutes plus at least two naps with early onset REM sleep supports a diagnosis of narcolepsy 8.
Cataplexy, sleep paralysis, and hypnagogic hallucinations can be treated with REM-suppressing antidepressants, such as venlafaxine (Effexor) or other selective serotonin reuptake inhibitors. Sleepiness may be managed with adequate sleep hygiene and scheduled daytime naps. Otherwise, modafinil (Provigil; 200 to 800 mg daily) or stimulants such as methylphenidate (Ritalin; 10 to 100 mg daily) or dextroamphetamine (5 to 60 mg daily) can be used. The U.S. Food and Drug Administration has approved gamma hydroxybutyric acid (sodium oxybate [Xyrem]) for use in patients with narcolepsy. Sodium oxybate is usually administered twice per night because of its short half-life, and is effective for both daytime sleepiness and cataplexy.
Obstructive Sleep Apnea
Obstructive Sleep Apnea affects 4% of men and 2% of women,30 with a higher prevalence in older persons. It is characterized by partial (hypopnea) or complete (apnea) closure of the upper airway despite ongoing respiratory efforts. obstructive sleep ppnea leads to excessive daytime sleepiness, cognitive impairment, occupational accidents, and motor vehicle crashes. Evidence suggests that obstructive sleep ppnea also causes hypertension 22. Treatment with continuous positive airway pressure (CPAP) decreases blood pressure in patients with obstructive sleep apnea 23, especially those with severe obstructive sleep apnea and daytime sleepiness 24.
Factors that should prompt evaluation for obstructive sleep apnea include excessive daytime sleepiness, obesity, treatment-refractory hypertension, need for bariatric surgery, atrial fibrillation, congestive heart failure, stroke, nocturnal cardiac dysrhythmias, type 2 diabetes mellitus, and pulmonary hypertension. If obstructive sleep apnea is suspected, a referral for polysomnography is indicated. An apnea-hypopnea index (number of apneas and hypopneas per hour of sleep) of five per hour or more establishes an obstructive sleep apnea diagnosis. An apnea-hypopnea index of five to 15 per hour indicates mild disease; 15 to 30 per hour, moderate disease; and greater than 30 per hour, severe disease.
Portable home monitoring devices may be used as an alternative to polysomnography for diagnosing obstructive sleep apnea in patients with high pretest probability of moderate to severe Obstructive Sleep Apnea 25. Portable home monitoring devices are less expensive than polysomnography and have the added advantage of patients being in their normal sleeping environment. Portable home monitoring devices may not be appropriate in patients who have congestive heart failure or chronic obstructive pulmonary disease because of lower accuracy, or in those with comorbid sleep disorders such as parasomnia 25.
The most effective treatment for Obstructive Sleep Apnea is CPAP 26, which serves as a pneumatic splint to the upper airway. Autotitrating CPAP is another option that automatically adjusts the pressure within a set range in response to apneas, hypopneas, snoring, or flow limitation. Patients who cannot tolerate CPAP may be treated with bilevel positive airway pressure. Compliance with CPAP therapy is challenging for many patients. Other treatment options include weight loss, positional therapy, surgical approaches, oral appliances, and Provent therapy (a nasal device that has an expiratory valve to create positive end-expiratory pressure to keep the upper airway open). Choosing one of these therapies depends on obstructive sleep ppnea severity, patient preference, and tolerance.
REM sleep behavior disorder
Rapid eye movement (REM) sleep behavior disorder is a sleep disorder in which you physically act out vivid, often unpleasant dreams with vocal sounds and sudden, often violent arm and leg movements during REM sleep — sometimes called dream-enacting behavior.
You normally don’t move during REM sleep, a normal stage of sleep that occurs many times during the night. About 20 percent of your sleep is spent in REM sleep, the usual time for dreaming, which occurs primarily during the second half of the night.
The onset of REM sleep behavior disorder is often gradual and it can get worse with time.
REM sleep behavior disorder may be associated with other neurological conditions, such as Lewy body dementia (also called dementia with Lewy bodies), Parkinson’s disease or multiple system atrophy.
Complications of REM sleep behavior disorder
Complications caused by REM sleep behavior disorder may include:
- Distress to your sleeping partner or other people living in your home
- Social isolation for fear that others may become aware of your sleep disruption
- Injury to yourself or your sleeping partner
Symptoms of REM sleep behavior disorder
With REM sleep behavior disorder, instead of experiencing the normal temporary paralysis of your arms and legs (atonia) during REM sleep, you physically act out your dreams.
The onset can be gradual or sudden, and episodes may occur occasionally or several times a night. The disorder often worsens with time.
Symptoms of REM sleep behavior disorder may include:
- Movement, such as kicking, punching, arm flailing or jumping from bed, in response to action-filled or violent dreams, such as being chased or defending yourself from an attack
- Noises, such as talking, laughing, shouting, emotional outcries or even cursing
- Being able to recall the dream if you awaken during the episode
Causes of REM sleep behavior disorder
Nerve pathways in the brain that prevent muscles from moving are active during normal REM or dreaming sleep, resulting in temporary paralysis of your body. In REM sleep behavior disorder, these pathways no longer work and you may physically act out your dreams.
Risk factors for of REM sleep behavior disorder
Factors associated with the development of REM sleep behavior disorder include:
- Being male and over 50 years old — however, more women are now being diagnosed with the disorder, especially under age 50, and young adults and children can develop the disorder, usually in association with narcolepsy, antidepressant use or brain tumors
- Having a certain type of neurodegenerative disorder, such as Parkinson’s disease, multiple system atrophy, stroke or dementia with Lewy bodies
- Having narcolepsy, a chronic sleep disorder characterized by overwhelming daytime drowsiness
- Taking certain medications, especially newer antidepressants, or the use or withdrawal of drugs or alcohol
Recent evidence suggests that there may also be several specific environmental or personal risk factors for REM sleep behavior disorder, including occupational pesticide exposure, farming, smoking or a previous head injury.
REM sleep behavior disorder Diagnosis
To diagnose REM sleep behavior disorder, your doctor reviews your medical history and your symptoms. Your evaluation may include:
- Physical and neurological exam. Your doctor conducts a physical and neurological exam and evaluates you for REM sleep behavior disorder and other sleep disorders. REM sleep behavior disorder may have symptoms similar to other sleep disorders, or it may coexist with other sleep disorders such as obstructive sleep apnea or narcolepsy.
- Talking with your sleeping partner. Your doctor may ask your sleeping partner whether he or she has ever seen you appear to act out your dreams while sleeping, such as punching, flailing your arms in the air, shouting or screaming. Your doctor may also ask your partner to fill out a questionnaire about your sleep behaviors.
- Nocturnal sleep study (polysomnogram). Doctors may recommend an overnight study in a sleep lab. During this test, sensors monitor your heart, lung and brain activity, breathing patterns, arm and leg movements, vocalizations, and blood oxygen levels while you sleep. Typically, you’ll be videotaped to document your behavior during REM sleep cycles.
Diagnostic criteria
To diagnose REM sleep behavior disorder, sleep medicine physicians typically use the symptom criteria in the International Classification of Sleep Disorders, Third Edition (ICSD-3).
For a diagnosis of REM sleep behavior disorder, criteria include the following:
- You have repeated times of arousal during sleep where you talk, make noises or perform complex motor behaviors, such as punching, kicking or running movements that often relate to the content of your dreams
- You recall dreams associated with these movements or sounds
- If you awaken during the episode, you are alert and not confused or disoriented
- A sleep study (polysomnogram) shows you have increased muscle activity during REM sleep
- Your sleep disturbance is not caused by another sleep disturbance, a mental health disorder, medication or substance abuse
REM sleep behavior disorder can be the first indication of development of a neurodegenerative disease, such as Parkinson’s disease, multiple system atrophy or dementia with Lewy bodies. So if you develop REM sleep behavior disorder, it’s important to follow up with your doctor.
REM sleep behavior disorder Treatment
Treatment for REM sleep behavior disorder may include physical safeguards and medications.
Physical safeguards
Your doctor may recommend that you make changes in your sleep environment to make it safer for you and your bed partner, including:
- Padding the floor near the bed
- Removing dangerous objects from the bedroom, such as sharp items and weapons
- Placing barriers on the side of the bed
- Moving furniture and clutter away from the bed
- Protecting bedroom windows
- Possibly sleeping in a separate bed or room from your bed partner until symptoms are controlled
Medications
Examples of treatment options for REM sleep behavior disorder include:
- Melatonin. Your doctor may prescribe a dietary supplement called melatonin, which may help reduce or eliminate your symptoms. Melatonin may be as effective as clonazepam and is usually well-tolerated with few side effects.
- Clonazepam (Klonopin). This prescription medication, often used to treat anxiety, is also the traditional choice for treating REM sleep behavior disorder, appearing to effectively reduce symptoms. Clonazepam may cause side effects such as daytime sleepiness, decreased balance and worsening of sleep apnea.
Doctors continue to study several other medications that may treat REM sleep behavior disorder. Talk with your doctor to determine the most appropriate treatment option for you.
Periodic limb movements
Periodic limb movements are characterized by periodic episodes of repetitive and stereotypic limb movements during sleep, predominantly in the lower extremities. If these movements are associated with sleep disturbance that results in daytime fatigue or sleepiness, it is then called periodic limb movement disorder. The disorder may be diagnosed only after other causes of daytime symptoms are excluded. Most patients with restless legs syndrome have periodic limb movements. These movements have also been associated with REM sleep behavior disorder and narcolepsy.
The diagnosis of periodic limb movement disorder is made using polysomnography when the periodic limb movement index is more than 15 per hour in adults and more than five per hour in children. The movements alone do not require treatment, but periodic limb movement disorder can be treated with pharmacologic agents used for restless legs syndrome.
Types of sleep disorders
Circadian rhythm sleep disorder
Circadian rhythm disorders are disorders in which there is a persistent or recurrent pattern of sleep disturbance due primarily to either alterations in the internal circadian timing system or misalignment between an individual’s internal circadian rhythm of sleep propensity and the 24-hour social and physical environments. The primary symptoms of circadian rhythm disorders are disorders are insomnia, excessive sleepiness during wake periods, or both. These disturbances are associated with impairment of social, occupational, or other areas of functioning.
There are five types of circadian rhythm sleep disorders
Delayed Sleep Phase Disorder
This is characterized by an inability to fall asleep until 2-6 hours after usual sleep onset times (10pm to midnight) resulting in extreme difficulty with waking. Delayed sleep-wake phase sleep disorder — is an internal sleep clock (circadian rhythm) sleep disorder in which your sleep pattern is delayed two hours or more from a conventional sleep pattern, causing you to go to sleep later and wake up later. When left to sleep, sleep length is generally normal or extended. Delayed sleep phase disorder has a prevalence of 7-16% in adolescents and young adults. Individuals with delayed sleep phase disorder may be misdiagnosed with chronic insomnia disorder, ADHD, depression, or other psychiatric conditions which can also co-exist. When forced to be out of bed at conventional wakeup times, patients with delayed sleep phase disorder experience a short-sleep duration and feel “jet lagged”. This potentially leads to impairments in performance, quality of life, and comorbid chronic medical and psychiatric illness.
Typical scenario
Usually an adolescent or young adult presenting with a history of taking “hours” to get to sleep, with difficulty waking in the morning for school, university or work. They often describe themselves as a “night owl”. Misunderstandings between parents and adolescents resleep timing and getting up in the morning may negatively impact on family dynamics.
Delayed sleep phase disorder clinical presentation
- Insomnia symptoms but with sleepiness especially during the early part of the day.
- Alertness improves in the afternoon or evening
Delayed sleep phase disorder diagnosis
- Sleep diary for at least 2 full weeks to assess sleep patterns during the working/school week and weekends.
- Consider actigraphy (e.g actiwatch) to document sleep-wake pattern objectively. In this test, you wear a small device that tracks your sleep-wake behavior at home.
- Polysomnogram. If your doctor suspects you have a different sleep disorder, he or she may order a polysomnogram. In this test, you stay in a sleep center overnight. A polysomnogram monitors your brain activity, heart rate, oxygen levels, eye movements and breathing function as you sleep.
- Commercial activity monitor may supplement sleep diary, but beware limited or no validation.
The sleep diary or actigraphy are used to determine the patient’s core body temperature minimum (CBTmin), usually about 2 hours before habitual wake time, which is the key to resetting the delayed clock with light exposure.
Delayed sleep phase disorder treatment plan
Your plan may include:
- Improving sleep habits. Your doctor may call this sleep hygiene. Good sleep hygiene involves maintaining a regular sleep schedule, avoiding caffeine and stimulating activities near bedtime, avoiding tobacco and alcohol, and only using your bedroom for sleeping and sex. It’s also helpful to exercise in the morning, and avoid moderate to vigorous exercise close to bedtime.
- Light therapy. Having light exposure in the morning may adjust your internal sleep clock (circadian rhythm).
- Chronotherapy. In chronotherapy, doctors may prescribe you a sleep schedule that delays your bedtime by one to 2.5 hours every six days, until the desired bedtime is reached. You should maintain your sleep schedule once it is established.
Educate
- Light after the core body temperature minimum (CBTmin) advances sleep and shortens the sleep wake cycle – no hat or sunglasses for 40 mins in outside light where possible. Where core body temperature minimum cannot be determined (or if getting up times are variable), a conservative approach would be to suggest light exposure after the latest getting up time, gradually moving this earlier every 3 days.
- Combine exercise with light and changing sleep patterns. It also improves mood.
- Avoid light before the core body temperature minimum as it will further delay sleep onset. Reduce afternoon and evening light exposure. Use ordinary sunglasses when outside, and if using electronic devices reduce the screen light or use amber glasses to block the blue spectrum.
- Discuss with the patient that it is highly unlikely that he/she will be able to have a sleep onset time earlier than 11pm to midnight.
- Avoid long sleep-ins on the weekend as this can easily shift the patient back into a more delayed pattern.
Medication
- Consider low dose evening Melatonin (1mg, immediate-release, from a compounding chemist) to aid in resetting the endogenous clock. Take approximately 4.5 hours prior to current bed time (eg. if patient is going to bed 3am suggest Melatonin at 10.30pm).
- Slowly advance the time of Melatonin administration by ½ hour every 3 days. When sleep onset becomes more stable around midnight, then a reduced dose of Melatonin (eg. 0.5mg) I to 2 hours before bedtime (ie 10pm) will be able to stabilise sleep onset times.
Future management
Review once stabilized after 3-6 months, or earlier if there are ongoing difficulties.
- May require Sleep Physician or Sleep Psychologist intervention, management often difficult, especially with co-morbid depression.
Advanced Sleep Phase Disorder
There is early evening sleepiness and accompanying early sleep onset at 6-8pm. Wake time is then in the early hours of the morning. Total sleep time can be shortened.
Advanced sleep phase disorder is usually seen in an older person, often living alone, who may have lost a lifelong partner. It may develop in winter with the person going to bed earlier due to darkness or the cold climate.
Advanced sleep phase disorder clinical presentation
- The patient describes being sleepy early in the evening and falls asleep on couch, or goes to bed around 8pm, because they are unable to stay awake.
- Early morning awakening generally unable to return to sleep and feeling wide awake. May be misinterpreted as insomnia with early morning awakening.
Advanced sleep phase disorder diagnosis
- A sleep diary of at least 2 weeks.
Advanced sleep phase disorder treatment plan
Educate
- The importance of afternoon or evening bright light to delay sleep onset. Encourage the patient to do activities outside at this time of the day such as walking, gardening or sitting and reading.
- Good to combine exercise with changing sleep patterns. Do this in the afternoon or early evening to improve alertness. Try light weight lifting exercises whilst watching TV/sitting down in the evening.
- In Winter, can use blue light (glasses or a light box) in the late afternoon or early evening.
- Discourage morning light after the core body temperature minimum (CBTmin) as this will advance sleep time and maintain the early evening sleep onset. Wear hats and sunglasses in the morning when outside.
- A shift back to an earlier bedtime on a regular basis will result in return of advanced sleep phase.
Future management
Review once stabilized after 3-6 months, or earlier if there are ongoing difficulties.
May require Sleep Physician or Sleep Psychologist intervention, management often difficult, especially with co-morbid depression.
Non-24-Hour Sleep-Wake Disorder (Non-24)
The sleep/wake cycle is longer than 24 hours, resulting in sleep onset times becoming progressively later to the point where there is continual forward sleep cycling with daytime sleep until it moves again to nighttime.
Most people have a 24-hour circadian rhythm that is largely driven by light and darkness. Every 24 hours, you make a full cycle, going from being awake, to sleepy, to awake again. And unless you force your circadian rhythm to move earlier or later (like if you are a shift worker), it usually syncs with daylight and nighttime. But this isn’t true for everyone.
If you have Non-24-Hour Sleep-Wake Disorder (Non-24), your circadian rhythm isn’t always matched up with daytime and nighttime because your cycle is longer than 24 hours. As a result, you go from being on the same sleep cycle as everyone else to slowly shifting your sleepiness later and later in the day. Eventually, you become most tired during the day and least tired at night. This shift continues until you’re back to where you started, but the cycle just keeps going. This disorder is often due to light not reaching a certain part of the brain, which is why it tends to affect people who are totally blind and can’t sense light. Between 50 and 75 percent of people who are totally blind have Non-24, which is between 65,000 and 95,000 Americans.
It can be tough to accurately diagnose Non-24 because the symptoms can look similar to other sleep disorders. One warning sign that you have Non-24 is that you are completely blind and have periods of healthy, high-quality sleep, followed by periods of poor sleep. For a diagnosis, see a sleep specialist. The specialist will likely ask you to track your sleep or will even monitor internal chemicals like melatonin and cortisol to confirm whether Non-24 is present.
There are a few different therapies and treatment options if you get diagnosed with Non-24. If you have some sight, you can use light exposure to get your body back in sync (morning light to move your circadian rhythm earlier, evening light to move it later). Otherwise, a medication called Tasimelteon or melatonin supplements might help.
Non-24-Hour Sleep-Wake Disorder (Non-24) Treatment
Treatments for Non-24-Hour Sleep Wake Disorder (Non-24) are aimed at resynchronizing the patient’s internal body clock to the 24-hour day-night cycle. While appropriately timed light exposure that resets the phase (i.e., timing) of the internal body clock to the 24-hour day-night cycle is important to most people and other mammals, light exposure is only effective in the sighted. Dietary melatonin is a commonly used treatment for Non-24, although no large-scale clinical trials of melatonin therapy for Non-24 have been conducted to date.
Phototherapy
Appropriately timed light exposure is important to non-blind humans and other mammals because it acts as the major environmental cue that resets the phase (i.e. timing) of the internal body clock to the 24-hour light-dark cycle Most sighted patients with Non-24 have a circadian rhythm period longer than 24 hours that creates a daily delay in their phase. Thus, they need to reset their rhythms with an appropriate phase advance. Under normal circumstances, morning light will cause a phase advance of the clock, and evening light will cause a phase delay. The ‘cross-over’ point, where delay responses switch to advance responses on this Phase Response Curve (PRC), occurs around the time of the core body temperature minimum, which is usually 2 to 3 hours before habitual wakeup time (typically about 5-6 am). For phase advance resetting to treat Non-24 properly, therefore, light exposure must occur after core body temperature reaches its minimum. In practice, if an individual maintains a fixed, stable light therapy time, the circadian clock should eventually reach the correct phase. Continued maintenance of a stable and regular schedule is required for continued therapeutic benefit.
Medication
- Tasimelteon
In 2014, the Food and Drug Administration (FDA) approved the medication tasimelteon (found under the brand name Hetlioz) for the treatment of Non 24-Hour Sleep Wake Disorder (Non-24) in blind individuals.
Tasimelteon is a melatonin receptor agonist. It works by targeting receptors in the brain that control the timing of the sleep-wake cycle. In clinical trials, totally blind individuals with Non-24 who were treated with tasimelteon slept longer during the night. Tasimelteon was also found to decrease the amount of time totally blind individuals with Non-24 slept during the day.
Side Effects
The most common side effects of tasimelteon include headache, elevated liver enzymes, nightmares or unusual dreams, disturbed night’s sleep, upper respiratory or urinary tract infection, and drowsiness.
Tasimelteon should be taken as prescribed by your physician. It should be taken at the same time every night before bedtime. Because it may impair activities that require complete mental alertness, people should not plan any activities other than preparing themselves for bed after taking it.
- Melatonin
Melatonin is a possible treatment for both blind and sighted individuals with Non-24. Melatonin is a hormone, produced by the pineal gland, and is the biochemical signal of darkness. Melatonin has a nighttime peak both in species that are active during the day and species active at night. It can be synthesized artificially and, as with light, melatonin treatment can shift the circadian clock earlier (an advance) or later (a delay) depending on the timing of administration. For those with a normal sleep-wake cycle, melatonin administration from ~1pm to 1am will cause an advance, with dosing at ~7 pm producing the maximum advance, prior to the natural melatonin rise.
Assessing the patient’s circadian phase prior to treatment is important to ensure appropriate timing of melatonin and successful results. The most important point to make about melatonin treatments is that it should be given on a strict 24-hour cycle; that is, at a fixed time each night.
Studies on the blind suggest that 0.5 mg/day is an effective dose, and research studies do suggest that it is a relatively safe drug in most patients. Since melatonin supplements sold over the counter are not rigorously tested for purity or quality, care should be taken to ensure melatonin is from a reliable source and of pharmaceutical grade. With melatonin therapy, a correct diagnosis, the initial and maintenance doses, and timing of administration must be determined while under the care of a sleep specialist.
Fatigue
Fatigue is a constant battle for people with Non 24-Hour Sleep Wake Disorder (Non-24). But, there are some things you can do to help fight fatigue. Here are some general tips for managing fatigue:
- Eat a healthy diet. Food is fuel for the body. Eating a balanced diet that includes lots of fruits and vegetables, whole grains, and lean proteins can keep your blood sugar in check, which will help you to feel more alert.
- Exercise during waking hours. Moderate exercise five times a week can increase your energy and help stabilize your blood sugar.
- Drink caffeine in moderation. Although caffeine can give you a brief burst of energy, too much of it can lead to anxiety or withdrawal symptoms, which may cause you to feel more fatigued.
- Drink plenty of water. Dehydration can cause fatigue. Make sure you drink plenty of water throughout the day, and before and after exercising.
- Limit alcohol consumption. Too much alcohol can disrupt your sleep and make you feel sluggish when you are awake, particularly on mornings when you have not had enough sleep.
- Lose excess weight. People who are overweight tend to tire more easily than those who are at a normal weight for their height.
Follow your treatment regimen. The best way to fight Non-24-related fatigue is to follow your doctor’s treatment plan and talk to your doctor if you’re still having difficulty.
Irregular Sleep-Wake Disorder
Irregular Sleep-Wake Disorder is a condition of irregular sleep and wake periods, containing at least three sleep periods per day.
Irregular Sleep-Wake Type Disorder is characterized by a lack of well-defined sleep-wake cycle; individuals with Irregular Sleep-Wake Type Disorder typically have three or more sleep episodes during a 24-hour period, rather than one major sleep period.
The total sleep time per 24-hour period is usually normal for the person’s age 27.
The prevalence of Irregular Sleep-Wake Disorder is unknown, but estimates suggest it is rare 28.
Irregular Sleep-Wake Type Disorder is most frequently observed as a co-morbidity with neurological dysfunction, such as in patients with Alzheimer’s disease or other forms of dementia 27.
Consequences of Irregular Sleep Wake Type Disorder
People with Irregular Sleep-Wake Type Disorder experience insomnia and excessive daytime sleepiness stemming from the abnormal synchronization between their circadian rhythms and normal sleep-wake cycles 27.
In addition, they also experience problems with social, emotional, and other areas of life, due to their condition 27. For example, they may have trouble maintaining employment, caring for their children, and managing their social lives. These challenges “in day-to-day activities can contribute to feelings of depression. For people who are already suffering from depression, these impairments in daytime alertness and nighttime sleep can make their depression worse.”
Risk Factors for Irregular Sleep Wake Type Disorder
Dysfunction of circadian regulation can lead to Irregular Sleep-Wake Type Disorder, such as in patients with neurological dysfunction and reduced exposure to external cues (e.g., light, activity, social schedule). These situations are commonly observed in retired, elderly, or chronically ill individuals 29.
Irregular Sleep Wake Type Disorder Diagnosis
Similar clinical assessment is required of all circadian rhythm sleep disorders. As with all suspected sleep disorders, proper assessment of circadian rhythm sleep disorders includes a complete sleep history. Specifically, analysis of the sleep-wake schedule by sleep diary or actigraph, for at least 7 days, should be used to uncover potential variations in sleep timing and to rule out other sleep disorders, such as insomnia, that are often mistaken for circadian rhythm sleep disorders 30.
Irregular Sleep Wake Type Disorder Treatment
Treatment is designed to improve the amplitude of the individual’s circadian rhythm and their alignment with external cues.
Consolidation of sleep-wake cycles has been effectively achieved using bright light therapy 31 and structured social and physical activity (i.e., from 9:00 AM to 10:30 AM and 7:00 PM to 8:30 PM daily for two weeks) 32.
In one study, 45 percent of patients showed improvement when treated with a combination of bright light therapy, vitamin B12, chronotherapy, and hypnotics 33. It is important to note that the use of hypnotics increases the risk of hip fractures and other injuries from falling.
Shift Work Disorder
Shift Work Disorder is a condition in which circadian rhythms are disturbed due to an inability to adapt to shift work schedules (working predominantly at night) with insomnia type symptoms. High levels of sleepiness are present which is generally uncommon in insomnia.
Generally, your body is programmed to sleep best overnight and to be most alert during the day and early evening. If you work night shift, it might not be easy to sleep enough or to sleep well during the day. If you start work very early in the morning, it might be hard to sleep in the evening. The average shiftworker sleeps one hour a day less than someone who doesn’t work shifts. Some shiftworkers sleep up to four hours a day less than normal, but this is not common.
Shiftworkers often complain of being tired, both on and off the job. It may be harder to concentrate and be alert while at work. This means there is more danger of accidents at work and on the road, driving to and from work. Sleep loss impairs performance: 17 hours without sleep is as dangerous as having a blood alcohol content of 0.05% and 24 hours without sleep, as dangerous as having a blood alcohol content of 0.08%.
Shift work sleep disorder consists of symptoms of insomnia or excessive sleepiness that occur as transient phenomena in relation to work schedules. A critical component in the diagnosis of shift work sleep disorder is the distinction between what might be considered ‘normal’ responses to the challenges of shift work, and a clinical response which may include issues that impact on the individual’s work and family life. Prevalence estimates suggest that 10% of shiftworkers likely suffer from shift work sleep disorder.
Shiftwork requires individuals to attempt sleep at biologically inappropriate times of the day, but most people are able to obtain some sleep. A key symptom of shift work sleep disorder is an inability to obtain sleep at these times. Shiftworkers without a diagnosis of shift work sleep disorder obtain an average of approximately 6.5 hours sleep in a 24-hour period whereas sufferers of shift work sleep disorder report obtaining far less. The other key diagnostic criterion is excessive sleepiness during periods of desired wakefulness (i.e. work periods). Again, while most people experience reduced alertness and increased sleepiness during the night hours, shift work sleep disorder is characterized by extreme sleepiness (Epworth Sleepiness Scale score >10).
Typical scenario
- Individual who works night shift or rotating shifts that involve night work
- Inability to get to sleep or stay asleep for desired period
- Excessive sleepiness associated work hours
- Prolonged symptoms (coincident with work schedule)
- Caffeine or other stimulant use to promote alertness, may also impact sleep.
Why does this happen?
The human body is designed to be active during the day and rest at night. There are many body hormones that work to keep this in balance. It is not always easy to switch to being active at night and resting during the day.
What can I do about it?
- Make time for enough sleep. Shiftworkers have to sleep when others are awake. Social and sporting events can sometimes be rearranged so that shiftworkers can still participate in these activities.
- Try to go to bed at the same time every day and get up at the same time also.
- Try to sleep in peace. Others in the house need to respect the need of the shiftworker to sleep. This may mean removing the telephone from the bedroom and having heavy carpet or curtains in the bedroom to help absorb any noise. Some shiftworkers find that wearing ear plugs to bed helps.
- A fan or “white noise” machine will help to muffle noise.
- Keep the bedroom cool and dark.
- Avoid caffeine, sleeping pills, alcohol or cigarettes before going to bed.
- If you can, sleep just before going to work. This is better than earlier in the day. If this is not possible, taking a nap before going to work may help.
- Some workers are allowed to take a break during their shift. This time can be used for a short nap.
What can my employer do about it?
- Avoid scheduling back-to-back shifts. After working double or triple shifts, the problems only get worse and safety will be reduced.
- Keep each worker on the same shift. The best thing to do is to go to bed at the same time every day. If this is possible, the body will adapt to shiftwork better. If shifts are rotated often, it makes it difficult to develop a good sleep pattern.
- When shifts do rotate, rotate them forwards (morning to afternoon to evening to night) instead of backwards. For example, if someone is working afternoon shift, it is easier for this person to rotate forwards to evening shift than backwards to a morning shift.
- Schedule the heaviest work that requires most concentration during day shifts.
- Schedule breaks during night shifts. This allows tired workers to take a nap.
How long should a nap be?
Fifteen minutes is best. Avoid napping for longer than this. When driving, pull over to a quiet spot and put the seat back. After the nap, walk around for 5 minutes to wake up properly before resuming other activities.
Clinical Presentation
- Likely to present extremely tired, depending on time of day and day in schedule
- May look fatigued with dark circles under the eyes but may equally look very alert and so called ‘normal’
- Mood may be depressed
Shift work sleep disorder diagnosis
- Sleep log or actigraphy monitoring (with sleep diaries) for at least 7 days demonstrates disturbed sleep (insomnia) and circadian and sleep-time misalignment. In this test (actigraphy), you wear a small device that tracks your sleep-wake behavior at home.
- Epworth sleepiness scale.
Shift work sleep disorder treatment plan
Educate
Discuss normal sleep with emphasis being placed on how most of our sleep is relatively light (45-55% whilst we only spend 20% of the night in deep sleep and 25% in dream or REM sleep; Waking is also normal and it is what we learn to do with the wake and how we manage it which is most important. Discuss the challenges presented by night work and sleep hygiene strategies.
What to do initially
Manage immediate risks to health and safety (e.g. drive home); encourage consistent sleep and wake times as far as possible; restrict caffeine and alcohol intake; set up bedroom for sleep (cool, dark, quiet); minimize screen time in hour prior to bed; recommend strategic napping to manage alertness.
Discuss options for change to work hours, even if temporarily to assist diagnosis.
Medication
Consider melatonin for sleep promotion and caffeine and modafinil for alertness promotion.
Try caffeine early in the shift, suggest the equivalent of 1-3 cups of coffee depending on habitual use. Recommend using caffeine only when need boost in alertness as tolerance can biuld up. Timing of caffeine is important as it can imapct sleep quality.
Melatonin may be used for its hypnotic effect, at higher doses, for example 3mg 1-2 h before intended sleep time. Recommend trial with dose and timing. Most shifts change too rapidly to allow melatonin to have an effect on adjusting circadian phase. Melatonin is not effective in everyone.
Modafinil, a wakefulness-promoting agent was first registered for treatment of narcolepsy but two additional indications were registered in 2007 including the treatment of excessive sleepiness associated with moderate to severe chronic shift work sleep disorder. The indication to treat was further revised to include only patients where non-pharmacological interventions have been unsuccessful or are inappropriate.
Future management
- Advise the patient to return for a follow-up visit.
- Consider referral to sleep psychologist or sleep physician.
Jet Lag Disorder
Jet lag disorder occurs when there is a temporary mismatch between sleep-wake cycle timing generated by the person’s internal circadian clock, and the external cues at the new destination 34. This is commonly known just as “jet lag.”
In general, eastward travel causes more severe jet lag than westward travel (i.e., moving the clock ahead is harder than moving it back); this is because the biological clock can adjust better when bedtime and waking occur at a later hour 35.
The ability and speed to adapt varies with the individual 36.
Jet lag can affect all age groups. However, the elderly may have more severe symptoms and require a longer recovery time 37.
Typical consequences of jet lag include disturbed sleep, decreased alertness, general malaise, and impaired daytime function; in addition, gastrointestinal distress is common, and occurs when travelers eat at a time that the body is unused to 38.
Jet lag is a temporary condition, with symptoms beginning after air travel across at least two time zones 34.
Severity of symptoms is dependent on the number of time zones traveled, the direction of travel (i.e., east vs. west), and individual differences. Symptoms dissipate as the circadian clock gradually adjusts to the new, local time 35.
Because jet lag is temporary, assessment and diagnosis is rarely needed. Since jet lag can precipitate psychophysiological insomnia, individuals who experience jet lag for more than two weeks should seek treatment.
Jet lag Treatment
Jet lag’s effects can be reduced through 39:
- Simulating the destination’s schedule before traveling and during the flight
- Staying hydrated and moving around during the flight
- Eating healthy and avoiding caffeine and alcohol
- Using melatonin and bright lights to reset the circadian rhythm (see below).
Light therapy
Carefully timed bright light therapy can be effective in treating jet lag.
Carefully timed bright light therapy can be effective in treating jet lag. Various three-day studies have found that three hours of bright light in the morning can help advance circadian rhythms before eastward travel 40.
Pharmacological options
Pharmacological options include melatonin and hypnotic agents.
A systematic review of randomized trials comparing the efficacy of melatonin vs. placebo in air travelers found that melatonin was highly effective in reducing or preventing jet lag 41.
Melatonin in doses of 0.5 to 5 mg promoted sleep and decreased jet lag in travelers crossing five or more time zones. Higher doses promoted more rapid sleep than lower doses, but doses greater than 5 mg had no additional benefit. For greatest efficacy, melatonin should be taken at the target (i.e. destination) bedtime, optimally starting several days before departure, depending on the number of time zones to be crossed. Although studies have looked at melatonin in addition to bright light, it is yet to be determined whether this can further accelerate adaptation to the new time zone 42.
Randomized controlled trials have demonstrated that inducing sleep during a long flight with short-acting hypnotics can facilitate adaptation of circadian rhythms 43.
Sleep disorders in children
Sleep is one of the most commonly discussed topics during well-child visits 44. Epidemiologic studies indicate that up to 50% of children experience a sleep problem 45 and about 4% have a formal sleep disorder diagnosis 46.
Normal Sleep in Infants and Children
Sleep is an opportunity for the body to conserve energy, restore its normal processes, promote physical growth, and support mental development. The most recognized consequence of inadequate sleep is daytime sleepiness. However, sleepiness in children commonly manifests as irritability, behavioral problems, learning difficulties, motor vehicle crashes in teenagers, and poor academic performance 47. Distinguishing significant sleep disruptions from normal age-related changes can be challenging and can ultimately delay treatment.
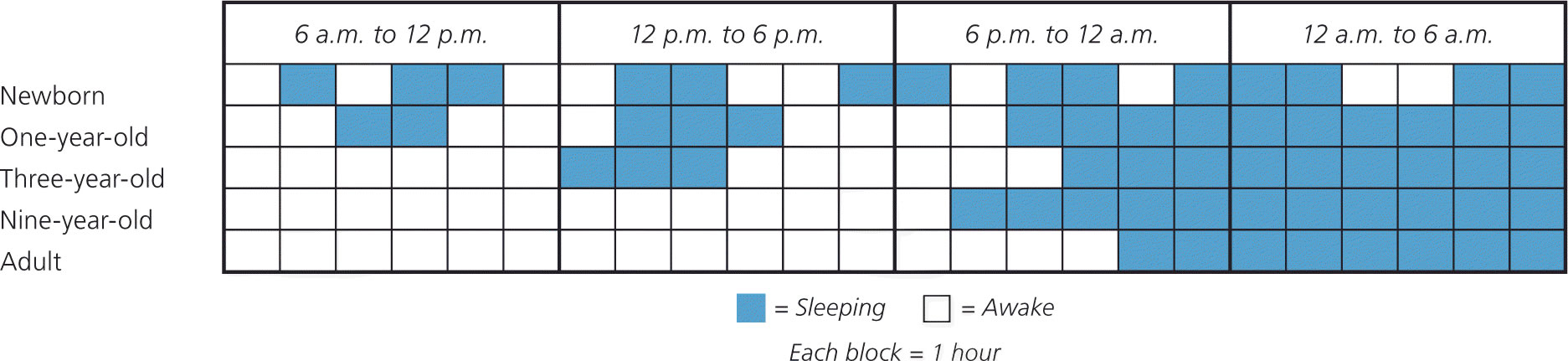
Sleep changes considerably during the first few years of life and parallels physical maturation and development. Newborns require the greatest total sleep time and have a fragmented sleep-wake pattern. Starting at five months of age, infants have the ability to sleep for longer periods. At six months of age, children are able to go without nighttime feedings, but significant variation exists. Additionally, breastfeeding infants have more frequent awakenings, shorter sleep periods, and slightly shorter total sleep times 48. As children age, sleep periods gradually lengthen and total sleep time decreases (Figure 4).
Figure 4. Sleep-Wake Cycle Development
Note: Generalization of sleep-wake cycle patterns at different stages during sleep pattern development. Newborns have a fragmented sleep-wake pattern with no significant sleep consolidation. Over time, daytime napping decreases, and nighttime sleep consolidation occurs.
The large variation in sleep behavior among children may be secondary to cultural or genetic differences; however, there are some general trends (Table 6). Ultimately, knowing the normal developmental stages of sleep will help differentiate between normal sleep and common sleep disorders, such as obstructive sleep apnea (OSA), parasomnias, behavioral insomnia of childhood, delayed sleep phase disorder, and restless legs syndrome. These disorders are summarized in Table 7 47.
Table 6. Summary of Normal Sleep Parameters in Children
| Age | Total sleep time | Naps (on average) |
|---|---|---|
0 to 2 months | 16 to 18 hours | 3.5 per day at 1 month of age |
2 to 12 months | 12 to 16 hours | 2 per day at 12 months of age |
Most children 6 to 9 months of age sleep through the night | ||
1 to 3 years | 10 to 16 hours | 1 per day at 18 months of age |
3 to 5 years | 11 to 15 hours | 50% of 3-year-olds do not nap |
5 to 14 years | 9 to 13 hours | 5% of whites and 39% of blacks nap at 8 years of age |
14 to 18 years | 7 to 10 hours | Napping in this age group suggests insufficient sleep or a possible sleep disorder |
Table 7. Summary of Common Sleep Disorders in Children
| Sleep disorder | Epidemiology | Clinical features | Diagnostic criteria | Treatment options | |||
|---|---|---|---|---|---|---|---|
Obstructive sleep apnea |
|
|
|
| |||
Parasomnias | |||||||
Sleepwalking (somnambulism) |
|
|
|
| |||
Confusional arousals |
|
|
|
| |||
Sleep terrors |
|
|
|
| |||
Nightmares |
|
|
|
| |||
Behavioral insomnia of childhood |
|
|
|
| |||
Delayed sleep phase disorder |
|
|
|
| |||
Restless legs syndrome |
|
|
|
| |||
PSG = polysomnography.
[Source 6]
Obstructive Sleep Apnea
Obstructive Sleep Apnea is characterized by upper airway obstruction, despite respiratory effort, that disrupts normal sleep patterns and ventilation. Obstructive Sleep Apnea can be associated with obesity, excessive soft tissue in the upper airway, decreased upper airway lumen size, or failure of pharyngeal dilator muscles. However, in children, the obstruction is primarily due to enlarged tonsils and adenoids 49. Onset usually occurs between two and eight years of age, coinciding with peak tonsil growth, but the condition can manifest at any age 50. The overall prevalence in children is 1% to 5%.13 Iccurs equally among males and females, but is more common in ethnic minorities 51.
Snoring and witnessed apneas are the classic symptoms of Obstructive Sleep Apnea, but not all snorers have the condition. The prevalence of habitual snoring in children is as high as 27%, which can complicate the recognition of Obstructive Sleep Apnea. Other common symptoms include unusual sleeping positions (e.g., hyperextended neck, seated with open mouth), sleep-related paradoxical breathing, nighttime diaphoresis or enuresis, morning headaches, and excessive daytime sleepiness. However, children are less likely than adults to present with daytime sleepiness. Sleepiness in children is more likely to manifest as depressed mood, poor concentration, decreased attention, or behavioral issues 52.
Weight and body mass index are usually normal in children with Obstructive Sleep Apnea; however, the incidence of obesity-related sleep apnea is steadily increasing 46. Physical examination findings can include enlarged tonsils, micrognathia, and pectus excavatum. However, subjective grading of tonsil size in children does not always correlate with objective findings 53.
Results of the history and physical examination alone correlate poorly with objective findings of Obstructive Sleep Apnea, and questionnaires have shown a sensitivity of only 78%. Therefore, children with suspected Obstructive Sleep Apnea should be referred for polysomnography. In addition, referral to a sleep medicine specialist should be considered for those with high-risk features (e.g., attention-deficit/hyperactivity disorder, cardiorespiratory failure, craniofacial abnormalities, congenital defects, Down syndrome).
Weight loss interventions have demonstrated benefits of reducing the severity of Obstructive Sleep Apnea and should be initiated in all children who are overweight or obese 51. Continuous positive airway pressure should be offered to those with residual Obstructive Sleep Apnea symptoms or if adenotonsillectomy was not performed. There is limited evidence to support the use of intranasal corticosteroids for children with mild Obstructive Sleep Apnea or with residual mild Obstructive Sleep Apnea following adenotonsil-lectomy 54. Although rapid maxillary expansion (i.e., use of an orthodontic device that widens the upper jaw) and montelukast (Singulair) are sometimes recommended, no clinical trial evidence supports the use of these treatments for Obstructive Sleep Apnea in children.
Untreated Obstructive Sleep Apnea is associated with neurobehavioral problems, decreased attention, disturbed emotional regulation, decreased academic performance, nighttime enuresis, impaired growth, and, rarely, systemic hypertension, pulmonary hypertension, and cor pulmonale.6,17–20,52,53 Adenotonsillectomy is the primary treatment for Obstructive Sleep Apnea in children 51. Following adenotonsillectomy, postoperative polysomnography demonstrates resolution of Obstructive Sleep Apnea in more than 70% of normal-weight children, but in less than one-half of obese children. Postoperative improvements in quality of life and behavior may also occur 55. Children being considered for adenotonsillectomy who are at high risk of postoperative complications (risk factors include age younger than three years, severe Obstructive Sleep Apnea, obesity, current respiratory infection, craniofacial abnormalities, failure to thrive, cardiac complications of Obstructive Sleep Apnea, and neuromuscular disorders) should undergo the procedure as an inpatient. Once treated, all children should have a clinical assessment six to eight weeks postoperatively, and polysomnography should be repeated to assess for residual Obstructive Sleep Apnea in those with obesity, moderate to severe Obstructive Sleep Apnea on initial testing, or persistent symptoms. If ordered, polysomnography should be performed after the pharynx has fully healed, usually no earlier than six weeks postoperatively.
Parasomnias
Parasomnias such as sleepwalking (somnambulism), sleep talking (somniloquy), confusional arousals, sleep terrors, and nightmares affect up to 50% of children 49. They are defined as undesirable events that accompany sleep and typically occur during sleep-wake transitions 49. They are additionally characterized by complex, awake-like activity by the child that appears purposeful but lacks meaningful interaction with his or her environment. Associated features include confusion, automatic behaviors, difficulty awakening, amnesia, and rapid return to full sleep after the event.
Most parasomnias, such as sleepwalking, sleep talking, confusional arousals, and sleep terrors, occur in the first half of the sleep period during slow wave sleep; children typically have no memory of the event. In contrast, nightmares typically occur in the last half of the sleep period during rapid eye movement sleep, with children able to remember the event. It is important to note that the symptoms and timing of nocturnal seizures can overlap with parasomnias. Physicians should inquire about repetitive stereotypic behaviors and odd posturing that could represent nocturnal seizures 49.
Genetically predisposed individuals are susceptible to precipitating factors, contributing to the development of parasomnias. Precipitating factors include insufficient sleep and disorders causing partial awakenings from sleep. Obstructive sleep apnea is a common trigger for parasomnias, and a review of studies showed that more than one-half of children referred for sleep terrors or sleepwalking also had Obstructive sleep apnea 56. Other triggers may include periodic limb movement disorder, gastroesophageal reflux disease, forced awakenings, and certain medications.
Parasomnias often resolve spontaneously by adolescence; however, 4% of persons will have recurring events 49. Treatment centers on reassurance, reducing precipitating factors, and increasing total sleep times 56. When it is appropriate, parents should be counseled about safety measures (e.g., locking doors and windows, using motion alarms, clearing the floor of toys, placing the mattress on the floor). Children who exhibit atypical, harmful, or violent behaviors or who are unresponsive to conservative treatments should be referred for further evaluation.
Behavioral Insomnia of Childhood
Behavioral insomnia of childhood is characterized by a learned inability to fall and/or stay asleep; the estimated prevalence is 10% to 30%. The condition is divided into the sleep-onset association type and the limit-setting type. The sleep-onset association type is characterized by the child’s inability or unwillingness to fall asleep or return to sleep in the absence of specific conditions, such as a parent rocking the child to sleep 49. The limit-setting type occurs when parents fail to set appropriate limits, such as when the parents allow the child to sleep in their bed when the child refuses to sleep. Most children with behavioral insomnia of childhood have features of both types.
Prevention is the best treatment for behavioral insomnia of childhood. Physicians should educate parents on normal sleep patterns, good sleep hygiene, realistic expectations, setting boundaries, and sleep plans. These plans should focus on regular and consistent feedings, nap times, bedtime routines, and sleep-wake times. Infants are more likely to become self-soothers (fall asleep on their own) when consistently placed in the crib awake vs. already asleep 57. Creating a regular routine will establish expectations, and the child will eventually learn how to fall asleep on his or her own. Extinction techniques (placing the child in bed and ignoring him or her until the morning, or for a set period) are effective in the treatment of this disorder. There are various extinction techniques, and no single method is superior. Techniques for managing behavioral insomnia of childhood are summarized in Table 8 58. Sleep or sedating medications are ineffective for the treatment of this disorder 59.
Table 8. Treatments for Behavioral Insomnia of Childhood
| Treatment technique | Description | |
|---|---|---|
Parental education | Parents are taught about good sleep practices, such as consistent feedings, nap times, bedtime routines, regular sleep-wake times, and placing the child in bed drowsy but awake. | |
Unmodified extinction | The child is placed in bed at a predetermined bedtime. | |
The child’s crying, calls for the parents, and tantrums are ignored until the following morning, although significant cries for suspected injuries or illnesses are not ignored. | ||
Cries are ignored to prevent reinforcing negative learned behavior (e.g., crying is rewarded with parental response/presence). | ||
This technique can be difficult and distressing for parents. | ||
Modified version for decreased parental distress: | ||
A parent stays in the child’s room, but follows the same technique. | ||
Graduated extinction | This is fundamentally the same as unmodified extinction, but with scheduled “check-ins.” | |
A parent checks on the child on a fixed schedule (e.g., every 10 minutes) or in gradually increased intervals (e.g., first check-in after five minutes, second check-in after 10 minutes). | ||
Parental interactions with the child are calming and positive, but last no more than one minute at a time. | ||
Positive bedtime routines/faded bedtime with response cost | Positive bedtime routines: Relaxing/calming activities are implemented before bedtime (e.g., bedtime stories). | |
Faded bedtime: Bedtime is delayed until the predicted time of sleep onset to decrease the time the child spends in bed awake. | ||
Response cost: The child is removed from bed for a specific amount of time if sleep onset does not occur within the desired period. | ||
Scheduled awakenings | Parents must document the pattern of nighttime awakenings. | |
The child is awakened before the normally predicted nighttime awakening, and the number of scheduled awakenings is slowly decreased over time. | ||
[Source 6]
Delayed Sleep Phase Disorder
The master circadian clock, located within the suprachiasmatic nucleus, controls the timing of sleep and cycles approximately every 24 hours in most individuals 60. The discrepancy between the internal clock and the external world requires continuous “resetting” by time cues, such as light, melatonin, physical activity, body temperature, and meals. Light is the most powerful of these entrainers. Inappropriate timing of light exposure can alter the circadian rhythm. For example, light exposure before bedtime can suppress melatonin and ultimately delay sleep onset.
In children with delayed sleep phase disorder, habitual sleep-wake times are delayed by at least two hours compared with socially acceptable times 49. The disorder is more common during adolescence when the circadian rhythm is thought to lengthen and the child becomes more social 60. The prevalence in adolescents is 7% to 16%.12,31,34 The disorder is diagnosed using patient history and documentation of sleep and wake times on a sleep diary or log. Parental concerns usually focus on late bedtimes (2 a.m. or later), sleeping in, difficulty awakening, and school tardiness. However, frequent nighttime awakenings are unusual, and sleep architecture is usually normal (Figure 5).
Figure 5. Time to day
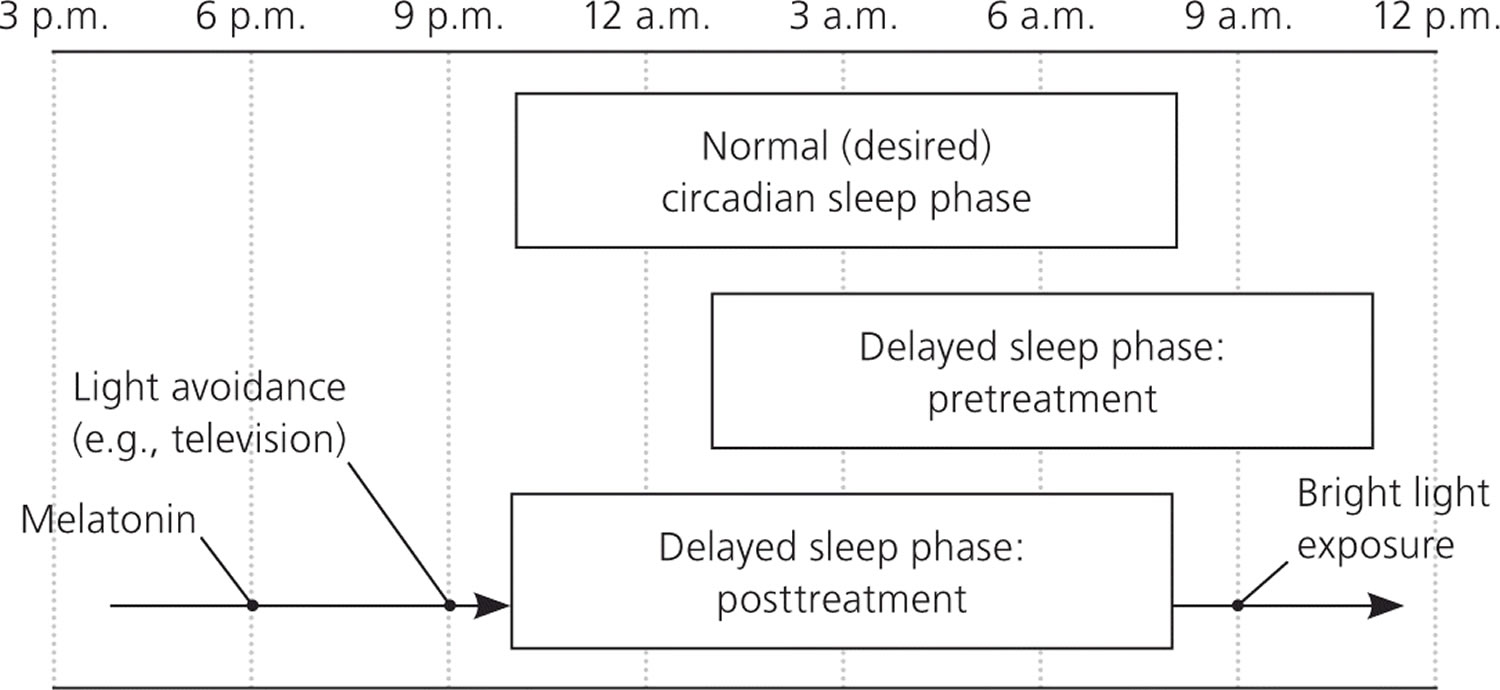
Note: Comparison of the sleep pattern associated with delayed sleep phase disorder (pre- and posttreatment) and that of normal (desired) sleep phase. Treatments such as melatonin, nighttime light avoidance, and early morning bright light therapy will slowly advance the individual’s sleep phase to the desired time.
Restless Legs Syndrome
The rate of restless legs syndrome in children is unclear, but limited studies suggest a prevalence of 2% 49. The condition is characterized by an unpleasant sensation in the legs, with the urge to move the legs starting in the evening. Rest worsens symptoms, and movement provides some relief. Other symptoms include difficulty falling asleep, bedtime resistance, “growing pains,” and symptoms similar to those of attention-deficit/hyperactivity disorder 61. The condition in children is associated with negative behavior and mood, and decreased cognition and attention, and it is more common in children with attention-deficit/hyperactivity disorder 62.
Dopamine dysfunction, genetics, and iron deficiency are thought to play a role in the pathogenesis of restless legs syndrome 63. Additionally, symptoms may be exacerbated by excessive or inadequate physical activity or the use of caffeine, nicotine, antihistamines, selective serotonin reuptake inhibitors, or tricyclic antidepressants 64.
Diagnosing restless legs syndrome in children can be challenging because they may be unable to describe the core symptoms. A diagnosis can be made if the history is consistent with the condition and at least two of the following are present: a sleep disturbance, a first-degree relative has the condition, or five or more periodic limb movements per hour of sleep during polysomnography 49. Once restless legs syndrome is diagnosed, conservative treatment includes avoiding exacerbating factors.47 Because iron deficiency is common in children, measuring the ferritin level is reasonable.49,50 Iron replacement should be initiated if ferritin levels are less than 50 mcg per L, and they should be rechecked in three months 65. There are no medications approved for treating restless legs syndrome in children. Patients with symptoms that do not respond to conservative treatments should be referred for further evaluation.
Treatment focuses on aligning the circadian rhythm with desired sleep-wake times. As in all sleep disorders, maintaining a regular sleep-wake cycle and practicing good sleep hygiene are the foundation of treatment. It is important to avoid bright lights before bedtime. Removing all light-emitting devices (e.g., electronics, portable media, tablet computers, cell phones) from the bedroom may be beneficial. Bright light therapy used for the first one to two hours after awakening may also be beneficial and will advance the circadian rhythm.31 There is strong evidence that melatonin supplementation (0.3 to 5 mg given 1.5 to 6.5 hours before desired bedtime) is an effective treatment for delayed sleep phase disorder, although the exact dose or timing has not been well established 66.
- National Sleep Foundation. Sleep in America polls. 2005 adult sleep habits and styles. https://sleepfoundation.org/sleep-polls-data/sleep-in-america-poll/2005-adult-sleep-habits-and-styles
[↩] - Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439.[↩]
- Patel SR, Malhotra A, Gao X, et al. A prospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35(1):97–101.[↩]
- Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27(3):440–444.[↩]
- Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163(2):205–209.[↩]
- Management of Common Sleep Disorders. Am Fam Physician. 2013 Aug 15;88(4):231-238. https://www.aafp.org/afp/2013/0815/p231.html[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ohayon MM, Reynolds CF III. Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD) [published correction appears in Sleep Med. 2010;11(2):227]. Sleep Med. 2009;10(9):952–960.[↩]
- The International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, Ill.: American Academy of Sleep Medicine; 2005.[↩][↩][↩]
- Littner M, Hirshkowitz M, Kramer M, et al.; American Academy of Sleep Medicine; Standards of Practice Committee. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26(6):754–760.[↩]
- Lande RG, Gragnani C. Nonpharmacologic approaches to the management of insomnia. J Am Osteopath Assoc. 2010;110(12):695–701.[↩]
- Soldatos CR, Dikeos DG, Whitehead A. Tolerance and rebound insomnia with rapidly eliminated hypnotics: a meta-analysis of sleep laboratory studies. Int Clin Psychopharmacol. 1999;14(5):287–303.[↩]
- Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29(11):1415–1419[↩]
- Brzezinski A, Vangel MG, Wurtman RJ, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9(1):41–50.[↩]
- Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66(4):469–476.[↩]
- Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of phar-macotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5–11.[↩]
- Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. 2004;164(17):1888–1896.[↩]
- Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295(24):2851–2858.[↩]
- Allen RP, Earley CJ. Restless legs syndrome: a review of clinical and pathophysiologic features. J Clin Neurophysiol. 2001;18(2):128–147.[↩]
- Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults–an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35(8):1039–1062.[↩]
- Coad J, Conlon C. Iron deficiency in women: assessment, causes and consequences. Curr Opin Clin Nutr Metab Care. 2011;14(6):625–634.[↩]
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369(9560):499–511.[↩]
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–2176.[↩]
- Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167(8):757–764.[↩]
- Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in non-sleepy hypertensive OSA patients. Eur Respir J. 2006;27(6):1229–1235.[↩]
- Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–747.[↩][↩]
- Kushida CA, Littner MR, Hirshkowitz M, et al.; American Academy of Sleep Medicine. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–380.[↩]
- Reid KJ and Zee PC. “Circadian disorders of the sleep-wake cycle,” in Kryger M, Roth T, Dement W (ed.), Principles and Practice of Sleep Medicine (5th Edition), St. Louis: Elsevier Saunders, 2011, pages 470-482.[↩][↩][↩][↩]
- Yamadera H, Takahashi K, Okawa M: A multicenter study of sleep-wake rhythm disorders: Clinical features of sleep-wake rhythm disorders. Psychiatry ClinNeurosci. 1996;50:195-201.[↩]
- Pollak CP, Stokes PE. Circadian rest-activity rhythms in demented and nondemented older community residents and their caregivers. J Am Geriatr Soc. 1997;45:446-452.[↩]
- Silber MH, Krahn LE, Morgenthaler TI, eds. Sleep medicine in clinical practice. London, Eng: Taylor and Francis; 2004:253-276.[↩]
- Ancoli-Israel S, Martin JL, Kripke DF, et al. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc. 2002;50:282-289.[↩]
- Naylor E, Penev PD, Orbeta L, et al. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep. 2000;23:87-95.[↩]
- Yamadera H, Takahashi K, Okawa M: A multicenter study of sleep-wake rhythm disorders: Clinical features of sleep-wake rhythm disorders. Psychiatry Clin Neurosci. 1996;50:195-201.[↩]
- Sack RL. Shift work and jet lag. In: Lee-Chiong TL, Sateia MJ, Carskadon MA, eds. Sleep Medicine. Philadelphia, Pa: Hanley and Belfus; 2002:255-263.[↩][↩]
- Drake CL, Wright KP, “Shift Work, Shift-Work Disorder, and Jet Lag,” in In Kryger M, Roth T, Dement W (ed.), Principles and Practice of Sleep Medicine (5th Edition), St. Louis: Elsevier Saunders, 2011, pages 784-798[↩][↩]
- Czeisler CA, Moore-Ede MC, Coleman RM. Rotating shift work schedules that disrupt sleep are improved by applying circadian principles. Science. 1982;217:460-464.[↩]
- American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, Ill: American Academy of Sleep Medicine; 2005.[↩]
- Drake CL, Wright KP, “Shift Work, Shift-Work Disorder, and Jet Lag,” in Kryger M, Roth T, Dement W (ed.), Principles and Practice of Sleep Medicine (5th Edition), St. Louis: Elsevier Saunders, 2011, pages 784-798[↩]
- Drake CL, Wright KP, “Shift Work, Shift-Work Disorder, and Jet Lag,” in In Kryger M, Roth T, Dement W (ed.), Principles and Practice of Sleep Medicine (5th Edition), St. Louis: Elsevier Saunders, 2011, pages 784-798.[↩]
- Eastman CI, Gazda CJ, Burgess HJ, Crowley SJ, Fogg LF. Advancing circadian rhythms before eastward flight: a strategy to prevent or reduce jet lag. Sleep. 2005;28:33-44.[↩]
- Caspi O. Melatonin for the prevention and treatment of jet lag. Altern Ther Health Med. 2004;10:74-78.[↩]
- Buscemi N, Vandermeer B, Hooton N, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ. 2006;332:385-393.[↩]
- Buxton OM, Copinschi G, Van Onderbergen A, Karrison TG, Van Cauter E. A benzodiazepine hypnotic facilitates adaptation of circadian rhythms and sleep-wake homeostasis to an eight hour delay shift simulating westward jet lag. Sleep. 2000;23:915-927.[↩]
- Olson LM, Inkelas M, Halfon N, Schuster MA, O’Connor KG, Mistry R. Overview of the content of health supervision for young children: reports from parents and pediatricians. Pediatrics. 2004;113(6 suppl):1907–1916.[↩]
- Pagel JF, Forister N, Kwiatkowki C. Adolescent sleep disturbance and school performance: the confounding variable of socioeconomics. J Clin Sleep Med. 2007;3(1):19–23.[↩]
- Meltzer LJ, Johnson C, Crosette J, Ramos M, Mindell JA. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics. 2010;125(6):e1410–e1418.[↩][↩]
- Beebe DW, Ris MD, Kramer ME, Long E, Amin R. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep. 2010;33(11):1447–1456.[↩][↩]
- Elias MF, Nicolson NA, Bora C, Johnston J. Sleep/wake patterns of breast-fed infants in the first 2 years of life. Pediatrics. 1986;77(3):322–329.[↩]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, Ill.: American Academy of Sleep Medicine; 2005.[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Akcay A, Kara CO, Dagdeviren E, Zencir M. Variation in tonsil size in 4- to 17-year-old schoolchildren. J Otolaryngol. 2006;35(4):270–274.[↩]
- Marcus CL, Brooks LJ, Draper KA, et al.; American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755.[↩][↩][↩]
- Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29(9):1115–1134.[↩]
- Wang JH, Chung YS, Jang YJ, Lee BJ. Palatine tonsil size and its correlation with subjective tonsil size in patients with sleep-disordered breathing. Otolaryngol Head Neck Surg. 2009;141(6):716–721[↩]
- Kuhle S, Urschitz MS. Anti-inflammatory medications for obstructive sleep apnea in children. Cochrane Database Syst Rev. 2011;(1):CD007074.[↩]
- Ye J, Liu H, Zhang GH, et al. Outcome of adenotonsillectomy for obstructive sleep apnea syndrome in children. Ann Otol Rhinol Laryngol. 2010;119(8):506–513.[↩]
- Guilleminault C, Palombini L, Pelayo R, Chervin RD. Sleepwalking and sleep terrors in prepubertal children: what triggers them? Pediatrics. 2003;111(1):e17–e25.[↩][↩]
- Goodlin-Jones BL, Burnham MM, Gaylor EE, Anders TF. Night waking, sleep-wake organization, and self-soothing in the first year of life. J Dev Behav Pediatr. 2001;22(4):226–233.[↩]
- Mindell JA, Kuhn B, Lewin DS, Meltzer LJ, Sadeh A; American Academy of Sleep Medicine. Behavioral treatment of bedtime problems and night wakings in infants and young children [published correction appears in Sleep. 2006;29(11):1380]. Sleep. 2006;29(10):1277–1281.[↩]
- Merenstein D, Diener-West M, Halbower AC, Krist A, Rubin HR. The trial of infant response to diphenhydramine: the TIRED study—a randomized, controlled, patient-oriented trial. Arch Pediatr Adolesc Med. 2006;160(7):707–712.[↩]
- Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30(11):1484–1501.[↩][↩]
- Picchietti DL, England SJ, Walters AS, Willis K, Verrico T. Periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. J Child Neurol. 1998;13(12):588–594.[↩]
- Picchietti D, Allen RP, Walters AS, Davidson JE, Myers A, Ferini-Strambi L. Restless legs syndrome: prevalence and impact in children and adolescents—the Peds REST study. Pediatrics. 2007;120(2):253–266.[↩]
- Winkelmann J, Muller-Myhsok B, Wittchen HU, et al. Complex segregation analysis of restless legs syndrome provides evidence for an autosomal dominant mode of inheritance in early age at onset families. Ann Neurol. 2002;52(3):297–302.[↩]
- Gamaldo CE, Earley CJ. Restless legs syndrome: a clinical update. Chest. 2006;130(5):1596–1604.[↩]
- Kryger MH, Otake K, Foerster J. Low body stores of iron and restless legs syndrome: a correctable cause of insomnia in adolescents and teenagers. Sleep Med. 2002;3(2):127–132.[↩]
- Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28(10):1271–1278.[↩]