Contents
What is telomerase
Telomerase is a ribonucleoprotein DNA polymerase complex that maintains telomere length. The complex comprises the telomerase reverse transcriptase protein (TERT, or hTERT in humans) and a catalytic telomerase RNA component (TERC) 1. Telomerase reverse transcriptase protein (TERT) assembles with the integral RNA template within catalytic telomerase RNA component (TERC) to repeatedly synthesize tandem repeats of DNA at the telomeric end of the chromosome. In the absence of telomerase activity telomeres progressively shorten. Telomerase is a master regulator for maintaining replicative potential in most eukaryotic cells. It does so by controlling telomere length at chromosome ends. In the absence of telomerase activity telomeres progressively shorten. Telomerase activity is absent in most normal human somatic cells because of the lack of expression of the protein telomerase reverse transcriptase (TERT); catalytic telomerase RNA component (TERC) is usually present. Without telomerase, telomere shortening eventually limits the growth of cells, either by aging, in cells with intact cell cycle checkpoints (a G1 cell cycle block), or by crisis in cells with inactivated checkpoints (telomeric end-to-end fusions cause chromosome breakage and mitotic catastrophe) 1. Expression of telomerase reverse transcriptase (TERT) in cells that otherwise lack telomerase activity causes cells to bypass senescence and crisis, and such cells are usually termed “immortalized.”
The level of telomerase activity is important in determining telomere length in aging cells and tissues. The enzyme telomerase elongates telomeres and thus prevents chromosome erosion and telomerase was found to be activated in over 85% of human cancers 2. Since telomerase seems closely associated with malignancy, telomerase is hoped to be an early indicator of cancer and a new therapeutic target common to most cancers.
Telomerase activity can be restored to human cells by telomerase reverse transcriptase protein (hTERT) gene transduction or potentially via drug therapy; such extended-lifespan cells could be useful in forms of cell therapy to be developed for age-related diseases. On the other hand, the absence of telomerase acts as a limitation on cancer growth unless telomerase becomes reactivated.
Replicative capacity of a cell is strongly correlated with telomere length regulation. Aberrant lengthening or reduction in the length of telomeres can lead to health anomalies, such as cancer or premature aging. Telomerase is a master regulator for maintaining replicative potential in most eukaryotic cells. It does so by controlling telomere length at chromosome ends. Akin to cancer cells, most single-cell eukaryotic pathogens are highly proliferative and require persistent telomerase activity to maintain constant length of telomere and propagation within their host.
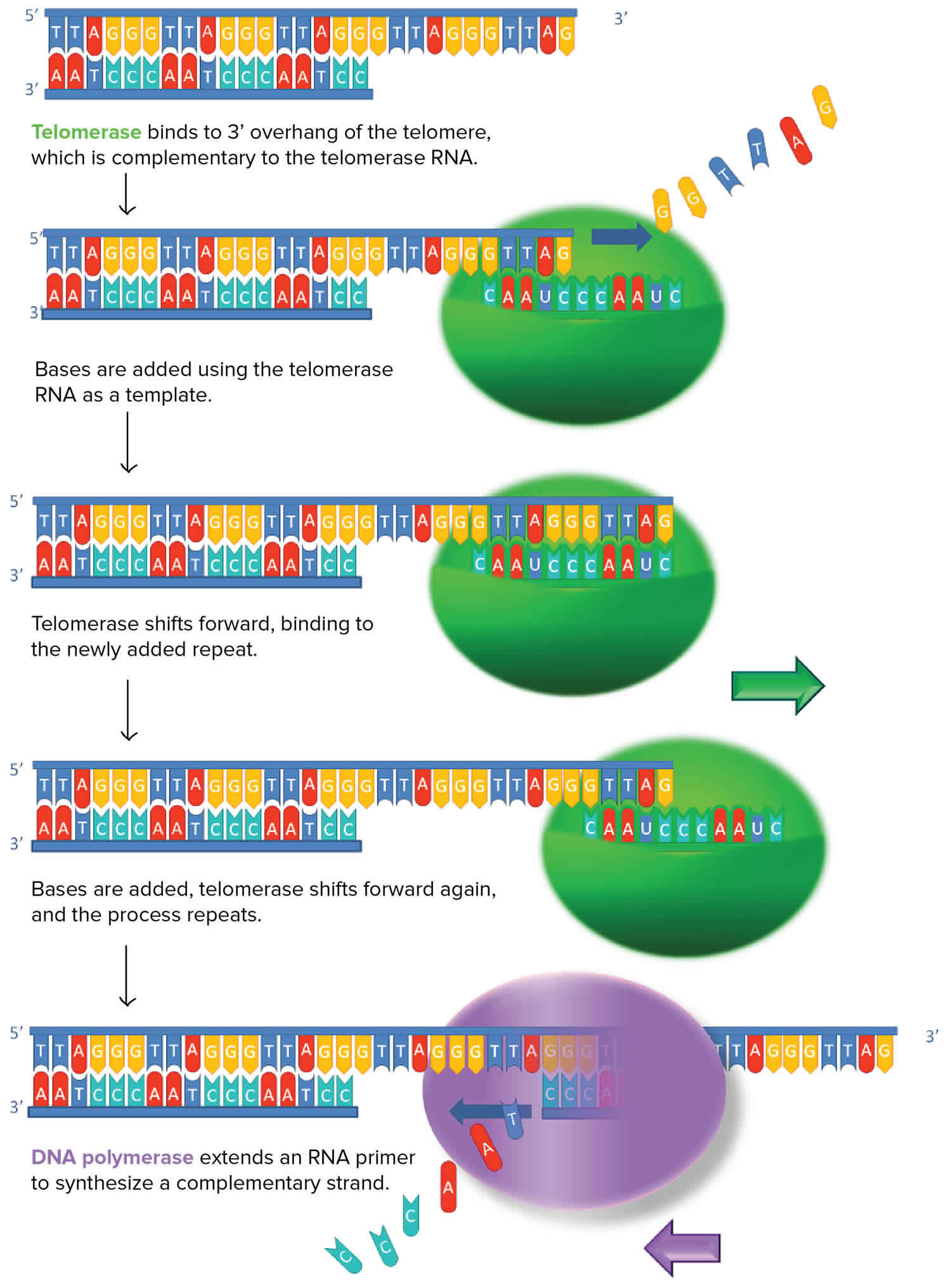
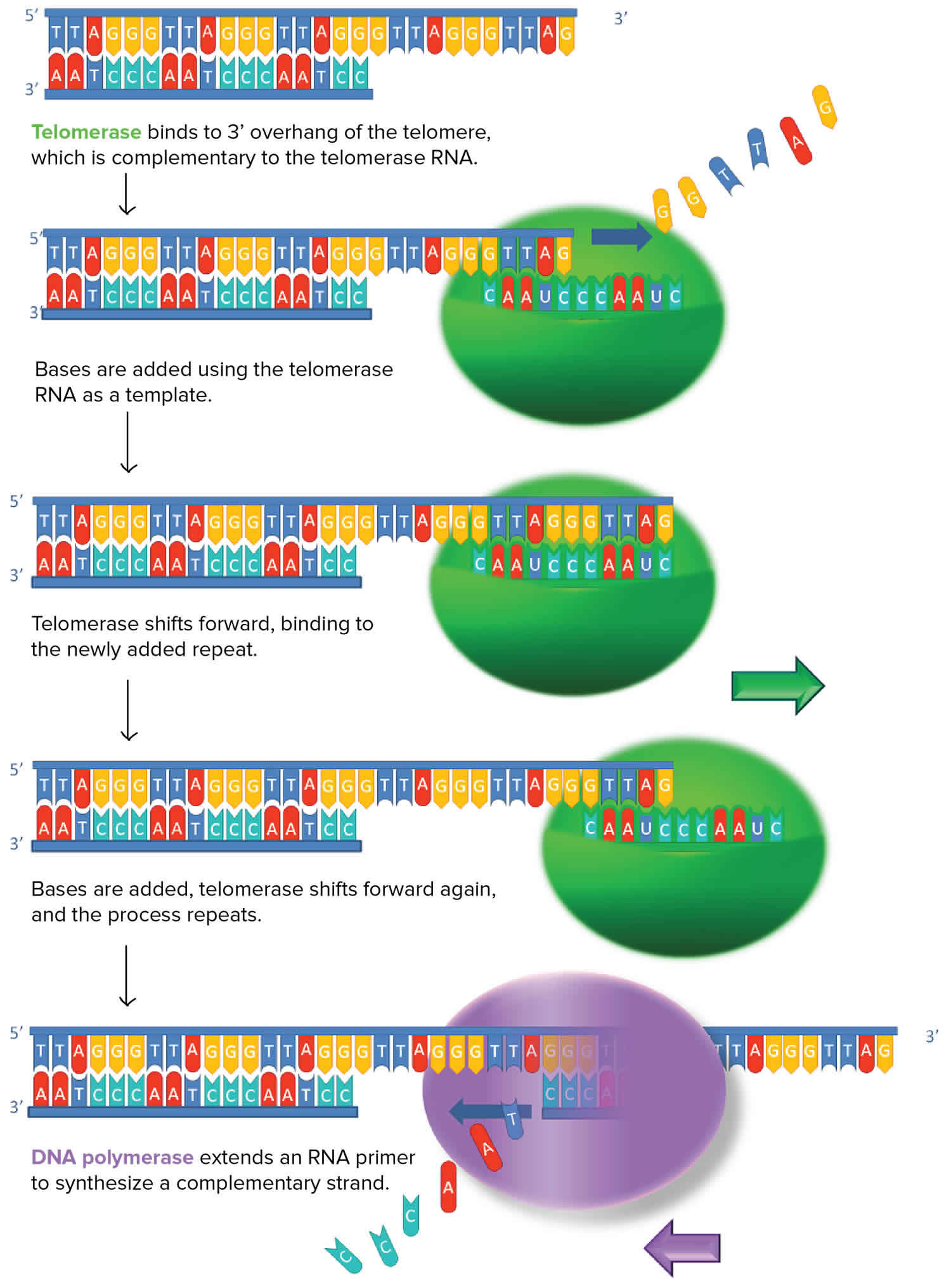
Figure 1. Telomerase
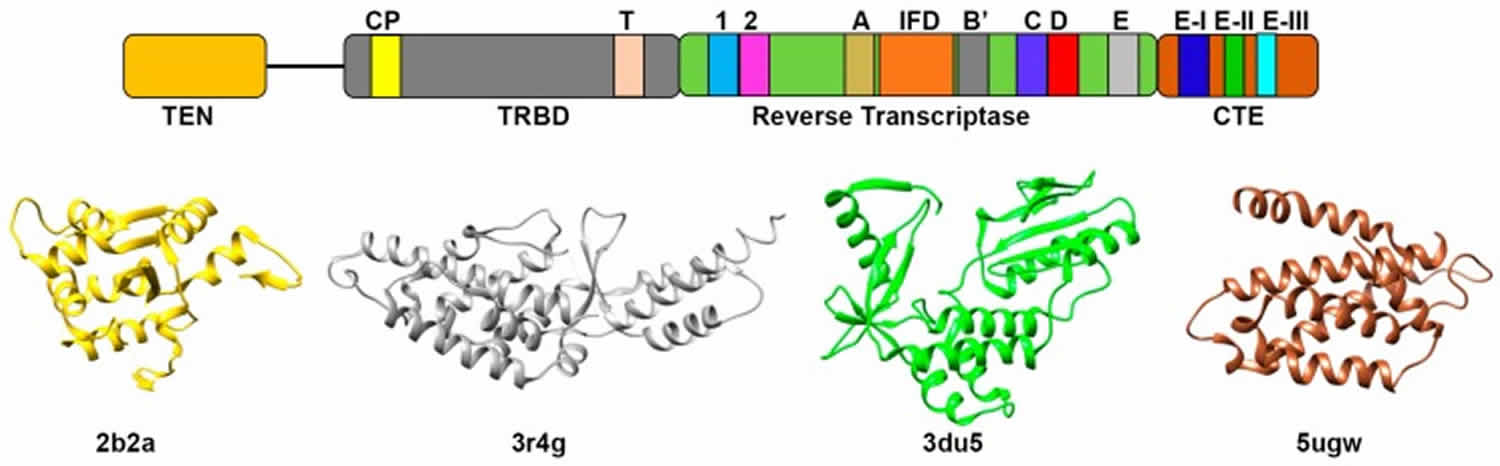
Figure 2. Telomerase structure
What is telomere
A telomere is the end of a chromosome, a specialized repetitive DNA sequences at the ends of the linear chromosomes, and associated proteins, that serve to maintain the integrity of the chromosomes. Telomeres are made of hundreds or thousands of repeats of the same short DNA sequence of non-coding DNA, which varies between organisms but is 5′-TTAGGG-3′ in humans, that protect the chromosome from damage. Each time a cell divides, the telomeres become shorter. Eventually, the telomeres become so short that the cell can no longer divide. Replicative capacity of a cell is strongly correlated with telomere length regulation. Aberrant lengthening or reduction in the length of telomeres can lead to health anomalies, such as cancer or premature aging. Telomerase is a ribonucleoprotein DNA polymerase complex that maintains telomere length. The complex comprises the protein telomerase reverse transcriptase (TERT, or hTERT in humans) and a catalytic telomerase RNA component (TERC) 1. Telomerase is a master regulator for maintaining replicative potential in most eukaryotic cells. It does so by controlling telomere length at chromosome ends. In the absence of telomerase activity telomeres progressively shorten. Telomerase activity is absent in most normal human somatic cells because of the lack of expression of the protein telomerase reverse transcriptase (TERT); catalytic telomerase RNA component (TERC) is usually present. Without telomerase, telomere shortening eventually limits the growth of cells, either by senescence, in cells with intact cell cycle checkpoints (a G1 cell cycle block), or by crisis in cells with inactivated checkpoints (telomeric end-to-end fusions cause chromosome breakage and mitotic catastrophe) 1. Expression of telomerase reverse transcriptase (TERT) in cells that otherwise lack telomerase activity causes cells to bypass senescence and crisis, and such cells are usually termed “immortalized.”
Modern interest in telomeres and telomerase has its roots in experiments carried out in the 1930s by two remarkable geneticists: Barbara McClintock, then at the University of Missouri at Columbia, and Hermann J. Muller, then at the University of Edinburgh. Working separately and with different organisms, both investigators realized that chromosomes bore a special component at their ends that provided stability. Muller coined the term “telomere,” from the Greek for “end” (telos) and “part” (meros). McClintock noted that without these end caps, chromosomes stick to one another, undergo structural changes and misbehave in other ways. These activities threaten the survival and faithful replication of chromosomes and, consequently, of the cells housing them.
The chromosome ends (telomeres) of mammalian cells contain tandemly arrayed hexanucleotide repeats with the sequence 5′-TTAGGG-3′ 3. This telomeric DNA is mostly double-stranded, but it terminates in a single-stranded 3′ overhang 4. In human somatic cells, each telomere is 4-12 kb long and the single-stranded overhang contains 100-200 nucleotides (Figure 1A). Telomeres need to be distinguished from double strand breaks (DSBs), to avoid being fused to each other by normal DNA repair mechanisms, because they have single-stranded overhangs, which “look like” damaged DNA. The overhang at the lagging strand end of the chromosome is due to incomplete end replication. The overhang at the leading strand end of the chromosome is actually generated by enzymes that cut away part of the DNA 5. In some species (including humans), the single-stranded overhangs bind to complementary repeats in the nearby double-stranded DNA, causing the telomere ends to form protective loops (Figure 1B) 6. Proteins associated with the telomere ends also help protect them and prevent them from triggering DNA repair pathways. Additionally, mammalian telomeres form a higher order structure by sequestering the 3′ overhang in cis within the duplex telomeric DNA, resulting in a telomere loop (t-loop) that likely contributes to the capping mechanism 7.
Because conventional DNA replication machinery cannot copy extreme terminal sequences of the lagging-strand during replication of linear chromosomes, 50-200 base pairs of telomeric DNA will be lost during each successive cell division 8. Due to the end-replication problem 9, the ends of linear chromosomes (repeats that make up a telomere) shorten with each round of DNA replication (a telomere are eaten away slowly over many division cycles) 10. Telomere shortening has been connected to the aging of cells, and the progressive loss of telomeres may explain why cells can only divide a certain number of times 11. In human somatic cells, the progressive telomere shortening that occurs with continued proliferation eventually results in the triggering of a replicative checkpoint. Telomere shortening and the structural changes that it presumably causes, leads to a DNA-damage checkpoint response at the telomere and induction of a permanent p53- and Rb-dependent growth arrest (i.e., replicative senescence) 12. Because this limits the proliferative capacity of somatic cells, including those that have accumulated oncogenic mutations, telomere shortening and replicative senescence are a potent tumor suppressor mechanism.
If senescence pathways are absent, due for example to loss of p53 and Rb function, cells will continue to divide until the telomeres become almost completely eroded, leading to crisis, a period characterized by rampant chromosome end-to-end fusions and cell death 13. Formation of tumors is, in most cases, dependent on the evolution of cells that escape from the barriers that senescence and crisis present to unlimited proliferation. Cells that achieve this are referred to as “immortalized” and in all cases this requires the activation of a mechanism for preventing telomere shortening. In most cases this is accomplished by upregulating the activity of telomerase 14, a ribonucleoprotein enzyme that adds new telomeric repeats to chromosome termini. Telomerase has an important role in cells of the germ line and in normal somatic biology, especially in those tissue compartments that depend upon extensive cellular proliferation. Nevertheless, in normal somatic cells telomerase is not expressed at sufficient levels to prevent telomere shortening and telomere length maintenance in many cancers requires dysregulated levels of telomerase. A substantial minority of immortalized cell lines and tumors are telomerase-negative, however, and in these cells telomere length maintenance can be achieved instead by a telomerase-independent mechanism, termed Alternative Lengthening of Telomeres (ALT) 15.
Alternative Lengthening of Telomeres may resemble (or represent) the earliest telomere maintenance mechanism (TMM), which preceded the evolution of telomerase-dependent maintenance of chromosomal termini. While the possibility cannot be excluded that a low level of Alternative Lengthening of Telomeres-like activity occurs at normal mammalian telomeres, the telomere phenotype seen in Alternative Lengthening of Telomeres-positive immortalized cells and tumors is not found in normal cells. The current data strongly support Alternative Lengthening of Telomeres being a homologous recombination (HR)-mediated DNA replication mechanism, which occurs in the context of telomere instability resulting from loss of several controls over telomere function.
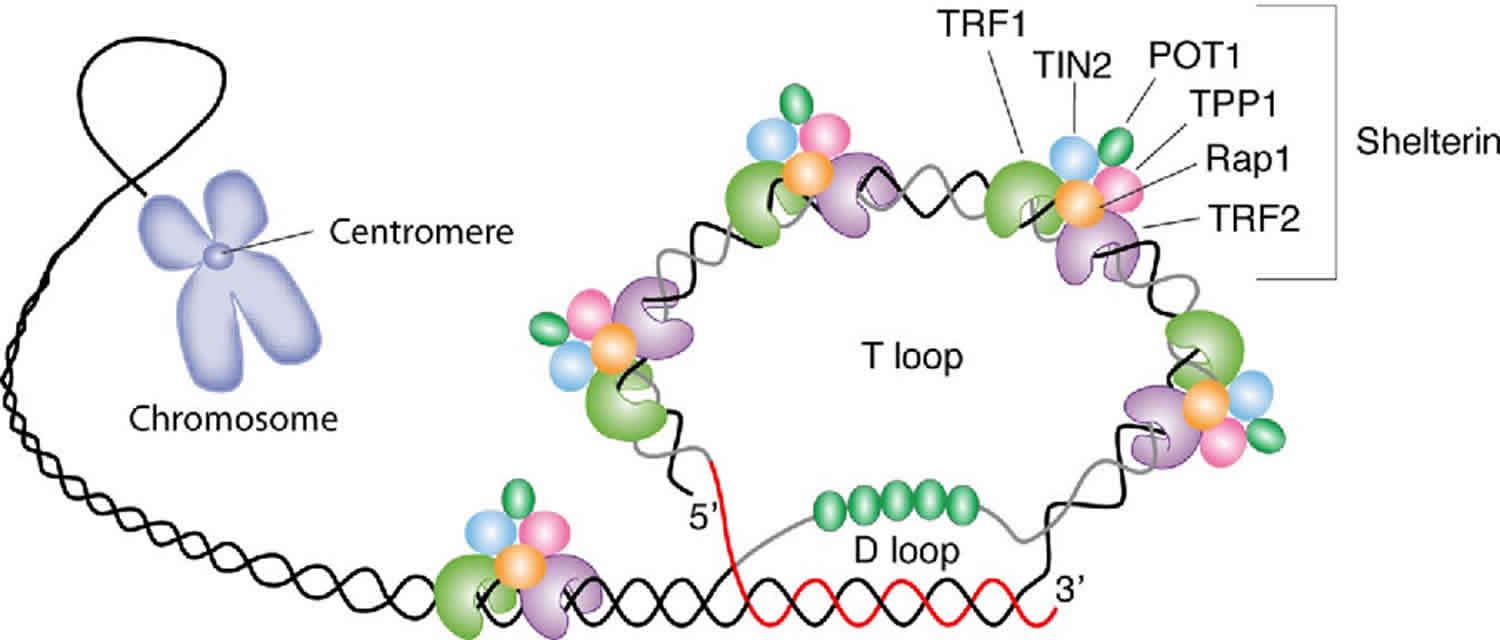
Figure 3. Telomere structure
Footnote: Schematic representation of telomere structure. Telomeres are at the extremities of chromosome DNA. The telomeric 3 end terminates as a single-stranded, G-rich overhang able to form the t-loop, in which the overhang invades the telomeric double helix, remodeling the DNA into a circle. Telomeres are capped by at least 6 proteins (TRF1, TRF2, TPP1, POT1, TIN2, and Rap1), collectively known as shelterin, that physically shield the DNA.19 TRF1, TRF2, and TPP1 specifically recognize and bind to doublestranded TTAGGG repeats; POT1 binds to the singlestranded telomeric overhang19,20; TIN2 and Rap1 complete the shelterin complex. Shelterin allows discrimination of telomeres from double-stranded DNA breaks; lack of shelterin allows telomeres to be identified as double-stranded DNA breaks and triggers DNAdamage pathways.
[Source 16 ]Telomerase function
Telomerase is a ribonucleoprotein DNA polymerase complex that maintains telomere length. Telomerase is a highly specialized reverse transcriptase that synthesizes telomeric DNA repeats at the ends of chromosome to confer cellular immortality. Unlike conventional reverse transcriptases, telomerase reverse transcriptase (TERT) contains an integral RNA component and uses only a very short region of the RNA as template. To add multiple DNA repeats processively, telomerase relies on a unique mechanism whereby the template RNA and the telomeric DNA dissociate and realign reiteratively during the telomere repeat synthesis. In spite of its essential role in tumorigenesis and aging, the detailed mechanism of telomerase action remains elusive.
Telomerase functions as a ribonucleoprotein (RNP) enzyme requiring minimally the catalytic telomerase reverse transcriptase (TERT) protein subunit and the telomerase RNA (TER) subunit. Assembly of functional telomerase ribonucleoprotein (RNP) relies on specific interactions between the telomerase reverse transcriptase (TERT) and telomerase RNA (TER) components.
Some cells have the ability to reverse telomere shortening by expressing telomerase, an enzyme that extends the telomeres of chromosomes. Telomerase is an RNA-dependent DNA polymerase, meaning an enzyme that can make DNA using RNA as a template.
The telomerase enzyme binds to a special RNA molecule that contains a sequence complementary to the telomeric repeat. It extends (adds nucleotides to) the overhanging strand of the telomere DNA using this complementary RNA as a template. When the overhang is long enough, a matching strand can be made by the normal DNA replication machinery (that is, using an RNA primer and DNA polymerase), producing double-stranded DNA. The primer may not be positioned right at the chromosome end and cannot be replaced with DNA, so an overhang will still be present. However, the overall length of the telomere will be greater.
Telomerase is not usually active in most somatic cells (cells of the body), but it’s active in germ cells (the cells that make sperm and eggs) and some adult stem cells. These are cell types that need to undergo many divisions, or, in the case of germ cells, give rise to a new organism with its telomeric “clock” 17.
Interestingly, many cancer cells have shortened telomeres, and telomerase is active in these cells. If telomerase could be inhibited by drugs as part of cancer therapy, their excess division (and thus, the growth of the cancerous tumor) could potentially be stopped.
Factors involved in telomerase assembly and activity
Telomerase activity can be modulated by its associated proteins that are involved in RNA maturation, telomerase assembly, telomerase recruitment and telomere binding. The telomerase core ribonucleoprotein (RNP) has a telomerase reverse transcriptase (TERT) catalytic protein subunit and an intrinsic telomerase RNA (TER) that provides the template for telomere DNA synthesis. Telomerase also often interacts with species-specific factors: in humans, Hsp90, p23 and TER binding proteins, L22 and human Staufen (hStau), are associated with telomerase activity 18. Dyskerin, Nucleolar protein family A member 2 (NHP2) and NOP10 bind the H/ACA snoRNA motif of mammalian telomerase RNA (TER) 19 and stabilize newly transcribed TER RNA, which is required for proper localization of telomerase. Dyskerin is one of the evolutionary conserved components of telomerase ribonucleoprotein complex as AtNAP57, the Arabidopsis dyskerin is also required for maintaining the Arabidopsis telomere 20. In S. cerevisiae, Sm proteins are necessary for telomerase RNA stability, while three other proteins, Est1, Est3 and Cdc13, are dispensable for in vitro telomerase activity but are required for telomere length maintenance 21. In S. pombe, sequential binding of Sm and Lsm proteins to telomerase RNA is important for telomerase RNA maturation 22.
In addition to telomerase-specific factors, telomere-binding proteins play pivotal roles in telomerase activity regulation 23. A six-protein ”shelterin” complex has several important contributions to this process controlling telomere length maintenance and telomerase activity. These proteins are: telomere repeat binding factors 1 and 2 (TRFs), repressor/activator protein 1 (RAP1, most evolutionary conserved shelterin, deficiency of which leads to increased telomere recombination), protection of telomeres 1 (POT1), TRF1 interacting nuclear factor 2 (TIN2) and TPP1 (Figure 4B). It is known that telomeric protein POT1 directly interacts with the 3′ end of telomere and binds TPP1 24. This POT1-TPP1 inhibits telomerase access to telomeres and provides a physical link between telomerase and the shelterin complex 25. Mutations identified in POT1–TPP1 complex are suspected to be associated with human diseases, such as familial glioma, melanoma and chronic lymphocytic leukemia 26.
Pathophysiology of Telomerase
For maintenance of the chromosome end structure and hence chromosome integrity, regulation of telomerase activity is necessary. However, several studies have demonstrated that any deviation from the regulated process involving telomerase and telomere preservation is linked to variety of human diseases ranging from cancers to age related disorders. Telomere binding proteins are also known to participate in disease progression caused by human parasites. In this section, we will briefly describe the association between telomerase and various diseases along with the necessity to understand the telomerase structural and functional characteristics for the development of telomerase targeted therapies.
Telomerase and Aging
Telomere elongation in human is a cell-cycle regulated process 27. Telomere is elongated by telomerase during the S-phase until it enters into the M-phase for cell division 28. Human germ line and stem cells that proliferate indefinitely and hence immortal have active telomerase which maintains the telomere length throughout the cell division 29. Conversely, in most of the differentiated somatic cells that have reduced life span, telomerase is found to be inactive and hence these cells are called mortal cells. In these cells, the telomere length decreases with each cell division as opposed to the immortal one and thus establishing the connection between telomerase, chromosomal stability and mortality of cells. Thus, telomere shortening and inactive/repressed telomerase in normal somatic cell may be indicative of some kind of ‘telomere clock mechanism’ that elicits cellular senescence 30, loss of proliferative capacity and ageing. The exact mechanism of telomere attrition is still obscure but it is thought that this may be due to the loss of TRF2 from the chromosomal termini. Studies have shown that TRF2 can promote the formation of T-loop (described above) in vitro and may be in vivo as well 31. Due to the loss of TRF2 the chromosomal termini continue to shorten, as they are unable to form T-loop. The exposed chromosomal end can then activate the p53 or Ataxia-Telangiectasia Mutated (ATM) kinase mediated cell apoptosis pathway 31. In the absence of telomerase activity, telomeres will shorten with each round of cell division until they reach a short length threshold known as “the Hayflick limit” 30. Once they reached this threshold, p53 and pRB dependent DNA damage response pathway is activated hence eliciting the non-proliferative state called replicative senescence 32. This state impedes the onset of tumorigenesis but cells with defective p53 and pRB (retinoblastoma) pathways have the ability to evade this barrier and can undergo additional cell division 33. However, during the period of additional cell division the telomeres will continue to shorten and ultimately have to undergo the breakage-fusion-bridge (chromosome end fusion) cycle which stimulates genomic instability and hence activates second proliferative blockage which is followed by cellular apoptosis 34. Sometimes, the cell can also evade this second barrier by either up-regulating the telomerase activity or by alternative telomere lengthening (ALT) mechanism which can stabilize the short telomere and allow the cells to divide again 35. This give rise to cellular immortality and eventually could lead to tumorigenesis. Most of the human cancer cells (80–90%) constantly express telomerase catalytic subunit and employ telomerase dependent telomere elongation mechanism to achieve the infinite growth potential, which make activation of telomerase holoenzyme as one of the most common pathway for cellular immortalization 36. The rest of the tumor cells use alternative telomere lengthening (ALT) pathway, which involves homologous recombination based for telomere maintenance 35. A better understanding of this alternative telomere lengthening (ALT) based pathway is now necessary to develop new strategies for combating cancer. As discussed above, telomere attrition results in aging and cellular senescence.
Cells that undergo cell division continue to have their telomeres shortened because most somatic cells do not make telomerase. This essentially means that telomere shortening is associated with aging. With the advent of modern medicine, preventative health care, and healthier lifestyles, the human life span has increased, and there is an increasing demand for people to look younger and have a better quality of life as they grow older.
In 2010, scientists found that telomerase can reverse some age-related conditions in mice. This may have potential in regenerative medicine 37. Telomerase-deficient mice were used in these studies; these mice have tissue atrophy, stem cell depletion, organ system failure, and impaired tissue injury responses. Telomerase reactivation in these mice caused extension of telomeres, reduced DNA damage, reversed neurodegeneration, and improved the function of the testes, spleen, and intestines. Thus, telomere reactivation may have potential for treating age-related diseases in humans.
Telomerase and Cancer
Cancer is characterized by uncontrolled cell division of abnormal cells. The cells accumulate mutations, proliferate uncontrollably, and can migrate to different parts of the body through a process called metastasis. Scientists have observed that cancerous cells have considerably shortened telomeres and that telomerase is active in these cells. Interestingly, only after the telomeres were shortened in the cancer cells did the telomerase become active. If the action of telomerase in these cells can be inhibited by drugs during cancer therapy, then the cancerous cells could potentially be stopped from further division.
Mechanism of telomerase activation in cancerous cells has been studied extensively. Among series of mutations that are identified in key regulatory regions, mutations in the promoter region of hTERT in most of the tumor cells are deemed important, since these mutations could be an early event in the onset of carcinogenesis 38. hTERT is a 40 kb long gene comprising of 16 exons and 15 introns and have a promoter region which is not only GC rich but also lacks both TATA and CAAT regions. Recent studies have shown the presence of two exceedingly frequent mutations, C→T at −124 bp and −146 bp upstream from ATG start site. These mutations, thus generates an E-twenty-six (ETS) transcription factor binding site which can up-regulate the hTERT expression in cancerous cells 39. One study reported that epidermal growth factor (EGF) mediated activation of telomerase activity in lung cancer is linked to the binding of ETS-2 to hTERT promoter 40. Furthermore, some less frequent hTERT mutations resulting in A→C and/or C→A transitions are also reported in some other cancer lines 41. Although these promoter mutations are being most frequent in cancer, their level and frequency differs with cancer types. Melanoma, skin carcinoma and liver cancer have highest frequencies of TERT promoter mutations while gastric cancer, pancreatic cancer and gastrointestinal stromal cancer reported to have lower frequency of these mutations 42. In prostate cancer cells, expression of both hTER and hTERT are found to be directly correlated to the expression of myelocytomatosis viral oncogene (MYC) as silencing of the later results in decreased expression of hTER thus causing reduced cell proliferation 43. The exact mechanism of this correlation is still under investigation but it is thought that mutations in the hTERT promoter enhances the binding of MYC via the increased binding of ETS/T-cell factor (TCF) 44. Nevertheless, highly activated telomerase becomes an appealing target for the development of novel anti-cancer therapeutics 35.
- Shay J, Wright W. Hallmarks of telomeres in ageing research. J. Pathol. 2007;211:114–23[↩][↩][↩][↩]
- Dhaene K. Telomerase in Mesothelioma: Diagnostic and Therapeutic Applications. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6010[↩]
- Moyzis RK, Buckingham JM, Cram LS. et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6626[↩]
- Makarov VL, Hirose Y, Langmore JP. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 1997;88:657–666[↩]
- Chow, T. T., Zhao, Y., Mak, S. S., Shay, J. W., and Wright, W. E. (2012). Early and late steps in telomere overhang processing in normal human cells: The position of the final RNA primer drives telomere shortening. Genes Dev., 26(11), 1168. http://dx.doi.org/10.1101/gad.187211.112.[↩]
- Vega, L. R., Mateyak, M. K., and Zakian, V. A. (2003). Getting to the end: Telomerase access in yeast and humans. Nature Reviews Molecular Cell Biology, 4, 951. http://dx.doi.org/10.1038/nrm1256[↩]
- Griffith JD, Comeau L, Rosenfield S. et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514.[↩]
- Greider, C.W. and E.H. Blackburn (1985) Cell 43:405.[↩]
- Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239:197–201[↩]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460[↩]
- Bartlett, Z. (2014, November 14). The Hayflick limit. In The embryo project encyclopedia. Retrieved from https://embryo.asu.edu/pages/hayflick-limit[↩]
- d’Adda di Fagagna F, Reaper PM, Clay-Farrace L. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426(6963):194–198.[↩]
- Counter CM, Avilion AA, LeFeuvre CE. et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929 [↩]
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791[↩]
- Bryan TM, Englezou A, Dalla-Pozza L. et al. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–1274[↩]
- Calado, Rodrigo T. and Neal S. Young. “Telomere maintenance and human bone marrow failure.” Blood 111 9 (2008): 4446-55. https://pdfs.semanticscholar.org/cc3e/b79d4c3054d3f740af751a29692a55f35c66.pdf[↩]
- Reece, J. B., Urry, L. A., Cain, M. L., Wasserman, S. A., Minorsky, P. V., and Jackson, R. B. (2011). Shortening of the ends of linear DNA molecules. In Campbell biology (10th ed., p. 327). San Francisco, CA: Pearson.[↩]
- Shay J.W., Zou Y., Hiyama E., Wright W.E. Telomerase and cancer. Hum. Mol. Genet. 2001;10:677–685. doi: 10.1093/hmg/10.7.677[↩]
- Wang C., Meier U.T. Architecture and assembly of mammalian H/ACA small nucleolar and telomerase ribonucleoproteins. EMBO J. 2004;23:1857–1867. doi: 10.1038/sj.emboj.7600181[↩]
- Kannan K., Nelson A.D., Shippen D.E. Dyskerin is a component of the Arabidopsis telomerase RNP required for telomere maintenance. Mol. Cell. Biol. 2008;28:2332–2341. doi: 10.1128/MCB.01490-07[↩]
- Livengood A.J., Zaug A.J., Cech T.R. Essential regions of Saccharomyces cerevisiae telomerase RNA: Separate elements for Est1p and Est2p interaction. Mol. Cell Biol. 2002;22:2366–2374. doi: 10.1128/MCB.22.7.2366-2374.2002[↩]
- Tang W., Kannan R., Blanchette M., Baumann P. Telomerase RNA biogenesis involves sequential binding by Sm and Lsm complexes. Nature. 2012;484:260–264. doi: 10.1038/nature10924[↩]
- Cifuentes-Rojas C., Shippen D.E. Telomerase regulation. Mutat. Res. 2012;730:20–27. doi: 10.1016/j.mrfmmm.2011.10.003[↩]
- Hockemeyer D., Collins K. Control of telomerase action at human telomeres. Nat. Struct. Mol. Biol. 2015;22:848–852. doi: 10.1038/nsmb.3083[↩]
- Zaug A.J., Podell E.R., Nandakumar J., Cech T.R. Functional interaction between telomere protein TPP1 and telomerase. Genes Dev. 2010;24:613–622. doi: 10.1101/gad.1881810[↩]
- Chen C., Gu P., Wu J., Chen X., Niu S., Sun H., Wu L., Li N., Peng J., Shi S., et al. Structural insights into POT1-TPP1 interaction and POT1 C-terminal mutations in human cancer. Nat. Commun. 2017;8:14929. doi: 10.1038/ncomms14929[↩]
- Marcand S., Brevet V., Mann C., Gilson E. Cell cycle restriction of telomere elongation. Curr. Biol. 2000;10:487–490. doi: 10.1016/S0960-9822(00)00450-4[↩]
- Diede S.J., Gottschling D.E. Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerases α and δ Cell. 1999;99:723–733. doi: 10.1016/S0092-8674(00)81670-0[↩]
- Allsopp R.C., Vaziri H., Patterson C., Goldstein S., Younglai E.V., Futcher A.B., Greider C.W., Harley C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114[↩]
- Harley C.B., Futcher A.B., Greider C.W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0[↩][↩]
- Stansel R.M., de Lange T., Griffith J.D. T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J. 2001;20:5532–5540. doi: 10.1093/emboj/20.19.5532[↩][↩]
- Herbig U., Jobling W.A., Chen B.P., Chen D.J., Sedivy J.M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53 and p21(CIP1) but not p16(INK4a) Mol. Cell. 2004;14:501–513. doi: 10.1016/S1097-2765(04)00256-4[↩]
- Shay J.W., Pereira-Smith O.M., Wright W.E. A role for both RB and p53 in the regulation of human cellular senescence. Exp. Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2[↩]
- Artandi S.E., DePinho R.A. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268[↩]
- Cesare A.J., Reddel R.R. Telomere uncapping and alternative lengthening of telomeres. Mech Ageing Dev. 2008;129:99–108. doi: 10.1016/j.mad.2007.11.006[↩][↩][↩]
- Kim N.W., Piatyszek M.A., Prowse K.R., Harley C.B., West M.D., Ho P.L., Coviello G.M., Wright W.E., Weinrich S.L., Shay J.W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428[↩]
- Jaskelioff et al., “Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice,” Nature 469 (2011): 102-7.[↩]
- Chiba K., Johnson J.Z., Vogan J.M., Wagner T., Boyle J.M., Hockemeyer D. Cancer-associated TERT promoter mutations abrogate telomerase silencing. Elife. 2015;4:e07918. doi: 10.7554/eLife.07918[↩]
- Horn S., Figl A., Rachakonda P.S., Fischer C., Sucker A., Gast A., Kadel S., Moll I., Nagore E., Hemminki K., et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062[↩]
- Hsu C.P., Lee L.W., Tang S.C., Hsin I.L., Lin Y.W., Ko J.L. Epidermal growth factor activates telomerase activity by direct binding of Ets-2 to hTERT promoter in lung cancer cells. Tumour Biol. 2015;36:5389–5398. doi: 10.1007/s13277-015-3204-x[↩]
- Heidenreich B., Rachakonda P.S., Hemminki K., Kumar R. TERT promoter mutations in cancer development. Curr. Opin. Genet. Dev. 2014;24:30–37. doi: 10.1016/j.gde.2013.11.005[↩]
- Huang D.S., Wang Z., He X.J., Diplas B.H., Yang R., Killela P.J., Meng Q., Ye Z.Y., Wang W., Jiang X.T., et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur. J. Ccancer. 2015;51:969–976. doi: 10.1016/j.ejca.2015.03.010[↩]
- Baena-Del Valle J.A., Zheng Q., Esopi D.M., Rubenstein M., Hubbard G.K., Moncaliano M.C., Hruszkewycz A., Vaghasia A., Yegnasubramanian S., Wheelan S.J., et al. MYC drives overexpression of telomerase RNA (hTR/TERC) in prostate cancer. J. Pathol. 2017 doi: 10.1002/path.4980[↩]
- Akincilar S.C., Unal B., Tergaonkar V. Reactivation of telomerase in cancer. Cell Mol. Life Sci. 2016;73:1659–1670. doi: 10.1007/s00018-016-2146-9[↩]