Contents
- What is testosterone replacement therapy
- What is testosterone?
- Normal testosterone levels
- What is the role of testosterone in men’s health?
- Low testosterone / testosterone deficiency
- What causes low testosterone?
- Age related low testosterone causes
- What happens to testosterone levels with age?
- Does a naturally declining testosterone level cause the signs and symptoms of aging?
- How is low testosterone diagnosed?
- How is low testosterone treated?
- Can testosterone replacement therapy promote youth and vitality?
- Should I talk to my doctor about testosterone replacement therapy?
- Benefits of testosterone replacement therapy for men
- Testosterone replacement therapy types
- Therapies that Increase Endogenous Testosterone
- Testosterone replacement therapy contraindications
- Testosterone replacement therapy dosage
- Testosterone replacement therapy monitoring
- Testosterone replacement therapy risks
What is testosterone replacement therapy
Testosterone replacement therapy also called TRT is testosterone therapy for male hypogonadism 1, 2, 3. The indication of testosterone-replacement therapy treatment requires the presence of low testosterone level, and symptoms and signs of low testosterone or hypogonadism 4. Hypogonadism is seen in 19% of men in their 60s, 28% of men in their 70s, and 49% of men in their 80s 5. Low testosterone (hypogonadism) can be treated with the use of doctor-prescribed testosterone replacement therapy. Testosterone-replacement therapy treatment is safe and can be effective for men who are diagnosed with consistently abnormal low testosterone production and symptoms that are associated with this type of androgen (hormone) deficiency.
Testosterone is US Food and Drug Administration (FDA) approved as replacement therapy in men who have low testosterone levels and those with symptoms of hypogonadism 6. It is important to distinguish between primary (testicular) and secondary (pituitary-hypothalamic) hypogonadism. Symptoms highly suggestive of hypogonadism include decreased spontaneous erections, decreased nocturnal penile tumescence, decreased libido, decreased beard growth, and shrinking testicles. The normal range for early morning testosterone in a male is between 300 ng/dL to 1000 ng/dL 7.
Testosterone therapy is not FDA-approved to treat low libido in women.
While testosterone replacement therapy is the primary treatment option for men with low testosterone or hypogonadism, some conditions that cause hypogonadism are reversible without testosterone therapy. These should be addressed before testosterone therapy is contemplated. If testosterone therapy is needed, goal of treatment is to improve symptoms associated with testosterone deficiency and maintain sex characteristics. There are many different types of testosterone therapy. You should discuss the different options with your physician “your partner in care” to find out which therapy is right for you.
Hypogonadism is a common condition in the male population, with a higher prevalence in older men, obese men, and men with type 2 diabetes. If you are concerned about your testosterone levels It is important to talk to doctor about ways to manage.
Currently, there are a variety of widely available testosterone formulations, including topical gels and patches, intramuscular injections, subcutaneous pellets, and oral/buccal formulations that provide clinicians and male patients the opportunity to personalize replacement therapy to reestablish testosterone levels and improve testosterone deficiency symptoms 8. Method of testosterone replacement therapy treatment depends on the cause of low testosterone, the patient’s preferences, cost, tolerance, and concern about fertility.
- Injections. Self or doctor administered in a muscle every 1–2 weeks; administered at a clinic every 10 weeks for longer-acting. Side effects: uncomfortable, fluctuating symptoms.
- Gels/Solutions. Applied to upper arm, shoulder, inner thigh, armpit. Side effects: may transfer to others via skin contact — must wait to absorb completely into skin.
- Patches. Adhere to skin every day to back, abdomen, upper arm, thigh; rotate locations to lessen skin reaction. Side effects: skin redness and rashes.
- Buccal Tablets. Sticky pill applied to gums twice a day, absorbs quickly into bloodstream through gums. Side effects: gum irritation.
- Pellets. Implanted under skin surgically every 3–6 months for consistent and long-term dosages. Side effects: pellet coming out through skin, site infection/ bleeding (rare), dose decreasing over time and hypogonadism symptoms possibly returning towards the end of dose period.
- Nasal Gel. Applied by pump into each nostril 3x a day. Side effects: nasal irritation or congestion.
Transdermal gels and intramuscular (IM) injections are the top 2 options 6.
Oral capsules and tablets of testosterone such as methyltestosterone should generally not be used to treat testosterone deficiency due to hepatic side effects and decreased efficacy when compared with other formulations 6. The buccal form should not be chewed or swallowed.
Transdermal formulations include testosterone gels, patches, solutions, and pellets. Testosterone gels are generally recommended due to patient preference, cost, convenience, and insurance coverage. The major advantage of gels is the maintenance of stable serum testosterone concentrations resulting in stable libido, energy, and mood. There are various formulations of testosterone gel. These gels should be applied to the shoulder, upper arms, or abdomen and should not be applied to the scrotum. A study showed the bioavailability of testosterone gel is 30% lower when applied to the abdomen as when compared with arms and shoulders 9.
A nasal testosterone gel is now approved in the United States. It should be given 3 times daily. Some patients may find this inconvenient. A testosterone patch should be applied to the back, abdomen, thigh or upper arm and should not be applied to the scrotum. A testosterone solution was discontinued by the FDA in 2017. Subcutaneous testosterone pellets are placed every 3 to 6 months into the subdermal fat of the buttocks, abdominal wall, or thigh but are not routinely recommended due to limited data on the serum testosterone concentrations during treatment.
Intramuscular injections of testosterone include testosterone enanthate and testosterone cypionate. These injections are generally recommended to be given at doses of 50 to 100 mg every week or 100 to 200 mg every 2 weeks 10. In 2014, the FDA approved an extra-long acting intramuscular injection form of testosterone called testosterone undecanoate, which is dosed at 750 mg followed by a second dose 4 weeks later and subsequent doses every 10 weeks 11. Testosterone undecanoate is not the first-line treatment of choice but generally used when patients do not have access to other forms of treatment.
The most controversial area for TRT is the issue of risk, especially possible stimulation of prostate cancer by testosterone, even though no evidence to support this risk exists. A 2021 study 12 concluded that compared to TRT-untreated patients, TRT-treated patients may not have increased risks for disease progression in prostate cancer. However, the quality of currently available evidence is extremely poor. TRT may be harmful in men with advanced prostate cancer, in those with untreated prostate cancer undergoing active surveillance, and in those with successfully treated prostate cancer but having high-risk disease 12. The United States Food and Drug Administration (FDA) stated, in all testosterone package inserts, that TRT is contraindicated in men with known or suspected prostate cancer, but it did not substantiate this contraindication 13. The clinical guidelines by Endocrine Society recommend against treating hypogonadism in men with prostate cancer, citing lack of sufficient data to make a general recommendation in men previously treated for prostate cancer (‘recommendation with low quality evidence’) 14. Meanwhile, the European Association of Urology stated that there is no conclusive evidence that TRT increases the risk of prostate cancer (‘level of evidence=4’), and that men with prostate cancer can receive TRT with careful monitoring for prostate safety (‘level of evidence=3’) 15. Similarly, the recent treatment guideline by the American Urologic Association stated that patients should be informed that there is inadequate evidence for TRT (‘expert opinion’), but TRT can be considered in men who have undergone radical prostatectomy with favorable pathology (e.g., negative margins, negative seminal vesicles, negative lymph nodes), without PSA recurrence 16.
What is testosterone?
Testosterone is the major male sex hormone and is produced by the male testes in men and to a lesser extent by the adrenal glands in both men and women. In men, testosterone is thought to regulate sex drive (libido), bone mass, fat distribution, promotes gains in muscle mass and strength when combined with resistance training, the production of red blood cells and controlling fertility and the development of sperm (spermatogenesis) 17, 18. Testosterone also plays an important part in the development of ‘male sex characteristics’ such as a deeper voice and certain patterns of muscle development and hair growth. Testosterone helps bring on the physical changes that turn a boy into a man. This time of life is called puberty. Adolescent boys with too little testosterone may not experience normal masculinization. For example, the genitals may not enlarge, facial and body hair may be scant and the voice may not deepen normally. In women or anyone with ovaries, testosterone impacts overall growth as well as development of muscle and reproductive tissue. Testosterone is produced in the Leydig cells in the testis in men and to a lesser extent by the adrenal glands in both men and women 19. In males, the normal range for early morning testosterone is between 300 ng/dL to 1000 ng/dL but may vary from laboratory to laboratory 20, 21, 22.
Blood levels of testosterone peak in 20 to 30 year-old males and then decline with age with average rate of decline of 1.6% per year 23, 24, 25, 26, 27, 28, 29. One study found that serum testosterone levels were below the normal range in 20% of men in their 60s and in close to 50% of men in their 80s 26. However, the prevalence of symptomatic low testosterone (hypogonadism) is estimated by some to be much lower in this population, at about 2% 30. Longitudinal studies demonstrated a gradual decline in total serum testosterone levels in men from the age of 40, with 20% and 30% of men meeting age-related low testosterone
criteria in their 60s and 70s 31, 32.
Testosterone in the blood can be either bound or free:
- Bound testosterone is attached to proteins such as sex-hormone-binding-globulin (SHBG) and albumin. Most testosterone (approximately 60–70%) is transported in the blood to target tissues bound to sex hormone-binding globulin (SHBG), which in men is also called testosterone-binding globulin. About 30–40% of testosterone is loosely bound to albumin. This majority supply of protein-bound testosterone acts as a surplus of testosterone hormone for the body 33.
- Free testosterone, the active form, is all the remaining testosterone that is not bound to other substances. The small amounts of free testosterone (approximately 0.5–2%) in the blood act at the level of the tissues, primarily the seminal vesicles, bone, muscle, and prostate gland 33.
Historically, only free testosterone was thought to be the biologically active component. However, testosterone is weakly bound to serum albumin and dissociates freely in the capillary bed, thereby becoming readily available for tissue uptake. All non-SHBG-bound testosterone is therefore considered bioavailable.
Low testosterone levels in men is defined as total testosterone < 350 ng/dL and free testosterone < 225 pmol/L and are associated with sexual dysfunction such as low sexual desire (low libido), erectile dysfunction (fewer or diminished spontaneous erections, decreased nocturnal penile tumescence), reduced skeletal muscle mass and strength, decreased bone mineral density, sparse beard growth, shrinking testes, increased cardiovascular risk and alterations of the glycometabolic profile 34, 20, 35, 36, 37. The European Urological Association recommends a total testosterone level < 300 ng/dL as the diagnosis of hypogonadism 38, the American Urological Association considers 3.5 ng/mL (12 nmol/L) total testosterone represents a reliable threshold to diagnose age-related hypogonadism 39, and the Endocrine Society recommends the reasonable cut-off of total testosterone in men as 264 ng/dL (9.2 nmol/L) 40. This variation total testosterone level diagnostic criteria underscores the lack of uniformity in defining age-related low testosterone across different medical governing bodies. Total testosterone 12 nmol/L (3.5 ng/mL) represents a reliable threshold to diagnose age-related low testosterone
3. Calculated free-testosterone of < 220 pmol/L has been suggested as a possible cut-off to diagnose age-related hypogonadism 3.
Testosterone levels are also potentially influenced by food intake 41; therefore, serum total testosterone should be measured in fasting conditions in the morning (between 7AM and 11AM). A confirmatory measurement should always be undertaken in the case of a abnormal value, and before starting any testosterone therapy.
In clinical conditions that may interfere with sex hormone-binding globulin (SHBG) levels, evaluation of free testosterone should be considered to better estimate actual androgen levels. Unfortunately, despite free testosterone potential clinical value 42, no validated thresholds for free testosterone are available from clinical studies and this represents an area of uncertainty; however, data from the European Male Aging Study indicated that free testosterone levels < 220 pmol/L (6.4 ng/dL) increased the likelihood to correct identify hypogonadism as compared with total testosterone level alone, particularly when total testosterone levels are between 8.0 and 11 nmol per liter 43, 44, 30.
The incidence of low testosterone in the United States is reported to be approximately 20% in men older than 60 years of age with 30% in those older than 70 years of age and 50% in those older than 80 years 26, although the prevalence of syndromic low testosterone defined as at least 3 sexual symptoms with a total testosterone level < 320 ng/dL (<11.1 nmol/L) is lower 30.
However, uncertainty exists as to whether nonspecific signs and symptoms associated with age-related low testosterone, such as sexual dysfunction, decreases in energy and muscle mass, mood disturbances, changes in bone mineral density, cardiovascular disease, depression, decreased libido, erectile dysfunction, decreased volume of ejaculate, loss of body and facial hair, weakness, and mortality, are a consequence of age-related low testosterone or whether they are a result of other factors, such as chronic illnesses or concomitant medications 45, 46, 47, 48, 49. Given the high prevalence of low testosterone and more limited correlation of low testosterone with symptoms in aging men, it is uncertain to what extent does low testosterone represents a physiologic or pathologic event 24, 26. Furthermore, symptoms typically associated with low testosterone are less specific in older men and may be caused by other diseases or medical conditions. For example, erectile dysfunction can be the result of vascular insufficiency, neurologic impairment, psychogenic causes or substance use 50. Conditions such as diabetes and atherosclerosis are more common in older men, with up to 40% of men over 50 years of age having evidence of vascular insufficiency as the primary cause of their erectile dysfunction 51. Low libido similarly can result from psychiatric or medical conditions that are more common in older men 52.
The role of testosterone treatment in managing age-related low testosterone is controversial 53. The U.S. Food and Drug Administration (FDA) requires the pharmaceutical industry to label all testosterone medications to clearly state that their products are approved for use only in persons with low testosterone levels due to known causes and the FDA cautions about using testosterone products for low testosterone due to aging; requiring labeling change to inform of possible increased risk of heart attack and stroke with testosterone use 54, 46.
Testosterone replacement therapy (TRT), a generally accepted method treating for age-related low testosterone, can improve the symptoms of age-related hypogonadism and is most usually administered by injection, oral administration and transdermal administration 55. Many studies have reported the benefit of testosterone replacement therapy (TRT) for age-related low testosterone 56. However, some studies revealed that symptoms related to age-related hypogonadism may not be correlated with serum total testosterone levels 57, 58.
The American College of Physicians (ACP) recently issued new guidelines on prescribing supplemental testosterone for men with age-related low testosterone 53:
- The American College of Physicians suggests that clinicians discuss whether to initiate testosterone treatment in men with age-related low testosterone with sexual dysfunction who want to improve sexual function (conditional recommendation; low-certainty evidence). The discussion should include the potential benefits, harms, costs, and patient’s preferences.
- The American College of Physicians suggests that clinicians should reevaluate symptoms within 12 months and periodically thereafter. Clinicians should discontinue testosterone treatment in men with age-related low testosterone with sexual dysfunction in whom there is no improvement in sexual function (conditional recommendation; low-certainty evidence).
- The American College of Physicians suggests that clinicians consider intramuscular rather than transdermal formulations when initiating testosterone treatment to improve sexual function in men with age-related low testosterone, as costs are considerably lower for the intramuscular formulation and clinical effectiveness and harms are similar.
- The evidence shows that men with age-related low testosterone may experience slight improvements in sexual function by 35% and erectile dysfunction by 27% if prescribed supplemental testosterone.
- The evidence does not support prescribing testosterone for men with concerns about energy, vitality, physical function, or cognition.
- The new guidelines point out that treating with supplemental testosterone is not risk free. Potential risks include blood clots, stroke, sleep apnea, acne and possibly enlarged breasts (gynecomastia). Men with these or other conditions may be advised against using supplemental testosterone even for sexual dysfunction.
- If men are treated with supplemental testosterone for sexual dysfunction, the decision to continue to treat should be reevaluated after a year and then periodically after that. Treatment should be stopped if sexual dysfunction doesn’t improve, according to the new guidelines.
Data from meta-analyses have shown that testosterone replacement therapy is ineffective when baseline testosterone levels are > 3.5 ng/mL (>12 nmol/L). Positive outcomes are documented when testosterone levels are < 3.5 ng/mL (<12 nmol/L), being higher in symptomatic patients with more severe forms of hypogonadism (< 8 nmol/L). Hence, 3.5 ng/mL (12 nmol/L) should be considered as a possible threshold for starting testosterone therapy in the presence of hypogonadal symptoms 59, 60.
You should not receive testosterone therapy if you have:
- Prostate or breast cancer (or suspected)
- Enlarged prostate causing difficulty with urination
- Elevated prostate specific antigen (PSA) levels
- High number of red blood cells
- Untreated sleep apnea (obstructed breathing during sleep)
- Planning to have children
- Heart attack or stroke within the last 6 months
- Blood clots
Possible risks of testosterone treatment include:
- Decreased sperm production
- A high red blood cell count
- Acne
- An increase in prostate size
- Sleep apnea—the occasional stopping of breathing during sleep (rarely)
You should discuss with your doctor how to monitor for prostate cancer and other risks to your prostate. Men with known or suspected prostate or breast cancer should not receive testosterone therapy. You should also talk to your doctor about the risks of testosterone therapy if you have, or are at risk for, heart disease or stroke. In addition, if you are planning fertility, you should not use testosterone therapy.
Figure 1. Testosterone
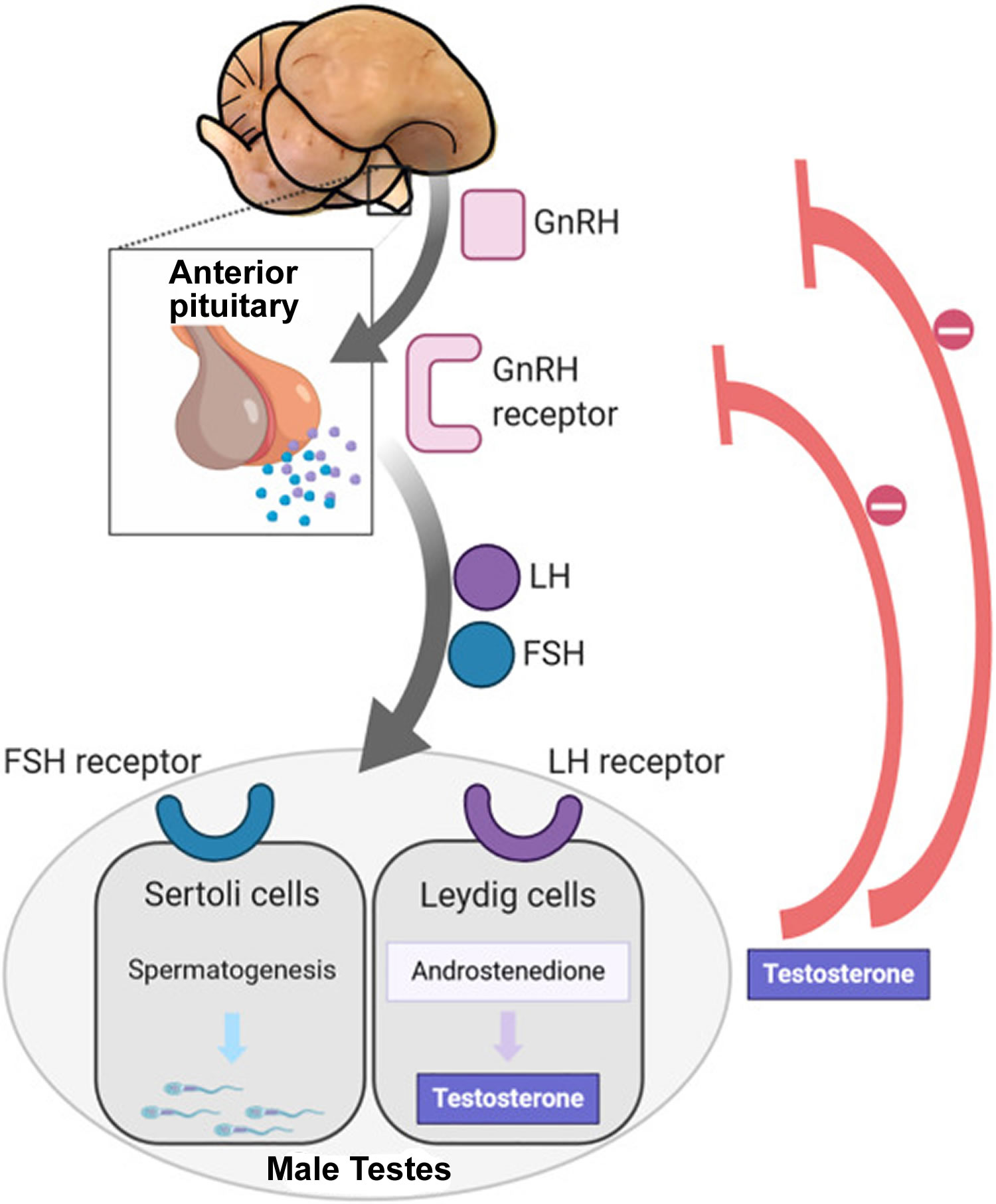
Figure 2. Hypothalamic–pituitary–gonadal axis
Footnotes: In puberty, the hypothalamic-pituitary-gonadal axis plays a major role in regulating testosterone levels and gonadal function. Gonadotropin-releasing hormone (GnRH) is secreted from the hypothalamus by GnRH-expressing neurons. The GnRH travels down the hypothalamohypophyseal portal system to the anterior pituitary, which secretes luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH are two gonadotropic hormones that travel through the blood and act on receptors in the gonads. The largest amounts of testosterone (>95%) are produced by the testes in men, while the adrenal glands account for most of the remainder. In the testes, testosterone is produced by the Leydig cells 61. The male testes also contain Sertoli cells, which require testosterone for spermatogenesis (sperm cell development). Luteinizing hormone (LH), in particular, acts on the Leydig cells to increase testosterone production. Testosterone limits its own secretion via negative feedback. High levels of testosterone in the blood feedback to the hypothalamus to suppress the secretion of GnRH and also feedback to the anterior pituitary, making it less responsive to GnRH stimuli 62. Throughout the reproductive life of males, the hypothalamus releases GnRH in pulses every 1 to 3 hours. Despite this pulsatile release, however, average plasma levels of FSH and LH remain fairly constant from the start of puberty, where levels spike, to the third decade of life, where levels peak and slowly begin to decline. Prior to puberty, testosterone levels are low, reflecting the low secretion of GnRH and gonadotropins. Changes in neuronal input to the hypothalamus and brain activity during puberty cause a dramatic rise in GnRH secretion.
Figure 3. Testosterone biosynthetic pathways
Footnotes: Biosynthetic pathways for testosterone and dihydrotestosterone (DHT) synthesis. The classic pathway show testosterone synthesized from cholesterol with further metabolism to dihydrotestosterone (DHT). The alternative or “backdoor” pathway shows DHT production without going through testosterone. Note only some of the enzymes are shown for clarity.
[Source 19 ]Figure 4. Age-related hypogonadism diagnostic algorithm
Abbreviations: TT = total testosterone; cFT = calculated free testosterone; PRL = prolactin; SHBG = sex hormone-binding globulin; LH = luteinising hormone; MRI = Magnetic resonance imaging.
[Source 3 ]Normal testosterone levels
A total testosterone test measures both bound and free testosterone in a sample of blood. Total testosterone is the most commonly used test for diagnostic and monitoring purposes and levels are commonly reported in nanograms per deciliter of blood (ng/dL). Less often, a testosterone test may be performed for free testosterone, which is reported in picograms per deciliter of blood (pg/dL). Another less common test is for bioavailable testosterone, which is testosterone that can be used more readily by the body. Bioavailable testosterone includes all testosterone that is not bound to sex-hormone-binding-globulin (SHBG) , including free testosterone and albumin-bound testosterone. Bioavailable testosterone is also commonly reported in nanograms per deciliter of blood (ng/dL).
To measure your testosterone level, your doctor can order a blood test. The blood test should be done in the morning between 7:00 AM and 10:00 AM.
Normal testosterone levels
- Normal total testosterone levels in males: 300 to 1,000 nanograms per deciliter (ng/dL) or 10 to 35 nanomoles per liter (nmol/L). The harmonized normal serum total testosterone range in a healthy nonobese population of European and American men, 19 to 39 years, is 264 to 916 ng/dL 63. Furthermore, in randomized testosterone trials, the men with testosterone levels below the harmonized threshold would be more likely to respond to testosterone therapy than those with testosterone levels above the harmonized threshold.
- 0-5 months: 75-400 ng/dL
- 6 months-9 years: <7-20 ng/dL
- 10-11 years: <7-130 ng/dL
- 12-13 years: <7-800 ng/dL
- 14 years: <7-1,200 ng/dL
- 15-16 years: 100-1,200 ng/dL
- 17-18 years: 300-1,200 ng/dL
- Over 19 years: 240-950 ng/dL
- Normal total testosterone levels in females: 15 to 70 ng/dL or 0.5 to 2.4 nmol/L
- 0-5 months: 20-80 ng/dL
- 6 months to 9 years: <7-20 ng/dL
- 10-11 years: <7-44 ng/dL
- 12-16 years: <7-75 ng/dL
- 17-18 years: 20-75 ng/dL
- Over 19 years: 8-60 ng/dL
- Normal free testosterone levels in males:
- 20-25 years: 5.25-20.7 ng/dL
- 25-30 years: 5.05-19.8 ng/dL
- 30-35 years: 4.85-19.0 ng/dL
- 35-40 years: 4.65-18.1 ng/dL
- 40-45 years: 4.46-17.1 ng/dL
- 45-50 years: 4.26-16.4 ng/dL
- 50-55 years: 4.06-15.6 ng/dL
- 55-60 years: 3.87-14.7 ng/dL
- 60-65 years: 3.67-13.9 ng/dL
- 65-70 years: 3.47-13.0 ng/dL
- 70-75 years: 3.28-12.2 ng/dL
- 75-80 years: 3.08-11.3 ng/dL
- 80-85 years: 2.88-10.5 ng/dL
- 85-90 years: 2.69-9.61 ng/dL
- 90-95 years: 2.49-8.76 ng/dL
- 95-100+ years: 2.29-7.91 ng/dL
- Normal free testosterone levels in females:
- 20-25 years: 0.06-1.08 ng/dL
- 25-30 years: 0.06-1.06 ng/dL
- 30-35 years: 0.06-1.03 ng/dL
- 35-40 years: 0.06-1.00 ng/dL
- 40-45 years: 0.06-0.98 ng/dL
- 45-50 years: 0.06-0.95 ng/dL
- 50-55 years: 0.06-0.92 ng/dL
- 55-60 years: 0.06-0.90 ng/dL
- 60-65 years: 0.06-0.87 ng/dL
- 65-70 years: 0.06-0.84 ng/dL
- 70-75 years: 0.06-0.82 ng/dL
- 75-80 years: 0.06-0.79 ng/dL
- 80-85 years: 0.06-0.76 ng/dL
- 85-90 years: 0.06-0.73 ng/dL
- 90-95 years: 0.06-0.71 ng/dL
- 95-100+ years: 0.06-0.68 ng/dL
Talk to your doctor about the meaning of your specific test results. Testosterone levels change from hour to hour. Testosterone levels tend to be highest in the morning and lowest at night. Testosterone levels are highest by age 20 to 30 and slowly go down after age 30 to 35. In addition, normal testosterone level ranges may vary slightly among different laboratories. Some labs use different measurements or test different specimens.
If the result is not normal, you should repeat the test to make sure of the result. In healthy men, testosterone levels can change a lot from day to day, so a second test could be normal.
What is the role of testosterone in men’s health?
Testosterone is a hormone produced primarily in the testicles. Testosterone is the most important sex hormone that men have. It is responsible for the typical male characteristics, such as facial, pubic, and body hair as well as muscle. Testosterone hormone also helps maintain sex drive, sperm production, and bone health. The brain and pituitary gland (a small gland at the base of the brain) control the production of testosterone by the testes.
Testosterone helps maintain men’s:
- Bone density
- Fat distribution
- Muscle strength and mass
- Facial and body hair
- Red blood cell production
- Sex drive
- Sperm production
Low testosterone / testosterone deficiency
Testosterone deficiency also known as male hypogonadism, is defined as having one or more symptoms (e.g. decreased libido, decreased erections, decreased energy, decreased physical stamina, decreased lean muscle mass) attributable to low circulating levels of testosterone (morning serum total testosterone less than 300 ng/dL on two separate occasions) 16, 64, 65. An estimated 25% of men have low levels of testosterone 66. Besides being fundamental for the development and maintenance of male characteristics and male sexual organs, testosterone also has effects on most major organs, such as the brain, muscle, kidney, bone, liver and skin 67. Recent studies have also suggested that testosterone deficiency is independently and robustly associated with various obesity-related chronic diseases in men, including type 2 diabetes, hypertension, cardiovascular disease, hyperlipidemia, asthma, chronic obstructive pulmonary disease (COPD) 68, 69. However, there is uncertainty as to what constitutes optimal physiological levels of total testosterone among men across different age categories, and to the effects that varying total testosterone levels have on disease risk 70. This debate is partially fueled by inconsistent findings in the literature and differences across expert clinical recommendations, as well as an incomplete understanding of the underlying mechanisms and temporal sequence of events leading to and stemming from testosterone deficiency. For example, the authors of a well-known systematic review 71 assert that although testosterone deficiency is robustly associated with obesity, insulin resistance, low high-density lipoprotein (HDL) cholesterol and elevated triglycerides, low-density lipoprotein (LDL) cholesterol, and plasminogen activator type 1, there is insufficient evidence to demonstrate a causal role of endogenous testosterone deficiency on coronary artery disease-the leading cause of preventable death among men in the U.S 72.
Incidence of hypogonadism (symptomatic low testosterone) increases with advancing male age such that levels of circulating testosterone decline annually by approximately 1% from the age of 40 years according to the European Male Ageing Study 30. When hypogonadism occurs in an older man, the condition is often called andropause or androgen deficiency of the aging male or late onset hypogonadism (LOH) 73. The most easily recognized clinical signs of late onset hypogonadism (andropause) in older men are a decrease in muscle mass and strength, a decrease in bone mass and osteoporosis, decreased libido, erectile dysfunction, anemia, increase in central body fat, and impaired memory, mood, concentration, and sleep 74, as well as increased mortality 75. Sexual symptoms and fatigue are the earliest and most common presentations 76. Other symptoms include depression, sleep alterations, poor concentration, and metabolic disorders are seen at borderline testosterone levels 77, 78. However, symptoms such as a decrease in libido and sexual desire, forgetfulness, loss of memory, anemia, difficulty in concentration, insomnia, and a decreased sense of well-being are more difficult to measure and differentiate from hormone-independent aging 79. Androgen deficiency of the aging male may result in significant detriment to quality of life and adversely affect the function of multiple organ systems 80.
The pathophysiology of male hypogonadism can be characterized into two types 81:
- Primary hypogonadism also known as primary testicular failure or hypergonadotropic hypogonadism. Primary hypogonadism is a testosterone deficiency due to a testicular abnormality impairing testosterone production; for example, Klinefelter syndrome, testicular tumors, infection, trauma, impaired Leydig cell function, or those caused by certain medications 82.
- Secondary hypogonadism also known as hypogonadotropic hypogonadism. Secondary hypogonadism is a testosterone deficiency due to a problem in the hypothalamus or the pituitary gland, parts of the brain that signal the testicles to produce testosterone (i.e., luteinizing hormone (LH) and follicle-stimulating hormone (FSH)); for example, hypothalamic or pituitary lesions, GnRH deficiency, or hyperprolactinemia 82. Another important cause of hypogonadism is late-onset hypogonadism, an age-related (i.e., >40 years) decline in testosterone accompanied by clinical symptoms that is often associated with obesity, diabetes, and other chronic health conditions 83.
Risk factors for hypogonadism include:
- HIV/AIDS
- Previous chemotherapy or radiation therapy
- Aging
- Obesity
- Malnutrition
Late-onset hypogonadism (andropause) has the combined features of both primary and secondary hypogonadism 84. The diagnosis of andropause (late-onset hypogonadism) requires the presence of characteristic signs and symptoms, in combination with decreased serum total testosterone. Based on the recent guidelines by the International Society for the Study of Aging Male (ISSAM), the European Association of Urology (EAU), the European Society of Endocrinology (ESE), the European Academy of Andrology (EAA), and the American Association of Urology (AUA), a total testosterone of 250–350 ng/dL is the proper threshold value to define low testosterone. The optimal indication for TRT in late-onset hypogonadism (andropause) is the presence of signs and symptoms of hypogonadism, and low testosterone without contraindications for TRT 85.
What causes low testosterone?
Testosterone deficiency also known as male hypogonadism, is defined as having one or more symptoms (e.g. decreased libido, decreased erections, decreased energy, decreased physical stamina, decreased lean muscle mass) attributable to low circulating levels of testosterone (morning serum total testosterone less than 300 ng/dL on two separate occasions) 16, 64, 65.
There are two basic types of hypogonadism:
- Primary. This type of hypogonadism — also known as primary testicular failure — originates from a problem in the testicles.
- Secondary. This type of hypogonadism indicates a problem in the hypothalamus or the pituitary gland — parts of the brain that signal the testicles to produce testosterone. The hypothalamus produces gonadotropin-releasing hormone, which signals the pituitary gland to make follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Luteinizing hormone then signals the testes to produce testosterone.
Either type of hypogonadism can be caused by an inherited (congenital) trait or something that happens later in life (acquired), such as an injury or an infection. At times, primary and secondary hypogonadism occur together.
Primary hypogonadism
Common causes of primary hypogonadism include:
- Klinefelter syndrome also known as 47,XXY or XXY male. Klinefelter syndrome results from a congenital abnormality of the sex chromosomes, X and Y. A male normally has one X and one Y chromosome. In Klinefelter syndrome, two or more X chromosomes are present in addition to one Y chromosome. The Y chromosome contains the genetic material that determines the sex of a child and related development. The extra X chromosome typically affects physical, neurodevelopmental, behavioral, and neurocognitive functioning. Common physical features may include tall stature, reduced muscle tone, abnormal development of the testicles (hypogonadism), which in turn results in underproduction of testosterone with delayed pubertal development and lack of secondary male sex characteristics such as decreased facial and body hair. Increased breast growth (gynecomastia) may occur later in puberty without appropriate biological care.
- Undescended testicles (cryptorchidism). Before birth, the testicles develop inside the abdomen and normally move down into their permanent place in the scrotum. Sometimes one or both of the testicles aren’t descended at birth. This condition often corrects itself within the first few years of life without treatment. If not corrected in early childhood, it can lead to malfunction of the testicles and reduced production of testosterone.
- Mumps orchitis. A mumps infection involving the testicles that occurs during adolescence or adulthood can damage the testicles, affecting the function of the testicles and testosterone production.
- Hemochromatosis. Too much iron in the blood can cause testicular failure or pituitary gland dysfunction, affecting testosterone production.
- Injury to the testicles. Because they’re outside the abdomen, the testicles are prone to injury. Damage to both testicles can cause hypogonadism. Damage to one testicle might not impair total testosterone production.
- Cancer treatment. Chemotherapy or radiation therapy for the treatment of cancer can interfere with testosterone and sperm production. The effects of both treatments often are temporary, but permanent infertility may occur. Although many men regain their fertility within a few months after treatment, preserving sperm before starting cancer therapy is an option for men.
Secondary hypogonadism
In secondary hypogonadism, the testicles are normal but don’t function properly due to a problem with the pituitary or hypothalamus. A number of conditions can cause secondary hypogonadism, including:
- Kallmann’s syndrome. This is an abnormal development of the area of the brain that controls the secretion of pituitary hormones (hypothalamus). This abnormality can also affect the ability to smell (anosmia) and cause red-green color blindness.
- Pituitary disorders. An abnormality in the pituitary gland can impair the release of hormones from the pituitary gland to the testicles, affecting normal testosterone production. A pituitary tumor or other type of brain tumor located near the pituitary gland may cause testosterone or other hormone deficiencies. Also, treatment for a brain tumor, such as surgery or radiation therapy, can affect the pituitary gland and cause hypogonadism.
- Inflammatory disease. Certain inflammatory diseases, such as sarcoidosis, histiocytosis and tuberculosis, involve the hypothalamus and pituitary gland and can affect testosterone production.
- HIV/AIDS. HIV/AIDS can cause low levels of testosterone by affecting the hypothalamus, the pituitary and the testes.
- Medications. The use of certain drugs, such as opiate pain medications and some hormones, can affect testosterone production.
- Obesity. Being significantly overweight at any age might be linked to hypogonadism.
- Aging. As men age, there’s a slow, progressive decrease in testosterone production. The rate varies greatly.
As men age, there’s a slow, progressive decrease in testosterone or “T” production. Blood levels of testosterone peak in 20 to 30 year-old males and then decline with age with average rate of decline of 1.6% per year 23, 24, 25, 26, 27, 28, 29. One study found that serum testosterone levels were below the normal range in 20% of men in their 60s and in close to 50% of men in their 80s 26. However, the prevalence of symptomatic low testosterone (hypogonadism) is estimated by some to be much lower in this population, at about 2% 30. Longitudinal studies demonstrated a gradual decline in total serum testosterone levels in men from the age of 40, with 20% and 30% of men meeting age-related low testosterone criteria in their 60s and 70s 31, 32.
Risk factors for hypogonadism include:
- HIV/AIDS. HIV/AIDS can cause low levels of testosterone by affecting the hypothalamus, the pituitary and the testes.
- Medications. The use of certain drugs, such as opiate pain medications and some hormones, can affect testosterone production.
- Previous chemotherapy or radiation therapy. Chemotherapy or radiation therapy for the treatment of cancer can interfere with testosterone and sperm production. The effects of both treatments often are temporary, but permanent infertility may occur. Although many men regain their fertility within a few months after treatment, preserving sperm before starting cancer therapy is an option for men.
- Obesity. Being significantly overweight at any age might be linked to hypogonadism.
- Undescended testicles (cryptorchidism). Before birth, the testicles develop inside the abdomen and normally move down into their permanent place in the scrotum. Sometimes one or both of the testicles aren’t descended at birth. This condition often corrects itself within the first few years of life without treatment. If not corrected in early childhood, it can lead to malfunction of the testicles and reduced production of testosterone.
- Mumps orchitis. A mumps infection involving the testicles that occurs during adolescence or adulthood can damage the testicles, affecting the function of the testicles and testosterone production.
- Hemochromatosis. Too much iron in the blood can cause testicular failure or pituitary gland dysfunction, affecting testosterone production.
Injury to the testicles. Because they’re outside the abdomen, the testicles are prone to injury. Damage to both testicles can cause hypogonadism. Damage to one testicle might not impair total testosterone production. - Malnutrition or nutritional deficiencies.
- Pituitary disorders. An abnormality in the pituitary gland can impair the release of hormones from the pituitary gland to the testicles, affecting normal testosterone production. A pituitary tumor or other type of brain tumor located near the pituitary gland may cause testosterone or other hormone deficiencies. Also, treatment for a brain tumor, such as surgery or radiation therapy, can affect the pituitary gland and cause hypogonadism.
- Inflammatory disease. Certain inflammatory diseases, such as sarcoidosis, histiocytosis and tuberculosis, involve the hypothalamus and pituitary gland and can affect testosterone production.
- Kallmann’s syndrome. This is an abnormal development of the area of the brain that controls the secretion of pituitary hormones (hypothalamus). This abnormality can also affect the ability to smell (anosmia) and cause red-green color blindness.
- Klinefelter syndrome. Klinefelter syndrome results from a congenital abnormality of the sex chromosomes, X and Y. A male normally has one X and one Y chromosome. In Klinefelter syndrome, two or more X chromosomes are present in addition to one Y chromosome. The Y chromosome contains the genetic material that determines the sex of a child and related development. The extra X chromosome that occurs in Klinefelter syndrome causes abnormal development of the testicles, which in turn results in underproduction of testosterone.
Hypogonadism can be inherited. If any of these risk factors are in your family health history, tell your doctor.
What happens to testosterone levels with age?
Testosterone levels generally peak during adolescence and early adulthood. As you get older, your testosterone level gradually declines — typically about 1 percent a year after age 30 or 40. It is important to determine in older men if a low testosterone level is simply due to the decline of normal aging or if it is due to a disease (hypogonadism).
Hypogonadism is a disease in which the body is unable to produce normal amounts of testosterone due to a problem with the testicles or with the pituitary gland that controls the testicles. Testosterone replacement therapy can improve the signs and symptoms of low testosterone in these men. Doctors may prescribe testosterone as injections, pellets, patches or gels.
In the short term, low testosterone also called hypogonadism can cause:
- A drop in sex drive
- Poor erections
- Low sperm count
- Enlarged breasts
Over time, low testosterone may cause a man to lose body hair, muscle bulk, and strength and to gain body fat. Chronic (long-term) low testosterone may also cause weak bones (osteoporosis), mood changes, less energy, and smaller testes. Signs and symptoms (what you see and feel) vary from person to person.
Does a naturally declining testosterone level cause the signs and symptoms of aging?
Not necessarily. Men can experience many signs and symptoms as they age. Some may occur as a result of lower testosterone levels and can include:
- Changes in sexual function. This may include reduced sexual desire, fewer spontaneous erections — such as during sleep — and infertility.
- Changes in sleep patterns. Sometimes low testosterone causes insomnia or other sleep disturbances.
- Physical changes. Various physical changes are possible, including increased body fat, reduced muscle bulk and strength, and decreased bone density.
- Swollen or tender breasts (gynecomastia) and body hair loss are possible. You may have less energy than you used to.
- Emotional changes. Low testosterone may contribute to a decrease in motivation or self-confidence. You may feel sad or depressed, or have trouble concentrating or remembering things.
Some of these signs and symptoms can be caused by various underlying factors, including medication side effects, obstructive sleep apnea, thyroid problems, diabetes and depression. It’s also possible that these conditions may be the cause of low testosterone levels, and treatment of these problems may cause testosterone levels to rise. A blood test is the only way to diagnose a low testosterone level.
How is low testosterone diagnosed?
During a physical exam, your doctor will examine your body hair, breasts, penis, and the size and consistency of the testes and scrotum. Your doctor may check for loss of side (peripheral) vision, which could indicate a pituitary tumor, a rare cause of low testosterone.
Your doctor will also use blood tests to see if your total testosterone level is low. The normal range is generally 300 to 1,000 ng/dL, but this depends on the lab that conducts the test. To get a diagnosis of low testosterone, you may need more than one early morning (7–10 AM) blood test and, sometimes, tests of pituitary gland hormones.
If you have symptoms of low testosterone, your doctor may suggest that you talk with an endocrinologist. This expert in hormones can help find the cause. Be open with your doctor about your medical history, all prescription and nonprescription drugs you are now taking, sexual problems, and any major changes in your life.
Hypogonadism is diagnosed when the morning serum testosterone level is less than 300 ng/dL 6. However, clinical judgment is required when making the diagnosis of hypogonadism in a patient who has testosterone levels in the normal range but has persistent symptoms of testosterone deficiency 86. Of note, total testosterone less than 405.9 ng/dL is below the fifth percentile 87. In older men, one should aim for testosterone levels between 500 and 800 ng/dL while young adults should aim for testosterone levels between 600 and 900 ng/dL 6.
Initial laboratory testing should include 2 early mornings (8 AM to 10 AM) measurements of serum testosterone. If both measurements are low, then certain studies should be ordered to rule out secondary hypogonadism. Further testing includes FSH (follicle-stimulating hormone), LH (luteinizing hormone), prolactin, TSH (thyroid-stimulating hormone), complete blood count (CBC), and comprehensive metabolic panel. In cases of low normal testosterone with clinical symptoms, further testing to assess free or bioavailable testosterone should be done. These tests include sex hormone binding globulin (SHBG) and albumin to calculate the bioavailable testosterone which can be affected by obesity, type 2 diabetes, hypothyroidism, and liver disease.
Furthermore, semen analysis, pituitary MRI, testicular ultrasound and biopsy, and genetic studies can be ordered, if there is clinical suspicion of a secondary cause.
Medical History
Specific symptoms associated with hypogonadism, including age-related low testosterone, are shown in Table 1. These symptoms are non-specific and need to be recorded and taken in context with the clinical and biochemical state. Several self-reported questionnaires or structural interviews have been developed for screening of hypogonadism. Although these case-history tools have demonstrated clinical utility in supporting the biochemical diagnosis of hypogonadism, or in the assessment of testosterone therapy outcomes, their specificity remains poor and they should not be used for a systematic screening of hypogonadal men 88. Headache and/or visual disturbance may indicate a pituitary-related disorder. History of surgical intervention for undescended testicle (cryptorchidism) or hypospadias (a condition in which the urethral opening of the penis is on the underside rather than at the tip) must be taken into account as possible signs of congenital defects. Chronic and systemic diseases or medical conditions must be comprehensively investigated in every patient. Use of drugs that potentially interfere with the hypothalamic-pituitary-gonadal axis (HPG axis) should be excluded. Acute diseases are associated with development of functional hypogonadism and determination of serum total testosterone levels should be avoided in these conditions; however, the role of testosterone in the case of acute illness remains to be clarified 89, 90, 91. Fertility issues should be always discussed.
Table 1. Age-related hypogonadism symptoms
| Sexual symptoms | Physical symptoms | Psychological symptoms | |
|---|---|---|---|
| More specific | Reduced libido Erectile dysfunction Decreased spontaneous/morning erections | Decreased vigorous activity Difficulty walking > 1 km Decreased bending | Low mood/mood deflection Decreased motivation Fatigue |
| Less specific | Reduced frequency of sexual intercourse Reduced frequency of masturbation Delayed ejaculation | Hot flushes Decreased energy Decreased physical strength/function/ activity | Concentration difficulties Sleep disturbances |
Physical Examination
Since obesity is frequently associated with hypogonadism (mostly functional), the determination of body mass index (BMI) and the measurement of waist circumference are strongly recommended in all individuals. Testicular and penile size, as well as the presence of sexual secondary characteristics can provide useful information regarding overall androgen status. In addition, upper segment/lower segment ratio (n.v. > 0.92) and arm-span to height ratio (n.v. < 1.0) can be useful to identify a eunuchoid body shape, especially in subjects with pre-pubertal hypogonadism or delayed puberty. Finally, digital rectal examination (DRE) should be performed in all subjects to exclude prostate abnormalities before testosterone therapy (any type) or to support suspicion of hypogonadism (in case of reduced volume) 92.
Laboratory tests
Testosterone levels are produced in a circadian variation being highest in the morning and lowest at night, which may persist in ageing men 93, 94. Testosterone levels are also potentially influenced by food intake 41. Therefore, serum total testosterone should be measured in fasting conditions in the morning (between 7 a.m. and 11 a.m.). Testosterone levels can also be temporarily lower due to too much exercise, poor nutrition, severe illness, and with certain medications. A confirmatory measurement should always be undertaken in the case of a abnormal value, and before starting any testosterone therapy.
Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) represents the most accurate method for sex steroid evaluation; however, standardised automated platform immuno-assays for total testosterone assessment demonstrate a good correlation with Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) 95. Available immuno-assays are not able to provide an accurate estimation of free testosterone; therefore, direct free testosterone evaluation with these methods is not recommended and should be avoided 96. Equilibrium dialysis is the most accurate method for total testosterone measurement and free testosterone calculation 97. Alternatively, free testosterone can be derived from specific mathematical calculations using total testosterone as derived by common immunoassays and taking into account serum sex hormone binding globulin (SHBG) and albumin levels 98.
Low testosterone levels in men is defined as total testosterone < 350 ng/dL and free testosterone < 225 pmol/L and are associated with sexual dysfunction such as low sexual desire (low libido), erectile dysfunction (fewer or diminished spontaneous erections, decreased nocturnal penile tumescence), reduced skeletal muscle mass and strength, decreased bone mineral density, sparse beard growth, shrinking testes, increased cardiovascular risk and alterations of the glycometabolic profile 34, 20, 35, 36, 37. The European Urological Association recommends a total testosterone level < 300 ng/dL as the diagnosis of hypogonadism 38, the American Urological Association considers 3.5 ng/mL (12 nmol/L) total testosterone represents a reliable threshold to diagnose age-related hypogonadism 39, and the Endocrine Society recommends the reasonable cut-off of total testosterone in men as 264 ng/dL (9.2 nmol/L) 40. This variation total testosterone level diagnostic criteria underscores the lack of uniformity in defining age-related low testosterone across different medical governing bodies.
Data from meta-analyses have shown that testosterone therapy is ineffective when baseline levels are > 3.5 ng/mL (>12 nmol/L). Positive outcomes are documented when testosterone levels are <3.5 ng/mL (<12 nmol/L), being higher in symptomatic patients with more severe forms of hypogonadism (< 8 nmol/L). Hence, 3.5 ng/mL (12 nmol/L) should be considered as a possible threshold for starting testosterone therapy in the presence of hypogonadal symptoms 59, 60.
In clinical conditions that may interfere with sex-hormone-binding-globulin (SHBG) levels, evaluation of free testosterone should be considered to better estimate actual androgen levels. Unfortunately, despite free testosterone potential clinical value 42, no validated thresholds for free testosterone are available from clinical studies and this represents an area of uncertainty; however, data from the European Male Aging Study indicated that free testosterone levels < 220 pmol/L (6.4 ng/dL) increased the likelihood to correct identify hypogonadism as compared with total testosterone level alone, particularly when total testosterone levels are between 8.0 and 11 nmol per liter 43, 44, 30.
The determination of luteinizing hormone (LH) must be performed along with prolactin (PRL) when pathological total testosterone levels are detected, in order to correctly define the underlying conditions and exclude possible organic causes. Follicle-stimulating hormone (FSH) determination can further support the diagnosis of primary or secondary hypogonadism 2, 99. Due to prolactin (PRL) negative influence on libido, prolactin (PRL) can also be considered as first-line screening in patients with reduced sexual desire. In addition, contrast-enhanced pituitary magnetic resonance imaging (MRI) scanning, as well as other pituitary hormone evaluations, is required in the presence of specific symptoms such as visual disturbances, headache and when hyperprolactinemia is confirmed 100, 101. Limited evidence suggests also performing pituitary MRI in the case of severe hypogonadism (< 6 nmol/L, 1.75 ng/mL) with inadequate luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels 100, 101, 102.
How is low testosterone treated?
Testosterone replacement therapy (TRT), a generally accepted method treating for age-related low testosterone, can improve the symptoms of age-related low testosterone and is most usually administered by injection, oral administration and transdermal administration 55. Many studies have reported the benefit of testosterone replacement therapy (TRT) for age-related hypogonadism 56. However, some studies revealed that symptoms related to age-related hypogonadism may not be correlated with serum total testosterone levels 57, 58.
The American College of Physicians (ACP) recently issued new guidelines on prescribing supplemental testosterone for men with age-related low testosterone 53:
- The American College of Physicians suggests that clinicians discuss whether to initiate testosterone treatment in men with age-related low testosterone with sexual dysfunction who want to improve sexual function (conditional recommendation; low-certainty evidence). The discussion should include the potential benefits, harms, costs, and patient’s preferences.
- The American College of Physicians suggests that clinicians should reevaluate symptoms within 12 months and periodically thereafter. Clinicians should discontinue testosterone treatment in men with age-related low testosterone with sexual dysfunction in whom there is no improvement in sexual function (conditional recommendation; low-certainty evidence).
- The American College of Physicians suggests that clinicians consider intramuscular rather than transdermal formulations when initiating testosterone treatment to improve sexual function in men with age-related low testosterone, as costs are considerably lower for the intramuscular formulation and clinical effectiveness and harms are similar.
- The evidence shows that men with age-related low testosterone may experience slight improvements in sexual function by 35% and erectile dysfunction by 27% if prescribed supplemental testosterone.
- The evidence does not support prescribing testosterone for men with concerns about energy, vitality, physical function, or cognition.
- The new guidelines point out that treating with supplemental testosterone is not risk free. Potential risks include blood clots, stroke, sleep apnea, acne and possibly enlarged breasts (gynecomastia). Men with these or other conditions may be advised against using supplemental testosterone even for sexual dysfunction.
- If men are treated with supplemental testosterone for sexual dysfunction, the decision to continue to treat should be reevaluated after a year and then periodically after that. Treatment should be stopped if sexual dysfunction doesn’t improve, according to the new guidelines.
Data from meta-analyses have shown that testosterone replacement therapy is ineffective when baseline testosterone levels are > 3.5 ng/mL (>12 nmol/L). Positive outcomes are documented when testosterone levels are < 3.5 ng/mL (<12 nmol/L), being higher in symptomatic patients with more severe forms of hypogonadism (< 8 nmol/L). Therefore, patients with symptomatic hypogonadism without specific contraindications where testosterone levels are less than 3.5 ng/mL (<12 nmol/L) should be considered for starting testosterone therapy 59, 60.
There are potential risks with long-term use of testosterone. You should discuss with your physician how to monitor for prostate cancer and other risks to your prostate. Men with known or suspected prostate cancer, or with breast cancer, should not receive testosterone treatment.
Other possible risks of testosterone treatment include:
Testosterone therapy carries various risks, including:
- Increased production of red blood cells
- Acne
- Enlarged breasts
- Sleep disturbances
- Prostate enlargement
- Limited sperm production
- Sleep apnea—the occasional stopping of breathing during sleep (rarely)
- Fluid buildup (edema) in ankles, feet and legs (rarely)
Can testosterone replacement therapy promote youth and vitality?
Testosterone replacement therapy can help reverse the effects of hypogonadism, but it’s unclear whether testosterone replacement therapy would have any benefit for older men who are otherwise healthy.
Although some men believe that taking testosterone medications may help them feel younger and more vigorous as they age, few rigorous studies have examined testosterone therapy in men who have healthy testosterone levels. And some small studies have revealed mixed results. For example, in one study healthy men who took testosterone medications increased muscle mass but didn’t gain strength.
Should I talk to my doctor about testosterone replacement therapy?
If you wonder whether testosterone therapy might be right for you, talk with your doctor about the risks and benefits. Your doctor will likely measure your testosterone levels at least twice before discussing whether testosterone replacement therapy is an option for you.
A medical condition that leads to an unusual decline in testosterone may be a reason to take supplemental testosterone. However, treating normal aging with testosterone therapy is not currently advisable.
Your doctor will also likely suggest natural ways to boost testosterone, such as losing weight and increasing muscle mass through resistance exercise.
Benefits of testosterone replacement therapy for men
- Low testosterone comes with age — testosterone levels naturally decrease by 1% each year after age 30, though don’t severely deplete, even in advanced age
- Testosterone production may be disrupted by disorders of the testicles, pituitary gland, or brain
- Testosterone levels change from hour to hour — highest in the morning; lowest at night
- Testosterone levels can temporarily lower due to too much exercise, poor nutrition, severe illness, and with certain medications
- Normal testosterone levels should be between 300–1,000 ng/dL (nanograms per deciliter), depending on age and lab used
- Testosterone must be measured more than once for accurate assessment
Testosterone therapy is only recommended for hypogonadism patients. Boosting testosterone is NOT approved by the US Food and Drug Administration (FDA) to help improve your strength, athletic performance, physical appearance, or to treat or prevent problems associated with aging. Using testosterone for these purposes may be harmful to your health.
Male hypogonadism is a combination of low testosterone levels and the presence of any of these symptoms:
- Drop in sex drive (libido)
- Erectile dysfunction (ED — inability to get or keep an erection) and loss of spontaneous erections
- Lowered sperm count and infertility (inability to have children)
- Breast enlargement or tenderness
- Reduced energy
- Reduced muscle mass
- Shrinkage of testes
- Increased irritability, inability to concentrate, and depressed mood
- Hot flashes (when testosterone levels are very low)
You should NOT receive testosterone therapy if you have:
- Prostate or breast cancer (or suspected)
- Enlarged prostate causing difficulty with urination
- Elevated prostate specific antigen (PSA) levels
- High number of red blood cells
- Untreated sleep apnea (obstructed breathing during sleep)
- Planning to have children
- Heart attack or stroke within the last 6 months
- Blood clots
Testosterone replacement therapy types
Several testosterone formulations are available. Direct comparisons among different testosterone products are still lacking. Patients who are considering testosterone therapy should be adequately informed about the possible risks and benefits of all available testosterone preparations. The final choice should be based on the clinical situation, testosterone formulation availability, and patient needs and expectations 103, 104.
One Food and Drug Administration-approved oral testosterone replacement preparation, testosterone undecanoate (Jatenzo), is absorbed by the lymph system. It might avoid the liver problems seen with other oral forms of testosterone.
Other preparations you might choose, depending on convenience, cost and your insurance coverage, include:
- Testosterone Gel. There are several gels and solutions available, with different ways of applying them. Depending on the brand, you rub the testosterone into your skin on your upper arm or shoulder (AndroGel, Testim, Vogelxo) or apply it to the front and inner thigh (Fortesta). Your body absorbs testosterone through your skin. Don’t shower or bathe for several hours after a gel application, to be sure it gets absorbed. Side effects include skin irritation and the possibility of transferring the medication to another person. Avoid skin-to-skin contact until the gel is completely dry, or cover the area after an application.
- Testosterone Injection. Testosterone cypionate (Depo-Testosterone) and testosterone enanthate are given in a muscle or under the skin. Your symptoms might waver between doses depending on the frequency of injections. You or a family member can learn to give testosterone injections at home. If you’re uncomfortable giving yourself injections, member of your care team can give the injections.
- Testosterone undecanoate (Aveed) is given by deep intramuscular injection, typically every 10 weeks. It must be given at your provider’s office and can have serious side effects.
- Testosterone Patch. A patch containing testosterone (Androderm) is applied each night to your thighs or torso. A possible side effect is severe skin reaction.
- Testosterone Pellets (Testopel): Testosterone-containing pellets are surgically implanted under the skin every three to six months for consistent and long-term dosages. Side effects: pellet coming out through skin, site infection/ bleeding (rare), dose decreasing over time and hypogonadism symptoms possibly returning towards the end of dose period.
- Testosterone Gum and Cheek (buccal cavity). A small putty-like substance, gum-and-cheek testosterone replacement delivers testosterone through the natural depression above your top teeth where your gum meets your upper lip (buccal cavity). This product, taken three times a day, sticks to your gumline and allows testosterone to be absorbed into your bloodstream. It can cause gum irritation.
- Testosterone Nasal. This testosterone gel (Natesto) can be pumped into the nostrils. This option reduces the risk that medication will be transferred to another person through skin contact. Nasal-delivered testosterone must be applied twice in each nostril, three times daily, which might be more inconvenient than other delivery methods.
Table 2. Testosterone replacement therapy formulations
| Names | Strengths | Limitations | Comparative Efficacy | References |
|---|---|---|---|---|
| Testosterone undecanoate |
|
|
| Nieschlag 2015 106, Swerdloff et al. 2020 107 |
| Striant (testosterone buccal) |
|
| Tsametis & Isidori 2018 110, Dinsmore & Wyllie 2012 111 | |
Long acting testosterone:
Extra-long acting testosterone:
|
|
|
| Nieschlag 2015 106, Tsametis & Isidori 2018 110, Middleton et al. 2015 113 |
Testosterone Gel:
Testosterone Solution:
Testosterone Patch:
|
|
|
| Tsametis & Isidori 2018 110 |
Testosterone implant:
|
|
| Tsametis & Isidori 2018 110 | |
Testosterone nasal:
|
|
|
| Tsametis & Isidori 2018 110, Gronski et al. 2019 118, Rogol et al. 2018 119 |
Testosterone Oral formulations
An oral formulation has been available in oleic acid since the 1970s, and has been recently reformulated in a mixture of castor oil and propylene glycol laureate (testosterone undecanoate [TU] caps), to allow the drug to be maintained at room temperature without degradation 103, 104. The main limitation is related to poor bioavailability, which is strongly dependent on dietary fat content 103, 104. The US Food and Drug Administration (FDA) approved a new formulation of oral testosterone undecanoate in a liquid-filled soft gel capsule, which improved oral availability 120. Available evidence showed that testosterone undecanoate capsule formulations can reach steady 24-hour average serum testosterone levels in more than 80% of hypogonadal men, thus resulting in a significant improvement of all sexual function domains at all time points when compared to baseline along with an excellent safety profile 120. More recently, the FDA has approved a new oral formulation which contains as carriers Vitamin E, phytosterol esters, polyoxyl 40 hydrogenated castor oil and propylene glycol monolaurate 120. For all new oral testosterone undecanoate formulations a mild increase in arterial blood pressure has been reported. Hence, the FDA has required a black box warning that these drugs can induce a blood pressure increase 120.
Mesterolone is a 5α-dihydrotestosterone (DHT) derivate available for oral administration. Along with DHT, mesterolone cannot be converted to estrogens and can only be used for a limited period and for specific indications, such as the presence of painful gynecomastia. However, the lack of a full spectrum of testosterone bioactivity strongly limits its long-term use 103.
Testosterone Injectable formulations
Injectable testosterone preparations can be classified according to their half-lives. Testosterone propionate is a short-term ester formulation requiring multiple fractionated doses (usually 50-100 mg, every two to three days), thus representing a major limitation for its use 103, 104. Cypionate and enanthate-testosterone esters are short-term formulations, requiring administration every two to four weeks. A formulation containing mixed testosterone esters (testosterone undecanoate, isocaproate, phenyl propionate, propionate) which has the benefit of a steady release of testosterone into the circulation, is available in some countries. The use of these older formulations is associated with wide fluctuations in plasma testosterone concentrations and is often reported as unpleasant by patients potentially resulting in adverse effects, such as polycythaemia 103, 104, 121. A longer-lasting testosterone undecanoate injectable formulation is widely available 103, 104, with a good safety/benefit profile allowing the maintenance of normal stable testosterone levels at a dose of 1,000 mg initially every twelve weeks, following a six-week loading dose, but can be adjusted to a frequency of ten to fourteen weeks dependent on the trough (pre-injection level) after three to five injections to maintain levels in the therapeutic range (usually > 12 and < 18 nmol/L) 103, 104, 122.
Testosterone Transdermal preparations
Among the available transdermal formulations, testosterone gels represent the most frequently used preparations. The testosterone gel is quickly absorbed by the stratum corneum, creating a reservoir within the subcutaneous tissues from where testosterone is continuously delivered for 24 hours, after a single daily application. These formulations have been shown to normalise serum testosterone levels with an excellent safety profile 103, 104, 122. The introduction of specific devices and skin enhancers has resulted in better skin penetration of the drugs, thus reducing potential adverse effects. Local skin adverse effects are limited when compared to those with traditional testosterone patches, but they potentially allow transference of testosterone during close contact with the skin surface. The risk can be reduced by wearing clothing or by applying the gel on skin surfaces not usually touched (e.g., the inner thigh surface) 103, 104. To reduce the total amount of gel applied and residual quantities remaining on the skin, new formulations of testosterone gel have been introduced with a testosterone concentration of 1.62-2% 103, 104. Another transdermal testosterone formulation includes a topical, alcohol-based testosterone (2%) solution, which must be applied to the underarm once daily, using a metered dose applicator 103, 104. Testosterone levels should be monitored to optimize the testosterone dose. Blood collection is best taken two to four hours after gel application to use the peak level of testosterone absorbed as a reference for adequate therapeutic levels. Levels of testosterone after application can vary and a repeat measurement may be indicated especially as sometimes, inadvertently, the skin over the venipuncture site can be contaminated by the gel, leading to falsely elevated results.
In some European countries, dihydrotestosterone (DHT) is available as a hydroalcoholic 2.5% gel. It is rapidly absorbed, reaching a steady state in two to three days 103, 104. Similar to that reported for mesterolone, dihydrotestosterone (DHT) is not aromatised but can be useful for treating particular conditions, such as gynecomastia and microphallus 103, 104.
Testosterone Transmucosal formulations
A testosterone buccal system is still available in several countries. It consists of a sustained-release muco-adhesive buccal-testosterone-tablet requiring twice-daily application to the upper gums. The tablet does not dissolve completely in the mouth and must be removed after twelve hours. This formulation has been proven to restore testosterone levels within the physiological range with minimal or transient local problems, including gum oedema, blistering and gingivitis 103, 104.
A gel for intranasal administration is available in some countries, including the USA and Canada. It requires administration two or three times daily using a specific metered-dose pump. The application is rapid, non-invasive, and convenient, and avoids secondary transference observed with other topical products 103, 104. Preliminary results suggest that intranasal testosterone is associated with lower suppression of gonadotropin levels and with a lower risk of hematocrit increases 123.
Testosterone Subdermal depots
The implantation of testosterone pellets, available in a limited number of countries, represents the longest available testosterone formulation lasting from four to seven months. The procedure is invasive and may be unattractive to patients 103, 104.
Therapies that Increase Endogenous Testosterone
Mechanism of action: Multiple mechanisms of action by affecting various points along the hypothalamic-pituitary-gonadal axis to increase
Types: Selective estrogen receptor modulators (SERMs), Gonadotropins, Aromatase inhibitors, Selective androgen receptor modulators (SARMs), Leydig stem cell transplantation
- Advantages:
- Able to increase serum and intratesticular testosterone levels
- Greater likelihood of preserving hypothalamic-pituitary-gonadal axis axis function and, subsequently, spermatogenesis and fertility
- Limitations:
- Potential unintended decline in quality of semen and complications related to changes in estrogen levels
- None have been approved by the US FDA specifically for men with hypogonadism or infertility.
Table 3. Therapies that increase endogenous testosterone
| Class | Mechanism of Action | Strengths | Limitations | References |
|---|---|---|---|---|
| Selective Estrogen Receptor Modulators (SERMs) |
|
|
| El Meliegy et al. 2018 124, Chehab et al. 2015 125 |
| Gonadotropins |
|
|
| Lee & Ramasamy 2018 126, El Meliegy et al. 2018 124, Dohle et al. 2016 127 |
| Aromatase Inhibitors |
|
|
| Crosnoe et al. 2013 128, El Meliegy et al. 2018 124, Ribeiro et al. 2016 129 |
| Selective Androgen Receptor Modulators (SARMs) |
|
|
| Narayanan et al. 2018 130, Solomon et al. 2018 131 |
| Leydig Stem Cell Transplantation |
|
|
| Zang et al. 2017 132, Beattie et al. 2015 133, Arora et al. 2019 134 |
Selective estrogen receptor modulators
Selective estrogen receptor modulators (SERMs) are a group of pharmaceuticals that act as competitive inhibitors of estrogen receptors in the hypothalamus and pituitary with the increasing release of GnRH and gonadotrophins, subsequently increasing production of intra-testicular testosterone and improving spermatogenesis 135. Clomiphene citrate is a SERM that has been used in the treatment of men with azoospermia, oligozoospermia, and unexplained infertility, in addition to treating male hypogonadism. It has been demonstrated that Clomiphene citrate leads to a moderate increase in FSH, LH, total testosterone, and the concentration of sperm in these men 136. Also, in men with late-onset hypogonadism, long-term Clomiphene citrate therapy can be prescribed as a testosterone replacement 135. Although thromboembolic events and carcinogenesis have been reported as adverse effects of SERMs 137, current ongoing research shows that this group of pharmaceuticals has great potential for future applications 138.
Gonadotropins
Gonadotropin therapy is used to manage infertile patients with low testosterone concentrations, with the aim of recovering normal sperm production. In such patients, stimulating spermatogenesis requires human chorionic gonadotropin (hCG) therapy alone or in combination with human menopausal gonadotrophins (hMGs), urinary FSH, or recombinant FSH 135. It has been recommended that TRT with concomitant use of hCG can be prescribed in hypogonadal men who seek fertility treatment 139. Treatment with hCG can preserve normal sperm production in men undergoing TRT by maintaining intratesticular testosterone concentrations 140. Potential side effects of gonadotropin therapy include gynecomastia, headache, fatigue, and mood changes 141. A retrospective study found that treatment of hypogonadal men with TRT and co-administration of low dose hCG maintains semen parameters in these men 142. Coviello et al. 143 evaluated intratesticular testosterone concentrations from men with normal reproductive physiology who received testosterone enanthate in combination with hCG (125, 250, or 500 IU). A linear increase in intratesticular testosterone concentrations with increased hCG confirms that moderately low dose hCG preserves testicular function in endogenous gonadotropin-suppressed healthy men 143. A systematic review showed that hypogonadal men desiring fertility preservation while benefiting from TRT can be prescribed SERMs or testosterone plus a low dose of hCG. Prescription of hCG alone or in combination with FSH preparations can be recommended to hypogonadotropic hypogonadal patients who need to be treated for infertility 135. In younger men with low serum testosterone levels, treatment with hCG or with Clomiphene citrate will increase serum and intra-testicular testosterone levels without resulting in declining gonadotropin levels via feedback inhibition 128. Overall, human chorionic gonadotropin (hCG) is an efficient and safe alternative or adjunct to TRT in men desiring both fertility and treatment for symptoms of hypogonadism. However, a drawback of hCG is that this is an injectable agent.
Aromatase inhibitors
Aromatase inhibitors are a class of drugs that act by blocking aromatase P450 enzymes, consequently normalizing the testosterone/E2 ratio and improving spermatogenesis 124. It has been shown that an aromatase inhibitor (Testolactone) is an effective drug for alleviating infertility in hypogonadotropic hypogonadal obese men 125. However, Testolactone is no longer commercially available. Some men with severe spermatogenic failure who have too much aromatase activity, decreased serum levels of testosterone, increased E2 levels, or impaired testosterone/E2 ratio might benefit from the use of aromatase inhibitors in treating infertility 135. Although no important side effects have been observed with consumption of an aromatase inhibitor for elevating serum testosterone concentrations (46), one study has reported a slight insignificant increase in the level of serum prostate-specific antigen (PSA) in elderly men with low or borderline hypogonadism 144. However, the use of aromatase inhibitors is not suggested due to the absence of long-term data and a limited amount of published literature 145.
Selective androgen receptor modulators
The pathological effects arising from testosterone has resulted in the search for tissue-selective agonists of the androgen receptor which could potentially trigger the androgen receptor in a tissue-selective manner 130. Selective androgen receptor modulators (SARMs) are a new class of androgen receptor ligands that bind to androgen receptors, depending on the chemical structure of each SARM. Consequently, SARMs lead to anabolic activity while avoiding most of the side effects caused by anabolic steroids 131. The tissue specificity of SARMs has led to the notion that they may be a promising treatment for a wide variety of diseases 131. SARMs have been studied in the treatment of male hypogonadism and their use is being investigated in clinical trials (6, 60). Miner et al. (2007), in a rat model of sexual behavior, showed that SARMs can lead to increased sexual function. In this model treatment of male rats with 100 mg/kg of a synthetic SARM, LGD226, increased number of mounts, intromissions, and ejaculation 127. Jones et al. 146 demonstrated that administration of S-23 with estradiol benzoate in male rats led to inhibition in spermatogenesis and the suppression of FSH and LH levels. However, these effects are completely reversible. Chen et al. 147 reported that C-6, a novel SARM, can mimic in vivo endocrine effects of testosterone. Administration of C-6 significantly inhibited spermatogenesis and decreased testosterone concentration, epididymal and testicular size (63). Another study showed that SARM-2f, a novel SARM compound, can restore the sexual behavior in castrated male rats 148. Although SARMs represent an attractive therapeutic approach, further studies are required for the safe use of these medications 131.
Leydig stem cell transplantation
Adult Leydig cells found in the interstitial space of the testis, are the primary source of testosterone production in males 149. Adult Leydig cells undergo a four-stage process of differentiation, beginning with undifferentiated mesenchymal cells (also known as Leydig stem cells [LSCs]) and progressing to progenitor Leydig cells, immature Leydig cells, and finally to adult Leydig cells 150. Leydig stem cells do not have steroidogenic capabilities; however, they are unique in that they are capable of replicating indefinitely and differentiating to functioning adult Leydig cells 150. This suggests the possibility of having an endless pool of progenitor cells capable of replacing dysfunctional adult Leydig cells and restoring testosterone production in men with low testosterone 151. Arora et al. 134 showed that Leydig stem cells in combination with Sertoli and myoid cells underwent self-renewal as well as differentiation into mature Leydig cells. Adult Leydig cells act in the hypothalamic-pituitary-gonadal axis: an increase in LH stimulates the first step of testosterone synthesis in adult Leydig cells; conversely, an increase in testosterone production by adult Leydig cells decreases LH production by the pituitary 152. Dysfunction at any point along the hypothalamic-pituitary-gonadal axis axis can lead to testosterone deficiency 132.
Beattie et al. 133 demonstrated increased testosterone production in rat models by directly stimulating aged adult Leydig cells with translocator protein, a ligand involved in transporting cholesterol from the cytosol to mitochondria. This process was independent of LH regulation and treated aged adult Leydig cells had equivalent testosterone production to young adult Leydig cells. Other studies have shown great promise in increasing testosterone production at more upstream regulation points. Jiang et al. 153 showed that transplantation of nestin-positive Leydig stem cells into the testis of mice with impaired adult Leydig cells caused differentiation into functional adult Leydig cells and an increase in testosterone production. Zhang et al. 108 identified that transplanting human p75+ Leydig stem cells into EDS-treated rats (inducing ALC dysfunction) successfully replaced the impaired adult Leydig cells and partially restored testosterone production. Arora et al. 134 demonstrated a novel form of Leydig stem cell transplantation by autografting Leydig stem cells in combination with Sertoli cells and peritubular myoid cells into the subcutaneous, rather than the testicular, tissue of hypogonadal mouse models. This also resulted in differentiation of Leydig stem cells into adult Leydig cells and increased testosterone production 134.
Testosterone replacement therapy contraindications
Main contraindications of testosterone therapy:
- Absolute contraindications
- Locally advanced or metastatic prostate cancer
- Male breast cancer
- Men with an active desire to have children
- Hematocrit ≥ 54%
- Uncontrolled or poorly controlled congestive heart failure
- Relative contraindication
- International Prostate Symptom Score (IPSS) > 19
- Baseline hematocrit 48-50%
- Familial history of venous thromboembolism
Absolute contraindications to testosterone replacement therapy are untreated breast and prostate cancer. Similarly, conditions such as cardiovascular diseases as well as uncontrolled or poorly controlled congestive heart failure should be considered when prescribing testosterone therapy 154. Severe lower urinary tract symptoms (LUTS) [International Prostate Symptom Score (IPSS) score > 19] represent a relative contraindication, as there is insufficient data on the long-term effects of testosterone therapy in these patients 96. A positive family history for venous thromboembolism requires further analysis to exclude a condition of undiagnosed thrombophilia-hypofibrinolysis 155. These patients need to be carefully counseled prior to testosterone therapy initiation. An elevated hematocrit (HCT) level is the most common adverse effect of testosterone therapy. Stimulation of erythropoiesis is a normal biological action that enhances the delivery of oxygen to testosterone-sensitive tissues (e.g., striated, smooth and cardiac muscle). Any elevation of hematocrit (HCT) above the normal range for hematocrit usually becomes evident between three and twelve months after testosterone therapy initiation. However, polycythemia can also occur after any subsequent increase in testosterone dose, switching from topical to parenteral administration and, development of comorbidity, which can be linked to an increase in hematocrit (e.g., respiratory or haematological diseases).
A hematocrit (HCT) > 54% should require testosterone therapy withdrawal, reduction in dose, change of formulation and venesection depending on the clinical situation to avoid any potential cardiovascular complications. Lower baseline hematocrit (HCT) (48-50%) should be carefully evaluated before testosterone therapy initiation, to avoid pathological increases during treatment, especially in high-risk men such as those with Chronic Obstructive Pulmonary Disease (COPD) or Obstructive Sleep Apnea (OSA). Accordingly, the Framingham Heart Study showed that hematocrit (HCT) > 48% represented a condition associated with increased risk of coronary artery disease (coronary heart disease) and mortality and was associated with cardiovascular disorders 156. Testosterone therapy suppresses gonadotropin (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]) and endogenous testosterone secretion as well as spermatogenesis 157. Therefore, testosterone therapy is contraindicated in individuals who desire fertility 158. Secondary hypogonadism is characterised by low or inappropriately normal gonadotropin levels; therefore, the rationale is to substitute the gonadotropin deficiency with simultaneously luteinizing hormone (LH) and follicle-stimulating hormone (FSH) analogues, if fertility is desired 159.
Testosterone replacement therapy dosage
Testosterone undecanoate injection (Aveed) may cause serious breathing problems and allergic reactions, during or immediately after the injection. The injection should be given by a doctor or nurse in a healthcare setting where these problems or reactions can be treated. You will need to remain in the healthcare setting for at least 30 minutes after you receive your injection. Tell your doctor or nurse immediately if you experience any of the following symptoms during or after your injection: tightening of your throat, difficulty breathing, difficulty swallowing, shortness of breath, cough or urge to cough, chest pain, dizziness, fainting, sweating, rash, hives, or itching.
A program has been set up to limit the use of testosterone undecanoate injection (Aveed) and to inform people about the increased risk of breathing problems and allergic reactions while receiving this medication. The program also makes sure that everyone who received this medication understands the risks and benefits from this medication and receives the medication in a setting where they can be monitored for serious reactions.
Your doctor or pharmacist will give you the manufacturer’s patient information sheet (Medication Guide) when you begin treatment with testosterone undecanoate injection. Read the information carefully and ask your doctor or pharmacist if you have any questions. You can also visit the Food and Drug Administration (FDA) website (http://www.fda.gov/Drugs/DrugSafety/ucm085729.htm) or the manufacturer’s website to obtain the Medication Guide.
Adult Male Dose for Hypogonadism
Uses:
- Primary hypogonadism (congenital or acquired): Testicular failure due to conditions such as cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter Syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations and gonadotropins (FSH, LH) above the normal range.
- Hypogonadotropic hypogonadism (congenital or acquired): Gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum concentrations but have gonadotropins in the normal or low range.
IM INJECTION:
- Testosterone Undecanoate: 750 mg (3 mL) IM injection followed by 750 mg (3 mL) injected after 4 weeks, then 750 mg (3 mL) every 10 weeks thereafter
- Testosterone Enanthate and Cypionate: 50 to 400 mg IM injection every 2 to 4 weeks
IMPLANT:
- 2 to 6 pellets (75 mg each) implanted subcutaneously every 3 to 6 months.
- The number of pellets to be implanted depends upon the minimal daily requirements of testosterone propionate administered parenterally. Thus, implant two 75 mg pellets for each 25 mg testosterone propionate required weekly.
Comments:
- The chronological and skeletal ages must be taken into consideration, both in determining the initial dose and in adjusting the dose.
- This drug should be used only if the benefits outweigh the serious risks of pulmonary oil microembolism and anaphylaxis.
- Injections more frequently than every two weeks are not recommended.
- Adequate effect of the implants (pellets) continues for three to four months, sometimes as long as six months.
BUCCAL:
- Mucoadhesive Oral Patch: Apply a 30 mg patch to the gum region twice a day; morning and evening (about 12 hours apart).
TOPICAL:
- Transdermal Film: 2 to 6 mg applied to the back, abdomen, upper arm, or upper thigh once a day, preferably at night.
- Gel (in tubes, packets or spray): 5 g applied once a day, preferably in the morning. Consult the manufacturer product information for specific dosage and additional instructions of use.
- Transdermal Solution: Initial dose is 60 mg of testosterone (1 pump actuation of 30 mg of testosterone to each axilla), applied once a day, at the same time each morning. Consult the manufacturer product information for specific dosage and additional instructions of use.
Comments: Prior to initiating therapy with this drug, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days, and that these serum testosterone concentrations are below the normal range.
Pediatric Male Dose for Delayed Puberty
Use: To stimulate puberty in selected males with clearly delayed puberty
IM INJECTION:
- Testosterone Enanthate: 50 to 200 mg every 2 to 4 weeks for 4 to 6 months
IMPLANT:
- 2 pellets (each pellet contain 75 mg of testosterone) implanted subcutaneously every 3 to 6 months
- Duration of therapy: 4 to 6 months
Comments:
- The chronological and skeletal ages should be taken into consideration when determining the initial dose and when adjusting the dose.
- An X-ray of the hand and wrist to determine bone age should be obtained every six months to assess the effect of treatment on the epiphyseal centers.
- Report frequent or persistent erections.
- Androgen therapy should be used very cautiously in children and only by specialists who are aware of the adverse effects on bone maturation.
Renal Dose Adjustments
- Testosterone Cypionate IM injection: Contraindicated in renal disease
Liver Dose Adjustments
- Testosterone Cypionate IM injection: Contraindicated in liver disease
Dose Adjustments
- The doses of this drug should be adjusted according to the patient’s response and the appearance of adverse reactions.
Testosterone replacement therapy monitoring
Testosterone replacement therapy alleviates symptoms and signs of hypogonadism in men in a specific time-dependent manner 3. The results of testosterone clinical trials showed that testosterone therapy improved sexual symptoms as early as 3 months after initiation 160, 155, 161. Table 2 summarises the clinical and biochemical parameters that should be monitored during testosterone therapy. The first evaluation should be planned after 3 months of testosterone treatment. Further evaluation may be scheduled at 6 months or 12 months, according to patient characteristics, as well as results of biochemical testing. Patients at high risk of developing elevated hematocrit should be evaluated every 3 months during the first year of testosterone therapy and at least every 6 months thereafter. Accordingly, current guidelines suggest that hematocrit should be maintained below 45% in patients with polycythemia vera to avoid thromboembolism risk 162. Similarly, data derived using a multi-institutional database including a large cohort of hypogonadal (total testosterone < 12 nmol/L) men who received testosterone therapy and subsequently did (n=5,887) or did not (n=4,2784) develop polycythemia (hematocrit > 52%) showed that men who had an increased hematocrit had a higher risk of major adverse cardiovascular events or venous thromboembolism mostly during the first year of therapy 163. The risk was even higher when a hematocrit threshold of 54% was considered whilst no risk was observed when a hematocrit 50% threshold was applied 163.
Testosterone Trials were designed to maintain the serum testosterone concentration within the normal range for young men (280–873 ng/dL or 9.6-30 nmol/L) 160. This approach resulted in a good benefit/risk ratio. A similar approach could be considered during follow-up. The correct timing for the evaluation of testosterone levels varies according to the type of testosterone preparation used. Testosterone is involved in the regulation of erythropoiesis 121 and prostate growth 92, hence measuring serum PSA and hematocrit should be mandatory before and during testosterone therapy. However, it is important to recognize that the risk of prostate cancer in men aged < 40 years is low. Similarly, the mortality risk for prostate cancer in men aged > 70 years has not been considered high enough to warrant monitoring in the general population 164. Therefore, any screening for prostate cancer through the determination of PSA and digital rectal exam (DRE) in men aged < 40 or > 70 years during testosterone therapy should be discussed with the patients.
Baseline and annual metabolic profile evaluation may be a reasonable consideration, particularly in the management of functional hypogonadism. Testosterone therapy may be beneficial for hypogonadal men with low or moderate fracture risk 165. Therefore, dual energy X-ray absorptiometry (DEXA) bone scan may also be considered at baseline and 18-24 months following testosterone therapy, particularly in patients with more severe hypogonadism 165.
Digital rectal examination (DRE) may detect prostate abnormalities that can be present even in men with normal PSA values. Hence, digital rectal exam (DRE) is mandatory in all men at baseline and is recommended to be performed at least annually during testosterone therapy, as long as there is no significant increase in PSA velocity.
The decision to stop testosterone therapy or to perform a prostate biopsy due to PSA increase or prostate abnormalities should be based on local prostate cancer guidelines. There is a large consensus that any increase of hematocrit > 54% during testosterone therapy requires testosterone withdrawal and phlebotomy to avoid potential adverse effects including venous-thromboembolism and cardiovascular disease, especially in high-risk individuals 3. In patients with lower risk of relevant clinical complications, the situation can be alternatively managed by reducing testosterone dose and switching formulation along with venesection 3. A positive family history of venous-thromboembolism should be carefully investigated and the patient counseled about testosterone therapy to avoid/prevent thrombophilia-hypofibrinolysis 155. Finally, caution should be exercised in men with pre-existing cardiovascular disease or at higher risk of cardiovascular disease 154.
Table 2. Clinical and biochemical parameters to be checked during testosterone therapy
| Parameters | Year 1 of treatment | After year 1 of treatment | ||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | Annually | 18-24 months | |
| Clinical | ||||||
| Symptoms | X | X | X | X | X | |
| Body Mass Index | X | X | X | |||
| Waist circumference | X | X | X | X | ||
| Digital rectal examination | X | X | X | |||
| Blood pressure | X | X | X | X | ||
| Biochemistry | ||||||
| PSA (ng/mL) | X | X | X2 | X | X | |
| Hematocrit (%) | X | X | X1,2 | X | X | |
| Testosterone | X | X | X | X | ||
| Lipid and glycaemic profile | X | X | X | |||
| Instrumental | ||||||
| DEXA | X | X | ||||
Footnotes: 1) Population with polycythemia vera or at high risk of secondary polycythemia (e.g., sleep apnea, morbidobesity, heavy smokers, chronic obstructive pulmonary disease); 2) Prostate cancer survivors.
[Source 3 ]Testosterone replacement therapy risks
Testosterone replacement therapy has various risks. For example, testosterone therapy may:
- Elevate red blood cell count (erythrocytosis), thereby increasing the risk of blood clots or venous thromboembolism 166
- Cause acne or other skin reactions
- Contribute to sleep apnea — a potentially serious sleep disorder in which breathing repeatedly stops and starts
- Stimulate noncancerous growth of the prostate (benign prostatic hyperplasia) and growth of existing prostate cancer
- Enlarge breasts
- Limit sperm production or cause testicle shrinkage
- Increase the risk of a blood clot forming in a deep vein (deep vein thrombosis), which could break loose, travel through your bloodstream and lodge in your lungs, blocking blood flow (pulmonary embolism)
In addition, testosterone therapy may impact your risk of heart disease. Research has had conflicting results, so the exact risk isn’t clear yet. Most notable are the TOM (Testosterone in Older Men) trial and the TEAAM (Testosterone’s Effects on Atherosclerosis Progression in Aging Men) trials. Low testosterone levels have been associated with increased risk of coronary artery disease 167. Published in JAMA 2017, a study 168 found that testosterone replacement was associated with a lower risk of cardiovascular outcomes. In 2015, the FDA concluded a possible increased cardiovascular risk associated with testosterone use requiring labeling change to inform the public. The FDA requires that you are made aware that the possibility of cardiovascular events may exist during treatment.
The American Association of Clinical Endocrinologists 169 issued a guideline in response to the 2015 FDA labeling requirement on cardiovascular risk and stated that there is no compelling evidence that testosterone therapy increases cardiovascular risk. On the other hand, testosterone deficiency has been associated with falls, sarcopenia, frailty, osteopenia, and osteoporosis 170.
Serum PSA levels can increase in response to testosterone treatment, so it is important to rule out prostate cancer before starting therapy as it can worsen the disease process. Patients on replacement therapy need to be reevaluated for prostate cancer at 3 months and 1 year after beginning treatment 171.
There is no firm scientific evidence that long-term testosterone replacement is associated with either prostate cancer or cardiovascular events. Prostate cells are stimulated by testosterone, so be extra vigilant about cancer screenings. African American men over age 45 — especially those with family history of cancer — are already at risk for prostate cancer.
- Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, Cocci A, Corona G, Dimitropoulos K, Gül M, Hatzichristodoulou G, Jones TH, Kadioglu A, Martínez Salamanca JI, Milenkovic U, Modgil V, Russo GI, Serefoglu EC, Tharakan T, Verze P, Minhas S; EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology Guidelines on Sexual and Reproductive Health-2021 Update: Male Sexual Dysfunction. Eur Urol. 2021 Sep;80(3):333-357. doi: 10.1016/j.eururo.2021.06.007[↩]
- Isidori AM, Aversa A, Calogero A, Ferlin A, Francavilla S, Lanfranco F, Pivonello R, Rochira V, Corona G, Maggi M. Adult- and late-onset male hypogonadism: the clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE). J Endocrinol Invest. 2022 Dec;45(12):2385-2403. doi: 10.1007/s40618-022-01859-7[↩][↩]
- MALE HYPOGONADISM. https://uroweb.org/guidelines/sexual-and-reproductive-health/chapter/male-hypogonadism[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009;5(3):427–448. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2701485/[↩]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR., Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab. 2001 Feb;86(2):724-31[↩]
- Sizar O, Pico J. Androgen Replacement. [Updated 2018 Dec 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534853[↩][↩][↩][↩][↩]
- Vermeulen A, Kaufman JM. Diagnosis of hypogonadism in the aging male. Aging Male. 2002 Sep;5(3):170-6.[↩]
- Khodamoradi, K., Khosravizadeh, Z., Parmar, M., Kuchakulla, M., Ramasamy, R., & Arora, H. (2021). Exogenous testosterone replacement therapy versus raising endogenous testosterone levels: current and future prospects. F&S reviews, 2(1), 32–42. https://doi.org/10.1016/j.xfnr.2020.11.001[↩]
- Miller J, Britto M, Fitzpatrick S, McWhirter C, Testino SA, Brennan JJ, Zumbrunnen TL. Pharmacokinetics and relative bioavailability of absorbed testosterone after administration of a 1.62% testosterone gel to different application sites in men with hypogonadism. Endocr Pract. 2011 Jul-Aug;17(4):574-83[↩]
- Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J. Clin. Endocrinol. Metab. 1980 Dec;51(6):1335-9.[↩]
- Schubert M, Minnemann T, Hübler D, Rouskova D, Christoph A, Oettel M, Ernst M, Mellinger U, Krone W, Jockenhövel F. Intramuscular testosterone undecanoate: pharmacokinetic aspects of a novel testosterone formulation during long-term treatment of men with hypogonadism. J. Clin. Endocrinol. Metab. 2004 Nov;89(11):5429-34.[↩]
- Kim, M., Byun, S. S., & Hong, S. K. (2021). Testosterone Replacement Therapy in Men with Untreated or Treated Prostate Cancer: Do We Have Enough Evidences?. The world journal of men’s health, 39(4), 705–723. https://doi.org/10.5534/wjmh.190158[↩][↩]
- Morgentaler A. Testosterone therapy for men at risk for or with history of prostate cancer. Curr Treat Options Oncol. 2006 Sep;7(5):363-9. doi: 10.1007/s11864-006-0004-y[↩]
- Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018 May 1;103(5):1715-1744. doi: 10.1210/jc.2018-00229[↩]
- Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, Morley JE, Schulman C, Thompson IM, Weidner W, Wu FC; International Society of Andrology; International Society for the Study of Aging Male; European Association of Urology; European Academy of Andrology; American Society of Andrology. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Eur Urol. 2009 Jan;55(1):121-30. doi: 10.1016/j.eururo.2008.08.033[↩]
- Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, Lightner DJ, Miner MM, Murad MH, Nelson CJ, Platz EA, Ramanathan LV, Lewis RW. Evaluation and Management of Testosterone Deficiency: AUA Guideline. J Urol. 2018 Aug;200(2):423-432. doi: 10.1016/j.juro.2018.03.115[↩][↩][↩]
- Nassar GN, Leslie SW. Physiology, Testosterone. [Updated 2023 Jan 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526128[↩]
- Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol (2013) 217(3):R25–45. doi: 10.1530/JOE-12-0455[↩]
- McEwan IJ, Brinkmann AO. Androgen Physiology: Receptor and Metabolic Disorders. [Updated 2021 Jul 2]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279028[↩][↩]
- Sizar O, Leslie SW, Pico J. Androgen Replacement. [Updated 2023 Nov 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534853[↩][↩][↩]
- Vermeulen A, Kaufman JM. Diagnosis of hypogonadism in the aging male. Aging Male. 2002 Sep;5(3):170-6. Erratum in: Aging Male. 2002 Dec;5(4):iv.[↩]
- Vermeulen A. Hormonal cut-offs of partial androgen deficiency: a survey of androgen assays. J Endocrinol Invest. 2005;28(3 Suppl):28-31.[↩]
- Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Vanderschueren D, Huhtaniemi IT, Wu FC; EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010 Apr;95(4):1810-8. doi: 10.1210/jc.2009-1796[↩][↩]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002 Feb;87(2):589-98. doi: 10.1210/jcem.87.2.8201[↩][↩][↩]
- Nieschlag E, Swerdloff R, Behre HM, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morley JE, Schulman C, Wang C, Weidner W, Wu FC. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, and EAU recommendations. Int J Androl. 2005 Jun;28(3):125-7. doi: 10.1111/j.1365-2605.2005.00553.x[↩][↩]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001 Feb;86(2):724-31. doi: 10.1210/jcem.86.2.7219[↩][↩][↩][↩][↩][↩]
- Stanworth RD, Jones TH. Testosterone for the aging male; current evidence and recommended practice. Clin Interv Aging. 2008;3(1):25-44. doi: 10.2147/cia.s190[↩][↩]
- Iwamoto T., Yanase T., Koh E., Horie H., Baba K., Namiki M., Nawata H. Reference ranges of total serum and free testosterone in Japanese male adults. Nihon Hinyokika Gakkai Zasshi. 2004;95:751–760. doi: 10.5980/jpnjurol1989.95.751[↩][↩]
- Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D; European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008 Jul;93(7):2737-45. doi: 10.1210/jc.2007-1972[↩][↩]
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Boonen S, Vanderschueren D, Labrie F, Huhtaniemi IT; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010 Jul 8;363(2):123-35. doi: 10.1056/NEJMoa0911101[↩][↩][↩][↩][↩][↩]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al.. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab (2002) 87(2):589–98. doi: 10.1210/jcem.87.2.8201[↩][↩]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of Aging . Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab (2001) 86(2):724–31. doi: 10.1210/jcem.86.2.7219[↩][↩]
- Winter, A. G., Zhao, F., & Lee, R. K. (2014). Androgen deficiency and metabolic syndrome in men. Translational andrology and urology, 3(1), 50–58. https://doi.org/10.3978/j.issn.2223-4683.2014.01.04[↩][↩]
- Abbasi A.A., Drinka P.J., Mattson D.E., Rudman D. Low circulating levels of insulin-like growth factors and testosterone in chronically institutionalized elderly men. J. Am. Geriatr. Soc. 1993;41:975–982. doi: 10.1111/j.1532-5415.1993.tb06764.x[↩][↩]
- Spaziani M, Mileno B, Rossi F, Granato S, Tahani N, Anzuini A, Lenzi A, Radicioni AF. Endocrine and metabolic evaluation of classic Klinefelter syndrome and high-grade aneuploidies of sexual chromosomes with male phenotype: are they different clinical conditions? Eur J Endocrinol. 2018 Apr;178(4):343-352. doi: 10.1530/EJE-17-0902[↩][↩]
- Bozzola M, Bozzola E, Montalbano C, Stamati FA, Ferrara P, Villani A. Delayed puberty versus hypogonadism: a challenge for the pediatrician. Ann Pediatr Endocrinol Metab. 2018 Jun;23(2):57-61. doi: 10.6065/apem.2018.23.2.57[↩][↩]
- Hauser LJ, Jensen EL, Mirsky DM, Chan KH. Pediatric anosmia: A case series. Int J Pediatr Otorhinolaryngol. 2018 Jul;110:135-139. doi: 10.1016/j.ijporl.2018.05.011[↩][↩]
- Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al.. EAU working group on male sexual and reproductive health. European association of urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol (2021) 80(3):333–57. doi: 10.1016/j.eururo.2021.06.007[↩][↩]
- Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al.. Evaluation and management of testosterone deficiency: AUA guideline. J Urol (2018) 200(2):423–32. doi: 10.1016/j.juro.2018.03.115[↩][↩]
- Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al.. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2018) 103(5):1715–44. doi: 10.1210/jc.2018-00229[↩][↩]
- Gagliano-Jucá T, Li Z, Pencina KM, Beleva YM, Carlson OD, Egan JM, Basaria S. Oral glucose load and mixed meal feeding lowers testosterone levels in healthy eugonadal men. Endocrine. 2019 Jan;63(1):149-156. doi: 10.1007/s12020-018-1741-y[↩][↩]
- Boeri L, Capogrosso P, Ventimiglia E, Cazzaniga W, Pederzoli F, Moretti D, Dehò F, Montanari E, Montorsi F, Salonia A. Does Calculated Free Testosterone Overcome Total Testosterone in Protecting From Sexual Symptom Impairment? Findings of a Cross-Sectional Study. J Sex Med. 2017 Dec;14(12):1549-1557. doi: 10.1016/j.jsxm.2017.10.070[↩][↩]
- Rastrelli G, O’Neill TW, Ahern T, Bártfai G, Casanueva FF, Forti G, Keevil B, Giwercman A, Han TS, Slowikowska-Hilczer J, Lean MEJ, Pendleton N, Punab M, Antonio L, Tournoy J, Vanderschueren D, Maggi M, Huhtaniemi IT, Wu FCW; EMAS study group. Symptomatic androgen deficiency develops only when both total and free testosterone decline in obese men who may have incident biochemical secondary hypogonadism: Prospective results from the EMAS. Clin Endocrinol (Oxf). 2018 Oct;89(4):459-469. https://eprints.gla.ac.uk/163468/7/163468.pdf[↩][↩]
- Antonio L, Wu FC, O’Neill TW, Pye SR, Ahern TB, Laurent MR, Huhtaniemi IT, Lean ME, Keevil BG, Rastrelli G, Forti G, Bartfai G, Casanueva FF, Kula K, Punab M, Giwercman A, Claessens F, Decallonne B, Vanderschueren D; European Male Ageing Study Study Group. Low Free Testosterone Is Associated with Hypogonadal Signs and Symptoms in Men with Normal Total Testosterone. J Clin Endocrinol Metab. 2016 Jul;101(7):2647-57. doi: 10.1210/jc.2015-4106[↩][↩]
- Bassil N, Morley JE. Late-life onset hypogonadism: a review. Clin Geriatr Med. 2010 May;26(2):197-222. doi: 10.1016/j.cger.2010.02.003[↩]
- Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, Joffe HV. Testosterone and “Age-Related Hypogonadism”–FDA Concerns. N Engl J Med. 2015 Aug 20;373(8):689-91. doi: 10.1056/NEJMp1506632[↩][↩]
- Maggi M, Schulman C, Quinton R, Langham S, Uhl-Hochgraeber K. The burden of testosterone deficiency syndrome in adult men: economic and quality-of-life impact. J Sex Med. 2007 Jul;4(4 Pt 1):1056-69. doi: 10.1111/j.1743-6109.2007.00531.x[↩]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006 Jul;60(7):762-9. doi: 10.1111/j.1742-1241.2006.00992.x[↩]
- Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez-Rigau LJ; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients–2002 update. Endocr Pract. 2002 Nov-Dec;8(6):440-56. Erratum in: Endocr Pract. 2008 Sep;14(6):802-3. Petak, Steven M [added]; Nankin, Howard R [added]; Spark, Richard F [added]; Swerdloff, Ronald S [added]; Rodriguez-Rigau, Luis J [added].[↩]
- McVary KT. Clinical practice. Erectile dysfunction. N Engl J Med. 2007 Dec 13;357(24):2472-81. doi: 10.1056/NEJMcp067261[↩]
- Kaiser FE, Viosca SP, Morley JE, Mooradian AD, Davis SS, Korenman SG. Impotence and aging: clinical and hormonal factors. J Am Geriatr Soc. 1988 Jun;36(6):511-9. doi: 10.1111/j.1532-5415.1988.tb04021.x[↩]
- Meuleman EJ, van Lankveld JJ. Hypoactive sexual desire disorder: an underestimated condition in men. BJU Int. 2005 Feb;95(3):291-6. doi: 10.1111/j.1464-410X.2005.05285.x[↩]
- Amir Qaseem, Carrie A. Horwitch, Sandeep Vijan, et al; for the Clinical Guidelines Committee of the American College of Physicians. Testosterone Treatment in Adult Men With Age-Related Low Testosterone: A Clinical Guideline From the American College of Physicians. Ann Intern Med.2020;172:126-133. doi:10.7326/M19-0882[↩][↩][↩]
- FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-cautions-about-using-testosterone-products-low-testosterone-due[↩]
- Xu Z, Chen X, Zhou H, Ren C, Wang Q, Pan Y, Liu L, Liu X. An updated systematic review and meta-analysis of the effects of testosterone replacement therapy on erectile function and prostate. Front Endocrinol (Lausanne). 2024 Jan 26;15:1335146. doi: 10.3389/fendo.2024.1335146[↩][↩]
- Hisanori Taniguchi, Seiji Shimada, Hidefumi Kinoshita; Testosterone Therapy for Late-Onset Hypogonadism Improves Erectile Function: A Systematic Review and Meta-Analysis. Urol Int 1 June 2022; 106 (6): 539–552. https://doi.org/10.1159/000520135[↩][↩]
- Kang S, Park HJ, Park NC. Serum total testosterone level and identification of late-onset hypogonadism: a communitybased study. Korean J Urol (2013) 54:619–23. doi: 10.4111/kju.2013.54.9.619[↩][↩]
- Lin YC, Hwang TI, Chiang HS, Yang CR, Wu HC, Wu TL, et al.. Correlations of androgen deficiency with clinical symptoms in Taiwanese males. Int J Impot Res (2006) 18:343–7. doi: 10.1038/sj.ijir.3901417[↩][↩]
- Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, Saad F, Mannucci E, Maggi M. THERAPY OF ENDOCRINE DISEASE: Testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016 Mar;174(3):R99-116. doi: 10.1530/EJE-15-0262[↩][↩][↩]
- Corona G, Rastrelli G, Morgentaler A, Sforza A, Mannucci E, Maggi M. Meta-analysis of Results of Testosterone Therapy on Sexual Function Based on International Index of Erectile Function Scores. Eur Urol. 2017 Dec;72(6):1000-1011. doi: 10.1016/j.eururo.2017.03.032[↩][↩][↩]
- Saez, J. M., Forest, M. G., Morera, A. M., & Bertrand, J. (1972). Metabolic clearance rate and blood production rate of testosterone and dihydrotestosterone in normal subjects, during pregnancy, and in hyperthyroidism. The Journal of clinical investigation, 51(5), 1226–1234. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC292254/pdf/jcinvest00201-0192.pdf[↩]
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001 Dec;22(6):764-86. doi: 10.1210/edrv.22.6.0446[↩]
- Travison, T. G., Vesper, H. W., Orwoll, E., Wu, F., Kaufman, J. M., Wang, Y., Lapauw, B., Fiers, T., Matsumoto, A. M., & Bhasin, S. (2017). Harmonized Reference Ranges for Circulating Testosterone Levels in Men of Four Cohort Studies in the United States and Europe. The Journal of clinical endocrinology and metabolism, 102(4), 1161–1173. https://doi.org/10.1210/jc.2016-2935[↩]
- Carrasquillo R, Chu K, Ramasamy R. Novel Therapy for Male Hypogonadism. Curr Urol Rep. 2018 Jun 9;19(8):63. doi: 10.1007/s11934-018-0816-x[↩][↩]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010 Jun;95(6):2536-59. doi: 10.1210/jc.2009-2354. Erratum in: J Clin Endocrinol Metab. 2021 Jun 16;106(7):e2848.[↩][↩]
- Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, Perry HM 3rd. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000 Sep;49(9):1239-42. doi: 10.1053/meta.2000.8625[↩]
- Grober E. D. (2014). Testosterone deficiency and replacement: Myths and realities. Canadian Urological Association journal = Journal de l’Association des urologues du Canada, 8(7-8 Suppl 5), S145–S147. https://doi.org/10.5489/cuaj.2309[↩]
- Peterson, M. D., Belakovskiy, A., McGrath, R., & Yarrow, J. F. (2018). Testosterone Deficiency, Weakness, and Multimorbidity in Men. Scientific reports, 8(1), 5897. https://doi.org/10.1038/s41598-018-24347-6[↩]
- Mulligan, T., Frick, M. F., Zuraw, Q. C., Stemhagen, A., & McWhirter, C. (2006). Prevalence of hypogonadism in males aged at least 45 years: the HIM study. International journal of clinical practice, 60(7), 762–769. https://doi.org/10.1111/j.1742-1241.2006.00992.x[↩]
- Aversa A, Morgentaler A. The practical management of testosterone deficiency in men. Nat Rev Urol. 2015 Nov;12(11):641-50. doi: 10.1038/nrurol.2015.238[↩]
- Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003 Apr;24(2):183-217. doi: 10.1210/er.2001-0025[↩]
- Heron M. Deaths: Leading Causes for 2014. Natl Vital Stat Rep. 2016 Jun;65(5):1-96.[↩]
- Nieschlag E, Swerdloff R, Behre HM, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morley JE, Schulman C, Wang C, Weidner W, Wu FC. Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2005 Jun;8(2):56-8. doi: 10.1080/13685530500130969[↩]
- Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med. 2011 Jul;124(7):578-87. doi: 10.1016/j.amjmed.2010.12.027[↩]
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006 Aug 14-28;166(15):1660-5. doi: 10.1001/archinte.166.15.1660[↩]
- Choi, S. W., Jeon, S. H., Kwon, E. B., Zhu, G. Q., Lee, K. W., Choi, J. B., Jeong, H. C., Kim, K. S., Bae, S. R., Bae, W. J., Kim, S. J., Cho, H. J., Ha, U. S., Hong, S. H., Hwang, S. Y., & Kim, S. W. (2019). Effect of Korean Herbal Formula (Modified Ojayeonjonghwan) on Androgen Receptor Expression in an Aging Rat Model of Late Onset Hypogonadism. The world journal of men’s health, 37(1), 105–112. https://doi.org/10.5534/wjmh.180051[↩]
- Hackett, G. Type 2 Diabetes and Testosterone Therapy. World J. Mens Health 2019, 37, 31–44.[↩]
- Nam, Y.S.; Lee, G.; Yun, J.M.; Cho, B. Testosterone Replacement, Muscle Strength, and Physical Function. World J. Mens Health 2018, 36, 110–122.[↩]
- Bassil, N., Alkaade, S., & Morley, J. E. (2009). The benefits and risks of testosterone replacement therapy: a review. Therapeutics and clinical risk management, 5(3), 427–448. https://doi.org/10.2147/tcrm.s3025[↩]
- Morales A, Schulman CC, Tostain J, C W Wu F. Testosterone Deficiency Syndrome (TDS) needs to be named appropriately–the importance of accurate terminology. Eur Urol. 2006 Sep;50(3):407-9. doi: 10.1016/j.eururo.2006.07.001[↩]
- Kumar, P., Kumar, N., Thakur, D. S., & Patidar, A. (2010). Male hypogonadism: Symptoms and treatment. Journal of advanced pharmaceutical technology & research, 1(3), 297–301. https://doi.org/10.4103/0110-5558.72420[↩]
- Seftel A. Male hypogonadism. Part II: etiology, pathophysiology, and diagnosis. Int J Impot Res. 2006 May-Jun;18(3):223-8. doi: 10.1038/sj.ijir.3901365[↩][↩]
- Naifar M, Rekik N, Messedi M, Chaabouni K, Lahiani A, Turki M, Abid M, Ayedi F, Jamoussi K. Male hypogonadism and metabolic syndrome. Andrologia. 2015 Jun;47(5):579-86. doi: 10.1111/and.12305[↩]
- Shin YS, Park JK. The Optimal Indication for Testosterone Replacement Therapy in Late Onset Hypogonadism. Journal of Clinical Medicine. 2019; 8(2):209. https://doi.org/10.3390/jcm8020209[↩]
- Shin, Y. S., & Park, J. K. (2019). The Optimal Indication for Testosterone Replacement Therapy in Late Onset Hypogonadism. Journal of clinical medicine, 8(2), 209. https://doi.org/10.3390/jcm8020209[↩]
- Carnegie C. Diagnosis of hypogonadism: clinical assessments and laboratory tests. Rev Urol. 2004;6 Suppl 6:S3-8.[↩]
- Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino R, Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J. Clin. Endocrinol. Metab. 2011 Aug;96(8):2430-9.[↩]
- Millar AC, Lau ANC, Tomlinson G, Kraguljac A, Simel DL, Detsky AS, Lipscombe LL. Predicting low testosterone in aging men: a systematic review. CMAJ. 2016 Sep 20;188(13):E321-E330. doi: 10.1503/cmaj.150262[↩]
- Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, Spreafico F, Greco GF, Cervi G, Pecoriello A, Magini A, Todisco T, Cipriani S, Maseroli E, Corona G, Salonia A, Lenzi A, Maggi M, De Donno G, Vignozzi L. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021 Jan;9(1):88-98. doi: 10.1111/andr.12821[↩]
- Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, Carenzi C, Abbate C, Cignoli D, Ferrara AM, Cazzaniga W, Rowe I, Ramirez GA, Tresoldi C, Mushtaq J, Locatelli M, Santoleri L, Castagna A, Zangrillo A, De Cobelli F, Tresoldi M, Landoni G, Rovere-Querini P, Ciceri F, Montorsi F. Severely low testosterone in males with COVID-19: A case-control study. Andrology. 2021 Jul;9(4):1043-1052. doi: 10.1111/andr.12993[↩]
- Corona G, Vena W, Pizzocaro A, Pallotti F, Paoli D, Rastrelli G, Baldi E, Cilloni N, Gacci M, Semeraro F, Salonia A, Minhas S, Pivonello R, Sforza A, Vignozzi L, Isidori AM, Lenzi A, Maggi M, Lombardo F. Andrological effects of SARS-Cov-2 infection: a systematic review and meta-analysis. J Endocrinol Invest. 2022 Dec;45(12):2207-2219. doi: 10.1007/s40618-022-01801-x[↩]
- Rastrelli G, Vignozzi L, Corona G, Maggi M. Testosterone and Benign Prostatic Hyperplasia. Sex Med Rev. 2019 Apr;7(2):259-271. doi: 10.1016/j.sxmr.2018.10.006[↩][↩]
- Guay A, Miller MG, McWhirter CL. Does early morning versus late morning draw time influence apparent testosterone concentration in men aged > or =45 years? Data from the Hypogonadism In Males study. Int J Impot Res. 2008 Mar-Apr;20(2):162-7. doi: 10.1038/sj.ijir.3901580[↩]
- Travison TG, Vesper HW, Orwoll E, Wu F, Kaufman JM, Wang Y, Lapauw B, Fiers T, Matsumoto AM, Bhasin S. Harmonized Reference Ranges for Circulating Testosterone Levels in Men of Four Cohort Studies in the United States and Europe. J Clin Endocrinol Metab. 2017 Apr 1;102(4):1161-1173. doi: 10.1210/jc.2016-2935[↩]
- Huhtaniemi IT, Tajar A, Lee DM, O’Neill TW, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Silman AJ, Vanderschueren D, Forti G, Wu FC; EMAS Group. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur J Endocrinol. 2012 Jun;166(6):983-91. doi: 10.1530/EJE-11-1051[↩]
- Rosner W, Vesper H; Endocrine Society; American Association for Clinical Chemistry; American Association of Clinical Endocrinologists; Androgen Excess/PCOS Society; American Society for Bone and Mineral Research; American Society for Reproductive Medicine; American Urological Association; Association of Public Health Laboratories; Endocrine Society; Laboratory Corporation of America; North American Menopause Society; Pediatric Endocrine Society. Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab. 2010 Oct;95(10):4542-8. doi: 10.1210/jc.2010-1314[↩][↩]
- Fiers T, Wu F, Moghetti P, Vanderschueren D, Lapauw B, Kaufman JM. Reassessing Free-Testosterone Calculation by Liquid Chromatography-Tandem Mass Spectrometry Direct Equilibrium Dialysis. J Clin Endocrinol Metab. 2018 Jun 1;103(6):2167-2174. doi: 10.1210/jc.2017-02360[↩]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999 Oct;84(10):3666-72. doi: 10.1210/jcem.84.10.6079[↩]
- Ferlin A, Calogero AE, Krausz C, Lombardo F, Paoli D, Rago R, Scarica C, Simoni M, Foresta C, Rochira V, Sbardella E, Francavilla S, Corona G. Management of male factor infertility: position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS) : Endorsing Organization: Italian Society of Embryology, Reproduction, and Research (SIERR). J Endocrinol Invest. 2022 May;45(5):1085-1113. doi: 10.1007/s40618-022-01741-6[↩]
- Dalvi M, Walker BR, Strachan MW, Zammitt NN, Gibb FW. The prevalence of structural pituitary abnormalities by MRI scanning in men presenting with isolated hypogonadotrophic hypogonadism. Clin Endocrinol (Oxf). 2016 Jun;84(6):858-61. doi: 10.1111/cen.13015[↩][↩]
- Molitch ME. Diagnosis and Treatment of Pituitary Adenomas: A Review. JAMA. 2017 Feb 7;317(5):516-524. doi: 10.1001/jama.2016.19699[↩][↩]
- Cipriani S, Todisco T, Ghiandai N, Vignozzi L, Corona G, Maggi M, Rastrelli G. Biochemical predictors of structural hypothalamus-pituitary abnormalities detected by magnetic resonance imaging in men with secondary hypogonadism. J Endocrinol Invest. 2021 Dec;44(12):2785-2797. doi: 10.1007/s40618-021-01586-5[↩]
- Rastrelli G, Vignozzi L, Corona G, Maggi M. Pharmacotherapy of male hypogonadism. Curr Opin Pharmacol. 2023 Feb;68:102323. doi: 10.1016/j.coph.2022.102323[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Rastrelli G, Maggi M, Corona G. Pharmacological management of late-onset hypogonadism. Expert Rev Clin Pharmacol. 2018 Apr;11(4):439-458. doi: 10.1080/17512433.2018[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet. 1977 Aug 6;2(8032):262-3.[↩]
- Korbonits M, Kipnes M, Grossman AB. Striant SR: a novel, effective and convenient testosterone therapy for male hypogonadism. Int J Clin Pract. 2004 Nov;58(11):1073-80. doi: 10.1111/j.1368-5031.2004.00383.x[↩][↩]
- Swerdloff, R. S., Wang, C., White, W. B., Kaminetsky, J., Gittelman, M. C., Longstreth, J. A., Dudley, R. E., & Danoff, T. M. (2020). A New Oral Testosterone Undecanoate Formulation Restores Testosterone to Normal Concentrations in Hypogonadal Men. The Journal of clinical endocrinology and metabolism, 105(8), 2515–2531. https://doi.org/10.1210/clinem/dgaa238[↩]
- Zhang, M., Wang, J., Deng, C., Jiang, M. H., Feng, X., Xia, K., Li, W., Lai, X., Xiao, H., Ge, R. S., Gao, Y., & Xiang, A. P. (2017). Transplanted human p75-positive stem Leydig cells replace disrupted Leydig cells for testosterone production. Cell death & disease, 8(10), e3123. https://doi.org/10.1038/cddis.2017.531[↩][↩]
- Dobs AS, Matsumoto AM, Wang C, Kipnes MS. Short-term pharmacokinetic comparison of a novel testosterone buccal system and a testosterone gel in testosterone deficient men. Curr Med Res Opin. 2004 May;20(5):729-38. doi: 10.1185/030079904125003494[↩][↩]
- Dinsmore WW, Wyllie MG. The long-term efficacy and safety of a testosterone mucoadhesive buccal tablet in testosterone-deficient men. BJU Int. 2012 Jul;110(2):162-9. doi: 10.1111/j.1464-410X.2011.10837.x[↩][↩][↩][↩][↩]
- de Ronde W. Hyperandrogenism after transfer of topical testosterone gel: case report and review of published and unpublished studies. Hum Reprod. 2009 Feb;24(2):425-8. doi: 10.1093/humrep/den372[↩]
- Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004 May;89(5):2085-98. doi: 10.1210/jc.2003-032006[↩][↩][↩]
- Fennell C, Sartorius G, Ly LP, Turner L, Liu PY, Conway AJ, Handelsman DJ. Randomized cross-over clinical trial of injectable vs. implantable depot testosterone for maintenance of testosterone replacement therapy in androgen deficient men. Clin Endocrinol (Oxf). 2010 Jul;73(1):102-9. doi: 10.1111/j.1365-2265.2009.03744.x[↩]
- Mazer N, Bell D, Wu J, Fischer J, Cosgrove M, Eilers B; BS, RN,. Comparison of the steady-state pharmacokinetics, metabolism, and variability of a transdermal testosterone patch versus a transdermal testosterone gel in hypogonadal men. J Sex Med. 2005 Mar;2(2):213-26. doi: 10.1111/j.1743-6109.2005.20231.x[↩]
- Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R; North American AA2500 T Gel Study Group. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003 Jun;88(6):2673-81. doi: 10.1210/jc.2002-021058[↩]
- Grober ED, Khera M, Soni SD, Espinoza MG, Lipshultz LI. Efficacy of changing testosterone gel preparations (Androgel or Testim) among suboptimally responsive hypogonadal men. Int J Impot Res. 2008 Mar-Apr;20(2):213-7. doi: 10.1038/sj.ijir.3901618[↩]
- Kim ED, McCullough A, Kaminetsky J. Oral enclomiphene citrate raises testosterone and preserves sperm counts in obese hypogonadal men, unlike topical testosterone: restoration instead of replacement. BJU Int. 2016 Apr;117(4):677-85. doi: 10.1111/bju.13337[↩]
- Rogol AD, Tkachenko N, Bryson N. Natesto™ , a novel testosterone nasal gel, normalizes androgen levels in hypogonadal men. Andrology. 2016 Jan;4(1):46-54. doi: 10.1111/andr.12137. Epub 2015 Dec 22. Erratum in: Andrology. 2017 Jul;5(4):844.[↩]
- Rogol, A. D., Tkachenko, N., Badorrek, P., Hohlfeld, J. M., & Bryson, N. (2018). Phase 1 pharmacokinetics and phase 3 efficacy of testosterone nasal gel in subjects with seasonal allergies. Canadian Urological Association journal = Journal de l’Association des urologues du Canada, 12(7), E349–E356. https://doi.org/10.5489/cuaj.4898[↩]
- Miller JA, Nguyen TT, Loeb C, Khera M, Yafi FA. Oral testosterone therapy: past, present, and future. Sex Med Rev. 2023 Apr 3;11(2):124-138. doi: 10.1093/sxmrev/qead003[↩][↩][↩][↩]
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis Following Testosterone Therapy. Sex Med Rev. 2018 Jan;6(1):77-85. doi: 10.1016/j.sxmr.2017.04.001[↩][↩]
- Rastrelli G, Corona G, Mannucci E, Maggi M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology. 2014 Nov;2(6):794-808. doi: 10.1111/andr.262[↩][↩]
- Rogol AD, Tkachenko N, Bryson N. Natesto™ , a novel testosterone nasal gel, normalizes androgen levels in hypogonadal men. Andrology. 2016 Jan;4(1):46-54. doi: 10.1111/andr.12137. Epub 2015 Dec 22. Erratum in: Andrology. 2017 Jul;5(4):844. doi: 10.1111/andr.12402[↩]
- Ribeiro MA, Gameiro LF, Scarano WR, Briton-Jones C, Kapoor A, Rosa MB, El Dib R. Aromatase inhibitors in the treatment of oligozoospermic or azoospermic men: a systematic review of randomized controlled trials. JBRA Assist Reprod. 2016 May 1;20(2):82-8. doi: 10.5935/1518-0557.20160019[↩][↩][↩][↩]
- Zumoff B, Miller LK, Strain GW. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism. 2003 Sep;52(9):1126-8. doi: 10.1016/s0026-0495(03)00186-0[↩][↩]
- Majzoub, A., & Sabanegh, E., Jr (2016). Testosterone replacement in the infertile man. Translational andrology and urology, 5(6), 859–865. https://doi.org/10.21037/tau.2016.08.03[↩]
- Miner JN, Chang W, Chapman MS, Finn PD, Hong MH, López FJ, Marschke KB, Rosen J, Schrader W, Turner R, van Oeveren A, Viveros H, Zhi L, Negro-Vilar A. An orally active selective androgen receptor modulator is efficacious on bone, muscle, and sex function with reduced impact on prostate. Endocrinology. 2007 Jan;148(1):363-73. doi: 10.1210/en.2006-0793[↩][↩]
- Pasqualotto FF, Lucon AM, Sobreiro BP, Pasqualotto EB, Arap S. Effects of medical therapy, alcohol, smoking, and endocrine disruptors on male infertility. Rev Hosp Clin Fac Med Sao Paulo. 2004 Dec;59(6):375-82. doi: 10.1590/s0041-87812004000600011[↩][↩]
- Bhasin, S., & Jasuja, R. (2009). Selective androgen receptor modulators as function promoting therapies. Current opinion in clinical nutrition and metabolic care, 12(3), 232–240. https://doi.org/10.1097/MCO.0b013e32832a3d79[↩]
- Narayanan, R., Coss, C. C., & Dalton, J. T. (2018). Development of selective androgen receptor modulators (SARMs). Molecular and cellular endocrinology, 465, 134–142. https://doi.org/10.1016/j.mce.2017.06.013[↩][↩]
- Solomon, Z. J., Mirabal, J. R., Mazur, D. J., Kohn, T. P., Lipshultz, L. I., & Pastuszak, A. W. (2019). Selective Androgen Receptor Modulators: Current Knowledge and Clinical Applications. Sexual medicine reviews, 7(1), 84–94. https://doi.org/10.1016/j.sxmr.2018.09.006[↩][↩][↩][↩]
- Winters SJ, Moore JP Jr, Clark BJ. Leydig cell insufficiency in hypospermatogenesis: a paracrine effect of activin-inhibin signaling? Andrology. 2018 Mar;6(2):262-271. doi: 10.1111/andr.12459[↩][↩]
- Beattie, M. C., Adekola, L., Papadopoulos, V., Chen, H., & Zirkin, B. R. (2015). Leydig cell aging and hypogonadism. Experimental gerontology, 68, 87–91. https://doi.org/10.1016/j.exger.2015.02.014[↩][↩]
- Arora, H., Zuttion, M., Nahar, B., Lamb, D., Hare, J. M., & Ramasamy, R. (2019). Subcutaneous Leydig Stem Cell Autograft: A Promising Strategy to Increase Serum Testosterone. Stem cells translational medicine, 8(1), 58–65. https://doi.org/10.1002/sctm.18-0069[↩][↩][↩][↩]
- El Meliegy, A., Motawi, A., & El Salam, M. (2017). Systematic review of hormone replacement therapy in the infertile man. Arab journal of urology, 16(1), 140–147. https://doi.org/10.1016/j.aju.2017.11.011[↩][↩][↩][↩][↩]
- Chehab M, Madala A, Trussell JC. On-label and off-label drugs used in the treatment of male infertility. Fertil Steril. 2015 Mar;103(3):595-604. doi: 10.1016/j.fertnstert.2014.12.122[↩]
- Martinkovich, S., Shah, D., Planey, S. L., & Arnott, J. A. (2014). Selective estrogen receptor modulators: tissue specificity and clinical utility. Clinical interventions in aging, 9, 1437–1452. https://doi.org/10.2147/CIA.S66690[↩]
- Pickar JH, MacNeil T, Ohleth K. SERMs: progress and future perspectives. Maturitas. 2010 Oct;67(2):129-38. doi: 10.1016/j.maturitas.2010.05.009[↩]
- Dohle G, Arver S, Bettocchi C, Jones T, Kliesch S, Punab M. EAU guidelines on male hypogonadism European Association of Urology, Arnhem, The Netherlands, accessed Mar 2016;21:2018.[↩]
- Lee, J. A., & Ramasamy, R. (2018). Indications for the use of human chorionic gonadotropic hormone for the management of infertility in hypogonadal men. Translational andrology and urology, 7(Suppl 3), S348–S352. https://doi.org/10.21037/tau.2018.04.11[↩]
- Crosnoe-Shipley, L. E., Elkelany, O. O., Rahnema, C. D., & Kim, E. D. (2015). Treatment of hypogonadotropic male hypogonadism: Case-based scenarios. World journal of nephrology, 4(2), 245–253. https://doi.org/10.5527/wjn.v4.i2.245[↩]
- Hsieh TC, Pastuszak AW, Hwang K, Lipshultz LI. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol. 2013 Feb;189(2):647-50. doi: 10.1016/j.juro.2012.09.043[↩]
- Coviello AD, Matsumoto AM, Bremner WJ, Herbst KL, Amory JK, Anawalt BD, Sutton PR, Wright WW, Brown TR, Yan X, Zirkin BR, Jarow JP. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab. 2005 May;90(5):2595-602. doi: 10.1210/jc.2004-0802[↩][↩]
- Leder BZ, Rohrer JL, Rubin SD, Gallo J, Longcope C. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. J Clin Endocrinol Metab. 2004 Mar;89(3):1174-80. doi: 10.1210/jc.2003-031467[↩]
- Crosnoe, L. E., Grober, E., Ohl, D., & Kim, E. D. (2013). Exogenous testosterone: a preventable cause of male infertility. Translational andrology and urology, 2(2), 106–113. https://doi.org/10.3978/j.issn.2223-4683.2013.06.01[↩]
- Jones, A., Chen, J., Hwang, D. J., Miller, D. D., & Dalton, J. T. (2009). Preclinical characterization of a (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro, 4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide: a selective androgen receptor modulator for hormonal male contraception. Endocrinology, 150(1), 385–395. https://doi.org/10.1210/en.2008-0674[↩]
- Chen J, Hwang DJ, Bohl CE, Miller DD, Dalton JT. A selective androgen receptor modulator for hormonal male contraception. J Pharmacol Exp Ther. 2005 Feb;312(2):546-53. doi: 10.1124/jpet.104.075424[↩]
- Morimoto, M., Amano, Y., Oka, M., Harada, A., Fujita, H., Hikichi, Y., Tozawa, R., Yamaoka, M., & Hara, T. (2017). Amelioration of sexual behavior and motor activity deficits in a castrated rodent model with a selective androgen receptor modulator SARM-2f. PloS one, 12(12), e0189480. https://doi.org/10.1371/journal.pone.0189480[↩]
- Zhou R, Wu J, Liu B, Jiang Y, Chen W, Li J, He Q, He Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell Mol Life Sci. 2019 Jul;76(14):2681-2695. doi: 10.1007/s00018-019-03101-9[↩]
- Chen, H., Ge, R. S., & Zirkin, B. R. (2009). Leydig cells: From stem cells to aging. Molecular and cellular endocrinology, 306(1-2), 9–16. https://doi.org/10.1016/j.mce.2009.01.023[↩][↩]
- Chen, H., Stanley, E., Jin, S., & Zirkin, B. R. (2010). Stem Leydig cells: from fetal to aged animals. Birth defects research. Part C, Embryo today : reviews, 90(4), 272–283. https://doi.org/10.1002/bdrc.20192[↩]
- Neaves WB. Leydig cells. Contraception. 1975 May;11(5):571-606. doi: 10.1016/0010-7824(75)90111-0[↩]
- Jiang, M. H., Cai, B., Tuo, Y., Wang, J., Zang, Z. J., Tu, X., Gao, Y., Su, Z., Li, W., Li, G., Zhang, M., Jiao, J., Wan, Z., Deng, C., Lahn, B. T., & Xiang, A. P. (2014). Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell research, 24(12), 1466–1485. https://doi.org/10.1038/cr.2014.149[↩]
- Lincoff AM, Bhasin S, Flevaris P, Mitchell LM, Basaria S, Boden WE, Cunningham GR, Granger CB, Khera M, Thompson IM Jr, Wang Q, Wolski K, Davey D, Kalahasti V, Khan N, Miller MG, Snabes MC, Chan A, Dubcenco E, Li X, Yi T, Huang B, Pencina KM, Travison TG, Nissen SE; TRAVERSE Study Investigators. Cardiovascular Safety of Testosterone-Replacement Therapy. N Engl J Med. 2023 Jul 13;389(2):107-117. doi: 10.1056/NEJMoa2215025[↩][↩]
- Corona G, Dicuio M, Rastrelli G, Maseroli E, Lotti F, Sforza A, Maggi M. Testosterone treatment and cardiovascular and venous thromboembolism risk: what is ‘new’? J Investig Med. 2017 Aug;65(6):964-973. doi: 10.1136/jim-2017-000411[↩][↩][↩]
- Gagnon DR, Zhang TJ, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease–the Framingham study: a 34-year follow-up. Am Heart J. 1994 Mar;127(3):674-82. doi: 10.1016/0002-8703(94)90679-3[↩]
- Corona G, Rastrelli G, Marchiani S, Filippi S, Morelli A, Sarchielli E, Sforza A, Vignozzi L, Maggi M. Consequences of Anabolic-Androgenic Steroid Abuse in Males; Sexual and Reproductive Perspective. World J Mens Health. 2022 Apr;40(2):165-178. doi: 10.5534/wjmh.210021[↩]
- Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, Goulis DG, Krausz C, Giwercman A. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology. 2018 Jul;6(4):513-524. doi: 10.1111/andr.12502[↩]
- Corona G, Rastrelli G, Maggi M. The pharmacotherapy of male hypogonadism besides androgens. Expert Opin Pharmacother. 2015 Feb;16(3):369-87. doi: 10.1517/14656566.2015.993607. Epub 2014 Dec 19. Erratum in: Expert Opin Pharmacother. 2015 Apr;16(6):941. doi: 10.1517/14656566.2015.1027864. Ratrelli, Giulia [corrected to Rastrelli, Giulia].[↩]
- Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Resnick SM, Budoff M, Mohler ER 3rd, Wenger NK, Cohen HJ, Schrier S, Keaveny TM, Kopperdahl D, Lee D, Cifelli D, Ellenberg SS. Lessons From the Testosterone Trials. Endocr Rev. 2018 Jun 1;39(3):369-386. doi: 10.1210/er.2017-00234[↩][↩]
- Isidori AM, Buvat J, Corona G, Goldstein I, Jannini EA, Lenzi A, Porst H, Salonia A, Traish AM, Maggi M. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment-a systematic review. Eur Urol. 2014 Jan;65(1):99-112. doi: 10.1016/j.eururo.2013.08.048[↩]
- Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R, Verstovsek S, Mesa R, Kiladjian JJ, Hehlmann R, Reiter A, Cervantes F, Harrison C, Mc Mullin MF, Hasselbalch HC, Koschmieder S, Marchetti M, Bacigalupo A, Finazzi G, Kroeger N, Griesshammer M, Birgegard G, Barosi G. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018 May;32(5):1057-1069. doi: 10.1038/s41375-018-0077-1[↩]
- Ory J, Nackeeran S, Balaji NC, Hare JM, Ramasamy AR. Secondary Polycythemia in Men Receiving Testosterone Therapy Increases Risk of Major Adverse Cardiovascular Events and Venous Thromboembolism in the First Year of Therapy. J Urol. 2022 Jun;207(6):1295-1301. doi: 10.1097/JU.0000000000002437[↩][↩]
- Mottet N, De Santis M, Briers E, Bourke L, Gillessen S, Grummet JP, Lam TB, van der Poel HG, Rouvière O, van den Bergh RCN, Cornford P. Updated Guidelines for Metastatic Hormone-sensitive Prostate Cancer: Abiraterone Acetate Combined with Castration Is Another Standard. Eur Urol. 2018 Mar;73(3):316-321. doi: 10.1016/j.eururo.2017.09.029[↩]
- Rochira V, Antonio L, Vanderschueren D. EAA clinical guideline on management of bone health in the andrological outpatient clinic. Andrology. 2018 Mar;6(2):272-285. doi: 10.1111/andr.12470[↩][↩]
- Ponce OJ, Spencer-Bonilla G, Alvarez-Villalobos N, Serrano V, Singh-Ospina N, Rodriguez-Gutierrez R, Salcido-Montenegro A, Benkhadra R, Prokop LJ, Bhasin S, Brito JP. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: A systematic review and meta-analysis of randomized, placebo-controlled trials. J. Clin. Endocrinol. Metab. 2018 Mar 17[↩]
- Rosano GM, Sheiban I, Massaro R, Pagnotta P, Marazzi G, Vitale C, Mercuro G, Volterrani M, Aversa A, Fini M. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int. J. Impot. Res. 2007 Mar-Apr;19(2):176-82[↩]
- Cheetham TC, An J, Jacobsen SJ, Niu F, Sidney S, Quesenberry CP, VanDenEeden SK. Association of Testosterone Replacement With Cardiovascular Outcomes Among Men With Androgen Deficiency. JAMA Intern Med. 2017 Apr 01;177(4):491-499.[↩]
- Goodman N, Guay A, Dandona P, Dhindsa S, Faiman C, Cunningham GR., AACE Reproductive Endocrinology Scientific Committee. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY POSITION STATEMENT ON THE ASSOCIATION OF TESTOSTERONE AND CARDIOVASCULAR RISK. Endocr Pract. 2015 Sep;21(9):1066-73.[↩]
- Hsu B, Cumming RG, Handelsman DJ. Testosterone, frailty and physical function in older men. Expert Rev Endocrinol Metab. 2018 May;13(3):159-165.[↩]
- Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018 May 01;103(5):1715-1744.[↩]