Contents
- Celiac Disease
- Long Term Health Effects of Celiac Disease
- Rare complications of celiac disease can include
- What are Symptoms of Celiac Disease
- Who Should Be Screened for Celiac Disease ?
- How Coeliac Disease is Diagnosed
- Confirming the Diagnosis of Celiac Disease
- What Follow Up is Needed for Celiac Disease ?
- What Celiac Disease Foods to Avoid
- What is Celiac Disease Diet
- What You Can Eat
- Celiac Disease Treatment
Celiac Disease
In 1950, Dr Willem Karel Dicke, of the Netherlands, discovered the harmful effect of dietary gluten in celiac disease 1. Celiac disease is also known as coeliac disease, celiac sprue, non-tropical sprue, autoimmune enteropathy and gluten sensitive enteropathy. Gluten is the term used to identify a mixture of proteins (prolamines) that occurs in the endosperm of wheat (gliadins) and other cereals such as barley (hordeins) and rye (secalins) 2. Celiac disease is an immune-mediated systemic disorder elicited by gluten and related prolamines in genetically susceptible individuals that is characterized by the presence of a variable combination of gluten-dependent clinical manifestations, veliac disease-specific antibodies, human leucocyte antigen (HLA)-DQ2 or HLA-DQ8 haplotypes, and enteropathy 3.
Celiac disease is a serious autoimmune disorder triggered by the ingestion of gluten, that can occur in genetically predisposed people where the ingestion of gluten (a major protein in wheat, rye, barley or of related proteins in other grains) leads to damage in the small intestine. Research into the root causes indicates that the disorder develops when a person exposed to gluten also has a genetic susceptibility to celiac disease and an unusually permeable intestinal wall. It is estimated to affect 1 -3 in 100 (1-3%) people worldwide, except for populations in which the HLA risk alleles (celiac disease genes) (HLA-DQ2 and/or DQ8) are rare such as in South East Asia 4, 5, 6, 7. Two and one-half million Americans are undiagnosed and are at risk for long-term health complications 8.
Celiac disease is also hereditary, meaning that it runs in families. People with a first-degree relative with celiac disease (parent, child, sibling) have a 1 in 10 risk of developing celiac disease 8. Furthermore, first-degree family members (parent, child, sibling) of patients with celiac disease have an increased risk for the disease, already at a young age, ranging from 5% to 30%, depending on their sex and HLA makeup 9, 10. Celiac disease also is more common among people with certain other diseases, such as Down syndrome, Turner syndrome and type 1 diabetes.
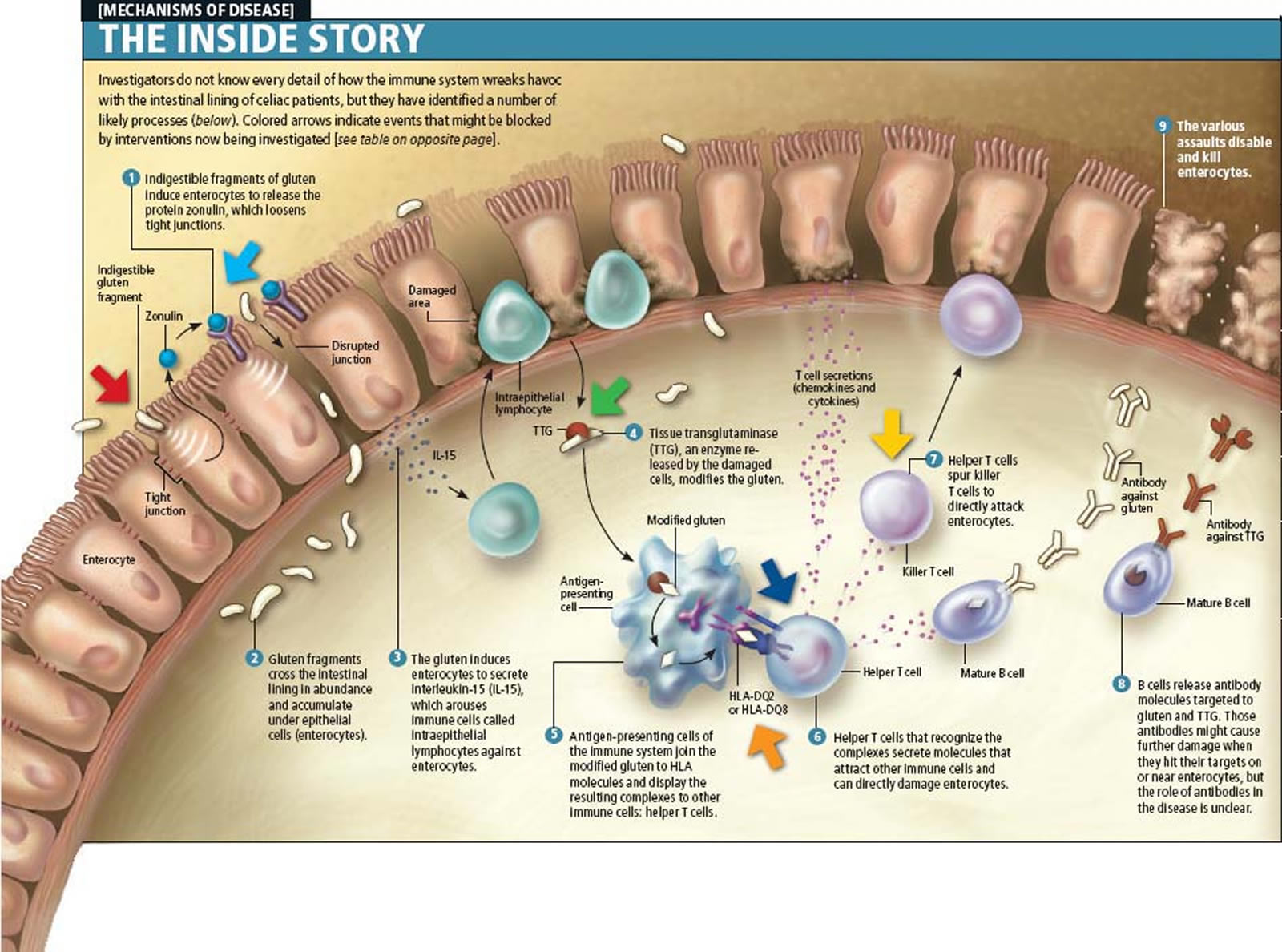
Patients with other autoimmune diseases, including type 1 diabetes mellitus and autoimmune thyroid disease, or patients with selective immunoglobulin A deficiency, and those with certain syndromes such as Down syndrome, Turner syndrome, and Williams syndrome, have an increased risk of coeliac disease 9. More than 95% of patients with coeliac disease carry the HLA-DQ2 or -DQ8 heterodimers, and the rest express HLA-DQ that contain “half” of the coeliac disease-associated molecules. In coeliac disease, gluten peptides, after crossing the small intestinal epithelium into the lamina propria, are deaminated by the enzyme tissue-transglutaminase and presented by HLA-DQ2–positive or HLA-DQ8–positive antigen-presenting cells to activated T cells. Once activated, the T cells produce interferon-γ and other cytokines, leading to a higher expression of the HLA-DQ molecules and thereby to increased gluten peptide presentation. This inflammatory process mediated by T cells leads to mucosal damage of the small bowel 11.
The incidence of coeliac disease is increasing worldwide, and many patients remain undiagnosed, probably because of the clinical picture being so diverse and because coeliac disease can affect any organ, and not just the gastrointestinal tract 12, 13, 14, 15. The development of coeliac disease and the onset of symptoms may occur at any age. The classical clinical picture of overt malabsorption with diarrhoea, abdominal distension, and weight loss is observed, but only in a minority of children. Nonspecific signs and symptoms such as iron deficiency anaemia, osteoporosis, or fatigue are now common and could be the only sign of coeliac disease 12. In addition, coeliac disease may be asymptomatic.
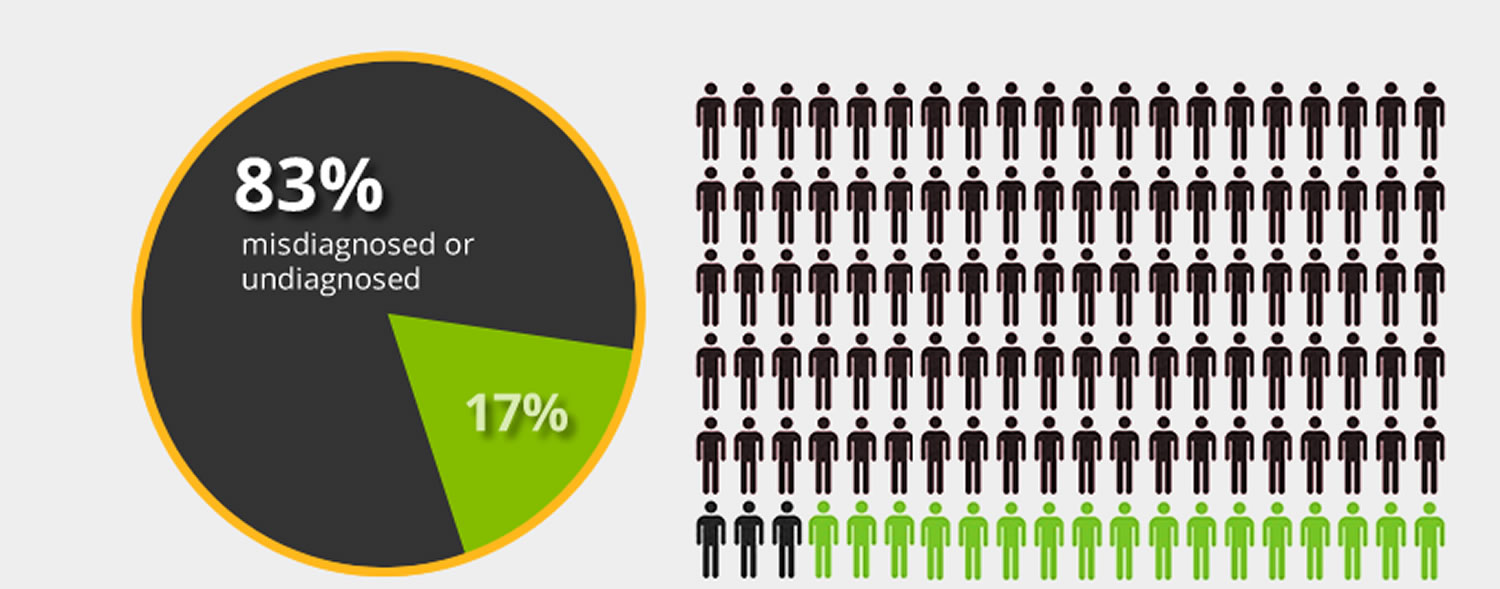
Figure 1. It is estimated that 83% of Americans who have celiac disease are undiagnosed or misdiagnosed with other conditions.
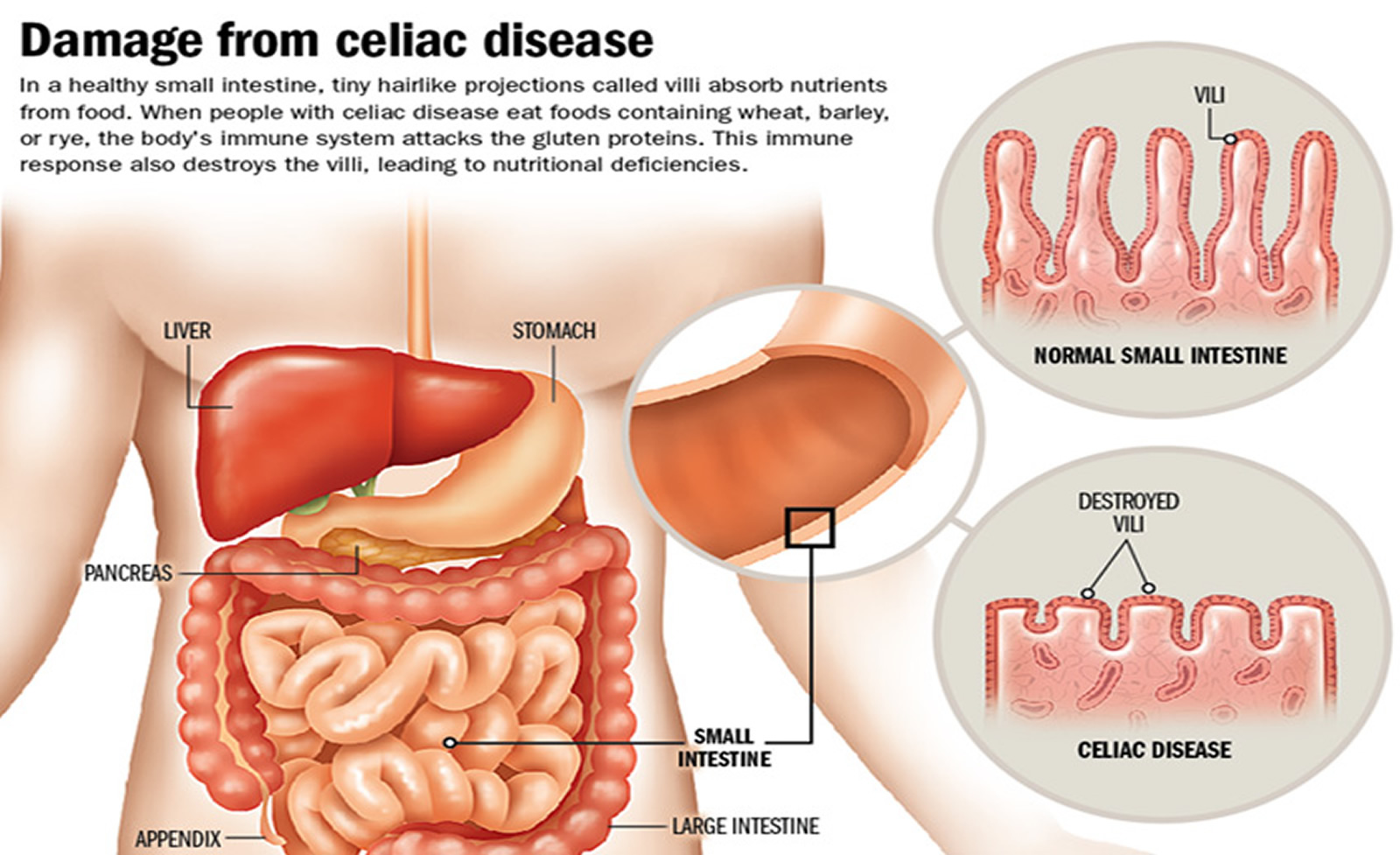
[Source: Beyond Celiac. Celiac Disease: Fast Facts 16]When people with celiac disease eat gluten (a protein found in wheat, rye and barley), their body mounts an immune response that attacks the small intestine. These attacks lead to damage on the villi, small fingerlike projections that line the small intestine, that promote nutrient absorption. When the villi get damaged, nutrients cannot be absorbed properly into the body 8.
Celiac disease can be very serious. The disease can cause long-lasting digestive problems and keep your body from getting all the nutrients it needs. Celiac disease can also affect the body outside the intestine 17.
Celiac disease is different from gluten sensitivity or wheat intolerance 17. If you have gluten sensitivity, you may have symptoms similar to those of celiac disease, such as abdominal pain and tiredness. Unlike celiac disease, gluten sensitivity does not damage the small intestine.
People with gluten sensitivity have problems with gluten 18. Gluten-sensitive individuals cannot tolerate gluten and may develop gastrointestinal symptoms similar to those in celiac disease, but the overall clinical picture is generally less severe and is not accompanied by the concurrence of tissue transglutaminase autoantibodies or autoimmune comorbidities. Gluten sensitivityas a condition associated with prevalent gluten-induced activation of innate, rather than adaptive, immune responses in the absence of detectable changes in mucosal barrier function 19. It is different from celiac disease, an immune disease in which people can’t eat gluten because it will damage their small intestine 19. Unlike celiac disease, gluten sensitivity is not associated with increased intestinal permeability and does not damage the small intestine like celiac disease. Some of the symptoms of gluten sensitivity are similar to celiac disease. They include tiredness and stomachaches. It can cause other symptoms too, including muscle cramps and leg numbness.
Celiac disease is also different from a wheat allergy 20, 11. In both cases, your body’s immune system reacts to wheat. However, some symptoms in wheat allergies, such as having itchy eyes or a hard time breathing, are different from celiac disease. Wheat allergy is an immunoglobulin E–mediated reaction to the insoluble gliadins, particularly ω-5 gliadin, the major allergen of wheat-dependent, exercise-induced anaphylaxis (“baker’s asthma”) 21. Usually, patients with wheat allergy are not allergic to other prolamines containing grains, such as rye or barley and their wheat-free diet is less restrictive than the strict gluten-free diet for patients with celiac disease. The symptoms of wheat allergy develop within minutes to hours after gluten ingestion and are typical for an immunoglobulin E–mediated allergy, including itching and swelling in the mouth, nose, eyes, and throat; rash and wheezing; and life-threatening anaphylaxis. The gastrointestinal manifestations of wheat allergy may be similar to those of coeliac disease, but wheat allergy does not cause (permanent) gastrointestinal damage 20. Those with wheat allergy also benefit from the gluten free diet, although these patients often do not need to restrict rye, barley, and oats from their diet 20.
Long Term Health Effects of Celiac Disease
Celiac disease can develop at any age after people start eating foods or medicines that contain gluten. Celiac disease affects people differently. Some people develop symptoms as children and others as adults. Symptoms vary and may or may not occur in the digestive system. They may include diarrhea, abdominal pain, weight loss, irritability, and depression, among others. Irritability is one of the most common symptoms among children. In some cases, a diagnosis of celiac disease is missed because the symptoms are so varied and may only flare up occasionally 22.
Children and adults with untreated celiac disease may become malnourished, leading to additional serious health problems. These include the development of other autoimmune disorders like Type I diabetes and multiple sclerosis (MS), dermatitis herpetiformis (an itchy skin rash), anemia, weight loss, osteoporosis, infertility and miscarriage, neurological conditions like epilepsy and migraines, intestinal cancers and in children, delayed growth and small stature 8. Among the possible complications of untreated celiac disease is the inability to develop optimal bone mass in children and the loss of bone in adults, both of which increase the risk of osteoporosis 22. Osteoporosis is a complication of untreated celiac disease. The small intestine is responsible for absorbing important nutrients, such as calcium. Calcium is essential for building and maintaining healthy bones. Even people with celiac disease who consume enough calcium are often deficient in this nutrient. And because calcium is needed to keep bones healthy, low bone density is common in both children and adults with untreated and newly diagnosed celiac disease 22.
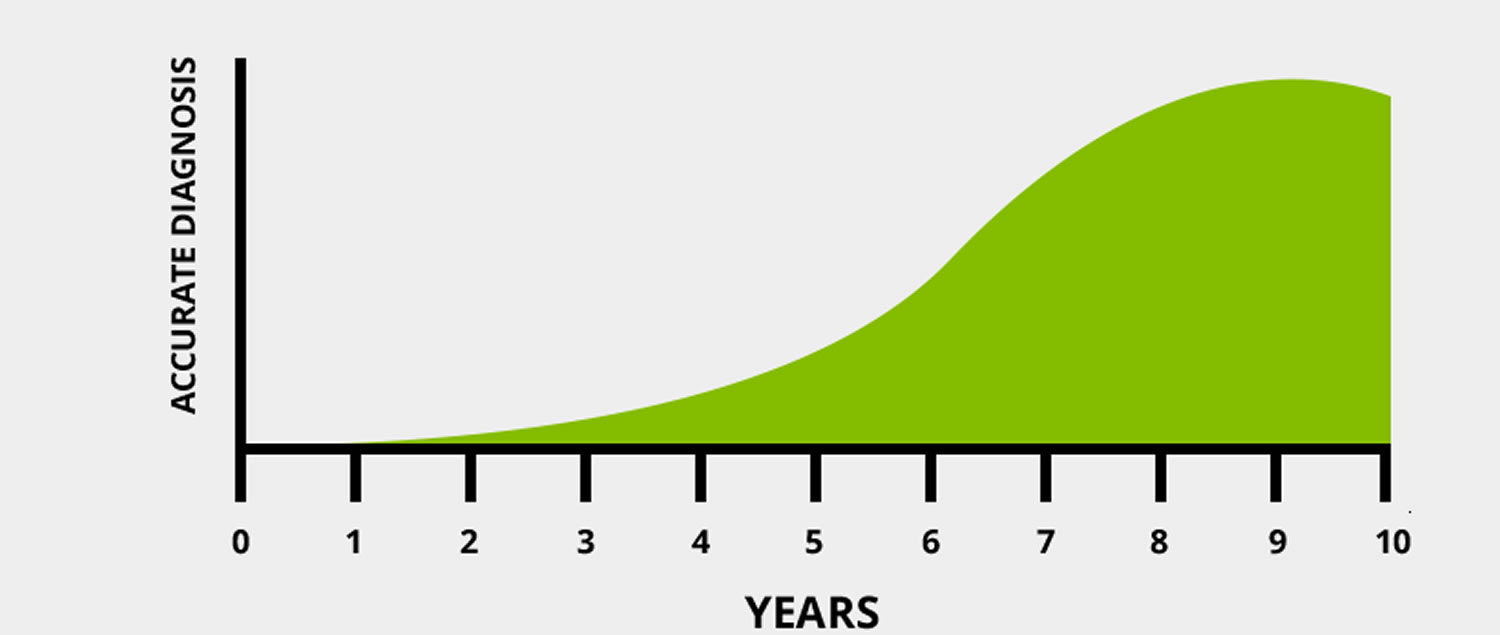
Figure 2. 6-10 years is the average time a person waits to be correctly diagnosed.
[Source: Beyond Celiac. Celiac Disease: Fast Facts 16]Long-Term Health Conditions of Undiagnosed or Untreated Celiac Disease
- Iron deficiency anemia
- Early onset osteoporosis or osteopenia
- Infertility and miscarriage
- Lactose intolerance
- Vitamin and mineral deficiencies
- Central and peripheral nervous system disorders
- Pancreatic insufficiency
- Intestinal lymphomas and other gastrointestinal cancers (malignancies)
- Gall bladder malfunction
- Neurological manifestations, including ataxia, epileptic seizures, dementia, migraine, neuropathy, myopathy and multifocal leucoencephalopathy
Rare complications of celiac disease can include
- Intestinal cancer
- Liver diseases
- Lymphoma, a cancer of part of the immune system called the lymph system that includes the gut.
In rare cases, you may continue to have trouble absorbing nutrients even though you have been following a strict gluten-free diet. If you have this condition, called refractory celiac disease, your intestines are severely damaged and can’t heal. You may need to receive nutrients intravenously 17.
In a 1999 a large Italian study, Ventura, et al. found a correlation between the duration of exposure to gluten and the prevalence of autoimmune disorder in patients with celiac disease 23. The incidence of autoimmune disorder varied inversely as to the age of diagnosis of celiac disease, implying that the longer the exposure to gluten, the more likely one is to develop an autoimmune disorder 23. This is in keeping with the conclusion found by Cosnes et al 24 that a risk factor for developing autoimmune disorder was the diagnosis of celiac disease at a young age. However, the conclusion that a gluten free diet is protective against the development of autoimmune disorder is still controversial 25.
Table 1. Autoimmune and Other Conditions Associated with Celiac Disease
| Autoimmune Condition | Prevalence in Celiac Disease Population |
| Anemia | 12-69% |
| Autoimmune Hepatitis | 2% |
| Autoimmune Thyroid Disease | 26% |
| Chronic fatigue syndrome | 2% |
| Dermatitis Herpetiformis | 25% |
| Down’s Syndrome | 12% |
| Gluten Ataxia | 10-12% |
| Idiopathic Dilated Cardiomyopathy | 5.7% |
| Juvenile Idiopathic Arthritis | 1.5-6.6% |
| Liver Disease | 10% |
| Lymphocytic Colitis | 15-27% |
| Microscopic Colitis | 4% |
| Peripheral Neuropathy | 10-12% |
| Primary Bilary Cirrhosis | 3% |
| Sjögren’s Syndrome | 3% |
| Type 1 Diabetes | 8-10% |
| Unexplained infertility | 12% |
(Source 8).
What are Symptoms of Celiac Disease
Celiac disease can be difficult to diagnose because it affects people differently. There are more than 200 known celiac disease symptoms which may occur in the digestive system or other parts of the body. Some people with celiac disease have no symptoms at all, but still test positive on the celiac disease blood test. A few others may have a negative blood test, but have a positive intestinal biopsy.
All people with celiac disease who have no symptoms can still develop long-term complications from the disease over time if they do not get treatment.
Some people develop celiac disease as a child, others as an adult. The reason for this is still unknown 8.
- Gastrointestinal symptoms are still prominent, particularly in younger children. The onset of celiac disease in infancy and very early childhood may have severe gastrointestinal manifestations resulting in malnutrition, failure to thrive, and in some patients a protein-losing enteropathy. Although these were relatively common presentations of celiac disease in the past, they are rare nowadays 26.
Here are the most common symptoms found in children 27:
- abdominal bloating and pain
- chronic diarrhea
- vomiting
- constipation
- pale, foul-smelling, or fatty stool
- weight loss
- fatigue
- irritability and behavioral issues
- dental enamel defects of the permanent teeth
- delayed growth and puberty
- short stature
- failure to thrive
- Attention Deficit Hyperactivity Disorder (ADHD)
Depending on how old you are when a doctor diagnoses your celiac disease, some symptoms, such as short height and tooth defects, will not improve.
Adults are less likely to have digestive symptoms, with only one-third experiencing diarrhea 27.
Adults are more likely to have:
- unexplained iron-deficiency anemia
- a red, smooth, shiny tongue
- fatigue
- bone or joint pain
- headaches
- arthritis
- osteoporosis or osteopenia (bone loss)
- liver and biliary tract disorders (transaminitis, fatty liver, primary sclerosing cholangitis, etc.)
- depression or anxiety
- peripheral neuropathy ( tingling, numbness or pain in the hands and feet)
- seizures or migraines
- missed menstrual periods
- infertility or recurrent miscarriage
- canker sores inside the mouth
- dermatitis herpetiformis (itchy skin rash)
- seizures
- tingling numbness in the hands and feet
- tiredness that lasts for long periods of time
- abdominal pain and bloating
- intestinal blockages
- ulcers, or sores on the stomach or lining of the intestine
Dermatitis herpetiformis is an itchy, blistering skin rash that usually appears on the elbows, knees, buttocks, back, or scalp. The rash affects about 10 percent of people with celiac disease. The rash can affect people of all ages but is most likely to appear for the first time between the ages of 30 and 40. Men who have the rash also may have oral or, rarely, genital sores. Some people with celiac disease may have the rash and no other symptoms. If a doctor suspects you have dermatitis herpetiformis, he or she will perform a skin biopsy. A doctor then examines the skin tissue and checks the tissue for antibodies common in celiac disease. If the skin tissue has the antibodies, a doctor will perform blood tests to confirm celiac disease. If the skin biopsy and blood tests both suggest celiac disease, you may not need an intestinal biopsy.
Celiac disease also can produce a reaction in which your immune system, or your body’s natural defense system, attacks healthy cells in your body. This reaction can spread outside your digestive tract to other areas of your body, including your
- bones
- joints
- nervous system
- skin
- spleen
Abdominal pain and distention with diarrhea or even frank steatorrhea are hallmarks of celiac disease, but severe forms of these manifestations have become progressively less frequent, and milder forms are more common at initial presentation. Counter intuitively, severe constipation related to delay in orocecal transit time (time needed by food eaten by mouth to reach the cecum which is frequently used as an indicator of small intestinal transit time) 28, 29, possibly aggravated by disordered upper gastrointestinal motor function 30, can be the presenting manifestation in a significant number of children. Although celiac disease is typically thought to be associated with weight loss or failure to gain weight, some children with celiac disease are initially overweight or obese 31. Less common presentations include acute electrolyte disturbances, hypotension, and lethargy, and recurrent intussusception occurs more frequently in children with celiac disease 32.
- There are also numerous extraintestinal manifestations, and almost any body system can be involved. Older children and adolescents are more likely to present with nongastrointestinal symptoms 33, 34, 35 and previously used terms of “typical” and “atypical” to describe gastrointestinal and extraintestinal symptoms, respectively, are now considered obsolete and no longer recommended 36. The variable nature of the clinical manifestations, and the fact that celiac disease may be asymptomatic, is believed to be largely responsible for the majority of people with celiac disease remaining undiagnosed.
A mild elevation of serum liver enzymes is also well described as a presenting manifestation of celiac disease in the pediatric age group and may account for up to 12% of children with unexplained hypertransaminasemia 37. The enzymes involved are alanine aminotransferase and aspartate aminotransferase, and typically these are elevated in the region of 2 to 3 times the upper limit of normal. Following institution of a gluten free diet, the majority of affected patients will have normal transaminase levels within 4 to 8 months 37. In a small number, hypertransaminasemia persists despite strict adherence to agluten free diet. In these, additional workup should be considered to look for other causes of liver disease, such as autoimmune hepatitis, which can be associated with celiac disease.
- Anemia, most commonly as a result of iron deficiency, has been reported in 12% to 69% of newly diagnosed patients 38, 39, 40, 41 and appears more prevalent in celiac patients with an atrophic mucosa compared with those with mild enteropathy 42.
- Linear growth failure as an isolated initial presentation of celiac disease is well described and can be found in up to 10% of children undergoing investigation for short stature 43, 44.
- Dermatitis herpetiformis is considered a skin presentation of celiac disease and is more common in adults or older teenagers. It is characterized by symmetrical, pruritic blisters followed by erosions, excoriations, and hyperpigmentation most commonly involving elbows (90%), knees (30%), shoulders, buttocks, sacral region, and face 45. The diagnosis of dermatitis herpetiformis depends on demonstrating typical immunoglobulin A (IgA) deposits on skin biopsies 46.
- Other manifestations include dental enamel hypoplasia (24), recurrent aphthous ulcers in the mouth, low–bone mineral density, and arthritis/arthralgia 47. Although children with low–bone mineral density appear better able to correct this deficiency after starting the gluten free diet, recovery can be delayed in some patients, whereas others are at risk for never achieving optimal bone density as they go through puberty 48, 49.
- There appears to be a slight increase in the frequency of neurological symptoms including headache, peripheral neuropathy, and seizures in celiac disease 50, 51, 52, 53. In 1 young adult with celiac disease and epilepsy refractory to antiepileptic drugs, seizures were controlled with a gluten free diet 54.
- Adolescents with celiac disease have been reported to have psychiatric issues including anxiety, recurrent panic attacks, hallucinations, depression, and an increased prevalence of suicidal behavior. There is some evidence that the gluten free diet may help alleviate depression in adolescents with celiac disease 55, 56.
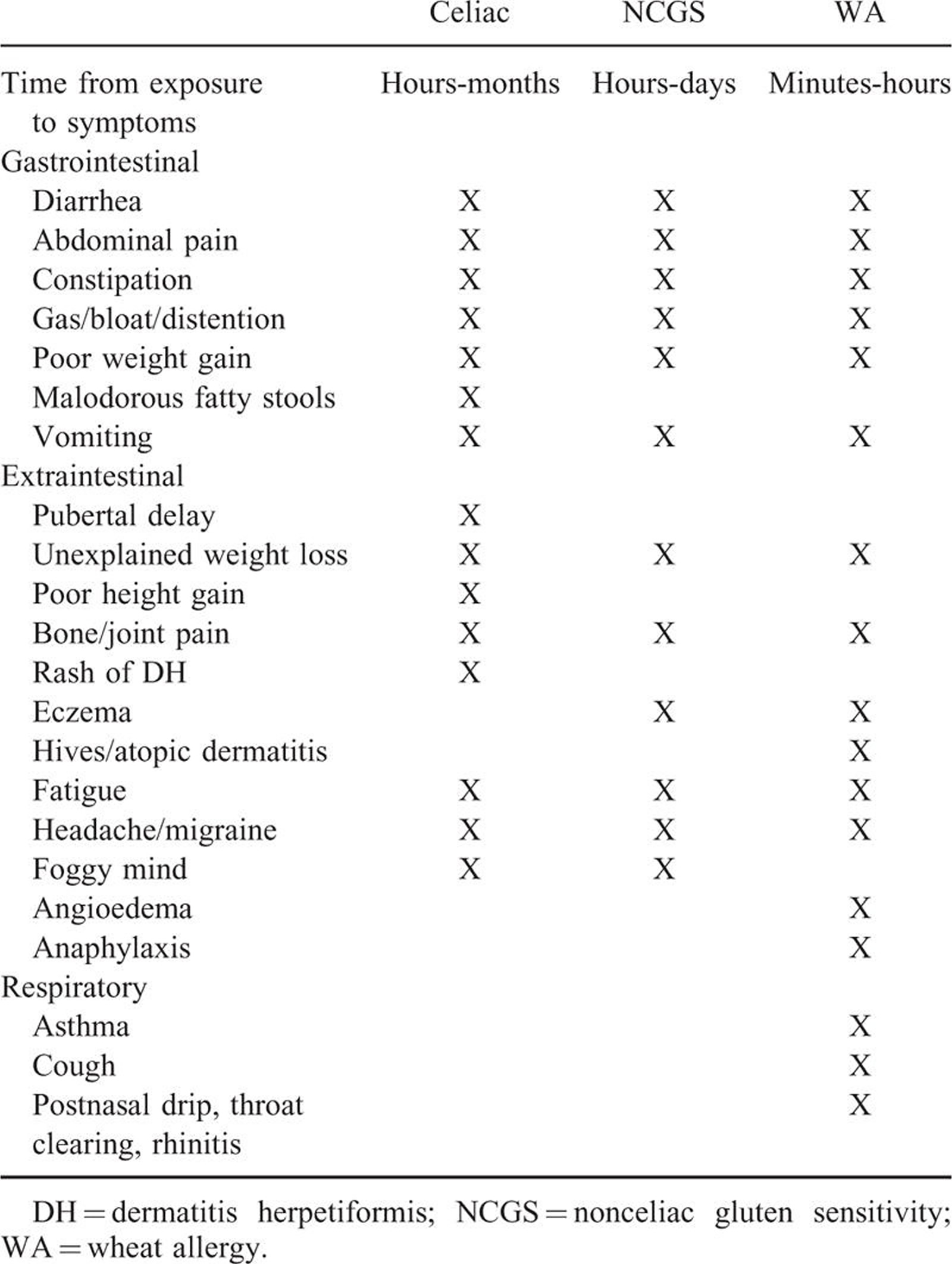
Table 2. The clinical manifestations of gluten-related disorders are numerous and complex in nature and involve multiple organ systems. There is considerable overlap of symptoms between these conditions, which makes differentiation impossible on clinical grounds alone. (Source 57).
Who Should Be Screened for Celiac Disease ?
According the the Celiac Disease Center at Columbia University Medical Center, “anyone who suffers from an unexplained, stubborn illness for several months, should consider celiac disease a possible cause and be properly screened for it.” 27
First-degree relatives (parent, child, sibling) should also be screened since they have a 1 in 10 risk of developing celiac disease compared to the general population risk of 1 in 100 58.
- Children older than 3 and adults experiencing symptoms of celiac disease.
- First-degree relatives of people with celiac disease (parent, child, sibling)
- Any individual with an associated autoimmune disorder or other condition, especially type 1 diabetes mellitus, autoimmune thyroid disease, autoimmune liver disease, Down syndrome, Turner syndrome, Williams syndrome, and selective immunoglobulin A (IgA) deficiency.
The First Step: tTG-IgA Test
For most children and adults, the best way to screen for celiac disease is with the Tissue Transglutaminase IgA antibody, plus an IgA antibody in order to ensure that the patient generates enough of this antibody to render the celiac disease test accurate. Total Serum IgA to test for IgA deficiency (this health condition can affect accuracy of antibody test). While it is rare, IgA deficiency can cause a negative blood test (or panel) but still experiencing symptom.
For young children (around age 2 years or below), Deamidated Gliadin IgA and IgG antibodies should also be included.
All celiac disease blood tests require that you be on a gluten-containing diet to be accurate.
- Tissue Transglutaminase Antibodies (tTG-IgA) – The tTG-IgA test will be positive in about 98% of patients with celiac disease who are on a gluten-containing diet. This is called the test’s sensitivity. The same test will come back negative in about 95% of healthy people without celiac disease. This is called the test’s specificity. Though rare, this means patients with celiac disease could have a negative antibody test result.
There is also a slight risk of a false positive test result, especially for people with associated autoimmune disorders like type 1 diabetes, autoimmune liver disease, Hashimoto’s thyroiditis, psoriatic or rheumatoid arthritis, and heart failure, who do not have celiac disease.
There are other antibody tests available to double-check for potential false positives or false negatives, but because of potential for false antibody test results, a biopsy of the small intestine is the only way to diagnose celiac disease.
Other tests:
- IgA Endomysial antibody (EMA): The EMA test has a specificity of almost 100%, but is not as sensitive as the tTG-IgA test. About 5-10% of people with celiac disease do not have a positive EMA test. It is also very expensive in comparison to the tTG-IgA and requires the use of primate esophagus or human umbilical cord. It is usually reserved for difficult to diagnose patients.
- Total serum IgA: This test is used to check for IgA deficiency, a condition associated with celiac disease that can cause a false negative tTG-IgA or EMA result. If you are IgA deficient, your doctor can order a DGP or tTG-IgG test.
- Deaminated gliadin peptide (DGP IgA and IgG): This test can be used to further screen for celiac disease in individuals with IgA deficiency or people who test negative for tTg or EMA antibodies.
While it is very rare, it is possible for someone with celiac disease to have negative antibody test results. If your tests were negative, but you continue to experience symptoms, consult your physician and undergo further medical evaluation.
Gluten Challenge
If you are currently on a gluten-free diet, your physician may recommend a gluten challenge to allow antibodies to build in your bloodstream prior to testing. The recommended gluten intake for the gluten challenge is two slices of wheat-based bread for 6-8 weeks. A gluten challenge should only be supervised by a physician trained in celiac disease, who can move you immediately to a biopsy if your symptoms are severe. Never undertake a gluten challenge when pregnant.
Genetic Testing
People with celiac disease carry one or both of the HLA DQ2 and DQ8 genes, but so does up to 25-30% of the general population. Carrying HLA DQ2 and/or DQ8 is not a diagnosis of celiac disease nor does it mean you will ever develop celiac disease. However, if you carry HLA DQ2 and/or DQ8, your risk of developing celiac disease is 3% instead of the general population risk of 1%.
Since celiac disease is genetic, this means it runs in families. First-degree family members (parents, siblings, children), who have the same genotype as the family member with celiac disease, have up to a 40% risk of developing celiac disease. The overall risk of developing celiac diseaes when the genotype is unknown is 7% to 20%.
A negative gene test excludes the possibility of later developing celiac disease, so this can be valuable information for first-degree family members. We recommend performing the genetic test for celiac disease in family members, especially children, to prevent future unnecessary screening. We recommend screening gene-positive first-degree relatives every 3-5 years.
Who Should Have Celiac HLA Testing ?
- Those on a gluten-free diet – celiac antibody blood testing is not accurate
When diagnosis of celiac disease is not clear
- ambiguous antibody testing results (especially in children under the age of 3)
- equivocal intestinal biopsy results
- discrepancy between antibody and biopsy findings
Family members of people with celiac disease to evaluate risk
- a negative result assures a 99% probability that the family member will NOT develop celiac disease
- a positive result indicates the family member should follow up with celiac antibody testing every 2-3 years or immediately if symptoms develop
How Do You Get Tested ?
Your physician should be able to order genetic testing. Genetic testing can be done by blood test, saliva test or cheek swab.
Genetic testing is expensive with the cost running in the hundreds of dollars, but may be covered by some insurance plans. First-degree family members unsure about the expense should weigh this against the time and expense of undergoing life-time serologic testing.
How Coeliac Disease is Diagnosed
Differentiating celiac disease from wheat allergy and gluten sensitivity is important because there are significant differences in potential long-term health consequences. People with celiac disease are at increased risk for other autoimmune diseases such as autoimmune thyroiditis and should be monitored for these. Those with symptomatic celiac disease who do not follow a strict gluten free diet have increased risk for mortality and relative increased risk for intestinal malignancies 26. On the contrary, those with wheat allergy and gluten sensitivity may be able to follow a less restrictive diet and have a lower risk for long-term adverse health outcomes. Although symptom relief following self-initiation of a gluten free diet is evidence for the role of gluten, this is not diagnostic by itself. A gluten challenge should be considered in those who initiate a gluten free diet before confirmatory diagnostic testing, given the significance of the long-term clinical implications. The decision to undertake a gluten challenge should be considered carefully in the context of each individual patient.
Coeliac disease is diagnosed by a combination of clinical suspicion, detection of specific autoantibodies to tissue-transglutaminase, endomysium, and deamidated forms of gliadin peptides, and histology of small bowel biopsies performed when the patient is on a gluten-containing diet 12. The characteristic histological alterations of the small bowel mucosa of patients with coeliac disease who consume gluten are partial to total villous atrophy with crypt hyperplasia and intraepithelial lymphocytic infiltration, rated according to the Marsh classification III and IV. At present, the only treatment of coeliac disease is lifelong adherence to a gluten-free diet that reduces the risk of complications and the increased mortality 11.
- Commercial serological tests for both immunoglobulin A (IgA) and immunoglobulin G (IgG) antibodies against gliadin (AGA), IgA Endomysial antibody (EMA), Tissue Transglutaminase Antibodies (tTG-IgA), and deamidated gliadin peptides (DGPs) are available. Serological tests for CD are dependent on the consumption of gluten, and avoidance of gluten before testing can result in a false-negative result. Although the exact duration of gluten consumption required before testing is not known, experts agree that the ingestion of ≥10 g of gluten (equivalent to 2 slices of whole wheat bread) per day for ≥8 weeks should allow for confident interpretation of the tissue transglutaminase antibody test result.
- Present guidelines recommend the tTG-IgA antibody as the most cost-effective and reliable test to identify people who may have celiac disease 59, 60, 61, 62. Obtaining a serum IgA level at the same time should be considered to identify those who have selective IgA deficiency.The tissue transglutaminase-IgA antibody is performed by means of an enzyme-linked immunosorbent assay or radio immune assay (RIA) method and is highly sensitive and specific . The EMA-IgA is less sensitive than the tissue transglutaminase-IgA but slightly more specific. The endomysium requires an immunofluorescent technique using monkey esophagus or human umbilical cord as the substrate. It is more expensive than the tTG and subject to interobserver variability, and thus is prone to false-negative results and, to a lesser extent, particularly at low titers, to false-positive results in inexperienced hands.The antibodies against gliadin tests are both poorly sensitive and specific compared with the tissue transglutaminase and endomysium, and prone to wide variability between laboratories. Therefore, antibodies against gliadin tests are not recommended for initial diagnosis of celiac disease 63, 60, 61. DGP tests detect antibodies against synthetically derived peptides and perform better than the AGA tests. The DGP-IgG has comparable specificity but lower sensitivity than the tissue transglutaminase-IgA and endomysium-IgA, whereas the DGP-IgA is both less sensitive and specific.Use of a panel of antibodies instead of a single tTG-IgA test is not recommended. Although this approach may be associated with a marginal increase in the sensitivity of the test, it decreases the specificity and significantly increases the costs 61, 64.
- HLA Tests: The HLA DQ heterodimers DQ2 and/or DQ8 are necessary for celiac disease but not specific to people with celiac disease, and can be found in up to 40% of the general population 26.Testing for HLA-DQ2/8 is best reserved for patients in whom there is a diagnostic dilemma, such as when there is a discrepancy between the serological and histologic findings or when a gluten free diet has been started before any testing. In such patients, if neither HLA-DQ2 nor DQ8 is present, celiac disease is highly unlikely, and an alternative diagnosis should be sought.It has been recommended that the HLA test should be used as a first test when screening asymptomatic people at increased risk for celiac disease such as family members of an index case 65. In those who are negative for both DQ2 and DQ8 alleles, no further testing for celiac disease is needed, whereas in all other patients testing for tTG/EMA antibodies is needed to identify those who require intestinal biopsies to confirm the diagnosis. Currently, the HLA tests are expensive, and the cost-effectiveness of such a strategy has not been determined 26.
- Point-of-Care Tests: Rapid tests for tTG antibodies that can be used at the point of care have accuracy similar to that of tTG tests by laboratory detection 65. These point of care tests do not allow for quantitative analysis of the antibody levels and therefore should not be used to replace laboratory testing. If a point of care test is positive, the test should be repeated by means of a standard laboratory test before diagnosis and treatment of celiac disease. In addition, a negative point of care test performed by someone who has not been specifically trained to perform the test should be ignored and repeated by means of a laboratory test.
Confirming the Diagnosis of Celiac Disease
The diagnosis of celiac disease is confirmed on demonstration of the characteristic changes in the histology of the small intestinal mucosa. Biopsies are obtained from the duodenum via an upper gastrointestinal endoscopy. Initially, the histologic changes may be patchy in distribution and confined to the duodenal bulb, so it is recommended that 1 or 2 biopsies be obtained from the bulb and ≥4 from the distal duodenum 59, 60.
Documentation of the characteristic histologic findings of celiac disease on small intestinal biopsy has been considered central to the diagnosis for decades. The cascade of immunologic events that follows ingestion of gluten in those predisposed to develop celiac disease result in an inflammatory state causing derangement of the mucosal architecture. The characteristic microscopic features include infiltration of lymphocytes in the epithelium, increased density and depth of the crypts, and progressive flattening of the villi. This progression was first described by Marsh 66 in 1992, and his scoring system, from stage 0 (normal) to stage 3 (villus blunting), subsequently modified by Oberhuber et al 67, is now widely used by pathologists to diagnose celiac disease.
It is important to note that these changes are not unique for celiac disease and can be seen in other disease processes such as autoimmune enteropathy, food allergies (in children, particularly allergies to cow’s milk and soy protein), Crohn disease, and a number of viral, bacterial, and parasitic infections. Therefore, in addition to the biopsy findings, the clinical history, results of the serological tests, and response to a strict gluten free diet are all essential considerations to confirm a diagnosis of celiac disease 68.
Although a nonbiopsy diagnosis of celiac disease is desirable, there are potential risks associated with skipping the biopsy. There is currently no standardization of serological tests for celiac disease in the United States, and marked variation in antibody levels between commercial assays when the same serum samples are tested has been documented 69, 70, 71. Consequently, it is possible that without biopsy confirmation, some children may be falsely diagnosed with celiac disease and placed on treatment. Because a strict gluten free diet is cumbersome, expensive, and has an adverse impact on the quality of life of the individual, it is important to confirm the diagnosis before recommending such a lifelong dietary change. Another possible disadvantage of a nonbiopsy diagnosis is the potential for missing additional gastrointestinal disorders (such as peptic esophagitis, EoE, Helicobacter pylori gastritis), which may occur as comorbidities in celiac patients and would not be diagnosed without an endoscopy 72.
What Follow Up is Needed for Celiac Disease ?
Repeat determination of celiac disease serological tests at 3- to 6-month intervals demonstrating a progressive decline in antibody levels suggests good compliance. Conversely, failure to demonstrate progressive decline in antibody levels, or an increase in these levels at any stage, requires careful review of the diet by a knowledgeable dietitian for continued overt or inadvertent gluten ingestion. Once you’re symptom free, and the antibody levels have returned to normal, follow-up visits may occur on an annual basis 26.
Although there are some recommendations regarding testing for nutrient deficiencies at the time of diagnosis, these are not based on any good evidence 73. The gluten free diet may be deficient in micronutrients such as iron, folate, and B vitamins, and monitoring for micronutrient deficiency is a consideration. As people with celiac disease are at increased risk for other autoimmune disorders such as autoimmune thyroiditis, monitoring for such conditions should also be considered 26.
What Celiac Disease Foods to Avoid
The Big 3: Wheat, Barley, Rye
1)Wheat is commonly found in:
- breads
- baked goods
- soups
- pasta
- cereals
- sauces
- salad dressings
- roux
Varieties and derivatives of wheat such as:
- wheatberries
- durum
- emmer
- semolina
- spelt
- farina
- farro
- graham
- KAMUT® khorasan wheat
- einkorn wheat
2) Barley is commonly found in:
- malt (malted barley flour, malted milk and milkshakes, malt extract, malt syrup, malt flavoring, malt vinegar)
- food coloring
- soups
- beer
- Brewer’s Yeast
3) Rye is commonly found in:rye
- rye bread, such as pumpernickel
- rye beer
- cereals
4) Triticale is a newer grain, specifically grown to have a similar quality as wheat, while being tolerant to a variety of growing conditions like rye. It can potentially be found in:
- breads
- pasta
- cereals
5) Malt in various forms including: malted barley flour, malted milk or milkshakes, malt extract, malt syrup, malt flavoring, malt vinegar
6) Brewer’s Yeast
7) Wheat Starch that has not been processed to remove the presence of gluten to below 20ppm and adhere to the FDA Labeling Law. According to the FDA, if a food contains wheat starch, it may only be labeled gluten-free if that product has been processed to remove gluten, and tests to below 20 parts per million of gluten. With the enactment of this law on August 5th, 2014, individuals with celiac disease or gluten intolerance can be assured that a food containing wheat starch and labeled gluten-free contains no more than 20ppm of gluten. If a product labeled gluten-free contains wheat starch in the ingredient list, it must be followed by an asterisk explaining that the wheat has been processed sufficiently to adhere to the FDA requirements for gluten-free labeling.
Common Foods That Contain Gluten
- Pastas: raviolis, dumplings, couscous, and gnocchi
- Noodles: ramen, udon, soba (those made with only a percentage of buckwheat flour) chow mein, and egg noodles. (Note: rice noodles and mung bean noodles are gluten free)
- Breads and Pastries: croissants, pita, naan, bagels, flatbreads, cornbread, potato bread, muffins, donuts, rolls
- Crackers: pretzels, goldfish, graham crackers
- Baked Goods: cakes, cookies, pie crusts, brownies
- Cereal & Granola: corn flakes and rice puffs often contain malt extract/flavoring, granola often made with regular oats, not gluten-free oats
- Breakfast Foods: pancakes, waffles, french toast, crepes, and biscuits.
- Breading & Coating Mixes: panko breadcrumbs
- Croutons: stuffings, dressings
- Sauces & Gravies (many use wheat flour as a thickener): traditional soy sauce, cream sauces made with a roux
- Flour tortillas
- Beer (unless explicitly gluten-free) and any malt beverages (see “Distilled Beverages and Vinegars” below for more information on alcoholic beverages)
- Brewer’s Yeast
- Anything else that uses “wheat flour” as an ingredient
Distilled Beverages And Vinegars
Most distilled alcoholic beverages and vinegars are gluten-free. These distilled products do not contain any harmful gluten peptides even if they are made from gluten-containing grains. Research indicates that the gluten peptide is too large to carry over in the distillation process, leaving the resulting liquid gluten-free.
Wines and hard liquor/distilled beverages are gluten-free. However, beers, ales, lagers, malt beverages and malt vinegars that are made from gluten-containing grains are not distilled and therefore are not gluten-free. There are several brands of gluten-free beers available in the United States and abroad.
Foods That May Contain Gluten
- Energy bars/granola bars – some bars may contain wheat as an ingredient, and most use oats that are not gluten-free
- French fries – be careful of batter containing wheat flour or cross-contact from fryers
- Potato chips – some potato chip seasonings may contain malt vinegar or wheat starch
- Processed lunch meats
- Candy and candy bars
- Soup – pay special attention to cream-based soups, which have flour as a thickener. Many soups also contain barley
- Multi-grain or “artisan” tortilla chips or tortillas that are not entirely corn-based may contain a wheat-based ingredient
- Salad dressings and marinades – may contain malt vinegar, soy sauce, flour
- Starch or dextrin if found on a meat or poultry product could be from any grain, including wheat
- Brown rice syrup – may be made with barley enzymes
- Meat substitutes made with seitan (wheat gluten) such as vegetarian burgers, vegetarian sausage, imitation bacon, imitation seafood (Note: tofu is gluten-free, but be cautious of soy sauce marinades and cross-contact when eating out, especially when the tofu is fried)
- Soy sauce (though tamari made without wheat is gluten-free)
- Self-basting poultry
- Pre-seasoned meats
- Cheesecake filling – some recipes include wheat flour
- Eggs served at restaurants – some restaurants put pancake batter in their scrambled eggs and omelets, but on their own, eggs are naturally gluten-free
Other Items That Must Be Verified By Reading The Label Or Checking With The Manufacturer
- Lipstick, lipgloss, and lip balm because they are unintentionally ingested
- Communion wafers
- Herbal or nutritional supplements
- Drugs and over-the-counter medications (Learn about Gluten in Medication)
- Vitamins and supplements (Learn about Vitamins and Supplements)
- Play-dough: children may touch their mouths or eat after handling wheat-based play-dough. For a safer alternative, make homemade play-dough with gluten-free flour.
Gluten in Medication 74
The true chances of getting a medication that contains gluten is extremely small, but you should still eliminate all risks by evaluating the ingredients in your medications by reading the medicine labels.
Medications are composed of many ingredients, both inside and outside of the product. These ingredients, also known as excipients, include the active component, absorbents (which absorb water to allow the tablet to swell and disintegrate), protectants, binders, coloring agents, lubricators, and bulking agents (which allow some products to dissolve slowly as they travel throughout the intestinal tract). Excipients can be synthetic or from natural sources that are derived from either plants or animals. Excipients are considered inactive and safe for human use by the FDA, but can be a potential source for unwanted reactions.
- Dr. Steve Plogsted (Associate Clinical Professor of Pharmacy, Ohio Northern University College of Pharmacy) maintains a website that provides information regarding gluten-free drugs 75. However, this site is for informational purposes only and may contain inaccuracies. Dr. Plogsted advises that, “All persons should interpret the information with caution and should seek medical advice when necessary.” 75
Vitamins & Supplements 76
Vitamin and mineral therapy can be used in addition to the standard gluten-free diet to hasten a patient’s recovery from nutritional deficiency. However, certain ingredients in vitamins and supplements – typically the inactive ingredients – can contain gluten, so extra care must be taken to avoid any gluten exposure.
People recently diagnosed with celiac disease are commonly deficient in fiber, iron, calcium, magnesium, zinc, folate, niacin, riboflavin, vitamin B12, and vitamin D, as well as in calories and protein. Deficiencies in copper and vitamin B6 are also possible, but less common. A study from 2002 by Bona et. al. indicated that the delay in puberty in children with celiac disease may partially be due to low amounts of B vitamins, iron, and folate.
However, after treatment with a strict gluten-free diet, most patients’ small intestines recover and are able to properly absorb nutrients again, and therefore do not require supplementation. For certain patients however, nutrient supplements may be beneficial.
It is also important to remember that “wheat-free” does not necessarily mean “gluten-free.” Be wary, as many products may appear to be gluten-free, but are not.
If In Doubt, Go Without !
When unable to verify ingredients for a food item or if the ingredient list is unavailable DO NOT EAT IT. Adopting a strict gluten-free diet is the only known treatment for those with gluten-related disorders.
What is Celiac Disease Diet
- Babies and Infants
Identifying preventive strategies that would reduce the prevalence of coeliac disease has been a major target of research in recent years 10, 77. Investigated preventive strategies relate to early infant feeding practices, namely to the possible protective effect of breast-feeding, the introduction of gluten while the infant is still being breast-fed, and the age when gluten is introduced into the infant’s diet.
The risk of inducing celiac disease through a gluten-containing diet exclusively concerns persons carrying at least one of the coeliac risk alleles (celiac disease genes). This applies to 30% to 40% of the general population in Europe and to ∼75% to 80% of the offspring of families in which at least one first degree relative (father, mother, sibling) is affected by celiac disease 10, 77. Because the genetic risk alleles are generally not known in an infant at the time of solid food introduction, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition propose that the following recommendations are applicable to all infants, although accepting that they may not be of importance to approximately two-thirds of the population without a genetic predisposition 78.
The European Society for Paediatric Gastroenterology, Hepatology and Nutrition recommended in 2016, based on observational data, gluten can be introduced into the infant’s diet between the ages of 4 and 12 completed months (*4 completed months = 17 weeks of age). The age of gluten introduction in infants in this age range does not seem to influence the absolute risk of developing coeliac disease autoimmunity (defined as positive serology) or celiac disease during childhood 78. Evidence from these randomised controlled trials showed that the age of gluten introduction into the infant’s diet, whether early or late, influences the incidence of each during the first 2 years, but not the cumulative incidence and prevalence of celiac disease during childhood, and, thus, indicated that primary prevention of celiac disease through nutritional interventions is not possible at the present time 10, 77. A systematic review that evaluated evidence from prospective observational studies published up until February 2015 also showed that breast feeding, any or at the time of gluten introduction, as compared to gluten introduction after weaning (i.e., cessation of breast feeding), has not been shown to reduce the risk of developing celiac disease during childhood and had no preventive effect on the development of coeliac disease autoimmunity or celiac disease during childhood 79. Introducing gluten while the infant is being breast-fed cannot be recommended as a means of reducing the risk of developing celiac disease 80. No recommendation can be made regarding the type of gluten to be used at introduction.
Neither the optimal amounts of gluten to be introduced at weaning nor the effects of different wheat preparations on the risks of developing celiac disease and coeliac disease autoimmunity have been established. Despite the limited evidence regarding the exact amounts and with no randomised controlled trials to support it, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition suggests that consumption of large amounts of gluten should be discouraged during the first months after gluten introduction 80.
- Gluten Introduction in Children From Families With a First-Degree Relative With Celiac Disease
The very early development of celiac disease and coeliac disease autoimmunity (<3–5 years of age) seems to affect preferentially children carrying the very high risk of celiac disease alleles (HLA-DQ2.5 homozygous), which are found in only 1% to 2% of the general population but in 10% to 15% of children with first-degree relatives having celiac disease 80.
It remains a matter of debate whether or not separate recommendations for gluten introduction should be formulated for children from families with first-degree relatives who have celiac disease that differ from the recommendations made for the general population. Although present evidence does not support separate recommendations, highlighting the available literature is, however, essential.
Because of a lack of consensus within the group on how to interpret the limited evidence available, no recommendations have been formulated 80. This may change when more data on the outcome of these children become available with long-term follow-up (symptoms, complications, final height). On one hand, it can be argued that delaying gluten introduction toward the end of the first year may be considered in all infants from coeliac disease families to reduce the risk of very early celiac disease manifestation with potential adverse effects on growth and development at a young age, even though delaying gluten introduction may only benefit the 10% to 15% who have the high risk alleles. An alternative approach could be intensive celiac disease screening such as HLA typing in all children born in families with a first-degree relative with celiac disease to assess the risk of coeliac disease by identifying infants with high risk alleles 81, and careful serological screening after gluten introduction to detect celiac disease before deficiencies of macro- and micronutrients develop 80.
What You Can Eat
Fortunately, there are many healthy and delicious foods that are naturally gluten-free.
The most cost-effective and healthy way to follow the gluten-free diet is to seek out these naturally gluten-free food groups, which include:
- Fruits
- Vegetables
- Meat and poultry
- Fish and seafood
- Dairy
- Beans, legumes, and nuts

There are many naturally gluten-free grains that you can enjoy in a variety of creative ways. Many of these grains can be found in your local grocery store, but some of the lesser-known grains may only be found in specialty or health food stores. It is not recommended to purchase grains from bulk bins because of the possibility for cross-contact with gluten.
The following grains and other starch-containing foods are naturally gluten-free:
- Rice
- Cassava
- Corn (maize)
- Soy
- Potato
- Tapioca
- Beans
- Sorghum
- Quinoa
- Millet
- Buckwheat groats (also known as kasha)
- Arrowroot
- Amaranth
- Teff
- Flax
- Chia
- Yucca
- Gluten-free oats
- Nut flours
There has been some research that some naturally gluten-free grains may contain gluten from cross-contact with gluten-containing grains through harvesting and processing. If you are concerned about the safety of a grain, purchase only versions that are tested for the presence of gluten and contain less than 20 ppm.
Celiac Disease Treatment
Currently, the only treatment for celiac disease is lifelong adherence to a strict gluten-free diet. On the diet, the small intestinal mucosal injury heals and gluten-induced symptoms and signs disappear 1. The mucosal healing is a prerequisite for sustaining health and is also obtained with a diet containing oats (100% pure uncontaminated oats) and trace industrially purified wheat starch-based gluten-free products 1. The small intestinal mucosa does not heal in noncompliant people, nor when a patient is inadvertently ingesting gluten.
People living gluten-free must avoid foods with wheat, rye and barley, such as bread and beer. Ingesting small amounts of gluten, like crumbs from a cutting board or toaster, can trigger small intestine damage 8.
In many European countries, industrially purified wheat starch–based gluten-free flours have been part of the gluten-free diet treatment in celiac disease. It was estimated that starch flours meant for patients with celiac disease had a content of <100 ppm (very low gluten) or <20 ppm (gluten-free) 1. As reviewed by Hischenhuber et al 82, evidence-based studies show that a diet including industrially purified gluten-free wheat starch–based flours is safe and the small-intestinal mucosa heals and stays long-term morphologically normal. No differences were observed when comparing the clinical and biopsy results to those patients on a diet that is gluten-free by nature.
The dogma on oats toxicity for patients with celiac disease was challenged by Janatuinen et al in 1995 83. Follow-up evidence-based clinical studies, in children and adults, confirmed avenin not to be harmful, neither clinically or at the mucosal level. This holds true also for dermatitis herpetiformis 84. All gluten-intolerant patients in Finland, both children and adults, have been allowed to consume oats for the last decade. Today 86% of all diagnosed patients have a gluten-free diet including oats. In Europe, an allowance of oats that does not contain wheat, rye, or barley and the gluten level does not exceed 20 ppm, may be determined at the national level. In the United States, oats are not included as one of the prohibited grains and can be labeled as gluten-free, as long as the oats contain <20 ppm gluten. Barley is the grain that often contaminates oats 85, 86. The US Food and Drug Administration has stated that for most individuals with celiac disease, oats can add whole-grain options, nutrient enrichment, and dietary variety. Health Canada in their 2015 review confirms the 2007 conclusions made by Health Canada on the safety of the introduction of uncontaminated oats into the gluten free diet of individuals with celiac disease. More recent information establishes no evidence to restrict such consumption to a limited daily amount 87.
Starch hydrolysates of wheat origin—wheat-based glucose syrups, dextrose, and maltodextrins—are found in >50% of European processed food. It was shown that these common foodstuffs and ingredients elicited no harmful effects to patients with celiac disease 88. The wheat origin of these ingredients does not have to be mentioned on the label of the final foodstuff intended to the end consumer (Commission Directive 2007/68/EC).
Development of adjunctive or alternative therapies is on its way. There are several novel treatment pipelines within academy and industry. Examples are the ideas of using glutenases as a drug to degrade the ingested gluten, polymers to bind and sequester the gluten to the feces, and also vaccine development for an immunotherapy to induce tolerance towards gluten 1.
Summary
Celiac disease is an autoimmune disorder that damages the small intestine and inhibits absorption of nutrients. People with celiac disease cannot tolerate gluten, a protein in wheat, rye, barley, and in some products such as medicines, vitamins, and lip balms. When affected people eat food with gluten, or use a product with gluten, the immune system reacts by damaging tiny parts of the lining of the small intestine called villi. Because villi normally allow the blood to absorb nutrients from food, affected individuals become malnourished 89. Classic signs and symptoms are caused by inflammation of the gastrointestinal tract and may include diarrhea, weight loss, abdominal pain, swelling, and food intolerance. However, many people have other symptoms involving many body systems, and some people have no symptoms 90, 91. While celiac disease tends to run in families, it does not follow a specific inheritance pattern. The risk to develop celiac disease is raised by having certain forms of the HLA-DQA1 and HLA-DQB1 genes 89. Treatment is a lifelong, gluten-free diet 91.
The long-term outlook (prognosis) for people with celiac disease can vary because some people have no symptoms, while others have severe malabsorption features. Overall, people with untreated or unresponsive celiac disease have increased early mortality compared to the general population 92. Without diagnosis and treatment, celiac disease is ultimately fatal in 10 to 30% of people. Currently this outcome is rare, as most people do well if they avoid gluten 93.
Following a gluten-free diet heals the damage to the intestines and prevents further damage. This healing most often occurs within 3-6 months in children and may take 2-3 years in adults. In rare cases there can be long-term damage to the lining of the intestines before the diagnosis is made 94. Strictly adhering to a gluten-free diet also significantly decreases the risk of cancer 93.
- Journal of Pediatric Gastroenterology & Nutrition: July 2014 – Volume 59 – Issue – p S15–S17. doi: 10.1097/01.mpg.0000450397.76521.f9. Celiac Disease Treatment: Gluten-Free Diet and Beyond. http://journals.lww.com/jpgn/Fulltext/2014/07001/Celiac_Disease_Treatment___Gluten_Free_Diet_and.9.aspx[↩][↩][↩][↩][↩]
- Journal of Pediatric Gastroenterology & Nutrition: April 2015 – Volume 60 – Issue 4 – p 429–432. doi: 10.1097/MPG.0000000000000708. Coeliac Disease and Noncoeliac Gluten Sensitivity. http://journals.lww.com/jpgn/Fulltext/2015/04000/Coeliac_Disease_and_Noncoeliac_Gluten_Sensitivity.7.aspx[↩]
- Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54:136–160[↩]
- Mustalahti K, Catassi C, Reunanen A, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med 2010; 42:587–595.[↩]
- Myléus A, Ivarsson A, Webb C, et al. Celiac disease revealed in 3% of Swedish 12-year-olds born during an epidemic. J Pediatr Gastroenterol Nutr 2009; 49:170–176.[↩]
- Catassi C, Gatti S, Fasano A. The new epidemiology of celiac disease. J Pediatr Gastroenterol Nutr 2014; 59 (suppl 1):S7–S9.[↩]
- Ivarsson A, Myleus A, Norstrom F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics 2013; 131:e687–e694.[↩]
- Celiac Disease Foundation. What is Celiac Disease ? https://celiac.org/celiac-disease/understanding-celiac-disease-2/what-is-celiac-disease/[↩][↩][↩][↩][↩][↩][↩]
- Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54:136–160. [↩][↩]
- Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med 2014; 371:1304–1315.[↩][↩][↩][↩]
- Journal of Pediatric Gastroenterology & Nutrition: April 2015 – Volume 60 – Issue 4 – p 429–432. doi: 10.1097/MPG.0000000000000708. Coeliac Disease and Noncoeliac Gluten Sensitivity. http://journals.lww.com/jpgn/Fulltext/2015/04000/Coeliac_Disease_and_Noncoeliac_Gluten_Sensitivity.7.aspx [↩][↩][↩]
- Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54:136–160.[↩][↩][↩]
- Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterololgy 2009; 137:88–93.[↩]
- Catassi C, Kryszak D, Bhatti B, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med 2010; 42:530–538.[↩]
- Burger JP, Roovers EA, Drenth JP, et al. Rising incidence of celiac disease in the Netherlands; an analysis of temporal trends from 1995 to 2010. Scand J Gastroenterol 2014; 49:933–941.[↩]
- Beyond Celiac. Celiac Disease: Fast Facts. https://www.beyondceliac.org/celiac-disease/facts-and-figures/[↩][↩]
- The National Institute of Diabetes and Digestive and Kidney Diseases Health Information Center. Celiac Disease. https://www.niddk.nih.gov/health-information/digestive-diseases/celiac-disease/all-content[↩][↩][↩]
- U.S. National Library of Medicine. Medline Plus. Gluten Sensitivity. https://medlineplus.gov/glutensensitivity.html[↩]
- BMC Med. 2011; 9: 23. Published online 2011 Mar 9. doi: 10.1186/1741-7015-9-23. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3065425/[↩][↩]
- Pietzak, M. Celiac Disease, Wheat Allergy, and Gluten Sensitivity: When Gluten Free Is Not a Fad. Journal of Parenteral and Enteral Nutrition. 2012;36:68S-75S.[↩][↩][↩]
- Mulder CJ, van Wanrooij RL, Bakker SF, et al. Gluten-free diet in gluten-related disorders. Dig Dis 2013; 31:57–62.[↩]
- The National Institutes of Health Osteoporosis and Related Bone Diseases National Resource Center. What People With Celiac Disease Need to Know About Osteoporosis. https://www.niams.nih.gov/Health_Info/Bone/Osteoporosis/Conditions_Behaviors/celiac.asp[↩][↩][↩]
- Ventura A, et al. Duration of exposure to gluten and the risk of autoimmune disorders in patients with celiac disease. Gastroenterology 1999; 117:297–303. https://www.ncbi.nlm.nih.gov/pubmed/10419909[↩][↩]
- Cosnes J, Cellier C, Viola S, et al. Incidence of autoimmune diseases in celiac disease: protective effect of the gluten-free diet. Clin Gastroenterol Hematol 2008; 6:753–758.[↩]
- Journal of Pediatric Gastroenterology & Nutrition: May 2009 – Volume 48 – Issue 5 – p 645–646. doi: 10.1097/MPG.0b013e31819770fe. Incidence of Autoimmune Diseases in Celiac Disease: Protective Effect of the Gluten-Free Diet. http://journals.lww.com/jpgn/Fulltext/2009/05000/Incidence_of_Autoimmune_Diseases_in_Celiac.23.aspx[↩]
- Journal of Pediatric Gastroenterology & Nutrition: July 2016 – Volume 63 – Issue 1 – p 156–165. doi: 10.1097/MPG.0000000000001216. NASPGHAN Clinical Report on the Diagnosis and Treatment of Gluten-related Disorders. http://journals.lww.com/jpgn/Fulltext/2016/07000/NASPGHAN_Clinical_Report_on_the_Diagnosis_and.28.aspx[↩][↩][↩][↩][↩][↩]
- Celiac Disease Foundation. Celiac Disease Symptoms. https://celiac.org/celiac-disease/understanding-celiac-disease-2/celiacdiseasesymptoms/[↩][↩][↩]
- Bai JC, Maurino E, Martinez C, et al Abnormal colonic transit time in untreated celiac sprue. Acta Gastroenterol Latinoam 1995; 25:277–284. https://www.ncbi.nlm.nih.gov/pubmed/8733253[↩]
- Sadik R, Abrahamsson H, Kilander A, et al Gut transit in celiac disease: delay of small bowel transit and acceleration after dietary treatment. Am J Gastroenterol 2004; 99:2429–2436. https://www.ncbi.nlm.nih.gov/pubmed/15571592[↩]
- Cucchiara S, Bassotti G, Castellucci G, et al Upper gastrointestinal motor abnormalities in children with active celiac disease. J Pediatr Gastroenterol Nutr 1995; 21:435–442. https://www.ncbi.nlm.nih.gov/pubmed/8583296[↩]
- Diamanti A, Capriati T, Basso MS, et al Celiac disease and overweight in children: an update. Nutrients 2014; 6:207–220.[↩]
- Reilly NR, Aguilar KM, Green PH. Should intussusception in children prompt screening for celiac disease? J Pediatr Gastroenterol Nutr 2013; 56:56–59. https://www.ncbi.nlm.nih.gov/pubmed/22832512 [↩]
- Garampazzi A, Rapa A, Mura S, et al Clinical pattern of celiac disease is still changing. J Pediatr Gastroenterol Nutr 2007; 45:611–614. https://www.ncbi.nlm.nih.gov/pubmed/18030243[↩]
- Maki M, Kallonen K, Lahdeaho ML, et al Changing pattern of childhood coeliac disease in Finland. Acta Paediatr Scand 1988; 77:408–412. https://www.ncbi.nlm.nih.gov/pubmed/3389134[↩]
- Roma E, Panayiotou J, Karantana H, et al Changing pattern in the clinical presentation of pediatric celiac disease: a 30-year study. Digestion 2009; 80:185–191. https://www.ncbi.nlm.nih.gov/pubmed/19776583[↩]
- Rostami Nejad M, Rostami K, Pourhoseingholi MA, et al Atypical presentation is dominant and typical for coeliac disease. J Gastrointestin Liver Dis 2009; 18:285–291.[↩]
- Vajro P, Paolella G, Maggiore G, et al Pediatric celiac disease, cryptogenic hypertransaminasemia, and autoimmune hepatitis. J Pediatr Gastroenterol Nutr 2013; 56:663–670. https://www.ncbi.nlm.nih.gov/pubmed/23434875[↩][↩]
- Bottaro G, Cataldo F, Rotolo N, et al The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol 1999; 94:691–696. https://www.ncbi.nlm.nih.gov/pubmed/10086653[↩]
- Harper JW, Holleran SF, Ramakrishnan R, et al Anemia in celiac disease is multifactorial in etiology. Am J Hematol 2007; 82:996–1000. https://www.ncbi.nlm.nih.gov/pubmed/17636474[↩]
- Kolho KL, Farkkila MA, Savilahti E. Undiagnosed coeliac disease is common in Finnish adults. Scand J Gastroenterol 1998; 33:1280–1283.[↩]
- Hin H, Bird G, Fisher P, et al Coeliac disease in primary care: case finding study. BMJ 1999; 318:164–167. https://www.ncbi.nlm.nih.gov/pubmed/9888912 [↩]
- Zanini B, Caselani F, Magni A, et al Celiac disease with mild enteropathy is not mild disease. Clin Gastroenterol Hepatol 2013; 11:253–258. https://www.ncbi.nlm.nih.gov/pubmed/23022697[↩]
- Troncone R, Kosova R. Short stature and catch-up growth in celiac disease. J Pediatr Gastroenterol Nutr 2010; 51:S137–S138. https://www.ncbi.nlm.nih.gov/pubmed/21088537[↩]
- Cacciari E, Salardi S, Lazzari R, et al Short stature and celiac disease: a relationship to consider even in patients with no gastrointestinal tract symptoms. J Pediatr 1983; 103:708–711. https://www.ncbi.nlm.nih.gov/pubmed/6631596[↩]
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. Part I. Epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol 2011; 64:1017–1024. https://www.ncbi.nlm.nih.gov/pubmed/21571167[↩]
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. Part II. Diagnosis, management, and prognosis. J Am Acad Dermatol 2011; 64:1027–1033. https://www.ncbi.nlm.nih.gov/pubmed/21571168[↩]
- Ghozzi M, Sakly W, Mankai A, et al Screening for celiac disease, by endomysial antibodies, in patients with unexplained articular manifestations. Rheumatol Int 2013; 34:637–642. https://www.ncbi.nlm.nih.gov/pubmed/24292850[↩]
- Margoni D, Chouliaras G, Duscas G, et al Bone health in children with celiac disease assessed by dual x-ray absorptiometry: effect of gluten-free diet and predictive value of serum biochemical indices. J Pediatr Gastroenterol Nutr 2012; 54:680–684. https://www.ncbi.nlm.nih.gov/pubmed/22094895[↩]
- Krupa-Kozak U. Pathologic bone alterations in celiac disease: etiology, epidemiology, and treatment. Nutrition 2014; 30:16–24. https://www.ncbi.nlm.nih.gov/pubmed/24290593[↩]
- Dimitrova AK, Ungaro RC, Lebwohl B, et al Prevalence of migraine in patients with celiac disease and inflammatory bowel disease. Headache 2013; 53:344–355. https://www.ncbi.nlm.nih.gov/pubmed/23126519[↩]
- Chin RL, Sander HW, Brannagan TH, et al Celiac neuropathy. Neurology 2003; 60:1581–1585. https://www.ncbi.nlm.nih.gov/pubmed/12771245[↩]
- Pengiran Tengah DS, Holmes GK, Wills AJ. The prevalence of epilepsy in patients with celiac disease. Epilepsia 2004; 45:1291–1293. https://www.ncbi.nlm.nih.gov/pubmed/15461685[↩]
- Ludvigsson JF, Zingone F, Tomson T, et al Increased risk of epilepsy in biopsy-verified celiac disease: a population-based cohort study. Neurology 2012; 78:1401–1407. https://www.ncbi.nlm.nih.gov/pubmed/22517096[↩]
- Canales P, Mery VP, Larrondo FJ, et al Epilepsy and celiac disease: favorable outcome with a gluten-free diet in a patient refractory to antiepileptic drugs. Neurologist 2006; 12:318–321. https://www.ncbi.nlm.nih.gov/pubmed/17122729[↩]
- Pynnonen P, Isometsa E, Aronen E, et al Mental disorders in adolescents with celiac disease. Psychosomatics 2004; 45:325–335. https://www.ncbi.nlm.nih.gov/pubmed/15232047[↩]
- Pynnönen P, Isometsä E, Verkasalo M, et al Gluten-free diet may alleviate depressive and behavioural symptoms in adolescents with coeliac disease: a prospective follow-up case-series study. BMC Psychiatr 2005; 5:14.[↩]
- Journal of Pediatric Gastroenterology and Nutrition. 63(1):156-165, July 2016. NASPGHAN Clinical Report on the Diagnosis and Treatment of Gluten-related Disorders. http://journals.lww.com/jpgn/Fulltext/2016/07000/NASPGHAN_Clinical_Report_on_the_Diagnosis_and.28.aspx[↩]
- Celiac Disease Foundation. Celiac Disease Screening. https://celiac.org/celiac-disease/understanding-celiac-disease-2/diagnosing-celiac-disease/screening/[↩]
- Husby S, Koletzko S, Korponay-Szabo IR, et al European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54:136–160. https://www.ncbi.nlm.nih.gov/pubmed/22197856[↩][↩]
- Hill I, Dirks M, Colletti R, et al Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2005; 40:1–19. https://www.ncbi.nlm.nih.gov/pubmed/15625418[↩][↩][↩]
- Rubio-Tapia A, Hill ID, Kelly CP, et al American College of Gastroenterology clinical guideline: diagnosis and management of celiac disease. Am J Gastroenterol 2013; 108:656–676.[↩][↩][↩]
- Kagnoff M. AGA Institute medical position statement on the diagnosis and management of celiac disease. Gastroenterology 2006; 131:1977–1980.[↩]
- Dickey W, Kearney N. Overweight in celiac disease: prevalence, clinical characteristics and effect of a gluten free diet. Am J Gastroenterol 2006; 101:2356–2359. https://www.ncbi.nlm.nih.gov/pubmed/17032202[↩]
- Rashtak S, Ettore M, Homburger H, et al Combination testing for antibodies in the diagnosis of coeliac disease: comparison of multiplex immunoassay and ELISA methods. Aliment Pharmacol Ther 2008; 28:805–813. www.ncbi.nlm.nih.gov/pubmed/19145736[↩]
- Husby S, Koletzko S, Korponay-Szabo IR, et al European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54:136–160.[↩][↩]
- Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (“celiac sprue”). Gastroenterology 1992; 102:330–354. https://www.ncbi.nlm.nih.gov/pubmed/1727768[↩]
- Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999; 11:1185–1194. https://www.ncbi.nlm.nih.gov/pubmed/10524652[↩]
- Dickson B, Streutker C, Chetty R. Coeliac disease: an update for pathologists. J Clin Pathol 2006; 59:1008–1016. https://www.ncbi.nlm.nih.gov/pubmed/17021129[↩]
- Naiyer A, Hernandez L, Ciaccio E, et al Comparison of commercially available serological kits for detection of celiac disease. J Clin Gastroenterol 2009; 43:225–232. https://www.ncbi.nlm.nih.gov/pubmed/18724250[↩]
- Van Meensel B, Hiele M, Hoffman I, et al Diagnostic accuracy of ten second-generation (human) tissue transglutaminase antibody assays in celiac disease. Clin Chem 2004; 50:2125–2135. https://www.ncbi.nlm.nih.gov/pubmed/15388634[↩]
- Egner W, Shrimpton A, Sargur R, et al ESPGHAN guidance on coeliac disease 2012: multiples of ULN for decision making do not harmonise assay performance across centres. J Pediatr Gastroenterol Nutr 2012; 55:733–735. https://www.ncbi.nlm.nih.gov/pubmed/22744189[↩]
- Guandalini S, Newland C. Can we really skip the biopsy in diagnosing symptomatic children with celiac disease? J Pediatr Gastroenterol Nutr 2013; 57:e24. https://www.ncbi.nlm.nih.gov/pubmed/23820401[↩]
- Rubio-Tapia A, Hill ID, Kelly CP, et al American College of Gastroenterology clinical guideline: diagnosis and management of celiac disease. Am J Gastroenterol 2013; 108:656–676[↩]
- Celiac Disease Foundation. Gluten in Medication. https://celiac.org/live-gluten-free/glutenfreediet/gluten-medication/[↩]
- Gluten Free Drugs. http://www.glutenfreedrugs.com/[↩][↩]
- Celiac Disease Foundation. Vitamins & Supplements. https://celiac.org/live-gluten-free/glutenfreediet/vitamins-and-supplements/[↩]
- Lionetti E, Castellaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 2014; 371:1295–1303.[↩][↩][↩]
- Journal of Pediatric Gastroenterology & Nutrition: March 2016 – Volume 62 – Issue 3 – p 507–513. doi: 10.1097/MPG.0000000000001105. Gluten Introduction and the Risk of Coeliac Disease: A Position Paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. http://journals.lww.com/jpgn/Fulltext/2016/03000/Gluten_Introduction_and_the_Risk_of_Coeliac.32.aspx [↩][↩]
- Szajewska H, Shamir R, Chmielewska A, et al. Systematic review with meta-analysis: early infant feeding and coeliac disease—update 2015. Aliment Pharmacol Ther 2015; 41:1038–1054.[↩]
- Journal of Pediatric Gastroenterology & Nutrition: March 2016 – Volume 62 – Issue 3 – p 507–513. doi: 10.1097/MPG.0000000000001105. Gluten Introduction and the Risk of Coeliac Disease: A Position Paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. http://journals.lww.com/jpgn/Fulltext/2016/03000/Gluten_Introduction_and_the_Risk_of_Coeliac.32.aspx[↩][↩][↩][↩][↩]
- Leonard MM, Serena G, Sturgeon C, et al. Genetics and celiac disease: the importance of screening. Expert Rev Gastroenterol Hepatol 2015; 9:209–215.[↩]
- Hischenhuber C, Crevel R, Jarry B, et al. Safe amounts of gluten for patients with wheat allergy or coeliac disease. Aliment Pharmacol Ther 2006; 23:559–575. https://www.ncbi.nlm.nih.gov/pubmed/16480395[↩]
- Janatuinen EK, Pikkarainem PH, Kemppainen TA, et al. A comparison of diets with and without oats in adults with celiac disease. N Engl J Med 1995; 333:1033–1037. https://www.ncbi.nlm.nih.gov/pubmed/7675045[↩]
- Reunala T, Collin P, Holm K, et al. Tolerance to oats in dermatitis herpetiformis. Gut 1998; 43:490–493. https://www.ncbi.nlm.nih.gov/pubmed/9824575[↩]
- Kanerva PM, Sonntag-Strohm TS, Ryöppy PH, et al. analysis of barley contamination in oats using R5 and omega-gliadin antibodies. J Cereal Sci 2006; 44:347–352.[↩]
- Food Chem. 2017 Feb 1;216:170-5. doi: 10.1016/j.foodchem.2016.08.031. Epub 2016 Aug 12. Gluten-containing grains skew gluten assessment in oats due to sample grind non-homogeneity. https://www.ncbi.nlm.nih.gov/pubmed/27596406[↩]
- Health Canada updated May 29, 2015. Celiac Disease and Gluten-Free Claims on Uncontaminated Oats. https://www.canada.ca/en/health-canada/services/food-nutrition/public-involvement-partnerships/consultation-celiac-disease-gluten-free-claims-uncontaminated-oats/consultation.html[↩]
- Kaukinen K, Salmi T, Collin P, et al. Clinical trial: gluten microchallenge with wheat-based starch hydrolysates in coeliac disease patients – a randomized, double-blind, placebo-controlled study to evaluate safety. Aliment Pharmacol Ther 2008; 28:1240–1248. https://www.ncbi.nlm.nih.gov/pubmed/18710436[↩]
- Celiac disease. NDDIC. January 27, 2012; http://digestive.niddk.nih.gov/ddiseases/pubs/celiac/[↩][↩]
- Celiac disease. Genetics Home Reference. October, 2011; http://ghr.nlm.nih.gov/condition/celiac-disease. [↩]
- Celiac Disease. MedlinePlus. May 6, 2013; http://www.nlm.nih.gov/medlineplus/celiacdisease.html[↩][↩]
- Taylor AK, Lebwohl B, & Snyder CL. Celiac Disease. GeneReviews. September 17, 2015; http://www.ncbi.nlm.nih.gov/books/NBK1727[↩]
- Atenodoro R. Ruiz, Jr. Celiac Disease. Merck Manuals. https://www.merckmanuals.com/professional/gastrointestinal-disorders/malabsorption-syndromes/celiac-disease[↩][↩]
- Celiac disease – sprue. MedlinePlus. February 21, 2014; http://www.nlm.nih.gov/medlineplus/ency/article/000233.htm[↩]