Lutein
Lutein is a oxygenated carotenoid found naturally in vegetables and fruits 1. Lutein is synthesized only by plants and like other xanthophylls (xanthophylls is part of the carotenoid group; the other group is formed by the carotenes), is found in high quantities in all dark green leafy vegetables such as kale and spinach which contain 40 mg and 12 mg lutein in 100 g serving, respectively 2. Other foods rich in lutein include egg yolks, yellow carrots, corn, orange pepper, kiwi fruit, grapes, zucchini and squash 3, 4. In green plants, xanthophylls act to modulate light energy and serve as non-photochemical quenching agents to deal with triplet chlorophyll (an excited form of chlorophyll), which is overproduced at very high light levels, during photosynthesis 5.
Lutein is among the most abundant carotenoids in the human diet 6 often found combined with zeaxanthin. Lutein and zeaxanthin combined represent approximately 20% of the total carotenoids 7, 8. Zeaxanthin is generally found in much lower amounts in vegetables and is the dominant xanthophyll in only a few food products, such as the goji berry and orange pepper. Lutein consumed from dietary sources exceeds the amounts of zeaxanthin being consumed typically at a dietary ratio of 5:1.
The human eye accumulates the dietary lutein and zeaxanthin, which are both of dietary origin, and meso-zeaxanthin, which is derived from the conversion from lutein in the body. The maximum concentration of lutein and zeaxanthin (about 70% of their total content in the eye and more than a thousand times their concentration in serum) is observed in the macula lutea of the human retina, a small area of the retina responsible for central vision, where together with zeaxanthin it forms the macular pigment 9. These three carotenoids are efficient absorbers of blue light. They may prevent a substantial amount of the blue light entering the eye from reaching the underlying structures involved in vision and protect against light-induced oxidative damage, which is thought to play a role in the pathology of age-related macular degeneration (AMD) 10. It is also possible, though not proven, that lutein, zeaxanthin, and meso-zeaxanthin, act directly to neutralize oxidants formed in the retina.
Among the dietary carotenoids, only lutein and zeaxanthin are selectively deposited in this pigmented area of the retina responsible for high acuity color vision 11. The fact that lutein and zeaxanthin are highly concentrated in the macula strongly suggests that they might play a vital physiologic role 12. Zeaxanthin, which is fully conjugated (lutein is not), may offer somewhat better protection than lutein against phototoxic damage caused by blue and near ultraviolet light radiation 13, 5.
Lutein in the retina is believed to act as a yellow filter, filtering out potentially phototoxic blue light and near-ultraviolet radiation from the macula. The protective effect is due in part, to the reactive oxygen species (ROS) quenching ability of this carotenoid. The hypothesis is that lutein helps protect from oxidative stress and high-energy light. The specific ability of lutein and zeaxanthin to absorb high-energy short wavelengths of visible light (blue light) have an impact on vision beyond the protection of photoreceptor from blue-light induced production of reactive oxygen species (ROS). Blue light absorption can positively influence visual function by attenuating chromatic aberration, light scatter in the eye associated with disability glare, blue haze and improve contrast sensitivity 14, 15, 16, 17, 18. Supplemental lutein, alone or with zeaxanthin, was found to improve contrast sensitivity and protect against visual fatigue in young and/or healthy individuals 19, 20, 21, 22. Lutein has also been suggested to improve visual function through stimulating neuronal signaling efficiency in the eye 23.
Several studies show that an increase in macula pigmentation decreases the risk for eye diseases such as age-related macular degeneration (AMD) 24. Studies conducted in patients suffering from age-related macular degeneration (AMD) have shown the benefits of supplementation with 10 mg lutein in combination with 2 mg zeaxanthin or with other amounts in improving visual function and delaying the progression of AMD to more advanced disease stages 25, 26, 27, 28. Additional studies conducted in a healthy young population or more senior adults have shown that 10 mg lutein + 2 mg zeaxanthin supplementation increases macular pigment optical density (MPOD) and provide benefits on visual and cognitive health and function 29, 16, 30, 31, 32, 33, 34. Although possible (10 mg of lutein are found in 80 g of spinach), getting these nutrients in the adequate amount through the diet alone can be difficult on a regular daily basis especially in childhood.
The term macular pigment optical density (MPOD) refers to a measurement of the attenuation of blue light by macular pigment (expressed in density units [du]) and provides an indication of the amount of lutein and zeaxanthin isomers in the macula. Typical macular pigment optical density (MPOD) levels vary between 0 and 1 density units [du] 15. The concentration of macular carotenoids can be measured in the macula of donor eyes with high performance liquid chromatography (HPLC) while in living eyes MPOD can be assessed non-invasively using subjective psychophysical techniques, such as heterochromatic flicker photometry, and objective optical methods, such as fundus autofluorescence and reflectometry 15. In children up to the age of 7 years, due to the difficulties in performing psychophysical task reliably, objective measurements are preferred, and blue light reflectometry offers the opportunity to image the MP of infants and children 35. Preterm infants, who are highly susceptible to oxidative stress and related newborn diseases, were reported to have significantly lower serum and brain lutein concentrations than full-term infants 36, 37. Additionally, formula-fed infants were found to have lower serum lutein concentrations than breastfed infants 38, 39. A previous study reported that lutein/zeaxanthin concentration increased from 48 µg/L at birth to 96 µg/L at one month in breastfed newborns, while it decreased from 49 µg/L to 33 µg/L in infants fed with a formula that was not fortified with lutein 39. Children with higher lutein and zeaxanthin status reflected by higher MPOD were found to have better cognitive performance then their low MPOD peers 40. Therefore, increasing lutein intake through supplementation or fortification could benefit infants, especially those at risk 41.
Lutein and zeaxanthin research has focused on the beneficial effects for eye and brain aging 25, 26, 31, 33, 42, 43, 44 and more recently researchers have started to explore their effect for vision and cognition in young adults and early life 16, 30, 34, 45, 46, 47, 48, 49, 50.
Lutein acts as an antioxidant, protecting cells against the damaging effects of free radicals. Lutein like other Xanthophylls also can inhibit peroxidation of membrane phospholipids and reduce lipofuscin formation, both of which contribute to their antioxidant properties.
Lutein is more stable to decomposition by pro-oxidants than are other carotenoids such as beta-carotene and lycopene.
Lutein is one of only two carotenoids that have been identified in the human lens, may be protective against age-related increases in lens density and cataract formation 5. This study showed dietary lutein and zeaxanthin intake is associated with a reduced risk of age-related cataract, especially nuclear cataract in a dose-response manner, indicating a beneficial effect of lutein and zeaxanthin in age-related cataract prevention 51. Again, the possible protection afforded by lutein may be accounted for, in part, by its reactive oxygen species scavenging abilities. Carotenoids also provide protection from cancer 52, 53. One of the mechanisms of this is by increasing the expression of the protein connexin-43, thereby stimulating gap junctional communication and preventing unrestrained cell proliferation 5.
Lutein in most fruit and vegetables is in the free form 54, whereas both free and esterified lutein are available in commercial dietary supplements. Earlier studies found esterified and free lutein to be similarly bioavailable 55, 56. More recently, it was found that serum lutein and macular pigment optical density (MPOD) responses were similar between either free or esterified lutein supplementation for 3 months 57. However, a larger 4-week supplementation study found greater serum lutein responses from free lutein than from lutein ester supplements 58.
Lutein supplementation increases blood, tissue, and breast-milk carotenoids in a dose-response manner. In a current 140-day lutein supplementation study, serum lutein and macular pigment optical density (MPOD) changed positively, reaching a plateau that was linearly dependent on dose, across doses of 0, 5, 10, or 20 mg/day 59. Lutein supplementation also increased breast milk as well as infant and maternal plasma lutein concentrations in a dose-dependent manner 60. A recent meta-analysis concluded that lutein and zeaxanthin supplementation increases macular pigment optical density (MPOD) in patients with age-related macular degeneration (AMD) and healthy individuals in a dose-response manner 61.
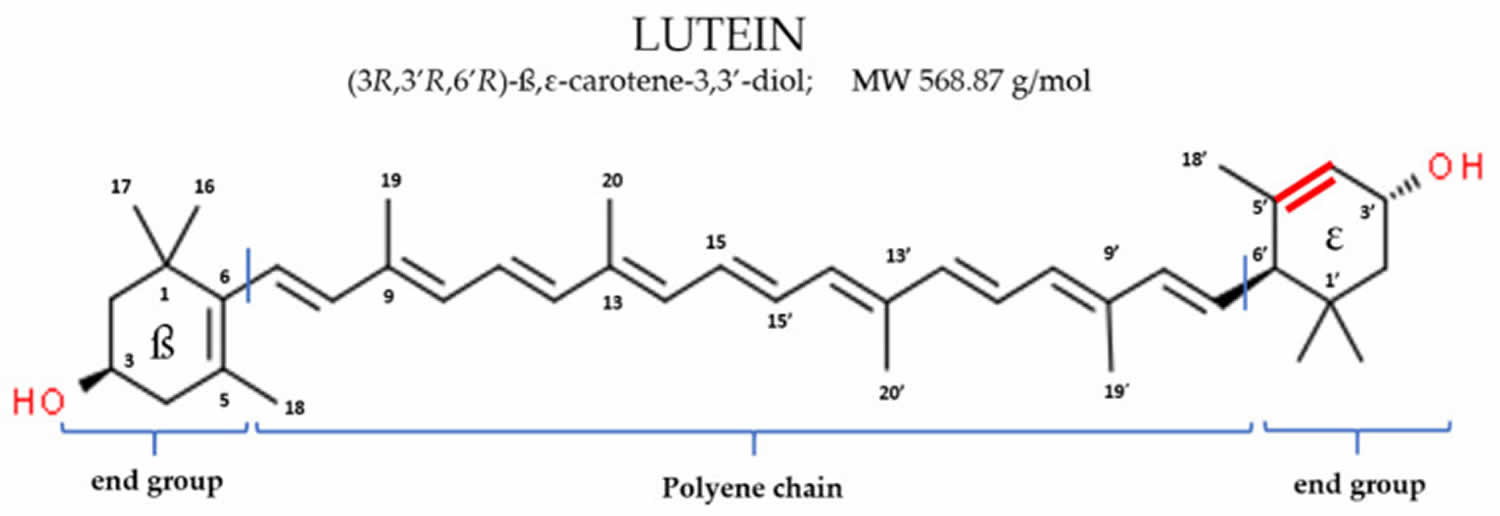
Figure 1. Lutein chemical structure
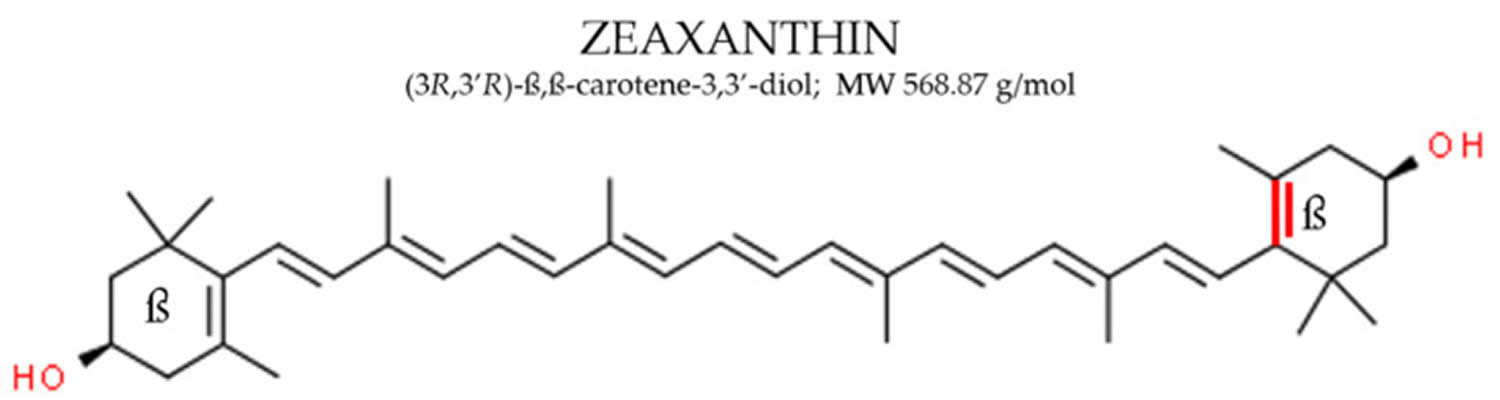
[Source 40 ]Figure 2. Zeaxanthin
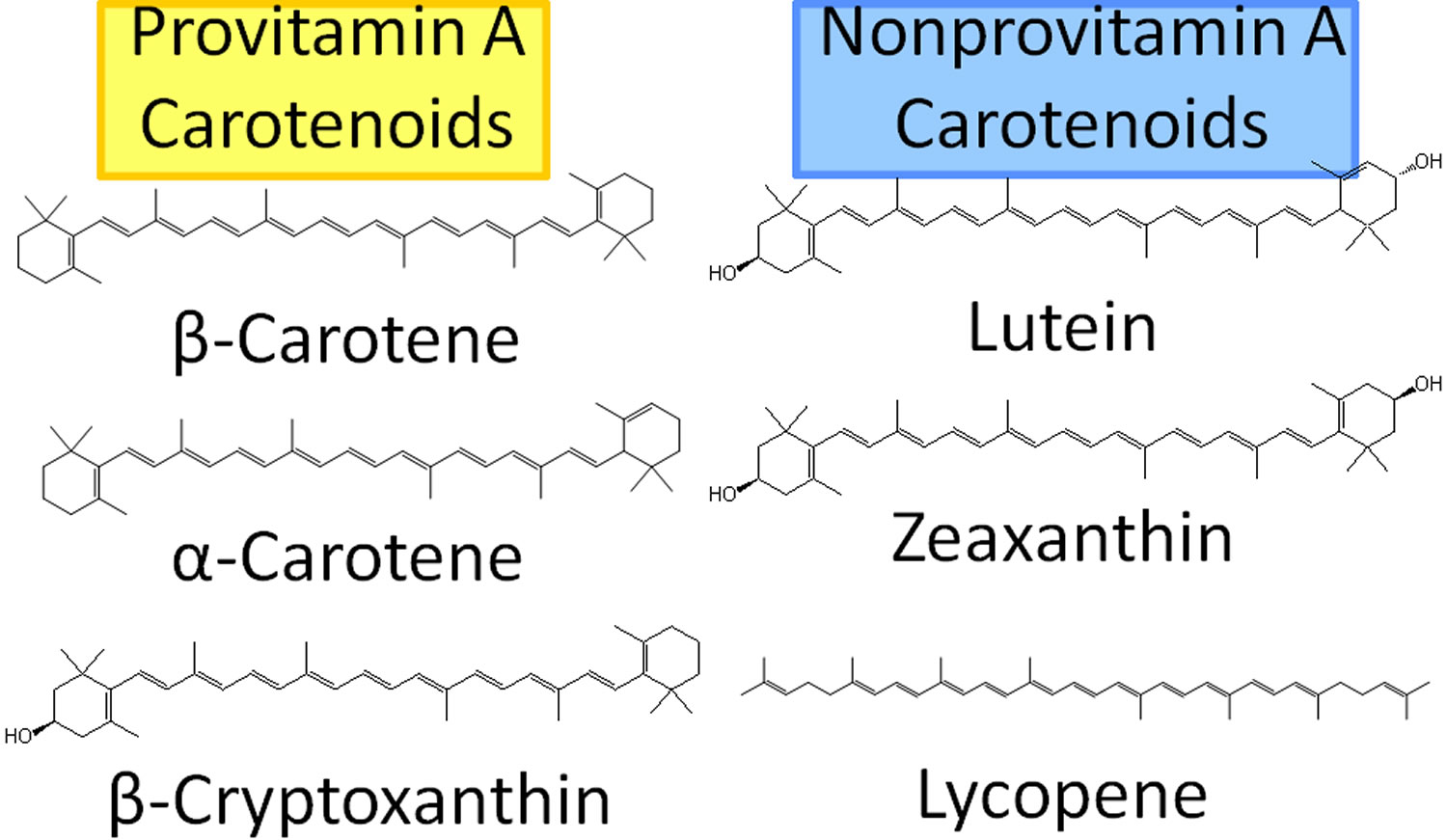
Figure 3. Carotenoids
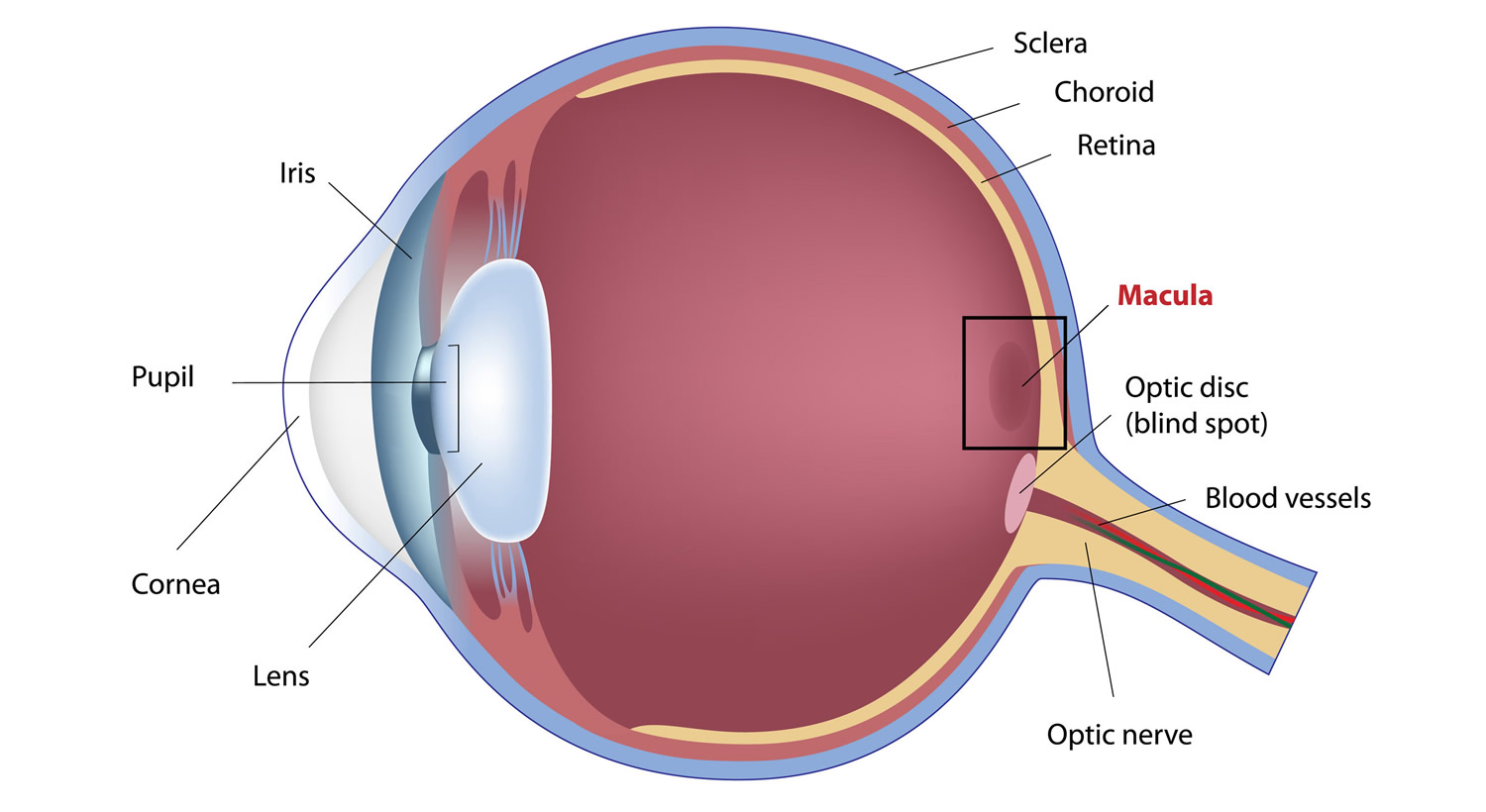
Figure 4. Eye Retina and Macula Lutea
Footnote: The macula lutea is located in the central and back portion of the retina and possesses the highest concentration of photoreceptors, which are responsible for central vision and high-resolution visual acuity. It is a circular area 5–6 mm in diameter that possesses a characteristic yellow pigment that is made up entirely of lutein and zeaxanthin
[Source 62 ]What are Carotenoids?
Carotenoids are yellow, orange, and red pigments synthesized by plants, algae, and photosynthetic bacteria found in colored fruit and vegetables, include carotenes (that animals, including humans, can convert to vitamin A), lycopene, lutein, and zeaxanthin 63, 64, 65, 66. Fruit and vegetables provide most of the 40 to 50 carotenoids found in the human diet. The most common carotenoids in North American diets are alpha-carotene, beta-carotene, beta-cryptoxanthin, lutein, zeaxanthin, and lycopene 67. Alpha-carotene, beta-carotene and beta-cryptoxanthin are provitamin A carotenoids, meaning they can be converted by the body to retinol (Figure 3). Lutein, zeaxanthin, and lycopene are nonprovitamin A carotenoids because they cannot be converted to retinol (Figure 3).
For dietary carotenoids to be absorbed intestinally, they must be released from the food matrix and incorporated into mixed micelles (mixtures of bile salts and several types of lipids). Food processing and cooking help release carotenoids embedded in their food matrix and increase intestinal absorption 65. Carotenoids are best absorbed with fat in a meal. As little as 3 to 5 g of fat in a meal appears sufficient to ensure carotenoid absorption 68, 69, although the minimum amount of dietary fat required may be different for each carotenoid. The type of fat (e.g., medium-chain vs. long-chain triglycerides), the presence of soluble fiber, and the type and amount of carotenoids (e.g., esterified vs. non-esterified) in the food also appear to influence the rate and extent of carotenoid absorption 70. Chopping, puréeing, and cooking carotenoid-containing vegetables in oil generally increase the bioavailability of the carotenoids they contain. Because they do not need to be released from the plant matrix, carotenoid supplements in oil are more efficiently absorbed than carotenoids in food 69, 71.
The human diet contains 2 sources for vitamin A: preformed vitamin A (retinol and retinyl esters) and provitamin A carotenoids (see Figure 3) 72, 73. Preformed vitamin A is found in foods from animal sources, including dairy products, eggs, fish, and organ meats 72, 74. Provitamin A carotenoids are plant pigments that include beta-carotene, alpha-carotene, and beta-cryptoxanthin 72, 73. Your body converts provitamin A carotenoids into vitamin A in the intestine via the beta-carotene monooxygenase type 1 BCMO1 enzyme 72, 75, although conversion rates may have genetic variability 76, 77, 78. Other carotenoids in food, such as lycopene, lutein, and zeaxanthin, are not converted into vitamin A and are referred to as non-provitamin A carotenoids; they might have other important activities not involving vitamin A formation 72. At present, it is unclear whether the biological effects of carotenoids in humans are related to their antioxidant activity and/or other non-antioxidant activities 63, 64. Lutein and zeaxanthin may be protective in eye disease because they absorb damaging blue light that enters the eye.
Carotenoids occur widely throughout the vegetable kingdom and are readily accumulated by vegetable-consuming animals, including humans 79. Food sources of these compounds include a variety of fruits and vegetables, although the primary sources of lycopene are tomato and tomato products. Additionally, egg yolk is a highly bioavailable source of lutein and zeaxanthin. These carotenoids are available in supplement form also. However, intervention trials with large doses of beta-carotene found an adverse effect on the incidence of lung cancer in smokers and workers exposed to asbestos 80, 81. Until the efficacy and safety of taking supplements containing these nutrients can be determined, current dietary recommendations of diets high in fruits and vegetables are advised.
The ultimate source of all carotenoids in the human diet is plant material, directly, or indirectly from ingesting carotenoids and their metabolites in animal products such as egg yolk, milk, and poultry. Adequate intake of carotenoids is purportedly important for the prevention of all manner of disease. Yet, whereas supplies of vegetables and fruit vary dramatically around the world, there is little clinical evidence that any sizeable population consumes inadequate amounts for normal physiologic function. Only relatively recently was vitamin A deficiency definitively recognized to influence immune competence and increase infectious morbidity and mortality 82, despite previous suspicions that this might be the case. Until such time as true, carotenoid “deficiency”–related clinical entities are discovered, the only natural physiologic role recognized to be important is that of the provitamin A activity of carotenes, especially β-carotene 79.
Unusually large dietary consumption of various carotenoids has been linked to a reduction in the risk of various chronic diseases, particularly cancer of the lung, gastrointestinal tract, and pancreas; cardiovascular disease; and both cataract and age-related macular degeneration 83, 84, 85. Most supportive data arise from observational epidemiologic studies, which compared the risk (prevalence or incidence) of these conditions among individuals consuming few if any vegetables with those consuming the most. Other analyses and observational studies have failed to support these purported relations.
More worrisome still are the outcomes of several large, particularly well-conducted randomized clinical trials. In 2 of these trials, which specifically enrolled participants at high risk of lung cancer (smokers and/or asbestos workers) the active agents appeared to increase the risk of developing lung cancer 86, 87. Subsequent systematic reviews of the literature confirm the potential for increased cancer risks from β-carotene supplementation 87, 88.
Why these apparently conflicting clinical and epidemiologic results? The most obvious reason is that purely observational studies are prone to suffer from bias. People who eat the most salad are likely to differ in many other ways from those who eat much less. Whereas these studies purportedly “adjust” for other differences in lifestyle and known risks, they cannot “adjust” for them all, nor necessarily for the most important. No study can collect data on every potentially important variable, and the most important variables may not even be known. Even if frequent consumption of salad, by itself, reduces the risk of certain diseases, salads contain an enormous number of different compounds, not just β-carotene or carotenoids in general.
Clearly, new and very different research designs are needed to begin to dissect out which dietary carotenoids (or combinations of carotenoids) are important for promoting health and preventing disease, if indeed there are diseases that increased carotenoid intake can help to prevent. The fact that lutein and zeaxanthin are highly concentrated in the macula strongly suggests that they might play a vital physiologic role 89. In the meantime, until definitive clinical evidence becomes available, we can only conclude that humans accumulate a variety of carotenoids, but their importance and roles remain uncertain. The only well-established pathophysiologic consequence of dietary carotenoid “deficiency” remains the provitamin A activity of carotene, especially β-carotene 79.
Lutein and Zeaxanthin
The human macula uniquely concentrates three carotenoids: lutein, zeaxanthin, and meso-zeaxanthin. Lutein and zeaxanthin must be obtained from dietary sources such as green leafy vegetables and orange and yellow fruits and vegetables, while meso-zeaxanthin is rarely found in diet and is believed to be formed at the macula by metabolic transformations of ingested carotenoids 90. Unlike the provitamin A carotenoids (α- and β-carotene and cryptoxanthin), they cannot be converted to vitamin A. Their presence in tissues is due entirely to ingestion of plant sources; they are not synthesized by animal tissues. However, a variety of metabolites may be found in animal sources, and several exist in human blood and milk 91. Meso-Zeaxanthin is rarely found in the human diet, but it has been detected in shrimp carapace, fish skin, and turtle fat, where all three isomers of zeaxanthin were found 92 and group of researchers have recently confirmed its presence in fish skin using more modern methods 93, 94. A significant amount of meso-zeaxanthin has been detected in commercially produced chicken eggs in Mexico where it is commonly added to the feed to achieve desirable coloration 95.
Approximately 30 to 50 carotenoids may exist in the diet, and about 20 may be measurable in the serum. The fact that only 2 of these, lutein and zeaxanthin, are present in the retina leads us to question why.
Lutein and zeaxanthin are especially concentrated in leafy green vegetables, many fruits, and colored vegetables such as squash, sweet peppers, sweet corn, and peas. Lutein is the dominant xanthophyll in almost all sources. Given the variability in food preferences among individuals and cultures, it is not surprising to have significant differences reported. African Americans on average consume twice as much lutein (about 3 mg/day) as Hispanic Americans and white Americans (1-2 mg/day) 96.
The macular pigment carotenoids, lutein, zeaxanthin, and meso-zeaxanthin are widely recommended as dietary supplements for the prevention of visual loss from age-related macular degeneration (AMD) and other ocular diseases. There is evidence that suggests that lutein and zeaxanthin may reduce risk for developing the two most common eye diseases in older people, i.e., cataract and macular degeneration. There is also the untested possibility that lutein and/or zeaxanthin may slow progression once these conditions are present. In addition, lutein may slow degeneration of vision in patients with retinitis pigmentosa 97, a heterogeneous group of slow retinal degenerations. However, only preliminary data in a very small number of patients have been published in which lutein slowed vision loss associated with retinitis pigmentosa in one but not another study.
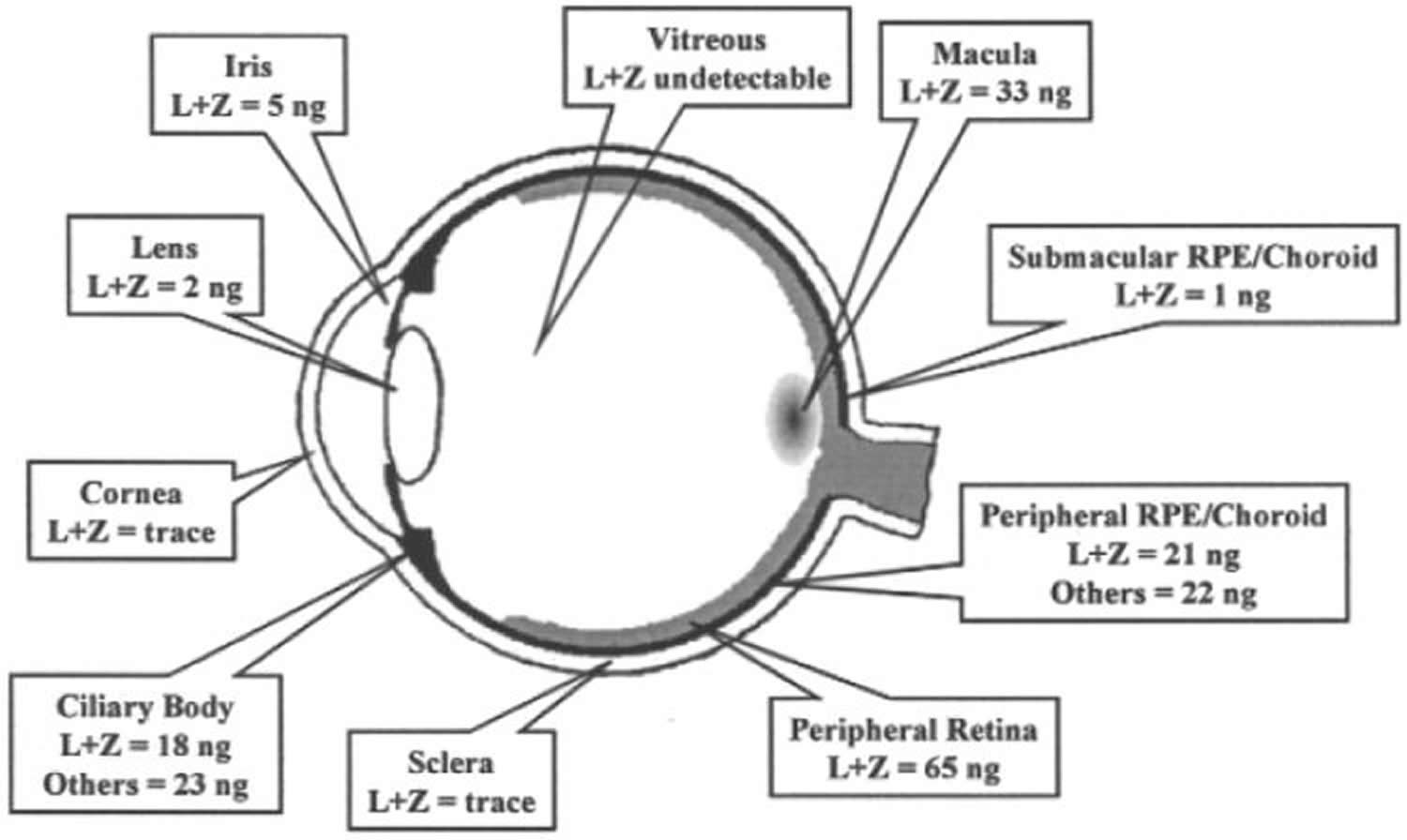
Figure 5. Lutein and Zeaxanthin levels in different parts of the eye
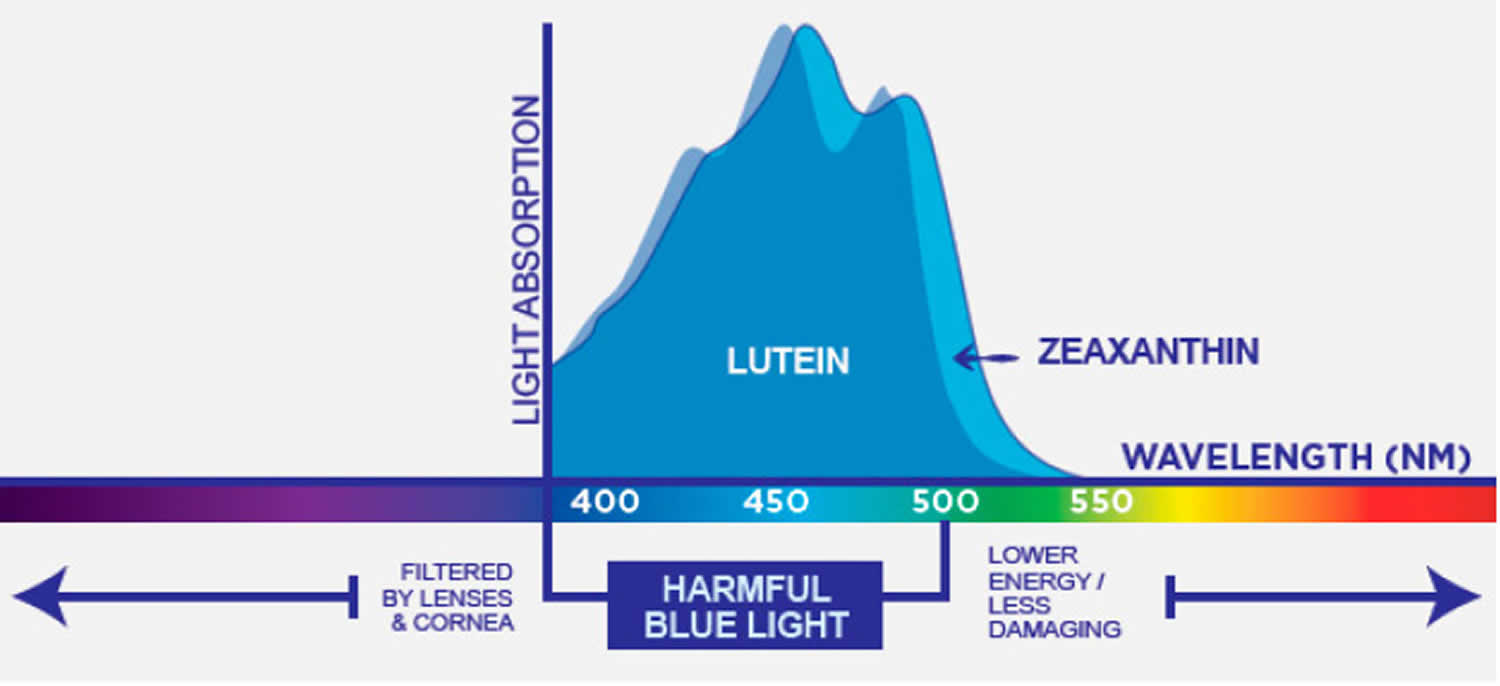
[Source 90]Figure 6. Absorption spectrum of blue (lutein) and light blue (zeaxanthin)
Footnote: Lutein and zeaxanthin light absorption spectra.
[Source 40 ]Lutein Health Benefits
Lutein is commonly taken by mouth to prevent eye diseases, including cataracts and a disease that leads to vision loss in older adults (age-related macular degeneration or AMD). Lutein is used for many other conditions, but there is no good scientific evidence to support these other uses. A systematic review and meta-analysis of literature published on lutein suggest that higher dietary intake and higher blood concentrations of lutein are generally associated with better cardiometabolic health 3. Currently, there are no dietary recommendations for lutein intake 3. The literature on risk factors of cardiometabolic diseases suggested that lutein might prevent atherosclerosis and reduce inflammatory markers, but there were inconsistent associations with blood pressure, adiposity, insulin resistance, and blood lipids 3. The majority of the studies were observational and performed in adults, and the effects of lutein on cardiometabolic health in children and during pregnancy remains largely unaddressed.
- Age-related macular degeneration (AMD). Taking lutein supplements by mouth for up to 36 months can improve some symptoms of AMD. More benefits might be seen when it’s taken for at least 3 months at doses above 5 mg, and when it’s combined with other carotenoid vitamins. But lutein doesn’t seem to keep AMD from becoming worse over time.
- Cataracts. Eating higher amounts of lutein in the diet is linked with a lower risk of developing cataracts. But it’s not clear if taking lutein supplements by mouth helps people who already have cataracts.
Age-related eye diseases are a burden for the economy and a major cause of moderate to severe visual impairment and blindness 98. Age-related macular degeneration (AMD) is the most common cause of irreversible vision loss and legal blindness among older Americans 99. It is a degenerative condition of the region of the retina that is responsible for central vision (the macula). Age-related macular degeneration (AMD) is a multifactorial disease. Among the important risk factors for AMD are age, genetic susceptibility, sunlight exposure, cigarette smoking, and poor nutritional status 62. This condition, which has been estimated to affect about 0.5 percent of Americans over age 40 years 100, steeply increases in prevalence with age, up to 36% after 85 years of age for early AMD 101. Estimates from one United States community indicate that as many as 7 percent of persons between ages 75 and 84 years have age-related macular degeneration (AMD) 102 and could affect 196 million people by 2020 and 288 million by 2040 103.
Increasing dietary consumption of lutein and zeaxanthin was shown to raise their serum concentration and macular pigment density 104, 105. Previous studies suggested that a consumption of lutein and zeaxanthin above 6-14 mg daily was considered to reduce the risk of eye diseases such as age-related macular degeneration (AMD) as well as in alleviating the symptoms if present 106, 107. Some, but not all, observational studies have provided evidence that higher intakes of lutein and zeaxanthin are associated with lower risk of age-related macular degeneration (AMD) 108. While cross-sectional and retrospective case-control studies found that higher levels of lutein and zeaxanthin in the diet 109, 110, 111, blood 112, 113 and retina 114, 115 were associated with a lower incidence of AMD, several prospective cohort studies found no relationship between baseline dietary intakes or serum concentrations of lutein and zeaxanthin and the risk of developing AMD over time 116, 117, 118, 119.
One report examined the association between the incidence of age-related macular degeneration (AMD) and calculated dietary intakes and predicted plasma concentrations of lutein and zeaxanthin in older adults (≥50 years) from two large prospective cohorts, the Nurses’ Health Study (63,443 women) and the Health Professionals Follow-up Study (38,603 men), followed for 26 years and 24 years, respectively 120. The highest versus lowest quintile of predicted plasma lutein and zeaxanthin scores was associated with a 41% lower risk of advanced AMD, yet no association was found with intermediate AMD 120. Evidence has suggested that the consumption of about 6 mg/day of lutein and zeaxanthin from fruit and vegetables (compared with less than 2 mg/day) may decrease the risk of advanced AMD 111, 120. In a small population-based prospective study among 609 older adults in France, followed for a median of 7.6 years, found an inverse association between plasma lutein concentration at baseline and advanced AMD 121. No association was observed for circulating zeaxanthin and advanced AMD 121.
The first randomized controlled trial in patients with atrophic AMD found that supplementation with 10 mg/day of lutein slightly improved visual acuity after one year compared to a placebo 122. The effects of long-term lutein supplementation on atrophic AMD were further investigated in combination with antioxidant vitamins and minerals in the Age-Related Eye Disease Study 2 (AREDS2), a multicenter, randomized, double-blind, placebo-controlled trial 123. In AREDS, oral supplementation with β-carotene (15 mg/day), vitamin C (500 mg/day), vitamin E (400 IU/day), zinc (80 mg/day as zinc oxide), and copper (2 mg/day as cupric oxide) for five years reduced the risk of developing advanced AMD by 25% 124. In the AREDS2 study conducted in 4,203 participants at risk for developing late AMD, supplemental lutein (10 mg/day) and zeaxanthin (2 mg/day), in combination with beta-carotene, vitamin C, vitamin E, zinc, and copper (the ‘AREDS’ formulation), did not slow the progression to advanced AMD, although subgroup analyses revealed a benefit in those with the lowest dietary intakes of lutein + zeaxanthin 123. A total of 3,036 subjects were further randomized to various combinations of carotenoids; supplementation with lutein and zeaxanthin significantly reduced the risk of progression to late AMD and to neovascular AMD compared to supplementation with beta-carotene 125.
Several smaller (n=30-433) randomized controlled trials also suggested that supplementation with lutein, zeaxanthin, and meso-zeaxanthin would be beneficial in the management of AMD 126. A meta-analysis of eight trials that examined the effect of supplemental lutein (6 to 20 mg/day) or/and zeaxanthin (0 to 10 mg/day) in 1,176 AMD subjects for up to 36 months found improvements in visual acuity and contrast sensitivity with increased levels of xanthophyll carotenoids 127. More recently, a randomized, placebo-controlled trial found daily consumption of a buttermilk drink with egg yolks enriched with lutein (1.4 mg), zeaxanthin (0.2 mg), and an omega-3 polyunsaturated fatty acid (DHA; 160 mg) for one year improved visual acuity and macular pigment optical density in subjects with drusen and/or retinal pigment abnormalities (about half of them being classified as having early AMD) 128. In a randomized, placebo-controlled trial in 74 patients with intermediate AMD, daily supplementation with lutein (10 mg/day) and zeaxanthin (2 mg) for two years, along with daily astaxanthin (4 mg), vitamin C (90 mg), vitamin E (30 mg), zinc (22.5 mg), copper (1 mg), and fish oil (containing 185 mg EPA and 140 mg DHA), resulted in a lower incidence of disease progression (2.1% of patients) when compared to placebo (15.4% of patients) 129.
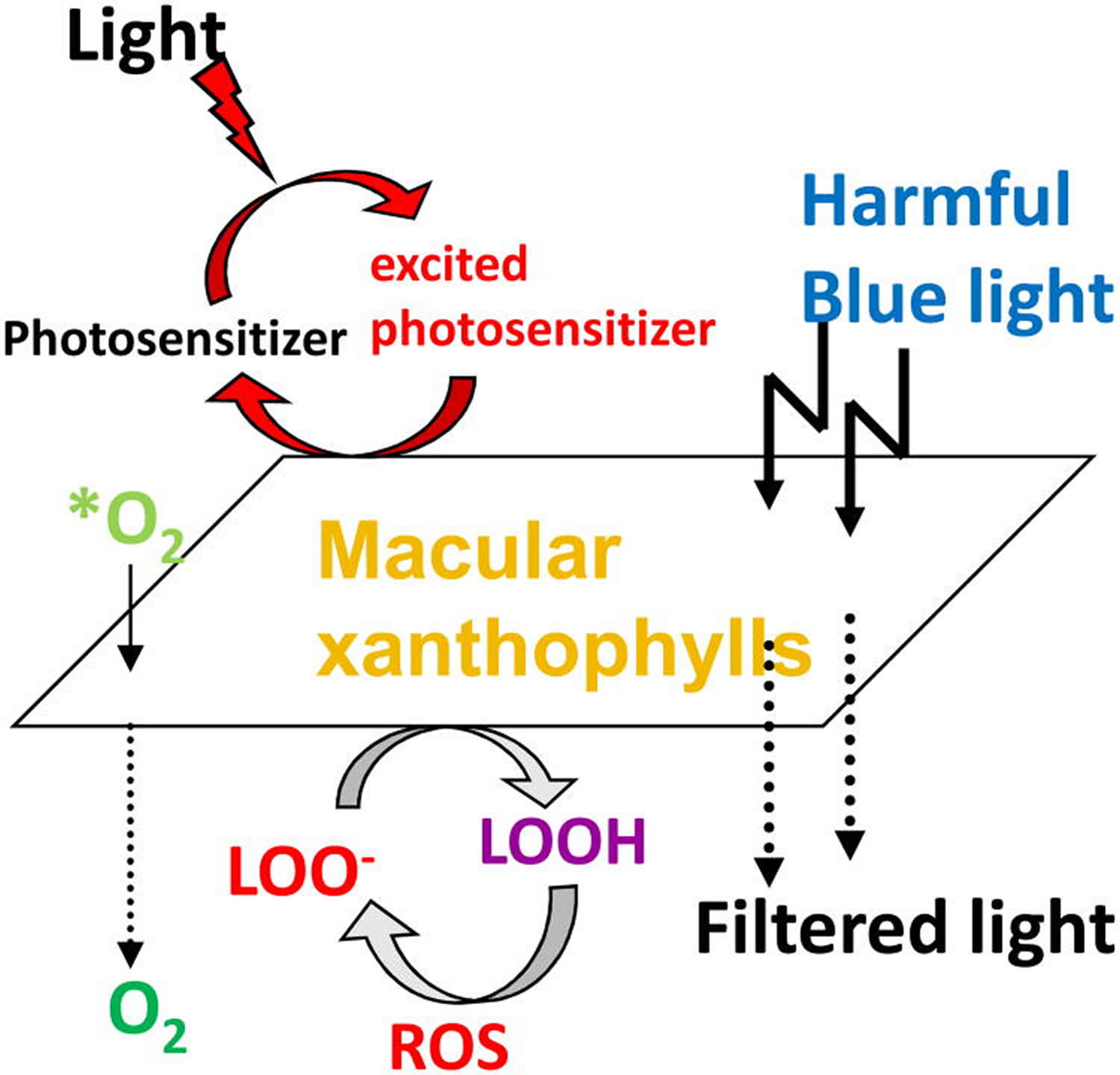
Figure 7. Protective roles of lutein and zeaxanthin, as an absorber of harmful light and as an antioxidant
Footnote: Protective roles of lutein and zeaxanthin, as an absorber of harmful light and as an antioxidant reacting with reactive oxygen species (ROS). *O2, singlet oxygen; LOO-, lipid peroxyl radicals ;LOOH, lipid peroxides.
[Source 90]Another major proposed function of macular pigment, and the one that is pertinent to age-related disease, is neutralization of reactive oxygen species. The antioxidant system in cells and tissues includes enzymes (catalase, glutathione peroxidase, and superoxide dismutase), primary water-soluble antioxidants (such as glutathione and vitamin C), and lipid-soluble antioxidants (xanthophylls, retinoids, and vitamin E). The potential for the creation of reactive oxygen species in the retina is high. Reactive oxygen species are produced by absorption of UV and blue light by a photosensitizing compound or molecule (lipofuscin, protoporphyrin, or cytochrome).These longer-lived molecules can then react with oxygen to produce reactive oxygen species, including superoxide anion, hydroxyl radical, hydrogen peroxide, and singlet oxygen. These in turn can cause lipid peroxidation by attacking polyunsaturated fatty acids, resulting in DNA damage, protein and transmembrane glycoprotein oxidation, and other forms of cellular vandalism 130. To counteract the deleterious effects of free radicals to the retina, macular pigment (MP) made up of three main carotenoids, lutein, zeaxanthin and meso-zeaxanthin, constitutes a barrier to blue-light damage and has antioxidant properties 131. Many epidemiological and interventional studies have assessed carotenoid intake and content through dietary questionnaires or plasma L and Z measurements, finding that the consumption and plasma levels of lutein are inversely correlated with the risk of AMD 132. Carotenoids are potent scavengers of free radicals (eg, superoxide anion and hydroxyl radical) and are particularly efficient at neutralizing singlet oxygen. Because their lipid-soluble nature relegates them to membranes, it is likely that they especially protect the polyunsaturated fatty acid–rich membranes of the outer retina.
Lutein benefits is known mostly for its effect on visual function and its preventive effect against cataracts and macular degeneration 133, potentially through protection against oxidative stress 134. Given its antioxidant properties, it is hypothesized that lutein may also have beneficial effects on metabolic and cardiovascular diseases. Although the larger group of carotenoids has been associated with cardiometabolic protection 135, research has focused mostly on other carotenoids, such as β-carotene 136. Nevertheless, interventional studies with the use of β-carotene supplements have failed to reproduce the beneficial effects that were seen in observational studies. Thus, what the substance is behind the previously published beneficial effects of carotenoids has yet to be determined. The xanthophyll lutein is of particular interest because lutein may be a more active antioxidant than β-carotene 79.
Eliminating lutein from the diet of experimental animals results in early degenerative signs in the retina while patients with an acquired condition of macular pigment loss (Macular Telangiectasia) show serious visual handicap indicating the importance of macular pigment. Whether lutein intake reduces the risk of age related macular degeneration (AMD) or cataract formation is currently a strong matter of debate and abundant research is carried out to unravel the biological properties of the lutein molecule 137. SR-B1 has recently been identified as a lutein binding protein in the retina and this same receptor plays a role in the selective uptake in the gut 137. In the blood lutein is transported via high-density lipoproteins (HDL) 137. Genes controlling SR-B1 and HDL levels predispose to AMD which supports the involvement of cholesterol/lutein transport pathways 137. Apart from beneficial effects of lutein intake on various visual function tests, recent findings show that lutein can affect immune responses and inflammation. Lutein diminishes the expression of various ocular inflammation models including endotoxin induced uveitis, laser induced choroidal neovascularization, streptozotocin induced diabetes and experimental retinal ischemia and reperfusion 137. In vitro studies show that lutein suppresses NF kappa-B activation as well as the expression of iNOS and COX-2. Since AMD has features of a chronic low-grade systemic inflammatory response, attention to the exact role of lutein in this disease has shifted from a local effect in the eye towards a possible systemic anti-inflammatory function 137.
Lutein and Diabetic retinopathy
Diabetic retinopathy is an eye disease that occurs when diabetes affects the blood vessels and nerve tissue in the retina.
There are two stages of diabetic retinopathy:
- Nonproliferative retinopathy is the early stage of the disease in which blood vessels swell and leak. In some cases, this can cause macular edema (swelling of the retina) which may result in mild vision loss but can be treated. There can also be early changes to the nerve cells in the retina that may affect vision, in part due loss of adequate blood supply.
- Proliferative retinopathy is the advanced stage where abnormal new blood vessels grow on the surface of the retina. These vessels may break and bleed into the vitreous, the clear watery gel that fills the eye, and cause severe vision loss. This stage of diabetic retinopathy typically requires urgent treatment.
Supplementation with lutein and zeaxanthin has been shown to help preserve retinal integrity in rats with diabetes by reducing oxidative stress and inflammatory mediators 138. A higher ratio of plasma nonprovitamin A carotenoids (lutein, zeaxanthin, and lycopene) to vitamin A carotenoids (alpha-carotene, beta-carotene, beta-cryptoxanthin) was associated with a reduced risk of diabetic retinopathy in a cross-sectional analysis in 111 individuals with type 2 diabetes mellitus 139. A small observational study also found lower macular pigment optical density in those with type 2 diabetes (n=17) or mild nonproliferative diabetic retinopathy (n=12) compared to those without either condition (n=14) 140. Data from 1,430 individuals participating in the Atherosclerosis Risk In Communities (ARIC) study found no association between lutein intake and diabetic retinopathy after adjustment for confounding variables 141. It is not known if supplementation with lutein and zeaxanthin might prevent or help treat diabetic retinopathy; well-designed, placebo-controlled studies would be needed to address these questions.
Lutein and Retinopathy of prematurity
Preterm infants have an immature retinal vascular system that places them at risk of developing retinopathy of prematurity. Basically, preterm birth halts the normal development of retinal vascular system, which results in a retina that is poorly vascularized and highly susceptible to hyperoxia. In order to meet metabolic demand, the hypoxic retina induces the production of proangiogenic factors like VEGF (vascular endothelial growth factor). These factors stimulate the development of new blood vessels (angiogenesis), causing aberrant vessels sprouting from the retina into the vitreous. It is thought that an imbalance between the production of reactive oxygen species (ROS) and the reduced levels of antioxidants in preterm infants contributes to ROP pathogenesis and causes additional damage to the retina 138.
A randomized controlled trial in 62 preterm infants (≤32 weeks of gestational age) failed to observe any benefits regarding retinopathy of prematurity incidence and severity with lutein (0.5 mg/kg/day) and zeaxanthin (0.02 mg/kg/day) supplemented from the seventh day post birth until about 10 weeks of age 142. In two other multicenter, placebo-controlled trials in a total of 343 preterm infants, a daily oral dose of 0.14 mg of lutein and 0.006 mg of zeaxanthin administered from the first week after birth did not significantly reduce retinopathy of prematurity incidence or the rate of progression from early to more advanced stages of retinopathy of prematurity occurrence 143, 144. A fourth multicenter, randomized controlled trial in 203 preterm infants found that administration of a formula containing carotenoids (lutein/zeaxanthin, lycopene, and beta-carotene) had no effect on retinopathy of prematurity incidence, but limited the progression to severe retinopathy of prematurity stages in infants with mild retinopathy of prematurity compared to a carotenoid-free formula 145. Compared with the control formula, preterm infants free of retinopathy of prematurity fed the carotenoid-containing formula had significantly increased plasma carotenoid concentrations, which were correlated with greater rod photoreceptor sensitivity 145. More research is needed to examine whether carotenoid supplementation might promote normal photoreceptor development and prevent retinopathy of prematurity in preterm infants
Lutein and zeaxanthin in Cataract
Lutein and zeaxanthin are the only carotenoids present in the crystalline lens 146, 147. Cataract is the opacification of the crystalline lens and is caused by precipitation of lens proteins. The development of cataract is facilitated by oxidative damage and often results in impaired vision or blindness.
The observation that lutein and zeaxanthin are the only carotenoids in the human lens has stimulated interest in the potential for increased intakes of lutein and zeaxanthin to prevent or slow the progression of cataracts 148. Large prospective cohort studies have found that men and women with the highest intakes of foods rich in lutein and zeaxanthin, particularly spinach, kale, and broccoli, were 18%-50% less likely to require cataract extraction 149, 150 or develop cataracts 151, 152, 153. Moreover, plasma concentrations of lutein and zeaxanthin have been found to be inversely associated to the progression of nuclear cataract. Additional research is required to determine whether these findings are related specifically to lutein and zeaxanthin intake or to other factors associated with diets high in carotenoid-rich foods 108.
The Age-related Eye Disease Study 2 (AREDS2) failed to show an effect of lutein (10 mg/day) and zeaxanthin (2 mg/day) supplementation for a median of 4.7 years on the risk of developing cataract, on the progression to severe cataract or to cataract surgery, and on visual acuity 154. However, the results might have been confounded by the fact that most participants were better nourished than the general population and/or used multivitamins that have been found to decrease the risk of developing cataract (116). Additional limitations to consider in interpreting the results have been reviewed elsewhere 155. Additional interventions are required to study whether supplemental lutein and zeaxanthin might be helpful in the prevention of cataract.
Lutein and coronary heart disease
Pooled study results show that the highest intake of lutein was associated with a lower risk of coronary heart disease, compared with the lowest intake 3. In a US national survey, National Health And Nutrition Examination Survey (NHANES) 2003-2006, serum total carotenoid concentration was inversely associated with blood concentrations of two cardiovascular risk factors, C-reactive protein (CRP) and total homocysteine 156. HDL-cholesterol (“good” cholesterol) concentration was found to be positively associated with alpha-carotene, beta-cryptoxanthin, and lutein/zeaxanthin concentrations, but only the latter was inversely associated with LDL-cholesterol (“bad” cholesterol) 156. NHANES 2007-2014 also found an inverse association between total dietary carotenoid intake (sum of alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene, lutein, and zeaxanthin) and risk of hypertension 157. Finally, a meta-analysis of observational studies reported lower risks of coronary heart disease (-12%) and stroke (-18%) in individuals in the highest versus lowest tertile of blood lutein concentration 158.
Lutein and Cognitive function
Among the carotenoids, lutein and its isomer zeaxanthin are the only two carotenoids that cross the blood-retina barrier to form macular pigment in the eye. Lutein also preferentially accumulates in the brain 159, 160. A few studies have suggested that lutein and zeaxanthin concentrations in the macula correlate with brain lutein and zeaxanthin status and therefore might be used as a biomarker of cognitive health 160, 161, 162, 163. Lutein and zeaxanthin account for 66–77% of the total carotenoid population in the brain, making them the main players in eye and brain health when compared to other members of the carotenoid family 164. Lutein and its isomer zeaxanthin have a neurological connection between the macula and the brain, implying a role in cognition 165. Lutein and zeaxanthin are possible biomarkers of brain xanthophyll concentrations 166 and therefore provide information on the concentrations of lutein in the brain, making them a critical biomarker 167. This includes the relationship with several measures of temporal processing speed 168, which is a necessary component of sensory and cognitive functions such as language, executive function, learning, and memory.
Observational studies have suggested that dietary lutein may be of benefit in maintaining cognitive health 169, 170, 171, 172 and a cross-sectional study of 4,076 older adults associated higher blood lutein concentrations with improved cognition, including memory and executive function 173.
In a small, four-month, double-blind, placebo-controlled study on the cognitive functions of older women (ages, 60 to 80 years) without cognitive impairment who were given lutein dose (12 mg/day), docosahexaenoic acid dose (800 mg/d), or their combination for the period of 4 months revealed the significant improvement in verbal fluency scores in all 3 groups with boost in memory scores and learning rate 174. Lutein and zeaxanthin have also been found to improve gap junctional communication 175, which is necessary for light processing and the development of neural circuitry in the visual system in the retina. These are also related with an increase in visual processing speed and a reduction in scotopic noise 176.
In the Georgia Centenarian Study, the analysis of cross-sectional data from 47 deceased centenarians showed a positive association between post-mortem measures of brain lutein concentrations and pre-mortem measures of cognitive function 159. Brain lutein concentrations were found to be significantly lower in individuals with mild cognitive impairment compared to those with normal cognitive function 159.
Two small trials in younger adults found supplementation with lutein (10 mg/day) and zeaxanthin (2 mg/day) for one year improved some measures of cognitive function, including memory 177, 178. However, the Age-related Eye Disease Study 2 (AREDS2) failed to show an effect of supplemental lutein (10 mg/day) and zeaxanthin (2 mg/day; supplementation for a median of 4.7 years) on the cognitive test scores of 3,741 older participants (mean age, 72.7 years) 179, perhaps because the trial was conducted in a highly educated and well-nourished population.
Lutein and stroke
Results of 3 longitudinal studies that reported on the associations between lutein and stroke were pooled 180, 181, 182. The pooled results showed that the highest intake of lutein was associated with a lower risk of stroke compared with the lowest intake 3.
Lutein and cardiovascular diseases
Five studies reported on lutein in relation to mortality from a combined outcome of any cardiovascular disease 183, 184, 185, 186, 187. Mean follow-up time ranged from 4.25 to 15 years and sample sizes ranged from 216 to 13,293. None of the studies found significant associations, but the effect estimates were mostly in the direction of higher lutein being associated with a lower risk of mortality from cardiovascular disease 183, 185, 186, 187 except for one study 184.
Two studies from a US cohort (one in male subjects and one in female subjects), both studies found no significant associations 188, 189.
Lutein and type 2 diabetes mellitus
The meta-analysis of the results of lutein in relation to type 2 diabetes mellitus included four studies 190, 191, 192, 193, all of which were longitudinal observational studies with follow-up periods between 10 and 23 years, including in total 35,242 participants (including 1661 cases). The pooled results showed no significant association between lutein and risk of diabetes.
Lutein and metabolic syndrome
Of the 6 studies on metabolic syndrome, one was in adolescents 194 and the others were in adults 195, 196, 197, 198, 199. All studies were cross-sectional, with a total of 8133 participants (including 1773 cases). The highest intake of lutein was associated with a lower risk of metabolic syndrome compared with the lowest intake of lutein 3.
Lutein Food Sources
Lutein and zeaxanthin are the most common xanthophylls in green leafy vegetables (e.g., kale, spinach, broccoli, peas and lettuce) and egg yolks (Table 1) 200. They are also found at relatively high levels in einkorn, Khorasan and durum wheat and corn and their food products (Table 1). The ratio of lutein and zeaxanthin in green vegetables has been reported to range between 12 to 63, highest being in kale, while in yellow-orange fruits and vegetable this ratio ranges between 0.1 and 1.4. Lutein is found in many natural products including broccoli, spinach, kale, corn, orange pepper, kiwi fruit, grapes, orange juice, zucchini, and squash. There is 44 mg of lutein per cup of cooked kale, 26 mg/cup of cooked spinach, and 3 mg/cup of broccoli. Dark green leafy vegetables like spinach and kale are particularly rich sources of lutein but poor sources of zeaxanthin 201. Although relatively low in lutein, egg yolks and avocados are highly bioavailable sources of lutein. Good sources of dietary zeaxanthin include yellow corn, corn-based products, bell peppers, and egg yolk 202. Some foods containing lutein and zeaxanthin are listed in Table 1 below.
They also quantified small amounts of lutein and zeaxanthin in breads prepared from modern wheat varieties, Pioneer and Catoctin, while breads prepared from green-harvested wheat, Freekeh, an ancient grain, contained considerably large amounts of lutein and zeaxanthin compared to the North American breads. Lutein to zeaxanthin ratio followed the order Pioneer > Catochtin > Freekeh 203. Chicken egg yolk is deemed a better source of lutein and zeaxanthin compared to fruits and vegetables because of its increased bioavailability due to the high fat content in eggs. The concentrations of lutein and zeaxanthin in chicken egg yolk are 292 ± 117 µg/yolk and 213 ± 85 µg/yolk (average weight of yolk is about 17–19 g), respectively and are likely dependent on the type of feed, found mainly in on-esterified form with minute amounts of lycopene and β-carotene 204. It is not surprising that egg noodle had almost 6 times more xanthophyll carotenoids than lasagne. Astaxanthin and fucoxanthin are abundant in green and brown algae, respectively, which are eaten by fish. Capsanthin is found mainly in pepper. β-Cryptoxanthin is a pro-vitamin A and found in many fruits and vegetables, but mainly in corn, oranges, peaches, papaya, watermelon, and egg yolk 205, 206.
In general carotenoids are very minor constituents in cereal grains except for einkorn and durum wheat and corn that contain relatively high levels of carotenoids or yellow pigments. The common carotenoids in cereal grains are alpha- and beta-carotene, beta-cryptoxanthin, lutein and zeaxanthin with lutein being the dominant carotenoid compound. In common wheat flour (low in carotenoids), the bran/gem fraction had 4-fold more lutein, 12-fold more zeaxanthin, and 2-fold more beta-cryptoxanthin than the endosperm fractions 207. Higher amounts of lutein were found in durum, Kamut and Khorasan (5.4–5.8 µg/g) compared with common bread and pastry wheat (2.0–2.1 µg/g). Einkorn, on the other hand, had the highest concentration of all-trans-lutein, which is influenced by environmental growing conditions and processing. Corn also contains exceptionally high levels of non-provitamin A carotenoids primarily lutein and zeaxanthin 208, 209.
Table 1. Commonly consumed foods as high sources of lutein and zeaxanthin
| Food | Lutein | Zeaxanthin |
|---|---|---|
| Vegetables | ||
| Basil a | 70.5 | in |
| Parsley a | 64–106.5 | in |
| Spinach a | 59.3–79.0 | in |
| Kale a | 48–114.7 | – |
| Leek a | 36.8 | in |
| Pea a | 19.1 | in |
| Lettuce a | 10–47.8 | – |
| Green pepper a | 8.8 | – |
| Broccoli a | 7.1–33 | in |
| Carrot a | 2.5–5.1 | in |
| Red pepper a | 2.5–85.1 | 5.9–13.5 |
| Eggs | ||
| Egg yolk a | 3.84–13.2 | – |
| Nuts | ||
| Pistachio a | 7.7–49 | – |
| Baked foods | ||
| High lutein bread b | 36.7 | 3.3 |
| High lutein cookie b | 21.3 | 2.9 |
| High lutein muffin b | 26.1 | 3.7 |
| Corn tortilla c | 72.5 | 105.3 |
| Corn chips c | 61.1 | 92.5 |
| Grains | ||
| Corn d | 21.9 | 10.3 |
| Einkorn wheat d | 7.4 | 0.9 |
| Khorasan wheat d | 5.5 | 0.7 |
| Durum wheat d | 5.4 | 0.5 |
Footnote: µg/g fresh weight except for corn tortilla and chips µg/g dry matter; in = included with lutein.
Data obtained from: a 210; b 211; c 212; d 213
[Source 214 ]Lutein supplements
Lutein and zeaxanthin are not provitamin A carotenoids. Lutein and zeaxanthin supplements are available as free carotenoids (non-esterified) or as esters (esterified to fatty acids). Both forms appear to have comparable bioavailability 56. Many commercially available lutein and zeaxanthin supplements have much higher amounts of lutein than zeaxanthin 201. Supplements containing only lutein or zeaxanthin are also available.
It has been suggested that 6 mg of lutein per day, either through diet or using supplements is likely effective in reducing the risk of cataracts and AMD. Although the optimal dose for lutein supplementation has not been established yet, the most common dose in commercial products is 10 mg/day.
Carotenoids such as lutein and zeaxanthin are generally recognized as safe (GRAS) for human consumption by the FDA, which allows food manufacturers to use them as additives 215, 216. Recently, the European Food Safety Authority (EFSA) Panel on Food Additives and Nutrient Sources added to Food established an acceptable daily intake of 1 mg / kg bodyweight / day for lutein preparations derived from marigold (Tagetes erecta) containing at least 80% carotenoids 217, 218. Based on the available data, EFSA concluded that an intake of 0.75 mg / kg bodyweight / day of synthetic zeaxanthin does not raise any safety concerns 219. These values correspond to a daily intake of 53 mg of zeaxanthin and 70 mg of lutein for a person weighing 70 kg. These numbers are much higher than the earlier claims that 20 mg/day/person was safe in dietary supplements 220, 221. Mutagenic studies have revealed that lutein and zeaxanthin are safe for human consumption 222, 223. The no observed-adverse-effect-level (NOAEL) for lutein/zeaxanthin concentrate was determined to be 400 mg/kg bodyweight/day, the highest dose tested in rats 224. The safety of supplemental meso-zeaxanthin was recently reviewed 225, and the NOAEL of meso-zeaxanthin in rats is 300 mg/kg bodyweight/day when administered orally for 13 consecutive weeks 226.
Lutein Side Effects
Lutein is LIKELY SAFE when taken by mouth with an extensive history of consumption from the diet at varying levels of intake 227. Consuming 6.9-11.7 mg/day of lutein as part of the diet appears to be safe. Lutein supplements have been used safely in studies in doses up to 15 mg daily for up to 2 years. The highest lutein dosage administered (40 mg/day for nine weeks) and the longest duration of supplementation (10 years supplementation with 10 mg lutein + 2 mg zeaxanthin) have been assessed in clinical trial on eye-related diseases 25, 28, 228. A risk assessment analysis of 11 human studies concluded that lutein is likely safe at intake levels below 20 mg/day 229. A more recent case report documented “foveal sparkles” (eye crystals) in an older woman with glaucoma taking 20 mg/day of lutein for eight years; the patient also had very high dietary lutein intake, and total daily intake of lutein was not known 230.
- Beta-carotene: Using beta-carotene along with lutein may reduce the amount of lutein or beta-carotene that the body can absorb. The results of metabolic studies suggested that high doses of ebat-carotene compete with lutein and lycopene for absorption when consumed at the same time 231, 232, 233.
- Vitamin E: Taking lutein supplements might decrease how much vitamin E the body absorbs. Taking lutein and vitamin E together might decrease the effects of vitamin E.
Pregnancy and breast-feeding
Lutein is LIKELY SAFE when used in the amounts found in food 234. Emerging data from research in non-human primates indicates that lutein supplementation lead to increased deposition of this carotenoid in the developing eye and brain tissues 235, 236 and mother-infant human observational studies suggest a role of lutein in visual and cognitive function 47, 48.
Lutein Summary
Lutein is a oxygenated carotenoid that is synthesized only by plants and like other xanthophylls, is found naturally in high quantities in green leafy vegetables such as spinach, kale, squash, sweet peppers, sweet corn, peas and yellow carrots. Lutein is also naturally present in a concentrated area of the macula of the human retina, a small area of the retina responsible for central vision, where together with zeaxanthin it forms the macular pigment. Several studies show that lutein, zeaxanthin and meso-zeaxanthin supplementation improved macular pigment optical density both in AMD patients and healthy subjects with a dose-response relationship, whereas the improvement in visual acuity was milder 237, 238. Dietary lutein and zeaxanthin intake is also associated with a reduced risk of age-related cataract, especially nuclear cataract in a dose-response manner, indicating a beneficial effect of lutein and zeaxanthin in age-related cataract prevention.
Studies have also shown that higher dietary intake of lutein and higher blood concentrations are associated with a lower risk of coronary heart disease, stroke, and metabolic syndrome, but not with risk of type 2 diabetes mellitus, possibly through less atherosclerosis and lower inflammatory activity 3.
- National Center for Biotechnology Information, U.S. National Library of Medicine. Lutein. https://pubchem.ncbi.nlm.nih.gov/compound/Lutein[↩]
- Holden J.M., Eldridge A.L., Beecher G.R., Marilyn Buzzard I., Bhagwat S., Davis C.S., Douglass L.W., Gebhardt S., Haytowitz D., Schakel S. Carotenoid Content of U.S. Foods: An Update of the Database. J. Food Compos. Anal. 1999;12:169–196. doi: 10.1006/jfca.1999.0827[↩]
- Am J Clin Nutr February 2016, vol. 103 no. 2 481-494. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. http://ajcn.nutrition.org/content/103/2/481.long[↩][↩][↩][↩][↩][↩][↩][↩]
- Khachik F., Spangler C.J., Smith J.C., Canfield L.M., Steck A., Pfander H. Identification, Quantification, and Relative Concentrations of Carotenoids and Their Metabolites in Human Milk and Serum. Anal. Chem. 1997;69:1873–1881. doi: 10.1021/ac961085i[↩]
- Canadian Institutes of Health Research. DrugBank. Lutein. https://www.drugbank.ca/drugs/DB00137[↩][↩][↩][↩]
- Stahl W. Macular Carotenoids: Lutein and Zeaxanthin. Dev. Ophthalmol. 2005;38:70–88. doi: 10.1159/000082768[↩]
- Widomsa J., Zareba M., Subczynski W.K. Can Xanthophyll-Membrane Interactions Explain Their Selective Presence in the Retina and the Brain? Foods. 2016;5:7. doi: 10.3390/foods5010007[↩]
- Böhm V., Lietz G., Olmedilla-Alonso B., Phelan D., Reboul E., Bánati D., Borel P., Corte-Real J., De Lera A.R., Desmarchelier C., et al. From Carotenoid Intake to Carotenoid Blood and Tissue Concentrations-Implications for Dietary Intake Recommendations. Nutr. Rev. 2021;79:544–573. doi: 10.1093/nutrit/nuaa008[↩]
- Yakovleva M.A., Panova I.G., Fel’dman T.B., Zak P.P., Tatikolov A.S., Sukhikh G.T., Ostrovsky M.A. Finding of Carotenoids in the Vitreous Body of Human Eye during Prenatal Development. Russ. J. Dev. Biol. 2007;38:317–321. doi: 10.1134/S1062360407050062[↩]
- Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171-201. doi: 10.1146/annurev.nutr.23.011702.073307[↩]
- Kijlstra A, Tian Y, Kelly ER, Berendschot TT. Lutein: more than just a filter for blue light. Prog Retin Eye Res. 2012 Jul;31(4):303-15. doi: 10.1016/j.preteyeres.2012.03.002[↩]
- Whitehead AJ, Mares JA, Danis RP. Macular pigment: a review of current knowledge. Arch Ophthalmol. 2006 Jul;124(7):1038-45. doi: 10.1001/archopht.124.7.1038[↩]
- Bernstein P.S., Li B., Vachali P.P., Gorusupudi A., Shyam R., Henriksen B.S., Nolan J.M. Lutein, Zeaxanthin, and Meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-Based Nutritional Interventions against Ocular Disease. Prog. Retin. Eye Res. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003[↩]
- Wooten B.R., Hammond B.R. Macular Pigment: Influences on Visual Acuity and Visibility. Prog. Retin. Eye Res. 2002;21:225–240. doi: 10.1016/S1350-9462(02)00003-4[↩]
- Bernstein P.S., Delori F.C., Richer S., van Kuijk F.J.M., Wenzel A.J. The Value of Measurement of Macular Carotenoid Pigment Optical Densities and Distributions in Age-Related Macular Degeneration and Other Retinal Disorders. Vision Res. 2010;50:716–728. doi: 10.1016/j.visres.2009.10.014[↩][↩][↩]
- Hammond B.R., Fletcher L.M., Roos F., Wittwer J., Schalch W. A Double-Blind, Placebo-Controlled Study on the Effects of Lutein and Zeaxanthin on Photostress Recovery, Glare Disability, and Chromatic Contrast. Investig. Ophthalmol. Vis. Sci. 2014;55:8583–8589. doi: 10.1167/iovs.14-15573[↩][↩][↩]
- Stringham J.M., Hammond B.R. The Glare Hypothesis of Macular Pigment Function. Optom. Vis. Sci. 2007;84:859–864. doi: 10.1097/OPX.0b013e3181559c2b[↩]
- Loughman J., Nolan J.M., Howard A.N., Connolly E., Meagher K., Beatty S. The Impact of Macular Pigment Augmentation on Visual Performance Using Different Carotenoid Formulations. Investig. Ophthalmol. Vis. Sci. 2012;53:7871–7880. doi: 10.1167/iovs.12-10690[↩]
- Kvansakul J, Rodriguez-Carmona M, Edgar DF, Barker FM, Köpcke W, Schalch W, Barbur JL. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic Physiol Opt. 2006 Jul;26(4):362-71. doi: 10.1111/j.1475-1313.2006.00387.x[↩]
- Ma L, Lin XM, Zou ZY, Xu XR, Li Y, Xu R. A 12-week lutein supplementation improves visual function in Chinese people with long-term computer display light exposure. Br J Nutr. 2009 Jul;102(2):186-90. doi: 10.1017/S0007114508163000[↩]
- Stringham JM, Hammond BR. Macular pigment and visual performance under glare conditions. Optom Vis Sci. 2008 Feb;85(2):82-8. doi: 10.1097/OPX.0b013e318162266e. Erratum in: Optom Vis Sci. 2008 Apr;85(4):285.[↩]
- Yagi A, Fujimoto K, Michihiro K, Goh B, Tsi D, Nagai H. The effect of lutein supplementation on visual fatigue: a psychophysiological analysis. Appl Ergon. 2009 Nov;40(6):1047-54. doi: 10.1016/j.apergo.2009.04.013[↩]
- Stringham JM, Hammond BR Jr. Dietary lutein and zeaxanthin: possible effects on visual function. Nutr Rev. 2005 Feb;63(2):59-64. doi: 10.1111/j.1753-4887.2005.tb00122.x[↩]
- National Center for Biotechnology Information, U.S. National Library of Medicine. PubChem. Lutein. https://pubchem.ncbi.nlm.nih.gov/compound/Lutein[↩]
- Chew E.Y., Clemons T.E., SanGiovanni J.P., Danis R.P., Ferris F.L., Elman M.J., Antoszyk A.N., Ruby A.J., Orth D., Bressler S.B., et al. Secondary Analyses of the Effects of Lutein/Zeaxanthin on Age-Related Macular Degeneration Progression AREDS2 Report No. 3. JAMA Ophthalmol. 2014;132:142–149. doi: 10.1001/jamaophthalmol.2013.7376[↩][↩][↩]
- Liu R., Wang T., Zhang B., Qin L., Wu C., Li Q., Ma L. Lutein and Zeaxanthin Supplementation and Association with Visual Function in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2015;56:252–258. doi: 10.1167/iovs.14-15553[↩][↩]
- Dawczynski J., Jentsch S., Schweitzer D., Hammer M., Lang G.E., Strobel J. Long Term Effects of Lutein, Zeaxanthin and Omega-3-LCPUFAs Supplementation on Optical Density of Macular Pigment in AMD Patients: The LUTEGA Study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013;251:2711–2723. doi: 10.1007/s00417-013-2376-6[↩]
- Chew E.Y., Clemons T.E., Keenan T.D.L., Agron E., Malley C.E., Domalpally A. The Results of the 10 Year Follow-on Study of the Age-Related Eye Disease Study 2 (AREDS2) Investig. Ophthalmol. Vis. Sci. 2001;62:1215.[↩][↩]
- Stringham J.M., Hammond B.R. Macular Pigment and Visual Performance under Glare Conditions. Optom. Vis. Sci. 2008;85:82–88. doi: 10.1097/OPX.0b013e318162266e[↩]
- Stringham J.M., O’Brien K.J., Stringham N.T. Macular Carotenoid Supplementation Improves Disability Glare Performance and Dynamics of Photostress Recovery. Eye Vis. 2016;3:1–8. doi: 10.1186/s40662-016-0060-8[↩][↩]
- Johnson E.J., McDonald K., Caldarella S.M., Chung H.Y., Troen A.M., Snodderly D.M. Cognitive Findings of an Exploratory Trial of Docosahexaenoic Acid and Lutein Supplementation in Older Women. Nutr. Neurosci. 2008;11:75–83. doi: 10.1179/147683008X301450[↩][↩]
- Hammond B.R., Stephen Miller L., Bello M.O., Lindbergh C.A., Mewborn C., Renzi-Hammond L.M. Effects of Lutein/Zeaxanthin Supplementation on the Cognitive Function of Community Dwelling Older Adults: A Randomized, Double-Masked, Placebo-Controlled Trial. Front. Aging Neurosci. 2017;9:1–9. doi: 10.3389/fnagi.2017.00254[↩]
- Lindbergh C.A., Renzi-Hammond L.M., Hammond B.R., Terry D.P., Mewborn C.M., Puente A.N., Miller L.S. Lutein and Zeaxanthin Influence Brain Function in Older Adults: A Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2018;24:77–90. doi: 10.1017/S1355617717000534[↩][↩]
- Renzi-Hammond L.M., Bovier E.R., Fletcher L.M., Miller L.S., Mewborn C.M., Lindbergh C.A., Baxter J.H., Hammond B.R. Effects of a Lutein and Zeaxanthin Intervention on Cognitive Function: A Randomized, Double-Masked, Placebo-Controlled Trial of Younger Healthy Adults. Nutrients. 2017;9:1246. doi: 10.3390/nu9111246[↩][↩]
- Bernstein P.S., Arunkumar R. The Emerging Roles of the Macular Pigment Carotenoids throughout the Lifespan and in Prenatal Supplementation. J. Lipid Res. 2021;62:1–33. doi: 10.1194/jlr.TR120000956[↩]
- Vishwanathan R., Kuchan M.J., Sen S., Johnson E.J. Lutein and preterm infants with decreased concentrations of brain carotenoids. J. Pediatr. Gastroenterol. Nutr. 2014;59:659–665. doi: 10.1097/MPG.0000000000000389[↩]
- Bernstein P.S., Sharifzadeh M., Liu A., Ermakov I., Nelson K., Sheng X., Panish C., Carlstrom B., Hoffman R.O., Gellermann W. Blue-light reflectance imaging of macular pigment in infants and children. Investig. Ophthalmol. Vis. Sci. 2013;54:4034–4040. doi: 10.1167/iovs.13-11891[↩]
- Bettler J., Zimmer J.P., Neuringer M., DeRusso P.A. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur. J. Nutr. 2010;49:45–51. doi: 10.1007/s00394-009-0047-5[↩]
- Johnson L., Norkus E., Abbasi S., Gerdes J., Bhutani V. Contribution of beta-carotene (BC) from BC enriched formulae to individual and total serum carotenoids in term infants. FASEB J. 1995;9:1869.[↩][↩]
- Gazzolo D, Picone S, Gaiero A, Bellettato M, Montrone G, Riccobene F, Lista G, Pellegrini G. Early Pediatric Benefit of Lutein for Maturing Eyes and Brain-An Overview. Nutrients. 2021 Sep 17;13(9):3239. doi: 10.3390/nu13093239[↩][↩][↩]
- Zhang Y, Kong L, Lawrence JC, Tan L. Utilization of Biopolymer-Based Lutein Emulsion as an Effective Delivery System to Improve Lutein Bioavailability in Neonatal Rats. Nutrients. 2024 Jan 31;16(3):422. doi: 10.3390/nu16030422[↩]
- Johnson E.J., Vishwanathan R., Johnson M.A., Hausman D.B., Davey A., Scott T.M., Green R.C., Miller L.S., Gearing M., Woodard J., et al. Relationship between Serum and Brain Carotenoids, α -Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. J. Aging Res. 2013;2013 doi: 10.1155/2013/951786[↩]
- Liu X.H., Yu R.B., Liu R., Hao Z.X., Han C.C., Zhu Z.H., Ma L. Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients. 2014;6:452–465. doi: 10.3390/nu6010452[↩]
- Lindbergh C.A., Mewborn C.M., Hammond B.R., Renzi-Hammond L.M., Curran-Celentano J.M., Miller L.S. Relationship of Lutein and Zeaxanthin Levels to Neurocognitive Functioning: An FMRI Study of Older Adults. J. Int. Neuropsychol. Soc. 2017;23:11–22. doi: 10.1017/S1355617716000850[↩]
- Bovier E.R., Hammond B.R. A Randomized Placebo-Controlled Study on the Effects of Lutein and Zeaxanthin on Visual Processing Speed in Young Healthy Subjects. Arch. Biochem. Biophys. 2015;572:54–57. doi: 10.1016/j.abb.2014.11.012[↩]
- Bovier E.R., Renzi L.M., Hammond B.R. A Double-Blind, Placebo-Controlled Study on the Effects of Lutein and Zeaxanthin on Neural Processing Speed and Efficiency. PLoS ONE. 2014;9:e108178. doi: 10.1371/journal.pone.0108178[↩]
- Lai J.S., Veetil V.O., Lanca C., Lee B.L., Godfrey K.M., Gluckman P.D., Shek L.P., Yap F., Tan K.H., Chong Y.S., et al. Maternal Lutein and Zeaxanthin Concentrations in Relation to Offspring Visual Acuity at 3 Years of Age: The GUSTO Study. Nutrients. 2020;12:274. doi: 10.3390/nu12020274[↩][↩]
- Mahmassani H.A., Switkowski K.M., Scott T.M., Johnson E.J., Rifas-Shiman S.L., Oken E., Jacques P.F. Maternal Intake of Lutein and Zeaxanthin during Pregnancy Is Positively Associated with Offspring Verbal Intelligence and Behavior Regulation in Mid-Childhood in the Project Viva Cohort. J. Nutr. 2021;151:615–627. doi: 10.1093/jn/nxaa348[↩][↩]
- Cheatham C.L., Sheppard K.W. Synergistic Effects of Human Milk Nutrients in the Support of Infant Recognition Memory: An Observational Study. Nutrients. 2015;7:9079–9095. doi: 10.3390/nu7115452[↩]
- Barnett S.M., Khan N.A., Walk A.M., Raine L.B., Moulton C., Cohen N.J., Kramer A.F., Hammond B.R., Renzi-Hammond L., Hillman C.H. Macular Pigment Optical Density Is Positively Associated with Academic Performance among Preadolescent Children. Nutr. Neurosci. 2018;21:632–640. doi: 10.1080/1028415X.2017.1329976[↩]
- Ma L, Hao ZX, Liu RR, Yu RB, Shi Q, Pan JP. Graefes Arch Clin Exp Ophthalmol. 2014 Jan;252(1):63-70. doi: 10.1007/s00417-013-2492-3. Epub 2013 Oct 23. A dose-response meta-analysis of dietary lutein and zeaxanthin intake in relation to risk of age-related cataract. https://www.ncbi.nlm.nih.gov/pubmed/24150707[↩]
- Bae J-M. Reinterpretation of the results of a pooled analysis of dietary carotenoid intake and breast cancer risk by using the interval collapsing method. Epidemiology and Health. 2016;38:e2016024. doi:10.4178/epih.e2016024. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4974449/[↩]
- Chen J, Jiang W, Shao L, Zhong D, Wu Y, Cai J. Int J Food Sci Nutr. 2016 Nov;67(7):744-53. doi: 10.1080/09637486.2016.1197892. Epub 2016 Jun 30. Association between intake of antioxidants and pancreatic cancer risk: a meta-analysis. https://www.ncbi.nlm.nih.gov/pubmed/27356952[↩]
- Perry A, Rasmussen H, Johnson EJ. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J Food Compos Anal 2009:9–15.[↩]
- Chung HY, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J Nutr. 2004 Aug;134(8):1887-93. doi: 10.1093/jn/134.8.1887[↩]
- Bowen PE, Herbst-Espinosa SM, Hussain EA, Stacewicz-Sapuntzakis M. Esterification does not impair lutein bioavailability in humans. J Nutr. 2002 Dec;132(12):3668-73. doi: 10.1093/jn/132.12.3668[↩][↩]
- Yoshizako H, Hara K, Takai Y, Kaidzu S, Obana A, Ohira A. Comparison of macular pigment and serum lutein concentration changes between free lutein and lutein esters supplements in Japanese subjects. Acta Ophthalmol. 2016 Sep;94(6):e411-6. doi: 10.1111/aos.13106[↩]
- Norkus EP, Norkus KL, Dharmarajan TS, Schierle J, Schalch W. Serum lutein response is greater from free lutein than from esterified lutein during 4 weeks of supplementation in healthy adults. J Am Coll Nutr. 2010 Dec;29(6):575-85. doi: 10.1080/07315724.2010.10719896[↩]
- Bone RA, Landrum JT. Dose-dependent response of serum lutein and macular pigment optical density to supplementation with lutein esters. Arch Biochem Biophys. 2010 Dec 1;504(1):50-5. doi: 10.1016/j.abb.2010.06.019[↩]
- Sherry CL, Oliver JS, Renzi LM, Marriage BJ. Lutein supplementation increases breast milk and plasma lutein concentrations in lactating women and infant plasma concentrations but does not affect other carotenoids. J Nutr. 2014 Aug;144(8):1256-63. doi: 10.3945/jn.114.192914[↩]
- Ma L, Liu R, Du JH, Liu T, Wu SS, Liu XH. Lutein, Zeaxanthin and Meso-zeaxanthin Supplementation Associated with Macular Pigment Optical Density. Nutrients. 2016 Jul 12;8(7):426. doi: 10.3390/nu8070426[↩]
- Koushan K, Rusovici R, Li W, Ferguson LR, Chalam KV. The Role of Lutein in Eye-Related Disease. Nutrients. 2013;5(5):1823-1839. doi:10.3390/nu5051823. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3708350/[↩][↩]
- Carotenoids. https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/carotenoids[↩][↩]
- Vitamin A and Carotenoids. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional[↩][↩]
- Wang XD. Carotenoids. In: Ross CA, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed: Lippincott Williams & Wilkins; 2014:427-439.[↩][↩]
- Sommer A, Vyas KS. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr. 2012 Nov;96(5):1204S-6S. https://ajcn.nutrition.org/article/S0002-9165(23)03029-0/fulltext[↩]
- Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005 Dec;26(6):459-516. doi: 10.1016/j.mam.2005.10.001[↩]
- Jalal F, Nesheim MC, Agus Z, Sanjur D, Habicht JP. Serum retinol concentrations in children are affected by food sources of beta-carotene, fat intake, and anthelmintic drug treatment. Am J Clin Nutr. 1998 Sep;68(3):623-9. doi: 10.1093/ajcn/68.3.623[↩]
- van Het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr. 2000 Mar;130(3):503-6. doi: 10.1093/jn/130.3.503[↩][↩]
- Priyadarshani AM. A review on factors influencing bioaccessibility and bioefficacy of carotenoids. Crit Rev Food Sci Nutr. 2017 May 24;57(8):1710-1717. doi: 10.1080/10408398.2015.1023431[↩]
- Moran NE, Mohn ES, Hason N, Erdman JW Jr, Johnson EJ. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Adv Nutr. 2018 Jul 1;9(4):465-492. doi: 10.1093/advances/nmy025[↩]
- Blaner WS. Vitamin A and Provitamin A Carotenoids. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition. 11th ed. Cambridge, Massachusetts: Wiley-Blackwell; 2020:73-91.[↩][↩][↩][↩][↩]
- Food and Nutrition Board, Institute of Medicine. Beta-carotene and other carotenoids. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, D.C.: National Academy Press; 2000:325-400. https://nap.nationalacademies.org/read/9810/chapter/10[↩][↩]
- Ross A. Vitamin A. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:260-77.[↩]
- Kedishvili NY. Enzymology of retinoic acid biosynthesis and degradation. J Lipid Res. 2013 Jul;54(7):1744-60. doi: 10.1194/jlr.R037028[↩]
- Zumaraga MPP, Arquiza JMRA, Concepcion MA, Perlas L, Alcudia-Catalma MN, Rodriguez M. Genotype Effects on β-Carotene Conversion to Vitamin A: Implications on Reducing Vitamin A Deficiency in the Philippines. Food Nutr Bull. 2022 Mar;43(1):25-34. doi: 10.1177/03795721211060229[↩]
- Lietz G, Oxley A, Leung W, Hesketh J. Single nucleotide polymorphisms upstream from the β-carotene 15,15′-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr. 2012 Jan;142(1):161S-5S. doi: 10.3945/jn.111.140756[↩]
- Leung WC, Hessel S, Méplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15′-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 2009 Apr;23(4):1041-53. doi: 10.1096/fj.08-121962[↩]
- Sommer A, Vyas KS. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr 2012;96:1204S–6S. http://ajcn.nutrition.org/content/96/5/1204S.full[↩][↩][↩][↩]
- N Engl J Med. 1996 May 2;334(18):1150-5. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. http://www.nejm.org/doi/full/10.1056/NEJM199605023341802[↩]
- Satia JA, Littman A, Slatore CG, Galanko JA, White E. Long-term Use of β-Carotene, Retinol, Lycopene, and Lutein Supplements and Lung Cancer Risk: Results From the VITamins And Lifestyle (VITAL) Study. American Journal of Epidemiology. 2009;169(7):815-828. doi:10.1093/aje/kwn409. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2842198/[↩]
- Sommer A, West KP Jr. Vitamin A deficiency: health, survival, and vision. New York, NY: Oxford University Press, 1996.[↩]
- Tapiero H, Townsend DM, Tew KD. The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother 2004;58:100–10. https://www.ncbi.nlm.nih.gov/pubmed/14992791?dopt=Abstract[↩]
- Ribaya-Mercado JD, Blumberg JB. Lutein and zeaxanthin and their potential roles in disease prevention. J Am Coll Nutr 2004;23:567S–87S. https://www.ncbi.nlm.nih.gov/pubmed/15640510?dopt=Abstract[↩]
- Mares-Perlman JA, Millen AE, Ficek TL, Hankinson SE. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease overview. J Nutr 2002;132(suppl):518S–24S. http://jn.nutrition.org/content/132/3/518S.full[↩]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150–5. http://www.nejm.org/doi/full/10.1056/NEJM199605023341802[↩]
- Albanes D. β-Carotene and lung cancer: a case study. Am J Clin Nutr 1999;69:1345S–50S. http://ajcn.nutrition.org/content/69/6/1345s.full[↩][↩]
- Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, Hercberg S. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer 2010;127:172–84. https://www.ncbi.nlm.nih.gov/pubmed/19876916?dopt=Abstract[↩]
- Whitehead AJ, Mares JA, Danis RP. Macular pigment: a review of current knowledge. Arch Ophthalmol 2006;124:1038–45. http://jamanetwork.com/journals/jamaophthalmology/fullarticle/417803[↩]
- Bernstein PS, Li B, Vachali PP, et al. Lutein, Zeaxanthin, and meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-based Nutritional Interventions against Ocular Disease. Progress in retinal and eye research. 2016;50:34-66. doi:10.1016/j.preteyeres.2015.10.003. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4698241/[↩][↩][↩]
- Khachik, F., Bernstein, P. & Garland, D. L. (1997) Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Investig. Ophthamol. Vis. Sci. 38:1802-1811.[↩]
- The first isolation of enantiomeric and meso-zeaxanthin in nature. Maoka T, Arai A, Shimizu M, Matsuno T. Comp Biochem Physiol B. 1986; 83(1):121-4. https://www.ncbi.nlm.nih.gov/pubmed/3943294/[↩]
- Verification of Meso-Zeaxanthin in Fish. Nolan JM, Beatty S, Meagher KA, Howard AN, Kelly D, Thurnham DI. J Food Process Technol. 2014 Jun 1; 5(6):335. https://www.ncbi.nlm.nih.gov/pubmed/25717420/[↩]
- Macular response to supplementation with differing xanthophyll formulations in subjects with and without age-related macular degeneration. Thurnham DI, Nolan JM, Howard AN, Beatty S. Graefes Arch Clin Exp Ophthalmol. 2015 Aug; 253(8):1231-43. https://www.ncbi.nlm.nih.gov/pubmed/25311651/[↩]
- The selective retention of lutein, meso-zeaxanthin and zeaxanthin in the retina of chicks fed a xanthophyll-free diet. Wang Y, Connor SL, Wang W, Johnson EJ, Connor WE. Exp Eye Res. 2007 Mar; 84(3):591-8. https://www.ncbi.nlm.nih.gov/pubmed/17227674/[↩]
- Mares-Perlman JAFisher AIKlein R et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2001;153424- 432. https://academic.oup.com/aje/article-lookup/doi/10.1093/aje/153.5.424[↩]
- Eye (Lond). 2017 Feb;31(2):273-285. doi: 10.1038/eye.2016.286. Epub 2016 Dec 9. Effectiveness and safety of nutritional supplements in the treatment of hereditary retinal dystrophies: a systematic review. https://www.ncbi.nlm.nih.gov/pubmed/27935602[↩]
- Bourne RR, Jonas JB, Flaxman SR, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990-2010. Br J Ophthalmol. 2014; 98: 629–638.[↩]
- National Advisory Eye Council. Vision research, a national plan, 1999–2003. Bethesda, MD: United States Department of Health and Human Services, National Institutes of Health, National Eye Institute, 1998. (NIH publication no. 98–4120).[↩]
- Klein R, Klein BEK, Jensen SC, et al. Age-related maculopathy in a multiracial United States population. The National Health and Nutrition Examination Survey III. Ophthalmology 1999;106:1056–65.[↩]
- Seydou Alassane, Christine Binquet, Vanessa Cottet, Olivier Fleck, Niyazi Acar, Sandrine Daniel, Cécile Delcourt, Lionel Bretillon, Alain M. Bron, Catherine Creuzot-Garcher; Relationships of Macular Pigment Optical Density With Plasma Lutein, Zeaxanthin, and Diet in an Elderly Population: The Montrachet Study. Invest. Ophthalmol. Vis. Sci. 2016;57(3):1160-1167. doi: 10.1167/iovs.15-18007. http://iovs.arvojournals.org/article.aspx?articleid=2503589[↩]
- Klein R, Klein BEK, Linton KLP. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992;99:933–43.[↩]
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014; 2: e106–e116.[↩]
- Hammond BR Jr, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, Edwards RB, Snodderly DM. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci. 1997 Aug;38(9):1795-801.[↩]
- Mares JA, LaRowe TL, Snodderly DM, Moeller SM, Gruber MJ, Klein ML, Wooten BR, Johnson EJ, Chappell RJ; CAREDS Macular Pigment Study Group and Investigators. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women’s Health Initiative. Am J Clin Nutr. 2006 Nov;84(5):1107-22. doi: 10.1093/ajcn/84.5.1107[↩]
- Intake of lutein and zeaxanthin differ with age, sex, and ethnicity.Johnson EJ, Maras JE, Rasmussen HM, Tucker KL. J Am Diet Assoc. 2010 Sep; 110(9):1357-62. https://www.ncbi.nlm.nih.gov/pubmed/20800129/[↩]
- Lutein and zeaxanthin intakes and risk of age-related macular degeneration and cataracts: an evaluation using the Food and Drug Administration’s evidence-based review system for health claims. Trumbo PR, Ellwood KC. Am J Clin Nutr. 2006 Nov; 84(5):971-4. https://www.ncbi.nlm.nih.gov/pubmed/17093145/[↩]
- Mares-Perlman JA, Millen AE, Ficek TL, Hankinson SE. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview. J Nutr. 2002 Mar;132(3):518S-524S. doi: 10.1093/jn/132.3.518S[↩][↩]
- Snellen EL, Verbeek AL, Van Den Hoogen GW, Cruysberg JR, Hoyng CB. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmol Scand. 2002 Aug;80(4):368-71. doi: 10.1034/j.1600-0420.2002.800404.x[↩]
- Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G, Millen AE, Wright JD. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol. 2001 Mar 1;153(5):424-32. doi: 10.1093/aje/153.5.424[↩]
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994 Nov 9;272(18):1413-20. Erratum in: JAMA 1995 Feb 22;273(8):622.[↩][↩]
- Antioxidant status and neovascular age-related macular degeneration. Eye Disease Case-Control Study Group. Arch Ophthalmol. 1993 Jan;111(1):104-9. doi: 10.1001/archopht.1993.01090010108035. Erratum in: Arch Ophthalmol 1993 Nov;111(11):1499. Erratum in: Arch Ophthalmol 1993 Sep;111(9):1228, 1993 Oct;111(10):1366[↩]
- Gale CR, Hall NF, Phillips DI, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003 Jun;44(6):2461-5. doi: 10.1167/iovs.02-0929[↩]
- Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci. 2001 Jan;42(1):235-40. Erratum in: Invest Ophthalmol Vis Sci 2001 Mar;42(3):548.[↩]
- Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci. 2001 Feb;42(2):439-46.[↩]
- Cho E, Seddon JM, Rosner B, Willett WC, Hankinson SE. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch Ophthalmol. 2004 Jun;122(6):883-92. doi: 10.1001/archopht.122.6.883[↩]
- Flood V, Smith W, Wang JJ, Manzi F, Webb K, Mitchell P. Dietary antioxidant intake and incidence of early age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 2002 Dec;109(12):2272-8. doi: 10.1016/s0161-6420(02)01263-0[↩]
- Mares-Perlman JA, Klein R, Klein BE, Greger JL, Brady WE, Palta M, Ritter LL. Association of zinc and antioxidant nutrients with age-related maculopathy. Arch Ophthalmol. 1996 Aug;114(8):991-7. doi: 10.1001/archopht.1996.01100140199014[↩]
- Mares-Perlman JA, Brady WE, Klein R, Klein BE, Bowen P, Stacewicz-Sapuntzakis M, Palta M. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol. 1995 Dec;113(12):1518-23. doi: 10.1001/archopht.1995.01100120048007[↩]
- Wu J, Cho E, Willett WC, Sastry SM, Schaumberg DA. Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration During 2 Decades of Prospective Follow-up. JAMA Ophthalmol. 2015 Dec;133(12):1415-24. doi: 10.1001/jamaophthalmol.2015.3590[↩][↩][↩]
- Merle BMJ, Cougnard-Grégoire A, Korobelnik JF, Schalch W, Etheve S, Rougier MB, Féart C, Samieri C, Delyfer MN, Delcourt C. Plasma Lutein, a Nutritional Biomarker for Development of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients. 2021 Jun 15;13(6):2047. doi: 10.3390/nu13062047[↩][↩]
- Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry. 2004 Apr;75(4):216-30. doi: 10.1016/s1529-1839(04)70049-4[↩]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013 May 15;309(19):2005-15. doi: 10.1001/jama.2013.4997. Erratum in: JAMA. 2013 Jul 10;310(2):208.[↩][↩]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36. doi: 10.1001/archopht.119.10.1417. Erratum in: Arch Ophthalmol. 2008 Sep;126(9):1251.[↩]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL 3rd, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agrón E, Toth CA, Bernstein PS, Sperduto RD. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014 Feb;132(2):142-9. doi: 10.1001/jamaophthalmol.2013.7376[↩]
- Scripsema NK, Hu DN, Rosen RB. Lutein, Zeaxanthin, and meso-Zeaxanthin in the Clinical Management of Eye Disease. J Ophthalmol. 2015;2015:865179. doi: 10.1155/2015/865179[↩]
- Liu R, Wang T, Zhang B, Qin L, Wu C, Li Q, Ma L. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014 Dec 16;56(1):252-8. doi: 10.1167/iovs.14-15553[↩]
- van der Made SM, Kelly ER, Kijlstra A, Plat J, Berendschot TT. Increased Macular Pigment Optical Density and Visual Acuity following Consumption of a Buttermilk Drink Containing Lutein-Enriched Egg Yolks: A Randomized, Double-Blind, Placebo-Controlled Trial. J Ophthalmol. 2016;2016:9035745. doi: 10.1155/2016/9035745[↩]
- Piatti A, Croce A, Mazzacane D, Traina G, Ambrosino L, Boni L, Lisi L, Cascella MC, Grunberger A. Effect of 2-year nutritional supplementation on progression of age-related macular degeneration. Eur J Ophthalmol. 2020 Mar;30(2):376-381. doi: 10.1177/1120672119836007[↩]
- Winkler BSBoulton MEGottsch JDSternberg P Oxidative damage and age-related macular degeneration. Mol Vis 1999;532 Review. https://www.ncbi.nlm.nih.gov/pubmed/10562656[↩]
- Kijlstra A, Tian Y, Kelly ER, Berendschot TT. Lutein: more than just a filter for blue light. Prog Retin Eye Res. 2012; 31: 303–315.[↩]
- Delcourt C, Carriere I, Delage M, Barberger-Gateau P, Schalch W. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci. 2006; 47: 2329–2335.[↩]
- Kijlstra A, Tian Y, Kelly ER, Berendschot TTJM. Lutein: More than just a filter for blue light. Prog Retin Eye Res 2012;31:303–15. https://www.ncbi.nlm.nih.gov/pubmed/22465791?dopt=Abstract[↩]
- Sujak A, Gabrielska J, Grudzinski W, Borc R, Mazurek P, Gruszecki WI. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch Biochem Biophys 1999;371:301–7. https://www.ncbi.nlm.nih.gov/pubmed/10545218?dopt=Abstract[↩]
- Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr 2006;83(6):1265–71. http://ajcn.nutrition.org/content/83/6/1265.abstract?ijkey=57ef21c4177b8442f40b357ae0266deeec20c77e[↩]
- Karppi J, Laukkanen JA, Makikallio TH, Ronkainen K, Kurl S. Low beta-carotene concentrations increase the risk of cardiovascular disease mortality among Finnish men with risk factors. Nutr Metab Cardiovasc Dis 2012;22:921–8. https://www.ncbi.nlm.nih.gov/pubmed/22494809?dopt=Abstract[↩]
- Progress in Retinal and Eye Research Volume 31, Issue 4, July 2012, Pages 303-315. Lutein: More than just a filter for blue light. http://www.sciencedirect.com/science/article/pii/S135094621200016X[↩][↩][↩][↩][↩][↩]
- Gong X, Rubin LP. Role of macular xanthophylls in prevention of common neovascular retinopathies: retinopathy of prematurity and diabetic retinopathy. Arch Biochem Biophys. 2015 Apr 15;572:40-48. doi: 10.1016/j.abb.2015.02.004[↩][↩]
- Brazionis L, Rowley K, Itsiopoulos C, O’Dea K. Plasma carotenoids and diabetic retinopathy. Br J Nutr. 2009 Jan;101(2):270-7. doi: 10.1017/S0007114508006545[↩]
- Lima VC, Rosen RB, Maia M, Prata TS, Dorairaj S, Farah ME, Sallum J. Macular pigment optical density measured by dual-wavelength autofluorescence imaging in diabetic and nondiabetic patients: a comparative study. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5840-5. doi: 10.1167/iovs.09-4695[↩]
- Sahli MW, Mares JA, Meyers KJ, Klein R, Brady WE, Klein BE, Ochs-Balcom HM, Donahue RP, Millen AE. Dietary Intake of Lutein and Diabetic Retinopathy in the Atherosclerosis Risk in Communities Study (ARIC). Ophthalmic Epidemiol. 2016;23(2):99-108. doi: 10.3109/09286586.2015.1129426[↩]
- Romagnoli C, Giannantonio C, Cota F, Papacci P, Vento G, Valente E, Purcaro V, Costa S. A prospective, randomized, double blind study comparing lutein to placebo for reducing occurrence and severity of retinopathy of prematurity. J Matern Fetal Neonatal Med. 2011 Oct;24 Suppl 1:147-50. doi: 10.3109/14767058.2011.607618[↩]
- Dani C, Lori I, Favelli F, Frosini S, Messner H, Wanker P, De Marini S, Oretti C, Boldrini A, Ciantelli M, Bragetti P, Germini C. Lutein and zeaxanthin supplementation in preterm infants to prevent retinopathy of prematurity: a randomized controlled study. J Matern Fetal Neonatal Med. 2012 May;25(5):523-7. doi: 10.3109/14767058.2011.629252[↩]
- Manzoni P, Guardione R, Bonetti P, Priolo C, Maestri A, Mansoldo C, Mostert M, Anselmetti G, Sardei D, Bellettato M, Biban P, Farina D. Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: a multicenter randomized controlled trial. Am J Perinatol. 2013 Jan;30(1):25-32. doi: 10.1055/s-0032-1321494[↩]
- Rubin LP, Chan GM, Barrett-Reis BM, Fulton AB, Hansen RM, Ashmeade TL, Oliver JS, Mackey AD, Dimmit RA, Hartmann EE, Adamkin DH. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J Perinatol. 2012 Jun;32(6):418-24. doi: 10.1038/jp.2011.87[↩][↩]
- Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Gao S, Qin T, Liu Z, Caceres MA, Ronchi CF, Chen CY, Yeum KJ, Taylor A, Blumberg JB, Liu Y, Shang F. Mol Vis. 2011; 17():3180-90. https://www.ncbi.nlm.nih.gov/pubmed/22194644/[↩]
- Difference in phototoxicity of cyclodextrin complexed fullerene [(gamma-CyD)2/C60] and its aggregated derivatives toward human lens epithelial cells. Zhao B, He YY, Chignell CF, Yin JJ, Andley U, Roberts JE. Chem Res Toxicol. 2009 Apr; 22(4):660-7. https://www.ncbi.nlm.nih.gov/pubmed/19281132/[↩]
- Yeum KJ, Shang FM, Schalch WM, Russell RM, Taylor A. Fat-soluble nutrient concentrations in different layers of human cataractous lens. Curr Eye Res. 1999 Dec;19(6):502-5. doi: 10.1076/ceyr.19.6.502.5282[↩]
- Brown L, Rimm EB, Seddon JM, Giovannucci EL, Chasan-Taber L, Spiegelman D, Willett WC, Hankinson SE. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am J Clin Nutr. 1999 Oct;70(4):517-24. doi: 10.1093/ajcn/70.4.517[↩]
- Chasan-Taber L, Willett WC, Seddon JM, Stampfer MJ, Rosner B, Colditz GA, Speizer FE, Hankinson SE. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. Am J Clin Nutr. 1999 Oct;70(4):509-16. doi: 10.1093/ajcn/70.4.509[↩]
- Lyle BJ, Mares-Perlman JA, Klein BE, Klein R, Greger JL. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am J Epidemiol. 1999 May 1;149(9):801-9. doi: 10.1093/oxfordjournals.aje.a009895[↩]
- Christen WG, Liu S, Glynn RJ, Gaziano JM, Buring JE. Dietary carotenoids, vitamins C and E, and risk of cataract in women: a prospective study. Arch Ophthalmol. 2008 Jan;126(1):102-9. doi: 10.1001/archopht.126.1.102[↩]
- Moeller SM, Voland R, Tinker L, Blodi BA, Klein ML, Gehrs KM, Johnson EJ, Snodderly DM, Wallace RB, Chappell RJ, Parekh N, Ritenbaugh C, Mares JA; CAREDS Study Group; Women’s Helath Initiative. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study, an Ancillary Study of the Women’s Health Initiative. Arch Ophthalmol. 2008 Mar;126(3):354-64. doi: 10.1001/archopht.126.3.354[↩]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew EY, SanGiovanni JP, Ferris FL, Wong WT, Agron E, Clemons TE, Sperduto R, Danis R, Chandra SR, Blodi BA, Domalpally A, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Friberg TR, Rosenfeld PJ, Toth CA, Bernstein P. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013 Jul;131(7):843-50. doi: 10.1001/jamaophthalmol.2013.4412[↩]
- Glaser TS, Doss LE, Shih G, Nigam D, Sperduto RD, Ferris FL 3rd, Agrón E, Clemons TE, Chew EY; Age-Related Eye Disease Study Research Group. The Association of Dietary Lutein plus Zeaxanthin and B Vitamins with Cataracts in the Age-Related Eye Disease Study: AREDS Report No. 37. Ophthalmology. 2015 Jul;122(7):1471-9. doi: 10.1016/j.ophtha.2015.04.007[↩]
- Wang Y, Chung SJ, McCullough ML, Song WO, Fernandez ML, Koo SI, Chun OK. Dietary carotenoids are associated with cardiovascular disease risk biomarkers mediated by serum carotenoid concentrations. J Nutr. 2014 Jul;144(7):1067-74. doi: 10.3945/jn.113.184317[↩][↩]
- Li Z, Chen J, Zhang D. Association between dietary carotenoid intakes and hypertension in adults: National Health and Nutrition Examination Survey 2007-2014. J Hypertens. 2019 Dec;37(12):2371-2379. doi: 10.1097/HJH.0000000000002200[↩]
- Leermakers ET, Darweesh SK, Baena CP, Moreira EM, Melo van Lent D, Tielemans MJ, Muka T, Vitezova A, Chowdhury R, Bramer WM, Kiefte-de Jong JC, Felix JF, Franco OH. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. Am J Clin Nutr. 2016 Feb;103(2):481-94. doi: 10.3945/ajcn.115.120931[↩]
- Johnson EJ, Vishwanathan R, Johnson MA, Hausman DB, Davey A, Scott TM, Green RC, Miller LS, Gearing M, Woodard J, Nelson PT, Chung HY, Schalch W, Wittwer J, Poon LW. Relationship between Serum and Brain Carotenoids, α-Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. J Aging Res. 2013;2013:951786. doi: 10.1155/2013/951786[↩][↩][↩]
- Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ. Lutein and preterm infants with decreased concentrations of brain carotenoids. J Pediatr Gastroenterol Nutr. 2014 Nov;59(5):659-65. doi: 10.1097/MPG.0000000000000389[↩][↩]
- Feeney J, Finucane C, Savva GM, Cronin H, Beatty S, Nolan JM, Kenny RA. Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiol Aging. 2013 Nov;34(11):2449-56. doi: 10.1016/j.neurobiolaging.2013.05.007[↩]
- Renzi LM, Dengler MJ, Puente A, Miller LS, Hammond BR Jr. Relationships between macular pigment optical density and cognitive function in unimpaired and mildly cognitively impaired older adults. Neurobiol Aging. 2014 Jul;35(7):1695-9. doi: 10.1016/j.neurobiolaging.2013.12.024[↩]
- Kelly D, Coen RF, Akuffo KO, Beatty S, Dennison J, Moran R, Stack J, Howard AN, Mulcahy R, Nolan JM. Cognitive Function and Its Relationship with Macular Pigment Optical Density and Serum Concentrations of its Constituent Carotenoids. J Alzheimers Dis. 2015;48(1):261-77. doi: 10.3233/JAD-150199[↩]
- Krinsky N.I., Johnson E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001[↩]
- Vishwanathan R., Iannaccone A., Scott T.M., Kritchevsky S.B., Jennings B.J., Carboni G., Forma G., Satterfield S., Harris T., Johnson K.C., et al. Macular pigment optical density is related to cognitive function in older people. Age Ageing. 2014;43:271–275. doi: 10.1093/ageing/aft210[↩]
- Johnson E.J., Vishwanathan R., Johnson M.A., Hausman D.B., Davey A., Scott T.M., Green R.C., Miller L.S., Gearing M., Woodard J. Relationship between serum and brain carotenoids, α-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the georgia centenarian study. J. Aging Res. 2013;2013:951786. doi: 10.1155/2013/951786[↩]
- Feeney J., Finucane C., Savva G.M., Cronin H., Beatty S., Nolan J.M., Kenny R.A. Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiol. Aging. 2013;34:2449–2456. doi: 10.1016/j.neurobiolaging.2013.05.007[↩]
- Bovier E.R., Renzi L.M., Hammond B.R. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on neural processing speed and efficiency. PLoS ONE. 2014;9:e108178. doi: 10.1371/journal.pone.0108178[↩]
- Edwards CG, Walk AM, Thompson SV, Reeser GE, Dilger RN, Erdman JW Jr, Burd NA, Holscher HD, Khan NA. Dietary lutein plus zeaxanthin and choline intake is interactively associated with cognitive flexibility in middle-adulthood in adults with overweight and obesity. Nutr Neurosci. 2022 Jul;25(7):1437-1452. doi: 10.1080/1028415X.2020.1866867[↩]
- Christensen K, Gleason CE, Mares JA. Dietary carotenoids and cognitive function among US adults, NHANES 2011-2014. Nutr Neurosci. 2020 Jul;23(7):554-562. doi: 10.1080/1028415X.2018.1533199[↩]
- Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006 Oct 24;67(8):1370-6. doi: 10.1212/01.wnl.0000240224.38978.d8[↩]
- Kang JH, Ascherio A, Grodstein F. Fruit and vegetable consumption and cognitive decline in aging women. Ann Neurol. 2005 May;57(5):713-20. doi: 10.1002/ana.20476[↩]
- Feeney J, O’Leary N, Moran R, O’Halloran AM, Nolan JM, Beatty S, Young IS, Kenny RA. Plasma Lutein and Zeaxanthin Are Associated With Better Cognitive Function Across Multiple Domains in a Large Population-Based Sample of Older Adults: Findings from The Irish Longitudinal Study on Aging. J Gerontol A Biol Sci Med Sci. 2017 Oct 1;72(10):1431-1436. doi: 10.1093/gerona/glw330[↩]
- Johnson E.J., McDonald K., Caldarella S.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008;11:75–83. doi: 10.1179/147683008X301450[↩]
- Stahl W., Seis H. Effects of carotenoids and retinoids on gap junctional communication. Biofactors. 2001;15:95–98. doi: 10.1002/biof.5520150209[↩]
- Hammond B.R., Jr., Wooten B.R. CFF Thresholds: Relation to macular pigment optical density. Ophthalmic Physiol. Opt. 2005;25:315–319. doi: 10.1111/j.1475-1313.2005.00271.x[↩]
- Renzi-Hammond LM, Bovier ER, Fletcher LM, Miller LS, Mewborn CM, Lindbergh CA, Baxter JH, Hammond BR. Effects of a Lutein and Zeaxanthin Intervention on Cognitive Function: A Randomized, Double-Masked, Placebo-Controlled Trial of Younger Healthy Adults. Nutrients. 2017 Nov 14;9(11):1246. doi: 10.3390/nu9111246[↩]
- Power R, Coen RF, Beatty S, Mulcahy R, Moran R, Stack J, Howard AN, Nolan JM. Supplemental Retinal Carotenoids Enhance Memory in Healthy Individuals with Low Levels of Macular Pigment in A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Alzheimers Dis. 2018;61(3):947-961. doi: 10.3233/JAD-170713[↩]
- Chew EY, Clemons TE, Agrón E, Launer LJ, Grodstein F, Bernstein PS; Age-Related Eye Disease Study 2 (AREDS2) Research Group. Effect of Omega-3 Fatty Acids, Lutein/Zeaxanthin, or Other Nutrient Supplementation on Cognitive Function: The AREDS2 Randomized Clinical Trial. JAMA. 2015 Aug 25;314(8):791-801. doi: 10.1001/jama.2015.9677[↩]
- Ascherio A, Rimm EB, Hernan MA, Giovannucci E, Kawachi I, Stampfer MJ, Willett WC. Relation of consumption of vitamin E, vitamin C, and carotenoids to risk for stroke among men in the United States. Ann Intern Med 1999;130:963–70. https://www.ncbi.nlm.nih.gov/pubmed/10383366?dopt=Abstract[↩]
- Hak AE, Ma J, Powell CB, Campos H, Gaziano JM, Willett WC, Stampfer MJ. Prospective study of plasma carotenoids and tocopherols in relation to risk of ischemic stroke. Stroke 2004;35:1584–8. http://stroke.ahajournals.org/content/35/7/1584[↩]
- Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke 2000;31:2301–6. http://stroke.ahajournals.org/content/31/10/2301[↩]
- Bates CJ, Hamer M, Mishra GD. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over. Br J Nutr 2011;105:123–32. https://www.ncbi.nlm.nih.gov/pubmed/20807458?dopt=Abstract[↩][↩]
- Buijsse B, Feskens EJM, Kwape L, Kok FJ, Kromhout D. Both (alpha)- and (beta)-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr 2008;138:344–50. http://jn.nutrition.org/content/138/2/344.full[↩][↩]
- Ito Y, Suzuki K, Ishii J, Hishida H, Tamakoshi A, Hamajima N, Aoki K. A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in Japanese inhabitants. Asian Pac J Cancer Prev 2006;7:533–46. https://www.ncbi.nlm.nih.gov/pubmed/17250424?dopt=Abstract[↩][↩]
- Mayne ST, Cartmel B, Lin HQ, Zhang TZ, Goodwin WJ. Low plasma lycopene concentration is associated with increased mortality in a cohort of patients with prior oral, pharynx or larynx cancers. J Am Coll Nutr 2004;23:34–42. https://www.ncbi.nlm.nih.gov/pubmed/14963051?dopt=Abstract[↩][↩]
- Shardell MD, Alley DE, Hicks GE, El-Kamary SS, Miller RR, Semba RD, Ferrucci L. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the Third National Health and Nutrition Examination Survey. Nutr Res 2011;31:178–89. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3081783/[↩][↩]
- Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am J Clin Nutr 2004;79:47–53. http://ajcn.nutrition.org/content/79/1/47.full[↩]
- Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in men. Am J Clin Nutr 2005;81:990–7. http://ajcn.nutrition.org/content/81/5/990.full[↩]
- Hozawa A, Jacobs DR, Steffes MW, Gross MD, Steffen LM, Lee DH. Associations of serum carotenoid concentrations with the development of diabetes and with insulin concentration: interaction with smoking – The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol 2006;163:929–37.[↩]
- Kataja-Tuomola MK, Kontto JP, Mannisto S, Albanes D, Virtamo J. Intake of antioxidants and risk of type 2 diabetes in a cohort of male smokers. Eur J Clin Nutr 2011;65:590–7. https://www.ncbi.nlm.nih.gov/pubmed/21245884?dopt=Abstract[↩]
- Montonen J, Knekt P, Jarvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care 2004;27:362–6. http://care.diabetesjournals.org/content/27/2/362[↩]
- Wang L, Liu SM, Pradhan AD, Manson JE, Buring JE, Gaziano JM, Sesso HD. Plasma lycopene, other carotenoids, and the risk of type 2 diabetes in women. Am J Epidemiol 2006;164:576–85.[↩]
- Beydoun MA, Canas JA, Beydoun HA, Chen XL, Shroff MR, Zonderman AB. Serum antioxidant concentrations and metabolic syndrome are associated among US adolescents in recent national surveys. J Nutr 2012;142:1693–704. http://jn.nutrition.org/content/142/9/1693.full[↩]
- Beydoun MA, Shroff MR, Chen X, Beydoun HA, Wang Y, Zonderman AB. Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys. J Nutr 2011;141:903–13. http://jn.nutrition.org/content/141/5/903.full[↩]
- Coyne T, Ibiebele TI, Baade PD, McClintock CS, Shaw JE. Metabolic syndrome and serum carotenoids: findings of a cross-sectional study in Queensland, Australia. Br J Nutr 2009;102:1668–77. https://www.ncbi.nlm.nih.gov/pubmed/19631019?dopt=Abstract[↩]
- Sluijs I, Beulens JWJ, Grobbee DE, van der Schouw YT. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J Nutr 2009;139:987–92. http://jn.nutrition.org/content/139/5/987.full[↩]
- Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Matsumoto H, Ando F, Shimokata H, Yano M. Associations of serum carotenoid concentrations with the metabolic syndrome: interaction with smoking. Br J Nutr 2008;100:1297–306. https://www.ncbi.nlm.nih.gov/pubmed/18445303?dopt=Abstract[↩]
- Suzuki K, Ito Y, Inoue T, Hamajima N. Inverse association of serum carotenoids with prevalence of metabolic syndrome among Japanese. Clin Nutr 2011;30:369–75. https://www.ncbi.nlm.nih.gov/pubmed/21216053?dopt=Abstract[↩]
- Perry A., Rasmussen H., Johnson E.J. Xanthophyll (lutein, zeaxanthin) content of fruits, vegetables and corn and egg products. J. Food Comp. Anal. 2009;22:9–15. doi: 10.1016/j.jfca.2008.07.006.[↩]
- Hendler SS, Rorvik DM, eds. PDR for Nutritional Supplements. 2nd ed. Thomson Reuters; 2008.[↩][↩]
- Tudor C, Pintea A. A Brief Overview of Dietary Zeaxanthin Occurrence and Bioaccessibility. Molecules. 2020 Sep 6;25(18):4067. doi: 10.3390/molecules25184067[↩]
- Distribution of lutein, zeaxanthin, and related geometrical isomers in fruit, vegetables, wheat, and pasta products. Humphries JM, Khachik F. J Agric Food Chem. 2003 Feb 26; 51(5):1322-7. https://www.ncbi.nlm.nih.gov/pubmed/12590476/[↩]
- Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Handelman GJ, Nightingale ZD, Lichtenstein AH, Schaefer EJ, Blumberg JB. Am J Clin Nutr. 1999 Aug; 70(2):247-51. https://www.ncbi.nlm.nih.gov/pubmed/10426702/[↩]
- Chandrika U.G., Jansz E.R., Wickranasinghe S.M.D.N., Warnasuriya N.D. Carotenoids in yellow and red-fleshed papaya (Carcia papaya L) J. Sci. Food Agric. 2003;83:1279–1282. doi: 10.1002/jsfa.1533.[↩]
- United States Department of Agriculture. USDA Nutritional database for standard reference release.[↩]
- Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. Adom KK, Sorrells ME, Liu RH. J Agric Food Chem. 2005 Mar 23; 53(6):2297-306. https://www.ncbi.nlm.nih.gov/pubmed/15769171/[↩]
- Identification and quantification of seed carotenoids in selected wheat species. Abdel-Aal el-SM, Young JC, Rabalski I, Hucl P, Fregeau-Reid J. J Agric Food Chem. 2007 Feb 7; 55(3):787-94. https://www.ncbi.nlm.nih.gov/pubmed/17263475/[↩]
- Analysis of xanthophylls in corn by HPLC. Moros EE, Darnoko D, Cheryan M, Perkins EG, Jerrell J. J Agric Food Chem. 2002 Oct 9; 50(21):5787-90. https://www.ncbi.nlm.nih.gov/pubmed/12358439/[↩]
- Maiani G., Periago Caston M.J., Catasta G., Toti E., Cambrodon I.G., Bysted A., Granado-Lorencio F., Olmedilla-Alonso B., Knuthsen P., Valoti M., et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009;53:S194–S218. doi: 10.1002/mnfr.200800053[↩]
- Abdel-Aal el-SM, Young JC, Akhtar H, Rabalski I. Stability of lutein in wholegrain bakery products naturally high in lutein or fortified with free lutein. J Agric Food Chem. 2010 Sep 22;58(18):10109-17. doi: 10.1021/jf102400t[↩]
- De La Parra C., Saldivar S.O.S., Lui R.H. Effect of processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas and tortilla chips. J. Agric. Food Chem. 2007;55:4177–4183. doi: 10.1021/jf063487p[↩]
- Abdel-Aal el-SM, Young JC, Rabalski I, Hucl P, Fregeau-Reid J. Identification and quantification of seed carotenoids in selected wheat species. J Agric Food Chem. 2007 Feb 7;55(3):787-94. doi: 10.1021/jf062764p[↩]
- Abdel-Aal E-SM, Akhtar H, Zaheer K, Ali R. Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients. 2013;5(4):1169-1185. doi:10.3390/nu5041169. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3705341[↩]
- U.S. Food and Drug Administration. Lutein. https://www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm439151.pdf[↩]
- U.S. Food and Drug Administration. Zeaxanthin. https://www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm514709.pdf[↩]
- EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific Opinion on the re-evaluation of lutein preparations other than lutein with high concentrations of total saponified carotenoids at levels of at least 80% EFSA Journal. 2011;9:2144.[↩]
- Agostoni C, Bresson J, Fairweather-Tait S, Flynn A, Golly I, Korhonen H, Lagiou P, Løvik M, Marchelli R, Martin A. Scientific Opinion on the substantiation of health claims related to lutein and protection of DNA, proteins and lipids from oxidative damage (ID 3427), protection of the skin from UV-induced (including photo-oxidative) damage (ID 1605, 1779) and maintenance of normal vision (ID 1779, 2080) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA journal. 2011;9:2030–2030. [↩]
- Agostoni C, Bresson J, Fairweather-Tait S, Flynn A, Golly I, Korhonen H, Lagiou P, Løvik M, Marchelli R, Martin A, Moseley B, Neuhäuser-Berthold M, Przyrembel H, Salminen S, Sanz Y, Strain S, Strobel S, Tetens I, Tome D, van Loveren H, Verhagen H. Scientific Opinion on the substantiation of health claims related to lutein and maintenance of normal vision (ID 1603, 1604, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2012;10.[↩]
- Agostoni C, Bresson J, Fairweather-Tait S, Flynn A, Golly I, Korhonen H, Lagiou P, Løvik M, Marchelli R, Martin A, Moseley B, Neuhäuser-Berthold M, Przyrembel H, Salminen S, Sanz Y, Strain S, Strobel S, Tetens I, Tome D, van Loveren H, Verhagen H. Scientific Opinion on the substantiation of health claims related to lutein and maintenance of normal vision (ID 1603, 1604, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2012;10[↩]
- European FoodSafetyAuthority. Technical specifications on the harmonised monitoring and reporting of antimicrobial resistance in methicillin-resistant Staphylococcus aureus in food-producing animals and food. EFSA Journal. 2012;10:2897.[↩]
- An innovative approach to the determination of safety for a dietary ingredient derived from a new source: case study using a crystalline lutein product. Kruger CL, Murphy M, DeFreitas Z, Pfannkuch F, Heimbach J. Food Chem Toxicol. 2002 Nov; 40(11):1535-49. https://www.ncbi.nlm.nih.gov/pubmed/12176081/[↩]
- Effects of lutein and zeaxanthin on aspects of eye health. Ma L, Lin XM. J Sci Food Agric. 2010 Jan 15; 90(1):2-12. https://www.ncbi.nlm.nih.gov/pubmed/20355006/[↩]
- Safety assessment of lutein and zeaxanthin (Lutemax 2020): subchronic toxicity and mutagenicity studies. Ravikrishnan R, Rusia S, Ilamurugan G, Salunkhe U, Deshpande J, Shankaranarayanan J, Shankaranarayana ML, Soni MG. Food Chem Toxicol. 2011 Nov; 49(11):2841-8. https://www.ncbi.nlm.nih.gov/pubmed/21872637/[↩]
- What is meso-zeaxanthin, and where does it come from? Nolan JM, Meagher K, Kashani S, Beatty S. Eye (Lond). 2013 Aug; 27(8):899-905. https://www.ncbi.nlm.nih.gov/pubmed/23703634/[↩]
- Xu X, Zhang L, Shao B, Sun X, Ho CT, Li S. Safety evaluation of meso-zeaxanthin. Food Control. 2013;32:678–686.[↩]
- Solomons NW. Vitamin A and carotenoids. In: Bowman BA, Russell RM, eds. Present Knowledge in Nutrition. 8th ed. Washington, D.C.: ILSI Press; 2001:127-145.[↩]
- Dagnelie G, Zorge IS, McDonald TM. Lutein improves visual function in some patients with retinal degeneration: a pilot study via the Internet. Optometry. 2000 Mar;71(3):147-64.[↩]
- Shao A, Hathcock JN. Risk assessment for the carotenoids lutein and lycopene. Regul Toxicol Pharmacol. 2006 Aug;45(3):289-98. doi: 10.1016/j.yrtph.2006.05.007[↩]
- Choi RY, Chortkoff SC, Gorusupudi A, Bernstein PS. Crystalline Maculopathy Associated With High-Dose Lutein Supplementation. JAMA Ophthalmol. 2016 Dec 1;134(12):1445-1448. doi: 10.1001/jamaophthalmol.2016.4117[↩]
- van den Berg H. Carotenoid interactions. Nutr Rev. 1999 Jan;57(1):1-10. doi: 10.1111/j.1753-4887.1999.tb01769.x[↩]
- Micozzi MS, Brown ED, Edwards BK, Bieri JG, Taylor PR, Khachik F, Beecher GR, Smith JC Jr. Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. Am J Clin Nutr. 1992 Jun;55(6):1120-5. doi: 10.1093/ajcn/55.6.1120[↩]
- Kostic D, White WS, Olson JA. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr. 1995 Sep;62(3):604-10. doi: 10.1093/ajcn/62.3.604[↩]
- Lutein. https://medlineplus.gov/druginfo/natural/754.html[↩]
- Jeon S., Neuringer M., Johnson E.E., Kuchan M.J., Pereira S.L., Johnson E.J., Erdman J.W. Effect of Carotenoid Supplemented Formula on Carotenoid Bioaccumulation in Tissues of Infant Rhesus Macaques: A Pilot Study Focused on Lutein. Nutrients. 2017;9:51. doi: 10.3390/nu9010051[↩]
- Jeon S., Ranard K.M., Neuringer M., Johnson E.E., Renner L., Kuchan M.J., Pereira S.L., Johnson E.J., Erdman J.W. Lutein Is Differentially Deposited across Brain Regions Following Formula or Breast Feeding of Infant Rhesus Macaques. J. Nutr. 2018;148:31–39. doi: 10.1093/jn/nxx023[↩]
- Ma L, Liu R, Du JH, Liu T, Wu SS, Liu XH. Lutein, Zeaxanthin and Meso-zeaxanthin Supplementation Associated with Macular Pigment Optical Density. Nutrients. 2016;8(7):426. doi:10.3390/nu8070426. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4963902/[↩]
- Wang X, Jiang C, Zhang Y, Gong Y, Chen X, Zhang M, Role of Lutein Supplementation in the Management of Age-Related Macular Degeneration: Meta-Analysis of Randomized Controlled Trials. Ophthalmic Res 2014;52:198-205. https://www.karger.com/Article/Abstract/363327[↩]