Contents
Finasteride
Finasteride an antiandrogen medicine, sold under the brand names Proscar and Propecia among others, is a specific type II 5-alpha reductase inhibitor used to treat male pattern hair loss (men with androgenetic alopecia) and in men with a benign (non-cancerous) enlargement of the prostate also called benign prostatic hyperplasia (BPH) 1, 2. Finasteride inhibits the enzyme, type II 5-alpha reductase, responsible for regulating the conversion of testosterone to dihydrotestosterone (DHT). By reducing DHT (dihydrotestosterone) levels in your scalp, finasteride decreases DHT’s effects on the hair follicles, reversing the process of hair loss. Finasteride has not been shown to treat thinning hair at the temples and is not used to treat hair loss in women or children 3. Finasteride is used alone or in combination with another medication (doxazosin) to treat benign enlargement of the prostate gland (BPH, benign prostatic hyperplasia). Finasteride is used to treat symptoms of benign enlargement of the prostate gland (BPH) such as frequent and difficult urination and may reduce the chance of acute urinary retention (sudden inability to urinate). It also may decrease the chance that prostate surgery will be needed 3. Finasteride treats BPH by blocking the body’s production of a male hormone that causes the prostate to enlarge. Finasteride treats male pattern hair loss by blocking the conversion of testosterone to dihydrotestosterone (DHT) in the scalp that stops hair growth. Finasteride may be prescribed for other uses; ask your doctor or pharmacist for more information.
Finasteride is not approved for use in females but has been used “off-label” in females to treat hair loss and hirsutism (extra hair growth on areas of the body such as the face, chest, and back), although these treatments are only partially effective at best 4, 5, 6, 7, 8, 9, 10. The effectiveness of finasteride treatment for women varies and only some studies concluded that finasteride may be considered for treatment of female pattern hair loss in patients who fail other treatment and adhere to reliable contraception during finasteride use 6, 7, 8, 4, 11. With particular regard to female alopecia, finasteride is considered both effective 12, 13, 14 and ineffective 15, 16, 17, 18. Moreover, indications for finasteride dosage in women hair loss are confusing. Studies have been published reporting dose of 1 mg, 1.25 mg, 2.5 mg or 5 mg 4, 15, 19, 16.
Topical finasteride (used on the skin only) has not been approved by the U.S. Food and Drug Administration (FDA), but has been approved in India to treat male and female pattern hair loss. Topical finasteride has been found to be as effective as oral finasteride 20, 21, 22.
Finasteride comes as a 1 mg tablet or a 5 mg tablet to be taken by mouth. Each dose has a different indication. Finasteride 1 mg orally once a day for male pattern hair loss (androgenetic alopecia) and finasteride 5 mg orally once a day for benign prostatic hyperplasia (BPH). For women with hirsutism (idiopathic, and related to polycystic ovary syndrome): 5 mg once daily or 2.5 mg once daily; this is an off-label use. Finasteride is usually taken once a day with or without food. Take finasteride at around the same time every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take finasteride exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor. Ask your pharmacist or doctor for a copy of the manufacturer’s information for the patient.
If you are taking finasteride to treat male pattern hair loss (androgenetic alopecia), it may take at least 3 months before you see any improvement because hair loss and growth happen slowly over time. However, you should expect to see improvement during the first 12 months of your treatment. If you have taken finasteride for 12 months and have not noticed any improvement, further treatment probably will not help. Talk to your doctor about whether you should continue your treatment.
Finasteride will only slow hair loss while you are taking the medication. Continue to take finasteride even if you have already noticed an improvement. Do not stop taking finasteride without talking to your doctor. You will probably lose any hair that grew back while you were taking finasteride during the first 12 months after you stop taking the medication..
If you are taking finasteride to treat BPH (benign prostatic hyperplasia), you should know that finasteride may control your condition, but will not cure it. It may take at least 6 months before your symptoms improve. Continue to take finasteride even if you feel well. Do not stop taking finasteride without talking to your doctor.
Decreased libido and erectile problems are recognized side-effects of treatment with finasteride in men 23. Some small differences have been seen in the semen of males who take finasteride, such as low sperm counts 24. Sperm levels improved when the medication was stopped. Taking finasteride may increase the risk that you will develop high-grade prostate cancer (a type of prostate cancer that spreads and grows more quickly than other types of prostate cancer) or male breast cancer 25, 26. Talk to your doctor about the risks of taking finasteride.
Finasteride is contraindicated in women when they are or may potentially be pregnant, because it may cause abnormalities of the external genitalia of a male fetus (Pregnancy Category X, i.e., drugs that have such a high risk of causing permanent damage to the fetus that they should not be used in pregnancy or when there is a possibility of pregnancy.) 27. Touching broken or crushed finasteride tablets may harm the fetus. If a woman who is pregnant or who could become pregnant touches broken or crushed finasteride tablets, she should wash the area with soap and water immediately and call her doctor. Animal studies have suggested that exposure to large doses of finasteride when the fetal sex organs are developing (8 to 12 weeks of pregnancy) could increase the chance for some birth defects of the sex organs in a male fetus 28, 27, 29. The animal studies have reported hypospadias (when the opening of the penis is on the underside of the penis instead of at the tip), a shorter distance from the anus to the genitals (anogenital distance), and lower weight of the prostate and seminal vesicles (glands that help make semen) 30, 31.
Also in case of female finasteride users, side effects have been reported during treatment 32. The top ten side effects in women were: abortion induction, abortion spontaneous, paternal drug affecting fetus, uterine cervix stenosis, menstruation irregularity, menorrhagia, endometrial hypertrophy, phalangeal agenesis, fatigue and arthritis 32. Regarding the adverse events on reproductive features, it is important to recall that finasteride is forbidden in pregnant women because it may cause abnormalities of the external genitalia of a male fetus, therefore, careful contraception is strongly suggested during finasteride treatment.
Other data described, in women treated for alopecia with 5 mg of finasteride, headache, menstrual irregularities, dizziness and increased body hair growth as the most common complaints 33. Moreover, decreased libido and gastrointestinal discomfort were also described 34. A drug-gene network analysis, in patients treated with finasteride, also revealed that “oocyte meiosis” and “progesterone-mediated oocyte maturation” pathways were affected by finasteride treatment 32. Few studies evaluated finasteride toxicity in female animal models. Of note, a paper by Alkahtane and collaborators showed altered serum biochemical parameters (e.g., alkaline phosphatase, cholesterol, glucose), increased DNA damage and histological abnormalities in the liver, spleen, kidney and heart of female Swiss albino mice treated with finasteride (0.5 or 1.5 mg/kg/day) 35.
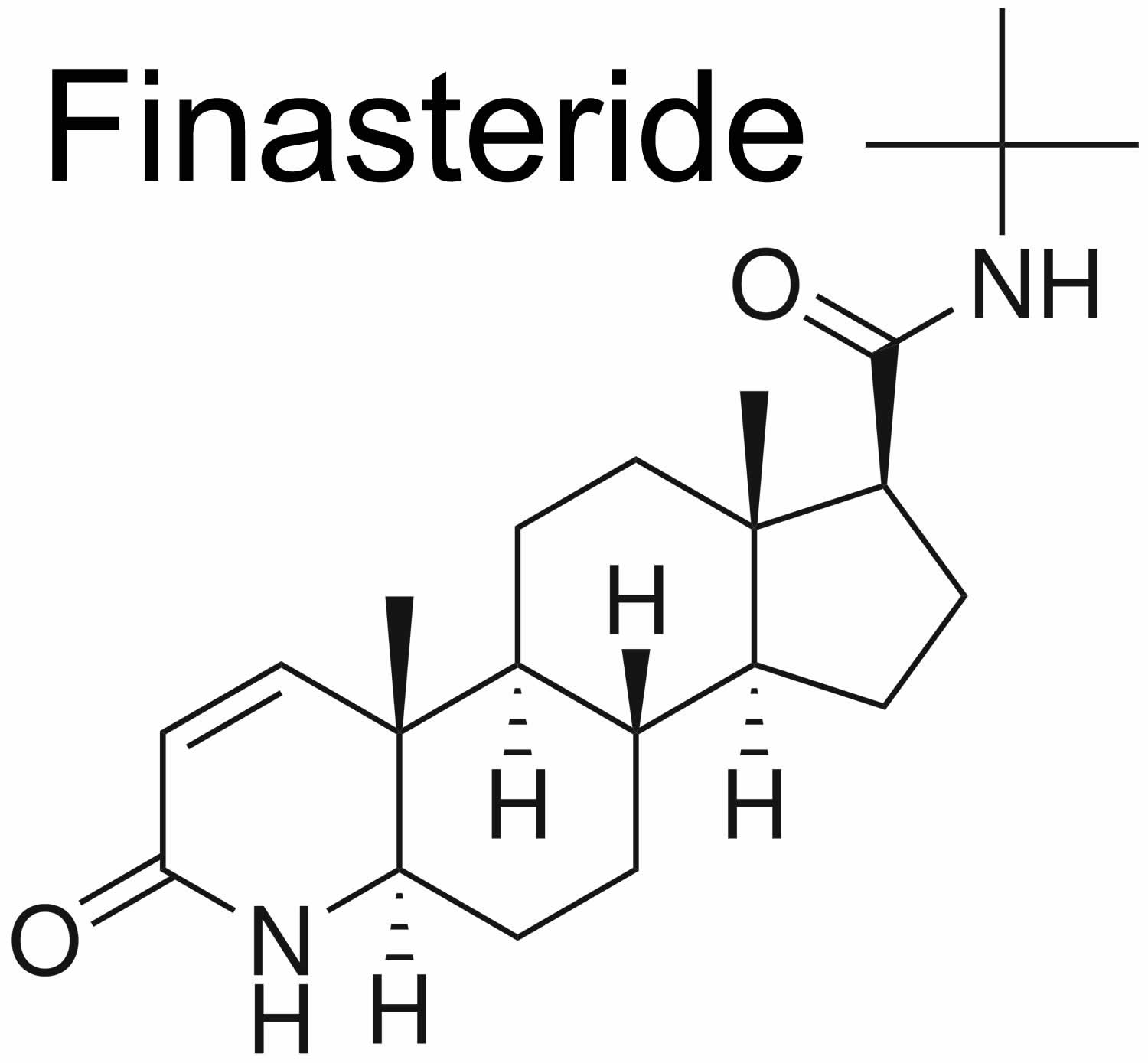
Figure 1. Finasteride chemical structure
How effective is finasteride for hair loss in men?
The data are from three large, multicenter, placebo-controlled studies of 1,879 men with mild-to-moderate, but not complete, male pattern hair loss 1, 36, 37. The men received either oral finasteride once daily or placebo for one year. The endpoints for the studies were objective hair counts taken from a 1-inch diameter circular area, and subjective assessments of improvement by patients, investigators, and an independent panel of dermatology experts who evaluated pre- and post-treatment photographs.
The trials showed that finasteride can prevent hair loss in men with mild-to-moderate male pattern hair loss. In two of the clinical studies involving men with mild-to-moderate male pattern hair loss, 86% of men treated with finasteride maintained or showed an increase in the amount of their hair based on hair counts during the course of the studies. Only 14% of men treated with finasteride had further hair loss after 12 months of treatment, compared with 58% of placebo patients.
On-going studies have demonstrated that finasteride halts hair loss or regrows hair in 9 out of 10 men taking it longterm every day.
Is finasteride safe for long-term use in men?
Although finasteride is generally well tolerated in a study of 3,200 men, including patients on therapy for up to two years. Recently many reports described adverse effects in men during finasteride treatment, such as sexual dysfunction and mood alteration 38, 39, 40, 41, 42. In addition, it has been also reported that persistent side effects may occur in some androgenetic alopecia patients. This condition, termed post-finasteride syndrome (PFS) represents a constellation of sexual, physical, and neurological symptoms that develop and persist during treatment and/or after finasteride discontinuation 43, 44, 45. Among the reported sexual and physical adverse effects associated with post-finasteride syndrome (PFS) are: loss of libido; erectile dysfunction; ejaculatory disorders; reduction in penis size; penile curvature; reduced sensation; decreased arousal and difficulty in achieving orgasm; gynecomastia; muscle atrophy; fatigue; and severely dry skin. Reported neurological (psychiatric) adverse events include: depression and anxiety; cognitive impairment; and suicidal ideation that are still present despite drug withdrawal 45. Furthermore, several case studies have linked finasteride with male infertility 46, 47, cataract and intraoperative floppy-iris syndrome 48, pseudoporphyria 49 and T cell–mediated acute localized exanthematous pustulosis 50. As a result, regulatory agencies in several countries generated warnings about finasteride (e.g., Swedish Medical Products Agency, the Medicines and Healthcare Products Regulatory Agency of UK and the U.S. Food and Drug Administration) required to include multiple persistent side effects within the finasteride labels 43.
Taking finasteride may increase the risk that you will develop high-grade prostate cancer (a type of prostate cancer that spreads and grows more quickly than other types of prostate cancer) or male breast cancer 25, 26.
Can women with hair loss use finasteride?
Finasteride is not indicated for use in women with hair loss (female pattern hair loss) but is occasionally used (off-label) in postmenopausal women with hair loss. Finasteride is contraindicated in women when they are or may potentially be pregnant, because it may cause abnormalities of the external genitalia of a male fetus (Pregnancy Category X, i.e., drugs that have such a high risk of causing permanent damage to the fetus that they should not be used in pregnancy or when there is a possibility of pregnancy.) 27
Are there any contraindications for finasteride?
Finasteride is contraindicated in those with hypersensitivity to any component of the formulation. Finasteride is not indicated for use in women or children. However, it is sometimes prescribed off-label for postmenopausal women without problems. Finasteride is contraindicated in women who are or may potentially be pregnant (Pregnancy Category X, i.e., drugs that have such a high risk of causing permanent damage to the fetus that they should not be used in pregnancy or when there is a possibility of pregnancy.) Women who are pregnant or may become pregnant should not use finasteride or handle the crushed or broken tablets. Finasteride can cause birth defects in male babies 27. Finasteride is contraindicated in patients who are hypersensitive to any component of the product.
Finasteride is not recommended in men that are subfertile. In men planning to have children, some doctors check sperm counts prior to starting finasteride and repeat it after 6 months of treatment. If the sperm count has reduced, finasteride should be stopped.
How does finasteride work?
Finasteride is in a class of medications called 5-alpha reductase inhibitors. Finasteride is a competitive inhibitor of types II and III 5-alpha-reductase isoenzyme, resulting in inhibition of testosterone conversion to dihydrotestosterone (DHT) 51. Finasteride has minimal selectivity for the type I 5-alpha-reductase enzyme. The type I 5-alpha-reductase isomer is present in sebaceous glands, sweat glands, dermal papillae cells, and epidermal and follicular keratinocytes. Type II 5-alpha reductase enzyme is in the outer root sheaths of hair follicles, the epididymis, vas deferens, seminal vesicles, and the prostate 52, 53. Finasteride inhibits expression of the enzyme, 5-alpha reductase, which regulates the production of dihydrotestosterone (DHT). By lowering DHT levels in the scalp, it reduces DHT’s harmful effect on hair follicles. Finasteride decreases DHT concentrations in the serum and the scalp by up to 90 and 70%, respectively 51. However, increasing finasteride dose does not necessarily result in greater serum reduction. Dutasteride (another 5-alpha reductase inhibitor), in comparison, inhibits all three 5-alpha-reductase isoenzymes leading to a 99% reduction in serum DHT levels 51. Continuous use for 3 to 6 months is required before a benefit is usually seen. It may take up to 6 months before you can tell if one of these medicines is working. When you stop taking these medicines, any beneficial effects on hair growth will be lost within 6 to 12 months of discontinuing treatment. Two one-year trials encompassing 1,553 men with male-pattern hair loss found 99% of subjects to show decreased progression or the reversal of hair loss with oral finasteride 54. In addition, authors observed clinically significant increases in hair count with oral finasteride treatment compared to placebo 54. Decreased libido and erectile problems are recognized side-effects of treatment with 5-alpha-reductase inhibitors 23.
When testosterone is present in the hair follicle and it combines with the enzyme 5-alpha-reductase type 2, it produces dihydrotestosterone (DHT). Dihydrotestosterone (DHT) is the most potent hormone among the androgens [i.e, dehydroepiandrosterone (DHEA), androstenedione, testosterone, and dihydrotestosterone (DHT)] and is considered a pure androgen as it cannot be converted into estrogen 23. DHT (dihydrotestosterone) attacks the hair follicle, causing it to shrink, finally causing the hair to fall out and not grow back and is implicated in male-pattern hair loss androgenetic alopecia pathophysiology 55. Hair follicle receptors are sensitive to DHT and thereby start the process of male or female pattern hair loss 56. Upon binding to androgen receptors in the hair follicle, dihydrotestosterone (DHT) promotes the shortening of the anagen phase and elongation of the telogen phase 57, resulting in enhanced apoptosis of hair cells and thus hair loss 58. A mouse-model study found that dihydrotestosterone (DHT) promoted premature hair regression, hair miniaturization, loss of hair density, and altered hair morphology in male mice, with partial reversal with an androgen receptor antagonist, bicalutamide 59. In addition, those with 5-alpha-reductase enzyme deficiencies are less likely to develop male-pattern hair loss androgenetic alopecia. The role of dihydrotestosterone (DHT) in the promotion of transition to telogen and male-pattern hair loss androgenetic alopecia pathophysiology justifies the use of oral 5-alpha-reductase inhibitors, such as finasteride, in the management of hair loss 55.
Before taking finasteride
- tell your doctor and pharmacist if you are allergic to finasteride, any other medications, or any of the ingredients in finasteride tablets. Ask your pharmacist or check the patient information for a list of the ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if you have or have ever had liver disease or prostate cancer.
- you should know that finasteride is only for use in men. Women, especially those who are or may become pregnant should not touch broken or crushed finasteride tablets. Touching broken or crushed finasteride tablets may harm the fetus. If a woman who is pregnant or who could become pregnant touches broken or crushed finasteride tablets, she should wash the area with soap and water immediately and call her doctor. Animal studies have suggested that exposure to large doses of finasteride when the fetal sex organs are developing (8 to 12 weeks of pregnancy) could increase the chance for some birth defects of the sex organs in a male fetus 28, 27, 29. The animal studies have reported hypospadias (when the opening of the penis is on the underside of the penis instead of at the tip), a shorter distance from the anus to the genitals (anogenital distance), and lower weight of the prostate and seminal vesicles (glands that help make semen) 30, 31.
- there are no adequate studies in women for determining infant risk when using finasteride during breastfeeding. Weigh the potential benefits against the potential risks before taking finasteride while breastfeeding.
- you should know that finasteride may decrease fertility in men; decreased sexual desire, ejaculation problems, and inability to have or maintain an erection may occur during and after your treatment is stopped. Talk to your doctor about the risks of taking finasteride.
- the presence of other medical problems may affect the use of finasteride. Make sure you tell your doctor if you have any other medical problems, especially liver disease. Use with caution. The effects may be increased because of slower removal of finasteride from the body.
Drug interactions
Although certain medicines should not be used together at all, in other cases two different medicines may be used together even if an interaction might occur. In these cases, your doctor may want to change the dose, or other precautions may be necessary. When you are taking finasteride, it is especially important that your healthcare professional know if you are taking any of the medicines listed below. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using finasteride with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. Discuss with your healthcare professional the use of your medicine with food, alcohol, or tobacco.
Finasteride uses
Oral finasteride (a pill taken by mouth) has been approved by the U.S. Food and Drug Administration (FDA) for use to treat male pattern hair loss in men and to treat symptoms of benign prostatic hyperplasia (BPH) in men with non-cancerous enlarged prostate 51.
Finasteride is not approved for use in females but has been used “off-label” in females to treat hair loss and hirsutism (extra hair growth on areas of the body such as the face, chest, and back), although these treatments are only partially effective at best 4, 5, 6, 7, 8, 9, 10. The effectiveness of finasteride treatment for women varies and only some studies concluded that finasteride may be considered for treatment of female pattern hair loss in patients who fail other treatment and adhere to reliable contraception during finasteride use 6, 7, 8, 4, 11. With particular regard to female alopecia, finasteride is considered both effective 12, 13, 14 and ineffective 15, 16, 17, 18. Moreover, indications for finasteride dosage in women hair loss are confusing. Studies have been published reporting dose of 1 mg, 1.25 mg, 2.5 mg or 5 mg 4, 15, 19, 16.
Topical finasteride (used on the skin only) has not been approved by the U.S. Food and Drug Administration (FDA), but has been approved in India to treat male and female pattern hair loss. Topical finasteride has been found to be as effective as oral finasteride 20, 21, 22.
Finasteride dosage
The dose of finasteride will be different for different patients. Take finasteride only as directed by your doctor. Do not take more of it, do not take it more often, and do not take it for a longer time than your doctor ordered. Finasteride comes with a patient information leaflet. Read and follow the instructions carefully. Ask your doctor if you have any questions.
You may take finasteride with or without food.
If you are taking finasteride for male pattern hair loss, it may take at least 3 months to see an effect. The medicine will not cure hair loss, but it will cause scalp hair to grow. The hair growth will only last as long as the medicine is used. The new hair will be lost within 1 year after the medicine is stopped.
If you are taking finasteride for benign prostatic hyperplasia (BPH), it may take up to 6 months to see the full effect. The medicine will not cure BPH, but it does help reduce the size of the prostate and improve symptoms. The effect on the prostate will only last as long as the medicine is used. When it is stopped, the prostate begins to grow again within a few months.
Swallow the tablet whole. Do not crush, break, or chew it. Take finasteride at the same time each day.
For oral dosage form (tablets)
- For male pattern hair loss:
- Adults—1 milligram (mg) once a day.
- Children—Use is not recommended.
- For benign prostatic hyperplasia (BPH):
- Adults—5 milligrams (mg) once a day.
- Children—Use is not recommended.
Adult dose for androgenetic alopecia (male pattern hair loss)
Use: For the treatment of male pattern hair loss (androgenetic alopecia) in men only. Safety and efficacy have been demonstrated in men between 18 to 41 years of age with mild to moderate hair loss of the vertex and anterior mid scalp area.
Dose: 1 mg orally once a day
Comments:
- Daily use for 3 months or more is necessary before benefit is observed. Continued use is recommended to sustain benefit.
- Withdrawal of treatment leads to reversal of effect within 12 months.
Adult dose for benign prostatic hyperplasia (BPH)
Use: For the treatment of benign prostatic hyperplasia (BPH).
Dose: 5 mg orally once a day
Comments:
- Dosing is the same both in monotherapy and in combination therapy.
- Not approved for the treatment of prostate cancer.
- 5-alpha reductase inhibitors reduce serum PSA levels by approximately 50%, even in the presence of prostate cancer. Health professionals should therefore be aware of how to monitor and interpret PSA levels in patients taking 5-alpha reductase inhibitors.
- Prior to the start of treatment, the risk of high-grade prostate cancer should be weighed against the benefits of 5-alpha reductase inhibitor therapy.
What should I do if I forget a dose?
Skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
Finasteride side effects
Finasteride may cause side effects. See your doctor if any of these symptoms are severe or do not go away:
- inability to have or maintain an erection
- decreased sexual desire
- problems with ejaculation (including decreased volume of ejaculate)
- pain in the testicles
- depression
In clinical trials, finasteride was very well tolerated in men, with most patients reporting no serious side effects. The principal side effects associated with finasteride were decreased libido (1.8% of finasteride patients versus 1.3% on placebo) and erectile dysfunction (1.3% finasteride versus 0.7% placebo). In addition, decreased volume of ejaculate was reported in 0.8% of men treated with finasteride and 0.4% of those on placebo. Gynaecomastia (breast enlargement) has also been reported. All of these side effects resolved upon discontinuation of therapy, and also resolved in many men who preferred to continue therapy. Postmarketing reports have included some patients complaining of depression, but the risk of this is likely very small.
Some side effects can be serious. If you experience any of these symptoms, see your doctor immediately:
- changes in the breasts such as increased size, lumps, pain, or nipple discharge
- rash
- itching
- hives
- swelling of the lips and face
- difficulty breathing or swallowing
Finasteride may cause other side effects. See your doctor if you have any unusual problems while taking finasteride.
Taking finasteride may increase the risk that you will develop high-grade prostate cancer (a type of prostate cancer that spreads and grows more quickly than other types of prostate cancer) or male breast cancer. Talk to your doctor about the risks of taking finasteride.
Finasteride toxicity
Single finasteride doses up to 400 mg and multiple doses up to 80 mg per day for three months are well-tolerated and did not result in any significant adverse reactions in the clinical studies. Until further experience is obtained, no specific treatment for an overdose with finasteride can be recommended. There are no reports of overdoses of finasteride, resulting in clinically significant toxicity 51. However, overdoses could occur as an extension of previously reported adverse drug effects, including orthostatic hypotension.
- Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, Price VH, Van Neste D, Roberts JL, Hordinsky M, Shapiro J, Binkowitz B, Gormley GJ. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998 Oct;39(4 Pt 1):578-89. doi: 10.1016/s0190-9622(98)70007-6[↩][↩]
- Tacklind J, Fink HA, Macdonald R, Rutks I, Wilt TJ. Finasteride for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2010 Oct 6;2010(10):CD006015. doi: 10.1002/14651858.CD006015.pub3[↩]
- Finasteride. https://medlineplus.gov/druginfo/meds/a698016.html[↩][↩]
- Boersma IH, Oranje AP, Grimalt R, Iorizzo M, Piraccini BM, Verdonschot EH. The effectiveness of finasteride and dutasteride used for 3 years in women with androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2014 Nov-Dec;80(6):521-5. doi: 10.4103/0378-6323.144162[↩][↩][↩][↩][↩][↩]
- Levy LL, Emer JJ. Female pattern alopecia: current perspectives. Int J Womens Health. 2013 Aug 29;5:541-56. doi: 10.2147/IJWH.S49337[↩][↩]
- Stout SM, Stumpf JL. Finasteride treatment of hair loss in women. Ann Pharmacother. 2010 Jun;44(6):1090-7. doi: 10.1345/aph.1M591[↩][↩][↩][↩]
- Oliveira-Soares R, E Silva JM, Correia MP, André MC. Finasteride 5 mg/day Treatment of Patterned Hair Loss in Normo-androgenetic Postmenopausal Women. Int J Trichology. 2013 Jan;5(1):22-5. doi: 10.4103/0974-7753.114709[↩][↩][↩][↩]
- Yeon, J., Jung, J., Choi, J., Kim, B., Youn, S., Park, K. and Huh, C. (2011), 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. Journal of the European Academy of Dermatology and Venereology, 25: 211-214. https://doi.org/10.1111/j.1468-3083.2010.03758.x[↩][↩][↩][↩]
- Hu A.C., Chapman L.W., Mesinkovska N.A. The efficacy and use of finasteride in women: a systematic review. Int. J. Dermatol. 2019;58:759–776. doi: 10.1111/ijd.14370[↩][↩]
- Camacho-Martinez F.M. Hair loss in women. Semin. Cutan. Med. Surg. 2009;28:19–32. doi: 10.1016/j.sder.2009.01.001[↩][↩]
- Trüeb RM; Swiss Trichology Study Group. Finasteride treatment of patterned hair loss in normoandrogenic postmenopausal women. Dermatology. 2004;209(3):202-7. doi: 10.1159/000079890[↩][↩]
- Boersma I.H., Oranje A.P., Grimalt R., Iorizzo M., Piraccini B.M., Verdonschot E.H. The effectiveness of finasteride and dutasteride used for 3 years in women with androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2014;80:521–525. doi: 10.4103/0378-6323.144162[↩][↩]
- Oliveira-Soares R., JM E.S., Correia M.P., Andre M.C. Finasteride 5 mg/day treatment of patterned hair loss in normo-androgenetic postmenopausal women. Int. J. Trichol. 2013;5:22–25. doi: 10.4103/0974-7753.114709[↩][↩]
- Won Y.Y., Lew B.L., Sim W.Y. Clinical efficacy of oral administration of finasteride at a dose of 2.5 mg/day in women with female pattern hair loss. Dermatol. Ther. 2018;31 doi: 10.1111/dth.12588[↩][↩]
- Kim W.J., Song M., Ko H.C., Kim B.S., Kim M.B. Efficacy of finasteride 1.25 mg on female pattern hair loss; pilot study. Ann. Dermatol. 2012;24:370–372. doi: 10.5021/ad.2012.24.3.370[↩][↩][↩][↩]
- Price V.H., Roberts J.L., Hordinsky M., Olsen E.A., Savin R., Bergfeld W., Fiedler V., Lucky A., Whiting D.A., Pappas F., Culbertson J., Kotey P., Meehan A., Waldstreicher J. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J. Am. Acad. Dermatol. 2000;43:768–776. doi: 10.1067/mjd.2000.107953[↩][↩][↩][↩]
- van Zuuren E.J., Fedorowicz Z., Schoones J. Interventions for female pattern hair loss. Cochrane Database Syst. Rev. 2016:CD007628. doi: 10.1002/14651858.CD007628.pub4[↩][↩]
- Varothai S., Bergfeld W.F. Androgenetic alopecia: an evidence-based treatment update. Am. J. Clin. Dermatol. 2014;15:217–230. doi: 10.1007/s40257-014-0077-5[↩][↩]
- Mervis J.S., Borda L.J., Miteva M. ‘Post-finasteride syndrome’: what to tell our female patients? Br. J. Dermatol. 2018;179:785–786. doi: 10.1111/bjd.16658[↩][↩]
- Lee SW, Juhasz M, Mobasher P, Ekelem C, Mesinkovska NA. A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. J Drugs Dermatol. 2018 Apr 1;17(4):457-463 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6609098[↩][↩]
- Rossi A, Magri F, D’Arino A, Pigliacelli F, Muscianese M, Leoncini P, Caro G, Federico A, Fortuna MC, Carlesimo M. Efficacy of Topical Finasteride 0.5% vs 17α-Estradiol 0.05% in the Treatment of Postmenopausal Female Pattern Hair Loss: A Retrospective, Single-Blind Study of 119 Patients. Dermatol Pract Concept. 2020 Apr 20;10(2):e2020039. doi: 10.5826/dpc.1002a39[↩][↩]
- Piraccini BM, Blume-Peytavi U, Scarci F, Jansat JM, Falqués M, Otero R, Tamarit ML, Galván J, Tebbs V, Massana E; Topical Finasteride Study Group. Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. J Eur Acad Dermatol Venereol. 2022 Feb;36(2):286-294. doi: 10.1111/jdv.17738. Epub 2021 Oct 25. Erratum in: J Eur Acad Dermatol Venereol. 2023 Feb;37(2):452.[↩][↩]
- Kinter KJ, Anekar AA. Biochemistry, Dihydrotestosterone. [Updated 2022 Mar 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557634[↩][↩][↩]
- Laborde, E. and Brannigan, R.E. (2010), Effect of 1-mg Dose of Finasteride on Spermatogenesis and Pregnancy. Journal of Andrology, 31: e1-e2. https://doi.org/10.2164/jandrol.109.009381[↩]
- FDA Drug Safety Communication: 5-alpha reductase inhibitors (5-ARIs) may increase the risk of a more serious form of prostate cancer. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-5-alpha-reductase-inhibitors-5-aris-may-increase-risk-more-serious[↩][↩]
- October – December 2009 | Potential Signals of Serious Risks/New Safety Information Identified by the Adverse Event Reporting System (AERS). https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/october-december-2009-potential-signals-serious-risksnew-safety-information-identified-adverse-event[↩][↩]
- AlSaad D, Lee BH, Al-Obaidly S. Finasteride use during pregnancy and early neonatal outcome: a case report. Int J Clin Pharm. 2018 Aug;40(4):803-805. doi: 10.1007/s11096-018-0661-5[↩][↩][↩][↩][↩]
- Ahn KH, et al. 2015. Pregnancy outcomes with paternal exposure to finasteride, a synthetic 5-alpha-reductase inhibitor: a case-series. J Clin Toxicol 5:2[↩][↩]
- Martínez AG, Pardo B, Gámez R, Mas R, Noa M, Marrero G, Valle M, García H, Curveco D, Mendoza N, Goicochea E. Effects of in utero exposure to D-004, a lipid extract from Roystonea regia fruits, in the male rat: a comparison with finasteride. J Med Food. 2011 Dec;14(12):1663-9. doi: 10.1089/jmf.2010.0279[↩][↩]
- Kurzrock EA, Jegatheesan P, Cunha GR, Baskin LS. Urethral development in the fetal rabbit and induction of hypospadias: a model for human development. J Urol. 2000 Nov;164(5):1786-92.[↩][↩]
- Clark RL, Antonello JM, Grossman SJ, Wise LD, Anderson C, Bagdon WJ, Prahalada S, MacDonald JS, Robertson RT. External genitalia abnormalities in male rats exposed in utero to finasteride, a 5 alpha-reductase inhibitor. Teratology. 1990 Jul;42(1):91-100. doi: 10.1002/tera.1420420111[↩][↩]
- Wu M., Yu Q., Li Q. Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 2016;7:82074–82084. doi: 10.18632/oncotarget.12617[↩][↩][↩]
- Yeon J.H., Jung J.Y., Choi J.W., Kim B.J., Youn S.W., Park K.C., Huh C.H. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J. Eur. Acad. Dermatol. Venereol. 2011;25:211–214. doi: 10.1111/j.1468-3083.2010.03758.x[↩]
- Valsecchi R, Leghissa P, Riva M. Female androgenetic alopecia treated by finasteride: a case forward. Acta Derm Venereol. 2004;84(6):488-9.[↩]
- Alkahtane A.A., Albasher G., Al-Sultan N.K., Alqahtani W.S., Alarifi S., Almeer R.S., Alghamdi J., Ali D., Alahmari A., Alkahtani S. Long-term treatment with finasteride induces apoptosis and pathological changes in female mice. Hum. Exp. Toxicol. 2019;38:762–774. doi: 10.1177/0960327119842195[↩]
- York K, Meah N, Bhoyrul B, Sinclair R. A review of the treatment of male pattern hair loss. Expert Opin Pharmacother. 2020 Apr;21(5):603-612. doi: 10.1080/14656566.2020.1721463[↩]
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol. 2017 Jul;77(1):136-141.e5. doi: 10.1016/j.jaad.2017.02.054[↩]
- Baas W.R., Butcher M.J., Lwin A., Holland B., Herberts M., Clemons J., Delfino K., Althof S., Kohler T.S., McVary K.T. A review of the FAERS data on 5-alpha reductase inhibitors: implications for postfinasteride syndrome. Urology. 2018;120:143–149. doi: 10.1016/j.urology.2018.06.022[↩]
- Gupta A.K., Carviel J., MacLeod M.A., Shear N. Assessing finasteride-associated sexual dysfunction using the FAERS database. J. Eur. Acad. Dermatol. Venereol. 2017;31:1069–1075. doi: 10.1111/jdv.14223[↩]
- Tsunemi Y., Irisawa R., Yoshiie H., Brotherton B., Ito H., Tsuboi R., Kawashima M., Manyak M., Group A.R.I.S. Long-term safety and efficacy of dutasteride in the treatment of male patients with androgenetic alopecia. J. Dermatol. 2016;43:1051–1058. doi: 10.1111/1346-8138.13310[↩]
- Traish A.M., Melcangi R.C., Bortolato M., Garcia-Segura L.M., Zitzmann M. Adverse effects of 5alpha-reductase inhibitors: what do we know, don’t know, and need to know? Rev. Endocr. Metab. Disord. 2015;16:177–198. doi: 10.1007/s11154-015-9319-y[↩]
- Kaplan S.A., Chung D.E., Lee R.K., Scofield S., Te A.E. A 5-year retrospective analysis of 5alpha-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. Int. J. Clin. Pract. 2012;66:1052–1055. doi: 10.1111/j.1742-1241.2012.03010.x[↩]
- Diviccaro S, Melcangi RC, Giatti S. Post-finasteride syndrome: An emerging clinical problem. Neurobiol Stress. 2019 Dec 26;12:100209. doi: 10.1016/j.ynstr.2019.100209[↩][↩]
- Traish A.M. The post-finasteride syndrome: clinical manifestation of drug-induced epigenetics due to endocrine disruption. Current Sexual Health Reports. 2018; 10: 88-103[↩]
- Traish AM. Post-finasteride syndrome: a surmountable challenge for clinicians. Fertil Steril. 2020 Jan;113(1):21-50. https://www.fertstert.org/article/S0015-0282(19)32599-3/fulltext[↩][↩]
- Tu H.Y., Zini A. Finasteride-induced secondary infertility associated with sperm DNA damage. Fertil Steril. 2011; 95: 2125.e13-2125.e14[↩]
- Chiba K., Yamaguchi K., Li F., Ando M., Fujisawa M. Finasteride-associated male infertility. Fertil Steril. 2011; 95: 1786.e9-1786.e11[↩]
- Wong A.C., Mak S.T. Finasteride-associated cataract and intraoperative floppy-iris syndrome. J Cataract Refract Surg. 2011; 37: 1351-1354[↩]
- Santo Domingo D., Stevenson M.L., Auerbach J., Lerman J. Finasteride-induced pseudoporphyria. Arch Dermatol. 2011; 147: 747-748[↩]
- Tresch S, Cozzio A, Kamarashev J, Harr T, Schmid-Grendelmeier P, French LE, Feldmeyer L. T cell-mediated acute localized exanthematous pustulosis caused by finasteride. J Allergy Clin Immunol. 2012 Feb;129(2):589-94. doi: 10.1016/j.jaci.2011.07.033[↩]
- Zito PM, Bistas KG, Syed K. Finasteride. [Updated 2022 Aug 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513329[↩][↩][↩][↩][↩]
- Shin JW, Chung EH, Kim MB, Kim TO, Kim WI, Huh CH. Evaluation of long-term efficacy of finasteride in Korean men with androgenetic alopecia using the basic and specific classification system. J Dermatol. 2019 Feb;46(2):139-143. doi: 10.1111/1346-8138.14719[↩]
- Azarchi S, Bienenfeld A, Lo Sicco K, Marchbein S, Shapiro J, Nagler AR. Androgens in women: Hormone-modulating therapies for skin disease. J Am Acad Dermatol. 2019 Jun;80(6):1509-1521. doi: 10.1016/j.jaad.2018.08.061[↩]
- Kaufman K.D., Olsen E.A., Whiting D., Savin R., DeVillez R., Bergfeld W., Price V.H., Van Neste D., Roberts J.L., Hordinsky M., et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J. Am. Acad. Dermatol. 1998;39:578–589. doi: 10.1016/S0190-9622(98)70007-6[↩][↩]
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J Clin Med. 2023 Jan 23;12(3):893. doi: 10.3390/jcm12030893[↩][↩]
- Girijala RL, Riahi RR, Cohen PR. Platelet-rich plasma for androgenic alopecia treatment: A comprehensive review. Dermatol Online J. 2018 Jul 15;24(7):13030/qt8s43026c https://doi.org/10.5070/D3247040910[↩]
- Thom E. Stress and the Hair Growth Cycle: Cortisol-Induced Hair Growth Disruption. J Drugs Dermatol. 2016 Aug 1;15(8):1001-4. https://jddonline.com/articles/stress-and-the-hair-growth-cycle-cortisol-induced-hair-growth-disruption-S1545961616P1001X/[↩]
- Bassino E, Gasparri F, Munaron L. Protective Role of Nutritional Plants Containing Flavonoids in Hair Follicle Disruption: A Review. Int J Mol Sci. 2020 Jan 14;21(2):523. doi: 10.3390/ijms21020523[↩]
- Fu D., Huang J., Li K., Chen Y., He Y., Sun Y., Guo Y., Du L., Qu Q., Miao Y., et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. Pharmacother. 2021;137:111247. doi: 10.1016/j.biopha.2021.111247[↩]