Contents
- Bacillary angiomatosis
Bacillary angiomatosis
Bacillary angiomatosis also known as epithelioid angiomatosis is a rare skin and internal organs infection caused by intracellular Gram-negative Bartonella quintana and Bartonella henselae bacteria that primarily occurs in immunocompromised persons and HIV-positive patients 1, 2, 3, 4, 5, 4, 6, 7, 8, 9, 10, 11. More recent case reports had brought into attention the probability that Bacillary angiomatosis may manifest in otherwise healthy individuals, triggered by trauma and skin burns 12. Cats are the most important reservoir for Bartonella henselae bacteria and the transmission between cats is through a flea vector (Ctenocephalides felis) 13. In healthy individuals, the infection with Bartonella bacterium can lead to the ‘cat scratch disease’ 14. In these situations, almost 90% of patients admit a recent contact with a cat (a scratch or a bite) 13. Although bacillary angiomatosis is treatable and curable, it may be life threatening if untreated.
Bartonella quintana bacterium has a human reservoir and among humans it can be transmitted by body lice (Pediculus humanus) and fleas as well, especially in the context of poverty, lack of hygienic conditions and homelessness 14, 15. Furthermore, Bartonella quintana bacterium was the causative organism of trench fever among militaries in World War 1 16.
In both immunodeficient and healthy patients, Bartonella henselae can produce bacillary angiomatosis, but in this case, fleas may play a major role in transmitting the infection to humans, considering the fact that only 20% of patients or less admit exposure to cats 13, 17. In order to sustain this hypothesis, Bartonella henselae DNA was detected in fleas 18, 19, 20.
Bacillary angiomatosis usually manifests as skin angioma-like tumor masses (vascular malformations or angiomas) or internal organs infection 21. Bacillary angiomatosis is the second-most-common cause of angiomatous skin lesions in persons infected with the human immunodeficiency virus (HIV). Multiple lesions often demonstrate more than 1 morphologic appearance. Black patients, in particular, may bear the plaque form. Bacillary angiomatosis lesions may be solitary or multiple and spreading to internal organs can occur. Bone lesions and subcutaneous masses are associated with Bartonella quintana, whereas hepatic peliosis (peliosis hepatis) and lymph node lesions are associated with Bartonella henselae 22.
Histologic examination of the lesions from the affected organs shows vascular proliferation with characteristic lobes, several mitoses with cell atypia, and leukocyte infiltration. In the majority of cases, the diagnosis is confirmed by histopathology with Warthin-Starry staining, which shows clamps of bacteria in the involved tissues, although the polymerase chain reaction test can be helpful. In differential diagnosis, the Kaposi sarcoma and pyogenic granuloma must be considered first 23, 24.
The drug of choice in the treatment of bacillary angiomatosis is erythromycin or doxycycline antibiotic. To avoid relapses, at least a 3-month therapy is recommended 25. For patients with multisystem bacillary angiomatosis involvement, a combination therapy with rifampicin can be helpful; however, interactions between the selected drugs must be carefully considered, especially in transplant recipients treated with tacrolimus 25. For such patients, the treatment with tacrolimus requires dose adjustment based on its blood concentration 26, 27.
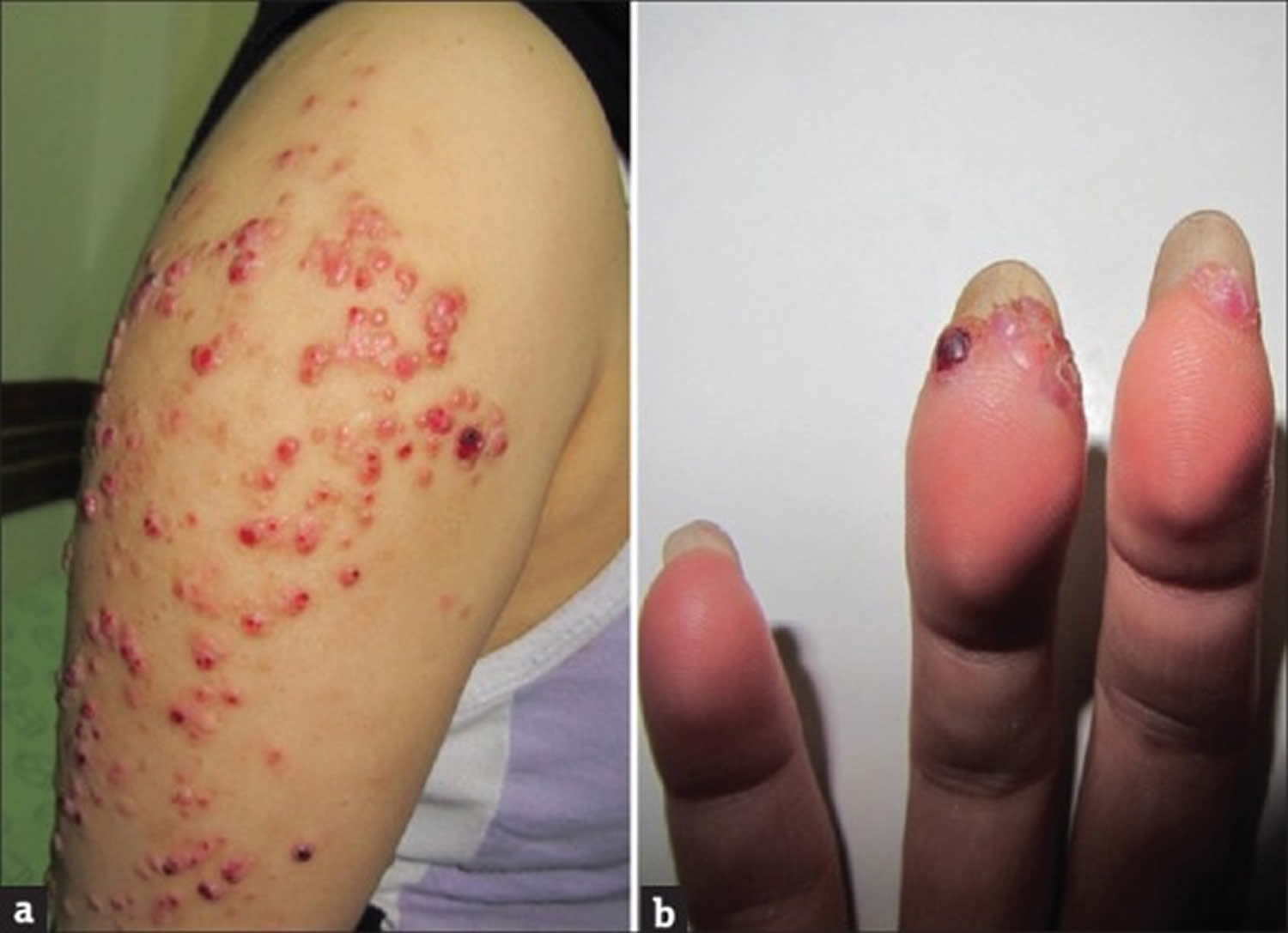
Figure 1. Bacillary angiomatosis
Footnotes: Bacillary angiomatosis clinical appearance. Angiomatous papule and nodules (a) arm and (b) fingers. A 26-year-old healthy woman had developed papules and nodules on the right arm 6 months ago. Despite treatment with short course systemic antibiotics the lesions had enlarged and gradually extended to the forearm and hand and also to her fingers.
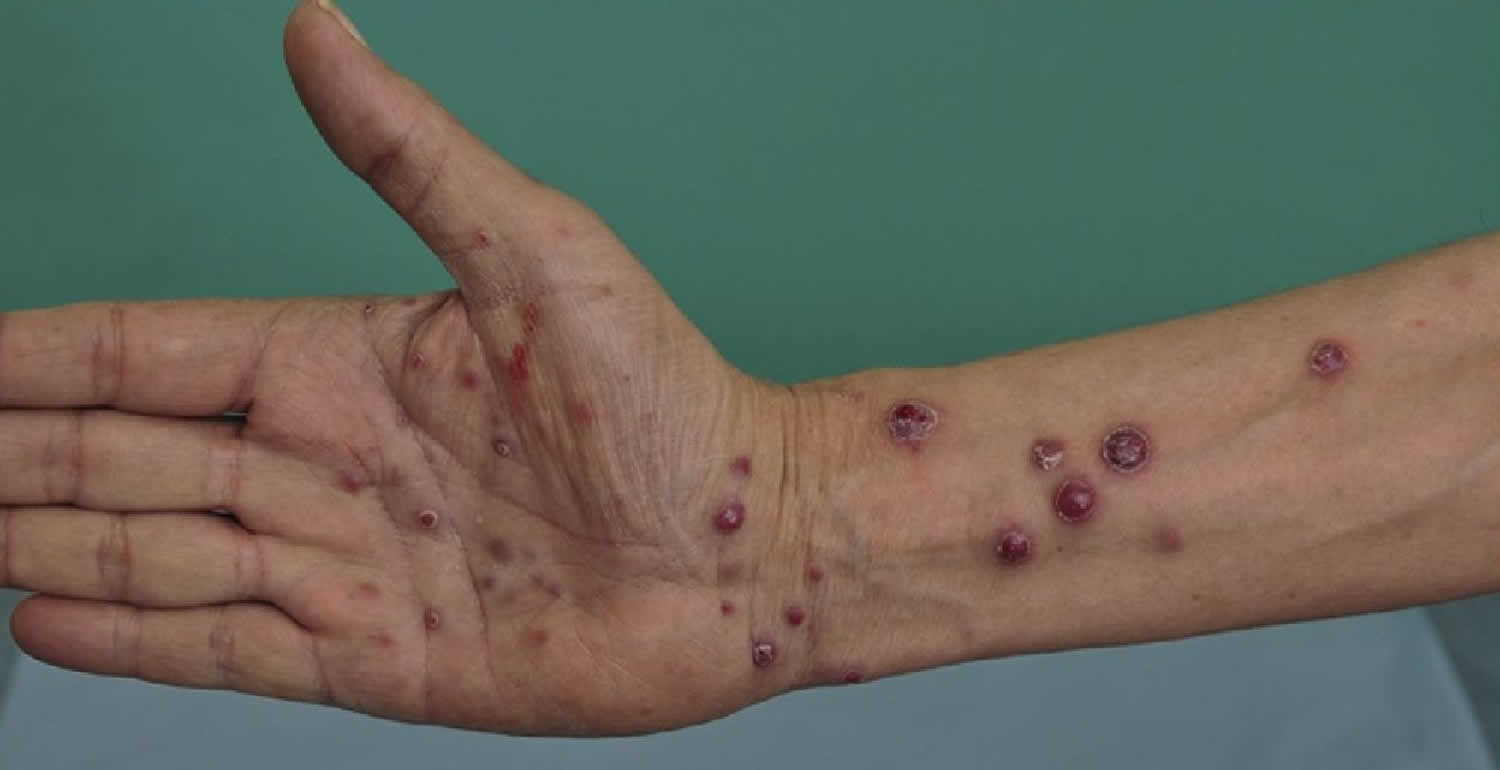
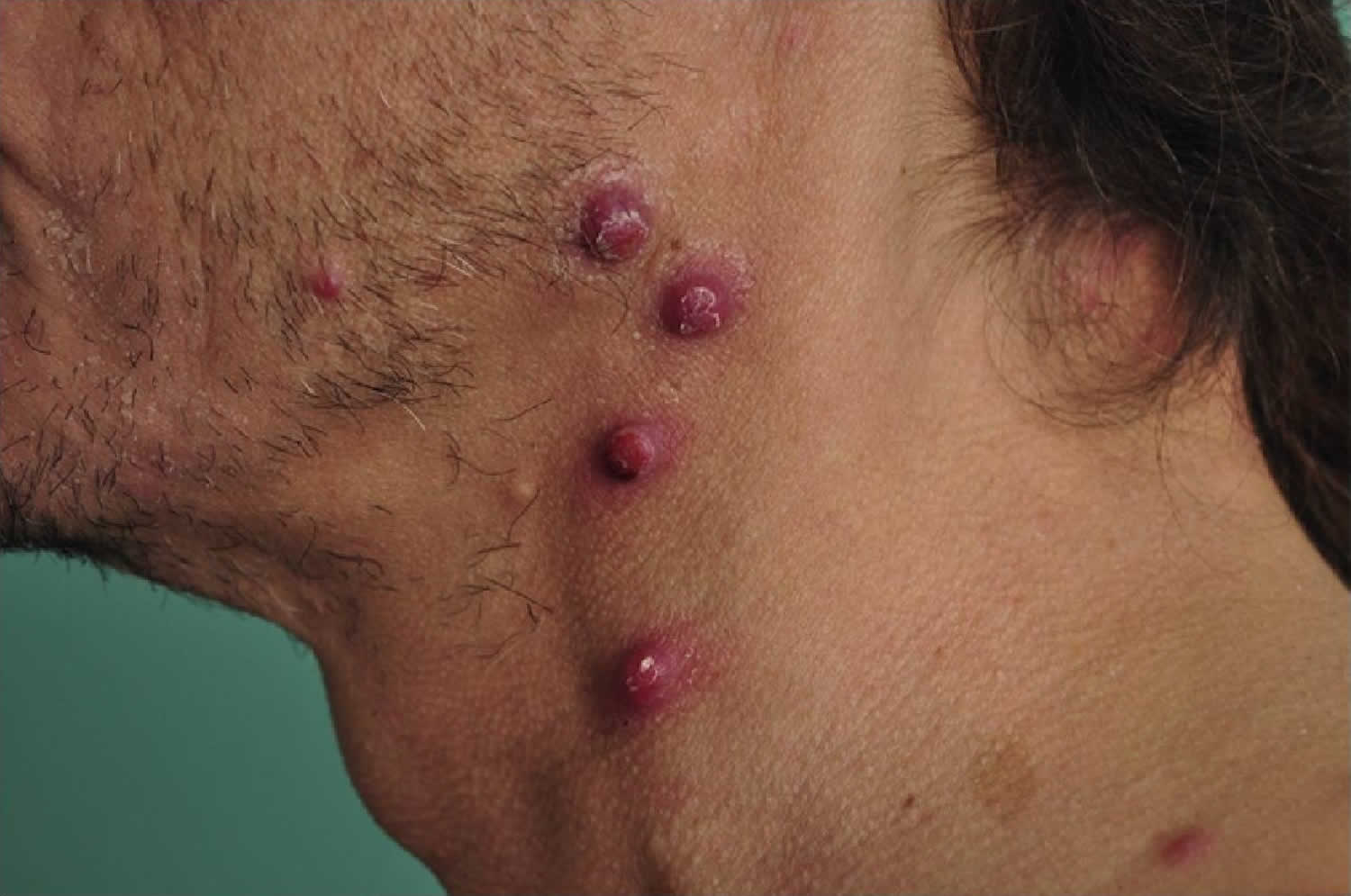
[Source 3 ]Figure 2. Bacillary angiomatosis
Footnote: Bacillary angiomatosis tumor-like skin lesions in a solid organ transplant recipient
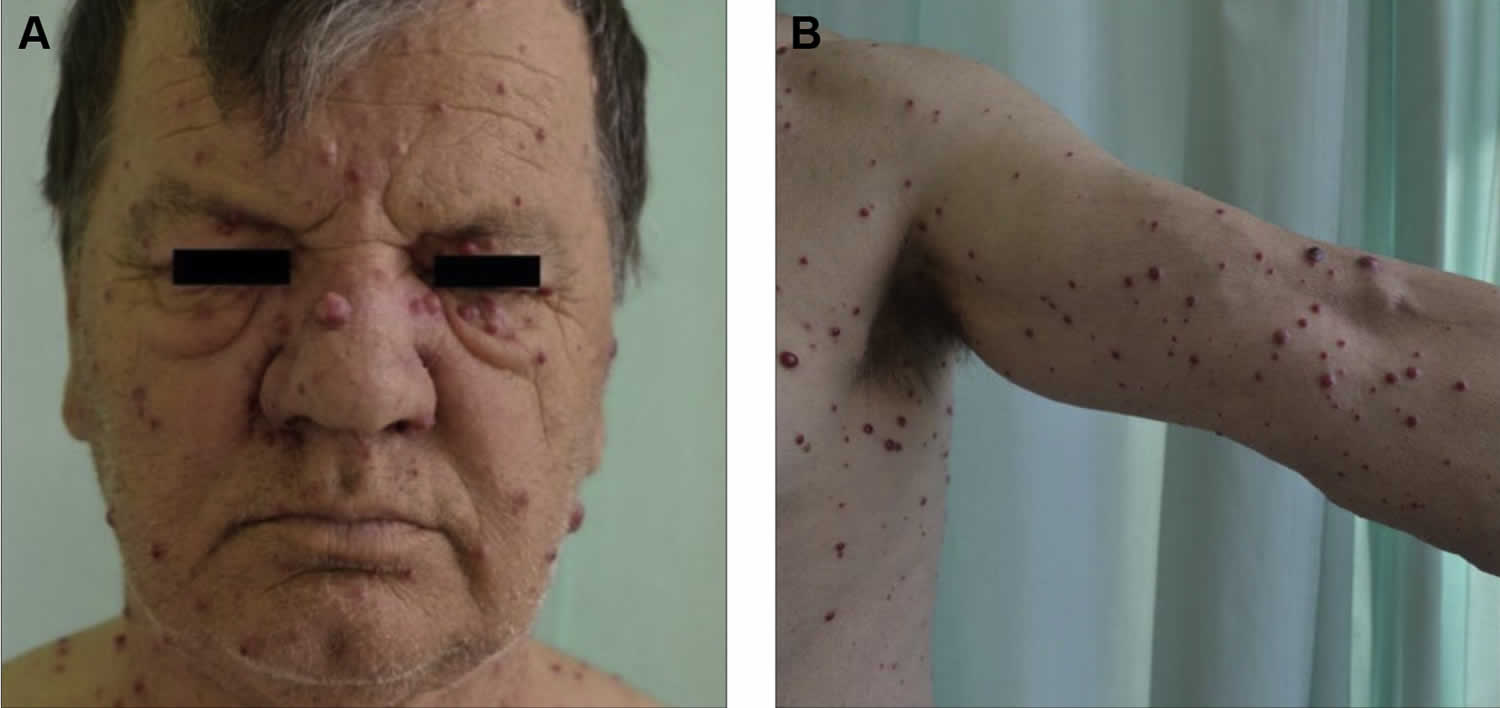
[Source 28 ]Figure 3. Bacillary angiomatosis skin lesions
Footnote: A 65-year-old man reported to the dermatology department with fever and numerous nodular skin lesions 5 months after kidney transplant. (A and B) The physical examination demonstrated multiple painless blood-red papules and nodules from 5 to 15 mm in diameter, localized on the arms, trunk, thighs, and face. Some papules and nodules were covered with a fine, tightly adherent scale. Oral and genital mucosa was free of lesions. The patient complained of high fever with chills and muscle pain in the evening.
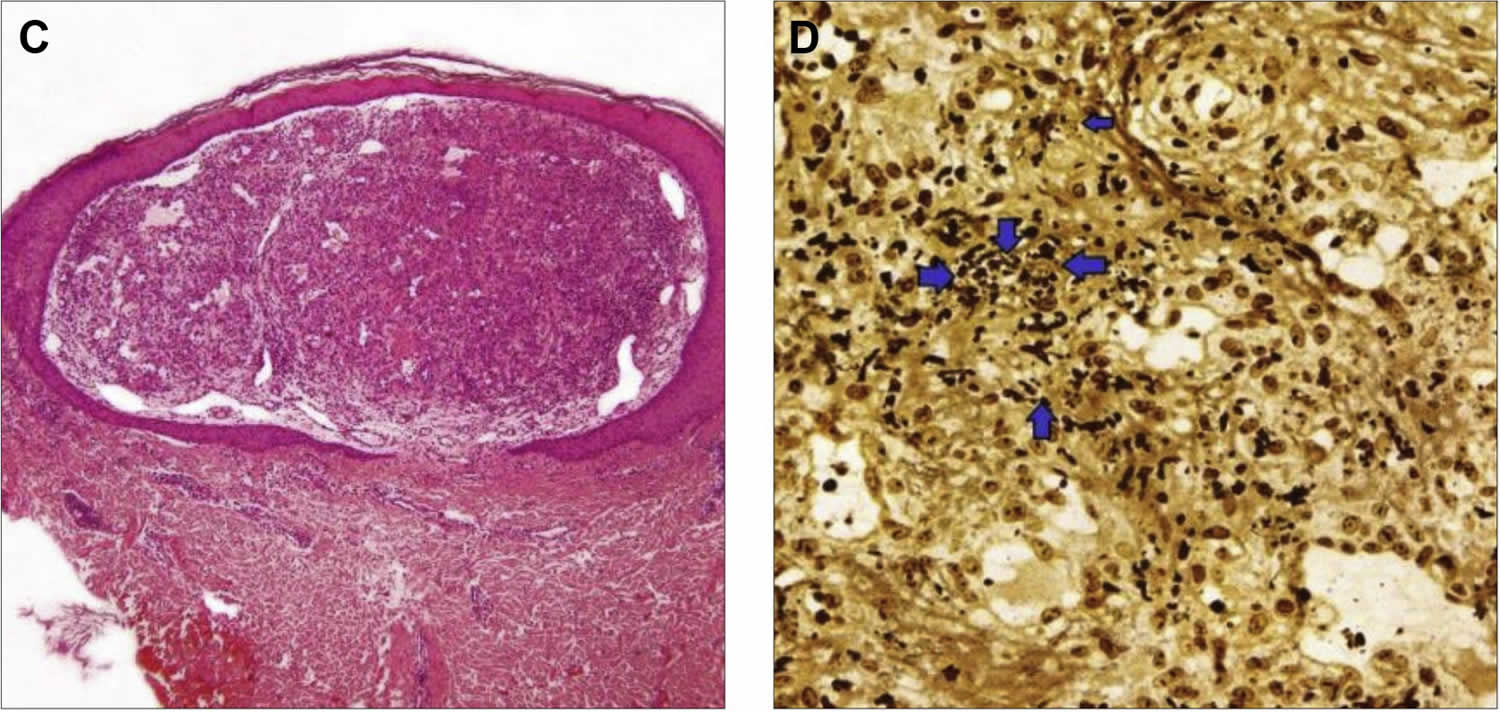
[Source 25 ]Figure 4. Bacillary angiomatosis histology
Footnote: Bacillary angiomatosis histology on skin biopsy. (C) Nodule under epidermis built of proliferating small blood vessels (hematoxylin and eosin stain). (D) High-power view of Warthin-Starry stain demostrating small extracellular aggregates of coccobacilli (arrowheads) confirming the diagnosis of bacillary angiomatosis.
[Source 25 ]Who gets bacillary angiomatosis?
Bacillary angiomatosis is seen predominantly with drug-induced immunosuppression, particularly organ transplant recipients, chronic lymphocytic leukemia, and those with HIV/AIDS. However, otherwise healthy individuals, including children, can also contract bacillary angiomatosis infection such as after major local trauma and skin burns 12. Cats are the most important reservoir for Bartonella henselae bacteria and the transmission between cats is through a flea vector (Ctenocephalides felis) 13.
Bacillary angiomatosis cause
Bacillary angiomatosis is caused by Bartonella quintana and Bartonella henselae, gram-negative intracellular bacteria. While both Bartonella species cause skin lesions, subcutaneous and bone lesions are more frequent with Bartonella quintana, and Bartonella henselae causes peliosis of the liver and spleen 10. Cats are the main reservoir for Bartonella henselae and humans for Bartonella quintana 11. Although bacillary angiomatosis infection may be spread via cat bites and scratches, fleas and body lice are the major route of transmission 29, 30, 31, 32, 33, 34. Bartonella quintana is transmitted by the human body louse (Pediculus humanus) and fleas.
In addition to bacillary angiomatosis, Bartonella henselae and Bartonella quintana cause a variety of other diseases, including endocarditis, cat scratch fever, trench fever, and peliosis hepatis 35, 36, 37, 38, 39, 6.
Once Bartonella bacteria are inoculated by blood-sucking arthropods or penetrate the skin after exposure to cat, the gram-negative bacteria attach themselves to a variety of host cells, which include red blood cells (erythrocytes), monocytes, macrophages, and dendritic cells. The endothelial cell is an important target for Bartonella quintana but not so much for Bartonella henselae.
There are several mechanisms employed by Bartonella species to enter the host cells and are the result of complex interactions at the cell surface between the bacterial and host cell proteins. Therefore, Bartonella species are highly host-specific. First, adhesion of the bacteria to the host cell membrane is followed by uptake of groups of Bartonella in a vacuole by the host cell. In a second mechanism, bacteria accumulate on the host cell surface and induce self-phagocytosis. This process results in phagosome where Bartonella species must survive the harsh environment resisting enzymes of oxidative stress. Both are well-equipped to deal with intracellular oxidative stress and produce heat shock proteins. A third mechanism is specific for entry into the erythrocytes, which involves secretion of a protein called deformin. Deformin causes, invaginations of the erythrocyte membrane providing entry points for these bacteria which are adept at rearranging the cell cytoskeleton once they are attached to the cell surface. These bacteria are the carpenters of the bacterial world 40.
The pathogenesis of bacillary angiomatosis is a proliferation of vascular endothelial cells stimulated by the angiogenic factor, what leads to the development of skin lesions, mostly described as violaceus papules, nodules, and plaques 23, 26..
Bartonella is the only genus known to release factors that stimulate endothelial cells to produce angiopoietin-2 and epidermal production of vascular endothelial growth factor 10. The physiopathology of the blood vessel proliferative process in bacillary angiomatosis is based on the production of angiogenetic molecules, such as vascular endothelial growth factor (VEGF) and interlukin-8 (IL-8) 2, 41, 42. BadA protein on the cell surface of Bartonella henselae is responsible for adhesion to endothelial cells and fibronectin 10. BadA protein is responsible for promoting angiogenesis in Bartonella henselae infections. BadA protein is also known to promote angiogenesis via inducible hypoxia factor-1 (IHF-1). Expression of outer membrane proteins (Vomp) is responsible for promoting vascular growth in the case of B. quintana.
The ability to enter the erythrocytes protects these Bartonella species from the host’s adaptive and innate immune response. The CD4 T-helper cells produce interferon gamma and TNF alpha, which are responsible for eliminating Bartonella bacteria. The Bartonella species are capable of attenuating the host immune response thereby establishing a chronic asymptomatic carrier state. An asymptomatic carrier state caused by Bartonella quintana exemplifies this in homeless patients 10.
Bacillary angiomatosis risk factors
Risk factors for bacillary angiomatosis include the following:
- HIV infection 43, 44
- Chronic lymphocytic leukemia
- Cytotoxic chemotherapy
- Organ transplantations 26, 45, 28.
Additional risk factors for bacillary angiomatosis associated with Bartonella henselae infection include the following:
- Cat ownership
- Cat bites
- Cat scratches
Additional risk factors for bacillary angiomatosis associated with Bartonella quintana infection include the following:
- Homelessness
- Low socioeconomic status
- Exposure to body and hair lice
Bacillary angiomatosis was reported in a patient who was HIV-seronegative but had idiopathic thrombocytopenic purpura (ITP), had undergone splenectomy, and had been administered long-term systemic prednisone 46.
Another report described an immunocompetent child with infected facial wound, in the vicinity of which bacillary angiomatosis lesions had developed. Similar lesions also appeared at the donor site of the skin graft, which was grafted on the facial wound 47. A case of bacillary angiomatosis presenting as a pyogenic granuloma of the hand in an otherwise apparently healthy man recently was reported from Saudi Arabia 48.
Multiple leg ulcers caused by bacillary angiomatosis without a history of direct contact with cats in healthy adult man has also been reported 49.
A case of bacillary angiomatosis in an HIV-negative patient who had chronic hepatitis B but no other immunosuppressive status was reported from Turkey, suggesting that immunologic differences secondary to chronic hepatitis B could have led to an increased risk for the disease 50. Bacillary angiomatosis may be triggered by trauma and skin burns immunocompetent individuals 1, 51.
Bacillary angiomatosis transmission
In nature, cats are the reservoir of Bartonella henselae. Cats may have episodes of asymptomatic bacteremia where the bacteria have adapted to life within the red blood cells (erythrocytes) 10. Bartonella infection is acquired by cats via cat fleas (Ctenocephalides felis) when they carry Bartonella henselae or Bartonella quintana 30. Transmission of Bartonella bacteria to humans by cats occur when cats scratch humans. The human body louse Pediculus humanus transmits Bartonella quintana to humans.
Transmission to humans via cat fleas has been hypothesized but not documented 10. Transmission to humans via ticks has been reported. Bartonella henselae has been isolated from both cat scratch disease and lesions in bacillary angiomatosis.
Humans are considered the only reservoir for Bartonella quintana. However, Japanese macaques (Macaca fuscata or snow monkey) have recently been found to be another reservoir of Bartonella quintana. Bartonella quintana has been implicated in bacillary angiomatosis but has never been associated with cat scratch disease. Body louse transmits Bartonella quintana in homeless, low socioeconomic and impoverished populations 52.
Infection sites
The specific Bartonella species, Bartonella henselae or Bartonella quintana, can affect the location of bacillary angiomatosis, as follows:
- Skin lesions: Result almost equally from Bartonella henselae and Bartonella quintana infections
- Subcutaneous lesions: Usually caused by Bartonella quintana infection
- Bone lesions: Usually caused by Bartonella quintana infection
- Visceral involvement: Almost exclusively caused by Bartonella henselae infection
- Neurologic disorders: Associated more frequently with Bartonella quintana infection than with Bartonella henselae
Disease reservoirs
Domestic cats (Felis domesticus) are the reservoirs of B henselae, which may be transmitted via cat bites or scratches or, potentially, by bites from cat fleas (Ctenocephalides felis). Kittens are more frequently associated with transmission of B henselae than are older cats. Humans appear to be the only reservoir of B quintana; the human body louse, Pediculus humanus, is the transmission vector.
Bacillary angiomatosis signs and symptoms
Bacillary angiomatosis skin lesions are usually the first sign of bacillary angiomatosis infection 11:
- Papules and nodules, range in size from pinpoint to 10 cm
- Purple, violaceous, or bright red in color
- Single lesion or many hundreds
- Any skin or mucosal site can be involved, although rarely the palms and soles
- In HIV, large subcutaneous tumors may form
- Lesions may bleed easily
- Overlying skin surface may become ulcerated or crusted
The typical bacillary angiomatosis skin lesions appear as single or multiple bright red to deep purple dome-shaped papules, nodules, or plaques 53, 54. As the number of skin lesions increase, the patient may develop high fever, tender swollen lymph nodes, nausea, vomiting, night sweats, chills, muscle aches and pain (myalgias), weakness and poor appetite 26, 11.
Bacillary angiomatosis can affect almost any organ system, although most commonly it affects skin and subcutaneous tissue. Subcutaneous lesions may erode into underlying bones (ie, osseous bacillary angiomatosis), especially the tibia, fibula, and radius. Involvement of ribs and vertebrae has been described. Rarely, skeletal muscles may be involved, resulting in pyomyositis. Mucous membranes of the conjunctiva, upper airway, and perineum (anus and penis) may also be affected. Bacillary angiomatosis may be accompanied by disseminated visceral disease (peliosis), mainly in the liver (peliosis hepatis), spleen, and lymph nodes.
Other internal organs that may be involved by bacillary angiomatosis include the following 55, 56, 10:
- Brain
- Bone marrow
- Heart
- Lungs
- Lymph noded
- Pleura
- Larynx
- Oropharynx
- Tongue
- Esophagus
- Stomach
- Duodenum
- Colon
- Peritoneum
- Diaphragm
- Liver
- Spleen
- Kidneys
- Adrenal glands
- Pancreas
- Uterine cervix
- Vulva 57
Angioproliferative lesions of the liver and spleen are called bacillary peliosis 27.
Extrinsic compression of the common bile duct by enlarged peripancreatic, celiac, and portohepatic nodes has been reported.
Bacillary angiomatosis is often clinically indistinguishable from Kaposi’s sarcoma, which similarly presents as red to purple papules, nodules, or plaques in immunocompromised patients. Differentiating bacillary angiomatosis from Kaposi’s sarcoma is largely reliant on skin biopsy with histopathologic examination, which, for bacillary angiomatosis, demonstrates protuberant endothelial cells surrounded by clumps of bacilli that are visible with Warthin-Starry staining 58.
Bacillary angiomatosis complications
Bacillary angiomatosis may affect the heart, brain, liver, spleen, larynx, lymph nodes and gastrointestinal tract. Complications include gastrointestinal bleeding, encephalopathy, endocarditis, laryngeal obstruction and disfigurement due to the extensive lesions 59, 60.
Bacillary angiomatosis diagnosis
Bacillary angiomatosis should be suspected clinically, particularly in an immunocompromised patient.
Skin biopsy is the preferred method of diagnosing bacillary angiomatosis 61. Skin biopsy shows lobular blood vessel proliferation with epithelioid endothelial cells, and an inflammatory infiltrate including neutrophils. The microorganisms can be seen as basophilic clumps in the stroma and confirmed on Giemsa or Warthin-Starry stain (Figure 4).
In routine clinical practice, histological methods are used to confirm the diagnose bacillary angiomatosis. The hematoxylin and eosin (H and E) stain demonstrate endothelial-lined peliosis spaces. Warthin-Starry stain shows clumps of bacteria. The histological methods cannot make a diagnosis to the species level.
Differentiating bacillary angiomatosis histologically from other benign and malignant vascular proliferations, including Kaposi’s sarcoma, is facilitated by the use of histochemical (ie, Warthin-Starry staining to detect Bartonella bacilli) and immunohistochemical (ie, anti- HHV-8) stains.
Culture and serology are insensitive 62, 63.
Although polymerase chain reaction (PCR) is sensitive, specific and can be used to identify the causative agent, it is expensive and is used primarily for research or in the rare clinical setting when no skin lesion is present to biopsy 64, 65.
In resource-limited settings, where Warthin-Starry and immunohistochemical stains may not be available, distinguishing between bacillary angiomatosis and Kaposi’s sarcoma can be challenging, as the pathologist must rely on routine histopathologic poorly clues 61. Both bacillary angiomatosis and Kaposi’s sarcoma may have nodular architecture on low power and are composed of thin-walled vessels. Bacillary angiomatosis lesions have well-developed capillaries and inflamed stroma that harbors clumps of basophilic bacilli and acute inflammatory cells. On the other hand, Kaposi’s sarcoma is composed of ill-developed vasculature with slit-like spaces, prominent spindle cells, many extravasated erythrocytes, and siderophages. These features are less commonly seen in bacillary angiomatosis. Additionally, Kaposi’s sarcoma often has an abundance of plasma cells, in contrast to the mixed infiltrate, including neutrophils, that serve as an additional clue to the diagnosis of bacillary angiomatosis.
Bacillary angiomatosis histology
Histologically, the lesions of bacillary angiomatosis closely resemble pyogenic granuloma. Typically, there are ulcerated papules or nodules composed of lobules of highly vascularized edematous connective tissue (Figure 4). There is typically a mixed-cell infiltrate and nuclear dust of degenerated inflammatory cells. Accompanying this peculiar background are diagnostic clumps of pink-purplish granular material (Figure 4C). This granular material consist of huge numbers of the gram-negative rods of Bartonella henselae (Figure 4D).
Bacillary angiomatosis differential diagnosis
The clinical differential diagnosis for bacillary angiomatosis skin lesions may include Kaposi’s sarcoma, Carrion disease, pyogenic granuloma, cherry angioma, dermatofibroma, hemangioma, mycobacterial infection such as tuberculosis, coccidioidomycosis, cryptococcosis, and histoplasmosis 66.
Bacillary angiomatosis treatment
Bacillary angiomatosis is treated with antibiotic. The antibiotic of choice is macrolide erythromycin (Erythromycin 500mg four times daily) or doxycycline (Doxycycline 100mg twice daily) 10, 25. In patients who are not able to tolerate erythromycin or doxycycline, azithromycin or clarithromycin may be used 67. Mild bacillary angiomatosis skin lesions can be treated for 12 weeks 10. The response is seen by three to four weeks 10. The nodule size and the number may be followed to determine the response to the antibiotic therapy. If the response is not satisfactory, the duration of the antibiotic therapy can be extended 10. Antibiotic therapy can cure most bacillary angiomatosis skin lesions. Even peliosis responds to antibiotic therapy 10. The duration of antibiotic therapy has not been determined and most likely longer in cases with invasive systemic disease. Antibiotics should be continued for 3-6 months to prevent recurrence 25. Infectious disease consultations should be obtained. Sometimes a surgical consultation may be required for adjunctive procedures like cryotherapy, electrodesiccation with curettage, and surgical excision of solitary cutaneous lesions.
For patients with multisystem bacillary angiomatosis involvement, a combination therapy with rifampicin can be helpful; however, interactions between the selected drugs must be carefully considered, especially in transplant recipients treated with tacrolimus 25. For such patients, the treatment with tacrolimus requires dose adjustment based on its blood concentration 26, 27.
In patients who are immunosuppressed, therapy should be continued till the CD4 T-helper cell count normalizes. In HIV patients, antiretroviral therapy is essential however a case of immune reconstitution inflammatory syndrome has been reported 68. Therapy in HIV patients should be continued until the CD4 count remains above 200 cells/microliter for over six months 10.
Large pus-filled lymph nodes or blisters may need to be drained. Supportive therapy includes hydration and analgesics for pain and fever. Warm moist compresses to affected lymph nodes may decrease swelling and tenderness 11.
Bacillary angiomatosis prognosis
Bacillary angiomatosis resolves rapidly when antibiotic therapy is instituted early, antibiotics are curative in most patients 10. Skin lesions of bacillary angiomatosis disappear with days to weeks of starting appropriate treatment, although response can be incomplete. Hyperpigmentation or slight induration at the site of a lesion may persist indefinitely.
Relapses are common in immunocompromised patients and can occur after cessation of antibiotic therapy. However, if systemic bacillary angiomatosis infection is not treated promptly it can be fatal 10.
Overall bacillary angiomatosis prognosis depends on early detection and treatment and on the degree of immunosuppression. Treatment may be more difficult and requires a longer duration of therapy if the diagnosis is delayed. Untreated bacillary angiomatosis may be progressive and life threatening 69.
- Balaban M, Ioana Nedelcu R, Balmes G, Adela Todorovic T, Brinzea A, Nichita L, Gabriela Popp C, Theodor Andrei R, Andrada Zurac S, Adriana Ion D, Turcu G. Bacillary angiomatosis triggered by severe trauma in a healthy Caucasian patient: A case report. Exp Ther Med. 2020 Jul;20(1):56-60. doi: 10.3892/etm.2019.8260[↩][↩]
- Blattner C, Jacobson-Dunlop E, Miller JH, Elston DM. A case of bacillary angiomatosis in a patient with pancreatic adenocarcinoma. J Cutan Pathol. 2014;41:277–280. doi: 10.1111/cup.12303[↩][↩]
- Iraji F, Pourazizi M, Abtahi-Naeini B, Meidani M, Rajabi P. Bacillary Angiomatosis in Immunocompetent Patient with Atypical Manifestations. Indian J Dermatol. 2015 Sep-Oct;60(5):523. doi: 10.4103/0019-5154.164444[↩][↩]
- Stoler MH, Bonfiglio TA, Steigbigel RT, Pereira M. An atypical subcutaneous infection associated with acquired immune deficiency syndrome. Am J Clin Pathol. 1983 Nov;80(5):714-8. doi: 10.1093/ajcp/80.5.714[↩][↩]
- Milde P, Brunner M, Borchard F, et al. Cutaneous Bacillary Angiomatosis in a Patient With Chronic Lymphocytic Leukemia. Arch Dermatol. 1995;131(8):933–936. doi:10.1001/archderm.1995.01690200071013[↩]
- Koehler JE, Tappero JW. Bacillary angiomatosis and bacillary peliosis in patients infected with human immunodeficiency virus. Clin Infect Dis. 1993 Oct;17(4):612-24. doi: 10.1093/clinids/17.4.612[↩][↩]
- Tappero JW, Koehler JE, Berger TG, Cockerell CJ, Lee TH, Busch MP, Stites DP, Mohle-Boetani J, Reingold AL, LeBoit PE. Bacillary angiomatosis and bacillary splenitis in immunocompetent adults. Ann Intern Med. 1993 Mar 1;118(5):363-5. doi: 10.7326/0003-4819-118-5-199303010-00007[↩]
- Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med. 1990 Dec 6;323(23):1573-80. doi: 10.1056/NEJM199012063232301[↩]
- Slater LN, Welch DF, Min K. Rochalimaea henselae Causes Bacillary Angiomatosis and Peliosis Hepatis. Arch Intern Med. 1992;152(3):602–606. doi:10.1001/archinte.1992.00400150114021[↩]
- Akram SM, Anwar MY, Thandra KC, et al. Bacillary Angiomatosis. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448092[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Bacillary angiomatosis. https://dermnetnz.org/topics/bacillary-angiomatosis[↩][↩][↩][↩][↩]
- Bacillary Angiomatosis. https://emedicine.medscape.com/article/212737-overview[↩][↩]
- Millet CR, Halpern AV, Reboli AC, Heymann WR. Bacterial diseases. In: Dermatology. Bolognia JL, Jorizzo JL and Schaffer JV (eds). 3rd edition. Elsevier Saunders, USA. 2012:pp1208–1210.[↩][↩][↩][↩]
- Markowicz M, Käser S, Müller A, Lang G, Lang S, Mayerhöfer M, Stanek G, Rieger A. Bacillary angiomatosis presenting with facial tumor and multiple abscesses: A case report. Medicine (Baltimore) 2016;95(e4155) doi: 10.1097/MD.0000000000004155[↩][↩]
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217–223. doi: 10.3201/eid1202.050874[↩]
- Fulchini R, Bloemberg G, Boggian K. Bacillary angiomatosis and bacteremia due to Bartonella quintana in a patient with chronic lymphocytic leukemia. Case Rep Infect Dis. 2013;2013(694765) doi: 10.1155/2013/694765[↩]
- Karakas M. Baba, VAksungur VL, Homan S, Memisoglu HR and Uguz A: Bacillary angiomatosis on a region of burned skin in a immunocompetent patient. Br J Dermatol. 2000;143:609–611. doi: 10.1111/j.1365-2133.2000.03719.x[↩]
- Nosal JM. Bacillary angiomatosis, cat-scratch disease, and bartonellosis: What’s the connection? Int J Dermatol. 1997;36:405–411. doi: 10.1046/j.1365-4362.1997.00246.x[↩]
- Zangwill KM, Hamilton DH, Perkins BA, Regnery RL, Plikaytis BD, Hadler JL, Cartter ML, Wenger JD. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med. 1993;329:8–13. doi: 10.1056/NEJM199307013290102[↩]
- Chomel BB, Kasten RW, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Gurfield AN, Abbott RC, Pedersen NC, Koehler JE. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol. 1996 Aug;34(8):1952-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC229161/pdf/341952.pdf[↩]
- Fulchini R, Bloemberg G, Boggian K. Bacillary Angiomatosis and Bacteremia due to Bartonella quintana in a Patient with Chronic Lymphocytic Leukemia. Case Rep Infect Dis. 2013;2013:694765. doi: 10.1155/2013/694765[↩]
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006 Feb;12(2):217-23. doi: 10.3201/eid1202.050874[↩]
- Blattner C, Jacobson-Dunlop E, Miller JH, Elston DM. A case of bacillary angiomatosis in a patient with pancreatic adenocarcinoma. J Cutan Pathol. 2014 Mar;41(3):277-80. doi: 10.1111/cup.12303[↩][↩]
- Wanat KA, Reid E, Kamiyango W, El-Mallawany NK, Kovarik CL. Tumoral bacillary angiomatosis in a child with human immunodeficiency virus. JAMA Dermatol. 2014 Sep;150(9):1015-6. doi: 10.1001/jamadermatol.2013.9128[↩]
- Brzewski P, Kwiecińska M, Sułowicz J, Podolec K, Obtułowicz A, Dyduch G, Wojas-Pelc A. Bacillary Angiomatosis in Renal Transplant Recipient: A Case Report. Transplant Proc. 2020 Oct;52(8):2524-2526. https://doi.org/10.1016/j.transproceed.2020.02.092[↩][↩][↩][↩][↩][↩][↩]
- Moulin C, Kanitakis J, Ranchin B, Chauvet C, Gillet Y, Morelon E, Euvrard S. Cutaneous bacillary angiomatosis in renal transplant recipients: report of three new cases and literature review. Transpl Infect Dis. 2012 Aug;14(4):403-9. doi: 10.1111/j.1399-3062.2011.00713.x[↩][↩][↩][↩][↩]
- Orsag J, Flodr P, Melter O, Tkadlec J, Sternbersky J, Hruby M, Klicova A, Zamboch K, Krejci K, Zadrazil J. Cutaneous bacillary angiomatosis due to Bartonella quintana in a renal transplant recipient. Transpl Int. 2015 May;28(5):626-31. doi: 10.1111/tri.12539[↩][↩][↩]
- Helleberg M. Bacillary angiomatosis in a solid organ transplant recipient. IDCases. 2019 Oct 31;18:e00649. doi: 10.1016/j.idcr.2019.e00649[↩][↩]
- Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol. 2008 Mar;22(1):1-15. doi: 10.1111/j.1365-2915.2008.00713.x[↩]
- Cotté V, Bonnet S, Le Rhun D, Le Naour E, Chauvin A, Boulouis HJ, Lecuelle B, Lilin T, Vayssier-Taussat M. Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis. 2008 Jul;14(7):1074-80. doi: 10.3201/eid1407.071110[↩][↩]
- Chang CC, Chomel BB, Kasten RW, Romano V, Tietze N. Molecular evidence of Bartonella spp. in questing adult Ixodes pacificus ticks in California. J Clin Microbiol. 2001 Apr;39(4):1221-6. doi: 10.1128/JCM.39.4.1221-1226.2001[↩]
- Eskow E, Rao RV, Mordechai E. Concurrent infection of the central nervous system by Borrelia burgdorferi and Bartonella henselae: evidence for a novel tick-borne disease complex. Arch Neurol. 2001;58:1357–63. 10.1001/archneur.58.9.1357[↩]
- Lucey D, Dolan MJ, Moss CW, Garcia M, Hollis DG, Wegner S, Morgan G, Almeida R, Leong D, Greisen KS, et al. Relapsing illness due to Rochalimaea henselae in immunocompetent hosts: implication for therapy and new epidemiological associations. Clin Infect Dis. 1992 Mar;14(3):683-8. doi: 10.1093/clinids/14.3.683[↩]
- Holden K, Boothby JT, Kasten RW, Chomel BB. Co-detection of Bartonella henselae, Borrelia burgdorferi, and Anaplasma phagocytophilum in Ixodes pacificus ticks from California, USA. Vector Borne Zoonotic Dis. 2006 Spring;6(1):99-102. doi: 10.1089/vbz.2006.6.99[↩]
- Frean J, Arndt S, Spencer D. High rate of Bartonella henselae infection in HIV-positive outpatients in Johannesburg, South Africa. Trans R Soc Trop Med Hyg. 2002 Sep-Oct;96(5):549-50. doi: 10.1016/s0035-9203(02)90437-2[↩]
- Benslimani A, Fenollar F, Lepidi H, Raoult D. Bacterial zoonoses and infective endocarditis, Algeria. Emerg Infect Dis. 2005 Feb;11(2):216-24. doi: 10.3201/eid1102.040668[↩]
- Znazen A, Rolain JM, Hammami N, Kammoun S, Hammami A, Raoult D. High prevalence of Bartonella quintana endocarditis in Sfax, Tunisia. Am J Trop Med Hyg. 2005 May;72(5):503-7.[↩]
- Kelly PJ, Rooney JJ, Marston EL, Jones DC, Regnery RL. Bartonella henselae isolated from cats in Zimbabwe. Lancet. 1998 Jun 6;351(9117):1706. doi: 10.1016/s0140-6736(05)77744-8[↩]
- Kelly PJ, Eoghain GN, Raoult D. Antibodies reactive with Bartonella henselae and Ehrlichia canis in dogs from the communal lands of Zimbabwe. J S Afr Vet Assoc. 2004 Sep;75(3):116-20. doi: 10.4102/jsava.v75i3.465[↩]
- Urbanowicz M, Kutzner H, Riveiro-Falkenbach E, Rodriguez-Peralto JL. Infectious Angiogenesis-Different Pathways, the Same Goal. Am J Dermatopathol. 2016 Nov;38(11):793-801. doi: 10.1097/DAD.0000000000000577[↩]
- Sommer LL, Reboli AC, Heyman WR. Bacterial diseases. In: Dermatology. Bolognia JL, Schaffer JV and Cerroni L (eds). 4th edition. Elsevier, USA. 2017:pp1282–1285.[↩]
- Kaçar N, Taşli L, Demirkan N, Ergin C, Ergin S. HIV-negative case of bacillary angiomatosis with chronic hepatitis. B. J Dermatol. 2010;37:722–725. doi: 10.1111/j.1346-8138.2010.00836.x[↩]
- Pons I, Sanfeliu I, Nogueras MM, Sala M, Cervantes M, Amengual MJ, Segura F. Seroprevalence of Bartonella spp. infection in HIV patients in Catalonia, Spain. BMC Infect Dis. 2008 May 1;8:58. doi: 10.1186/1471-2334-8-58[↩]
- Pape M, Kollaras P, Mandraveli K, Tsona A, Metallidis S, Nikolaidis P, Alexiou-Daniel S. Occurrence of Bartonella henselae and Bartonella quintana among human immunodeficiency virus-infected patients. Ann N Y Acad Sci. 2005 Dec;1063:299-301. doi: 10.1196/annals.1355.047[↩]
- Vassallo C, Ardigò M, Brazzelli V, Zecca M, Locatelli F, Alessandrino PE, Lazzarino M, Corona S, Lanzerini P, Benazzo M, Fabbi M, Borroni G. Bartonella-related pseudomembranous angiomatous papillomatosis of the oral cavity associated with allogeneic bone marrow transplantation and oral graft-versus-host disease. Br J Dermatol. 2007 Jul;157(1):174-8. doi: 10.1111/j.1365-2133.2007.07968.x[↩]
- Schwartz RA, Gallardo MA, Kapila R, Gascón P, Herscu J, Siegel I, Lambert WC. Bacillary angiomatosis in an HIV seronegative patient on systemic steroid therapy. Br J Dermatol. 1996 Dec;135(6):982-7. doi: 10.1046/j.1365-2133.1996.d01-1107.x[↩]
- Turgut M, Alabaz D, Karakaş M, Kavak M, Aksaray N, Alhan E, Cevlik F, Tuncer I. Bacillary angiomatosis in an immunocompetent child with a grafted traumatic wound. J Dermatol. 2004 Oct;31(10):844-7. doi: 10.1111/j.1346-8138.2004.tb00613.x[↩]
- Al-Thunayan A, Al-Rehaili M, Al-Meshal O, Al-Qattan MM. Bacillary angiomatosis presenting as a pyogenic granuloma of the hand in an otherwise apparently healthy patient. Ann Plast Surg. 2013 Jun;70(6):652-3. doi: 10.1097/SAP.0b013e31823b6866[↩]
- Karakaş M, Baba M, Homan S, Akman A, Acar MA, Memişoğlu HR, Gümürdülü D. A case of bacillary angiomatosis presenting as leg ulcers. J Eur Acad Dermatol Venereol. 2003 Jan;17(1):65-7. doi: 10.1046/j.1468-3083.2003.00513.x[↩]
- Kaçar N, Taşli L, Demirkan N, Ergin C, Ergin S. HIV-negative case of bacillary angiomatosis with chronic hepatitis B. J Dermatol. 2010 Aug;37(8):722-5. doi: 10.1111/j.1346-8138.2010.00836.x[↩]
- Karakaş M, Baba M, Aksungur VL, Homan S, Memisoğlu HR, Uğuz A. Bacillary angiomatosis on a region of burned skin in a immunocompetent patient. Br J Dermatol. 2000 Sep;143(3):609-11. doi: 10.1111/j.1365-2133.2000.03719.x[↩]
- Anstead GM. The centenary of the discovery of trench fever, an emerging infectious disease of World War 1. Lancet Infect Dis. 2016 Aug;16(8):e164-72. doi: 10.1016/S1473-3099(16)30003-2[↩]
- Tappero JW, Perkins BA, Wenger JD, Berger TG. Cutaneous manifestations of opportunistic infections in patients infected with human immunodeficiency virus. Clin Microbiol Rev. 1995 Jul;8(3):440-50. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC174635/pdf/080440.pdf[↩]
- Mohle-Boetani JC, Koehler JE, Berger TG, LeBoit PE, Kemper CA, Reingold AL, Plikaytis BD, Wenger JD, Tappero JW. Bacillary angiomatosis and bacillary peliosis in patients infected with human immunodeficiency virus: clinical characteristics in a case-control study. Clin Infect Dis. 1996 May;22(5):794-800. doi: 10.1093/clinids/22.5.794[↩]
- Bacillary Angiomatosis. https://emedicine.medscape.com/article/212737-overview#a4[↩]
- Diniz LM, Medeiros KB, Landeiro LG, Lucas EA. Bacillary angiomatosis with bone invasion. An Bras Dermatol. 2016 Nov-Dec;91(6):811-814. doi: 10.1590/abd1806-4841.20165436[↩]
- Ramdial PK, Sing Y, Ramburan A, Dlova NC, Bagratee JS, Calonje E. Bartonella quintana-induced vulval bacillary angiomatosis. Int J Gynecol Pathol. 2012 Jul;31(4):390-4. doi: 10.1097/PGP.0b013e31823f8463[↩]
- LeBoit PE, Berger TG, Egbert BM, Beckstead JH, Yen TS, Stoler MH. Bacillary angiomatosis. The histopathology and differential diagnosis of a pseudoneoplastic infection in patients with human immunodeficiency virus disease. Am J Surg Pathol. 1989 Nov;13(11):909-20.[↩]
- Marra CM. Neurologic complications of Bartonella henselae infection. Curr Opin Neurol. 1995 Jun;8(3):164-9. doi: 10.1097/00019052-199506000-00002[↩]
- Chetty R, Sabaratnam RM. Upper gastrointestinal bacillary angiomatosis causing hematemesis: a case report. Int J Surg Pathol. 2003 Jul;11(3):241-4. doi: 10.1177/106689690301100316[↩]
- Forrestel AK, Naujokas A, Martin JN, Maurer TA, McCalmont TH, Laker-Opwonya MO, Mulyowa G, Busakhala N, Amerson EH. Bacillary angiomatosis masquerading as Kaposi’s sarcoma in East Africa. J Int Assoc Provid AIDS Care. 2015 Jan-Feb;14(1):21-5. doi: 10.1177/2325957414521497[↩][↩]
- Comer JA, Flynn C, Regnery RL, Vlahov D, Childs JE. Antibodies to Bartonella Species in Inner-city Intravenous Drug Users in Baltimore, Md. Arch Intern Med. 1996;156(21):2491–2495. doi:10.1001/archinte.1996.00440200111014[↩]
- Koehler JE, Sanchez MA, Tye S, Garrido-Rowland CS, Chen FM, Maurer T, Cooper JL, Olson JG, Reingold AL, Hadley WK, Regnery RR, Tappero JW. Prevalence of Bartonella infection among human immunodeficiency virus-infected patients with fever. Clin Infect Dis. 2003 Aug 15;37(4):559-66. doi: 10.1086/375586[↩]
- Sander A, Penno S. Semiquantitative species-specific detection of Bartonella henselae and Bartonella quintana by PCR-enzyme immunoassay. J Clin Microbiol. 1999 Oct;37(10):3097-101. doi: 10.1128/JCM.37.10.3097-3101.1999[↩]
- Buffoni F. Nature of the organic cofactor of pig plasma benzylamine oxidase. Biochim Biophys Acta. 1990 Aug 1;1040(1):77-83. doi: 10.1016/0167-4838(90)90148-9[↩]
- Cortes EE, Saraceni V, Medeiros D, Ribeiro I. Bacillary angiomatosis and Kaposi’s sarcoma in AIDS. AIDS Patient Care STDS. 2000 Apr;14(4):179-82. doi: 10.1089/108729100317777[↩]
- Rodriguez O, Campbell LR, Bacha JM, Kovarik CL. Successful treatment of bacillary angiomatosis with oral doxycycline in an HIV-infected child with skin lesions mimicking Kaposi sarcoma. JAAD Case Rep. 2016 Feb 4;2(1):77-9. doi: 10.1016/j.jdcr.2015.12.002[↩]
- DallaPiazza M, Akiyama MJ. The First Report of Bartonella quintana Immune Reconstitution Inflammatory Syndrome Complicated by Jarisch-Herxheimer Reaction. J Int Assoc Provid AIDS Care. 2017 Jul/Aug;16(4):321-323. doi: 10.1177/2325957417702484[↩]
- Bacillary Angiomatosis. https://emedicine.medscape.com/article/212737-overview#a7[↩]