Contents
What is calcium gluconate
Calcium gluconate also called D-gluconic acid calcium salt, is the calcium salt of gluconic acid or gluconate salt of calcium. Calcium gluconate is used to prevent or to treat calcium deficiencies. Calcium gluconate exists in both an anhydrous form (empirical formula C12H22O14Ca) and as a monohydrate (empirical formula C12H22O14CaH2O). Each form is an odorless, white, free-flowing powder that is soluble in water, insoluble in alcohol and most organic solvents, and stable in air. Calcium gluconate has a variety of uses, including its use as a calcium replenisher in hypocalcemic states. Calcium as the gluconate salt helps to maintain calcium balance and prevent bone loss when taken orally. Calcium gluconate use in food in general as a source of calcium and the U.S. Food and Drug Administration (FDA) consider calcium gluconate as Generally Recognized as Safe (GRAS) 1.
Calcium, the most abundant mineral in the body, is found in some foods, added to others, available as a dietary supplement, and present in some medicines (such as antacids). Calcium is required for vascular contraction and vasodilation, muscle function, nerve transmission, intracellular signaling and hormonal secretion, though less than 1% of total body calcium is needed to support these critical metabolic functions 2. Serum calcium is very tightly regulated and does not fluctuate with changes in dietary intakes; the body uses bone tissue as a reservoir for, and source of calcium, to maintain constant concentrations of calcium in blood, muscle, and intercellular fluids 2.
The remaining 99% of the body’s calcium supply is stored in the bones and teeth as calcium hydroxyapatite, where it supports their structure and function 2. Bone itself undergoes continuous remodeling, with constant resorption and deposition of calcium into new bone. The balance between bone resorption and deposition changes with age. Bone formation exceeds resorption in periods of growth in children and adolescents, whereas in early and middle adulthood both processes are relatively equal. In aging adults, particularly among postmenopausal women, bone breakdown exceeds formation, resulting in bone loss that increases the risk of osteoporosis over time 2.
Intestinal calcium absorption occurs through both an active, saturable, transcellular process and a non-saturable, passive process. Active transport is controlled by 1,25-dihydroxyvitamin D [1,25(OH)2D] or calcitriol, and passive transport is paracellular. Calcium absorption varies considerably throughout the lifespan, being higher during periods of rapid growth and lower in old age. Calcium absorption is affected by vitamin D status; it has been shown to be low in patients with vitamin D deficiency, but there is uncertainty about the serum concentration of 25-hydroxyvitamin D [25(OH)D] or calcidiol, that is required for optimal calcium absorption. Unabsorbed dietary calcium is lost in the feces. The main routes of obligatory (endogenous) calcium loss are urine, feces, and skin and sweat (dermal losses).
Table 1: Recommended Dietary Allowances (RDAs) for Calcium
| Age | Male | Female | Pregnant | Lactating |
|---|---|---|---|---|
| 0–6 months* | 200 mg | 200 mg | ||
| 7–12 months* | 260 mg | 260 mg | ||
| 1–3 years | 700 mg | 700 mg | ||
| 4–8 years | 1,000 mg | 1,000 mg | ||
| 9–13 years | 1,300 mg | 1,300 mg | ||
| 14–18 years | 1,300 mg | 1,300 mg | 1,300 mg | 1,300 mg |
| 19–50 years | 1,000 mg | 1,000 mg | 1,000 mg | 1,000 mg |
| 51–70 years | 1,000 mg | 1,200 mg | ||
| 71+ years | 1,200 mg | 1,200 mg |
Footnotes:
Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
* Adequate Intake (AI)
[Source 3]National Academy of Sciences has recommended 1000 – 1300 mg per day as an adequate intake for adults, with an “upper limit” of 2500 mg per day to limit the potential for adverse effects that can be associated with excessive consumption of calcium, such as kidney stones, hypercalcemia, renal insufficiency, and the possibility of reduced absorption of other minerals.
If the dietary supply of calcium is insufficient to meet physiological requirements, calcium is resorbed from the skeleton to maintain blood concentrations within the range required for normal cellular and tissue functions. This causes a reduction in bone mass, which leads to osteopenia and osteoporosis, and an associated increased risk of fracture.
Excessively high levels of calcium in the blood known as hypercalcemia is defined by serum calcium concentrations > 2.75 mmol/L (11 mg/dL). Although very high calcium intakes have the potential to cause hypercalcemia 4, it is unlikely to occur with high intake of calcium from the diet alone but can be caused by high-dose calcium supplements, especially when accompanied by vitamin D supplements, as these can increase calcium absorption and hypercalcemia is most commonly associated with primary hyperparathyroidism or malignancy 2.
Excessively high levels of calcium in the blood (hypercalcemia) can cause renal insufficiency, vascular and soft tissue calcification, hypercalciuria (high levels of calcium in the urine) and kidney stones 2.
High calcium intake can cause constipation. It might also interfere with the absorption of iron and zinc, though this effect is not well established 2. High intake of calcium from supplements, but not foods, has been associated with increased risk of kidney stones 2. Some evidence links higher calcium intake with increased risk of prostate cancer, but this effect is not well understood, in part because it is challenging to separate the potential effect of dairy products from that of calcium 2. Some studies also link high calcium intake, particularly from supplements, with increased risk of cardiovascular disease 4.
Milk, yogurt, and cheese are rich natural sources of calcium and are the major food contributors of this nutrient to people in the United States 2. Nondairy sources include vegetables, such as Chinese cabbage, kale, and broccoli. Spinach provides calcium, but its bioavailability is poor. Most grains do not have high amounts of calcium unless they are fortified; however, they contribute calcium to the diet because they contain small amounts of calcium and people consume them frequently. Foods fortified with calcium include many fruit juices and drinks, tofu, and cereals. Selected food sources of calcium are listed in Table 2.
Table 2: Selected Food Sources of Calcium
| Food | Milligrams (mg) per serving | Percent DV* |
|---|---|---|
| Yogurt, plain, low fat, 8 ounces | 415 | 42 |
| Mozzarella, part skim, 1.5 ounces | 333 | 33 |
| Sardines, canned in oil, with bones, 3 ounces | 325 | 33 |
| Yogurt, fruit, low fat, 8 ounces | 313–384 | 31–38 |
| Cheddar cheese, 1.5 ounces | 307 | 31 |
| Milk, nonfat, 8 ounces** | 299 | 30 |
| Soymilk, calcium-fortified, 8 ounces | 299 | 30 |
| Milk, reduced-fat (2% milk fat), 8 ounces | 293 | 29 |
| Milk, buttermilk, lowfat, 8 ounces | 284 | 28 |
| Milk, whole (3.25% milk fat), 8 ounces | 276 | 28 |
| Orange juice, calcium-fortified, 6 ounces | 261 | 26 |
| Tofu, firm, made with calcium sulfate, ½ cup*** | 253 | 25 |
| Salmon, pink, canned, solids with bone, 3 ounces | 181 | 18 |
| Cottage cheese, 1% milk fat, 1 cup | 138 | 14 |
| Tofu, soft, made with calcium sulfate, ½ cup*** | 138 | 14 |
| Ready-to-eat cereal, calcium-fortified, 1 cup | 100–1,000 | 10–100 |
| Frozen yogurt, vanilla, soft serve, ½ cup | 103 | 10 |
| Turnip greens, fresh, boiled, ½ cup | 99 | 10 |
| Kale, fresh, cooked, 1 cup | 94 | 9 |
| Ice cream, vanilla, ½ cup | 84 | 8 |
| Chinese cabbage, bok choi, raw, shredded, 1 cup | 74 | 7 |
| Bread, white, 1 slice | 73 | 7 |
| Pudding, chocolate, ready to eat, refrigerated, 4 ounces | 55 | 6 |
| Tortilla, corn, ready-to-bake/fry, one 6” diameter | 46 | 5 |
| Tortilla, flour, ready-to-bake/fry, one 6” diameter | 32 | 3 |
| Sour cream, reduced fat, cultured, 2 tablespoons | 31 | 3 |
| Bread, whole-wheat, 1 slice | 30 | 3 |
| Kale, raw, chopped, 1 cup | 24 | 2 |
| Broccoli, raw, ½ cup | 21 | 2 |
| Cheese, cream, regular, 1 tablespoon | 14 | 1 |
Footnotes:
* DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration to help consumers compare the nutrient contents among products within the context of a total daily diet. The DV for calcium is 1,000 mg for adults and children aged 4 years and older. Foods providing 20% of more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet. The U.S. Department of Agriculture’s (USDA’s) Nutrient Database site lists the nutrient content of many foods and provides comprehensive list of foods containing calcium arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Calcium-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/Calcium-Food.pdf).
** Calcium content varies slightly by fat content; the more fat, the less calcium the food contains.
*** Calcium content is for tofu processed with a calcium salt. Tofu processed with other salts does not provide significant amounts of calcium.
Calcium and Health
Many claims are made about calcium’s potential benefits in health promotion and disease prevention and treatment.
Bone health and osteoporosis
Bones increase in size and mass during periods of growth in childhood and adolescence, reaching peak bone mass around age 30. The greater the peak bone mass, the longer one can delay serious bone loss with increasing age. Everyone should therefore consume adequate amounts of calcium and vitamin D throughout childhood, adolescence, and early adulthood. Osteoporosis, a disorder characterized by porous and fragile bones, is a serious public health problem for more than 10 million U.S. adults, 80% of whom are women. Another 34 million have osteopenia, or low bone mass, which precedes osteoporosis. Osteoporosis is most associated with fractures of the hip, vertebrae, wrist, pelvis, ribs, and other bones 5. An estimated 1.5 million fractures occur each year in the United States due to osteoporosis 6.
When calcium intake is low or ingested calcium is poorly absorbed, bone breakdown occurs as the body uses its stored calcium to maintain normal biological functions. Bone loss also occurs as part of the normal aging process, particularly in postmenopausal women due to decreased amounts of estrogen. Many factors increase the risk of developing osteoporosis, including being female, thin, inactive, or of advanced age; smoking cigarettes; drinking excessive amounts of alcohol; and having a family history of osteoporosis 7.
Various bone mineral density (BMD) tests are available. The T-score from these tests compares an individual’s bone mineral density to an optimal bone mineral density (that of a healthy 30-year old adult). A T-score of -1.0 or above indicates normal bone density, -1.0 to -2.5 indicates low bone mass (osteopenia), and lower than -2.5 indicates osteoporosis 8. Although osteoporosis affects individuals of all races, ethnicities, and both genders, women are at highest risk because their skeletons are smaller than those of men and because of the accelerated bone loss that accompanies menopause. Regular exercise and adequate intakes of calcium and vitamin D are critical to the development and maintenance of healthy bones throughout the life cycle. Both weight-bearing exercises (such as walking, running, and activities where one’s feet leave and hit the ground and work against gravity) and resistance exercises (such as calisthenics and that involve weights) support bone health.
Supplementation with calcium plus vitamin D has been shown to be effective in reducing fractures and falls (which can cause fractures) in institutionalized older adults 9. However, among community-dwelling older adults over age 50, the benefits of supplementation with these nutrients on fracture resistance are much less clear. A recent systematic review of 26 randomized controlled trials found that calcium supplements, with or without vitamin D, modestly but significantly reduced the risk of total and vertebral fractures, but not fractures of the hip or forearm 10. But the four trials with the lowest risk of bias, involving a total of 44,505 individuals, showed no effect of supplementation on risk of fracture at any site. A related meta-analysis of calcium intake on bone mineral density found that calcium supplementation produced only a small, initial, and non-progressive increase in bone mineral density that was unlikely to result in a clinically significant reduction in the risk of bone fractures 11. The U.S. Preventive Services Task Force concluded that the current evidence is insufficient to assess the balance of benefits and harms of combined vitamin D and calcium supplementation to prevent bone fractures in premenopausal women or in men 12. For non-institutionalized postmenopausal women, the U.S. Preventive Services Task Force concluded that while current evidence was insufficient to assess the balance of benefits and harms of combined supplementation with vitamin D (at more than 400 IU/day) and calcium (at more than 1,000 mg/day) to prevent bone fractures, there was clearly no benefit in supplementing with smaller doses of these nutrients for this purpose.
In 1993, the U.S. Food and Drug Administration authorized a health claim related to calcium and osteoporosis for foods and supplements. In January 2010, this health claim was expanded to include vitamin D. Model health claims include the following: “Adequate calcium throughout life, as part of a well-balanced diet, may reduce the risk of osteoporosis” and “Adequate calcium and vitamin D as part of a healthful diet, along with physical activity, may reduce the risk of osteoporosis in later life” 13.
Cancer of the colon and rectum
Data from observational and experimental studies on the potential role of calcium in preventing colorectal cancer, though somewhat inconsistent, are highly suggestive of a protective effect 2. Several studies have found that higher intakes of calcium from foods (low-fat dairy sources) and/or supplements are associated with a decreased risk of colon cancer 14. In a follow-up study to the Calcium Polyp Prevention Study, supplementation with calcium carbonate led to reductions in the risk of adenoma (a nonmalignant tumor) in the colon, a precursor to cancer 15, even as long as 5 years after the subjects stopped taking the supplement 16. In two large prospective epidemiological trials, men and women who consumed 700–800 mg per day of calcium had a 40%–50% lower risk of developing left-side colon cancer 17. But other observational studies have found the associations to be inconclusive 18.
In the Women’s Health Initiative, a clinical trial involving 36,282 postmenopausal women, daily supplementation with 1,000 mg of calcium and 400 International Units (IU) of vitamin D3 for 7 years produced no significant differences in the risk of invasive colorectal cancer compared to placebo 19. The authors of a Cochrane systematic review concluded that calcium supplementation might moderately help prevent colorectal adenomas, but there is not enough evidence to recommend routine use of calcium supplements to prevent colorectal cancer 20. Given the long latency period for colon cancer development, long-term studies are needed to fully understand whether calcium intakes affect colorectal cancer risk.
Cancer of the prostate
Several epidemiological studies have found an association between high intakes of calcium, dairy foods or both and an increased risk of developing prostate cancer 21. However, others have found only a weak relationship, no relationship, or a negative association between calcium intake and prostate cancer risk 22. The authors of a meta-analysis of prospective studies concluded that high intakes of dairy products and calcium might slightly increase prostate cancer risk 23.
Interpretation of the available evidence is complicated by the difficulty in separating the effects of dairy products from that of calcium. But overall, results from observational studies suggest that total calcium intakes >1,500 mg/day or >2,000 mg/day may be associated with increased prostate cancer risk (particularly advanced and metastatic cancer) compared with lower amounts of calcium (500–1,000 mg/day 24. Additional research is needed to clarify the effects of calcium and/or dairy products on prostate cancer risk and elucidate potential biological mechanisms.
Cardiovascular disease
Calcium has been proposed to help reduce cardiovascular disease risk by decreasing intestinal absorption of lipids, increasing lipid excretion, lowering cholesterol levels in the blood, and promoting calcium influx into cells 2. However, data from prospective studies of calcium’s effects on cardiovascular disease risk are inconsistent, and whether dietary calcium has different effects on the cardiovascular system than supplemental calcium is not clear. In the Iowa Women’s Health Study, higher calcium intake from diet and/or supplements was associated with reduced ischemic heart disease mortality in postmenopausal women 25. Conversely, in a cohort of older Swedish women, both total and dietary calcium intakes of 1,400 mg/day and higher were associated with higher rates of death from cardiovascular disease and ischemic heart disease than intakes of 600–1,000 mg/day [85]. Other prospective studies have shown no significant associations between calcium intake and cardiac events or cardiovascular mortality 26. Data for stroke are mixed, with some studies linking higher calcium intakes to lower risk of stroke, and others finding no associations or trends in the opposite direction 26, 4.
Several studies have raised concerns that calcium from supplements might increase the risk of cardiovascular disease, including myocardial infarction and coronary heart disease 27. For example, Xiao and colleagues 28 reported that men who took more than 1,000 mg/day supplemental calcium had a 20% higher risk of total cardiovascular disease death than men who did not take supplemental calcium, but supplemental calcium intake in women was unrelated to cardiovascular disease mortality. A reanalysis of data from the Women’s Health Initiative found that calcium supplements (1,000 mg/day) taken with or without vitamin D (400 IU/day) increased the risk of cardiovascular events in women who were not taking calcium supplements when they entered the study 29. While there is no established biological mechanism to support an association between calcium and cardiovascular disease, some scientists hypothesize that excessively high calcium intakes from supplements might override normal homeostatic controls of serum calcium levels and produce a temporary hypercalcemia 29. Hypercalcemia is associated with increased blood coagulation, vascular calcification, and arterial stiffness, all of which raise cardiovascular disease risk 29.
Many scientists question the strength of the available evidence linking supplemental calcium intake with cardiovascular disease risk, noting that no clinical trials were designed primarily to evaluate this potential relationship, so researchers have only considered cardiovascular disease outcomes in secondary analyses of trial data 30. Based on a 2016 systematic review and meta-analysis of 4 randomized trials and 27 observational studies 31, the American Society for Preventive Cardiology and the National Osteoporosis Foundation concluded that there is “moderate-quality evidence” that calcium with or without vitamin D (from supplements or foods) “has no relationship (beneficial or harmful) with the risk for cardiovascular and cerebrovascular disease, mortality, or all-cause mortality in generally healthy adults” 32. They added that based on the evidence to date, “calcium intake from food and supplements that does not exceed the [UL] should be considered safe from a cardiovascular standpoint.”
Blood pressure and hypertension
Several clinical trials have demonstrated a relationship between increased calcium intakes and both lower blood pressure and risk of hypertension 33, although the reductions are inconsistent. In the Women’s Health Study, calcium intake was inversely associated with risk of hypertension in middle-aged and older women 34. However, other studies have found no association between calcium intake and incidence of hypertension 26. The authors of a systematic review of the effects of calcium supplements for hypertension found any link to be weak at best, largely due to the poor quality of most studies and differences in methodologies 35.
Calcium’s effects on blood pressure might depend upon the population being studied. In hypertensive subjects, calcium supplementation appears to lower systolic blood pressure by 2–4 mmHg, whereas in normotensive subjects, calcium appears to have no significant effect on systolic or diastolic blood pressure 26.
Other observational and experimental studies suggest that individuals who eat a vegetarian diet high in minerals (such as calcium, magnesium, and potassium) and fiber and low in fat tend to have lower blood pressure 36. The Dietary Approaches to Stop Hypertension (DASH) study was conducted to test the effects of three different eating patterns on blood pressure: a control “typical” American diet; one high in fruits and vegetables; and a third diet high in fruits, vegetables, and low-fat dairy products. The diet containing dairy products resulted in the greatest decrease in blood pressure 37, although the contribution of calcium to this effect was not evaluated.
Preeclampsia
Preeclampsia is a serious medical condition in which a pregnant woman develops hypertension and proteinuria, usually after 20 weeks’ gestation 38. It is a leading cause of maternal and neonatal morbidity and mortality, affecting about 5–8% of pregnancies in the United States and up to 14% of pregnancies worldwide 38.
Studies suggest that calcium supplementation during pregnancy reduces the risk of preeclampsia, but the benefits may apply only to populations with inadequate calcium intakes 39. For example, in a randomized clinical trial among 524 healthy women in India with mean baseline calcium intakes of only 314 mg/day, daily supplementation with 2,000 mg calcium starting between 12 and 25 weeks’ gestation and continuing until delivery significantly reduced the risk of preeclampsia, as well as preterm birth, compared to placebo 40. Conversely, in a randomized trial of 4,589 healthy women in the United States, daily supplementation with 2,000 mg calcium from 13–21 weeks’ gestation through the remainder of pregnancy did not reduce the incidence of preeclampsia, pregnancy-induced hypertension, or other adverse perinatal outcomes compared to placebo [112]. The mean baseline calcium intake among these women, however, was about 1,100 mg/day. The authors of a 2014 Cochrane review of 13 clinical trials concluded that daily supplementation with 1,000 mg or more of calcium during pregnancy reduced the risk of preeclampsia by 55% 41. The reduction in risk was greatest for women at high risk of preeclampsia and those with low baseline calcium intakes (less than about 900 mg/day). For women with higher dietary calcium intakes, however, the reduction in preeclampsia risk was not statistically significant.
Several professional organizations recommend calcium supplements during pregnancy for women with low calcium intakes to reduce the risk of preeclampsia. For example, the American College of Obstetrics and Gynecology (ACOG) states that daily supplementation with 1,500–2,000 mg calcium may reduce the severity of preeclampsia in pregnant women who have calcium intakes less than 600 mg/day 42. Similarly, the World Health Organization (WHO) recommends 1,500–2,000 mg calcium for pregnant women with low dietary calcium intakes, particularly those at higher risk of gestational hypertension 39. The WHO recommends dividing the total daily dose into three doses, preferably to be taken at mealtimes, and taking the supplements from 20 weeks’ gestation until delivery. The WHO also recommends separating calcium and prenatal iron supplements by several hours to minimize the inhibitory effects of calcium on iron absorption. But some researchers argue that this interaction has minimal clinical significance and suggest that providers not counsel patients to separate the supplements to simplify the supplement regimen and facilitate adherence 43. The Canadian Hypertensive Disorders of Pregnancy Working Group 44, the International Society for the Study of Hypertension in Pregnancy 45, and the Society of Obstetric Medicine of Australia and New Zealand 46 have all issued similar recommendations to ACOG and the WHO.
Kidney stones
Kidney stones in the urinary tract are most commonly composed of calcium oxalate. Some, but not all, studies suggest a positive association between supplemental calcium intake and the risk of kidney stones, and these findings were used as the basis for setting the calcium upper limit intake in adults 2. In the Women’s Health Initiative, postmenopausal women who consumed 1,000 mg of supplemental calcium and 400 IU of vitamin D per day for 7 years had a 17% higher risk of kidney stones than subjects taking a placebo 47. The Nurses’ Health Study also showed a positive association between supplemental calcium intake and kidney stone formation 46. High intakes of dietary calcium, on the other hand, do not appear to cause kidney stones and may actually protect against developing them 48. For most individuals, other risk factors for kidney stones, such as high intakes of oxalates from food and low intakes of fluid, probably play a bigger role than calcium intake 49.
Weight management
Several studies have linked higher calcium intakes to lower body weight or less weight gain over time 50. Two explanations have been proposed. First, high calcium intakes might reduce calcium concentrations in fat cells by decreasing the production of parathyroid hormone and the active form of vitamin D. Decreased intracellular calcium concentrations in turn increase fat breakdown and discourage fat accumulation in these cells 51. Secondly, calcium from food or supplements might bind to small amounts of dietary fat in the digestive tract and prevent its absorption 51. Dairy products, in particular, might contain additional components that have even greater effects on body weight than their calcium content alone would suggest 52.
Despite these findings, the results from clinical trials have been largely negative. For example, dietary supplementation with 1,500 mg/day of calcium (from calcium carbonate) for 2 years was found to have no clinically significant effects on weight in 340 overweight and obese adults as compared with placebo 53. Three reviews of published studies on calcium from supplements or dairy products on weight management came to similar conclusions 54. A meta-analysis of 13 randomized controlled trials published in 2006 concluded that neither calcium supplementation nor increased dairy product consumption had a statistically significant effect on weight reduction [136]. More recently, a 2009 evidence report from the Agency for Healthcare Research and Quality concluded that, overall, clinical trial results do not support an effect of calcium supplementation on weight loss 26. Also, a 2012 meta-analysis of 29 randomized controlled trials found no benefit of an increased consumption of dairy products on body weight and fat loss in long-term studies 54. Overall, the results from clinical trials do not support a link between higher calcium intakes and lower body weight or weight loss.
Calcium gluconate vs calcium chloride
10% Calcium Chloride injection is indicated for the treatment of hypocalcemia and conditions secondary to hypocalcemia (eg, tetany, seizures, arrhythmias); emergent treatment of severe hypermagnesemia, requiring a prompt increase in plasma calcium levels. Calcium chloride in water dissociates to provide calcium (Ca++) and chloride (Cl−) ions. They are normal constituents of the body fluids and are dependent on various physiological mechanisms for maintenance of balance between intake and output.
Based on the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care 55, calcium chloride is an effective and recommended treatment to stabilize the myocardial cell membrane in patients with severe hyperkalemia (K+ >6.5 mEq/L with toxic ECG changes).
Based on the American Heart Association (AHA) Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care 55, calcium chloride is effective and recommended for the treatment of malignant arrhythmias (including cardiac arrest) in patients with hypermagnesemia.
Based on the American Heart Association (AHA) Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care 55 and the American Academy of Pediatrics Committee on Drugs, calcium chloride, although based on limited evidence, is an effective and recommended treatment in the setting of beta-blocker overdose (refractory to glucagon and high-dose inotropes/vasopressors).
Based on the American Heart Association (AHA) Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care 55 and the American Academy of Pediatrics Committee on Drugs, calcium chloride, although based on limited evidence, is an effective and recommended treatment in the setting of calcium channel blocker overdose.
Calcium chloride injection also contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Calcium chloride is contraindications
Calcium chloride is contraindicated for cardiac resuscitation in the presence of ventricular fibrillation or in patients with the risk of existing digitalis toxicity.
Calcium chloride is not recommended in the treatment of asystole and electromechanical dissociation.
Calcium chloride dosage
10% Calcium Chloride injection consists of 1 gram of calcium chloride in a 10 mL vial, or 100 mg/mL. This concentration represents 27 mg or 1.4 mEq of elemental calcium per mL. Thus, one 10 mL syringe provides 270 mg of elemental calcium. The dosage recommendation in various references is given either as amount of calcium chloride or amount of elemental calcium, and often it is not specified. Ionized calcium concentrations should be measured, to assist in dosage adjustment.
10% Calcium Chloride injection is administered only by slow intravenous injection (not to exceed 1 mL/min), preferably in a central or deep vein.
The usual precautions for intravenous therapy should be observed. If time permits, the solution should be warmed to body temperature. The injection should be halted if the patient complains of any discomfort; it may be resumed when symptoms disappear. Following injection, the patient should remain recumbent for a short time.
The usual adult dosage in hypocalcemic disorders ranges from 200 mg to 1 g (2 to 10 mL) at intervals of 1 to 3 days depending on the response of the patient and/or results of serum ionized calcium determinations. Repeated injections may be required because of rapid excretion of calcium.
The pediatric dosage in hypocalcemic disorders ranges from 2.7 to 5.0 mg/kg hydrated Calcium Chloride (or 0.136 to 0.252 mEq elemental calcium per kg, or 0.027 to 0.05 mL of 10% Calcium Chloride injection per kg). No data from clinical trials is available about repeated dosages, though textbook references recommend repeat dosages q 4 to 6 hours.
Calcium gluconate uses
Parenteral calcium salts (ie, chloride, glubionate, gluceptate, and gluconate) are indicated in the treatment of hypocalcemia in conditions that require a rapid increase in serum calcium-ion concentration, such as in neonatal hypocalcemia due to “hungry bones” syndrome (remineralization hypocalcemia) following surgery for hyperparathyroidis, vitamin D deficiency; and alkalosis. Calcium gluconate have been used as adjunctive therapy for insect bites or stings, such as Black Widow Spider bites, and sensitivity reactions, especially when characterized by urticaria; and as an aid in the management of acute symptoms of lead colic, overdose of magnesium or certain heart medicines, and rickets. Calcium gluconate is also used for life support and life-threatening heart conditions. Parenteral calcuim gluconate and calcium gluceptate are also used for the prevention of hypocalcemia during exchange transfusions 56.
Calcium gluconate is also used in sewage purification, industrial cleaning, metal surface treatment, textile bleach stabilizing, aluminum processing, and as a chelating agent in cement set retarding, cleaning products, personal care products, pharmaceuticals, coffee processing (anti-caking agent), and food and drugs (sequestering, buffering, and gelling agent). Calcium gluconate also has veterinary use for hypocalcemic conditions.
Calcium gluconate precautions and warnings
Calcium Gluconate Injection Contraindications
Calcium Gluconate Injection is contraindicated in:
- Hypercalcemia
- Neonates (28 days of age or younger) receiving ceftriaxone
Arrhythmias with concomitant cardiac glycoside use
Cardiac arrhythmias may occur if calcium and cardiac glycosides are administered together. Hypercalcemia increases the risk of digoxin toxicity. Administration of Calcium Gluconate Injection should be avoided in patients receiving cardiac glycosides. If concomitant therapy is necessary, Calcium Gluconate Injection should be given slowly in small amounts and with close ECG monitoring.
End-Organ damage due to intravascular ceftriaxone-calcium precipitates
Concomitant use of ceftriaxone and Calcium Gluconate Injection is contraindicated in neonates (28 days of age or younger) due to cases of fatal outcomes in neonates in which a crystalline material was observed in the lungs and kidneys at autopsy after ceftriaxone and calcium were administrated simultaneously through the same intravenous line. Concomitant administration can lead to the formation of ceftriaxone-calcium precipitates that may act as emboli, resulting in vascular spasm or infarction.
In patients older than 28 days of age, ceftriaxone and Calcium Gluconate Injection may be administered sequentially, provided the infusion lines are thoroughly flushed between infusions with a compatible fluid. Do not administer Ceftriaxone simultaneously with Calcium Gluconate Injection via a Y-site in any age group.
Tissue Necrosis and Calcinosis
Intravenous administration of Calcium Gluconate Injection and local trauma may result in calcinosis cutis due to transient increase in local calcium concentration. Calcinosis cutis can occur with or without extravasation of Calcium Gluconate Injection, is characterized by abnormal dermal deposits of calcium salts, and clinically manifests as papules, plaques, or nodules that may be associated with erythema, swelling, or induration. Tissue necrosis, ulceration, and secondary infection are the most serious complications.
A case report on an infant with severe asphyxia and persistent pulmonary hypertension as a newborn. The baby received prolonged intravenous calcium gluconate therapy for hypocalcemia 57. At 5 weeks of age, multiple firm, indurated areas (armor-like lesions) were palpable in the subcutaneous tissues of the trunk, arms, legs, and face, particularly in skin folds. Roentgenographic study showed generalized soft-tissue calcifications throughout the body, extremities, and face. Calcinosis cutis occurs through a variety of pathogenetic mechanisms. Case reports on calcinosis cutis in infants are uncommon, and the calcifications are mostly localized. In this patient, they are generalized 57.
If extravasation occurs or clinical manifestations of calcinosis cutis are noted, immediately discontinue intravenous administration at that site and treat as needed.
Hypotension, bradycardia, and cardiac arrhythmias with rapid administration
Rapid injection of Calcium Gluconate Injection may cause vasodilation, decreased blood pressure, bradycardia, cardiac arrhythmias, syncope and cardiac arrest. To avoid adverse reactions that may follow rapid intravenous administration, Calcium Gluconate Injection should be diluted with 5% dextrose or normal saline and infused slowly. If rapid intravenous bolus of Calcium Gluconate Injection is required, the rate of intravenous administration should not exceed 200 mg/minute in adults and 100 mg/minute in pediatric patients and ECG monitoring during administration is recommended
Aluminum Toxicity
Calcium Gluconate Injection contains aluminum, up to 400 mcg per liter, that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum. Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 mcg/kg/day to 5 mcg/kg/day accumulate aluminum levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Drug Incompatibilities
- Do not mix Calcium Gluconate Injection with ceftriaxone. Concurrent use of intravenous ceftriaxone and Calcium Gluconate Injection can lead to the formation of ceftriaxone-calcium precipitates. Concomitant use of ceftriaxone and intravenous calcium-containing products is contraindicated in neonates (28 days of age or younger). In patients older than 28 days of age, ceftriaxone and calcium-containing products may be administered sequentially, provided the infusion lines are thoroughly flushed between infusions with a compatible fluid. Ceftriaxone must not be administered simultaneously with intravenous calcium-containing solutions via a Y-site in any age group.
- Do not mix Calcium Gluconate Injection with fluids containing bicarbonate or phosphate. Calcium Gluconate Injection is not physically compatible with fluids containing phosphate or bicarbonate. Precipitation may result if mixed.
- Do not mix Calcium Gluconate Injection with minocycline injection. Calcium complexes minocycline rendering it inactive.
- Vitamin D, vitamin A, thiazide diuretics, estrogen, calcipotriene and teriparatide administration may cause hypercalcemia. Monitor plasma calcium concentrations in patients taking these drugs concurrently.
Calcium gluconate in pregnancy
FDA Pregnancy Risk Category C: Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk 58.
Risk summary
Limited available data with Calcium Gluconate Injection use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. There are risks to the mother and the fetus associated with hypocalcemia in pregnancy.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal risk: Maternal hypocalcemia can result in an increased rate of spontaneous abortion, premature and dysfunctional labor, and possibly preeclampsia.
Fetal/Neonatal adverse reactions: Infants born to mothers with hypocalcemia can have associated fetal and neonatal hyperparathyroidism, which in turn can cause fetal and neonatal skeletal demineralization, subperiosteal bone resorption, osteitis fibrosa cystica and neonatal seizures. Infants born to mothers with hypocalcemia should be carefully monitored for signs of hypocalcemia or hypercalcemia, including neuromuscular irritability, apnea, cyanosis and cardiac rhythm disorders.
Calcium gluconate in breastfeeding
Risk summary
Calcium is present in human milk as a natural component of human milk. It is not known whether intravenous administration of Calcium Gluconate Injection can alter calcium concentration in human milk. There are no data on the effects of Calcium Gluconate Injection on the breastfed infant, or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Calcium Gluconate Injection and any potential adverse effects on the breastfed child from Calcium Gluconate Injection or from the underlying maternal condition.
Calcium gluconate for pediatric use
The safety and effectiveness of Calcium Gluconate Injection have been established in pediatric patients for the treatment of acute, symptomatic hypocalcemia.
Pediatric approval for Calcium Gluconate Injection, including doses, is not based on adequate and well-controlled clinical studies. Safety and dosing recommendations in pediatric patients are based on published literature and clinical experience.
Concomitant use of ceftriaxone and Calcium Gluconate Injection is contraindicated in neonates (28 days of age or younger) due to reports of fatal outcomes associated with the presence of lung and kidney ceftriaxone-calcium precipitates. In patients older than 28 days of age, ceftriaxone and Calcium Gluconate Injection may be administered sequentially, provided the infusion lines are thoroughly flushed between infusions with a compatible fluid. Calcium Gluconate Injection contains up to 400 mcg/L aluminum which may be toxic, particularly for premature neonates due to immature renal function. Parenteral administration of aluminum greater than 4 to 5 mcg/kg/day is associated with central nervous system and bone toxicity.
Calcium gluconate for geriatric use
In general dose selection for an elderly patient should start at the lowest dose of the recommended dose range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Calcium gluconate in people with kidney impairment
For patients with renal impairment, initiate Calcium Gluconate Injection at the lowest dose of the recommended dose ranges across all age groups. Monitor serum calcium levels every 4 hours.
Calcium Gluconate in people with liver impairment
Hepatic function does not impact the availability of ionized calcium after calcium gluconate intravenous administration. Dose adjustment in hepatically impaired patients may not be necessary.
Calcium gluconate dose
- Calcium gluconate is contraindicated in patients with hypercalcemia, ventricular fibrillation, and in digitalized patients. Some medicines can affect how calcium gluconate works. Tell your doctor if you are taking digoxin or antibiotics.
Calcium gluconate injection contains aluminum which may be toxic. Patients with impaired renal function, undergoing prolonged parenteral administration of calcium gluconate, should be monitored for clinical signs of aluminum toxicity. Clinicians should be aware these patients may achieve a level of aluminum accumulation capable of causing central nervous system and bone toxicity, as well as, tissue loading.
Ask a doctor or pharmacist if it is safe for you to take calcium gluconate if you have ever had:
- kidney disease;
- kidney stones;
- cancer;
- a parathyroid gland disorder; or
- high levels of calcium in your blood.
Ask a doctor before using calcium gluconate if you are pregnant or breast-feeding. Your dose needs may be different during pregnancy or while you are nursing.
Calcium can make it harder for your body to absorb certain medicines. If you take other medications, take them at least 2 hours before or 4 or 6 hours after you take calcium gluconate.
Other drugs may interact with calcium gluconate, including prescription and over-the-counter medicines, vitamins, and herbal products. Tell your doctor about all your current medicines and any medicine you start or stop using.
Calcium Gluconate Injection Dosage and Administration
- Your doctor will prescribe your dose and schedule. This medicine is given through a needle placed in a vein.
- A nurse or other health provider will give you this medicine.
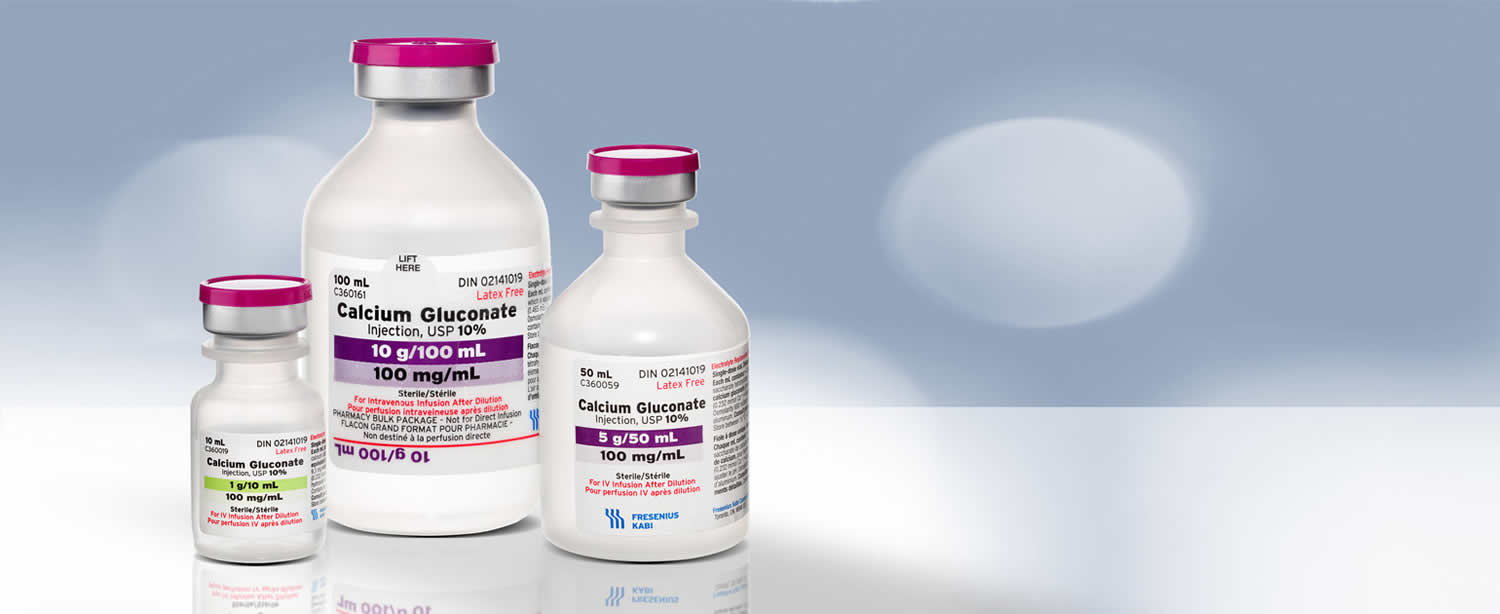
Calcium Gluconate Injection USP is a clear, colorless to slightly yellow, solution available in the following:
- Single dose vial: 1,000 mg per 10 mL (100 mg per mL)
- Single dose vial: 5,000 mg per 50 mL (100 mg per mL)
- Pharmacy bulk package: 10,000 mg per 100 mL (100 mg per mL)
Each mL of Calcium Gluconate Injection USP contains 9.3 mg (0.465 mEq) of elemental calcium.
Important Administration Instructions
- Calcium Gluconate Injection contains 100 mg of calcium gluconate per mL which contains 9.3 mg (i.e., 0.465 mEq) of elemental calcium.
- Dilute Calcium Gluconate Injection prior to use in 5% dextrose or normal saline and assess for potential drug or IV fluid incompatibilities.
- Inspect Calcium Gluconate Injection visually prior to administration. The solution should appear clear and colorless to slightly yellow. Do not administer if there is particulate matter or discoloration.
- Use the diluted solution immediately after preparation.
- Administer Calcium Gluconate Injection intravenously via a secure intravenous line to avoid calcinosis cutis and tissue necrosis.
- Administer Calcium Gluconate Injection by bolus administration or continuous infusion:
For bolus intravenous administration:
- Dilute the dose of Calcium Gluconate Injection in 5% dextrose or normal saline to a concentration of 10-50 mg/mL prior to administration. Administer the dose slowly and DO NOT exceed an infusion rate of 200 mg/minute in adults or 100 mg/minute in pediatric patients, including neonates. Monitor patients, vitals and electrocardiograph (ECG) during administration.
For continuous intravenous infusion:
- Dilute Calcium Gluconate Injection in 5% dextrose or normal saline to a concentration of 5.8-10 mg/mL prior to administration. Administer at the rate recommended in Table 3 and monitor patients, vitals, calcium and ECG during the infusion.
- Calcium Gluconate Injection is supplied in single-dose vials and pharmacy bulk packages.
Table 3. Dosing Recommendations in mg of Calcium Gluconate for Neonate, Pediatric, and Adult Patients
| Patient Population | Initial Dose | Subsequent Doses (if needed) | |
| Bolus | Continuous Infusion | ||
| Neonate (≤ 1 month) | 100 – 200 mg/kg | 100 – 200 mg/kg every 6 hours | Initiate at 17-33 mg/kg/hour |
| Pediatric (> 1 month to < 17 years) | 29 – 60 mg/kg | 29 – 60 mg/kg every 6 hours | Initiate at 8-13 mg/kg/hour |
| Adult | 1000 – 2000 mg | 1000 – 2000 mg every 6 hours | Initiate at 5.4 – 21.5 mg/kg/hour |
For bolus administration, DO NOT exceed an infusion rate of:
For continuous infusions, adjust rate as needed based on serum calcium levels | |||
Serum Calcium Monitoring
- Measure serum calcium every 4 to 6 hours during intermittent infusions with Calcium Gluconate Injection and measure serum calcium every 1 to 4 hours during continuous infusion.
Dosage in Renal Impairment
For patients with renal impairment, initiate Calcium Gluconate Injection at the lowest dose of the recommended dose ranges for all age groups and monitor serum calcium levels every 4 hours.
Adult dose for hypocalcemia
Calcium gluconate intravenous:
- 500 to 2000 mg (5 to 20 mL) IV one time at a rate not to exceed 0.5 to 2 mL/min. The dose may be increased as needed. The usual daily dosage ranges from 1000 to 15,000 mg (10 to 150 mL) in divided doses or as a continuous infusion. Doses may be repeated every 1 to 3 days as needed and tolerated to normalize the serum calcium level.
Calcium gluconate oral:
- 500 to 2000 mg orally 2 to 4 times a day.
Adult dose for hypermagnesemia
1000 to 2000 mg (10 to 20 mL) IV one time at a rate not to exceed 0.5 to 2 mL/min. This dose may be repeated as necessary in severe cases of hypermagnesemia (where discontinuation of exogenous magnesium is inadequate) to temporarily reverse many of the toxic effects of magnesium in the central nervous system.
Adult dose for hyperkalemia
500 to 3000 mg (5 to 30 mL) IV one time at a rate not to exceed 0.5 to 2 mL/min. This dose may be repeated as necessary in cases of extreme hyperkalemia cardiotoxicity when P waves are absent, the QRS complexes are widened, and when continuous ECG monitoring is available. The use of calcium does not reduce the serum potassium level, but counteracts the effects of hyperkalemia on cardiac excitability.
Adult dose for exchange transfusion
300 mg (3 mL) IV one time with each 100 mL of citrated blood at a rate not to exceed 0.5 to 2 mL/min.
Adult dose for osteoporosis
1000 to 1500 mg/day orally in divided doses.
Pediatric dose for hypocalcemia
Neonatal:
Recommended daily allowance (RDA): (Dosage is in terms of elemental calcium):
- Oral: 400 mg/day
- Daily maintenance calcium:
- IV: 3 to 4 mEq/kg/day
Cardiac arrest in the presence of hyperkalemia or hypocalcemia, magnesium toxicity, or calcium antagonist toxicity: Dosage expressed in mg of calcium gluconate:
IV or intraosseous IO:
60 to 100 mg/kg/dose; may repeat in 10 minutes if necessary. If effective, consider IV infusion.
Hypocalcemia (dose depends on clinical condition and serum calcium level):
- IV: (Dose expressed in mg of calcium gluconate): 200 to 800 mg/kg/day as a continuous infusion or in 4 divided doses
- Oral: (Dosage expressed in mg of elemental calcium): 50 to 150 mg/kg/day in 4 to 6 divided doses
- Do not exceed 1 g/day
Dose expressed in mg of calcium gluconate:
- 500 to 1500 mg/kg/day in 4 to 6 divided doses
Hypocalcemia secondary to citrated blood infusion:
- IV: Give 0.45 mEq elemental calcium for each 100 mL citrated blood infused
Tetany: (Dose expressed in mg of calcium gluconate):
- IV: 100 to 200 mg/kg/dose over 5 to 10 minutes; may repeat after 6 hours or follow with an infusion with a maximum dose of 500 mg/kg/day
Dosing: Usual
Adequate intake (AI): (Dosage is in terms of elemental calcium):
Oral:
- 1 to 6 months: 210 mg/day
- 7 to 12 months: 270 mg/day
- 1 to 3 years: 500 mg/day
- 4 to 8 years: 800 mg/day
- 9 to 18 years: 1300 mg/day
Recommended daily allowance (RDA): (Dosage is in terms of elemental calcium):
Oral:
- 1 to 6 months: 400 mg/day
- 6 to 12 months: 600 mg/day
- 1 to 10 years: 800 mg/day
- 11 to 24 years: 1200 mg/day
Hypocalcemia (dose depends on clinical condition and serum calcium level):
Oral: (Dose expressed in mg of elemental calcium):
- Children: 45 to 65 mg/kg/day in 4 divided doses
- Dose expressed in mg of calcium gluconate: Infants and Children: 500 to 725 mg/kg/day in 3 to 4 divided doses
Hypocalcemia (dose depends on clinical condition and serum calcium level):
IV: (Dose expressed in mg of calcium gluconate):
- Infants and Children: 200 to 500 mg/kg/day as a continuous infusion or in 4 divided doses
Cardiac arrest in the presence of hyperkalemia or hypocalcemia, magnesium toxicity, or calcium antagonist toxicity:
IV, IO: (Dosage expressed in mg of calcium gluconate):
- Infants and Children: 60 to 100 mg/kg/dose (maximum: 3 g/dose); may repeat in 10 minutes if necessary; if effective, consider IV infusion.
Hypocalcemia secondary to citrated blood infusion:
- IV: Give 0.45 mEq elemental calcium for each 100 mL citrated blood infused
Tetany:
- IV: (Dose expressed in mg of calcium gluconate): Infants and Children: 100 to 200 mg/kg/dose; over 5 to 10 minutes; may repeat after 6 hours or follow with an infusion with a maximum dose of 500 mg/kg/day.
Daily maintenance calcium:
IV:
- Infants and Children 25 kg and less: 1 to 2 mEq/kg/day
- Children 25 to 45 kg: 0.5 to 1.5 mEq/kg/day
- Children greater than 45 kg: 0.2 to 0.3 mEq/kg/day or 10 to 20 mEq/day
Renal dose adjustments
Patients with renal dysfunction have an increased risk of hypercalcemia. Periodically checking the serum calcium level, especially if signs or symptoms of hypercalcemia are detected, is recommended.
Liver dose adjustments
Data not available
Dialysis
Calcium is removed by hemodialysis. To ensure a positive net calcium flux into the patient during dialysis, a dialysate calcium concentration of 3.0 to 3.5 mEq/L is usually required. Mid-dialysis hypercalcemia is not uncommon when this concentration is used.
Calcium is removed by peritoneal dialysis. The standard peritoneal dialysate contains 3.5 mEq/L of calcium (in 1.5% dextrose) to maintain a positive calcium balance and to prevent calcium losses. When higher concentrations of dextrose are used, the net calcium balance may be negative because of a greater convective removal of calcium during ultrafiltration, which counterbalances the diffusion of calcium from the dialysate to the patient.
Calcium gluconate side effects
Do not use calcium gluconate if you had an allergic reaction to calcium gluconate.
Call your doctor right away if you notice any of these side effects:
- Allergic reaction: Itching or hives, swelling in your face or hands, swelling or tingling in your mouth or throat, chest tightness, trouble breathing
- Feeling of warmth, tingling, or confusion
- Lightheadedness or fainting
- Slow or irregular heartbeat
- Little or no urinating
- Swelling, rapid weight gain
- High levels of calcium in your blood–nausea, vomiting, constipation, increased thirst or urination, muscle weakness, bone pain, confusion, lack of energy, or feeling tired.
If you notice these less serious side effects, talk with your doctor:
- Constipation, loss of appetite, nausea, vomiting, stomach pain
- Dry mouth, thirst, chalky taste in your mouth
- Increase in how much or how often you urinate
- Redness, swelling, irritation, or infection on the skin where the injection is given
Common side effects may include:
- upset stomach, gas; or
- constipation.
If you notice other side effects that you think are caused by calcium gluconate, tell your doctor.
Call your doctor for medical advice about side effects.
Calcium gluconate injection contains aluminum, up to 400 mcg per liter, that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum. Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 mcg/kg/day to 5 mcg/kg/day accumulate aluminum levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Psychiatric
Psychiatric side effects have been reported rarely. They have included a single case of mania possibly associated with changes in cerebrospinal fluid and serum calcium following calcium gluconate administration.
A 35-year-old woman with an organic mental disorder associated with hypocalcemia and hypomagnesemia (secondary to short bowel syndrome) became noncompliant with her intramuscular injections of magnesium (Mg). Upon evaluation for progressive confusion and dysphoria, her serum Mg and calcium were found to be 0.3 mEq/L and 7.1 mg/dl, respectively. Intravenous Mg sulfate and calcium gluconate (6,000 mg) were given, with subsequent clearing of her sensorium. Within 12 hours the patient became manic and grandiose; associated Mg and calcium levels were 1.4 mEq/L and 8.2 mg/L, respectively. The symptoms of mania resolved without psychotropic medications and with discontinuation of calcium gluconate. This case report and other limited data suggest that mania can be precipitated by the rapid intravenous administration of calcium gluconate, particularly in persons who are predisposed to affective disorders.
Genitourinary
Genitourinary side effects have rarely included calcium nephrolithiasis. This effect has been reported more commonly with coadministration of loop diuretics.
Cardiovascular
A 51-year-old man with no history of cardiac disease was referred for calcium gluconate/pentagastrin testing for early detection of medullary thyroid carcinoma. He became pulseless and hypotensive within 15 seconds after receiving calcium gluconate 2 mg/kg and pentagastrin 0.5 mcg/kg. Atrial fibrillation and a ventricular rate response of 110/min ensued after a precordial thump. A complete evaluation for the cause of his atrial fibrillation was negative.
Cardiovascular side effects have included peripheral vasodilation, hypotension, syncope, vasospastic angina, serious cardiac arrhythmias, AV dissociation, and shock. Extreme caution is advised when parenteral calcium is given to a patient who has received digitalis since calcium may unmask digitalis intoxication. A single case of new atrial fibrillation has been reported.
Renal
Renal side effects have included significant increases in renal plasma flow, glomerular filtration rate, diuresis, natriuresis, and prostaglandin E2 and F1-alpha levels. However, the renal side effects do not appear to be clinically significant.
The renal effects of calcium gluconate include significant increases in renal plasma flow, glomerular filtration rate, diuresis, natriuresis, and prostaglandin E2 and F1-alpha levels. Data indicate that calcium gluconate infusions at subpressor doses have renal vasodilating, diuretic and natriuretic properties that appear to be facilitated by an increase in the renal production of prostaglandins.
Nervous system
Nervous system side effects have included tingling sensations, a “chalky” taste and a sense of oppression or “heat wave.”
Calcium gluconate antidote
There is no specific antidote for calcium salts poisoning. Calcium is rapidly cleared by hemodialysis. However, dialysis is rarely indicated unless the patient has renal failure.
A serum calcium concentration exceeding 2.6 mmol per liter (10.5 mg per 100 mL) is considered a hypercalcemic condition. Withholding additional administration of calcium and any other medications that may cause hypercalcemia usually resolves mild hypercalcemia in asymptomatic patients, when patient renal function is adequate 59.
Most cases of hypercalcemia can be treated with saline hydration. Gastrointestinal or skin irritation is usually self-limited and does not require specific treatment beyond decontamination. There is no role for bisphosphonates or calcitonin in the treatment of hypercalcemia due to calcium salt exposure.
Management of severe toxicity
Most cases of hypercalcemia will resolve with hydration in patients with normal renal function. Hemodialysis can be used if emergent clearance is required of if the patient’s renal function is impaired. There is no role for bisphosphonates or calcitonin in the treatment of hypercalcemia due to calcium salt exposure.
When serum calcium concentration are greater than 2.9 mmol per liter (12 mg per 100 mL), immediate measures may be required with possible use of the following: Hydrating with intravenous 0.9% sodium chloride injection 59. Forcing diuresis with furosemide or ethacrynic acid may be used to rapidly increase calcium and sodium excretion when saline overload occurs. Monitoring of potassium and magnesium serum concentrations and starting replacement early to prevent complications of therapy. ECG monitoring and the possible use of beta-adrenergic blocking agents to protect the heart against serious arrhythmias. Possibly including hemodialysis, calcitonin, and adrenocorticoids in the treatment. Determining serum calcium concentration at frequent intervals to guide therapy adjustments 59.
- Calcium gluconate. GRN No. 136 https://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=136[↩]
- Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Calcium. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/[↩][↩]
- Michaelsson K, Melhus H, Warensjo Lemming E, Wold A, Byberg L. Long term calcium intake and rates of all cause and cardiovascular mortality: community based prospective longitudinal cohort study. BMJ 2013;12;346:f228[↩][↩][↩]
- National Institutes of Health. Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement Online 2000:1-45[↩]
- Riggs BL, Melton L. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 1995;17:505S-11S.[↩]
- National Osteoporosis Foundation. https://www.nof.org/interdisciplinary-symposium-on-osteoporosis/[↩]
- National Osteoporosis Foundation. https://www.nof.org/patients/diagnosis-information/[↩]
- Cranney A, Horsley T, O’Donnell S, Weiler HA, Puil L, Ooi DS, Atkinson SA, Ward LM, Moher D, Hanley DA, Fang M, Yazdi F, Garritty C, Sampson M, Barrowman N, Tsertsvadze A, Mamaladze V. Effectiveness and Safety of Vitamin D in Relation to Bone Health. Evidence Report/Technology Assessment No. 158 (Prepared by the University of Ottawa Evidence-based Practice Center (UO-EPC) under Contract No. 290-02-0021. AHRQ Publication No. 07-E013. Rockville, MD: Agency for Healthcare Research and Quality. August 2007.[↩]
- Bolland MJ, Leung W, Tai V, Bastin S, Gamble GD, Grey A, Reid IR. Calcium intake and risk of fracture: systematic review. BMJ 2015;351:h4580 doi: 10.1136/bmj.h4580.[↩]
- Tai V, Leung W, Grey A, Reid IR, Bolland MJ. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 2015;351:h4183 doi: 10.1136/bmj.h4183.[↩]
- Moyer VA. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013;158:691-6.[↩]
- Guidance for Industry: Health Claims on Calcium and Osteoporosis; and Calcium, Vitamin D, and Osteoporosis. https://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/ucm152626.htm[↩]
- Biasco G, Paganelli M. European trials on dietary supplementation for cancer prevention. Ann N Y Acad Sci 1999;889:152-6.[↩]
- Bonithon-Kopp C, Kronborg O, Giacosa A, Rath U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet. 2000;356:1300-6.[↩]
- Grau MV, Baron JA, Sandler RS, Wallace K, Haile RW, Church TR, et al. Prolonged effect of calcium supplementation on risk of colorectal adenomas in a randomized trial. J Natl Cancer Inst 2007;99:129-36.[↩]
- Wu K, Willett WC, Fuchs CS, Colditz GA, Giovannucci EL. Calcium intake and risk of colon cancer in women and men. J Natl Cancer Inst 2002;94:437-46.[↩]
- Cascinu S, Del Ferro E, Cioccolini P. Effects of calcium and vitamin supplementation on colon cancer cell proliferation in colorectal cancer. Cancer Invest 2000;18:411-6.[↩]
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684-96[↩]
- Weingarten MA, Zalmanovici A, Yaphe J. Dietary calcium supplementation for preventing colorectal cancer and adenomatous polyps. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD003548[↩]
- Mitrou PN, Albanes D, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, et al. A prospective study of dietary calcium, dairy products and prostate cancer risk (Finland). Int J Cancer 2007;120:2466-73.[↩]
- Chan JM, Pietinen P, Virtanen M, Chan JM, Pietinen P, Virtanen M, et al. Diet and prostate cancer risk in a cohort of smokers, with a specific focus on calcium and phosphorus (Finland). Cancer Causes Control 2000;11:859-67.[↩]
- Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst 2005;97:1768-77.[↩]
- Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, Lichtenstein A, Patel K, Raman G, Tatsioni A, Terasawa T, Trikalinos TA. Vitamin D and Calcium: Systematic Review of Health Outcomes. Evidence Report/Technology Assessment No. 183. (Prepared by Tufts Evidence-based Practice Center under Contract No. 290-2007-10055-I). AHRQ Publication No. 09-E015, Rockville, MD: Agency for Healthcare Research and Quality. August 2009[↩]
- Bostick RM, Kushi LH, Wu Y, Meyer KA, Sellers TA, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol. 1999 Jan 15;149(2):151-61.[↩]
- Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, Lichtenstein A, Patel K, Raman G, Tatsioni A, Terasawa T, Trikalinos TA. Vitamin D and Calcium: Systematic Review of Health Outcomes. Evidence Report/Technology Assessment No. 183. (Prepared by Tufts Evidence-based Practice Center under Contract No. 290-2007-10055-I). AHRQ Publication No. 09-E015, Rockville, MD: Agency for Healthcare Research and Quality. August 2009.[↩][↩][↩][↩][↩]
- Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, Reid IR.Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010 Jul 29;341:c3691[↩]
- Xiao Q, Murphy RA, Houston DK, Harris TB, Chow WH, Park Y. Dietary and Supplemental Calcium Intake and Cardiovascular Disease Mortality: The National Institutes of Health-AARP Diet and Health Study. JAMA Intern Med. 2013 Feb 4:1-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3756477/[↩]
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011 Apr 19;342:d2040 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3079822/[↩][↩][↩]
- Wang L, Manson JE, Sesso HD. Calcium intake and risk of cardiovascular disease: a review of prospective studies and randomized clinical trials. Am J Cardiovasc Drugs 2012;12:105-16[↩]
- Chung M, Tang AM, Fu Z, Wang DD, Newberry SJ. Calcium intake and cardiovascular disease risk: An updated systematic review and meta-analysis. Ann Intern Med. Published online ahead of print on October 25, 2016.[↩]
- Kopecky SL, Bauer DC, Giulati M, Nieves JW, Singer AJ, Toth PP, UInderberg JA, Wallace TC, Weaver C. Lack of evidence linking calcium with or without vitamin D supplementation to cardiovascular disease in generally healthy adults: A clinical guideline from the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Ann Intern Med. Published online ahead of print on October 25, 2016.[↩]
- McCarron D, Reusser M. Finding consensus in the dietary calcium-blood pressure debate. J Am Coll Nutr 1999;18:398S-405S.[↩]
- Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008 Apr;51(4):1073-9.[↩]
- Dickinson HO, Nicolson DJ, Cook JV, Campbell F, Beyer FR, Ford GA, et al. Calcium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev 2006;(2):CD004639[↩]
- Berkow SE, Barnard ND. Blood pressure regulation and vegetarian diets. Nutr Rev 2005;63:1-8.[↩]
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117-24[↩]
- Cudihy D, Lee RV. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynaecol. 2009;29:576-82.[↩][↩]
- World Health Organization. Guideline: Calcium supplementation in pregnant women. http://apps.who.int/iris/bitstream/handle/10665/85120/9789241505376_eng.pdf[↩][↩]
- Kumar A, Devi SG, Batra S, Singh C, Shukla DK. Calcium supplementation for the prevention of pre-eclampsia. Int J Gynaecol Obstet. 2009;104:32-6.[↩]
- Levine RJ, Hauth JC, Curet LB, Sibai BM, Catalano PM, Morris CD, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337:69-76.[↩]
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy[↩]
- Omotayo MO, Dickin KL, O’Brien KO, Neufeld LM, De Regil LM, Stoltzfus RJ. Calcium supplementation to prevent preeclampsia: translating guidelines into practice in low-income countries. Adv Nutr. 2016;7:275-8.[↩]
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36:416-41.[↩]
- Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97-104.[↩]
- Lowe SA, Bowyer L, Lust K, McMahon LP, Morton M, North RA, et al. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol. 2015;55:e1-29[↩][↩]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669-83.[↩]
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997 Apr 1;126(7):497-504.[↩]
- Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002;346:77-84.[↩]
- Davies KM, Heaney RP, Recker RR, Lappe JM, Barger-Lux MJ, Rafferty K, et al. Calcium intake and body weight. J Clin Endocrinol Metab 2000;85:4635-8.[↩]
- Parikh SJ, Yanovski JA. Calcium intake and adiposity. Am J Clin Nutr 2003;77:281-7[↩][↩]
- Zemel MB, Shi H, Greer B, DiRienzo D, Zemel P. Regulation of adiposity by dietary calcium. FASEB J 2000;14:1132-8.[↩]
- Zemel MB, Richards J, Mathis S, Milstead A, Gebhardt L, Silva E. Dairy augmentation of total and central fat loss in obese subjects. Int J Obes 2005;29:391-7.[↩]
- Chen M, Pan A, Malik VS, Hu FB. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:735-747.[↩][↩]
- 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. https://www.ahajournals.org/doi/pdf/10.1161/circulationaha.110.971069[↩][↩][↩][↩]
- Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 741[↩]
- Puvabanditsin S et al; Pediatric Radiology 35 (5): 539-42, 2005 https://www.ncbi.nlm.nih.gov/pubmed/15565339[↩][↩]
- Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 742[↩]
- Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 746[↩][↩][↩]