Contents
- What is Osteosarcoma
- Types of osteosarcoma
- Osteosarcoma causes
- Osteosarcoma symptoms
- Osteosarcoma complications
- Osteosarcoma diagnosis
- Osteosarcoma staging
- Osteosarcoma treatment
- Osteosarcoma prognosis
What is Osteosarcoma
Osteosarcoma also called osteogenic sarcoma is a rare bone cancer that usually affects the large bones of the arm or leg (more common in the knee and upper arm), but can affect other bones too. In very rare instances, osteosarcoma occurs in soft tissue outside the bone. Osteosarcoma occurs most commonly in children, teens, and young adults and affects more males than females. Osteosarcoma usually develops during the adolescent growth spurt usually ages 13 to 16 in boys but a little younger in girls. Average age at diagnosis is 15. Osteosarcoma is also common in people over age 60. Osteosarcoma causes pain in the bone, which may be worse during exercise or at night. A lump or swelling may form.
Osteosarcoma tends to occur in the bones of the:
- Shin (near the knee)
- Thigh (near the knee)
- Upper arm (near the shoulder)
Osteosarcoma occurs most commonly in large bones in the area of bone with the fastest growth rate. However, it can occur in any bone.
Pain at the site of the tumor in the bone is the most common symptom of osteosarcoma 1. The most common sites for these tumors in younger people are around the knee or in the upper arm, but they can occur in other bones as well. At first, the pain might not be constant and might be worse at night. The pain often increases with activity and might result in a limp if the tumor is in a leg bone.
Swelling in the area is another common symptom, although it might not occur until later 1. Depending on where the tumor is, it might be possible to feel a lump or mass 1.
Limb pain and swelling are very common in normal, active children and teens. They are much more likely to be caused by normal bumps and bruises, so they might not prompt a doctor visit right away. This can delay a diagnosis. If your child has these symptoms and they don’t go away within a few weeks (or they get worse), see a doctor so that the cause can be found and treated, if needed.
These symptoms are less common in adults, so they should be a sign to see a doctor even sooner 1.
Osteosarcoma is not a common cancer. Each year, about 1,000 new cases of osteosarcoma are diagnosed in the United States 2. About half of these are in children and teens. Most osteosarcomas occur in children, teens, and young adults between the ages of 10 and 30 2. Teens are the most commonly affected age group, but people of any age can develop osteosarcoma. About 1 in 10 osteosarcomas occur in people older than 60 2.
About 2% of childhood cancers are osteosarcomas, but they make up a much smaller percentage of adult cancers 2.
The cause of osteosarcoma is not known. In some cases, osteosarcoma runs in families. At least one gene has been linked to an increased risk. This gene is also associated with familial retinoblastoma. Retinoblastoma is a cancer of the eye that occurs in children.
If your symptoms are pointing to a bone tumor such as an osteosarcoma, here are some of the tests that might be done to find out more:
- Bone x-ray: This is often the first test done if a doctor suspects a bone tumor.
- MRI (magnetic resonance imaging) scan: MRIs use radio waves and strong magnets instead of x-rays to take pictures. MRIs can show details about the bone and nearby areas. This helps the doctor plan for a biopsy (see below) and surgery.
- CT scan (CAT scan): This test uses x-rays to make detailed pictures of a person’s insides. A CT scan of the chest may be done to see if cancer has spread to the lungs.
- Bone scan: A bone scan can help show if a cancer has spread to other bones. This test is useful because it can show all of the bones in the body at once.
- PET scan: PET scans use a special kind of sugar that can be seen inside the body with a special camera. If there is cancer, this sugar shows up as “hot spots” where the cancer is found. This test can help show if the cancer has spread, as well as if the cancer is being helped by treatment. PET scans are sometimes done at the same time as a CT scan (known as a PET/CT scan).
- Bone biopsy. In a bone biopsy, the doctor takes out a small piece of bone to check it for cancer cells. A biopsy is the only way to tell for sure if a person has a bone cancer like osteosarcoma. For tumors in a bone, the biopsy should be done by doctors who often treat bone tumors. The biopsy and surgery to remove the tumor should be planned at the same time, and the same doctors should do both. This can help prevent problems later on. The biopsy can be done either by surgery, or by putting a hollow needle into the bone. Ask your doctor what kind will be done.
Osteosarcoma treatment usually starts after a biopsy of the tumor is done. Treatment usually involves chemotherapy, surgery and, sometimes, radiation therapy (radiotherapy). Doctors select treatment options based on where the osteosarcoma starts, the size of the cancer, the type and grade of the osteosarcoma, and whether the cancer has spread beyond the bone.
Before surgery to remove the tumor, chemotherapy, radiation therapy or both is usually given. This can shrink the tumor and make surgery easier. It may also kill any cancer cells that have spread to other parts of the body.
Surgery is used after chemotherapy to remove any remaining tumor. In most cases, surgery can remove the tumor while saving the affected limb. This is called limb-sparing surgery. In rare cases, more extensive surgery such as amputation is necessary.
Treatment innovations for osteosarcoma have greatly improved the prognosis (outlook) for people with osteosarcoma over the years, but depends on many factors, including where the tumor is, if the cancer has already spread (metastasized) when it’s first found, and the person’s age. After completion of osteosarcoma treatment, lifelong monitoring is recommended to watch for potential late effects of intense treatments.
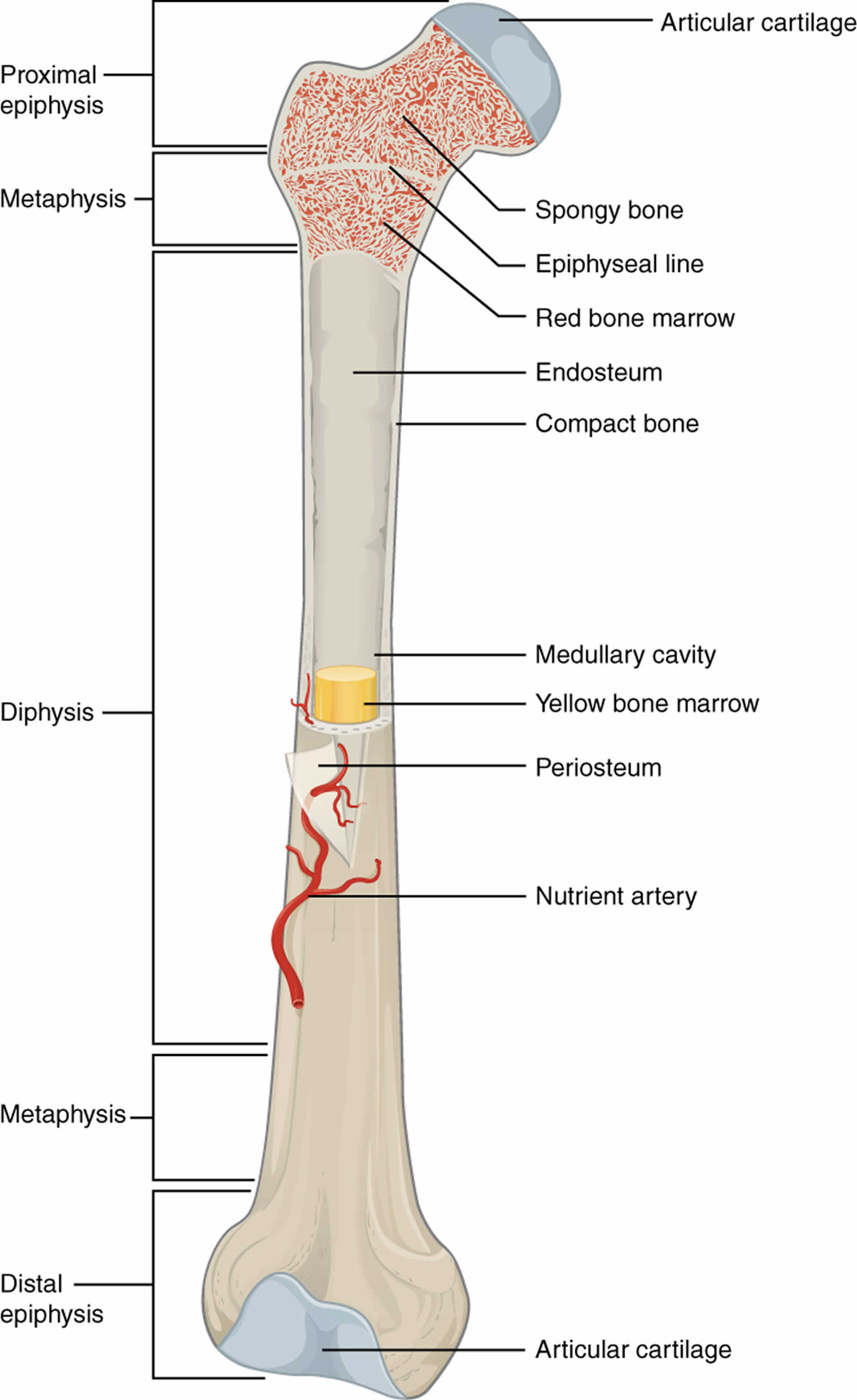
Figure 1. Long bone anatomy
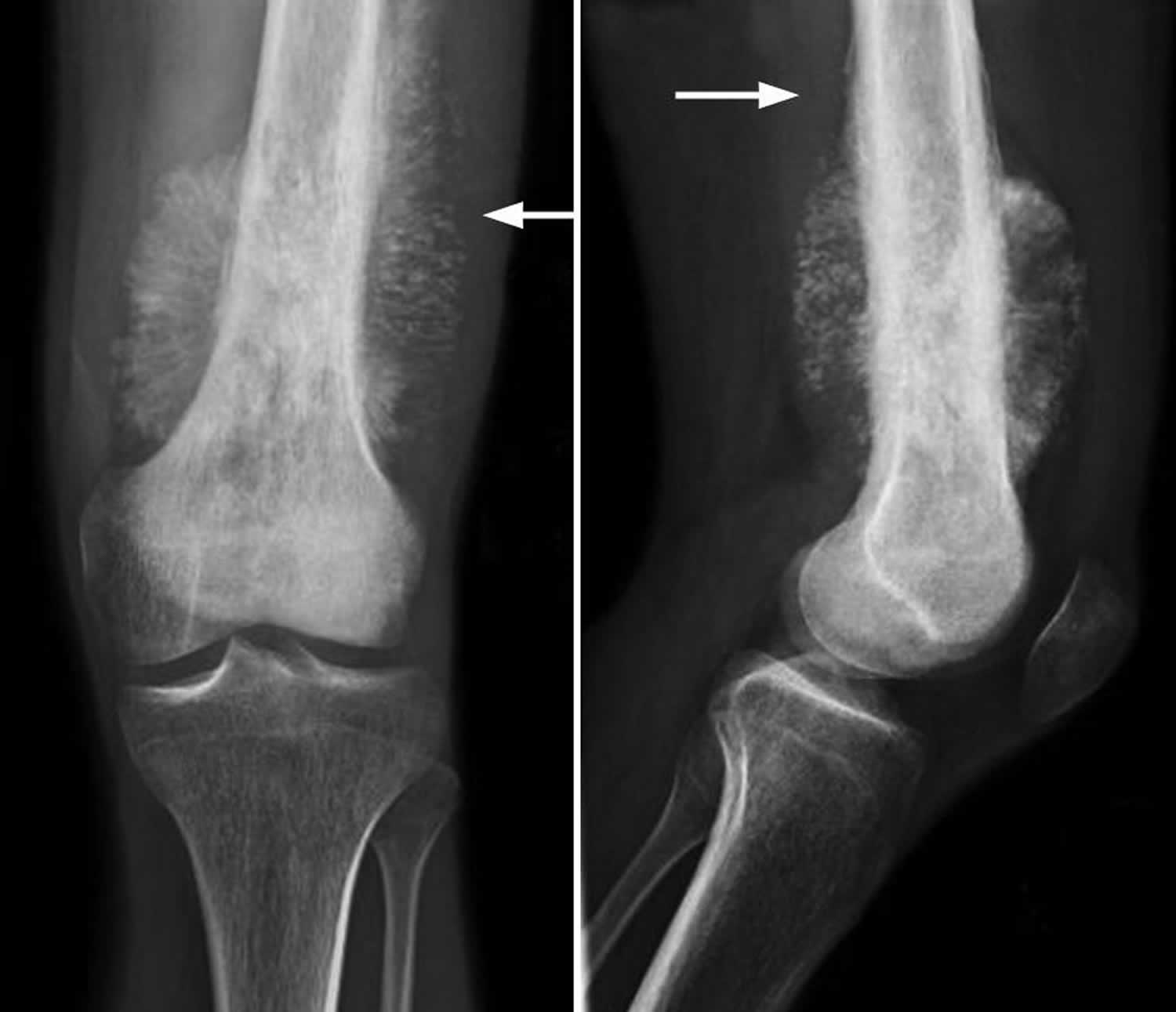
Figure 2. Osteosarcoma knee
Footnote: (Left) X-ray shows an osteosarcoma in the femur (thighbone). Note the formation of new bone in a typical “sunburst” pattern. (Right) When viewed from the side, a Codman triangle can also be seen rising from the bone.
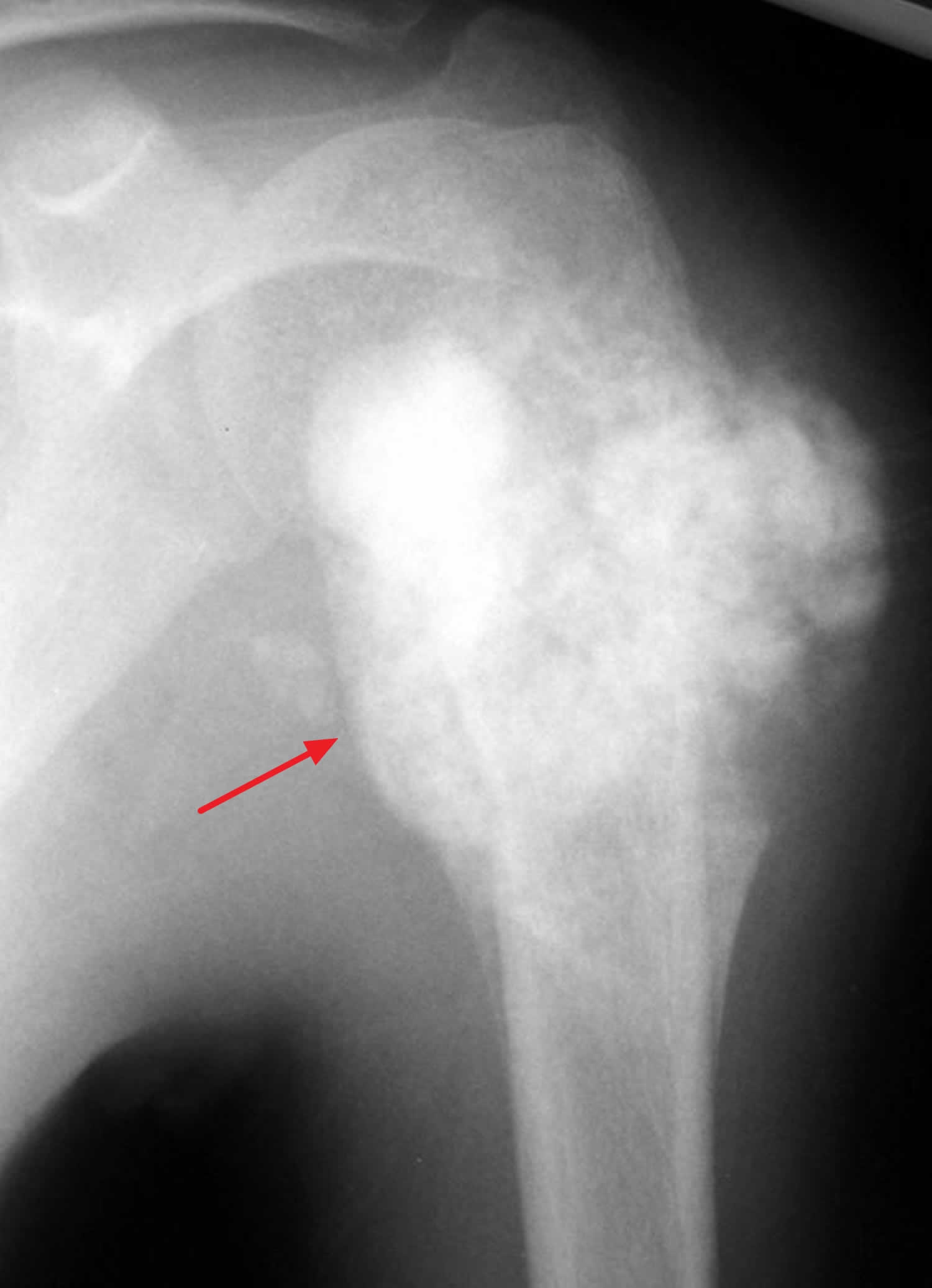
[Source 3 ]Figure 3. Osteosarcoma humerus
Footnote: An osteosarcoma in the upper humerus (arm bone). The tumors often appear as cloud-like lesions on X-ray.
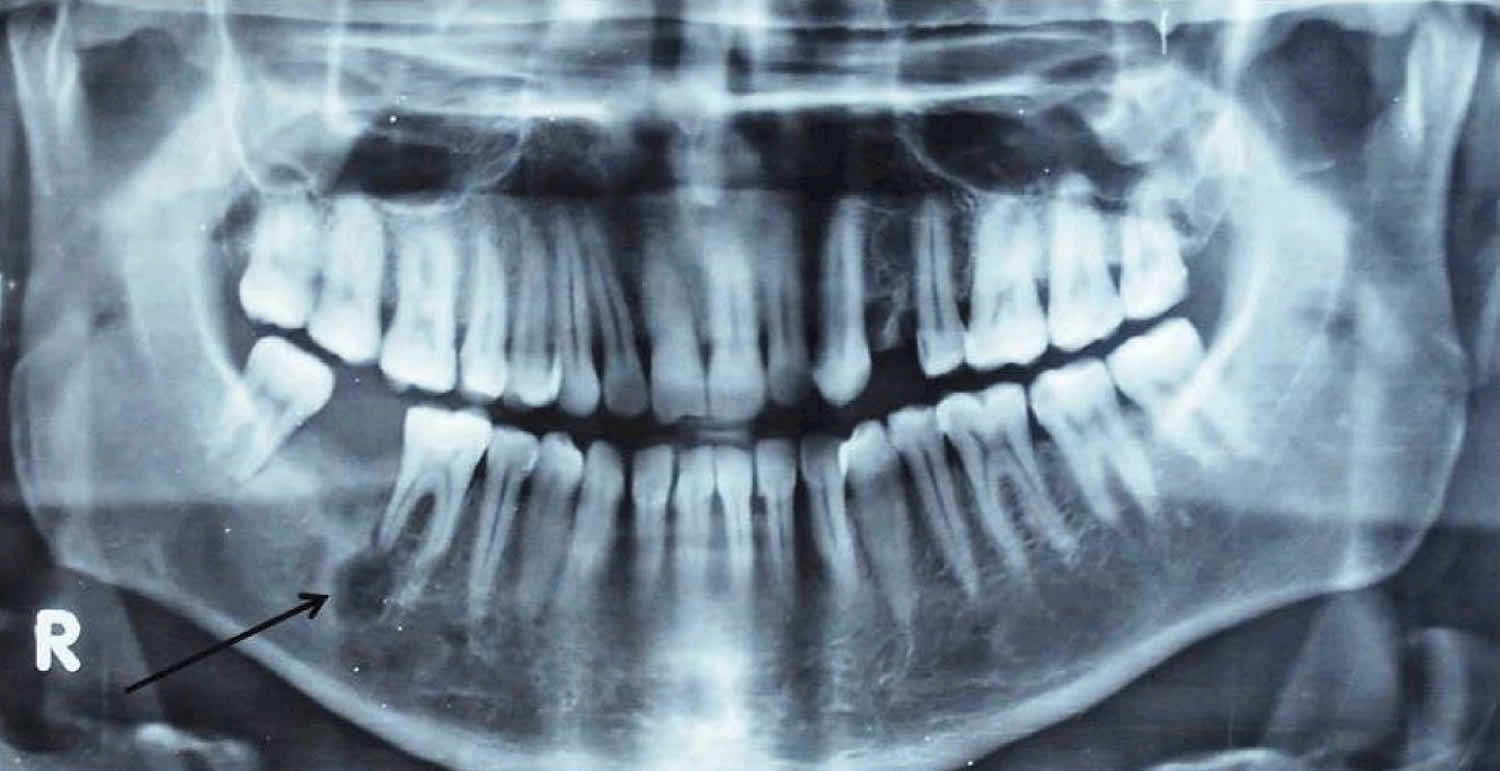
[Source 4 ]Figure 4. Osteosarcoma mandibular (osteosarcoma jaw)
Footnote: Orthopantomogram (OPG) showing 0.5 x 0.5 cm radiolucent area at root apex of mandibular right first molar.
[Source 5 ]Figure 5. Osteosarcoma skull
Footnote: Skull X-ray showing tumour bone formation with a sunburst-pattern.
[Source 6 ]Where does osteosarcoma start?
In children, teens, and young adults, osteosarcoma usually starts in areas where the bone is growing quickly, such as near the ends of the leg or arm bones:
- Most tumors develop in the bones around the knee, either in the lower part of the thigh bone (distal femur) or the upper part of the shinbone (proximal tibia).
- The upper arm bone close to the shoulder (proximal humerus) is the next most common site.
Still, osteosarcoma can develop in any bone, including the bones of the pelvis (hips), shoulder, and jaw. This is especially true in older adults.
Can osteosarcoma be found early?
At this time, there are no widely recommended screening tests for osteosarcoma in people who aren’t known to be at increased risk. Screening is testing for cancer in people without any symptoms.
Still, most osteosarcomas are found at an early stage, before they have clearly spread to other parts of the body. Symptoms such as bone pain or swelling often prompt a visit to a doctor.
For some people who are at increased risk for osteosarcoma because they have certain bone diseases or inherited conditions, doctors might recommend increased monitoring for bone cancer. Osteosarcoma usually does not run in families, but watching for early signs and symptoms is important if it is to be treated successfully.
Make an appointment with your child’s doctor if your child has any persistent signs and symptoms that worry you. Osteosarcoma symptoms are similar to many more-common conditions, such as sports injuries, so your doctor may investigate those causes first.
Types of osteosarcoma
Based on how the cancer cells look under the microscope, osteosarcomas can be classified as high grade, intermediate grade, or low grade. The grade of the tumor tells doctors how likely it is that the cancer will grow quickly and spread to other parts of the body. The grade of the tumor plays a role in determining its stage and the type of treatment used.
High-grade osteosarcomas
High-grade osteosarcomas are the fastest growing types of osteosarcoma. When seen with a microscope, high-grade osteosarcomas do not look like normal bone, and many of the cancer cells are in the process of dividing into new cells. Most osteosarcomas that occur in children and teens are High-grade osteosarcomas. There are many types of high-grade osteosarcomas (although the first 3 are the most common).
- Osteoblastic
- Chondroblastic
- Fibroblastic
- Small cell
- Telangiectatic
- High-grade surface (juxtacortical high grade)
Other high-grade osteosarcomas include:
- Pagetoid osteosarcoma: a tumor that develops in someone with Paget disease of the bone
- Extraskeletal osteosarcoma: a tumor that starts in a part of the body other than a bone (but still makes bone tissue)
- Post-radiation osteosarcoma: a tumor that starts in a bone that had once been exposed to radiation
Intermediate-grade osteosarcomas
Intermediate-grade osteosarcomas are uncommon tumors that fall between high-grade and low-grade osteosarcomas. Intermediate-grade osteosarcomas are usually treated the same way as low-grade osteosarcomas.
- Periosteal (juxtacortical intermediate grade)
Low-grade osteosarcomas
Low-grade osteosarcomas are the slowest-growing osteosarcomas. Low-grade osteosarcomas look more like normal bone and have few dividing cells when seen with a microscope.
- Parosteal (juxtacortical low grade)
- Intramedullary or intraosseous well differentiated (low-grade central)
Parosteal osteosarcoma
Parosteal osteosarcoma is a subtype of osteosarcoma that arises from the outer fibrous layer of the periosteum 7. Parosteal osteosarcoma is the most common type of juxtacortical or surface osteosarcoma and accounts for ~4% of all osteosarcomas 8, 9, 10. Parosteal osteosarcoma typically presents in early adulthood and middle age with a peak incidence in the third decade. It affects females slightly more than males 8, 10.
Parosteal osteosarcomas might progress to a high-grade sarcoma including other osteosarcoma variants, undifferentiated spindle cell sarcoma or rhabdomyosarcoma 8.
Immunohistochemistry stains are usually reactive to MDM2 and CDK4 2,3 and might help in the differentiation to a variety of benign fibrous and fibro-osseous lesions such as reactive periostitis, juxtacortical myositis ossificans, fibrous dysplasia protuberans or desmoplastic fibroma as well in the diagnosis of dedifferentiated parosteal osteosarcoma 8, 10.
As parosteal osteosarcomas tend to be low-grade lesions, they are usually treated with surgical resection, without chemotherapy or radiation. As they are frequently metaphyseal in location, large parosteal osteosarcomas or those with deep medullary invasion may require limb salvage, including joint replacement.
Parosteal osteosarcomas have an excellent prognosis (80-95% long-term survival) 9.
Figure 6. Parosteal osteosarcoma
Footnotes: Parosteal osteosarcoma in a 24-year-old patient: (a) Anteroposterior radiograph of the right femur shows an ossified exophytic tumor on the surface of the femur. A lucent cleavage plane (arrow) is seen between the tumor and the underlying cortex. (b) Transversal CT image in soft tissue algorithm: ossified lesion developed at the surface of bone cortex with hypodense peripheral rim. (c) Transversal T1 MR image after Gadolinium administration and (d) sagittal T2 image. The lesion shows low T1 and T2 intensity with peripheral enhancement but no adjacent bone signal abnormality. (e) Gross specimen showing a large, gritty white mass pasted on the underlying cortex with no medullary invasion. The cut surface displayed a homogeneous appearance with lucent areas. A thin lucent line ( arrow) is seen between the tumor and bone and corresponds to the periosteum. (f) Lower-power photomicrograph showing a well-formed bony trabeculae in a hypocellular spindle cell stroma. The tumor is low grade attested by the slight nuclear pleomorphism of the cellular component (hematoxylin and eosin 25×).
[Source 11 ]Periosteal osteosarcoma
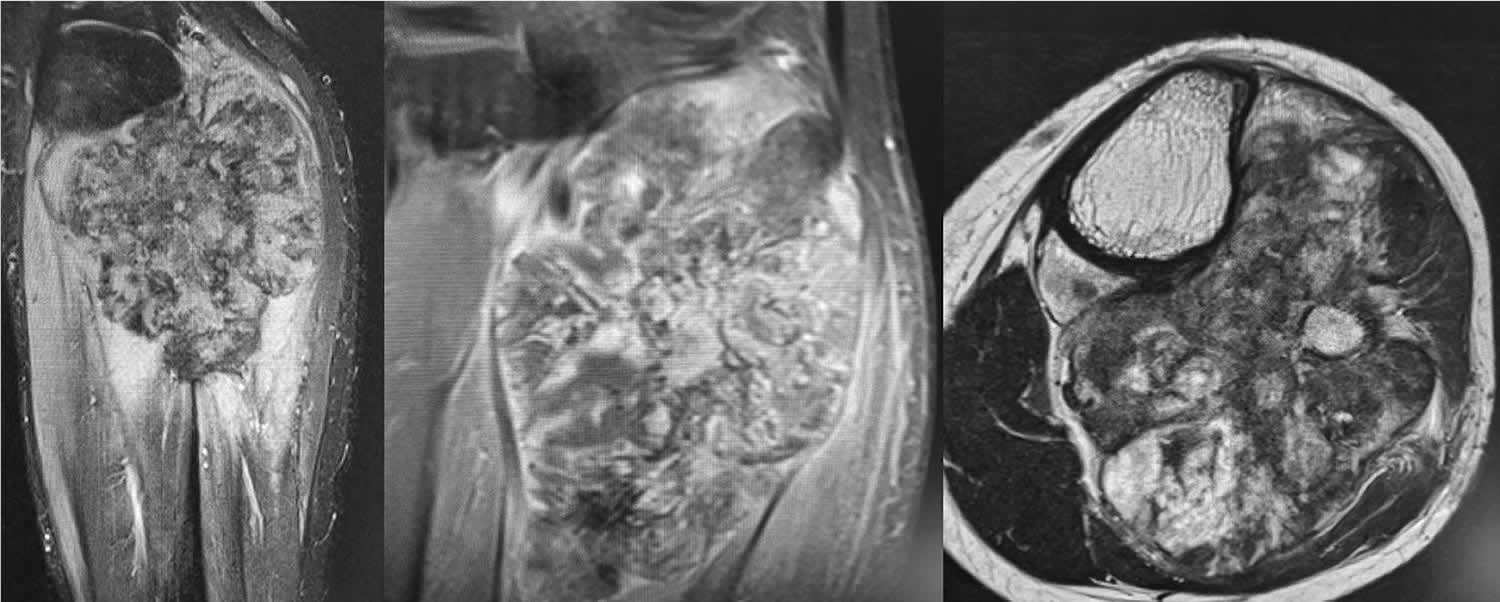
Periosteal osteosarcoma is the second most common type of surface-based osteosarcoma after parosteal osteosarcoma and account for 1-2% of all osteosarcomas 12, 13, 14, 15, 16, 17, 18, 19, 20. Periosteal osteosarcomas are intermediate-grade chondroblastic osteosarcoma originating from the surface of the bones typically underneath the periosteum that most commonly diagnosed in young patients in their second and third decades of life with a median age of 18 years 12. Periosteal osteosarcomas are seen in a wide age range from the first to the seventh decade with a peak in the second decade of life 21, 15, 22, 17.
Periosteal osteosarcoma usually affects the periosteum of long bones of the femur and tibia 23, 24, 25, 26. Distant metastases occur more commonly in the lungs and pleura; however, isolated cases of bone skip lesions and diaphragmatic metastases have also been reported 27, 28. Eventhough periosteal osteosarcomas have distinct specific radiological and pathological features, the current management strategies are based on only several case reports and series, without any solid recommendations 29, 30, 31, 32.
Controversy remains about the appropriate treatment of periosteal osteosarcoma. Most experts agree on the crucial role of wide surgical excision as mandatory for local control. With the lack of available reports, the role of chemotherapy and its timing remain debatable 12. As periosteal osteosarcoma is an intermediate-grade with the prognosis being better than conventional osteosarcoma, sometimes chemotherapy is included in the treatment protocol 14. Periosteal osteosarcoma is considered to have a good prognosis with an overall 5 and 10 year survival of 89% and 83%, respectively, with approximately 15% of patients developing metastasis 33, 34. Although the literature shows that adjuvant and neoadjuvant chemotherapy has increased the survival of patients with conventional osteosarcomas, the benefit of chemotherapy in periosteal osteosarcoma is doubtful, and several studies concluded that it does not improve the outcome or survival 33, 34, 13.
The important factor which affects metastasis-free survival is the radical resection of the tumor, preventing local recurrence, which is known to be a risk factor for the subsequent development of metastatic disease. Grimer et al. 34 recommended chemotherapy when the tumor is of high grade. They reported 17 cases treated over 16 years 34. No patient has developed metastases, and no deaths have resulted from recurrence or metastasis of the tumor 34. They suggested that chemotherapy was valuable but emphasized the importance of complete surgical excision 34.
Bone marrow invovement is rare but if present is associated with less favorable outcome 17. Treatment usually consists in wide excision. Chemotherapy does not seem to influence prognosis or survival and has been associated with the occurance of secondary malignancies in occasional cases 12. Local recurrences, metatsases and death usually occur within a period of 3 years after the diagnosis 17, 34.
Figure 7. Periosteal osteosarcoma
Footnotes: MRI findings of periosteal osteosarcoma: a high signal of heterogeneous intensity with a mass of the posterior fossa of the leg in contact with the posterior and medial cortical bone of the proximal metaphysis of the tibia; hypersignal T2-weighted images with a predominant similar signal intensity for the muscle on the T1-weighted images and heterogeneous enhancement. Focal areas of marrow replacement without continuity with the soft-mass component and the lesion margins are poly-lobulated.
[Source 12 ]Telangiectatic osteosarcoma
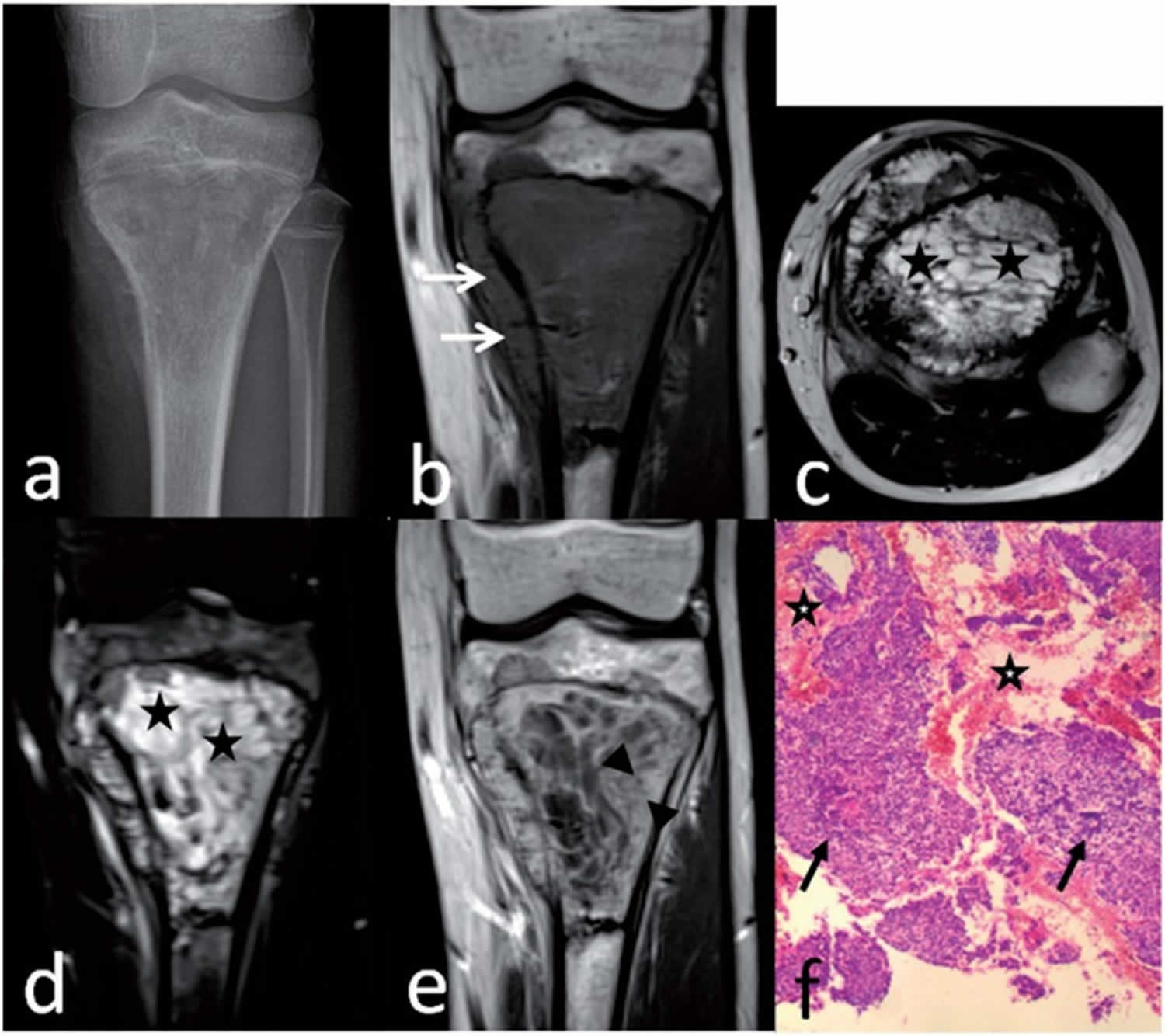
Telangiectatic osteosarcoma is a rare subtype of osteosarcoma that represents from 2% to 12% of all cases osteosarcoma 35, 36, 37, 38, 39, 40, 41, 42, 36, 37, 43. Telangiectatic osteosarcoma is characterized by multiple, aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells in the peripheral rim and septae 44. Telangiectatic osteosarcomas make up for 2-12% of high-grade osteosarcomas with varying frequencies between studies 36, 37, 43, 45, 46, 47. Telangiectatic osteosarcoma are associated with a high rate of pathological fractures and fracture risk is higher than conventional osteosarcoma 47, 48.
Telangiectatic osteosarcoma have similar demographics to that of conventional osteosarcoma and typically occur in adolescents and young adults (reported age range of 3-67 years ref with a median age of 15- 20 years) 45, 46, 46. There is a recognized male preference 45, 46, 46. To date detailed evaluation of imaging characteristics of telangiectatic osteosarcomas is limited 44, 49.
Identification of the unique histologic and radiographic features of telangiectatic osteosarcoma is crucial for correct diagnosis. Nevertheless, in many cases telangiectatic osteosarcoma is misdiagnosed. For example, telangiectatic osteosarcoma can be easily confused with aneurysmal bone cyst 43, both radiologically and pathologically. Misdiagnosis always results in delay in initiation of proper therapy and potentially affects the prognosis 50, 49, 51, 52. In previous studies of telangiectatic osteosarcoma, the clinical significance of the serum alkaline phosphatase (ALP) and preoperative biopsy pathology has been unclear. The prognosis for patients with telangiectatic osteosarcoma has been debated in the literature for many decades 53, 54, 55.
Telangiectatic osteosarcoma destroys surrounding bone tissue and enlarges within several months 41. Extraosseous involvement into subcutaneous or dura mater is not common. Distant metastases are observed in 10% of patients, and distant metastases to the lungs are reportedly more common than metastases to other locations 56. As risk factors for distant metastases, onset in patients older than 30 years and large tumor size have been identified 56, 57.
The treatment of telangiectatic osteosarcoma is often similar to that of conventional osteosarcoma: chemotherapy followed by wide surgical resection and limb salvage or amputation.
The survival rate of telangiectatic osteosarcoma (estimated at ~70%) is similar to that of conventional osteosarcoma.
Figure 8. Telangiectatic osteosarcoma
Footnotes: Telangiectatic osteosarcoma of the proximal tibia in a 14-year-old boy. Anteroposterior radiograph (a) shows a geographic destruction with a wide transition. Coronal T1-weighted image (b) shows marrow replacement by heterogeneous tissue with cortical destruction and associated soft-tissue mass (arrows). Axial T2-weighted image (c) and coronal T2-weighted fat-suppressed image (d) shows predominately multiple small cystic spaces (stars). Coronal T1-weighted enhanced image (e) shows a thick rim and septa with nodularity (between arrowheads). Preoperative biopsy pathology (f) shows some blood spaces (stars), anaplastic malignant tumor cells (arrows), and neoplastic osteoid tissue, suggesting the diagnosis of telangiectatic osteosarcoma (hematoxylin–eosin stain; original magnification ×40).
[Source 35 ]Extraskeletal osteosarcoma
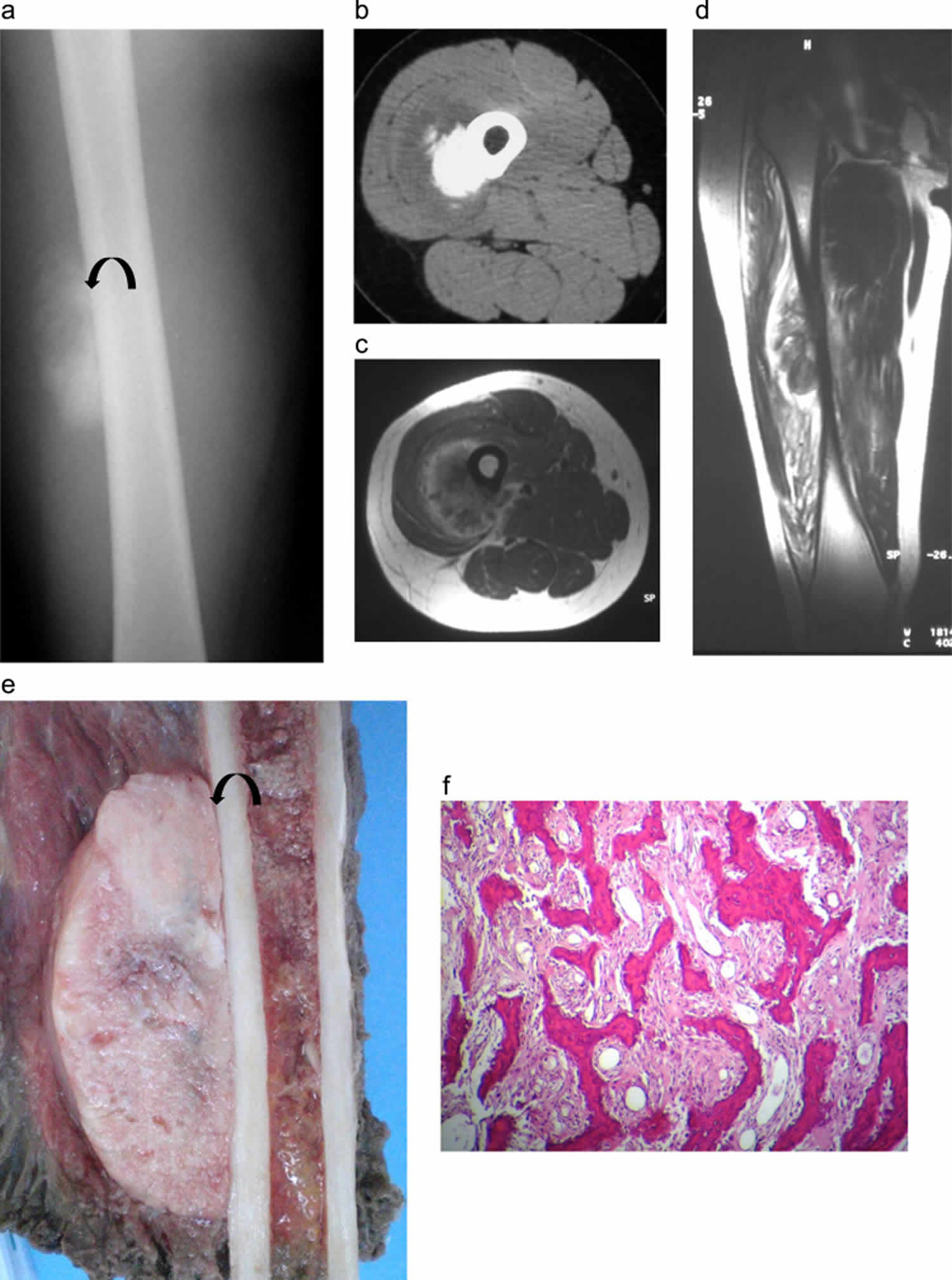
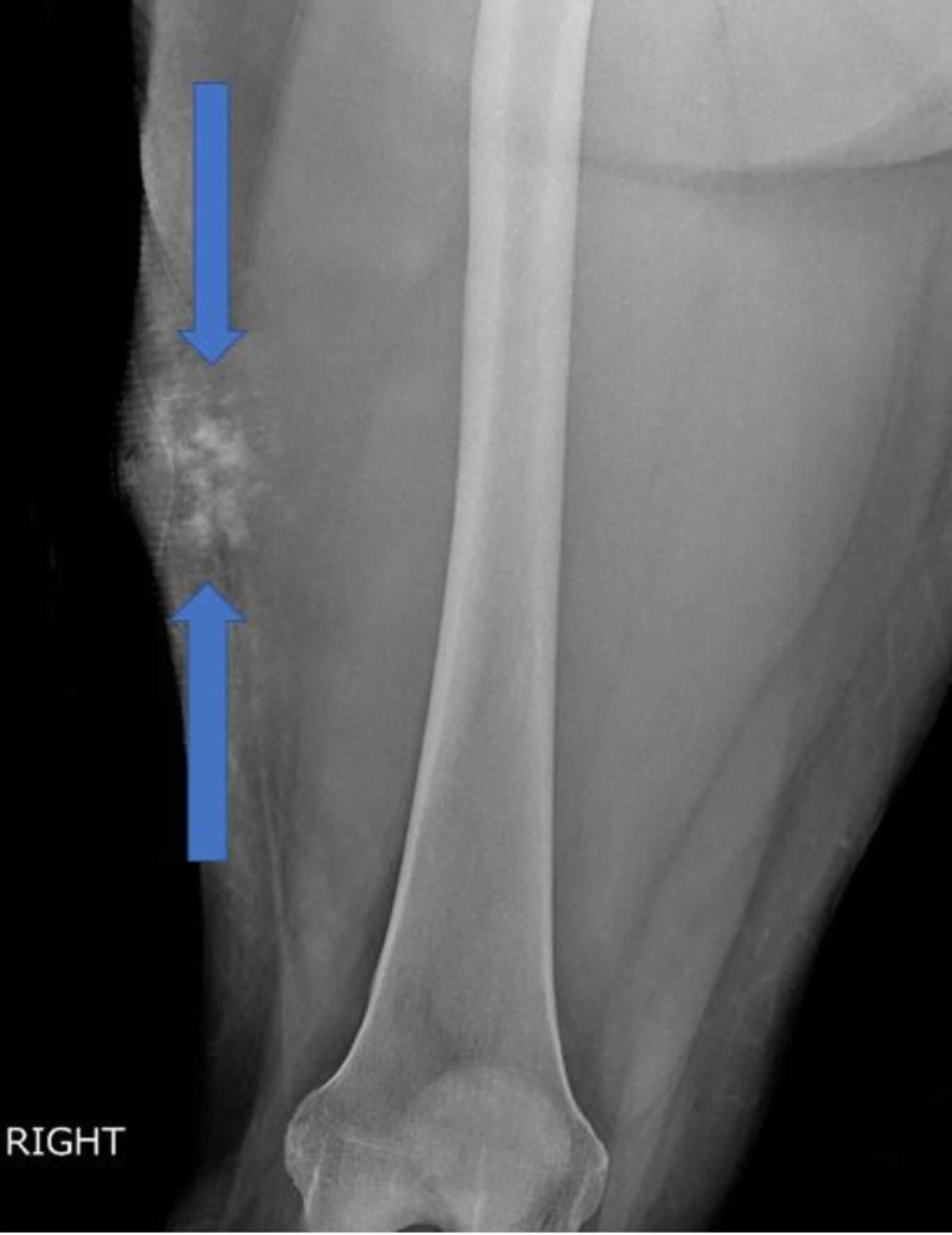
Extraskeletal osteosarcoma is an extremely rare highly aggressive sarcoma of the soft tissue that produces neoplastic osteoid or bone that occurs in the retroperitoneum and soft tissue of extremities without any attachment to bone 58, 59, 60, 61, 62. Extraskeletal osteosarcoma accounts for approximately 1-2% of all soft tissue sarcomas and 2-5% of all osteosarcomas 62. Extraskeletal osteosarcoma typically appears as a solid mass with variable mineralization and seldom has a cystic lesion. Extraskeletal osteosarcoma typically presents as a palpable heterogenous mineralized soft tissue mass, most commonly arising in the thigh which may be associated with a history of prior trauma or radiation therapy. Patients most commonly present with an enlarging soft-tissue mass either with or without pain 61.
Extraskeletal osteosarcoma in contrast to other subtypes of osteosarcoma occurs infrequently in individuals under 40 years of age, most commonly occurring in individuals 40 to 80 years of age with a median age of presentation of 61 years 58. Males are more commonly affected than females. Radiation exposure is a well-documented risk factor for the development of extraskeletal osteosarcoma. The role of trauma in development of extraskeletal osteosarcoma is unclear.
Extraskeletal osteosarcoma location 58:
- lower extremity (thigh): 50-60%
- upper extremity: 10-20%
- retroperitoneum: 10-15%
- trunk: 10-15%
Grossly, extraskeletal osteosarcoma is a well defined lesion with hemorrhagic and necrotic areas within 58. Microscopically extraskeletal osteosarcoma is typically a high grade spindle cell malignancy with osteoid and chondroid matrix. The histologic appearance of extraskeletal osteosarcoma resembles malignant fibrous histiocytoma, osteoblastic osteosarcoma and chondroblastic osteosarcoma.
Five pathologic subtypes of extraskeletal osteosarcoma are recognized, similar to conventional osteosarcoma:
- osteoblastic
- chondroblastic
- fibroblastic
- telangiectatic
- small cell
There is a lack of evidence regarding standardized treatment options for extraskeletal osteosarcoma given the rarity of the cancer 63. Treatment plans are typically based on standard treatment options for conventional osteosarcoma, and include wide surgical resection or amputation with doxorubicin and ifosfamide-based chemotherapy regimens 64, 65. Recent retrospective evaluation of patients with extraskeletal osteosarcoma favor more polychemotherapeutic regimens in addition to wide resection, and found 5-year overall survival rates of 77% and 66%. Radiation therapy has been postulated to have a positive effect on local tumor control 65.
The prognosis of extraskeletal osteosarcoma is poor, even when compared with conventional osteosarcoma 61. Metastasis and local recurrence are quite common with extraskeletal osteosarcoma. Up to 90% patients develop metastases at the time of presentation which makes the overall prognosis poor. The most common site for distant metastasis is lungs, followed by liver, bones, regional lymph nodes, peritoneum, adrenals, and rarely the brain 66. The duration from primary tumor resection to subsequent tumor recurrence can vary from 2 months up to 10 years 64. Tumor size helps predict outcomes with poorer survival for tumors greater than 5 cm; however, Roller et. al 67 suggest that lower grade tumors and absence of metastatic disease are more important prognostic features 68. Reported five-year survival varies, from as low as 28% in older studies to close to 47% in more recent studies 69, 70.
Figure 9. Extraskeletal osteosarcoma
Footnote: 46-year-old female with extraskeletal osteosarcoma of the right thigh. Anteroposterior radiograph of the distal right thigh demonstrates a soft tissue mass (arrows) involving the lateral thigh with amorphous and cloud-like internal mineralization. The mass extends beyond the skin border. The mineralized portion of the mass does not appear to extend to the underlying femur.
[Source 61 ]Intracortical osteosarcoma
Intracortical osteosarcoma is the rarest variety of osteosarcoma and represents less than 1% of all osteosarcoma cases. Like other subtypes of osteosarcoma, it also typically presents in adolescents and young adults (mean age 19 years). Intracortical osteosarcoma has a slight male predominance. Only 18 intracortical osteosarcoma cases have been reported in the literature, and the most common sites are the tibia (nine cases) and the femur (nine cases) 71. The sex ratio of females to males is 5:13, and the median age is 19 years (range, nine to 43 years) 71.
Intracortical osteosarcoma is a low-grade tumor of cortical bones which typically does not extend into the medullary canal and surrounding soft tissue until the late stages of the disease 72, 71. Histologically, intracortical osteosarcoma is characterized as a sclerosing variant of the osteosarcoma and contains osteoid matrix with few fibroblastic foci and a mild degree of cellular atypia.
Although most reported cases have shown slow progression and a low-grade histological appearance, recurrence and distant metastases have been reported 72, 73, 74, 75, 76. The ideal treatment is surgical resection with an adequate margin; adjuvant chemotherapy can be considered. Following surgical resection and adjuvant chemotherapy, the overall prognosis is good.
Figure 10. Intracortical osteosarcoma
Footnotes: Tibial intracortical osteosarcoma. (a) Anteroposterior radiograph of the right tibia indicating an intra-cortical lytic bone lesion. (b) Longitudinal section of the en bloc histological specimen demonstrating a well-circumscribed intra-cortical mass enclosed by periosteum. (c) A tissue histological specimen (first episode) shows a tumor consisting of small nests and cords of cells (arrow) surrounded by thick anastomosing branches of osteoid without any chondrosarcomatous or fibrosarcomatous matrix (hematoxylin and eosin stain; original magnification, ×400). (d) The tibia was reconstructed by using an Ilizarov external fixator for bone transportation. (e) Radiographs showing the patient’s tibia six months after Ilizarov removal (two years after bone resection). (f) Good functional activity of the patient’s knee and ankle was shown after treatment.
[Source 71 ]Gnathic osteosarcoma
Gnathic osteosarcoma is a subtype of osteosarcoma that primarily affects the mandible and maxilla (alveolar ridge, sinus floor, and palate) 77, 78, 79, 80, 81. Primary osteosarcoma of the jaw bones accounts for approximately 10% of all osteosarcoma cases and 1% of all malignant tumors of the head and neck 82, 83, 84.
Males are more commonly affected than females 78. Swelling, loss and displacement of local teeth, spasm, and local parasthesia are common presenting complaints and are often misdiagnosed as chronic pulpitis or chronic periodontitis 78. Gnathic osteosarcoma is usually not associated with pain. Most patients present after dental treatment and mostly relate the symptoms to previous tooth extraction 85, 86.
Previous studies have proposed that gnathic osteosarcomas occur in older ages compared to those affecting the long bones, showing favorable prognoses 87, 82. However, details regarding the histological feature of gnathic osteosarcoma are still obscured. Some studies have indicated that conventional osteosarcoma is the predominant histological type 88, 89, 90, 91, 83. Other studies have shown a predominance of low‐grade histology 92, 77. Bennett et al. 89 indicated that the majority of tumors showed focal chondroid formation.
Gnathic osteosarcoma treatment plan generally includes preoperative chemotherapy followed by surgical excision and postoperative chemotherapy.
Osteosarcoma of the mandible has a more favorable prognosis than that of the maxilla.
Local recurrence and metastasis from jaw osteosarcoma are less common than in osteosarcoma of the long bones, and the proposed routes of metastatic spread include microscopically through the marrow spaces, via the mandibular canal or mental foramen and spread along the inferior alveolar nerve or mental nerve, and via the connective tissue that connects the intraosseous components and soft tissues such as the periodontal ligament 78. This may explain the facilitation of spread of an intraosseous lesion into the adjacent soft tissues. Metastases are relatively higher in postradiation osteosarcoma of the jaws and tend to occur within 2 to 3 years 78. The 5-year survival rates of mandibular and maxillofacial osteosarcoma are 34.8% and 25.8%, respectively, with a median survival time of 2.9 years for maxillary osteosarcoma and 6.5 years for mandibular osteosarcoma 84, 93, 82. Older age at onset carries a better prognosis because older age is associated with better resistance 93, 82. The higher local recurrence rate (range, 33% to 39%) is attributed to the complex anatomy associated with the maxillofacial region that interferes with achieving adequate margins 84.
Unlike osteosarcoma in the long bones, for unknown reasons, chemotherapy has shown negligible impact on the outcome of craniofacial osteosarcoma 94. Some studies have used radiotherapy and/or chemotherapy in conjunction with local radical surgery 95. Early diagnosis and appropriate surgery with adequate margins are the mainstay of treatment 84, 94, 95.
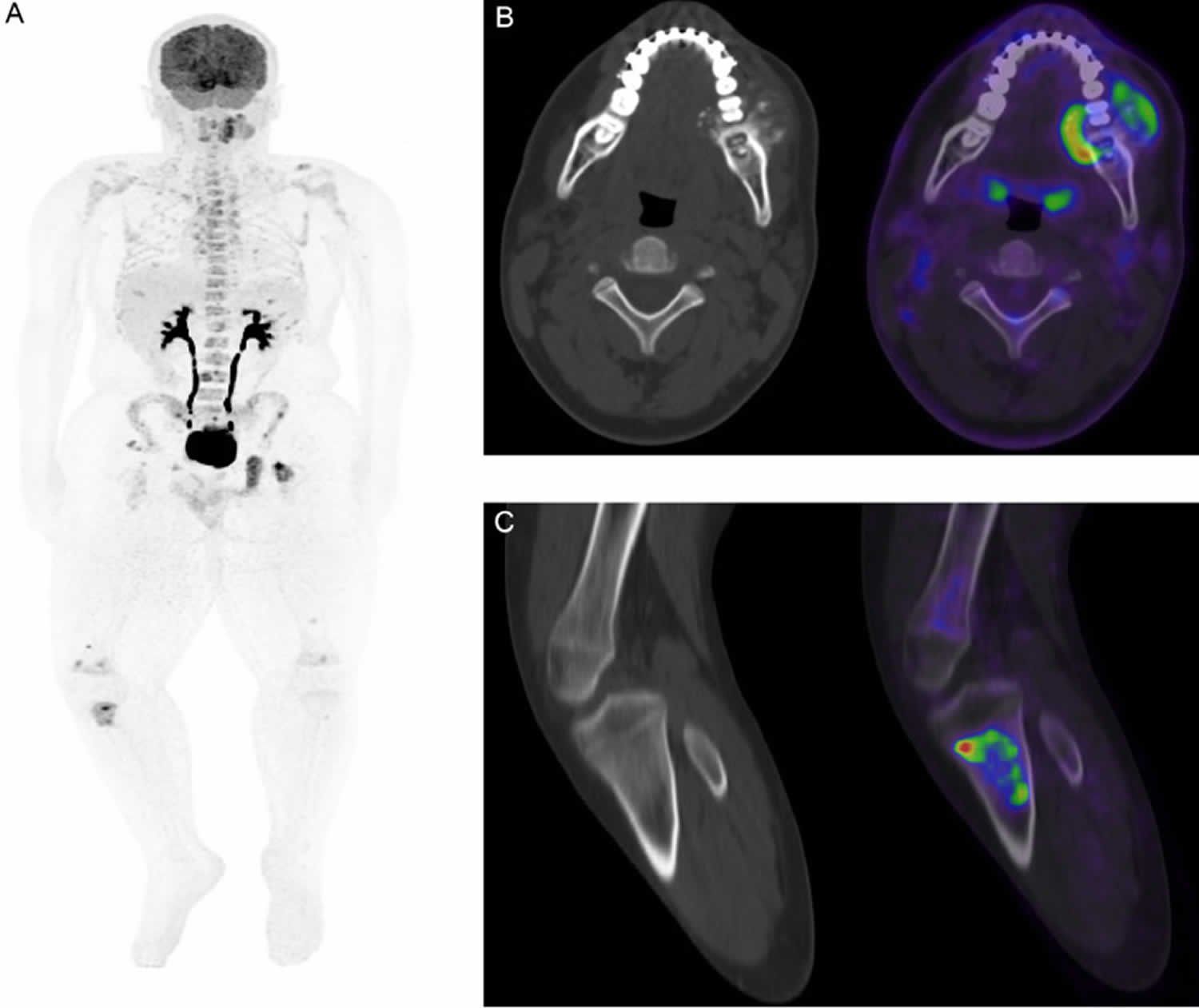
Figure 11. Gnathic osteosarcoma
Footnotes: (A) Whole-body FDG PET-CT revealed intense uptake in the lingual part of the tumor and heterogeneous increased uptake in the left mandibular lesion, SUV max: 13. (B) Whole-body FDG PET-CT shows extensive hypermetabolic lesions scattered throughout the skeleton, mostly within the bone marrow, suggestive of metastasis, SUV max: 12. (C) Whole-body FDG PET CT reveals unusual focal intense tracer uptake seen within the proximal right tibia at the level of the tibial tuberosity involving both the medulla and cortex; non-concordant with metastasis pattern.
[Source 80 ]Osteosarcoma causes
It’s not clear what causes osteosarcoma. Doctors know this cancer forms when something goes wrong in one of the cells that are responsible for making new bone.
Osteosarcoma begins when a healthy bone cell develops changes in its DNA. A cell’s DNA contains the instructions that tell a cell what to do. The changes tell the cell to start making new bone when it isn’t needed. The result is a mass (tumor) of poorly formed bone cells that can invade and destroy healthy body tissue. Cells can break away and spread (metastasize) throughout the body.
Inherited gene changes
Some inherited DNA mutations result in syndromes that are linked with an increased risk of osteosarcoma. For example:
- Li-Fraumeni syndrome is usually caused by inherited mutations that turn off the TP53 tumor suppressor gene. These mutations give a person a very high risk of developing one or more types of cancer, including breast cancer, brain tumors, and osteosarcoma.
- Inherited changes in the retinoblastoma (RB1) tumor suppressor gene increase the risk of developing retinoblastoma, a type of eye cancer that affects children. Children with this gene change also have an increased risk of osteosarcoma, especially if they are treated with radiation.
If you are concerned you or your child might possibly have an inherited gene change, talk with your doctor about whether genetic testing might be helpful.
Acquired gene changes
Most osteosarcomas are not caused by inherited gene mutations, but instead are the result of gene changes acquired during the person’s lifetime.
Sometimes these gene changes are caused by radiation therapy used to treat another form of cancer, because radiation can damage the DNA inside cells.
But many gene changes are probably just random events that sometimes happen inside a cell, without having an outside cause. Cells that are dividing quickly are more likely to create new cells with mistakes in their DNA, which increases the risk that a cancer such as osteosarcoma may develop. This may be why some normal situations (such as the teenage growth spurt) and some diseases (such as Paget disease of bone) that cause rapid bone growth increase the risk of osteosarcoma.
Other than radiation, there are no known lifestyle-related or environmental causes of osteosarcoma, so it’s important to remember that in most cases people with these cancers could have done nothing to prevent them.
Researchers now understand some of the gene changes that occur in osteosarcomas, but it’s not always clear what causes these changes. As we learn more about what causes osteosarcoma, hopefully we will be able to use this knowledge to develop ways to better prevent and treat it.
Risk factors for osteosarcoma
These factors increase the risk of osteosarcoma:
- Previous treatment with radiation therapy. People who were treated with radiation therapy for another cancer appear to have a higher risk of later developing osteosarcoma in the area that was exposed to radiation. Being treated at a younger age and being treated with higher doses of radiation both increase this risk. It’s not clear if imaging tests that use radiation, such as x-rays, CT scans, and nuclear medicine scans (such as PET scans or bone scans), raise the risk of developing osteosarcoma. The amount of radiation used for these tests is many times lower than that used for radiation therapy. If there is any increased risk it is likely to be very small, but doctors try to limit the use of these types of tests whenever possible, especially in children, just in case.
- Certain bone disorders, such as Paget’s disease of bone and fibrous dysplasia
- Paget’s disease of the bone: In Paget’s disease of the bone, abnormal bone tissue forms in one or more bones. Paget’s disease of the bone mostly affects people older than 50. The affected bones are heavy and thick but are weaker than normal bones and are more likely to break. Usually Paget’s disease of the bone by itself is not life-threatening. But bone sarcomas mostly osteosarcomas develop in about 1% of people with Paget’s disease of the bone, usually when many bones are affected.
- Hereditary multiple osteochondromas: Osteochondromas are benign tumors formed of bone and cartilage. Each osteochondroma has a very small risk of developing into a bone sarcoma most often a chondrosarcoma, but less often it can be an osteosarcoma. Most osteochondromas can be removed completely by surgery. However, some people inherit a tendency to develop many osteochondromas starting when they are young, and it may not be possible to remove them all. The more osteochondromas a person has, the greater the risk of developing a bone sarcoma.
- Fibrous dysplasia: This is an uncommon condition in which cells in a certain part of a bone make too much fibrous (scar-like) tissue, which replaces the normal bone in the area. In some people this happens in only one bone, while in others it affects more than one. It is sometimes seen as part of a condition called McCune-Albright syndrome. There is a small risk that each area of fibrous dysplasia might transform into an osteosarcoma.
- Certain inherited or genetic conditions, including hereditary retinoblastoma, Bloom syndrome, Li-Fraumeni syndrome, Rothmund-Thomson syndrome, Werner syndrome, and Diamond-Blackfan anemia
- Retinoblastoma is a rare eye cancer in children. Some children have the inherited form of retinoblastoma (hereditary retinoblastoma), in which all the cells of the body have a mutation (change) in the RB1 gene. These children also have an increased risk of developing bone or soft tissue sarcomas, including osteosarcoma. If radiation therapy is used to treat retinoblastoma, the risk of osteosarcoma in the bones around the eye is even higher.
- People with Li-Fraumeni syndrome are much more likely to develop certain types of cancer, including breast cancer, brain tumors, osteosarcoma, and other types of sarcoma. This syndrome is usually caused by a mutation of the TP53 gene.
- Children with Rothmund-Thomson syndrome tend to be short and to have skin and skeletal problems. They also are more likely to develop osteosarcoma. This syndrome is usually caused by abnormal changes in the REQL4 gene.
- Race or ethnicity. In the United States, osteosarcoma is slightly more common in African American, Hispanics, and Latino children than in White children.
Osteosarcoma symptoms
Osteosarcoma first symptom is usually bone pain or joint pain. This symptom may be overlooked, especially in young people, because of other more common causes of joint pain.
Signs and symptoms of osteosarcoma may include any of the following:
- Bone fracture may occur after a routine movement for no clear reason
- Limitation of motion
- Limping if the tumor is in the leg
- Pain when lifting if the tumor is in the arm
- Tenderness, swelling, or redness at the site of the tumor
Although osteosarcoma might weaken the bone it develops in, fractures (breaks) are not common. Exceptions are rare telangiectatic osteosarcomas, which tend to weaken bones more than other forms of osteosarcoma and are more likely to cause breaks where the tumor is.
People with a fracture next to or through an osteosarcoma often describe a limb that was sore for a few months and suddenly became very painful when the fracture occurred.
Osteosarcoma complications
Complications of osteosarcoma and its treatment may include:
- Limb removal or limb amputation. Surgery that removes the tumor and spares the limb is used whenever possible. But sometimes it’s necessary to remove part of the affected limb in order to remove all of the cancer. Learning to use an artificial limb (prosthesis) will take time, practice and patience. Experts can help you adapt.
- Cancer that spreads (metastasizes). Osteosarcoma can spread from where it started to other areas, making treatment and recovery more difficult. Osteosarcoma that spreads most often spreads to the lungs (pulmonary metastasis) and to other bones.
- Side effects of chemotherapy. The aggressive chemotherapy needed to control osteosarcoma can cause substantial side effects, both in the short and long term. Your health care team can help you manage the side effects that happen during treatment and provide you with a list of side effects to watch for in the years after treatment.
Osteosarcoma diagnosis
To diagnose osteosarcoma, your doctor will perform a physical exam and ask about your medical history and symptoms.
Tests that may be done include:
- Biopsy (at time of surgery for diagnosis)
- Blood tests
- Bone scan to see if the cancer has spread to other bones
- Computerized tomography (CT) scan of the chest to see if the cancer has spread to the lungs
- Magnetic resonance imaging (MRI) scan
- Positron emission tomography (PET) scan
- X-ray
Imaging tests
Imaging tests use x-rays, magnetic fields, or radioactive substances to help your doctor investigate your bone symptoms, look for cancer and look for signs that the cancer has spread. Imaging tests might be done for a number of reasons, including:
- To help find out if a suspicious area might be cancer
- To help determine if a cancer might have started in another part of the body
- To learn how far cancer has spread
- To help determine if treatment is working
- To look for signs that the cancer might have come back
People who have or might have bone cancer will have one or more of these tests.
Imaging tests may include:
- X-ray. An x-ray of the bone is often the first test done if some type of bone tumor is suspected. Tumors might look “ragged” instead of solid on an x-ray, or they might look like a hole in the bone. Sometimes doctors can see a tumor that might extend into nearby tissues (such as muscle or fat). Doctors might strongly suspect an abnormal area is a bone cancer by the way it appears on an x-ray, but usually a biopsy is needed to tell for sure. Adults with bone tumors might have a chest x-ray done to see if the cancer has spread to the lungs. But this test isn’t needed if a chest CT scan has been done.
- Magnetic resonance imaging (MRI). MRI scans create detailed images of the inside of the body using radio waves and strong magnets instead of x-rays, so no radiation is involved. A contrast material called gadolinium is often injected into a vein before the scan to better see details. An MRI is often done to get a more detailed look at an abnormal area of bone seen on an x-ray. MRIs can usually show if it’s likely to be a tumor, an infection, or some type of bone damage from another cause. MRIs can help determine the exact extent of a tumor, as they can show the marrow inside bones and the soft tissues around the tumor, including nearby blood vessels and nerves. MRIs can also show any small bone tumors several inches away from the main tumor (called skip metastases). Knowing the extent of tumor is very important when planning surgery.
- Computerized tomography (CT). A CT scan combines many x-ray pictures to make detailed cross-sectional images of parts of the body. CT scans aren’t usually as helpful as MRIs in showing the detail in and around bone tumors. But they are often done to look for possible cancer spread in other parts of the body, such as the lungs, liver, or other organs. CT scans can also be used to guide a biopsy needle into a tumor (a CT-guided needle biopsy). For this test, you stay on the CT scanning table while the doctor moves a biopsy needle toward the tumor. CT scans are repeated until the tip of the needle is within the mass.
- Positron emission tomography (PET). For a PET scan, a form of radioactive sugar known as F-18 fluorodeoxyglucose (FDG) is injected into the blood. Because cancer cells in the body are growing quickly, they absorb large amounts of the sugar. A special camera then creates a picture of areas of radioactivity in the body. The picture is not detailed like a CT or MRI scan, but it provides useful information about the whole body. PET scans can help show the spread of bone cancer to the lungs, other bones, or other parts of the body. They can also be used to see how well the cancer is responding to treatment. Many machines can do a PET and CT scan at the same time (PET/CT scan). This lets the doctor compare areas of higher radioactivity on the PET scan with the more detailed appearance of that area on the CT scan.
- Bone scan. A bone scan can show if a cancer has spread to other bones, and is often part of the workup for people with bone cancer. A bone scan is useful because it can show the entire skeleton at once. A positron emission tomography (PET) scan, can often provide similar information, so a bone scan might not be needed if a PET scan is done. For this test, a small amount of low-level radioactive material is injected into the blood and travels to the bones. A special camera that can detect the radioactivity then creates a picture of the skeleton. Areas of active bone changes attract the radioactivity and appear as “hot spots” on the skeleton. Hot spots may suggest areas of cancer, but other bone diseases can also cause the same pattern. To make an accurate diagnosis, other tests such as plain x-rays, MRI scans, or even a bone biopsy might be needed.
Biopsy (removing a sample of cells for testing)
The results of imaging tests might strongly suggest that a person has bone cancer, but a biopsy (removing some of the abnormal area and checking it under a microscope and with other laboratory testing) is usually the only way to be certain. Tests can show whether the cells are cancerous. Lab tests can determine the type of cancer and whether it’s aggressive (the grade).
Types of biopsy procedures used to diagnose osteosarcoma include:
- Needle biopsy. For needle biopsies, the doctor uses a hollow needle to remove a small cylinder of tissue from the tumor. The needle biopsy is usually done with local anesthesia, where numbing medicine is injected into the skin and other tissues over the biopsy site. In some cases, the patient might need sedation or general anesthesia (where the patient is asleep). Often, the doctor can aim the needle by feeling the suspicious area if it’s near the surface of the body. If the tumor can’t be felt because it’s too deep, the doctor can guide the needle into the tumor using an imaging test such as an ultrasound or CT scan. These types of image-guided biopsies are usually done by a doctor who is an interventional radiologist. There are 2 types of needle biopsies:
- A core needle biopsy uses a large needle to remove a cylinder of tissue. This is the most common type of needle biopsy used for bone tumors.
- A fine needle aspiration (FNA) biopsy uses a very thin needle on the end of a syringe to suck out a small amount of fluid and some cells from the tumor. This type of biopsy is less likely to be helpful for bone tumors, as the smaller needle might not be able to get through the bone. And even if it can be done, it might not remove enough of a sample for testing. But FNA can sometimes be useful for checking abnormal areas in other parts of the body for cancer cells.
- Surgical biopsy. The doctor makes an incision through the skin and removes either the entire tumor (excisional biopsy) or a portion of the tumor (incisional biopsy).
It’s important that the biopsy is done by an expert in bone tumors, or it could result in problems later on. For example, if the tumor is on the arm or leg and the biopsy isn’t done properly, it might lower the chances of saving the limb. If possible, the incision for the biopsy should be lengthwise along the arm or leg because this is the way the incision will be made during the operation to remove the cancer. The entire scar of the original biopsy will also most likely need to be removed, so making the biopsy incision this way means less tissue will need to be removed later on.
Determining the type of biopsy needed and the specifics of how it should be performed requires careful planning by the medical team. Doctors need to perform the biopsy in a way that won’t interfere with future surgery to remove the cancer. For this reason, ask your doctor for a referral to a team of experts with extensive experience in treating osteosarcoma before the biopsy.
Testing the biopsy samples
All samples removed by biopsy are sent to a pathologist (a doctor specializing in lab tests) to be looked at with a microscope. If cancer cells are seen, other types of lab tests might also be done to learn more about the exact type of cancer.
The pathologist will also assign the cancer a grade, which is a measure of how quickly it is likely to grow and spread, based on how the tumor cells look. Cancers that look somewhat like normal bone tissue are described as low grade (and tend to grow more slowly), while those that look very abnormal are called high grade.
Blood tests
Blood tests are not needed to diagnose bone cancer, but they may be helpful once a diagnosis is made. For example, high levels of chemicals in the blood such as alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) can suggest that the cancer may be more advanced.
Other tests such as blood cell counts and blood chemistry tests are done before surgery and other treatments to get a sense of a person’s overall health. These tests can also be used to monitor the person’s health while they are getting treatments such as chemotherapy.
Osteosarcoma pathology
Primary osteosarcomas typically occur at the metadiaphysis of long bones in the appendicular skeleton, most commonly at the following sites 96:
- Femur: 40% approx. (especially distal femur)
- Tibia: 16% approx. (especially proximal tibia)
- Humerus: 15% approx.
Other sites are less common:
- Fibula
- Innominate bone (i.e. os coxae)
- Mandible (gnathic osteosarcoma)
- Maxilla
- Vertebrae
Secondary tumors, on the other hand, have a much wider distribution, largely mirroring the combined incidence of their underlying conditions, and thus much have a higher incidence in flat bones, especially the pelvis (a favorite site of Paget disease).
Osteosarcomas can be further categorized by anatomic relationship to the bone 97, 11:
- Intramedullary or central: ~80%
- Surface: ~10-15%
- Intracortical osteosarcoma: rare
- Extraskeletal osteosarcoma: ~5%
High-grade osteosarcomas may occasionally present with skip metastases, which are non-contiguous smaller tumor foci in the same bone or in adjacent bone 98.
Osteosarcoma staging
After someone is diagnosed with osteosarcoma, doctors will try to figure out if it has spread, and if so, how far. This process is called staging. The stage describes how much cancer is in the body. It helps determine how serious the cancer is and how best to treat it. Doctors also use a cancer’s stage when talking about survival statistics.
The stage of an osteosarcoma is based on the results of physical exams, imaging tests, and any biopsies that have been done.
Doctors use formal staging systems to describe the extent of an osteosarcoma in detail. A staging system is a standard way to describe the extent of the cancer. But when trying to figure out the best treatment, doctors often use a simpler system that divides osteosarcomas into 2 main groups: localized and metastatic.
Cancer staging can be complex and confusing. If you have any questions about the stage of your cancer, ask a member of your cancer care team to explain it to you in a way you understand.
Localized osteosarcoma
A localized osteosarcoma is seen only in the bone it started in and possibly the tissues next to the bone, such as muscle, tendon, or fat.
About 4 out of 5 osteosarcomas appear to be localized osteosarcomas when they are first found. But even when imaging tests don’t show that the cancer has spread to distant areas, there are likely to be very small areas of cancer spread that can’t be detected with tests. This is why chemotherapy is an important part of treatment for most osteosarcomas. If it is not given, the cancer is more likely to come back after surgery.
Doctors further divide localized osteosarcomas into 2 groups:
- Resectable osteosarcomas are those in which all of the visible tumor can be removed (resected) by surgery.
- Non-resectable or unresectable osteosarcomas can’t be removed completely by surgery.
Metastatic osteosarcoma
A metastatic osteosarcoma has clearly spread to other parts of the body. Most often it spreads to the lungs, but it can also spread to other bones, the brain, or other organs.
About 1 out of 5 osteosarcomas have spread already when they are first diagnosed. Metastatic osteosarcomas are harder to treat, but some can be cured if the metastases can be removed by surgery. The cure rate for these cancers improves markedly if chemotherapy is also given.
Musculoskeletal Tumor Society (MSTS) staging system
A system commonly used to stage osteosarcoma is the Musculoskeletal Tumor Society (MSTS) system also known as the Enneking system. It is based on 3 key pieces of information:
- The grade (G) of the tumor, which is a measure of how likely it is to grow and spread, based on how it looks under the microscope. Tumors are either low grade (G1) or high grade (G2). Low-grade tumor cells look more like normal cells and are less likely to grow and spread quickly, while high-grade tumor cells look more abnormal.
- The extent of the primary tumor (T), which is classified as either intracompartmental (T1), meaning it has basically remained within the bone, or extracompartmental (T2), meaning it has extended beyond the bone into other nearby structures.
- If the tumor has metastasized (M), which means it has spread to other areas, either to nearby lymph nodes (bean-sized collections of immune system cells) or other organs. Tumors that have not spread to the lymph nodes or other organs are considered M0, while those that have spread are M1.
These factors are combined to give an overall stage, using Roman numerals from I (1) to III (3). Stages I (1) and II (2) are further divided into A for intracompartmental tumors or B for extracompartmental tumors.
Musculoskeletal Tumor Society (MSTS) osteosarcoma staging system:
- Low-grade, localized tumors are stage I (1).
- High-grade, localized tumors are stage II (2).
- Metastatic tumors (regardless of grade) are stage III (3).
Table 1. Musculoskeletal Tumor Society (MSTS) osteosarcoma staging system
| Stage | Grade | Tumor | Metastasis |
|---|---|---|---|
| IA (1A) | G1 | T1 | M0 |
| IB (1B) | G1 | T2 | M0 |
| IIA (2A) | G2 | T1 | M0 |
| IIB (2A) | G2 | T2 | M0 |
| III (3) | G1 or G2 | T1 orT2 | M1 |
TNM staging system
Another system sometimes used to stage bone cancers including osteosarcomas is the American Joint Commission on Cancer (AJCC) TNM system. This system is based on 4 key pieces of information:
- The extent (size) of the main (primary) tumor (T): How large is the tumor and/or has it reached nearby bones? Is it in more than one spot in the bone?
- The spread to nearby lymph nodes (N): Has the cancer spread to nearby lymph nodes? Bone tumors rarely spread to the lymph nodes.
- The spread (metastasis) to distant sites (M): Has the cancer spread to distant parts of the body, such as the lungs, other bones, or the liver? The most common sites of spread are to the lungs or other bones.
- The grade of the cancer (G): How abnormal do the cells look under a microscope? Low-grade tumor cells look more like normal cells and are less likely to grow and spread quickly, while high-grade tumor cells look more abnormal.
Numbers after T, N, M, and G give more details about each of these factors. Higher numbers generally mean the cancer has more concerning features.
For example, the scale used for grading bone cancer in this system ranges from 1 to 3. Low-grade cancers (G1) tend to grow and spread more slowly than high-grade (G2 or G3) cancers.
- Grade 1 (G1) means the cancer looks much like normal bone tissue.
- Grade 2 (G2) means the cancer looks more abnormal.
- Grade 3 (G3) means the cancer looks very abnormal.
Once the T, N, and M categories and the grade of the bone cancer have been determined, the information is combined into an overall stage. These stages (which are different from those of the MSTS system) are described using Roman numerals from I to IV (1 to 4), and are sometimes divided further.
Where the cancer started is another important factor in the AJCC system. In the current edition of the AJCC system (which came into use in 2018), the T categories are different for bone cancers that start in the arms, legs, trunk, skull, or facial bones, as opposed to cancers that start in the pelvis or spine. The T categories in the table below do not apply to cancers that start in the pelvis or spine. If you have a cancer that starts in one of these areas, it’s best to speak with your doctor about your cancer’s stage.
Two types of stages can be assigned to bone cancers in the TNM system:
- The clinical stage is based on the results of the exams and tests that have been done before the cancer has been treated with surgery. This stage can be used to help plan treatment.
- Once surgery has been done, the pathological stage also called the surgical stage can be determined, based on the results of exams and imaging tests, as well as what was found during surgery.
Sometimes, the clinical and pathological stages can be different; for example, if surgery finds that the cancer has spread farther than could be seen on imaging tests. The table below describes the pathological stage of the cancer.
Table 2. TNM osteosarcoma staging system
| American Joint Commission on Cancer (AJCC) stage | Stage grouping | Stage description* (8 centimeters = about 3 inches) |
|---|---|---|
| IA (1A) | T1 N0 M0 G1 or GX | The main tumor is no more than 8 centimeters across (T1). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0). The cancer is low grade (G1), or the grade cannot be determined (GX). |
| IB (1B) | T2 N0 M0 G1 or GX | The main tumor is more than 8 centimeters across (T2). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0). The cancer is low grade (G1), or the grade cannot be determined (GX). |
| OR | ||
| T3 N0 M0 G1 or GX | There is more than one tumor in the same bone (T3). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0). The cancer is low grade (G1), or the grade cannot be determined (GX). | |
| IIA (2B) | T1 N0 M0 G2 or G3 | The main tumor is no more than 8 centimeters across (T1). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0). The cancer is high grade (G2 or G3). |
| IIB (2B) | T2 N0 M0 G2 or G3 | The main tumor is more than 8 centimeters across (T2). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0). The cancer is high grade (G2 or G3). |
| III (3) | T3 N0 M0 G2 or G3 | There is more than one tumor in the same bone (T3). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0). The cancer is high grade (G2 or G3). |
| IVA (4A) | Any T N0 M1a Any G | The main tumor can be any size, and there may be more than one in the bone (Any T). The cancer has not spread to nearby lymph nodes (N0). It has spread only to the lungs (M1a). The cancer can be any grade (Any G). |
| IVB (4B) | Any T N1 Any M Any G | The main tumor can be any size, and there may be more than one in the bone (Any T). The cancer has spread to nearby lymph nodes (N1). It may or may not have spread to distant organs like the lungs or other bones (Any M). The cancer can be any grade (Any G). |
| OR | ||
| Any T Any N M1b Any G | The main tumor can be any size, and there may be more than one in the bone (Any T). The cancer might or might not have spread to nearby lymph nodes (Any N). It has spread to distant parts of the body, such as other bones, the liver, or the brain (M1b). The cancer can be any grade (Any G). | |
Footnotes: * The following additional categories are not listed on the table above:
- TX: Main tumor cannot be assessed due to lack of information.
- T0: No evidence of a primary tumor.
- NX: Regional lymph nodes cannot be assessed due to lack of information.
Osteosarcoma treatment
Osteosarcoma treatment typically involves surgery and chemotherapy. Radiation therapy might be an option in certain situations.
Surgery
Surgery is an important part of treatment for almost all osteosarcomas. It includes:
- The biopsy to diagnose the cancer
- The removal of the tumor(s)
Whenever possible, it’s very important that the biopsy and the surgery to remove the tumor be planned together, and that an experienced orthopedic surgeon does both the biopsy and the surgery to remove the tumor. The biopsy should be done in a certain way to give the best chance that less extensive surgery will be needed later on.
The main goal of surgery is to remove all of the cancer cells. If even a small amount of cancer is left behind, it might continue to grow and make a new tumor, and might even spread to other parts of the body. To lower the risk of this happening, surgeons remove the tumor plus some of the normal tissue that surrounds it. This is known as a wide excision.
A doctor called a pathologist will look at the removed tissue under a microscope to see if there are cancer cells at the margins (outer edges).
- If cancer cells are seen at the edges of the tissue, the margins are called positive. Positive margins can mean that some cancer was left behind.
- When no cancer cells are seen at the edges of the tissue, the margins are said to be negative, clean, or clear. A wide excision with clean margins helps limit the risk that the cancer will come back in the place where it started.
The type of surgery done depends mainly on the location and size of the tumor. Although all operations to remove osteosarcomas are complex, tumors in the limbs (arms or legs) are generally not as hard to remove as those in the jaw bone, at the base of the skull, in the spine, or in the pelvic (hip) bone.
Operations used to treat osteosarcoma include:
- Surgery to remove the cancer only (limb-sparing surgery). Most osteosarcoma operations can be done in a way that removes all of the cancer and spares the limb so that function can be maintained. Whether this procedure is an option depends, in part, on the extent of the cancer and how much muscle and tissue need to be removed. If a section of bone is removed, the surgeon will reconstruct the bone. The method of reconstruction depends on your particular situation, but options include metal prosthetics or bone grafts.
- Surgery to remove the affected limb (amputation). With advancements in limb-sparing surgery, the need for amputation — removing a limb or part of a limb — has greatly reduced over the years. If amputation is necessary, advances in prosthetic joints can significantly improve outcomes and function.
- Surgery to remove the lower portion of the leg (rotationplasty). In this surgery, sometimes used for children who are still growing, the surgeon removes the cancer and surrounding area, including the knee joint. The foot and ankle are then rotated, and the ankle functions as a knee. A prosthesis is used for the lower leg and foot. Results typically enable the person to function very well in physical activities, sports and daily living.
Osteosarcoma in the arms or legs
Osteosarcoma in the arms or legs might be treated with either:
- Limb-salvage (limb-sparing) surgery: removing the cancer and some surrounding normal tissue but leaving the limb basically intact
- Surgery to remove the affected limb (amputation): removing the cancer and all or part of an arm or leg
Limb-salvage surgery
Most patients with tumors in the arms or legs can have limb-sparing surgery, but this depends on where the tumor is, how big it is, and whether it has grown into nearby structures.
Limb-salvage surgery is a very complex operation. The surgeons who do this type of operation must have special skills and experience. The challenge is to remove the entire tumor while still saving the nearby tendons, nerves, and blood vessels to keep as much of the limb’s function and appearance as possible. If the cancer has grown into these structures, they will need to be removed along with the tumor. In such cases, amputation may sometimes be the best option.
The section of bone that is removed along with the osteosarcoma is replaced with a piece of bone from another part of the body or from another person (a bone graft) or with a man-made device made of metal and other materials that replaces part or all of a bone (an internal prosthesis). Some newer devices combine a graft and a prosthesis.
Complications of limb-salvage surgery can include infections and grafts or rods that become loose or broken. Patients who have limb-salvage surgery might need more surgery in the following years, and some might still eventually need an amputation.
Using an internal prosthesis in growing children is especially challenging. In the past, it required occasional operations to replace the prosthesis with a longer one as the child grew. Newer prostheses have become very sophisticated and often can be made longer without any extra surgery. They have tiny devices in them that can lengthen the prosthesis when needed to make room for a child’s growth. But even these prostheses may need to be replaced with a stronger adult prosthesis once the child’s body stops growing.
It takes about a year, on average, for patients to learn to walk after limb-salvage surgery on a leg. Physical rehabilitation after limb-salvage surgery is more intense than after amputation, and it’s extremely important. If the patient doesn’t actively take part in the rehabilitation program, the salvaged arm or leg might become useless.
Surgery to remove the affected limb (amputation)
For some patients, amputation may be the best option. For example, if the tumor is very large or if it extends into the nerves and/or the blood vessels, it might not be possible to save the limb.
The surgeon determines how much of the arm or leg needs to be amputated based on the results of MRI scans and an examination of removed tissue by the pathologist during the surgery.
Surgery is usually planned so that muscles and the skin will form a cuff around the remaining bone. This cuff will fit into the end of a prosthetic (artificial) limb. Another option might be to implant a prosthesis into the remaining bone, with the end of the prosthesis remaining outside the skin. This can then be attached to an external prosthesis.
Reconstructive surgery can help some patients who lose a limb to function as well as possible. For example, if the leg must be amputated mid-thigh (including the knee joint), the lower leg and foot can be rotated and attached to the thigh bone, so that the ankle functions as a new knee joint. This surgery is called rotationplasty. Of course, the patient would still need a prosthetic limb to replace the lower part of the leg.
With proper physical therapy, a person is often able to walk on his/her own 3 to 6 months after a leg amputation.
If the osteosarcoma is in the shoulder or upper arm and amputation is needed, in some cases the area with the tumor can be removed and the lower arm reattached so that the patient has a functional, but much shorter, arm.
Rehabilitation after surgery
This may be the hardest part of treatment, and it cannot be described here completely. Patients and parents should meet with a rehabilitation specialist before surgery to learn about their options and what might be required after surgery.
If a limb is amputated, the patient will need to learn to live with and use a prosthetic limb. This can be particularly hard for growing children if the prosthetic limb needs to be changed to keep up with their growth.
When only the tumor and part of the bone is removed in a limb-sparing operation, the situation can sometimes be even more complicated, especially in growing children. More surgery might be needed in the future to replace an internal prosthesis with one more suited to their growing body size.
Both amputation and limb-sparing surgery can have pros and cons. For example, limb-sparing surgery, although often more acceptable than amputation, tends to lead to more complications because of its complexity. Growing children who have limb-sparing surgery are also more likely to need further surgery later.
When researchers have looked at the results of the different surgeries in terms of quality of life, there has been little difference between them. Perhaps the biggest problem can be for teens, who may worry about the social effects of their operation. Emotional issues can be very important, and support and encouragement are needed for all patients.
Osteosarcoma that start in other areas
Osteosarcoma in the pelvic (hip) bones can often be hard to remove completely with surgery. But if the tumor responds well to chemotherapy first, surgery (sometimes followed by radiation therapy) may get rid of all of the cancer. Pelvic bones can sometimes be reconstructed after surgery, but in some cases pelvic bones and the leg they are attached to might need to be removed.
For osteosarcoma in the lower jaw bone, the entire lower half of the jaw may be removed and later replaced with bones from other parts of the body. If the surgeon can’t remove all of the tumor, radiation therapy may be used as well.
For osteosarcoma in areas like the spine or the skull, it may not be possible to remove all of the tumor safely. Cancers in these bones may require a combination of treatments such as chemotherapy, surgery, and radiation.
Joint fusion (arthrodesis)
Sometimes, after the removal of a tumor that involves a joint (an area where two bones come together), it might not be possible to reconstruct the joint. In this case, surgery might be done to fuse the two bones together. This is most often used for tumors in the spine, but it might also be used in other parts of the body, such as a shoulder or hip. While it can help stabilize the joint, the person will have to learn to compensate for the resulting loss of motion.
Chemotherapy
Chemotherapy (chemo) is the use of drugs to kill cancer cells. The drugs are usually given into a vein and can reach and destroy cancer cells throughout the body.
The chemo drugs used most often to treat osteosarcoma include:
- Methotrexate (given in high doses, along with leucovorin to help limit side effects)
- Doxorubicin (Adriamycin)
- Cisplatin or carboplatin
- Ifosfamide
- Cyclophosphamide
- Etoposide
- Gemcitabine
Usually, 2 or more chemo drugs are given together. Some common combinations of drugs include:
- High-dose methotrexate, doxorubicin, and cisplatin (known as the MAP regimen), sometimes with ifosfamide. This is used more often in children, teens, and young adults.
- Doxorubicin and cisplatin. This is used more often in older adults.
- Ifosfamide and etoposide
- Ifosfamide, cisplatin (or carboplatin), and epirubicin
Many experts recommend that the drugs be given in very high doses when possible.
Chemo is an important part of the treatment for most people with osteosarcoma (although some patients with low-grade osteosarcoma might not need it). Most osteosarcomas don’t appear to have spread beyond the main tumor when they are first found. But in the past, when these cancers were treated with surgery alone, the cancer would often come back in other parts of the body, where it would be very hard to control. Giving chemo along with surgery helps lower the risk of these cancers coming back.
Most osteosarcomas are treated with chemo before surgery also known as neoadjuvant chemotherapy for about 10 weeks. In some people with osteosarcoma in an arm or leg bone, this can shrink the tumor, which might help make surgery easier. Chemo is then given again after surgery also known as adjuvant chemotherapy for up to a year.
Chemo in is given in cycles, with each period of treatment followed by a rest period to give the body time to recover. Each cycle typically lasts for a few weeks.
Before starting chemo, the doctor might advise putting a catheter (a thin, soft tube) into a large vein in the chest. This is sometimes called a venous access device (VAD) or central venous catheter (CVC). The catheter is inserted surgically while the patient is sedated (sleepy) or under general anesthesia (in a deep sleep). One end of the catheter stays in the vein, while the other end lies just under or outside the skin. This lets the health care team give chemo and other drugs and draw blood samples without having to stick needles into the veins each time. The catheter usually remains in place for several months and can make having chemo less painful. If such a device is used, the health care team will teach you how to care for it to reduce the risk of problems such as infections.
Doctors monitor how the cancer cells respond to the chemotherapy in order to plan further treatments.
If the osteosarcoma shrinks in response to the chemotherapy, it may make limb-sparing surgery possible.
If the osteosarcoma doesn’t respond to treatment, it may indicate the cancer is very aggressive. Doctors may recommend a different combination of chemotherapy drugs or suggest a more aggressive operation to ensure all the cancer is removed.
Chemotherapy can also be used after surgery to kill any cancer cells that might remain.
If osteosarcoma returns after surgery or spreads to other areas of the body, chemotherapy might be recommended to try to slow the growth of the disease.
Chemotherapy side effects
Chemotherapy (chemo) drugs can cause side effects. Children tend to have less severe side effects from chemo than adults and often recover from side effects more quickly. Because of this, doctors can give them higher doses of chemo to try to kill the tumor.
The side effects of chemo drugs depend on the type, dose, and the length of time they are taken.
General side effects of chemo:
- Nausea and vomiting
- Loss of appetite
- Diarrhea
- Hair loss
- Mouth sores
Chemo can damage the bone marrow, where new blood cells are made. This can lead to low blood cell counts, which can result in:
- Increased chance of infection (from a shortage of white blood cells)
- Bleeding or bruising after minor cuts or injuries (from a shortage of platelets)
- Fatigue or shortness of breath (from low red blood cell counts)
A major concern with chemo used to treat osteosarcoma is that it can lead to dangerously low white blood cell levels and an increased risk of serious infections. Because of this, drugs called growth factors (such as filgrastim, also known as G-CSF) may be given along with the chemo to help the body make new white blood cells as quickly as possible.
Most of the side effects above tend to go away after treatment is finished. Often there are ways to make these side effects less severe. For example, drugs can be given to help prevent or reduce nausea and vomiting, or to help get blood counts back to normal levels. Be sure to discuss any questions you have about side effects with the cancer care team, and tell them about any side effects so that they can be controlled.
Side effects of certain chemo drugs:
Some side effects are specific to certain drugs. Many of these side effects are uncommon, but they are possible. Before treatment, ask your cancer care team about the possible side effects of the drugs you or your child will be getting.
- Ifosfamide and cyclophosphamide can damage the lining of the bladder, which can cause blood in the urine. The chance of this happening can be lowered by giving a drug called mesna during chemotherapy, along with plenty of fluids.
- Cisplatin and carboplatin can cause nerve damage (called neuropathy) leading to numbness, tingling, or pain in the hands and feet. This often goes away or gets better once treatment is stopped, but it might last a long time in some people. These drugs can sometimes affect hearing, especially of high-pitched sounds. Kidney damage can also occur after treatment. Giving lots of fluid before and after the drug is infused can help prevent this.
- Etoposide can also cause nerve damage. It can also increase the risk of later developing acute myeloid leukemia (AML), a cancer of white blood cells. Fortunately, this is not common.
- High-dose methotrexate can damage the white matter of the brain (called leukoencephalopathy) and can also affect the liver and kidneys. Before starting high-dose methotrexate, medicines are given to help protect the kidneys. Methotrexate blood levels may be checked to see how much leucovorin (also called folinic acid) should be given to help limit any damage to normal tissues.
- Doxorubicin (Adriamycin) can damage the heart muscle. The risk of this goes up with the total amount of the drug that is given, so doctors are careful to limit the total dose. Your (child’s) doctor may order a heart function test (such as an echocardiogram) before and during treatment to see if this drug is affecting the heart. A drug called dexrazoxane may be given along with the chemo to help lessen the possible damage.
Some chemo drugs can affect your (child’s) ability to have children (fertility) later in life. Ask the cancer care team about the possible effects of treatment on fertility, and ask if there are options for preserving fertility, such as sperm banking or egg preservation.
The doctors and nurses will watch closely for chemo side effects. Don’t hesitate to ask the cancer care team any questions about side effects.
Tests to check for side effects of chemo: Before each treatment, lab test results will be checked to be sure the liver, kidneys, and bone marrow are working well. Other tests might be done during and after treatment as well.
- The complete blood count (CBC) checks levels of white blood cells, red blood cells, and blood platelets. These will be watched closely during and after chemo. The white blood cells and platelets usually reach their lowest point about 2 weeks after chemo is given, though this can occur earlier with high-dose regimens.
- Blood chemistry tests measure certain blood chemicals that tell doctors how well the liver and kidneys are working. Some chemo drugs can damage these organs.
- An audiogram might be done to check hearing, which can be affected by certain chemo drugs.
- If doxorubicin is to be given, tests such as an echocardiogram (an ultrasound of the heart) may be done before and during treatment to check heart function.
Targeted therapy drugs
Doctors are now studying newer drugs that target specific parts of tumor cells or nearby cells as a way to treat osteosarcoma. These targeted drugs work differently from standard chemo drugs. Targeted therapy drugs might be helpful sometimes when chemo drugs are no longer working.
For example, regorafenib (Stivarga), sorafenib (Nexavar), and cabozantinib (Cabometyx) are drugs that affect a tumor’s ability to develop new blood vessels, which it needs to grow. These drugs have been shown to help some people with osteosarcoma in early studies. Although these drugs are not yet FDA-approved to treat osteosarcoma, they are approved to treat other types of cancer, and trying one of them might be an option if standard chemo drugs are no longer helpful.
Common side effects of these drugs can include fatigue, loss of appetite, hand-foot syndrome (redness and irritation of the hands and feet), high blood pressure, weight loss, diarrhea, and abdominal (belly) pain. Less common but more serious side effects can include problems with blood flow to the heart, bleeding, abnormal thyroid test results, and perforations (holes) in the stomach or intestines.
Radiation therapy
Radiation therapy uses high-energy beams, such as X-rays and protons, to kill cancer cells. Radiation might be an option in certain situations, such as when surgery isn’t possible or if surgeons can’t remove all of the cancer during an operation.
During radiation therapy, the beams of energy are delivered from a machine that moves around you as you lie on a table. The beams are carefully directed to the area of the osteosarcoma in order to reduce the risk of damage to surrounding healthy cells.
External beam radiation therapy
External beam radiation therapy (EBRT) is most often used to treat osteosarcoma. A machine outside the body focuses high-energy beams on the tumor to kill the cancer cells.
Before treatments start, the radiation team takes careful measurements with imaging tests such as MRI scans to determine the correct angles for aiming the radiation beams and the proper dose of radiation. This planning session is called simulation.
Most often, radiation treatments are given 5 days a week for several weeks. Each treatment is much like getting an x-ray, although the dose of radiation is much higher. The treatment is not painful. For each session, you (or your child) will lie on a special table while a machine delivers the radiation from precise angles.
Each treatment lasts only a few minutes, although the setup time – getting into place for treatment – usually takes longer. Young children may be given medicine to make them sleep so they will not move during the treatment.
Newer techniques, such as intensity modulated radiation therapy (IMRT), conformal proton beam therapy, and stereotactic radiosurgery (SRS), let doctors aim the radiation at the tumor more precisely while reducing how much nearby healthy tissues get. This may offer a better chance of increasing the success rate and reducing side effects. Many doctors now recommend using these approaches when they are available.
Radiation therapy side effects
The side effects of external radiation therapy depend on the dose of radiation and where it is aimed.
Short-term problems can include effects on skin areas that receive radiation, which can range from mild sunburn-like changes and hair loss to more severe skin reactions. Radiation to the abdomen or pelvis can cause nausea, diarrhea, and urinary problems. Talk with your (child’s) health care team about the possible side effects because there may be ways to relieve some of them.
In children, radiation therapy can slow bone growth. For example, radiation to the bones in one leg might result in it being shorter than the other. Radiation to the facial bones may cause uneven growth, which might affect how a child looks. But if a child is fully or almost fully grown, this is less likely to be an issue.
Depending on where the radiation is aimed, it can also damage other organs:
- Radiation to the chest wall or lungs can affect lung and heart function.
- Radiation to the jaw area might affect the salivary glands, which could lead to dry mouth and tooth problems.
- Radiation therapy to the spine or skull might affect the nerves in the spinal cord or brain. This could lead to nerve damage, headaches, and trouble thinking, which usually become most serious 1 or 2 years after treatment. Radiation to the spine might cause numbness or weakness in part of the body.
- Radiation to the pelvis can damage the bladder or intestines, which can lead to problems with urination or bowel movements. It can also damage reproductive organs, which could affect a child’s fertility later in life, so doctors do their best to protect these organs by shielding them from the radiation or moving them out of the way whenever possible.
Another major concern with radiation therapy is that it might cause a new cancer to form in the part of the body that was treated with the radiation. The higher the dose of radiation, the more likely this is to occur, but the overall risk is small and should not keep children who need radiation from getting it.
To lower the risk of serious long-term effects from radiation, doctors try to use the lowest dose of radiation therapy that is still effective. Still, it’s important to continue follow-up visits with your (child’s) doctor so that if problems come up they can be found and treated as early as possible.
Radioactive drugs (radiopharmaceuticals)
Drugs that include a radioactive element can sometimes be helpful in people with advanced osteosarcoma. Bone-seeking radioactive drugs such as samarium-153-EDTMP (Quadramet) or radium-233 (Xofigo) are sometimes used to slow tumor growth and treat symptoms such as pain in people with advanced osteosarcoma. These drugs are injected into a vein and collect in the bones. Once there, the radiation they give off kills the cancer cells.
These drugs are especially helpful when cancer has spread to many bones, since external beam radiation would need to be aimed at each affected bone. In some cases, these drugs are used together with external beam radiation aimed at the most painful bone metastases.
The major side effect of these drugs is a lowering of blood cell counts, which could increase the risk for infections or bleeding, especially if the blood counts are already low.
Follow-up care
After your treatment, it’s very important to go to all follow-up appointments. During these visits, doctors will ask about symptoms and do physical exams, and might order blood tests or imaging tests such as MRI, CT scans or x-rays. Follow-up visits are needed to check for cancer recurrence or spread, as well as possible side effects of treatment. This is a good time for you to ask the health care team any questions and to discuss any concerns you might have.
You or your child will probably see the oncologist and the orthopedic surgeon and get imaging tests every few months during the first couple of years after treatment, and less often after that if there are no issues.
Physical therapy and rehabilitation are typically a very important part of recovery after treatment for osteosarcoma, and your doctors and other health providers will continue to monitor your (child’s) progress as time goes on.
Some chemotherapy drugs can cause problems with hearing or heart damage. People who get these drugs may also have tests to check hearing or heart function.
Almost any cancer treatment can have side effects. Some may last for only a short time, but others can last longer or might not show up until months or even years later. For example, in younger people, treatment might affect fertility (the ability to have children) later in life. It’s important to talk to the cancer care team to learn about what to look for, and to tell them about any symptoms or side effects so they can help manage them.
Talk with the treatment team about developing a survivorship care plan. This plan might include:
- A suggested schedule for follow-up exams and tests
- A schedule for other tests that might be needed in the future, such as early detection (screening) tests for other types of cancer, or tests to look for long-term health effects from the cancer or its treatment
- A list of possible late- or long-term side effects from treatment, including what to watch for and when to contact the doctor
Metastatic osteosarcoma
Metastatic osteosarcoma have already spread to distant parts of the body when they are diagnosed. Most often they have spread to the lungs. As with other osteosarcomas, a biopsy is needed first to establish the diagnosis.
Chemotherapy is usually the first treatment for metastatic osteosarcomas. If all of the tumors are thought to be resectable after chemotherapy, they are removed with surgery, sometimes in more than one operation. This is followed by more chemo for up to a year.
If some of the tumors remain unresectable after chemo, radiation therapy can often be used to try to keep them under control and to help relieve symptoms. More chemo might be another option, either instead of or after radiation therapy. If the first chemo regimen didn’t work very well, different chemo drugs might be tried.
Newer targeted therapy drugs such as regorafenib (Stivarga), sorafenib (Nexavar), or cabozantinib (Cabometyx) might also be an option at some point as well, although more research will be needed to see how effective these drugs are.
Because metastatic osteosarcomas can be hard to treat, clinical trials of newer treatments may be a good option in many cases.
Surgery of metastatic osteosarcoma
If the osteosarcoma has spread to other parts of the body, these tumors need to be removed to have a chance at curing the cancer.
Osteosarcoma most often spreads to your lungs. If surgery can be done to remove these metastases, it must be planned very carefully. Things to be considered before the operation include:
- The number of tumors
- The location of the tumor(s) (one lung or both lungs)
- The size of the tumor(s)
- How well the tumor(s) responded to chemotherapy
- The person’s overall health
Since the chest CT scan done before surgery might not show all of the lung tumors, the surgeon will have a treatment plan in case more tumors are found during the operation.
Patients who have tumors in both lungs and respond well to chemotherapy can have surgery on one lung at a time. Removing tumors from both lungs at the same time may be another option.
Some lung metastases may not be able to be removed because they are too big or are too close to important structures in the chest (such as large blood vessels). Patients whose overall health isn’t good (for example, because of heart, liver, or kidney problems) might not be able to withstand the stress of anesthesia and surgery to remove the metastases.
A small number of osteosarcomas spread to other bones or to organs like the kidneys, liver, or brain. Whether these tumors can be removed with surgery depends on their size, location, and other factors.
Recurrent osteosarcoma
Recurrent osteosarcoma means that the cancer has come back after treatment. It may come back locally (near where the first tumor was) or in other parts of the body. Most of the time, if osteosarcoma recurs it will be in the lungs.
If possible, surgery to remove the tumor(s) is an important part of treatment, as it offers the best chance for long-term survival. If the cancer recurs at the original site on an arm or leg after limb-sparing surgery, amputation of the limb may be recommended.
Chemotherapy is often part of the treatment for recurrent cancers as well. If the cancer is not resectable, chemo might be used to try to shrink the tumor(s), which might then allow surgery to be done. If the cancer is resectable, chemo might be given after surgery. For more advanced cancers, chemo might be used to try to help relieve symptoms.
Radiation therapy might be part of treatment as well. It can sometimes help keep tumor growth in check and help relieve symptoms.
If the cancer is still growing, newer targeted therapy drugs such as regorafenib (Stivarga), sorafenib (Nexavar), or cabozantinib (Cabometyx) might be an option at some point as well, although more research is needed to see how effective these drugs are.
Because recurrent osteosarcomas can be hard to treat, clinical trials of newer treatments may be a good option.
Clinical trials
Clinical trials are studies to investigate new ways of treating cancer. Ask your doctor or your child’s doctor about whether you may be eligible to join a trial.
Each clinical trial has benefits and risks. Before you decide to be in one, you need to have all your questions answered. Some people take notes, record meetings with the clinical team, or bring a friend with them to help remember the answers and think of other questions.
Risks versus benefits
Each clinical trial has its own benefits and risks. But for the most part, clinical trials (other than phase 0) have some of the same potential benefits:
- You might help others who have the same disease by helping to advance cancer research.
- You could get a treatment that’s not available outside of the trial. This treatment might be safer or work better than current options.
- This may increase the number of your treatment options.
- You may feel more control by taking a more active role in your health care.
- You’ll likely see your cancer care team more often so that they can monitor your disease and check for side effects of the new treatment.
- Some study sponsors may pay for part or all your medical care and other expenses during the trial. (This isn’t true for all clinical trials.)
Some possible risks of being in a clinical trial can include:
- The new treatment may have unknown side effects or other risks which might be worse than those from standard treatments.
- The new treatment may not work for you even if it helps others.
- You may need to have more doctor visits or testing which may require more time and travel.
- If you take part in a randomized clinical trial , you may not have a choice about which treatment you get. If the study is blinded, you (and maybe your doctor) won’t know which treatment you’re getting. (This information will be available to the clinical trial team as needed for your safety).
- Insurers may not cover all costs of the clinical trial. They usually do cover the costs of what would normally be standard care. Be sure to talk to your insurance provider and someone involved with the clinical trial before you decide to take part.
Common concerns about clinical trials
Most people have some concerns about taking part in a clinical trial because they’re not really sure what it will mean for them. Get as much information as you need to make the choice that’s right for you.
- Will there be risks? Yes, all clinical trials have risks. But any medical test, treatment, or procedure has risks. The risk may be higher in a clinical trial because there are more unknowns. This is especially true of phase I and II clinical trials, where the treatment has been studied in fewer people. Perhaps a bigger question is if the possible benefits outweigh the risks. People with cancer are often willing to accept a certain amount of risk for a chance to be helped. But it’s always important to be clear on what this chance is. Ask your doctor to give you an idea of what the benefits may be and which is likely for you. Some people may decide that any chance of being helped is worth the risk, while others may not. Others may be willing to take certain risks to help others.
- Will I be a “guinea pig?” You will not be a guinea pig, but it’s true that the purpose of a clinical trial is to answer a medical question. People who take part in clinical trials may need to do extra things or have certain tests done as part of the clinical trial. But this doesn’t mean that you won’t get excellent care while in the study. In fact, most people enrolled in clinical trials welcome the extra attention they get from their cancer care team. Studies have shown that people with cancer who felt well informed before they took part in a clinical trial had less regret after the study than those who felt unsure. That’s why it’s important to take your time, ask questions, and feel good about your decision.
- Will I get a placebo? A placebo is a fake pill or treatment used in some types of clinical trials to help make sure results are from the new treatment or drug. A placebo pill is sometimes called a “sugar pill.” Placebos are rarely used alone in clinical trials unless there is no known effective treatment. Most cancer clinical trials do not use placebos unless they are given along with an active drug. It’s unethical to give someone a placebo instead of a treatment that’s known to work. There are some types of cancer that no treatments have proven to help. In rare cases, testing a new treatment against a placebo might be needed to prove that the treatment is better than nothing at all. The very least you should expect from any clinical trial is to be offered the treatment standard of care.
- Can my doctor or I pick which group I’m in? Not for studies that are randomized. This means that each person in the study gets assigned by chance to either the treatment group or the control group (who get the best current treatment). Randomization helps decrease the chance that the people in one group will be so different from the other that it could affect outcomes. Randomization helps to make sure that the groups have people in similar states of health, so the results are not affected by differences between the groups. If people could choose which treatment they got, the study results might not be as accurate. Some people find the concept of randomized clinical trials distressing, since neither the patient nor the doctor can choose which group the patient is in. This can be especially true if a trial is looking at totally different treatments and a person believes that one is better than the other. But remember, doctors are doing the study because they really don’t know which one is better. And sometimes taking part in such a study is the only way a person has a chance of getting a new treatment.
- Will I know which group I’m in? Will my doctor know? Each study is different. In a blinded study, the patient doesn’t know which treatment they’re getting. In a double-blinded study, neither the patient nor the doctor knows which treatment is being used. Not knowing what you’re getting can be hard. Your doctor can always find out which group you’re in if there’s an important medical reason (such as a possible drug reaction), but it may result in your being removed from the study. Blinding reduces the risk that the doctor or patient’s beliefs about the new treatment will affect their evaluation of response or side effects.
- Will my information be kept confidential? As much as possible, your personal and medical information will be kept confidential. Of course your cancer care team needs this data to give you the best possible care, just as they would if you were not in a clinical trial. Information that’s needed for the clinical trial, such as test results, is put on special forms and into computer systems. This is only shared with the people who analyze the study results. Your data is given a number or code – your name isn’t on the forms or in the study system. Sometimes, members from the research team or from the FDA might need to look at your records to be sure the data they were given is correct. But your personal information isn’t given to them and is never used in any published clinical trial results.
- What about cost? Will my insurance cover it? In most cases, the study sponsor provides the new treatment at no cost and pays for any special tests or extra doctor visits. Some sponsors may pay for more. For example, some might pay you back for travel time and mileage. It’s important to find out what will be paid for before you decide to get involved in any clinical trial. The Affordable Care Act requires that health insurance covers the routine patient care costs for people who are in approved clinical trials. “Routine patient care costs” are costs that would normally be covered for anyone being treated for your kind of cancer. Insurers are not allowed to drop or limit coverage because a person chooses to take part in a clinical trial. This applies to all clinical trials unless the insurance plan is “grandfathered.” (Grandfathered plans are those that a person was enrolled in on or before March 23, 2010 and which has not decreased benefits or increased costs.) The law requires insurance coverage for phase I, II, III, or IV clinical trials related to prevention, detection, or treatment of cancer or other life-threatening disease if the clinical trial meets one of these requirements:
- It’s federally funded (any US federal agency such as the National Cancer Institute, Centers for Disease Control, Department of Defense, etc.)
- It’s covered under an investigational new drug application (IND) that’s reviewed by the FDA
- It’s does not need an IND application.
- Insurers do not need to pay for:
- The treatment, device, or service that’s being studied. This is usually paid for by the trial’s sponsor
- Items and services only needed for data collection and analysis and not used in direct patient care
- Any service that’s clearly not in line with standards of care for a certain type of cancer. This is why it is important to know what the clinical trial may not pay for so you can check if your insurance will.
Medicare coverage for clinical trials
If you have Medicare, it pays for many of the routine patient care costs for people with cancer who are in approved clinical trials. This is true no matter where in the United States you live. Medicare normally covers any cancer care when it’s part of either:
- A clinical trial for the diagnosis and treatment of cancer; or
- A clinical trial funded by the National Cancer Institute (NCI), NCI-Designated Cancer Centers, NCI-Sponsored Clinical Trials Cooperative Groups, or another federal agency that funds cancer research
If you’re not sure if your trial meets all the requirements, discuss these concerns with your doctor.
Osteosarcoma prognosis
If the osteosarcoma has not spread to the lungs (pulmonary metastasis), long-term survival rates are better. If the osteosarcoma has spread to other parts of the body, the outlook is worse. However, there is still a chance of cure with effective treatment.
Survival rates can give you an idea of what percentage of people with the same type and stage of cancer are still alive a certain amount of time (usually 5 years) after they were diagnosed. They can’t tell you how long a person will live, but they may help give you a better understanding of how likely it is that treatment will be successful.
Keep in mind that survival rates are estimates and are often based on previous outcomes of large numbers of people who had a specific cancer, but they can’t predict what will happen in any particular person’s case. These statistics can be confusing and may lead you to have more questions. Ask your doctor how these numbers might apply to you (or your child).
The Surveillance, Epidemiology, and End Results (SEER) database, maintained by the National Cancer Institute, tracks 5-year relative survival rates for osteosarcoma in the United States, based on how far the cancer has spread. The SEER database, however, does not group cancers by MSTS or TNM stages (stage 1, stage 2, stage 3, etc.). Instead, it groups cancers into localized, regional, and distant stages:
- Localized: There is no sign that the cancer has spread outside of the bone where it started.
- Regional: The cancer has spread outside the bone and into nearby structures, or it has reached nearby lymph nodes.
- Distant: The cancer has spread to distant parts of the body, such as to the lungs or to bones in other parts of the body.
Table 3. Osteosarcoma 5-year relative survival rates
| Surveillance, Epidemiology, and End Results (SEER)* stage | 5-year relative survival rate |
|---|---|
| Localized | 76% |
| Regional | 64% |
| Distant | 24% |
| All SEER stages combined | 59% |
Footnotes:
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread. But other factors, such as those listed below, can also affect a person’s outlook.
- People now being diagnosed with osteosarcoma may have a better outlook than these numbers show. Treatments improve over time, and these numbers are based on people who were diagnosed and treated at least 5 years earlier.
Factors that can affect a person’s prognosis (outlook)
Factors other than the stage of the cancer can also affect survival rates. For example, factors that have been linked with a better prognosis include:
- Being younger (child or teen, as opposed to an adult)
- Being female
- The tumor being on an arm or leg (as opposed to the hip or other bones)
- The tumor(s) being completely resectable (removable with surgery)
- Normal blood alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) levels
- The tumor responding well to chemotherapy.
- Signs and Symptoms of Osteosarcoma. https://www.cancer.org/cancer/types/osteosarcoma/detection-diagnosis-staging/signs-and-symptoms.html[↩][↩][↩][↩]
- Key Statistics for Osteosarcoma. https://www.cancer.org/cancer/types/osteosarcoma/about/key-statistics.html[↩][↩][↩][↩]
- Biermann JS, ed: Orthopaedic Knowledge Update: Musculoskeletal Tumors 3. Rosemont, IL, American Academy of Orthopaedic Surgeons, 2014, p. 161.[↩]
- Weisstein JS, Goldsby RE, O’Donnell RJ. Oncologic approaches to pediatric limb preservation. J Am Acad Orthop Surg. 2005 Dec;13(8):544-54. doi: 10.5435/00124635-200512000-00007[↩]
- Halder GC, Patsa S, Jadav RB, Ray JG. Osteosarcoma of mandible: A case report. Int J Case Rep Images 2015;6(5):280–285. https://www.ijcasereportsandimages.com/archive/2015/005-2015-ijcri/CR-10506-05-2015-halder/ijcri-1050605201506-halder.pdf[↩]
- Vasquez L, Tejada V, Maza I, Mendoza R. Primary osteosarcoma of the skull in teenager. BMJ Case Rep. 2019 Sep 16;12(9):e229585. doi: 10.1136/bcr-2019-229585[↩]
- Campanacci M. 2nd edition. Springer-Verlag; Wien, New York: 1999. Bone and Soft Tissue Tumors.[↩]
- Wang J, Nord KH, O’Donell PG, Yoshida A. Parosteal osteosarcoma. In: WHO Classification of Tumours Editorial Board. Soft tissue and bone tumours. Lyon (France): International Agency for Research on Cancer; 2020. WHO classification of tumours series, 5th ed.; vol. 3 https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Soft-Tissue-And-Bone-Tumours-2020[↩][↩][↩][↩]
- Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007 Jun;459:40-7. doi: 10.1097/BLO.0b013e318059b8c9[↩][↩]
- Hang JF, Chen PC. Parosteal osteosarcoma. Arch Pathol Lab Med. 2014 May;138(5):694-9. doi: 10.5858/arpa.2013-0030-RS[↩][↩][↩]
- Nouri H, Ben Maitigue M, Abid L, Nouri N, Abdelkader A, Bouaziz M, Mestiri M. Surface osteosarcoma: Clinical features and therapeutic implications. J Bone Oncol. 2015 Nov 10;4(4):115-23. doi: 10.1016/j.jbo.2015.07.002[↩][↩]
- Assi T, Kattan J, Nassereddine H, Rassy E, Briand S, Court C, Verret B, Le Cesne A, Mir O. Chemotherapy in the management of periosteal osteosarcoma: A narrative review. J Bone Oncol. 2021 Sep 10;30:100389. doi: 10.1016/j.jbo.2021.100389[↩][↩][↩][↩][↩]
- Rose PS, Dickey ID, Wenger DE, Unni KK, Sim FH. Periosteal osteosarcoma: long-term outcome and risk of late recurrence. Clin Orthop Relat Res. 2006 Dec;453:314-7. doi: 10.1097/01.blo.0000229341.18974.95[↩][↩]
- Babu M, Chacko A, S ST, Abraham L, George D. Periosteal Osteosarcoma of the Distal Shaft of Fibula: Case Report on Rare Entity. J Orthop Case Rep. 2023 Jun;13(6):5-10. doi: 10.13107/jocr.2023.v13.i06.3674[↩][↩]
- Liu XW, Zi Y, Xiang LB, Han TY. Periosteal osteosarcoma: a review of clinical evidence. Int J Clin Exp Med. 2015 Jan 15;8(1):37-44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4358427[↩][↩]
- Gulia A, Puri A, Pruthi M, Desai S. Oncological and functional outcome of periosteal osteosarcoma. Indian J Orthop. 2014 May;48(3):279-84. doi: 10.4103/0019-5413.132518[↩]
- Bonar SFM, Klein MJ, O’Donell PG. Periosteal osteosarcoma. In: WHO Classification of Tumours Editorial Board. Soft tissue and bone tumours. Lyon (France): International Agency for Research on Cancer; 2020. WHO classification of tumours series, 5th ed.; vol. 3 https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Soft-Tissue-And-Bone-Tumours-2020[↩][↩][↩][↩]
- Yoshida A. Osteosarcoma: Old and New Challenges. Surg Pathol Clin. 2021 Dec;14(4):567-583. doi: 10.1016/j.path.2021.06.003[↩]
- Fox MG, Trotta BM. Osteosarcoma: review of the various types with emphasis on recent advancements in imaging. Semin Musculoskelet Radiol. 2013 Apr;17(2):123-36. doi: 10.1055/s-0033-1342969[↩]
- Harper K, Sathiadoss P, Saifuddin A, Sheikh A. A review of imaging of surface sarcomas of bone. Skeletal Radiol. 2021 Jan;50(1):9-28. doi: 10.1007/s00256-020-03546-1[↩]
- Okada K, Unni KK, Swee RG, Sim FH. High grade surface osteosarcoma: a clinicopathologic study of 46 cases. Cancer. 1999 Mar 1;85(5):1044-54. doi: 10.1002/(sici)1097-0142(19990301)85:5<1044::aid-cncr6>3.0.co;2-a[↩]
- Ritts GD, Pritchard DJ, Unni KK, Beabout JW, Eckardt JJ. Periosteal osteosarcoma. Clin Orthop Relat Res. 1987 Jun;(219):299-307.[↩]
- Campanacci M. Springer; New York: 1999. Bone and Soft Tissue Tumors. Padova: Piccin Nuova Libraria and Wien.[↩]
- Lichtenstein L. Tumors of periosteal origin. Cancer. 1955;8(5):1060–1069. doi: 10.1002/1097-0142(1955)8:5<1060::aid-cncr2820080533>3.0.co;2-7[↩]
- Unni KK, Dahlin DC, Beabout JW. Periosteal osteogenic sarcoma. Cancer. 1976 May;37(5):2476-85. doi: 10.1002/1097-0142(197605)37:5<2476::aid-cncr2820370541>3.0.co;2-c[↩]
- Campanacci M, Giunti A. Periosteal osteosarcoma. Review of 41 cases, 22 with long-term follow-up. Ital J Orthop Traumatol. 1976 Apr;2(1):23-35.[↩]
- Zhao L, Qin Y, Ma D, Wang W, Li S, Liu H. Isolated diaphragmatic metastasis from periosteal osteosarcoma of the humerus: a case report. J Cardiothorac Surg. 2020 Apr 16;15(1):61. doi: 10.1186/s13019-020-01103-4[↩]
- Chan CM, Lindsay AD, Spiguel ARV, Gibbs CP Jr, Scarborough MT. Periosteal Osteosarcoma: A Single-Institutional Study of Factors Related to Oncologic Outcomes. Sarcoma. 2018 Sep 27;2018:8631237. doi: 10.1155/2018/8631237[↩]
- Barakat MJ, Collins C, Dixon JH. Distal recurrence of periosteal osteosarcoma after complete excision of proximal primary tumour with good excision margins. Sarcoma. 2003;7(2):79-80. doi: 10.1080/13577140310001607310[↩]
- Chano T, Matsumoto K, Ishizawa M, Morimoto S, Hukuda S, Okabe H. Periosteal osteosarcoma and parosteal chondrosarcoma evaluated by double immunohistochemical staining. Report of 2 cases. Acta Orthop Scand. 1994 Jun;65(3):355-8. doi: 10.3109/17453679408995471[↩]
- Dash H, Little JR, Zaino R, Colao DJ, Chaurushiya P, Schoolwerth AC, O’Neill MJ, Hart JC, Martin GB. Metastatic periosteal osteosarcoma causing cardiac and renal failure. Am J Med. 1983 Jul;75(1):145-9. doi: 10.1016/0002-9343(83)91178-6[↩]
- Hoshi M, Matsumoto S, Manabe J, Tanizawa T, Shigemitsu T, Takeuchi K, Kawaguchi N. Three cases with periosteal osteosarcoma arising from the femur. J Orthop Sci. 2006 Mar;11(2):224-8. doi: 10.1007/s00776-005-0981-x[↩]
- Cesari M, Alberghini M, Vanel D, Palmerini E, Staals EL, Longhi A, Abate M, Ferrari C, Balladelli A, Ferrari S. Periosteal osteosarcoma: a single-institution experience. Cancer. 2011 Apr 15;117(8):1731-5. doi: 10.1002/cncr.25718[↩][↩]
- Grimer RJ, Bielack S, Flege S, Cannon SR, Foleras G, Andreeff I, Sokolov T, Taminiau A, Dominkus M, San-Julian M, Kollender Y, Gosheger G; European Musculo Skeletal Oncology Society. Periosteal osteosarcoma–a European review of outcome. Eur J Cancer. 2005 Dec;41(18):2806-11. doi: 10.1016/j.ejca.2005.04.052[↩][↩][↩][↩][↩][↩][↩]
- Gao ZH, Yin JQ, Liu DW, Meng QF, Li JP. Preoperative easily misdiagnosed telangiectatic osteosarcoma: clinical-radiologic-pathologic correlations. Cancer Imaging. 2013 Dec 11;13(4):520-6. doi: 10.1102/1470-7330.2013.0042[↩][↩]
- Farr GH, Huvos AG, Marcove RC, Higinbotham NL, Foote FW Jr. Telangiectatic osteogenic sarcoma. A review of twenty-eight cases. Cancer. 1974 Oct;34(4):1150-8. doi: 10.1002/1097-0142(197410)34:4<1150::aid-cncr2820340426>3.0.co;2-s[↩][↩][↩]
- Matsuno T, Unni KK, McLeod RA, Dahlin DC. Telangiectatic osteogenic sarcoma. Cancer. 1976 Dec;38(6):2538-47. doi: 10.1002/1097-0142(197612)38:6<2538::aid-cncr2820380643>3.0.co;2-1[↩][↩][↩]
- Rosen G, Huvos AG, Marcove R, Nirenberg A. Telangiectatic osteogenic sarcoma. Improved survival with combination chemotherapy. Clin Orthop Relat Res. 1986 Jun;(207):164-73.[↩]
- Bacci G, Ferrari S, Ruggieri P, Biagini R, Fabbri N, Campanacci L, Bacchini P, Longhi A, Forni C, Bertoni F. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001 Apr;72(2):167-72. doi: 10.1080/000164701317323426[↩]
- Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini G, Müller C, Tienghi A, Wiebe T, Comandone A, Böhling T, Del Prever AB, Brosjö O, Bacci G, Saeter G; Italian and Scandinavian Sarcoma Groups. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005 Dec 1;23(34):8845-52. doi: 10.1200/JCO.2004.00.5785[↩]
- Takeuchi Y, Sonobe S, Iwabuchi N, Yoshida M, Tominaga T. A Case of Telangiectatic Osteosarcoma in the Frontal Bone. NMC Case Rep J. 2021 Jun 5;8(1):159-165. doi: 10.2176/nmccrj.cr.2019-0217[↩][↩]
- Xu M, Dai N, Yang X, Guan W, Pu Y, Wang D, Yin L, Nie M. Characteristics and prognosis of telangiectatic osteosarcoma: a population-based study using the Surveillance, Epidemiology and End Results (SEER) database. Ann Transl Med. 2021 May;9(9):796. doi: 10.21037/atm-20-8001[↩]
- Huvos AG, Rosen G, Bretsky SS, Butler A. Telangiectatic osteogenic sarcoma: a clinicopathologic study of 124 patients. Cancer. 1982 Apr 15;49(8):1679-89. doi: 10.1002/1097-0142(19820415)49:8<1679::aid-cncr2820490824>3.0.co;2-2[↩][↩][↩]
- Murphey MD, wan Jaovisidha S, Temple HT, Gannon FH, Jelinek JS, Malawer MM. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003 Nov;229(2):545-53. doi: 10.1148/radiol.2292021130[↩][↩]
- Baumhoer D, Böhling TO, Cates JM, Cleton-Janson AM, Hogendoorn PC, O’Donell PG, Rosenberg AE. Osteosarcoma. In: WHO Classification of Tumours Editorial Board. Soft tissue and bone tumours. Lyon (France): International Agency for Research on Cancer; 2020. WHO classification of tumours series, 5th ed.; vol. 3 https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Soft-Tissue-And-Bone-Tumours-2020[↩][↩][↩]
- Sangle NA, Layfield LJ. Telangiectatic osteosarcoma. Arch Pathol Lab Med. 2012 May;136(5):572-6. doi: 10.5858/arpa.2011-0204-RS[↩][↩][↩][↩][↩]
- Colomina J, Peiro A, Trullols L, Garcia I. Telangiectatic osteosarcoma. J Orthop Surg (Hong Kong). 2013 Apr;21(1):96-9. doi: 10.1177/230949901302100124[↩][↩]
- Weiss A, Khoury JD, Hoffer FA, Wu J, Billups CA, Heck RK, Quintana J, Poe D, Rao BN, Daw NC. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007 Apr 15;109(8):1627-37. doi: 10.1002/cncr.22574[↩]
- Vanel D, Tcheng S, Contesso G, Zafrani B, Kalifa C, Dubousset J, Kron P. The radiological appearances of telangiectatic osteosarcoma. A study of 14 cases. Skeletal Radiol. 1987;16(3):196-200. doi: 10.1007/BF00356952[↩][↩]
- Chawla SP, Benjamin RS. Effectiveness of chemotherapy in the management of metastatic telangiectatic osteosarcoma. Am J Clin Oncol. 1988 Apr;11(2):177-80. doi: 10.1097/00000421-198804000-00018[↩]
- Gomes H, Menanteau B, Gaillard D, Behar C. Telangiectatic osteosarcoma. Pediatr Radiol. 1986;16(2):140-3. doi: 10.1007/BF02386639[↩]
- Suh YL, Chi JG. Telangiectatic osteosarcoma–a case report. J Korean Med Sci. 1989 Jun;4(2):97-101. doi: 10.3346/jkms.1989.4.2.97[↩]
- Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;(270):135-9.[↩]
- Bacci G, Bertoni F, Longhi A, Ferrari S, Forni C, Biagini R, Bacchini P, Donati D, Manfrini M, Bernini G, Lari S. Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity. Histologic response to preoperative chemotherapy correlates with histologic subtype of the tumor. Cancer. 2003 Jun 15;97(12):3068-75. doi: 10.1002/cncr.11456[↩]
- Bacci G, Picci P, Ferrari S, Sangiorgi L, Zanone A, Brach del Prever A. Primary chemotherapy and delayed surgery for non-metastatic telangiectatic osteosarcoma of the extremities. Results in 28 patients. Eur J Cancer. 1994;30A(5):620-6. doi: 10.1016/0959-8049(94)90532-0[↩]
- Yin JQ, Fu YW, Xie XB, Cheng XY, Yang XY, Liu WH, Tu J, Gao ZH, Shen JN. Telangiectatic osteosarcoma: Outcome analyses and a diagnostic model for differentiation from aneurysmal bone cyst. J Bone Oncol. 2017 Nov 26;11:10-16. doi: 10.1016/j.jbo.2017.11.003[↩][↩]
- Huang X, Zhao J, Bai J, Shen H, Zhang B, Deng L, Sun C, Liu Y, Zhang J, Zheng J. Risk and clinicopathological features of osteosarcoma metastasis to the lung: A population-based study. J Bone Oncol. 2019 Mar 7;16:100230. doi: 10.1016/j.jbo.2019.100230[↩]
- Extraskeletal osteosarcoma. https://radiopaedia.org/articles/extraskeletal-osteosarcoma?lang=us[↩][↩][↩][↩]
- Tong GY, Leow KS, Gunasekaran S, Srinavasan S, Hue SS. Primary extraskeletal osteosarcoma of small bowel mesentery presenting with acute bowel obstruction. J Radiol Case Rep. 2021 Dec 1;15(12):10-19. doi: 10.3941/jrcr.v15i12.4329[↩]
- Ito S, Terado Y, Shimojima R, Hara Y, Narita K, Tachimori Y, Goto M. Primary extraskeletal osteosarcoma of the mesentery: A case report. Int J Surg Case Rep. 2019;60:111-114. doi: 10.1016/j.ijscr.2019.05.058[↩]
- Nelson J, Mousa MS, Diaz J, Bui MM, Caracciolo JT. Unusual Presentation of Widely Metastatic Extraskeletal Osteosarcoma: Case Report. J Radiol Case Rep. 2021 Apr 30;15(4):7-16. doi: 10.3941/jrcr.v15i4.4147[↩][↩][↩][↩]
- Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F (eds) Extraskeletal osteosarcoma. In: WHO Classification of Tumours of Soft Tissue and Bone. 4th edition. IARC Press, Lyon. 2013:pp161–162.[↩][↩]
- Goldstein-Jackson SY, Gosheger G, Delling G, Berdel WE, Exner GU, Jundt G, Machatschek JN, Zoubek A, Jürgens H, Bielack SS; Cooperative Osteosarcoma Study Group COSS. Extraskeletal osteosarcoma has a favourable prognosis when treated like conventional osteosarcoma. J Cancer Res Clin Oncol. 2005 Aug;131(8):520-6. doi: 10.1007/s00432-005-0687-7[↩]
- Hoch M, Ali S, Agrawal S, Wang C, Khurana JS. Extraskeletal osteosarcoma: a case report and review of the literature. J Radiol Case Rep. 2013 Jul 1;7(7):15-23. doi: 10.3941/jrcr.v7i7.1245[↩][↩]
- Fan Z, Patel S, Lewis VO, Guadagnolo BA, Lin PP. Should High-grade Extraosseous Osteosarcoma Be Treated With Multimodality Therapy Like Other Soft Tissue Sarcomas? Clin Orthop Relat Res. 2015 Nov;473(11):3604-11. doi: 10.1007/s11999-015-4463-y[↩][↩]
- Mc Auley G, Jagannathan J, O’Regan K, Krajewski KM, Hornick JL, Butrynski J, Ramaiya N. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR Am J Roentgenol. 2012 Jan;198(1):W31-7. doi: 10.2214/AJR.11.6927[↩]
- Roller LA, Chebib I, Bredella MA, Chang CY. Clinical, radiological, and pathological features of extraskeletal osteosarcoma. Skeletal Radiol. 2018 Sep;47(9):1213-1220. doi: 10.1007/s00256-018-2908-6[↩]
- Bane BL, Evans HL, Ro JY, Carrasco CH, Grignon DJ, Benjamin RS, Ayala AG. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990 Jun 15;65(12):2762-70. doi: 10.1002/1097-0142(19900615)65:12<2762::aid-cncr2820651226>3.0.co;2-k[↩]
- Narayanan ST, Gopalakrishnan MK, Ibrahim SS, Sankar R. Extraskeletal Osteosarcoma- A Case Report. J Clin Diagn Res. 2016 Jan;10(1):ED03-4. doi: 10.7860/JCDR/2016/16183.7065[↩]
- Longhi A, Bielack SS, Grimer R, et al. Extraskeletal osteosarcoma: A European Musculoskeletal Oncology Society study on 266 patients. Eur J Cancer. 2017 Mar;74:9-16. doi: 10.1016/j.ejca.2016.12.016[↩]
- Arpornchayanon, O., Leerapun, T., Sivasomboon, C. et al. Recurrent tibial intra-cortical osteosarcoma: a case report and review of the literature. J Med Case Reports 5, 93 (2011). https://doi.org/10.1186/1752-1947-5-93[↩][↩][↩][↩]
- Intracortical osteosarcoma. https://radiopaedia.org/articles/intracortical-osteosarcoma?lang=us[↩][↩]
- Picci P, Gherlinzoni F, Guerra A. Intracortical osteosarcoma: rare entity or early manifestation of classical osteosarcoma? Skeletal Radiol. 1983;9(4):255-8. doi: 10.1007/BF00354127[↩]
- Jaffe HL: Intracortical osteogenic sarcoma. Bull Hosp Joint Dis. 1960, 21: 189-197.[↩]
- Scranton PE Jr, DeCicco FA, Totten RS, Yunis EJ. Prognostic factors in osteosarcoma. A review of 20 year’s experience at the University of Pittsburgh Health Center Hospitals. Cancer. 1975 Dec;36(6):2179-91. doi: 10.1002/cncr.2820360936[↩]
- Banic A, Hertel R. Double vascularized fibulas for reconstruction of large tibial defects. J Reconstr Microsurg. 1993 Nov;9(6):421-8. doi: 10.1055/s-2007-1006751[↩]
- Padilla RJ, Murrah VA. The spectrum of gnathic osteosarcoma: caveats for the clinician and the pathologist. Head Neck Pathol. 2011 Mar;5(1):92-9. doi: 10.1007/s12105-010-0218-y[↩][↩]
- Mahajan A, Vaish R, Desai S, Arya S, Sable N, K D’cruz A. Gnathic Osteosarcoma: Clinical, Radiologic, and Pathologic Review of Bone Beard Tumor. J Glob Oncol. 2017 Dec;3(6):823-827. doi: 10.1200/JGO.2016.006494[↩][↩][↩][↩][↩]
- Sasaki A, Miyashita H, Kawaida M, Kameyama K. Low-grade osteosarcoma is predominant in gnathic osteosarcomas: A report of seven cases of osteosarcoma of the jaw. Clin Exp Dent Res. 2021 Dec;7(6):1175-1182. doi: 10.1002/cre2.442[↩]
- Elawad MF, Sibira DM, Ammar A, Szabados L, Ibrahem RE. Synchronous gnathic osteosarcoma and B-cell lymphoblastic lymphoma/leukemia: A rare case presentation. Radiol Case Rep. 2023 Sep 6;18(11):4085-4090. doi: 10.1016/j.radcr.2023.08.028[↩][↩]
- Gnathic osteosarcoma. https://radiopaedia.org/articles/gnathic-osteosarcoma?lang=us[↩]
- Paparella ML, Olvi LG, Brandizzi D, Keszler A, Santini-Araujo E, Cabrini RL. Osteosarcoma of the jaw: an analysis of a series of 74 cases. Histopathology. 2013 Oct;63(4):551-7. doi: 10.1111/his.12191[↩][↩][↩][↩]
- van den Berg H, Schreuder WH, de Lange J. Osteosarcoma: A Comparison of Jaw versus Nonjaw Localizations and Review of the Literature. Sarcoma. 2013;2013:316123. doi: 10.1155/2013/316123[↩][↩]
- Nissanka EH, Amaratunge EA, Tilakaratne WM. Clinicopathological analysis of osteosarcoma of jaw bones. Oral Dis. 2007 Jan;13(1):82-7. doi: 10.1111/j.1601-0825.2006.01251.x[↩][↩][↩][↩]
- Granowski-LeCornu M, Chuang SK, Kaban LB, August M. Osteosarcoma of the jaws: factors influencing prognosis. J Oral Maxillofac Surg. 2011 Sep;69(9):2368-75. doi: 10.1016/j.joms.2010.10.023[↩]
- Demicco EG, Deshpande V, Nielsen GP, Kattapuram SV, Rosenberg AE. Well-differentiated osteosarcoma of the jaw bones: a clinicopathologic study of 15 cases. Am J Surg Pathol. 2010 Nov;34(11):1647-55. doi: 10.1097/PAS.0b013e3181f7dac6[↩]
- Lee RJ, Arshi A, Schwartz HC, Christensen RE. Characteristics and prognostic factors of osteosarcoma of the jaws: a retrospective cohort study. JAMA Otolaryngol Head Neck Surg. 2015 May 1;141(5):470-7. doi: 10.1001/jamaoto.2015.0340[↩]
- Baumhoer D, Brunner P, Eppenberger-Castori S, Smida J, Nathrath M, Jundt G. Osteosarcomas of the jaws differ from their peripheral counterparts and require a distinct treatment approach. Experiences from the DOESAK Registry. Oral Oncol. 2014 Feb;50(2):147-53. doi: 10.1016/j.oraloncology.2013.10.017[↩]
- Bennett JH, Thomas G, Evans AW, Speight PM. Osteosarcoma of the jaws: a 30-year retrospective review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Sep;90(3):323-32. doi: 10.1067/moe.2000.108274[↩][↩]
- Jasnau S, Meyer U, Potratz J, Jundt G, Kevric M, Joos UK, Jürgens H, Bielack SS; Cooperative Osteosarcoma Study Group COSS. Craniofacial osteosarcoma Experience of the cooperative German-Austrian-Swiss osteosarcoma study group. Oral Oncol. 2008 Mar;44(3):286-94. doi: 10.1016/j.oraloncology.2007.03.001[↩]
- Thariat J, Schouman T, Brouchet A, Sarini J, Miller RC, Reychler H, Ray-Coquard I, Italiano A, Verite C, Sohawon S, Bompas E, Dassonville O, Salas S, Aldabbagh K, Maingon P, de La MotteRouge T, Kurtz JE, Usseglio J, Kerbrat P, Raoul G, Lotz JP, Bar-Sela G, Brugières L, Chaigneau L, Saada E, Odin G, Marcy PY, Thyss A, Julieron M. Osteosarcomas of the mandible: multidisciplinary management of a rare tumor of the young adult a cooperative study of the GSF-GETO, Rare Cancer Network, GETTEC/REFCOR and SFCE. Ann Oncol. 2013 Mar;24(3):824-31. doi: 10.1093/annonc/mds507[↩]
- Lopes MA, Nikitakis NG, Ord RA, Sauk J Jr. Amplification and protein expression of chromosome 12q13-15 genes in osteosarcomas of the jaws. Oral Oncol. 2001 Oct;37(7):566-71. doi: 10.1016/s1368-8375(00)00130-5[↩]
- Guadagnolo BA, Zagars GK, Raymond AK, Benjamin RS, Sturgis EM. Osteosarcoma of the jaw/craniofacial region: outcomes after multimodality treatment. Cancer. 2009 Jul 15;115(14):3262-70. doi: 10.1002/cncr.24297[↩][↩]
- Smeele LE, Kostense PJ, van der Waal I, Snow GB. Effect of chemotherapy on survival of craniofacial osteosarcoma: a systematic review of 201 patients. J Clin Oncol. 1997 Jan;15(1):363-7. doi: 10.1200/JCO.1997.15.1.363[↩][↩]
- Ferrari D, Codecà C, Battisti N, Broggio F, Crepaldi F, Violati M, Bertuzzi C, Dottorini L, Caldiera S, Luciani A, Moneghini L, Biglioli F, Cassinelli G, Morabito A, Foa P. Multimodality treatment of osteosarcoma of the jaw: a single institution experience. Med Oncol. 2014 Sep;31(9):171. doi: 10.1007/s12032-014-0171-9[↩][↩]
- Osteosarcoma. https://radiopaedia.org/articles/osteosarcoma?lang=us[↩]
- Kumar V, Abbas AK, Fausto N et-al. Robbins and Cotran pathologic basis of disease. W B Saunders Co. (2005) ISBN:0721601871[↩]
- Kager L, Zoubek A, Kastner U, Kempf-Bielack B, Potratz J, Kotz R, Exner GU, Franzius C, Lang S, Maas R, Jürgens H, Gadner H, Bielack S; Cooperative Osteosarcoma Study Group. Skip metastases in osteosarcoma: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2006 Apr 1;24(10):1535-41. doi: 10.1200/JCO.2005.04.2978[↩]
- Osteosarcoma Stages. https://www.cancer.org/cancer/types/osteosarcoma/detection-diagnosis-staging/staging.html[↩]
- Bone Cancer Stages. https://www.cancer.org/cancer/types/bone-cancer/detection-diagnosis-staging/staging.html[↩]
- Survival Rates for Osteosarcoma. https://www.cancer.org/cancer/types/osteosarcoma/detection-diagnosis-staging/survival-rates.html[↩]