Contents
What is pouchitis
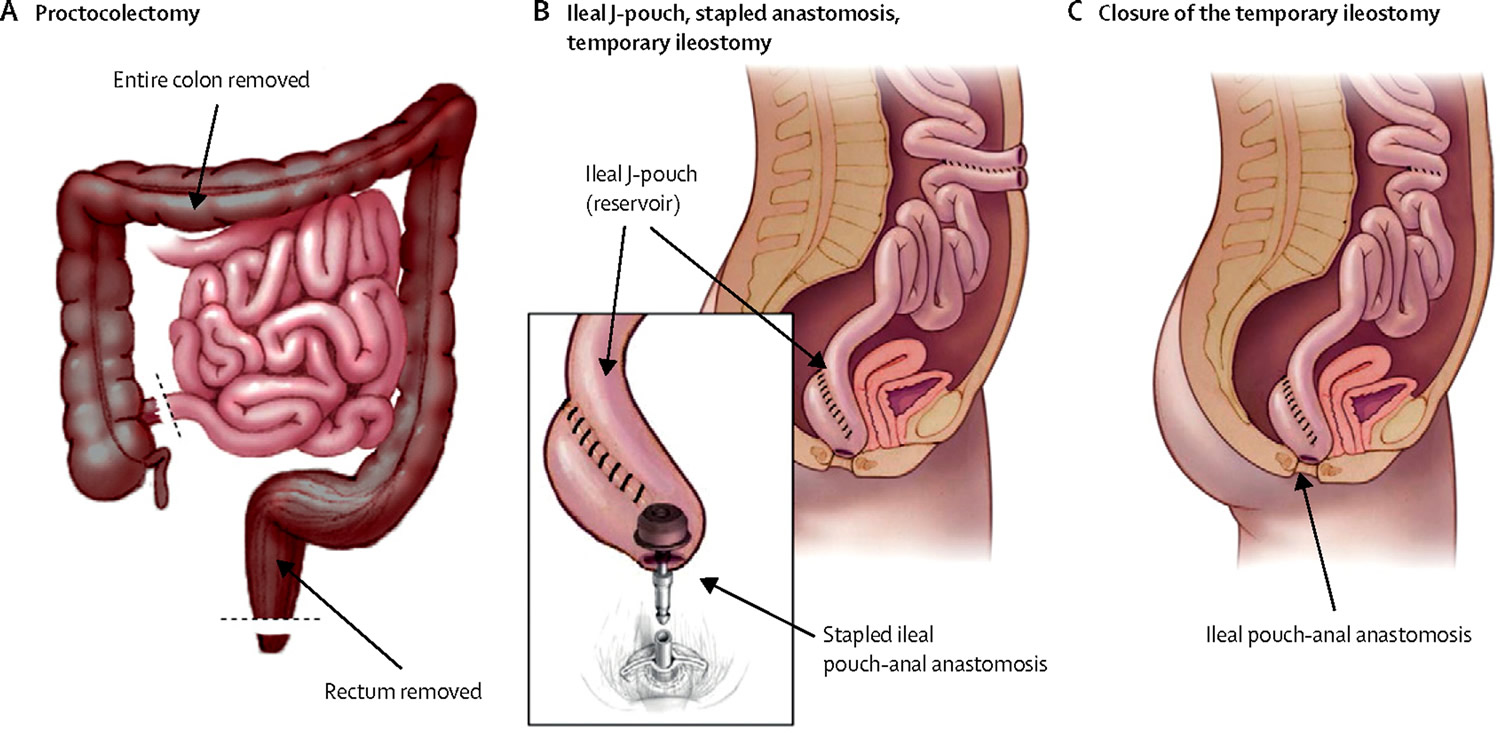
Pouchitis is inflammation that occurs in the lining of a pouch created during surgery to treat ulcerative colitis or certain other diseases (e.g. familial adenomatous polyposis) 1. Many people with ulcerative colitis need to have their diseased colon removed and the bowel reconnected with a procedure called J pouch surgery (ileoanal anastomosis) (see Figure 1 below).
Surgeons use the end of the small intestine (ileum) to create a pouch shaped like the letter J. The pouch is attached internally to the area just above the anus to hold waste before it’s eliminated.
Pouchitis is a common complication of J pouch surgery. About 5-35% of ulcerative colitis patients and 0-11% of familial adenomatous polyposis patients develop subsequent inflammation of the ileal pouch termed pouchitis 1. Pouchitis is the most common complication of ileal pouch-anal anastomosis but its pathogenesis is still largely unknown 1.
The diagnosis of pouchitis is based on the presence of symptoms plus endoscopic and histological evidence of inflammation of the pouch. In general, pouchitis can present in 3 forms – acute, relapsing or chronic.
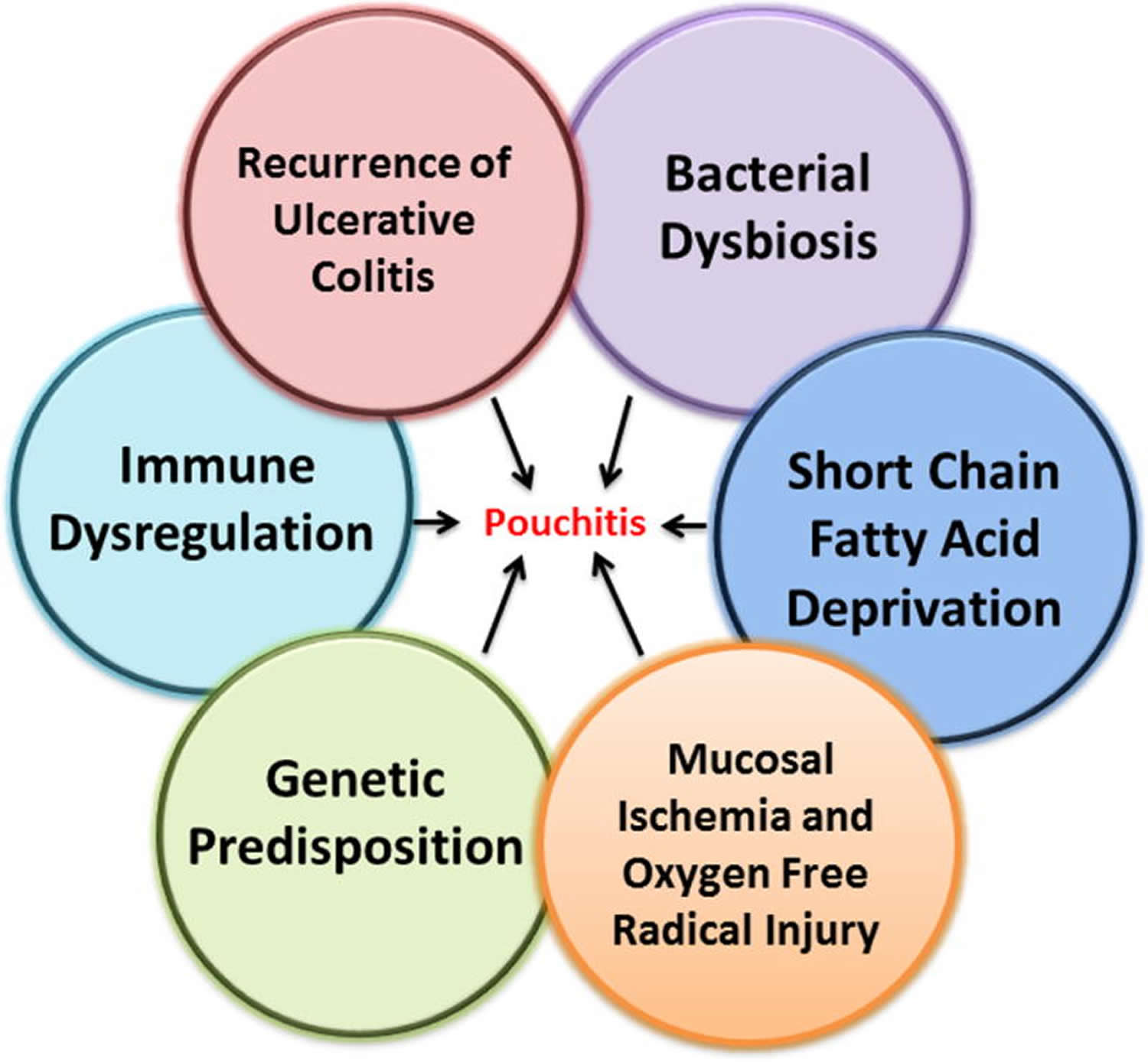
Current hypotheses suggest that the development of pouchitis might be caused by: (1) recurrence of ulcerative colitis in the colon-like ileal reservoir, (2) dysbiosis of ileal pouch microbiota, (3) short chain fatty acid (SCFA) deprivation, (4) mucosal ischemia and oxygen free radical injury, (5) genetic susceptibility, and (6) immune dysregulation. Signs and symptoms of pouchitis are similar to those of ulcerative colitis can include diarrhea, which is often bloody, abdominal pain, joint pain, cramps, a high temperature (fever), an increased number of bowel movements, nighttime fecal seepage, fecal incontinence, dehydration and a strong urge to have a bowel movement.
Speak to your doctor if you have symptoms of pouchitis. The condition can usually be successfully treated with a course of antibiotics.
- The risk of pouchitis increases the longer the J pouch is in place, and also if you smoke or have arthritis or certain skin diseases.
Sometimes pouchitis doesn’t respond to treatment. Then surgeons may need to remove the pouch and construct an ileostomy.
Most people can resume their normal activities, including work and sports, after J-pouch surgery. The surgery doesn’t affect a woman’s ability to have a normal pregnancy and delivery, but may affect fertility.
Figure 1. Ileoanal anastomosis (J pouch surgery)
Note: During the J pouch surgery, the surgeon will:
- A) Proctocolectomy: Remove the entire colon and rectum, preserving the muscles (sphincter) and opening (anus) at the end of the rectum.
- B) Construct a pouch shaped like the letter J from the end of the small intestine and attach it to the anus (opening at the end of the rectum).
- B) Construct a temporary opening in the abdominal wall (ileostomy) for eliminating waste.
- C) Remove the ileostomy a few months later. Then you can pass bowel movements through the anus, with only a slight increase in bowel movement frequency.
After about 3 months of healing, the surgeon does a second procedure to close the ileostomy, allowing you to pass stool normally.
[Source 2]The J pouch surgery
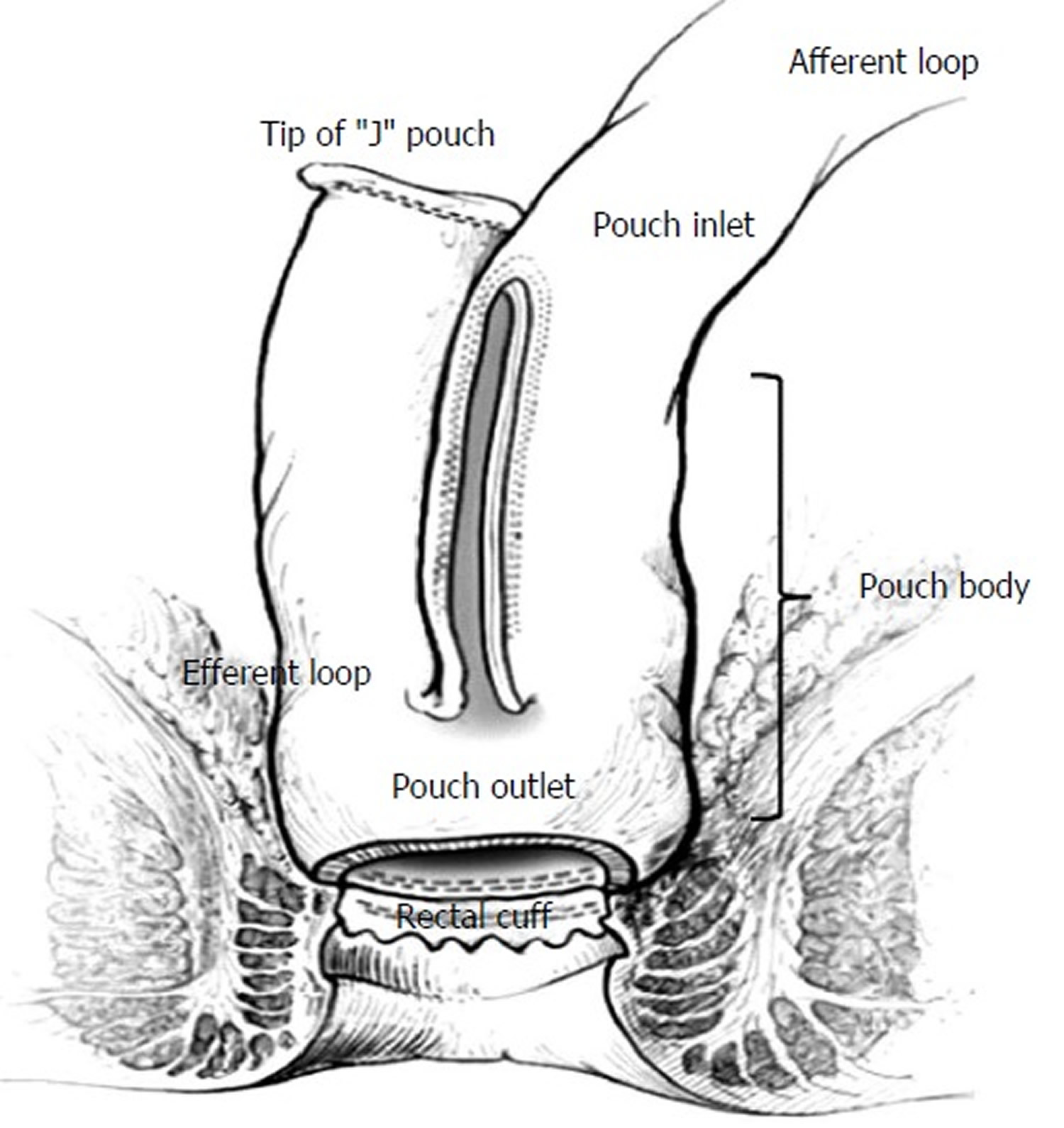
First developed by Sir Alan Parks at the St. Mark’s Hospital in London in 1978 3, an ileal pouch is formed from the terminal ileum and connected to the remnant anorectal canal, resulting in a new fecal reservoir. Currently, a J shaped pouch (“J-pouch”) is the most common type of ileal pouch, but S- or W-pouches may also be constructed 1. The surgery may take place in one-, two-, or three-stages, with the two- and three-stage surgeries including the creation of a temporary upstream ileostomy to allow healing of the ileal pouch reconstruction (see Figure 1 B). Theoretically, once the colon and rectum are removed, so is the disease (ulcerative colitis or familial adenomatous polyposis); however, about 50% of patients with an ileal pouch-anal anastomosis for ulcerative colitis develop at least one episode of subsequent inflammation of the ileal pouch, termed “pouchitis” 4. The symptomology of pouchitis is similar to that of ulcerative colitis and may include fecal urgency, incontinence, straining during defecation, hematochezia, abdominal or pelvic discomfort, fever, and malaise. Unlike ulcerative colitis which is treated with biologics, immunosuppressives, or aminosalicylates, pouchitis is most commonly and very effectively treated with a course of antibiotics. Interestingly, the incidence of pouchitis in familial adenomatous polyposis patients is considerably less (0-11%) 5, suggesting that the underlying inflammatory pathogenesis associated with ulcerative colitis may predispose those individuals to pouchitis, more so than patients with familial adenomatous polyposis.
Normally, the terminal ileum is the site of vitamin B12 and bile salt absorption, as well as other digestion products not absorbed more proximally in the small bowel. However, the ileal pouch, created during the ileal pouch-anal anastomosis surgery, functions as a fecal reservoir akin to the rectum in healthy individuals. Over time, morphological changes occur which transform the ileal mucosa to a more colonic-type mucosa in response to the new microenvironment associated with fecal stasis. Villus atrophy and crypt hyperplasia (termed “colonic metaplasia”) are two of the most common histological changes that occur in most, if not all, ileal pouches 6. In fact, some studies have shown that the degree of colonic metaplasia correlates with pouchitis 7.
Pouchitis causes
Classification of “pouchitis” according to the underlying causes
- Idiopathic pouchitis
- Secondary pouchitis
- Infectious
- Bacterial pathogens
- Clostridium difficile, Campylobacter jejuni, Salmonella typhi, Escherichia coli, Klebsiella, Pseudomonas, others
- Fungi: Candida
- Viruses: CMV
- Ischemic
- NSAID: Non-steroidal anti-inflammatory drug associated
- Collagenous
- Autoimmune-associated
- Crohn’s disease-associated
- Other diagnoses
- Cuffitis
- Irritable pouch syndrome
Based on the causes, scientists can identify 2 main diagnostic pouchitis groups – idiopathic and secondary. In “idiopathic” pouchitis, the cause and pathogenesis are unclear, while in “secondary” pouchitis, there is an association with a specific causative or pathogenetic factor 8. Secondary pouchitis occurs in up to 30% of cases and can be infectious, ischemic, non-steroidal anti-inflammatory drug (NSAID)-induced, collagenous, autoimmune-associated, or due to Crohn’s disease. Sometimes, cuffitis or irritable pouch syndrome are misdiagnosed as pouchitis. Furthermore, idiopathic pouchitis can be sub-classified in types based on the clinical pattern, presentation, and responsiveness to antibiotic treatment.
Idiopathic pouchitis
In the majority of patients with pouchitis, the cause and pathogenesis are not clear and the disease is identified as idiopathic pouchitis. But multiple hypotheses have arisen which may predispose certain individuals to disease. Clinical, endoscopic, and histological presentation is often dissimilar from patient to patient, presumably reflecting variation in host susceptibility and possibly varying exposure to other factors affecting the disease. Pouchitis is probably a widely variable disease process and the term “pouchitis” might be viewed better as an umbrella term used to describe multiple differing pathologies that end commonly with inflammation of the ileal pouch. There are six main proposed pathogenic mechanisms of pouchitis: (1) recurrence of ulcerative colitis in the colon-like ileal reservoir, (2) dysbiosis of the ileal pouch microbiota, (3) short chain fatty acid (SCFA) deprivation, (4) mucosal ischemia and oxygen free radical injury, (5) genetic predisposition, and (6) immune dysregulation (Figure 2). Development of pouchitis probably results from an interplay of several of these factors. Importantly, the relative contribution of these multiple processes may differ from individual to individual.
Figure 2. Proposed Factors Associated with Pouchitis
 Idiopathic pouchitis can be further categorized as acute, acute relapsing, or chronic. It can also be classified as antibiotic-responsive, antibiotic-dependent and antibiotic-refractory 9. It is important to emphasize that approximately 20%-30% of patients with chronic antibiotic-refractory pouchitis are mis-classified, and actually have secondary pouchitis. The management of these conditions differs from that for idiopathic pouchitis and is specific to the underlying etiology.
Idiopathic pouchitis can be further categorized as acute, acute relapsing, or chronic. It can also be classified as antibiotic-responsive, antibiotic-dependent and antibiotic-refractory 9. It is important to emphasize that approximately 20%-30% of patients with chronic antibiotic-refractory pouchitis are mis-classified, and actually have secondary pouchitis. The management of these conditions differs from that for idiopathic pouchitis and is specific to the underlying etiology.
The variety of idiopathic pouchitis classifications
- Activity
- Active
- Inactive
- Presentation
- Acute < 4 wk duration
- Chronic > 4 wk duration
- Clinical pattern
- Single episode
- Infrequent < 4 episodes a year
- Relapsing > 4 episodes a year
- Continuous
- Response to treatment
- Responsive
- Refractory
- Response to antibiotics
- Antibiotic-responsive:
- Infrequent episodes (< 4 episodes per year) responding to a 2-wk course of a single antibiotic
- Antibiotic-dependent
- Frequent episodes or persistent episodes of pouchitis requiring long-term, continuous therapy for maintaining remission
- Chronic antibiotic-refractory
- Not responding to a 4-wk course of metronidazole or ciprofloxacin, requiring prolonged therapy of ≥ 4 wk consisting of 2 or more antibiotics, oral or topical 5-ASA, corticosteroids, AZA/6-MP, or biologics
The initial treatment regimen for pouchitis is antibiotics, suggesting that the disease is at least partially mediated by a bacterial component, at least for those who respond to antibiotics. Increases in the bacterial populations that are commonly considered pathogenic are seen in the uninflamed ulcerative colitis pouch compared to familial adenomatous polyposis pouches. Increased abundance of beneficial bacteria, such as Bacteroidetes and Faecalibacterium prausnitzii are seen in familial adenomatous polyposis pouches and may be a potential reason why familial adenomatous polyposis patients rarely develop pouchitis. Probiotics do not seem to benefit induction of remission but some data is available to indicate that it may be helpful to maintain remission after standard antibiotic therapy in some patients. Longitudinal studies in the same patients would be of interest since multiple factors can influence the gut microbiota and many studies do not account for differences in diet, medication usage, and miscellaneous environmental or host genetic factors in different patients.
Secondary pouchitis
Secondary pouchitis is defined as an identifiable cause of pouchitis, distinct from the conventional or idiopathic pouchitis that has been previously discussed. In general, about 20-30% of patients with chronic pouchitis that do not respond to antibiotics can be diagnosed with some form of secondary pouchitis 10. This may include various etiologies or triggers that cause onset of disease, including strip pouchitis, Clostridium difficile infection, cytomegalovirus (CMV) infection, non-steroidal anti-inflammatory drugs (NSAIDs), or eosinophilia.
Strip pouchitis
Another variant “strip pouchitis” is actually a misnomer in that this represents inflammation of the retained rectum below the pouch anastomosis. The pouch may not be inflamed at all but the urgency, bleeding, and frequent stooling comes from an exacerbation of the underlying ulcerative colitis of the remnant rectal tissue. Treatment is usually transanal medications such as steroid suppositories or in cases with inappropriately long segments, re-operation.
Clostridium difficile-associated pouchitis
The presence of Clostridium difficile in the stool is commonly found with antibiotic-associated pseudomembranous colitis in patients with recent hospitalization and antibiotic therapy. Antibiotic-associated dysbiosis of the normal gut microbiota allows overgrowth of the opportunistic pathogen Clostridium difficile. As described in Navaneethan et al. 10, Clostridium difficile infection has been reported in ileal pouch patients and should be a consideration for patients who either do not respond to standard antibiotic therapy (metronidazole and/or ciprofloxacin) or those known to have chronic antibiotic-refractory pouchitis. Risk factors for Clostridium difficile infection-associated pouchitis may include recent hospitalization 11, weight loss 11, increased stool frequency 12, male gender 12, pre-operative comorbidities, pre-operative antibiotic use 12 and low serum IgG 13. Pre-operative Clostridium difficile infection was not found to be associated with post-operative Clostridium difficile infection-associated pouchitis 12. Identification of C. difficile toxin in the stool defines a diagnosis of Clostridium difficile infection-associated pouchitis and administration of appropriate medical therapy (e.g. vancomycin). Fecal microbiota transplant has been shown to be efficacious in treating patients with Clostridium difficile infection who are unresponsive to antibiotics. The use of fecal microbiota transplant to treat Clostridium difficile infection-associated pouchitis has not be widely studied; however, a case study presented evidence that fecal microbiota transplant was capable of treating a patient with Clostridium difficile infection-associated pouchitis 14.
Cytomegalovirus pouchitis
Cytomegalovirus is another possible infective cause of pouchitis, although very rare. Cytomegalovirus is a β-herpes virus that usually remains dormant in infected individuals; however, infants and immunosuppressed individuals are at risk for developing reactivated Cytomegalovirus infection. If suspected, the virus may be identified by Cytomegalovirus DNA PCR or the detection of viral inclusion bodies on hematoxylin and eosin stained histopathology slides. A retrospective study of 2,559 ileal pouch patients identified seven that had positive Cytomegalovirus infection in the ileal pouch. Of the seven patients, four were on immunosuppressive agents following liver transplant for primary sclerosing cholangitis, one was on azathioprine and steroids for refractory pouchitis, and two had no immunosuppressive therapy. Logistic regression analysis identified that individuals of the female gender or on immunosuppressives were at highest risk for Cytomegalovirus pouchitis 15. Whether Cytomegalovirus is the cause of pouchitis or simply a bystander is unknown. Nonetheless, if Cytomegalovirus pouchitis is encountered, treatment with ganciclovir was shown to reduce viral load and improve symptoms 15.
NSAID-associated pouchitis
Regular usage of nonsteroidal anti-inflammatory drugs (NSAIDs) has been shown to promote development of pouchitis. Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit cyclooxygenase-1 (COX-1) and COX-2 which are normally increased during inflammation to promote mucosal healing. NSAID use is a known risk factor for pouchitis as well as inflammatory bowel disease (IBD). Symptoms of NSAID-associated pouchitis are similar to that of ulcerative colitis, including bloody stools and abdominal pain. If the patient is believed to have NSAID-associated pouchitis, the most appropriate treatment is to withdraw NSAID use and prescribe antibiotics 16.
Eosinophilic pouchitis
Eosinophils are inflammatory cells whose levels are normally increased during allergic reaction and parasitic infection. Mucosal eosinophils have been found in intestinal tissue of patients with inflammatory bowel disease (IBD); but, the exact contribution of these cells to inflammatory bowel disease (IBD) has yet to be established. However, eosinophils are known to affect inflammation and the normal gastrointestinal microenvironment 17. Eosinophilia of the ileal pouch was associated with left-sided colitis compared to those with pancolitis. Individuals with no history of NSAID use were at higher risk for eosinophilia of the afferent pouch limb 18. It is not known if ileal pouch eosinophilia is associated with allergy or specific microbiota. If associated with allergy, antihistamines would be a more appropriate treatment than antibiotic therapy.
Risk factors for pouchitis
Disease-associated risk factors
Defining the risk factors associated with pouchitis may help to potentially gain insight into pathogenesis, and to possibly identify those individuals which may be difficult to treat and require long-term maintenance therapy. The presence of extraintestinal manifestations, including arthritis, primary sclerosing cholangitis, reflux ileitis, and osteoporosis are positively associated with the subsequent onset of pouchitis 19. Pouchitis can also be influenced by pre-surgical, surgical, and post-surgical factors (Table 1). Patients with a concomitant autoimmune disorder have a higher risk for developing antibiotic-refractory pouchitis 20. Factors that are not associated with pouchitis include anal transition zone inflammatory features 21, straight pull-through vs. J-pouch 22, fecal alpha 1-antitrypsin levels 23 and patients with portal vein thrombi 24. Pancolitis and extensive disease are significant risk factors for pouchitis 25, although this association was not seen in all studies 26. Some of these risk factors may suggest differing pathologies, including fecal stasis, technical failures, ischemia, systemic immune dysregulation or other metabolic factors (e.g. anemia).
Table 1. Disease-associated predictive factors for development of pouchitis
| Pre-surgical | Surgical | Post-surgical |
|---|---|---|
| Pre-operative steroid use27 | Hand sewn anastomosis28 | Positive fecal lactoferrin23 |
| Primary sclerosing cholangitis29 | Anastomosis placed <0.5cm from pectinate line28 | Non-steroidal anti-inflammatory drug (NSAID) use30 |
| Pulmonary comorbidity31 | S-pouch construction31 | Iron deficiency anemia32 |
| Extraintestinal manifestations19 | Thrombocytosis33 | |
| Backwash ileitis34 | Longer duration of follow-up/time since pouch construction33 | |
| Pancolitis/extensive disease35 | Ulcerative gastroduodenal lesions36 | |
| Ulcerative colitis Disease severity37 | Non-daily proton pump inhibitor (PPI)/H2 antagonist use38 | |
| Steroid dependency37 | ||
| First-degree relative with IBD39 | ||
| Chronic active inflammation of the appendix40 | ||
| Presence of a concomitant autoimmune disorder20 |
Dietary factors and smoking
Diet is potentially one of the most influential factors that determines the composition of the gut microbiota and it therefore potentially represents a significant risk factor for the development of pouchitis 41. A small retrospective pilot study examined whether consuming the FODMAP (fermentable oligo-, di-, and mono-saccharides and polyols) diet influenced pouchitis in seven patients with either ileal pouch or ileorectal anastomosis. This diet avoids foods that are poorly absorbed and thus increase fecal output, such as high lactose dairy, gluten, wheat, rye, barley, beans, some fruits and vegetables, and high fructose corn syrup. Five (71%) patients adhered to the diet and displayed noticeable improvement of pouchitis symptoms, while two (29%) patients who deviated from the diet manifested chronic pouchitis 42. Furthermore, this study also included a small prospective analysis of an additional eight patients. However, the results were inconclusive due to the high withdrawal rate and poor adherence to the diet 42. In a second study reported by an Israeli group, pouchitis-free patients consumed twice as many servings of fruit, more liposoluble antioxidants (e.g. cryptoxanthin commonly found in orange/yellow fruits and vegetables and lycopene found in tomatoes and other red fruits), and more vitamins A and C compared to those who had a history of pouchitis 43. A third study reported that supplementing the diet of ileal pouch patients without clinical signs of pouchitis with 24 g/day of the dietary fiber inulin found a 62% increase in the luminal content of the short chain fatty acid (SCFA) butyrate. Additionally, Bacteroides fragilis abundance was decreased and PDAI endoscopic and histologic scores were reduced compared to individuals consuming a placebo 44. Short chain fatty acids (SCFAs) are important to maintaining colonic health and in particular, butyrate is the primary fuel source for the colon-like ileal pouch 45.
Smoking is a recognized protective factor for the development of ulcerative colitis 46, but its role in pouchitis is not as well-defined. In a study reported in 2007, acute pouchitis was defined as episodes that were antibiotic-responsive occurring at least four months apart while chronic pouchitis were those patients who were antibiotic-dependent and antibiotic-refractory. Multivariate analysis found that smoking increased the risk of developing acute pouchitis, but reduced the risk for chronic pouchitis 33. The risk of pouchitis for never smokers is similarly conflicting. Some studies have found that individuals that have never smoked had a higher risk for developing pouchitis 30; however, this finding was not confirmed in subsequent studies 47.
Pouchitis symptoms
The symptoms of pouchitis is similar to that of ulcerative colitis and may include fecal urgency, incontinence, straining during defecation, hematochezia, abdominal or pelvic discomfort, fever, and malaise.
Signs and symptoms of pouchitis include:
- an urgent need to have a bowel movement (tenesmus)
- inability to defecate despite urgency
- feeling tired
- nausea or loss of appetite
- weight loss
- fever
- anemia—a condition in which the body has fewer red blood cells than normal
- rectal pain
- rectal bleeding — passing small amount of blood with stool
- in children, failure to grow
Less common symptoms include:
- joint pain or soreness
- eye irritation
- certain rashes.
Pouchitis treatment
Standard medical therapy includes 10-14 days of oral antibiotics most commonly consisting of ciprofloxacin and/or metronidazole. Ciprofloxacin may be preferable since it eradicates potentially pathogenic bacteria while maintaining normal anaerobic bacterial populations. Ciprofloxacin also lowers fecal pH to a level that is more comparable to that seen in uninflamed ileal pouch patients 48.
Pouchitis is classified into three categories based on the response to standard antibiotic-based medical therapy 4:
- Antibiotic-responsive,
- Antibiotic-dependent, and
- Antibiotic-refractory.
Patients who respond favorably to a 10-14 day course of antibiotics are considered to be antibiotic-responsive. Of those patients with acute pouchitis, it is estimated that about 39% will have only one acute episode that will respond to treatment with antibiotics while 61% will have at least one subsequent episode 19. About 7-19% of pouchitis patients will require long-term continuous use of antibiotics to maintain pouchitis remission and these individuals are considered antibiotic-dependent 49. A small percentage of patients (<5%) may be classified as antibiotic-refractory 50. These patients do not respond to antibiotic therapy and in many cases, require aminosalicylates (e.g. mesalamine), immunosuppressive (e.g. 6-mercaptopurine), or biologics to induce or maintain remission. Secondary causes of pouchitis (e.g. Clostridium difficile, viral, or medication-induced) and the presence of Crohn’s disease, another type of inflammatory bowel disease (IBD), in the ileal pouch should be considered in the differential diagnosis for antibiotic-refractory patients.
Primary prophylaxis of a first episode of pouchitis
The prevention of pouchitis begins in the operative room during surgical construction of the pouch. A suitable-sized, not too long pouch, is less susceptible to pouchitis 51. Excessive weight gain postoperatively has been associated with an increased risk for worse pouch outcomes, including pouchitis 52. Moreover, the increase in fruit consumption and intake of antioxidants, vitamin A, and vitamin C may protect from pouchitis 53. The use of probiotics, i.e., VSL#3, has been shown to be beneficial in the primary prevention of pouchitis 54. The administration of Lactobacillus rhamnosus GG has also shown to be effective in the primary prophylaxis 55. However, these treatments are expensive and the long-term benefit or safety is as yet unknown 56.
Treatment of acute idiopathic pouchitis
Patients with a first episode of acute pouchitis typically respond rapidly to antibiotic therapy. Metronidazole, ciprofloxacin, tinidazole, and rifaximin have all been used in the treatment of acute pouchitis in clinical practice[62]. First-line therapy includes a 2-wk treatment with metronidazole (15-20 mg/kg per day) or ciprofloxacin (1 g/d). High-dose VSL#3 has been reported to be effective for treating mild acute pouchitis 57. It is noteworthy that patients who experience pouchitis symptoms immediately post-ileoanal anastomosis and do not respond to the antibiotic therapy, surgery-associated complications, such as pouch anastomotic leaks, should be suspected 58.
Secondary prophylaxis of subsequent episodes of pouchitis
Relapse of pouchitis or recurrent pouchitis is common (60%) after treatment and resolution of the initial episode, and some of the patients will develop treatment-refractory disease. Long-term administration of the probiotic VSL#3 has been shown to be effective in maintaining antibiotic-induced pouchitis remission in 85% of treated patients in a 9-month period 57. However, other studies have failed to confirm this beneficial effect of VSL#3 59. Rifaximin may be an alternative maintenance treatment 60.
Treatment of chronic antibiotic-refractory pouchitis
Chronic antibiotic-refractory pouchitis may respond to longer courses of antibiotic combinations such as ciprofloxacin and rifaximin 61, ciprofloxacin and metronidazole 62 or ciprofloxacin and tinidazole 63. In patients with antibiotic-resistant pouchitis, a thorough investigation is recommended. Fecal cultures and antibiotic sensitivity testing may be needed to choose the appropriate effective antibiotics. Additionally, the investigation should aim to identify and treat causes of secondary pouchitis.
Despite the atypical endoscopic findings of C. difficile infection in pouch patients, management choices should reflect standard practices for the treatment of this infection 64. Finally, recent studies have suggested that fecal microbiota transplantation might be an alternative or adjunctive treatment for refractory C. difficile infection pouchitis 65. CMV-pouchitis can be treated with oral or intravenous anti-CMV agents (ganciclovir) 66. Management should reflect current practice.
If NSAID-associated pouchitis is suspected, a trial of discontinuation of NSAIDs is recommended, and should result in prompt resolution. The safety of selective COX-2 inhibitors in ileoanal anastomosis patients is still unknown and if those suffering from arthralgias do not respond to acetaminophen (paracetamol), they can be tried on sulfasalazine 67.
Ischemic pouchitis may be treated with allopurinol. Allopurinol is a scavenger of oxygen-derived free radicals and previous studies have shown its beneficial effect both in an animal model and in treating active pouchitis 68.
Autoimmune pouchitis, both PSC- and IgG4-associated, can be treated with corticosteroids (prednisone or budesonide), immunomodulators (azathioprine, 6-mercaptopurine), or anti-TNF biologics (infliximab, adalimumab, others). The same treatment options apply for Crohn’s pouchitis together with endoscopic or surgical interventions for fistulizing or stenosing disease 69. With a view to pouch preservation, management should be relatively aggressive; unless antibiotics are highly effective, immunomodulators and/or biologics should be used, with the goal of mucosal healing.
Cuffitis can be treated similarly to ulcerative proctitis with topical 5-ASA or topical steroids; suppositories should be sufficient. Cuffitis refractory to topical treatment should raise the suspicion of other conditions in the perianal and peri-pouch area. Therefore, investigation with the appropriate additional imaging studies is recommended to rule out surgical complications or Clostridium difficile manifestations including fistulas, sinus tracts or abscesses 70.
Finally, the treatment of irritable pouch syndrome is empiric similar to the irritable bowel syndrome, and therapies can include dietary modifications, anti-diarrheals, antispasmodics, and tricyclic antidepressants.
- Schieffer KM, Williams ED, Yochum GS, Koltun WA. Review article: the pathogenesis of pouchitis. Alimentary pharmacology & therapeutics. 2016;44(8):817-835. doi:10.1111/apt.13780. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5785099/[↩][↩][↩][↩]
- Ulcerative colitis. Ordás, Ingrid et al. The Lancet , Volume 380 , Issue 9853 , 1606 – 1619 http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(12)60150-0/fulltext[↩]
- Parks A, Nicholls R. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978;2:85–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1605901/pdf/brmedj00134-0015.pdf[↩]
- Shen B, Lashner BA. Diagnosis and treatment of pouchitis. Gastroenterol Hepatol (N Y) 2008;4:355–61. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3093723/[↩][↩]
- McLaughlin SD, Clark SK, Tekkis PP, Nicholls RJ, Ciclitira PJ. The bacterial pathogenesis and treatment of pouchitis. Therap Adv Gastroenterol. 2010;3:335–48 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3002591/[↩]
- Arashiro RT, Teixeira MG, Rawet V, et al. Histopathological evaluation and risk factors related to the development of pouchitis in patients with ileal pouches for ulcerative colitis. Clinics (Sao Paulo, Brazil) 2012;67:705–10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3400158/[↩]
- Fruin AB, El-Zammer O, Stucchi AF, O’Brien M, Becker JM. Colonic metaplasia in the ileal pouch is associated with inflammation and is not the result of long-term adaptation. J Gastrointest Surg. 2003;7:246–53. discussion 253-4. https://www.ncbi.nlm.nih.gov/pubmed/12600449[↩]
- Secondary pouchitis: those with identifiable etiopathogenetic or triggering factors. Navaneethan U, Shen B. Am J Gastroenterol. 2010 Jan; 105(1):51-64. https://www.ncbi.nlm.nih.gov/pubmed/19755972/[↩]
- Acute and chronic pouchitis–pathogenesis, diagnosis and treatment. Shen B. Nat Rev Gastroenterol Hepatol. 2012 Apr 17; 9(6):323-33. https://www.ncbi.nlm.nih.gov/pubmed/22508158/[↩]
- Navaneethan U, Shen B. Secondary pouchitis: those with identifiable etiopathogenetic or triggering factors. Am J Gastroenterol. 2010;105:51–64 https://www.ncbi.nlm.nih.gov/pubmed/19755972[↩][↩]
- Li Y, Qian J, Queener E, Shen B. Risk factors and outcome of PCR-detected Clostridium difficile infection in ileal pouch patients. Inflamm Bowel Dis. 2013;19:397–403 https://www.ncbi.nlm.nih.gov/pubmed/23328770[↩][↩]
- Sun C, Du P, Wu XR, Queener E, Shen B. Preoperative Clostridium difficile infection is not associated with an increased risk for the infection in ileal pouch patients. Dig Dis Sci. 2014;59:1262–8. https://www.ncbi.nlm.nih.gov/pubmed/24504594[↩][↩][↩][↩]
- Seril DN, Ashburn JH, Lian L, Shen B. Risk factors and management of refractory or recurrent clostridium difficile infection in ileal pouch patients. Inflamm Bowel Dis. 2014;20:2226–33 https://www.ncbi.nlm.nih.gov/pubmed/25222656[↩]
- Patel LN, Schairer J, Shen B. Fecal transplantation therapy for Clostridium difficile-associated pouchitis. Int J Colorectal Dis. 2014;29:263–4 https://www.ncbi.nlm.nih.gov/pubmed/24132529[↩]
- McCurdy JD, Loftus EV, Jr, Tremaine WJ, et al. Cytomegalovirus infection of the ileoanal pouch: clinical characteristics and outcomes. Inflamm Bowel Dis. 2013;19:2394–9 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4085480/[↩][↩]
- Tonolini M. Acute nonsteroidal anti-inflammatory drug-induced colitis. J Emerg Trauma Shock. 2013;6:301–3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3841543/[↩]
- Woodruff SA, Masterson JC, Fillon S, Robinson ZD, Furuta GT. Role of eosinophils in inflammatory bowel and gastrointestinal diseases. J Pediatr Gastroenterol Nutr. 2011;52:650–61. https://www.ncbi.nlm.nih.gov/pubmed/21593640[↩]
- Shen B, Plesec T, Remzi FH, et al. Evaluation of tissue eosinophilia in the pouch and afferent limb in patients with restorative proctocolectomy. Inflamm Bowel Dis. 2008;14:744–9 https://www.ncbi.nlm.nih.gov/pubmed/18286609[↩]
- Lohmuller JL, Pemberton JH, Dozois RR, Ilstrup D, van Heerden J. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosis. Ann Surg. 1990;211:622–9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1358238/[↩][↩][↩]
- Shen B, Remzi FH, Nutter B, et al. Association between immune-associated disorders and adverse outcomes of ileal pouch-anal anastomosis. Am J Gastroenterol. 2009;104:655–64 https://www.ncbi.nlm.nih.gov/pubmed/19262522[↩][↩]
- Wasserman M, Hyman N, Iyer A, Wilcox R, Osler T. The natural history of anal transition zone inflammation and possible relationship to pouchitis: a long-term longitudinal study. Colorectal Dis. 2013;15:1493–8 https://www.ncbi.nlm.nih.gov/pubmed/23777389[↩]
- Rintala RJ, Lindahl H. Restorative proctocolectomy for ulcerative colitis in children–is the J-pouch better than straight pull-through? J Pediatr Surg. 1996;31:530–3 https://www.ncbi.nlm.nih.gov/pubmed/8801306[↩]
- Parsi MA, Shen B, Achkar JP, et al. Fecal lactoferrin for diagnosis of symptomatic patients with ileal pouch-anal anastomosis. Gastroenterology. 2004;126:1280–6 https://www.ncbi.nlm.nih.gov/pubmed/15131788[↩][↩]
- Ball CG, MacLean AR, Buie WD, Smith DF, Raber EL. Portal vein thrombi after ileal pouch-anal anastomosis: its incidence and association with pouchitis. Surg Today. 2007;37:552–7. https://www.ncbi.nlm.nih.gov/pubmed/17593473[↩]
- Hashavia E, Dotan I, Rabau M, Klausner JM, Halpern Z, Tulchinsky H. Risk factors for chronic pouchitis after ileal pouch-anal anastomosis: a prospective cohort study. Colorectal Dis. 2012;14:1365–71. https://www.ncbi.nlm.nih.gov/pubmed/22339717[↩]
- Samarasekera DN, Stebbing JF, Kettlewell MG, Jewell DP, Mortensen NJ. Outcome of restorative proctocolectomy with ileal reservoir for ulcerative colitis: comparison of distal colitis with more proximal disease. Gut. 1996;38:574–7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1383117/[↩]
- Okita Y, Araki T, Tanaka K, et al. Characteristics of extremely early-onset pouchitis after proctocolectomy with ileal pouch-anal anastomosis. J Gastrointest Surg. 2013;17:533–9 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3568199/[↩]
- Gozzetti G, Poggioli G, Marchetti F, et al. Functional outcome in handsewn versus stapled ileal pouch-anal anastomosis. Am J Surg. 1994;168:325–9 https://www.ncbi.nlm.nih.gov/pubmed/7943588[↩][↩]
- Wasmuth H, Tranø G, Endreseth B, Wibe A, Rydning A, Myrvold H. Primary sclerosing cholangitis and extraintestinal manifestations in patients with ulcerative colitis and ileal pouch-anal anastomosis. J Gastrointest Surg. 2010;14:1099–104 https://www.ncbi.nlm.nih.gov/pubmed/20480253[↩]
- Shen B, Fazio VW, Remzi FH, et al. Risk factors for diseases of ileal pouch-anal anastomosis after restorative proctocolectomy for ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4:81–9. https://www.ncbi.nlm.nih.gov/pubmed/16431309[↩][↩]
- Lipman JM, Kiran RP, Shen B, Remzi F, Fazio VW. Perioperative factors during ileal pouch-anal anastomosis predict pouchitis. Dis Colon Rectum. 2011;54:311–7 https://www.ncbi.nlm.nih.gov/pubmed/21304302[↩][↩]
- Pastrana RJ, Torres EA, Arroyo JM, Rivera CE, Sanchez CJ, Morales L. Iron-deficiency anemia as presentation of pouchitis. J Clin Gastroenterol. 2007;41:41–4 https://www.ncbi.nlm.nih.gov/pubmed/17198064[↩]
- Fleshner P, Ippoliti A, Dubinsky M, et al. A prospective multivariate analysis of clinical factors associated with pouchitis after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2007;5:952–8. https://www.ncbi.nlm.nih.gov/pubmed/17544871[↩][↩][↩]
- Abdelrazeq AS, K N, BI D, et al. Predictors for acute and chronic pouchitis following restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2008;10:805–13 https://www.ncbi.nlm.nih.gov/pubmed/18005192[↩]
- Lipman JM, Kiran RP, Shen B, Remzi F, Fazio VW. Perioperative factors during ileal pouch-anal anastomosis predict pouchitis. Dis Colon Rectum. 2011;54:311–7. https://www.ncbi.nlm.nih.gov/pubmed/21304302[↩]
- Hisabe T, Matsui T, Miyaoka M, et al. Diagnosis and clinical course of ulcerative gastroduodenal lesion associated with ulcerative colitis: possible relationship with pouchitis. Dig Endosc. 2010;22:268–74. https://www.ncbi.nlm.nih.gov/pubmed/21175478[↩]
- Kalkan IH, Dağli Ü, Önder F, et al. Evaluation of preoperative predictors of development of pouchitis after ileal-pouch-anastomosis in ulcerative colitis. Clin Res Hepatol Gastroenterol. 2012;36:622–7. https://www.ncbi.nlm.nih.gov/pubmed/22705025[↩][↩]
- Poritz LS, Sehgal R, Berg AS, Laufenberg L, Choi C, Williams ED. Chronic use of PPI and H2 antagonists decreases the risk of pouchitis after IPAA for ulcerative colitis. J Gastrointest Surg. 2013;17:1027–31. https://www.ncbi.nlm.nih.gov/pubmed/23532599[↩]
- Stahlberg D, Gullberg K, Liljeqvist L, Hellers G, Lofberg R. Pouchitis following pelvic pouch operation for ulcerative colitis. Incidence, cumulative risk, and risk factors. Dis Colon Rectum. 1996;39:1012–8. https://www.ncbi.nlm.nih.gov/pubmed/8797652[↩]
- Yantiss RK, Sapp HL, Farraye FA, et al. Histologic predictors of pouchitis in patients with chronic ulcerative colitis. Am J Surg Pathol. 2004;28:999–1006 https://www.ncbi.nlm.nih.gov/pubmed/15252305[↩]
- Graf D, Di Cagno R, Fåk F, et al. Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis. 2015;26:26164 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4318938/[↩]
- Croagh C, Shepherd SJ, Berryman M, Muir JG, Gibson PR. Pilot study on the effect of reducing dietary FODMAP intake on bowel function in patients without a colon. Inflamm Bowel Dis. 2007;13:1522–8 https://www.ncbi.nlm.nih.gov/pubmed/17828776[↩][↩]
- Ianco O, Tulchinsky H, Lusthaus M, et al. Diet of patients after pouch surgery may affect pouch inflammation. World J Gastroenterol. 2013;19:6458–64. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3801317/[↩]
- Welters C, Heineman E, Thunnissen F, van den Bogaard A, Soeters P, Baeten C. Effect of dietary inulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis Colon Rectum. 2002;45:621–7. https://www.ncbi.nlm.nih.gov/pubmed/12004211[↩]
- Chapman MA, Hutton M, Grahn MF, Williams NS. Metabolic adaptation of terminal ileal mucosa after construction of an ileoanal pouch. Br J Surg. 1997;84:71–3. https://www.ncbi.nlm.nih.gov/pubmed/9043459[↩]
- Bastida G, Beltran B. Ulcerative colitis in smokers, non-smokers and ex-smokers. World J Gastroenterol. 2011;17:2740–7 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3122262/[↩]
- Joelsson M, Benoni C, Oresland T. Does smoking influence the risk of pouchitis following ileal pouch anal anastomosis for ulcerative colitis? Scand J Gastroenterol. 2006;41:929–33 https://www.ncbi.nlm.nih.gov/pubmed/16803691[↩]
- Gosselink MP, Schouten WR, van Lieshout LM, Hop WC, Laman JD, Ruseler-van Embden J. Eradication of pathogenic bacteria and restoration of normal pouch flora: comparison of metronidazole and ciprofloxacin in the treatment of pouchitis. Dis Colon Rectum. 2004;47:1519–25. https://www.ncbi.nlm.nih.gov/pubmed/15486751[↩]
- Mowschenson PM, Critchlow JF, Peppercorn MA. Ileoanal pouch operation: long-term outcome with or without diverting ileostomy. Arch Surg. 2000;135:463–5. discussion 465-6. https://www.ncbi.nlm.nih.gov/pubmed/10768713[↩]
- Madiba TE, Bartolo DC. Pouchitis following restorative proctocolectomy for ulcerative colitis: incidence and therapeutic outcome. J R Coll Surg Edinb. 2001;46:334–7. https://www.ncbi.nlm.nih.gov/pubmed/11768572[↩]
- Brisinda G, Vanella S, Valenza V, Crocco A, Perotti G, Di Giuda D, Maria G. Surgical prophylaxis of pouchitis in ulcerative colitis. Dig Dis Sci. 2011;56:1257–1265 https://www.ncbi.nlm.nih.gov/pubmed/21127981[↩]
- Liu Z, Song H, Shen B. Pouchitis: prevention and treatment. Curr Opin Clin Nutr Metab Care. 2014;17:489–495. https://www.ncbi.nlm.nih.gov/pubmed/25023187[↩]
- Ianco O, Tulchinsky H, Lusthaus M, Ofer A, Santo E, Vaisman N, Dotan I. Diet of patients after pouch surgery may affect pouch inflammation. World J Gastroenterol. 2013;19:6458–6464 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3801317/[↩]
- Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209 https://www.ncbi.nlm.nih.gov/pubmed/12730861[↩]
- Gosselink MP, Schouten WR, van Lieshout LM, Hop WC, Laman JD, Ruseler-van Embden JG. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum. 2004;47:876–884 https://www.ncbi.nlm.nih.gov/pubmed/15108026[↩]
- Liu Z, Song H, Shen B. Pouchitis: prevention and treatment. Curr Opin Clin Nutr Metab Care. 2014;17:489–495 https://www.ncbi.nlm.nih.gov/pubmed/25023187[↩]
- Holubar SD, Cima RR, Sandborn WJ, Pardi DS. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2010;(6):CD001176. https://www.ncbi.nlm.nih.gov/pubmed/20556748[↩][↩]
- Shen B, Remzi FH, Lavery IC, Lashner BA, Fazio VW. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin Gastroenterol Hepatol. 2008;6:145–158; quiz 124 https://www.ncbi.nlm.nih.gov/pubmed/18237865[↩]
- Shen B, Brzezinski A, Fazio VW, Remzi FH, Achkar JP, Bennett AE, Sherman K, Lashner BA. Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practice. Aliment Pharmacol Ther. 2005;22:721–728 https://www.ncbi.nlm.nih.gov/pubmed/16197493[↩]
- Shen B, Remzi FH, Lopez AR, Queener E. Rifaximin for maintenance therapy in antibiotic-dependent pouchitis. BMC Gastroenterol. 2008;8:26 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2442097/[↩]
- Abdelrazeq AS, Kelly SM, Lund JN, Leveson SH. Rifaximin-ciprofloxacin combination therapy is effective in chronic active refractory pouchitis. Colorectal Dis. 2005;7:182–186 https://www.ncbi.nlm.nih.gov/pubmed/15720360[↩]
- Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment Pharmacol Ther. 2002;16:909–917. https://www.ncbi.nlm.nih.gov/pubmed/11966499[↩]
- Shen B, Fazio VW, Remzi FH, Bennett AE, Lopez R, Brzezinski A, Oikonomou I, Sherman KK, Lashner BA. Combined ciprofloxacin and tinidazole therapy in the treatment of chronic refractory pouchitis. Dis Colon Rectum. 2007;50:498–508 https://www.ncbi.nlm.nih.gov/pubmed/17279300[↩]
- Nitzan O, Elias M, Chazan B, Raz R, Saliba W. Clostridium difficile and inflammatory bowel disease: role in pathogenesis and implications in treatment. World J Gastroenterol. 2013;19:7577–7585. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3837256/[↩]
- Patel LN, Schairer J, Shen B. Fecal transplantation therapy for Clostridium difficile-associated pouchitis. Int J Colorectal Dis. 2014;29:263–264. https://www.ncbi.nlm.nih.gov/pubmed/24132529[↩]
- McCurdy JD, Loftus EV, Tremaine WJ, Smyrk TC, Bruining DH, Pardi DS, Raffals LE, Kisiel JB, Coelho-Prabhu N, Kane SV, et al. Cytomegalovirus infection of the ileoanal pouch: clinical characteristics and outcomes. Inflamm Bowel Dis. 2013;19:2394–2399. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4085480/[↩]
- Abi Karam G, Awada H, Nasr F, Uthman I. Ileal pouchitis and arthritis. Semin Arthritis Rheum. 2003;33:215 https://www.ncbi.nlm.nih.gov/pubmed/14723216[↩]
- Levin KE, Pemberton JH, Phillips SF, Zinsmeister AR, Pezim ME. Role of oxygen free radicals in the etiology of pouchitis. Dis Colon Rectum. 1992;35:452–456 https://www.ncbi.nlm.nih.gov/pubmed/1568395[↩]
- Haveran LA, Sehgal R, Poritz LS, McKenna KJ, Stewart DB, Koltun WA. Infliximab and/or azathioprine in the treatment of Crohn’s disease-like complications after IPAA. Dis Colon Rectum. 2011;54:15–20 https://www.ncbi.nlm.nih.gov/pubmed/21160308[↩]
- Shen B, Lashner BA, Bennett AE, Remzi FH, Brzezinski A, Achkar JP, Bast J, Bambrick ML, Fazio VW. Treatment of rectal cuff inflammation (cuffitis) in patients with ulcerative colitis following restorative proctocolectomy and ileal pouch-anal anastomosis. Am J Gastroenterol. 2004;99:1527–1531. https://www.ncbi.nlm.nih.gov/pubmed/15307872[↩]