Contents
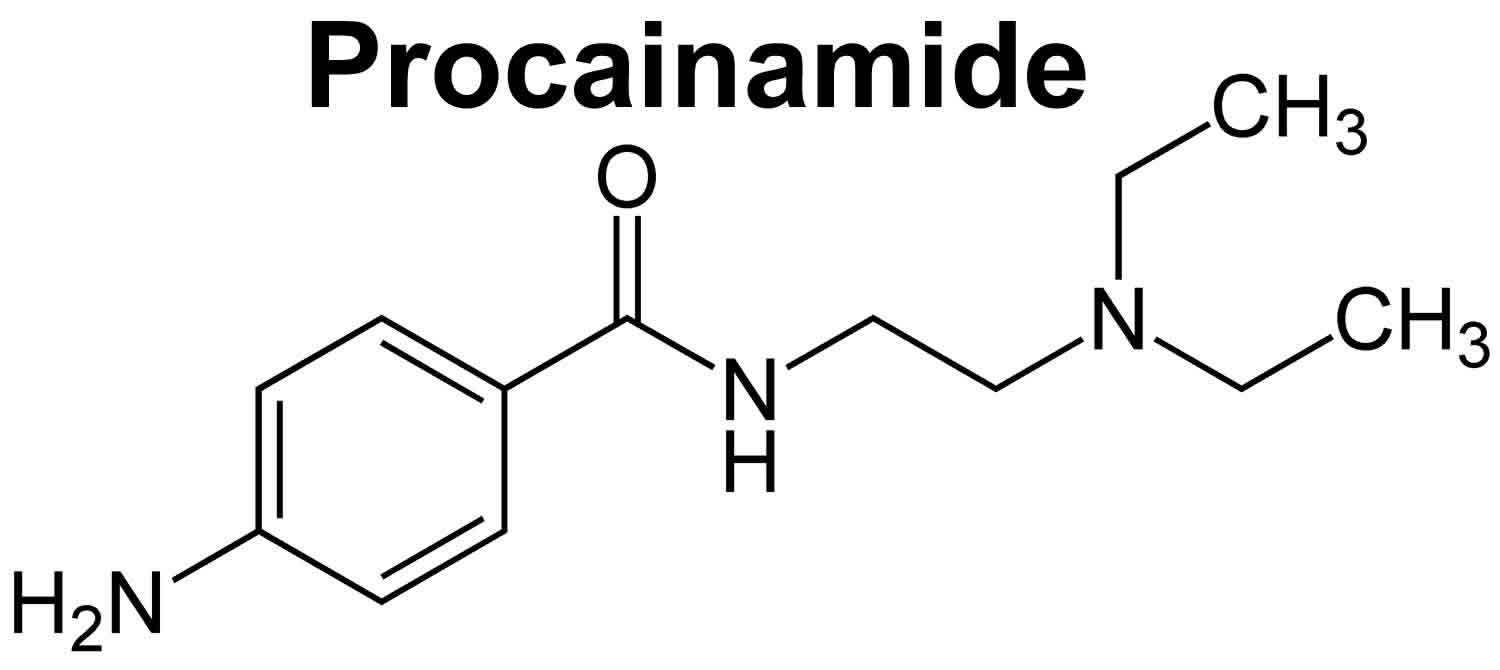
Procainamide
Procainamide is an oral antiarrhythmic agent (antiarrhythmic Class 1A) that has been in wide use for more than 60 years 1, 2. Procainamide is used to treat abnormal heart rhythms such as ventricular arrhythmias, supraventricular arrhythmias, atrial flutter, atrial fibrillation, and Wolf-Parkinson-White syndrome. Procainamide works by making your heart more resistant to abnormal activity by blocking open sodium channels and outward potassium channels. As a consequence, it decreases cardiac automaticity, increases refractory periods and slows conduction. Procainamide was approved for use in the United States in 1950, and current indications include suppression of symptomatic premature ventricular contractions and life threatening ventricular tachycardia, as well as maintenance of normal sinus rhythm after conversion of atrial fibrillation or flutter. Because of its safety profile, procainamide is now rarely used. Procainamide is available only with your doctor’s prescription.
The oral dosage forms of procainamide are no longer available in the United States but is available in Canada. Procainamide is available in capsules and tablets of 250, 375 and 500 mg generically as well as under the brand name Pronestyl; it is also available as extended release forms of 250, 500, 750 and 1,000 mg under the brand name Procanbid. Immediate-acting procainamide usually is taken every 3 or 4 hours. The long-acting product is usually taken every 6 or 12 hours. Do not cut, crush, or chew extended-release (long-acting) tablets; swallow them whole. You may see a waxy core in your stool if you are taking the extended-release product; this is normal. Procainamide is currently available in the United States as a solution for intravenous infusion. The usual maintenance dose in adults is 500 to 1000 mg every 4 to 6 hours.
Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take procainamide exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
Procainamide helps control your condition but will not cure it. Continue to take procainamide even if you feel well. Do not stop taking procainamide without talking to your doctor.
Procainamide is broken down by your liver via acetylation to form N-acetyl procainamide (NAPA) via a substrate of CYP2D6. This compound is then excreted as N-acetyl procainamide (NAPA). The half-life of procainamide is 2.5 to 5 hours, and the maximum dose in current recommendations is 17 mg/kg. As such, clinicians may consider decreasing the dosing or frequency of procainamide in cases of hepatic impairment 3.
Procainamide most common side effects include headache, nervousness, anxiety, nausea, decreased appetite, palpitations and disturbed sleep. Rare but potentially severe adverse effects include cardiac toxicity, bradycardia, hypotension, aplastic anemia, agranulocytosis, drug hypersensitivity reactions, induction of autoantibodies including antinuclear antibody and drug-induced lupus erythematosus-like syndrome 2. QRS, QTc, and PR prolongation are the most potentially harmful cardiac side effects of procainamide and may become worse when levels of procainamide rise. Serial electrocardiograms are useful for monitoring these toxic effects during treatment with procainamide. Procainamide infusion may also increase the number of premature ventricular contractions in patients.
Another side effect of procainamide is hypotension, more commonly seen at doses of 20 mg/min. Drug-induced lupus erythematosus-like syndrome is rare and occurs due to the creation of positive ANA titers when taking the medication chronically. The symptoms of chronic use may include arthritis, arthralgias, and pleuritis and commonly resolve when usage stops.
Lastly, procainamide is known to cause certain blood dyscrasias. Procainamide has been known to cause bone marrow toxicity, leading to pancytopenia or agranulocytosis; this is usually due to hypersensitivity or varied immunologic mechanisms 4, 5.
Antiarrhythmic drugs, including procainamide, may increase the risk of death. Tell your doctor if you have had a heart attack within the past two years. Procainamide should be used only to treat life-threatening arrhythmias (irregular heartbeats).
Procainamide may cause a decrease in the number of cells in your bone marrow. Procainamide may also cause symptoms of lupus.
Keep all appointments with your doctor and the laboratory. Your doctor will order certain lab tests to check your response to procainamide.
If you experience any of the following symptoms, call your doctor immediately: fever, chills, sore throat, bruising, bleeding, muscle aches or weakness, stomach or chest pain, skin rash, or blisters on the cheek, tongue and lips.
Talk to your doctor about the risks of taking procainamide.
Procainamide mechanism of action
Procainamide is an analogue of the local anesthetic procaine and has electrophysiological effects that resemble quinidine. Procainamide is a class 1A anti-arrhythmic that appears to act by blocking fast sodium channels and outward potassium channels inhibiting recovery after repolarization. Procainamide also prolongs the action potential and reduces the speed of impulse conduction. This action results in decreased myocardial excitability, slowed conduction velocity, and reduced myocardial contractility. It is possible that it acts as a negative inotrope and may cause peripheral vasodilation and hypotension, which may require cardioversion.
Procainamide special precautions
Before taking procainamide:
- tell your doctor and pharmacist if you are allergic to procainamide, anesthetics, aspirin, or any other drugs.
- tell your doctor and pharmacist what prescription and nonprescription medications you are taking, especially digoxin (Lanoxin) or drugs for high blood pressure, and vitamins.
- in addition to the condition listed in the IMPORTANT WARNING section, tell your doctor if you have or have ever had lupus, heart disease, high blood pressure, kidney or liver disease, or myasthenia gravis.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking procainamide, call your doctor.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking procainamide.
- you should know that this drug may make you dizzy. Do not drive a car or operate machinery until you know how this drug affects you.
- remember that alcohol can add to the dizziness caused by this drug.
- talk to your doctor about the use of cigarettes and caffeine-containing beverages. These products may increase the irritability of your heart and interfere with the action of procainamide.
It is important that your doctor check your progress carefully while you are receiving procainamide to make sure it is working properly. This will allow necessary changes in the amount of medicine you receive and may also help reduce side effects.
Dizziness or lightheadedness may occur with procainamide, especially in elderly patients and when large doses are used. Patients should use extra care to avoid falling. Make sure you know how you react to procainamide before you drive, use machines, or do anything else that could be dangerous if you are dizzy or not alert.
Procainamide in Pregnancy
Pregnancy Category C: Animal studies have shown an adverse effect and there are no adequate studies in pregnant women OR no animal studies have been conducted and there are no adequate studies in pregnant women.
Procainamide in Breastfeeding
There are no adequate studies in women for determining infant risk when using this medication during breastfeeding. Weigh the potential benefits against the potential risks before taking this medication while breastfeeding.
Procainamide Drug Interactions
Although certain medicines should not be used together at all, in other cases two different medicines may be used together even if an interaction might occur. In these cases, your doctor may want to change the dose, or other precautions may be necessary. When you are taking procainamide, it is especially important that your healthcare professional know if you are taking any of the medicines listed below. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using procainamide with any of the following medicines is not recommended. Your doctor may decide not to treat you with this medication or change some of the other medicines you take.
- Amisulpride
- Bepridil
- Cisapride
- Dronedarone
- Fingolimod
- Grepafloxacin
- Levomethadyl
- Mesoridazine
- Pimozide
- Piperaquine
- Saquinavir
- Sparfloxacin
- Terfenadine
- Thioridazine
- Vernakalant
- Ziprasidone
Using procainamide with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Acecainide
- Ajmaline
- Alcuronium
- Alfuzosin
- Amiodarone
- Amitriptyline
- Amoxapine
- Anagrelide
- Apomorphine
- Aprindine
- Aripiprazole
- Aripiprazole Lauroxil
- Arsenic Trioxide
- Artemether
- Asenapine
- Astemizole
- Atracurium
- Azithromycin
- Buprenorphine
- Bupropion
- Buserelin
- Ceritinib
- Chloral Hydrate
- Chloroquine
- Chlorpromazine
- Ciprofloxacin
- Cisatracurium
- Citalopram
- Clarithromycin
- Clofazimine
- Clomipramine
- Clozapine
- Crizotinib
- Dabrafenib
- Dasatinib
- Degarelix
- Delamanid
- Desipramine
- Deslorelin
- Deutetrabenazine
- Disopyramide
- Dofetilide
- Dolasetron
- Domperidone
- Donepezil
- Doxacurium
- Doxepin
- Droperidol
- Efavirenz
- Eliglustat
- Encorafenib
- Enflurane
- Erythromycin
- Escitalopram
- Flecainide
- Fluconazole
- Fluoxetine
- Foscarnet
- Gallamine
- Gatifloxacin
- Gemifloxacin
- Glasdegib
- Gonadorelin
- Goserelin
- Granisetron
- Halofantrine
- Haloperidol
- Halothane
- Hexafluorenium
- Histrelin
- Hydroquinidine
- Hydroxychloroquine
- Hydroxyzine
- Ibutilide
- Iloperidone
- Imipramine
- Inotuzumab Ozogamicin
- Isoflurane
- Isradipine
- Ivabradine
- Ivosidenib
- Ketoconazole
- Lacosamide
- Lapatinib
- Leuprolide
- Levofloxacin
- Lidocaine
- Lidoflazine
- Lofexidine
- Lopinavir
- Lorcainide
- Lumefantrine
- Macimorelin
- Mefloquine
- Methadone
- Metocurine
- Metronidazole
- Mifepristone
- Mivacurium
- Moricizine
- Moxifloxacin
- Nafarelin
- Nalidixic Acid
- Nilotinib
- Norfloxacin
- Nortriptyline
- Octreotide
- Ofloxacin
- Ondansetron
- Osimertinib
- Paliperidone
- Pancuronium
- Pasireotide
- Pazopanib
- Pentamidine
- Pimavanserin
- Pipecuronium
- Pirmenol
- Pitolisant
- Posaconazole
- Prilocaine
- Probucol
- Promethazine
- Propafenone
- Protriptyline
- Quetiapine
- Quinidine
- Quinine
- Ribociclib
- Risperidone
- Rocuronium
- Salmeterol
- Sertindole
- Sertraline
- Sevoflurane
- Siponimod
- Sodium Phosphate
- Sodium Phosphate, Dibasic
- Sodium Phosphate, Monobasic
- Solifenacin
- Sorafenib
- Sotalol
- Spiramycin
- Succinylcholine
- Sulfamethoxazole
- Sulpiride
- Sultopride
- Sunitinib
- Tacrolimus
- Telavancin
- Telithromycin
- Tetrabenazine
- Tizanidine
- Toremifene
- Trazodone
- Triclabendazole
- Trifluoperazine
- Trimethoprim
- Trimipramine
- Triptorelin
- Tubocurarine
- Vandetanib
- Vardenafil
- Vasopressin
- Vecuronium
- Vemurafenib
- Vinflunine
- Voriconazole
- Zolmitriptan
- Zotepine
- Zuclopenthixol
Using procainamide with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Cimetidine
Other Interactions
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. Discuss with your healthcare professional the use of your medicine with food, alcohol, or tobacco.
Other Medical Problems
The presence of other medical problems may affect the use of procainamide. Make sure you tell your doctor if you have any other medical problems, especially:
- Heart block or
- Heart rhythm problem (e.g., QT prolongation) or
- Lupus erythematosus, history of—Should not use in patients with these conditions.
- Heart failure, congestive or
- Myasthenia gravis—May make these conditions worse.
- Kidney disease or
- Liver disease—Use with caution. The effects may be increased because of slower removal from the body.
Procainamide contraindications
Hypersensitivity to procainamide, procaine, other ester-type local anesthetics, or any component of the formulation; complete heart block; second-degree AV block or various types of hemiblock (without a functional artificial pacemaker); systemic lupus erythematosus (SLE); Torsade de Pointes.
Use with caution in patients with heart failure, electrolyte imbalances (particularly hypokalemia and hypomagnesemia), myasthenia gravis patients, and in hepatic or renal impairment. Procainamide also crosses the placenta and may be present in the milk of breastfeeding mothers, and as such, chronic use requires caution in this population 6.
Canadian labeling: Additional contraindications (not in US labeling):
- Myasthenia gravis;
- Severe heart failure (IV);
- Renal failure (IV);
- Shock (IV).
Procainamide uses
Procainamide is used to treat abnormal heart rhythms. It works by making your heart more resistant to abnormal activity.
Ventricular arrhythmias: Intravenous: Treatment of life-threatening ventricular arrhythmias
Supraventricular arrhythmias: Oral [Canadian product]: Treatment of supraventricular arrhythmias.
Note: In the treatment of atrial fibrillation (AF), use only when preferred treatment is ineffective or cannot be used. Use in paroxysmal atrial tachycardia when reflex stimulation or other measures are ineffective.
Procainamide is indicated in patients with Wolf-Parkinson-White syndrome as it is important for acute termination of antidromic AV re-entrant tachycardia in stable patients 1. In particular, because the use of an AV nodal blocking agent in this patient population may enhance conduction down the accessory pathway and therefore induce ventricular tachycardia or ventricular fibrillation 7.
Procainamide has also historically been used in diagnostic testing for Brugada syndrome; this was known as the “procainamide challenge,” which produces the standard Brugada-like pattern on ECG, which may have been otherwise unnoticed and thus identifying patients at risk for sudden cardiac death 1. However, this usage has fallen out of favor, as it has low sensitivity for detecting Brugada-like patterns and may also put the patient at risk of going into ventricular arrhythmia 8.
Procainamide has been used for chemical cardioversion in atrial flutter and atrial fibrillation. These are common arrhythmias seen in emergency department patients, but there is no consensus for optimal management. Stiell et al. looked at the usage of IV procainamide in 341 patients over five years. Adverse events were infrequent and included hypotension, bradycardia, atrioventricular block, and ventricular tachycardia. There were no cases of torsades de pointes, cerebrovascular accidents, or death. Most patients (94.4%) received a discharge to home. IV procainamide had a 52% conversion rate of atrial fibrillation to normal sinus and a 28% conversion rate from atrial flutter to normal sinus 9.
Researchers have also studied the utility of procainamide compared to lidocaine in terminating sustained ventricular tachycardia in patients with structural heart defects. In this retrospective study from Circulation Journal, procainamide was found to be more effective than lidocaine in the termination of the arrhythmia 10.
One recent major study looked at the difference between amiodarone and procainamide in stable ventricular tachycardia. The PROCAMIO study 11 has concluded that in patients with stable ventricular tachycardia, procainamide should be considered over the traditionally used amiodarone due to faster resolution of the arrhythmia, fewer major cardiac events, and is more efficacious in a subgroup of patients with structural heart disease.
Off Label Uses
Atrial fibrillation (preexcited)
Based on the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science and the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society Guideline (AHA/ACC/HRS) for the Management of Patients With Atrial Fibrillation (AF), procainamide may be considered for the treatment of hemodynamically stable preexcited AF with rapid ventricular response in adults with preserved left ventricular function.
Junctional tachycardia
Based on the American Heart Association, American College of Clinical Cardiology and Heart Rhythm Society Guideline for the Management of Patients with Supraventricular Tachycardia, procainamide may be considered for the treatment of hemodynamically, acute junctional tachycardia when beta-blocker therapy is ineffective. Dosing not specified.
Stable monomorphic ventricular tachycardia
Based on the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science, procainamide is an effective and recommended treatment alternative for hemodynamically stable monomorphic ventricular tachycardia in adults with preserved left ventricular function. Use should be avoided in those with a prolonged QT interval.
Procainamide dose
Procainamide is given IV or Oral with the onset of action in 10 to 30 minutes 1. The loading dose of IV procainamide is 10 to 17 mg/kg and administered at a rate of 20 to 50 mg/min 1. Alternatively, IV procainamide may be dosed at 100 mg every 5 minutes in adult patients. The administration of this maintenance dose is from 1 to 4 mg/minute; however, the manufacturer labeling recommends 2 to 6 mg/minute.
Administration of oral procainamide dosing for supraventricular arrhythmia is at 50 mg/kg/24 hours divided into doses every 6 hours.
In children, IV procainamide dosing divides into those less than 12 months in which a bolus dose of 7 to 10 mg/kg given over 15 to 30 minutes and those older than 12 months in which a bolus dose of 10 to 15 mg/kg is the regimen. An infusion rate of 20 to 50 mcg/kg/min follows the initial bolus 12.
Adult dose for Arrhythmias
IV:
- Loading dose: 15 to 18 mg/kg administered as slow infusion over 25 to 30 minutes or 100 mg/dose at a rate not to exceed 50 mg/minute repeated every 5 minutes as needed to a total dose of 1 gram.
- Maintenance dose: 1 to 4 mg/minute by continuous infusion. Maintenance infusions should be reduced by one-third in patients with moderate renal or cardiac impairment and by two-thirds in patients with severe renal or cardiac impairment.
ACLS guidelines: Loading dose: Infuse 20 mg/minute (up to 50 mg/minute for more urgent situations) until arrhythmia is controlled, hypotension occurs, QRS complex widens by 50% of its original width, or total of 17 mg/kg is given. Note: Not recommended for use in ongoing ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT) due to prolonged administration time and uncertain efficacy. Follow with maintenance dose as continuous infusion.
IM:
- 50 mg/kg divided into fractional amounts of 1/8 to 1/4 and injected every 3 to 6 hours or 0.5 to 1 gram every 4 to 8 hours.
Oral: ORAL procainamide is not available in the US but is available in Canada.
40 to 50 kg:
- Immediate-release: 250 mg orally every 3 hours.
- Sustained-release: 500 mg every 6 hours.
- Twice daily formulation: 1000 mg every 12 hours.
60 to 70 kg:
- Immediate-release: 375 mg every 3 hours.
- Sustained-release: 750 mg every 6 hours.
- Twice daily formulation: 1500 mg every 12 hours.
80 to 90 kg:
- Immediate-release: 500 mg every 3 hours.
- Sustained-release: 1000 mg every 6 hours.
- Twice daily formulation: 2000 mg every 12 hours.
100 kg or more
- Immediate-release: 625 mg every 3 hours.
- Sustained-release: 1250 mg every 6 hours.
- Twice daily formulation: 2500 mg every 12 hours.
Pediatric dose for Arrhythmias
Less than 1 month:
- Loading dose: 7 to 10 mg/kg IV infused over 60 minutes followed by a continuous IV infusion of 20 to 80 mcg/kg/minute; a retrospective study of 20 neonates (GA: 25 weeks or older) reported a mean loading dose of 9.6 ± 1.5 mg/kg and a mean continuous infusion rate of 37.56 ± 13.52 mcg/kg/minute.
- Note: Procainamide serum concentrations were supratherapeutic in five neonates studied; four of the five were less than 36 weeks GA and all five had Clcr less than 30 mL/minute/1.73 m2; these results indicate that doses may need to be decreased in preterm neonates and in those with renal impairment.
1 year or older:
- Oral: (ORAL procainamide is not available in the US but is available in Canada.)
- 15 to 50 mg/kg/day divided every 3 to 6 hours. Maximum 4 g/day.
IV:
- Loading dose: 3 to 6 mg/kg over 5 minutes (not to exceed 100 mg per dose), may repeat every 5 to 10 minutes to maximum total loading dose of 15 mg/kg; do not exceed 500 mg in 30 minutes.
- Maintenance dose: continuous IV infusion: 20 to 80 mcg/kg/minute; maximum dose: 2 g/day.
IM:
- 20 to 30 mg/kg/day divided every 4 to 6 hours. Maximum 4 g/day.
Stable wide complex tachycardia of unknown origin (atrial or ventricular) or SVT (PALS, 2010): Note: Avoid or use extreme caution when administering procainamide with other drugs that prolong QT interval (e.g., amiodarone); consider consulting with expert.
Loading dose: 15 mg/kg infused intravenously over 30 to 60 minutes; monitor ECG and blood pressure; stop the infusion if hypotension occurs or QRS complex widens by more than 50% of baseline
Renal Dose Adjustments
Oral:
- CrCl less than 10 mL/min: A dosing interval of every 8 to 24 hours is recommended.
- CrCl 10 to 50 mL/min: A dosing interval of every 6 to 12 hours is recommended.
IV:
- Reduce loading dose to 12 mg/kg in severe renal impairment.
- Maintenance infusions should be reduced by one-third in patients with moderate renal impairment and by two-thirds in patients with severe renal impairment.
Liver Dose Adjustments
A 50% reduction in the dose is recommended.
What should I do if I forget a dose?
Take the missed dose as soon as you remember it. However, if it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
Procainamide side effects
Procainamide may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- dizziness or lightheadedness
- loss of appetite
- upset stomach
- vomiting
- bitter taste
If you experience the following symptom or any of those listed in the IMPORTANT WARNING section, call your doctor immediately:
- irregular heartbeat
Less common
- fever and chills
- joint pain or swelling
- pains with breathing
- skin rash or itching
Rare
- Bleeding, blistering, burning, coldness, discoloration of skin, feeling of pressure, hives, infection, inflammation, itching, lumps, numbness, pain, rash, redness, scarring, soreness, stinging, swelling, tenderness, tingling, or warmth at the injection site
- Confusion
- Fever or sore mouth, gums, or throat
- Hallucinations (seeing, hearing, or feeling things that are not there)
- Mental depression
- Unusual bleeding or bruising
- Unusual tiredness or weakness
Get emergency help immediately if any of the following symptoms of overdose occur:
Symptoms of procainamide overdose
- decrease in urination
- dizziness (severe) or fainting
- drowsiness
- fast or irregular heartbeat
- nausea and vomiting
Some side effects may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects. Check with your health care professional if any of the following side effects continue or are bothersome or if you have any questions about them:
More common
- diarrhea
- hardening or thickening of the skin where the needle is placed
- loss of appetite
Less common
- dizziness or lightheadedness
Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional.
Procainamide injection side effects
Get emergency medical help if you have any of these signs of an allergic reaction: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
Tell your caregivers at once if you have a serious side effect such as:
- a new or a worsening irregular heartbeat pattern;
- chest pain, wheezing, trouble breathing;
- feeling like you might pass out;
- signs of infection such as fever, chills, sore throat, flu symptoms, pale skin, easy bruising or bleeding (nosebleeds, bleeding gums), loss of appetite, nausea and vomiting, sores in your mouth and throat, unusual weakness;
- depressed mood, hallucinations, severe dizziness;
- upper stomach pain, itching, dark urine, clay-colored stools, jaundice (yellowing of the skin or eyes); or
- joint pain or swelling with fever, swollen glands, muscle pain or weakness, unusual thoughts or behavior, patchy skin color, red spots.
Less serious side effects may include:
- mild dizziness or tired feeling;
- flushing (warmth, redness, or tingly feeling); or
- mild itching or rash.
- Pritchard B, Thompson H. Procainamide. [Updated 2023 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557788[↩][↩][↩][↩][↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Procainamide. [Updated 2020 Jul 10]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548477[↩][↩]
- Klotz U. Antiarrhythmics: elimination and dosage considerations in hepatic impairment. Clin Pharmacokinet. 2007;46(12):985-96. doi: 10.2165/00003088-200746120-00002[↩]
- Lawson DH, Jick H. Adverse reactions to procainamide. Br J Clin Pharmacol. 1977 Oct;4(5):507-11. doi: 10.1111/j.1365-2125.1977.tb00777.x[↩]
- Danielly J, DeJong R, Radke-Mitchell LC, Uprichard AC. Procainamide-associated blood dyscrasias. Am J Cardiol. 1994 Dec 1;74(11):1179-80. doi: 10.1016/0002-9149(94)90478-2[↩]
- Pittard WB 3rd, Glazier H. Procainamide excretion in human milk. J Pediatr. 1983 Apr;102(4):631-3. doi: 10.1016/s0022-3476(83)80210-8[↩]
- Correction to: 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016 Sep 13;134(11):e232-3. doi: 10.1161/CIR.0000000000000447. Erratum for: Circulation. 2016 Apr 5;133(14):e471-505.[↩]
- Obeyesekere MN, Klein GJ. Preventing Sudden Death in Asymptomatic Wolf-Parkinson-White Patients. JACC Clin Electrophysiol. 2018 Apr;4(4):445-447. doi: 10.1016/j.jacep.2017.11.014[↩]
- Stiell IG, Sivilotti MLA, Taljaard M, Birnie D, Vadeboncoeur A, Hohl CM, McRae AD, Rowe BH, Brison RJ, Thiruganasambandamoorthy V, Macle L, Borgundvaag B, Morris J, Mercier E, Clement CM, Brinkhurst J, Sheehan C, Brown E, Nemnom MJ, Wells GA, Perry JJ. Electrical versus pharmacological cardioversion for emergency department patients with acute atrial fibrillation (RAFF2): a partial factorial randomised trial. Lancet. 2020 Feb 1;395(10221):339-349. doi: 10.1016/S0140-6736(19)32994-0[↩]
- Komura S, Chinushi M, Furushima H, Hosaka Y, Izumi D, Iijima K, Watanabe H, Yagihara N, Aizawa Y. Efficacy of procainamide and lidocaine in terminating sustained monomorphic ventricular tachycardia. Circ J. 2010 May;74(5):864-9. doi: 10.1253/circj.cj-09-0932[↩]
- Ortiz M, Martín A, Arribas F, Coll-Vinent B, Del Arco C, Peinado R, Almendral J; PROCAMIO Study Investigators. Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study. Eur Heart J. 2017 May 1;38(17):1329-1335. doi: 10.1093/eurheartj/ehw230[↩]
- Guerrier K, Shamszad P, Czosek RJ, Spar DS, Knilans TK, Anderson JB. Variation in Antiarrhythmic Management of Infants Hospitalized with Supraventricular Tachycardia: A Multi-Institutional Analysis. Pediatr Cardiol. 2016 Jun;37(5):946-52. doi: 10.1007/s00246-016-1375-x[↩]