Contents
What is serotonin
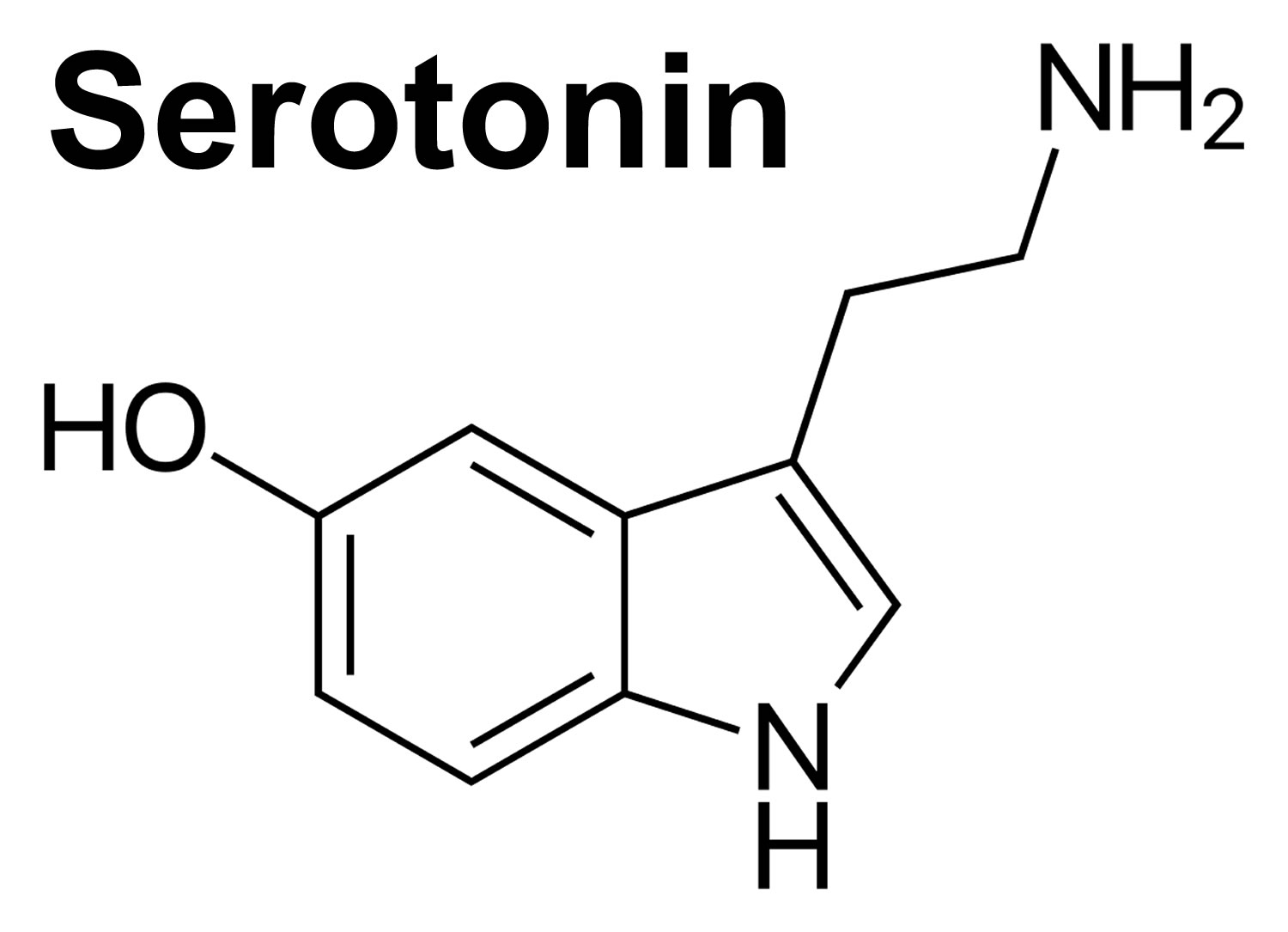
Serotonin also known as 5-hydroxytryptamine or 5-HT, is one of the a biochemical messengers and regulators, synthesized from the essential amino acid L-Tryptophan that nerve cells in the brain use to communicate. Serotonin helps transmit nerve impulses and constrict blood vessels, is a participant in the wake-sleep cycle, and affects mood. Serotonin is metabolized by the liver and its metabolites, primarily 5-HIAA (5-hydroxyindoleacetic acid), are eliminated in the urine. In healthy volunteers, the transient reduction of serotonergic neurotransmission by means of dietary depletion of tryptophan has often been associated with mood-lowering 1). Serotonin deficiency causes disorders such as depression, obsessive-compulsive disorder (OCD), phobias, posttraumatic stress disorder (PTSD), epilepsy, and generalized anxiety disorder (GAD). Modifying serotonin levels is one way that antidepressant and anti-anxiety medications are thought to work and help people feel better. selective serotonin reuptake inhibitors, whose mechanism of action is believed to rely on the enhancement of serotonergic function in the brain, are effective antidepressants 2). In vivo measurements of various components of serotonin neurotransmission in patients with major depressive disorder and their relatives all support the theory of an absolute or relative deficit in cortico-limbic pathways believed to regulate the expression of mood 3). Serotonin has three different modes of action in the nervous system: as neurotransmitter, acting locally at synaptic boutons; upon diffusion at a distance from its release sites, producing paracrine (also called volume) effects, and by circulating in the blood stream, producing hormonal effects. The three modes can affect a single neuronal circuit 4). However, the precise nature of serotonin’s role in the brain is largely unknown.
In humans serotonin is found primarily in the central nervous system (CNS), gastrointestinal tract, special cells in the bronchial tubes (lungs) and blood platelets. More than 90% of serotonin in the blood is found in the platelets. Serotonin mediates several important physiological functions including neurotransmission, gastrointestinal motility, hemostasis, and cardiovascular integrity. Multiple receptor families (serotonin receptors the serotonin receptor 1AR and serotonin receptor 2AR) explain the broad physiological actions and distribution of this biochemical mediator 5).
A significantly increased level of serotonin in a person with carcinoid syndrome symptoms is suggestive but not diagnostic of a carcinoid tumor. In order to diagnose the condition, the tumor itself must be located, biopsied, and examined by a pathologist. The healthcare practitioner will frequently follow an abnormal test result with an order for an imaging scan to help locate any tumor(s) that may be present. Someone may still have a carcinoid tumor even if the concentrations of serotonin and 5-HIAA are normal. Some carcinoid tumors do not produce serotonin or only produce it intermittently. A person with no symptoms and normal levels of serotonin and 5-HIAA is unlikely to have a serotonin-secreting carcinoid tumor.
Serotonin is produced as needed by the nervous system, mainly the brain, but also by carcinoid tumors are slow-growing masses that can form in the gastrointestinal tract (especially in the appendix) and in the lungs, although they may affect other organs as well. They are one of several types of tumors that arise from cells in the neuroendocrine system. These cells, which secrete hormones in response to signals from the nervous system, are found in organs throughout the body. The serotonin produced by carcinoid tumors may cause flushing of the face, diarrhea, a rapid heart rate, and wheezing, especially when the tumor has spread to the liver. This group of signs and symptoms is referred to as the carcinoid syndrome. According to the American Cancer Society, there are about 8,000 gastrointestinal and 4,000 lung carcinoid tumors diagnosed each year in the United States. Many more of these tumors may exist, but most remain small and do not cause any symptoms. When carcinoid tumors are discovered in asymptomatic patients during surgical procedures performed for other reasons, they are called “incidental” tumors. A small percentage of these tumors may eventually grow large enough to cause obstructions in the intestines or bronchial tubes of the lungs.
Serotonin in the nervous system acts as a local transmitter at synapses, and as a paracrine or hormonal modulator of circuits upon diffusion, allowing a wide variety of “state-dependent” behavioral responses to different stimuli 6). Such is the case of aggressive behavior and rhythmic motor patterns , including those responsible for feeding. In vertebrates, which display a wider and much more sophisticated behavioral repertoire, serotonin also modulates sleep, the arousal state, sexual behavior, and others 7).
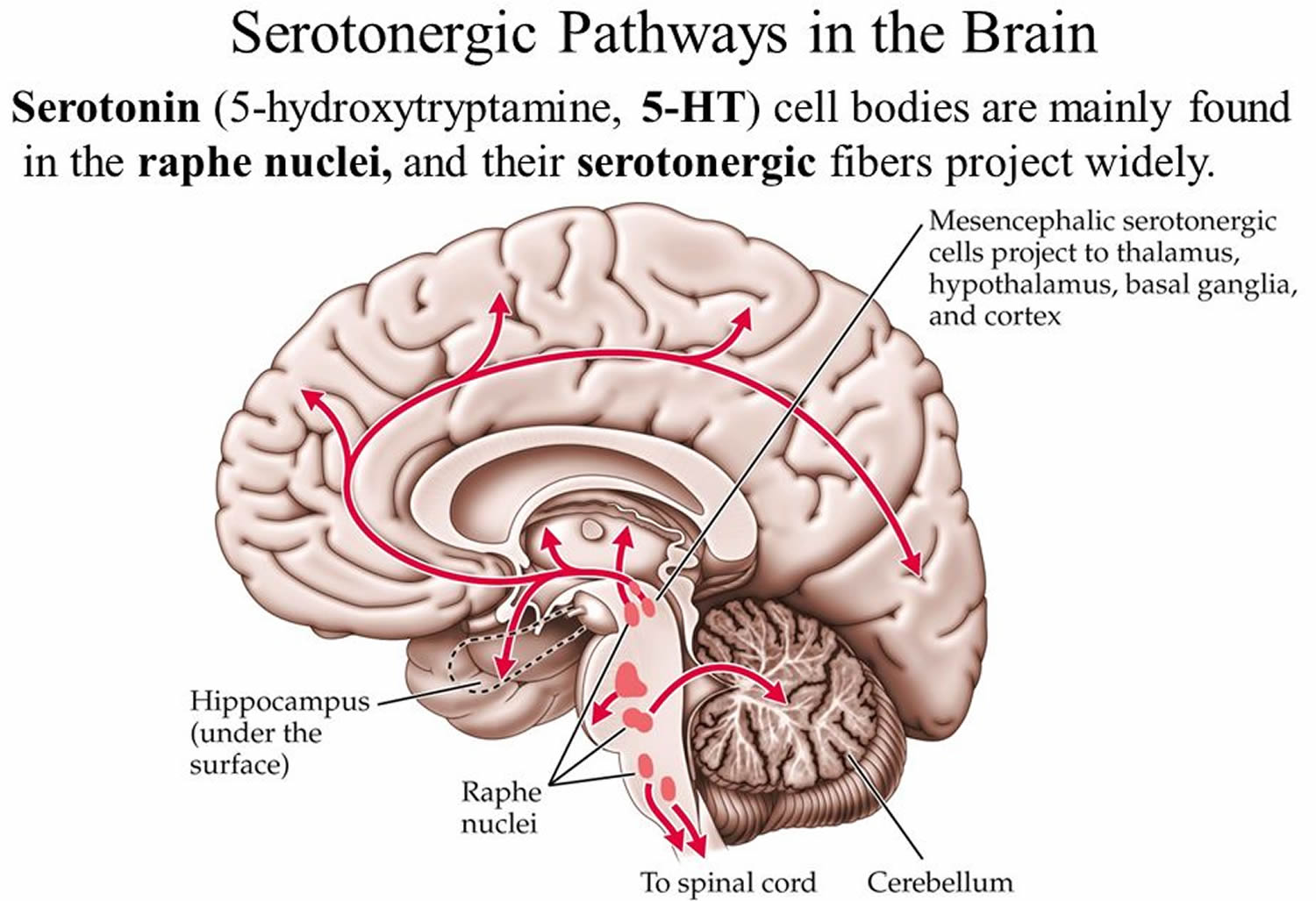
Serotonin-containing neuronal cell bodies are restricted to discrete clusters or groups of cells located along the midline of the brainstem. Their axons, however, innervate nearly every area of the CNS (Figures 1 and 2). In 1964, Dahlstrom and Fuxe 8), using the Falck-Hillarp technique of histofluorescence, observed that the majority of serotonergic soma are found in cell body groups, which previously had been designated as the raphe nuclei. This earlier description of the raphe nuclei was based on cell body structural characteristics and organization. Dahlstrom and Fuxe described nine groups of serotonin-containing cell bodies, which they designated B1 through B9, and which correspond for the most part with the raphe nuclei 9) (Table 1). Some serotonergic neuronal cell bodies, however, are found outside the raphe nuclei, and not all of the cell bodies in the raphe nuclei are serotonergic. In most of the raphe nuclei, the majority of neurons are nonserotonergic. For example, the dorsal raphe contains the largest number of serotonergic neurons; however, only 40 to 50% of the cell bodies in the dorsal raphe are serotonergic 10).
Figure 1. Serotonergic pathway in the brain
Figure 2. Location of the serotonergic cell body in the brain
Footnotes: Schematic drawing depicting the location of the serotonergic cell body groups in a sagittal section of the rat central nervous system and their major projections.
Abbreviations: OT= olfactory tuberculum; Sept = septum; C. Put = nucleus caudate-putamen; G. Pal = globus pallidus; T = thalamus; H = habenula; S. Nigra = substantia nigra.
[Source 11)]Table 1. Classification of Serotonergic Cell Body Groups According to Dahlstrom and Fuxe and Corresponding Anatomical Structure
| roups of serotonin-containing cell bodies | Anatomical structure |
|---|---|
| B1 | Raphe pallidus nucleus, caudal ventrolateral medulla |
| B2 | Raphe obscurus nucleus |
| B3 | Raphe magnus nucleus, rostral ventrolateral medulla, lateral paragigantocellular reticular nucleus |
| B4 | Raphe obscurus nucleus, dorsolateral part |
| B5 | Median raphe nucleus, caudal part |
| B6 | Dorsal raphe nucleus, caudal part |
| B7 | Dorsal raphe nucleus principal, rostral part |
| B8 | Median raphe nucleus, rostral main part; caudal linear nucleus; nucleus pontis oralis |
| B9 | Nucleus pontis oralis, supralemniscal region |
Serotonin biosynthesis
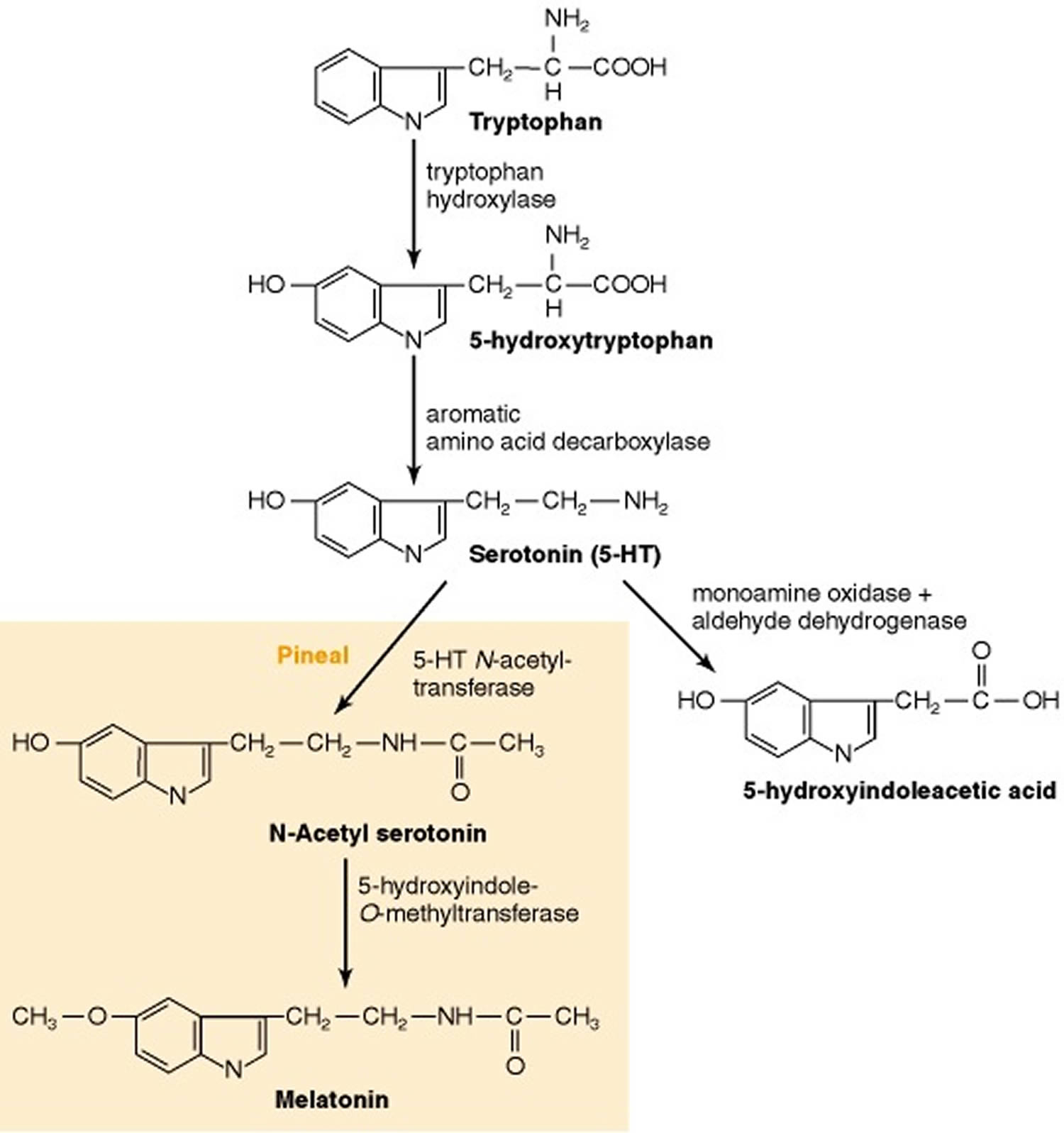
The essential amino acid L-tryptophan serves as the precursor for the synthesis of serotonin (5-hydroxytryptamine). Not all cells that contain serotonin (5-HT) synthesize it. For example, platelets do not synthesize serotonin (5-HT); rather, they accumulate serotonin (5-hydroxytryptamine) from plasma by an active-transport mechanism found on the platelet membrane 13). Certain brain cells do synthesize serotonin. The synthesis and primary metabolic pathways of serotonin are shown in Figure 3. The initial step in the synthesis of serotonin is the facilitated transport of the amino acid l-tryptophan from blood into brain. The primary source of tryptophan is dietary protein. Certain other neutral amino acids, such as phenylalanine, leucine and methionine, are transported into brain by the same carrier. The entry of tryptophan into brain is related not only to its concentration in blood but is also a function of its concentration in relation to the concentrations of other neutral amino acids. Consequently, lowering the dietary intake of tryptophan while raising intake of the amino acids that tryptophan competes with for transport into brain lowers the content of serotonin in brain and changes certain behaviors associated with serotonin function. This strategy for lowering the brain content of serotonin has been used clinically to evaluate the importance of brain serotonin in the mechanism of action of psychotherapeutic drugs 14).
According to some evidence, tryptophan, which increases brain serotonin in humans as in experimental animals 15), is an effective antidepressant in mild-to-moderate depression 16). Further, in healthy people with high trait irritability, it increases agreeableness, decreases quarrelsomeness and improves mood 17).
Interestingly, some researchers stated that purified tryptophan and foods containing tryptophan have different effects on brain serotonin. Although purified tryptophan increases brain serotonin, foods containing tryptophan do not 18). This is because tryptophan is transported into the brain by a transport system that is active toward all the large neutral amino acids and tryptophan is the least abundant amino acid in protein. There is competition between the various amino acids for the transport system, so after the ingestion of a meal containing protein, the rise in the plasma level of the other large neutral amino acids will prevent the rise in plasma tryptophan from increasing brain tryptophan. The idea, common in popular culture, that a high-protein food such as turkey will raise brain tryptophan and serotonin is, unfortunately, false 19). Another popular myth that is widespread on the internet is that bananas improve mood because of their serotonin content. Although it is true that bananas contain serotonin, it does not cross the blood–brain barrier 20). α-Lactalbumin, a minor constituent of milk, is one protein that contains relatively more tryptophan than most proteins. Acute ingestion of α-lactalbumin by humans can improve mood and cognition in some circumstances, presumably owing to increased serotonin 21).
Serotonergic neurons contain the enzyme l-tryptophan-5-monooxygenase, more commonly termed tryptophan hydroxylase, which converts tryptophan to 5-hydroxytryptophan (5-HTP) (Figure 3). Tryptophan hydroxylase enzyme is synthesized in serotonergic cell bodies of the raphe nuclei and is found only in cells that synthesize serotonin; its distribution in brain is similar to that of serotonin itself. Tryptophan hydroxylase enzyme requires both molecular oxygen and a reduced pteridine cofactor, such as l-erythro-tetrahydrobiopterin (BH4), for activity. In the enzymatic reaction, one atom of oxygen is used to form 5-HTP (5-hydroxytryptophan) and the other is reduced to water. The pteridine cofactor donates electrons, and the unstable quinonoid dihydrobiopterin that results is regenerated immediately to the tetrahydrobiopterin form by a NADPH-linked enzymatic reaction. If the concentration of tryptophan in serotonergic neurons is assumed to be comparable to that in whole brain, the enzyme would not be saturated with substrate and the formation of serotonin (5-HT) in brain would be expected to rise as the brain concentration of tryptophan increases. This occurs specifically in response to raising the dietary intake of tryptophan. However, the relationship among tryptophan availability, total tissue serotonin (5-HT) concentration and serotonin (5-HT) release is not fully understood.
The other enzyme involved in the synthesis of serotonin, aromatic l-amino acid decarboxylase (AADC), is a soluble pyridoxal-5′-phosphate-dependent enzyme which converts 5-hydroxytryptophan (5-HTP) to serotonin (5-HT) (Figure 3). It has been demonstrated that administration of pyridoxine increases the rate of synthesis of serotonin (5-HT) in monkey brain, as revealed using position emission tomography 22). This presumably reflects a regulatory effect of pyridoxine on aromatic l-amino acid decarboxylase (AADC) activity and raises the interesting issue of the use of pyridoxine supplementation in situations associated with serotonin deficiency.
Aromatic l-amino acid decarboxylase (AADC) is present not only in serotonergic neurons but also in catecholaminergic neurons, where it converts 3,4-dihydroxyphenylalanine (DOPA) to dopamine. However, different pH optima or concentrations of substrate or cofactor are required for optimal activity of the enzyme in brain homogenates when using either 5-hydroxytryptophan (5-HTP) or DOPA (3,4-dihydroxyphenylalanine) as the substrate. cDNAs encoding AADC have been cloned from various species. The encoded protein contains 480 amino acids and has a molecular weight of 54,000 but it appears to exist as a dimer. Characterization of the protein expressed in cells transfected with the cDNA shows that it decarboxylates either DOPA (3,4-dihydroxyphenylalanine) or 5-hydroxytryptophan (5-HTP). Also, in situ hybridization of the mRNA for the enzyme revealed its presence both in serotonergic cells in the dorsal raphe nucleus and in catecholaminergic cells in brain regions containing catecholaminergic nerve cell soma 23). Taken together, these results support the idea that the enzymatic decarboxylation of both DOPA and 5-hydroxytryptophan (5-HTP) is catalyzed by the same enzyme.
Because the decarboxylase enzyme is not saturated with 5-hydroxytryptophan (5-HTP) under physiological conditions, that is, the concentration of 5-hydroxytryptophan (5-HTP) is much less than the Km of 10 μM, it is possible to raise the content of serotonin (5-HT) in brain not only by increasing the dietary intake of tryptophan but also by raising the intake of 5-hydroxytryptophan (5-HTP). This procedure, though, results in the formation of serotonin (5-HT) in cells that would not normally contain it, such as catecholaminergic neurons, because of the nonselective nature of aromatic l-amino acid decarboxylase (AADC).
The initial hydroxylation of tryptophan, rather than the decarboxylation of 5-hydroxytryptophan (5-HTP), appears to be the rate-limiting step in serotonin synthesis. Evidence in support of this view includes the fact that 5-hydroxytryptophan (5-HTP) is found only in trace amounts in brain, presumably because it is decarboxylated about as rapidly as it is formed. As might be expected if the hydroxylation reaction is rate-limiting, inhibition of this reaction results in a marked depletion of the content of serotonin (5-HT) in brain. The enzyme inhibitor most widely used in experiments is parachlorophenylalanine. In vivo, parachlorophenylalanine irreversibly inhibits tryptophan hydroxylase, presumably by incorporating itself into the enzyme to produce an inactive protein. This results in a long-lasting reduction of serotonin (5-HT) levels. Recovery of enzyme activity and serotonin (5-HT) biosynthesis requires the synthesis of new enzyme. Marked increases in levels of mRNA for tryptophan hydroxylase are found in the raphe nuclei 1 to 3 days after administration of parachlorophenylalanine 24).
Figure 3. Serotonin biosynthesis and catabolism
Footnote: In the pineal gland, serotonin is converted enzymatically to melatonin.
[Source 25)]The synthesis of serotonin can increase markedly under conditions requiring a continuous supply of the neurotransmitter
Plasticity is an important concept in neurobiology. In general, this refers to the ability of neuronal systems to conform to either short- or long-term demands placed upon their activity or function. One of the processes contributing to neuronal plasticity is the ability to increase the rate of neurotransmitter synthesis and release in response to increased neuronal activity. Serotonergic neurons have this capability; the synthesis of serotonin from tryptophan is increased in a frequency-dependent manner in response to electrical stimulation of serotonergic soma 26). The increase in synthesis results from the enhanced conversion of tryptophan to 5-hydroxytryptophan (5-HTP) and has an absolute dependence on extracellular Ca2+. It is likely that the increased synthesis results in part from alterations in the kinetic properties of tryptophan hydroxylase, perhaps due to calcium-dependent phosphorylation of the enzyme. The enzyme can be phosphorylated directly by the action of calmodulin-dependent protein kinase II; an activator protein appears to be required for this interaction. In the presence of the activator, tryptophan hydroxylase also may be a substrate for cAMP-dependent protein kinase (PKA). The increased activity of tryptophan hydroxylase does not result from the removal of enzyme inhibition caused by either serotonin or 5-hydroxytryptophan (5-HTP).
Short-term requirements for increases in the synthesis of serotonin can be met by processes that change the kinetic properties of tryptophan hydroxylase, such as phosphorylation, without necessitating the synthesis of more molecules of tryptophan hydroxylase. By contrast, situations requiring long-term increases in the synthesis and release of serotonin result in the synthesis of tryptophan hydroxylase protein. For example, partial but substantial destruction of >60% of central serotonergic neurons results in an increase in the synthesis of serotonin in residual terminals. The increase in synthesis initially results from activation of existing tryptophan hydroxylase molecules, but the increased synthesis of serotonin seen weeks after the lesion results from more tryptophan hydroxylase being present in the residual terminals. An increase in tryptophan hydroxylase mRNA has been reported in residual raphe serotonergic neurons after partial lesioning, consistent with the idea of an increase in the synthesis of tryptophan hydroxylase molecules in residual neurons.
As with other biogenic amine transmitters, serotonin is stored primarily in vesicles and released by an exocytotic mechanism.
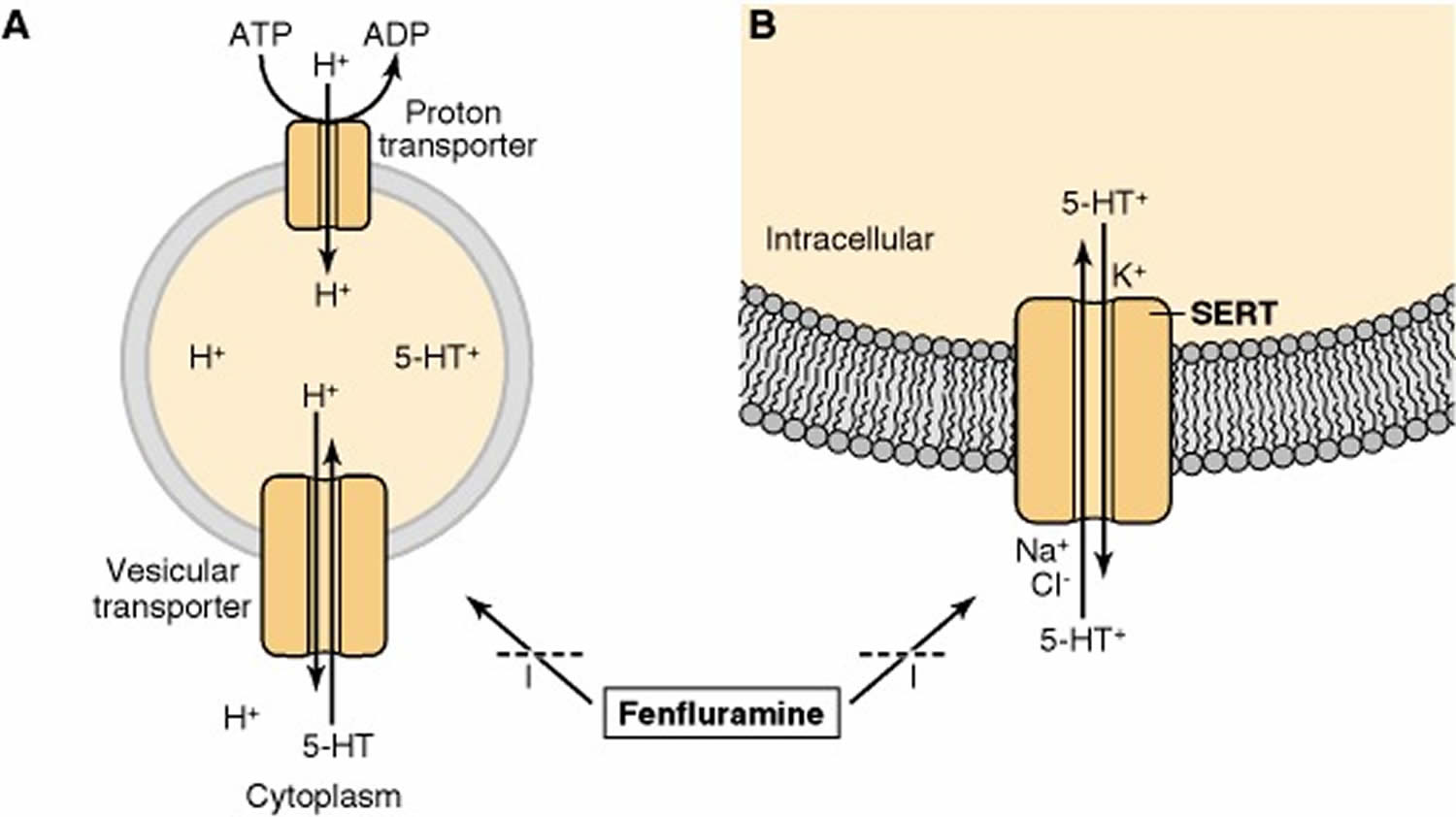
Peripheral sources of monoamine-containing cells have been utilized to study the properties of storage vesicles, such as chromaffin cells of the adrenal medulla for catecholamines and parafollicular cells of the thyroid gland for serotonin. In some respects, the vesicles that store serotonin (5-HT) resemble those that store catecholamines. For example, drugs such as reserpine and tetrabenazine, which inhibit the activity of the transporter localized to the vesicular membrane, deplete the brain content of serotonin as well as of catecholamines. Storage of serotonin in vesicles requires its active transport from the cytoplasm. The vesicular transporter uses the electrochemical gradient generated by a vesicular H+-ATPase to drive transport, such that a cytoplasmic amine is exchanged for a luminal proton; that is, uptake of serotonin (5-HT) is coupled to efflux of H+ (Figure 4).
Vesicles storing 5-HT exhibit some differences from those storing catecholamines. In contrast to catecholamine-containing vesicles, there is virtually no ATP in serotonin vesicles. Also, serotonergic synaptic vesicles, but not chromaffin granules, contain a specific protein that binds serotonin (5-HT) with high affinity. This serotonin-binding protein is present in serotonergic cells derived ontogenetically from the neuroectoderm 27). It binds serotonin (5-HT) with high affinity in the presence of Fe2+. There are three isoforms of serotonin-binding protein. The 45-kDa isoform appears to be packaged in secretory vesicles along with serotonin (5-HT), which probably accounts for the observation that newly taken up [3H]serotonin (5-HT) is rapidly complexed with this isoform in brain in situ. This isoform is secreted along with serotonin (5-HT) by a calcium-dependent process.
There is considerable evidence that the release of serotonin (5-HT) occurs by exocytosis, that is, by the discharge from the cell of the entire contents of individual storage vesicles. First, 5-HT is ionized sufficiently at physiological pH so that it does not cross plasma membranes by simple diffusion. Second, most intraneuronal 5-HT is contained in storage vesicles and other contents of the vesicle, including serotonin-binding protein (SPB), are released together with serotonin. By contrast, cytosolic proteins do not accompany electrical stimulation-elicited release of 5-HT. Third, the depolarization-induced release of serotonin (5-HT) occurs by a calcium-dependent process; indeed, it appears that the influx of Ca2+ with or without membrane depolarization can increase the release of serotonin (5-HT). Ca2+ has been reported to stimulate the fusion of vesicular membranes with the plasma membrane.
The rate of serotonin release is dependent on the firing rate of serotonergic soma in the raphe nuclei. Numerous studies have revealed that an increase in raphe cell firing enhances the release of serotonin (5-HT) in terminal fields. The opposite effect is observed when raphe cell firing decreases. This means that drugs that change the firing rate of serotonergic soma modify the release of serotonin as well. An important target for such drugs is the somatodendritic autoreceptor, which, as discussed later, is the 5-HT1A receptor subtype (see Figure 5). Administration of 5-HT1A agonists, such as 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT), into the dorsal raphe nucleus slows the rate of firing of serotonergic soma. Using the technique of in vivo microdialysis, application of 8-OH-DPAT in the dorsal raphe nucleus decreases the release of 5-HT in the striatum. Depending on the species, serotonergic autoreceptors in terminal fields appear to be either the 5-HT1B or the 5-HT1D subtype. Administration of agonists of these receptors into areas receiving serotonergic innervation decreases the synthesis and release of 5-HT measured in vitro or in situ, using the technique of microdialysis. However, in contrast to the activation of somatodendritic autoreceptors, such effects are not due to decreases in the firing rate of serotonergic soma.
Figure 4. Serotonin release and storage
Footnote: The substituted amphetamine fenfluramine inhibits the transport of serotonin (5-HT) by both (A) the vesicular transporter and (B) the serotonin transporter (SERT). Substituted amphetamines, such as fenfluramine and 3,4-methylenedioxymethamphetamine (MDMA), stimulate the release of serotonin (5-HT) from serotonergic terminals. These drugs block the vesicular transporter and disrupt the proton gradient across the vesicle membrane. The increase in intracellular serotonin (5-HT) favors the release of serotonin (5-HT) by the reverse action of the SERT. These drugs also act as substrates for the SERT so as to inhibit the transport of serotonin (5-HT) into cells.
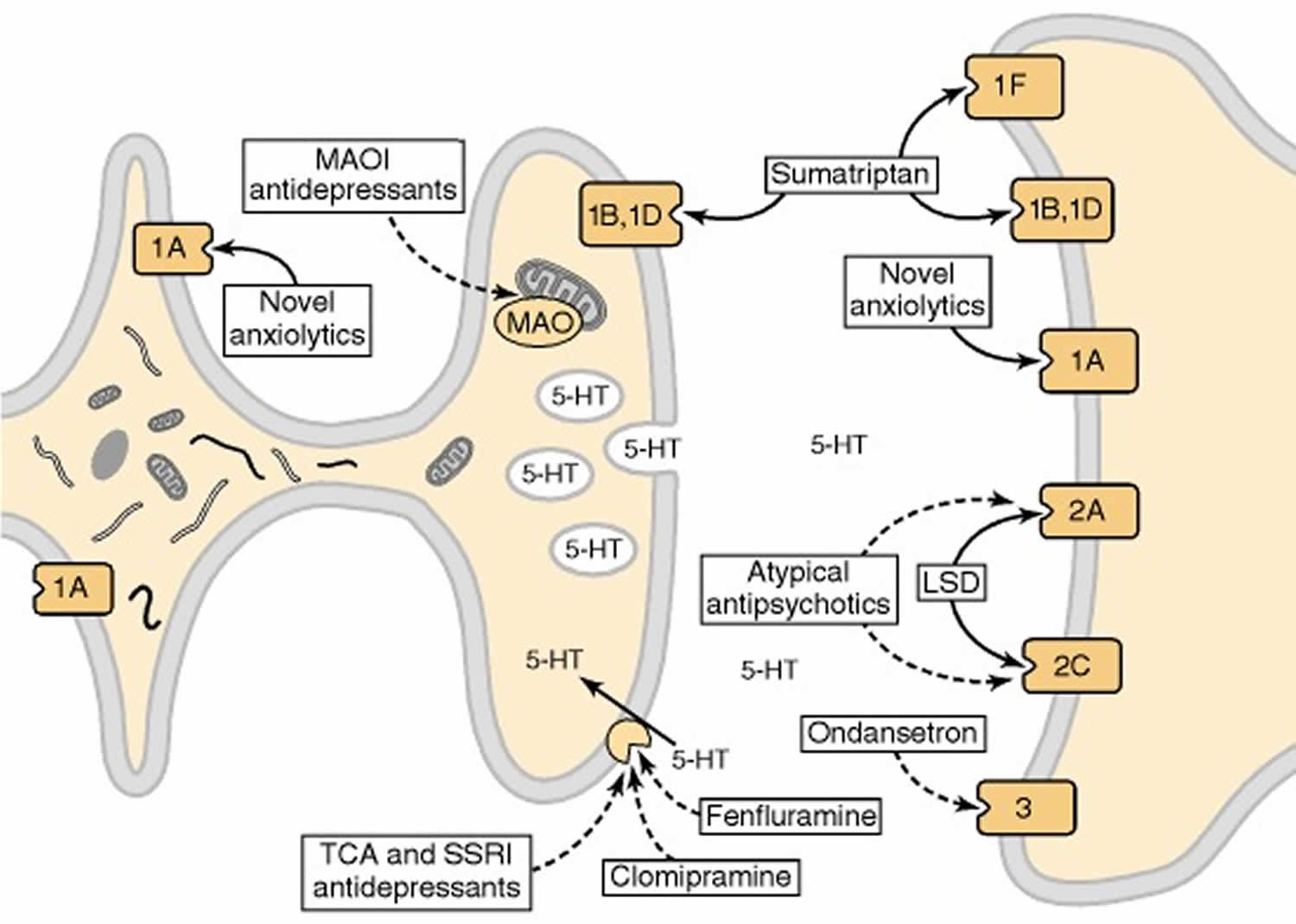
[Source 28)]Figure 5. Effects of psychoactive drugs on serotonergic neurotransmission
Footnote: Drugs that act as agonists are indicated by solid-line arrows, whereas antagonists or inhibitors are shown with broken-line arrows. The serotonin receptor 1A receptor (5-HT1AR) acts as both the somatodendritic autoreceptor and a postsynaptic receptor. Anxiolytic drugs, such as buspirone, are agonists at this receptor. In terminal fields, the autoreceptor is either the serotonin receptor 1B receptor (5-HT1B) or serotonin receptor 1D receptor (5-HT1D) subtype; these receptors also function as postsynaptic receptors. The antimigraine drug sumatriptan is an agonist at these receptors as well as at the 5-HT1F receptor. Hallucinogenic drugs, such as LSD, are agonists at serotonin receptor 2A receptor (5-HT2A) and serotonin receptor 2C receptor (5-HT2C) receptors, whereas atypical antipsychotic drugs, such as clozapine and olanzapine, are antagonists. The 5-HT3 receptor, a ligand-gated ion channel, is blocked by drugs effective in the treatment of chemotherapy-induced nausea and emesis, such as ondansetron. Another important target for psychotherapeutic drugs is the serotonin transporter, which is blocked by drugs effective in the treatment of depression or obsessive-compulsive disorder, such as clomipramine. The enzyme responsible for the catabolism of serotonin, monoamine oxidase (MAO), is inhibited by another class of antidepressants. MAOI (monoamine oxidase inhibitor); TCA (tricyclic antidepressant); SSRI (selective serotonin reuptake inhibitor).
[Source 29)]The activity of serotonin in the synapse is terminated primarily by its re-uptake into serotonergic terminals.
Synaptic effects of many amino acid and monoaminergic neurotransmitters, including serotonin, are terminated by binding of these molecules to specific transporter proteins. The serotonin transporter (SERT) is located on serotonergic neurons. Evidence for this comes from studies showing that the selective lesioning of serotonergic neurons in brain markedly reduces both the high-affinity uptake of [3H]5-HT in areas of brain receiving serotonergic innervation and the specific binding of radioligands to the serotonin transporter. Activity of the serotonin transporter (SERT) regulates the concentration of serotonin in the synapse, thereby influencing synaptic transmission.
The uptake system for serotonin is saturable and of high affinity, with a Km value for serotonin of approximately 0.2 to 0.5 μM. Uptake of serotonin is an active process that is temperature-dependent and has an absolute requirement for external Na+ and C1−; it is inhibited by metabolic inhibitors as well as by inhibitors of Na/K ATPase activity. From these and other data, it has been inferred that the energy requirement for serotonin uptake is not used directly to transport serotonin but rather is necessary to maintain the gradient of Na+ across the plasma membrane, upon which serotonin uptake is dependent. The current model of transport has one Na+, one C1− and one protonated serotonin binding to the transporter extracellularly prior to translocation to form a quaternary complex that subsequently undergoes a conformational change to release the neurotransmitter and the ions into the cytoplasm. The conformational change may involve the “opening” of a pore formed by some portion of the transmembrane domains of the serotonin transporter (SERT). In the cytoplasm, K+ associates with the serotonin transporter (SERT) to promote reorientation of the unloaded carrier for another transport cycle (Figure 4).
Serotonin breakdown
The primary catabolic pathway for 5-hydroxytryptamine is oxidative deamination by the enzyme monoamine oxidase.
Monoamine oxidase (MAO) converts serotonin to 5-hydroxyindoleacetaldehyde, and this product is oxidized by an NAD+-dependent aldehyde dehydrogenase to form 5-hydroxyindoleacetic acid (5-HIAA) (Figure 3). The intermediate acetaldehyde also can be reduced by an NADH-dependent aldehyde reductase to form the alcohol 5-hydroxytryptophol. Whether oxidation or reduction takes place depends on the ratio of NAD+ to NADH in the tissue. In brain, 5-HIAA is the primary metabolite of serotonin.
There are at least two isoenzymes of MAO (monoamine oxidase), referred to as MAO-A (monoamine oxidase type A) and MAO-B (monoamine oxidase type B). These isoenzymes are integral flavoproteins of outer mitochondrial membranes in neurons, glia and other cells. Evidence for the existence of isoenzymes was based initially on differing substrate specificities and sensitivities to inhibitors of MAO. For example, both serotonin and norepinephrine are metabolized preferentially by MAO-A. Selective inhibitors of each form of MAO exist: clorgyline or moclobemide for type A and deprenyl for type B. Definitive proof of the existence of these two forms of MAO comes from the cloning of cDNAs encoding subunits of MAO-A and MAO-B from human liver 30). The deduced amino acid sequences of MAO-A and MAO-B show about 70% homology and have masses of 59.7 and 58.8 kDa, respectively. When each cDNA was cloned into an expression vector and transfected independently into a cell line, the activity of the proteins expressed resembled that of the endogenous enzymes from human brain, such that the expressed MAO-A preferred serotonin as a substrate and was inhibited preferentially by clorgyline. From such data it was inferred that the functional differences between these two enzymes exist in their primary structures.
The use of transgenic mice permits the selective elimination, or “knockout,” of either MAO type 31). In the brains of mice deficient in MAO-A, the content of serotonin is elevated markedly for about 12 days after birth and then slowly declines, reaching values comparable to those in normal mice after about 7 months. In MAO-A-deficient mice, the selective inhibitor of MAO-B, deprenyl, had a greater effect on serotonin metabolism than it did in normal mice. Such observations indicate that, in the absence of MAO-A, MAO-B can metabolize serotonin in vivo. However, mice lacking the MAO-B isoenzyme do not have elevated levels of serotonin in brain. Of interest are the aggressive behaviors exhibited by mice deficient in MAO-A, consistent with a postulated role of serotonergic neurons in human aggressive behaviors.
Serotonin function
The function of brain serotonin remains an enigma 32). There have been several attempts to identify a unifying function of serotonin 33), 34). Most researchers acknowledge that the function of the serotonin system remains ‘elusive’ 35) and ‘a puzzle’ 36) and it is argued that this may be due to the special diversity and complexity of the serotonin system with its many receptor subtypes 37), extensive innervation of the brain and paracrine style of transmission 38). The notion that serotonin is an enigma among neuromodulators [said to be ‘involved in everything but responsible for nothing’ 39)] and it is argued that the riddle of serotonin can only be solved by focusing on its individual receptor subtypes 40).
Accordingly, given the inherent complexity of the serotonin system, one strategy for understanding its functioning is to focus on a select number of receptor subtypes that have been particularly well characterized. From this foundation, one might then consider whether other serotonin receptor subtypes can be incorporated into the associated model, or whether one or more additional models are required to cover the full range of functions associated with brain serotonin transmission. Broadly consistent with prior theories 41), Carhart-Harris and Nutt 42) believed that a key function of brain serotonin (5-HT) is to moderate anxiety and stress, and promote patience and coping 43) via (postsynaptic) 5-HT1AR (serotonin receptor 1AR) signalling. Moreover, Carhart-Harris and Nutt 44) also extend on this by proposing that a second major function of brain serotonin is to open a window of plasticity for greater adaptation 45), mediated in large part by 5-HT2AR (serotonin receptor 2AR) signalling. This bipartite model is consistent with a ‘flexible coping’ model of brain serotonin function, in which postsynaptic 5-HT1ARs mediate so-called ‘passive coping’ (i.e. tolerating but not necessarily dealing with a source of psychological pain) and 5-HT2ARs mediate ‘active coping’ (actively dealing with a source of psychological pain by changing one’s relationship to it) 46).
The brain serotonin (5-hydroxytryptamine) system is thought to play an essential role in the control of anxiety-, fear- and panic-like responses in rodents 47). In humans, several lines of evidence link alterations in serotonin (5-hydroxytryptamine) signaling to panic attacks through a defensive behavioral system activated by acute threats 48). Panic attacks represent abrupt surges of intense fear or extreme discomfort that reach a peak within minutes. Unexpected panic attacks with at least one of the attacks followed by persistent concern or worry about additional panic attacks or their consequences define panic disorder 49). Maladaptive changes in behavior related to the attack, such as agoraphobia, describe anticipatory anxiety and/or marked fear about apparently threatening situations.
Serotonin (5-HT) may influence anxiety-, fear- and panic-like responses within the basolateral complex of the amygdala. In humans, alteration in the Tryptophan hydroxylase 2 gene (TPH2) were associated with alterations in amygdala responsiveness to anxiety-related stimuli 50) as well as anxiety 51) and panic disorders 52). This has been linked recently to alterations in the GABAergic system 53). In mice, pharmacological depletion of serotonin (5-HT) in the basolateral complex of the amygdala reduces anxiety and interferes with fear conditioning 54). Furthermore, stress-induced enhancement of fear memory is dependent on serotonin (5-HT) action within the basolateral complex of the amygdala 55).
Exaggerated fear accompanied by a panic-like state and escape behavior resulting from lifelong absence of serotonin synthesis involves dysfunction of the amygdalo-dorsal raphe circuitry controlling fear-related behavioral responses 56). This is due to alterations in GABAergic transmission prohibiting increased activity of the basolateral nucleus in aversive inescapable contexts. Increased escape responses reflect characteristic syndromal dimensions of panic disorder and agoraphobia. Thus, constitutive lack of serotonin synthesis influences the risk for anxiety- and stressor-related disorders including panic disorder and comorbid agoraphobia through the absence of GABAergic-dependent compensatory mechanisms in the basolateral complex of the amygdala.
Serotonin and depression
The “serotonin hypothesis” of clinical depression is almost 50 years old 57). At its simplest, the hypothesis proposes that diminished activity of serotonin pathways plays a causal role in the pathophysiology of depression. This notion was based on the depressogenic effects of amine depleting agents such as reserpine, as well as the actions of antidepressant drugs such as monoamine oxidase inhibitors and tricyclic antidepressants, discovered by clinical serendipity, but later found in animal experimental studies to potentiate the effects of serotonin and other monoamines at the synapse 58).
The best evidence that serotonin plays a role in the pathophysiology of depression comes from studies of “tryptophan depletion”, where an acute dietary manipulation is employed to produce a transient lowering in brain serotonin activity through diminishing availability of its precursor amino acid, tryptophan 59). In healthy participants with no risk factors for depression, tryptophan depletion does not produce clinically significant changes in mood; however, recovered depressed patients free of medication can show brief, clinically relevant, depressive symptomatology 60). Interestingly, the same is true of recovered depressed patients undergoing catecholamine depletion with alpha-methyl-para-tyrosine 61).
Overall, this evidence suggests that impairing serotonin function can cause clinical depression in some circumstances, but is neither necessary nor sufficient. In addition, the depressogenic effects of tryptophan depletion are much more apparent in people who have experienced prior episodes of depression than in those simply at high risk of illness, for example by virtue of a strong family history 62). This suggests that low serotonin function may compromise mechanisms involved in maintaining recovery from depression rather than having a primary effect to lower mood in all vulnerable people.
These findings also hint at a role for diminished tryptophan availability in triggering depression, particularly in people with a previous history of illness. Interestingly, lower plasma levels of tryptophan are one of the few reasonably robust findings in patients with more severe forms of depression 63) and, more recently, have been linked to peripheral inflammation and consequent induction of the tryptophan metabolizing enzyme indoleamine 2,3-dioxygenase 64). Inflammation could therefore produce depression in vulnerable individuals by lowering plasma tryptophan and diminishing brain serotonin activity. Conceivably, such an effect could explain the diminished efficacy of SSRIs in depressed patients with high levels of inflammatory biomarkers 65).
Serotonin and antidepressant action
Undoubtedly, a major reason for the continuing interest in serotonin and depression is the fact that SSRIs are useful antidepressant drugs for some patients. Elegant basic studies have revealed intriguing molecular and cellular consequences of repeated SSRI administration in animals, for example increases in hippocampal cell proliferation and enhanced expression of neuroplasticity related proteins such as brain derived neurotrophic factor (BDNF) 66). However, linking such changes to resolution of the clinical depressive syndrome is challenging. More pertinent in this respect are neuropsychological studies which show that, in both healthy participants and depressed patients, administration of SSRIs leads to positive shifts in the way the brain appraises emotionally-valenced information. This effect occurs very early in treatment, prior to clinical antidepressant effects, and appears to be mediated via serotonergic innervation to limbic circuitry, particularly the amygdala 67).
This work gives a new insight into how serotonin pathways may influence mood in depressed patients, that is by altering the way the brain appraises emotionally-laden information at an implicit level. Unlike mood, emotions are relatively short-lived, automatic responses to internal or external stimuli, and in depressed patients emotional responses are reliably negatively biased 68). Thus, from this viewpoint, increasing serotonin activity in depressed people does not influence subjective mood directly but, rather, as a secondary consequence of positive shifts in automatic emotional responses.
Over time, it is suggested, this positive biasing of automatic processing would, in an appropriate interpersonal environment, lead to changes in the strategic processing associated with conscious emotional experience. This psychological process is likely to involve re-learning a range of emotional associations, which might account for the gradual onset of clinical antidepressant activity 69). In addition, the notion that “re-learning” is involved in subjective improvement in depression sits well with the finding noted above that antidepressants such as SSRIs promote synaptic plasticity, an effect classically associated with learning 70).
How to increase serotonin
Perreau-Linck and colleagues 71) provides an initial lead about one possible strategy for raising brain serotonin. Using positron emission tomography (PET), they obtained a measure of serotonin synthesis in the brains of healthy participants who underwent positive, negative and neutral mood inductions. Reported levels of happiness were positively correlated and reported levels of sadness were negatively correlated with serotonin synthesis in the right anterior cingulate cortex 72). The idea that alterations in thought, either self-induced or due to psychotherapy, can alter brain metabolism is not new. Numerous studies have demonstrated changes in blood flow in such circumstances. However, reports related to specific transmitters are much less common. In one recent study, meditation was reported to increase release of dopamine 73). The study by Perreau-Linck and colleagues 74) is the first to report that self-induced changes in mood can influence serotonin synthesis. This raises the possibility that the interaction between serotonin synthesis and mood may be 2-way, with serotonin influencing mood and mood influencing serotonin. Obviously, more work is needed to answer questions in this area. For example, is the improvement in mood associated with psychotherapy accompanied by increases in serotonin synthesis? If more precise information is obtained about the mental states that increase serotonin synthesis, will this help to enhance therapy techniques?
Exposure to bright light is a second possible approach to increasing serotonin without drugs. Bright light is, of course, a standard treatment for seasonal affective disorder (SAD) or seasonal depression, but a few studies also suggest that it is an effective treatment for nonseasonal depression 75) and also reduces depressed mood in women with premenstrual dysphoric disorder 76) and in pregnant women suffering from depression (antepartum depression) 77). The evidence relating these effects to serotonin is indirect. In human postmortem brain, serotonin levels are higher in those who died in summer than in those who died in winter 78). A similar conclusion came from a study on healthy volunteers, in which serotonin synthesis was assessed by measurements of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the venous outflow from the brain 79). There was also a positive correlation between serotonin synthesis and the hours of sunlight on the day the measurements were made, independent of season. In rats, serotonin is highest during the light part of the light–dark cycle, and this state is driven by the photic cycle rather than the circadian rhythm 80). The existence of a retinoraphe tract may help explain why, in experimental animals, neuronal firing rates, c-fos expression and the serotonin content in the raphe nuclei are responsive to retinal light exposure 81). In humans, there is certainly an interaction between bright light and the serotonin system. The mood-lowering effect of acute tryptophan depletion in healthy women is completely blocked by carrying out the study in bright light (3000 lux) instead of dim light 82).
Relatively few generations ago, most of the world population was involved in agriculture and was outdoors for much of the day. This would have resulted in high levels of bright light exposure even in winter. Even on a cloudy day, the light outside can be greater than 1000 lux, a level never normally achieved indoors. In a recent study carried out at around latitude 45° North, daily exposure to light greater than 1000 lux averaged about 30 minutes in winter and only about 90 minutes in summer 83) among people working at least 30 hours weekly; weekends were included. In this group, summer bright light exposure was probably considerably less than the winter exposure of our agricultural ancestors. Today we may be living in a bright light–deprived society. A large literature exists on the beneficial effect of bright light exposure in healthy individuals 84). Lamps designed for the treatment of seasonal affective disorder (SAD), which provide more lux than is ever achieved by normal indoor lighting, are readily available, although incorporating their use into a daily routine may be a challenge for some. “Light cafes” pioneered in Scandinavia have come to the United Kingdom 85) and an Austrian village that receives no sunshine in the winter because of its surrounding mountains is building a series of giant mirrors to reflect sunlight into the valley 86). Better use of daylight in buildings is an issue that architects are increasingly aware of. Working indoors does not have to be associated with suboptimal exposure to bright light.
A third strategy that may raise brain serotonin is exercise. A comprehensive review of the relation between exercise and mood concluded that antidepressant and anxiolytic effects have been clearly demonstrated 87). In the United Kingdom the National Institute for Health and Clinical Excellence (NICE), which works on behalf of the National Health Service and makes recommendations on treatments according to the best available evidence, has published a guide on the treatment of depression 88). The guidance recommends treating mild clinical depression with various strategies, including exercise rather than antidepressants, because the risk–benefit ratio is poor for antidepressant use in patients with mild depression. Exercise improves mood in subclinical populations as well as in patients. The most consistent effect is seen when regular exercisers undertake aerobic exercise at a level with which they are familiar 89). In support of the National Institute for Health and Clinical Excellence recommendations, the National Institute of Mental Health in the United States funded clinical trial called Treatment with Exercise Augmentation for Depression (TREAD) study found “symptoms improve with exercise treatment for depression, and this change correlates well with overall outcome” 90). And “changes in motivation and energy were larger, as measured by effect size, than changes in anhedonia” 91). Anhedonia is the inability to feel pleasure in normally pleasurable activities.
Several lines of research suggest that exercise increases brain serotonin function in the human brain. Post and colleagues 92) measured biogenic amine metabolites in cerebrospinal fluid (CSF) of patients with depression before and after they increased their physical activity to simulate mania. Physical activity increased 5-hydroxyindoleacetic acid (5-HIAA), but it is not clear that this was due to increased serotonin turnover or to mixing of CSF from higher regions, which contain higher levels of 5-hydroxyindoleacetic acid (5-HIAA), with lumbar CSF (or to a combination of both mechanisms). Nonetheless, this finding stimulated many animal studies on the effects of exercise. For example, Chaouloff and colleagues 93) showed that exercise increased tryptophan and 5-hydroxyindoleacetic acid (5-HIAA) in rat ventricles. More recent studies using intracerebral dialysis have shown that exercise increases extracellular serotonin and 5-HIAA in various brain areas, including the hippocampus and cortex 94). Two different mechanisms may be involved in this effect. As reviewed by Jacobs and Fornal 95), motor activity increases the firing rates of serotonin neurons, and this results in increased release and synthesis of serotonin 96). In addition, there is an increase in the brain of the serotonin precursor tryptophan that persists after exercise 97).
The largest body of work in humans looking at the effect of exercise on tryptophan availability to the brain is concerned with the hypothesis that fatigue during exercise is associated with elevated brain tryptophan and serotonin synthesis. A large body of evidence supports the idea that exercise, including exercise to fatigue, is associated with an increase in plasma tryptophan and a decrease in the plasma level of the branched chain amino acids (BCAAs) leucine, isoleucine and valine 98). The branched chain amino acids (BCAAs) inhibit tryptophan transport into the brain 99). Because of the increase in plasma tryptophan and decrease in BCAA, there is a substantial increase in tryptophan availability to the brain. Tryptophan is an effective mild hypnotic 100), a fact that stimulated the hypothesis that it may be involved in fatigue. It is notable that several clinical trials of branched chain amino acid (BCAA) investigated whether it was possible to counter fatigue by lowering brain tryptophan, with results that provided little support for the hypothesis. Furthermore, exercise results in an increase in the plasma ratio of tryptophan to the branched chain amino acids (BCAAs) before the onset of fatigue 101). The conclusion of these studies is that, in humans, a rise in precursor availability should increase serotonin synthesis during and after exercise and that this is not related to fatigue, although it may be related to improved mood. Whether motor activity increases the firing rate of serotonin neurons in humans, as in animals, is not known. However, it is clear that aerobic exercise can improve mood.
As with exposure to bright light, there has been a large change in the level of vigorous physical exercise experienced since humans were hunter-gatherers or engaged primarily in agriculture. Lambert 102) argued that the decline in vigorous physical exercise and in particular, in effort-based rewards may contribute to the high level of depression in today’s society. The effect of exercise on serotonin suggests that the exercise itself, not the rewards that stem from exercise, may be important. If trials of exercise to prevent depression are successful, then prevention of depression can be added to the numerous other benefits of exercise.
The fourth factor that could play a role in raising brain serotonin is diet. According to some evidence, tryptophan, which increases brain serotonin in humans as in experimental animals 103), is an effective antidepressant in mild-to-moderate depression 104). Further, in healthy people with high trait irritability, it increases agreeableness, decreases quarrelsomeness and improves mood 105). Treating tryptophan as a drug is reasonable because, first, there is normally no situation in which purified tryptophan is needed for dietary reasons, and second, purified tryptophan and foods containing tryptophan have different effects on brain serotonin. Although purified tryptophan increases brain serotonin, foods containing tryptophan do not 106). This is because tryptophan is transported into the brain by a transport system that is active toward all the large neutral amino acids and tryptophan is the least abundant amino acid in protein. There is competition between the various amino acids for the transport system, so after the ingestion of a meal containing protein, the rise in the plasma level of the other large neutral amino acids will prevent the rise in plasma tryptophan from increasing brain tryptophan. The idea, common in popular culture, that a high-protein food such as turkey will raise brain tryptophan and serotonin is, unfortunately, false 107). Another popular myth that is widespread on the internet is that bananas improve mood because of their serotonin content. Although it is true that bananas contain serotonin, it does not cross the blood–brain barrier 108).
α-Lactalbumin, a minor constituent of milk, is one protein that contains relatively more tryptophan than most proteins. Acute ingestion of α-lactalbumin by humans can improve mood and cognition in some circumstances, presumably owing to increased serotonin 109). Enhancing the tryptophan content of the diet chronically with α-lactalbumin is probably not practical. However, increasing the tryptophan content of the diet relative to that of the other amino acids is something that possibly occurred in the past and could occur again in the future. Kerem and colleagues 110) studied the tryptophan content of both wild chickpeas and the domesticated chickpeas that were bred from them in the Near East in neolithic times. The mean protein content (per mg dry seed) was similar for 73 cultivars and 15 wild varieties. In the cultivated group, however, the tryptophan content was almost twice that of the wild seeds. Interestingly, the greater part of the increase was due to an increase in the free tryptophan content (i.e., not part of the protein). In cultivated chickpeas, almost two-thirds of the tryptophan was in the free form. Kerem and colleagues 111) argue that there was probably selection for seeds with a higher tryptophan content. This is plausible, given another example of an early strategy to increase the available tryptophan content of an important food source. Pellagra is a disorder caused by niacin (vitamin B3) deficiency, usually owing to poverty and a diet relying heavily on corn (maize), which has a low level of niacin and its precursor tryptophan. Cultures in the Americas that relied greatly on corn used alkali during its processing (e.g., boiling the corn in lime when making tortillas). This enhanced the nutritional quality of the corn by increasing the bioavailability of both niacin and tryptophan, a practice that prevented pellagra 112). The Europeans transported corn around the world but did not transport the traditional alkali-processing methods, thereby causing epidemics of pellagra in past centuries. Breeding corn with a higher tryptophan content was shown in the 1980s to prevent pellagra 113); presumably, it also raised brain serotonin. In a recent issue of Nature Biotechnology, Morris and Sands 114) argue that plant breeders should be focusing more on nutrition than on yield. They ask, “Could consumption of tryptophan-rich foods play a role in reducing the prevalence of depression and aggression in society?” Cross-national studies have reported a positive association between corn consumption and homicide rates 115) and a negative association between dietary tryptophan and suicide rates 116). Although the idea behind such studies is interesting, any causal attribution must remain speculative, given the possible confounders. Nonetheless, the possibility that the mental health of a population could be improved by increasing the dietary intake of tryptophan relative to the dietary intake of other amino acids remains an interesting idea that should be explored.
Serotonin foods
The following fruits had a high serotonin concentration expressed in µg/g weight:
- butternuts 398 ± 90;
- black walnuts 304 ± 46;
- shagbark hickory nuts 143 ± 23;
- English walnuts 87 ± 20;
- mockemut hickory nuts 67 ± 13;
- plantain 30.3 ± 7.5;
- pecans 29 ± 4;
- sweet pignuts 25 ± 8.;
- pineapple 17.0 ± 5.1;
- banana 15.0 ± 2.4;
- Kiwi fruit 5.8 ± 0.9;
- plums 4.7 ± 0.8; and
- tomatoes 3.2 ± 0.6.
Table 2. Foods high in tryptophan (ordered from highest to low)
| Description | Tryptophan (g) Value Per 100 gramm |
| Egg, white, dried, stabilized, glucose reduced | 1.43 |
| Egg, white, dried, powder, stabilized, glucose reduced | 1.27 |
| Egg, white, dried, flakes, stabilized, glucose reduced | 1.18 |
| Soy protein isolate | 1.12 |
| Soy protein isolate, potassium type | 1.12 |
| Seeds, sesame flour, low-fat | 1.1 |
| Egg, white, dried | 1 |
| Seaweed, spirulina, dried | 0.93 |
| Seeds, sesame flour, partially defatted | 0.88 |
| Soy protein concentrate, produced by alcohol extraction | 0.83 |
| Soy protein concentrate, produced by acid wash | 0.83 |
| Whale, beluga, meat, dried (Alaska Native) | 0.8 |
| Egg, whole, dried | 0.78 |
| Egg, whole, dried, stabilized, glucose reduced | 0.77 |
| Winged beans, mature seeds, raw | 0.76 |
| Seeds, cottonseed flour, low fat (glandless) | 0.75 |
| Tofu, dried-frozen (koyadofu) | 0.75 |
| Tofu, dried-frozen (koyadofu), prepared with calcium sulfate | 0.75 |
| Seeds, cottonseed meal, partially defatted (glandless) | 0.74 |
| Seeds, sunflower seed flour, partially defatted | 0.73 |
| Beverages, Protein powder soy based | 0.72 |
| Fish, cod, Atlantic, dried and salted | 0.7 |
| Soy flour, defatted | 0.68 |
| Seeds, sesame flour, high-fat | 0.67 |
| Soy meal, defatted, raw | 0.65 |
| Pork, fresh, variety meats and by-products, pancreas, cooked, braised | 0.62 |
| Seeds, cottonseed flour, partially defatted (glandless) | 0.62 |
| Mollusks, whelk, unspecified, cooked, moist heat | 0.62 |
| Soybeans, mature seeds, raw | 0.59 |
| Seeds, pumpkin and squash seed kernels, dried | 0.58 |
| Soybeans, mature seeds, dry roasted | 0.57 |
| Meat extender | 0.57 |
| Seeds, pumpkin and squash seed kernels, roasted, without salt | 0.57 |
| Seeds, pumpkin and squash seed kernels, roasted, with salt added | 0.57 |
| Cheese, parmesan, shredded | 0.56 |
| Cheese, mozzarella, low moisture, part-skim | 0.55 |
| Cheese, cheddar (Includes foods for USDA’s Food Distribution Program) | 0.55 |
| Game meat, elk, cooked, roasted | 0.55 |
| Leavening agents, yeast, baker’s, active dry | 0.54 |
| Parsley, freeze-dried | 0.52 |
| Cheese, mozzarella, whole milk | 0.52 |
| Soybeans, mature seeds, roasted, salted | 0.51 |
| Soybeans, mature seeds, roasted, no salt added | 0.51 |
| Milk, dry, nonfat, regular, without added vitamin A and vitamin D | 0.51 |
| Milk, dry, nonfat, regular, with added vitamin A and vitamin D | 0.51 |
| Peanut flour, defatted | 0.51 |
| Soy flour, full-fat, roasted | 0.51 |
| Soy flour, full-fat, raw | 0.5 |
| Milk, dry, nonfat, calcium reduced | 0.5 |
| Milk, dry, nonfat, instant, with added vitamin A and vitamin D | 0.49 |
| Milk, dry, nonfat, instant, without added vitamin A and vitamin D | 0.49 |
| Seeds, cottonseed kernels, roasted (glandless) | 0.49 |
| Milk, buttermilk, dried | 0.48 |
| Cheese, parmesan, hard | 0.48 |
| Spices, parsley, dried | 0.47 |
| Pork, cured, bacon, cooked, microwaved | 0.46 |
| Game meat, caribou, cooked, roasted | 0.46 |
| Seeds, chia seeds, dried | 0.44 |
| Game meat, rabbit, wild, cooked, stewed | 0.44 |
| Cheese, romano | 0.43 |
| Cheese, gruyere | 0.42 |
| Lamb, shoulder, arm, separable lean only, trimmed to 1/4″ fat, choice, cooked, braised | 0.41 |
| T.G.I. FRIDAY’S, classic sirloin steak (10 oz) | 0.41 |
| Game meat, elk, raw | 0.41 |
| CRACKER BARREL, grilled sirloin steak | 0.41 |
| Pork, ground, 96% lean / 4% fat, cooked, pan-broiled | 0.41 |
| Beef, round, top round roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 0.41 |
| Pork, fresh, variety meats and by-products, pancreas, raw | 0.41 |
| Pork, cured, bacon, pre-sliced, cooked, pan-fried | 0.41 |
| Beef, round, eye of round roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 0.41 |
| Beef, round, top round, separable lean only, trimmed to 0″ fat, choice, cooked, braised | 0.41 |
| Beef, round, top round, separable lean only, trimmed to 0″ fat, select, cooked, braised | 0.41 |
| Chicken, broiler or fryers, breast, skinless, boneless, meat only, cooked, braised | 0.4 |
| Goose, domesticated, meat only, cooked, roasted | 0.4 |
| Seeds, safflower seed meal, partially defatted | 0.4 |
| Game meat, goat, cooked, roasted | 0.4 |
| Beef, round, top round steak, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, grilled | 0.4 |
| Cheese, swiss | 0.4 |
| Game meat, rabbit, domesticated, composite of cuts, cooked, stewed | 0.4 |
| Beef, loin, top sirloin filet, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.4 |
| Duck, young duckling, domesticated, White Pekin, leg, meat only, bone in, cooked without skin, braised | 0.4 |
| Beef, plate steak, boneless, inside skirt, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.4 |
| Beef, round, top round, separable lean and fat, trimmed to 0″ fat, choice, cooked, braised | 0.4 |
| Beef, round, top round, separable lean and fat, trimmed to 0″ fat, select, cooked, braised | 0.4 |
| Lamb, Australian, imported, fresh, shoulder, arm, separable lean only, trimmed to 1/8″ fat, cooked, braised | 0.4 |
| Cereals ready-to-eat, wheat germ, toasted, plain | 0.4 |
| Lamb, New Zealand, imported, frozen, shoulder, whole (arm and blade), separable lean only, cooked, braised | 0.4 |
| Seeds, sesame butter, paste | 0.4 |
| Pork, ground, 96% lean / 4% fat, cooked, crumbles | 0.39 |
| Beef, round, top round steak, boneless, separable lean only, trimmed to 0″ fat, choice, cooked, grilled | 0.39 |
| Lamb, cubed for stew or kabob (leg and shoulder), separable lean only, trimmed to 1/4″ fat, cooked, braised | 0.39 |
| Seeds, sesame butter, tahini, from unroasted kernels (non-chemically removed seed coat) | 0.39 |
| Restaurant, family style, sirloin steak | 0.39 |
| Spices, fenugreek seed | 0.39 |
| Egg, yolk, dried | 0.39 |
| Chicken, broilers or fryers, breast, meat only, cooked, fried | 0.39 |
| Seeds, watermelon seed kernels, dried | 0.39 |
| Seeds, sesame butter, tahini, from raw and stone ground kernels | 0.39 |
| Beef, top loin filet, boneless, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 0.39 |
| Seeds, sesame seeds, whole, dried | 0.39 |
| Beef, round, top round, separable lean and fat, trimmed to 1/8″ fat, select, cooked, braised | 0.39 |
| Beef, loin, top loin steak, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 0.39 |
| Chicken, stewing, light meat, meat only, cooked, stewed | 0.39 |
| Pork, fresh, loin, tenderloin, separable lean only, cooked, broiled | 0.39 |
| Beef, loin, top loin steak, boneless, lip off, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.39 |
| Chicken, broiler or fryers, breast, skinless, boneless, meat only, cooked, grilled | 0.39 |
| Beef, round, top round, separable lean and fat, trimmed to 1/8″ fat, all grades, cooked, braised | 0.39 |
| Beef, chuck, mock tender steak, boneless, separable lean only, trimmed to 0″ fat, choice, cooked, braised | 0.39 |
| Beef, round, eye of round steak, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.39 |
| Game meat, rabbit, domesticated, composite of cuts, cooked, roasted | 0.38 |
| Cheese, parmesan, grated | 0.38 |
| Chicken, broilers or fryers, light meat, meat only, cooked, fried | 0.38 |
| Lamb, shoulder, whole (arm and blade), separable lean only, trimmed to 1/4″ fat, choice, cooked, braised | 0.38 |
| Beef, loin, top sirloin petite roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 0.38 |
| Beef, round, top round, separable lean and fat, trimmed to 1/8″ fat, choice, cooked, braised | 0.38 |
| Beef, chuck, mock tender steak, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, braised | 0.38 |
| Beef, rib, back ribs, bone-in, separable lean only, trimmed to 0″ fat, select, cooked, braised | 0.38 |
| Beef, round, top round roast, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, roasted | 0.38 |
| Duck, young duckling, domesticated, White Pekin, breast, meat only, boneless, cooked without skin, broiled | 0.38 |
| Game meat, boar, wild, cooked, roasted | 0.38 |
| DENNY’S, top sirloin steak | 0.38 |
| Lamb, shoulder, blade, separable lean only, trimmed to 1/4″ fat, choice, cooked, braised | 0.38 |
| Beef, chuck, mock tender steak, boneless, separable lean only, trimmed to 0″ fat, select, cooked, braised | 0.38 |
| Beef, rib eye steak, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 0.38 |
| Beef, rib eye steak, boneless, lip off, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.38 |
| Beef, shank crosscuts, separable lean only, trimmed to 1/4″ fat, choice, cooked, simmered | 0.38 |
| Beef, round, eye of round roast, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, roasted | 0.38 |
| Pork, fresh, loin, tenderloin, separable lean and fat, cooked, broiled | 0.38 |
| Snacks, soy chips or crisps, salted | 0.38 |
| Fish, roe, mixed species, cooked, dry heat | 0.38 |
| Beef, rib eye roast, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, roasted | 0.38 |
| Beef, loin, top sirloin filet, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, grilled | 0.38 |
| Pork, fresh, leg (ham), whole, separable lean only, cooked, roasted | 0.37 |
| Pork, fresh, loin, center rib (chops), boneless, separable lean only, cooked, broiled | 0.37 |
| Pork, fresh, composite of trimmed retail cuts (loin and shoulder blade), separable lean only, cooked | 0.37 |
| Beef, plate steak, boneless, outside skirt, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.37 |
| Chicken, broilers or fryers, giblets, cooked, fried | 0.37 |
| Beef, chuck for stew, separable lean and fat, choice, cooked, braised | 0.37 |
| Turkey, retail parts, wing, meat only, cooked, roasted | 0.37 |
| Chicken, broiler or fryers, breast, skinless, boneless, meat only, with added solution, cooked, grilled | 0.37 |
| Ham and cheese spread | 0.37 |
| Seeds, sesame butter, tahini, from roasted and toasted kernels (most common type) | 0.37 |
| Veal, leg (top round), separable lean only, cooked, braised | 0.37 |
| Beef, chuck for stew, separable lean and fat, all grades, cooked, braised | 0.37 |
| Milk, dry, whole, with added vitamin D | 0.37 |
| Milk, dry, whole, without added vitamin D | 0.37 |
| Seeds, sesame seeds, whole, roasted and toasted | 0.37 |
| Seeds, sesame seed kernels, toasted, without salt added (decorticated) | 0.37 |
| Seeds, sesame meal, partially defatted | 0.37 |
| Seeds, sesame seed kernels, toasted, with salt added (decorticated) | 0.37 |
References [ + ]