What are Gut Microbiome
For years, we thought of bacteria as organisms to avoid. It turns out our bodies are already loaded with trillions of bacteria. They help digest food and play an important role in your well-being. Recently, thanks to two revolutions in biology, it has become apparent that our DNA does not tell the whole story of our individuality and other factors, environmental factors, play an important role in human health and disease. First, there was epigenetics, where diet and lifestyle changes have been shown to turn genes on and off. And the second, our unfolding understanding of our microbiome, how changes in our gut flora appear to impact greatly on human biology. And, what we learned is that we can each be thought of as a superorganism, a kind of human-microbe hybrid. We have trillions of bacteria living inside us, that there are more bacterial cells and genes in our own body than there are human cells and genes and most of those bacteria live in our gut. Researchers are still in the process of figuring out which bacteria are which.
Your gut flora can be considered like a forgotten organ. Health-promoting effects of our good bacteria include boosting your immune system, improving digestion and absorption, making vitamins, inhibiting the growth of potential pathogens, and keeping you from feeling bloated, but should bad bacteria take roost, they can produce carcinogens, putrefy protein in our gut, produce toxins, mess up our bowel function, and cause infections. Research suggests your gut bacteria are tied to your probability of things like diabetes, obesity, depression, heart disease and colon cancer.
The human microbiome is the collection of all the microorganisms living in association with the human body. These communities consist of a variety of microorganisms including eukaryotes, archaea, bacteria and viruses. Bacteria in an average human body number ten times more than human cells, for a total of about 1000 more genes than are present in the human genome. Because of their small size, however, microorganisms make up only about 1 to 3 percent of our body mass (that’s 2 to 6 pounds of bacteria in a 200-pound adult). These microbes are generally not harmful to us, in fact they are essential for maintaining health. For example, they produce some vitamins that we do not have the genes to make, break down our food to extract nutrients we need to survive, teach our immune systems how to recognize dangerous invaders and even produce helpful anti-inflammatory compounds that fight off other disease-causing microbes. An ever-growing number of studies have demonstrated that changes in the composition of our microbiomes correlate with numerous disease states, raising the possibility that manipulation of these communities could be used to treat disease.
Traditional microbiology has focused on the study of individual species as isolated units. However the vast majority of microbial species have never been successfully isolated as viable specimens for analysis, presumably because their growth is dependent upon a specific micro-environment that has not been, or cannot be, reproduced experimentally. Advances in DNA sequencing technologies have created a new field of research, called metagenomics, allowing comprehensive examination of microbial communities, without the need for cultivation. Instead of examining the genome of an individual bacterial strain that has been grown in a laboratory, the metagenomic approach examines the collection of genomes derived from microbial communities sampled from natural environments. In the Human Microbiome Project, this method complemented genetic analyses of known isolated strains, providing unprecedented information about the complexity of human microbial communities.
(Source 1).
The Intestinal Microbiome Revealed as a Source of Human Genetic and Metabolic Diversity
A recent study 2 found that the composition of bacterial species that populate the human gut—the gut microbiota—evolves with age, particularly in the first years of life, and it differs among people from diverse geographic regions, potentially reflecting varying nutrition.
Scientists sequenced the gut microbiomes (microbiota DNA) of healthy individuals of different ages from the Amazonas of Venezuela, the African nation of the Republic of Malawi, and metropolitan areas of the United States to determine whether differences could be discerned in the diversity of bacterial communities and in the metabolic and nutritional functionality of the genes they contained. Microbiome DNA was obtained from fecal samples donated by members of the study cohort, which included parents, children, siblings, and identical and fraternal twins.
A broad spectrum of information was obtained from the analysis of the microbiome data. Of particular importance, microbiota bacterial diversity increased with age in all populations, and bacterial species composition evolved from an infant microbiota into an adult microbiota during the first 3 years of life. In addition, the repertory of microbiome genes involved in vitamin biosynthesis, carbohydrate metabolism, and other metabolic functions also changed with age and differed among the countries. There were greater differences in bacterial community composition among the children than among the adults, and there were significant differences in the types of bacteria represented by the microbiomes of the three geographically representative populations. The greatest differences among populations were seen between the United States and the other two countries, in terms of their bacterial capacities for metabolizing vitamins, carbohydrates, proteins, and other substances, which closely reflect dietary patterns in these countries. This study reveals significant differences in the gut microbiome among young children and adults and among cultures with different diets, underscoring the importance of considering microbiome contributions in studies and nutrition-related policies involving human development, nutrition, physiology, and the impact of westernization. (Source 2).
The Placenta’s Microbiome
You’re probably not thinking that the placenta has bacteria. Until recently, the uterus and the placenta were thought to be germ-free and sterile—to keep the baby safe from infection. But at just one week old, babies have a complex collection of microbes in their guts.
Where do those bacteria come from ? Dr Kjersti Aagaard 3, a 2007 recipient of a National Institute of Health Director’s New Innovator Award and an obstetrician and associate professor of gynecology at Baylor and the Texas Children’s Hospital, began to suspect there was more to the story 4.
To test the idea, she and her team collected tissue samples from 320 placentas and isolated the bacteria present in each.When they compared the placental microbes with those in the mouth, nose, skin, vagina and gut of non-pregnant women, they found they most closely resembled the microbes that lived in the mouth 3. The finding was unexpected. How would oral microbes from the mother end up in the placenta ? Aagaard turned to earlier animal studies for possible answers. A mouse study suggested that one of the oral microbes, Fusobacterium nucleatum, could alter the structure of blood vessels, enabling the infiltration of other microbes into the blood 5. Once in the bloodstream, these oral microbes could presumably find their way to the placenta. Aagaard reasoned that in humans, oral microbes would similarly enter the bloodstream from the oral cavity and “seed” the placental microbiome. Her team provided further support for this idea by showing that women who had suffered a bacterial infection (such as a bladder infection) during the first or second trimester had “microbial fingerprints” of this infection in their placenta.
They also found that the microbes from placentas from babies born earlier than 37 weeks (preterm births) had a significantly different collection of microbes compared to those of babies carried for the full term of 40 weeks. That’s an intriguing observation, with possible clinical significance. Every year, 500,000 babies—1 in 8—are born preterm. These early arrivals can be dangerous, because the baby is still developing vital brain and lung functions in the womb until 39 weeks. While most preterm babies do well with the kind of support now available in hospitals, they are at greater risk of breathing and feeding difficulties, cerebral palsy and vision deficits, and neurological problems later in life.
Aagaard and her colleagues are now beginning a National Institute of Health-sponsored longitudinal study of 526 women to determine whether microbes may actually play a causative role in preterm births. It may be that specific microbes, or predictable shifts in the bacterial community, may explain why some infants are more likely to be born preterm. If these possibilities are confirmed, early diagnostic testing may help identify women at risk—and ultimately there might even be ways to reset the microbiome to encourage full term pregnancies.
How What You Eat Can Affect How Much You Eat
Scientists 6 identified one way the gut microbiome influences obesity and metabolism. A link between the bacteria that populate the intestines (part of the gut microbiome) and obesity had been previously discovered, but the details of how the microbiome influenced body weight were not known. To delve into this question, researchers built on a previous observation: changes in the amount of short-chain fatty acids (by-products of digestion in the gut) can be associated with overfeeding, obesity, and metabolic syndrome (factors that increase risk of heart disease and diabetes). In this new study, the scientists found that male rats fed a high-fat diet showed a striking increase in the amount of acetate, a short-chain fatty acid, in their bodies, and became insulin resistant, a condition associated with metabolic syndrome. Determining the origin and consequences of the increase in acetate resulted in an exciting discovery of how the gut microbiome affects metabolism.
By measuring the acetate in tissues of the rat, the scientists found the highest amount in the gut; treating the rats with antibiotics to kill the gut bacteria, or removing the colon (part of the gut), reduced the amount of acetate dramatically. Consistent with previous research, they also found that rats fed a high-fat diet had a mix of bacteria in their microbiome that was somewhat different from the gut bacteria of rats fed a normal diet. A fecal transfer—transplanting the gut microbiome from rats eating the high-fat diet into rats on a normal diet—also transferred the increase in acetate production. Together, these observations indicate that the gut microbiome was responsible for generating the increased acetate. To determine the chronic effects of increased acetate, rats on a normal diet received acetate infusions for 10 days. After this period, the rats had increased insulin secretion by the pancreatic β (beta) cells in response to insulin, were insulin resistant, and more than doubled their daily caloric intake and weight gain. Interestingly, the researchers discovered that the acetate stimulated the parasympathetic nervous system through the brain. These results suggest a model: exposure to a diet high in calories leads to increased acetate production by bacteria in the gut. The acetate enters the blood and travels to the brain. As a result, the brain signals to the pancreas to increase insulin secretion and storage of fat, and signals to the stomach to release the hunger hormone ghrelin. This process appears to lead to overfeeding and insulin resistance, creating a feedback loop. Additional research will be necessary to determine whether the same mechanism operates in humans and to identify which bacteria in the gut microbiome contribute to the production of acetate. Nevertheless this study describes a novel link between the gut microbiome, obesity, and metabolic syndrome that could be targeted in the development of therapeutics for obesity and diabetes. (Source 6).
In another research project, the scientists used combinations of gut microbes harvested from human stool samples and tested them in male mice raised under sterile conditions to be free of any microbes. Two weeks after transplanting the human gut microbes into the mice, they measured increases in a type of immune cell that prevents inappropriate inflammation in the gut, but they also saw an increase in fat deposits (adiposity). Using one of the human donors’ samples as a representative, the researchers sequenced the bacterial genomes present. They identified 17 unique bacterial strains that, when given to mice, showed effects on immune cells and adiposity similar to the effects of the initial bacterial transplants. To find out which specific combinations of bacterial strain subsets were responsible for these effects, the researchers gave 94 different combinations of the bacteria as well as single bacterial strains to the mice. They then measured immune cells, adiposity, and products of nutrient metabolism, such as bile acids, fatty acids, and amino acids, and compared the results to measurements of the same elements in control mice that remained bacteria-free. Through these experiments, they identified which bacteria, alone or in combination, promoted these immune and metabolic functions. For example, they found several bacterial strains were associated with increased adiposity, including five strains in the bacterial group Bacteroides, two strains of Bacteroidetes, and Escherichia coli. Many of the same Bacteroides strains were also associated with expansion of the population of immune cells called regulatory T cells in the intestine. (Source 7, 8).
Functional Role of Microbiome in Obesity
Scientists have discovered that bacteria that dwell in the human gut are associated with their host’s obesity or leanness 9. Bacteria that inhabit the gut—the gut microbiota—perform important functions, including breaking down food that could not otherwise be digested. Mouse studies have also suggested that bacterial diversity in the gut may influence whether animal “hosts” are lean or obese, based on differences in the efficiency of specific types of bacteria to extract energy (calories) from food. Recent data indicate that the gut microbiome can predict body composition (lean or obese) with 90 percent accuracy compared to 60 percent accuracy with genetics alone. Moreover, evidence suggests the microbiome plays a critical role in weight loss interventions, but the precise functional nature of this role has not been clearly established 10.
- Gut Microbes from People Can Transmit Obese or Lean Body Types to Mice
- These studies 11, 9 provide convincing evidence that the gut microbiome, in conjunction with diet, can strongly affect the ability to gain or lose weight in mice, and may lead to insights into the role of the gut microbiome in regulating weight in humans. A study in mice has found that gut microbes obtained from obese or lean people, within certain dietary contexts, can transmit obesity or leanness to mice in the lab. While there is ample evidence that genetics and other factors play an important role in the development of obesity, there is also evidence that the community of bacteria living in the gut and their collective bacterial genomes, or “gut microbiome,” may affect and relect a person’s health and nutritional status. For example, recent research raised the possibility that differences in the gut microbiome may explain why twins with identical genetic makeups can have very different disease and nutritional states. To examine this possibility, scientists transferred the gut microbiomes from twins discordant (which means one twin was obese while the other was not) for obesity into mice previously raised in sterile conditions and initially free of any gut microbes. Even though all mice were fed the same diet, only the mice that received the obese twin’s microbiome gained weight, while the mice that received gut microbes from the lean twin did not.When the scientists compared the microbiome from the lean mice to that of the obese mice, they found differences in genes that regulate metabolism, including the metabolism of certain amino acids (components of proteins) and effects on fats and starches, suggesting that metabolic changes are responsible for the microbiome’s effects on weight. However, the protective effects of the lean twin’s microbiome were only seen when the mice were fed a healthy diet with high amounts of fruits and vegetables and low amounts of saturated fat, meaning that changes in weight were not dependent on the microbiome alone, but were also dependent on diet. (Source 11).
- In a recent study 12 of obese and lean adult twin sets and their mothers, researchers studied the human microbiota using fecal samples to determine whether host obesity, genetics, or environment is associated with the bacterial composition of the microbiota. To determine which types of bacteria were present in the gut, the researchers analyzed DNA sequences in a particular gene common to all bacteria to identify sequence variations unique to each type. Comparisons revealed that the proportion of different types of bacteria in the guts of obese twins differed from that in the lean twins. Actinobacteria were more abundant than Bacteriodetes bacteria in the obese twins. Conversely, Bacteriodetes were more numerous in the lean twins. Obesity was also associated with significantly less bacterial diversity overall than leanness. Additional analysis revealed that the microbiota of family members are more similar in bacterial composition than unrelated individuals. Surprisingly, the identical twins were not more similar in their gut microbes than fraternal twins, suggesting that composition of the gut microbiota is influenced more strongly by environmental factors than by an individual’s genes. An analysis of bacterial genes represented in the “microbiome”—the combined DNA of the microbiota—found that although the precise composition of the types of bacteria in the gut differs among individuals, people share a “core microbiome” of common microbial genes harbored by the various bacteria. Additionally, comparison of noncore microbiome genes identified over 350 genes that were either enriched or depleted in the microbiomes of obese individuals. Among the genes enriched in the obese gut microbiome, many of which are involved in processing carbohydrates and other metabolic pathways, most were from Actinobacteria and others were from another group of bacteria, Firmicutes.While this study does not demonstrate cause and effect—whether differences in human microbiota help cause obesity or leanness, or whether obesity or leanness leads to changes in gut microbes—earlier research has shown that the composition of gut microbiota can influence weight gain in mice. This study does demonstrate a significant link between obesity and the gut microbiome, including the identification of several hundred genes that represent biomarkers of unique gut bacterial activity in obese individuals. These biomarkers may lead to more personalized healthcare and potential probiotic interventions to modify the microbial content of the human gut. (Source 12).
- Gut microbial communities shaped by human genetic factors
A collaborative research group 13 studying a large population of twins in the United Kingdom has shown that human genetic factors shape the composition of the gut microbial community, and some gut microbes may in turn affect the metabolism of their human “hosts.” The first members of the gut microbiome, the collection of all microbes present in the gut and/or their genetic material, are acquired from the maternal “environment” at birth, or possibly even earlier in the womb. Similar gut microbiomes in related adults are often attributed largely to a shared environment, including common diets. However, previous studies also hinted at the possibility that other determinants of an individual’s unique gut microbiome over time may lie in one’s own human genome. Researchers based at institutions in the United States and the United Kingdom set out to analyze a sufficiently large number of people to test this idea, using the TwinsUK study. They collected fecal samples from 416 pairs of identical or fraternal twins. By sequencing a portion of the microbial genetic material from each of those samples, they were able to identify and quantify specific gut microbial species present in the twins’ samples. Pairs of genetically identical twins had more similar gut microbiomes than pairs of fraternal twins did. Within the identical twin pairs, winning the prize for the group of gut microbes whose abundance was most closely tied to human genetic influences were members of the Christensenallaceae family—a family of bacteria that has only been described since 2012. The varying abundance of these bacteria in different twin pairs may actually have a larger impact on their gut microbial communities, as they tended to coincide with certain other gut microbes. The researchers also noticed that the Christensenallaceae family microbes were more abundant in lean study participants than in those who were obese. They tested whether these microbes might protect against weight gain by introducing microbes from obese participants into mice that had been raised up to that point in a sterile environment (free of microbes). To some of the mice they also introduced bacteria from one species of Christensenallaceae. After 3 weeks, the mice that received Christensenallaceae family microbes along with the obese donor’s gut microbes were leaner than those that received only the obese donor’s microbes. These findings point to members of this microbial family as important contributors to human metabolism, with potential effects on weight gain, and show that its abundance, and in some cases presence or absence, is strongly affected by one’s genes. This may be one way in which genes affect human metabolic health: by influencing the capacity to host beneficial microorganisms. Future studies will explore which human genes influence the components of the gut microbiome. Such knowledge could inform future health-promoting interventions, potentially suggesting ways to adjust gut microbial levels depending on an individual’s genetic background.
- Foods affect our health in two ways:
- Through the fats, carbohydrates, proteins, and other nutrients they provide, and through the bacteria that break them down in the intestine. Early research indicates that a diet high in saturated fat increases the proportion of one phylum (major group) of bacteria, Firmicutes, to that of another phylum, Bacteriodetes. A high ratio of Firmicutes to Bacteriodetes has been linked to obesity. If we eat a lot of processed foods, populations of fiber-loving Bifidobacterium, which is linked with lower rates of inflammation, will shrink.
- Our intestinal bacteria may be key to understanding why the traditional “Western diet” hasn’t served us very well. Studies comparing populations of intestinal microbes have indicated that people eating a traditional Mediterranean diet or a traditional Asian diet—both of which are plant-based—have a greater diversity of intestinal bacteria and a higher proportion of beneficial bacteria than Americans and Europeans whose diets are heavier in red meat, sugars, and other refined carbohydrates, and lighter in fruits and vegetables. Westerners also have a higher rate of obesity, cardiovascular disease, and colon cancer. (Source 14, 15).
How Gut Bacteria may affect the Heart
Scientists are learning more about how the trillions of bacteria (gut microbiota) dwelling deep inside your digestive tract can affect your risk of cardiovascular disease 16.
Over the past decade, scientists have uncovered compelling connections between different types of gut microbes and the development of obesity and diabetes — two factors closely tied to a higher risk of heart disease. Recently, several studies have explored how our gut microbes interact with the food we eat to spur artery-damaging inflammation and narrowing. These findings are still preliminary, but experts hope they’ll one day lead to personalized diet recommendations or other therapies to lower the risk of heart disease.
- Artery damage
The initial discovery connecting the gut microbiota to cardiovascular disease came from researchers at the Cleveland Clinic. They discovered that when gut microbes feed on a chemical called choline (found in eggs, red meat, and dairy products), they produce a compound called TMA. In the liver, TMA is converted to TMAO, which causes hardening of the arteries (atherosclerosis) in mice and is linked to a higher risk of heart disease in humans.
For the first time, they showed how the relationship between a dietary component, bacterial metabolism, and human metabolism can have adverse consequences for blood vessels.
- Avoiding arterial blockages
The investigators then tested a molecule that blocks the production of TMA, which they gave to mice prone to atherosclerosis, thanks to their genes and a high-fat diet. The molecule, called DMB, occurs naturally in olive oil and red wine. The mice that got DMB in their water had healthier, clearer arteries than those that didn’t.
Earlier this year, Chinese researchers described a different but related approach to preventing blood vessel injury in atherosclerosis-prone mice. They found that by giving the mice a specific strain of bacteria called Akkermansia muciniphila, they could prevent inflammation — the chronic, persistent immune response that contributes to the buildup of fatty plaque in arteries. The effect was largely due to a protein that was able to “tighten up” the communication between cells in the inner lining of the gut. As a result, fewer toxins from the diet could pass from the gut into the bloodstream, which in turn dampened inflammation.
Together, these findings suggest that altering the gut microbiota in different ways might minimize blood vessel damage. There’s also some evidence that the gut microbiota may influence the levels of cholesterol and other fats in the bloodstream, as well as blood pressure.
But for now, it’s far too early to offer any specific advice based on this research. The human microbiome is unique, which makes it hard to define exactly what constitutes a healthy gut environment. However, a more diverse mix of bacteria seems to be healthier than a limited one. People who eat a traditional, plant-based Mediterranean or Asian diet tend to have a greater diversity of intestinal bacteria than Americans and Europeans, whose diets are heavier in red meat, sugars, and other refined carbohydrates, and lighter in fruits and vegetables.
Can Gut Bacteria improve your Health ?
Initial research suggests certain bacteria in your gut can prevent and treat many common diseases 17.
About 100 trillion bacteria, both good and bad, live inside your digestive system. Collectively, they’re known as the gut microbiota. The microorganisms that inhabit your body can be valuable allies in reducing inflammation and treating disease.
The more we learn about the human microbiome—the trillions of single-celled organisms that colonize our skin, nose, digestive system, and vagina—the more we realize that the microscopic critters that live on us and in us may be as important to our health as our body cells 14. Science has begun to look more closely at how this enormous system of organisms influences—and even improves—health conditions, from heart disease to arthritis to cancer. Knowing which sorts of microbes are normally found in healthy people can help us understand the roles that changes in microbe populations play in disease.
How microbes may influence your health
Microbes function a lot like your body cells. They take in nutrients and break them down to supply the energy they need to grow and reproduce. In the process, they secrete molecules that are taken in by our body cells.
The effects of those microbial products can be either harmful or beneficial. For example, Clostridium tetani—the bacterium responsible for tetanus—secretes a toxin that acts on nerve cells to create the powerful muscle contractions responsible for lockjaw. On the other hand, Bifidobacterium, which digests dietary fiber in the colon, produces short-chain fatty acids that stimulate the growth of the immune cells that control inflammation. A vaginal microbe, Lactobacillus, feeds on sugars and produces lactic acid, which discourages other bacteria responsible for vaginal infections.
What Are Gut Bacteria ?
Living inside of your gut are 300 to 500 different kinds of bacteria containing nearly 2 million genes. Paired with other tiny organisms like viruses and fungi, they make what’s known as the microbiota, or the microbiome.
Like a fingerprint, each person’s microbiome is unique: The mix of bacteria in your body is different from everyone else’s mix. It’s determined partly by your mother’s microbiome — the environment that you’re exposed to at birth — and partly from your diet and lifestyle.
The bacteria live throughout your body, but the ones in your gut may have the biggest impact on your well-being. They line your entire digestive system. Most live in your intestines and colon. They affect everything from your metabolism to your mood to your immune system.
Diet is extremely important because the vast majority of the body’s microbes live in the digestive system. In effect, they eat what you eat. Certain types of bacteria thrive on vegetable fiber while others flourish on sugars, so changing your diet can have a big impact on our microbial mix.
How you can help your bacteria
As the discoveries of the Human Microbiome Project mount, there are bound to be new insights into the role of microbes and ideas for new ways to help the good bacteria and hinder the bad ones. In the meantime, the following health advice is still in effect:
- Eat a Mediterranean-style diet. You’ve probably seen a list of inflammation-fighting foods that is likely to include fruits, green leafy vegetables, olive oil, tomatoes, and fish. These are all part of the Mediterranean diet. However, eating one—or even all—of these foods may not help foster your inflammation-fighting gut bacteria if you’re also consuming quantities of sugar, refined carbohydrates, and red meat, which boost other, less friendly microbes.
- Practice good hygiene. This doesn’t mean being squeaky clean or lathering up with antimicrobial soap. Overusing antibacterial agents can upset the bacterial balance on your skin and promote the development of antibiotic-resistant bacteria. But it is a good idea to wash your hands before you prepare meals and after you use the toilet. Brushing and flossing your teeth after meals is also a good way to keep decay-causing bacteria from invading the biofilm of healthy bacteria that protect your teeth and gums.
- Don’t douche. Douching disrupts colonies of Lactobacillus in the vagina, making it vulnerable to infection by other bacteria or yeast.
- Take antibiotics only when needed. These medications don’t distinguish between helpful and harmful bacteria—they attack all bacteria. The price of eradicating an infection is losing a lot of friendly bacteria in the process.
Using bacteria to fight disease
There is increasing evidence that probiotics—preparations of helpful bacteria—can help treat the following conditions by restoring microbial balance. Since there are so many types of probiotics on the shelves, you might want to ask your clinician for a recommendation if you’re considering using one.
- Irritable bowel syndrome. Bifidobacterium, but not Lactobacillus, has been shown to reduce bloating, cramping, diarrhea, and other symptoms.
- Diarrhea caused by antibiotics. Probiotics containing Saccharomyces boulardii, Lactobacillus, and Bifidobacterium may prevent or minimize diarrhea.
- Diarrhea due to Clostridium difficile. This type of diarrhea, which is difficult to control, has been alleviated with oral capsules containing freeze-dried fecal samples from healthy people who have a diverse population of intestinal bacteria. This treatment eradicated the diarrhea in more than 90% of people in clinical trials, but hasn’t been approved by the FDA yet.
Gut Bacteria and Disease
Research suggests the gut bacteria in healthy people are different from those with certain diseases. People who are sick may have too little or too much of a certain type. Or they may lack a wide variety of bacteria. It’s thought some kinds may protect against ailments, while others may raise the risk.
Scientists have begun to draw links between the following illnesses and the bacteria in your gut:
Obesity, type 2 diabetes, and heart disease: Your gut bacteria affect your body’s metabolism. They determine things like how many calories you get from food and what kinds of nutrients you draw from it. Too much gut bacteria can make you turn fiber into fatty acids. This may cause fat deposits in your liver, which can lead to something called “metabolic syndrome” — a condition that often leads to type 2 diabetes, heart disease, and obesity.
- Cardiovascular Disease. Research are being conducted to determine a relationship between a person’s gut microbiome (the intestinal microbiome) and their risk of cardiovascular disease. Investigators will look at inflammatory markers in the blood and also look at the genome of the bacteria in the gut. This research is being done because Investigators believe that there is a connection between the way food is digested by a person’s gut bacteria and the development of atherosclerosis (hardening of the arteries) and cardiovascular disease. The ultimate goal of this research is to eventually determine if changes to the gut bacteria can prevent cardiovascular disease or disease progression.
- Type 2 diabetes mellitus is a significant health problem facing our nation and our study is likely to provide important insights into this disease. Close to 20 million individuals in the United States have type 2 diabetes, which cost $174 billion in 2007 to treat, a figure projected to double by 2050. Currently, there are an estimated 79M prediabetics in the United States who have a lifetime risk of diabetes conversion of 50%. Differences in the gut microbiome have been noted between diabetics and healthy individuals, and direct alteration of the microbiome in mice has been shown to lower glucose levels. To better understand the biological changes that occur type 2 diabetes disease acquisition, researchers plan to perform a detailed analysis of the biological processes that occur in patients at risk for type 2 diabetes and their microbiomes by longitudinal profiling of patients during healthy periods and periods of stress.
- Inflammatory bowel diseases, including Crohn’s disease and ulcerative colitis: People with these conditions are believed to have lower levels of certain anti-inflammatory gut bacteria. The exact connection is still unclear. But it’s thought that some bacteria may make your body attack your intestines and set the stage for these diseases. Inflammatory bowel disease (IBD), which includes both Crohn’s Disease (CD) and ulcerative colitis (UC), affects approximately 1.5 million Americans and is one of the most-studied imbalances between microbes and the immune system. Genetic and environmental risk factors exist that are associated with inflammatory bowel disease, however they are inadequate to explain the dramatic (more than 400%) increase in IBD over the past 50 years. Rather, a comprehensive body of evidence has linked inflammatory bowel disease to the gut microbiota (the bacteria, viruses, archaea, and fungi resident in the gut). In contrast to traditional disease models, no single pathogen seems to cause inflammatory bowel disease. Rather, many studies have found an association between inflammatory bowel disease and an overall disrupted gut ecology and decrease in diversity 18.
- Colon cancer: Studies show that people with it have a different gut microbiota, including higher levels of disease-causing bacteria, than healthy people.
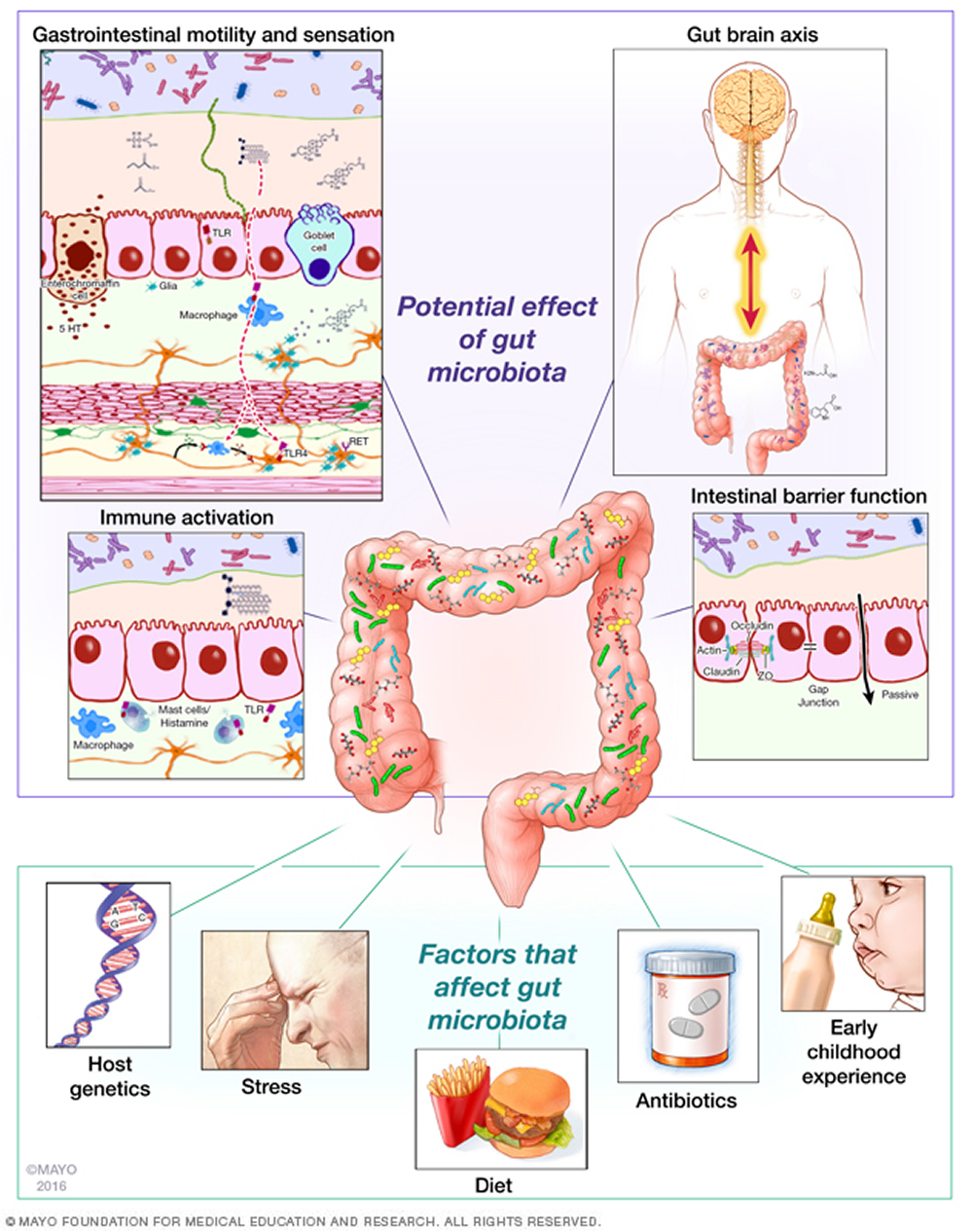
- Anxiety, depression, and autism: The gut is packed with nerve endings that communicate with the brain. Your doctor may call this connection the “gut-brain axis.” Studies have suggested a link between gut bacteria and disorders of the central nervous system, like anxiety, depression, and autism.
- Arthritis: It’s thought that people with rheumatoid arthritis may have greater amounts of a bacteria linked to inflammation than people without it.
The hope of impacting health through diet may be one of the oldest concepts in medicine; however, only in recent years has our understanding of human physiology grown to the point where we can begin to understand how individual dietary components affect specific illnesses through our gut bacteria.
What Can You Do ?
How can you get healthy gut bacteria ?
Start by eating a nutritious diet high in fiber-rich foods, like fruits, vegetables, and whole grains. A “western diet” that’s high in fat and sugar and low in fiber can kill certain types of gut bacteria, making your microbiome less diverse.
Exercise can also encourage the growth of a variety of gut bacteria. Having a more varied gut microbiota may promote better health and in turn, reduce your risk of disease.
You can’t just take probiotics to stave off diabetes or treat arthritis. Experts say that more research needs to be done to pinpoint the exact types of bacteria that lead to certain ailments.
You may soon be able to take a medication or supplement made of a certain strain of gut bacteria to reduce your risk of — or even cure — certain diseases.
Summary
Unraveling the mystery of which microbes are present in the gut, how they come to reside there, and their health implications for the host is the subject of recent and exciting research. These new studies will add to the storm of new knowledge about the mammalian gut microbial community, in terms of understanding the succession of species during colonization and teasing out effects of individual bacterial strains. These works also provide a new means for scientists to identify which resident gut microbes are helping or hindering their human hosts, in terms of key health indicators such as immune function, nutrient metabolism, and fat mass. These studies could be used in the future to identify probiotics or prebiotics—beneficial bacteria or the nutrients they rely on—to enhance human health and limit disease.
- The Gut Microbiome Laboratory at Mayo Clinic. http://www.mayo.edu/research/labs/gut-microbiome/projects/gut-microbiota-host-physiology[↩]
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 486: 222-227, 2012.[↩][↩]
- The Placenta Harbors a Unique Microbiome. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic . J Sci Transl Med. 2014 May 21;6(237). https://www.ncbi.nlm.nih.gov/pubmed/24848255[↩][↩]
- National Institute of Health. Posted on May 28, 2014 by Dr. Francis Collins. – Not Sterile, After All: The Placenta’s Microbiome – https://directorsblog.nih.gov/2014/05/28/not-sterile-after-all-the-placentas-microbiome/[↩]
- Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Fardini Y, Chung P, Dumm R, Joshi N, Han YW. Infect Immun. 2010 Apr;78(4):1789-96. https://www.ncbi.nlm.nih.gov/pubmed/20123706[↩]
- Perry RJ, Peng L, Barry NA, Shulman GI. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534: 213-217, 2016.[↩][↩]
- Seedorf H, Griffin NW, Ridaura VK, Reyes A, Cheng J, Rey FE, Smith MI, Simon GM, Scheffrahn RH, Woebken D, Spormann AM, Van Treuren W, Ursell LK, Pirrung M, Robbins-Pianka A, Cantarel BL, Lombard V, Henrissat B, Knight R, Gordon JI. Bacteria from diverse habitats colonize and compete in the mouse gut. Cell 159: 253-266, 2014.[↩]
- Faith JJ, Ahern PP, Ridaura VK, Cheng J, and Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med 6: 220ra11, 2014.[↩]
- Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 457: 480-484, 2009. https://www.niddk.nih.gov/news/research-updates/pages/science-news-obesity-linked-unique-mix-intestinal-bacteria-bacterial-genes.aspx[↩][↩]
- U.S. Department of Health and Human Services, National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/Documents/2017_NIDDK_RecentAdvancesEmergingOpps_Obesity.pdf[↩]
- Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341: 1241214, 2013. https://www.niddk.nih.gov/news/research-updates/pages/gut-microbes-people-transmit-obese-lean-body-types-mice.aspx[↩][↩]
- Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 457: 480-484, 2009.[↩][↩]
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, and Ley RE. Human genetics shape the gut microbiome. Cell 159: 789-799, 2014. https://www.niddk.nih.gov/news/research-updates/pages/gut-microbial-communities-shaped-human-genetic-factors.aspx[↩]
- Harvard University, Harvard Health Publications January, 2016. – Making peace with your germs – http://www.health.harvard.edu/staying-healthy/making-peace-with-your-germs[↩][↩]
- The Gut Microbiome Laboratory at Mayo Clinic. – Individualized Responses of Gut Microbiome to Diet and Their Role in Gastrointestinal Physiology – http://www.mayo.edu/research/labs/gut-microbiome/projects/diet-gastrointestinal-physiology[↩]
- Harvard University, Harvard Health Publications July 22, 2016. – Gut reaction: How bacteria in your belly may affect your heart – http://www.health.harvard.edu/blog/gut-reaction-bacteria-belly-may-affect-heart-2016072210005[↩]
- Harvard University, Harvard Health Publications October, 2016. – Can gut bacteria improve your health ? – http://www.health.harvard.edu/staying-healthy/can-gut-bacteria-improve-your-health[↩]
- The Gut Microbiome Laboratory at Mayo Clinic. – Temporal Changes in Gut Microbiome in Patients With Irritable Bowel Syndrome and the Relationship to Host Genetics and Diet – http://www.mayo.edu/research/labs/gut-microbiome/projects/irritable-bowel-syndrome-genetics-diet[↩]