Contents
- What is Parkinson’s disease ?
- What Causes Parkinson’s Disease ?
- Symptoms of Parkinson’s Disease
- Diagnosis of Parkinson’s Disease
- Treatment of Parkinson’s Disease
What is Parkinson’s disease ?
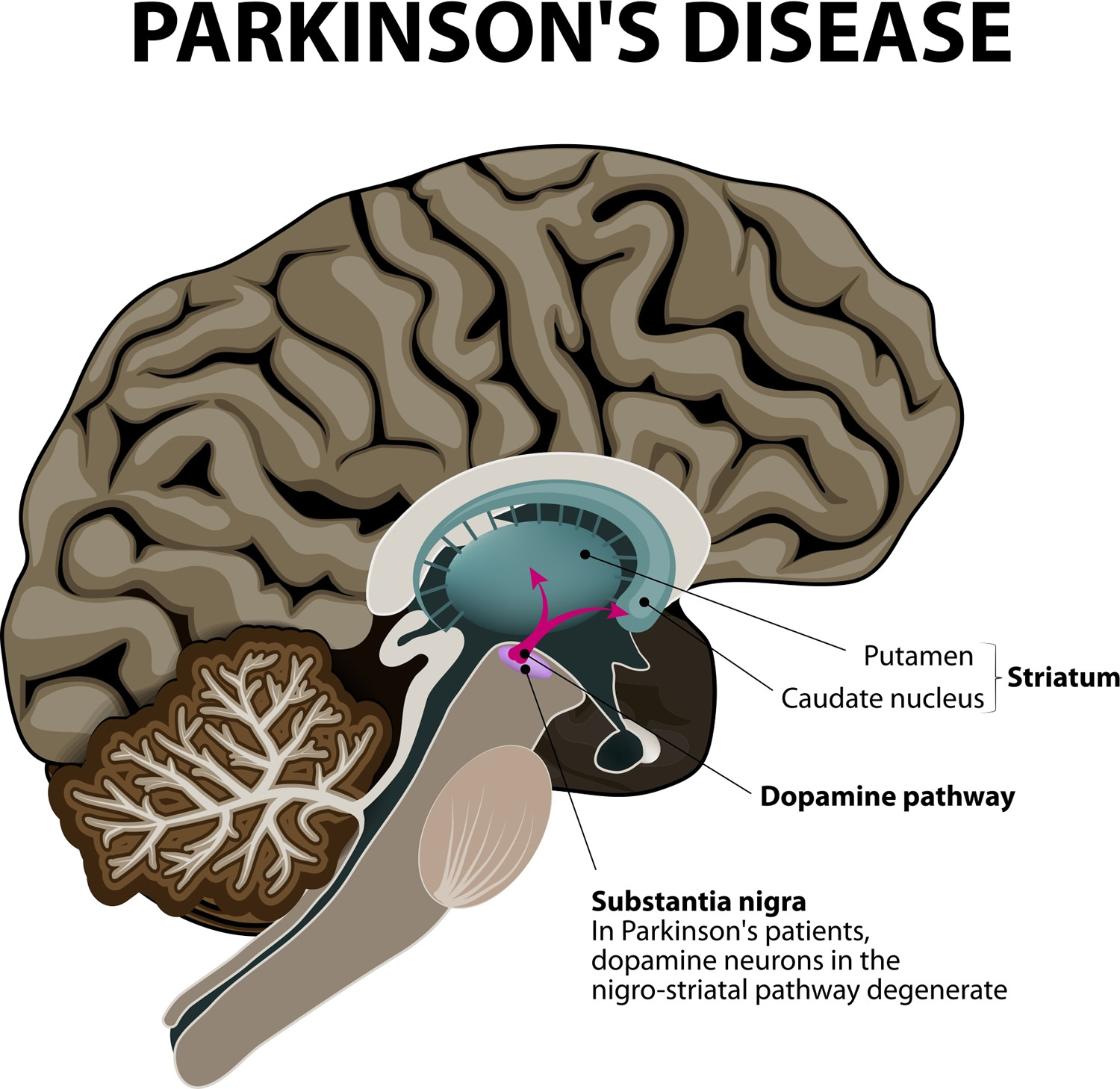
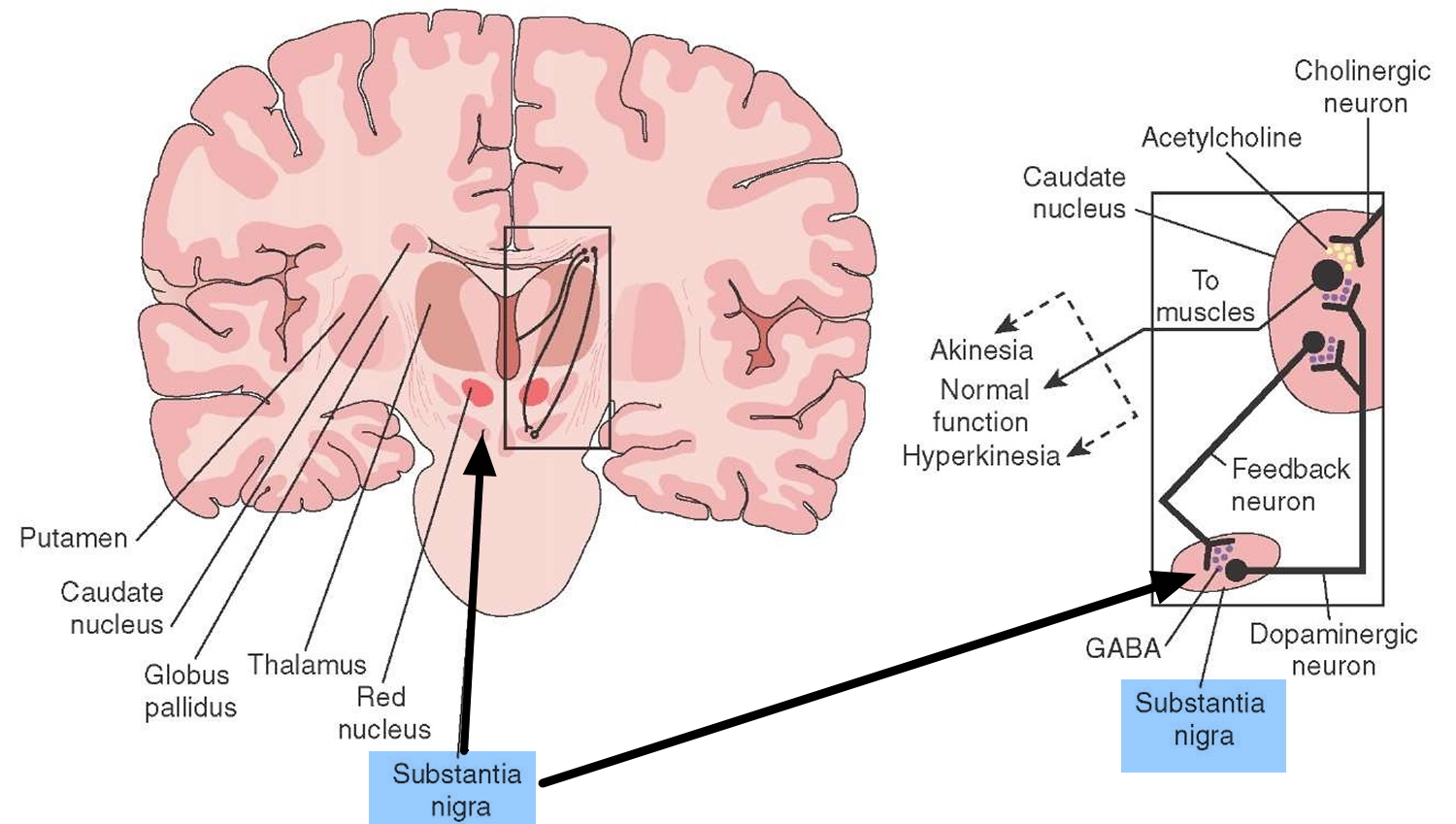
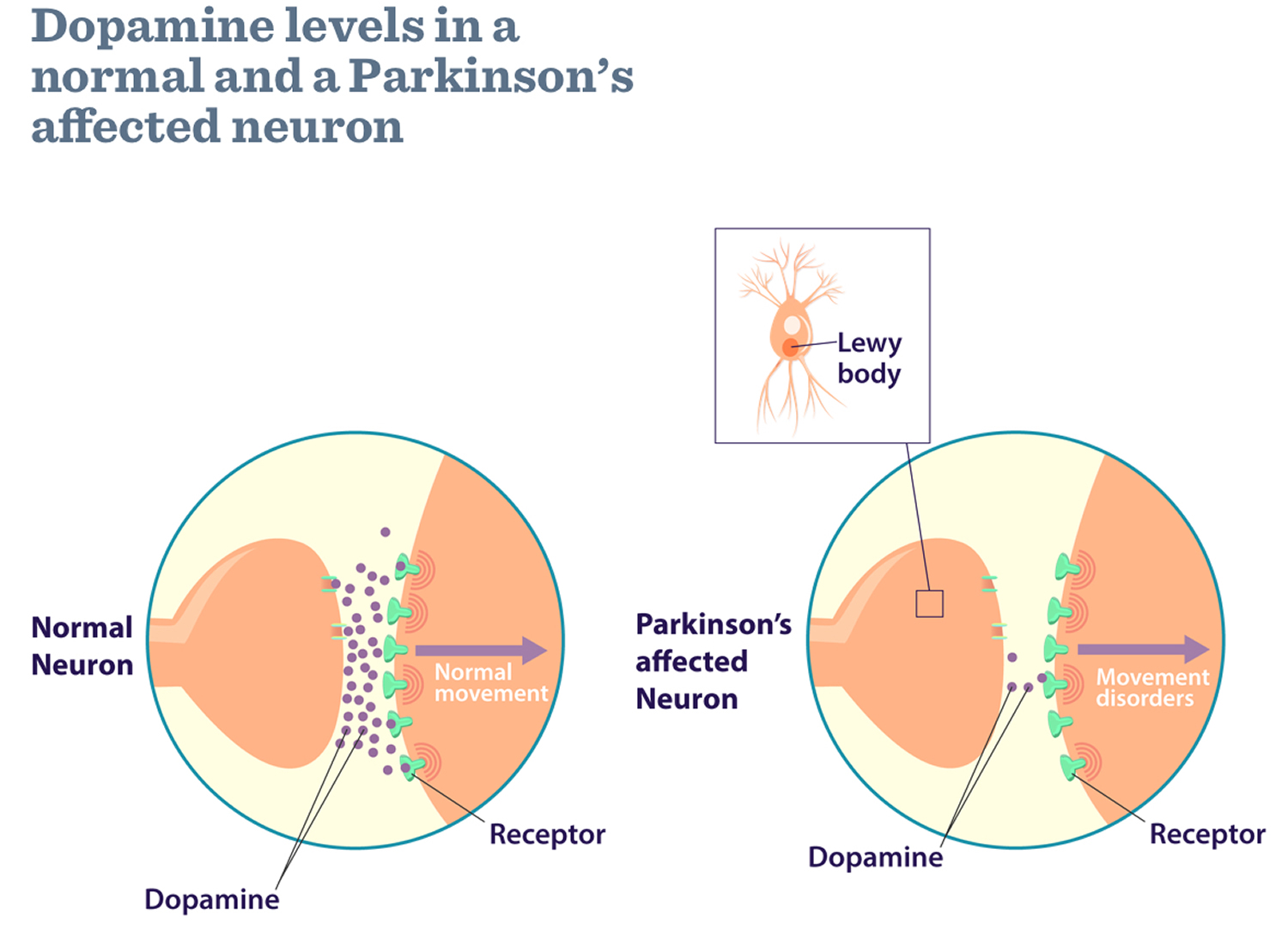
Parkinson’s disease is a brain disorder which is the result of the loss of dopaminergic neurons in the midbrain die (dopamine-producing brain cells) that leads to shaking, stiffness, and difficulty with walking, balance, and coordination 1. Parkinson’s disease progresses slowly as small clusters of dopaminergic neurons in the midbrain die. The gradual loss of these neurons results in reduction of a critical neurotransmitter called dopamine, a chemical responsible for transmitting messages to parts of the brain that coordinate muscle movement 2. Studies have shown that the symptoms of Parkinson’s usually appear when 50 percent or more of the dopamine neurons in the midbrain have been lost 2. Symptoms begin gradually and typically worsen over time.
Parkinson’s disease is a progressive neurodegenerative disease, the second most common disorder of this type after Alzheimer’s disease. It is difficult to know exactly how many people have Parkinson’s disease, since there is no national registry, but it is estimated that at least 500,000 people in the U.S. currently have the disease 3. However, Parkinson’s disease affects about 1 to 2 percent of people over the age of 60 years and the chance of developing Parkinson’s disease increases as we age 4. Most people affected with Parkinson’s disease are not aware of any relatives with the condition but in a number of families, there is a family history. When three or more people are affected in a family, especially if they are diagnosed at an early age (under 50 years) scientists suspect that there may be a gene making this family more likely to develop the condition 4.
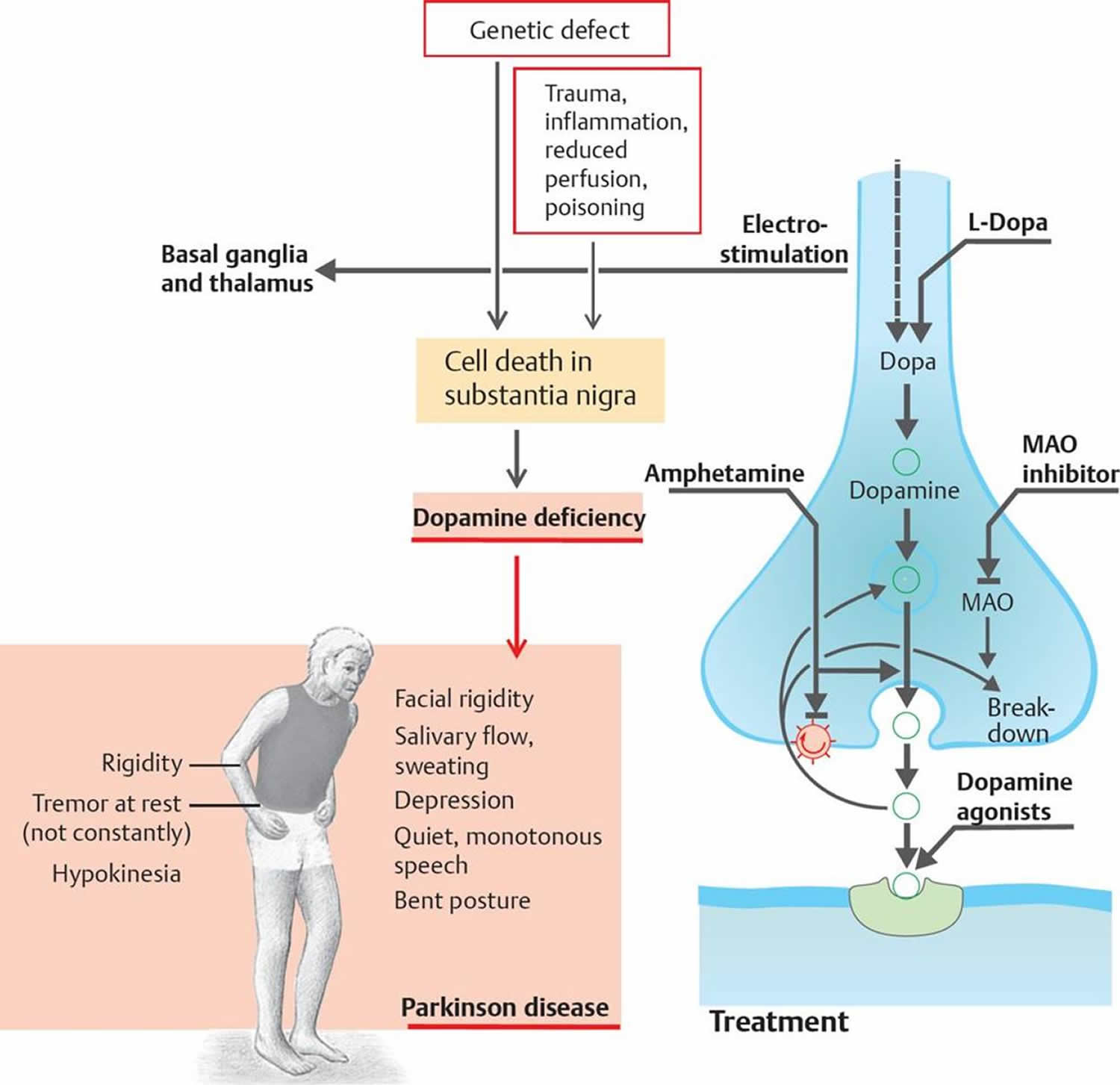
Parkinson’s disease belongs to a group of conditions called motor system disorders 5. The four primary symptoms of Parkinson’s disease are tremor, or trembling in hands, arms, legs, jaw, and face; rigidity, or stiffness of the limbs and trunk; bradykinesia, or slowness of movement; and postural instability, or impaired balance and coordination 5. As these symptoms become more pronounced, patients may have difficulty walking, talking, or completing other simple tasks.
Parkinson’s disease usually affects people over the age of 60. Early symptoms of Parkinson’s disease are subtle and occur gradually and get worse over time. In some people the disease progresses more quickly than in others. As the disease progresses, people may have difficulty walking and talking. And the shaking, or tremor, which affects the majority of people with Parkinson’s disease may begin to interfere with daily activities. People with Parkinson’s disease may also have mental and behavioral changes, sleep problems, depression, memory difficulties, and fatigue. Other symptoms may include depression and other emotional changes; difficulty in swallowing, chewing, and speaking; urinary problems or constipation; skin problems; and sleep disruptions. There are currently no blood or laboratory tests that have been proven to help in diagnosing sporadic Parkinson’s disease. Therefore the diagnosis is based on medical history and a neurological examination. The disease can be difficult to diagnose accurately. Doctors may sometimes request brain scans or laboratory tests in order to rule out other diseases.
Both men and women can have Parkinson’s disease. However, the disease affects about 50 percent more men than women.
One clear risk factor for Parkinson’s is age. Although most people with Parkinson’s first develop the disease at about age 60, about 5 to 10 percent of people with Parkinson’s have “early-onset” disease, which begins before the age of 30 4. Early-onset forms of Parkinson’s are often, but not always, inherited, and some forms have been linked to specific gene mutations.
What Causes Parkinson’s Disease ?
The exact cause of Parkinson’s disease is unknown. Most researchers agree that the disease is caused by both genetic and environmental factors, and by interactions among these factors. Although some cases of Parkinson’s disease appear to be hereditary, and a few can be traced to specific genetic mutations, in most cases the disease occurs randomly and does not seem to run in families. Many researchers now believe that Parkinson’s disease results from a combination of genetic factors and environmental factors such as exposure to toxins.
Parkinson’s disease occurs when nerve cells, or neurons, in an area of the brain that controls movement become impaired and/or die. Normally, these neurons produce an important brain chemical known as dopamine. When the neurons die or become impaired, they produce less dopamine, which causes the movement problems of Parkinson’s. Scientists still do not know what causes cells that produce dopamine to die.
People with Parkinson’s also lose the nerve endings that produce norepinephrine, the main chemical messenger of the sympathetic nervous system, which controls many automatic functions of the body, such as heart rate and blood pressure. The loss of norepinephrine might help explain some of the non-movement features of Parkinson’s, such as fatigue, irregular blood pressure, decreased movement of food through the digestive tract, and sudden drop in blood pressure when a person stands up from a sitting or lying-down position.
Many brain cells of people with Parkinson’s disease contain Lewy bodies, unusual clumps of the protein alpha-synuclein. Scientists are trying to better understand the normal and abnormal functions of alpha-synuclein and its relationship to genetic mutations that impact Parkinson’s disease and Lewy body dementia.
What genes are linked to Parkinson’s disease ?
In 1997, the National Human Genome Research Institute studied a large family that came from a small town in Southern Italy in which Parkinson’s disease was inherited from parent to child (dominant inheritance) 4. The National Human Genome Research Institute found the gene that caused their inherited Parkinson’s Disease and it coded for a protein called alpha-synuclein. If one studies the brains of people with Parkinson’s disease after they die, one can see tiny little accumulations of protein called Lewy Bodies (named after the doctor who first found them). Research has shown that there is a large amount of alpha-synuclein protein in the Lewy Bodies of people who have non-inherited Parkinson’s disease as well as in the brains of people who have inherited Parkinson’s disease. This immediately told the researchers at the National Human Genome Research Institute that alpha-synuclein played an important role in all forms of Parkinson’s disease and they are still doing a lot of research to better understand this role.
Currently, seven genes that cause some form of Parkinson’s disease have been identified. Mutations (changes) in three known genes called SNCA (PARK1), UCHL1 (PARK 5), and LRRK2 (PARK8) and another mapped gene (PARK3) have been reported in families with dominant inheritance. Mutations in three known genes, PARK2 (PARK2), PARK7 (PARK7), and PINK1 (PARK6) have been found in affected individuals who had siblings with the condition but whose parents did not have Parkinson’s disease (recessive inheritance). There is some research to suggest that these genes are also involved in early-onset Parkinson’s disease (diagnosed before the age of 30) or in dominantly inherited Parkinson’s disease but it is too early yet to be certain 4.
New research studies, called genome-wide association studies (GWAS) are an approach that involves rapidly scanning markers across the complete sets of DNA, or genomes, of many people to find genetic variations associated with a particular disease. GWAS have been able to identify genetic variations that contribute to common diseases including Parkinson’s disease.
What determines who gets Parkinson’s disease ?
In most cases inheriting a non-working copy of a single gene will not cause someone to develop Parkinson’s disease. Scientists believe that many other complicating factors such as additional genes and environmental factors determine who will get the condition, when they get it and how it affects them. In the families we have studied, some people who inherit the gene develop the condition and others live their entire lives without showing any symptoms. There is a lot of research on genes and the environment that is attempting to understand how all these factors interact.
Genetic Testing in Parkinson’s Disease
Genetic testing has recently become available for the parkin and PINK1 genes. Parkin is a large gene and testing is difficult. At the current stage of understanding, testing is likely to give a meaningful result only for people who develop the condition before the age of 30 years 4. PINK1 appears to be a rare cause of inherited Parkinson’s disease. A small percentage (~2 percent) of those developing the condition at an early age appear to carry mutations in the PINK1 gene. Genetic testing for the PARK7, SNCA and LRRK2 genes is also available.
Individuals and families who are interested in having genetic testing can learn more about their risk for Parkinson’s disease and the availability and accuracy of genetic testing for this disease by setting up an appointment with a genetics health professional. Genetic professionals work as members of health care teams providing information and support to individuals or families who have genetic disorders or may be at risk for inherited conditions. Genetic professionals can discuss the risks, benefits and limitations of available genetic testing for Parkinson’s disease.
Symptoms of Parkinson’s Disease
Parkinson’s disease has four main symptoms 6:
- Tremor (trembling) in hands, arms, legs, jaw, or head. Shaking that often begins in a hand, although sometimes a foot or the jaw is affected first. Characteristically, it is a rhythmic back-and-forth “pill rolling” motion of the thumb and forefinger. It is most obvious when the hand is at rest or when a person is under stress. Tremor usually disappears during sleep or improves with intentional movement.
- Stiffness of the limbs and trunk (Rigidity). The muscles remain tensed and contracted; the person aches or feels stiff. The arm moves in short, jerky measures, like a cogwheel.
- Slowness of movement (Bradykinesia). Bradykinesia is the slowing of spontaneous and automatic movement. For example, washing, dressing, and other daily activities become difficult and take longer than normal.
- Postural instability. Impaired balance and coordination, sometimes leading to falls
Other symptoms may include depression and other emotional changes; difficulty swallowing, chewing, poor sense of smell and speaking; urinary problems or constipation; skin problems; and sleep disruptions.
Symptoms of Parkinson’s and the rate of progression differ among individuals 1. Sometimes people dismiss early symptoms of Parkinson’s as the effects of normal aging. In most cases, there are no medical tests to definitively detect the disease, so it can be difficult to diagnose accurately.
Early symptoms of Parkinson’s disease are subtle and occur gradually. For example, affected people may feel mild tremors or have difficulty getting out of a chair 1. They may notice that they speak too softly, or that their handwriting is slow and looks cramped or small 1. Friends or family members may be the first to notice changes in someone with early Parkinson’s. They may see that the person’s face lacks expression and animation, or that the person does not move an arm or leg normally.
People with Parkinson’s often develop a parkinsonian gait that includes a tendency to lean forward, small quick steps as if hurrying forward, and reduced swinging of the arms. They also may have trouble initiating or continuing movement.
Symptoms often begin on one side of the body or even in one limb on one side of the body. As the disease progresses, it eventually affects both sides. However, the symptoms may still be more severe on one side than on the other.
Many people with Parkinson’s note that prior to experiencing stiffness and tremor, they had sleep problems, constipation, decreased ability to smell, and restless legs.
Diagnosis of Parkinson’s Disease
A number of disorders can cause symptoms similar to those of Parkinson’s disease 1. People with Parkinson’s-like symptoms that result from other causes are sometimes said to have parkinsonism. While these disorders initially may be misdiagnosed as Parkinson’s, certain medical tests, as well as response to drug treatment, may help to distinguish them from Parkinson’s. Since many other diseases have similar features but require different treatments, it is important to make an exact diagnosis as soon as possible.
There are currently no blood or laboratory tests to diagnose non-Postural instability in genetic cases of Parkinson’s disease 1. Diagnosis is based on a person’s medical history and a neurological examination. Also, MRI or CT brain scans of people with Parkinson’s disease usually appear normal. Since other diseases have similar features but require different treatments, making a diagnosis is important for properly treating Parkinson’s disease. Improvement after initiating medication is another important hallmark of Parkinson’s disease.
Treatment of Parkinson’s Disease
Although there is no cure for Parkinson’s disease, medicines, surgical treatment, and other therapies can often relieve some symptoms.
Medicines for Parkinson’s Disease
Medicines prescribed for Parkinson’s include:
- Drugs that increase the level of dopamine in the brain
- Drugs that affect other brain chemicals in the body
- Drugs that help control nonmotor symptoms
The main therapy for Parkinson’s is Levodopa, also called L-dopa. Nerve cells use levodopa to make dopamine to replenish the brain’s dwindling supply. Usually, people take levodopa along with another medication called carbidopa. Carbidopa delays the conversion of levodopa into dopamine until it reaches the brain. Carbidopa prevents or reduces some of the side effects of levodopa therapy—such as nausea, vomiting, low blood pressure, and restlessness—and reduces the amount of levodopa needed to improve symptoms.
When a tablet of levodopa is swallowed, the drug doesn’t make its way directly to the brain where it is needed. It is absorbed in the small intestine and makes its way into the blood stream. From there, the blood takes the drug all around your body. And it is here that the early levodopa tablets had their unwanted effects.
Outside the brain, your body contain proteins that break down levodopa. This means much of the drug (around 60–80%) is deactivated before it even has the chance to get into your brain. The first of these proteins, called DOPA decarboxylase, is the protein that turns levodopa into dopamine. Inside the brain this protein vital for the drug to work, but when levodopa is turned into dopamine outside the brain it causes people to feel nauseous.
To counteract this, today’s medications for Parkinson’s combine levodopa with other drugs — such as carbidopa, which blocks DOPA decarboxylase outside the brain— that stop this break down, allowing more of the levodopa to get into the brain and reducing the side effects from having dopamine in our bodies outside the brain.
Levodopa was first combined with other drugs in the 1970s, and the first levodopa-carbidopa medications (trade name Sinemet) were made commercially available. Later attention was turned to Catechol-O-methyltransferase (COMT), another protein that breaks down levodopa outside the brain, and drugs like entacapone (trade name Comtess) and tocapone (trade name Tasmar) were developed to block Catechol-O-methyltransferase (COMT) and allow more levodopa to get into the brain.
If you’re prescribed levodopa, the initial dose is usually very small and will be gradually increased until it takes effect.
At first, levodopa can cause a dramatic improvement in the symptoms. However, its effects can be less long-lasting over the following years – as more nerve cells in the brain are lost, there are fewer of them to absorb the medicine. This means the dose may need to be increased from time to time.
Long-term use of levodopa is also linked to problems such as uncontrollable, jerky muscle movements (dyskinesias) and “on-off” effects, where the person rapidly switches between being able to move (on) and being immobile (off).
L-dopa does not stop Parkinson’s from progressing and is not a cure. Over long periods people commonly develop involuntary twisting and writhing (called dyskinesias). Although L-dopa can be reduced, doctors and patients must work together closely to find a tolerable balance between L-dopa’s benefits and side effects. Surgery may be considered for severe dyskinesias. Because dyskinesias tend to be associated with long-term L-dopa use, doctors often start patients on other dopamine-increasing drugs and add L-dopa later.
People with Parkinson’s should never stop taking levodopa without telling their doctor. Suddenly stopping the drug may have serious side effects, such as being unable to move or having difficulty breathing.
Although levodopa helps at least three-quarters of parkinsonian cases, not all symptoms respond equally to the drug. Bradykinesia and rigidity respond best, while tremor may be only marginally reduced. Problems with balance and other symptoms may not be alleviated at all. Anticholinergics may help control tremor and rigidity. Other drugs, such as bromocriptine, pramipexole, and ropinirole, mimic the role of dopamine in the brain, causing the neurons to react as they would to dopamine. An antiviral drug, amantadine, also appears to reduce symptoms. In some cases, surgery called Deep Brain Stimulation (DBS) may be appropriate if the disease doesn’t respond to drugs.
Making dopamine stick around
Once levodopa is in the brain it can be converted to dopamine. Dopamine plays a vital function allowing brain cells in the substantia nigra to communicate, to do this the brains cells release this ‘neurotransmitter’ into a space between the cells called the synapse. Another trick to treating Parkinson’s is to make sure the dopamine sticks around as long as possible in this space where it can continue to work. To do this researchers have developed drugs that stop dopamine that has been released into the synapses being recycled.
The protein Catechol-O-methyltransferase (COMT), as well as being outside the brain stopping levodopa getting in, is also found in the brain. Here is has an additional function to recycle dopamine. As tocapone can cross the blood brain barrier, this drug can also block Catechol-O-methyltransferase (COMT) in the brain and allow the dopamine to stick around longer.
The second type of protein that recycles dopamine is MAO-B (monoamine oxidase-B inhibitor). While drugs that act to block MAO (monoamine oxidase) date back to the 1950s, it wasn’t until the 1970s that researchers in Vienna worked out that a drug called selegiline, could be used in Parkinson’s. Selegiline specifically blocked one of the two types of MAO (the B variety, MAO-B). It was 1993, when this drug (trade name Eldepryl) became the first MAO-B inhibitor (monoamine oxidase-B inhibitor) drug to be made available in the UK for the treatment of Parkinson’s, 3 years before it was made available in the US.
Other medicines used to treat Parkinson’s symptoms include 7 :
- Dopamine agonists to mimic the role of dopamine in the brain. These drugs include apomorphine, pramipexole, ropinirole, and rotigotine. They mimic dopamine in the brain and can be given alone or with L-dopa.
- MAO-B inhibitors to slow down an enzyme that breaks down dopamine in the brain. These drugs cause dopamine to accumulate in surviving nerve cells and reduce the symptoms of Parkinson’s. They include selegiline (also called deprenyl) and rasagiline.
- COMT inhibitors help prevent the break down of dopamine. These drugs prolong L-dopa’s effects by preventing the breakdown of dopamine. They include entacapone and tolcapone.
- Amantadine, an old antiviral drug, to reduce involuntary movements. It is often used alone in Parkinson’s early stages. It’s not known exactly how amantadine drug works for Parkinson’s. It may affect how the brain reacts to certain chemicals. There is not enough scientific evidence to support amantadine as a first choice in early Parkinson’s. But for some people, amantadine may reduce dyskinesia (involuntary movements) caused by your other Parkinson’s drugs, without making your Parkinson’s symptoms worse.
- Amantadine can be used to treat tremor and rigidity. It isn’t used very often and is unlikely to be prescribed alone. It’s usually given with other drug treatments and can be used at all stages of Parkinson’s. It is started at a lower dose and the amount is stepped up gradually.
- Anticholinergic drugs to reduce tremors and muscle rigidity. These drugs include trihexyphenidyl, benztropine, and ethopropazine. These drugs block the action of acetylcholine, which helps to send messages in the brain and from your nerves to your muscles. These drugs are old and are now not used very often for Parkinson’s.
- In March 2017, the FDA approved Safinamide tablets as an add-on treatment for individuals with Parkinson’s disease who are currently taking levodopa/carbisopa and experiencing “off” episodes (when the person’s medications are not working well, causing an increase in Parkinson’s disease symptoms).
Medications for Non-Motor Symptoms. Doctors also may prescribe a variety of medications to treat such non-motor Parkinson’s symptoms as depression and anxiety.
Non-oral therapies
When Parkinson’s symptoms become difficult to control with tablets alone, a number of other treatments can be considered.
Apomorphine
A dopamine agonist called apomorphine can be injected under the skin (subcutaneously) either by:
- a single injection, when required
- a continuous infusion using a small pump carried around on your belt, under your clothing, or in a bag
Duodopa
If you have severe on-off fluctuations, a type of levodopa called duodopa may be used.
This medication comes as a gel that’s continuously pumped into your gut through a tube inserted through your abdominal wall.
There’s an external pump attached to the end of the tube, which you carry around with you.
This treatment is only available if you have very severe on-off fluctuations or involuntary movements.
Dopamine agonists
Dopamine agonists act as a substitute for dopamine in the brain and have a similar but milder effect compared with levodopa. They can often be given less frequently than levodopa.
Some dopamine agonists are now available as one-a-day tablets, which can be a convenient option for people.
Pramipexole
- Mirapexin (tablets)
- Mirapexin prolonged release (prolonged release tablets)
- Pipexus (modified release tablets)
Ropinirole
- Unbranded (tablets)
- Adartrel (tablets)
- Ralnea XL (prolonged release tablets)
- Requip (tablets)
- Requip XL (prolonged release tablets)
- Spiroco XL (prolonged release tablets)
- Ipinnia XL (prolonged release tablets)
- Raponer XL (prolonged release tablets)
- Ropilynz XL (prolonged release tablets)
Rotigotine
- Neupro (skin patch)
Apomorphine
- APO-go PEN (pre-filled pen for intermittent injection)
- APO-go PFS (pre-filled syringe for infusion. Can be used with a continuous infusion pump)
Bromocriptine
- Unbranded (tablets)
- Parlodel (tablets, capsules)
Cabergoline
- Unbranded (tablets)
- Cabaser (tablets)
- Dostinex (tablets)
Pergolide
- Unbranded (tablets)
They are often taken as a tablet, but are also available as a skin patch (rotigotine ‘Neupro’).
A skin patch (rotigotine ‘Neupro’) may be useful if you have trouble swallowing tablets. The patch should be held in place for 30 seconds, but left on for 24 hours. It may cause a skin reaction, such as reddening or itching, but this is usually mild or moderate. It should only affect the area of skin where the patch has been and will normally disappear a few hours when you remove the patch. Moving the patch to a different part of your body every day will help to avoid irritation. Some, but not all, patches may need to be stored in a refrigerator – check the instructions included with your medication or speak to your pharmacist.
Sometimes dopamine agonists are taken at the same time as levodopa, as this allows lower doses of levodopa to be used.
Possible side effects of dopamine agonists include:
- nausea or vomiting
- tiredness and sleepiness
- dizziness
Dopamine agonists can also cause hallucinations and increased confusion, so they need to be used with caution, particularly in elderly patients, who are more susceptible.
For some people, dopamine agonists have been linked to the development of compulsive behaviors, especially at high doses, including addictive gambling and an excessively increased libido.
Talk to your healthcare specialist if you think you may be experiencing these problems.
As the person themselves may not realize the problem, it’s key that carers and family members also note any abnormal behavior and discuss it with an appropriate professional at the earliest opportunity.
If you’re prescribed a course of dopamine agonists, the initial dose is usually very small to prevent nausea and other side effects.
The dosage is gradually increased over a few weeks. If nausea becomes a problem, your doctor may prescribe anti-sickness medication.
A potentially serious, but uncommon, complication of dopamine agonist therapy is sudden onset of sleep.
This generally happens as the dose is being increased and tends to settle once the dose is stable.
People are usually advised to avoid driving while the dose is being increased in case this complication occurs.
Benefits of dopamine agonists
Delaying levodopa treatment
Dopamine agonists may be an effective treatment for several years when used alone. However, this will not be the case for everyone.
Lowers levodopa needs
Taking dopamine agonists may mean you can take lower doses of levodopa as your condition progresses. This can reduce the risk of experiencing involuntary movements (dyskinesia) or reduce how severe they are.
Fewer movement problems
Your symptoms may be controlled for longer than is usually the case with levodopa. You may also be less prone to long-term side effects such as dyskinesia.
Helping levodopa work better
Dopamine agonists can also be taken with levodopa, at all stages of Parkinson’s. They can help when the effect of levodopa wears off or doesn’t work as well.
Dopamine agonists may help to smooth out the ‘on/off’ effect that you may have with levodopa.
Fewer tablets to take (in some cases)
There are now some once-daily preparations that could make a big difference by reducing the number of tablets you need to take. But this option may not suit everyone.
Positive effects on non-movement symptoms
Some dopamine agonists have recently been shown to have a good effect on the symptoms of Parkinson’s not related to movement, including sleep problems, pain and mood.
When are dopamine agonists used?
Dopamine agonists are used at all stages of Parkinson’s.
You might take them on their own or alongside levodopa to help the levodopa work better.
Treatment with dopamine agonists has to be started carefully. The dose is gradually increased until you and your specialist or Parkinson’s nurse are happy that your symptoms are under control.
Risks and side effects of dopamine agonists
Heart problems
Some of the older dopamine agonists increase the risk of heart problems. As a group, these are known as the ‘ergot’ types and they include bromocriptine, pergolide and cabergoline. Because of this risk, these Parkinson’s drugs are not commonly used.
All newer dopamine agonists are non-ergot in type. These are apomorphine, pramipexole, ropinirole and rotigotine. They have not been associated with an increased risk of heart damage. Doctors prefer to use these if possible.
Sleepiness and fainting
Dopamine agonist drugs can make you feel very sleepy, faint or dizzy. This is most likely to happen when you start taking the drugs. Once a stable dose is reached, this effect often wears off.
Sudden onset of sleep, without any warning, has been reported. If this happens, it’s important that you tell your specialist or Parkinson’s nurse.
Sore skin
If you are having apomorphine injections, soreness or nodules can develop at the place where the needle enters your skin.
If this happens, do not stop the treatment, but make sure you get advice from your specialist or Parkinson’s nurse.
It is important to change the injection site each time to give your skin a chance to heal. Simple massage, silicone gel patches or ultrasound can help to reduce any nodules that form.
Other side effects
The patient information leaflet that comes with your medication will tell you the full range of side effects that you may experience.
Some of the possible side effects include:
- nausea – Apomorphine can cause severe short-term nausea, so you may also be given an anti-sickness drug called domperidone (Motilium). It needs to be started at least two days before your apomorphine treatment begins, and may be gradually reduced over time
- constipation
- impulsive and compulsive behaviour
- low blood pressure (hypotension)
- headaches
- psychological problems
- hallucinations
- movement problems
How do injections and infusion pumps (Apomorphine) work?
Apomorphine is a strong dopamine agonist that is given by injection under the skin (subcutaneously) either by:
- a single injection, when required
- a continuous infusion using a small infusion pump carried around on your belt, under your clothing, or in a bag
It is saved for people with more advanced Parkinson’s who do not respond as well to oral drug treatments, or when most oral drug treatments become less effective or stop working. If you continue to have unpredictable changes in your symptoms that aren’t controlled by other Parkinson’s medications, apomorphine may help.
Apomorphine injections are taken in a similar way to insulin for diabetes. There is a ready-to-use injection pen that works within 5-10 minutes and is often used as a ‘rescue’ measure. This is very useful if you have a sudden ‘off’ period.
If you need more than 7-10 injections a day, you may be changed to a syringe driver. This is a small, battery-driven pump that delivers a continuous dose of medication from a syringe through a needle under the skin.
Ideally, apomorphine will be started in hospital, under the guidance of a specialist or Parkinson’s nurse. Once you are settled on this treatment, you and your carer (if you have one) can be trained in how to use it. If you or a carer are unable to do this, district nurses will be able to help you with this treatment.
APO-go nurse advisors can also start you on treatment. They are registered general nurses with specialist experience in APO-go injections and infusions, as well as the general management of Parkinson’s.
Monoamine oxidase-B inhibitors
Monoamine oxidase-B (MAO-B) inhibitors, including selegiline and rasagiline, are another alternative to levodopa for treating early Parkinson’s disease.
Monoamine oxidase-B (MAO-B) inhibitors block the effects of an enzyme or brain substance that breaks down dopamine (monoamine oxidase-B), increasing dopamine levels.
Types of MAO–B inhibitors:
Rasagiline
- Azilect (tablets)
Selegiline
- Unbranded (tablets)
- Eldepryl (tablets)
- Zelapar (tablets that dissolve on the tongue)
Both selegiline and rasagiline can improve the symptoms of Parkinson’s disease, although their effects are small compared with levodopa. They can be used alongside levodopa or dopamine agonists.
Monoamine oxidase-B (MAO-B) inhibitors inhibitors are generally very well tolerated, but can occasionally cause side effects, including:
- nausea
- headache
- abdominal pain
- high blood pressure
Benefits of MAO–B inhibitors
Improving Parkinson’s disease symptoms
On its own, an MAO–B inhibitor can help with some Parkinson’s symptoms, but the effects are modest and it may become less effective over time.
You don’t have to wait to get the maximum dose
Many drug treatments for Parkinson’s have to be started gradually, with the dose slowly being increased over time. This is not the case for MAO-B inhibitors.
Delaying levodopa treatment
By taking an MAO–B inhibitor at an early stage of Parkinson’s, you may be able to delay taking levodopa until your symptoms become more difficult to manage.
Helping levodopa work better
When you have been taking levodopa for a while, you may find that its effects wear off too quickly. An MAO–B inhibitor can help with this.
Taking an MAO–B inhibitor may also reduce the dose of levodopa you need and lengthen the time between your doses of levodopa.
When are MAO–B inhibitors used?
An MAO–B inhibitor can be used on its own in early Parkinson’s disease, or in combination with other drugs at all stages of Parkinson’s disease.
These drugs are mainly available as tablets.
Risks and side effects of MAO–B inhibitors
Combination with antidepressants
If you’re taking some types of antidepressant, you might not be able to take MAO–B inhibitors, as these drugs can interact with each other to raise blood pressure to a dangerous level.
If you are taking antidepressants, your specialist should be able to advise you on how to take these alongside your Parkinson’s medication.
Combination with decongestants
Decongestants or cold remedies can affect some types of MAO–B inhibitors. If you need to use one of these products, check with your pharmacist to find out which one is safest to use.
Worse levodopa side effects
Because MAO–B inhibitors strengthen levodopa, the side effects of levodopa, including involuntary movements and sickness, may get worse too.
If this happens, your specialist or Parkinson’s nurse can reduce your dose of levodopa.
Other side effects
The patient information leaflet that comes with your medication will tell you the full range of side effects that you may experience.
Some of the possible side effects include:
- headaches
- aching joints
- indigestion
- flu-like symptoms
- depression
Catechol-O-methyltransferase inhibitors
Catechol-O-methyltransferase (COMT) inhibitors are prescribed for people in later stages of Parkinson’s disease. They prevent levodopa from being broken down by the enzyme COMT (Catechol-O-methyltransferase).
Types of COMT inhibitor:
Entacapone
- Comtess (tablets)
Co-careldopa plus entacapone
- Stalevo (tablets)
- Sastravi (tablets)
Tolcapone
- Tasmar (tablets)
Opicapone
- Ongentys (tablets)
Side effects of COMT inhibitors include:
- nausea or vomiting
- diarrhea
- abdominal pain
Benefits of COMT inhibitors
Helping levodopa work more smoothly
COMT inhibitors can help when levodopa is not working for long enough and starts to wear off between doses.
They can help to reduce your ‘off’ time, when you have more trouble with your Parkinson’s symptoms, and increase the amount of ‘on’ time, when your symptoms are better controlled.
Taking less levodopa
In many cases, levodopa can be taken less often and at a lower dose.
Improving Parkinson’s symptoms
Sometimes, adding a COMT inhibitor to your medication regime may help your Parkinson’s symptoms.
When are COMT inhibitors used?
These drugs are used alongside levodopa, to help it work more smoothly. They can be tried if your dose of levodopa is not working for long enough.
COMT inhibitors do not help to manage the symptoms of Parkinson’s on their own – they have to be used with levodopa.
COMT inhibitors come as tablets. If you take entacapone, you should take it at exactly the same time as your levodopa medication to get the best results.
There is also a combined tablet that includes levodopa, carbidopa (one of the ‘helper’ drugs that are always given alongside levodopa) and the COMT inhibitor entacapone.
Risks and side effects of COMT inhibitors
Liver damage
With tolcapone, there is a risk of liver damage that can be fatal. It is rare, but for this reason, it is only used if you can’t take entacapone, where the risk of liver damage has not been seen.
If you take tolcapone, you will need regular blood tests to check the health of your liver.
Worse levodopa side effects
Because COMT inhibitors strengthen levodopa, the side effects of levodopa, including involuntary movements and sickness, can get worse too.
If this happens, your specialist or Parkinson’s nurse can reduce your dose of levodopa.
Other side effects
The patient information leaflet that comes with your medication will tell you the full range of side effects that you may experience.
Some of the possible side effects include:
- sleeping problems
- loss of appetite
- diarrhea
- dizziness
- fainting
- falls
- hallucinations
- headaches
- confusion
- dry mouth
- chest pain
- sleepiness
These drugs can also discolor your urine.
Amantadine
Amantadine is a glutamate antagonist drug that can be prescribed to treat Parkinson’s symptoms.
It’s not known exactly how this drug works for Parkinson’s. It may affect how the brain reacts to certain chemicals.
There is not enough scientific evidence to support this drug as a first choice in early Parkinson’s. But for some people, amantadine may reduce dyskinesia (involuntary movements) caused by your other Parkinson’s drugs, without making your Parkinson’s symptoms worse.
When is amantadine used?
Amantadine can be used to treat tremor and rigidity.
It isn’t used very often and is unlikely to be prescribed alone. It’s usually given with other drug treatments and can be used at all stages of Parkinson’s.
It is started at a lower dose and the amount is stepped up gradually.
Risks and side effects of amantadine
Limited effect on Parkinson’s
Amantadine is not a first choice for the treatment of Parkinson’s and it may have only a mild effect. Over time, amantadine can become less effective.
Other side effects
The patient information leaflet that comes with your medication will tell you the full range of side effects that you may experience.
Some of the possible side effects include:
- feeling nervous, anxious or overexcited
- blurred vision, fainting, confusion or dizziness – If you have these side effects, it is not safe to drive or use machinery.
- poor concentration
- headaches
- hallucinations
- movement problems
- sleep problems
- fast or irregular heartbeat
- loss of appetite
- nausea and vomiting
- constipation
- sweating
- swollen hands and ankles
- skin reactions
Anticholinergics
Anticholinergics block acetylcholine – a chemical messenger found in your brain and body. They are not used very often to treat Parkinson’s.
Sometimes they are prescribed for reducing tremor and muscle stiffness.
Older medical studies have found some benefits from taking anticholinergics for Parkinson’s symptoms. They can be effective for younger people in the early stages of Parkinson’s. Anticholinergics may also be used to reduce saliva production, if you have a problem with drooling.
Risks and side effects of anticholinergics
Limited effect on movement symptoms
Some doctors say the advantages do not outweigh the disadvantages.
Memory problems
Anticholinergics are not very often given to people with Parkinson’s because they can cause memory problems or make them worse.
This can happen at any age, but particularly for older people. If you’re taking anticholinergics, your specialist or Parkinson’s nurse must closely monitor your situation.
Other side effects
The patient information leaflet that comes with your medication will tell you the full range of side effects that you may experience.
Some of the possible side effects include:
- dry mouth
- blurred vision
- constipation
- dizziness
- trouble urinating
- confusion
- hallucinations
- forgetfulness
Deep Brain Stimulation and Parkinson’s Disease
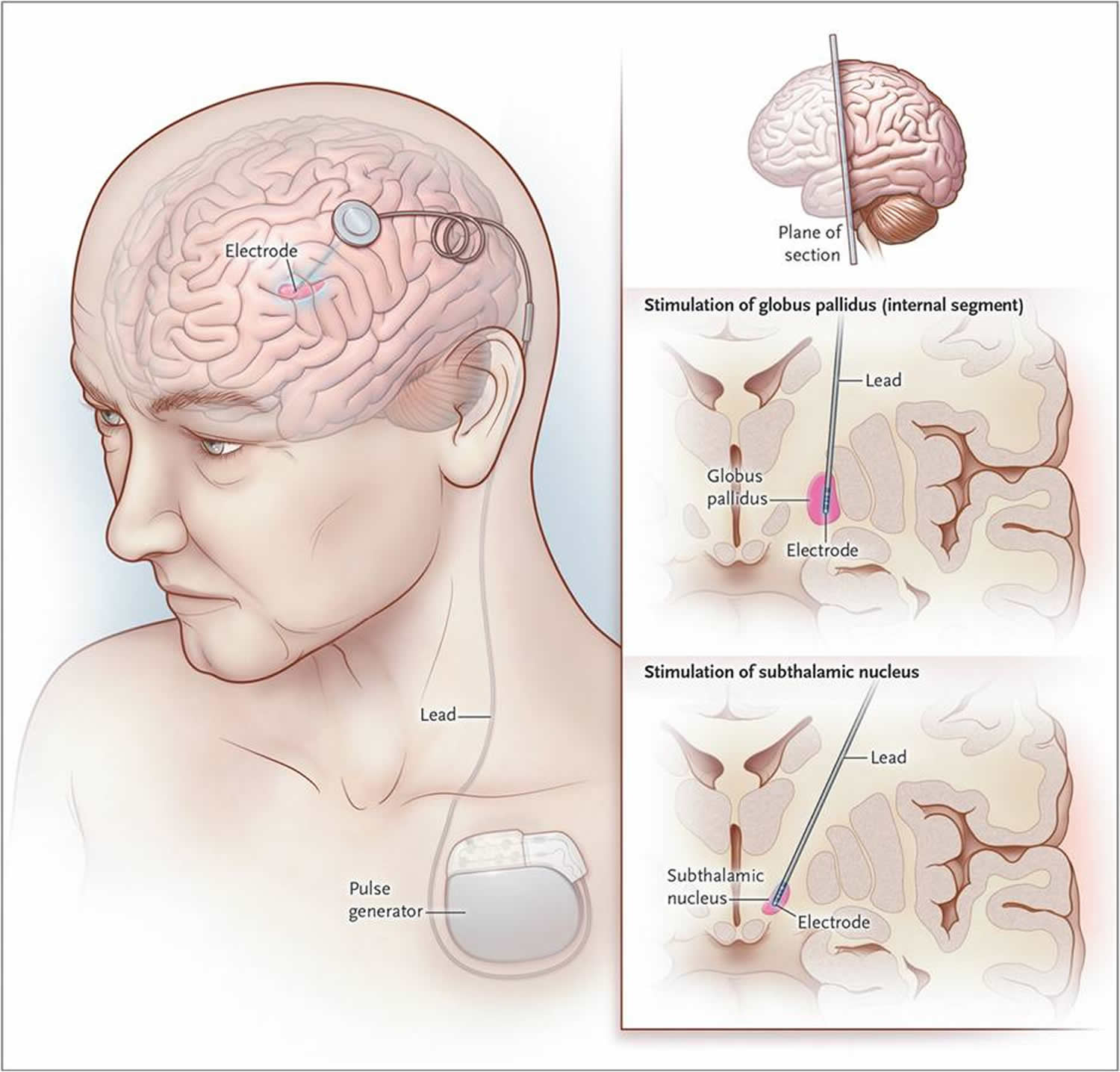
For people with Parkinson’s disease who do not respond well to medications, Deep brain stimulation (DBS), may be appropriate. Deep brain stimulation (DBS) is a surgical procedure used to treat several disabling neurological symptoms—most commonly the debilitating motor symptoms of Parkinson’s disease, such as tremor, rigidity, stiffness, slowed movement, and walking problems 8. The Deep brain stimulation (DBS) has been approved by the U.S. Food and Drug Administration and is widely used today. The procedure is also used to treat essential tremor and dystonia. At present, the procedure is used only for individuals whose symptoms cannot be adequately controlled with medications 8. However, only individuals who improve to some degree after taking medication for Parkinson’s benefit from Deep brain stimulation (DBS). A variety of conditions may mimic Parkinson’s disease but do not respond to medications or deep brain stimulation. Deep brain stimulation uses a surgically implanted, battery-operated medical device called an implantable pulse generator (IPG) – similar to a heart pacemaker and approximately the size of a stopwatch to – deliver electrical stimulation to specific areas in the brain that control movement, thus blocking the abnormal nerve signals that cause Parkinson’s disease symptoms. The device and electrodes painlessly stimulate the brain in a way that helps stop many of the movement-related symptoms of Parkinson’s, such as tremor, slowness of movement, and rigidity.
Before the procedure, a neurosurgeon uses magnetic resonance imaging (MRI) or computed tomography (CT) scanning to identify and locate the exact target within the brain for surgical intervention. Some surgeons may use microelectrode recording – which involves a small wire that monitors the activity of nerve cells in the target area – to more specifically identify the precise brain area that will be stimulated. Generally, these areas are the thalamus, subthalamic nucleus, and globus pallidus. There is a low chance that placement of the stimulator may cause bleeding or infection in the brain.
The Deep brain stimulation system consists of three components: the lead, the extension, and the implantable pulse generator (IPG). The lead (also called an electrode)—a thin, insulated wire—is inserted through a small opening in the skull and implanted in the brain. The tip of the electrode is positioned within the specific brain area.
The extension is an insulated wire that is passed under the skin of the head, neck, and shoulder, connecting the lead to the implantable pulse generator. The implantable pulse generator (IPG) (the “battery pack”) is the third component and is usually implanted under the skin near the collarbone. In some cases it may be implanted lower in the chest or under the skin over the abdomen.
Once the system is in place, electrical impulses are sent from the implantable pulse generator (IPG) up along the extension wire and the lead and into the brain. These impulses block abnormal electrical signals and alleviate Parkinson’s disease motor symptoms.
Other Therapies
Other therapies may be used to help with Parkinson’s disease symptoms. They include physical, occupational, and speech therapies, which help with gait and voice disorders, tremors and rigidity, and decline in mental functions. Other supportive therapies include a healthy diet and exercises to strengthen muscles and improve balance.
There currently are no specific vitamins, minerals, or other nutrients of proven therapeutic value in Parkinson’s disease. While there is no proof that any specific dietary factor is beneficial, a normal, healthy diet can promote overall well being for people with Parkinson’s, just as it would for anyone else. For example, a fiber-rich diet and drinking plenty of fluids can help alleviate constipation.
Exercise. Exercise’s effects on Parkinson’s disease are not known, but it may improve body strength so that the person is less disabled. Exercises also improve balance, helping people minimize gait problems, and can strengthen certain muscles so that people can speak and swallow better. Walking, gardening, swimming, calisthenics, and other general physical activity improve emotional wellbeing. Parkinson’s disease patients should always check with their doctors before beginning a new exercise program.
Other complementary therapies for Parkinson’s include massage, yoga, tai chi, hypnosis, acupuncture, and the Alexander technique, which optimizes posture and muscle activity. Another important approach involves speech and swallowing evaluation and therapy. Certain techniques can help with the low voice volume that Parkinson’s patients often experience.
Parkinson’s disease prognosis
Parkinson’s disease is both chronic, meaning it persists over a long period of time, and progressive, meaning its symptoms grow worse over time. Although some people become severely disabled, others experience only minor motor disruptions. Tremor is the major symptom for some individuals, while for others tremor is only a minor complaint and other symptoms are more troublesome. It is currently not possible to predict which symptoms will affect an individual, and the intensity of the symptoms also varies from person to person.
As the condition progresses, the symptoms of Parkinson’s disease can get worse and it can become increasingly difficult to carry out everyday activities without assistance.
Many people respond well to treatment and only experience mild to moderate disability, whereas the minority may not respond as well and can, in time, become more severely disabled.
Parkinson’s disease doesn’t directly cause people to die, but the condition can place great strain on the body, and can make some people more vulnerable to serious and life-threatening infections.
However, with advances in treatment, most people with Parkinson’s disease now have a normal or near-normal life expectancy.
- National Institutes of Health. National Institute on Aging. Parkinson’s Disease. https://www.nia.nih.gov/health/parkinsons-disease[↩][↩][↩][↩][↩][↩]
- National Institute of Environmental Health Sciences. Parkinson’s Disease. https://www.niehs.nih.gov/health/topics/conditions/parkinson/[↩][↩]
- NINDS (National Institute of Neorological Disorders and Stroke). Parkinson’s Disease Information Page. https://www.ninds.nih.gov/Disorders/All-Disorders/Parkinsons-Disease-Information-Page[↩]
- National Human Genome Research Institute. Learning About Parkinson’s Disease. https://www.genome.gov/page.cfm?pageID=10001217[↩][↩][↩][↩][↩][↩]
- The National Institute of Neurological Disorders and Stroke. Parkinson’s Disease Information Page. https://www.ninds.nih.gov/Disorders/All-Disorders/Parkinsons-Disease-Information-Page[↩][↩]
- National Institute of Health. MedlinePlus. What Are the Symptoms of Parkinson’s Disease? https://medlineplus.gov/magazine/issues/winter14/articles/winter14pg6.html[↩]
- National Institutes of Health. MedlinePlus. Parkinson’s Disease: Diagnosis and Treatment. https://medlineplus.gov/magazine/issues/winter14/articles/winter14pg8-10.html [↩]
- The National Institute of Neurological Disorders and Stroke. Deep Brain Stimulation for Parkinson’s Disease. https://www.ninds.nih.gov/Disorders/All-Disorders/Deep-Brain-Stimulation-Parkinsons-Disease-Information-Page[↩][↩]