Contents
- What is bone cancer
- Types of bone cancer
- Malignant bone tumors
- What Causes Bone Cancer?
- Risk Factors for Bone Cancer?
- Bone cancer signs and symptoms
- How is Bone Cancer Diagnosed?
- Bone Cancer Stages
- Bone cancer survival rate
- Bone cancer treatment
What is bone cancer
Bone cancer is a malignant (cancerous) tumor of the bone that destroys normal bone tissue 1. Not all bone tumors are malignant. In fact, benign (noncancerous) bone tumors are more common than malignant ones. Both malignant and benign bone tumors may grow and compress healthy bone tissue, but benign tumors do not spread, do not destroy bone tissue, and are rarely a threat to life.
Malignant tumors that begin in bone tissue are called primary bone cancer. Cancer that metastasizes (spreads) to the bones from other parts of the body, such as the breast, lung, or prostate, is called metastatic bone cancer and is named for the organ or tissue in which it began. Primary bone cancer is far less common than cancer that spreads to the bones.
The American Cancer Society’s estimates for cancer of the bones and joints for 2018 are 2:
- About 3,450 new cases will be diagnosed.
- About 1,590 deaths from these cancers are expected.
Primary cancers of bones account for less than 0.2% of all cancers.
In adults, over 40% of primary bone cancers are chondrosarcomas. This is followed by osteosarcomas (28%), chordomas (10%), Ewing tumors (8%), and malignant fibrous histiocytoma/fibrosarcomas (4%). The remainder of cases are several rare types of bone cancers.
In children and teenagers (those younger than 20 years), osteosarcoma (56%) and Ewing tumors (34%) are much more common than chondrosarcoma (6%).
Chondrosarcomas develop most often in adults, with an average age at diagnosis of 51. Less than 5% of cases occur in patients younger than 20.
Chordomas are also more common in adults. Less than 5% of cases occur in patients younger than 20.
Both osteosarcomas and Ewing tumors occur most often in children and teens.
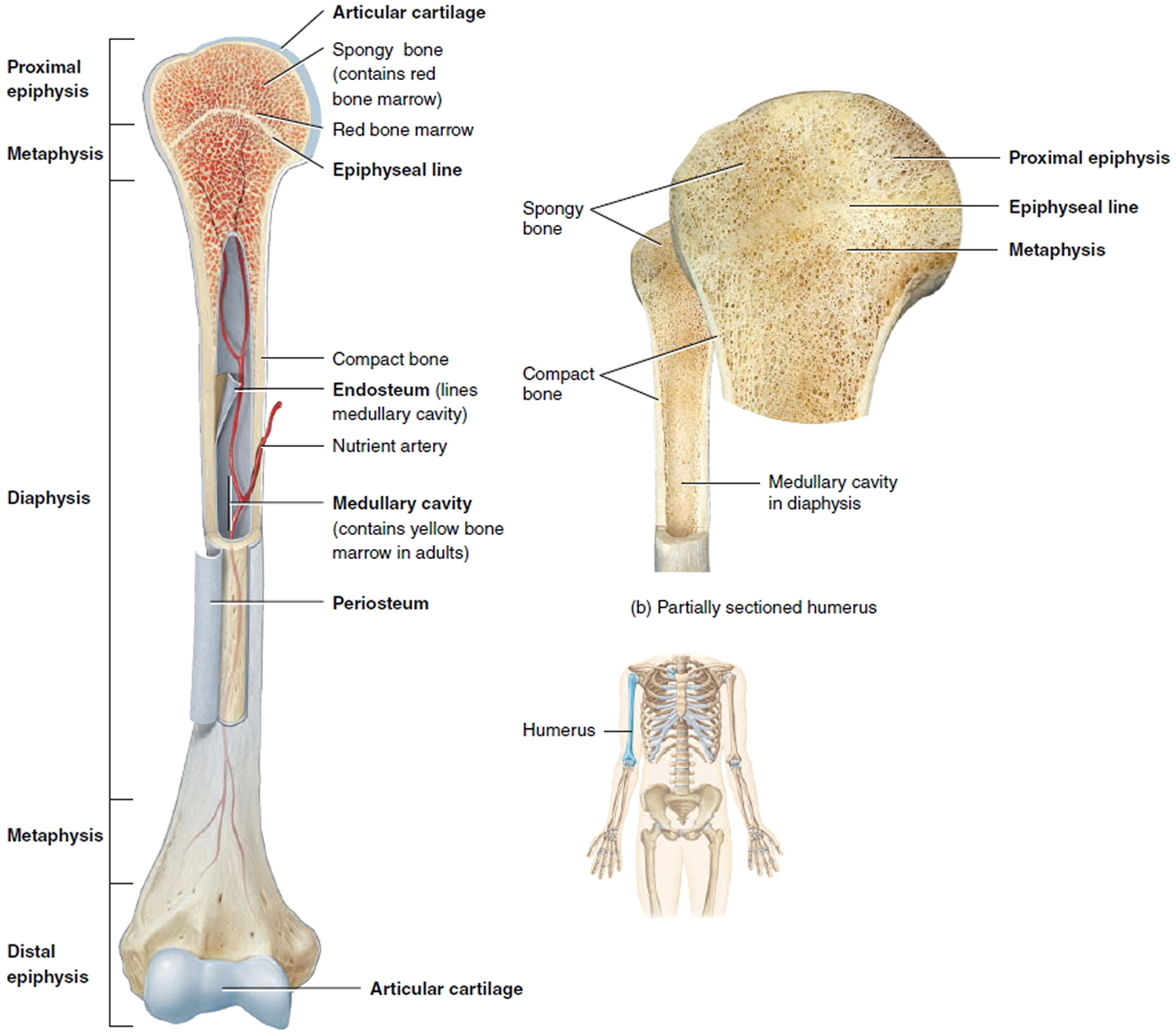
Normal bone tissue
To understand bone cancer, it helps to understand a little about normal bone tissue.
Bone is the supporting framework of your body. Most bones are hollow. The outer part of bones is a network of fibrous tissue called matrix onto which calcium salts are laid down.
The hard outer layer of bones is made of compact (cortical) bone, which covers the lighter spongy (trabecular) bone inside. The outside of the bone is covered with a layer of fibrous tissue called periosteum. Some bones are hollow and have a space called the medullary cavity which contains the soft tissue called bone marrow (discussed below). The tissue lining the medullary cavity is called endosteum. At each end of the bone is a zone of a softer form of bone-like tissue called cartilage.
Cartilage is softer than bone but more firm than most tissues. It is made of a fibrous tissue matrix mixed with a gel-like substance that does not contain much calcium.
Most bones start out as cartilage. The body then lays calcium down onto the cartilage to form bone. After the bone is formed, some cartilage may remain at the ends to act as a cushion between bones. This cartilage, along with ligaments and some other tissues connect bones to form a joint. In adults, cartilage is mainly found at the end of some bones as part of a joint. It is also seen at the place in the chest where the ribs meet the sternum (breastbone) and in parts of the face. The trachea (windpipe), larynx (voice box), and the outer part of the ear are other structures that contain cartilage.
Bone itself is very hard and strong. Some bone is able to support as much as 12,000 pounds per square inch. It takes as much as 1,200 to 1,800 pounds of pressure to break the femur (thigh bone). The bone itself contains 2 kinds of cells. The osteoblast is the cell that lays down new bone, and the osteoclast is the cell that dissolves old bone. Bone often looks as if it doesn’t change much, but the truth is that it is very active. Throughout our bodies, new bone is always forming while old bone is dissolving.
In some bones the marrow is only fatty tissue. The marrow in other bones is a mixture of fat cells and blood-forming cells. The blood-forming cells produce red blood cells, white blood cells, and blood platelets. Other cells in the marrow include plasma cells, fibroblasts, and reticuloendothelial cells.
Cells from any of these tissues can develop into a cancer.
Figure 1. Parts of a long bone
Note: The spongy bone tissue of the epiphyses and metaphyses contains red bone marrow, and the medullary cavity of the diaphysis contains yellow bone marrow (in adults).
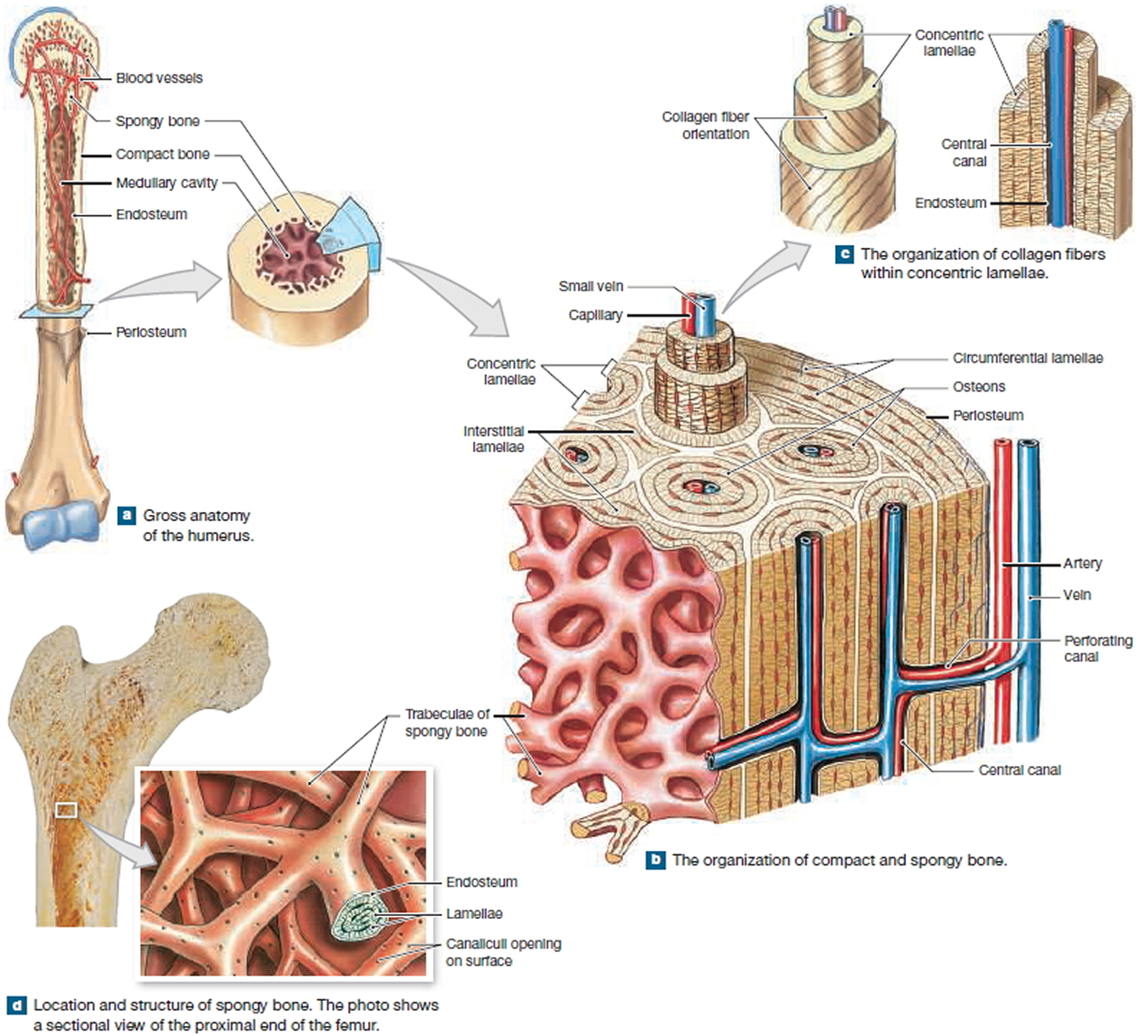
Figure 2. Internal organization of bones
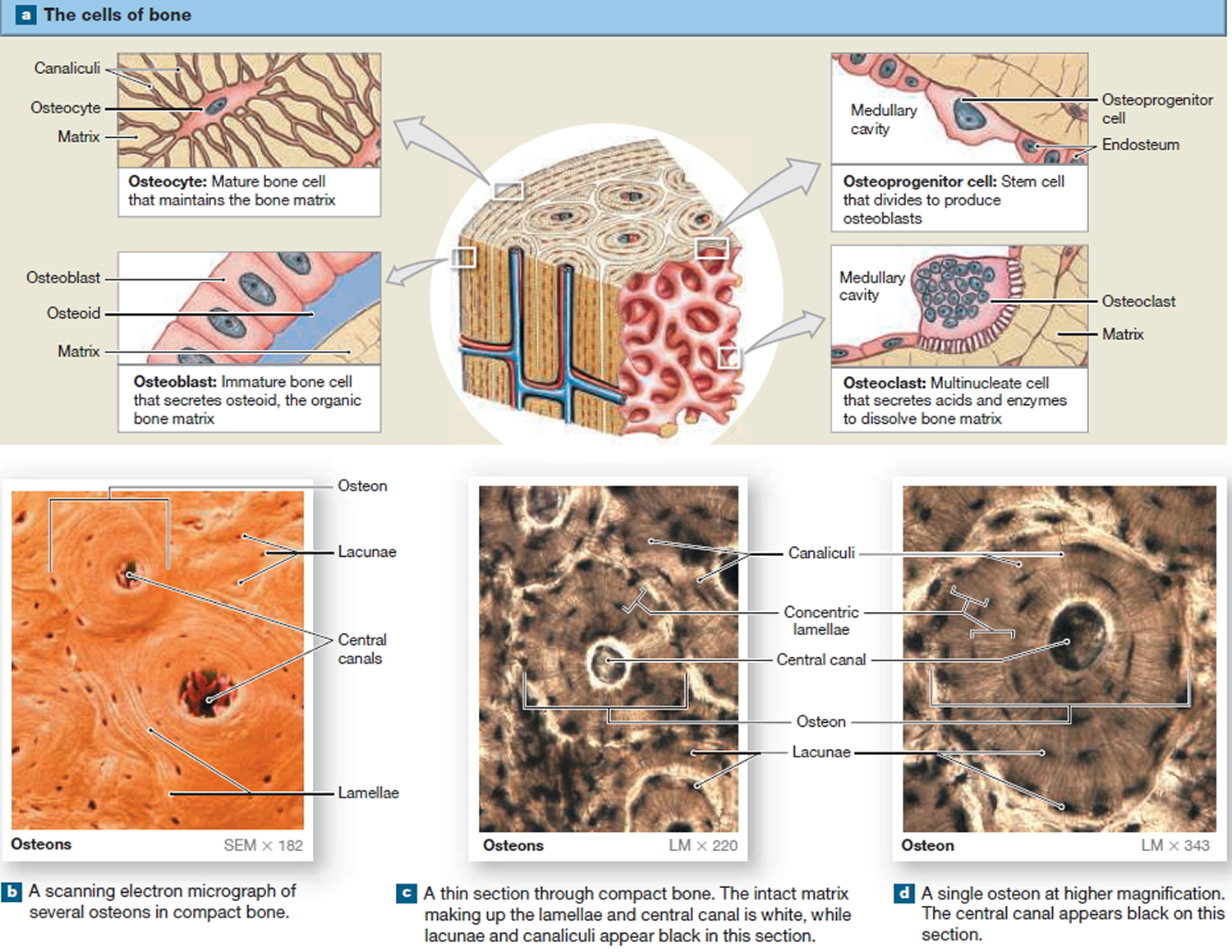
The Cells of Mature Bone
Bone contains four cell types: osteoblasts, osteocytes, osteoprogenitor cells, and osteoclasts.
Osteocytes
Mature bone cells are osteocytes. They maintain and monitor the protein and mineral content of the surrounding matrix. The minerals in the matrix are continually recycled. Each osteocyte directs the release of calcium from bone into blood and the deposition of calcium salts into the surrounding matrix. Osteocytes occupy small chambers, called lacunae, that are sandwiched between layers of calcified matrix. These matrix layers are called lamellae (singular, lamella) (Figure 3 b–d). Channels called canaliculi (“little canals”) radiate through the matrix from lacuna to lacuna and toward free surfaces and adjacent blood vessels. The canaliculi connect adjacent lacunae and bring the processes of neighboring osteocytes into close contact. Tight junctions interconnect these processes and provide a route for the diffusion of nutrients and waste products from one osteocyte to another across gap junctions.
Osteoblasts
Cuboidal cells found in a single layer on the inner or outer surfaces of a bone are called osteoblasts (precursor). These cells secrete the organic components of the bone matrix. This material, called osteoid, later becomes mineralized through a complicated, multistep mechanism. Osteoblasts are responsible for the production of new bone—a process called osteogenesis. It is thought that osteoblasts respond to a variety of different stimuli, including mechanical and hormonal, to initiate osteogenesis. If an osteoblast becomes surrounded by matrix, it differentiates into an osteocyte.
Osteoprogenitor Cells
Bone tissue also contains small numbers of stem cells termed osteoprogenitor cells (ancestor). Osteoprogenitor cells differentiate from mesenchyme and are found in numerous locations, including the innermost layer of the periosteum and the endosteum lining the medullary cavities. Osteoprogenitor cells divide to produce daughter cells that differentiate into osteoblasts. The ability to produce additional osteoblasts becomes extremely important after a bone is cracked or broken.
Osteoclasts
Large, multinucleate cells found at sites where bone is being removed are termed osteoclasts. They are derived from the same stem cells that produce monocytes and neutrophils. They secrete acids through a process involving the exocytosis of lysosomes. The acids dissolve the bony matrix and release amino acids and the stored calcium and phosphate. This erosion process, called osteolysis, increases the calcium and phosphate concentrations in body fluids. Osteoclasts are always removing matrix and releasing minerals, and osteoblasts are always producing matrix that quickly binds minerals. The balance between the activities of osteoblasts and osteoclasts is very important; when osteoclasts remove calcium salts faster than osteoblasts deposit them, bones become weaker. When osteoblasts are more active than osteoclasts, bones become stronger and more massive. New research indicates that osteoclasts may also be involved in osteoblast
differentiation, immune system activation, and the proliferation of tumor cells in bone.
Figure 3. Microscopic Structure of a Typical Bone
Types of bone cancer
Most of the time when someone with cancer is told they have cancer in the bones, the doctor is talking about a cancer that has spread to the bones from somewhere else. This is called metastatic cancer. It can be seen in many different types of advanced cancer, like breast cancer, prostate cancer, and lung cancer. When these cancers in the bone are looked at under a microscope, they look like the tissue they came from. For example, if someone has lung cancer that has spread to bone, the cells of the cancer in the bone still look and act like lung cancer cells. They do not look or act like bone cancer cells, even though they are in the bones. Since these cancer cells still act like lung cancer cells, they still need to be treated with drugs that are used for lung cancer.
Other kinds of cancers that are sometimes called “bone cancers” start in the blood forming cells of the bone marrow − not in the bone itself. The most common cancer that starts in the bone marrow and causes bone tumors is called multiple myeloma. Another cancer that starts in the bone marrow is leukemia, but it is generally considered a blood cancer rather than a bone cancer. Sometimes lymphomas, which more often start in lymph nodes, can start in bone marrow. Multiple myeloma, lymphoma, and leukemia are not discussed in this document. For more information on these cancers, refer to the individual document for each.
A primary bone tumor starts in the bone itself. True (or primary) bone cancers are called sarcomas. Sarcomas are cancers that start in bone, muscle, fibrous tissue, blood vessels, fat tissue, as well as some other tissues. They can develop anywhere in the body.
There are several different types of bone tumors. Their names are based on the area of bone or surrounding tissue that is affected and the kind of cells forming the tumor. Some primary bone tumors are benign (not cancerous), and others are malignant (cancerous). Most bone cancers are sarcomas.
Malignant bone tumors
Osteosarcoma
Osteosarcoma (also called osteogenic sarcoma) is the most common primary bone cancer. This cancer starts in the bone cells. It most often occurs in young people between the ages of 10 and 30, but about 10% of osteosarcoma cases develop in people in their 60s and 70s. It is rare in middle-aged people, and is more common in males than females. These tumors develop most often in bones of the arms, legs, or pelvis.
Osteosarcoma is not a common cancer. Each year, about 1,000 new cases of osteosarcoma are diagnosed in the United States. About 450 of these are in children and teens.
Most osteosarcomas occur in children and young adults between the ages of 10 and 30. Teens are the most commonly affected age group, but osteosarcoma can occur in people of any age. About 10% of all osteosarcomas occur in people over the age of 60.
Osteosarcomas account for about 2% of childhood cancers, but they make up a much smaller percentage of adult cancers.
In children and young adults, osteosarcoma usually develops in areas where the bone is growing quickly, such as near the ends of the long bones. Most tumors develop in the bones around the knee, either in the distal femur (the lower part of the thigh bone) or the proximal tibia (the upper part of the shinbone). The proximal humerus (the part of the upper arm bone close to the shoulder) is the next most common site. However, osteosarcoma can develop in any bone, including the bones of the pelvis (hips), shoulder, and jaw. This is especially true in older adults.
Like the osteoblasts in normal bone, the cells that form this cancer make bone matrix. But the bone matrix of an osteosarcoma is not as strong as that of normal bones.
The prognosis (outlook) for people with osteosarcoma depends on many factors, including the location of the tumor, whether the cancer has spread (metastasized) when it’s first found, and the person’s age.
Subtypes of osteosarcoma
Several subtypes of osteosarcoma can be identified by how they look on x-rays and under the microscope. Some of these subtypes have a better prognosis (outlook) than others.
Based on how they look under the microscope, osteosarcomas can be classified as high grade, intermediate grade, or low grade. The grade of the tumor tells doctors how likely it is that the cancer will grow and spread to other parts of the body.
High-grade osteosarcomas: These are the fastest growing types of osteosarcoma. When seen under a microscope, they do not look like normal bone and have many cells in the process of dividing into new cells. Most osteosarcomas that occur in children and teens are high grade. There are many types of high-grade osteosarcomas (although the first 3 are the most common).
- Osteoblastic
- Chondroblastic
- Fibroblastic
- Mixed
- Small cell
- Telangiectatic
- High-grade surface (juxtacortical high grade)
Other high-grade osteosarcomas include:
- Pagetoid: a tumor that develops in someone with Paget disease of the bone
- Extra-skeletal: a tumor that starts in a part of the body other than a bone
- Post-radiation: a tumor that starts in a bone that had once received radiation therapy
Intermediate-grade osteosarcomas: These uncommon tumors fall in between high-grade and low-grade osteosarcomas. (They are usually treated as if they are low-grade osteosarcomas.)
- Periosteal (juxtacortical intermediate grade)
Low-grade osteosarcomas: These are the slowest growing osteosarcomas. The tumors look more like normal bone and have few dividing cells when seen under a microscope.
- Parosteal (juxtacortical low grade)
- Intramedullary or intraosseous well differentiated (low-grade central)
The grade of the tumor plays a role in determining its stage and the type of treatment used.
Osteosarcoma survival rate
The 5-year survival rate refers to the percentage of patients who live at least 5 years after their cancer is diagnosed. Of course, many people live much longer than 5 years (and many are cured). Survival rates are often based on previous outcomes of large numbers of people who had the disease, but they can’t predict what will happen in any particular person’s case. Many other factors can affect a person’s outlook, such as the subtype and location of the osteosarcoma and how well the cancer responds to treatment. Your (child’s) doctor can tell you if the numbers below may apply, as he or she is familiar with the aspects of your (child’s) situation.
Localized tumors
With current treatment, the 5-year survival rate for people with localized osteosarcoma is in the range of 60% to 80% 3. These cancers are more likely to be cured if they are resectable; that is, if all of the visible tumor can be removed (resected) by surgery. For high-grade osteosarcomas that can be resected completely, chemotherapy is still an essential part of treatment. Without it, the cancer is still very likely to come back.
Metastatic tumors
If the osteosarcoma has already spread when it is first found, the 5-year survival rate is about 15% to 30% 3. The survival rate is closer to 40% if the cancer has spread only to the lungs (as opposed to having reached other organs), or if all of the tumors (including metastases) can be removed with surgery.
Other factors that may affect prognosis
As noted above, factors other than the stage of the cancer can also affect survival rates. For example, factors that have been linked with a better prognosis include:
- Being younger (child or young adult, as opposed to older adult)
- Being female
- The tumor being on an arm or leg (as opposed to the hip bones)
- The tumor(s) being completely resectable
- Normal blood alkaline phosphatase and LDH levels
- The tumor having a good response to chemotherapy
What Causes Osteosarcoma?
Researchers have found that osteosarcoma is linked with a number of other conditions, which were described in the Risk Factors for Osteosarcoma. But the cause of most osteosarcomas is not clear at this time.
Scientists have learned how certain changes in your DNA can cause cells to become cancerous. DNA is the chemical in each of your cells that makes up your genes – the instructions for nearly everything our cells do. You usually look like your parents because they are the source of your DNA. But DNA affects more than how you look. It influences your risks for developing certain diseases, including some kinds of cancer.
Some genes (parts of your DNA) control when your cells grow, divide to make new cells, and die. Genes that help cells grow, divide, or stay alive are called oncogenes. Others that slow down cell division or make cells die at the right time are called tumor suppressor genes. Cancers can be caused by DNA changes that turn on oncogenes or turn off tumor suppressor genes.
Some people inherit DNA mutations (changes) from a parent that increase their risk of cancer. In this situation, all of the cells in the body carry the same gene change. These are called germline or inherited mutations. Usually, however, cancer-causing changes are acquired during life rather than inherited before birth. In this case, the change occurs only in the cells that will develop the cancer. These are called somatic or acquired gene changes.
Inherited gene changes
Some inherited DNA mutations cause syndromes that are linked with an increased risk of osteosarcoma. For example:
The Li-Fraumeni syndrome is usually caused by inherited mutations that turn off the TP53 tumor suppressor gene. These mutations give a person a very high risk of developing one or more types of cancer, including breast cancer, brain tumors, osteosarcoma, and other cancers.
Inherited changes in the retinoblastoma (RB1) tumor suppressor gene increase the risk of developing retinoblastoma, a type of eye cancer that affects children. Children with this gene change also have an increased risk for developing osteosarcoma.
If you are concerned you or your child might possibly have an inherited gene change, talk with your doctor about whether genetic testing might be helpful.
Acquired gene changes
Most osteosarcomas are not caused by inherited DNA mutations. They are the result of gene changes acquired during the person’s lifetime. These changes are present only in the cancer cells and are not passed on to children.
Although radiation therapy is very useful in treating some forms of cancer, it can also cause cancer by damaging DNA. This is why people who get radiation therapy to treat another cancer are more likely to later develop osteosarcoma in the treated site.
Other DNA changes have no clear cause. They may result from random errors that occur when cells reproduce. Before a cell divides, it must copy its DNA so that both new cells have the same set of instructions. Sometimes mistakes are made during this copying process. Cells that are dividing quickly are more likely to create new cells with mistakes in their DNA, which increases the risk that a cancer such as osteosarcoma may develop. This may be why some normal situations (such as the teenage growth spurt) and diseases (such as Paget disease of bone) that cause rapid bone growth increase the risk of osteosarcoma.
Other than radiation, there are no known lifestyle-related or environmental causes of osteosarcoma, so it is important to remember that there is nothing people with these cancers could have done to prevent them.
Researchers now understand some of the gene changes that occur in osteosarcomas, but it’s not always clear what causes these changes. As we learn more about what causes osteosarcoma, hopefully we will be able to use this knowledge to develop ways to better prevent and treat it.
Risk Factors for Osteosarcoma
A risk factor is anything that affects your chance of getting a disease such as cancer. Different cancers have different risk factors.
Lifestyle-related risk factors such as body weight, physical activity, diet, and tobacco use play a major role in many adult cancers. But these factors usually take many years to influence cancer risk, and they are not thought to play much of a role in childhood cancers, including childhood osteosarcomas. So far, lifestyle-related factors have not been linked to osteosarcomas in adults, either.
Age
The risk of osteosarcoma is highest for those between the ages of 10 and 30, especially during the teenage growth spurt. This suggests there may be a link between rapid bone growth and risk of tumor formation. The risk goes down in middle age, but rises again in older adults (usually over the age of 60). Osteosarcoma in older adults is often linked to another cause, such as a long-standing bone disease.
Height
Children with osteosarcoma are usually tall for their age. This also suggests that osteosarcoma may be related to rapid bone growth.
Gender
Osteosarcoma is more common in males than in females. Females tend to develop it at a slightly earlier age, possibly because they tend to have their growth spurts earlier.
Race/ethnicity
Osteosarcoma is slightly more common in African Americans than in whites.
Radiation to bones
People who were treated with radiation therapy for another cancer might have a higher risk of later developing osteosarcoma in the area that was treated. Being treated at a younger age and being treated with higher doses of radiation both increase the risk of developing osteosarcoma.
It is not clear if imaging tests that use radiation, such as x-rays, CT scans, and bone scans, raise the risk of developing osteosarcoma. The amount of radiation used for these tests is many times lower than that used for radiation therapy. If there is any increased risk it is likely to be very small, but doctors try to limit the use of these types of tests in children whenever possible, just in case.
Certain bone diseases
People with certain non-cancerous bone diseases have an increased risk of developing osteosarcoma.
- Paget disease of the bone: In this condition, abnormal bone tissue forms in one or more bones. It mostly affects people older than 50. The affected bones are heavy and thick but are weaker than normal bones and are more likely to break. Usually this condition by itself is not life-threatening. But bone sarcomas (mostly osteosarcoma) develop in about 1% of people with Paget disease, usually when many bones are affected.
- Hereditary multiple osteochondromas: Osteochondromas are benign bone tumors formed by bone and cartilage. Each osteochondroma has a very small risk of developing into a bone sarcoma (most often a chondrosarcoma, but less often it can be an osteosarcoma). Most osteochondromas can be cured by surgery. However, some people inherit a tendency to develop many osteochondromas starting at a young age, and it may not be possible to remove them all. The more osteochondromas a person has, the greater the risk of developing a bone sarcoma.
Inherited cancer syndromes
People with certain rare, inherited cancer syndromes have an increased risk of developing osteosarcoma.
- Retinoblastoma is a rare eye cancer of children. Some children have the inherited form of retinoblastoma (hereditary retinoblastoma), in which all the cells of the body have a mutation (change) in the RB1 gene. These children also have an increased risk of developing bone or soft tissue sarcomas, including osteosarcoma. If radiation therapy is used to treat the retinoblastoma, the risk of osteosarcoma in the bones around the eye is even higher.
- The Li-Fraumeni syndrome makes people much more likely to develop certain types of cancer, including breast cancer, brain tumors, osteosarcoma, and other types of sarcoma. This syndrome is usually caused by a mutation of the TP53 tumor suppressor gene.
- Children with Rothmund-Thomson syndrome are short and tend to have skeletal problems and rashes. They also are more likely to develop osteosarcoma. This syndrome is caused by abnormal changes in the REQL4 gene.
- Other rare inherited conditions, including Bloom syndrome, Werner syndrome, and Diamond-Blackfan anemia, have also been linked to an increased risk of osteosarcoma.
Can Osteosarcoma be found early?
At this time, there are no widely recommended screening tests for this cancer. (Screening is testing for cancer in people without any symptoms.)
Still, most osteosarcomas are found at an early stage, before they have clearly spread to other parts of the body. Symptoms such as bone pain or swelling often prompt a visit to a doctor.
Osteosarcoma Signs and Symptoms
Osteosarcomas are usually found because of the symptoms they cause.
Pain and swelling
Pain in the affected bone (usually around the knee or in the upper arm) is the most common symptom of osteosarcoma. At first, the pain might not be constant and may be worse at night. The pain often increases with activity and may result in a limp if the tumor is in a leg bone.
Swelling in the area is another common symptom, although it may not occur until several weeks after the pain starts. Depending on where the tumor is, it may be possible to feel a lump or mass.
Limb pain and/or swelling are very common in normal, active children and teens. They are much more likely to be caused by normal bumps and bruises, so they might not prompt a doctor visit right away. This can delay a diagnosis. If your child has these symptoms and they do not go away within a few weeks (or they get worse), see a doctor so that the cause can be found and treated, if needed.
These symptoms are less common in adults, so they should be a sign to see a doctor even sooner.
Bone fractures (breaks)
Although osteosarcoma might weaken the bone it develops in, the bones often do not break. Telangiectatic osteosarcomas, which are rare, tend to weaken bones more than other forms of osteosarcoma and are more likely to cause a fracture at the tumor site.
People with a fracture next to or through an osteosarcoma often describe a limb that was sore for a few months and suddenly became very painful when the fracture occurred.
Osteosarcoma Diagnosis
Osteosarcomas are usually found when a person goes to the doctor because of signs or symptoms they are having. If a bone tumor is suspected, tests will be needed to find out for sure.
Medical history and physical exam
If a person has signs or symptoms that suggest a tumor in or around a bone, the doctor will want to take a complete medical history to find out more about the symptoms. A physical exam can provide information about a possible tumor, as well as other health problems. For example, the doctor may be able to see or feel an abnormal mass.
The doctor may also look for problems in other parts of the body. When people (especially adults) do have cancer in the bones, it’s often the result of cancer that started somewhere else and then spread to the bones.
If the doctor suspects a person could have osteosarcoma (or another type of bone tumor), more tests will be done. These might include imaging tests, biopsies, and/or lab tests.
Imaging tests
Imaging tests use x-rays, magnetic fields, or radioactive substances to create pictures of the inside of the body. Imaging tests may be done for a number of reasons, including:
- To help find out if a suspicious area might be cancer
- To help determine if a cancer may have started in another part of the body
- To learn how far cancer has spread
- To help determine if treatment is working
- To look for signs that the cancer may have come back
Patients who have or may have osteosarcoma will have one or more of these tests.
Bone x-ray
This is often the first test done if a doctor suspects a bone tumor. Doctors can often recognize a bone tumor such as osteosarcoma based on plain x-rays of the bone. But other imaging tests might be needed as well.
Even if results of an x-ray strongly suggest a person has osteosarcoma, a biopsy will still be needed to confirm that it is cancer rather than some other problem, such as an infection.
Magnetic resonance imaging (MRI) scan
MRI scans provide detailed images of soft tissues in the body. These scans make detailed images using radio waves and strong magnets instead of x-rays, so no radiation is involved. A contrast material called gadolinium may be injected into a vein before the scan to better see details.
Often, an MRI scan is done to get a more detailed look at a bone mass seen on an x-ray. MRI scans can usually show if the mass is likely to be a tumor, an infection, or some type of bone damage from other causes. MRI scans can also help show the exact extent of a tumor, as they provide a detailed view of the marrow inside bones and the soft tissues around the tumor. Sometimes, the MRI can help find small bone tumors several inches away from the main tumor (called skip metastases). Knowing the extent of an osteosarcoma is very important when planning surgery. An MRI scan usually gives better details than a CT scan.
An MRI scan can take up to an hour. You (or your child) may have to lie on a table that slides inside a narrow tube, which is confining and can be distressing. Newer, more open MRI machines can help with these feelings, but the test still requires staying still for long periods of time. The machines also make buzzing and clicking noises that may be disturbing. Sometimes, younger children are given medicine to help keep them calm or even asleep during the test.
Computed tomography (CT) scan
The CT scan uses x-rays to make detailed cross-sectional images of parts of the body. If a bone x-ray shows a tumor, CT scans are sometimes used to see if the tumor has grown into nearby muscle, fat, or tendons, although MRI is often better for this. A CT scan of the chest is often done to look for spread of the cancer to the lungs. CT scans may also be done to look for the spread of the cancer to other parts of the body.
Instead of taking one picture, like a regular x-ray, a CT scanner takes many pictures as it rotates around a person lying on a table. A computer then combines these pictures into detailed images of slices of the part of the body being studied.
Before the test, you (or your child) may be asked to drink a contrast solution and/or get an intravenous (IV) injection of a contrast dye that helps better outline abnormal areas in the body. If the contrast dye is to be injected, you (or your child) may need an IV line. The contrast may cause some flushing (a feeling of warmth, especially in the face). Some people are allergic and get hives. Rarely, more serious reactions like trouble breathing or low blood pressure can occur. Be sure to tell the doctor if you (or your child) have any allergies or have ever had a reaction to any contrast material used for x-rays.
A CT scanner has been described as a large donut, with a narrow table in the middle opening. During the test, the table slides in and out of the scanner. You (or your child) will need to lie still on the table while the scan is being done. CT scans take longer than regular x-rays, and you might feel a bit confined by the ring while the pictures are being taken. In some cases, children may need to be sedated before the test to stay still and help make sure the pictures come out well.
Chest x-ray
This test is sometimes done to see if cancer has spread to the lungs. It can find larger tumors, but it is not as good as a CT scan for spotting smaller tumors. If a CT scan of the chest is done, a chest x-ray probably won’t be needed.
Bone scan
A bone scan can help show if a cancer has spread to other bones, and is often part of the workup for people with osteosarcoma. This test is useful because it can show the entire skeleton at once. (A positron emission tomography [PET] scan, described below, can often provide similar information, so a bone scan might not be needed if a PET scan is done.)
For this test, a small amount of low-level radioactive material is injected into a vein (intravenously, or IV). (The amount of radioactivity used is very low and will pass out of the body within a day or so.) The substance settles in areas of damaged bone throughout the entire skeleton over the course of a couple of hours. You (or your child) then lie on a table for about 30 minutes while a special camera detects the radioactivity and creates a picture of the skeleton. Younger children may be given medicine to help keep them calm or even asleep during the test.
Areas of active bone changes appear as “hot spots” on the skeleton because they attract the radioactivity. Hot spots may suggest areas of cancer, but other bone diseases can also cause the same pattern. To make an accurate diagnosis, other tests such as plain x-rays, MRI scans, or even a bone biopsy might be needed.
Positron emission tomography (PET) scan
For a PET scan, a form of radioactive sugar (known as FDG) is injected into the blood. The amount of radioactivity used is very low and will pass out of the body within a day or so. Because cancer cells in the body are growing quickly, they absorb large amounts of the sugar. After about an hour, you (or your child) will lie on a table in the PET scanner for about 30 minutes while a special camera creates a picture of areas of radioactivity in the body. The picture is not detailed like a CT or MRI scan, but it provides useful information about the whole body.
PET scans can help show the spread of osteosarcomas to the lungs, other bones, or other parts of the body, and can also help in following the response to treatment.
Some newer machines can do a PET and CT scan at the same time (PET/CT scan). This lets the doctor compare areas of higher radioactivity on the PET scan with the more detailed appearance of that area on the CT scan.
Osteosarcoma Staging
The stage of a cancer is a standard summary of how far a cancer has spread. The treatment and prognosis (outlook) for osteosarcoma depend, to a large extent, on the stage of the cancer when it is first diagnosed.
The stage of an osteosarcoma is based on the results of physical exams, imaging tests, and any biopsies that have been done.
A staging system is a standard way for the cancer care team to sum up the extent of the cancer. When trying to figure out the best course of treatment, doctors often use a simple system that divides osteosarcomas into 2 groups – localized and metastatic. Doctors can also use more formal staging systems, described below, to describe the extent of an osteosarcoma in more detail.
Staging can be confusing. If you have any questions about the stage of the cancer, ask your (child’s) doctor to explain it to you in a way you understand.
Localized versus metastatic osteosarcoma
Localized osteosarcoma
A localized osteosarcoma is seen only in the bone it started in and possibly the tissues next to the bone, such as muscle, tendon, or fat.
About 4 out of 5 osteosarcomas are thought to be localized when they are first found. But even when imaging tests don’t show that the cancer has spread to distant areas, most patients are likely to have very small areas of cancer spread that can’t be detected with tests. This is why chemotherapy is an important part of treatment for most osteosarcomas. If it isn’t given, the cancer is more likely to come back after surgery.
Doctors further divide localized osteosarcomas into 2 groups:
- Resectable cancers are those in which all of the visible tumor can be removed by surgery.
- Non-resectable (or unresectable) osteosarcomas can’t be removed completely by surgery.
Metastatic osteosarcoma
A metastatic osteosarcoma has clearly spread to other parts of the body. Most often it spreads to the lungs, but it can also spread to other bones, the brain, or other organs.
About 1 out of 5 osteosarcoma patients has metastatic spread at the time of diagnosis. These patients are harder to treat, but some can be cured if the metastases can be removed by surgery. The cure rate for these patients improves markedly if chemotherapy is also given.
Musculoskeletal Tumor Society Staging System
One system commonly used to stage osteosarcoma is the Musculoskeletal Tumor Society Staging system, also known as the Enneking system. It is based on 3 key pieces of information:
- The grade of the tumor (G)
- The extent of the main (primary) tumor (T)
- If the tumor has metastasized (spread) to nearby lymph nodes (bean-sized collections of immune system cells) or other organs (M)
The grade of a tumor is a measure of how likely it is to grow and spread, based on how it looks under the microscope. Tumors are either low grade (G1) or high grade (G2). Low-grade tumor cells look more like normal cells and are less likely to grow and spread quickly, while high-grade tumor cells look more abnormal.
The extent of the primary tumor is classified as either intracompartmental (T1), meaning it has basically remained within the bone, or extracompartmental (T2), meaning it has extended beyond the bone into other nearby structures.
Tumors that have not spread to the lymph nodes or other organs are considered M0, while those that have spread are M1.
These factors are combined to give an overall stage, using Roman numerals from I to III. Stages I and II are further divided into A for intracompartmental tumors or B for extracompartmental tumors.
In summary:
- Low-grade, localized tumors are stage I.
- High-grade, localized tumors are stage II.
- Metastatic tumors (regardless of grade) are stage III.
Table 1. Musculoskeletal Tumor Society Staging System (Osteosarcoma)
| Stage | Grade | Tumor | Metastasis |
| IA | G1 | T1 | M0 |
| IB | G1 | T2 | M0 |
| IIA | G2 | T1 | M0 |
| IIB | G2 | T2 | M0 |
| III | G1 or G2 | T1 orT2 | M1 |
American Joint Commission on Cancer staging system
Another system sometimes used to stage bone cancers is the American Joint Commission on Cancer (AJCC) system. The AJCC uses one system to describe all bone cancers, including osteosarcomas. The AJCC staging system for bone cancers is based on 4 key pieces of information:
- T describes the size of the main (primary) tumor and whether it appears in different areas of the bone.
- N describes the extent of spread to nearby (regional) lymph nodes (small bean-sized collections of immune system cells). Bone tumors rarely spread to the lymph nodes.
- M indicates whether the cancer has metastasized (spread) to other organs of the body. (The most common sites of spread are to the lungs or other bones.)
- G stands for the grade of the tumor, which describes how the cells look under a microscope. Low-grade tumor cells look more like normal cells and are less likely to grow and spread quickly, while high-grade tumor cells look more abnormal.
Numbers after T, N, M, and G provide more details about each of these factors.
T categories of bone cancer
T0: There is no evidence of a main (primary) tumor.
T1: The tumor is 8 cm (around 3 inches) across or less.
T2: The tumor is larger than 8 cm across.
T3: The tumor has “skipped” to another site or sites within the same bone.
N categories of bone cancer
N0: The cancer has not spread to regional (nearby) lymph nodes.
N1: The cancer has spread to nearby lymph nodes.
M categories of bone cancer
M0: There is no spread (metastasis) to distant organs.
M1a: The cancer has spread only to the lung.
M1b: The cancer has spread to other distant sites in the body.
Grades of bone cancer
Note: The grades used for the AJCC system are different from those in the Musculoskeletal Tumor Society Staging system. There are other differences between the systems as well. To avoid confusion, it may help to ask your (child’s) doctor which staging system he or she uses.
GX: Grade can’t be assessed
G1, G2: Low grade
G3, G4: High grade
Stage grouping
Once the T, N, and M categories and the grade of the bone cancer have been determined, the information is combined into an overall stage. The process of assigning a stage number is called stage grouping. The stages are described in Roman numerals from I to IV (1 to 4), and are sometimes divided further.
Stage IA
T1, N0, M0, G1 or G2 (or GX): The tumor is 8 cm across or less and is low grade (or the grade can’t be assessed). It has not spread to nearby lymph nodes or to distant parts of the body.
Stage IB
T2-T3, N0, M0, G1 or G2 (or GX): The tumor is larger than 8 cm across or has “skipped” to other sites in the same bone. It is low grade (or the grade can’t be assessed). It has not spread to nearby lymph nodes or to distant parts of the body.
Stage IIA
T1, N0, M0, G3 or G4: The tumor is 8 cm across or less and is high grade. It has not spread to nearby lymph nodes or to distant parts of the body.
Stage IIB
T2, N0, M0, G3 or G4: The tumor is larger than 8 cm across and is high grade. It has not spread to nearby lymph nodes or to distant parts of the body.
Stage III
T3, N0, M0, G3 or G4: The tumor has “skipped” to other sites in the same bone. It is high grade. It has not spread to nearby lymph nodes or to distant parts of the body.
Stage IVA
Any T, N0, M1a, any G: The tumor has spread only to the lungs. It has not spread to the lymph nodes or to other distant sites. (It can be any size or grade.)
Stage IVB (if either of these applies)
Any T, N1, any M, any G: The tumor has spread to lymph nodes. It can be any size or grade, and might or might not have spread to other distant sites.
Any T, any N, M1b, any G: The tumor has spread to distant sites other than the lung. It can be any size or grade.
Osteosarcoma treatment
Because osteosarcoma is rare, only doctors in major cancer centers have a lot of experience treating these cancers.
The types of treatment used for osteosarcomas include:
- Surgery
- Chemotherapy
- Radiation therapy (in certain cases)
Most often, both chemotherapy and surgery are needed.
All of these treatments may have side effects, but many of them can be made less troublesome. Your medical team will help you take care of the side effects and can help you work closely with nutritionists, psychologists, social workers, and other professionals to understand and deal with medical problems, stress, and other issues related to the treatment.
Because many of these issues can be more complex for cancer in children and teens, many people will be involved in your child’s overall care. As a parent, taking care of a child with cancer can be a very big job. It’s important to remember that you will have a lot of help. It’s also important for you to know that the health professionals who treat children with osteosarcoma are using the experience and knowledge gained from many decades of studying the treatment of this disease.
Chondrosarcoma
Chondrosarcoma is a cancer of cartilage cells. It is the second most common primary bone cancer. This cancer is rare in people younger than 20. After age 20, the risk of getting a chondrosarcoma goes up until about age 75. Women get this cancer as often as men.
Chondrosarcomas can develop anywhere there is cartilage. Most develop in bones such as the pelvis, leg bone or arm bone. Occasionally, chondrosarcoma will develop in the trachea, larynx, and chest wall. Other sites are the scapula (shoulder blade), ribs, or skull.
Benign (non-cancerous) tumors of cartilage are more common than malignant ones. These are called enchondromas. Another type of benign tumor that has cartilage is a bony projection capped by cartilage called an osteochondroma. These benign tumors rarely turn into cancer. There is a slightly higher chance of cancer developing in people who have many of these tumors, but this is still not common.
Chondrosarcomas are classified by grade, which measures how fast they grow. The grade is assigned by the pathologist (a doctor specially trained to examine and diagnose tissue samples under a microscope). The lower the grade, the slower the cancer grows. When a cancer is slow growing, the chance that it will spread is lower and so the outlook is better. Most chondrosarcomas are either low grade (grade I) or intermediate grade (grade II). High-grade (grade III) chondrosarcomas, which are the most likely to spread, are less common.
Some chondrosarcomas have distinctive features under a microscope. These variants of chondrosarcoma can have a different prognosis (outlook) than usual chondrosarcomas.
- Dedifferentiated chondrosarcomas start out as typical chondrosarcomas but then some parts of the tumor change into cells like those of a high-grade sarcoma (such as high grade forms of malignant fibrous histiocytoma, osteosarcoma, or fibrosarcoma). This variant of chondrosarcoma tends to occur in older patients and is more aggressive than usual chondrosarcomas.
- Clear cell chondrosarcomas are rare and grow slowly. They rarely spread to other parts of the body unless they have already come back several times in the original location.
- Mesenchymal chondrosarcomas can grow rapidly, but like Ewing tumor, are sensitive to treatment with radiation and chemotherapy.
Ewing tumor
Ewing tumor is the third most common primary bone cancer and the second most common in children, adolescents, and young adults. This cancer (also called Ewing sarcoma) is named after the doctor who first described it in 1921, Dr. James Ewing. Most Ewing tumors develop in bones, but they can start in other tissues and organs. The most common sites for this cancer are the pelvis, the chest wall (such as the ribs or shoulder blades), and the long bones of the legs or arms. This cancer is most common in children and teenagers and is rare in adults older than 30. Ewing tumors occur most often in white people and are very rare among African Americans and Asian Americans.
The main types of Ewing tumors are:
- Ewing sarcoma of bone: Ewing sarcoma that starts in a bone is the most common tumor in this family. This type of tumor was first described by Dr. James Ewing in 1921, who found it was different from the more common bone tumor, osteosarcoma. Seen under a microscope, its cells looked different from osteosarcoma cells. It was also more likely to respond to radiation therapy.
- Extraosseous Ewing tumor: Extraosseous Ewing tumors start in soft tissues around bones, but they look and act very much like Ewing sarcomas in bones. They are also known as extraskeletal Ewing sarcomas.
- Peripheral primitive neuroectodermal tumor: This rare childhood cancer also starts in bone or soft tissue and shares many features with Ewing sarcoma of bone and extraosseous Ewing tumor. Peripheral primitive neuroectodermal tumors that start in the chest wall are known as Askin tumors. (Peripheral primitive neuroectodermal tumors are similar to, but not quite the same as, primitive neuroectodermal tumors of the brain and spinal cord.
Researchers have found that the cells that make up Ewing sarcoma, extraosseous Ewing tumor, and peripheral primitive neuroectodermal tumor are very similar. They tend to have the same DNA (gene) abnormalities and share similar proteins, which are rarely found in other types of cancers. That’s why these 3 cancers are thought to develop from the same type of cells in the body. There are slight differences among these tumors, but they all get the same treatment.
Most Ewing tumors occur in the bones. The most common sites are:
- The pelvis (hip bones)
- The chest wall (such as the ribs or shoulder blades)
- The legs, mainly in the middle of the long bones
In contrast, osteosarcoma usually occurs at the ends of the long bones, especially around the knees. Extraosseous Ewing tumors can occur almost anywhere.
Ewing Tumors Signs and Symptoms
Ewing tumors are often found because of the symptoms they cause.
Pain
Most children and teens with Ewing tumors will have pain in the area of the tumor. Bone pain can be caused by the tumor spreading under the outer covering of the bone (periosteum), or the pain can be from a break (fracture) in a bone that has been weakened by the tumor.
Lump or swelling
Over time, most Ewing bone tumors and almost all non-bone (soft tissue) Ewing tumors cause a lump or swelling, which is more likely to be noticed in tumors in the arms or legs. The lump is often soft and feels warm. Tumors in the chest wall or pelvis (hip bones) might not be noticed until they have grown quite large.
Other symptoms
Ewing tumors can also cause other symptoms, some of which are more common in tumors that have spread:
- Fever
- Feeling tired
- Weight loss
Rarely, tumors near the spine can cause weakness, numbness, or paralysis in the arms or legs, while tumors that have spread to the lungs can cause shortness of breath.
Many of the signs and symptoms of Ewing tumors are more likely to be caused by something else. Still, if your child has any of these symptoms, see a doctor so that the cause can be found and treated, if needed.
Because many of these signs and symptoms can be confused with normal bumps and bruises or bone infections, Ewing tumors might not be recognized right away. For example, the doctor might try giving antibiotics first if an infection is suspected. The correct diagnosis might not be made until the signs and symptoms don’t go away (or get worse) and the bone is then x-rayed.
Ewing Tumors Diagnosis
Ewing tumors are usually found because of signs or symptoms a child or teen is having. If a tumor is suspected, tests will be needed to find out for sure.
Medical history and physical exam
If your child has signs or symptoms that could be from a tumor, the doctor will want to get a complete medical history to find out more about the symptoms and how long they have been present. The doctor will also do a complete physical exam, paying special attention to any areas causing pain or swelling.
If a doctor suspects the child might have a bone tumor (or another type of tumor), more tests will be done to find out. These might include imaging tests, biopsies, and/or lab tests.
Imaging tests
Imaging tests (such as x-rays, MRI scans, CT scans, bone scans, and PET scans) create pictures of the inside of the body. Imaging tests can be done for many reasons, including:
- To help find out if a suspicious area might be cancer
- To determine the extent of a tumor or learn how far a cancer may have spread
- To help determine if treatment is working
Patients who have or may have a Ewing tumor will have one or more of these tests.
X-rays
If a bone lump doesn’t go away or the doctor suspects a bone tumor for some other reason, an x-ray of the area will probably be the first test done. A radiologist (doctor who specializes in reading imaging tests) can usually spot a bone tumor on an x-ray and can often tell if it is likely to be a Ewing tumor. But other imaging tests may be needed as well.
Even if an x-ray strongly suggests a Ewing bone tumor, a biopsy (described below) is still needed to confirm that it is cancer rather than some other problem, such as an infection.
Magnetic resonance imaging (MRI) scan
Often, an MRI scan is done to get a better look an abnormal area seen on an x-ray. MRI scans usually can show if it is likely to be a tumor, an infection, or some type of bone damage from other causes. MRI scans can also help determine the extent of a tumor, as they show in detail the marrow inside bones and the muscle, fat, and connective tissue around the tumor. Knowing the extent of the tumor is very important when planning surgery or radiation therapy.
MRI scans might also be done to see if the cancer has spread to other areas, such as the spine or pelvis (hip area). MRI scans can also be used during and after treatment to see how well the tumor is responding.
MRI scans create detailed images using radio waves and strong magnets instead of x-rays, so there is no radiation involved. A contrast material called gadolinium may be injected into a vein before the scan to help see details better.
MRI scans may take up to an hour. Your child may have to lie on a table that slides inside a narrow tube, which is confining and can be distressing. The test also requires a person to stay still for several minutes at a time. Open MRI machines, which are less confining, might be another option, but they still require staying still for long periods of time. The machines also make buzzing and clicking noises that may be disturbing. Sometimes, younger children are given medicine to help keep them calm or even asleep during the test.
Computed tomography (CT or CAT) scan
CT scans of the chest are often used to see if a Ewing tumor has spread to the lungs. MRI scans are usually a bit better at defining the extent of the main tumor itself, but a CT scan of the tumor may be done as well.
The CT scan uses x-rays to make detailed cross-sectional images of parts of the body, including soft tissues such as muscles. Instead of taking one picture, like a regular x-ray, a CT scanner takes many pictures as it rotates around your child while he or she lies on a table. A computer then combines these pictures into images of slices of the part of the body being studied.
Before the scan, your child may be asked to drink a contrast solution and/or get an intravenous (IV) injection of a contrast dye that helps better outline abnormal areas in the body. If a contrast dye is to be injected, your child may need an IV line. The contrast can cause some flushing (a feeling of warmth, especially in the face). Some people are allergic and get hives. Rarely, more serious reactions like trouble breathing or low blood pressure can occur. Be sure to tell the doctor if your child has any allergies (especially to iodine or shellfish) or has ever had a reaction to any contrast material used for x-rays.
CT scans take longer than regular x-rays, but not as long as MRI scans. A CT scanner has been described as a large donut, with a narrow table that slides in and out of the middle opening. Your child will need to lie still on the table while the scan is being done. Some people feel a bit confined while the pictures are being taken, although it is not as narrow as an MRI tube. Some children may need to be sedated before the test to stay still and help make sure the pictures come out well.
Bone scan
A bone scan can help show if a cancer has metastasized (spread) to bones in other parts of the body, and might be part of the workup for a child with a Ewing tumor. This test is useful because it can show the entire skeleton at once. (A positron emission tomography [PET] scan can often provide similar information, so a bone scan might not be needed if a PET scan is done.)
For this test, a small amount of low-level radioactive material is injected into a vein (intravenously, or IV). (The amount of radioactivity used is very low and will pass out of the body within a day or so.) The substance settles in abnormal areas of bone throughout the body over the course of a couple of hours. Your child then lies on a table for about 30 minutes while a special camera detects the radioactivity and creates a picture of the skeleton. Younger children might be given medicine to help keep them calm or even asleep during the test.
Areas of active bone changes appear as “hot spots” on the skeleton because they attract the radioactivity. These areas may suggest the presence of cancer, but other bone diseases can also cause the same pattern. To be sure, other tests such as plain x-rays or MRI scans, or even a bone biopsy might be needed.
Positron emission tomography (PET) scan
For a PET scan, a form of radioactive sugar (known as fluorodeoxyglucose or FDG) is injected into the blood. The amount of radioactivity used is very low and will pass out of the body within a day or so. Because cancer cells in the body are growing quickly, they absorb large amounts of the sugar. After about an hour, your child will lie on a table in the PET scanner for about 30 minutes while a special camera creates a picture of areas of radioactivity in the body. The picture is not detailed like a CT or MRI scan, but it provides helpful information about the whole body.
PET scans can be very helpful in showing the spread of Ewing tumors and in finding out whether abnormal areas seen on other imaging tests (such as a bone scan or CT scan) are tumors. PET scans can also be repeated during treatment to monitor the cancer over time.
Some newer machines can do a PET and CT scan at the same time (PET/CT scan). This lets the doctor compare areas of higher radioactivity on the PET scan with the more detailed appearance of that area on the CT scan.
Biopsy of the tumor
The results of imaging tests might strongly suggest a Ewing tumor, but a biopsy (removing some of the tumor for viewing under a microscope and other lab testing) is the only way to be certain. A biopsy is also the best way to tell Ewing tumors from other types of cancer.
If the tumor is in a bone, it is very important that a surgeon experienced in treating bone tumors does the biopsy. Whenever possible, the biopsy and the surgery to treat the cancer should be planned together, and the same surgeon should do both. Proper planning of the biopsy can help prevent later complications and might reduce the amount of surgery needed later on.
There are a few ways to get a sample of the tumor to diagnose Ewing tumors.
Excisional biopsy
In very rare cases, if the tumor is small enough and in a good location, the surgeon can completely remove it while the child is under general anesthesia (asleep). This is called an excisional biopsy.
Incisional biopsy
In most cases of suspected Ewing tumors, an incisional biopsy (taking only a piece of the tumor) is more likely to be done. This can be done in a couple of ways:
- Surgical (open) biopsy: For this type of biopsy, the surgeon cuts away a piece of the tumor through an opening on the skin.
- Needle (closed) biopsy: In this type of biopsy, the surgeon puts a large, hollow needle through the skin and into the tumor to remove a piece of it.
Incisional biopsies are often done while the patient is under general anesthesia (in a deep sleep), but in older teens and adults they are sometimes done using sedation and a local anesthetic (numbing medicine).
If general anesthesia is going to be used for the biopsy, the surgeon may also plan other procedures while the child is asleep to avoid having to do them as separate operations later on. For example, if the tumor is thought to have spread to the chest or elsewhere, the surgeon may take biopsy samples of these suspected tumors when the child is still asleep. The doctor might also do a bone marrow biopsy (see next section) at this time to see if the cancer has spread to the bone marrow spaces.
During the biopsy (while the child is still asleep), a pathologist (a doctor specializing in lab tests to diagnose diseases) can take a quick look at the biopsy samples under a microscope. If it looks like a Ewing tumor, the child will very likely need chemotherapy as part of treatment, so the surgeon may place a small flexible tube, known as a central venous catheter, into a large vein in the chest area during the same operation. The catheter end lies just under or outside on the skin. It can stay in place for several months during treatment. The catheter gives doctors and nurses easier access to the vein, which is allows the child to get fewer needle sticks when chemotherapy is given or blood needs to be drawn at a later time.
Bone marrow aspiration and biopsy
These tests are used to see if the cancer cells have spread into the bone marrow, the soft inner parts of certain bones. The tests aren’t usually done to diagnose Ewing tumors, but they may be done once the diagnosis is made because it is important to know if the tumor has spread to the bone marrow.
Bone marrow aspiration and biopsy are usually done at the same time. In most cases the marrow samples are taken from the back of both of the pelvic (hip) bones.
These tests may be done during the surgery to biopsy or treat the main tumor (while the child is still under anesthesia), or they may be done as a separate procedure.
If the bone marrow aspiration is being done as a separate procedure, the child lies on a table (on his or her side or belly). The area over the hip is cleaned, and the skin and the surface of the bone are numbed with a local anesthetic, which may cause a brief stinging or burning sensation. In most cases, the child is also given other medicines to make them sleepy, or they might even be asleep during the procedure. A thin, hollow needle is then inserted into the bone, and a syringe is used to suck out a small amount of liquid bone marrow.
A bone marrow biopsy is usually done just after the aspiration. A small piece of bone and marrow is removed with a slightly larger needle that is pushed down into the bone. Once the biopsy is done, pressure is applied to the site to help stop any bleeding.
Samples from the bone marrow are sent to a pathology lab, where they are looked at and tested for cancer cells.
Testing biopsy samples
A doctor called a pathologist looks at all biopsy specimens under a microscope to see if they contain cancer cells. If cancer is found, the specific type of cancer can often be determined as well. But because cells from Ewing tumors share many of the same features as cells from other types of childhood cancer, more lab tests are often needed.
Immunohistochemistry
For this test, a portion of the biopsy sample is treated with special proteins (antibodies) that attach to substances found on Ewing tumor cells but not on other cancers. Chemicals (stains) are then added so that cells containing these substances change color and can be seen under a microscope. This lets the pathologist know that the cells are from a Ewing tumor.
Cytogenetics
For this test, chromosomes (pieces of DNA) from the tumor cells are looked at under a microscope to detect any changes. Ewing tumor cells almost always have chromosome translocations, where 2 chromosomes swap pieces of their DNA. In most cases, the cells have translocations between chromosomes 22 and 11. Less often, the translocation is between other chromosomes. Finding these changes can help doctors tell Ewing tumors from other types of cancer. Other types of chromosome changes can also be found in some Ewing tumors.
Getting the results of cytogenetic testing usually takes about 2 to 3 weeks because the cancer cells must be grown in lab dishes for a couple of weeks before their chromosomes can be seen under the microscope.
Fluorescence in situ hybridization (FISH) is a type of cytogenetic test that uses special fluorescent dyes to spot specific chromosome changes in Ewing tumors. FISH can find most chromosome changes (such as translocations) that are visible under a microscope in standard cytogenetic tests, as well as some changes too small to be seen with usual cytogenetic testing.
FISH can be used to look for specific changes in chromosomes. It is very accurate and can usually provide results within a couple of days.
Reverse transcription polymerase chain reaction (RT-PCR)
This test is another way to find translocations in tumor cells to confirm the type of tumor. RT-PCR is a very sensitive test that is often able to detect very small numbers of cells with translocations, which wouldn’t be detected by cytogenetics.
RT-PCR is also useful in looking for leftover or recurrent cancer after treatment. For example, if RT-PCR testing of a bone marrow sample after treatment finds cells with a typical Ewing tumor translocation, it is likely that the cancer has not been cured, so more treatment is needed.
Blood tests
No blood test can be used to diagnose Ewing tumors. But certain blood tests may be helpful once a diagnosis has been made.
A complete blood count (CBC) measures the levels of white blood cells, red blood cells, and platelets in the blood. An abnormal CBC result at the time of diagnosis might suggest the cancer has spread to the bone marrow, where these blood cells are made.
A blood test for levels of an enzyme called lactate dehydrogenase (LDH) is typically done at diagnosis. A high LDH level indicates the cancer may be harder to treat.
Standard blood tests are done often to check a child’s general health both before treatment (especially before surgery) and during treatment (such as chemotherapy) to look for possible problems or side effects. These tests often include a CBC to monitor bone marrow function and blood chemistry tests to measure how well the liver and kidneys are working.
How Are Ewing Tumors Staged?
Once a Ewing tumor has been diagnosed, tests are done to determine the stage (extent of spread) of the cancer. The stage of a Ewing tumor is one of the most important factors determining a person’s outlook (prognosis) and in choosing treatment.
The stage is based on results of imaging tests and biopsies of the main tumor and other tissues.
American Joint Committee on Cancer (AJCC) staging system for bone cancer
The American Joint Committee on Cancer (AJCC) uses one system to describe all bone cancers, including Ewing tumors that start in bone. Extraosseous Ewing (EOE) tumors (Ewing tumors that don’t start in bones) are staged differently. They are staged like soft-tissue sarcomas.
The American Joint Committee on Cancer (AJCC) staging system for bone cancers is based on 4 key pieces of information:
- T describes the size of the main (primary) tumor and whether it appears in different areas of the bone.
- N describes the extent of spread to nearby (regional) lymph nodes (small bean-sized collections of immune system cells). Bone tumors rarely spread to the lymph nodes.
- M indicates whether the cancer has metastasized (spread) to other organs of the body. (The most common sites of spread are to the lungs or other bones.)
- G stands for the grade of the tumor, which describes how the cells from biopsy samples look. Low-grade tumor cells look more like normal cells and are less likely to grow and spread quickly, while high-grade tumor cells look more abnormal. (All Ewing tumors are considered high-grade tumors.)
Numbers or letters after T, N, M, and G provide more details about each of these factors.
T categories of bone cancer
T0: There is no evidence of a main (primary) tumor.
T1: The tumor is 8 cm (around 3 inches) across or less.
T2: The tumor is larger than 8 cm across.
T3: The tumor is in more than one site in the same bone.
N categories of bone cancer
N0: There is no spread to regional (nearby) lymph nodes.
N1: The cancer has spread to nearby lymph nodes.
M categories of bone cancer
M0: There is no spread (metastasis) to distant organs.
M1a: The cancer has spread only to the lungs.
M1b: The cancer has spread to other distant sites in the body.
Grades of bone cancer
GX: Grade can’t be assessed
G1-G2: Low grade
G3-G4: High grade
(All Ewing tumors are considered G4.)
Stage grouping
Once the T, N, and M categories and the grade of the bone cancer have been determined, the information is combined and expressed as an overall stage. The process of assigning a stage number is called stage grouping. The stages are described in Roman numerals from I to IV (1-4), and are sometimes divided further.
Stage IA*
T1, N0, M0, G1 to G2 (or GX): The tumor is 8 cm across or less (T1) and is low grade (or the grade can’t be assessed). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0).
Stage IB*
T2 or T3, N0, M0, G1 to G2 (or GX): The tumor is either larger than 8 cm across (T2) or it is in more than one place in the same bone (T3). It is low grade (or the grade can’t be assessed). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0).
Stage IIA
T1, N0, M0, G3 to G4: The tumor is 8 cm across or less (T1) and is high grade (G3 or G4). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0).
Stage IIB
T2, N0, M0, G3 to G4: The tumor is larger than 8 cm across (T2) and is high grade (G3 or G4). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0).
Stage III
T3, N0, M0, G3 to G4: The tumor is in more than one place in the same bone (T3). It is high grade (G3 or G4). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0).
Stage IVA
Any T, N0, M1a, any G: The tumor has spread only to the lungs (M1a). It has not spread to the lymph nodes or to other distant sites. (It can be any size or grade.)
Stage IVB (if either of these applies)
Any T, N1, any M, any G: The tumor has spread to lymph nodes (N1). It can be any size or grade, and may or may not have spread to other distant sites.
Any T, any N, M1b, any G: The tumor has spread to distant sites other than the lungs (M1b). It can be any size or grade.
*All Ewing tumors are classified as G4 (high grade), so they are never stage I bone cancers.
Localized vs. metastatic stages
Doctors use a simpler system for staging Ewing tumors to determine how best to treat them. In this system, the cancers are classified as either localized or metastatic.
Localized Ewing tumors
A localized Ewing tumor is thought to be confined to the area where it started and may also have reached nearby tissues such as muscle or tendons. A Ewing tumor is considered localized only after all of the imaging tests (x-rays, CT or MRI scans, and PET or bone scans) and the bone marrow biopsy and aspirate (if done) do not find it has spread to other distant areas.
Even when imaging tests do not show that the cancer has spread to distant areas, many patients are likely to have micrometastases (very small areas of cancer spread that can’t be detected with tests). This is why chemotherapy, which can reach all parts of the body, is an important part of treatment for all Ewing tumors.
Metastatic Ewing tumors
A metastatic Ewing tumor has clearly spread from where it started to distant parts of the body. Most of the time, it spreads to the lungs or to other bones or the bone marrow. Less commonly, it spreads to the liver or lymph nodes.
About 1 in 4 patients will have obvious spread that is found by imaging tests. But as mentioned above, many other patients are likely to have small amounts of cancer spread to other parts of the body that can’t be seen on imaging tests.
Survival Rates for Ewing Tumors by Stage
Localized tumors
With current treatment, the overall 5-year survival rate for patients with Ewing tumors that are still localized when they are first found is around 70%.
Metastatic tumors
When the cancer has already spread when it is diagnosed, the 5-year survival rate is about 15% to 30%. The survival rate is slightly better if the cancer has only spread to the lungs as opposed to having reached other organs.
Other factors affecting prognosis
Factors other than the stage of the cancer can also affect survival rates. Factors that have been linked with a better prognosis include:
- Smaller tumor size
- Main tumor is on an arm or leg (as opposed to chest wall or pelvis)
- Normal blood LDH level
- Good tumor response to chemotherapy
- Age younger than 10
Even when taking these other factors into account, survival rates are at best rough estimates. Your child’s doctor is your best source of information on this topic, as he or she is familiar with your situation.
Malignant fibrous histiocytoma
Malignant fibrous histiocytoma more often starts in soft tissue (connective tissues such as ligaments, tendons, fat, and muscle) than in bones. This cancer is also known as pleomorphic undifferentiated sarcoma, especially when it starts in soft tissues. When malignant fibrous histiocytoma occurs in bones, it usually affects the legs (often around the knees) or arms. This cancer most often occurs in elderly and middle-aged adults and is rare among children. Malignant fibrous histiocytoma mostly tends to grow locally, but it can spread to distant sites, like the lungs.
Fibrosarcoma
This is another type of cancer that develops more often in soft tissues than it does in bones. Fibrosarcoma usually occurs in elderly and middle-aged adults. Bones in the legs, arms, and jaw are most often affected.
Giant cell tumor of bone
This type of primary bone tumor has benign and malignant forms. The benign (non-cancerous) form is most common. Giant cell bone tumors typically affect the leg (usually near the knees) or arm bones of young and middle-aged adults. They don’t often spread to distant sites, but tend to come back where they started after surgery (this is called local recurrence). This can happen several times. With each recurrence, the tumor becomes more likely to spread to other parts of the body. Rarely, a malignant giant cell bone tumor spreads to other parts of the body without first recurring locally.
Chordoma
This primary tumor of bone usually occurs in the base of the skull and bones of the spine. It develops most often in adults older than 30, and is about twice as common in men as in women. Chordomas tend to grow slowly and often do not spread to other parts of the body, but they often come back in the same area if they are not removed completely. The lymph nodes, the lungs, and the liver are the most common areas for secondary tumor spread.
Other cancers that develop in bones
Non-Hodgkin lymphomas
Non-Hodgkin lymphoma generally develops in lymph nodes but sometimes starts in the bone. Primary non-Hodgkin lymphoma of the bone is often a widespread disease because multiple sites in the body are usually involved. The outlook is similar to other non-Hodgkin lymphomas of the same subtype and stage. Primary lymphoma of the bone is given the same treatment as lymphomas that start in lymph nodes − it is not treated like a primary bone sarcoma.
Multiple myelomas
Multiple myeloma almost always develops in bones, but doctors do not consider it a primary bone cancer because it develops from the plasma cells of the bone marrow (the soft inner part of some bones). Although it causes bone destruction, it is no more a bone cancer than is leukemia. It is treated as a widespread disease. At times, myeloma can be first found as a single tumor (called a plasmacytoma) in a single bone, but most of the time it will spread to the marrow of other bones.
What’s the difference between primary bone cancer and bone metastasis?
Some cancers start in the bone, rather than spreading to the bones from somewhere else. Cancers that start in the bone are called primary bone cancers. These cancers are very different from bone metastases. Bone metastasis is much more common than primary bone cancers, especially in adults.
Metastatic bone cancer
A bone metastasis is an area of bone that contains cancer that spread there from somewhere else.
Cancer can spread to any bone in the body, but metastases are most often found in bones near the center of the body. The spine is the most common site. Other common sites are the hip bone (pelvis), upper leg bone (femur), upper arm bone (humerus), ribs, and the skull.
Once cancer has spread to the bones or to other parts of the body it’s rarely able to be cured. Still, it often can be treated to shrink, stop, or slow its growth. Even if a cure is no longer possible, treating the cancer may be able to help you live longer and feel better.
How does bone metastasis cause bone changes and other problems?
Bone is the supporting framework of the body. Bones are made of a network of fibrous tissue called matrix, minerals such as calcium that attach to the matrix and give the bone its strength and hardness, and 2 main kinds of bone cells are osteoblasts and osteoclasts.
Knowing a little about these 2 kinds of cells can help you understand how bone metastases grow, and how some medicines work to treat bone metastases. The osteoblast is the cell that forms new bone, and the osteoclast is the cell that dissolves old bone. When these cells are both working right, new bone is always forming while old bone is dissolving. This helps keep the bones strong.
Cancer cells can affect the bones by interfering with osteoblasts and osteoclasts:
- Often, the cancer cells make substances that turn on the osteoclasts. This leads to bone being broken down without new bone being made. This weakens the bones. The holes that develop when parts of bones dissolve are called osteolytic or lytic lesions. Lytic lesions are so weak that they can cause the bone to easily break.
- Sometimes, the cancer cells release substances that turn on the osteoblasts. This leads to new bone being made without breaking down the old bone broken down first. This makes areas of the bones harder, a condition called sclerosis. The areas of bone where this occurs are called osteoblastic or blastic lesions. Although these blastic areas are harder, the structure of the bone is not normal and these areas actually break more easily than normal bone.
Bone metastasis can cause other problems as well:
- When cancer spreads to the bones of the spine, it can press on the spinal cord. This can cause nerve damage that may even lead to paralysis if not treated.
- As cancer cells damage the bones, calcium from the bones is released into the blood. This can lead to problems caused by high blood calcium levels (hypercalcemia).
Why do cancers metastasize to bones?
For cancer cells to spread to other parts of the body, they have to go through many changes:
- They have to be able to break away from the original (primary) tumor and get into the bloodstream or lymph system, which can carry them to another part of the body.
- At some point they need to attach to the wall of a blood or lymph vessel and move through it, out into a new organ.
- They then need to be able to grow and thrive in their new location.
All the while, the cancer cells need to be able to avoid attacks from the body’s immune system. Going through all these steps means the cells that start new tumors may no longer be exactly the same as the ones in the tumor where they started, but they will still be called the same name. For instance, breast cancer that spreads to the bone is called metastatic breast cancer, not bone cancer.
Benign bone tumors
Benign tumors do not spread to other tissues and organs and so are not usually life threatening. They are generally cured by surgery. Types of benign bone tumors include:
- Osteoid osteoma
- Osteoblastoma
- Osteochondroma
- Enchondroma
- Chondromyxoid fibroma.
These benign tumors are not discussed further here.
What Causes Bone Cancer?
The exact cause of most bone cancers is not known. However, scientists have found that bone cancers are associated with a number of other conditions, which are described in the section on risk factors. Still, most people with bone cancers do not have any known risk factors. Research is underway to learn more about the causes of these cancers.
Scientists have made great progress in understanding how certain changes in a person’s DNA can cause normal cells to become cancerous. DNA carries the instructions for nearly everything our cells do. We usually look like our parents because they are the source of our DNA. However, DNA affects more than our outward appearance. It may influence our risks for developing certain diseases, including some kinds of cancer.
DNA is divided into units called genes. Genes carry the recipes for making proteins, the molecules that determine all cell functions. Some genes contain instructions to control when our cells grow and divide. Genes that promote cell division are called oncogenes. Others that slow down cell division or make cells die at the right time are called tumor suppressor genes. Cancers can be caused by DNA mutations (defects) that activate oncogenes or inactivate tumor suppressor genes. Some people with cancer have DNA mutations that they inherited from a parent. These mutations increase their risk for the disease.
The DNA mutations that cause some inherited forms of bone cancers are known. In many cases, genetic testing can be used to see if someone has one of these mutations.
Most bone cancers are not caused by inherited DNA mutations. They are the result of mutations acquired during the person’s lifetime. These mutations may result from exposure to radiation or cancer-causing chemicals, but most often they occur for no apparent reason. These mutations are present only in the cancer cells and so cannot be passed on to the patient’s children.
Scientists are making progress in understanding this process, but there are still some points that are not completely understood. As their knowledge increases, they hope to develop ways to better prevent and treat bone cancers.
Risk Factors for Bone Cancer?
A risk factor is anything that affects your chance of getting a disease such as cancer. Different cancers have different risk factors. For example, exposing skin to strong sunlight is a risk factor for skin cancer. Smoking is a risk factor for cancers of the lung, mouth, larynx, bladder, kidney, and several other organs. But having a risk factor, or even several, does not mean that you will get the disease. Most people with bone cancers do not have any apparent risk factors.
Genetic disorders
A very small number of bone cancers (especially osteosarcomas) appear to be hereditary and are caused by defects (mutations) in certain genes.
Osteosarcomas
Children with certain rare inherited syndromes have an increased risk of developing osteosarcoma.
- The Li-Fraumeni syndrome makes people much more likely to develop several types of cancer, including breast cancer, brain cancer, osteosarcoma, and other types of sarcoma. Most of those cases are caused by a mutation of the p53 tumor suppressor gene, but some are caused by mutations in the gene CHEK2.
- Another syndrome that includes bone cancer is the Rothmund-Thomson syndrome. Children with this syndrome are short, have skeletal problems, and rashes. They also are more likely to develop osteosarcoma. This syndrome is caused by abnormal changes in the gene REQL4.
- Retinoblastoma is a rare eye cancer of children that can be hereditary. The inherited form of retinoblastoma is caused by a mutation (abnormal copy) of the RB1 gene. Those with this mutation also have an increased risk of developing bone or soft tissue sarcomas. Also, if radiation therapy is used to treat the retinoblastoma, the risk of osteosarcoma in the bones around the eye is even higher.
Finally, there are families with several members who have developed osteosarcoma without inherited changes in any of the known genes. The gene defects that may cause cancers in these families haven’t been discovered yet.
Chondrosarcomas
Multiple exostoses (sometimes called multiple osteochondromas) syndrome is an inherited condition that causes many bumps on a person’s bones. These bumps are made mostly of cartilage. They can be painful and deform and/or fracture bones. This disorder is caused by a mutation in any one of the 3 genes EXT1, EXT2, or EXT3. Patients with this condition have an increased risk of chondrosarcoma.
An enchondroma is a benign cartilage tumor that grows into the bone. People who get many of these tumors have a condition called multiple enchondromatosis. They have an increased risk of developing chondrosarcomas.
Chordomas
Chordomas seem to run in some families. The genes responsible have not yet been found, but familial chordoma has been linked to changes on chromosome 7.
Patients with the inherited syndrome tuberous sclerosis, which can be caused by defects (mutations) in either of the genes TSC1 and TSC2, seem to have a high risk of chordomas during childhood.
Paget disease
Paget (PA-jet) disease is a benign (non-cancerous) but pre-cancerous condition that affects one or more bones. It results in formation of abnormal bone tissue and is mostly a disease of people older than 50. Affected bones are heavy, thick, and brittle. They are weaker than normal bones and more likely to fracture (break). Most of the time Paget disease is not life threatening. Bone cancer (usually osteosarcoma) develops in about 1% of those with Paget disease, usually when many bones are affected.
Radiation
Bones that have been exposed to ionizing radiation may also have a higher risk of developing bone cancer. A typical x-ray of a bone is not dangerous, but exposure to large doses of radiation does pose a risk. For example, radiation therapy to treat cancer can cause a new cancer to develop in one of the bones in the treatment area. Being treated when you are younger and/or being treated with higher doses of radiation (usually over 60 Gy) increases the risk of developing bone cancer.
Exposure to radioactive materials such as radium and strontium can also cause bone cancer because these minerals build up in bones.
Non-ionizing radiation, like microwaves, electromagnetic fields from power lines, cellular phones, and household appliances, does not increase bone cancer risk.
Bone marrow transplantation
Osteosarcoma has been reported in a few patients who have undergone bone marrow (stem cell) transplantation.
Injuries
People have wondered whether injury to a bone can cause cancer, but this has never been proven. Many people with bone cancer remember having hurt that part of their bone. Most doctors believe that this did not cause the cancer, but rather that the cancer caused them to remember the incident or that the injury drew their attention to that bone and caused them to notice a problem that had already been present for some time.
Bone cancer signs and symptoms
Pain
Pain in the affected bone is the most common complaint of patients with bone cancer. At first, the pain is not constant. It may be worse at night or when the bone is used (for example, leg pain when walking). As the cancer grows, the pain will be there all the time. The pain increases with activity and the person might limp if a leg is involved.
Swelling
Swelling in the area of the pain may not occur until weeks later. It might be possible to feel a lump or mass depending on the location of the tumor.
Cancers in the bones of the neck can cause a lump in the back of the throat that can lead to trouble swallowing or make it hard to breathe.
Fractures
Bone cancer can weaken the bone it develops in, but most of the time the bones do not fracture (break). People with a fracture next to or through a bone cancer usually describe sudden severe pain in a limb that had been sore for a few months.
Other symptoms
Cancer in the bones of the spine can press on nerves, leading to numbness and tingling or even weakness.
Cancer can cause weight loss and fatigue. If the cancer spreads to internal organs it may cause other symptoms, too. For example, if the cancer spreads to the lung, you may have trouble breathing.
Any of these symptoms are more often due to conditions other than cancer, such as injuries or arthritis. Still, if these problems go on for a long time without a known reason, you should see your doctor.
How is Bone Cancer Diagnosed?
A patient’s symptoms, physical exam, and results of imaging tests, and blood tests may suggest that bone cancer is present. But in most cases, doctors must confirm this suspicion by examining a tissue or cell sample under a microscope (a procedure known as a biopsy).
Other diseases, such as bone infections, can cause symptoms and imaging results that could be confused with bone cancer. Accurate diagnosis of a bone tumor often depends on combining information about its location (what bone is affected and even which part of the bone is involved), appearance on x-rays, and appearance under a microscope.
Since a single bone metastasis can have the same signs and symptoms as a primary bone tumor, many doctors require a biopsy to diagnose a patient’s first bone metastasis. After that, additional bone metastases can usually be diagnosed based on x-rays and other imaging tests.
Imaging tests to detect bone cancer
X-rays
Most bone cancers show up on x-rays of the bone. The bone at the site of the cancer may appear “ragged” instead of solid. The cancer can also appear as a hole in the bone. Sometimes doctors can see a tumor around the defect in the bone that might extend into nearby tissues (such as muscle or fat). The radiologist (doctor who specializes in reading x-rays) can often tell if a tumor is malignant by the way it appears on the x-ray, but only a biopsy can absolutely determine that.
A chest x-ray is often done to see if bone cancer has spread to the lungs.
Computed tomography (CT) scans
CT scans are helpful in staging cancer. They help tell if your bone cancer has spread into your lungs, liver, or other organs. These scans also show the lymph nodes and distant organs where metastatic cancer might be present.
CT scans can also be used to precisely guide a biopsy needle into a suspected metastasis. For this procedure, called a CT-guided needle biopsy, the patient remains on the CT scanning table while a radiologist advances a biopsy needle toward the location of the mass. CT scans are repeated until the doctors are confident that the needle is within the mass.
Magnetic resonance imaging (MRI) scans
MRI scans are often the best test for outlining a bone tumor. They are also particularly helpful for looking at the brain and spinal cord. MRI scans are a little more uncomfortable than CT scans. First, they take longer — often up to an hour. Also, you have to be placed inside a tube, which is confining and can upset people with claustrophobia (fear of enclosed spaces). The machine also makes a thumping noise that you may find disturbing. Some places provide headphones with music to block this out.
Radionuclide bone scans
This procedure helps show if a cancer has spread to other bones. It can find metastases earlier than regular x-rays. Bone scans also can show how much damage the primary cancer has caused in the bone.
For this test, the patient receives an injection of radioactive material called technetium diphosphonate . The amount of radioactivity used is very low and causes no long-term effects. This substance is attracted to diseased bone cells throughout the entire skeleton. Areas of diseased bone will be seen on the bone scan image as dense, gray to black areas, called “hot spots.” These areas suggest metastatic cancer is present, but arthritis, infection, or other bone diseases can also cause a similar pattern. To distinguish among these conditions, the cancer care team may use other imaging tests or take bone biopsies.
Positron emission tomography (PET or PET) scans
PET scans use glucose (a form of sugar) that contains a radioactive atom. A special camera can detect the radioactivity. Cancer cells absorb a lot of the radioactive sugar because of their high rate of metabolism. PET scans are useful in looking for cancer throughout your entire body. It can sometimes help tell if a tumor is cancerous or benign. It is being combined with CT scans to better pinpoint some kinds of cancer.
Biopsy
A biopsy is a sample of tissue taken from a tumor so that it can be looked at under a microscope. This is the only way to know that the tumor is cancer and not some other bone disease. If cancer is present, the biopsy can tell the doctor if it is a primary bone cancer or cancer that started somewhere else and spread to the bone (metastasis). Several types of tissue and cell samples are used to diagnose bone cancer. It is very important a surgeon with experience in diagnosing and treating bone tumors do the biopsy procedure.
The surgeon will choose a biopsy method based on whether the tumor looks benign or malignant and exactly what type of tumor is most likely (based on the bone x-rays, the patient’s age, and the location of the tumor). Some kinds of bone tumors can be recognized from needle biopsy samples, but larger samples (from a surgical biopsy) are often needed to diagnose other types. Whether the surgeon plans to remove the entire tumor at the time of the biopsy will also influence the choice of biopsy type. The wrong kind of biopsy can sometimes make it hard later for the surgeon to remove all of the cancer without having to also remove all or part of the arm or leg containing the tumor. It also may cause the cancer to spread.
Needle biopsy
There are 2 types of needle biopsies: fine needle biopsies and core needle biopsies. For both types, a local anesthetic is first used to numb the area for the biopsy. For fine needle aspiration (FNA), the doctor uses a very thin needle attached to a syringe to withdraw a small amount of fluid and some cells from the tumor mass. Sometimes, the doctor can aim the needle by feeling the suspicious tumor or area that is near the surface of the body. If the tumor cannot be felt because it is too deep, the doctor can guide the needle while viewing a CT scan. This is called a CT guided needle biopsy and it is often done by an x-ray specialist known as an interventional radiologist. In a core needle biopsy, the doctor uses a larger needle to remove a small cylinder of tissue (about 1/16 inch in diameter and 1/2 inch long). Many experts feel that a core needle biopsy is better than FNA to diagnose a primary bone cancer.
Surgical bone biopsy
In this procedure, a surgeon needs to cut through the skin to reach the tumor in order to remove a small piece of tissue. This is also called an incisional biopsy. If the entire tumor is removed (not just a small piece), it is called an excisional biopsy. These biopsies are often done with the patient under general anesthesia (asleep). They can also be done using a nerve block, which numbs a large area. If this type of biopsy is needed, it is important that the surgeon who will later remove the cancer also be the one to do the biopsy.
Bone Cancer Stages
After someone is diagnosed with bone cancer, doctors will try to figure out if it has spread, and if so, how far. This process is called staging. The stage of a cancer describes how much cancer is in the body. It helps determine how serious the cancer is and how best to treat it. Doctors also use a cancer’s stage when talking about survival statistics.
Bone cancers range from stages I (1) through IV (4). As a rule, the lower the number, the less the cancer has spread. A higher number, such as stage IV, means cancer has spread more. And within a stage, an earlier letter means a lower stage. Although each person’s cancer experience is unique, cancers with similar stages tend to have a similar outlook and are often treated in much the same way.
How is the stage determined?
The staging system most often used for bone cancer is the American Joint Committee on Cancer (AJCC) TNM system, which is based on 4 key pieces of information:
- The extent (size) of the tumor (T): How large is the cancer? Is it in more than one spot in the bone?
- The spread to nearby lymph nodes (N): Has the cancer spread to nearby lymph nodes?
- The spread (metastasis) to distant sites (M): Has the cancer spread to the lungs only or to distant sites such as other bones or the liver?
- The grade of the cancer (G): How abnormal do the cells look when seen under a microscope?
The scale used for grading bone cancer is from 1 to 3. Low-grade cancers (G1) tend to grow and spread more slowly than high-grade (G2 or G3) cancers.
- Grade 1 (G1) means the cancer looks much like normal bone tissue.
- Grade 3 (G3) means the cancer looks very abnormal.
- Grade 2 (G2) falls somewhere in between.
The staging system described below is the most recent AJCC system effective January 2018 and applies to bone cancers of the appendicular skeleton (such as bones in the arms and legs), trunk, skull, and facial bones. Bone cancers of the pelvis and spine use different T categories and it is best to speak with your doctor about your stage for these specific cancers.
Numbers or letters after T, N, and M provide more details about each of these factors. Higher numbers mean the cancer is more advanced. Once a person’s T, N, and M categories have been determined, this information is combined in a process called stage grouping to assign an overall stage.
The staging system in the table below uses the pathologic stage (also called the surgical stage). It is determined by examining tissue removed during an operation. Sometimes, if surgery is not possible right away or at all, the cancer will be given a clinical stage instead. This is based on the results of a physical exam, biopsy, and imaging tests. The clinical stage will be used to help plan treatment. Sometimes, though, the cancer has spread further than the clinical stage estimates, and may not predict the patient’s outlook as accurately as a pathologic stage.
Cancer staging can be complex, so ask your doctor to explain it to you in a way you understand.
Table 2. American Joint Committee on Cancer Bone Cancer Staging System
| AJCC Stage | Stage grouping | Stage description* |
| IA | T1 N0 M0 G1 or GX | The cancer is 8 centimeters (cm) across (about 3 inches) or smaller(T1). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). The cancer is low grade (G1) or the grade cannot be determined (GX). |
|
IB | T2 N0 M0 G1 or GX | The cancer is larger than 8 cm (3 inches) across (T2). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). The cancer is low grade (G1) or the grade cannot be determined (GX). |
| OR | ||
| T3 N0 M0 G1 or GX | The cancer is in more than one place on the same bone (T3). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). The cancer is low grade (G1) or the grade cannot be determined (GX). | |
| IIA
| T1 N0 M0 G2 or G3 | The cancer is 8 centimeters (cm) across (about 3 inches) or less (T1). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). The cancer is high grade (G2 or G3). |
| IIB
| T2 N0 M0 G2 or G3 | The cancer is larger than 8 cm (3 inches) across (T2). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). The cancer is high grade (G2 or G3). |
| III | T3 N0 M0 G2 or G3 | The cancer is in more than one place on the same bone (T3). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). The cancer is high grade (G2 or G3). |
| IVA | Any T N0 M1a Any G | The cancer can be any size and may be in more than one place in the bone (Any T) AND has not spread to nearby lymph nodes (N0). It has spread only to the lungs (M1a). The cancer can be any grade (Any G). |
| IVB | Any T N1 Any M Any G | The cancer can be any size and may be in more than one place in the bone (Any T) AND it has spread to nearby lymph nodes (N1). It may or may not have has spread to distant organs like the lungs or other bones (Any M). The cancer can be any grade (Any G). |
| OR | ||
| Any T Any N M1b Any G | The cancer can be any size and may be in more than one place in the bone (Any T) and it might or might not have spread to nearby lymph nodes (Any N). It has spread to distant sites like other bones, the liver or brain (M1b). The cancer can be any grade (Any G). | |
* The following additional categories are not listed on the table above:
- TX: Main tumor cannot be assessed due to lack of information.
- T0: No evidence of a primary tumor.
- NX: Regional lymph nodes cannot be assessed due to lack of information.
Bone cancer survival rate
Survival rates are often based on previous outcomes of large numbers of people who had the disease, but they cannot predict what will happen in any particular person’s case. Many factors may affect a person’s outlook, such as the type and grade of the cancer, the patient’s age, where the cancer is located, the size of the tumor, and the treatment received. Your doctor can tell you how the numbers below may apply to you, as he or she is familiar with the aspects of your particular situation.
For all cases of bone cancer combined (in both adults and children), the 5-year relative survival is about 70%. For adults, the most common bone cancer is chondrosarcoma, which has a 5-year relative survival of about 80%.
Bone cancer treatment
Depending on the type and stage of your cancer, you may need more than one type of treatment. Doctors on your cancer treatment team may include:
- An orthopedic surgeon: a doctor who uses surgery to treat bone and joint problems
- An orthopedic oncologist: an orthopedic surgeon that specializes in treating cancer of the bones and joints
- A radiation oncologist: a doctor who uses radiation to treat cancer
- A medical oncologist: a doctor who uses chemotherapy and other medicines to treat cancer
Many other specialists may be involved in your care as well, including nurse practitioners, nurses, psychologists, social workers, rehabilitation specialists, and other health professionals.
The main types of treatment for bone cancer are:
- Surgery
- Radiation
- Chemotherapy
- Targeted therapy
Often, more than one type of treatment is used.
Chondrosarcomas
After a biopsy confirms the diagnosis, surgery is done to remove the tumor. Again, it is important that the biopsy be done by the same surgeon who will remove the tumor. For a low-grade chondrosarcoma in an arm or leg, curettage with cryotherapy is an option. If the tumor is high-grade, limb-sparing surgery will be done if possible. Sometimes amputation is needed to completely remove the cancer. If the chondrosarcoma has spread to the lung and there are only a few metastases, they may be removed surgically.
Chondrosarcomas in the skull are hard to treat. Complete surgical removal is difficult, and may cause serious side effects. Some low-grade tumors are treated with curettage and cryosurgery.
Sometimes the patient is treated with radiation therapy. Since chondrosarcomas are resistant to radiation, high doses are required. Proton-beam radiation works well for these tumors.
Chemotherapy (chemo) is not often used to treat chondrosarcoma, because most types of chondrosarcoma are resistant to chemo. Chemo can be used to treat some special types of chondrosarcoma. For example, dedifferentiated chondrosarcoma may be treated like osteosarcoma, with chemo followed by surgery and then more chemo. Patients with mesenchymal chondrosarcomas also get chemo before surgery. These tumors are treated the same as Ewing tumors or soft tissue sarcomas.
Malignant fibrous histiocytomas
Malignant fibrous histiocytomas is treated the same way osteosarcoma is treated. Often the patient is first treated with chemotherapy to shrink the tumor. Then the tumor and some surrounding normal tissue is removed (wide-excision). After resection, the bone may be reconstructed with a bone graft or a prosthesis (metallic rod). Amputation is rarely needed. In some cases, chemotherapy is also given after surgery.
Fibrosarcomas
Surgery is the main treatment for this kind of cancer, with the goal of removing the tumor and a margin of surrounding normal bone. Radiation is sometimes given after surgery if the doctor suspects that some cancer has been left behind. Radiation therapy is sometimes used instead of surgery if the tumor cannot be removed completely. Radiation is also used if a fibrosarcoma returns after surgery.
Giant cell tumors of bone
These are treated mainly with surgery. Different surgeries are used, depending on the size and location of the tumor. One option is wide-excision. This often means removing the part of the bone containing the tumor, and replacing it with a bone graft or prosthesis (such as a metal rod). If this operation can be done without seriously affecting the movement of the limb or without causing serious damage to nearby tissues, this approach provides a good likelihood of success.
Another option is curettage followed by cryosurgery. The defect (hole) in the bone can then be filled in with bone cement or a bone graft.
Radiation therapy may sometimes be used for giant cell tumors in bones where surgery may be difficult to perform without damaging nearby sensitive tissues − such as the skull and the spine. Radiation is not often used to treat giant cell tumors because if the tumor is not killed completely it may increase the chance that it comes back in the malignant form.
Amputation is rarely needed to treat a giant cell tumor.
If a giant cell bone tumor spreads to other organs, the lungs are most commonly affected. If there are only a few metastatic tumors in the lungs, it may be possible to remove them surgically. Metastases that can’t be removed can be treated with radiation or with the drug denosumab (Xgeva).
Chordomas
This primary tumor of bone most often occurs in the base of the skull or the bones of the spine. The best treatment is a wide excision to remove the tumor completely with some nearby normal tissue. This is not always possible because the spinal cord and nerves nearby may be involved. Still, as much of the tumor as possible will be removed.
Radiation is often given after surgery to lower the chance that the tumor will grow back. Proton-beam radiation, either alone or with intensity-modulated radiation therapy, is often used.
Imatinib (Gleevec) is often used for a chordoma that has spread widely. It may rarely shrink the tumors, but often can stop them from growing for a while. Studies are looking at adding other drugs to imatinib when it stops working. Chemo may be tried as well, but so far it hasn’t worked well by itself. Chordomas can come back, even 10 or more years after treatment, so long-term follow-up is important.
- Malawer MM, Helman LJ, O’Sullivan B. Sarcomas of bone. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Vol. 2. 7th ed. Philadelphia: Lippincott Williams and Wilkins, 2004[↩]
- https://cancerstatisticscenter.cancer.org[↩]
- What Are the Survival Rates for Osteosarcoma? https://www.cancer.org/cancer/osteosarcoma/detection-diagnosis-staging/survival-rates.html[↩][↩]