Contents
Cyclophosphamide
Cyclophosphamide also called Cytoxan or CTX is a drug used to treat many types of cancer and nephrotic syndrome (a certain type of kidney disease) in children. Cyclophosphamide damages the cell’s DNA and may kill cancer cells. Cyclophosphamide is used alone or in combination with other medications to treat Hodgkin’s lymphoma (Hodgkin’s disease) and non-Hodgkin’s lymphoma (types of cancer that begin in a type of white blood cells that normally fights infection); cutaneous T-cell lymphoma (CTCL, a group of cancers of the immune system that first appear as skin rashes); multiple myeloma (a type of cancer of the bone marrow); certain types of leukemia (cancer of the white blood cells), including chronic lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML), acute myeloid leukemia (AML, ANLL), and acute lymphoblastic leukemia (ALL); certain type of lung cancer (small cell lung cancer; SCLC); rhabdomyosarcoma (a type of cancer of the muscles) and Ewing’s sarcoma (a type of bone cancer) in children. Cyclophosphamide is also used to treat retinoblastoma (cancer in the eye), neuroblastoma (a cancer that begins in nerve cells and occurs mainly in children), ovarian cancer (cancer that begins in the female reproductive organs where eggs are formed), and breast cancer. When cyclophosphamide is used to treat cancer, it works by slowing or stopping the growth of cancer cells in your body.
Cyclophosphamide is also a potent immunosuppressive drug that works by suppressing your body’s immune system that is used in the treatment of severe forms of vasculitis, multiple sclerosis and nephrotic syndrome (a disease that is caused by damage to the kidneys), as well as other autoimmune diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus). Cyclophosphamide is also occasionally used in the prevention of rejection after organ transplantation 1, 2.
Cyclophosphamide comes as a tablets or capsules of 25 and 50 mg and as a powder or in liquid solution for intravenous infusion given every few weeks. The specific dosing used depends on several factors, including your age, your weight, how well your body responds to it, and the type of cancer or condition you have. The recommended cyclophosphamide dosage varies from 1 to 5 mg/kg per day with the patient age, body weight, mode of administration and disease entity.
When oral cyclophosphamide is used, it is important for patients to take cyclophosphamide early in the morning with a large glass of water, and to stay well hydrated during the day. Swallow the tablets whole; do not split, chew, or crush them. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take cyclophosphamide exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
Cyclophosphamide injection comes as a powder to be added to fluid and injected intravenously (into a vein) by a doctor or nurse in a medical office or hospital outpatient clinic. It may also be injected intramuscularly (into a muscle), intraperitoneally (into the abdominal cavity), or intrapleurally (into the chest cavity).
Your doctor may need to delay your treatment or adjust your dose of cyclophosphamide depending on your response to treatment and any side effects that you experience. Talk to your doctor about how you are feeling during your treatment. Do not stop taking cyclophosphamide without talking to your doctor.
Cyclophosphamide is a strong immunosuppressant and puts patients at risk of infections. It is extremely important that you report any signs of infection while using cyclophosphamide. Doctors often need to prescribe an antibiotic (Bactrim) to prevent fungal pneumonia in patients taking cyclophosphamide. Patients need to have frequent lab testing to monitor blood counts, kidney function, and urine for the presence of blood while taking cyclophosphamide. Because cyclophosphamide can cause injury to your bladder, it is important for you to drink plenty of fluids while using cyclophosphamide.
All immunosuppressants require regular monitoring in the form of blood tests, in-person assessments, and vigilance for signs of infection. The monitoring of complete blood counts in patients is an essential part of the management of cyclophosphamide toxicity. As cyclophosphamide can cause bone marrow suppression also known as myelosuppression, it should not be used in patients with lab values of neutrophils of 1500/mm³ or less and platelets less than 50,000/mm³. Complete blood count values should be monitored in addition to the administration of granulocyte colony stimulating factor (G-CSF) to reduce the effects of neutropenia (abnormally low count of neutrophils, a type of white blood cell) and infection risk 2, 3, 4. Before the induction of cyclophosphamide treatment, any urinary obstructions should be corrected or excluded. Urinalysis is also a recommendation to evaluate for the presence of blood in urine (hematuria), protein in urine (proteinuria) or bacterial infections. Patients also require monitoring for signs and symptoms of cardiotoxicity (heart muslcle damage due to side effects of cyclophosphamide), pulmonary toxicity (lung damage due to side effects of cyclophosphamide), and history of pre-existing heart disease. These tests may include tests for cholesterol, lipids, and triglycerides. The gold-standard test is generally considered to be an angiogram taken during cardiac catheterization or non-invasively via computed tomography 5, 6, 7, 8, 9.
Cyclophosphamide common side effects of include hair loss (alopecia), nausea, vomiting, diarrhea, gastrointestinal upset, cystitis, oral ulcers and bone marrow suppression. Rare, but potentially severe adverse events include severe neutropenia, sepsis, heart toxicity (cardiotoxicity), hemorrhagic cystitis, embryo-fetal toxicity and secondary cancers. Patients experiencing severe symptoms of cyclophosphamide toxicity may be taken off the medication or have their dose reduced by their physician 6, 10, 1, 4.
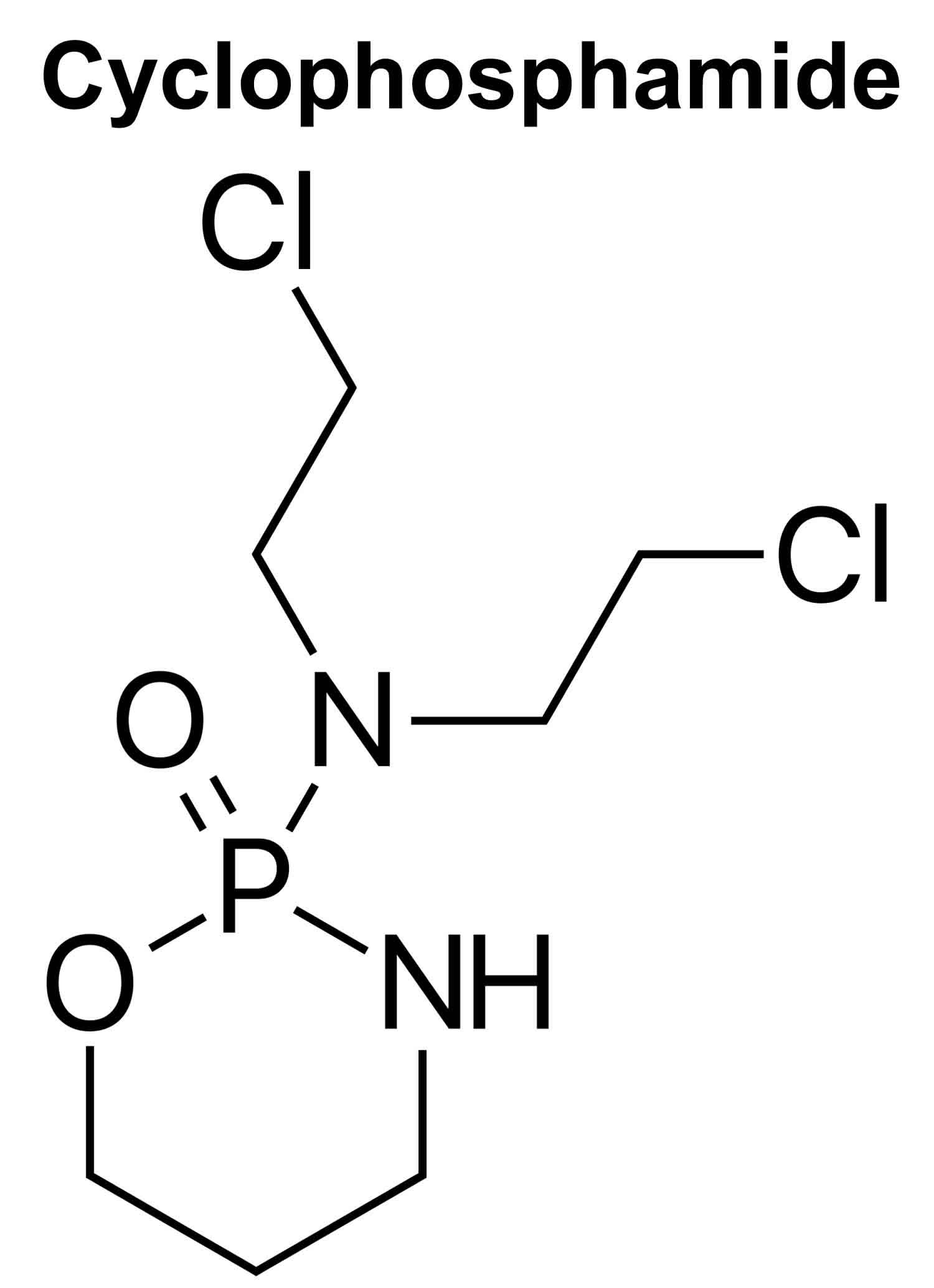
Figure 1. Cyclophosphamide chemical structure
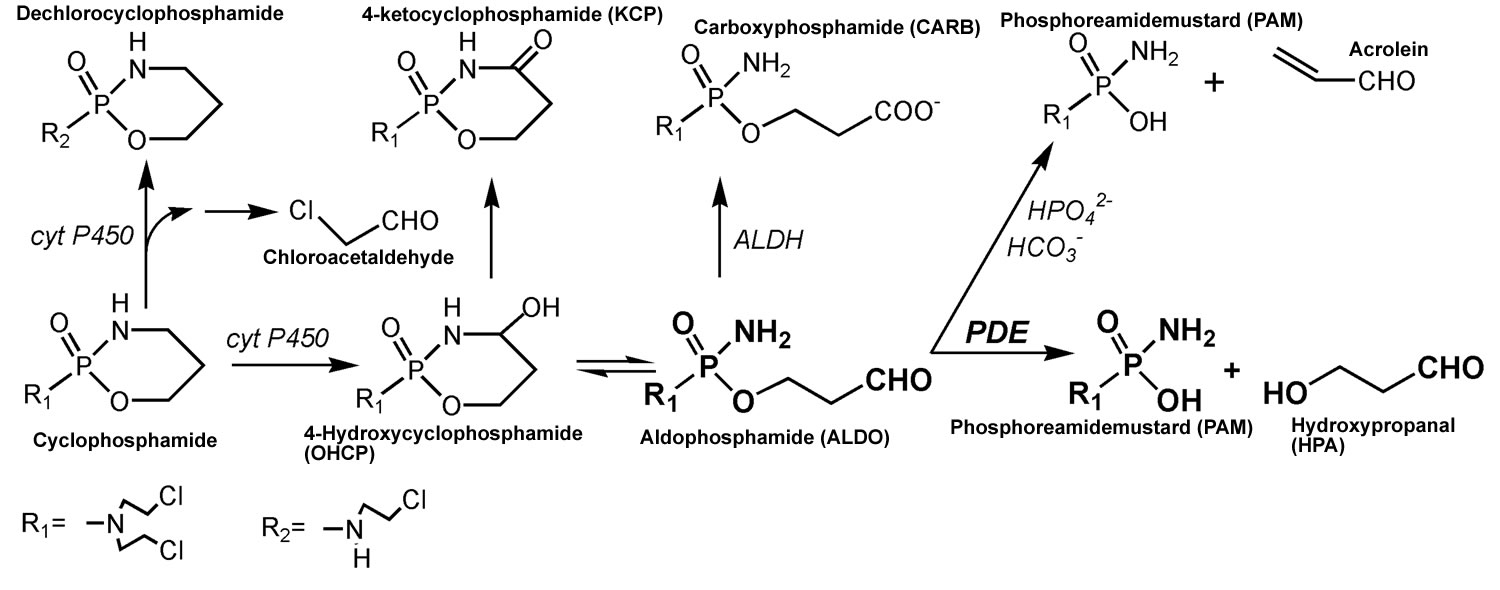
Figure 2. Cyclophosphamide metabolic pathways
Footnotes: Cyclophosphamide metabolic pathways. After the hydroxylation of cyclophosphamide by hepatic P450 enzymes, the resulting 4-hydroxycyclophosphamide (OHCP) forms an equilibrium with his tautomeric aldehyde aldophosphamide (ALDO). 4-hydroxycyclophosphamide (OHCP) and aldophosphamide (ALDO) are not cytotoxic. In vivo the tautomers 4-hydroxycyclophosphamide (OHCP) and aldophosphamide (ALDO) are irreversibly inactivated by liver enzymes. 4-hydroxycyclophosphamide (OHCP) is converted by enzymatic oxidation into the non-cytotoxic 4-ketocyclophosphamide (KCP), and aldophosphamide (ALDO) is detoxified by aldehyde dehydrogenases into the cytotoxically inactive carboxyphosphamide (CARB). In cell culture experiments the part of aldophosphamide (ALDO) that has not been detoxified to carboxyphosphamide (CARB) is decomposed by β elimination of acrolein to phosphoreamidemustard (PAM). This reaction is catalyzed by secondary phosphate ions and to a lesser extent by bicarbonate ions 11. In vivo, however, aldophosphamide (ALDO) is enzymatically decomposed by phosphodiesterases (PDE) to hydroxypropanal (HPA) and phosphoreamidemustard (PAM) 12. Phosphoreamidemustard (PAM) is the alkylating moiety which is thought to unfold cell toxicity by DNA alkylation. A small part of cyclophosphamide is converted to dechlorocyclophosphamide by side-chain hydroxylation. Toxic chloroacetaldehyde is produced as a by-product.
[Source 13 ]Cyclophosphamide special precautions
Before taking cyclophosphamide:
- tell your doctor and pharmacist if you are allergic to cyclophosphamide, other alkylating agents such as bendamustine (Treanda), busulfan (Myerlan, Busulfex), carmustine (BiCNU, Gliadel Wafer), chlorambucil (Leukeran), ifosfamide (Ifex), lomustine (CeeNU), melphalan (Alkeran), procarbazine (Mutalane), or temozolomide (Temodar), any other medications, or any of the ingredients in cyclophosphamide tablets. Ask your pharmacist for a list of the ingredients.
- tell your doctor and pharmacist what other prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention any of the following: allopurinol (Zyloprim), cortisone acetate, doxorubicin (Adriamycin, Doxil), hydrocortisone (Cortef), or phenobarbital (Luminal Sodium). Your doctor may need to change the doses of your medications or monitor you carefully for side effects. Many other medications may also interact with cyclophosphamide, so be sure to tell your doctor about all the medications you are taking, even those that do not appear on this list.
- tell your doctor if you have previously received treatment with other chemotherapy medications or if you have had x-rays recently. Also tell your doctor if you have or have ever had kidney or liver disease.
- you should know that cyclophosphamide may interfere with the normal menstrual cycle (period) in women and may stop sperm production in men. Cyclophosphamide may cause permanent infertility (difficulty becoming pregnant); however, you should not assume that you cannot get pregnant or that you cannot get someone else pregnant. Women who are pregnant or breast-feeding should tell their doctors before they begin taking this drug. You should not plan to have children while receiving chemotherapy or for a while after treatments. Talk to your doctor for further details. Use a reliable method of birth control to prevent pregnancy. Cyclophosphamide may harm the fetus.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking cyclophosphamide.
Cyclophosphamide contraindications
As with any drug, the use of cyclophosphamide is not recommended in patients with allergies or hypersensitivity reactions to cyclophosphamide or any of its metabolites. Deaths from anaphylactic reactions, as well as adverse interactions with other chemotherapy drugs like busulfan or chlorambucil, have been reported. Patients with urinary flow obstructive conditions should not take cyclophosphamide due to an increased risk of developing hemorrhagic clot retention 6, 14, 15, 9.
Pregnant or nursing women should not take cyclophosphamide as the use of cyclophosphamide has been associated with embryo-fetal toxicity. Cyclophosphamide is present in breast milk, and exposure may cause congenital disabilities, delayed development, and fetal death. It is a strong recommendation that female patients receiving treatment with cyclophosphamide avoid pregnancy during treatment and use contraceptive measures during treatment and up to a full year following completion of treatment 6, 16, 7.
Cyclophosphamide drug interactions
Tell your doctor about all your current medicines. Many drugs can affect cyclophosphamide, especially:
- medicine to prevent organ transplant rejection
- medicine to treat an infection
- medicine to treat multiple sclerosis, psoriasis, rheumatoid arthritis, or other autoimmune disorders
- other cancer medicine (especially tamoxifen).
This list is not complete and many other drugs may affect cyclophosphamide. This includes prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible drug interactions are listed here.
Cyclophosphamide disease interactions
There are 6 disease interactions with cyclophosphamide which include:
- myelosuppression
- urinary tract obstruction
- heart disease/cardiotoxicity
- liver dysfunction
- lung impairment
- kidney dysfunction
Cyclophosphamide mechanism of action
Cyclophosphamide is a synthetic nitrogen mustard-like alkylating agent that is widely used in the therapy of cancer and in severe forms of autoimmune disease 17. Cyclophosphamide is an inactive prodrug that requires activation in the liver by cytochrome P450-mediated microsomal oxidation to form its active form 4-hydroxycyclophosphamide (OHCP) which act by modifying and cross linking purine bases in DNA, thus inhibiting DNA, RNA and protein synthesis and causing cell death in rapidly dividing cells 13, 18, 15, 19, 20, 21, 22, 23. Cyclophosphamide is not cell-cycle phase-specific 19.
The majority of the anti-cancer effects of cyclophosphamide are due to the phosphoramide mustard formed from the metabolism of cyclophosphamide by liver enzymes like cytochrome P-450 19. Liver enzymes first convert cyclophosphamide to hydroxycyclophosphamide and then subsequently metabolized to aldophosphamide. Aldophosphamide is cleaved to the active alkylating agent phosphoramide mustard and acrolein 19. The phosphoramide metabolite forms cross-linkages within and between adjacent DNA strands at the guanine N-7 position. These modifications are permanent and eventually lead to programmed cell death 2. Acrolein has no antitumor activity but is the principal-agent responsible for the adverse effect of hemorrhagic cystitis.
In addition to antimitotic and anti-cancer effects, cyclophosphamide has immunosuppressive effects and selectivity for T cells 19. High-dose cyclophosphamide is used in eradication therapy of malignant hematopoietic cells, while lower dosages have shown merit for use in selective immunomodulation of regulatory T cells 19. Cyclophosphamide decreases the secretion of interferon‐gamma (IFN‐γ) and interleukin 12 (IL-12) while increasing the secretion of Th2 cytokines like interleukin 4 (IL-4) and interleukin 10 (IL-10) in the CSF and peripheral blood 5, 24. Due to these effects, cyclophosphamide is considered as a valuable addition to tumor vaccination protocols, post-transplant alloreactivity management, and the treatment of immune-mediated conditions and some forms of vasculitis 16, 18, 15. While the precise mechanism with which cyclophosphamide exerts its immunomodulatory effects are not completely clear, several studies have suggested a few probable modes of action. These include the elimination of regulatory T cells in naïve or malignant host cells, the induction of T cell growth factors like type 1 interferons, and preconditioning host cells for donor T cells, thus reducing alloreactivity 5, 24, 16.
Cyclophosphamide uses
Cyclophosphamide was approved for use in the United States in 1959 and its current indications include treatment of breast, head, neck, lung, cervix, testis and ovarian cancer, acute and chronic lymphocytic leukemia, Hodgkin’s and non-Hodgkin’s lymphoma, malignant histiocytosis, multiple myeloma, soft tissue sarcoma, mycosis fungoides, neuroblastoma, and retinoblastoma. Cyclophosphamide is also used in severe autoimmune disorders including minimal change nephrotic syndrome not responsive to conventional therapy. Cyclophosphamide is also occasionally used in the prevention of rejection after organ transplantation.
Cancer
In the treatment of a variety of cancers, doctors use cyclophosphamide in combination with other chemotherapeutic agents. Cyclophosphamide is often included in standard treatment regimens for Hodgkin and non-Hodgkin lymphoma as a component of CHOP along with hydroxydaunorubicin, oncovin, and prednisone. If the cancer has B-cell involvement, rituximab, and an anti-CD-20 monoclonal antibody, it is added to the CHOP regimen (called R-CHOP) 5, 25, 26. Cyclophosphamide has also been used in combination with corticosteroids in the treatment of multiple myeloma, chronic lymphocytic leukemia (CLL), cutaneous T-cell lymphoma, neuroblastoma, retinoblastoma, small cell lung cancer, and various types of sarcoma. As cyclophosphamide exerts immunosuppressive properties in addition to its anti-neoplastic effects, it is indicated in the management of other immune conditions such as severe multiple sclerosis and nephrotic syndrome 14, 27.
Cyclophosphamide is FDA approved to be used alone or with other drugs to treat 18, 15:
- Acute lymphoblastic leukemia (ALL) in children.
- Acute monocytic leukemia.
- Acute myeloid leukemia (AML).
- Breast cancer.
- Chronic granulocytic leukemia.
- Chronic lymphocytic leukemia (CLL).
- Chronic myelogenous leukemia (CML).
- Hodgkin lymphoma. It is used in patients with stage III or stage IV disease.
- Multiple myeloma.
- Mycosis fungoides (a type of cutaneous T-cell lymphoma) that is advanced.
- Neuroblastoma that is disseminated.
- Non-Hodgkin lymphoma (NHL). It is used in patients with stage III or stage IV NHL, including the following types:
- Lymphocytic lymphoma (nodular or diffuse).
- Mixed-cell type lymphoma.
- Histiocytic lymphoma.
- Burkitt lymphoma.
- Ovarian cancer.
- Retinoblastoma.
Cyclophosphamide is also being studied in the treatment of other types of cancer.
Immunosuppressive agent
Cyclophosphamide is a strong immunosuppressant, cyclophosphamide multiple studies have found cyclophosphamide useful in the treatment of autoimmune diseases such as multiple sclerosis. Cyclophosphamide has also been prescribed pretransplant as an immunosuppressant to prevent transplant rejection and graft-vs-host complications 1, 2.
Cyclophosphamide dosage
Cyclophosphamide is often orally administered, and the length of treatment and dosage depends upon individual factors such as metabolism, drug interactions, body mass, and age of the patient. There are standard guidelines available for the appropriate dosage and indications depending upon the goal of treatment. The general recommendation for patients without hematological deficiencies is an induction dose of IV cyclophosphamide of 40 to 50 mg/kg divided into dosages over 2 to 5 days. The maintenance dosage is dependent upon individual tolerance but is generally 1 to 5 mg/kg taken orally every day. A daily oral dose of 2 to 3 mg/kg is recommended for children. Cyclophosphamide should not be taken for more than 90 days to avoid hemorrhagic cystitis and sterility risk in males 19. The recommendation for the oral cyclophosphamide formulation is that it be taken at the same time every day, usually in the morning, with liquid. If gastrointestinal irritation is noted, the tablet can be taken with food 14, 2. While taking cyclophosphamide, an adequate intake of fluids is necessary to precipitate diuresis (production of urine) to reduce the risk of urinary tract side effects. To avoid the side effect of hemorrhagic cystitis, maintain a minimum urinary output of 100 mL/hr during therapy 26.
Mesna is a prophylactic cytoprotective drug administered orally or by IV to reduce the effects of hemorrhagic cystitis in patients treated with high-dose cyclophosphamide or ifosfamide. The recommended dosage of Mesna is generally as a fractionated dosing schedule of three IV bolus injections of 240 mg per meter cubed every 4 hours 1, 2.
What should I do if I forget a dose?
Take the missed dose as soon as you remember it. However, if it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
Cyclophosphamide side effects
Cyclophosphamide may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- nausea
- vomiting
- loss of appetite or weight
- abdominal pain
- diarrhea
- hair loss
- sores on the mouth or tongue
- changes in skin color
- changes in color or growth of finger or toe nails
Some side effects can be serious. If you experience any of these symptoms, see your doctor immediately:
- sore throat, fever, chills, or other signs of infection
- poor or slow wound healing
- unusual bruising or bleeding
- black, tarry stools
- painful urination or red urine
- rash
- hives
- itching
- difficulty breathing or swallowing
- shortness of breath
- cough
- swelling in the legs, ankles, or feet
- chest pain
- yellowing of the skin or eyes
Cyclophosphamide may increase the risk that you will develop other cancers. Talk to your doctor about the risks of taking cyclophosphamide.
Cyclophosphamide may cause other side effects. Call your doctor if you have any unusual problems while taking cyclophosphamide.
Cyclophosphamide overdose
Cyclophosphamide overdose may include the following signs and symptoms:
- black, tarry stools
- red urine
- unusual bruising or bleeding
- unusual tiredness or weakness
- sore throat, cough, fever, or other signs of infection
- swelling in the legs, ankles, or feet
- chest pain.
As an immunosuppressive agent, cyclophosphamide overdose correlates with the development of leukopenia (low white blood cell count), thrombocytopenia (low blood platelet count), and anemia (low red blood cell count or low hemoglobin concentration). These conditions may lead to the occurrence of recurrent infections and may interfere with wound healing. Other symptoms of cyclophosphamide toxicity include GI disturbances, alopecia, hemorrhagic cystitis, renal tubular necrosis, infertility, pulmonary fibrosis, and cardiotoxicity. The manifestation of cyclophosphamide toxicity becomes aggravated by concurrent use of phenobarbital, other myelosuppressives, radiation, and succinylcholine 28, 29.
To minimize the negative effects of cyclophosphamide toxicity, recommendations include monitoring the patient’s complete blood counts and modification of treatment as needed. Patients should maintain adequate hydration, and Mesna can be prescribed as prophylaxis against the development of hemorrhagic cystitis. If overdose is suspected, the patient or caregiver must seek emergency medical attention immediately 29.
- Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009 Nov;6(11):638-47. doi: 10.1038/nrclinonc.2009.146[↩][↩][↩][↩]
- Colvin OM. An overview of cyclophosphamide development and clinical applications. Curr Pharm Des. 1999 Aug;5(8):555-60.[↩][↩][↩][↩][↩][↩]
- Campagne O, Zhong B, Nair S, Lin T, Huang J, Onar-Thomas A, Robinson G, Gajjar A, Stewart CF. Exposure-Toxicity Association of Cyclophosphamide and Its Metabolites in Infants and Young Children with Primary Brain Tumors: Implications for Dosing. Clin Cancer Res. 2020 Apr 1;26(7):1563-1573. doi: 10.1158/1078-0432.CCR-19-2685[↩]
- Gregory SA, Trümper L. Chemotherapy dose intensity in non-Hodgkin’s lymphoma: is dose intensity an emerging paradigm for better outcomes? Ann Oncol. 2005 Sep;16(9):1413-24. doi: 10.1093/annonc/mdi264[↩][↩]
- Chatelanat O, Van Delden C, Adler D, Guerne PA, Nendaz M, Serratrice J. Facteurs de risque et prophylaxie de la pneumonie à Pneumocystis jirovecii chez les patients non VIH [Risk factors and prophylaxis of Pneumocystis jirovecii pneumonia in HIV-negative patients]. Rev Med Suisse. 2018 Oct 17;14(623):1829-1833. French.[↩][↩][↩][↩]
- Dan D, Fischer R, Adler S, Förger F, Villiger PM. Cyclophosphamide: As bad as its reputation? Long-term single centre experience of cyclophosphamide side effects in the treatment of systemic autoimmune diseases. Swiss Med Wkly. 2014 Oct 23;144:w14030. doi: 10.4414/smw.2014.14030[↩][↩][↩][↩]
- Martin F, Lauwerys B, Lefèbvre C, Devogelaer JP, Houssiau FA. Side-effects of intravenous cyclophosphamide pulse therapy. Lupus. 1997;6(3):254-7. doi: 10.1177/096120339700600307[↩][↩]
- Hirshman NA, Hughes FM Jr, Jin H, Harrison WT, White SW, Doan I, Harper SN, Leidig PD, Purves JT. Cyclophosphamide-induced cystitis results in NLRP3-mediated inflammation in the hippocampus and symptoms of depression in rats. Am J Physiol Renal Physiol. 2020 Feb 1;318(2):F354-F362. doi: 10.1152/ajprenal.00408.2019[↩]
- Tabchi S, Nair R, Kunacheewa C, Patel KK, Lee HC, Thomas SK, Amini B, Ahmed S, Mehta RS, Bashir Q, Qazilbash MH, Weber DM, Orlowski RZ, Alexanian R, Feng L, Manasanch EE. Retrospective Review of the Use of High-Dose Cyclophosphamide, Bortezomib, Doxorubicin, and Dexamethasone for the Treatment of Multiple Myeloma and Plasma Cell Leukemia. Clin Lymphoma Myeloma Leuk. 2019 Sep;19(9):560-569. doi: 10.1016/j.clml.2019.05.001[↩][↩]
- Evens AM, Carter J, Loh KP, David KA. Management of older Hodgkin lymphoma patients. Hematology Am Soc Hematol Educ Program. 2019 Dec 6;2019(1):233-242. doi: 10.1182/hematology.2019000028[↩]
- Low, J.E.; Borch, R.F.; Sladek, N.E. Conversion of 4-hydroperoxycyclophosphamide and 4-hydroxycyclophosphamide to phosphoramide mustard and acrolein mediated by bifunctional catalysis. Cancer Res. 1982, 42, 830–837[↩]
- Voelcker, G. Enzyme Catalyzed Decomposition of 4-Hydroxycyclophosphamide. Open Conf. Proceeding J. 2017, 8, 44–51.[↩]
- Voelcker G. The Mechanism of Action of Cyclophosphamide and Its Consequences for the Development of a New Generation of Oxazaphosphorine Cytostatics. Scientia Pharmaceutica. 2020; 88(4):42. https://doi.org/10.3390/scipharm88040042[↩][↩]
- Atilla E, Ateş C, Uslu A, Ataca Atilla P, Dolapçı I, Tekeli A, Topçuoğlu P. Prospective Analysis of Hemorrhagic Cystitis and BK Viremia in Allogeneic Hematopoietic Stem Cell Transplantation. Turk J Haematol. 2020 Aug 28;37(3):186-192. doi: 10.4274/tjh.galenos.2019.2019.0296[↩][↩][↩]
- Korkmaz A, Topal T, Oter S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol Toxicol. 2007 Sep;23(5):303-12. doi: 10.1007/s10565-006-0078-0[↩][↩][↩][↩]
- Cavallasca JA, Costa CA, Maliandi Mdel R, Contini LE, Fernandez de Carrera E, Musuruana JL. Severe infections in patients with autoimmune diseases treated with cyclophosphamide. Reumatol Clin. 2015 Jul-Aug;11(4):221-3. English, Spanish. doi: 10.1016/j.reuma.2014.09.003[↩][↩][↩]
- Colvin M. Alkylating Agents. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003. Available from: https://www.ncbi.nlm.nih.gov/books/NBK12772[↩]
- Mills KA, Chess-Williams R, McDermott C. Novel insights into the mechanism of cyclophosphamide-induced bladder toxicity: chloroacetaldehyde’s contribution to urothelial dysfunction in vitro. Arch Toxicol. 2019 Nov;93(11):3291-3303. doi: 10.1007/s00204-019-02589-1[↩][↩][↩]
- Ogino MH, Tadi P. Cyclophosphamide. [Updated 2023 Jul 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553087[↩][↩][↩][↩][↩][↩][↩]
- Colvin M, Chabner BA. Alkylating agents in cancer chemotherapy: principles and practice. In: Chabner BA, Collins JM, editors. Philadelphia: Lippincott; 1990. p. 276–313.[↩]
- Fenselau C, Kan MN, Rao SS, Myles A, Friedman OM, Colvin M. Identification of aldophosphamide as a metabolite of cyclophosphamide in vitro and in vivo in humans. Cancer Res. 1977 Aug;37(8 Pt 1):2538-43.[↩]
- Zon G, Ludeman SM, Brandt JA, Boyd VL, Ozkan G, Egan W, Shao KL. NMR spectroscopic studies of intermediary metabolites of cyclophosphamide. A comprehensive kinetic analysis of the interconversion of cis- and trans-4-hydroxycyclophosphamide with aldophosphamide and the concomitant partitioning of aldophosphamide between irreversible fragmentation and reversible conjugation pathways. J Med Chem. 1984 Apr;27(4):466-85. doi: 10.1021/jm00370a008[↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Cyclophosphamide. [Updated 2017 Nov 5]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548059[↩]
- Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol. 2016 Oct;78(4):661-71. doi: 10.1007/s00280-016-3152-1[↩][↩]
- Nishimura M, Onoe T, Sakai H, Arase M, Watanabe S, Soyama M, Hashimoto K, Miki M, Tane K, Hirokaga K, Takao S, Matsumoto K. Safety and Relative Dose Intensity of Dose-dense Doxorubicin and Cyclophosphamide Followed by Dose-dense Paclitaxel. Anticancer Res. 2019 Aug;39(8):4379-4383. doi: 10.21873/anticanres.13607[↩]
- Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, Reiser M, Metzner B, Harder H, Hegewisch-Becker S, Fischer T, Kropff M, Reis HE, Freund M, Wörmann B, Fuchs R, Planker M, Schimke J, Eimermacher H, Trümper L, Aldaoud A, Parwaresch R, Unterhalt M. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005 Dec 1;106(12):3725-32. doi: 10.1182/blood-2005-01-0016[↩][↩]
- Siddhartha G, Vijay P. R-CHOP versus R-CVP in the treatment of follicular lymphoma: a meta-analysis and critical appraisal of current literature. J Hematol Oncol. 2009 Mar 24;2:14. doi: 10.1186/1756-8722-2-14[↩]
- Fraiser LH, Kanekal S, Kehrer JP. Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs. 1991 Nov;42(5):781-95. doi: 10.2165/00003495-199142050-00005[↩]
- Ahmed AR, Hombal SM. Cyclophosphamide (Cytoxan). A review on relevant pharmacology and clinical uses. J Am Acad Dermatol. 1984 Dec;11(6):1115-26. doi: 10.1016/s0190-9622(84)80193-0[↩][↩]