Contents
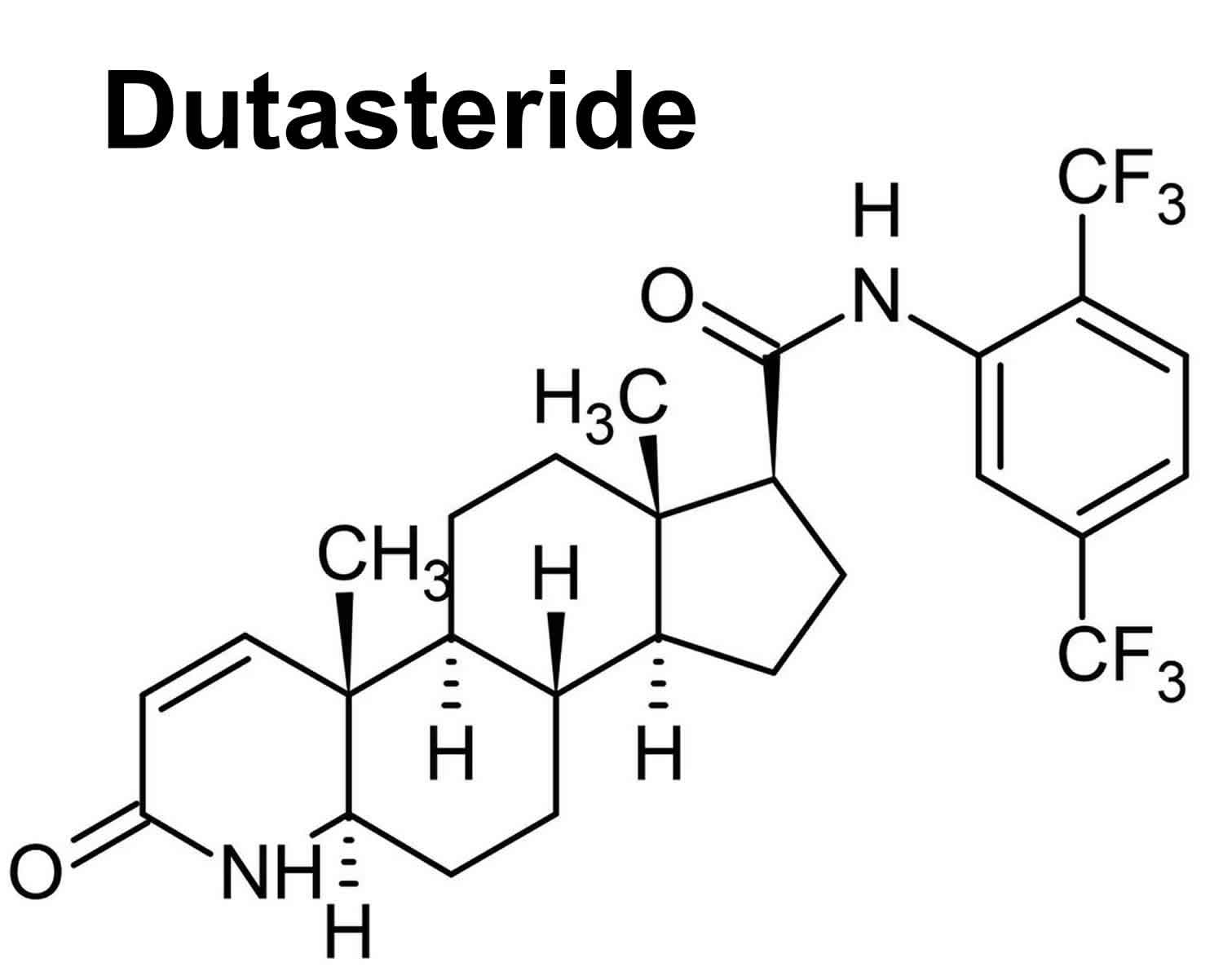
Dutasteride
Dutasteride (Avodart) is an 5-alpha-reductase inhibitor (similar to finasteride) that is used to treat benign prostatic hyperplasia (BPH; enlargement of the prostate gland) and male pattern hair loss (androgenic alopecia) 1, 2, 3, 4. Dutasteride works by blocking the production of a natural substance that enlarges the prostate in benign prostatic hyperplasia (BPH) and in male pattern hair loss (androgenic alopecia) dutasteride blocks the 5-alpha reductase enzyme responsible for regulating the conversion of testosterone to dihydrotestosterone (DHT). By reducing dihydrotestosterone (DHT) levels in your scalp, dutasteride decreases dihydrotestosterone (DHT) effects on your hair follicles, reversing the process of hair loss in men. The use of oral 5-alpha-reductase inhibitors (dutasteride and finasteride) in women with female pattern hair loss (FPHL) is not approved by the FDA, and they are contraindicated for pregnant women because of their teratogenicity in the male fetus 5.
Women and children should not use dutasteride. Pregnant women or women who may become pregnant should NOT use or handle dutasteride capsules. Dutasteride can be absorbed through the skin and can cause birth defects in male fetuses. Dutasteride can cause birth defects in male fetuses (Pregnancy Category X, i.e., drugs that have such a high risk of causing permanent damage to the fetus that they should not be used in pregnancy or when there is a possibility of pregnancy).
Dutasteride is available only with your doctor’s prescription.
In men with benign prostatic hyperplasia (BPH) Dutasteride is used alone or in combination with tamsulosin (Flomax) to treat men who have symptoms of an enlarged prostate gland, which is also known as benign prostatic hyperplasia (BPH). Benign enlargement of the prostate is a problem that can occur in men as they get older. The prostate gland is located below your bladder. As the prostate gland enlarges, certain muscles in the prostate gland may become tight and get in the way of the tube that drains urine from the bladder. This can cause problems with urinating, such as a need to urinate often, a weak stream when urinating, or a feeling of not being able to empty the bladder completely. Dutasteride may reduce your chance of developing acute urinary retention (sudden inability to urinate) and may also decrease the chance that prostate surgery will be needed.
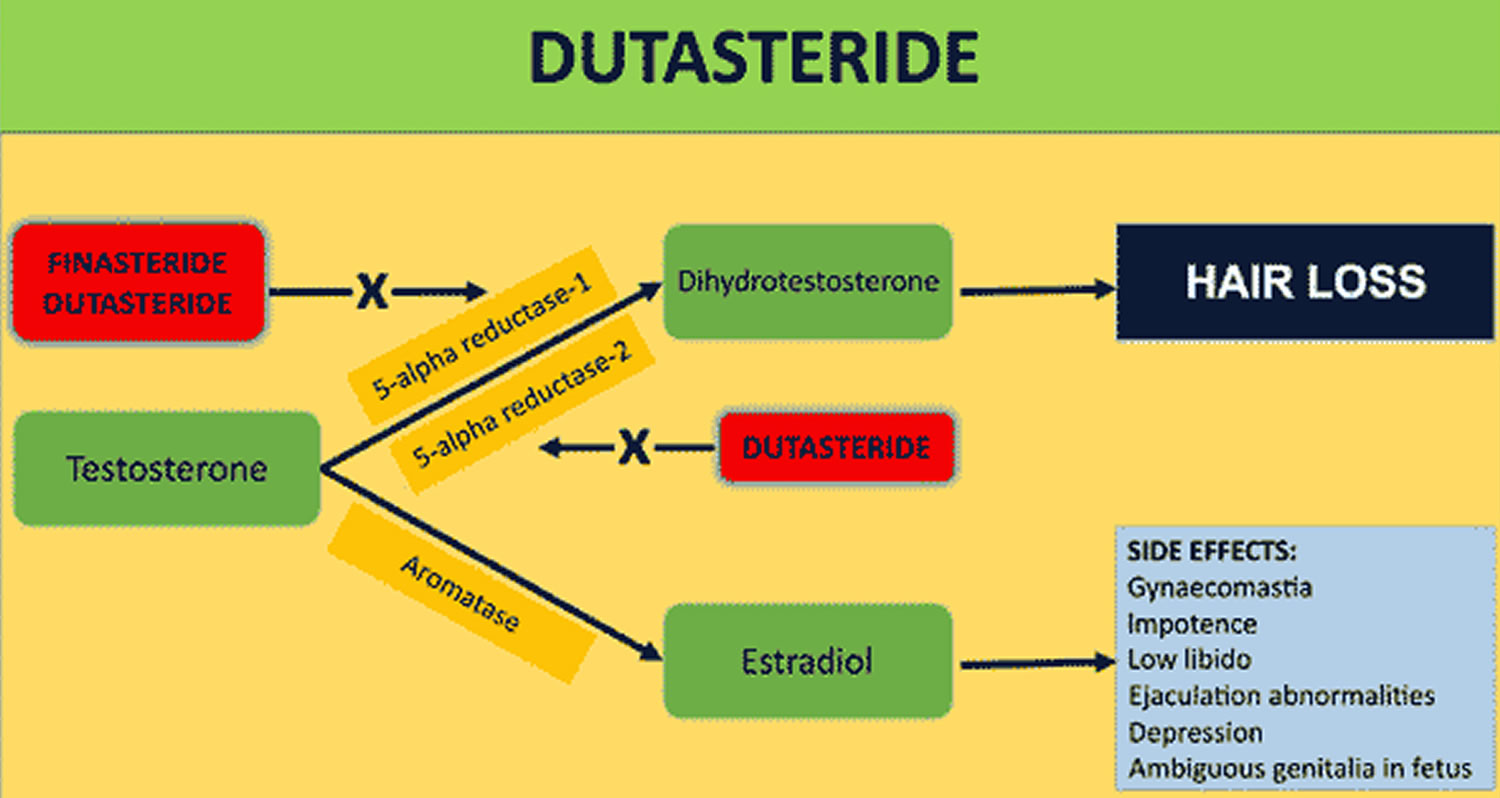
In the prostate gland Dutasteride blocks the action of an enzyme called 5-alpha-reductase. The 5-alpha-reductase enzyme changes testosterone to another hormone that causes the prostate to grow. As a result, the size of the prostate is decreased. The effect of dutasteride lasts only as long as the medicine is taken. If it is stopped, the prostate begins to grow again.
The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
Dutasteride was approved for use in the United States in 2001 and is available in 0.5 mg capsules generically and under the trade name Avodart to be taken by mouth. Swallow the capsules whole; do not open chew, or crush them. A fixed dose combination of dutasteride (0.5 mg) with tamsulosin (0.4 mg: an alpha blocker) is available under the trade name Jalyn. The recommended dose of dutasteride is 0.5 mg once daily with or without food. Take dutasteride at around the same time every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take dutasteride exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
Dutasteride is usually given long term and an effect is usually not seen until 3 to 6 months of therapy. Your symptoms may improve after you have taken dutasteride for 3 months, but it may take 6 months or longer for you to see the full benefit of dutasteride. Talk to your doctor about how you are feeling during your treatment. Dutasteride may control your symptoms but will not cure your condition. Continue to take dutasteride even if you feel well. Do not stop taking dutasteride without talking to your doctor.
Dutasteride side effects are uncommon, but include impotence and decreased libido, ejaculation disorders, overdevelopment or enlargement of the breast tissue in men (gynecomastia), dizziness and fatigue. Dutasteride also decreases serum prostate specific antigen (PSA) levels which should be monitored during therapy.
Ask your pharmacist or doctor for a copy of the manufacturer’s information for the patient.
Figure 1. Dutasteride mechanism of action
[Source 2 ]Finasteride versus Dutasteride safety profile
A study comparing the safety profile of dutasteride (0.5 mg/day) and finasteride (5 mg/day) for treating benign prostatic hyperplasia (BPH) was performed by Andriole et al 6. In the dutasteride group of 813 subjects, impotence occurred in 7%, decreased libido in 5%, and ejaculation disorder in 1% 6. Impotence was experienced by 8%, decreased libido by 6%, and ejaculation disorder by 1% in the finasteride 5mg group (n=817) 6. These differences were not considered significant and the safety profile of dutasteride was considered no different than finasteride by the authors based on their parallel group, comparator trial 6.
Kaplan et al. 7 conducted a retrospective analysis of 378 consecutive men treated at a single clinic with 5 alpha-reductase inhibitor monotherapy for lower urinary tract symptoms related to benign prostatic hyperplasia (BPH). Treatment duration was five years. Of those enrolled, 197 subjects were treated with finasteride 5 mg and 211 with dutasteride 0.5 mg 7. At 5 years, 57.4% of men in the finasteride group and 42.5% of men in the dutasteride group remained on treatment 7. Dutasteride resulted in more sexual side effects leading to discontinuation compared to finasteride, with the incidence of erectile dysfunction, ejaculatory dysfunction, and decreased libido being significantly higher in the dutasteride group (5.1%, 2.4%, 2.7%, respectively) compared with the finasteride group (2.1%, 1.8%, 1.4%, respectively) 7. Of those subjects who remained on treatment for five years, dutasteride resulted in significantly greater erectile dysfunction than finasteride. At year 5, subjects on dutasteride therapy had significantly worsened International Index of Erectile Function scores relative to baseline than did those on finasteride 7. This suggests that dutasteride may have stronger negative adverse sexual effects compared to finasteride 7.
How effective is dutasteride for hair loss in men?
In Gubelin Harcha et al 8 multicenter, randomized, double-blind, placebo-controlled study also compared dutasteride (0.02, 0.1, or 0.5 mg), finasteride (1 mg), and placebo in the treatment of androgenetic alopecia for 917 men (20-50 years old). Dutasteride 0.5mg was found to significantly increase hair growth compared to finasteride 1 mg and placebo with a similar rate of adverse side effects. Altered libido, impotence, and ejaculation disorders were listed for all groups (dutasteride 0.5mg: 4.9%, 5.4%, and 3.3%, respectively; finasteride 1mg: 6.7%, 6.1%, 3.9%, respectively; placebo: 1.7%, 3.9%, 3.3%, respectively) 8. There was no significant difference found between finasteride and dutasteride sexual side effects and additionally, a dose dependent response of sexual side effects for any of the treatment doses of dutasteride was absent 8. Sexual side effects were found to decrease over time.
In Gupta et al 9 study comparing of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia, dutasteride 0.5 mg/day is probably more effective than finasteride 5 mg/day, minoxidil 5 mg/day, finasteride 1 mg/day, followed by minoxidil 0.25 mg/d. Oral minoxidil predominantly causes hypertrichosis and cardiovascular system (CVS) symptoms/signs in a dose-dependent manner, whereas oral finasteride and dutasteride are associated with sexual dysfunction and neuropsychiatric side effects 9.
In the Olsen et al 10 androgenetic alopecia study comparing multiple doses of dutasteride (0.05 mg, 0.1 mg, 0.5 mg, 2.5 mg) versus finasteride 5 mg, dutasteride 0.5 mg was shown to be significantly superior at promoting hair growth compared to finasteride 5 mg after 24 weeks 10. Dutasteride 2.5mg was also shown to be significantly more effective than dutasteride 0.5 mg at increasing hair growth 10. Rates of decreased libido, ejaculation disorders, and impotence were 13%, 1%, and 0% for dutasteride 2.5 mg, respectively; 1%, 1%, and 0% for dutasteride 0.5mg, respectively; 4%, 3%, and 1% for finasteride 5mg, respectively; and 3%, 0%, and 5% for placebo, respectively 10. Of the 13% of subjects who reported decreased libido in the dutasteride 2.5 mg treatment group, 4 resolved during treatment, 1 within 3 weeks of stopping therapy, and 1 within 8 weeks of stopping; 1 patient had decreased libido that persisted after stopping but was considered by the patient to be unrelated to the drug 10. Based on these findings the authors suggest that while dutasteride 0.5 mg is the recommended daily dose for BPH (dutasteride is not FDA approved for androgenetic alopecia but it is prescribed for such as an off label use), dutasteride 2.5mg is significantly more effective at reducing DHT levels and thus could have a greater therapeutic impact at these levels.
Can women with hair loss use dutasteride?
The use of oral 5-alpha-reductase inhibitors (dutasteride and finasteride) in women with female pattern hair loss (FPHL) is not approved by the FDA, and they are contraindicated for pregnant women because of their teratogenicity in the male fetus 5.
Women and children should not use dutasteride. Pregnant women or women who may become pregnant should NOT use or handle dutasteride capsules. Dutasteride can be absorbed through the skin and can cause birth defects in male fetuses. Dutasteride can cause birth defects in male fetuses (Pregnancy Category X, i.e., drugs that have such a high risk of causing permanent damage to the fetus that they should not be used in pregnancy or when there is a possibility of pregnancy).
However, doctors have prescribed dutasteride off-label as a first-line treatment in post-menopausal women with female pattern hair loss (FPHL) 11, 12, 13, 8, 14. The androgen dihydrotestosterone (DHT) is thought to play a large role in inducing androgenetic alopecia and is formed from the conversion of circulating testosterone to DHT by 5-alpha reductase.
Dutasteride can reduce serum DHT levels by 90% and has been reported to treat female pattern hair loss successfully with no side effects at doses that range from 0.25 to 0.5 mg/day 10. However, Dutasteride should not be given to women of childbearing age unless they are using birth control measures due to potential feminizing effects on the male fetus or to patients with impaired liver function
A recent network meta-analysis demonstrated that the increase in total hair count at 24 weeks with 0.5 mg/day of dutasteride was more efficacious compared to that with 1 mg/day finasteride with the similar adverse events profile 15, 16. Furthermore, a Korean group analyzed the long-term effectiveness and safety profiles of finasteride and dutasteride; they reported that dutasteride-treated patients showed more significant improvement in hair growth compared to finasteride-treated patients and both medications were analogously safe (possibly with a lower incidence of sexual adverse events than previously reported; dutasteride = 1.6%, and finasteride = 1.1%) 17. Accordingly, oral 5-alpha reductase inhibitors represent the first-line treatment modality for androgenetic alopecia because of their effectiveness and tolerability, and dutasteride further widened therapeutic options 11, 17.
Are there any contraindications for dutasteride?
Dutasteride results in a decrease in dihydrotestosterone (DHT). Since DHT is an important androgen in sexual development, children and women who are pregnant or planning on getting pregnant should avoid use. Dutasteride should also be avoided in any persons who have had a hypersensitivity to this medication 18.
Does dutasteride cause any interactions with other drugs?
Using dutasteride with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Ceritinib
- Clarithromycin
- Itraconazole
Using dutasteride with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Cimetidine
- Ciprofloxacin
- Diltiazem
- Ketoconazole
- Ritonavir
- Verapamil
Other interactions
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. Discuss with your healthcare professional the use of your medicine with food, alcohol, or tobacco.
Is dutasteride safe for long-term use in men?
Although dutasteride and finasteride have been established to be effective for the treatment of benign prostatic hyperplasia (BPH) and male pattern hair loss (androgenetic alopecia) by substantially reducing the levels of dihydrotestosterone (DHT) 19, 20, it has also been documented that dutasteride and finasteride may increase the incidence of sexual dysfunction 21. Decreased libido, ejaculation disorder, and impotence are among the most commonly reported drug related side effects 22. Erectile dysfunction, or impotence, has been cited as the most common side effect in multiple studies for both finasteride 5 mg/day and dutasteride 0.5 mg/day, followed by decreased libido 6, 23, 24, 25, 26, 27. In the largest meta-analysis to date on5-alpha-reductase inhibitors, there was found to be a significantly increased risk of sexual dysfunction (156% increase) for men being treated with finasteride or dutasteride for benign prostatic hyperplasia (BPH), whereas there was not a significant association for those treated for androgenetic alopecia 21. Dutasteride 0.5 mg/day has also been shown to have a greater incidence of adverse sexual effects than finasteride 5 mg/day in the treatment of BPH 21. Unfortunately, there is no consensus regarding the relation between 5-alpha-reductase inhibitors dosage and the likelihood of sexual dysfunction and further study is needed in this area 21, 28, 29. It is based on these findings that experts recommend doctors consider and discuss the possible sexual side effects and risk of depression with their patients prior to selecting a drug therapy 3.
How does dutasteride work?
Dutasteride is the successor to finasteride acting as a second‐generation 5‐alpha‐reductase inhibitor and functioning as a selective competitive inhibitor of type 1 and type 2 isoenzymes of 5‐alpha‐reductase designed to decrease the production of dihydrotestosterone (DHT) 11, 30, 31, 4. 32. Dutasteride is reported to be three times more potent at inhibiting the type 1 5‐alpha‐reductase enzyme and 100 times more potent at inhibiting the type 2I 5‐alpha‐reductase enzyme than finasteride 33. Dutasteride comes in 2.5 and 5 mg doses, both of which have shown superior efficacy to finasteride 5 mg 10. Due to dutasteride’s large molecular size, it is difficult to formulate and deliver as a topical agent. However, its large size and fat soluble (lipophilic) nature contribute to it remaining on the scalp and preventing absorption into your body. If requested by clinicians, compounding pharmacies may formulate dutasteride topical solutions, although literature is sparse regarding its utility in treating androgenetic alopecia.
Dutasteride 0.5 mg/day can reduce dihydrotestosterone (DHT) serum levels by upwards of 90% in a dose dependent manner 10. By reducing dihydrotestosterone (DHT) levels in your scalp and your prostate gland, dutasteride decreases dihydrotestosterone (DHT) effects in your hair follicles and prostate gland, reversing the process of hair loss in men (androgenic alopecia) and reducing the size of your prostate in benign prostatic hyperplasia (BPH) 34.
Olszewska and Rudnicka 35 reported a case of a female patient with androgenetic alopecia who did not respond to minoxidil and initially benefited from finasteride. Given her persistent androgenetic alopecia, the patient was started on oral dutasteride. After 6 months of treatment, clinical and trichogram assessments revealed significant improvement in hair density 35. Several randomized, double‐blind, placebo‐controlled clinical studies have demonstrated dutasteride’s effectiveness for treating androgenetic alopecia 32, 36. Intradermal (injection into the skin) dutasteride was also reported in order to decrease the systemic side effects. Saceda‐Corralo et al. 37 administered 1 mL intradermal dutasteride 0.01% injections every 3 months for a total of three sessions to six subjects. Trichoscopy assessments revealed increased hair diameter and density, in addition to clinical improvement in androgenetic alopecia. There were no statistically significant differences in serum levels of total and free testosterone, 3 alpha androstanediol glucuronide, and dihydrotestosterone before and after treatment 37. Similar studies injecting dutasteride mesotherapy yielded promising results 38, 39, 40. Overall, oral dutasteride appears to be superior to the intradermal route. However, more studies are warranted 36.
Overall, dutasteride has shown superior efficacy both in blocking DHT and promoting hair growth compared to finasteride. In a study of 399 patients, dutasteride was found to block 98.4% of DHT, while finasteride blocked about 70% 33. In another study of 416 men between 21 and 45 years of age, dutasteride was found to produce better hair count results than finasteride over a period of 12–24 weeks 10. Despite the greater efficacy demonstrated by dutasteride, finasteride is still likely to be prescribed more often as a first‐line agent in treating male pattern baldness due to FDA approval and insurance coverage.
Dutasteride special precautions
In deciding to use a medicine, the risks of taking the medicine must be weighed against the good it will do. This is a decision you and your doctor will make. For this medicine, the following should be considered:
Men who have taken dutasteride should not donate blood until 6 months have passed since the last dose. Dutasteride can remain in your blood for a long time and be passed on to a pregnant woman who receives a blood transfusion.
Dutasteride may affect the results of the prostate specific antigen (PSA) test, which may be used to detect prostate cancer. Make sure you tell all of your doctors that you are using dutasteride.
Dutasteride does not usually affect normal sexual abilities for most men. You may notice that you ejaculate less fluid when you have sex.
Before taking dutasteride:
- tell your doctor and pharmacist if you are allergic to dutasteride, finasteride (Propecia, Proscar), any other medications, or any of the ingredients in dutasteride capsules. Ask your doctor or check the manufacturer’s patient information for a list of the ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention any of the following: antifungals such as ketoconazole (Nizoral); cimetidine (Tagamet); ciprofloxacin (Cipro); diltiazem (Cardizem, Dilacor, Tiazac); ritonavir, (Norvir), troleandomycin (TAO); and verapamil (Calan, Covera, Isoptin, Verelan). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- Dutasteride may increase your risk of developing high-grade prostate cancer. Tell your doctor if you have or have ever had liver disease or prostate cancer.
- you should know that dutasteride is for use only in men. Women, especially those who are or may become pregnant, should not handle dutasteride capsules. Touching the contents of the capsules may harm the fetus. If a woman who is pregnant or who could become pregnant accidentally touches leaking capsules, she should wash the area with soap and water immediately and call her doctor.
- you should know that you should not donate blood while you are taking dutasteride and for 6 months after you stop taking this medication.
Allergies
Tell your doctor if you have ever had any unusual or allergic reaction to this medicine or any other medicines. Also tell your health care professional if you have any other types of allergies, such as to foods, dyes, preservatives, or animals. For non-prescription products, read the label or package ingredients carefully.
Children
Dutasteride is not indicated for use in children. Safety and efficacy have not been established.
Elderly people
Appropriate studies performed to date have not demonstrated geriatric-specific problems that would limit the usefulness of dutasteride in the elderly.
Breastfeeding women
There are no adequate studies in women for determining infant risk when using dutasteride during breastfeeding. Weigh the potential benefits against the potential risks before taking dutasteride while breastfeeding.
Drug Interactions
Although certain medicines should not be used together at all, in other cases two different medicines may be used together even if an interaction might occur. In these cases, your doctor may want to change the dose, or other precautions may be necessary. When you are taking dutasteride, it is especially important that your healthcare professional know if you are taking any of the medicines listed below. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using dutasteride with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Ceritinib
- Clarithromycin
- Itraconazole
Using dutasteride with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Cimetidine
- Ciprofloxacin
- Diltiazem
- Ketoconazole
- Ritonavir
- Verapamil
Other interactions
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. Discuss with your healthcare professional the use of your medicine with food, alcohol, or tobacco.
Medical Problems
The presence of other medical problems may affect the use of dutasteride. Make sure you tell your doctor if you have any other medical problems, especially:
- Liver disease: Use with caution. The effects may be increased because of slower removal of dutasteride from the body.
Dutasteride uses
Dutasteride is a 5-alpha reductase inhibitor that is used alone or with another medication (tamsulosin [Flomax]) to treat symptomatic benign prostatic hypertrophy (BPH) 41, 42, 43, 44, 45, 46. Dutasteride inhibits the conversion of testosterone to dihydrotestosterone (DHT), which is important in the development and maintenance of prostatic hyperplasia. Dihydrotesterone (DHT) levels decrease during dutasteride therapy, but serum testosterone levels do not. Dutasteride usually takes several months to have an effect on prostate size and the symptoms of prostatic hypertrophy (urinary hesitancy and poor stream), unlike the alpha-1 adrenergic receptor blockers (alpha blockers) which have a more immediate effect.
Doctors also use finasteride and dutasteride off-label as a first-line treatment for male pattern hair loss (androgenetic alopecia) and in post-menopausal women with female pattern hair loss (FPHL) 11, 2, 3, 9, 12, 13, 8, 14. The androgen dihydrotestosterone (DHT) is thought to play a large role in inducing androgenetic alopecia and is formed from the conversion of circulating testosterone to DHT by 5-alpha reductase.
Dutasteride can reduce serum DHT levels by 90% and has been reported to treat female pattern hair loss successfully with no side effects at doses that range from 0.25 to 0.5 mg/day 10. However, Dutasteride should not be given to women of childbearing age unless they are using birth control measures due to potential feminizing effects on the male fetus or to patients with impaired liver function
A recent network meta-analysis demonstrated that the increase in total hair count at 24 weeks with 0.5 mg/day of dutasteride was more efficacious compared to that with 1 mg/day finasteride with the similar adverse events profile 15, 16. Furthermore, a Korean group analyzed the long-term effectiveness and safety profiles of finasteride and dutasteride; they reported that dutasteride-treated patients showed more significant improvement in hair growth compared to finasteride-treated patients and both medications were analogously safe (possibly with a lower incidence of sexual adverse events than previously reported; dutasteride = 1.6%, and finasteride = 1.1%) 17. Accordingly, oral 5-alpha reductase inhibitors represent the first-line treatment modality for androgenetic alopecia because of their effectiveness and tolerability, and dutasteride further widened therapeutic options 11, 17.
Dutasteride dosage
The dose of dutasteride will be different for different patients. Take dutasteride exactly as directed by your doctor. Do not take more of it, do not take it more often, and do not take it for a longer time than your doctor ordered. The following information includes only the average doses of dutasteride. If your dose is different, do not change it unless your doctor tells you to do so.
You may take dutasteride with or without food.
Swallow the capsule whole. Do not crush, chew, or open it. The contents of the capsule may irritate your lips, mouth, or throat.
Dutasteride comes with a patient information leaflet. Read and follow these instructions carefully. Ask your doctor if you have any questions.
Benign Prostatic Hyperplasia
Treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate to reduce the risk of acute urinary retention and BPH-related surgery.
- Adults: 0.5 milligram (mg) once a day.
What should I do if I forget a dose?
If you miss a dose of dutasteride, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule. Do not double doses.
Dutasteride side effects
Dutasteride may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- inability to have or maintain an erection
- decrease in sex drive or interest in sexual intercourse (libido)
- problems with ejaculation
- decreased sexual performance or desire
- impotence (inability in a man to achieve an erection or orgasm)
- inability to have or keep an erection (persistent erectile dysfunction)
- loss in sexual ability, desire, drive, or performance
- pain, soreness, swelling, or discharge from the breast or breasts
Rare side effects:
- chest pain or discomfort
- dilated neck veins
- extreme fatigue
- irregular breathing
- irregular heartbeat
- swelling of the face, fingers, feet, or lower legs
- trouble breathing
- weight gain
Incidence not known:
- blistering, flaking, or peeling of the skin
- cough
- difficulty with swallowing
- dizziness
- fast heartbeat
- hives or welts, itching skin, rash
- large, hive-like swelling on the face, eyelids, lips, tongue, throat, hands, legs, feet, or sex organs
- puffiness or swelling of the eyelids or around the eyes, face, lips, or tongue
- redness of the skin
- tightness in the chest
- unusual tiredness or weakness
Some side effects may be serious. If you experience any of these symptoms, call your doctor immediately or get emergency medical help:
- changes in the breasts such as increased size, lumps, pain, or nipple discharge
- swelling of the face, tongue, or throat
- difficulty breathing or swallowing
- peeling skin
Taking dutasteride may increase the risk that you will develop high-grade prostate cancer (a type of prostate cancer that spreads and grows more quickly than other types of prostate cancer). Talk to your doctor about the risks of taking dutasteride.
Dutasteride may cause other side effects. See your doctor if you have any unusual problems while taking dutasteride.
Dutasteride toxicity
In general, dutasteride appears to be a fairly safe drug, and so its use is recommended 30. A literature review by Hirshburg et al. 1 analyzed studies related to adverse events of 5-alpha reductase inhibitors in relation to prostate cancer, psychological effects, their use in women and sexual health.
In large and representative populations, an increase in the incidence of prostate cancer, or an increase in high-grade prostate cancers upon detection, or a variation in the survival rate were not associated with dutasteride 1. A direct link between the use of 5-alpha reductase inhibitors and depression was not established either 1. The same revision indicated that there were not many studies on the use of 5-alpha reductase inhibitors in women, but the current known risks to women include birth defects in male fetuses if taken during pregnancy, a decreased libido, headache, gastrointestinal problems, isolated cases of menstrual changes, acne and dizziness. Finally, it was reported that erectile dysfunction, a decreased libido and ejaculation disorders occurred in a fairly low percentage of men 1.
In addition, the long-term effect of dutasteride on sexual alterations is interesting as it was associated with a significant increase in impotence, a decreased libido and ejaculation disorders during the first year when compared with a healthy control, but there were no significant differences between the two groups in the second year 47.
Currently, there are no long-term studies evaluating finasteride or dutasteride use in women 1. In a recent study, Price et al 48 followed 137 women aged 41 to 60 years for 12 months in a double-blind, placebo-controlled, randomized study conducted at eight investigational sites in United States and found no statistical difference in side effects of finasteride 1mg daily versus placebo 48. The only adverse event reported in the finasteride group was one case of folliculitis, which resolved with continued treatment 48. Shum et al 49 presented a case series of four women treated with finasteride 1.25 mg daily for up to 2.5 8. years without reported side effects. The longest trial of finasteride and dutasteride in women found during the literature search was three years by Boersma et al 50. Unfortunately, side effects were not discussed 50.
- Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS. Adverse Effects and Safety of 5-alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review. J Clin Aesthet Dermatol. 2016 Jul;9(7):56-62. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5023004[↩][↩][↩][↩][↩][↩]
- Arif T, Dorjay K, Adil M, Sami M. Dutasteride in Androgenetic Alopecia: An Update. Curr Clin Pharmacol. 2017;12(1):31-35. https://dx.doi.org/10.2174/1574884712666170310111125[↩][↩][↩]
- Fertig, R. M, Gamret, A. C, Darwin, E., & Gaudi, S. (2017). Sexual side effects of 5-α-reductase inhibitors finasteride and dutasteride: A comprehensive review. Dermatology Online Journal, 23(11). http://dx.doi.org/10.5070/D32311037240 Retrieved from https://escholarship.org/uc/item/24k8q743[↩][↩][↩]
- Rhie A, Son HY, Kwak SJ, Lee S, Kim DY, Lew BL, Sim WY, Seo JS, Kwon O, Kim JI, Jo SJ. Genetic variations associated with response to dutasteride in the treatment of male subjects with androgenetic alopecia. PLoS One. 2019 Sep 16;14(9):e0222533. doi: 10.1371/journal.pone.0222533[↩][↩]
- Bhat YJ, Saqib NU, Latif I, Hassan I. Female Pattern Hair Loss-An Update. Indian Dermatol Online J. 2020 Jul 13;11(4):493-501. doi: 10.4103/idoj.IDOJ_334_19[↩][↩]
- Andriole GL, Kirby R. Safety and tolerability of the dual 5alpha-reductase inhibitor dutasteride in the treatment of benign prostatic hyperplasia. Eur Urol. 2003 Jul;44(1):82-8. doi: 10.1016/s0302-2838(03)00198-2[↩][↩][↩][↩][↩]
- Kaplan SA, Chung DE, Lee RK, Scofield S, Te AE. A 5-year retrospective analysis of 5α-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. Int J Clin Pract. 2012 Nov;66(11):1052-5. doi: 10.1111/j.1742-1241.2012.03010.x[↩][↩][↩][↩][↩][↩]
- Gubelin Harcha W, Barboza Martínez J, Tsai TF, Katsuoka K, Kawashima M, Tsuboi R, Barnes A, Ferron-Brady G, Chetty D. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol. 2014 Mar;70(3):489-498.e3. doi: 10.1016/j.jaad.2013.10.049[↩][↩][↩][↩][↩]
- Gupta AK, Talukder M, Williams G. Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia. J Dermatolog Treat. 2022 Nov;33(7):2946-2962. doi: 10.1080/09546634.2022.2109567[↩][↩][↩]
- Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, Wilson T, Rittmaster RS; Dutasteride Alopecia Research Team. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006 Dec;55(6):1014-23. doi: 10.1016/j.jaad.2006.05.007[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Manabe M., Tsuboi R., Itami S., Osada S.I., Amoh Y., Ito T., Inui S., Ueki R., Ohyama M., Kurata S., et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version. J. Dermatol. 2018;45:1031–1043. doi: 10.1111/1346-8138.14470[↩][↩][↩][↩][↩]
- Sperling LC, Sinclair RD, El Shabrawi-Caelen L. Alopecias. In: Bolognia J, Jorizzo J, Rapini R, editors. Dermatology. Philadelphia: Elsevier; 2013. pp. 1093–1114. 3rd ed. Vol. 1.[↩][↩]
- Traish AM, Melcangi RC, Bortolato M, Garcia-Segura LM, Zitzmann M. Adverse effects of 5α-reductase inhibitors: What do we know, don’t know, and need to know? Rev Endocr Metab Disord. 2015 Sep;16(3):177-98. doi: 10.1007/s11154-015-9319-y[↩][↩]
- Han SH, Byun JW, Lee WS, Kang H, Kye YC, Kim KH, Kim DW, Kim MB, Kim SJ, Kim HO, Sim WY, Yoon TY, Huh CH, Hwang SS, Ro BI, Choi GS. Quality of life assessment in male patients with androgenetic alopecia: result of a prospective, multicenter study. Ann Dermatol. 2012 Aug;24(3):311-8. doi: 10.5021/ad.2012.24.3.311[↩][↩]
- Dominguez-Santas M., Diaz-Guimaraens B., Saceda-Corralo D., Hermosa-Gelbard A., Muñoz-Moreno Arrones O., Pindado-Ortega C., Fernandez-Nieto D., Jimenez-Cauhe J., Ortega-Quijano D., Suarez-Valle A., et al. The state-of-the-art in the management of androgenetic alopecia: A review of new therapies and treatment algorithms. JEADV Clin. Pract. 2022;1:176–185. doi: 10.1002/jvc2.53[↩][↩]
- Gupta A.K., Venkataraman M., Talukder M., Bamimore M.A. Relative Efficacy of Minoxidil and the 5-α Reductase Inhibitors in Androgenetic Alopecia Treatment of Male Patients: A Network Meta-analysis. JAMA Dermatol. 2022;158:266–274. doi: 10.1001/jamadermatol.2021.5743[↩][↩]
- Choi G.S., Sim W.Y., Kang H., Huh C.H., Lee Y.W., Shantakumar S., Ho Y.F., Oh E.J., Duh M.S., Cheng W.Y., et al. Long-Term Effectiveness and Safety of Dutasteride versus Finasteride in Patients with Male Androgenic Alopecia in South Korea: A Multicentre Chart Review Study. Ann. Dermatol. 2022;34:349–359. doi: 10.5021/ad.22.027[↩][↩][↩][↩]
- Zito PM, Bistas KG, Syed K. Finasteride. [Updated 2022 Aug 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513329[↩]
- McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, Foster HE Jr, Gonzalez CM, Kaplan SA, Penson DF, Ulchaker JC, Wei JT. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011 May;185(5):1793-803. doi: 10.1016/j.juro.2011.01.074[↩]
- Tsuboi R, Itami S, Inui S, Ueki R, Katsuoka K, Kurata S, Kono T, Saito N, Manabe M, Yamazaki M; Guidelines Planning Committee for the Management of Androgenetic Alopecia. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012 Feb;39(2):113-20. doi: 10.1111/j.1346-8138.2011.01361.x[↩]
- Liu L, Zhao S, Li F, Li E, Kang R, Luo L, Luo J, Wan S, Zhao Z. Effect of 5α-Reductase Inhibitors on Sexual Function: A Meta-Analysis and Systematic Review of Randomized Controlled Trials. J Sex Med. 2016 Sep;13(9):1297-1310. doi: 10.1016/j.jsxm.2016.07.006[↩][↩][↩][↩]
- Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011 Mar;8(3):872-84. doi: 10.1111/j.1743-6109.2010.02157.x[↩]
- Moinpour CM, Darke AK, Donaldson GW, Thompson IM Jr, Langley C, Ankerst DP, Patrick DL, Ware JE Jr, Ganz PA, Shumaker SA, Lippman SM, Coltman CA Jr. Longitudinal analysis of sexual function reported by men in the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2007 Jul 4;99(13):1025-35. doi: 10.1093/jnci/djm023[↩]
- Fwu CW, Eggers PW, Kirkali Z, McVary KT, Burrows PK, Kusek JW. Change in sexual function in men with lower urinary tract symptoms/benign prostatic hyperplasia associated with long-term treatment with doxazosin, finasteride and combined therapy. J Urol. 2014 Jun;191(6):1828-34. doi: 10.1016/j.juro.2013.12.014[↩]
- Nickel JC, Fradet Y, Boake RC, Pommerville PJ, Perreault JP, Afridi SK, Elhilali MM. Efficacy and safety of finasteride therapy for benign prostatic hyperplasia: results of a 2-year randomized controlled trial (the PROSPECT study). PROscar Safety Plus Efficacy Canadian Two year Study. CMAJ. 1996 Nov 1;155(9):1251-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1335066/pdf/cmaj00105-0029.pdf[↩]
- Wilton L, Pearce G, Edet E, Freemantle S, Stephens MD, Mann RD. The safety of finasteride used in benign prostatic hypertrophy: a non-interventional observational cohort study in 14,772 patients. Br J Urol. 1996 Sep;78(3):379-84. doi: 10.1046/j.1464-410x.1996.00091.x[↩]
- Irwig MS. Persistent sexual side effects of finasteride: could they be permanent? J Sex Med. 2012 Nov;9(11):2927-32. doi: 10.1111/j.1743-6109.2012.02846.x[↩]
- Eun HC, Kwon OS, Yeon JH, Shin HS, Kim BY, Ro BI, Cho HK, Sim WY, Lew BL, Lee WS, Park HY, Hong SP, Ji JH. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010 Aug;63(2):252-8. doi: 10.1016/j.jaad.2009.09.018[↩]
- Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride induced depression: a prospective study. BMC Clin Pharmacol. 2006 Oct 7;6:7. doi: 10.1186/1472-6904-6-7[↩]
- Proaño B, Casani-Cubel J, Benlloch M, Rodriguez-Mateos A, Navarro-Illana E, Lajara-Romance JM, de la Rubia Ortí JE. Is Dutasteride a Therapeutic Alternative for Amyotrophic Lateral Sclerosis? Biomedicines. 2022 Aug 25;10(9):2084. doi: 10.3390/biomedicines10092084[↩][↩]
- Melcangi R.C., Garcia-Segura L.M., Mensah-Nyagan A.G. Neuroactive Steroids: State of the Art and New Perspectives. Cell. Mol. Life Sci. 2008;65:777–797. doi: 10.1007/s00018-007-7403-5[↩]
- Arif T, Dorjay K, Adil M, Sami M. Dutasteride in Androgenetic Alopecia: An Update. Curr Clin Pharmacol. 2017;12(1):31-35. doi: 10.2174/1574884712666170310111125[↩][↩]
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5α‐reductase inhibitor. J Clin Endocrinol Metab. 2004;89(5):2179‐2184. 10.1210/jc.2003-030330[↩][↩]
- Nassar GN, Leslie SW. Physiology, Testosterone. [Updated 2023 Jan 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526128[↩]
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005 Sep-Oct;4(5):637-40.[↩][↩]
- Herz-Ruelas ME, Álvarez-Villalobos NA, Millán-Alanís JM, de León-Gutiérrez H, Ocampo-Garza SS, Gómez-Flores M, Grimalt R. Efficacy of Intralesional and Oral Dutasteride in the Treatment of Androgenetic Alopecia: A Systematic Review. Skin Appendage Disord. 2020 Nov;6(6):338-345. doi: 10.1159/000510697[↩][↩]
- Saceda-Corralo D, Rodrigues-Barata AR, Vañó-Galván S, Jaén-Olasolo P. Mesotherapy with Dutasteride in the Treatment of Androgenetic Alopecia. Int J Trichology. 2017 Jul-Sep;9(3):143-145. doi: 10.4103/ijt.ijt_73_16[↩][↩]
- Abdallah M, El‐Zawahry KA, Besar H. Mesotherapy using dutasteride‐containing solution in male pattern hair loss: a controlled pilot study. J Pan Arab Leag Dermatol. 2009;20:137‐145.[↩]
- Moftah N, Moftah N, Abd‐Elaziz G, et al. Mesotherapy using dutasteride‐containing preparation in treatment of female pattern hair loss: photographic, morphometric and ultrustructural evaluation. J Eur Acad Dermatol Venereol. 2013;27(6):686‐693. 10.1111/j.1468-3083.2012.04535.x[↩]
- Sobhy N, Aly H, El Shafee A, El Deeb M. Evaluation of the effect of injection of dutasteride as mesotherapeutic tool in treatment of androgenetic alopecia in males. Our Derm Online. 2013;4(1):40‐45.[↩]
- Clark R.V., Hermann D.J., Cunningham G.R., Wilson T.H., Morrill B.B., Hobbs S. Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual 5α-Reductase Inhibitor. J. Clin. Endocrinol. Metab. 2004;89:2179–2184. doi: 10.1210/jc.2003-030330[↩]
- Lulic Z, Son H, Yoo SB, Cunnington M, Kapse P, Miller D, Cortes V, Park S, Bhak RH, Duh MS. Free combination of dutasteride plus tamsulosin for the treatment of benign prostatic hyperplasia in South Korea: analysis of drug utilization and adverse events using the National Health Insurance Review and Assessment Service database. BMC Urol. 2021 Dec 21;21(1):178. doi: 10.1186/s12894-021-00941-1[↩]
- Andriole G, Bostwick D, Brawley O, Gomella L, Marberger M, Tindall D, Breed S, Somerville M, Rittmaster R; REDUCE Study Group. Chemoprevention of prostate cancer in men at high risk: rationale and design of the reduction by dutasteride of prostate cancer events (REDUCE) trial. J Urol. 2004 Oct;172(4 Pt 1):1314-7. doi: 10.1097/01.ju.0000139320.78673.2a[↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Dutasteride. [Updated 2018 Jan 9]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548058[↩]
- Salisbury BH, Tadi P. 5-Alpha-Reductase Inhibitors. [Updated 2023 Apr 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555930[↩]
- Yance S, Montes P. Hepatotoxicidad por tamsulosina / dutasterida: reporte de un caso probable [Hepatotoxicity by tamsulosin / dutasteride: report of a probable case]. Rev Gastroenterol Peru. 2023 Jul-Sep;43(3):269-272. Spanish.[↩]
- Roehrborn C.G., Boyle P., Nickel J.C., Hoefner K., Andriole G. Efficacy and Safety of a Dual Inhibitor of 5-Alpha-Reductase Types 1 and 2 (Dutasteride) in Men with Benign Prostatic Hyperplasia. Urology. 2002;60:434–441. doi: 10.1016/S0090-4295(02)01905-2[↩]
- Price VH, Roberts JL, Hordinsky M, Olsen EA, Savin R, Bergfeld W, Fiedler V, Lucky A, Whiting DA, Pappas F, Culbertson J, Kotey P, Meehan A, Waldstreicher J. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000 Nov;43(5 Pt 1):768-76. doi: 10.1067/mjd.2000.107953[↩][↩][↩]
- Shum KW, Cullen DR, Messenger AG. Hair loss in women with hyperandrogenism: four cases responding to finasteride. J Am Acad Dermatol. 2002 Nov;47(5):733-9. doi: 10.1067/mjd.2002.124608[↩]
- Boersma IH, Oranje AP, Grimalt R, Iorizzo M, Piraccini BM, Verdonschot EH. The effectiveness of finasteride and dutasteride used for 3 years in women with androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2014 Nov-Dec;80(6):521-5. doi: 10.4103/0378-6323.144162[↩][↩]