Contents
- What is milk?
What is milk?
Whole milk is cow’s milk, containing about 3.25% milk fat, and is most similar to its original state when it comes from the cow. The main difference between whole milk and other milks is the fat content. Milk and dairy products are great nutritional sources because they provide essential macronutrients such as carbohydrates, fats and proteins and micronutrients such as vitamins, and minerals. Every serving of milk provides vitamins, minerals, protein, and energy (calories). All milk, whether whole milk or fat-free milk, lactose-free milk or chocolate milk, comes with 13 essential nutrients, such as protein, calcium, vitamin A and vitamin D, vitamin B12, riboflavin (vitamin B2), niacin (vitamin B3), phosphorus, pantothenic acid (vitamin B5), zinc, selenium, iodine and potassium. However, milk does not provide sufficient iron (Fe) and folate (vitamin B9), especially to meet the nutritional needs of growing infants 1. A study that evaluated the global supply of nutrients to the human population found that milk contributes to 28 of the 29 nutrients included in the human nutritional needs 2. Milk is the largest single source of protein for the human population, releasing a variety of peptides and essential amino acids of high biological value 2, 3. Cow milk proteins, as well as milk proteins from other mammals (goat, sheep, buffalo and camel), can be divided into two groups: caseins (alpha, beta, kappa-casein), which accounts for approximately 80% of the total protein content in milk, and whey proteins (alpha-lactalbumin, beta-lactoglobulin, transferrin, albumin, and lactoferrin), representing 20% of the total protein content in milk 4, 5, 6, 7, 8.
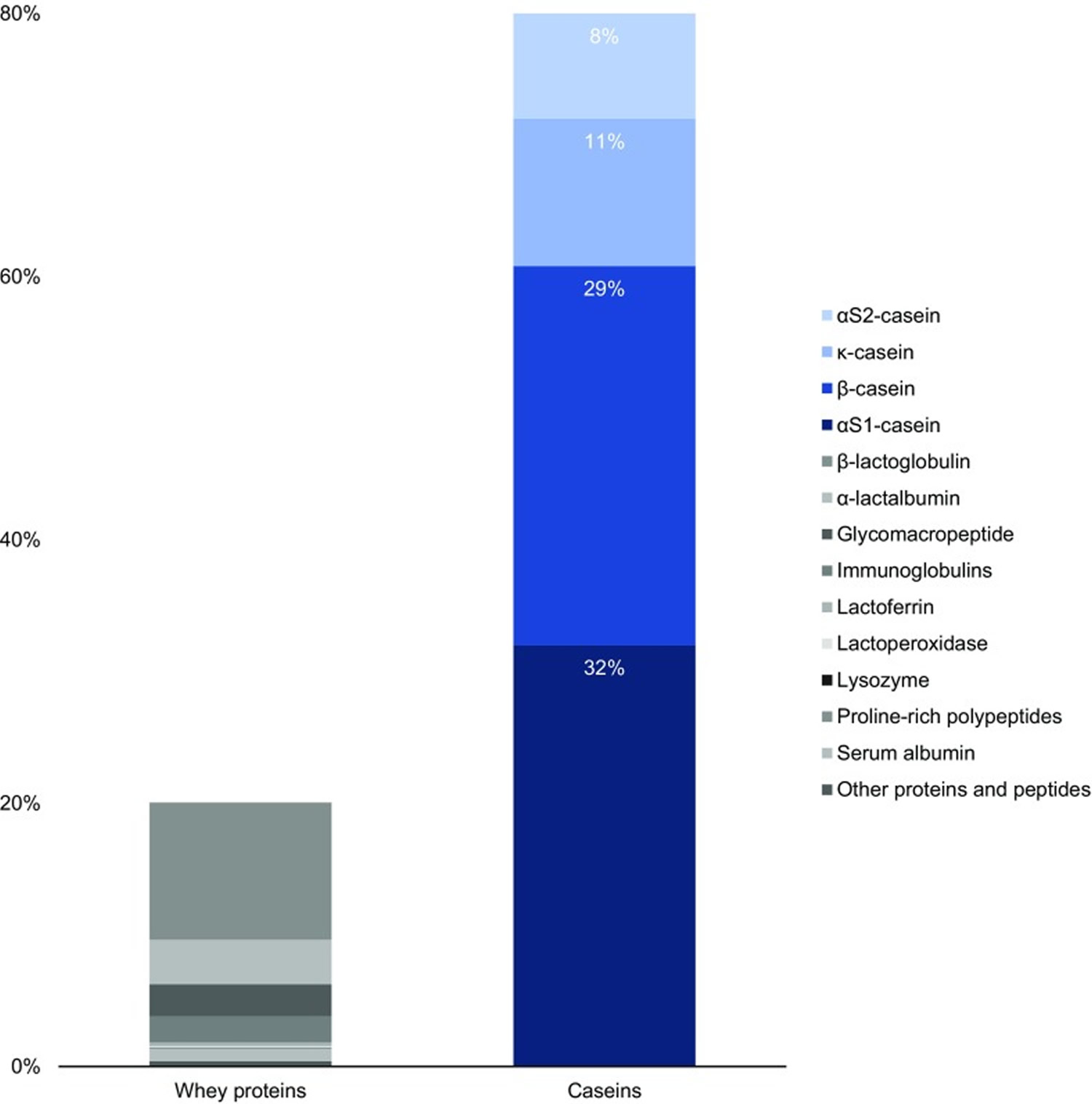
The main constituent of milk protein is casein, which accounts for around 80% of its content, is classified as phosphoproteins and are characterized into 4 isoforms: alphaS1-casein (αS1-CN) (40%), alphaS2-casein (αS2-CN) (10%), beta-casein (β-CN) (36%) and kappa-casein (k-CN) (14%), organized in a micellar format according to their electrophobic interactions 9. Beta-casein (β-CN) represents over 30% of total casein and is gaining importance in nutrition science due to its potential to release bioactive peptides, some of which have opioid characteristics 10. The other major milk protein is whey proteins, make up the remaining 20% of the total protein content in milk and are characterized into alpha-lactoglobulin, alpha-lactalbumin, lactoglobulin (7.8%), serum albumin (1.0%), lactoferrin (7.3%), immunoglobulin (17.1%), and enzymes (0.2%) 11, 12.
The beta-casein (β-CN) protein is a 209-amino-acid-long peptide chain, with thirteen identified genetic variants (A1, A2, A3, A4, B, C, D, E, F, G, H1, H2 and I), resulting from changes in the 209 amino acids in the sequence 9. These genetic variations have characteristics of codominance, which means that alleles may be present in either homozygous, i.e., A1A1 or A2A2, etc., or heterozygous, i.e., A1A2, A1B, etc., forms, where both alleles are expressed for each of the 13 variants 13. The A1A2 and A2A2 beta-casein genotypes are the most frequent, while other genotypes are considered rare or of very low prevalence, such as the A1A1 genotype 14. Regular milk (A1/A2 milk) contains both A1 and A2 beta-casein, whereas A2 milk contains A2 beta-casein only (A2/A2 milk).
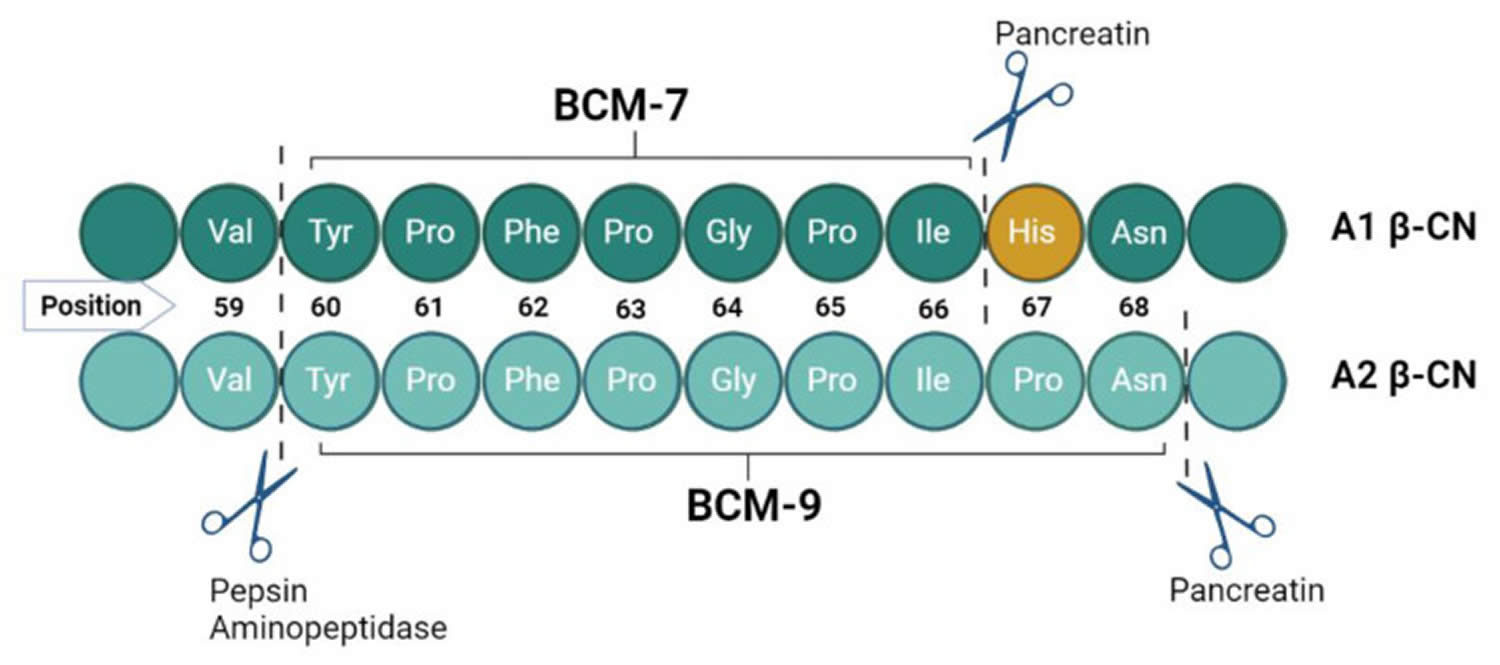
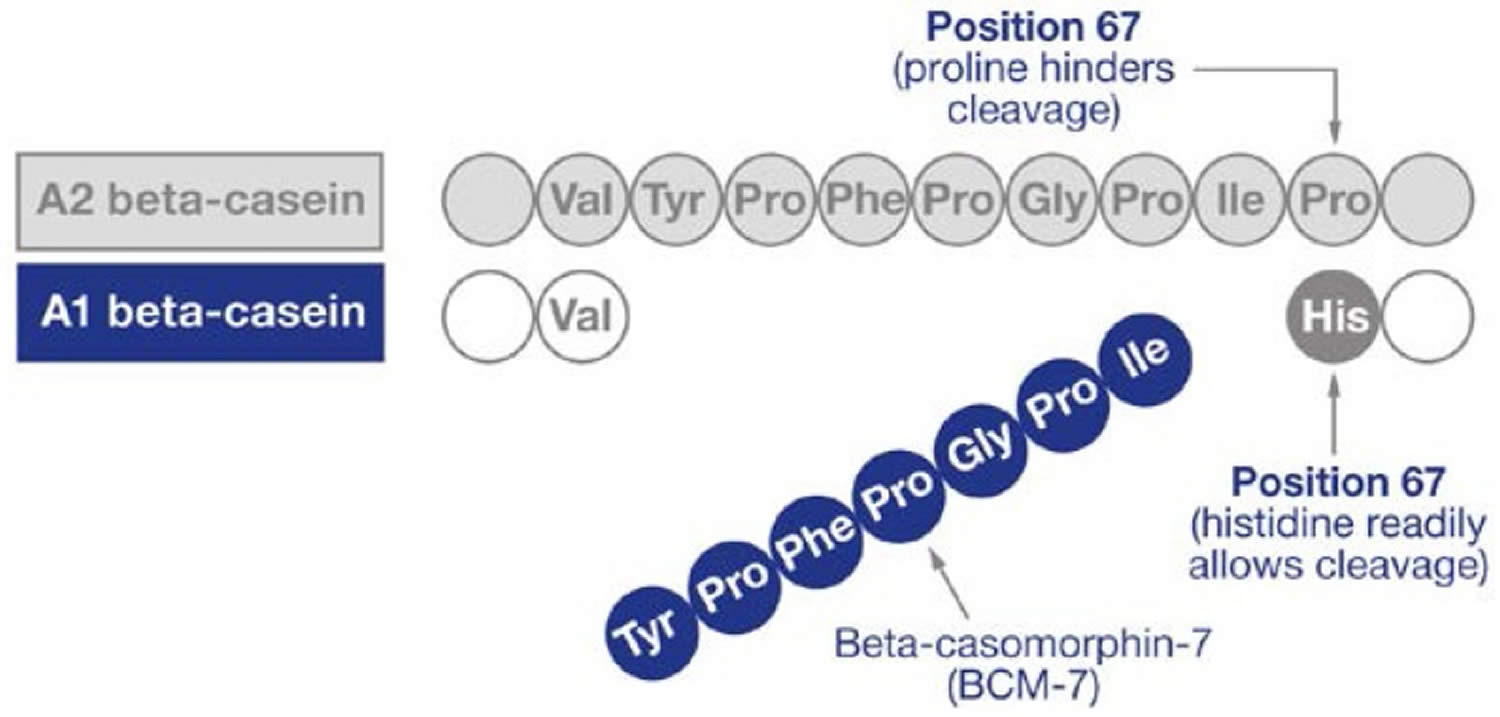
Approximately 30% of the total protein in cow’s milk is beta-casein (β-CN), which is comprised of both A1 beta-casein protein and A2 beta-casein protein 15, 16. These beta-casein proteins differ by the amino acid residue present at position 67, where A1 beta-casein has a histidine residue at position 67 (His67), and A2 beta-casein retains a proline residue at position 67 (Pro67) 17, 18, 19. The presence of histidine amino acid at position 67 (His67) in the A1 beta-casein increases the protein’s susceptibility to enzymatic cleavage of the preceding seven amino acids in the small intestine by pepsin and leucine aminopeptidase, this results in higher opioid peptide beta-casomorphin-7 (BCM-7) levels with moderate activity on mu (μ) opioid receptors 9, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. In contrast, A2 beta-casein peptide bond between isoleucine and proline has more enzymatic resistance, which makes it difficult for proteases to cut between positions 66 and 67, thus releasing much less and probably minimal amounts of the opioid peptide beta-casomorphin-7 (BCM-7) under normal gut conditions 31. In addition, in the A2 beta-casein, protein chain cleavage occurs in the nine amino acid long peptide known as β-Casomorphin-9 (BCM-9) (Tyr60-Pro61-Phe62-Pro63-Gly64-Pro65-Ile66-Pro67-Asn68), which is considered a potentially bioactive peptide with antihypertensive and antioxidant properties 20, 32.
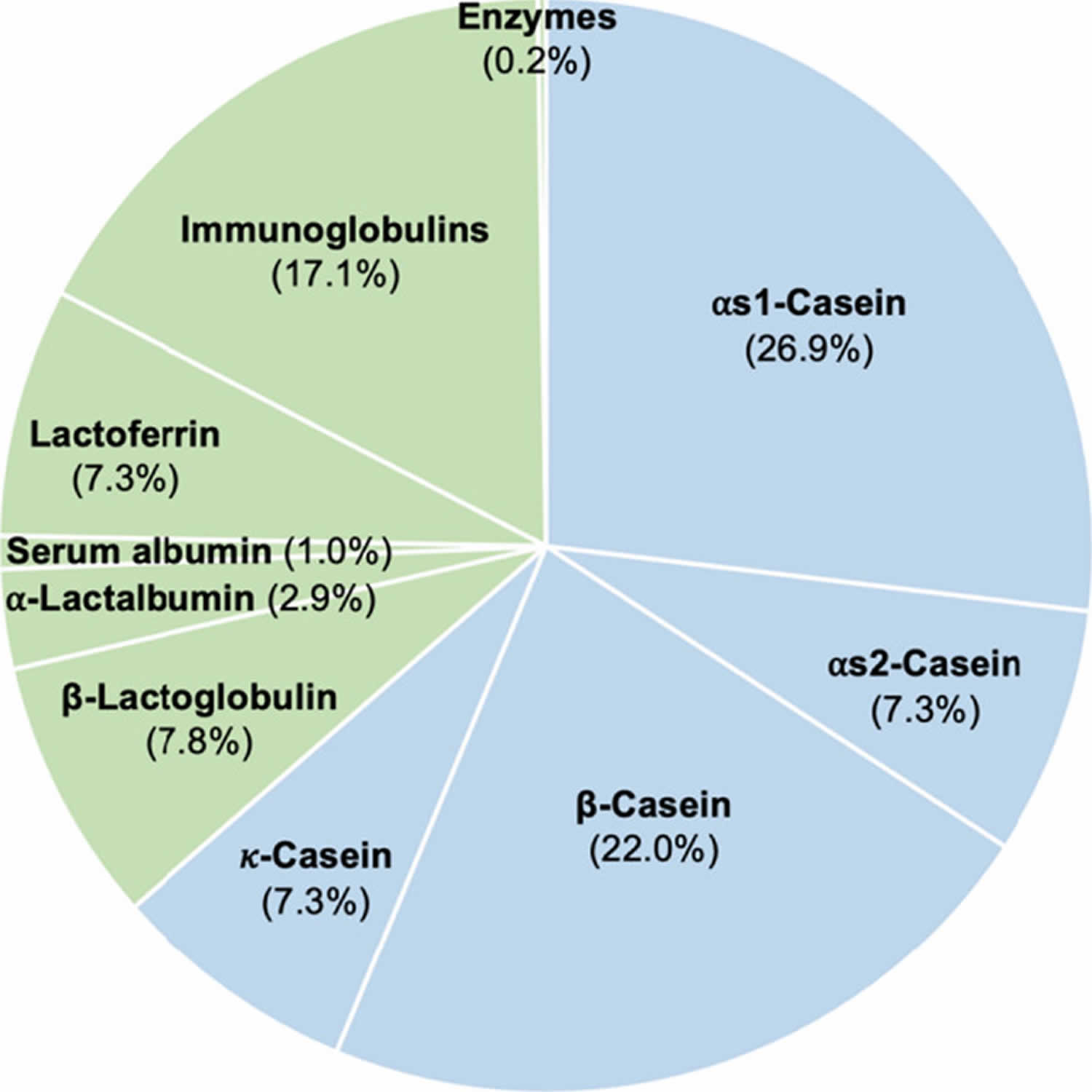
Figure 1. Cow milk protein composition
Footnotes: Protein composition in cow milk. Sky blue and yellowish-green areas indicate caseins and whey proteins, respectively. The numbers show the percentage of each protein in the milk based on protein content of 100%.
[Source 33 ]Figure 2. Standard protein content in cow milk
Table 1. Whole milk nutritional value per 100 Gram
| Name | Amount | Unit |
|---|---|---|
| Water | 88.1 | g |
| Energy | 61 | kcal |
| Protein | 3.27 | g |
| Total lipid (fat) | 3.2 | g |
| Carbohydrate, by difference | 4.63 | g |
| Fiber, total dietary | 0 | g |
| Total Sugars | 4.81 | g |
| Calcium, Ca | 123 | mg |
| Iron, Fe | 0 | mg |
| Magnesium, Mg | 12 | mg |
| Phosphorus, P | 101 | mg |
| Potassium, K | 150 | mg |
| Sodium, Na | 38 | mg |
| Zinc, Zn | 0.42 | mg |
| Copper, Cu | 0.001 | mg |
| Selenium, Se | 1.9 | µg |
| Vitamin C (total ascorbic acid) | 0 | mg |
| Thiamin | 0.056 | mg |
| Riboflavin | 0.138 | mg |
| Niacin | 0.105 | mg |
| Vitamin B6 | 0.061 | mg |
| Folate, total | 0 | µg |
| Folic acid | 0 | µg |
| Folate, food | 0 | µg |
| Folate, DFE | 0 | µg |
| Choline, total | 17.8 | mg |
| Vitamin B12 | 0.54 | µg |
| Vitamin B12, added | 0 | µg |
| Vitamin A, RAE | 32 | µg |
| Retinol | 31 | µg |
| Carotene, beta | 7 | µg |
| Carotene, alpha | 0 | µg |
| Cryptoxanthin, beta | 0 | µg |
| Lycopene | 0 | µg |
| Lutein + zeaxanthin | 0 | µg |
| Vitamin E (alpha-tocopherol) | 0.05 | mg |
| Vitamin E, added | 0 | mg |
| Vitamin D (D2 + D3) | 1.1 | µg |
| Vitamin K (phylloquinone) | 0.3 | µg |
| Fatty acids, total saturated | 1.86 | g |
| SFA 4:0 | 0.067 | g |
| SFA 6:0 | 0.054 | g |
| SFA 8:0 | 0.034 | g |
| SFA 10:0 | 0.084 | g |
| SFA 12:0 | 0.097 | g |
| SFA 14:0 | 0.303 | g |
| SFA 16:0 | 0.857 | g |
| SFA 18:0 | 0.309 | g |
| Fatty acids, total monounsaturated | 0.688 | g |
| MUFA 16:1 | 0.047 | g |
| MUFA 18:1 | 0.694 | g |
| MUFA 20:1 | 0.004 | g |
| MUFA 22:1 | 0 | g |
| Fatty acids, total polyunsaturated | 0.108 | g |
| PUFA 18:2 | 0.115 | g |
| PUFA 18:3 | 0.013 | g |
| PUFA 18:4 | 0 | g |
| PUFA 20:4 | 0.004 | g |
| PUFA 20:5 n-3 (EPA) | 0.001 | g |
| PUFA 22:5 n-3 (DPA) | 0.002 | g |
| PUFA 22:6 n-3 (DHA) | 0 | g |
| Cholesterol | 12 | mg |
| Alcohol, ethyl | 0 | g |
| Caffeine | 0 | mg |
| Theobromine | 0 | mg |
Footnotes: Whole milk 3.25% milk fat, with added vitamin D.
[Source 34 ]Table 2. A1 and A2 milk nutritional content
| Component | A2 Milk | A1 Milk | References |
|---|---|---|---|
| Energy (kJ/100 mL) | 278 | 270 | 35 |
| Protein (mg/mL) | 33 | 33 | 35 |
| αs-casein | 16.37 | 16.08 | 36 |
| β-casein | 8.02 | 8.59 | 36 |
| κ-casein | 2.44 | 2.41 | 36 |
| β-lactoglobulin | 4.5 | 4.49 | 36 |
| α-lactalbumin | 1.46 | 1.43 | 36 |
| Serum albumin | 0.45 | 0.46 | 36 |
| Immunoglobulins | 0.47 | 0.48 | 36 |
| Fat (mg/mL) | 37 | 35 | 35 |
| Carbohydrate (mg/mL) | 50 | 48 | 35 |
| Sodium (mg/mL) | 0.37 | 0.45 | 35 |
| Calcium (mg/mL) | 1.17 | 1.2 | 35 |
Table 3. Human breast-milk nutritional value per 100 Gram
| Name | Amount | Unit |
|---|---|---|
| Water | 87.5 | g |
| Energy | 70 | kcal |
| Energy | 291 | kJ |
| Protein | 1.03 | g |
| Total lipid (fat) | 4.38 | g |
| Ash | 0.2 | g |
| Carbohydrate, by difference | 6.89 | g |
| Fiber, total dietary | 0 | g |
| Total Sugars | 6.89 | g |
| Calcium, Ca | 32 | mg |
| Iron, Fe | 0.03 | mg |
| Magnesium, Mg | 3 | mg |
| Phosphorus, P | 14 | mg |
| Potassium, K | 51 | mg |
| Sodium, Na | 17 | mg |
| Zinc, Zn | 0.17 | mg |
| Copper, Cu | 0.052 | mg |
| Manganese, Mn | 0.026 | mg |
| Selenium, Se | 1.8 | µg |
| Vitamin C, total ascorbic acid | 5 | mg |
| Thiamin | 0.014 | mg |
| Riboflavin | 0.036 | mg |
| Niacin | 0.177 | mg |
| Pantothenic acid | 0.223 | mg |
| Vitamin B-6 | 0.011 | mg |
| Folate, total | 5 | µg |
| Folic acid | 0 | µg |
| Folate, food | 5 | µg |
| Folate, DFE | 5 | µg |
| Choline, total | 16 | mg |
| Vitamin B-12 | 0.05 | µg |
| Vitamin B-12, added | 0 | µg |
| Vitamin A, RAE | 61 | µg |
| Retinol | 60 | µg |
| Carotene, beta | 7 | µg |
| Carotene, alpha | 0 | µg |
| Cryptoxanthin, beta | 0 | µg |
| Vitamin A, IU | 212 | IU |
| Lycopene | 0 | µg |
| Lutein + zeaxanthin | 0 | µg |
| Vitamin E (alpha-tocopherol) | 0.08 | mg |
| Vitamin E, added | 0 | mg |
| Vitamin D (D2 + D3), International Units | 3 | IU |
| Vitamin D (D2 + D3) | 0.1 | µg |
| Vitamin D3 (cholecalciferol) | 0.1 | µg |
| Vitamin K (phylloquinone) | 0.3 | µg |
| Fatty acids, total saturated | 2.01 | g |
| SFA 4:0 | 0 | g |
| SFA 6:0 | 0 | g |
| SFA 8:0 | 0 | g |
| SFA 10:0 | 0.063 | g |
| SFA 12:0 | 0.256 | g |
| SFA 14:0 | 0.321 | g |
| SFA 16:0 | 0.919 | g |
| SFA 18:0 | 0.293 | g |
| Fatty acids, total monounsaturated | 1.66 | g |
| MUFA 16:1 | 0.129 | g |
| MUFA 18:1 | 1.48 | g |
| MUFA 20:1 | 0.04 | g |
| MUFA 22:1 | 0 | g |
| Fatty acids, total polyunsaturated | 0.497 | g |
| PUFA 18:2 | 0.374 | g |
| PUFA 18:3 | 0.052 | g |
| PUFA 18:4 | 0 | g |
| PUFA 20:4 | 0.026 | g |
| PUFA 20:5 n-3 (EPA) | 0 | g |
| PUFA 22:5 n-3 (DPA) | 0 | g |
| PUFA 22:6 n-3 (DHA) | 0 | g |
| Cholesterol | 14 | mg |
| Tryptophan | 0.017 | g |
| Threonine | 0.046 | g |
| Isoleucine | 0.056 | g |
| Leucine | 0.095 | g |
| Lysine | 0.068 | g |
| Methionine | 0.021 | g |
| Cystine | 0.019 | g |
| Phenylalanine | 0.046 | g |
| Tyrosine | 0.053 | g |
| Valine | 0.063 | g |
| Arginine | 0.043 | g |
| Histidine | 0.023 | g |
| Alanine | 0.036 | g |
| Aspartic acid | 0.082 | g |
| Glutamic acid | 0.168 | g |
| Glycine | 0.026 | g |
| Proline | 0.082 | g |
| Serine | 0.043 | g |
| Alcohol, ethyl | 0 | g |
| Caffeine | 0 | mg |
| Theobromine | 0 | mg |
Footnotes: The composition of human breast milk is dynamic and it has evolved to provide optimal infant nutrition. Human breast milk contains macronutrients including proteins, lipids, carbohydrates, and micronutrients such as vitamins and minerals. The macronutrient composition of human milk ranges from 9 to 12 g/L protein, 32 to 36 g/L lipids, 67 to 78 g/L lactose, and 5 to 15 g/L human milk oligosaccharides (HMOs) 38, 39, 40. Human milk also contains non-nutritional bioactive components, growth factors, hormones, immunological factors, noncoding RNAs, and microorganisms 41. Human Milk Oligosaccharides (HMOs) are non-nutritive, functional, and complex carbohydrates in human milk. The composition of human milk oligosaccharides (HMOs) in human milk is influenced by maternal genetic and lactation stage 42. Nearly 200 distinct oligosaccharides have been described to date 43. Human Milk Oligosaccharides (HMOs) are hypothesized to have many important roles in infant innate defense, metabolic health, and neural development 44, 45, 46. One of the most well-characterized functions of human milk oligosaccharides (HMOs) is to serve as a prebiotic source and shape the microbial community of the infant gastrointestinal tract 47. Human Milk Oligosaccharides (HMOs) reach the large intestine undigested where they are utilized by specialized gut microbes that possess the necessary molecular machinery for transport and metabolization of these complex structures 48. Specific species of infant-adapted bifidobacteria [Bifidobacterium longum subsp. infantis (B. infantis), Bifidobacterium bifidum (B. bifidum), Bifidobacterium breve (B. breve), and Bifidobacterium longum subsp. longum (B. longum)] have the capability to degrade and utilize oligosaccharides and thus often become the most dominant species in the breastfed infant gut 49, 50, 51, 52. Short-chain fatty acids (SCFA) (acetate, propionate, and butyrate) are produced as a result of fermentation of human milk oligosaccharide (HMO) in the large intestine. These molecules create an acidic environment (low pH) which favors the growth of strains of bifidobacteria while concomitantly creating an unfavorable environment for the growth of pH-sensitive pathogens 53, 54, 55. As human milk oligosaccharides (HMOs) have structural features that mimic epithelial surface carbohydrates, they are thought to also serve as decoy receptors for pathogens 56, 57, 58. Human milk oligosaccharides (HMOs) are also thought to promote several intracellular processes like differentiation and apoptosis of intestinal epithelial cells 59. Human milk oligosaccharides (HMOs) can also have direct bactericidal or bacteriostatic effects. For instance, some human milk oligosaccharides (HMOs) can directly inhibit the growth of Streptococcus agalactiae in test tube studies, a known invasive bacterial pathogen in newborns 60, 61; other human milk oligosaccharides (HMOs) have been demonstrated to reduce pathogen adherence to colonic cells in test tube study 62. Specific components present in human milk oligosaccharides (HMOs) (e.g., sialic acid) are also critical for the development of neurons and brain development, as well as neuronal transmission, cognitive ability and synaptogenesis 46, 63, 64.
[Source 65 ]What is casein protein?
Casein is the collective name for a family of milk proteins. Caseins, in contrast to the second milk protein fraction, whey proteins, are insoluble and account for 80% of total cow milk proteins 66, which translates to 2.75% of total milk components. Caseins, representing about 80% of the protein content in cow’s milk, are isolated from milk by acid or by rennet precipitation. The acid or isoelectric, precipitation is performed at pH 4.6, where the caseins precipitate and the whey proteins remain soluble. Caseins are flexible and heat stable proteins.
In cow milk, casein accounts for around 80% of its protein content and are characterized into 4 peptides: alphaS1-casein (αS1-CN) (40%), alphaS2-casein (αS2-CN) (10%), beta-casein (β-CN) (36%) and kappa-casein (k-CN) (14%), organized in a micellar format according to their electrophobic interactions 9. Casein phosphoproteins are arranged together according to their hydrophobicity, electrostatic interactions and interact with minerals collectively; they are referred to as casein-calcium-phosphate (CCP) 4. Beta-casein (β-CN) is the second most abundant protein in cow’s milk representing 30% of total protein 67. Beta-casein (β-CN) is encoded by the CSN2 gene mapped on chromosome 6q31 and consists of 209-amino-acid single polypeptide chain, and molecular mass of about 24 kDa 68.
Beta-casein (β-CN) is gaining importance in nutrition science due to its potential to release bioactive peptides, some of which have opioid characteristics 10. Beta-casein has consistently demonstrated important biological activities on immune, cardiovascular, gastrointestinal and central nervous systems 69.
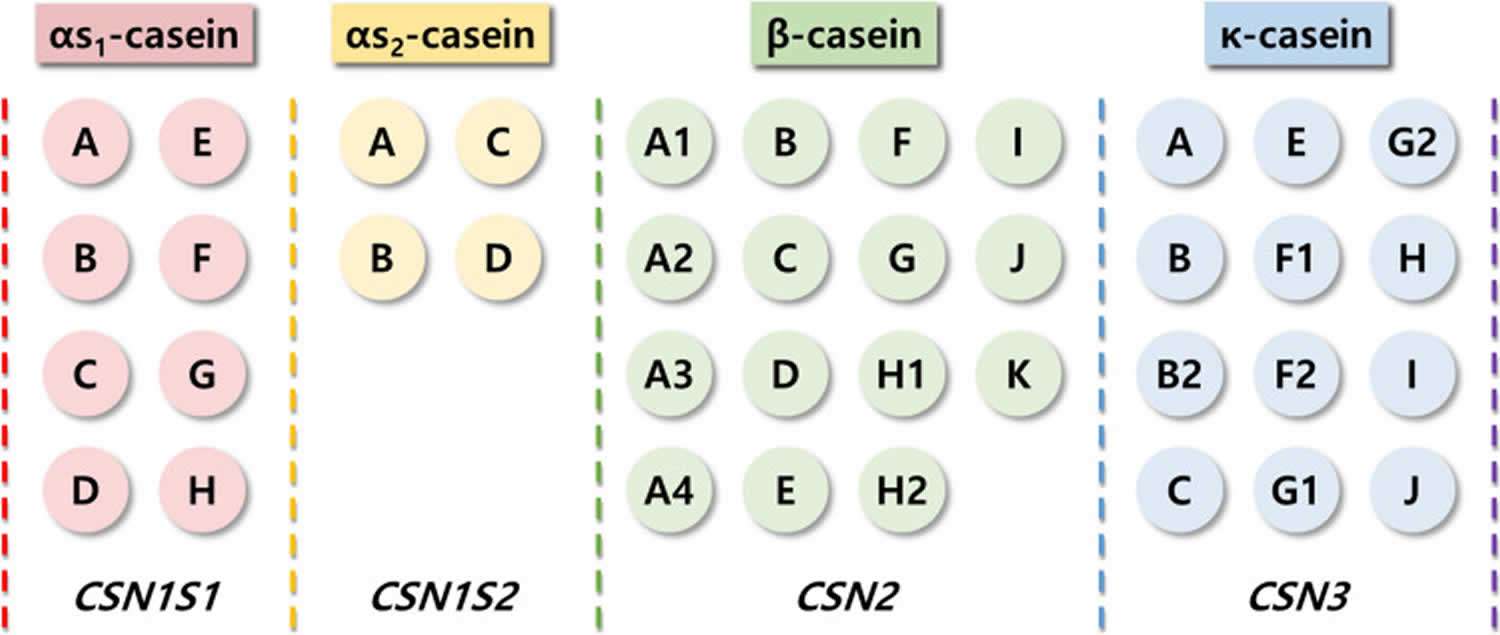
Beta-casein consists of 209 amino acids. Currently, cow milk contains 13 genetic variants of casein: A1, A2, A3, B, C, D, E, F, G, H1/H2, I, and J (Figure 3). The A1 and A2 variants are the most common 16. The distinguishing structure between the A1 and A2 beta-casein is the presence of either histidine (His67) in A1 or proline (Pro67) in A2 at position 67 of this 209–amino acid protein, with A1 being consequential to a point mutation from Pro67 to His67 occurring in ancestors to modern European-type cattle 70. A2 beta-casein is recognized as the original beta-casein variant because it existed before a proline67 to histidine67 point mutation caused the appearance of A1 beta-casein in some European herds some 5000–10,000 years ago 71. Studies have shown that humans can digest His67 in casein using digestive and microbial enzymes; this results in the release of a heptapeptide, beta-casomorphin-7 (BCM-7), during digestion as well as during the ripening of cheese. Beta-casomorphin-7 (BCM-7) belongs to a group of peptides with opioid properties, commonly called casomorphins 72. Among beta-casomorphins, only beta-casomorphin-7 (BCM-7) has been studied extensively. Recent epidemiological evidence has suggested that the consumption of milk containing the A1 beta-casein is associated with higher risk of developing type 1 diabetes and heart disease 73, 74. Beta-casomorphin-7 (BCM-7) can bind to opioid receptors in the central nervous system (brain and spinal cord), as well as in the gastrointestinal tract. In contrast, A2 milk with proline (Pro67) substitution hampers protein digestion in humans. Researchers have focused on the potential effects of beta-casomorphin-7 (BCM-7) and its associated peptides on human health. In 2009, the European Food Safety Authority (EFSA) concluded that beta-casomorphin-7 (BCM-7) is not directly related to non-communicable diseases such as cardiovascular diseases, autism, and insulin-dependent diabetes 75, 76. Nevertheless, beta-casomorphin-7 (BCM-7)-like peptides have been identified in raw milk, which requires further research because of their possible link to an elevated risk of developing chronic diseases 77. Whether beta-casomorphins are formed and the amount of beta-casomorphins formed at different steps of milk processing or fermentation remain highly intriguing.
Milk proteins have long been known for their nutritional and technological value. Proteins are important constituents of the human diet, since they comprise a principal source of nitrogen and essential amino acids. Milk proteins have high nutritional value compared to other proteins because of their relatively high content of essential amino acids and good digestibility 78. This has led to the use of cow’s milk proteins as an essential ingredient in the manufacturing of specialized foods in the pharmaceutical and food industries 79.
In human milk, beta-casein (~68% of total casein) is of the A2 type, with a proline at the equivalent position on the beta-casein protein chain 80. Human bioactive opioid peptide beta-casomorphin-7 (BCM-7) has a different amino acid sequence to cow bioactive opioid peptide beta-casomorphin-7 (BCM-7), with homology in five of seven amino acids (differing amino acids at positions four and five) 81 and considerably weaker opioid activity 82. Wada and Lonnerdal 81 examined non-digested and in vitro-digested human milk, and reported the presence of human BCM-9 (which has a proline at position eight), but not human BCM-7 or BCM-5 (i.e., BCM-5 is the truncated form of BCM-7). However, Jarmolowska et al. reported the presence of both human BCM-5 and BCM-7 in colostrum (averaging 5 and 3 μg/mL respectively), but at 2 months into the lactation period, the authors reported much lower quantities 83. It has been postulated that casomorphin functionality in neonates may relate to maternal bonding, gastrointestinal function, mucosal development and sleep induction 83.
A1 beta-casein has only been found in cattle of European origin. Purebred Asian and African cattle produce milk containing only the A2 beta-casein type, although some cattle presenting phenotypically as Asian or African cattle may produce A1 beta-casein as a consequence of crossbred ancestry. The relative prevalence of A1 and A2 beta-casein in cattle is breed-dependent, with Northern European breeds generally having higher levels of A1 beta-casein than Southern European breeds. Guernsey and Fleckvieh breeds are generally considered to have a particularly high A2 allele frequency. However, within any specific herd, basing the estimation of allele frequency on breed category is not reliable. In the herds in many Western countries, the ratio of A1:A2 is approximately 1:1 31. Herd testing for beta-casein alleles can be undertaken using DNA analysis, which is available commercially in some countries. Converting a specific herd by selective breeding to eliminate all A1 beta-casein from the milk can be achieved within 4 years using intensive methods of animal selection that incorporate the use of sex-selected semen, but more typically this will take 5–8 years or longer 84.
There is increasing evidence that A1 beta-casein, a protein produced by a major proportion of European-origin cattle but not purebred Asian or African cattle, is also associated with cows’ milk intolerance 85. In humans, digestion of bovine A1 beta-casein, but not the alternative A2 beta-casein, releases beta-casomorphin-7 (BCM-7), which activates mu (μ) opioid receptors expressed throughout the gastrointestinal tract and the body. Studies in rodents show that milk containing A1 beta-casein significantly increases gastrointestinal transit time, production of dipeptidyl peptidase-4 (DPP4) and the inflammatory marker myeloperoxidase compared with milk containing A2 beta-casein 85. Co-administration of the opioid receptor antagonist naloxone blocks the myeloperoxidase and gastrointestinal motility effects, indicating opioid signaling pathway involvement. In humans, a double-blind, randomized cross-over study showed that participants consuming A1 beta-casein type cows’ milk experienced statistically significantly higher Bristol stool values compared with those receiving A2 beta-casein milk. The Bristol stool scale, is a diagnostic medical tool designed to classify the form of human faeces into seven categories. It is used in both clinical and experimental fields. Additionally, a statistically significant positive association between abdominal pain and stool consistency was observed when participants consumed the A1 but not the A2 diet. However, further studies of the role of A1 beta-casein in milk intolerance are needed.
Figure 3. Casein protein
Footnotes: Different casein forms and their genetic variants and genes
[Source 33 ]Figure 4. Beta-casein bioactive products
Footnotes: Peptide binding of A1 and A2 beta-casein and cleavage sites with BCM-7 and BCM-9 release during proteolysis. In humans, casein digestion starts in the stomach, where enzymes hydrolyze peptides of various lengths, continues in the duodenum, and, finally, proceeds to jejunal digestion, where hydrolysis and the absorption of smaller peptides occurs 86.
[Source 9 ]Figure 5. Release of beta-casomorphin-7 (BCM-7) from A1 milk protein
Footnote: Difference in the amino acid sequences of A1 and A2 beta-casein and formation of beta-casomorphin-7 (BCM-7). The histidine residue in position 67 in A1 beta-casein protein is cleaved to release the preceding 7 amino acids resulting in the product peptide, beta-casomorphin-7 (BCM-7). DPPIV (dipeptidyl peptidase IV) can inhibit BCM-7 activity.
[Source 17 ]What is whey protein?
Whey proteins comprise approximately 20% of the total milk proteins 87. Whey proteins or cow’s milk serum proteins are defined as proteins in milk that remain soluble after acid precipitation 88 or after rennet casein precipitation 89. Acid precipitation yields acid whey, while rennet casein precipitation yields sweet or rennet whey 90. Whey consists of a heterogeneous group of proteins, including beta-lactoglobulin (35%), alpha-lactalbumin (12%), proteose peptone (12%), immunoglobulins (8%), and bovine serum albumin (5%) 91, 92. When chymosin is used in the cheese-making process, glycomacropeptide – which is high in branched chain amino acids – accounts for about 12% of total protein in whey 93. Up to 1% of the total protein content of whey comprises “low abundance” proteins, including lactoferrin, and lactoperoxidase 92. All these proteins have been reported to have nutritional and/or physiological functions 94.
Whey proteins are considered globular proteins that are soluble over a broad pH range between pH 2 and 10 95.
The amino acid composition is the most important factor in defining food protein quality, followed by the digestibility of the protein and the bioavailability of its amino acids 87. Because of their amino acid composition the main cow’s milk proteins, caseins and whey proteins, can be regarded as a complete source of amino acids. Milk proteins are currently the main source of a range of biologically active peptides and the occurrence of their specific physiological properties which might have nutritional implication is another aspect of their nutritive value.
From the nutritional point of view, milk whey proteins have been considered superior to casein in various aspects. They present amino acid profile superior to casein, being similar to human milk, is what recommends whey proteins for the formulation of humanized milk products for replacement of bovine milk in infant nutrition 96. Whey protein from cow’s milk is also a rich source of essential and branched chain amino acids 97. Some publications 98, 99 reported on important differential properties between caseins and the milk whey proteins. It was observed that the caseins undergo much lower digestion and absorption than the whey proteins.
Whey protein is one of the highest-quality proteins given its amino acid content (high essential, branched-chain, and leucine amino acid content) and rapid digestibility. Consumption of whey protein has a strong ability to stimulate muscle protein synthesis 100. In fact, whey protein has been found to stimulate muscle protein synthesis to a greater degree than other proteins such as casein and soy.
Table 4. Whey proteins component and their biological activities
| Components of whey protein | Biological Function | Species | References |
|---|---|---|---|
| Alpha-lactalbumin | Enhancement of antibody response to systematic antigen stimulation and used in manufacturing of infant food | Camel, bovine, and human | 101 |
| Lactoferrin | Antimicrobial activities against microorganisms, anticancer, anti- inflammatory | Camel, bovine, and human | 102 |
| Beta-lactoglobulin | Source of essential and branched chain amino acid, responsible for child allergy | Bovine, buffalo, caprine, and equine | 103 |
| Lysozymes | Antibacterial protein present in milk, tears, and saliva, and thus plays an important role in enhancing innate immunity | Camel and bovine | 104 |
| Immunoglobulin | Enhances immune functions | Camel, bovine, and human | 105 |
| Lactoperoxidase | Suppression of bacterial growth | Camel and bovine | 106 |
| Glycomacropeptide | Has an inhibitory effect on acid gastric secretion and modifies the concentration of blood which regulates digestive peptides | Camel and bovine | 107 |
What is lactose intolerance?
Lactose intolerance means that you cannot digest foods with lactose in them. Lactose is sometimes referred to as “milk sugar” because it is only found naturally in the milk of mammals including cows, goats and human breast milk and other dairy products. Milk from cows and goats is used to make cheese and yogurt, but not all milk products contain the same amount of lactose. For example, hard cheeses such as cheddar, parmesan and Swiss contain very little or no lactose, whereas milk, ice cream and yogurt contain higher amounts of lactose. Hard cheeses are lower in lactose due to the removal of whey (a liquid solution of fat, lactose and protein) while they are being made as well as the continued breakdown of lactose by bacteria during the aging process.
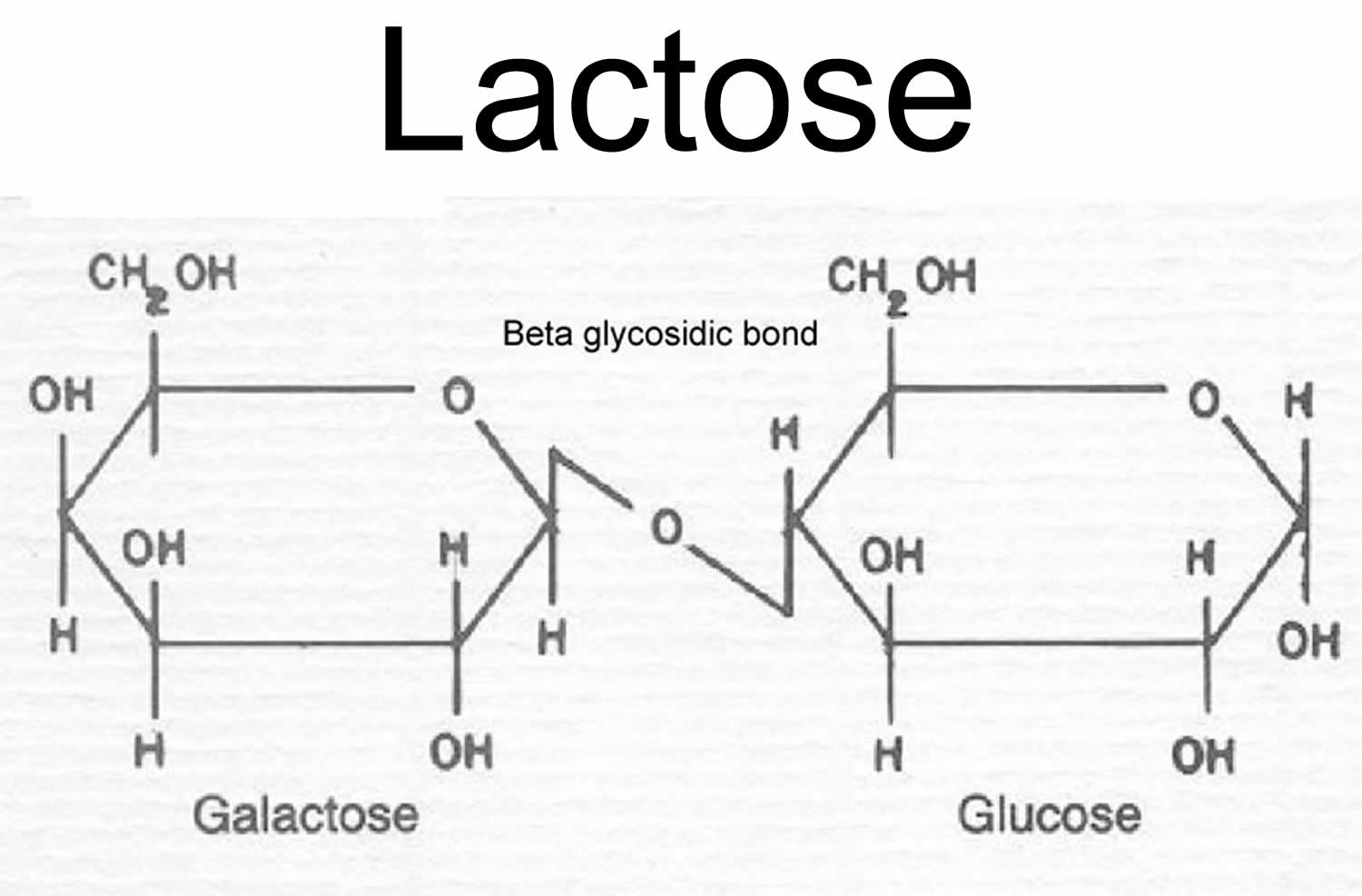
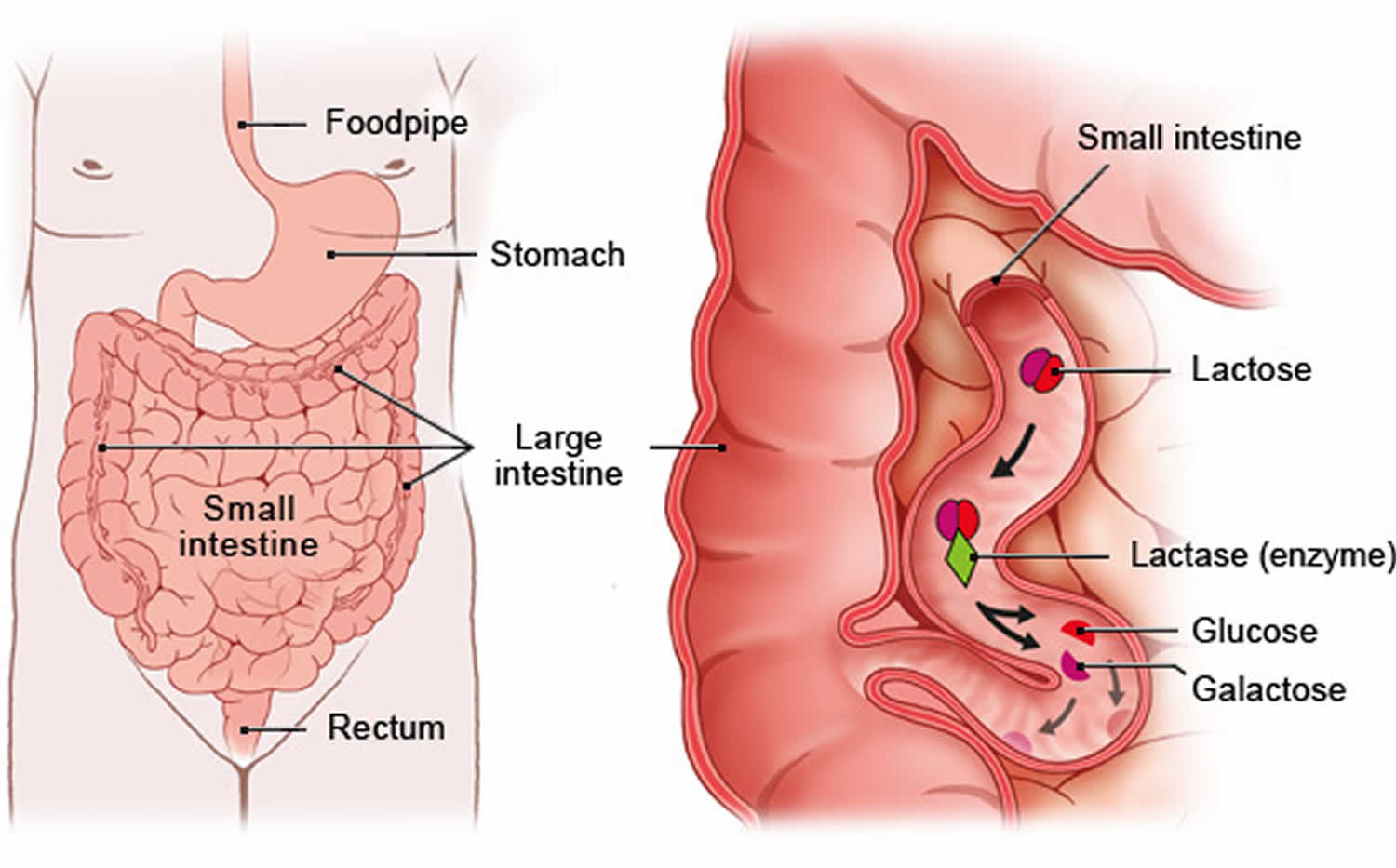
Lactose is a disaccharide or “double sugar”, which is a class of sugars whose molecules contain 2 simple sugars or two monosaccharides (glucose and galactose) linked together by a glycosidic bond 109. To be used by your body, lactose, a disaccharide, a natural sugar found in milk and other dairy products (and added in some foods as an ingredient during manufacturing), must be broken down into their constituent monosaccharides, glucose and galactose, through hydrolysis, a process that adds a water molecule to break the bond. An enzyme called lactase in the small intestine perform this breakdown into glucose and galactose, which are then absorbed into your bloodstream 110, 111. Some people lack sufficient lactase enzyme in their gut, a condition called lactose intolerance, which can cause digestive discomfort when they consume dairy.
People who lack sufficient lactase enzyme in their gut, after eating foods with lactose in them, may feel sick in their stomach. The symptoms of lactose intolerance usually begin within a few hours after eating or drinking foods that contain lactose. It takes at least half an hour for symptoms to occur after the person has eaten or drunk something containing lactose 112. The symptoms are at their worst after around 1.5 to 2 hours, but they can last longer 112.
Common symptoms of lactose intolerance include 113, 114, 115, 116, 117, 112:
- Diarrhea.
- Nausea, and sometimes, vomiting.
- Stomach cramps or pain in the lower belly.
- A bloated belly.
- Gas.
- Feeling full.
- Sometimes constipation too.
Lactose intolerance is usually passed on to children in their parents’ genes. In these cases, it’s referred to as “primary lactose intolerance” or “lactase non-persistence” 112, 118, 119, 120. People who develop “primary lactose intolerance”, the most common lactose intolerance type, produce enough lactase at birth. Babies’ digestive systems are designed to survive only on breast milk. In order to digest breast milk, babies make an enzyme called lactase. Lactase breaks down the natural sugar found in breast milk (lactose) in the bowel so that the body can process it further. When a child is weaned off breast milk, their digestive system gradually adapts to digest and process other foods. Their body then produces less lactase. When this happens, some people don’t have enough lactase to break down the lactose in their food. As a result, they don’t tolerate lactose-containing foods and drinks as well as other people do. If an adult consumes more lactose than their body can break down with the available lactase, some lactose is left over in the small intestine. It passes on into the large intestine, where it is digested by intestinal bacteria through fermentation. As a result, more gases like carbon dioxide (CO2) and hydrogen, and other byproducts like fluids and fatty acids are produced in the bowel, causing the typical symptoms of lactose intolerance. Primary lactose intolerance or lactase non-persistence is most prevalent in people of East Asian descent, with 70 to 100 percent of people affected in these communities 116. Primary lactose intolerance or lactase non-persistence is also very common in people of West African, Arab, Jewish, Greek, and Italian descent 116. Only about 5 percent of people of Northern European descent are primary lactose intolerant 116.
Other causes of lactose intolerance include:
- Developmental Lactase Deficiency. Developmental lactase deficiency is observed in premature infants born at 28 to 37 weeks of gestation 121. The infant’s intestine is underdeveloped, resulting in inadequate lactase production. Developmental lactase deficiency improves with age due to the maturation of the intestine, which results in adequate lactase activity.
- Congenital Lactase Deficiency. It’s possible, but rare, for babies to be born with lactose intolerance caused by a lack of lactase. Congenital lactase deficiency is a rare autosomal recessive genetic condition due to mutations in the LCT gene, with only about 40 cases reported worldwide 118. The LCT gene provides instructions for making the lactase enzyme. Mutations in the LCT gene that cause congenital lactase deficiency are believed to interfere with the processing and function of lactase, causing affected infants to have a severely impaired ability to digest the lactose in breast milk or formula. Congenital lactase deficiency is a rare disorder, its exact incidence is unknown 116. Congenital lactase deficiency is most common in Finland, where it affects an estimated 1 in 60,000 newborns 116. An autosomal recessive genetic condition is a disorder that occurs when an individual inherits two copies of a mutated gene, one from each parent. “Autosomal” means the gene is on a numbered, non-sex chromosome, affecting males and females equally. For a child to have the condition, both the mother and the father must be carriers of the gene, though they typically do not show symptoms themselves 122. Congenital lactase deficiency results in decreased or absent lactase enzyme activity 123, 124. Symptoms appear shortly after birth with the ingestion of milk 125.
- Secondary Lactase Deficiency. In some people, injury to the intestinal mucosa can lead to “secondary lactase deficiency“, which is a type of lactose intolerance that happens when your small intestine decreases lactase production after an illness, injury or surgery involving your small intestine 112, 115, 126.
Diseases or factors that can make someone more prone to secondary lactose intolerance include 112, 115, 126:
- Gastrointestinal infections, such as those caused by rotavirus in children and Giardia lamblia
- Celiac disease
- Crohn’s disease
- Antibiotics
- Bacterial overgrowth
- Small intestinal surgery
- Increasing age. Lactose intolerance usually appears in adulthood. The condition is not common in babies and young children.
- Ethnicity. Lactose intolerance is most common in people of African, Asian, Hispanic and American Indian descent.
- Premature birth. Infants born prematurely might have reduced levels of lactase because the small intestine doesn’t develop lactase-producing cells until late in the third trimester.
- Diseases affecting the small intestine. Small intestine problems that can cause lactose intolerance include bacterial overgrowth, celiac disease and Crohn’s disease.
- Certain cancer treatments. If you’ve had radiation therapy for cancer in your stomach or the small intestine or you have intestinal complications from chemotherapy (cancer treatment that uses one or more anti-cancer drugs), your risk of developing lactose intolerance increases.
Treatment of the underlying condition or resolution of the underlying cause may restore lactase levels and improve symptoms, though it can take time.
Your doctor might suspect lactose intolerance based on your symptoms and response to reducing the amount of dairy foods in your diet.
Your doctor may do a blood test, hydrogen breath test or stool test to find out if your problems are due to lactose intolerance. You don’t have to do all of these tests. In most countries, the standard test is the hydrogen breath test.
- Hydrogen breath test. Hydrogen breath test measures your breath hydrogen after lactose ingestion. Hydrogen breath test involves drinking a liquid that contains high levels of lactose. The amount of hydrogen in your breath is then measured at regular intervals. An increase >20 ppm above baseline means that you aren’t fully digesting and absorbing lactose (lactose malabsorption) 127. Proper test protocols are essential for accuracy 127.
- Stool acidity test: This test detects low stool pH due to fermentation of unabsorbed lactose into lactic acid.
- Milk tolerance test: After administering 500 mL of milk, a rise in blood glucose <9 mg/dL suggests lactose malabsorption 128.
- Lactose tolerance test: This test involves ingestion of 50 g lactose with serial glucose measurements at 0, 60, and 120 minutes 127. A rise <20 mg/dL suggests lactose intolerance. Sensitivity is 75%; specificity is 96%. False-negative results may occur in patients with diabetes mellitus or small intestinal bacterial overgrowth. The results are also affected by delayed gastric emptying 129.
- Small bowel biopsy: This test is invasive and rarely used; reserved for excluding secondary causes such as celiac disease.
- Elimination diet: This involves avoiding lactose-containing foods and drinks for a while and then consuming a certain amount of lactose afterwards to see how your body reacts.
- Lactase gene testing or genotyping: Lactase genotyping is emerging as a highly sensitive and specific test. This test is currently more common in Germany and Nordic countries, but not widely adopted elsewhere 130.
Lactose intolerance is not serious. Most people with lactose intolerance can manage the condition without having to give up all dairy foods. Eating less food with lactose, or using pills or drops to help you digest lactose usually helps. You may need to take a calcium supplement if you don’t get enough of it from your diet, since milk and foods made with milk are the most common source of calcium for most people.
In people with lactose intolerance caused by an underlying condition or secondary lactase deficiency, treating the condition might restore their body’s ability to digest lactose, although that process can take months 131.
People with genetic lactose intolerance or primary lactose intolerance can usually lead a symptom-free life if they make some changes to their diet. Research findings show that the best known way to reduce the symptoms is to eat and drink small amounts of lactose and to only ever drink dairy milk together with other foods. There is no treatment that can “cure” genetic lactose intolerance or primary lactose intolerance.
To lower the amount of lactose in your diet:
- Limit milk and other dairy products.
- Cow, goat, and sheep milk
- Ice cream and sherbet
- Butter and margarine
- Buttermilk, whipping cream, sour cream, and coffee creamers
- Soft cheeses, such as cream cheese, cottage cheese, ricotta, and mascarpone
- Evaporated milk, condensed milk, and milk powder
- Pudding and custard
- Whey protein, especially whey concentrate
- Pancakes and waffles, if prepared with milk or butter
- Include small servings of dairy products in your regular meals.
- Eat and drink lactose-reduced ice cream and milk.
- Add a liquid or powder lactase enzyme to milk to break down the lactose. Lactase enzyme supplements extracted from yeasts and molds are available as tablets or drops. In most cases, these supplements should be taken just before eating a high-lactose product or with the first bite. However, when drops are added to milk, they should be shaken or stirred and refrigerated for 24 hours before ingestion to allow the lactose to break down.
You don’t usually need to cut lactose-containing foods out of your diet completely. The following amounts are normally well tolerated especially if consumed together with a meal or other foods 112:
- Up to 12 grams of lactose at once (for example 250 milliliters of milk. or about one glass)
- Up to 24 grams of lactose over the course of the day (for example, 500 milliliters of milk, or about two glasses)
Reducing the dairy products doesn’t mean you can’t get enough calcium. Calcium is found in many other foods, such as:
- Broccoli and leafy green vegetables.
- Calcium-fortified products, such as cereals and juices.
- Canned salmon or sardines.
- Milk substitutes, such as soy milk and rice milk.
- Oranges.
- Almonds, Brazil nuts and dried beans.
Also make sure you get enough vitamin D, which is typically supplied in fortified milk. Eggs, liver and yogurt also contain vitamin D. Your body also makes vitamin D when you spend time in the sun.
Yogurt contains variable amounts of lactose and may trigger symptoms in some individuals. Greek yogurt has the lowest lactose content. Live yogurt cultures produce beta-galactosidase, which may enhance lactose digestion 132. Plant-based milk alternatives, such as almond, coconut, and soy milk, are lactose-free options, although concerns remain regarding palatability, nutritional adequacy, and processing challenges 133, 134.
Probiotics, such as the Lactobacillus acidophilus DDS-1 strain, have shown potential in alleviating symptoms 135, 136. Calcium and vitamin D supplementation are recommended to maintain bone health.
Untreated lactose malabsorption may contribute to osteopenia, a condition characterized by lower-than-normal bone density that increases the risk of fractures, but it is not as severe as osteoporosis 137, 138, 139. Vitamin D deficiency is linked to the LCT-13910C>T gene mutation of lactose intolerance among Whites 139.
Figure 6. Chemical Structure of Lactose
Footnotes: Lactose is a disaccharide sugar or two monosaccharides (glucose and galactose) linked together by a glycosidic bond.
[Source 140 ]Figure 7. Lactose digestion
Footnotes: Lactose sometimes referred to as “milk sugar” because it is only found naturally in the milk of mammals—including cows, goats and human breast milk, is hydrolyzed (broken down) into glucose and galactose by the enzyme lactase, located in the small intestinal border 110, 111.
[Source 112 ]What is milk allergy?
Milk allergy is an immune system response to proteins (whey and casein) in cow’s milk, causing symptoms like hives (a red, raised, and itchy rash), swelling, vomiting, and diarrhea, which can range from mild or moderate allergic reactions such as swelling of lips, face eyes, hives (a red, raised, and itchy rash) or welts on the skin, stomach (abdominal) pain, vomiting and diarrhea to a life-threatening reaction called anaphylaxis that include noisy breathing or wheeze, swelling or tightness in throat, or young children may be pale and floppy. Milk allergy is one of the most common food allergies in children and babies, affecting more than 2% of babies 141. Cow’s milk is the usual cause of milk allergy, but milk from sheep, goats, buffalo and other mammals also can cause milk allergy. A milk allergy is different from lactose intolerance, which is an inability to digest the sugar lactose, not the proteins in milk. A milk allergy can be deadly. If you have severe allergic reaction symptoms, such as trouble breathing, call your local emergency number or go to your nearest emergency room (ER) immediately. Milk allergy diagnosis is made by an allergy specialist using tests like skin prick or oral challenges, and management involves strict milk avoidance and carrying an adrenaline autoinjector for severe reactions.
In all allergies, the immune system reacts to triggers, also known as allergens. Your immune system produces antibodies that detect the allergen, causing inflammatory reactions and the release of chemicals, including histamine, which cause allergic symptoms. In the case of milk, the triggers are milk proteins including whey and casein. You or your child may be allergic to either one of these proteins, or both.
If you have milk allergy, your immune system identifies certain milk proteins as harmful, triggering the production of immunoglobulin E (IgE) antibodies to neutralize the protein (allergen) 142. The next time you come in contact with these proteins, IgE (immunoglobulin E) antibodies recognize them and signal your immune system to release histamine and other immune mediators. This release of chemicals causes the signs and symptoms of cow’s milk allergy.
Cow’s milk contains more than 20 protein fractions. There are 2 main proteins in cow’s milk that can cause an allergic reaction 143, 142, 144:
- Casein (alpha-s1-, alpha-s2-, beta-, and kappa-casein) found in the solid part (curd) of milk that curdles.
- Whey (alpha-lactalbumin and beta-lactoglobulin) found in the liquid part of milk that remains after milk curdles.
You or your child may be allergic to only one milk protein or to both. Most individuals with cow’s milk allergies have a sensitivity to both caseins and whey proteins 145. These milk proteins may be hard to avoid because they’re also in some processed foods. And most people who react to cow’s milk will react to sheep, goat and buffalo milk.
Certain factors may increase your risk of developing milk allergy:
- Other allergies. Many children who are allergic to milk also have other allergies. Milk allergy may develop before other allergies.
- Atopic dermatitis also known as eczema, is a common, chronic, and non-contagious skin condition that causes dry, itchy, and inflamed skin that can sometimes crack and ooze. Children who have atopic dermatitis, a common, chronic inflammation of the skin, are much more likely to develop a food allergy. It is often genetic and linked to other atopic conditions like asthma and hay fever, and its severity can be managed with moisturizers and topical corticosteroids, although there is no cure. Treatment focuses on managing symptoms, preventing flares, and avoiding triggers, with management similar for both adults and children.
- Family history. A person’s risk of a food allergy increases if one or both parents have a food allergy or another type of allergy or allergic disease, including hay fever, asthma, hives or eczema.
- Age. Milk allergy is more common in children. As they age, their digestive systems mature, and their bodies are less likely to react to milk.
An allergic reaction to milk can be rapid usually happens soon after you or your child consumes milk, appearing within minutes to two hours after consumption, or delayed, occurring several hours or even days later. Symptoms of milk allergy range from mild to severe and can include wheezing, vomiting, hives and digestive problems. Milk allergy also can cause anaphylaxis, a severe, life-threatening reaction.
Immediate symptoms of milk allergy might include:
- Hives (a red, raised, and itchy rash).
- Itching.
- Wheezing.
- Itching or tingling feeling around the lips, mouth, tongue or throat.
- Swelling of the lips, tongue or throat.
- Coughing or shortness of breath.
- Nausea.
- Vomiting.
Signs and symptoms of milk allergy that may take more time to develop include:
- Loose stools or diarrhea, which may contain blood.
- Abdominal cramps.
- Runny nose.
- Watery eyes.
- Colic, in babies.
- Milk allergy can cause anaphylaxis, a life-threatening reaction that narrows the airways and can block breathing. Milk is the third most common food after peanuts and tree nuts to cause anaphylaxis. Anaphylaxis is a medical emergency and requires treatment with an epinephrine (adrenaline) shot (EpiPen, Adrenaclick, others) and a trip to the emergency room.
Anaphylaxis symptoms start soon after milk consumption and can include:
- Constriction of airways, including a swollen throat that makes it difficult to breathe.
- Chest tightness
- Shortness of breath (dyspnea).
- Difficulty breathing.
- Wheezing.
- Difficulty swallowing (dysphagia)
- Rash.
- Facial flushing.
- Itching.
- Dizziness.
- Shock, with a marked drop in blood pressure (hypotension).
- Loss of consciousness (syncope).
Diagnosis of allergic reactions is usually obvious if symptoms occur soon after your child has consumed cow’s milk or other dairy foods. This can be confirmed by your doctor after taking a medical history and using allergy tests. Diagnosis should be made in consultation with a allergy specialist and/or specialist pediatrician. This usually involves excluding cow’s milk and other dairy foods from the diet for a trial period of one to four weeks to check for a clear improvement. A planned reintroduction of cow’s milk and other dairy foods should occur to confirm diagnosis before longer term exclusion is advised. Allergy tests (skin tests or blood tests), that measure allergen specific antibodies called immunoglobulin E (IgE), to cow’s milk are usually positive for rapid onset reactions. This is known as IgE-mediated cow’s milk allergy.
- Skin prick test. In this test, the skin is pricked and exposed to small amounts of the proteins found in milk. If someone has an allergy, a raised bump called a hive forms at the test location on the skin. Allergy specialists usually are best equipped to perform and interpret allergy skin tests. Keep in mind that this type of test isn’t completely accurate for detecting milk allergy.
- Blood test. A blood test can measure the immune system’s response to milk by measuring the amount of immunoglobulin E (IgE) antibodies in the blood. But this test isn’t completely accurate in identifying milk allergy.
- Diet elimination. If suspected, an infant should receive a diet free of cow’s milk protein for a month. If symptoms improve following elimination of the suspected food, then an oral food challenge is the gold standard test 146.
- Oral food challenge. If your examination and test results can’t confirm milk allergy, a allergy specialist might administer an oral food challenge. In this test, you are fed different foods that may or may not contain milk in increasing amounts to see if you react to the ones that contain milk. It’s a good idea to have allergy tests administered by an allergist who’s been trained to manage serious immunoglobulin E (IgE) mediated reaction. Patients should undergo reevaluation every 6 to 12 months to determine if they have developed a tolerance to cow’s milk protein 147.
If your allergy specialist suspects that your symptoms are caused by something other than a food allergy, you may need other tests to identify or rule out other medical problems.
There is no place in the diagnosis of cow’s milk allergy for non evidence-based tests such as cytotoxic food testing, kinesiology, hair analysis, vega testing (electro-diagnostic), electrodermal testing, pulse testing, reflexology, Bryan’s or Alcat tests, and Immunoglobulin G (IgG) to foods.
Milk allergy diagnostic algorithm 142:
- If there are signs of anaphylaxis or immediate reaction, then diet elimination is recommended, and testing for serum IgE should follow. If serum-specific IgE is positive, then the child is diagnosed with a cow’s milk allergy. If IgE is negative and symptoms improve after diet elimination, an oral challenge should be next. If the symptoms reoccur, the diagnosis is confirmed. If the symptoms do not reoccur, then the diagnosis of cow’s milk allergy is excluded 148.
- If the symptoms are not consistent with anaphylaxis or immediate reaction, then an elimination diet is recommended. If symptoms improve, then an oral challenge should be done, and if symptoms reoccur, the diagnosis is confirmed. If the symptoms do not reoccur, it excludes the diagnosis of cow’s milk allergy 148, 149.
- If symptoms do not improve after the elimination diet, this eliminates the diagnosis of cow’s milk allergy, and further evaluation should be done to assess the patient 148.
The only way to prevent an allergic reaction is to avoid milk and milk products and it is the primary treatment for milk allergy. This can be difficult because milk is a common ingredient in many foods. Also, some people with milk allergy can tolerate milk in some forms, such as milk that’s heated in baked goods (muffins, cakes or biscuits), or in some processed foods, such as yogurt. However, unless you are already certain that cooked or baked cow’s milk is tolerated, you should discuss this with your allergy specialist before introducing these foods at home. Talk to your allergy specialist about what to avoid. People with cow’s milk (dairy) allergy must avoid medicated toothpastes, chewing gums and any other dental products containing Recaldent™ which is made from cow’s milk protein.
Sources of allergy-causing milk proteins are found in dairy products, including:
- Whole milk, low-fat milk, skim milk and buttermilk.
- Butter.
- Yogurt.
- Ice cream, gelato.
- Cheese and anything that contains cheese.
- Half-and-half.
Milk can be harder to identify when it’s used as an ingredient in processed foods, including baked goods and processed meats.
Hidden sources of milk include:
- Whey.
- Casein.
- Ingredients spelled with the prefix “lact” — such as lactose and lactate.
- Candies, such as chocolate, nougat and caramel.
- Protein powders.
- Artificial butter flavor.
- Artificial cheese flavor.
- Hydrolysates.
Even if a food is labeled “milk-free” or “nondairy”, it may contain allergy-causing milk proteins. It’s important to read the label carefully. When in doubt, contact the manufacturer to be sure a product doesn’t contain milk ingredients.
Milk substitutes such as sheep’s and goat’s milk generally are not acceptable because of a high degree of cross-reactivity with cow’s milk protein. However, research shows that there have been decreased incidents of cross-reactivity to camel’s milk 150.
When eating out, ask how foods have been prepared. Does your steak have melted butter on it? Was your seafood dipped in milk before cooking?
Rates of cow’s milk allergy in breastfeeding infants is lower than formula-fed infants and have been reported to be about 0.5% 151. Breastfeeding is recommended, particularly if the infant is at high risk of developing milk allergy. Cow’s milk proteins passed through breastmilk to the child and may cause an allergic reaction. If the child has a cow’s milk allergy, then the mother should eliminate all foods containing cow’s milk protein, including cheese, yogurt, and butter from her diet 148.
As many as 50% of children affected by cow’s milk protein intolerance also develop soy protein intolerance if fed with soy-based formulas. Therefore, soy-based formulas are not generally a viable option for the treatment of cow’s milk protein intolerance 147, 152.
If you or your child has a serious allergic reaction (anaphylaxis), talk to your allergy specialist about carrying and using emergency epinephrine (adrenaline) 153, 154. Have your doctor or pharmacist demonstrate how to use injectable epinephrine (EpiPen, Adrenaclick, others) device so that you’re prepared for an emergency. If you have already had a severe reaction, wear a medical alert bracelet or necklace that lets others know you have a food allergy.
Oral immunotherapy may also be an option for some people with milk allergy. Oral immunotherapy is a special treatment program sometimes offered and supervised by allergy specialists. Speak with your allergy specialist about whether oral immunotherapy may be an option for you.
Fortunately, around 80% of children will outgrow their cow’s milk allergy by the age of three to five years and by the age of 6, the prevalence of cow’s milk allergy has fallen to less than 1% 155. Those who don’t outgrow it may need to continue to avoid milk products. Your doctor should advise if further allergy testing and food allergen challenges are needed. These are usually performed in hospital clinics and supervised by a allergy specialist.
What is A2 milk?
A2 milk is milk that contains 100% A2 beta-casein in contrast to conventional milk that contain 30% A1 and 70% A2 beta-casein. The main constituent of milk protein is casein, which accounts for around 80% of its content, is classified as phosphoproteins and are characterized into 4 isoforms: alphaS1-casein (αS1-CN), alphaS2-casein (αS2-CN), beta-casein (β-CN) and kappa-casein (k-CN), organized in a micellar format according to their electrophobic interactions 9. Casein phosphoproteins are arranged together according to their hydrophobicity, electrostatic interactions and interact with minerals collectively; they are referred to as casein-calcium-phosphate (CCP) 4. Beta-casein (β-CN) represents over 30% of total casein and is gaining importance in nutrition science due to its potential to release bioactive peptides, some of which have opioid characteristics 10.
The beta-casein (β-CN) protein is a 209-amino-acid-long peptide chain, with thirteen identified genetic variants (A1, A2, A3, A4, B, C, D, E, F, G, H1, H2 and I), resulting from changes in the 209 amino acids in the sequence 9. These genetic variations have characteristics of codominance, which means that alleles may be present in either homozygous, i.e., A1A1 or A2A2, etc., or heterozygous, i.e., A1A2, A1B, etc., forms, where both alleles are expressed for each of the 13 variants 13. The A1A2 and A2A2 beta-casein genotypes are the most frequent, while other genotypes are considered rare or of very low prevalence, such as the A1A1 genotype 14.
Approximately 30% of the total protein in cow’s milk is beta-casein (β-CN), which is comprised of both A1 beta-casein protein and A2 beta-casein protein 15, 16. These beta-casein proteins differ by the amino acid residue present at position 67, where A1 beta-casein has a histidine residue at position 67 (His67), and A2 beta-casein retains a proline residue at position 67 (Pro67) 17, 18, 19. The presence of histidine amino acid at position 67 (His67) in the A1 beta-casein increases the protein’s susceptibility to enzymatic cleavage of the preceding seven amino acids in the small intestine by pepsin and leucine aminopeptidase, this results in higher opioid peptide beta-casomorphin-7 (BCM-7) levels with moderate activity on mu (μ) opioid receptors 9, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. In contrast, A2 beta-casein peptide bond between isoleucine and proline has more enzymatic resistance, which makes it difficult for proteases to cut between positions 66 and 67, thus releasing much less and probably minimal amounts of the opioid peptide beta-casomorphin-7 (BCM-7) under normal gut conditions 31. In addition, in the A2 beta-casein, protein chain cleavage occurs in the nine amino acid long peptide known as β-Casomorphin-9 (BCM-9) (Tyr60-Pro61-Phe62-Pro63-Gly64-Pro65-Ile66-Pro67-Asn68), which is considered a potentially bioactive peptide with antihypertensive and antioxidant properties 20, 32.
It has been reported that casein and its derivatives, particularly beta-casomorphin-7 (BCM-7), exert a variety of effects on gastrointestinal function in animal models, including reducing the frequency and amplitude of intestinal contractions, increasing mucus secretion, and suppressing lymphocyte proliferation 156, 157, 158, 159, 160, 161, 162, 163. Beta-casomorphin-7 (BCM-7) is classified as an exorphin because it is an exogenous opioid peptide, with the same classification as that of morphine 164. Since beta-casomorphin-7 (BCM-7) is released into the intestinal lumen in about 30 minute to 6 hour after ingestion of beta-casein and because it is an exorphin, numerous studies show concern about its physiological activity as an opioid agonist, potential to activate the mu (μ) opioid receptor 165. The mu (μ) opioid receptors are located in various body parts, such as in the central nervous system (brain and spinal cord), the gastrointestinal tract, bladder, and in the cells of the immune and endocrine system 17. The opioid system has the following functions: inhibition of pain stimuli, endocrine and autonomic nervous system functions, emotions and cognitive ability, learning and modulation of memory and gastrointestinal functions 166, 86. The main potential effect of beta-casomorphin-7 (BCM-7) is the modulation of motility that delays gastrointestinal transit time and increases mucus production in humans 167.
Some studies have been conducted in humans in order to investigate the possible effects of beta-casomorphin-7 (BCM-7). Beta-casomorphin-7 (BCM-7) results in gastrointestinal discomfort, pro-inflammatory responses, and reduced antioxidant glutathione (GSH) levels 24, 9, 168. In contrast, the A2-type beta-casein releases almost no beta-casomorphin-7 (BCM-7) and consumption of milk without A1-type β-casein (A1PF milk) results in relatively fewer gastrointestinal symptoms and inflammatory markers versus milk containing both A1-type and A2-type β-casein (conventional milk) 169, 170, 171, 172, 173, 174, 175.
There is increasing evidence in the literature that the effects of dairy products on gastrointestinal dysfunction may be at least be partially attributed to the proteolytic release of the bioactive peptide beta-casomorphin-7 (BCM-7) from beta-casein rather than lactose intolerance 118, 176, 169. Recent studies report that consumption of A1 beta-casein induces inflammation in the small intestine, which may potentiate lactose intolerance symptoms by downregulating lactase enzyme expression or activity 177, 178. The A2 variant of beta-casein, however, has a much slower rate of proteolytic digestion; therefore, its consumption results in a nil or much lower and not physiologically relevant BCM-7 level 179, 180.
Two recent studies concluded that in lactose-intolerant patients, after consuming A2A2 milk, gastrointestinal discomforts were reduced compared to the case of conventional milk (A1A2) consumption; and symptoms of pain and fecal urgency were the main findings 181, 182. He et al. 178 observed acute gastrointestinal symptoms associated with milk consumption, such as abdominal pain, bloating, stool frequency and consistency measured by Bristol Stool Scale after the consumption of conventional (A1A2) milk and A2A2 milk after 1 to 12 hour by lactose-intolerant individuals. Among the results, it was observed that abdominal pain, distension and stool frequency were reduced after the consumption of A2 milk for 12 hour by lactose-intolerant Chinese adults. Those studies found a correlation between improved gastrointestinal discomfort and reduced exposure to BCM-7 through the consumption of A2A2 milk, although the peptide was not quantified in these studies. These suggest that A2A2 milk may result in higher tolerance to dairy consumption. He et al. concluded that milk containing A2 beta-casein reduce acute gastrointestinal symptoms of milk intolerance in Chinese adults, while conventional milk containing A1 beta-casein reduced lactase activity and increased gastrointestinal symptoms compared with milk containing A2 beta-casein 178. Therefore, milk-related gastrointestinal symptoms may result from the ingestion of A1 beta-casein rather than lactose in some individuals 178. These studies suggest that A2A2 milk may result in higher tolerance to dairy consumption.
Ho et al. 183 also identified an increase in abdominal pain in healthy individuals after the consumption of conventional milk (A1A2) without lactose intolerance when compared to a group that consumed A2A2 milk. Significant changes in stool consistency were also observed between groups, as well as a significant positive correlation between abdominal pain and softer stool after the consumption of A1A2 milk, raising the hypothesis that these adverse factors were induced by proinflammatory cytokines 183. These preliminary results suggest differences in gastrointestinal responses in some adult humans consuming milk containing beta-casein of either the A1 or the A2 beta-casein type, but require confirmation in a larger study of participants with perceived intolerance to ordinary A1 beta-casein-containing milk.
In contrast, the study conducted by Crowley et al. 184 showed no significant difference in patients after the intake of 400 mL of conventional milk (A1A2 milk) and the same amount of A2A2 milk. A two week period was considered in children with chronic constipation, the measurements of which were performed by bowel movements in relation to transit time. Therefore, under these experimental conditions, the different milk genotypes, conventional milk (A1A2) and A2A2 milk, were not significantly associated with better resolution of chronic constipation symptoms 184.
A double-blind, randomized, crossover study in 45 Chinese individuals (lactose-intolerant and lactose-tolerant [control group]) by Sun et al 185 showed consumption of milk containing A1 beta-casein (A1A2 milk) was associated with increased gastrointestinal inflammation in the small intestine and stomach, worsening of post-dairy digestive discomfort symptoms, delayed transit, and decreased cognitive processing speed and accuracy. Because elimination of A1 beta-casein attenuated these effects, some symptoms of lactose intolerance may stem from inflammation it triggers (the systemic increase in serum biomarkers such as interleukin-4 [IL-4] and immunoglobulins [IgG, IgE and IgG1] in the lactose-intolerant group), and can be avoided by consuming milk containing only the A2 type of beta casein (A2A2 milk) 185.
Recently, another double-blind, randomized, crossover study involving Chinese children aged 5 to 6 years who consumed 300 mL/day of conventional milk (A1A2 milk) versus milk containing only A2 beta-casein (A2A2 milk) for five days 25. The study found that Chinese children aged 5 to 6 years who consumed milk containing only A2 beta-casein (A2A2 milk) had significantly less severe gastrointestinal symptoms as measured by visual analog scales, reduced stool frequency, and improvements in stool consistency, compared with subjects consuming conventional milk (A1A2 milk) 25. There were significant increases from baseline in serum immunologic and inflammatory biomarkers such as interleukin-4 (IL-4), immunoglobulins G (IgG), E (IgE), and G1 (IgG1), and beta-casomorphin-7 (BCM-7) coupled to lower glutathione levels, in subjects consuming conventional milk (A1A2 milk) compared with milk containing only A2 beta-casein (A2A2 milk). Subtle Cognitive Impairment Test analysis showed significant improvements in test accuracy after consumption of milk containing only A2 beta-casein (A2A2 milk). There were no severe adverse events related to consumption of either milk product 25.
Overall, the few human studies do not provide conclusive results that confirm the adverse effects of beta-casomorphin-7 (BCM-7) due to conventional milk (A1A2 milk) consumption. These human studies highlighted the potential effects of beta-casomorphin-7 (BCM-7) to promote greater gastrointestinal discomfort such as gastric disorders and abdominal discomfort similar to the effects caused by lactose intolerance, increased mucus secretion, reduced motility, pain and abdominal distention 9. Studies on the action of beta-casomorphin-7 (BCM-7) in the human central nervous system (brain and spinal cord) reported the activation of the gut–brain axis due to gastric discomfort, and not directly by the binding of BCM-7 to mu (µ) opioid receptors in the central nervous system. This relationship is evidenced in the study conducted by Sheng et al 25 where, after the consumption of A2A2 milk by school-aged children with lactose intolerance, the subtle cognitive impairment test showed improvements in response accuracy and greater processing efficiency. This association with the cognitive system was correlated with the reduction in the effects on the gastrointestinal system caused by BCM-7 released due to the consumption of conventional milk (A1A2 milk). This is a potentially important result, in which exposure to beta-casomorphin-7 (BCM-7) briefly causes side effects in the central nervous system (brain and spinal cord), since direct consequences of BCM-7 to the central nervous system (brain and spinal cord) or other organs are difficult to investigate. In addition, the potential of beta-casomorphin-7 (BCM-7) to increase levels of some immune–inflammatory markers and causing gastrointestinal inflammation condition, which can trigger secondary effects in the abdominal region such as pain, distension, flatulence, and diarrhea.
Another potential emerging field is the possibility of beta-casomorphin-7 (BCM-7) indirectly influencing the central nervous system (brain and spinal cord) through stress to the intestinal microbiota. The gut–brain axis theory was expanded to the microbiota–gut–brain axis to explain the influence of microorganisms on cognitive development and functioning 186. The intestinal microbiota also called gut microbiota, gut microbiome, or gut flora, are the microorganisms, including bacteria, archaea, fungi, and viruses, that live in the digestive tracts and this microbiota is critically involved in the communication of the intestine–brain axis, influencing the brain and behavior modulation, also presenting analgesia effects 187. Gut microbiome disorders may also be associated with excessive mucin production in the intestine triggered by BCM-7 188. Intestinal microbiota disorders can influence the central nervous system by the deficiency in the absorption of molecules used as a substrate for its proper functioning, for example, the absorption of tryptophan, precursor of serotonin, an important neurotransmitter in the central nervous system involved in the regulation of mood, appetite, memory, learning and sleep 189. Some diseases and disorders present as characteristics the imbalance of intestinal microorganisms, such as in gastrointestinal disorders, celiac disease, obesity, diabetes, as well as in mental disorders, eating disorders, autism spectrum disorders and mood disorders 189. These data reinforce the hypothesis raised by Osman et al 190, who observed changes in the microbiota of mice accompanied by changes in mood through gut–brain communication, suggesting that this effect was due to the effect of BCM-7 released from the consumption of conventional milk (A1A2 milk).
In general, adverse reactions to milk consumption are usually referred to as lactose intolerance 188. However, recent studies have theorized that gastrointestinal discomforts can be exclusively attributed to the release of beta-casomorphin-7 (BCM-7) from the hydrolysis of A1 beta-casein 185, 191, 183. Among the groups of individuals considered more susceptible to beta-casomorphin-7 (BCM-7), the following unique factors stand out: Lactose intolerance, autism, type 1 diabetes, cardiovascular diseases, sudden infant death syndrome and chronic childhood constipation 86, 192, 188. Diet can play a key role in the exacerbation of symptoms related to these health conditions 188. However, the European Food Safety Authority (EFSA), after a review of papers published until 2009, published an opinion informing the population that no clear evidence of a cause/effect relationship was found between beta-casomorphin-7 (BCM-7) and the development of some non-communicable diseases 75. The European Food Safety Authority (EFSA) subsequently concluded that the reviewed studies were not sufficient to claim the A1 beta-casein as a cause or risk factor for the onset of these diseases, but reported that beta-Casomorphins may cause gastrointestinal tract disorders, such as bloating, flatulence and abdominal pain. In addition, there is need for further studies to verify the implication of the A1 beta-casein in the development or worsening of the symptoms of non-communicable diseases 86.
In summary, due to the few studies in humans, results from clinical trials are inconclusive 86. Although clinical trials have not yet been sufficient to establish a clear relationship of the adverse effects of beta-casomorphin-7 (BCM-7) on different physiological responses, the likelihood that this mechanism may initiate or exacerbate some gastrointestinal symptoms seems to be high, the evidence of which has been reported in most studies and explored in this review 9. Research is expected to continue to evolve as new studies are being developed and more results are released in the very near future.
Is A2 milk healthier than normal milk?
Systematic reviews on the effects of A1 or A2 beta-casein intake on human health are limited. Four reviews have mentioned that the intake of A2 milk may favor digestion compared to that of A1 milk 193, 1, 194, 195. The studies reported thus far showed the potential of beta-casomorphin-7 (BCM-7) to promote greater gastrointestinal discomfort and increased levels of some immune–inflammatory markers as observed in animal studies. In addition, a gastrointestinal inflammation condition is suggested, which can trigger secondary effects in the abdominal region such as pain, distension, flatulence, and diarrhea 86. However, evidence regarding A2 milk health benefits is limited 9, 33, 196, 197, 33, 37, 198, 177, 199, 200, 201. The findings of this randomized, double-blind, cross-over studies suggest that A2 milk consumption may serve as a beneficial dietary alternative for those with lactose intolerance or milk-related gastrointestinal discomfort, potentially improving gut health and alleviating gastrointestinal discomfort, potentially improving gut health 202, 201, 203.
Table 5. Summary of health effects of A1 and A2 beta-casein consumption
| Health Condition | Effects of A1 beta-casein consumption | Effects of A2 beta-casein consumption | References |
|---|---|---|---|
| Type 1 diabetes | Associated with increased risk of T1D | – | 204, 205, 73 |
| Cardiovascular diseases (CVD) | Associated with increased risk of CVD, atherosclerosis, and IHD | – | 76, 206, 207, 208, 209, 73 |
| Gastrointestinal disease | Associated with chronic constipation, altered gastrointestinal transit, inflammation, and lactose intolerance symptoms | A2 milk is easier to digest and better absorbed than A1 milk Milk containing only A2 beta-casein induces fewer symptoms of lactose intolerance than milk containing both A1 and A2 beta-caseins | 210, 193, 156, 211, 212, 213 |
| Autism and neurological disorders | BCM-7 is implicated in the pathogenesis of autism and other neurological disorders | – | 214, 215, 216, 217, 218, 219, 220, 221, 218, 222, 223 |
| Sudden infant death syndrome (SIDS) | BCM-7 may inhibit the respiratory center in infants leading to apnea and SIDS | – | 218, 222, 223 |
| Allergy | BCM-7 may influence inflammation and allergies | – | 224, 225, 226, 227 |
| Glutathione production | Consumption of milk with both A1 and A2 β-casein led to a decrease in plasma glutathione concentration | Consumption of milk with only A2 β-casein led to an increase in plasma glutathione concentration | 228, 229, 230 |
Gastrointestinal Discomfort
Several studies have shown that A1 milk causes some gastrointestinal discomfort problems during its digestion. Several studies have suggested a possible association between A1 beta-casein consumption and the incidence of chronic constipation in infants and children. Andiran et al 210 found higher intake of cow’s milk, specifically A1 beta-casein, in children diagnosed with chronic constipation and anal fissures. Accordingly, shorter breastfeeding duration, coupled with an early transition to bottle feeding with cow’s milk, was associated with the onset of constipation, suggesting a possible role for A1 beta-casein in the cause of constipation. Experimental research by Brooke-Taylor et al 193 showed that A1 beta-casein may affect gastrointestinal transit time and function. Unlike A2 beta-casein, A1 beta-casein has been shown to prolong the gastrointestinal transit time and alter the gut microbiota composition in rat models. Results from human studies corroborate these findings, suggesting that A1 beta-casein intake correlates with prolonged intestinal transit and less firm stool consistency, which could lead to digestive discomfort. It has been reported that the A2 milk is easier to digest and better absorb than A1 or other types of milk 211. Numerous studies have examined the interplay between A1 beta-casein and opioid receptors in the gastrointestinal tract. Beta-casomorphin-7 (BCM-7), the metabolic by-products of A1 beta-casein digestion, have been shown to bind to peripheral opiate receptors and thereby alter gastrointestinal motility in animal models 156. These interactions may explain the observed effects on gastrointestinal transit and discomfort. In addition, Summer et al 213 found a possible correlation between A1 beta-casein intake and gastrointestinal inflammation, suggesting the involvement of A1 beta-casein in inducing intestinal inflammation. For certain lactose-intolerant individuals, consumption of A1 beta-casein may exacerbate their symptoms. According to Ramakrishnan et al 212 milk containing only A2 beta-casein induces fewer symptoms of lactose intolerance than milk containing both A1 and A2 beta-caseins. This highlights the possibility that A1 beta-casein exacerbates lactose intolerance symptoms, possibly via its interaction with gastrointestinal function and inflammation.
A study has indicated that A1 milk causes higher stool consistency according to Bristol Scale in comparison to A2 milk 231. The study also showed that abdominal pain and stool consistency is positively associated with A1 milk consumption, but this is not observed for A2 milk digestion 231. A study also showed that A2 milk consumption by lactose-intolerant individuals significantly diminished the intolerance symptoms 232. In another study, lactose intolerant individuals consumed A2 milk (100% A2 β-casein), A1 milk (30% A1 and 70% A2 β-casein), regular milk without lactose, and Jersey milk (25% A1 and 75% A2 β-casein) to evaluate their gastrointestinal symptoms and hydrogen production during digestion 233. They showed that A2 milk causes considerably fewer gastrointestinal symptoms and pain 233. Furthermore, studies report reduced breath hydrogen levels, a marker of lactose malabsorption, and improved stool consistency, stool frequency, and inflammatory markers following A2 milk consumption 234.
Another study also examined lactose intolerance symptoms after participants consume A1A2 milk and only A2 milk 235. The study showed that the group which consumes A2 milk presents fewer intolerance symptoms 235. The consumption of A1A2 milk was linked with post-dairy digestive discomfort and a high proportion of inflammation markers and beta-casomorphin-7 (BCM-7) 235. A study performed on animals similarly showed that beta-casomorphin-7 (BCM-7) exerts different impacts on gastrointestinal function such as declining the frequency and amplitude of intestinal contractions. Barnett et al. 236 showed that A1 milk feeding on rats has an increment on myeloperoxidase which is an inflammatory marker with 65%. Generally, it is mainly shown that A1 milk consumption causes systemic inflammation and gastrointestinal mobility related to BCM-7 formation during its digestion 236.
In contrast, A2 milk consumption was not correlated with post-dairy discomfort, and it can be easily consumed without any gastrointestinal discomfort 237. A2 milk consumption by 10 individuals who are not tolerant to A1 milk did not cause any gastrointestinal problems. Another study also showed that A2 milk diminished gastrointestinal-related symptoms of lactose intolerant, whereas A1 milk decreased lactase activity and enhanced symptoms 35. In a study related to A2 milk and athlete health, the effect of A2, regular milk, and placebo on exercise-induced muscle damage is evaluated in a group including 21 men who regularly run. The results showed that A2 milk consumption diminishes muscle function loss and improves the recovery period 238. Randomized controlled trials have demonstrated that individuals consuming A2 milk experience fewer gastrointestinal symptoms, such as nausea, abdominal pain, and fecal urgency, compared to those consuming conventional milk, which contains both A1 and A2 β-casein 239, 240. Therefore, A2 milk may be a favorable drink without causing gastrointestinal discomfort.
Type 1 Diabetes
The relationship between type 1 diabetes and A1 milk is a subject of ongoing research and debate 241, 242, 243, 244. Type 1 diabetes is an autoimmune disease that results from the destruction of insulin-producing cells in the pancreas, leading to insulin deficiency 245, 246. The incidence of type 1 diabetes is increasing worldwide, and environmental factors, including diet, play a significant role in its cause 247. The consumption of cow’s milk, specifically A1 beta-casein, is one of the dietary factors implicated in the development of type 1 diabetes 205. A1 beta-casein has been shown to be diabetogenic in animal studies 204. Furthermore, Laugesen and Elliott 73 showed a positive correlation between A1 milk supply by cows per capita and the incidence of type 1 diabetes in 19 countries. Interestingly, geographical variations have been observed in the incidence of type 1 diabetes, which may be related to differences in A1 milk consumption. For example, Iceland has lower incidence of type 1 diabetes than Scandinavia, which may be due to the lower consumption of A1 beta-casein in Iceland 248. And Finland and Sweden’s highest A1 milk intake per capita had a higher incidence rate of type 1 diabetes, while low type 1 diabetes were in Venezuela and Japan where the lowest A1 milk consumption takes place per capita 73. This suggests that the type of cow’s milk consumed during infancy may influence the risk of developing type 1 diabetes. In addition, recent research has shown that individuals with type 1 diabetes have increased small intestinal permeability, also known as “leaky gut syndrome” 249. This condition, which is characterized by the passage of harmful substances from the gut into the bloodstream, has been linked to autoimmune diseases, including type 1 diabetes. It has been hypothesized that A1 beta-casein may contribute to increased intestinal permeability, thereby promoting the development of type 1 diabetes 250. However, the relationship between A1 milk and type 1 diabetes is complex and may be influenced by other factors. For example, genetic predisposition plays a critical role in the development of type 1 diabetes 251. Additionally, other environmental triggers such as viral infections may interact with dietary factors and influence the risk of developing type 1 diabetes 252.
In conclusion, despite evidence regarding a potential association between A1 milk consumption and type 1 diabetes, further research is required to completely understand this relationship and its implications in disease prevention. Considering the increasing incidence of type 1 diabetes, particularly in children and adolescents, it is critical to identify modifiable risk factors such as diet that can be targeted in strategies to prevent this disease 253.
Autism and neurological disorders
Autism Spectrum Disorder (ASD) also called autism is a lifelong neurodevelopmental disorder affecting social communication, interaction, and behavior 197. ‘Spectrum’ means that autism symptoms and severity vary greatly between individuals. Numerous studies have highlighted a significant role of BCM-7 in the pathogenesis of autism spectrum disorder (ASD) and other neurological disorders. Dohan 216 was one of the first to propose a correlation between autism and food-derived opioids. They postulated that schizophrenia and autism could be associated with the absorption of exorphins, such as beta-casomorphins, from gluten and casein. This hypothesis was supported by Cade et al 214 who discovered elevated levels of IgG antibodies against gliadin (a gluten component) and bovine casein in patients with autism and schizophrenia compared to in control subjects. Their study also revealed improvements in schizophrenia and autism symptoms following a gluten- and casein-free dietary regimen. Cieslinska et al 215 investigated the impact of polymorphisms in the dipeptidyl peptidase IV (DPPIV) and mu-opioid receptor (MOR) genes, which play crucial roles in the modulation of BCM-7 activity. Although they did not find any connection between specific single-nucleotide polymorphisms in these genes and heightened incidence of autism, they did discern differences in the expression of dipeptidyl peptidase IV (DPPIV) and mu-opioid receptor (MOR) when exposed to BCM-7 between healthy children and those with autism spectrum disorder (ASD), implying an interaction between BCM-7 and these genes. Further research by Jarmolowska et al 217 observed higher serum concentrations of BCM-7 and dipeptidyl peptidase IV (DPPIV) in children with autism spectrum disorder (ASD) than in healthy controls. Similarly, Sokolov et al 221 identified direct correlation between autism and BCM-7 levels in both the blood and urine of children with autism.
Beta-casomorphins have also been implicated in other neurological and behavioral disorders. Lindstrom et al 219 detected elevated levels of BCM-7-like opioid peptides in the cerebrospinal fluid (CSF) and plasma of women with postpartum psychosis. Kost et al 218 reported higher levels of BCM-7 in formula-fed infants, who exhibited delayed psychomotor development and increased muscle tone. Moreover, a study by Osman et al 220 revealed that the intake of A1 β-casein milk, a significant source of BCM-7, beyond the weaning age resulted in depressive-like behavior in rats and alterations in brain-opioid and oxytocin receptors. This suggests a potential effect of A1 beta-casein and hence BCM-7 on mood, potentially via the gut-brain axis.
In conclusion, although the precise mechanisms remain to be completely elucidated and require additional investigation, the current body of evidence supports the hypothesis that beta-casomorphins, specifically beta-casomorphin-7 (BCM-7), contribute to the pathogenesis of autism, as well as other neurological and behavioral disorders 33.
Cardiovascular Diseases
The relationship between A1 beta-casein consumption and cardiovascular disease has been extensively investigated for several decades. Several studies have shown that A1 milk consumption was correlated with ischemic heart disease mortality in France, Northern Ireland, and West Germany 74, 73. Similarly, A1 β-casein per capita by milk and cream was strongly associated with ischemic heart disease in 20 different countries. BCM-7 formed after A1 milk consumption is also linked with the oxidation process of low-density lipoprotein (LDL). Macrophages absorb oxidized low-density lipoprotein (LDL) molecules with surface receptors and converted them into foam cells, which promotes atherosclerosis in the heart 254. Tailford et al 208 investigated the direct effect of consuming A1 beta-casein versus A2 beta-casein on atherosclerosis progression and observed that rabbits fed a diet containing A1 beta-casein exhibited significantly elevated levels of serum cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides compared to rabbits fed A2 β-casein or whey protein. Additionally, the consumption of A1 β-casein results in increase in the surface area of aorta covered with fatty streaks, an early indication of atherosclerosis, and thicker lesions in the aortic arch. In another investigation, Torreilles and Guerin 209 discovered that casein-derived peptides may promote the oxidation of human low-density lipoprotein (LDL) cholesterol, a crucial process in atherosclerotic plaque formation. This oxidation of low-density lipoprotein (LDL) occurs via peroxidase-dependent and metal-independent processes, suggesting a distinct and direct pathway via which casein-derived peptides, including those from A1 β-casein, contribute to atherosclerosis. In addition, Heinecke 206 found that LDL oxidation in the human arterial wall leads to an increase in the production of specific oxidized amino acids in proteins. These findings suggested that oxidized LDL, which is potentially influenced by A1 β-casein consumption, contributes to the progression of atherosclerosis.
To broaden the research scope, De Noni et al 76 examined the potential health effects of BCM-7 peptides. Their research suggests that BCM-7 peptides may affect cardiovascular health by adding complexity to the relationship between A1 β-casein and cardiovascular disease. In a broader epidemiological context, McLachlan 207 presented substantial evidence linking A1 β-casein consumption to mortality associated with ischemic heart disease. This study revealed that the regions with higher A1 β-casein consumption had higher ischemic heart disease mortality rates. Similarly, Laugesen and Elliott 73 identified an association between A1 β-casein consumption and ischemic heart disease. However, cautionary notes by Allison and Clarke 200 and Elliott et al 205 emphasize the necessity for further research to completely comprehend this complex relationship. In fact, Elliott et al 205 suggested a potentially common causative risk factor for type 1 diabetes and cardiovascular disease, adding another dimension to the health implications of A1 β-casein consumption.
Some studies suggest that the relation between consumption of A1/A2 β-casein and increased risk of cardiovascular disease is inconclusive. In a randomized crossover human trial in New Zealand, researchers gave 62 subjects β-casein A1 and A2 variants for 2- to 5-week periods without washout and compared blood samples for any changes in plasma lipid concentrations 255. There were no significant differences in the mean plasma concentrations of triacylglycerols and total LDL and HDL cholesterol between β-casein A1 and A2 subjects. This suggests that there may be no atherosclerotic effects induced by A1 β-casein in humans 256. It was pointed out that the other possibility of atherogenic changes by A1 β-casein could be due to digestive release of BCM-7 from either casein A1 or B in the small intestine 256. This peptide is hypothesized to have the ability to oxidize LDL and thus increase the circulating serum lipid concentrations. However, it is challenging to conclude its specificity to casein A1 256.
A double-blind crossover human study investigating measures of the cardioprotective effects of A1/A2 β-casein demonstrated similar results 257. In this study, 15 asymptomatic subjects at high risk of developing cardiovascular disease were given daily supplementation of either casein A1 or A2 for 12 weeks, and the effects on vascular function such as endothelial and arterial change, resting blood pressure, plasma lipids, and inflammatory biomarkers were examined. At the end of the trial, total plasma lipid concentration change, C-reactive protein, fibrinogen, and Von Willebrand factor concentrations between β-casein A1 and A2 showed no difference 257. Furthermore, there were no significant differences in terms of endothelial function, blood pressure, or artery stiffness. This study suggests that beta-casein does not contribute to atherosclerotic changes in human arteries and plays no significant role in increasing adverse cardiovascular events 256. Further studies are warranted to decipher the role of milk A1 β-casein in predisposition to cardiovascular diseases.
Sudden Infant Death Syndrome (SIDS)
Sudden Infant Death Syndrome (SIDS) also called cot death or crib death, is the sudden unexplained death of a child of less than one year of age. Despite extensive research, the exact causes of SIDS remain unclear. Recent studies have begun to investigate a possible link between SIDS and A1 milk consumption. A key finding in this area of research is the potential effect of BCM-7 on the immature central nervous system of infants. In a review, Sun et al 222 proposed that BCM-7 could potentially inhibit the respiratory center in the brainstem of infants, leading to apnea (temporary cessation of breathing) and ultimately death. This hypothesis is supported by empirical evidence from a study conducted by Wasilewska et al 223 on an association between elevated levels of bovine BCM-7, infant apnea, and cow milk consumption. Researchers have found that infants who experienced an apneic event had higher serum levels of BCM-7 than healthy infants. In addition, decreased activity of DPPIV, an enzyme responsible for degrading beta-casomorphins, was observed in all infants after an apneic event. These findings suggest that the consumption of A1 milk may contribute to apneic events in infants, which is a known risk factor for Sudden Infant Death Syndrome (SIDS).
In addition, Kost et al 218 conducted a study showing that breastfed infants had higher levels of human beta-casomorphin-7 (HCM-7), while formula-fed infants consuming cow’s milk-based formula had higher levels of bovine β-casomorphin-7 (irBCM). Furthermore, elevated human beta-casomorphin-7 (BCM-7) levels were associated with delayed psychomotor development and increased muscle tone in formula-fed infants. This suggests that the consumption of A1 milk may contribute to developmental delays in infants, further supporting the potential link between A1 milk and Sudden Infant Death Syndrome (SIDS).
Allergy
The potential impact of BCM-7 on human health and its role in triggering allergic reactions have been studied extensively. BCM-7, an opioid peptide, has the ability to cross the intestinal-blood barrier and influence various functions in humans. Fiedorowicz et al 224 investigated the effects of BCM-7 on proliferation and cytokine secretion by human peripheral blood mononuclear cells, a population of blood cells with a single, round nucleus, primarily consisting of lymphocytes (T cells, B cells, and NK cells) and monocytes. These cells are crucial for the immune system. The results suggested that BCM-7 may induce changes in peripheral blood mononuclear cell proliferation and cytokine secretion, thereby shedding light on the immunomodulatory effects of food-derived opioid peptides. These findings have significant implications, particularly in the context of allergic diseases in newborns. Fiedorowicz et al 225 investigated the effects of BCM-7 in children diagnosed with severe atopic dermatitis (eczema). Their results showed that incubation of peripheral blood mononuclear cells with peptide extracts from cow milk resulted in the upregulation of opioid receptor (MOR) gene expression and concomitant downregulation of DPPIV expression. This study advances our understanding regarding the role of BCM-7 in the pathogenesis of atopic dermatitis (eczema) and provides a valuable diagnostic tool. The effects of BCM-7 are not limited to allergies. According to Fiedorowicz et al 226, the presence of BCM-7 in human milk and infant formula leads to changes in intestinal epithelial proliferation and increased interleukin-8 secretion. These findings improve our understanding regarding the pathogenesis of inflammation and food allergy in infants. In another study, Trivedi et al 227 found that BCM-7 reduced cysteine uptake, decreased the levels of the antioxidant glutathione (GSH), and decreased the levels of the methyl donor S-adenosylmethionine (SAM) in SH-SY5Y human neuroblastoma cells. The epigenetic effects of milk-derived opioid peptides may contribute to gastrointestinal dysfunction and inflammation in susceptible individuals. In conclusion, milk-derived opioid peptides, particularly BCM-7, influence inflammation and allergies, possibly via their effects on peripheral blood mononuclear cell proliferation, cytokine secretion, and gene expression. Further studies are required to completely understand the mechanisms underlying these effects and their clinical significance.
Glutathione production
Glutathione is a tripeptide (cysteine, glycine, and glutamic acid) found in relatively high concentrations in many bodily tissues 258, 259. Glutathione plays a pivotal role in reducing oxidative stress, maintaining redox balance, enhancing metabolic detoxification, and regulating the immune system 258, 259. Empirical evidence suggests a significant increase in plasma glutathione concentration following the consumption of milk containing only A2 β-casein, contrary to that observed after the consumption of milk containing a combination of A1 and A2 β-casein types. This indicates a potential advantage of A2 β-casein-specific milk in promoting the production of antioxidant glutathione in humans. In 2016, a double-blind randomized controlled crossover study on Chinese preschool children diagnosed with mild to moderate milk intolerance was conducted to investigate the plasma glutathione concentrations in healthy subjects 229. Sheng et al 229 postulated that replacing conventional milk, characterized by the presence of both A1 and A2 β-casein proteins, with milk containing only A2 β-casein would lead to reduction in gastrointestinal symptoms and improvement in cognitive performance. Interestingly, their findings also indicated a decrease in glutathione levels following the consumption of conventional milk, as opposed to the consumption of milk containing only A2 β-casein 229. To further expand the scope of this research area, Trivedi et al 230 investigated the effects of food-derived opioid peptides on cysteine uptake, a critical determinant of glutathione production. Their investigation revealed that peptides derived from the hydrolytic digestion of casein and gliadin exerted a modulatory effect on cysteine uptake in human neuronal and gastrointestinal cells. This modulation was associated with changes in the levels of glutathione and SAM. These findings underscore the profound influence of beta-casein variants in milk on glutathione biosynthesis in humans.
- Kaskous S. A1- and A2- milk and their effect on human health. Journal of Food Engineering and Technology. 2020;9:15–21. doi: 10.32732/jfet.2020.9.1.15[↩][↩]
- Smith N.W., Fletcher A.J., Hill J.P., McNabb W.C. Modeling the Contribution of Milk to Global Nutrition. Front. Nutr. 2022;8:716100. doi: 10.3389/fnut.2021.716100[↩][↩]
- Tulipano G. Role of Bioactive Peptide Sequences in the Potential Impact of Dairy Protein Intake on Metabolic Health. Int. J. Mol. Sci. 2020;21:8881. doi: 10.3390/ijms21228881[↩]
- Uzma S., Gill H., Chandrapala J. Casein Micelles as an Emerging Delivery System for Bioactive Food Components. Foods. 2021;10:1965. doi: 10.3390/foods10081965[↩][↩][↩]
- Holland JW, Deeth HC, Alewood PF. Resolution and characterisation of multiple isoforms of bovine kappa-casein by 2-DE following a reversible cysteine-tagging enrichment strategy. Proteomics. (2006) 6:3087–95. 10.1002/pmic.200500780[↩]
- Karav S, Le Parc A, Leite Nobrega de Moura Bell JM, Frese SA, Kirmiz N, Block DE, et al. Oligosaccharides released from milk glycoproteins are selective growth substrates for infant-associated bifidobacteria. Appl Environ Microbiol. (2016) 82:3622–30. 10.1128/AEM.00547-16[↩]
- Barłowska J., Szwajkowska M., Litwińczuk Z., Król J. Nutritional Value and Technological Suitability of Milk from Various Animal Species Used for Dairy Production. Compr. Rev. Food Sci. Food Saf. 2011;10:291–302. doi: 10.1111/j.1541-4337.2011.00163.x[↩]
- Asledottir T., Le T.T., Poulsen N.A., Devold T.G., Larsen L.B., Vegarud G.E. Release of β-Casomorphin-7 from Bovine Milk of Different β-Casein Variants after Ex Vivo Gastrointestinal Digestion. Int. Dairy J. 2018;81:8–11. doi: 10.1016/j.idairyj.2017.12.014[↩]
- de Vasconcelos ML, Oliveira LMFS, Hill JP, Vidal AMC. Difficulties in Establishing the Adverse Effects of β-Casomorphin-7 Released from β-Casein Variants-A Review. Foods. 2023 Aug 22;12(17):3151. doi: 10.3390/foods12173151[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Hannelore D., Vohwinkel M., Rehner G. Effect of Casein and β-Casomorphins on Gastrointestinal Motility in Rats. J. Nutr. 1990;120:252–257. doi: 10.1093/jn/120.3.252[↩][↩][↩]
- Lucey JA, Horne DS. Perspectives on casein interactions. International Dairy Journal. 2018;85:56–65. doi: 10.1016/j.idairyj.2018.04.010[↩]
- Franzoi M, Niero G, Visentin G, Penasa M, Cassandro M, De Marchi M. Variation of detailed protein composition of cow milk predicted from a large database of mid-infrared spectra. Animals. 2019;9:176. doi: 10.3390/ani9040176[↩]
- Shiven P., Shah T., Sabara P., Bhatia D., Panchal K., Italiya J., Koringa P., Rank D.N. Understanding Functional Implication of β-Casein Gene Variants in Four Cattle Breeds Characterized Using AmpliSeq Approach. 3 Biotech. 2020;10:414. doi: 10.1007/s13205-020-02410-2[↩][↩]
- Nan G., Uniacke-Lowe T., O’Regan J., Faulkner H., Alan, Kelly L. Effect of Protein Genotypes on Physicochemical Properties and Protein Functionality of Bovine Milk: A Review. Foods. 2021;10:2409. doi: 10.3390/foods10102409[↩][↩]
- Phelan M., Aherne A., FitzGerald R.J., O’Brien N.M. Casein-derived bioactive peptides: Biological effects, industrial uses, safety aspects and regulatory status. Int. Dairy J. 2009;19:643–654. doi: 10.1016/j.idairyj.2009.06.001[↩][↩]
- Formaggioni P., Summer A., Malacarne M., Mariani P. Milk protein polymorphism: Detection and diffusion of the genetic variants in Bos genus. Ann. Fac. Med. Vet. Univ. Parma. 1999;19:127–165.[↩][↩][↩]
- Pal S, Woodford K, Kukuljan S, Ho S. Milk Intolerance, Beta-Casein and Lactose. Nutrients. 2015 Aug 31;7(9):7285-97. doi: 10.3390/nu7095339[↩][↩][↩][↩]
- Jaiswal K., De S., Sarsavan A. Detection of Single Nucleotide Polymorphism by T-ARMS PCR of Cross Bred Cattle Karan Fries for A1, A2 Beta Casein Types. Int. J. Sci. Res. Biol. Sci. 2014;1:18–22.[↩][↩]
- Nguyen H.T., Schwendel H., Harland D., Day L. Differences in the Yoghurt Gel Microstructure and Physicochemical Properties of Bovine Milk Containing A1A1 and A2A2 β-Casein Phenotypes. Food Res. Int. 2018;112:217–224. doi: 10.1016/j.foodres.2018.06.043[↩][↩]
- Teagan E., Krista, Dawson L., Jacqueline, Keenan I., Andrew, Day S. A Simple Method to Generate β-Casomorphin-7 by in Vitro Digestion of Casein from Bovine Milk. J. Funct. Foods. 2021;85:104631. doi: 10.1016/j.jff.2021.104631[↩][↩][↩][↩]
- Cieslinska A, Kostyra E, Kostyra H, Olenski K, Fiedorowicz E, Kaminski S. Milk from cows of different beta-casein genotypes as a source of beta-casomorphin-7. Int J Food Sci Nutr. 2012;63:426–430. doi: 10.3109/09637486.2011.634785[↩][↩]
- Jinsmaa Y, Yoshikawa M. Enzymatic release of neocasomorphin and beta-casomorphin from bovine beta-casein. Peptides. 1999;20:957–962. doi: 10.1016/S0196-9781(99)00088-1[↩][↩]
- Noni ID. Release of beta-casomorphins 5 and 7 during simulated gastro-intestinal digestion of bovine beta-casein variants and milk-based infant formulas. Food Chem. 2008;110:897–903. doi: 10.1016/j.foodchem.2008.02.077[↩][↩]
- Asledottir T, Le TT, Poulsen NA, Devold TG, Larsen LB, Vegarud GE. Release of β-casomorphin-7 from bovine milk of different β-casein variants after exvivo gastrointestinal digestion. Int Dairy J. 2018; 81: 8-11. doi:10.1016/j.idairyj.2017.12.014[↩][↩][↩]
- Sheng X, Li Z, Ni J, Yelland G. Effects of Conventional Milk Versus Milk Containing Only A2 β-Casein on Digestion in Chinese Children: A Randomized Study. J Pediatr Gastroenterol Nutr. 2019 Sep;69(3):375-382. doi: 10.1097/MPG.0000000000002437[↩][↩][↩][↩][↩][↩]
- Asledottir T, Le TT, Petrat-Melin B, Devold TG, Larsen LB, Vegarud GE. Identification of bioactive peptides and quantification of β-casomorphin-7 from bovine β-casein A1, A2 and I after ex vivo gastrointestinal digestion. Int Dairy J. 2017; 71: 98-106. doi:10.1016/j.idairyj.2017.03.008[↩][↩]
- Boutrou R., Gaudichon C., Dupont D., Jardin J., Airinei G., Marsset-Baglieri A., Benamouzig R., Tomé D., Leonil J. Sequential release of milk protein-derived bioactive peptides in the jejunum in healthy humans. Am. J. Clin. Nutr. 2013;97:1314–1323. doi: 10.3945/ajcn.112.055202[↩][↩]
- De Noni I., Cattaneo S. Occurrence of β-casomorphins 5 and 7 in commercial dairy products and in their digests following in vitro simulated gastro-intestinal digestion. Food Chem. 2010;119:560–566. doi: 10.1016/j.foodchem.2009.06.058[↩][↩]
- De Noni I. Release of β-casomorphins 5 and 7 during simulated gastro-intestinal digestion of bovine β-casein variants and milk-based infant formulas. Food Chem. 2008;110:897–903. doi: 10.1016/j.foodchem.2008.02.077[↩][↩]
- Ul Haq M.R., Kapila R., Kapila S. Release of β-casomorphin-7/5 during simulated gastrointestinal digestion of milk β-casein variants from Indian crossbred cattle (Karan Fries) Food Chem. 2015;168:70–79. doi: 10.1016/j.foodchem.2014.07.024[↩][↩]
- De Noni R.J., FitzGerald H.J.T., Korhonen Y., Le Roux C.T., Livesey I., Thorsdottir D., Tomé R.W. Scientific Report of EFSA prepared by a DATEX Working Group on the potential health impact of β-casomorphins and related peptides. EFSA Sci. Rep. 2009;231:1–107.[↩][↩][↩]
- Woodford K.B. Casomorphins and Gliadorphins Have Diverse Systemic Effects Spanning Gut, Brain and Internal Organs. Int. J. Environ. Res. Public Health. 2021;18:7911. doi: 10.3390/ijerph18157911[↩][↩]
- Jeong H, Park YS, Yoon SS. A2 milk consumption and its health benefits: an update. Food Sci Biotechnol. 2023 Oct 25;33(3):491-503. doi: 10.1007/s10068-023-01428-5[↩][↩][↩][↩][↩][↩]
- Whole milk. https://fdc.nal.usda.gov/food-details/2705385/nutrients[↩]
- He M, Sun J, Jiang ZQ, Yang YX. Effects of cow’s milk beta-casein variants on symptoms of milk intolerance in Chinese adults: a multicentre, randomised controlled study. Nutr J. (2017) 16:72. 10.1186/s12937-017-0275-0[↩][↩][↩][↩][↩][↩][↩]
- Ng-Kwai-Hang KF, Hayes JF, Moxley JE, Monardes HG. Variation in milk protein concentrations associated with genetic polymorphism and environmental factors. J Dairy Sci. (1987) 70:563–70. 10.3168/jds.S0022-0302(87)80042-5[↩][↩][↩][↩][↩][↩][↩]
- Kaplan M, Baydemir B, Günar BB, Arslan A, Duman H, Karav S. Benefits of A2 Milk for Sports Nutrition, Health and Performance. Front Nutr. 2022 Jul 13;9:935344. doi: 10.3389/fnut.2022.935344[↩][↩]
- Ballard O, Morrow AL. Human milk composition. Nutrients and bioactive factors. Pediatr Clin North Am. (2013) 60:49–74. 10.1016/j.pcl.2012.10.002[↩]
- Thomson P, Medina DA, Garrido D. Human milk oligosaccharides and infant gut bifidobacteria: molecular strategies for their utilization. Food Microbiol. (2018) 75:37–46. 10.1016/j.fm.2017.09.001[↩]
- Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. (2015) 91:619–22. 10.1016/j.earlhumdev.2015.09.001[↩]
- Hennet T, Borsig L. Breastfed at Tiffany’s. Trends Biochem Sci. 2016 Jun;41(6):508-518. doi: 10.1016/j.tibs.2016.02.008[↩]
- Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, et al. A strategy for annotating the human milk glycome. J Agric Food Chem. (2006) 54:7471–80. 10.1021/jf0615810[↩]
- Triantis V, Bode L, van Neerven RJJ. Immunological effects of human milk oligosaccharides. Front Pediatr. (2018) 6:190. 10.3389/fped.2018.00190[↩]
- Wiciński M, Sawicka E, Gebalski J, Kubiak K, Malinowski B. Human milk oligosaccharides: health benefits, potential applications in infant formulas, and pharmacology. Nutrients. (2020) 12:266. 10.3390/nu12010266[↩]
- Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. (2012) 22:1147–62. 10.1093/glycob/cws074[↩]
- Bienenstock J, Buck RH, Linke H, Forsythe P, Stanisz AM, Kunze WA. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS One. (2013) 8:e76236. 10.1371/journal.pone.0076236[↩][↩]
- Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci USA. (2011) 108:4653–8. 10.1073/pnas.1000083107[↩]
- Thongaram T, Hoeflinger JL, Chow J, Miller MJ. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J Dairy Sci. (2017) 100:7825–33. 10.3168/jds.2017-12753[↩]
- Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, et al. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem. (2010) 58:5334–40. 10.1021/jf9044205[↩]
- Gotoh A, Katoh T, Sakanaka M, Ling Y, Yamada C, Asakuma S, et al. Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum. Sci Rep. (2018) 8:13958. 10.1038/s41598-018-32080-3[↩]
- Asakuma S, Hatakeyama E, Urashima T, Yoshida E, Katayama T, Yamamoto K, et al. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J Biol Chem. (2011) 286:34583–92. 10.1074/jbc.M111.248138[↩]
- LoCascio RG, Niñonuevo MR, Kronewitter SR, Freeman SL, German JB, Lebrilla CB, et al. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb Biotechnol. (2009) 2:333–42. 10.1111/j.1751-7915.2008.00072.x[↩]
- Frese SA, Hutton AA, Contreras LN, Shaw CA, Palumbo MC, Casaburi G, et al. Persistence of supplemented Bifidobacterium longum subsp. infantis EVC001 in breastfed infants. mSphere. (2017) 2:e00501–17 10.1128/mSphere.00501-17[↩]
- Duar RM, Casaburi G, Mitchell RD, Scofield LNC, Ortega Ramirez CA, Barile D, et al. Comparative genome analysis of Bifidobacterium longum subsp. infantis strains reveals variation in human milk oligosaccharide utilization genes among commercial probiotics. Nutrients. (2020) 12:3247. 10.3390/nu12113247[↩]
- Henrick BM, Hutton AA, Palumbo MC, Casaburi G, Mitchell RD, Underwood MA, et al. Elevated fecal pH indicates a profound change in the breastfed infant gut microbiome due to reduction of bifidobacterium over the past century. mSphere. (2018) 3:e00041-18 10.1128/mSphere.00041-18[↩]
- Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Lourdes Guerrero M, Meinzen-Derr JK, et al. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. (2004) 145:297–303. 10.1016/j.jpeds.2004.04.054[↩]
- Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fucα1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. (2003) 278:14112–20. 10.1074/jbc.M207744200[↩]
- Leach JL, Garber SA, Marcon AA, Prieto PA. In vitro and in vivo effects of soluble, monovalent globotriose on bacterial attachment and colonization. Antimicrob Agents Chemother. (2005) 49:3842–6. 10.1128/AAC.49.9.3842-3846.2005[↩]
- Kuntz S, Rudloff S, Kunz C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br J Nutr. (2008) 99:462–71. 10.1017/S0007114507824068[↩]
- Lin AE, Autran CA, Szyszka A, Escajadillo T, Huang M, Godula K, et al. Human milk oligosaccharides inhibit growth of group B Streptococcus. J Biol Chem. (2017) 292:11243–9. 10.1074/jbc.M117.789974[↩]
- Ackerman DL, Doster RS, Weitkamp JH, Aronoff DM, Gaddy JA, Townsend SD. Human milk oligosaccharides exhibit antimicrobial and antibiofilm properties against group b Streptococcus. ACS Infect Dis. (2017) 3:595–605. 10.1021/acsinfecdis.7b00064[↩]
- Coppa GV, Zampini L, Galeazzi T, Facinelli B, Ferrante L, Capretti R, et al. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr Res. (2006) 59:377–82. 10.1203/01.pdr.0000200805.45593.17[↩]
- Jacobi SK, Yatsunenko T, Li D, Dasgupta S, Yu RK, Berg BM, et al. Dietary isomers of sialyllactose increase ganglioside sialic acid concentrations in the corpus callosum and cerebellum and modulate the colonic microbiota of formula-fed piglets. J Nutr. (2016) 146:200–8. 10.3945/jn.115.220152[↩]
- Vázquez E, Barranco A, Ramírez M, Gruart A, Delgado-García JM, Martínez-Lara E, et al. Effects of a human milk oligosaccharide, 2′-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J Nutr Biochem. (2015) 26:455–65. 10.1016/j.jnutbio.2014.11.016[↩]
- Milk, human, mature, fluid. https://fdc.nal.usda.gov/food-details/171279/nutrients[↩]
- Milk nutritional composition and its role in human health. Pereira PC. Nutrition. 2014 Jun; 30(6):619-27. https://www.ncbi.nlm.nih.gov/pubmed/24800664/[↩]
- Phelan M., Aherne A., FitzGerald R.J., O’Brien N.M. Casein-derived bioactive peptides: Biological effects, industrial uses, safety aspects and regulatory status. Int. Dairy J. 2009;19:643–654. doi: 10.1016/j.idairyj.2009.06.001.[↩]
- Swaisgood H.E. Chemistry of the caseins. In: Fox P.F, editor. Advanced Dairy Chemistry. 1st ed. Volume 1. Elsevier Applied Science; London, UK: 1992. pp. 63–110.[↩]
- Raies M.H., Kapila R., Shandilya U.K., Kapila S. Impact of milk derived β-casomorphins on physiological functions and trends in research: A review. Int. J. Food Prop. 2014;17:1726–1741.[↩]
- Kamiński S, Cieslińska A, Kostyra E. Polymorphism of bovine beta-casein and its potential effect on human health. J Appl Genet 2007;48:189–98. https://www.ncbi.nlm.nih.gov/pubmed/17666771[↩]
- Ng-Kwai-Hang K.F., Grosclaude F. Genetic polymorphism of milk proteins. In: Fox P.F., McSweeney P.L.H., editors. Advanced Dairy Chemistry: Volume 1: Proteins, Parts A & B. Kluwer Academic/Plenum Publishers; New York, NY, USA: 2002. pp. 739–816.[↩]
- De Noni I, Cattaneo S. Occurrence of β-casomorphins 5 and 7 in commercial dairy products and in their digests following in vitro simulated gastro-intestinal digestion. Food Chemistry. 2010;119:560–566. doi: 10.1016/j.foodchem.2009.06.058[↩]
- Laugesen M, Elliott R. Ischaemic heart disease, Type 1 diabetes, and cow milk A1 beta-casein. N Z Med J. 2003 Jan 24;116(1168):U295.[↩][↩][↩][↩][↩][↩][↩]
- McLachlan CN. beta-casein A1, ischaemic heart disease mortality, and other illnesses. Med Hypotheses. 2001 Feb;56(2):262-72. doi: 10.1054/mehy.2000.1265[↩][↩]
- Scientific Report of EFSA prepared by a DATEX Working Group on the potential health impact of β-casomorphins and related peptides. EFSA Scientific Report (2009) 231, 1–107. https://doi.org/10.2903/j.efsa.2009.231r[↩][↩]
- De Noni I, FitzGerald RJ, Korhonen HJ, Le Roux Y, Livesey CT, Thorsdottir I, Tomé D, Witkamp R. Review of the potential health impact of β-casomorphins and related peptides. EFSA Journal. 2009;231:1–107.[↩][↩][↩]
- Cieslinska A, Fiedorowicz E, Rozmus D, Sienkiewicz-Szlapka E, Jarmolowska B, Kaminski S. Does a Little Difference Make a Big Difference? Bovine beta-casein A1 and A2 variants and human health-an update. International Journal of Molecular Sciences. 2022;23:15637. doi: 10.3390/ijms232415637[↩]
- Hambraeus L. Nutritional Aspects of Milk Proteins. In: Fox PF, editor. Advanced Dairy Chemistry–1: Proteins. London: Elsevier Applied Science; 1992. pp. 457–490.[↩]
- Mulvihill D.M., Ennis M.P. Functional milk proteins: Production and utilization. In: Fox P.F., McSweeney P.L.H., editors. Advanced Dairy Chemistry Proteins. 3rd ed. Volume 1. Springer International Publishing AG; New York, NY, USA: 2003. pp. 1175–1228.[↩]
- Hamosh M., Hong H., Hamosh P. Beta-Casomorphins: Milk-β-casein derived opioid peptides. In: Lebenthal E., editor. Textbook of Gastroenterology and Nutrition in Infancy. 2nd ed. Raven Press; New York, NY, USA: 1989. pp. 143–150.[↩]
- Bioactive peptides released from in vitro digestion of human milk with or without pasteurization. Wada Y, Lönnerdal B. Pediatr Res. 2015 Apr; 77(4):546-53. https://www.ncbi.nlm.nih.gov/pubmed/25580741/[↩][↩]
- Novel opioid peptides derived from human beta-casein: human beta-casomorphins. Brantl V. Eur J Pharmacol. 1984 Oct 30; 106(1):213-4. https://www.ncbi.nlm.nih.gov/pubmed/6529969/[↩]
- Changes of beta-casomorphin content in human milk during lactation. Jarmołowska B, Sidor K, Iwan M, Bielikowicz K, Kaczmarski M, Kostyra E, Kostyra H. Peptides. 2007 Oct; 28(10):1982-6. https://www.ncbi.nlm.nih.gov/pubmed/17869380/[↩][↩]
- Mencarini I.R., Woodford K.B., Old K.M. Comparing herd selection strategies for A2 beta-casein. Proc. N. Z. Soc. Anim. Prod. 2013;73:149–154.[↩]
- Pal S, Woodford K, Kukuljan S, Ho S. Milk Intolerance, Beta-Casein and Lactose. Nutrients. 2015;7(9):7285-7297. doi:10.3390/nu7095339. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4586534/[↩][↩]
- Andrea S., Di Frangia F., Marsan P.A., De Noni I., Malacarne M. Occurrence, Biological Properties and Potential Effects on Human Health of β-Casomorphin 7: Current Knowledge and Concerns. Crit. Rev. Food Sci. Nutr. 2020;60:3705–3723. doi: 10.1080/10408398.2019.1707157[↩][↩][↩][↩][↩][↩]
- Sindayikengera S, Xia W. Nutritional evaluation of caseins and whey proteins and their hydrolysates from Protamex . Journal of Zhejiang University Science B. 2006;7(2):90-98. doi:10.1631/jzus.2006.B0090. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1363751/[↩][↩]
- Walstra P, Jenness R. Dairy Chemistry and Physics. New York: John Willey & Sons Inc; 1984. pp. 114–122.[↩]
- [Nutritional physiology of whey and whey components]. Barth CA, Behnke U. Nahrung. 1997 Feb; 41(1):2-12. https://www.ncbi.nlm.nih.gov/pubmed/9157293/[↩]
- Fuente M.A., Hemar Y., Tamehana M., Munro P.A., Singh H. Process-induced changes in whey proteins during the manufacture of whey protein concentrates. Int. Dairy J. 2002;12:361–369. doi: 10.1016/S0958-6946(02)00031-6.[↩]
- Marschall Rhône-Poulenc Award Lecture. Nutritional and functional characteristics of whey proteins in food products. de Wit JN. J Dairy Sci. 1998 Mar; 81(3):597-608. https://www.ncbi.nlm.nih.gov/pubmed/9565865/[↩]
- Emerging health properties of whey proteins and their clinical implications. Krissansen GW. J Am Coll Nutr. 2007 Dec; 26(6):713S-23S. https://www.ncbi.nlm.nih.gov/pubmed/18187438/[↩][↩]
- Therapeutic applications of whey protein. Marshall K. Altern Med Rev. 2004 Jun; 9(2):136-56. https://www.ncbi.nlm.nih.gov/pubmed/15253675/[↩]
- Smithers GW. Whey and whey proteins – from ‘gutter to gold’ Int Dairy J. 2008;18:695–704.[↩]
- Mulvihill DM. Production, Functional Properties and Utilization of Milk Protein Products. In: Fox PF, editor. Advanced Dairy Chemistry–1: Proteins. London: Elsevier Science Publishers; 1992. pp. 369–404.[↩]
- Hambraeus L. Nutritional Aspects of Milk Proteins. In: Fox PF, editor. Developments in Dairy Chemistry–1: Proteins. London: Applied Science Publishers; 1982. pp. 289–313.[↩]
- Piccolomini AF, Kubow S, Lands LC. Clinical Potential of Hyperbaric Pressure-Treated Whey Protein. Samman S, Darnton-Hill I, eds. Healthcare. 2015;3(2):452-465. doi:10.3390/healthcare3020452. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4939533/[↩]
- Boirie Y, Dangin M, Gachon P, Vasson M-P, Maubois J-L, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14930-14935. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC25140/[↩]
- Protein metabolism. Slow and fast dietary proteins. Frühbeck G. Nature. 1998 Feb 26; 391(6670):843, 845. https://www.ncbi.nlm.nih.gov/pubmed/9495333/[↩]
- Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. 2015 Mar;80 Suppl 1:A8-A15. doi: 10.1111/1750-3841.12802[↩]
- Bounous G, Batist G, Gold P. Immunoenhancing property of dietary whey protein in mice: role of glutathione. Clin Invest Med. 1989 Jun;12(3):154-61.[↩]
- Legrand D, Pierce A, Elass E, Carpentier M, Mariller C, Mazurier J. Lactoferrin structure and functions. In Bioactive components of milk. Springer; 2008. pp. 163–194.[↩]
- Guimont C, Marchall E, Girardet JM, Linden G. Biologically active factors in bovine milk and dairy byproducts: influence on cell culture. Crit Rev Food Sci Nutr. 1997 Jun;37(4):393-410. doi: 10.1080/10408399709527780[↩]
- El-Agamy EI, Nawar M, Shamsia SM, Awad S, Haenlein GF. Are camel milk proteins convenient to the nutrition of cow milk allergic children?Small Rumin Res. 2009;82:1–6.[↩]
- Laleye LC, Jobe B, Wasesa AA. Comparative study on heat stability and functionality of camel and bovine milk whey proteins. J Dairy Sci. 2008 Dec;91(12):4527-34. doi: 10.3168/jds.2008-1446[↩]
- Konuspayeva G, Faye B, Loiseau G, Levieux D. Lactoferrin and immunoglobulin contents in camel’s milk (Camelus bactrianus, Camelus dromedarius, and Hybrids) from Kazakhstan. J Dairy Sci. 2007 Jan;90(1):38-46. doi: 10.3168/jds.S0022-0302(07)72606-1[↩]
- El-Hatmi H, Girardet JM, Gaillard JL, Yahyaoui MH, Attia H. Characterisation of whey proteins of camel (camelus dromedarius) milk and colostrum. SmallRumin Res. 2007;70:267–271.[↩]
- Badr G, Ramadan NK, Sayed LH, Badr BM, Omar HM, Selamoglu Z. Why whey? Camel whey protein as a new dietary approach to the management of free radicals and for the treatment of different health disorders. Iranian Journal of Basic Medical Sciences. 2017;20(4):338-349. doi:10.22038/IJBMS.2017.8573. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5425915/[↩]
- What Is Lactose? https://ific.org/resources/articles/what-is-lactose[↩]
- Vesa TH, Marteau P, Korpela R. Lactose intolerance. J Am Coll Nutr. 2000 Apr;19(2 Suppl):165S-175S. doi: 10.1080/07315724.2000.10718086[↩][↩]
- Mattar R, de Campos Mazo DF, Carrilho FJ. Lactose intolerance: diagnosis, genetic, and clinical factors. Clin Exp Gastroenterol. 2012;5:113-21. doi: 10.2147/CEG.S32368[↩][↩]
- InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. Overview: Lactose intolerance. [Updated 2024 Nov 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK310267[↩][↩][↩][↩][↩][↩][↩][↩]
- Bayless TM, Rothfeld B, Massa C, Wise L, Paige D, Bedine MS. Lactose and milk intolerance: clinical implications. N Engl J Med. 1975 May 29;292(22):1156-9. doi: 10.1056/NEJM197505292922205[↩]
- Deng Y, Misselwitz B, Dai N, Fox M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients. 2015 Sep 18;7(9):8020-35. doi: 10.3390/nu7095380[↩]
- Lactose intolerance. https://www.mayoclinic.org/diseases-conditions/lactose-intolerance/symptoms-causes/syc-20374232[↩][↩][↩]
- Lactose intolerance. https://medlineplus.gov/genetics/condition/lactose-intolerance[↩][↩][↩][↩][↩][↩]
- Goosenberg E, Afzal M. Lactose Intolerance. [Updated 2025 Aug 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532285[↩]
- Lomer MC, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice–myths and realities. Aliment Pharmacol Ther. 2008 Jan 15;27(2):93-103. doi: 10.1111/j.1365-2036.2007.03557.x[↩][↩][↩]
- Kretchmer N. Lactose and lactase. Sci Am. 1972 Oct;227(4):71-8.[↩]
- Beja-Pereira A, Luikart G, England PR, Bradley DG, Jann OC, Bertorelle G, Chamberlain AT, Nunes TP, Metodiev S, Ferrand N, Erhardt G. Gene-culture coevolution between cattle milk protein genes and human lactase genes. Nat Genet. 2003 Dec;35(4):311-3. doi: 10.1038/ng1263. Epub 2003 Nov 23. Erratum in: Nat Genet. 2004 Jan;36(1):106.[↩]
- Mobassaleh M, Montgomery RK, Biller JA, Grand RJ. Development of carbohydrate absorption in the fetus and neonate. Pediatrics. 1985 Jan;75(1 Pt 2):160-6.[↩]
- Autosomal Recessive Disorder. https://www.genome.gov/genetics-glossary/Autosomal-Recessive-Disorder[↩]
- AURICCHIO S, RUBINO A, LANDOLT M, SEMENZA G, PRADER A. ISOLATED INTESTINAL LACTASE DEFICIENCY IN THE ADULT. Lancet. 1963 Aug 17;2(7303):324-6. doi: 10.1016/s0140-6736(63)92991-x[↩]
- Upton J, Mackay R, George P. A simple gene test for lactose intolerance/adult hypolactasia. N Z Med J. 2007 Nov 9;120(1265):U2817[↩]
- Swallow DM. Genetics of lactase persistence and lactose intolerance. Annu Rev Genet. 2003;37:197-219. doi: 10.1146/annurev.genet.37.110801.143820[↩]
- Srinivasan R, Minocha A. When to suspect lactose intolerance. Symptomatic, ethnic, and laboratory clues. Postgrad Med. 1998 Sep;104(3):109-11, 115-6, 122-3. doi: 10.3810/pgm.1998.09.577[↩][↩]
- Beyerlein L, Pohl D, Delco F, Stutz B, Fried M, Tutuian R. Correlation between symptoms developed after the oral ingestion of 50 g lactose and results of hydrogen breath testing for lactose intolerance. Aliment Pharmacol Ther. 2008 Apr;27(8):659-65. doi: 10.1111/j.1365-2036.2008.03623.x[↩][↩][↩]
- Arola H. Diagnosis of hypolactasia and lactose malabsorption. Scand J Gastroenterol Suppl. 1994;202:26-35. doi: 10.3109/00365529409091742[↩]
- Hermans MM, Brummer RJ, Ruijgers AM, Stockbrügger RW. The relationship between lactose tolerance test results and symptoms of lactose intolerance. Am J Gastroenterol. 1997 Jun;92(6):981-4.[↩]
- Waud JP, Matthews SB, Campbell AK. Measurement of breath hydrogen and methane, together with lactase genotype, defines the current best practice for investigation of lactose sensitivity. Ann Clin Biochem. 2008 Jan;45(Pt 1):50-8. doi: 10.1258/acb.2007.007147[↩]
- Vonk RJ, Priebe MG, Koetse HA, Stellaard F, Lenoir-Wijnkoop I, Antoine JM, Zhong Y, Huang CY. Lactose intolerance: analysis of underlying factors. Eur J Clin Invest. 2003 Jan;33(1):70-5. doi: 10.1046/j.1365-2362.2003.01099.x[↩]
- Facioni MS, Raspini B, Pivari F, Dogliotti E, Cena H. Nutritional management of lactose intolerance: the importance of diet and food labelling. J Transl Med. 2020 Jun 26;18(1):260. doi: 10.1186/s12967-020-02429-2[↩]
- Sethi S, Tyagi SK, Anurag RK. Plant-based milk alternatives an emerging segment of functional beverages: a review. J Food Sci Technol. 2016 Sep;53(9):3408-3423. doi: 10.1007/s13197-016-2328-3[↩]
- Paul AA, Kumar S, Kumar V, Sharma R. Milk Analog: Plant based alternatives to conventional milk, production, potential and health concerns. Crit Rev Food Sci Nutr. 2020;60(18):3005-3023. doi: 10.1080/10408398.2019.1674243[↩]
- Pakdaman MN, Udani JK, Molina JP, Shahani M. The effects of the DDS-1 strain of lactobacillus on symptomatic relief for lactose intolerance – a randomized, double-blind, placebo-controlled, crossover clinical trial. Nutr J. 2016 May 20;15(1):56. doi: 10.1186/s12937-016-0172-y[↩]
- Shi LH, Balakrishnan K, Thiagarajah K, Mohd Ismail NI, Yin OS. Beneficial Properties of Probiotics. Trop Life Sci Res. 2016 Aug;27(2):73-90. doi: 10.21315/tlsr2016.27.2.6[↩]
- Kudlacek S, Freudenthaler O, Weissböeck H, Schneider B, Willvonseder R. Lactose intolerance: a risk factor for reduced bone mineral density and vertebral fractures? J Gastroenterol. 2002;37(12):1014-9. doi: 10.1007/s005350200171[↩]
- Di Stefano M, Veneto G, Malservisi S, Cecchetti L, Minguzzi L, Strocchi A, Corazza GR. Lactose malabsorption and intolerance and peak bone mass. Gastroenterology. 2002 Jun;122(7):1793-9. doi: 10.1053/gast.2002.33600[↩]
- Alharbi O, El-Sohemy A. Lactose Intolerance (LCT-13910C>T) Genotype Is Associated with Plasma 25-Hydroxyvitamin D Concentrations in Caucasians: A Mendelian Randomization Study. J Nutr. 2017 Jun;147(6):1063-1069. doi: 10.3945/jn.116.246108[↩][↩]
- Zewdie, Awoke. (2015). ANIMAL PRODUCTS PROCESSING AND MARKETING. https://www.researchgate.net/publication/277013033_ANIMAL_PRODUCTS_PROCESSING_AND_MARKETING[↩]
- Lifschitz C, Szajewska H. Cow’s milk allergy: evidence-based diagnosis and management for the practitioner. Eur J Pediatr. 2015 Feb;174(2):141-50. doi: 10.1007/s00431-014-2422-3[↩]
- Edwards CW, Younus MA. Cow Milk Allergy. [Updated 2024 Oct 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542243[↩][↩][↩]
- Wood RA, Sicherer SH, Vickery BP, Jones SM, Liu AH, Fleischer DM, Henning AK, Mayer L, Burks AW, Grishin A, Stablein D, Sampson HA. The natural history of milk allergy in an observational cohort. J Allergy Clin Immunol. 2013 Mar;131(3):805-12. doi: 10.1016/j.jaci.2012.10.060[↩]
- Milk allergy. https://www.mayoclinic.org/diseases-conditions/milk-allergy/symptoms-causes/syc-20375101[↩]
- Bartuzi Z, Cocco RR, Muraro A, Nowak-Węgrzyn A. Contribution of Molecular Allergen Analysis in Diagnosis of Milk Allergy. Curr Allergy Asthma Rep. 2017 Jul;17(7):46. doi: 10.1007/s11882-017-0716-z[↩]
- Caffarelli C, Ricò S, Rinaldi L, Povesi Dascola C, Terzi C, Bernasconi S. Blood pressure monitoring in children undergoing food challenge: association with anaphylaxis. Ann Allergy Asthma Immunol. 2012 Apr;108(4):285-6. doi: 10.1016/j.anai.2012.02.001[↩]
- Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, Mearin ML, Papadopoulou A, Ruemmele FM, Staiano A, Schäppi MG, Vandenplas Y; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012 Aug;55(2):221-9. doi: 10.1097/MPG.0b013e31825c9482[↩][↩]
- Kansu A, Yüce A, Dalgıç B, Şekerel BE, Çullu-Çokuğraş F, Çokuğraş H. Consensus statement on diagnosis, treatment and follow-up of cow’s milk protein allergy among infants and children in Turkey. Turk J Pediatr. 2016;58(1):1-11. doi: 10.24953/turkjped.2016.01.001[↩][↩][↩][↩]
- Pensabene L, Salvatore S, D’Auria E, Parisi F, Concolino D, Borrelli O, Thapar N, Staiano A, Vandenplas Y, Saps M. Cow’s Milk Protein Allergy in Infancy: A Risk Factor for Functional Gastrointestinal Disorders in Children? Nutrients. 2018 Nov 9;10(11):1716. doi: 10.3390/nu10111716[↩]
- Restani P, Gaiaschi A, Plebani A, Beretta B, Cavagni G, Fiocchi A, Poiesi C, Velonà T, Ugazio AG, Galli CL. Cross-reactivity between milk proteins from different animal species. Clin Exp Allergy. 1999 Jul;29(7):997-1004. doi: 10.1046/j.1365-2222.1999.00563.x[↩]
- Høst, A. (1994), Cow’s milk protein allergy and intolerance in infancy Some clinical, epidemiological and immunological aspects. Pediatric Allergy and Immunology, 5: 5-36. https://doi.org/10.1111/j.1399-3038.1994.tb00352.x[↩]
- Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM; NIAID-Sponsored Expert Panel. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010 Dec;126(6):1105-18. doi: 10.1016/j.jaci.2010.10.008[↩]
- Yue D, Ciccolini A, Avilla E, Waserman S. Food allergy and anaphylaxis. J Asthma Allergy. 2018 Jun 20;11:111-120. doi: 10.2147/JAA.S162456[↩]
- Anagnostou K. Anaphylaxis in Children: Epidemiology, Risk Factors and Management. Curr Pediatr Rev. 2018;14(3):180-186. doi: 10.2174/1573396314666180507115115[↩]
- Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002;13(s15):23-8. doi: 10.1034/j.1399-3038.13.s.15.7.x[↩]
- Defilippi C, Gomez E, Charlin V, Silva C. Inhibition of small intestinal motility by casein: a role of beta casomorphins? Nutrition. 1995 Nov-Dec;11(6):751-4.[↩][↩][↩]
- Daniel H, Vohwinkel M, Rehner G. Effect of casein and beta-casomorphins on gastrointestinal motility in rats. J Nutr. 1990 Mar;120(3):252-7. doi: 10.1093/jn/120.3.252[↩]
- Becker A, Hempel G, Grecksch G, Matthies H. Effects of beta-casomorphin derivatives on gastrointestinal transit in mice. Biomed Biochim Acta. 1990;49(11):1203-7.[↩]
- Claustre J, Toumi F, Trompette A, Jourdan G, Guignard H, Chayvialle JA, et al. Effects of peptides derived from dietary proteins on mucus secretion in rat jejunum. Am J Physiol Gastrointest Liver Physiol. 2002;283:G521–8. doi: 10.1152/ajpgi.00535.2001[↩]
- Trompette A, Claustre J, Caillon F, Jourdan G, Chayvialle JA, Plaisancie P. Milk bioactive peptides and beta-casomorphins induce mucus release in rat jejunum. J Nutr. 2003;133:3499–503. doi: 10.1093/jn/133.11.3499[↩]
- Zoghbi S, Trompette A, Claustre J, El Homsi M, Garzon J, Jourdan G, et al. beta-Casomorphin-7 regulates the secretion and expression of gastrointestinal mucins through a mu-opioid pathway. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1105–13. doi: 10.1152/ajpgi.00455.2005[↩]
- Elitsur Y, Luk GD. Beta-casomorphin (BCM) and human colonic lamina propria lymphocyte proliferation. Clin Exp Immunol. 1991;85:493–7. doi: 10.1111/j.1365-2249.1991.tb05755.x[↩]
- Kayser H, Meisel H. Stimulation of human peripheral blood lymphocytes by bioactive peptides derived from bovine milk proteins. FEBS Lett. 1996;383:18–20. doi: 10.1016/0014-5793(96)00207-4[↩]
- Richard D., Clarke A., Ni J., Trivedi M. Clinical Evaluation of Glutathione Concentrations after Consumption of Milk Containing Different Subtypes of β-Casein: Results from a Randomized, Cross-over Clinical Trial. Nutr. J. 2015;15:82. doi: 10.1186/s12937-016-0201-x[↩]
- Boutrou R., Gaudichon C., Dupont D., Jardin J., Airinei G., Marsset-Baglieri A., Benamouzig R., Tomé D., Leonil J. Sequential Release of Milk Protein-Derived Bioactive Peptides in the Jejunum in Healthy Humans. Am. J. Clin. Nutr. 2013;97:1314–1323. doi: 10.3945/ajcn.112.055202[↩]
- Sheng X., Li Z., Ni J., Yelland G. Effects of Conventional Milk Versus Milk Containing Only A2 β-Casein on Digestion in Chinese Children: A Randomized Study. J. Pediatr. Gastroenterol. Nutr. 2019;69:375–382. doi: 10.1097/MPG.0000000000002437[↩]
- Sebely P., Woodford K., Kukuljan S., Ho S. Milk Intolerance, Beta-Casein and Lactose. Nutrients. 2015;7:7285–7297. doi: 10.3390/nu7095339[↩]
- Morris D, Khurasany M, Nguyen T, et al. Glutathione and infection. Biochim Biophys Acta. 2013; 1830(5): 3329-3349. doi:10.1016/j.bbagen.2012.10.012[↩]
- Jinsmaa Y, Yoshikawa M. Enzymatic release of neocasomorphin and beta-casomorphin from bovine beta-casein. Peptides. 1999;20(8):957-62. doi: 10.1016/s0196-9781(99)00088-1[↩][↩]
- Sheng X, Li Z, Ni J, Yelland G. Effects of conventional milk versus milk containing only A2 β-casein on digestion in Chinese children: a randomized study. J Pediatr Gastroenterol Nutr. 2019; 69(3): 375-382. doi:10.1097/MPG.0000000000002437[↩]
- Jianqin S, Leiming X, Lu X, Yelland GW, Ni J, Clarke AJ. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr J. 2016; 15: 35. doi:10.1186/s12937-016-0147-z[↩]
- Duarte-Vázquez M, García-Ugalde C, Villegas-Gutiérrez L, García-Almendárez B, Rosado J. Production of cow’s milk free from beta-casein A1 and its application in the manufacturing of specialized foods for early infant nutrition. Foods. 2017; 6(7):50. doi:10.3390/foods6070050[↩]
- He M, Sun J, Jiang ZQ, Yang YX. Effects of cow’s milk beta-casein variants on symptoms of milk intolerance in Chinese adults: a multicentre, randomised controlled study. Nutr J. 2017; 16: 72. doi:10.1186/s12937-017-0275-0[↩]
- Ho S, Woodford K, Kukuljan S, Pal S. Comparative effects of A1 versus A2 beta-casein on gastrointestinal measures: a blinded randomised cross-over pilot study. Eur J Clin Nutr. 2014; 68(9): 994-1000. doi:10.1038/ejcn.2014.127[↩]
- Park YW, Haenlein GFW. A2 bovine milk and caprine milk as a means of remedy for milk protein allergy. Dairy. 2021; 2(2): 191-201. doi:10.3390/dairy2020017[↩]
- Savaiano DA, Boushey CJ, McCabe GP. Lactose intolerance symptoms assessed by meta-analysis: a grain of truth that leads to exaggeration. J Nutr. 2006 Apr;136(4):1107-13. doi: 10.1093/jn/136.4.1107[↩]
- Jianqin S, Leiming X, Lu X, Yelland GW, Ni J, Clarke AJ. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr J. 2016 Apr 2;15:35. doi: 10.1186/s12937-016-0147-z. Erratum in: Nutr J. 2016 Apr 29;15(1):45. doi: 10.1186/s12937-016-0164-y[↩][↩]
- He M, Sun J, Jiang ZQ, Yang YX. Effects of cow’s milk beta-casein variants on symptoms of milk intolerance in Chinese adults: a multicentre, randomised controlled study. Nutr J. 2017 Oct 25;16(1):72. doi: 10.1186/s12937-017-0275-0[↩][↩][↩][↩]
- Boutrou R, Gaudichon C, Dupont D, Jardin J, Airinei G, Marsset-Baglieri A, Benamouzig R, Tomé D, Leonil J. Sequential release of milk protein-derived bioactive peptides in the jejunum in healthy humans. Am J Clin Nutr. 2013 Jun;97(6):1314-23. doi: 10.3945/ajcn.112.055202[↩]
- Ul Haq MR, Kapila R, Kapila S. Release of β-casomorphin-7/5 during simulated gastrointestinal digestion of milk β-casein variants from Indian crossbred cattle (Karan Fries). Food Chem. 2015 Feb 1;168:70-9. doi: 10.1016/j.foodchem.2014.07.024[↩]
- Ramakrishnan M., Eaton T.K., Sermet O.M., Savaiano D.A. Milk Containing A2 β-Casein ONLY, as a Single Meal, Causes Fewer Symptoms of Lactose Intolerance than Milk Containing A1 and A2 β-Caseins in Subjects with Lactose Maldigestion and Intolerance: A Randomized, Double-Blind, Crossover Trial. Nutrients. 2020;12:3855. doi: 10.3390/nu12123855[↩]
- Milan A.M., Shrestha A., Karlström H.J., Martinsson J.A., Nilsson N.J., Perry J.K., Day L., Barnett M.P.G., Cameron-Smith D. Comparison of the Impact of Bovine Milk β-Casein Variants on Digestive Comfort in Females Self-Reporting Dairy Intolerance: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2020;111:149–160. doi: 10.1093/ajcn/nqz279[↩]
- Ho S., Woodford K., Kukuljan S., Pal S. Comparative Effects of A1 versus A2 Beta-Casein on Gastrointestinal Measures: A Blinded Randomised Cross-over Pilot Study. Eur. J. Clin. Nutr. 2014;68:994–1000. doi: 10.1038/ejcn.2014.127[↩][↩][↩]
- Crowley E., Williams L., Roberts T., Dunstan R., Jones P. Does Milk Cause Constipation? A Crossover Dietary Trial. Nutrients. 2013;5:253–266. doi: 10.3390/nu5010253[↩][↩]
- Jianqin S., Leiming X., Lu X., Yelland G.W., Ni J., Clarke A.J. Effects of Milk Containing Only A2 Beta Casein versus Milk Containing Both A1 and A2 Beta Casein Proteins on Gastrointestinal Physiology, Symptoms of Discomfort, and Cognitive Behavior of People with Self-Reported Intolerance to Traditional Cows’ Milk. Nutr. J. 2015;15:35. doi: 10.1186/s12937-016-0147-z[↩][↩][↩]
- Elisa K., Zukerman R., Eshraghi R.S., Mittal J., Deth R.C., Castejon A.M., Trivedi M., Mittal R., Eshraghi A.A. Nutritional Interventions for Autism Spectrum Disorder. Nutr. Rev. 2020;78:515–531. doi: 10.1093/nutrit/nuz092[↩]
- Cryan J.F., O’Riordan K.J., Cowan C.S., Sandhu K.V., Bastiaanssen T.F., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018[↩]
- Kay SI S., Delgado S., Mittal J., Eshraghi R.S., Mittal R., Eshraghi A.A. Beneficial Effects of Milk Having A2 β-Casein Protein: Myth or Reality? J. Nutr. 2021;151:1061–1072. doi: 10.1093/jn/nxaa454[↩][↩][↩][↩]
- Boushra D., Van Oudenhove L., Vervliet B., Verbeke K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3[↩][↩]
- Aya O., Zuffa S., Walton G., Fagbodun E., Zanos P., Georgiou P., Kitchen I., Swann J., Bailey A. Post-Weaning A1/A2 β-Casein Milk Intake Modulates Depressive-like Behavior, Brain μ-Opioid Receptors, and the Metabolome of Rats. IScience. 2021;24:103048. doi: 10.1016/j.isci.2021.103048[↩]
- Beata J., Bukało M., Fiedorowicz E., Cieślińska A., Kordulewska N.K., Moszyńska M., Świątecki A., Kostyra E. Role of Milk-Derived Opioid Peptides and Proline Dipeptidyl Peptidase-4 in Autism Spectrum Disorders. Nutrients. 2019;11:87. doi: 10.3390/nu11010087[↩]
- Davor D., Noel, McCarthy A., Vasiljevic T. Bovine β-Casomorphins: Friends or Foes? A Comprehensive Assessment of Evidence from in Vitro and Ex Vivo Studies. Trends Food Sci. Technol. 2021;116:681–700. doi: 10.1016/j.tifs.2021.08.003[↩]
- Brooke-Taylor S, Dwyer K, Woodford K, Kost N. Systematic review of the gastrointestinal effects of A1 compared with A2 beta-casein. Advances in Nutrition. 2017;8:739–748. doi: 10.3945/an.116.013953[↩][↩][↩]
- Kay SS, Delgado S, Mittal J, Eshraghi RS, Mittal R, Eshraghi AA. Beneficial effects of milk having a2 beta-casein protein: Myth or reality? Journal of Nutrition. 2021;151:1061–1072. doi: 10.1093/jn/nxaa454[↩]
- Kullenberg de Gaudry D, Lohner S, Schmucker C, Kapp P, Motschall E, Horrlein S, Roger C, Meerpohl JJ. Milk A1 beta-casein and health-related outcomes in humans: A systematic review. Nutrition Reviews. 2019;77:278–306. doi: 10.1093/nutrit/nuy063[↩]
- Borş A, Borş SI, Floriștean VC. Health-Related Outcomes and Molecular Methods for the Characterization of A1 and A2 Cow’s Milk: Review and Update. Vet Sci. 2024 Apr 12;11(4):172. doi: 10.3390/vetsci11040172[↩]
- Fernández-Rico S, Mondragón ADC, López-Santamarina A, Cardelle-Cobas A, Regal P, Lamas A, Ibarra IS, Cepeda A, Miranda JM. A2 Milk: New Perspectives for Food Technology and Human Health. Foods. 2022 Aug 9;11(16):2387. doi: 10.3390/foods11162387[↩][↩]
- Kay SS, Delgado S, Mittal J, Eshraghi RS, Mittal R, Eshraghi AA. Beneficial Effects of Milk Having A2 β-Casein Protein: Myth or Reality? J Nutr. 2021 May 11;151(5):1061-1072. doi: 10.1093/jn/nxaa454[↩]
- Truswell, A. The A2 milk case: a critical review. Eur J Clin Nutr 59, 623–631 (2005). https://doi.org/10.1038/sj.ejcn.1602104[↩]
- Allison AJ, Clarke AJ. Further research for consideration in ‘the A2 milk case’. Eur J Clin Nutr. 2006 Jul;60(7):921-4; reply 924-5. doi: 10.1038/sj.ejcn.1602276[↩][↩]
- Choi Y, Kim N, Song CH, Kim S, Lee DH. The Effect of A2 Milk on Gastrointestinal Symptoms in Comparison to A1/A2 Milk: A Single-center, Randomized, Double-blind, Cross-over Study. J Cancer Prev. 2024 Jun 30;29(2):45-53. doi: 10.15430/JCP.24.007[↩][↩]
- Song CH, Kim N, Choi Y, Kim S, Kim KS, Park MH, Lee SH, Lee DH. Beneficial effect of consuming milk containing only A2 beta-casein on gut microbiota: A single-center, randomized, double-blind, cross-over study. PLoS One. 2025 May 8;20(5):e0323016. doi: 10.1371/journal.pone.0323016[↩]
- Ramakrishnan M, Mysore Saiprasad S, Savaiano DA. Prolonged Consumption of A2 β-Casein Milk Reduces Symptoms Compared to A1 and A2 β-Casein Milk in Lactose Maldigesters: A Two-Week Adaptation Study. Nutrients. 2024 Jun 20;16(12):1963. doi: 10.3390/nu16121963[↩]
- Chia JSJ, McRae JL, Enjapoori AK, Lefevre CM, Kukuljan S, Dwyer KM. Dietary cows’ milk protein A1 beta-casein increases the incidence of T1D in NOD mice. Nutrients. 2018;10:1291. doi: 10.3390/nu10091291[↩][↩]
- Elliott RB, Harris DP, Hill JP, Bibby NJ, Wasmuth HE. Type I (insulin-dependent) diabetes mellitus and cow milk: Casein variant consumption. Diabetologia. 1999;42:292–296. doi: 10.1007/s001250051153[↩][↩][↩][↩]
- Heinecke JW. Mass spectrometric quantification of amino acid oxidation products in proteins: insights into pathways that promote LDL oxidation in the human artery wall. FASEB Journal. 1999;13:1113–1120. doi: 10.1096/fasebj.13.10.1113[↩][↩]
- McLachlan CN. Beta-casein A1, ischaemic heart disease mortality, and other illnesses. Medical Hypotheses. 2001;56:262–272. doi: 10.1054/mehy.2000.1265[↩][↩]
- Tailford KA, Berry CL, Thomas AC, Campbell JH. A casein variant in cow’s milk is atherogenic. Atherosclerosis. 2003;170:13–19. doi: 10.1016/S0021-9150(03)00131-X[↩][↩]
- Torreilles J, Guérin MC. Des peptides issus de la caséine peuvent provoquer l’oxydation des LDL humaines par un processus dépendant des peroxydases et indépendant des métaux [Casein-derived peptides can promote human LDL oxidation by a peroxidase-dependent and metal-independent process]. C R Seances Soc Biol Fil. 1995;189(5):933-42. French.[↩][↩]
- Andiran F, Dayi S, Mete E. Cows milk consumption in constipation and anal fissure in infants and young children. Journal of Paediatrics and Child Health. 2003;39:329–331. doi: 10.1046/j.1440-1754.2003.00152.x[↩][↩]
- Park YW, Haenlein GFW. A2 bovine milk and caprine milk as a means of remedy for milk protein allergy. Dairy. 2021;2:191–201. doi: 10.3390/dairy2020017[↩][↩]
- Ramakrishnan M, Eaton TK, Sermet OM, Savaiano DA. Milk containing A2 β-casein only, as a single meal, causes fewer symptoms of lactose intolerance than milk containing A1 and A2 β-caseins in subjects with lactose maldigestion and intolerance: A randomized, double-blind, crossover trial. Nutrients. 2020;12:3855. doi: 10.3390/nu12123855[↩][↩]
- Summer A, Di Frangia F, Ajmone Marsan P, De Noni I, Malacarne M. Occurrence, biological properties and potential effects on human health of beta-casomorphin 7: Current knowledge and concerns. Critical Reviews in Food Science and Nutrition. 2020;60:3705–3723. doi: 10.1080/10408398.2019.1707157[↩][↩]
- Cade R, Privette M, Fregly M, Rowland N, Sun Z, Zele V, Wagemaker H, Edelstein C. Autism and schizophrenia: intestinal disorders. Nutritional Neuroscience. 2000;3:57–72. doi: 10.1080/1028415X.2000.11747303[↩][↩]
- Cieslinska A, Sienkiewicz-Szlapka E, Wasilewska J, Fiedorowicz E, Chwala B, Moszynska-Dumara M, Cieslinski T, Bukalo M, Kostyra E. Influence of candidate polymorphisms on the dipeptidyl peptidase IV and mu-opioid receptor genes expression in aspect of the beta-casomorphin-7 modulation functions in autism. Peptides. 2015;65:6–11. doi: 10.1016/j.peptides.2014.11.012[↩][↩]
- Dohan FC. Hypothesis: genes and neuroactive peptides from food as cause of schizophrenia. Adv Biochem Psychopharmacol. 1980;22:535-48.[↩][↩]
- Jarmolowska B, Bukalo M, Fiedorowicz E, Cieslinska A, Kordulewska NK, Moszynska M, Swiatecki A, Kostyra E. Role of milk-derived opioid peptides and proline dipeptidyl peptidase-4 in autism spectrum disorders. Nutrients. 2019;11:87. doi: 10.3390/nu11010087[↩][↩]
- Kost NV, Sokolov OY, Kurasova OB, Dmitriev AD, Tarakanova JN, Gabaeva MV, Zolotarev YA, Dadayan AK, Grachev SA, Korneeva EV, Mikheeva IG, Zozulya AA. Beta-casomorphins-7 in infants on different type of feeding and different levels of psychomotor development. Peptides. 2009;30:1854–1860. doi: 10.1016/j.peptides.2009.06.025[↩][↩][↩][↩][↩]
- Lindstrom LH, Nyberg F, Terenius L, Bauer K, Besev G, Gunne LM, Lyrenas S, Willdeck-Lund G, Lindberg B. CSF and plasma beta-casomorphin-like opioid peptides in postpartum psychosis. American Journal of Psychiatry. 1984;141:1059–1066. doi: 10.1176/ajp.141.9.1059[↩][↩]
- Osman A, Zuffa S, Walton G, Fagbodun E, Zanos P, Georgiou P, Kitchen I, Swann J, Bailey A. Post-weaning A1/A2 beta-casein milk intake modulates depressive-like behavior, brain mu-opioid receptors, and the metabolome of rats. iScience. 2021;24:103048. doi: 10.1016/j.isci.2021.103048[↩][↩]
- Sokolov O, Kost N, Andreeva O, Korneeva E, Meshavkin V, Tarakanova Y, Dadayan A, Zolotarev Y, Grachev S, Mikheeva I, Varlamov O, Zozulya A. Autistic children display elevated urine levels of bovine casomorphin-7 immunoreactivity. Peptides. 2014;56:68–71. doi: 10.1016/j.peptides.2014.03.007[↩][↩]
- Sun Z, Zhang Z, Wang X, Cade R, Elmir Z, Fregly M. Relation of beta-casomorphin to apnea in sudden infant death syndrome. Peptides. 2003;24:937–943. doi: 10.1016/S0196-9781(03)00156-6[↩][↩][↩]
- Wasilewska J, Sienkiewicz-Szlapka E, Kuzbida E, Jarmolowska B, Kaczmarski M, Kostyra E. The exogenous opioid peptides and DPPIV serum activity in infants with apnoea expressed as apparent life threatening events (ALTE) Neuropeptides. 2011;45:189–195. doi: 10.1016/j.npep.2011.01.005[↩][↩][↩]
- Fiedorowicz E, Jarmolowska B, Iwan M, Kostyra E, Obuchowicz R, Obuchowicz M. The influence of mu-opioid receptor agonist and antagonist peptides on peripheral blood mononuclear cells (PBMCs) Peptides. 2011;32:707–712. doi: 10.1016/j.peptides.2010.12.003[↩][↩]
- Fiedorowicz E, Kaczmarski M, Cieslinska A, Sienkiewicz-Szlapka E, Jarmolowska B, Chwala B, Kostyra E. Beta-casomorphin-7 alters mu-opioid receptor and dipeptidyl peptidase IV genes expression in children with atopic dermatitis. Peptides. 2014;62:144–149. doi: 10.1016/j.peptides.2014.09.020[↩][↩]
- Fiedorowicz E, Markiewicz LH, Sidor K, Swiatecka D, Cieslinska A, Matysiewicz M, Piskorz-Ogorek K, Sienkiewicz-Szlapka E, Teodorowicz M, Swiatecki A, Kostyra E. The influence of breast milk and infant formulae hydrolysates on bacterial adhesion and Caco-2 cells functioning. Food Research International. 2016;89:679–688. doi: 10.1016/j.foodres.2016.09.022[↩][↩]
- Trivedi MS, Hodgson NW, Walker SJ, Trooskens G, Nair V, Deth RC. Epigenetic effects of casein-derived opioid peptides in SH-SY5Y human neuroblastoma cells. Nutrition & Metabolism. 2015;12:54. doi: 10.1186/s12986-015-0050-1[↩][↩]
- Deth R, Clarke A, Ni J, Trivedi M. Clinical evaluation of glutathione concentrations after consumption of milk containing different subtypes of beta-casein: Results from a randomized, cross-over clinical trial. Nutrition Journal. 2016;15:82. doi: 10.1186/s12937-016-0201-x[↩]
- Sheng X, Li Z, Ni J, Yelland G. Effects of conventional milk versus milk containing only a2 beta-casein on digestion in Chinese children: a randomized study. Journal of Pediatric Gastroenterology and Nutrition. 2019;69:375–382. doi: 10.1097/MPG.0000000000002437[↩][↩][↩][↩]
- Trivedi MS, Shah JS, Al-Mughairy S, Hodgson NW, Simms B, Trooskens GA, Van Criekinge W, Deth RC. Food-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequences. The Journal of Nutritional Biochemistry. 2014;25:1011–1018. doi: 10.1016/j.jnutbio.2014.05.004[↩][↩]
- Ho S, Woodford K, Kukuljan S, Pal S. Comparative effects of A1 versus A2 beta-casein on gastrointestinal measures: a blinded randomised cross-over pilot study. Eur J Clin Nutr. (2014) 68:994–1000. 10.1038/ejcn.2014.127[↩][↩]
- Rivera-Brown AM, Gutiérrez R, Gutiérrez JC, Frontera WR, Bar-Or O. Drink composition, voluntary drinking, and fluid balance in exercising, trained, heat-acclimatized boys. J Appl Physiol. (1999) 86:78–84. 10.1152/jappl.1999.86.1.78[↩]
- Ramakrishnan M, Eaton T, Sermet O, Savaiano D. A single meal of milk containing A2 β-casein causes fewer symptoms and lower gas production than milk containing both A1 and A2 β-casein among lactose intolerant individuals. Curr Dev Nutr. (2020) 4:772–772. 10.1093/cdn/nzaa052_041[↩][↩]
- Ramakrishnan M., Eaton T.K., Sermet O.M., Savaiano D.A. Milk containing A2 β-casein only, as a single meal, causes fewer symptoms of lactose intolerance than milk containing A1 and A2 β-caseins in subjects with lactose maldigestion and intolerance: A randomized, double-blind, crossover trial. Nutrients. 2020;12:3855. doi: 10.3390/nu12123855[↩]
- Massella E, Piva S, Giacometti F, Liuzzo G, Zambrini AV, Serraino A. Evaluation of bovine beta casein polymorphism in two dairy farms located in northern Italy. Ital J Food Saf . (2017) 6:6904. 10.4081/ijfs.2017.6904[↩][↩][↩]
- Barnett MPG, Mcnabb WC, Roy NC, Woodford KB, Clarke AJ. Dietary A1 β-casein affects gastrointestinal transit time, dipeptidyl peptidase-4 activity, and inflammatory status relative to A2 β-casein in Wistar rats. Int J Food Sci Nutr. (2014) 65:720–7. 10.3109/09637486.2014.898260[↩][↩]
- Roy BD. Milk: the new sports drink? a review. J Int Soc Sports Nutr. (2008) 5:15. 10.1186/1550-2783-5-15[↩]
- Kirk B, Mitchell J, Jackson M, Amirabdollahian F, Alizadehkhaiyat O, Clifford T. A2 Milk enhances dynamic muscle function following repeated sprint exercise, a possible ergogenic aid for A1-protein intolerant athletes? Nutrients. (2017) 9:94. 10.3390/nu9020094[↩]
- Milan A.M., Shrestha A., Karlström H.J., Martinsson J.A., Nilsson N.J., Perry J.K., Day L., Barnett M.P., Cameron-Smith D. Comparison of the impact of bovine milk β-casein variants on digestive comfort in females self-reporting dairy intolerance: A randomized controlled trial. Am. J. Clin. Nutr. 2020;111:149–160. doi: 10.1093/ajcn/nqz279[↩]
- Sheng X., Li Z., Ni J., Yelland G. Effects of conventional milk versus milk containing only A2 β-casein on digestion in Chinese children: A randomized study. J. Pediatr. Gastroenterol. Nutr. 2019;69:375. doi: 10.1097/MPG.0000000000002437[↩]
- Reddy PRK, Reddy AN, Ramadevi A, Kumar DS. Nutritional significance of indigenous cow milk with regard to a2 β-casein – an overview. (2016) 5:3376–80.[↩]
- Gustavsson F, Buitenhuis AJ, Johansson M, Bertelsen HP, Glantz M, Poulsen NA, et al. Effects of breed and casein genetic variants on protein profile in milk from Swedish Red, Danish Holstein, and Danish Jersey cows. J Dairy Sci. (2014) 97:3866–77. 10.3168/jds.2013-7312[↩]
- McLachlan CNS. beta-casein A1, ischaemic heart disease mortality, and other illnesses. Med Hypotheses. (2001) 56:262–72. 10.1054/mehy.2000.1265[↩]
- Birgisdottir BE, Hill JP, Thorsson AV, Thorsdottir I. Lower consumption of cow milk protein A1 β-Casein at 2 years of age, rather than consumption among 11-to 14-year-old adolescents, may explain the lower incidence of type 1 diabetes in iceland than in scandinavia on JSTOR. Ann Nutr Metab. (2006) 50:117–83. 10.1159/000090738[↩]
- DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018 Jun 16;391(10138):2449-2462. doi: 10.1016/S0140-6736(18)31320-5[↩]
- Pozzilli P. Beta-casein in cow’s milk: A major antigenic determinant for type 1 diabetes? Journal of Endocrinological Investigation. 1999;22:562–567. doi: 10.1007/BF03343610[↩]
- Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–2348. doi: 10.1016/S0140-6736(16)30507-4[↩]
- Thorsdottir I, Birgisdottir BE, Johannsdottir IM, Harris DP, Hill J, Steingrimsdottir L, Thorsson AV. Different beta-casein fractions in Icelandic versus Scandinavian cow’s milk may influence diabetogenicity of cow’s milk in infancy and explain low incidence of insulin-dependent diabetes mellitus in Iceland. Pediatrics. 2000;106:719–724. doi: 10.1542/peds.106.4.719[↩]
- Vaarala O. Leaking gut in type 1 diabetes. Current Opinion in Gastroenterology. 2008;24:701–706. doi: 10.1097/MOG.0b013e32830e6d98[↩]
- Chia JSJ, McRae JL, Kukuljan S, Woodford K, Elliott RB, Swinburn B, Dwyer KM. A1 beta-casein milk protein and other environmental pre-disposing factors for type 1 diabetes. Nutrition & Diabetes. 2017;7:e274. doi: 10.1038/nutd.2017.16[↩]
- Pociot F, Lernmark A. Genetic risk factors for type 1 diabetes. Lancet. 2016;387:2331–2339. doi: 10.1016/S0140-6736(16)30582-7[↩]
- Lempainen J, Tauriainen S, Vaarala O, Makela M, Honkanen H, Marttila J, Veijola R, Simell O, Hyoty H, Knip M, Ilonen J. Interaction of enterovirus infection and cow’s milk-based formula nutrition in type 1 diabetes-associated autoimmunity. Diabetes/Metabolism Research and Reviews. 2012;28:177–185. doi: 10.1002/dmrr.1294[↩]
- Wu HB, Zhong JM, Hu RY, Wang H, Gong WW, Pan J, Fei FR, Wang M, Guo LH, Yang L, Yu M. Rapidly rising incidence of type 1 diabetes in children and adolescents aged 0–19 years in Zhejiang, China, 2007 to 2013. Diabetic Medicine. 2016;33:1339–1346. doi: 10.1111/dme.13010[↩]
- Allison AJ, Clarke AJ. Further research for consideration in ‘the A2 milk case. Eur J Clin Nutr. (2005) 60:921–4. 10.1038/sj.ejcn.1602276[↩]
- Venn BJ, Skeaff CM, Brown R, Mann JI, Green TJ. A comparison of the effects of A1 and A2 beta-casein protein variants on blood cholesterol concentrations in New Zealand adults. Atherosclerosis. 2006 Sep;188(1):175-8. doi: 10.1016/j.atherosclerosis.2005.10.020[↩]
- Kay SS, Delgado S, Mittal J, Eshraghi RS, Mittal R, Eshraghi AA. Beneficial Effects of Milk Having A2 β-Casein Protein: Myth or Reality? J Nutr. 2021 May 11;151(5):1061-1072. https://doi.org/10.1093/jn/nxaa454[↩][↩][↩][↩]
- Chin-Dusting J, Shennan J, Jones E, Williams C, Kingwell B, Dart A. Effect of dietary supplementation with beta-casein A1 or A2 on markers of disease development in individuals at high risk of cardiovascular disease. Br J Nutr. 2006 Jan;95(1):136-44. doi: 10.1079/bjn20051599[↩][↩]
- Minich DM, Brown BI. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients. 2019 Sep 3;11(9):2073. doi: 10.3390/nu11092073[↩][↩]
- Pizzorno J. Glutathione! Integr Med (Encinitas). 2014 Feb;13(1):8-12. https://pmc.ncbi.nlm.nih.gov/articles/PMC4684116[↩][↩]