Contents
What is lime essential oil
A lime (Citrus Aurantifolia) also known as the Key Lime in the USA and the Mexican Lime, is a hybrid citrus fruit, which is any of several species and hybrids of trees and shrubs in the rue family (Rutaceae), widely grown in tropical and subtropical areas for their edible acidic fruits. The difficulty in identifying exactly which species of fruit are called “lime” in different parts of the English-speaking world is increased by the botanical complexity of the citrus genus itself, to which the majority of limes belong. Species of this genus hybridize readily, and it is only recently that genetic studies have started to throw light on the structure of the genus. The majority of cultivated species are in reality hybrids, produced from the citron (Citrus medica), the mandarin orange (Citrus reticulata), the pomelo (Citrus maxima) and in particular with many lime varieties, the micrantha (Citrus micrantha). Although the precise origin is uncertain, wild limes are believed to have first grown in Indonesia or Southeast Asia, and then were transported to the Mediterranean region and north Africa around 1000 BC.
Figure 1. Lime
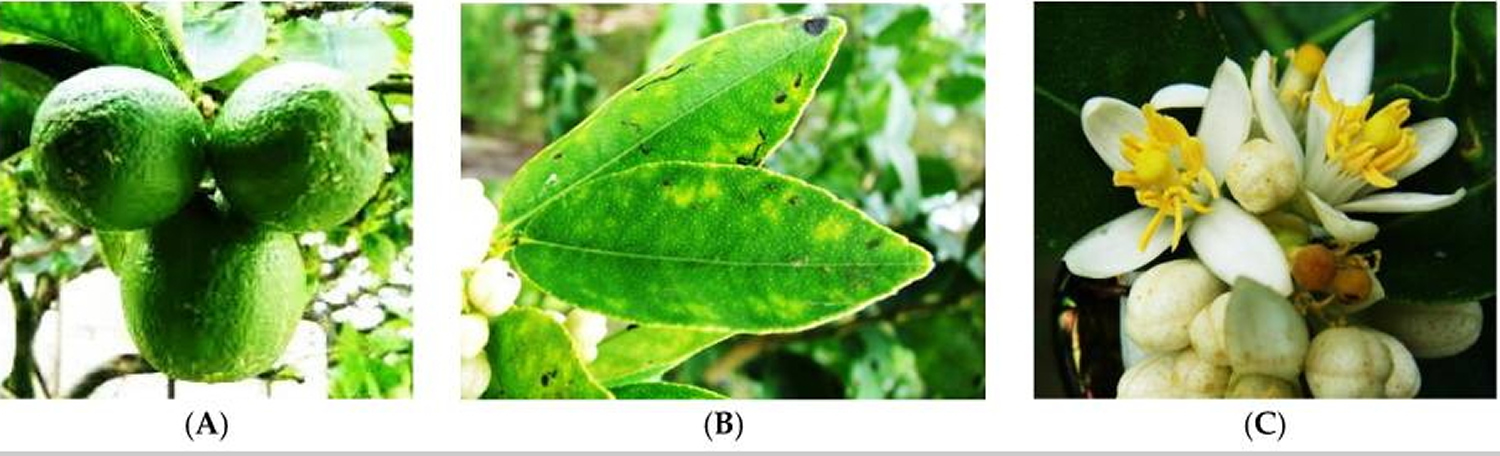
Figure 2. Key Lime or Mexican Lime (Citrus Aurantifolia) – (A) fruit; (B) leaf; and (C) flower of Citrus Aurantifolia.
Figure 3. Kaffir Lime (Citrus Hystrix) – (A) fruit; (B) leaf; and (C) flower of Citrus Hystrix.
Figure 4. Kassia Lime or Kalamansi (Citrus Microcarpa) – (A) fruit; (B) leaf; and (C) flower of Citrus Microcarpa
Lime is typically round, lime green, 3–6 centimeters (1.2–2.4 in) in diameter, and contains acidic juice vesicles. The Persian lime (Citrus latifolia) is one of the most common commercial varieties, though the smaller key lime, or Mexican lime (Citrus Aurantifolia), is also economically important in many places. Lime oil, from the peel of the fruit, is processed mainly in the West Indies. Citrate of lime and citric acid are also prepared from the fruit.
There are several species of citrus trees whose fruits are called limes, including the Key lime (Citrus Aurantifolia), Persian lime, kaffir lime, and desert lime. Limes are a rich source of vitamin C, sour and are often used to accent the flavors of foods and beverages. They are grown year-round. Plants with fruit called “limes” have diverse genetic origins; limes do not form a monophyletic group.
Lime oil production processes
Several processes are used to produce lime oil. Expressed lime oil are produced by pressing the outer rind of the ripe fruits by sponge-press (i.e., by hand) or machines 1. Lime oil may be produced more economically using an integrated juice-oil procedure such as the Brown Oil Extractor 2, where citrus fruit is partially submerged in water and abraded by metal discs. The oil is separated from the juice as a water-emulsion, and further separated using centrifugation to obtain the oil. The yield of expressed oil is approximately 4% for lemons (somewhat less for limes) based on the weight of whole fruit 3. Lime oil is also produced by distillation of expressed oil or direct distillation of fruit 2. Lime oil is distilled (rectified) for removal of terpenes in order to improve solubility and permit use for flavoring carbonated beverages 3. Lime oil can also be steam distilled to remove nonvolatile furocoumarins and are subsequently marketed as “psoralen free” (LOLI Global Industries). The types of lemon oil and lime oil added to cosmetics are not regulated by law, nor limited by recommendations of appropriate trade organizations.
Lime essential oil phototoxicity
Lime oil are added as fragrances to a number of perfumed cosmetic products 4. Many of these products are intended for use on sun-exposed skin. Clinical and experimental studies have found that lime oil are phototoxic. A phototoxic reaction may occur when certain chemicals are applied to the skin and subsequently exposed to the sun. Phototoxic reactions result from direct damage to tissue caused by light activation of the photosensitising agent. This is called photocontact dermatitis. Photodermatitis is a self-limited problem that resolves spontaneously once the offending agent is removed or avoided. If avoiding the offending agent is not practical, then affected individuals should follow sun protection strategies, including wearing sun protective clothing and using sunscreen. The U.S. Food and Drug Administration (FDA) has reported the oil from the Persian lime (Citrus aurantifolia, var. Swingle) is presented as a cause of photodermatitis, and 11 cases are reported in which this reaction was observed 5. Because of their phototoxicity, the International Fragrance Association has recommended safe use levels for “leave on products” applied to sun-exposed skin. These safe use levels are intended to avoid short-term phototoxicity. However, long-term effects have not been addressed.
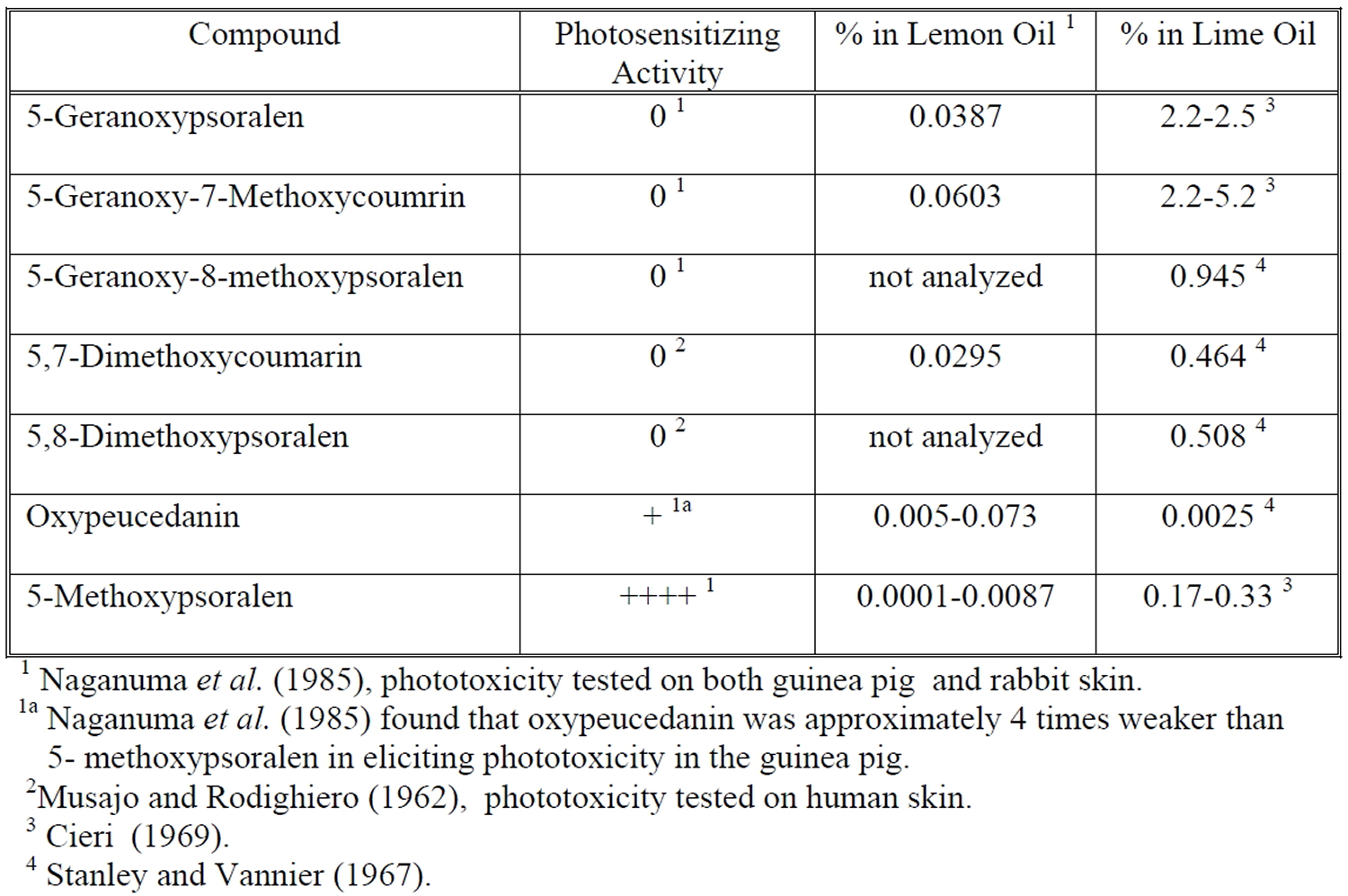
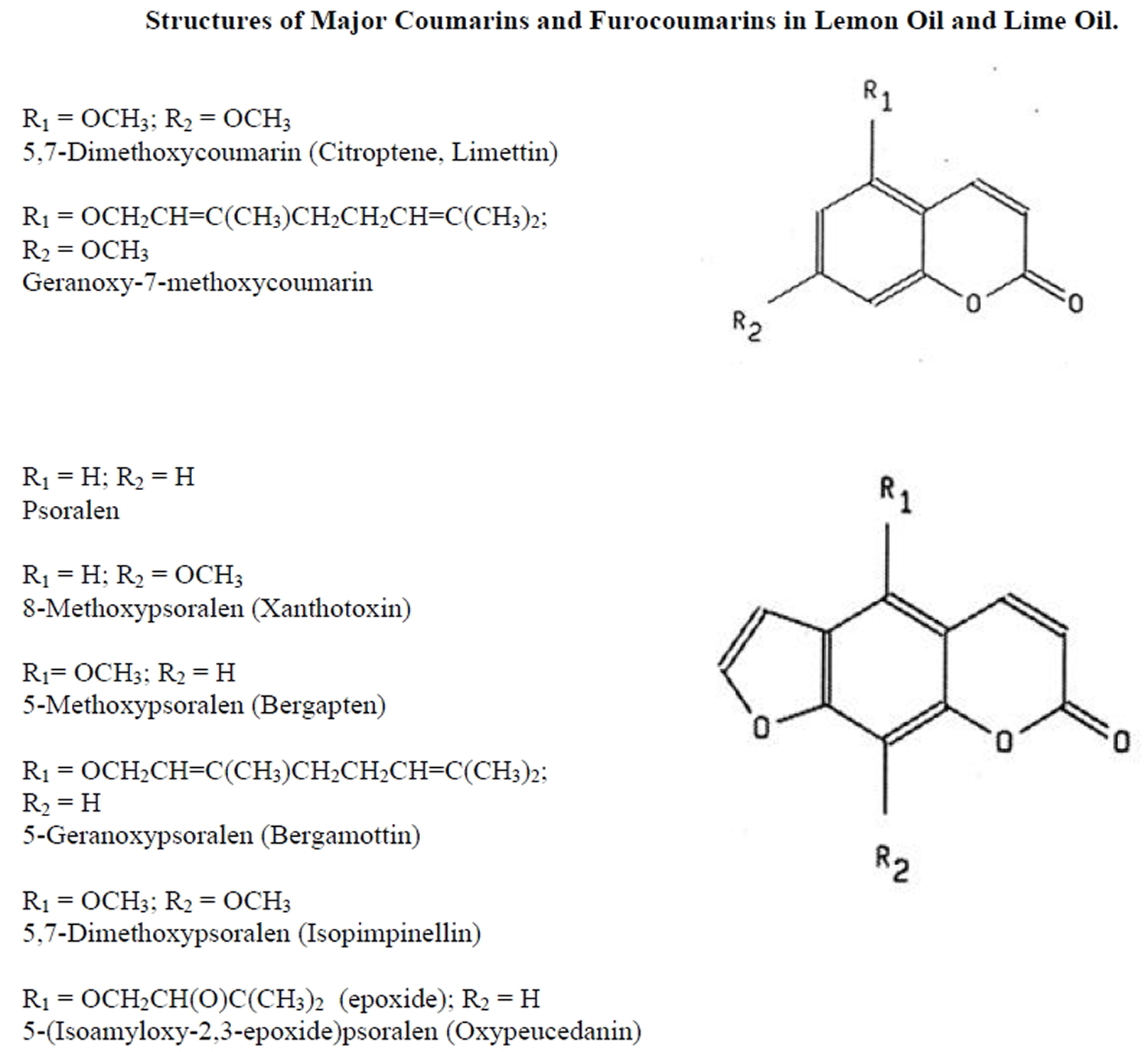
The phototoxicity elicited by lime oil has been associated with naturally occurring furocoumarins. Two of these furocoumarins, 5-methoxypsoralen (bergapten) and oxypeucedanin, have been identified as the probable phototoxins in both lemon oil and lime oil 4. The levels of these furocoumarins have been found to vary significantly with growing conditions. The phototoxic potency of oxypeucedanin was only one-quarter of that of 5-methoxypsoralen (bergapten). However, the amounts of these two phototoxic compounds present in lemon oils produced in different regions of the world varied by a factor of more than 20 (5-methoxypsoralen, 4-87 ppm; oxypeucedanin, 26-728 ppm), and their ratio was not constant. The two compounds accounted for essentially all of the phototoxic activity of all lemon-oil samples. Among various other citrus-essential oils investigated, lime oil and bitter-orange oil also contained large amounts of oxypeucedanin. Oxypeucedanin was found to elicit photopigmentation on colored-guinea-pig skin without preceding visible erythema.
While the photogenotoxicity of 5-methoxypsoralen is well established, the photogenotoxicity of oxypeucedanin is unknown 4. Bacterial mutagenesis studies indicated that oxypeucedanin is not mutagenic, however photogenotoxicity studies of oxypeucedanin are not available. Additionally, the mutagenicity and photomutagenicity of mixtures of coumarins and furocoumarins in lemon oil and lime oil have not been assessed 4. The photogenotoxicity of oxypeucedanin alone or in mixtures of compounds representative of lemon oil and lime oil has not been assessed. These photogenotoxicity studies are needed. Additional studies on photocarcinogenicity should be conducted if coumarins or furocoumarins are significantly photogenotoxic as individual compounds or in complex mixtures (e.g. lemon or lime oil).
Lemon oil and lime oil were selected by the Center for Food Safety and Applied Nutrition for photogenotoxicity and photocarcinogenesis studies since these phototoxic oils contain furocoumarins. Furocoumarins such as 5-methoxypsoralen have been shown to be photocarcinogenic and are present in lemon and lime oils 4.
The phototoxicity of lemon oil and lime oil has been demonstrated in both experimental models and humans. Naganuma et al. 6, studied the phototoxicity of lemon oil obtained from different geographic locations. Lemon oil was diluted in ethanol (100% lemon oil, 50% lemon oil, and 20% lemon oil) and applied to backs of shaved, albino guinea pigs. Animals were then exposed to UVA radiation (320-400 nm, 13 J/cm²). Erythema was evaluated at 24, 48 and 72 hr after irradiation. Undiluted lemon oil and 50% lemon oil elicited phototoxicity for most of the samples tested. Lemon oil from Australia and Brazil elicited a weaker phototoxic than lemon oil from the Ivory Coast, Sicily, California, or Argentina. In an effort to identify the phototoxic components in lemon oil, these investigators fractionated samples by solvent extraction and subsequent phototoxicity testing of isolated components. Two phototoxic components were identified: oxypeucedanin and 5-methoxypsoralen (see Figure 5).
Figure 5. Major Coumarins and Furocoumarins (Phototoxic Components) in Lime oil
Forbes et al. 7, have investigated the phototoxicity of a large number of fragrance raw materials including lemon oil and lime oil. Fragrance raw materials were tested using humans, pigs and albino, hairless mice. Several irradiation sources were used including sunlight, a solar simulator and a UVA radiation source (black light). Expressed lime oil was phototoxic in all three species under all three radiation sources. Distilled lime oil usually elicited no phototoxic response in any species. Two samples of distilled lime oil (derived from expressed lime oil), however, were found to be phototoxic. Lemon oil from Greece, Italy and from Florida (Persian limes) was reported to be phototoxic.
Coumarins and Furocoumarins Occurring in Lemon Oil and Lime Oil
Investigators have examined the level of coumarins and furocoumarins in lemon oil and lime oil (Table 1). A large body of evidence indicates that these secondary metabolites are photoactivated phytoalexins, produced to protect plants from bacterial, fungal, parasitic, and insectile infestations 8. Consistent with their protective function, levels of coumarins and furocoumarins vary widely. Naganuma et al. 6, found that levels of oxypeudedanin doubled in lemon oil derived from the same growing area in Australia between 1979 and 1980. The levels of oxypeudedanin in lemon oil from the Ivory Coast, Sicily and California diminished nearly three-fold in the same time period.
The photogenotoxicity and photocarcinogenicity of several linear furocoumarins (psoralens) have been well documented 9. 5-Methoxypsoralen (bergapten), a component of lemon and lime oil, is photogenototic in both prokaryotic and eukaryotic test systems. In addition, 5-methoxypsoralen (bergapten) has been found to be photocarcinogenic in an experimental model 10. Little is known about the photogenotoxicity and photocarcinogenicity of other furocoumarins found in lemon oil and lime oil. The mutagenicity of oxypeucedanin has been assessed in 6 tester strains of Salmonella typhimurium by Uwaifo 11. Oxypeucedanin was not mutagenic, however, photomutagenicity was not assessed.
Table 1. Levels of Major Coumarins and Furocoumarins in Lemon Oil and Lime Oil
Lime essential oil uses
Usually the Key lime or Mexican lime (Citrus Aurantifolia) is used in cuisine and traditional medicine. Key lime (Citrus Aurantifolia) is a spiny stem plant which is about 3–5 m tall. This plant has ovate-shaped 5–9 cm long leaves with 3–5 cm thickness. The flowers of lime (Citrus Aurantifolia) are white and the fruits are green and turn yellow after ripe with a diameter of 3–6 cm 13. The essential oil from lime (Citrus Aurantifolia) contains a variety of monoterpene and sesquiterpene hydrocarbons, and limonene 14 is the most abundant one. Traditionally, lime (Citrus Aurantifolia) is used to aid digestion process, and to reduce sugar, fat, and cholesterol in blood 15. The oil extracted from the fruits can be used for cold, asthma, arthritis, and bronchitis 16. The fruit juice is used as a facial wash to refresh the skin and prevent pimples, increase stamina, treat dysfunctional uterine bleeding, and act as an antidote for poison 17. Lime juice also has been found to be an excellent cough reliever when added with sugar and honey 18. Moreover, it can also reduce body temperature, remove body smell and act as a softener for meat 13. Additionally, it also has been useful as mosquito, cat, and moth repellants 19, 20. Lime (Citrus Aurantifolia) has been reported to have biological activities, e.g., antioxidant and anti-inflammatory 15.
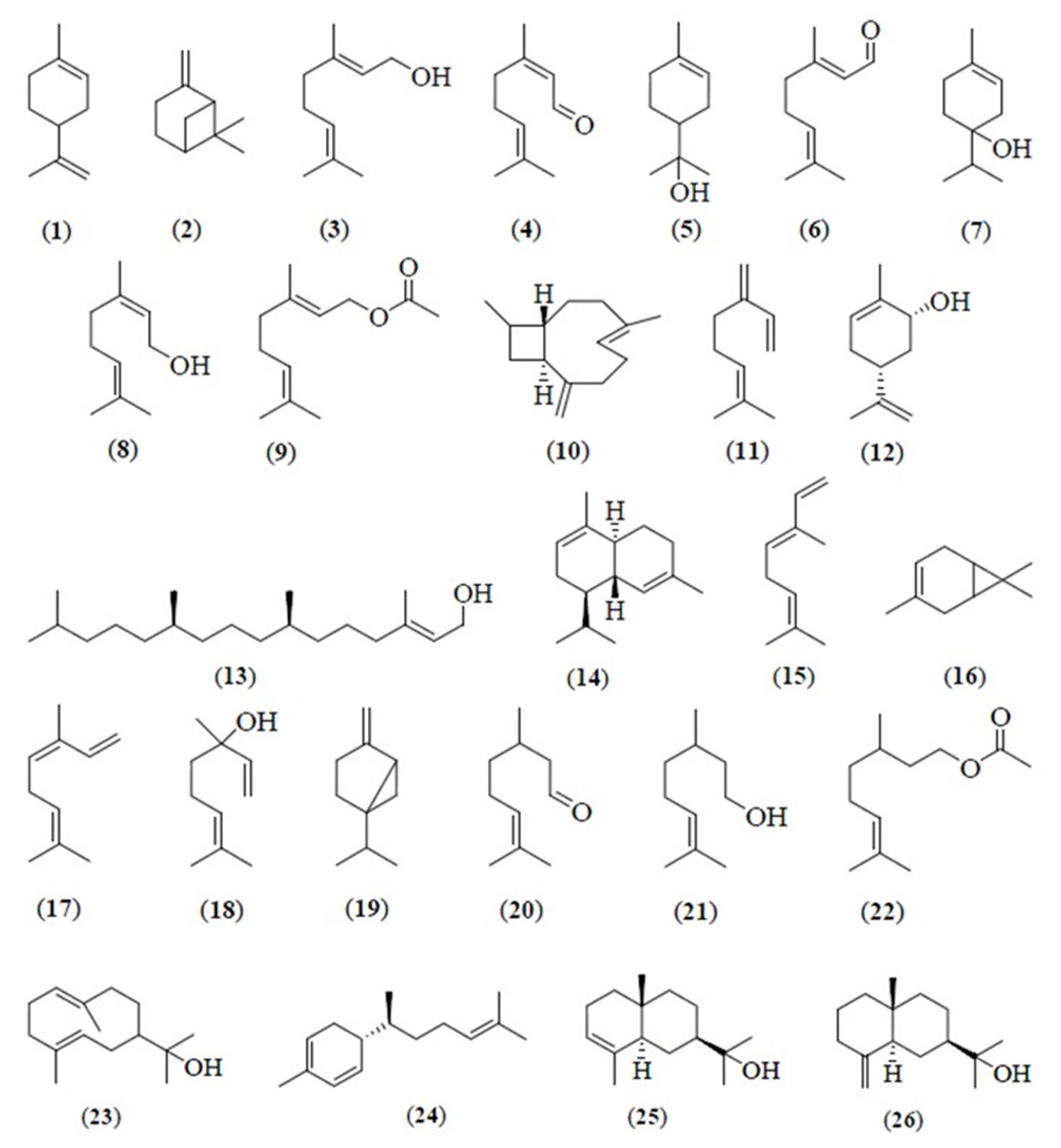
The essential oil of lime (Citrus aurantifolia) peels from Malaysia 21, was reported to contain limonene (1) (39.3%), β-pinene (2) (28.4%), geraniol (3) (7.5%), neral (4) (5.3%), α-terpineol (5) (2.4%), geranial (6) (2.1%), and terpinen-4-ol (7) (2.0%) (Table 2). Geranial (6) (19.4%), limonene (1) (16.4%), neral (4) (11.4), nerol (8) (9.5%), geraniol (3) (7.5%), geranyl acetate (9) (6.6%), and β-caryophyllene (10) (5.7%) (Figure 7) were the major compounds in the essential oil of the leaves of lime (Citrus Aurantifolia) 21.
Figure 6. Lime essential oil chemical components
[Source 18]Table 2. Composition of Essential Oil of Citrus Species in Malaysia
| Plant | Method | Part | Components | Ref. | |||||||
| Monoterpene Hydrocarbon | % | Oxygenated Monoterpene | % | Sesquiterpene Hydrocarbon | % | Oxygenated Sesquiterpene | % | ||||
| Citrus aurantifolia (Cristm.) Swingle | Water Distillation | Peel | α-Pinene | 1.5 | Terpinen-4-ol | 2 | β-Caryophyllene | 0.8 | (Z)-Nerolidol | 0.6 | 21 |
| β-Pinene | 28.4 | α-Terpineol | 2.4 | α-Bergamotene | 0.4 | α-Eudesmol | 0.1 | ||||
| β-Myrcene | 1 | Neral | 5.3 | α-Humulene | 0.1 | β-Eudesmol | 0.1 | ||||
| δ-3-Carene | 0.5 | Geraniol | 7.5 | (Z)-β-Farnesene | 0.4 | Elemol | 0.1 | ||||
| Limonene | 39.3 | Geranial | 2.1 | (E)-β-Farnesene | 1.5 | ||||||
| γ-Terpinene | 0.8 | Geranyl acetate | 0.6 | ||||||||
| Leaves | Sabinene | 0.1 | Linalool | 1.1 | (Z)-β-Farnesene | 0.1 | Phytol | 1 | |||
| β-Pinene | 0.9 | Nerol | 9.5 | α-Humulene | 0.8 | (Z)-Nerolidol | 2.1 | ||||
| β-Myrcene | 0.8 | Neral | 11.4 | α-Guaiene | 0.9 | α-Eudesmol | 0.3 | ||||
| Limonene | 16.4 | Geraniol | 7.5 | β-Caryophyllene | 5.7 | β-Eudesmol | 0.3 | ||||
| cis-β-Ocimene | 0.4 | Geranial | 19.4 | (E)-β-Farnesene | 1.8 | Elemol | 0.5 | ||||
| trans-β-Ocimene | 1.9 | Geranyl acetate | 6.6 | β-Bisabolene | 0.1 | ||||||
| Citrus grandis L. Osbek | Steam Distillation | Peel | α-Pinene | 0.3 | Linalool | 0.8 | δ-Guaiene | tr | Caryophyllene oxide | tr | 22 |
| Sabinene | 0.1 | Terpinen-4-ol | 0.1 | α-Cubebene | 0.1 | Muurolol | 0.1 | ||||
| β-Pinene | 0.1 | cis-Carveol | 1.5 | trans-Caryophyllene | tr | Farnesol | 0.3 | ||||
| β-Myrcene | 2.2 | trans-Carveol | 0.6 | Aromadendrene | tr | Nootkatone | 0.3 | ||||
| Limonene | 81.6 | 1-α-Terpineol | 1.2 | δ-Cadinene | tr | ||||||
| β-Cymene | 0.1 | Carvone | 0.9 | ||||||||
| Likens-Nikerson Extraction | Peel | α-Pinene | 0.1 | Linalool | 0.5 | δ-Guaiene | tr | Caryophyllene oxide | tr | ||
| Sabinene | 0.1 | Terpinen-4-ol | 0.3 | α-Cubebene | tr | Muurolol | tr | ||||
| β-Pinene | 0.1 | cis-Carveol | 1.4 | trans-Caryophyllene | 0.1 | Farnesol | 0.3 | ||||
| β-Myrcene | 1.6 | trans-Carveol | 0.4 | Aromadendrene | tr | Nootkatone | 0.3 | ||||
| Limonene | 86.8 | 1-α-Terpineol | 1.1 | δ-Cadinene | 0.1 | ||||||
| β-Cymene | tr | Carvone | 0.7 | ||||||||
| Water Distillation | Peel | β-Pinene | 0.6 | Linalool | 0.2 | (Z)-β-Farnesene | 0.3 | α-Eudesmol | tr | 21 | |
| α-Pinene | 0.3 | Terpinen-4-ol | 0.1 | α-Guaiene | 0.1 | Phytol | 0.1 | ||||
| β-Myrcene | 1.6 | α-Terpineol | 0.2 | (E)-β-Farnesene | 0.1 | ||||||
| α-Phellandrene | 0.1 | Neral | 0.1 | Aromadendrene | tr | ||||||
| Limonene | 95.1 | Geraniol | 0.1 | β-Caryophyllene | 0.1 | ||||||
| γ-Terpinene | 0.1 | Geranial | 0.1 | ||||||||
| Leaves | Sabinene | 0.1 | Terpinolene | 1.6 | β-Caryophyllene | 15.4 | Hedycaryol | 2.4 | |||
| β-Pinene | 4.9 | Nerol | 1.5 | α-Humulene | 1.8 | (Z)-Nerolidol | 0.8 | ||||
| δ-3-Carene | 3.9 | Neral | 4.5 | (Z)-β-Farnesene | 2.2 | α-Eudesmol | 1.8 | ||||
| Limonene | 1.4 | Geraniol | 1.4 | α-Cadinene | 7.1 | β-Eudesmol | 1.6 | ||||
| cis-β-Ocimene | 0.8 | Geranial | 4.5 | γ-Cadinene | 0.9 | Phytol | 23.1 | ||||
| trans-β-Ocimene | 9.9 | Citronellyl acetate | 1.8 | ||||||||
| Citrus grandis L. Osbek (white pomelo) | HS-SPME | Blossom | β-Pinene | 0.3 | Linalool | 9.2 | β-Caryophyllene | 0.1 | trans-Nerolidol | 0.1 | 23 |
| δ-3-Carene | 0.6 | α-Terpineol | tr | α-Humulene | 0.1 | cis-Farnesol | 0.4 | ||||
| α-Terpinene | 2.8 | Citronellol | 0.3 | β-Farnesene | 0.1 | Spathulenol | tr | ||||
| Limonene | 48.2 | Nerol | 1.5 | Germacrene D | 0.1 | ||||||
| cis-β-Ocimene | 12 | Geraniol | 1.3 | β-Bisabolene | 0.1 | ||||||
| allo-Ocimene | 1.6 | Carveol | 0.1 | α-Farnesene | 0.1 | ||||||
| HS-SPME | Peel | β-Pinene | 0.1 | Linalool | 0.2 | β-Caryophyllene | 0.1 | trans-Nerolidol | tr | ||
| δ-3-Carene | 0.1 | α-Terpineol | tr | α-Humulene | tr | cis-Farnesol | tr | ||||
| α-Terpinene | tr | Citronellol | tr | β-Farnesene | 0.1 | Elemol | 0.1 | ||||
| Limonene | 96.9 | Nerol | 0.1 | Germacrene D | tr | ||||||
| cis-β-Ocimene | 0.1 | Geraniol | 0.1 | β-Bisabolene | tr | ||||||
| β-Myrcene | 0.2 | Carveol | tr | α-Farnesene | 0.1 | ||||||
| Solvent extraction | Peel | β-Pinene | 0.1 | Linalool | 0.2 | β-Caryophyllene | 0.1 | trans-Nerolidol | 0.1 | ||
| δ-3-Carene | 0.1 | α-Terpineol | 0.1 | α-Humulene | 0.1 | cis-Farnesol | 0.1 | ||||
| α-Terpinene | 0.1 | Citronellol | tr | β-Farnesene | 0.1 | Elemol | 0.1 | ||||
| Limonene | 95.4 | Nerol | 0.2 | Germacrene D | 0.1 | ||||||
| cis-β-Ocimene | 0.1 | Geraniol | 0.3 | β-Bisabolene | 0.1 | ||||||
| β-Myrcene | 0.1 | Carveol | 0.1 | α-Farnesene | 0.1 | ||||||
| Citrus grandis L. Osbek (pink pomelo) | Headspace solid phase microextraction (HS-SPME) | Blossom | β-pinene | 0.1 | Linalool | 56.5 | β-Caryophyllene | 0.1 | trans-Nerolidol | 0.1 | 23 |
| δ-3-carene | 0.2 | α-Terpineol | tr | α-Humulene | 0.1 | cis-Farnesol | 1.8 | ||||
| α-Terpinene | 2.5 | Citronellol | 0.2 | β-Farnesene | 0.1 | Spathulenol | tr | ||||
| Limonene | 15.5 | Nerol | 0.4 | Germacrene D | 0.1 | ||||||
| cis-β-Ocimene | 4 | Geraniol | 0.4 | β-Bisabolene | 0.1 | ||||||
| allo-Ocimene | 1.1 | Carveol | 0.1 | α-Farnesene | 0.2 | ||||||
| HS-SPME | Peel | β-Pinene | 0.1 | Linalool | 0.1 | β-Caryophyllene | 0.1 | trans-Nerolidol | 0.1 | ||
| δ-3-Carene | 0.1 | α-Terpineol | 0.1 | α-Humulene | tr | cis-Farnesol | tr | ||||
| α-Terpinene | tr | Citronellol | 0.1 | β-Farnesene | 0.1 | Elemol | 0.1 | ||||
| Limonene | 96.1 | Nerol | 0.1 | Germacrene D | tr | ||||||
| cis-β-Ocimene | 0.1 | Geraniol | 0.1 | β-Bisabolene | tr | ||||||
| β-Myrcene | 0.4 | Carveol | tr | α-Farnesene | 0.1 | ||||||
| Solvent extraction | Peel | β-Pinene | 0.1 | Linalool | 0.3 | β-Caryophyllene | 0.2 | trans-Nerolidol | 0.1 | ||
| δ-3-Carene | tr | α-Terpineol | 0.2 | α-Humulene | 0.1 | cis-Farnesol | 0.1 | ||||
| α-Terpinene | 0.1 | Citronellol | 0.1 | β-Farnesene | 0.1 | Elemol | 0.1 | ||||
| Limonene | 93.1 | Nerol | 0.2 | Germacrene D | 0.2 | ||||||
| cis-β-Ocimene | 0.1 | Geraniol | 0.3 | β-Bisabolene | tr | ||||||
| β-Myrcene | 0.1 | Carveol | 0.1 | α-Farnesene | 0.1 | ||||||
| Citrus hystrix D.C. | Hydro-difusion steam distillation system | Peel | α-Thujene | 0.2 | Linalool | 0.8 | α-Copaene | 0.5 | 24 | ||
| α-Pinene | 1.8 | Citronellal | 10.8 | Caryophyllene | 0.3 | ||||||
| Sabinene | 36.4 | Terpinen-4-ol | 1.1 | α-Humulene | 0.1 | ||||||
| β-Pinene | 8.6 | α-Terpineol | 0.6 | Germacrene D | 0.4 | ||||||
| β-Myrcene | 1.7 | Citronellol | 1.8 | β-Selinene | 0.1 | ||||||
| Limonene | 32.5 | δ-Cadinene | 0.4 | ||||||||
| Steam distillation with induction heating system | Peel | α-Thujene | 0.1 | Linalool | 0.1 | α-Copaene | 0.1 | 25 | |||
| α-Pinene | 3.2 | Citronellal | 3.3 | Germacrene D | 0.2 | ||||||
| Sabinene | 48.5 | Terpinen-4-ol | 0.5 | δ-Cadinene | 0.1 | ||||||
| β-Pinene | 10.1 | α-Terpineol | 0.2 | ||||||||
| β-Myrcene | 1.5 | Citronellyl acetate | 0.1 | ||||||||
| Limonene | 27.7 | ||||||||||
| Automated steam distillation process | Peel | α-thujene | 0.2 | Linalool | 1.2 | 26 | |||||
| α-pinene | 3.3 | Citronellal | 7.8 | ||||||||
| Sabinene | 46.6 | Terpinen-4-ol | 2.4 | ||||||||
| β-pinene | 13.5 | α-Terpineol | 0.9 | ||||||||
| β-Myrcene | 1.8 | ||||||||||
| Limonene | 17.2 | ||||||||||
| Hydro-distillation | Leaves | Sabinene | 0.2 | Linalool | 3.9 | Nerolidol | 0.1 | 27 | |||
| β-Myrcene | 0.1 | Citronellal | 66.9 | ||||||||
| (E)-2,5-Dimethyl-1,6-octadine | 0.1 | Isopregol | 0.7 | ||||||||
| cis-2,6-Dimethyl-2,6-octadine | 0.3 | β-Citronellol | 6.6 | ||||||||
| Citronellol | 1.8 | ||||||||||
| Geraniol | 0.4 | ||||||||||
| Steam Distillation | Fresh Leaves | α-Pinene | 0.1 | Linalool | 1 | trans-Caryophyllene | tr | Elemol | tr | 28 | |
| Sabinene | 1.6 | Citronellal | 61.7 | β-Elemene | tr | Nerolidol | 1.2 | ||||
| β-Pinene | 0.1 | β-Citronellol | 13.4 | α-Muurolene | tr | Guaiol | 0.2 | ||||
| β-Myrcene | 0.7 | iso-Pulegol | 0.9 | β-Bisabolene | tr | Caryophyllene oxide | tr | ||||
| Limonene | 5.9 | Citronellyl acetate | 2 | δ-Cadinene | tr | ||||||
| p-Cymene | 0.1 | ||||||||||
| Likens-Nikerson Extraction | Fresh Leaves | α-Pinene | 0.1 | Linalool | 1.6 | δ-Cadinene | tr | Elemol | tr | 28 | |
| Sabinene | 2 | Citronellal | 72.5 | Nerolidol | tr | ||||||
| β-Pinene | 0.1 | β-Citronellol | 10.3 | Guaiol | tr | ||||||
| β-Myrcene | 0.6 | iso-Pulegol | 1.2 | ||||||||
| Limonene | 6.8 | Citronellyl acetate | 1.2 | ||||||||
| p-Cymene | tr | ||||||||||
| Likens-Nikerson Extraction | Peel | α-Pinene | 1.7 | Linalool | 1.8 | β-Bisabolene | 1.2 | Elemol | tr | 28 | |
| Sabinene | 20 | Citronellal | 12.6 | δ-Cadinene | 0.6 | Nerolidol | 0.2 | ||||
| β-Pinene | 23.5 | β-Citronellol | 3.3 | Guaiol | 0.1 | ||||||
| β-Myrcene | 1 | iso-pulegol | 0.5 | Caryophyllene oxide | 0.1 | ||||||
| Limonene | 11.8 | Citronellyl acetate | 1.7 | ||||||||
| p-cymene | 0.3 | ||||||||||
| Water Distillation | Peel | α-Pinene | 2 | cis-Linalool oxide | 1.9 | β-caryophyllene | 0.4 | Hedycaryol | 0.3 | 21 | |
| β-Pinene | 39.3 | Terpinolene | 1.6 | α-Humulene | 0.1 | (Z)-Nerolidol | 0.1 | ||||
| Limonene | 14.2 | Linalool | 1.9 | (Z)-β-Farnesene | 0.2 | α-Eudesmol | 0.2 | ||||
| β-Myrcene | 1.3 | Terpinen-4-ol | 8.9 | α-Cadinene | 0.1 | β-Eudesmol | 0.2 | ||||
| δ-3-Carene | 1.4 | Citronellal | 11.7 | (E)-β-Farnesene | 0.1 | Phytol | 0.1 | ||||
| γ-Terpinene | 2.4 | δ-Cadinene | 0.5 | α-Sinensal | 0.1 | ||||||
| Leaves | β-Pinene | 1.9 | trans-Sabinene hydrate | 1.5 | β-Cubebene | 0.2 | Hedycaryol | 0.3 | 21 | ||
| β-Myrcene | 0.9 | Linalool | 1.7 | β-Caryophyllene | 0.9 | (Z)-Nerolidol | 0.9 | ||||
| δ-3-Carene | 0.1 | Citronellal | 72.4 | α-Cadinene | 0.4 | α-Eudesmol | 0.2 | ||||
| Limonene | 0.1 | Citronellol | 6.7 | α-Humulene | 0.2 | β-Eudesmol | 0.2 | ||||
| trans-β-Ocimene | 0.5 | Citronellyl acetate | 4.1 | (E)-β-Farnesene | 0.2 | Elemol | 0.3 | ||||
| γ-Terpinene | 0.3 | Geranyl acetate | 0.8 | δ-Cadinene | 0.4 | ||||||
| Citrus microcarpa (Bunge) Wijnands | Water Distillation | Peel | α-Pinene | 0.5 | δ-Elemene | 0.1 | β-Caryophyllene | tr | Elemol | 0.1 | 21 |
| β-Pinene | 0.1 | Linalool | 0.4 | (Z)-β-Farnesene | 0.7 | β-Eudesmol | 0.2 | ||||
| Myrcene | 1.8 | Terpinen-4-ol | 0.1 | Aromadendrene | 0.1 | ||||||
| α-Phellandrene | 0.1 | α-Terpineol | 0.3 | (E)-β-Farnesene | 0.1 | ||||||
| Limonene | 94 | Terpinolene | 0.1 | α-Guaiene | 0.1 | ||||||
| γ-Terpinene | 0.1 | Geranyl acetate | 0.2 | ||||||||
| Leaves | α-Pinene | 0.8 | δ-Elemene | 2.7 | β-Caryophyllene | 2.8 | Hedycaryol | 19 | 21 | ||
| β-Pinene | 13.4 | Linalool | 6.1 | α-Humulene | 0.6 | (Z)-Nerolidol | 1.2 | ||||
| Myrcene | 0.2 | Terpinen-4-ol | 0.4 | α-Sesqui-phellandrene | 18.3 | α-Eudesmol | 14.4 | ||||
| α-Phellandrene | 0.8 | α-Terpineol | 0.3 | α-Selinene | 1.8 | β-Eudesmol | 8.6 | ||||
| Limonene | 0.7 | β-Elemene | 1.1 | δ-Cadinene | 0.5 | Elemol | 0.6 | ||||
| trans-β-Ocimene | 2 | Geranyl acetate | 0.1 | Phytol | 0.4 | ||||||
Kaffir lime (Citrus hystrix) (Figure 3) is known as ‘wild lime’. Kaffir lime (Citrus hystrix) leaves and fruits are widely used as spices in preparation of ‘tomyam’, either white or red, and it is famous dish in Malaysia and Thailand. The height of this plant is about 3–5 m and the fragrant green leaves are 7.5–10 cm long. It has white flowers with 4–6 petals. The diameter of pear-shaped fruits is about 5.0–7.5 cm with wrinkle on the surface of fruit. The fruit is dark green, and yellow when ripe 29. The essential oil of Kaffir lime (Citrus hystrix) is used in aromatherapy and an essential ingredient of various cosmetic and beauty products 27.
In traditional medicine, Kaffir lime (Citrus hystrix) (Figure 3) is used to treat flu, fever, hypertension, abdominal pains, and diarrhea in infants 30. The fruits are used as a digestive stimulant, blood purifier, and reduce high blood pressure 31. Additionally, the fruits are used in cooking for flavoring and also in the production of shampoo as an insecticide for washing the head 32. In addition, the fruit juice is used in softening the skin and the mixture of the fruit juice with bath water can be used to eliminate body odor 33. Furthermore, the essential oil of Kaffir lime (Citrus hystrix) has been reported to have various bioactivities such as antioxidant, antibacterial, antileukimic, and antitussive 31.
The essential oil of kaffir lime peel from Malaysia, contained sabinene (19) (36.0%–49.0%), limonene (1) (17.0%–33.0%), citronellal (20) (3.0%–11.0%) (Figure 6) and β-pinene (2) (8.0%–14.0%) as major components. Three methods were used to extract kaffir lime peel essential oil, e.g., hydro-diffusion steam distillation system, steam distillation with induction heating system, and automated steam distillation process with optimized temperature at 90 °C 26. However, citronellal (20) (66.9%) and β-citronellol (21) (6.6%) (Figure 6) were the major components in kaffir lime peel oil from Malaysia, obtained using the hydro-distillation method 34.
The essential oil of Kaffir lime (Citrus hystrix) fresh leaves from Malaysia, extracted by the steam distillation and the Likens-Nikerson extraction methods, was found to be dominated by citronellal (20) (61.0%–73.0%), β-citronellol (21) (10.0%–14.0%), and limonene (1) (5.0%–7.0%) as major components (Table 2). β-Pinene (2) (23.5%) and sabinene (19) (20.1%) appeared as the major components of Kaffir lime (Citrus hystrix) peel, followed by citronellal (20) (12.6%), limonene (1) (11.8%), and β-citronellol (21) (3.3%) 28. Moreover, β-pinene (2) (39.3%), limonene (1) (14.2%), citronellal (20) (11.7%), and terpinen-4-ol (7) (8.9%) were identified as the principal components in kaffir lime peels from Melaka. However, citronellal (20) (72.4%), β-citronellol (21) (6.7%), and citronellyl acetate (22) (4.1%) (Figure 6) were reported to be the major components in kaffir lime leaves, followed by β-pinene (2) (1.9%) and limonene (1) (0.1%) as minor components. Water distillation was used as a method to extract kafir lime peels and leaves from Melaka 21.
The antibacterial susceptibility of the essential oils and oil emulsions of Malaysian Kaffir lime (Citrus hystrix) was evaluated against Escherichia coli, Bacillus subtilis, and Staphylococcus aureus using the disc diffusion method. Pure essential oil with a percentage of 2% by weight exhibited a strong inhibitory effect against Escherichia coli and Bacillus subtilis with the zones of inhibition of 16.0 and 15.0 mm, respectively. Meanwhile, the formulated emulsions with surfactant mixture of Tween 80 and Span 80 (90:10) with 2% (by weight) essential oil displayed the most potential antibacterial activity against Escherichia coli with the zones of inhibition ranging between 11.0 to 18.0 mm 35.
The topical application bioassay on uniform weighted second instar larvae in the laboratory was carried out to determine the insecticidal properties of the essential oil from the leaves of the Malaysian Kaffir lime (Citrus hystrix) against Spodoptera litura (tobacco army worm). The study demonstrated considerable repellant activity of Kaffir lime essential oil against the Spodoptera litura larvae after 24 and 48 h of treatment with LD50 values of 29.25 and 26.75 μg/mL, respectively 34. LD50 is an abbreviation for “Lethal Dose, 50%” or median lethal dose. It is the amount of the substance required (usually per body weight) to kill 50% of the test population.
Kassia lime (Citrus microcarpa) also known as Kalamansi (Figure 4), is used in the preparation of beverages. Kassia lime (Citrus microcarpa) is 3–5 m tall with abundant of long spine on the stem, branches and twigs. The dark green leaves of Kassia lime (Citrus microcarpa) are between 2.5–6.8 cm long and 2–3 cm thick. The round or oblong-shaped green leaves of this plant are 2.5–3.8 cm in diameter. This plant is used to treat fever, cough, and pharyngitis 36. The juice is traditionally used to prevent respiratory diseases, strengthen the bones and act as growth stimulant for children 18. The juice is also commonly used in cooking as flavoring ingredients and additives. The Kassia lime (Citrus microcarpa) leaves of this plant can be used in the treatment of skin diseases, relieve headache and also act as a mouth wash to treat sore throat 37. Essential oil from Kassia lime (Citrus microcarpa) is used commercially in perfumes, food, cosmetics and detergents. It is one of the ingredients in pharmaceutical, aromatherapy and antiseptic products 38.
The essential oil from Kassia lime (Citrus microcarpa) peels was reported to be rich in limonene (1) (94.0%) similar to C. aurantifolia. β-Myrcene (11) (1.8%), linalool (18) (0.4%), and α-terpineol (5) (0.3%) were detected as the minor components (Table 2) in the oil. Sesquiterpene hydrocarbons were the most abundant in the leaves of Kassia lime (Citrus microcarpa). These include hedycaryol (23) (19.0%), α-sesquiphellandrene (24) (18.3%), α-eudesmol (25) (14.4%) and β-eudesmol (26) (8.6%) (Figure 6). The essential oil was extracted by hydrodistillation for 8 hours similar to that of Key Lime (Citrus Aurantifolia) oils 21.
- Poucher, W. A. (1991) Poucher’s Perfumes, Cosmetics and Soaps-Vol. 1. The Raw Materials of Perfumery, 9th Edition. Ed.: A. J. Jouhar. Chapman & Hall, NY. pp. 199-201.[↩]
- Swaine, R. L. and R. L. Swaine Jr. (1988) Citrus Oils: Processing, Technology, and Applications. Perfume & Flavorist 13: 2-20.[↩][↩]
- Burdock, G. A. (Ed.) (1995) Fenaroli’s Handbook of Flavor Ingredients, Volume 1, 3rd Edition. CRC Press, Boca Raton, FL[↩][↩]
- Lemon Oil. https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/lemonlimeoils_508.pdf[↩][↩][↩][↩][↩]
- FDA Poisonous Plant Database. https://www.accessdata.fda.gov/scripts/plantox/detail.cfm?id=23780[↩]
- Naganuma, M., S. Hirose, Y. Nakayama, K. Nakajima and T. Someya (1985) A Study of the Phototoxicity of Lemon Oil. Arch. Dermatol. Res. 278: 31-36.[↩][↩]
- Forbes, P. D., F. Urbach and R. E. Davies (1977) Phototoxicity Testing of Fragrance Raw Materials. Fd. Cosmet. Toxicol. 15: 55-60.[↩]
- Downum, K. R. (1992) Light-Activated Plant Defense. New Phytolog. 122: 401-420.[↩]
- Saffran, W. A. (1988) Genotoxic Effects of Psoralen. In: Psoralen DNA Photobiology. Volume II. Ed.: F. P. Gasparro, CRC Press, Boca Raton, FL. pp 73-86.[↩]
- Zajdela, F. and E. Bisagni (1981) 5-Methoxypsoralen, the Melanogenic Additive in Suntan Preparations, Is Tumorigenic in Mice Exposed to 365 nm UV Radiation. Carcinogenesis 2: 121-127.[↩]
- Uwaifo, A. O. (1984) The Mutagenicities of Seven Coumarin Derivatives and a Furan Derivative (Nimbolide) Isolated from Three Medicinal Plants. J. Toxicol. Environ. Health 13: 521-530.[↩]
- https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/lemonlimeoils_508.pdf[↩]
- Saidan I. Dalam Dusun Melayu (in Malay Orchard) Dewan Bahasa dan Pustaka; Kuala Lumpur, Malaysia: 2013. pp. 243–244.[↩][↩]
- Manner H.I., Buker R.S., Smith V.E., Ward D., Elevitch C.R. Citrus (citrus) and Fortunella (kumquat) Species Profile Pac. Isl. Agrofor. 2006;2:1–35.[↩]
- Samah B. Serangan Jantung: Punca, Pencegahan & Kaedah Meredakannya. (Heart Attack: The Cause, Prevention & Treatment) Alaf 21; Selangor, Malaysia: 2009. pp. 104–105.[↩][↩]
- Kunow M.A. Maya Medicine: Traditional Healing in Yucatan. University of New Mexico Press; Albuquerque, NM, USA: 2003. p. 117.[↩]
- Aibinu I., Adenipekun T., Adelowotan T., Ogunsanya T., Odugbemi T. Evaluation of the antimicrobial properties of different parts of Citrus aurantifolia (lime fruit) as used locally. Afr. J. Tradit. Complemnet. Altern. Med. 2007;4:185–190 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2816438/[↩]
- Md Othman SNA, Hassan MA, Nahar L, Basar N, Jamil S, Sarker SD. Essential Oils from the Malaysian Citrus (Rutaceae) Medicinal Plants. Batista R, ed. Medicines. 2016;3(2):13. doi:10.3390/medicines3020013. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5456223/[↩][↩][↩][↩]
- Effiom O.E., Avoaja D.A., Ohaeri C.C. Mosquito repellent activity of phytochemical extracts from fruit peels of Citrus fruit species. Glob. J. Sci. Front. Res. 2012;12:5–8.[↩]
- Lyle S. Discovering Fruit and Nuts. David Bateman Ltd.; Auckland, New Zealand: 2006. How to Use Citrus Fruit peels in the Home and Garden; pp. 130–142.[↩]
- Jantan I., Ahmad A.S., Ahmad A.R., Ali N.A.M., Ayop N. Chemical composition of some Citrus oils from Malaysia. J. Essent. Oil Res. 1996;8:627–632. doi: 10.1080/10412905.1996.9701030[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Nor O.M. Sebatian aroma dalam minyak Citrus grandis (Aromatic compounds from Citrus grandis Oil) J. Trop. Agric. Food Sci. 1999;27:231–236.[↩]
- Cheong M.-W., Loke X.-Q., Liu S.-Q., Pramudya K., Curran P., Yu B. Characterization of volatile compounds and aroma profiles of Malaysian pomelo (Citrus grandis (L.) Osbeck) blossom and peel. J. Essent. Oil Res. 2011;23:34–44. doi: 10.1080/10412905.2011.9712279[↩][↩]
- Mohd-Yusoff Z., Muhammad Z., Kasuan N., Rahiman M.H.F., Taib M.N. Effect of temperature on kaffir lime oil by using Hydro-diffusion steam distillation system. Malays. J. Anal. Sci. 2013;17:326–339[↩]
- Muhammad Z., Mohd Yusoff Z., Nordin M.N.N., Kasuan N., Taib M.N., Rahiman M.H.F., Haiyee Z.A. Steam distillation with induction heating system: Analysis of Kaffir Lime oil compound and production yield at various temperatures. Malays. J. Anal. Sci. 2013;17:340–347.[↩]
- Kasuan N., Muhammad Z., Yusoff Z., Rahiman M.H.F., Taib M.N., Haiyee Z.A. Extraction of Citrus hystrix DC (Kaffir Lime) essential oil using automated steam distillation process: Analysis of volatile compounds. Malays. J. Anal. Sci. 2013;17:359–369.[↩][↩]
- Waikedre J., Dugay A., Barrachina I., Herrenknecht C., Cabalion P., Fournet A. Chemical composition and antimicrobial activity of the essential oils from New Caledonian Citrus macroptera and Citrus hystrix. Chem. Biodivers. 2010;7:871–877. doi: 10.1002/cbdv.200900196[↩][↩]
- Nor O.M. Volatile aroma compounds in Citrus hystrix oil. J. Trop. Agric. Food Sci. 1999;27:225–229.[↩][↩][↩][↩]
- Awang C.R.C. Master’s Thesis. Universiti Sains Malaysia; Pulau Pinang, Malaysia: May 1, 2007. Kesan Minyak Esensial Citrus spp Ke Atas Sistem Kardiovaskular Tikus Serta Kesan Antiresah dan Antidepresi Pada Mencit. Anti-Anxious and Anti-Depression Effects of Citrus spp Essential Oils towards Cardiovascular System of Mice[↩]
- Fortin H., Vigora C., Lohezic-Le F., Robina V., le Bosse B., Boustiea J., Arnoros M. In vitro antiviral activity of thirty-six plants from La Reunion Island. Fitoterapia. 2002;73:346–350. doi: 10.1016/S0367-326X(02)00080-1 https://www.ncbi.nlm.nih.gov/pubmed/12234582[↩]
- Norkaew O., Pitija K., Pripdeevech P., Sookwong P., Wongpornchai S. Supercritical fluid extraction and gas chromatographic-mass spectrometric analysis of terpenoids in fresh Kaffir lime leaf oil. Chiang Mai J. Sci. 2013;40:240–247.[↩][↩]
- Koh D., Ong C.N. Phytophotodermatitis due to the application of Citrus hystrix as a folk remedy. Br. J. Dermatol. 1999;140:737–738. doi: 10.1046/j.1365-2133.1999.02782.x https://www.ncbi.nlm.nih.gov/pubmed/10233333[↩]
- Dassanayake M.D. A Revised Handbook to the Flora of Ceylon. Vol. V. Amerind Publishing Co Ltd.; New Delhi, India: 1985. pp. 432–433.[↩]
- Loh F.S., Awang R.M., Omar D., Rahmani M. Insecticidal properties of Citrus hystrix DC leaves essential oil against Spodoptera litura fabricius. J. Med. Plants Res. 2011;5:3739–3744.[↩][↩]
- Ng D.S.H., Rose L.C., Suhaimi H., Mohammad H., Rozaini M.Z.H., Taib M. Preliminary evaluation on the antibacterial activities of Citrus hystrix oil emulsions stabilized by tween 80 and span 80. Int. J. Pharm. Pharm. Sci. 2011;3:209–211.[↩]
- Ong H.C. Buah: Khasiat Makanan & Ubatan. Utusan Publications & Distributors; Kuala Lumpur, Malaysia: 2004. p. 90.[↩]
- Morton J.F. Fruits of Warm Climates. 1st ed. Creative Resource Systems; Winterville, NC, USA: 1987. Mexican Lime; pp. 168–172.[↩]
- Manaf Y.N., Osman A., Lai O.M., Long K., Ghazali H.M. Characterisation of musk lime (Citrus microcarpa) seed oil. J. Sci. Food Agric. 2008;88:676–683. doi: 10.1002/jsfa.3134[↩]