Contents
- What is rheumatoid arthritis
- Rheumatoid arthritis causes
- Rheumatoid arthritis Complications
- Signs and symptoms of rheumatoid arthritis
- How Rheumatoid arthritis is Diagnosed
- Rheumatoid arthritis diet
- Rheumatoid arthritis treatment
- American College of Rheumatology and the European Alliance of Associations for Rheumatology Treatment Guidelines for Rheumatoid Arthritis
- Table 4. Disease-modifying antirheumatic drugs (DMARDs) initiation (American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis 2021)
- Low Disease Activity
- Moderate to High Disease Activity
- Treat-to-Target Therapy
- Adjusting Antirheumatic Therapy
- Tapering or Discontinuing DMARD Therapy
- Patients with rheumatoid arthritis with Coexisting Conditions
- Corticosteroids
- Rheumatoid arthritis medications
- What are DMARDs?
- What are corticosteroids?
- What does research say about combining medicines?
- What should you think about when deciding which medicine is right for you?
- What are the costs of rheumatoid arthritis medications?
- Psychological treatment
- Surgery
- Lifestyle and home remedies for rheumatoid arthritis
- Coping and support
- American College of Rheumatology and the European Alliance of Associations for Rheumatology Treatment Guidelines for Rheumatoid Arthritis
- Rheumatoid arthritis prognosis
- Living with rheumatoid arthritis
What is rheumatoid arthritis
Rheumatoid arthritis is a chronic autoimmune inflammatory disease in which your body’s own immune system attacks the lining of the synovial membranes that surround the joints. Scientists don’t know the cause of rheumatoid arthritis. But it’s a condition in which the immune system attacks healthy joint tissue by mistake, called autoimmune disease. The cause is likely a mix of genetic changes and environmental factors from outside the body 9, 10. Hormones may play a role. There are also theories about certain viruses or bacteria causing autoimmune responses in people whose genes make them more likely to get it. Abnormal protein citrullination and the formation of anti-cyclic citrullinated peptide (anti-CCP) antibodies are critical pathogenic mechanisms in rheumatoid arthritis and are associated with severe joint lesions and extra-articular organ damage 11, 12, 13.
You are more likely to get rheumatoid arthritis (RA) if you have certain risk factors that include 14, 15, 16, 17, 18, 9, 19, 14, 20, 21, 22, 23:
- Age. Rheumatoid arthritis can happen at any age; however, the risk for developing rheumatoid arthritis increases with older age often begins in middle age from 30 to 50 years of age. Children and younger teenagers may be diagnosed with juvenile idiopathic arthritis (a group of autoimmune diseases causing joint inflammation in children and teenagers under 16 years old), a condition related to rheumatoid arthritis.
- Sex. Rheumatoid arthritis is more common among women than men with approximately 70–80% of rheumatoid arthritis cases in women. Women are 2 to 3 times more likely than men to get rheumatoid arthritis, and do so about ten years earlier on average. Rheumatoid arthritis (RA) is also often more severe in women than in men. Researchers think that reproductive and hormonal factors may play a role in the development of rheumatoid arthritis for some women 24.
- Family history and genetics. Having a family member with rheumatoid arthritis or other autoimmune conditions may raise your risk of the condition. There are several genetic factors that slightly increase the risk of getting rheumatoid arthritis.
- Smoking. Research shows that people who smoke cigarette over a long period of time are at an increased risk of getting rheumatoid arthritis. Smoking also seems to make the condition worse in people who keep smoking. For people who continue to smoke, rheumatoid arthritis may be more severe.
- Obesity or Excess weight. Some research shows that being obese or overweight may increase your risk for getting rheumatoid arthritis as well as limit how much the disease can be improved.
- Periodontitis (gum infection or periodontal disease). A serious gum infection or periodontal disease can damage the soft tissue around your teeth and raise youre risk of getting rheumatoid arthritis.
- Lung diseases. Diseases of the lungs and airways may also be associated with developing rheumatoid arthritis.
Typical symptoms of rheumatoid arthritis are 25:
- Warm, swollen and painful joints
- Stiff joints in the morning after you wake up. They usually only become more flexible again after more than an hour.
- Weakness: Painful, stiff joints often end up not getting as much use, which can cause the muscles to get weaker over time.
- Exhaustion: Rheumatoid arthritis affects the whole body, so it often causes tiredness and general physical weakness.
- Rheumatoid nodules: As the disease progresses, small firm lumps called rheumatoid nodules sometimes develop under the skin. They’re usually not sensitive to pressure or touch.
Additional features of rheumatoid arthritis can include the following 6, 25:
- Fatigue, mild fevers, and a loss of appetite.
- Rheumatoid arthritis may cause other medical problems outside of the joints, in areas such as your heart, lungs, blood, nerves, eyes, and skin.
Rheumatoid arthritis can increase your risk of getting other medical problems such as 26, 25:
- Osteoporosis. Rheumatoid arthritis itself, and some medicines used to treat it, can increase your risk of osteoporosis, a condition where bones become weak and brittle, increasing the risk of fractures. Osteoporosis weakens bones and makes them more likely to break.
- Rheumatoid nodules. Rheumatoid nodules that are firm lumps just below the skin most often form around pressure points, such as the hands and elbows. But these nodules can form anywhere in your body, including the heart and lungs.
- Dry eyes and mouth called secondary Sjogren’s syndrome (a disease primarily affecting the glands that produce tears and saliva, leading to dryness of the eyes and mouth). People who have rheumatoid arthritis are much more likely to get a condition that lowers the amount of moisture in the eyes and mouth.
- Infections. Rheumatoid arthritis and many of the medicines used to treat it can harm the immune system. This can lead to more infections.
- Anemia due to low red blood cell counts.
- Carpal tunnel syndrome. If rheumatoid arthritis affects the wrists, the swelling can press on the median nerve to the hand and fingers.
- Heart problems. Rheumatoid arthritis can raise the risk of hardened and blocked arteries. It also can raise the risk of swelling and irritation, called inflammation of the sac enclosing the heart (pericarditis).
- Lung disease. People with rheumatoid arthritis have a higher risk of swelling and irritation, called inflammation of lung tissues. This can cause scarring and lead to shortness of breath that gets worse over time.
- Lymphoma. Rheumatoid arthritis raises the risk of a group of blood cancers that happen in the lymph system. This is called lymphoma. People with rheumatoid arthritis may have a higher risk of other cancers, as well.
Symptoms may gradually worsen, or they might not change for a long time. Sometimes the symptoms come and go in episodes, so the inflammation and pain may suddenly get worse and then improve again after a while. During phases when the symptoms are more severe, or at more advanced stages of the disease, people might sometimes feel extremely exhausted. This is known as “fatigue”.
Rheumatoid arthritis can progress in very different ways. In one study involving people with rheumatoid arthritis, ten years after they had developed the condition 25:
- Just under 50% of the participants reported minor limitations,
- A good 40% reported moderate limitations, and
- About 10% reported severe limitations in their everyday life.
These limitations include difficulties with things like getting up in the morning, getting dressed or preparing food – for example, opening packages, bottles or jars.
The late stages of rheumatoid arthritis can lead to major joint damage. Especially the joints in the hands can become very deformed, weak and stiff.
The mortality rate is 2.5 times higher in rheumatoid arthritis patients in comparison to healthy control 2. Management in rheumatoid arthritis patients increased their survival rate. However, rheumatoid arthritis patients have a lifespan of 5 to 10 years lower than healthy people 27.
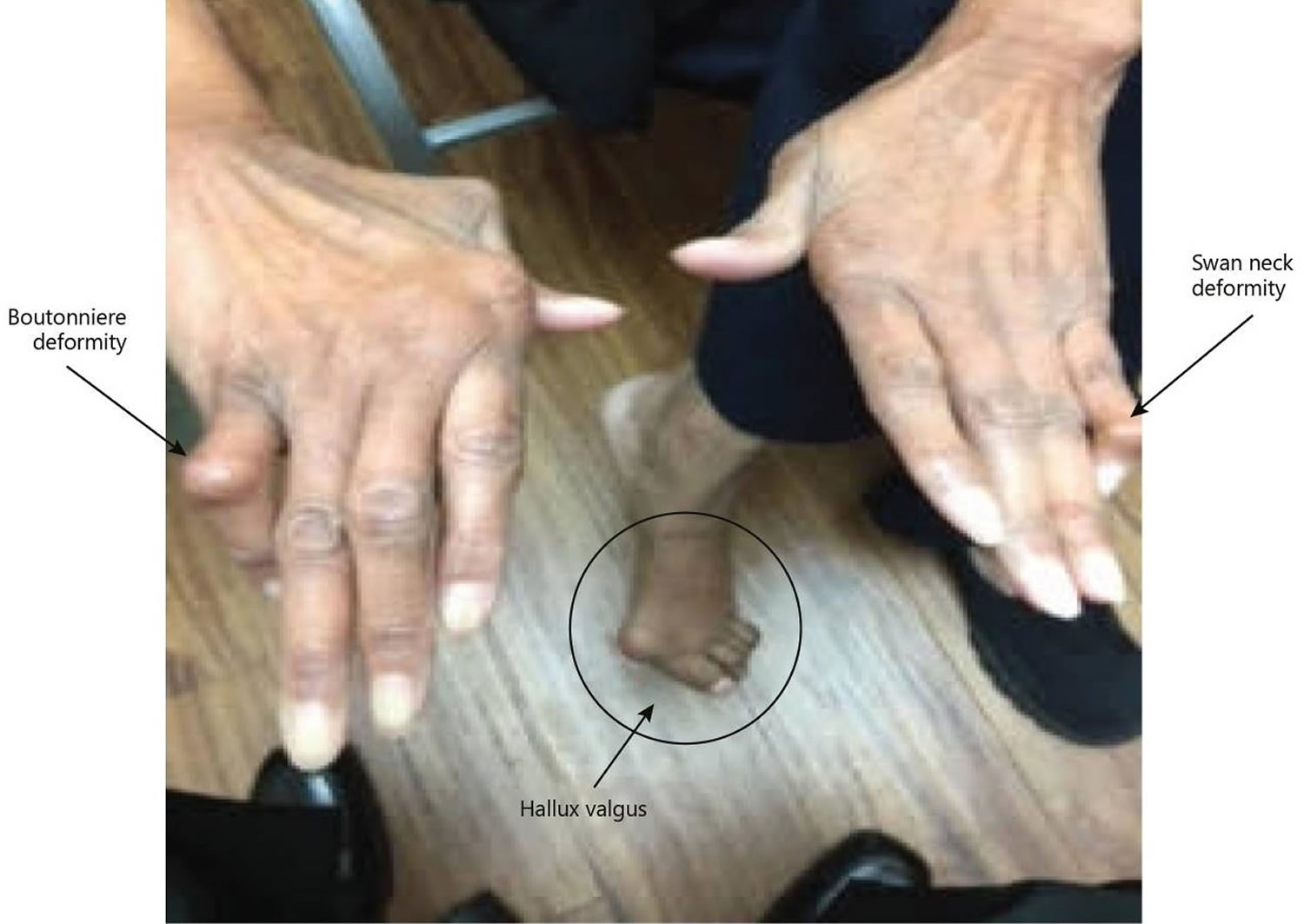
Figure 1. Rheumatoid arthritis

Figure 2. Rheumatoid arthritis
Footnote: A classic example of joint deformities associated with rheumatoid arthritis. Boutonniere deformity is visible in the 5th digit of the right hand, Swan neck deformity in the 5th digit of the left hand, and hallux valgus can be seen in the foot.
[Source 28 ]There is currently no cure for rheumatoid arthritis. Joint damage can happen quickly without treatment. But clinical studies show that easing of symptoms, called remission, is more likely with early treatment with medicines called disease-modifying antirheumatic drugs (DMARDs). Rheumatoid arthritis can also be treated with physical therapy and occupational therapy. There are also various support aids that can make some everyday tasks easier. People with rheumatoid arthritis are advised to do regular exercise or sports too.
Rheumatoid arthritis treatment options will depend on things like:
- how severe the inflammation and symptoms are,
- how far the disease has progressed,
- the predicted further course of the disease, and
- how well previous treatments have worked.
Your rheumatologist will suggest medicines based on how bad your symptoms are and how long you’ve had rheumatoid arthritis. You and your rheumatologist will decide on treatment. Medicines might include 29, 30:
- Nonsteroidal anti-inflammatory drugs (NSAIDs). Nonsteroidal anti-inflammatory drugs (NSAIDs) can relieve pain and ease swelling and irritation. NSAIDs you can get without a prescription include ibuprofen (Advil, Motrin IB, others) and naproxen sodium (Aleve). There also are stronger prescription NSAIDs. Side effects for all NSAIDs may include stomach upset, heart problems and kidney damage.
- Glucocorticoids. Corticosteroid medicines, such as prednisone (Rayos), ease inflammation and pain and slow joint damage. There can be serious side effects. The risk of side effects rises when taken at high doses over a long time. Side effects may include thinning of bones, fractures, easy bruising from skin thinning, weight gain, diabetes, cataracts and glaucoma, among others. Rheumatologists often prescribe a corticosteroid for quick symptom relief. The goal is to taper off the medicine when the condition is under control.
- Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). These drugs can slow the progression of rheumatoid arthritis and save the joints and other tissues from long-term damage. Common disease-modifying antirheumatic drugs (DMARDs) include methotrexate (Trexall, Otrexup, others), leflunomide (Arava), hydroxychloroquine (Plaquenil, Sovuna) and sulfasalazine (Azulfidine). Side effects of disease-modifying antirheumatic drugs (DMARDs) vary but may include liver damage and severe lung infections.
- Biological DMARDs (bDMARDs) also known as biologic response modifiers, this newer class of DMARDs includes abatacept (Orencia), adalimumab (Humira), anakinra (Kineret), certolizumab (Cimzia), etanercept (Enbrel), golimumab (Simponi), infliximab (Remicade), rituximab (Rituxan), sarilumab (Kevzara) and tocilizumab (Actemra). Biologic DMARDs most often work best when used with a conventional DMARD, such as methotrexate. Biologic agents also raise the risk of rare infections such as tuberculosis, also called TB, or fungal infections. If you take biologic agents, you need to be watched closely.
- Targeted synthetic DMARDs (tsDMARDs). Your rheumatologist may prescribe these man-made medicines if conventional DMARDs and biological DMARDs (bDMARDs) haven’t worked. They include baricitinib (Olumiant), tofacitinib (Xeljanz) and upadacitinib (Rinvoq). Higher doses of tofacitinib may raise the risk of blood clots in the lungs, serious heart-related events and cancer.
Some common complementary and alternative treatments that have shown promise for rheumatoid arthritis include:
- Fish oil. Fish oils contain the omega−3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) that are known to reduce inflammation in the body and improve hypertriglyceridemia. Some studies have found that fish oil supplements may ease rheumatoid arthritis pain and stiffness. Side effects can include nausea, belching and a fishy taste in the mouth. Fish oil can get in the way of medicines you take. So check with your rheumatologist before trying it.
- Tai chi. This movement therapy involves gentle exercises and stretches and deep breathing. Many people use tai chi to relieve stress. Small studies have found that tai chi may improve mood and quality of life in people with rheumatoid arthritis. When led by a trained leader, tai chi is safe. But don’t do any moves that cause pain or make it worse.
Physical Therapy and Occupational Therapy
- Physical therapy and sports can help improve or maintain mobility, strength and joint function. Examples of suitable types of sports include cycling, brisk walking, dancing, doing exercises (e.g. gentle strengthening exercises), swimming and aqua aerobics.
- The main aim of occupational therapy is to maintain your mobility and hand strength, and to learn how to get by with rheumatoid arthritis in daily life.
- Assistive devices can make it easier to keep from stressing painful joints. For instance, a kitchen knife with a hand grip helps protect finger and wrist joints. Certain tools, such as buttonhooks, can make it easier to get dressed. Look for ideas in medical supply brochures and stores.
- In advanced arthritis, various aids can compensate for many physical limitations and help you to carry out everyday activities. These include orthopedic shoe inserts, grabbing aids and specially designed cutlery.
Psychological treatments can help relieve pain and minimize the impact it has on everyday life. They are also supposed to help relieve disease-related anxiety and depression that some people develop.
Surgery
Better drugs to treat rheumatoid arthritis have lowered the need for surgery. But if medicines fail to prevent or slow joint damage or your medications are not able to relieve your symptoms and the arthritis keeps getting worse, the thin layer that lines the joint (synovium) can be surgically removed. Surgery can reduce the pain and symptoms caused by severe joint damage resulting from rheumatoid arthritis. The type of surgery may depend on the joint involved. Your surgery may involve implanting an artificial joint or fusing the joint (arthrodesis), for example.
How common is rheumatoid arthritis ?
- Rheumatoid arthritis is less common than other kinds of arthritis such as osteoarthritis.
- More than 1 million people in the United States have rheumatoid arthritis.
- Women are more likely to have rheumatoid arthritis than men. About 7 out of every 10 people with rheumatoid arthritis are women.
- Although rheumatoid arthritis can happen at any age, it usually develops between ages 30 and 50.
When to see a doctor
Make an appointment with your doctor if you have persistent discomfort and swelling in your joints.
Rheumatoid arthritis causes
Rheumatoid arthritis is a chronic autoimmune inflammatory disease in which your body’s own immune system attacks the lining of the synovial membranes that surround the joints. Scientists don’t know the cause of rheumatoid arthritis, but researchers think the condition may be passed down in families 31. Rheumatoid arthritis is a condition in which your immune system (the system of the body that helps defend you from germs) attacks healthy joint tissue by mistake, called autoimmune disease. The cause is likely a mix of genetic changes and environmental factors from outside the body 9, 10. Hormones may play a role because women are 2 to 3 times more likely than men to get rheumatoid arthritis with approximately 70–80% of rheumatoid arthritis cases in women, and do so about ten years earlier on average and rheumatoid arthritis (RA) is also often more severe in women than in men 24. There are also theories about certain viruses or bacteria causing autoimmune responses in people whose genes make them more likely to get it 32, 33, 34, 35. Abnormal protein citrullination and the formation of anti-cyclic citrullinated peptide (anti-CCP) antibodies are critical pathogenic mechanisms in rheumatoid arthritis and are associated with severe joint lesions and extra-articular organ damage 11, 12, 13.
- Rheumatoid arthritis occurs when your immune system attacks the synovium — the lining of the membranes that surround your joints.
- The resulting inflammation thickens the synovium, which can eventually destroy the cartilage and bone within the joint.
- The tendons and ligaments that hold the joint together weaken and stretch. Gradually, the joint loses its shape and alignment.
Risk factors
Factors that may increase your risk of rheumatoid arthritis include:
- Your sex. Women are more likely than men to develop rheumatoid arthritis.
- Age. Rheumatoid arthritis can occur at any age, but it most commonly begins between the ages of 40 and 60 36.
- Family history. If a member of your family has rheumatoid arthritis, you may have an increased risk of the disease.
- Smoking. Cigarette smoking increases your risk of developing rheumatoid arthritis, particularly if you have a genetic predisposition for developing the disease. Smoking also appears to be associated with greater disease severity.
- Environmental exposures. Although uncertain and poorly understood, some exposures such as asbestos or silica may increase the risk for developing rheumatoid arthritis. Emergency workers exposed to dust from the collapse of the World Trade Center are at higher risk of autoimmune diseases such as rheumatoid arthritis.
- Obesity. People who are overweight or obese appear to be at somewhat higher risk of developing rheumatoid arthritis, especially in women diagnosed with the disease when they were 55 or younger.
Rheumatoid arthritis Complications
Rheumatoid arthritis increases your risk of developing 37, 38, 39:
- Osteoporosis. Rheumatoid arthritis itself, along with some medications used for treating rheumatoid arthritis, can increase your risk of osteoporosis — a condition that weakens your bones and makes them more prone to fracture.
- Rheumatoid nodules. These firm bumps of tissue most commonly form around pressure points, such as the elbows. However, these nodules can form anywhere in the body, including the lungs.
- Dry eyes and mouth. People who have rheumatoid arthritis are much more likely to experience Sjogren’s syndrome, a disorder that decreases the amount of moisture in your eyes and mouth.
- Infections. The disease itself and many of the medications used to combat rheumatoid arthritis can impair the immune system, leading to increased infections.
- Abnormal body composition. The proportion of fat compared to lean mass is often higher in people who have rheumatoid arthritis, even in people who have a normal body mass index (BMI).
- Carpal tunnel syndrome. If rheumatoid arthritis affects your wrists, the inflammation can compress the nerve that serves most of your hand and fingers.
- Heart problems. Rheumatoid arthritis can increase your risk of hardened and blocked arteries, as well as inflammation of the sac that encloses your heart.
- Lung disease. People with rheumatoid arthritis have an increased risk of inflammation and scarring of the lung tissues, which can lead to progressive shortness of breath.
- Lymphoma. Rheumatoid arthritis increases the risk of lymphoma, a group of blood cancers that develop in the lymph system.
Signs and symptoms of rheumatoid arthritis
Signs and symptoms of rheumatoid arthritis may include:
- Tender, warm, swollen joints
- Joint stiffness that is usually worse in the mornings and after inactivity
- Fatigue, fever and weight loss
Early rheumatoid arthritis tends to affect your smaller joints first — particularly the joints that attach your fingers to your hands and your toes to your feet.
As the disease progresses, symptoms often spread to the wrists, knees, ankles, elbows, hips and shoulders. In most cases, symptoms occur in the same joints on both sides of your body.
About 40 percent of the people who have rheumatoid arthritis also experience signs and symptoms that don’t involve the joints. Rheumatoid arthritis can affect many non-joint structures, including:
- Skin
- Eyes
- Lungs
- Heart
- Kidneys
- Salivary glands
- Nerve tissue
- Bone marrow
- Blood vessels
Rheumatoid arthritis signs and symptoms may vary in severity and may even come and go. Periods of increased disease activity, called flares, alternate with periods of relative remission — when the swelling and pain fade or disappear. Over time, rheumatoid arthritis can cause joints to deform and shift out of place.
How Rheumatoid arthritis is Diagnosed
Rheumatoid arthritis can be difficult to diagnose in its early stages because the early signs and symptoms mimic those of many other diseases. There is no one blood test or physical finding to confirm the diagnosis of rheumatoid arthritis. When trying to find out if you have rheumatoid arthritis, your doctor will first ask about your symptoms such as painful joints, stiff joints in the morning and general symptoms like tiredness or exhaustion (fatigue).
During the physical exam, your doctor will check your joints for swelling, redness and warmth. He or she may also check your reflexes and muscle strength.
It can be difficult to diagnose rheumatoid arthritis at an early stage because the symptoms are often very mild in the first few weeks and months, and may not be typical. It is easier to diagnose rheumatoid arthritis (RA) in someone who has had it for a longer time. This is because, in addition to the typical physical symptoms, it’s often already easy to see changes in the joints.
If it’s thought that someone might have rheumatoid arthritis, specialized doctors known as rheumatologists (a doctor who specializes in arthritis care) can be consulted for tests, diagnosis, and care.
It can be difficult to diagnose rheumatoid arthritis at an early stage because the symptoms are often very mild in the first few weeks and months, and may not be typical. It is easier to diagnose rheumatoid arthritis (RA) in someone who has had it for a longer time. This is because, in addition to the typical physical symptoms, it’s often already easy to see changes in the joints.
Traditionally the presence of at least 4 of the following criteria for at least six weeks would classify the patient as having rheumatoid arthritis. These criteria were:
- morning stiffness,
- arthritis of three or more joints,
- arthritis of the hands,
- symmetric arthritis,
- elevated acute phase reactants,
- elevated rheumatoid factor,
- radiologic evidence of rheumatoid arthritis.
These criteria separated inflammatory from non-inflammatory arthritis but were not very specific for rheumatoid arthritis. It was also not sensitive for early-stage rheumatoid arthritis, which was a significant drawback 40.
So specialist criteria have been developed jointly by American and European experts to try to help make a diagnosis of rheumatoid arthritis in people presenting with new-onset swollen, painful joints (called synovitis) with no obvious cause (American College of Rheumatology and the European League Against Rheumatism 2010 Rheumatoid Arthritis Classification Criteria). The 2010 American College of Rheumatology and the European League Against Rheumatism diagnostic criteria for rheumatoid arthritis are outlined below. It includes four different domains, which are as follows 41:
2010 American College of Rheumatology and the European League Against Rheumatism Diagnostic Criteria for rheumatoid arthritis
- Number and site of involved joints
- 2 to 10 large joints = 1 point (shoulders, elbows, hips, knees, and ankles)
- 1 to 3 small joints = 2 points (metacarpophalangeal joints, proximal interphalangeal joints, second through fifth metatarsophalangeal joints, thumb interphalangeal joints, and wrists)
- 4 to 10 small joints = 3 points
- Greater than 10 joints (including at least 1 small joint) = 5 points
- Serological testing for rheumatoid factor (RF) or anti-citrullinated peptide/protein antibody (anti-CCP)
- Low positive = 2 points
- High positive = 3 points
- Elevated acute phase reactant (erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP]) = 1 point
- Symptom duration at least six weeks = 1 point
A total score of greater than or equal to 6 classifies the patient as having rheumatoid arthritis 41. It is important to note that joint involvement refers to any swollen or tender joint on examination. Imaging studies may also be used to determine the presence of synovitis/joint involvement. The 2010 American College of Rheumatology and the European League Against Rheumatism criteria excluded distal interphalangeal joints, first carpometacarpal joints, and first metatarsophalangeal joints from this criteria. Also, this criteria may only be applied to those patients where the joint involvement is not better explained by other inflammatory diseases, such as systemic lupus erythematosus or psoriasis. Specific testing must be obtained to rule out these diseases. The new criteria were noted to better predict the probability of rheumatoid arthritis, have the same sensitivity as the previous criteria for the diagnosis of rheumatoid arthritis and have a higher specificity as well as higher negative predictive value 40.
The 2010 American College of Rheumatology and the European League Against Rheumatism Diagnostic Criteria for rheumatoid arthritis should be used with care though as people with osteoarthritis (OA) or a crystal arthritis (gout) could meet the criteria and end up being incorrectly diagnosed with rheumatoid arthritis, which could have significant consequences for treatment. They have also been developed to classify, not diagnose, rheumatoid arthritis and so should not be used to decide who gets referred.
Blood tests
Your doctor may order these tests:
- Blood tests: Blood tests are used to detect certain antibodies and signs of inflammation in the body. People with rheumatoid arthritis (RA) often have an elevated erythrocyte sedimentation rate (ESR), also called sed rate, or C-reactive protein (CRP) level. This may show a higher level of inflammation in the body. Other blood tests look for rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies. Rheumatoid factor (RF) is an autoantibody that primarily targets the Fc fragment of IgG antibodies 42. Seropositive rheumatoid arthritis refers to rheumatoid arthritis where patients exhibit the presence of Rheumatoid factor (RF) or anti-cyclic citrullinated peptide (anti-CCP) antibodies, often involving early damage to lung tissue 43, 44. Anti-CCP antibodies and rheumatoid factor (RF) are two extensively utilized biomarkers in the diagnosis and prognosis of rheumatoid arthritis. Anti-MCV antibodies also known as anti-Sa antibodies have been proposed as an alternative due to their high specificity, which often outperforms anti-CCP antibody testing; nevertheless, it is unclear whether their benefits are significant enough to warrant routine clinical testing 21. The quest for novel biomarkers with significant therapeutic applications and their significance in rheumatoid arthritis (RA) remain a hot research area.
- Rheumatoid factor (RF) is detectable in about 60% of people diagnosed with rheumatoid arthritis (sensitivity); similarly, only about 60% specific, also occurring in older individuals, other immune mediated diseases, and in the context of infection 29. Typically pentameric IgM autoantibodies that bind the Fc portion of IgG (although can also occur in IgG and IgA isoforms). Rheumatoid factor (RF) likely has a role in perpetuating disease via immune complex formation and complement activation, leading to increased vascular permeability and immune cell chemotaxis to the joint.
- Anti-cyclic citrullinated peptide antibodies (ACPAs) is measured in routine practice using anti-cyclic citrullinated peptide assays. Anti-cyclic citrullinated peptide antibodies (ACPAs) are present in 60-80% of patients with rheumatoid arthritis 29. Anti-cyclic citrullinated peptide antibodies (ACPAs) are >90% specific in the setting of suspected rheumatoid arthritis (less specific at low titers and in the general, asymptomatic population) 29. Citrullination is a ubiquitous biochemical process catalyzed by the enzyme peptidyl arginine deiminase, leading to the post-translational modification of arginine amino acids; the presence of citrullinated auto-antigen is not associated with pathology, but the presence of anti-cyclic citrullinated peptide antibodies (ACPAs) is.
- Antiperinuclear factor (APF) and antikeratin antibodies (AKA). Antikeratin antibodies (AKA) and antiperinuclear factor (APF) have both demonstrated specificity for the diagnosis of rheumatoid arthritis 44. Antiperinuclear factor (APF) is found in 49 to 91% of rheumatoid arthritis patients, with a sensitivity range of 73 to 99%. Antikeratin antibodies are also seen in rheumatoid arthritis patients, with positive results occurring in 36 to 59% of cases 21, 45. Antikeratin antibodies (AKA) and antiperinuclear factor (APF) levels stay stable independent of disease duration. Antikeratin antibodies (AKA) and antiperinuclear factor (APF) can appear as early as the first stages of rheumatoid arthritis 44, 46. As a result, antikeratin antibodies (AKA) and antiperinuclear factor (APF) may aid in the early identification of rheumatoid arthritis, allowing for prompt intervention and drug delivery. These antibodies exhibit correlations with the presence of rheumatoid factor (RF), the activity and severity of rheumatoid arthritis, and with each other 44. Additional research has shown the utility of assessing anti-cyclic citrullinated peptide antibodies (ACPAs), antikeratin antibodies (AKA), antiperinuclear factor (APF), and certain rheumatoid factor (RF) isotypes for predicting early structural damage in the illness course 47. The best rheumatoid arthritis diagnostic marker among antikeratin antibodies (AKA), anti-CCP, and antiperinuclear factor (APF) remains unknown. According to a published study, antikeratin antibodies (AKA) and anti-CCP are both more effective than antiperinuclear factor (APF) in rheumatoid arthritis diagnosis. As a result, clinicians may choose antikeratin antibodies (AKA) or anti-CCP testing to aid with the diagnosis of rheumatoid arthritis 48.
- Anti-modified protein autoantibodies (AMPAs) aside from anti-cyclic citrullinated peptide antibodies (ACPAs), autoantibodies to carbamylated and acetylated protein antibodies (AMPAs) are well described and associated with rheumatoid arthritis; being unlikely to add diagnostic value, they are not routinely tested for, but remain of pathophysiological interest 29.
- Anti-mutated citrullinated vimentin (anti-MCV) also known as anti-Sa antibodies. Anti-mutated citrullinated vimentin (anti-MCV) is an important auto-antigen found in synovial tissue 21. In the diagnosis of rheumatoid arthritis, anti-Sa antibodies or anti-mutated citrullinated vimentin (anti-MCV) have a sensitivity of 20% to 40% and a specificity of 98% 21. Furthermore, anti-Sa antibodies or anti-mutated citrullinated vimentin (anti-MCV) strongly predict major joint issues and extra-articular rheumatoid arthritis symptoms 49. A new anti-MCV ELISA test was recently released, representing a step forward in rheumatoid arthritis diagnosis. According to Bang et al. 50, this test has the same specificity and sensitivity as anti-CCP antibodies. A published study evaluated the baseline antibodies against mutated citrullinated vimentin (MCV), CCP types 2 and 3 (both IgG isotype), and 3.1 (both IgG and IgA isotype) in 210 early rheumatoid arthritis patients over a two-year period. The Larsen score was used to assess disease activity at baseline and monthly during 24 months using radiographs of the hands and feet. Anti-MCV antibodies were associated with more severe illness, as seen by higher DAS28, ESR, and IgG levels, as compared to anti-CCP2, CCP3, and CCP3.1 antibodies 51. Another study looked at anti-MCV antibody levels at the start of treatment, one year later, and two years later in 162 individuals with early arthritis. When the indicated limit of 20 U/mL was used, the results showed that anti-MCV antibodies exhibited 92.3% specificity and 59.3% sensitivity. Patients with positive anti-MCV outcomes had higher Sharp-van der Heijde scores, higher ESR levels, and higher CRP levels at all assessment time points. 105 According to the published studies, anti-MCV antibodies may be a more sensitive and specific alternative to anti-CCP testing in diagnosing rheumatoid arthritis 52, 53.
- Antip68. Antibodies against the stress protein immunoglobulin heavy-chain binding protein (anti-BiP or antip68) are found in more than 60% of rheumatoid arthritis patients 21. These antibodies have also been found in experimental arthritis animal models 54. Furthermore, human stress protein BiP (immunoglobulin binding protein) levels in the rheumatoid joint are raised in sera from people in the early and pre-disease stages of rheumatoid arthritis 55. These data showed that human stress protein BiP (immunoglobulin binding protein) may be a significant auto-antigen in rheumatoid arthritis, but more study is needed to assess its usefulness as a biomarker.
- Other blood tests. Your doctor may also use other tests to check your kidney function, electrolytes, liver function, thyroid function, muscle markers, other autoimmune markers, and markers of infection to evaluate for your overall health and evaluate for other diagnoses. Other specific tests for rheumatoid arthritis, are sometimes considered.
Synovial fluid examination
Synovial fluid examination usually reveals a leukocyte count between 1500 to 25,000/mm³ and is predominantly polymorphonuclear cells. Cell counts higher than 25000/mm³ are rare and can be seen with very active disease; however, they warrant workup to rule out underlying infection. The synovial fluid in rheumatoid arthritis will also reveal low C3 and C4 levels despite elevated serum levels 56.
Imaging tests
Your doctor may recommend X-rays to help track the progression of rheumatoid arthritis in your joints over time. MRI and ultrasound tests can help your doctor judge the severity of the disease in your body. MRI scans is where strong magnetic fields and radio waves are used to produce detailed images of your joints.
With advanced disease, joint involvement on plain X-rays will reveal periarticular osteopenia, joint space narrowing, and bony erosions. Erosions of cartilage and bone are considered pathognomonic findings for rheumatoid arthritis. However, these findings are consistent with advanced disease 57. Magnetic resonance imaging (MRI) and ultrasonography are useful in early disease before radiographic evidence of bone erosion occurs 58. A decreased signal from the bone marrow on T1-weighted images and gadolinium-enhanced images indicates bone marrow edema. MRI can also reveal synovial thickening, which has been shown to predict the future presence of bony erosions 59. The clinical utility of MRI and its incorporation into the diagnostic criteria for rheumatoid arthritis remains to be determined.
Assessing your physical ability
If you have been diagnosed with rheumatoid arthritis, your rheumatologist will do an assessment to see how well you’re coping with everyday tasks.
You may be asked to fill in a questionnaire on how well you can do things like dress, walk and eat, and how good your grip strength is.
This assessment may be repeated after your treatment, to see if you have made any improvements.
Multiple clinical assessment tools have been developed to assist clinicians in determining the disease activity of patients with rheumatoid arthritis. An updated recommendation from the American College of Rheumatology (ACR) in 2019 recommended using the following assessment tools because they met the minimum standard for evaluation per their recommendation 60:
- Clinical Disease Activity Index (CDAI)
- Disease Activity Score (DAS)
- Disease Activity Score 28 Joints (DAS28-ESR/CRP)
- Patient-Derived DAS28
- Hospital Universitario La Princesa Index (HUPI)
- Multi-Biomarker Disease Activity Score (MBDA score, VECTRA DA)
- Rheumatoid Arthritis Disease Activity Index (RADAI)
- Rheumatoid Arthritis Disease Activity Index 5 (RADAI-5)
- Routine Assessment of Patient Index Data 3 (RAPID3)
- Routine Assessment of Patient Index Data 5 (RAPID5)
- Simplified Disease Activity Index (SDAI)
Rheumatoid arthritis diet
With the increasing evidence of altered microbiota (gut microbiome) in the gut of rheumatoid arthritis patients being responsible for pathogenesis as well as disease progression 61, it should be desirable for doctors to advocate a supplemental “diet therapy” to rheumatoid arthritis patients. Various dietary plans for rheumatoid arthritis have been reported since 62 and are being repeatedly projected 63, such as medically supervised 7–10 days fasting 64, vegan 65 or Mediterranean diets 66. We hereby discuss the reported dietary interventions that clearly indicate clinically and statistically significant and beneficial long-term effects for relieving symptoms, delay in disease progression and associated damages in rheumatoid arthritis patients. The outcomes of published randomized clinical trials performed on rheumatoid arthritis patients to observe the effect of various dietary interventions have been summarized in Table 1. A pictorial representation of effects put by various factors on progression/remission of rheumatoid arthritis is depicted in Figure 3.
Based on findings discussed in Khanna et al review 67, they have designed an anti-inflammatory food chart (Table 2) that may aid in reducing signs and symptoms of rheumatoid arthritis. This may not cure the patients; however, an effective incorporation of these food items in the daily food plan may help to reduce their disease activity, delay disease progression, and reduce joint damage, and eventually a decreased dose of drugs administered for therapeutic treatment of patients. The believe that an ideal meal can include raw or moderately cooked vegetables (lots of greens, legumes), with addition of spices like turmeric and ginger 68, seasonal fruits 69, probiotic yogurt 70; all of which are good sources of natural antioxidants and deliver anti-inflammatory effects. The patient should avoid any processed food, high salt 71, oils, butter, sugar, and animal products 72. Dietary supplements like vitamin D 73, cod liver oil 74, and multivitamins 75 can also help in managing rheumatoid arthritis. This diet therapy with low impact aerobic exercises can be used for a better degree of self-management of rheumatoid arthritis with minimal financial burden 76. A better patient compliance is, however, always necessary for effective care and management of rheumatoid arthritis.
Figure 3. Factors contributing to severity of rheumatoid arthritis

Note: The picture summarizes various factors contributing to severity of rheumatoid arthritis (RA) and diets which cause remission of symptoms (left side of image). The effects of various factors on state of disease are shown at the right side of the image. The upper half of the image shows highly inflamed joints and synovial membrane, increased infiltration of immune cells in joints on exposure to environmental factors or food antigens. Lower half of the image shows effect of various diets in reducing inflammation, immune cell infiltration, and reducing the severity of disease.
[Source 67]Table 1. Summary of clinical trials of various dietary interventions in rheumatoid arthritis (RA)
| Reference | Subjects, duration, and diet | Outcome |
|---|---|---|
| Kjeldsen-Kragh et al. 77 | Diet group—27 patients 7–10 days subtotal fasting (limited amount of nutritional supplements) 3.5 months on individually adjusted gluten-free vegan diet followed by lactovegetarian diet Control group—26 patients Ordinary diet throughout the study | After 1 month of diet Reduction in number of tender (p < 0.0002) and swollen joints (p < 0.04), Ritchie articular index (RAI) (p < 0.0004), pain (p < 0.0001), morning stiffness duration (p < 0.0002), grip strength, HAQ score, erythrocyte sedimentation rate (ESR) (p < 0.002), C-reactive protein (CRP) (p < 0.005), and WBC count (p < 0.0001) which were maintained even after 1 year of administration of diet Key note: Improvement can be maintained by continuing with individually adjusted diet |
| Kjeldsen-Kragh et al. 78 | Diet group—27 patients 7–10 days subtotal fasting 3.5 months on individually adjusted gluten-free vegan diet followed by lactovegetarian diet Control group—26 patients Ordinary diet throughout the study | After 1 month of treatment Significant decrease in leukocyte and platelet count (p < 0.003), IgM rheumatoid factors (p < 0.02), IgG, C3 (p < 0.04) and C4 complement components (p < 0.01), calprotectin (p < 0.03) and C3 activation products in diet responders in vegetarian diet group Key note: Dietary interventions can help in improvement of disease in some RA patients |
| Peltonen et al. 79 | Diet group—27 patients 7–10 days subtotal fasting 3.5 months on individually adjusted gluten-free vegan diet followed by 9 months lactovegetarian diet administration Control group—26 patients Ordinary diet throughout the study | Significant difference in fecal fatty acid profile at different times during the dietary intervention as compared to baseline in diet group was observed (p < 0.005). Fecal flora was significantly different between vegan diet (post 1 month treatment) and lactovegetarian diet period (p < 0.001). Significant difference in fecal flora was also observed between high improvement to low improvement groups (p < 0.001). This difference was also found at 1 month (vegan diet) and 13 months (lactovegetarian diet) Key note: Study finds association between disease activity and intestinal flora indicating impact of diet on disease progression |
| Haugen et al. 80 | Diet group—27 patients 7–10 days subtotal fasting 3.5 months on individually adjusted gluten-free vegan diet followed by lactovegetarian diet Control group—26 patients Ordinary diet throughout the study | Post 3.5 months of vegan diet Significant reduction in plasma fatty acid 20:3n-6 (p < 0.0001) and 20:4n-6 (p < 0.01) was observed which reversed to baseline concentration after lactovegetarian diet Significant reduction in 20:5n-3 post-vegan diet (p < 0.0001) and lactovegetarian diet (p < 0.01) No significant difference in fatty acid concentration between diet responders and non-responders after vegan or lactovegetarian Key note: Change in fatty acid profile could not explain disease improvement |
| Haugen et al. 81 | Diet group—17 patients 7–10 days fasting 3.5 months on gluten-free vegan diet followed by 9 months lactovegetarian diet administration Control group—17 patients Ordinary diet throughout the study | After 1 month Significant reduction in body mass index (BMI) and triceps skin fold thickness in diet group as compared with baseline (post 1 month) (p < 0.001) and controls (post study) (p = 0.04; p < 0.01) Key note: One year of dietary intervention had a minor impact on nutritional status of patients. No significant differences in other clinical variables studied were observed between the two groups |
| Kjeldsen-Kragh et al. 82 | Patients of above study were called for follow-up; 1 year post-trial. All responders and half non-responders were still on diet. Most of the patients eliminated those food which they thought aggravated their disease | Diet responders showed greatest change in clinical variables including HAQ (p < 0.04) and RAI (p < 0.02) from the baseline. Significant improvements were observed in all clinical variables including pain (p < 0.005), morning stiffness duration (p < 0.005), tender joint (p < 0.0003), RAI (p < 0.0001) and swollen joints (p < 0.05) except grip strength as compared to non-responders and controls Key note: Patients gained benefit from manipulation of diet which can be maintained for long term |
| Kjeldsen-Kragh et al. 83 | Diet group—26 RA patients 7–10 days fasting followed by 3.5 months of gluten-free vegetarian diet | Agalactosyl IgG antibodies reduced in RA patients and correlated significantly (p = 0.04) with clinical improvement post fasting which was not observed after administration of vegetarian diet Key note: IgG glycosylation may improve disease status during fasting |
| Fraser et al. 84 | Diet group—10 patients 7 days subtotal fasting 13 patients—ketogenic diet for 7 days All patients followed 2 weeks period of re-feeding on lactovegetarian diet | Post 7 days fasting Significant decrease in serum IL-6 levels in fasting group (p < 0.03) on seventh day as compared to baseline and after re-feeding. Improvement was observed in ESR, CRP, and tender joint counts post 7 days fasting Key note: Fasting improves disease activity in RA patients |
| Michalsen et al. 85 | 16 RA patients and 35 fibromyalgia patients 21 patients—vegetarian Mediterranean diet (MD) 30 patients—intermittent modified 8 days fasting therapy | No difference in the fecal bacterial counts, concentration of secretory immunoglobulin or pH of the stool within or between the two diet groups. Post 2 weeks of study, fasting RA patients showed more clinical improvement as compared to non-fasting patients Key note: Clinical improvement is not related to intestinal flora |
| Abendroth et al. 86 | 22 patients—medical fasting for 7 days 28 patient—MD | Both groups observed significant decrease in disease activity score (DAS) (p < 0.001). Significantly higher decrease in pain in fasting group on seventh day (p = 0.049). No significant difference was observed in total fatty acid profile, butyrate and propionate but acetate increased significantly (p = 0.044) in fasting group and decreased significantly in MD group. No significant correlation between diet induced changes in short chain fatty acids and disease activity changes was observed Key note: Change of intestinal microflora and relation with diet needs further studies |
| Sköldstam et al. 66 | Diet group—26 patients—MD Control group—25 patients | After 12 weeks of study, MD group showed significant reduction in DAS28 score (p < 0.001), decrease in HAQ (p = 0.020), and improvement in SF-36 health survey in two dimensions (p = 0.018). Out of 14 efficacy variables, 9 had shown improvement in diet group Key note: MD administration reduced disease activity in RA patients |
| Hafström et al. 87 | Diet group—38 patients—gluten-free vegan diet Control group—28 patients | Vegan group showed higher response rate and significant improvement in all variables except CRP. The diet responders have significant improvement in CRP (p < 0.05). Levels of IgG anti-gliadin (p = 0.0183) and anti-β-lactoglobulin (p = 0.0162) levels have significantly reduced from baselines in vegan diet groups. After 6 and 12 months, there was significant increase in Larsen score, number of erosions and joint count in both groups Key note: Diet change may reduce immunoreactivity to certain food antigens and some RA patients and may have certain clinical benefits |
| Peltonen et al. 88 | Diet group—uncooked vegan diet rich in lactobacilli Control group—normal omnivorous diet. | Diet group had significant change in fecal microflora from pre-test and post-test samples (p < 0.001) but not in control group. Significant difference was found on comparison of test group with control group at 1 month (p < 0.001). Significant difference in microflora was observed between low and high improvement index group after 1 month (p = 0.001) and after intervention (p = 0.029) but not in pre-test samples Key note: Fecal microflora changes with diet and helps in improvement of RA |
| McDougall et al. 65 | 24 RA patients—very low fat vegan diet | Significant decrease in energy intake (p < 0.001), fats (p < 0.001) and proteins (p < 0.001) and significant increase in carbohydrate intake (p < 0.001) with decrease in weight. RA symptoms decreased including pain (p < 0.004), morning stiffness (p < 0.04), joint swelling (p < 0.02), and tenderness (p < 0.01) with increased joint mobility (p < 0.001) Key note: RA symptoms significantly decrease in moderate or severe RA patients on administration of very low fat vegan diet |
| Elkan et al. 89 | Diet group—38 patients—gluten-free vegan diet Control group—28 patients | After 12 months, vegan group showed decreased BMI, LDL, and weight. DAS28 (p = 0.002) and HAQ scores (p = 0.010) decreased significantly in at least 3 months when compared to baseline and CRP decreased (p = 0.008) at 12 months. In vegan group, at least in 3 months, total cholesterol (p < 0.001), LDL (p < 0.001) and LDL/HDL ratio (p < 0.001) significantly decreased but TGs and HDL did not change. OxLDL significantly decreased (p = 0.021) after 3 months in responders group. IgM anti-phosphorylcholine increased significantly trend wise and was significant at twelfth month (p = 0.057) Key note: Vegan diet (gluten free) is anti-inflammatory and atheroprotective |
| Sköldstam et al. 90 | Study 1: Diet group—14 patients—lactovegetarian diet Control group—10 patients Study 2: 13 patients—control period of 2 months 7 patients—control period of 5 months followed by vegan diet for following 4 months Study 3: Diet group—26 patients—Cretan MD Control group—25 patients | Study 1: At end of study, diet group reported reduction in pain with a significant weight loss (p < 0.001) but no change in disease outcome and no change in control subjects were observed Study 2: During vegan diet, all 20 patients were reported to have significant reduction in pain score, increased functional capacity, and significant weight loss (p < 0.001), which was not observed during the control period Study 3: 9 out of 14 disease outcome measures were improved with a significant loss in weight (p < 0.001) and decreased pain when compared to controls Statistically significant correlation was found between diet and three disease outcome variables including ΔAcute-Phase Response (p = 0.007), ΔPain Score (p = 0.005), and ΔPhysical Function (p = 0.002) Key note: Improvement of RA on administration of Vegan, Mediterranean, or lactovegetarian diet is not related to reduction of body weight |
| Ågren et al. 91 | Diet group—16 patients—vegan diet Control group—13 patients | Significant reduction (p < 0.001) of serum total, LDL cholesterol, and phospholipid concentrations were observed in vegan diet group. Sitosterol concentration increased and that of campesterol decreased giving a significant greater ratio of sitosterol: campestrol (p < 0.001) in vegan diet group when compared to control group Key note: Serum cholesterol, cholestanol, phospholipids, and lathosterol decrease in uncooked vegan diet |
| Hänninen et al. 92 | 42 patients divided in two groups—Uncooked vegan diet for 3 months and omnivorous control groups | The RA symptoms reduced in diet group and reverted on restarting omnivorous diet. There was a significant negative correlation between degree of subjective adaptation system and decreased activity of RA (p = 0.003) Key note: Vegan diet rich in fibers, antioxidants, and lactobacilli improved RA in some patients |
| Vaghef-Mehrabany et al. 93 | Diet group—22 patients—108 colony-forming unit (CFU) of Lactobacillus casei 01 for 8 weeks 24 patients—placebo with maltodextrin for 8 weeks | Number of tender and swollen joints, serum hs-CRP levels, DAS, visual analog scale (VAS) score, tumor necrosis factor (TNF)-α, and IL-12 decreased significantly in probiotic group. Significant increase in IL-10 (p = 0.02), IL-10/IL-12 (p = 0.01), and IL-10/TNF-α (p = 0.03) was observed in the probiotic group Key note: Disease activity and inflammatory status improved in patients on L. casei 01 supplementation |
| Vaghef-Mehrabany et al. 94 | Diet group—22 patients—108 CFU of L. casei 01 for 8 weeks 24 patients—placebo with maltodextrin for 8 weeks | No significant difference was observed within or between probiotic and placebo group in serum malondialdehyde, total antioxidant capacity, and catalase activity. Erythrocyte superoxide dismutase activity decreased significantly in probiotic group and glutathione peroxidase activity decreased in both groups. Difference between two groups was insignificant for both groups at the end of the study Key note: Probiotic supplementation does not have significant effect on oxidative status of RA patients |
| Hatakka et al. 95 | Diet group—8 patients—L. rhamnosusGG (LGG) (≥5 × 109 CFU/capsule), twice a day for 12 months 13 patients—placebo group | Mean number of tender and swollen joints decreased in probiotic group. A 71% reduction in disease activity was observed in probiotic group and 30% in placebo group. Serum IL-1β increased in probiotic group and decreased in placebo group. At the end of the study, fecal recovery of LGG was increased from 25 to 86% in probiotic from baseline and decreased from 23 to 0% in placebo group Key note: More patients administered with LGG reported subjective well-being |
| Zamani et al. 96 | Diet group—30 patients—L. acidophilus (2 × 109 CFU/g), L. casei (2 × 109 CFU/g), and Bifidobacterium bifidum (2 × 109 CFU/g) 30 patients—placebo group received capsule filled with cellulose | Probiotic group observed significant decrease in DAS28 score (p = 0.01), serum insulin levels (p = 0.03), HOMA-B (p = 0.03), serum hs-CRP concentrations (p < 0.001), LDL cholesterol (p = 0.07), and total cholesterol (p = 0.09) compared to placebo group. No significant effect was observed in tender and swollen joints, VAS pain, glucose homeostasis parameters, biomarkers of oxidative stress, and lipid profiles after probiotic administration Key note: Patients had significant benefit by incorporating probiotic supplements in diet |
| Vaghef-Mehrabany et al. 97 | Diet group—22 patients—108 CFU of L. casei 01 24 patients—placebo group received similar capsules with maltodextrin | No significant difference within or between group for anthropometric and demographic parameters, physical activity was observed. Serum lipid did not change within any group significantly or in between the groups Key note: L. casei 01 could not improve serum lipid in patients |
| Alipour et al. 98 | Diet group—22 patients—108 CFU of L. casei 01 24 patients—placebo group | Probiotic decreased serum high sensitivity CRP levels (p = 0.009), counts of swollen (p = 0.003) and tender joints (p = 0.03), DAS (p < 0.05), and global health score (p = 0.00). Global health score decreased significantly in placebo group as well. At the end of study, more patients in probiotic group showed moderate response to the supplementation according to EULAR criteria but all were non-responders in placebo group. The difference of IL-6, IL-12 (0.00), TNF-α (p = 0.002), and IL-10 (p = 0.007) cytokines between the two groups was statistically significant Key note: Probiotic can be an adjunct therapy for relieving symptoms |
| de los Angeles Pineda et al. 99 | Diet group—15 patients—L. rhamnosus GR-1 and L. reuteri RC-14 with 2 billion CFU viable bacteria 14 patients—placebo | Significant difference was observed in HAQ score (p = 0.02) in probiotic group when compared to baseline but not between groups. The pro-inflammatory cytokines including GM-CSF, IL-6, IL-1α, TNF-α, and IL-15 decreased but not significantly in the probiotic group. No difference was observed in cytokine levels and DAS Key note: Probiotics did not improve RA but functional improvements were reported |
| Mandel et al. 100 | Diet group—22 patients—Bacillus coagulans GBI-30, 6086 (2 billion CFU) with green tea extract, methylsulfonylmethane, and vitamins and minerals (including vitamins A, B, C, D, E, folic acid, and selenium) 22 patients—placebo group received microcrystaline cellulose | Probiotic group showed statistically significant improvement in patient pain assessment score (p = 0.052) and pain scale (p = 0.046) as compared to baseline. Improvement was observed in patient global assessment, patient self-assessed disability, and reduction in total CRP but statistical difference was not found in physician global assessment or physician assessment of painful and swollen joints. Ability to walk 2 miles was marginally significant (p = 0.072) and ability to participate in daily activities was more in probiotic group Key note: Adjunctive therapy with probiotics serves effective for RA patients |
| Kavanagh et al. 101 | Diet group—24 patients—elemental diet 028 (E028) (4 weeks) followed by food reintroduction where food unlikely to cause intolerance were introduced first followed by those which were known to cause intolerance one at a time. Food worsening RA was eliminated 23 patients—control groups were given E028 as a substitute to any drink along with normal diet | After 4 weeks of elemental diet, the diet group showed significant increase in grip strength (p = 0.008), decrease in RAI (p = 0.006), and loss of weight as compared to control diet group. CRP concentrations were different between the two groups but not significant. Statistically significant correlation was observed between loss of weight and grip strength at 1 week (p = 0.009) and 4 weeks (p = 0.027) in the diet group Key note: Elemental diet may improve some parameters in RA patients |
| Podas et al. 102 | Diet group—21 patients—elemental diet E028 9 patients—oral prednisolone 15 mg/day | All clinical parameters of RA including early morning stiffness, VAS, RAI, and HAQ improved significantly (p < 0.05) in both groups. Clinical parameters were improved by 20% in 72% patients in elemental diet group as compared to 78% in steroid group Key note: A 2 week treatment with elemental diet is as effective as 15 mg/day of prednisolone in improvement of clinical parameters. RA may start within the intestine due to reaction to various food antigens |
| Holst-Jensen et al. 103 | Diet group—15 patients—commercial liquid diet (TU). TU contains hydrolyzed soy protein, triglycerides and carbohydrates, methionine, tryptophan, vitamins, and trace elements and is lactose free Control group—15 patients | 4 weeks of treatment caused statistical significant improvements in pain (p = 0.02), HAQ score (p = 0.03) and reduction in BMI (p = 0.001). After the study, the number of swollen joints, ESR and General assessment of health, average during the last week lowered but not statistically significant. No difference was observed in the control group. Only one patient in the diet group achieved complete remission Key note: Peptide diet can improve some subjective and objective parameters of the disease. This diet may help those patients who have diet aggravated RA |
| Van de Laar and Van der Korst 104 | Diet group—45 patients—allergen free diet 49 patients—allergen restricted with lactoproteins and yellow dyes During first 4 weeks, patients followed their normal diets followed by 4 weeks of assigned diets and then administration of normal diet for 4 weeks | No significant difference could be found in clinical effects between the allergen free and allergen restricted diet. Only 9 out of 94 patients enrolled in the study showed favorable response but the disease relapsed after readministration of usual diets Key note: Some patients have food-aggravated RA, and they can be controlled by administering allergen-free food |
| Karatay et al. 105 | 20 patients—positive skin prick test (SPT) to food extracts 20 patients—negative SPT All patients first fasted to most common allergenic food for 12 days. Food challenge was performed for PPG with allergenic food and for PNG with corn and rice for 12 days. Followed which allergenic foods were removed from respective groups | On food challenge in PPG, ESR (p < 0.05), CRP (p = 0.001), TNF-α (p < 0.01), and IL-1β (p < 0.05) increased and was also observed on re-elimination of food. In PNG, pain decreased significantly (p < 0.05) on food challenge. At end of re-elimination phase, differences were observed in between two groups in pain, duration of stiffness, number of tender and swollen joints, CRP levels, and RAI but not in HAQ and ESR levels. 72% patients in PPG group and 18% in PNG group suffered from disease aggravation on food challenge which continued in re-elimination phase Key note: Diet changes on individual level may change disease activity in patients |
Table 2. Dietary interventions and their impact on rheumatoid arthritis
| Diet Type | Impact on rheumatoid arthritis (Enhance/Suppress) | Mechanism Associated with rheumatoid arthritis | References |
|---|---|---|---|
| High-fiber diet | Suppresses, and in the case of dysbiosis, it worsens. | Reduces inflammation, improves quality of life; in the case of concurrent colonisation with Prevotella copri, it may lead to rheumatoid arthritis exacerbation. | 106, 107, 108 |
| Mediterranean diet | Suppresses/no effect. | Positive effect on overall health (prevention of cancer and cardiovascular diseases), positive effect on mental health (prevention of depression), reduces or has no effect on rheumatoid arthritis activity. | 109, 110, 111, 112 |
| Anti-inflammatory diet | Suppresses/has no effect. | Reduces or has no effect on rheumatoid arthritis activity. | 113, 114 |
| Mediterranean–DASH intervention for neurodegenerative delay (MIND) diet | Suppresses. | Prevention of cardiovascular diseases, improvement in cognitive function, reduces rheumatoid arthritis activity. | 115, 116 |
Table 3. Recommended anti-inflammatory food chart
| Fruits | Dried plums, grapefruits, grapes, blueberries, pomegranate, mango (seasonal fruit), banana, peaches, apples |
| Cereals | Whole oatmeal, whole wheat bread, whole flattened rice |
| Legumes | Black soybean, black gram |
| Whole grains | Wheat, rice, oats, corn, rye, barley, millets, sorghum, canary seed |
| Spices | Ginger, turmeric |
| Herbs | Sallaki, ashwagandha |
| Oils | Olive oil, fish oil, borage seed oil (in encapsulated form) |
| Miscellaneous | Yogurt (curd), green tea, basil (tulsi) tea |
Rheumatoid arthritis treatment
Although there is no cure for rheumatoid arthritis, treatment can:
- Relieve pain and swelling
- Slow down or stop joint damage
- Help lower the number of symptom “flareups” (times when pain or swelling is the worst)
- Improve your ability to do daily activities such as bathing, getting dressed, doing chores, reaching, and lifting.
Although there’s no cure for rheumatoid arthritis, early treatment and support (including medicine, lifestyle changes, supportive treatments and surgery) can reduce the risk of joint damage and limit the impact of rheumatoid arthritis.
The International Task Force Guidelines published in 2014 make the following recommendations regarding treatment of rheumatoid arthritis 118:
- The primary goal of treatment is to achieve long-term clinical remission and optimize quality of life with the absence of signs and symptoms associated with inflammatory disease activity.
- If clinical remission cannot be achieved, low disease activity is an acceptable alternative.
- Disease activity should be assessed every month in patients with moderate to severe disease activity.
- In patients with low disease activity or clinical remission, disease activity should be assessed every 3 to 6 months.
Clinical studies indicate that remission of symptoms is more likely when treatment begins early with medications known as disease-modifying antirheumatic drugs (DMARDs).
Disease-modifying antirheumatic drugs (DMARDs) typically used in treating rheumatoid arthritis include methotrexate, hydroxychloroquine, sulfasalazine, and leflunomide. Anti-TNF-alpha agents include etanercept, infliximab, adalimumab, golimumab, and certolizumab pegol. Non-TNF biologic DMARDs include interleukin (IL) 6 receptor antagonists such as tocilizumab and sarilumab, T-cell blockers such as abatacept (CTLA4-Ig), and the anti-CD20 B-cell depleting monoclonal antibody such as rituximab. Other synthetic DMARDs include Janus kinases (JAK) inhibitors such as tofacitinib, baricitinib, and upadacitinib.
DMARD therapy, including biologic agents and targeted therapy agents (tofacitinib), should be temporarily held in patients with a serious active infection. They can be resumed after the infection has resolved and antimicrobial treatment has been completed 119. It is essential to remember that all patients starting treatment for rheumatoid arthritis should be screened for hepatitis B and C and tuberculosis. Methotrexate should be avoided in patients with liver damage 119. Patients with latent tuberculosis should complete treatment for at least one month before the initiation of biologic agents. If patients cannot take or complete treatment for latent tuberculosis, conventional DMARD therapy should be used 119. In patients with underlying skin cancer and lymphoproliferative disorders, biologic agents should be avoided except for rituximab in patients with lymphoproliferative disorders as there is evidence of benefit from B-cell suppression in these cases 119. The American College of Rheumatology also recommends that before starting therapy for rheumatoid arthritis, patients should receive vaccination for pneumococcus, hepatitis, influenza, human papillomavirus (HPV), and herpes zoster virus (HZV).
American College of Rheumatology and the European Alliance of Associations for Rheumatology Treatment Guidelines for Rheumatoid Arthritis
The American College of Rheumatology (ACR) has published its 2021 guideline for rheumatoid arthritis treatments 120. The 2020 European Alliance of Associations for Rheumatology (EULAR) recommendations regarding the management of rheumatoid arthritis highlight the importance of early intervention, with the aim of achieving remission or low disease activity 121. Emerging evidence have shown that early diagnosis and treatment of rheumatoid arthritis can reduce the incidence of joint damage and disability and ultimately improve prognosis 122, 123, 124. Treatment for rheumatoid arthritis should be initiated as soon as possible after the diagnosis is confirmed—preferably within the first three months from the onset of symptoms—as the early therapeutic window offers the best chance of controlling the disease and preventing irreversible joint damage 121. For patients without previous DMARD use, treatment recommendations are stratified by disease activity using patient ratings and inflammation measures. Treatment decisions should be reevaluated within a minimum of 3 months based on efficacy and tolerability of the DMARD(s) chosen 120.
American College of Rheumatology and the European League Against Rheumatism Treatment Guidelines 119:
- According to the American College of Rheumatology treatment guidelines for early rheumatoid arthritis, patients who have not taken disease-modifying antirheumatic drug (DMARD) therapy should start DMARD therapy regardless of the activity level.
- In patients with low disease activity and early disease, monotherapy with methotrexate is the preferred treatment.
- Leflunomide or sulfasalazine are the first-line treatment in patients with a contraindication to methotrexate or intolerance to it.
- If monotherapy with DMARD does not control disease activity (regardless of concomitant glucocorticoid use), therapy should be altered. Methotrexate can be continued or discontinued at this point. Additional therapy options after failed monotherapy with DMARD are recommended as either dual traditional/nonbiologic DMARD therapy, tumor necrosis factor (TNF) inhibitors, or non-tumor necrosis factor biologic agents.
- In patients with established rheumatoid arthritis, who are DMARD naive, methotrexate is the preferred agent for initial monotherapy, regardless of the disease activity level.
- If monotherapy with DMARD does not control disease activity in established rheumatoid arthritis (regardless of concomitant glucocorticoid use), dual DMARD therapy, a TNF inhibitor, a non-TNF biologic agent, or tofacitinib therapy can be added.
- If disease activity remains high on TNF inhibitor monotherapy, DMARD therapy should be added in addition to the TNF inhibitor.
- If disease activity remains high despite anti-TNF inhibitor switch to a non-TNF biologic agent with or without methotrexate
- If disease activity remains high despite a trial of anti-TNF and non-TNF agents, use another non-TNF biologic agent before considering tofacitinib.
- If still uncontrolled despite the above trials, use tofacitinib
- If disease activity remains high despite the above combination therapies, short-term low-dose glucocorticoid therapy should be added.
- TNF inhibitors should be avoided in patients with congestive heart failure.
- Patients with hepatitis C who have not been treated or are currently not on treatment for it should receive nonbiologic DMARD therapy rather than TNF inhibitors.
Table 4. Disease-modifying antirheumatic drugs (DMARDs) initiation (American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis 2021)
| Recommendations | Certainty of evidence |
|---|---|
| Initiation of treatment in DMARD-naive patients with moderate-to-high disease activity | |
| Methotrexate monotherapy is strongly recommended over: | |
| Very low/low ‡ |
| Very low/moderate |
| Low/very low |
| Methotrexate monotherapy is conditionally recommended over: | |
| Low |
| Moderate |
| Low |
| Very low |
| Moderate |
| Initiation of treatment in DMARD-naive patients with low disease activity | |
| Very low |
| Very low |
| Very low |
| Initiation of treatment in csDMARD-treated, but methotrexate-naive, patients with moderate-to-high disease activity # | |
| Moderate/very low |
Footnotes:
† The closest matching population, intervention, comparator, and outcomes (PICO) questions to each recommendation are provided.
‡ The first certainty of evidence applies to the first listed option; the second certainty of evidence applies to the second listed option.
§ The original population, intervention, comparator, and outcomes (PICO) included individual DMARDs as comparators. The recommendation considers bDMARDs as a group.
¶ The direction of the beneficial effect is in favor of the nonpreferred option.
# Other recommendations for this patient population are the same as those for DMARD-naive patients.
** The direction of the beneficial effect is in favor of the nonpreferred option. The certainty of evidence is high for the combination of methotrexate plus a TNF inhibitor and moderate for other bDMARDs.
Abbreviations: bDMARD = biologic DMARD; tsDMARD = targeted synthetic DMARD; TNF = tumor necrosis factor; csDMARD = conventional synthetic DMARD.
[Source 120 ]Low Disease Activity
Patients with rheumatoid arthritis with low disease activity (see Clinical Disease Activity Index (CDAI) for Rheumatoid Arthritis calculator), consider, in this order, initial treatment with hydroxychloroquine (Plaquenil), sulfasalazine (Azulfidine), methotrexate, and leflunomide (Arava). Hydroxychloroquine is better tolerated and has a better risk profile than the others 125. Sulfasalazine is recommended over methotrexate and leflunomide because it causes less immunosuppression. Methotrexate is recommended over leflunomide because of lower cost and better dosing flexibility 125.
Moderate to High Disease Activity
Patients with rheumatoid arthritis with moderate to high disease activity (see Clinical Disease Activity Index (CDAI) for Rheumatoid Arthritis calculator), methotrexate is the best initial treatment for rheumatoid arthritis 125. Methotrexate has stronger evidence of disease-modifying activity than the other conventional synthetic DMARDs (csDMARDs), hydroxychloroquine and sulfasalazine. Although some biologic DMARDs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) have evidence of better outcomes, methotrexate is safe and effective, with convenient dosing and low cost 125. Combination therapy is not recommended for initial treatment because it is unnecessary for many patients and incurs higher toxicity and cost.
Methotrexate is strongly recommended over hydroxychloroquine or sulfasalazine for DMARD-naive patients with moderate-to-high disease activity 120. This recommendation is strongly in favor of methotrexate despite very low-certainty evidence for hydroxychloroquine and low-certainty evidence for sulfasalazine based on the amount of data supporting the disease-modifying properties of methotrexate monotherapy compared to hydroxychloroquine or sulfasalazine and concerns over the long-term tolerability of sulfasalazine 126, 127.
Methotrexate is conditionally recommended over leflunomide for DMARD-naive patients with moderate-to-high disease activity 120. Despite low-certainty evidence of comparable efficacy, methotrexate is preferred over leflunomide because of the evidence supporting its value as an anchor DMARD in combination regimens. Additional advantages of methotrexate include its greater dosing flexibility and lower cost.
Methotrexate monotherapy is strongly recommended over biologic DMARD (bDMARD) or targeted synthetic DMARD (tsDMARD) monotherapy for DMARD-naive patients with moderate-to-high disease activity 120. There is low-certainty evidence suggesting superiority of tocilizumab monotherapy over methotrexate monotherapy and moderate-certainty evidence suggesting greater efficacy of JAK inhibitor monotherapy over methotrexate monotherapy 128. However, methotrexate monotherapy is preferred because of its established efficacy and safety as a first-line DMARD and low cost 120. Moreover, tocilizumab and JAK inhibitors are not approved by the US Food and Drug Administration (FDA) for use in csDMARD-naive patients 120. Safety concerns released in early 2021 associated with JAK inhibitors further support the recommendation of methotrexate monotherapy over tsDMARDs as initial DMARD therapy at this time 120.
Methotrexate monotherapy is conditionally recommended over dual or triple csDMARD therapy for DMARD-naive patients with moderate-to-high disease activity 120. The recommendation favors methotrexate monotherapy because the higher burden of combination therapy (e.g., multiple medications, higher cost) outweighs the moderate-quality evidence suggesting greater improvements in disease activity associated with combination csDMARDs 129. The recommendation is conditional because some patients may choose csDMARD combination therapy for an increased probability of obtaining a better response despite the added burden of taking multiple medications 120.
Methotrexate monotherapy is conditionally recommended over methotrexate plus a tumor necrosis factor (TNF) inhibitor for DMARD-naive patients with moderate-to-high disease activity 120. Despite low-certainty evidence supporting greater improvement in disease activity with methotrexate plus a TNF inhibitor, methotrexate monotherapy is preferred over the combination because many patients will reach their goal on methotrexate monotherapy and because of the additional risks of toxicity and higher costs associated with TNF inhibitors 120. The recommendation is conditional because some patients, especially those with poor prognostic factors, may prioritize more rapid onset of action and greater chance of improvement associated with combination therapy over the additional risks and costs associated with initial use of methotrexate in combination with a TNF inhibitor 130, 131, 132.
Methotrexate monotherapy is strongly recommended over methotrexate plus a non–TNF inhibitor bDMARD or tsDMARD for DMARD-naive patients with moderate-to-high disease activity 120. There is very low-certainty evidence supporting the superiority of methotrexate plus a non–TNF inhibitor bDMARD or tsDMARD over methotrexate monotherapy in DMARD-naive patients; thus, methotrexate monotherapy is strongly preferred given the lack of proven benefit and additional risks and costs associated with the addition of a non–TNF inhibitor bDMARD or tsDMARD in this patient population 120.
Recommendations for methotrexate therapy are as follow 125:
- Start with oral methotrexate at 15mg weekly, if tolerated. Methotrexate should be avoided in patients with liver disease 133. If oral Methotrexate is started at less than 15mg weekly, titrate to 15mg weekly within 6 weeks. Give folic acid or folinic acid to reduce adverse effects. If patients do not tolerate oral methotrexate at sufficient dose, split the dose over 24 hours or use weekly subcutaneous methotrexate injections or increase the dose of folic acid or folinic acid. If symptoms do not improve with oral Methotrexate, switch to subcutaneous methotrexate before changing or adding medications.
Neither short- nor long-term glucocorticoid therapy is recommended as standard initial treatment, although some patients may require short-term glucocorticoid therapy in addition to DMARDs to aid with symptom control 125.
Treat-to-Target Therapy
Treat-to-target refers to a systematic approach involving frequent monitoring of disease activity using validated instruments and modification of treatment to minimize disease activity with the goal of reaching a predefined target (low disease activity or remission) 120. Target refers to low disease activity or remission. Recommendations specify that patients be at target (low disease activity or remission) for at least 6 months prior to tapering 120. Because initial treatment depends on disease activity measured by a standard scale, optimal doses should be determined by target disease activity. Although the evidence is strongest for patients treated with conventional synthetic DMARDs (csDMARDs) alone, treat-to-target therapy is recommended for all patients. An initial target of low disease activity is recommended because remission may not be achievable for many patients.
Adjusting Antirheumatic Therapy
Patients with rheumatoid arthritis without adequate symptom control on methotrexate alone, another medication should be added 125. Studies demonstrate that triple therapy, a combination of methotrexate, hydroxychloroquine, and sulfasalazine, is as effective as adding a bDMARD or tsDMARD 125. The ACR recommends adding a biologic DMARD (bDMARD) or targeted synthetic DMARD (tsDMARD) based on a more rapid improvement in symptoms and the greater persistence of therapy in observational studies. Yet, the cost of triple therapy is significantly less and may be appropriate for some patients 125.
If adding a biologic DMARD (bDMARD) or targeted synthetic DMARD (tsDMARD) does not improve symptoms to the targeted disease activity level, switching to a different class of bDMARD or tsDMARD is recommended based on very low-quality evidence 125. Switching DMARD medications or adding another DMARD is preferred to adding or continuing glucocorticoids, including intraarticular glucocorticoids 125.
Tapering or Discontinuing DMARD Therapy
Because stopping all DMARDs is associated with a moderate to high risk of a symptom flare-up and potential for irreversible damage, a minimum of one DMARD is recommended in all patients 125. For patients with symptoms at the targeted level for less than six months, continuing all DMARDs at their current dose is recommended 125. Because the risk of a flare-up is highest when lowering the dose or stopping DMARDs, any dose reductions should be gradual. For patients taking triple therapy, discontinuing sulfasalazine should be considered before hydroxychloroquine. If patients are taking methotrexate and a bDMARD or tsDMARD, tapering methotrexate should be considered first because the bDMARD or tsDMARD was added after methotrexate alone was ineffective 125.
Patients with rheumatoid arthritis with Coexisting Conditions
Patients with rheumatoid arthritis with subcutaneous rheumatoid nodules, methotrexate is recommended as initial therapy unless nodules progress 125. Switching to a different DMARD is then recommended 125.
Although preexisting lung disease is a risk factor for methotrexate-related pneumonitis, the risk is uncertain and other therapies can also worsen lung disease. Methotrexate is recommended with careful monitoring 125.
For patients with nonalcoholic fatty liver disease (NAFLD), methotrexate should be restricted to those with normal liver enzymes and no history of fibrosis 125.
For patients with preexisting heart failure or who develop heart failure, tumor necrosis factor blocker therapy should be avoided because of risk of worsening heart failure 125.
In patients who have had a serious infection within the previous 12 months, bDMARDs, tsDMARDs, and glucocorticoids should be avoided 125.
Corticosteroids
In an effort to greatly limit the use of corticosteroids, even as a bridge treatment, the guidelines strongly recommend against long-term steroids and conditionally recommend against short-term steroid use 134. For patients who need steroids to reach a treatment goal, such as low disease activity, adding or switching DMARDs instead of continuing with steroids is conditionally recommended 134.
There are several situations in which corticosteroids may be considered. In a new patient with very active rheumatoid arthritis, corticosteroids can be used as bridge therapy while DMARD therapy is instituted 135. Some studies show that using corticosteroids early in rheumatoid arthritis patients improves outcomes and has disease-modifying effects, including radiographic progression 136, 137. Another study did not show long-term benefits from corticosteroid bridge therapy 138. Short courses of corticosteroids can be used for mild flares of rheumatoid arthritis. Intra-articular corticosteroids can be used for single-joint flares 135. About 50% of patients with rheumatoid arthritis require low-dose corticosteroids (prednisone 2.5 to 7.5 mg daily to maintain control of their disease 135. Long-term corticosteroids are associated with numerous toxicities, including weight gain, osteoporosis, and increased risk of infections.
Rheumatoid arthritis medications
The types of medications recommended by your doctor will depend on the severity of your symptoms and how long you’ve had rheumatoid arthritis.
- Non-steroidal anti-inflammatory drugs (NSAIDs) or cyclo-oxygenase-2 selective (COX-2) inhibitors. Nonsteroidal anti-inflammatory drugs (NSAIDs) can relieve pain and reduce inflammation. Over-the-counter NSAIDs include ibuprofen (Advil, Motrin IB) and naproxen sodium (Aleve). Stronger NSAIDs are available by prescription. Side effects may include ringing in your ears, stomach irritation, heart problems, and liver and kidney damage.
- Glucocorticoids. Corticosteroid medications, such as prednisone, reduce inflammation and pain and slow joint damage. Side effects may include thinning of bones, weight gain and diabetes. Doctors often prescribe a corticosteroid to relieve acute symptoms, with the goal of gradually tapering off the medication.
- Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). These drugs can slow the progression of rheumatoid arthritis and save the joints and other tissues from permanent damage. Common DMARDs include methotrexate (Trexall, Otrexup, Rasuvo), leflunomide (Arava), hydroxychloroquine (Plaquenil) and sulfasalazine (Azulfidine). Side effects vary but may include liver damage and severe lung infections.
- Biological DMARDs (bDMARDs) also known as biologic response modifiers, this newer class of DMARDs includes abatacept (Orencia), adalimumab (Humira), anakinra (Kineret), certolizumab (Cimzia), etanercept (Enbrel), golimumab (Simponi), infliximab (Remicade), rituximab (Rituxan), sarilumab (Kevzara) and tocilizumab (Actemra). Biologic DMARDs are usually most effective when paired with a conventional DMARD, such as methotrexate. This type of drug also increases the risk of infections.
- Targeted synthetic DMARDs (tsDMARDs) also known as JAK inhibitors. Baricitinib (Olumiant), tofacitinib (Xeljanz) and upadacitinib (Rinvoq) may be used if conventional DMARDs and biologics haven’t been effective. Higher doses of tofacitinib can increase the risk of blood clots in the lungs, serious heart-related events and cancer.
Sometimes DMARDs and corticosteroids are taken together to treat rheumatoid arthritis.
What are DMARDs?
Disease-modifying antirheumatic drugs (DMARDs) are a family of medicines that stop your body’s immune system from attacking and destroying joints 30. If you have severe rheumatoid arthritis symptoms or are not getting enough relief from pain relievers or corticosteroids, your doctor may suggest a disease-modifying antirheumatic drug (DMARD). Disease-modifying antirheumatic drugs (DMARDs) may be taken with each other or together with pain relievers and corticosteroids.
Because disease-modifying antirheumatic drugs (DMARDs) suppress your immune system to control inflammation, all of them will increase your risk of infection. If you have signs of infection – chills, fever, sore throat or painful urination, for example – report them to your doctor immediately. Disease-modifying antirheumatic drugs (DMARDs) also make receiving live vaccines dangerous. Use extra care to avoid infection and discuss vaccines with your doctor.
Several DMARDs can damage the fetus but others are safe during pregnancy. Discuss your family plans with your doctor before taking any medication.
Each DMARD has different specific benefits and risks. Be sure to talk over any concerns with your doctor.
There are 3 kinds of disease-modifying antirheumatic drugs (DMARDs):
- Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs)
- Biological DMARDs (bDMARDs) also known as biologic response modifiers
- Targeted synthetic DMARDs (tsDMARDs) also known as JAK inhibitors
Nonbiologic Disease-modifying antirheumatic drugs (DMARDs)
Like most medicines, nonbiologic DMARDs are produced from chemicals. They are usually taken daily or weekly as pills, but some can also be given as shots. Nonbiologic DMARDs include:
- Hydroxychloroquine (Plaquenil®)
- Leflunomide (Arava®)
- Methotrexate (Folex®, Rheumatrex®, Trexall®)
- Sulfasalazine (Azulfidine EN-Tabs®, Sulfazine®)
What does research say about nonbiologic DMARDs?
How well they work to treat rheumatoid arthritis:
- Methotrexate (Folex®, Rheumatrex®, Trexall®) and sulfasalazine (Azulfidine EN-Tabs®, Sulfazine®) work about the same to reduce symptoms, reduce the number of joints affected, improve the ability to do daily activities, and slow down or stop joint damage.
- Leflunomide (Arava®) appears to work about as well as methotrexate, but there is not enough research to know this for certain.
Side effects:
- All nonbiologic DMARDs appear to cause about the same amount of side effects, but there is not enough research to know this for certain.
Biologic Disease-modifying antirheumatic drugs (DMARDs)
Biologic agents also known as biologic response modifiers, this newer class of DMARDs includes abatacept (Orencia), adalimumab (Humira), anakinra (Kineret), certolizumab (Cimzia), etanercept (Enbrel), golimumab (Simponi), infliximab (Remicade), rituximab (Rituxan), tocilizumab (Actemra) and tofacitinib (Xeljanz).
These drugs can target parts of the immune system that trigger inflammation that causes joint and tissue damage. These types of drugs also increase the risk of infections.
Biologic DMARDs are usually most effective when paired with a nonbiologic DMARD, such as methotrexate.
Biologic DMARDs are proteins similar to those made in your body, but these proteins are created in laboratories. Biologic DMARDs must be given as shots or through an IV (intravenous) tube into a vein in your arm. Biologic DMARDs include:
- Abatacept (Orencia®)
- Adalimumab (Humira®)
- Anakinra (Kineret®)
- Certolizumab pegol (Cimzia®)
- Etanercept (Enbrel®)
- Golimumab (Simponi®)
- Infliximab (Remicade®)
- Rituximab (Rituxan®)
- Tocilizumab (Actemra®)
Some biologic DMARDs such as infliximab (Remicade®), rituximab (Rituxan®), and tocilizumab (Actemra®) must be given through an IV tube at a doctor’s office or clinic. This could take up to 2 hours. Other biologic DMARDs come in injection pens that you can use at home.
Most biologic DMARDs are given once a month, once every other week, or once a week. Your doctor may change your schedule depending on how well you are doing.
Side effects vary but may include liver damage, bone marrow suppression and severe lung infections.
What does research say about biologic DMARDs?
How well they work to treat rheumatoid arthritis:
- Biologic DMARDS work to decrease or completely stop symptoms, improve the ability to do daily activities, and slow down or stop joint damage.
Side effects:
- Taking a biologic DMARD increases the risk of developing serious infections.
- Taking biologic DMARDs for long periods of time does not increase the risk of having serious side effects.
What does research say about how nonbiologic and biologic DMARDs compare to each other ?
How well they work to treat rheumatoid arthritis:
- The biologic DMARDs adalimumab (Humira®) and etanercept (Enbrel®) help decrease symptoms about the same as the nonbiologic DMARD methotrexate.
Side effects:
- There is not enough research to know if certain side effects happen more often with nonbiologic or biologic DMARDs.
Table 5. Possible Side Effects of Disease-modifying antirheumatic drugs (DMARDs)
| Nonbiologic DMARDs | Biologic DMARDs |
|---|---|
| Upset stomach Nausea Diarrhea Hair loss Mouth sores Rash or serious skin reactions Liver, kidney, or lung problems | Redness, swelling, itching, bruising, or pain in the area where the shot was given Sinus infection (sore throat, runny nose, hoarseness) Headache Nausea Diarrhea |
| Possible Serious Side Effects | |
| In rare cases, the nonbiologic DMARD methotrexate and some biologic DMARDs (including adalimumab [Humira®], etanercept [Enbrel®], golimumab [Simponi®], and infliximab [Remicade®]) have been associated with: Serious infections such as tuberculosis (called “TB”), fungal infections such as yeast, pneumonia, or food-borne illnesses such as listeria Cancer, usually lymphoma (cancer in the lymph glands, which are part of the immune system) The risk of serious infections or cancer is increased by taking two or more biologic DMARDs together or by taking a biologic DMARD with a nonbiologic DMARD. The exact risk to people with RA who are taking a DMARD is not known. Rituximab (Rituxan®) can cause a severe reaction the first time you take it. It could also cause a life-threatening rash. | |
What are corticosteroids?
Corticosteroids are a kind of medicine that works like a certain type of hormone in your body. Corticosteroids can help reduce swelling and stop the body’s immune system from attacking healthy joints. Corticosteroids are taken as pills, liquids, or shots and include:
- Methylprednisolone (Depo Medrol®, Medrol®, Solu-Medrol®)
- Prednisolone (Delta-Cortef ®, Econopred®, Orapred®, Pediapred®, Prelone®)
- Prednisone (Liquidpred®, Deltasone®, Sterapred®)
What are the possible side effects of corticosteroids?
Possible side effects of corticosteroids listed by the FDA include:
- Swelling in the lower legs
- Weight gain
- Increased blood pressure
- Mood swings
- Increased pressure in the eyes
Possible side effects from taking corticosteroids for longer than a few days or weeks include:
- High blood sugar, which can cause or worsen diabetes
- Increased risk of infections
- Loss of calcium from bones, which can make it easier to break a bone
- Irregular menstrual periods
- Thin skin that bruises easily
- Longer time for wounds to heal.
What does research say about combining medicines?
How well they work to treat rheumatoid arthritis:
- If treatment with one DMARD does not relieve symptoms, taking a biologic DMARD together with the nonbiologic DMARD methotrexate works better than taking only one to improve the ability to do daily activities and slow down or stop joint damage.
- Adding a corticosteroid to treatment with a nonbiologic DMARD improves the ability to do daily activities more than taking a nonbiologic DMARD alone.
- Adding a corticosteroid may also slow down or stop joint damage more than taking a nonbiologic DMARD alone, but there is not enough research to know this for certain.
- For people who have had rheumatoid arthritis for less than 3 years, taking two or three nonbiologic DMARDs plus a corticosteroid works better than taking only one nonbiologic DMARD.
- For people who have had rheumatoid arthritis for less than 3 years, taking the nonbiologic DMARD methotrexate together with a biologic DMARD decreases or completely stops symptoms and slows down or stops joint damage in people whose rheumatoid arthritis was quickly getting worse.
- For people who have had rheumatoid arthritis for a long time without any improvement, taking three nonbiologic DMARDs together reduces symptoms and the number of joints affected more than taking one or two nonbiologic DMARDs.
Side effects:
- Taking a nonbiologic DMARD with a biologic DMARD does not cause more side effects than taking the biologic DMARD alone.
- Taking two or more biologic DMARDs together can cause more serious side effects than taking only one.
- Taking a corticosteroid together with a nonbiologic DMARD does not make treatment more difficult to tolerate.
- In people who have had RA for less than 3 years, taking two or three nonbiologic DMARDs plus a corticosteroid does not make treatment more difficult to tolerate than taking only a nonbiologic DMARD.
What should you think about when deciding which medicine is right for you?
More research is needed to know which rheumatoid arthritis medicines might work best for different people. There are several things to consider when choosing a medicine to treat your rheumatoid arthritis:
- The trade-offs between the possible benefits and side effects for each medicine
- Which medicine best fits your lifestyle, what is important to you (your values), and your preferences
- You may want to think about how comfortable you are with taking pills, getting shots, or taking the medicine through an IV tube. You may also want to consider how often you are able to go to the doctor’s office or clinic and how much time you are able to spend there.
- The cost of each medicine
What are the trade-offs ?
Only you and your doctor can decide whether taking a DMARD for your rheumatoid arthritis is worth the risk of possible side effects. You and your doctor should discuss:
- The amount of pain or joint damage you have and whether treatment with a DMARD can help
- The risk of serious side effects from DMARDs
- Signs to look for to help you notice serious side effects so they can be treated or so your medicine can be changed
- Whether adding a corticosteroid to your treatment with DMARDs might help
- Other options besides DMARDs that might help your rheumatoid arthritis
What are the costs of rheumatoid arthritis medications?
The costs to you for nonbiologic and biologic DMARDs and corticosteroids depend on:
- Your health insurance plan
- The amount (dose) you need
- Whether you take the medicine as a pill, as a shot, or through an IV tube
- Whether a generic form of the medicine is available
- Whether the company that makes the medicine offers financial help to lower the cost
Corticosteroids can be taken for short periods of time (30 days) or longer, depending on your specific needs. The cost for corticosteroids is around $3 to $15 a month. The cost to you depends on how much of the medicine you need and how long you will need to take it.
Table 6. Wholesale Prices: Nonbiologic and Biologic DMARDs
| Drug Name | Brand Name | Price per Month* | Form | Dose |
|---|---|---|---|---|
| Nonbiologic DMARDs | ||||
| Hydroxychloroquine | Generic | $35–$75 | Tablet | 200–400 mg daily |
| Plaquenil® | $110–$215 | |||
| Leflunomide | Generic | $490.00 | Tablet | 20 mg daily |
| Arava® | $910.00 | |||
| Methotrexate | Generic | $45–$90 | Tablet | 7.5–15 mg weekly |
| Folex®, Rheumatrex®, Trexall® | $125–$140 | |||
| Sulfasalazine | Generic | $40.00 | Tablet | 2,000 mg daily |
| Azulfidine EN-Tabs® | $120.00 | |||
| Sulfazine® | $30.00 | |||
| Biologic DMARDs(Generic versions of these medicines are not available.) | ||||
| Abatacept | Orencia® | $1,430–$2,860 | IV | 500–1,000 mg monthly |
| $2,530.00 | Shot | 125 mg weekly | ||
| Adalimumab | Humira® | $2,450.00 | Shot | 40 mg twice a month |
| Anakinra | Kineret® | $2,760.00 | Shot | 100 mg daily |
| Certolizumab pegol | Cimzia® | $2,360.00 | Shot | 400 mg monthly |
| Etanercept | Enbrel® | $2,475.00 | Shot | 50 mg weekly |
| Golimumab | Simponi® | $2,650.00 | Shot | 50 mg monthly |
| Infliximab | Remicade® | $3,725–$9,300 | IV | 200–500 mg twice a month (depending on your weight) |
| Rituximab | Rituxan® | $15,180.00 | IV | 1,000 mg twice a month |
| Tocilizumab | Actemra® | $1,660–$3,320 | IV | 400–800 mg monthly (depending on your weight) |
Ask your doctor
- Do you think a DMARD could help my rheumatoid arthritis ?
- What serious side effects should I look for?
- Would adding a corticosteroid to a DMARD help my rheumatoid arthritis ?
- How long will it take until I start to feel better?
- Is there a less expensive medicine that I could take?
- What else can I do to help my rheumatoid arthritis ?
- Are there specific lifestyle changes you can suggest that might help?
- Physical and Occupational Therapy
Your doctor may send you to a physical or occupational therapist who can teach you exercises to help keep your joints flexible. The therapist may also suggest new ways to do daily tasks, which will be easier on your joints. For example, if your fingers are sore, you may want to pick up an object using your forearms.
Physical therapy and sports can help improve or maintain mobility, strength and joint function. Examples of suitable types of sports include cycling, brisk walking, dancing, doing exercises (e.g. gentle strengthening exercises), swimming and aqua aerobics.
The main aim of occupational therapy is to maintain your mobility and hand strength, and to learn how to get by with rheumatoid arthritis in daily life.
Assistive devices can make it easier to avoid stressing your painful joints. For instance, a kitchen knife equipped with a saw handle helps protect your finger and wrist joints. Certain tools, such as buttonhooks, can make it easier to get dressed. Catalogs and medical supply stores are good places to look for ideas.
In advanced arthritis, various aids can compensate for many physical limitations and help you to carry out everyday activities. These include orthopedic shoe inserts, grabbing aids and specially designed cutlery.
Psychological treatment
Psychological treatments can help relieve pain and minimize the impact it has on everyday life. They are also supposed to help relieve disease-related anxiety and depression that some people develop.
Surgery
If medications fail to prevent or slow joint damage, you and your doctor may consider surgery to repair damaged joints. Surgery may help restore your ability to use your joint. It can also reduce pain and correct deformities.
Rheumatoid arthritis surgery may involve one or more of the following procedures:
- Synovectomy. Surgery to remove the inflamed synovium (lining of the joint). Synovectomy can be performed on knees, elbows, wrists, fingers and hips.
- Tendon repair. Inflammation and joint damage may cause tendons around your joint to loosen or rupture. Your surgeon may be able to repair the tendons around your joint.
- Joint fusion. Surgically fusing a joint may be recommended to stabilize or realign a joint and for pain relief when a joint replacement isn’t an option.
- Total joint replacement. During joint replacement surgery, your surgeon removes the damaged parts of your joint and inserts a prosthesis made of metal and plastic.
Surgery carries a risk of bleeding, infection and pain. Discuss the benefits and risks with your doctor.
Lifestyle and home remedies for rheumatoid arthritis
You can take steps to care for your body if you have rheumatoid arthritis. These self-care measures, when used along with your rheumatoid arthritis medications, can help you manage your signs and symptoms:
- Exercise regularly. Gentle exercise can help strengthen the muscles around your joints, and it can help fight fatigue you might feel. Check with your doctor before you start exercising. If you’re just getting started, begin by taking a walk. Try swimming or gentle water aerobics. Avoid exercising tender, injured or severely inflamed joints.
- Apply heat or cold. Heat can help ease your pain and relax tense, painful muscles. Cold may dull the sensation of pain. Cold also has a numbing effect and decreases muscle spasms.
- Relax. Find ways to cope with pain by reducing stress in your life. Techniques such as guided imagery, distraction and muscle relaxation can all be used to control pain.
Alternative medicine
Some common complementary and alternative treatments that have shown promise for rheumatoid arthritis include:
- Fish oil. Fish oils contain the omega−3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) that are known to reduce inflammation in the body and improve hypertriglyceridemia. Some studies have found that fish oil supplements may ease rheumatoid arthritis pain and stiffness. Side effects can include nausea, belching and a fishy taste in the mouth. Fish oil can get in the way of medicines you take. So check with your rheumatologist before trying it.
- Plant oils. The seeds of evening primrose, borage and black currant contain a type of fatty acid that may help with rheumatoid arthritis pain and morning stiffness. Side effects may include nausea, diarrhea and gas. Some plant oils can cause liver damage or interfere with medications, so check with your doctor first.
- Tai chi. This movement therapy involves gentle exercises and stretches combined with deep breathing. Many people use tai chi to relieve stress in their lives. Small studies have found that tai chi may reduce rheumatoid arthritis pain. When led by a knowledgeable instructor, tai chi is safe. But don’t do any moves that cause pain.
Coping and support
The pain and disability associated with rheumatoid arthritis can affect a person’s work and family life. Depression and anxiety are common, as are feelings of helplessness and low self-esteem.
The degree to which rheumatoid arthritis affects your daily activities depends in part on how well you cope with the disease. Talk to your doctor or nurse about strategies for coping. With time you’ll learn what strategies work best for you. In the meantime, try to:
- Take control. With your doctor, make a plan for managing your arthritis. This will help you feel in charge of your disease.
- Know your limits. Rest when you’re tired. Rheumatoid arthritis can make you prone to fatigue and muscle weakness. A rest or short nap that doesn’t interfere with nighttime sleep may help.
- Connect with others. Keep your family aware of how you’re feeling. They may be worried about you but might not feel comfortable asking about your pain.
- Find a family member or friend you can talk to when you’re feeling especially overwhelmed. Also connect with other people who have rheumatoid arthritis — whether through a support group in your community or online.
- Take time for yourself. It’s easy to get busy and not take time for yourself. Find time for what you like, whether it’s time to write in a journal, go for a walk or listen to music. Use this time to relieve stress and reflect on your feelings.
Rheumatoid arthritis prognosis
Rheumatoid arthritis has no cure and is a progressive disease 139. All individuals will experience multiple exacerbations (flare-ups) and without treatment tend to have poor outcomes with increased disability and mortality 140. Early treatment (within six months of symptom onset) has shown improved functional capacity and decreased disease activity as measured by swollen joint count and tender joint count. However, the mortality rate was similar in patients receiving early treatment and late treatment (after six months of symptoms), and both were significantly improved from no treatment 140. Approximately 40% of patients with rheumatoid arthritis will have a functional disability affecting their ability to work and complete activities of daily living within ten years of the diagnosis 141. Patients with rheumatoid arthritis also develop other chronic medical conditions/complications which drastically affect their outcomes. The most notable of this correlation is the relationship between rheumatoid arthritis and atherosclerotic cardiovascular disease leading to accelerated coronary artery disease 142. rheumatoid arthritis increases the risk of cardiovascular disease, lung diseases, and malignancies, which in turn increases the risk of premature mortality in these patients 143.
Living with rheumatoid arthritis
Rheumatoid arthritis can be life changing. You may need long-term treatment to control the symptoms and joint damage. Depending on how much pain and stiffness you feel and how much joint damage you have, simple daily tasks may become difficult or take longer to do. You may need to adapt the way you do everyday tasks, or make changes to your lifestyle, to help you manage your condition.
Here are some things you can do to help.
Self care
Self care is an important part of daily life. It involves taking responsibility for your own health and wellbeing with support from people involved in your care.
It includes what you do every day to stay fit and maintain good physical and mental health, prevent illness and accidents, and manage minor ailments and long-term conditions.
People who have a long-term condition, such as rheumatoid arthritis, can benefit enormously from being supported to care for themselves. They can live longer, have a better quality of life, and be more active and independent.
Healthy eating and exercise
Regular exercise and a healthy diet are recommended for everyone, not just people with rheumatoid arthritis. Regular exercise and a healthy diet can help reduce your risk of many conditions, including heart disease and many forms of cancer.
Exercising regularly can help relieve stress, help keep your joints mobile, and strengthen the muscles supporting your joints.
Exercise can also help you lose weight if you’re overweight, which can put extra strain on your joints. But it’s important to find a balance between rest and exercise. Rest will make inflamed joints feel more comfortable, but without movement your joints will stiffen and your muscles will become weaker.
Find the best activities and the right balance for you. It’s usually best to increase the amount of exercise you do gradually.
If a particular activity causes your joints to become warm and swollen, or it causes severe pain, then stop and rest. If it does not cause problems, then it is usually fine to continue.
If a particular activity always causes a flare-up, it’s best to avoid it and find an alternative.
High-impact activities, such as running or contact sports like rugby and football, are more likely to cause problems.
Try low-impact activities that put less strain on your joints, such as swimming, cycling, walking and aqua aerobics.
If you need more guidance, a physiotherapist is a good person to advise you on suitable types of exercise.
Preventing falls
Rheumatoid arthritis can make your joints stiffer and less flexible, increasing your risk of falling. Some examples of how to reduce your risk of falling at home include:
- good lighting — ensure your home and outdoor areas are well lit
- removing trip hazards such as loose wires or rugs
- placing rubber mats in the bathroom to prevent slipping
An occupational therapist can give you personalised advice on how to avoid falls and keep safe at home.
Regular reviews
As rheumatoid arthritis is a long-term condition, you’ll be in contact with your healthcare team regularly so they can check if your condition is being well controlled and if your treatment is right for you.
You may have a disease activity score (DAS) measured regularly, which can help your healthcare team to decide on the best treatment.
The more your healthcare team know, the more they can help you, so discuss any concerns you have with them.
Take your medicine
It’s important to take your medicine as instructed, even if you start to feel better, as medicine can help prevent flare-ups and reduce the risk of further problems, such as joint damage.
If you have any questions or concerns about the medicine you’re taking or side effects, talk to your healthcare team.
It may also be useful to read the information leaflet that comes with the medicine, as this tells you about possible interactions with other medicines or supplements.
Check with your healthcare team before taking any over-the-counter remedies, such as painkillers or nutritional supplements. These may interfere with your medicine.
Reducing your medicine
Some people find their symptoms go away or get much better. If your symptoms stay better for at least 1 year without needing to take steroids, your treatment can be reviewed.
Your doctor may suggest slowly reducing your dose of medicine, then seeing if you can stop taking it.
You’ll be monitored during this time. If your symptoms come back, you’ll need to start taking disease-modifying anti-rheumatic drugs (DMARDs) straight away.
Talk to others
It can be hard to deal with the unpredictable nature of rheumatoid arthritis. Many people find it helpful to talk to others in a similar position. There may be a local support group where you can meet others with the same condition as you.
Some days the pain and stiffness will be much worse than others, and there may be no way of knowing when a flare-up will happen.
The difficult nature of rheumatoid arthritis can mean some people develop depression or feelings of stress and anxiety. Sometimes these feelings can be related to poorly controlled pain or fatigue.
Living with a long-term condition makes you more likely to have emotions such as frustration, fear, anger and resentment.
Speak to your healthcare team if you’re struggling to deal with your feelings about your condition. They may be able to offer medicine or suggest mental health services that may help.
Starting and raising a family
If you’re taking medicines for rheumatoid arthritis, let your healthcare team know if you want to start a family or you’re worried about becoming pregnant while taking medicine.
Some medicines, such as methotrexate, leflunomide and biological treatments should not be taken by men or women while they’re trying for a baby. Your heathcare team will work with you to try to keep your rheumatoid arthritis under control while you’re trying to get pregnant.
Babies and young children are physically and mentally demanding for any parent, but particularly if you have rheumatoid arthritis.
If you’re struggling to cope, it may help to talk to other people in the same situation as you.
You may also be able to get extra support from your health visitor or occupational therapist to help you manage your young family.
Sex and relationships
Pain, discomfort and changes in the way you feel can affect your sex life.
Your self-esteem or thoughts about how you look may affect your confidence.
Although many people find it difficult to talk about such private issues, there are resources that may help you.
Talking to your partner or doctor about the impact of rheumatoid arthritis on your sexuality and sexual relationships may help.
- Vlad AL, Popazu C, Lescai AM, Voinescu DC, Baltă AAȘ. Where Do We Stand in the Management of Rheumatoid Arthritis Ahead of EULAR/ACR 2025? Clin Pract. 2025 May 28;15(6):103. doi: 10.3390/clinpract15060103[↩]
- Masoumi M, Solaymani M, Abbasifard M, Houshmandfar S, Iravani P, Saeedi-Boroujeni A, Karami J. The genetic puzzle of rheumatoid arthritis: Causes, progression, and treatment. Biochem Biophys Rep. 2025 Jul 21;43:102148. doi: 10.1016/j.bbrep.2025.102148[↩][↩]
- Poznyak A.V., Kirichenko T.V., Beloyartsev D.F., Churov A.V., Kovyanova T.I., Starodubtseva I.A., Sukhorukov V.N., Antonov S.A., Orekhov A.N. Rheumatoid Arthritis: What Inflammation Do We Face? J. Mol. Pathol. 2024;5:454–465. doi: 10.3390/jmp5040030[↩]
- Di Matteo A, Bathon JM, Emery P. Rheumatoid arthritis. Lancet. (2023) 402:2019–33. doi: 10.1016/S0140-6736(23)01525-8[↩]
- Yang P, Song Y, Li M. Biological mechanisms of pulmonary inflammation and its association with seropositive rheumatoid arthritis. Front Immunol. 2025 May 23;16:1530753. doi: 10.3389/fimmu.2025.1530753[↩]
- Rheumatoid Arthritis. https://www.niams.nih.gov/health-topics/rheumatoid-arthritis[↩][↩]
- Lin Y.-J., Anzaghe M., Schülke S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells. 2020;9:880. doi: 10.3390/cells9040880[↩]
- Sundas A., Contreras I., Mujahid O., Beneyto A., Vehi J. The Effects of Environmental Factors on General Human Health: A Scoping Review. Healthcare. 2024;12:2123. doi: 10.3390/healthcare12212123[↩]
- Bax M., van Heemst J., Huizinga T.W., Toes R.E. Genetics of rheumatoid arthritis: what have we learned? Immunogenetics. 2011;63:459–466. doi: 10.1007/s00251-011-0528-6[↩][↩][↩]
- Weyand C. New insights into the pathogenesis of rheumatoid arthritis. Rheumatology. 2000;39(suppl_1):3–8. doi: 10.1093/oxfordjournals.rheumatology.a031491[↩][↩]
- Mena-Vazquez N, Perez AL, Manrique-Arija S, Romero BC, Gomez CC, Urena GI, et al. Analysis of clinical-analytical characteristics in patients with rheumatoid arthritis and interstitial lung disease: case-control study. Reumatol Clin (Engl Ed). (2021) 17:197–202. doi: 10.1016/j.reuma.2019.06.001[↩][↩]
- Liu YCLF. High levels of antibodies to citrullinated alpha-enolase peptide-1 (CEP-1) identify erosions and interstitial lung disease (ILD) in a Chinese rheumatoid arthritis cohort. Clin Immunol: Off J Clin Immunol Soc. (2019) 200:10–5. doi: 10.1016/j.clim.2019.01.001[↩][↩]
- Giles JT, Danoff SK, Sokolove J, Wagner CA, Winchester R, Pappas DA, et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis. (2014) 73:1487–94. doi: 10.1136/annrheumdis-2012-203160[↩][↩]
- Gravallese E.M., Firestein G.S. Rheumatoid arthritis—common origins, divergent mechanisms. N. Engl. J. Med. 2023;388(6):529–542. doi: 10.1056/NEJMra2103726[↩][↩]
- Masoumi M., Bashiri H., Khorramdelazad H., Barzaman K., Hashemi N., Sereshki H.A., et al. Destructive roles of fibroblast-like synoviocytes in chronic inflammation and joint damage in rheumatoid arthritis. Inflammation. 2021;44:466–479. doi: 10.1007/s10753-020-01371-1[↩]
- Masoumi M., Mehrabzadeh M., Mahmoudzehi S., Mousavi M.J., Jamalzehi S., Sahebkar A., Karami J. Role of glucose metabolism in aggressive phenotype of fibroblast-like synoviocytes: latest evidence and therapeutic approaches in rheumatoid arthritis. Int. Immunopharmacol. 2020;89 doi: 10.1016/j.intimp.2020.107064[↩]
- Okada Y., Eyre S., Suzuki A., Kochi Y., Yamamoto K. Genetics of rheumatoid arthritis: 2018 status. Ann. Rheum. Dis. 2019;78(4):446–453. doi: 10.1136/annrheumdis-2018-213678[↩]
- Zhao J., Guo S., Schrodi S.J., He D. Cuproptosis and cuproptosis–related genes in rheumatoid arthritis: implication, prospects, and perspectives. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.930278[↩]
- Scherer H.U., Häupl T., Burmester G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020;110 doi: 10.1016/j.jaut.2019.102400[↩]
- Silman A.J., Pearson J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. Ther. 2002;4:1–8. doi: 10.1186/ar578[↩]
- Riaz M, Rasool G, Yousaf R, Fatima H, Munir N, Ejaz H. Anti-Rheumatic potential of biological DMARDS and protagonistic role of bio-markers in early detection and management of rheumatoid arthritis. Innate Immun. 2025 Jan-Dec;31:17534259251324820. doi: 10.1177/17534259251324820[↩][↩][↩][↩][↩][↩]
- Ogbodo J.O., Arazu A.V., Iguh T.C., Onwodi N.J., Ezike T.C. Volatile organic compounds: A proinflammatory activator in autoimmune diseases. Front. Immunol. 2022;13:928379. doi: 10.3389/fimmu.2022.928379[↩]
- Celen H., Dens A.C., Ronsmans S., Michiels S., De Langhe E. Airborne pollutants as potential triggers of systemic autoimmune rheumatic diseases: A narrative review. Acta Clin. Belg. 2022;77:874–882. doi: 10.1080/17843286.2021.1992582[↩]
- Carmona L., Aurrecoechea E., García de Yébenes M.J. Tailoring Rheumatoid Arthritis Treatment through a Sex and Gender Lens. J. Clin. Med. 2024;13:55. doi: 10.3390/jcm13010055[↩][↩]
- InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. Overview: Rheumatoid arthritis. [Updated 2024 Jan 11]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK384455[↩][↩][↩][↩]
- Rheumatoid arthritis. https://www.mayoclinic.org/diseases-conditions/rheumatoid-arthritis/symptoms-causes/syc-20353648[↩]
- Rustamovich T.D., Alisherovna K.M., Djamshedovna K.D., Nizamitdinovich K.S. Features of the psychoemotional status of patients with rheumatoid arthritis. Miasto Przyszłości. 2023;32:23–30.[↩]
- Bullock J, Rizvi SAA, Saleh AM, Ahmed SS, Do DP, Ansari RA, Ahmed J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med Princ Pract. 2018;27(6):501-507. doi: 10.1159/000493390[↩]
- Brown P, Pratt AG, Hyrich KL. Therapeutic advances in rheumatoid arthritis. BMJ. 2024 Jan 17;384:e070856. https://www.bmj.com/content/384/bmj-2022-070856.long[↩][↩][↩][↩][↩]
- Zanwar A, Ahmed S, Misra DP, et al. Recent evidence comparing combination of conventional synthetic disease-modifying antirheumatic drugs with biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Indian J Rheumatol 2017; 12: 104–109.[↩][↩]
- Khan S., Mohan K., Muzammil S., Alam M.A., Khayyam K.U. Current Prospects in Rheumatoid Arthritis: Pathophysiology, Genetics, and Treatments. Recent Adv. Anti-Infect. Drug Discov. 2024;19:36–55. doi: 10.2174/2772434418666230406083149[↩]
- Kostara M., Chondrou V., Sgourou A., Douros K., Tsabouri S. HLA Polymorphisms and Food Allergy Predisposition. J. Pediatr. Genet. 2020;9:77–86. doi: 10.1055/s-0040-1708521[↩]
- Jang S., Kwon E.-J., Lee J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022;23:905. doi: 10.3390/ijms23020905[↩]
- Arleevskaya M., Takha E., Petrov S., Kazarian G., Renaudineau Y., Brooks W., Larionova R., Korovina M., Valeeva A., Shuralev E., et al. Interplay of Environmental, Individual and Genetic Factors in Rheumatoid Arthritis Provocation. Int. J. Mol. Sci. 2022;23:8140. doi: 10.3390/ijms23158140[↩]
- Romão V.C., Fonseca J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021;8:689698. doi: 10.3389/fmed.2021.689698[↩]
- Serhal L., Lwin M.N., Holroyd C., Edwards C.J. Rheumatoid Arthritis in the Elderly: Characteristics and Treatment Considerations. Autoimmun. Rev. 2020;19:102528. doi: 10.1016/j.autrev.2020.102528[↩]
- Azam A.T., Odeyinka O., Alhashimi R., Thoota S., Ashok T., Palyam V., Sange I. Rheumatoid Arthritis and Associated Lung Diseases: A Comprehensive Review. Cureus. 2022;14:e21914. doi: 10.7759/cureus.22367[↩]
- Hysa E., Cutolo C.A., Gotelli E., Paolino S., Cimmino M.A., Pacini G., Cutolo M. Ocular Microvascular Damage in Autoimmune Rheumatic Diseases: The Pathophysiological Role of the Immune System. Autoimmun. Rev. 2021;20:102796. doi: 10.1016/j.autrev.2021.102796[↩]
- Rothschild B. Subcutaneous Nodules as Manifestations of Systemic Disease. Rheumato. 2024;4:75–87. doi: 10.3390/rheumato4020007[↩]
- Cornec D, Varache S, Morvan J, Devauchelle-Pensec V, Berthelot JM, Le Henaff-Bourhis C, Hoang S, Martin A, Chalès G, Jousse-Joulin S, Saraux A. Comparison of ACR 1987 and ACR/EULAR 2010 criteria for predicting a 10-year diagnosis of rheumatoid arthritis. Joint Bone Spine. 2012 Dec;79(6):581-5. doi: 10.1016/j.jbspin.2012.01.015[↩][↩]
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010 Sep;62(9):2569-81. https://onlinelibrary.wiley.com/doi/epdf/10.1002/art.27584[↩][↩]
- Wu F, Gao J, Kang J, Wang X, Niu Q, Liu J, et al. B cells in rheumatoid arthritis:Pathogenic mechanisms and treatment prospects. Front Immunol. (2021) 12:750753. doi: 10.3389/fimmu.2021.750753[↩]
- Malmstrom V, Catrina AI, Klareskog L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat Rev Immunol. (2017) 17:60–75. doi: 10.1038/nri.2016.124[↩]
- Khudhair HAA. A study of the roles of some immunological biomarkers in the diagnosis of rheumatoid arthritis. J Med Life. 2023 Aug;16(8):1194-1200. doi: 10.25122/jml-2023-0158[↩][↩][↩][↩]
- Abedian Z, Sagafi M, Kenari SA, Abedian F. Anti-perinuclear factor as diagnostic marker in rheumatoid arthritis. J Clin Diagn Res. 2015;9(9):OC13–OC16. doi: 10.7860/JCDR/2015/14962.6456[↩]
- Kurki P, Aho K, Palosuo T, Heliövaara M. Immunopathology of rheumatoid arthritis. Antikeratin antibodies precede the clinical disease. Arthritis Rheum. 1992 Aug;35(8):914-7. doi: 10.1002/art.1780350810[↩]
- Vencovský J, Machácek S, Sedová L, Kafková J, Gatterová J, Pesáková V, Růzicková S. Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis. 2003 May;62(5):427-30. https://pmc.ncbi.nlm.nih.gov/articles/instance/1754544/pdf/v062p00427.pdf[↩]
- Saraux A, Berthelot JM, Devauchelle V, Bendaoud B, Chalès G, Le Henaff C, Thorel JB, Hoang S, Jousse S, Baron D, Le Goff P, Youinou P. Value of antibodies to citrulline-containing peptides for diagnosing early rheumatoid arthritis. J Rheumatol. 2003 Dec;30(12):2535-9. https://www.jrheum.org/content/jrheum/30/12/2535.full.pdf[↩]
- Vossenaar ER, Després N, Lapointe E, van der Heijden A, Lora M, Senshu T, van Venrooij WJ, Ménard HA. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004;6(2):R142-50. doi: 10.1186/ar1149[↩]
- Renger F, Bang H, Feist E, Fredenhagen G, Natusch A, Backhaus M, Burmester GR, Egerer K. Immediate determination of ACPA and rheumatoid factor–a novel point of care test for detection of anti-MCV antibodies and rheumatoid factor using a lateral-flow immunoassay. Arthritis Res Ther. 2010;12(3):R120. doi: 10.1186/ar3057[↩]
- Innala L, Kokkonen H, Eriksson C, Jidell E, Berglin E, Dahlqvst SR. Antibodies against mutated citrullinated vimentin are a better predictor of disease activity at 24 months in early rheumatoid arthritis than antibodies against cyclic citrullinated peptides. J Rheumatol. 2008 Jun;35(6):1002-8. https://www.jrheum.org/content/jrheum/35/6/1002.full.pdf[↩]
- Luime J, Colin E, Hazes J, et al. Does anti-MCV has additional value as serological marker in the diagnostic and prognostic work-up of patients with rheumatoid arthritis? A systematic review. Ann Rheum Dis 2009. 10.1136/ard.2008.103283[↩]
- Wagner E, Skoumal M, Bayer PM, Klaushofer K. Antibody against mutated citrullinated vimentin: a new sensitive marker in the diagnosis of rheumatoid arthritis. Rheumatol Int. 2009 Sep;29(11):1315-21. doi: 10.1007/s00296-009-0854-2[↩]
- Corrigall VM, Bodman-Smith MD, Fife MS, Canas B, Myers LK, Wooley P, Soh C, Staines NA, Pappin DJ, Berlo SE, van Eden W, van Der Zee R, Lanchbury JS, Panayi GS. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J Immunol. 2001 Feb 1;166(3):1492-8. doi: 10.4049/jimmunol.166.3.1492[↩]
- Bodman-Smith MD, Corrigall VM, Berglin E, Cornell HR, Tzioufas AG, Mavragani CP, Chan C, Rantapää-Dahlqvist S, Panayi GS. Antibody response to the human stress protein BiP in rheumatoid arthritis. Rheumatology (Oxford). 2004 Oct;43(10):1283-7. doi: 10.1093/rheumatology/keh312[↩]
- Kortekangas P, Aro HT, Tuominen J, Toivanen A. Synovial fluid leukocytosis in bacterial arthritis vs. reactive arthritis and rheumatoid arthritis in the adult knee. Scand J Rheumatol. 1992;21(6):283-8. doi: 10.3109/03009749209099243[↩]
- Fuchs HA, Kaye JJ, Callahan LF, Nance EP, Pincus T. Evidence of significant radiographic damage in rheumatoid arthritis within the first 2 years of disease. J Rheumatol. 1989 May;16(5):585-91.[↩]
- F. M. McQueen, The use of MRI in early RA, Rheumatology, Volume 47, Issue 11, November 2008, Pages 1597–1599, https://doi.org/10.1093/rheumatology/ken332[↩]
- McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PL, McLean L. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis. 1999 Mar;58(3):156-63. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1752839/pdf/v058p00156.pdf[↩]
- England BR, Tiong BK, Bergman MJ, Curtis JR, Kazi S, Mikuls TR, O’Dell JR, Ranganath VK, Limanni A, Suter LG, Michaud K. 2019 Update of the American College of Rheumatology Recommended Rheumatoid Arthritis Disease Activity Measures. Arthritis Care Res (Hoboken). 2019 Dec;71(12):1540-1555. doi: 10.1002/acr.24042[↩]
- Maeda Y, Matsushita M, Yura A, Teshigawara S, Katayama M, Yoshimura M, et al. OP0191 the fecal microbiota of rheumatoid arthritis patients differs from that of healthy volunteers and is considerably altered by treatment with biologics. Ann Rheum Dis (2013) 72(Suppl 3):A117.10.1136/annrheumdis-2013-eular.396[↩]
- Panush RS, Carter RL, Katz P, Kowsari B, Longley S, Finnie S. Diet therapy for rheumatoid arthritis. Arthritis Rheum (1983) 26(4):462–71.10.1002/art.1780260403 https://www.ncbi.nlm.nih.gov/pubmed/6838671[↩]
- Vitetta L, Coulson S, Schloss J, Beck SL, Allen R, Sali A. Dietary recommendations for patients with rheumatoid arthritis: a review. Nutr Diet Suppl (2012) 4(4):1–15.10.2147/NDS.S6922[↩]
- Kjeldsen-Kragh J, Borchgrevink C, Laerum E, Haugen M, Eek M, Forre O, et al. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet (1991) 338(8772):899–902.10.1016/0140-6736(91)91770-U https://www.ncbi.nlm.nih.gov/pubmed/1681264[↩]
- McDougall J, Bruce B, Spiller G, Westerdahl J, McDougall M. Effects of a very low-fat, vegan diet in subjects with rheumatoid arthritis. J Altern Complement Med (2002) 8(1):71–5.10.1089/107555302753507195 https://www.ncbi.nlm.nih.gov/pubmed/11890437[↩][↩]
- Sköldstam L, Hagfors L, Johansson G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann Rheum Dis (2003) 62(3):208–14.10.1136/ard.62.3.208 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1754463/[↩][↩]
- Khanna S, Jaiswal KS, Gupta B. Managing Rheumatoid Arthritis with Dietary Interventions. Frontiers in Nutrition. 2017;4:52. doi:10.3389/fnut.2017.00052. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5682732/[↩][↩][↩][↩]
- Ramadan G, Al-Kahtani MA, El-Sayed WM. Anti-inflammatory and anti-oxidant properties of Curcuma longa (turmeric) versus Zingiber officinale (ginger) rhizomes in rat adjuvant-induced arthritis. Inflammation (2011) 34(4):291–301.10.1007/s10753-010-9278-0. https://www.ncbi.nlm.nih.gov/pubmed/21120596[↩]
- Balbir-Gurman A, Fuhrman B, Braun-Moscovici Y, Markovits D, Aviram M. Consumption of pomegranate decreases serum oxidative stress and reduces disease activity in patients with active rheumatoid arthritis: a pilot study. Isr Med Assoc J (2011) 13(8):474–9.[↩]
- Shadnoush M, Shaker Hosseini R, Mehrabi Y, Delpisheh A, Alipoor E, Faghfoori Z, et al. Probiotic yogurt affects pro-and anti-inflammatory factors in patients with inflammatory bowel disease. Iran J Pharm Res (2013) 12(4):929–36.[↩]
- van der Meer JW, Netea MG. A salty taste to autoimmunity. N Engl J Med (2013) 368(26):2520–1.10.1056/NEJMcibr1303292[↩]
- Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep (2014) 14(1):404.10.1007/s11882-013-0404-6[↩]
- Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B. Vitamin D in rheumatoid arthritis. Autoimmun Rev (2007) 7(1):59–64.10.1016/j.autrev.2007.07.001[↩]
- Galarraga B, Ho M, Youssef H, Hill A, McMahon H, Hall C, et al. Cod liver oil (n-3 fatty acids) as an non-steroidal anti-inflammatory drug sparing agent in rheumatoid arthritis. Rheumatology (2008) 47(5):665–9.10.1093/rheumatology/ken024[↩]
- Martin RH. The role of nutrition and diet in rheumatoid arthritis. Proc Nutr Soc (1998) 57(02):231–4.10.1079/PNS19980036[↩]
- Neuberger GB, Aaronson LS, Gajewski B, Embretson SE, Cagle PE, Loudon JK, et al. Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness, and disease outcomes of rheumatoid arthritis. Arthritis Care Res (2007) 57(6):943–52.10.1002/art.22903[↩]
- Kjeldsen-Kragh J, Borchgrevink C, Laerum E, Haugen M, Eek M, Forre O, et al. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet (1991) 338(8772):899–902.10.1016/0140-6736(91)91770-U http://www.thelancet.com/journals/lancet/article/PII0140-6736(91)91770-U/abstract[↩]
- Kjeldsen-Kragh J, Mellbye O, Haugen M, Mollnes T, Hammer H, Sioud M, et al. Changes in laboratory variables in rheumatoid arthritis patients during a trial of fasting and one-year vegetarian diet. Scand J Rheumatol (1995) 24(2):85–93.10.3109/03009749509099290 https://www.ncbi.nlm.nih.gov/pubmed/774714 [↩]
- Peltonen R, Kjeldsen-Kragh J, Haugen M, Tuominen J, Toivanen P, Förre Ö, et al. Changes of faecal flora in rheumatoid arthritis during fasting and one-year vegetarian diet. Rheumatology (1994) 33(7):638–43.10.1093/rheumatology/33.7.638 https://www.ncbi.nlm.nih.gov/pubmed/8019792[↩]
- Haugen MA, Kjeldsen-Kragh J, Bjervea KS, Høstmark AT, Førre Ø. Changes in plasma phospholipid fatty acids and their relationship to disease activity in rheumatoid arthritis patients treated with a vegetarian diet. Br J Nutr (1994) 72(4):555–66.10.1079/BJN19940059 https://www.ncbi.nlm.nih.gov/pubmed/7986787[↩]
- Haugen M, Kjeldsen-Kragh J, Skakkebaek N, Landaas S, Sjaastad Ø, Movinkel P, et al. The influence of fast and vegetarian diet on parameters of nutritional status in patients with rheumatoid arthritis. Clin Rheumatol (1993) 12(1):62–9.10.1007/BF02231561 https://www.ncbi.nlm.nih.gov/pubmed/8467614[↩]
- Kjeldsen-Kragh J, Haugen M, Borchgrevink C, Førre Ø. Vegetarian diet for patients with rheumatoid arthritis-status: two years after introduction of the diet. Clin Rheumatol (1994) 13(3):475–82.10.1007/BF02242946 https://www.ncbi.nlm.nih.gov/pubmed/7835013[↩]
- Kjeldsen-Kragh J, Sumar N, Bodman-Smith K, Brostoff J. Changes in glycosylation of IgG during fasting in patients with rheumatoid arthritis. Rheumatology (1996) 35(2):117–9.10.1093/rheumatology/35.2.117 https://www.ncbi.nlm.nih.gov/pubmed/8612020[↩]
- Fraser D, Thoen J, Dioseland O, Forre O, Kjeldsen-Kragh J. Serum levels of interleukin-6 and dehydroepiandrosterone sulphate in response to either fasting or a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol (2000) 18(3):357–62. https://www.ncbi.nlm.nih.gov/pubmed/10895373[↩]
- Michalsen A, Riegert M, Lüdtke R, Bäcker M, Langhorst J, Schwickert M, et al. Mediterranean diet or extended fasting’s influence on changing the intestinal microflora, immunoglobulin A secretion and clinical outcome in patients with rheumatoid arthritis and fibromyalgia: an observational study. BMC Complement Altern Med (2005) 5(1):22.10.1186/1472-6882-5-22 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1352378/[↩]
- Abendroth A, Michalsen A, Luedtke R, Rueffer A, Musial F, Dobos GJ, et al. Changes of intestinal microflora in patients with rheumatoid arthritis during fasting or a Mediterranean diet. Forsch Komplementmed (2010) 17(6):307–13.10.1159/000322313 https://www.ncbi.nlm.nih.gov/pubmed/21196744[↩]
- Hafström I, Ringertz B, Spångberg A, Von Zweigbergk L, Brannemark S, Nylander I, et al. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: the effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology (2001) 40(10):1175–9.10.1093/rheumatology/40.10.1175 https://www.ncbi.nlm.nih.gov/pubmed/11600749[↩]
- Peltonen R, Nenonen M, Helve T, Hänninen O, Toivanen P, Eerola E. Faecal microbial flora and disease activity in rheumatoid arthritis during a vegan diet. Rheumatology (1997) 36(1):64–8.10.1093/rheumatology/36.1.64 https://www.ncbi.nlm.nih.gov/pubmed/9117178[↩]
- Elkan A-C, Sjöberg B, Kolsrud B, Ringertz B, Hafström I, Frostegård J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: a randomized study. Arthritis Res Ther (2008) 10(2):R34.10.1186/ar2388 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2453753/[↩]
- Sköldstam L, Brudin L, Hagfors L, Johansson G. Weight reduction is not a major reason for improvement in rheumatoid arthritis from lacto-vegetarian, vegan or Mediterranean diets. Nutr J (2005) 4(1):15.10.1186/1475-2891-4-15 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1156940/[↩]
- Ågren J, Tvrzicka E, Nenonen M, Helve T, Hänninen O. Divergent changes in serum sterols during a strict uncooked vegan diet in patients with rheumatoid arthritis. Br J Nutr (2001) 85(02):137–9.10.1079/BJN2000234 https://www.ncbi.nlm.nih.gov/pubmed/11242480[↩]
- Hänninen O, Kaartinen K, Rauma A-L, Nenonen M, Törrönen R, Häkkinen S, et al. Antioxidants in vegan diet and rheumatic disorders. Toxicology (2000) 155(1):45–53.10.1016/S0300-483X(00)00276-6 https://www.ncbi.nlm.nih.gov/pubmed/11156742[↩]
- Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A, Sharif S-K, Asghari-Jafarabadi M, Zavvari S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition (2014) 30(4):430–5.10.1016/j.nut.2013.09.007 https://www.ncbi.nlm.nih.gov/pubmed/24355439[↩]
- Vaghef-Mehrabany E, Homayouni-Rad A, Alipour B, Sharif S-K, Vaghef-Mehrabany L, Alipour-Ajiry S. Effects of probiotic supplementation on oxidative stress indices in women with rheumatoid arthritis: a randomized double-blind clinical trial. J Am Coll Nutr (2016) 35(4):291–9.10.1080/07315724.2014.959208 https://www.ncbi.nlm.nih.gov/pubmed/25856220[↩]
- Hatakka K, Martio J, Korpela M, Herranen M, Poussa T, Laasanen T, et al. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis – a pilot study. Scand J Rheumatol (2003) 32(4):211–5.10.1080/03009740310003695 https://www.ncbi.nlm.nih.gov/pubmed/14626627[↩]
- Zamani B, Golkar HR, Farshbaf S, Emadi-Baygi M, Tajabadi-Ebrahimi M, Jafari P, et al. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. Int J Rheum Dis (2016) 19(9):869–79.10.1111/1756-185X.12888 https://www.ncbi.nlm.nih.gov/pubmed/27135916[↩]
- Vaghef-Mehrabany E, Vaghef-Mehrabany L, Asghari-Jafarabadi M, Homayouni-Rad A, Issazadeh K, Alipour B. Effects of probiotic supplementation on lipid profile of women with rheumatoid arthritis: a randomized placebo-controlled clinical trial. Health Promot Perspect (2017) 7(2):95.10.15171/hpp.2017.17 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5350556/[↩]
- Alipour B, Homayouni-Rad A, Vaghef-Mehrabany E, Sharif SK, Vaghef-Mehrabany L, Asghari-Jafarabadi M, et al. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. Int J Rheum Dis (2014) 17(5):519–27.10.1111/1756-185X.12333 https://www.ncbi.nlm.nih.gov/pubmed/24673738[↩]
- de los Angeles Pineda M, Thompson SF, Summers K, de Leon F, Pope J, Reid G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med Sci Monit (2011) 17(6):CR347–54.10.12659/MSM.881808 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3539551/[↩]
- Mandel DR, Eichas K, Holmes J. Bacillus coagulans: a viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complementary and Alternative Medicine. 2010;10:1. doi:10.1186/1472-6882-10-1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2826289/[↩]
- Kavanagh R, Workman E, Nash P, Smith M, Hazleman B, Hunter J. The effects of elemental diet and subsequent food reintroduction on rheumatoid arthritis. Rheumatology (1995) 34(3):270–3.10.1093/rheumatology/34.3.270 https://www.ncbi.nlm.nih.gov/pubmed/7728405[↩]
- Podas T, Nightingale JM, Oldham R, Roy S, Sheehan NJ, Mayberry JF. Is rheumatoid arthritis a disease that starts in the intestine? A pilot study comparing an elemental diet with oral prednisolone. Postgrad Med J (2007) 83(976):128–31.10.1136/pgmj.2006.050245 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2805936/[↩]
- Holst-Jensen SE, Pfeiffer-Jensen M, Monsrud M, Tarp U, Buus A, Hessov I, et al. Treatment of rheumatoid arthritis with a peptide diet: a randomized, controlled trial. Scand J Rheumatol (1998) 27(5):329–36.10.1080/03009749850154339 https://www.ncbi.nlm.nih.gov/pubmed/9808394[↩]
- Van de Laar M, Van der Korst J. Food intolerance in rheumatoid arthritis. I. A double blind, controlled trial of the clinical effects of elimination of milk allergens and AZO dyes. Ann Rheum Dis (1992) 51(3):298–302.10.1136/ard.51.3.298 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1004647/[↩]
- Karatay S, Erdem T, Yildirim K, Melikoglu M, Ugur M, Cakir E, et al. The effect of individualized diet challenges consisting of allergenic foods on TNF-α and IL-1β levels in patients with rheumatoid arthritis. Rheumatology (2004) 43(11):1429–33.10.1093/rheumatology/keh366 https://www.ncbi.nlm.nih.gov/pubmed/15304675[↩]
- Häger J., Bang H., Hagen M., Frech M., Träger P., Sokolova M.V., Steffen U., Tascilar K., Sarter K., Schett G., et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients. 2019;11:2392. doi: 10.3390/nu11102392[↩]
- Liu L., Xie S. Dietary fiber intake associated with risk of rheumatoid arthritis among U.S. adults: NHANES 2010–2020. Medicine. 2023;102:e33357. doi: 10.1097/MD.0000000000033357[↩]
- Jiang L., Shang M., Yu S., Liu Y., Zhang H., Zhou Y., Wang M., Wang T., Li H., Liu Z., et al. A high-fiber diet synergizes with Prevotella copri and exacerbates rheumatoid arthritis. Cell. Mol. Immunol. 2022;19:1414–1424. doi: 10.1038/s41423-022-00934-6[↩]
- Alawadhi B., Alsaber A., Shatawan I., Al-Herz A., Setiya P., Saleh K., Al-Awadhi A., Hasan E., Al-Kandari W., Mokaddem K., et al. Adherence to the Mediterranean diet is associated with a reduced DAS28 index among patients with rheumatoid arthritis: Case study from KRRD. Int. J. Rheum. Dis. 2023;26:2430–2440. doi: 10.1111/1756-185X.14928[↩]
- Nguyen Y., Salliot C., Gelot A., Gambaretti J., Mariette X., Boutron-Ruault M.C., Seror R. Mediterranean Diet and Risk of Rheumatoid Arthritis: Findings from the French E3N-EPIC Cohort Study. Arthritis Rheumatol. 2021;73:69–77. doi: 10.1002/art.41487[↩]
- Mostafaei R., Elahi N., Moludi J., Moradi F., Solouki L., Nachvak S.M., Behrooz M. Association of Mediterranean diet pattern with disease activity in the patients with rheumatoid arthritis: A cross-sectional study on Iranian patients. Clin. Nutr. ESPEN. 2024;60:95–101. doi: 10.1016/j.clnesp.2024.01.012[↩]
- Ma L., Yuan J., Yang X., Yan M., Li Y., Niu M. Association between the adherence to Mediterranean diet and depression in rheumatoid arthritis patients: A cross-sectional study from the NHANES database. J. Health Popul. Nutr. 2024;43:103. doi: 10.1186/s41043-024-00572-w[↩]
- Vadell A.K.E., Bärebring L., Hulander E., Gjertsson I., Lindqvist H.M., Winkvist A. Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)-a randomized, controlled crossover trial indicating effects on disease activity. Am. J. Clin. Nutr. 2020;111:1203–1213. doi: 10.1093/ajcn/nqaa019[↩]
- Ghaseminasab-Parizi M., Nazarinia M.A., Akhlaghi M. The effect of flaxseed with or without anti-inflammatory diet in patients with rheumatoid arthritis, a randomized controlled trial. Eur. J. Nutr. 2022;61:1377–1389. doi: 10.1007/s00394-021-02707-9[↩]
- Safaei M., Kheirouri S., Alizadeh M., Pirovi A.H. Association between Mediterranean-dietary approaches to stop hypertension intervention for neurodegenerative delay diet and biomarkers of oxidative stress, metabolic factors, disease severity, and odds of disease in rheumatoid arthritis patients. Food Sci. Nutr. 2024;12:3973–3981. doi: 10.1002/fsn3.4055[↩]
- Morris M.C., Tangney C.C., Wang Y., Sacks F.M., Bennett D.A., Aggarwal N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11:1007–1014. doi: 10.1016/j.jalz.2014.11.009[↩]
- Bakinowska E, Stańska W, Kiełbowski K, Szwedkowicz A, Boboryko D, Pawlik A. Gut Dysbiosis and Dietary Interventions in Rheumatoid Arthritis-A Narrative Review. Nutrients. 2024 Sep 23;16(18):3215. doi: 10.3390/nu16183215[↩]
- Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016 Jan;75(1):3-15. doi: 10.1136/annrheumdis-2015-207524[↩]
- Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, Curtis JR, Furst DE, Parks D, Kavanaugh A, O’Dell J, King C, Leong A, Matteson EL, Schousboe JT, Drevlow B, Ginsberg S, Grober J, St Clair EW, Tindall E, Miller AS, McAlindon T. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016 Jan;68(1):1-26. https://onlinelibrary.wiley.com/doi/epdf/10.1002/art.39480[↩][↩][↩][↩][↩]
- Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2021 Jul;73(7):924-939. https://pmc.ncbi.nlm.nih.gov/articles/PMC9273041[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kerschbaumer A, Sepriano A, Smolen JS, van der Heijde D, Dougados M, van Vollenhoven R, McInnes IB, Bijlsma JWJ, Burmester GR, de Wit M, Falzon L, Landewé R. Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2020 Jun;79(6):744-759. https://pmc.ncbi.nlm.nih.gov/articles/PMC7286044[↩][↩]
- van der Heide A, Jacobs JW, Bijlsma JW, Heurkens AH, van Booma-Frankfort C, van der Veen MJ, Haanen HC, Hofman DM, van Albada-Kuipers GA, ter Borg EJ, Brus HL, Dinant HJ, Kruize AA, Schenk Y. The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med. 1996 Apr 15;124(8):699-707. doi: 10.7326/0003-4819-124-8-199604150-00001[↩]
- Bukhari MA, Wiles NJ, Lunt M, Harrison BJ, Scott DG, Symmons DP, Silman AJ. Influence of disease-modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: results from a large observational inception study. Arthritis Rheum. 2003 Jan;48(1):46-53. doi: 10.1002/art.10727[↩]
- van Dongen H, van Aken J, Lard LR, Visser K, Ronday HK, Hulsmans HM, Speyer I, Westedt ML, Peeters AJ, Allaart CF, Toes RE, Breedveld FC, Huizinga TW. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007 May;56(5):1424-32. doi: 10.1002/art.22525[↩]
- Management of Rheumatoid Arthritis: Update From ACR. Am Fam Physician. 2022;106(3):340-342. https://www.aafp.org/pubs/afp/issues/2022/0900/practice-guidelines-rheumatoid-arthritis.html[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Curtis JR, Palmer JL, Reed GW, Greenberg J, Pappas DA, Harrold LR, Kremer JM. Real-World Outcomes Associated With Methotrexate, Sulfasalazine, and Hydroxychloroquine Triple Therapy Versus Tumor Necrosis Factor Inhibitor/Methotrexate Combination Therapy in Patients With Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2021 Aug;73(8):1114-1124. doi: 10.1002/acr.24253[↩]
- Erhardt DP, Cannon GW, Teng CC, Mikuls TR, Curtis JR, Sauer BC. Low Persistence Rates in Patients With Rheumatoid Arthritis Treated With Triple Therapy and Adverse Drug Events Associated With Sulfasalazine. Arthritis Care Res (Hoboken). 2019 Oct;71(10):1326-1335. doi: 10.1002/acr.23759[↩]
- Burmester GR, Rigby WF, van Vollenhoven RF, Kay J, Rubbert-Roth A, Kelman A, Dimonaco S, Mitchell N. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016 Jun;75(6):1081-91. doi: 10.1136/annrheumdis-2015-207628[↩]
- Katchamart W, Trudeau J, Phumethum V, Bombardier C. Efficacy and toxicity of methotrexate (MTX) monotherapy versus MTX combination therapy with non-biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2009 Jul;68(7):1105-12. doi: 10.1136/ard.2008.099861[↩]
- Emery P, Bingham CO 3rd, Burmester GR, Bykerk VP, Furst DE, Mariette X, van der Heijde D, van Vollenhoven R, Arendt C, Mountian I, Purcaru O, Tatla D, VanLunen B, Weinblatt ME. Certolizumab pegol in combination with dose-optimised methotrexate in DMARD-naïve patients with early, active rheumatoid arthritis with poor prognostic factors: 1-year results from C-EARLY, a randomised, double-blind, placebo-controlled phase III study. Ann Rheum Dis. 2017 Jan;76(1):96-104. doi: 10.1136/annrheumdis-2015-209057[↩]
- Detert J, Bastian H, Listing J, Weiß A, Wassenberg S, Liebhaber A, Rockwitz K, Alten R, Krüger K, Rau R, Simon C, Gremmelsbacher E, Braun T, Marsmann B, Höhne-Zimmer V, Egerer K, Buttgereit F, Burmester GR. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis. 2013 Jun;72(6):844-50. doi: 10.1136/annrheumdis-2012-201612[↩]
- Nam JL, Villeneuve E, Hensor EM, Wakefield RJ, Conaghan PG, Green MJ, Gough A, Quinn M, Reece R, Cox SR, Buch MH, van der Heijde DM, Emery P. A randomised controlled trial of etanercept and methotrexate to induce remission in early inflammatory arthritis: the EMPIRE trial. Ann Rheum Dis. 2014 Jun;73(6):1027-36. doi: 10.1136/annrheumdis-2013-204882[↩]
- Singh JA, Saag KG, Bridges SL Jr, Akl EA, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016 Jan;68(1):1-26. doi: 10.1002/art.39480[↩]
- Rheumatoid Arthritis Treatment Guidelines. https://www.arthritis.org/diseases/more-about/rheumatoid-arthritis-treatment-guidelines[↩][↩]
- Chauhan K, Jandu JS, Brent LH, et al. Rheumatoid Arthritis. [Updated 2023 May 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441999[↩][↩][↩]
- van Everdingen AA, Jacobs JW, Siewertsz Van Reesema DR, Bijlsma JW. Low-dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects: a randomized, double-blind, placebo-controlled clinical trial. Ann Intern Med. 2002 Jan 1;136(1):1-12. doi: 10.7326/0003-4819-136-1-200201010-00006[↩]
- Bakker MF, Jacobs JW, Welsing PM, Verstappen SM, Tekstra J, Ton E, Geurts MA, van der Werf JH, van Albada-Kuipers GA, Jahangier-de Veen ZN, van der Veen MJ, Verhoef CM, Lafeber FP, Bijlsma JW; Utrecht Rheumatoid Arthritis Cohort Study Group. Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2012 Mar 6;156(5):329-39. doi: 10.7326/0003-4819-156-5-201203060-00004[↩]
- Krause D, Mai A, Klaassen-Mielke R, Timmesfeld N, Trampisch U, Rudolf H, Baraliakos X, Schmitz E, Fendler C, Klink C, Boeddeker S, Saracbasi-Zender E, Christoph HJ, Igelmann M, Menne HJ, Schmid A, Rau R, Wassenberg S, Sonuc N, Ose C, Schade-Brittinger C, Trampisch HJ, Braun J. The Efficacy of Short-Term Bridging Strategies With High- and Low-Dose Prednisolone on Radiographic and Clinical Outcomes in Active Early Rheumatoid Arthritis: A Double-Blind, Randomized, Placebo-Controlled Trial. Arthritis Rheumatol. 2022 Oct;74(10):1628-1637. doi: 10.1002/art.42245[↩]
- Chauhan K, Jandu JS, Goyal A, et al. Rheumatoid Arthritis. [Updated 2022 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441999[↩]
- Gwinnutt JM, Symmons DPM, MacGregor AJ, Chipping JR, Marshall T, Lunt M, Verstappen SMM. Twenty-Year Outcome and Association Between Early Treatment and Mortality and Disability in an Inception Cohort of Patients With Rheumatoid Arthritis: Results From the Norfolk Arthritis Register. Arthritis Rheumatol. 2017 Aug;69(8):1566-1575. doi: 10.1002/art.40090[↩][↩]
- Paul BJ, Kandy HI, Krishnan V. Pre-rheumatoid arthritis and its prevention. Eur J Rheumatol. 2017 Jun;4(2):161-165. doi: 10.5152/eurjrheum.2017.16006[↩]
- Holmqvist, M., Mantel, Ä., Wållberg-Jonsson, S., James, S., Jernberg, T. and Askling, J. (2021), Findings on Coronary Angiographies in Patients With Rheumatoid Arthritis and Ischemic Heart Disease: Are They Different From Patients Without Rheumatoid Arthritis?. Arthritis Care Res, 73: 658-665. https://doi.org/10.1002/acr.24214[↩]
- Martin-Trujillo A, van Rietschoten JG, Timmer TC, Rodríguez FM, Huizinga TW, Tak PP, Marsal S, Ibrahim SM, Dijkmans BA, van der Pouw Kraan TC, Verweij CL. Loss of imprinting of IGF2 characterises high IGF2 mRNA-expressing type of fibroblast-like synoviocytes in rheumatoid arthritis. Ann Rheum Dis. 2010 Jun;69(6):1239-42. doi: 10.1136/ard.2008.106195[↩]