Contents
- Pulmonary hypertension in newborns
- Pulmonary hypertension in newborns causes
- Pulmonary hypertension in newborns pathophysiology
- Persistent pulmonary hypertension in the newborn types
- Pulmonary hypertension in newborns signs and symptoms
- Pulmonary hypertension in newborns complications
- Pulmonary hypertension in newborns diagnosis
- Pulmonary hypertension in newborns differential diagnosis
- Pulmonary hypertension in newborns treatment
- Inhaled nitric oxide (iNO)
- Bosentan (Endothelin-1 receptor blocker)
- Surfactant Therapy
- Prostaglandin E1 (PGE1)
- Inhaled Prostacyclin (PGI2)
- Corticosteroids
- Oxygen therapy and mechanical ventilation (breathing machine)
- High-frequency oscillatory ventilation (HFOV)
- Inotropes
- Extra-corporeal membrane oxygenation (ECMO)
- Moving forward and discharge from intensive care
- Pulmonary hypertension in newborns prognosis
Pulmonary hypertension in newborns
Pulmonary hypertension in newborns also called persistent pulmonary hypertension of the newborn (PPHN) is a potentially life-threatening condition where a baby’s blood vessels to the lungs don’t open fully after birth, leading to high blood pressure in the lungs and reduced oxygen reaching the body 1, 2, 3, 4, 5, 6, 7. Persistent pulmonary hypertension of the newborn (PPHN) occurs when a newborn fails to make the normal transition from fetal to newborn circulation, leading to persistent high pressure in the pulmonary arteries (the blood vessels that carry deoxygenated blood from the heart to the lungs) 8. In the mother’s womb, before babies are born, they do not need to use their lungs to breathe, because they receive oxygen via the umbilical cord and placenta from their mother 9, 10. The placenta is an organ that develops in the uterus during pregnancy, connected to the developing baby by the umbilical cord, which delivers oxygen and nutrients to the fetus and removes waste products. In the mother’s womb, babies’ lungs are filled with amniotic fluid, and the blood vessels (arteries and veins) which take blood from the heart to the lungs are constricted, or closed 9, 10. This means that the pressure inside the blood vessels of the babies’ lungs is high – you will often hear doctors talk about ‘high pulmonary pressures’. When babies are born, they take a big breath or cry, and their lungs fill with air instead of fluid. When babies’ lungs fill with air, the blood vessels which take blood from the heart to the lungs open up (dilate), and this means that oxygen can be carried from the lungs, back to the heart, and pumped to the brain and the rest of the body once the umbilical cord is cut 11. Delaying cord clamping until ventilation is established may increase blood flow into the lungs and improve the cardiopulmonary transition at birth 12, 13. The most important stimulus to promote pulmonary blood vessels to open up (vasodilate) appears to be ventilation of the lungs and an increase in oxygen tension 14. The pressure inside the lungs and the blood vessels is now low. Persistent pulmonary hypertension of the newborn (PPHN) occurs due to failure to make the transition from fetal circulation to normal newborn circulation. If there is a problem around the time of birth which interferes with this process, the blood vessels may not open up properly so the pressure inside them remains high 1. This is called persistent pulmonary hypertension of the newborn (PPHN). As a result of the pulmonary arteries (the blood vessels that carry deoxygenated blood from the heart to the lungs) not opening up, blood cannot get into the lungs to pick up oxygen and then the body will not have enough oxygen for the brain and other organs, and this can make a baby unwell. The severity of persistent pulmonary hypertension of the newborn (PPHN) can range from mild hypoxemia (low blood oxygen levels) with minimal respiratory distress to severe hypoxemic respiratory failure and cardiopulmonary instability. General management principles for the newborn with persistent pulmonary hypertension of the newborn (PPHN) include maintenance of normal temperature, electrolytes particularly calcium and magnesium, glucose, nutritional support, avoidance of stress, maintaining good hemoglobin level (Hb >140 g/L), and handling with sedation and analgesia as needed. In severe persistent pulmonary hypertension of the newborn (PPHN) cases treatment in an intensive care unit may be needed if simple measures such as keeping the baby warm and giving oxygen don’t increase oxygen levels to normal. Persistent pulmonary hypertension of the newborn (PPHN) can lead to severe complications, including heart failure, stroke, and long-term oxygen dependence.
Persistent pulmonary hypertension of the newborn (PPHN) incidence has been reported at 1.9 per 1,000 live births (0.4–6.8 per 1,000 live births) in the United States and 0.43 to 6 per 1,000 live births in the United Kingdom, with mortality rates ranging from 4% to 33% 15, 16. Persistent pulmonary hypertension of the newborn usually occurs in babies that are born at fullterm (at nine months) but can occasionally occur in premature (born too early) babies as well.
Persistent pulmonary hypertension of the newborn (PPHN) can be caused by a variety of factors, including lung disease like meconium aspiration syndrome, lung hypoplasia (underdeveloped lungs), congenital diaphragmatic hernia (a developmental defect in the diaphragm that separates the thorax and the abdomen and is the most important cause of pulmonary hypoplasia resulting in PPHN). Congenital diaphragmatic hernia has a mortality rate of 20% to 30%, and the degree of associated lung hypoplasia and the severity of pulmonary hypertension remain the major determinants of survival 3. Despite marked improvement in survival of PPHN resulting from other causes, the mortality and need for extracorporeal membrane oxygenation (ECMO) remain high in infants with congenital diaphragmatic hernia 3. Certain conditions in the mother like maternal high blood sugar (hyperglycemia), non-steroidal anti-inflammatory drugs (NSAIDs) or selective serotonin reuptake inhibitors (SSRIs) use, Cesarean section (C-section), chorioamnionitis (a serious infection of the placenta, amniotic fluid, and fetal membranes (chorion and amnion) during pregnancy), and neonatal conditions like meconium aspiration syndrome and neonatal respiratory distress syndrome (also known as hyaline membrane disease, is a breathing problem in newborns, primarily affecting premature infants, due to a lack of surfactant in the lungs, which is a substance that helps keep the air sacs open) can increase the risk of PPHN. When the cause of persistent pulmonary hypertension of the newborn (PPHN) is unknown it’s called idiopathic PPHN.
Persistent pulmonary hypertension of the newborn (PPHN) diagnosis is based on clinical signs such as hypoxemia, cyanosis (bluish or greyish color of baby’s skin due to a lack of oxygen in her blood), difficulty breathing or rapid breathing (respiratory distress) and confirmed by echocardiography (an ultrasound of the heart).
In contrast to adult primary pulmonary hypertension, persistent pulmonary hypertension of the newborn (PPHN) is not defined by a specific pressure of the pulmonary circulation but rather an abnormally elevated pulmonary vascular resistance (PVR). Persistent pulmonary hypertension of the newborn (PPHN) diagnosis is confirmed regardless of the mean pulmonary arterial pressure, as long as it is accompanied by a right-to-left shunt and absence of congenital heart disease 17.
Evaluation of an infant with suspected persistent pulmonary hypertension in the newborn (PPHN) includes obtaining arterial blood gas (arterial blood gas test measures oxygen, carbon dioxide, and pH levels in a blood sample taken from an artery, helping assess lung function and overall acid-base balance), chest X-ray, and echocardiogram. Blood tests to look for signs of infection with complete blood count with differential (CBC with diff), C- reactive protein (CRP), and blood culture. In sepsis, leucocytosis or leucopenia may be seen. CRP may be high in sepsis. Chest radiographs may show signs of underlying lung parenchymal disease. Brain natriuretic peptide (BNP) is a hormone produced by stressed right ventricles. Brain natriuretic peptide (BNP) levels are elevated in babies with persistent pulmonary hypertension in the newborn (PPHN). BNP level of more than 550pg/ml is predictive of persistent pulmonary hypertension 18.
- Oxygenation Index (OI) = Mean airway pressure (MAP) x fraction of inspired oxygen (FiO2) x 100 / partial pressure of arterial oxygen (PaO2)
- Fraction of inspired oxygen (FiO2) is the concentration of oxygen in the gas mixture a person inhale
- Partial pressure of arterial oxygen (PaO2) is the pressure of oxygen dissolved in arterial blood, a key indicator of oxygenation and gas exchange in the lungs, typically measured during an arterial blood gas (ABG) test. A normal PaO2 value at sea level is typically between 80 and 100 mmHg
- Oxygenation Index >15, along with pre-post ductal saturation difference of >10%, are suggestive of high pulmonary vascular resistance 19.
- The oxygenation index is also used to assess the severity of the disease. Persistent Oxygenation Index > 40 is often used to indicate the severity and the need for extracorporeal membrane oxygenation (ECMO). If ECMO support is anticipated, coagulation studies and a head ultrasound should be done before cannulation.
Persistent pulmonary hypertension of the newborn (PPHN) treatment focuses on improving oxygenation, reducing pulmonary vascular resistance, and addressing any underlying causes 21.
- Inhaled Nitric Oxide (iNO): Inhaled Nitric Oxide (iNO) is a crucial medication that helps relax the blood vessels in the lungs, improving blood flow and oxygenation.
- Ventilation: Gentle ventilation with optimal use of positive end-expiratory pressure or mean airway pressure and/or surfactant is important.
- Other management strategies include optimal oxygenation, avoiding respiratory and metabolic acidosis, blood pressure stabilization, sedation, and pulmonary vasodilator therapy.
- In severe cases, extracorporeal membrane oxygenation (ECMO) a heart-lung bypass machine may be needed to support the baby’s lungs and heart.
Persistent pulmonary hypertension of the newborn (PPHN) is a serious condition, and if a child has been moved to the intensive care unit, that is because the baby is doing very poorly. The mortality is thought to be under 10 per cent (fewer than 1 in 10 babies affected will die). Mortality ranges from 7.6 to 10.7%, depending on the severity of the condition. Boys had a higher risk than girls with an adjusted risk ratio of 0.8 4. African American babies had the highest risk, followed closely by Hispanic and Asian infants.
There are also some after-effects from the lack of oxygen to the brain during the illness, and up to a quarter of babies affected will have some impairment because of their illness as they grow older. This includes difficulties such as learning problems and deafness.
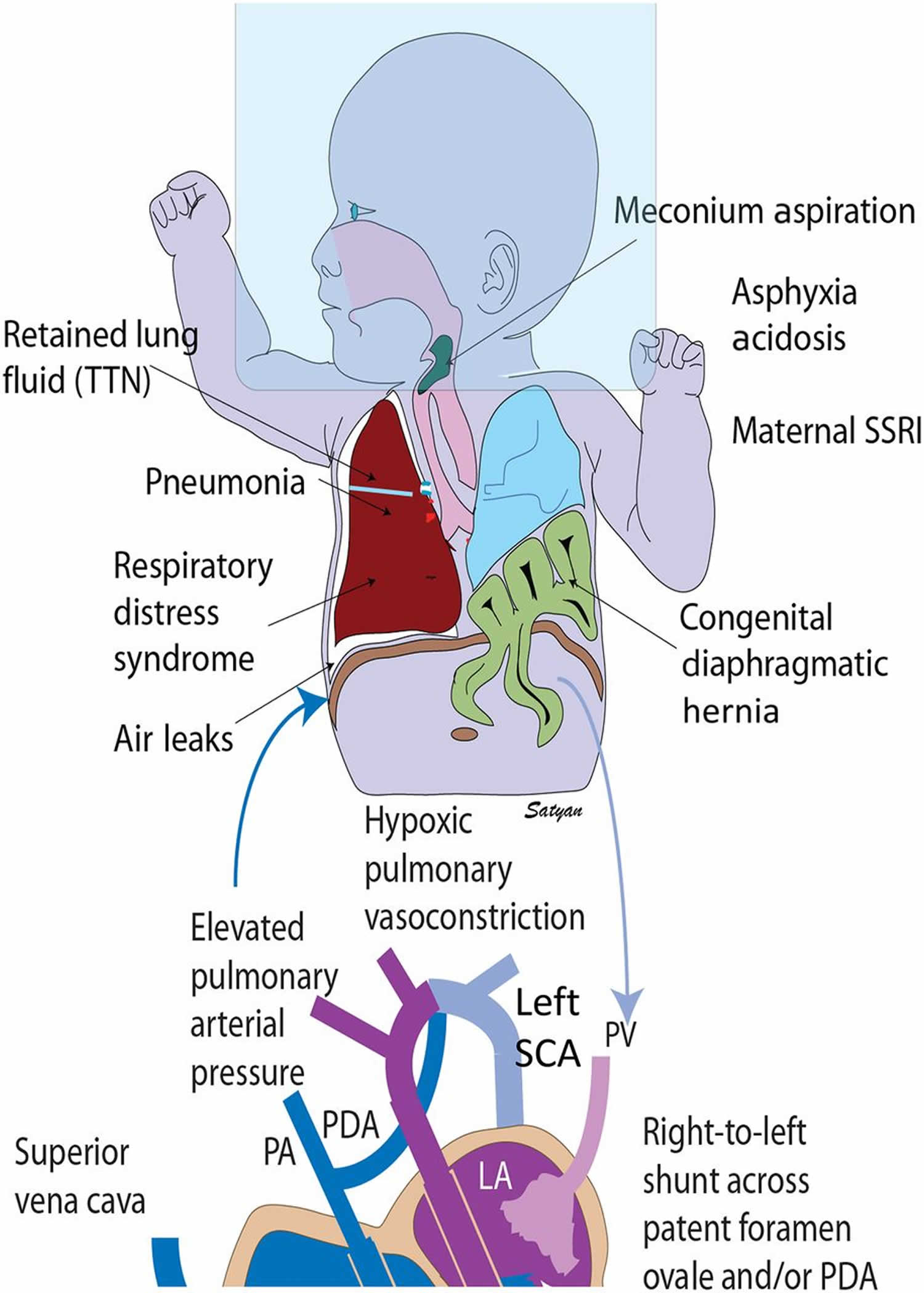
Figure 1. Persistent pulmonary hypertension of the newborn causes
Footnotes: Persistent pulmonary hypertension of the newborn (PPHN) causes and pathophysiology. Secondary persistent pulmonary hypertension of the newborn (PPHN) can be due to various lung diseases, such as retained lung fluid or transient tachypnea of newborn (TTN), pneumonia, aspiration syndromes, respiratory distress syndrome (RDS), and congenital diaphragmatic hernia with lung hypoplasia. Use of high concentrations of inspired oxygen (approximately 100%) without positive pressure (oxygen hood) can lead to absorption atelectasis and worsening of ventilation-perfusion mismatch. Lung disease and V/Q mismatch result in hypoxemia. Increased pulmonary vascular resistance results in reduced pulmonary blood flow and right-to-left shunt through patent ductus arteriosus (PDA) and/or patent foramen ovale (PFO). Pulmonary hypertension is often associated with systemic hypotension with deviation of the interventricular septum to the left. The right subclavian artery (SCA) (and blood flowing to the right upper extremity) is always preductal. The left subclavian artery (SCA) may be preductal, juxtaductal, or postductal. Hence, preductal oxygen saturations should be obtained from the right upper extremity and compared with lower extremity to assess differential cyanosis.
Abbreviations: LA = left atrium; LV = left ventricle; PA = pulmonary artery; RA = right atrium RV = right ventricle; TR = tricuspid regurgitation; PROM = premature rupture of membranes; CDH = congenital diaphragmatic hernia, MAS = meconium aspiration syndrome, PPHN = persistent pulmonary hypertension of the newborn.
[Sources 3, 20 ]Pulmonary hypertension in newborns causes
Persistent pulmonary hypertension in the newborn can occur due to several reasons. Doctors do not always know the cause of persistent pulmonary hypertension of the newborn (PPHN), but they do know that the following may be factors 22:
- Meconium aspiration: This is when a baby has passed feces (poop) while still in the womb, and because the poop becomes mixed with the amniotic fluid (the fluid inside the womb), the baby can breathe it into their lungs.
Infection - Infections such as pneumonia (lung or chest infection) and bloodstream infections (sepsis) can make persistent pulmonary hypertension of the newborn more likely, and there may be an increased risk of these conditions if the waters broke a long time before the baby was delivered, or if there was a group B strep infection present.
- Congenital abnormalities of the heart and lungs: A small number of babies who get persistent pulmonary hypertension of the newborn will have it because they have been born with an abnormality of their heart or lungs such as a diaphragmatic hernia, or a blocked heart valve, or lungs that are smaller than they should be.
Other causal factors include oligohydramnios (a condition during pregnancy where there’s less amniotic fluid than expected for the baby’s gestational age, which can lead to complications), pulmonary hypoplasia (also called lung hypoplasia, is a condition where the lungs don’t develop properly during fetal development, resulting in abnormally small and underdeveloped lungs), infants of diabetic mothers, in utero closure of ductus arteriosus, small and large for gestational age status 23.
Maternal risk factors such as obesity, diabetes, pre-eclampsia, chorioamnionitis, smoking, selective serotonin reuptake inhibitors (SSRIs), and non-steroidal anti-inflammatory drugs (NSAIDs) use during pregnancy can also contribute to persistent pulmonary hypertension in the newborn 24, 25.
Congenital heart defects (structural heart problems that are present at birth) such as transposition of great arteries (TGA) and congenital diaphragmatic hernia (CDH) are also associated with persistent fetal circulation in the immediate neonatal period.
A few genetic risk factors have been identified in patients who have developed PPHN including trisomy 21 (Down syndrome) 26, genetic mutations leading to surfactant protein B deficiency (SFTPB), and mutations in the ATP-binding cassette transporter 3 gene (ABCA3) 27.
The hallmark of persistent pulmonary hypertension of the newborn (PPHN) is a sustained elevation of pulmonary vascular resistance (PVR), meaning the blood vessels to the lungs are constricted and don’t allow enough blood flow. The high pulmonary vascular resistance (PVR) causes blood to bypass the lungs and flow through shunts (openings) in the heart and blood vessels, further reducing oxygen delivery. This leads to hypoxemia (low blood oxygen levels) and hypoxemic respiratory failure, a life-threatening condition.
Pulmonary hypertension in newborns pathophysiology
High pulmonary vascular resistance (PVR) relative to systemic vascular resistance is essential to normal intrauterine fetal circulation. Fluid-filled alveoli and hypoxia-induced pulmonary vasoconstriction in the presence of circulating vasoconstrictors such as endothelin-1 and thromboxane maintain high pulmonary vascular resistance (PVR) in the fetal phase 14. Conversely, circulating levels of vasodilators such as nitric oxide (NO) and prostaglandins are low 14. Pulmonary reactivity to vasodilators increases with increasing gestational age.
During normal transition after birth, several events occur simultaneously, which results in a smooth transition to extrauterine life. A drastic fall in pulmonary arterial pressure (PAP) following the first breath accompanies increased pulmonary blood flow when the umbilical cord is clamped. The increased partial pressure of oxygen and the initiation of ventilation also stimulate the production of vasodilators such as nitric oxide and prostacyclins; cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP) mediate pulmonary vasodilation through endothelial nitric oxide 28.
Cyclooxygenase (COX) enzyme mediates the conversion of arachidonic acid to prostacyclin and is a rate-limiting enzyme. Cyclooxygenase 1 (COX-1) is found in the lung and is upregulated when the fetus reaches term gestation. Prostacyclins increase cyclic adenosine monophosphate (cAMP) levels which in turn cause vasorelaxation by decreasing intracellular calcium concentration. Nitric oxide – cyclic guanosine monophosphate (NO- cGMP) and nitric oxide- cyclic adenosine monophosphate (NO-cAMP) pathways are extensively studied in the pathophysiology of persistent fetal circulation 29, 30. Subsequent clinical studies supported the widely accepted inhaled nitric oxide (iNO) therapy in persistent pulmonary hypertension in newborns.
High pulmonary vascular resistance (PVR) decreases pulmonary blood flow. This causes ventilation-perfusion (V/Q) mismatch and right to left shunting of blood across the foramen ovale and ductus arteriosus resulting in refractory hypoxemia. Extra-cardiac shunting across patent ductus arteriosus (PDA) results in more than a 10% differential between pre and post-ductal saturations 31.
Infants with persistent pulmonary hypertension are vulnerable to wide swings in oxygen saturation and increased left to right shunting with minimal stimulation and agitation.
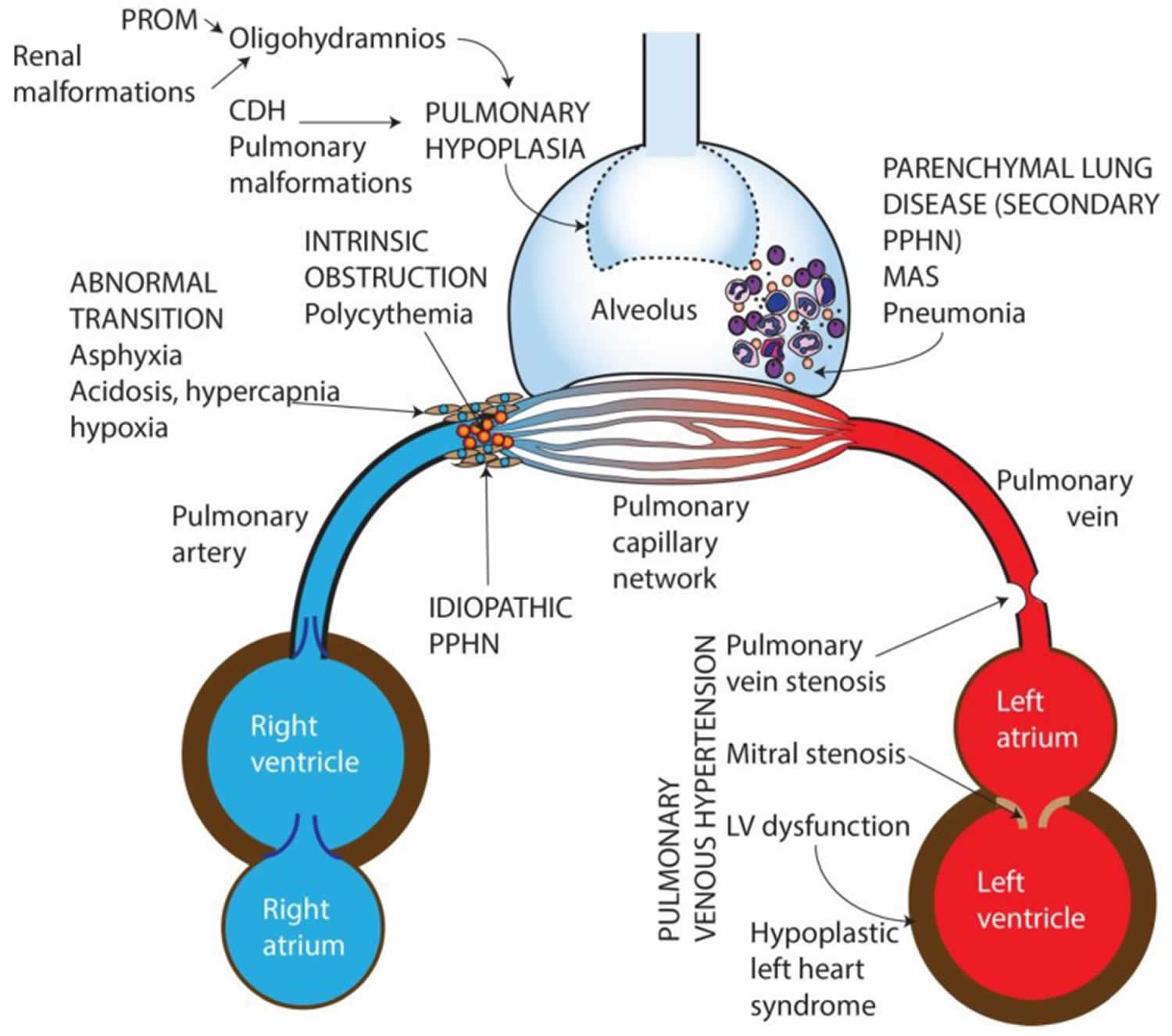
Persistent pulmonary hypertension in the newborn types
Persistent pulmonary hypertension in the newborn (PPHN) can be characterized into 6 types:
- Parenchymal lung diseases: Persistent pulmonary hypertension in the newborn (PPHN) secondary to lung parenchymal diseases such as meconium aspiration syndrome (MAS), respiratory distress syndrome (RDS), or pneumonia.
- Idiopathic pulmonary hypertension or idiopathic PPHN: Lung with normal parenchyma and remodeled pulmonary vasculature. Idiopathic persistent pulmonary hypertension in the newborn is caused by impaired pulmonary relaxation after birth in the absence of parenchymal lung disease. One cause of idiopathic PPHN is constriction, or premature closure of the ductus arteriosus in utero, which can occur after exposure to aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) (eg, ibuprofen, naproxen) during the third trimester. Evaluation of infants at autopsy shows significant remodeling of their pulmonary vasculature, with vascular wall thickening and smooth muscle hyperplasia 32. Furthermore, the smooth muscle extends to the level of the intra-acinar arteries, which does not normally occur until late in the postnatal period. As a result, infants do not vasodilate their pulmonary vessels adequately in response to birth-related stimuli, and they present with hypoxemia and hyperlucent lung fields on radiography, which is termed black lung PPHN.
- Hypoplastic vasculature and Pulmonary hypoplasia: Persistent pulmonary hypertension in the newborn (PPHN) secondary to hypoplastic vasculature as seen in congenital diaphragmatic hernia and other causes of lung hypoplasia (oligohydramnios secondary to Potter’s syndrome, kidney disease or chronic leakage of amniotic fluid)
- Intravascular obstruction due to hyperviscosity—polycythemia: Persistent pulmonary hypertension in the newborn (PPHN) secondary to high blood viscosity due to polycythemia (an increase in the absolute red blood cell in the body, this is reflected by an increase in hemoglobin levels, or hematocrit) resulting in intravascular obstruction and elevated pulmonary vascular resistance (PVR).
- Abnormal pulmonary transition: Abnormal pulmonary transition at birth, due to perinatal asphyxia can lead to hypoxemia, hypercarbia and metabolic acidosis, all of which cause pulmonary vasoconstriction and increased intra- and extra-pulmonary shunting. Decreased alveolar recruitment and lung volume can cause physical constriction of the intrapulmonary blood vessels further impeding pulmonary blood flow. Optimizing lung recruitment to functional residual capacity (FRC) and effective ventilation often lead to reversal of the pathophysiological process associated with these conditions. Preterm infants with severe respiratory distress syndrome (RDS) and infants born by cesarean section without labor experience a slower decrease in pulmonary vascular resistance (PVR) compared to term infants born by vaginal delivery.
- Pulmonary venous hypertension: Pulmonary venous hypertension present with hypoxic respiratory failure and can be clinically indistinguishable from pulmonary arterial hypertension 33, 34. The chest X-ray appearance shows increased pulmonary vascularity and fluid congestion. The decrease in pulmonary blood flow is due to backpressure from impaired venous drainage into the left atrium. Left-sided obstructive congenital heart disease (mitral atresia, hypoplastic left heart syndrome, critical coarctation of aorta), total anomalous pulmonary venous connection with obstruction 35, pulmonary vein stenosis can all present with varying degrees of pulmonary venous hypertension. Left ventricular dysfunction due to asphyxia, sepsis or congenital diaphragmatic hernia can also present with pulmonary venous hypertension. The diagnosis is made on echocardiography by evaluating the direction of fetal shunts. Inhaled nitric oxide (NO) is contraindicated in these conditions as it increases pulmonary edema and causes deterioration of gas exchange 36.
These categories can overlap in any given condition 2.
Maladaption/Parenchymal lung diseases resulting in secondary PPHN
Meconium aspiration syndrome
Meconium aspiration syndrome in newborns leads to acute respiratory failure with a mortality of up to 10% 37. Meconium causes chemical pneumonitis and surfactant inactivation that leads to ventilation-perfusion mismatch. Resulting low level of oxygen in the blood (hypoxemia) and elevated levels of carbon dioxide in the blood (hypercarbia) cause pulmonary vasoconstriction and persistent pulmonary hypertension in the newborn (PPHN). Structural studies of the lung at postmortem in fatal cases of meconium aspiration suggest that antecedent pulmonary vascular abnormalities exacerbated the postnatal pulmonary hypertension 38. Decreased endothelial nitric oxide synthase (eNOS) expression has also been reported in umbilical venous endothelial cell cultures from human infants with meconium staining who develop persistent pulmonary hypertension in the newborn (PPHN) 39. Meconium components incite an inflammatory response with release of cytokines and increase the production of vasoconstrictors including endothelin and thromboxane 40. The incidence of meconium aspiration syndrome has decreased in developed countries but continues to be prevalent in resource-limited settings often associated with asphyxia 41.
Management of a neonate born through meconium stained amniotic fluid has changed dramatically over the last decade. Amnioinfusion, suctioning at the perineum and tracheal suctioning in vigorous infants did not alter the incidence of meconium aspiration syndrome in multicenter randomized trials 42, 43, 44. The current guidelines recommend tracheal suctioning only if the infant born through meconium stained amniotic fluid is not vigorous at birth 45. Recent data from a randomized trial and a translational study have pushed further to question the benefit of tracheal suctioning of meconium at birth even if the newborn is not vigorous 46, 47. Additional studies are required to evaluate the effect of tracheal suctioning in meconium aspiration syndrome and the incidence and severity of persistent pulmonary hypertension in the newborn (PPHN).
Pneumonia and sepsis
Pneumonia and sepsis often present with elevated pulmonary vascular resistance (PVR) associated with systemic hypotension and decreased systemic vascular resistance (SVR). In addition, some infants with sepsis have myocardial dysfunction resulting in pulmonary venous hypertension due to elevated left atrial pressures 48.
Pulmonary hypertension in premature infants
Although persistent pulmonary hypertension in the newborn (PPHN) is traditionally considered a disease of fullterm and late preterm infants, it is increasingly being diagnosed in extremely preterm infants 49. Some preterm infants with respiratory distress syndrome (RDS) present with persistent pulmonary hypertension in the newborn (PPHN) in the first few days of life 23, while preterm infants with bronchopulmonary dysplasia (BPD) may be diagnosed with pulmonary hypertension later in the hospital course or after discharge from the Neonatal Intensive Care Unit (NICU). Preterm infants with fetal growth restriction and born after prolonged rupture of membranes are at higher risk for developing pulmonary hypertension 50. Pulmonary vascular disease significantly increases morbidity and mortality in bronchopulmonary dysplasia 51.
Idiopathic persistent pulmonary hypertension in the newborn (Black-lung PPHN)
Some cases of persistent pulmonary hypertension in the newborn (PPHN) are not secondary to parenchymal lung disease and are referred to as idiopathic or “black-lung” (referring to paucity of pulmonary vascularity and absence of lung disease) persistent pulmonary hypertension in the newborn (PPHN) 5. Idiopathic pulmonary hypertension is secondary to remodeled pulmonary arteries, characterized by smooth muscle hyperplasia and extension of smooth muscle in intra-acinar arteries 5. The abnormal structural remodeling of the pulmonary circulation as seen in persistent pulmonary hypertension in the newborn (PPHN) affects the responsiveness to vasodilator stimuli, and may prevent the access of nitric oxide (NO) to the vascular smooth muscle cells 52. Maternal use of nonsteroidal anti-inflammatory drugs (NSAIDs) during third trimester of pregnancy can lead to premature closure of ductus arteriosus 53 and “black-lung” persistent pulmonary hypertension in the newborn (PPHN). Ovine fetal ligation or constriction of ductus arteriosus can produce similar vascular changes and is widely used to replicate persistent pulmonary hypertension in the newborn (PPHN) in newborn lambs, showing the same pulmonary vascular findings as idiopathic pulmonary hypertension 54.
Hypoplastic vasculature and Pulmonary hypoplasia
Congenital diaphragmatic hernia (CDH) is developmental defect in the diaphragm separating the thorax and the abdomen and is the most important cause of pulmonary hypoplasia resulting in persistent pulmonary hypertension in the newborn (PPHN). Congenital diaphragmatic hernia defect leads to a herniation of the abdominal viscera into the thoracic cavity. Congenital diaphragmatic hernia occurs in 1/2,500 to 5,000 live births. Congenital diaphragmatic hernia has a mortality rate of 20-30% and the degree of associated pulmonary hypoplasia and the severity of pulmonary hypertension remain the major determinants of survival 55. Pulmonary vascular abnormalities in congenital diaphragmatic hernia include a decreased number of pulmonary arteries per unit lung volume and peripheral muscularization of small arteries, with medial and adventitial thickening 56. In spite of marked improvement in survival of persistent pulmonary hypertension in the newborn (PPHN) resulting from other causes, the mortality and need for ECMO remain high in infants with congenital diaphragmatic hernia.
Pulmonary hypoplasia secondary to renal dysfunction and oligohydramnios or thoracic dystrophy can be associated with pulmonary hypertension 57, 58, 59. Prolonged rupture of membranes is also a risk factor for pulmonary hypertension in preterm infants 60.
Alveolar capillary dysplasia
Alveolar capillary dysplasia is generally associated with malalignment of the pulmonary veins and produces respiratory failure early in life and carries a mortality rate that approaches 100% 61. Recent reports of infants presenting with fulminant symptoms of alveolar capillary dysplasia with malalignment of the pulmonary veins well beyond the neonatal period, even as late as 7 months of age, have begun to emerge, challenging the established phenotype and offering the possibility that long-term survivors with milder forms of the disease may exist 62. FOXF1 transcription factor gene or deletions upstream to FOXF1 gene were identified in 40% of cases 63. Blood testing to screen for these defects is now available, but a negative result does not preclude the diagnosis, and histological examination of lung tissue remains the gold standard for diagnosis. A lung biopsy to rule out alveolar capillary dysplasia should be considered for neonates who do not respond to conventional medical management or fail attempts at ECMO decannulation.
Pulmonary hypertension in newborns signs and symptoms
The main feature of persistent pulmonary hypertension of the newborn (PPHN) is that not enough oxygen is getting to the heart, brain and other organs (hypoxia). This causes babies to look blue (cyanosis) or pale and to have difficulty in breathing. Doctors, nurses and midwives will use oxygen saturation monitoring to measure the amount of oxygen in the blood, expressed as a percentage. Monitoring involves putting a small probe which looks like a sticky plaster around the baby’s hand or foot, then displaying a number on a screen. If this number is low (below 92 per cent), and does not come up to 100 per cent easily then doctors treat problems with breathing using oxygen or breathing machines, they may diagnose persistent pulmonary hypertension of the newborn.
Oximeter probes can be placed on preductal (right hand) and postductal (feet) sites to assess for right-to-left shunting at the level of the foramen ovale and ductus arteriosus. A difference greater than 10% between preductal and postductal oxygen saturations correlates to right-to-left ductal shunting. Sites on the left hand should be avoided, because it may be preductal or postductal. Significant right-to-left shunting at the level of the foramen ovale may result in lower-than-expected preductal oxygen saturations (right hand), although a significant differential should still be evident when compared to postductal oxygen saturations. It is important to rule out the presence of an aberrant right subclavian artery arising from the descending aorta when the aortic arch is left sided since this would adversely effect the ability to assess for a pre and postductal oxygen saturation differential (right arm vs lower extremity).
Persistent pulmonary hypertension of the newborn (PPHN) is often associated with the following signs and symptoms during perinatal period:
- Trouble breathing
- Fast breathing, respiratory distress. Respiratory distress may include tachypnea (rapid breathing), grunting, nasal flaring, and retractions.
- Respiratory acidosis
- Loud, single second heart sound (S2) or a harsh systolic murmur (secondary to tricuspid regurgitation)
- Low Apgar scores
- Meconium staining
- Poor cardiac function and perfusion
- Systemic hypotension
- Symptoms of shock
Persistent pulmonary hypertension of the newborn (PPHN) should be suspected in any newborn with profound and often labile hypoxemia. Labile hypoxemia refers to unstable or fluctuating low blood oxygen levels (hypoxemia) in newborns, often associated with right-to-left shunting of blood due to elevated pulmonary vascular resistance (PVR) 3. Infants will have a preductal and postductal oxygen saturation gradient difference of at least 10%, which is dependent on the magnitude of the right-to-left shunting at the foramen ovale (with preductal saturations being higher). These findings are not specific to PPHN and, therefore, it is important to rule out structural heart disease with an echocardiogram.
A thorough history is important to identify causes that may increase the risk for having PPHN such as meconium aspiration syndrome, respiratory distress syndrome, congenital diaphragmatic hernia, pulmonary hypoplasia, pneumonia, and sepsis. Infants of mothers who have diabetes, asthma, or obesity are also reported to be at increased risk for PPHN 64.
Pulmonary hypertension in newborns complications
Complications of persistent pulmonary hypertension of newborns are related to the underlying cause. In meconium aspiration syndrome (when a newborn has breathing difficulties due to inhaling meconium (first stool) into their lungs, potentially leading to airway obstruction, lung damage, and other complications), air leaks such as pneumothorax and pneumomediastinum occur very frequently due to the ball-valve phenomenon or the need for high ventilator settings 65. In infants with profound hypoxemia, multiorgan dysfunction can manifest as low urine output (oliguria) and easy bleeding due to disseminated intravascular coagulation (also called DIC, which is a serious condition where the body’s blood clotting system becomes overactive, leading to both excessive clotting and bleeding) 19.
Pulmonary hypertension in newborns diagnosis
Evaluation of an infant with suspected persistent pulmonary hypertension in the newborn (PPHN) includes obtaining arterial blood gas (arterial blood gas test measures oxygen, carbon dioxide, and pH levels in a blood sample taken from an artery, helping assess lung function and overall acid-base balance), chest X-ray, and echocardiogram. Blood tests to look for signs of infection with complete blood count with differential (CBC with diff), C- reactive protein (CRP), and blood culture. In sepsis, leucocytosis or leucopenia may be seen. CRP may be high in sepsis. Chest radiographs may show signs of underlying lung parenchymal disease. Brain natriuretic peptide (BNP) is a hormone produced by stressed right ventricles. Brain natriuretic peptide (BNP) levels are elevated in babies with persistent pulmonary hypertension in the newborn (PPHN). BNP level of more than 550 pg/ml is predictive of persistent pulmonary hypertension 18.
Severity of PPHN is commonly assessed by oxygenation index (OI) and alveolar-arterial oxygen difference (AaDO2) 5. Oxygenation Index (OI) is more commonly used during medical management of PPHN since it takes ventilator support into the consideration and is calculated as:
- Oxygenation Index (OI) = Mean airway pressure (MAP) x fraction of inspired oxygen (FiO2) x 100 / partial pressure of arterial oxygen (PaO2)
- Mean airway pressure (MAP) is the average pressure measured at the airway opening throughout a respiratory cycle, reflecting the stress applied to the lung parenchyma. Under normal conditions, mean airway pressure (MAP) closely reflects mean alveolar pressure, indicating the stress on the lung tissue during ventilation. Typical mean airway pressure (MAP) values for fully ventilated patients are 5 to 10 cmH2O for patients with normal lungs, 10 to 20 cmH2O for patients with airflow obstruction, and 15 to 30 cmH2O for patients with Acute Respiratory Distress Syndrome (ARDS) 66.
- Fraction of inspired oxygen (FiO2) is the concentration of oxygen in the gas mixture a person inhale
- Partial pressure of arterial oxygen (PaO2) is the pressure of oxygen dissolved in arterial blood, a key indicator of oxygenation and gas exchange in the lungs, typically measured during an arterial blood gas (ABG) test. A normal PaO2 value at sea level is typically between 80 and 100 mmHg
- Alveolar-arterial oxygen difference (AaDO2) is the difference between alveolar partial pressure of oxygen (PAO2) and arterial partial pressure of oxygen (PaO2) and is calculated using the following formula 67:
- AaDO2 = Alveolar partial pressure of oxygen (PAO2) – Arterial partial pressure of oxygen (PaO2) = [ (Patm-PH2O) x FiO2 – PaCO2/RQ ] – PaO2
- PAO2 = (Patm-PH2O) x FiO2 – PaCO2/RQ
- Patm is the atmospheric pressure (760 mmHg at sea level)
- PH2O is the partial pressure of water (approximately 45 mm Hg)
- PaCO2 = Partial pressure of carbon dioxide in arterial blood (around 40-45 mmHg under normal physiological conditions)
- RQ = Respiratory quotient (equal to 1 if the energy source is purely carbohydrate or equal to 0.8 when the nutritional source is a combination of carbohydrate, protein, and lipid)
- Normal A-a gradient = (Age in years + 10) / 4
- Oxygenation Index (OI) >15, along with pre-post ductal saturation difference of >10%, are suggestive of high pulmonary vascular resistance 19.
- The oxygenation index (OI) is also used to assess the severity of the disease. Persistent Oxygenation Index > 40 is often used to indicate the severity and the need for extracorporeal membrane oxygenation (ECMO). If ECMO support is anticipated, coagulation studies and a head ultrasound should be done before cannulation.
Echocardiography (ultrasound of the baby’s heart), which can look to see if the blood vessels in the lungs are closed or constricted (pulmonary hypertension, sometimes called ‘high pulmonary pressures’) and also rule out any abnormality of the heart which may be causing the low oxygen levels, is the gold standard for confirming the diagnosis. It is also used to follow the effectiveness of treatment. The direction of flow across patent ductus arteriosus (also called PDA, a congenital heart defect where a blood vessel that connects the aorta and pulmonary artery in the fetus remains open after birth, causing extra blood flow to the lungs) and patent foramen ovale (also called PFO, is a condition where a flap-like opening, normally present between the heart’s upper chambers (atria) before birth, fails to close after birth, potentially increasing the risk of stroke), interventricular septal deviation or flattening, and regurgitation across the tricuspid valve (TR jet) are used to estimate right ventricular and/or pulmonary vascular pressure.
The tricuspid valve is located between the right atrium and right ventricle, a heart chamber that pumps blood to the lungs. On echocardiograms, a tricuspid regurgitant (TR) jet, visualized using color Doppler, indicates blood flowing backward through the tricuspid valve, and its velocity can be measured using continuous wave Doppler to estimate right ventricular systolic pressure (RVSP). TR jet may not be accurate in 30% of cases due to poor right ventricular dysfunction.
Echocardiography also provides information about right and left ventricular function, which is vital in treating persistent pulmonary hypertension 3, 31. Poor right ventricular function coupled with low right and left ventricular output is predictive of poor outcomes 68.
Pulmonary hypertension in newborns differential diagnosis
Pulmonary hypertension in newborns differential diagnosis includes cyanotic congenital heart disease, which presents with cyanosis. Inhaled Nitric Oxide (iNO) treatment worsens the clinical condition in total anomalous pulmonary venous return ( is a heart disease in which the 4 veins that take blood from the lungs to the heart do not attach normally to the left atrium (left upper chamber of the heart), instead they attach to another blood vessel or the wrong part of the heart) and should be avoided. Infants with patent ductus arteriosus (a congenital heart defect where a fetal blood vessel, the ductus arteriosus, fails to close after birth, allowing blood to flow between the aorta and pulmonary artery, potentially straining the heart and lungs), transposition of great vessels (also known as transposition of the great vessels, is a congenital heart defect where the aorta and pulmonary artery are switched, leading to a separation of oxygenated and deoxygenated blood circulation) and coarctation of the aorta can present with cyanosis 3.
Pulmonary hypertension in newborns treatment
Initial treatment of persistent pulmonary hypertension of the newborn (PPHN) will consist of simple measures such as keeping the baby warm (but not too hot) and giving oxygen, usually through small prongs (short plastic tubes) in the nostrils, or in an incubator 2. Doctors will usually insert a cannula or drip into the baby’s hand or foot, and use this to give some antibiotics.
Agitation can increase pulmonary vascular resistance and further worsen persistent pulmonary hypertension. Sedatives and anti-anxiolytic are used in infants with PPHN as add-on (adjunctive) therapy to reduce wide swings in oxygen saturations 69. These medications can be given as continuous infusions or on an as-needed basis 4. Induced paralysis: with paralytic agents are typically reserved for newborns who cannot be treated with sedatives alone. Note: paralysis, especially with pancuronium, may promote atelectasis of dependent lung regions and promote ventilation-perfusion mismatch.
As a baby is not likely to feed well while he/she have persistent pulmonary hypertension of the newborn, he/she will receive fluids containing sugar for energy through a drip. If these simple measures do not bring the oxygen levels up easily, the baby is likely to need to be moved to a neonatal intensive care unit or NICU.
Treatment of persistent pulmonary hypertension of the newborn in the intensive care unit (PICU or NICU)
Inhaled nitric oxide (iNO)
Inhaled nitric oxide (iNO) gas is a combination of nitrogen and oxygen which is given to a baby through the breathing machine, straight into the lungs. Inhaled nitric oxide (iNO) works by opening up the closed blood vessels so that more blood flows into the lungs, and the high pressures are reduced. Inhaled nitric oxide (iNO) stimulates guanylate cyclase in vascular smooth muscle cells, increasing cyclic guanosine monophosphate (cGMP) levels, leading to pulmonary vasodilation 70. Inhaled nitric oxide (iNO) is the only United States FDA-approved vasodilator agent for persistent pulmonary hypertension. Inhaled nitric oxide (iNO) is a rapid and potent vasodilator used in inhaled form. Inhaled nitric oxide (iNO) treatment should always start at 20 ppm to evaluate for a response. Large trials showed the efficacy of inhaled nitric oxide (iNO) in decreasing the need for extra-corporeal membrane oxygenation (ECMO) 71, 72. Approximately 30-40% of infants may not respond to inhaled nitric oxide (iNO) 21. Possible side effects of inhaled nitric oxide (iNO) include methemoglobinemia and rebound pulmonary hypertension upon discontinuation 21.
While inhaled nitric oxide (iNO) is the mainstay of treatment for persistent fetal circulation, it did not decrease mortality, hospital stay, or risk of neurologic impairment 4. Early initiation of inhaled nitric oxide (iNO) did not affect the need for extra-corporeal membrane oxygenation (ECMO) or the risk of death or neurodevelopmental outcomes 73. Inhaled nitric oxide (iNO) may not work in the presence of severe left ventricular dysfunction or sub-optimal management of severe lung parenchymal disease. Inhaled nitric oxide (iNO) improves clinical outcomes when Oxygenation Index (OI) is between 15 and 40 4. Inhaled nitric oxide (iNO) is rarely helppul in Oxygenation Index (OI) greater than 40, and aggressive measures such as ECMO are important to prevent death 74.
Milrinone
Milrinone is an inotropic vasodilator that inhibits phosphodiesterase 3 that works by increasing intracellular cAMP, leading to vasodilation and improved cardiac function 75, 4. Milrinone improves right and left ventricular function and can improve oxygenation in infants with severe pulmonary hypertension 19. Milrinone reduces systemic vascular and pulmonary venous pressure, thereby improving left ventricular performance. Some studies show beneficial effects in reducing pulmonary hypertension and improving cardiac output in neonates with PPHN 21. Milrinone is used as add-on therapy to inhaled nitric oxide (iNO) 76. A loading dose (50 mcg/kg over 30–60 min) followed by a maintenance dose (0.33 mcg/kg/min and escalated to 0.66 and then to 1 mcg/kg/min based on response) is commonly used 5. The loading dose is not recommended in the presence of systemic hypotension and in premature neonates 77. As with any systemic vasodilator, hypotension is a clinical concern and blood pressure needs to be closely monitored. Milrinone is non-selective and can cause systemic hypotension and arrhythmias, which may negatively affect heart perfusion and heart function 78. A fluid bolus (10 ml/kg of Lactated Ringer’s solution) prior to loading dose may decrease the risk of hypotension. In addition, one case series described an increased incidence of intracranial hemorrhage with the use of milrinone in PPHN 79. Milrinone is still under investigation for routine use in PPHN 21. Milrinone may be the pulmonary vasodilator of choice in the presence of PPHN with left ventricular dysfunction.
Sildenafil
Sildenafil is a phosphodiesterase 5 (PDE-5) inhibitor that inhibits phosphodiesterase type 5 (PDE-5), increasing cGMP, leading to enhanced vasodilation in pulmonary vessels 80. Though not yet approved by the FDA for the treatment of persistent fetal circulation, clinical data suggest beneficial effects of sildenafil in infants who did not respond to inhaled nitric oxide (iNO) 4. Sildenafil is used as an add-on or in cases unresponsive to inhaled nitric oxide (iNO). Sildenafil is usually administered orally since the intravenous form is rarely available. Studies showed improvement in oxygenation with oral and intravenous forms of sildenafil 81, 82. Sildenafil has been shown to improve oxygenation and decrease ECMO need in PPHN 21. Ongoing clinical trials are evaluating long-term efficacy.
Risk of systemic hypotension, and the long-term safety in neonates remains unclear 21. Gastrointestinal disturbances have been reported 21.
Bosentan (Endothelin-1 receptor blocker)
Endothelin receptor antagonists, such as bosentan, which block endothelin-1-mediated vasoconstriction, are also under investigation, though clinical trial results have been mixed. Endothelin receptor antagonists are beneficial and well tolerated in adult patients with pulmonary arterial hypertension 83. Initial reports suggested that bosentan was an effective drug in the management of PPHN 84. The results of a multi-center, randomized, double-blind, placebo-controlled exploratory trial of bosentan in PPHN was recently reported. Bosentan (2 mg/kg/dose BID) did not show any additive effect on top of iNO in term neonates with PPHN 85. However, endothelin receptor antagonists may have a role in the management of chronic pulmonary hypertension associated with bronchopulmonary dysplasia or congenital diaphragmatic hernia.
Surfactant Therapy
Exogenous surfactant therapy improved oxygenation and reduced the need for extracorporeal membrane oxygenation (ECMO) in neonates with PPHN secondary to parenchymal lung disease such as respiratory distress syndrome (RDS), pneumonia/sepsis or meconium aspiration syndrome 5, 86. This multicenter trial 87 also demonstrated that this benefit was greatest for infants with mild to moderate disease, and with an Oxygenation Index (OI) of 15–25. A post-hoc analysis of the randomized trial of early nitric oxide use showed that early use of surfactant prior to randomization decreased the risk of death/ECMO especially in infants with parenchymal lung disease 88. Over the past decade, the use of surfactant in treating secondary PPHN and respiratory failure has increased and might have contributed to improved effectiveness of iNO with reduced need for ECMO. Surfactant inactivation and deficiency are observed in many neonatal respiratory disorders such as pneumonia, respiratory distress syndrome (RDS) and meconium aspiration syndrome. Some experts recommend that infants with PPHN secondary to parenchymal lung disease receive a dose of surfactant rich in surfactant protein-B (SP-B), such as calfactant – Infasurf® (ONY Inc, Amherst NY) or poractant-α – Curosurf® (Chiesi Farmaceutici, S.p.A, Parma, Italy) 5. Synthetic surfactant (lucinactant, Surfaxin, Discovery laboratories, Inc. Warrington PA) rich in surfactant protein-B (SP-B) mimetics has been shown to be resistant to inactivation and effective in animal studies 89, 90 and appears to be safe in children with hypoxemic respiratory failure 91 but its efficacy in term infants with hypoxic respiratory failure is not known.
It is not clear if surfactant therapy is beneficial in infants with congenital diaphragmatic hernia. Animal studies show benefit 92, 93, 94 but a review of the congenital diaphragmatic hernia registry did not support the use of surfactant 95. Some experts recommend administration of surfactant only in the presence of clinical, radiological or biochemical evidence of surfactant deficiency in congenital diaphragmatic hernia and administer only 50% of the dose because of pulmonary hypoplasia 5.
Prostaglandin E1 (PGE1)
Aerosolized prostaglandin E1 (Alprostadil) has been used to treat pulmonary hypertension in adults. In a small pilot phase I-II study, Sood et al 96 suggested that inhaled prostaglandin E1 (PGE1) was a safe and selective pulmonary vasodilator in hypoxemic respiratory failure with or without use of iNO. PGE1 solution for aerosolization is prepared from Alprostadil® (Prostin VR 500, Pfizer, New York NY) and administered as a continuous nebulization through a MiniHeart low flow jet nebulizer (WestMed Inc, Tuczon, AZ) at 150–300 ng/kg/min diluted in saline to provide 4 ml/hr 97.
Intravenous PGE1 has also been used in patients with congenital diaphragmatic hernia in combination with iNO to promote pulmonary vasodilation and to maintain ductal patency and reduce right ventricular afterload 98.
Inhaled Prostacyclin (PGI2)
Prostacyclin administered intravenously is a common therapy in adults with pulmonary arterial hypertension. Inhaled Prostacyclin (PGI2) has been used in PPHN resistant to iNO at a dose of 50 ng/kg/min 99. The intravenous formulation Flolan° (Glaxo-Wellcome, Middlesex, UK) is dissolved in 20 ml of manufacturer’s diluent (a glycine buffer, pH −10). Fresh solution is added to the nebulization chamber every 4 hours 99. The effect of such alkaline pH on neonatal respiratory tract is not known.
Corticosteroids
Antenatal betamethasone attenuated oxidative stress and improved in vitro response to vasodilators in a fetal lamb model of pulmonary hypertension 100. Glucocorticoids have been found to improve oxygenation and attenuate the pulmonary hypertensive response in animal models of meconium aspiration syndrome, which is a common cause of PPHN 101. Steroids reduces inflammation and oxidative stress that contribute to pulmonary vascular remodeling and hypoxemia 75. Steroids have been reported to decrease hospital stay and duration of oxygen use in infants with meconium aspiration 102, 103. Steroids may be used as adjunct therapy in specific cases of inflammation-related PPHN. Risk of immunosuppression, impaired growth, and neurodevelopmental issues, especially with prolonged use.
Oxygen therapy and mechanical ventilation (breathing machine)
The mainstay of treatment is improving oxygenation by optimizing mechanical ventilatory support. It is likely that doctors will pass a breathing tube into the baby’s airway through their mouth or nose, which is connected to a breathing machine or ventilator. This will breathe for the baby while they are unwell. The machine reduces the amount of effort and energy needed to breathe, which in turn reduces the amount of oxygen that the baby needs and it also delivers the maximum possible amount of oxygen into the lungs. When the baby is connected to the breathing machine, doctors will give them some medicines to make them sleepy so that they do not feel any discomfort as well as some medicines to stop them from moving. As the breathing tube passes into their airway, the baby will not be able to cry or make noises while they are on the machine. Though oxygen is a potent vasodilator, fraction of inspired oxygen (FiO2, the concentration of oxygen in the gas mixture a person inhale) over 50% is rarely beneficial 104.
Achieving adequate lung volume improves oxygenation and pulmonary vascular resistance in newborns with parenchymal lung disease. A conventional or high-frequency ventilator can be used to correct any ventilation-perfusion mismatch 105.
High-frequency oscillatory ventilation (HFOV)
This is another type of breathing machine that doctors use to help deliver oxygen into the lungs of babies with persistent pulmonary hypertension of the newborn. This machine pushes air in and out of the lungs very quickly, so it is very noisy. In newborns with severe lung disease, high frequency ventilation is frequently used to optimize lung inflation and minimize lung injury 106.
In clinical studies using inhaled nitric oxide (iNO), the combination of high frequency ventilation and inhaled nitric oxide (iNO) resulted in the greatest improvement in oxygenation in PPHN associated with diffuse parenchymal lung disease such as respiratory distress syndrome and pneumonia 107, 108.
Inotropes
These are medicines that are given directly into the bloodstream via a drip to help keep the baby’s blood pressure high, as this helps the heart to pump blood into the lungs. Dopamine and milrinone are commonly used medications. Dopamine is a non-selective vasoconstrictor and should be used with caution in higher doses 109. Increasing systemic blood pressure helps reduce the extra-cardiac shunting across the patent ductus arteriosus, although the ideal blood pressure values are not known yet.
Extra-corporeal membrane oxygenation (ECMO)
If doctors have tried all of the treatments described above, and the baby’s oxygen saturations are still low, they may consider a treatment called extracorporeal membrane oxygenation (ECMO), which is like a heart and lung bypass machine during heart surgery. Extra-corporeal membrane oxygenation (ECMO) is life-saving but is invasive, labor intensive, and associated with severe complications such as intracranial hemorrhage 110. If ECMO support is anticipated, coagulation studies and a head ultrasound should be done before cannulation. ECMO takes over the work of the heart and lungs, putting oxygen directly into a baby’s blood rather than relying on the lungs to add oxygen. For ECMO treatment a baby will be moved to the Cardiac Intensive Care Unit.
In severe respiratory failure, ECMO use was associated with improved survival 111. The results of ECMO use in persistent pulmonary hypertension depend on the underlying cause. The best outcomes in survival are seen in infants with meconium aspiration syndrome (MAS). ECMO use in other conditions such as trisomy 21 or congenital diaphragmatic hernia had post-ECMO morbidity or a low survival rate of one year 112. Very few infants survived with long-term complications (12%) such as long-term physical and neurodevelopmental morbidity 112.
Though ECMO use in persistent fetal circulation drastically decreased since improved ventilation strategies and the introduction of iNO, it is still an effective rescue therapy. ECMO use remains controversial in the sickest babies with congenital diaphragmatic hernia 112.
Moving forward and discharge from intensive care
Once the baby’s oxygen saturation levels are normal, the doctors and nurses will start to reduce the amount of nitric oxide delivered though the breathing machine, until it is turned off altogether. They will then gradually reduce the amount of oxygen used, and the amount of breathing work being done by the ventilator. You may hear them referring to this as ‘weaning’. During this time the doctors will reduce the medicine that keeps the baby still and sleepy so that they will become more awake and aware of their surroundings.
At the same time, the doctors will gradually reduce the inotropes (medicines being given to raise blood pressure) and might consider stopping antibiotics if a full course has been given. At this time the nurses may use a feeding tube, passed into one of the baby’s nostrils and down the throat to the stomach, to start some milk feeds. These can be expressed breast milk or formula, depending on the parents’ preference.
Once the baby is doing most of the work of breathing themselves, with only a small amount of help from the breathing machine, doctors will try to take the baby off the breathing machine altogether. Once this has happened, it is likely that they will be ready to go back to their local hospital, where the baby will finish any antibiotics that are still needed, continue oxygen treatment until it is no longer needed, and help parents to establish feeding before they are discharged home.
Pulmonary hypertension in newborns prognosis
Persistent pulmonary hypertension of the newborn prognosis (outlook) are dependent on the underlying cause. Surviving infants with persistent pulmonary hypertension have long-term neurodevelopmental, cognitive, and hearing problems 113, 114, 115. Approximately one in four infants with persistent pulmonary hypertension have neurodevelopmental delays and hearing impairment 116, 4. The risk is higher in infants with underlying conditions such as congenital diaphragmatic hernia or genetic disorders 4. In addition to the severity and cause of persistent pulmonary hypertension in newborns, small for gestational age and Hispanic ethnicity are independent risk factors for higher mortality and morbidity in the first year after discharge 117.
Infants with mild persistent pulmonary hypertension of the newborn (PPHN) are at higher risk for hospital readmission than fullterm infants with no persistent pulmonary hypertension of the newborn 4.
A 2019 report of a retrospective California population-based study (2005-2012) found a large postdischarge morbidity burden of infants with PPHN in their first year of life, including those with mild PPHN and causes involving pulmonary vascular changes that have been believed to be of short duration and recoverable 117. The investigators also identified the following as risk factors for postdischarge mortality and morbidity in the first year of life of newborns with PPHN 117:
- Hispanic ethnicity
- Small for gestational age
- Severe PPHN
- PPHN cause (eg, congenital diaphragmatic hernia, meconium aspiration syndrome).
- Sankaran D, Lakshminrusimha S. Pulmonary hypertension in the newborn- etiology and pathogenesis. Semin Fetal Neonatal Med. 2022 Aug;27(4):101381. doi: 10.1016/j.siny.2022.101381[↩][↩]
- Mandell E, Kinsella JP, Abman SH. Persistent pulmonary hypertension of the newborn. Pediatr Pulmonol. 2021 Mar;56(3):661-669. doi: 10.1002/ppul.25073[↩][↩][↩]
- Lakshminrusimha S, Keszler M. Persistent Pulmonary Hypertension of the Newborn. Neoreviews. 2015 Dec;16(12):e680-e692. doi: 10.1542/neo.16-12-e680[↩][↩][↩][↩][↩][↩][↩]
- Nandula PS, Shah SD. Persistent Pulmonary Hypertension of the Newborn. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK585100[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Sharma, V., Berkelhamer, S. & Lakshminrusimha, S. Persistent pulmonary hypertension of the newborn. matern health, neonatol and perinatol 1, 14 (2015). https://doi.org/10.1186/s40748-015-0015-4[↩][↩][↩][↩][↩][↩][↩][↩]
- Puthiyachirakkal M, Mhanna MJ. Pathophysiology, management, and outcome of persistent pulmonary hypertension of the newborn: a clinical review. Front Pediatr. 2013 Sep 2;1:23. doi: 10.3389/fped.2013.00023[↩]
- Lakshminrusimha, S., Konduri, G. & Steinhorn, R. Considerations in the management of hypoxemic respiratory failure and persistent pulmonary hypertension in term and late preterm neonates. J Perinatol 36 (Suppl 2), S12–S19 (2016). https://doi.org/10.1038/jp.2016.44[↩]
- Persistent Pulmonary Hypertension of the Newborn (PPHN). https://emedicine.medscape.com/article/898437-overview[↩]
- Dawes GS. Pulmonary circulation in the foetus and new-born. Br Med Bull. 1966;22(1):61–65. doi: 10.1093/oxfordjournals.bmb.a070439[↩][↩]
- Rasanen J, Wood DC, Weiner S, Ludomirski A, Huhta JC. Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation. 1996;94(5):1068–1073. doi: 10.1161/01.cir.94.5.1068[↩][↩]
- Lakshminrusimha S, Steinhorn RH. 155 – pathophysiology of persistent pulmonary hypertension of the newborn. In: Polin RA, Abman SH, Rowitch DH, Benitz WE, Fox WW, editors. Fetal and neonatal physiology. fifth ed. Elsevier; 2017. 1576–1588.e4.[↩]
- Smolich JJ, Kenna KR. Divergent effects of initial ventilation with delayed cord clamping on systemic and pulmonary arterial flows in the birth transition of preterm lambs. J Physiol. 2022 Aug;600(15):3585-3601. doi: 10.1113/JP282934[↩]
- Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, te Pas AB, Morley CJ, Polglase GR, Hooper SB. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol. 2013 Apr 15;591(8):2113-26. doi: 10.1113/jphysiol.2012.250084[↩]
- Lakshminrusimha S, Steinhorn RH. Pulmonary vascular biology during neonatal transition. Clin Perinatol. 1999 Sep;26(3):601-19. https://doi.org/10.1016/S0095-5108(18)30039-3[↩][↩][↩]
- Walsh-Sukys MC, Tyson JE, Wright LL, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105(1, pt 1):14–20. doi: 10.1542/peds.105.1.14[↩]
- Bendapudi P, Rao GG, Greenough A. Diagnosis and management of persistent pulmonary hypertension of the newborn. Paediatr Respir Rev. 2015;16(3):157–161. doi: 10.1016/j.prrv.2015.02.001[↩]
- Cabral JE, Belik J. Persistent pulmonary hypertension of the newborn: recent advances in pathophysiology and treatment. J Pediatr (Rio J). 2013 May-Jun;89(3):226-42. doi: 10.1016/j.jped.2012.11.009[↩]
- Reynolds EW, Ellington JG, Vranicar M, Bada HS. Brain-type natriuretic peptide in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatrics. 2004 Nov;114(5):1297-304. doi: 10.1542/peds.2004-0525. Erratum in: Pediatrics. 2005 May;115(5):1454.[↩][↩]
- Bendapudi P, Rao GG, Greenough A. Diagnosis and management of persistent pulmonary hypertension of the newborn. Paediatr Respir Rev. 2015 Jun;16(3):157-61. doi: 10.1016/j.prrv.2015.02.001[↩][↩][↩][↩]
- Mathew B, Lakshminrusimha S. Persistent Pulmonary Hypertension in the Newborn. Children (Basel). 2017 Jul 28;4(8):63. doi: 10.3390/children4080063[↩][↩]
- Kaplish D, Vagha JD, Rathod S, Jain A. Current Pharmaceutical Strategies in the Management of Persistent Pulmonary Hypertension of the Newborn (PPHN): A Comprehensive Review of Therapeutic Agents. Cureus. 2024 Sep 27;16(9):e70307. doi: 10.7759/cureus.70307[↩][↩][↩][↩][↩][↩][↩][↩]
- Hernández-Díaz S, Van Marter LJ, Werler MM, Louik C, Mitchell AA. Risk factors for persistent pulmonary hypertension of the newborn. Pediatrics. 2007 Aug;120(2):e272-82. doi: 10.1542/peds.2006-3037[↩]
- Kumar VH, Hutchison AA, Lakshminrusimha S, Morin FC 3rd, Wynn RJ, Ryan RM. Characteristics of pulmonary hypertension in preterm neonates. J Perinatol. 2007 Apr;27(4):214-9. doi: 10.1038/sj.jp.7211673[↩][↩]
- Huybrechts KF, Bateman BT, Palmsten K, Desai RJ, Patorno E, Gopalakrishnan C, Levin R, Mogun H, Hernandez-Diaz S. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015 Jun 2;313(21):2142-51. doi: 10.1001/jama.2015.5605[↩]
- Alano MA, Ngougmna E, Ostrea EM Jr, Konduri GG. Analysis of nonsteroidal antiinflammatory drugs in meconium and its relation to persistent pulmonary hypertension of the newborn. Pediatrics. 2001 Mar;107(3):519-23. doi: 10.1542/peds.107.3.519[↩]
- Weijerman ME, van Furth AM, van der Mooren MD, van Weissenbruch MM, Rammeloo L, Broers CJ, Gemke RJ. Prevalence of congenital heart defects and persistent pulmonary hypertension of the neonate with Down syndrome. Eur J Pediatr. 2010 Oct;169(10):1195-9. doi: 10.1007/s00431-010-1200-0[↩]
- Fuloria M, Aschner JL. Persistent pulmonary hypertension of the newborn. Semin Fetal Neonatal Med. 2017 Aug;22(4):220-226. doi: 10.1016/j.siny.2017.03.004[↩]
- Farrow KN, Lee KJ, Perez M, Schriewer JM, Wedgwood S, Lakshminrusimha S, Smith CL, Steinhorn RH, Schumacker PT. Brief hyperoxia increases mitochondrial oxidation and increases phosphodiesterase 5 activity in fetal pulmonary artery smooth muscle cells. Antioxid Redox Signal. 2012 Aug 1;17(3):460-70. doi: 10.1089/ars.2011.4184[↩]
- Gaston BM, Carver J, Doctor A, Palmer LA. S-nitrosylation signaling in cell biology. Mol Interv. 2003 Aug;3(5):253-63. doi: 10.1124/mi.3.5.253[↩]
- Mata-Greenwood E, Jenkins C, Farrow KN, Konduri GG, Russell JA, Lakshminrusimha S, Black SM, Steinhorn RH. eNOS function is developmentally regulated: uncoupling of eNOS occurs postnatally. Am J Physiol Lung Cell Mol Physiol. 2006 Feb;290(2):L232-41. doi: 10.1152/ajplung.00393.2004[↩]
- Singh Y, Lakshminrusimha S. Pathophysiology and Management of Persistent Pulmonary Hypertension of the Newborn. Clin Perinatol. 2021 Aug;48(3):595-618. doi: 10.1016/j.clp.2021.05.009[↩][↩]
- Robin H Steinhorn, Kathryn N Farrow; Pulmonary Hypertension in the Neonate. Neoreviews January 2007; 8 (1): e14–e21. https://doi.org/10.1542/neo.8-1-e14[↩]
- Holcomb RG, Tyson RW, Ivy DD, Abman SH, Kinsella JP. Congenital pulmonary venous stenosis presenting as persistent pulmonary hypertension of the newborn. Pediatr Pulmonol. 1999 Oct;28(4):301-6. doi: 10.1002/(sici)1099-0496(199910)28:4<301::aid-ppul10>3.0.co;2-m[↩]
- Swier NL, Richards B, Cua CL, Lynch SK, Yin H, Nelin LD, Smith CV, Backes CH. Pulmonary Vein Stenosis in Neonates with Severe Bronchopulmonary Dysplasia. Am J Perinatol. 2016 Jun;33(7):671-7. doi: 10.1055/s-0035-1571201[↩]
- Lakshminrusimha S., Wynn R.J., Youssfi M., Pabalan M.J., Bommaraju M., Kirmani K., Carrion V. Use of ct angiography in the diagnosis of total anomalous venous return. J. Perinatol. 2009;29:458–461. doi: 10.1038/jp.2008.240[↩]
- Baird J.S., Havalad V., Aponte-Patel L., Ravindranath T.M., October T.W., Starc T.J., Smerling A.J. Nitric oxide-associated pulmonary edema in children with pulmonary venous hypertension. Pediatr. Cardiol. 2012;34:817–825. doi: 10.1007/s00246-012-0538-7[↩]
- Yoder BA, Kirsch EA, Barth WH, Gordon MC. Changing obstetric practices associated with decreasing incidence of meconium aspiration syndrome. Obstet Gynecol. 2002 May;99(5 Pt 1):731-9. doi: 10.1016/s0029-7844(02)01942-7[↩]
- Murphy JD, Vawter GF, Reid LM. Pulmonary vascular disease in fatal meconium aspiration. J Pediatr. 1984 May;104(5):758-62. doi: 10.1016/s0022-3476(84)80962-2[↩]
- Villanueva ME, Zaher FM, Svinarich DM, Konduri GG. Decreased gene expression of endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr Res. 1998 Sep;44(3):338-43. doi: 10.1203/00006450-199809000-00012[↩]
- Soukka H, Jalonen J, Kero P, Kääpä P. Endothelin-1, atrial natriuretic peptide and pathophysiology of pulmonary hypertension in porcine meconium aspiration. Acta Paediatr. 1998 Apr;87(4):424-8. doi: 10.1080/08035259850157039[↩]
- Velaphi S, Van Kwawegen A. Meconium aspiration syndrome requiring assisted ventilation: perspective in a setting with limited resources. J Perinatol. 2008 Dec;28 Suppl 3:S36-42. doi: 10.1038/jp.2008.155[↩]
- Fraser WD, Hofmeyr J, Lede R, Faron G, Alexander S, Goffinet F, Ohlsson A, Goulet C, Turcot-Lemay L, Prendiville W, Marcoux S, Laperrière L, Roy C, Petrou S, Xu HR, Wei B; Amnioinfusion Trial Group. Amnioinfusion for the prevention of the meconium aspiration syndrome. N Engl J Med. 2005 Sep 1;353(9):909-17. doi: 10.1056/NEJMoa050223[↩]
- Vain NE, Szyld EG, Prudent LM, Wiswell TE, Aguilar AM, Vivas NI. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet. 2004 Aug 14-20;364(9434):597-602. doi: 10.1016/S0140-6736(04)16852-9[↩]
- Wiswell TE, Gannon CM, Jacob J, Goldsmith L, Szyld E, Weiss K, Schutzman D, Cleary GM, Filipov P, Kurlat I, Caballero CL, Abassi S, Sprague D, Oltorf C, Padula M. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics. 2000 Jan;105(1 Pt 1):1-7. doi: 10.1542/peds.105.1.1[↩]
- Kattwinkel J, Perlman JM, Aziz K, Colby C, Fairchild K, Gallagher J, Hazinski MF, Halamek LP, Kumar P, Little G, McGowan JE, Nightengale B, Ramirez MM, Ringer S, Simon WM, Weiner GM, Wyckoff M, Zaichkin J. Part 15: neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010 Nov 2;122(18 Suppl 3):S909-19. doi: 10.1161/CIRCULATIONAHA.110.971119. Erratum in: Circulation. 2011 Oct 11;124(15):e406.[↩]
- Lakshminrusimha S, Mathew B, Nair J, Gugino SF, Koenigsknecht C, Rawat M, Nielsen L, Swartz DD. Tracheal suctioning improves gas exchange but not hemodynamics in asphyxiated lambs with meconium aspiration. Pediatr Res. 2015 Feb;77(2):347-55. doi: 10.1038/pr.2014.186[↩]
- Kumar A, Kumar P, Basu S. Endotracheal suctioning for prevention of meconium aspiration syndrome: a randomized controlled trial. Eur J Pediatr. 2019 Dec;178(12):1825-1832. doi: 10.1007/s00431-019-03463-z[↩]
- Sehgal A, Athikarisamy SE, Adamopoulos M. Global myocardial function is compromised in infants with pulmonary hypertension. Acta Paediatr. 2012 Apr;101(4):410-3. doi: 10.1111/j.1651-2227.2011.02572.x[↩]
- Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, Perritt R, Higgins RD, Oh W, Hudak ML, Laptook AR, Shankaran S, Finer NN, Carlo WA, Kennedy KA, Fridriksson JH, Steinhorn RH, Sokol GM, Konduri GG, Aschner JL, Stoll BJ, D’Angio CT, Stevenson DK; Preemie Inhaled Nitric Oxide Study. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med. 2005 Jul 7;353(1):13-22. doi: 10.1056/NEJMoa043927[↩]
- Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, Mestan KK. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. 2013 Jul;33(7):553-7. doi: 10.1038/jp.2012.164[↩]
- Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr Opin Pediatr. 2013 Jun;25(3):329-37. doi: 10.1097/MOP.0b013e328360a3f6[↩]
- Steinhorn RH, Morin FC 3rd, Russell JA. The adventitia may be a barrier specific to nitric oxide in rabbit pulmonary artery. J Clin Invest. 1994 Nov;94(5):1883-8. https://pmc.ncbi.nlm.nih.gov/articles/instance/294594/pdf/jcinvest00036-0181.pdf[↩]
- Levin DL, Mills LJ, Parkey M, Garriott J, Campbell W. Constriction of the fetal ductus arteriosus after administration of indomethacin to the pregnant ewe. J Pediatr. 1979 Apr;94(4):647-50. doi: 10.1016/s0022-3476(79)80043-8[↩]
- Wild LM, Nickerson PA, Morin FC 3rd. Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res. 1989 Mar;25(3):251-7. doi: 10.1203/00006450-198903000-00006[↩]
- Skari H, Bjornland K, Haugen G, Egeland T, Emblem R. Congenital diaphragmatic hernia: a meta-analysis of mortality factors. J Pediatr Surg. 2000 Aug;35(8):1187-97. doi: 10.1053/jpsu.2000.8725[↩]
- Schachtner SK, Wang Y, Scott Baldwin H. Qualitative and quantitative analysis of embryonic pulmonary vessel formation. Am J Respir Cell Mol Biol. 2000 Feb;22(2):157-65. doi: 10.1165/ajrcmb.22.2.3766[↩]
- Semidotskaia ZhD, Bil’chenko OS, Andon’eva NM, Geletko MO. O sochetanii mukopolisakharidoza s pervichnoĭ legochnoĭ gipertonieĭ i gipoplazieĭ pochek [Association of mucopolysaccharidosis with primary pulmonary hypertension and renal hypoplasia]. Klin Med (Mosk). 1985 Jun;63(6):126-7. Russian.[↩]
- Günthard J, Fliegel C, Ohnacker H, Rutishauser M, Bühler E. Lung hypoplasia and severe pulmonary hypertension in an infant with double heterozygosity for spondyloepiphyseal dysplasia congenita and achondroplasia. Clin Genet. 1995 Jul;48(1):35-40. doi: 10.1111/j.1399-0004.1995.tb04051.x[↩]
- Bizzarro MJ, Copel JA, Pearson HA, Pober B, Bhandari V. Pulmonary hypoplasia and persistent pulmonary hypertension in the newborn with homozygous alpha-thalassemia: a case report and review of the literature. J Matern Fetal Neonatal Med. 2003 Dec;14(6):411-6. doi: 10.1080/14767050412331312280[↩]
- Chock VY, Van Meurs KP, Hintz SR, Ehrenkranz RA, Lemons JA, Kendrick DE, Stevenson DK; NICHD Neonatal Research Network. Inhaled nitric oxide for preterm premature rupture of membranes, oligohydramnios, and pulmonary hypoplasia. Am J Perinatol. 2009 Apr;26(4):317-22. doi: 10.1055/s-0028-1104743[↩]
- Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, Brody AS, Nogee LM, Trapnell BC, Langston C; Pathology Cooperative Group; Albright EA, Askin FB, Baker P, Chou PM, Cool CM, Coventry SC, Cutz E, Davis MM, Dishop MK, Galambos C, Patterson K, Travis WD, Wert SE, White FV; ChILD Research Co-operative. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007 Dec 1;176(11):1120-8. doi: 10.1164/rccm.200703-393OC[↩]
- Ahmed S, Ackerman V, Faught P, Langston C. Profound hypoxemia and pulmonary hypertension in a 7-month-old infant: late presentation of alveolar capillary dysplasia. Pediatr Crit Care Med. 2008 Nov;9(6):e43-6. doi: 10.1097/PCC.0b013e31818e383e[↩]
- Stankiewicz P, Sen P, Bhatt SS, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009 Jun;84(6):780-91. doi: 10.1016/j.ajhg.2009.05.005. Epub 2009 Jun 4. Erratum in: Am J Hum Genet. 2009 Oct;85(4):537. multiple author names added.[↩]
- Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, Partridge JC, Rogers EE, Keller RL. Persistent Pulmonary Hypertension of the Newborn in Late Preterm and Term Infants in California. Pediatrics. 2017 Jan;139(1):e20161165. doi: 10.1542/peds.2016-1165[↩]
- Reuter S, Moser C, Baack M. Respiratory distress in the newborn. Pediatr Rev. 2014 Oct;35(10):417-28; quiz 429. doi: 10.1542/pir.35-10-417[↩]
- Mean Airway Pressure. https://www.sciencedirect.com/topics/medicine-and-dentistry/mean-airway-pressure[↩]
- Sharma S, Hashmi MF, Hendrix JM, et al. Alveolar Gas Equation. [Updated 2024 Sep 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482268[↩]
- Peterson AL, Deatsman S, Frommelt MA, Mussatto K, Frommelt PC. Correlation of echocardiographic markers and therapy in persistent pulmonary hypertension of the newborn. Pediatr Cardiol. 2009 Feb;30(2):160-5. doi: 10.1007/s00246-008-9303-3[↩]
- Lai MY, Chu SM, Lakshminrusimha S, Lin HC. Beyond the inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Pediatr Neonatol. 2018 Feb;59(1):15-23. doi: 10.1016/j.pedneo.2016.09.011[↩]
- Witek J, Lakhkar AD. Nitric Oxide. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554485[↩]
- Roberts JD Jr, Fineman JR, Morin FC 3rd, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, Heymann MA, Zapol WM. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med. 1997 Feb 27;336(9):605-10. doi: 10.1056/NEJM199702273360902[↩]
- Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000 Feb 17;342(7):469-74. doi: 10.1056/NEJM200002173420704[↩]
- Konduri GG, Solimano A, Sokol GM, Singer J, Ehrenkranz RA, Singhal N, Wright LL, Van Meurs K, Stork E, Kirpalani H, Peliowski A; Neonatal Inhaled Nitric Oxide Study Group. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics. 2004 Mar;113(3 Pt 1):559-64. doi: 10.1542/peds.113.3.559[↩]
- Jain A, McNamara PJ. Persistent pulmonary hypertension of the newborn: Advances in diagnosis and treatment. Semin Fetal Neonatal Med. 2015 Aug;20(4):262-71. doi: 10.1016/j.siny.2015.03.001[↩]
- Extra corporeal membrane oxygenation (ecmo) review of a lifesaving technology. Makdisi G, Wang IW. J Thorac Dis. 2015;7:0–76. doi: 10.3978/j.issn.2072-1439.2015.07.17[↩][↩]
- McNamara PJ, Shivananda SP, Sahni M, Freeman D, Taddio A. Pharmacology of milrinone in neonates with persistent pulmonary hypertension of the newborn and suboptimal response to inhaled nitric oxide. Pediatr Crit Care Med. 2013 Jan;14(1):74-84. doi: 10.1097/PCC.0b013e31824ea2cd[↩]
- James AT, Bee C, Corcoran JD, McNamara PJ, Franklin O, El-Khuffash AF. Treatment of premature infants with pulmonary hypertension and right ventricular dysfunction with milrinone: a case series. J Perinatol. 2015 Apr;35(4):268-73. doi: 10.1038/jp.2014.208[↩]
- James AT, Corcoran JD, McNamara PJ, Franklin O, El-Khuffash AF. The effect of milrinone on right and left ventricular function when used as a rescue therapy for term infants with pulmonary hypertension. Cardiol Young. 2016 Jan;26(1):90-9. doi: 10.1017/S1047951114002698[↩]
- Bassler D, Choong K, McNamara P, Kirpalani H. Neonatal persistent pulmonary hypertension treated with milrinone: four case reports. Biol Neonate. 2006;89(1):1-5. doi: 10.1159/000088192[↩]
- Endothelin receptor antagonists for pulmonary arterial hypertension. Liu C, Chen J, Gao Y, Deng B, Liu K. Cochrane Database Syst Rev. 2013;2013:0. doi: 10.1002/14651858.CD004434.pub5[↩]
- Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006 Apr;117(4):1077-83. doi: 10.1542/peds.2005-0523[↩]
- Steinhorn RH, Kinsella JP, Pierce C, Butrous G, Dilleen M, Oakes M, Wessel DL. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr. 2009 Dec;155(6):841-847.e1. doi: 10.1016/j.jpeds.2009.06.012[↩]
- Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002 Mar 21;346(12):896-903. doi: 10.1056/NEJMoa012212. Erratum in: N Engl J Med 2002 Apr 18;346(16):1258.[↩]
- Mohamed WA, Ismail M. A randomized, double-blind, placebo-controlled, prospective study of bosentan for the treatment of persistent pulmonary hypertension of the newborn. J Perinatol. 2012 Aug;32(8):608-13. doi: 10.1038/jp.2011.157[↩]
- Steinhorn RH, Fineman J, Kusic-Pajic A, Cornelisse P, Gehin M, Nowbakht P, Pierce CM, Beghetti M; FUTURE-4 study investigators. Bosentan as Adjunctive Therapy for Persistent Pulmonary Hypertension of the Newborn: Results of the Randomized Multicenter Placebo-Controlled Exploratory Trial. J Pediatr. 2016 Oct;177:90-96.e3. doi: 10.1016/j.jpeds.2016.06.078[↩]
- Khawar H, Marwaha K. Surfactant. [Updated 2023 Jun 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546600[↩]
- Lotze A, Mitchell BR, Bulas DI, Zola EM, Shalwitz RA, Gunkel JH. Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. Survanta in Term Infants Study Group. J Pediatr. 1998 Jan;132(1):40-7. doi: 10.1016/s0022-3476(98)70482-2[↩]
- Konduri GG, Sokol GM, Van Meurs KP, Singer J, Ambalavanan N, Lee T, Solimano A. Impact of early surfactant and inhaled nitric oxide therapies on outcomes in term/late preterm neonates with moderate hypoxic respiratory failure. J Perinatol. 2013 Dec;33(12):944-9. doi: 10.1038/jp.2013.83[↩]
- Wolfson MR, Wu J, Hubert TL, Gregory TJ, Mazela J, Shaffer TH. Lucinactant attenuates pulmonary inflammatory response, preserves lung structure, and improves physiologic outcomes in a preterm lamb model of RDS. Pediatr Res. 2012 Oct;72(4):375-83. doi: 10.1038/pr.2012.96[↩]
- Salvesen B, Curstedt T, Mollnes TE, Saugstad OD. Effects of Natural versus Synthetic Surfactant with SP-B and SP-C Analogs in a Porcine Model of Meconium Aspiration Syndrome. Neonatology. 2014;105(2):128-35. doi: 10.1159/000356065[↩]
- Thomas NJ, Guardia CG, Moya FR, Cheifetz IM, Markovitz B, Cruces P, Barton P, Segal R, Simmons P, Randolph AG; PALISI Network. A pilot, randomized, controlled clinical trial of lucinactant, a peptide-containing synthetic surfactant, in infants with acute hypoxemic respiratory failure. Pediatr Crit Care Med. 2012 Nov;13(6):646-53. doi: 10.1097/PCC.0b013e3182517bec.[↩]
- Davey MG, Biard JM, Robinson L, Tsai J, Schwarz U, Danzer E, Adzick NS, Flake AW, Hedrick HL. Surfactant protein expression is increased in the ipsilateral but not contralateral lungs of fetal sheep with left-sided diaphragmatic hernia. Pediatr Pulmonol. 2005 Apr;39(4):359-67. doi: 10.1002/ppul.20175[↩]
- Davey MG, Danzer E, Schwarz U, Adzick NS, Flake AW, Hedrick HL. Prenatal glucocorticoids and exogenous surfactant therapy improve respiratory function in lambs with severe diaphragmatic hernia following fetal tracheal occlusion. Pediatr Res. 2006 Aug;60(2):131-5. doi: 10.1203/01.pdr.0000227509.94069.ae[↩]
- O’Toole SJ, Karamanoukian HL, Morin FC 3rd, Holm BA, Egan EA, Azizkhan RG, Glick PL. Surfactant decreases pulmonary vascular resistance and increases pulmonary blood flow in the fetal lamb model of congenital diaphragmatic hernia. J Pediatr Surg. 1996 Apr;31(4):507-11. doi: 10.1016/s0022-3468(96)90484-4[↩]
- Van Meurs K; Congenital Diaphragmatic Hernia Study Group. Is surfactant therapy beneficial in the treatment of the term newborn infant with congenital diaphragmatic hernia? J Pediatr. 2004 Sep;145(3):312-6. doi: 10.1016/j.jpeds.2004.04.056[↩]
- Sood BG, Delaney-Black V, Aranda JV, Shankaran S. Aerosolized PGE1: a selective pulmonary vasodilator in neonatal hypoxemic respiratory failure results of a Phase I/II open label clinical trial. Pediatr Res. 2004 Oct;56(4):579-85. doi: 10.1203/01.PDR.0000139927.86617.B6[↩]
- Sood BG, Keszler M, Garg M, Klein JM, Ohls R, Ambalavanan N, Cotten CM, Malian M, Sanchez PJ, Lakshminrusimha S, Nelin LD, Van Meurs KP, Bara R, Saha S, Das A, Wallace D, Higgins RD, Shankaran S; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Inhaled PGE1 in neonates with hypoxemic respiratory failure: two pilot feasibility randomized clinical trials. Trials. 2014 Dec 12;15:486. doi: 10.1186/1745-6215-15-486[↩]
- Shiyanagi S, Okazaki T, Shoji H, Shimizu T, Tanaka T, Takeda S, Kawashima K, Lane GJ, Yamataka A. Management of pulmonary hypertension in congenital diaphragmatic hernia: nitric oxide with prostaglandin-E1 versus nitric oxide alone. Pediatr Surg Int. 2008 Oct;24(10):1101-4. doi: 10.1007/s00383-008-2225-6[↩]
- Kelly LK, Porta NF, Goodman DM, Carroll CL, Steinhorn RH. Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. J Pediatr. 2002 Dec;141(6):830-2. doi: 10.1067/mpd.2002.129849[↩][↩]
- Chandrasekar I, Eis A, Konduri GG. Betamethasone attenuates oxidant stress in endothelial cells from fetal lambs with persistent pulmonary hypertension. Pediatr Res. 2008 Jan;63(1):67-72. doi: 10.1203/PDR.0b013e31815b43ee[↩]
- Soukka H, Halkola L, Aho H, Rautanen M, Kero P, Kääpä P. Methylprednisolone attenuates the pulmonary hypertensive response in porcine meconium aspiration. Pediatr Res. 1997 Aug;42(2):145-50. doi: 10.1203/00006450-199708000-00003[↩]
- Ward M, Sinn J. Steroid therapy for meconium aspiration syndrome in newborn infants. Cochrane Database Syst Rev. 2003;2003(4):CD003485. doi: 10.1002/14651858.CD003485[↩]
- Tripathi S, Saili A. The effect of steroids on the clinical course and outcome of neonates with meconium aspiration syndrome. J Trop Pediatr. 2007 Feb;53(1):8-12. doi: 10.1093/tropej/fml018[↩]
- Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF, Wynn KA, Kumar VH, Mathew B, Kirmani K, Morin FC 3rd. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res. 2007 Sep;62(3):313-8. doi: 10.1203/PDR.0b013e3180db29fe[↩]
- Clark RH, Yoder BA, Sell MS. Prospective, randomized comparison of high-frequency oscillation and conventional ventilation in candidates for extracorporeal membrane oxygenation. J Pediatr. 1994 Mar;124(3):447-54. doi: 10.1016/s0022-3476(94)70374-4[↩]
- Kinsella JP, Abman SH. Clinical approaches to the use of high-frequency oscillatory ventilation in neonatal respiratory failure. J Perinatol. 1996 Mar-Apr;16(2 Pt 2 Su):S52-5.[↩]
- Kinsella JP, Abman SH. High-frequency oscillatory ventilation augments the response to inhaled nitric oxide in persistent pulmonary hypertension of the newborn: Nitric Oxide Study Group. Chest. 1998 Jul;114(1 Suppl):100S. doi: 10.1378/chest.114.1_supplement.100s[↩]
- Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Mayock DE, Redding GJ, deLemos RA, Sardesai S, McCurnin DC, Moreland SG, Cutter GR, Abman SH. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr. 1997 Jul;131(1 Pt 1):55-62. doi: 10.1016/s0022-3476(97)70124-0[↩]
- Lakshminrusimha S, Steinhorn RH. Inodilators in nitric oxide resistant persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med. 2013 Jan;14(1):107-9. doi: 10.1097/PCC.0b013e318250af44[↩]
- UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. UK Collaborative ECMO Trail Group. Lancet. 1996 Jul 13;348(9020):75-82. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(96)04100-1/abstract[↩]
- Mugford M, Elbourne D, Field D. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001340. doi: 10.1002/14651858.CD001340.pub2[↩]
- Davis PJ, Firmin RK, Manktelow B, Goldman AP, Davis CF, Smith JH, Cassidy JV, Shekerdemian LS. Long-term outcome following extracorporeal membrane oxygenation for congenital diaphragmatic hernia: the UK experience. J Pediatr. 2004 Mar;144(3):309-15. doi: 10.1016/j.jpeds.2003.11.031[↩][↩][↩]
- Konduri GG, Vohr B, Robertson C, Sokol GM, Solimano A, Singer J, Ehrenkranz RA, Singhal N, Wright LL, Van Meurs K, Stork E, Kirpalani H, Peliowski A, Johnson Y; Neonatal Inhaled Nitric Oxide Study Group. Early inhaled nitric oxide therapy for term and near-term newborn infants with hypoxic respiratory failure: neurodevelopmental follow-up. J Pediatr. 2007 Mar;150(3):235-40, 240.e1. doi: 10.1016/j.jpeds.2006.11.065[↩]
- Robertson CM, Tyebkhan JM, Hagler ME, Cheung PY, Peliowski A, Etches PC. Late-onset, progressive sensorineural hearing loss after severe neonatal respiratory failure. Otol Neurotol. 2002 May;23(3):353-6. doi: 10.1097/00129492-200205000-00022[↩]
- Lipkin PH, Davidson D, Spivak L, Straube R, Rhines J, Chang CT. Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with nitric oxide. J Pediatr. 2002 Mar;140(3):306-10. doi: 10.1067/mpd.2002.122730[↩]
- Berti A, Janes A, Furlan R, Macagno F. High prevalence of minor neurologic deficits in a long-term neurodevelopmental follow-up of children with severe persistent pulmonary hypertension of the newborn: a cohort study. Ital J Pediatr. 2010 Jun 13;36:45. doi: 10.1186/1824-7288-36-45[↩]
- Steurer MA, Baer RJ, Oltman S, Ryckman KK, Feuer SK, Rogers E, Keller RL, Jelliffe-Pawlowski LL. Morbidity of Persistent Pulmonary Hypertension of the Newborn in the First Year of Life. J Pediatr. 2019 Oct;213:58-65.e4. doi: 10.1016/j.jpeds.2019.06.053. Epub 2019 Aug 6. Erratum in: J Pediatr. 2019 Dec;215:291. doi: 10.1016/j.jpeds.2019.09.064[↩][↩][↩]