Contents
- What is Dementia
- Who gets dementia ?
- What causes dementia ?
- Can dementia be prevented ?
- What is the first step to diagnose dementia ?
- How is Dementia Diagnosed ?
- Do Memory Problems Always Mean Alzheimer’s Disease ?
- What Is Alzheimer’s Disease ?
- What Happens to the Brain in Alzheimer’s Disease ?
- Figure 1. Alzheimer’s disease brain
- Figure 2. Alzheimer’s disease brain with amyloid plaques and neurofibrillary or tau, tangles
- Figure 3. Glial (neuroglial) cells in the brain (astrocytes, oligodendrocytes, and microglial cells)
- How Does Alzheimer’s Disease Affect the Brain ?
- Figure 4. Brain showing Entorhinal cortex (parahippocampal gyrus) and Hippocampus
- What Are the Main Characteristics of the Brain with Alzheimer’s ?
- How Many Americans Have Alzheimer’s Disease ?
- What Does Alzheimer’s Disease Look Like ?
- How Long Can a Person Live with Alzheimer’s Disease ?
- What Causes Alzheimer’s Disease ?
- Alzheimer’s Disease Genetics
- Table 1. Some Differences Between Late-Onset and Early-Onset Alzheimer’s Disease
- Late-Onset Alzheimer’s Disease
- Early-Onset Alzheimer’s Disease
- Health, Environmental, and Lifestyle Factors
- How Is Alzheimer’s Disease Diagnosed ?
- What Are the Benefits of Early Alzheimer’s Disease Diagnosis ?
- How Is Alzheimer’s Disease Treated ?
- Table 1. Medicines used to treat Alzheimer’s Disease
- Dosage and Side Effects
- Managing Behavior
- Medicines to be Used with Caution
- Tips for Dealing with Memory Problems and Forgetfulness
- What is HIV associated dementia ?
- What is alcohol related dementia ?
- What is Creutzfeldt-Jakob disease ?
- What is Huntington’s disease ?
What is Dementia
Dementia is not a disease, but a collection of symptoms that result from damage to the brain or disorders affecting the brain 1. Dementia is not a specific disease 2. Dementia affects thinking, behavior, remembering, reasoning and behavioral abilities to such an extent that it interferes with a person’s daily life and activities 3. In dementia, the brain function is affected enough to interfere with the person’s normal social or working life. People with dementia may not be able to think well enough to do normal activities, such as getting dressed or eating. They may lose their ability to solve problems or control their emotions 2. Their personalities may change 2. They may become agitated or see things (hallucinate) that are not there 2.
Memory loss is a common symptom of dementia 2. However, memory loss by itself does not mean you have dementia 2. People with dementia have serious problems with two or more brain functions, such as memory and language 2. People with advanced dementia may not recognise close family and friends, they may not remember where they live or know where they are. They may find it impossible to understand simple pieces of information, carry out basic tasks or follow instructions.
Although dementia is common in very elderly people, up to half of all people age 85 or older may have some form of dementia. Dementia is not a normal part of aging 2, 3. Many people live into their 90s and beyond without any signs of dementia. One type of dementia, fronto-temporal disorders, is more common in middle-aged than older adults.
Dementia ranges in severity from the mildest stage, when it is just beginning to affect a person’s functioning, to the most severe stage, when the person must depend completely on others for basic activities of living 4. As dementia progresses, memory loss and difficulties with communication often become very severe. It’s common for people with dementia to have increasing difficulty speaking and they may eventually lose the ability to speak altogether. It’s important to keep trying to communicate with them and to recognise and use other, non-verbal means of communication, such as expression, touch and gestures. In the later stages, the person is likely to neglect their own health and require constant care and attention.
Many people with dementia gradually become less able to move about unaided and may appear increasingly clumsy when carrying out everyday tasks. Some people may eventually be unable to walk and may become bedbound. Bladder incontinence is common in the later stages of dementia and some people will also experience bowel incontinence.
Loss of appetite and weight loss are common in the later stages of dementia. It’s important that people with dementia get help at mealtimes to ensure they eat enough.
Many people have trouble eating or swallowing and this can lead to choking, chest infections and other problems.
Signs and symptoms of dementia result when once-healthy neurons (nerve cells) in the brain stop working, lose connections with other brain cells, and die. While everyone loses some neurons as they age, people with dementia experience far greater loss 4.
Memory loss, though common, is not the only sign of dementia. For a person to have dementia, he or she must have 4:
- Two or more core mental functions that are impaired. These functions include memory, language skills, visual perception, and the ability to focus and pay attention. These also include cognitive skills such as the ability to reason and solve problems.
- A loss of brain function severe enough that a person cannot do normal, everyday tasks.
In addition, some people with dementia cannot control their emotions. Their personalities may change. They can have delusions, which are strong beliefs without proof, such as the idea that someone is stealing from them. They also may hallucinate, seeing or otherwise experiencing things that are not real.
The causes of dementia can vary, depending on the types of brain changes that may be taking place. Many different diseases can cause dementia, including Alzheimer’s disease and stroke 2. Other dementias include Lewy body dementia, frontotemporal disorders, and vascular dementia 4. It is common for people to have mixed dementia—a combination of two or more disorders, at least one of which is dementia 4. For example, some people have both Alzheimer’s disease and vascular dementia. Drugs are available to treat some of these diseases. While these drugs cannot cure dementia or repair brain damage, they may improve symptoms or slow down the disease 2.
Who gets dementia ?
Most people with dementia are older, but it is important to remember that not all older people get dementia. It is not a normal part of ageing.
Dementia can happen to anybody, but it is more common after the age of 65 years. People in their 40s and 50s can also have dementia.
What causes dementia ?
Dementia symptoms can be caused by a number of conditions and each has its own causes.
The most common types of dementia symptoms are caused by 5 :
- Alzheimer’s disease,
- Vascular dementia and vascular cognitive impairment (to learn more about Vascular Dementia),
- Parkinson’s disease (to learn more about Parkinson’s Disease Dementia),
- Dementia with Lewy bodies (to learn more about Lewy Body Dementia),
- Fronto Temporal Lobar Degeneration (to learn more about Frontotemporal dementia),
- Huntington’s disease,
- Alcohol related dementia (Korsakoff’s syndrome),
- HIV Associated Dementia (AIDS Dementia Complex),
- Creutzfeldt-Jacob disease and
- Mixed dementia, a combination of two or more disorders, at least one of which is dementia 6.
Can dementia be prevented ?
There is no certain way to prevent all types of dementia.
However, a healthy lifestyle can help lower your risk of developing dementia when you are older. It can also prevent cardiovascular diseases, such as strokes and heart attacks.
To reduce your risk of developing dementia and other serious health conditions, it’s recommended that you:
- eat a healthy diet
- maintain a healthy weight
- exercise regularly
- don’t drink too much alcohol
- stop smoking (if you smoke)
- make sure to keep your blood pressure at a healthy level
Diet and dementia
A low-fat, high-fibre diet including plenty of fresh fruit and vegetables and wholegrains can help reduce your risk of some kinds of dementia.
Limiting the amount of salt in your diet to no more than 1.5 grams a day can also help. Too much salt will increase your blood pressure, which puts you at risk of developing some types of dementia.
High cholesterol levels may also put you at risk of developing some kinds of dementia, so try to limit the amount of food you eat that is high in saturated fat.
How weight affects dementia risk
Being overweight can increase your blood pressure, which increases your risk of getting some kinds of dementia. The risk is higher if you are obese. The most scientific way to measure your weight is to calculate your body mass index (BMI).
You can calculate your BMI using the BMI healthy weight calculator. People with a BMI of 25-30 are overweight, and those with a BMI above 30 are obese. People with a BMI of 40 or more are morbidly obese.
Exercise to reduce dementia risk
Exercising regularly will make your heart and blood circulatory system more efficient. It will also help to lower your cholesterol and keep your blood pressure at a healthy level, decreasing your risk of developing some kinds of dementia.
For most people, a minimum of 150 minutes (2 hours and 30 minutes) of moderate-intensity aerobic activity each week, such as cycling or fast walking, is recommended.
Alcohol and dementia
Drinking excessive amounts of alcohol will cause your blood pressure to rise, as well as raising the level of cholesterol in your blood.
Stick to the recommended limits for alcohol consumption to reduce your risk of high blood pressure, cardiovascular disease and dementia.
The recommended daily limit for alcohol consumption is three to four units of alcohol a day for men, and two to three units a day for women. A unit of alcohol is equal to about half a pint of normal-strength lager, a small glass of wine or a pub measure (25ml) of spirits.
Stopping smoking could reduce dementia risk
Smoking can cause your arteries to narrow, which can lead to a rise in your blood pressure. It also increases your risk of developing cardiovascular diseases, cancer and dementia.
What is the first step to diagnose dementia ?
Visiting a family doctor is often the first step for people who are experiencing changes in thinking, movement, or behavior 7. However, neurologists—doctors who specialize in disorders of the brain and nervous system—generally have the expertise needed to diagnose dementia 7. Geriatric psychiatrists, neuropsychologists, and geriatricians may also be skilled in diagnosing the condition 7.
If a specialist cannot be found in your community, ask the neurology department of the nearest medical school for a referral. A hospital affiliated with a medical school may also have a dementia or movement disorders clinic that provides expert evaluation.
How is Dementia Diagnosed ?
To diagnose dementia, doctors first assess whether a person has an underlying treatable condition such as depression, abnormal thyroid function, normal pressure hydrocephalus, or vitamin B12 deficiency 7. Early diagnosis is important, as some causes for symptoms can be treated. In many cases, the specific type of dementia a person has may not be confirmed until after the person has died and the brain is examined.
A medical assessment for dementia generally includes:
- Patient history. Typical questions about a person’s medical and family history might include asking about whether dementia runs in the family, how and when symptoms began, changes in behavior and personality, and if the person is taking certain medications that might cause or worsen symptoms.
- Physical exam. Measuring blood pressure and other vital signs may help physicians detect conditions that might cause or occur with dementia. Such conditions may be treatable.
- Neurological tests. Assessing balance, sensory function, reflexes, vision, eye movements, and other functions helps identify conditions that may affect the diagnosis or are treatable with drugs.
Tests Used to Diagnose Dementia
These tests for dementia are mainly tests of mental abilities, blood tests and brain scans. The following procedures also may be used to diagnose dementia:
- Cognitive and neuropsychological tests. These tests measure memory, problem solving, attention, counting, language skills, and other abilities related to mental functioning.
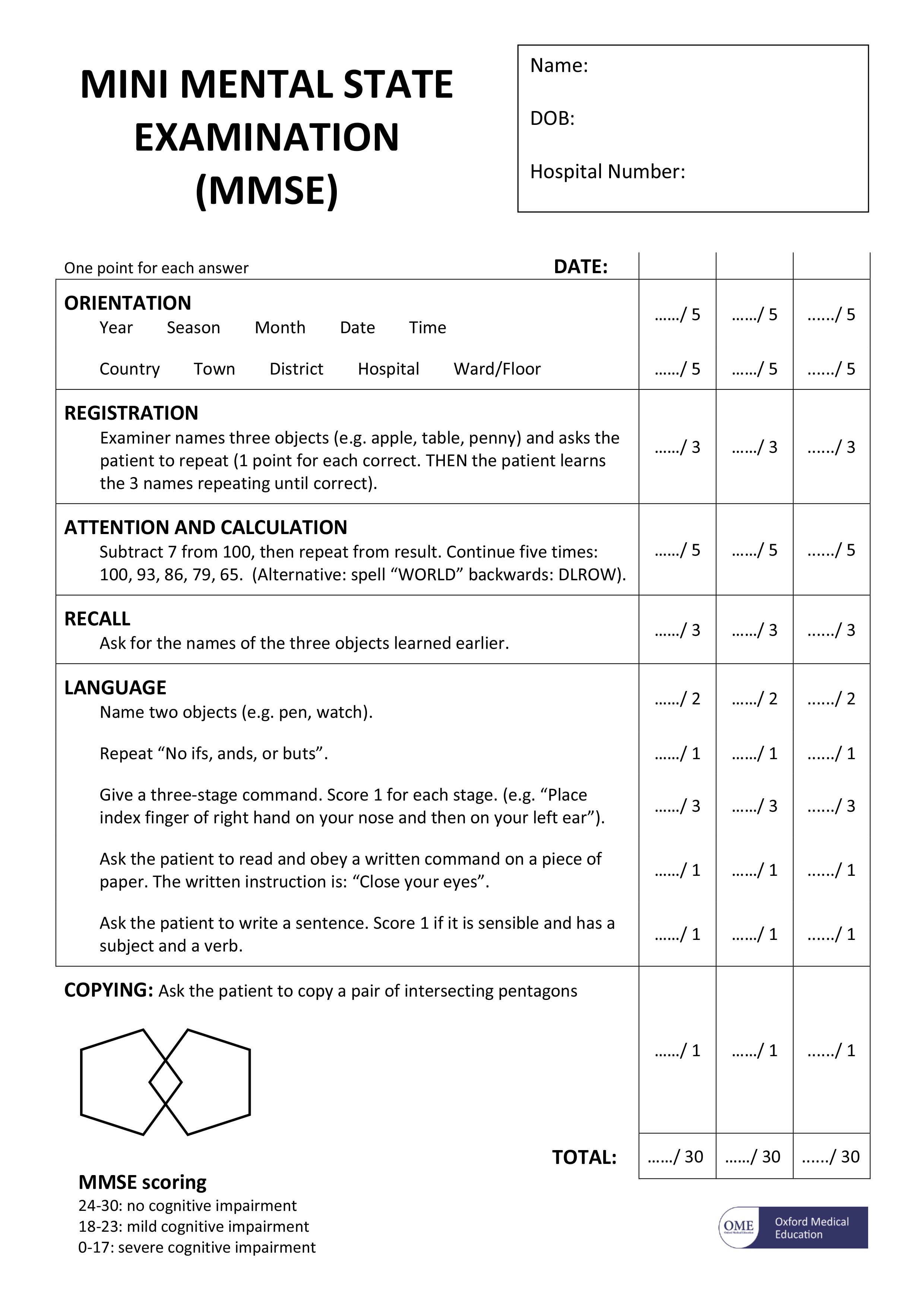
People with symptoms of dementia are often given questionnaires to help test their mental abilities, to see how severe any memory problems may be. One widely used test is called the mini mental state examination (MMSE) 8.
The mini mental state examination (MMSE) assesses a number of different mental abilities, including:
- short- and long-term memory
- attention span
- concentration
- language and communication skills
- ability to plan
- ability to understand instructions
The mini mental state examination (MMSE) is a series of exercises, each carrying a score with a maximum of 30 points. These exercises include:
- memorising a short list of objects and then repeating the list
- writing a short sentence that is grammatically correct, such as “the dog sat on the floor”
- correctly answering time-orientation questions, such as identifying the day of the week, the date or the year
The Mini Mental State Examination (MMSE)
[Source 9]Note: The mini mental state examination (MMSE) is not a test to diagnose dementia. However, it is useful for assessing the level of mental impairment that a person with dementia may have. Test scores may be influenced by a person’s level of education. For example, someone who cannot read or write very well may have a lower score, but they may not have dementia. Similarly, someone with a higher level of education may achieve a higher score, but still have dementia.
The mini mental state examination (MMSE) is a 30-point test
Advantages
- Relatively quick and easy to perform
- Requires no additional equipment
- Can provide a method of monitoring deterioration over time
Disadvantages
- Biased against people with poor education due to elements of language and mathematical testing
- Bias against visually impaired
- Limited examination of visuospatial cognitive ability
- Poor sensitivity at detected mild/early dementia
- Laboratory tests. Blood and urine tests can help rule out possible causes of symptoms.
Blood tests (in roughly descending order of importance)
- Thyroid function tests: Rule out hypothyroidism as a cause of a dementia-like presentation
- B12: Low levels can cause memory impairment and mood changes
- Blood glucose: Independent risk factor for dementia. Unclear if high blood sugars are direct cause. Iatrogenic hypoglycaemic episodes can also cause worsening dementia. Indeed, tightly controlled blood sugars in the elderly has a negative impact on mortality.
- Urea and electrolytes: Severe disturbances can cause cognitive impairment (e.g. renal failure and hyperuraemia). Sodium and calcium are particularly important.
- Liver function tests: Metabolic causes in liver dysfunction (e.g. hyperammonaemia in cirrhosis). Rarer causes (e.g. Wilson’s disease).
- Infective screen: FBC, ESR, CRP to look for superimposed infection as cause of confusion. HIV-associated or neurosyphilis dementia.
- Autoimmune screen: Should be considered in rapidly progressive dementia.
- Further tests: More specific tests are rarely needed. However, if there is diagnostic uncertainly they may include: serum and urinary copper and caeruloplasmin (Wilsons disease); ammonia (liver disease and inherited metabolic abnormalities); HIV; syphilis serology.
Brain scans.
- Brain scans are often used for diagnosing dementia once other simpler tests have ruled out other problems. They are needed to check for evidence of other possible problems that could explain a person’s symptoms, such as a major stroke or a brain tumor and other problems that can cause dementia. Scans also identify changes in the brain’s structure and function. The most common scans are:
- Computed tomography (CT) scan, which uses X-rays to produce images of the brain and other organs. A computerised tomography (CT) scan can be used to check for signs of stroke or a brain tumor. However, unlike an MRI scan, a CT scan cannot provide detailed information about the structure of the brain. The National Institute for Health recommends using a magnetic resonance imaging (MRI) scan to help confirm a diagnosis of dementia 8.CT scans are quick, painless and generally safe. However, there’s a small risk you could have an allergic reaction to the contrast dye used and you will be exposed to X-ray radiation. The amount of radiation you’re exposed to during a CT scan varies, depending on how much of your body is scanned. CT scanners are designed to make sure you’re not exposed to unnecessarily high levels. Generally, the amount of radiation you’re exposed to during each scan is the equivalent to between a few months and a few years of exposure to natural radiation from the environment.
- Magnetic resonance imaging (MRI), which uses magnetic fields and radio waves to produce detailed images of body structures, including tissues, organs, bones, and nerves. An MRI scan can provide detailed information about the blood vessel damage that occurs in vascular dementia, plus any shrinking of the brain. In frontotemporal dementia, the frontal and temporal lobes are mainly affected by shrinking 8. MRI scans don’t involve exposing the body to X-ray radiation. This means people who may be particularly vulnerable to the effects of radiation, such as pregnant women and babies, can use them if necessary. An MRI scan is a painless and safe procedure. You may find it uncomfortable if you have claustrophobia, but most people find this manageable with support from the radiographer. Extensive research has been carried out into whether the magnetic fields and radio waves used during MRI scans could pose a risk to the human body. No evidence has been found to suggest there’s a risk, which means MRI scans are one of the safest medical procedures currently available. However, not everyone can have an MRI scan. For example, they’re not always possible for people who have certain types of implants fitted, such as a pacemaker (a battery-operated device that helps to control an irregular heartbeat).
- Positron emission tomography (PET) scan or a single photon-emission computed tomography (SPECT) scan, which uses radiation to provide pictures of brain activity may be recommended if the result of your CT or MRI scan is uncertain. These scans look at how the brain functions and can pick up abnormalities with the blood flow in the brain. They’re particularly helpful for investigating confirmed cases of cancer, to determine how far the cancer has spread and how well it’s responding to treatment. They can also help diagnose some conditions that affect the normal workings of the brain, such as dementia. PET scanners work by detecting the radiation given off by a substance called a radiotracer as it collects in different parts of your body.In most PET scans a radiotracer called fluorodeoxyglucose (FDG) is used, which is similar to naturally occurring glucose (a type of sugar) so your body treats it in a similar way. Before the scan, the radiotracer is injected into a vein in your arm or hand. You will need to wait quietly for about an hour, to give it time to be absorbed by the cells in your body. By analysing the areas where the radiotracer does and doesn’t build up, it’s possible to work out how well certain body functions are working and identify any abnormalities. For example, a concentration of fluorodeoxyglucose (FDG) in the body’s tissues can help identify cancerous cells because cancer cells use glucose at a much faster rate than normal cells.In a standard PET scan the amount of radiation you’re exposed to is small – about the same as the amount you get from natural sources, such as the sun, over three years.The radiotracer becomes quickly less radioactive over time and will usually be passed out of your body naturally within a few hours. Drinking plenty of fluid after the scan can help flush it from your body.As a precaution, you may be advised to avoid prolonged close contact with pregnant women, babies or young children for a few hours after a PET scan, as you will be slightly radioactive during this time.
- In some cases, an electroencephalogram (EEG) may be taken to record the brain’s electrical signals (brain activity).
- Psychiatric evaluation. This evaluation will help determine if depression or another mental health condition is causing or contributing to a person’s symptoms.
- Genetic tests. Some dementias are caused by a known gene defect. In these cases, a genetic test can help people know if they are at risk for dementia. People should talk with family members, a primary care doctor, and a genetic counselor before getting tested.
Do Memory Problems Always Mean Alzheimer’s Disease ?
Many people worry about becoming forgetful. They think forgetfulness is the first sign of Alzheimer’s disease. But not all people with memory problems have Alzheimer’s disease 10.
Other causes for memory problems can include aging, medical conditions, emotional problems, mild cognitive impairment, or another type of dementia.
What Is Mild Cognitive Impairment ?
Some forgetfulness can be a normal part of aging. However, some people have more memory problems than other people their age. This condition is called mild cognitive impairment 11. People with mild cognitive impairment can take care of themselves and do their normal activities 12. Mild cognitive impairment has several types. The type most associated with memory loss is called amnestic mild cognitive impairment.
Symptoms of Mild Cognitive Impairment
People with amnestic mild cognitive impairment have more memory problems than normal for people their age, but their symptoms are not as severe as those of people with Alzheimer’s disease 12. For example, they do not experience the personality changes or other problems that are characteristic of Alzheimer’s disease 12. People with mild cognitive impairment are still able to carry out their normal daily activities 12.
Signs of Mild Cognitive Impairment include 12:
- Losing things often.
- Forgetting to go to events or appointments.
- Having more trouble coming up with words than other people of the same age.
Movement difficulties and problems with the sense of smell have also been linked to mild cognitive impairment.
Diagnosing Mild Cognitive Impairment
Memory problems can also have other causes, including certain medicines and diseases that affect the blood vessels that supply the brain 11. Some of the problems brought on by these conditions can be managed or reversed.
Family and friends may notice memory lapses, and the person with mild cognitive impairment may worry about losing his or her memory. These worries may prompt the person to see a doctor for diagnosis.
Researchers have found that more people with mild cognitive impairment than those without it go on to develop Alzheimer’s disease. However, not everyone who has mild cognitive impairment develops Alzheimer’s disease. About 8 of every 10 people who fit the definition of amnestic mild cognitive impairment go on to develop Alzheimer’s disease within 7 years 12. In contrast, 1 to 3 percent of people older than 65 who have normal cognition will develop Alzheimer’s disease in any one year 12.
Research suggests genetic factors may play a role in who will develop mild cognitive impairment, as they do in Alzheimer’s disease 12. Studies are underway to learn why some people with mild cognitive impairment progress to Alzheimer’s disease and others do not.
A doctor can do thinking, memory, and language tests to see if a person has mild cognitive impairment. He or she also may suggest that the person see a specialist for more tests. There currently is no standard treatment for mild cognitive impairment, but there are things a person can do to try and keep his or her memory sharp.
Because mild cognitive impairment may be an early sign of Alzheimer’s disease, it’s important to see a doctor or specialist every 6 to 12 months 11.
What Is Alzheimer’s Disease ?
Alzheimer’s disease is an irreversible, progressive brain disorder that slowly destroys memory and thinking skills and eventually, the ability to carry out the simplest tasks 13. In most people with the disease—those with the late-onset type—symptoms first appear in their mid-60s. Early-onset Alzheimer’s occurs between a person’s 30s and mid-60s and is very rare. Alzheimer’s disease is the most common cause of dementia among older adults 14.
Alzheimer’s disease begins slowly and it usually begins after age 60 14. The risk goes up as you get older. Your risk is also higher if a family member has had the disease 14. It first involves the parts of the brain that control thought, memory and language. People with Alzheimer’s disease may have trouble remembering things that happened recently or names of people they know. A related problem, mild cognitive impairment, causes more memory problems than normal for people of the same age. Many, but not all, people with mild cognitive impairment will develop Alzheimer’s Disease 14.
In Alzheimer’s disease, over time, symptoms get worse. People may not recognize family members. They may have trouble speaking, reading or writing. They may forget how to brush their teeth or comb their hair. Later on, they may become anxious or aggressive, or wander away from home. Eventually, they need total care. This can cause great stress for family members who must care for them.
No treatment can stop the disease. However, some drugs may help keep symptoms from getting worse for a limited time.
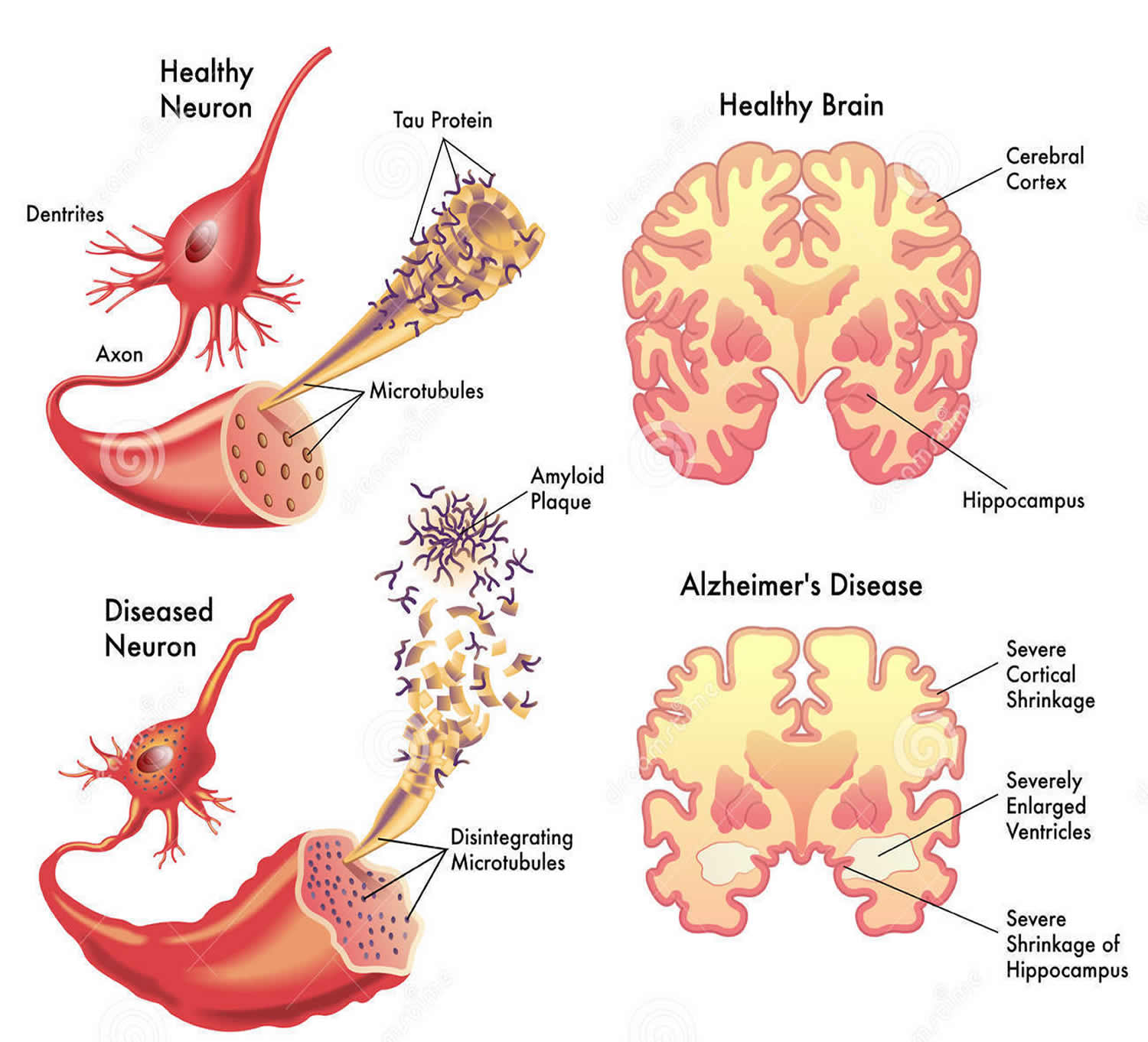
Alzheimer’s disease is named after Dr. Alois Alzheimer. In 1906, Dr. Alzheimer noticed changes in the brain tissue of a woman who had died of an unusual mental illness 13. Her symptoms included memory loss, language problems, and unpredictable behavior. After she died, he examined her brain and found many abnormal clumps (now called amyloid plaques) and tangled bundles of fibers (now called neurofibrillary, or tau, tangles).
These plaques and tangles in the brain are still considered some of the main features of Alzheimer’s disease 13. Another feature is the loss of connections between nerve cells (neurons) in the brain. Neurons transmit messages between different parts of the brain and from the brain to muscles and organs in the body. Many other complex brain changes are thought to play a role in Alzheimer’s disease, too.
This damage initially appears to take place in the hippocampus, the part of the brain essential in forming memories. As neurons die, additional parts of the brain are affected. By the final stage of Alzheimer’s disease, damage is widespread, and brain tissue has shrunk significantly.
What Happens to the Brain in Alzheimer’s Disease ?
The healthy human brain contains tens of billions of neurons—specialized cells that process and transmit information via electrical and chemical signals. They send messages between different parts of the brain, and from the brain to the muscles and organs of the body. Alzheimer’s disease disrupts this communication among neurons, resulting in loss of function and cell death.
Key Biological Processes in the Brain
Most neurons have three basic parts: a cell body, multiple dendrites, and an axon (see Figure 2).
- The cell body contains the nucleus, which houses the genetic blueprint that directs and regulates the cell’s activities.
- Dendrites are branch-like structures that extend from the cell body and collect information from other neurons.
- The axon is a cable-like structure at the end of the cell body opposite the dendrites and transmits messages to other neurons.
The function and survival of neurons depend on several key biological processes:
- Communication. Neurons are constantly in touch with neighboring brain cells. When a neuron receives signals from other neurons, it generates an electrical charge that travels down the length of its axon and releases neurotransmitter chemicals across a tiny gap, called a synapse. Like a key fitting into a lock, each neurotransmitter molecule then binds to specific receptor sites on a dendrite of a nearby neuron. This process triggers chemical or electrical signals that either stimulate or inhibit activity in the neuron receiving the signal. Communication often occurs across networks of brain cells. In fact, scientists estimate that in the brain’s communications network, one neuron may have as many as 7,000 synaptic connections with other neurons.
- Metabolism. Metabolism—the breaking down of chemicals and nutrients within a cell—is critical to healthy cell function and survival. To perform this function, cells require energy in the form of oxygen and glucose, which are supplied by blood circulating through the brain. The brain has one of the richest blood supplies of any organ and consumes up to 20 percent of the energy used by the human body—more than any other organ.
- Repair, remodeling, and regeneration. Unlike many cells in the body, which are relatively short-lived, neurons have evolved to live a long time—more than 100 years in humans. As a result, neurons must constantly maintain and repair themselves. Neurons also continuously adjust, or “remodel,” their synaptic connections depending on how much stimulation they receive from other neurons. For example, they may strengthen or weaken synaptic connections, or even break down connections with one group of neurons and build new connections with a different group. Adult brains may even generate new neurons—a process called neurogenesis. Remodeling of synaptic connections and neurogenesis are important for learning, memory, and possibly brain repair.
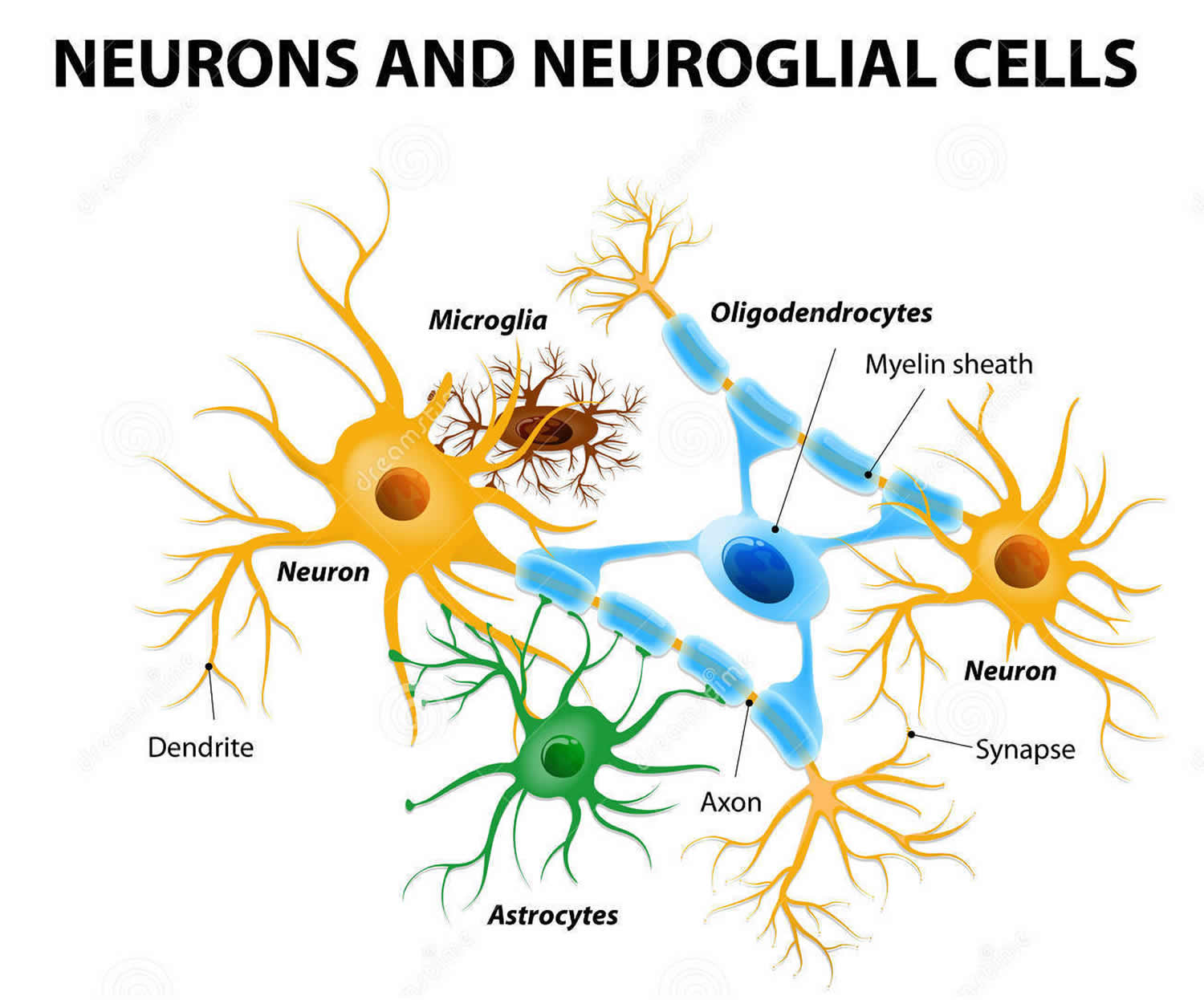
Neurons are a major player in the central nervous system, but other cell types are also key to healthy brain function. In fact, glial cells are by far the most numerous cells in the brain, outnumbering neurons by about 10 to 1 (see Figure 3). These cells, which come in various forms—such as microglia, astrocytes, and oligodendrocytes—surround and support the function and healthy of neurons. For example, microglia protect neurons from physical and chemical damage and are responsible for clearing foreign substances and cellular debris from the brain. To carry out these functions, glial cells often collaborate with blood vessels in the brain. Together, glial and blood vessel cells regulate the delicate balance within the brain to ensure that it functions at its best.
Figure 1. Alzheimer’s disease brain
Figure 2. Alzheimer’s disease brain with amyloid plaques and neurofibrillary or tau, tangles
Figure 3. Glial (neuroglial) cells in the brain (astrocytes, oligodendrocytes, and microglial cells)
Note: Glial (Neuroglial) cells do not conduct nerve impulses, but, instead, support, nourish, and protect the neurons. Glial cells (glia) are far more numerous than neurons and, unlike neurons, are capable of mitosis. Glial roles that are well-established include maintaining the ionic milieu of nerve cells, modulating the rate of nerve signal propagation, modulating synaptic action by controlling the uptake of neurotransmitters, providing a scaffold for some aspects of neural development, and aiding in (or preventing, in some instances) recovery from neural injury 15.
How Does Alzheimer’s Disease Affect the Brain ?
The brain typically shrinks to some degree in healthy aging but, surprisingly, does not lose neurons in large numbers. In Alzheimer’s disease, however, damage is widespread, as many neurons stop functioning, lose connections with other neurons, and die. Alzheimer’s disrupts processes vital to neurons and their networks, including communication, metabolism, and repair 16.
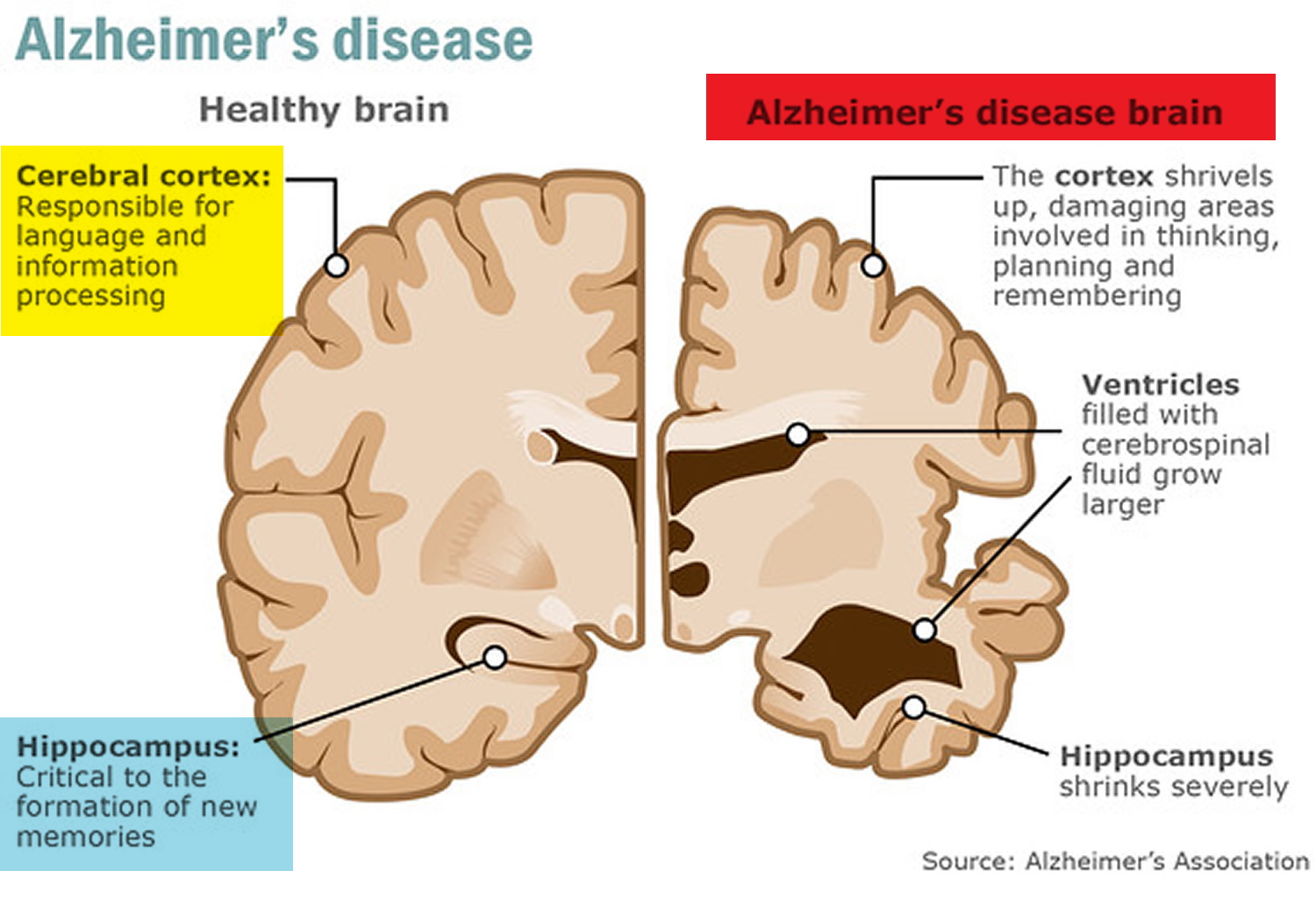
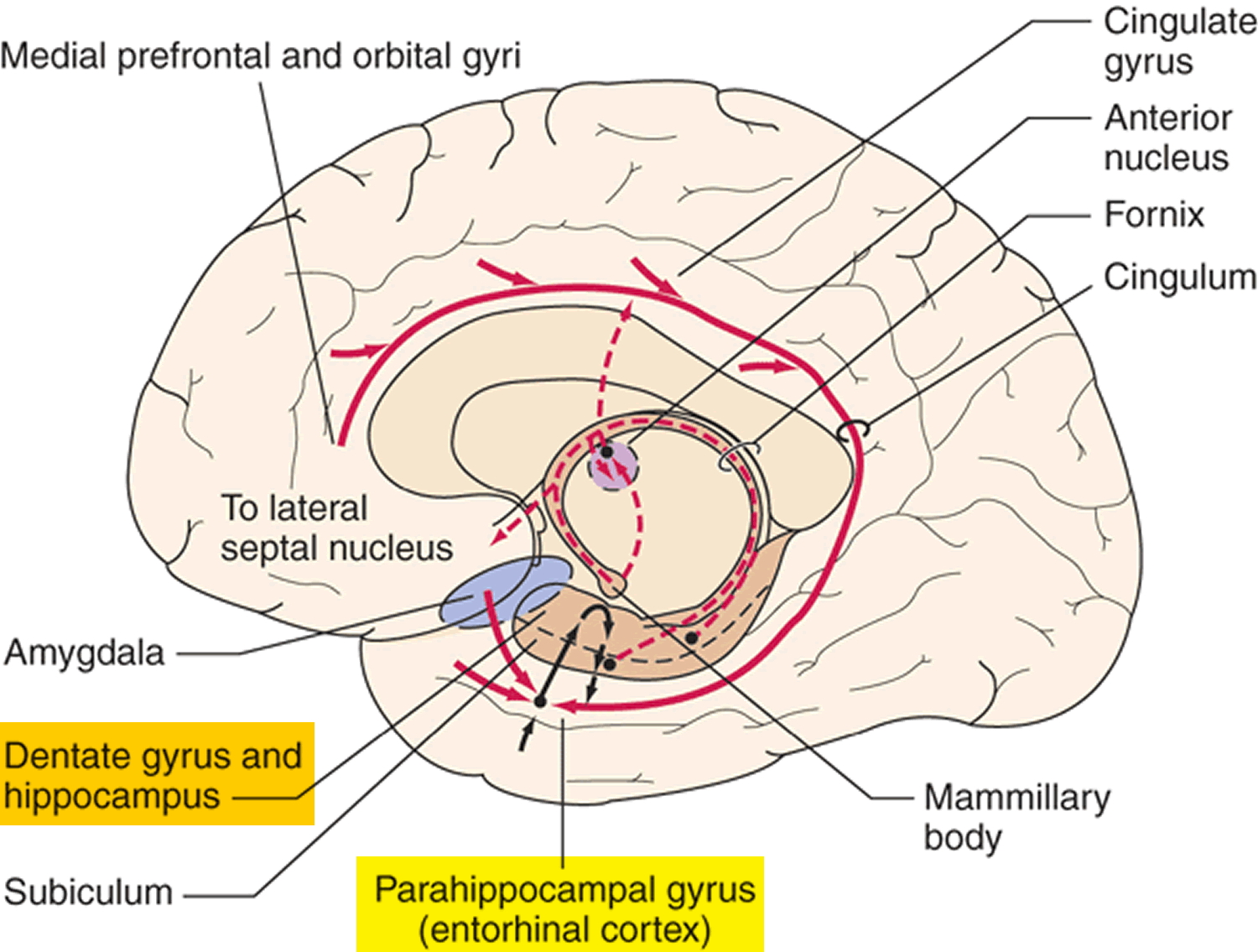
At first, Alzheimer’s disease typically destroys neurons and their connections in parts of the brain involved in memory, including the entorhinal cortex and hippocampus. It later affects areas in the cerebral cortex responsible for language, reasoning, and social behavior. Eventually, many other areas of the brain are damaged. Over time, a person with Alzheimer’s gradually loses his or her ability to live and function independently. Ultimately, the disease is fatal 16.
Figure 4. Brain showing Entorhinal cortex (parahippocampal gyrus) and Hippocampus
What Are the Main Characteristics of the Brain with Alzheimer’s ?
Many molecular and cellular changes take place in the brain of a person with Alzheimer’s disease. These changes can be observed in brain tissue under the microscope after death. Investigations are underway to determine which changes may cause Alzheimer’s and which may be a result of the disease.
Amyloid Plaques
The beta-amyloid protein involved in Alzheimer’s comes in several different molecular forms that collect between neurons. It is formed from the breakdown of a larger protein, called amyloid precursor protein. One form, beta-amyloid 42, is thought to be especially toxic. In the Alzheimer’s brain, abnormal levels of this naturally occurring protein clump together to form plaques that collect between neurons and disrupt cell function. Research is ongoing to better understand how, and at what stage of the disease, the various forms of beta-amyloid influence Alzheimer’s.
Neurofibrillary Tangles
Neurofibrillary tangles are abnormal accumulations of a protein called tau that collect inside neurons. Healthy neurons, in part, are supported internally by structures called microtubules, which help guide nutrients and molecules from the cell body to the axon and dendrites. In healthy neurons, tau normally binds to and stabilizes microtubules. In Alzheimer’s disease, however, abnormal chemical changes cause tau to detach from microtubules and stick to other tau molecules, forming threads that eventually join to form tangles inside neurons. These tangles block the neuron’s transport system, which harms the synaptic communication between neurons.
Emerging evidence suggests that Alzheimer’s-related brain changes may result from a complex interplay among abnormal tau and beta-amyloid proteins and several other factors. It appears that abnormal tau accumulates in specific brain regions involved in memory. Beta-amyloid clumps into plaques between neurons. As the level of beta-amyloid reaches a tipping point, there is a rapid spread of tau throughout the brain.
Chronic Inflammation
Research suggests that chronic inflammation may be caused by the buildup of glial cells normally meant to help keep the brain free of debris. One type of glial cell, microglia, engulfs and destroys waste and toxins in a healthy brain. In Alzheimer’s, microglia fail to clear away waste, debris, and protein collections, including beta-amyloid plaques. Researchers are trying to find out why microglia fail to perform this vital function in Alzheimer’s.
One focus of study is a gene called TREM2. Normally, TREM2 tells the microglia cells to clear beta-amyloid plaques from the brain and helps fight inflammation in the brain. In the brains of people where this gene does not function normally, plaques build up between neurons. Astrocytes—another type of glial cell—are signaled to help clear the buildup of plaques and other cellular debris left behind. These microglia and astrocytes collect around the neurons but fail to perform their debris-clearing function. In addition, they release chemicals that cause chronic inflammation and further damage the neurons they are meant to protect.
Vascular Contributions to Alzheimer’s Disease
People with dementia seldom have only Alzheimer’s-related changes in their brains. Any number of vascular issues—problems that affect blood vessels, such as beta-amyloid deposits in brain arteries, atherosclerosis (hardening of the arteries), and mini-strokes—may also be at play.
Vascular problems may lead to reduced blood flow and oxygen to the brain, as well as a breakdown of the blood-brain barrier, which usually protects the brain from harmful agents while allowing in glucose and other necessary factors. In a person with Alzheimer’s, a faulty blood-brain barrier prevents glucose from reaching the brain and prevents the clearing away of toxic beta-amyloid and tau proteins. This results in inflammation, which adds to vascular problems in the brain. Because it appears that Alzheimer’s is both a cause and consequence of vascular problems in the brain, researchers are seeking interventions to disrupt this complicated and destructive cycle.
Loss of Neuronal Connections and Cell Death
In Alzheimer’s disease, as neurons are injured and die throughout the brain, connections between networks of neurons may break down, and many brain regions begin to shrink. By the final stages of Alzheimer’s, this process—called brain atrophy—is widespread, causing significant loss of brain volume.
How Many Americans Have Alzheimer’s Disease ?
Estimates vary, but experts suggest that more than 5 million Americans may have Alzheimer’s 13. Unless the disease can be effectively treated or prevented, the number of people with it will increase significantly if current population trends continue. This is because increasing age is the most important known risk factor for Alzheimer’s disease 13.
What Does Alzheimer’s Disease Look Like ?
Memory problems are typically one of the first signs of Alzheimer’s, though initial symptoms may vary from person to person. A decline in other aspects of thinking, such as finding the right words, vision/spatial issues, and impaired reasoning or judgment, may also signal the very early stages of Alzheimer’s disease. Mild cognitive impairment is a condition that can be an early sign of Alzheimer’s, but not everyone with mild cognitive impairment will develop the disease.
People with Alzheimer’s have trouble doing everyday things like driving a car, cooking a meal, or paying bills. They may ask the same questions over and over, get lost easily, lose things or put them in odd places, and find even simple things confusing. As the disease progresses, some people become worried, angry, or violent. Later on, they may become anxious or aggressive, or wander away from home. Eventually, they need total care. This can cause great stress for family members who must care for them.
No treatment can stop the disease. However, some drugs may help keep symptoms from getting worse for a limited time.
How Long Can a Person Live with Alzheimer’s Disease ?
The time from diagnosis to death varies—as little as 3 or 4 years if the person is older than 80 when diagnosed, to as long as 10 or more years if the person is younger 13.
Alzheimer’s disease is currently ranked as the sixth leading cause of death in the United States, but recent estimates indicate that the disorder may rank third, just behind heart disease and cancer, as a cause of death for older people 13.
Although treatment can help manage symptoms in some people, currently there is no cure for this devastating disease 13.
What Causes Alzheimer’s Disease ?
Alzheimer’s disease is an irreversible, progressive brain disorder that slowly destroys memory and thinking skills, and eventually the ability to carry out the simplest tasks 17.
Scientists believe that many factors influence when Alzheimer’s disease begins and how it progresses.
Increasing age is the most important known risk factor for Alzheimer’s disease 17. The number of people with the disease doubles every 5 years beyond age 65. About one-third of all people age 85 and older may have Alzheimer’s disease 17.
The causes of late-onset Alzheimer’s, the most common form of the disease, probably include a combination of genetic, lifestyle, and environmental factors 17. The importance of any one of these factors in increasing or decreasing the risk of development Alzheimer’s may differ from person to person.
Scientists are learning how age-related changes in the brain may harm nerve cells and contribute to Alzheimer’s damage. These age-related changes include atrophy (shrinking) of certain parts of the brain, inflammation, production of unstable molecules called free radicals, and breakdown of energy production within cells.
As scientists learn more about this devastating disease, they realize that genes also play an important role.
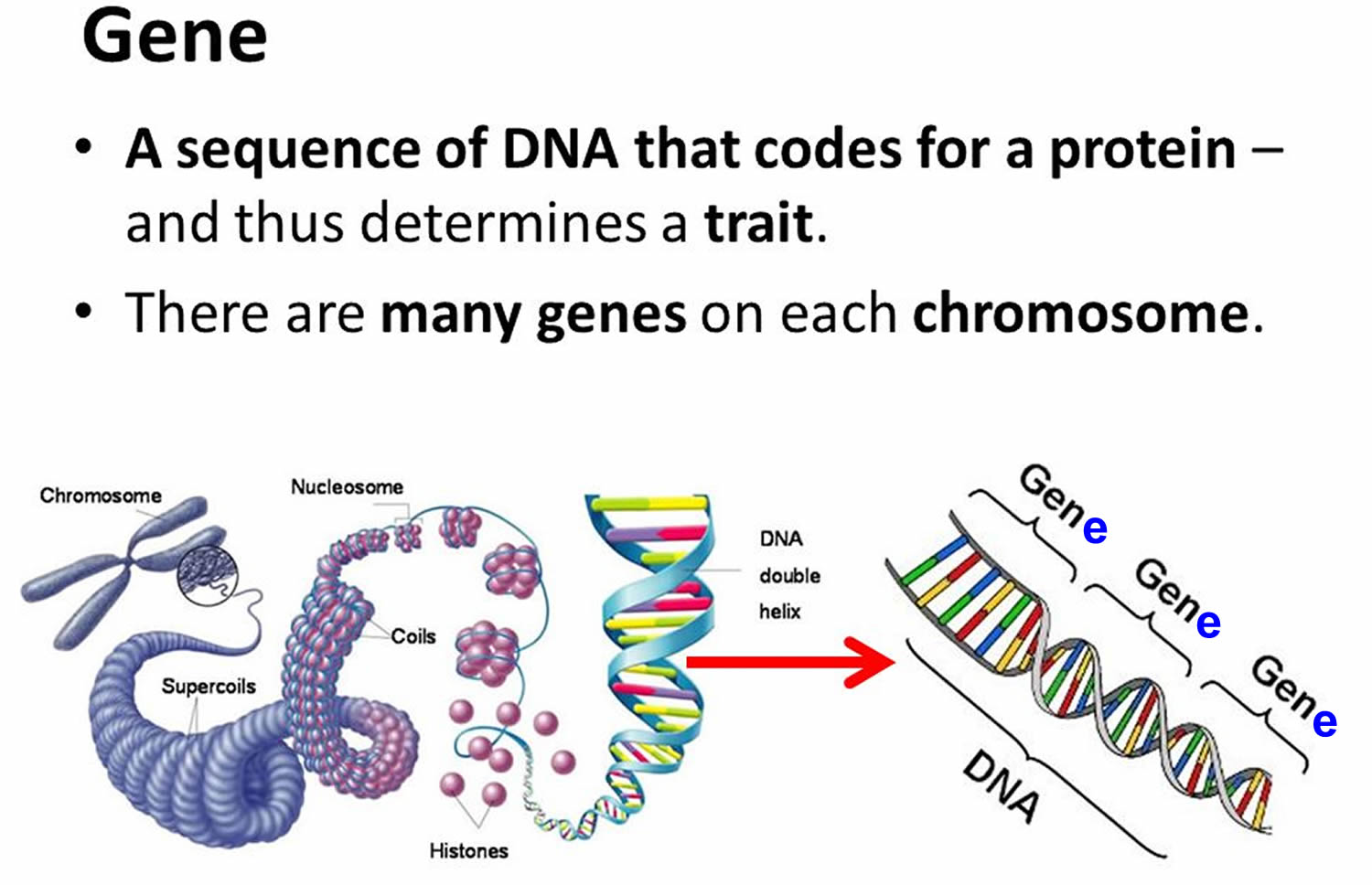
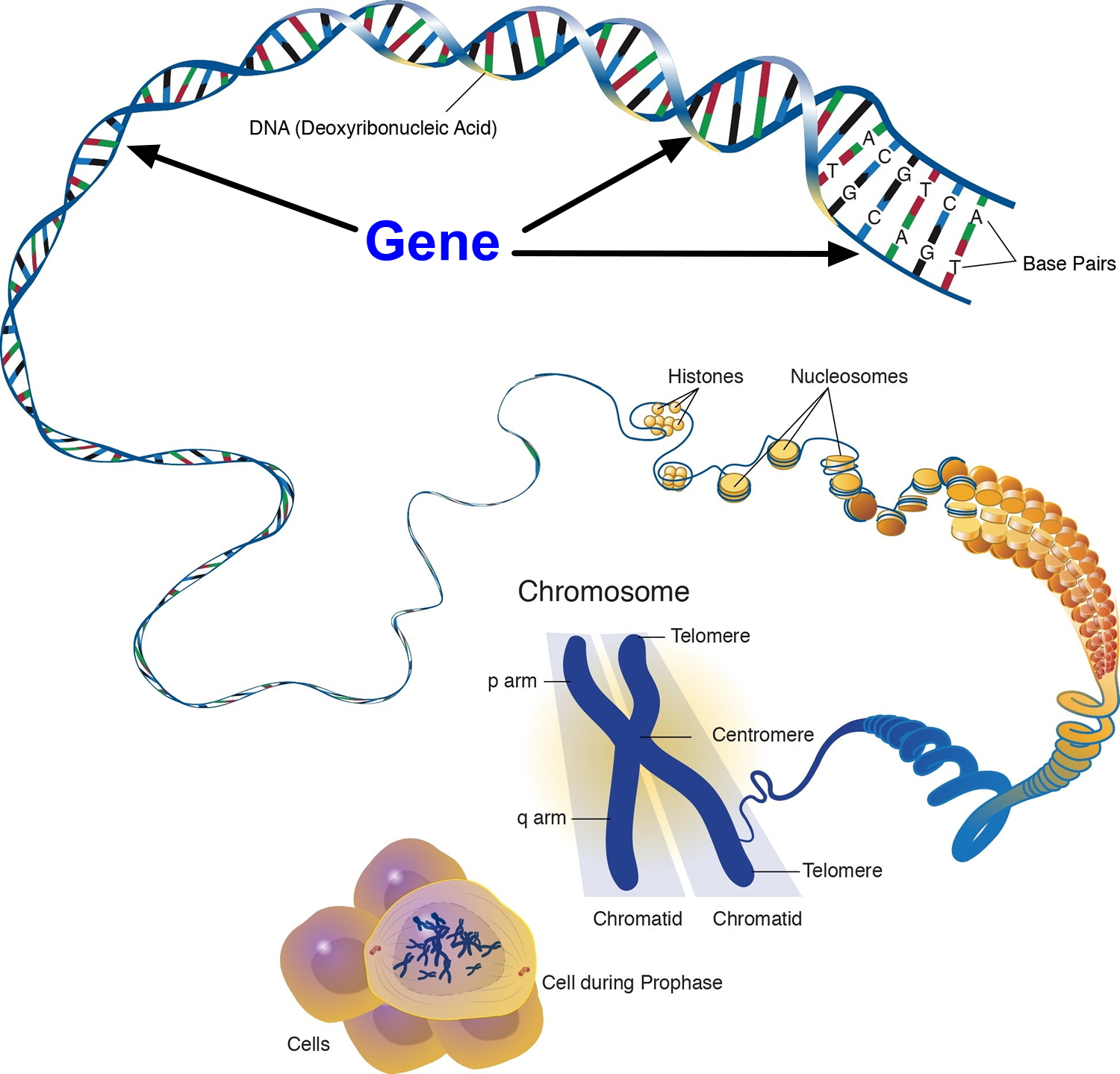
Each human cell contains the instructions a cell needs to do its job. These instructions are made up of DNA, which is packed tightly into structures called chromosomes. Each chromosome has thousands of segments called genes.
Genes are passed down from a person’s birth parents. They carry information that defines traits such as eye color and height. Genes also play a role in keeping the body’s cells healthy. Problems with genes—even small changes to a gene—can cause diseases like Alzheimer’s disease.
Figure 5. Genes
Figure 6. Genes (DNA) come from Chromosomes that reside inside your cells nucleus
Some diseases are caused by a genetic mutation, or permanent change in one or more specific genes. If a person inherits from a parent a genetic mutation that causes a certain disease, then he or she will usually get the disease. Sickle cell anemia, cystic fibrosis, and early-onset familial Alzheimer’s disease are examples of inherited genetic disorders.
In other diseases, a genetic variant may occur. A single gene can have many variants. Sometimes, this difference in a gene can cause a disease directly. More often, a variant plays a role in increasing or decreasing a person’s risk of developing a disease or condition. When a genetic variant increases disease risk but does not directly cause a disease, it is called a genetic risk factor.
Alzheimer’s Disease Genetics
There are two types of Alzheimer’s—early-onset and late-onset. Both types have a genetic component.
Table 1. Some Differences Between Late-Onset and Early-Onset Alzheimer’s Disease
| Late-Onset Alzheimer’s | Early-Onset Alzheimer’s |
|---|---|
| Signs first appear in a person’s mid-60s | Signs first appear between a person’s 30s and mid-60s |
| Most common type (over 90% of Alzheimer’s) | Very rare (under 10% of Alzheimer’s) |
| May involve a gene called APOE ɛ4 | Usually caused by gene changes passed down from parent to child |
Late-Onset Alzheimer’s Disease
Most people with Alzheimer’s disease have the late-onset form of the disease, in which symptoms become apparent in the mid-60s.
Researchers have not found a specific gene that directly causes the late-onset form of the disease 17. However, one genetic risk factor—having one form of the apolipoprotein E (APOE) gene on chromosome 19—does increase a person’s risk. APOE comes in several different forms, or alleles:
- APOE ɛ2 is relatively rare and may provide some protection against the disease. If Alzheimer’s disease occurs in a person with this allele, it usually develops later in life than it would in someone with the APOE ɛ4 gene.
- APOE ɛ3, the most common allele, is believed to play a neutral role in the disease—neither decreasing nor increasing risk.
- APOE ɛ4 increases risk for Alzheimer’s disease and is also associated with an earlier age of disease onset. A person has zero, one, or two APOE ɛ4 alleles. Having more APOE ɛ4 alleles increases the risk of developing Alzheimer’s.
APOE ɛ4 is called a risk-factor gene because it increases a person’s risk of developing the disease. However, inheriting an APOE ɛ4 allele does not mean that a person will definitely develop Alzheimer’s disease. Some people with an APOE ɛ4 allele never get the disease, and others who develop Alzheimer’s disease do not have any APOE ɛ4 alleles.
Early-Onset Alzheimer’s Disease
Early-onset Alzheimer’s disease occurs between a person’s 30s to mid-60s and represents less than 10 percent of all people with Alzheimer’s disease 17. Some cases are caused by an inherited change in one of three genes, resulting in a type known as early-onset Familial Alzheimer’s disease. For other cases of early-onset Alzheimer’s disease, research suggests there may be a genetic component related to factors other than these three genes.
A child whose biological mother or father carries a genetic mutation for early-onset Familial Alzheimer’s disease has a 50/50 chance of inheriting that mutation. If the mutation is in fact inherited, the child has a very strong probability of developing early-onset Familial Alzheimer’s disease.
Early-onset Familial Alzheimer’s disease is caused by any one of a number of different single gene mutations on chromosomes 21, 14, and 1 17. Each of these mutations causes abnormal proteins to be formed. Mutations on chromosome 21 cause the formation of abnormal amyloid precursor protein (APP) 17. A mutation on chromosome 14 causes abnormal presenilin 1 to be made, and a mutation on chromosome 1 leads to abnormal presenilin 2 17.
Each of these mutations plays a role in the breakdown of APP, a protein whose precise function is not yet fully understood. This breakdown is part of a process that generates harmful forms of amyloid plaques, a hallmark of Alzheimer’s disease.
Health, Environmental, and Lifestyle Factors
Research suggests that a host of factors beyond genetics may play a role in the development and course of Alzheimer’s disease. There is a great deal of interest, for example, in the relationship between cognitive decline and vascular conditions such as heart disease, stroke, and high blood pressure, as well as metabolic conditions such as diabetes and obesity. Ongoing research will help us understand whether and how reducing risk factors for these conditions may also reduce the risk of Alzheimer’s.
A nutritious diet, physical activity, social engagement, and mentally stimulating pursuits have all been associated with helping people stay healthy as they age. These factors might also help reduce the risk of cognitive decline and Alzheimer’s disease. Clinical trials are testing some of these possibilities.
How Is Alzheimer’s Disease Diagnosed ?
Doctors use several methods and tools to help determine whether a person who is having memory problems has “possible Alzheimer’s dementia” (dementia may be due to another cause), “probable Alzheimer’s dementia” (no other cause for dementia can be found), or some other problem 18.
To diagnose Alzheimer’s, doctors may 18:
- Ask the person and a family member or friend questions about overall health, use of prescription and over-the-counter medicines, diet, past medical problems, ability to carry out daily activities, and changes in behavior and personality
- Conduct tests of memory, problem solving, attention, counting, and language
- Carry out standard medical tests, such as blood and urine tests, to identify other possible causes of the problem
- Perform brain scans, such as computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET), to rule out other possible causes for symptoms
These tests may be repeated to give doctors information about how the person’s memory and other cognitive functions are changing over time. They can also help diagnose other causes of memory problems, such as stroke, tumor, Parkinson’s disease, sleep disturbances, side effects of medication, an infection, mild cognitive impairment, or a non-Alzheimer’s dementia, including vascular dementia. Some of these conditions may be treatable and possibly reversible.
People with memory problems should return to the doctor every 6 to 12 months 18.
- It’s important to note that Alzheimer’s disease can be definitively diagnosed only after death, by linking clinical measures with an examination of brain tissue in an autopsy 18.
What Happens Next ?
If a primary care doctor suspects mild cognitive impairment or possible Alzheimer’s, he or she may refer the patient to a specialist who can provide a detailed diagnosis or further assessment. Specialists include:
- Geriatricians, who manage health care in older adults and know how the body changes as it ages and whether symptoms indicate a serious problem
- Geriatric psychiatrists, who specialize in the mental and emotional problems of older adults and can assess memory and thinking problems
- Neurologists, who specialize in abnormalities of the brain and central nervous system and can conduct and review brain scans
- Neuropsychologists, who can conduct tests of memory and thinking
Memory clinics and centers, including Alzheimer’s Disease Research Centers, offer teams of specialists who work together to diagnose the problem. Tests often are done at the clinic or center, which can speed up diagnosis.
What Are the Benefits of Early Alzheimer’s Disease Diagnosis ?
Early, accurate diagnosis is beneficial for several reasons. Beginning treatment early in the disease process may help preserve daily functioning for some time, even though the underlying Alzheimer’s process cannot be stopped or reversed.
Having an early diagnosis helps people with Alzheimer’s Disease and their families:
- Plan for the future
- Take care of financial and legal matters
- Address potential safety issues
- Learn about living arrangements
- Develop support networks
In addition, an early diagnosis gives people greater opportunities to participate in clinical trials that are testing possible new treatments for Alzheimer’s disease or in other research studies.
How Is Alzheimer’s Disease Treated ?
Alzheimer’s disease is complex, and it is unlikely that any one drug or other intervention will successfully treat it 19. Current approaches focus on helping people maintain mental function, manage behavioral symptoms, and slow or delay the symptoms of disease.
Several prescription drugs are currently approved by the U.S. Food and Drug Administration (FDA) to treat people who have been diagnosed with Alzheimer’s disease. Treating the symptoms of Alzheimer’s can provide patients with comfort, dignity, and independence for a longer period of time and can encourage and assist their caregivers as well.
Most medicines work best for people in the early or middle stages of Alzheimer’s. For example, they can keep memory loss from getting worse over time. It is important to understand that none of these medications stops the disease itself.
Treatment for Mild to Moderate Alzheimer’s disease
Medications called cholinesterase inhibitors are prescribed for mild to moderate Alzheimer’s disease. These drugs may help delay or prevent symptoms from becoming worse for a limited time and may help control some behavioral symptoms. The medications are Razadyne® (galantamine), Exelon® (rivastigmine), and Aricept® (donepezil) 19.
Scientists do not yet fully understand how cholinesterase inhibitors work to treat Alzheimer’s disease, but research indicates that they prevent the breakdown of acetylcholine, a brain chemical believed to be important for memory and thinking 19. As Alzheimer’s progresses, the brain produces less and less acetylcholine; therefore, cholinesterase inhibitors may eventually lose their effect.
No published study directly compares these drugs. Because they work in a similar way, switching from one of these drugs to another probably will not produce significantly different results. However, an Alzheimer’s patient may respond better to one drug than another.
Treatment for Moderate to Severe Alzheimer’s disease
A medication known as Namenda® (memantine), an N-methyl D-aspartate (NMDA) antagonist, is prescribed to treat moderate to severe Alzheimer’s disease 19. This drug’s main effect is to delay progression of some of the symptoms of moderate to severe Alzheimer’s. It may allow patients to maintain certain daily functions a little longer than they would without the medication. For example, Namenda® may help a patient in the later stages of the disease maintain his or her ability to use the bathroom independently for several more months, a benefit for both patients and caregivers.
The FDA has also approved Aricept® and Namzaric®, a combination of Namenda® and Aricept®, for the treatment of moderate to severe Alzheimer’s disease 19.
Namenda® is believed to work by regulating glutamate, an important brain chemical. When produced in excessive amounts, glutamate may lead to brain cell death. Because NMDA antagonists work very differently from cholinesterase inhibitors, the two types of drugs can be prescribed in combination 19.
Table 1. Medicines used to treat Alzheimer’s Disease
| Drug Name | Drug Type and Use | How It Works | Common Side Effects |
|---|---|---|---|
Aricept® (donepezil) | Cholinesterase inhibitor prescribed to treat symptoms of mild, moderate, and severe Alzheimer’s | Prevents the breakdown of acetylcholine in the brain | Nausea, vomiting, diarrhea, muscle cramps, fatigue, weight loss |
Exelon® (rivastigmine) | Cholinesterase inhibitor prescribed to treat symptoms of mild to moderate Alzheimer’s (patch is also for severe Alzheimer’s) | Prevents the breakdown of acetylcholine and butyrylcholine (a brain chemical similar to acetylcholine) in the brain | Nausea, vomiting, diarrhea, weight loss, indigestion, muscle weakness |
Namenda® (memantine) | N-methyl D-aspartate (NMDA) antagonist prescribed to treat symptoms of moderate to severe Alzheimer’s | Blocks the toxic effects associated with excess glutamate and regulates glutamate activation | Dizziness, headache, diarrhea, constipation, confusion |
Namzaric® (memantine extended-release and donepezil) | NMDA antagonist and cholinesterase inhibitor prescribed to treat symptoms of moderate to severe Alzheimer’s (for patients stabilized on both memantine and donepezil taken separately) | Blocks the toxic effects associated with excess glutamate and prevents the breakdown of acetylcholine in the brain | Headache, nausea, vomiting, diarrhea, dizziness |
Razadyne® (galantamine) | Cholinesterase inhibitor prescribed to treat symptoms of mild to moderate Alzheimer’s | Prevents the breakdown of acetylcholine and stimulates nicotinic receptors to release more acetylcholine in the brain | Nausea, vomiting, diarrhea, decreased appetite, dizziness, headache |
Table 2. Drug Manufacturer’s Recommended Dosage
Drug Name | Manufacturer’s Recommended Dosage | For More Information |
|---|---|---|
Aricept® (donepezil)* |
| For current information about this drug’s safety and use, visit www.aricept.com/prescribing-and-patient-info. |
Exelon® (rivastigmine)* |
| For current information about this drug’s safety and use, visit the www.fda.gov/Drugs. Click on “Drugs @ FDA,” search for Exelon, and click on drug-name links to see “Label Information.” |
Namenda® (memantine)* |
| For current information about this drug’s safety and use, visit www.namenda.com and www.namendaxr.com . See “Full Prescribing Information.” |
Namzaric® (memantine extended-release and donepezil) |
| For current information about this drug’s safety and use, visit www.namzaric.com . Click on “Full Prescribing Information” to see the drug label. |
Razadyne® (galantamine)* |
| For current information about this drug’s safety and use, visit www.janssenmd.com/razadyne. Click on “full Prescribing Information” to see the drug label. |
Note: * Available as a generic drug.
Dosage and Side Effects
Doctors usually start patients at low drug doses and gradually increase the dosage based on how well a patient tolerates the drug. There is some evidence that certain patients may benefit from higher doses of the cholinesterase inhibitors. However, the higher the dose, the more likely side effects are to occur.
Patients should be monitored when a drug is started. All of these medicines have possible side effects, including nausea, vomiting, diarrhea, and loss of appetite. Report any unusual symptoms to the prescribing doctor right away. It is important to follow the doctor’s instructions when taking any medication, including vitamins and herbal supplements. Also, let the doctor know before adding or changing any medications.
Managing Behavior
Common behavioral symptoms of Alzheimer’s include sleeplessness, wandering, agitation, anxiety, aggression, restlessness, and depression 19. Scientists are learning why these symptoms occur and are studying new treatments—drug and nondrug—to manage them. Research has shown that treating behavioral symptoms can make people with Alzheimer’s disease more comfortable and makes things easier for caregivers.
Examples of medicines used to help with depression, aggression, restlessness, and anxiety include:
- Celexa® (citalopram)
- Remeron® (mirtazapine)
- Zoloft® (sertraline)
- Wellbutrin® (bupropion)
- Cymbalta® (duloxetine)
- Tofranil® (imipramine)
Experts agree that medicines to treat these behavior problems should be used only after other strategies that don’t use medicine have been tried 19.
Medicines to be Used with Caution
There are some medicines, such as sleep aids, anti-anxiety drugs, anticonvulsants, and antipsychotics, that a person with Alzheimer’s disease should take only:
- After the doctor has explained all the risks and side effects of the medicine
- After other, safer non-medication options have not helped treat the problem
You will need to watch closely for side effects from these medications.
Sleep aids are used to help people get to sleep and stay asleep. People with Alzheimer’s disease should NOT use these drugs regularly because they make the person more confused and more likely to fall. Examples of these medicines include:
- Ambien® (zolpidem)
- Lunesta® (eszopiclone)
- Sonata® (zaleplon)
Anti-anxiety drugs are used to treat agitation. These drugs can cause sleepiness, dizziness, falls, and confusion. For this reason, doctors recommend using them only for short periods of time. Examples of these medicines include:
- Ativan® (lorazepam)
- Klonopin® (clonazepam)
Anticonvulsants are drugs sometimes used to treat severe aggression. Side effects may cause sleepiness, dizziness, mood swings, and confusion. Examples of these medicines include:
- Depakote® (sodium valproate)
- Tegretol® (carbamazepine)
- Trileptal® (oxcarbazepine)
Antipsychotics are drugs used to treat paranoia, hallucinations, agitation, and aggression. Side effects of using these drugs can be serious, including increased risk of death in some older people with dementia. They should only be given to people with Alzheimer’s disease when the doctor agrees that the symptoms are severe. Examples of these medicines include:
- Risperdal® (risperidone)
- Seroquel® (quetiapine)
- Zyprexa® (olanzapine)
Tips for Dealing with Memory Problems and Forgetfulness
We’ve all forgotten a name, where we put our keys, or if we locked the front door. It’s normal to forget things once in a while. But serious memory problems make it hard to do everyday things. Forgetting how to make change, use the telephone, or find your way home may be signs of a more serious memory problem.
For some older people, memory problems are a sign of mild cognitive impairment, Alzheimer’s disease, or a related dementia. People who are worried about memory problems should see a doctor. Signs that it might be time to talk to a doctor include:
- Asking the same questions over and over again.
- Getting lost in places a person knows well.
- Not being able to follow directions.
- Becoming more confused about time, people, and places.
- Not taking care of oneself—eating poorly, not bathing, or being unsafe.
People with memory complaints should make a follow-up appointment to check their memory after 6 months to a year. They can ask a family member, friend, or the doctor’s office to remind them if they’re worried they’ll forget.
People with some forgetfulness can use a variety of techniques that may help them stay healthy and deal with changes in their memory and mental skills 20. Here are some tips:
- Learn a new skill.
- Stay involved in activities that can help both the mind and body.
- Volunteer in your community, at a school, or at your place of worship.
- Spend time with friends and family.
- Use memory tools such as big calendars, to-do lists, and notes to yourself.
- Put your wallet or purse, keys, and glasses in the same place each day.
- Get lots of rest.
- Exercise and eat well.
- Don’t drink a lot of alcohol.
- Get help if you feel depressed for weeks at a time.
Take Care of Your Health
Taking care of your physical health may help your cognitive health. Cognitive health—the ability to clearly think, learn, and remember—is an important component of your brain health. You can:
- Get recommended health screenings.
- Manage chronic health problems like diabetes, high blood pressure, depression, and high cholesterol.
- Consult with your healthcare provider about the medicines you take and possible side effects on memory, sleep, and brain function.
- Reduce risk for brain injuries due to falls and other accidents.
- Limit use of alcohol (some medicines can be dangerous when mixed with alcohol).
- Quit smoking, if you smoke.
- Get enough sleep, generally 7-8 hours each night.
Eat Healthy Foods
A healthy diet can help reduce the risk of many chronic diseases, such as heart disease or diabetes. It may also help keep your brain healthy.
In general, a healthy diet consists of fruits and vegetables; whole grains; lean meats, fish, and poultry; and low-fat or non-fat dairy products. You should also limit solid fats, sugar, and salt. Be sure to control portion sizes and drink enough water and other fluids.
Researchers are looking at whether a healthy diet can help preserve cognitive function or reduce the risk of Alzheimer’s. For example, there is some evidence that people who eat a “Mediterranean diet” have a lower risk of developing mild cognitive impairment.
Researchers have developed and are testing another diet, called MIND, a combination of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets. One study suggests that a combination of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets may affect the risk of Alzheimer’s disease 21.
Be Physically Active
Being physically active—through regular exercise, household chores, or other activities—has many benefits. It can help you:
- Keep and improve your strength.
- Have more energy.
- Improve your balance.
- Prevent or delay heart disease, diabetes, and other diseases.
- Perk up your mood and reduce depression.
Studies link ongoing physical activity with benefits for the brain, too. In one study, exercise stimulated the human brain’s ability to maintain old network connections and make new ones that are vital to cognitive health 21. Other studies 22 have shown that exercise increased the size of a brain structure important to memory and learning, improving spatial memory.
Aerobic exercise, such as brisk walking, is thought to be more beneficial to cognitive health than non-aerobic stretching and toning exercise. Studies are ongoing.
Federal guidelines recommend that all adults get at least 150 minutes of physical activity each week. Aim to move about 30 minutes on most days. Walking is a good start. You can also join programs that teach you to move safely and prevent falls, which can lead to brain and other injuries. Check with your healthcare provider if you haven’t been active and want to start a vigorous exercise program.
Keep Your Mind Active
Being intellectually engaged may benefit the brain. People who engage in meaningful activities, like volunteering or hobbies, say they feel happier and healthier. Learning new skills may improve your thinking ability, too. For example, one study found that older adults who learned quilting or digital photography had more memory improvement than those who only socialized or did less cognitively demanding activities.
Lots of activities can keep your mind active. For example, read books and magazines. Play games. Take or teach a class. Learn a new skill or hobby. Work or volunteer. These types of mentally stimulating activities have not been proven to prevent serious cognitive impairment or Alzheimer’s disease, but they can be fun!
Scientists think that such activities may protect the brain by establishing “cognitive reserve.” They may help the brain become more adaptable in some mental functions, so it can compensate for age–related brain changes and health conditions that affect the brain.
Formal cognitive training also seems to have benefits. In the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial, healthy adults 65 and older participated in 10 sessions of memory training, reasoning training, or processing–speed training. The sessions improved participants’ mental skills in the area in which they were trained. Most of these improvements persisted 10 years after the training was completed.
Be wary of claims that playing certain computer and online games can improve your memory and other types of thinking. Evidence to back up such claims is evolving. National Institute of Aging and others are supporting research to determine if different types of cognitive training have lasting effects.
Stay Connected
Connecting with other people through social activities and community programs can keep your brain active and help you feel less isolated and more engaged with the world around you. Participating in social activities may lower the risk for some health problems and improve well-being.
So, visit with family and friends. Join programs through your Area Agency on Aging , senior center, or other community organizations.
We don’t know for sure yet if any of these actions can prevent or delay Alzheimer’s disease and age–related cognitive decline. But some of them have been associated with reduced risk of cognitive impairment and dementia.
What is HIV associated dementia ?
When someone has the human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (AIDS) they may develop a complication to the disease which is known as HIV associated dementia, or as AIDS Dementia Complex 23.
AIDS Dementia Complex is a complicated syndrome made up of different nervous system and mental symptoms that can develop in some people with HIV disease. The incidence of ADC is uncommon in people with the early stages of the disease, but may increase as the disease advances to around 7% in people not taking anti-HIV drugs.
Not everyone who has HIV/AIDS will develop AIDS Dementia Complex, but some will.
What is the cause of HIV associated dementia ?
While it has been shown that HIV does not directly infect nerve cells, it is thought that it can somehow infect them indirectly. Immune cells that are present in the brain act as HIV reservoirs and are the primary source of indirect damage to nerve cells.
Symptoms of HIV associated dementia
The following is a list of possible AIDS Dementia Complex symptoms that could also be related to other problems that are not AIDS Dementia Complex.
Possible symptoms of early stage AIDS Dementia Complex are:
- Difficulty concentrating
- Difficulty remembering phone numbers or appointments
- Slowed thinking
- Taking longer to complete complicated tasks
- Difficulty keeping track of daily activities
- Irritability
- Unsteady gait or difficulty keeping balance
- Poor coordination and a change in handwriting
- Depression
Results of mental status tests and other mental capabilities may be normal in the early stages. Symptoms usually develop slowly. In the later stages of ADC they become worse. They may also worsen temporarily when the person is sick with other illnesses.
Possible symptoms of middle stage AIDS Dementia Complex are:
- Symptoms of motor dysfunction, such as muscle weakness
- Poor performance on regular tasks
- Increased concentration and attention required
- Reversing of numbers or words
- Slower responses and frequently dropping objects
- General feelings of indifference or apathy
- Slowness in normal activities, such as eating and writing
- Walking, balance, and coordination requires an increased effort.
Possible symptoms of late stage AIDS Dementia Complex are:
- Loss of bladder or bowel control
- Spastic gait, making walking increasingly difficult
- Loss of initiative or interest
- Withdrawal
- Psychosis or mania
- Confinement to bed.
These symptoms can leave the person confused and unable to make sense of the world. This frequently results in depression.
How is HIV associated dementia diagnosed ?
AIDS Dementia Complex should be diagnosed by someone with knowledge and experience with HIV patients, such as an HIV general practitioner or a medical specialist. The diagnosis of AIDS Dementia Complex is usually made by excluding other possible causes of the symptoms.
However, the main way to diagnose and evaluate AIDS Dementia Complex is a test called the mental status examination. Also, certain laboratory tests, including an examination of cerebrospinal fluid (CSF) can be useful. In addition, the amount of HIV in the CSF seems to correlate with progressive dementia in children. Other tests which can help in the differential diagnosis of AIDS Dementia Complex are CT scans, MRI scans and SPECT scans. These tests help differentiate AIDS Dementia Complex from other brain disorders such as cryptococcal meningitis, toxoplasmosis, lymphoma. An early diagnosis is important as many of the symptoms can be caused by other conditions and illnesses common to people with HIV/AIDS, many of which may be treatable. Some of the symptoms typical of AIDS Dementia Complex are also seen in psychiatric illnesses such as anxiety or depression. If an early diagnosis of AIDS Dementia Complex is made, appropriate treatment and management can be started.
What is the progress of AIDS Dementia Complex ?
The rate of progression varies from person to person. However the disease can lead to complete dependence and death.
Is there treatment available AIDS Dementia Complex ?
AIDS Dementia Complex can be treated to some degree and may even be preventable. The best treatments seem to be the anti-HIV drugs. Initially it was feared that highly active antiretroviral therapy (HAART) would not be effective against HIV in the brain, because many of these drugs do not cross the blood-brain barrier. However recent research has shown evidence of improvements in dementia and other neurological problems due to HAART. Despite these encouraging results, there is evidence that HAART is not as effective against dementia as it is against other opportunistic infections, as dementia is related more to tissue damage rather than removal of an infective organism.
Much of the evidence for the effectiveness of anti-HIV drugs against dementia relates to the drug AZT, mainly because for many years it was the only available anti-HIV drug which crossed the blood-brain barrier to any appreciable extent.
Some of the newer drugs such as d4T, abacavir, nevirapine, indinavir and efavirenz also cross the blood-brain barrier and reduce the amount of HIV in the CSF. However, by treating the HIV outside of the brain, the immune system can recover and fight the HIV inside the brain to help reduce or prevent AIDS Dementia Complex.
Medications that can also relieve some of the symptoms of AIDS Dementia Complex include antipsychotics, antidepressants and anticonvulsants.
Who gets AIDS Dementia Complex ?
People who have HIV/AIDS are at risk of developing AIDS Dementia Complex. Because HIV/AIDS affects so many young people who are enjoying a full and independent lifestyle, there are particular issues such as employment, identity and sexuality which may have to be addressed. HIV/AIDS is still a disease that has a stigma attached to it by many people, and the effect of dementia on top of that can be enormously difficult for all concerned. AIDS Dementia Complex can cause great isolation and loneliness which adds to the daily struggles with the disease.
Alcohol related dementia is, as the name suggests, a form of dementia related to the excessive drinking of alcohol. This affects memory, learning and other mental functions. Korsakoff’s syndrome and Wernicke/Korsakoff syndrome are particular forms of alcohol related brain injury which may be related to alcohol related dementia.
It is currently unclear as to whether alcohol has a direct toxic effect on the brain cells, or whether the damage is due to lack of thiamine, vitamin B1. Nutritional problems, which often accompany consistent or episodic heavy use of alcohol, are thought to be contributing factors. Key parts of the brain may suffer damage through vitamin deficiencies, particularly marked levels of thiamine deficiency and the direct effect that alcohol has on the absorption and use of thiamine.
This can vary from person to person, but generally symptoms will include:
- Impaired ability to learn things
- Personality changes
- Problems with memory
- Difficulty with clear and logical thinking on tasks which require planning, organising, common sense judgement and social skills
- Problems with balance
- Decreased initiative and spontaneity.
Generally skills learned earlier in life and old habits such as language and gestures tend to be relatively unaffected.
Anyone who drinks excessive amounts of alcohol over a period of years may get alcohol related dementia. Males who drink more than six standard alcoholic drinks a day, and women who drink more than four, seem to be at increased risk of developing alcohol related dementia. The risk clearly increases for people who drink high levels of alcohol on a regular basis. The U.S. Department of Health and Human Services Dietary Guidelines for Americans recommends that for health reasons related to the prevention of brain and liver damage adult males should drink no more than two standard drinks per day and adult females should drink no more than one standard drinks per day. One standard drink-equivalent contains 14 grams of pure alcohol.
Some people who drink at high levels do not develop alcohol related dementia, but it is not currently possible to understand and predict who will and who won’t develop alcohol related dementia. Some people who develop alcohol related dementia might also show some degree of recovery over time if they reduce alcohol intake to safe levels or abstain from alcohol and maintain good health. Alcohol related dementia can affect both men and women of any age.
At an early stage of the disease, problems may be reduced or reversed if the person abstains from alcohol, improves their diet and replace vitamins especially thiamine and vitamin B1. Thiamine is important to limit some of the toxic effects of alcohol, and is an important supplement for heavy drinkers.
Community support is available for the person with dementia, their family and carers. This support can make a positive difference to managing dementia.
Many people who develop alcohol related dementia are young, and this can mean that they and their family and carers will need extra consideration.
What is Creutzfeldt-Jakob disease ?
Creutzfeldt-Jakob disease (CJD) is a rare, degenerative, invariably fatal brain disorder that is characterized by rapidly progressive dementia 24. It affects about one person in every one million people per year worldwide; in the United States there are about 300 cases per year. Creutzfeldt-Jakob disease usually appears in later life and runs a rapid course. Typically, onset of symptoms occurs about age 60, and about 90 percent of individuals die within 1 year. In the early stages of disease, people may have failing memory, behavioral changes, lack of coordination and visual disturbances. As the illness progresses, mental deterioration becomes pronounced and involuntary movements, blindness, weakness of extremities, and coma may occur 25. This condition often leads to death within a few weeks or months after symptoms begin. About 90 percent of patients do not survive for more than one year.
There are three major categories of Creutzfeldt-Jakob disease:
- In sporadic Creutzfeldt-Jakob disease, the disease appears even though the person has no known risk factors for the disease. This is by far the most common type of Creutzfeldt-Jakob disease and accounts for at least 85 percent of cases.
- In hereditary Creutzfeldt-Jakob disease, the person has a family history of the disease and/or tests positive for a genetic mutation associated with Creutzfeldt-Jakob disease. About 5 to 10 percent of cases of Creutzfeldt-Jakob disease in the United States are hereditary.
- In acquired Creutzfeldt-Jakob disease, the disease is transmitted by exposure to brain or nervous system tissue, usually through certain medical procedures. There is no evidence that Creutzfeldt-Jakob disease is contagious through casual contact with a Creutzfeldt-Jakob disease patient. Since Creutzfeldt-Jakob disease was first described in 1920, fewer than 1 percent of cases have been acquired Creutzfeldt-Jakob disease.
Creutzfeldt-Jakob disease belongs to a family of human and animal diseases known as the transmissible spongiform encephalopathies (TSEs). Spongiform refers to the characteristic appearance of infected brains, which become filled with holes until they resemble sponges under a microscope. Creutzfeldt-Jakob disease is the most common of the known human TSEs. Other human TSEs include kuru, fatal familial insomnia (FFI), and Gerstmann-Straussler-Scheinker disease (GSS). Kuru was identified in people of an isolated tribe in Papua New Guinea and has now almost disappeared. FFI and GSS are extremely rare hereditary diseases, found in just a few families around the world. Other TSEs are found in specific kinds of animals. These include bovine spongiform encephalopathy (BSE), which is found in cows and is often referred to as “mad cow” disease; scrapie, which affects sheep and goats; mink encephalopathy; and feline encephalopathy. Similar diseases have occurred in elk, deer, and exotic zoo animals.
Creutzfeldt-Jakob disease can be very difficult to diagnose because it is similar to other forms of dementia 25. The only way to confirm the diagnosis is to test a small sample of brain tissue, which can be done by brain biopsy or autopsy. Creutzfeldt-Jakob disease is caused by the build up of abnormal prion proteins in the brain. For most patients, the reason for the abnormal prions is unknown (sporadic Creutzfeldt-Jakob disease). About 5 to 10 percent of cases are due to an inherited genetic mutation associated with Creutzfeldt-Jakob disease (familial Creutzfeldt-Jakob disease). This condition can also be acquired through contact with infected brain tissue (iatrogenic Creutzfeldt-Jakob disease) or consuming infected beef (variant Creutzfeldt-Jakob disease). There is no specific treatment for Creutzfeldt-Jakob disease, so the goal is to make a person as comfortable as possible 24, 26.
What causes Creutzfeldt-Jakob disease ?
Some researchers believe an unusual “slow virus” or another organism causes Creutzfeldt-Jakob disease. However, they have never been able to isolate a virus or other organism in people with the disease. Furthermore, the agent that causes Creutzfeldt-Jakob disease has several characteristics that are unusual for known organisms such as viruses and bacteria. It is difficult to kill, it does not appear to contain any genetic information in the form of nucleic acids (DNA or RNA), and it usually has a long incubation period before symptoms appear. In some cases, the incubation period may be as long as 50 years. The leading scientific theory at this time maintains that Creutzfeldt-Jakob disease and the other TSEs are caused by a type of protein called a prion.
Prion proteins occur in both a normal form, which is a harmless protein found in the body’s cells, and in an infectious form, which causes disease. The harmless and infectious forms of the prion protein have the same sequence of amino acids (the “building blocks” of proteins) but the infectious form of the protein takes a different folded shape than the normal protein. Sporadic Creutzfeldt-Jakob disease may develop because some of a person’s normal prions spontaneously change into the infectious form of the protein and then alter the prions in other cells in a chain reaction.
Once they appear, abnormal prion proteins aggregate, or clump together. Investigators think these protein aggregates may lead to the neuron loss and other brain damage seen in Creutzfeldt-Jakob disease. However, they do not know exactly how this damage occurs.
About 5 to 10 percent of all Creutzfeldt-Jakob disease cases are inherited. These cases arise from a mutation, or change, in the gene that controls formation of the normal prion protein. While prions themselves do not contain genetic information and do not require genes to reproduce themselves, infectious prions can arise if a mutation occurs in the gene for the body’s normal prion protein. If the prion protein gene is altered in a person’s sperm or egg cells, the mutation can be transmitted to the person’s offspring. All mutations in the prion protein gene are inherited as dominant traits. Therefore, family history is helpful in considering the diagnosis. Several different mutations in the prion gene have been identified. The particular mutation found in each family affects how frequently the disease appears and what symptoms are most noticeable. However, not all people with mutations in the prion protein gene develop Creutzfeldt-Jakob disease.
Symptoms of Creutzfeldt-Jakob disease
Initially, patients experience problems with muscular coordination; personality changes, including impaired memory, judgment, and thinking; and impaired vision. People with the disease also may experience insomnia, depression, or unusual sensations. Creutzfeldt-Jakob disease does not cause a fever or other flu-like symptoms. As the illness progresses, the patients’ mental impairment becomes severe. They often develop involuntary muscle jerks called myoclonus, and they may go blind. They eventually lose the ability to move and speak and enter a coma. Pneumonia and other infections often occur in these patients and can lead to death 24.
There are several known variants of Creutzfeldt-Jakob disease. These variants differ somewhat in the symptoms and course of the disease. For example, a variant form of the disease-called new variant or variant (nv-Creutzfeldt-Jakob disease, v-Creutzfeldt-Jakob disease), described in Great Britain and France-begins primarily with psychiatric symptoms, affects younger patients than other types of Creutzfeldt-Jakob disease, and has a longer than usual duration from onset of symptoms to death. Another variant, called the panencephalopathic form, occurs primarily in Japan and has a relatively long course, with symptoms often progressing for several years. Scientists are trying to learn what causes these variations in the symptoms and course of the disease 24.
Some symptoms of Creutzfeldt-Jakob disease can be similar to symptoms of other progressive neurological disorders, such as Alzheimer’s or Huntington’s disease. However, Creutzfeldt-Jakob disease causes unique changes in brain tissue which can be seen at autopsy. It also tends to cause more rapid deterioration of a person’s abilities than Alzheimer’s disease or most other types of dementia.
How is Creutzfeldt-Jakob disease diagnosed ?
There is currently no single diagnostic test for Creutzfeldt-Jakob disease. When a doctor suspects Creutzfeldt-Jakob disease, the first concern is to rule out treatable forms of dementia such as encephalitis (inflammation of the brain) or chronic meningitis. A neurological examination will be performed and the doctor may seek consultation with other physicians. Standard diagnostic tests will include a spinal tap to rule out more common causes of dementia and an electroencephalogram (EEG) to record the brain’s electrical pattern, which can be particularly valuable because it shows a specific type of abnormality in Creutzfeldt-Jakob disease. Computerized tomography of the brain can help rule out the possibility that the symptoms result from other problems such as stroke or a brain tumor. Magnetic resonance imaging (MRI) brain scans also can reveal characteristic patterns of brain degeneration that can help diagnose Creutzfeldt-Jakob disease.
The only way to confirm a diagnosis of Creutzfeldt-Jakob disease is by brain biopsy or autopsy. In a brain biopsy, a neurosurgeon removes a small piece of tissue from the patient’s brain so that it can be examined by a neuropathologist. This procedure may be dangerous for the individual, and the operation does not always obtain tissue from the affected part of the brain. Because a correct diagnosis of Creutzfeldt-Jakob disease does not help the person, a brain biopsy is discouraged unless it is needed to rule out a treatable disorder. In an autopsy, the whole brain is examined after death. Both brain biopsy and autopsy pose a small, but definite, risk that the surgeon or others who handle the brain tissue may become accidentally infected by self-inoculation. Special surgical and disinfection procedures can minimize this risk. A fact sheet with guidance on these procedures is available from the National Institute of Neurological Disorders and Stroke and the World Health Organization.
Scientists are working to develop laboratory tests for Creutzfeldt-Jakob disease. One such test, developed at the National Institute of Neurological Disorders and Stroke, is performed on a person’s cerebrospinal fluid and detects a protein marker that indicates neuronal degeneration. This can help diagnose Creutzfeldt-Jakob disease in people who already show the clinical symptoms of the disease. This test is much easier and safer than a brain biopsy. The false positive rate is about 5 to 10 percent. Scientists are working to develop this test for use in commercial laboratories. They are also working to develop other tests for this disorder.
How is Creutzfeldt-Jakob disease treated ?
There is no treatment that can cure or control Creutzfeldt-Jakob disease. Researchers have tested many drugs, including amantadine, steroids, interferon, acyclovir, antiviral agents, and antibiotics. Studies of a variety of other drugs are now in progress. However, so far none of these treatments has shown any consistent benefit in humans.
Current treatment for Creutzfeldt-Jakob disease is aimed at alleviating symptoms and making the individual as comfortable as possible. Opiate drugs can help relieve pain if it occurs, and the drugs clonazepam and sodium valproate may help relieve myoclonus. During later stages of the disease, changing the person’s position frequently can keep him or her comfortable and helps prevent bedsores. A catheter can be used to drain urine if the individual cannot control bladder function, and intravenous fluids and artificial feeding also may be used.
How is Creutzfeldt-Jakob disease transmitted ?
CJD cannot be transmitted through the air or through touching or most other forms of casual contact. Spouses and other household members of sporadic CJD patients have no higher risk of contracting the disease than the general population. However, exposure to brain tissue and spinal cord fluid from infected individuals should be avoided to prevent transmission of the disease through these materials.
In some cases, CJD has spread to other people from grafts of dura mater (a tissue that covers the brain), transplanted corneas, implantation of inadequately sterilized electrodes in the brain, and injections of contaminated pituitary growth hormone derived from human pituitary glands taken from cadavers. Doctors call these cases that are linked to medical procedures iatrogenic cases. Since 1985, all human growth hormone used in the United States has been synthesized by recombinant DNA procedures, which eliminates the risk of transmitting CJD by this route.
The appearance of the new variant of Creutzfeldt-Jakob disease (nv-CJD or v-CJD) in several younger than average people in Great Britain and France has led to concern that BSE may be transmitted to humans through consumption of contaminated beef. Although laboratory tests have shown a strong similarity between the prions causing BSE and v-CJD, there is no direct proof to support this theory.
Many people are concerned that it may be possible to transmit Creutzfeldt-Jakob disease through blood and related blood products such as plasma. Some animal studies suggest that contaminated blood and related products may transmit the disease, although this has never been shown in humans. If there are infectious agents in these fluids, they are probably in very low concentrations. Scientists do not know how many abnormal prions a person must receive before he or she develops CJD, so they do not know whether these fluids are potentially infectious or not. They do know that, even though millions of people receive blood transfusions each year, there are no reported cases of someone contracting CJD from a transfusion. Even among people with hemophilia, who sometimes receive blood plasma concentrated from thousands of donors, there are no reported cases of CJD.
While there is no evidence that blood from people with sporadic CJD is infectious, studies have found that infectious prions from BSE and vCJD may accumulate in the lymph nodes (which produce white blood cells), the spleen, and the tonsils. These findings suggest that blood transfusions from people with vCJD might transmit the disease. The possibility that blood from people with vCJD may be infectious has led to a policy preventing people in the United States from donating blood if they have resided for more than 3 months in a country or countries where BSE is common.`
How can people avoid spreading Creutzfeldt-Jakob disease ?
To reduce the already very low risk of CJD transmission from one person to another, people should never donate blood, tissues, or organs if they have suspected or confirmed CJD, or if they are at increased risk because of a family history of the disease, a dura mater graft, or other factor.
Normal sterilization procedures such as cooking, washing, and boiling do not destroy prions. Caregivers, healthcare workers, and undertakers should take the following precautions when they are working with a person with CJD:
- Cover cuts and abrasions with waterproof dressings.
- Wear surgical gloves when handling a patient’s tissues and fluids or dressing the patient’s wounds.
- Avoid cutting or sticking themselves with instruments contaminated by the patient’s blood or other tissues.
- Use disposable bedclothes and other cloth for contact with the patient. If disposable materials are not available, regular cloth should be soaked in undiluted chlorine bleach for an hour or more, and then washed in a normal fashion after each use.
- Use face protection if there is a risk of splashing contaminated material such as blood or cerebrospinal fluid.
- Soak instruments that have come in contact with the patient in undiluted chlorine bleach for an hour or more, then use an autoclave (pressure cooker) to sterilize them in distilled water for at least one hour at 132 – 134 degrees Centigrade.
What is Huntington’s disease ?
Huntington’s disease is an inherited disorder that causes degeneration of brain cells, called neurons, in motor control regions of the brain, as well as other areas 27. Symptoms of Huntington’s disease, which gets progressively worse, include uncontrolled movements (called chorea), abnormal body postures, and changes in behavior, emotion, judgment, and cognition. People with Huntington’s disease also develop impaired coordination, slurred speech, and difficulty feeding and swallowing. Huntington’s disease typically begins between ages 30 and 50 27. An earlier onset form called juvenile Huntington’s disease, occurs under age 20 27. Symptoms of juvenile Huntington’s disease differ somewhat from adult onset Huntington’s disease and include unsteadiness, rigidity, difficulty at school, and seizures. More than 30,000 Americans have Huntington’s disease 27.
Huntington’s disease is caused by a mutation in the gene for a protein called huntingtin. The defect causes the cytosine, adenine, and guanine (CAG) building blocks of DNA to repeat many more times than is normal. Each child of a parent with Huntington’s disease has a 50-50 chance of inheriting the Huntington’s disease gene 27. If a child does not inherit the Huntington’s disease gene, he or she will not develop the disease and generally cannot pass it to subsequent generations. There is a small risk that someone who has a parent with the mutated gene but who did not inherit the Huntington’s disease gene may pass a possibly harmful genetic sequence to her/his children. A person who inherits the Huntington’s disease gene will eventually develop the disease 27. A genetic test, coupled with a complete medical history and neurological and laboratory tests, helps physicians diagnose Huntington’s disease 27.
Is there treatment for Huntington’s disease ?
There is no treatment that can stop or reverse the course of Huntington’s disease. Tetrabenazine and deuterabenazine are prescribed for treating the chorea associated with Huntington’s disease. Antipsychotic drugs may help to alleviate chorea and may also be used to help control hallucinations, delusions, and violent outbursts. Drugs may be prescribed to treat depression and anxiety. Drugs used to treat the symptoms of Huntington’s disease may have side effects such as fatigue, sedation, decreased concentration, restlessness, or hyperexcitability, and should be only used when symptoms create problems for the individual.
What is the prognosis for Huntington’s disease ?
Huntington’s disease causes disability that gets worse over time. People with this disease usually die within 15 to 20 years following diagnosis. At this time, no treatment is available to slow, stop or reverse the course of Huntington’s disease.
- National Health Service of UK. Symptoms of dementia. http://www.nhs.uk/Conditions/dementia-guide/Pages/symptoms-of-dementia.aspx[↩]
- U.S. National Library of Medicine. MedlinePlus. Dementia. https://medlineplus.gov/dementia.html[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- National Institutes of Health. National Institute on Aging. What Is Dementia ? https://www.nia.nih.gov/health/what-dementia[↩][↩]
- National Institutes of Health. National Institute on Aging. What Is Dementia ? https://www.nia.nih.gov/health/what-dementia[↩][↩][↩][↩][↩]
- Alzheimer’s Australia. What is dementia ? https://www.fightdementia.org.au/about-dementia/what-is-dementia[↩]
- National Institutes of Health. National Institute on Aging. Types of Dementia. https://www.nia.nih.gov/health/what-dementia[↩]
- National Institutes of Health. National Institute on Aging. Diagnosing Dementia. https://www.nia.nih.gov/health/diagnosing-dementia[↩][↩][↩][↩]
- National Health Services of U.K. Tests for diagnosing dementia. http://www.nhs.uk/Conditions/dementia-guide/Pages/dementia-diagnosis-tests.aspx[↩][↩][↩]
- Oxford Medical Education. Mini-mental state examination (MMSE). http://www.oxfordmedicaleducation.com/geriatrics/mini-mental-state-examination-mmse/[↩]
- National Institutes of Health. National Institute on Aging. Do Memory Problems Always Mean Alzheimer’s Disease ? https://www.nia.nih.gov/health/do-memory-problems-always-mean-alzheimers-disease[↩]
- U.S. National Library of Medicine. MedlinePlus. Mild Cognitive Impairment. https://medlineplus.gov/mildcognitiveimpairment.html[↩][↩][↩]
- National Institutes of Health. National Institute on Aging. What Is Mild Cognitive Impairment ? https://www.nia.nih.gov/health/what-mild-cognitive-impairment[↩][↩][↩][↩][↩][↩][↩][↩]
- National Institutes of Health. National Institute on Aging. What Is Alzheimer’s Disease ? https://www.nia.nih.gov/health/what-alzheimers-disease[↩][↩][↩][↩][↩][↩][↩][↩]
- U.S. National Library of Medicine. MedlinePlus. Alzheimer’s Disease. https://medlineplus.gov/alzheimersdisease.html[↩][↩][↩][↩]
- Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001. Neuroglial Cells. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10869/[↩]
- National Institutes of Health. National Institute on Aging. What Happens to the Brain in Alzheimer’s Disease ? https://www.nia.nih.gov/health/what-happens-brain-alzheimers-disease[↩][↩]
- National Institutes of Health. National Institute on Aging. What Causes Alzheimer’s Disease ? https://www.nia.nih.gov/health/what-causes-alzheimers-disease[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- National Institutes of Health. National Institute on Aging. How Is Alzheimer’s Disease Diagnosed ? https://www.nia.nih.gov/health/how-alzheimers-disease-diagnosed[↩][↩][↩][↩]
- National Institutes of Health. National Institute on Aging. How Is Alzheimer’s Disease Treated ? https://www.nia.nih.gov/health/how-alzheimers-disease-treated[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- National Institutes of Health. National Institute on Aging. Noticing Memory Problems ? What to Do Next. https://www.nia.nih.gov/health/noticing-memory-problems-what-do-next[↩]
- National Institutes of Health. National Institute on Aging. Cognitive Health and Older Adults. https://www.nia.nih.gov/health/cognitive-health-and-older-adults[↩][↩]
- Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3017-3022. doi:10.1073/pnas.1015950108. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3041121/[↩]
- Alzheimer’s Australia. HIV associated dementia. https://www.fightdementia.org.au/about-dementia/types-of-dementia/aids-related-dementia[↩]
- Creutzfeldt-Jakob Disease Fact Sheet. National Institute of Neurological Disorders and Stroke (NINDS). February 2, 2016; https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Creutzfeldt-Jakob-Disease-Fact-Sheet[↩][↩][↩][↩]
- National Institutes of Health. Genetic and Rare Diseases Information Center. Creutzfeldt-Jakob disease. https://rarediseases.info.nih.gov/diseases/6956/creutzfeldt-jakob-disease[↩][↩]
- CJD Fact Sheet. Creutzfeld-Jakob Disease Foundation. http://www.cjdfoundation.org/webfm_send/76.[↩]
- National Institutes of Neurological Disorders and Stroke. Huntington’s disease. https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page[↩][↩][↩][↩][↩][↩][↩]