Contents
- What is azoospermia
- Does having azoospermia mean that the testis makes no sperm?
- Is azoospermia common?
- Can a man have normal testosterone and azoospermia?
- Can I get pregnant naturally if my husband has azoospermia?
- I had a semen analysis showing azoospermia, what should I do?
- What is the role of varicocelectomy in men with azoospermia?
- What is the role of hormone therapy for men with azoospermia?
- Is IVF the only option if my partner has azoospermia?
- Do insurance plans cover infertility treatment?
- Male Reproductive System
- Azoospermia causes
- Azoospermia prevention
- Azoospermia symptoms
- Azoospermia complications
- Azoospermia diagnosis
- Azoospermia treatment
- Azoospermia Prognosis
What is azoospermia
Azoospermia defined as the total absence of spermatozoa (sperm) in a man ejaculates (semen) in two successive semen examinations 1. If no spermatozoa are observed in the wet preparation, the World Health Organization (WHO) recommends an examination of the centrifuged sample (3000 X g or greater for 15 minutes) 2. If no sperm are observed in the centrifuged sample, the semen analysis should be repeated. The presence of a small number of spermatozoa in either of the centrifuged samples is defined as cryptozoospermia, and the complete absence of spermatozoa is defined as azoospermia 2. Azoospermia accounts for around 10% of cases of male infertility, and affects about 1% of the men in the general population 3. This may be because the man does not make sperm or because the sperm is blocked from entering the semen. Azoospermia can be classified as non-obstructive azoospermia associated with spermatogenesis failure and obstructive azoospermia characterized by an obstruction in the seminal tract and normal spermatogenesis. Whereas non-obstructive azoospermia accounts for 60% of azoospermic patients, obstructive azoospermia accounts for around 40% 4.
Azoospermia may be caused by hormone problems, obstructive or nonobstructive blockages in the reproductive tract, certain genetic conditions, previous vasectomy or other surgery, or other conditions. Azoospermia may also be caused by certain cancer treatments. Azoospermia can cause infertility (the inability to produce children).
Of the men presenting for fertility investigation, up to 20% are found to be azoospermic. These men can be categorized as having either 5:
- Pre-testicular azoospermia (2% of men with azoospermia, due to a hypothalamic or pituitary abnormality diagnosed with hypo-gonadotropic-hypogonadism),
- Testicular failure or non-obstructive azoospermia (49% to 93%, while the term testicular failure would seem to indicate a complete absence of spermatogenesis, actually men with testicular failure have either reduced spermatogenesis [hypospermatogenesis], maturation arrest at either an early or late stage of spermatogenesis or a complete failure of spermatogenesis noted with Sertoli-cell only syndrome),1–5
- Post-testicular obstruction or retrograde ejaculation (7% to 51%, normal spermatogenesis but obstructive azoospermia or retrograde ejaculation) 6.
A further group of men have a failure to ejaculate. These may be men with spinal cord injury, psychogenic failure to ejaculate or neurological damage (sympathetic nerve damage from a retroperitoneal lymph node dissection for example).
To understand the management of azoospermia, it is important to also understand the assisted reproductive technologies (ARTs) (e.g., in-vitro fertilization [IVF]). Since the 1970s, breakthroughs in the assisted reproductive technologies (ARTs) have allowed the ART clinics to offer potentially successful treatments for up to 98% of couples with male factor infertility 7. These significant advances had little to do with techniques to improve the sperm quality but relied on ARTs. These programs used techniques to increase the number of mature eggs produced by the women by manipulating the hormonal environment in the women using exogenous hormones (ovulation induction) followed by:
- Timed insemination: timed to optimize the pregnancy rates: either through intercourse or intra-uterine insemination of the partners washed sperm; or
- In-vitro fertilization (IVF): oocytes are retrieved from the ovaries then are either incubated with the sperm in a dish; or
- Intra-cytoplasmic sperm injection (ICSI): injecting the sperm directly into the cytoplasm of the oocyte.
All of the above techniques are widely used to treat couples with male factor infertility.
Using intra-cytoplasmic sperm injection (ICSI), it is now possible to produce a pregnancy with any live sperm (moving or not), from either the semen or any site within the male reproductive tract. Even men with azoospermia can now be offered sperm retrieval with ICSI. Sperm could be retrieved from any site in the reproductive tract and used for ICSI. These are the men who previously had very limited chances to ever have biologically related children. Pregnancy rates of close to 50% per cycle of ICSI (women under 35 years old) are expected, with the pregnancy rates independent of the site of the origin of the sperm 7.
Azoospermia key facts
- a) Azoospermia is defined as the absence of sperm in at least two different ejaculate samples (including the centrifuged sediment) 8. Ten to fifteen percent of couples in the general population suffer from infertility issues 9. Approximately 50% of these cases can be attributable to a male issue. Ten to twenty percent of these men (or 1% of men in the general population) suffer from azoospermia-induced infertility 9.
- b) A complete medical history, physical examination and hormonal investigation are the principal components of the initial evaluation of the azoospermic male 10.
- c) Sperm production is controlled by the hypothalamo-pituitary-gonadal axis.
- d) Scrotal ultrasonography, transrectal ultrasound (TRUS), TRUS-guided seminal vesiculography, seminal tract washout, vasography, endorectal magnetic resonance imaging, abdominal ultrasound and cranial imaging studies can be performed when evaluating the azoospermic male.
- e) In the evaluation of azoospermic patients who have normal-sized testes and a normal hormone profile, testicular biopsy has a critical role in distinguishing between obstructive azoospermia and non-obstructive azoospermia. It is best to plan for the cryopreservation of sperm at the time of the biopsy, if feasible.
- f) Diagnostic testicular biopsies are of limited value in men with small testes and elevated FSH levels (greater than twice the upper limit), which support a diagnosis of non-obstructive azoospermia. It is recommended that such patients undergo a microTESE (testicular sperm extraction) combined with cryopreservation and subsequent in vitro fertilization via intracytoplasmic sperm injection (ICSI); this procedure would enable several samples to be taken 10.
- g) Genetic factors are important in the evaluation and management of azoospermic males. These factors can be pretesticular (Kallmann syndrome), testicular (Klinefelter’s syndrome or Y chromosome microdeletions) or post-testicular (CBAVD). Genetic counseling provides couples with information about the nature, inheritance patterns, and implications of genetic disorders to help them make informed medical and personal decisions.
Does having azoospermia mean that the testis makes no sperm?
Not necessarily. The testis can be making sperm, but it might not be enough to have any noticeable amount come out in the ejaculate.
Is azoospermia common?
Yes. Around 10 percent of infertile men and 1 percent of all men have azoospermia.
Can a man have normal testosterone and azoospermia?
Yes and yes. Sperm come from “germ cells” in small tubules within the testis. Testosterone comes from “Leydig” or “interstitial” cells in between the tubules. Since Leydig cells are more resilient than germ cells, they will often function partially or fully, even in a damaged or poorly formed testicle.
Can I get pregnant naturally if my husband has azoospermia?
Yes, you still may be able to get pregnant naturally. This depends on the cause of azoospermia your partner has and if it’s treatable. Your doctor is the best person to discuss treatment and family planning with.
I had a semen analysis showing azoospermia, what should I do?
Aside from seeing a specialist in male infertility, the first step would be to get a repeat semen analysis at a lab that has a lot of experience doing semen and sperm tests, because results can vary a lot from test to test and lab to lab. Also, having small numbers of sperm can change the management/treatment options drastically, so the first step should be getting proper confirmation of the finding.
What is the role of varicocelectomy in men with azoospermia?
This remains controversial. There is some evidence that a small percentage of men with azoospermia due to testicular failure may benefit from treatment of a clinical varicocele 11. It is considered reasonable to offer men with clinical varicoceles and testicular failure a varicocele repair, but it is important to warn men that there is a low probability that this will result in any improvement in his semen parameters.
What is the role of hormone therapy for men with azoospermia?
Apart from the management of men with hypogonadotropic hypogonadism, the use of hormones to treat men with azoospermia should be discouraged. The use of androgens is contraindicated.
Is IVF the only option if my partner has azoospermia?
It depends on the cause of azoospermia. If the cause is testicular and requires surgical sperm retrieval, then in-vitro fertilization (IVF) is the only option. If the azoospermia is treatable, couples may be able to avoid IVF.
Do insurance plans cover infertility treatment?
The degree of services covered depends on where you live and the type of insurance plan you have. Several states currently have laws that require insurers to either cover or offer to cover some form of infertility diagnosis and treatment. However, the laws vary greatly in their scope of what is and is not required to be covered. For more information about the specific laws for each of those states, please call your state’s Insurance Commissioner’s office or to learn about pending insurance legislation in your state, please contact your State Representatives.
Whether or not you live in a state with an infertility insurance law, you may want to consult with your employer’s director of human resources to determine the exact coverage your plan provides. Another good source of assistance is RESOLVE, an infertility patient advocacy and information organization.
Male Reproductive System
The male reproductive system creates sperm cells that are produced in the seminiferous tubules within each testicle. The sperm have to reach the uterus and the fallopian tube in order to fertilize a woman’s egg.
The male reproductive system includes the testicles, epididymis, vas deferens, seminal vesicles, prostate gland, urethra, and penis.
Figure 1. Male reproductive system
Sperm
Sperm are male reproductive cells. They are produced in the two testicles (testes). The testes are contained in the scrotal sac (scrotum).
Structure of sperm
A mature sperm cell has three parts:
- The head consists of the nucleus, which is surrounded by the acrosome. The nucleus contains genetic material. The acrosome contains enzymes that penetrate the egg and allow the sperm to enter.
- The middle part (body) contains mitochondria, which supply energy for the sperm to swim into the female genital tract.
- The tail of the sperm also known as the flagellum. The tail moves with whip-like movements back and forth to propel the sperm toward the egg.
Figure 2. Sperm (spermatozoa) structure
Footnotes: Sperm (spermatozoa) are composed of two main parts: head and tail (also called flagellum). The sperm head is constituted basically by the acrosome and nucleus. The sperm tail includes: the neck that contains mainly the proximal centriole; the midpiece which is composed by mitochondria, outer dense fibers (ODF) and axoneme; principal piece containing the fibrous sheath and axoneme; and terminal piece.
[Source 12 ]Sperm head
Within the sperm head is a limited quantity of cytoplasm, highly condensed DNA, and a well-delimited acrosome. A substantial portion of the cytoplasm is lost during the final steps of spermatogenesis, specifically during spermiogenesis, the process during which round spermatids differentiate into elongated spermatids and then spermatozoa 13. The remaining cytoplasm from intercellular bridges, called the cytoplasmic droplet, is lost during sperm transit through the epididymis 14. During spermiogenesis (the process of transformation of spermatids into fully developed sperm cells with a head, neck middle piece and a tail), the sequential replacement of histones by transitional proteins and then by protamines within sperm chromatin triggers genome condensation 15, 13. The acrosome, located at the top of the sperm head, contains specific enzymes that promote specific functions trailing sperm capacitation and acrosome reaction: (1) exposure of acrosome zona pellucida binding proteins during sperm capacitation; (2) sperm ability to cross cumulus cells that surrounds the oocytes (female eggs) (3) sperm binding to zona pellucida and acrosome reaction, and (4) migration of IZUMO1 protein from the outer acrosomal membrane to the equatorial segment of sperm surface to ensure its binding to JUNO receptor on the oocyte 16, 17. Since acrosome reacted sperm retain the ability to penetrate the zona pellucida, the previous paradigm supporting the role of enzymes released during acrosome reaction in the digestion of the zona pellucida has been revisited in different species 18.
Sperm tail (flagellum)
The sperm tail or flagellum includes the neck, midpiece (body), principal piece and terminal piece. The sperm centrioles are important to early embryo development and are localized in the sperm neck 19. The axoneme, located internally along the entire flagellum, is composed of nine peripheral doublets and two central single microtubules (9 + 2 structure) integrated by the intraflagellar transport (IFT) system. Surrounding the axoneme, there is the outer dense fibers (ODF) and mitochondria in the sperm midpiece and the fibrous sheath (FS), formed by nine bundles of fibers of different lengths, in the principal piece 20. Depending on the species, approximately 22 to 75 mitochondria are present in the midpiece to produce enough energy necessary for a sperm to transit along the female reproductive tract and to reach the fertilization site in the oviduct 21. All of these sperm structural characteristics are essential to ensure the ability of a sperm to cross the muco-cervical and uterine barriers and reach the oviduct, where they bind to and penetrate the oocyte (egg) to deliver their DNA content.
Healthy sperm
Healthy sperm also known as “viable spermatozoa” should possess the ability to reach the fertilization site, bind to and fertilize the oocytes (female eggs), and properly contribute to initiation of early embryo development 22. These abilities are strictly dependent on sperm’s structural, morpho-functional, and intrinsic features.
Morpho-functional sperm features such as sperm motility/kinetics, morphological abnormalities, integrity of plasma and acrosome membranes, mitochondrial activity production of reactive oxygen species (ROS), DNA fragmentation and capacitation status are essential in determining male fertility potential 23, 12. A high proportion of sperm morphological abnormalities often referred to as teratozoospermia includes sperms with large, small or piriform heads as well as coiled-tails, is associated with male infertility 24. This common cause of male infertility is routinely assessed by light microscopic analysis of semen in fertility clinics.
Figure 3. Healthy (viable) sperm concept
[Source 12 ]Figure 4. Sperm morpho-functional features
Footnotes: Drawings representing the sperms with satisfactory (left) and unsatisfactory (right) morpho-functional features. Sperm acrosome membrane integrity, sperm plasma membrane integrity, sperm DNA integrity, low quantity of reactive oxygen species (ROS), sperm mitochondrial membrane high activity, high sperm motility and normal sperm morphology characterize the satisfactory morpho-functional sperm features. Sperm acrosome membrane damage, sperm plasma membrane damage, sperm DNA fragmentation, high quantity of reactive oxygen species (ROS), sperm mitochondrial membrane low activity, low sperm motility and abnormal sperm morphology characterize the unsatisfactory morpho-functional sperm features.
[Source 12 ]Hormonal regulation
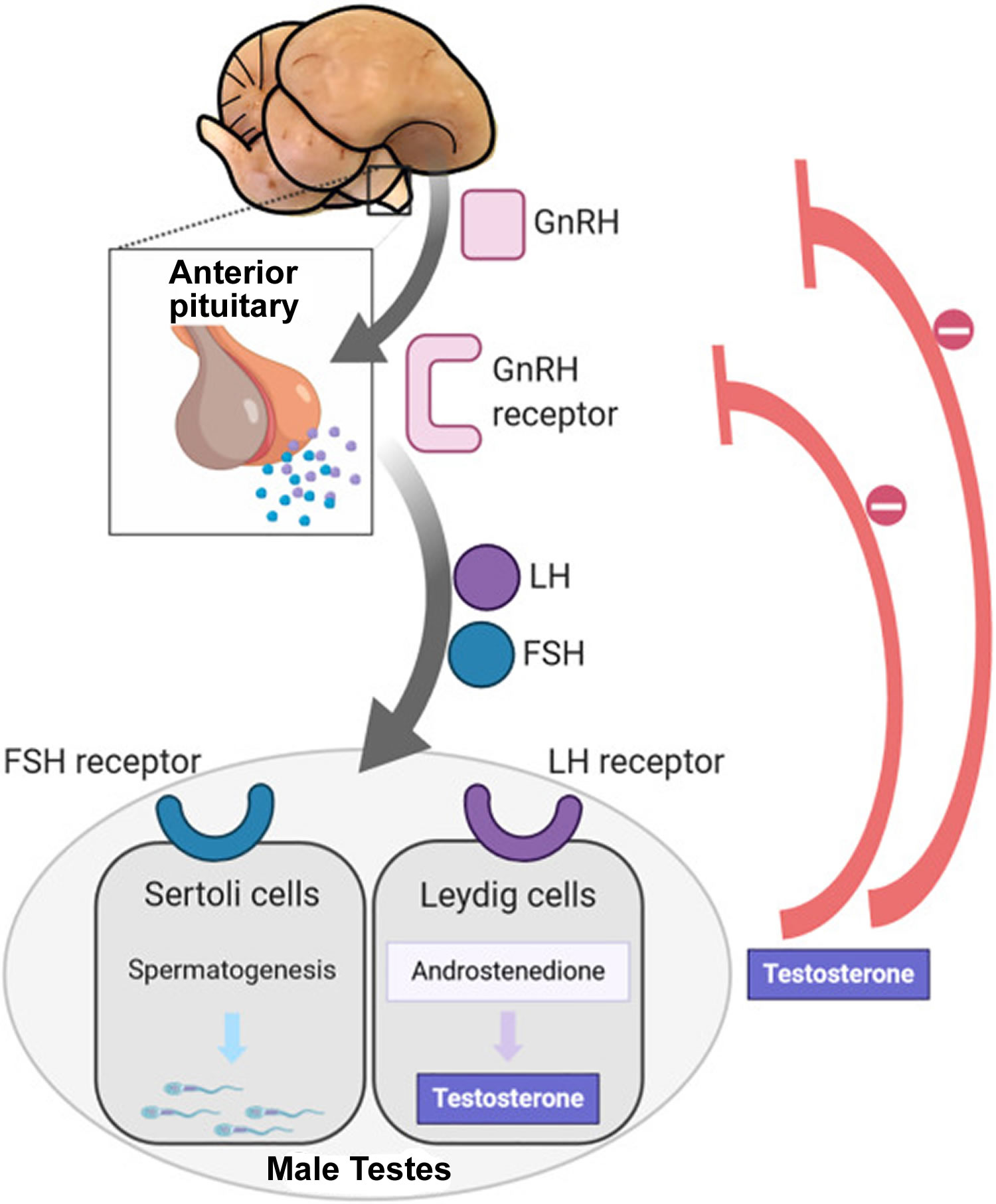
Sperm production depends on three major hormones:
- Follicle-stimulating hormone (FSH) stimulates the production of sperm in the Sertoli cells, which are located inside the testicles’ seminiferous tubules. The testicles contain hundreds of these microscopic tubules.
- Luteinizing hormone (LH) stimulates receptors in Leydig cells to produce testosterone. Leydig cells surround the seminiferous tubules.
- Testosterone is the most important male hormone.
Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are produced in the brain’s anterior pituitary gland. These hormones are also important for female reproduction. Testosterone is produced in the testicles.
Figure 5. Hypothalamic–pituitary–gonadal axis
Footnotes: In puberty, the hypothalamic-pituitary-gonadal axis plays a major role in regulating testosterone levels and gonadal function. Gonadotropin-releasing hormone (GnRH) is secreted from the hypothalamus by GnRH-expressing neurons. The GnRH travels down the hypothalamohypophyseal portal system to the anterior pituitary, which secretes luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH are two gonadotropic hormones that travel through the blood and act on receptors in the gonads. The largest amounts of testosterone (>95%) are produced by the testes in men, while the adrenal glands account for most of the remainder. In the testes, testosterone is produced by the Leydig cells 25. The male testes also contain Sertoli cells, which require testosterone for spermatogenesis (sperm cell development). Luteinizing hormone (LH), in particular, acts on the Leydig cells to increase testosterone production. Testosterone limits its own secretion via negative feedback. High levels of testosterone in the blood feedback to the hypothalamus to suppress the secretion of GnRH and also feedback to the anterior pituitary, making it less responsive to GnRH stimuli 26. Throughout the reproductive life of males, the hypothalamus releases GnRH in pulses every 1 to 3 hours. Despite this pulsatile release, however, average plasma levels of FSH and LH remain fairly constant from the start of puberty, where levels spike, to the third decade of life, where levels peak and slowly begin to decline. Prior to puberty, testosterone levels are low, reflecting the low secretion of GnRH and gonadotropins. Changes in neuronal input to the hypothalamus and brain activity during puberty cause a dramatic rise in GnRH secretion.
Sperm development
The scientific word for sperm development is spermatogenesis. This process begins in the testicle (testes):
- The testes are composed of coiled structures called seminiferous tubules, which are the sites of sperm production.
- Sperm are produced within nurturing Sertoli cells, which are located in the lower parts of the seminiferous tubules.
- Once sperm are produced, they pass through the seminiferous tubules and are collected in a structure of tightly coiled tubes called the epididymis. An epididymis lies along the back side of each testicle.
- Sperm mature in the epididymis and are stored there to await ejaculation.
Figure 6. Testicle anatomy
Ejaculation
When a man experiences sexual excitement, his penis fills with blood and becomes erect. Nerves stimulate muscle contractions, which force the sperm out from the epididymis through the penis’ urethra, where they are expelled:
- The sperm pass through the epididymis into a muscular tube called the vas deferens. The vas deferens connects the epididymis to the seminal vesicle and urethra.
- Muscle contractions in the vas deferens propel the sperm into the ampulla, where secretions from the seminal vesicle are added to form seminal fluid (semen). The seminal vesicles are sac-like glands attached to the vas deferens. They produce fructose, which provide energy for sperm movement.
- Each vas deferens and seminal vesicle join together to form an ejaculatory duct. The two ejaculatory ducts (left and right) converge into the prostate gland, which adds milky protein secretions to the seminal fluid.
- Semen is composed mainly of fluid from the seminal vesicles and the prostate gland. Sperm make up only a small percentage of semen.
- The ejaculatory ducts open into the urethra. The urethra is the same channel in the penis through which a man urinates. During orgasm, muscles close the bladder neck so that urine cannot enter the urethra and the semen cannot enter the bladder.
- The semen is forced through the urethra during ejaculation, the final stage of orgasm when the sperm is literally ejected from the penis.
- A man’s ejaculate contains over 100 million sperm. Only 1 sperm fertilizes the egg (see Figure 7 below).
Figure 7. Fertilization
[Source 12 ]Azoospermia causes
The many causes of azoospermia is divided into three primary categories: pretesticular, testicular and post-testicular categories. Although the pretesticular and post-testicular causes of azoospermia are generally curable, the testicular causes of azoospermia are generally not.
Pretesticular (central) causes of azoospermia are endocrine abnormalities that are characterized by low levels of sex steroids and abnormal gonadotropin levels and include hypogonadotropic hypogonadism, hyperprolactinemia, and androgen resistance. These abnormalities can be congenital (e.g., Kallmann syndrome), acquired (e.g., hypothalamic or pituitary disorders) or secondary (e.g., an adverse effect from a medication such as gonadotropins, anabolic steroids, testosterone supplementation).
In contrast, testicular causes are characterized by disorders of spermatogenesis inside the testes, such as varicocele-induced testicular damage, undescended testes (cryptorchidism), testicular torsion, mumps orchitis, gonadotoxic effects of medications, genetic abnormalities, and idiopathic causes. Most cases of non-obstructive azoospermia have a pretesticular or testicular cause. Lastly, post-testicular causes due to ejaculatory dysfunction or genital tract outflow obstruction are the major contributors to obstructive azoospermia 27. De novo or familial chromosomal or gene abnormalities constitute well-established genetic causes of azoospermia. Congenital testicular causes consist of anorchia (absence of testes in a newborn with male external genitalia and 46,XY chromosome), testicular dysgenesis (cryptorchidism), genetic abnormalities (Y chromosome deletions), germ cell aplasia (Sertoli cell-only syndrome) and spermatogenic arrest (maturation arrest). Acquired testicular causes include trauma, torsion, infection (mumps orchitis), testicular tumors, medications, irradiation, surgery (compromising vascularization of testis), systemic diseases (cirrhosis, renal failure) and varicocele 28.
Post-testicular causes include ejaculatory disorders or obstructions, which impair the transport of spermatozoa from the testis. These obstructions can also be congenital, caused by a congenital bilateral absence of the vas deferens (CBAVD), or acquired because of infection or surgery (vasectomy or an iatrogenic injury). Obstructive azoospermia (OA) is also classified according to the localization of the obstruction: epididymal (postinfection), vasal (vasectomy, congenital bilateral absence of the vas deferens) or ductal (Müllerian cysts) 28.
Other causes of azoospermia include XYY syndrome, myotonic dystrophy, Noonan syndrome, 5 alpha-reductase deficiency, androgen insensitivity syndrome, and vanishing testis syndrome. There is sufficient evidence to confirm that varicoceles have a deleterious impact on the semen and testes, while varicocelectomy (a surgical procedure that treats a varicocele) has been shown to improve sperm parameters and testicular function 29, 30, 31. However, only a small percentage of men with azoospermia due to testicular failure will benefit from surgical treatment of their clinical varicocele 32.
Azoospermia may also be clinically classified as obstructive (post-testicular) azoospermia and nonobstructive (pretesticular or testicular) azoospermia. Obstructive azoospermia is less common than nonobstructive azoospermia and occurs in 15 to 20% of men with azoospermia 28. Although nonobstructive azoospermia indicates impaired sperm production of the entire testis by definition, it has been observed that focal normal spermatogenesis can be observed in 50 to 60% of men with nonobstructive azoospermia 33.
- Obstructive azoospermia patients tend to have normal follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels along with normal-sized testes. Testicular ultrasound may demonstrate an increased intratesticular resistive index or some dilated ducts in the testes, epididymis, or proximal vas deferens. Dilation of the epididymis, hydrocele presence, or vas deferens’ absence suggest obstruction. Azoospermia due to vas deferens obstruction or epididymal obstruction has a normal biochemical profile in the seminal fluid. Testicular biopsy is indicated in azoospermic men with normal testicular examinations and hormonal profiles. These patients do not require a karyotype evaluation. However, an assessment for the cystic fibrosis gene (CFTR) mutation should be performed to rule out cystic fibrosis.
- Nonobstructive azoospermia patients may have normal follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels but will often show elevated levels. The size of the testes may be normal, hypotrophic, or atrophic. If the differentiation is still unclear, a testis biopsy may be required. In nonobstructive azoospermia, the abnormal hormonal profile suggests a significant spermatogenic abnormality, as evidenced by an elevated FSH level. In cases of a normal hormonal profile, a testicular biopsy can be used to evaluate spermatogenesis further, as it would be the definitive way to diagnose azoospermia from causes like maturational arrest and Sertoli cell-only syndrome. A karyotype analysis, genetic testing, and Y chromosome microdeletion test should be performed to assess genetic abnormalities. Endocrinological abnormalities are diagnosed and then managed accordingly. In general, maturational arrest, Sertoli cell-only syndrome, and Y chromosome microdeletions of AZFa, AZFb, or AZFc subregions are permanent and untreatable forms of nonobstructive azoospermia. However, microdissection testicular sperm extraction (microTESE) may allow sperm retrieval in about 50% of cases.
- Men with nonobstructive azoospermia tend to have a higher incidence of other health-related disorders, including pituitary prolactinomas, various tumors including Sertoli cell, Leydig cell, and germ-cell tumors and have 3 times the overall risk of developing a future cancer compared to infertile men without azoospermia 34. About 30% of men with nonobstructive azoospermia also have testosterone deficiency, usually due to Leydig cell dysfunction 35, 36.
The overall incidence of chromosomal abnormalities in infertile men is about 6% 37. The highest risk is found in nonobstructive azoospermic men 38, 39. The absence of 1 or both vas deferens and/or the seminal vesicles on transrectal ultrasound is highly suggestive of a genetic interaction associated with cystic fibrosis, which will be present about 80% of the time 40. It is recommended that both the man and his female partner be tested for the cystic fibrosis gene (CFTR). Other abnormalities include Robertsonian and reciprocal translocations as well as chromosomal inversions 41.
All men with primary testicular failure should be given karyotype and Y chromosome microdeletion testing 41. About 6% of men with nonobstructive azoospermia will have microdeletions of the Y chromosome involving the AZFa and/or AZFb subregions. Men with such microdeletions typically have extremely poor sperm retrieval rates and should be counseled to consider using donor sperm for intracytoplasmic sperm injection (ICSI) or adoption 42.
Around four percent of Y chromosome microdeletion will have a microdeletion of the AZFc subregion, which is not quite as severe a problem with infertility but will be inherited by male offspring 41. Up to 30% of nonobstructive azoospermic men will have Y chromosome microdeletions 43. Complete deletions of the AZFa, AZFb, or AZFc subregions generally result in nonobstructive azoospermia. Histologically, about 46% of men with AZFc microdeletions will demonstrate Sertoli cell-only syndrome, and 38% of the men will have maturational arrest 44. There are also rare cases where atypical Y chromosome microdeletions will be found. Individualized counseling and treatment should be offered to such patients.
Men with hypogonadotropic hypogonadism should also be considered for genetic testing. About a third will demonstrate a genetic lesion with variable inheritance. For example, 13 genes contributing to maturational arrest resulting in nonobstructive azoospermia have been identified 45.
Genetic testing in human azoospermia was initially restricted to karyotype analyses 46. With technical progress, genetic screening has been broadened to the analysis of the gene coding for cystic fibrosis transmembrane conductance regulator (CFTR) in patients with obstructive azoospermia 47 and Y chromosome microdeletions in patients with non-obstructive azoospermia 48. Over the last 5 years, emergence of whole-genome techniques has led to the identification of many other supposedly causal genetic defects. Table 1 provides an up-to-date overview of all the types of genetic defects known to be linked to human azoospermia, including (i) chromosome abnormalities, (ii) causative gene mutations in obstructive azoospermia, (iii) causative gene mutations in non-obstructive azoospermia, (iv) polymorphisms and (v) epigenetic alterations.
Table 1. Genetic abnormalities observed in cases of obstructive or non-obstructive azoospermia
| Genetic abnormality | Type of azoospermia | Sterility phenotype |
|---|---|---|
| Chromosome abnormalities | ||
| Klinefelter syndrome | Non-obstructive azoospermia | Variable |
| 47,XYY | Variable | |
| 46,XX | Sertoli-cell-only syndrome | |
| Chromosome rearrangements | Variable | |
| Y chromosome microdeletions | ||
| AZFa | Non-obstructive azoospermia | Sertoli-cell-only syndrome |
| AZFb | Meiotic arrest | |
| AZFc | Variable | |
| Gene mutations | ||
| CFTR | Obstructive azoospermia | Congenital bilateral absence of the vas deferens |
| ADGRG2 | Congenital bilateral absence of the vas deferens | |
| PANK2 | Congenital bilateral absence of the vas deferens | |
| SLC9A3 | Congenital bilateral absence of the vas deferens | |
| TEX11 | Non-obstructive azoospermia | Meiotic arrest |
| DMC1 | Meiotic arrest | |
| DNAH6 | Meiotic arrest | |
| MAGEB4 | Sertoli-cell-only syndrome | |
| MCM8 | Unknown | |
| MEIOB | Meiotic arrest | |
| MEI1 | Meiotic arrest | |
| NPAS2 | Unknown | |
| PSMC3IP | Unknown | |
| SPINK2 | Post-meiotic arrest | |
| STX2 | Meiotic arrest | |
| SYCE1 | Meiotic arrest | |
| TAF4B | Unknown | |
| TDRD7 | Post-meiotic arrest | |
| TDRD9 | Meiotic arrest | |
| TEX14 | Meiotic arrest | |
| TEX15 | Meiotic arrest | |
| XRCC2 | Meiotic arrest | |
| ZMYND15 | Meiotic arrest | |
Abbreviations: CBAVD = congenital bilateral absence of the vas deferens; SCOS = Sertoli-cell-only syndrome
[Source 1 ]Kallmann syndrome
Kallmann syndrome is an X chromosome-linked disorder characterized by isolated gonadotropin releasing hormone (GnRH) deficiency accompanied by complete or partial anosmia. Six X chromosome-linked autosomal dominant and recessive genes have been identified; of these, KAL1 is the gene that is most commonly associated with Kallmann syndrome. Kallmann syndrome is essentially a hormonal disorder in which the lack of GnRH secretion leads to testicular insufficiency (i.e., hypogonadotropic hypogonadism) 49.
Kallmann syndrome is diagnosed clinically in the presence of anosmia, micropenis, cryptorchidism, diminished libido, erectile dysfunction and the absence of secondary sex characters. While serum testosterone level is low (<100 ng/ml) in patients with Kallmann syndrome, pituitary and hypothalamus imaging studies are normal. Adult males with Kallmann syndrome tend to exhibit prepubertal testicular volume (<4 ml) and have eunuchoid body habitus caused by delayed skeletal maturation 49. One study recently demonstrated that testicular morphology in patients with Kallmann syndrome can vary 50. However, spermatogenesis can be easily induced by hormonal stimulation 51. Depending on the type of gene mutation, nonreproductive phenotypes in men with Kallmann syndrome can include unilateral renal agenesis, dyskinesia and/or skeletal abnormalities, cleft lip/palate, ear/hearing defects, coloboma (eye defect) and hyperlaxity of the joints.
Klinefelter’s syndrome
Klinefelter’s syndrome is a chromosomal disorder in which at least one additional X chromosome is observed in the male karyotype (47, XXY). Although there are several mosaic forms of Klinefelter’s syndrome, most cases are of the nonmosaic form, 47, XXY. Klinefelter’s syndrome is the most common chromosome aneuploidy in human beings and the most common form of male hypogonadism, with a prevalence of 0.1 to 0.2% in the general population and up to 3.1% in the infertile population and in approximately 5% of men with nonobstructive azoospermia 52. The presence of the extra X chromosome sets in motion several undefined events that lead to spermatogenic and androgenic failure, gynecomastia, and learning difficulties 53. The extra X chromosome may originate from either the maternal or paternal side.
Klinefelter’s syndrome has a wide spectrum of clinical presentations. Individuals with Klinefelter’s syndrome are phenotypically male. Many patients are unaware they have the condition until they present with infertility. It is believed that about 50% of all patients are never diagnosed. The overall incidence is about 1 in every 500 to 800 men, accounting for 3% of all infertile men. Klinefelter syndrome patients typically exhibit Sertoli cell-only syndrome on histological examination. Some of the characteristics of Klinefelter syndrome include the following 54:
- Taller than average stature
- Longer legs, shorter torso, and broader hips compared to other boys
- Poor muscle tone
- Poor fine motor skills, dexterity, and coordination
- Absent, delayed, or incomplete puberty
- After puberty, less muscle as well as less facial and body hair compared with other teens
- Small, firm testicles
- Small penis
- Enlarged breast tissue (gynecomastia)
- Infertility
The clinical presentation of Klinefelter’s syndrome varies according to the age at diagnosis and the severity of the mosaicism. It is difficult to differentiate prepubertal boys with Klinefelter’s syndrome from normal boys based solely on their phenotype. Small, firm testes and varying symptoms of androgen deficiency characterize Klinefelter’s syndrome in adolescence and after puberty 55. On one end of the spectrum are boys who are identified as having Klinefelter’s syndrome because they have failed to undergo puberty and virilization as a result of nearly complete androgenic malfunction. These boys exhibit a eunuchoid appearance. On the opposite end of the spectrum are phenotypically normal boys who are diagnosed with Klinefelter’s syndrome during an evaluation for azoospermia 56.
Adult men with Klinefelter’s syndrome also have high rates of hypogonadism, diabetes, thromboses, metabolic syndrome, various cancers (breast, blood, and extragonadal germ cell tumors), and cardiovascular disease. Adult men with Klinefelter’s syndrome should receive appropriate counseling and referrals 57.
Although the exact mechanism of androgen deficiency is unknown, most patients with Klinefelter’s syndrome exhibit low serum testosterone concentrations and elevated follicle-stimulating hormone (FSH) levels. This reflects spermatogenic compromise and the compensatory elevation of luteinizing hormone (LH) levels that results because the Leydig cells are being maximally stimulated and have a small reserve capacity 56. The majority of these patients also suffer from decreased libido (low sex drive) and erectile dysfunction (the inability to get or maintain an erection long enough to have sexual intercourse). Generally, the patients’ ejaculate presents with azoospermia. A testis biopsy reveals extensive fibrosis, hyalinization of seminiferous tubules and hyperplasia of the interstitium. However, the tubules may exhibit residual foci of spermatogenesis 58.
Microscopic testicular sperm extraction (micro-TESE) can retrieve viable sperm in 50% of men with Klinefelter syndrome 59. The data is conflicting on the use of testosterone levels to help predict successful sperm retrieval in men with Klinefelter syndrome 60, 61, 62. Sperm retrieval surgical procedures should be limited to adults and are not currently recommended in boys and adolescents with Klinefelter syndrome 63, 64.
Congenital bilateral absence of vas deference
Congenital bilateral absence of vas deference (CBAVD) is observed in 2 to 6% of men with obstructive azoospermia and is responsible for infertility in approximately 1% of infertile men 65. A strong association between congenital bilateral absence of vas deference (CBAVD) and the cystic fibrosis transmembrane conductance regulator (CFTR) gene has been demonstrated 66. This gene is located on the short arm of chromosome 7 and encodes the CFTR protein, which is crucial for maintaining proper sodium/chloride balance in epithelial secretions. This balance is necessary to optimize the viscosity and fluidity of these secretions. Approximately 1,500 different mutations of the CFTR gene have been described. Nearly all male patients with clinically diagnosed cystic fibrosis have CBAVD, and approximately 80% of patients with CBAVD have mutations in at least one CFTR allele. The inability to identify a second mutation is presumed to result from the fact that these mutations are located elsewhere in the noncoding regions of the CFTR gene 67.
Cystic fibrosis (CF) is characterized by elevated concentrations of electrolytes in the sweat, chronic pulmonary disease resulting from thickened respiratory epithelial secretions and pancreatic exocrine insufficiency secondary to thickened and occlusive ductal secretions. Both maternal and paternal mutant alleles must be present to cause clinical cystic fibrosis. However, the clinical presentation of cystic fibrosis depends on the severity of the mutations in each CFTR allele and/or in the noncoding regions of the genes (e.g., the 5T alleles). Thus, whereas a subset of patients with CFTR mutations suffer from severe pulmonary disease and pancreatic dysfunctions, the bilateral absence of the vas deferens may be the only observable effect in other patients 67.
The physical examination of the patients with CBAVD reveals normal-sized and full testes, a full and firm caput epididymis caused by efferent ducts that are distended with sperm, a present or absent distal two-thirds of the epididymis and a bilateral absence of the vas deferens. Semen analysis reveals a low-volume (0.5 ml), acidic ejaculate that is devoid of fructose and seminal vesicle fluid because of atrophic, dysfunctional or absent seminal vesicles. The seminal vesicle anomalies can be confirmed with TRUS imaging 56. Most men with CBAVD exhibit normal spermatogenesis, but it has been observed that a large proportion exhibit impaired spermatogenesis. Prior to sperm harvesting, other potential coexisting causes of impaired spermatogenesis should be investigated 68. A careful abdominal US should be performed because as many as 10% of patients with CBAVD may also exhibit renal agenesis 69.
Y chromosome microdeletions
The relationship between deletions on the Y chromosome and azoospermia was first recognized in 1976. With the elucidation of the molecular anatomy of the Y chromosome, specific microdeletions that are associated with azoospermia or severe oligospermia were discovered in the 1990s 70. Since this time, several case series have been published. A study with a large number of participants demonstrated that the prevalence of microdeletions was approximately 3% in unselected infertile men, 8% in men with non-obstructive azoospermia and 5.5% in men with severe obstructive azoospermia 71. Although rare, these microdeletions have also been reported to occur in fertile men 72.
Three microdeletions have been mapped to three different regions on the long arm of the Y chromosome. These regions are referred to as azoospermia factors (AZF)a, AZFb and AZFc and are observed proximally, centrally and distally on Yq11, respectively 73. Multiple genes are distributed throughout these regions; most are involved in spermatogenesis but are still poorly characterized. For example, the deleted-in-azoospermia (DAZ) gene is located in the AZFc region. This gene encodes a transcription factor that is generally present in men with normal fertility 74. The most frequently deleted region is AZFc (65-70%), and the least frequently deleted region is AZFa (5%). AZFb, AZFb+c and AZFa+b+c deletions are responsible for approximately 25 to 30% of Y microdeletions. It has been reported that Y microdeletions are observed nearly exclusively in patients with severe oligospermia (<1 million spermatozoa/ml) and are extremely rare in patients with sperm concentrations >5 million spermatozoa/ml 75.
Genetic testing for Y microdeletions may predict the outcome of sperm retrieval techniques. One study has reported that sperm retrieval is possible in approximately 50% of patients with AZFc and partial AZFb deletions. The same study reported that the possibility of detecting mature spermatozoa in patients with complete AZFb deletions is virtually zero 76. In another study, Kamp et al. demonstrated a strong association between AZFa deletions and Sertoli cell-only syndrome 77.
It is also important to know whether AZFc microdeletions are present in patients with oligospermia given the evidence of a progressive decrease in sperm count over time in such men. The cryopreservation of spermatozoa in these cases may avoid invasive sperm retrieval procedures in the future 76.
Hormonal analysis (FSH and inhibin B levels) studies have not been reliable in discriminating between patients with idiopathic and microdeletion-associated oligospermia and azoospermia 75.
The male offspring of men with Y chromosome microdeletions are likely to inherit the same abnormality and may also be infertile. It is unclear whether Y chromosome microdeletions can cause additional health risks or affect the results of assisted reproductive techniques.
Genetic counseling may be offered whenever a genetic abnormality is suspected in either the male or the female partner. Men with non-obstructive azoospermia should receive genetic counseling and should be offered karyotyping and Y chromosome microdeletion analysis before their sperm is used in assisted reproductive techniques 78.
Azoospermia prevention
There’s no known way to prevent the genetic conditions that cause azoospermia. If your azoospermia isn’t a genetic problem, doing the following can help lessen the chance of azoospermia:
- Avoid activities that could injure your reproductive organs or wear protection (like a cup when playing baseball).
- Avoid exposure to radiation when possible.
- Know the risks and benefits of medications that could harm sperm production.
- Avoid lengthy exposure of your testes to hot temperatures.
While it remains unclear exactly how much influence these factors have in male infertility, it is reasonable to expect that avoiding potentially spermatotoxic activities and adopting a healthier lifestyle will improve overall male fertility 79.
Azoospermia symptoms
Azoospermia accounts for around 10% of cases of male infertility, and affects about 1% of the men in the general population 3. You usually don’t realize you have azoospermia or decrease in male fertility until you begin trying to conceive a child with your female partner without success. Some men have symptoms related to the underlying cause. For example, you could have low sex drive (libido) due to hormonal imbalance or a lump on your testicles from an infection. Determining whether a man’s semen is lacking sperm cannot be accomplished simply by viewing the semen with the naked eye. A semen analysis by an experienced laboratory is required to determine if sperm are present or not.
Couples are generally advised to seek medical help if they are unable to achieve pregnancy after a year of unprotected intercourse. Your doctor will conduct a physical examination of both your and your female partner to determine your general state of health and to evaluate physical disorders that may be causing infertility. Usually, both partners are interviewed about their sexual habits in order to determine whether intercourse is taking place properly for conception.
Azoospermia complications
Complications of azoospermia and/or associated surgical treatment include the following:
- Hematoma formation due to surgery
- Infection
- Parenchymal fibrosis of testis
- Testicular atrophy
Additionally, azoospermia can lead to significant emotional distress, impacting the mental health of affected individuals and straining relationships. The pursuit of fertility treatments, while offering hope for conception, may also cause financial burdens and emotional stress. The psychological toll of grappling with infertility and potential societal stigmas surrounding male reproductive health further compounds the challenges and complications associated with azoospermia.
Azoospermia diagnosis
The initial step in the evaluation of an infertile male is to obtain a thorough medical, sexual and urologic history that might affect fertility. Such a history should include the following:
- Infertility history, such as duration of infertility, whether the infertility is primary or secondary, any treatments to date, libido and sexual activity for both partners
- Previous fertility in the patient and the partner
- Timing of puberty (early, normal, or delayed)
- Childhood urologic disorders or surgical procedures and any childhood problems in development
- Current or recent acute or chronic medical illnesses such as diabetes, respiratory infections, allergies, or cancer
- Sexual history including any sexually transmitted infections. Frequency and timing of sexual intercourse.
- History of proven or suspected genito-urinary infections
- History of any genetic abnormalities in the patient or his family
- Testicular cancer and its treatment
- Social history (e.g., alcohol, tobacco, marijuana, anabolic steroid, or recreational drug use)
- Medication use which might have an adverse impact on spermatogenesis, including but not limited to medical agents like hormone/steroid therapy, antibiotics (sulphasalazine), alpha-blockers, 5 alpha-reductase inhibitors, chemotherapeutic agents
- Family history of reproductive problems
- Respiratory disease
- Environmental or occupational exposure. Any exposure to toxins, such as chemicals, pesticides or radiation, excessive heat on the testicles
- Spinal cord injury
- History of surgeries or trauma to the genital area (hydrocelectomies, varicocelectomies etc)
If the man has had exposure to any of the above agents, the agents should be discontinued and the semen retested in 3 to 6 months. If the man has had a recent serious medical illness or injury or he has evidence of a recent reproductive tract infection, semen testing should be repeated at least 3 months following recovery from the illness.
Physical examination should include a thorough general examination with particular attention paid to the scrotal exam (size and consistency of the testis, presence and grade of varicoceles and palpable vas deferens). The initial testing will depend on these findings.
At least 2 separate semen analyses should be done with 3 days of sexual abstinence preceding each specimen. In patients with azoospermia who have normal ejaculate volume, there is either spermatogenic failure or obstruction between the testes and the seminal vesicles. Patients with azoospermia having low semen volume and normal-sized testicles may either have ejaculatory duct obstruction or ejaculatory dysfunction. Therefore, all patients displaying absent ejaculate (aspermia) or low-volume ejaculation (< 1.5 mL) should be requested to repeat the semen investigations and also give a post-ejaculation urine example.
Physical Exam
Your doctor will carefully examine your scrotum and testicles. Varicoceles can be felt during examination of the scrotum. They are described as feeling like “a bag of worms”. Your doctor may also check your prostate gland. Your doctor will examine your penis for any signs of infection or anatomical abnormalities.
The physical examination should include a thorough inspection of the following:
- Testicles (for bilateral presence, size, consistency, symmetry)
- Epididymis (for presence bilaterally, as well as any induration, cystic changes, enlargement, tenderness)
- Vas deferens (for presence bilaterally, defects, segmental dysplasia, induration, nodularity, swelling)
- Spermatic cord (for varicocele)
- Penis (for anatomic abnormalities, strictures, or plaques)
- Rectum (for abnormalities of the prostate or seminal vesicles)
- Body habitus
Depending on the findings from the history, detailed examination of other body functions may also be warranted.
In addition to a medical history and physical exam, specific tests for male infertility may include:
- Semen analysis to evaluate the quantity and quality of sperm
- Blood tests to evaluate hormone levels
- Imaging tests to look for structural problems
- Genetic testing to identify sperm DNA fragmentation, chromosomal defects, or genetic diseases
The sperm count test is performed if a man’s fertility is in question. It is helpful in determining if there is a problem in sperm production or quality of the sperm as a cause of infertility. The sperm count test may also be used after a vasectomy to make sure there are no sperm in the semen.
Semen analysis
Semen analysis is the cornerstone of the male infertility diagnostic workup.
The sperm collection involves the following steps:
- Abstain from ejaculation for several days before (but no more than 5 days) the test because each ejaculation can reduce sperm count.
- Collect a sample of your semen either by masturbating into a sterilized cup or having sexual intercourse using a special collection condom.
- Keep the sample at room or body temperature and deliver it promptly to the laboratory or medical office. The sperm should be analyzed within 1 hour of collection.
- Because sperm counts can fluctuate, collection and analysis may need to be repeated over several months.
A semen analysis will provide information on:
- Semen volume (normal 1.5 to 5 mL). The amount of semen is important. Most men ejaculate between 2.5 to 5 mL of semen. Either significantly high or low amounts can indicate problems. Low amounts (less than 1.5 milliliters) are a sign of fewer sperm. High amounts may dilute the concentration of sperm.
- Semen quality
- Semen density or concentration (normal > 15 million sperm/mL). Semen concentration refers to the amount of sperm found in 1 mL of semen. A normal concentration is at least 20 million sperm per milliliter.
- Total number of sperm in the sample (sperm count). Sperm count refers to the number of sperm found in the entire sample of semen. In general, a normal sperm count is considered to be at least 40 million.
- Percent of live sperm (sperm viability). At least 50% of the sperm in the semen sample should be alive (viable).
- Percentage of moving sperm (sperm motility) (normal > 40% of sperm having normal movement). Sperm motility (the speed and quality of movement) is graded on a scale from 0 (worst) to 4 (best). Grades 3 and 4 indicate good mobility. At least 40% of the sperm should be motile.
- Sperm size, shape, and structure (sperm morphology). Sperm morphology is the size, shape, and structure of the sperm. At least 50% of the sperm should be of normal appearance.
- Signs of infection – An increased number of white blood cells (WBCs) in the semen may be observed in patients with infectious or inflammatory processes
- Other variables (eg, levels of zinc, citric acid, acid phosphatase, or alpha-glucosidase). The acidity of the semen is tested (pH should be 7.2 or greater). The lab will test for presence or absence of certain chemicals and enzymes, such as fructose. The semen will be tested for how thick it is and how quickly it liquefies, and whether it clumps together.
An abnormal semen analysis test result done after sexual intercourse (postcoital) is observed in 10% of infertile couples. Indications for performing a postcoital test include semen hyperviscosity, increased or decreased semen volume with good sperm density, or unexplained infertility.
If the postcoital semen analysis test result is normal, consider sperm function tests, such as the following:
- Capacitation assay
- Acrosome reaction assay
- Sperm penetration assay
- Hypoosmotic swelling test
- Sperm-cervical mucus test
- Inhibin B level
- Vitality stains
Although less commonly performed, sperm penetration tests may be used to evaluate function. For example, the sperm penetration assay measure the sperm’s ability to penetrate specially prepared hamster eggs. The exact role of these tests for most people with infertility remains unclear.
A post-ejaculatory urine sample can detect presence of sperm, which may indicate retrograde ejaculation.
A laboratory sperm-cervical mucus test (Kurzrock-Miller or Kremer test) may also be used to evaluate how well sperm move in cervical mucus. Either the female partner’s mucus or donated midcycle mucus can be used in this test.
Reduced semen volume
If the semen volume is reduced (<1.5 mL) and documented on repeat testing, careful questioning should elicit whether this is an artifact (missed the container, difficulty providing specimen, etc.) or truly a low semen volume. Low semen volume could be due to:
- absence/abnormalities of the vas deferens/seminal vesicles,
- retrograde ejaculation, or
- failure of emission.
Azoospermia patients with semen volumes consistently below 1.5 ml are likely to have retrograde ejaculation, an ejaculatory duct cyst, or congenital absence of the vas or seminal vesicle 41. Examination of the post-ejaculation urine sample can identify patients with retrograde ejaculation. The physical examination can determine if there is an absent vas deferens. Transrectal ultrasonography or cystoscopy can be used to identify an ejaculatory duct cyst. The seminal vesicles can be evaluated with transrectal ultrasonography.
Occasionally, an alpha agonist (use pseudoephedrine or other just before the semen testing) will convert retrograde into antegrade ejaculation. Diabetic men often have retrograde ejaculation or failure of emission.
Physical examination will help determine if the vas deferens is present in the scrotum and a transrectal ultrasound (TRUS) will determine if the seminal vesicles and vas deferens close to the prostate are normal. If absence of the vas deferens and/or the seminal vesicle is identified, the man has about an 80% chance to carry a genetic alteration associated with cystic fibrosis 80. Cystic fibrosis testing should be performed on all men with absence of the vas deferens/seminal vesicles.
Obstruction of the ejaculatory duct is detected by transrectal ultrasound (TRUS) and is usually accompanied by dilation of the seminal vesicles (typically >1.5 cm). Vasography is not required and should be discouraged for men with an ejaculatory duct obstruction. If an ejaculatory duct obstruction is identified, the man has about a 25% chance to carry a genetic alteration associated with cystic fibrosis 80. Cystic fibrosis testing should be performed on all men with ejaculatory duct cysts.
As mentioned above, the categories of the causes of azoospermia are:
- Pre-testicular azoospermia (2%: hypothalamic or pituitary etiology)
- Testicular failure or non-obstructive azoospermia (49% to 93%)
- Post-testicular obstruction (7% to 51%: normal spermatogenesis but obstructive azoospermia).
The category of azoospermia can often be determined by the luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels. The diagnosis of pre-testicular azoospermia is relatively uncomplicated: LH and FSH levels will be low and the testosterone levels will be either low or normal. Men with elevated FSH and LH and small testis bilaterally have non-obstructive azoospermia. However, men with normal levels of FSH and LH could have either non-obstructive or obstructive azoospermia 81. Unfortunately, there is no non-invasive method to differentiate obstructive from non-obstructive azoospermia in this group of men. A testicular biopsy is usually required to provide a definitive diagnosis.
There have been several recent publications about the use of biomarkers in the semen and serum to differentiate obstructive from non-obstructive azoospermia 82. A number of authors report on the use of inhibin B serum levels to determine testicular function 5. While inhibin B levels are generally lower in those men with more severe testicular dysfunction and is undetectable in those with a Sertoli cell only pattern on testis biopsy, inhibin B levels in men with maturation arrest or hyopospermatogenesis patterns on testis biopsies may be identical to those found in men with full spermatogenesis. At present, serum inhibin B levels do not provide significant clinical benefit: with high FSH the inhibin B levels are generally low (both indicating testicular failure), while with normal FSH the inhibin B levels are generally normal (both indicating either obstructive or non-obstructive azoospermia).
At present, for most men, there are no non-invasive methods to differentiate obstructive from non-obstructive azoospermia. As noted above, about 60% of men with azoospermia will require a testicular biopsy to provide a definitive diagnosis.
Blood tests
Other laboratory tests that may be helpful in male infertility diagnostic workup include the following:
- Antisperm antibody test
- Hormonal analysis (follicle-stimulating hormone [FSH], luteinizing hormone [LH], thyroid stimulating hormone [TSH], testosterone, prolactin)
- Genetic testing (karyotype, CFTR, AZF deletions if severe oligospermia (< 5 million sperm/mL)
Blood tests may be used to check for hormone levels of testosterone, FSH, and LH. A blood test can also check for evidence of sexually transmitted infections.
In evaluating azoospermia, especially with normal testicle size and consistency on physical examination, a detailed endocrinological evaluation is necessary to make a specific diagnosis and strategize the treatment protocol 83. Hormonal profiles, including testosterone level (total and free testosterone), follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin, and estradiol levels, should be obtained in addition to a thyroid function test (thyroid stimulating hormone [TSH]) 41. There is usually a decreased or normal follicle-stimulating hormone (FSH) level in patients with obstructive azoospermia 41. In contrast, FSH levels are typically high in nonobstructive azoospermia, especially if the testes are below normal size 41. However, some overlap exists, so FSH levels alone may not be sufficient for differentiation 41. As a general rule, the higher the FSH, the more likely a significant spermatogenic failure will be. Therefore, a testicular biopsy may need to be performed to make a definitive diagnosis. In modern practice, testicular biopsy is rarely performed alone 84.
Hypogonadotropic hypogonadism is a relatively uncommon cause of nonobstructive azoospermia. Hypogonadotropic hypogonadism accounts for no more than 2% of all infertile males. Possible causes include Kallmann syndrome, androgen-induced hypogonadism from testosterone supplementation, brain tumors, radiation, trauma, radiation, etc 85. The incidence of azoospermia from testosterone replacement therapy is increasing as anabolic steroid abuse is increasing worldwide, and most patients and even many physicians are unaware or uninformed about the negative effects of testosterone therapy on sperm counts and spermatogenesis 86, 87, 88, 89.
Imaging studies
Imaging studies in male infertility diagnostic workup may include the following:
- Transrectal ultrasound
- Scrotal ultrasound
- Vasography
- Brain MRI (magnetic resonance imaging) if your doctor believe your hypothalamus or pituitary gland is playing a role.
Ultrasound examinations are painless and inexpensive, but a discussion with the diagnostic radiologist on which factors to look for and evaluate when a scrotal ultrasound is ordered for a male infertility patient will be very helpful. Therefore, the recommendation is for the routine use of a comprehensive sonographic evaluation to help diagnose azoospermia and differentiate obstructive from nonobstructive azoospermia.
Ultrasound uses sound waves to produce an image. Ultrasound imaging may be used to check for abnormalities or blockages in the testicles, or to find varicoceles that are too small for physical detection.
In transrectal ultrasound, a probe is inserted into the rectum to provide images of the prostate gland, vas deferens, and seminal vesicles.
The role of scrotal ultrasound in evaluating male infertility is evolving and is becoming more routine. Ultrasound allows for a more precise measurement of testicular size and can identify cysts, varicoceles, spermatoceles, and lesions that might not otherwise be detectable by other means 41. Ultrasound can also help differentiate obstructive from nonobstructive azoospermia. Scrotal ultrasound will tend to show ectasia of the epididymal tubules and/or the rete testis, abnormal epididymal echogenicity, or dilated proximal vas deferens in obstructive azoospermia. Testicular volume, epididymal head size, and the resistive index of intratesticular vessels all tend to be increased compared to patients with nonobstructive azoospermia 90. In addition, transrectal ultrasound can be used to evaluate the seminal vesicles and to screen for ejaculatory duct cysts. The absence of the seminal vesicles suggests a possible cystic fibrosis genetic mutation (CFTR) 41. If the seminal vesicles are absent or dilated/enlarged, an obstructive azoospermia can be diagnosed 41.
Testicular biopsy
A small tissue sample of the testicle may be taken using a thin needle. A biopsy may be performed for diagnostic purposes to evaluate sperm production function. A biopsy may also be used to collect sperm that will be used in an intracytoplasmic sperm injection (ICSI) fertility procedure.
Testicular biopsy is usually indicated in a complete absence of sperm in semen (azoospermia) with a normal-sized testis and normal findings on hormonal studies to evaluate for ductal obstruction, to further evaluate idiopathic infertility, and to retrieve sperm.
As a rule, testicular characteristics and diagnostic lab findings are good but imperfect indicators of nonobstructive azoospermia. Hence, testicular sperm extraction (TESE) can be performed in a specialized assisted reproduction center, which permits cryopreservation of sperm and avoids testicular biopsy. A normal testicular biopsy result is suggestive of obstruction, and vasography is required to identify the precise location of the blockage. A formal surgical repair can then be performed or sperm retrieval as appropriate. Depending on the severity of the defect, serum testosterone levels can be low, normal, or high. Only a single testis needs to be biopsied for a histological diagnosis. The larger of the 2 testes should be selected for the procedure.
In modern practice, testicular biopsy is rarely performed as a diagnostic tool because the design of the testicular tissue is heterogeneous, and spermatogenesis occurs in the foci 91. In most cases, doctors can predict with high accuracy whether or not a man has an obstructive cause of azoospermia. Since doctors have started performing testicular dissections to search for sperm, they have learned that different areas of the testis might show different patterns of nonobstructive azoospermia. For example, one area might show decreased production of mature sperm (hypo spermatogenesis or maturation arrest), while another area might show the complete absence of sperm precursor cells (Sertoli-cell-only syndrome). Therefore, in the modern era, doing a diagnostic testicular biopsy does not often change the ultimate management for men with nonobstructive azoospermia. For those men, doctors offer microdissection testicular sperm extraction (microTESE), which gives the best chance of finding sperm that can be used for assisted reproductive technology (ART). Sometimes, at the time of the microTESE, doctors will send a tiny specimen for pathological evaluation to rule out a precursor to cancer called intratubular germ cell neoplasia (ITGCN).
When should the azoospermic man have a testis biopsy?
As mentioned above, close to 60% of the men would need a testicular biopsy to document whether the azoospermia is post-testicular (normal spermatogenesis) or testicular (testicular failure with either Sertoli cell only syndrome, Maturation arrest pattern or hypospermatogenesis). However, a testis biopsy should only be offered to men in whom this diagnosis would alter management. As an example, male fertility experts would discourage a man from having a testis biopsy if the couple is not interested in any of the potential management options that follow (e.g., things like sperm aspiration plus ICSI, vaso-epididymostomy). If the couple is interested in considering the other fertility treatments mentioned above, then the biopsy could be performed by either of these 2 procedures.
The biopsy can be performed as a diagnostic procedure alone (either a percutaneous or an open biopsy are acceptable methods of testicular biopsies). The biopsy results then guide the next treatments.
The biopsy can be the initial part of the larger fertility treatment. Once the biopsy results are available as a quick section, the surgery would then proceed with a reconstruction and/or sperm retrieval (if active spermatogenesis is detected) or a testicular sperm extraction (if a pattern of testicular failure is detected).
A bilateral diagnostic testicular biopsy is generally not required. If there is a discrepancy in testicular size, the larger of the 2 testes should be biopsied.
Failure to ejaculate
In men with a clear neurological cause (spinal cord injury, retroperitoneal lymph node surgery, etc.), no further investigations are required prior to treatment. Men with idiopathic failure to ejaculate (particularly those with a failure to orgasm) should be seen by a sex therapist.
Genetic investigations for men with azoospermia
Genetic factors occupy an important place in the evaluation and management of the azoospermic male. Such factors can be pretesticular (e.g., Kallmann syndrome), testicular (e.g., Klinefelter’s syndrome or Y chromosome microdeletions) or post-testicular (congenital bilateral absence of vas deference [CBAVD]). Genetic counseling provides couples with information about the nature, inheritance pattern, and implications of genetic disorders to help them make informed medical and personal decisions. The common genetic disorders that are associated with azoospermia are reviewed below.
All men with hypogonadotropic hypogonadism should be referred for genetics counseling as almost all of the congenital abnormalities of the hypothalamus are due to a genetic alteration.
All men with absence (absence of the vas deferens) or obstruction (epididymal or ejaculatory duct) of the reproductive tract ductal structures are at an elevated risk to carry a genetic alteration associated with cystic fibrosis. Experts recommend that not only the man but his partner should be offered cystic fibrosis testing in this situation. If a genetic alteration is identified, then genetic counselling is suggested.
All men with testicular failure should be offered karyotype and Y-micro-deletion testing then referred for genetics counselling if an abnormality is identified 92.
Azoospermia treatment
Treatment for azoospermia depends on the cause of azoospermia, and whether a cause is found. Depending on the suspected causes, many treatments may be available. Treatment for male infertility should first address any underlying medical conditions that may be contributing to fertility problems. If there is a blockage or history of vasectomy, reconstruction might be the best treatment for some men. In others, removing offending agents such as medications or recreational drugs might be the first step. Sometimes there may be hormonal abnormalities that need to be addressed, and in a fraction of men, treatment could increase sperm production. In some men, surgery to fix anatomical abnormalities or varicoceles can be pursued, and in others the best option is to go directly into the testicle to attempt retrieval of sperm that could be used for assisted reproductive technology (ART). It is very important that these procedures are performed by the few physicians with proper training, expertise and experience to optimize outcomes and chances of retrieving sperm. Finally, men with azoospermia should always remember that countless couples across the world have formed families with love by becoming parents by using donor sperm or by adopting an infant or child. After being evaluated and counseled by a specialist, these are acceptable paths for couples to choose. Being evaluated by a specialist is imperative to rule out any dangerous underlying medical conditions, to help couples optimize their chances of building the family they desire and to give guidance regarding risk and screening for medical conditions later in life.
Hypogonadotropic-hypogonadism or pre-testicular azoospermia
This is best treated with the use of FSH/LH or GnRH analogues to stimulate spermatogenesis. In over 90% of the cases, spermatogenesis is induced and the men have ejaculated sperm. However, therapy may take more than 6 months to be effective.
Retrograde ejaculation
Use of pseudoephedrine or a similar alpha agonist may convert retrograde ejaculation into antegrade ejaculation. If this is not successful, it is often possible to retrieve sperm from the bladder (either using a post-ejaculatory voided or catherized urine specimen). This sperm could then be used for one of the assisted reproductive technologies (ARTs). To optimize the sperm quality, it is often necessary to ask the men to alkalinize (pH of 6.5 – 8) the urine using standard medications.
Obstructive azoospermia
The main aim of managing obstructive azoospermia is to correct the site of obstruction by using reconstructive surgical techniques, eg, vasoepididymostomy and vasovasostomy. Assisted reproductive techniques (ARTs) are useful in patients suffering from congenital absence of vas deferens, as surgical reconstruction in these patients is not feasible. In cases of ejaculatory ductal obstruction, surgical correction with transurethral resection is performed to achieve the patency of the ejaculatory ducts. Intraoperative vasography gives better results than vasoepididyostomy. Transurethral resection (TUR) improves semen parameters in about 50% to 70% of cases.
Obstructive azoospermia may be managed with:
- Sperm retrieved from the reproductive tract (close to 100% chance of finding sperm) then the sperm is used in an intra-cytoplasmic sperm injection (ICSI) program. The type of sperm retrieval used could be a percutaneous or an open microscopic aspiration of sperm from the epididymis (MESA) or a percutaneous or open biopsy of the testis. Any of the types of retrievals listed above are acceptable.
- Bypass/repair of the obstructed area of the reproductive tract is possible in less than half of the men with obstructive azoospermia 92. The most common area of obstruction is within the epididymis. With the present micro-surgical techniques, centres with expertise in performing vaso-epididymostomies report over 85% patency of the anastomosis (sperm in the ejaculate is the measure of patency) with over a 50% spontaneous pregnancy rate. However, this is surgery requiring micro-surgical expertise and experience and should only be performed in centres with this kind of expertise. We recommend that all men be offered the option to cryo-bank sperm retrieved during the course of the operation in case the surgery is not successful.
- Men with an ejaculatory duct obstruction may be candidates for a transurethral resection (TUR) ejaculatory duct. This is best performed using a transrectal ultrasound guidance to allow the transurethral resection to precisely unroof the ejaculatory duct cyst. It is important to warn men of the potential complications associated with a transurethral resection of the prostate.
Sperm retrieval for assisted reproduction is an excellent option for managing obstructive azoospermia, as successful sperm recovery is close to 100%. Intracytoplasmic sperm injection (ICSI) has increased pregnancy rates as the sperm obtained are motile, and samples can also be cryopreserved 93, 94. In patients suffering from obstructive azoospermia, if surgical repair is not possible or the female factor is a major contributor to infertility in couples, sperm retrieval for assisted reproduction is an excellent option for managing the infertile state. Surgical bypass or repair of sperm obstructions is generally possible in less than 50% of men with obstructive azoospermia 95.
The area of obstruction most commonly found is in the epididymis. Current success rates for vasoepididymostomies are 85% patency rates with a 50% spontaneous pregnancy rate. Collecting and cryopreserving sperm at the time of surgery is recommended if the outcome is unsuccessful. Inguinal approaches for microscopic vasovasostomy have been described, and laparoscopic and robotic techniques for vas isolation before a microsurgical anastomosis 96.
In obstructive azoospermia, microsurgical epididymal sperm aspiration (MESA) is optimally used for sperm retrieval when possible. This is almost always successful for extra-testicular obstructions. If the blockage is intratesticular, then testicular sperm extraction (TESE) or testicular sperm aspiration (TESA) will be required. Post-vasectomy obstructions can be treated with microscopic vasectomy reversals, which currently achieve a reported postoperative patency rate of 90% to 97% 63. These surgeries may also be done robotically with similar success rates 97.
Obstruction from scarring, inflammation, or ejaculatory duct cysts is typically treated with transurethral resection of the duct. Reported pregnancy rates following such procedures are 12.5% to 31%, while side effects include failure, incontinence, reflux into the seminal vesicles and ejaculatory ducts, as well as epididymitis. Intraoperative transrectal ultrasound and using methylene blue to verify patency can help reduce complications and increase the procedure’s safety 98. The procedure can also be done with a transurethral laser incision of the ejaculatory duct along with seminal vesiculoscopy. Still, it is unclear if this offers any significant advantages over the standard procedure 99.
Percutaneous procedures, including testicular sperm extraction (TESE), percutaneous epididymal aspiration of sperm, and percutaneous testicular biopsy, can be performed to obtain sperm in couples desiring fertility. However, the highest rates of successful sperm retrieval are reported with microdissection testicular sperm extraction techniques.
Procedures are used to retrieve sperm
For men with obstructive azoospermia, there is often an abundance of sperm within the reproductive structures, and various procedures can be used to obtain sperm. These include testicular sperm extraction (TESE), testicular sperm aspiration (TESA), microsurgical epididymal sperm aspiration and others. The choice is based on both patient factors, patient priorities and the preferences of the reproductive endocrinologists. For men with nonobstructive azoospermia, various approaches are available, but the procedure most likely to find usable sperm for use with in vitro fertilization and intracytoplasmic sperm injection (ICSI) is microsurgical testicular sperm extraction (microTESE). When performed by an experienced expert in the field, microsurgical testicular sperm extraction (microTESE) procedure involves careful dissection through the tubules of the testis to search for the tissue most likely to be actively making sperm. This allows for maximum yield of sperm with maximum preservation of other tissues in the testis, including the Leydig cells that produce testosterone.
Non-obstructive azoospermia
Advanced assisted reproductive techniques (ARTs) are required for most patients with non-obstructive azoospermia. Microscopic testicular sperm extraction (micro-TESE) may be used to identify sperm (reported success up to 75%, mean 52%) which could then be processed for use in an intra-cytoplasmic sperm injection (ICSI) program 100. At present, the optimum way to identify these pockets of sperm is to perform an extensive, surgical dissection of the seminiferous tubules (a testicular sperm extraction). Large sections of the seminiferous tubules of the testis are examined with an operating microscope. Those tubules which are larger in size are more likely to have spermatogenesis than smaller diameter tubules. The advantage of this technique over the regular random biopsy method is the ability to identify areas of the seminiferous tubules, which are more likely to contain sperm before the tissue is removed from the testicle. Using this technique the chance of finding sperm is higher than the older technique of taking random testicular biopsies alone (in one series 63% compared to 45%), and while the procedure is laborious (surgical time may exceed 3 hours) the damage to the testicle is minimal due to the minimal amount of testis tissue eventually taken 101. ICSI pregnancy rates using sperm from a testicular sperm extraction program are reported to be between 19% to 50% 92. The testicular sperm extraction (TESE) procedure should be offered to all men with nonobstructive azoospermia but should only be undertaken in a centre where intra-cytoplasmic sperm injection (ICSI) is available.
In testicular sperm aspiration (TESA), a needle is inserted into the testicular tissue percutaneously. The failure rate of sperm retrieval in nonobstructive azoospermia is high and varies with the underlying cause. Overall success in sperm retrieval in these patients is reportedly as high as 75% but averages about 50%. There is also a significant risk of blood vessel injury. In addition, it has been observed that the incidence of chromosomal abnormalities and DNA damage in the sperm is relatively high in patients with nonobstructive azoospermia, and potentially inheritable infertility-related genes may be passed on to male offspring. Repeat micro-TESE procedures can be performed successfully, if necessary, to retrieve sperm 102.
The only absolute contraindications for an attempt at sperm retrieval in male nonobstructive azoospermic patients are Y chromosomal microdeletions of the AZFa or AZFb subregions and post bilateral orchiectomy as the sperm retrieval rates will be zero. Even in cases of Sertoli cell-only syndrome, there is a reported sperm retrieval success rate with micro-TESE of at least 24%, with a mean average rate of about 50% 84, 103. No degree of testicular atrophy or FSH elevation can determine the success or failure of a sperm retrieval procedure.
Good predictive indicators of successful sperm retrieval with microscopic testicular sperm extraction (micro-TESE) in nonobstructive azoospermic men include focal type Sertoli cell-only syndrome, late-stage maturation arrest (compared to early maturation arrest), hypospermatogenesis (as opposed to maturation arrest or Sertoli cell-only syndrome), and viable sperm visible in the seminiferous tubules on testis biopsy 41. With micro-TESE, the successful sperm retrieval rate is about 50%, while ICSI also has approximately a 50% success rate, giving an overall pregnancy rate of only 25% 41. Given the associated costs and potential genetic consequences, couples should seriously consider artificial insemination or intra-cytoplasmic sperm injection (ICSI) using donor sperm or adoption before starting advanced reproduction treatments.
Men who become azoospermic as a result of testosterone supplementation therapy have an excellent chance of recovery of spermatogenesis just by stopping the hormonal treatment and waiting 104. Most men will recover 85% of their pretreatment sperm counts after 1 year and almost all in 2 years of stopping testosterone use 105, 106.
To stimulate spermatogenesis in cases of hypogonadotropic hypogonadism or pre-testicular azoospermia (secondary hypogonadism, testosterone therapy), gonadotropin analogs or FSH and human chorionic gonadotropin (hCG) are used. The preferred therapy includes human chorionic gonadotropin (hCG) (3,000 IU to 10,000 IU injections 2 to 3 times per week) plus either anastrozole, clomiphene, FSH, or tamoxifen 104. This therapy has demonstrated success in achieving at least some sperm in the ejaculate of 75% to 77% of men with nonobstructive azoospermia due to hypogonadotropic hypogonadism, but treatment may require as long as 6 months 107. Pulsed gonadotropin hormone-releasing hormone (GnRH) is an option but is usually more expensive and does not offer any substantial benefit over standard treatment. Testosterone therapy is specifically not recommended.
While up to about 11% of azoospermic men who received hormone therapy (usually clomiphene) have shown improvement with the presence of sperm in the ejaculate, there is no standardization of this therapy and no good-quality randomized trials. Therefore, many experts and the European Association of Urology do not recommend the general use of hormone therapy in men with nonobstructive azoospermia and primary hypogonadism (hypergonadotropic hypogonadism) 63. The main treatment for these men is microscopic testicular sperm extraction when feasible and ICSI. Overall success with these techniques resulting in a pregnancy is no more than 25%, and they are costly.
The role of estrogen receptor modulators, gonadotropins, and aromatase inhibitors in men with primary hypogonadism (hypergonadotropic hypogonadism) and nonobstructive azoospermia is much more controversial. These are frequently used to improve sperm parameters in infertile oligozoospermic men, and some evidence supports their use 108, 109, 110. However, their effectiveness in improving sperm retrieval rates through testicular sperm extraction (TESE) or testicular sperm aspiration (TESA) is somewhat uncertain and has not been definitively proven 63. The optimal protocol and dosing schedule have not yet been determined, although a progressive protocol starting with clomiphene and escalating to hCG has been proposed 110. There are also potential side effects from the therapy. However, despite these limitations, and since no other treatment is available, the use of hormone stimulation therapy remains a common clinical practice 111.
Treatment of hypogonadotropic hypogonadism is relatively effective. One regimen for azoospermic men due to hypogonadotropic hypogonadism is the pulsatile administration of 5 mcg to 20 mcg of gonadotropin hormone-releasing hormone (GnRH) every 2 hours using a portable infusion pump. The return of sperm in the semen was generally noted after 6 months of therapy, and 77% of initially azoospermic men were found to have spermatogenesis after 12 to 24 months of treatment 112.
FSH stimulation before gonadotropin hormone-releasing hormone (GnRH) therapy seems to improve the outcomes. GnRH is only effective in men with normal pituitary activity. Gonadotropin treatment with hCG (with or without FSH) is preferred in patients with decreased or absent pituitary function. The suggested dosage is 1000 IU to 3000 IU 2 to 3 times weekly. This typically leads to sperm production after 3 to 6 months. If unsuccessful, FSH is added at a 75 IU to 150 IU dose twice a week 113. The overall success rate of spermatogenesis from medical therapy of hypogonadotropic hypogonadism is about 75%. If medical treatment is unsuccessful, assisted reproductive techniques are recommended 111, 114.
Failure to ejaculate
Men with a neurological cause for a failure to ejaculate should be offered either vibro-stimulation or electro-ejaculation. Both of these procedures may cause autonomic dysreflexia in men with high spinal cord injuries. The semen specimen may be used for one of the ARTs. It is common that multiple (2 to 3) procedures several weeks apart may be needed to optimize the semen quality. These men may also have a concomitant obstruction in the epididymis, so occasionally sperm aspiration is required.
Azoospermia Prognosis
Every cause of oligospermia has a different prognosis depending on the underlying cause. Obstructive azoospermia often resulting from surgical correctable conditions such as congenital absence of the vas deferens or post-surgical blockages, generally offers a favorable prognosis. Surgical interventions or assisted reproductive techniques can be effective in achieving conception for individuals with obstructive azoospermia.
In contrast, nonobstructive azoospermia, primarily due to testicular failure, presents a more challenging prognosis. While advanced reproductive technologies (ARTs) like testicular sperm extraction (TESE) and intracytoplasmic sperm injection (ICSI) may enable conception, success rates can vary, and the condition may remain untreatable in some cases. Spermatogenesis may be so disrupted that no viable sperm can be collected. Accurate diagnosis, tailored treatment plans, and ongoing support are crucial factors influencing the prognosis, emphasizing the importance of a comprehensive and individualized approach to azoospermia management.
- Ghieh F, Mitchell V, Mandon-Pepin B, Vialard F. Genetic defects in human azoospermia. Basic Clin Androl. 2019;29:4. Published 2019 Apr 23. doi:10.1186/s12610-019-0086-6 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6477738[↩][↩]
- World Health Organization. WHO laboratory manual for the examination and processing of human semen. Geneva: World Health Organization; 2010. 5th ed.[↩][↩]
- Foresta C, Ferlin A, Bettella A, Rossato M, Varotto A. Diagnostic and clinical features in azoospermia. Clin Endocrinol. 1995;43:537–543. doi: 10.1111/j.1365-2265.1995.tb02917.x.[↩][↩]
- Matsumiya K, Namiki M, Takahara S, Kondoh N, Takada S, Kiyohara H, et al. Clinical study of azoospermia. Int J Androl. 1994;17:140–142. doi: 10.1111/j.1365-2605.1994.tb01233.x[↩]
- Jarvi K, Lo K, Fischer A, et al. CUA Guideline: The workup of azoospermic males. Can Urol Assoc J. 2010;4(3):163–167. doi:10.5489/cuaj.10050 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2874589[↩][↩]
- Fogle RH, Steiner AZ, Marshall FE, et al. Etiology of azoospermia in a large nonreferral inner-city population. Fertil Steril. 2006;86:197–9[↩]
- Assisted Reproductive Technology Success Rates. US Department of Health and Human Services, eds. 2005[↩][↩]
- World Health Organization. WHO laboratory manual for the examination and processing of human semen. Geneva: World Health Organization; 2010. 5th ed[↩]
- Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142(1):62–5.[↩][↩]
- Evaluation of the azoospermic male. Fertil Steril. 2008;90(5 Suppl):S74–7.[↩][↩]
- Schlegel PN, Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril. 2004;81:1585–8[↩]
- Alves MBR, Celeghini ECC, Belleannée C. From Sperm Motility to Sperm-Borne microRNA Signatures: New Approaches to Predict Male Fertility Potential. Front Cell Dev Biol. 2020 Aug 21;8:791. doi: 10.3389/fcell.2020.00791[↩][↩][↩][↩][↩]
- Gadea J., Parrington J., Kashir J., Coward K. (2013). “The male reproductive tract and spermatogenesis,” in Textbook of Clinical Embryology, eds Coward K., Wells D. (Cambridge: Cambridge University Press; ), 18–26. 10.1017/cbo9781139192736.005[↩][↩]
- Cooper T. G., Yeung C.-H. (2003). Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc. Res. Tech. 61 28–38. 10.1002/jemt.10314[↩]
- Zini A., Agarwal A. (2011). Sperm Chromatin. New York, NY: Springer.[↩]
- Satouh Y., Inoue N., Ikawa M., Okabe M. (2012). Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J. Cell Sci. 125 4985–4990. 10.1242/jcs.100867[↩]
- Bianchi E., Doe B., Goulding D., Wright G. J. (2014). Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 508 483–487. 10.1038/nature13203[↩]
- Hirohashi N., Yanagimachi R. (2018). Sperm acrosome reaction: its site and role in fertilization. Biol. Reprod. 99 127–133. 10.1093/biolre/ioy045[↩]
- Avidor-Reiss T., Fishman E. L. (2019). It takes two (centrioles) to tango. Reproduction 157 R33–R51. 10.1530/REP-18-0350[↩]
- Inaba K. (2003). Molecular architecture of the sperm flagella: molecules for motility and signaling. Zool. Sci. 20 1043–1056. 10.2108/zsj.20.1043[↩]
- Song G. J., Lewis V. (2008). Mitochondrial DNA integrity and copy number in sperm from infertile men. Fertil. Steril. 90 2238–2244. 10.1016/j.fertnstert.2007.10.059[↩]
- Krawetz S. A. (2005). Paternal contribution: new insights and future challenges. Nat. Rev. Genet. 6 633–642. 10.1038/nrg1654[↩]
- Amann R. P., Hammerstedt R. H. (1993). In vitro evaluation of sperm quality: an opinion. J. Androl. 14 397–406. 10.1002/j.1939-4640.1993.tb03247.x[↩]
- Gillan L., Kroetsch T., Chis Maxwell W. M., Evans G. (2008). Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls. Anim. Reprod. Sci. 103 201–214. 10.1016/j.anireprosci.2006.12.010[↩]
- Saez, J. M., Forest, M. G., Morera, A. M., & Bertrand, J. (1972). Metabolic clearance rate and blood production rate of testosterone and dihydrotestosterone in normal subjects, during pregnancy, and in hyperthyroidism. The Journal of clinical investigation, 51(5), 1226–1234. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC292254/pdf/jcinvest00201-0192.pdf[↩]
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001 Dec;22(6):764-86. doi: 10.1210/edrv.22.6.0446[↩]
- Clements KM, Shipley CF, Coleman DA, Ehrhart EJ, Haschek WM, Clark SG. Azoospermia in an 8-month-old boar due to bilateral obstruction at the testis/epididymis interface. Can Vet J Rev Veterinaire Can. 2010;51:1130–1134[↩]
- European Association of Urology guidelines on Male Infertility: the 2012 update. Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, Krausz C, European Association of Urology Working Group on Male Infertility. Eur Urol. 2012 Aug; 62(2):324-32.[↩][↩][↩]
- Marmar JL, Benoff S. Varicoceles. J Urol. 2006 Mar;175(3 Pt 1):818-9. doi: 10.1016/S0022-5347(05)00831-1[↩]
- Cocuzza M, Cocuzza MA, Bragais FM, Agarwal A. The role of varicocele repair in the new era of assisted reproductive technology. Clinics (Sao Paulo). 2008 Jun;63(3):395-404. doi: 10.1590/s1807-59322008000300018[↩]
- Cocuzza M, Athayde KS, Agarwal A, Pagani R, Sikka SC, Lucon AM, Srougi M, Hallak J. Impact of clinical varicocele and testis size on seminal reactive oxygen species levels in a fertile population: a prospective controlled study. Fertil Steril. 2008 Oct;90(4):1103-8. doi: 10.1016/j.fertnstert.2007.07.1377[↩]
- Schlegel PN, Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril. 2004 Jun;81(6):1585-8. doi: 10.1016/j.fertnstert.2003.10.036[↩]
- Sperm retrieval techniques for assisted reproduction. Esteves SC, Miyaoka R, Agarwal A. Int Braz J Urol. 2011 Sep-Oct; 37(5):570-83.[↩]
- Eisenberg ML, Betts P, Herder D, Lamb DJ, Lipshultz LI. Increased risk of cancer among azoospermic men. Fertil Steril. 2013 Sep;100(3):681-5. doi: 10.1016/j.fertnstert.2013.05.022[↩]
- Bobjer J, Naumovska M, Giwercman YL, Giwercman A. High prevalence of androgen deficiency and abnormal lipid profile in infertile men with non-obstructive azoospermia. Int J Androl. 2012 Oct;35(5):688-94. doi: 10.1111/j.1365-2605.2012.01277.x[↩]
- Patel DP, Brant WO, Myers JB, Zhang C, Presson AP, Johnstone EB, Dorais JA, Aston KI, Carrell DT, Hotaling JM. Sperm concentration is poorly associated with hypoandrogenism in infertile men. Urology. 2015 May;85(5):1062-1067. doi: 10.1016/j.urology.2015.01.014[↩]
- Johnson MD. Genetic risks of intracytoplasmic sperm injection in the treatment of male infertility: recommendations for genetic counseling and screening. Fertil Steril. 1998 Sep;70(3):397-411. doi: 10.1016/s0015-0282(98)00209-x[↩]
- Clementini E, Palka C, Iezzi I, Stuppia L, Guanciali-Franchi P, Tiboni GM. Prevalence of chromosomal abnormalities in 2078 infertile couples referred for assisted reproductive techniques. Hum Reprod. 2005 Feb;20(2):437-42. doi: 10.1093/humrep/deh626[↩]
- Vincent MC, Daudin M, De MP, Massat G, Mieusset R, Pontonnier F, Calvas P, Bujan L, Bourrouillout G. Cytogenetic investigations of infertile men with low sperm counts: a 25-year experience. J Androl. 2002 Jan-Feb;23(1):18-22; discussion 44-5. doi: 10.1002/j.1939-4640.2002.tb02597.x[↩]
- Mak V, Jarvi KA. The genetics of male infertility. J Urol. 1996 Oct;156(4):1245-56; discussion 1256-7.[↩]
- Sharma M, Leslie SW. Azoospermia. [Updated 2023 Nov 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK578191[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Stahl PJ, Masson P, Mielnik A, Marean MB, Schlegel PN, Paduch DA. A decade of experience emphasizes that testing for Y microdeletions is essential in American men with azoospermia and severe oligozoospermia. Fertil Steril. 2010 Oct;94(5):1753-6. doi: 10.1016/j.fertnstert.2009.09.006[↩]
- Jarvi K, Lo K, Fischer A, Grantmyre J, Zini A, Chow V, Mak V. CUA Guideline: The workup of azoospermic males. Can Urol Assoc J. 2010 Jun;4(3):163-7. doi: 10.5489/cuaj.10050[↩]
- Yuen W, Golin AP, Flannigan R, Schlegel PN. Histology and sperm retrieval among men with Y chromosome microdeletions. Transl Androl Urol. 2021 Mar;10(3):1442-1456. doi: 10.21037/tau.2020.03.35[↩]
- Sudhakar DVS, Shah R, Gajbhiye RK. Genetics of Male Infertility – Present and Future: A Narrative Review. J Hum Reprod Sci. 2021 Jul-Sep;14(3):217-227. doi: 10.4103/jhrs.jhrs_115_21[↩]
- Diaz-Castaños LR, Rivera H, Gonzalez-Montes RM, Diaz M. Translocation (Y;19)(q12;q13) and azoospermia. Ann Genet. 1991;34:27–29[↩]
- Stuppia L, Antonucci I, Binni F, Brandi A, Grifone N, Colosimo A, et al. Screening of mutations in the CFTR gene in 1195 couples entering assisted reproduction technique programs. Eur J Hum Genet EJHG. 2005;13:959–964. doi: 10.1038/sj.ejhg.5201437[↩]
- Quilter CR, Svennevik EC, Serhal P, Ralph D, Bahadur G, Stanhope R, et al. Cytogenetic and Y chromosome microdeletion screening of a random group of infertile males. Fertil Steril. 2003;79:301–307. doi: 10.1016/S0015-0282(02)04692-7[↩]
- Pallais JC, Au M, Pitteloud N, Seminara S, Crowley WF. Pagon R A, Bird T D, Dolan C R, Stephens K, Adam M P, editors. , Kallmann Syndrome GeneReviews 1993 Seattle, WA[↩][↩]
- Nishio H, Mizuno K, Moritoki Y, Kamisawa H, Kojima Y, Mizuno H, et al. Clinical features and testicular morphology in patients with Kallmann syndrome. Urology. 2012;79(3):684–6.[↩]
- Jungwirth A, Diemer T, Dohle GR, Giwercman A, Kopa Z, Krausz C, et al. European Association of Urology Guidelines on Male Infertility. 2012[↩]
- Morel F, Douet-Guilbert N, Le Bris MJ, Amice V, Le Martelot MT, Roche S, Valéri A, Derrien V, Amice J, De Braekeleer M. Chromosomal abnormalities in couples undergoing intracytoplasmic sperm injection. A study of 370 couples and review of the literature. Int J Androl. 2004 Jun;27(3):178-82. doi: 10.1111/j.1365-2605.2004.00472.x[↩]
- Visootsak J, Graham JM., Jr Klinefelter syndrome and other sex chromosomal aneuploidies. Orphanet J Rare Dis. 2006;1:42.[↩]
- Groth KA, Skakkebæk A, Høst C, Gravholt CH, Bojesen A. Clinical review: Klinefelter syndrome–a clinical update. J Clin Endocrinol Metab. 2013 Jan;98(1):20-30. doi: 10.1210/jc.2012-2382[↩]
- Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter’s syndrome. Lancet. 2004;364(9430):273–83[↩]
- Oates RD. The genetic basis of male reproductive failure. Urol Clin North Am. 2008;35(2):257, ix–70.[↩][↩][↩]
- Kanakis GA, Nieschlag E. Klinefelter syndrome: more than hypogonadism. Metabolism. 2018 Sep;86:135-144. doi: 10.1016/j.metabol.2017.09.017[↩]
- Wikstrom AM, Dunkel L. Klinefelter syndrome. Best Pract Res Clin Endocrinol Metab. 2011;25(2):239–50.[↩]
- Corona G, Pizzocaro A, Lanfranco F, Garolla A, Pelliccione F, Vignozzi L, Ferlin A, Foresta C, Jannini EA, Maggi M, Lenzi A, Pasquali D, Francavilla S; Klinefelter ItaliaN Group (KING). Sperm recovery and ICSI outcomes in Klinefelter syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2017 May 1;23(3):265-275. doi: 10.1093/humupd/dmx008[↩]
- Yang Q, Huang YP, Wang HX, Hu K, Wang YX, Huang YR, Chen B. Follicle-stimulating hormone as a predictor for sperm retrieval rate in patients with nonobstructive azoospermia: a systematic review and meta-analysis. Asian J Androl. 2015 Mar-Apr;17(2):281-4. doi: 10.4103/1008-682X.139259[↩]
- Rohayem J, Fricke R, Czeloth K, Mallidis C, Wistuba J, Krallmann C, Zitzmann M, Kliesch S. Age and markers of Leydig cell function, but not of Sertoli cell function predict the success of sperm retrieval in adolescents and adults with Klinefelter’s syndrome. Andrology. 2015 Sep;3(5):868-75. doi: 10.1111/andr.12067[↩]
- Okada H, Goda K, Yamamoto Y, Sofikitis N, Miyagawa I, Mio Y, Koshida M, Horie S. Age as a limiting factor for successful sperm retrieval in patients with nonmosaic Klinefelter’s syndrome. Fertil Steril. 2005 Dec;84(6):1662-4. doi: 10.1016/j.fertnstert.2005.05.053[↩]
- Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, Cocci A, Corona G, Dimitropoulos K, Gül M, Hatzichristodoulou G, Jones TH, Kadioglu A, Martínez Salamanca JI, Milenkovic U, Modgil V, Russo GI, Serefoglu EC, Tharakan T, Verze P, Salonia A; EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur Urol. 2021 Nov;80(5):603-620. doi: 10.1016/j.eururo.2021.08.014[↩][↩][↩][↩]
- Franik S, Hoeijmakers Y, D’Hauwers K, Braat DD, Nelen WL, Smeets D, Claahsen-van der Grinten HL, Ramos L, Fleischer K. Klinefelter syndrome and fertility: sperm preservation should not be offered to children with Klinefelter syndrome. Hum Reprod. 2016 Sep;31(9):1952-9. doi: 10.1093/humrep/dew179[↩]
- Donat R, McNeill AS, Fitzpatrick DR, Hargreave TB. The incidence of cystic fibrosis gene mutations in patients with congenital bilateral absence of the vas deferens in Scotland. Br J Urol. 1997;79(1):74–7[↩]
- Anguiano A, Oates RD, Amos JA, Dean M, Gerrard B, Stewart C, et al. Congenital bilateral absence of the vas deferens.A primarily genital form of cystic fibrosis. JAMA. 1992;267(13):1794–7.[↩]
- Chillon M, Casals T, Mercier B, Bassas L, Lissens W, Silber S, et al. Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med. 1995;332(22):1475–80.[↩][↩]
- Meng MV, Black LD, Cha I, Ljung BM, Pera RA, Turek PJ. Impaired spermatogenesis in men with congenital absence of the vas deferens. Hum Reprod. 2001;16(3):529–33.[↩]
- Schlegel PN, Shin D, Goldstein M. Urogenital anomalies in men with congenital absence of the vas deferens. J Urol. 1996;155(5):1644–8.[↩]
- Tiepolo L, Zuffardi O. Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum Genet. 1976;34(2):119–24.[↩]
- Ferlin A, Arredi B, Speltra E, Cazzadore C, Selice R, Garolla A, et al. Molecular and clinical characterization of Y chromosome microdeletions in infertile men: a 10-year experience in Italy. J Clin Endocrinol Metab. 2007;92(3):762–70[↩]
- Pryor JL, Kent-First M, Muallem A, Van Bergen AH, Nolten WE, Meisner L, et al. Microdeletions in the Y chromosome of infertile men. N Engl J Med. 1997;336(8):534–9[↩]
- Vogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann P, Kiesewetter F, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet. 1996;5(7):933–43[↩]
- Jobling MA, Tyler-Smith C. The human Y chromosome: an evolutionary marker comes of age. Nat Rev Genet. 2003;4(8):598–612.[↩]
- Krausz C, Forti G, McElreavey K. The Y chromosome and male fertility and infertility. Int J Androl. 2003;26(2):70–5.[↩][↩]
- Krausz C, Quintana-Murci L, McElreavey K. Prognostic value of Y deletion analysis: what is the clinical prognostic value of Y chromosome microdeletion analysis. Hum Reprod. 2000;15(7):1431–4.[↩][↩]
- Kamp C, Huellen K, Fernandes S, Sousa M, Schlegel PN, Mielnik A, et al. High deletion frequency of the complete AZFa sequence in men with Sertoli-cell-only syndrome. Molecular Hum Reprod. 2001;7(10):987–94[↩]
- Evaluation of the azoospermic male. Fertil Steril. 2008;90(5 Suppl):S74–7[↩]
- Sharma R, Biedenharn KR, Fedor JM, Agarwal A. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013 Jul 16;11:66. doi: 10.1186/1477-7827-11-66[↩]
- Mak V, Jarvi KA. The genetics of male infertility J Urol 19961561245–56.; discussion 1256–7.[↩][↩]
- von Eckardstein S, Simoni M, Bergmann M, et al. Serum inhibin B in combination with serum follicles-timulating hormone (FSH) is a more sensitive marker than serum FSH alone for impaired spermatogenesis in men, but cannot predict the presence of sperm in testicular tissue samples. J Clin Endocrinol Metab. 1999;84:2496–501[↩]
- Chen DY, Wang JJ, Huang YF, et al. Relationship between lipocalin-type prostaglandin D synthase and alpha-glucosidase in azoospermia seminal plasma. Clin Chim Acta. 2005;354:69–76.[↩]
- Kim HH, Schlegel PN. Endocrine manipulation in male infertility. Urol Clin North Am. 2008 May;35(2):303-18, x. doi: 10.1016/j.ucl.2008.01.003[↩]
- Schoor RA, Elhanbly S, Niederberger CS, Ross LS. The role of testicular biopsy in the modern management of male infertility. J Urol. 2002 Jan;167(1):197-200.[↩][↩]
- Büchter D, Behre HM, Kliesch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol. 1998 Sep;139(3):298-303. doi: 10.1530/eje.0.1390298[↩]
- Al Hashimi M, Farahat Y, Kandil H, Al Khalidi I. Androgenic-anabolic steroid abuse trend and management: A prospective, cross-sectional, questionnaire-based survey. Health Sci Rep. 2023 Jan 9;6(1):e1032. doi: 10.1002/hsr2.1032[↩]
- Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES Jr. Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012 Mar;187(3):973-8. doi: 10.1016/j.juro.2011.10.137[↩]
- Kolettis PN, Purcell ML, Parker W, Poston T, Nangia AK. Medical testosterone: an iatrogenic cause of male infertility and a growing problem. Urology. 2015 May;85(5):1068-1073. doi: 10.1016/j.urology.2014.12.052[↩]
- McBride JA, Coward RM. Recovery of spermatogenesis following testosterone replacement therapy or anabolic-androgenic steroid use. Asian J Androl. 2016 May-Jun;18(3):373-80. doi: 10.4103/1008-682X.173938[↩]
- Sihag P, Tandon A, Pal R, Bhatt S, Sinha A, Sumbul M. Sonography in male infertility: a useful yet underutilized diagnostic tool. J Ultrasound. 2022 Sep;25(3):675-685. doi: 10.1007/s40477-021-00646-z[↩]
- Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999 Jan;14(1):131-5. doi: 10.1093/humrep/14.1.131[↩]
- Schlegel PN. Causes of azoospermia and their management. Reprod Fertil Dev. 2004;16:561–72.[↩][↩][↩]
- Schlegel PN, Palermo GD, Alikani M, Adler A, Reing AM, Cohen J, Rosenwaks Z. Micropuncture retrieval of epididymal sperm with in vitro fertilization: importance of in vitro micromanipulation techniques. Urology. 1995 Aug;46(2):238-41. doi: 10.1016/s0090-4295(99)80199-x[↩]
- Janzen N, Goldstein M, Schlegel PN, Palermo GD, Rosenwaks Z, Hariprashad J. Use of electively cryopreserved microsurgically aspirated epididymal sperm with IVF and intracytoplasmic sperm injection for obstructive azoospermia. Fertil Steril. 2000 Oct;74(4):696-701. doi: 10.1016/s0015-0282(00)01496-5. Erratum in: Fertil Steril 2001 Jan;75(1):230.[↩]
- Schlegel PN. Causes of azoospermia and their management. Reprod Fertil Dev. 2004;16(5):561-72. doi: 10.10371/RD03087[↩]
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Male Reproduction and Urology. Electronic address: [email protected]. The management of obstructive azoospermia: a committee opinion. Fertil Steril. 2019 May;111(5):873-880. doi: 10.1016/j.fertnstert.2019.02.013[↩]
- Etafy M, Gudeloglu A, Brahmbhatt JV, Parekattil SJ. Review of the role of robotic surgery in male infertility. Arab J Urol. 2017 Dec 13;16(1):148-156. doi: 10.1016/j.aju.2017.11.006[↩]
- Avellino GJ, Lipshultz LI, Sigman M, Hwang K. Transurethral resection of the ejaculatory ducts: etiology of obstruction and surgical treatment options. Fertil Steril. 2019 Mar;111(3):427-443. doi: 10.1016/j.fertnstert.2019.01.001[↩]
- Jiang HT, Yuan Q, Liu Y, Liu ZQ, Zhou ZY, Xiao KF, Yang JG. Multiple advanced surgical techniques to treat acquired seminal duct obstruction. Asian J Androl. 2014 Nov-Dec;16(6):912-6. doi: 10.4103/1008-682X.139256[↩]
- Schiff JD, Palermo GD, Veeck LL, et al. Success of testicular sperm extraction [corrected] and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab. 2005;90:6263–7[↩]
- Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–5[↩]
- Ghalayini IF, Alazab R, Halalsheh O, Al-Mohtaseb AH, Al-Ghazo MA. Repeated microdissection testicular sperm extraction in patients with non-obstructive azoospermia: Outcome and predictive factors. Arab J Urol. 2022 Jan 24;20(3):137-143. doi: 10.1080/2090598X.2022.2028066[↩]
- Kalsi J, Thum MY, Muneer A, Abdullah H, Minhas S. In the era of micro-dissection sperm retrieval (m-TESE) is an isolated testicular biopsy necessary in the management of men with non-obstructive azoospermia? BJU Int. 2012 Feb;109(3):418-24. doi: 10.1111/j.1464-410X.2011.10399.x[↩]
- Kohn TP, Louis MR, Pickett SM, Lindgren MC, Kohn JR, Pastuszak AW, Lipshultz LI. Age and duration of testosterone therapy predict time to return of sperm count after human chorionic gonadotropin therapy. Fertil Steril. 2017 Feb;107(2):351-357.e1. doi: 10.1016/j.fertnstert.2016.10.004[↩][↩]
- Ly LP, Liu PY, Handelsman DJ. Rates of suppression and recovery of human sperm output in testosterone-based hormonal contraceptive regimens. Hum Reprod. 2005 Jun;20(6):1733-40. doi: 10.1093/humrep/deh834[↩]
- Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C; Hormonal Male Contraception Summit Group. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet. 2006 Apr 29;367(9520):1412-20. doi: 10.1016/S0140-6736(06)68614-5[↩]
- Rastrelli G, Corona G, Mannucci E, Maggi M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology. 2014 Nov;2(6):794-808. doi: 10.1111/andr.262[↩]
- Selman H, De Santo M, Sterzik K, Cipollone G, Aragona C, El-Danasouri I. Rescue of spermatogenesis arrest in azoospermic men after long-term gonadotropin treatment. Fertil Steril. 2006 Aug;86(2):466-8. doi: 10.1016/j.fertnstert.2005.12.055[↩]
- Shiraishi K, Ohmi C, Shimabukuro T, Matsuyama H. Human chorionic gonadotrophin treatment prior to microdissection testicular sperm extraction in non-obstructive azoospermia. Hum Reprod. 2012 Feb;27(2):331-9. doi: 10.1093/humrep/der404[↩]
- Hussein A, Ozgok Y, Ross L, Rao P, Niederberger C. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU Int. 2013 Mar;111(3 Pt B):E110-4. doi: 10.1111/j.1464-410X.2012.11485.x[↩][↩]
- Tharakan T, Salonia A, Corona G, Dhillo W, Minhas S, Jayasena C. The Role of Hormone Stimulation in Men With Nonobstructive Azoospermia Undergoing Surgical Sperm Retrieval. J Clin Endocrinol Metab. 2020 Dec 1;105(12):dgaa556. doi: 10.1210/clinem/dgaa556[↩][↩]
- Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, Crowley WF Jr. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002 Sep;87(9):4128-36. doi: 10.1210/jc.2002-020518[↩]
- Bhasin S. Approach to the infertile man. J Clin Endocrinol Metab. 2007 Jun;92(6):1995-2004. doi: 10.1210/jc.2007-0634[↩]
- Resorlu B, Abdulmajed MI, Kara C, Unsal A, Aydos K. Is intracytoplasmic sperm injection essential for the treatment of hypogonadotrophic hypogonadism? A comparison between idiopathic and secondary hypogonadotrophic hypogonadism. Hum Fertil (Camb). 2009 Dec;12(4):204-8. doi: 10.3109/14647270903331139[↩]