Contents
Manganese toxicity
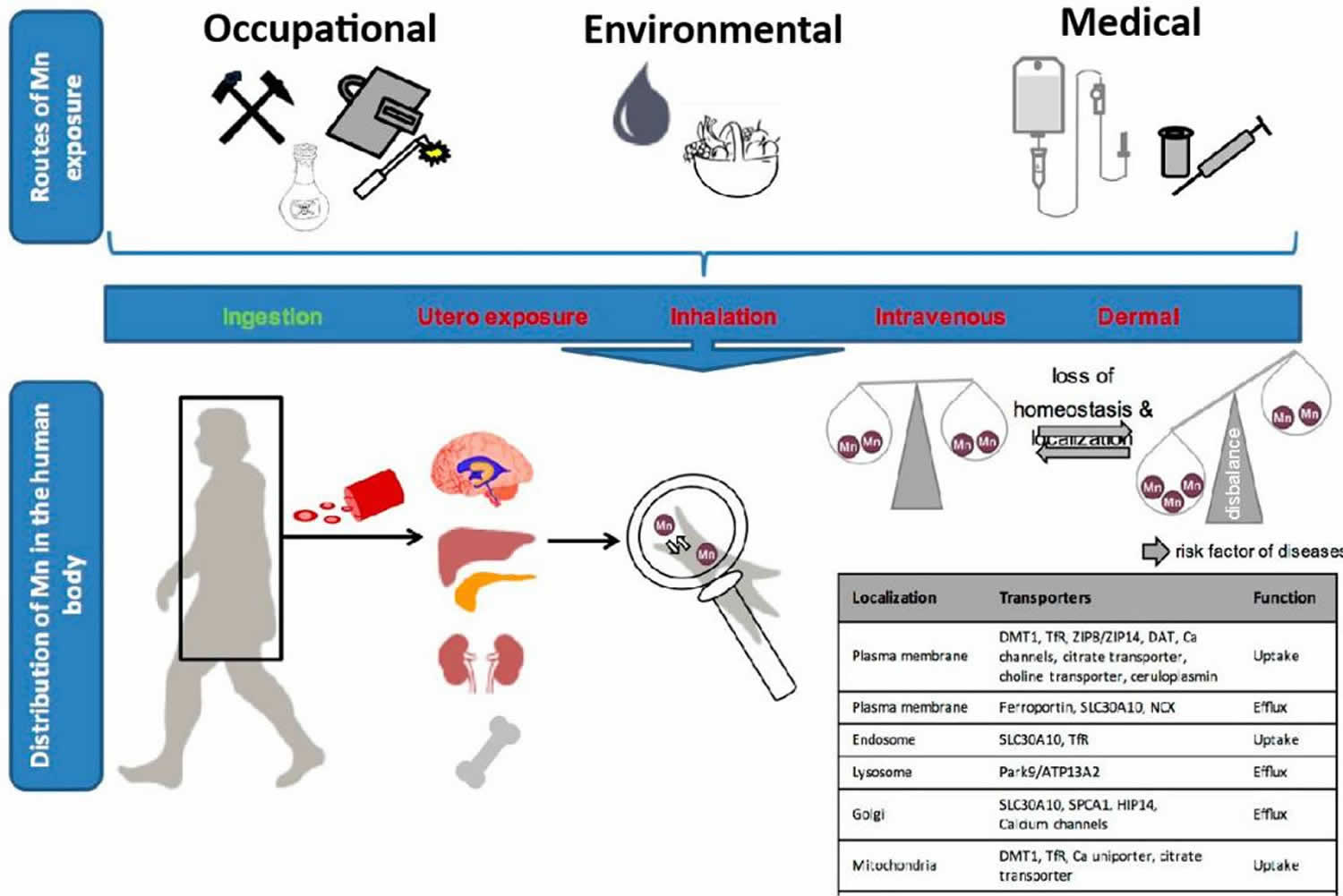
Exposure to excessive amount of manganese can result in manganese toxicity also known as “manganism” or “manganese-induced neurotoxicity”, a neurological disorder with symptoms similar to those of Parkinson’s disease 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11. Manganese toxicity classically results from elevated exposure levels in drinking water or air. Manganese is widely used in industrial processes and commercial products. Excessive occupational exposure to manganese is most common in mining, welding, ore processing, dry battery manufacture, and organochemical fungicide use 12, 13, 14, 15. Manganese toxicity can also arise from an impaired or under-developed excretion system, including in patients receiving total parenteral nutrition (TPN) therapy 16, 17, patients with hepatic encephalopathy 18 and abusers of ephedrone (methcathinone) 19.
Manganese toxicity is caused by the preferential accumulation of manganese in brain areas rich in dopaminergic neurons such as the caudate nucleus, putamen, globus pallidus, substantia nigra, and subthalamic nuclei (the extrapyramidal system) 4, 6, 7, 5, 1, 2, 3. The extrapyramidal system is a part of your brain that controls involuntary movements, maintains posture, and regulates muscle tone. Visual reaction time, hand steadiness, and eye-hand coordination were affected in chronically-exposed workers.
The most common health problems in workers exposed to high levels of manganese involve the central nervous system (brain and spinal cord). The early stages of manganese toxicity include psychiatric symptoms such as emotional liability, mania, compulsive or aggressive behavior, irritability, movements that may become slow and clumsy, hallucinations, feeding and sex dysfunctions, intellectual deficits, humor changes and mild motor impairment. This combination of symptoms when sufficiently severe is referred to as “manganism” or “manganese-induced neurotoxicity”. Other less severe nervous system effects such as slowed hand movements have been observed in some workers exposed to lower concentrations in the work place. In situations of established manganese-induced neurotoxicity, the characteristic extrapyramidal symptoms (motor symptoms), such as a mask-like face, become evident 20.
The inhalation of a large quantity of dust or fumes containing manganese may cause irritation of the lungs which could lead to pneumonia. Loss of sex drive and sperm damage has also been observed in men exposed to high levels of manganese in workplace air.
The manganese concentrations that cause effects such as slowed hand movements in some workers are approximately twenty thousand times higher than the concentrations normally found in the environment. Manganism has been found in some workers exposed to manganese concentrations about a million times higher than normal air concentrations of manganese.
Manganese neurotoxicity can occur following high-dose oral, inhalation, or parenteral exposure to manganese 5. The development of manganese neurotoxicity following different routes of exposure indicates that the manganese dose to target tissue is the critical determinant of manganese toxicity, regardless of route 5. The association between manganese and neurotoxicity (damage to the brain) was first noted by Couper in 1837 who reported abnormal neurologic effects in workers at an ore-grinding plant where “black oxide of manganese” was processed 21. Most epidemiologic research on manganese conducted during the late 20th century focused on occupational inhalation exposure. Manganese toxicity may result in multiple neurologic problems and is a well-recognized health hazard for people who inhale manganese dust. Subsequent epidemiologic studies of welders, manganese miners, battery producers, smelters and other manganese workers have clearly established a causal association between chronic high-dose-manganese exposure via inhalation and neurotoxicity 22, 23, 24, 25. Unlike ingested manganese, inhaled manganese is transported directly to your brain before it can be brokendown in your liver 26, 27. Hallmarks of manganese neurotoxicity in adults include behavioral changes, cognitive deficits, progressive bradykinesia, dystonia, and other gait abnormalities 28, 29, 30. There has been increasing concern regarding the role of environmental manganese exposure and children’s health 31. Manganese has been identified as a risk factor for the development of aggressive behavior, attention deficit, cognitive decline resulting in lowered IQ, and learning deficits in infants and children 32, 33, 34, 35.
Manganese neurotoxicity can occur following high-dose oral, inhalation, or parenteral exposure to manganese 5. The symptoms of manganese neurotoxicity generally appear slowly over a period of months to years. In its worst form, manganese neurotoxicity can result in a permanent neurological disorder with symptoms similar to those of Parkinson’s disease (also known as manganese-induced parkinsonism) with symptoms such as tremors, difficulty walking, and facial muscle spasms. Excessive manganese accumulation in the central nervous system (brain and spinal cord) is an established clinical entity, referred to as manganism. Manganism resembles idiopathic Parkinson’s disease (the term “idiopathic” means that the cause is unknown) in its clinical features, resulting in adverse neurological effects both in laboratory animals and humans 4, 22. Manganism is sometimes preceded by psychiatric symptoms, such as irritability, aggressiveness, and even hallucinations 36, 37.
Analysis of brain samples have shown that manganese accumulates within the human striatum, globus pallidus, and substantia nigra 38, 39. Manganese accumulation in these brain regions is associated with the presence of the divalent metal transporter 1 (DMT1) although additional transporters may play a role in brain uptake of manganese 40, 41, 42. Brain imaging studies that rely on the paramagnetic properties of manganese that result in increased signal intensity seen with T1-weighted magnetic resonance imaging (MRI), allow for visual inspection of the brain for evidence of manganese accumulation at this site. Brain MRI studies of highly exposed people reveal signal intensity changes in the globus pallidus, striatum, and midbrain consistent with manganese accumulation at these sites 43, 44. Studies performed in nonhuman primates have shown that changes in the T1-weighted image correlate with manganese tissue concentration 45. The primary neuropathologic target of manganese neurotoxicity is the globus pallidus (particularly the internal segment) with sparing of the substantia nigra pars compacta and an absence of Lewy bodies 46. Studies of a manganese-exposed South African mine worker have revealed reduced astrocyte and neuron density in both the caudate and putamen 47. Chronic manganese neurotoxicity in people is also associated with decreased gamma-aminobutyric acid (GABA) neurons, reduced myelinated fibers, and moderate astrocytic proliferation in the medial segment of the globus pallidus 46.
Figure 1. Manganese exposure (pathways of human manganese exposure)
[Source 48 ]Several studies have examined neurochemical changes following high-dose-manganese exposure. Because manganese neurotoxicity results in dysregulation of motor control, many studies have focused on the striatal and pallidal dopaminergic system. Manganese reacts with dopamine and other biogenic amines resulting in oxidative damage to the neurotransmitters 49. One pathway involves manganese catalyzed oxidation of the alpha hydroxyl group of dopamine forming a semi-quinone radical. The semi-quinone radical then reacts with oxygen to generate superoxide anion radical [O2•−] and a quinone. Oxygen can reoxidize the quinone to quinol to generate hydrogen peroxide 49. Manganese-catalyzed dopamine auto-oxidation may also involve semiquinone and aminochrome intermediates, l-cysteine or copper, and NADH facilitation 50, 51. Excess manganese may also alter glutamate homeostasis in the basal ganglia 52. Changes in glutamate homeostasis have been associated with excitotoxicity in the brain 52.
Limited evidence suggests that high manganese intakes from drinking water may be associated with neurological symptoms similar to those of Parkinson’s disease. Severe neurological symptoms were reported in 25 people who drank water contaminated with manganese and probably other contaminants from dry cell batteries for two to three months 53. Water manganese concentrations were found to be 14 mg/L almost two months after symptoms began and may have already been declining 23. A study of older adults in Greece found a high prevalence of neurological symptoms in those exposed to water manganese levels of 1.8 to 2.3 mg/L 54, while a study in Germany found no evidence of increased neurological symptoms in people drinking water with manganese levels ranging from 0.3 to 2.2 mg/L compared to those drinking water containing less than 0.05 mg/L of manganese 55. Manganese in drinking water may be more bioavailable than manganese in food. However, none of the studies measured dietary manganese, so total manganese intake in these cases is unknown. In the US, the Environmental Protection Agency (EPA) recommends 0.05 mg/L as the maximum allowable manganese concentration in drinking water 56 and the World Health Organization (WHO) health-based guideline value for manganese in drinking water is 0.08 mg/L 57, 58. Current acceptable levels of manganese in drinking water are 0.04 mg/L and a tolerable intake of manganese from dietary sources should not exceed 0.06 milligrams/kg 59
Additionally, several cross-sectional studies have associated high levels of manganese in drinking water with cognitive and behavioral deficits in children 60. For example, a cross-sectional study of 362 children (ages 6-13 years) in Canada found children with the highest manganese concentrations in home tap water (median of 216 microgram/L) had a 6.2-point lower Full Scale IQ (lower Performance IQ but not Verbal IQ) than those with lowest manganese levels in home tap water (median of 1 μg/L) 61. A cohort study that followed 287 of these children for a mean of 4.4 years found that exposure to higher concentrations of manganese in drinking water was linked to a lower Performance IQ among girls but a higher Performance IQ among boys 62. Additionally, a prospective cohort study among 1,265 children in Bangladesh did not find manganese concentration in drinking water (medians of 0.20 mg/L during pregnancy and 0.34 mg/L at 10 years) to be associated with any measure of cognitive ability (i.e., IQ, verbal comprehension, perceptual reasoning, working memory, processing speed) when assessed at age 10 63. Yet, this study associated manganese in drinking water with higher risks of conduct problems among boys and low prosocial scores among girls 63. In a population-based cohort study in Denmark that followed 643,401 children, exposure to higher manganese concentrations in drinking water was linked to a heightened risk of one subtype of attention-deficit hyperactive disorder (ADHD) 64. Specifically, exposure to a manganese concentration in drinking water of at least 100 mcg/L was associated with a 51% higher risk of the ADHD-Inattentive subtype in girls and a 20% higher risk in boys, in comparison to exposure of <5 mcg/L — using these exposure comparisons, a 9% increased risk of ADHD-Overall was observed in girls and no difference found in boys 64.
Only a few adverse effects of manganese intake from supplements have been documented. A single case of manganese toxicity was reported in a person who took large amounts of mineral supplements for years 65, while another case was reported as a result of a person taking a Chinese herbal supplement 36. More recently, Parkinson’s disease was reported in a woman taking 100 mg/day of manganese chloride for at least two years, followed by 30 mg/day for two months 66.
Manganese neurotoxicity has been observed in individuals receiving total parenteral nutrition (TPN), both as a result of excessive manganese in the solution and as an incidental contaminant 67. Total parenteral nutrition (TPN) is a method of feeding that involves delivering nutrients directly into the bloodstream through a vein (IV). Total parenteral nutrition (TPN) is used when the digestive system isn’t working properly, or when a person can’t or shouldn’t receive oral or tube feedings. Neonates (newborn babies) are especially vulnerable to manganese-related neurotoxicity 68. Infants receiving manganese-containing TPN can be exposed to manganese concentrations about 100-fold higher than breast-fed infants 69. Because of potential manganese neurotoxicities, some argue against including manganese in parenteral nutrition 70.
Individuals with increased susceptibility to manganese neurotoxicity:
- Chronic liver disease: Manganese is eliminated from the body mainly in bile. Therefore, impaired liver function may lead to decreased manganese excretion. Manganese accumulation in individuals with cirrhosis or liver failure may contribute to neurological problems and Parkinson’s disease-like symptoms 71, 23.
- Infants and children: Compared to adults, infants and children have higher intestinal absorption of manganese, as well as lower biliary excretion of manganese 72, 59. Therefore, infants and children are especially susceptible to any negative, neurotoxic effects of manganese. Several studies in school-aged children have reported deleterious cognitive and behavioral effects following excessive manganese exposure 73, 74, 75, 76, 77, 78, 79, 61. Additional studies have associated higher manganese exposures during pregnancy with cognitive and motor deficits in children under six years of age 60.
- Iron-deficient populations: Iron deficiency has been shown to increase the risk of manganese accumulation in the brain 80.
- Individuals with occupational exposures to airborne manganese, such as welders, miners, and smelters 69.
- Abusers of the illicit drug, methcathinone (ephedrone): Intravenous use of manganese-contaminated methcathinone (i.e., when the drug is synthesized with potassium permanganate as the oxidant) can cause lasting neurological damage and a parkinsonism disorder 81, 82.
Manganese toxicity resulting from food alone has not been reported in humans, even though certain vegetarian diets could provide up to 20 mg/day of manganese 65, 83.
Additionally, environmental or occupational inhalation of manganese can cause an inflammatory response in your lungs 84, with clinical symptoms including cough, acute bronchitis, and decreased lung function 85.
Due to the severe implications of manganese neurotoxicity, the Food and Nutrition Board of the Institute of Medicine set very conservative tolerable upper intake levels (UL) for manganese; the tolerable upper intake levels (ULs) are listed in Table 1 according to age 83. The Tolerable Upper Intake Level (UL) is the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects in almost all individuals 83. The Tolerable Upper Intake Levels (ULs) for Manganese do not apply to individuals who are taking supplemental manganese under medical supervision.
Older adults (>50 years)
The requirement for manganese is not known to be higher for older adults. However, liver disease is more common in older adults and may increase the risk of manganese toxicity by decreasing the elimination of manganese from the body. Manganese supplementation beyond 100% of the Daily Value (DV) (2 mg/day) is not recommended.
Table 1. Tolerable Upper Intake Levels (ULs) for Manganese
| Age Group | UL (mg/day) |
|---|---|
| Infants 0-12 months | Not possible to establish* |
| Children 1-3 years | 2 mg/day |
| Children 4-8 years | 3 mg/day |
| Children 9-13 years | 6 mg/day |
| Adolescents 14-18 years | 9 mg/day |
| Adults 19 years and older | 11 mg/day |
Footnote: *Breast milk, formula, and food should be the only sources of manganese for infants.
[Source 86 ]What is manganese?
Manganese (Mn) is a mineral element that is both nutritionally essential at low levels in your diet and at the same time it can also be potentially neurotoxic and scientists are still working to understand the diverse effects of manganese deficiency and manganese neurotoxicity in living organisms 23, 87, 24. Manganese (Mn) is the 10th most common mineral in the Earth’s crust usually occurring with iron (Fe) in the environment where it can be found in rocks and soil and your body needs trace amounts of manganese to stay healthy. Manganese (Mn) can exist in 11 oxidation states, often as chloride, oxides and sulfates 58. The most common oxidation states for manganese in natural water are Mn2+ and Mn4+ 88, 58. Divalent manganese (Mn2+) is absorbed by clay minerals and organic material, and this form is the most significant in plant nutrition 89. Manganese is used principally in the manufacture of iron and steel alloys, and manganese compounds such as potassium and sodium permanganate are ingredients in various products used for cleaning, bleaching and disinfection 58. Manganese compounds are additionally used in some locations for potable water treatment and can also be an impurity in coagulants used during water treatment 58. Manganese occurs naturally in many surface water and groundwater sources; although naturally occurring manganese is usually the most important source for drinking-water, human activities can also contribute to high levels of manganese in water 58. Manganese also occurs naturally in many food sources, and the greatest exposure to manganese is usually from food. Manganese is also available as a dietary supplement.
Your body uses manganese to make energy and protect your cells from damage. Your body also needs manganese for strong bones, reproduction, blood clotting, and a healthy immune system 90. Manganese is a compound that is essential for the activity for many enzymes serving as an enzyme cofactor and is incorporated into several metalloenzymes, including manganese superoxide dismutase (MnSOD), arginase, glutamine synthetase, phosphoenolpyruvate decarboxylase, and pyruvate carboxylase 91, 92, 93, 94. Through the action of these enzymes, manganese is involved in amino acid (a building block of protein), cholesterol, glucose, and carbohydrate metabolism (the process by which the body converts carbohydrate into energy through chemical reactions in cells); reactive oxygen species scavenging; bone formation; reproduction; and immune response 83, 95, 96, 97, 69. Manganese also plays a role in blood clotting and stopping bleeding in combination with vitamin K 96.
The human body’s nutritional requirements for manganese are normally met through dietary intake via food and drinking water. The Adequate Intake (AI) for manganese in adult men is 2.3 mg/day, women is 1.8 mg/day and 1.5 mg/day for children 4 to 8 years of age 83. A Tolerable Upper Intake Level (maximum daily intake unlikely to cause adverse health effects) for manganese was set for adults at 11 mg/day based on a no-observed-adverse-effect level (NOAEL) for Western diets 83.
Only a small percentage of dietary manganese is absorbed. Manganese is absorbed in the small intestine through an active transport system and, possibly, through diffusion when intakes are high 94. After absorption, some manganese remains free, but most is bound to transferrin, albumin, and plasma alpha-2-macroglobulin. Manganese is taken up by your liver and other tissues, but the mechanism of this process is not well understood 94, 93. Manganese inhibited iron absorption, both from a solution and from a hamburger meal 98.
How much manganese do I need?
The amount of manganese you need depends on your age and sex. Average daily recommended amounts are listed below in milligrams (mg). Intake recommendations for manganese and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by an expert committee of the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine 83. The Dietary Reference Intake (DRI) is the general term for a set of reference values used for planning and assessing nutrient intakes of healthy people. These values vary by age and sex and they include:
- Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals
- Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA).
- Estimated Average Requirement (EAR): Average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals
- Tolerable Upper Intake Level (UL): Maximum daily intake unlikely to cause adverse health effects
In its 2001 evaluation, the Food and Nutrition Board found the existing data insufficient to derive an Estimated Average Requirement (EAR) for manganese. The Food and Nutrition Board therefore established Adequate Intakes (AIs) for all ages based on usual manganese intakes in healthy populations 83. The Adequate Intake (AI) for manganese in adult men is 2.3 mg/day and women is 1.8 mg/day 83. A Tolerable Upper Intake Level (maximum daily intake unlikely to cause adverse health effects) for manganese was set for adults at 11 mg/day based on a no-observed-adverse-effect level (NOAEL) for Western diets 83. Table 3 lists the current Adequate Intakes (AIs) for manganese.
Table 2. Adequate Intakes (AIs) for Manganese
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 6 months* | 0.003 mg | 0.003 mg | ||
| 7–12 months | 0.6 mg | 0.6 mg | ||
| 1–3 years | 1.2 mg | 1.2 mg | ||
| 4–8 years | 1.5 mg | 1.5 mg | ||
| 9–13 years | 1.9 mg | 1.6 mg | ||
| 14–18 years | 2.2 mg | 1.6 mg | 2.0 mg | 2.6 mg |
| 19–50 years | 2.3 mg | 1.8 mg | 2.0 mg | 2.6 mg |
| 51+ years | 2.3 mg | 1.8 mg |
Footnote: *For infants from birth to age 6 months, the Adequate Intake (AI) is based on mean manganese intakes of infants fed primarily human milk.
[Source 86 ]Manganese food sources
Many foods contain manganese. You can get recommended amounts of manganese by eating a variety of foods, including the following 90:
- Whole grains, such as brown rice, oatmeal, and whole-wheat bread
- Clams, oysters, and mussels
- Nuts, such as hazelnuts and pecans
- Legumes, such as soybeans and lentils
- Leafy vegetables, such as spinach and kale
- Some fruits, such as pineapple and blueberries
- Tea
- Many spices, such as black pepper
In the US, estimated average dietary manganese intakes range from 2.1 to 2.3 mg/day for men and 1.6 to 1.8 mg/day for women. People eating vegetarian diets and Western-type diets may have manganese intakes as high as 10.9 mg/day 99. Rich sources of manganese include whole grains, nuts, leafy vegetables, and teas. Foods high in phytic acid, such as beans, seeds, nuts, whole grains, and soy products, or foods high in oxalic acid, such as cabbage, spinach, and sweet potatoes, may slightly inhibit manganese absorption. Although teas are rich sources of manganese, the tannins present in tea may moderately reduce the absorption of manganese 100. Intake of other minerals, including iron, calcium, and phosphorus, have been found to limit retention of manganese 99. The manganese content of some manganese-rich foods is listed in milligrams (mg) in Tables 3 and 4. For more information on the nutrient content of foods, search the USDA food composition database 101.
Humans absorb only about 1% to 5% of dietary manganese 94, 96, 69. Infants and children tend to absorb greater amounts of manganese than adults 69. In addition, manganese absorption efficiency increases with low manganese intakes and decreases with higher intakes, but little is known about the mechanisms that control absorption 93, 94. Systemic manganese regulation is achieved through intestinal control of manganese absorption and hepatic excretion of manganese into bile 96.
Dietary iron intakes and iron status (measured by serum ferritin concentration) appear to be inversely associated with manganese absorption 102, 103. The mechanism for this effect is unknown, but the shared transporter of iron and manganese in the intestine might play a role 94. In addition, men appear to absorb dietary manganese less efficiently than women, possibly because men usually have higher iron status 69, 104. Infants absorb higher proportions of manganese than adults; limited research shows that formula-fed infants retain about 20% of the manganese they consume 96.
Table 3. Manganese content of selected foods
| Food | Milligrams (mg) per serving | Percent Daily Value (DV)* |
|---|---|---|
| Mussels, blue, cooked, 3 ounces | 5.8 | 252 |
| Hazelnuts, dry roasted, 1 ounce | 1.6 | 70 |
| Pecans, dry roasted, 1 ounce | 1.1 | 48 |
| Brown rice, medium grain, cooked, ½ cup | 1.1 | 48 |
| Oysters, Pacific, cooked, 3 ounces | 1 | 43 |
| Clams, cooked, 3 ounces 0.9 | 0.9 | 39 |
| Chickpeas, cooked, ½ cup | 0.9 | 39 |
| Spinach, boiled, ½ cup | 0.8 | 35 |
| Pineapple, raw, chunks, ½ cup | 0.8 | 35 |
| Soybeans, boiled, ½ cup | 0.7 | 30 |
| Bread, whole wheat, 1 slice | 0.7 | 30 |
| Oatmeal, cooked, ½ cup | 0.7 | 30 |
| Peanuts, oil-roasted, 1 ounce | 0.5 | 22 |
| Tea, black, brewed, 1 cup | 0.5 | 22 |
| Lentils, cooked, ½ cup | 0.5 | 22 |
| Potato, flesh and skin, baked, 1 medium | 0.3 | 13 |
| White rice, long grain, cooked, ½ cup | 0.3 | 13 |
| Kidney beans, canned, drained, rinsed, ½ cup | 0.3 | 13 |
| Squash, acorn, cooked, cubed, ½ cup | 0.3 | 13 |
| Blueberries, raw, ½ cup | 0.3 | 13 |
| Sesame seeds, dried, 1 tablespoon | 0.2 | 9 |
| Kale, raw, 1 cup | 0.2 | 9 |
| Black pepper, 1 gram (about ½ tsp) | 0.2 | 9 |
| Asparagus, boiled, ½ cup | 0.1 | 4 |
| Apple, raw, with skin, 1 medium | 0.1 | 4 |
| Lettuce, romaine, raw, shredded, 1 cup | 0.1 | 4 |
| Coffee, brewed, 1 cup | 0.1 | 4 |
| Shrimp, cooked, 3 ounces | 0 | 0 |
| Tuna, white, canned in water, drained, 3 ounces | 0 | 0 |
| Chicken, breast, roasted, 3 ounces | 0 | 0 |
| Ground beef, cooked, 3 ounces | 0 | 0 |
| Egg, whole, hard-boiled, 1 large | 0 | 0 |
| Milk, 1%, 1 cup | 0 | 0 |
| Yogurt, low-fat, plain, 1 cup | 0 | 0 |
Footnotes: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed Daily Values (DVs) to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The Daily Value (DV) for manganese is 2.3 mg for adults and children age 4 years and older. FDA does not require food labels to list manganese content unless manganese has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Sources 105, 86, 83 ]Table 4. Food sources of Manganese
| Food | Serving | Manganese (mg) |
|---|---|---|
| Pineapple, raw | ½ cup, chunks | 0.77 |
| Pineapple juice | ½ cup (4 fl. oz.) | 0.63 |
| Pecans | 1 ounce (19 halves) | 1.28 |
| Almonds | 1 ounce (23 whole kernels) | 0.65 |
| Peanuts | 1 ounce | 0.55 |
| Instant oatmeal (prepared with water) | 1 packet | 0.99 |
| Raisin bran cereal | 1 cup | 0.78-3.02 |
| Brown rice, cooked | ½ cup | 1.07 |
| Whole wheat bread | 1 slice | 0.6 |
| Pinto beans, cooked | ½ cup | 0.39 |
| Lima beans, cooked | ½ cup | 0.49 |
| Navy beans, cooked | ½ cup | 0.48 |
| Spinach, cooked | ½ cup | 0.84 |
| Sweet potato, cooked | ½ cup, mashed | 0.44 |
| Tea (green) | 1 cup (8 ounces) | 0.41-1.58 |
| Tea (black) | 1 cup (8 ounces) | 0.18-0.77 |
Breast milk and infant formulas
Infants are exposed to varying amounts of manganese depending on their source of nutrition. Manganese concentrations in breast milk, cow’s milk–based infant formulas, and soy-based formula range from 3 to 10 mcg/L, 30 to 50 mcg/L, and 200 to 300 mcg/L, respectively 96, 106. Limited research suggests that the absorption rate of manganese from human milk (8.2%) is much higher than that from soy formula (0.7%) and cow’s milk formula (3.1%) 107. However, manganese deficiencies in breast-fed infants or toxicities in formula-fed infants have not been reported 108.
Water
Manganese concentrations in drinking water range from 1 to 100 micrograms/liter (μg/L), but most sources contain less than 10 μg/L 109. The US Environmental Protection Agency (EPA) recommends 0.05 mg (50 mcg)/L as the maximum allowable manganese concentration in drinking water 110.
Manganese Deficiency
Manganese deficiency is very rare in humans and there is more concern for toxicity related to manganese overexposure. Manganese deficiency has been observed in a number of animal species. Signs of manganese deficiency include impaired growth, impaired reproductive function, skeletal abnormalities, impaired glucose tolerance, and altered carbohydrate and lipid metabolism. In humans, demonstration of a manganese deficiency syndrome has been less clear 111, 112. The very limited evidence in humans suggests that manganese deficiency might cause bone demineralization and poor growth in children; skin rashes, hair depigmentation, decreased serum cholesterol, and increased alkaline phosphatase activity in men; and altered mood and increased premenstrual pain in women 94, 83. Manganese deficiency might also alter lipid and carbohydrate metabolism and cause abnormal glucose tolerance 95.
A child on long-term total parenteral nutrition (TPN) lacking manganese developed bone demineralization and impaired growth that were corrected by manganese supplementation 113, 114, 115. Young men who were fed a low-manganese diet developed decreased serum cholesterol levels and a transient skin rash 116. Blood calcium, phosphorus, and alkaline phosphatase levels were also elevated, which may indicate increased bone remodeling as a consequence of insufficient dietary manganese 116. Young women fed a manganese-poor diet developed mildly abnormal glucose tolerance in response to an intravenous (IV) infusion of glucose 117. Overall, manganese deficiency is quite rare, and there is more concern for toxicity related to manganese overexposure.
Causes of manganese toxicity
Manganese (Mn) is an essential mineral in human nutrition, playing a crucial role in several physiological processes. Manganese is necessary for the functioning of enzymes involved in metabolism, bone formation, and regulation of blood sugar levels. Manganese also supports the antioxidant defense system by contributing to the activity of superoxide dismutase. While manganese is important for overall health, your body requires only small amounts of manganese, with the recommended intake for adults being around 1.8 to 2.3 mg per day (1.8 mg per day for women, and 2.3 mg for men) 118, 119, 86. Manganese neurotoxicity can occur following high-dose oral, inhalation, or parenteral exposure to manganese 5. However, the mechanism of action of manganese neurotoxicity remains the subject of ongoing research 120. Most clinically recorded instances of manganese poisoning are the result of occupational exposure. The main route of exposure in occupational manganese poisoning is inhalation of airborne manganese. Manganese toxicity causes include occupational manganese exposure from welding or working in a factory where steel is made, battery manufacturing, mining, consumption of contaminated well water, tap water, infant formula, or total parenteral nutrition (TPN) leads to excessive absorption and cellular uptake of manganese 121, 122, 123, 119. Because manganese is a natural component of the environment, you are always exposed to low levels of it in water, air, soil, and food. Manganese is routinely contained in groundwater, drinking water and soil at low levels. Drinking water containing manganese or swimming or bathing in water containing manganese may expose you to low levels of this metal 119.
Air also contains low levels of manganese, and breathing air may expose you to manganese. Releases of manganese into the air occur from:
- Industries using or manufacturing products containing manganese.
- Mining activities.
- Automobile exhaust. Methylcyclopentadienyl manganese tricarbonyl (MMT) is a manganese-containing compound used in gasoline as an anti-knock additive. Although it has been used for this purpose in Canada for more than 20 years, uncertainty about adverse health effects from inhaled exhaust emissions kept the US EPA from approving its use in unleaded gasoline. In 1995, a US court decision made MMT available for widespread use in unleaded gasoline 124. A study in Montreal, where MMT had been used for more than 10 years, found airborne manganese levels to be similar to those in areas where MMT was not used 125. A more recent Canadian study found higher concentrations of respirable manganese in an urban versus a rural area, but average concentrations in both areas were below the safe level set by the US EPA 126. Manganese exposure via the skin is also a concern for those who come into contact with organic forms of manganese, such as the gasoline additive methylcyclopentadienyl manganese tricarbonyl (MMT) 119. The impact of long-term exposure to low levels of MMT combustion products, however, has not been thoroughly evaluated and will require additional study 127. A single case of reversible neurotoxicity and seizures following unintentional MMT ingestion has been documented: a 54-year old man accidentally drank an MMT-containing anti-knock agent that he assumed was an energy drink due so similar product labeling 128.
Lifestyle traits may also lead to exposure to manganese. People who smoke tobacco or inhale second-hand smoke are typically exposed to manganese at levels higher than those not exposed to tobacco smoke.
Manganese toxicity is caused by the preferential accumulation of manganese in brain areas rich in dopaminergic neurons such as the caudate nucleus, putamen, globus pallidus, substantia nigra, and subthalamic nuclei (the extrapyramidal system) 4, 6, 7, 5, 1, 2, 3.
Analysis of brain samples have shown that manganese accumulates within the human striatum, globus pallidus, and substantia nigra 38, 39. Manganese accumulation in these brain regions is associated with the presence of the divalent metal transporter 1 (DMT1) although additional transporters may play a role in brain uptake of manganese 40, 41, 42. Brain imaging studies that rely on the paramagnetic properties of manganese that result in increased signal intensity seen with T1-weighted magnetic resonance imaging (MRI), allow for visual inspection of the brain for evidence of manganese accumulation at this site. Brain MRI studies of highly exposed people reveal signal intensity changes in the globus pallidus, striatum, and midbrain consistent with manganese accumulation at these sites 43, 44. Studies performed in nonhuman primates have shown that changes in the T1-weighted image correlate with manganese tissue concentration 45. The primary neuropathologic target of manganese neurotoxicity is the globus pallidus (particularly the internal segment) with sparing of the substantia nigra pars compacta and an absence of Lewy bodies 46. Studies of a manganese-exposed South African mine worker have revealed reduced astrocyte and neuron density in both the caudate and putamen 47. Chronic manganese neurotoxicity in people is also associated with decreased gamma-aminobutyric acid (GABA) neurons, reduced myelinated fibers, and moderate astrocytic proliferation in the medial segment of the globus pallidus 46.
Several studies have examined neurochemical changes following high-dose-manganese exposure. Because manganese neurotoxicity results in dysregulation of motor control, many studies have focused on the striatal and pallidal dopaminergic system. Manganese reacts with dopamine (DA) and other biogenic amines resulting in oxidative damage to the neurotransmitters 49, 129. The primary neuropathologic target of manganese neurotoxicity is the globus pallidus (particularly the internal segment) with sparing of the substantia nigra pars compacta and an absence of Lewy bodies 46. Studies of a manganese-exposed South African mine worker have revealed reduced astrocyte and neuron density in both the caudate and putamen 47. Chronic manganese neurotoxicity in people is also associated with decreased gamma-aminobutyric acid (GABA) neurons, reduced myelinated fibers, and moderate astrocytic proliferation in the medial segment of the globus pallidus 46.
One pathway involves manganese catalyzed oxidation of the alpha hydroxyl group of dopamine forming a semi-quinone radical. The semi-quinone radical then reacts with oxygen to generate superoxide anion radical [O2•−] and a quinone. Oxygen can reoxidize the quinone to quinol to generate hydrogen peroxide 49. Manganese-catalyzed dopamine auto-oxidation may also involve semiquinone and aminochrome intermediates, l-cysteine or copper, and NADH facilitation 50, 51. Excess manganese may also alter glutamate homeostasis in the basal ganglia 52. Changes in glutamate homeostasis have been associated with excitotoxicity in the brain 52.
The biphasic condition seen in patients with manganese toxicity is most likely explained by changes in striatal dopamine (DA) levels. An early phase of elevated dopamine (DA) production has been linked to psychotic episodes in psychiatric patients 130. Catecholamine levels fall as manganese poisoning progresses, most likely owing to the death of nigrostriatal dopaminergic neurons, and Parkinson-like symptoms follow 129, 131, 132. As a result, in the early phases of manganese toxicity, symptoms may be reversed by discontinuing manganese exposure, but manganese toxicity is permanent in individuals with motor abnormalities 133.
Inhaled manganese
Manganese toxicity may result in multiple neurological problems and is a well-recognized health hazard for people who inhale manganese dust, such as welders and smelters 134,99. Unlike ingested manganese, inhaled manganese is transported directly to the brain before it can be metabolized in the liver 124. The symptoms of manganese toxicity generally appear slowly over a period of months to years. In its worst form, manganese toxicity can result in a permanent neurological disorder with symptoms similar to those of Parkinson’s disease, including tremors, difficulty walking, and facial muscle spasms. This syndrome, often called manganism, is sometimes preceded by psychiatric symptoms, such as irritability, aggressiveness, and even hallucinations 135, 136. Additionally, environmental or occupational inhalation of manganese can cause an inflammatory response in the lungs 137. Clinical symptoms of effects to the lung include cough, acute bronchitis, and decreased lung function 138.
Ingested manganese
Diet is the primary source of manganese in the general population 139. Manganese is abundant in plant-based foods, including whole grains, rice, nuts, and leafy vegetables. Animal foods, including meat, fish, poultry, eggs, and dairy, lack manganese 140. Daily intakes of manganese typically range from 2 to 6 mg, of which about 1 to 5% is normally absorbed 141. Due to the dual role of manganese as an essential nutrient and a potent toxin, whole-body manganese levels are tightly controlled by regulating intestinal absorption and excretion of the metal through homeostatic mechanisms 96. Thus far, manganese toxicity from high dietary manganese intakes in humans has not been reported 142.
Limited evidence suggests that high manganese intakes from drinking water may be associated with neurological symptoms similar to those of Parkinson’s disease. Severe neurological symptoms were reported in 25 people who drank water contaminated with manganese, and probably other contaminants, from dry cell batteries for two to three months 143. Water manganese levels were found to be 14 mg/liter (mg/L) almost two months after symptoms began and may have already been declining. A study of older adults in Greece found a high prevalence of neurological symptoms in those exposed to water manganese levels of 1.8 to 2.3 mg/L 144, while a study in Germany found no evidence of increased neurological symptoms in people drinking water with manganese levels ranging from 0.3 to 2.2 mg/L compared to those drinking water containing less than 0.05 mg/L 145. Manganese in drinking water may be more bioavailable than manganese in food. However, none of the studies measured dietary manganese, so total manganese intake in these cases is unknown. In the US, the EPA recommends 0.05 mg/L as the maximum allowable manganese concentration in drinking water 146.
Additionally, more recent studies have shown that children exposed to high levels of manganese through drinking water experience cognitive and behavioral deficits 147. For instance, a cross-sectional study in 142, 10-year old children, who were exposed to a mean manganese water concentration of 0.8 mg/L, found that children exposed to higher manganese levels had significantly lower scores on three tests of intellectual function 148. Another study associated high levels of manganese in tap water with hyperactive behavioral disorders in children 149. These and other recent reports have raised concern over the neurobehavioral effects of manganese exposure in children 147.
A single case of manganese toxicity was reported in a person who took large amounts of mineral supplements for years 150, while another case was reported as a result of a person taking a Chinese herbal supplement 135. Manganese toxicity resulting from foods alone has not been reported in humans, even though certain vegetarian diets could provide up to 20 mg/day of manganese 99, 109.
Intravenous manganese
Intravenous delivery of drugs containing high quantities of manganese is another manganese exposure route that bypasses gastrointestinal tract control, resulting in 100% manganese metal absorption 151, 152. Manganese neurotoxicity has been observed in individuals receiving total parenteral nutrition (TPN), both as a result of excessive manganese in the solution and as an incidental contaminant 153. Neonates are especially vulnerable to manganese-related neurotoxicity 154. Premature newborns, for example, do not absorb adequate nutrition owing to an underdeveloped gastrointestinal tract or certain disorders. As a result, newborns are often supplemented with total parenteral nutrition (TPN) by intravenous injection, which includes several trace elements necessary for life support. Infants on total parenteral nutrition (TPN) are more vulnerable to manganese toxicity. Infants receiving manganese-containing total parenteral nutrition (TPN) can be exposed to manganese concentrations about 100-fold higher than breast-fed infants 108. Furthermore, manganism has been described because of intravenous consumption of methcathinone, which contains manganese dioxide as a by-product from production 155, 156. The absorbed quantity of manganese may range between 60 and 180 mg per day, greatly above the typical dietary intake 156, 157. Because of potential manganese toxicities, some argue against including manganese in parenteral nutrition 158.
Manganese prenatal exposure
In utero, manganese exposure is often overlooked since the actual relationship between manganese exposure and health consequences is unclear 88. However, there has been an increase in the number of studies examining the relationship between in utero manganese exposure and newborn health. The average manganese concentration (78.8 mg/L) in umbilical cord blood is higher than in the mother’s whole blood (55.0 mcg/L), and an inverted U-shaped curve has been observed between manganese levels in mother’s whole blood and birth weights, as well as between manganese levels in umbilical cord blood and birth weights 159. Other research has shown that both low and high maternal blood manganese levels are related to poor newborn health 160, 161.
Manganese toxicity symptoms
Early symptoms of manganese toxicity involves changes in the person’s psychiatric and emotional state 71. A person with manganese toxicity may experience personality changes with periods of rapid emotional fluctuations, which need to be qualitatively differentiated from that person’s baseline personality 162. Psychiatric symptoms include hallucinations and psychosis 163. Other symptoms reported in a case study from China included memory impairment and insomnia 164. In a group of California welders, manganese exposure during their everyday duties was analyzed over their work period from 2003 to 2004; neuropsychiatric symptoms developed in these welders included a measured decrease in IQ score, decreased sex drive, depression, and anxiety 165.
Early neurological dysfunction was also a hallmark in manganese toxicity patients, characteristic of the basal ganglia/nigrostriatal system involvement 71. Symptoms discovered included tremor, gait abnormalities, headaches, dysfunctional speech, hyperreflexia, hypertonicity, and tremors 164. Other acute neurological signs and symptoms in the study introduced above from California included bradykinesia, postural instability, decreased motor dexterity and speed, and olfactory dysfunction 165. Severe symptoms include the “cock-walk” described in the earliest manganese studies, which is characterized by patients walking on their toes with a forward tilt as they move 166. Other severe symptoms are worsening tremor and dystonic movements of arms and legs 71.
Other systems affected by manganese exposure include the heart, blood vessels and pulmonary systems. A form of obstructive lung disease formed in approximately one-third of the welders involved in the California study by Bower et. al 165. Animal studies have shown that overexposure to manganese causes hemodynamic changes, including hypotension and bradycardia, concomitant with a prolonged PR and QRS interval. These studies, however, have not been validated in humans, and often the reverse findings are present (tachycardia, hypertension, shortened PR interval) 167, 168.
Manganese toxicity diagnosis
Manganese (Mn) is the fifth most abundant metal in the environment and twelfth most abundant element as a whole in the environment where it can be found in rocks and soil and your body needs trace amounts of manganese to stay healthy. Every year, natural earth erosion releases tonnes of manganese into the air, soil, and rivers for microbes, plants, and animals to absorb 169. Manganese (Mn) can exist in 11 oxidation states, often as chloride, oxides and sulfates 58. The most common oxidation states for manganese in natural water are manganese2+(Mn2+) and manganese4+ (Mn4+) 88, 58. Manganese is used principally in the manufacture of iron and steel alloys, and manganese compounds such as potassium and sodium permanganate are ingredients in various products used for cleaning, bleaching and disinfection 58. Manganese compounds are additionally used in some locations for potable water treatment and can also be an impurity in coagulants used during water treatment 58. Manganese occurs naturally in many surface water and groundwater sources; although naturally occurring manganese is usually the most important source for drinking-water, human activities can also contribute to high levels of manganese in water 58. Manganese also occurs naturally in many food sources, and the greatest exposure to manganese is usually from food. Manganese is also available as a dietary supplement.
When suspecting a person of manganese toxicity, patient’s occupational history can confirm the diagnosis in patients suspected of having manganese toxicity. Patients will have an occupational history consistent with exposure, including welding, metal manufacturing, mining, battery manufacturing, smelting, those intimately exposed to gasoline combustion, and steelworkers 27, 170. Inquire about personal protective equipment the patients utilize daily, as those without appropriate respiratory protection are more at risk for the development of symptoms 165. If the patient recently immigrated or is traveling from a country with sub-standard public drinking water conditions, they may have been chronically ingesting high amounts of manganese-toxic water 59, 73. Identify patients whose water source is well-water, as their well may be spoiled by leaching from mineral-laden soil 78, 171
Query the patient about drug use, as recent studies have shown, bath salts use intravenously puts patients at risk for manganese overload due to poor purification processes 172. Chronic intravenous TPN also puts patients at risk of manganese toxicity, therefore inquiring about recent hospitalizations may be helpful.
Further discussion of patients’ past medical histories should include known or presumed liver insufficiency, as patients with decreased liver function are at increased risk for developing manganese toxicity 173. Male patients who note difficulty conceiving (male infertility) with an unremarkable maternal work-up coincident with a known occupational history may also be a clue, as manganese toxicity results in decreased fertility 174.
Manganese toxicity tests
Several tests are available to measure manganese in blood, urine, hair, or feces. Because manganese is normally present in your body, some is always found in tissues or fluids.
Normal ranges of manganese levels in the general population is 4–15 mcg/L in blood, 1–8 mcg/L in urine, and 0.4–0.85 mcg/L in serum (the fluid portion of the blood), though the usefulness of urine and serum manganese as biomarkers for exposure is limited 175, 176. Blood manganese levels have a half-life of approximately 40 days 177 and are higher with female sex, younger ages, and Asian origins 178, 179.
Because excess manganese is usually removed from the body within a few days, past exposures are difficult to measure with common laboratory tests.
A medical test known as magnetic resonance imaging (MRI), can detect the presence of increased amounts of manganese in the brain. Brain imaging studies that rely on the paramagnetic properties of manganese that result in increased signal intensity seen with T1-weighted magnetic resonance imaging (MRI), allow for visual inspection of the brain for evidence of manganese accumulation at this site. Brain MRI studies of highly exposed people reveal signal intensity changes in the globus pallidus, striatum, and midbrain consistent with manganese accumulation at these sites 43, 44. Studies performed in nonhuman primates have shown that changes in the T1-weighted image correlate with manganese tissue concentration 45. However, magnetic resonance imaging (MRI) test is qualitative, and has not been shown to reliably reflect or predict toxicologically meaningful exposures.
The latest research presented at the 2016 conference on manganese neurotoxicity described new methodologies of identification of manganism, including the use of fMRI, PET scans, and new methods of using blood manganese and ferritin levels to detect globus pallidus accumulation of toxic metal concentrations 180, 181, 182.
In summary, manganese neurotoxicity diagnosis requires a high clinical suspicion alongside recognition of the risk factors placing patients at risk for manganese toxicity 71. Ideal evaluation for the determination of manganese toxicity includes a team-based approach, based on early recognition and outpatient referral to neurology for definitive care 71. Early consultation with a clinical toxicologist may aid in the identification of the cause of the patient’s symptoms. Usage of MRI or serum-based studies should be done at the request of specialists familiar with heavy metals toxicity and the latest research.
Manganese toxicity differential diagnosis
The following conditions can be considered in the differential diagnosis of manganese toxicity 71:
- Idiopathic Parkinson disease: Idiopathic Parkinson disease is characterized by bradykinesia, shuffling gait, postural instability, and resting pill-rolling tremor. Parkinson disease is most clinically similar to manganese toxicity. Distinguishing differences between Parkinson disease and manganese neurotoxicity include less prominent tremors in manganese neurotoxicity, the reversal of psychiatric symptom onset (later in Parkinson disease, earlier in manganese neurotoxicity), the age of onset is typically younger in manganese neurotoxicity as compared to Parkinson disease and clear occupational correlation with manganese neurotoxicity 183.
- Dementia with Lewy bodies: Patients with dementia with Lewy bodies develop a parkinsonian syndrome in combination with many cognitive dysfunctions in the 5th to 6th decade of life. Early cognitive deficits include short term memory difficulties, multi-tasking deficits, and visuospatial deficiencies. These symptoms will develop alongside visual hallucinations and motor difficulties consistent with Parkinson disease. Lewy Body dementias process can be differentiated from both Parkinson’s disease and manganese neurotoxicity by the presence of characteristic hallucinations and concurrent development of neuropsychiatric and motor dysfunction 184.
- Frontotemporal dementia: Frontotemporal dementia is characterized by several different phenotypes, which include a behavioral/executive dysfunction type and two primary language deficiency types. Clinical features of the behavioral frontotemporal dementia subtype include disinhibition, apathy, compulsive behaviors, hyper-orality, and overall diminished executive function skills. Features of the language dysfunction variants include difficulties with daily life due to speech deficits, aphasia, speech apraxia, and other speech and comprehension impairments. Frontotemporal dementia can easily be mistaken for manganese neurotoxicity in its early stages if behavioral symptoms predominate, given manganese neurotoxicity’s early neuropsychiatric manifestations. An adequate history-taking can differentiate these two distinct conditions 185.
- Essential tremor: Essential tremor is a chronic, progressive neurologic disorder characterized by a high-frequency action tremor. Essential tremor can affect the neck, jaw, and other body regions alongside that of the arms and hands. Manganese neurotoxicity will produce tremors early on in the disease process, typically after the development of psychiatric dysfunction. Differentiation of essential tremor from manganese neurotoxicity involves elucidating occupational history, lack of family history, and identification of concomitant neuropsychiatric features in manganese toxicity 186.
- Multiple system atrophy: As its name denotes, multiple system atrophy (MSA) is a complex disorder characterized by autonomic dysfunction, orthostatic hypotension, cerebellar dysfunction, parkinsonian symptoms unresponsive to L-dopa, and earlier age in onset (greater than 30 years old). Multiple system atrophy (MSA) can be distinguished from manganese neurotoxicity due to its multiple accompanying system dysfunction, autonomic dysfunction, and cerebellar findings 187.
- Corticobasal degeneration: As the name denotes, corticobasal degeneration affects predominantly the brain cortices and basal ganglia, resulting in apraxia (a neurological disorder that makes it difficult to perform tasks or movements, even though the person understands the request and is willing to do it), hemineglect symptoms, alterations in behavior and emotion, rigidity, bradykinesia, and myoclonus. Corticobasal degeneration signs and symptoms are very similar to manganese neurotoxicity; however, it can be differentiated based on history and chronicity in relation to exposure risk for manganese 188.
- Progressive supranuclear palsy: Progressive supranuclear palsy (PSP) is an levodopa (L-dopa) responsive parkinsonian syndrome similar to corticobasilar degeneration. Features of progressive supranuclear palsy include vertical supranuclear palsy and parkinsonian symptoms. Progressive supranuclear palsy can be differentiated from manganese neurotoxicity by their responsiveness to L-dopa therapy 188.
- Drug-induced Parkinsonism: Drug-induced Parkinsonism is most common cause of parkinsonian symptoms and is an often under-appreciated disease process. Common precipitants include antipsychotic medications, anti-emetics, anti-motility agents, some rarely prescribed calcium channel blockers, and dopamine depleting medications. A clear and concise medication history can lead to the diagnosis in drug-induced Parkinsonism cases 189.
- Methcathinone (ephedrone). Methcathinone (also called Bathtub speed, Cadillac express, cat, crank, ephedrone, gag- ers, go-fast, goob, qat, slick superspeed, star, the C, tweeker, wild cat and wonder star) is a monoamine alkaloid and psychoactive stimulant, a substituted cathinone 190. Methcathinone is used as a recreational drug due to its potent stimulant and euphoric effects and is considered to be addictive, with both physical and psychological withdrawal occurring if its use is discontinued after prolonged or high-dosage administration 190. It is usually snorted, but can be smoked, injected, or taken orally. Case reports have suggested chronic use of methcathinone is linked to the development of manganese toxicity, substantiated by the evidence of parkinsonian features and MRI correlate abnormalities in the basal ganglia. Drug use and medication history may lead to the discovery of this diagnosis 191.
- Other neurodegenerative disorders, including Huntington’s disease, repeated and chronic head trauma, several infections such as syphilis, HIV, progressive multifocal leukoencephalopathy, structural brain disorders such as intraparenchymal masses or hydrocephalus, and metabolic disorders such as Wilson disease, hypoparathyroidism, chronic liver failure, and hemochromatosis 192.
Manganese toxicity treatment
Manganese toxicity treatment involves the treatment of the acute threats from the manganese toxicity and the management of chronic manganese exposure. The most accessible form of treatment for manganese toxicity is the removal of the individual from the source exposure, whether the source is occupational, environmental, or iatrogenic 71.
Chelation therapy for manganism involves the use of calcium sodium ethylenediaminetetraacetate (CaNa2EDTA) and para-aminosalicylic acid (PAS) 193, 71. The use of calcium sodium ethylenediaminetetraacetate (CaNa2EDTA) has been shown to effectively increase urine concentration of manganese and decrease blood levels of manganese 71. However, these findings did not coincide with the finding of decreased observed clinical neurotoxicity, owing to the chronic nature of manganese toxicity and incomplete reversibility 164. Furthermore, calcium sodium ethylenediaminetetraacetate (CaNa2EDTA) prevents further manganese from crossing the blood-brain barrier, deactivating manganese ability to enter into the brain and spinal cord to exert its toxic effects 194, 195, 196. Unfortunately, calcium sodium ethylenediaminetetraacetate (CaNa2EDTA) has a poor bioavailability for brain tissue and does not appear to be able to completely reverse the neurotoxic effects of manganese 71. Therefore, in patients with chronic manganese toxicity or advanced manganism, chelation therapy is likely to not reverse the significant clinical deterioration 197.
Another chelation molecule called para-aminosalicylic acid (PAS) – typically used as an anti-tuberculosis medication – has shown clinical benefit for use in patients with manganese toxicity 71. Para-aminosalicylic acid (PAS) and its metabolites concentrate within the choroid plexus, brain tissue and cerebrospinal fluid (CSF), making para-aminosalicylic acid (PAS) an ideal chelator for manganese toxicity 198. Para-aminosalicylic acid (PAS) has the additional benefit of chelating both the manganese2+ (Mn2+) and manganese3+ (Mn3+) molecules 199. The anti-inflammatory properties of the salicylic acid component may also produce a beneficial effect, as studies have illustrated neuroprotective benefits from the use of other nonsteroidal anti-inflammatory drugs (NSAIDs) in Alzheimer disease, Parkinson disease, and amyotrophic lateral sclerosis 200, 201.
Previous attempted treatments included levodopa (L-dopa) supplementation 71. Initial studies supported a benefit with L-dopa administration, but more recent data suggests prolonged L-dopa treatment results in poor clinical response 202. Also, after the cessation of L-dopa use, patients had a progression of manganese toxicity disease despite initial therapy 203
Several vitamins and supplements appear to improve clinical response and provide a means of prevention for patients. In addition to chelation therapy, iron supplementation was shown to improve neurological symptom improvement compared to a treatment group that received chelation alone 204. Vitamin E was shown to prevent the supposed oxidative stress brought on by manganese-induced toxicity 205. Glutathione and N-acetylcysteine have also shown to be beneficial in test tube study for decreasing the downstream effects of manganese-induced cell toxicity 206. Several plants and plant extracts have also been shown to be beneficial, including Silymarin, Acai, Lycopene, Nicotine, and Lemon balm (Melissa officinalis) 207, 208, 209, 210.
Newer therapies are currently being investigated include the use of taurine and Rasagiline (a medication which is used in the treatment of Parkinson’s disease) 71. Taurine use decreases the toxic effects of manganese in test tube study, mostly through the preservation of mitochondrial functionality in brain tissues 211, 212. Taurine also appears to improve the learning and memory impairments associated with chronic manganese toxicity 213. Rasagiline (a medication which is used in the treatment of Parkinson’s disease) is a monoamine oxidase inhibitor (MAOI) used to block the breakdown of dopamine in patients with Parkinson disease. Rasagiline appeared to offer a benefit through protection from reactive oxygen species (ROS) created by manganese toxicity 214.
Manganese toxicity prognosis
There are very small number of studies on the prognosis of patients suffering ongoing manganese exposure and of patients who have successfully removed themselves from manganese exposure after the development of manganese toxicity. What little literature exists suggests that, with removal from manganese source exposure, recovery in some neuropsychiatric symptoms is possible while improbable in others 71. Bouchard et al. 215 followed a group of workers exposed to manganese (Mn) over the course of 15 years and observed signs of recovery approximately 14 years after their documented exposure. Specific neurological deficits that remained included poor performance on simple and alternating movements, drawing ability, and diminished hand stability 215. These findings are validated by prior studies by Hochberg et al and Pal et al 216, 36. Bouchard et al. 215 also found a trend towards more feelings of anger, hostility, confusion, and a sensation of fatigue. This finding was correlated with greater cumulative doses of manganese. Finally, their group identified a relationship between manganese exposure and respiratory tract pathology, possibly secondary to the induced inflammatory state 215.
In addition, older patients who are exposed to manganese often develop more significant neurotoxicity than younger patients, owing to possible pre-exposure neurological degenerative changes. This is theorized to lead to the observed age-related cognitive deficits that persist after manganese exposures cease. Research by Bouchard et al in 2005 and 2007 217, 218 showed that individuals who were exposed that were at least greater than or equal to 45 years old had persistent cognitive deficits after cessation of toxic exposure to manganese.
In another follow-up study by Roels et al. 219, workers from a battery manufacturing plant were followed for 8 years after cessation of manganese exposure. Variables measured included hand steadiness and reaction time. Analysis of their study group (workers from a battery manufacturing plant) yielded no improved steadiness of the hands and no improvement in reaction time 219. The study group (workers from a battery manufacturing plant) did, however, observe an improvement in hand-forearm movements beyond that of their baseline, which suggested some partial recovery from their toxicity 219. Lucchini et al. 220 measured improvement in manganse toxic patients 5 years after exposure was decreased and found similar results during and after their exposures. This lack of improvement may be due to the fact that manganese exposure was reduced but not definitively eliminated.
The trend following cessation of manganese exposure appears to be that of mostly recovery, with residual deficits remaining in the behavioral and neurologic symptoms 71. This lends credence to the mainstay of therapy, which is to abstain from any further activity, which puts individuals at risk for further mangnese toxicity. Overall, the prognosis is more related to the illness than death and is overall favorable once the individual is removed from manganese exposure setting. More research will be needed to further outline the expected clinical course following manganese toxicity, especially with the addition of new treatment modalities such as supplementation and chelators 71.
- Bornhorst J., Wehe C.A., Huwel S., Karst U., Galla H.J., Schwerdtle T. Impact of manganese on and transfer across blood-brain and blood-cerebrospinal fluid barrier in vitro. J. Biol. Chem. 2012;287:17140–17151. doi: 10.1074/jbc.M112.344093[↩][↩][↩]
- Rubin L.L., Staddon J.M. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11[↩][↩][↩]
- Yoon M., Schroeter J.D., Nong A., Taylor M.D., Dorman D.C., Andersen M.E., Clewell H.J. Physiologically based pharmacokinetic modeling of fetal and neonatal manganese exposure in humans: Describing manganese homeostasis during development. Toxicol. Sci. 2011;122:297–316. doi: 10.1093/toxsci/kfr141[↩][↩][↩]
- Andruska K.M., Racette A.B. Neuromythology of manganism. Curr. Epidemiol. Rep. 2015;2:143–148. doi: 10.1007/s40471-015-0040-x[↩][↩][↩][↩]
- Dorman DC. The Role of Oxidative Stress in Manganese Neurotoxicity: A Literature Review Focused on Contributions Made by Professor Michael Aschner. Biomolecules. 2023 Jul 28;13(8):1176. doi: 10.3390/biom13081176[↩][↩][↩][↩][↩][↩][↩]
- Dorman D.C. Extrapyramidal system neurotoxicity: Animal models. Handb. Clin. Neurol. 2015;131:207–223. doi: 10.1016/B978-0-444-62627-1.00012-3[↩][↩][↩]
- Kulshreshtha D., Ganguly J., Jog M. Manganese and movement disorders: A review. J. Mov. Disord. 2021;14:93–102. doi: 10.14802/jmd.20123[↩][↩][↩]
- Bowler RM, Adams SW, Wright CW, Kim Y, Booty A, Colledge M, Gocheva VV, Lobdell DT. Medication use associated with exposure to manganese in two Ohio towns. Int J Environ Health Res. 2016 Oct-Dec;26(5-6):483-96. doi: 10.1080/09603123.2016.1194381[↩]
- Fulk F, Succop P, Hilbert TJ, Beidler C, Brown D, Reponen T, Haynes EN. Pathways of inhalation exposure to manganese in children living near a ferromanganese refinery: A structural equation modeling approach. Sci Total Environ. 2017 Feb 1;579:768-775. doi: 10.1016/j.scitotenv.2016.11.030[↩]
- Haynes EN, Sucharew H, Kuhnell P, Alden J, Barnas M, Wright RO, Parsons PJ, Aldous KM, Praamsma ML, Beidler C, Dietrich KN. Manganese Exposure and Neurocognitive Outcomes in Rural School-Age Children: The Communities Actively Researching Exposure Study (Ohio, USA). Environ Health Perspect. 2015 Oct;123(10):1066-71. doi: 10.1289/ehp.1408993[↩]
- Li Y, Jiao Q, Xu H, Du X, Shi L, Jia F, Jiang H. Biometal Dyshomeostasis and Toxic Metal Accumulations in the Development of Alzheimer’s Disease. Front Mol Neurosci. 2017 Oct 24;10:339. doi: 10.3389/fnmol.2017.00339[↩]
- Myers JE, teWaterNaude J, Fourie M, Zogoe HB, Naik I, Theodorou P, Tassel H, Daya A, Thompson ML. Nervous system effects of occupational manganese exposure on South African manganese mineworkers. Neurotoxicology. 2003 Aug;24(4-5):649-56. doi: 10.1016/S0161-813X(03)00035-4[↩]
- Lucchini R, Bergamaschi E, Smargiassi A, Festa D, Apostoli P. Motor function, olfactory threshold, and hematological indices in manganese-exposed ferroalloy workers. Environ Res. 1997;73(1-2):175-80. doi: 10.1006/enrs.1997.3702. Erratum in: Environ Res 1997 Nov;75(2):187.[↩]
- Bader M, Dietz MC, Ihrig A, Triebig G. Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int Arch Occup Environ Health. 1999 Nov;72(8):521-7. doi: 10.1007/s004200050410[↩]
- Srivastava AK, Gupta BN, Mathur N, Murty RC, Garg N, Chandra SV. An investigation of metal concentrations in blood of industrial workers. Vet Hum Toxicol. 1991 Jun;33(3):280-2.[↩]
- Nagatomo S, Umehara F, Hanada K, Nobuhara Y, Takenaga S, Arimura K, Osame M. Manganese intoxication during total parenteral nutrition: report of two cases and review of the literature. J Neurol Sci. 1999 Jan 1;162(1):102-5. doi: 10.1016/s0022-510x(98)00289-5[↩]
- Aschner JL, Anderson A, Slaughter JC, Aschner M, Steele S, Beller A, Mouvery A, Furlong HM, Maitre NL. Neuroimaging identifies increased manganese deposition in infants receiving parenteral nutrition. Am J Clin Nutr. 2015 Dec;102(6):1482-9. doi: 10.3945/ajcn.115.116285[↩]
- Butterworth RF, Spahr L, Fontaine S, Layrargues GP. Manganese toxicity, dopaminergic dysfunction and hepatic encephalopathy. Metab Brain Dis. 1995 Dec;10(4):259-67. doi: 10.1007/BF02109357[↩]
- Janocha-Litwin J, Marianska K, Serafinska S, Simon K. Manganese Encephalopathy among Ephedron Abusers. J Neuroimaging. 2015 Sep-Oct;25(5):832-5. doi: 10.1111/jon.12173[↩]
- Avila D.S., Puntel R.L., Aschner M. Manganese in health and disease. Met. Ions Life Sci. 2013;13:199–227. doi: 10.1007/978-94-007-7500-8_7[↩]
- Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Br. Ann. Med. Pharm. Vital Stat. Gen. Sci. 1837;1:41.[↩]
- Lucchini R.G., Martin C.J., Doney B.C. From manganism to manganese-induced parkinsonism: A conceptual model based on the evolution of exposure. Neuromol. Med. 2009;11:311–321. doi: 10.1007/s12017-009-8108-8[↩][↩]
- Keen CL, Ensunsa JL, Watson MH, Baly DL, Donovan SM, Monaco MH, Clegg MS. Nutritional aspects of manganese from experimental studies. Neurotoxicology. 1999 Apr-Jun;20(2-3):213-23.[↩][↩][↩][↩]
- Studying Manganese Exposure in Eastern Ohio. https://www.epa.gov/healthresearch/studying-manganese-exposure-eastern-ohio[↩][↩]
- Drinking Water Health Advisory for Manganese. EPA-822-R-04-003 January, 2004. https://nepis.epa.gov/Exe/ZyPDF.cgi/P100537R.PDF?Dockey=P100537R.PDF[↩]
- Davis JM. Methylcyclopentadienyl manganese tricarbonyl: health risk uncertainties and research directions. Environ Health Perspect. 1998 Feb;106 Suppl 1(Suppl 1):191-201. doi: 10.1289/ehp.98106s1191[↩]
- O’Neal SL, Zheng W. Manganese Toxicity Upon Overexposure: a Decade in Review. Curr Environ Health Rep. 2015 Sep;2(3):315-28. doi: 10.1007/s40572-015-0056-x[↩][↩]
- Bowler R.M., Gysens S., Diamond E., Nakagawa S., Drezgic M., Roels H.A. Manganese exposure: Neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–326. doi: 10.1016/j.neuro.2005.10.007[↩]
- Roels H.A., Bowler R.M., Kim Y., Claus Henn B., Mergler D., Hoet P., Gocheva V.V., Bellinger D.C., Wright R.O., Harris M.G., et al. Manganese exposure and cognitive deficits: A growing concern for manganese neurotoxicity. Neurotoxicology. 2012;33:872–880. doi: 10.1016/j.neuro.2012.03.009[↩]
- Ruiz-Azcona L., Fernández-Olmo I., Expósito A., Markiv B., Paz-Zulueta M., Parás-Bravo P., Sarabia-Cobo C., Santibáñez M. Impact of environmental airborne manganese exposure on cognitive and motor functions in adults: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2021;18:4075. doi: 10.3390/ijerph18084075[↩]
- Zoni S., Lucchini R.G. Manganese exposure: Cognitive, motor and behavioral effects on children: A review of recent findings. Curr. Opin. Pediatr. 2013;25:255–260. doi: 10.1097/MOP.0b013e32835e906b[↩]
- Bjørklund G., Chartrand M.S., Aaseth J. Manganese exposure and neurotoxic effects in children. Environ. Res. 2017;155:380–384. doi: 10.1016/j.envres.2017.03.003[↩]
- Carvalho C.F., Menezes-Filho J.A., de Matos V.P., Bessa J.R., Coelho-Santos J., Viana G.F., Argollo N., Abreu N. Elevated airborne manganese and low executive function in school-aged children in Brazil. Neurotoxicology. 2014;45:301–308. doi: 10.1016/j.neuro.2013.11.006[↩]
- Menezes-Filho J.A., de Carvalho-Vivas C.F., Viana G.F., Ferreira J.R., Nunes L.S., Mergler D., Abreu N. Elevated manganese exposure and school-aged children’s behavior: A gender-stratified analysis. Neurotoxicology. 2014;45:293–300. doi: 10.1016/j.neuro.2013.09.006[↩]
- Rodrigues J.L.G., Araújo C.F.S., Dos Santos N.R., Bandeira M.J., Anjos A.L.S., Carvalho C.F., Lima C.S., Abreu J.N.S., Mergler D., Menezes-Filho J.A. Airborne manganese exposure and neurobehavior in school-aged children living near a ferro-manganese alloy plant. Environ. Res. 2018;167:66–77. doi: 10.1016/j.envres.2018.07.007[↩]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999 Apr-Jun;20(2-3):227-38.[↩][↩][↩]
- Aschner M, Aschner JL. Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Neurosci Biobehav Rev. 1991 Fall;15(3):333-40. doi: 10.1016/s0149-7634(05)80026-0[↩]
- Aschner M., Erikson K.M., Dorman D.C. Manganese dosimetry: Species differences and implications for neurotoxicity. Crit. Rev. Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920[↩][↩]
- Nagatomo S., Umehara F., Hanada K., Nobuhara Y., Takenaga S., Arimura K., Osame M. Manganese intoxication during total parenteral nutrition: Report of two cases and review of the literature. J. Neurol. Sci. 1999;162:102–105. doi: 10.1016/S0022-510X(98)00289-5[↩][↩]
- Crossgrove J.S., Yokel R.A. Manganese distribution across the blood-brain barrier III. The divalent metal transporter-1 is not the major mechanism mediating brain manganese uptake. Neurotoxicology. 2004;25:451–460. doi: 10.1016/j.neuro.2003.10.005[↩][↩]
- Fitsanakis V.A., Piccola G., Marreilha dos Santos A.P., Aschner J.L., Aschner M. Putative proteins involved in manganese transport across the blood-brain barrier. Hum. Exp. Toxicol. 2007;26:295–302. doi: 10.1177/0960327107070496[↩][↩]
- Nyarko-Danquah I., Pajarillo E., Digman A., Soliman K.F.A., Aschner M., Lee E. Manganese accumulation in the brain via various transporters and its neurotoxicity mechanisms. Molecules. 2020;25:5880. doi: 10.3390/molecules25245880[↩][↩]
- Fitsanakis V.A., Zhang N., Avison M.J., Gore J.C., Aschner J.L., Aschner M. The use of magnetic resonance imaging (MRI) in the study of manganese neurotoxicity. Neurotoxicology. 2006;27:798–806. doi: 10.1016/j.neuro.2006.03.001[↩][↩][↩]
- Tuschl K., Mills P.B., Clayton P.T. Manganese and the brain. Int. Rev. Neurobiol. 2013;110:277–312. doi: 10.1016/B978-0-12-410502-7.00013-2[↩][↩][↩]
- Dorman D.C., Struve M.F., Wong B.A., Dye J.A., Robertson I.D. Correlation of brain magnetic resonance imaging changes with pallidal manganese concentrations in rhesus monkeys following subchronic manganese inhalation. Toxicol. Sci. 2006;92:219–227. doi: 10.1093/toxsci/kfj209[↩][↩][↩]
- Yamada M., Ohno S., Okayasu I., Okeda R., Hatakeyama S., Watanabe H., Ushio K., Tsukagoshi H. Chronic manganese poisoning: A neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. 1986;70:273–278. doi: 10.1007/BF00686083[↩][↩][↩][↩][↩][↩]
- Gonzalez-Cuyar L.F., Nelson G., Criswell S.R., Ho P., Lonzanida J.A., Checkoway H., Seixas N., Gelman B.B., Evanoff B.A., Murray J., et al. Quantitative neuropathology associated with chronic manganese exposure in South African mine workers. Neurotoxicology. 2014;45:260–266. doi: 10.1016/j.neuro.2013.12.008[↩][↩][↩]
- Chen P., Bornhorst J., Aschner M. Manganese metabolism in humans. Front. Biosci. (Landmark Ed). 2018;23:1655–1679. doi: 10.2741/4665[↩]
- Cobley J.N., Fiorello M.L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox. Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008[↩][↩][↩][↩]
- Benedetto A., Au C., Aschner M. Manganese-induced dopaminergic neurodegeneration: Insights into mechanisms and genetics shared with Parkinson’s disease. Chem. Rev. 2009;109:4862–4884. doi: 10.1021/cr800536y[↩][↩]
- Martinez-Finley E.J., Gavin C.E., Aschner M., Gunter T.E. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic. Biol. Med. 2013;62:65–75. doi: 10.1016/j.freeradbiomed.2013.01.032[↩][↩]
- Sidoryk-Wegrzynowicz M., Aschner M. Role of astrocytes in manganese mediated neurotoxicity. BMC Pharmacol. Toxicol. 2013;14:23. doi: 10.1186/2050-6511-14-23[↩][↩][↩][↩]
- Kawamura R. Intoxication by manganese in well water. Kisasato Arch Exp Med. 1941;18:145-169.[↩]
- Kondakis XG, Makris N, Leotsinidis M, Prinou M, Papapetropoulos T. Possible health effects of high manganese concentration in drinking water. Arch Environ Health. 1989 May-Jun;44(3):175-8. doi: 10.1080/00039896.1989.9935883[↩]
- Vieregge P, Heinzow B, Korf G, Teichert HM, Schleifenbaum P, Mösinger HU. Long term exposure to manganese in rural well water has no neurological effects. Can J Neurol Sci. 1995 Nov;22(4):286-9. doi: 10.1017/s0317167100039482[↩]
- Secondary Drinking Water Standards: Guidance for Nuisance Chemicals. https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals[↩]
- Manganese in drinking-water. Background document for development of WHO Guidelines for drinking-water quality Geneva: World Health Organization; 2021 https://iris.who.int/bitstream/handle/10665/350933/WHO-HEP-ECH-WSH-2021.5-eng.pdf[↩]
- Guidelines for drinking-water quality. Fourth edition incorporating the first and second addenda. World Health Organization 2022. https://iris.who.int/bitstream/handle/10665/352532/9789240045064-eng.pdf[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ljung K, Vahter M. Time to re-evaluate the guideline value for manganese in drinking water? Environ Health Perspect. 2007 Nov;115(11):1533-8. doi: 10.1289/ehp.10316[↩][↩][↩]
- Liu W, Xin Y, Li Q, Shang Y, Ping Z, Min J, Cahill CM, Rogers JT, Wang F. Biomarkers of environmental manganese exposure and associations with childhood neurodevelopment: a systematic review and meta-analysis. Environ Health. 2020 Oct 2;19(1):104. doi: 10.1186/s12940-020-00659-x[↩][↩]
- Bouchard MF, Sauvé S, Barbeau B, Legrand M, Brodeur MÈ, Bouffard T, Limoges E, Bellinger DC, Mergler D. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011 Jan;119(1):138-43. doi: 10.1289/ehp.1002321[↩][↩]
- Dion LA, Saint-Amour D, Sauvé S, Barbeau B, Mergler D, Bouchard MF. Changes in water manganese levels and longitudinal assessment of intellectual function in children exposed through drinking water. Neurotoxicology. 2018 Jan;64:118-125. doi: 10.1016/j.neuro.2017.08.015[↩]
- Rahman SM, Kippler M, Tofail F, Bölte S, Hamadani JD, Vahter M. Manganese in Drinking Water and Cognitive Abilities and Behavior at 10 Years of Age: A Prospective Cohort Study. Environ Health Perspect. 2017 May 26;125(5):057003. doi: 10.1289/EHP631[↩][↩]
- Schullehner J, Thygesen M, Kristiansen SM, Hansen B, Pedersen CB, Dalsgaard S. Exposure to Manganese in Drinking Water during Childhood and Association with Attention-Deficit Hyperactivity Disorder: A Nationwide Cohort Study. Environ Health Perspect. 2020 Sep;128(9):97004. doi: 10.1289/EHP6391[↩][↩]
- Keen CL, Zidenberg-Cherr S. Manganese toxicity in humans and experimental animals. In: Klimis-Tavantzis DJ, ed. Manganese in Health and Disease. Boca Raton: CRC Press, Inc.; 1994:193-205.[↩][↩]
- Schuh MJ. Possible Parkinson’s Disease Induced by Chronic Manganese Supplement Ingestion. Consult Pharm. 2016 Dec 1;31(12):698-703. doi: 10.4140/TCP.n.2016.698[↩]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004 Mar;1012:115-28. doi: 10.1196/annals.1306.009[↩]
- Erikson KM, Thompson K, Aschner J, Aschner M. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther. 2007 Feb;113(2):369-77. doi: 10.1016/j.pharmthera.2006.09.002[↩]
- Chen P, Bornhorst J, Aschner M. Manganese metabolism in humans. Front Biosci (Landmark Ed). 2018 Mar 1;23(9):1655-1679. doi: 10.2741/4665[↩][↩][↩][↩][↩][↩]
- Hardy IJ, Gillanders L, Hardy G. Is manganese an essential supplement for parenteral nutrition? Curr Opin Clin Nutr Metab Care. 2008 May;11(3):289-96. doi: 10.1097/MCO.0b013e3282f9e889[↩]
- Evans GR, Masullo LN. Manganese Toxicity. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560903[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Roth JA. Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biol Res. 2006;39(1):45-57. doi: 10.4067/s0716-97602006000100006[↩]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Zheng Y, Graziano JH. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006 Jan;114(1):124-9. doi: 10.1289/ehp.8030[↩][↩]
- Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007 Jan;115(1):122-7. doi: 10.1289/ehp.9504[↩]
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006 Mar;27(2):210-6. doi: 10.1016/j.neuro.2005.10.001[↩]
- Menezes-Filho JA, Novaes Cde O, Moreira JC, Sarcinelli PN, Mergler D. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ Res. 2011 Jan;111(1):156-63. doi: 10.1016/j.envres.2010.09.006[↩]
- Carvalho CF, Menezes-Filho JA, de Matos VP, Bessa JR, Coelho-Santos J, Viana GF, Argollo N, Abreu N. Elevated airborne manganese and low executive function in school-aged children in Brazil. Neurotoxicology. 2014 Dec;45:301-8. doi: 10.1016/j.neuro.2013.11.006[↩]
- Oulhote Y, Mergler D, Barbeau B, Bellinger DC, Bouffard T, Brodeur MÈ, Saint-Amour D, Legrand M, Sauvé S, Bouchard MF. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ Health Perspect. 2014 Dec;122(12):1343-50. doi: 10.1289/ehp.1307918[↩][↩]
- Khan K, Factor-Litvak P, Wasserman GA, Liu X, Ahmed E, Parvez F, Slavkovich V, Levy D, Mey J, van Geen A, Graziano JH. Manganese exposure from drinking water and children’s classroom behavior in Bangladesh. Environ Health Perspect. 2011 Oct;119(10):1501-6. doi: 10.1289/ehp.1003397[↩]
- Aschner M, Dorman DC. Manganese: pharmacokinetics and molecular mechanisms of brain uptake. Toxicol Rev. 2006;25(3):147-54. doi: 10.2165/00139709-200625030-00002[↩]
- de Bie RM, Gladstone RM, Strafella AP, Ko JH, Lang AE. Manganese-induced Parkinsonism associated with methcathinone (Ephedrone) abuse. Arch Neurol. 2007 Jun;64(6):886-9. doi: 10.1001/archneur.64.6.886[↩]
- Sikk K, Haldre S, Aquilonius SM, Taba P. Manganese-Induced Parkinsonism due to Ephedrone Abuse. Parkinsons Dis. 2011 Feb 17;2011:865319. doi: 10.4061/2011/865319[↩]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001. 10, Manganese. Available from: https://www.ncbi.nlm.nih.gov/books/NBK222332[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Han J, Lee JS, Choi D, Lee Y, Hong S, Choi J, Han S, Ko Y, Kim JA, Kim YM, Jung Y. Manganese (II) induces chemical hypoxia by inhibiting HIF-prolyl hydroxylase: implication in manganese-induced pulmonary inflammation. Toxicol Appl Pharmacol. 2009 Mar 15;235(3):261-7. doi: 10.1016/j.taap.2009.01.003[↩]
- Roels H, Lauwerys R, Buchet JP, Genet P, Sarhan MJ, Hanotiau I, de Fays M, Bernard A, Stanescu D. Epidemiological survey among workers exposed to manganese: effects on lung, central nervous system, and some biological indices. Am J Ind Med. 1987;11(3):307-27. doi: 10.1002/ajim.4700110308. Erratum in: Am J Ind Med 1987;12(1):119-20[↩]
- Manganese. https://ods.od.nih.gov/factsheets/Manganese-HealthProfessional[↩][↩][↩][↩]
- Manganese. https://lpi.oregonstate.edu/mic/minerals/manganese[↩]
- Obeng SK, Kulhánek M, Balík J, Černý J, Sedlář O. Manganese: From Soil to Human Health-A Comprehensive Overview of Its Biological and Environmental Significance. Nutrients. 2024 Oct 11;16(20):3455. doi: 10.3390/nu16203455[↩][↩][↩]
- Malakouti M.J., Tehrani M.H. Effect of Micronutrients on the Yield and Quality of Agricultural Products: Micro-Nutrients with Macro-Effects. Tarbiat Modares University; Tehran, Iran: 1999.[↩]
- Manganese. https://ods.od.nih.gov/factsheets/Manganese-Consumer[↩][↩]
- Balachandran R.C., Mukhopadhyay S., McBride D., Veevers J., Harrison F.E., Aschner M., Haynes E.N., Bowman A.B. Brain manganese and the balance between essential roles and neurotoxicity. J. Biol. Chem. 2020;295:6312–6329. doi: 10.1074/jbc.REV119.009453[↩]
- Horning K.J., Caito S.W., Tipps K.G., Bowman A.B., Aschner M. Manganese is essential for neuronal health. Annu. Rev. Nutr. 2015;35:71–108. doi: 10.1146/annurev-nutr-071714-034419[↩]
- Buchman AR. Manganese. In: A. Catharine Ross BC, Robert J. Cousins, Katherine L. Tucker, Thomas R. Ziegler ed. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:238-44.[↩][↩][↩]
- Nielsen FH. Manganese, Molybdenum, Boron, Chromium, and Other Trace Elements. In: John W. Erdman Jr. IAM, Steven H. Zeisel, ed. Present Knowledge in Nutrition. 10th ed: Wiley-Blackwell; 2012:586-607.[↩][↩][↩][↩][↩][↩][↩]
- Li L, Yang X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid Med Cell Longev. 2018 Apr 5;2018:7580707. doi: 10.1155/2018/7580707[↩][↩]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005 Aug-Oct;26(4-5):353-62. doi: 10.1016/j.mam.2005.07.003[↩][↩][↩][↩][↩][↩][↩]
- Palacios C. The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr. 2006;46(8):621-8. doi: 10.1080/10408390500466174[↩]
- Rossander-Hultén L, Brune M, Sandström B, Lönnerdal B, Hallberg L. Competitive inhibition of iron absorption by manganese and zinc in humans. Am J Clin Nutr. 1991 Jul;54(1):152-6. doi: 10.1093/ajcn/54.1.152[↩]
- Food and Nutrition Board, Institute of Medicine. Manganese. Dietary reference intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C.: National Academy Press; 2001:394-419. https://www.nap.edu/read/10026/chapter/1[↩][↩][↩][↩]
- Kies C. Bioavailability of manganese. In: Klimis-Tavantzis DL, ed. Manganese in health and disease. Boca Raton: CRC Press, Inc; 1994:39-58.[↩]
- US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 22. 2009. https://ndb.nal.usda.gov/ndb/[↩]
- Finley JW. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am J Clin Nutr. 1999 Jul;70(1):37-43. doi: 10.1093/ajcn/70.1.37[↩]
- Finley JW, Davis CD. Manganese deficiency and toxicity: are high or low dietary amounts of manganese cause for concern? Biofactors. 1999;10(1):15-24. doi: 10.1002/biof.5520100102[↩]
- Finley JW, Johnson PE, Johnson LK. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am J Clin Nutr. 1994 Dec;60(6):949-55. doi: 10.1093/ajcn/60.6.949[↩]
- T. M. Ansari, N. Ikram, M. Najam-ul-Haq, I. Fayyaz, Q. Fayyaz, I. Ghafoor and N. Khalid, 2004. Essential Trace Metal (Zinc, Manganese, Copper and Iron) Levels in Plants of Medicinal Importance. Journal of Biological Sciences, 4: 95-99. https://scialert.net/abstract/?doi=jbs.2004.95.99[↩]
- Lönnerdal B. Nutritional aspects of soy formula. Acta Paediatr Suppl. 1994 Sep;402:105-8. doi: 10.1111/j.1651-2227.1994.tb13371.x[↩]
- Davidsson L, Cederblad A, Lönnerdal B, Sandström B. Manganese absorption from human milk, cow’s milk, and infant formulas in humans. Am J Dis Child. 1989 Jul;143(7):823-7. doi: 10.1001/archpedi.1989.02150190073024[↩]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26(4-5):353-362. https://www.ncbi.nlm.nih.gov/pubmed/16099026[↩][↩]
- Keen CL, Zidenberg-Cherr S. Manganese toxicity in humans and experimental animals. In: Klimis-Tavantzis DL, ed. Manganese in health and disease. Boca Raton: CRC Press, Inc; 1994:193-205.[↩][↩]
- EPA Office of Water. Current Drinking Water Standards. Environmental Protection Agency. https://safewater.zendesk.com/hc/en-us/categories/201454937[↩]
- Nielsen FH. Ultratrace minerals. In: Shils M, Olson JA, Shike M, Ross AC, eds. Modern Nutrition in Health and Disease. 9th ed. Baltimore: Williams & Wilkins; 1999:283-303.[↩]
- Keen CL, Zidenberg-Cherr S. Manganese. In: Ziegler EE, Filer LJ, eds. Present Knowledge in Nutrition. 7th ed. Washington D.C.: ILSI Press; 1996:334-343.[↩]
- Norose N, Terai M, Norose K. Manganese deficiency in a child with very short bowel syndrome receiving long-term parenteral nutrition. J Trace Elem Exp Med. 1992;5:100-101 (abstract).[↩]
- Suita S, Masumoto K, Yamanouchi T, Nagano M, Nakamura M. Complications in neonates with short bowel syndrome and long-term parenteral nutrition. JPEN J Parenter Enteral Nutr. 1999 Sep-Oct;23(5 Suppl):S106-9. doi: 10.1177/014860719902300526[↩]
- Dahlstrom KA, Ament ME, Medhin MG, Meurling S. Serum trace elements in children receiving long-term parenteral nutrition. J Pediatr. 1986 Oct;109(4):625-30. doi: 10.1016/s0022-3476(86)80225-6[↩]
- Friedman BJ, Freeland-Graves JH, Bales CW, et al. Manganese balance and clinical observations in young men fed a manganese-deficient diet. J Nutr. 1987;117(1):133-143. https://www.ncbi.nlm.nih.gov/pubmed/3819860[↩][↩]
- Johnson PE, Lykken GI. Manganese and calcium absorption and balance in young women fed diets with varying amounts of manganese and calcium. J Trace Elem Exp Med. 1991;4:19-35.[↩]
- Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001 Mar;101(3):294-301. doi: 10.1016/S0002-8223(01)00078-5[↩]
- Williams M, Todd GD, Roney N, et al. Toxicological Profile for Manganese. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2012 Sep. Available from: https://www.ncbi.nlm.nih.gov/books/NBK158872[↩][↩][↩][↩]
- Pajarillo E., Nyarko-Danquah I., Adinew G., Rizor A., Aschner M., Lee E. Neurotoxicity mechanisms of manganese in the central nervous system. Adv. Neurotoxicol. 2021;5:215–238. doi: 10.1016/bs.ant.2020.11.003[↩]
- Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Br. Ann. Med. Pharm. Vital Stat. Gen. Sci. 1837;1:41–42.[↩]
- Huang C.C., Chu N.S., Lu C.S., Wang J.D., Tsai J.L., Tzeng J.L., Wolters E.C., Calne D.B. Chronic manganese intoxication. Arch. Neurol. 1989;46:1104–1106. doi: 10.1001/archneur.1989.00520460090018[↩]
- Bowler R.M., Gocheva V., Harris M., Ngo L., Abdelouahab N., Wilkinson J., Doty R.L., Park R., Roles H.A. Prospective study on neurotoxic effects in manganese-exposed bridge construction welders. Neurotoxicology. 2011;32:596–605. doi: 10.1016/j.neuro.2011.06.004[↩]
- Davis JM. Methylcyclopentadienyl manganese tricarbonyl: health risk uncertainties and research directions. Environ Health Perspect. 1998;106 Suppl 1:191-201. https://www.ncbi.nlm.nih.gov/pubmed/9539013[↩][↩]
- Zayed J, Thibault C, Gareau L, Kennedy G. Airborne manganese particulates and methylcyclopentadienyl manganese tricarbonyl (MMT) at selected outdoor sites in Montreal. Neurotoxicology. 1999;20(2-3):151-157. https://www.ncbi.nlm.nih.gov/pubmed/10385879[↩]
- Bolte S, Normandin L, Kennedy G, Zayed J. Human exposure to respirable manganese in outdoor and indoor air in urban and rural areas. J Toxicol Environ Health A. 2004;67(6):459-467. https://www.ncbi.nlm.nih.gov/pubmed/15000130[↩]
- Aschner M. Manganese: brain transport and emerging research needs. Environ Health Perspect. 2000;108 Suppl 3:429-432. https://www.ncbi.nlm.nih.gov/pubmed/10852840[↩]
- Nemanich A, Chen B, Valento M. Toxic boost: Acute, reversible neurotoxicity after ingestion of methylcyclopentadienyl manganese tricarbonyl (MMT) mistaken for an energy drink. Am J Emerg Med. 2021 Apr;42:261.e3-261.e5. doi: 10.1016/j.ajem.2020.08.087[↩]
- Fujishiro H., Yano Y., Takada Y., Tanihara M., Himeno S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics. 2012;4:700–708. doi: 10.1039/c2mt20024d[↩][↩]
- Leyva-Illades D., Chen P., Zogzas C.E., Hutchens S., Mercado J.M., Swaim C.D., Morrisett R.A., Bowman A.B., Aschner M., Mukhopadhyay S. SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J. Neurosci. 2014;34:14079–14095. doi: 10.1523/JNEUROSCI.2329-14.2014[↩]
- Russell R., Beard J.L., Cousins R.J., Dunn J.T., Ferland G., Hambidge K.M., Lynch S., Penland J.G., Ross A.C., Stoecker B.J., et al. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Food and Nutrition Board, Institute of Medicine. National Academy Press; Washington, DC, USA: 2001.[↩]
- Yokel R.A. Manganese flux across the blood–brain barrier. NeuroMolecular Med. 2009;11:297–310. doi: 10.1007/s12017-009-8101-2[↩]
- Quadri M., Federico A., Zhao T., Breedveld G.J., Battisti C., Delnooz C., Severijnen L.-A., Di Toro Mammarella L., Mignarri A., Monti L., et al. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet. 2012;90:467–477. doi: 10.1016/j.ajhg.2012.01.017[↩]
- Keen CL, Ensunsa JL, Watson MH, et al. Nutritional aspects of manganese from experimental studies. Neurotoxicology. 1999;20(2-3):213-223. https://www.ncbi.nlm.nih.gov/pubmed/10385885[↩]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20(2-3):227-238. https://www.ncbi.nlm.nih.gov/pubmed/10385886[↩][↩]
- Aschner M, Aschner JL. Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Neurosci Biobehav Rev. 1991;15(3):333-340. https://www.ncbi.nlm.nih.gov/pubmed/1956602[↩]
- Han J, Lee JS, Choi D, et al. Manganese (II) induces chemical hypoxia by inhibiting HIF-prolyl hydroxylase: implication in manganese-induced pulmonary inflammation. Toxicol Appl Pharmacol. 2009;235(3):261-267. https://www.ncbi.nlm.nih.gov/pubmed/19263519[↩]
- Roels H, Lauwerys R, Buchet JP, et al. Epidemiological survey among workers exposed to manganese: effects on lung, central nervous system, and some biological indices. Am J Ind Med. 1987;11(3):307-327. https://www.ncbi.nlm.nih.gov/pubmed/3578289[↩]
- Bakulski KM, Seo YA, Hickman RC, Brandt D, Vadari HS, Hu H, Park SK. Heavy Metals Exposure and Alzheimer’s Disease and Related Dementias. J Alzheimers Dis. 2020;76(4):1215-1242. doi: 10.3233/JAD-200282[↩]
- Freeland-Graves JH, Mousa TY, Kim S. International variability in diet and requirements of manganese: Causes and consequences. J Trace Elem Med Biol. 2016 Dec;38:24-32. doi: 10.1016/j.jtemb.2016.05.004[↩]
- Davis CD, Zech L, Greger JL. Manganese metabolism in rats: an improved methodology for assessing gut endogenous losses. Proc Soc Exp Biol Med. 1993 Jan;202(1):103-8. doi: 10.3181/00379727-202-43518[↩]
- Finley JW, Penland JG, Pettit RE, Davis CD. Dietary manganese intake and type of lipid do not affect clinical or neuropsychological measures in healthy young women. J Nutr. 2003 Sep;133(9):2849-56. doi: 10.1093/jn/133.9.2849[↩]
- Kawamura R. Intoxication by manganese in well water. Kisasato Archives of Experimental Medicine. 1941;18:145-169.[↩]
- Kondakis XG, Makris N, Leotsinidis M, Prinou M, Papapetropoulos T. Possible health effects of high manganese concentration in drinking water. Arch Environ Health. 1989;44(3):175-178. https://www.ncbi.nlm.nih.gov/pubmed/2751354[↩]
- Vieregge P, Heinzow B, Korf G, Teichert HM, Schleifenbaum P, Mosinger HU. Long term exposure to manganese in rural well water has no neurological effects. Can J Neurol Sci. 1995;22(4):286-289. https://www.ncbi.nlm.nih.gov/pubmed/8599771[↩]
- EPA Office of Water. Current Drinking Water Standards. Environmental Protection Agency. https://www.nap.edu/read/10026/chapter/1[↩]
- Ljung K, Vahter M. Time to re-evaluate the guideline value for manganese in drinking water? Environ Health Perspect. 2007;115(11):1533-1538. https://www.ncbi.nlm.nih.gov/pubmed/18007980[↩][↩]
- Wasserman GA, Liu X, Parvez F, et al. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114(1):124-129. https://www.ncbi.nlm.nih.gov/pubmed/16393669[↩]
- Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115(1):122-127. https://www.ncbi.nlm.nih.gov/pubmed/17366831[↩]
- Keen C, Zidenberg-Cherr S. Manganese toxicity in humans and experimental animals. In: Klimis-Tavantzis D (ed). Manganese in health and disease. Boca Raton: CRC Press, Inc.; 1994.[↩]
- Aschner J.L., Aschner M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003[↩]
- Chen P., Chakraborty S., Mukhopadhyay S., Lee E., Paoliello M.M., Bowman A.B., Aschner M. Manganese homeostasis in the nervous system. J. Neurochem. 2015;134:601–610. doi: 10.1111/jnc.13170[↩]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann NY Acad Sci. 2004;1012:115-128. https://www.ncbi.nlm.nih.gov/pubmed/15105259[↩]
- Erikson KM, Thompson K, Aschner J, Aschner M. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther. 2007;113(2):369-377. https://www.ncbi.nlm.nih.gov/pubmed/17084903[↩]
- de Bie R.M., Gladstone R.M., Strafella A.P., Ko J.-H., Lang A.E. Manganese-induced Parkinsonism associated with methcathinone (Ephedrone) abuse. Arch. Neurol. 2007;64:886–889. doi: 10.1001/archneur.64.6.886[↩]
- Sikk K., Taba P., Haldre S., Bergquist J., Nyholm D., Askmark H., Danfors T., Sörensen J., Thurfjell L., Raininko R. Clinical, neuroimaging and neurophysiological features in addicts with manganese-ephedrone exposure. Acta Neurol. Scand. 2010;121:237–243. doi: 10.1111/j.1600-0404.2009.01189.x[↩][↩]
- Sikk K., Haldre S., Aquilonius S.M., Asser A., Paris M., Roose Ä., Petterson J., Eriksson S.L., Bergquist J., Taba P. Manganese-induced parkinsonism in methcathinone abusers: Biomarkers of exposure and follow-up. Eur. J. Neurol. 2013;20:915–920. doi: 10.1111/ene.12088[↩]
- Hardy IJ, Gillanders L, Hardy G. Is manganese an essential supplement for parenteral nutrition? Curr Opin Clin Nutr Metab Care. 2008;11(3):289-296. https://www.ncbi.nlm.nih.gov/pubmed/18403926[↩]
- Guan H., Wang M., Li X., Piao F., Li Q., Xu L., Kitamura F., Yokoyama K. Manganese concentrations in maternal and umbilical cord blood: Related to birth size and environmental factors. Eur. J. Public Health. 2013;24:150–157. doi: 10.1093/eurpub/ckt033[↩]
- Zota A.R., Ettinger A.S., Bouchard M., Amarasiriwardena C.J., Schwartz J., Hu H., Wright R.O. Maternal blood manganese levels and infant birth weight. Epidemiology. 2009;20:367–373. doi: 10.1097/EDE.0b013e31819b93c0[↩]
- Chen L., Ding G., Gao Y., Wang P., Shi R., Huang H., Tian Y. Manganese concentrations in maternal–infant blood and birth weight. Environ. Sci. Pollut. Res. 2014;21:6170–6175. doi: 10.1007/s11356-013-2465-4[↩]
- Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology. 2005 Jun 28;64(12):2021-8. doi: 10.1212/01.WNL.0000166916.40902.63[↩]
- Olanow CW. Manganese-induced parkinsonism and Parkinson’s disease. Ann N Y Acad Sci. 2004 Mar;1012:209-23. doi: 10.1196/annals.1306.018[↩]
- Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004 Dec;17(8):544-53. doi: 10.1002/nbm.931[↩][↩][↩]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, Koller W, Bowler RP, Mergler D, Bouchard M, Smith D, Gwiazda R, Doty RL. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med. 2007 Mar;64(3):167-77. doi: 10.1136/oem.2006.028761[↩][↩][↩][↩]
- Huang CC, Chu NS, Lu CS, Calne DB. Cock gait in manganese intoxication. Mov Disord. 1997 Sep;12(5):807-8. doi: 10.1002/mds.870120532[↩]
- Charash B, Placek E, Sos TA, Kligfield P. Dose-related effects of manganese on the canine electrocardiogram. J Electrocardiol. 1982 Apr;15(2):149-52. doi: 10.1016/s0022-0736(82)80009-5[↩]
- Vander Elst L, Colet JM, Muller RN. Spectroscopic and metabolic effects of MnCl2 and MnDPDP on the isolated and perfused rat heart. Invest Radiol. 1997 Oct;32(10):581-8. doi: 10.1097/00004424-199710000-00001[↩]
- Dey S., Tripathy B., Kumar M.S., Das A.P. Ecotoxicological consequences of manganese mining pollutants and their biological remediation. Environ. Chem. Ecotoxicol. 2023;5:55–61. doi: 10.1016/j.enceco.2023.01.001[↩]
- Michalke B, Fernsebner K. New insights into manganese toxicity and speciation. J Trace Elem Med Biol. 2014 Apr;28(2):106-116. doi: 10.1016/j.jtemb.2013.08.005[↩]
- Kullar SS, Shao K, Surette C, Foucher D, Mergler D, Cormier P, Bellinger DC, Barbeau B, Sauvé S, Bouchard MF. A benchmark concentration analysis for manganese in drinking water and IQ deficits in children. Environ Int. 2019 Sep;130:104889. doi: 10.1016/j.envint.2019.05.083[↩]
- Angoa-Pérez M, Anneken JH, Kuhn DM. Neurotoxicology of Synthetic Cathinone Analogs. Curr Top Behav Neurosci. 2017;32:209-230. doi: 10.1007/7854_2016_21[↩]
- Squitti R, Gorgone G, Panetta V, Lucchini R, Bucossi S, Albini E, Alessio L, Alberici A, Melgari JM, Benussi L, Binetti G, Rossini PM, Draicchio F. Implications of metal exposure and liver function in Parkinsonian patients resident in the vicinities of ferroalloy plants. J Neural Transm (Vienna). 2009 Oct;116(10):1281-7. doi: 10.1007/s00702-009-0283-0[↩]
- Lauwerys R, Roels H, Genet P, Toussaint G, Bouckaert A, De Cooman S. Fertility of male workers exposed to mercury vapor or to manganese dust: a questionnaire study. Am J Ind Med. 1985;7(2):171-6. doi: 10.1002/ajim.4700070208[↩]
- ATSDR (2012) Toxicological profile for manganese. US Department of Health and Human Services (HHS), Atlanta, GA, https://www.atsdr.cdc.gov/toxprofiles/tp151.pdf[↩]
- Baker MG, Simpson CD, Sheppard L, Stover B, Morton J, Cocker J, Seixas N. Variance components of short-term biomarkers of manganese exposure in an inception cohort of welding trainees. J Trace Elem Med Biol. 2015 Jan;29:123-9. doi: 10.1016/j.jtemb.2014.05.004[↩]
- Mahoney JP, Small WJ. Studies on manganese. 3. The biological half-life of radiomanganese in man and factors which affect this half-life. J Clin Invest. 1968 Mar;47(3):643-53. https://pmc.ncbi.nlm.nih.gov/articles/instance/297210/pdf/jcinvest00238-0231.pdf[↩]
- Zhang LL, Lu L, Pan YJ, Ding CG, Xu DY, Huang CF, Pan XF, Zheng W. Baseline blood levels of manganese, lead, cadmium, copper, and zinc in residents of Beijing suburb. Environ Res. 2015 Jul;140:10-7. doi: 10.1016/j.envres.2015.03.008[↩]
- Oulhote Y, Mergler D, Bouchard MF. Sex- and age-differences in blood manganese levels in the U.S. general population: national health and nutrition examination survey 2011-2012. Environ Health. 2014 Oct 24;13:87. doi: 10.1186/1476-069X-13-87[↩]
- de Water E, Proal E, Wang V, Medina SM, Schnaas L, Téllez-Rojo MM, Wright RO, Tang CY, Horton MK. Prenatal manganese exposure and intrinsic functional connectivity of emotional brain areas in children. Neurotoxicology. 2018 Jan;64:85-93. doi: 10.1016/j.neuro.2017.06.006[↩]
- Yang HJ, Park SH, Seo M, Weon YC, Kim Y. 18F-FP-CIT dopamine transporter PET findings in cirrhotic patients with parkinsonism. Neurotoxicology. 2018 Jan;64:78-84. doi: 10.1016/j.neuro.2017.02.014[↩]
- Casjens S, Dydak U, Dharmadhikari S, Lotz A, Lehnert M, Quetscher C, Stewig C, Glaubitz B, Schmidt-Wilcke T, Edmondson D, Yeh CL, Weiss T, Thriel CV, Herrmann L, Muhlack S, Woitalla D, Aschner M, Brüning T, Pesch B. Association of exposure to manganese and iron with striatal and thalamic GABA and other neurometabolites – Neuroimaging results from the WELDOX II study. Neurotoxicology. 2018 Jan;64:60-67. doi: 10.1016/j.neuro.2017.08.004[↩]
- Antony PM, Diederich NJ, Krüger R, Balling R. The hallmarks of Parkinson’s disease. FEBS J. 2013 Dec;280(23):5981-93. doi: 10.1111/febs.12335[↩]
- Gomperts SN. Lewy Body Dementias: Dementia With Lewy Bodies and Parkinson Disease Dementia. Continuum (Minneap Minn). 2016 Apr;22(2 Dementia):435-63. doi: 10.1212/CON.0000000000000309[↩]
- Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015 Oct 24;386(10004):1672-82. doi: 10.1016/S0140-6736(15)00461-4[↩]
- Clark LN, Louis ED. Essential tremor. Handb Clin Neurol. 2018;147:229-239. doi: 10.1016/B978-0-444-63233-3.00015-4[↩]
- Palma JA, Norcliffe-Kaufmann L, Kaufmann H. Diagnosis of multiple system atrophy. Auton Neurosci. 2018 May;211:15-25. doi: 10.1016/j.autneu.2017.10.007[↩]
- Levin J, Kurz A, Arzberger T, Giese A, Höglinger GU. The Differential Diagnosis and Treatment of Atypical Parkinsonism. Dtsch Arztebl Int. 2016 Feb 5;113(5):61-9. doi: 10.3238/arztebl.2016.0061[↩][↩]
- Brigo F, Erro R, Marangi A, Bhatia K, Tinazzi M. Differentiating drug-induced parkinsonism from Parkinson’s disease: an update on non-motor symptoms and investigations. Parkinsonism Relat Disord. 2014 Aug;20(8):808-14. doi: 10.1016/j.parkreldis.2014.05.011[↩]
- Calkins RF, Aktan GB, Hussain KL. Methcathinone: the next illicit stimulant epidemic? J Psychoactive Drugs. 1995 Jul-Sep;27(3):277-85. https://doi.org/10.1080/02791072.1995.10472472[↩][↩]
- Iqbal M, Monaghan T, Redmond J. Manganese toxicity with ephedrone abuse manifesting as parkinsonism: a case report. J Med Case Rep. 2012 Feb 7;6:52. doi: 10.1186/1752-1947-6-52[↩]
- Koh J, Ito H. Differential diagnosis of Parkinson’s disease and other neurodegenerative disorders. Nihon Rinsho. 2017 Jan;75(1):56-62. English, Japanese.[↩]
- Herrero Hernandez E, Discalzi G, Valentini C, Venturi F, Chio A, Carmellino C, Rossi L, Sacchetti A, Pira E. Follow-up of patients affected by manganese-induced Parkinsonism after treatment with CaNa2EDTA. Neurotoxicol. 2006;27:333–339. doi: 10.1016/j.neuro.2005.09.003[↩]
- Fenstermacher J, Kaye T. Drug “diffusion” within the brain. Ann N Y Acad Sci. 1988;531:29-39. doi: 10.1111/j.1749-6632.1988.tb31809.x[↩]
- Schlageter NL, Carson RE, Rapoport SI. Examination of blood-brain barrier permeability in dementia of the Alzheimer type with [68Ga]EDTA and positron emission tomography. J Cereb Blood Flow Metab. 1987 Feb;7(1):1-8. doi: 10.1038/jcbfm.1987.1[↩]
- von Holst H, Ericson K, Edner G. Positron emission tomography with 68-Ga-EDTA and computed tomography in patients with subarachnoid haemorrhage. Acta Neurochir (Wien). 1989;97(3-4):146-9. doi: 10.1007/BF01772827[↩]
- Bleich S, Degner D, Sprung R, Riegel A, Poser W, Rüther E. Chronic manganism: fourteen years of follow-up. J Neuropsychiatry Clin Neurosci. 1999 Winter;11(1):117. doi: 10.1176/jnp.11.1.117[↩]
- Hong L, Jiang W, Zheng W, Zeng S. HPLC analysis of para-aminosalicylic acid and its metabolite in plasma, cerebrospinal fluid and brain tissues. J Pharm Biomed Anal. 2011 Apr 5;54(5):1101-9. doi: 10.1016/j.jpba.2010.11.031[↩]
- Jiang YM, Mo XA, Du FQ, Fu X, Zhu XY, Gao HY, Xie JL, Liao FL, Pira E, Zheng W. Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med. 2006 Jun;48(6):644-9. doi: 10.1097/01.jom.0000204114.01893.3e[↩]
- Asanuma M, Miyazaki I, Ogawa N. Neuroprotective effects of nonsteroidal anti-inflammatory drugs on neurodegenerative diseases. Curr Pharm Des. 2004;10(6):695-700. doi: 10.2174/1381612043453072[↩]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005 Jan 6;433(7021):73-7. doi: 10.1038/nature03180[↩]
- Huang CC, Lu CS, Chu NS, Hochberg F, Lilienfeld D, Olanow W, Calne DB. Progression after chronic manganese exposure. Neurology. 1993 Aug;43(8):1479-83. doi: 10.1212/wnl.43.8.1479[↩]
- Huang CC, Chu NS, Lu CS, Chen RS, Calne DB. Long-term progression in chronic manganism: ten years of follow-up. Neurology. 1998 Mar;50(3):698-700. doi: 10.1212/wnl.50.3.698[↩]
- Tuschl K, Mills PB, Clayton PT. Manganese and the brain. Int Rev Neurobiol. 2013;110:277-312. doi: 10.1016/B978-0-12-410502-7.00013-2[↩]
- Cordova FM, Aguiar AS Jr, Peres TV, Lopes MW, Gonçalves FM, Pedro DZ, Lopes SC, Pilati C, Prediger RD, Farina M, Erikson KM, Aschner M, Leal RB. Manganese-exposed developing rats display motor deficits and striatal oxidative stress that are reversed by Trolox. Arch Toxicol. 2013 Jul;87(7):1231-44. doi: 10.1007/s00204-013-1017-5[↩]
- Stephenson AP, Schneider JA, Nelson BC, Atha DH, Jain A, Soliman KF, Aschner M, Mazzio E, Renee Reams R. Manganese-induced oxidative DNA damage in neuronal SH-SY5Y cells: attenuation of thymine base lesions by glutathione and N-acetylcysteine. Toxicol Lett. 2013 Apr 26;218(3):299-307. doi: 10.1016/j.toxlet.2012.12.024[↩]
- Peres TV, Schettinger MR, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M. “Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies”. BMC Pharmacol Toxicol. 2016 Nov 4;17(1):57. doi: 10.1186/s40360-016-0099-0[↩]
- da Silva Santos V, Bisen-Hersh E, Yu Y, Cabral IS, Nardini V, Culbreth M, Teixeira da Rocha JB, Barbosa F Jr, Aschner M. Anthocyanin-rich açaí (Euterpe oleracea Mart.) extract attenuates manganese-induced oxidative stress in rat primary astrocyte cultures. J Toxicol Environ Health A. 2014;77(7):390-404. doi: 10.1080/15287394.2014.880392[↩]
- Martins EN, Pessano NT, Leal L, Roos DH, Folmer V, Puntel GO, Rocha JB, Aschner M, Ávila DS, Puntel RL. Protective effect of Melissa officinalis aqueous extract against Mn-induced oxidative stress in chronically exposed mice. Brain Res Bull. 2012 Jan 4;87(1):74-9. doi: 10.1016/j.brainresbull.2011.10.003[↩]
- Getachew B, Csoka AB, Aschner M, Tizabi Y. Nicotine protects against manganese and iron-induced toxicity in SH-SY5Y cells: Implication for Parkinson’s disease. Neurochem Int. 2019 Mar;124:19-24. doi: 10.1016/j.neuint.2018.12.003[↩]
- Ommati MM, Heidari R, Ghanbarinejad V, Abdoli N, Niknahad H. Taurine Treatment Provides Neuroprotection in a Mouse Model of Manganism. Biol Trace Elem Res. 2019 Aug;190(2):384-395. doi: 10.1007/s12011-018-1552-2[↩]
- Ahmadi N, Ghanbarinejad V, Ommati MM, Jamshidzadeh A, Heidari R. Taurine prevents mitochondrial membrane permeabilization and swelling upon interaction with manganese: Implication in the treatment of cirrhosis-associated central nervous system complications. J Biochem Mol Toxicol. 2018 Nov;32(11):e22216. doi: 10.1002/jbt.22216[↩]
- Lu CL, Tang S, Meng ZJ, He YY, Song LY, Liu YP, Ma N, Li XY, Guo SC. Taurine improves the spatial learning and memory ability impaired by sub-chronic manganese exposure. J Biomed Sci. 2014 May 24;21(1):51. doi: 10.1186/1423-0127-21-51[↩]
- Neely MD, Davison CA, Aschner M, Bowman AB. From the Cover: Manganese and Rotenone-Induced Oxidative Stress Signatures Differ in iPSC-Derived Human Dopamine Neurons. Toxicol Sci. 2017 Oct 1;159(2):366-379. doi: 10.1093/toxsci/kfx145[↩]
- Bouchard, M., Mergler, D., Baldwin, M., Panisset, M., Bowler, R. and Roels, H.A. (2007), Neurobehavioral functioning after cessation of manganese exposure: A follow-up after 14 years†. Am. J. Ind. Med., 50: 831-840. https://doi.org/10.1002/ajim.20407[↩][↩][↩][↩]
- Hochberg F, Miller G, Valenzuela R, McNelis S, Crump KS, Covington T, Valdivia G, Hochberg B, Trustman JW. Late motor deficits of Chilean manganese miners: a blinded control study. Neurology. 1996 Sep;47(3):788-95. doi: 10.1212/wnl.47.3.788[↩]
- Bouchard M, Mergler D, Baldwin M. Manganese exposure and age: neurobehavioral performance among alloy production workers. Environ Toxicol Pharmacol. 2005 May;19(3):687-94. doi: 10.1016/j.etap.2004.12.037[↩]
- Bouchard M, Mergler D, Baldwin M, Panisset M, Roels HA. Neuropsychiatric symptoms and past manganese exposure in a ferro-alloy plant. Neurotoxicology. 2007 Mar;28(2):290-7. doi: 10.1016/j.neuro.2006.08.002[↩]
- Roels HA, Ortega Eslava MI, Ceulemans E, Robert A, Lison D. Prospective study on the reversibility of neurobehavioral effects in workers exposed to manganese dioxide. Neurotoxicology. 1999 Apr-Jun;20(2-3):255-71.[↩][↩][↩]
- Lucchini R, Selis L, Folli D, Apostoli P, Mutti A, Vanoni O, Iregren A, Alessio L. Neurobehavioral effects of manganese in workers from a ferroalloy plant after temporary cessation of exposure. Scand J Work Environ Health. 1995 Apr;21(2):143-9. doi: 10.5271/sjweh.1369[↩]