Contents
Thyrotoxic crisis
Thyrotoxic crisis also called thyroid storm or thyroid crisis is a rare life-threatening condition that can affect people with hyperthyroidism (overactive thyroid) 1, 2, 3, 4. Thyrotoxic crisis happens when your thyroid gland releases a large amount of thyroid hormones called triiodothyronine (T3) and thyroxine (T4) in a short amount of time. Thyrotoxic crisis is a medical emergency that can be fatal if left untreated requiring rapid diagnosis and aggressive treatment in a hospital. The mortality associated with thyrotoxic crisis is estimated to be 8 to 25% despite modern advancements in its treatment and supportive measures 5.
Thyrotoxic crisis occurs in people with poor compliance with the medications for hyperthyroidism and is triggered by major stressors such as:
- Trauma
- Heart attack
- Infection
- Surgical procedures
Thyrotoxic crisis is rarely caused by radioactive iodine (radioiodine) treatment for Graves disease 6, 7. This can occur even a week or more after treatment.
In the past, thyrotoxic crisis was commonly observed during thyroid surgery, especially in older children and adults, but improved preoperative management has markedly decreased the incidence of this complication. Today, thyrotoxic crisis occurs more commonly as a medical crisis rather than a surgical crisis.
Thyrotoxic crisis may also be the initial presentation of undiagnosed thyrotoxicosis in children, especially in neonates. Thyrotoxicosis is a clinical state of inappropriately high levels of circulating thyroid hormones (T3 and/or T4) in the body either from endogenous (within the body) or exogenous (external) source 8, 9. On the other hand, hyperthyroidism, which is a form of thyrotoxicosis, is caused by excessive endogenous (within the body) thyroid hormone production.
Thyrotoxic crisis accounts for about 1% to 2% of admissions for hyperthyroidism. As per the United States survey, the incidence of thyroid storms ranged from 0.57 to 0.76 cases per 100,000 population per year in the normal population and 4.8 to 5.6 cases per 100,000 per year in hospitalized patients 10. Per the Japanese National Survey, the incidence of thyroid storms was 0.2 per 100,000 people per year, about 0.22% of all thyrotoxicosis patients and 5.4% of hospitalized thyrotoxicosis patients 11. The average age of people with thyroid storm was 42 to 43 years, which was similar to people with thyrotoxicosis without thyroid storm. The male to female ratio for incidence of thyroid storm was about 1:3, similar to thyrotoxicosis without storm group 11.
Individuals at a risk of developing thyrotoxic crisis are usually the elderly, individuals with mental health issues, pregnant women, individuals with Addison’s disease and individuals with alcohol abuse 12.
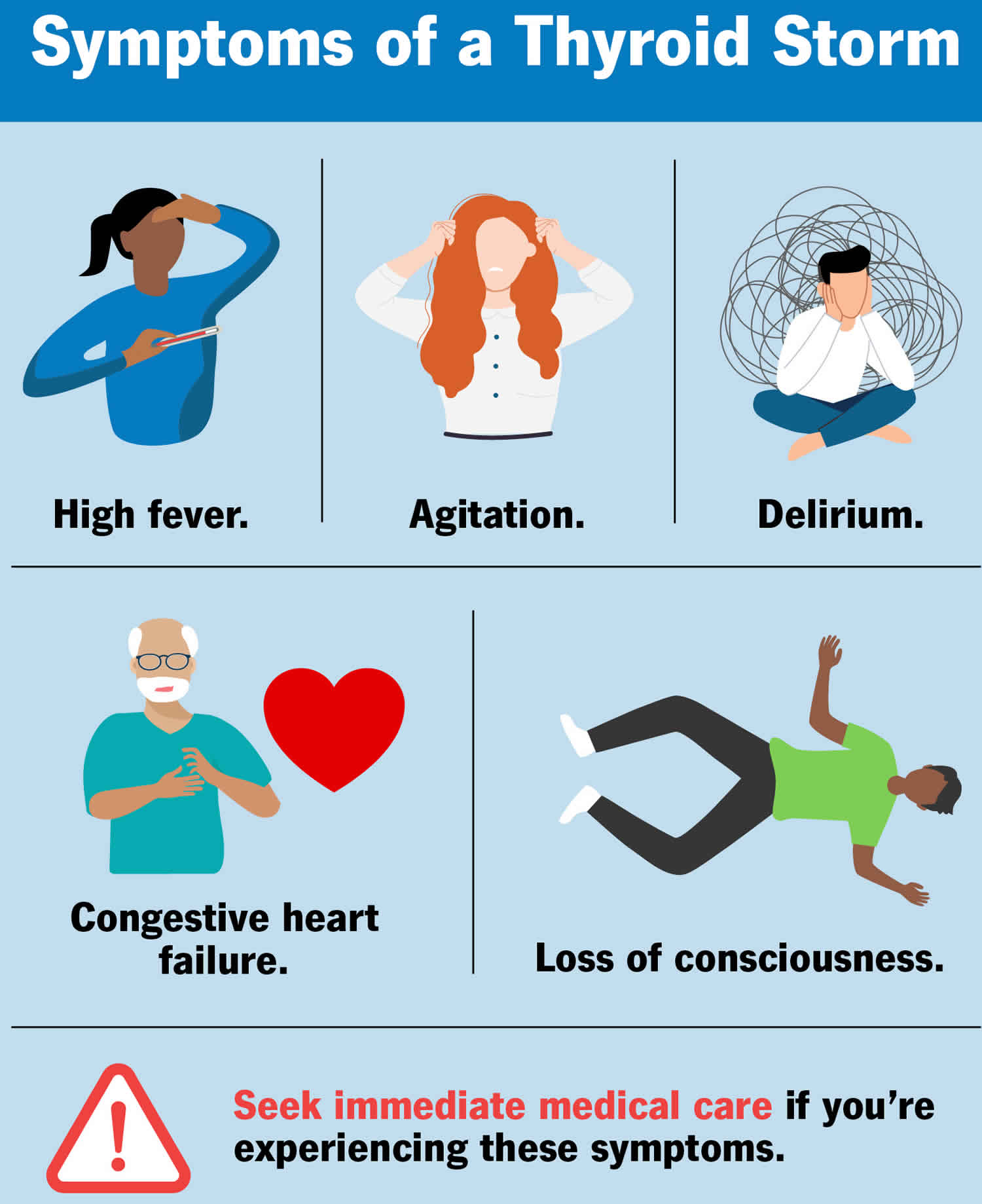
Patients may have a known history of thyrotoxicosis or hyperthyroidism. Thyrotoxic crisis symptoms include high fever, rapid heart rate (tachycardia), high blood pressure, heart failure, neurological (irritability, agitation, emotional lability, unconsciousness) and gastrointestinal (nausea, vomiting, diarrhea, jaundice, abdominal pain) symptoms 13.
If left untreated, thyrotoxic crisis can lead to the following complications:
- Arrhythmias (irregular heartbeats)
- High-output heart failure
- Seizures, delirium and coma
- Elevated liver enzymes and jaundice
- Abdominal cramps, vomiting, diarrhea
- Atrial fibrillation and thromboembolism 12
The diagnosis of thyrotoxic crisis is primarily clinical, and no specific laboratory tests are available.
In 1993, the following scoring system for the diagnosis of thyroid storm was introduced. Burch-Wartofsky Point Scale (BWPS) 14:
- Temperature: 5 points per 1 Fahrenheit above 99 °F (maximum 30 points)
- Central nervous system dysfunction: 10 points for mild (agitation), 20 for moderate (delirium, psychosis, or extreme lethargy), and 30 for severe (seizure or coma)
- Tachycardia: 5 (99-109 beats per minute), 10 (110 -119 beats per minute), 15 (120 -129 beats per minute), 20 (130 -139 beats per minute) and 25 (greater than 140 beats per minute)
- Presence of atrial fibrillation: 10
- Heart failure: 5 for mild (pedal edema), 10 for moderate (bi-basilar rales), 15 for severe (pulmonary edema)
- Gastrointestinal dysfunction: 10 for moderate (diarrhea, nausea/vomiting, or abdominal pain) and 20 for severe (unexplained jaundice)
- Presence of Precipitating factor: 10 points
Thyrotoxic crisis diagnosis: A score of more than 45 highly suggests thyroid storm, 25 to 44 supports the diagnosis, and less than 25 makes the diagnosis unlikely 14.
Thyrotoxic crisis patients are best managed in the intensive care unit (ICU) by a multidisciplinary team that includes an intensive care physician, cardiologist, endocrinologist, internist, emergency department physician and infectious disease expert.
If the patient’s clinical picture is consistent with thyroid storm, do not delay treatment pending laboratory confirmation of thyrotoxicosis 13. Results of thyroid studies are usually consistent with hyperthyroidism and are useful only if the patient has not been previously diagnosed 13. Usual findings include elevated triiodothyronine (T3), thyroxine (T4), and free T4 levels; increased T3 resin uptake; suppressed thyroid-stimulating hormone (TSH) levels; and an elevated 24-hour iodine uptake. TSH levels are not suppressed and in the rare instances of excess TSH secretion 13.
Chest radiography may reveal heart enlargement due to congestive heart failure. Radiography may also reveal pulmonary edema caused by heart failure and/or evidence of pulmonary infection 13.
Head computed tomography (head CT) scanning may be necessary to exclude other neurologic conditions if diagnosis is uncertain after the initial stabilization of a patient who presents with altered mental status 13.
The approach to treatment of thyrotoxic crisis includes the following:
- Supportive measures
- Antiadrenergic drugs
- Thionamides (a class of antithyroid drugs that inhibit thyroid hormone synthesis)
- Iodine preparations
- Glucocorticoids
- Bile acid sequestrants
- Treatment of the underlying condition
- Rarely plasmapheresis
Patients with contraindications to thionamides (antithyroid drugs that inhibit thyroid hormone synthesis) need to be managed with supportive measures, aggressive beta blockade, iodine preparations, glucocorticoids, and bile acid sequestrants for about a week in preparation for a thyroidectomy. Plasmapheresis may be attempted if other measures are not effective.
Treatment of thyrotoxic crisis
- Propylthiouracil: 600 mg orally given before iodine, then 400 mg every 6 hours
- Iodine: 5 drops saturated solution of potassium iodide orally 3 times a day OR 10 drops Lugol solution orally 3 times a day OR 1g sodium iodide slowly by intravenous drip over 24 hours
- Propranolol: 40 mg orally 4 times a day OR 1 mg slowly intravenously every 4 hours (not to exceed 1 mg/min) under close monitoring. A repeat 1-mg dose given after 2 minutes, if needed, or esmolol
- Intravenous dextrose solutions
- Correction of dehydration and electrolyte imbalance
- Cooling blanket for hyperthermia
- Antiarrhythmics (eg, calcium channel blockers, adenosine, beta-blockers) if necessary for atrial fibrillation
- Treatment of underlying disorder, such as infection
- Corticosteroids: Hydrocortisone 100 mg intravenously every 8 hours OR dexamethasone 8 mg intravenously once a day.
If untreated, thyrotoxic crisis is almost invariably fatal in adults (90% mortality rate) and is likely to cause a similarly severe outcome in children, although the condition is so rare in children that these data are not available. Death from thyrotoxic crisis may be a consequence of cardiac arrhythmia, congestive heart failure, hyperthermia, multiple organ failure or other factors 15, though the precipitating factor is often the cause of death.
With adequate thyroid-suppressive therapy and sympathetic blockade, clinical improvement should occur within 24 hours. Adequate therapy should resolve the crisis within a week. Treatment for adults has reduced mortality to less than 20%. In one retrospective study from Japan of 1324 patients who were diagnosed with thyrotoxic crisis, the overall mortality was 10% 16. In the same study, the following factors were associated with increased mortality risk in thyroid storm 16:
- Age 60 years or older
- Central nervous system (CNS) dysfunction at admission
- Lack of antithyroid drug and beta-blockade use
- Need for mechanical ventilation and plasma exchange along with hemodialysis
In addition, a study by Swee et al 17 of 28 patients with thyroid storm reported that central nervous system (CNS) dysfunction of greater than mild severity appeared to be a risk factor for mortality.
What does my thyroid do?
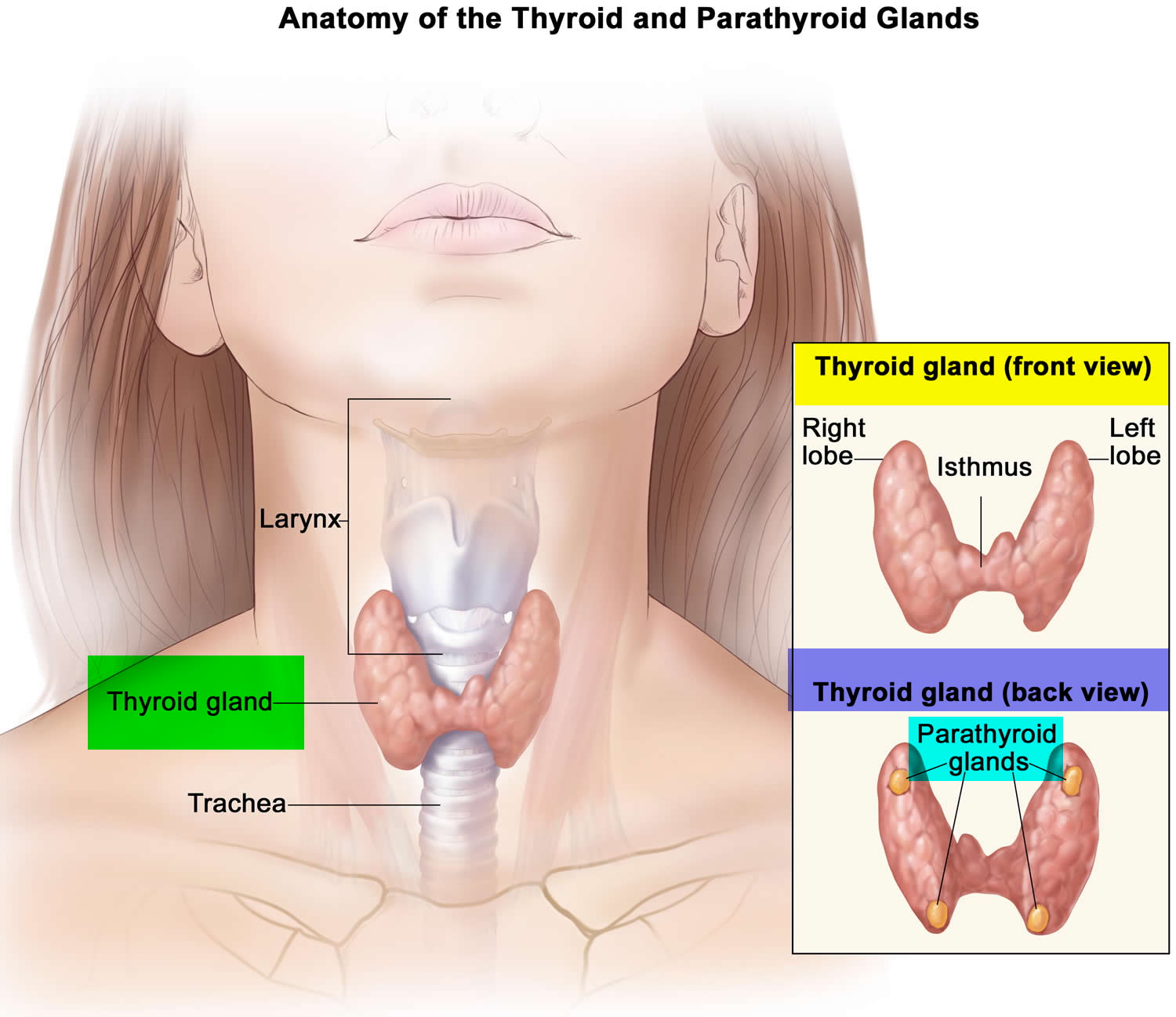
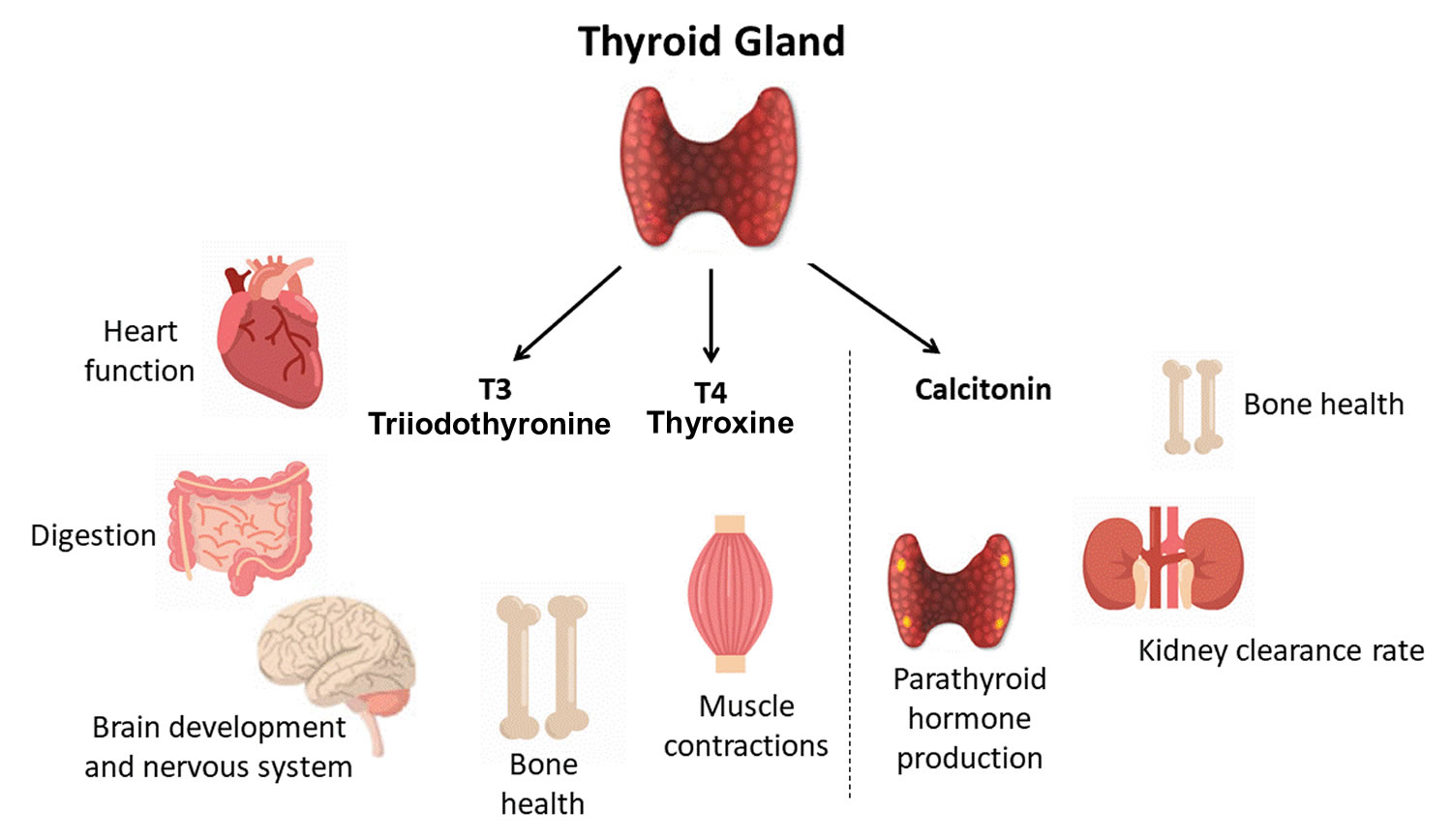
The thyroid gland is the largest adult gland to have a purely endocrine function, weighing about 25-30 g. The thyroid gland is a small butterfly shaped gland with 2 lobes, the right lobe and the left lobe joined by a narrow piece of the thyroid gland called the isthmus, that is located in front of your neck near the base of your throat, beneath the larynx (voice box or Adam’s apple). About 50% of thyroid glands have a small third lobe, called the pyramidal lobe. It extends superiorly from the isthmus. The thyroid gland makes and releases hormones. You can’t usually feel a thyroid gland that is normal.
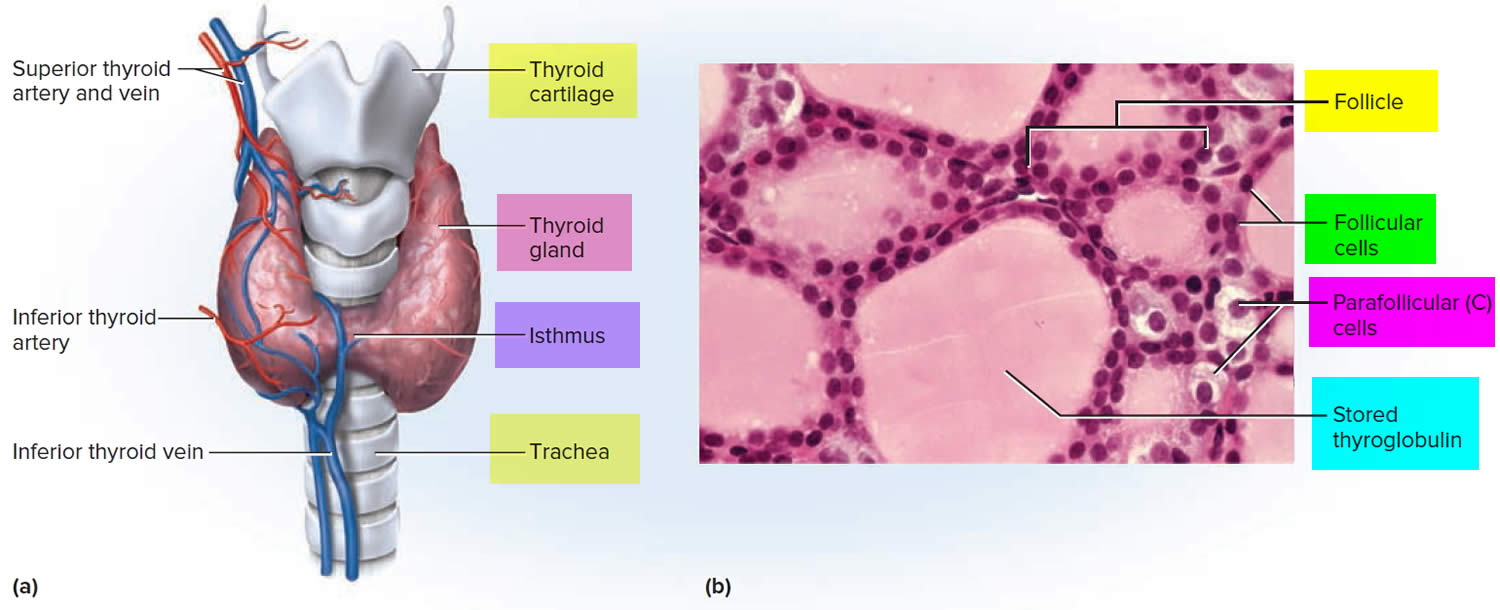
The thyroid gland has 2 main types of cells:
- Follicular cells use iodine from the blood to make thyroid hormones, which help regulate a person’s metabolism. Having too much thyroid hormone (hyperthyroidism) can cause a fast or irregular heartbeat, trouble sleeping, nervousness, hunger, weight loss, and a feeling of being too warm. Having too little thyroid hormone (hypothyroidism) causes a person to slow down, feel tired, and gain weight. The amount of thyroid hormone released by the thyroid gland is regulated by the pituitary gland at the base of the brain, which makes a substance called thyroid-stimulating hormone (TSH) (see Figure 4).
- C cells also called parafollicular cells at the periphery of the follicles that make calcitonin, a hormone that helps control how your body uses calcium. The parafollicular cells (C cells) respond to rising levels of blood calcium by secreting the hormone calcitonin. Calcitonin antagonizes (blocks) parathyroid hormone (PTH) and stimulates osteoblast activity, thus promoting calcium deposition and bone formation. It is important mainly in children, having relatively little effect in adults. Parathyroid hormone is made by parathyroid glands. These sit behind and are attached to the thyroid gland (see Figure 1).
Other, less common cells in the thyroid gland include immune system cells (lymphocytes) and supportive (stromal) cells.
Thyroid hormone is secreted or inhibited in response to fluctuations in metabolic rate. The brain monitors the body’s metabolic rate and stimulates thyroid hormone secretion through the action of thyrotropin-releasing hormone (TRH) and thyroid stimulating hormone (TSH) as depicted in figure 4.
The primary effect of thyroid hormone (TH) is to increase one’s metabolic rate. As a result, it raises oxygen consumption and has a calorigenic effect—it increases heat production. To ensure an adequate blood and oxygen supply to meet this increased metabolic demand, thyroid hormone also raises the breathing (respiratory) rate, heart rate, and strength of the heartbeat. It stimulates the appetite and accelerates the breakdown of carbohydrates, fats, and protein for fuel. Thyroid hormone also promotes alertness and quicker reflexes; growth hormone secretion; growth of the bones, skin, hair, nails, and teeth; and development of the fetal nervous system.
Figure 1. Thyroid gland location and parathyroid gland
Footnotes: Anatomy of the thyroid and parathyroid glands. The thyroid gland lies at the base of the throat near the trachea. It is shaped like a butterfly, with the right lobe and left lobe connected by a thin piece of tissue called the isthmus. The parathyroid glands are four pea-sized organs found in the neck near the thyroid. The thyroid and parathyroid glands make hormones.
Figure 2. Thyroid gland anatomy
Footnote: (a) Gross anatomy, anterior view. (b) Histology, showing the saccular thyroid follicles (the source of thyroid hormone) and nests of C cells (the source of calcitonin).
Formation, storage, and release of thyroid hormones
The thyroid gland is the only endocrine gland that stores its secretory product in large quantities—normally about a 100-day supply. Synthesis and secretion of triiodothyronine (T3) and thyroxine or tetraiodothyronine (T4) occurs as follows:
- Iodide trapping. Thyroid follicular cells trap iodide ions (I −) by actively transporting them from the blood into the cytosol. As a result, the thyroid gland normally contains most of the iodide in the body.
- Synthesis of thyroglobulin. While the follicular cells are trapping I −, they are also synthesizing thyroglobulin (TG), a large glycoprotein that is produced in the rough endoplasmic reticulum, modified in the Golgi complex, and packaged into secretory vesicles. The vesicles then undergo exocytosis, which releases thyroglobulin into the lumen of the follicle.
- Oxidation of iodide. Some of the amino acids in thyroglobulin are tyrosines that will become iodinated. However, negatively charged iodide (I −) ions cannot bind to tyrosine until they undergo oxidation (removal of electrons) to iodine: I −→ I. As the iodide ions are being oxidized, they pass through the membrane into the lumen of the follicle.
- Iodination of tyrosine. As iodine atoms (I) form, they react with tyrosines that are part of thyroglobulin molecules. Binding of one iodine atom yields monoiodotyrosine (T1), and a second iodination produces diiodotyrosine (T2). The thyroglobulin with attached iodine atoms, a sticky material that accumulates and is stored in the lumen of the thyroid follicle, is termed colloid.
- Coupling of monoiodotyrosine (T1) and diiodotyrosine (T2). During the last step in the synthesis of thyroid hormone, two diiodotyrosine (T2) molecules join to form thyroxine (T4) or one T1 and one T2 join to form triiodothyronine (T3).
- Pinocytosis and digestion of colloid. Droplets of colloid reenter follicular cells by pinocytosis and merge with lysosomes. Digestive enzymes in the lysosomes break down thyroglobulin, cleaving off molecules of triiodothyronine (T3) and thyroxine (T4).
- Secretion of thyroid hormones. Because T3 and T4 are lipid soluble, they diffuse through the plasma membrane into interstitial fluid and then into the blood. T4 normally is secreted in greater quantity than T3, but T3 is several times more potent. Moreover, after T4 enters a body cell, most of it is converted to T3 by removal of one iodine.
- Transport thyroid hormones in the blood. More than 99% of both the T3 and the T4 combine with transport proteins in the blood, mainly thyroxine binding globulin (TBG).
Figure 3. Thyroid hormones
Actions of thyroid hormones
Because most body cells have receptors for thyroid hormones, triiodothyronine (T3) and thyroxine (T4) affect tissues throughout the body. Thyroid hormones act on their target cells mainly by inducing gene transcription and protein synthesis. The newly formed proteins in turn carry out the cellular response.
Functions of thyroid hormones include the following:
- Increase basal metabolic rate. Thyroid hormones raise the basal metabolic rate (BMR), the rate of energy expenditure under standard or basal conditions (awake, at rest, and fasting). When basal metabolic rate increases, cellular metabolism of carbohydrates, lipids, and proteins increases. Thyroid hormones increase BMR in several ways: (1) They stimulate synthesis of additional Na+/K+ ATPases, which use large amounts of ATP to continually eject sodium ions (Na+) from cytosol into extracellular fluid and potassium ions (K+) from extracellular fluid into cytosol; (2) they increase the concentrations of enzymes involved in cellular respiration, which increases the breakdown of organic fuels and ATP production; and (3) they increase the number and activity of mitochondria in cells, which also increases ATP production. As cells produce and use more ATP, basal metabolic rate increases, more heat is given off and body temperature rises, a phenomenon called the calorigenic effect. In this way, thyroid hormones play an important role in the maintenance of normal body temperature. Normal mammals can survive in freezing temperatures, but those whose thyroid glands have been removed cannot.
- Enhance actions of catechlolamines. Thyroid hormones have permissive effects on the catecholamines (epinephrine and norepinephrine) because they up-regulate β-adrenergic receptors. Catecholamines bind to β-adrenergic receptors, promoting sympathetic responses. Therefore, symptoms of excess levels of thyroid hormone include increased heart rate, more forceful heartbeats, and increased blood pressure.
- Regulate development and growth of nervous tissue and bones. Thyroid hormones are necessary for the development of the nervous system: They promote synapse formation, myelin production, and growth of dendrites. Thyroid hormones are also required for growth of the skeletal system: They promote formation of ossification centers in developing bones, synthesis of many bone proteins, and secretion of growth hormone (GH) and insulin-like growth factors (IGFs). Deficiency of thyroid hormones during fetal development, infancy, or childhood causes severe mental retardation and stunted bone growth.
Control of thyroid hormone secretion
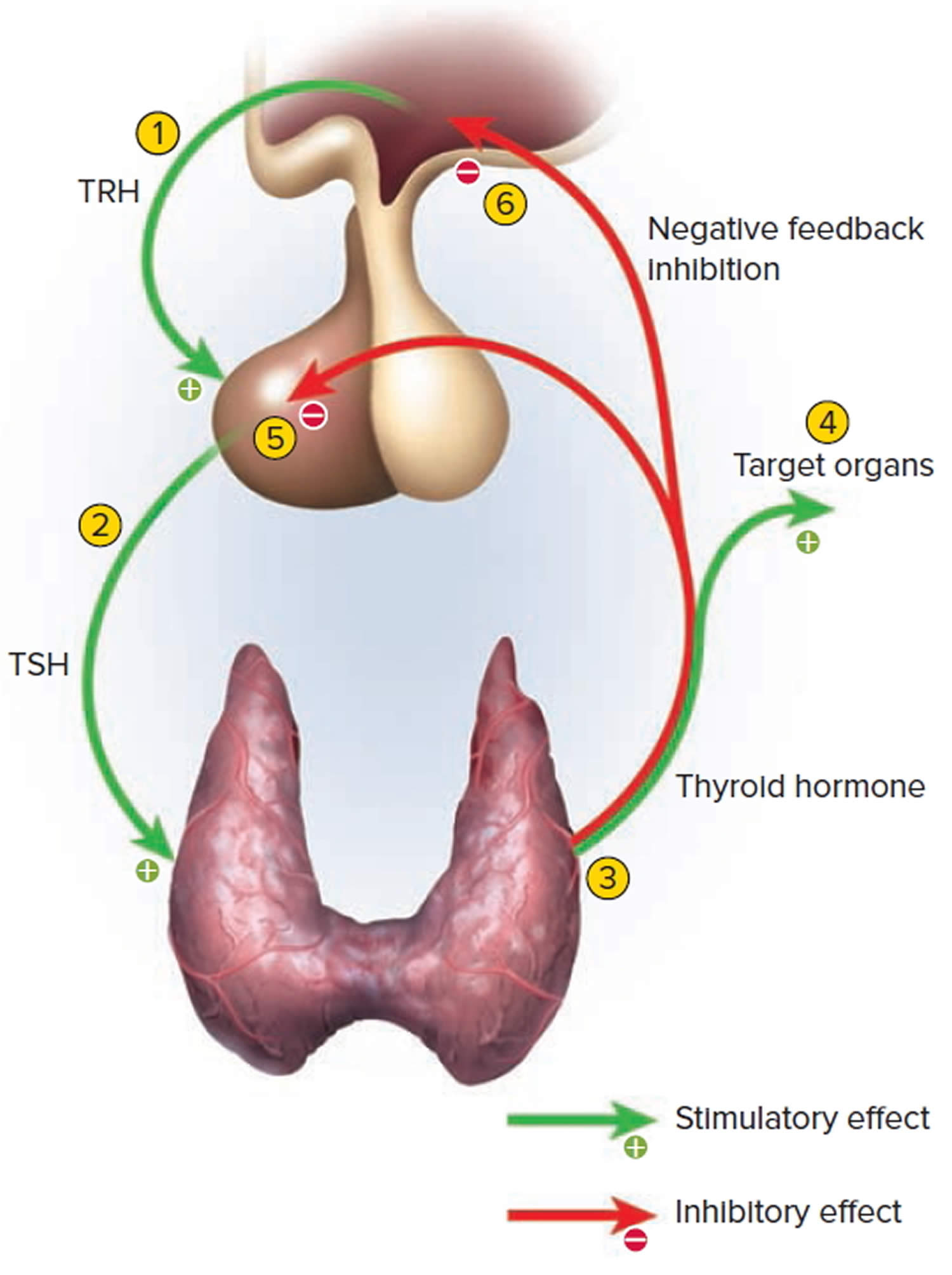
Thyrotropin-releasing hormone (TRH) from the hypothalamus and thyroid-stimulating hormone (TSH) from the anterior pituitary stimulate secretion of thyroid hormones, as shown in Figure 4:
- Low blood levels of T3 and T4 or low metabolic rate stimulate the hypothalamus to secrete thyrotropin-releasing hormone (TRH).
- Thyrotropin-releasing hormone (TRH) enters the hypothalamic–hypophyseal portal system and flows to the anterior pituitary, where it stimulates thyrotrophs to secrete thyroid stimulating hormone (TSH).
- Thyroid stimulating hormone (TSH) stimulates virtually all aspects of thyroid follicular cell activity, including iodide trapping, hormone synthesis and secretion, and growth of the follicular cells.
- The thyroid follicular cells release T3 and T4 into the blood until the metabolic rate returns to normal.
- An elevated level of T3 inhibits release of TRH and TSH (negative feedback inhibition).
Conditions that increase ATP demand—a cold environment, hypoglycemia, high altitude, and pregnancy—increase the secretion of the thyroid hormones.
Figure 4. Control of thyroid hormone secretion
Footnote: Negative Feedback Inhibition of the Anterior Pituitary Gland by the Thyroid Gland
Control of calcium balance
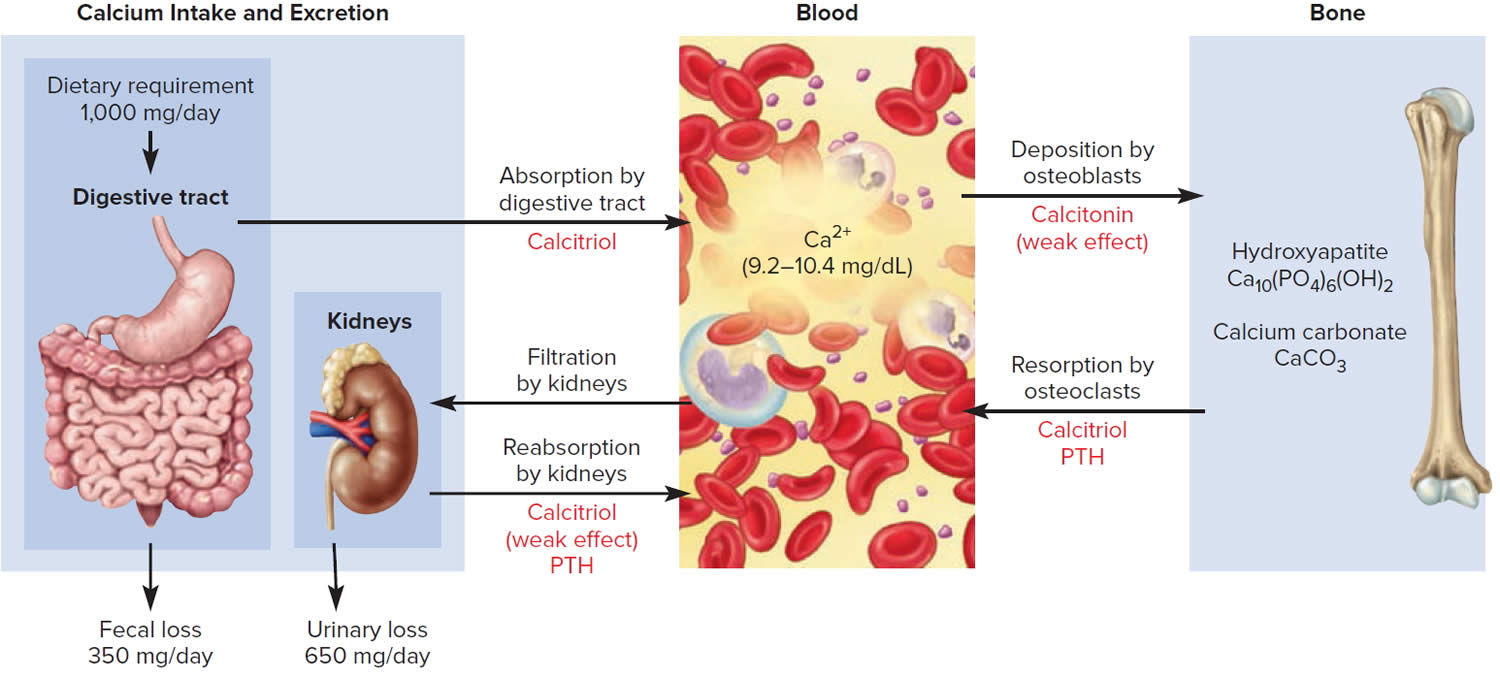
The hormone produced by the parafollicular cells of the thyroid gland is calcitonin. Calcitonin can decrease the level of calcium in the blood by inhibiting the action of osteoclasts, the cells that break down bone extracellular matrix. The secretion of calcitonin is controlled by a negative feedback system (see Figure 5).
Calcitonin is produced by C cells (clear cells) of the thyroid gland. It is secreted when the blood calcium concentration rises too high, and it lowers the concentration by two principal mechanisms:
- Osteoclast inhibition. Within 15 minutes after it is secreted, calcitonin reduces osteoclast activity by as much as 70%, so osteoclasts liberate less calcium from the skeleton.
- Osteoblast stimulation. Within an hour, calcitonin increases the number and activity of osteoblasts, which deposit calcium into the skeleton.
Calcitonin plays an important role in children but has only a weak effect in most adults. The osteoclasts of children are highly active in skeletal remodeling and release 5 g or more of calcium into the blood each day. By inhibiting this activity, calcitonin can significantly lower the blood calcium level in children. In adults, however, the osteoclasts release only about 0.8 g of calcium per day. Calcitonin cannot change adult blood calcium very much by suppressing this lesser contribution. Calcitonin deficiency is not known to cause any adult disease. Calcitonin may, however, inhibit bone loss in pregnant and lactating women. Miacalcin, a calcitonin extract derived from salmon that is 10 times more potent than human calcitonin, is prescribed to treat osteoporosis.
Figure 5. Hormonal control of calcium balance
Footnote: The central panel represents the blood reservoir of calcium and shows its normal (safe) range. Calcitriol and Parathyroid Hormone (PTH) regulate calcium exchanges between the blood and the small intestine and kidneys (left). Calcitonin, calcitriol, and Parathyroid Hormone (PTH) regulate calcium exchanges between blood and bone (right).
Thyrotoxic crisis causes
Thyrotoxic crisis is more common in people with Graves disease but can occur with other causes of hyperthyroidism, such as toxic multinodular goiter and toxic thyroid adenoma 14.
Thyrotoxic crisis is precipitated by the following factors in individuals with thyrotoxicosis:
- Abrupt discontinuation or noncompliance with antithyroid medicine
- Thyroid surgery
- Non-thyroid surgery
- Direct trauma to the thyroid gland
- Trauma
- Acute illness like infections, diabetic ketoacidosis, acute myocardial infarction, cardiovascular accident, cardiac failure, drug reaction
- Childbirth
- Recent use of iodinated contrast medium
- Radioactive iodine (RAI) therapy (rare) 18
- Burns
- Sepsis
- Surgery
- Anesthesia induction 19
- Drugs (anticholinergic and adrenergic drugs, eg, pseudoephedrine; salicylates; nonsteroidal anti-inflammatory drugs [NSAIDs]; chemotherapy
- Medication side effects, eg, amiodarone, anesthetics, salicylates 20
- Excessive thyroid hormone ingestion
- Diabetic ketoacidosis 21
- Vigorous palpation of an enlarged thyroid
- Hyperemesis gravidarum in pregnancy 22
- Toxemia of pregnancy and labor in older adolescents; molar pregnancy
- Stroke or traumatic brain injury 23
- Intense exercise
- Ischemic heart disease
- Adrenocortical insufficiency
- Extraction of teeth
- Severe emotional stress
- Infection
Thyrotoxic crisis can occur in children with thyrotoxicosis from any cause but is most commonly associated with Graves disease. Other reported causes of thyrotoxicosis associated with thyroid storm include the following:
- Transplacental passage of maternal thyroid-stimulating immunoglobulins in neonates
- McCune-Albright syndrome with autonomous thyroid function 24
- Hyperfunctioning thyroid nodule
- Hyperfunctioning multinodular goiter
- Thyroid-stimulating hormone (TSH)-secreting tumor
Thyrotoxic crisis pathophysiology
The pathophysiological basis for the precipitation of thyrotoxic crisis in patients with thyrotoxicosis is not clear 1. But, as mentioned above, a precipitating factor is always required to cause a thyroid storm. Several hypotheses have been proposed. One theory suggests the incidence of thyroid storm is due to the rapid increase in thyroid hormone levels rather than the absolute hormone level that occurs during thyroid surgery, following radioactive iodine treatment, after sudden discontinuation of the antithyroid drug, or after administration of the large dose of iodine in contrast studies 1. The hyperactivity of the sympathetic nervous system with increased response to catecholamine and an increased cellular response to thyroid hormone during acute stress or infections, causing cytokines release and altered immunological disturbances, are other possible mechanisms of thyroid storm. Most studies have failed to relate higher thyroid hormone levels as a cause of thyroid storm, except for the study by Brooks and others, which reported higher free thyroid hormone among the patients with thyroid storm 25. In other words, the degree of thyroid hormone level is not directly related to a higher incidence of thyroid storm 14.
The clinical features are due to the exaggerated effects of the thyroid hormone. There is an intense metabolic activity that increases oxygen requirements. The resulting tachycardia to meet the oxygen requirements can induce heart failure and predispose the patient to arrhythmias. Similarly, central nervous system symptoms include irritability, seizures, delirium, and eventually coma.
Thyrotoxic crisis signs and symptoms
Thyrotoxic crisis signs and symptoms include 14:
- Fever
- Profuse sweating
- Poor feeding and weight loss
- Respiratory distress
- Fatigue (more common in older adolescents)
Thyrotoxic crisis gastrointestinal signs and symptoms include 14:
- Nausea and vomiting
- Diarrhea
- Abdominal pain
- Jaundice 26
Thyrotoxic crisis neurologic signs and symptoms include the following 14:
- Anxiety (more common in older adolescents)
- Altered behavior
- Seizures, coma
Presentation of thyrotoxic crisis is an exaggerated manifestation of hyperthyroidism, with the presence of an acute precipitating factor. Fever, cardiovascular involvement (including tachycardia, heart failure, arrhythmia), central nervous system (CNS) manifestations, and gastrointestinal symptoms are common. Fever of 104° F to 106°F (40 °C to 41.1 °C) with excessive sweating is a key presenting feature. Cardiovascular manifestations include tachycardia of more than 140 beats/minute, heart failure with pulmonary edema and peripheral edema, hypotension, arrhythmia, and death from cardiac arrest. Central nervous system (CNS) involvement includes agitation, delirium, anxiety, psychosis, or coma. Gastrointestinal (GI) symptoms include nausea, vomiting, diarrhea, abdominal pain, intestinal obstruction, and acute hepatic failure 27. A Japanese study found the central nervous system (CNS) involvement to be a poor prognostic factor for increased mortality 11.
Physical examination findings may include high temperature, tachycardia, orbitopathy, goiter, hand tremors, moist and warm skin, hyperreflexia, systolic hypertension, and jaundice.
Physical findings include the following:
Fever
- Temperature consistently exceeds 101.3 °F (38.5°C).
- Patients may progress to hyperpyrexia.
- Temperature frequently exceeds 105.8 °F (41°C).
Excessive sweating
Cardiovascular signs
- Hypertension with wide pulse pressure
- Hypotension in later stages with shock
- Tachycardia disproportionate to fever
- Signs of high-output heart failure
- Cardiac arrhythmia (Supraventricular arrhythmias are more common, [eg, atrial flutter and fibrillation], but ventricular tachycardia may also occur.)
Neurologic signs
- Agitation and confusion
- Hyperreflexia and transient pyramidal signs
- Tremors, seizures
- Coma
Signs of thyrotoxicosis
- Orbital signs
- Goiter
- Rhabdomyolysis – Rare cases have been reported following a diagnosis of thyroid storm in adults 28
Thyrotoxic crisis complications
Complications of thyrotoxic crisis include:
- High output cardiac failure
- Cardiac arrhythmias
- Delirium, seizures, coma
- Abdominal pain, diarrhea, vomiting, jaundice
- Elevation of transaminases
A study by Mohananey et al 29 found that out of 41,835 US patients with thyroid storm, 1% developed cardiogenic shock, with the incidence of this complication in these patients rising between 2003 and 2011 from 0.5% to 3%. However, during this same period the mortality rate from cardiogenic shock in patients with thyroid storm decreased from 60.5% to 20.9%. The highest likelihood of cardiogenic shock was in male patients with preexisting atherosclerotic or structural heart disease 29.
Thyrotoxic crisis diagnosis
The diagnosis of thyrotoxic crisis is primarily clinical, and no specific laboratory tests are available.
Medical history
Patients may have a known history of thyrotoxicosis. In the absence of previously diagnosed thyrotoxicosis, the history may include symptoms such as irritability, agitation, emotional lability, a voracious appetite with poor weight gain, excessive sweating and heat intolerance, and poor school performance caused by decreased attention span.
Thyrotoxic Crisis Diagnostic Criteria
In 1993, Burch and Wartofsky published diagnostic criteria and a scoring system for the diagnosis of thyroid storm based on clinical features 14. Burch and Wartofsky diagnostic criteria for the diagnosis of thyroid storm (Table 1) include fever, tachycardia, arrhythmias, congestive heart failure, agitation, delirium, psychosis, stupor, and coma, as well as nausea, vomiting, diarrhea, hepatic failure, and the presence of an identified precipitant 14. Points in the Burch and Wartofsky diagnostic criteria are based on the severity of individual manifestations, with a score total of ≥45 consistent with thyroid storm, a score between 25 and 44 is suggestive of impending thyroid storm, and <25 points making thyroid storm unlikely (see Table 1 below).
More recently, an additional empirically defined diagnostic system has been proposed by the Japanese Thyroid Association (JTA) 11. The JTA system uses combinations of similar clinical features to assign patients to the diagnostic categories Definite Thyroid Storm (TS1) or Suspected Thyroid Storm (TS2). The Japanese Thyroid Association (JTA) 11 have formulated diagnostic criteria based on the review of clinical manifestations, namely central nervous system, cardiovascular, gastrointestinal and hepatic signs and symptoms and multi-organ failure. The prerequisite for the diagnosis of thyroid storm is a definite biochemical evidence of thyrotoxicosis.
These scoring systems are just guidelines. The actual diagnosis of thyroid storm is based on clinical judgment. Data comparing these two diagnostic systems suggest an overall agreement, but a tendency toward underdiagnosis using the Japanese Thyroid Association (JTA) categories of TS1 and TS2, compared to a Burch and Wartofsky Point Scale ≥45 11, 30, 27, 31.
Based on the Burch and Wartofsky Point Scale scoring system, a score of 45 or more is more sensitive but less specific than the Japanese Thyroid Association (JTA) scoring systems TS1 or TS2 to detect thyroid storm cases. A Burch and Wartofsky Point Scale score of 25 to 45 may suggest an impending storm. A chest X-ray may help assess heart failure, and a head CT may help exclude a neurological cause in some patients. An ECG is often done to monitor for arrhythmias.
Table 1. Burch and Wartofsky thyroid storm diagnostic criteria
| Criteria | Points | Criteria | Points |
|---|---|---|---|
| Thermoregulatory dysfunction | Gastrointestinal–hepatic dysfunction | ||
| Temperature (°F) b | Manifestation | ||
| 99.0–99.9 | 5 | Absent | 0 |
| 100.0–100.9 | 10 | Moderate (diarrhea, abdominal pain, nausea/vomiting) | 10 |
| 101.0–101.9 | 15 | Severe (jaundice) | 20 |
| 102.0–102.9 | 20 | ||
| 103.0–103.9 | 25 | ||
| ≥104.0 | 30 | ||
| Cardiovascular | Central nervous system disturbance | ||
| Tachycardia (beats per minute) | Manifestation | ||
| 100–109 | 5 | Absent | 0 |
| 110–119 | 10 | Mild (agitation) | 10 |
| 120–129 | 15 | Moderate (delirium, psychosis, extreme lethargy) | 20 |
| 130–139 | 20 | Severe (seizure, coma) | 30 |
| ≥140 | 25 | ||
| Atrial fibrillation | |||
| Absent | 0 | ||
| Present | 10 | ||
| Congestive heart failure | Precipitant history | ||
| Absent | 0 | Status | |
| Mild | 5 | Positive | 0 |
| Moderate | 10 | Negative | 10 |
| Severe | 20 | ||
| Scores totaled | |||
| >45 | Thyroid storm | ||
| Impending storm | |||
| <25 | Storm unlikely | ||
Footnotes: b Celsius 37.2–37.7 (5), 37.8–38.3 (10), 38.3–38.8 (15), 38.9–39.4 (20), 39.4–39.9 (25), ≥40 (30 points).
[Source 14 ]Japanese Thyroid Association (JTA) thyroid storm diagnostic criteria
The Japanese Thyroid Association (JTA) thyroid storm diagnostic criteria is a different scoring system based on similar clinical findings 11. Thyrotoxicosis (elevated free T3 and/or free T4) is a prerequisite, and it requires various combinations of the following symptoms 11:
- Central nervous system manifestation (restlessness, delirium, psychosis/mental aberration, lethargy/somnolence, coma)
- Fever (38 °C/100.4 °F or greater)
- Tachycardia (130/min or higher)
- Congestive heart failure (pulmonary edema, rales, cardiogenic shock, or NYHA class 4)
- Gastrointestinal and liver manifestation (Nausea, vomiting, diarrhea, total Bilirubin 3 mg/dl or more
Thyroid storm diagnosis 11:
- Definite Thyroid Storm (TS1): Thyrotoxicosis (elevated free T3 and/or free T4) plus at least 1 central nervous system manifestation plus 1 or more other symptoms (fever, tachycardia, congestive heart failure, gastrointestinal/liver) ‘OR’ A combination of at least 3 features among fever, gastrointestinal/liver, congestive heart failure, or tachycardia
- Suspected Thyroid Storm (TS2): Thyrotoxicosis (elevated free T3 and/or free T4) plus a combination of at least 2 features among tachycardia, congestive heart failure, gastrointestinal/liver, fever ‘OR’ a patient with thyroid disease, presence of goiter and exophthalmos who meets criteria for TS1 but thyroid function tests not available.
Thyrotoxic crisis differential diagnosis
Thyrotoxic crisis should be differentiated from other diseases with similar symptoms and signs 32. Fever is the most common presentation of multiple conditions; therefore, it can be misdiagnosed.
Thyrotoxic crisis differential diagnoses include 1:
- Sepsis
- Infection
- Psychosis
- Cocaine use
- Pheochromocytoma
- Neuroleptic malignant syndrome
- Hyperthermia
Thyrotoxic crisis treatment
Treatment of thyrotoxic crisis consists of supportive measures like intravenous (IV) fluids, oxygen, cooling blankets, acetaminophen, as well as specific measures to treat hyperthyroidism. If any precipitating factors, for example, infection, are present, that needs to be taken care. Patients with thyroid storm must be admitted to the intensive care unit (ICU) with close cardiac monitoring and ventilatory support if needed 5, 33.
Specific strategic steps for thyrotoxic crisis treatment 1:
- Therapy to control increased adrenergic tone: Beta-blocker
- Therapy to reduce thyroid hormone synthesis: Thionamide
- Therapy to reduce release of thyroid hormone: Iodine solution
- Therapy to block peripheral conversion of T4 to T3: Iodinated radiocontrast agent, glucocorticoid, propylthiouracil (PTU), propranolol
- Therapy to reduce enterohepatic recycling of thyroid hormone: Bile acid sequestrant
After initial supportive measures, a beta blocker should be started for any case of suspected thyroid storm. Typically, propranolol 40 mg to 80 mg is given every 4 to 6 hours. Then, either a loading dose of propylthiouracil (PTU) 500 mg to 1000 mg followed by 250 mg every 4 hours or Methimazole (MMI) 20 mg every 4 to 6 hours should be given. Propylthiouracil is favored because it has a small but additional effect of blocking the peripheral conversion of T4 to T3. An hour after the administration of propylthiouracil or Methimazole, give five drops of SSKI (super saturated potassium iodide) by mouth every 6 hours. Always administer thionamide before starting iodine solution (SSKI) therapy 34. This prevents the eminent increase in thyroid hormone synthesis due to increased iodine load from super saturated potassium iodide. Hydrocortisone 100 mg IV every eight hours (or Dexamethasone 2 mg every 6 hours) should also be started. If available, oral cholestyramine 4 grams four times daily can be started for severe cases. One should look for precipitating factors and treat them accordingly. Use of aspirin should be avoided due to its potential risk of increasing free thyroid hormone levels by interfering with thyroid binding protein.
In the first 24 hours of treatment, propylthiouracil decreases T3 level by 45%, but Methimazole drops T3 level by only 10 % to 15%. Methimazole, whereas, causes more rapid normalization of serum T3 level after few weeks of treatment and it has less hepatotoxicity compared to propylthiouracil. Therefore, after initial stabilization, we should treat with Methimazole (if propylthiouracil was started at the beginning, it should be changed to Methimazole). For patients who cannot take oral antithyroid medicine, liquid preparation (pharmacist may have to compound) can be given as enemas. Sometimes, pharmacists can prepare IV form of antithyroid medicine by dissolving the tablet 35, 36, 37.
Esmolol, a short-acting beta blocker, at a loading dose of 250 mcg/kg to 500 mcg/kg followed by 50 mcg/kg to 100 mcg/kg/minute can be given in ICU setting. Cardiovascular beta-blockers like atenolol or metoprolol should be chosen for patients with reactive airway disease. If there is a contraindication for using beta-blockers, diltiazem is an alternative.
If thionamide therapy is contraindicated because of an allergic reaction, thyroidectomy is needed after treatment with a beta-blocker, hydrocortisone, cholestyramine, and iodine solution. Plasmapheresis is the last resort if all other measures fail.
Once patients’ clinical conditions improve, the iodine solution should be stopped, glucocorticoids can be tapered and stopped, and beta-blockers should be adjusted. Thionamide therapy should be titrated, and if propylthiouracil is used initially, it should be switched to methimazole. Patients should be recommended for definitive treatment with radioiodine (RAI) therapy or thyroidectomy. Surgery may be required in patients with Graves disease to treat hyperthyroidism. These patients must be pretreated with beta-blockers, glucocorticoids, and iodine formulas. Surgery is usually done after 5 to 7 days. As recently reported, therapeutic plasma exchange is potentially helpful in resistant cases that do not respond to conventional treatments 38, 35.
Surgery
If toxic adenoma or multinodular goiter is causing thyroid storm, then surgical resection and radioactive iodine ablation are the mainstays of treatment 1. Thyroidectomy is preferred if a patient has compressive symptoms. Patients who refuse ablation or have contraindications to surgery can be treated with long-term antithyroid medicines 39. Grave’s disease with intrathoracic mass causes severe compressive symptoms, and thyroidectomy is preferred in these cases. Thyroidectomy has other adverse effects, like recurrent laryngeal nerve damage and hypoparathyroidism 40, 41.
Radioactive iodine (I-131) therapy
Radioactive iodine (I-131) therapy is helpful in hyperfunctioning nodules, but the risk of hypothyroidism is 60% after a 20-year follow-up in a retrospective study 42. Other factors increasing the risk of hypothyroidism in these patients are:
- Methimazole therapy
- Age
Thyrotoxic crisis prognosis
Thyrotoxic crisis is a rare life-threatening condition that can affect people with hyperthyroidism (overactive thyroid). A thyrotoxic crisis is a medical emergency that is fatal if left untreated. However, despite modern advancements in its treatment and supportive measures, the mortality associated with thyroid storm is estimated to be 8 to 25% 5, 1. In the Japan Thyroid Association study carried out by Akamizu et al. 11, a 10% mortality rate was reported for thyroid storm and the presence of multi-organ failure was an independent prognostic factor for death. A higher incidence of irreversible neurological defects including psychosis was observed in this cohort 11.
The causes of death in people with thyroid storm include be heart failure, irregular heartbeats (arrhythmias) or multiple organ failure. However, with treatment, most patients see an improvement within 24 hours. Risk factors for poor prognosis include 1:
- Advanced age
- Neurological deficits on admission 27
- Failure to use beta-blockers and antithyroid medications
- Need for dialysis and/or mechanical ventilation
- Pokhrel B, Aiman W, Bhusal K. Thyroid Storm. [Updated 2022 Oct 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448095[↩][↩][↩][↩][↩][↩][↩][↩]
- Wartofsky L. Thyrotoxic storm. In: Braverman L, editor; Utiger R, editor. Werner & Ingbar’s the Thyroid. 9th. Williams & Wilkins; Philadelphia, PA: 2005. pp. 651–657.[↩]
- Gavin LA. Thyroid crises. Med Clin North Am. 1991 Jan;75(1):179-93. doi: 10.1016/s0025-7125(16)30478-3[↩]
- Tietgens ST, Leinung MC. Thyroid storm. Med Clin North Am. 1995 Jan;79(1):169-84. doi: 10.1016/s0025-7125(16)30090-6[↩]
- Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016 Oct;26(10):1343-1421. doi: 10.1089/thy.2016.0229. Erratum in: Thyroid. 2017 Nov;27(11):1462. doi: 10.1089/thy.2016.0229.correx[↩][↩][↩]
- McDermott MT, Kidd GS, Dodson LE Jr, Hofeldt FD. Radioiodine-induced thyroid storm. Case report and literature review. Am J Med. 1983 Aug;75(2):353-9. doi: 10.1016/0002-9343(83)91217-2[↩]
- Walter MA, Briel M, Christ-Crain M, Bonnema SJ, Connell J, Cooper DS, Bucher HC, Müller-Brand J, Müller B. Effects of antithyroid drugs on radioiodine treatment: systematic review and meta-analysis of randomised controlled trials. BMJ. 2007 Mar 10;334(7592):514. doi: 10.1136/bmj.39114.670150.BE[↩]
- Blick C, Nguyen M, Jialal I. Thyrotoxicosis. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482216[↩]
- Bartalena L, Fatourechi V. Extrathyroidal manifestations of Graves’ disease: a 2014 update. J Endocrinol Invest. 2014 Aug;37(8):691-700. doi: 10.1007/s40618-014-0097-2[↩]
- Galindo RJ, Hurtado CR, Pasquel FJ, García Tome R, Peng L, Umpierrez GE. National Trends in Incidence, Mortality, and Clinical Outcomes of Patients Hospitalized for Thyrotoxicosis With and Without Thyroid Storm in the United States, 2004-2013. Thyroid. 2019 Jan;29(1):36-43. doi: 10.1089/thy.2018.0275[↩]
- Akamizu T, Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, Tsuboi K, Monden T, Kouki T, Otani H, Teramukai S, Uehara R, Nakamura Y, Nagai M, Mori M; Japan Thyroid Association. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012 Jul;22(7):661-79. doi: 10.1089/thy.2011.0334 Erratum in: Thyroid. 2012 Sep;22(9):979.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Min T, Benjamin S, Cozma L. Thromboembolic complications of thyroid storm. Endocrinol Diabetes Metab Case Rep. 2014;2014:130060. doi: 10.1530/EDM-13-0060[↩][↩]
- Thyroid Storm. https://emedicine.medscape.com/article/925147-overview[↩][↩][↩][↩][↩][↩]
- Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am. 1993 Jun;22(2):263-77.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- De Groot LJ, Bartalena L, De Groot LJ, Chrousos G, Dungan K, Feingold KR, et al. Thyroid Storm. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. SourceEndotext [Internet]. South Dartmouth (MA): MDText.com, Inc. 2000.[↩]
- Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Factors Associated With Mortality of Thyroid Storm: Analysis Using a National Inpatient Database in Japan. Medicine (Baltimore). 2016 Feb. 95 (7):e2848.[↩][↩]
- Swee du S, Chng CL, Lim A. Clinical characteristics and outcome of thyroid storm: a case series and review of neuropsychiatric derangements in thyrotoxicosis. Endocr Pract. 2015 Feb 1. 21 (2):182-9.[↩]
- Kadmon PM, Noto RB, Boney CM, et al. Thyroid storm in a child following radioactive iodine (RAI) therapy: a consequence of RAI versus withdrawal of antithyroid medication. J Clin Endocrinol Metab. 2001 May. 86(5):1865-7[↩]
- Hirvonen EA, Niskanen LK, Niskanen MM. Thyroid storm prior to induction of anaesthesia. Anaesthesia. 2004 Oct. 59(10):1020-2[↩]
- Raza MA, Jain A, Mumtaz M, Mehmood T. Thyroid Storm in a Patient on Chronic Amiodarone Treatment. Cureus. 2022 Apr 15;14(4):e24164. doi: 10.7759/cureus.24164[↩]
- Ikeoka T, Otsuka H, Fujita N, et al. Thyroid Storm Precipitated by Diabetic Ketoacidosis and Influenza A: A Case Report and Literature Review. Intern Med. 2017. 56 (2):181-5.[↩]
- Zimmerman CF, Ilstad-Minnihan AB, Bruggeman BS, Bruggeman BJ, Dayton KJ, Joseph N, Moas DI, Rohrs HJ. Thyroid Storm Caused by Hyperemesis Gravidarum. AACE Clin Case Rep. 2022 Jan 3;8(3):124-127. doi: 10.1016/j.aace.2021.12.005[↩]
- Gong S, Hong W, Wu J, Xu J, Zhao J, Zhang X, Liu Y, Yu RG. Cerebral venous sinus thrombosis caused by traumatic brain injury complicating thyroid storm: a case report and discussion. BMC Neurol. 2022 Jul 7;22(1):248. doi: 10.1186/s12883-022-02777-0[↩]
- Lawless ST, Reeves G, Bowen JR. The development of thyroid storm in a child with McCune-Albright syndrome after orthopedic surgery. Am J Dis Child. 1992 Sep. 146(9):1099-102[↩]
- Brooks MH, Waldstein SS. Free thyroxine concentrations in thyroid storm. Ann Intern Med. 1980 Nov;93(5):694-7. doi: 10.7326/0003-4819-93-5-694[↩]
- Hasan MK, Tierney WM, Baker MZ. Severe cholestatic jaundice in hyperthyroidism after treatment with 131-iodine. Am J Med Sci. 2004 Dec;328(6):348-50. doi: 10.1016/s0002-9629(15)33945-8[↩]
- Angell TE, Lechner MG, Nguyen CT, Salvato VL, Nicoloff JT, LoPresti JS. Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study. J Clin Endocrinol Metab. 2015 Feb;100(2):451-9. doi: 10.1210/jc.2014-2850[↩][↩][↩]
- Umezu T, Ashitani K, Toda T, Yanagawa T. A patient who experienced thyroid storm complicated by rhabdomyolysis, deep vein thrombosis, and a silent pulmonary embolism: a case report. BMC Res Notes. 2013 May 20. 6(1):198.[↩]
- Mohananey D, Smilowitz N, Villablanca PA, et al. Trends in the Incidence and In-Hospital Outcomes of Cardiogenic Shock Complicating Thyroid Storm. Am J Med Sci. 2017 Aug. 354 (2):159-64.[↩][↩]
- Warmock A, Cooper DS, Burch HB 2014 Life-threatening thyrotoxicosis: thyroid storm and adverse effects of anti-thyroid drugs. In: Matflin D, ed. Medical Emergencies in Endocrinology. Endocrine Press, Endocrine Society, Washington, DC.[↩]
- Swee du S, Chng CL, Lim A. Clinical characteristics and outcome of thyroid storm: a case series and review of neuropsychiatric derangements in thyrotoxicosis. Endocr Pract. 2015 Feb;21(2):182-9. doi: 10.4158/EP14023.OR[↩]
- Idrose AM. Acute and emergency care for thyrotoxicosis and thyroid storm. Acute Med Surg. 2015 May 12;2(3):147-157. doi: 10.1002/ams2.104[↩]
- Sarlis NJ, Gourgiotis L. Thyroid emergencies. Rev Endocr Metab Disord. 2003 May;4(2):129-36. doi: 10.1023/a:1022933918182[↩]
- Goodier CG. Endocrine Emergencies in Obstetrics. Clin Obstet Gynecol. 2019 Jun;62(2):339-346.[↩]
- Ba JH, Wu BQ, Wang YH, Shi YF. Therapeutic plasma exchange and continuous renal replacement therapy for severe hyperthyroidism and multi-organ failure: A case report. World J Clin Cases. 2019 Feb 26;7(4):500-507. doi: 10.12998/wjcc.v7.i4.500[↩][↩]
- Kalpakam H, Dhooria S, Agarwal R, Mukherjee S, Sehgal IS. A rare complication of bedside tracheotomy: Thyroid crisis. Lung India. 2019 Jan-Feb;36(1):77-79. doi: 10.4103/lungindia.lungindia_238_18[↩]
- Andrade Luz I, Pereira T, Catorze N. Thyroid storm: a case of haemodynamic failure promptly reversed by aggressive medical therapy with antithyroid agents and steroid pulse. BMJ Case Rep. 2018 Dec 14;11(1):e226669. doi: 10.1136/bcr-2018-226669[↩]
- Tan AWK, Lim BSP, Hoe JKM, Hoi WH, Leow MKS. Therapeutic plasma exchange for control of thyroid storm. J Clin Apher. 2021 Feb;36(1):189-195. doi: 10.1002/jca.21832[↩]
- Vidal-Trecan GM, Stahl JE, Eckman MH. Radioiodine or surgery for toxic thyroid adenoma: dissecting an important decision. A cost-effectiveness analysis. Thyroid. 2004 Nov;14(11):933-45. doi: 10.1089/thy.2004.14.933[↩]
- Randolph GW, Shin JJ, Grillo HC, Mathisen D, Katlic MR, Kamani D, Zurakowski D. The surgical management of goiter: Part II. Surgical treatment and results. Laryngoscope. 2011 Jan;121(1):68-76. doi: 10.1002/lary.21091[↩]
- Watt T, Cramon P, Bjorner JB, Bonnema SJ, Feldt-Rasmussen U, Gluud C, Gram J, Hansen JL, Hegedüs L, Knudsen N, Bach-Mortensen P, Nolsøe R, Nygaard B, Pociot F, Skoog M, Winkel P, Rasmussen AK. Selenium supplementation for patients with Graves’ hyperthyroidism (the GRASS trial): study protocol for a randomized controlled trial. Trials. 2013 Apr 30;14:119. doi: 10.1186/1745-6215-14-119[↩]
- Ceccarelli C, Bencivelli W, Vitti P, Grasso L, Pinchera A. Outcome of radioiodine-131 therapy in hyperfunctioning thyroid nodules: a 20 years’ retrospective study. Clin Endocrinol (Oxf). 2005 Mar;62(3):331-5. doi: 10.1111/j.1365-2265.2005.02218.x[↩]