Contents
- What are muscles?
- Muscle fiber types
- Table 1. Muscle fiber types
- Type 1 muscle fibers (slow-twitch muscle fibers)
- Fast-Twitch muscle fibers
- Changes in muscle fiber types
- Determinants of muscle fiber type
- The cell biology of muscle fiber size
- Metabolic pathways for ATP production in skeletal myofibers
- Exercise-Induced Adaptation
- Exercise and skeletal muscle mass
- What is the best way to build muscle?
- The science behind muscle building
- What builds muscle the most?
- How to many calories I need?
- What should bodybuilders eat?
- Protein supplement
- What is the best protein powder supplement?
- Do performance-enhancing dietary supplements work?
- Nutritional Supplements
- Safety
- Micronutrients
- Caffeine
- Beta-alanine
- Beta-hydroxy-beta-methylbutyrate
- Creatine
- Creatine supplementation Responders vs. Non-responders
- Effects of creatine supplementation on Muscle Mass
- Effects of creatine ingestion to improve recovery from injury, muscle damage and oxidative stress induced by exercise
- Effects of creatine supplementation on glycogen stores
- Effects of creatine supplementation on predominantly aerobic exercise
- Documented effects of creatine supplementation on physical performance
- Effects of creatine supplementation on predominantly anaerobic exercise
- Effects of creatine supplementation on predominantly aerobic exercise
- Effects of creatine supplementation on range of motion
- Creatine use in children and adolescents
- Safety and side effects of creatine supplementation
- Commercially available forms of creatine supplements
- Creatine in combination with other supplements
- Branched-Chain Amino Acid Supplement
- Are bodybuilding supplements safe?
- Nutritional Supplements
- What are Anabolic Steroids?
- What are steroidal supplements?
- Commonly Abused Steroids
- Possible Health Consequences of Anabolic Steroid Abuse
What are muscles?
Did you know you have more than 600 muscles in your body ? These muscles help you move, lift things, pump blood through your body, and even help you breathe.
When you think about your muscles, you probably think most about the ones you can control. These are your voluntary muscles, which means you can control their movements. They are also called skeletal muscles, because they attach to your bones and work together with your bones to help you walk, run, pick up things, play an instrument, throw a baseball, kick a soccer ball, push a lawnmower, or ride a bicycle. The muscles of your mouth and throat even help you talk and eat.
Smooth muscles are also called involuntary muscles since you have no control over them. Smooth muscles work in your digestive system to move food along and push waste out of your body. They also help keep your eyes focused without your having to think about it.
Did you know your heart is also a muscle ? Cardiac muscle is a specialized type of involuntary muscle. It pumps blood through your body, changing its speed to keep up with the demands you put on it. It pumps more slowly when you’re sitting or lying down, and faster when you’re running or playing sports and your skeletal muscles need more blood to help them do their work.
Skeletal muscles are connected to your bones by tough cords of tissue called tendons. As the muscle contracts, it pulls on the tendon, which moves the bone. Bones are connected to other bones by ligaments, which are like tendons and help hold your skeleton together.
Skeletal muscle is a highly plastic tissue. The ability of adult muscle fibres to change in response to external stimuli has been called muscle plasticity. Force, contraction speed, endurance and oxidative/glycolytic capacity are all examples of muscle properties that are plastic 1. Skeletal muscle is a permanent, post-mitotic tissue, and unless the muscle is damaged there is little turnover of cells 2, 3. Thus, it has been demonstrated that dramatic changes in gene expression, protein composition and physiological properties can occur in pre-existing fibres without de- or regeneration 4, 5. The plastic changes occur mainly by reprogramming the cell by turning on and off sets of relevant genes.
Exercise evokes signaling pathways that strongly modify myofiber metabolism and physiological and contractile properties of skeletal muscle. Regular physical activity is beneficial for health and is highly recommended for the prevention of several chronic conditions 6.
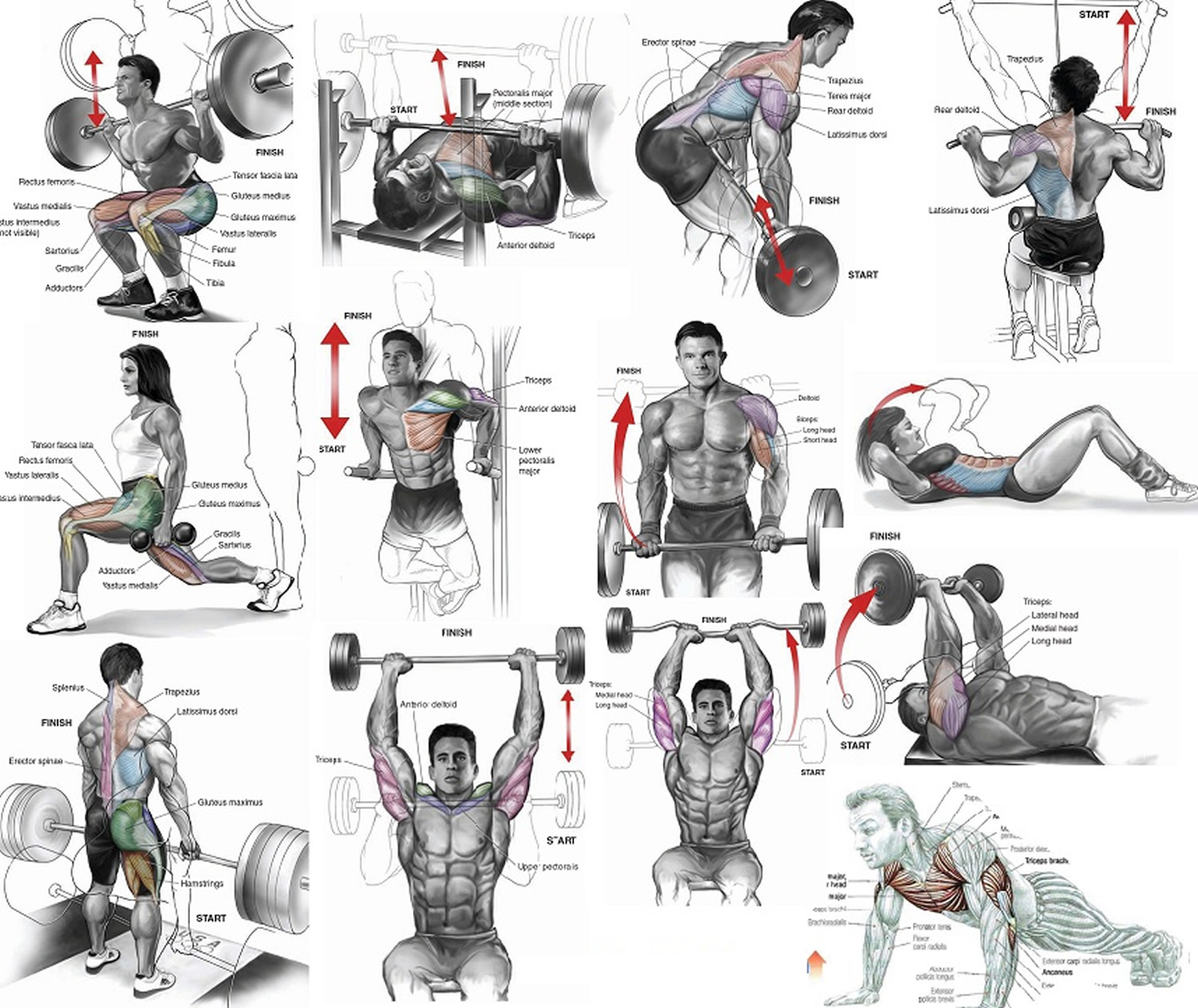
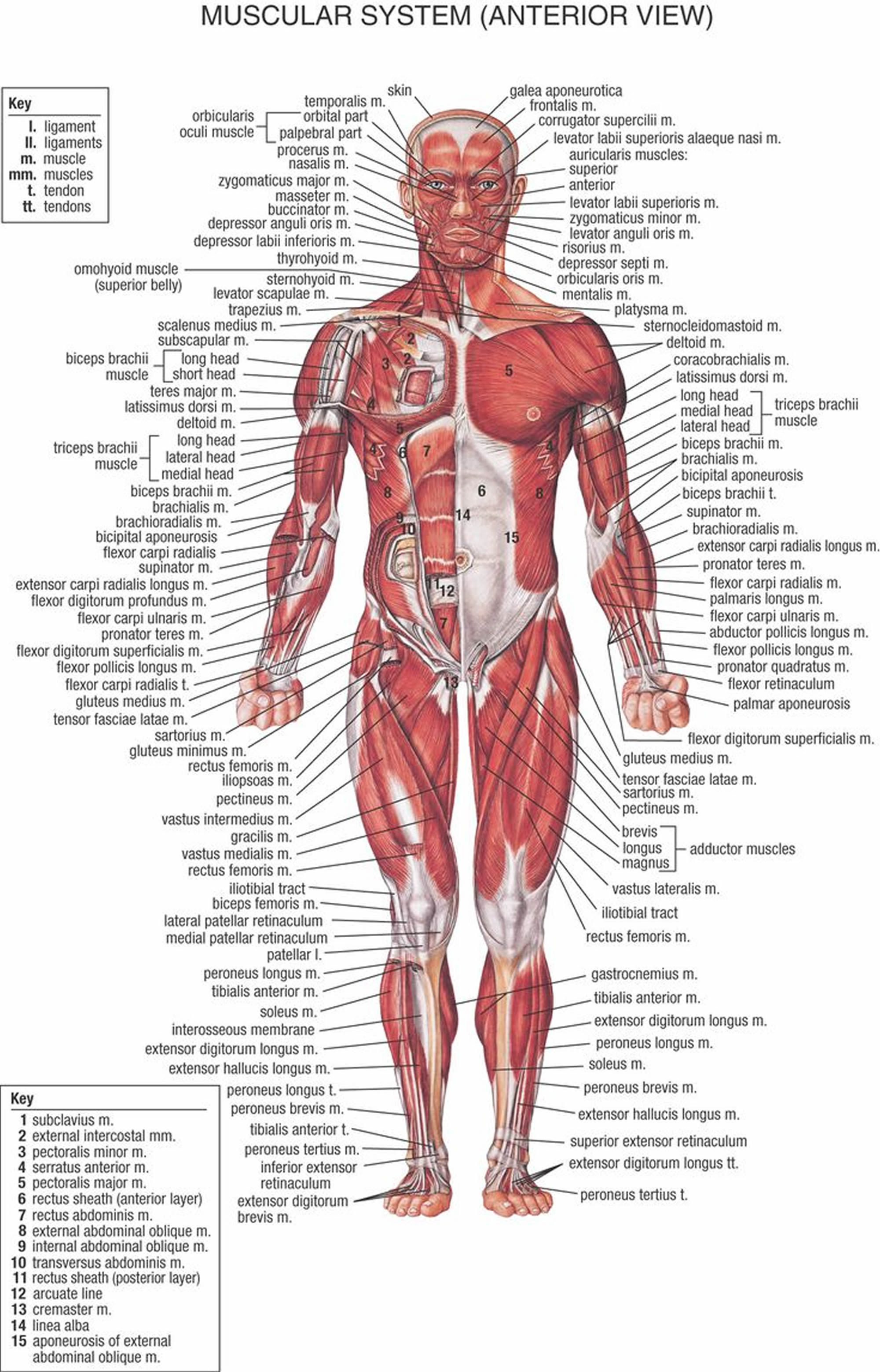
Figure 1. Muscles anatomy front
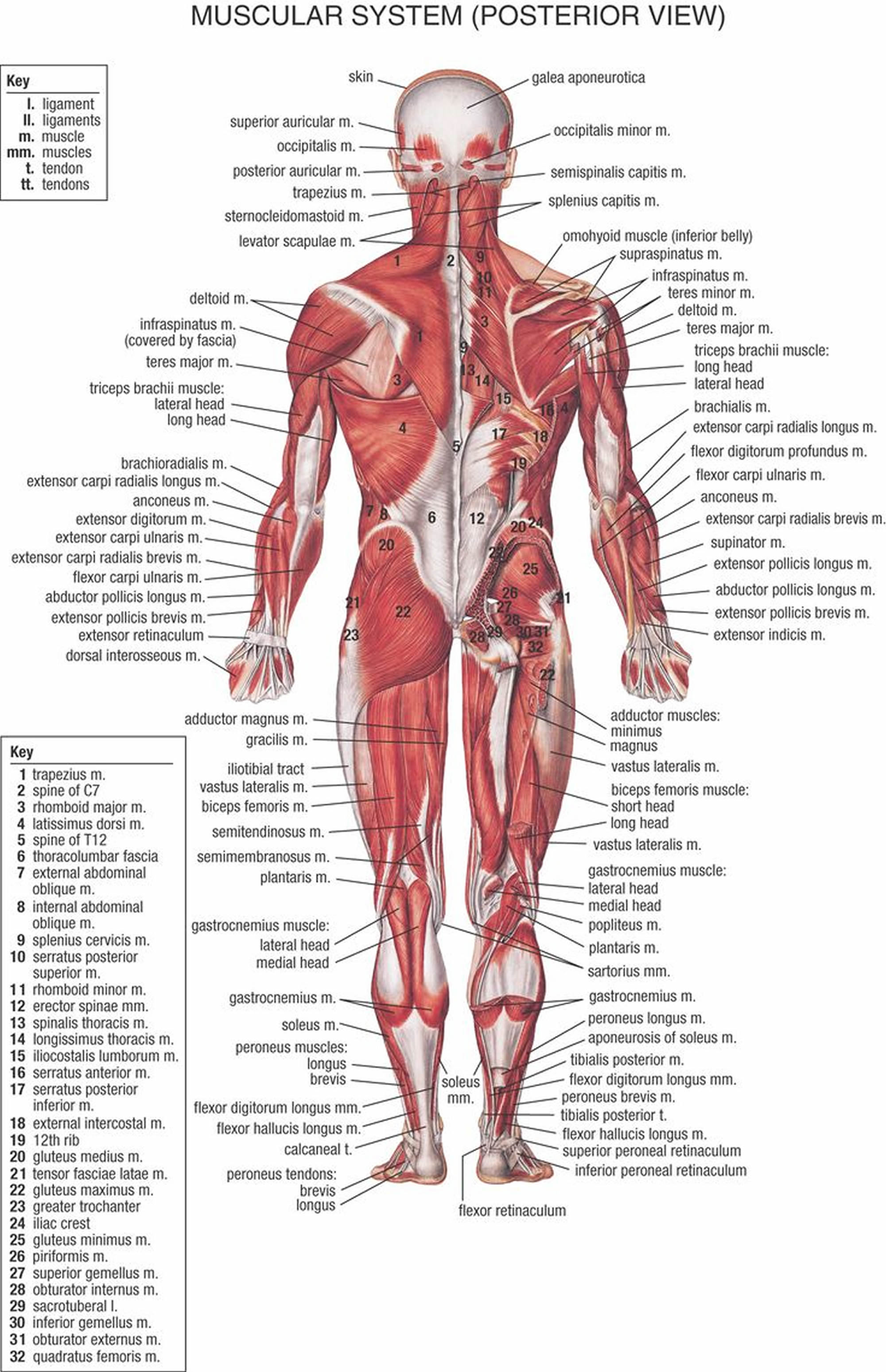
Figure 2. Muscles anatomy back
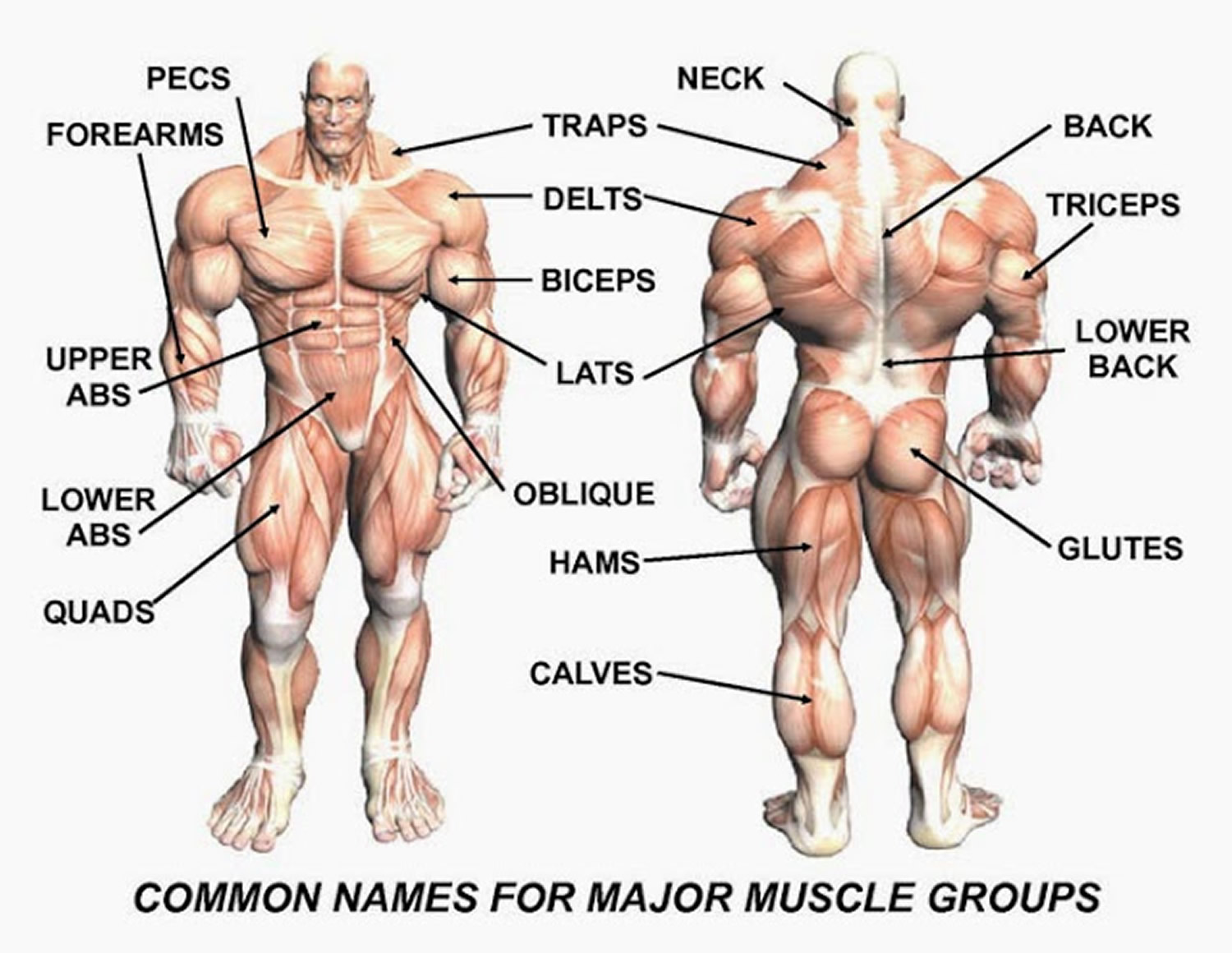
Figure 3. Muscle anatomy – simplified for bodybuilding and body builders
Muscle fiber types
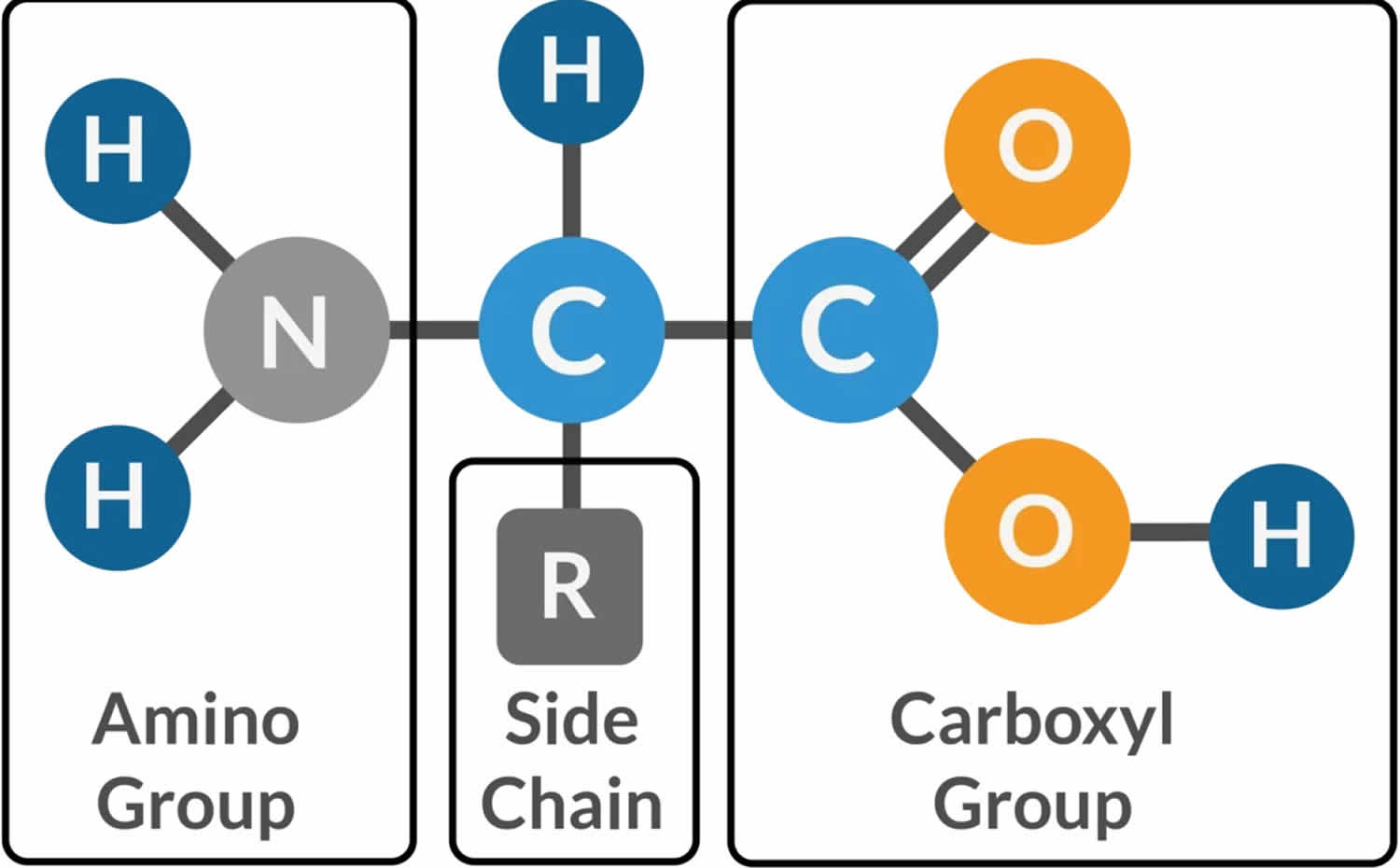
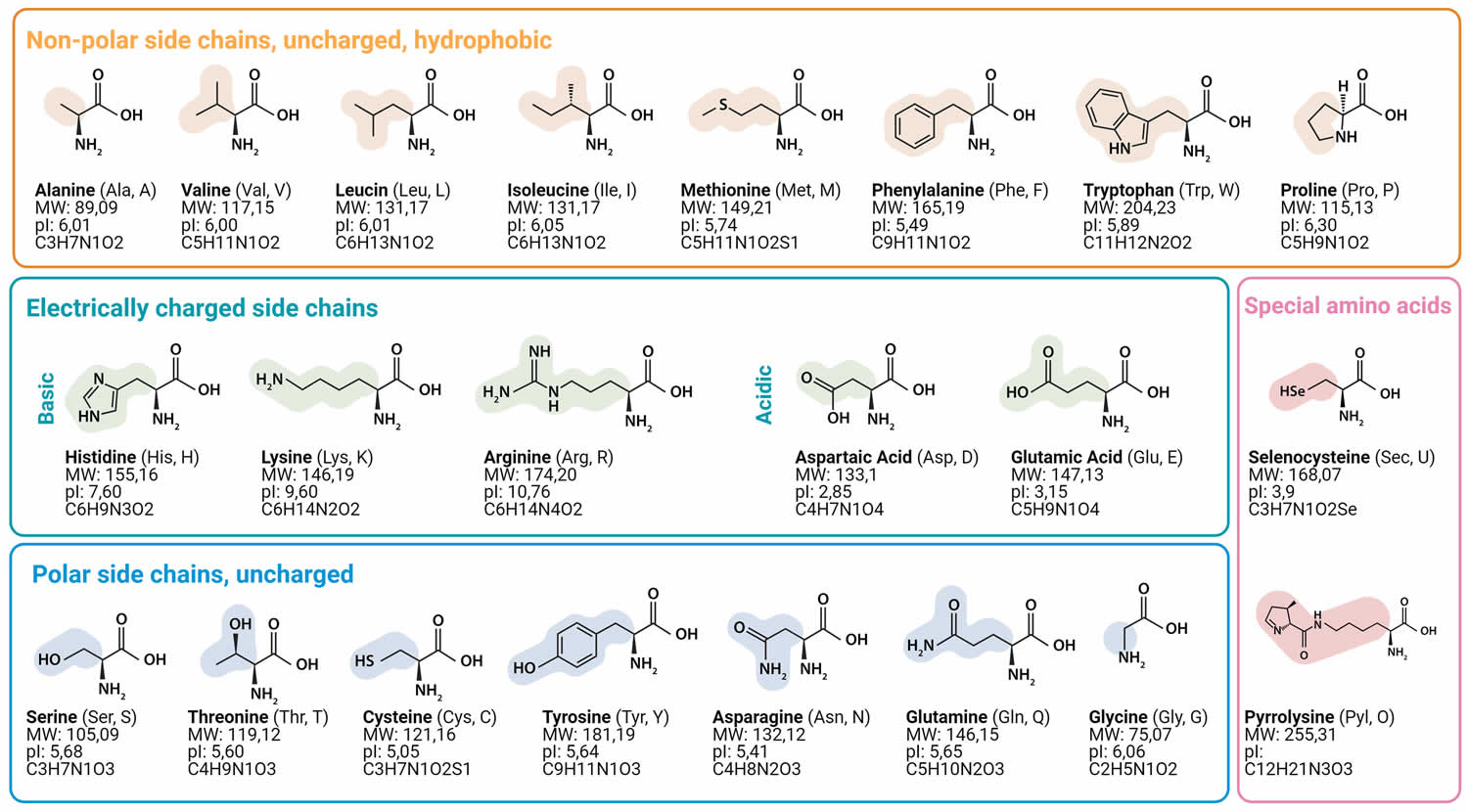
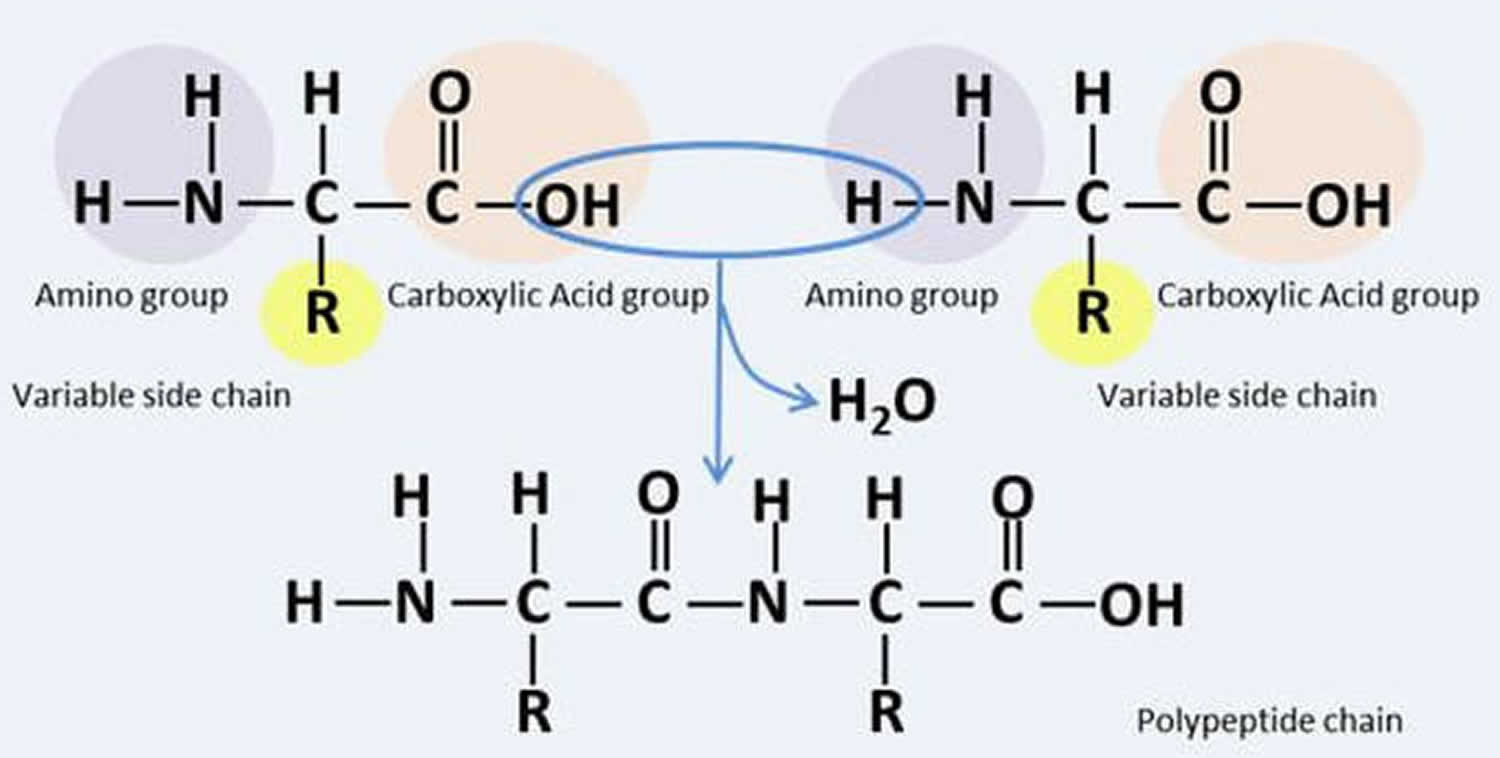
Muscle fibers (myofibers) are single muscle cells that help your body perform a specific physical function 7. Mammalian skeletal muscles are composed of muscle fibers (myofibers) with various contractile properties such as force production, endurance, twitch duration, and shortening velocity and differing metabolism. Like muscles themselves, not all muscle fibers are the same. There are 7 primary types of skeletal muscle fibers, including fast-twitch and slow-twitch based on the maximal speed of shortening. They each have different functions that are important to understand when it comes to movement and exercise programming 8.
Most experts agree that the distribution of muscle fiber types depend on the primary function of the muscle in question, as well as:
- Your Activity level. Your activity level and the types of activities that you do can affect how much you have of each muscle fiber type. For example, endurance athletes usually have a higher proportion of slow-twitch muscle fibers. And strength or power athletes usually have a higher amount of fast-twitch muscle fibers. But the exact proportion of each muscle fiber type can range from 15 to 85% of one type or the other, and the distribution also highly depends on the muscle. There’s also a theory that people who genetically have a higher percentage of slow-twitch fibers might be drawn to endurance activities, and people with more fast-twitch are drawn to power-based activities 9.
- Your Age. Muscle fiber type is also heavily influenced by the aging process. The percentage of type 2 fast-twitch muscle fibers tend to decline with age. People usually reach peak muscle mass by the age of 30, which means they have a higher percentage of type 2 fast-twitch muscle fibers. Women experience a rapid decline in muscle mass post-menopause. Men have a more gradual decline in muscle mass during and after their 40s. That means that as most people age, they have a higher number of slow-twitch type 1 muscle fibers. However, humans still need to have some muscle strength as they age, which is why most experts recommend that older people continue to do strength training exercises 10.
- Your Genetics.
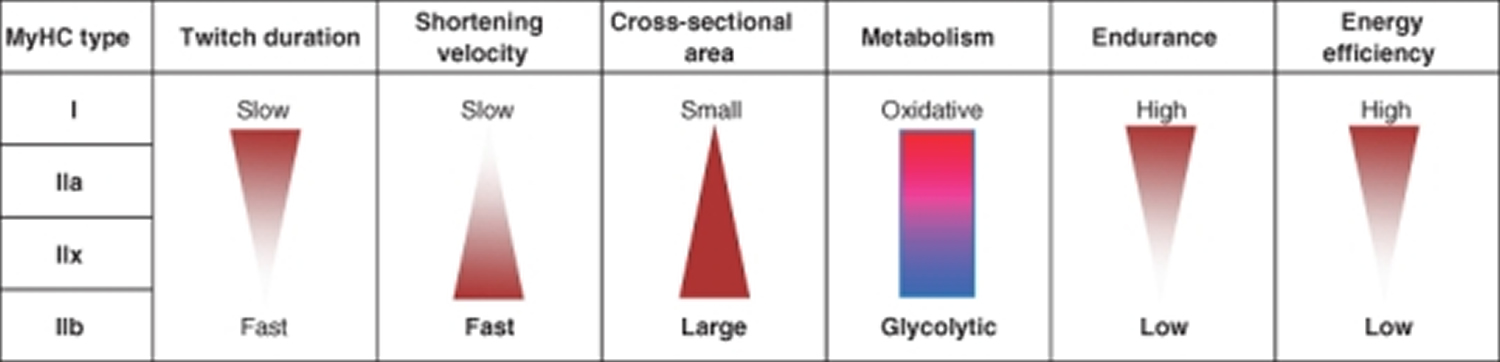
In human 3 muscle fiber subtypes are recognized based on their contractile and metabolic properties 11. Fast-twitch fatigable fibers rely predominantly on glycolytic metabolism and are designated FG (fast glycolytic), whereas, fast-twitch fatigue-resistant and slow-twitch fibres have relatively greater mitochondrial content and are designated FOG (fast oxidative glycolytic) and SO (slow oxidative), respectively 12. The fibre type-specific differences in contractile function are due to differential expression of a diverse range of isoforms of each myofibrillar protein 13. Myosin heavy chain (MyHC) isoforms are intimately associated with myofibre contractile and energetic properties and are commonly used molecular markers: FG fibres express MyHC IIx/d, FOG fibres express MyHC IIa and SO fibres express MyHC I, which is also the predominant isoform in adult human myocardium. Indeed, myosin ATPase activity determines the sliding velocity between actin and myosin, thereby shortening the velocity of the fiber 14. Myosin ATPase type I histochemical staining identifies slow-twitch fibers, while myosin ATPase type II (which has the highest ATPase activity) stains fast-twitch myofibers. Based on the expression of the predominant isoforms of MyHC protein expressed, myofibers are mainly classified as type I fibers, type IIx/d fibers, and type IIa fibers 8, 15 (Figure 1).
In addition to the three or four major MyHC genes expressed in adult limb muscles there are specialized forms expressed during development and in gill-arch-derived muscles. In total 10 different MyHC genes have been connected to skeletal muscle 16.
While histochemical or immunohistochemical staining might give the impression that the vast majority of fibres are positive only for one MyHC, single-fibre gel electrophoresis has revealed that 11–67% of the fibres from various limb muscles express more than one MyHC isoform even under steady-state activity conditions 17. It can be concluded that the concept of universal fibre types throughout the body is an oversimplification.
Table 1. Muscle fiber types
| Characteristic | Slow-Twitch Type 1 | Fast-Twitch Type 2A | Fast-Twitch Type 2X or 2B |
|---|---|---|---|
| Activities | Marathons, distance running, swimming, cycling, power walking, endurance training | Powerlifting, sprinting, jumping, strength and agility training | Powerlifting, sprinting, jumping, strength and agility training |
| Muscle Fiber Size | Small | Large | Large |
| Force Production | Low | High | Very high |
| Resistance to Fatigue | Slow | Quick | Very quick |
| Contraction Speed | Slow | Quick | Very quick |
| Mitochondria | High | Medium | Low |
| Capillaries | High | Medium | Low |
| Myoglobin | High | Medium | Low |
| ATPase Level | Low | Medium | High |
| Oxidative Capacity | High | Medium | Low |
Footnotes: *ATP (adenosine triphosphate) is the body’s energy currency. ATP provides energy for your muscle cell to contract. Type 2 muscle fibers have more readily available ATP. Type 1 fibers rely on aerobic respiration (oxygen delivery) to produce ATP in the muscle cells.
** Oxidative capacity refers to how much oxygen a gram of muscle uses in an hour.
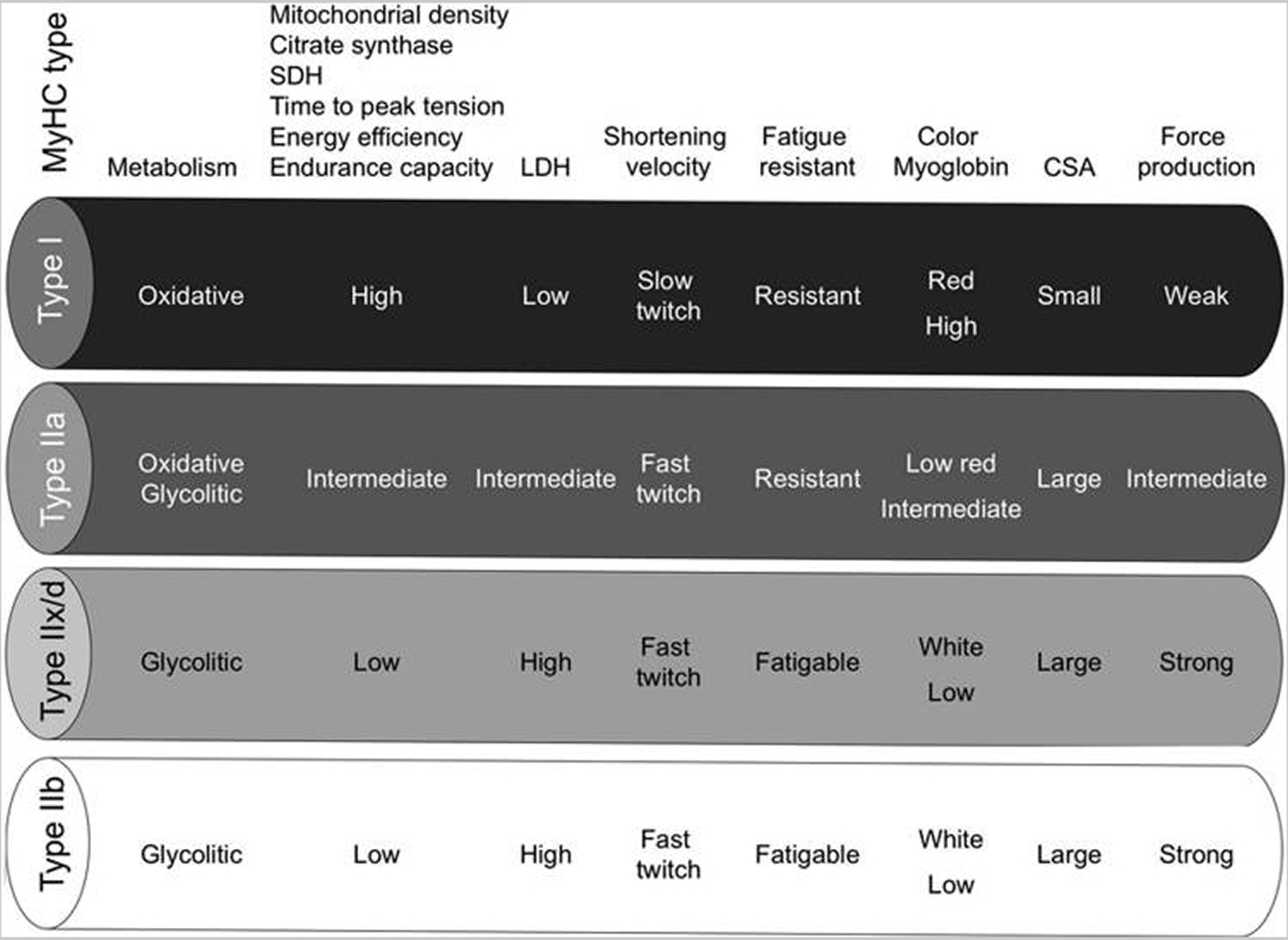
[Source 18 ]Table 2. Characteristics of mammalian skeletal muscle fiber types. The red color is associated with a high content of myoglobin
Abbreviations: MyHC = myosin heavy-chain; SDH = succinate dehydrogenase; LDH = lactate dehydrogenase; CSA = cross-sectional area.
[Source 19 ]Type 1 muscle fibers (slow-twitch muscle fibers)
Type 1 fibers (slow-twitch fibers) contain the slow isoform of myosin heavy-chain (MyHC) and slow isoforms of other contractile proteins. Force production depends on the time the myosin head spends bound to actin, on the myosin head density and on the duty ratio 20. Slow-twitch muscle fibers (type 1 muscle fibers) are the muscle cells responsible for endurance movements 18, 21. They are characterized by high mitochondrial content, high capillary density and express mainly glucose and fatty acid oxidative enzymes. Type 1 fibers are rich in myoglobin and are red colored. They develop a slow contractile force and are resistant to fatigue. For example, the story of the tortoise and the hare. Slow-twitch or type 1 muscle fibers are like the tortoise. They don’t produce a lot of power, but they’re also resistant to fatigue and can contract for a long time 22. Slow-twitch type 1 muscle fibers are involved in continuous tonic activity. Slow-twitch type 1 muscle fibers help with a lot of your daily movements, like walking, cleaning your house, or sitting upright in a chair.
Type 1 muscle fibers get most of their energy (ATP) from aerobic respiration, meaning they need oxygen to function. The oxygen makes the muscle fibers look red, which is why slow-twitch fibers are sometimes called red fibers. Type 1 muscle fibers have a much better blood supply and ability to receive oxygen than type 2 fibers. They also have a high concentration of mitochondria which is the powerhouse of a cell where aerobic respiration takes place.
Because slow-twitch muscle fibers use oxygen to produce energy, they are more resistant to fatigue. Type 1 muscle fibers are responsible for endurance activities such as distance running, swimming, cycling, hiking, low-to-moderate intensity dancing, and walking.
Fast-Twitch muscle fibers
Fast-twitch muscle fibers are the muscle cells responsible for short, powerful movements 18, 21. Going back to the story between the tortoise and hare, your fast-twitch or type 2 fibers are like the hare. They can produce a lot more force and power for a short time, but they get fatigued fast 23, 24.
Type 2 muscle fibers are subdivided into type 2X and 2A 18.
Type 2X muscle fibers produce force that’s much greater than type 1 muscle fibers 18. However, they use anaerobic (without oxygen) metabolic pathways to get their energy (ATP) 18. That means they receive less blood flow and oxygen and can only produce force for short periods of time and are highly fatigable 25.
Type 2A muscle fibers are like a hybrid of type 1 and type 2X muscle fibers 18. Type 2A muscle fibers have elements of both type 1 and type 2X muscle fiber types. For example, type 2A muscle fibers use both aerobic and anaerobic pathways and produce a medium amount of power for a medium amount of time.
Most people have high numbers of type 2A muscle fibers that produce a medium amount of power and have medium fatigue resistance 18. Type 2A muscle fibers tend to be influenced more by training because they operate as fast-twitch fibers in untrained people and slow-twitch fibers in endurance-trained people. Rather than specifically trying to target type 2A muscle fibers with training, train for your sport or activity and allow these muscle fibers to adjust automatically 21.
When your body moves, it will use slow-twitch type 1 muscle fibers first. Then, if type 1 muscle fibers can’t produce enough force, the body will use fast-twitch type 2X and 2A muscle fibers to get more power.
So, if your fitness goals involve strength and power, you’ll want to focus on training type 2 muscle fibers. Technically, any resistance training will train both type 1and type 2 muscle fibers, but training with heavier loads at least 70% of one-repetition maximum (1RM) or lighter weights with explosive tempos are the best ways to activate and train type 2 fibers. These muscle fibers also tend to achieve muscle growth easily, which can be important for bodybuilders.
Note that 1 repetition maximum (1RM) is the heaviest weight a person can lift once while using proper form and performing a full range of motion. 1RM (1 repetition maximum) is a reliable way to measure your overall muscular strength and is often used by strength and conditioning coaches. 1RM is used to determine the appropriate load and intensity for resistance training. For example, if you want to do 5 back squats, you can calculate the weight to use by taking 85–90% of your 1RM.
Strength- and power-based activities typically use more type 2X and some 2A muscle fibers. These activities require a large amount of force to be produced at once with little need for fatigue resistance. Some activities that use type 2 muscle fibers include 18:
- Sprints. A sprint workout is a training routine that involves alternating short, high-intensity bursts of exercise with rest or low-intensity exercise. Sprinting is an anaerobic exercise that involves running at top speed for a short time. Your body can’t bring in enough oxygen quickly enough to provide energy for the movement. This produces lactic acid, which builds up in your blood and limits how long you can sprint. Sprinting helps you run faster and for longer by increasing your lactate threshold. Sprinting also builds muscle in your legs and stimulates growth throughout your body.
- Olympic weightlifting. Olympic weightlifting is an Olympic sport where athletes attempt to lift a barbell loaded with weight plates in a single lift. The two lifts in Olympic weightlifting are the snatch and the clean and jerk
- Powerlifting. Powerlifting focuses on lifting the most weight possible in three lifts: bench press, squat and deadlift.
- Plyometrics. Plyometrics are a type of exercise that uses explosive movements to build muscle power and improve physical performance. Plyometric exercises can include jumping, running, kicking, and throwing. Some examples of plyometric exercises include:
- Box jumps: Jump up and onto a box while lifting your arms for momentum, then jump back down
- Squat thrusters: Start in a high plank position, then jump your feet forward into a squat
- Jumping lunges: Stand with your feet shoulder-width apart, then jump while bringing one leg in front of you and the other behind you
- Squat jumps: Start standing on your toes, then flex your hips and jump up
To stimulate fast-twitch type 2 muscle fibers, lift higher loads (more than 70% one-repetition maximum [1RM]) at lower repetitions (1 to 12) or use lighter weight with explosive tempos. Some examples of fast-twitch stimulating exercises include 18:
- Heavy barbell squats. Heavy barbell squats are a compound exercise that involves holding a weighted barbell and performing a squat. To do a barbell squat stand with your feet shoulder-width apart, unrack the barbell, and hold it on your upper back. Keep your chest up and back straight, then hinge your hips and knees to lower your body into a squat position.
- Heavy barbell bench presses. A heavy barbell bench press is a weight training exercise that involves using a barbell to press a heavy weight upwards while lying on a bench. Remember to always have a spotter to help you lift safely.
- Medicine ball slams. A medicine ball slam is a full-body exercise that involves lifting a weighted ball overhead and slamming it into the ground. To do a medicine ball slam:
- Stand with your feet shoulder-width apart.
- Hold the ball in both hands at your torso.
- Squat down slightly.
- Inhale and press through your heels to stand up on the balls of your feet.
- Extend your knees and hips as you rise to lift the ball overhead.
- Slam the ball down between your feet with as much force as you can.
- Catch the ball on the rebound or pick it up for repetition.
- Chest pass. A chest pass is a passing technique in basketball and netball where a player holds the ball at chest level and throws it to another player, usually without the ball touching the floor.
- Box jumps. A box jump is a type of exercise that involves jumping from the ground onto an elevated surface, such as a box. Box jumps are a high-impact exercise that can help improve your lower body strength and speed, as well as your vertical jump range.
Fast-twitch fibers can also recruit slow-twitch fibers: endurance training at high-intensity intervals can be effective in improving aerobic power 26, 27.
Tapering during training programs (reducing volume and intensity), can also improve the strength and power of type 2A fibers without decreasing type 1 performance 28.
One study investigated muscle fiber changes in recreational runners training for a marathon 28. After 13 weeks of increasing mileage and a three-week tapering cycle, not only did the functions of type 1 and type 2A fibers improve, but type 2A continued to improve significantly during the tapering cycle 28.
Changes in muscle fiber types
The physiological properties (shortening velocity, twitch duration, endurance, etc.) that are linked in a muscle fiber type are related to highly different molecular families (MyHC, SERCA, metabolic enzymes, etc.). Coupling regulation of different physiological properties may be beneficial from an energy conservation point of view, and/or it might reflect common signaling systems diverging to regulate several sets of diverse genes. To some extent however, different properties can be uncoupled and regulated independently during plastic changes. For example, some degree of uncoupling has been observed between twitch speed and shortening velocity 29. More importantly, endurance-exercise in man and in other animals can lead to pronounced changes in metabolic properties without MyHC fibre-type conversion 30, 31, 32, although exercise can also change MyHC type in particular within type II (e.g. from type IIb/IIx to IIa) 33, 34, 35 and under more severe experimental conditions fibre-type conversions are frequent.
When fibre-type conversions occur, it usually happens in a sequential order 36, 13: I ↔ IIa ↔ IIx ↔ IIb. During transitions hybrids between the “nearest neighbour” fibre type in this flow chart (e.g. I+IIa, IIa+IIx) are common, but aberrant hybrids such as I+IIb, I+IIa+IIb and I+IIx+IIb can also be seen under some experimental conditions 37.
Determinants of muscle fiber type
The factors that determine the molecular make-up of already formed adult muscles, and how that make-up can change. At any point in time, a muscle fiber’s make-up appears to depend on previous: (1) cell history/lineage; (2) nerve-evoked electrical activity; (3) mechanical conditions; (4) para-/autocrine conditions; and (5) circulating hormones.
There is a consensus that changes in muscle usage will transform muscle phenotype, but the precise biological signaling mechanisms responsible for such changes are less clear.
Muscle properties are strongly influenced by hormones such as testosterone and thyroid hormones, as reviewed previously 38, 39, 40. The link between external factors related to activity and usage (points 2 and 3 above) and gene expression and the ability to change is, however, constrained by the muscle’s cell lineage.
The importance of cell lineage
Developmental studies suggest that initial fibre-type differentiation might be determined by myoblast cell lineage independent of external influences such as innervation or usage.
In adult rats, when different muscles are regenerating from myoblasts after myofibre destruction, the various regenerated muscles express different MyHC types reminiscent of the muscle of origin. This happens even if the muscles receive similar experimental patterns of activity. Thus, when regenerating soleus and extensor digitorum longus (EDL) were stimulated by the same slow pattern, the EDL failed to express the large amount of slow MyHC that was observed in the soleus under the same conditions 41.
While it seems clear that cell lineage limits the adaptive range of muscle plasticity, it is equally clear that external signals can change muscle phenotype in the adult. In particular signals from the nerve appear to be important.
It can be concluded that muscle fibre pedigree matters, and that there is a cell line component to the resulting adult phenotype of a fibre. The relationship is, however, not simple, since experiments with genetically marked myoblasts suggest that single myoblast clones can contribute to both fast and slow fibres, clones are not restricted to contribute to subsets of fibre types, and clones show no detectable preference for fusion to a particular fibre type 42.
What are the signals from the nerve?
There is currently no compelling evidence to suggest that there are any relevant sources of neural influence on the muscle other than activity, and in spite of intense searching for several decades, no neurotrophic substances have been identified that prevent atrophy or mimic other effects of normal innervation or cross-innervation outside the synaptic zone 1.
The importance of nerve-evoked muscle activity
Generally type I motor units seem to receive high amounts of impulses delivered in long low-frequency trains, while type II units seem to receive short bursts of high-frequency activity. The total amount of impulses delivered to type II units is lower, but the amount seems to vary among the type II subtypes.
Mechanical stress
It is widely assumed that contraction against a resistance leads to larger muscles than contraction against lower resistance, but this does not necessarily have a direct bearing on the importance of mechanical factors as such.
The most compelling evidence for a mechano-dependent mechanism comes from experiments where limbs have been immobilized by a cast. This leads to atrophy, but studies over almost 100 years have shown that atrophy can be partly counteracted when muscles are immobilized in a lengthened position rather than a shortened position. There are also studies suggesting that muscle length influences contraction speed such that chronic stretch makes a muscle slower; immobilization of fast muscles in a lengthened position thus increases the fraction of slow fibres. In most experimental conditions it is hard to separate electrical activity and mechanical stretch, but some experimental data point to the presence of an activity-independent mechanical mechanism influencing muscle size and perhaps contraction speed.
Myostatin
Myostatin (previously called GDF-8) is a member of the transforming growth factor β (TGF-β) superfamily and plays a major role during development where it acts as an inhibitor of muscle growth. Disruption of the myostatin gene leads to development of grossly enlarged muscles in mice 43, farm animals 44, and man 45. The enlargement is caused both by an increase in the number of fibres (hyperplasia) and in fibre size (hypertrophy). Importantly, muscle enlargement obtained by myostatin deficiency is peculiar because it does not increase force in proportion to size, thus the amount of contractile proteins may not be properly regulated 46, 47. Thus, reducing myostatin alone might not mimic effects of strength training, although strength training in adults has been shown to be associated with reduced levels of myostatin in muscle and plasma 48, 49. In adult animals inhibition of myostatin with antibodies leads to hypertrophy without an increase in the number of fibres 50; conversely, overexpression of myostatin in muscle fibres after electroporation leads to muscle atrophy without loss of muscle fibres 51. In the latter study it was suggested that myostatin acted by reducing muscle gene expression of myofibrillar proteins perhaps by reducing expression of MyoD and myogenin. In addition, myostatin might activate the ubiquitin-proteasome pathway for proteolysis 52.
Insulin-like growth factor I (IGF-1)
IGF-1 has been implicated as a factor promoting hypertrophy in the adult animal. The liver supplies approximately 75% of the circulating IGF-1 (Schwander et al., 1983), and a selective abolishment of IGF-1 production in hepatocytes leads to a 75% reduction in circulating IGF-1 levels but without growth impairment 53. In humans increasing the circulating level of IGF-1 does not promote muscle protein synthesis 54. IGF-1 is also expressed locally in several tissues including muscle where it is induced by stretch or high-resistance exercise. IGF-1 seems to work as a local hormone that promotes hypertrophy in adult muscle. It might do so both by interfering with protein balance in muscle fibres and by activating satellite cells, but for the latter there is still little information in adult muscles.
The cell biology of muscle fiber size
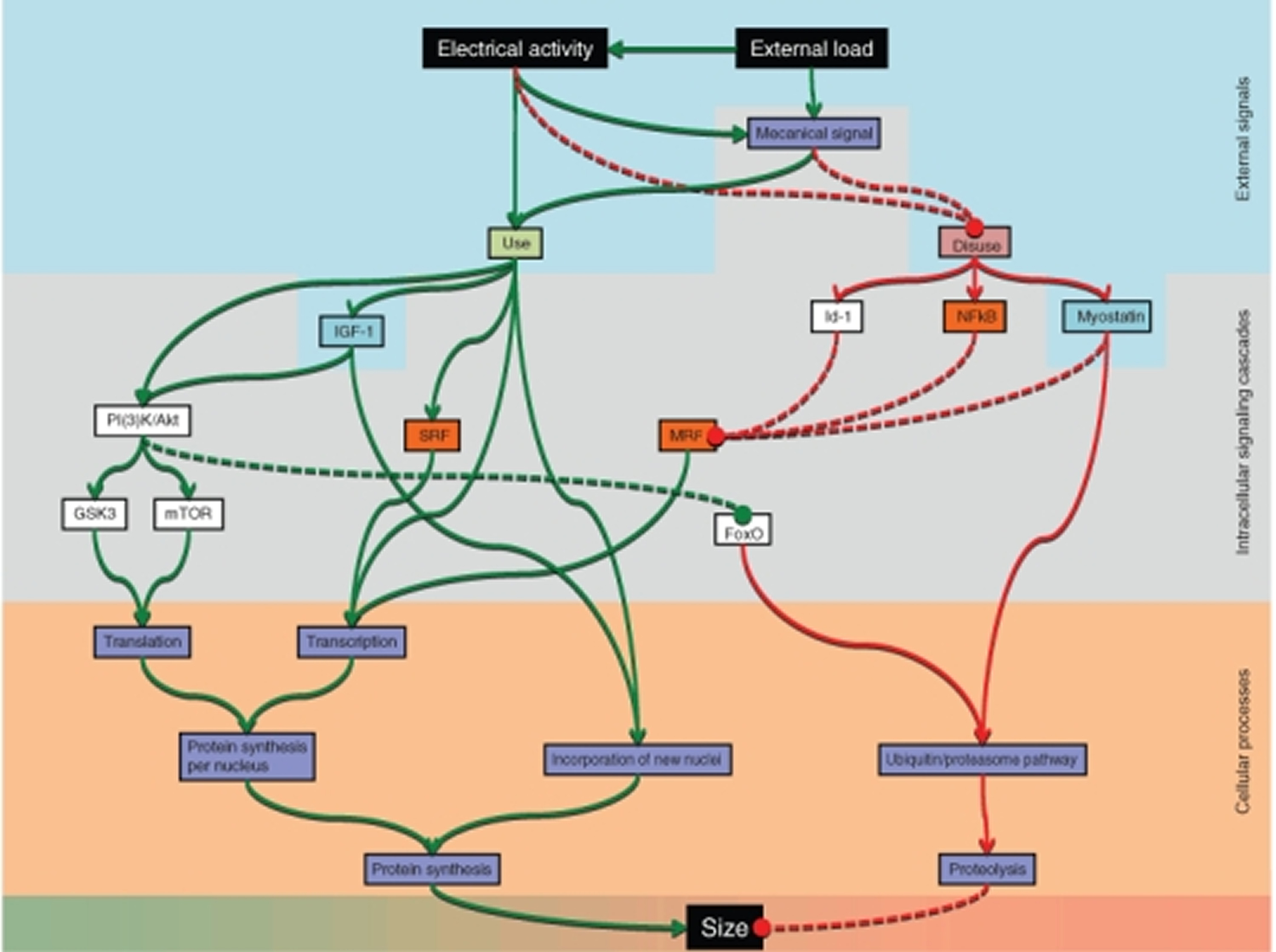
Regulation of force is mainly a question of regulating muscle fiber size, and ultimately fiber size is regulated by altering the balance between protein synthesis and degradation in each muscle fibre. Change in fiber size can be achieved by regulating three major conditions: (1) the number of nuclei; (2) the rate of protein synthesis for each nucleus; and (3) the rate of protein degradation.
Figure 4. Pathways currently believed to be involved in regulating muscle fiber size
[Source 1]The pathways have different degrees of scientific support, and their relative importance is still poorly understood. Abbreviations: forkhead box O (FoxO), glycogen synthase kinase 3 (GSK3), inhibition-of-DNA-binding-protein 1 (Id-1), insulin-like growth factor I (IGF-1), mammalian target of rapamycin (mTOR), myogenic regulatory factor (MRF), phosphatidylinositol 3-kinases/Akt (PI(3)K/Akt), serum response factor (SRF), κ light polypeptide gene enhancer in B-cells (NFκB).
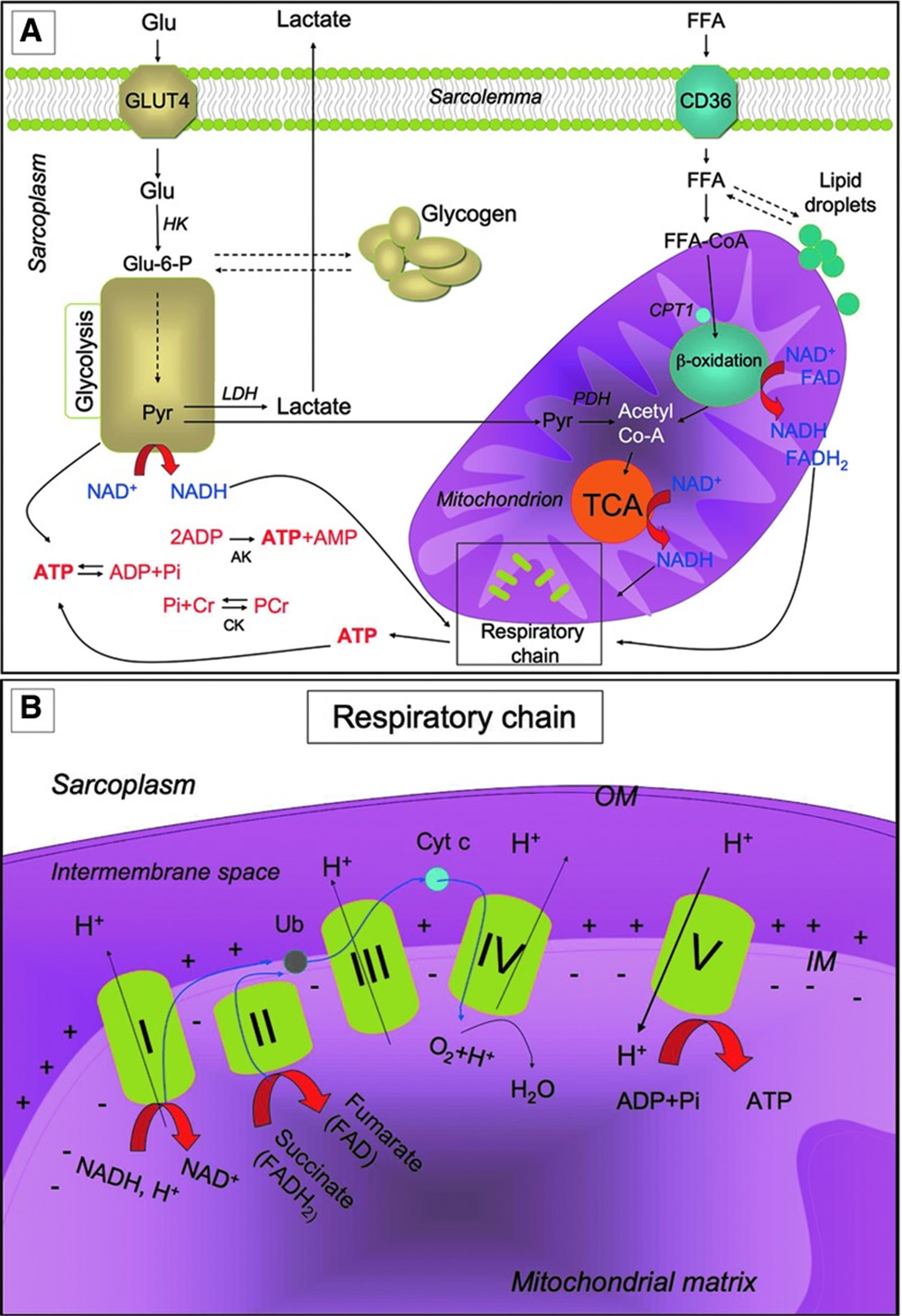
Metabolic pathways for ATP production in skeletal myofibers
Metabolic pathways for ATP production in skeletal myofibers. (A) Skeletal muscles require a high amount of ATP for contraction. The main sources of energy are Glu and FFA. Glu uptake into the sarcoplasm from blood occurs, among other things, through the GLUT4. Once in the cytosol, Glu is phosphorylated by HK and forms Glu-6-P. One molecule of Glu-6-P can be converted into two molecules of Pyr through glycolysis, a metabolic anaerobic pathway involving 10 enzymes (the enzyme phosphofructokinase is an important control point in the glycolytic pathway). Depending on the energy needs, Glu-6-P can also be stored as glycogen. In anaerobic conditions Pyr is reduced to lactate by LDH. Alternatively, in aerobic conditions, Pyr might be transferred into the mitochondria matrix, where it is decarboxylated into acetyl-CoA by the PDH complex. Acetyl-CoA is then metabolized through the TCA cycle. The first enzyme acting in the TCA cycle is the citrate synthase that forms citrate from acetyl-CoA and oxaloacetate. The TCA cycle produces reducing equivalents (NADH, FADH2) and CO2. In addition to Pyr, another important source of acetyl-CoA is the β-oxidation of FFA. FFA enter the myofiber through a passive flip-flop or through a protein-mediated mechanism such as the FAT/CD36. In the cytosol, FFA undergo esterification and form triglycerides stored as lipid droplets that are surrounded by mitochondria. Alternatively, at the mitochondrial OM, they can be condensed with CoA to form FFA-CoA and, through the CPT1, they can cross the mitochondrial IM and reach the mitochondrial matrix where they undergo β-oxidation. β-oxidation is a cycle of four reactions. Each cycle produces a molecule of acetyl-CoA which, in turn, enters the TCA cycle. Along with acetyl-CoA, during β-oxidation, FADH2 and NADH are also formed. In skeletal muscles, at rest, excess of ATP produced is stored as PCr. ATP is converted into ADP and Pi by ATPase, and the Pi is used to convert Cr in PCr whose amount is roughly 10 times higher than the amount of ATP. During intense activity, PCr can anaerobically donate a phosphate group to ADP and form ATP for quick regeneration of ATP. PCr is, therefore, a rapid system to supply energy during contraction. The reversible phosphorylation of Cr is catalyzed by several CK. Once ATP also produced by PCr is consumed, the AK (myokinase) catalyzes the formation of ATP and AMP from two ADP molecules. During exercise, the amount of ATP produced by the myofiber increases enormously. However, the stores of ATP that can be detected in the myofiber are not as high, as ATP is stored in the form of PCr. (B) Reducing equivalents (NADH and FADH2) generated mainly during TCA, β-oxidation, and glycolysis are oxidized by the complexes of the respiratory chain (Complex I, II, III, and IV) in the oxidative phosphorylation pathway. Electrons are transferred from NADH and FADH2 to oxygen (which is reduced to H2O) by means of the enzyme complexes and by the electron carriers Ub and Cyt c of the respiratory chain. The energy released by reducing equivalent oxidation as electrons pass from one complex to the next is used to pump protons (H+) across the IM into the intermembrane space. This creates an electrochemical proton gradient across the IM, which is highly energetic. Protons can flow along this gradient through ATP synthase (ATPase or complex V); this backflow releases the energy of the proton gradient, which is used by ATP synthase to phosphorylate ADP and to form ATP. This phosphorylation of ADP is called oxidative, as it is coupled to the presence of oxygen that enables the oxidation of reducing equivalents. By this mechanism, nutrients are oxidated and their energy is stored in usable energy as ATP. ATP is also produced in a lower amount during glycolysis. OM, outer membrane; Glu, glucose; FFAs, free fatty acids; Glu-6-P, glucose-6-phosphate; Pyr, pyruvate; PDH, pyruvate dehydrogenase; TCA, tricarboxylic acid; FAT/CD36, fatty acyl translocase; CPT, carnitine palmitoyltransferase; CK, creatine kinases; AK, adenylate kinase; Ub, ubiquinon; IM, inner membrane; Cr, creatine; Cyt c, cytochrome c; GLUT4, glucose transporter 4; HK, hexokinase; PCr, phosphocreatine.
Figure 5. Muscle metabolism
[Source 6]Exercise-Induced Adaptation
Skeletal muscle is extremely adaptable to environmental changes and is characterized by a high metabolic flexibility: It is able to rapidly modify the rate of ATP synthesis, the blood flow, and the kind of substrate used, depending on needs 55. Skeletal muscle is also extremely adaptable to changes in contractile activity: Physical exercise strongly modifies metabolic potential, morphology, and physiology of skeletal muscle, thus producing a strong beneficial effect on health 56. All pathways of ATP generation are active during exercise, but the relative contribution of each is determined by the intensity and duration of contraction. Indeed, exercise might be performed with different modalities, thus producing different effects on muscles 57.
Physical exercise might be grossly classified as “endurance training” and “resistance training.” Endurance training is based on endurance and is aerobic, while resistance training is based on strength. Endurance exercise (e.g., performed by marathon runners, swimmers, and cyclists) is generally characterized by high-frequency, long duration, and low power output. Resistance exercise (e.g., body building and throwing events) is, in general, characterized by low frequency, high resistance, high intensity, and short duration. Along with the modality of exercise, other parameters such as duration, frequency, and intensity of the exercise influence the effect of physical training on the muscle 58.
Exercise triggers a metabolic and structural remodeling in skeletal muscle, thus leading to changes in contractile properties and to increased angiogenesis in order to reduce muscle fatigue. These adaptations improve skeletal muscle performance. The specific features of skeletal muscle adaptation to exercise depend on the modality of exercise performed. Resistance exercise acts mainly by increasing muscle mass and strength (see “Exercise and skeletal muscle mass” section). On the other hand, endurance exercise stimulates mitochondrial biogenesis and expression of mitochondrial respiration and FFA β-oxidation genes, thereby providing a phenotypic adaptation toward a more oxidative phenotype. Submaximal aerobic activities increase insulin-independent glucose uptake and utilization in skeletal muscle, along with insulin sensitivity and redistribution of GLUT4 to the plasma membrane 59. With regard to the contractile properties, endurance exercise promotes fiber type transformation toward the slow-twitch contractile apparatus by inducing a dramatic modification of gene expression and physiological properties of the myofiber. The muscle used frequently needs to be more energy efficient, with both longer twitches and slower MyHC types contributing to higher energy efficiency 60.
Exercise provides numerous beneficial effects on skeletal muscle and, in general, on health. Although both exercise modalities are beneficial for health, endurance exercise is more effective for preventing cardiovascular diseases; while resistance training (mostly inducing muscle hypertrophy) is more effective for the maintenance of muscle mass contrasting atrophy and age-related muscle wasting 61.
Exercise and skeletal muscle mass
As stated earlier, while endurance exercise acts by up-regulating mitochondrial metabolism and fiber-type transformation, the beneficial effects of resistance exercise mainly depend on its ability to increase muscle mass. Muscle fiber hypertrophy was a determinant of overall muscle enlargement as a result of resistance training. Although many training variables contribute to the performance, cellular and molecular adaptations to resistance exercise, relative intensity (% 1 repetition maximum [%1RM]) appears to be an important factor 62. This review summarises and analyses data from numerous resistance exercise training studies that have monitored percentage fibre type, fibre type cross-sectional areas, percentage cross-sectional areas, and myosin heavy chain (MyHC) isoform expression. In general, relative intensity appears to account for 18-35% of the variance for the muscle hypertrophy response to resistance exercise. On the other hand, fibre type and MyHC transitions were not related to the relative intensity used for training. When competitive lifters were compared, those typically utilising the heaviest loads (> or =90% 1RM), that is weightlifters and powerlifters, exhibited a preferential hypertrophy of type II fibres when compared with body builders who appear to equally hypertrophy both type I and type II fibres. These data suggest that maximal hypertrophy occurs with loads from 80-95% 1RM 62.
Skeletal muscle mass depends on a delicate balance between protein synthesis and protein degradation: Resistance exercise influences both these processes by activating the phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling 63. The kinase mTOR exists in two independent complexes: mTOR complex 1 (mTORC1) and mTORC2. Raptor and Rictor are specific functional components of TORC1 and TORC2, respectively. mTORC1 controls protein translation by phosphorylating the eukaryotic translation initiation factor 4E-binding protein-1 (4E-BP1) and p70 ribosomal protein S6 kinase (p70S6K). p70S6K phosphorylates the ribosomal subunit S6 and up-regulates protein synthesis. mTORC2 prevents protein degradation by phosphorylating and inhibiting the forkhead box (FoxO) class of transcription factors. Indeed, FoxO transcription factors induce the expression of atrogin-1/muscle atrophy F-box (MAFbx) and muscle ring finger protein 1 (MuRF-1), two E3 ubiquitin ligases, which promote the ubiquitination and the proteasome-mediated degradation of critical sarcomeric proteins. The ubiquitin-proteasome system mediates muscle atrophy in several conditions, and the oxidative stress plays a key role in the regulation of the proteasome proteolytic activity. Mechanosensory regulation of protein synthesis is determined by high-force contractions that damage the sarcolemma and activate the membrane phospholipid phosphatidic acid, which, in turn, activates mTOR. During resistance exercise, mechanosensory regulation of protein synthesis also involves some transmembrane receptors called focal adhesion kinase (FAK) proteins, which transmit the contractile force through the skeletal muscle architecture and trigger protein synthesis by inducing mTOR activation.
The influence of exercise on muscle mass also involves muscle stem cells. As such, exercise induction of hypertrophy is accompanied by satellite cell fusion to myofibers. Mitochondria are considered as being involved in the regulation of myoblast proliferation/differentiation; therefore, PGC-1α-mediated mitochondrial biogenesis triggered by endurance exercise might possibly influence satellite cell fusion. Interestingly, PGC-1α up-regulation occurs during differentiation. Other signalings triggered by endurance exercise, such as p38 MAPK and Akt, contribute to satellite cell differentiation. Therefore, muscle wasting might be counteracted by endurance training through enhancement of myoblast differentiation and fusion. In addition, it has been suggested that PGC-1α might control muscle wasting pathways. It reduces the FoxO3-associated muscle atrophy, and mice overexpressing PGC-1α are protected from sarcopenia and have an increased lifespan. Moreover, increasing mitochondrial oxidative metabolism and biogenesis protects from atrophy, and this might be achieved by endurance exercise-induced PGC-1α. Regular submaximal aerobic activities have also been found to be beneficial for patients afflicted with Duchenne muscular dystrophy (DMD), while “exercise mimetics” decrease muscle inflammation and inhibit FoxO1 signaling. It has also been shown that exercise inhibits MuRF up-regulation due to diabetes and that this might mediate exercise’s beneficial effects on this disease.
At baseline levels, autophagy is a housekeeping mechanism cleaning cells of aberrant and dysfunctional molecules and organelles, thereby maintaining cell homeostasis. Autophagy is a multi-step process during which a part of the cytoplasm (including intracellular organelles) is sequestered within double-membraned autophagic vacuoles (autophagosomes), which then fuse to lysosomes and become autophagolysosomes (Figure 7). By this mechanism, defective organelles and proteins are digested by lysosomal hydrolases 64. Under stress conditions, autophagy increases and promotes temporary cellular adaptation to unfavorable conditions. It primarily favors survival during nutritional stress imposed by decreased nutrients; the degradation of intracellular material through autophagy becomes an alternative source of energy 65.
Autophagy and skeletal muscle mass maintenance
The role of autophagy in the maintenance of muscle mass is controversial. While excessive autophagy is detrimental to skeletal muscle and contributes to muscle wasting, basal autophagy is required for the maintenance of skeletal muscle homeostasis and integrity 66. The autophagy-lysosome system is activated in several atrophy conditions such as fasting, caloric restriction, cancer cachexia, aging, disuse, and denervation 67. Conversely, the key role of autophagy in skeletal muscle homeostasis maintenance is supported by the fact that muscle-specific ablation of key autophagy proteins such as Atg7 or Atg5 produces myofiber degeneration and muscle weakness 68. On the other hand, the autophagy-lysosome system is activated in several atrophy conditions such as fasting, caloric restriction, cancer cachexia, aging, disuse, and denervation 66. Moreover, the phenotype of some transgenic mice suggests that autophagy may favor muscle atrophy.
Increasing evidence suggests that exercise triggers autophagy in skeletal muscle and that autophagy mediates some beneficial effects due to exercise. Grumati et al. 69 have revealed an important connection between autophagy and exercise physiology. They have shown that physical training stimulates autophagy in mice skeletal muscles, and that autophagy was able to prevent the accumulation of damaged organelles and to maintain myofiber homeostasis.
It is currently believed that a correct balance between activation and inhibition of autophagy is critical for muscle homeostasis. Too much autophagy causes an excessive removal of crucial cellular components, which leads to muscle atrophy. On the other hand, insufficient autophagy leads to the accumulation of dysfunctional organelles, thus impairing myofiber homeostasis 66.
The involvement of autophagy in exercise-induced remodeling might be related to the two most important functions of autophagy: providing new sources of energy and removing dysfunctional organelles. During exercise, more energy is needed; the requirement of energy generally induces autophagy and it is possible that, as stated earlier, the increase of glucose uptake triggered by exercise depends on autophagy. Moreover, autophagy is the main mechanism for the removal of damaged mitochondria that is necessary to protect myfibers from atrophy. Damaged mitochondria removal is especially needed during exercise when oxidative metabolism and turnover of mitochondria increase. Interestingly, many of the sensors and pathways triggered by exercise in skeletal muscle are involved in the modulation of autophagy.
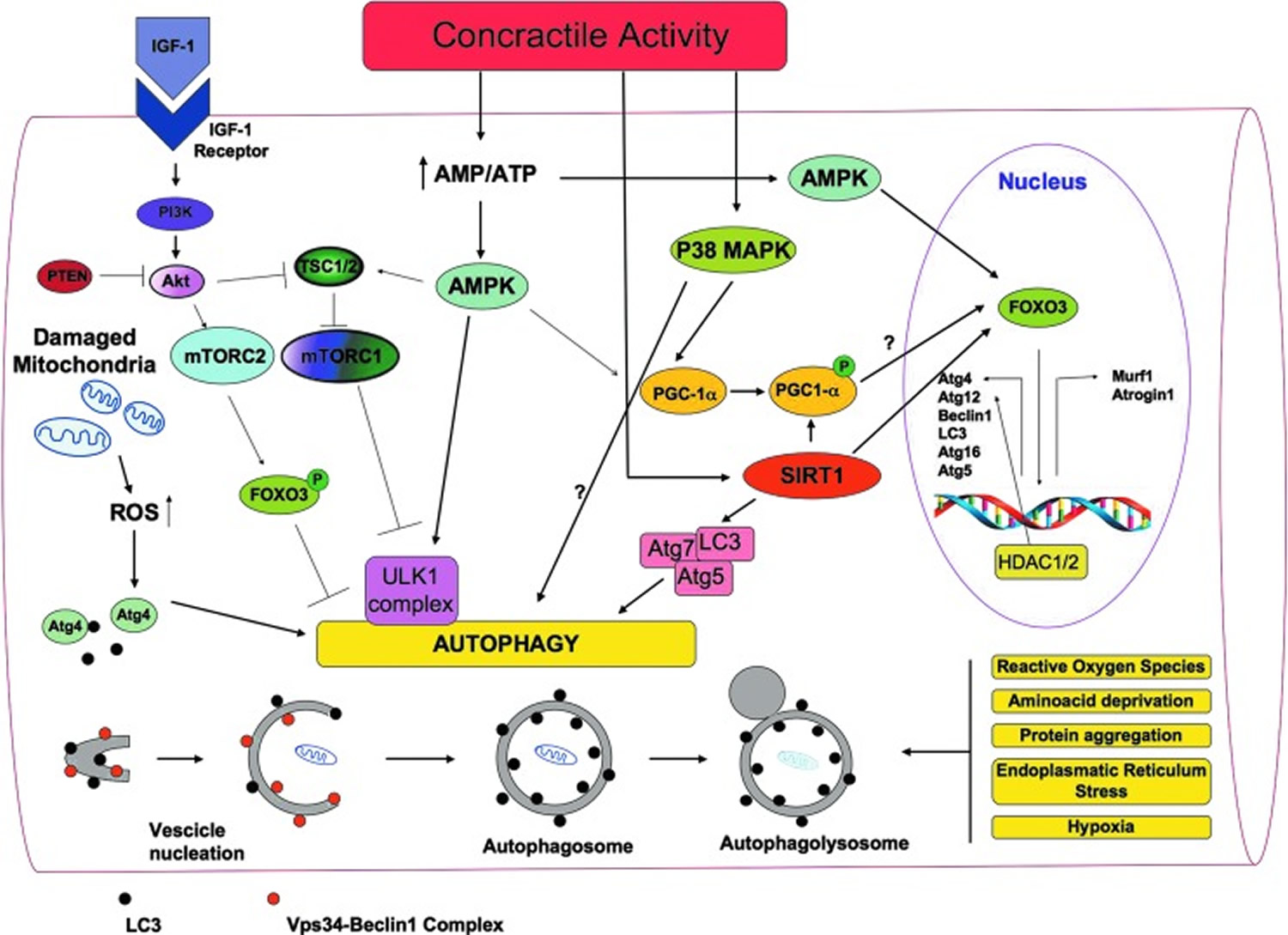
Figure 6. A general overview of the signaling molecules involved in the regulation of autophagy in skeletal muscles during exercise
[Source 19]What is the best way to build muscle?
Bodybuilders size and shape depend largely on their genetic factors, which is why it is difficult for a naturally thin person to put on muscles. The human body can change to a limited extent through weight training and increased food intake. Gaining or regaining weight can be just as difficult as losing weight. When done in a smart, healthful way, many of the same basic principles apply to both gaining and losing weight.
Lean muscle mass naturally diminishes with age. Your body fat percentage will increase over time if you don’t do anything to replace the lean muscle you lose over time. Strength training (see below) can help you preserve and enhance your muscle mass at any age.
Lifting weights or doing physical activities such as push-ups, pull-ups and squats 2 or 3 days a week will help you build strong muscles. Only intense strength training, along with certain genes, can build large muscles. Like other kinds of physical activity, muscle-strengthening activities will help improve your health and also may help you control your weight by increasing the amount of energy-burning muscle.
Muscle building also called muscle hypertrophy is defined as an increase in skeletal muscle size is the process of increasing muscle size, density, and shape, typically through weightlifting and resistance training 70, 71, 72, 73, 74, 75, 76. Research has shown that in order to increase muscle mass, stress must be put on the body, leading to increased hormone release, and increased flow of nutrients into the muscle, and with rest, muscles will grow 77, 78, 79, 80, 81, 82, 83, 84.
To get a bigger muscle, you can:
- Use a reps-and-rest cycle. Aim for 6–12 reps per set, with 60–90 seconds of rest between sets.
- Lift heavy weight. Lifting too light a weight won’t lead to the same definition gains.
- Vary your exercises. This will help you work different muscle fibers.
- Progressively increase the resistance over time.
- Muscle growth is typically experienced after 6 to 7 weeks of resistance training 85. Muscle growth is more common in fast-twitch than in slow-twitch muscles. Type 2A fibers exhibit the greatest growth, more so than type 2B and type 1 fibers.
- Eat a healthy diet rich in macronutrients, especially protein 86, 87.
However, gaining lean body weight is a slow process that takes months and years rather than days and weeks. Most muscle tissue is made up of different kinds of proteins. When you lift heavy loads, your muscles tear and your body experiences metabolic stress 88. In response to this, your body tells the proteins to increase, and the muscles slowly grow 88. Then, to keep growing your muscles, you have to keep increasing weightlifting volumes over time 88. A beginner new to weightlifting who uses full body workouts three times a week can expect to build 1/2 to 1 pound per week or 6 to 12 pounds of muscle in 3 months. An experienced lifter can build 1/4 to 1/2 pound per week or 3 to 6 pounds of muscle in 3 months.
There are several ways you can train to make your muscles bigger. Most hypertrophy training plans focus on lifting heavier loads for a smaller number of reps and sets. However, different bodies might respond differently to the same programs, so there is usually some trial and error when finding your optimal training plan.
According to the National Academy of Sports Medicine (NASM), muscle building training can sometimes result in overuse injuries like tendonitis or tendinosis or low-grade muscle tears, especially when you don’t properly rest and recover 88. Lifters who try to lift too much or have poor form can get more serious acute injuries like ruptured discs, ligament tears, fractures, or high-grade muscle tears 88.
Most of these risks can be avoided if you follow a structured program from a qualified trainer who knows your capabilities 88. In fact, muscle building is a more advanced form of strength training. According to the Optimum Performance Training Model (OPT Model), a 5-phased fitness training system developed by Dr. Mike Clark to guide National Academy of Sports Medicine (NASM) personal trainers to help their clients improve their performance, training, and recovery 89. The Optimum Performance Training Model (OPT Model) is based on human movement science principles, including biomechanics, kinesiology, and exercise physiology. It combines a variety of exercises, including:
- Flexibility training
- Cardiorespiratory training
- Core training
- Balance training
- Plyometric training
- Speed, agility, and quickness training
- Resistance training
- Stabilization endurance training
The Optimum Performance Training Model (OPT Model) progresses people through 5 phases 89:
- Phase 1 Stabilization and Endurance. Stabilization and Endurance is the foundation of the entire OPT Model. Before you start building training, you should have good stability, muscle endurance, and optimal movement patterns to prevent injury. During this first phase, you will perform 12 to 20 repetitions per set, your movement speed will slow down, and the intensity/weight used for exercises reduced to promote muscular endurance and ensure correct form and technique. Phase 1 is a great starting point for those who are new to training and is an opportune time to do questionnaires and fitness assessments to determine goals, establish baselines for training, and identify any movement compensations, respectively. And, for more experienced clients, Stabilization and Endurance phase is a great to include in their program to add different stresses and challenges to their body and will also become a critical phase to cycle back through between training periods in the other phases. Reinforcing correct movements in this phase can lead to strength gains — even with lighter weights — because of enhanced joint and postural control, and coordination. When progressing in this phase, a primary focus is on increasing proprioceptive demand (controlled instability) of the exercises, rather than just increasing the amount of weight you use.
- Phase 2 Strength Endurance. The Strength Endurance Phase gives you the chance to acclimate to heavier weights and higher training intensities. Workouts in the Strength Endurance Phase use superset techniques in which you will follow a more traditional strength exercise such as a bench press with an exercise that has similar biomechanical motions but requires more stabilization to perform (like a stability ball push-up). The Strength Endurance Phase is the logical next step from Phase 1 for increasing the intensity of your workouts. Sets increase to 2 to 4, repetitions will stay high (8-12 per exercise / 16-24 per superset). The supersets combined with decreased rest periods will elevate the challenge considerably leading not only to noticeable improvements in your strength and endurance but more significant your calorie expenditure too.
- Phase 3 Muscular Development/Hypertrophy. Phase 3 of the OPT Model is all about building strength and developing muscle. Muscular Development/Hypertrophy training is ideal for the adaptation of maximal muscle growth, by focusing on higher volumes of work at moderate-to-high intensity levels and with minimal rest periods between exercise sets. These training variables contribute to cellular changes that result in an overall increase in muscle size. If caloric intake is appropriate, the increased intensities and training volumes, and decreased rest periods experienced in this phase also make it great for those who aspire to change their body composition through fat/weight loss. Typically, workouts in this phase involve performing 3 to 6 sets of 6 to 12 reps per resistance exercise at intensities ranging from 75 to 85% of your one-rep max (the heaviest weight you can lift for just one go or for a single lift) or 1RM. You’ll typically be lifting at 85 to 100% of your one-rep max (1RM), so knowing your limits ensures your workouts remain challenging yet safe. And, don’t make the mistake of thinking your max on one lift applies to another – your bench press one-rep max (1RM) might be worlds apart from your back squat. Go here for a free online One Rep Max (1RM) calculator (https://www.nasm.org/resources/one-rep-max-calculator). You can also use the Standard One-Rep Max (1RM) table below. The “standard” one-rep max (1RM) values can differ based on age, gender, weight, training history, and other factors. Below is a generalized range for an average adult male, assuming he’s in decent shape but not necessarily an elite athlete.
- Phase 4 Maximal Strength. Phase 4 is geared towards enhancing your ability to produce maximal muscular force. Accomplishing this requires maximal efforts and lifting near-max/maximal loads during resistance training—ranging anywhere from 85 to 100% of your one-rep max for 1 to 5 repetitions. While similar to Muscular Development training in scope, developing maximal strength largely depends on neuromuscular adaptations resulting from consistently and progressively overloading muscles with higher intensities (loads). Because you will be lifting very heavy weights (near-max/maximal loads) in this phase, longer rest periods between exercise sets and higher volumes of training are usually required to optimize strength gains.
- Phase 5 Power. The 5th phase of the OPT Model focuses on using high force and high velocity exercises to increase power. One method to improve power is to perform supersets with contrasting loads. Like the supersets outlined and used in Phase 2 of the OPT Model, supersets in 5th phase will consist of two biomechanically similar exercises performed back-to-back. The first exercise should challenge near-max/maximal strength for 1 to 5 reps, and the second exercise should involve and challenge moving relatively low loads as fast and explosively as possible for 8 to 10 reps. The rationale for this sequence is to activate and tap into as many muscle fibers as possible with the maximal lift, while utilizing explosive exercises directly after to improve how quickly and efficiently those muscle fibers contract. Keeping with the upper body exercise theme used previously, an example Phase 5 superset is performing a bench press followed by a medicine ball chest pass.
Table 3. Standard One-Rep Maximum (1RM)
| Experience Level | Deadlift (kg) | Bench press (kg) | Squat |
|---|---|---|---|
| Beginner | 60-100 | 40-70 | 50-85 |
| Intermediate | 100-140 | 70-100 | 85-125 |
| Advanced | 140-180 | 100-130> | 125-170 |
| Elite | 180 | 130 | 170 |
Footnote: Always consult with a fitness professional to set realistic and safe goals.
[Source 90 ]The Optimum Performance Training Model (OPT Model) should be thought of as a staircase, guiding you through different physical adaptation levels. This journey will involve going up and down the stairs, stopping at different steps, and moving to various heights, depending on your goals, needs, and abilities.
Table 4. Optimum Performance Training Model (OPT Model)
| Summary of the Optimum Performance Training Model (OPT Model) | |||
|---|---|---|---|
| Level | Phase | Primary Adaptations | Primary Methods of Progression |
| Stabilization | 1. Stabilization Endurance Training |
|
|
| Strength | 2. Strength Endurance Training |
|
|
| 3. Hypertrophy Training (Muscular Development Training) |
|
| |
| 4. Maximal Strength training |
|
| |
| Power | 5. Power Training |
|
|
To build muscle, you can:
- Train consistently: Train 2 or 3 times per week to give your muscles time to recover. Commit to a regular training routine and don’t take weeks off.
Make your workouts short and intense rather than long and leisurely. - Eat a protein-rich diet: Eat lean protein sources like chicken, fish, lean meat, and plant-based protein powder. The recommended protein intake is 0.8 to 1 gram per kilogram of body weight per day 91. For strength training athletes adequate protein intake should range between 1.2 and 1.7 grams of protein per kilogram of body weight per day or 0.5 to 0.8 grams per pound of body weight 92, 93, 94, 95. Try to eat protein within 30 minutes of a workout.
- Get enough rest: Your body releases growth hormones during sleep and rest, which helps muscles grow and repair.
- Do resistance training: Use weights, resistance bands, or your own body weight to build muscle. You can try:
- Compound exercises that work multiple muscle groups, like squats and bench presses
- Body weight exercises like pushups, pullups, lunges, and planks
- Progress your strength training: Increase the amount of weight you lift.
- Find a qualified training partner: A gym instructor or personal trainer can help you do exercises correctly and reduce your risk of injury.
- Mix in cardio: Short, sharp cardio can help burn fat.
You can expect to see noticeable muscle growth after 8 to 12 weeks, but it depends on many factors, including: nutrition, intensity, frequency, age, genetics, and sex.
The science behind muscle building
It is hypothesized that there are 3 three main mechanisms involved in the process of inducing muscle hypertrophy to resistance exercise 96, 97, 98, 99. These are:
- Muscular Damage
- Metabolic Stress
- Muscular tension.
Muscular Damage
Exercise training can result in localized damage to muscle tissue, which under certain conditions is theorized to generate a hypertrophic response 96, 100. When you perform an activity that is harder in some way than your current ability, that activity produces stress within one or more body systems that consequently requires these systems to adapt. It does this in many ways, from chemical to structural alterations, but the underlying principle remains the same 101, 102, 103, 101, 100, 104.
Stress causes an adaptation within a particular body system, which the body then responds to by reorganising and repairing itself to be better prepared for next time 105. In order to drive further adaptation, higher stress must be applied to signal to the system that an adaptation is required. The adaptation of the body system is specific to the stress applied, in other words, you adapt in a way that is directly related to the stress experienced 105. For example, calluses form on your hands as an adaptation to picking things up. They develop on your hands and not on your face because that is the area where the stress was applied. Gradual and planned increases in this cycle of stress, recovery and adaptation are what scientists refer to as progressive overload and it forms the basic principle of almost all human performance-based training around the world 105. The goal of building muscle is no different.
When you stress muscle tissue appropriately, either through the application of load, volume and time under tension, this creates a certain level of structural and systemic damage within the muscular tissue itself. As a result of that damage, the muscular system reorganises and repairs that tissue to a level above what previously existed. Muscular damage, often as a side effect, creates soreness and inflammation within the tissue and for years it was assumed that more soreness equated to more growth. Thankfully, scientists now understand that not to be the case, despite this misconception still being repeated in many places. That being said, while the level of soreness does not directly correlate to more muscular growth, the basic concept of stress, recovery and adaptation of the muscle tissue is still a factor that must be considered in the process. For years, muscular damage was the be-all and end-all of training, but we now understand several other important factors that contribute to the process of inducing muscle hypertrophy – metabolic stress and muscle tension.
Metabolic Stress
Metabolic stress is the concept of eliciting an influx of metabolic products into the muscle through manipulating reps, sets and rest time in an exercise. More commonly referred to as the “burn” or “pump”, this concept has existed for many decades in bodybuilding styles of training but has only more recently been researched and understood on a scientific level. The hypothesis that currently exists reports that increased metabolic activity in the muscle tissue (specifically metabolite accumulation) improves motor unit recruitment and drives the release of anabolic hormones accelerating muscle hypertrophy 106, 103, 107, 108, 109, 110, 111.
This concept was established through numerous studies where various set and rep range protocols were manipulated at the results closely studied. It was established that the primary driver of metabolic stress is:
- Higher volume exercises of between 10 to 12 repetitions.
- Performed at 70-80% 1RM (1 repetition maximum) for multiple sets. 1 repetition maximum (1RM) is the heaviest weight a person can lift once while using proper form and performing a full range of motion. 1RM (1 repetition maximum) is a reliable way to measure your overall muscular strength and is often used by strength and conditioning coaches. 1RM is used to determine the appropriate load and intensity for resistance training. For example, if you want to do 5 back squats, you can calculate the weight to use by taking 85–90% of your 1RM.
- With only 30 seconds to 1 minute of rest between each set.
While this might seem simple on the surface, how can this be applied on a practical level to your training? The fundamental problem with the metabolic stress model (apart from turning each workout into a hellish nightmare) is that it is very difficult to practically apply during large compound lifts, which should always form the foundation of any good program. You simply cannot perform 6-8 sets of 12 reps with only 1 minute of rest with any meaningful amount of weight (certainly not a true 80% of a 1RM which is advocated in several studies), at the very least not without compromising the form and safety of the trainee. This means that this type of training typically limits itself to isolation-based exercises that neither utilize as much muscle mass nor provide the systemic stress that compound lifts do. This reduces their overall ability to make any substantial change to muscle mass. The danger with this is when programs are designed based purely on this principle and forget to factor in the other primary factors of muscle hypertrophy, you are leaving a huge amount of untapped potential in your training, especially for beginners as well as leaving yourself open to possible injury, overtraining and chronic soreness 105.
Muscle Tension
The previously mentioned mechanisms of muscle hypertrophy cannot happen without the third being present. Increases in muscular tension are the only reliable and constant factor across all demographics that must be in place for hypertrophy to occur. Mechanically induced tension produced both by force generation and stretch is considered essential to muscle growth, and the combination of these stimuli appears to have a pronounced additive effect 112, 113, 99. More specifically, muscular tension is the contraction of the sarcomeres within the muscle tissue to produce force. Yet it should be noted that hypertrophy from muscular tension can be produced in the absence of both significant metabolic stress and muscular damage. How much muscular tension and under what conditions are where the debate lies 105.
Theoretically, muscular tension is produced whenever a muscle is under contraction, but in the gym, you typically obtain muscle tension under two conditions.
- When heavy weight is lifted for lower repetitions, or
- When lighter weights are lifted for higher repetitions but taken very close to failure.
These two events are similar in a sense that the repetitions involuntarily slow down the further through the set you move and both events can, and will, create fatigue forcing you to exert more force and effort into finishing the set 105. However, there are some less obvious differences between the two in terms of their performance.
A heavy set of say 5 at 90% 1RM (1 repetition maximum), requires more motor unit recruitment from the start of the set, due to the outright force production necessary to lift the weight in the first place. The more force you must produce, the more motor units must be recruited (particularly the larger type 2 fibres). In a lighter, but still taxing 12RM set (a repetition maximum of 12, which is the most weight you can lift and perform 12 repetitions of an exercise with proper form), the first 6 reps are submaximal, meaning that they do not require close to the maximal effort to move. However, as the set continues, you must then call into recruitment of more motor units (type 2) to complete the set. A moderate repetition scheme, like 8 to 12 repetitions per set with 60% to 80% of 1RM, is best for optimizing hypertrophic gains 114. Some research suggests that alternating between blocks of 10-12 reps at 70% and blocks of higher intensity, like 3-5 reps with 90% 1RM, can achieve similar muscular gains.
The question is then placed as to what method do you choose? Both can have significant results in the production of muscle hypertrophy and can generally be safely used by the most individuals. There are several things such as time, specific goals, and access to equipment, that all play into this. You want to choose the method that give you the most bang for your buck in the most efficient way possible.
Firstly, heavy sets feel heavy because they ARE heavy. They also give the added benefit of calling into contraction a higher number of motor units in order to move the weight. Secondly, you know that hypertrophy occurs as a result of more motor units being used, but also the ability to produce maximal force increases in those muscle fibers when they are called into contraction. In other words, the adaptation occurs in both the size of the muscle but also in its ability to produce force.
In contrast, lighter weights taken close to failure may feel difficult, and in some cases, will produce a good hypertrophic response. However, this method pales in comparison to the increase in overall force production of heavier weights completed for lower reps. The problem here is that due to the limited amount of adaptation towards more force production, the weight quickly becomes the bottleneck to continued progress. If you are getting bigger, but are not able to go up in weight, how do you continue to drive progress without now changing the rep ranges away from those best suited to continue to build muscle?
The point being made here is that a lot of time beginners looking to gain muscle will jump immediately into a highly complicated multi-factor program that is backed by “science” only to spend the next year on a hamster wheel making little to no progress. Getting the foundations right in any training regime is fundamental before you start debating the ins and outs or finer details, and anyone who has been training for any amount of time generally arrives at the same conclusion eventually.
The reality is that all you need at the beginning is a simple program that focuses on large compound lifts which utilize and progressively overload as much muscle mass as possible over the full range of motion.
Many people have wasted years in the gym only to find out that the answer was simple, not complexity. As your training progresses and goals become more clearly defined, then complexity can be added, but don’t waste precious time by trying to get complicated before you have to.
What builds muscle the most?
The best way to build muscle is to lift heavy weights and have proper nutrition. Weight training is the best way to keep the muscle mass you have and even increase your muscle mass. You’ll also need to consume more protein than your body removes to build muscle. Getting enough sleep is also important for muscle growth because your muscles recover and grow while you’re asleep. Try to get 8 to 10 hours of sleep per night. The National Sleep Foundation, an organization of doctors and researchers who specialize in sleep, recommends that adults (between the ages of 18 and 64) achieve between 7 to 9 hours of sleep per night 115. If you’re older than 65, you may need a little less: seven to eight hours is recommended.
For both men and women, sleeping less than 6 hours per night could result in higher belly fat levels. A lack of sleep can elevate the sympathetic nervous system (SNS), responsible for stimulating the metabolism to produce the energy for physical activity. Insufficient sleep could boost the hormones cortisol and epinephrine (adrenaline), which help release free fatty acids that you use for energy. When there is low physical activity, the free fatty acids can deposit in the adipose (fat) tissue of your abdominal region resulting in additional belly fat 116.
Another way that insufficient sleep could lead to weight gain is through the production of specific hormones. Grehlin is a hormone responsible for stimulating hunger. Leptin performs the opposite function and tells the body when it has had enough food intake. Poor sleep is associated with leptin levels decrease and ghrelin rises, potentially resulting in an increase in appetite and over-eating 117. In addition, staying awake late into the evening allows you more opportunities for mindless snacking on calorically-dense food. Furthermore, the added fatigue from lack of sleep may also lead you to skipping out on exercising, another setback for reaching your weight loss goals.
Insufficient sleep could also impair your body’s ability to properly recover from a challenging strength training workout designed to promote muscle growth. Growth hormone (GH), an anabolic hormone responsible for repairing muscle tissue damaged during exercise, is produced during stage 3 of Non-Rapid Eye Movement (NREM) sleep; achieving optimal sleep could be helping muscles grow 118.
While sleeping, your body will experience multiple cycles of sleep, each of which can last between 70 to 120 minutes; there are three stages of Non-Rapid Eye Movement (NREM) sleep and a fourth stage of Rapid Eye Movement (REM) sleep and over the course of one night, your body goes through the sleep stages every 90 minutes or so 119.
The Sleep Stages 120:
- Stage 1. Stage 1 of the sleep cycle is the lightest phase of sleep and generally lasts about seven minutes. The sleeper is somewhat alert and can be woken up easily. During this stage, your heartbeat and breathing slow down while your muscles begin to relax. Your brain produces alpha and theta waves.
- Stage 2. In Stage 2, your brain creates brief bursts of electrical activity known as “sleep spindles” that create a distinct sawtooth pattern on recordings of brain activity. Eventually, the waves continue to slow down. Stage 2 is still considered a light phase of sleep, but the sleeper is less likely to be awakened. Heart rate and breathing slow down even more, and the body temperature drops. Stage 2 lasts around 25 minutes.
- Stage 3. Stage 3 represents your body falling into a deep sleep, where slow wave sleep occurs. Your brain produces slower delta waves, and there’s no eye movement or muscle activity. As your brain produces even more delta waves, you enter an important restorative sleep stage from which it’s difficult to be awakened. This phase of deep sleep is what helps you feel refreshed in the morning. It’s also the phase in which your body repairs muscle and tissue, encourages growth and development, and improves immune function.
- Rapid Eye Movement (REM) Sleep. About 90 minutes after falling asleep, your body enters REM (Rapid Eye Movement) sleep and is named so for the way your eyes quickly move back and forth behind your eyelids. REM sleep is thought to play a role in central nervous system (brain and spinal cord) development in infants, which might explain why infants need more REM sleep than adults. REM sleep pattern is characterized by dreaming, since your brain is very active during this stage. Physically, your body experiences faster and irregular breathing, increased heart rate, and increased blood pressure; however, your arm and leg muscles become temporarily paralyzed, stopping you from acting out your dreams. REM sleep increases with each new sleep cycle, starting at about ten minutes during the first cycle and lasting up to an hour in the final cycle. Stage 4 is the last stage before the cycle repeats. This sleep stage is critical for learning, memory, daytime concentration, and your mood.
While all sleep stages are important, Stage 3 and REM sleep have unique benefits. One to two hours of Stage 3 deep sleep per night will keep the average adult feeling restored and healthy 120. If you’re regularly waking up tired, it could be that you’re not spending enough time in that deep sleep phase. Meanwhile, REM sleep helps your brain consolidate new information and maintain your mood – both critical for daily life 120. Talk to your doctor if you feel you are not getting the restful sleep that you need.
How to many calories I need?
You can calculate your basal metabolic rate (BMR) or resting metabolic rate (RMR) using the Mifflin-St Jeor equation 121, which is considered more accurate than the Harris-Benedict equation, especially for lean people. According to the Academy of Nutrition and Dietetics Evidence Analysis Library (EAL), the Mifflin-St. Jeor equation accurately predicted resting metabolic rate (RMR) using actual body weight within +/- 10% of measured RMR in 70% of obese individuals 122. Of the remaining 30%, 9% were overestimations and 21% were underestimations. The individual error range was a maximum overestimate of 15% to a maximum underestimate of 20%” 123. While the Harris-Benedict and WHO equations are often used in clinical practice with reasonable accuracy, results have been mixed regarding their applications to individuals who are overweight or obese 124.
The Mifflin-St Jeor formula for calculating your basal metabolic rate (BMR) or resting metabolic rate (RMR):
- Males Basal metabolic rate [BMR] (kcal/day) = (10 X weight in kilograms) + (6.25 X height in centimeters) – (5 X age in years) + 5 (kcal/day)
- Females Basal metabolic rate [BMR] (kcal/day) = (10 X weight in kilograms) + (6.25 X height in centimeters) – (5 X age in years) – 161 (kcal/day)
You can also use the free online Basal Metabolic Rate (BMR) calculator here: https://www.nasm.org/resources/calorie-calculator
Or the Body Weight Planner (https://www.niddk.nih.gov/health-information/weight-management/body-weight-planner).
The Body Weight Planner allows you to make personalized calorie and physical activity plans to reach a goal weight within a specific time period and to maintain it afterwards.
The Basal Metabolic Rate (BMR) calculator factor in your activity levels, overall goals, and calorie usage to help you craft a weight-loss plan.
Once you have found your basal metabolic rate (BMR), multiply your BMR by your Physical Activity Levels to provide a baseline daily caloric level for weight maintenance:
- Sedentary (light physical activity associated with typical day-to-day life) = 1
- Low Active (walking about 1.5 to 3 miles per day at 3 to 4 miles per hour, in addition to the light physical activity associated with typical day-to-day life), For males = 1.11 and females = 1.20
- Active (walking more than 3 miles per day at 3 to 4 miles per hour, in addition to light physical activity associated with typical day-to-day life: 60 minutes of at least moderate intensity physical activity). For males = 1.25 and females = 1.27
- Very Active (walking more than 7.5 miles per day at 3 to 4 miles per hour, in addition to light physical activity associated with typical day-to-day life: 60 minutes of at least moderate to vigorous intensity physical activity). For males = 1.48 and females = 1.45
Your Total Daily Energy Expenditure (TDEE) gives you the estimated number of calories you need to maintain your current weight based on your activity level.
To find your Total Daily Energy Expenditure (TDEE) multiply your Basal Metabolic Rate (BMR) by your Physical Activity Levels
For example:
- Sedentary (little to no exercise): BMR x 1
- Lightly active (walking about 1.5 to 3 miles per day at 3 to 4 miles per hour, in addition to the light physical activity associated with typical day-to-day life): BMR x For males = 1.11 and females = 1.20
- Moderately active (moderate exercise/sports 3-5 days/week): BMR x 1.55
- Very active (walking more than 7.5 miles per day at 3 to 4 miles per hour, in addition to light physical activity associated with typical day-to-day life: 60 minutes of at least moderate to vigorous intensity physical activity): BMR x For males = 1.48 and females = 1.45
- Super active (very hard exercise & physical job or 2x training): BMR x 1.9
You can increase your basal metabolic rate (BMR) by:
- Exercising more, especially interval training
- Weight training to build muscle mass
- Eating fat-burning foods
- Getting enough sleep
After calculating your basal metabolic rate (BMR) or resting metabolic rate (RMR), your RMR should be multiplied by an appropriate physical activity factor to provide your baseline daily caloric level for weight maintenance. Once your baseline caloric level is known, your recommended calorie intake should be reduced to facilitate your weight loss.
If you want to lose weight, subtract 500 to 1000 calories from your Total Daily Energy Expenditure (TDEE) to get a daily intake goal. For weight gain, add extra calories. Reducing your calorie intake by 500 calories is a common strategy to yield a weight loss of approximately one pound per week, although reductions of up to 750 calories per day are sometimes used 125.
Another approach is to reduce your current caloric intake by 30% 125. Diets that reduce caloric intake relative to energy expenditure result in weight loss, regardless of macronutrient composition 125.
Here’s how to estimate how long it will take to reach your goal:
Jessie’s current weight is 150 lbs. She wants to lose 20 lbs.
- 150lbs – 20lbs = 130lbs.
- 20lbs loss at 2lbs/week = 10 weeks.
- It will take Jessie about 10 week to lose the weight.
Remember, these are general guidelines only. It’s crucial to monitor your progress and adjust as necessary. Consulting with a nutritionist or health professional is always recommended for personalized advice.
What happens when your calories are too low?
Consuming calories below your body’s needs for an extended period can lead to various physiological and psychological consequences. Here’s what can happen when your caloric intake is too low:
- Slower metabolism: Your body might slow down its metabolic rate as a defense mechanism to conserve energy. This can make weight loss harder over time and weight regain more likely once normal eating resumes.
- Nutrient deficiencies: Low calorie intake can lead to inadequate intake of essential vitamins and minerals. Over time, this can result in conditions like anemia, osteoporosis, and impaired immune function.
- Loss of muscle mass: Your body might start breaking down muscle tissue for energy, especially if protein intake is inadequate. This can further slow down metabolism and lead to weakness.
- Hormonal changes: Reduced calorie intake can affect hormone levels, leading to disruptions in menstrual cycles for women, reduced bone density, and other hormonal imbalances.
- Reduced energy and fatigue: You might feel constantly tired or find it difficult to concentrate.
- Mood changes: Low caloric intake can influence mood. This can result in irritability, depression, or anxiety.
- Impaired Immune Function: Your body might become more susceptible to infections due to a weakened immune system.
- Hair and skin problems: You might experience hair loss, dry skin, or brittle nails due to inadequate nutrient intake.
- Digestive problems: Constipation or other digestive issues can occur as a result of reduced fiber and fluid intake.
- Fertility issues: Low calorie and nutrient intake can lead to fertility problems in both men and women.
- Cardiovascular problems: Chronic low calorie intake can affect heart health, leading to low blood pressure, irregular heart rhythms, or other cardiovascular issues.
- Increased risk of gallstones: Rapid weight loss from very low-calorie diets can lead to the development of gallstones.
What should bodybuilders eat?
Most of body builders can meet all of their nutritional needs from food. Tips for making healthy eating choices:
- Eat a variety of foods. Eat a variety of foods from each of the five food groups daily. Healthy choices include fruits, vegetables, whole grains, protein foods, and fat-free or low-fat dairy. Foods are grouped together because they provide similar amounts of key nutrients. For example, key nutrients of the milk, yoghurt, cheese and alternatives group include calcium and protein, while the fruit group is a good source of vitamins, minerals, antioxidants. As a bonus, choosing a variety of foods will help to make your meals interesting, so that you don’t get bored with your diet.
- Eat fruit instead of drinking it. Eating fruit is linked to a reduced risk of several health conditions, but fruit juices are more likely to spike blood sugar levels.
- Add healthy fats. Healthy fats like monounsaturated and polyunsaturated fats can help lower cholesterol and protect your heart. You can find healthy fats in foods like olive oil, nuts, avocados, and some types of fish.
- Drink water. Sipping water throughout the day can help keep you full and hydrated. Sometimes thirst is mistaken for hunger.
- Reduce added sugar. Too much added sugar in your diet can contribute to weight gain, obesity, type 2 diabetes, and heart disease.
- Chew your food well. Chewing your food well can help you make healthier food choices.
- Sit at the table to eat. Sitting at the table to eat can help you focus on your food and internal cues for hunger or fullness.
Some bodybuilders and athletes use dietary supplements to try to improve their strength, muscle mass, and energy. However, many of these types of products contain harmful ingredients. Also, for some substances, including glutamine, choline, methoxyisoflavone, quercetin, zinc/magnesium aspartate, nitric oxide, and L-arginine, there’s no clear evidence that they improve athletic performance.
Carbohydrates
Carbohydrates are your body’s fuel. Carbohydrates also play roles in gut health and immune function 126. For healthy children and adults, carbohydrates should make up approximately 45 to 65% of energy intake based on the minimum required glucose for brain function 126. However, some carbohydrates are more nutritious than others.
Foods that contain the most carbohydrates include:
- Fruit.
- Vegetables, especially potatoes and corn.
- Legumes, including dried beans, peas and lentils.
- Grains.
- Bread.
- Breakfast cereal.
- Rice, pasta and noodles.
- Low-fat milk and yoghurt.
These foods are rich in vitamins, minerals and antioxidants and are generally low in fat. This makes them well suited to a healthy eating plan. Some are excellent sources of dietary fibre, including wholegrain varieties, legumes, fruit and vegetables.
Foods with lots of added sugar like soft drinks, alcohol and sweets are another source of carbohydrates, but these contribute extra kilojoules with few vitamins and minerals.
Optimal carbohydrate intake should consist of high fiber, nutrient-dense whole grains, fruits, vegetables and legumes, without the added sugar 127.
The Dietary Guidelines for Americans recommends that carbohydrates should make up 45 to 65% of one’s daily calories 128. To calculate how many grams of carbohydrates you need, multiply your daily calorie requirements by 0.45 and 0.65 to obtain calories from carbohydrates.
- (A) 0.45 x 2000 = 900 calories
- (B) 0.65 x 2000 = 1300 calories
Divide answers in step 1 by 4 since there are 4 calories per 1 gram of carbohydrate
- (A) 900/4 = 225g of carbohydrate
- (B) 1300/4 = 325g of carbohydrate
Carbohydrates are eaten in the form of simple carbohydrates, like monosaccharides and disaccharides, or complex carbohydrates, like oligosaccharides and polysaccharides 126. Monosaccharides are the basic building blocks of all carbohydrates and include glucose, fructose, and galactose. Glucose is the simplest type of carbohydrates and is the major source of energy for your body’s cells 126. Glycogen is the storage form of glucose in animals and is present in the liver and muscle, but there is little to none in the diet.
Disaccharides contain two sugar units and include lactose, sucrose, and maltose. Lactose is a carbohydrate found in milk, and sucrose is basic table sugar.
Oligosaccharides consist of 3 to 10 sugar units and include raffinose and stachyose, which are in legumes.
Polysaccharides include greater than ten sugar units and consist of starches, glycogen, and fibers, like pectin and cellulose. Starches like amylose are in grains, starchy vegetables, and legumes and consist of glucose monomers.
Fibers are plant polysaccharides like pectin and cellulose found in whole grains, fruits, vegetables, and legumes but are not digestible by humans. However, fibers play a major role in gut health and function and can be digested by microbiota (microorganisms) in the large intestine 129. The recommended fiber intake is greater than 38 g for men and 25 g for women, which is the intake that research has observed to lower the risk of coronary artery disease (a heart disease that affects the main blood vessels that supply blood to the heart).
Does eating more carbohydrates cause body fat?
If carbohydrates control body fat, then you would expect that low-carb diets are less “fattening” than higher carbohydrate diets. This means that low carb diets should add less body fat to people than higher carbohydrate diets do. This is true in the most basic sense in that when you overconsume calories (Calorie IN more than Calorie OUT). You do store at least some of the excess calories as body fat. But studies don’t support that view that low-carb diets are less “fattening” than higher carbohydrate diets. It turns out that excess carbohydrates are relatively difficult to store as body fat, at least compared to fats.
In one study where people were overfed carbohydrates and fats, fats were stored ~20% more efficiently than carbs 130. In another study where people were overfed carbohydrates, there was a very minimal conversion of carbohydrates to stored body fat, indicating that it is very inefficient to turn carbohydrates into body fat 131.
Furthermore, low-carb diets are not necessary for weight loss, nor are they alone sufficient for weight loss. Carbs don’t necessarily control body fat after all. Over the last several decades there have been dozens of scientific studies comparing low-carb diets to other diets to examine their efficacy 132. There are plenty of studies whose results do not support the idea that carbohydrates per se control body fat. For example, in one study of 4,451 people, there was a lower risk of being obese or overweight if you consumed a moderate to high carbohydrate diet when compared to a lower carbohydrate diet 133. That study found consuming a low-carbohydrate (approximately <47% energy) diet is associated with greater likelihood of being overweight or obese among healthy, free-living adults 133.
Another study found that there was no real association between BMI and daily carbohydrate intake, suggesting that if carbohydrates did control body fat, it would be a relatively minor effect 134.
Ultimately, science tells us that carbohydrates are not more fattening than fats; in fact, it would make more sense to eat a few too many carbohydrates than a few too many fats. Indeed, this is what you see when you follow people who over-consume carbohydrates versus fats – they tend to gain a little less body fat 135.
Are low-carb diets are better for weight loss than other diets?
The majority of the clinical trials that have examined whether low-carb diets are better than other diets for fat loss show that low-carb diets result in the same amount of weight loss as other diets 136.
When you tightly control people’s diets and measure virtually every part of their metabolism, it is apparent that low-carb diets are not better for weight loss 137. They might be slightly worse for body fat loss than low-fat diets. This holds true even if you go to very low levels of carbohydrate intake 138.
Furthermore, when people adopt a low-carb diet in the real world and over more extended periods, they still see very similar results for weight-loss.
The primary findings from these studies have been:
- Low-carb diets are not necessary for weight loss. Virtually all types of diets can and do result in weight loss when there is a negative energy balance (i.e. an energy deficit).
- From a body fat mass perspective, low-carb diets may result in inferior fat mass reductions in shorter term diets.
- Adherence to low-carb diets is no better or worse compared to most other forms of dieting.
- Low-carb diets often result in more immediate water weight loss and glycogen depletion than moderate-carb diets.
In conclusion, although the idea that carbohydrates control body fat has been popular in the media, there is little scientific evidence to support it. Unless you have extreme levels of carbohydrate intake (Calorie IN more than Calorie OUT), there is no real link between carbohydrates and body fat. From scientific studies, it turns out that carbohydrates are less fattening than dietary fat. When followed in the real-world, low-carb diets can be useful for weight loss, but they are not any more effective than other low calorie diets.
Very low-carb diets can often result in a state called ketosis. This occurs when dietary carbohydrates are low enough, or fat is high enough, such that the body begins producing ketones at a level that allows them to accumulate.
It is often touted that being in a state of ketosis increases fat loss, but there is no good evidence to suggest that is true. In fact, one recent study showed that fat loss is similar, if not inferior, in a state of ketosis 139. If you choose to adopt a low-carb diet, ketosis may be a result of that process, but should not be the primary focus.
Furthermore, there is some evidence that if someone is an athlete engaging in higher intensity, higher volume exercise, ketogenic diets should be avoided as they can impair performance 140, 141.
Very low carbohydrate diets can come with unwanted and potentially dangerous side effects when followed for extended periods. For example, cardiac dysfunction, impairment of physical activity, hair loss, nausea, digestive issues, and lipid abnormalities are all common side effects.
How many carbs should you eat a day to lose weight?
For otherwise healthy individuals with no underlying medical conditions, there does not appear to be a truly minimal amount of carbohydrates that need to be consumed daily.
Your daily recommendations for carb intake are based on two primary criteria:
- Your total daily calorie requirements (your basal metabolic rate [BMR] or resting metabolic rate [RMR])
- Your intensity or volume of physical activity.
Higher total daily calorie needs come with higher recommendations for total daily carb intake, while lower total daily calorie needs come with lower recommendations. Furthermore, as your body relies heavily on carbohydrate intake for moderate to higher intensity physical activity, carb recommendations will increase as your total volume and intensity of activity increase.
Your total daily calorie intake can be estimated using the Mifflin-St Jeor formula above. However, there are also online tools that can be used that can help you determine how many calories you should consume daily. Such as the free online Basal Metabolic Rate (BMR) calculator here: https://www.nasm.org/resources/calorie-calculator
Or the Body Weight Planner (https://www.niddk.nih.gov/health-information/weight-management/body-weight-planner).
The Body Weight Planner allows you to make personalized calorie and physical activity plans to reach a goal weight within a specific time period and to maintain it afterwards.
After that, the number of carbs grams per unit of body weight can be estimated based on current guidelines from the American College of Sports Medicine and the Academy of Nutrition and Dietetics. These recommendations are generalized as follows 142:
- Light Activity: 3-5 g carb/kg/day
- Moderate Activity (1 hour of moderate exercise): 5-7 g carb/kg/day
- High Activity (1-3 hours of intense, endurance exercise): 6-10 g carb/kg/day
- Very High (4-5 hours of intense, endurance exercise): 8-12 g carb/kg/day
It is often recommended that more moderate carbohydrate intakes (1 to 3 g/kg/day) are consumed even in the context of weight loss.
Your muscles need carbs to fuel and recover from your workouts. At least 40% of your total daily calories should come from good carbs. Eat carbs 60 to 90 minutes prior to your workout, and then eat a combo of carbs and protein (2:1 ratio) within an hour after you finish.
Although many nutrition labels count all carbohydrates toward calorie intake, the truth is not all carbohydrates provide a meaningful number of calories as the human body does not digest and extract energy from all forms of carbohydrates.
In most situations, dietary fiber is considered a non-digestible carb and does not contribute to the total carbohydrate intake. As such, in many cases, fiber is subtracted from total carb intake. The grams of fiber is often subtracted from the total carbs grams to yield a total of usable carbs grams. For example, a food label may list 34 g total carbohydrate consisting of 4 g fiber and 6 g added sugar. By subtracting dietary fiber (4 grams of fiber) from total carbohydrates (34 grams) = 30 grams of usable carbs.
Protein
Protein is an essential nutrient that you need throughout life. Proteins are the building blocks of life. Protein is in every cell in your body. The basic structure of protein is a chain of amino acids called a polypeptide 143, 144, 145. A protein is a chain of amino acids bound to one another via peptide bonds (chemical bond linking amino acids together to form a protein). When someone eats protein, it is broken down into its amino acids. There are hundreds of amino acids exist in nature, but humans use only 20 amino acids, each with distinct chemical and physical characteristics 143, 146. All amino acids contain an amino group (-NH2) and a carboxyl group (-COOH). Amino acids that serve as the building blocks of proteins are alpha amino acids (α-amino acids), having both the amino group (-NH2) and carboxyl group (-COOH) linked to the same carbon atom. In alpha-amino acids (α-amino acids), the two functional groups are bound to a central carbon atom known as the alpha carbon. At the alpha carbon is also a hydrogen atom and a variable side chain, often referred to as the R-group, which gives each amino acid its unique chemical properties. These amino acids are alpha amino acids and classified based on the properties of their R-groups. Amino acids are not only the building blocks of proteins, but some are also precursors for neurotransmitters (e.g., serotonin, dopamine), signaling molecules (e.g., nitric oxide), and metabolic intermediates (e.g., α-ketoglutarate, oxaloacetate).
Your body needs protein to make, maintain, repair and renew bones, muscles, cartilage, hormones, enzymes, neurotransmitters, vitamins, blood and skin cells 126, 147, 146. Proteins provide energy (calories) if necessary, the others are fat and carbohydrates. Proteins do everything from fighting infections to helping cells divide. Protein is also important for growth and development in children, teens, and pregnant women.
Excess or deficiency of protein can lead to disease, resulting in nervous system defects, metabolic problems, organ failure, and even death 143. Clinical symptoms of inadequate intake of essential amino acids may include depression, anxiety, insomnia, fatigue, weakness, and growth stunting in the young. These symptoms are mostly caused by a lack of protein synthesis in the body because of the lack of essential amino acids 146. Kwashiorkor and marasmus are examples of more severe clinical disorders caused by malnutrition and inadequate intake of essential amino acids as a macronutrient 146.
High protein diets can promote weight loss via increased insulin sensitivity, fatty acid oxidation, appetite suppression, and feeling full. However, caution is necessary for people with diabetes who have gout because protein can elevate niacin levels, which may exacerbate gout-related symptoms.
The protein in your food is broken down into parts called amino acids during digestion. Your body needs a number of amino acids in large enough amounts to maintain good health. While there are hundreds of amino acids, humans use only 20 amino acids 143, 146.
Amino acids are classified into three groups 143:
- Essential amino acids. Essential amino acids cannot be made by your body, and must be supplied by food. Essential amino acids do not need to be eaten at every meal. The balance over the whole day is more important. There are 9 essential amino acids:
- Histidine
- Isoleucine
- Leucine
- Lysine
- Methionine
- Phenylalanine
- Threonine
- Tryptophan
- Valine
- Nonessential amino acids. Nonessential amino acids are made by your body from essential amino acids or in the normal breakdown of proteins. There are 5 amino acids that are termed non-essential amino acids:
- Alanine
- Asparagine
- Aspartic acid
- Glutamic acid
- Serine
- Conditionally Essential amino acids. Conditionally Essential amino acids are needed in times of illness, stress, starvation or inborn errors of metabolism. A healthy body can make conditionally essential amino acids under normal physiologic conditions. There are 6 amino acids that are called conditionally essential amino acids:
- Arginine
- Cysteine
- Glutamine
- Glycine
- Proline
- Tyrosine
You get protein (amino acids) in your diet from animal and plant-based foods such as meat, fish, eggs, dairy products, nuts, and certain grains, beans, peas, and lentils 148. Proteins from meat and other animal products are complete proteins. This means they supply all of the amino acids your body can’t make on its own. Most plant proteins are incomplete. So you should eat different types of plant proteins every day to get all nine essential amino acids your body needs. For example, pairing protein sources like rice and beans, hummus, pita bread, or oatmeal topped with almond butter. Regarding volume, it may be necessary to eat more plant-based foods to get a similar amount of protein and amino acid profile provided by animal-based proteins 149.
The Dietary Guidelines for Americans recommend a variety of protein foods from both animal and plant sources in healthy dietary patterns 128. The Dietary Guidelines for Americans also recommend a variety of nutrient-dense protein foods from both plant (beans, peas, lentils, and cereal grains) and animal sources (lean meat, poultry, fish, eggs, as well as low-fat dairy products) to ensure adequate nutrient intake 128. You should try to include at least one protein-rich food with every meal. Eating foods high in protein can help you build strength and recover more quickly if you’ve been sick.
Protein foods include 150, 151, 152, 153:
- Animal-based protein foods: Red and white meat (pork, beef, chicken, turkey, duck), seafood (fish, shrimp, oysters, clams, and scallops), eggs, milk, yogurt, and cheese. Animal-based protein sources, such as meat and dairy products, contribute zinc, vitamin B12, vitamin D, calcium, phosphorus, and iron. Meat and poultry foods should be lean or low-fat, like 93% lean ground beef, pork loin, and skinless chicken breasts. Fish is nutritious, providing energy (calories), protein, selenium, zinc, iodine and vitamins A and D (some species only). Fish is also an excellent source of omega-3 fatty acids (good fats), which are well known for their health benefits and are essential for life. Eating fish regularly can reduce the risk of a range of diseases from childhood asthma to heart disease, cardiovascular diseases and prostate cancer. Choose seafood options that are higher in healthy fatty acids called omega-3s fatty acid and lower in methylmercury, such as salmon, anchovies, and trout. And stay away from processed meats or artificial (fake) meat.
- Plant-based protein foods: Nuts and nut spreads such as almond butter, peanut butter, soy nut butter, seeds (sunflower seeds), beans, peas, legumes, lentils, soy products, soy milk, and tofu. Plant protein foods, such as legumes (including soybeans and pulses), nuts, seeds, and cereal grains contribute to dietary fiber, potassium, folate, vitamin E, and magnesium. Peanuts and peanut butter are high in protein, folate, magnesium, and vitamin E. Legumes can also contribute significant non-heme iron to diets 150.
Good protein choices include:
- Soy protein
- Beans
- Nuts
- Fish
- Lean chicken with no skin
- Lean beef
- Pork
- Salmon
- Anchovies
- Trout
- Low-fat dairy products
It is important to get enough dietary protein. You need to eat protein every day, because your body doesn’t store it the way it stores fats or carbohydrates. Furthermore, protein foods provide nutrients important for maintaining your health and body. How much protein you need depends on your age, sex, height, weight, health, and level of physical activity. The amount can also depend on whether or not you are pregnant or breastfeeding. The good news is most Americans eat enough protein and some eat more than they need 154. In the US, about a one-third of protein comes from plant sources, which are primarily derived from grain foods 148.
Choosing animal-based sources can be beneficial, as they’re considered complete proteins, meaning they contain all 9 essential amino acids. Animal-based protein sources also contain vitamins and minerals such as vitamin B12 and iron. However, some animal proteins, such as processed meats and certain cuts of meat that are high in saturated fat, can affect your health negatively. It’s best to choose leaner protein sources and cut back on red meat (e.g., pork, beef, lamb) and processed meat (i.e., meat preserved by smoking, salting, curing or adding other preservatives). Because diets high in red meat and diets high in processed meat have been linked to increased risk of colorectal cancer. If you do choose to eat red meat, choose leaner sources. Look for sources that are low in saturated fat, are unprocessed, or are high in heart-healthy unsaturated fats and omega-3 fatty acids. Some good examples are:
- White-meat poultry, such as chicken or turkey breasts
- Fish, especially fatty fish like salmon, lake trout, mackerel, herring, sardines and tuna
- Pork tenderloin
- Lean or extra-lean cuts of beef such as sirloin or round cuts, greater than 93% lean ground beef
- Eggs and egg whites
- Non-fat/low-fat Greek yogurt, cottage cheese, milk
Remember that it’s still important to eat a balanced diet that includes all food groups and a variety of both plant and animal protein sources, in addition to plenty of fruits, vegetables and whole grains 155, 156. Although individual plant protein sources (e.g., beans, nuts and seeds) don’t contain all 9 essential amino acids, plant sources offer more fiber and a different variety of vitamins and minerals than animal sources of protein. And even though individual plants don’t contain all 9 essential amino acids on their own, when eaten in combination throughout the day, they do provide enough of the essential amino acids to meet your body’s needs. An ideal human diet would consist of both animal- and plant-source foods in appropriate amounts and proportions to ensure intake of sufficient quantity and quality of proteins, while consuming adequate dietary fiber 157.
The recommended protein intake is 0.8 to 1 gram per kilogram of body weight per day 91. For strength training athletes adequate protein intake should range between 1.2 and 1.7 grams of protein per kilogram of body weight per day or 0.5 to 0.8 grams per pound of body weight 92, 93, 94, 95. Recently, the American Dietetic Association, and Dieticians of Canada recommended that endurance-training (moderate exercise) athletes and strength-training (intense exercise) athletes consume 1.3 (ranging from 1.2 to 1.4) and 1.6 (ranging from 1.2 to 1.7) g protein per kg body weight per day, respectively 158. There is evidence that the inclusion of high-quality animal protein or combinations of high-quality plant-based proteins can stimulate muscle growth 159. Recent data also indicate that adequate intake of protein at each meal of the day has an advantage over a large amount of protein in a single meal to support skeletal-muscle mass and function 158.
Timing of protein or amino acids consumption is also important for muscle recovery after exercise. Skeletal muscle takes up nutrients (e.g., amino acids, glucose and fatty acids) from the blood circulation most efficiently within the first 30 to 60 minutes after an exercise program is completed, followed by great reductions several hours later 160. Therefore, the response of muscle protein synthesis to exercise-induced growth is much greater when amino acids intake is initiated immediately after the end of exercise, as compared to 3 hour after the end of exercise 161. The proportions and amounts of all amino acids in diets should be considered when specific essential amino acids are supplemented to subjects after exercise. For example, consuming individual branched-chain amino acids (BCAA) alone cannot enhance muscle protein synthesis when the availability of other amino acids is limited 162. This is because protein synthesis requires all 20 different amino acids as the building blocks.
For healthy children ages 1 to 3, approximately 5 to 20% and children ages 4 to 18 approximately 10 to 30% of daily energy intake should come from protein. The daily recommended intake of protein for healthy adults is 10% to 35% of your daily energy intake based on the adequate amount needed for nitrogen equilibrium 127. One gram of protein supplies 4 calories. Therefore, if you consume 2,000 calories per day, this would work out to be between 200 to 700 calories of protein per day, you could eat 100 grams of protein, or 400 calories from protein, which would supply 20% of your total daily calories.
The recommended daily intakes (RDIs) can also be calculated by your body weight. The Academy of Nutrition and Dietetics recommends that the average individual should consume 0.8 grams of protein per kilogram or 0.35 grams per pound of body weight per day for general health. So a person that weighs 75 kg (165 pounds) should consume an average of 60 grams of protein per day. Since there are approximately four calories per gram of protein, 60 grams of protein would result in the intake of 240 calories.
If you’re looking for ways to get more protein into your diet, here are some suggestions:
- Try a peanut butter sandwich. Remember to use natural peanut butter (or any other nut paste) with no added salt, sugar or other fillers.
- Low-fat cottage or ricotta cheese is high in protein and can go in your scrambled eggs, casserole, mashed potato or pasta dish. Or spread it on your toast in the morning.
- Nuts and seeds are fantastic in salads, with vegetables and served on top of curries. Try toasting some pine nuts or flaked almonds and putting them in your green salad.
- Beans are great in soups, casseroles, and pasta sauces. Try tipping a drained can of cannellini beans into your favorite vegetable soup recipe or casserole.
- A plate of hummus and freshly cut vegetable sticks as a snack or hummus spread on your sandwich will give you easy extra protein at lunchtime.
- Greek yogurt is a protein rich food that you can use throughout the day. Add some on your favorite breakfast cereal, put a spoonful on top of a bowl of pumpkin soup or serve it as dessert with some fresh fruit.
- Eggs are a versatile and easy option that can be enjoyed on their own or mixed in a variety of dishes.
Figure 7. Amino acid structure
Footnotes: Amino acids are the fundamental building blocks of proteins, essential for the structure, function, and regulation of biological systems. Amino acid basic structure has 4 components linked together with a central carbon atom called alpha carbon (α–carbon). All amino acids contain an amino group (-NH2) and a carboxyl group (-COOH). In alpha-amino acids (α-amino acids), the two functional groups are bound to a central carbon atom known as the alpha carbon (α–carbon). At the alpha carbon is also a hydrogen atom (H) and a variable side chain, often referred to as the R-group (R), which varies with each amino acid and gives each amino acid its unique chemical properties. The R groups (R) may be: Hydrophobic, Hydrophilic, Charged R-groups: positive or negative charged and Special R-groups: conjugated with other molecules
[Source 163 ]Figure 8. Amino Acid Chart
Footnotes: All amino acids contain an amino group (-NH2) and a carboxyl group (-COOH). In alpha-amino acids (α-amino acids), the two functional groups are bound to a central carbon atom known as the alpha carbon. At the alpha carbon is also a hydrogen atom and a variable side chain, often referred to as the R-group, which gives each amino acid its unique chemical properties. Nonpolar amino acids (hydrophobic amino acids) have R-groups that are mostly composed of hydrocarbons and are hydrophobic. Examples include glycine (Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), methionine (Met), and phenylalanine (Phe). Polar amino acids (uncharged amino acids) have R-groups that contain polar functional groups but do not ionize under physiological conditions. Examples include serine (Ser), threonine (Thr), cysteine (Cys), asparagine (Asn), and glutamine (Gln). Basic amino acids (positively charged amino acids) have R-groups that contain positively charged functional groups, which can form ionic bonds with negatively charged groups. Examples include lysine (Lys), arginine (Arg), and histidine (His). Often charged side chains appear at the protein surface to enable solubility in water. Neighboring side chains with positive and negative charges can form electrostatic contacts called salt bridges that maintain structures within a single protein or between interfacing proteins. Acidic amino acids (negatively charged amino acids) have R-groups that contain negatively charged functional groups, which can form ionic bonds with positively charged groups. Examples include aspartic acid (Asp) and glutamic acid (Glu). Some proteins use charged side chains to bind metals that are important for the proteins function.
[Source 164 ]Figure 9. Peptide bond
Footnote: Amino acids link together in a reaction known as peptide bond to form proteins.
[Source 165 ]Figure 10. Protein structure
Footnotes: A protein is a chain of amino acids bound to one another via peptide bonds. Like a string of beads, these strings can twist and fold into a final protein shape. When someone eats protein, it breaks down into its amino acids. These amino acids are composed of a central carbon atom bonded to an amino group (-NH2) or nitrogen-containing group and a carboxylic “acid” group (-COOH), hence the name “amino acid”. The carbon also has a single hydrogen atom and a side chain or “R-group,” unique to each amino acid. The exception to this is Proline, which is a ring structure.
[Source 166 ]Figure 11. Protein structures
[Source 167 ]Table 5. Amino acid content of various milk
| Amino acids (g/100 g) | Cow milk | Goat milk | Buffalo milk |
|---|---|---|---|
| Aspartic acid | 7.8 | 7.4 | 7.13 |
| Serine | 4.8 | 7.4 | 7.13 |
| Threonine | 4.5 | 5.7 | 5.7 |
| Glutamic acid | 23.2 | 19.3 | 21.4 |
| Proline | 9.6 | 14.6 | 12 |
| Cystine | 0.6 | 0.6 | 0.58 |
| Glycine | 1.8 | 2.1 | 1.9 |
| Alanine | 3 | 3.5 | 3.03 |
| Valine | 4.8 | 5.4 | 6.7 |
| Methionine | 1.8 | 3.5 | 0.9 |
| Isoleucine | 4.5 | 7 | 5.7 |
| Leucine | 8.5 | 8.2 | 9.5 |
| Tyrosine | 4.3 | 4.8 | 3.8 |
| Phenylalanine | 4.8 | 6 | 4.7 |
| Histidine | 3 | 5 | 2.7 |
| Lysine | 8 | 8 | 7.3 |
How much protein do I need?
How much protein you need depends on your age, sex, height, weight, health, and level of physical activity. The amount can also depend on whether or not you are pregnant or breastfeeding. The recommended protein intake is 0.8 to 1 gram per kilogram of body weight per day 91. For strength training athletes adequate protein intake should range between 1.2 and 1.7 grams of protein per kilogram of body weight per day or 0.5 to 0.8 grams per pound of body weight 92, 93, 94, 95.
How to calculate your daily protein needs:
Convert body weight in pounds to kilograms (round to the nearest 10th). Multiply weight in kilograms by the range that best fits your activity levels.
Let’s look at an example:
- Convert pounds into kilograms 150lbs / 2.2 = 68.2kg
The recommended protein intake is 0.8 to 1 gram per kilogram of body weight per day
- 68.2kg (0.8g grams of protein per kilogram) = 54.6g
- 68.2kg (1g grams of protein per kilogram) = 68.2g
For strength training athletes adequate protein intake should range between 1.2 and 1.7 grams of protein per kilogram of body weight per day.
- 68.2kg (1.2g grams of protein per kilogram) = 81.8g
- 68.2kg (1.7g grams of protein per kilogram) = 115.9g
Here are some practical protein equivalents in common foods. One ounce (30 grams) of most protein-rich foods contains 7 grams of protein. An ounce (30 grams) equals:
- 1 oz (30 g) of meat fish or poultry
- 1 large egg has six grams of protein
- ¼ cup (60 milliliters) tofu
- ½ cup (65 grams) cooked beans or lentils
- 1 cup of dry beans has about 16 grams of protein
- 1 cup of milk has eight grams of protein
- 1 cup of soy milk has about seven grams of protein
Low fat dairy is also a good source of protein. An eight ounce container of yogurt has about 11 grams of protein
Most Americans eat enough protein in their diet but need to select leaner varieties of meat and poultry. Americans may also need to increase the variety of protein foods selected and choose meats less often. However, if you are vegetarian or vegan, the advice to eat meat, poultry, and seafood does not apply to you. Vegetarian protein options include beans, peas, lentils, nuts, seeds, and soy products.
What counts as an ounce-equivalent in the protein foods group?
The following examples count as 1 ounce-equivalent from the protein foods group 147:
- 1 ounce of meat, poultry, or fish
- ¼ cup cooked beans
- 1 egg
- 1 tablespoon of peanut butter
- ½ ounce of nuts or seeds
- ¼ cup (about 2 ounces) of tofu
- 1 ounce tempeh, cooked
The table below lists amounts that count as 1 ounce-equivalent in the protein foods group towards your daily recommended amount.
Table 6. Daily protein foods general recommendations by age
| Daily Protein Recommendation* in Ounce-Equivalents | ||
|---|---|---|
| Toddlers | 12 to 23 months | 2 ounce-equivalent |
| Children | 2-3 yrs 4-8 yrs | 2 to 4 ounce-equivalent 3 to 5½ ounce-equivalent |
| Girls | 9-13 yrs 14-18 yrs | 4 to 6 ounce-equivalent 5 to 6½ ounce-equivalent |
| Boys | 9-13 yrs 14-18 yrs | 5 to 6½ ounce-equivalent 5½ to 7 ounce-equivalent |
| Women | 19-30 yrs 31-59 yrs 60+ yrs | 5 to 6½ ounce-equivalent 5 to 6 ounce-equivalent 5 to 6 ounce-equivalent |
| Men | 19-30 yrs 31-59 yrs 60+ yrs | 6½ to 7 ounce-equivalent 6 to 7 ounce-equivalent 5½ to 6½ ounce-equivalent |
How much protein do you need for optimal muscle maintenance?
The recommended protein intake is 0.8 to 1 gram per kilogram of body weight per day 91. For strength training athletes adequate protein intake should range between 1.2 and 1.7 grams of protein per kilogram of body weight per day or 0.5 to 0.8 grams per pound of body weight 92, 93, 94, 95, 169, 170. That’s because your skeletal muscle is made up of 75 percent water and 20 percent protein, with the remainder from other materials including fat, glycogen, inorganic salts, and minerals 171. Given the protein content of your skeletal muscle, it is not surprising resistance trained athletes emphasize the importance of dietary protein in their meal plans 81. This is also reflected in the scientific literature with significant attention given to protein focused nutritional interventions to facilitate resistance training induced adaptations 172, including manipulation of total daily dietary protein intake 173, protein dosage per meal 174, 175, 176, protein quality 177 and protein distribution 178.
Higher-protein diets have been shown to 86, 87, 169, 170:
- Promote gains in muscle mass, especially when paired with resistance training;
- Spare muscle mass loss during caloric restriction; and
- Reduce the natural loss of muscle mass that accompanies aging.
Protein quality is also important to the gain and maintenance of muscle mass 179. Protein quality is a function of protein digestibility, amino acid content, and the resulting amino acid availability to support metabolic function 179. Whey protein is one of the highest-quality proteins given its amino acid content (high essential, branched-chain, and leucine amino acid content) and rapid digestibility. Consumption of whey protein has a strong ability to stimulate muscle protein synthesis 179. In fact, whey protein has been found to stimulate muscle protein synthesis to a greater degree than other proteins such as casein and soy.
A recent meta-analysis suggested dietary protein supplementation enhances resistance training induced gains in muscle mass and strength, at least when dietary protein intake is suboptimal (<1.6 g per kg body weight daily) 180, resistance training alone provides a far greater stimulus than whey protein supplementation 175.
How to calculate your daily protein needs:
Convert body weight in pounds to kilograms (round to the nearest 10th). Multiply weight in kilograms by the range that best fits your activity levels.
Let’s look at an example:
- Convert pounds into kilograms 150lbs / 2.2 = 68.2kg
The recommended protein intake is 0.8 to 1 gram per kilogram of body weight per day
- 68.2kg (0.8g grams of protein per kilogram) = 54.6g
- 68.2kg (1g grams of protein per kilogram) = 68.2g
For strength training athletes adequate protein intake should range between 1.2 and 1.7 grams of protein per kilogram of body weight per day.
- 68.2kg (1.2g grams of protein per kilogram) = 81.8g
- 68.2kg (1.7g grams of protein per kilogram) = 115.9g
Muscle mass is built when the net protein balance is positive: that is muscle protein synthesis exceeds muscle protein breakdown. Research shows muscle protein turnover is the greatest after working out. Additionally, it has been shown that muscle mass increases over time when resistance exercise (i.e. weight lifting, body weight exercises, etc) is combined with nutrient intake.
However, as you age, you need to increase your protein intake 92. Around 50 years of age, you need to increase the protein in your diets to 1 gram per kilogram of your body weight to maintain muscle mass 92. People that exercise regularly also need to eat more protein than the recommended daily intake 92.
Several studies performed by the group of Philip and others showed that protein supplementation did not further increase muscle strength among individuals who consumed adequate amounts of dietary protein 95, 181, 93, 182. However, with the aim of maximizing performance, individuals seeking to gain muscle mass are likely to consume more protein with the misconceived belief that large quantities of protein consumption might generate more muscle protein 183.
To increase muscle mass in combination with physical activity, it is recommended that a person that lifts weights regularly or is training for a running or cycling event eat a range of 1.2 to 1.7 grams of protein per kilogram of body weight per day, or 0.5 to 0.8 grams per pound of body weight 92. Consequently, the same 75
kilogram individual should increase their protein intake to 75 grams (300 calories) to 128 grams (512 calories) in order to gain muscle mass. This level of intake can generally be met through diet alone and without additional protein and amino acid supplementation 92.
When should I consume protein?
The process of protein turnover is increased with resistance training and can remain elevated for up to 48 hours in people beginning a new resistance training program 92. Therefore it is important to provide enough energy including protein so there is a sufficient pool of amino acids available to repair and build new muscle. You do not want to exercise on an empty stomach. In fact, exercising in an unfed state leads to an increase in protein loss making it more difficult for your body to both repair and build muscle 92. Your body can only use approximately 20–40 g of protein per meal. For best results, eat around this much protein every 3 to 4 hours.
Research suggests there are several benefits to pre-exercise protein supplementation 92. Pre-exercise protein supplementation helps to improve body composition by increasing resting energy expenditure up to 48 hours after exercise 92. This is important because it suggests that pre-exercise protein ingestion will not only help increase lean muscle mass and strength, but will also simultaneously reduce fat mass 92. However, the most scientifically supported and most significant benefits of consuming protein prior to exercise may be improved recovery and hypertrophy. This is thought to occur because of improved amino acid delivery 92.
Make sure you have a healthy diet that meets the current protein intake recommendations and then use supplements to add anything else you might need. A good diet will not make a mediocre athlete into a champion, but poor food choices can turn a champion into a mediocre athlete. The International Olympic Committee (IOC) position stand is that “the use of supplements does not compensate for poor food choices and an inadequate diet”. Reinforcing this importance of food, researchers have found that athletes eating a diet rich in nitrates from vegetables (not supplements) for just 10 days were able to enhance their exercise performance, compared to when they were eating their usual diet 184.
Fat
You need to eat some fat even when you are trying to lose weight. The human body needs small amounts (3 to 6 grams) of essential fatty acids (Omega-6 and Omega-3 fatty acids). Fat is important for many body processes. Fat helps your body absorb nutrients and move nutrients around your body. Some fat is necessary as a carrier for the fat-soluble vitamins A, D, E, and K. Fat is the main source of energy storage in your body, fat contributes to cellular structure and function, fat keeps you warm, and protects your organs 185, 186, 187. Fat also helps with hormone production. Therefore your diet should not be devoid of fat. However, because fat is calorically dense (1 gram of fat has 9 calories of energy), it is often decreased on weight-loss diets to reduce energy intake.
Fat needs will vary by individual and will depend largely on your body composition goals and body types. For example, dietary fat recommendations are slightly higher in competitive athletes than non-athletes to promote health, maintain healthy hormone function, and maintain energy balance. Typical recommendations for athletes are 30 to 50% of total energy intake.
There are 4 main types of fats:
- Unsaturated fats are those that are liquid at room temperature. The two kinds of unsaturated fats are monounsaturated fat and polyunsaturated fat. Both of these unsaturated fats are typically liquid at room temperature. Unsaturated fats are in fish, such as salmon, trout and herring, and plant-based foods such as avocados, olives and walnuts. Liquid vegetable oils, such as soybean oil, corn oil, safflower oil, canola oil, olive oil, peanut oil, canola oil and sunflower oil, also contain unsaturated fats. Eaten in moderation, both kinds of unsaturated fats may help improve your blood cholesterol when used in place of saturated and trans fats. You want to include as many unsaturated fats in your diet because they can decrease bad cholesterol, contain high amounts of antioxidants such as Vitamin E, and contain essential omega-3 and omega-6 fatty acids. Unsaturated fats are typically classified by how many hydrogen bonds they have in their structure: either 1 (mono) or two or more (poly).
- Monounsaturated fats. Monounsaturated fat is a type of unsaturated fat. Monounsaturated fats are liquid at room temperature but start to harden when chilled. Monounsaturated fats is one of the healthy fats, along with polyunsaturated fat. Monounsaturated fats are good for your health in several ways:
- Monounsaturated fats can help lower your LDL (bad) cholesterol level. Cholesterol is a soft, waxy substance that can cause clogged, or blocked, arteries (blood vessels). Keeping your LDL level low reduces your risk for heart disease and stroke.
- Eating plant foods high in monounsaturated fats, particularly extra virgin olive oil and tree nuts, may benefit heart health and blood sugar regulation. Monounsaturated fats from plants may lower bad cholesterol and raise good cholesterol. They also may improve the control of blood sugar levels. Replacing saturated fats with monounsaturated fats in your diet may lower the level of bad cholesterol and triglycerides in your blood. Triglycerides are fat cells that circulate in the bloodstream and are stored in the body’s fat cells. A high level of triglycerides in the blood increases the risk of diseases of the heart and blood vessels.
- Monounsaturated fats help develop and maintain your cells.
- Monounsaturated fats are found in plant foods, such as nuts, avocados, and vegetable oils. Monounsaturated fats are found in red meats and dairy products. About half the fats in these foods are saturated and half monounsaturated. Many plants and plant oils are high in monounsaturated fats but low in saturated fats. These include:
- Oils from olives, peanuts, canola seeds, safflower seeds, and sunflower seeds.
- Avocadoes.
- Pumpkin seeds.
- Sesame seeds.
- Almonds.
- Cashews.
- Peanuts and peanut butter.
- Pecans.
- Polyunsaturated fats. Polyunsaturated fat is a type of unsaturated fat. Polyunsaturated fats are liquid at room temperature but start to harden when chilled. Polyunsaturated fats include omega-3 and omega-6 fats. These are essential fatty acids that your body needs for brain function and cell growth. Your body does not make essential fatty acids, so you must get them from food. Polyunsaturated fats can help lower your LDL (bad) cholesterol. Cholesterol is a soft, waxy substance that can cause clogged or blocked arteries (blood vessels). Having low LDL cholesterol reduces your risk for heart disease. Polyunsaturated fats is one of the healthy fats, along with monunsaturated fat. Polyunsaturated fat is found in plant and animal foods, such as salmon, vegetable oils, and some nuts and seeds.
- Omega-3 fatty acids are good for your heart in several ways. They help:
- Reduce triglycerides, a type of fat in your blood
- Reduce the risk of developing an irregular heartbeat (arrhythmia)
- Slow the buildup of plaque, a substance comprising fat, cholesterol, and calcium, which can harden and clog your arteries
- Slightly lower your blood pressure
- Sources of omega-3 fatty acids include:
- Fish such as salmon, anchovies, mackerel, herring, sardines and tuna.
- Oils from canola seeds, soybeans, walnuts and flaxseed.
- Soybeans.
- Chia seeds.
- Flaxseed.
- Walnuts.
- Omega-6 fatty acids may help:
- Control your blood sugar
- Reduce your risk for diabetes
- Lower your blood pressure
- Sources of omega-6 fatty acids include:
- Corn oil.
- Cottonseed oil.
- Peanut oil.
- Soybean oil.
- Sunflower oil.
- Omega-3 fatty acids are good for your heart in several ways. They help:
- Saturated fats. Saturated fats are those that are solid at room temperature. Examples include margarine, butter, whole fat dairy products, the fat marbling in meats, and coconut oil. Saturated fats don’t need to be avoided entirely, but diets high in saturated fats can increase bad cholesterol and triglycerides, increasing the risk for heart disease. The Dietary Guidelines for Americans suggest that less than 10% of calories a day should be from saturated fats. The American Heart Association recommends that saturated fats only make up 5 to 6% of your daily calories. For a 2,000 calorie diet, that is a total of 100 calories, or 11 grams a day. Foods high in saturated fats include:
- Foods baked or fried using saturated fats.
- Meats, including beef, lamb, pork as well as poultry, especially with skin.
- Lard.
- Dairy products like butter and cream.
- Whole or 2% milk.
- Whole-milk cheese or yogurt.
- Oils from coconuts, palm fruits, or palm kernels.
- Trans fats. Trans fatty acids are unhealthy fats that form when vegetable oil goes through a process called hydrogenation. This leads the fat to harden and become solid at room temperature. Hydrogenated fats, partially hydrogenated oils (PHOs) or “trans fats,” are often used to keep some foods fresh for a long time. Trans fats are unsaturated fats that are artificially turned into saturated fats and increase heart disease and stroke risk by raising bad LDL cholesterol and decreasing good HDL cholesterol levels. High LDL (bad) cholesterol along with low HDL (good) cholesterol levels can cause cholesterol to build up in your arteries (blood vessels). This increases your risk for heart disease and stroke. Trans fats have also been known to increase the risk of developing type 2 diabetes. Trans fats are most commonly found in fried foods, frozen baked products such as pizza, non-dairy coffee creamers, vegetable shortenings, some margarines, crackers, cookies, snack foods, and other foods made with or fried in partially hydrogenated oils (PHOs). Because of the health risks from trans fats, the United States Food and Drug Administration (FDA) has banned food manufacturers from adding partially hydrogenated oils (PHOs) to foods. Although the food industry has greatly reduced the use of trans fat in recent years, trans fat may still be found in many fried, packaged, or processed foods. There are very small amounts of naturally occurring trans fat in meats and dairy from grazing animals, such as cows, sheep and goats. You should avoid foods made with hydrogenated and partially hydrogenated oils (such as hard butter and margarine). They may contain high levels of trans fatty acids. It is important to read nutrition labels on foods. This will help you know what kinds of fats, and how much, your food contains.
How much fat do I need?
To prevent any fatty acid deficiencies it is recommended that you consume at minimum 1g of fat per kg of body weight per day. According to the Dietary Guidelines for Americans 128, fats should make up 20 to 35% of your total daily calorie intake. For those attempting to lose body fat, 0.5 to 1 fat per kg of body weight per day should be consumed per day to avoid essential fatty acid deficiency. For healthy children ages 1 to 3, ages 4 to 18, and adults, approximately 30 to 40%, 25 to 15%, and 20 to 35% of daily energy intake should come from fat, respectively 126. Approximately 5 to 10% of your daily fat energy intake should consist of Omega−6 fatty acids (linoleic acid) and 0.6 to 1.2% of Omega−3 fatty acids (alpha-linolenic acid, eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) 127. Both omega−6 fatty acids (linoleic acid) and omega−3 fatty acids (alpha-linolenic acid, eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) are considered essential fatty acids, meaning that they must be obtained from your diet 188. Apha-linolenic acid can be converted into eicosapentaenoic acid (EPA) and then to docosahexaenoic acid (DHA), but the conversion (which occurs primarily in your liver) is very limited, with reported rates of less than 15% 189, 190. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are essential fatty acids, meaning the body can’t produce them and they must come from your diet 191. Therefore, consuming eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) directly from foods and/or dietary supplements is the only practical way to increase levels of these fatty acids in your body.
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are omega-3 fatty acids found in varying amounts in seafood such as cold-water fish like salmon, tuna, herring, and mackerel, as well as in fish oil supplements and seaweed. Eating 8 ounces per week of seafood may help reduce the risk for heart disease.
Some researchers propose that the relative intakes of omega-6s and omega-3s, the omega-6/omega-3 ratio, may have important implications for the cause of many chronic diseases, such as heart and blood vessels disease (cardiovascular disease) and cancer 192, but the optimal ratio, if any, has not been defined 193. Others have concluded that the omega-6/omega-3 ratios are too nonspecific and are insensitive to individual fatty acid levels 194, 195, 196.. Most agree that raising EPA and DHA blood levels is far more important than lowering linoleic acid or arachidonic acid levels 190.
For someone who weighs 150lbs (68kg), this would equate to 34-68g fat per day. Using both of these references you can calculate your daily fat needs:
To calculate your daily fat needs:
- Convert body weight in pounds to kilograms (round to the nearest 10th). Multiply weight in kilograms by 1.
Let’s look at an example:
- Convert pounds into kilograms 150lbs / 2.2 = 68.2kg
The recommended fat intake is 1 gram per kilogram of body weight per day
- 68.2kg (1g grams of fat per kilogram) = 68.2g of fat per day
Weight loss diets that are moderate to low in fat (20 to 30% of calories) are called “balanced deficit” diets because they maintain a reasonable balance among macronutrients similar to that recommended in MyPlate, DASH, and the Dietary Guidelines for Americans 128, 197. They tend to achieve most of the caloric deficit by reducing fat from the typical level in North American Diets of about 34% or more of calories to 20 to 30% fat, 15% protein, and 55 to 65% of calories from carbohydrates. Some examples of low fat diets are the Weight Watchers Diet (25% fat, 20% protein, and 55% carbohydrate, with 26 grams of dietary fiber), Jenny Craig, the National Cholesterol Education Program Step 1 diet (25% fat), diets based on the MyPlate, the DASH diet, the Shape up and Drop 10 diet of Shape Up! America and the Nutrisystem diet 124. Popular diet books using this approach include The Biggest Loser Diet, The Mayo Clinic Diet and The Engine 2 Die 124. These dietary patterns have been extensively reviewed and appear to be effective for weight reduction on low calorie diets for most individuals 124.
Very low-fat diets such as the Pritikin Diet 198, the Ornish Diet 199 and The Spark Solution Diet 200 have been advocated not only for weight reduction, but also for improving cardiovascular risk profiles. The Ornish Diet 199, which is very low in fat (13% of calories) and saturated fat, very high in carbohydrate (81% of calories) and very high in fiber (38 grams), is part of a program that includes nonsmoking, exercise and behavior modification. The Ornish Diet 199 was shown to reduce some cardiovascular risk factors in a limited long term study. For those who can adhere to the Ornish regime it may be helpful. However, it may not be appropriate for all populations, such as diabetics.
What are Healthy fats?
Healthy fats include:
- Monounsaturated fats
- Polyunsaturated fats (omega-3 and omega-6).
The healthier fats are unsaturated fats. They can be found in sunflower oil, safflower oil, peanut and olive oils, poly- and mono-unsaturated margarine spreads, nuts, seeds and avocado. These are much better for you than the saturated fat found in butter, cream, fatty meats, sausages, biscuits, cakes and fried foods.
Foods that contain healthy monounsaturated fats include:
- Avocados and their oils/spreads
- Unsalted nuts such as almonds, cashews and peanuts and their butters/spreads
- Olives and their oils/spreads
- Cooking oils made from plants or seeds, including: olive, canola, peanut, sunflower, soybean, sesame and safflower.
Foods that contain healthy polyunsaturated fats (omega-3 and omega-6) include:
- Oily fish like salmon, mackerel and sardines
- Tahini (sesame seed spread)
- Linseed (flaxseed) and chia seeds
- Soybean, sunflower, safflower, canola oil and margarine spreads made from these oils
- Pine nuts, walnuts and brazil nuts.
It is important to choose foods with the healthiest type of fat such as avocados, olives, nuts and seeds, and use healthy oils for cooking, for example, olive, canola, sunflower, peanut and soybean oil.
Reduce the amount of highly processed food you eat such as baked goods including cakes, biscuits and pastries, along with processed meat, and fried and takeaway foods. These foods are high in saturated and trans fats, added sugar and salt, and are not part of a heart-healthy eating pattern. Try to have these foods only sometimes and in small amounts.
Many Americans eat more fat than they need, which can lead to weight gain and heart disease.
What are Unhealthy fats?
Unhealthy fats include:
- Saturated fat
- Trans fat
Foods rich in unhealthy fats include:
- Animal fats including butter, ghee and lard along with the visible fat/skin on meat
- Hydrogenated plant oils like copha, vegetable shortening and some margarines
- Coconut oil
- Processed foods such as baked goods (cakes, biscuits and pastries), processed meat ( bacon, sausages, salami) and fried and takeaway foods.
Replace foods rich in saturated and trans fats with foods rich in healthy unsaturated fats as part of a healthy diet.
It is important to choose foods with the healthiest type of fat such as avocados, olives, nuts and seeds, and use healthy oils for cooking, for example, olive, canola, sunflower, peanut and soybean oil.
Reduce the amount of highly processed food you eat such as baked goods including cakes, biscuits and pastries, along with processed meat, and fried and takeaway foods. These foods are high in saturated and trans fats, added sugar and salt, and are not part of a heart-healthy eating pattern. Try to have these foods only sometimes and in small amounts.
Many Americans eat more fat than they need, which can lead to weight gain and heart disease.
Protein supplement
Protein supplementation has been shown to improve muscle building with regular exercise training. Protein supplementation should contain a high amount of the amino acid leucine, which is responsible for muscle protein synthesis. Whey protein is a great option for leucine. Eating less protein may not be enough to rebuild muscles, and eating more doesn’t usually give you more benefits. Furthermore, a combination of whey (a rapidly digested protein) with casein (a slow digested protein) seems to be an effective formula for skeletal-muscle protein synthesis after exercise 145.
Whey protein is beneficial in supporting muscle adaptations due to its rapid absorption rate in addition to casein that has a slower and more sustained rate of amino acid absorption over a few hours 92. Branched chain amino acids are similarly beneficial and have been shown to aid in recovery from exercise with respect to not only protein synthesis but also aiding in replacing your muscle glycogen and delaying fatigue associated with exercise.
Protein supplementation after exercise may have a more profound impact on skeletal muscle growth. Several studies have demonstrated that protein ingestion following an acute bout of resistance training stimulates muscle protein synthesis for up to three hours 92. In contrast, failing to eat after exercise may limit protein synthesis and therefore limit potential progress in lean muscle tissue development. Research actually suggests there may be an “anabolic window” such that protein intake within an hour of exercise has the greatest influence on resistance training adaptations 92.
Generally, naturally occurring animal proteins contain 2:1:1 ratio of leucine, isoleucine and valine. These proteins have been identified as providing optimal support of muscle adaptations with exercise training. In order to meet the recommended RDA a consumption of approximately 45 mg/kg/day of leucine and 22.5 mg/kg/day of isoleucine and valine is suggested 92.
Protein powder supplement
When people think of protein supplement, they might think of powder mixed with water in a shaker bottle. However, as we mentioned above, protein comes from many different sources and also does more for the body than just repair muscle. Protein is made up of amino acids and is required for nearly everything that your body does to function properly. The protein in your food is broken down into parts called amino acids during digestion. Amino acids are referred to as the building blocks of protein – imagine the structure of a brick wall. One brick by itself only has so much strength, but many stacked on top of one another can create an entire wall, build houses or even buildings. The same goes for amino acids – individually, they’re not as “effective” – but when strung together to form an entire protein, they can ultimately help build and repair muscle along with assisting with their many other “jobs” in the body. High protein diets can promote weight loss via increased insulin sensitivity, fatty acid oxidation, appetite suppression, and feeling full. However, caution is necessary for people with diabetes who have gout because protein can elevate niacin levels, which may exacerbate gout-related symptoms.
Your body needs a number of amino acids in large enough amounts to maintain good health. While there are hundreds of amino acids, humans use only 20 amino acids 143, 146.
Amino acids are classified into three groups 143:
- Essential amino acids. Essential amino acids cannot be made by your body, and must be supplied by food. Essential amino acids do not need to be eaten at every meal. The balance over the whole day is more important. There are 9 essential amino acids:
- Histidine
- Isoleucine
- Leucine
- Lysine
- Methionine
- Phenylalanine
- Threonine
- Tryptophan
- Valine
- Nonessential amino acids. Nonessential amino acids are made by your body from essential amino acids or in the normal breakdown of proteins. There are 5 amino acids that are termed non-essential amino acids:
- Alanine
- Asparagine
- Aspartic acid
- Glutamic acid
- Serine
- Conditionally Essential amino acids. Conditionally Essential amino acids are needed in times of illness, stress, starvation or inborn errors of metabolism. A healthy body can make conditionally essential amino acids under normal physiologic conditions. There are 6 amino acids that are called conditionally essential amino acids:
- Arginine
- Cysteine
- Glutamine
- Glycine
- Proline
- Tyrosine
There are 3 main types of protein powders:
- Whey. Whey is the liquid remaining after milk has been curdled and strained. It is very fast absorbing and is generally the type of protein that is recommended after exercise.
- Casein. Casein protein is also a by-product of milk production and is a slower digesting protein. This protein is generally best to consume at night or as a snack.
- Plant protein powders. Plant protein powders are generally a combination of protein derived from wheat, pea, hemp, or soy products. Plant proteins generally contain a combination of various protein sources to include all the essential amino acids needed to build new tissue.
Protein shakes, powders and supplements are unnecessary for most Americans’ health needs. According to the most recent national nutrition survey, 99% of Americans get enough protein through the food they eat 148. Any protein you eat on top of what your body needs will either be excreted from your body as waste, or stored as weight gain. The best way for you to get the protein you need is to eat a wide variety of protein-rich foods as outlined in the American dietary guidelines, as part of a balanced diet. But if you are still interested in using protein shakes, powders and supplements, talk to your doctor 128. Protein powders especially whey or casein protein powders are complete protein sources. Furthermore, they are in an elemental form so your body will absorb and utilize those proteins quickly. This makes protein powders an excellent source of protein in the diet for fitness enthusiasts or athletes alike. However, unlike protein from food source, when it comes to protein powders, it’s important to be aware of additional ingredients that can be placed in them like heavy metals, artificial sweeteners, fillers, and sugar alcohols.
Cadmium (Cd), Arsenic (As), Mercury (Hg), and lead (Pb) are among the 4 most common heavy metals found in protein powders 201. In 2010, the US Consumer Reports measured heavy metal concentrations in 15 commercially available protein powder supplements, and reported that all of the examined products contained “detectable concentrations” of at least one heavy metal 202. In a separate evaluation in 2018, the Clean Label Project tested 133 protein powder supplements, and found that all of the tested products similarly contained “detectable concentrations” of heavy metals 203. Specifically, the Clean Label Project reported that 70 % and 74 % of the test products contained “measurable levels” of lead (Pb) and xadmium (Cd), respectively 203. These studies are cited by the media as evidence for possible adverse health effects following consumption of protein powder supplements.
When ingested in sufficient quantities, cadmium (Cd), arsenic (As), mercury (Hg), and lead (Pb) have been associated with adverse human health effects, potentially including cancers, nerve damage, kidney damage and fertitlity issues 204, 205, 206, 207, 208, 209, 210. For example, chronic exposure to cadmium (Cd) is associated with kidney disease, thyroid disruption, and weakened bones, while chronic exposure to arsenic (As) is associated with skin lesions and cancers 211, 212, 213, 214. Additionally, high doses of ingested lead (Pb) compete with calcium in your body, affecting neurotransmitter release and heme synthesis, which may result in nervous, blood, reproductive, and kidney problems 206, 215, 216. Sufficient mercury (Hg) exposure can elicit neurological, motor, kidney, cardiovascular, immune and reproductive dysfunction 217.
To add flavor without adding extra sugar or calories, sugar alcohols or artificial sweeteners are commonly used in protein powders. Some common sweeteners include sucralose, aspartame, erythritol, sorbitol, and xylitol. There is some debate concerning the effects of artificial sweeteners on the gut microbiome 218, 219, 220, 221, 222, 223. Some studies have shown that in mice, artificial sweeteners had negative effects on glucose metabolism which lead to weight gain. In human studies, there has been evidence to show that artificial sweeteners disrupt the learning process associated with recognizing “real sugar” and decreased hormone signaling responsible for feelings of fullness which lead to weight gain. While the results are varied and warrant more research, excessive consumption of artificial sweeteners does seem to impact the bacteria within the gut 218, 219, 220, 221, 222, 223.
In general, people who exercise vigorously or are trying to put on muscle mass do not need to consume protein shakes, powders and supplements. Protein shakes, powders and supplements do not lead to increased muscle mass 224, 225, 226, 227, 228, 229. It’s the stimulation of muscle tissue through weightlifting, resistance training (to strengthen muscles and stabilize joints to support more-efficient movement) and proper nutrition and not extra dietary protein, which leads to muscle growth. Studies show that weight-trainers who do not eat extra protein (either in food or protein powders) still gain muscle at the same rate as weight-trainers who supplement their diets with protein. A sedentary lifestyle has a profound negative effect on skeletal muscle. For example, a 7-day bed rest in young healthy males can decrease leg muscle mass by 3% and muscle oxygen consumption by 4% 230. Much evidence shows that moderate exercise is beneficial for improving skeletal muscle mass as well as muscle and whole-body health, while reducing the risk of metabolic syndrome 160.
Strength training or muscle-strengthening exercise is a key component of overall health and fitness for everyone. Strength training or muscle-strengthening exercise can reduce your body fat, increase lean muscle mass and burn calories more efficiently. Strength training will make you stronger, leaner and healthier.
Strength training involves lifting free weights, using stationary weight machines, resistance bands, or your own body weight such as push-ups, pull-ups and squats to make your muscles stronger. Strength training classes that incorporate some or all of the above activities will improve your balance and prevent falls.
Strength training may help you:
- build and maintain strong muscles as you get older
- continue to perform activities of daily living, such as carrying groceries or moving furniture
- keep your bones strong, which may help prevent osteoporosis and fractures.
As you incorporate strength training exercises into your fitness routine, you may notice improvement in your strength over time. As your muscle mass increases, you’ll likely be able to lift weight more easily and for longer periods of time. If you keep it up, you can continue to increase your strength, even if you’re not in shape when you begin.
Strength-training tips:
- Aim for at least 2 days per week of strengthen-training activities.
- Try to perform each exercise 8 to 12 times. If that’s too hard, the weight you are lifting is too heavy. If it’s too easy, your weight is too light.
- Try to exercise all the major muscle groups. These groups include the muscles of your legs, hips, chest, back, abdomen, shoulders, and arms.
- Don’t work the same muscles 2 days in a row. Your muscles need time to recover.
If you are just starting out, using a weightlifting machine may be safer than dumbbells. As you get fit, you may want to add free-weight exercises with dumbbells.
You do not need a weight bench or large dumbbells to do strength training at home. You can use a pair of hand weights to do bicep curls. You can also use your own body weight: for example, push-ups, pull-ups and squats.
Proper form is very important when lifting weights. You may hurt yourself if you don’t lift weights properly. You may want to schedule a session with a certified fitness professional to learn which exercises to do and how to do them safely.
If you decide to buy a home gym, check how much weight it can support to make sure it is safe for you.
Whey Protein Powder
Whey is the liquid remaining after milk has been curdled and strained to make cheese or casein production 231, 168, 232, 233. Only 20% of milk is made up of whey. Other components of milk are lactose, protein, fat, and water. In general, liquid whey obtained from cheese-making has 94.3% water, and 50% total solids, from which 4.3% lactose, 0.8% whey proteins, 0.5% minerals, and 0.1% fat can be extracted 234. A large cheese factory may create more than 1 million liters of whey every day. Nine liters of whey are produced as the residue of each kilogram of cheese produced 168.
Whey can be divided into 2 types 235:
- Sweet whey, which is a by-product during the production of cheddar cheese or other types of cheese coagulated with rennet at pH 6–7.
- Acid whey, which is a by-product of the production of fresh and cream cheese, Greek yogurt, and caseinates by coagulating casein at pH < 5.
Whey protein can be prepared through the clarification, ultrafiltration, nanofiltration and drying process of whey 236.
Whey protein contains a range of proteins, including 50–60% beta-lactoglobulin (β-lactoglobulin), 15–25% alpha-lactalbumin (α-lactalbumin), bovine serum albumin, 10% immunoglobulins (IGs), < 3% bovine lactoferrin (BLF), ≥ 15% glycomacropeptide, and bovine lactoperoxidase (LP) 237, 238, 168. When whey is not consumed by humans, it is supplied to pigs and various other cattle, used as a fertilizer, or discarded 239.
Whey protein biological components like beta-lactoglobulin (β-lactoglobulin), glycomacropeptide, alpha-lactalbumin (α-lactalbumin), lactoferrin, and immunoglobulins (IGs) exhibit a range of immune-boosting characteristics 240. Whey has anti-cancer, anti-inflammatory, anti-high blood pressure, lipid lowering, antiviral, and antibacterial properties in addition to chelating 241. The intracellular metabolism of cysteine, an amino acid, to glutathione, a powerful intracellular antioxidant, is the main mechanism through which whey is believed to provide its benefits 168. Numerous clinical studies have shown the use of whey in the resistance of diseases like cancer, hepatitis B, osteoporosis, HIV, cardiovascular disease, and as an antibacterial agent 242.
All the essential amino acids such as histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine are present in high-quality proteins known as bovine whey proteins 168. These proteins also contain bioactive peptides, which facilitate easy digestion 234.
Whey protein is very fast absorbing and is generally the type of protein that is recommended after exercise 243, 244, 245, 246.
Whey protein can be distinguished into whey protein concentrate (WPC), whey protein isolate (WPI), and whey protein hydrolysates (WPH) according to the difference in components and processing procedures 247.
Ultrafiltration is employed to separate lactose and whey protein, resulting in whey protein concentrate (WPC) with protein content ranging from 20 to 80% and have more carbohydrates and fat. To further increase protein content and eliminate any carbohydrate or fat sources, microfiltration is utilized to obtain whey protein isolate (WPI) 248. Ideally, whey protein isolate (WPI) will digest and absorb faster than a whey protein concentrate (WPC), but both are equal in terms of protein quality.
Whey is a soluble protein that empties rapidly from your stomach, therefore becoming rapidly assimilated into your body (whey protein isolates (WPI) can enter your blood within 15 to 20-minutes after ingestion on an empty stomach). Whey is mother nature’s ‘fastest protein’ (whey protein isolate [WPI] and whey protein hydrolysates [WPH]) that supports muscle protein synthesis in the immediate hours following a workout. Whey protein is a richer source of branched chain amino acids (BCAAs) and glutamine, containing approximately 24 to 25% more leucine than casein. Leucine is considered the all-important essential amino acid which acts as the engine to drive muscle protein synthesis.
Since whey is a by-product of milk, you can source it naturally from dairy products. Consuming whey protein is extremely beneficial because whey is a complete protein — meaning it contains all the amino acids necessary for muscle growth. In fact, in terms of protein quality, milk proteins have the highest digestibility score. However, whey protein contains a myriad of additional benefits aside from enhancing muscle growth. Studies have shown that consumption of whey can also improve sleep quality and enhance immune system responses.
Several studies have evaluated the effects of whey protein on training and performance and have found that consumption of whey in doses of 20-40g/day or more over 8-12 weeks showed increases in lean body mass, strength, and decreases in fat mass.
So, what does this mean if you’re trying to build muscle? Whey is a great, complete source of protein that will offer the most benefit if consumed every 3-4 hours (if not consuming additional sources of protein via food), or within 2 hours after exercise in doses of 20-40g.
Figure 12. Physiological effect of whey proteins inside the human body
[Source 168 ]Table 7. Therapeutic applications of whey protein in humans
| Whey components | Whey protein (%) | Benefits |
|---|---|---|
| Beta-lactoglobulin | 50 to 55 | Source of branched-chain amino acids (BCAAs) and essential amino acids |
| Alpha-lactalbumin | 20 to 25 | Sources of branched-chain amino acids (BCAAs) and essential amino acids |
| Immunoglobulins | 10 to 15 | Immune modulating benefits |
| Lactoferrin | 1 to 2 | Antibacterial, antiviral, antifungal, and antioxidant properties encourage the development of necessary microorganisms |
| Lactoperoxidase | 0.5 | Inhibits the bacterial growth |
| Bovine serum | 5 to 10 | A source of vital amino acids hefty protein |
| Glycomacropeptide | 10 to 15 | Branched-chain amino acid source |
Casein protein
Casein is a milk protein, representing 75 to 80% (w/w) of all milk proteins present in milk, that’s sometimes found in non-dairy products such as soymilk, soy cheese and non-dairy creamer 249. Casein protein component of milk is made up of four casein protein families proteins (κ-, β-, αS1-, and αS2-caseins) that have evolved in different mammalian species to maintain specialized roles in milk and their primary function is the provision of nutrients and minerals, especially calcium, to offspring while maintaining fluidity in mammary glands 249, 250.
A1 β-casein milk also called A1 milk is the most abundant milk, it is obtained from cows varieties, such as like Holstein Friesian and Jersey, while A2 β-casein milk also called A2 milk is mostly obtained from cows, such as Gir and Sahiwal, and milk from camels and goats 251, 252. Compared to A2 milk, A1 milk is relatively cheaper and easier to find. Recently, there has been a difference of opinion about the health effects of the A1 milk type 253, 254, 255. The digestion of regular cow’s milk, which includes A1 β-casein, can cause lactose intolerance and potential health problems mainly linked to its A1 β-casein and its derived peptide BCM-7 (Beta-casomorphine-7) which is derived from the A1 β-casein digestion of A1 milk, which is not present in A2 milk 256. Moreover, BCM-7 (Beta-casomorphine-7) peptide formed during the digestion of A1 milk has been linked with several undesirable health effects from lactose intolerance to diabetes 257, 258, 259, 260. BCM-7 (Beta-casomorphine-7) morphine-like small peptide cannot be digested by human-associated enzymes, which causes indigestion problems. Several studies including epidemiological and clinical research have supported that the BCM-7 (Beta-casomorphine-7) is a risk for diseases such as gastrointestinal discomfort, type 1 diabetes, ischemic heart, and, neurological diseases 261, 262, 263, 264, 265. Unfortunately, there are many conflicting reports on this subject in the scientific literature. The numerous studies and reports that have appeared in recent years do not provide a definitive answer of whether milk containing the A1 variant of β-casein has a negative effect on the human body, and whether there are grounds for avoiding the A1 milk 266, 267, 268. Furthermore, the report of the European Food Safety Authority “Scientific Report—Review of the potential health impact of β-casomorphins and related peptides” has not supported the hypothesis of the causal relationship between BCM-7 (Beta-casomorphine-7) exposure and the cause of human diseases, it also does not resolve the credibility hypothesis of the negative impact of the A1 β-casein variant, arguing that there is insufficient evidence and suggests further research in this field 269. In summary, although the hypothesis on the influence of the A1 β-casein milk in A1 milk on human health was expressed almost 20 years ago, it still lacks conclusive evidence for its confirmation.
A1 β-casein in A1 milk is formed as a result of a point mutation in the position of 67th in the amino acid sequence A2 β-casein by changing proline to histidine. Therefore, this mutated form of β-casein in regular milk cannot easily be digested by the human-associated digestion enzymes 270.
Because of the presumed undesirable health effects from lactose intolerance to diabetes of regular A1 milk and A1 milk based products have been excluded from the sports diet even though they benefit athlete health in several ways. The lack of cow’s milk in the diet of athletes means that they miss out on essential nutrients including protein, vitamin D, calcium, and potassium for their performance 271, 253. An alternative for athletes who have gut discomfort or other health problems due to the consumption of regular milk is A2 milk, which lacks A1 β-casein and related BCM-7 (Beta-casomorphine-7) protein, is considered to be a promising alternative to regular A1 milk for athletes who cannot consume it 272, 273, 274.
Casein represents a group of insoluble proteins that form a gel (clot) in your stomach when it mixes with stomach acids (much like milk curdles when bacteria feast on milk sugars and release acids) – this slows gastric emptying significantly providing your body with a true ‘slow protein’ 275, 276. This provides a sustained (slow) release of amino acids into your blood which can last several hours and prolong muscle protein synthesis long after the completion of a workout. Casein is a good source of branched chain amino acids (BCAAs) and glutamine, both of which are believed to contribute positively to muscle protein synthesis and muscle recovery.
Figure 13. Casein molecular structure
Footnote: Caseins are single-chain polypeptides differing in length and amino acid sequence.
[Source 277 ]Collagen protein
Collagen belong to the family of fibrous or fiber-forming proteins that self-assemble into fibrils that define their mechanical properties and biological functions 278. Collagen consists of protein fibrils wound in a strong triple helical structure. Collagen accounts for 30% of your body’s protein. Collagen is the main protein in many structural supportive connective tissues in your body such as skin, bones, ligaments, tendons, cartilage and muscle. Collagen is also found in your organs, blood vessels and intestinal lining. Collagen constitutes at least 70% of the dry weight of human skin.
More than 28 different types of collagen have been identified, but nearly 90% of all collagen in the human body comes from types 1 (I) to 3 (III). They differ by how the molecules are assembled, the cell components that are added and where the collagen is used in your body. All collagen fibrils have at least one triple helix structure.
The main 5 types of collagen and what they do are:
- Collagen type 1. Collagen type 1 makes up 90% of your body’s collagen. Collagen type 1 is densely packed and used to provide structure to your skin, bones, tendons and ligaments. Collagen type 1 is the major form of collagen found in the dermis (the middle layer of the skin, located beneath the epidermis), providing tensile strength in your skin.
- Collagen type 2. Collagen type 2 is found in elastic cartilage, which provides joint support.
- Collagen type 3. Collagen type 3 is found in muscles, arteries and organs. Collagen type 3 is also the main dermal collagen particularly in fetal skin.
- Collagen type 4. Collagen type 4 is found in the basement membrane zone of your skin.
- Collagen type 5. Collagen type 5 is found in the cornea of your eyes, some layers of skin, hair and tissue of the placenta.
- Collagen type 7. Collagen type 7 is also found in the basement membrane zone of your skin.
Collagen’s main role is to provide structure, strength and support throughout your body.
Collagen’s specific roles include:
- Helping fibroblasts form in your dermis (middle skin layer), which helps new cells grow.
- Playing a role in replacing dead skin cells.
- Providing a protective covering for organs.
- Giving structure, strength and elasticity to your skin.
- Helping your blood to clot.
Collagen deficiency can’t be measured with a blood test — but there are signs that your collagen level is decreasing. These signs and symptoms include:
- Skin that’s wrinkled, crepey or sagging.
- Hallowing in and around your eyes and face.
- Shrinking, weakening muscles and muscle aches.
- Stiffer, less flexible tendons and ligaments.
- Joint pain or osteoarthritis due to worn cartilage.
- Loss of mobility due to joint damage or stiffness.
- Gut problems due to thinning of the lining of your digestive tract.
- Problems with blood flow.
Collagen is formed in the human body by “assembling” collagen fibrils from 3 amino acids: proline, glycine, and hydroxyproline. These amino acids group together to form protein fibrils in a triple helix structure. These fibrils are then bundled into larger groups, much like muscle tissue, to make collagen fibers. As such, collagen has a cable-like structure in that it is comprised of many smaller bundles of proteins that are bundled together, which gives it much of its physical properties, including great tensile strength.
Collagen is synthesized in your skin by dermal fibroblasts, linking amino acids into a sequence of either glycine-proline-X or glycine-X-hydroxyproline where X can be any other amino acid. Every third amino acid in collagen is glycine and 20% are either proline or hydroxyproline. Glycine is the smallest amino acid, enabling the protein alpha chain to form a strong tight helical configuration. Hydroxylase enzymes add hydroxyl groups to proline and lysine using cofactor vitamin C. Hydroxyproline is unique to collagen. Glucose and galactose molecules are attached to selected lysine hydroxyl groups in a process called glycosylation. Three alpha helix chains coil upon each other into a triple helical structure. Outside the fibroblast, this propeptide collagen is trimmed by collagen peptidases to form a shortened tropocollagen molecule. In the final step, a copper-dependent enzyme, lysyl oxidase, facilitates the formation of stable crosslinks between lysine and hydroxylysine on separate tropocollagen molecules to give tensile strength to the collagen fibril.
Collagen in your body is continuously turned over. Collagen fibrils break down in response to oxidative cell damage and in the course of normal cellular metabolism involving the enzyme collagenase. A loss of balance between production and destruction of collagen results in reduced tensile strength and formation of skin wrinkles. Collagen degradation is accelerated by poor diet and excessive sun exposure. Nutritional deficiencies, such as scurvy, and genetic mutations, as in Ehlers-Danlos syndrome and osteogenesis imperfecta, result in construction errors in collagen.
Foods rich in collagen include bones, red meats (eg, lamb, beef, pork), and white meats (eg, poultry, fish, shellfish). Egg white, marine algae, and spirulina are also sources of collagen. Collagen lacks the essential amino acid tryptophan, so other protein sources such as beans and legumes are required for optimal health.
Proline is found in mushrooms, cabbage, asparagus, peanuts, wheat, fish, egg whites and meat. Glycine is found in red meats, turkey, chicken and pork skin, peanuts and granola.
Your body also needs the proper amount of vitamin C, zinc, copper and manganese to make the collagen triple helix. Vitamin C is a cofactor (a compound that is essential for the activity of an enzyme) essential for the synthesis of collagen. Fresh fruit and vegetables including citrus fruit, berries, kiwifruit, and beetroot, are rich sources of vitamin C.
Minerals including copper are also important in collagen production. Natural food sources of copper include shellfish, liver, lobster, oysters, shiitake mushrooms, nuts such as cashews, grains and seeds, fruit and vegetables including avocado, chickpeas, sweet potatoes, mushrooms, tofu and dark chocolate.
Zinc is found in oysters, red meat, poultry, pork, beans, chickpeas, nuts, broccoli, green leafy vegetables, whole grains and milk products.
Nutrients such as vitamins and minerals are best absorbed from fresh food sources compared to manufactured foods or supplements. Protein-deficient diets may benefit from collagen supplements.
Ingested collagen-rich foods are broken down to 2 to 3 amino acid oligopeptides (collagen hydrosylates) in your gut. Oligopeptides and single amino acids are easily absorbed from the large intestine into the bloodstream, from where they can be used to construct any required protein. Although the amino acid hydroxyproline is unique to collagen, it may be used to make collagen in any connective tissue, not just the skin.
While you can make the amino acids necessary to produce more collagen from other foods in your diet, consuming dietary collagen appears to be far more effective for replacing collagen than making collagen from other sources. The reason for this is that not only does eating dietary collagen provide more of the amino acids necessary when you consume collagen. It also tells specific cells in your skin to make more collagen. This has been shown to work in humans when sufficiently large quantities of collagen supplements are consumed (around 15 grams). Collagen supplements have been studied over the last several decades, and there are two main areas where there is good evidence to support collagen supplementation: skin and joint pain.
Collagen peptides are small pieces of animal collagen. Collagen can’t be absorbed in a whole form. It has to be broken down into smaller peptides or amino acids. Oral collagen supplements come in the form of pills and powders. They usually contain two or three amino acids. They are sold as collagen peptides or hydrolyzed collagen. Collagen peptides are made by breaking down whole collagen proteins into smaller pieces. Collagen peptides are absorbed through your gastrointestinal tract. When taken by mouth, collagen peptides seem to build up in your skin and cartilage. This might help improve some skin and joint conditions.
Collagen peptides are used for dry skin, aging skin and osteoarthritis. They are also used for osteoporosis, brittle nails, muscle strength, and many other purposes, but there is no good scientific evidence to support most of these uses.
- Aging skin. Skin aging is associated with a progressive reduction in collagen production and increased collagen breakdown. The three-dimensional spatial arrangement of collagen fibrils seen in young skin progressively becomes two-dimensional and the collagen bundles thin and become fragmented and clumped as the skin ages. These changes are accelerated by external factors including ultraviolet (UV) light, pollution, smoking and poor nutrition, resulting in skin dryness and wrinkling. Taking collagen peptides by mouth seems to improve skin hydration and skin elasticity in older people. It might also help reduce wrinkles, but it’s not clear if it helps enough to be noticeable.
- Dry skin. Taking collagen peptides by mouth seems to improve skin hydration and skin elasticity in people with dry skin.
- Pain associated with osteoarthritis. Collagen is one of the primary proteins in your joint connective tissue and another similar protein called elastin. Loss of collagen is one common reason for joint pain in some people. As such, it has been hypothesized that supplementation with collagen might be able to improve joint pain. The overall evidence from several randomized trials appear to be low, so making blanket recommendations for collagen supplementation in the treatment of osteoarthritis does not appear to be prudent at this time.
- Muscle strength. Taking collagen peptides by mouth does not seem to improve leg muscle strength. However, collagen peptides may improve hand-grip strength in the elderly.
There is interest in using collagen peptides for a number of other purposes, but there isn’t enough reliable information to say whether it might be helpful.
Collagen peptides have most often been used by adults in doses of 2.5 to 10 grams daily for up to 6 months. Speak with your doctor to find out what dose might be best for your specific condition.
Based on the scientific literature, there is no universally accepted time frame for how long it takes for collagen supplementation to show efficacy. However, most studies show benefits on outcome measures between 4-8 weeks, suggesting that a 1 to 2 months time frame appears to be a rough time frame for collagen supplements to show benefits.
Figure 14. Collagen structures
Footnotes: Collagens molecular structures. (a) Schematic representation of the most abundant collagen Pro-Hyp-Gly and Pro-Lys-Gly triplets. (b) Crystal structure of a collagen model (Gly-Pro-Hyp)10 single chain helix. (c) The collagen triple helix of the (Pro-Pro-Gly)10 model. Crystal structures are depicted in colored ball-and-stick representations.
[Source 278 ]Is collagen peptide safe?
When taken by mouth collagen peptides are possibly safe. Collagen peptides have been safely used in doses up to 10 grams daily for up to 6 months. Side effects are rare.
Collagen supplements in moderate doses (<30 grams per day) appear to be safe for humans. The Lethal Dose 50 (LD50) is an estimate of the amount of a substance that will kill half of a group of test animals when administered under controlled conditions. LD50 for oral collagen supplements in rodents is around 5 grams per kg, which would equate to about 350 grams a day for a 70 kg human being.
What is the best protein powder supplement?
Creatine (N-[aminoiminomethyl]-N-methyl glycine) is a non-essential amino acid–like compound that is produced in your liver, kidney, pancreas, and possibly your brain from the biosynthesis of the essential amino acids methionine, glycine, and arginine (with folate and vitamin B 12 as catalysts), or obtained from dietary sources 279. The primary dietary sources of creatine are high-protein foods including red meats, fish, and poultry. Once synthesized or ingested, creatine is transferred from the plasma through the intestinal wall into other tissues by specific creatine transporters located in skeletal muscles, the kidney, heart, liver, and brain. An average 150lb male has a creatine pool of approximately 120-140gms. Typically, humans manufacture about 1 gram/day of creatine, obtains 1 gram from food (muscle meats contain ~300-500 mg per 100gm serving), and loses about 2 grams per day. Therefore, under normal circumstances, creatine levels are fairly constant.
Creatine is mainly stored in your muscles with a small amount stored in your brain as well. It’s naturally found in foods such as red meats, fish, and poultry. In a normal diet that contains about 1-2 grams of creatine per day, muscle stores are only about 60-80% saturated. Vegans/vegetarians will likely have lower stores since natural sources mainly exist in animal meats.
The phosphorylated form creatine, creatine phosphate, provides an immediate energy source for your brain and muscles, and therefore, the primary reasons for supplementation are to increase, rapidly replete, and prolong this energy source to increase the metabolic capacity of these target tissues, such as the capability of a muscle to contract more powerfully longer and heal faster.
Creatine is one of the most widely studied supplements namely for its ability to increase muscle mass! Research has shown that increases in muscle mass can occur in as little as 4 weeks by supplementing with creatine in the diet.
Creatine as a dietary supplement is a tasteless, crystalline powder that readily dissolves in liquids and is marketed as creatine monohydrate or as a combination with phosphorous (creatine phosphate) 280. The majority of creatine (95%) is stored in skeletal muscle (fast twitch, type 2): two-thirds in a phosphorylated form and one-third as free creatine 281. Creatine serves as an energy substrate for the contraction of skeletal muscle. The intention of creatine supplementation is to increase resting phosphocreatine levels in your muscles, as well as free creatine, with the goal of postponing fatigue, even briefly, for sports-enhancing results 282. The goal of creatine monohydrate supplementation is to increase your muscle levels of creatine and speed the regeneration of creatine phosphate beyond what can practically be accomplished by diet alone. Creatine monohydrate supplementation has been shown to increase skeletal muscle total creatine content >15% and up to 24%, and >9% in the brain.
Creatine monohydrate is currently the most effective performance enhancement supplement for people seeking to improve their high-intensity exercise capacity (i.e., acute performance enhancement including the quality of each training session and production on game/competition day), and/or increase exercise-induced lean body mass. Creatine monohydrate is generally safe and can help you build more muscle mass 282, 283, 284, 285, 286, 287. However, always check with your doctor before starting any supplement.
Creatine is one of the most widely used dietary supplements. Athletes, body builders, and military personnel use creatine to enhance muscle mass and increase strength. Creatine is also used as an ergogenic aid to improve performance of high-intensity exercise of short duration 288, 289, 290. Creatine’s popularity as a dietary supplement was further increased by a 2006 study demonstrating its positive effect on cognitive and psychomotor performance 291.
Experiments among athletes and military personnel indicate that creatine taken at levels commonly available in supplements produces minimal, if any, side effects 290, 292. Using evidence from well-designed, randomized controlled human clinical trials of creatine, Shao and Hathcock 292 concluded that chronic intake of 5 g/ day of creatine was safe and posed no significant health risks.
Muscle creatine concentrations are increased by 20% with creatine monohydrate supplementation 281. Creatine monohydrate supplements increase lean body mass, as well as strength, power and effectiveness in short-duration, high-intensity exercises 293. The increase in body mass may be a result of the increase in intracellular water related to the osmotic properties of creatine 294. Studies on creatine monohydrate supplementation have shown short-duration improvements in sports performance and strength: specifically, in maximum-intensity exercises, muscle power, number of repetitions, muscle endurance, speed and total strength 295.
The use of creatine monohydrate can yield increases in power during short sprints of maximum intensity, which can be even more evident when repeated sprints are accompanied by short recovery periods 287. Furthermore, with creatine monohydrate supplementation, effects are also observed in muscle glycogen stores 294. This is important because the availability of muscle glycogen is the main determinant of sports performance in resistance exercises, and its depletion can lead to muscle fatigue 296. In addition, creatine monohydrate is one of the few sports foods supplements or ergogenic aids (substance used for the purpose of enhancing performance) with health claims authorised by the EFSA and the European Commission (EC), due to its evident effects on the health and sports performance of athletes 297, 298.
The approved health claims are ‘Creatine increases physical performance in repeated bursts of high-intensity exercise in the short term’ and ‘Daily creatine consumption can enhance the effect of resistance training on muscle strength in adults over the age of 55’. These health claims refer to the 3-g dose of creatine monohydrate 298. Resistance training should be performed at least three times per week for several weeks, at an intensity of at least 65–75% of one repetition maximum (1RM). The target population is adults over the age of 55, who are engaged in regular resistance training 298. Creatine in combination with resistance training and improvement in muscle strength 298.
The goal of creatine monohydrate supplementation is to deliver a greater and prolonged accrual of gains, as opposed to a non-supplemented state, that can translate to the “field of play” (specific sport activities) because continuous better workouts allow greater and continuous improved muscular adaptations. To see the fastest results, a loading protocol for creatine is often recommended. For most individuals, supplementing 5 grams of creatine per day (or about 0.3g/kg) four times daily for 5-7 days can fully saturate stores. After a loading protocol, stores can be maintained by ingesting about 5 grams per day (for larger individuals, doses of 10g per day may be needed).
In regard to timing, creatine offers the most benefit when consumed after exercise since it can help facilitate water and carbohydrates back into the muscles more quickly (faster recovery).
How does creatine monohydrate supplement improve performance and muscle growth?
Creatine monohydrate supplementation works by enhancing your body’s natural ability to create adenosine-triphosphate (ATP) is the energy molecule produced in your body that allows you to perform work.
Adenosine-triphosphate (ATP) is often called the “energy currency” of the cell because it provides energy for many processes, including:
- Muscle contraction
- Nerve impulse transmission
- Protein synthesis
- Biosynthesis
- Motility
- Maintenance functions
The human body can store ~100 grams of creatine at a given time, which is a relatively small amount considering how important it is to generating ATP (adenosine-triphosphate). Supplementing with creatine can increase your body’s creatine stores by ~30%, which increases the overall capacity of your phosphocreatine pathway (ATP production pathway). Creatine monohydrate supplement helps you produce more energy for better workouts and recover faster/better.
During all-out high-intensity activities lasting 4 to 15 seconds (e.g., jumping, sprinting, weightlifting, etc.) ATP is rapidly depleted but declines very little until stores of creatine phosphates are used. Therefore, creatine phosphates, with its high-energy phosphoryl transfer potential, serves to maintain intracellular adenosine triphosphate (ATP) levels. Creatine monohydrate supplementation significantly increases anaerobic capacity by raising the natural levels of creatine phosphates allowing intracellular concentrations of ATP to be maintained at higher levels for longer periods, permitting athletes to maintain greater training intensity and quality/quantity of each workout. Maintaining higher quality workouts throughout an entire training period leads to greater overall performance gains immediately that also compound over time, including skeletal muscle growth based on training protocols.
Creatine monohydrate supplementation also delays fatigue by reducing exercise-induced decreases in muscle pH, thereby buffering lactate and/or allowing less reliance on glycolysis.
Creatine monohydrate supplementation has been shown to:
- Improve body composition among resistance training athletes
- Improve performance over high intensity repeated bouts of exercise
- Increase strength in short-time domain exercises
The best way for athletes to take creatine is to take between 3 to 7 grams per day, with ~5 grams per day being the appropriate average dose for most people. Individuals who are smaller can consume closer to 3 grams per day, while individuals who are larger can consume closer to 7 grams per day. Typical creatine monohydrate supplementation using standard dosing protocol (.045 g/pound body weight per day) in exercisers for 10 weeks: improved strength, body composition (lean body mass) and muscle size versus no supplementation.
Creatine monohydrate supplementat may also work through other unique muscle building mechanisms related to recovery, development and muscular adaptations during the supplementing phase. These related mechanisms of creatine monohydrate supplementat may include changes in gene expression, satellite cell proliferation, insulin-like growth factor signaling, increase in growth hormone, alterations in myogenic transcription factors leading to a reduction in serum myostatin (muscle growth inhibitor), improved neuromuscular function (facilitating the reuptake of calcium ion into sarcoplasmic reticulum), and as mentioned above, reduced exercise induced blood lactate.
Finally, creatine monohydrate supplementation may also participate in reducing certain types of muscle damage (not all) from high intensity resistance training and endurance exercise allowing more complete recovery before subsequent exercise bouts.
Creatine monohydrate supplementation increases the metabolic capacity of the target tissues, such as the capability of a muscle to contract more powerfully longer and also helps with faster complete recovery.
Can creatine monohydrate supplement improve aerobic performance?
Possibly through energy partitioning (where energy is drawn from), heat regulation and recovery. Relatively little has been studied or benefits quantified using creatine monohydrate supplementation in aerobic activities due to creatine monohydrate supplementation targeting the ATP-phosphocreatine energy system. If there is a benefit, it is probably related to a change in energy substrate utilization (when creatine phosphates levels are increased by creatine monohydrate supplementation), at least during the early phase of aerobic activity that might help decrease time to exhaustion.
In aerobic/endurance activities, creatine monohydrate supplementation may improve energy usage, thermoregulation and overall recovery including glycogen restoration. Roberts et al. 299 found creatine monohydrate supplementation to augment muscle glycogen stores post exercise to potentially enhance the next bout of endurance activities. And finally, another mechanism in which creatine monohydrate supplementation may indirectly benefit aerobic activities is the ability of creatine to attenuate cardiovascular and thermoregulatory responses during prolonged exercise in the heat.
How do I use creatine monohydrate supplement to get maximum results?
- Effective Creatine dosing: Load 5g four times per day for first 5 to 7 days; then 3 to 10g per day maintenance till end of training phase cycle.
- Creatine Loading phase: The most common and successful creatine monohydrate supplementation protocol starts with a loading phase of 20g of creatine monohydrate per day or 0.14 g of creatine monohydrate per pound of body weight per day split into four daily doses of 5g each for 5 to 7 days ingesting each dose with meal/shakes to improve creatine skeletal retention. Following the loading period, continue with the maintenance phase of 3 to 5g per day or for larger athletes 5-10 g per day (or 0.04 g of creatine monohydrate per pound of body weight per day), for the duration of the supplementation period. The length of the supplementation period would be based on the goal, but generally last 12 to 16 weeks and cycled throughout the year in combination with intense training for competitive athletes.
- Each creatine monohydrate supplementation dose should be accompanied with some form of carbohydrate or protein to maximize skeletal muscle uptake/retention.
On training days, use one dose before workout and one after with meals/drinks. May mix with your pre/post training formula. On non-training days, take one dose with a morning meal and one dose with an evening meal if using 2 doses for maintenance, otherwise one dose with any meal. To maximize uptake and using multiple doses, creatine monohydrate supplementation intake should be spread as evenly as possible throughout the day with carbohydrate and/or protein containing meals or shakes.
The safety of short and long-term creatine monohydrate supplementation use is well established in healthy users using correct dosing. In 25 years with over 1000 clinical trials, creatine monohydrate supplementation has been shown to be safe and effective when taken as directed in all healthy adult populations.
Is creatine safe?
There have been reports that creatine may impair liver and kidney function. Creatine has also been linked to an increased risk of compartment syndrome, a condition where pressure builds in a muscle compartment and prevents blood flow. People at risk of kidney problems should check with their doctor before using creatine and be carefully monitored while using it.
There are no data documenting the safety of creatine in children or adolescents. The American Academy of Pediatrics and the American College of Sports Medicine warn that teens should not use performance-enhancing supplements, including creatine, because of the possible health risks 300.
Do performance-enhancing dietary supplements work?
Some bodybuilders and athletes use dietary supplements to try to improve their strength, muscle mass, and energy. However, many of these types of products contain harmful ingredients. Also, for some substances, including glutamine, choline, methoxyisoflavone, quercetin, zinc/magnesium aspartate, nitric oxide, and L-arginine, there’s no clear evidence that they improve athletic performance. Studies have looked at a variety of supplements used for bodybuilding or to improve physical performance, including glutamine, choline, methoxyisoflavone, quercetin, zinc/magnesium aspartate, nitric oxide, and L-arginine 301, 302, 303, 304, 305, 306. There’s no clear evidence these supplements improve athletic performance 301.
The results of studies on beta-alanine, an amino acid found in food and dietary supplements, are mixed but generally don’t show that it improves athletic performance significantly 307.
Nutritional Supplements
Some bodybuilders and athletes turn to dietary supplements to help them increase muscle size and definition 308. However, many bodybuilding products marketed as dietary supplements have been found to contain other ingredients that can be harmful. Use caution and talk with your health care provider before you begin taking any supplement to gain strength or muscle size. Furthermore, natural bodybuilding federations have extensive banned substance lists 309. It should be noted that there are considerably more supplements that are used by bodybuilders and sold on the market. However, an exhaustive review of all of the supplements commonly used by bodybuilders that often lack supporting data is beyond the scope of this article.
- Multivitamin and mineral supplements are unnecessary for athletes or other physically active people who eat a well-balanced diet and enough calories. The safety of supplements used for bodybuilding remains an issue of concern (see Safety below).
- There is no scientific evidence that other dietary supplements, such as choline, methoxyisoflavone, zinc/magnesium aspartate, nitric oxide precursors, and chromium, are effective for building strength and muscle mass.
- Evidence suggests that creatine, a popular dietary supplement, may enhance the effects of vigorous exercise on strength, muscle mass, and endurance, but it may also cause fluid weight gain, nausea, cramping, and diarrhea.
Safety
- Many bodybuilding products marketed as dietary supplements have been found to be deceptively labeled and to contain hidden ingredients that can be harmful, such as anabolic steroids, compounds chemically similar to them, or other substances that don’t qualify as dietary ingredients.
- In April 2013, the U.S. Food and Drug Administration issued a warning to consumers to avoid products containing the stimulant dimethylamylamine (DMAA). DMAA can elevate blood pressure and lead to other problems, such as a heart attack.
- Evidence suggests that creatine (an amino acid produced by the body) supplements may be safe for short-term use in healthy adults, but the American College of Sports Medicine recommends against anyone younger than age 18 using it to enhance athletic performance.
- Some dietary supplements may have side effects and some may interact with drugs or other supplements. Some vitamins and minerals are harmful at high doses. Talk with your health care provider before using a dietary supplement to increase muscle size and strength.
Micronutrients
Several previous studies have observed deficiencies in intakes of micronutrients, such as vitamin D, calcium, zinc, magnesium, and iron, in dieting bodybuilders 310, 311. However, it should be noted that these studies were all published nearly 2 decades ago and that micronutrient deficiencies likely occurred due to elimination of foods or food groups and monotony of food selection 312, 311. Therefore, future studies are needed to determine if these deficiencies would present while eating a variety of foods and using the contest preparation approach described herein. Although the current prevalence of micronutrient deficiencies in competitive bodybuilders is unknown, based on the previous literature, a low-dose micronutrient supplement may be beneficial for natural bodybuilders during contest preparation; however, future studies are needed to verify this recommendation.
Caffeine
Caffeine is perhaps the most common pre-workout stimulant consumed by bodybuilders. Numerous studies support the use of caffeine to improve performance during endurance training 313, 314, sprinting 315, 316, and strength training 317, 318, 319. However, not all studies support use of caffeine to improve performance in strength training 320, 321. It should be noted that many of the studies that found increases in strength training performance supplemented with larger (5–6 mg/kg) dosages of caffeine. However, this dosage of caffeine is at the end of dosages that are considered safe (6 mg/kg/day) 322. Additionally, it appears that regular consumption of caffeine may result in a reduction of ergogenic effects 323. Therefore, it appears that 5–6 mg/kg caffeine taken prior to exercise is effective in improving exercise performance; however, caffeine use may need to be cycled in order for athletes to obtain the maximum performance enhancing effect.
Beta-alanine
Beta-alanine (BA) is becoming an increasingly popular supplement among bodybuilders. Once consumed, BA enters the circulation and is up-taken by skeletal muscle where it is used to synthesize carnosine, a pH buffer in muscle that is particularly important during anaerobic exercise such as sprinting or weightlifting 324. Indeed, consumption of 6.4 g BA daily for four weeks has been shown to increase muscle carnosine levels by 64.2% 325. Moreover, supplementation with BA for 4–10 weeks has been shown to increase knee extension torque by up to 6%, improve workload and time to fatigue during high intensity cardio, improve muscle resistance to fatigue during strength training, increase lean mass by approximately 1 kg and significantly reduce perceptions of fatigue. Additionally, the combination of BA and CM may increase performance of high intensity endurance exercise and has been shown to increase lean mass and decrease body fat percentage more than CM alone. However, not all studies have shown improvements in performance with BA supplementation. To clarify these discrepancies, Hobson et al. 326 conducted a meta-analysis of 15 studies on BA supplementation and concluded that BA significantly increased exercise capacity and improved exercise performance on 60-240 seconds and >240 seconds exercise bouts.
Although BA appears to improve exercise performance, the long-term safety of BA has only been partially explored. Currently, the only known side effect of BA is unpleasant symptoms of parasthesia reported after consumption of large dosages; however, this can be minimized through consumption of smaller dosages throughout the day 325. While BA appears to be relatively safe in the short-term, the long-term safety is unknown. In cats, an addition of 5 percent BA to drinking water for 20 weeks has been shown to deplete taurine and result in damage to the brain; however, taurine is an essential amino acid for cats but not for humans and it is unknown if the smaller dosages consumed by humans could result in similar effects 327. BA may increase exercise performance and increase lean mass in bodybuilders and currently appears to be safe; however, studies are needed to determine the long-term safety of BA consumption.
Beta-hydroxy-beta-methylbutyrate
Beta-hydroxy-beta-methylbutyrate (HMB) is a metabolite of the amino acid leucine that has been shown to decrease muscle protein catabolism and increase muscle protein synthesis 328, 329. The safety of HMB supplementation has been widely studied and no adverse effects on liver enzymes, kidney function, cholesterol, white blood cells, hemoglobin, or blood glucose have been observed 330, 331. Furthermore, two meta-analyses on HMB supplementation have concluded that HMB is safe and does not result in any major side effects 330. HMB may actually decrease blood pressure, total and LDL cholesterol, especially in hypercholesterolemic individuals.
HMB is particularly effective in catabolic populations such as the elderly and patients with chronic disease 332. However, studies on the effectiveness of HMB in trained, non-calorically restricted populations have been mixed. Reasons for discrepancies in the results of HMB supplementation studies in healthy populations may be due to many factors including clustering of data in these meta-analysis to include many studies from similar groups, poorly designed, non-periodized training protocols, small sample sizes, and lack of specificity between training and testing conditions 333. However, as a whole HMB appears to be effective in a majority of studies with longer-duration, more intense, periodized training protocols and may be beneficial to bodybuilders, particularly during planned over-reaching phases of training 334. While the authors hypothesize that HMB may be effective in periods of increased catabolism, such as during contest preparation, the efficacy of HMB on maintenance of lean mass in dieting athletes has not been investigated in a long-term study. Therefore, future studies are needed to determine the effectiveness of HMB during caloric restriction in healthy, lean, trained athletes.
Creatine
Creatine is a naturally-occurring amino acid (protein building block) that’s found in meat and fish, and also made by the human body predominately in the liver, kidneys, and to a lesser extent in the pancreas 335. Creatine is produced in your body at an amount of about 1 g/day 336. The remainder of the creatine available to the body is obtained through the diet at about 1 g/day for an omnivorous diet 336. 95% of the bodies creatine stores are found in the skeletal muscle and the remaining 5% is distributed in the brain, liver, kidney, and testes 337.
Creatine is chemically known as a non-protein nitrogen; a compound which contains nitrogen but is not a protein per se 338. It is synthesized in the liver and pancreas from the amino acids arginine, glycine, and methionine 338, 339. Approximately 95% of the body’s creatine is stored in skeletal muscle. Additionally, small amounts of creatine are also found in the brain and testes 340, 341. About two thirds of the creatine found in skeletal muscle is stored as phosphocreatine (PCr) while the remaining amount of creatine is stored as free creatine 340. The total creatine pool (PCr + free creatine) in skeletal muscle averages about 120-140 grams for a 70 kg individual 342, depending on the skeletal muscle fiber type 337 and quantity of muscle mass 343. However, the average human has the capacity to store up to 160 grams of creatine under certain conditions 344, 345. The body breaks down about 1 – 2% of the creatine pool per day (about 1–2 grams/day) into creatinine in the skeletal muscle 338. The body production and dietary intake matches the rate of creatinine production from the degradation of phosphocreatine and creatine at 2.6% and 1.1%/d respectively. In general, oral creatine supplementation leads to an increase of creatine levels within the body. Creatine can be cleared from the blood by saturation into various organs and cells or by renal filtration then excreted in urine 337.
Creatine stores can be replenished by obtaining creatine in the diet or through endogenous synthesis of creatine from glycine, arginine, and methionine 346, 347. Dietary sources of creatine include meats and fish. Large amounts of fish and meat must be consumed in order to obtain gram quantities of creatine. Whereas dietary supplementation of creatine provides an inexpensive and efficient means of increasing dietary availability of creatine without excessive fat and/or protein intake.
Three amino acids (glycine, arginine and methionine) and three enzymes (L-arginine:glycine amidinotransferase, guanidinoacetate methyltransferase and methionine adenosyltransferase) are required for creatine synthesis. The impact creatine synthesis has on glycine metabolism in adults is low, however the demand is more appreciable on the metabolism of arginine and methionine 343.
As creatine is predominately present in the diet from meats, vegetarians have lower resting creatine concentrations 348. It is converted into creatine phosphate or phosphocreatine and stored in the muscles, where it is used for energy 335. During high-intensity, short-duration exercise, such as lifting weights or sprinting, phosphocreatine is converted into ATP, a major source of energy within the human body.
Biochemically speaking, the energy supplied to rephosphorylate adenosine diphosphate (ADP) to adenosine triphosphate (ATP) during and following intense exercise is largely dependent on the amount of phosphocreatine (PCr) stored in the muscle 349, 344. As phosphocreatine stores become depleted during intense exercise, energy availability diminishes due to the inability to resynthesize ATP at the rate required to sustained high-intensity exercise 349, 344. Consequently, the ability to maintain maximal-effort exercise declines. The availability of phosphocreatine in the muscle may significantly influence the amount of energy generated during brief periods of high-intensity exercise. Furthermore, it has been hypothesized that increasing muscle creatine content, via creatine supplementation, may increase the availability of phosphocreatine allowing for an accelerated rate of resynthesis of ATP during and following high-intensity, short-duration exercise 349, 344. Theoretically, creatine supplementation during training may lead to greater training adaptations due to an enhanced quality and volume of work performed. In terms of potential medical applications, creatine is intimately involved in a number of metabolic pathways. For this reason, medical researchers have been investigating the potential therapeutic role of creatine supplementation in a variety of patient populations.
Creatine supplements are popular among body builders and competitive athletes. As an oral supplement, the most widely used and researched form is creatine monohydrate 336. When orally ingested, creatine monohydrate has shown to improve exercise performance and increase fat free mass 350, 351, 352, 353, 354. The attraction of creatine is that it may increase lean muscle mass and enhance athletic performance, particularly during high-intensity, short-duration sports (like high jumping and weight lifting).
However, not all human studies show that creatine improves athletic performance 335. Nor does every person seem to respond the same way to creatine supplements. For example, people who tend to have naturally high stores of creatine in their muscles don’t get an energy-boosting effect from extra creatine. Preliminary clinical studies also suggest that creatine’s ability to increase muscle mass and strength may help fight muscle weakness associated with illnesses, such as heart failure and muscular dystrophy.
Creatine supplementation Responders vs. Non-responders
Syrotuik and Bell 355 investigated the physical characteristics of responder and non-responder subjects to creatine supplementation in recreationally resistance trained men with no history of creatine monohydrate usage. The supplement group was asked to ingest a loading dosage of 0.3 g/kg/d for 5 days. The physiological characteristics of responders were classified using Greenhaff et al 356 criterion of >20 mmol/kg dry weight increase in total intramuscular creatine and phosphocreatine and non responders as <10 mmol/kg dry weight increase, a third group labeled quasi responders were also used to classify participants who fell in between the previously mentioned groups (10-20 mmol/kg dry weight). Overall, the supplemented group showed a mean increase in total resting muscle creatine and phosphocreatine of 14.5% (from 111.12 ± 8.87 mmol/kg dry weight to 127.30 ± 9.69 mmol/kg dry weight) whilst the placebo group remained relatively unaffected (from 115.70 ± 14.99 mmol/kg dry weight to 111.74 ± 12.95 mmol/kg dry weight). However when looking at individual cases from the creatine group the results showed a variance in response. From the 11 males in the supplemented group, 3 participants were responders (mean increase of 29.5 mmol/kg dry weight or 27%), 5 quasi responders (mean increase of 14.9 mmol/kg dry weight or 13.6%) and 3 non-responders (mean increase of 5.1 mmol/kg dry weight or 4.8%). Using muscle biopsies of the vastus lateralis, a descending trend for groups and mean percentage fiber type was observed. Responders showed the greatest percentage of type II fibers followed by quasi responders and non-responders. The responder and quasi responder groups had an initial larger cross sectional area for type I, type IIa and type IIx fibers. The responder group also had the greatest mean increase in the cross sectional area of all the muscle fiber types measured (type I, type IIa and type IIx increased 320, 971 and 840 μm2 respectively) and non-responders the least (type I, type IIa and type IIx increased 60, 46 and 78 μm2 respectively). There was evidence of a descending trend for responders to have the highest percentage of type II fibers; furthermore, responders and quasi responders possessed the largest initial cross sectional area of type I, IIa and IIx fibers. Responders were seen to have the lowest initial levels of creatine and phosphocreatine. This has also been observed in a previous study 357 which found that subjects whose creatine levels were around 150 mmol/Kg dry mass did not have any increments in their creatine saturation due to creatine supplementation, neither did they experience any increases of creatine uptake, phosphocreatine resynthesis and performance. This would indicate a limit maximum size of the creatine pool.
In summary responders are those individuals with a lower initial level of total muscle creatine content, greater population of type II fibers and possess higher potential to improve performance in response to creatine supplementation.
Effects of creatine supplementation on Muscle Mass
Cribb et al (2007) 358 observed greater improvements on 1RM, lean body mass, fiber cross sectional area and contractile protein in trained young males when resistance training was combined with a multi-nutrient supplement containing 0.1 g/kg/d of creatine, 1.5 g/kg/d of protein and carbohydrate compared with protein alone or a protein carbohydrate supplement without the creatine. These findings were novel because at the time no other research had noted such improvements in body composition at the cellular and sub cellular level in resistance trained participants supplementing with creatine. The amount of creatine consumed in the study by Cribb et al 358 was greater than the amount typically reported in previous studies (a loading dose of around 20 g/d followed by a maintenance dose of 3-5 g/d is generally equivalent to approximately 0.3 g/kg/d and 0.03 g/kg/d respectively) and the length of the supplementation period or absence of resistance exercise may explain the observed transcriptional level changes that were absent in previous studies 359, 360.
Deldicque et al 361 found a 250%, 45% and 70% increase for collagen mRNA, glucose transporter 4 (GLUT4) and Myosin heavy chain IIA, respectively after 5 days creatine loading protocol (21 g/d). The authors speculated that creatine in addition to a single bout of resistance training can favor an anabolic environment by inducing changes in gene expression after only 5 days of supplementation.
When creatine supplementation is combined with heavy resistance training, muscle insulin like growth factor (IGF-1) concentration has been shown to increase. Burke et al 362 examined the effects of an 8 week heavy resistance training protocol combined with a 7 day creatine loading protocol (0.25 g/d/kg lean body mass) followed by a 49 day maintenance phase (0.06 g/kg lean mass) in a group of vegetarian and non-vegetarian, novice, resistance trained men and women. Compared to placebo, creatine groups produced greater increments in IGF-1 (78% Vs 55%) and body mass (2.2 Vs 0.6 kg). Additionally, vegetarians within the supplemented group had the largest increase of lean mass compared to non vegetarian (2.4 and 1.9 kg respectively). Changes in lean mass were positively correlated to the modifications in intramuscular total creatine stores which were also correlated with the modified levels of intramuscular IGF-1. The authors suggested that the rise in muscle IGF-1 content in the creatine group could be due to the higher metabolic demand created by a more intensely performed training session. These amplifying effects could be caused by the increased total creatine store in working muscles. Even though vegetarians had a greater increase in high energy phosphate content, the IGF-1 levels were similar to the amount observed in the non vegetarian groups. These findings do not support the observed correlation pattern by which a low essential amino acid content of a typical vegetarian diet should reduce IGF-1 production 363. According to authors opinions it is possible that the addition of creatine and subsequent increase in total creatine and phosphocreatine storage might have directly or indirectly stimulated production of muscle IGF-I and muscle protein synthesis, leading to an increased muscle hypertrophy 362.
Effects of creatine ingestion to improve recovery from injury, muscle damage and oxidative stress induced by exercise
Creatine supplementation may also be of benefit to injured athletes. Op’t Eijnde et al 364 noted that the expected decline in GLUT4 content after being observed during a immobilization period can be offset by a common loading creatine (20g/d) supplementation protocol. In addition, combining creatine monohydrate 15g/d for 3 weeks following 5 g/d for the following 7 weeks positively enhances GLUT4 content, glycogen, and total muscle creatine storage 364.
Bassit et al (Bassit RA, Pinheiro CH, Vitzel KF, Sproesser AJ, Silveira LR, Curi R. Effect of short-term creatine supplementation on markers of skeletal muscle damage after strenuous contractile activity. Eur J Appl Physiol. 2010;108:945–955. doi: 10.1007/s00421-009-1305-1. https://www.ncbi.nlm.nih.gov/pubmed/19956970()) observed a decrease in several markers of muscle damage (creatine kinase, lactate dehydrogenase, aldolase, glutamic oxaloacetic acid transaminase and glutamic pyruvic acid transaminase) in 4 athletes after an iron man competition who supplemented with 20 g/d plus 50 g maltodextrin during a 5 day period prior to the competition.
Cooke et al 365 observed positive effects of a prior (0.3 g/d kg body weight) loading and a post maintenance protocol (0.1 g/d kg body weight) to attenuate the loss of strength and muscle damage after an acute supramaximal (3 set x 10 rep with 120% 1RM) eccentric resistance training session in young males. The authors speculate that creatine ingestion prior to exercise may enhance calcium buffering capacity of the muscle and reduce calcium-activated proteases which in turn minimize sarcolemma and further influxes of calcium into the muscle. In addition creatine ingestion post exercise would enhance regenerative responses, favoring a more anabolic environment to avoid severe muscle damage and improve the recovery process. In addition, in vitro studies have demonstrated the antioxidant effects of creatine to remove superoxide anion radicals and peroxinitrite radicals 366. This antioxidant effect of creatine has been associated with the presence of Arginine in its molecule. Arginine is also a substrate for nitric oxide synthesis and can increase the production of nitric oxide which has higher vasodilatation properties, and acts as a free radical that modulates metabolism, contractibility and glucose uptake in skeletal muscle. Other amino acids contained in the creatine molecule such as glycine and methinine may be especially susceptible to free radical oxidation because of sulfhydryl groups 366. A more recent in vitro study showed that creatine exerts direct antioxidant activity via a scavenging mechanism in oxidatively injured cultured mammalian cells 367. In a recent in vivo study Rhaini et al 368 showed a positive effect of 7 days of creatine supplementation (4 x 5 g CM 20 g total) on 27 recreational resistance trained males to attenuate the oxidation of DNA and lipid peroxidation after a strenuous resistance training protocol.
Collectively the above investigations indicate that creatine supplementation can be an effective strategy to maintain total creatine pool during a rehabilitation period after injury as well as to attenuate muscle damage induced by a prolonged endurance training session. In addition, it seems that creatine can act as an effective antioxidant agent after more intense resistance training sessions 336.
Effects of creatine supplementation on glycogen stores
It is suggested 369, 370 that another mechanism for the effect of creatine could be enhanced muscle glycogen accumulation and GLUT4 expression, when creatine supplementation is combined with a glycogen depleting exercise. Whereas it has been observed 371 that creatine supplementation alone does not enhance muscle glycogen storage. Hickner et al 372 observed positive effects of creatine supplementation for enhancing initial and maintaining a higher level of muscle glycogen during 2 hours of cycling. In general, it is accepted that glycogen depleting exercises, such as high intensity or long duration exercise should combine high carbohydrate diets with creatine supplementation to achieve heightened muscle glycogen stores 364.
Effects of creatine supplementation on predominantly aerobic exercise
Although creatine supplementation has been shown to be more effective on predominantly anaerobic intermittent exercise, there is some evidence of its positive effects on endurance activities. Branch 373 highlights that endurance activities lasting more than 150 seconds rely on oxidative phosphorylation as primary energy system supplier. From this meta analysis 373, it would appear that the ergogenic potential for creatine supplementation on predominantly aerobic endurance exercise diminishes as the duration of the activity increases over 150s. However it is suggested that creatine supplementation may cause a change in substrate utilization during aerobic activity possibly leading to an increase in steady state endurance performance.
Chwalbinska-Monteta 374 observed a significant decrease in blood lactate accumulation when exercising at lower intensities as well as an increase in lactate threshold in elite male endurance rowers after consuming a short loading (5 days 20 g/d) creatine monohydrate protocol. However, the effects of creatine supplementation on endurance performance have been questioned by some studies. Graef et al 375 examined the effects of four weeks of creatine citrate supplementation and high-intensity interval training on cardio respiratory fitness. A greater increase of the ventilatory threshold was observed in the creatine group respect to placebo; however, oxygen consumption showed no significant differences between the groups. The total work presented no interaction and no main effect for time for any of the groups. Thompson et al 376 reported no effects of a 6 week 2 g creatine monohydrate/day in aerobic and anaerobic endurance performance in female swimmers.
Documented effects of creatine supplementation on physical performance
The majority of studies focusing on creatine supplementation report an increase in the body’s’ creatine pool 372, 369, 357. There is a positive relationship between muscle creatine uptake and exercise performance 357. Volek et al. 377 observed a significant increase in strength performance after 12 weeks creatine supplementation with a concurrent periodized heavy resistance training protocol. The creatine supplementation protocol consisted of a weeklong loading period of 25 g/day followed by a 5 gram maintenance dose for the remainder of the training. These positive effects were attributed to an increased total creatine pool resulting in more rapid adenosine triphosphate (ATP) regeneration between resistance training sets allowing athletes to maintain a higher training intensity and improve the quality of the workouts along the entire training period.
It is regularly reported that creatine supplementation, when combined with heavy resistance training leads to enhanced physical performance, fat free mass, and muscle morphology 378, 379, 380. A 2003 meta analysis 353 showed individuals ingesting creatine, combined with resistance training, obtain on average +8% and +14% more performance on maximum (1RM) or endurance strength (maximal repetitions at a given percent of 1RM) respectively than the placebo groups. However, contradicting studies have reported no effects of creatine supplementation on strength performance. Jakobi et al 381 found no effects of a short term creatine loading protocol upon isometric elbow flexion force, muscle activation, and recovery process. However, this study did not clearly state if creatine supplementation was administered concurrent with resistance training. Bemben et al 382 have shown no additional benefits of creatine alone or combined with whey protein for improving strength and muscle mass after a progressive 14 weeks (3 days per week) resistance training program in older men. These conflicting results can be explained by the possibility that the supplemented groups were formed by a greater amount of non-responders or even because creatine supplementation was administered on the training days only (3 times a week). This strategy has not been adequately tested as effective in middle aged and older men for maintaining post loading elevated creatine stores 350.
A quantitative, comprehensive scientific summary and view of knowledge up to 2007 on the effects of creatine supplementation in athletes and active people was published in a 100 citation review position paper by the International Society of Sports Nutrition 350. More recent literature has provided greater insight into the anabolic/performance enhancing mechanisms of creatine supplementation 372, 383 suggesting that these effects may be due to satellite cell proliferation, myogenic transcription factors and insulin-like growth factor-1 signalling 369. Saremi et al 384 reported a change in myogenic transcription factors when creatine supplementation and resistance training are combined in young healthy males. It was found that serum levels of myostatin, a muscle growth inhibitor, were decreased in the creatine group.
Collectively, in spite of a few controversial results, it seems that creatine supplementation combined with resistance training would amplify performance enhancement on maximum and endurance strength as well muscle hypertrophy 336.
Effects of creatine supplementation on predominantly anaerobic exercise
Creatine has demonstrated neuromuscular performance enhancing properties on short duration, predominantly anaerobic, intermittent exercises. Bazzucch et al 385 observed enhanced neuromuscular function of the elbow flexors in both electrically induced and voluntary contractions but not on endurance performance after 4 loading doses of 5 g creatine plus 15 g maltodextrin for 5/d in young, moderately trained men. Creatine supplementation may facilitate the reuptake of Ca2+ into the sacroplasmic reticulum by the action of the Ca2+ adenosine triphosphatase pump, which could enable force to be produced more rapidly through the faster detachment of the actomyosin bridges.
A previous meta-analysis 373 reported an overall creatine supplementation effect size (ES) of 0.24 ± 0.02 for activities lasting ≤30 s. (primarily using the ATP- phosphocreatine energy system). For this short high-intensity exercise, creatine supplementation resulted in a 7.5 ± 0.7% increase from base line which was greater than the 4.3 ± 0.6% improvement observed for placebo groups. When looking at the individual selected measures for anaerobic performance the greatest effect of creatine supplementation was observed on the number of repetitions which showed an ES of 0.64 ± 0.18. Furthermore, an increase from base line of 45.4 ± 7.2% compared to 22.9 ± 7.3% for the placebo group was observed. The second greatest ES was on the weight lifted at 0.51 ± 0.16 with an increase from base line of 13.4 ± 2.7% for the placebo group and 24.7 ± 3.9% for the creatine group. Other measures improved by creatine with a mean ES greater than 0 were for the amount of work accomplished, weight lifted, time, force production, cycle ergometer revolutions/min and power. The possible effect of creatine supplementation on multiple high intensity short duration bouts (<30 s) have shown an ES not statistically significant from 0. This would indicate that creatine supplementation might be useful to attenuate fatigue symptoms over multiple bouts of high-intensity, short duration exercise. The ES of creatine on anaerobic endurance exercise (>30 – 150s), primarily using the anaerobic glycolysis energy system, was 0.19 ± 0.05 with an improvement from baseline of 4.9 ± 1.5 % for creatine and -2.0 ± 0.6% for the placebo. The specific aspects of anaerobic endurance performance improved by creatine supplementation were work and power, both of which had a mean ES greater than 0. From the findings of this previous meta-analysis 373 it would appear that creatine supplementation has the most pronounced effect on short duration (<30s) high intensity intermittent exercises.
Effects of creatine supplementation on predominantly aerobic exercise
Although creatine supplementation has been shown to be more effective on predominantly anaerobic intermittent exercise, there is some evidence of its positive effects on endurance activities. Branch 373 highlights that endurance activities lasting more than 150 seconds rely on oxidative phosphorylation as primary energy system supplier. From this meta analysis 373, it would appear that the ergogenic potential for creatine supplementation on predominantly aerobic endurance exercise diminishes as the duration of the activity increases over 150s. However it is suggested that creatine supplementation may cause a change in substrate utilization during aerobic activity possibly leading to an increase in steady state endurance performance.
Chwalbinska-Monteta 374 observed a significant decrease in blood lactate accumulation when exercising at lower intensities as well as an increase in lactate threshold in elite male endurance rowers after consuming a short loading (5 days 20 g/d) CM protocol. However, the effects of creatine supplementation on endurance performance have been questioned by some studies. Graef et al 375 examined the effects of four weeks of creatine citrate supplementation and high-intensity interval training on cardio respiratory fitness. A greater increase of the ventilatory threshold was observed in the creatine group respect to placebo; however, oxygen consumption showed no significant differences between the groups. The total work presented no interaction and no main effect for time for any of the groups. Thompson et al 376 reported no effects of a 6 week 2 g CM/d in aerobic and anaerobic endurance performance in female swimmers. In addition, of the concern related to the dosage used in these studies, it could be possible that the potential benefits of creatine supplementation on endurance performance were more related to effects of anaerobic threshold localization.
Effects of creatine supplementation on range of motion
Sculthorpe et al 386 has shown that a 5 day (25g/d) loading protocol of creatine supplementation followed by a further 3 days of 5 g/d negatively influence both active ankle dorsiflexion and shoulder abduction and extension range of movement (ROM) in young men. There are two possible theories to explain these effects: 1) Creatine supplementation increases intracellular water content resulting in increased muscle stiffness and resistance to stretch; 2) Neural outflow from the muscle spindles is affected due to an increased volume of the muscle cell. The authors highlight that the active ROM measures were taken immediately after the loading phase and the reduced active ROM may not be seen after several weeks of maintenance phase 386. Hile et al 387 observed an increase in compartment pressure in the anterior compartment of the lower leg, which may also have been responsible for a reduced active ROM.
Creatine use in children and adolescents
Creatine supplementation in the under 18 population has not received a great deal of attention, especially in regards to sports/exercise performance. Despite this, creatine is being supplemented in young, <18 years old, athletes 388, 389. In a 2001 report 388 conducted on pupils from middle and high school (aged 10 – 18) in Westchester County (USA) 62 of the 1103 pupils surveyed were using creatine. The authors found this concerning for 2 main reasons: firstly, the safety of creatine supplementation is not established for this age group and is therefore not recommended. Secondly, it was speculated that taking creatine would lead on to more dangerous performance enhancing products such as anabolic steroids. It is important to point out that this potential escalation is speculation. Furthermore, a questionnaire was used to determine creatine use amongst this age group and does not necessarily reflect the truth.
A child’s ability to regenerate high energy phosphates during high intensity exercise is less than that of an adult. Due to this, creatine supplementation may benefit the rate and use of creatine phosphate and ATP rephosporylation. However, performance in short duration high-intensity exercise can be improved through training therefore supplementation may not be necessary 390.
Based on the limited data on performance and safety, some authors have not identified any conclusions and do not recommend its consumption in regards to creatine supplementation in children and adolescents 389, 390. Conversely, according to the view of the International Society of Sports Nutrition 350, younger athletes should consider a creatine supplement under certain conditions: puberty is past and he/she is involved in serious competitive training; the athlete is eating a well-balanced caloric adequate diet; he/she as well as the parents approve and understand the truth concerning the effects of creatine supplementation; supplement protocols are supervised by qualified professionals; recommended doses must not be exceeded; quality supplements are administered.
Within this framework, creatine supplementation in young, post puberty athletes can be considered a high quality type of “food” that can offer additional benefits to optimise training outcomes.
Safety and side effects of creatine supplementation
There have been a few reported renal health disorders associated with creatine supplementation 391, 392. These are isolated reports in which recommended dosages are not followed or there is a history of previous health complaints, such as renal disease or those taking nephrotoxic medication aggravated by creatine supplementation 392. Specific studies into creatine supplementation, renal function and/or safety conclude that although creatine does slightly raise creatinine levels there is no progressive effect to cause negative consequences to renal function and health in already healthy individuals when proper dosage recommendations are followed 393, 394, 395, 392. Urinary methylamine and formaldehyde have been shown to increase due to creatine supplementation of 20 g/d; this however did not bring the production outside of normal healthy range and did not impact on kidney function 396, 397. It has been advised that further research be carried out into the effects of creatine supplementation and health in the elderly and adolescent 391, 395. More recently, a randomized, double blind, 6 month resistance exercise and supplementation intervention 398 was performed on elderly men and women (age >65 years) in which subjects were assigned to either a supplement or placebo group. The supplement group was given 5 g creatine monohydrate, 2 g dextrose and 6 g conjugated linoleic acid/d, whilst the placebo group consumed 7 g dextrose and 6 g safflower oil/d. Creatine monohydrate administration showed significantly greater effects to improve muscular endurance, isokinetic knee extension strength, fat free mass and to reduce fat mass compared to placebo. Furthermore the supplement group had an increase in serum creatinine but not creatinine clearance suggesting no negative effect on renal function.
Cornelissen et al 399 analyzed the effects of 1 week loading protocol (3 X 5 g/d creatine monohydrate) followed by a 3 month maintenance period (5 g/d) on cardiac patients involved in an endurance and resistance training program. Although creatine monohydrate supplementation did not significantly enhance performance, markers of renal and liver function were within normal ranges indicating the safety of the applied creatine supplementation protocol.
A retrospective study 400, that examined the effects of long lasting (0.8 to 4 years) creatine monohydrate supplementation on health markers and prescribed training benefits, suggested that there is no negative health effects (including muscle cramp or injuries) caused by long term creatine monohydrate consumption. In addition, despite many anecdotal claims, it appears that creatine supplementation would have positive influences on muscle cramps and dehydration 401. Creatine was found to increase total body water possibly by decreasing the risk of dehydration, reducing sweat rate, lowering core body temperature and exercising heart rate. Furthermore, creatine supplementation does not increase symptoms nor negatively affect hydration or thermoregulation status of athletes exercising in the heat 402, 403. Additionally, CM ingestion has been shown to reduce the rate of perceived exertion when training in the heat 404.
It is prudent to note that creatine supplementation has been shown to reduce the body’s endogenous production of creatine, however levels return to normal after a brief period of time when supplementation ceases 405, 351. Despite this creatine supplementation has not been studied/supplemented with for a relatively long period. Due to this, long term effects are unknown, therefore safety cannot be guaranteed. Whilst the long term effects of creatine supplementation remain unclear, no definitive certainty of either a negative or a positive effect upon the body has been determined for many health professionals and national agencies 406, 396. For example the French Sanitary Agency has banned the buying of creatine due to the unproven allegation that a potential effect of creatine supplementation could be that of mutagenicity and carcinogenicity from the production of heterocyclic amines 396. Long term and epidemiological data should continue to be produced and collected to determine the safety of creatine in all healthy individuals under all conditions 396.
Commercially available forms of creatine supplements
There are several different available forms of creatine: creatine anhydrous which is creatine with the water molecule removed in order to increase the concentration of creatine to a greater amount than that found in creatine monohydrate. Creatine has been manufactured in salt form: creatine pyruvate, creatine citrate, creatine malate, creatine phosphate, magnesium creatine, creatine oroate, Kre Alkalyn (creatine with baking soda). Creatine can also be manufactured in an ester form. Creatine ethyl ester (hydrochloride) is an example of this, as is creatine gluconate which is creatine bound to glucose. Another form is creatine effervescent which is creatine citrate or creatine monohydrate with citric acid and bicarbonate. The citric acid and bicarbonate react to produce an effervescent effect. When mixed with water the creatine separates from its carrier leaving a neutrally charged creatine, allowing it to dissolve to a higher degree in water. Manufacturers claim that creatine effervescent has a longer and more stable life in solution. When di-creatine citrate effervescent was studied 407 for stability in solution it was found that the di-creatine citrate dissociates to citric acid and creatine in aqueous solutions which in turn forms creatine monohydrate and eventually crystallises out of the solution due to its low solubility. Some of the creatine may also convert to creatinine.
Jager et al 408 observed 1.17 and 1.29 greater peak plasma creatine concentration 1 hour after ingesting creatine pyruvate compared to isomolar amount of creatine monohydrate and creatine citrate respectively. However time to peak concentration, and velocity constants of absorption and elimination, was the same for all three forms of creatine. Although not measured in this study it is questionable that these small differences in plasma creatine concentrations would have any effect on the increase of muscle creatine uptake. Jäger et al 409 investigated the effects of 28-days of creatine pyruvate and citrate supplementation on endurance capacity and power measured during an intermittent handgrip (15 seconds effort per 45 seconds rest) exercise in healthy young athletes. The authors used a daily dose protocol with the intention to slowly saturate muscle creatine stores. Both forms of creatine showed slightly different effects on plasma creatine absorption and kinetics. The two creatine salts significantly increased mean power but only pyruvate forms showed significant effects for increasing force and attenuating fatigability during all intervals. These effects can be attributed to an enhanced contraction and relaxation velocity as well as a higher blood flow and muscle oxygen uptake. On the other hand, the power performance measured with the citrate forms decreases with time and improvements were not significant during the later intervals. In spite of these positive trends further research is required about the effects of these forms of creatine as there is little or no evidence for their safety and efficacy. Furthermore the regularity status of the novel forms of creatine vary from country to country and are often found to be unclear when compared to that of creatine monohydrate 410.
In summary, creatine salts have been show to be less stable than creatine monohydrate. However the addition of carbohydrates could increase their stability 410. The potential advantages of creatine salts over creatine monohydrate include enhanced aqueous solubility and bioavailability which would reduce their possible gastrointestinal adverse effects 411. The possibility for new additional formulation such as tablets or capsules is interesting for its therapeutic application due to its attributed better dissolution kinetics and oral absorption compared to creatine monohydrate 411. However more complete in vivo pharmaceutical analysis of creatine salts are required to fully elucidate their potential advantages/disadvantages over the currently available supplement formulations.
Creatine is a hydrophilic polar molecule that consists of a negatively charged carboxyl group and a positively charged functional group 412. The hydrophilic nature of creatine limits its bioavailability [65]. In an attempt to increase creatines bioavailability creatine has been esterified to reduce the hydrophilicity; this product is known as creatine ethyl ester. Manufacturers of creatine ethyl ester promote their product as being able to by-pass the creatine transporter due to improved sarcolemmal permeability toward creatine 413. Spillane et al 413 analyzed the effects of a 5 days loading protocol (0.30 g/kg lean mass) followed by a 42 days maintenance phase (0.075 g/kg lean mass) of CM or ethyl ester both combined with a resistance training program in 30 novice males with no previous resistance training experience. The results of this study 413 showed that ethyl ester was not as effective as creatine monohydrate to enhance serum and muscle creatine stores. Furthermore creatine ethyl ester offered no additional benefit for improving body composition, muscle mass, strength, and power. This research did not support the claims of the creatine ethyl ester manufacturers.
Polyethylene glycol is a non-toxic, water-soluble polymer that is capable of enhancing the absorption of creatine and various other substances 414. Polyethylene glycol can be bound with creatine monohydrate to form polyethylene glycosylated creatine. One study 415 found that 5 g/d for 28 days of polyethylene glycosylated creatine was capable of increasing 1RM bench press in 22 untrained young men but not for lower body strength or muscular power. Body weight also did not significantly change in the creatine group which may be of particular interest to athletes in weight categories that require upper body strength. Herda et al 416 analyzed the effects of 5 g of creatine monohydrate and two smaller doses of polyethylene glycosylated creatine (containing 1.25 g and 2.5 g of creatine) administered over 30 days on muscular strength, endurance, and power output in fifty-eight healthy men. Creatine monohydrate produced a significantly greater improvement in mean power and body weight meanwhile both creatine monohydrate and polyethylene glycosylated form showed a significantly greater improvement for strength when compared with control group. These strength increases were similar even though the dose of creatine in the polyethylene glycosylated creatine groups was up to 75% less than that of creatine monohydrate. These results seem to indicate that the addition of polyethylene glycol could increase the absorption efficiency of creatine but further research is needed before a definitive recommendation can be reached.
Creatine in combination with other supplements
Although creatine can be bought commercially as a standalone product it is often found in combination with other nutrients. A prime example is the combination of creatine with carbohydrate or protein and carbohydrate for augmenting creatine muscle retention 350 mediated through an insulin response from the pancreas 417. Steenge et al 418 found that body creatine retention of 5 g CM was increased by 25% with the addition of 50 g of protein and 47 g of carbohydrate or 96 g carbohydrate when compared to a placebo treatment of 5 g carbohydrate. The addition of 10g of creatine to 75 g of dextrose, 2 g of taurine, vitamins and minerals, induced a change in cellular osmolarity which in addition to the expected increase in body mass, seems to produce an up regulation of large scale gene expression (mRNA content of genes and protein content of kinases involved in osmosensing and signal transduction, cytoskeleton remodelling, protein and glycogen synthesis regulation, satellite cell proliferation and differentiation, DNA replication and repair, RNA transcription control, and cell survival) 383. Similar findings have also been reported for creatine monohydrate supplementation alone when combined with resistance training 419.
A commercially available pre-workout formula comprised of 2.05 g of caffeine, taurine and glucuronolactone, 7.9 g of L-leucine, L-valine, L-arginine and L-glutamine, 5 g of di-creatine citrate and 2.5 g of β-alanine mixed with 500 ml of water taken 10 minutes prior to exercise has been shown to enhance time to exhaustion during moderate intensity endurance exercise and to increase feelings of focus, energy and reduce subjective feelings of fatigue before and during endurance exercise due to a synergistic effect of the before mentioned ingredients 420. The role of creatine in this formulation is to provide a neuroprotective function by enhancing the energy metabolism in the brain tissue, promoting antioxidant activities, improving cerebral vasculation and protecting the brain from hyperosmotic shock by acting as a brain cell osmolyte. Creatine can provide other neuroprotective benefits through stabilisation of mitochondrial membranes, stimulation of glutamate uptake into synaptic vesicles and balance of intracellular calcium homeostasis 420.
Summary
The above review indicates that creatine supplementation has positive effects on:
- Amplifying the effects of resistance training for enhancing strength and muscle mass.
- Improving the quality and benefits of high intensity intermittent speed training.
- Improving aerobic endurance performance in trials lasting more than 150s.
- Seems to produce positive effects on strength, power, fat free mass, daily living performance and neurological function in young and older people.
- Regarding predominantly aerobic endurance performance, the increased bodies’ creatine stores, seems to amplify favorable physiological adaptations such as: increased plasma volume, glycogen storage, improvements of ventilatory threshold and a possible reduction of oxygen consumption in sub maximal exercise.
A typical creatine supplementation protocol of either a loading phase of 20 to 25 g CM/d or 0.3 g CM/kg/d split into 4 to 5 daily intakes of 5 g each have been recommended to quickly saturate creatine stores in the skeletal muscle. However a more moderate protocol where several smaller doses of creatine are ingested along the day (20 intakes of 1 g every 30 min) could be a better approach to get a maximal saturation of the intramuscular creatine store. In order to keep the maximal saturation of body creatine, the loading phase must be followed by a maintenance period of 3-5 g CM/d or 0.03 g CM/kg/d. These strategies appear to be the most efficient way of saturating the muscles and benefitting from CM supplementation. However more recent research has shown CM supplementation at doses of 0.1 g/kg body weight combined with resistance training improves training adaptations at a cellular and sub-cellular level. Creatine retention by the body from supplementation appears to be promoted by about 25% from the simultaneous ingestion of carbohydrate and/or protein mediated through an increase in insulin secretion. This combination would produce a faster saturation rate but has not been shown to have a greater effect on performance.
The available evidence indicates that creatine consumption is safe. This perception of safety cannot be guaranteed especially that of the long term safety of creatine supplementation and the various forms of creatine which are administered to different populations (athletes, sedentary, patient, active, young or elderly) throughout the world.
Branched-Chain Amino Acid Supplement
It is well-established that ingestion of essential amino acids following resistance exercise stimulates an increased response of muscle protein synthesis (MPS) in humans 421, 422, 423. Indeed, the stimulation of muscle protein synthesis in humans can be achieved by supplying essential amino acids only (i.e., the non-EAAs necessary for MPS may be supplied by endogenous sources) 424. More recent evidence from studies in rodents and cell culture models suggest that the stimulation of muscle protein synthesis by essential amino acids may be mediated by a few amino acids rather than a combination of all essential amino acids 425. The branched-chain amino acid (BCAA), leucine, has been shown to play a unique role in stimulating MPS 425. Leucine serves as substrate for the synthesis of new muscle proteins and as a signal to initiate the rate-limiting translation initiation step of muscle protein synthesis 426. Accordingly, the response of MPS to leucine provision has been extensively investigated over the past two decades, both in cell culture studies and in vivo rodent and human 427 studies.
The stimulation of muscle protein synthesis is accompanied by an increased activation of intracellular signaling proteins that regulate the translational activity of muscle protein synthesis 428. In particular, the mechanistic/mammalian target of rapamycin complex-1 (mTORC1) signaling, often assessed as the phosphorylation status of the ribosomal S6 protein kinase (S6K1), is stimulated by ingestion of EAA following resistance exercise 429. There is current debate over whether leucine alone 430, or the BCAAs combined 429, provide the most important component of an exogenous EAA source for stimulating the mTORC1-S6K1 signaling pathway. Whereas, the inclusion of leucine is necessary for the maximal activation of mTORC1 signaling 431, recent results from the same research group show that mTORC1 signaling is enhanced with the addition of the other two BCAAs, valine and isoleucine 429. Moreover, there often is a disconnect between the response of mTORC1 signaling and muscle protein synthesis 432, 433. Thus, the response of MPS to BCAA ingestion is still uncertain.
Branched chain amino acids
Branched chain amino acids (BCAA’s) make up 14-18% of amino acids in skeletal muscle proteins and are quite possibly the most widely used supplements among natural bodybuilders 434. Of the BCAA’s, leucine is of particular interest because it has been shown to stimulate protein synthesis to an equal extent as a mixture of all amino acids 435. However, ingestion of leucine alone can lead to depletion of plasma valine and isoleucine; therefore, all three amino acids need to be consumed to prevent plasma depletion of any one of the BCAA’s 436. Recently, the safe upper limit of leucine was set at 550 mg/kg bodyweight/day in adult men; however, future studies are needed to determine the safe upper limit for both other populations and a mixture of all 3 BCAA’s 437.
Numerous acute studies in animals and humans have shown that consumption of either essential amino acids, BCAA’s, or leucine either at rest or following exercise increases skeletal muscle protein synthesis, decreases muscle protein degradation, or both 438, 439; however, there are few long-term studies of BCAA supplementation in resistance-trained athletes. Stoppani et al. 440 supplemented trained subjects with either 14 g BCAAs, whey protein, or a carbohydrate placebo for eight weeks during a periodized strength training routine. After training the BCAA group had a 4 kg increase in lean mass, 2% decrease in body fat percentage, and 6 kg increase in bench press 10 repetition maximum. All changes were significant compared to the other groups. However, it should be noted that this data is only available as an abstract and has yet to undergo the rigors of peer-review.
The use of BCAA’s between meals may also be beneficial to keep protein synthesis elevated. Recent data from animal models suggest that consumption of BCAA’s between meals can overcome the refractory response in protein synthesis that occurs when plasma amino acids are elevated, yet protein synthesis is reduced 441. However, long-term human studies examining the effects of a diet in which BCAA’s are consumed between meals on lean mass and strength have not been done to date. It should also be noted that BCAA metabolism in humans and rodents differ and the results from rodent studies with BCAA’s may not translate in human models 442. Therefore, long-term studies are needed in humans to determine the effectiveness of this practice.
Based on the current evidence, it is clear BCAA’s stimulate protein synthesis acutely and one study 440 has indicated that BCAA’s may be able to increase lean mass and strength when added to a strength training routine; however, additional long-term studies are needed to determine the effects of BCAA’s on lean mass and strength in trained athletes. In addition, studies are needed on the effectiveness of BCAA supplementation in individuals following a vegetarian diet in which consumption of high-quality proteins are low as this may be population that may benefit from BCAA consumption. Furthermore, the effects of BCAA ingestion between meals needs to be further investigated in a long-term human study.
Arginine
“NO supplements” containing arginine are consumed by bodybuilders pre-workout in an attempt to increase blood flow to the muscle during exercise, increase protein synthesis, and improve exercise performance. However, there is little scientific evidence to back these claims. Fahs et al. 443 supplemented healthy young men with 7 g arginine or a placebo prior to exercise and observed no significant change in blood flow following exercise. Additionally, Tang et al. 444 supplemented either 10 g arginine or a placebo prior to exercise and found no significant increase in blood flow or protein synthesis following exercise. Moreover, arginine is a non essential amino acid and prior work has established that essential amino acids alone stimulate protein synthesis 445. Based on these findings, it appears that arginine does not significantly increase blood flow or enhance protein synthesis following exercise.
The effects of arginine supplementation on performance are controversial. Approximately one-half of acute and chronic studies on arginine and exercise performance have found significant benefits with arginine supplementation, while the other one-half has found no significant benefits 446. Moreover, Greer et al. 447 found that arginine supplementation significantly reduced muscular endurance by 2–4 repetitions on chin up and push up endurance tests. Based on these results, the authors of a recent review concluded that arginine supplementation had little impact on exercise performance in healthy individuals 448. Although the effects of arginine on blood flow, protein synthesis, and exercise performance require further investigation, dosages commonly consumed by athletes are well below the observed safe level of 20 g/d and do not appear to be harmful 449.
Citrulline malate
Citrulline malate (CitM) has recently become a popular supplement among bodybuilders; however, there has been little scientific research in healthy humans with this compound. CitM is hypothesized to improve performance through three mechanisms: 1) citrulline is important part of the urea cycle and may participate in ammonia clearance, 2) malate is a tricarboxylic acid cycle intermediate that may reduce lactic acid accumulation, and 3) citrulline can be converted to arginine; however, as discussed previously, arginine does not appear to have an ergogenic effect in young healthy athletes so it is unlikely CitM exerts an perfromance enhancing effect through this mechanism 450.
Supplementation with CitM for 15 days has been shown to increase ATP production by 34% during exercise, increase the rate of phosphocreatine recovery after exercise by 20%, and reduce perceptions of fatigue [184]. Moreover, ingestion of 8 g CitM prior to a chest workout significantly increased repetitions performed by approximately 53% and decreased soreness by 40% at 24 and 48 hours post-workout 450. Furthermore, Stoppani et al. 440 in an abstract reported a 4 kg increase in lean mass, 2 kg decrease in body fat percentage, and a 6 kg increase in 10 repetition maximum bench press after consumption of a drink containing 14 g BCAA, glutamine, and CitM during workouts for eight weeks; although, it is not clear to what degree CitM contributed to the outcomes observed. However, not all studies have supported ergogenic effects of CitM. Sureda et al. 451 found no significant difference in race time when either 6 g CitM or a placebo were consumed prior to a 137 km cycling stage. Hickner et al. 452 found that treadmill time to exhaustion was significantly impaired, with the time taken to reach exhaustion occurring on average seven seconds earlier following CitM consumption.
Additionally, the long-term safety of CitM is unknown. Therefore, based on the current literature a decision on the efficacy of CitM cannot be made. Future studies are needed to conclusively determine if CitM is ergogenic and to determine its long term safety.
Glutamine
Glutamine is the most abundant non-essential amino acid in muscle and is commonly consumed as a nutritional supplement. Glutamine supplementation in quantities below 14 g/d appear to be safe in healthy adults 449; however, at present there is little scientific evidence to support the use of glutamine in healthy athletes 453. Acutely, glutamine supplementation has not been shown to significantly improve exercise performance 454, 455, improve buffering capacity 455, help to maintain immune function or reduce muscle soreness after exercise 453. Long-term supplementation studies including glutamine in cocktails along with creatine monohydrate, whey protein, BCAA’s, and/or CitM have shown 1.5 – 2 kg increases in lean mass and 6 kg increase in 10RM bench press strength 440, 456. However, the role of glutamine in these changes is unclear. Only one study 457 has investigated the effects of glutamine supplementation alone in conjunction with a six week strength training program. No significant differences in muscle size, strength, or muscle protein degradation were observed between groups. Although the previous studies do not support the use of glutamine in bodybuilders during contest preparation, it should be noted that glutamine may be beneficial for gastrointestinal health and peptide uptake in stressed populations 458; therefore, it may be beneficial in dieting bodybuilders who represent a stressed population. As a whole, the results of previous studies do not support use of glutamine as an ergogenic supplement; however, future studies are needed to determine the role of glutamine on gastrointestinal health and peptide transport in dieting bodybuilders.
Whey protein
Milk protein is mostly composed of whey protein 20% and casein 80% 459. During cheese manufacturing, whey protein is generated as a by-product of casein precipitation. Whey protein is the most popular protein supplement sold in powder format. It contains valuable food ingredients because of its nutritional value and functional bioactivity. Whey protein contains β-lactoglobulin, α-lactalbumin, immunoglobulins, bovine serum albumin, lactoferrin, lactoperoxidase, phospholipoprotein, bioactive factors, and enzymes in order of abundance 460. The biological components of whey protein and its isolates have been reported to benefit antioxidation 461 and regulation of lipid metabolism 462 and have antifatigue 463 and antidiabetic properties 464.
Whey protein isolates contain enriched essential amino acids, including branched chain amino acids, which the body needs for tissue synthesis, energy, and health. The high leucine content (50%–75% more than other protein sources), one of the branched chain amino acids, in whey protein could explain its ability to stimulate muscle protein synthesis 465 and upregulate mammalian target of rapamycin signaling in high concentration. With whey protein supplementation, resistance exercises can result in muscle adaption and hypertrophy, regardless of the contraction mode (Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Farup J, Rahbek SK, Vendelbo MH, Matzon A, Hindhede J, Bejder A, Ringgard S, Vissing K. Scand J Med Sci Sports. 2014 Oct; 24(5):788-98. https://www.ncbi.nlm.nih.gov/pubmed/23647357/()). Whey protein is marketed as a dietary supplement and as an aid for muscle development with resistance training. Because of its rapid rate of digestion, whey protein provides a rapid source of amino acids that can be taken up by the muscles to repair and rebuild muscular tissue. The use of whey protein to enhance aerobic exercises and swimming training has only been reported in terms of glycogen storage 466, antioxidation 467, and lipid metabolism. A combination of resistant exercise and WP benefitted the lipid profile, especially plasma triglycerides and cholesterol 468. Few reports have shown the beneficial synergistic effects of whey protein and long-term aerobic exercise training on biochemical profiles in specific tissues.
Resistance exercise, eccentric (muscle lengthening), isometric (non-lengthening) and concentric (shortening) contractions cause skeletal muscle damage and generate inflammatory markers (muscle proteins in blood) (The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Morton JP, Kayani AC, McArdle A, Drust B. Sports Med. 2009; 39(8):643-62. https://www.ncbi.nlm.nih.gov/pubmed/19769414/()). Anabolic interventions with protein hydrolysates and amino acid supplements have been evidenced to expedite the repair. Leucine-derived metabolite β-hydroxy-β-methylbutyrate ingestion has proved beneficial in recovery from the soreness. Resistance exercise (weight-lifting) elevates oxidation products in plasma, perturbs leukocyte redistribution and leukocyte functionality. Whey protein isolate nanoparticles were prepared using ethanol desolvation and their capacity to incorporate ZnCl2 was analysed. The amount of zinc incorporated in the particle suspensions was within the range of daily zinc requirements for healthy adults. Also, the nanoparticles remained stable after 30 days of storage at 22 °C. Cell surface glucose transporter 4 (GLUT 4) is the major glucose transporter isoform expressed in skeletal muscle that determines the rate of muscle glucose transport in the cell membrane, in response to insulin and muscle contraction. Whey protein hydrolysate was evaluated for its ability to translocate GLUT 4 and accumulate them in the membrane thereby augmenting glucose trapping by skeletal muscle. The amino acid l-isoleucine and the peptide l-leucyl-l-isoleucine in the hydrolysate contributed the most 469. The effect of whey supplementation in comparison to casein diet, on the recovery of muscle functional properties such as contractility, extensibility, elasticity and excitability was investigated in rats. The whey protein diet promoted a faster recovery from injury sustained due to isometric as well as concentric exercise in comparison to the casein diet 470. The effect of the supplementation of a beverage with varying doses of leucine or a mixture of branched chain amino acids on myofibrillar protein synthesis after resistance exercise was assessed. Results showed that low-protein (6.25 g) beverage can be as effective as a high-protein dose (25 g) at stimulating myofibrillar protein synthesis rates when supplemented with a high (5 g) leucine content. As leucine comprises 10 % of the total whey amino acid, the latter appears important for augmenting muscle hypertrophy. Health parameters, performance and body composition effects produced by 12 week intake of hydrolysed whey protein were compared in players. Intervention with the hydrolysed whey protein resulted in significant reduction in the muscle damage markers (creatine kinase and lactate dehydrogenase). Muscle mass growth by daily consumption of whey protein was compared with that of soy protein, using a randomized study on subjects undergoing resistance exercise 471. Lean body mass gains were significantly high in whey protein than soy protein group and the remarkable response was correlated with the elevated levels of leucine and faster absorption.
Concerning lean body mass, many studies reported that protein synthesis could be upregulated by the branched chain amino acids of whey protein, especially leucine 472. The combination of daily supplementation with whey protein and resistance exercise training was effective in promoting muscle hypertrophy 473.
Whey protein consumption at intake levels up to 3 g/kg per day had a no-observed-adverse-effect level 474 and the hydrolysate of whey protein at 2 g/kg as a food additive resulted in no adverse effects or mortality 475. In this study, the whey protein dose was 4.1 g/kg, which is equivalent to 20 g of whey protein per 60 kg body weight for humans did not reveal any adverse effects 476.
Based on their concentration and attributes, whey proteins are marketed in various forms such as whey protein concentrate (has fat and lactose along with proteins (29–89 %)), whey protein isolate (90 % protein) and whey protein hydrolysate (partially digested for ease of metabolism and hypo-allergenicity) 477. A broad range of functionality has been assigned to whey protein and its derivatives, such as reduction of oxidative stress, promotion of muscle growth and lean body mass, appetite suppression, hypoglycemia, cardiovascular risk mitigation, phenylketonuria management and protection from ultraviolet (UV) radiation 478. Further, its role in food processing such as emulsifier, texturizer, fat-replacer, encapsulating agent, delivery vehicle and antimicrobial film are being recognized 479.
Are bodybuilding supplements safe?
Dangerous hidden ingredients are an increasing problem in products promoted for bodybuilding, the U.S. Food and Drug Administration (FDA) warns 480, 481, 482. Consumers may unknowingly take products laced with prescription drug ingredients, controlled substances, and other ingredients.
- Bodybuilding supplements often are adulterated with anabolic steroids that are modified variants of male hormones designed to increase muscle mass.
- Liver injury from taking bodybuilding dietary supplements has increased in recent years. Bodybuilding products are the most common cause of liver injury linked to herbal and dietary supplement use.
- Products containing the stimulants beta-methylphenethylamine (BMPEA) or 1,3-dimethylamylamine (DMAA) an an amphetamine derivative can cause serious health problems.
- Supplements labeled as containing the herb Acacia rigidula often contain beta-methylphenethylamine (BMPEA), although BMPEA isn’t in the herb and isn’t a dietary ingredient.
- 1,3-dimethylamylamine (DMAA) containing products marketed as dietary supplements are illegal. In 2013, the FDA began taking action to remove these products from the market 482. However, DMAA is still found in some products marketed as supplements, including under different names, such as geranium oil.
- Some dietary supplements may interact with drugs or other supplements. Some vitamins and minerals are harmful at high doses. Talk with your health care provider before using a dietary supplement for bodybuilding or endurance.
What are Anabolic Steroids?
Anabolic steroids are synthetic substances related to the male sex hormone testosterone. The proper term for these compounds is anabolic-androgenic steroids. “Anabolic” refers to muscle building, and “androgenic” refers to increased male sex characteristics. Some common names for anabolic steroids are Gear, Juice, Roids, and Stackers. They promote the growth of skeletal muscle (anabolic effects) and the development of male sexual characteristics (androgenic effects) in both males and females 483. The term “anabolic steroids” will be used throughout this report because of its familiarity, although the proper term for these compounds is “anabolic-androgenic steroids.”
Anabolic steroids were developed in the late 1930s primarily to treat hypogonadism, a condition in which the testes do not produce sufficient testosterone for normal growth, development, and sexual functioning. The primary medical uses of these compounds are to treat delayed puberty, some types of impotence, and wasting of the body caused by HIV infection or other diseases.
During the 1930s, scientists discovered that anabolic steroids could facilitate the growth of skeletal muscle in laboratory animals, which led to abuse of the compounds first by bodybuilders and weightlifters and then by athletes in other sports. Steroid abuse has become so widespread in athletics that it can affect the outcome of sports contests.
Illicit steroids are often sold at gyms, competitions, and through mail order operations after being smuggled into this country. Most illegal steroids in the United States are smuggled from countries that do not require a prescription for the purchase of steroids. Steroids are also illegally diverted from U.S. pharmacies or synthesized in clandestine laboratories.
What are steroidal supplements?
In the United States, supplements such as tetrahydrogestrinone (THG) and androstenedione (street name “Andro”) previously could be purchased legally without a prescription through many commercial sources, including health food stores 484. Steroidal supplements can be converted into testosterone or a similar compound in the body. Less is known about the side effects of steroidal supplements, but if large quantities of these compounds substantially increase testosterone levels in the body, then they also are likely to produce the same side effects as anabolic steroids themselves. The purchase of these supplements, with the notable exception of dehydroepiandrosterone (DHEA), became illegal after the passage in 2004 of amendments to the Controlled Substances Act.
Commonly Abused Steroids
Oral Steroids
- Anadrol (oxymetholone)
- Oxandrin (oxandrolone)
- Dianabol (methandrostenolone)
- Winstrol (stanozolol)
Injectable Steroids
- Deca-Durabolin (nandrolone decanoate)
- Durabolin (nandrolone phenpropionate)
- Depo-Testosterone (testosterone cypionate)
- Equipoise (boldenone undecylenate)
- Tetrahydrogestrinone (THG).
How are anabolic steroids abused?
Some anabolic steroids are taken orally, others are injected intramuscularly and still others are provided in gels or creams that are applied to the skin 485. Doses taken by abusers can be 10 to 100 times higher than the doses used for medical conditions 485.
Cycling, stacking, and pyramiding
Steroids are often abused in patterns called “cycling,” which involve taking multiple doses of steroids over a specific period of time, stopping for a period, and starting again 485. Users also frequently combine several different types of steroids in a process known as “stacking.” Steroid abusers typically “stack” the drugs, meaning that they take two or more different anabolic steroids, mixing oral and/or injectable types, and sometimes even including compounds that are designed for veterinary use 485. Abusers think that the different steroids interact to produce an effect on muscle size that is greater than the effects of each drug individually, a theory that has not been tested scientifically 485.
Another mode of steroid abuse is referred to as “pyramiding.” This is a process in which users slowly escalate steroid abuse (increasing the number of steroids or the dose and frequency of one or more steroids used at one time), reaching a peak amount at mid-cycle and gradually tapering the dose toward the end of the cycle 485. Often, steroid abusers pyramid their doses in cycles of 6 to 12 weeks. At the beginning of a cycle, the person starts with low doses of the drugs being stacked and then slowly increases the doses. In the second half of the cycle, the doses are slowly decreased to zero. This is sometimes followed by a second cycle in which the person continues to train but without drugs. Abusers believe that pyramiding allows the body time to adjust to the high doses, and the drug-free cycle allows the body’s hormonal system time to recuperate. As with stacking, the perceived benefits of pyramiding and cycling have not been substantiated scientifically 485.
Why do people abuse anabolic steroids?
One of the main reasons people give for abusing steroids is to improve their athletic performance 486. Among athletes, steroid abuse has been estimated to be less that 6 percent according to surveys, but anecdotal information suggests more widespread abuse. Although testing procedures are now in place to deter steroid abuse among professional and Olympic athletes, new designer drugs constantly become available that can escape detection and put athletes willing to cheat one step ahead of testing efforts. This dynamic, however, may be about to shift if the saving of urine and blood samples for retesting at a future date becomes the standard. The high probability of eventual detection of the newer designer steroids, once the technology becomes available, plus the fear of retroactive sanctions, should give athletes pause.
Another reason people give for taking steroids is to increase their muscle size or to reduce their body fat 486. This group includes people suffering from the behavioral syndrome called muscle dysmorphia, which causes them to have a distorted image of their bodies. Men with muscle dysmorphia think that they look small and weak, even if they are large and muscular. Similarly, women with this condition think that they look fat and flabby, even though they are actually lean and muscular.
Some people who abuse steroids to boost muscle size have experienced physical or sexual abuse 486. In one series of interviews with male weightlifters, 25 percent who abused steroids reported memories of childhood physical or sexual abuse. Similarly, female weightlifters who had been raped were found to be twice as likely to report use of anabolic steroids or another purported musclebuilding drug, compared with those who had not been raped. Moreover, almost all of those who had been raped reported that they markedly increased their bodybuilding activities after the attack. They believed that being bigger and stronger would discourage further attacks because men would find them either intimidating or unattractive.
Finally, some adolescents abuse steroids as part of a pattern of high-risk behaviors 486. These adolescents also take risks such as drinking and driving, carrying a gun, driving a motorcycle without a helmet, and abusing other illicit drugs. Conditions such as muscle dysmorphia, a history of physical or sexual abuse, or a history of engaging in high-risk behaviors have all been associated with an increased risk of initiating or continuing steroid abuse.
Are anabolic steroids addictive?
An undetermined percentage of steroid abusers may become addicted to the drugs, as evidenced by their continued abuse despite physical problems and negative effects on social relations. Also, steroid abusers typically spend large amounts of time and money obtaining the drugs, which is another indication that they may be addicted. Individuals who abuse steroids can experience withdrawal symptoms when they stop taking steroids, such as mood swings, fatigue, restlessness, loss of appetite, insomnia, reduced sex drive, and steroid cravings. The most dangerous of the withdrawal symptoms is depression, because it sometimes leads to suicide attempts. If left untreated, some depressive symptoms associated with anabolic steroid withdrawal have been known to persist for a year or more after the abuser stops taking the drugs.
People who abuse steroids may experience withdrawal symptoms when they stop use, including:
- mood swings
- fatigue
- restlessness
- loss of appetite
- sleep problems
- decreased sex drive
- steroid cravings.
One of the more serious withdrawal symptoms is depression, which can sometimes lead to suicide attempts.
What are the health consequences of steroid abuse?
Anabolic steroid abuse has been associated with a wide range of adverse side effects ranging from some that are physically unattractive, such as acne and breast development in men, to others that are life threatening, such as heart attacks and liver cancer. Most are reversible if the abuser stops taking the drugs, but some are permanent, such as voice deepening in females.
Most data on the long-term effects of anabolic steroids in humans come from case reports rather than formal epidemiological studies. From the case reports, the incidence of lifethreatening effects appears to be low, but serious adverse effects may be underrecognized or underreported, especially since they may occur many years later. Data from animal studies seem to support this possibility. One study found that exposing male mice for one-fifth of their lifespan to steroid doses comparable to those taken by human athletes caused a high frequency of early deaths.
Anabolic steroids affect the brain
Anabolic steroids work differently from other drugs of abuse; they do not have the same short-term effects on the brain. The most important difference is that steroids do not trigger rapid increases in the brain chemical dopamine, which causes the “high” that drives people to abuse other substances. However, long-term steroid abuse can act on some of the same brain pathways and chemicals—including dopamine, serotonin, and opioid systems—that are affected by other drugs. This may result in a significant effect on mood and behavior.
Short-Term Effects
Abuse of anabolic steroids may lead to mental problems, such as:
- paranoid (extreme, unreasonable) jealousy
- extreme irritability
- delusions—false beliefs or ideas
- impaired judgment
Extreme mood swings can also occur, including “roid rage”—angry feelings and behavior that may lead to violence.
Aside from mental problems, steroid use commonly causes severe acne. It also causes the body to swell, especially in the hands and feet.
Long-Term Effects
Anabolic steroid abuse may lead to serious, even permanent, health problems such as:
- kidney problems or failure
- liver damage
- enlarged heart, high blood pressure, and changes in blood cholesterol, all of which increase the risk of stroke and heart attack, even in young people
Several other effects are gender- and age-specific:
In men:
- shrinking testicles
- decreased sperm count
- baldness
- development of breasts
- increased risk for prostate cancer.
In women:
- growth of facial hair or excess body hair
- male-pattern baldness
- changes in or stop in the menstrual cycle
- enlarged clitoris
- deepened voice.
In teens:
- stunted growth (when high hormone levels from steroids signal to the body to stop bone growth too early)
- stunted height (if teens use steroids before their growth spurt).
Some of these physical changes, such as shrinking sex organs in men, can add to mental side effects such as mood disorders.
Hormonal system
Steroid abuse disrupts the normal production of hormones in the body, causing both reversible and irreversible changes. Changes that can be reversed include reduced sperm production and shrinking of the testicles (testicular atrophy). Irreversible changes include male-pattern baldness and breast development (gynecomastia) in men. In one study of male bodybuilders, more than half had testicular atrophy and/or gynecomastia.
In the female body, anabolic steroids cause masculinization. Breast size and body fat decrease, the skin becomes coarse, the clitoris enlarges, and the voice deepens. Women may experience excessive growth of body hair but lose scalp hair. With continued administration of steroids, some of these effects become irreversible.
Musculoskeletal system
Rising levels of testosterone and other sex hormones normally trigger the growth spurt that occurs during puberty and adolescence and provide the signals to stop growth as well. When a child or adolescent takes anabolic steroids, the resulting artificially high sex hormone levels can prematurely signal the bones to stop growing.
Cardiovascular system
Steroid abuse has been associated with cardiovascular diseases (CVD), including heart attacks and strokes, even in athletes younger than 30. Steroids contribute to the development of CVD, partly by changing the levels of lipoproteins that carry cholesterol in the blood. Steroids, particularly oral steroids, increase the level of low-density lipoprotein (LDL) and decrease the level of high-density lipoprotein (HDL). High LDL and low HDL levels increase the risk of atherosclerosis, a condition in which fatty substances are deposited inside arteries and disrupt blood flow. If blood is prevented from reaching the heart, the result can be a heart attack. If blood is prevented from reaching the brain, the result can be a stroke.
Steroids also increase the risk that blood clots will form in blood vessels, potentially disrupting blood flow and damaging the heart muscle so that it does not pump blood effectively.
Liver
Steroid abuse has been associated with liver tumors and a rare condition called peliosis hepatis, in which blood-filled cysts form in the liver. Both the tumors and the cysts can rupture, causing internal bleeding.
Skin
Steroid abuse can cause acne, cysts, and oily hair and skin.
Infections
Many abusers who inject anabolic steroids may use nonsterile injection techniques or share contaminated needles with other abusers. In addition, some steroid preparations are manufactured illegally under nonsterile conditions. These factors put abusers at risk for acquiring lifethreatening viral infections, such as HIV and hepatitis B and C. Abusers also can develop endocarditis, a bacterial infection that causes a potentially fatal inflammation of the inner lining of the heart. Bacterial infections also can cause pain and abscess formation at injection sites.
What effects do anabolic steroids have on behavior ?
Case reports and small studies indicate that anabolic steroids, when used in high doses, increase irritability and aggression. Some steroid abusers report that they have committed aggressive acts, such as physical fighting or armed robbery, theft, vandalism, or burglary. Abusers who have committed aggressive acts or property crimes generally report that they engage in these behaviors more often when they take steroids than when they are drug free. A recent study suggests that the mood and behavioral effects seen during anabolic-androgenic steroid abuse may result from secondary hormonal changes.
Scientists have attempted to test the association between anabolic steroids and aggression by administering high steroid doses or placebo for days or weeks to human volunteers and then asking the people to report on their behavioral symptoms. To date, four such studies have been conducted. In three, high steroid doses did produce greater feelings of irritability and aggression than did placebo, although the effects appear to be highly variable across individuals. In one study, the drugs did not have that effect. One possible explanation, according to the researchers, is that some but not all anabolic steroids increase irritability and aggression. Recent animal studies show an increase in aggression after steroid administration.
In a few controlled studies, aggression or adverse, overt behaviors resulting from the administration of anabolic steroid use have been reported by a minority of volunteers.
In summary, the extent to which steroid abuse contributes to violence and behavioral disorders is unknown. As with the health complications of steroid abuse, the prevalence of extreme cases of violence and behavioral disorders seems to be low, but it may be underreported or underrecognized.
Research also indicates that some users might turn to other drugs to alleviate some of the negative effects of anabolic steroids. For example, a study of 227 men admitted in 1999 to a private treatment center for addiction to heroin or other opioids found that 9.3 percent had abused anabolic steroids before trying any other illicit drug. Of these 9.3 percent, 86 percent first used opioids to counteract insomnia and irritability resulting from anabolic steroids.
What treatments are effective for anabolic steroid abuse?
Few studies of treatments for anabolic steroid abuse have been conducted. Current knowledge is based largely on the experiences of a small number of physicians who have worked with patients undergoing steroid withdrawal. The physicians have found that supportive therapy is sufficient in some cases. Patients are educated about what they may experience during withdrawal and are evaluated for suicidal thoughts. If symptoms are severe or prolonged, medications or hospitalization may be needed.
Some medications that have been used for treating steroid withdrawal restore the hormonal system after its disruption by steroid abuse. Other medications target specific withdrawal symptoms—for example, antidepressants to treat depression and analgesics for headaches and muscle and joint pains.
Some patients require assistance beyond pharmacological treatment of withdrawal symptoms and are treated with behavioral therapies.
Summary
Abuse of anabolic steroids differs from the abuse of other illicit substances because the initial abuse of anabolic steroids is not driven by the immediate euphoria that accompanies most drugs of abuse, such as cocaine, heroin, and marijuana, but by the desire of abusers to change their appearance and performance, characteristics of great importance to adolescents. The effects of steroids can boost confidence and strength, leading abusers to overlook the potential serious and long-term damage that these substances can cause.
While anabolic steroids can enhance certain types of performance or appearance, they are dangerous drugs, and when used inappropriately they can cause a host of severe, long-lasting, and in some cases, irreversible negative health consequences. Anabolic steroids can lead to early heart attacks, strokes, liver tumors, kidney failure, and serious psychiatric problems. In addition, because steroids are often injected, users who share needles or use nonsterile techniques when they inject steroids are at risk for contracting dangerous infections, such as HIV/AIDS and hepatitis B and C.
Possible Health Consequences of Anabolic Steroid Abuse
Hormonal system
Men
- infertility
- breast development
- shrinking of the testicles
- male-pattern baldness
Women
- enlargement of the clitoris
- excessive growth of body hair
- male-pattern baldness
Musculoskeletal system
- short stature (if taken by adolescents)
- tendon rupture
Cardiovascular system
- increases in LDL (bad) cholesterol;
- decreases in HDL (good) cholesterol;
- high blood pressure
- heart attacks
- enlargement of the heart’s left ventricle
Liver
- cancer
- peliosis hepatis (blood-filled cysts form in the liver)
- tumors
- Both the tumors and the cysts can rupture, causing internal bleeding.
Skin
- severe acne and cysts
- oily scalp
- jaundice
- fluid retention
Infection
- HIV/AIDS
- hepatitis B and C.
Psychiatric effects
- rage, aggression
- mania
- delusions
- Gundersen K. Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biological Reviews of the Cambridge Philosophical Society. 2011;86(3):564-600. doi:10.1111/j.1469-185X.2010.00161.x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3170710/[↩][↩][↩]
- Bintliff S, Walker BE. Radioautographic study of skeletal muscle regeneration. American Journal of Anatomy. 1960;106:233–245.[↩]
- Nature of dividing nuclei in skeletal muscle of growing rats. Moss FP, Leblond CP. J Cell Biol. 1970 Feb; 44(2):459-62. https://www.ncbi.nlm.nih.gov/pubmed/5411085/[↩]
- Morphological changes during fiber type transitions in low-frequency-stimulated rat fast-twitch muscle. Delp MD, Pette D. Cell Tissue Res. 1994 Aug; 277(2):363-71. https://www.ncbi.nlm.nih.gov/pubmed/7521794/[↩]
- Fast to slow transformation of denervated and electrically stimulated rat muscle. Windisch A, Gundersen K, Szabolcs MJ, Gruber H, Lømo T. J Physiol. 1998 Jul 15; 510 ( Pt 2)():623-32. https://www.ncbi.nlm.nih.gov/pubmed/9706009/[↩]
- Ferraro E, Giammarioli AM, Chiandotto S, Spoletini I, Rosano G. Exercise-Induced Skeletal Muscle Remodeling and Metabolic Adaptation: Redox Signaling and Role of Autophagy. Antioxidants & Redox Signaling. 2014;21(1):154-176. doi:10.1089/ars.2013.5773. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4048572/[↩][↩]
- Tihanyi J., Apor P., Fekete G. Force-Velocity-Power Characteristics and Fiber Composition in Human Knee Extensor Muscles. Eur. J. Appl. Physiol. Occup. Physiol. 1982;48:331–343. doi: 10.1007/BF00430223[↩]
- Schiaffino S. Fibre types in skeletal muscle: a personal account. Acta Physiol (Oxf) 199: 451–463, 2010. https://www.ncbi.nlm.nih.gov/pubmed/20353491[↩][↩]
- Lievens E, Klass M, Bex T, Derave W. Muscle fiber typology substantially influences time to recover from high-intensity exercise. J Appl Physiol (1985). 2020 Mar 1;128(3):648-659. doi: 10.1152/japplphysiol.00636.2019[↩]
- Miljkovic N, Lim JY, Miljkovic I, Frontera WR. Aging of skeletal muscle fibers. Ann Rehabil Med. 2015 Apr;39(2):155-62. doi: 10.5535/arm.2015.39.2.155[↩]
- Fibre types in skeletal muscle: a personal account. Schiaffino S. Acta Physiol (Oxf). 2010 Aug; 199(4):451-63. https://www.ncbi.nlm.nih.gov/pubmed/20353491/[↩]
- Burniston JG, Hoffman EP. Proteomic responses of skeletal and cardiac muscle to exercise. Expert review of proteomics. 2011;8(3):361-377. doi:10.1586/epr.11.17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4948674/[↩]
- Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Schiaffino S, Reggiani C. Physiol Rev. 1996 Apr; 76(2):371-423. https://www.ncbi.nlm.nih.gov/pubmed/8618961/[↩][↩]
- Barany M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol 50Suppl: 197–218, 1967. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2225740/pdf/197.pdf[↩]
- Schiaffino S. and Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76: 371–423, 1996. https://www.ncbi.nlm.nih.gov/pubmed/8618961[↩]
- Evolutionary implications of three novel members of the human sarcomeric myosin heavy chain gene family. Desjardins PR, Burkman JM, Shrager JB, Allmond LA, Stedman HH. Mol Biol Evol. 2002 Apr; 19(4):375-93. https://www.ncbi.nlm.nih.gov/pubmed/11919279/[↩]
- Hybrid skeletal muscle fibres: a rare or common phenomenon ? Stephenson GM. Clin Exp Pharmacol Physiol. 2001 Aug; 28(8):692-702. https://www.ncbi.nlm.nih.gov/pubmed/11473538/[↩]
- Fast-Twitch Vs. Slow-Twitch Muscle Fiber Types + Training Tips. https://blog.nasm.org/fitness/understanding-fast-twitch-vs-slow-twitch-mucle-fibers[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ferraro E, Giammarioli AM, Chiandotto S, Spoletini I, Rosano G. Exercise-Induced Skeletal Muscle Remodeling and Metabolic Adaptation: Redox Signaling and Role of Autophagy. Antioxidants & Redox Signaling. 2014;21(1):154-176. doi:10.1089/ars.2013.5773. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4048572[↩][↩]
- Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story? Bottinelli R. Pflugers Arch. 2001 Oct; 443(1):6-17. https://www.ncbi.nlm.nih.gov/pubmed/11692261/[↩]
- Plotkin DL, Roberts MD, Haun CT, Schoenfeld BJ. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports (Basel). 2021 Sep 10;9(9):127. doi: 10.3390/sports9090127[↩][↩][↩]
- Tesch P.A., Karlsson J. Muscle Fiber Types and Size in Trained and Untrained Muscles of Elite Athletes. J. Appl. Physiol. (1985) 1985;59:1716–1720. doi: 10.1152/jappl.1985.59.6.1716[↩]
- Serrano N., Colenso-Semple L.M., Lazauskus K.K., Siu J.W., Bagley J.R., Lockie R.G., Costa P.B., Galpin A.J. Extraordinary Fast-Twitch Fiber Abundance in Elite Weightlifters. PLoS ONE. 2019;14:e0207975. doi: 10.1371/journal.pone.0207975[↩]
- Trappe S., Luden N., Minchev K., Raue U., Jemiolo B., Trappe T.A. Skeletal Muscle Signature of a Champion Sprint Runner. J. Appl. Physiol. (1985) 2015;118:1460–1466. doi: 10.1152/japplphysiol.00037.2015[↩]
- Smerdu V., Karsch-Mizrachi I., Campione M., Leinwand L., Schiaffino S. Type IIx Myosin Heavy Chain Transcripts Are Expressed in Type IIb Fibers of Human Skeletal Muscle. Am. J. Physiol. 1994;267:C1723–C1728. doi: 10.1152/ajpcell.1994.267.6.C1723[↩]
- Powers SK, and Howley ET. (2012). Exercise Physiology: Theory and Application to Fitness and Performance, (8th Edition). New York, NY: McGraw Hill.[↩]
- Vanhatalo A, Poole DC, DiMenna FJ, Bailey SJ, Jones AM. Muscle fiber recruitment and the slow component of O2 uptake: constant work rate vs. all-out sprint exercise. Am J Physiol Regul Integr Comp Physiol. 2011 Mar;300(3):R700-7. doi: 10.1152/ajpregu.00761.2010[↩]
- Trappe S, Harber M, Creer A, Gallagher P, Slivka D, Minchev K, Whitsett D. Single muscle fiber adaptations with marathon training. J Appl Physiol (1985). 2006 Sep;101(3):721-7. doi: 10.1152/japplphysiol.01595.2005[↩][↩][↩]
- Electrical stimulation resembling normal motor-unit activity: effects on denervated fast and slow rat muscles. Eken T, Gundersen K. J Physiol. 1988 Aug; 402():651-69. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1191914/[↩]
- Henriksson J, Hickner J. Training-induced adaptions in skeletal muscle. In: Harries M, editor. Oxford textbook of sports medicine. Oxford: Oxford University Press; 1994.[↩]
- Saltin B, Gollnick PD. Skeletal muscle adaptability: significance for metabolism and performance. In: Peachey LD, Adrian RH, Geiger SR, editors. Handbook of Physiology. Vol. 10. Bethesda: American Physiological Society; 1983. pp. 555–631[↩]
- Muscle mechanics: adaptations with exercise-training. Fitts RH, Widrick JJ. Exerc Sport Sci Rev. 1996; 24():427-73. https://www.ncbi.nlm.nih.gov/pubmed/8744258/[↩]
- Aerobic capacity and skeletal muscle properties of normoxic and hypoxic rats in response to training. Abdelmalki A, Fimbel S, Mayet-Sornay MH, Sempore B, Favier R. Pflugers Arch. 1996 Mar; 431(5):671-9.[↩]
- Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. Kraemer WJ, Patton JF, Gordon SE, Harman EA, Deschenes MR, Reynolds K, Newton RU, Triplett NT, Dziados JE. J Appl Physiol (1985). 1995 Mar; 78(3):976-89. https://www.ncbi.nlm.nih.gov/pubmed/7775344/[↩]
- Myosin heavy chain isoforms in single fibres from m. vastus lateralis of sprinters: influence of training. Andersen JL, Klitgaard H, Saltin B. Acta Physiol Scand. 1994 Jun; 151(2):135-42. https://www.ncbi.nlm.nih.gov/pubmed/7942047/[↩]
- Myosin isoforms, muscle fiber types, and transitions. Pette D, Staron RS. Microsc Res Tech. 2000 Sep 15; 50(6):500-9. https://www.ncbi.nlm.nih.gov/pubmed/10998639/[↩]
- Novel transitions in MHC isoforms: separate and combined effects of thyroid hormone and mechanical unloading. Caiozzo VJ, Baker MJ, Baldwin KM. J Appl Physiol (1985). 1998 Dec; 85(6):2237-48. https://www.ncbi.nlm.nih.gov/pubmed/9843548/[↩]
- Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Kadi F. Br J Pharmacol. 2008 Jun; 154(3):522-8. https://www.ncbi.nlm.nih.gov/pubmed/18414389/[↩]
- Transitions of muscle fiber phenotypic profiles. Pette D, Staron RS. Histochem Cell Biol. 2001 May; 115(5):359-72. https://www.ncbi.nlm.nih.gov/pubmed/11449884/[↩]
- Fiber type composition of the vastus lateralis muscle of young men and women. Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K. J Histochem Cytochem. 2000 May; 48(5):623-9. https://www.ncbi.nlm.nih.gov/pubmed/10769046/[↩]
- “Fast” and “slow” muscle fibres in hindlimb muscles of adult rats regenerate from intrinsically different satellite cells. Kalhovde JM, Jerkovic R, Sefland I, Cordonnier C, Calabria E, Schiaffino S, Lømo T. J Physiol. 2005 Feb 1; 562(Pt 3):847-57. https://www.ncbi.nlm.nih.gov/pubmed/15564285/[↩]
- Muscle fiber pattern is independent of cell lineage in postnatal rodent development. Hughes SM, Blau HM. Cell. 1992 Feb 21; 68(4):659-71. https://www.ncbi.nlm.nih.gov/pubmed/1531450/[↩]
- Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. McPherron AC, Lawler AM, Lee SJ. Nature. 1997 May 1; 387(6628):83-90. https://www.ncbi.nlm.nih.gov/pubmed/9139826/[↩]
- Double muscling in cattle due to mutations in the myostatin gene. McPherron AC, Lee SJ. Proc Natl Acad Sci U S A. 1997 Nov 11; 94(23):12457-61. https://www.ncbi.nlm.nih.gov/pubmed/9356471/[↩]
- Myostatin mutation associated with gross muscle hypertrophy in a child. Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, Braun T, Tobin JF, Lee SJ. N Engl J Med. 2004 Jun 24; 350(26):2682-8. https://www.ncbi.nlm.nih.gov/pubmed/15215484/[↩]
- Lack of myostatin results in excessive muscle growth but impaired force generation. Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbóva G, Partridge T, Zammit P, Bunger L, Patel K. Proc Natl Acad Sci U S A. 2007 Feb 6; 104(6):1835-40. https://www.ncbi.nlm.nih.gov/pubmed/17267614/[↩]
- Muscle hypertrophy induced by the Ski protein: cyto-architecture and ultrastructure. Bruusgaard JC, Brack AS, Hughes SM, Gundersen K. Acta Physiol Scand. 2005 Oct; 185(2):141-9. https://www.ncbi.nlm.nih.gov/pubmed/16168008/[↩]
- Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. J Appl Physiol (1985). 2007 Feb; 102(2):573-81. https://www.ncbi.nlm.nih.gov/pubmed/17038487/[↩]
- Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight. Lalani R, Bhasin S, Byhower F, Tarnuzzer R, Grant M, Shen R, Asa S, Ezzat S, Gonzalez-Cadavid NF. J Endocrinol. 2000 Dec; 167(3):417-28. https://www.ncbi.nlm.nih.gov/pubmed/11115768/[↩]
- Functional improvement of dystrophic muscle by myostatin blockade. Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Nature. 2002 Nov 28; 420(6914):418-21. https://www.ncbi.nlm.nih.gov/pubmed/12459784/[↩]
- Ectopic expression of myostatin induces atrophy of adult skeletal muscle by decreasing muscle gene expression. Durieux AC, Amirouche A, Banzet S, Koulmann N, Bonnefoy R, Pasdeloup M, Mouret C, Bigard X, Peinnequin A, Freyssenet D. Endocrinology. 2007 Jul; 148(7):3140-7. https://www.ncbi.nlm.nih.gov/pubmed/17395701/[↩]
- Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Gilson H, Schakman O, Combaret L, Lause P, Grobet L, Attaix D, Ketelslegers JM, Thissen JP. Endocrinology. 2007 Jan; 148(1):452-60. https://www.ncbi.nlm.nih.gov/pubmed/17038559/[↩]
- Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Sjögren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Törnell J, Isaksson OG, Jansson JO, Ohlsson C. Proc Natl Acad Sci U S A. 1999 Jun 8; 96(12):7088-92. https://www.ncbi.nlm.nih.gov/pubmed/10359843/[↩]
- Short-term growth hormone treatment does not increase muscle protein synthesis in experienced weight lifters. Yarasheski KE, Zachweija JJ, Angelopoulos TJ, Bier DM. J Appl Physiol (1985). 1993 Jun; 74(6):3073-6. https://www.ncbi.nlm.nih.gov/pubmed/8366011/[↩]
- PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Lira VA, Benton CR, Yan Z, Bonen A. Am J Physiol Endocrinol Metab. 2010 Aug; 299(2):E145-61. https://www.ncbi.nlm.nih.gov/pubmed/20371735/[↩]
- Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJ, Grant SM. Am J Physiol. 1996 Feb; 270(2 Pt 1):E265-72. https://www.ncbi.nlm.nih.gov/pubmed/8779948/[↩]
- Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Egan B, Zierath JR. Cell Metab. 2013 Feb 5; 17(2):162-84. https://www.ncbi.nlm.nih.gov/pubmed/23395166/[↩]
- Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. Nader GA, Esser KA. J Appl Physiol (1985). 2001 May; 90(5):1936-42. http://jap.physiology.org/content/90/5/1936.long[↩]
- Mikines KJ, Sonne B, Farrell PA, Tronier B, and Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol 254: E248–E259, 1988. https://www.ncbi.nlm.nih.gov/pubmed/3126668[↩]
- Gundersen K. Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev Camb Philos Soc 86: 564–600, 2011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3170710/[↩]
- Borst SE. Interventions for sarcopenia and muscle weakness in older people. Age Ageing 33: 548–555, 2004. https://www.ncbi.nlm.nih.gov/pubmed/15385272[↩]
- Fry AC. Sports Med. 2004;34(10):663-79. The role of resistance exercise intensity on muscle fibre adaptations. https://www.ncbi.nlm.nih.gov/pubmed/15335243[↩][↩]
- PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Glass DJ. Curr Top Microbiol Immunol. 2010; 346():267-78. https://www.ncbi.nlm.nih.gov/pubmed/20593312/[↩]
- Autophagy: renovation of cells and tissues. Mizushima N, Komatsu M. Cell. 2011 Nov 11; 147(4):728-41. https://www.ncbi.nlm.nih.gov/pubmed/22078875/[↩]
- Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Kuma A, Mizushima N. Semin Cell Dev Biol. 2010 Sep; 21(7):683-90. https://www.ncbi.nlm.nih.gov/pubmed/20223289/[↩]
- Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Sandri M. Int J Biochem Cell Biol. 2013 Oct; 45(10):2121-9. https://www.ncbi.nlm.nih.gov/pubmed/23665154/[↩][↩][↩]
- FoxO3 controls autophagy in skeletal muscle in vivo. Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. Cell Metab. 2007 Dec; 6(6):458-71. https://www.ncbi.nlm.nih.gov/pubmed/18054315/[↩]
- Cellular and molecular mechanisms of muscle atrophy. Bonaldo P, Sandri M. Dis Model Mech. 2013 Jan; 6(1):25-39. https://www.ncbi.nlm.nih.gov/pubmed/23268536/[↩]
- Grumati P, Coletto L, Schiavinato A, Castagnaro S, Bertaggia E, Sandri M, and Bonaldo P. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy 7: 1415–1423, 2011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3288016/[↩]
- Resistance Training Recommendations to Maximize Muscle Hypertrophy in an Athletic Population: Position Stand of the IUSCA. https://journal.iusca.org/index.php/Journal/article/view/81/140[↩]
- Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev. 1991;71(2):541–585. doi: 10.1152/physrev.1991.71.2.541[↩]
- Ratamess NA, Alvar BA, Evetoch TK, Housh TJ, Kibler WB, Kraemer WJ. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670[↩]
- Refalo MC, Helms ER, Robinson ZP, Hamilton DL, Fyfe JJ. Similar muscle hypertrophy following eight weeks of resistance training to momentary muscular failure or with repetitions-in-reserve in resistance-trained individuals. J Sports Sci. 2024 Jan;42(1):85-101. https://www.tandfonline.com/doi/full/10.1080/02640414.2024.2321021[↩]
- Hickmott LM, Chilibeck PD, Shaw KA, Butcher SJ. The Effect of Load and Volume Autoregulation on Muscular Strength and Hypertrophy: A Systematic Review and Meta-Analysis. Sports Med Open. 2022 Jan 15;8(1):9. doi: 10.1186/s40798-021-00404-9[↩]
- Refalo MC, Helms ER, Trexler ET, Hamilton DL, Fyfe JJ. Influence of Resistance Training Proximity-to-Failure on Skeletal Muscle Hypertrophy: A Systematic Review with Meta-analysis. Sports Med. 2023 Mar;53(3):649-665. doi: 10.1007/s40279-022-01784-y[↩]
- Wackerhage H, Schoenfeld BJ, Hamilton DL, Lehti M, Hulmi JJ. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J Appl Physiol (1985). 2019 Jan 1;126(1):30-43. doi: 10.1152/japplphysiol.00685.2018[↩]
- Haun CT, Vann CG, Roberts BM, Vigotsky AD, Schoenfeld BJ, Roberts MD. A Critical Evaluation of the Biological Construct Skeletal Muscle Hypertrophy: Size Matters but So Does the Measurement. Front Physiol. 2019 Mar 12;10:247. doi: 10.3389/fphys.2019.00247[↩]
- Brechue WF, Abe T. The role of FFM accumulation and skeletal muscle architecture in powerlifting performance. Eur J Appl Physiol. (2002) 86:327–36. doi: 10.1007/s00421-001-0543-7[↩]
- Siahkouhian M, Hedayatneja M. Correlations of anthropometric and body composition variables with the performance of young elite weightlifters. J Hum Kinet. (2010) 25:125–31. doi: 10.2478/v10078-010-0040-3[↩]
- Alway SE, MacDougall JD, Sale DG, Sutton JR, McComas AJ. Functional and structural adaptations in skeletal muscle of trained athletes. J Appl Physiol. (1988) 64:1114–20. doi: 10.1152/jappl.1988.64.3.1114[↩]
- Mitchell L, Hackett D, Gifford J, Estermann F, O’Connor H. Do Bodybuilders Use Evidence-Based Nutrition Strategies to Manipulate Physique? Sports (Basel). 2017 Sep 29;5(4):76. doi: 10.3390/sports5040076[↩][↩]
- Hackett D.A., Johnson N.A., Chow C.M. Training practices and ergogenic aids used by male bodybuilders. J. Strength Cond. Res. 2013;27:1609–1617. doi: 10.1519/JSC.0b013e318271272a[↩]
- Sartori, R., Romanello, V. & Sandri, M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun 12, 330 (2021). https://doi.org/10.1038/s41467-020-20123-1[↩]
- Schoenfeld, Brad J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. Journal of Strength and Conditioning Research 24(10):p 2857-2872, October 2010. | DOI: 10.1519/JSC.0b013e3181e840f3[↩]
- 15 – Therapeutic Exercise. David X. Cifu, Braddom’s Physical Medicine and Rehabilitation (Sixth Edition), Elsevier, 2021, Pages 291-315.e4, ISBN 780323625395 https://doi.org/10.1016/B978-0-323-62539-5.00015-1[↩]
- Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. 2015 Mar;80 Suppl 1:A8-A15. Doi: 10.1111/1750-3841.12802[↩][↩]
- Spendlove J., Mitchell L., Gifford J., Hackett D., Slater G., Cobley S., O’Connor H. Dietary intake of competitive bodybuilders. Sports Med. 2015;45:1041–1063. doi: 10.1007/s40279-015-0329-4[↩][↩]
- Defining Muscular Hypertrophy & Growth Training Best Practices. https://blog.nasm.org/sports-performance/defining-muscular-hypertrophy-and-training-growth-best-practices[↩][↩][↩][↩][↩][↩]
- The Optimum Performance Training® Model. https://www.nasm.org/certified-personal-trainer/the-opt-model[↩][↩][↩]
- One Rep calculator. https://www.nasm.org/resources/one-rep-max-calculator[↩]
- Acheson KJ. Diets for body weight control and health: the potential of changing the macronutrient composition. Eur J Clin Nutr. 2013 May;67(5):462-6. doi: 10.1038/ejcn.2012.194[↩][↩][↩][↩]
- Protein Intake for Optimal Muscle Maintenance. https://www.acsm.org/docs/default-source/files-for-resource-library/protein-intake-for-optimal-muscle-maintenance.pdf[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Hector A. J., Phillips S. M. Protein recommendations for weight loss in elite athletes: a focus on body composition and performance. International Journal of Sport Nutrition and Exercise Metabolism . 2018;28(2):170–177. doi: 10.1123/ijsnem.2017-0273[↩][↩][↩][↩][↩]
- Passariello C. L., Marchionni S., Carcuro M., et al. The Mediterranean athlete’s nutrition: are protein supplements necessary? Nutrients . 2020;12(12):p. 3681. doi: 10.3390/nu12123681[↩][↩][↩][↩]
- Stokes T., Hector A., Morton R., McGlory C., Phillips S. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients . 2018;10(2):p. 180. doi: 10.3390/nu10020180[↩][↩][↩][↩][↩]
- Evans WJ. Effects of exercise on senescent muscle. Clin Orthop Relat Res. 2002 Oct;(403 Suppl):S211-20. doi: 10.1097/00003086-200210001-00025[↩][↩]
- Jones DA, Rutherford OM. Human muscle strength training: the effects of three different regimens and the nature of the resultant changes. J Physiol. 1987 Oct;391:1-11. doi: 10.1113/jphysiol.1987.sp016721[↩]
- Shinohara M, Kouzaki M, Yoshihisa T, Fukunaga T. Efficacy of tourniquet ischemia for strength training with low resistance. Eur J Appl Physiol Occup Physiol. 1998;77(1-2):189-91. doi: 10.1007/s004210050319[↩]
- Vandenburgh HH. Motion into mass: how does tension stimulate muscle growth? Med Sci Sports Exerc. 1987 Oct;19(5 Suppl):S142-9.[↩][↩]
- Hill M, Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol. 2003 Jun 1;549(Pt 2):409-18. doi: 10.1113/jphysiol.2002.035832[↩][↩]
- Vierck J, O’Reilly B, Hossner K, Antonio J, Byrne K, Bucci L, Dodson M. Satellite cell regulation following myotrauma caused by resistance exercise. Cell Biol Int. 2000;24(5):263-72. doi: 10.1006/cbir.2000.0499[↩][↩]
- Allen DG, Whitehead NP, Yeung EW. Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: role of ionic changes. J Physiol. 2005 Sep 15;567(Pt 3):723-35. doi: 10.1113/jphysiol.2005.091694[↩]
- Toigo M, Boutellier U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol. 2006 Aug;97(6):643-63. doi: 10.1007/s00421-006-0238-1[↩][↩]
- Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab. 2006 Aug;91(8):3024-33. doi: 10.1210/jc.2006-0357[↩]
- The Fitness Zone. https://fitness.edu.au/the-fitness-zone/muscle-hypertrophy-a-practical-guide[↩][↩][↩][↩][↩][↩]
- Pierce JR, Clark BC, Ploutz-Snyder LL, Kanaley JA. Growth hormone and muscle function responses to skeletal muscle ischemia. J Appl Physiol (1985). 2006 Dec;101(6):1588-95. doi: 10.1152/japplphysiol.00585.2006[↩]
- Rooney KJ, Herbert RD, Balnave RJ. Fatigue contributes to the strength training stimulus. Med Sci Sports Exerc. 1994 Sep;26(9):1160-4.[↩]
- Schott J, McCully K, Rutherford OM. The role of metabolites in strength training. II. Short versus long isometric contractions. Eur J Appl Physiol Occup Physiol. 1995;71(4):337-41. doi: 10.1007/BF00240414[↩]
- Smith RC, Rutherford OM. The role of metabolites in strength training. I. A comparison of eccentric and concentric contractions. Eur J Appl Physiol Occup Physiol. 1995;71(4):332-6. doi: 10.1007/BF00240413[↩]
- Suga T, Okita K, Morita N, Yokota T, Hirabayashi K, Horiuchi M, Takada S, Takahashi T, Omokawa M, Kinugawa S, Tsutsui H. Intramuscular metabolism during low-intensity resistance exercise with blood flow restriction. J Appl Physiol (1985). 2009 Apr;106(4):1119-24. doi: 10.1152/japplphysiol.90368.2008[↩]
- Tesch PA, Colliander EB, Kaiser P. Muscle metabolism during intense, heavy-resistance exercise. Eur J Appl Physiol Occup Physiol. 1986;55(4):362-6. doi: 10.1007/BF00422734[↩]
- Goldspink G. Gene expression in skeletal muscle. Biochem Soc Trans. 2002 Apr;30(2):285-90.[↩]
- Hornberger TA, Chien S. Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. J Cell Biochem. 2006 Apr 15;97(6):1207-16. doi: 10.1002/jcb.20671[↩]
- Schoenfeld BJ, Grgic J, Van Every DW, Plotkin DL. Loading Recommendations for Muscle Strength, Hypertrophy, and Local Endurance: A Re-Examination of the Repetition Continuum. Sports (Basel). 2021 Feb 22;9(2):32. doi: 10.3390/sports9020032[↩]
- How Much Sleep Do You Really Need? https://www.thensf.org/how-many-hours-of-sleep-do-you-really-need[↩]
- Kenney, W., Wilmore, J. And Costill, D. (2015) Physiology of Sport and Exercise, 6th edition. Human Kinetics: Champaign, IL.[↩]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004 Dec;1(3):e62. doi: 10.1371/journal.pmed.0010062[↩]
- Chennaoui M, Arnal PJ, Sauvet F, Léger D. Sleep and exercise: a reciprocal issue? Sleep Med Rev. 2015 Apr;20:59-72. doi: 10.1016/j.smrv.2014.06.008[↩]
- Bushman, Barbara A. Ph.D., FACSM. Exercise and Sleep. ACSM’s Health & Fitness Journal 17(5):p 5-8, September/October 2013. | DOI: 10.1249/FIT.0b013e3182a05fce[↩]
- What Are the Sleep Stages? https://www.thensf.org/what-are-the-sleep-stages/[↩][↩][↩]
- Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990 Feb;51(2):241-7. doi: 10.1093/ajcn/51.2.241[↩]
- American Dietetic Association (ADA). Adult Weight Management Guideline: Major Recommendations. ADA Evidence Analysis Library 2014. https://www.andeal.org/vault/pq132.pdf[↩]
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults–The Evidence Report. National Institutes of Health, 6: 51S-179S. https://doi.org/10.1002/j.1550-8528.1998.tb00690.x[↩]
- Dwyer JT, Melanson KJ, Sriprachy-anunt U, et al. Dietary Treatment of Obesity. [Updated 2015 Feb 28]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278991[↩][↩][↩][↩]
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel, 2013. Expert Panel Report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity (Silver Spring). 2014 Jul;22 Suppl 2:S41-410. doi: 10.1002/oby.20660[↩][↩][↩]
- Morris AL, Mohiuddin SS. Biochemistry, Nutrients. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554545[↩][↩][↩][↩][↩][↩]
- Trumbo P, Schlicker S, Yates AA, Poos M; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002 Nov;102(11):1621-30. doi: 10.1016/s0002-8223(02)90346-9. Erratum in: J Am Diet Assoc. 2003 May;103(5):563.[↩][↩][↩]
- Dietary Guidelines for Americans. https://www.dietaryguidelines.gov/[↩][↩][↩][↩][↩][↩]
- Cummings JH, Stephen AM. Carbohydrate terminology and classification. Eur J Clin Nutr. 2007 Dec;61 Suppl 1:S5-18. doi: 10.1038/sj.ejcn.1602936[↩]
- Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. 1995 Jul;62(1):19-29. doi: 10.1093/ajcn/62.1.19[↩]
- Minehira K, Vega N, Vidal H, Acheson K, Tappy L. Effect of carbohydrate overfeeding on whole body macronutrient metabolism and expression of lipogenic enzymes in adipose tissue of lean and overweight humans. Int J Obes Relat Metab Disord. 2004 Oct;28(10):1291-8. doi: 10.1038/sj.ijo.0802760[↩]
- Hall KD. A review of the carbohydrate-insulin model of obesity. Eur J Clin Nutr. 2017 Mar;71(3):323-326. doi: 10.1038/ejcn.2016.260. Epub 2017 Jan 11. Erratum in: Eur J Clin Nutr. 2017 May;71(5):679. doi: 10.1038/ejcn.2017.21[↩]
- Merchant AT, Vatanparast H, Barlas S, Dehghan M, Shah SM, De Koning L, Steck SE. Carbohydrate intake and overweight and obesity among healthy adults. J Am Diet Assoc. 2009 Jul;109(7):1165-72. doi: 10.1016/j.jada.2009.04.002[↩][↩]
- Yunsheng Ma, Barbara Olendzki, David Chiriboga, James R. Hebert, Youfu Li, Wenjun Li, MaryJane Campbell, Katherine Gendreau, Ira S. Ockene, Association between Dietary Carbohydrates and Body Weight, American Journal of Epidemiology, Volume 161, Issue 4, 15 February 2005, Pages 359–367, https://doi.org/10.1093/aje/kwi051[↩]
- Do Carbohydrates Control Body Fat? https://blog.nasm.org/do-carbohydrates-control-body-fat[↩]
- Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS Jr, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006 Feb 13;166(3):285-93. doi: 10.1001/archinte.166.3.285. Erratum in: Arch Intern Med. 2006 Apr 24;166(8):932.[↩]
- Hall KD, Bemis T, Brychta R, et al. Calorie for Calorie, Dietary Fat Restriction Results in More Body Fat Loss than Carbohydrate Restriction in People with Obesity. Cell Metab. 2015 Sep 1;22(3):427-36. doi: 10.1016/j.cmet.2015.07.021[↩]
- Hall KD, Chen KY, Guo J, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016 Aug;104(2):324-33. doi: 10.3945/ajcn.116.133561[↩]
- Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BT, Ravussin E. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016 Aug;104(2):324-33. doi: 10.3945/ajcn.116.133561[↩]
- Burke, L.M. (2021), Ketogenic low-CHO, high-fat diet: the future of elite endurance sport?. J Physiol, 599: 819-843. https://doi.org/10.1113/JP278928[↩]
- Burke LM, Ross ML, Garvican-Lewis LA, Welvaert M, Heikura IA, Forbes SG, Mirtschin JG, Cato LE, Strobel N, Sharma AP, Hawley JA. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol. 2017 May 1;595(9):2785-2807. doi: 10.1113/JP273230[↩]
- How Many Carbs Should You Eat a Day to Lose Weight? https://blog.nasm.org/how-many-carbs-should-you-eat-a-day-to-lose-weight[↩]
- LaPelusa A, Kaushik R. Physiology, Proteins. [Updated 2022 Nov 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555990[↩][↩][↩][↩][↩][↩][↩]
- Cena H, Calder PC. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients. 2020 Jan 27;12(2):334. doi: 10.3390/nu12020334[↩]
- Wu G. Dietary protein intake and human health. Food Funct. 2016 Mar;7(3):1251-65. https://pubs.rsc.org/en/content/articlelanding/2016/fo/c5fo01530h[↩][↩]
- Lopez MJ, Mohiuddin SS. Biochemistry, Essential Amino Acids. [Updated 2024 Apr 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557845[↩][↩][↩][↩][↩][↩]
- Protein Foods. https://www.myplate.gov/eat-healthy/protein-foods[↩][↩]
- Pasiakos SM, Agarwal S, Lieberman HR, Fulgoni VL 3rd. Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007-2010. Nutrients. 2015 Aug 21;7(8):7058-69. doi: 10.3390/nu7085322[↩][↩][↩]
- O’Neil CE, Keast DR, Fulgoni VL, Nicklas TA. Food sources of energy and nutrients among adults in the US: NHANES 2003–2006. Nutrients. 2012 Dec 19;4(12):2097-120. doi: 10.3390/nu4122097[↩]
- Phillips S.M., Fulgoni V.L., Heaney R.P., Nicklas T.A., Slavin J.L., Weaver C.M. Commonly consumed protein foods contribute to nutrient intake, diet quality, and nutrient adequacy. Am. J. Clin. Nutr. 2015;101:1346S–1352S. doi: 10.3945/ajcn.114.084079[↩][↩]
- Salome M., Huneau J.F., Le Baron C., Kesse-Guyot E., Fouillet H., Mariotti F. Substituting Meat or Dairy Products with Plant-Based Substitutes Has Small and Heterogeneous Effects on Diet Quality and Nutrient Security: A Simulation Study in French Adults (INCA3) J. Nutr. 2021;151:2435–2445. doi: 10.1093/jn/nxab146[↩]
- Hoy M.K., Murayi T., Moshfegh A.J. Effect of Animal Protein Intake on Meeting Recommendations for Nutrient Intake among US Adults, What We Eat in America, NHANES 2015–2018. Curr. Dev. Nutr. 2022;7:100027. doi: 10.1016/j.cdnut.2022.100027[↩]
- Fouillet H., Dussiot A., Perraud E., Wang J., Huneau J.F., Kesse-Guyot E., Mariotti F. Plant to animal protein ratio in the diet: Nutrient adequacy, long-term health and environmental pressure. Front. Nutr. 2023;10:1178121. doi: 10.3389/fnut.2023.1178121[↩]
- Dietary Guidelines Advisory Committee. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010, to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington (DC): US Department of Agriculture, Agricultural Research Service; 2010.[↩]
- FAO Food-Based Dietary Guidelines. 2023. https://www.fao.org/nutrition/education/food-based-dietary-guidelines[↩]
- Cámara M, Giner RM, González-Fandos E, López-García E, Mañes J, Portillo MP, Rafecas M, Domínguez L, Martínez JA. Food-Based Dietary Guidelines around the World: A Comparative Analysis to Update AESAN Scientific Committee Dietary Recommendations. Nutrients. 2021 Sep 8;13(9):3131. doi: 10.3390/nu13093131[↩]
- Wu G, Fanzo J, Miller DD, Pingali P, Post M, Steiner JL, Thalacker-Mercer AE. Production and supply of high-quality food protein for human consumption: sustainability, challenges, and innovations. Ann N Y Acad Sci. 2014 Aug;1321:1-19. doi: 10.1111/nyas.12500[↩]
- Layman DK, Anthony TG, Rasmussen BB, Adams SH, Lynch CJ, Brinkworth GD, Davis TA. Defining meal requirements for protein to optimize metabolic roles of amino acids. Am J Clin Nutr. 2015 Jun;101(6):1330S-1338S. doi: 10.3945/ajcn.114.084053[↩][↩]
- van Vliet S, Burd NA, van Loon LJ. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J Nutr. 2015 Sep;145(9):1981-91. doi: 10.3945/jn.114.204305[↩]
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006 Sep;84(3):475-82. doi: 10.1093/ajcn/84.3.475[↩][↩]
- Levenhagen DK, Gresham JD, Carlson MG, Maron DJ, Borel MJ, Flakoll PJ. Postexercise nutrient intake timing in humans is critical to recovery of leg glucose and protein homeostasis. Am J Physiol Endocrinol Metab. 2001 Jun;280(6):E982-93. doi: 10.1152/ajpendo.2001.280.6.E982[↩]
- Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, Saris WH, van Loon LJ. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr. 2011 Jun;141(6):1070-6. doi: 10.3945/jn.111.138495[↩]
- What Are Amino Acids? https://www.reagent.co.uk/blog/what-are-amino-acids/[↩]
- What are Amino acids? https://www.jpt.com/support-contact/resources/amino-acids/[↩]
- Biochemistry and molecular genetics. https://www.toxmsdt.com/topic-11-biochemistry.html[↩]
- Amino Acid Analysis: Unveiling the Secrets of Protein Composition and Metabolism. https://www.creative-proteomics.com/resource/exploring-amino-acid-analysis-of-protein-composition-metabolism.htm[↩]
- Protein. https://www.genome.gov/genetics-glossary/Protein[↩]
- Pillai AT, Morya S, Kasankala LM. Emerging Trends in Bioavailability and Pharma-Nutraceutical Potential of Whey Bioactives. J Nutr Metab. 2024 Apr 10;2024:8455666. doi: 10.1155/2024/8455666[↩][↩][↩][↩][↩][↩][↩][↩]
- Areta J.L., Burke L.M., Ross M.L., Camera D.M., West D.W., Broad E.M., Jeacocke N.A., Moore D.R., Stellingwerff T., Phillips S.M., et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 2013;591:2319–2331. doi: 10.1113/jphysiol.2012.244897[↩][↩]
- Cribb P.J., Williams A.D., Carey M.F., Hayes A. The effect of whey isolate and resistance training on strength, body composition, and plasma glutamine. Int. J. Sport Nutr. Exerc. Metab. 2006;16:494–509. doi: 10.1123/ijsnem.16.5.494[↩][↩]
- Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015 Mar;96(3):183-95. doi: 10.1007/s00223-014-9915-y[↩]
- Morton RW, McGlory C, Phillips SM. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front Physiol. (2015) 6:245. doi: 10.3389/fphys.2015.00245[↩]
- Tarnopolsky MA, Atkinson SA, MacDougall JD, Chesley A, Phillips S, Schwarcz HP. Evaluation of protein requirements for trained strength athletes. J Appl Physiol. (1992) 73:1986–95. doi: 10.1152/jappl.1992.73.5.1986[↩]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. (2009) 89:161–8. doi: 10.3945/ajcn.2008.26401[↩]
- Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr. (2014) 99:86–95. doi: 10.3945/ajcn.112.055517[↩][↩]
- Macnaughton LS, Wardle SL, Witard OC, McGlory C, Hamilton DL, Jeromson S, et al. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol Rep. (2016) 4:e12893. doi: 10.14814/phy2.12893[↩]
- Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. (2015) 80(Suppl. 1):A8–15. doi: 10.1111/1750-3841.12802[↩]
- Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol. (2013) 591:2319–31. doi: 10.1113/jphysiol.2012.244897[↩]
- Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. 2015 Mar;80 Suppl 1:A8-A15. doi: 10.1111/1750-3841.12802[↩][↩][↩]
- Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. (2018) 52:376–84. doi: 10.1136/bjsports-2017-097608[↩]
- Finger D., Goltz F. R., Umpierre D., Meyer E., Rosa L. H. T., Schneider C. D. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Medicine . 2015;45(2):245–255. doi: 10.1007/s40279-014-0269-4[↩]
- Morton R. W., Murphy K. T., McKellar S. R., et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. British Journal of Sports Medicine . 2018;52(6):376–384. doi: 10.1136/bjsports-2017-097608[↩]
- Phillips S. M., Van Loon L. J. C. Dietary protein for athletes: from requirements to optimum adaptation. Journal of Sports Sciences . 2011;29(1):S29–S38. doi: 10.1080/02640414.2011.619204[↩]
- Porcelli S, Pugliese L, Rejc E, Pavei G, Bonato M, Montorsi M, La Torre A, Rasica L, Marzorati M. Effects of a Short-Term High-Nitrate Diet on Exercise Performance. Nutrients. 2016 Aug 31;8(9):534. doi: 10.3390/nu8090534[↩]
- Del Razo Olvera FM, Melgarejo Hernández MA, Mehta R, Aguilar Salinas CA. Setting the Lipid Component of the Diet: A Work in Process. Adv Nutr. 2017 Jan 17;8(1):165S-172S. doi: 10.3945/an.116.013672[↩]
- Tvrzicka E, Kremmyda LS, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, health and disease–a review. Part 1: classification, dietary sources and biological functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011 Jun;155(2):117-30. doi: 10.5507/bp.2011.038[↩]
- Jacobs DR, Tapsell LC. Food synergy: the key to a healthy diet. Proc Nutr Soc. 2013 May;72(2):200-6. doi: 10.1017/S0029665112003011[↩]
- Jones PJH, Papamandjaris AA. Lipids: cellular metabolism. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:132-48.[↩]
- Harris WS. Omega-3 fatty acids. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:577-86.[↩]
- Omega-3 Fatty Acids, https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional[↩][↩]
- Jones PJH, Rideout T. Lipids, sterols, and their metabolites. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014.[↩]
- Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008 Jun;233(6):674-88. doi: 10.3181/0711-MR-311[↩]
- Wang C, Chung M, Lichtenstein A, Balk E, Kupelnick B, DeVine D, et al. Effects of omega-3 fatty acids on cardiovascular disease. Summary, evidence report/technology assessment no. 94. https://archive.ahrq.gov/downloads/pub/evidence/pdf/o3cardio/o3cardio.pdf[↩]
- Stanley JC, Elsom RL, Calder PC, Griffin BA, Harris WS, Jebb SA, Lovegrove JA, Moore CS, Riemersma RA, Sanders TA. UK Food Standards Agency Workshop Report: the effects of the dietary n-6:n-3 fatty acid ratio on cardiovascular health. Br J Nutr. 2007 Dec;98(6):1305-10. doi: 10.1017/S000711450784284X[↩]
- Harris WS, Davidson MH. RE: Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2014 Apr;106(4):dju019. doi: 10.1093/jnci/dju019[↩]
- Fritsche KL. Too much linoleic acid promotes inflammation-doesn’t it? Prostaglandins Leukot Essent Fatty Acids. 2008 Sep-Nov;79(3-5):173-5. doi: 10.1016/j.plefa.2008.09.019[↩]
- Very Low-Calorie Diets. https://www.govinfo.gov/content/pkg/GOVPUB-HE20-PURL-LPS78142/pdf/GOVPUB-HE20-PURL-LPS78142.pdf[↩]
- Pritikin M, McGrady PM. The Pritikin Program for Diet and Exercise. New York: Bantum Books, 1980.[↩]
- Ornish D. Can lifestyle changes reverse coronary heart disease? World Rev Nutr Diet. 1993;72:38-48. doi: 10.1159/000422326[↩][↩][↩]
- Hand B and Romine S. The Spark Solution. HarperOne, 2014.[↩]
- Bandara SB, Towle KM, Monnot AD. A human health risk assessment of heavy metal ingestion among consumers of protein powder supplements. Toxicol Rep. 2020 Aug 21;7:1255-1262. doi: 10.1016/j.toxrep.2020.08.001[↩]
- Consumer Reports . Consumer Reports; Yonkers, NY: 2010. Investigation: Test Reveal Contaminants in Many Protein Drinks, Unclear Labeling May Lead to Excessive Protein Consumption Which Can Pose Health Problems. https://www.consumerreports.org/media-room/press-releases/2010/06/investigation-tests-reveal-contaminants-in-many-protein-drinks/[↩]
- Clean Label . Clean Label Project; Broomfield, CO: 2018. 2018 Protein Powder Study. https://cleanlabelproject.org/protein-powder-infographic/[↩][↩]
- Bernhoft R.A. Mercury toxicity and treatment: a review of the literature. J. Environ. Public Health. 2012;2012 doi: 10.1155/2012/460508[↩]
- Godt J., Scheidig F., Grosse-Siestrup C., Esche V., Brandenburg P., Reich A., Groneberg D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006;1:22. doi: 10.1186/1745-6673-1-2[↩]
- Needleman H. Lead poisoning. Annu. Rev. Med. 2004;55:209–222. doi: 10.1146/annurev.med.55.091902.103653[↩][↩]
- Robles-Osorio M.L., Sabath-Silva E., Sabath E. Arsenic-mediated nephrotoxicity. Ren. Fail. 2015;37(4):542–547. doi: 10.3109/0886022X.2015.1013419[↩]
- Vahidnia A., van der Voet G.B., de Wolff F.A. Arsenic neurotoxicity–a review. Hum. Exp. Toxicol. 2007;26(10):823–832. doi: 10.1177/0960327107084539[↩]
- Wallace D.R., Taalab Y.M., Heinze S., Tariba Lovakovic B., Pizent A., Renieri E., Tsatsakis A., Farooqi A.A., Javorac D., Andjelkovic M., Bulat Z., Antonijevic B., Buha Djordjevic A. Toxic-metal-induced alteration in miRNA expression profile as a proposed mechanism for disease development. Cells. 2020;9(4) doi: 10.3390/cells9040901[↩]
- Patrick L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern Med Rev. 2006 Mar;11(1):2-22.[↩]
- Buha A., Matovic V., Antonijevic B., Bulat Z., Curcic M., Renieri E.A., Tsatsakis A.M., Schweitzer A., Wallace D. Overview of cadmium thyroid disrupting effects and mechanisms. Int. J. Mol. Sci. 2018;19(5) doi: 10.3390/ijms19051501[↩]
- Johri N., Jacquillet G., Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals. 2010;23(5):783–792. doi: 10.1007/s10534-010-9328-y[↩]
- Rodriguez J., Mandalunis P.M. A review of metal exposure and its effects on bone health. J. Toxicol. 2018;2018 doi: 10.1155/2018/4854152[↩]
- Zhou Q., Xi S. A review on arsenic carcinogenesis: epidemiology, metabolism, genotoxicity and epigenetic changes. Regul. Toxicol. Pharmacol. 2018;99:78–88. doi: 10.1016/j.yrtph.2018.09.010[↩]
- Bellinger DC. Lead. Pediatrics. 2004 Apr;113(4 Suppl):1016-22.[↩]
- Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM. Lead toxicity update. A brief review. Med Sci Monit. 2005 Oct;11(10):RA329-36. https://medscimonit.com/abstract/index/idArt/430340[↩]
- Zahir F., Rizwi S.J., Haq S.K., Khan R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005;20(2):351–360. doi: 10.1016/j.etap.2005.03.007[↩]
- Swithers SE. Artificial sweeteners are not the answer to childhood obesity. Appetite. 2015 Oct;93:85-90. doi: 10.1016/j.appet.2015.03.027[↩][↩]
- Suez J, Korem T, Zilberman-Schapira G, Segal E, Elinav E. Non-caloric artificial sweeteners and the microbiome: findings and challenges. Gut Microbes. 2015;6(2):149-55. doi: 10.1080/19490976.2015.1017700[↩][↩]
- Pearlman M, Obert J, Casey L. The Association Between Artificial Sweeteners and Obesity. Curr Gastroenterol Rep. 2017 Nov 21;19(12):64. doi: 10.1007/s11894-017-0602-9[↩][↩]
- Debras C, Chazelas E, Srour B, et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med. 2022 Mar 24;19(3):e1003950. doi: 10.1371/journal.pmed.1003950[↩][↩]
- Christofides EA. POINT: Artificial Sweeteners and Obesity-Not the Solution and Potentially a Problem. Endocr Pract. 2021 Oct;27(10):1052-1055. doi: 10.1016/j.eprac.2021.08.001[↩][↩]
- Pavanello S, Moretto A, La Vecchia C, Alicandro G. Non-sugar sweeteners and cancer: Toxicological and epidemiological evidence. Regul Toxicol Pharmacol. 2023 Mar;139:105369. doi: 10.1016/j.yrtph.2023.105369[↩][↩]
- Backx EM, Tieland M, Borgonjen-van den Berg KJ, Claessen PR, van Loon LJ, de Groot LC. Protein intake and lean body mass preservation during energy intake restriction in overweight older adults. Int J Obes (Lond). 2016 Feb;40(2):299-304. doi: 10.1038/ijo.2015.182[↩]
- Kerstetter JE, Bihuniak JD, Brindisi J, Sullivan RR, Mangano KM, Larocque S, Kotler BM, Simpson CA, Cusano AM, Gaffney-Stomberg E, Kleppinger A, Reynolds J, Dziura J, Kenny AM, Insogna KL. The Effect of a Whey Protein Supplement on Bone Mass in Older Caucasian Adults. J Clin Endocrinol Metab. 2015 Jun;100(6):2214-22. doi: 10.1210/jc.2014-3792[↩]
- Zhu K, Kerr DA, Meng X, Devine A, Solah V, Binns CW, Prince RL. Two-Year Whey Protein Supplementation Did Not Enhance Muscle Mass and Physical Function in Well-Nourished Healthy Older Postmenopausal Women. J Nutr. 2015 Nov;145(11):2520-6. doi: 10.3945/jn.115.218297[↩]
- Smith GI, Commean PK, Reeds DN, Klein S, Mittendorfer B. Effect of Protein Supplementation During Diet-Induced Weight Loss on Muscle Mass and Strength: A Randomized Controlled Study. Obesity (Silver Spring). 2018 May;26(5):854-861. doi: 10.1002/oby.22169[↩]
- Haghighat N, Ashtary-Larky D, Bagheri R, Wong A, Cheraghloo N, Moradpour G, Nordvall M, Asbaghi O, Moeinvaziri N, Amini M, Sohrabi Z, Dutheil F. Effects of 6 Months of Soy-Enriched High Protein Compared to Eucaloric Low Protein Snack Replacement on Appetite, Dietary Intake, and Body Composition in Normal-Weight Obese Women: A Randomized Controlled Trial. Nutrients. 2021 Jun 30;13(7):2266. doi: 10.3390/nu13072266[↩]
- Reinders I, Visser M, Jyväkorpi SK, Niskanen RT, Bosmans JE, Jornada Ben Â, Brouwer IA, Kuijper LD, Olthof MR, Pitkälä KH, Vijlbrief R, Suominen MH, Wijnhoven HAH. The cost effectiveness of personalized dietary advice to increase protein intake in older adults with lower habitual protein intake: a randomized controlled trial. Eur J Nutr. 2022 Feb;61(1):505-520. doi: 10.1007/s00394-021-02675-0. Epub 2021 Oct 5. Erratum in: Eur J Nutr. 2022 Feb;61(1):521. doi: 10.1007/s00394-021-02778-8[↩]
- Ringholm S, Biensø RS, Kiilerich K, Guadalupe-Grau A, Aachmann-Andersen NJ, Saltin B, Plomgaard P, Lundby C, Wojtaszewski JF, Calbet JA, Pilegaard H. Bed rest reduces metabolic protein content and abolishes exercise-induced mRNA responses in human skeletal muscle. Am J Physiol Endocrinol Metab. 2011 Oct;301(4):E649-58. doi: 10.1152/ajpendo.00230.2011[↩]
- Barone G, O’Regan J, Kelly AL, O’Mahony JA. Interactions between whey proteins and calcium salts and implications for the formulation of dairy protein-based nutritional beverage products: A review. Comprehensive Reviews in Food Science and Food Safety. 2022;21:1254–1274. doi: 10.1111/1541-4337.12884[↩]
- Li C, Meng H, Wu S, Fang A, Liao G, Tan X, Chen P, Wang X, Chen S, Zhu H. Daily Supplementation With Whey, Soy, or Whey-Soy Blended Protein for 6 Months Maintained Lean Muscle Mass and Physical Performance in Older Adults With Low Lean Mass. J Acad Nutr Diet. 2021 Jun;121(6):1035-1048.e6. doi: 10.1016/j.jand.2021.01.006[↩]
- Lamina T, Brandt S, Abdi HI, et al. The Effect of Protein Intake on Health: A Systematic Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2024 Nov. 3, Results. Available from: https://www.ncbi.nlm.nih.gov/books/NBK610209[↩]
- González-Weller D., Paz-Montelongo S., Bethencourt-Barbuzano E., et al. Proteins and minerals in whey protein supplements. Foods . 2023;12(11):p. 2238. doi: 10.3390/foods12112238[↩][↩]
- Zandona E, Blazic M, Jambrak AR. Whey utilisation: Sustainable uses and environmental approach. Food Technology and Biotechnology. 2021;59:147–161. doi: 10.17113/ftb.59.02.21.6968[↩]
- Nicolas P, Lujan Ferreira M, Lassalle V. A review of magnetic separation of whey proteins and potential application to whey proteins recovery, isolation and utilization. Journal of Food Engineering. 2019;246:7–15. doi: 10.1016/j.jfoodeng.2018.10.021[↩]
- Arbizu S, Chew B, Mertens-Talcott SU, Noratto G. Commercial whey products promote intestinal barrier function with glycomacropeptide enhanced activity in downregulating bacterial endotoxin lipopolysaccharides (LPS)-induced inflammationin vitro. Food & Function. 2020;11:5842–5852. doi: 10.1039/D0FO00487A[↩]
- Anirudh J., Dhineshkumar V., Sangeetha T. Whey as crucial component in rejuvenating athlete health-a review. Journal of Postharvest Technology . 2022;10(4):135–155.[↩]
- Corrochano A. R., Arranz E., De Noni I., et al. Intestinal health benefits of bovine whey proteins after simulated gastrointestinal digestion. Journal of Functional Foods . 2018;49:526–535. doi: 10.1016/j.jff.2018.08.043[↩]
- Sandhu D., Morya S. A review on the comparative study of nutraceutically activated fruits and herbs-based wines. Journal of Applied and Natural Science . 2022;14(2):500–511. doi: 10.31018/jans.v14i2.3429[↩]
- Bintsis T., Papademas P. Sustainable approaches in whey cheese production: a review. Dairy . 2023;4(2):249–270. doi: 10.3390/dairy4020018[↩]
- Zhang L., Zhou R., Zhang J., Zhou P. Heat-induced denaturation and bioactivity changes of whey proteins. International Dairy Journal . 2021;123 doi: 10.1016/j.idairyj.2021.105175[↩]
- Guefai F. Z., Martínez-Rodríguez A., Grindlay G., Mora J., Gras L. Elemental bioavailability in whey protein supplements. Journal of Food Composition and Analysis . 2022;112 doi: 10.1016/j.jfca.2022.104696[↩]
- Ha H. K., Rankin S. A., Lee M. R., Lee W. J. Development and characterization of whey protein-based nano-delivery systems: a review. Molecules . 2019;24(18):p. 3254. doi: 10.3390/molecules24183254[↩]
- Kelly P. In Whey Proteins . MA, USA: Academic Press; 2019. Manufacture of whey protein products: concentrates, isolate, whey protein fractions and microparticulated; pp. 97–122.[↩]
- Pehlivanoğlu H., Bardakci H. F., Yaman M. Protein quality assessment of commercial whey protein supplements commonly consumed in Turkey by in vitro protein digestibility-corrected amino acid score (PDCAAS) Food Science and Technology . 2022;42 doi: 10.1590/fst.64720[↩]
- Mehra R, Kumar H, Kumar N, Ranvir S, Jana A, Buttar HS, Telessy IG, Awuchi CG, Okpala COR, Korzeniowska M, Guiné RPF. Whey proteins processing and emergent derivatives: An insight perspective from constituents, bioactivities, functionalities to therapeutic applications. Journal of Functional Foods. 2021;87:104760. doi: 10.1016/j.jff.2021.104760[↩]
- Meng Y, Liang Z, Zhang C, Hao S, Han H, Du P, Li A, Shao H, Li C, Liu L. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. LWT-Food Science and Technology. 2021;152:112272. doi: 10.1016/j.lwt.2021.112272[↩]
- Bhat, M. Y., Dar, T. A., & Singh, L. R. (2016). Casein Proteins: Structural and Functional Aspects. InTech. doi: 10.5772/64187[↩][↩]
- Runthala A, Mbye M, Ayyash M, Xu Y, Kamal-Eldin A. Caseins: Versatility of Their Micellar Organization in Relation to the Functional and Nutritional Properties of Milk. Molecules. 2023 Feb 21;28(5):2023. doi: 10.3390/molecules28052023[↩]
- Sodhi M., Mukesh M., Kataria R.S., Mishra B.P., Joshii B.K. Milk proteins and human health: A1/A2 milk hypothesis. Indian J. Endocrinol. Metab. 2012;16:856. doi: 10.4103/2230-8210.100685[↩]
- Kaplan M., Baydemir B., Günar B.B., Arslan A., Duman H., Karav S. Benefits of A2 Milk for Sports Nutrition, Health and Performance. Front. Nutr. 2022;9:1500. doi: 10.3389/fnut.2022.935344[↩]
- Roy BD. Milk: the new sports drink? a review. J Int Soc Sports Nutr. (2008) 5:15. 10.1186/1550-2783-5-15[↩][↩]
- Ferguson-Stegall L, McCleave E, Ding Z, Doerner PG, III, Liu Y, Wang B, et al. Aerobic exercise training adaptations are increased by postexercise carbohydrate-protein supplementation. J Nutr Metab. (2011) 2011:1–11. 10.1155/2011/623182[↩]
- Wilkinson SB, Tarnopolsky MA, MacDonald MJ, MacDonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. (2007) 85:1031–40. 10.1093/ajcn/85.4.1031[↩]
- Giribaldi M., Lamberti C., Cirrincione S., Giuffrida M.G., Cavallarin L. A2 milk and BCM-7 peptide as emerging parameters of milk quality. Front. Nutrition. 2022;9:65. doi: 10.3389/fnut.2022.842375[↩]
- He M, Sun J, Jiang ZQ, Yang YX. Effects of cow’s milk beta-casein variants on symptoms of milk intolerance in Chinese adults: a multicentre, randomised controlled study. Nutr J. (2017) 16:72. 10.1186/s12937-017-0275-0[↩]
- Çak B. Discussions of effect A1 and A2 milk beta-casein gene on health. Approaches Poult Dairy Vet Sci. (2018) 3. 10.31031/APDV.2018.03.000556[↩]
- Ul Haq MR, Kapila R, Kapila S. Release of β-casomorphin-7/5 during simulated gastrointestinal digestion of milk β-casein variants from Indian crossbred cattle (Karan Fries). Food Chem. (2015) 168:70–9. 10.1016/j.foodchem.2014.07.024[↩]
- Fiedorowicz E, Kaczmarski M, Cieślińska A, Sienkiewicz-Szłapka E, Jarmołowska B, Chwała B, et al. β-casomorphin-7 alters μ-opioid receptor and dipeptidyl peptidase IV genes expression in children with atopic dermatitis. Peptides. (2014) 62:144–9. 10.1016/j.peptides.2014.09.020[↩]
- Gustavsson F, Buitenhuis AJ, Johansson M, Bertelsen HP, Glantz M, Poulsen NA, et al. Effects of breed and casein genetic variants on protein profile in milk from Swedish Red, Danish Holstein, and Danish Jersey cows. J Dairy Sci. (2014) 97:3866–77. 10.3168/jds.2013-7312[↩]
- McLachlan CNS. beta-casein A1, ischaemic heart disease mortality, and other illnesses. Med Hypotheses. (2001) 56:262–72. 10.1054/mehy.2000.1265[↩]
- Muehlenkamp MR, Warthesen JJ. Beta-casomorphins: analysis in cheese and susceptibility to proteolytic enzymes from Lactococcus lactis ssp. cremoris. J Dairy Sci. (1996) 79:20–6. 10.3168/jds.S0022-0302(96)76329-4[↩]
- Jianqin S, Leiming X, Lu X, Yelland GW Ni J, Clarke AJ. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr J. (2016) 15:1–16. 10.1186/s12937-016-0164-y[↩]
- Laugesen M, Elliott R. Ischaemic heart disease, Type 1 diabetes, and cow milk A1 beta-casein. N Z Med J. 2003 Jan 24;116(1168):U295.[↩]
- Kaminski S., Cieslinska A., Kostyra E. Polymorphism of Bovine Beta-Casein and Its Potential Effect on Human Health. J. Appl. Genet. 2007;48:189–198. doi: 10.1007/BF03195213[↩]
- Sodhi M., Mukesh M., Kataria R.S., Mishra B.P., Joshii B.K. Milk Proteins and Human Health: A1/A2 Milk Hypothesis. Indian J. Endocrinol. Metab. 2012;16:856. doi: 10.4103/2230-8210.100685[↩]
- Thiruvengadam M., Venkidasamy B., Thirupathi P., Chung I.-M., Subramanian U. β-Casomorphin: A Complete Health Perspective. Food Chem. 2021;337:127765. doi: 10.1016/j.foodchem.2020.127765[↩]
- Scientific Report of EFSA prepared by a DATEX Working Group on the potential health impact of β-casomorphins and related peptides. EFSA Scientific Report (2009) 231, 1–107. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2009.231r[↩]
- Kaplan M, Baydemir B, Günar BB, Arslan A, Duman H, Karav S. Benefits of A2 Milk for Sports Nutrition, Health and Performance. Front Nutr. 2022 Jul 13;9:935344. doi: 10.3389/fnut.2022.935344[↩]
- Rankin P, Lawlor MJ, Hills FA, Bell PG, Cockburn E, Lawlor M, et al. The effect of milk on recovery from repeat-sprint cycling in female team-sport athletes. Appl Physiol Nutr Metab. (2018) 43:113–22. 10.1139/apnm-2017-0275[↩]
- Reddy PRK, Reddy AN, Ramadevi A, Kumar DS. Nutritional significance of indigenous cow milk with regard to a2 β-casein – an overview. (2016) 5:3376–80.[↩]
- Ramakrishnan M, Eaton T, Sermet O, Savaiano D. A single meal of milk containing A2 β-casein causes fewer symptoms and lower gas production than milk containing both A1 and A2 β-casein among lactose intolerant individuals. Curr Dev Nutr. (2020) 4:772–772. 10.1093/cdn/nzaa052_041[↩]
- Chitra P. Bovine milk: A1 and A2 beta casein milk proteins and their impact on human health: a review. Agric Rev. (2021). 10.18805/ag.R-2126[↩]
- Lucey J.A. Advanced Dairy Chemistry. Springer; New York, NY, USA: 2016. Acid coagulation of milk; pp. 309–328.[↩]
- Mohamed H., Johansson M., Lundh Å., Nagy P., Kamal-Eldin A. Short communication: Caseins and α-lactalbumin content of camel milk (Camelus dromedarius) determined by capillary electrophoresis. J. Dairy Sci. 2020;103:11094–11099. doi: 10.3168/jds.2020-19122[↩]
- Dhasmana, S., Das, S., & Shrivastava, S. (2022). Potential nutraceuticals from the casein fraction of goat’s milk. Journal of Food Biochemistry, 46, e13982. https://doi.org/10.1111/jfbc.13982[↩]
- Tvaroška I. Glycosylation Modulates the Structure and Functions of Collagen: A Review. Molecules. 2024 Mar 22;29(7):1417. doi: 10.3390/molecules29071417[↩][↩]
- Institute of Medicine (US) Committee on Nutrition, Trauma, and the Brain; Erdman J, Oria M, Pillsbury L, editors. Nutrition and Traumatic Brain Injury: Improving Acute and Subacute Health Outcomes in Military Personnel. Washington (DC): National Academies Press (US); 2011. 10, Creatine. Available from: https://www.ncbi.nlm.nih.gov/books/NBK209321[↩]
- Stricker PR. Other ergogenic agents. Clin Sports Med. 1998 Apr;17(2):283-97. doi: 10.1016/s0278-5919(05)70081-8[↩]
- Butts J, Jacobs B, Silvis M. Creatine Use in Sports. Sports Health. 2018 Jan/Feb;10(1):31-34. doi: 10.1177/1941738117737248[↩][↩]
- Butts J, Jacobs B, Silvis M. Creatine Use in Sports. Sports Health. 2018 Jan/Feb;10(1):31-34. Doi: 10.1177/1941738117737248[↩][↩]
- Bird SP. Creatine supplementation and exercise performance: a brief review. J Sports Sci Med. 2003 Dec 1;2(4):123-32. https://pmc.ncbi.nlm.nih.gov/articles/PMC3963244[↩]
- Cooke WH, Grandjean PW, Barnes WS. Effect of oral creatine supplementation on power output and fatigue during bicycle ergometry. J Appl Physiol (1985). 1995 Feb;78(2):670-3. doi: 10.1152/jappl.1995.78.2.670[↩]
- Rawson ES, Gunn B, Clarkson PM. The effects of creatine supplementation on exercise-induced muscle damage. J Strength Cond Res. 2001 May;15(2):178-84.[↩]
- Ellery SJ, Walker DW, Dickinson H. Creatine for women: a review of the relationship between creatine and the reproductive cycle and female-specific benefits of creatine therapy. Amino Acids. 2016 Aug;48(8):1807-17. doi: 10.1007/s00726-016-2199-y[↩]
- Molina Juan L, Sospedra I, Perales A, González-Díaz C, Gil-Izquierdo A, Martínez-Sanz JM. Analysis of health claims regarding creatine monohydrate present in commercial communications for a sample of European sports foods supplements. Public Health Nutr. 2021 Jan 20;24(4):1-9. doi: 10.1017/S1368980020005121[↩][↩]
- Bemben MG, Lamont HS. Creatine supplementation and exercise performance: recent findings. Sports Med. 2005;35(2):107-25. doi: 10.2165/00007256-200535020-00002[↩]
- Branch JD. Effect of creatine supplementation on body composition and performance: a meta-analysis. Int J Sport Nutr Exerc Metab. 2003 Jun;13(2):198-226. doi: 10.1123/ijsnem.13.2.198[↩]
- Institute of Medicine (US) Committee on Dietary Supplement Use by Military Personnel; Greenwood MRC, Oria M, editors. Use of Dietary Supplements by Military Personnel. Washington (DC): National Academies Press (US); 2008. Available from: https://www.ncbi.nlm.nih.gov/books/NBK3977[↩][↩]
- McMorris T, Harris RC, Howard AN, Langridge G, Hall B, Corbett J, Dicks M, Hodgson C. Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol Behav. 2007 Jan 30;90(1):21-8. doi: 10.1016/j.physbeh.2006.08.024[↩]
- Shao A, Hathcock JN. Risk assessment for creatine monohydrate. Regul Toxicol Pharmacol. 2006 Aug;45(3):242-51. doi: 10.1016/j.yrtph.2006.05.005[↩][↩]
- Burke LM. Practical Issues in Evidence-Based Use of Performance Supplements: Supplement Interactions, Repeated Use and Individual Responses. Sports Med. 2017 Mar;47(Suppl 1):79-100. doi: 10.1007/s40279-017-0687-1[↩]
- Rawson ES, Miles MP, Larson-Meyer DE. Dietary Supplements for Health, Adaptation, and Recovery in Athletes. Int J Sport Nutr Exerc Metab. 2018 Mar 1;28(2):188-199. doi: 10.1123/ijsnem.2017-0340[↩][↩]
- Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, Candow DG, Kleiner SM, Almada AL, Lopez HL. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr. 2017 Jun 13;14:18. doi: 10.1186/s12970-017-0173-z[↩]
- Maughan RJ, Greenhaff PL, Hespel P. Dietary supplements for athletes: emerging trends and recurring themes. J Sports Sci. 2011;29 Suppl 1:S57-66. doi: 10.1080/02640414.2011.587446[↩]
- European Comission (2018) EU Register on Nutrition and Health Claims. Brussels: European Commission.[↩]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2016. Scientific opinion on creatine in combination with resistance training and improvement in muscle strength: evaluation of a health claim pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA Journal 2016; 14(2):4400, 17 pp. doi:10.2903/j.efsa.2016.4400[↩][↩][↩][↩]
- Roberts PA, Fox J, Peirce N, Jones SW, Casey A, Greenhaff PL. Creatine ingestion augments dietary carbohydrate mediated muscle glycogen supercompensation during the initial 24 h of recovery following prolonged exhaustive exercise in humans. Amino Acids. 2016 Aug;48(8):1831-42. doi: 10.1007/s00726-016-2252-x[↩]
- Herriman M, Fletcher L, Tchaconas A, Adesman A, Milanaik R. Dietary Supplements and Young Teens: Misinformation and Access Provided by Retailers. Pediatrics. 2017 Feb;139(2):e20161257. doi: 10.1542/peds.2016-1257[↩]
- Bodybuilding and Performance Enhancement Supplements: What You Need To Know. https://www.nccih.nih.gov/health/bodybuilding-and-performance-enhancement-supplements[↩][↩]
- Alvares TS, Conte-Junior CA, Silva JT, Paschoalin VM. L-arginine does not improve biochemical and hormonal response in trained runners after 4 weeks of supplementation. Nutr Res. 2014 Jan;34(1):31-9. doi: 10.1016/j.nutres.2013.10.006[↩]
- Austin KG, McLellan TM, Farina EK, McGraw SM, Lieberman HR. Soldier use of dietary supplements, including protein and body building supplements, in a combat zone is different than use in garrison. Appl Physiol Nutr Metab. 2016 Jan;41(1):88-95. doi: 10.1139/apnm-2015-0387[↩]
- Candow DG, Chilibeck PD, Forbes SC. Creatine supplementation and aging musculoskeletal health. Endocrine. 2014 Apr;45(3):354-61. doi: 10.1007/s12020-013-0070-4[↩]
- Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, Reddy KR, Seeff LB, Serrano J, Sherker AH, Stolz A, Talwalkar J, Vega M, Vuppalanchi R. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014 Oct;60(4):1399-408. doi: 10.1002/hep.27317[↩]
- Ronis MJJ, Pedersen KB, Watt J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu Rev Pharmacol Toxicol. 2018 Jan 6;58:583-601. doi: 10.1146/annurev-pharmtox-010617-052844[↩]
- Bellinger PM. β-Alanine supplementation for athletic performance: an update. J Strength Cond Res. 2014 Jun;28(6):1751-70. doi: 10.1519/JSC.0000000000000327[↩]
- National Center for Complementary and Integrative Health. Bodybuilding. https://nccih.nih.gov/health/bodybuilding[↩]
- http://ocbonline.com/[↩]
- Nutrient intake, body fat, and lipid profiles of competitive male and female bodybuilders. Bazzarre TL, Kleiner SM, Litchford MD. J Am Coll Nutr. 1990 Apr; 9(2):136-42. https://www.ncbi.nlm.nih.gov/pubmed/2338462/[↩]
- Nutritional status of nationally ranked elite bodybuilders. Kleiner SM, Bazzarre TL, Ainsworth BE. Int J Sport Nutr. 1994 Mar; 4(1):54-69. https://www.ncbi.nlm.nih.gov/pubmed/8167655/[↩][↩]
- Food selection patterns of bodybuilders. Sandoval WM, Heyward VH. Int J Sport Nutr. 1991 Mar; 1(1):61-8. https://www.ncbi.nlm.nih.gov/pubmed/1844403/[↩]
- Improved cycling time-trial performance after ingestion of a caffeine energy drink. Ivy JL, Kammer L, Ding Z, Wang B, Bernard JR, Liao YH, Hwang J. Int J Sport Nutr Exerc Metab. 2009 Feb; 19(1):61-78. https://www.ncbi.nlm.nih.gov/pubmed/19403954/[↩]
- The effects of caffeine ingestion on time trial cycling performance. McNaughton LR, Lovell RJ, Siegler J, Midgley AW, Moore L, Bentley DJ. Int J Sports Physiol Perform. 2008 Jun; 3(2):157-63. https://www.ncbi.nlm.nih.gov/pubmed/19208924/[↩]
- Carr A, Dawson B, Schneiker K, Goodman C, Lay B. Effect of caffeine supplementation on repeated sprint running performance. J Sports Med Phys Fitness. 2008;48:472–478. https://www.ncbi.nlm.nih.gov/pubmed/18997650[↩]
- Glaister M, Howatson G, Abraham CS, Lockey RA, Goodwin JE, Foley P, McInnes G. Caffeine supplementation and multiple sprint running performance. Med Sci Sports Exerc. 2008;40:1835–1840. doi: 10.1249/MSS.0b013e31817a8ad2. https://www.ncbi.nlm.nih.gov/pubmed/18799995[↩]
- Green JM, Wickwire PJ, McLester JR, Gendle S, Hudson G, Pritchett RC, Laurent CM. Effects of caffeine on repetitions to failure and ratings of perceived exertion during resistance training. Int J Sports Physiol Perform. 2007;2:250–259. https://www.ncbi.nlm.nih.gov/pubmed/19168925[↩]
- Woolf K, Bidwell WK, Carlson AG. The effect of caffeine as an ergogenic aid in anaerobic exercise. Int J Sport Nutr Exerc Metab. 2008;18:412–429. https://www.ncbi.nlm.nih.gov/pubmed/18708685[↩]
- Duncan MJ, Oxford SW. The effect of caffeine ingestion on mood state and bench press performance to failure. J Strength Cond Res. 2011;25:178–185. doi: 10.1519/JSC.0b013e318201bddb. https://www.ncbi.nlm.nih.gov/pubmed/21157384[↩]
- Williams AD, Cribb PJ, Cooke MB, Hayes A. The effect of ephedra and caffeine on maximal strength and power in resistance-trained athletes. J Strength Cond Res. 2008;22:464–470. doi: 10.1519/JSC.0b013e3181660320. https://www.ncbi.nlm.nih.gov/pubmed/18550961[↩]
- Hendrix CR, Housh TJ, Mielke M, Zuniga JM, Camic CL, Johnson GO, Schmidt RJ, Housh DJ. Acute effects of a caffeine-containing supplement on bench press and leg extension strength and time to exhaustion during cycle ergometry. J Strength Cond Res. 2010;24:859–865. doi: 10.1519/JSC.0b013e3181ae7976. https://www.ncbi.nlm.nih.gov/pubmed/19834348[↩]
- Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M. Effects of caffeine on human health. Food Addit Contam. 2003;20:1–30. https://www.ncbi.nlm.nih.gov/pubmed/12519715[↩]
- Tarnopolsky MA, Atkinson SA, MacDougall JD, Sale DG, Sutton JR. Physiological responses to caffeine during endurance running in habitual caffeine users. Med Sci Sports Exerc. 1989;21:418–424. https://www.ncbi.nlm.nih.gov/pubmed/2674593[↩]
- Artioli GG, Gualano B, Smith A, Stout J, Lancha AH Jr. Role of beta-alanine supplementation on muscle carnosine and exercise performance. Med Sci Sports Exerc. 2010;42:1162–1173. https://www.ncbi.nlm.nih.gov/pubmed/20479615[↩]
- Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006;30:279–289. doi: 10.1007/s00726-006-0299-9. https://www.ncbi.nlm.nih.gov/pubmed/16554972[↩][↩]
- Hobson RM, Saunders B, Ball G, Harris RC, Sale C. Effects of beta-alanine supplementation on exercise performance: a meta-analysis. . Amino Acids. 2012;43:25–37. doi: 10.1007/s00726-011-1200-z. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3374095/[↩]
- Lu P, Xu W, Sturman JA. Dietary beta-alanine results in taurine depletion and cerebellar damage in adult cats. J Neurosci Res. 1996;43:112–119. doi: 10.1002/jnr.490430115. https://www.ncbi.nlm.nih.gov/pubmed/8838582[↩]
- Smith HJ, Mukerji P, Tisdale MJ. Attenuation of proteasome-induced proteolysis in skeletal muscle by {beta}-hydroxy-{beta}-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005;65:277–283. https://www.ncbi.nlm.nih.gov/pubmed/15665304[↩]
- Eley HL, Russell ST, Baxter JH, Mukerji P, Tisdale MJ. Signaling pathways initiated by beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab. 2007;293:E923–E931. doi: 10.1152/ajpendo.00314.2007. https://www.ncbi.nlm.nih.gov/pubmed/17609254[↩]
- Rathmacher JA, Nissen S, Panton L, Clark RH, Eubanks May P, Barber AE, D’Olimpio J, Abumrad NN. Supplementation with a combination of beta-hydroxy-beta-methylbutyrate (HMB), arginine, and glutamine is safe and could improve hematological parameters. JPEN J Parenter Enteral Nutr. 2004;28:65–75. doi: 10.1177/014860710402800265. https://www.ncbi.nlm.nih.gov/pubmed/15080599[↩][↩]
- Gallagher PM, Carrithers JA, Godard MP, Schulze KE, Trappe SW. Beta-hydroxy-beta-methylbutyrate ingestion, part II: effects on hematology, hepatic and renal function. Med Sci Sports Exerc. 2000;32:2116–2119. doi: 10.1097/00005768-200012000-00023. https://www.ncbi.nlm.nih.gov/pubmed/11128860[↩]
- Fitschen PJ, Wilson GJ, Wilson JM, Wilund KR. Efficacy of beta-hydroxy-beta-methylbutyrate supplementation in elderly and clinical populations. Nutrition. 2013;29:29–36. doi: 10.1016/j.nut.2012.05.005. https://www.ncbi.nlm.nih.gov/pubmed/23085015[↩]
- Wilson GJ, Wilson JM, Manninen AH. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: a review. Nutr Metab (Lond) 2008;5:1. doi: 10.1186/1743-7075-5-1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2245953/[↩]
- Wilson J, Fitschen P, Campbell B, Wilson G, Zanchi N, Taylor L, Wilborn C, Kalman D, Stout J, Hoffman J, Ziegenfuss T, Lopez H, Kreider R, Smith-Ryan A, Antonio J. International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB) J Int Soc Sports Nutr. 2013;10:6. doi: 10.1186/1550-2783-10-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3568064/[↩]
- University of Maryland Medical Center. Creatine. http://www.umm.edu/health/medical/altmed/supplement/creatine[↩][↩][↩]
- Cooper R, Naclerio F, Allgrove J, Jimenez A. Creatine supplementation with specific view to exercise/sports performance: an update. Journal of the International Society of Sports Nutrition. 2012;9:33. doi:10.1186/1550-2783-9-33. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3407788/[↩][↩][↩][↩][↩]
- Clinical pharmacology of the dietary supplement creatine monohydrate. Persky AM, Brazeau GA. Pharmacol Rev. 2001 Jun; 53(2):161-76. https://www.ncbi.nlm.nih.gov/pubmed/11356982/[↩][↩][↩]
- Brunzel NA. Renal function: Nonprotein nitrogen compounds, function tests, and renal disease. In: Scardiglia J, Brown M, McCullough K, Davis K, editor. Clinical Chemistry. McGraw-Hill: New York, NY; 2003. pp. 373–399.[↩][↩][↩]
- Potential ergogenic effects of arginine and creatine supplementation. Paddon-Jones D, Børsheim E, Wolfe RR. J Nutr. 2004 Oct; 134(10 Suppl):2888S-2894S; discussion 2895S. http://jn.nutrition.org/content/134/10/2888S.long[↩]
- Creatine in humans with special reference to creatine supplementation. Balsom PD, Söderlund K, Ekblom B. Sports Med. 1994 Oct; 18(4):268-80. https://www.ncbi.nlm.nih.gov/pubmed/7817065/[↩][↩]
- Muscle creatine loading in men. Hultman E, Söderlund K, Timmons JA, Cederblad G, Greenhaff PL. J Appl Physiol (1985). 1996 Jul; 81(1):232-7. https://www.ncbi.nlm.nih.gov/pubmed/8828669/[↩]
- Buford TW, Kreider RB, Stout JR, et al. International Society of Sports Nutrition position stand: creatine supplementation and exercise. Journal of the International Society of Sports Nutrition. 2007;4:6. doi:10.1186/1550-2783-4-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2048496/[↩]
- The metabolic burden of creatine synthesis. Brosnan JT, da Silva RP, Brosnan ME. Amino Acids. 2011 May; 40(5):1325-31. https://www.ncbi.nlm.nih.gov/pubmed/21387089/[↩][↩]
- Hultman E, Bergstrom J, Spreit L, Soderlund K. Energy metabolism and fatigue. In: Taylor A, Gollnick PD, Green H, editor. Biochemistry of Exercise VII. Human Kinetics: Champaign, IL; 1990. pp. 73–92.[↩][↩][↩][↩]
- Greenhaff P. The nutritional biochemistry of creatine. J Nutrit Biochem. 1997;11:610–618. doi: 10.1016/S0955-2863(97)00116-2.[↩]
- Creatine supplementation and exercise performance: an update. Williams MH, Branch JD. J Am Coll Nutr. 1998 Jun; 17(3):216-34. https://www.ncbi.nlm.nih.gov/pubmed/9627907/[↩]
- Williams MH, Kreider R, Branch JD. Creatine: The power supplement. Champaign, IL: Human Kinetics Publishers; 1999. p. 252.[↩]
- Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Burke DG, Candow DG, Chilibeck PD, MacNeil LG, Roy BD, Tarnopolsky MA, Ziegenfuss T. Int J Sport Nutr Exerc Metab. 2008 Aug; 18(4):389-98. https://www.ncbi.nlm.nih.gov/pubmed/18708688/[↩]
- Chanutin A. The fate of creatine when administered to man. J Biol Chem. 1926;67:29–34.[↩][↩][↩]
- Buford T, Kreider R, Stout J, Greenwood M, Campbell B, Spano M, Ziegenfuss T, Lopez H, Landis J, Antonio J. International Society of Sports Nutrition position stand: creatine supplementation and exercise. J Int Soc Sports Nutr. 2007;4:6. doi: 10.1186/1550-2783-4-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2048496/[↩][↩][↩][↩][↩]
- American College of Sport Medicine. Round Table, the physiological and health effects of oral creatine supplementation. Med Sci Sports Exc. 2000;32:706–717. https://www.ncbi.nlm.nih.gov/pubmed/10731017[↩][↩]
- Branch JD. Effects of creatine supplementation on body composition and performace: a meta análisis. Int J Sports Nutr Exerc Metabol. 2003;13:I198-122. https://www.ncbi.nlm.nih.gov/pubmed/12945830[↩]
- Rawson ES, Volek JS. Effects of creatine supplementation and resistance training on muscle strength and weightlifting performance. J Strength Cond Res. 2003;17:822–831. https://www.ncbi.nlm.nih.gov/pubmed/14636102[↩][↩]
- Volek JS, Kraemer WJ. Creatine suplemetation: its effects on human muscular performance and body composition. J Strength Cond Res. 1996;10:200–210.[↩]
- Syrotuik DG, Bell GJ. Acute creatine monohydrate supplementation: a descriptive physiological profile of responders vs. nonresponders. J Strength Cond Res. 2004;18:610–617. https://www.ncbi.nlm.nih.gov/pubmed/15320650[↩]
- Greenhaff PL, Bodin K, Soderlund K, Hultman E. Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. Am J Physiol. 1994;266:E725–E730. https://www.ncbi.nlm.nih.gov/pubmed/8203511[↩]
- Casey A, Greenhaff P. Does dietary creatine supplementation play a role in skeletal muscle metabolism and performance? Am J Clin Nutr. 2000;72:607S–617S. https://www.ncbi.nlm.nih.gov/pubmed/10919967[↩][↩][↩]
- Cribb PJ, Williams AD, Hayes A. A creatine-protein-carbohydrate supplement enhances responses to resistance training. Med Sci Sports Exerc. 2007;39:1960–1968. doi: 10.1249/mss.0b013e31814fb52a. https://www.ncbi.nlm.nih.gov/pubmed/17986903[↩][↩]
- Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol. 2001;91:1041–1047. https://www.ncbi.nlm.nih.gov/pubmed/11509496[↩]
- Louis M, Poortmans JR, Francaux M, Hultman E, Berre J, Boisseau N, Young VR, Smith K, Meier-Augenstein W, Babraj JA. et al. Creatine supplementation has no effect on human muscle protein turnover at rest in the postabsorptive or fed states. Am J Physiol Endocrinol Metab. 2003;284:E764–E770. https://www.ncbi.nlm.nih.gov/pubmed/12475751[↩]
- Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie M, Francaux M. Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol. 2008;104:371–378. https://www.ncbi.nlm.nih.gov/pubmed/18048590[↩]
- Burke DG, Candow DG, Chilibeck PD, MacNeil LG, Roy BD, Tarnopolsky MA, Ziegenfuss T. Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Int J Sport Nutr Exerc Metab. 2008;18:389–398. https://www.ncbi.nlm.nih.gov/pubmed/18708688[↩][↩]
- Harp JB, Goldstein S, Phillips LS. Nutrition and somatomedin. XXIII. Molecular regulation of IGF-I by amino acid availability in cultured hepatocytes. Diabetes. 1991;40:95–101. doi: 10.2337/diabetes.40.1.95. https://www.ncbi.nlm.nih.gov/pubmed/1901809[↩]
- Op ‘t Eijnde B, Urso B, Richter EA, Greenhaff PL, Hespel P. Effect of oral creatine supplementation on human muscle GLUT4 protein content after immobilization. Diabetes. 2001;50:18–23. doi: 10.2337/diabetes.50.1.18. https://www.ncbi.nlm.nih.gov/pubmed/11147785[↩][↩][↩]
- Cooke MB, Rybalka E, Williams AD, Cribb PJ, Hayes A. Creatine supplementation enhances muscle force recovery after eccentrically-induced muscle damage in healthy individuals. J Int Soc Sports Nutr. 2009;6:13. doi: 10.1186/1550-2783-6-13. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2697134/[↩]
- Lawler JM, Barnes WS, Wu G, Song W, Demaree S. Direct antioxidant properties of creatine. Biochem Biophys Res Commun. 2002;290:47–52. doi: 10.1006/bbrc.2001.6164. https://www.ncbi.nlm.nih.gov/pubmed/11779131[↩][↩]
- Sestili P, Martinelli C, Bravi G, Piccoli G, Curci R, Battistelli M, Falcieri E, Agostini D, Gioacchini AM, Stocchi V. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med. 2006;40:837–849. doi: 10.1016/j.freeradbiomed.2005.10.035. https://www.ncbi.nlm.nih.gov/pubmed/16520236[↩]
- Rahimi R. Creatine supplementation decreases oxidative DNA damage and lipid peroxidation induced by a single bout of resistance exercise. J Strength Cond Res. 2011;25:3448–3455. doi: 10.1519/JSC.0b013e3182162f2b. https://www.ncbi.nlm.nih.gov/pubmed/22080314[↩]
- Hespel P, Derave W. Ergogenic effects of creatine in sports and rehabilitation. Subcell Biochem. 2007;46:245–259. doi: 10.1021/bi061646s. https://www.ncbi.nlm.nih.gov/pubmed/18652080[↩][↩][↩]
- Nelson A, Arnall D, Kokkonen J, Day R, Evans J. Muscle glycogen supercompensation is enhanced by prior creatine supplementation. Med Sci Sports Exerc. 2001;33:1096–1100. https://www.ncbi.nlm.nih.gov/pubmed/11445755[↩]
- Sewell D, Robinson T, Greenhaff P. Creatine supplementation does not affect human skeletal muscle glycogen content in the absence of prior exercise. J Appl Physiol. 2008;104:508–512. https://www.ncbi.nlm.nih.gov/pubmed/18032580[↩]
- Hickner R, Dyck D, Sklar J, Hatley H, Byrd P. Effect of 28 days of creatine ingestion on muscle metabolism and performance of a simulated cycling road race. J Int Soc Sports Nutr. 2010;7:26. doi: 10.1186/1550-2783-7-26. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2909923/[↩][↩][↩]
- Branch J. Effect of creatine supplementation on body composition and performance: a meta-analysis. Int J Sport Nutr Exerc Metab. 2003;13:198–226. https://www.ncbi.nlm.nih.gov/pubmed/12945830[↩][↩][↩][↩][↩][↩]
- Chwalbiñska-Moneta J. Effect of creatine supplementation on aerobic performance and anaerobic capacity in elite rowers in the course of endurance training. Int J Sport Nutr Exerc Metab. 2003;13:173–183. https://www.ncbi.nlm.nih.gov/pubmed/12945828[↩][↩]
- Graef J, Smith A, Kendall K, Fukuda D, Moon J, Beck T, Cramer J, Stout J. The effects of four weeks of creatine supplementation and high-intensity interval training on cardiorespiratory fitness: a randomized controlled trial. J Int Soc Sports Nutr. 2009;6:18. doi: 10.1186/1550-2783-6-18. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2780977/[↩][↩]
- Thompson C, Kemp G, Sanderson A, Dixon R, Styles P, Taylor D, Radda G. Effect of creatine on aerobic and anaerobic metabolism in skeletal muscle in swimmers. Br J Sports Med. 1996;30:222–225. doi: 10.1136/bjsm.30.3.222. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1332335/[↩][↩]
- Volek J, Duncan N, Mazzetti S, Staron R, Putukian M, Gómez A, Pearson D, Fink W, Kraemer W. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Exerc. 1999;31:1147–1156. doi: 10.1097/00005768-199908000-00011. https://www.ncbi.nlm.nih.gov/pubmed/10449017[↩]
- Volek J, Rawson E. Scientific basis and practical aspects of creatine supplementation for athletes. Nutrition. 2004;20:609–614. doi: 10.1016/j.nut.2004.04.014. https://www.ncbi.nlm.nih.gov/pubmed/15212742[↩]
- van Loon L, Oosterlaar A, Hartgens F, Hesselink M, Snow R, Wagenmakers A. Effects of creatine loading and prolonged creatine supplementation on body composition, fuel selection, sprint and endurance performance in humans. Clin Sci (Lond) 2003;104:153–162. doi: 10.1042/CS20020159. https://www.ncbi.nlm.nih.gov/pubmed/12546637[↩]
- Kreider RB. Effects of creatine supplementation on performance and training adaptations. Mol Cell Biochem. 2003;244:89–94. doi: 10.1023/A:1022465203458. https://www.ncbi.nlm.nih.gov/pubmed/12701815[↩]
- Jakobi J, Rice C, Curtin S, Marsh G. Contractile properties, fatigue and recovery are not influenced by short-term creatine supplementation in human muscle. Exp Physiol. 2000;85:451–460. doi: 10.1017/S0958067000020212. https://www.ncbi.nlm.nih.gov/pubmed/10918084[↩]
- Bemben MG, Witten MS, Carter JM, Eliot KA, Knehans AW, Bemben DA. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J Nutr Health Aging. 2010;14:155–159. doi: 10.1007/s12603-009-0124-8. https://www.ncbi.nlm.nih.gov/pubmed/20126965[↩]
- Safdar A, Yardley N, Snow R, Melov S, Tarnopolsky M. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol Genomics. 2008;32:219–228. https://www.ncbi.nlm.nih.gov/pubmed/17957000[↩][↩]
- Saremi A, Gharakhanloo R, Sharghi S, Gharaati M, Larijani B, Omidfar K. Effects of oral creatine and resistance training on serum myostatin and GASP-1. Mol Cell Endocrinol. 2010;317:25–30. doi: 10.1016/j.mce.2009.12.019. https://www.ncbi.nlm.nih.gov/pubmed/20026378[↩]
- Bazzucchi I, Felici F, Sacchetti M. Effect of short-term creatine supplementation on neuromuscular function. Med Sci Sports Exerc. 2009;41:1934–41. doi: 10.1249/MSS.0b013e3181a2c05c. https://www.ncbi.nlm.nih.gov/pubmed/19727018[↩]
- Sculthorpe N, Grace F, Jones P, Fletcher I. The effect of short-term creatine loading on active range of movement. Appl Physiol Nutr Metab. 2010;35:507–511. doi: 10.1139/H10-036. https://www.ncbi.nlm.nih.gov/pubmed/20725117[↩][↩]
- Hile A, Anderson J, Fiala K, Stevenson J, Casa D, Maresh C. Creatine supplementation and anterior compartment pressure during exercise in the heat in dehydrated men. J Athl Train. 2006;41:30–35. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1421498/[↩]
- Metzl JD, Small E, Levine SR, Gershel JC. Creatine use among young athletes. Pediatrics. 2001;108:421–425. doi: 10.1542/peds.108.2.421. https://www.ncbi.nlm.nih.gov/pubmed/11483809[↩][↩]
- Evans MW, Ndetan H, Perko M, Williams R, Walker C. Dietary supplement use by children and adolescents in the United States to Enhance sport performance: results of the national health interview survey. J Prim Prev. 2012;33:3–12. doi: 10.1007/s10935-012-0261-4. https://www.ncbi.nlm.nih.gov/pubmed/22297456[↩][↩]
- Unnithan VB, Veehof SH, Vella CA, Kern M. Is there a physiologic basis for creatine use in children and adolescents? J Strength Cond Res. 2001;15:524–528. https://www.ncbi.nlm.nih.gov/pubmed/11726268[↩][↩]
- Yoshizumi W, Tsourounis C. Effects of creatine supplementation on renal function. J Herb Pharmacother. 2004;4:1–7. https://www.ncbi.nlm.nih.gov/pubmed/15273072[↩][↩]
- Thorsteinsdottir B, Grande J, Garovic V. Acute renal failure in a young weight lifter taking multiple food supplements, including creatine monohydrate. J Ren Nutr. 2006;16:341–345. doi: 10.1053/j.jrn.2006.04.025. https://www.ncbi.nlm.nih.gov/pubmed/17046619[↩][↩][↩]
- Bizzarini E, De Angelis L. Is the use of oral creatine supplementation safe? J Sports Med Phys Fitness. 2004;44:411–416. https://www.ncbi.nlm.nih.gov/pubmed/15758854[↩]
- Poortmans J, Francaux M. Adverse effects of creatine supplementation: fact or fiction? Sports Med. 2000;30:155–170. doi: 10.2165/00007256-200030030-00002. https://www.ncbi.nlm.nih.gov/pubmed/10999421[↩]
- Pline K, Smith C. The effect of creatine intake on renal function. Ann Pharmacother. 2005;39:1093–1096. doi: 10.1345/aph.1E628. https://www.ncbi.nlm.nih.gov/pubmed/15886291[↩][↩]
- Kim HJ, Kim CK, Carpentier A, Poortmans JR. Studies on the safety of creatine supplementation. Amino Acids. 2011;40:1409–1418. doi: 10.1007/s00726-011-0878-2. https://www.ncbi.nlm.nih.gov/pubmed/21399917[↩][↩][↩][↩]
- Sale C, Harris RC, Florance J, Kumps A, Sanvura R, Poortmans JR. Urinary creatine and methylamine excretion following 4 x 5 g x day(-1) or 20 x 1 g x day(-1) of creatine monohydrate for 5 days. J Sports Sci. 2009;27:759–766. doi: 10.1080/02640410902838237. https://www.ncbi.nlm.nih.gov/pubmed/19437189[↩]
- Tarnopolsky M, Zimmer A, Paikin J, Safdar A, Aboud A, Pearce E, Roy B, Doherty T. Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS One. 2007;2:e991. doi: 10.1371/journal.pone.0000991. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1994592/[↩]
- Cornelissen VA, Defoor JG, Stevens A, Schepers D, Hespel P, Decramer M, Mortelmans L, Dobbels F, Vanhaecke J, Fagard RH, Vanhees L. Effect of creatine supplementation as a potential adjuvant therapy to exercise training in cardiac patients: a randomized controlled trial. Clin Rehabil. 2010;24:988–999. doi: 10.1177/0269215510367995. https://www.ncbi.nlm.nih.gov/pubmed/20576665[↩]
- Schilling B, Stone M, Utter A, Kearney J, Johnson M, Coglianese R, Smith L, O’Bryant H, Fry A, Starks M. et al. Creatine supplementation and health variables: a retrospective study. Med Sci Sports Exerc. 2001;33:183–188. https://www.ncbi.nlm.nih.gov/pubmed/11224803[↩]
- Dalbo V, Roberts M, Stout J, Kerksick C. Putting to rest the myth of creatine supplementation leading to muscle cramps and dehydration. Br J Sports Med. 2008;42:567–573. doi: 10.1136/bjsm.2007.042473. https://www.ncbi.nlm.nih.gov/pubmed/18184753[↩]
- Watson G, Casa D, Fiala K, Hile A, Roti M, Healey J, Armstrong L, Maresh C. Creatine use and exercise heat tolerance in dehydrated men. J Athl Train. 2006;41:18–29. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1421496/[↩]
- Lopez R, Casa D, McDermott B, Ganio M, Armstrong L, Maresh C. Does creatine supplementation hinder exercise heat tolerance or hydration status? A systematic review with meta-analyses. J Athl Train. 2009;44:215–223. doi: 10.4085/1062-6050-44.2.215. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2657025/[↩]
- Hadjicharalambous M, Kilduff L, Pitsiladis Y. Brain serotonin and dopamine modulators, perceptual responses and endurance performance during exercise in the heat following creatine supplementation. J Int Soc Sports Nutr. 2008;5:14. doi: 10.1186/1550-2783-5-14. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2570654/[↩]
- Persky A, Brazeau G. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev. 2001;53:161–176. https://www.ncbi.nlm.nih.gov/pubmed/11356982[↩]
- Dempsey R, Mazzone M, Meurer L. Does oral creatine supplementation improve strength? A meta-analysis. J Fam Pract. 2002;51:945–951. https://www.ncbi.nlm.nih.gov/pubmed/12485548[↩]
- Ganguly S, Jayappa S, Dash AK. Evaluation of the stability of creatine in solution prepared from effervescent creatine formulations. AAPS PharmSciTech. 2003;4:E25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2750587/[↩]
- Jäger R, Harris RC, Purpura M, Francaux M. Comparison of new forms of creatine in raising plasma creatine levels. J Int Soc Sports Nutr. 2007;4:17. doi: 10.1186/1550-2783-4-17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2206055/[↩]
- Jäger R, Metzger J, Lautmann K, Shushakov V, Purpura M, Geiss K, Maassen N. The effects of creatine pyruvate and creatine citrate on performance during high intensity exercise. J Int Soc Sports Nutr. 2008;5:4. doi: 10.1186/1550-2783-5-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2276184/[↩]
- Jäger R, Purpura M, Shao A, Inoue T, Kreider RB. Analysis of the efficacy, safety, and regulatory status of novel forms of creatine. Amino Acids. 2011;40:1369–83. doi: 10.1007/s00726-011-0874-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3080578/[↩][↩]
- Gufford BT, Sriraghavan K, Miller N, Miller D, Gu X, Vennerstrom J, Robinson D. Physicochemical characterization of creatine N-methylguanidinium salts. Journal of Dietary Supplements. 2010;7:240–252. doi: 10.3109/19390211.2010.491507. https://www.ncbi.nlm.nih.gov/pubmed/22432515[↩][↩]
- Persky AM, Brazeau GA, Hochhaus G. Pharmacokinetics of the dietary supplement creatine. Clin Pharmacokinet. 2003;42:557–574. doi: 10.2165/00003088-200342060-00005. https://www.ncbi.nlm.nih.gov/pubmed/12793840[↩]
- Spillane M, Schoch R, Cooke M, Harvey T, Greenwood M, Kreider R, Willoughby DS. The effects of creatine ethyl ester supplementation combined with heavy resistance training on body composition, muscle performance, and serum and muscle creatine levels. J Int Soc Sports Nutr. 2009;6:6. doi: 10.1186/1550-2783-6-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2649889/[↩][↩][↩]
- Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49:6288–6308. doi: 10.1002/anie.200902672. https://www.ncbi.nlm.nih.gov/pubmed/20648499[↩]
- Camic CL, Hendrix CR, Housh TJ, Zuniga JM, Mielke M, Johnson GO, Schmidt RJ, Housh DJ. The effects of polyethylene glycosylated creatine supplementation on muscular strength and power. J Strength Cond Res. 2010;24:3343–3351. doi: 10.1519/JSC.0b013e3181fc5c5c. https://www.ncbi.nlm.nih.gov/pubmed/21068676[↩]
- Herda TJ, Beck TW, Ryan ED, Smith AE, Walter AA, Hartman MJ, Stout JR, Cramer JT. Effects of creatine monohydrate and polyethylene glycosylated creatine supplementation on muscular strength, endurance, and power output. J Strength Cond Res. 2009;23:818–826. doi: 10.1519/JSC.0b013e3181a2ed11. https://www.ncbi.nlm.nih.gov/pubmed/19387397[↩]
- Steenge GR, Lambourne J, Casey A, Macdonald IA, Greenhaff PL. Stimulatory effect of insulin on creatine accumulation in human skeletal muscle. Am J Physiol. 1998;275:E974–E979. https://www.ncbi.nlm.nih.gov/pubmed/9843739[↩]
- Steenge G, Simpson E, Greenhaff P. Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans. J Appl Physiol. 2000;89:1165–1171. https://www.ncbi.nlm.nih.gov/pubmed/10956365[↩]
- Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. 2006;573:525–534. doi: 10.1113/jphysiol.2006.107359. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1779717/[↩]
- Walsh AL, Gonzalez AM, Ratamess NA, Kang J, Hoffman JR. Improved time to exhaustion following ingestion of the energy drink Amino Impact. J Int Soc Sports Nutr. 2010;7:14. doi: 10.1186/1550-2783-7-14. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2861014/[↩][↩]
- Jackman SR, Witard OC, Philp A, Wallis GA, Baar K, Tipton KD. Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans. Frontiers in Physiology. 2017;8:390. doi:10.3389/fphys.2017.00390. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5461297/[↩]
- Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Am J Physiol. 1998 Jul; 275(1 Pt 1):E73-8. https://www.ncbi.nlm.nih.gov/pubmed/9688876/[↩]
- Postexercise net protein synthesis in human muscle from orally administered amino acids. Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR. Am J Physiol. 1999 Apr; 276(4 Pt 1):E628-34. https://www.ncbi.nlm.nih.gov/pubmed/10198297/[↩]
- Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. Tipton KD, Gurkin BE, Matin S, Wolfe RR. J Nutr Biochem. 1999 Feb; 10(2):89-95. https://www.ncbi.nlm.nih.gov/pubmed/15539275/[↩]
- Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. Kimball SR, Jefferson LS. J Nutr. 2006 Jan; 136(1 Suppl):227S-31S. https://www.ncbi.nlm.nih.gov/pubmed/16365087/[↩][↩]
- Oral leucine administration stimulates protein synthesis in rat skeletal muscle. Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. J Nutr. 2005 Mar; 135(3):376-82. https://www.ncbi.nlm.nih.gov/pubmed/15735066/[↩]
- Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ. J Physiol. 2013 Jun 1; 591(11):2911-23. https://www.ncbi.nlm.nih.gov/pubmed/23551944/[↩]
- Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. Philp A, Hamilton DL, Baar K. J Appl Physiol (1985). 2011 Feb; 110(2):561-8. https://www.ncbi.nlm.nih.gov/pubmed/21071597/[↩]
- Activation of mTORC1 by leucine is potentiated by branched-chain amino acids and even more so by essential amino acids following resistance exercise. Moberg M, Apró W, Ekblom B, van Hall G, Holmberg HC, Blomstrand E. Am J Physiol Cell Physiol. 2016 Jun 1; 310(11):C874-84. https://www.ncbi.nlm.nih.gov/pubmed/27053525/[↩][↩][↩]
- Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, Phillips SM. Am J Clin Nutr. 2014 Feb; 99(2):276-86. https://www.ncbi.nlm.nih.gov/pubmed/24284442/[↩]
- Absence of leucine in an essential amino acid supplement reduces activation of mTORC1 signalling following resistance exercise in young females. Moberg M, Apró W, Ohlsson I, Pontén M, Villanueva A, Ekblom B, Blomstrand E. Appl Physiol Nutr Metab. 2014 Feb; 39(2):183-94. https://www.ncbi.nlm.nih.gov/pubmed/24476474/[↩]
- The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Macnaughton LS, Wardle SL, Witard OC, McGlory C, Hamilton DL, Jeromson S, Lawrence CE, Wallis GA, Tipton KD. Physiol Rep. 2016 Aug; 4(15): https://www.ncbi.nlm.nih.gov/pubmed/27511985/[↩]
- Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. McGlory C, Wardle SL, Macnaughton LS, Witard OC, Scott F, Dick J, Bell JG, Phillips SM, Galloway SD, Hamilton DL, Tipton KD. Physiol Rep. 2016 Mar; 4(6): https://www.ncbi.nlm.nih.gov/pubmed/27009278/[↩]
- Shimomura Y, Yamamoto Y, Bajotto G, Sato J, Murakami T, Shimomura N, Kobayashi H, Mawatari K. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J Nutr. 2006;136:529S–532S. https://www.ncbi.nlm.nih.gov/pubmed/16424141[↩]
- Garlick PJ, Grant I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J. 1988;254:579–584. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1135117/[↩]
- Balage M, Dardevet D. Long-term effects of leucine supplementation on body composition. Curr Opin Clin Nutr Metab Care. 2010;13:265–270. doi: 10.1097/MCO.0b013e328336f6b8. https://www.ncbi.nlm.nih.gov/pubmed/20110810[↩]
- Pencharz PB, Elango R, Ball RO. Determination of the tolerable upper intake level of leucine in adult men. J Nutr. 2012;142:2220S–2224S. doi: 10.3945/jn.112.160259. https://www.ncbi.nlm.nih.gov/pubmed/23077191[↩]
- Louard RJ, Barrett EJ, Gelfand RA. Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin Sci. 1990;79:457–466. https://www.ncbi.nlm.nih.gov/pubmed/2174312[↩]
- Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–E657. https://www.ncbi.nlm.nih.gov/pubmed/12217881[↩]
- Stoppani J, Scheett T, Pena J, Rudolph C, Charlebois D. Consuming a supplement containing branched-chain amino acids during a resistance-traning program increases lean mass, muscle strength, and fat loss. J Int Soc Sports Nutr. 2009;6:P1. doi: 10.1186/1550-2783-6-S1-P1.[↩][↩][↩][↩]
- Wilson GJ, Layman DK, Moulton CJ, Norton LE, Anthony TG, Proud CG, Rupassara SI, Garlick PJ. Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab. 2011;301:E1236–E1242. doi: 10.1152/ajpendo.00242.2011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4395871/[↩]
- Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68:72–81. https://www.ncbi.nlm.nih.gov/pubmed/9665099[↩]
- Fahs CA, Heffernan KS, Fernhall B. Hemodynamic and vascular response to resistance exercise with L-arginine. Med Sci Sports Exerc. 2009;41:773–779. doi: 10.1249/MSS.0b013e3181909d9d. https://www.ncbi.nlm.nih.gov/pubmed/19276857[↩]
- Tang JE, Lysecki PJ, Manolakos JJ, MacDonald MJ, Tarnopolsky MA, Phillips SM. Bolus arginine supplementation affects neither muscle blood flow nor muscle protein synthesis in young men at rest or after resistance exercise. J Nutr. 2011;141:195–200. doi: 10.3945/jn.110.130138. https://www.ncbi.nlm.nih.gov/pubmed/21191143[↩]
- Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3192452/[↩]
- Alvares TS, Meirelles CM, Bhambhani YN, Paschoalin VM, Gomes PS. L-Arginine as a potential ergogenic aid in healthy subjects. Sports Med. 2011;41:233–248. doi: 10.2165/11538590-000000000-00000. https://www.ncbi.nlm.nih.gov/pubmed/21395365[↩]
- Greer BK, Jones BT. Acute arginine supplementation fails to improve muscle endurance or affect blood pressure responses to resistance training. J Strength Cond Res. 2011;25:1789–1794. doi: 10.1519/JSC.0b013e3181e07569. https://www.ncbi.nlm.nih.gov/pubmed/21399536[↩]
- McConell GK. Effects of L-arginine supplementation on exercise metabolism. Curr Opin Clin Nutr Metab Care. 2007;10:46–51. doi: 10.1097/MCO.0b013e32801162fa. https://www.ncbi.nlm.nih.gov/pubmed/17143054[↩]
- Shao A, Hathcock JN. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul Toxicol Pharmacol. 2008;50:376–399. doi: 10.1016/j.yrtph.2008.01.004. https://www.ncbi.nlm.nih.gov/pubmed/18325648[↩][↩]
- Perez-Guisado J, Jakeman PM. Citrulline malate enhances athletic anaerobic performance and relieves muscle soreness. J Strength Cond Res. 2010;24:1215–1222. doi: 10.1519/JSC.0b013e3181cb28e0. https://www.ncbi.nlm.nih.gov/pubmed/20386132[↩][↩]
- Sureda A, Cordova A, Ferrer MD, Perez G, Tur JA, Pons A. L-citrulline-malate influence over branched chain amino acid utilization during exercise. Eur J Appl Physiol. 2010;110:341–351. doi: 10.1007/s00421-010-1509-4. https://www.ncbi.nlm.nih.gov/pubmed/20499249[↩]
- Hickner RC, Tanner CJ, Evans CA, Clark PD, Haddock A, Fortune C, Geddis H, Waugh W, McCammon M. L-citrulline reduces time to exhaustion and insulin response to a graded exercise test. Med Sci Sports Exerc. 2006;38:660–666. doi: 10.1249/01.mss.0000210197.02576.da. https://www.ncbi.nlm.nih.gov/pubmed/16679980[↩]
- Gleeson M. Dosing and efficacy of glutamine supplementation in human exercise and sport training. J Nutr. 2008;138:2045S–2049S. https://www.ncbi.nlm.nih.gov/pubmed/18806122[↩][↩]
- Antonio J, Sanders MS, Kalman D, Woodgate D, Street C. The effects of high-dose glutamine ingestion on weightlifting performance. J Strength Cond Res. 2002;16:157–160. https://www.ncbi.nlm.nih.gov/pubmed/11834123[↩]
- Haub MD, Potteiger JA, Nau KL, Webster MJ, Zebas CJ. Acute L-glutamine ingestion does not improve maximal effort exercise. J Sports Med Phys Fitness. 1998;38:240–244. https://www.ncbi.nlm.nih.gov/pubmed/9830832[↩][↩]
- Colker CM, Swain MA, Fabrucini B, Shi Q, Kalman DS. Effects of supplemental protein on body composition and muscular strength in healthy athletic male adults. Curr Ther Res. 2000;61:19–28. doi: 10.1016/S0011-393X(00)88492-1.[↩]
- Candow DG, Chilibeck PD, Burke DG, Davison KS, Smith-Palmer T. Effect of glutamine supplementation combined with resistance training in young adults. Eur J Appl Physiol. 2001;86:142–149. doi: 10.1007/s00421-001-0523-y. https://www.ncbi.nlm.nih.gov/pubmed/11822473[↩]
- Camilleri M, Madsen K, Spiller R, Van Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. https://www.ncbi.nlm.nih.gov/pubmed/22583600[↩]
- Emerging health properties of whey proteins and their clinical implications. Krissansen GW. J Am Coll Nutr. 2007 Dec; 26(6):713S-23S. https://www.ncbi.nlm.nih.gov/pubmed/18187438/[↩]
- Invited review: physiological properties of bioactive peptides obtained from whey proteins. Madureira AR, Tavares T, Gomes AM, Pintado ME, Malcata FX. J Dairy Sci. 2010 Feb; 93(2):437-55. https://www.ncbi.nlm.nih.gov/pubmed/20105516/[↩]
- Protective effect of whey protein hydrolysates on H₂O₂-induced PC12 cells oxidative stress via a mitochondria-mediated pathway. Jin MM, Zhang L, Yu HX, Meng J, Sun Z, Lu RR. Food Chem. 2013 Nov 15; 141(2):847-52. https://www.ncbi.nlm.nih.gov/pubmed/23790857/[↩]
- Whey protein isolate counteracts the effects of a high-fat diet on energy intake and hypothalamic and adipose tissue expression of energy balance-related genes. McAllan L, Keane D, Schellekens H, Roche HM, Korpela R, Cryan JF, Nilaweera KN. Br J Nutr. 2013 Dec 14; 110(11):2114-26. https://www.ncbi.nlm.nih.gov/pubmed/23731955/[↩]
- Effect of whey protein hydrolysates with different molecular weight on fatigue induced by swimming exercise in mice. Liu J, Wang X, Zhao Z. J Sci Food Agric. 2014 Jan 15; 94(1):126-30. https://www.ncbi.nlm.nih.gov/pubmed/23653310/[↩]
- Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. Jakubowicz D, Froy O. J Nutr Biochem. 2013 Jan; 24(1):1-5. https://www.ncbi.nlm.nih.gov/pubmed/22995389/[↩]
- The role of leucine in the regulation of protein metabolism. Garlick PJ. J Nutr. 2005 Jun; 135(6 Suppl):1553S-6S. https://www.ncbi.nlm.nih.gov/pubmed/15930468/[↩]
- Post-exercise carbohydrate plus whey protein hydrolysates supplementation increases skeletal muscle glycogen level in rats. Morifuji M, Kanda A, Koga J, Kawanaka K, Higuchi M. Amino Acids. 2010 Apr; 38(4):1109-15. https://www.ncbi.nlm.nih.gov/pubmed/19593593/[↩]
- Effect of soy- and whey protein-isolate supplemented diet on the redox parameters of trained mice. Elia D, Stadler K, Horváth V, Jakus J. Eur J Nutr. 2006 Aug; 45(5):259-66. https://www.ncbi.nlm.nih.gov/pubmed/16575496/[↩]
- Effects of the dietary amount and source of protein, resistance training and anabolic-androgenic steroids on body weight and lipid profile of rats. Aparicio VA, Sánchez C, Ortega FB, Nebot E, Kapravelou G, Porres JM, Aranda P. Nutr Hosp. 2013 Jan-Feb; 28(1):127-36. https://www.ncbi.nlm.nih.gov/pubmed/23808440/[↩]
- A dipeptide and an amino acid present in whey protein hydrolysate increase translocation of GLUT-4 to the plasma membrane in Wistar rats. Morato PN, Lollo PC, Moura CS, Batista TM, Carneiro EM, Amaya-Farfan J. Food Chem. 2013 Aug 15; 139(1-4):853-9. https://www.ncbi.nlm.nih.gov/pubmed/23561181/[↩]
- Whey proteins are more efficient than casein in the recovery of muscle functional properties following a casting induced muscle atrophy. Martin V, Ratel S, Siracusa J, Le Ruyet P, Savary-Auzeloux I, Combaret L, Guillet C, Dardevet D. PLoS One. 2013; 8(9):e75408. https://www.ncbi.nlm.nih.gov/pubmed/24069411/[↩]
- Whey protein supplementation during resistance training augments lean body mass. Volek JS, Volk BM, Gómez AL, Kunces LJ, Kupchak BR, Freidenreich DJ, Aristizabal JC, Saenz C, Dunn-Lewis C, Ballard KD, Quann EE, Kawiecki DL, Flanagan SD, Comstock BA, Fragala MS, Earp JE, Fernandez ML, Bruno RS, Ptolemy AS, Kellogg MD, Maresh CM, Kraemer WJ. J Am Coll Nutr. 2013; 32(2):122-35. https://www.ncbi.nlm.nih.gov/pubmed/24015719/[↩]
- Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. Appuhamy JA, Knoebel NA, Nayananjalie WA, Escobar J, Hanigan MD. J Nutr. 2012 Mar; 142(3):484-91. https://www.ncbi.nlm.nih.gov/pubmed/22298573/[↩]
- Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Farup J, Rahbek SK, Vendelbo MH, Matzon A, Hindhede J, Bejder A, Ringgard S, Vissing K. Scand J Med Sci Sports. 2014 Oct; 24(5):788-98. https://www.ncbi.nlm.nih.gov/pubmed/23647357/[↩]
- In vitro and in vivo safety studies of a proprietary whey extract. Dyer AR, Burdock GA, Carabin IG, Haas MC, Boyce J, Alsaker R, Read LC. Food Chem Toxicol. 2008 May; 46(5):1659-65. https://www.ncbi.nlm.nih.gov/pubmed/18295388/[↩]
- Acute and repeated dose (28 days) oral safety studies of ALIBIRD in rats. Anadón A, Martínez MA, Ares I, Castellano V, Martínez-Larrañaga MR, Corzo N, Olano A, Montilla A, Recio I, Martínez-Maqueda D, Miralles B, Fornari T, García-Risco MR, Gonzalez M, Reglero G. J Food Prot. 2013 Jul; 76(7):1226-39. https://www.ncbi.nlm.nih.gov/pubmed/23834798/[↩]
- CHEN W-C, HUANG W-C, CHIU C-C, CHANG Y-K, HUANG C-C. Whey Protein Improves Exercise Performance and Biochemical Profiles in Trained Mice. Medicine and Science in Sports and Exercise. 2014;46(8):1517-1524. doi:10.1249/MSS.0000000000000272. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4186725/[↩]
- Nutritional physiology of whey and whey components. Barth CA, Behnke U. Nahrung. 1997 Feb; 41(1):2-12. https://www.ncbi.nlm.nih.gov/pubmed/9157293/[↩]
- Dietary whey protein lessens several risk factors for metabolic diseases: a review. Sousa GT, Lira FS, Rosa JC, de Oliveira EP, Oyama LM, Santos RV, Pimentel GD. Lipids Health Dis. 2012 Jul 10; 11():67. https://www.ncbi.nlm.nih.gov/pubmed/22676328/[↩]
- Antimicrobial activity of whey protein isolate edible films with essential oils against food spoilers and foodborne pathogens. Fernández-Pan I, Royo M, Ignacio Maté J. J Food Sci. 2012 Jul; 77(7):M383-90. https://www.ncbi.nlm.nih.gov/pubmed/22671770/[↩]
- U.S. Food and Drug Administration website. BMPEA in Dietary Supplements. https://www.fda.gov/food/information-select-dietary-supplement-ingredients-and-other-substances/bmpea-dietary-supplements[↩]
- U.S. Food and Drug Administration website. Tainted Body Building Products. https://www.fda.gov/drugs/medication-health-fraud/tainted-body-building-products[↩]
- U.S. Food and Drug Administration website. DMAA in Products Marketed as Dietary Supplements. https://www.fda.gov/food/information-select-dietary-supplement-ingredients-and-other-substances/dmaa-products-marketed-dietary-supplements[↩][↩]
- National Institute on Drug Abuse. What are anabolic steroids ? https://www.drugabuse.gov/publications/research-reports/anabolic-steroid-abuse/what-are-anabolic-steroids[↩]
- National Institute on Drug Abuse. What are steroidal supplements ? https://www.drugabuse.gov/publications/research-reports/anabolic-steroid-abuse/what-are-steroidal-supplements[↩]
- National Institute on Drug Abuse. How are anabolic steroids abused ? https://www.drugabuse.gov/publications/research-reports/anabolic-steroid-abuse/how-are-anabolic-steroids-abused[↩][↩][↩][↩][↩][↩][↩]
- National Institute on Drug Abuse. Why do people abuse anabolic steroids ? https://www.drugabuse.gov/publications/research-reports/anabolic-steroid-abuse/why-do-people-abuse-anabolic-steroids[↩][↩][↩][↩]