Contents
What is endocarditis
Endocarditis also called infective endocarditis, bacterial endocarditis or septic endocarditis is a rare life-threatening inflammation of the inner lining of the heart’s chambers and valves called the endocardium 1, 2. There are 3 forms of endocarditis 3:
- Acute infective endocarditis also called acute bacterial endocarditis develops suddenly and may become life threatening within days. Cases of acute bacterial endocarditis present with far more aggressive symptoms, including the following 4:
- Sepsis
- Congestive heart failure
- Kidney failure
- Stroke
- Septic emboli
- Subacute infective endocarditis also called subacute bacterial endocarditis or chronic infective endocarditis develops slowly over a period of weeks to several months. Fever, often low-grade and intermittent, is present in up to 90% of cases of subacute infective endocarditis. Heart murmurs are documented in approximately 85% of patients 4. 50% of patients may exhibit 1 or more classic signs and symptoms of subacute infective endocarditis. Signs and symptoms of subacute bacterial endocarditis include the following 4:
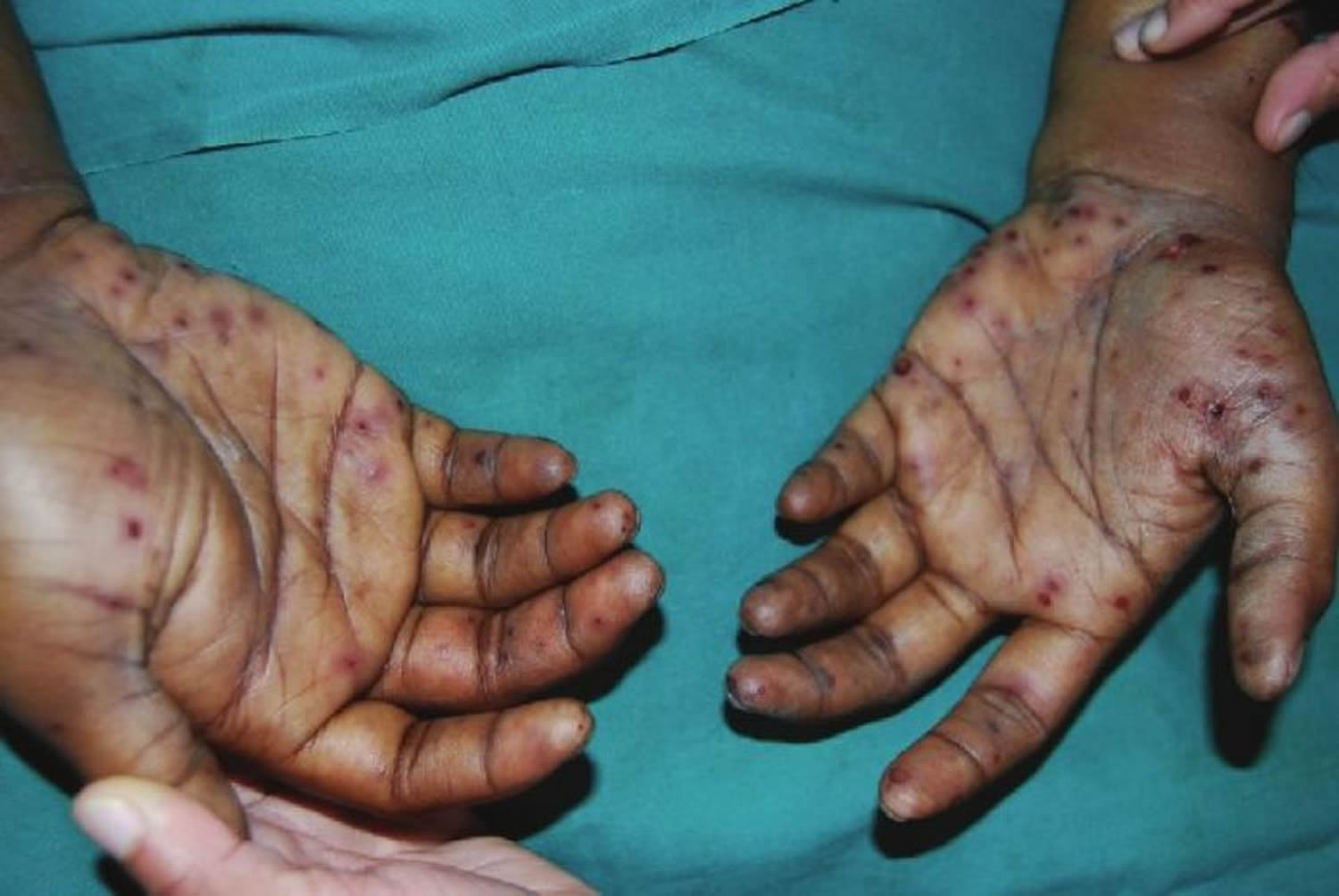
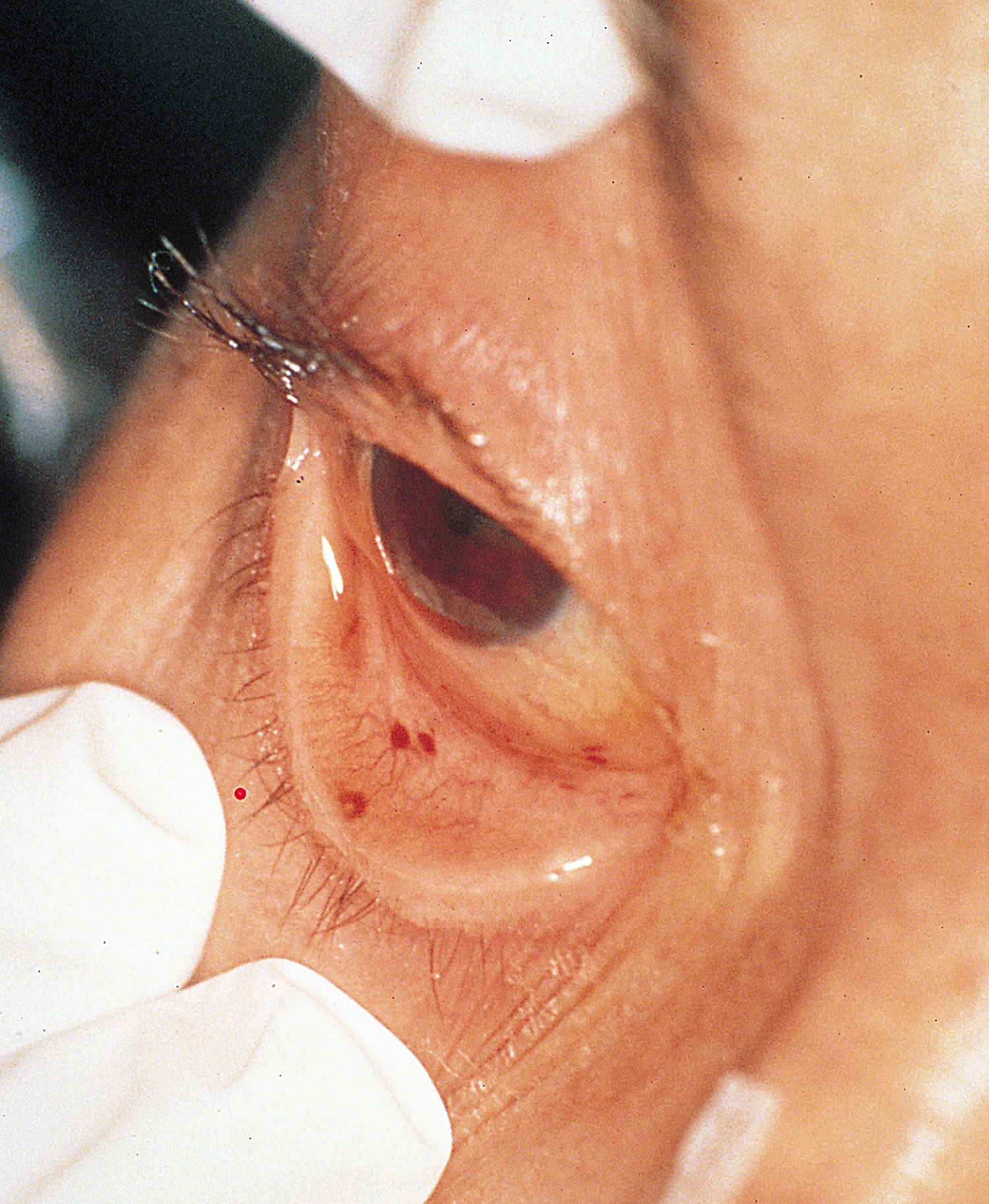
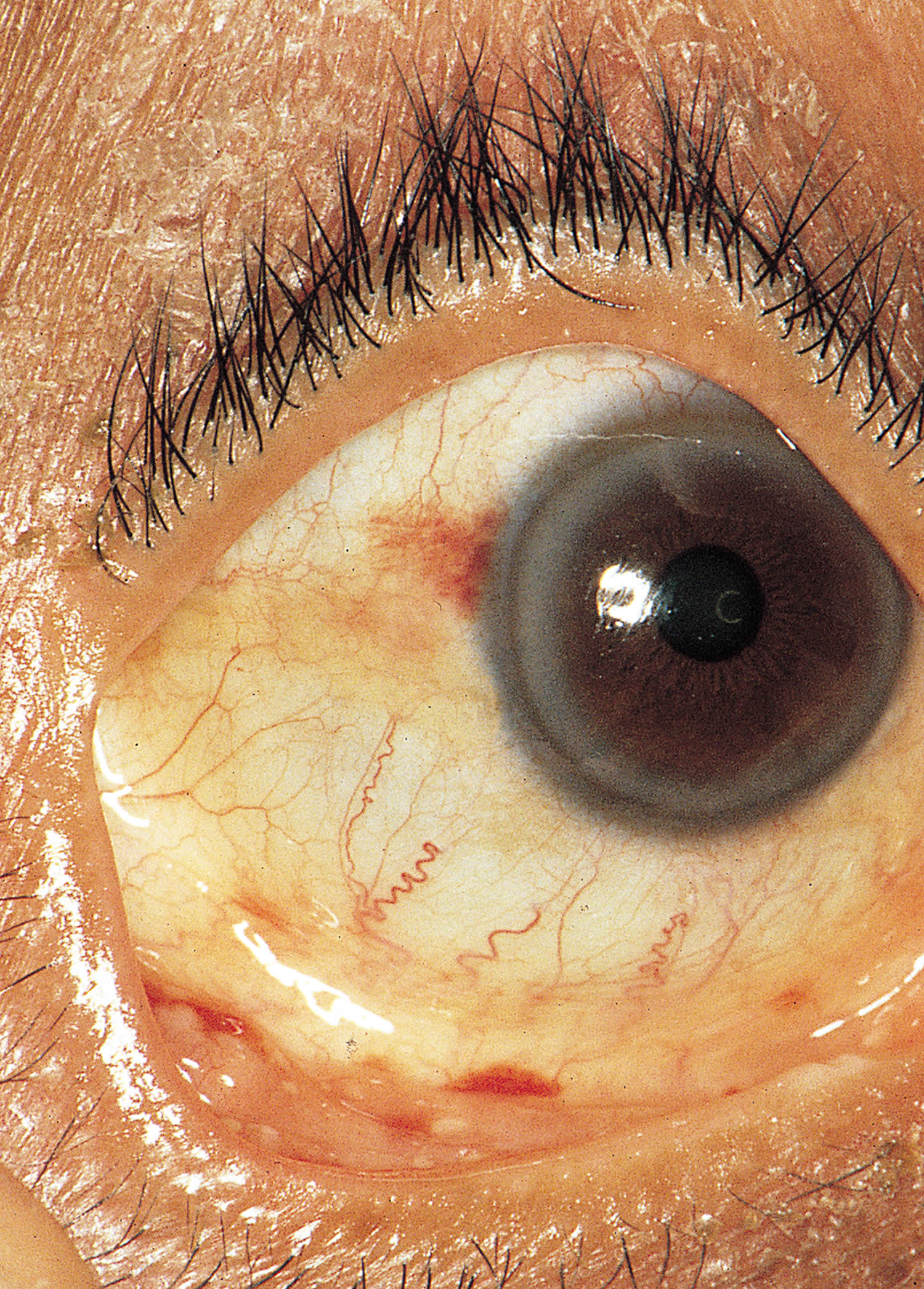
- Petechiae. Conjunctival petechiae (tiny purple, red or brown round spots on the skin [petechiae], in the whites of the eyes or inside the mouth).
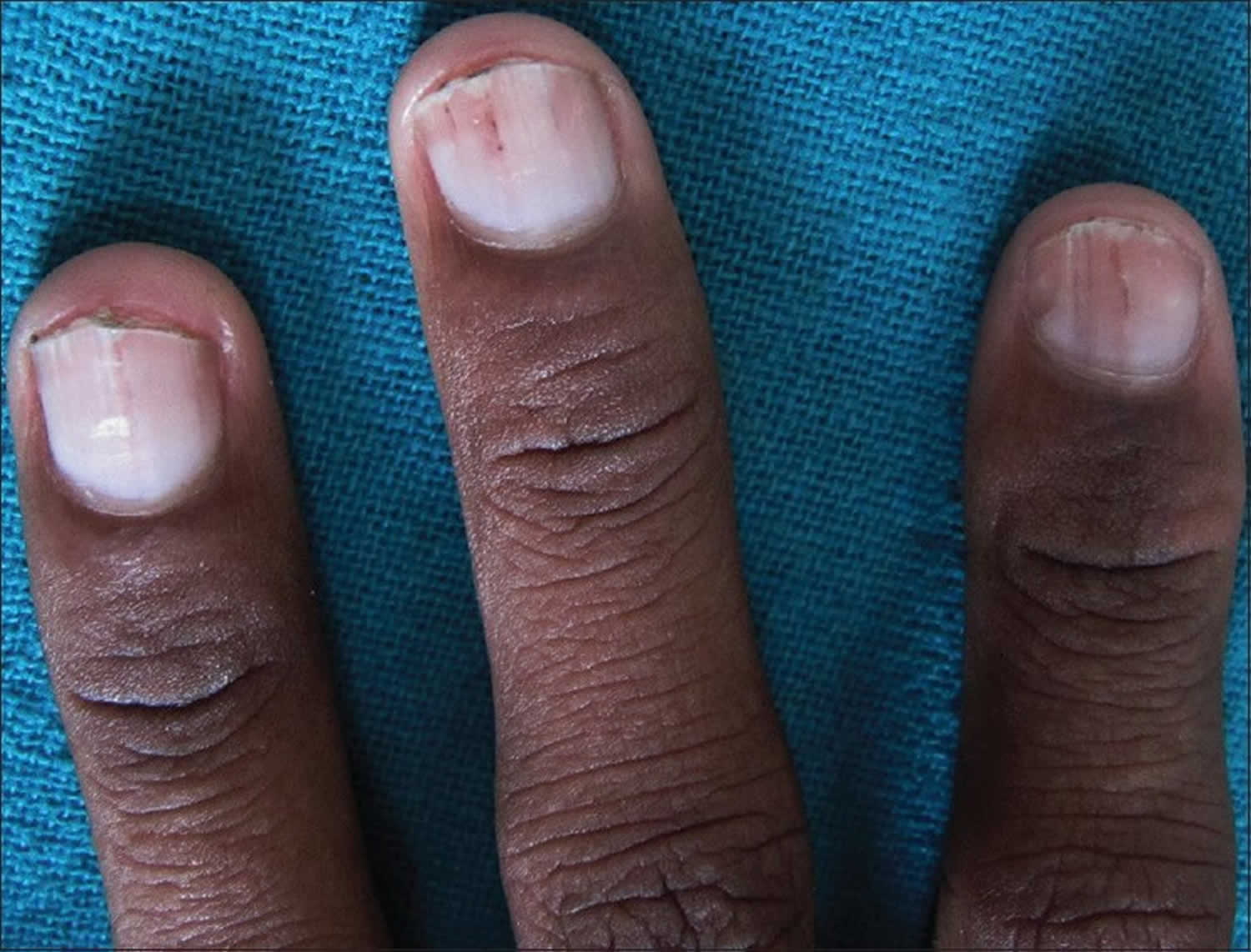
- Splinter hemorrhages: Dark red, linear lesions in the nail beds
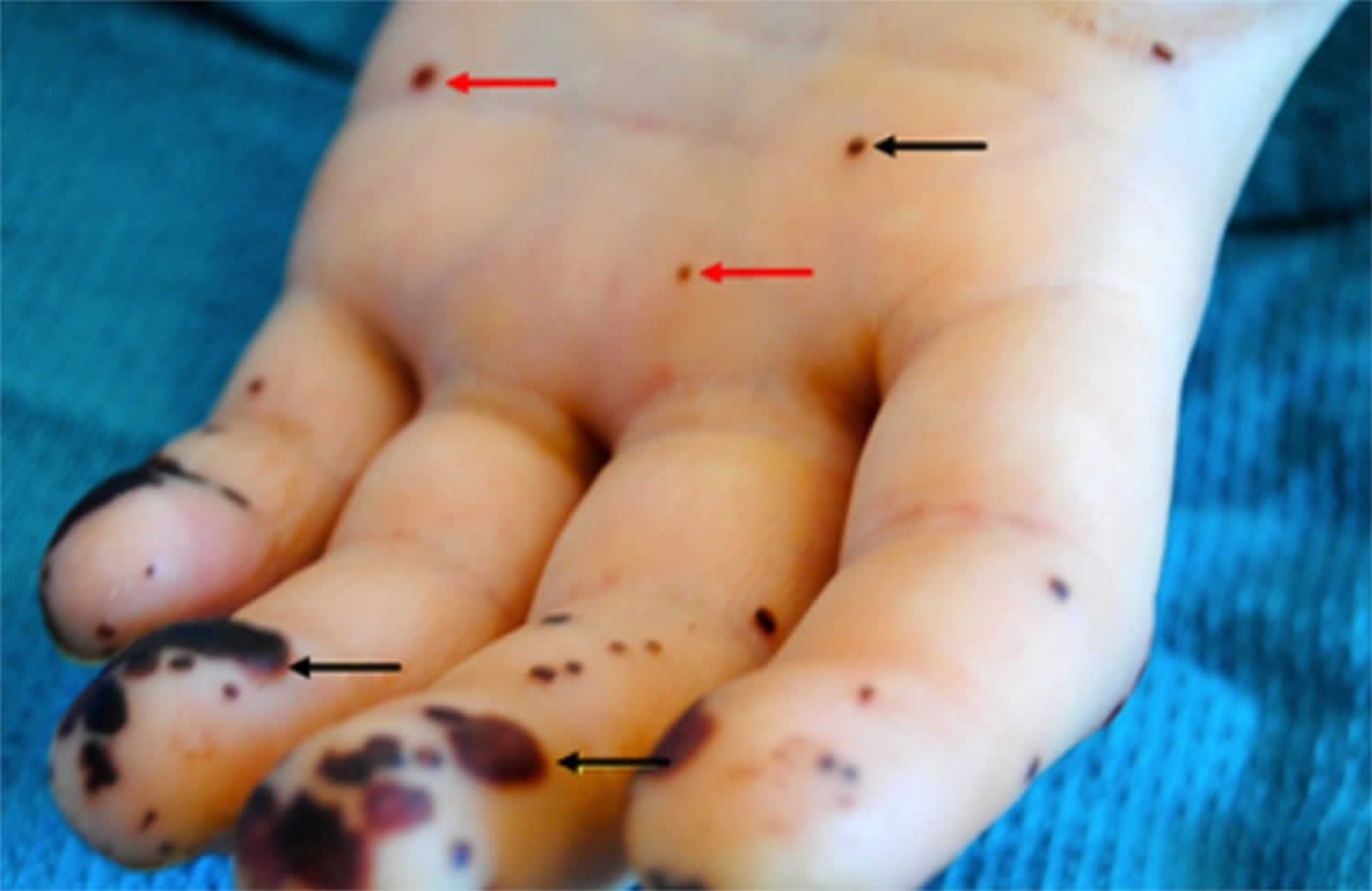
- Janeway lesions (painless red, purple or brown flat spots on the soles bottom of the feet or the palms of the hands).
- Osler’s nodes (painful red or purple bumps or patches of darkened skin [hyperpigmented] on the tips of the fingers or toes).
- Roth spots: Retinal hemorrhages with small, clear centers (rare)
- Signs of neurologic disease include the following 5, 6, 7, 8, 9.:
- Embolic stroke with focal neurologic deficits (the most common neurologic sign)
- Intracerebral hemorrhage
- Multiple microabscesses
- Prosthetic valvular infective endocarditis develops within a year after heart valve replacement.
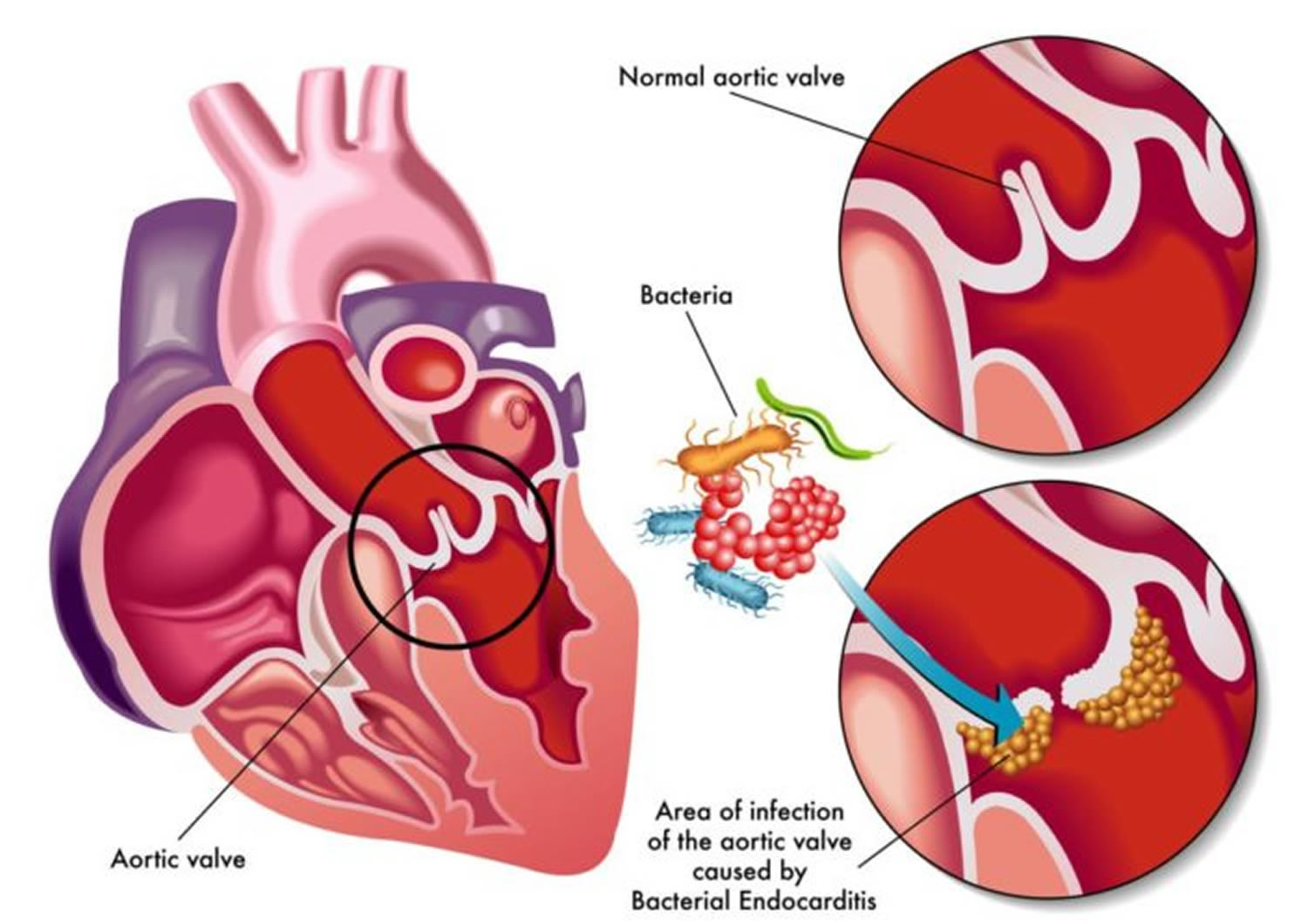
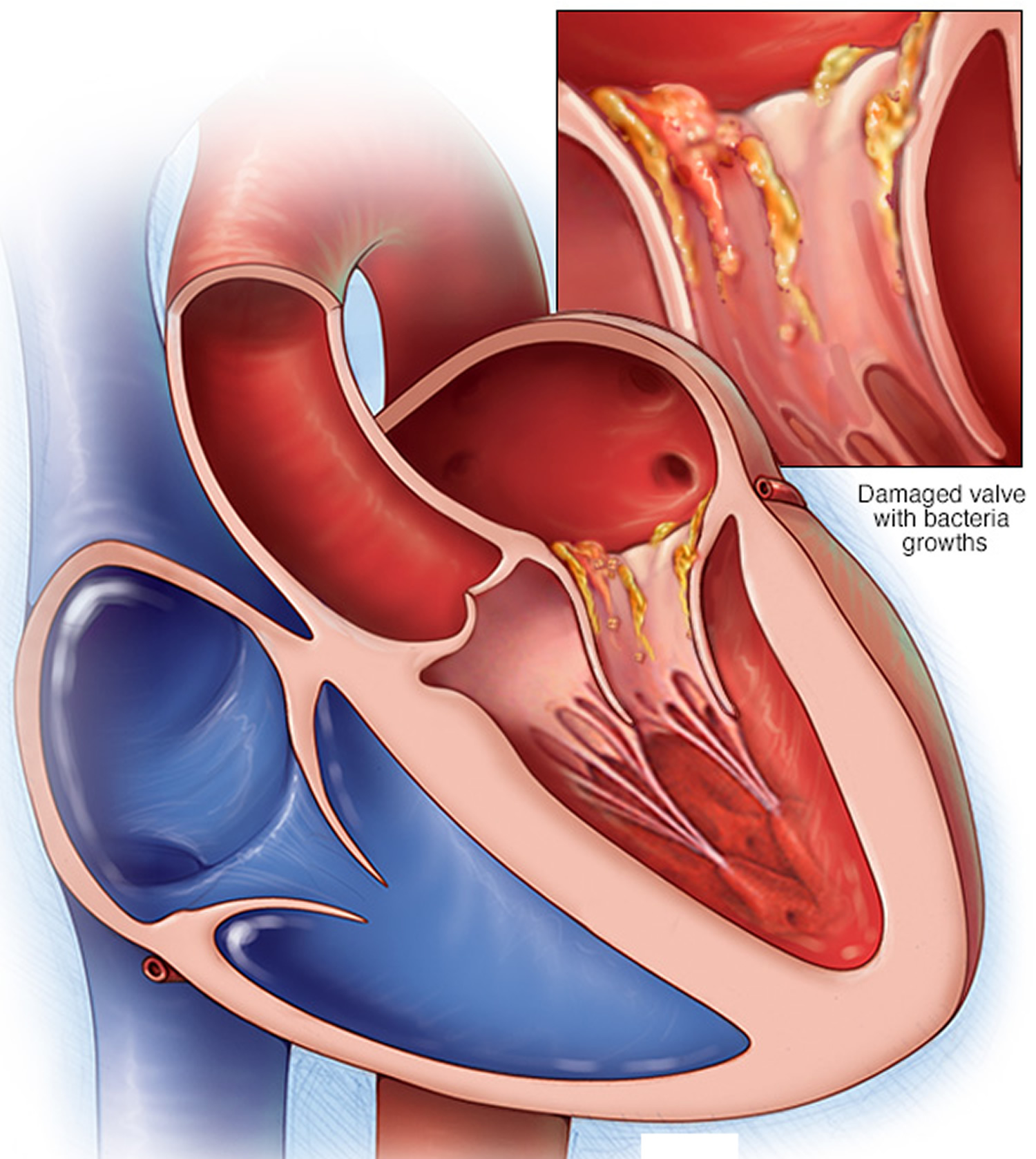
Endocarditis is usually caused by bacteria, fungi or other germs from another part of your body, such as your mouth that get into your bloodstream and travel to your heart and lodge on abnormal heart valves or damaged heart tissue. Once germs get inside your heart, the germs can attach to the lining or get trapped in the valves (the endocardium). They start to grow, causing an infection. Abnormal growths (vegetations) that contain collections of bacteria may form in your heart at the site of the infection and damage the heart valves, which can cause them to leak. If not treated quickly, the infection can cause damage to the heart and lead to serious health problems.
Bacterial endocarditis is an infection of the lining of the heart (the endocardium) caused by various bacteria. The responsible bacteria include various species of staphylococcus, streptococcus, pseudomonas, bartonella and several other organisms. Streptococci (viridans-group streptococci) and staphylococci (Staphylococcus aureus) have collectively accounted for approximately 80% of infective endocarditis cases 10.
Bacterial endocarditis most often affects the heart valves. Most commonly, infective endocarditis affects the aortic and mitral valves, less commonly the tricuspid vale, and in approximately 10% of cases affects more than one valve 5. The bacteria gain access to the heart via the bloodstream; an infection elsewhere in the body may or may not be apparent. While some bacteria can cause infection in normal heart valves, bacterial endocarditis more commonly affects patients with abnormal valves as a result of previous rheumatic fever, valve surgery/replacement, or congenital abnormalities.
Infective endocarditis is an uncommon infectious disease with an annual incidence ranging from 1.5 to 11.6 per 100 000 person-years in the most contemporary population surveys 11. In the United States, there are up to 34,000 hospital discharges related to infective endocarditis each year. Men, women and children of all racial and ethnic groups can get endocarditis.
Your risk of endocarditis is increased by having:
- Congenital heart disease
- Previous endocarditis. Endocarditis can damage heart tissue, which increases your risk of getting it again.
- Heart valve replacement
- Heart valve disease – either stenosis (a narrowed valve) or regurgitation (a leaking valve)
- Heart devices (such as a pacemaker or ICD)
- Intravenous drug use (intravenous users of heroin, cocaine or amphetamine) 12.
If you have a problem with a heart valve or you have man-made materials in your heart, it is easier for bacteria to lodge there, and your body may not be able to fight off the infection. Intravenous drug users are at risk even if they don’t have heart problems.
The factors predisposing to infective endocarditis are an elderly age of > 65 years, male gender, history of a rheumatic disease, presence of a prosthetic heart valve, diabetes mellitus, HIV infection, long-term presence of catheters in central veins, dialysis treatment, immunosuppression, and intravenous drug use by addicts 5. The most common complications of infective endocarditis include heart failure, renal failure, ischemic stroke, interstitial bleeding, and septic shock 5.
Bacterial endocarditis is often divided into ‘acute bacterial endocarditis’ and ‘subacute bacterial endocarditis’ depending on the speed that it progresses. The symptoms may include fever, lethargy, shortness of breath, chest pain or palpitations. These symptoms require prompt assessment and investigation by a doctor.
Despite recent advances in diagnostic and therapeutic strategies, the mortality of infective endocarditis remains high, with more than one-third of patients affected dying within a year following diagnosis 13.
Globally, in 2010, infective endocarditis was associated with 1.58 million disability-adjusted life-years or years of healthy life lost as a result of death and nonfatal illness or impairment 14.
Endocarditis is a rare, but potentially fatal condition that can cause damage your heart, so it requires early diagnosis and treatment. If it’s not treated quickly, endocarditis can damage or destroy your heart valves and can lead to life-threatening complications. Treatments for endocarditis include antibiotics and, in certain cases, surgery.
Since there are many ways to develop endocarditis, your doctor might not be able to pinpoint the exact cause of your condition. However, people at greatest risk of endocarditis usually have damaged heart valves, artificial heart valves or other heart defects.
Although the heart is usually well protected against infection, it may be easier for bacteria to bypass the immune system in people who have:
- a prosthetic (artificial) heart valve – valve replacement surgery is increasingly being used when people experience narrowing of one of their heart valves
- congenital heart disease – where a person is born with heart defects
- hypertrophic cardiomyopathy – where the heart muscle cells have enlarged and the walls of the heart chambers thicken
- damaged heart valves – because of infection or heart disease
People who inject drugs are also more likely to develop endocarditis.
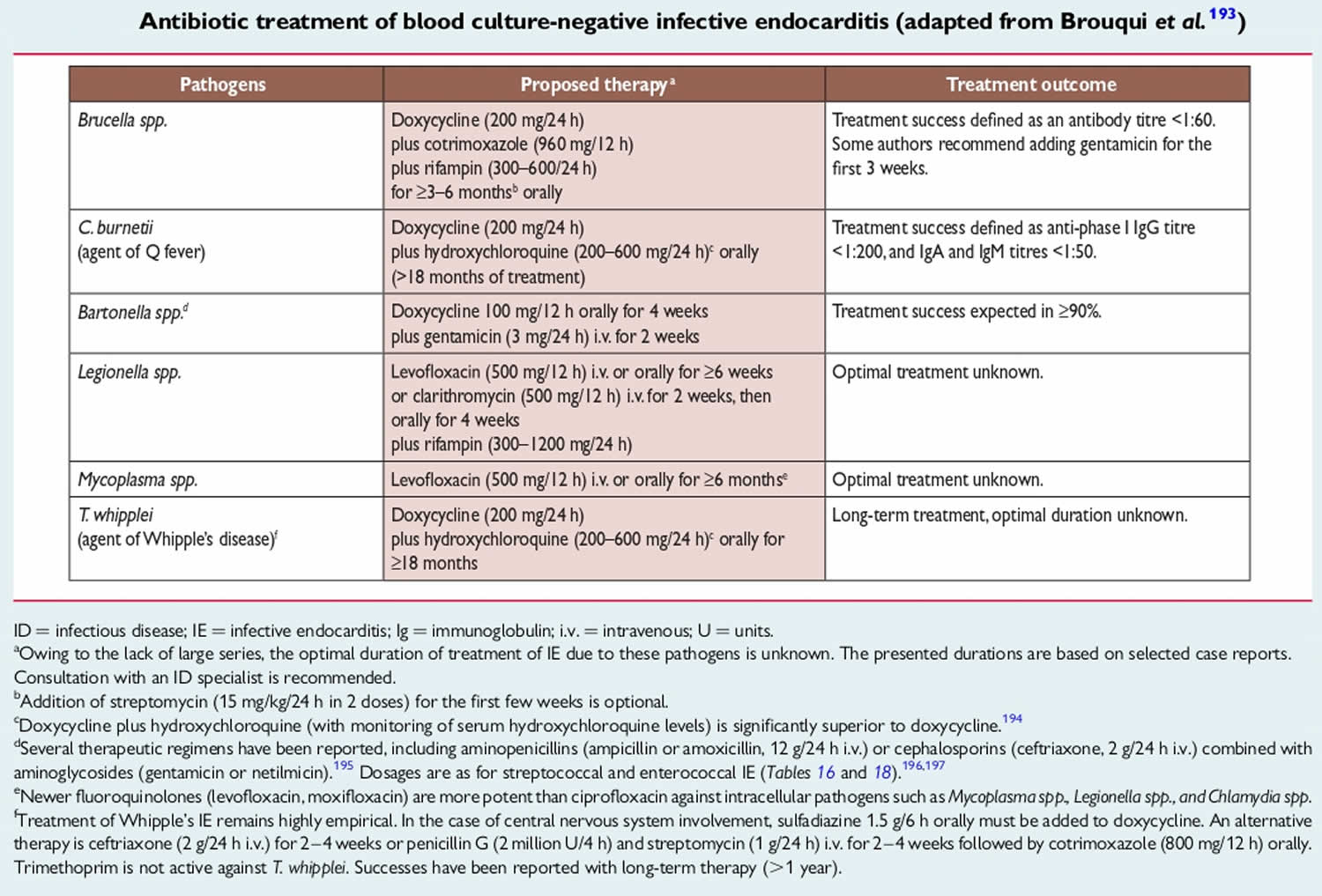
In approximately 10% of cases of infective endocarditis, blood cultures are negative, most commonly due to patient receipt of antibiotics prior to the diagnostic work-up. ‘True’ culture-negative infective endocarditis is caused by fastidious microorganisms that are difficult to isolate with conventional microbiological techniques. Highly specialized assays such as serologic testing and polymerase-chain-reaction (PCR) using blood or valve biopsies can ultimately suggest a causative pathogen in up to 60% of such cases 15. Although the aetiology of true culture-negative infective endocarditis varies with geographic and epidemiologic factors, important causes include Coxiella burnetii (the causative agent of Q fever), Bartonella species, Brucella species, and Tropheryma whipplei 16. Specific risk factors such as contact with livestock or abattoirs (for Brucella and Coxiella), homelessness or alcoholism (for Bartonella quintana), travel to the Middle East or Mediterranean or consumption of unpasteurized dairy products (for Brucella), contact with cats (for Bartonella henselae) or extensive healthcare contact in a patient with a prosthetic valve and negative blood cultures (for Aspergillus) may be useful clues when evaluating potential infective endocarditis cases.
Infective endocarditis disease process can follow one of several courses resulting in the frequently made clinical distinction between sub-acute bacterial endocarditis and acute bacterial endocarditis. Sub-acute bacterial endocarditis usually occurs on normal valves and follows a smouldering course of many weeks. Micro-organisms from a source of sepsis attach to the heart valves and proliferate there, inducing further thrombosis and the formation of vegetations. The presence of bacteremia and generalized sepsis may also cause a peripheral syndrome of vasculitic changes. The significant outcomes of sub-acute bacterial endocarditis include heart failure, embolisms of vegetation fragments and abscess formation. Acute bacterial endocarditis occurs more commonly in intravenous drug abusers and on prosthetic valves and or after acute suppurative illness such as pneumonia and meningitis and follows a much more fulminant course with vasculitis and abscesses in other organs. The vasculitic syndromes (inflammation of blood vessels) can produce renal failure. infective endocarditis can also produce almost any valvular heart disorder. Sometimes, endocarditis can cause severe damage to your heart which can lead to heart failure.
Infective endocarditis is almost universally fatal if untreated. Untreated, the average time from onset of disease to death in sub-acute bacterial endocarditis is about 6 months, 4 weeks in acute bacterial endocarditis. Heart Failure is by far the strongest negative prognostic indicator. Despite advances in antibacterial therapies, early and late mortality rates in patients with infective endocarditis remain high, due to co-morbidities and late detection of the disease.
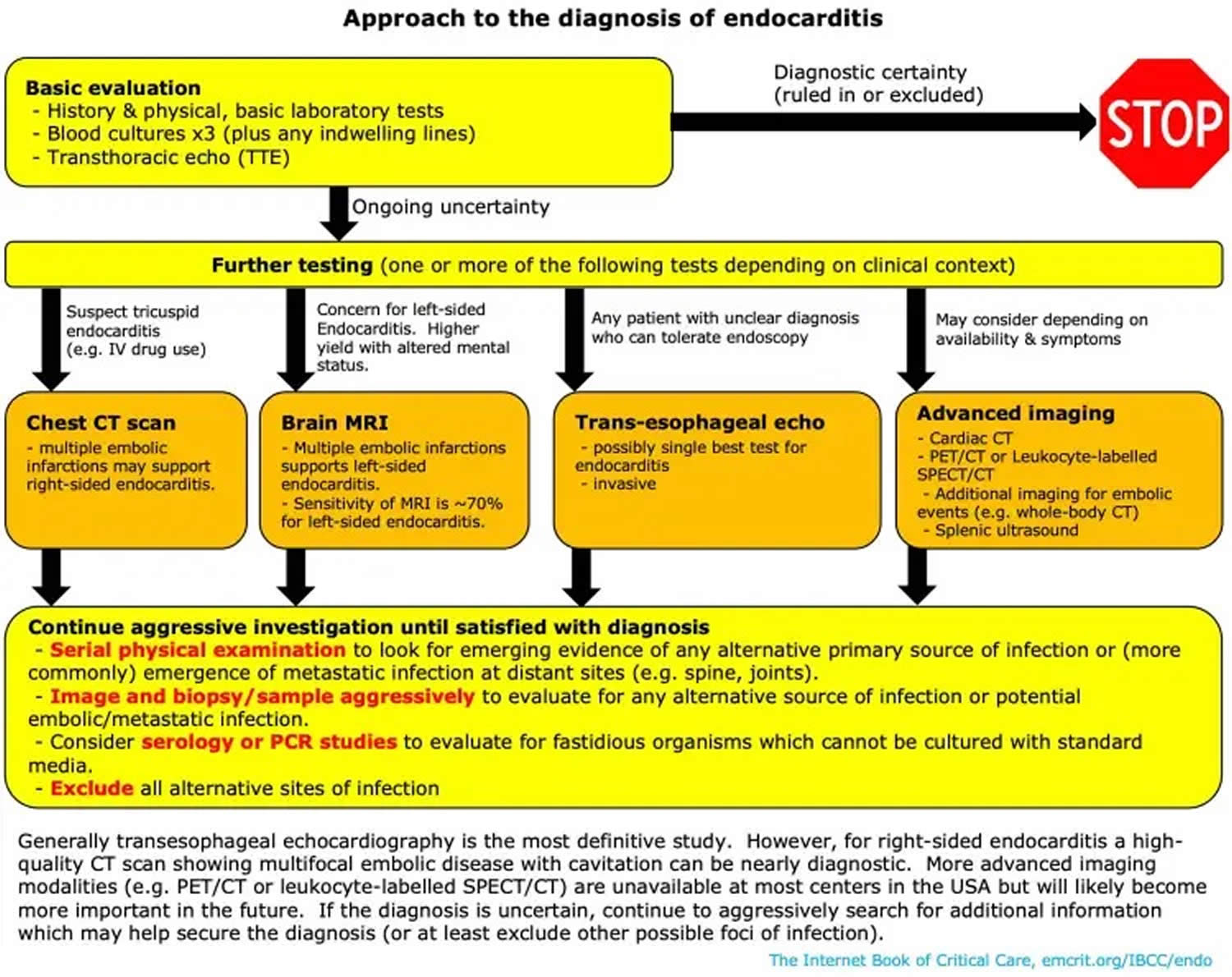
Infective endocarditis diagnostic tests include:
- Blood cultures that show bacteria or microorganisms. Blood cultures — blood tests taken over time — allow a laboratory to isolate the specific bacteria that are causing your infection. To secure a diagnosis, the lab must take blood cultures before you start taking antibiotics.
- Complete blood count, which can tell your doctor if you have an unusually high number of white blood cells. This can mean you may have an infection.
- Blood tests for substances like C-reactive protein (CRP) can show you have inflammation.
- Echocardiogram (ultrasound of the heart), which may show growths (vegetations on your valve), abscesses (holes), new regurgitation (leaking) or stenosis (narrowing), or an artificial heart valve that has begun to pull away from your heart tissue. Sometimes providers insert an ultrasound probe into your esophagus or “food pipe” (transesophageal echo) to get a closer, more detailed look at your heart.
- Bopsy of heart valve tissue to find out which kind of microbe you have.
- Positron emission tomography (PET) or nuclear medicine scans to create images using radioactive material that can show an infection’s location.
The diagnosis of infective endocarditis requires the integration of clinical, laboratory, and echocardiographic data. Nonspecific laboratory abnormalities may be present, including anemia, leukocytosis, abnormal urinalysis results, and an elevated erythrocyte sedimentation rate and C-reactive protein level.
Duke criteria for diagnosing infective endocarditis 17:
Major criteria
- Blood culture typical of infective endocarditis, single positive culture for C. burnetti, or immunoglobulin G antiphase 1 level of ≥1/800. Major blood culture criteria for infective endocarditis include the following:
- 2 blood cultures positive for organisms typically found in patients with infective endocarditis
- Blood cultures persistently positive for 1 of these organisms, from cultures drawn more than 12 hours apart
- Three or more separate blood cultures drawn at least 1 hour apart
- Echocardiogram positive for infective endocarditis. Major echocardiographic criteria include the following:
- Echocardiogram positive for infective endocarditis, documented by an oscillating intracardiac mass on a valve or on supporting structures, in the path of regurgitant jets, or on implanted material, in the absence of an alternative anatomic explanation
- Myocardial abscess
- Development of partial dehiscence of a prosthetic valve
- New-onset valvular regurgitation
Minor criteria
- Predisposing heart condition or injection drug use
- Fever (temperature of >100.4°F [>38°C])
- Vascular phenomena, including major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhage, conjunctival hemorrhage, or Janeway lesions
- Immunologic phenomena such as glomerulonephritis, Osler nodes, Roth spots, or rheumatoid factor
- Positive blood culture results not meeting major criteria or serologic evidence of active infection with an organism consistent with infective endocarditis
- Echocardiogram results consistent with infective endocarditis but not meeting major echocardiographic criteria
Definite endocarditis
- (i) 2 major clinical criteria OR
- (ii) 1 major clinical criterion and 3 minor clinical criteria OR
- (iii) 5 minor clinical criteria
Possible endocarditis
- (i) 1 major clinical criterion and 1 minor clinical criterion OR
- (ii) 3 minor clinical criteria
Rejected endocarditis
- (i) Firm alternative diagnosis is made explaining evidence of infective endocarditis;
- (ii) Resolution of infective endocarditis syndrome with antibiotic therapy for ≤4 days ;
- (iii) No pathologic evidence of infective endocarditis at surgery or autopsy, with antibiotic therapy for ≤4 days;
- (iv) Fail to meet criteria for for possible or definite infective endocarditis
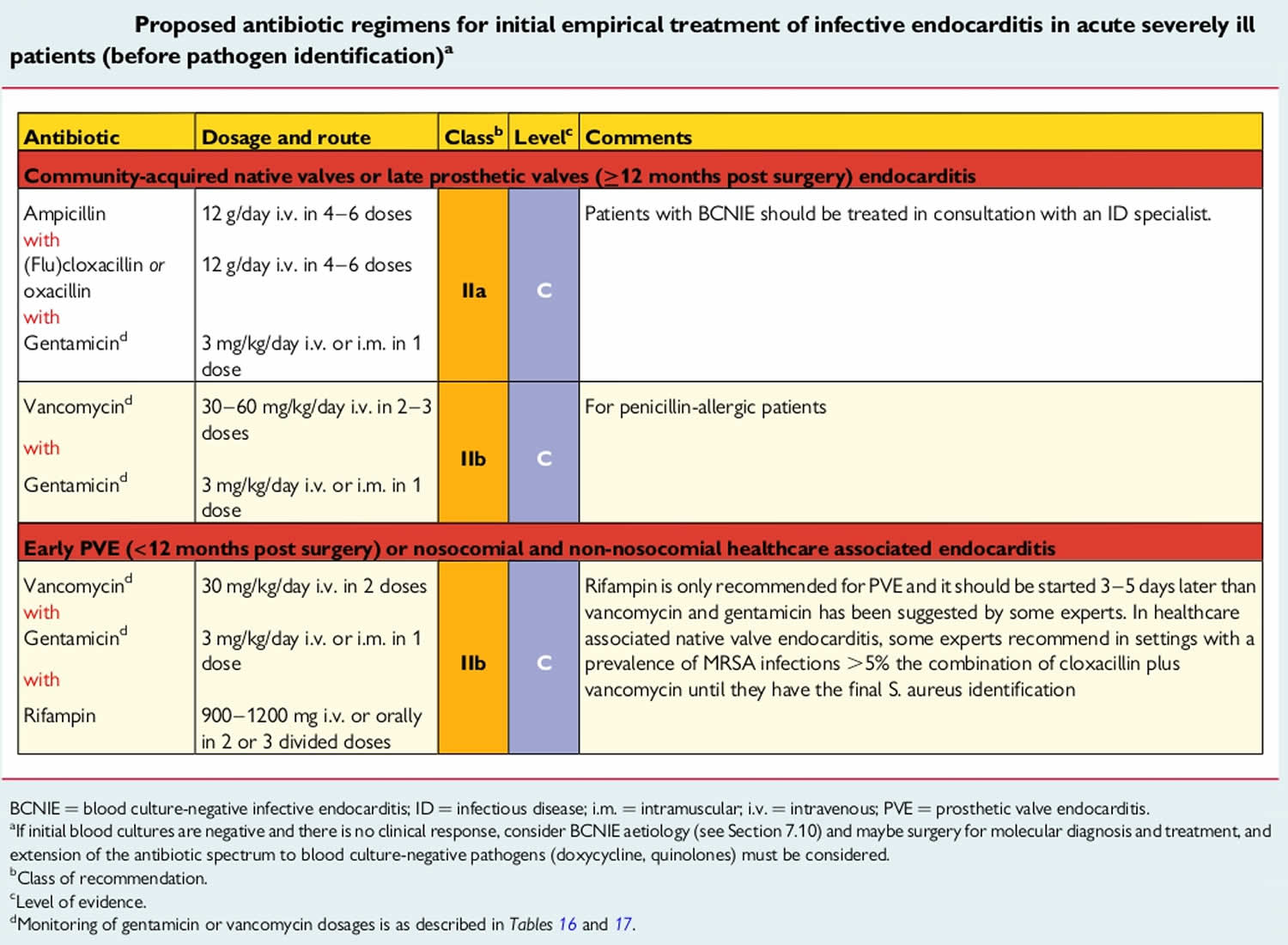
Once you get endocarditis, you’ll need quick treatment to prevent damage to your heart valves and more serious complications. After taking your blood cultures, your doctor will start you on intravenous (IV) antibiotic therapy. They’ll use a broad-spectrum antibiotic to cover as many suspected bacterial species as possible. As soon as they know which specific type of organism you have, they’ll adjust your antibiotics to target it. Usually, you’ll receive IV antibiotics for as long as six weeks to cure your infection. Your doctor will monitor your symptoms throughout your therapy to see if your treatment is effective. They’ll also repeat your blood cultures.
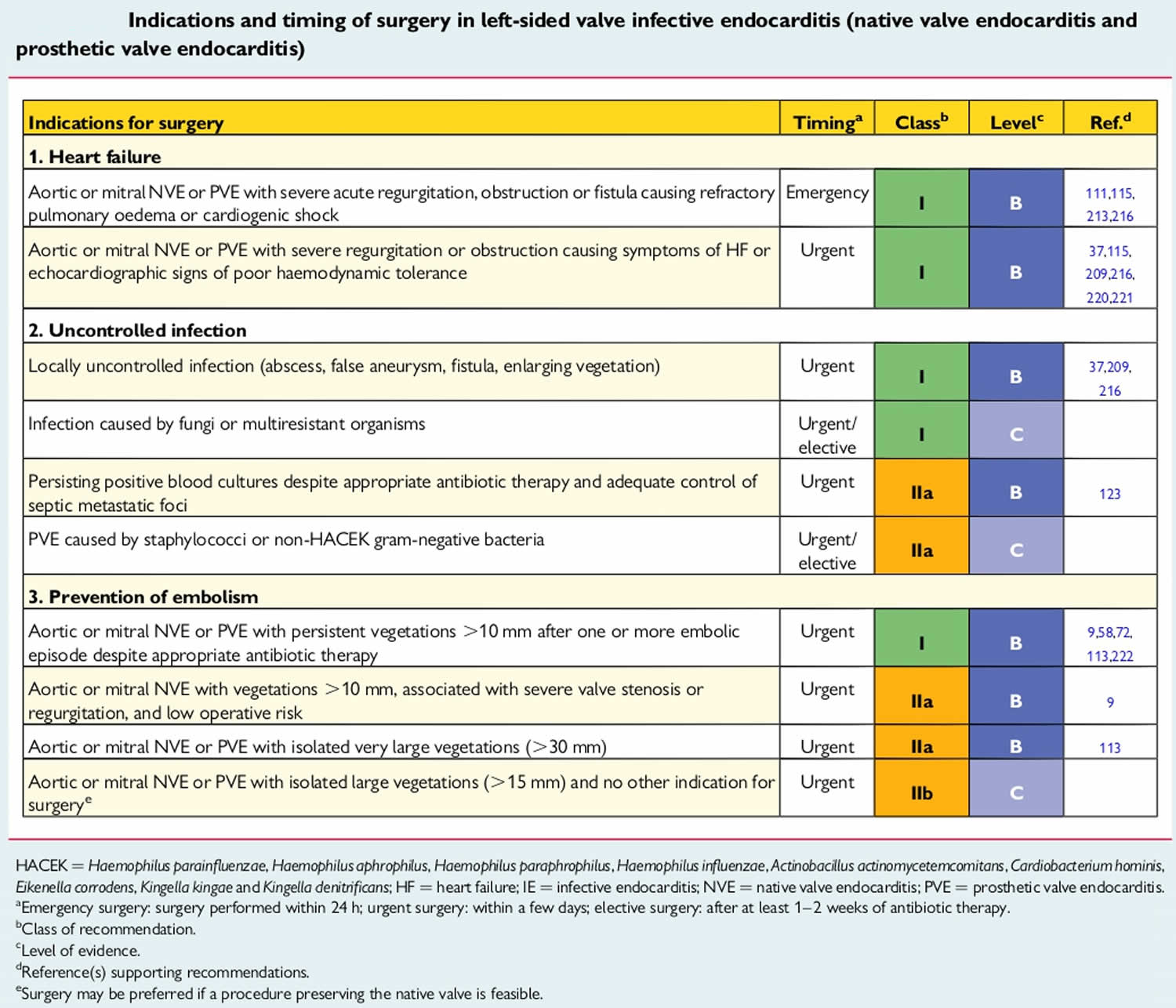
If endocarditis damages your heart valve and any other part of your heart, you may need surgery to fix your heart valve and improve your heart function.
After you complete your treatment, your doctor will determine the sources of bacteria in your blood (for example, dental infections) and treat them. In the future, you should take preventive or prophylactic antibiotics according to the national guidelines.
However, despite recent advances in diagnostic and treatment strategies for infective endocarditis, 1 year mortality has not improved and remains at 30%, which is worse than for many cancers 18, 19, 20.
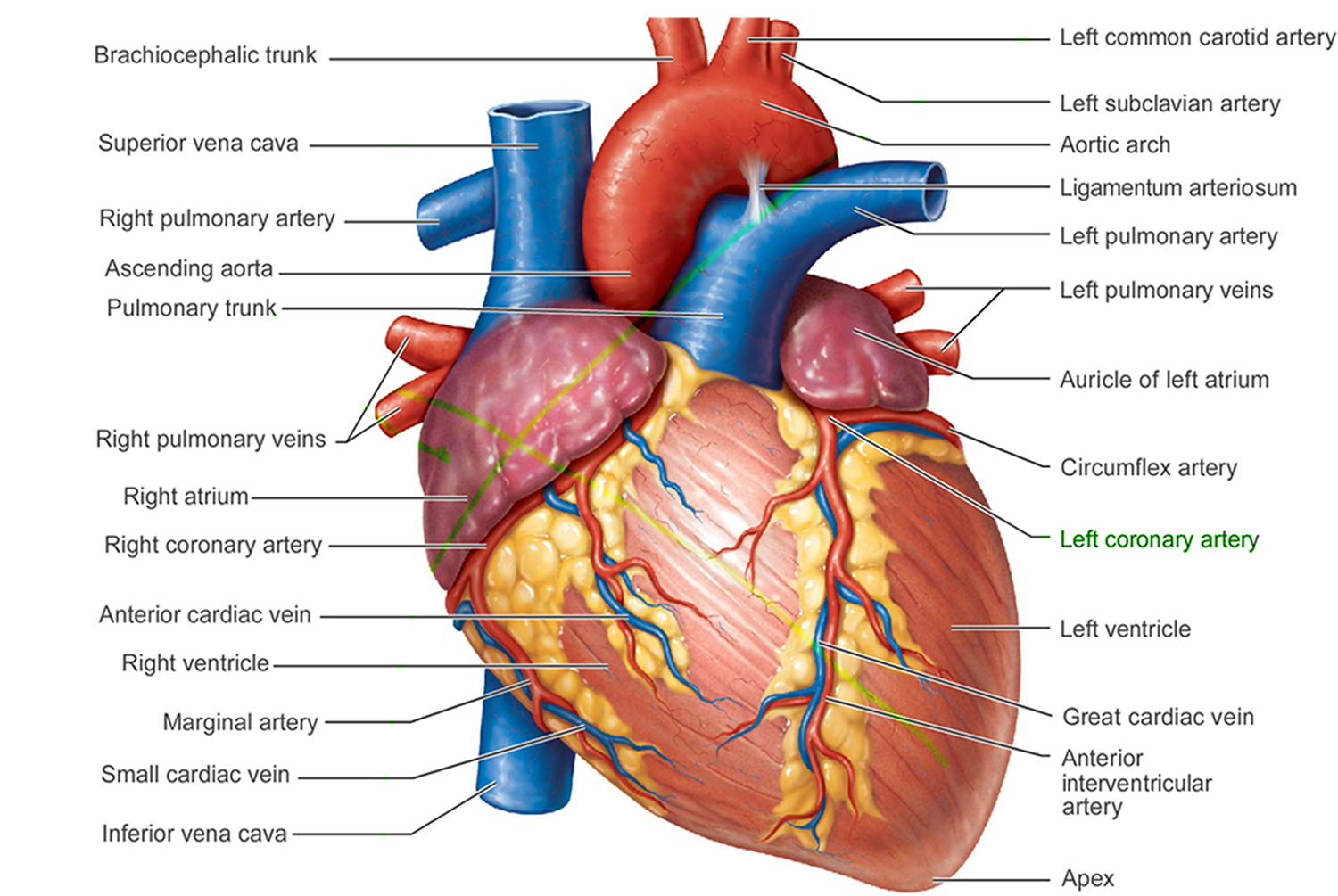
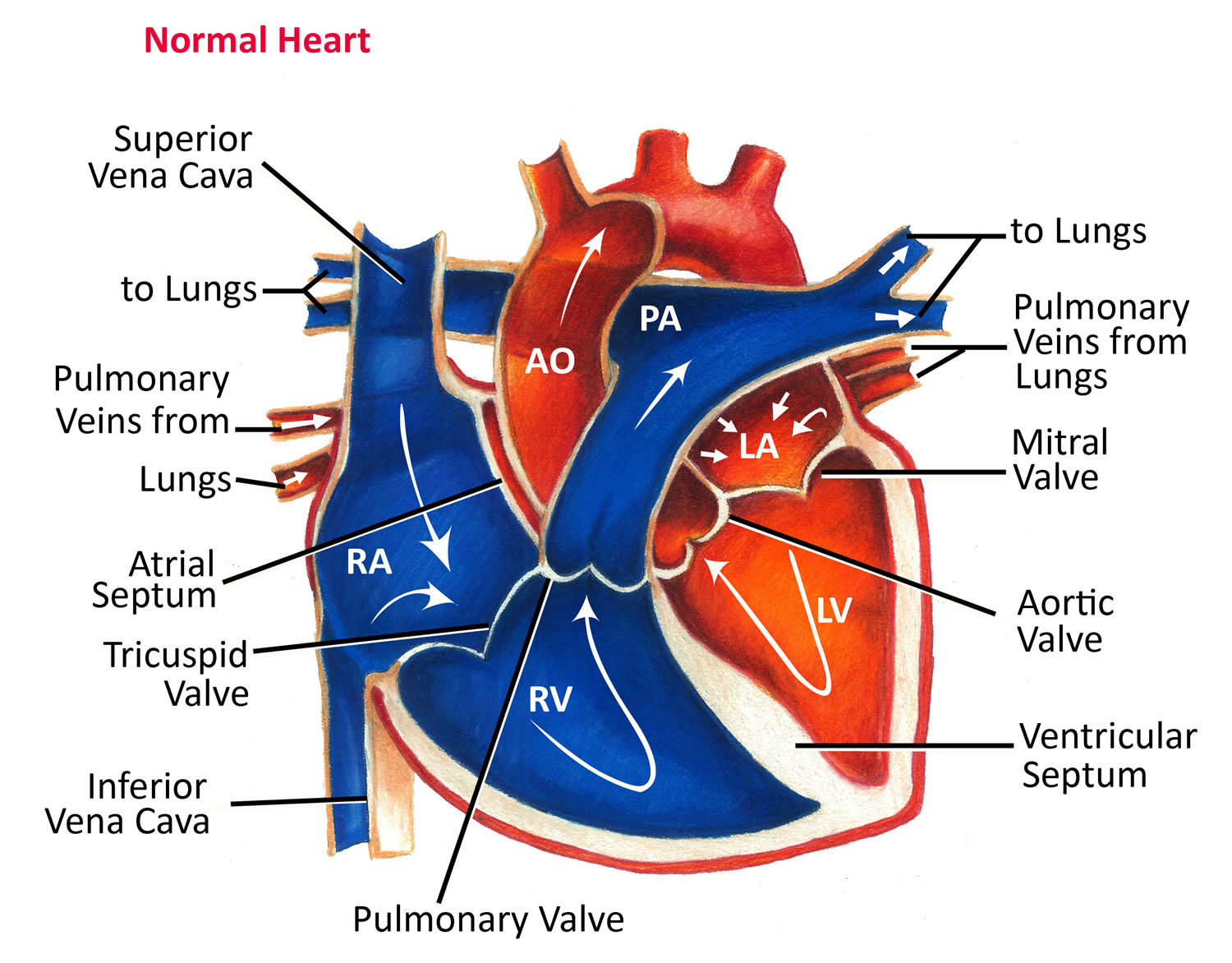
Figure 1. The anatomy of the heart
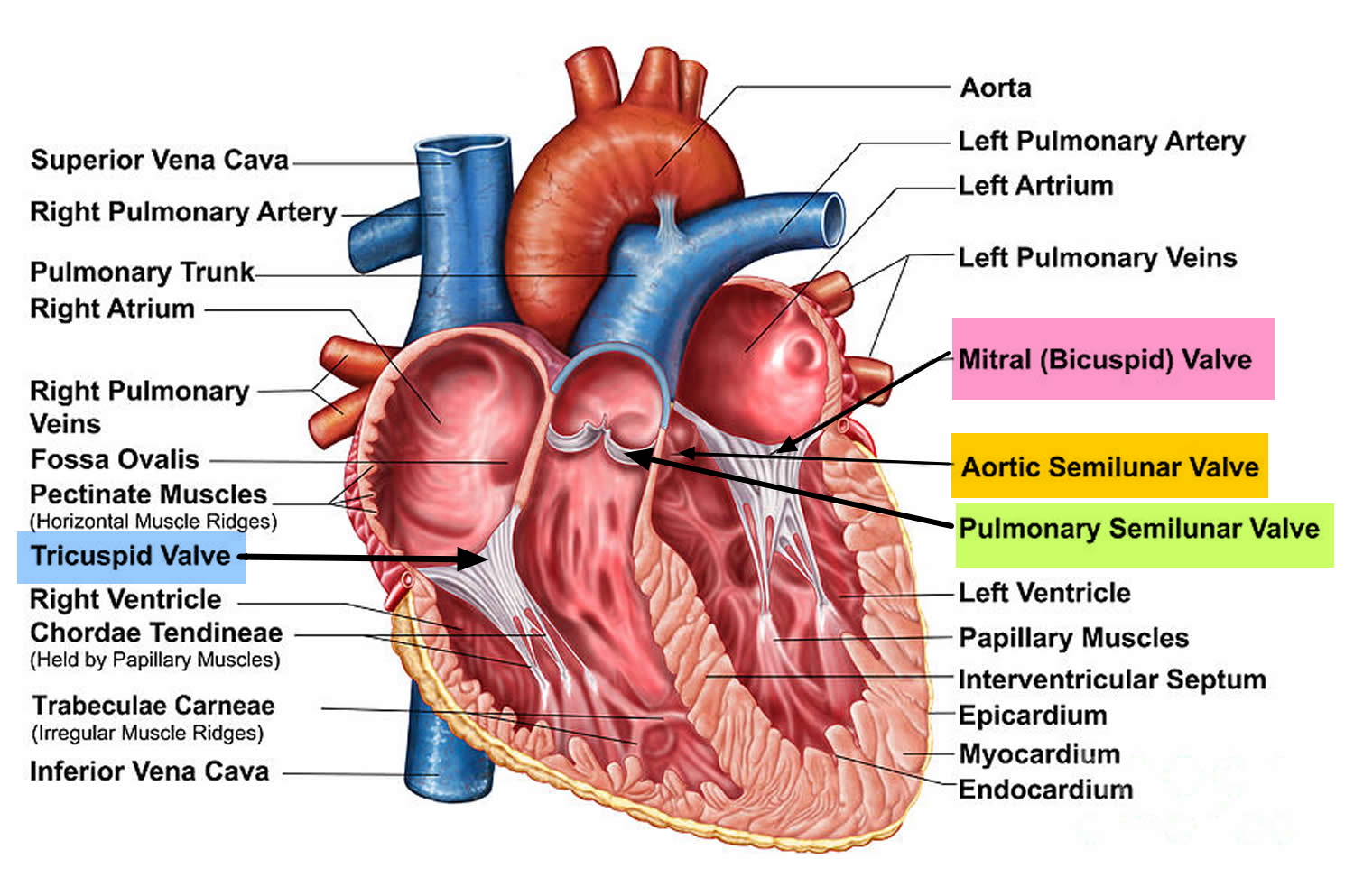
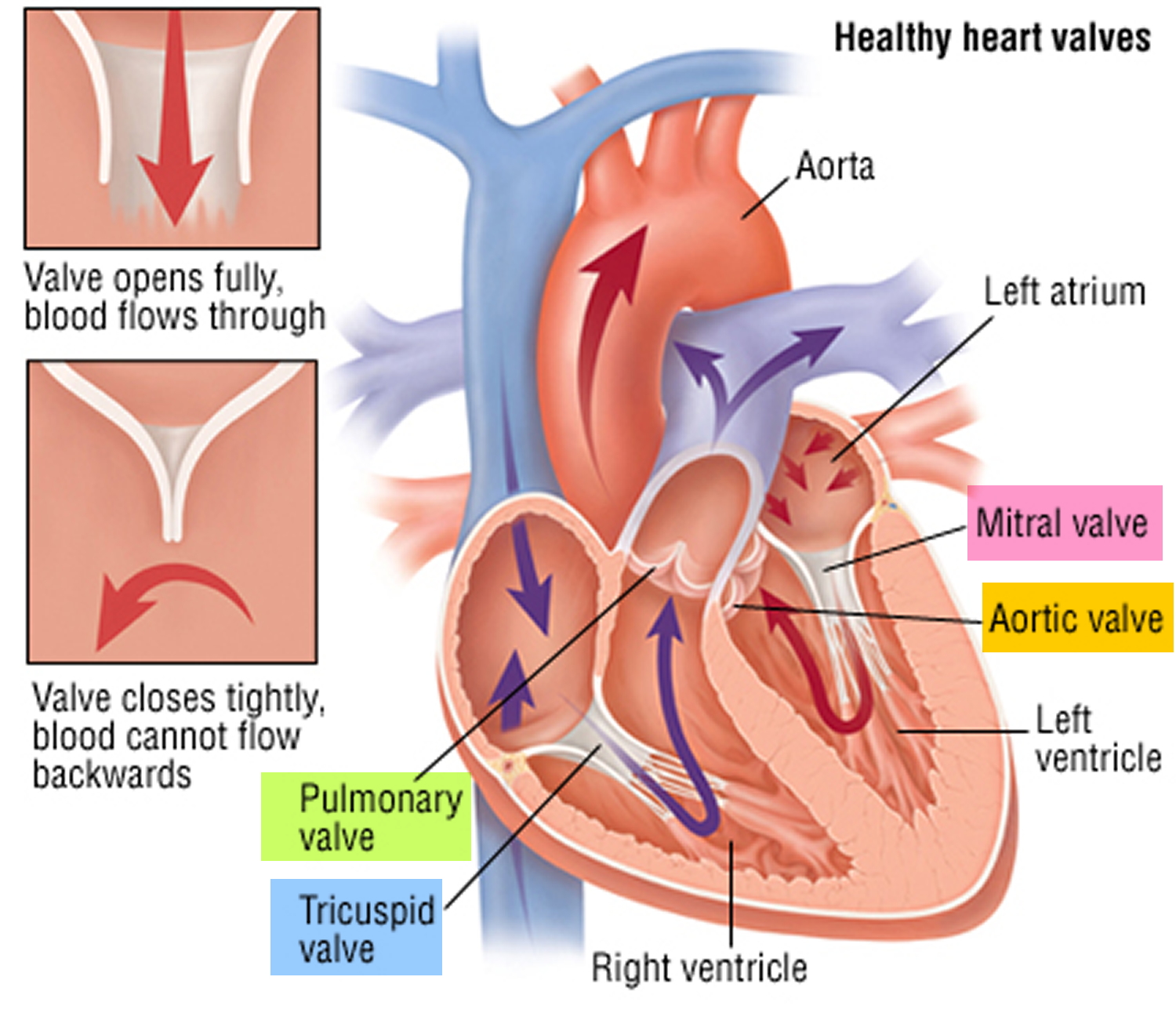
Figure 2. The anatomy of the heart valves
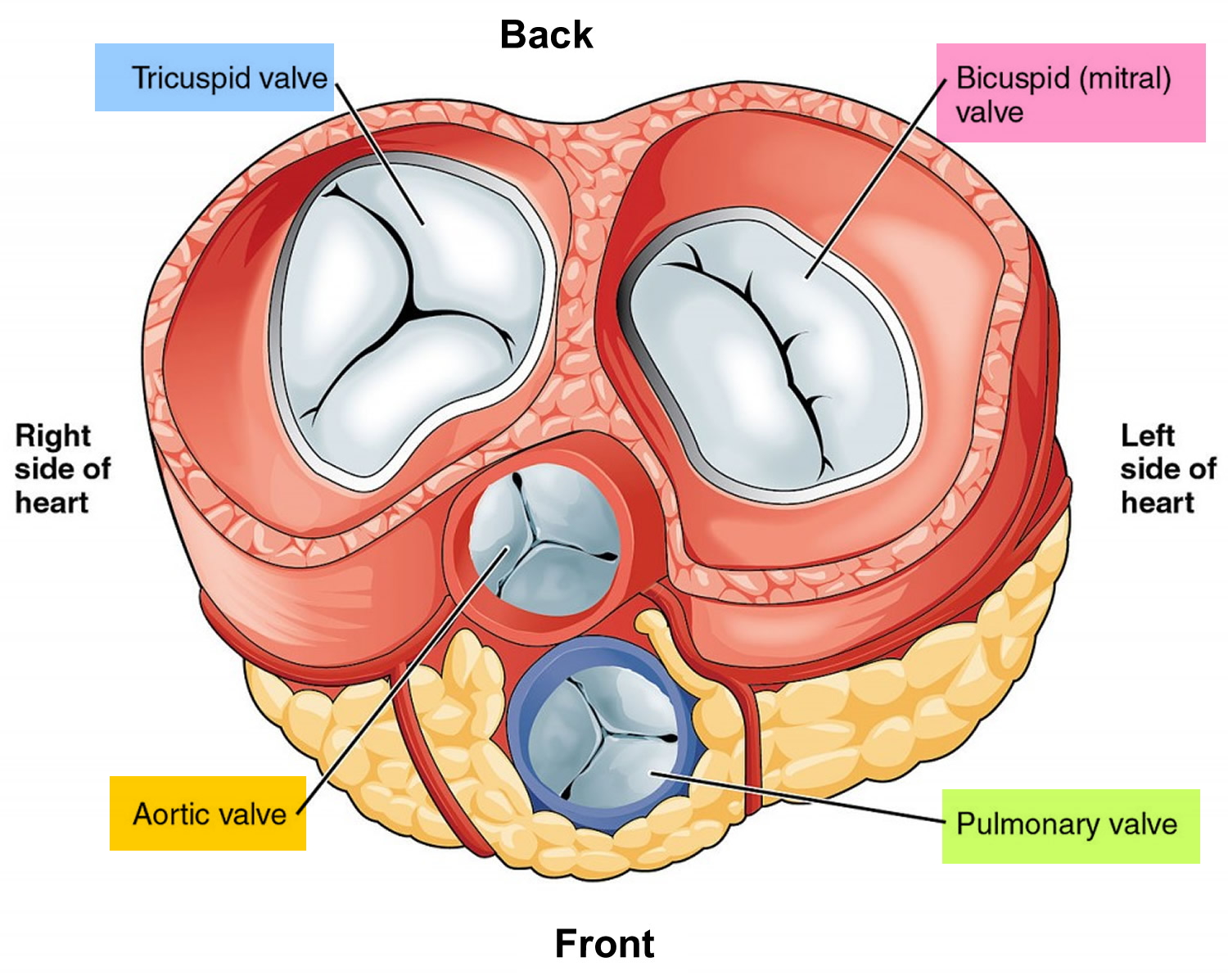
Figure 3. Top view of the 4 heart valves
Figure 4. Normal heart blood flow
Figure 5. Heart valves function
Figure 6. Endocarditis
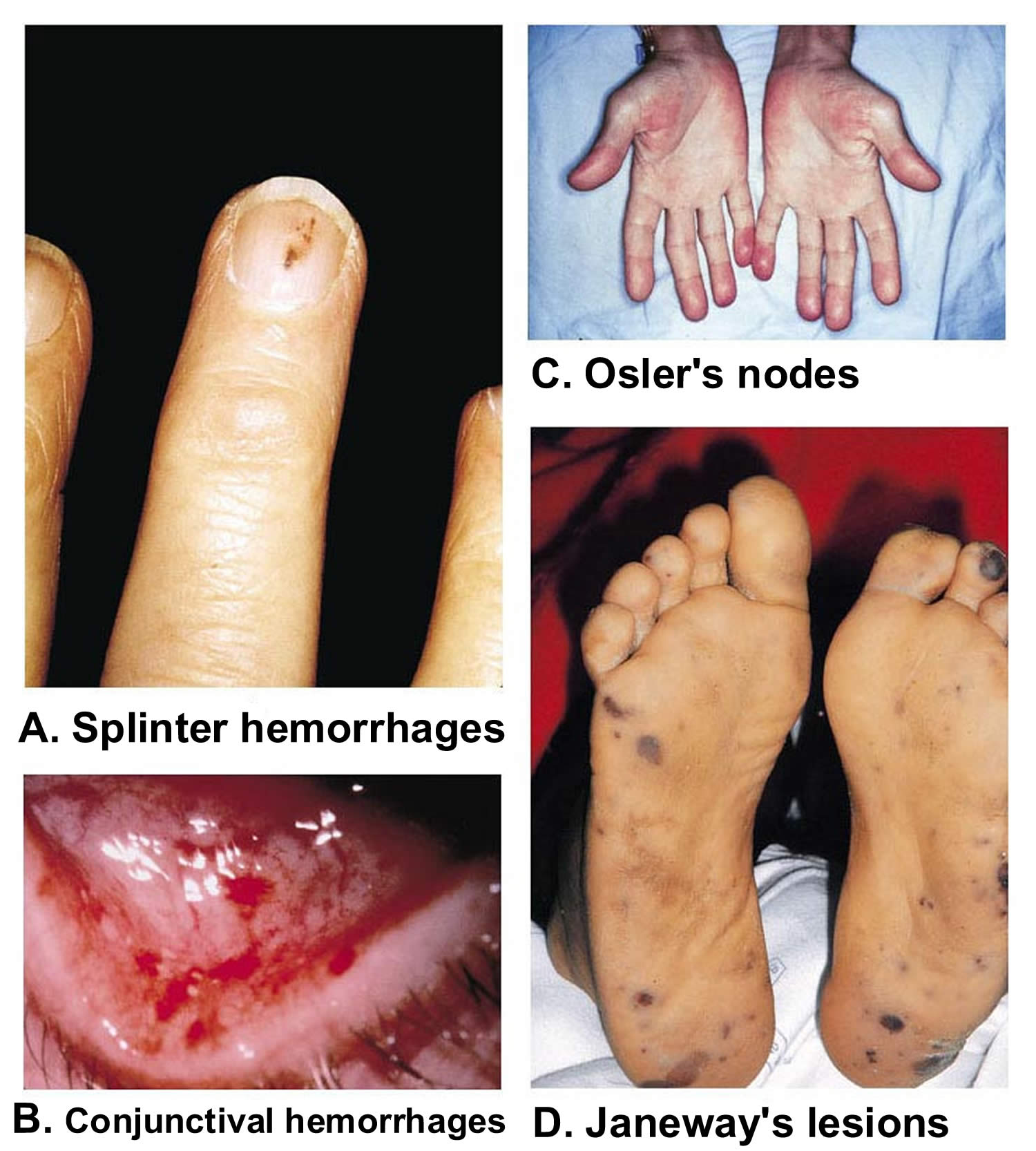
Figure 7. Infective endocarditis signs and symptoms
Footnotes: (A) Splinter hemorrhages are normally seen under the fingernails or toenails. They are usually linear and red for the first two to three days and brownish thereafter. (B) Shows conjunctival hemorrhages (petechiae). (C) Osler’s nodes are tender, subcutaneous nodules, often in the pulp of the digits or the thenar eminence. (D) Janeway’s lesions are nontender erythematous, hemorrhagic, or pustular lesions, often on the palms or soles.
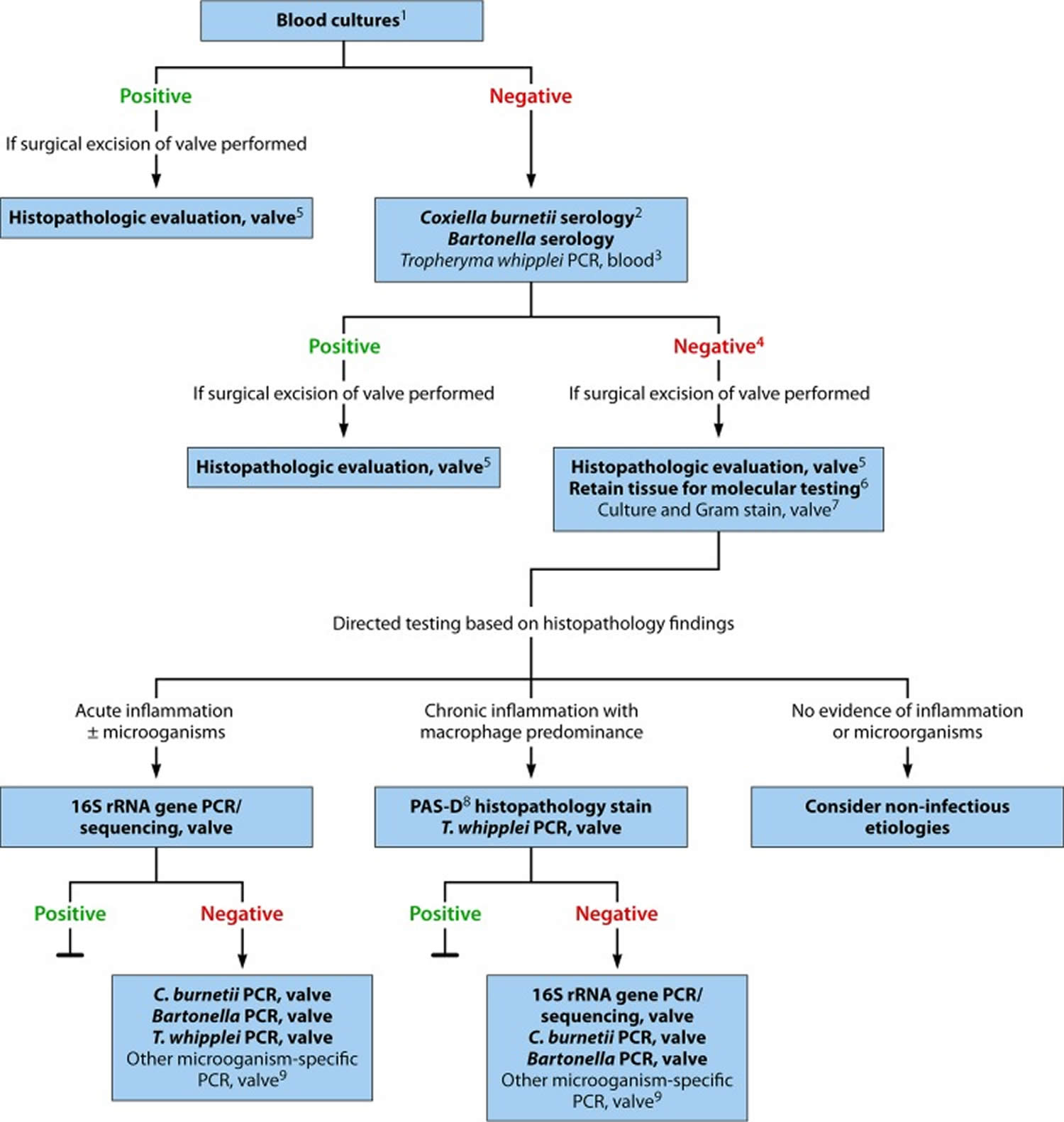
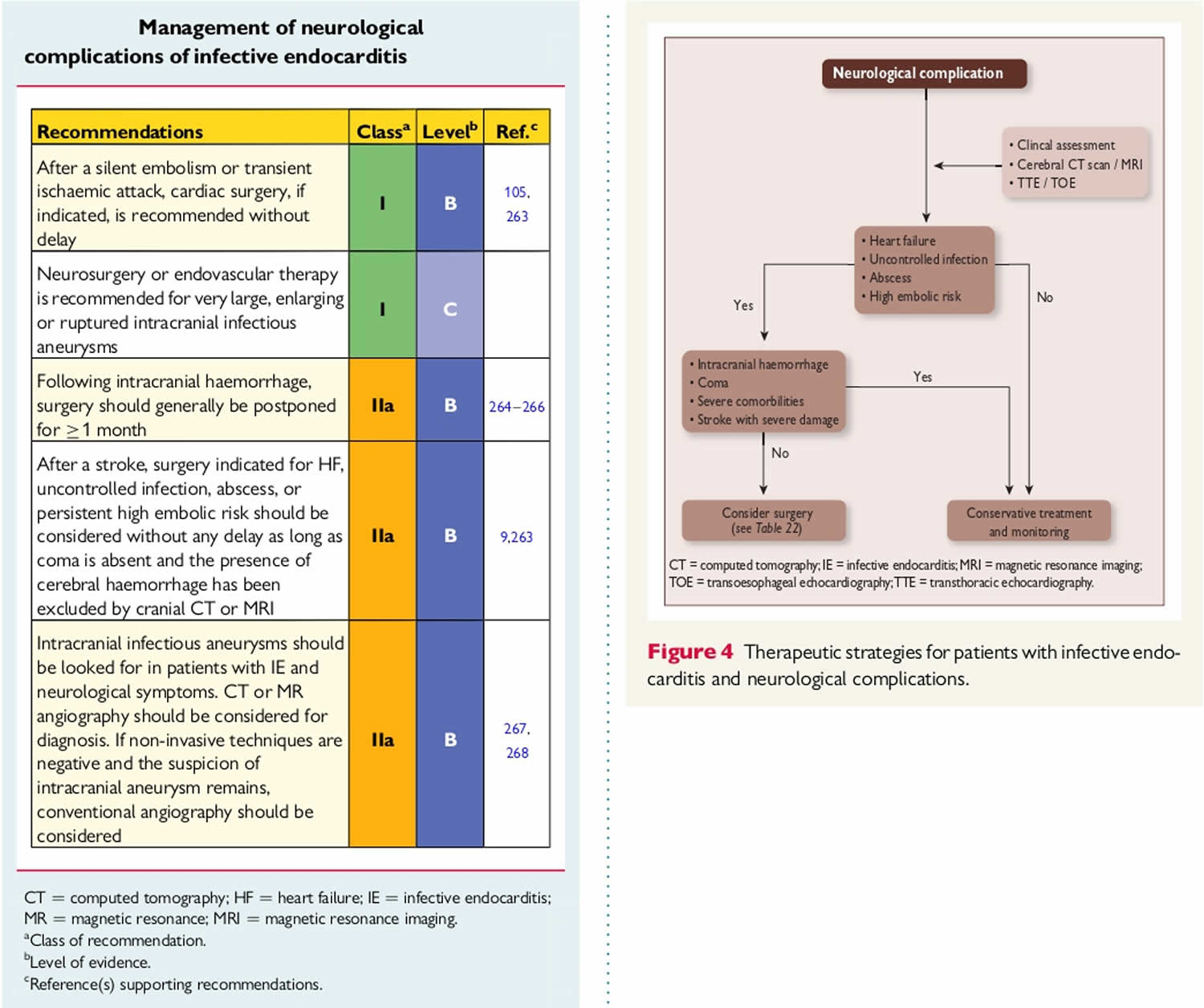
[Source 21 ]Figure 8. Infective endocarditis diagnostic algorithm
Footnotes: Diagnostic testing for identification of the microbiological origin of infective endocarditis. Peripheral blood cultures is the single most important test to order for suspected infective endocarditis. Cultures must be obtained prior to starting antibiotics in suspected endocarditis, even if this causes a short treatment delay. This diagnostic algorithm is intended for use in patients with clinical and/or echocardiographic findings suggestive of infective endocarditis based on the modified Duke criteria 22. Strong recommendations appear in boldface, with other diagnostic testing considerations shown in standard typeface. (1) Details on blood culture collection. (2) Coxiella burnetii anti-phase I IgG antibody titer of ≥1:800 is considered positive. (3) The sensitivity of Tropheryma whipplei PCR from blood in endocarditis is unknown; a negative result should not be used to rule out Tropheryma whipplei endocarditis. (4) If surgery is not performed, consider testing for noninfectious etiologies. (5) Histologic evaluation is used to evaluate for infectious and noninfectious etiologies and for correlation with microbiology test results. (6) Ideally, a representative sample of valvular tissue should be specifically collected for molecular testing in a sterile fashion in the operating room. (7) If sufficient valvular tissue is available after sampling for histopathological and molecular (microorganism-specific and broad-range) testing, consider culture and microbiology Gram stain. Due to the low sensitivity and specificity of culture, molecular testing should be prioritized over culture. (8) PAS-D, periodic acid-Schiff with diastase. Macrophages infected with Tropheryma whipplei will stain PAS positive following diastase digestion. (9) Examples include Mycoplasma hominis and Cutibacterium (formerly Propionibacterium) acnes PCR.
[Sources 23, 24 ]Endocarditis complications
In endocarditis, irregular growths made of germs and cell pieces form a mass in the heart called vegetations can break loose and travel to your brain, lungs, kidneys and other organs. They can also travel to the arms and legs.
Most common complications of infective endocarditis may include:
- Heart failure
- Heart valve damage
- Ischemic stroke
- Pockets of collected pus (abscesses) that develop in the heart, brain, lungs and other organs
- Blood clot in a lung artery (pulmonary embolism)
- Kidney damage and failure
- Enlarged spleen
- Interstitial bleeding
- Septic shock
Endocarditis may sometimes lead to sepsis, a medical emergency that happens when your body has an extreme response to the infection.
Endocarditis causes
Endocarditis occurs when germs enter your bloodstream, travel to your heart, and attach to abnormal heart valves or damaged heart tissue. Certain types of bacteria cause most cases, but fungi or other microorganisms also may be responsible. Usually, your immune system destroys harmful bacteria that make it into your bloodstream. Even if bacteria reach your heart, they may pass through without causing an infection. However, bacteria that live in your mouth, throat or other parts of your body, such as your skin or your gut, can sometimes cause serious infections like endocarditis under the right circumstances.
Bacteria, fungi or other germs that cause endocarditis might enter your bloodstream through:
- Everyday oral activities. Activities such as brushing your teeth, or other activities that could cause your gums to bleed, can allow bacteria to enter your bloodstream — especially if you don’t floss or your teeth and gums aren’t healthy.
- An infection or other medical condition. Bacteria may spread from an infected area, such as a skin sore. Other medical conditions, such as gum disease, a sexually transmitted infection or certain intestinal disorders — such as inflammatory bowel disease — can also give bacteria the opportunity to enter your bloodstream.
- Catheters. Bacteria can enter your body through a catheter — a thin tube that doctors sometimes use to inject or remove fluid from the body. This is more likely to occur if the catheter is in place for a long period of time.
- Needles used for tattoos and body piercing. The bacteria that can cause endocarditis can also enter your bloodstream through the needles used for tattooing or body piercing.
- Intravenous (IV) illegal drug use. Contaminated needles and syringes are a special concern for people who use illegal intravenous (IV) drugs, such as heroin or cocaine. Often, individuals who use these types of drugs don’t have access to clean, unused needles or syringes.
- Certain dental procedures. Some dental procedures that can cut your gums may allow bacteria to enter your bloodstream.
Bacteria can more easily attach to the lining of your heart (endocardium), if the lining’s surface is rough. You’re also more likely to develop endocarditis if you have faulty, diseased or damaged heart valves. However, endocarditis does occasionally occur in previously healthy individuals.
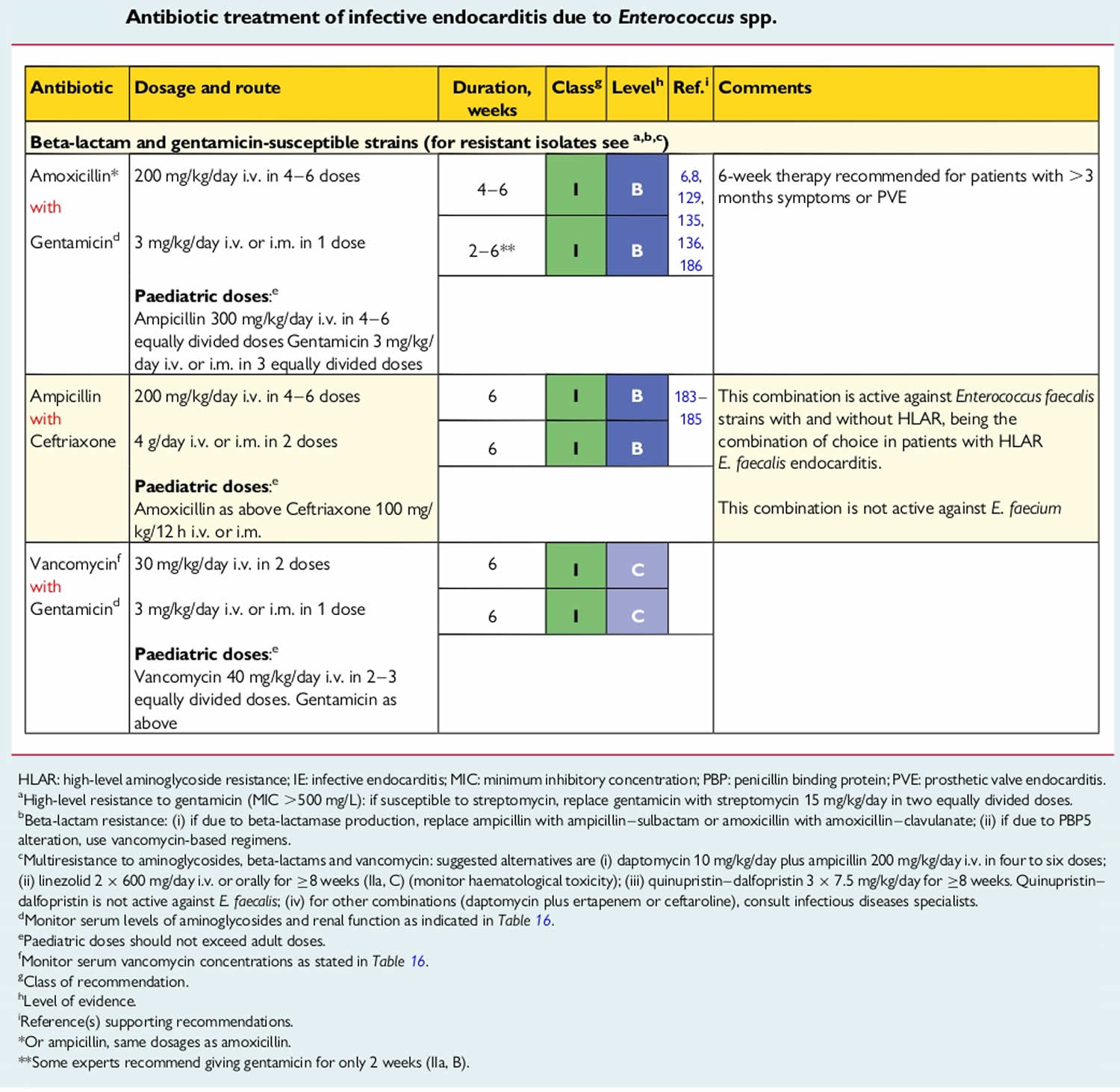
Infective endocarditis is primarily an inflammation of the endocardium caused by Gram-positive Streptococci, Staphylococci, and Enterococci bacteria 1. Together, these three groups of bacteria account for 80% to 90% of all infective endocarditis cases, with Staphylococcus aureus specifically responsible for around 30% of cases, while coagulase-negative Staphylococci accounting for ∼11% of cases in the developed world 25, 26, 27. Streptococci, primarily viridans group Streptococci, cause ∼30% of cases, with Streptococcus gallolyticus (a Streptococcus bovis group member) being involved in ∼20% to ∼50% of streptococcal cases 26, 27. Enterococci, especially Enterococcus faecalis, account for ∼10% of cases 26, 27. In addition to various Streptococci, Staphylococci, and Enterococci bacteria, other common colonizers of the oropharynx, such as the Haemophilus aphrophilus, Aggregatibacter actinomycetemcomitans (formerly Actinobacillus actinomycetemcomitans), Cardiobacterium hominis, Eikenella corrodens, Kingella kingae (HACEK organisms) can less frequently be the culprit bacteria 1. Less commonly, non-HACEK Gram-negative bacilli, such as the Enterobacteriaceae and nonfermenting Gram-negative bacilli have been previously identified as well but comprise only about 6% of total cases 24. Fungi are rare endocarditis causes, with Candida and Aspergillus infection in people with weakened immune system (immunocompromised patients) 1. A number of uncultivable or challenging to cultivate organisms cause endocarditis, the most common of which are Coxiella burnetii, Bartonella species, and Tropheryma whipplei 24.
Infective endocarditis most often involves the aortic or mitral valves, with tricuspid valve involvement accounting for fewer than 10% of cases often in association with injection drug use 26, 28, 29. Endocarditis associated with artificial heart valves or cardiovascular implantable electronic devices accounts for approximately one-third of cases and is most commonly caused by staphylococci 26, 29. Coagulase-negative Staphylococci are more frequent causes of prosthetic versus native valve endocarditis, while viridans group Streptococci more commonly cause native than prosthetic valve endocarditis 24. Although the majority of endocarditis cases are community acquired, health care-associated endocarditis is increasing and now accounts for approximately one-third of endocarditis cases in North America 26. Staphylococcus aureus, coagulase-negative Staphylococcus species, and Enterococci are most frequently detected in health care-associated cases 24. Organisms acquired in health care settings are notable for being increasingly resistant to antibacterial agents; methicillin-resistant Staphylococcus aureus (MRSA), for example, is more frequently associated with health care-acquired than community-acquired endocarditis, and most cases of endocarditis caused by non-HACEK Gram-negative bacilli are health care associated 30.
Table 1. Endocarditis Clinical Features According to Causative Organism
| Causative Organism(s) | Clinical Features of infective endocarditis |
|---|---|
| Staphylococcus aureus | Overall, Staphylococcus aureus infection is the most common cause of infective endocarditis, including prosthetic valve endocarditis, acute infective endocarditis, and intravenous drug abuser infective endocarditis. Approximately 35-60.5% of Staphylococcal bacteremias are complicated by infective endocarditis. More than half of cases are not associated with underlying valvular disease. The mortality rate for Staphylococcus aureus infective endocarditis is 40-50%. Staphylococcus aureus infection is the second most common cause of nosocomial bloodstream infections, second only to coagulase-negative staphylococci infection. The incidence of MRSA infections, both the hospital- and community-acquired varieties, has dramatically increased (50% of isolates). Sixty percent of individuals are intermittent carriers of MRSA or MSSA . The primary risk factor for Staphylococcus aureus bloodstream infection is the presence of intravascular lines. Other risk factors include cancer, diabetes, corticosteroid use, intravenous drug abuser, alcoholism, and renal failure. The realization that approximately 50% of hospital- and community-acquired staphylococcal bacteremias arise from infected vascular catheters has led to the reclassification of staphylococcal bloodstream infections. Bloodstream infections are carried not only in the hospital but also in any type of healthcare facility (eg, nursing home, dialysis center). Of Staphylococcus aureus bacteremia cases in the United States, 7.8% (200,000) per year are associated with intravascular catheters. |
| Streptococcus viridans | This organism accounts for approximately 50-60% of cases of subacute disease. Most clinical signs and symptoms are mediated immunologically. Streptococcus gordonii has migrated from Norway to the United States over the last 15 years. It characteristically is associated with a sterile inflammatory response manifest after the bacterial component has been successfully treated. |
| Streptococcus intermedius group | These infections may be acute or subacute. Streptococcus intermedius infection accounts for 15% of streptococcal infective endocarditis cases. Members of the Streptococcus intermedius group, especially Streptococcus anginosus, are unique among the streptococci in that they can actively invade tissue and form abscesses, often in the central nervous system (CNS). |
| Abiotrophia | Approximately 5% of subacute cases of infective endocarditis are due to infection with Abiotrophia species. They require metabolically active forms of vitamin B6 for growth. This type of infective endocarditis is associated with large vegetations that lead to embolization and a high rate of posttreatment relapse. |
| Group D streptococci | Most cases are subacute. The source is the gastrointestinal or genitourinary tract. It is the third most common cause of infective endocarditis. They pose major resistance problems for antibiotics. |
| Nonenterococcal group D | The clinical course is subacute. Infection often reflects underlying abnormalities of the large bowel (eg, ulcerative colitis, polyps, cancer). The organisms are sensitive to penicillin. |
| Group B streptococci | Acute disease develops in pregnant patients and older patients with underlying diseases (eg, cancer, diabetes, alcoholism). The mortality rate is 40%. Complications include metastatic infection, arterial thrombi, and congestive heart failure. It often requires valve replacement for cure. |
| Group A, C, and G streptococci | Acute disease resembles that of S aureus infective endocarditis (30-70% mortality rate), with suppurative complications. Group A organisms respond to penicillin alone. Group C and G organisms require a combination of synergistic antibiotics (as with enterococci). |
| Coagulase-negative S aureus | This causes subacute disease. It behaves similarly to S viridans infection. It accounts for approximately 30% of prosthetic valve endocarditis cases and less than 5% of native valve endocarditis cases. |
| Staphylococcus lugdunensis | Staphylococcus lugdunensis is another coagulase-negative Staphylococcus species but is extremely aggressive compared to coagulase-positive Staphylococcus aureus. Staphylococcus lugdunensis frequently causes infective endocarditis. |
| Pseudomonas aeruginosa | This is usually acute, except when it involves the right side of the heart in intravenous drug abuser infective endocarditis. Surgery is commonly required for cure. |
| HACEK (ie, Haemophilus aphrophilus, Aggregatibacter actinomycetemcomitans (formerly Actinobacillus actinomycetemcomitans), Cardiobacterium hominis, Eikenella corrodens, Kingella kingae) | These organisms usually cause subacute disease. They account for approximately 5% of infective endocarditis cases. They are the most common gram-negative organisms isolated from patients with infective endocarditis. Complications may include massive arterial emboli and congestive heart failure. Cure requires ampicillin, gentamicin, and surgery. |
| Fungal | These usually cause subacute disease. The most common organism of both fungal native valve endocarditis and fungal prosthetic valve endocarditis is Candida albicans. Fungal intravenous drug abuser infective endocarditis is usually caused by Candida parapsilosis or Candida tropicalis. Aspergillus species are observed in fungal prosthetic valve endocarditis and native valve infective endocarditis. Candida auris is progressively contaminating all types of healthcare facilities worldwide |
| Bartonella | The most involved species is Bartonella quintana. Infective endocarditis typically develops in homeless males who have extremely substandard hygiene. Bartonella must be considered in cases of culture-negative endocarditis among homeless individuals. |
| Multiple pathogens (polymicrobial | Pseudomonas and enterococci are the most common combination of organisms . It is observed in cases of intravenous drug abuser infective endocarditis The cardiac surgery mortality rate is twice that associated with single-agent infective endocarditis. |
Risk factors for endocarditis
Endocarditis rarely happens in people whose hearts are healthy. Having a faulty, diseased or damaged heart valve increases the risk of endocarditis. However, endocarditis may occur in those without heart valve problems. According to the American Heart Association, about 47,000 people in the United States get an endocarditis diagnosis each year. Historically, infective endocarditis has a tendency for males, with a male to female ratio of nearly 2 to 1 1. The average age of infectious endocarditis patients is now greater than 65 years old 1. This tendency for the elderly likely corresponds to the increased prevalence of risk factors such as prosthetic valves, indwelling cardiac devices, acquired valvular disease, hemodialysis, and diabetes within this age group 32. Although previously a major risk factor, rheumatic heart disease now only affects less than 5% of all cases in the modern antibiotic era 1. Recreational intravenous drug use represents a growing risk factor that now accounts for about 10% of all infectious endocarditis cases 33.
People most at risk of developing bacterial endocarditis include those who have:
- Acquired heart valve disease (for example, rheumatic heart disease) including mitral valve prolapse with valve regurgitation (leaking) and/or thickened valve leaflets.
- An artificial (prosthetic) heart valve, including bioprosthetic and homograft valves. Germs are more likely to attach to an artificial (prosthetic) heart valve than to a regular heart valve.
- Transcatheter aortic valve replacement (TAVR).
- Previous bacterial endocarditis. A history of endocarditis increases the risk of infection.
- Certain congenital (present at birth) heart defects. Being born with certain types of heart defects, such as an irregular heart or damaged heart valves, raises the risk of heart infections.
- Implanted heart device such as a pacemaker or implantable cardioverter-defibrillator (ICD), PICC line or subcutaneous port (e.g., used for chemotherapy) and left ventricular assist device (LVAD). Bacteria can attach to an implanted device, such as a pacemaker, causing an infection of the heart’s lining.
- A suppressed immune system.
- Illegal intravenous drug abuse habit. Using dirty needles can lead to infections such as endocarditis. Contaminated needles and syringes are a special concern for people who use illegal drugs, such as heroin or cocaine.
- Aortic stenosis.
- Hypertrophic cardiomyopathy (HCM).
- Hemodialysis (17 fold increase in endocarditis risk)
- Older age. Endocarditis occurs most often in adults over age 60.
- Recent hospitalization.
- Long-term catheter use. A catheter is a thin tube that’s used to do some medical procedures. Having a catheter in place for a long period of time (indwelling catheter) increases the risk of endocarditis.
- Poor care of the teeth and gums. A healthy mouth and healthy gums are essential for good health. If you don’t brush and floss regularly, bacteria can grow inside your mouth and may enter your bloodstream through a cut on your gums. Some dental procedures that can cut the gums also may allow bacteria to get in the bloodstream.
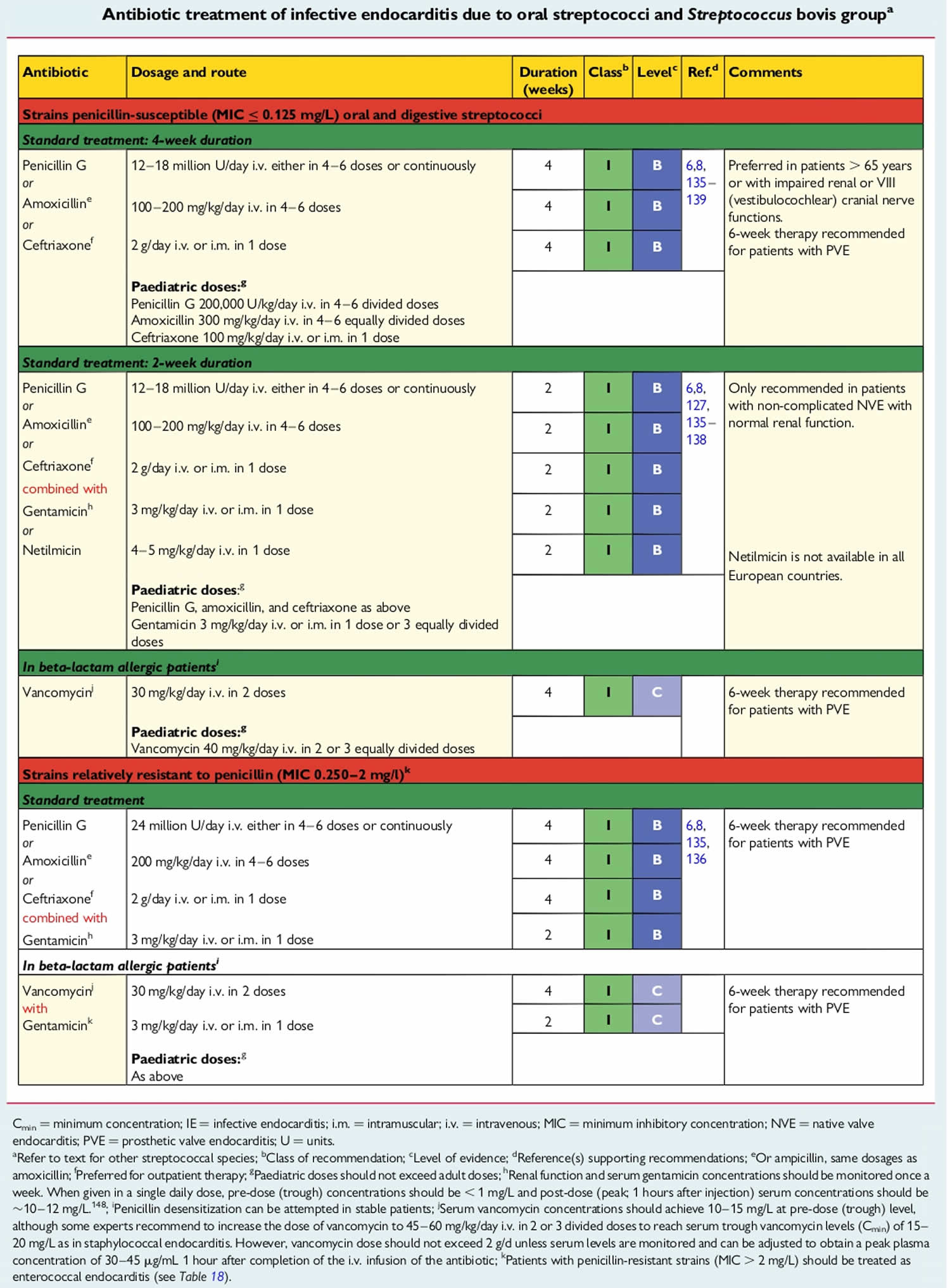
Native valve endocarditis
The following are the main underlying causes of native valve endocarditis:
- Rheumatic valvular disease (30% of native valve endocarditis) – Primarily involves the mitral valve
- Congenital heart disease (15% of native valve endocarditis) – Underlying causes include a patent ductus arteriosus, ventricular septal defect, tetralogy of Fallot, or any native or surgical high-flow lesion
- Mitral valve prolapse with an associated murmur (20% of native valve endocarditis)
- Degenerative heart disease – Including calcific aortic stenosis resulting from a bicuspid valve, Marfan syndrome, or syphilitic disease
Approximately 70% of infections in native valve endocarditis are caused by Streptococcus species, including Streptococcus viridans, Streptococcus bovis, and enterococci. Staphylococcus species cause 25% of cases and generally demonstrate a more aggressive acute course.
Prosthetic valve endocarditis
Early prosthetic valve endocarditis (PVE), which presents shortly after surgery, has a different bacteriology and prognosis than late prosthetic valve endocarditis, which presents in a subacute fashion similar to native valve endocarditis. Early prosthetic valve endocarditis may be caused by a variety of pathogens, including Staphylococcus aureus and Staphylococcus epidermidis. These hospital-acquired organisms often are methicillin-resistant Staphylococcus aureus (eg, MRSA) 34. Late disease most commonly is caused by Streptococci. Overall, coagulase-negative Staphylococci are the most frequent cause of prosthetic valve endocarditis (30%).
Staphylococcus aureus causes 17% of early prosthetic valve endocarditis and 12% of late prosthetic valve endocarditis.
Corynebacterium, nonenterococcal streptococci, fungi (eg, Candida albicans, Candida stellatoidea, Aspergillus species), Legionella, and the HACEK (ie, Haemophilus aphrophilus, Aggregatibacter actinomycetemcomitans (formerly Actinobacillus actinomycetemcomitans), Cardiobacterium hominis, Eikenella corrodens, Kingella kingae) organisms cause the remaining cases.
Infection associated with aortic valve prostheses is particularly associated with local abscess and fistula formation, and valvular dehiscence. This may lead to shock, heart failure, heart block, shunting of blood to the right atrium, pericardial tamponade, and peripheral emboli to the central nervous system and elsewhere.
Opioid use disorder infective endocarditis
Diagnosis of endocarditis in those who abuse IV drugs can be difficult and requires a high index of suspicion. Two thirds of patients have no previous history of heart disease or murmur on admission. A murmur may be absent in those with tricuspid disease, owing to the relatively small pressure gradient across this valve. Pulmonary manifestations may be prominent in patients with tricuspid infection: one third have pleuritic chest pain, and three quarters demonstrate chest radiographic abnormalities
Staphylococcus aureus is the most common (< 50% of cases) etiologic organism in patients with intravenous drug abuser infective endocarditis. MRSA accounts for an increasing portion of S aureus infections and has been associated with previous hospitalizations, long-term addiction, and nonprescribed antibiotic use. Groups A, C, and G streptococci and enterococci also are recovered from patients with intravenous drug abuser infective endocarditis.
Gram-negative organisms are involved infrequently. Pseudomonas aeruginosa and the HACEK family (Haemophilus aphrophilus, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae) are the most common examples 35.
Healthcare-associated infective endocarditis
Endocarditis may be associated with therapeutic modalities involving intravascular devices such as central or peripheral intravenous catheters, rhythm control devices such as pacemakers and defibrillators, hemodialysis shunts and catheters, and chemotherapeutic and hyperalimentation lines. These patients tend to have significant comorbidities, more advanced age, and predominant infection with Staphylococcus aureus. The mortality rate is high in this group.
The gram-positive cocci (ie, Staphylococcus aureus, coagulase-negative staphylococci, enterococci, nonenterococcal streptococci) are the most common pathogens of non-nosocomial healthcare-associated infective endocarditis (HCIE). Non-nosocomial healthcare-associated infective endocarditis (HCIE) occurs when patients acquire the infection outside the hospital but following contact with the healthcare system.
Fungal endocarditis
Fungal endocarditis is found in IV drug users and intensive care unit patients who receive broad-spectrum antibiotics 5. Blood cultures may be negative. Microscopic examination of large emboli may detect the organism.
Candida auris is particularly concerning since most infections are recognized in healthcare facilities and can rapidly spread throughout a given facility and between that facility and previously uninfected sites. There were at least 8200 cases in the United States in 2022. Cleaning or disinfecting infected surfaces, including patient’s skin, is ineffective. Candida auris typically is resistant to many antifungals 36, 37.
Culture-Negative Endocarditis
Many cases of culture-negative endocarditis are due to inappropriate institution antibiotics prior to obtaining adequately drawn blood cultures 38. Blood cultures are negative in 2% to 40% of cases of endocarditis, with some studies reporting blood culture-negative rates up to 71% 26, 27, 39, 40. The causes of so-called “culture-negative endocarditis” fall into two categories: negative blood cultures due to concomitant or antecedent antibacterial therapy or the presence of an organism that does not grow in routine blood cultures, with concomitant or antecedent antibacterial therapy being more common 24. Even if considered necessary, antibacterial agents should not be started in patients with suspected endocarditis until after blood cultures have been collected 24. For cases in which antibiotics have been administered prior to blood culture collection, consideration may be given to stopping antibiotics if possible, with recollection of blood cultures after an antibiotic-free period 24. While 7 to 10 days off antimicrobial therapy has been recommended, the ideal length of time needed off therapy is unknown and may vary depending on the infecting organism, antibiotics used, and duration of therapy administered 41. Nevertheless, many patients with bacterial endocarditis receive antibiotics without blood cultures having been appropriately collected, obscuring subsequent microbiologic diagnosis of endocarditis 24.
In patients who have not received antibiotics, the most common causes of culture-negative endocarditis are Coxiella burnetii and Bartonella species, with the former accounting for 28% to 37% and the latter accounting for 12% to 28% of cases 29, 42. Tropheryma whipplei causes up to 6% of cases of culture-negative endocarditis 29, 42, 43. Cutibacterium acnes, a rare cause of endocarditis, may cause culture-negative endocarditis due to the requirement for prolonged blood culture incubation for growth of some strains 44; in addition, some strains may not grow in blood cultures. Mycoplasmal endocarditis, while rare, is primarily caused by Mycoplasma hominis and is usually diagnosed using molecular methods. Traditionally, Mycoplasma pneumoniae has been considered an important cause of culture-negative endocarditis, but reported cases have relied primarily on serologic testing, rendering these historical diagnoses questionable. The incidence of extremely rare causes of endocarditis, such as those caused by Legionella species, Chlamydia/Chlamydophila species, and Mycoplasma species other than M. hominis, is unclear and requires further study, especially in light of evolving diagnostics.
A number of microbiologic tools have been developed to facilitate identification of an infectious agent in patients with suspected endocarditis and negative blood cultures. These technologies should be incorporated into a multimodal strategy to optimize detection of the etiological agent in culture-negative endocarditis.
Endocarditis pathophysiology
The normal heart valve endothelium is resistant to bacterial colonization upon intravascular challenge 45. Thus, the development of infective endocarditis requires the simultaneous occurrence of several independent factors: alteration of the cardiac valve surface to produce a suitable site for bacterial attachment and colonization; bacteraemia with an organism capable of attaching to and colonizing valve tissue; and creation of the infected mass or ‘vegetation’ by ‘burying’ of the proliferating organism within a protective matrix of serum molecules (for example, fibrin) and platelets (Figure 7).
Figure 7. Pathogenesis of endocarditis
Note: a. Pathogens gain access to the bloodstream, for example via an intravenous catheter, injection drug use or from a dental source. b Pathogens adhere to an area of abnormal cardiac valve surface. c Some pathogens, such as S. aureus, obtain intracellular access to the valve endothelium. d The infected vegetation is created by burying of the proliferating organism within a protective matrix of serum molecules. e –Vegetation particles can detach and disseminate to form emboli. These may lead to complications such as ischemic stroke, mycotic aneurysms and infarcts or abscesses at remote sites.
Marantic endocarditis
Marantic endocarditis, otherwise known as nonbacterial thrombotic endocarditis, is a well-documented phenomenon due to hypercoagulability from an underlying cause. While any of the cardiac valves may be involved, the mitral valve and/or aortic valve are the most common sites at which this process occurs. Marantic endocarditis has been associated with a variety of inflammatory states including malignancy 46. Marantic endocarditis is the result of a prothrombotic condition characterized by deposition of sterile fibrin and platelet-rich thrombi on previously undamaged heart valves. Cardiac function is generally unaffected.
The likelihood of systemic embolism secondary to marantic endocarditis is high (42%–50%) 47. Clinical presentation is most often a sudden neurological deficit. Associated morbidity and mortality are due to increased frequency of arterial embolic events, specifically in the central nervous system 48. The incidence of stroke due to marantic endocarditis (33%) is higher compared to infective endocarditis (19%) 47. Infarcts (strokes) due to marantic endocarditis tend to be multiple and variable-sized, with different characteristics from infarcts due to intracranial stenosis; the latter are commonly encountered in Asians and follow particular arterial territories 47.
The preferred diagnostic test for marantic endocarditis is a transesophageal echocardiography (TEE), which is more sensitive in the detection of valvular vegetations than the transthoracic approach 49. Recommended treatment is therapeutic anticoagulation with unfractionated heparin or LMWH (low molecular weight heparin) and treatment of the underlying cause 48. However, Pons et al 50 reported that anticoagulation with intravenous heparin does not play a role in the prevention of strokes in marantic endocarditis. Furthermore, there have been no studies defining the optimal treatment of marantic endocarditis. Also, the benefit of heparin compared to warfarin in the treatment of marantic endocarditis has not been studied.
A review of the literature from the past 3 decades reveals multiple case reports associating marantic endocarditis with various malignancies, most commonly of gynecologic origin 51. Surprisingly, although hypercoagulability is most often seen in patients with pancreatic cancer, marantic endocarditis has rarely been reported antemortem in this population 52. A prospective echocardiographic study of 200 living patients with solid tumors demonstrated a high prevalence of cardiac valvular vegetations with an overall incidence of 19%, including 3 of 6 pancreatic cancer patients (50%) 53. Recently, Smeglin et al. 54 documented the first case of an antemortem diagnosis of marantic endocarditis as the initial presentation of an underlying pancreatic cancer.
This study 46 suggests that prompt recognition and treatment of marantic endocarditis along with effective treatment of the underlying malignancy may improve the dismal prognosis of this syndrome.
Libman Sacks endocarditis
Libman-Sacks endocarditis, best characterized by Libman-Sacks vegetations, is common in systemic lupus erythematosus (SLE) and antiphospholipid syndrome 55. Libman-Sacks vegetations are sterile abnormal growths of tissue around the heart valves with an autoimmune-mediated inflammatory and thrombotic pathogenesis 56. The pathologic changes of Libman-Sacks endocarditis involve the formation of fibrin-platelet thrombi on the altered valve, the organization of which leads to valve fibrosis, edema, diffuse thickening, mild inflammatory changes, valve distortion, scarring, and subsequent valvular dysfunction such like stenosis or regurgitation 57. Libman-Sacks endocarditis often involve left heart valves, tricuspid lesions were very rare. And most Libman-Sacks endocarditis can be treated with medicine therapy while very few need surgical treatment.
Libman-Sacks vegetations are strong independent risk or pathogenic factors for stroke or TIA (transient ischemic attack or mini-stroke), focal brain lesions on MRI, or cognitive disability and can be further complicated with acute or chronic severe valve regurgitation, superimposed infective endocarditis, need for high risk valve surgery, and ultimately with death 58.
Endocarditis prevention
You can help prevent endocarditis in several ways, including:
- Know the signs and symptoms of endocarditis. See your doctor immediately if you develop any signs or symptoms, especially a fever that won’t go away, unexplained fatigue, any type of skin infection, or open cuts or sores that don’t heal properly.
- Pay special attention to your dental health — brush and floss your teeth and gums often, and have regular dental checkups.
- Have dental exams and cleaning at your dentist’s office every 6 months.
- Avoid procedures that may lead to skin infections, such as body piercings or tattoos.
- Make heart-healthy habits part of your daily life to help prevent heart disease.
- See your doctor right away if you have symptoms that could be endocarditis.
- Don’t use illegal IV drugs. Dirty needles can send bacteria into the bloodstream, increasing the risk of endocarditis.
People with the highest risk for bacterial endocarditis need antibiotics before dental visits or certain medical or surgical procedures. You may want to request an endocarditis wallet card from the American Heart Association (https://www.heart.org/-/media/files/health-topics/infective-endocarditis/infective-endocarditis-wallet-card.pdf?la=en). Ask your doctor if you’re part of the highest risk group. If so, let all your healthcare providers know about your risk.
If you’re at high risk of endocarditis, the American Heart Association recommends taking antibiotics an hour before having any dental work done (https://www.heart.org/-/media/files/health-topics/infective-endocarditis/infective-endocarditis-wallet-card.pdf?la=en).
Antibiotic prophylaxis is reasonable before dental procedures that involve manipulation of the gingival tissue, manipulation of the periapical region of teeth, or perforation of the oral mucosa in patients with valvular heart disease who have any of the following (https://www.heart.org/-/media/files/health-topics/infective-endocarditis/infective-endocarditis-wallet-card.pdf?la=en):
- Prosthetic cardiac valves, including transcatheter-implanted prostheses and homografts
- Prosthetic material used for cardiac valve repair, such as annuloplasty rings, chords or clips
- Previous bacterial endocarditis
- Unrepaired cyanotic congenital heart defect (CHD) or repaired congenital heart defect, with residual shunts or valvular regurgitation at the site of or adjacent to the site of a prosthetic patch or prosthetic device*
- A heart transplant with valve regurgitation due to a structurally abnormal valve
*Except for the conditions listed above, antibiotic prophylaxis before dental procedures is not recommended for any other types of congenital heart defect.
If you have endocarditis or any type of congenital heart disease, talk to your dentist and other care providers about your risks and whether you need preventive antibiotics.
Preventive antibiotics
Certain dental and medical procedures may allow bacteria to enter your bloodstream. For some people with heart disease or damaged or diseased heart valves, taking antibiotics before these procedures can help destroy or control the harmful bacteria that may lead to endocarditis. This is because these people are more at risk of developing endocarditis after having these procedures.
In the past, doctors gave antibiotics to many people before dental or other surgical procedures, such as procedures involving the intestinal or urinary tracts, even if they weren’t at high risk of developing endocarditis. However, antibiotics are no longer recommended before all dental or other surgical procedures, or for all people. As doctors have learned more about endocarditis prevention, they’ve realized endocarditis is much more likely to occur from exposure to random germs than from a standard dental exam or surgery.
For patients whose heart conditions put them at the highest risk for adverse events from infective endocarditis, the American Heart Association 59 recommends antibiotics before certain dental procedures. These include procedures that involve manipulation of gingival tissue or the periapical region of teeth, or perforation of the oral mucosa.
The American Heart Association has an endocarditis wallet card in English and Spanish. People who have been told that they need to take antibiotics should carry it. You can get it from here: http://www.heart.org/idc/groups/heart-public/@wcm/@hcm/documents/downloadable/ucm_307644.pdf
Show the card to your dentist or physician. It will help them take the precautions needed to protect your health.
If you’re at risk of endocarditis, let your doctor and dentist know before having any dental work. They will decide whether you need antibiotics before any dental procedures.
It’s still important to take good care of your teeth through brushing and flossing, since doctors have some concern that infections in your mouth from poor oral hygiene might increase the risk of germs entering your bloodstream. In addition to brushing and flossing, regular dental exams are an important part of maintaining good oral health.
Endocarditis guidelines
Endocarditis prophylaxis – American Heart Association Guidelines
Dental Procedures and Infective Endocarditis
In the past, patients with nearly every type of congenital heart defect needed to receive antibiotics one hour before dental procedures or operations on the mouth, throat, gastrointestinal genital, or urinary tract. However, in 2007 the American Heart Association simplified its recommendations. Today, antibiotics before dental procedures are only recommended for patients with the highest risk of infective endocarditis, those who have 59:
- A prosthetic heart valve or who have had a heart valve repaired with prosthetic material.
- A history of endocarditis.
- A heart transplant with abnormal heart valve function
- Certain congenital heart defects including:
- Cyanotic congenital heart disease (birth defects with oxygen levels lower than normal), that has not been fully repaired, including children who have had a surgical shunts and conduits.
- A congenital heart defect that’s been completely repaired with prosthetic material or a device for the first six months after the repair procedure. †
- Repaired congenital heart disease with residual defects, such as persisting leaks or abnormal flow at or adjacent to a prosthetic patch or prosthetic device.
Key changes for patients with congenital heart defects
Preventive antibiotics are no longer recommended for any other congenital heart disease than these:
- Cyanotic congenital heart disease (birth defects with oxygen levels lower than normal), that has not been fully repaired, including children who have had a surgical shunts and conduits.
- A congenital heart defect that’s been completely repaired with prosthetic material or a device for the first six months after the repair procedure. †
- Repaired congenital heart disease with residual defects, such as persisting leaks or abnormal flow at or adjacent to a prosthetic patch or prosthetic device.
Additionally, taking antibiotics just to prevent endocarditis is not recommended for patients who have procedures involving the reproductive, urinary or gastrointestinal tracts.
Note: † Prophylaxis is reasonable because endothelialization of prosthetic material occurs within six months after the procedure.
Antibiotic prophylaxis against infective endocarditis is not recommended routinely:
- for people undergoing dental procedures
- for people undergoing non‑dental procedures at the following sites:
- upper and lower gastrointestinal tract
- genitourinary tract; this includes urological, gynaecological and obstetric procedures, and childbirth
- upper and lower respiratory tract; this includes ear, nose and throat procedures and bronchoscopy.
Chlorhexidine mouthwash should not be offered as prophylaxis against infective endocarditis to people at risk of infective endocarditis undergoing dental procedures.
Adults and children with structural cardiac defects at risk of developing infective endocarditis
The following cardiac conditions as being at increased risk of developing infective endocarditis:
- acquired valvular heart disease with stenosis or regurgitation
- hypertrophic cardiomyopathy
- previous infective endocarditis
- structural congenital heart disease, including surgically corrected or palliated structural conditions, but excluding isolated atrial septal defect, fully repaired ventricular septal defect or fully repaired patent ductus arteriosus, and closure devices that are judged to be endothelialised
- valve replacement.
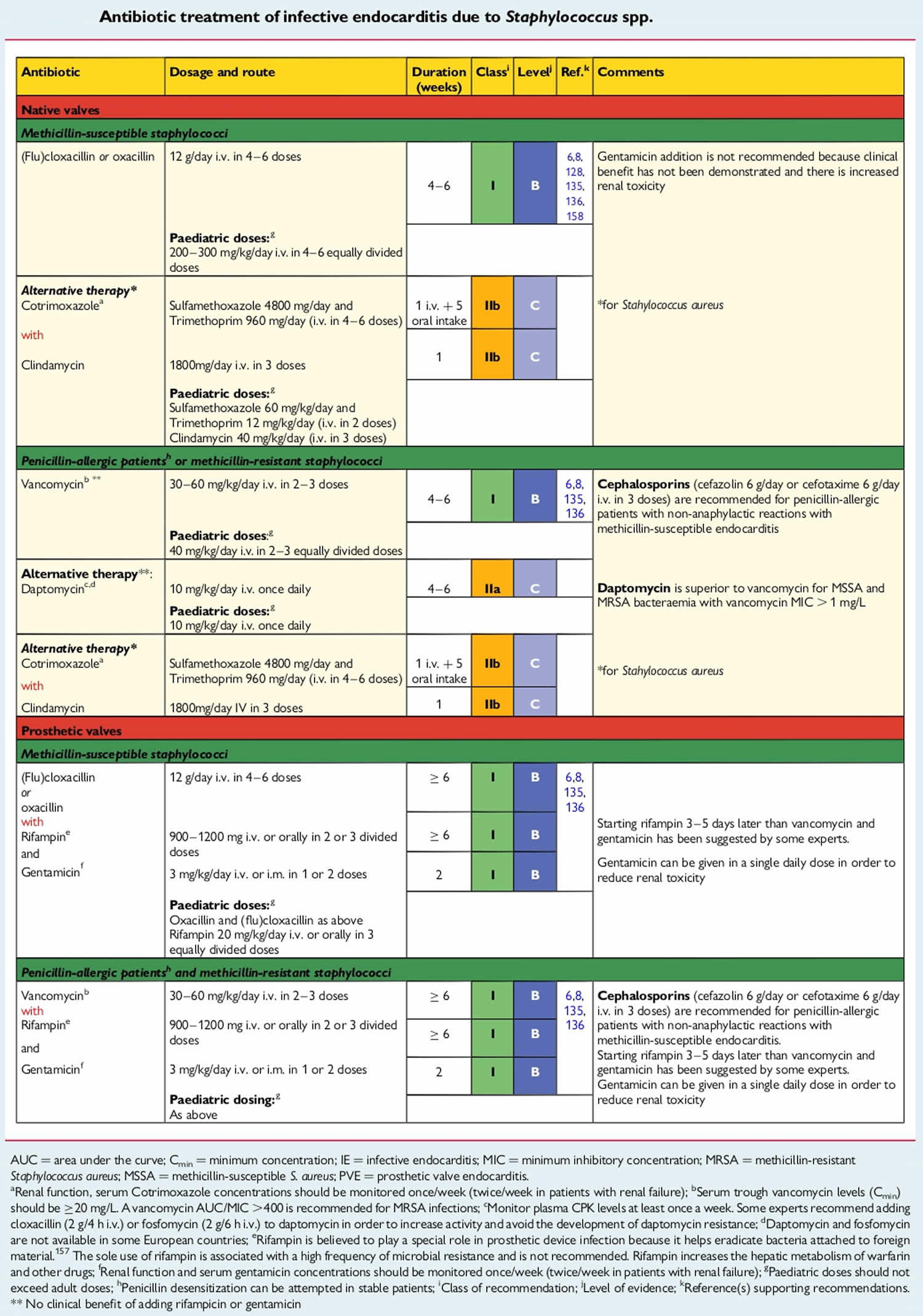
Antibiotic Regimens
An antibiotic for prophylaxis should be administered in a single dose before the procedure. If the dosage of antibiotic is inadvertently not administered before the procedure, the dosage may be administered up to 2 hours after the procedure. However, administration of the dosage after the procedure should be considered only when the patient did not receive the pre-procedure dose. Some patients who are scheduled for an invasive procedure may have a coincidental endocarditis. The presence of fever or other manifestations of systemic infection should alert the provider to the possibility of infective endocarditis. In these circumstances, it is important to obtain blood cultures and other relevant tests before administration of antibiotics intended to prevent infective endocarditis. Failure to do so may result in delay in diagnosis or treatment of a concomitant case of infective endocarditis.
Table 2. Antibiotic Regimens for a Dental Procedure
| Situation | Agent | Regimen: Single Dose 30 to 60 min Before Procedure | |
|---|---|---|---|
| Adults | Children | ||
| Oral | Amoxicillin | 2 g | 50 mg/kg |
| Unable to take oral medication | Ampicillin OR Cefazolin or ceftriaxone | 2 g IM or IV | 50 mg/kg IM or IV |
| 1 g IM or IV | 50 mg/kg IM or IV | ||
| Allergic to penicillins or ampicillin—oral | Cephalexin*† OR Clindamycin OR Azithromycin or clarithromycin | 2 g | 50 mg/kg |
| 600 mg | 20 mg/kg | ||
| 500 mg | 15 mg/kg | ||
| Allergic to penicillins or ampicillin and unable to take oral medication | Cefazolin or ceftriaxone† OR Clindamycin | 1 g IM or IV | 50 mg/kg IM or IV |
| 600 mg IM or IV | 20 mg/kg IM or IV | ||
Footnotes:
* Other first- or second-generation oral cephalosporin in equivalent adult or pediatric dosage.
† Cephalosporins should not be used in an individual with a history of anaphylaxis, angioedema, or urticaria with penicillins or ampicillin.
Abbreviations: IM = intramuscular; IV = intravenous.
[Source 59 ]Infective endocarditis signs and symptoms
Symptoms of endocarditis can vary from person to person. Infective endocarditis may develop slowly or suddenly. It depends on the type of germs causing the infection and whether there are other heart problems. The symptoms of acute infective endocarditis usually begin with fever, chills, fast heart rate, fatigue, night sweats, aching joints and muscles, persistent cough or swelling in the feet, legs or abdomen. The symptoms of chronic infective endocarditis may include fatigue, mild fever, a moderately fast heart rate, weight loss, sweating and a low red blood cell count (anemia).
Common symptoms of infective endocarditis include:
- Fever above 100°F (38.4°C).
- Aching joints and muscles
- Chest pain when you breathe
- Fatigue
- Flu-like symptoms, such as fever and chills
- Sweats or chills, particularly night sweats.
- Shortness of breath
- Swelling in the feet, legs or belly
- A new or changed whooshing sound in your heart (heart murmur)
Less common endocarditis symptoms can include:
- Poor appetite or unexplained weight loss
- Blood in the urine
- Tenderness under the left rib cage (spleen)
- Painless red, purple or brown flat spots on the soles bottom of the feet or the palms of the hands (Janeway lesions)
- Painful red or purple bumps or patches of darkened skin (hyperpigmented) on the tips of the fingers or toes (Osler nodes)
- Tiny purple, red or brown round spots on the skin (petechiae), in the whites of the eyes or inside the mouth.
- Wound or cut that won’t heal.
- Red, warm or draining sore.
- Sore throat, scratchy throat or pain when swallowing.
- Sinus drainage, nasal congestion, headaches or tenderness along your upper cheekbones.
- Persistent dry or a moist cough that lasts more than two days.
- White patches in your mouth or on your tongue.
- Nausea, vomiting or diarrhea.
- Emboli (small blood clots), hemorrhages (internal bleeding) or stroke.
Classic findings for endocarditis (<5% sensitive, mostly seen in subacute endocarditis) (Figure 5) 21:
- Splinter hemorrhages.
- Conjunctival petechiae.
- Janeway lesions (painless red/blue macules on palms and soles).
- Osler’s nodes (painful swelling in pulp of fingers).
Left-sided endocarditis signs and symptoms 23:
- Fever above 100°F (38.4°C) (~80% sensitivity)
- Fever plus IV drug use indicates ~15% risk of endocarditis.
- Flu-like, nonspecific illness (e.g., chills, night sweats, headache).
- Heart failure from valve regurgitation (~25%).
- Septic shock (e.g., Staph aureus causing acute bacterial endocarditis).
- Systemic emboli (e.g., ischemic stroke, kidney infarction; 25%):
- Stroke in a young patient is a classic endocarditis presentation.
- Delirium due to multifocal emboli (with no clinically obvious focal neurologic lesion).
Right-sided endocarditis signs and symptoms 23:
- Fever above 100°F (38.4°C).
- Septic pulmonary emboli:
- Will often initially mimic pneumonia (with a constellation of respiratory failure, pulmonary infiltrates, and fever) or pulmonary embolism.
- May eventually lead to hemoptysis, pneumothorax, or empyema.
- The key radiologic finding is multiple pulmonary nodules that eventually cavitate.
Signs of endocarditis
There are four peripheral signs of infective endocarditis: Roth spots, Osler nodes, Janeway lesions, and splinter hemorrhages 60. Sandre and Shafran 61 reported the incidence of peripheral signs of endocarditis as follows: 39% for splinter hemorrhages, 6.7% for Osler nodes, 2.2% for Janeway lesions, and 3% for Roth spots. Patients with dermatological manifestations of infective endocarditis have significantly higher complication rates 62.
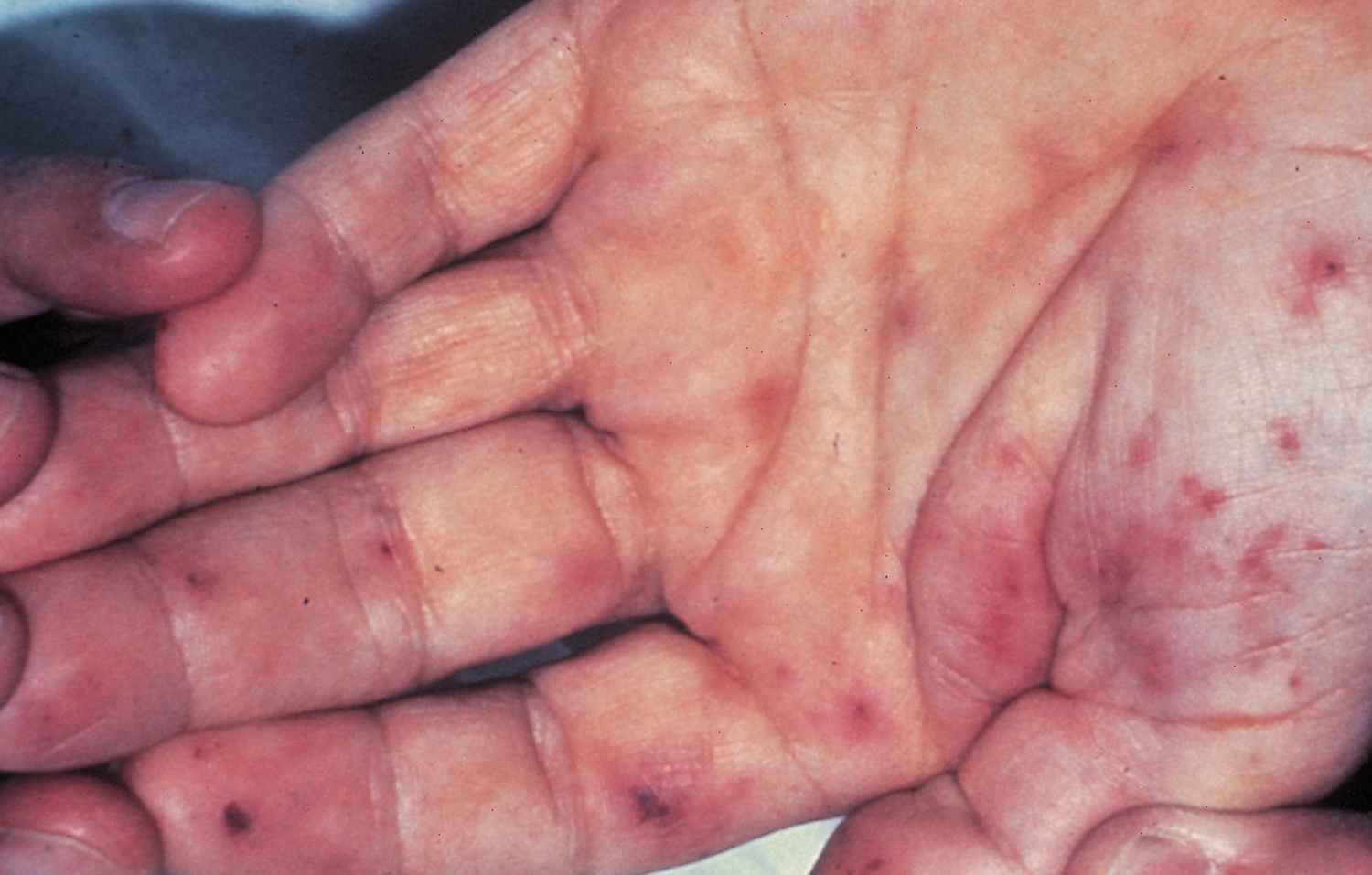
Osler nodes
Osler nodes also called Osler’s node are red-purple, slightly raised, tender or painful lumps, often with a pale center typically found on the tip of the fingers and/or toes 63, 64, 65. Pain often precedes the development of the visible lesion by up to 24 hours. Osler’s nodes can occur at any time during the course of bacterial endocarditis, usually subacute and last from hours to several days. Osler’s node was first described by William Osler 65.
Figure 9. Osler’s nodes (tender red spots or nodules under the skin of the toes)
Figure 10. Osler nodes and Janeway lesions
Footnote: Osler nodes (black arrow) seen in the palm of the left hand, and Janeway lesions (red arrow) seen on the finger pulp and palm of the left hand.
[Source 66 ]Janeway lesions
Janeway lesions are painless (non-tender) often hemorrhagic (bleeding into the skin) slightly nodular lesions that occur mostly on palms and soles on the thenar and hypothenar eminences (at the base of the thumb and little finger respectively) 67. Janeway lesions tend to last days to weeks before healing totally 68. Janeway lesions are more commonly seen in acute form of bacterial endocarditis, when bacteria such as Staphylococcus aureus may be cultured from them 68. The histology is usually consistent with microabscesses in the dermis (i.e. bacteria may be found within the blood in the capillaries with neutrophil infiltration) with thrombosis of small vessels, without evidence of vasculitis 69, 70, 71. Janeway lesions are associated with embolic events other than cerebral emboli 72.
Figure 11. Janeway lesions (A, Nontender, small purpuric spots on all toes. B, Linear reddish-brown streaks on the distal portions of the nail plates of all fingers)
Figure 12. Janeway lesions (non-tender hemorrhagic macules and papules over the palms and volar aspect of the fingers)
Figure 13. Conjunctival Petechiae
Figure 14. Conjunctival Hemorrhage
Splinter hemorrhages
Splinter hemorrhage in the proximal nail plate is also a sign of bacterial endocarditis. Splinter hemorrhages are small areas of longitudinal bleeding (hemorrhage) under the fingernails or toenails and look like a wood splinter. Seen end-on, splinter hemorrhage is in the lower part of the nail plate or underneath it. Splinter hemorrhages in nails are formed by the hemorrhage of blood from the rupture of the longitudinally oriented blood vessels of the nail bed. Splinter hemorrhages look like thin, red to reddish-brown lines of blood under the nails. Splinter hemorrhages run in the direction of nail growth. The underlying blood will attach to the nail plate and seems to move up as the nail grows. Splinter hemorrhages can be single or multiple.
Figure 15. Splinter hemorrhage with bacterial endocarditis
Roth spots
Roth spots appear as white-centered retinal hemorrhages on a fundoscopic examination 73, 74, 74. The white center of a Roth spot is thought to be a platelet-fibrin plug 75, 76, 76. These platelet-fibrin plugs presumably form in response to vascular leaks brought about by elevated intravascular pressure, ischemia, increased capillary fragility, or combinations thereof 76. In most cases, emboli appear to play no role 77. Although Roth spots are a classic peripheral sign of endocarditis, they are also present in other conditions, including hematologic malignancies, connective tissue diseases, vasculitis, anemia, hypertension, diabetes mellitus, human immunodeficiency virus infections, and intracranial hemorrhages 77.
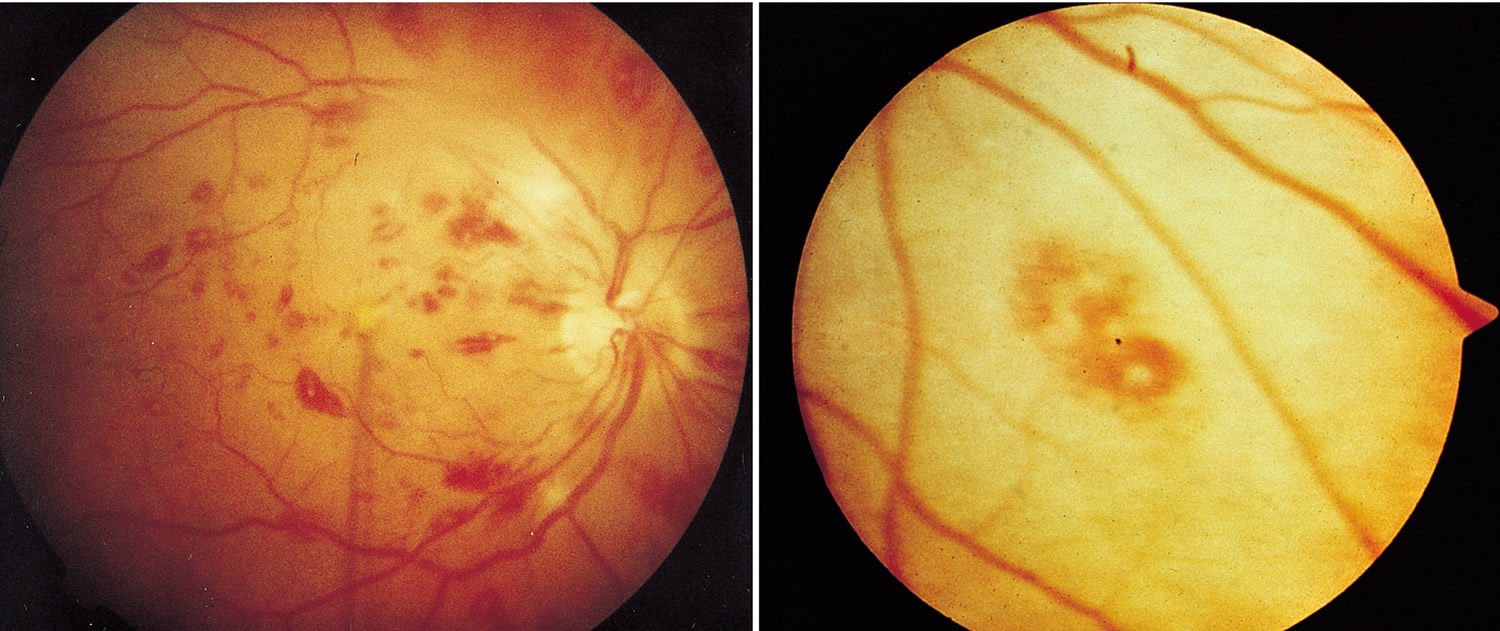
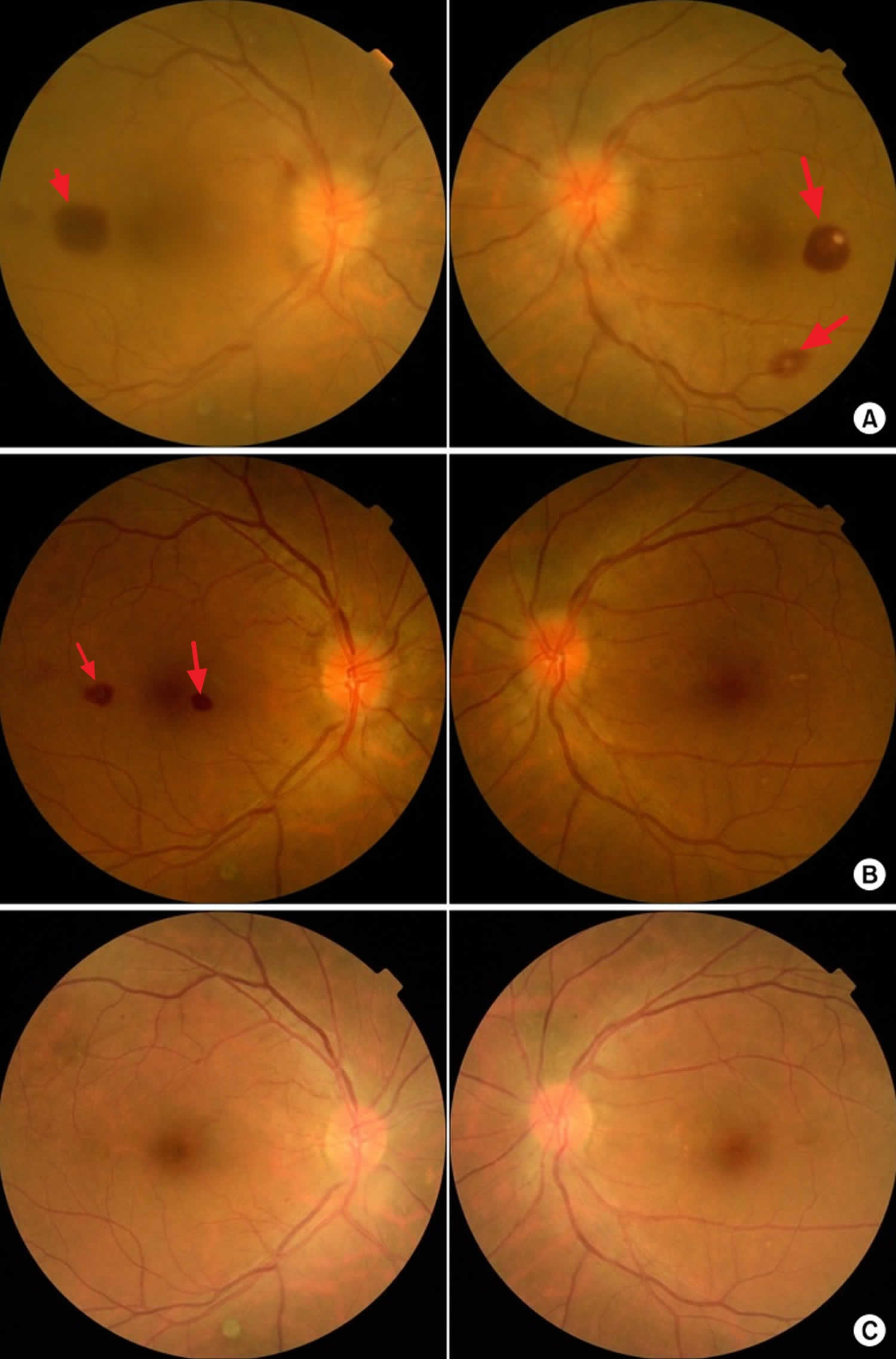
Figure 16. Infective Endocarditis – Retinal Roth Spots (retinal hemorrhages) – image on the right is a close up image of a Roth spot showing a central clearing
Figure 17. Roth spots
Footnote: Fundoscopic findings of Roth spots. (A) Preoperative findings. (B) 2 weeks postoperatively. (C) 6 weeks postoperatively.
[Source 75 ]Endocarditis diagnosis
Your doctor may suspect endocarditis based on your medical history, signs and symptoms you’re experiencing, and your test results. A diagnosis of endocarditis is usually based on several factors instead of a single positive test result or symptom.
Your doctor may order several tests to help make a positive diagnosis, including:
- Blood tests. A blood culture test is used to identify any bacteria or fungi in your bloodstream, and it’s the most important test your doctor will perform. Blood tests can also help your doctor identify certain conditions that can be a sign of endocarditis, such as anemia — a shortage of healthy red blood cells.
- Echocardiogram. An echocardiogram (heart ultrasound scan) uses sound waves to produce images of your heart while it’s beating. An echocardiogram can show an abnormal bacterial growth on the heart valve. Your doctor may use two different types of echocardiograms to help diagnose endocarditis.
- In a standard (transthoracic) echocardiogram (TTE), a wandlike device (transducer) is moved over the chest area. The device directs sound waves at the heart and records them as they bounce back.
- In a transesophageal echocardiogram (TEE), a flexible tube containing a transducer is guided down your throat and into the tube connecting the mouth to the stomach (esophagus). A transesophageal echocardiogram provides much more detailed pictures of the heart than is possible with a standard transthoracic echocardiogram.
- Electrocardiogram (ECG). While an ECG isn’t specifically used to diagnose endocarditis, it can show your doctor if something is affecting your heart’s electrical activity. During an ECG, sensors that can detect your heart’s electrical activity are attached to your chest, arms and legs. This test is used to measure the timing and duration of each electrical phase in your heartbeat.
- Chest X-ray. X-ray images help your doctor see the condition of your lungs and heart. Your doctor can use X-ray images to see if endocarditis has caused your heart to enlarge or if any infection has spread to your lungs.
- Computerized tomography (CT) scan or magnetic resonance imaging (MRI). You may need a CT scan or an MRI scan of your brain, chest or other parts of your body if your doctor thinks that infection has spread to these areas.
Duke Criteria for the diagnosis of infective endocarditis
The Duke criteria are used to rule in or rule out endocarditis. In 1994, a group at Duke University proposed standardized criteria for assessing patients with suspected infective endocarditis 78. The Duke criteria combine the clinical, microbiologic, pathologic, and echocardiographic findings of a specific case 78. The usefulness of Duke criteria in assessing patients with potential infective endocarditis has been validated in several subsequent studies 17, 79, 80, 81, 82, 83, 84, 85, 86, 87. The specificity of the initially proposed Duke criteria (the ability to reject the diagnosis correctly) was high 87 and the negative predictive value was greater than 92 percent 88. Also, a retrospective study of 410 patients with diagnosed endocarditis found that the Duke criteria had good (72 to 90 percent) agreement with clinical assessment by infectious-disease experts 85. Most discrepancies occurred when the experts rejected cases categorized as possible endocarditis according to the Duke criteria. Misclassification of culture-negative cases, the increasing role of transesophageal echocardiography, the relative risk of endocarditis in Staph. aureus bacteremia, and the overly broad categorization of cases as “possible” were problems with the original criteria. A modified version of the Duke criteria has recently been proposed 89.
Duke criteria for diagnosing infective endocarditis 17:
Major criteria
- Blood culture typical of infective endocarditis, single positive culture for C. burnetti, or immunoglobulin G antiphase 1 level of ≥1/800. Major blood culture criteria for infective endocarditis include the following:
- 2 blood cultures positive for organisms typically found in patients with infective endocarditis
- Blood cultures persistently positive for 1 of these organisms, from cultures drawn more than 12 hours apart
- Three or more separate blood cultures drawn at least 1 hour apart
- Echocardiogram positive for infective endocarditis. Major echocardiographic criteria include the following:
- Echocardiogram positive for infective endocarditis, documented by an oscillating intracardiac mass on a valve or on supporting structures, in the path of regurgitant jets, or on implanted material, in the absence of an alternative anatomic explanation
- Myocardial abscess
- Development of partial dehiscence of a prosthetic valve
- New-onset valvular regurgitation
Minor criteria
- Predisposing heart condition or injection drug use
- Fever (temperature of >100.4°F [>38°C])
- Vascular phenomena, including major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhage, conjunctival hemorrhage, or Janeway lesions
- Immunologic phenomena such as glomerulonephritis, Osler nodes, Roth spots, or rheumatoid factor
- Positive blood culture results not meeting major criteria or serologic evidence of active infection with an organism consistent with infective endocarditis
- Echocardiogram results consistent with infective endocarditis but not meeting major echocardiographic criteria
Definite endocarditis
- (i) 2 major clinical criteria OR
- (ii) 1 major clinical criterion and 3 minor clinical criteria OR
- (iii) 5 minor clinical criteria
Possible endocarditis
- (i) 1 major clinical criterion and 1 minor clinical criterion OR
- (ii) 3 minor clinical criteria
Rejected endocarditis
- (i) Firm alternative diagnosis is made explaining evidence of infective endocarditis;
- (ii) Resolution of infective endocarditis syndrome with antibiotic therapy for ≤4 days ;
- (iii) No pathologic evidence of infective endocarditis at surgery or autopsy, with antibiotic therapy for ≤4 days;
- (iv) Fail to meet criteria for for possible or definite infective endocarditis.
Peripheral blood cultures
Since endocarditis is associated with the persistent presence of infecting microorganisms in your blood, for this reason, blood cultures is the single most important test to order for suspected endocarditis to find the germs in the bloodstream that is causing infective endocarditis. Cultures must be obtained prior to starting antibiotics in suspected endocarditis, even if this causes a short treatment delay.
Routine blood cultures incubated on modern automated, continuous-monitoring blood culture systems allow recovery of almost all easily cultivable agents of endocarditis without additional specialized testing, such as prolonged incubation or terminal subculture. Recommendations regarding the number and timing of blood cultures differ by guideline set.
- The American Heart Association and the European Society of Cardiology recommend at least 3 sets of blood cultures collected from different venipuncture sites, with at least 1 hour between the first and last draw 19, 28.
- The British Society for Antimicrobial Chemotherapy (BSAC) recommends collection of 2 sets of blood cultures within 1 hour of each other in patients with suspected endocarditis and acute sepsis and 3 sets of blood cultures spaced ≥6 hours apart in cases of suspected subacute or chronic endocarditis 41.
3 sets of blood cultures, with each set including one aerobic and one anaerobic bottle, are collected. Alternatively, 2 sets may be collected, with 2 aerobic and 1 anaerobic bottle per set (i.e., a total of six blood culture bottles) 90. Yield of blood cultures is directly related to volume of blood cultured, with properly filled blood culture bottles (i.e., 10 ml of blood per Bactec or BacT/Alert bottle) being essential. Most, if not all, blood cultures from patients with endocarditis caused by microorganisms able to grow in blood culture systems should be positive, provided that blood cultures are appropriately collected and drawn prior to the administration of antimicrobial therapy; a single positive blood culture does not typically represent an endocarditis pathogen. Although the concept of spacing blood culture draws to detect continuous bacteremia is promulgated in the above-referenced guidelines, separation of blood culture draws over time is not the norm for routine blood culture draws. We are not aware of evidence supporting the value of spaced blood culture draws for etiologic diagnosis of endocarditis; for these reasons, we do not recommend routinely spacing blood culture draws in cases of suspected endocarditis.
Standard blood culture incubation times of 5 days are adequate for recovery of almost all cultivable causes of endocarditis, including Candida species. The Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, and Kingella species (HACEK organisms) were classically considered challenging to detect in blood cultures due to their fastidious nature; accordantly, in the past, prolonged incubation times were advised. With current blood culture systems, extended incubation (and terminal blind subculture) is unnecessary for recovery of these organisms, as they are easily grown and detected within the standard 5-day incubation period 91, 92. Current blood culture systems also contain sufficient supplements to support growth of Abiotrophia and Granulicatella species (nutritionally variant streptococci). Brucella species are infrequent causes of endocarditis in the United States, and detection in routine blood cultures is typically achieved within the standard 5-day incubation period 93; serologic testing may be helpful if exposures are suggestive of Brucella endocarditis. Cutibacterium (formerly Propionibacterium) acnes deserves special consideration, however, as some strains of this species may require prolonged blood culture incubation (e.g., 14 days) 44. The Clinical and Laboratory Standards Institute (CLSI) guidelines recommend terminal subculture to chocolate agar if blood cultures are negative at 5 days and an endocarditis diagnosis is under consideration 94. However, current evidence fails to support the usefulness of blind subculture, and this practice is not recommended in the British Society for Antimicrobial Chemotherapy (BSAC) guidelines 41, 95. Fungal endocarditis is most commonly caused by Candida species, which should grow in routine blood cultures. Noncandidal fungal causes of endocarditis (e.g., Histoplasma capsulatum, Aspergillus species) are rare, require specialized testing (e.g., antigen detection, specialized fungal blood cultures), and should only be considered in patients with specific risks for these types of endocarditis (e.g., malignancy, injection drug use, prolonged health care exposure, presence of a prosthetic heart valve) after more common causes have been excluded 24.
Number of cultures:
- One “set” of cultures = two bottles (anaerobic & aerobic) drawn from a single location.
- Ideally three sets should be obtained from three different locations (two sets are OK if this isn’t possible).
Location of cultures:
- Ideally cultures should be obtained from a fresh peripheral stick.
- If this isn’t feasible, obtain blood wherever you can get it. For example, obtaining blood from a freshly placed central line is OK.
Timing
- In endocarditis, bacteremia is generally constant, so there is little rationale to space cultures out over time.
- Don’t worry about the timing of cultures – the key thing is to get a lot of cultures and fill the culture bottles fully. (Get a lot of blood; more blood removed = higher likelihood of capturing a causative pathogen).
Serologic testing
For organisms that do not grow in routine bacterial cultures (e.g., Coxiella burnetii) or are especially fastidious (e.g., Bartonella species), serologic evaluation may aid in diagnosis. In one study, when evaluated in conjunction with blood cultures, systematic serologic testing established an etiological diagnosis in an additional 8% (34/425) of patients 27. In a separate investigation focused on culture-negative endocarditis, serology provided a diagnosis in 77% (268/348) of cases 42. Organisms for which serologic tests have been shown to aid in the diagnosis of endocarditis include Coxiella burnetii and Bartonella species and, in areas where Brucella endocarditis occurs, Brucella species. Generally, these pathogens cause subacute endocarditis resulting in elevated IgG titers. Serology for Coxiella burnetii is the best established serologic test for the diagnosis of endocarditis and is included as a major criterion in the modified Duke criteria 19, 22. In chronic Q fever with endocarditis, anti-phase I IgG Coxiella burnetii titers of ≥1:800 are diagnostic. Aside from that for Coxiella burnetii, specific serological criteria for the diagnosis of endocarditis have not been incorporated into the modified Duke criteria, although Bartonella endocarditis is often diagnosed by serologic testing. Dependence on antibody detection for etiological diagnosis of endocarditis may be complicated by serologic cross-reactivity; most notably, Chlamydia or Chlamydophila serologic assays demonstrate high level cross-reactivity with Bartonella species, possibly leading to erroneous diagnoses of chlamydial endocarditis 96. Low-level cross-reactivity has also been demonstrated between Bartonella and Coxiella, although in cases of endocarditis, antibody titers against the “true” agent are typically more elevated than those against the cross-reacting organism. Seroreactivity resulting from prior exposure to organisms unrelated to the episode of endocarditis under evaluation may confound interpretation. Serologic testing for extremely rare causes of endocarditis (e.g., Legionella species, Chlamydia/Chlamydophila species) is not recommended due to challenges with falsely positive results 24.
Other lab tests
- Urinalysis consistent with glomerulonephritis is seen in ~60% of patients (proteinuria, microscopic hematuria).
- Rheumatoid factor (may help satisfy the diagnostic criteria for endocarditis).
- Acute-phase reactants (C-reactive protein):
- More than 95% sensitive for endocarditis.
- May be useful for subsequent follow-up, to help determine if the infection is responding to therapy.
Endocarditis treatment
If you have endocarditis, it’s important to get treatment quickly. Treatments may include 97, 98:
- Medicines
- Antibiotics to treat bacterial infections. Antibiotics are usually started through an intravenous (IV) line in the hospital.
- Antifungal medicine to treat fungal infections. Your doctor may suggest taking antifungal medicine for the rest of your life to prevent the infection from coming back.
- Heart surgery may be needed to repair or replace damaged valves and heart tissue. Surgery may also be done to remove infected tissue.
- Dental care, especially cleanings, can help reduce the amount of bacteria that grows in your mouth.
Treatment may last weeks, and you may need tests to make sure it’s working. Your doctor will also check you for problems that could develop from endocarditis, such as heart failure or an irregular heartbeat.
Many people with endocarditis are successfully treated with antibiotics. Sometimes, surgery may be needed to fix or replace damaged heart valves and clean up any remaining signs of the infection.
Antibiotics
If you have endocarditis, your doctor might recommend high doses of intravenous (IV) antibiotics in the hospital. Your doctor will use blood culture tests to help identify the organism that’s causing your infection. Based on the results of your blood tests, your doctor will choose the most appropriate antibiotic or combination of antibiotics to fight the infection.
You’ll generally spend a week or more in the hospital when you start taking IV antibiotics. This gives your doctor time to see if the antibiotics are working against your infection. You’ll usually take antibiotics for several weeks to clear up the infection.
Once your fever and the worst of your signs and symptoms have passed, you might be able to leave the hospital and continue IV antibiotic therapy with visits to your doctor’s office or at home with home-based care. You’ll still need to see your doctor regularly to make sure your treatment is working.
It’s important to tell your doctor about any signs or symptoms that may mean your infection is getting worse, such as:
- Fever
- Chills
- Headaches
- Joint pain
- Shortness of breath
Also, if you develop diarrhea, a rash, itching or joint pain, let your doctor know as soon as possible. These signs and symptoms may indicate you’re having a reaction to your prescribed antibiotic.
If you have shortness of breath or swelling in your legs, ankles or feet after you start antibiotic treatment, see your doctor immediately. These signs and symptoms can be indicators of heart failure.
Table 3. Pathogen-specific therapy of infective endocarditis
| Pathogen | Regimen(s) | Comments |
|---|---|---|
| Penicillin-susceptible (MIC ≤0.12 mcg per ml) viridans streptococci and S. bovis | Penicillin | Adverse effects include hypersensitivity and seizures |
| Ceftriaxone | Generally well tolerated, once daily administration may enable outpatient therapy | |
| Penicillin (or ceftriaxone) + gentamicin | Addition of an aminoglycoside enables a shorter treatment duration (2 weeks vs 4 weeks) at the expense of potential aminoglycoside adverse effects (renal, vestibular and cochlear toxicity) | |
| Vancomycin | Use should be limited to those with true penicillin allergy | |
| Penicillin-intermediate (MIC >0.12 and ≤0.5 mcg per ml) viridans streptococci | Penicillin (or ceftriaxone) + gentamicin | 4 weeks of therapy recommended |
| Vancomycin | For penicillin-allergic patients or to avoid gentamicin | |
| Enterococci and penicillin-resistant (MIC >0.5 mcg per ml) viridans streptococci | Penicillin (or ampicillin) + gentamicin | Extended therapy (6 weeks) recommended for prosthetic valves and prolonged duration of symptoms prior to diagnosis |
| Ampicillin + ceftriaxone | Favoured in patients with renal insufficiency or high-level aminoglycoside resistance | |
| Vancomycin + gentamicin | Nephrotoxic regimen; role of gentamicin is uncertain | |
| Daptomycin | For vancomycin-resistant and penicillin-resistant enterococci; may combine with β-lactam | |
| Linezolid | May be used for vancomycin- and penicillin-resistant enterococci, although adverse events including bone marrow suppression and neuropathy are of concern with extended treatment courses | |
| Staphylococci | Nafcillin | For MSSA; adverse effects include rash, interstitial nephritis |
| Cefazolin | For MSSA; better tolerated than nafcillin | |
| Vancomycin | For MRSA | |
| Nafcillin + gentamicin | 2 week regimen for IV drug users with uncomplicated right-sided IE* | |
| Nafcillin + gentamicin + rifampin | For prosthetic valve IE; substitute vancomycin for nafcillin in patients with MRSA | |
| Daptomycin | FDA-approved for right-sided S. aureus IE; observational data supports use in left-sided IE as well; may combine with β-lactam | |
| HACEK strains | Ceftriaxone | Effective for β-lactamase producing strains |
| Ampicillin/sulbactam | For β-lactamase producing strains | |
| Ciprofloxacin | For patients intolerant of β-lactam therapy | |
| Enterobacteriaceae | Extended-spectrum penicillin or cephalosporin + aminoglycoside (or fluoroquinolone) | Rare cause of IE and may require a tailored approach depending on the pathogen |
| Pseudomonas aeruginosa | An anti-pseudomonal β-lactam (such as ticarcillin, piperacillin, ceftazidime, cefepime or imipenem) + tobramycin (or fluoroquinolone) | Typically requires prolonged therapy and valve surgery |
| Fungi | Parenteral antifungal agent (most commonly an amphotericin product) | Long-term suppressive therapy with an oral antifungal agent is often required |
Footnotes: *defined as infective endocarditis involving only the tricuspid valve, with no renal insufficiency and no extrapulmonary infection.
Abbreviations: FDA = Food and Drug Administration; HACEK = Haemophilus species, Aggregatibacter species, Cardiobacterium hominis, Eikenella corrodens and Kingella species; IE = infective endocarditis; IV = intravenous; MIC = Minimum inhibitory concentration; MRSA = methicillin-resistant S. aureus; MSSA = methicillin-susceptible S. aureus.
[Source 99 ]Figure 18. Staphylococcus endocarditis antibiotics
[Source 100 ]Figure 19. Streptococcus endocarditis antibiotics
[Source 100 ]Figure 20. Enterococcus endocarditis antibiotics
[Source 100 ]Figure 21. Culture-negative infective endocarditis antibiotics
[Source 100 ]Figure 22. Infective endocarditis in acute severely ill patients antibiotic regimens before pathogen identification of pathogen
[Source 100 ]Duration of antibiotic therapy
The duration of therapy must be sufficient to completely eradicate microorganisms within cardiac vegetations. Due to poor penetration of antibiotics into these vegetations and the slowly bactericidal properties of some of the commonly used drugs (such as vancomycin), extended courses of antibiotics are usually required. When bactericidal activity is rapid, shorter courses may be feasible. For example, combination therapy with penicillin or ceftriaxone and an aminoglycoside is synergistic for viridans-group streptococci-associated infective endocarditis, enabling effective courses as short as two weeks for susceptible strains 101. Right-sided vegetations tend to have lower bacterial densities and may also be amenable to shorter course therapy.
Duration of antimicrobial therapy is generally calculated from the first day on which blood cultures are negative. Blood cultures should be obtained every 24–72 hours until it is demonstrated that the bloodstream infection has cleared 102. If operative valve tissue cultures are positive, an entire antimicrobial course should be considered following cardiovascular surgery.
Surgery
If the infection damages your heart valves, you may have symptoms and complications for years after treatment. Sometimes surgery is needed to treat persistent infections or to replace a damaged valve. Surgery is also sometimes needed to treat endocarditis that’s caused by a fungal infection.
Depending on your condition, your doctor may recommend either repairing your damaged valve or replacing it with an artificial valve made of cow, pig or human heart tissue (biological tissue valve) or man-made materials (mechanical valve).
The three main surgical procedures that are used to treat endocarditis are:
- Repair of the damaged heart valve
- Replacement of the damaged heart valves with prosthetic ones
- Draining of any abscesses and the repair of any fistulas that may have developed in the heart muscle
Surgery for endocarditis can be very challenging, not least because a person who needs surgery will usually be very ill to begin with. Despite the best efforts of their surgical teams, approximately one in 10 people will die during or shortly after surgery for endocarditis.
The principal consensus indications for valve surgery are heart failure, uncontrolled infection and prevention of embolic events in patients at high risk 99. Uncontrolled infection may be related to paravalvular complications, such as abscess, an enlarging vegetation or dehiscence of a prosthetic valve. In addition, uncontrolled infection may be manifested by ongoing systemic illness with persistent fevers and positive blood cultures despite appropriate antibiotic therapy. As larger left-sided vegetations are more likely to lead to embolic events, infective endocarditis with a vegetation of >10 mm in length is a relative indication for surgical intervention.
The timing of cardiac surgery for patients with infective endocarditis and neurovascular complications remains controversial. A large prospective cohort study of 857 patients with infective endocarditis complicated by ischemic stroke without hemorrhagic conversion found that no patient benefit was gained from delaying surgery 103. By contrast, patients with embolic stroke complicated by hemorrhagic conversion sustained higher mortality when surgery was performed within 4 weeks of the hemorrhagic event compared with later surgery (75% versus 40%, respectively) 104. On the basis of these observational data, the American Heart Association 101 currently recommends that valve surgery may be considered in patients with infective endocarditis who also have stroke or subclinical cerebral emboli without delay if intracranial hemorrhage has been excluded by imaging studies and neurological damage is not severe (such as coma). In patients with major ischemic stroke or intracranial haemorrhage, American Heart Association guidelines currently state that delaying valve surgery for at least 4 weeks is reasonable 101.
Valve surgery was traditionally recommended for difficult-to-treat pathogens such as Pseudomonas aeruginosa, fungal organisms and β-lactam resistant staphylococci. However, these pathogen-specific recommendations for surgery have been recently called into question in favor of an individualized decision-making approach based upon hemodynamic and structural indications 105.
Figure 23. Indications and timing of surgery in left-sided valve infective endocarditis (native valve endocarditis and prosthetic valve endocarditis)
[Source 100 ]Other adjunctive therapies
Anticoagulation
Patients with prosthetic valve infective endocarditis who are receiving oral anticoagulants may be at an increased risk of death from cerebral hemorrhage 106. Antiplatelet therapies are not currently recommended for infective endocarditis. A single randomized trial examined the role of 325 mg of aspirin daily for patients with infective endocarditis. The incidence of embolic events was similar in between aspirin- and placebo-treated patients, and there was a non-significant increase in the rate of cerebral bleeding episodes 107. There are several limitations to this study, however, that include dose of aspirin used and delayed initiation of aspirin. For patients with another indication for antiplatelet therapy, it may be reasonable to continue the antiplatelet agent unless bleeding complications develop. Similarly, it is not recommended to initiate anticoagulant therapy such as warfarin for the purpose of treating infective endocarditis. In patients with infective endocarditis who have another indication for anticoagulation therapy, such as a mechanical valve, data are contradictory on whether to continue anticoagulation during acute therapy 106 and bridging therapy with heparin products has not been studied.
Management of metastatic foci
Metastatic foci of infection frequently complicate infective endocarditis. As with any infection, recognition of these foci of infection is important so that targeted interventions, such as drainage of abscesses or removal of infected prosthetic material, may be undertaken. This is of critical importance in patients who require valve surgery because a persistent source of infection may serve as a source from which a recently placed prosthetic valve or annuloplasty ring becomes infected 101. Some metastatic foci, such as vertebral osteomyelitis, may require additional antibiotic therapy beyond what is typically indicated for infective endocarditis 108. There is currently insufficient evidence to recommend specific imaging strategies to look for metastatic foci in all patients with infective endocarditis.
Neurological complications of infective endocarditis treatment
Symptomatic neurological complications occur in 15–30% of patients with infective endocarditis and are mainly the consequence of embolism from vegetations 109, 110, 111. Neurological manifestations occur before or at infective endocarditis diagnosis in a majority of cases, but new or recurrent events can also take place later in the course of infective endocarditis. Clinical presentation is variable and may
include multiple symptoms or signs in the same patient, but focal signs predominate and ischemic strokes are most commonly diagnosed. Transient ischemic attack, intracerebral or subarachnoidal hemorrhage, brain abscess, meningitis and toxic encephalopathy are also seen, and firm evidence supports that additional clinically silent cerebral embolisms occur in 35–60% of infective endocarditis patients 112, 113, 114.
Staphylococcus aureus infective endocarditis is more frequently associated with neurological complications compared with infective endocarditis caused by other bacteria. Vegetation length and mobility also correlate with embolic tendency 115, 116. Neurological complications are associated with an excess mortality, as well as complications, particularly in the case of stroke 110, 111. Rapid diagnosis and initiation of appropriate antibiotics are of major importance to prevent a first or recurrent neurological complication 117. Early surgery in high-risk patients is the second mainstay of embolism prevention, while antithrombotic drugs have no role.
Figure 24. Neurological complications of infective endocarditis treatment
[Source 100 ]Endocarditis prognosis
Without treatment, infective endocarditis is a fatal disease. Early detection and appropriate treatment of this endocarditis can be lifesaving. However, despite recent advances in diagnostic and treatment strategies for infective endocarditis, 1 year mortality has not improved and remains at 30%, which is worse than for many cancers 18, 19, 20. Infective endocarditis prognosis largely depends on whether you develop any complications 118. Increased mortality rates are associated with older age, infection involving the aortic valve, development of congestive heart failure, central nervous system complications, and underlying disease such as diabetes mellitus. Catastrophic neurological events of all types resulting from infective endocarditis are highly predictive of morbidity and mortality.
Predictors of poor outcome in patients with infective endocarditis 100:
- Patient characteristics
- Older age
- Prosthetic valve endocarditis
- Diabetes mellitus
- Comorbidity (e.g., frailty, immunosuppression, kidney or lung disease)
- Clinical complications of endocarditis
- Heart failure
- Kidney failure
- Moderate area of ischemic stroke
- Brain hemorrhage
- Septic shock
- What type of organism made you sick
- Staphylococcus aureus
- Fungi
- Non-HACEK Gram-negative bacilli
- Echocardiographic findings
- Periannular complications
- Severe left-sided valve regurgitation
- Low left ventricular ejection fraction
- Pulmonary hypertension
- Large vegetations
- Severe prosthetic valve dysfunction
- Premature mitral valve closure and other signs of elevated diastolic pressures.
Cure rates for appropriately managed (including both medical and surgical therapies) native valve endocarditis are as follows 118:
- For Streptococcus viridans and Streptococcus bovis infection, the rate is 98%.
- For enterococci and Staphylococcus aureus infection in individuals who abuse intravenous drugs, the rate is 90%.
- For community-acquired Staphylococcus aureus infection in individuals who do not abuse intravenous drugs, the rate is 60-70%.
- For infection with aerobic gram-negative organisms, the rate is 40-60%.
- For infection with fungal organisms, the rate is lower than 50%.
For prosthetic valve endocarditis, the cure rates are as follows 118:
- Rates are 10-15% lower for each of the above categories, for both early and late prosthetic valve endocarditis.
- Surgery is required far more frequently.
- Approximately 60% of early coagulase-negative Staphylococci prosthetic valve endocarditis cases and 70% of late coagulase-negative Staphylococci prosthetic valve endocarditis cases are curable.
The overall mortality rate has remained stable at 14.5% and there has been no overall improvement in outcomes of infective endocarditis of congenital heart disease 118. The in-hospital mortality rate hovers around 18%, with one-year mortality reaching up to 40% 119. Mortality rates in native valve endocarditis range from 16-27%, and mortality rates in patients with prosthetic valve endocarditis are higher. More than 50% of these infections occur within 2 months after surgery. The fatality rate of pacemaker infective endocarditis ranges up to 34% 116, 120, 121, 122, 123.
In general, cases of artificial (prosthetic) valve endocarditis occurring within the first 60 days of surgery demonstrate the highest in-hospital mortality rates of about 30%. A large, Japanese prospective cohort study found a Staphylococcal infection and heart failure to be the greatest predictors of in-hospital mortality 124. Although nearly 50% of infectious endocarditis cases now undergo surgical intervention, in of itself, the surgical intervention does not appear to elevate the in-hospital mortality risk 26. The largest study to date indicates that in cases of infective endocarditis complicated by heart failure, valvular surgery reduces the 1-year mortality rate. More recent studies document that early surgery in patients, especially those with large vegetations, significantly reduces the risk for death from any cause 125, 126, 127, 128.
Mortality rates also vary with the infecting organism. Acute endocarditis caused by Staphylococcus aureus is associated with a high mortality rate (30-40%), except when it is associated with IV drug use 129, whereas infective endocarditis resulting from Streptococci has a mortality rate of approximately 10% 130.
- Yallowitz AW, Decker LC. Infectious Endocarditis. [Updated 2023 Apr 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557641[↩][↩][↩][↩][↩][↩][↩]
- Que, YA., Moreillon, P. Infective endocarditis. Nat Rev Cardiol 8, 322–336 (2011). https://doi.org/10.1038/nrcardio.2011.43[↩]
- Heart Valves and Infective Endocarditis. https://www.heart.org/en/health-topics/heart-valve-problems-and-disease/heart-valve-problems-and-causes/heart-valves-and-infective-endocarditis[↩]
- Infective Endocarditis. https://emedicine.medscape.com/article/216650-overview[↩][↩][↩]
- Guzek A, Braksator W, Gąsior Z, Kuśmierczyk M, Różański J, Rybicki Z. Infective endocarditis – can we treat it more effectively? Kardiochir Torakochirurgia Pol. 2020 Mar;17(1):8-14. doi: 10.5114/kitp.2020.94184[↩][↩][↩][↩][↩]
- Hackett AJ, Stuart J. Infective endocarditis: identification and management in the emergency department. Emerg Med Pract. 2020 Sep;22(9):1-24. https://www.ebmedicine.net/topics/cardiovascular/infective-endocarditis[↩]
- Fowler VG, Durak DT, Selton-Suty C, et al. The 2023 Duke-ISCVID Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. CID. May 2023. 200.[↩]
- Brusch JL. Infective endocarditis and its mimics in the critical care unit. Cunha BA, ed. Infectious Diseases in Critical Care. 2nd ed. New York, NY: Informa Healthcare; 2007. 261-2.[↩]
- Popovich KJ, Weinstein RA. Questioning Old Staphylococcus aureus Beliefs With New Technology. J Infect Dis. 2023 Apr 26;227(9):1028-1030. doi: 10.1093/infdis/jiac439[↩]
- Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Federspiel JJ, Stearns SC, Peppercorn AF, Chu VH, Fowler VG Jr. Arch Intern Med. 2012 Feb 27; 172(4):363-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3314241/[↩]
- Global and regional burden of infective endocarditis, 1990-2010: a systematic review of the literature. Bin Abdulhak AA, Baddour LM, Erwin PJ, Hoen B, Chu VH, Mensah GA, Tleyjeh IM. Glob Heart. 2014 Mar; 9(1):131-43. https://www.sciencedirect.com/science/article/pii/S2211816014000143[↩]
- Yanagawa B, Bahji A, Lamba W, Tan DH, Cheema A, Syed I, Verma S. Endocarditis in the setting of IDU: multidisciplinary management. Curr Opin Cardiol. 2018 Mar;33(2):140-147. doi: 10.1097/HCO.0000000000000493[↩]
- Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O’Gara P, Taubert KA. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi:10.1161/CIR.0000000000000296. http://circ.ahajournals.org/content/132/15/1435.long[↩]
- Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2013;381:628]. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(12)61689-4/fulltext[↩]
- Fournier PE, et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;51:131–140. https://www.ncbi.nlm.nih.gov/pubmed/20540619[↩]
- Murdoch DR, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Archives of internal medicine. 2009;169:463–473. This prospective cohort study of 2781 adults with definite endocarditis demonstrated that IE had shifted from a subacute disease of younger people with rheumatic valvular abnormalities, to one in which the presentation is more acute and is characterized by a high rate of S. aureus infection in patients with previous health care exposure. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3625651/[↩]
- Raoult D, Casalta JP, Richet H, Khan M, Bernit E, Rovery C, Branger S, Gouriet F, Imbert G, Bothello E, Collart F, Habib G. Contribution of systematic serological testing in diagnosis of infective endocarditis. J Clin Microbiol. 2005 Oct;43(10):5238-42. doi: 10.1128/JCM.43.10.5238-5242.2005[↩][↩][↩]
- Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016 Feb 27;387(10021):882-93. https://doi.org/10.1016/S0140-6736(15)00067-7[↩][↩]
- Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O’Gara P, Taubert KA. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296[↩][↩][↩][↩]
- Thuny F, Grisoli D, Collart F, Habib G, Raoult D. 2012. Management of infective endocarditis: challenges and perspectives. Lancet 379:965–975. doi: 10.1016/S0140-6736(11)60755-1[↩][↩]
- Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001 Nov 1;345(18):1318-30. doi: 10.1056/NEJMra010082[↩][↩]
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633–638. doi: 10.1086/313753[↩][↩]
- Endocarditis. https://emcrit.org/ibcc/endo/[↩][↩][↩]
- Liesman RM, Pritt BS, Maleszewski JJ, Patel R. Laboratory Diagnosis of Infective Endocarditis. J Clin Microbiol. 2017 Sep;55(9):2599-2608. doi: 10.1128/JCM.00635-17[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Barnett R. Infective endocarditis. Lancet. 2016 Sep 17;388(10050):1148. https://doi.org/10.1016/S0140-6736(16)31602-6[↩]
- Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falco V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort study. Arch Intern Med 169:463–473. doi: 10.1001/archinternmed.2008.603[↩][↩][↩][↩][↩][↩][↩][↩]
- Raoult D, Casalta JP, Richet H, Khan M, Bernit E, Rovery C, Branger S, Gouriet F, Imbert G, Bothello E, Collart F, Habib G. 2005. Contribution of systematic serological testing in diagnosis of infective endocarditis. J Clin Microbiol 43:5238–5242. doi: 10.1128/JCM.43.10.5238-5242.2005[↩][↩][↩][↩][↩]
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015 Nov 21;36(44):3075-3128. doi: 10.1093/eurheartj/ehv319[↩][↩]
- Fournier PE, Thuny F, Richet H, Lepidi H, Casalta JP, Arzouni JP, Maurin M, Celard M, Mainardi JL, Caus T, Collart F, Habib G, Raoult D. 2010. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis 51:131–140. doi: 10.1086/653675[↩][↩][↩][↩]
- Morpeth S, Murdoch D, Cabell CH, Karchmer AW, Pappas P, Levine D, Nacinovich F, Tattevin P, Fernandez-Hidalgo N, Dickerman S, Bouza E, del Rio A, Lejko-Zupanc T, de Oliveira Ramos A, Iarussi D, Klein J, Chirouze C, Bedimo R, Corey GR, Fowler VG Jr. 2007. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med 147:829–835. doi: 10.7326/0003-4819-147-12-200712180-00002[↩]
- Infective Endocarditis. https://emedicine.medscape.com/article/216650-overview#a4[↩]
- Correa de Sa DD, Tleyjeh IM, Anavekar NS, Schultz JC, Thomas JM, Lahr BD, Bachuwar A, Pazdernik M, Steckelberg JM, Wilson WR, Baddour LM. Epidemiological trends of infective endocarditis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2010 May;85(5):422-6. doi: 10.4065/mcp.2009.0585. Erratum in: Mayo Clin Proc. 2010 Aug;85(8):772.[↩]
- Murdoch DR, Corey GR, Hoen B, Miró JM, et al. International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009 Mar 9;169(5):463-73. doi: 10.1001/archinternmed.2008.603[↩]
- Reyes MP, Ali A, Mendes RE, Biedenbach DJ. Resurgence of Pseudomonas endocarditis in Detroit, 2006-2008. Medicine (Baltimore). 2009 Sep;88(5):294-301. doi: 10.1097/MD.0b013e3181b8bedc[↩]
- Wang A, Athan E, Pappas PA, Fowler VG Jr, Olaison L, Paré C, Almirante B, Muñoz P, Rizzi M, Naber C, Logar M, Tattevin P, Iarussi DL, Selton-Suty C, Jones SB, Casabé J, Morris A, Corey GR, Cabell CH; International Collaboration on Endocarditis-Prospective Cohort Study Investigators. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007 Mar 28;297(12):1354-61. doi: 10.1001/jama.297.12.1354[↩]
- Alvarez-Moreno CA, Morales-López S, Rodriguez GJ, Rodriguez JY, Robert E, Picot C, Ceballos-Garzon A, Parra-Giraldo CM, Le Pape P. The Mortality Attributable to Candidemia in C. auris Is Higher than That in Other Candida Species: Myth or Reality? J Fungi (Basel). 2023 Mar 31;9(4):430. doi: 10.3390/jof9040430[↩]
- Fuller R, Jacobs SE. Candida Infectious Endocarditis and Implantable Cardiac Device Infections. Mycopathologia. 2023 Dec;188(6):893-905. doi: 10.1007/s11046-023-00745-x[↩]
- Lin KP, Yeh TK, Chuang YC, Wang LA, Fu YC, Liu PY. Blood Culture Negative Endocarditis: A Review of Laboratory Diagnostic Approaches. Int J Gen Med. 2023 Jan 24;16:317-327. doi: 10.2147/IJGM.S393329[↩]
- Morris AJ, Drinkovic D, Pottumarthy S, Strickett MG, MacCulloch D, Lambie N, Kerr AR. 2003. Gram stain, culture, and histopathological examination findings for heart valves removed because of infective endocarditis. Clin Infect Dis 36:697–704. doi: 10.1086/367842[↩]
- Topan A, Carstina D, Slavcovici A, Rancea R, Capalneanu R, Lupse M. 2015. Assesment of the Duke criteria for the diagnosis of infective endocarditis after twenty-years. An analysis of 241 cases. Clujul Med 88:321–326. doi: 10.15386/cjmed-469[↩]
- Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, Sandoe JA, Spry MJ, Watkin RW, Working Party of the British Society for Antimicrobial Chemotherapy. 2012. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 67:269–289. doi: 10.1093/jac/dkr450[↩][↩][↩]
- Houpikian P, Raoult D. 2005. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 84:162–173. doi: 10.1097/01.md.0000165658.82869.17[↩][↩][↩]
- Geissdorfer W, Moos V, Moter A, Loddenkemper C, Jansen A, Tandler R, Morguet AJ, Fenollar F, Raoult D, Bogdan C, Schneider T. 2012. High frequency of Tropheryma whipplei in culture-negative endocarditis. J Clin Microbiol 50:216–222. doi: 10.1128/JCM.05531-11[↩]
- Sohail MR, Gray AL, Baddour LM, Tleyjeh IM, Virk A. 2009. Infective endocarditis due to Propionibacterium species. Clin Microbiol Infect 15:387–394. doi: 10.1111/j.1469-0691.2009.02703.x[↩][↩]
- Durack DT, Beeson PB, Petersdorf RG. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. British journal of experimental pathology. 1973;54:142–151. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2072580/pdf/brjexppathol00410-0029.pdf[↩]
- Jameson GS, Ramanathan RK, Borad MJ, Downhour M, Korn R, Von Hoff D. Marantic Endocarditis Associated with Pancreatic Cancer: A Case Series. Case Reports in Gastroenterology. 2009;3(1):67-71. doi:10.1159/000207195. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2895179/[↩][↩]
- Lee V, Gilbert JD, Byard RW. Marantic endocarditis – a not so benign entity. J Forensic Leg Med. 2012;19:312–5. https://www.ncbi.nlm.nih.gov/pubmed/22847046[↩][↩][↩]
- El-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis and treatment. Oncologist. 2007;12:518–523 https://www.ncbi.nlm.nih.gov/pubmed/17522239[↩][↩]
- Dutta T, Karas MG, Segal AZ, Kizer JR. Yield of transesophageal echocardiography for nonbacterial thrombotic endocarditis and other cardiac sources of embolism in cancer patients with cerebral ischemia. Am J Cardiol. 2006;97:894–898. https://www.ncbi.nlm.nih.gov/pubmed/16516597[↩]
- Pons F, Poyet R, Daranda E, et al. [Ischemic strokes as a presenting feature of marantic endocarditis despite heparin treatment] Ann Cardiol Angeiol (Paris) 2011;60:233–5. French. https://www.ncbi.nlm.nih.gov/pubmed/20723881[↩]
- Aryana A, Esterbrooks DJ, Morris PC. Nonbacterial thrombotic endocarditis with recurrent embolic events as manifestation of ovarian neoplasm. J Gen Intern Med. 2006;21:C12–C15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1924740/[↩]
- Chen L, Li Y, Gebre W, Lin J. Myocardial and cerebral infarction due to nonbacterial thrombotic endocarditis as an initial presentation of pancreatic adenocarcinoma. Arch Pathol Lab Med. 2004;128:1307–1308. http://www.archivesofpathology.org/doi/pdf/10.1043/1543-2165%282004%29128%3C1307%3AMACIDT%3E2.0.CO%3B2[↩]
- Edoute Y, Haim N, Rinkevich D, Brenner B, Reisner S. Cardiac valvular vegetations in cancer patients: a prospective echocardiographic study of 200 patients. Am J Med. 1997;102:252–258. https://www.ncbi.nlm.nih.gov/pubmed/9217593[↩]
- Smeglin A, Ansari M, Skali H, Oo TH, Maysky M. Marantic endocarditis and disseminated intravascular coagulation with systemic emboli in presentation of pancreatic cancer. J Clin Oncol. 2008;26:1383–1385. https://www.ncbi.nlm.nih.gov/pubmed/18323562[↩]
- Bai Z, Hou J, Ren W, Guo Y. Diagnosis and surgical treatment for isolated tricuspid libman-sacks endocarditis: a rare case report and literatures review. Journal of Cardiothoracic Surgery. 2015;10:93. doi:10.1186/s13019-015-0302-1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4494164/[↩]
- Bulkley BH, Roberts WC. The heart in systemic lupus erythematosus and the changes induced in it by corticosteroid therapy: a study of 36 necropsy patients. Am J Med. 1975;58:243–264. https://www.ncbi.nlm.nih.gov/pubmed/1115070[↩]
- Ziporen L, Goldberg I, Arad M, et al. Libman-sacks endocarditis in the antiphospholipid syndrome: immunopathologic findings in deformed heart valves. Lupus. 1996;5(3):196–205. doi: 10.1177/096120339600500306 https://www.ncbi.nlm.nih.gov/pubmed/8803890[↩]
- Avgoustidis N, Bourantas CV, Anastasiadis GP, Sipsas N, Pikazis D. Endocarditis due to Gemella haemolysans in a patient with systemic lupus erythematosus. J Heart Valve Dis. 2011;20:107–109. https://www.ncbi.nlm.nih.gov/pubmed/21404908[↩]
- Prevention of Infective Endocarditis. Circulation. 2007;116:1736-1754 http://circ.ahajournals.org/content/116/15/1736[↩][↩][↩]
- Habib G, Lancellotti P, Iung B. 2015 ESC Guidelines on the management of infective endocarditis: a big step forward for an old disease. Heart. 2016 Jul 1;102(13):992-4. doi: 10.1136/heartjnl-2015-308791[↩]
- Sandre RM, Shafran SD. Infective endocarditis: review of 135 cases over 9 years. Clin Infect Dis. 1996;22:276–86. doi: 10.1093/clinids/22.2.276[↩]
- Servy A, Valeyrie-Allanore L, Alla F, Lechiche C, Nazeyrollas P, Chidiac C, Hoen B, Chosidow O, Duval X; Association Pour l’Etude et la Prévention de l’Endocardite Infectieuse Study Group. Prognostic value of skin manifestations of infective endocarditis. JAMA Dermatol. 2014 May;150(5):494-500. doi: 10.1001/jamadermatol.2013.8727[↩]
- Hirai T, Koster M. Osler’s nodes, Janeway lesions and splinter haemorrhages. Case Reports 2013;2013:bcr2013009759[↩]
- Marrie TJ. Osler’s nodes and Janeway lesions. Am J Med 2008;2013:105–6.[↩]
- Osler W. Chronic infectious endocarditis. Q J Med 1909;2:219–30.[↩][↩]
- Tikoo M, Bardia A, Gupta A, Pandey A. Dermatological Manifestations of Infective Endocarditis. J Gen Intern Med. 2015 Aug;30(8):1229-30. doi: 10.1007/s11606-015-3256-z[↩]
- Silverman ME, Upshaw CB., Jr Extracardiac manifestations of infective endocarditis and their historical descriptions. Am J Cardiol. 2007;100(12):1802–7. doi: 10.1016/j.amjcard.2007.07.034[↩]
- Beaulieu A, Rehman HU. Janeway lesions. CMAJ. 2010 Jul 13;182(10):1075. doi: 10.1503/cmaj.091528[↩][↩]
- Gunson, T.H. and Oliver, G.F. (2007), Osler’s nodes and Janeway lesions. Australasian Journal of Dermatology, 48: 251-255. https://doi.org/10.1111/j.1440-0960.2007.00397.x[↩]
- Sethi K, Buckley J, de Wolff J. Splinter haemorrhages, Osler’s nodes, Janeway lesions and Roth spots: the peripheral stigmata of endocarditis. Br J Hosp Med (Lond). 2013 Sep;74(9):C139-42.[↩]
- KERR, A., JR. and TAN, J.S. (1979), Biopsies of the Janeway Lesion of Infective Endocarditis. Journal of Cutaneous Pathology, 6: 124-129. https://doi.org/10.1111/j.1600-0560.1979.tb01113.x[↩]
- Misin A, Di Bella S, Priolo L, Luzzati R. Image of the month: ‘Diagnostic hands’: Janeway lesions. Clin Med (Lond). 2017 Jul;17(4):373-374. doi: 10.7861/clinmedicine.17-4-373[↩]
- Sapira JD. The art & science of bedside diagnosis. Baltimore: Urban & Schwarzenberg, Inc.; 1990. p. 197–8.[↩]
- Van Uitert RL, Solomon GE. White-centered retinal hemorrhages: a sign of intracranial hemorrhage. Neurology. 1979 Feb;29(2):236-9. doi: 10.1212/wnl.29.2.236[↩][↩]
- Chong Y, Han SJ, Rhee YJ, Kang SK, Yu JH, Na MH. Classic Peripheral Signs of Subacute Bacterial Endocarditis. Korean J Thorac Cardiovasc Surg. 2016 Oct;49(5):408-412. doi: 10.5090/kjtcs.2016.49.5.408[↩][↩]
- Duane TD, Osher RH, Green WR. White centered hemorrhages: their significance. Ophthalmology. 1980 Jan;87(1):66-9. doi: 10.1016/s0161-6420(80)35277-9[↩][↩][↩]
- Fred HL. Little black bags, ophthalmoscopy, and the Roth spot. Tex Heart Inst J. 2013;40(2):115-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3649782[↩][↩]
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994 Mar;96(3):200-9. https://doi.org/10.1016/0002-9343(94)90143-0[↩][↩]
- Hoen B, Béguinot I, Rabaud C, Jaussaud R, Selton-Suty C, May T, Canton P. The Duke criteria for diagnosing infective endocarditis are specific: analysis of 100 patients with acute fever or fever of unknown origin. Clin Infect Dis. 1996 Aug;23(2):298-302. doi: 10.1093/clinids/23.2.298[↩]
- Hoen B, Selton-Suty C, Danchin N, Weber M, Villemot JP, Mathieu P, Floquet J, Canton P. Evaluation of the Duke criteria versus the Beth Israel criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 1995 Oct;21(4):905-9. doi: 10.1093/clinids/21.4.905[↩]
- Millar B, Moore J, Mallon P, Xu J, Crowe M, Mcclurg R, Raoult D, Earle J, Hone R, Murphy P. Molecular diagnosis of infective endocarditis–a new Duke’s criterion. Scand J Infect Dis. 2001;33(9):673-80. doi: 10.1080/00365540110026764[↩]
- Bayer AS, Ward JI, Ginzton LE, Shapiro SM. Evaluation of new clinical criteria for the diagnosis of infective endocarditis. Am J Med. 1994 Mar;96(3):211-9. doi: 10.1016/0002-9343(94)90144-9[↩]
- Gagliardi JP, Nettles RE, McCarty DE, Sanders LL, Corey GR, Sexton DJ. Native valve infective endocarditis in elderly and younger adult patients: comparison of clinical features and outcomes with use of the Duke criteria and the Duke Endocarditis Database. Clin Infect Dis. 1998 May;26(5):1165-8. doi: 10.1086/520304[↩]
- Nettles RE, McCarty DE, Corey GR, Li J, Sexton DJ. An evaluation of the Duke criteria in 25 pathologically confirmed cases of prosthetic valve endocarditis. Clin Infect Dis. 1997 Dec;25(6):1401-3. doi: 10.1086/516145[↩]
- Sekeres MA, Abrutyn E, Berlin JA, Kaye D, Kinman JL, Korzeniowski OM, Levison ME, Feldman RS, Strom BL. An assessment of the usefulness of the Duke criteria for diagnosing active infective endocarditis. Clin Infect Dis. 1997 Jun;24(6):1185-90. doi: 10.1086/513657[↩][↩]
- Habib G, Derumeaux G, Avierinos JF, Casalta JP, Jamal F, Volot F, Garcia M, Lefevre J, Biou F, Maximovitch-Rodaminoff A, Fournier PE, Ambrosi P, Velut JG, Cribier A, Harle JR, Weiller PJ, Raoult D, Luccioni R. Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol. 1999 Jun;33(7):2023-9. doi: 10.1016/s0735-1097(99)00116-3[↩]
- Pérez-Vázquez A, Fariñas MC, García-Palomo JD, Bernal JM, Revuelta JM, González-Macías J. Evaluation of the Duke criteria in 93 episodes of prosthetic valve endocarditis: could sensitivity be improved? Arch Intern Med. 2000 Apr 24;160(8):1185-91. doi: 10.1001/archinte.160.8.1185[↩][↩]
- Dodds GA, Sexton DJ, Durack DT, Bashore TM, Corey GR, Kisslo J. Negative predictive value of the Duke criteria for infective endocarditis. Am J Cardiol. 1996 Feb 15;77(5):403-7. doi: 10.1016/s0002-9149(97)89372-1[↩]
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000 Apr;30(4):633-8. doi: 10.1086/313753[↩]
- Patel R, Vetter EA, Harmsen WS, Schleck CD, Fadel HJ, Cockerill FR III. 2011. Optimized pathogen detection with 30- compared to 20-milliliter blood culture draws. J Clin Microbiol 49:4047–4051. doi: 10.1128/JCM.01314-11[↩]
- Petti CA, Bhally HS, Weinstein MP, Joho K, Wakefield T, Reller LB, Carroll KC. 2006. Utility of extended blood culture incubation for isolation of Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella organisms: a retrospective multicenter evaluation. J Clin Microbiol 44:257–259. doi: 10.1128/JCM.44.1.257-259.2006[↩]
- Chambers ST, Murdoch D, Morris A, Holland D, Pappas P, Almela M, Fernandez-Hidalgo N, Almirante B, Bouza E, Forno D, del Rio A, Hannan MM, Harkness J, Kanafani ZA, Lalani T, Lang S, Raymond N, Read K, Vinogradova T, Woods CW, Wray D, Corey GR, Chu VH. 2013. HACEK infective endocarditis: characteristics and outcomes from a large, multi-national cohort. PLoS One 8:e63181. doi: 10.1371/journal.pone.0063181[↩]
- Sagi M, Nesher L, Yagupsky P. 2017. The Bactec FX blood culture system detects Brucella melitensis bacteremia in adult patients within the routine 1-week incubation period. J Clin Microbiol 55:942–946. doi: 10.1128/JCM.02320-16[↩]
- Clinical and Laboratory Standards Institute. 2007. Principles and procedures for blood cultures; approved standard—1st ed CLSI document M47-A. Clinical and Laboratory Standards Institute, Wayne, PA.[↩]
- Baron EJ, Scott JD, Tompkins LS. 2005. Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures. Clin Infect Dis 41:1677–1680. doi: 10.1086/497595[↩]
- Maurin M, Eb F, Etienne J, Raoult D. Serological cross-reactions between Bartonella and Chlamydia species: implications for diagnosis. J Clin Microbiol. 1997 Sep;35(9):2283-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC229955/pdf/352283.pdf[↩]
- Matthew E. Falagas, Katerina G. Manta, Fotinie Ntziora, Konstantinos Z. Vardakas, Linezolid for the treatment of patients with endocarditis: a systematic review of the published evidence, Journal of Antimicrobial Chemotherapy, Volume 58, Issue 2, August 2006, Pages 273–280, https://doi.org/10.1093/jac/dkl219[↩]
- Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO; American College of Cardiology; American Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation. 2003 Sep 2;108(9):1146-62. doi: 10.1161/01.CIR.0000073597.57414.A9[↩]
- Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG. Infective endocarditis. Nature reviews Disease primers. 2016;2:16059. doi:10.1038/nrdp.2016.59. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5240923/[↩][↩]
- Habib G, Lancellotti P, Antunes MJ, et al. ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015 Nov 21;36(44):3075-3128. https://orbi.uliege.be/bitstream/2268/191313/1/eur%20h%20j%202015%2036%203075.pdf[↩][↩][↩][↩][↩][↩][↩][↩]
- Baddour LM, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132:1435–1486. This guideline from the American Heart Association updates the previous 2005 guideline for diagnosis and management of infective endocarditis, including detailed antibiotic guidance. http://circ.ahajournals.org/content/132/15/1435.long[↩][↩][↩][↩]
- Habib G, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. This guideline from the European Society of Cardiology updates the previous 2009 guidance for management of infective endocarditis, including prevention, diagnosis, and treatment. https://academic.oup.com/eurheartj/article/36/44/3075/2293384[↩]
- Barsic B, et al. Influence of the timing of cardiac surgery on the outcome of patients with infective endocarditis and stroke. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56:209–217. This study of 857 patient patients with endocarditis complicated by ischemic stroke without haemorrhagic conversion found that no survival benefit was gained from delaying surgery. https://www.ncbi.nlm.nih.gov/pubmed/23074311[↩]
- Garcia-Cabrera E, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation. 2013;127:2272–2284. http://circ.ahajournals.org/content/127/23/2272.long[↩]
- Arnold CJ, et al. Candida infective endocarditis: an observational cohort study with a focus on therapy. Antimicrobial agents and chemotherapy. 2015;59:2365–2373. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4356766/[↩]
- Tornos P, et al. Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Archives of internal medicine. 1999;159:473–475 https://www.ncbi.nlm.nih.gov/pubmed/10074955[↩][↩]
- Chan KL, et al. Effect of long-term aspirin use on embolic events in infective endocarditis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;46:37–41. https://www.ncbi.nlm.nih.gov/pubmed/18171211[↩]
- Park KH, et al. Optimal Duration of Antibiotic Therapy in Patients With Hematogenous Vertebral Osteomyelitis at Low Risk and High Risk of Recurrence. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;62:1262–1269. https://www.ncbi.nlm.nih.gov/pubmed/26917813[↩]
- Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falcó V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009 Mar 9;169(5):463-73. doi: 10.1001/archinternmed.2008.603[↩]
- García-Cabrera E, Fernández-Hidalgo N, Almirante B, Ivanova-Georgieva R, Noureddine M, Plata A, Lomas JM, Gálvez-Acebal J, Hidalgo-Tenorio C, Ruíz-Morales J, Martínez-Marcos FJ, Reguera JM, de la Torre-Lima J, de Alarcón González A; Group for the Study of Cardiovascular Infections of the Andalusian Society of Infectious Diseases; Spanish Network for Research in Infectious Diseases. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation. 2013 Jun 11;127(23):2272-84. doi: 10.1161/CIRCULATIONAHA.112.000813[↩][↩]
- Heiro M, Nikoskelainen J, Engblom E, Kotilainen E, Marttila R, Kotilainen P. Neurologic manifestations of infective endocarditis: a 17-year experience in a teaching hospital in Finland. Arch Intern Med. 2000 Oct 9;160(18):2781-7. doi: 10.1001/archinte.160.18.2781[↩][↩]
- Huang JS, Ho AS, Ahmed A, Bhalla S, Menias CO. Borne identity: CT imaging of vascular infections. Emerg Radiol. 2011 Aug;18(4):335-43. doi: 10.1007/s10140-011-0946-7[↩]
- Duval X, Iung B, Klein I, Brochet E, Thabut G, Arnoult F, Lepage L, Laissy JP, Wolff M, Leport C; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med. 2010 Apr 20;152(8):497-504, W175. doi: 10.7326/0003-4819-152-8-201004200-00006[↩]
- Hess A, Klein I, Iung B, Lavallée P, Ilic-Habensus E, Dornic Q, Arnoult F, Mimoun L, Wolff M, Duval X, Laissy JP. Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. AJNR Am J Neuroradiol. 2013 Aug;34(8):1579-84. doi: 10.3174/ajnr.A3582[↩]
- Iung B, Tubiana S, Klein I, Messika-Zeitoun D, Brochet E, Lepage L, Al-Attar N, Ruimy R, Leport C, Wolff M, Duval X; ECHO-IMAGE Study Group. Determinants of cerebral lesions in endocarditis on systematic cerebral magnetic resonance imaging: a prospective study. Stroke. 2013 Nov;44(11):3056-62. doi: 10.1161/STROKEAHA.113.001470[↩]
- Di Salvo G, Habib G, Pergola V, Avierinos JF, Philip E, Casalta JP, Vailloud JM, Derumeaux G, Gouvernet J, Ambrosi P, Lambert M, Ferracci A, Raoult D, Luccioni R. Echocardiography predicts embolic events in infective endocarditis. J Am Coll Cardiol. 2001 Mar 15;37(4):1069-76. doi: 10.1016/s0735-1097(00)01206-7[↩][↩]
- Dickerman SA, Abrutyn E, Barsic B, Bouza E, Cecchi E, Moreno A, Doco-Lecompte T, Eisen DP, Fortes CQ, Fowler VG Jr, Lerakis S, Miro JM, Pappas P, Peterson GE, Rubinstein E, Sexton DJ, Suter F, Tornos P, Verhagen DW, Cabell CH; ICE Investigators. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE-PCS). Am Heart J. 2007 Dec;154(6):1086-94. doi: 10.1016/j.ahj.2007.07.023[↩]
- Infective Endocarditis. https://emedicine.medscape.com/article/216650-overview#a6[↩][↩][↩][↩]
- Cabell CH, Jollis JG, Peterson GE, et al. Changing Patient Characteristics and the Effect on Mortality in Endocarditis. Arch Intern Med. 2002;162(1):90–94. doi:10.1001/archinte.162.1.9[↩]
- Ortiz-Bautista C, López J, García-Granja PE, Vilacosta I, Sevilla T, Sarriá C, Olmos C, Ferrera C, Sáez C, Puerto A, San Román JA. Right-sided infective endocarditis in cardiac device carriers: Clinical profile and prognosis. Med Clin (Barc). 2017 Dec 7;149(11):477-482. English, Spanish. doi: 10.1016/j.medcli.2017.03.055[↩]
- Özcan C, Raunsø J, Lamberts M, Køber L, Lindhardt TB, Bruun NE, Laursen ML, Torp-Pedersen C, Gislason GH, Hansen ML. Infective endocarditis and risk of death after cardiac implantable electronic device implantation: a nationwide cohort study. Europace. 2017 Jun 1;19(6):1007-1014. doi: 10.1093/europace/euw404[↩]
- Cresti A, Chiavarelli M, Scalese M, Nencioni C, Valentini S, Guerrini F, D’Aiello I, Picchi A, De Sensi F, Habib G. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther. 2017 Feb;7(1):27-35. doi: 10.21037/cdt.2016.08.09[↩]
- Janga KC, Sinha A, Greenberg S, Sharma K. Nephrologists Hate the Dialysis Catheters: A Systemic Review of Dialysis Catheter Associated Infective Endocarditis. Case Rep Nephrol. 2017;2017:9460671. doi: 10.1155/2017/9460671[↩]
- Ohara T, Nakatani S, Kokubo Y, Yamamoto H, Mitsutake K, Hanai S; CADRE investigators. Clinical predictors of in-hospital death and early surgery for infective endocarditis: results of CArdiac Disease REgistration (CADRE), a nation-wide survey in Japan. Int J Cardiol. 2013 Sep 10;167(6):2688-94. doi: 10.1016/j.ijcard.2012.06.117[↩]
- Baddley JW, Benjamin DK Jr, Patel M, Miró J, Athan E, Barsic B, Bouza E, Clara L, Elliott T, Kanafani Z, Klein J, Lerakis S, Levine D, Spelman D, Rubinstein E, Tornos P, Morris AJ, Pappas P, Fowler VG Jr, Chu VH, Cabell C; International Collaboration on Endocarditis-Prospective Cohort Study Group (ICE-PCS). Candida infective endocarditis. Eur J Clin Microbiol Infect Dis. 2008 Jul;27(7):519-29. doi: 10.1007/s10096-008-0466-x[↩]
- Hill EE, Herijgers P, Claus P, Vanderschueren S, Herregods MC, Peetermans WE. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J. 2007 Jan;28(2):196-203. doi: 10.1093/eurheartj/ehl427[↩]
- Liu PY, Huang YF, Tang CW, Chen YY, Hsieh KS, Ger LP, Chen YS, Liu YC. Staphylococcus lugdunensis infective endocarditis: a literature review and analysis of risk factors. J Microbiol Immunol Infect. 2010 Dec;43(6):478-84. doi: 10.1016/S1684-1182(10)60074-6[↩]
- Wallace SM, Walton BI, Kharbanda RK, Hardy R, Wilson AP, Swanton RH. Mortality from infective endocarditis: clinical predictors of outcome. Heart. 2002 Jul;88(1):53-60. doi: 10.1136/heart.88.1.53[↩]
- Soong G, Chun J, Parker D, Prince A. Staphylococcus aureus activation of caspase 1/calpain signaling mediates invasion through human keratinocytes. J Infect Dis. 2012 May 15;205(10):1571-9. doi: 10.1093/infdis/jis244[↩]
- Hémar V, Camou F, Roubaud-Baudron C, Ternacle J, Pernot M, Greib C, Dijos M, Wirth G, Chaussade H, Peuchant O, Bonnet F, Issa N; MoISE Study Group. The Mortality of Infective endocarditis with and without Surgery in Elderly (MoISE) Study. Clin Infect Dis. 2023 Nov 17;77(10):1440-1448. doi: 10.1093/cid/ciad384[↩]