Contents
Oligospermia
Oligospermia also called oligozoospermia is a medical term for ‘low sperm count’ and is a common cause of infertility or low fertility in men 1, 2, 3. A sperm count of less than 15 million/mL is considered low sperm count (oligospermia or oligozoospermia). Whereas, azoospermia means complete absence of sperm cells in a man’s semen or ejaculate. Most cases of male infertility cases are due to oligospermia (low sperm counts), poor sperm quality, or both.
Typically, men testicles produce sperm. Sperm travels through parts of your reproductive system and mixes with fluids to form semen. Semen is the fluid that your penis releases during ejaculation. If you have oligospermia, you may have semen — but there’s few sperm in it. Sometimes, oligospermia is referred to as having “low sperm count”. The most common male infertility factors include azoospermia (no sperm cells are produced) and oligospermia (few sperm cells are produced) 4. Sometimes, sperm cells are malformed or they die before they can reach the egg. In rare cases, infertility in men is caused by a genetic disease such as cystic fibrosis or a chromosomal abnormality.
Fertility experts recommend that both male and female partners get tested for infertility if pregnancy fails to occur after 1 year of regular unprotected sexual intercourse 5. Fertility testing should be done earlier if a woman is over age 35 or if either partner has known risk factors for infertility. If an evaluation of both male and female partners is possible, semen analysis should be done before more invasive testing of the female partner.
Infertility in men can be caused by disruption of testicular or ejaculatory function, as well as by hormonal and genetic disorders. Your doctor may refer you to a fertility specialist, usually a urologist, for specific tests. Infertility in men is typically evaluated by a semen analysis, complete medical history, and physical examination. This assessment helps determine if and how male factors are contributing to infertility. A semen analysis assesses the number (concentration), motility (movement), and morphology (shape) of sperm. The results are then evaluated by a specialist. If semen is found to be slightly abnormal, it does not necessarily mean that a man is infertile. The next step would be to get a repeat semen analysis at a lab that has a lot of experience doing semen and sperm tests, because results can vary a lot from test to test and lab to lab. Also, having small numbers of sperm can change the management/treatment options drastically, so the next step should be getting proper confirmation of the finding and seeing a specialist.
Treatment for male infertility should first address any underlying medical conditions that may be contributing to fertility problems. Depending on the suspected causes, many treatments may be available. If there is a blockage or history of vasectomy, reconstruction might be the best treatment for some men. In others, removing offending agents such as medications or recreational drugs might be the first step. Sometimes there may be hormonal abnormalities that need to be addressed, and in a fraction of men, treatment could increase sperm production. In some men, surgery to fix anatomical abnormalities or varicoceles can be pursued, and in others the best option is to go directly into the testicle to attempt retrieval of sperm that could be used for assisted reproductive technology (ART). It is very important that these procedures are performed by specialist doctors with proper training, expertise and experience to optimize outcomes and chances of retrieving sperm. Finally, men with oligospermia should always remember that countless couples across the world have formed families with love by becoming parents by using donor sperm or by adopting an infant or child. After being evaluated and counseled by a specialist, these are completely acceptable paths for couples to choose.
Can I get pregnant naturally if my husband has oligospermia?
Yes, you still may be able to get pregnant naturally. This depends on the cause of oligospermia your partner has and if it’s treatable. Your doctor is the best person to discuss treatment and family planning with.
Is IVF the only option if my partner has oligospermia?
It depends on the cause of oligospermia. If the cause is testicular and requires surgical sperm retrieval, then in-vitro fertilization (IVF) is the only option. If the oligospermia is treatable, couples may be able to avoid IVF.
Do insurance plans cover infertility treatment?
The degree of services covered depends on where you live and the type of insurance plan you have. Several states currently have laws that require insurers to either cover or offer to cover some form of infertility diagnosis and treatment. However, the laws vary greatly in their scope of what is and is not required to be covered. For more information about the specific laws for each of those states, please call your state’s Insurance Commissioner’s office or to learn about pending insurance legislation in your state, please contact your State Representatives.
Whether or not you live in a state with an infertility insurance law, you may want to consult with your employer’s director of human resources to determine the exact coverage your plan provides. Another good source of assistance is RESOLVE, an infertility patient advocacy and information organization.
Male Reproductive System
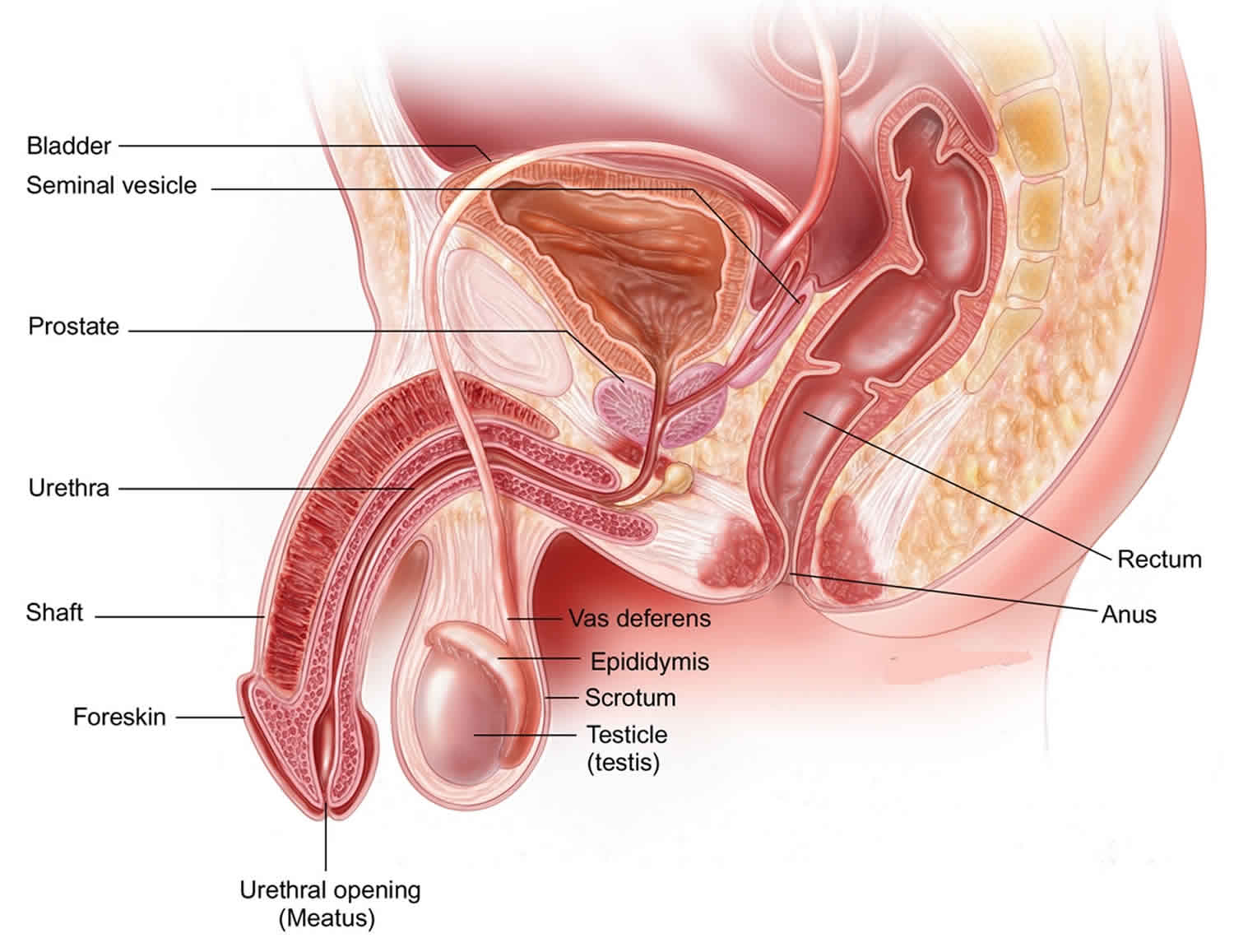
The male reproductive system creates sperm cells that are produced in the seminiferous tubules within each testicle. The sperm have to reach the uterus and the fallopian tube in order to fertilize a woman’s egg.
The male reproductive system includes the testicles, epididymis, vas deferens, seminal vesicles, prostate gland, urethra, and penis.
Figure 1. Male reproductive system
Sperm
Sperm are male reproductive cells. They are produced in the two testicles (testes). The testes are contained in the scrotal sac (scrotum).
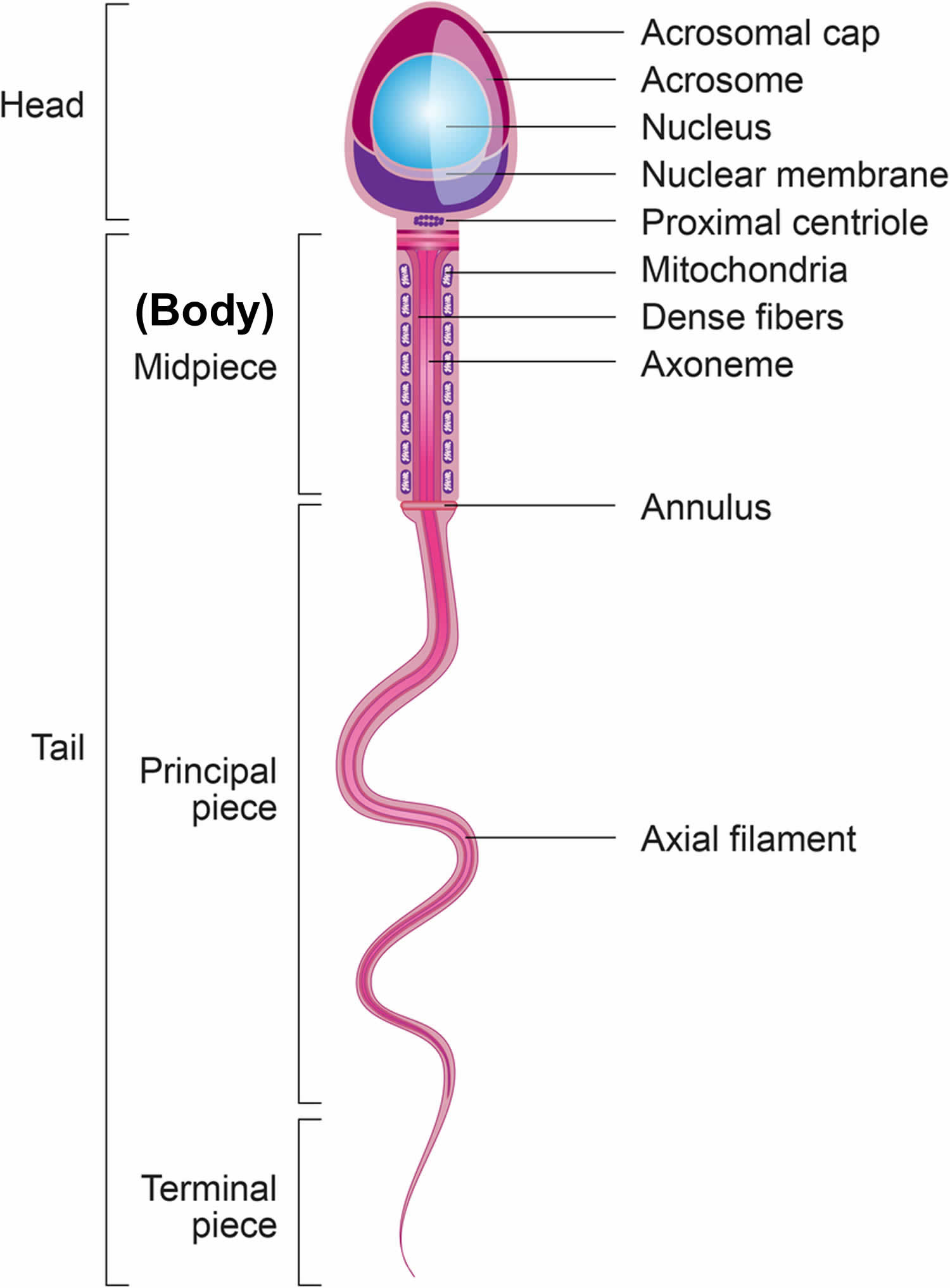
Structure of sperm
A mature sperm cell has three parts:
- The head consists of the nucleus, which is surrounded by the acrosome. The nucleus contains genetic material. The acrosome contains enzymes that penetrate the egg and allow the sperm to enter.
- The middle part (body) contains mitochondria, which supply energy for the sperm to swim into the female genital tract.
- The tail of the sperm also known as the flagellum. The tail moves with whip-like movements back and forth to propel the sperm toward the egg.
Figure 2. Sperm (spermatozoa) structure
Footnotes: Sperm (spermatozoa) are composed of two main parts: head and tail (also called flagellum). The sperm head is constituted basically by the acrosome and nucleus. The sperm tail includes: the neck that contains mainly the proximal centriole; the midpiece which is composed by mitochondria, outer dense fibers (ODF) and axoneme; principal piece containing the fibrous sheath and axoneme; and terminal piece.
[Source 6 ]Sperm head
Within the sperm head is a limited quantity of cytoplasm, highly condensed DNA, and a well-delimited acrosome. A substantial portion of the cytoplasm is lost during the final steps of spermatogenesis, specifically during spermiogenesis, the process during which round spermatids differentiate into elongated spermatids and then spermatozoa 7. The remaining cytoplasm from intercellular bridges, called the cytoplasmic droplet, is lost during sperm transit through the epididymis 8. During spermiogenesis (the process of transformation of spermatids into fully developed sperm cells with a head, neck middle piece and a tail), the sequential replacement of histones by transitional proteins and then by protamines within sperm chromatin triggers genome condensation 9, 7. The acrosome, located at the top of the sperm head, contains specific enzymes that promote specific functions trailing sperm capacitation and acrosome reaction: (1) exposure of acrosome zona pellucida binding proteins during sperm capacitation; (2) sperm ability to cross cumulus cells that surrounds the oocytes (female eggs) (3) sperm binding to zona pellucida and acrosome reaction, and (4) migration of IZUMO1 protein from the outer acrosomal membrane to the equatorial segment of sperm surface to ensure its binding to JUNO receptor on the oocyte 10, 11. Since acrosome reacted sperm retain the ability to penetrate the zona pellucida, the previous paradigm supporting the role of enzymes released during acrosome reaction in the digestion of the zona pellucida has been revisited in different species 12.
Sperm tail (flagellum)
The sperm tail or flagellum includes the neck, midpiece (body), principal piece and terminal piece. The sperm centrioles are important to early embryo development and are localized in the sperm neck 13. The axoneme, located internally along the entire flagellum, is composed of nine peripheral doublets and two central single microtubules (9 + 2 structure) integrated by the intraflagellar transport (IFT) system. Surrounding the axoneme, there is the outer dense fibers (ODF) and mitochondria in the sperm midpiece and the fibrous sheath (FS), formed by nine bundles of fibers of different lengths, in the principal piece 14. Depending on the species, approximately 22 to 75 mitochondria are present in the midpiece to produce enough energy necessary for a sperm to transit along the female reproductive tract and to reach the fertilization site in the oviduct 15. All of these sperm structural characteristics are essential to ensure the ability of a sperm to cross the muco-cervical and uterine barriers and reach the oviduct, where they bind to and penetrate the oocyte (egg) to deliver their DNA content.
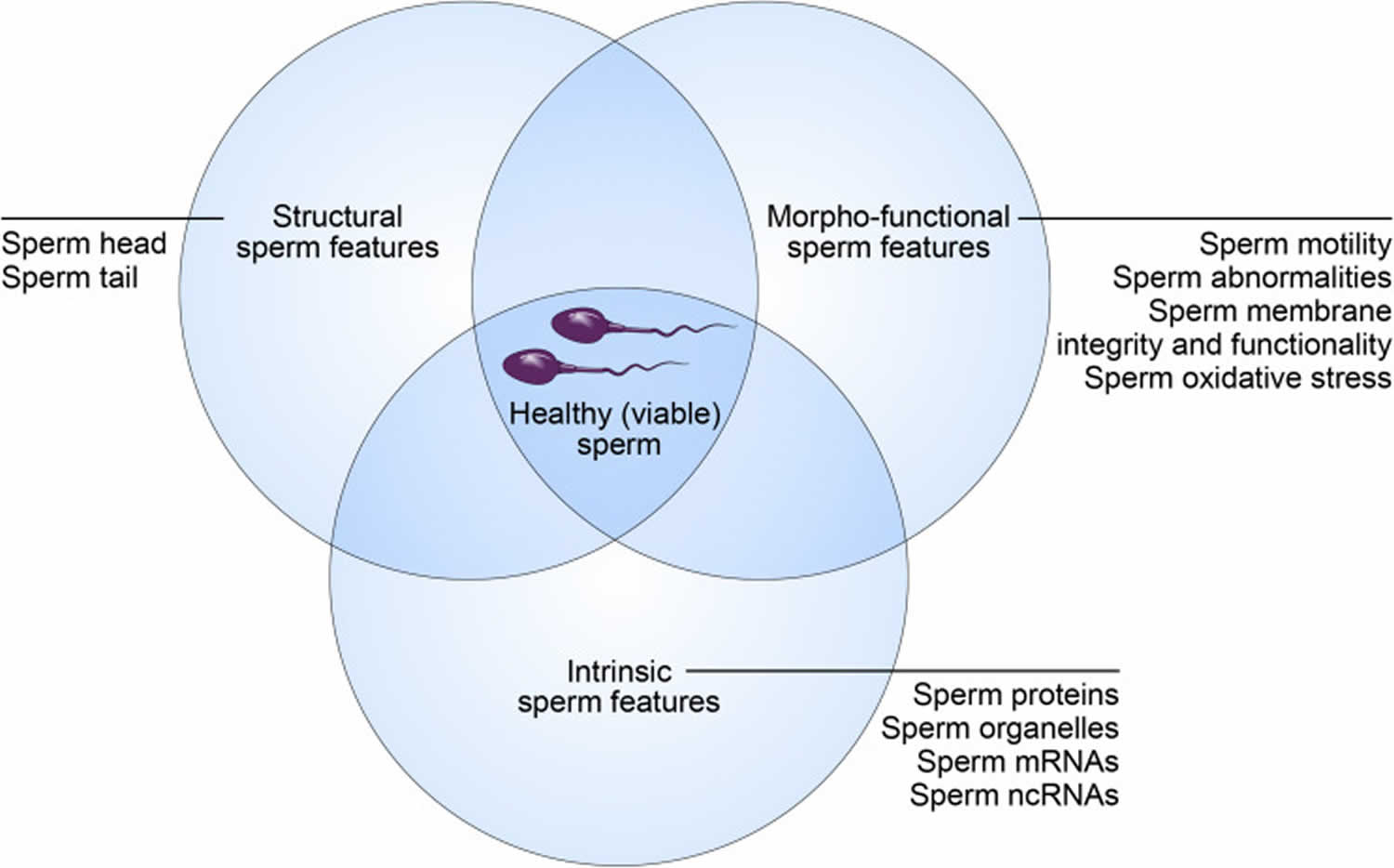
Healthy sperm
Healthy sperm also known as “viable spermatozoa” should possess the ability to reach the fertilization site, bind to and fertilize the oocytes (female eggs), and properly contribute to initiation of early embryo development 16. These abilities are strictly dependent on sperm’s structural, morpho-functional, and intrinsic features.
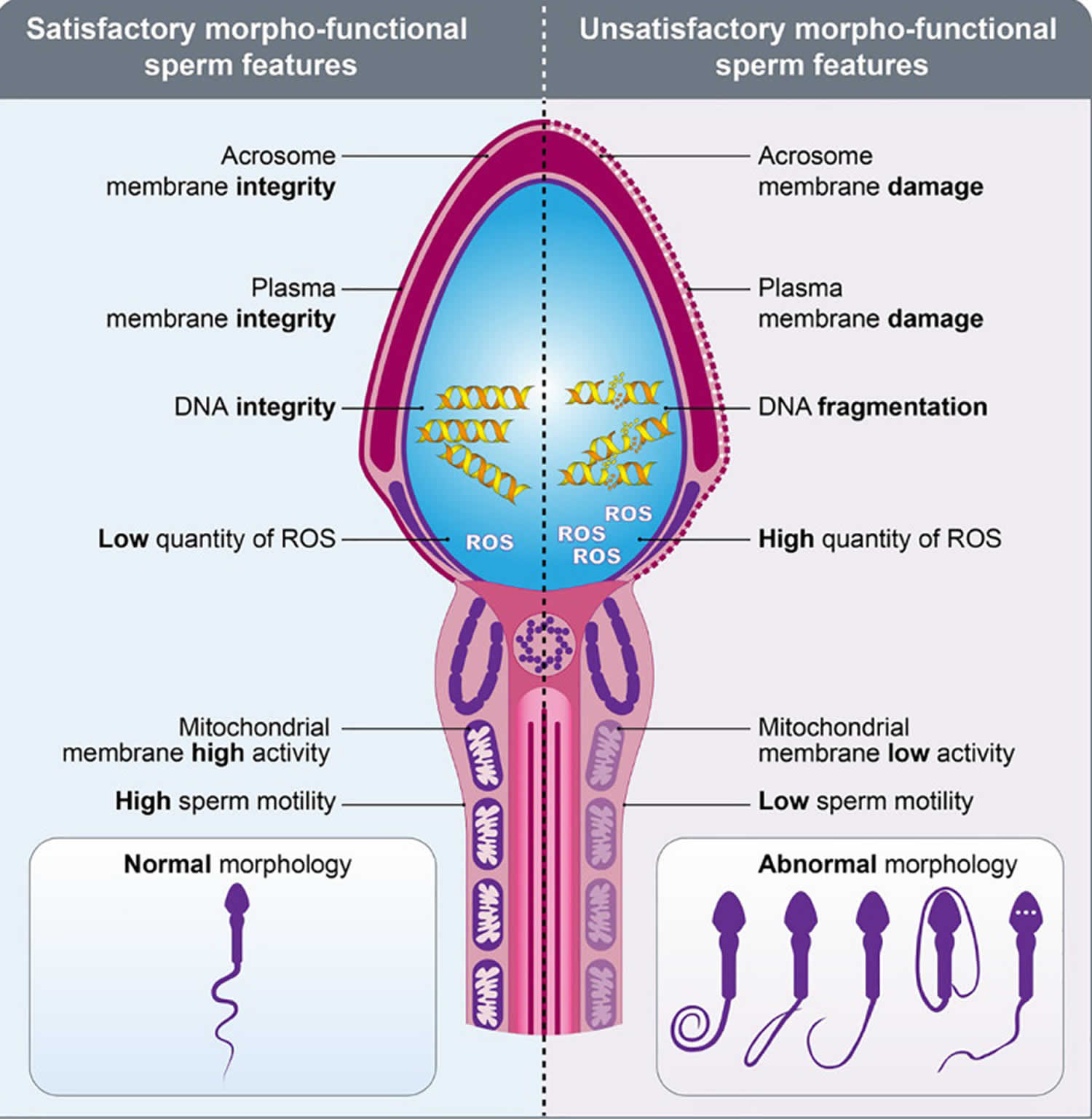
Morpho-functional sperm features such as sperm motility/kinetics, morphological abnormalities, integrity of plasma and acrosome membranes, mitochondrial activity production of reactive oxygen species (ROS), DNA fragmentation and capacitation status are essential in determining male fertility potential 17, 6. A high proportion of sperm morphological abnormalities often referred to as teratozoospermia includes sperms with large, small or piriform heads as well as coiled-tails, is associated with male infertility 18. This common cause of male infertility is routinely assessed by light microscopic analysis of semen in fertility clinics.
Figure 3. Healthy (viable) sperm concept
[Source 6 ]Figure 4. Sperm morpho-functional features
Footnotes: Drawings representing the sperms with satisfactory (left) and unsatisfactory (right) morpho-functional features. Sperm acrosome membrane integrity, sperm plasma membrane integrity, sperm DNA integrity, low quantity of reactive oxygen species (ROS), sperm mitochondrial membrane high activity, high sperm motility and normal sperm morphology characterize the satisfactory morpho-functional sperm features. Sperm acrosome membrane damage, sperm plasma membrane damage, sperm DNA fragmentation, high quantity of reactive oxygen species (ROS), sperm mitochondrial membrane low activity, low sperm motility and abnormal sperm morphology characterize the unsatisfactory morpho-functional sperm features.
[Source 6 ]Hormonal regulation
Sperm production depends on three major hormones:
- Follicle-stimulating hormone (FSH) stimulates the production of sperm in the Sertoli cells, which are located inside the testicles’ seminiferous tubules. The testicles contain hundreds of these microscopic tubules.
- Luteinizing hormone (LH) stimulates receptors in Leydig cells to produce testosterone. Leydig cells surround the seminiferous tubules.
- Testosterone is the most important male hormone.
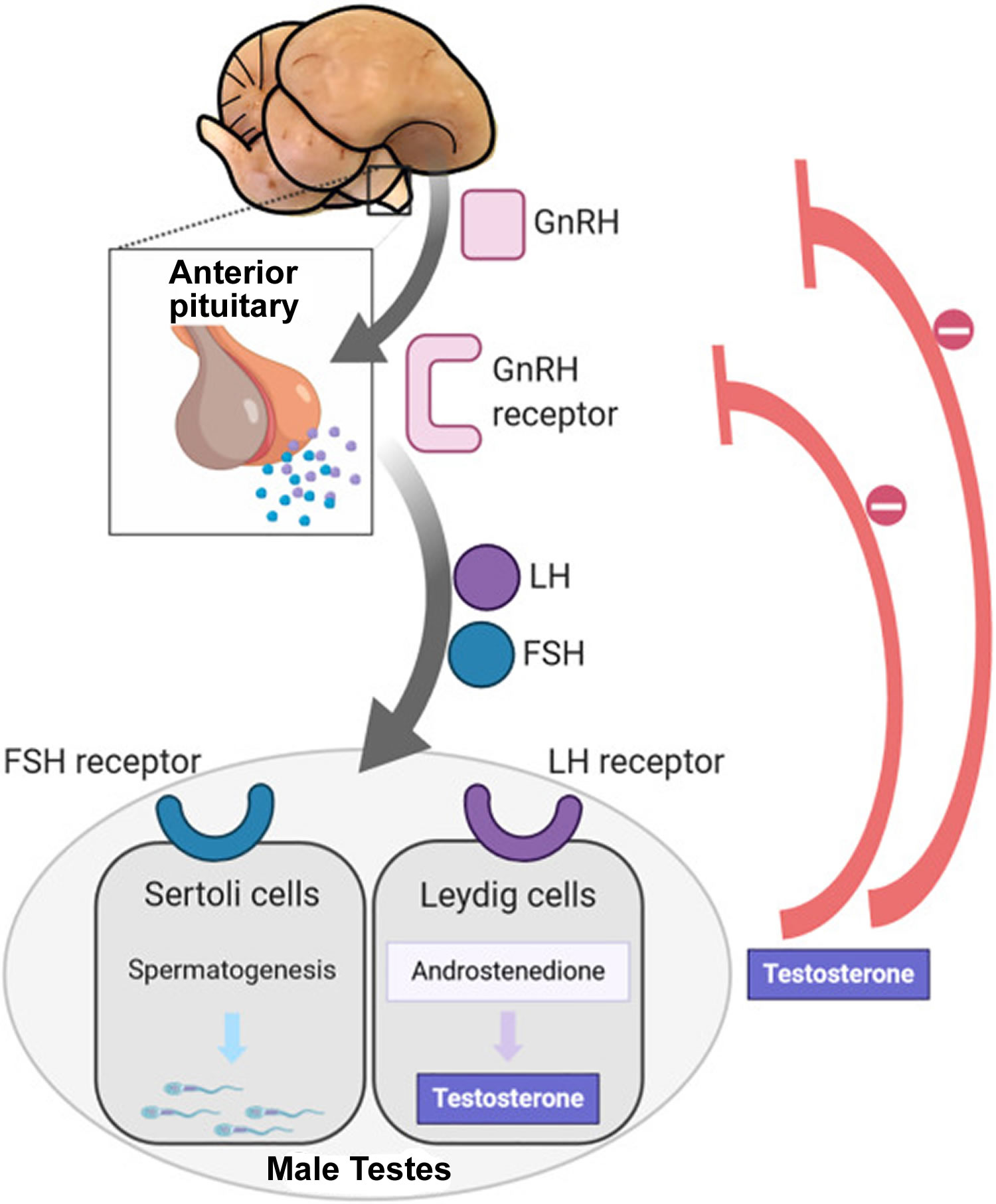
Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are produced in the brain’s anterior pituitary gland. These hormones are also important for female reproduction. Testosterone is produced in the testicles.
Figure 5. Hypothalamic–pituitary–gonadal axis
Footnotes: In puberty, the hypothalamic-pituitary-gonadal axis plays a major role in regulating testosterone levels and gonadal function. Gonadotropin-releasing hormone (GnRH) is secreted from the hypothalamus by GnRH-expressing neurons. The GnRH travels down the hypothalamohypophyseal portal system to the anterior pituitary, which secretes luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH are two gonadotropic hormones that travel through the blood and act on receptors in the gonads. The largest amounts of testosterone (>95%) are produced by the testes in men, while the adrenal glands account for most of the remainder. In the testes, testosterone is produced by the Leydig cells 19. The male testes also contain Sertoli cells, which require testosterone for spermatogenesis (sperm cell development). Luteinizing hormone (LH), in particular, acts on the Leydig cells to increase testosterone production. Testosterone limits its own secretion via negative feedback. High levels of testosterone in the blood feedback to the hypothalamus to suppress the secretion of GnRH and also feedback to the anterior pituitary, making it less responsive to GnRH stimuli 20. Throughout the reproductive life of males, the hypothalamus releases GnRH in pulses every 1 to 3 hours. Despite this pulsatile release, however, average plasma levels of FSH and LH remain fairly constant from the start of puberty, where levels spike, to the third decade of life, where levels peak and slowly begin to decline. Prior to puberty, testosterone levels are low, reflecting the low secretion of GnRH and gonadotropins. Changes in neuronal input to the hypothalamus and brain activity during puberty cause a dramatic rise in GnRH secretion.
Sperm development
The scientific word for sperm development is spermatogenesis. This process begins in the testicle (testes):
- The testes are composed of coiled structures called seminiferous tubules, which are the sites of sperm production.
- Sperm are produced within nurturing Sertoli cells, which are located in the lower parts of the seminiferous tubules.
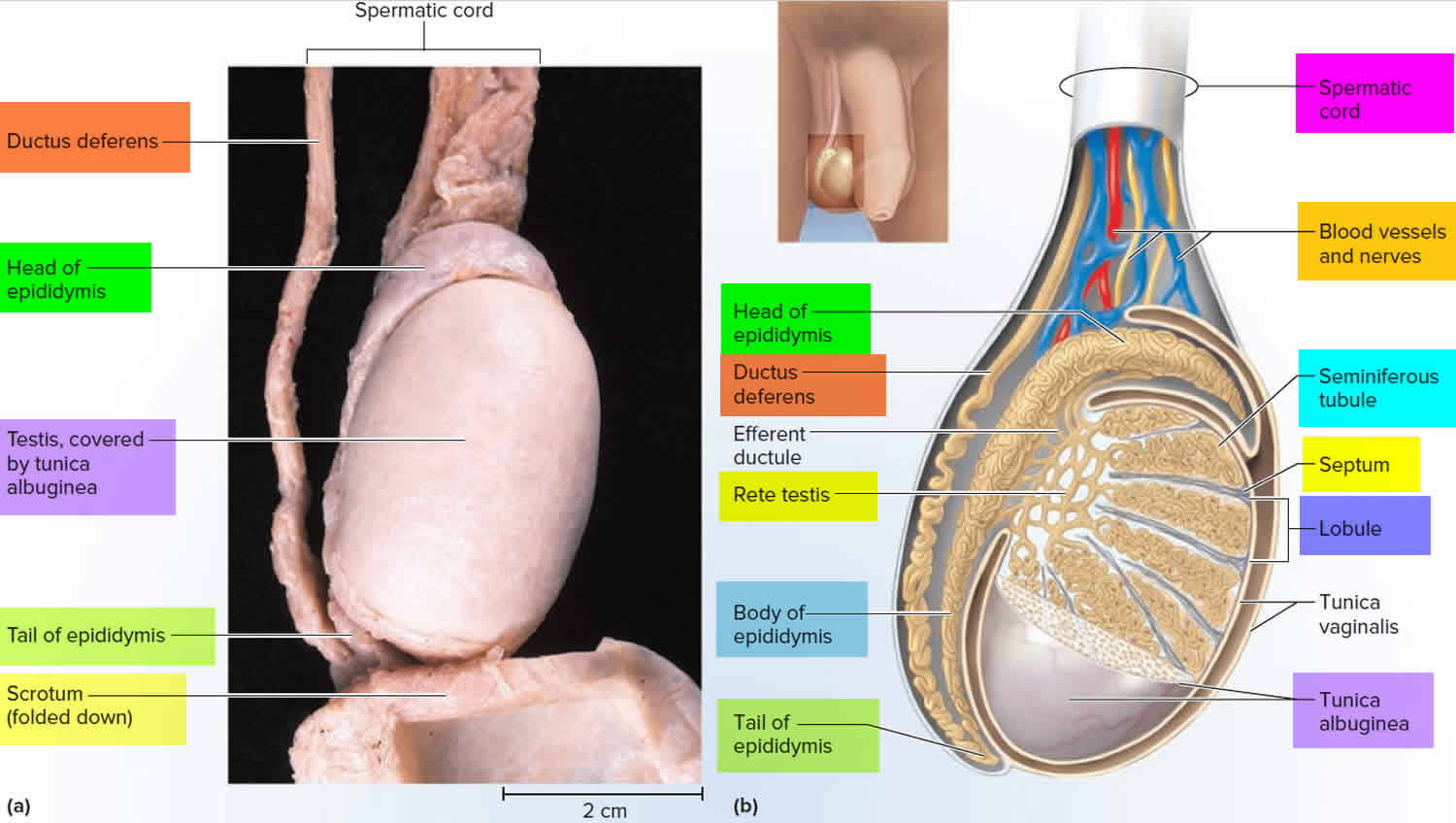
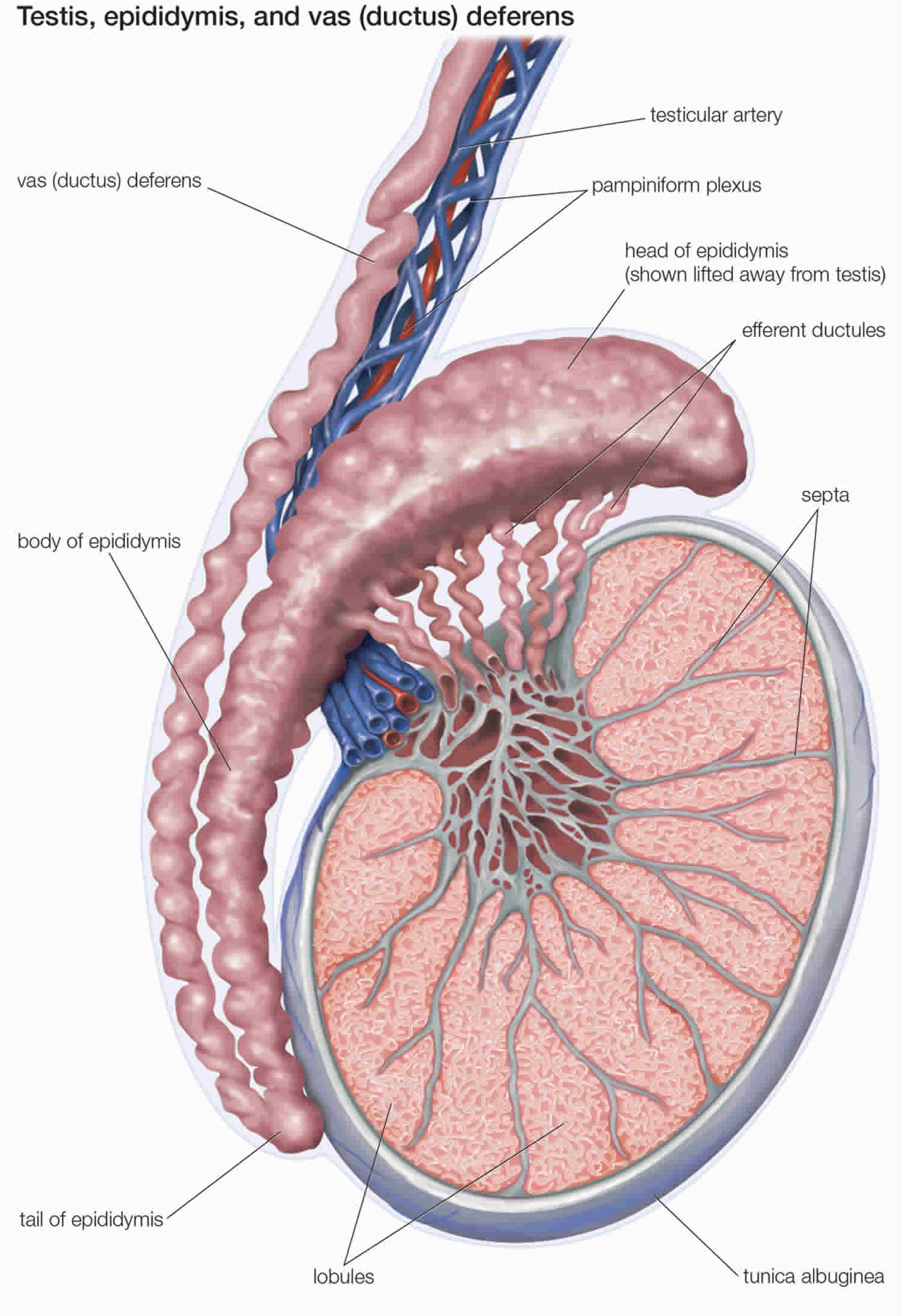
- Once sperm are produced, they pass through the seminiferous tubules and are collected in a structure of tightly coiled tubes called the epididymis. An epididymis lies along the back side of each testicle.
- Sperm mature in the epididymis and are stored there to await ejaculation.
Figure 6. Testicle anatomy
Ejaculation
When a man experiences sexual excitement, his penis fills with blood and becomes erect. Nerves stimulate muscle contractions, which force the sperm out from the epididymis through the penis’ urethra, where they are expelled:
- The sperm pass through the epididymis into a muscular tube called the vas deferens. The vas deferens connects the epididymis to the seminal vesicle and urethra.
- Muscle contractions in the vas deferens propel the sperm into the ampulla, where secretions from the seminal vesicle are added to form seminal fluid (semen). The seminal vesicles are sac-like glands attached to the vas deferens. They produce fructose, which provide energy for sperm movement.
- Each vas deferens and seminal vesicle join together to form an ejaculatory duct. The two ejaculatory ducts (left and right) converge into the prostate gland, which adds milky protein secretions to the seminal fluid.
- Semen is composed mainly of fluid from the seminal vesicles and the prostate gland. Sperm make up only a small percentage of semen.
- The ejaculatory ducts open into the urethra. The urethra is the same channel in the penis through which a man urinates. During orgasm, muscles close the bladder neck so that urine cannot enter the urethra and the semen cannot enter the bladder.
- The semen is forced through the urethra during ejaculation, the final stage of orgasm when the sperm is literally ejected from the penis.
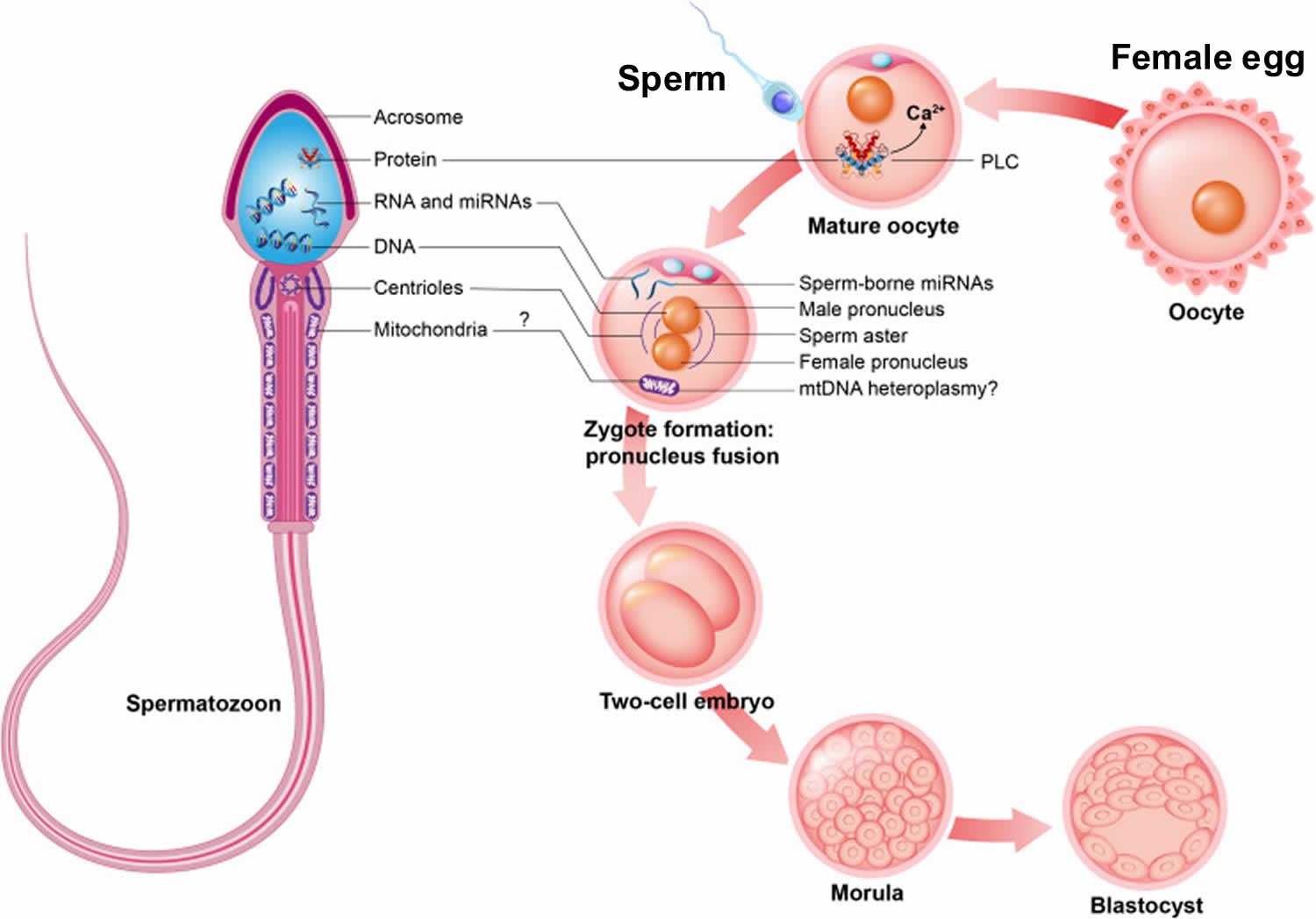
- A man’s ejaculate contains over 100 million sperm. Only 1 sperm fertilizes the egg (see Figure 7 below).
Figure 7. Fertilization
[Source 6 ]Oligospermia causes
Most cases of male infertility cases are due to low sperm count (oligospermia), poor sperm motility (movement), abnormal sperm structure and shape (morphology) or poor sperm quality. The remaining cases of male infertility are caused by a variety of factors including anatomical problems, hormonal imbalances, and genetic defects.
Sperm abnormalities are categorized by whether they affect sperm count, sperm movement, or sperm shape:
- Oligospermia or low sperm count. A sperm count of less than 20 million/mL is considered low sperm count (oligospermia). Azoospermia is the complete absence of sperm cells in the ejaculate. Obstructions in the vas deferens (such as from vasectomy) or other areas can block the passage of sperm and reduce sperm counts.
- Poor Sperm Motility (Asthenospermia). Sperm motility is the sperm’s ability to move. If movement is slow or not in a straight line, the sperm have difficulty invading the cervical mucus or penetrating the hard outer shell of the egg. Sperm that move sluggishly may have genetic or other defects that render them incapable of fertilizing the egg. Poor sperm motility may be associated with DNA fragmentation (damage) and may increase the risk for passing on genetic diseases.
- Abnormal Sperm Morphology (Teratospermia). Morphology refers to shape and structure. Abnormally shaped sperm cannot fertilize an egg. The perfect sperm structure is an oval head and long tail.
The following are possible causes of sperm abnormalities in males:
- Hormonal disorders. Any condition that affects the production of the hormones testosterone, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) can affect sperm production. Improper function of the hypothalamus or pituitary glands are hormonal disorders that can cause infertility. These glands produce hormones that maintain normal testicular function. Too much of these hormones, especially prolactin, can result in infertility. Other conditions that damage or impair the hypothalamus or the pituitary gland may also result in low or no sperm production.
- Low levels of gonadotropins FSH and LH can be caused by pituitary gland tumors or other problems in the pituitary or hypothalamus
- Testosterone deficiency (hypogonadism) can result from genetic disorders, thyroid disorders, medications, or cancer treatments
- Malignant (cancerous) pituitary tumors
- Congenital adrenal hyperplasia (CAH)
- Exposure to too much estrogen
- Exposure to too much testosterone
- Hyperprolactinemia is the excessive production of the hormone prolactin (which produces milk in pregnancy and that suppresses ovulation). In men, abnormal prolactin levels can lead to sexual dysfunction. In men, hyperprolactinemia may be associated with impotence, visual disturbances, sudden weight loss or gain, fatigue or depression.
- Cushing’s syndrome
- Chronic use of medications called glucocorticoids
- Antisperm antibodies. Sometimes a man’s immune system mistakenly identifies sperm as a foreign invader and produces antibodies that attack and destroy the sperm. Antisperm antibodies may result from surgery, infections, or trauma or twisting of the testicles.
- Retrograde ejaculation. Retrograde ejaculation occurs when the muscles of the bladder wall do not function properly during orgasm and sperm are forced backward into the bladder instead of forward out of the urethra. Sperm number and quality are often impaired. Retrograde ejaculation can result from several conditions:
- Surgery to the lower part of the bladder or prostate is the most common cause of retrograde ejaculation
- Nerve damage caused by spinal cord injury or surgery, or diseases such as diabetes and multiple sclerosis
- Medications such as alpha blockers used to treat prostate gland enlargement
- Birth defects. Any anatomical abnormalities that damage or block the testes, tubes, or other reproductive structures can affect sperm and reduce male fertility. These conditions are often birth defects such as:
- Cryptorchidism (undescended testes) is a condition in which the testicles fail to descend from the abdomen into the scrotum.
- Hypospadias is a condition in which the urinary opening is on the underside of the penis.
- Blockages or other problems in the epididymis or ejaculatory ducts are also birth defects. Some men are born lacking the vas deferens, the tube that carries sperm from the testicles.
- Congenital bilateral absence of the vas deferens (CBAVD). Congenital bilateral absence of the vas deferens (CBAVD) is a condition present from birth in which the vas deferens is missing. This greatly affects a man’s fertility since the sperm are essentially stuck in the testicles, with no way of reaching the urethra and out of the body.
- Genetic disorders. Certain inherited disorders can impair fertility. Examples include:
- Cystic fibrosis can cause missing or obstructed vas deferens.
- Klinefelter syndrome results when a man has one or more extra X chromosomes. It causes low testosterone levels and abnormalities of the seminiferous tubules
- Kartagener syndrome causes problems with sperm motility.
- Y chromosome microdeletion can result in loss of genes that are important for sperm formation. A significant proportion of men with azoospermia and oligospermia also have Y chromosome microdeletions.
- Myotonic dystrophy
- Poor semen quality or number
- Lifestyle habits. A number of lifestyle factors may affect sperm and contribute to infertility. They include:
- Cigarette smoking
- Drug abuse with cocaine, opioids, or marijuana
- Prolonged bicycling or horseback riding
- Excessive exercise
- Hot tubs and saunas
- Emotional stress
- Infections e.g., sexually transmitted disease (STD)
- Chemical exposure. Occupational or other long-term exposure to certain types of toxins and chemicals (such as herbicides and pesticides) may reduce sperm count by either affecting testicular function or altering hormone systems. Estrogen-like and hormone-disrupting chemicals such as bisphenol A, phthalates, and organochlorines are particular concerns. Long-term (chronic) exposure to heavy metals such as lead, cadmium, or arsenic may also affect sperm quality. These chemicals usually affect men who have long-term and intense occupational exposure to them.
- Cancer treatment involving the use of certain types of chemotherapy, radiation, or surgery to remove one or both testicles may affect sperm production or the ability of sperm to fertilize an egg.
- Medical conditions such as diabetes, cystic fibrosis, certain types of autoimmune disorders, and certain types of infections may cause testicular failure.
- Testicular trauma
In more than 30% of patients, the causes of sperm abnormalities are unknown 21, 1.
Risk Factors for oligospermia
Risk factors for sperm abnormalities in males include:
- Varicocele. A varicocele is an abnormally enlarged and twisted varicose vein in the spermatic cord that connects to the testicle. Varicoceles are found more often in men who report infertility problems. However, most men with a varicocele are fertile. It is not clear how much varicocele affects fertility or by what mechanisms. Varicoceles can raise testicular temperature, which may have effects on sperm production, movement, and shape.
- Aging. Age-related sperm changes in men are not abrupt, but are a gradual process. Aging can adversely affect sperm counts and sperm motility (the sperm’s ability to swim quickly and move in a straight line). The genetic quality of sperm declines as a man ages.
- Sexually transmitted infections (STDs), which can cause scarring in the male reproductive system or impair sperm function.
- Infections in the prostate gland, testicles, or urethra
- Surgery or injury to the testicles
- Uncontrolled high blood pressure or diabetes
- Obesity
- Thyroid disorders
- Kidney failure especially end-stage kidney disease (ESKD) 22
- Certain types of drugs such as testosterone replacement therapy and anabolic steroids (current or previous use).
- Chemotherapy and radiation cancer treatments
- Lifestyle factors such as smoking and substance abuse
- Long-term or intensive exposure to certain types of chemicals, toxins, or medications
- Frequent exposure of the testes to high temperatures.
Oligospermia prevention
There’s no known way to prevent the genetic conditions that cause oligospermia. If your oligospermia isn’t a genetic problem, doing the following can help lessen the chance of oligospermia:
- Avoid activities that could injure your reproductive organs or wear protection (like a cup when playing baseball).
- Avoid exposure to radiation when possible.
- Know the risks and benefits of medications that could harm sperm production.
- Avoid lengthy exposure of your testes to hot temperatures.
While it remains unclear exactly how much influence these factors have in male infertility, it is reasonable to expect that avoiding potentially spermatotoxic activities and adopting a healthier lifestyle will improve overall male fertility 23.
Oligospermia symptoms
You usually don’t realize you have oligospermia or decrease in male fertility until you begin trying to conceive a child with your female partner without success. Some men have symptoms related to the underlying cause. For example, you could have low sex drive (libido) due to hormonal imbalance or a lump on your testicles from an infection. Determining whether a man’s semen is lacking sperm cannot be accomplished simply by viewing the semen with the naked eye. A semen analysis by an experienced laboratory is required to determine if sperm are present or not.
Couples are generally advised to seek medical help if they are unable to achieve pregnancy after a year of unprotected intercourse. Your doctor will conduct a physical examination of both your and your female partner to determine your general state of health and to evaluate physical disorders that may be causing infertility. Usually, both partners are interviewed about their sexual habits in order to determine whether intercourse is taking place properly for conception.
Oligospermia diagnosis
The initial step in the evaluation of an infertile male is to obtain a thorough medical, sexual and urologic history that might affect fertility. Such a history should include the following:
- Duration of infertility and any prior problems with fertility
- Previous fertility in the patient and the partner
- Timing of puberty (early, normal, or delayed)
- Childhood urologic disorders or surgical procedures and any childhood problems in development
- Current or recent acute or chronic medical illnesses such as diabetes, respiratory infections, allergies, or cancer
- Sexual history including any sexually transmitted infections. Frequency and timing of sexual intercourse.
- Testicular cancer and its treatment
- Social history (e.g., alcohol, tobacco, anabolic steroid, or recreational drug use)
- Medication use
- Family history
- Respiratory disease
- Environmental or occupational exposure. Any exposure to toxins, such as chemicals or radiation
- Spinal cord injury
- History of surgeries or trauma to the genital area
- Any family history of reproductive problems
Physical Exam
Your doctor will carefully examine your scrotum and testicles. Varicoceles can be felt during examination of the scrotum. They are described as feeling like “a bag of worms”. Your doctor may also check your prostate gland. Your doctor will examine your penis for any signs of infection or anatomical abnormalities.
The physical examination should include a thorough inspection of the following:
- Testicles (for bilateral presence, size, consistency, symmetry)
- Epididymis (for presence bilaterally, as well as any induration, cystic changes, enlargement, tenderness)
- Vas deferens (for presence bilaterally, defects, segmental dysplasia, induration, nodularity, swelling)
- Spermatic cord (for varicocele)
- Penis (for anatomic abnormalities, strictures, or plaques)
- Rectum (for abnormalities of the prostate or seminal vesicles)
- Body habitus
Depending on the findings from the history, detailed examination of other body functions may also be warranted.
In addition to a medical history and physical exam, specific tests for male infertility may include:
- Semen analysis to evaluate the quantity and quality of sperm
- Blood tests to evaluate hormone levels
- Imaging tests to look for structural problems
- Genetic testing to identify sperm DNA fragmentation, chromosomal defects, or genetic diseases
The sperm count test is performed if a man’s fertility is in question. It is helpful in determining if there is a problem in sperm production or quality of the sperm as a cause of infertility. The sperm count test may also be used after a vasectomy to make sure there are no sperm in the semen.
Semen analysis
Semen analysis is the cornerstone of the male infertility diagnostic workup.
The sperm collection involves the following steps:
- Abstain from ejaculation for several days before (but no more than 5 days) the test because each ejaculation can reduce sperm count.
- Collect a sample of your semen either by masturbating into a sterilized cup or having sexual intercourse using a special collection condom.
- Keep the sample at room or body temperature and deliver it promptly to the laboratory or medical office. The sperm should be analyzed within 1 hour of collection.
- Because sperm counts can fluctuate, collection and analysis may need to be repeated over several months.
A semen analysis will provide information on:
- Semen volume (normal 1.5 to 5 mL). The amount of semen is important. Most men ejaculate between 2.5 to 5 mL of semen. Either significantly high or low amounts can indicate problems. Low amounts (less than 1.5 milliliters) are a sign of fewer sperm. High amounts may dilute the concentration of sperm.
- Semen quality
- Semen density or concentration (normal > 15 million sperm/mL). Semen concentration refers to the amount of sperm found in 1 mL of semen. A normal concentration is at least 20 million sperm per milliliter.
- Total number of sperm in the sample (sperm count). Sperm count refers to the number of sperm found in the entire sample of semen. In general, a normal sperm count is considered to be at least 40 million.
- Percent of live sperm (sperm viability). At least 50% of the sperm in the semen sample should be alive (viable).
- Percentage of moving sperm (sperm motility) (normal > 40% of sperm having normal movement). Sperm motility (the speed and quality of movement) is graded on a scale from 0 (worst) to 4 (best). Grades 3 and 4 indicate good mobility. At least 40% of the sperm should be motile.
- Sperm size, shape, and structure (sperm morphology). Sperm morphology is the size, shape, and structure of the sperm. At least 50% of the sperm should be of normal appearance.
- Signs of infection – An increased number of white blood cells (WBCs) in the semen may be observed in patients with infectious or inflammatory processes
- Other variables (eg, levels of zinc, citric acid, acid phosphatase, or alpha-glucosidase). The acidity of the semen is tested (pH should be 7.2 or greater). The lab will test for presence or absence of certain chemicals and enzymes, such as fructose. The semen will be tested for how thick it is and how quickly it liquefies, and whether it clumps together.
An abnormal semen analysis test result done after sexual intercourse (postcoital) is observed in 10% of infertile couples. Indications for performing a postcoital test include semen hyperviscosity, increased or decreased semen volume with good sperm density, or unexplained infertility.
If the postcoital semen analysis test result is normal, consider sperm function tests, such as the following:
- Capacitation assay
- Acrosome reaction assay
- Sperm penetration assay
- Hypoosmotic swelling test
- Sperm-cervical mucus test
- Inhibin B level
- Vitality stains
Although less commonly performed, sperm penetration tests may be used to evaluate function. For example, the sperm penetration assay measure the sperm’s ability to penetrate specially prepared hamster eggs. The exact role of these tests for most people with infertility remains unclear.
A post-ejaculatory urine sample can detect presence of sperm, which may indicate retrograde ejaculation.
A laboratory sperm-cervical mucus test (Kurzrock-Miller or Kremer test) may also be used to evaluate how well sperm move in cervical mucus. Either the female partner’s mucus or donated midcycle mucus can be used in this test.
Blood tests
Other laboratory tests that may be helpful in male infertility diagnostic workup include the following:
- Antisperm antibody test
- Hormonal analysis (FSH, LH, TSH, testosterone, prolactin)
- Genetic testing (karyotype, CFTR, AZF deletions if severe oligospermia (< 5 million sperm/mL)
Blood tests may be used to check for hormone levels of testosterone, FSH, and LH. A blood test can also check for evidence of sexually transmitted infections.
Imaging studies
Imaging studies in male infertility diagnostic workup may include the following:
- Transrectal ultrasound
- Scrotal ultrasound
- Vasography
- Brain MRI (magnetic resonance imaging) if your doctor believe your hypothalamus or pituitary gland is playing a role.
Ultrasound uses sound waves to produce an image. Ultrasound imaging may be used to check for abnormalities or blockages in the testicles, or to find varicoceles that are too small for physical detection.
In transrectal ultrasound, a probe is inserted into the rectum to provide images of the prostate gland, vas deferens, and seminal vesicles.
Testicular biopsy
A small tissue sample of the testicle may be taken using a thin needle. A biopsy may be performed for diagnostic purposes to evaluate sperm production function. A biopsy may also be used to collect sperm that will be used in an intracytoplasmic sperm injection (ICSI) fertility procedure.
Testicular biopsy is usually indicated in a complete absence of sperm in semen (azoospermia) with a normal-sized testis and normal findings on hormonal studies to evaluate for ductal obstruction, to further evaluate idiopathic infertility, and to retrieve sperm.
In modern practice, testicular biopsy is rarely performed alone. In most cases, doctors can predict with high accuracy whether or not a man has an obstructive cause of azoospermia. Since doctors have started performing testicular dissections to search for sperm, they have learned that different areas of the testis might show different patterns of nonobstructive azoospermia. For example, one area might show decreased production of mature sperm (hypo spermatogenesis or maturation arrest), while another area might show the complete absence of sperm precursor cells (Sertoli-cell-only syndrome). Therefore, in the modern era, doing a diagnostic testicular biopsy does not often change the ultimate management for men with nonobstructive azoospermia. For those men, doctors offer microdissection testicular sperm extraction (microTESE), which gives the best chance of finding sperm that can be used for assisted reproductive technology (ART). Sometimes, at the time of the microTESE, doctors will send a tiny specimen for pathological evaluation to rule out a precursor to cancer called intratubular germ cell neoplasia (ITGCN).
Genetic testing
Genetic testing may be recommended for some men, particularly those who will use intracytoplasmic sperm injection (ICSI). Genetic testing can help identify sperm DNA fragmentation, chromosomal defects, or the possibility of genetic diseases that can be passed on to children.
Oligospermia treatment
Treatment for male infertility depends on the cause of infertility, and whether a cause is found. Treatment for male infertility should first address any underlying medical conditions that may be contributing to fertility problems. Drug therapy may be used to treat hormonal disorders or infections. Surgery may be used to repair varicoceles in some men and correct any obstructions in the reproductive tract.
Male infertility treatments may include:
- Drug therapy to treat hormone imbalances or infections.
- Surgery to repair varicoceles and correct any obstructions in the reproductive tract. However, there is some controversy over whether varicocele treatment actually improves fertility or the chance for conception.
- Fertility treatments with testicular sperm extraction (TESE), intrauterine insemination (IUI), or intracytoplasmic sperm injection (ICSI).
If fertility issues remain unresolved, intracytoplasmic sperm injection (ICSI) is commonly used in combination with in vitro fertilization (IVF) to achieve pregnancy when male infertility is a factor. Intracytoplasmic sperm injection (ICSI) involves injecting a single sperm into an egg obtained through IVF. The fertilized egg is then implanted back into the woman. Testicular sperm extraction (TESE) may sometimes be used to get viable sperm from the testes. Testicular sperm extraction (TESE) involves placing an anesthetic on the scrotum and passing a needle into the testicle to obtain some tissue. Sperm then can be found within this tissue. This sperm can sometimes be used for ICSI. Pregnancy success rates depend on many different factors.
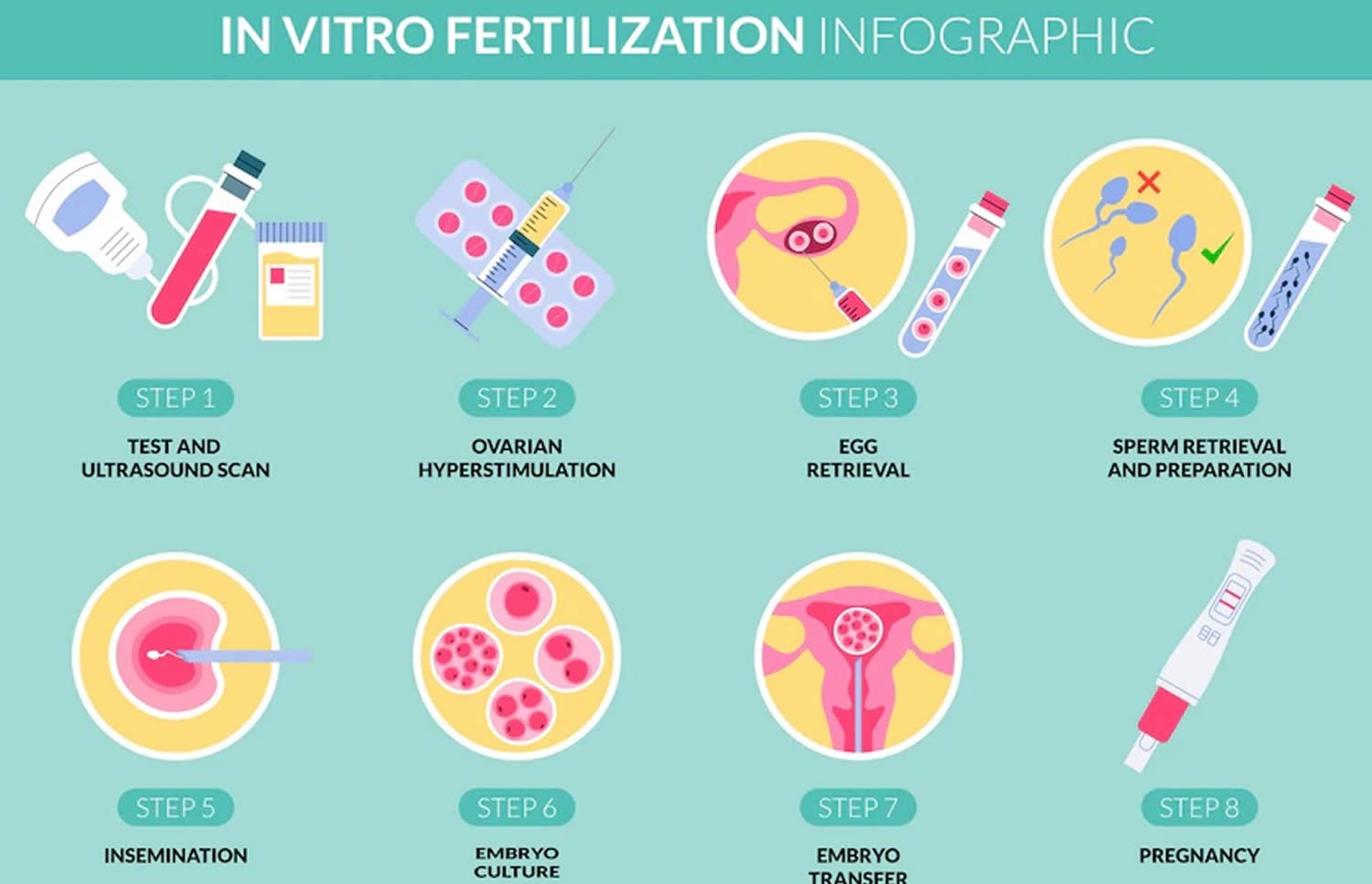
Figure 8. In vitro fertilization (IVF)
[Source 24 ]Oligospermia treatment medicine
Antioxidants are man-made or natural substances that may prevent or delay some types of cell damage. There is some thought that these agents may improve sperm function and therefore fertility. Common antioxidants include carnitines, lycopene, glutathione, vitamin E, vitamin C, vitamin A, Folate (folic acid), Selenium, Zinc, Coenzyme Q10 (CoQ10) and N-acetyl cysteine (NAC). Antioxidants are found in many foods, such as fruits and vegetables. They may also be found as dietary supplements. The evidence that these substances improve pregnancy rates is poor 25. A 3-year, multi-institutional study from 9 fertility centers that included 174 infertile male patients using antioxidant therapy alone, without L-carnitine, showed no benefit in improving semen parameters or pregnancies for the 6 months the patients were followed 26. However, significant other data supports antioxidant use for male infertility 27, 28, 29, 30, 31, 32, 33.
A 2020 single-blinded study 34 involving 50 idiopathic infertile men with abnormal semen analyses (oligospermia and reduced sperm motility with less than 50% sperm motility or less than 25% with rapid progressive motility [asthenozoospermia]) showed that a proprietary daily antioxidant mixture of coenzyme Q10 (30 mg), zinc (8 mg), vitamins C (100 mg), and E (400 IU), folic acid (400 mcg) and selenium (200 mcg) taken for 3 months resulted in statistically significant improvements in sperm count, concentration, motility, progressive motility, and morphology as well as better semen volume and pH.
A similar trial using just daily coenzyme Q10 (200 mg) compared to placebo in 114 infertile men followed for 26 weeks found statistically significant improvements in sperm motility, count, and strict morphology. Interestingly, these improved sperm parameters returned to pretreatment levels 12 weeks after coenzyme Q10 was discontinued 35. Pregnancy rates were not reported 35.
A randomized trial using just zinc and folic acid supplementation failed to show any significant benefit to male semen parameters or pregnancy rates 36. This is contradicted by several examination of data from a number of independent multiple studies of the same subject suggesting that vitamin C and vitamin E can significantly improve multiple semen parameters, including sperm counts, motility, concentration, and morphology 37, 38, 39.
The most studied vitamins, minerals, and antioxidants used for male infertility include coenzyme Q10, folic acid, L-carnitine, lycopene, N-acetyl cysteine, vitamin C, vitamin E, folic acid, selenium, and zinc 28, 40, 41.
Coenzyme Q10 (CoQ10) appears to have a beneficial effect on sperm quality. Coenzyme Q10 reduces organic peroxides in the semen, which decreases sperm cell oxidative stress 42, 43, 44, 45. Coenzyme Q10 reportedly improves sperm motility, morphology, and concentration 35, 46, 47, 48. The usual recommended Coenzyme Q10 dose is 300 mg daily 49.
Folic acid also known as folate or vitamin B-9, is a very effective antioxidant intimately involved in numerous cellular functions, including cell division and the synthesizing and repairing of DNA and RNA 50, 51, 52. Folic acid improves spermatogenesis by enhancing the methylation of DNA, limiting the activity of apoptotic genes in the testes, reducing reactive oxygen species through its antioxidant activity, and managing abnormal testicular methylenetetrahydrofolate reductase (an enzyme involved in DNA methylation) 50, 53, 54, 55. Improvements in semen parameters, especially sperm motility, have been noted in patients on folic acid supplementation 50, 56, 57. Folic acid is often used together with zinc supplementation 50. The recommended daily dose of folic acid is 500 to 1000 mcg 57. Higher doses are not recommended and may actually decrease sperm quality by downregulating DNA methylation 58.
L-carnitine is an amino acid and antioxidant that is typically found in high concentrations in the epididymis and has long been suggested as a possible, nontoxic general therapy for male infertility 37, 59, 60, 61. L-carnitine is known to increase fatty acid transport into sperm mitochondria, which is needed for epididymal sperm energy production. L-carnitine also appears to increase sperm motility, morphology, and maturation while reducing apoptosis 60, 61, 62, 63, 64. Recently, 180 infertile male patients with idiopathic oligo-astheno-teratozoospermia (low sperm counts with decreased motility and poor morphology, a “stress pattern”) were given an L-carnitine supplement and demonstrated significant improvements in sperm count, concentration, and morphology. However, motility was not affected, and the study was of insufficient duration to determine if pregnancy rates were affected 65, 66. A daily dose of 3 gm per day has been suggested.
Lycopene is a carotenoid antioxidant and the organic pigment that makes fruits and vegetables yellow, orange, or red. Lycopene is naturally found in carrots, pink watermelon, grapefruit, apricots, and especially tomatoes. Lycopene is a powerful antioxidant shown to increase male fertility and significantly improve semen parameters 67. Sperm counts can increase up to 70% and progressive motility up to 54%, while morphology improves by up to 40% in various studies 68, 69, 70, 71, 72, 73. The recommended dosage of lycopene used as a supplement for male infertility is 6 mg daily 72.
N-acetylcysteine (NAC) is a dietary supplement and mucolytic agent sometimes used to treat overdoses of acetaminophen and paracetamol 74. A thiol-based derivative of the amino acid L-cysteine and a precursor to glutathione peroxidase, N-acetylcysteine (NAC) possesses significant anti-inflammatory, mucolytic, and antioxidant properties 74. When used in infertile men, N-Acetylcysteine (NAC) therapy increases sperm counts, enhances motility, reduces abnormal morphology, decreases DNA fragmentation, improves acrosomal activity, and acts as an effective semen antioxidant 63, 29, 30, 75, 76, 77. The usual dose of N-acetylcysteine (NAC) is 600 mg to 1200 mg daily; no prescription is required. However, since it was previously available as a prescription medication, the FDA is reviewing its status as an OTC dietary supplement 78.
Selenium, especially when used together with N-acetylcysteine (NAC), appears to help improve sperm concentration, motility, and morphology by acting to enhance enzymatic antioxidant activity 28, 79, 80, 81. Selenium is critical for the biosynthesis of testosterone and sperm production 82. Selenium can also reverse the negative effects of heavy metal exposure on spermatogenesis and male fertility 83. The recommended Selenium dosage when used as a supplement is 200 mcg per day and is often used together with 400 IU of vitamin E 80, 84, 85.
Vitamin C (ascorbic acid) is a potent antioxidant normally found in large amounts in the semen, which protects against DNA damage and may improve semen viscosity 86, 87, 88. Supplemental vitamin C also appears to improve the hormonal profile of subfertile men and their semen parameters 89. Patients given vitamin C tended to have improved progressive motility, sperm counts, concentration, morphology, and pregnancy rates 38. Vitamin C supplementation for male infertility is often given with 400 IU of vitamin E daily 38, 37, 39. The suggested dosage of supplemental vitamin C is 500 mg to 1000 mg daily 90, 88.
Vitamin D supplementation may help with sperm motility 91, 92, 93. There is also evidence that men with unexplained infertility and low vitamin D levels may suffer increased sperm DNA damage 91, 94.
Zinc is the most abundant metal in the human body after iron. According to the Food and Agriculture Organization of the United Nations, over 17% of the global population is zinc deficient 95. Zinc is important for sperm maturation and testicular development, protecting spermatozoa from damage by oxidized thiols, improving sperm function, and maintaining fertilization capacity 28, 96, 97, 98. Zinc is often given together with folate, which seems to improve the results 99. Seminal zinc levels appear important in maintaining sperm counts, but excessive levels can adversely affect motility 100. The best results were found when used together with folic acid 50. Zinc also appears able to reverse damage from heavy metal exposure 83. The suggested daily dosage of supplemental zinc is 200 mg (25-400 mg). Note that zinc sulfate 220 mg has only 50 mg of elemental zinc 101.
Fish oil supplements have also been suggested as helpful in male fertility, but there is insufficient evidence to make a recommendation 102.
Endocrine treatments may be tried in selected men with identified endocrine dysfunction as a possible cause. The goal of these treatments is to increase the level of testosterone within the testes. These include:
- Clomiphene and tamoxifen
- Aromatase inhibitors
Clomiphene is an antiestrogen and, in small doses (25 mg every other day up to 50 mg daily, with 25 mg daily being recommended most often), can increase gonadotropins (FSH and LH) and stimulate spermatogenesis, making it potentially useful in idiopathic cases of male infertility. Clomiphene works by inhibiting the estradiol negative feedback response to the hypothalamus, which results in a higher release of LH, causing higher testosterone levels but also resulting in higher estradiol levels 103. Most patients will notice an improvement in semen analyses in 3 months, but some will need 6 months or longer 104. This benefit can be increased by adding tamoxifen (10 mg twice daily), which also acts as an estrogen receptor blocker (antagonist) 105, 106, 107. Clomiphene is also possibly helpful in hypogonadotropic hypogonadism, although there is limited data showing any significant improvement in pregnancy rates 108, 109.
Tamoxifen is a selective estrogen receptor modulator (SERM) that competes with the hormone for binding sites, acting as a competitive inhibitor, and is most often used in estrogen-receptor-positive breast cancer 110. By selectively blocking hypothalamic estrogen receptors, tamoxifen stimulates GnRH secretion, greatly increasing FSH and LH levels and ultimately promoting spermatogenesis 106, 111, 112. Tamoxifen is best used for idiopathic oligospermia as it tends to be most effective in boosting sperm count and concentration 106, 105, 107, 112. Motility, viability, and morphology may also improve, but generally not to the same degree 112, 106, 107. In a trial, 68 infertile men were treated with either a placebo, folate, tamoxifen, or a combination of folate and tamoxifen for 3 months. Sperm concentrations and counts were improved by tamoxifen therapy, while adding folate increased motility, suggesting the 2 supplements are complementary when used together 57. The recommended Tamoxifen dose is 10 mg twice a day 110.
Aromatase inhibitors have shown an ability to improve semen parameters but have not been conclusively proven to improve pregnancy rates, as most of the available studies are either case reports, anecdotal, or of low quality 113. A recent review and meta-analysis of aromatase inhibitors for male infertility have suggested that these drugs can statistically improve abnormal semen and hormonal parameters, plus they appear to be safe 114. They are most useful when testosterone levels are normal but estradiols (estrogens) are relatively high 102. Also, they can be used together with clomiphene, which is recommended. Both the steroid-based (testolactone) and nonsteroidal (anastrozole and letrozole) drugs appear to have equivalent efficacy. Still, it is difficult to recommend an aromatase inhibitor without adequate, prospective, randomized, placebo-controlled multicenter trials to determine their efficacy and optimal dosage definitively 113, 115, 114. Anastrozole and letrozole are inexpensive, with minimal adverse effects. The recommended dose of anastrozole for male infertility is 1 mg 3 times a week, while the dose for letrozole is 2.5 mg also 3 times weekly. The most common adverse effects of these medications are joint pain and stiffness 116.
Varicocelectomy (Varicocele Removal)
Conditions for treating a varicocele that can be felt on physical exam include both of the following:
- Female partner has either normal fertility or has a fertility problem that can be treated.
- Abnormal semen findings in semen analysis.
Depending on semen analysis findings, other fertility treatments such as intrauterine insemination (IUI), in vitro fertilization (IVF), or intracytoplasmic sperm injection (ICSI) are other options before surgical treatment of the varicocele.
Invasive varicocele treatment options include:
- A surgical approach, most often through the inguinal area.
- A percutaneous (through the skin) approach injecting an agent or device into the widened vein which feeds the varicocele to obstruct it.
In general, surgical invasive treatment of varicoceles has been found to increase sperm counts by around 12 million and improve sperm motility by around 10%. These improvements take 3 to 6 months to be evident.
Studies that report on pregnancy rates after treatment appeared to indicate an improvement for couples with otherwise unexplained infertility. The quality of these studies is generally not considered to be high.
Low fertility in males home remedies
The American Society for Reproductive Medicine recommends:
- If you smoke, stop. Smoking may impair your sperm quality, as can excessive alcohol use, recreational drugs, and anabolic steroids. It is also important to take care of your overall health including managing any long-term (chronic) health conditions such as high blood pressure, obesity and diabetes.
- A couple’s best chance for conception is to have sex 2 to 3 times throughout a woman’s cycle and every 1 to 2 days during a women’s “fertile window” (the 6 days leading up to and including ovulation). Abstaining from sex for more than 5 to 10 days may adversely affect sperm health. But a couple’s decision on how frequently to have sex is a personal choice.
- Avoid using water-based sexual lubricants as they can interfere with the sperm’s ability to swim. Use instead canola oil, mineral oil, or commercial “fertility-friendly” labeled products.
Healthy lifestyle changes should be recommended to all male infertility patients 102. These changes include stopping smoking, limiting or eliminating alcohol intake, adopting a more nutritious diet, weight loss measures if obese, increased exercise, avoiding potentially toxic artificial lubricants during sexual activity, reducing stress, eliminating illegal and recreational drug use (such as marijuana), minimizing prescription drugs, avoiding exposure to pesticides and heavy metals (such as lead, mercury, boron, and cadmium), and eliminating any unnecessary chemical exposures 117, 23, 118, 27. Low body weight and obesity are also possible risk factors for male infertility 119, 120.
Clothing selection, “boxers or briefs”, might play a role in male infertility due to possible alterations in scrotal temperature, with “boxers” being preferred, although the evidence is not compelling or definitive 121. While avoiding hot baths, saunas, and tight-fitting underwear has not been conclusively demonstrated to improve male fertility significantly, it is not unreasonable to discuss these suggestions with patients.
Fertile Window
Couples can optimize their chances for conception by planning sexual intercourse during the woman’s fertile window:
- The “fertile window” is 6 days long, starts 5 days before a woman’s ovulation, and ends the day of ovulation. (Ovulation is when an egg is released to await fertilization. The egg lives only 12 to 24 hours after ovulation and must be fertilized by a sperm during this time in order for pregnancy to occur.)
- In a woman with a 28-day menstrual cycle, ovulation usually occurs around day 14 (14 days after the start of her menstrual period), which means the fertile window occurs from days 9 to 14. However, the length of menstrual cycles, and fertile windows, vary from woman to woman.
- The chance that intercourse will result in pregnancy is highest during the fertile window, during the 3-day interval that ends on the day of ovulation.
Frequency of Intercourse
Evidence indicates that chances are best for conception when sexual intercourse occurs every 1 to 2 days, during the “fertile window”. Less frequent regular sexual intercourse such as 2 to 3 times a week also achieves good results. However, couples need to make their own personal choices about what amount of sexual activity is appropriate, comfortable, and desirable. Data suggest that abstinence (not having sex) for more than 5 to 10 days may adversely affect sperm health.
Sexual Practices
There is no evidence that any particular sexual positions, or resting after sex, help increase the chances for pregnancy. There is also no evidence that whether or not a woman achieves orgasm affects the likelihood of her becoming pregnant. Couples should be aware that water-based sexual lubricants (Astroglide, KY Jelly, saliva) can damage sperm’s ability to swim and should not be used. For lubrication, try mineral oil, canola oil, or commercially available “fertility-friendly” products such as Pre-Seed, which do not harm sperm.
Fertility and Seasonal Changes
Some studies have reported higher sperm counts and better motility in the winter than in the summer.
Healthy Diet
Everyone should eat a healthy diet rich in fresh fruits, vegetables, and whole grains. Replace animal fats with monounsaturated oils, such as olive oil. Certain specific nutrients and vitamins have been studied for their effects on male infertility and sperm health. They include antioxidant vitamins (vitamin C, vitamin E) and the dietary supplements L-carnitine and L-acetylcarnitine. To date, there is no conclusive evidence that they are effective.
Dealing with Stress
Stress is how your brain and body respond to a challenge or demand. When you are stressed, your body releases certain hormones. The hormones that are released when you are stressed make you alert and ready to act. They can raise your blood pressure, heart rate, and blood glucose (blood sugar) levels. This response is sometimes called a “fight or flight” response.
The first step in managing stress is recognizing it in your life. Everyone feels stress in a different way. You may get angry or irritable, lose sleep, or have headaches or stomach upset. What are your signs of stress? Once you know what signals to look for, you can start to manage it. Also identify the situations that cause you stress. These are called stressors. Your stressors could be family, school, work, relationships, money, or health problems. Once you understand where your stress is coming from, you can come up with ways to deal with your stressors.
When you feel stressed, you may fall back on unhealthy behaviors to help you relax. These may include:
- Eating too much
- Smoking cigarettes
- Drinking alcohol or using drugs
- Sleeping too much or not sleeping enough
These behaviors may help you feel better at first, but they may hurt you more than they help. Instead, use the tips below to find healthy ways to reduce your stress.
Healthy ways to manage stress
- Recognize the things you can’t change. Accepting that you can’t change certain things allows you to let go and not get upset. For instance, you cannot change the fact that you have to drive during rush hour. But you can look for ways to relax during your commute, such as listening to a podcast or book.
- Avoid stressful situations. When you can, remove yourself from the source of stress. For example, if your family squabbles during the holidays, give yourself a breather and go out for a walk or drive.
- Exercise regularly. Getting physical activity every day is one of the easiest and best ways to cope with stress. When you exercise, your brain releases chemicals that make you feel good. It can also help you release built-up energy or frustration. Find something you enjoy, whether it is walking, cycling, softball, swimming, or dancing, and do it for at least 30 minutes on most days.
- Change your outlook. Try to develop a more positive attitude toward challenges. You can do this by replacing negative thoughts with more positive ones. For example, rather than thinking, “Why does everything always go wrong?” change this thought to, “I can find a way to get through this.” It may seem hard or silly at first, but with practice, you may find it helps turn your outlook around.
- Do something you enjoy. When stress has you down, do something you enjoy to help pick you up. It could be as simple as reading a good book, listening to music, watching a favorite movie, or having dinner with a friend. Or, take up a new hobby or class. Whatever you choose, try to do at least one thing a day that’s just for you.
- Learn new ways to relax. Practicing relaxation techniques is a great way to handle daily stress. Relaxation techniques help slow your heart rate and lower your blood pressure. There are many types, from deep breathing and meditation to yoga and tai chi. Take a class, or try learning from books, videos, or online sources.
- Connect with loved ones. Do not let stress get in the way of being social. Spending time with family and friends can help you feel better and forget about your stress. Confiding in a friend may also help you work out your problems.
- Get enough sleep. Getting a good night’s sleep can help you think more clearly and have more energy. This will make it easier to handle any problems that crop up. Aim for about 7 to 9 hours each night.
- Maintain a healthy diet. Eating healthy foods helps fuel your body and mind. Skip the high-sugar snack foods and load up on vegetables, fruits, whole grains, low-fat or nonfat dairy, and lean proteins.
- Learn to say no. If your stress comes from taking on too much at home or work, learn to set limits. Ask others for help when you need it.
If you can’t manage stress on your own, you may want to talk with your doctor or consider seeing a therapist or counselor who can help you find other ways to deal with your stress. Depending on the cause of your stress, you also may find it helps to join a support group.
Other Lifestyle Changes
Other tips for helping fertility include:
- Control any long-term (chronic) medical conditions you may have such as high blood pressure and diabetes.
- Overweight men should try to reduce their weight as obesity may be associated with infertility. However, good clinical studies to support an improvement in fertility have not been done.
- Avoid cigarettes and any drugs that may affect sperm count or reduce sexual function.
- Get sufficient rest and exercise moderately but regularly. (Excessive exercise may impair fertility.)
- Although there is no conclusive evidence that tight underwear or pants pose a threat to male fertility, there is no harm in wearing looser clothing (in other words, choose boxers over briefs).
- To prevent overheating of the testes, men should avoid hot baths, prolonged showers, and steam rooms. Similarly, avoid using a laptop on your lap for long periods of time.
- Stress may affect hormones that contribute to sperm quality. It is not known if stress reduction techniques can improve fertility, but they may help couples endure the difficult processes involved in fertility treatments.
Oligospermia prognosis
Every cause of oligospermia has a different prognosis. Many causes of oligospermia can be reversed. You and your doctor will work together to determine the cause of your oligospermia and treatment options. Hormonal problems and obstructive causes of oligospermia are usually treatable, and fertility can potentially be restored. If testicular disorders are the cause, it’s still possible to retrieve live sperm to be used in assisted reproductive techniques like IVF.
- Agarwal A., Mulgund A., Hamada A., Chyatte M. R. (2015). A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 13 1–9. 10.1186/s12958-015-0032-1[↩][↩]
- Zegers-Hochschild F., Adamson G. D., Dyer S., Racowsky C., De Mouzon J., Sokol R., et al. (2017). The international glossary on infertility and fertility care, 2017. Hum. Reprod. 32 1786–1801. 10.1093/humrep/dex234[↩]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The International Glossary on Infertility and Fertility Care, 2017. Hum Reprod. 2017 Sep 1;32(9):1786-1801. doi: 10.1093/humrep/dex234[↩]
- Frequently Asked Questions About Infertility. https://www.reproductivefacts.org/browse-resources/frequently-asked-questions/faq-about-infertility[↩]
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013 Jan;99(1):63. doi: 10.1016/j.fertnstert.2012.09.023[↩]
- Alves MBR, Celeghini ECC, Belleannée C. From Sperm Motility to Sperm-Borne microRNA Signatures: New Approaches to Predict Male Fertility Potential. Front Cell Dev Biol. 2020 Aug 21;8:791. doi: 10.3389/fcell.2020.00791[↩][↩][↩][↩][↩]
- Gadea J., Parrington J., Kashir J., Coward K. (2013). “The male reproductive tract and spermatogenesis,” in Textbook of Clinical Embryology, eds Coward K., Wells D. (Cambridge: Cambridge University Press; ), 18–26. 10.1017/cbo9781139192736.005[↩][↩]
- Cooper T. G., Yeung C.-H. (2003). Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc. Res. Tech. 61 28–38. 10.1002/jemt.10314[↩]
- Zini A., Agarwal A. (2011). Sperm Chromatin. New York, NY: Springer.[↩]
- Satouh Y., Inoue N., Ikawa M., Okabe M. (2012). Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J. Cell Sci. 125 4985–4990. 10.1242/jcs.100867[↩]
- Bianchi E., Doe B., Goulding D., Wright G. J. (2014). Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 508 483–487. 10.1038/nature13203[↩]
- Hirohashi N., Yanagimachi R. (2018). Sperm acrosome reaction: its site and role in fertilization. Biol. Reprod. 99 127–133. 10.1093/biolre/ioy045[↩]
- Avidor-Reiss T., Fishman E. L. (2019). It takes two (centrioles) to tango. Reproduction 157 R33–R51. 10.1530/REP-18-0350[↩]
- Inaba K. (2003). Molecular architecture of the sperm flagella: molecules for motility and signaling. Zool. Sci. 20 1043–1056. 10.2108/zsj.20.1043[↩]
- Song G. J., Lewis V. (2008). Mitochondrial DNA integrity and copy number in sperm from infertile men. Fertil. Steril. 90 2238–2244. 10.1016/j.fertnstert.2007.10.059[↩]
- Krawetz S. A. (2005). Paternal contribution: new insights and future challenges. Nat. Rev. Genet. 6 633–642. 10.1038/nrg1654[↩]
- Amann R. P., Hammerstedt R. H. (1993). In vitro evaluation of sperm quality: an opinion. J. Androl. 14 397–406. 10.1002/j.1939-4640.1993.tb03247.x[↩]
- Gillan L., Kroetsch T., Chis Maxwell W. M., Evans G. (2008). Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls. Anim. Reprod. Sci. 103 201–214. 10.1016/j.anireprosci.2006.12.010[↩]
- Saez, J. M., Forest, M. G., Morera, A. M., & Bertrand, J. (1972). Metabolic clearance rate and blood production rate of testosterone and dihydrotestosterone in normal subjects, during pregnancy, and in hyperthyroidism. The Journal of clinical investigation, 51(5), 1226–1234. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC292254/pdf/jcinvest00201-0192.pdf[↩]
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001 Dec;22(6):764-86. doi: 10.1210/edrv.22.6.0446[↩]
- Irvine D. S. (1998). Epidemiology and aetiology of male infertility. Hum. Reprod. 13 33–38. 10.1093/humrep/13.1.33[↩]
- Lundy SD, Vij SC. Male infertility in renal failure and transplantation. Transl Androl Urol. 2019 Apr;8(2):173-181. doi: 10.21037/tau.2018.07.16[↩]
- Sharma R, Biedenharn KR, Fedor JM, Agarwal A. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013 Jul 16;11:66. doi: 10.1186/1477-7827-11-66[↩][↩]
- In-Vitro Fertilization (IVF). https://www.yashodahealthcare.com/blogs/in-vitro-fertilization-ivf/[↩]
- Qamar AY, Naveed MI, Raza S, Fang X, Roy PK, Bang S, Tanga BM, Saadeldin IM, Lee S, Cho J. Role of antioxidants in fertility preservation of sperm – A narrative review. Anim Biosci. 2023 Mar;36(3):385-403. doi: 10.5713/ab.22.0325[↩]
- Steiner AZ, Hansen KR, Barnhart KT, Cedars MI, Legro RS, Diamond MP, Krawetz SA, Usadi R, Baker VL, Coward RM, Huang H, Wild R, Masson P, Smith JF, Santoro N, Eisenberg E, Zhang H; Reproductive Medicine Network. The effect of antioxidants on male factor infertility: the Males, Antioxidants, and Infertility (MOXI) randomized clinical trial. Fertil Steril. 2020 Mar;113(3):552-560.e3. doi: 10.1016/j.fertnstert.2019.11.008[↩]
- Benatta M, Kettache R, Buchholz N, Trinchieri A. The impact of nutrition and lifestyle on male fertility. Arch Ital Urol Androl. 2020 Jun 24;92(2). doi: 10.4081/aiua.2020.2.121[↩][↩]
- Dimitriadis F, Borgmann H, Struck JP, Salem J, Kuru TH. Antioxidant Supplementation on Male Fertility-A Systematic Review. Antioxidants (Basel). 2023 Mar 30;12(4):836. doi: 10.3390/antiox12040836[↩][↩][↩][↩]
- Jannatifar R, Parivar K, Roodbari NH, Nasr-Esfahani MH. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod Biol Endocrinol. 2019 Feb 16;17(1):24. doi: 10.1186/s12958-019-0468-9[↩][↩]
- Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, Depuydt CE. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukot Essent Fatty Acids. 2000 Sep;63(3):159-65. doi: 10.1054/plef.2000.0174[↩][↩]
- Hajjar T, Soleymani F, Vatanchian M. Protective Effect of Vitamin C and Zinc as an Antioxidant Against Chemotherapy-Induced Male Reproductive Toxicity. J Med Life. 2020 Apr-Jun;13(2):138-143. doi: 10.25122/jml-2019-0107[↩]
- Ali M, Martinez M, Parekh N. Are antioxidants a viable treatment option for male infertility? Andrologia. 2021 Feb;53(1):e13644. doi: 10.1111/and.13644[↩]
- Alahmar AT, Sengupta P. Impact of Coenzyme Q10 and Selenium on Seminal Fluid Parameters and Antioxidant Status in Men with Idiopathic Infertility. Biol Trace Elem Res. 2021 Apr;199(4):1246-1252. doi: 10.1007/s12011-020-02251-3[↩]
- Sadaghiani S, Fallahi S, Heshmati H, Teshnizi SH, Chaijan HA, Ebrahimi FFA, Khorrami F, Poorrezaeian M, Alizadeh F. Effect of antioxidant supplements on sperm parameters in infertile male smokers: a single-blinded clinical trial. AIMS Public Health. 2020 Feb 11;7(1):92-99. doi: 10.3934/publichealth.2020009[↩]
- Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol. 2012 Aug;188(2):526-31. doi: 10.1016/j.juro.2012.03.131[↩][↩][↩]
- Schisterman EF, Sjaarda LA, Clemons T, Carrell DT, Perkins NJ, Johnstone E, Lamb D, Chaney K, Van Voorhis BJ, Ryan G, Summers K, Hotaling J, Robins J, Mills JL, Mendola P, Chen Z, DeVilbiss EA, Peterson CM, Mumford SL. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment: A Randomized Clinical Trial. JAMA. 2020 Jan 7;323(1):35-48. doi: 10.1001/jama.2019.18714. Erratum in: JAMA. 2020 Mar 24;323(12):1194. doi: 10.1001/jama.2020.2066[↩]
- Li KP, Yang XS, Wu T. The Effect of Antioxidants on Sperm Quality Parameters and Pregnancy Rates for Idiopathic Male Infertility: A Network Meta-Analysis of Randomized Controlled Trials. Front Endocrinol (Lausanne). 2022 Feb 21;13:810242. doi: 10.3389/fendo.2022.810242[↩][↩][↩]
- Zhou X, Shi H, Zhu S, Wang H, Sun S. Effects of vitamin E and vitamin C on male infertility: a meta-analysis. Int Urol Nephrol. 2022 Aug;54(8):1793-1805. doi: 10.1007/s11255-022-03237-x[↩][↩][↩]
- Wang R, Wang S, Song Y, Zhou H, Pan Y, Liu L, Niu S, Liu X. Effect of vitamin E on Semen Quality Parameters: A Meta-Analysis of a Randomized Controlled Trial. Urol J. 2022 Nov 8;19(5):343-351. doi: 10.22037/uj.v19i.7160[↩][↩]
- Ring JD, Lwin AA, Köhler TS. Current medical management of endocrine-related male infertility. Asian J Androl. 2016 May-Jun;18(3):357-63. doi: 10.4103/1008-682X.179252[↩]
- Walke G, Gaurkar SS, Prasad R, Lohakare T, Wanjari M. The Impact of Oxidative Stress on Male Reproductive Function: Exploring the Role of Antioxidant Supplementation. Cureus. 2023 Jul 27;15(7):e42583. doi: 10.7759/cureus.42583[↩]
- Tirabassi G, Vignini A, Tiano L, Buldreghini E, Brugè F, Silvestri S, Orlando P, D’Aniello A, Mazzanti L, Lenzi A, Balercia G. Protective effects of coenzyme Q10 and aspartic acid on oxidative stress and DNA damage in subjects affected by idiopathic asthenozoospermia. Endocrine. 2015 Jun;49(2):549-52. doi: 10.1007/s12020-014-0432-6[↩]
- Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F, Amoroso S, Ricciardo-Lamonica G, Boscaro M, Lenzi A, Littarru G. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril. 2009 May;91(5):1785-92. doi: 10.1016/j.fertnstert.2008.02.119[↩]
- Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 2009 Jul;182(1):237-48. doi: 10.1016/j.juro.2009.02.121[↩]
- Safarinejad MR. Expression of Concern: Efficacy of Coenzyme Q10 on Semen Parameters, Sperm Function and Reproductive Hormones in Infertile Men. J Urol. 2023 Jan 10:101097JU0000000000003110. doi: 10.1097/JU.0000000000003110[↩]
- Lafuente R, González-Comadrán M, Solà I, López G, Brassesco M, Carreras R, Checa MA. Coenzyme Q10 and male infertility: a meta-analysis. J Assist Reprod Genet. 2013 Sep;30(9):1147-56. doi: 10.1007/s10815-013-0047-5[↩]
- Thakur AS, Littarru GP, Funahashi I, Painkara US, Dange NS, Chauhan P. Effect of Ubiquinol Therapy on Sperm Parameters and Serum Testosterone Levels in Oligoasthenozoospermic Infertile Men. J Clin Diagn Res. 2015 Sep;9(9):BC01-3. doi: 10.7860/JCDR/2015/13617.6424[↩]
- Vishvkarma R, Alahmar AT, Gupta G, Rajender S. Coenzyme Q10 effect on semen parameters: Profound or meagre? Andrologia. 2020 Jul;52(6):e13570. doi: 10.1111/and.13570[↩]
- Sood B, Patel P, Keenaghan M. Coenzyme Q10. [Updated 2024 Jan 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531491[↩]
- Li X, Zeng YM, Luo YD, He J, Luo BW, Lu XC, Zhu LL. Effects of folic acid and folic acid plus zinc supplements on the sperm characteristics and pregnancy outcomes of infertile men: A systematic review and meta-analysis. Heliyon. 2023 Jul 13;9(7):e18224. doi: 10.1016/j.heliyon.2023.e18224[↩][↩][↩][↩][↩]
- Ebara S. Nutritional role of folate. Congenit Anom (Kyoto). 2017 Sep;57(5):138-141. doi: 10.1111/cga.12233[↩]
- Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol. 2018 Apr;218(4):379-389. doi: 10.1016/j.ajog.2017.08.010[↩]
- Cassuto NG, Montjean D, Siffroi JP, Bouret D, Marzouk F, Copin H, Benkhalifa M. Different Levels of DNA Methylation Detected in Human Sperms after Morphological Selection Using High Magnification Microscopy. Biomed Res Int. 2016;2016:6372171. doi: 10.1155/2016/6372171[↩]
- Afedo SY, Cui Y, Yu S, Liao B, Zhao Z, Li H, Zhang H, Zou S, Li H, Zhang P. Histological Analysis, Bioinformatics Profile, and Expression of Methylenetetrahydrofolate Reductase (MTHFR) in Bovine Testes. Animals (Basel). 2020 Sep 23;10(10):1731. doi: 10.3390/ani10101731[↩]
- Benedetti S, Catalani S, De Stefani S, Primiterra M, Fraternale A, Palma F, Palini S. A microplate-based DCFH-DA assay for the evaluation of oxidative stress in whole semen. Heliyon. 2022 Sep 15;8(9):e10642. doi: 10.1016/j.heliyon.2022.e10642[↩]
- Raigani M, Yaghmaei B, Amirjannti N, Lakpour N, Akhondi MM, Zeraati H, Hajihosseinal M, Sadeghi MR. The micronutrient supplements, zinc sulphate and folic acid, did not ameliorate sperm functional parameters in oligoasthenoteratozoospermic men. Andrologia. 2014;46(9):956-62. doi: 10.1111/and.12180[↩]
- Boonyarangkul A, Vinayanuvattikhun N, Chiamchanya C, Visutakul P. Comparative Study of the Effects of Tamoxifen Citrate and Folate on Semen Quality of the Infertile Male with Semen Abnormality. J Med Assoc Thai. 2015 Nov;98(11):1057-63.[↩][↩][↩]
- Aarabi M, San Gabriel MC, Chan D, Behan NA, Caron M, Pastinen T, Bourque G, MacFarlane AJ, Zini A, Trasler J. High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. Hum Mol Genet. 2015 Nov 15;24(22):6301-13. doi: 10.1093/hmg/ddv338[↩]
- Kopets R, Kuibida I, Chernyavska I, Cherepanyn V, Mazo R, Fedevych V, Gerasymov S. Dietary supplementation with a novel l-carnitine multi-micronutrient in idiopathic male subfertility involving oligo-, astheno-, teratozoospermia: A randomized clinical study. Andrology. 2020 Sep;8(5):1184-1193. doi: 10.1111/andr.12805[↩]
- Szymański M, Wandtke T, Wasilow K, Andryszczyk M, Janicki R, Domaracki P. Comparison of 3- and 6-Month Outcomes of Combined Oral L-Carnitine Fumarate and Acetyl-L-Carnitine Therapy, Included in an Antioxidant Formulation, in Patients with Idiopathic Infertility. Am J Mens Health. 2021 Sep-Oct;15(5):15579883211036790. doi: 10.1177/15579883211036790[↩][↩]
- Su L, Qu H, Cao Y, Zhu J, Zhang SZ, Wu J, Jiao YZ. Effect of Antioxidants on Sperm Quality Parameters in Subfertile Men: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Adv Nutr. 2022 Mar;13(2):586-594. doi: 10.1093/advances/nmab127[↩][↩]
- Zafar MI, Mills KE, Baird CD, Jiang H, Li H. Effectiveness of Nutritional Therapies in Male Factor Infertility Treatment: A Systematic Review and Network Meta-analysis. Drugs. 2023 Apr;83(6):531-546. doi: 10.1007/s40265-023-01853-0[↩]
- Wei G, Zhou Z, Cui Y, Huang Y, Wan Z, Che X, Chai Y, Zhang Y. A Meta-Analysis of the Efficacy of L-Carnitine/L-Acetyl-Carnitine or N-Acetyl-Cysteine in Men With Idiopathic Asthenozoospermia. Am J Mens Health. 2021 Mar-Apr;15(2):15579883211011371. doi: 10.1177/15579883211011371[↩][↩]
- Agarwal A, Said TM. Carnitines and male infertility. Reprod Biomed Online. 2004 Apr;8(4):376-84. doi: 10.1016/s1472-6483(10)60920-0[↩]
- Tsampoukas G, Dellis A, Papatsoris A. Bilateral disease and intratesticular haemodynamics as markers of dyspermia in patients with subclinical varicocele: A prospective study. Arab J Urol. 2019 Aug 1;17(4):298-304. doi: 10.1080/2090598X.2019.1647676[↩]
- Nazari L, Salehpour S, Hosseini S, Allameh F, Jahanmardi F, Azizi E, Ghodssi-Ghassemabadi R, Hashemi T. Effect of antioxidant supplementation containing L-carnitine on semen parameters: a prospective interventional study. JBRA Assist Reprod. 2021 Feb 2;25(1):76-80. doi: 10.5935/1518-0557.20200043[↩]
- Durairajanayagam D, Agarwal A, Ong C, Prashast P. Lycopene and male infertility. Asian J Androl. 2014 May-Jun;16(3):420-5. doi: 10.4103/1008-682X.126384[↩]
- Chen Z, Hong Z, Wang S, Qiu J, Wang Q, Zeng Y, Weng H. Effectiveness of non-pharmaceutical intervention on sperm quality: a systematic review and network meta-analysis. Aging (Albany NY). 2023 May 17;15(10):4253-4268. doi: 10.18632/aging.204727[↩]
- Mangiagalli MG, Martino PA, Smajlovic T, Guidobono Cavalchini L, Marelli SP. Effect of lycopene on semen quality, fertility and native immunity of broiler breeder. Br Poult Sci. 2010 Feb;51(1):152-7. doi: 10.1080/00071660903401540[↩]
- Nouri M, Amani R, Nasr-Esfahani M, Tarrahi MJ. The effects of lycopene supplement on the spermatogram and seminal oxidative stress in infertile men: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2019 Dec;33(12):3203-3211. doi: 10.1002/ptr.6493[↩]
- Gupta NP, Kumar R. Lycopene therapy in idiopathic male infertility–a preliminary report. Int Urol Nephrol. 2002;34(3):369-72. doi: 10.1023/a:1024483520560[↩]
- Hekimoglu A, Kurcer Z, Aral F, Baba F, Sahna E, Atessahin A. Lycopene, an antioxidant carotenoid, attenuates testicular injury caused by ischemia/reperfusion in rats. Tohoku J Exp Med. 2009 Jun;218(2):141-7. doi: 10.1620/tjem.218.141[↩][↩]
- Zhai LL, Tang ZG. Lycopene improves sperm motility and morphology: a promising agent for treating male infertility. Eur J Nutr. 2020 Mar;59(2):835-836. doi: 10.1007/s00394-019-02173-4[↩]
- Tenório MCDS, Graciliano NG, Moura FA, Oliveira ACM, Goulart MOF. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants (Basel). 2021 Jun 16;10(6):967. doi: 10.3390/antiox10060967[↩][↩]
- Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology. 2009 Jul;74(1):73-6. doi: 10.1016/j.urology.2009.02.034[↩]
- Oeda T, Henkel R, Ohmori H, Schill WB. Scavenging effect of N-acetyl-L-cysteine against reactive oxygen species in human semen: a possible therapeutic modality for male factor infertility? Andrologia. 1997 May-Jun;29(3):125-31. doi: 10.1111/j.1439-0272.1997.tb00305.x[↩]
- Genazzani AD, Petraglia F, Battaglia C, Gamba O, Volpe A, Genazzani AR. A long-term treatment with gonadotropin-releasing hormone agonist plus a low-dose oral contraceptive improves the recovery of the ovulatory function in patients with polycystic ovary syndrome. Fertil Steril. 1997 Mar;67(3):463-8. doi: 10.1016/s0015-0282(97)80070-2[↩]
- Ershad M, Naji A, Patel P, et al. N-Acetylcysteine. [Updated 2024 Feb 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537183[↩]
- Safarinejad MR, Safarinejad S. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study. J Urol. 2009 Feb;181(2):741-51. doi: 10.1016/j.juro.2008.10.015[↩]
- Moslemi MK, Tavanbakhsh S. Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int J Gen Med. 2011 Jan 23;4:99-104. doi: 10.2147/IJGM.S16275[↩][↩]
- Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, Zghal K, Fki H, Damak J, Bahloul A. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch Androl. 2003 Mar-Apr;49(2):83-94. doi: 10.1080/01485010390129269[↩]
- Flohé L. Selenium in mammalian spermiogenesis. Biol Chem. 2007 Oct;388(10):987-95. doi: 10.1515/BC.2007.112[↩]
- Ozoani H, Ezejiofor AN, Okolo KO, Orish CN, Cirovic A, Cirovic A, Orisakwe OE. Zinc and selenium attenuate quaternary heavy metal mixture-induced testicular damage via amplification of the antioxidant system, reduction in metal accumulation, inflammatory and apoptotic biomarkers. Toxicol Res. 2023 May 18;39(3):497-515. doi: 10.1007/s43188-023-00187-z[↩][↩]
- Medina J, Gupta V. Vitamin E. [Updated 2023 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557737[↩]
- Nessel TA, Gupta V. Selenium. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557551[↩]
- Dawson EB, Harris WA, Rankin WE, Charpentier LA, McGanity WJ. Effect of ascorbic acid on male fertility. Ann N Y Acad Sci. 1987;498:312-23. doi: 10.1111/j.1749-6632.1987.tb23770.x[↩]
- Colagar AH, Marzony ET. Ascorbic Acid in human seminal plasma: determination and its relationship to sperm quality. J Clin Biochem Nutr. 2009 Sep;45(2):144-9. doi: 10.3164/jcbn.08-251[↩]
- Ko EY, Sabanegh ES Jr. The role of over-the-counter supplements for the treatment of male infertility–fact or fiction? J Androl. 2012 May-Jun;33(3):292-308. doi: 10.2164/jandrol.111.013730[↩][↩]
- Rastegar Panah M, Tahir I, Garcia-Bailo B, Lo K, Jarvi K, El-Sohemy A. Ascorbic acid is associated with favourable hormonal profiles among infertile males. Front Reprod Health. 2023 Jun 8;5:1143579. doi: 10.3389/frph.2023.1143579[↩]
- Abdullah M, Jamil RT, Attia FN. Vitamin C (Ascorbic Acid) [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499877[↩]
- de Angelis C, Galdiero M, Pivonello C, Garifalos F, Menafra D, Cariati F, Salzano C, Galdiero G, Piscopo M, Vece A, Colao A, Pivonello R. The role of vitamin D in male fertility: A focus on the testis. Rev Endocr Metab Disord. 2017 Sep;18(3):285-305. doi: 10.1007/s11154-017-9425-0[↩][↩]
- Simpson S, Pal L. Vitamin D and infertility. Curr Opin Obstet Gynecol. 2023 Aug 1;35(4):300-305. doi: 10.1097/GCO.0000000000000887[↩]
- Adamczewska D, Słowikowska-Hilczer J, Walczak-Jędrzejowska R. The Association between Vitamin D and the Components of Male Fertility: A Systematic Review. Biomedicines. 2022 Dec 29;11(1):90. doi: 10.3390/biomedicines11010090[↩]
- Güngör K, Güngör ND, Başar MM, Cengiz F, Erşahin SS, Çil K. Relationship between serum vitamin D levels semen parameters and sperm DNA damage in men with unexplained infertility. Eur Rev Med Pharmacol Sci. 2022 Jan;26(2):499-505. doi: 10.26355/eurrev_202201_27875[↩]
- Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. 2012;7(11):e50568. doi: 10.1371/journal.pone.0050568[↩]
- Alsalman ARS, Almashhedy LA, Hadwan MH. Effect of Oral Zinc Supplementation on the Thiol Oxido-Reductive Index and Thiol-Related Enzymes in Seminal Plasma and Spermatozoa of Iraqi Asthenospermic Patients. Biol Trace Elem Res. 2018 Aug;184(2):340-349. doi: 10.1007/s12011-017-1215-8[↩]
- Elgazar V, Razanov V, Stoltenberg M, Hershfinkel M, Huleihel M, Nitzan YB, Lunenfeld E, Sekler I, Silverman WF. Zinc-regulating proteins, ZnT-1, and metallothionein I/II are present in different cell populations in the mouse testis. J Histochem Cytochem. 2005 Jul;53(7):905-12. doi: 10.1369/jhc.4A6482.2005[↩]
- Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2007 Mar-Apr;13(2):163-74. doi: 10.1093/humupd/dml054[↩]
- Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielhuis GA, Steegers-Theunissen RP. Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Fertil Steril. 2002 Mar;77(3):491-8. doi: 10.1016/s0015-0282(01)03229-0[↩]
- Khan MS, Zaman S, Sajjad M, Shoaib M, Gilani G. Assessment of the level of trace element zinc in seminal plasma of males and evaluation of its role in male infertility. Int J Appl Basic Med Res. 2011 Jul;1(2):93-6. doi: 10.4103/2229-516X.91152[↩]
- Rabinovich D, Smadi Y. Zinc. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547698[↩]
- Leslie SW, Soon-Sutton TL, Khan MAB. Male Infertility. [Updated 2024 Feb 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562258[↩][↩][↩]
- Krzastek SC, Sharma D, Abdullah N, Sultan M, Machen GL, Wenzel JL, Ells A, Chen X, Kavoussi M, Costabile RA, Smith RP, Kavoussi PK. Long-Term Safety and Efficacy of Clomiphene Citrate for the Treatment of Hypogonadism. J Urol. 2019 Nov;202(5):1029-1035. doi: 10.1097/JU.0000000000000396[↩]
- Ross LS, Kandel GL, Prinz LM, Auletta F. Clomiphene treatment of the idiopathic hypofertile male: high-dose, alternate-day therapy. Fertil Steril. 1980 Jun;33(6):618-23. doi: 10.1016/s0015-0282(16)44775-8[↩]
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology. 2013 Sep;1(5):749-57. doi: 10.1111/j.2047-2927.2013.00107.x[↩][↩]
- Kotoulas IG, Cardamakis E, Michopoulos J, Mitropoulos D, Dounis A. Tamoxifen treatment in male infertility. I. Effect on spermatozoa. Fertil Steril. 1994 May;61(5):911-4. doi: 10.1016/s0015-0282(16)56705-3[↩][↩][↩][↩]
- Paffenholz P, Votteler S, Nazari S, Nestler T, Salem J, Grabbert M, von Brandenstein M, Loosen SH, Zey S, Heidenreich A, Herden J, Denil J. Efficacy of the Oestrogen Antagonist Tamoxifen on Sperm Parameters in Patients with Idiopathic Oligoathenoteratozoospermia. Urol Int. 2019;103(1):108-115. doi: 10.1159/000500301[↩][↩][↩]
- Whitten SJ, Nangia AK, Kolettis PN. Select patients with hypogonadotropic hypogonadism may respond to treatment with clomiphene citrate. Fertil Steril. 2006 Dec;86(6):1664-8. doi: 10.1016/j.fertnstert.2006.05.042[↩]
- Willets AE, Corbo JM, Brown JN. Clomiphene for the treatment of male infertility. Reprod Sci. 2013 Jul;20(7):739-44. doi: 10.1177/1933719112466304[↩]
- Farrar MC, Jacobs TF. Tamoxifen. [Updated 2023 Apr 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532905[↩][↩]
- Shahid MN, Khan TM, Neoh CF, Lean QY, Bukhsh A, Karuppannan M. Effectiveness of Pharmacological Intervention Among Men with Infertility: A Systematic Review and Network Meta-Analysis. Front Pharmacol. 2021 Aug 16;12:638628. doi: 10.3389/fphar.2021.638628[↩]
- Dimakopoulou A, Foran D, Jayasena CN, Minhas S. Stimulation of Leydig and Sertoli Cellular Secretory Function by Anti-Oestrogens: Tamoxifen. Curr Pharm Des. 2021;27(23):2682-2691. doi: 10.2174/1381612826666200213095228[↩][↩][↩]
- Schlegel PN. Aromatase inhibitors for male infertility. Fertil Steril. 2012 Dec;98(6):1359-62. doi: 10.1016/j.fertnstert.2012.10.023[↩][↩]
- Del Giudice F, Busetto GM, De Berardinis E, Sperduti I, Ferro M, Maggi M, Gross MS, Sciarra A, Eisenberg ML. A systematic review and meta-analysis of clinical trials implementing aromatase inhibitors to treat male infertility. Asian J Androl. 2020 Jul-Aug;22(4):360-367. doi: 10.4103/aja.aja_101_19[↩][↩]
- Pavlovich CP, King P, Goldstein M, Schlegel PN. Evidence of a treatable endocrinopathy in infertile men. J Urol. 2001 Mar;165(3):837-41.[↩]
- Peters A, Tadi P. Aromatase Inhibitors. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557856[↩]
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: [email protected]; Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2018 Sep;110(4):611-618. doi: 10.1016/j.fertnstert.2018.06.016[↩]
- Jung A, Schuppe HC. Influence of genital heat stress on semen quality in humans. Andrologia. 2007 Dec;39(6):203-15. doi: 10.1111/j.1439-0272.2007.00794.x[↩]
- Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010 May 1;93(7):2222-31. doi: 10.1016/j.fertnstert.2009.01.100[↩]
- Maskey S, Rijal H. Correlation of Body Mass Index on Semen Parameters. J Nepal Health Res Counc. 2022 Mar 13;19(4):838-843. doi: 10.33314/jnhrc.v19i04.3919[↩]
- Jung A, Leonhardt F, Schill WB, Schuppe HC. Influence of the type of undertrousers and physical activity on scrotal temperature. Hum Reprod. 2005 Apr;20(4):1022-7. doi: 10.1093/humrep/deh697[↩]