Contents
What is optic atrophy
Optic atrophy also called optic nerve atrophy or optic neuropathy, is an eye condition where your eye’s optic nerve also called cranial nerve number 2 (CN II), which connects your eye to your brain, is damaged or degenerates, potentially leading to irreversible vision loss and blindness 1, 2, 3, 4. Optic atrophy refers to the death of the retinal ganglion cell nerve fibers (neurons) that comprise the optic nerve with the resulting picture of a pale optic nerve or white appearance of the optic disc on an examination of the back of the eye using a specialized instrument called an ophthalmoscope (Figures 2 to 4). Optic atrophy is an end stage that arises from myriad causes of optic nerve damage that can be caused by various factors, including glaucoma, optic neuritis, papilledema, tumor, trauma, injury, infection, inflammation, lack of blood flow, or even inherited genetic conditions such as Leber’s hereditary optic neuropathy (LHON) and autosomal dominant optic atrophy or Kjer syndrome – the most common inherited optic atrophies, accounting for 30–50% of inherited optic neuropathies 5, 6, 7, 8, 9. Since the optic nerve transmits retinal information to the brain, optic atrophy is associated with vision loss. Clinically, optic atrophy manifests as changes in the color and the structure of the optic disc (cupping) associated with variable degrees of visual dysfunction. The term “atrophy” is a misnomer, since, in its strict histologic definition, atrophy implies the wasting away or decrease in size of a body part, tissue, cell, or organ, often due to lack of use, disease, or other causes. Optic atrophy is somewhat of a misnomer as atrophy implies disuse and optic nerve damage is better termed “optic neuropathy” 1.
Since the optic nerve is the conduit for information from the retina to your brain, a damaged optic nerve will result in vision loss. Subtle optic nerve damage might not affect visual acuity (how sharply you can see, the clarity and detail you can perceive) but may lead to a loss of contrast or color vision 1. Severe optic nerve damage may cause legal blindness to no light perception 1. Damage to a part of the optic nerve results in loss of vision in the corresponding visual field. Occasionally, if the process causing the optic nerve damage is removed before the death of nerve cells occurs (e.g., removal of a pituitary tumor compressing the optic chiasm – an X-shaped structure formed at the point below the brain where the two optic nerves cross over each other or reducing inflammation in a sarcoidosis [a condition that causes tiny collections of immune system cells in any part of the body]), some improvement in visual function may be noted.
According to Tielsch et al. the prevalence of blindness attributable to optic atrophy was 0.8% 10. According to Munoz et al. the prevalence of visual impairment and blindness attributable to optic atrophy was 0.04% and 0.12%, respectively 11.
The optic nerve comprises approximately 1.2 million axons (nerve fibers) that originate at the ganglion cell layer of the retina (see Figure 2). The axons of the optic nerve are heavily myelinated by oligodendrocytes, and the axons, once damaged, do not regenerate. Thus, the optic nerve behaves more like a white matter tract rather than a true peripheral nerve.
The optic nerve is divided into the following 4 parts:
- Intraocular part (1 mm) (optic nerve head)
- Intraorbital part (25 mm)
- Intracanalicular part (5-9 mm)
- Intracranial part (10-16 mm)
The average optic nerve head is 1 mm deep, 1.5 mm wide, 1.8 mm high at the retinal level, and a little wider posteriorly. The optic nerve head sits at a major transition between an area of high pressure to an area of low pressure (intracranial pressure) and is composed of 4 types of cells: ganglion cell axons, astrocytes, capillary-associated cells, and fibroblasts.
Light incident from the ophthalmoscope undergoes total internal reflection through the axonal fibers, and subsequent reflection from the capillaries on the disc surface gives rise to the characteristic yellow-pink color of a healthy optic disc. Degenerated axons lose this optical property, explaining the pallor in optic atrophy.
The blood supply at the optic nerve head is provided by pial capillaries arising from the circle of Zinn-Haller. These capillaries exhibit autoregulation and are not leaky. Alternatively, the loss of these capillaries leads to a pale-appearing disc. The Kestenbaum capillary number index is the number of capillaries observed on the optic disc. The normal count is approximately 10. In optic atrophy, the number of these capillaries reduces to less than 6, while more than 12 suggests a hyperemic disc.
Histopathologic changes noted in optic atrophy include the following:
- Shrinkage or loss of both myelin and axis cylinders
- Gliosis
- Deepening of the physiologic cup with barring of the lamina cribrosa
- Widening of the subarachnoid space with redundant dura
- Widening of the pial septa
- Severed nerve leads to bulbous axonal swellings (Cajal end bulbs); may be observed at the anterior cut end of the fibers.
A comprehensive eye exam is necessary to determine the cause of optic atrophy. This includes a complete medical history, assessment of visual acuity, color vision, visual field, contrast vision and pupil reaction. By looking in the back of your eye with an instrument called the ophthalmoscope, your ophthalmologist may determine that the optic nerve appears pale, indicating a loss of nerve fibers. Additional testing such as MRI of the orbits (the bony cavity or socket within your skull that houses the eyeball and its nerves and muscles) and brain and blood tests may be necessary.
Optic atrophy treatment involves treating the underlying cause. Unfortunately, once the nerve fibers in the optic nerve are lost they never heal or grow back. However, early diagnosis and treatment of the underlying causes of optic atrophy can help prevent further damage from the disease.
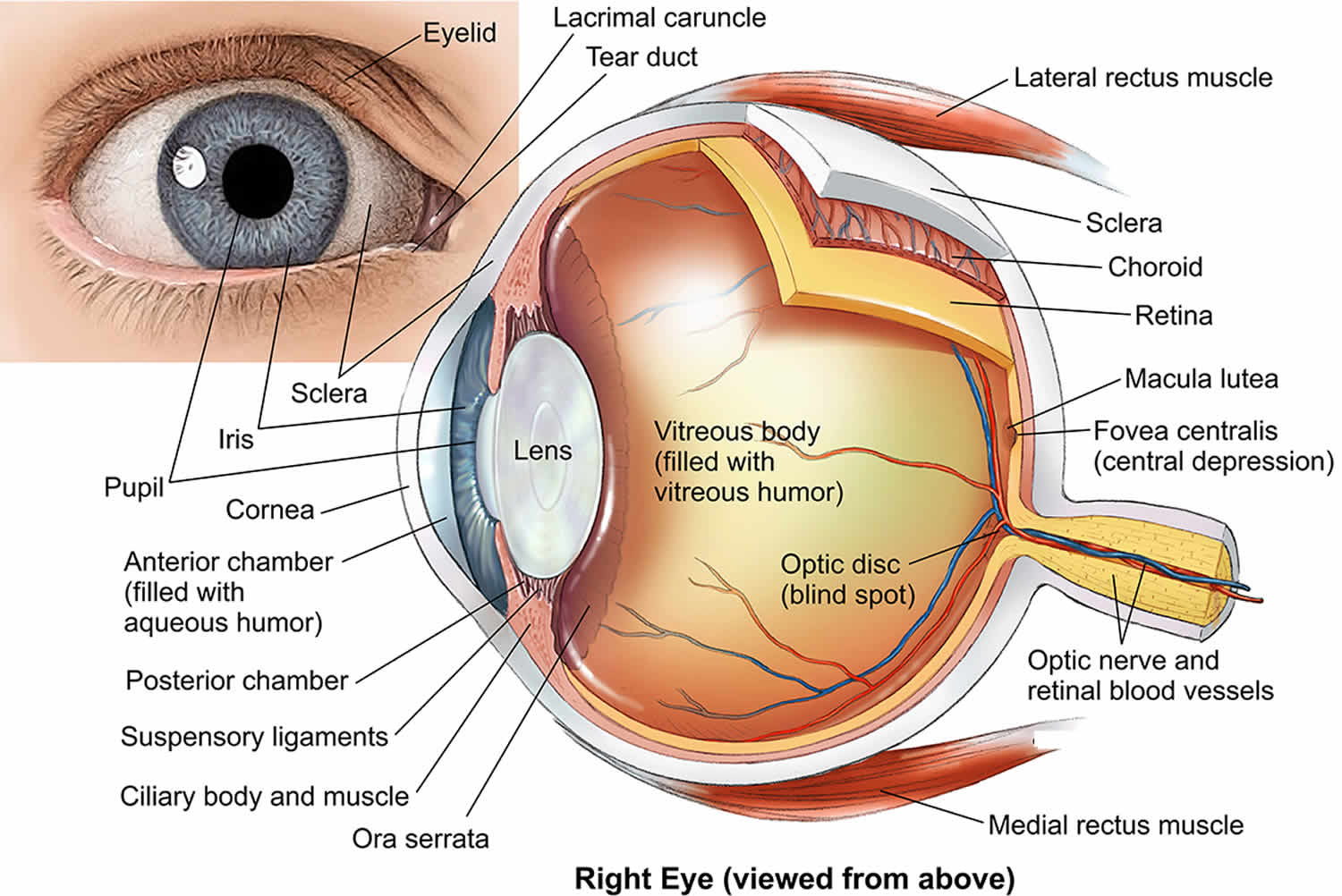
Figure 1. Human eye anatomy
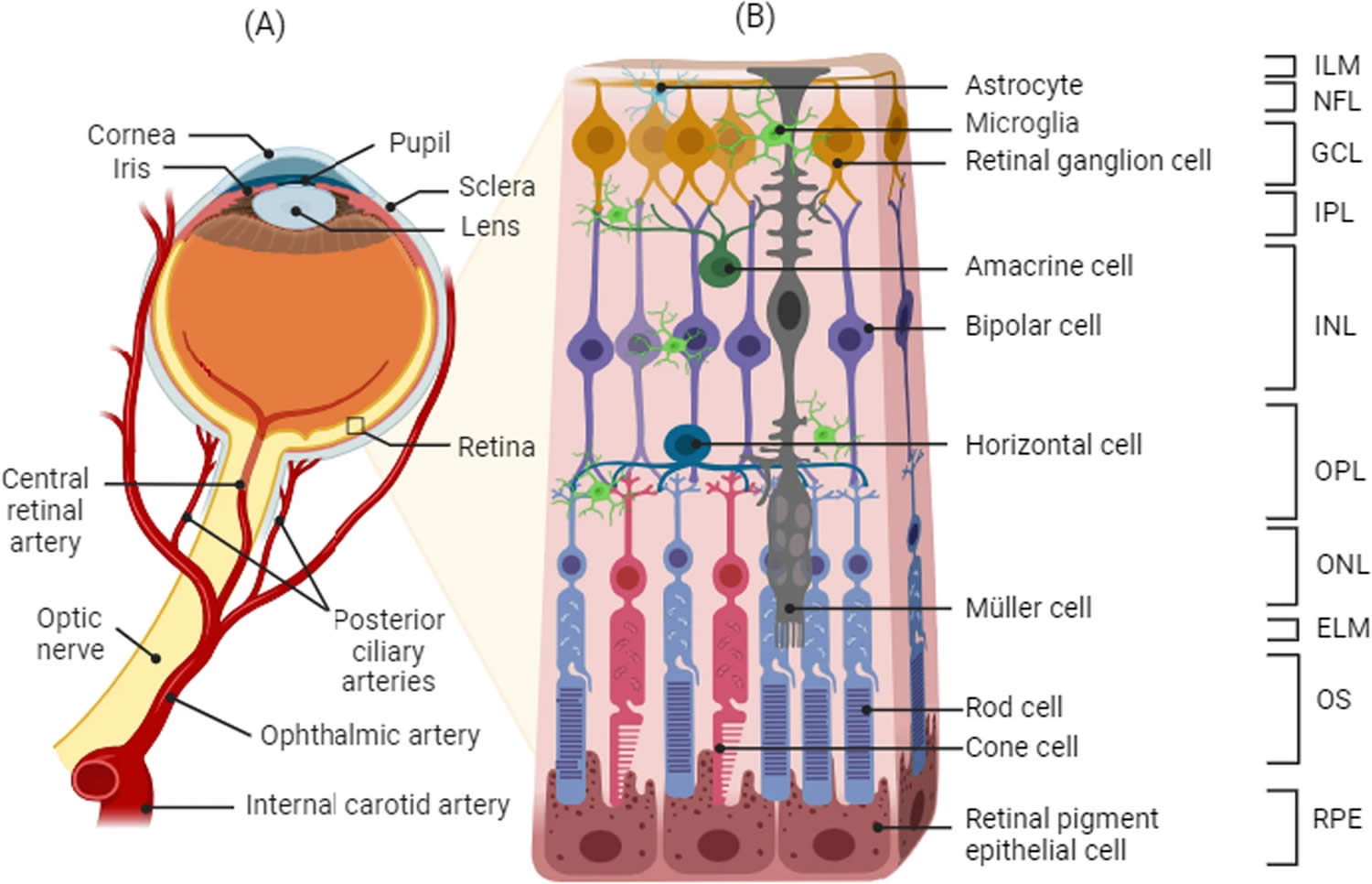
Figure 2. Optic nerve showing retinal ganglion cells
Footnotes: Schematic representation of the retina and the retinal cell layers. (A) Blood supply and (B) structure of the retina. The retina is a layered structure lining the back of the eye consisting of a pigmented layer called retinal pigment epithelium (RPE), and a multilayered neuroretina. The retinal pigment epithelium (RPE) is in close contact with the outer segments of the photosensitive rod and cone cells of the neuroretina. The connecting cilium connects the photoreceptor outer segments with the cell bodies, which constitute a layer known as the outer nuclear layer (ONL). The axons of the photoreceptors synapse with the neuronal (bipolar, amacrine, and horizontal) cells of the inner nuclear layer (INL) via the outer plexiform layer (OPL). The axons of the inner nuclear layer (INL) cells in turn synapse with the ganglion cell layer (GCL) via the inner plexiform layer (IPL). The axons of the ganglion cells converge to form the optic nerve. Approximately 1.2 million nerve fibers, or axons, make up each human optic nerve. A retinal ganglion cell (RGC) is a type of neuron located near the inner surface (ganglion cell layer [GCL]) of the retina of the eye. A retinal ganglion cell (RGC) receives visual information from photoreceptors via two intermediate neuron types: bipolar cells and retina amacrine cells. Retina amacrine cells, particularly narrow field cells, are important for creating functional subunits within the ganglion cell layer and making it so that ganglion cells can observe a small dot moving a small distance. Retinal ganglion cells collectively transmit image-forming and non-image forming visual information from the retina in the form of action potential to several regions in the thalamus, hypothalamus, and mesencephalon, or midbrain. Visual images from the retina travel through the optic nerve, optic tract, and eventually to the visual part of the brain (the occipital lobe). There the images are processed and interpreted by the brain. Any disease process which affects the optic nerve could disrupt this input, leading to visual loss.
Abbreviations: ILM: internal limiting membrane, NFL: nerve fiber layer, GCL: ganglion cell layer, IPL: inner plexiform layer, INL: inner nuclear layer, OPL: outer plexiform layer, ONL: outer nuclear layer, ELM: external limiting membrane, OS: photoreceptor outer segment, RPE: retinal pigment epithelium
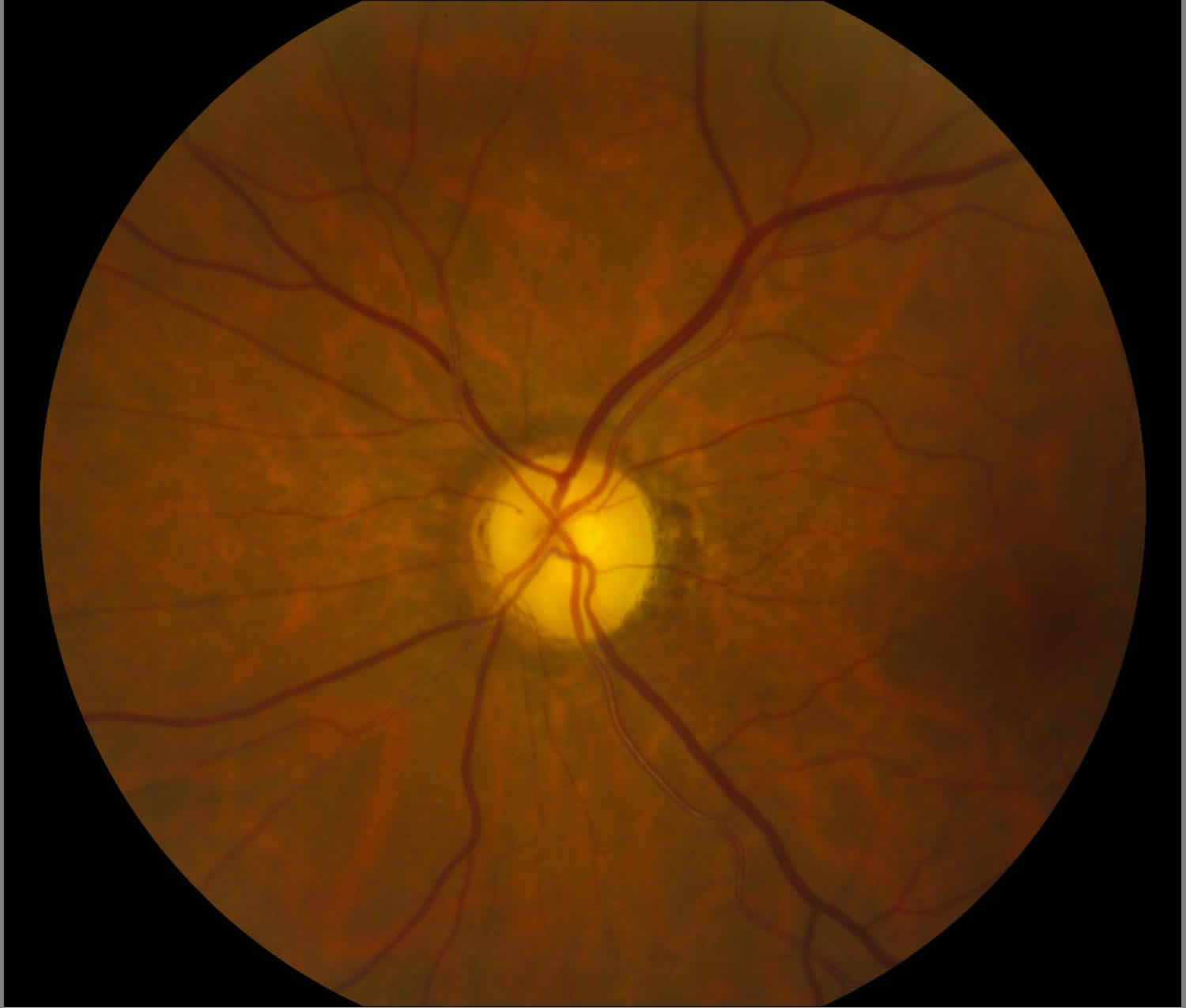
[Source 12 ]Figure 3. Optic atrophy
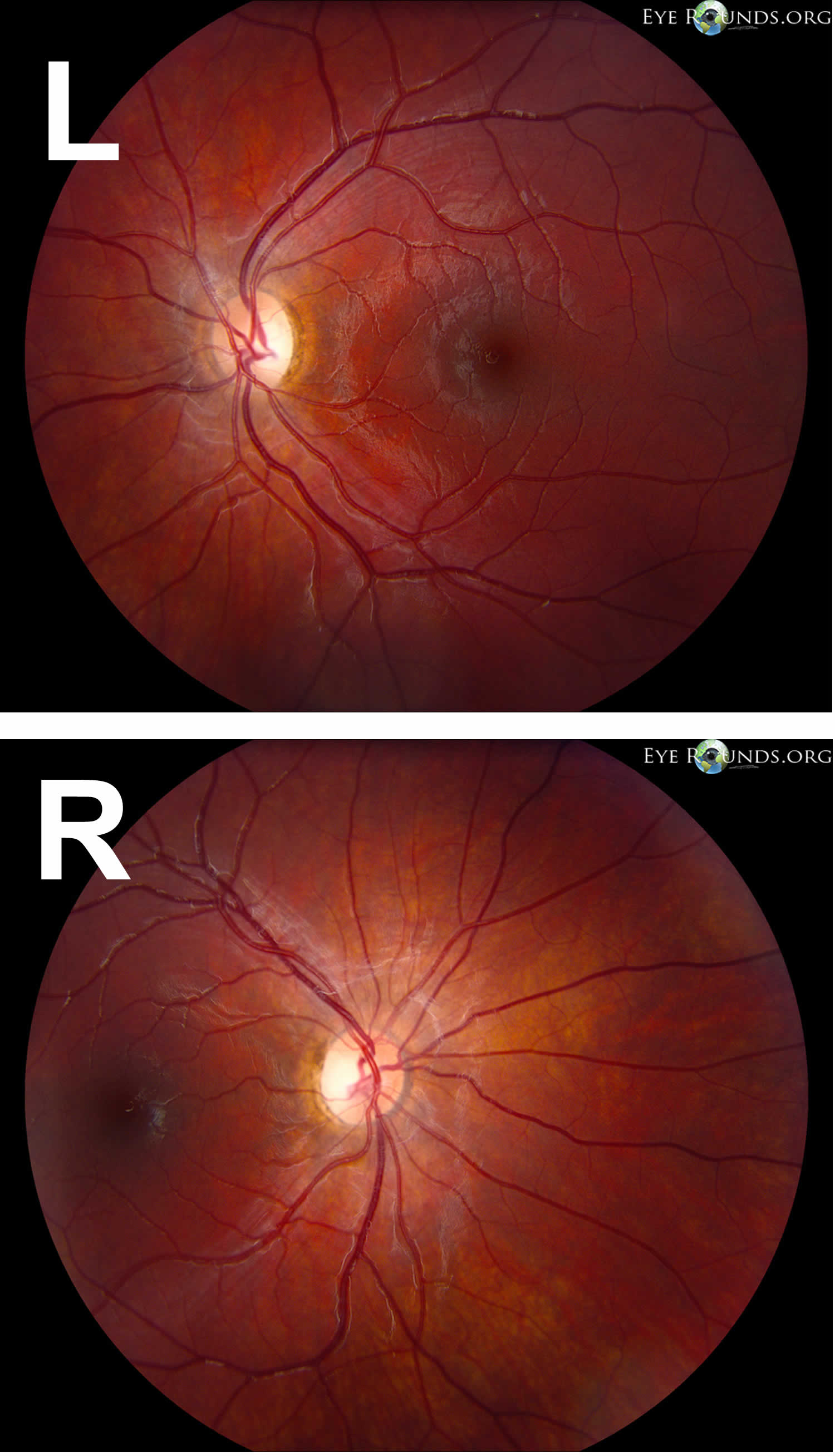
Figure 4. Autosomal dominant optic atrophy or Kjer syndrome
Footnotes: Autosomal dominant optic atrophy or Kjer syndrome, the most common inherited optic atrophy caused by a mutation in the OPA1 gene. Autosomal dominant optic atrophy typically results in mild to moderate bilateral vision loss with an insidious onset beginning in childhood. Note the symmetric temporal wedges of disc pallor in the photographs.
[Source 13 ]Optic atrophy causes
Many diseases and disorders can lead to optic atrophy or damage to the optic nerve. Anything that can compromise ganglion cell function can cause (over time) optic atrophy and more broadly optic neuropathy. Optic atrophy can occur in cases where the optic nerve did not develop properly. It may also result from inflammation or infection of the optic nerve or from glaucoma when the pressure inside the eye remains too high (increased intraocular pressure). In unusual cases, poisons, vitamin deficiencies, ischemia, or tumors compression may be responsible. Most commonly, optic atrophy simply occurs without a known or proven cause also called idiopathic.
Causes for optic atrophy include 1:
- Compressive – secondary to papilledema, tumor, bony growth (fibrous dysplasia, osteopetrosis), thyroid eye disease (Graves orbitopathy), chiasmal (pituitary, etc), optic nerve sheath meningioma, disc drusen, increased intraocular pressure (IOP) in glaucoma
- Glaucoma has a worldwide prevalence of approximately 3.54% in the population aged 40–80 years 14. Glaucoma is one of the leading causes of irreversible blindness globally 15
- Thyroid optic neuropathies occur in 3–7% of individuals with Graves orbitopathy, which itself has a prevalence of 90 to 300 in 100,000 16, 17, 18
- Vascular – arteritic and nonarteritic ischemic optic neuropathy, diabetes
- Anterior ischemic optic neuropathies (AION) includes arteritic forms, like giant cell arteritis (GCA), which has a pooled prevalence of approximately 51.74 in 100,000 for individuals over the age of 50 19. Nonarteritic forms have a reported prevalence of approximately 102.87 in 100,000 in the general population over the age of 40 in the Republic of Korea
- 20, 21;
- Inflammatory – sarcoidosis, systemic lupus erythematosus (SLE), Behçet’s disease, demyelination (multiple sclerosis), optic neuritis
- Infectious – herpes, tuberculosis, bartonella, etc.
- Toxic and nutritional – medications (e.g., ethambutol, amiodarone, methanol), vitamin deficiency, alcohol consumption 26, 27, 28, 29, 30, 31, 32, 33
- Metabolic – diabetes mellitus
- Neoplastic infiltrative optic neuropathies – lymphoma, leukemia, tumor, glioma
- Leukemic optic neuropathy presents in approximately 16% and 18% of all chronic and acute leukemia cases, respectively 34
- Genetic – autosomal dominant optic atrophy (OPA1 or Kjer syndrome), Leber hereditary optic atrophy, Leber hereditary optic neuropathy (LHON), late complications of retinal degeneration.
- Leber’s hereditary optic neuropathy (LHON) has a prevalence of 2–4 in 100,000 for complete penetrance 35, 36, 37, 38, 39. In cases of incomplete penetrance, the prevalence can reach 1 in 800 40, 41
- Congenital anomalies of the optic nerve, such as optic nerve hypoplasia is between approximately 10.9 and 17.3 in 100,000 individuals under the age of 18 42, 43
- Radiation – optic neuropathy
- Traumatic – optic neuropathy. The overall incidence of traumatic optic neuropathies ranges from 0.7% to 2.5% 44, 45, 46, 47
Optic atrophy is also classified as pathologic, ophthalmoscopic, or etiologic.
Pathologic optic atrophy
Anterograde degeneration (Wallerian degeneration)
- Degeneration begins in the retina and proceeds toward the lateral geniculate body (eg, toxic retinopathy, chronic simple glaucoma). Larger axons disintegrate more rapidly than smaller axons.
Retrograde degeneration
- Degeneration starts from the proximal portion of the axon and proceeds toward the optic disc (eg, optic nerve compression via intracranial tumor).
Trans-synaptic degeneration
- In trans-synaptic degeneration, a neuron on one side of a synapse degenerates as a consequence of the loss of a neuron on the other side (eg, in individuals with occipital damage incurred either in utero or during early infancy).
Ophthalmoscopic optic atrophy
Primary optic atrophy
In conditions with primary optic atrophy (eg, pituitary tumor, optic nerve tumor, traumatic optic neuropathy, multiple sclerosis), optic nerve fibers degenerate in an orderly manner and are replaced by columns of glial cells without alteration in the architecture of the optic nerve head. The disc is chalky white and sharply demarcated, and the retinal vessels are normal. Lamina cribrosa is well defined.
Secondary optic atrophy
In conditions with secondary optic atrophy (eg, papilledema, papillitis), the atrophy is secondary to papilledema (shown in the image below). Optic nerve fibers exhibit marked degeneration, with excessive proliferation of glial tissue. The architecture is lost, resulting in indistinct margins. The disc is grey or dirty grey, the margins are poorly defined, and the lamina cribrosa is obscured due to proliferating fibroglial tissue. Hyaline bodies (corpora amylacea) or drusen may be observed. Peripapillary sheathing of arteries as well as tortuous veins may be observed. On visual fields, progressive contraction of visual fields may be seen.
Consecutive optic atrophy
In consecutive optic atrophy (eg, retinitis pigmentosa, myopia, central retinal artery occlusion), the disc is waxy pale with a normal disc margin, marked attenuation of arteries, and a normal physiologic cup.
Glaucomatous optic atrophy
Also known as cavernous optic atrophy, marked cupping of the disc is observed in glaucomatous optic atrophy. Characteristics include vertical enlargement of cup, visibility of the laminar pores (laminar dot sign), backward bowing of the lamina cribrosa, bayoneting and nasal shifting of the retinal vessels, and peripapillary halo and atrophy. Splinter hemorrhage at the disc margin may be observed.
Temporal pallor
Temporal pallor may be observed in traumatic or nutritional optic neuropathy, and it is most commonly seen in patients with multiple sclerosis, particularly in those with a history of optic neuritis. The disc is pale with a clear, demarcated margin and normal vessels, and the physiologic pallor temporally is more distinctly pale.
Etiologic optic atrophy
Hereditary atrophy
This is divided into congenital or infantile optic atrophy (recessive or dominant form), Behr hereditary optic atrophy (autosomal recessive), and Leber optic atrophy 48. Several hereditary optic neuropathies, including optic atrophy type 1 and Leber optic atrophy, have been attributed to mitochondrial dysfunction in retinal ganglion cells.
Autosomal-dominant optic atrophy type 1 is caused by mutations in the OPA1 gene on chromosome 3q29. The OPA1 protein produced plays a key role in a process called oxidative phosphorylation and in self-destruction of cells (apoptosis). OPA1 is an integral pro-fusion protein within the internal mitochondrial membrane. Mutations in the OPA1 gene lead to vision problems experienced by people with breakdown of structures that transmit visual information from the eyes to the brain. Affected individuals first experience a progressive loss of nerve cells within the retina, called retinal ganglion cells. The loss of these cells is followed by the degeneration (atrophy) of the optic nerve.
X-linked optic atrophy type 2 is caused by mutation in the OPA2 gene with cytogenetic location Xp11.4-p11.21. The patient presents with early-onset childhood vision loss with slow progression of loss.
Hereditary optic atrophy type 3 is caused by mutation in the OPA3 gene with cytogenetic location 19q13.32. The mutation in this gene is associated with childhood-onset vision loss with cataract. It can also be associated with type III methylglutaconic aciduria.
Leber hereditary optic neuropathy results from mitochondrial point mutations in mtDNA 11778G>A, 14484T>C, or 3460G>A mutations.
Consecutive atrophy
Consecutive atrophy is an ascending type of atrophy (eg, chorioretinitis, pigmentary retinal dystrophy, cerebromacular degeneration) that usually follows diseases of the choroid or the retina.
Circulatory atrophy (vascular)
Circulatory is an ischemic optic neuropathy observed when the perfusion pressure of the ciliary body falls below the intraocular pressure. Circulatory atrophy is observed in central retinal artery occlusion, carotid artery occlusion, and cranial arteritis.
Metabolic atrophy
It is observed in disorders such as thyroid ophthalmopathy, juvenile diabetes mellitus, nutritional amblyopia, toxic amblyopia, tobacco, methyl alcohol, and drugs (eg, ethambutol, sulphonamides).
Demyelinating atrophy
It is observed in diseases such as multiple sclerosis and Devic disease.
Pressure or traction atrophy
It is observed in diseases such as glaucoma and papilledema.
Postinflammatory atrophy
It is observed in diseases such as optic neuritis, perineuritis secondary to inflammation of the meninges, and sinus and orbital cellulites.
Traumatic optic neuropathy
The exact pathophysiology of traumatic optic neuropathy is poorly understood, although optic nerve avulsion and transection, optic nerve sheath hematoma, and optic nerve impingement from a penetrating foreign body or bony fragment all reflect traumatic forms of optic nerve dysfunction that can lead to optic atrophy.
Regardless of etiology, optic atrophy is associated with variable degrees of visual dysfunction, which may be detected by one or all of the optic nerve function tests.
Radiation optic neuropathy
Radiation optic neuropathy more frequently occurs with radiation doses of at least 5,000 centigray. It may be result from radiation damage to the optic nerve vasculature or the optic nerve parenchyma itself.
Optic atrophy prevention
Optic atrophy is the end stage of a process causing damage to the optic nerve. Medical practice is currently unable to return function (regrow axons) to an atrophic optic nerve, and at best is able to stabilize whatever function remains. Primary prevention (removal of the process causing the damage) is the goal to prevent loss of axons and optic atrophy (neuropathy).
Optic atrophy symptoms
The main symptom of optic atrophy is vision loss. Any other symptoms are attributable to the underlying process that caused the optic disc damage such as pain with acute angle closure glaucoma.
Symptoms of optic atrophy:
- Blurred vision
- Abnormal side vision
- Abnormal color vision
- Decreased brightness in one eye relative to the other
The symptoms described above may not necessarily mean that you have optic atrophy. However, if you experience one or more of these symptoms, see an eye specialist (ophthalmologist) for a complete eye exam.
Optic atrophy is a sign and typically is noted as optic nerve pallor. This is the end stage of a process resulting in optic nerve damage. Because the optic nerve fiber layer is thinned or absent the disc margins appear sharp and the disc is pale, probably reflecting absence of small vessels in the disc head.
Optic atrophy diagnosis
History is critical in the diagnosis of optic atrophy since the physician needs to know how the eye arrived at this juncture. A careful history with attention to past medical history including all medications, time course of vision loss, associated symptoms etc is critical for arriving at a correct diagnosis. A complete eye exam including visual field, assessing color and contrast vision, intraocular pressures, looking for afferent pupil defect, and funduscopy should be done.
Optic atrophy is usually not difficult to diagnose due to its characteristic pale optic disc, but the cause may be difficult to ascertain. Sometimes it may be hard to differentiate between subtle optic neuropathy and disease of the retina or both. Electrophysiology (ERG, mERG) and OCT can be helpful for to assessing the thickness of the nerve fiber layers in such cases.
Characteristic visual field patterns include papillomacular defect (cecocentral scotoma), arcuate defect (include altitudinal) and/or temporal wedge defect (nasal fibers) for pre=chiasmal lesions, bitemporal (superior) field defects for chiasmal lesions, and hemianopsia for post-chiasmal lesions.
The following diagnostic work up should be considered for patients presenting with unexplained optic atrophy 1:
- Check for afferent pupil
- Visual fields 30-2, color vision
- MRI of brain and orbit with contrast
- CT with contrast (check bony disease, sinuses)
- Blood pressure and check of cardiovascular health (carotids with carotid doppler ultrasound),
- Serum glucose for the diagnosis of diabetes mellitus, as well as collecting a baseline level before initiating steroid therapy.
Screen for these if history or examination are suggestive 49:
- Heavy metals,
- Vitamin B12,
- Folate (vitamin B9),
- Homocysteine test measures the amount of the amino acid homocysteine in the blood. It’s often used to assess for vitamin B deficiencies (vitamin B6, B12, and folate) and can be a marker for increased risk of cardiovascular disease.
- FTA-ABS test (Fluorescent Treponemal Antibody-Absorption test) is a blood test used to detect antibodies to Treponema pallidum, the bacterium that causes syphilis
- VDRL (Venereal Disease Research Laboratory) test is a blood test used to screen for syphilis, a sexually transmitted infection caused by the bacteria Treponema pallidum. It detects antibodies in the blood that the body produces in response to the Treponema pallidum bacteria.
- ANA (antinuclear antibody) test is a blood test used to detect antinuclear antibodies, which are proteins that the immune system produces when it mistakenly attacks its own tissues. A positive ANA test can indicate an autoimmune disorder, where the body’s immune system attacks its own healthy cells.
- ACE (angiotensin-converting enzyme) blood test measures the amount of angiotensin-converting enzyme (ACE) in your blood. It’s primarily used to help diagnose and monitor sarcoidosis, a condition where abnormal knots of immune cells form in various parts of the body. Elevated angiotensin-converting enzyme (ACE) levels are often found in people with active sarcoidosis, and the test can be used to track disease progression and the effectiveness of treatment.
- Antiphospholipid antibody blood tests used to detect specific autoantibodies that target phospholipids, which are normal components of cell membranes and platelets. Antiphospholipid antibodies can interfere with blood clotting and are associated with conditions like Antiphospholipid Syndrome (APS), which increases the risk of blood clots, miscarriages, and other complications.
- TORCH panel test is a group of blood tests used to screen for infections that can harm a developing fetus during pregnancy. The acronym TORCH stands for Toxoplasmosis, Others (like syphilis, HIV, hepatitis B), Rubella, Cytomegalovirus, and Herpes simplex.
Diagnostic procedures
- Visual Field Testing (Humphrey 30-2, Tangent Screen) – to help localize the location of the lesion as well as follow-up of the patient’s condition. The 30-2 program is most useful in the investigation of optic atrophy. Visual field changes can include enlargement of the blind spot and paracentral scotoma, altitudinal defects (as seen in AION and optic neuritis), and bitemporal defects (seen in compressive lesions, similar to optic chiasma tumors).
- Optical Coherence Tomography (OCT)– to assess the thickness of peripapillary retinal nerve fiber layer and/or ganglion cell layer.
- Electroretinography (ERG) and multifocal electroretinogram (MERG) to rule out retinal disease.
- Electroretinography (ERG) results may show abnormalities such as 2:
- Subnormal: Potential less than 0.08 µv, seen in toxic neuropathy
- Negative: When a large a-wave is seen, it may be due to giant cell arteritis, central retinal artery occlusion, or central retinal vein occlusion.
- Extinguished: This is the response seen in complete optic atrophy.
- Electroretinography (ERG) results may show abnormalities such as 2:
- Neuro-imaging (MRI, CT).
- Magnetic resonance imaging (MRI) scan uses a magnetic field and pulses of radio wave energy to make pictures of your body. Magnetic Resonance Imaging (MRI) of the brain and orbits with contrast is useful in the diagnosis and should be ordered in all patients with optic atrophy 2. Imaging techniques may demonstrate space-occupying lesions, sinusitis, hyperpneumatized sinuses, fibrous dysplasia, and fractures. An MRI is important to determine whether there are damaged areas (lesions) in your brain. Such lesions indicate a high risk of developing multiple sclerosis. On MRI images, multiple sclerosis plaques are typically seen located in the infratentorial region, in the deep white matter, periventricular, juxtacortical, or mixed white matter-grey matter lesions. The lesions appear iso- or hypointense on T1 images (T1 black holes), whereas on T2 images, they are hyperintense. Fluid-attenuated inversion recovery (FLAIR) sequence also shows hyperintense lesions. Other sequences, such as T1-weighted post-contrast (gadolinium), MR spectroscopy, and double-inversion recovery (DIR), can also be used. MRI to check for optic neuritis, you might receive an injection of a contrast solution to make the optic nerve and other parts of your brain more visible on the images. An MRI can also rule out other causes of visual loss, such as a tumor.
- Computed Tomography (CT) Scan: In suspected fractures, a noncontrast computed tomography (CT) scan is preferable.
- Magnetic resonance imaging (MRI) scan uses a magnetic field and pulses of radio wave energy to make pictures of your body. Magnetic Resonance Imaging (MRI) of the brain and orbits with contrast is useful in the diagnosis and should be ordered in all patients with optic atrophy 2. Imaging techniques may demonstrate space-occupying lesions, sinusitis, hyperpneumatized sinuses, fibrous dysplasia, and fractures. An MRI is important to determine whether there are damaged areas (lesions) in your brain. Such lesions indicate a high risk of developing multiple sclerosis. On MRI images, multiple sclerosis plaques are typically seen located in the infratentorial region, in the deep white matter, periventricular, juxtacortical, or mixed white matter-grey matter lesions. The lesions appear iso- or hypointense on T1 images (T1 black holes), whereas on T2 images, they are hyperintense. Fluid-attenuated inversion recovery (FLAIR) sequence also shows hyperintense lesions. Other sequences, such as T1-weighted post-contrast (gadolinium), MR spectroscopy, and double-inversion recovery (DIR), can also be used. MRI to check for optic neuritis, you might receive an injection of a contrast solution to make the optic nerve and other parts of your brain more visible on the images. An MRI can also rule out other causes of visual loss, such as a tumor.
- Ultrasonography (B-scan) is recommended for orbital tumors. B-scan in papilledema may demonstrate nerve sheath dilatation.
- Visual evoked response (VER). During this test, you sit before a screen on which an alternating checkerboard pattern is displayed. Attached to your head are wires with small patches to record your brain’s responses to what you see on the screen. This type of test tells your doctor if the electrical signals to your brain are slower than normal as a result of optic nerve damage. In optic neuritis, Visually Evoked Response (VER) or Visually Evoked Potential (VEP) has an increased latency period and decreased amplitude compared with the normal eye. Compressive optic lesions tend to reduce the amplitude of visually evoked response (VER) while producing a minimal shift in the latency.
- Fundus Fluorescein Angiography (FFA). Fundus fluorescein angiography (FFA) can be performed in cases of retino-choroiditis and diabetic retinopathy to look for areas of capillary drop-out, neovascularization, and other defects. In optic disc drusen, fundus fluorescein angiography (FFA) shows fundus autofluorescence.
Optical Coherence Tomography (OCT) has become a valuable tool to verify the status of the nerve fiber layer/ganglion axons. Quantification of the nerve fiber layer height and comparison with normative data can document axon loss and differentiate between optic nerve and retinal disease as a cause for vision loss.
Since the optic nerve is the conduit for information from the retina to the brain, a damaged optic nerve will result in vision loss. Subtle damage might not affect acuity but may lead to a loss of contrast or color vision. Severe damage may lead from legal blindness to no light perception. Damage to a part of the optic nerve results in loss of vision in the corresponding visual field. Occasionally if the process causing damage is removed before apoptosis occurs (as for instance removal of a pituitary tumor compressing the chiasm or reducing inflammation in sarcoid) some improvement in visual function may be noted. A complete diagnosis is based on optic nerve appearance, tests of visual function (visual field, contrast, color, acuity), identifying the causative factor of the damage, and ruling out other causes for vision loss (such as retinal causes).
Certain disc appearances can help to determine the cause for the optic nerve damage. Sector disc pallor in an older individual could have been caused by non-arteritic anterior ischemic optic neuropathy due to loss of blood flow to the optic nerve. Non-arteritic anterior ischemic optic neuropathy typically causes sudden vision loss in one eye, without any pain. Severe optic atrophy with gliosis again in an elderly person could have been due to giant cell arteritis. Damage from papilledema may leave retinal folds and sometimes glistening bodies in the optic nerve head. Cupping is suggestive of glaucoma.
When examining a patient with a pale disc, determine primarily if the pallor is physiologic. Nonpathologic disc pallor is observed in the following:
- Axial myopia: The optic disc has a segmental whitish appearance due to an oblique angle of insertion of the optic nerve and nasal displacement of the optic nerve contents.
- Myelinated nerve fibers: Feathery margins are due to the superficial location, usually adjacent to the disc.
- Optic nerve pit: Small colobomas are most often located in the inferotemporal portion of the disc.
- Tilted disc can cause confusion.
- Optic nerve hypoplasia is characterized by a small disc and peripapillary double ring sign, and the inner ring is actually the optic disc margin.
- Scleral crescent areas are devoid of retinal pigment epithelium.
- Optic disc drusen
- Fundus viewing through an intraocular lens implant
- Brighter-than-normal luminosity: The luminosity of an indirect ophthalmoscope is approximately 2000 lux and that of a direct ophthalmoscope is up to 900 lux. A disc appears pale if the luminosity of the instrument is brighter than normal.
Optic atrophy in young individuals
Hereditary and congenital optic atrophy generally presents in the first or second decade of life. They can be broadly classified into the following 3 major groups:
- Optic atrophy with generalized white matter disease (eg, adrenoleukodystrophy)
- Optic atrophy with seemingly unrelated systemic features (generally associated with OPA1 gene mutation)
- Isolated optic atrophy (may be autosomal dominant or recessive mitochondrial inheritance; eg, Leber hereditary optic neuropathy or or Kjer syndrome).
Optic atrophy differential diagnosis
Optic atrophy is not usually difficult to diagnose, but it might be confused with optic nerve hypoplasia, myelinated nerve fibers, myopic or scleral crescent, or tilted disc 1.
Optic atrophy treatment
No proven treatment reverses optic atrophy. However, treatment that is initiated before the development of optic atrophy can be helpful in saving remaining visual function. Early diagnosis and prompt treatment can help patients with compressive and toxic neuropathies.
The role of pulse intravenous methylprednisolone is proven in a case of optic neuritis, arteritic anterior ischemic optic neuropathy, and traumatic optic neuropathy with successful outcomes 2. The optic neuritis treatment trial recommended doses of 500-1000 mg/day of IV methylprednisolone for three days, followed by oral prednisolone 1 mg/Kg of body weight for 11 days. Beta-interferons and glatiramer acetate have been used in the treatment of multiple sclerosis (MS) and related optic neuritis to reduce the occurrence of clinical lesions seen on MRI as well as the number of recurrences 50, 51.
Idebenone, a quinone analog, has been used and is the only clinically proven drug in the treatment of Leber hereditary optic neuropathy. The drug molecule bypasses the defective mitochondrial complex I, leading to improved energy supply and a functional recovery of retinal ganglion cells during the acute stage of the disease, thereby preventing further vision loss and promoting vision recovery 52. So far, the results were noted to be modest and the treatment is quite expensive. Klopstock et al conducted a 24-week multicenter double-blind, randomized, placebo-controlled trial in 85 patients with Leber hereditary optic neuropathy. They did not find a statistically significant visual recovery in the intention-to-treat population. They did find, however, evidence that patients with discordant visual acuities are the most likely to benefit from idebenone treatment, which is safe and well tolerated 53.
Research in stem cell treatment can hold a key in the future treatment of neuronal disorders. Currently, there are no approved treatments for mitochondrial disease, including optic neuropathies caused by primary or secondary mitochondrial dysfunction 54.
de Lima et al were able to restore some depth perception in mice with severe optic nerve damage. In addition, they found that the mice regained the ability to detect overall movement of the visual field and were able to perceive light. They found that using adequate stimulus, the fibers (1) are able to find their way to the correct visual centers in the brain, (2) are wrapped in the conducting insulation known as myelin, and (3) can make connections (synapses) with other neurons, allowing visual circuits to re-form. At present, the best defense is an early diagnosis because if the cause can be found and corrected, further damage can be prevented. de Lima et al discovered a molecule called oncomodulin. They achieved neuroregeneration in mice by simultaneously targeting the protein oncomodulin, elevating levels of the small signaling molecule cyclic adenosine monophosphate (cAMP) and deleting the gene that encodes the enzyme PTEN 55.
The optic nerve fiber is made of axons from the retinal ganglion cells, which usually do not regenerate after injury, resulting in lifelong visual loss. In recent studies using hamster models, anterograde tracing and electrophysiologic responses reveal that a small number of axons can regenerate all the way back to the superior colliculus 56. In other studies, remapping of the retina was noted in the superior colliculus following axon regeneration 57. These findings have given hope to clinically meaningful regeneration of axons, which may become a reality in the near future.
At present, the best defense is early diagnosis, because, if the cause can be found and corrected, further damage can be prevented.
Low-vision aids for patients with some useful vision should be considered for occupational rehabilitation.
Optic atrophy prognosis
Early and intensive treatment in nutritional optic neuropathy can provide patients with near-normal vision. Studies in glaucoma (based on Optical Coherence Tomography (OCT) nerve fiber layer measurements and other methods) have shown that the optic nerve has some reserve (axons) before vision loss is appreciated. After that reserve is depleted small changes in nerve fiber loss lead to significant decrease in vision. Early detection is key since you cannot replace dead axons.
- Optic Atrophy. https://eyewiki.org/Optic_Atrophy[↩][↩][↩][↩][↩][↩][↩]
- Ahmad SS, Blair K, Kanukollu VM. Optic Atrophy. [Updated 2024 Mar 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559130[↩][↩][↩][↩]
- Biousse V, Newman NJ. Diagnosis and clinical features of common optic neuropathies. Lancet Neurol 2016;15:1355–67 10.1016/S1474-4422(16)30237-X[↩]
- Trip SA, Schlottmann PG, Jones SJ, et al. Optic nerve atrophy and retinal nerve fibre layer thinning following optic neuritis: evidence that axonal loss is a substrate of MRI-detected atrophy. Neuroimage 2006;31:286–93 10.1016/j.neuroimage.2005.11.051[↩]
- Yu-Wai-Man P, Chinnery PF. Leber Hereditary Optic Neuropathy. 2000 Oct 26 [Updated 2021 Mar 11]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1174[↩]
- Biousse V, Newman NJ. Hereditary optic neuropathies. Ophthalmol. Clin. N. Am. 2001;14:547–568. doi: 10.1016/S0896-1549(05)70252-2[↩]
- Newman NJ, Biousse V. Hereditary optic neuropathies. Eye (Lond). 2004;18:1144–1160. doi: 10.1038/sj.eye.6701591[↩]
- Yu-Wai-Man P, et al. Genetic screening for OPA1 and OPA3 mutations in patients with suspected inherited optic neuropathies. Ophthalmology. 2011;118:558–563. doi: 10.1016/j.ophtha.2010.07.029[↩]
- Neuhann T, Rautenstrauss B. Genetic and phenotypic variability of optic neuropathies. Expert Rev. Neurother. 2013;13:357–367. doi: 10.1586/ern.13.19[↩]
- Tielsch JM, Javitt JC, Coleman A, et al. The prevalence of blindness and visual impairment among nursing home residents in Baltimore. N Engl J Med. 1995 May 4. 332(18):1205-9.[↩]
- Munoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000 Jun. 118(6):819-25.[↩]
- Shahror, R.A., Morris, C.A., Mohammed, A.A. et al. Role of myeloid cells in ischemic retinopathies: recent advances and unanswered questions. J Neuroinflammation 21, 65 (2024). https://doi.org/10.1186/s12974-024-03058-y[↩]
- Dominant optic atrophy. https://eyerounds.org/atlas/pages/Dominant-optic-atrophy.htm#gsc.tab=0[↩]
- Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013[↩]
- Stingl J.V., Wagner F.M., Liebezeit S., Baumgartner R., Spät H., Schuster A.K., Prokosch V., Grehn F., Hoffmann E.M. Long-Term Efficacy and Safety of Modified Canaloplasty Versus Trabeculectomy in Open-Angle Glaucoma. Life. 2023;13:516. doi: 10.3390/life13020516[↩]
- Blandford A.D., Zhang D., Chundury R.V., Perry J.D. Dysthyroid optic neuropathy: Update on pathogenesis, diagnosis, and management. Expert Rev. Ophthalmol. 2017;12:111–121. doi: 10.1080/17469899.2017.1276444[↩]
- Neigel J.M., Rootman J., Belkin R.I., Nugent R.A., Drance S.M., Beattie C.W., Spinelli J.A. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology. 1988;95:1515–1521. doi: 10.1016/S0161-6420(88)32978-7[↩]
- Bartalena L., Piantanida E., Gallo D., Lai A., Tanda M.L. Epidemiology, Natural History, Risk Factors, and Prevention of Graves’ Orbitopathy. Front. Endocrinol. 2020;11:615993. doi: 10.3389/fendo.2020.615993[↩]
- Li K.J., Semenov D., Turk M., Pope J. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res. Ther. 2021;23:82. doi: 10.1186/s13075-021-02450-w[↩]
- Salvetat ML, Pellegrini F, Spadea L, Salati C, Zeppieri M. Non-Arteritic Anterior Ischemic Optic Neuropathy (NA-AION): A Comprehensive Overview. Vision (Basel). 2023 Nov 9;7(4):72. doi: 10.3390/vision7040072[↩]
- Lee J.Y., Park K.A., Oh S.Y. Prevalence and incidence of non-arteritic anterior ischaemic optic neuropathy in South Korea: A nationwide population-based study. Br. J. Ophthalmol. 2018;102:936–941. doi: 10.1136/bjophthalmol-2017-311140[↩]
- Toosy A.T., Mason D.F., Miller D.H. Optic neuritis. Lancet Neurol. 2014;13:83–99. doi: 10.1016/S1474-4422(13)70259-X[↩]
- Braithwaite T., Subramanian A., Petzold A., Galloway J., Adderley N.J., Mollan S.P., Plant G.T., Nirantharakumar K., Denniston A.K. Trends in Optic Neuritis Incidence and Prevalence in the UK and Association With Systemic and Neurologic Disease. JAMA Neurol. 2020;77:1514–1523. doi: 10.1001/jamaneurol.2020.3502[↩]
- Rodriguez M., Siva A., Cross S.A., O’Brien P.C., Kurland L.T. Optic neuritis: A population-based study in Olmsted County, Minnesota. Neurology. 1995;45:244–250. doi: 10.1212/WNL.45.2.244[↩]
- Percy A.K., Nobrega F.T., Kurland L.T. Optic neuritis and multiple sclerosis. An epidemiologic study. Arch. Ophthalmol. 1972;87:135–139. doi: 10.1001/archopht.1972.01000020137004[↩]
- Delibes C, Ferré M, Rozet M, Desquiret-Dumas V, Descatha A, Gohier B, Gohier P, Amati-Bonneau P, Milea D, Reynier P. Genetic susceptibility to optic neuropathy in patients with alcohol use disorder. J Transl Med. 2024 May 25;22(1):495. doi: 10.1186/s12967-024-05334-0[↩]
- Xie X, Feng K, Wang J, Zhang M, Hong J, Zhang H. Comprehensive visual electrophysiological measurements discover crucial changes caused by alcohol addiction in humans: clinical values in early prevention of alcoholic vision decline. Front Neural Circuits. 2022;16:912883. doi: 10.3389/fncir.2022.912883[↩]
- Lessell S. Nutritional amblyopia. J Neuroophthalmol. 1998;18:106–111. doi: 10.1097/00041327-199806000-00006[↩]
- Milea D, Cassoux N, LeHoang P. Blindness in a strict vegan. N Engl J Med. 2000;342:897–898. doi: 10.1056/NEJM200003233421217[↩]
- The Cuba Neuropathy Field Investigation Team Epidemic optic neuropathy in Cuba clinical characterization and risk factors. New Engl J Med. 1995;333:1176–1182. doi: 10.1056/NEJM199511023331803[↩]
- Sadun AA, Martone JF. Cuba: response of medical science to a crisis of optic and peripheral neuropathy. Int Ophthalmol. 1995;18:373–378. doi: 10.1007/BF00930318[↩]
- Syed S, Lioutas V. Tobacco-alcohol amblyopia: a diagnosis dilemma. J Neurol Sci. 2013;15(327):41–45. doi: 10.1016/j.jns.2013.02.004[↩]
- González-Quevedo A, Santiesteban-Frexas R, Eells JT, Lima L, Sadun AA. Cuban epidemic neuropathy: insights into the toxic-nutritional hypothesis through international collaboration. MEDICC Rev. 2018;20:27–31. doi: 10.37757/MR2018.V20.N2.6[↩]
- Kincaid M.C., Green W.R. Ocular and orbital involvement in leukemia. Surv. Ophthalmol. 1983;27:211–232. doi: 10.1016/0039-6257(83)90123-6[↩]
- Puomila A., Hämäläinen P., Kivioja S., Savontaus M.L., Koivumäki S., Huoponen K., Nikoskelainen E. Epidemiology and penetrance of Leber hereditary optic neuropathy in Finland. Eur. J. Hum. Genet. 2007;15:1079–1089. doi: 10.1038/sj.ejhg.5201828[↩]
- Rosenberg T., Nørby S., Schwartz M., Saillard J., Magalhães P.J., Leroy D., Kann E.C., Duno M. Prevalence and Genetics of Leber Hereditary Optic Neuropathy in the Danish Population. Investig. Ophthalmol. Vis. Sci. 2016;57:1370–1375. doi: 10.1167/iovs.15-18306[↩]
- Mascialino B., Leinonen M., Meier T. Meta-analysis of the prevalence of Leber hereditary optic neuropathy mtDNA mutations in Europe. Eur. J. Ophthalmol. 2012;22:461–465. doi: 10.5301/ejo.5000055[↩]
- Spruijt L., Kolbach D.N., de Coo R.F., Plomp A.S., Bauer N.J., Smeets H.J., de Die-Smulders C.E. Influence of mutation type on clinical expression of Leber hereditary optic neuropathy. Am. J. Ophthalmol. 2006;141:676–682. doi: 10.1016/j.ajo.2005.11.007[↩]
- Yu-Wai-Man P., Griffiths P.G., Brown D.T., Howell N., Turnbull D.M., Chinnery P.F. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am. J. Hum. Genet. 2003;72:333–339. doi: 10.1016/j.ajhg.2016.05.015[↩]
- Watson E.C., Davis R.L., Ravishankar S., Copty J., Kummerfeld S., Sue C.M. Low disease risk and penetrance in Leber hereditary optic neuropathy. Am. J. Hum. Genet. 2023;110:166–169. doi: 10.1016/j.ajhg.2022.11.013[↩]
- Mackey D.A., Ong J.S., MacGregor S., Whiteman D.C., Craig J.E., Lopez Sanchez M.I.G., Kearns L.S., Staffieri S.E., Clarke L., McGuinness M.B., et al. Is the disease risk and penetrance in Leber hereditary optic neuropathy actually low? Am. J. Hum. Genet. 2023;110:170–176. doi: 10.1016/j.ajhg.2022.11.014[↩]
- Patel L., McNally R.J., Harrison E., Lloyd I.C., Clayton P.E. Geographical distribution of optic nerve hypoplasia and septo-optic dysplasia in Northwest England. J. Pediatr. 2006;148:85–88. doi: 10.1016/j.jpeds.2005.07.031[↩]
- Tear Fahnehjelm K., Dahl S., Martin L., Ek U. Optic nerve hypoplasia in children and adolescents; prevalence, ocular characteristics and behavioural problems. Acta Ophthalmol. 2014;92:563–570. doi: 10.1111/aos.12270[↩]
- Cockerham G.C., Goodrich G.L., Weichel E.D., Orcutt J.C., Rizzo J.F., Bower K.S., Schuchard R.A. Eye and visual function in traumatic brain injury. J. Rehabil. Res. Dev. 2009;46:811–818. doi: 10.1682/JRRD.2008.08.0109[↩]
- Karimi S., Arabi A., Ansari I., Shahraki T., Safi S. A Systematic Literature Review on Traumatic Optic Neuropathy. J. Ophthalmol. 2021;2021:5553885. doi: 10.1155/2021/5553885[↩]
- Pirouzmand F. Epidemiological trends of traumatic optic nerve injuries in the largest Canadian adult trauma center. J. Craniofac. Surg. 2012;23:516–520. doi: 10.1097/SCS.0b013e31824cd4a7[↩]
- Miller N.R. Traumatic Optic Neuropathy. J. Neurol. Surg. B Skull Base. 2021;82:107–115. doi: 10.1055/s-0040-1722632[↩]
- Nakaso K, Adachi Y, Fusayasu E, Doi K, Imamura K, Yasui K, et al. Leber’s Hereditary Optic Neuropathy with Olivocerebellar Degeneration due to G11778A and T3394C Mutations in the Mitochondrial DNA. J Clin Neurol. 2012 Sep. 8(3):230-4.[↩]
- Lee AG, Chau FY, Golnik KC, Kardon RH, Wall M. The diagnostic yield of the evaluation for isolated unexplained optic atrophy. Ophthalmology. 2005 May;112(5):757-9. doi: 10.1016/j.ophtha.2004.12.009[↩]
- Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev. 2015 Aug 14;2015(8):CD001430. doi: 10.1002/14651858.CD001430.pub4[↩]
- Wilhelm H, Schabet M. The Diagnosis and Treatment of Optic Neuritis. Dtsch Arztebl Int. 2015 Sep 11;112(37):616-25; quiz 626. doi: 10.3238/arztebl.2015.0616[↩]
- Gueven N. Idebenone for Leber’s hereditary optic neuropathy. Drugs Today (Barc). 2016. 52(3):173-81.[↩]
- Klopstock T, Yu-Wai-Man P, Dimitriadis K, et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011 Sep. 134:2677-86.[↩]
- Lopez Sanchez MI, Crowston JG, Mackey DA, Trounce IA. Emerging Mitochondrial Therapeutic Targets in Optic Neuropathies. Pharmacol Ther. 2016. Sep 165:132-52[↩]
- de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, et al. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci U S A. 2012 Jun 5. 109(23):9149-54.[↩]
- Keirstead SA, Rasminsky M, Fukuda Y, Carter DA, Aguayo AJ, Vidal-Sanz M. Electrophysiologic responses in hamster superior colliculus evoked by regenerating retinal axons. Science. 1989 Oct 13. 246(4927):255-7.[↩]
- Fischer D, Heiduschka P, Thanos S. Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol. 2001 Dec. 172(2):257-72.[↩]