Contents
- Assisted reproductive technology
- Assisted Reproductive Technology clinic near me
- Assisted reproductive technology types
- Assisted reproductive technology risks
- Assisted reproductive technology success rates
- Assisted reproductive technology cost
- When to end ART treatment?
Assisted reproductive technology
Assisted reproductive technology also known as “ART” is a complex series of treatments, procedures and techniques to treat infertility, a condition in which you can’t get pregnant after at least a year of trying for most couples. Assisted reproductive technology (ART) includes in-vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), gamete intrafallopian transfer (GIFT), zygote intrafallopian transfer (ZIFT), pronuclear stage tubal transfer (PROST), tubal embryo transfer (TET), egg and embryo cryopreservation (freezing), egg and embryo donation, and gestational surrogacy 1. In general, assisted reproductive technology (ART) procedures involve removing eggs from a woman’s ovaries using a needle. These eggs are then combined with sperm in the laboratory to create embryos. The embryos are returned to the woman’s body, frozen for future use, or donated to another woman. The main type of ART is in vitro fertilization (IVF). IVF involves extracting a woman’s eggs, fertilizing the eggs in the laboratory, and then transferring the resulting embryos into the woman’s uterus through the cervix. Assisted reproductive technology (ART) procedures sometimes also involve the use of donor eggs, donor sperm, or donated embryos. Donor eggs are sometimes used for women who cannot produce eggs. Donor eggs or donor sperm are sometimes used for other reasons, such as a genetic disease that can be passed on to the baby. An infertile couple may also use donated embryos that were created by other couples in infertility treatment and were not used. When donated embryos are used, the child will not be genetically related to either parent. Donor eggs, donor sperm, or donated embryos may also be used by same-sex couples. A gestational carrier (surrogate) carries an embryo that was formed from the egg of another woman with the expectation of returning the infant to its intended parents. Women who have no uterus may be able to use a gestational carrier. Women who cannot become pregnant, or who have a serious health problem and should not become pregnant may also consider this option.
Preimplantation genetic testing (PGT) can also be used during ART to identify genetic disorders or chromosomal abnormalities in embryos. Preimplantation genetic testing (PGT) is a technique in which one or more cells is taken from an egg or embryo (fertilized egg) for testing to provide information about the genetic make-up of the rest of the cells in that embryo. In order to use preimplantation genetic testing (PGT), couples must undergo in vitro fertilization (IVF), where the eggs (oocytes) are removed from a woman’s body and mixed with her partner’s sperm in a laboratory. The embryos which are created can be tested on Day 3 after egg harvest and then implanted back into the uterus on Day 5. Alternatively, the embryos can be frozen after the cells are removed for testing and implanted in a subsequent menstrual cycle. While preimplantation genetic testing (PGT) can reduce the likelihood of conceiving a pregnancy with an affected child, it cannot eliminate the risk. Confirmation with chorionic villus sampling (CVS), amniocentesis, or other testing is still necessary.
ART can be expensive and time-consuming. The most common complication of ART is a multiple pregnancy. This can be prevented or minimized by limiting the number of embryos that are transferred back to the uterus. For example, transfer of a single embryo greatly reduces the chances of a multiple pregnancy. Using a single embryo, rather than multiple embryos, lowers risks such as preterm birth. Despite these challenges, assisted reproductive technology (ART) has helped many couples have children that otherwise would not have been conceived.
What is an assisted reproductive technology (ART) cycle?
Because Assisted Reproductive Technology (ART) consists of several steps, an ART procedure is typically referred to as a cycle of treatment rather than a procedure at a single point in time. The start of an ART cycle is usually when a woman begins taking medication to stimulate egg production or begins monitoring with the intent of having embryos transferred. If eggs are produced, the cycle progresses to egg retrieval. Retrieved eggs can be combined with sperm to create embryos or frozen for future use. If fertilization is successful, embryos can be selected for transfer in the same cycle or frozen for future use. If embryo transfer results in implantation, the cycle may progress to clinical pregnancy and possibly a live-birth delivery. For the purposes of ART reporting, data on all cycles that were started, even those that were discontinued before all steps were undertaken, are counted in the clinic’s success rates.
What are my chances of getting pregnant using assisted reproductive technology (ART)?
Assisted Reproductive Technology (ART) success rates vary according to patient and treatment characteristics. These characteristics include age, type of infertility diagnosis, number of embryos transferred, type of ART procedure, use of techniques such as intracytoplasmic sperm injection (ICSI), and history of previous births, miscarriages, and ART cycles. With ICSI, a single sperm is injected into a mature egg. The age of the woman is the most important factor in determining ART success rates, when women are using their own eggs. Success rates decline as women age, specifically after the mid-30’s. Part of this decline is due to a lower chance of getting pregnant from ART, and part is due to a higher risk of miscarriage with increasing age, especially over age 40.
Assisted Reproductive Technology (ART) success rates also vary with the number of embryos transferred. However, transferring more and more embryos at one time does not increase the chance of live birth significantly, but may only increase your risk of a multiple pregnancy, and its associated risks. The impact of the number of embryos that are transferred also varies with your age (woman’s age).
You can increase your chance of achieving a pregnancy, having a healthy pregnancy, and avoiding complications that could affect your health and the outcome of your pregnancy by following these actions:
- Start and continue taking 400 mcg of folic acid (folate) daily, in the form of a vitamin supplement or enriched foods.
- Stop smoking cigarettes. Quitting smoking can improve your fertility. Smokers are more likely to be infertile than non-smokers. The negative effects of smoking are generally gone one year after you give up smoking.
- Eliminate alcohol and recreational drugs consumption. Alcohol and recreational drugs (like cocaine, heroin, cannabis, and ecstasy) have also been shown to affect fertility. They should be avoided if you want the best chance of getting pregnant.
- Reduce or eliminate caffeine intake.
- Start or continue an exercise regimen that helps control weight and provides relaxation and stress reduction benefits.
- Control your chronic conditions under medical supervision (e.g., high blood pressure, diabetes, reproductive tract infections, dental disease, anxiety, lupus, arthritis, epilepsy.)
- Develop eating habits that can continue into pregnancy and beyond, consider smaller portions of high quality foods providing sound nutritional value.
- Be sure your immunization records are up-to-date. Vaccines protect you from diseases such as rubella, tetanus, influenza, and whooping cough.
- Avoid exposure to environmental and workplace hazards. These can include products such as pesticides, solvents, and even prescription medicines that you may handle or touch. This includes clothing or equipment used by a household member in their work or as part of a hobby. If a pregnancy occurs, these exposures could be dangerous during the first trimester.
What is intrauterine insemination (IUI)?
Intrauterine insemination (IUI) is the placement of a man’s sperm into a woman’s uterus using a long, narrow tube similar to a thin straw 2.
Intrauterine insemination (IUI) includes the following 2:
- Intrauterine insemination (IUI) is most effective for treating infertility in:
- Women who have scarring or defects of the cervix
- Men who have low sperm counts
- Men who have sperm with low mobility
- Men who cannot get erections
- Men who have retrograde ejaculation, a condition in which sperm are ejaculated into the bladder instead of out of the penis
- Couples who have difficulty having intercourse
- Intrauterine insemination (IUI) can be used in combination with medications that stimulate ovulation. This combination can increase the chance of pregnancy in some cases.
- The success of intrauterine insemination (IUI) depends on the cause of the couple’s infertility. If inseminations are performed monthly with fresh or frozen sperm, success rates can be as high as 20% per cycle. These outcomes depend on whether fertility medications are used, the age of the female partner, and the infertility diagnosis, as well as on other factors that could affect the success of the cycle.
What causes infertility in men?
Infertility in men can be caused by disruption of testicular or ejaculatory function, as well as by hormonal and genetic disorders. It is typically evaluated by a semen analysis, complete medical history, and physical examination. This assessment helps determine if and how male factors are contributing to infertility. A semen analysis assesses the number (concentration), motility (movement), and morphology (shape) of sperm. The results are then evaluated by a specialist. If semen is found to be slightly abnormal, it does not necessarily mean that a man is infertile.
Disruption of testicular or ejaculatory function. Some conditions that can disrupt testicular or ejaculatory function include the following:
- Varicocele is a condition in which the veins in the testicles are enlarged, which may affect the number or shape of the sperm.
- Trauma to the testes may affect sperm production and result in lower number of sperm.
- Unhealthy habits, such as heavy alcohol use, smoking, anabolic steroid use, and illicit drug use, may decrease sperm production.
- Cancer treatment involving the use of certain types of chemotherapy, radiation, or surgery to remove one or both testicles may affect sperm production or the ability of sperm to fertilize an egg.
- Medical conditions such as diabetes, cystic fibrosis, certain types of autoimmune disorders, and certain types of infections may cause testicular failure.
Hormonal disorders. Improper function of the hypothalamus or pituitary glands are hormonal disorders that can cause infertility. These glands produce hormones that maintain normal testicular function. Too much of these hormones, especially prolactin, can result in infertility. Other conditions that damage or impair the hypothalamus or the pituitary gland may also result in low or no sperm production.
Examples of hormonal disorders include:
- Malignant (cancerous) pituitary tumors
- Congenital adrenal hyperplasia (CAH)
- Exposure to too much estrogen
- Exposure to too much testosterone
- Cushing’s syndrome
- Chronic use of medications called glucocorticoids
Genetic disorders. Genetic conditions can cause no sperm or low numbers of sperm to be produced.
Examples of genetic disorders include:
- Klinefelter’s syndrome
- Y-chromosome microdeletion
- Myotonic dystrophy
Men’s fertility is also known to decline with:
- Age. Couples in which the male partner is 40 or older are more likely to report difficulty conceiving.
- Having overweight or obesity.
- Smoking.
- Excessive use of alcohol or drug use (such as opioids or marijuana).
- A history of trauma to the testes.
- Exposure to testosterone, radiation, certain medicines, or certain environmental toxins.
- Frequent exposure of the testes to high temperatures.
What causes infertility in women?
Women need functioning ovaries, fallopian tubes, and a uterus to get pregnant. Conditions affecting any one of these organs can contribute to female infertility 3.
Disruption of ovarian function. A woman’s menstrual cycle is on average 28 days long. Regular, predictable periods that occur every 21 to 35 days likely reflect ovulation. A woman with irregular periods is likely not ovulating. Ovulation can be predicted by using an ovulation test or blood test. Disruption in ovarian function may be caused by several conditions and calls for an evaluation by a health care provider. No single test is a perfect predictor of fertility, but there are tests for common markers of ovarian function. These include follicle-stimulating hormone (FSH), anti-müllerian hormone (AMH), and antral follicle count (AFC) tests. Some conditions that may cause issues related to ovarian function include the following:
- Polycystic ovary syndrome (PCOS) is the most common cause of infertility. PCOS is a condition that causes women to not ovulate, or to ovulate irregularly.
- Diminished ovarian reserve is a condition where there are fewer eggs remaining in the ovaries than expected at a specific age. Diminished ovarian reserve can be due to congenital, medical, surgical, or unexplained causes.
- Impaired function of the hypothalamus and pituitary glands can affect ovarian function. If impaired, these glands can cause too much of the hormones that maintain normal ovarian function to be produced.
- Functional hypothalamic amenorrhea (FHA) is a condition that can affect ovarian function. It can be caused by excessive exercise, weight loss, or stress, or by a combination of these factors.
- Primary ovarian insufficiency (POI) is sometimes referred to as premature menopause. It occurs when a woman’s ovaries fail before age 40.
Fallopian tube obstruction. Fallopian tubes can be evaluated by various methods, including with a hysterosalpingogram or by chromopertubation. Hysterosalpingogram is an X-ray of the uterus and fallopian tubes. These images are used to see if dye moves freely through the fallopian tubes indicating they are open. Chromopertubation is similar to a hysterosalpingogram but is done in the operating room at the time of a laparoscopy. This test is used to evaluate if the fallopian tubes are open and to assess if they are dilated.
Risk factors for fallopian tube obstruction include:
- History of pelvic infection
- Ruptured appendix
- Gonorrhea
- Chlamydia
- Endometriosis
- Prior abdominal surgery
Abnormalities of the uterus. The uterus may be evaluated by transvaginal ultrasound to look for various problems. A sonohystogram or hysteroscopy may also be performed to evaluate the uterine environment.
Problems that affect the physical characteristics of the uterus include:
- Intrauterine adhesions
- Endometrial polyps
- Adenomyosis
- Congenital anomalies of the uterus
- Fibroids
Women’s fertility is also known to decline with:
- Age. Fertility declines with age primarily because egg quality declines over time. Older women also have fewer eggs left, and they are more likely to have health conditions that can cause fertility problems. Aging also increases a woman’s chances of miscarriage and of having a child with a genetic abnormality.
- Smoking.
- Excessive alcohol use.
- Having overweight, obesity, or low body weight.
- Extreme weight gain or loss.
- Excessive physical or emotional stress that results in amenorrhea (absent periods).
Should we have genetic testing?
Preimplantation genetic testing (PGT) is a technique in which one or more cells is taken from an egg or embryo (fertilized egg) for testing to provide information about the genetic make-up of the rest of the cells in that embryo. In order to use preimplantation genetic testing (PGT), couples must undergo in vitro fertilization (IVF), where the eggs (oocytes) are removed from a woman’s body and mixed with her partner’s sperm in a laboratory. The embryos which are created can be tested on Day 3 after egg harvest and then implanted back into the uterus on Day 5. Alternatively, the embryos can be frozen after the cells are removed for testing and implanted in a subsequent menstrual cycle.
Patients with many inherited familial diseases can have their embryos tested to determine its genetic make-up. Specifically, this would include patients with a history of single-gene disorders such as cystic fibrosis or sickle cell anemia and patients with a history of sex-linked disorders such as Duchenne muscular dystrophy and Fragile X syndrome. In addition, even families in search of a bone marrow donor may be able to use preimplantation genetic testing (PGT) to bring a child into the world that can provide matching stem cells for an affected sibling.
Other patients may also decide to use genetic screening. For some patients with recurrent pregnancy loss, severe male factor infertility, advanced reproductive age or recurrent IVF treatment failures, genetic screening may be used. Genetic screening is different than other types of genetic testing because the testing is looking for any abnormality instead of a specific disease, and as a result is associated with higher rates of false results. At this time, the American Society for Reproductive Medicine considers genetic screening for this indication experimental. If you are uncertain about genetic testing for you, speak with your doctor about whether preimplantation genetic testing is right for you. While preimplantation genetic testing (PGT) can reduce the likelihood of conceiving a pregnancy with an affected child, it cannot eliminate the risk. Confirmation with chorionic villus sampling (CVS), amniocentesis, or other testing is still necessary.
Assisted Reproductive Technology clinic near me
The links below contain all practicing members of American Society for Reproductive Medicine (ASRM) and Society for Assisted Reproductive Technology (SART). Find a Assisted Reproductive Technology Clinic near you here:
https://www.sartcorsonline.com/members/Search
https://www.reproductivefacts.org/find-a-health-professional/
Assisted reproductive technology types
Assisted Reproductive Technology (ART) includes all fertility treatments in which either eggs or embryos are handled outside a woman’s body. In general, ART procedures involve surgically removing eggs from a woman’s ovaries, combining them with sperm in the laboratory, and returning them to a female patient or a gestational carrier or donating them to another patient. Assisted Reproductive Technology (ART) does NOT include treatments in which only sperm are handled such as intrauterine insemination (IUI) or procedures in which a woman takes drugs only to stimulate egg production without the intention of having eggs surgically retrieved. ART may be recommended when other treatments such as intrauterine insemination (IUI) have not been successful or when there is severe male factor infertility, severe endometriosis or tubal obstruction. In addition, ART is often categorized according to whether the procedure involved freezing all eggs or embryos (banking), whether the procedure used a patient’s own eggs or eggs from another woman (donor), whether the eggs were frozen and thawed before use, and whether the embryos used were newly fertilized (fresh) or previously fertilized, frozen, and then thawed.
Assisted Reproductive Technology (ART) includes in vitro fertilization (IVF), gamete intrafallopian transfer (GIFT), zygote intrafallopian transfer (ZIFT), and frozen embryo transfer (FET). These techniques also apply to egg donation and gestational carriers.
Approximately 99 percent of ART cycles performed are in vitro fertilization (IVF). For some IVF procedures, fertilization involves a specialized technique known as intracytoplasmic sperm injection (ICSI). In ICSI, a single sperm is injected directly into a woman’s egg.
Other types of ART exist but are rarely performed. Gamete intrafallopian transfer (GIFT) involves using a fiber optic instrument called a laparoscope to guide the transfer of unfertilized eggs and sperm (gametes) into a woman’s fallopian tubes through small incisions in her abdomen. Zygote intrafallopian transfer (ZIFT) involves fertilizing a woman’s eggs in the laboratory and then using a laparoscope to guide the transfer of the fertilized eggs (zygotes) into a woman’s fallopian tubes.
In vitro fertilization (IVF)
In vitro fertilization (IVF) is the most common type of assisted reproductive technology (ART). During in vitro fertilization (IVF), eggs and sperm from the couple are incubated together in a petri dish to produce an embryo. A doctor then places the embryo into the woman’s uterus, where it may implant and result in a successful pregnancy.
To get started, you’ll want to find a reputable fertility clinic. If you live in the United States, the Centers for Disease Control and Prevention (CDC) and the Society for Assisted Reproductive Technology provide information online about clinics’ individual pregnancy and live birth rates. A fertility clinic’s success rate depends on many things. These include the ages and medical issues of people they treat, as well as the clinic’s treatment approaches. When you talk with a representative at a clinic, also ask for detailed information about the costs of each step of the procedure.
Before you start a cycle of IVF using your own eggs and sperm, you and your partner will likely need various screening tests. These include 4:
- Ovarian reserve testing. This involves getting blood tests to find out how many eggs are available in your body. This is also called egg supply. The results of the blood tests, often used together with an ultrasound of the ovaries, can help predict how your ovaries will respond to fertility medicines. The woman’s ovarian reserve is evaluated using either cycle day three follicle-stimulating hormone (FSH) and estradiol (E2), anti-Mullerian hormone (AMH), or antral follicle count. If the woman is determined to have poor ovarian reserve based on any of these values, she can still pursue IVF but may need to consider the use of donor oocytes.
- Semen analysis. Semen is the fluid that contains sperm. An analysis of it can check the amount of sperm, their shape and how they move. This testing may be part of an initial fertility evaluation. Or it might be done shortly before the start of an IVF treatment cycle.
Infectious disease screening. You and your partner will both be screened for diseases such as HIV. - Practice embryo transfer. This test doesn’t place a real embryo in the uterus. It may be done to figure out the depth of your uterus. It also helps determine the technique that’s most likely to work well when one or more actual embryos are inserted.
- Uterine exam. The inside lining of the uterus is checked before you start IVF. This might involve getting a test called sonohysterography. Fluid is sent through the cervix into the uterus using a thin plastic tube. The fluid helps make more-detailed ultrasound images of the uterine lining. Or the uterine exam might include a test called hysteroscopy. A thin, flexible, lighted telescope is inserted through the vagina and cervix into the uterus to see inside it. Uterine cavity issues such as endometrial polyps or fibroids, adhesions, or septa may interfere with embryo implantation.
- Infectious disease screening for HIV, hepatitis B, and hepatitis C and syphilis is recommended for both partners.
Before you begin a cycle of IVF, think about some key questions, including 4:
- How many embryos will be transferred? The number of embryos placed in the uterus often is based on age and the number of eggs collected. Since the rate of fertilized eggs attaching to the lining of uterus is lower for older people, usually more embryos are transferred — except for people who use donor eggs from a young person, genetically tested embryos or in certain other cases. Most fertility specialists follow specific guidelines to prevent a multiple pregnancy with triplets or more. In some countries, legislation limits the number of embryos that can be transferred. Make sure you and your fertility specialist agree on the number of embryos that will be placed in the uterus before the transfer procedure.
- What will you do with any extra embryos? Extra embryos can be frozen and stored for future use for many years. Not all embryos will survive the freezing and thawing process, but most will. Having frozen embryos can make future cycles of IVF less expensive and less invasive. Or you might be able to donate unused frozen embryos to another couple or a research facility. You also might choose to discard unused embryos. Make sure you feel comfortable making decisions about extra embryos before they are created.
- How will you handle a multiple pregnancy? If more than one embryo is placed in your uterus, IVF can cause you to have a multiple pregnancy. This poses health risks for you and your babies. In some cases, a surgery called fetal reduction can be used to help a person deliver fewer babies with lower health risks. Getting fetal reduction is a major decision with ethical, emotional and mental risks.
- Have you thought through the risks linked with using donor eggs, sperm or embryos, or a gestational carrier? A trained counselor with expertise in donor issues can help you understand the concerns, such as the legal rights of the donor. You also may need an attorney to file court papers to help you become legal parents of an embryo that’s developing in the uterus.
After the above IVF preparations are completed,
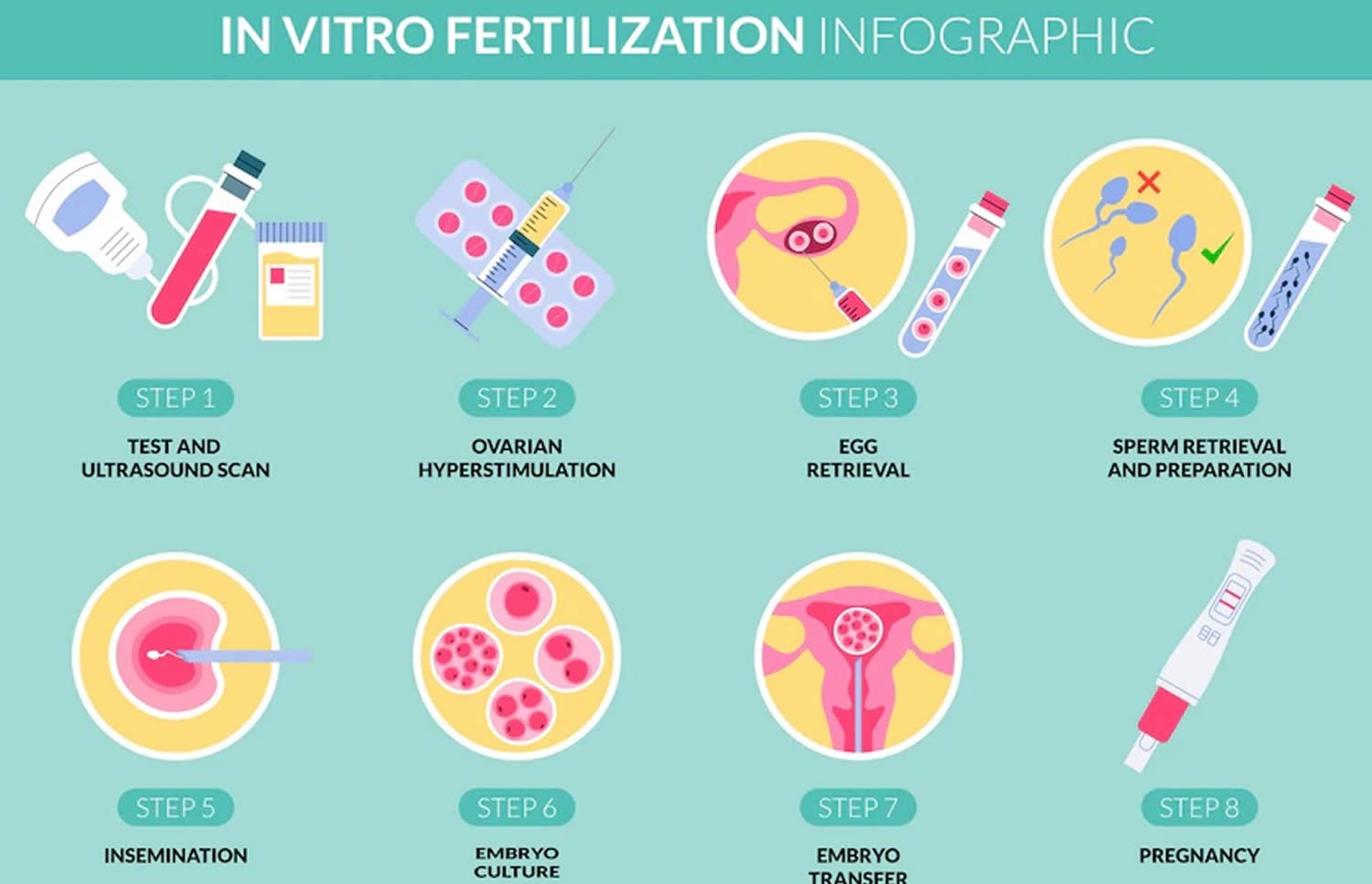
The steps of IVF are:
- Ovarian stimulation. Ovarian stimulation also known as treatment to make mature eggs, ovulation induction, stimulation of egg maturation or superovulation, a woman takes medication to stimulate the ovaries to make many mature eggs at one time. These medications are given by injection for 8 to 14 days. A doctor closely monitors the development of the eggs using transvaginal ultrasound and blood tests to assess follicle growth and estrogen production by the ovaries. When the eggs are mature—as determined by the size of the ovarian follicles and the level of estrogen—an injection of the hormone human chorionic gonadotropin (hCG) initiates the ovulation process. A doctor takes out (egg retrieval) the eggs 34 to 36 hours after the human chorionic gonadotropin (hCG) injection.
- Egg Retrieval. Egg retrieval is the process used to remove the eggs from the ovaries so they can be fertilized. The procedure is performed in a doctor’s office as an outpatient procedure. A mild sedative and painkiller are often used during the procedure, and it normally takes about 30 minutes. The steps for egg retrieval are as follows:
- An ultrasound probe is inserted into the vagina to visualize the ovaries and the follicles, which contain the eggs.
- A needle is inserted through the wall of the vagina to the ovaries. Generally, ultrasound is used to guide the placement of the needle.
- Suction is used to pull the eggs from the ovaries into the needle.
- Fertilization. A man provides a semen sample. If the sperm are healthy, they are centrifuged to concentrate them and reduce the volume, placed in a dish with the egg, and left overnight in an incubator. Fertilization usually occurs on its own. However, sometimes sperm are not able to fertilize the egg on their own. When this is the case, a single sperm is injected into an egg using a needle. This process is called intracytoplasmic sperm injection (ICSI). About 60% of IVF in the Unites States is performed with ICSI 5. The pregnancy rate is about the same for IVF using natural fertilization or intracytoplasmic sperm injection (ICSI). If sperm cannot fertilize the egg without assistance, couples should consider genetic testing. Genetic testing can determine whether the sperm have chromosome problems that might cause development problems in the resulting embryos. Embryos that develop from IVF are placed into the uterus 1 to 6 days after retrieval.
- Embryo Transfer. Embryo transfer is performed in a doctor’s office. Embryo transfer procedure is normally painless, but some women may experience cramping. A doctor inserts a long, thin tube through the vagina and into the uterus and injects the embryo into the uterus. The embryo should implant into the lining of the uterus 6 to 10 days after retrieval. Sometimes the embryos are frozen and thawed at a later date for embryo transfer. This is often done when fresh embryos fail to implant or when a woman wants to preserve her eggs in order to become pregnant years later. Women either time implantation with their ovulation cycle or receive estrogen and progesterone medications to prepare their uterine linings for implantation.
Figure 1. In vitro fertilization (IVF)
Evaluation before IVF
Before starting IVF, each patient is evaluated to help maximize her chances for success and a healthy pregnancy. Good preconception health is essential to achieving pregnancy with IVF. Chronic medical conditions such as diabetes, high blood pressure and asthma should be well controlled before attempting to conceive. In addition, women planning an IVF cycle should optimize their weight. Obesity has been associated with infertility, a reduced chance of success with IVF, and an increase in the risk of miscarriage and preterm birth. Your doctor can help you determine your ideal weight and refer you to appropriate resources for weight management.
Blood tests
Prior to starting IVF, the woman’s blood type should be verified, and she should be screened for conditions that could affect the health of a pregnancy. Documentation of immunity to rubella (German measles) and varicella (chicken pox) may also require a blood test. Vaccination can be offered before pregnancy if immunity is not present. The patient and her partner will also be tested for:
Female
- HIV
- Hepatitis B antigen
- Hepatitis C antibody
- RPR (rapid plasma reagin) a screening test for syphilis
- Pap smear
- Blood group, Rh, and antibody screen
Male
- HIV
- Hepatitis B antigen
- Hepatitis C antibody
- RPR (rapid plasma reagin) a screening test for syphilis
- Complex semen analysis, antisperm antibodies, and strict morphology
If you are an egg donor, several additional tests are required. These include blood testing for evidence of previous or current cytomegalovirus (CMV infection), a genetic consultation to evaluate your family medical history, and a psychological screening interview.
An option for couples to consider is Universal Genetic Carrier Screening. This testing offers the additional advantages of identifying before pregnancy couples at risk of having children with genetic diseases. They can then be offered appropriate testing to optimize patient education, counseling, and options for achieving pregnancy. Couples at risk of having children with specific genetic diseases can be counseled about the disease inheritance and course and offered referral for potential interventions, such as preimplantation genetic testing (PGT).
Ovarian Reserve Testing
As women age they have a decreased ability to conceive and an increased risk of miscarriage. The reproductive potential of the ovaries, termed ovarian reserve, represents the number of eggs (oocytes) available for potential fertilization at that point in time and may be assessed by blood tests or ultrasound. The presence of decreased ovarian reserve predicts future response to ovarian stimulation. The results of ovarian reserve tests should be considered in the context of the patient’s age. Ovarian reserve tests are good predictors of response to ovarian stimulation, but poor results do not necessarily predict inability to achieve a live birth.
- Anti-Mullerian Hormone Test (AMH): AMH levels remain relatively stable throughout the menstrual cycle and can be assessed on any day of the menstrual cycle. An AMH value less than 1.0 can predict a low response to stimulation.
- Day 2-5 Levels of Follicle-Stimulating Hormone (FSH), and Estradiol (E2): Follicle stimulating hormone (FSH) values greater than 10 IU/L are associated with a less robust response to ovarian stimulation. Estradiol (E2) serves as an aid for interpreting FSH results. Basal estradiol levels typically should be less than 60–80 pg/mL; elevated estradiol levels may have a suppressive effect on FSH levels and may be indicative of decreased ovarian reserve.
- Ultrasonographic assessment of the antral follicle count: determines the number of follicles that measure 2–10 mm in both ovaries. Low antral follicle count may be defined as fewer than 5–7 follicles and is associated with poor response to ovarian stimulation. However, antral follicle count is a relatively poor predictor of future ability to become pregnant.
Semen analysis
A semen analysis should be reviewed. Changes in sperm quality may occur over time that could affect IVF success. Semen parameters can help determine whether standard insemination of eggs or intracytoplasmic sperm injection (ICSI) may be advised.
Uterus exam
The uterus is usually evaluated prior to an IVF. Three methods can be used: a hysterosalpingogram, a saline infusion sonohysterography or a hysteroscopy.
Prior to IVF, a trial or “mock” transfer may be done. The purpose of this procedure is to determine the length and direction of the uterus. This enables the physician to anticipate any difficulties with the embryo transfer.
Ovarian stimulation (treatment to make mature eggs)
The cycle of IVF begins with ovarian stimulation. The start of an IVF cycle begins by using man-made hormones to help the ovaries to make eggs — rather than the single egg that usually develops each month. Multiple eggs are needed because some eggs won’t fertilize or develop correctly after they’re combined with sperm. One cycle of IVF can take about 2 to 3 weeks. More than one cycle may be needed.
During ovarian stimulation, also known as treatment to make mature eggs, ovulation induction or superovulation, “fertility drugs” or “medications for inducing ovulation” are used to stimulate multiple eggs to grow in the ovaries rather than the single egg that normally develops each month. Several medications are used in a typical IVF cycle. These medicines belong to several categories and each are an important part of a stimulated cycle. The types and amount of medications used vary according to the medication protocol prescribed by your physician.
- Medications for Ovarian Stimulation
- Human menopausal gonadotropin (hMG)
- Follicle-stimulating hormone (FSH)
- Luteinizing hormone (LH) (used in conjunction with FSH)
- Human chorionic gonadotropin (hCG)
- Clomiphene citrate
- Letrozole
- Medications to Prevent Premature Ovulation
- Gonadatropin-releasing hormone (GnRH) agonists
- GnRH antagonists
Clomiphene citrate and letrozole are administered orally while the other medications listed are given by injection. Clomiphene citrate and letrozole oral medications are less potent than injectable medications and are not as commonly used in ART cycles. There is no evidence that one injectable medication is superior to any other.
Timing is crucial in an IVF cycle. The ovaries are evaluated during treatment with vaginal ultrasound examinations to monitor the development of ovarian follicles. Blood samples are drawn to measure the response to ovarian stimulation medications. Normally, estrogen levels increase as the follicles develop, and progesterone levels are low until after ovulation.
Using ultrasound examinations and blood testing, the physician can determine when the follicles are ready for egg retrieval. Generally, 8 to 14 days of stimulation are required. When the follicles are ready, human chorionic gonadotropin (hCG) or other medications are given. The human chorionic gonadotropin (hCG) replaces the woman’s natural luteinizing hormone (LH) surge and causes the final stage of egg maturation so the eggs are capable of being fertilized. The eggs are retrieved before ovulation occurs, usually 34 to 36 hours after the hCG injection is given.
Up to 20% of IVF cycles may be cancelled prior to egg retrieval. IVF cycles may be cancelled for a variety of reasons, usually due to an inadequate number of follicles developing. Cancellation rates due to low response to the ovulation drugs increase with a woman’s age, especially after age 35. When IVF cycles are cancelled due to a poor response, alternate drug strategies may be helpful to
promote a better response in a future attempt. Occasionally, a IVF cycle may be cancelled to reduce the risk of ovarian hyperstimulation syndrome (OHSS).
Treatment with a gonadotropin releasing hormone (GnRH) agonist or antagonist reduces the possibility of premature LH surges from the pituitary gland, and thereby reduces the risk of premature ovulation. However, LH surges and ovulation occur prematurely in a small percentage of ART cycles despite the use of these drugs. When this occurs, since it is unknown when the LH surges began and eggs will mature, the cycle is usually cancelled. Collection of eggs from the peritoneal cavity after ovulation is not efficient.
Gonadotropin releasing hormone (GnRH) Agonists
Gonadotropin releasing hormone (GnRH) is a hormone produced in the brain (hypothalamus) that indirectly stimulates ovarian function. Gonadotropin releasing hormone (GnRH) causes the anterior pituitary gland to release other hormones (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]). Agonists of GnRH are synthetic forms of this hormone which do not directly induce follicle development or ovulation but which have become very important in ART therapy.
GnRH agonists such as Leuprolide (Lupron®) initially stimulate the anterior pituitary gland to release all the stored gonadotropins (LH and FSH -the hormones that normally stimulate ovarian function). Over the course of a week to 10 days, GnRH analogs suppress the production of any new LH and FSH. This effect appears to prevent the ovaries from receiving mixed signals from the patient’s own LH and FSH and from the medications that are administered to stimulate follicle development. The result for many patients is a more synchronized development of mature eggs (oocytes).
There are several advantages to using GnRH agonists:
- They make ovarian stimulation easier to regulate, since the patient’s own hormone production (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]) is suppressed.
- Patients who are treated with GnRH agonists tend to produce a greater proportion of mature eggs than patients who do not receive them.
- GnRH agonists markedly decrease the risk of cycle cancellation for most patients. Prior to their use, 20-50 percent of IVF cycles were canceled because patients would have a premature luteinizing hormone (LH) surge with spontaneous ovulation. Using GnRH agonists, the risk of cycle cancellation is less than 5 percent.
- Ovarian function can be suspended with GnRH agonists for variable periods of time if necessary, which allows for flexibility in cycle scheduling.
GnRH agonists Dosage and Monitoring
The GnRH agonist used most commonly is leuprolide acetate (Lupron®). Lupron® is not US Food and Drug Adminstration (FDA) approved for use in IVF treatment but has been used successfully in IVF for 20 years. Lupron® must be injected to be active. In ART therapy, a formulation of Lupron® is used, which can be injected just under the skin, in a manner similar to insulin injections for diabetes therapy.
The usual dosage of Lupron® is 0.1 or 0.2 ml daily as a single injection. Menstruation usually occurs four to 10 days later. During the time of actual ovarian stimulation, the dosage of Lupron® is halved (e.g., 0.1 ml to 0.05 ml daily). Lupron is usually administered until the day of human chorionic gonadotropin (hCG) administration. Some patients, because of their history or condition, are treated with a different dosage or schedule of Lupron®.
In some protocols, a GnRH analog can be used to trigger ovulation of mature follicles in place of human chorionic gonadotropin (hCG). This is usually reserved for patients with a risk of ovarian hyperstimulation.
Another GnRH analog used in ART therapy is nafarelin acetate (Synarel®). Synarel® is administered as a nasal spray. The usual starting dose is two sprays twice a day. The timing of administration is identical to Lupron®. The dosage of Synarel® is usually halved (e.g., from two sprays twice a day to one spray twice a day) when ovarian stimulation is begun.
Gonadotropin releasing hormone (GnRH) Antagonists
Gonadotropin releasing hormone (GnRH) Antagonists also called “anti- gonadotropin-releasing hormones” bind to the receptor for gonoadotropin releasing hormone on the anterior pituitary gland, preventing the natural luteinizing hormone (LH) surge and ovulation. This is important to the success of an IVF cycle, when premature ovulation could reduce the number of eggs retrieved.
Gonadotropin releasing hormone (GnRH) Antagonists such as cetrorelix acetate (Cetrotide®) and ganirelix acetate (Antagon®) are started later in the cycle than leuprolide acetate (Lupron®) and directly and immediately inhibit luteinizing hormone (LH) and follicle-stimulating hormone (FSH) production. Protocols that use GnRH Antagonists may require fewer injections. Ultrasound measurements of follicular growth are used to determine when to start these medicines.
Gonadotropins
To increase likelihood of pregnancy through ART, multiple eggs must be produced. This is accomplished through the administration of gonadotropins, hormonal medications that directly stimulate the ovaries. Stimulation can be achieved with a variety of drug regimens. Gonadotropin medications come in several forms; Repronex® (menotropins for injection) and Menopur® (menotropins for injection) are a combination of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). They replace a woman’s own LH and FSH which are normally produced by the anterior pituitary gland. Bravelle®, Follistim® AQ Cartridge for use with Follistim Pen®, Follistim® AQ Vial, Gonal-F®, and Gonal-F® RFF Pen are preparations that contain only FSH. Follistim® AQ Cartridge for use with Follistim Pen®, Follistim® AQ Vial, Gonal-F®, and Gonal-F® RFF Pen are recombinant products which are made by genetically engineered cells. This process ensures uniform purity and potency. Because the dose of hormones that are used in ART is greater than what the body normally produces, the ovaries typically develop more than one oocyte as occurs in a natural cycle.
Gonadotropins act directly on the ovary to stimulate the growth of follicles (the structures in the ovaries which contain eggs). Granulosa cells within the follicles grow and develop which cause the follicles to enlarge and fill with follicular fluid. These developing follicles can be counted and measured using transvaginal ultrasound. As the follicles grow, they produce increasing amounts of estrogen, which can be measured with a laboratory blood test. Some physicians prefer one formulation or another. Your doctor can discuss this with you in more detail.
Gonadotropins Dosage and Monitoring
Gonadotropins are packaged in vials containing 37.5, 75 or 150 International Units (IU). Follistim AQ Pens and Gonal-F RFF Pens are packaged in pre-mixed injectable pens. Multi-dose vials of some medications are also available. Dosage may vary depending on the patient’s history. Patients will then have regularly scheduled transvaginal ultrasound examinations and serum estradiol tests. The dose of gonadotropins is then determined by the result of the ultrasound and estradiol tests. Most women require between seven to 10 days of gonadotropin therapy.
Bravelle®, and Repronex® are administered subcutaneously or by intramuscular injection, usually into the muscles of the buttocks. Gonal-F,® Follistim,® Follistim AQ Pens, and Gonal-F RFF Pens are administered subcutaneously, like an insulin or allergy shot.
Human Chorionic Gonadotropin (hCG)
Human chorionic gonadotropin (hCG) is an injectable medication that is administered to complete egg maturation. Human chorionic gonadotropin is structurally similar to the LH that is produced by a woman’s pituitary gland. It acts on the ovary in a manner similar to a woman’s own LH. Human chorionic gonadotropin, like LH, stimulates the final maturation of the oocytes in the follicle. It also stimulates progesterone production from the ovary after egg retrieval. This progesterone is important to prepare the uterus for implantation of the embryo.
Dosage and Administration
Human chorionic gonadotropin (hCG) are Profasi®, Ovidrel®, Novarel®,and Pregnyl® can be administered several different ways. The commonly administered dose is a single injection of 10,000 units. Once hCG is administered, ovulation usually occurs in approximately 36 to 40 hours. Therefore oocyte retrieval is routinely scheduled at 34-36 hours after hCG. This helps ensure maximal egg maturity, which is important for fertilization and embryo development. Occasionally, several doses of 2,500 units (usually every three days) are administered after egg retrieval to stimulate progesterone production. If your response to stimulation is particularly exuberant, the dose of hCG can be reduced to 5,000 units in an attempt to reduce the risk of ovarian hyperstimulation syndrome.
It typically takes 8-10 days for single injection of 10,000 units of hCG to be cleared from the blood stream. As hCG is the same hormone that is produced by a developing pregnancy, patients should not have a blood or urine pregnancy test sooner than 10 days following the hCG injection. If a pregnancy test is performed earlier, it may measure the hCG that was given by injection rather than measure hCG produced by a pregnancy.
What if I don’t respond to the drugs for ovarian stimulation?
A response to ovarian stimulation depends on a number of different factors, the most important include available eggs, appropriate hormone levels, proper administration of any medications and lifestyle/environmental factors. In order to respond to ovarian stimulation, a woman must have eggs available to respond; this is sometimes referred to as ovarian reserve. If a woman has diminished ovarian reserve (identified by a high blood levels of follicle stimulation hormone (FSH), low blood levels of anti Müllerian hormone (AMH) or a low antral follicle count on ultrasound), she may not have as robust (or any) response to ovarian stimulation. For these patients, an alternate stimulation protocol may be tried or donated eggs may be used (from a woman known or unknown to the patient).
It is possible that a woman does have the necessary eggs but lacks the appropriate pituitary hormones to respond. In this case, using a different medication- one which may contain both follicle stimulation hormone (FSH) and luteinizing hormone (LH) may allow for an optimal response. Lifestyle factors can also affect a woman’s response to ovarian stimulation. Optimizing your weight, diet and stress and cessation of use of tobacco, alcohol and recreational substances can also improve a response to ovarian stimulation. Speak to your doctor regarding improving your particular response to ovarian stimulation.
Egg Retrieval
Egg retrieval is the procedure to collect the eggs from one or both of your ovaries. It takes place in your doctor’s office or a clinic. The procedure is done 34 to 36 hours after the final shot of fertility medicine human chorionic gonadotropin (hCG) and before ovulation.
- Before egg retrieval, you’ll be given medicine to help you relax and keep you from feeling pain.
- An ultrasound device is placed into your vagina to find follicles, which are the sacs in the ovaries that each contain an egg. Then a thin needle is inserted into an ultrasound guide to go through the vagina and into the follicles to collect the eggs. This process is called transvaginal ultrasound aspiration.
- If your ovaries can’t be reached through the vagina this way, an ultrasound of the stomach area may be used to guide the needle through the stomach and into the ovaries.
- The eggs are removed from the follicles through a needle connected to a suction device. Multiple eggs can be removed in about 20 minutes.
- After the procedure, you may have cramping and feelings of fullness or pressure.
- Mature eggs are placed in a liquid that helps them develop. Eggs that appear healthy and mature will be mixed with sperm to attempt to create embryos. But not all eggs are able to be fertilized with success.
Egg retrieval is usually accomplished by transvaginal ultrasound aspiration, a minor surgical procedure that can be performed in the doctor’s office or an outpatient center. Some form of pain medication is generally administered. An ultrasound probe is inserted into the vagina to identify the follicles, and a needle is guided through the vagina and into the follicles.
The eggs are aspirated (removed) from the follicles through the needle connected to a suction device. Removal of multiple eggs can usually be completed in less than 30 minutes. Some women experience cramping on the day of the retrieval, but this sensation usually subsides by the next day. Feelings of fullness and/or pressure may last for several weeks following the procedure because the ovaries remain enlarged. In some circumstances, one or both ovaries may not be accessible by transvaginal ultrasound. Laparoscopy may then be used to retrieve the eggs using a small telescope placed in the umbilicus.
Fertilization
After the eggs are retrieved, they are examined in the laboratory for maturity and quality. Mature eggs are placed in an IVF culture medium and transferred to an incubator to await fertilization by the sperm.
Sperm is separated from semen usually obtained by masturbation or in a special condom used during intercourse. Alternatively, sperm may be obtained from the testicle, epididymis, or vas deferens from men whose semen is void of sperm either due to an obstruction or lack of production.
Two common methods can be used to try to fertilize eggs with sperm:
- Conventional insemination. Healthy sperm and mature eggs are mixed and kept in a controlled environment called an incubator.
- Intracytoplasmic sperm injection (ICSI). A single healthy sperm is injected right into each mature egg. Often, ICSI is used when semen quality or number is an issue. Or it might be used if fertilization attempts during prior IVF cycles didn’t work.
In certain situations, other procedures may be recommended before embryos are placed in the uterus. These include:
- Assisted hatching. About 5 to 6 days after fertilization, an embryo “hatches” from the thin layer that surrounds it, called a membrane. This lets the embryo attach to the lining of the uterus. If you’re older and you want to get pregnant, or if you have had past IVF attempts that didn’t work, a technique called assisted hatching might be recommended. With assisted hatching a hole is made in the embryo’s membrane just before the embryo is placed in the uterus. This helps the embryo hatch and attach to the lining of the uterus. Assisted hatching is also useful for eggs or embryos that were frozen, as that process can harden the membrane.
- Preimplantation genetic testing (PGT). Embryos are allowed to develop in the incubator until they reach a stage where a small sample can be removed. The sample is tested for certain genetic diseases or the correct number of threadlike structures of DNA, called chromosomes. There are usually 46 chromosomes in each cell. Embryos that don’t contain affected genes or chromosomes can be transferred to the uterus. Preimplantation genetic testing can lower the chances that a parent will pass on a genetic problem. It can’t get rid of the risk completely. Prenatal testing may still be recommended during pregnancy.
Fertilization may be accomplished by insemination, where motile sperm are placed together with the eggs and incubated overnight or by intracytoplasmic sperm injection (ICSI), where a single sperm is directly injected into each mature egg. In the United States, ICSI is performed in approximately 60% of ART cycles. ICSI is usually performed when there is a likelihood of reduced fertilization e.g., poor semen quality, history of failed fertilization in a prior IVF cycle. Overall, pregnancy and delivery rates with ICSI are similar to the rates seen with traditional IVF. Genetic counseling is advisable before ICSI if inherited abnormalities are identified that may be passed from father to son.
Usually 65% to 75% of mature eggs will fertilize after insemination or intracytoplasmic sperm injection (ICSI). Lower rates may occur if the sperm and/or egg quality are poor. Occasionally, fertilization does not occur at all, even if ICSI was used.
Two days after the egg retrieval, the fertilized egg has divided to become a 2- to 4-cell embryo. By the third day, a normally developing embryo will contain approximately 6 to 10 cells. By the fifth day, a fluid cavity forms in the embryo, and the placenta and fetal tissues begin to separate. An embryo at this stage is called a blastocyst. Embryos may be transferred to the uterus at any time between one and six days after the egg retrieval. If successful development continues in the uterus, the embryo hatches from the surrounding zona pellucida and implants into the lining of the uterus approximately 6 to 10 days after the egg retrieval.
Assisted hatching
Assisted hatching is a micromanipulation procedure in which a hole is made in the zona pellucida just prior to embryo transfer to facilitate hatching of the embryo. Although assisted hatching has not been demonstrated definitively to improve live birth rates, assisted hatching may be used for older women or couples who have had unsuccessful prior IVF attempts. There is no clear benefit of assisted hatching to improve pregnancy or live birth rates in other groups of IVF patients.
Preimplantation genetic testing (PGT)
Preimplantation genetic testing (PGT) also called preimplantation genetic diagnosis (PGD) is performed at some centers to screen for inherited diseases. In preimplantation genetic testing (PGT), one or two cells are removed from the developing embryo and tested for a specific genetic disease. Embryos that do not have the gene associated with the disease are selected for transfer to the uterus.
Preimplantation genetic testing (PGT) procedures require specialized equipment and experience together with IVF (in a couple who may otherwise not need IVF to conceive). Some couples, especially those who are carriers of genetic diseases, consider embryo screening beneficial in reducing the risk of having an affected child. While preimplantation genetic testing (PGT) can reduce the likelihood of conceiving a pregnancy with an affected child, it cannot eliminate the risk. Confirmation with chorionic villus sampling (CVS), amniocentesis, or other testing is still necessary.
Embryo Transfer
The next step in the IVF process is the embryo transfer. No anesthesia is necessary, although some women may wish to have a mild sedative. Your doctor identifies your cervix using a vaginal speculum. One or more embryos suspended in a drop of culture medium are drawn into a transfer catheter (a long, thin sterile tube) with a syringe on one end. Your doctor gently guide the tip of the transfer catheter through the cervix and places the fluid containing the embryos into the uterine cavity. The embryo transfer procedure is usually painless, although some women experience mild cramping.
The number of embryos transferred will depend on the embryo stage, embryo quality, maternal age, and patient preference. Since each embryo has a fair probability of implantation and development, the number of embryos to be transferred should be determined for each patient, taking into account the odds of achieving a pregnancy based on the number of embryos transferred weighed against the risk of multiple pregnancy. The American Society for Reproductive Medicine recommends no more than two embryos to be transferred in women 37 years-old or less, no more than three embryos in 38 to 40 year-olds as well as in women 41 to 42 years of age 7. A higher number of cleavage stage embryos can be transferred due to the lower likelihood of successful implantation; no more than two embryos in women < 35 years of age, no more than three embryos in women 35 to 37 years of age, no more than four embryos in women 38 to 40 and in women 41 to 42 years of age, five or fewer embryos. These guidelines have been effective in helping US ART programs maintain their high success rates while significantly decreasing the number of high-order multiple pregnancies (triplets and higher). The reproductive endocrinologist or embryologist will discuss this with you prior to the transfer.
To optimize embryo implantation and a continuing pregnancy, progesterone supplementation is initiated on the day of egg retrieval or embryo transfer. Excess good quality embryos are cryopreserved for future use.
If the procedure works, an embryo will attach to the lining of your uterus about 6 to 10 days after egg retrieval.
After the embryo transfer, you can get back to your usual daily routine. Your ovaries may still be enlarged, so vigorous activities or sex might cause discomfort. Ask your doctor how long you should stay away from these activities.
Typical side effects of embryo transfer include:
- Passing a small amount of clear or bloody fluid shortly after the procedure. This is due to the swabbing of the cervix before the embryo transfer.
- Breast tenderness due to high estrogen levels.
- Mild bloating.
- Mild cramping.
- Constipation.
Call your doctor if you have moderate or severe pain, or heavy bleeding from the vagina after the embryo transfer. You’ll likely to need to get checked for complications such as infection, twisting of an ovary and ovarian hyperstimulation syndrome (OHSS).
Should I have my IVF center perform Assisted Hatching on my embryos before transfer?
In vivo hatching of the embryo is a critical component of the physiologic events culminating in implantation. Conversely, the failure to hatch may be one of the many factors limiting human reproductive efficiency. The clinical application of assisted hatching has been proposed as one approach toward the enhancement of implantation and pregnancy rates following in vitro fertilization (IVF). The assisted hatching procedure entails the creation of a gap in the outer area of the embryo called the zona. This is done either by drilling with an acid medium, by laser, or by using a piezomicromanipulator. Success rates following the use of assisted hatching in different ART programs have varied considerably. Well-designed studies suggest that assisted hatching might best be used in patients > 38 years old or with multiple prior failed IVF cycles.
How many embryos should I have transferred back?
The objective of infertility treatment should be the birth of a single, healthy child. Many of the treatment options presented to infertile couples, however, are associated with high risks of multiple births. Moreover, many couples view multiple gestation as desirable and are unaware of the risks they pose to both mother and babies. Couples should understand these potential risks before starting treatment.
The ability to limit the number of embryos or eggs transferred is an effective approach to limit multiple pregnancies. The Society for Assisted Reproductive Technology and the American Society for Reproductive Medicine have published guidelines recommending an optimal number of embryos for transfer based on patient age, embryo quality, and other criteria. In the United States, the decision regarding the number of embryos to transfer is made jointly by the doctor and the patients. This decision should be based upon the best interests of the patient and the future offspring. However, in England no more than three embryos may be transferred in most circumstances. In Canada, a recent Royal Commission recommended the transfer of a maximum of three embryos.
The ultimate goal is to achieve a high pregnancy rate while transferring a single embryo. Recent laboratory improvements have allowed programs to transfer two embryos while maintaining acceptable pregnancy rates. Eventually, the transfer of one embryo will resolve the issues surrounding multiple pregnancies.
Some clinics see more than the average number of patients with difficult infertility problems. Some clinics are willing to offer assisted reproductive technology (ART) to most potential users, even those who have a low probability of success. Others discourage such patients or encourage them to use donor eggs, a practice that results in higher success rates among older women. Clinics that accept a higher percentage of women who previously have had multiple unsuccessful ART cycles will generally have lower success rates than clinics that do not. In contrast, clinics that offer ART procedures to patients who might have become pregnant with less technologically advanced treatment will have higher success rates than clinics that do not.
What is the difference between a day 3 and day 5 transfer?
Usually embryos are transferred on day 3 or 5. The day of transfer depends on embryo quantity quality, patient characteristics, and laboratory practices. You should discuss this decision with your doctor.
What are my options if I decide not to use my stored embryos?
If you have stored embryos that you have decided not to transfer into your uterus to attempt pregnancy, you have four options for their final disposition. First, you can donate your embryos to another woman with fertility problems that you don’t know so that she can attempt pregnancy through a process called “anonymous embryo donation”. Second, you can donate your embryos to another woman that you do know so that she can attempt pregnancy though a process called “directed embryo donation”. Third, you can donate your embryos for laboratory research to help improve pregnancy rates for infertile couples in the future. Finally, you can ask that your embryos be thawed and discarded. In both of these last two situations, your embryos will not be transferred into another person and no child will be born as a result.
Embryos Freezing
Extra embryos remaining after the embryo transfer may be frozen or cryopreserved for future transfer. Embryos Freezing or cryopreservation makes future ART cycles simpler, less expensive, and less invasive than the initial IVF cycle, since the woman does not require ovarian stimulation or egg retrieval. Once frozen, embryos may be stored for prolonged periods, and live births have been
reported using embryos that have been frozen for almost 20 years. However, not all embryos survive the freezing and thawing process, and the live birth rate is lower with cryopreserved embryo transfer. Couples should decide if they are going to cryopreserve extra embryos before undergoing IVF.
There are two methods used to cryopreserve embryos: conventional (slow) freezing and “vitrification” or fast freezing. Your center will determine which method is best to use based on their experience and the developmental stage at which the embryos are frozen. Although some reports claim that fast freezing (vitrification) may have higher success rates after thawing or warming, this is not the case at all centers.
It should also be noted that more and more ART centers are cryopreserving eggs (oocytes) prior to fertilization. This is done most commonly in young women who are about to undergo treatments or procedures that may affect their future fertility, such as chemotherapy for cancer. However, it is also used for couples who do not wish to freeze embryos because of concerns over their survival during freezing and thawing or the dilemma of what to do with remaining embryos after they have completed their families. Clinic success rates may vary.
Finally, it should be noted that although there are theoretical risks, freezing of sperm, eggs, and embryos is very safe. There have been no documented cases of infectious disease transmission, nor do the risks of birth defects, chromosomal anomalies, or pregnancy complications appear to be increased compared with using fresh sperm, eggs, or embryos.
Gamete intrafallopian transfer (GIFT)
Gamete intrafallopian transfer (GIFT) is similar to IVF, but the gametes (egg and sperm) are transferred to the woman’s fallopian tubes rather than her uterus, and fertilization takes place in the fallopian tubes rather than in the lab. Another difference is that laparoscopy, a surgical procedure, is necessary to transfer the sperm and egg to the tubes. Gamete intrafallopian transfer (GIFT) is an option only for women who have normal fallopian tubes. Some couples may consider GIFT for religious reasons because eggs are not fertilized outside the body. One limitation of GIFT is
that fertilization cannot be confirmed as with IVF. Today, GIFT comprises less than 1% of ART procedures performed in the United States.
Zygote intrafallopian transfer (ZIFT)
Zygote intrafallopian transfer (ZIFT) technique differs from gamete intrafallopian transfer (GIFT) in that fertilization takes place in the lab rather than the woman’s fallopian tube, but is similar in that the fertilized egg is transferred to the fallopian tube rather than the uterus. Zygote intrafallopian transfer (ZIFT) procedure also requires a laparoscopy. Today, ZIFT comprises less than 1% of ART procedures performed in the United States.
Third-Party Assisted Reproductive Technology
Third-party assisted reproductive technology also called “third-party reproduction” refers to involving someone other than the individual or couple that plans to raise the child (intended parent[s]) in the process to get pregnant 8. This includes using donated eggs, sperm, or embryos and gestational-carrier arrangements, in which the pregnancy is carried by someone other than the intended parent(s). Surrogacy, also sometimes referred to as traditional gestational carrier, is a particular type of gestational-carrier arrangement where the woman who carries the pregnancy also provides the egg. Unless specifically indicated, the term gestational carrier in this booklet will refer to a woman who carries a pregnancy but has no genetic link to the fetus, meaning her egg was not used to achieve the pregnancy.
Third-party assisted reproductive technology can consist of:
- Sperm Donation. Couples can opt for donated sperm if a man does not produce sperm, produces very low numbers of sperm, or has a genetic disease. Donated sperm can be used with intrauterine insemination (IUI) or with IVF.
- Egg Donation. Egg donation may be an option when a woman does not produce healthy eggs that can be fertilized. An egg donor undergoes the superovulation and egg retrieval steps of IVF. The donated egg can then be fertilized by sperm from the woman’s partner. The resulting embryo is placed into the woman’s uterus, which is receptive for implantation because of hormone treatments. Egg donation may be particularly helpful for women who:
- Have primary ovary insufficiency
- Have had chemotherapy or radiation therapy
- Have had surgical removal of the ovaries
- Were born without ovaries
- Are carriers of known genetic diseases
- Are infertile because of poor egg quality
- Are menopausal
- Surrogates and Gestational Carriers. If a woman is unable to carry a pregnancy to term, she and her partner may choose a surrogate or gestational carrier.
- A surrogate is a woman inseminated with sperm from the male partner of the couple. The resulting child will be biologically related to the surrogate and to the male partner. Surrogacy can be used when the female of the couple does not produce healthy eggs that can be fertilized.
- A gestational carrier is implanted with an embryo that is not biologically related to her. This alternative can be used when a woman produces healthy eggs but is unable to carry a pregnancy to term. If needed, egg or sperm donation can also be used in this situation.
- Embryo Donation. Embryo donation also called embryo adoption, allows the recipient mother to experience pregnancy and give birth to her adopted child. Couples who have undergone IVF and completed their families sometimes choose to donate their remaining embryos. An embryo donation agency, such as the National Embryo Donation Center, stores these frozen embryos and mediates adoption with the recipient woman or couple. Communication between the donating and adoptive couple can range from anonymous to a fully open relationship. Reasons a woman may choose embryo adoption include:
- She or her partner is infertile and looking for alternatives to other ART.
- IVF has repeatedly failed.
- She or her partner is concerned about or at high risk for passing on genetic disorders.
- The donated embryo is transferred to the recipient’s uterus. According to the Centers for Disease Control and Prevention (CDC), 50% of transfers with donated frozen embryos result in pregnancy, and 40% result in a live birth 9.
However, third-party reproduction can be socially, ethically, and legally complex. As egg donation has become more common, there has been a reconsideration of the social and ethical impact this technology has had on prospective parents, their offspring, and the egg donors themselves. Surrogacy arrangements are controversial and are subject to both legal and psychosocial scrutiny 8.
Donor sperm, eggs and embryos
IVF may be performed with a couple’s own eggs and sperm or with donor eggs, sperm, or both. A couple may choose to use a donor if there is a problem with their own sperm or eggs, or if they have a genetic disease that could be passed on to a child. Donors may be known or anonymous. In most cases, donor sperm is obtained from a sperm bank. Both sperm and egg donors undergo extensive medical and genetic screening, as well as testing for infectious diseases. Sexually transmitted disease screening and testing for both sperm and egg donation are highly regulated by the US Food and Drug Adminstration (FDA).
Donor sperm is frozen and quarantined for six months, the donor is re-tested for infectious diseases including the human immunodeficiency virus (HIV), and sperm are only released for use if all tests are negative. Donor sperm may be used for insemination or in an ART cycle. Unlike intrauterine insemination (IUI) cycles, the use of frozen sperm in IVF cycles does not lower the chance of pregnancy.
Donor eggs are an option for women with a uterus who are unlikely or unable to conceive with their own eggs. Egg donors undergo much the same medical and genetic screening as sperm donors. Until recently, it has not been possible to freeze and quarantine eggs like sperm. Recent advances in egg freezing have made this a possibility, and there are a few companies and clinics that are using such an approach. The egg donor may be chosen by the infertile couple or the ART program.
Egg donors assume more risk and inconvenience than sperm donors. In the United States, egg donors selected by ART programs generally receive monetary compensation for their participation. Egg donation is more complex than sperm donation and is done as part of an IVF procedure. The egg donor must undergo ovarian stimulation and egg retrieval. During this time, the recipient (the woman who will receive the eggs after they are fertilized) receives hormonal medications to prepare her uterus for implantation. After the egg retrieval, the donor’s eggs are fertilized by sperm from the recipient’s partner and transferred to the recipient’s uterus. The recipient will not be genetically related to the child, but she is a biologic parent in the sense that she will carry the pregnancy and give birth.
Egg donation is expensive because donor selection, screening, and treatment add additional costs to the IVF procedure. However, the relatively high live birth rate for egg donation, over 50% nationally, provides many couples with their best chance for success. Overall, donor eggs are used in nearly 10% of all ART cycles in the United States.
In some cases, when both the man and woman are infertile, both donor sperm and eggs have been used. Donor embryos may also be used in these cases. Some IVF programs allow couples to donate their unused frozen embryos to other infertile couples. Appropriate screening of the individuals whose genetic embryos are used should adhere to federal and state guidelines. The use of donor sperm, eggs, or embryos is a complicated issue that has lifelong implications. Talking with a trained counselor who understands donor issues can be very helpful in the decision-making process. Many programs have a mental health professional on staff or the physician may recommend one. If a couple knows the donor, their physician may suggest that both the couple and the donor speak with a counselor and an attorney. Some states require and most IVF centers recommend an attorney to file paperwork for the couple with the court when donor gametes or embryos are used.
Surrogacy and gestational carrier
A pregnancy may be carried by the egg donor (traditional surrogate) or by another woman who has no genetic relationship to the baby (gestational carrier). If the embryo is to be carried by a surrogate, pregnancy may be achieved through insemination alone or through ART. The surrogate will be biologically related to the child. If the embryo is to be carried by a gestational carrier, the eggs are removed from the infertile woman, fertilized with her partner’s sperm, and transferred into the gestational carrier’s uterus. The gestational carrier will not be genetically related to the child. All parties benefit from psychological and legal counseling before pursuing surrogacy or a gestational carrier.
Assisted reproductive technology risks
Assisted reproductive technology (ART) risks depend upon each specific step of the procedure.
The following are some of the primary risks of ART procedures:
- Ovarian hyperstimulation syndrome (OHSS). Ovarian stimulation carries a risk of hyperstimulation, where the ovaries become swollen and painful. Fluid may accumulate in the abdominal cavity (ascites) and chest (pleural effusion), and the woman may feel bloated, nauseated, and experience vomiting or lack of appetite. Up to 30% of women undergoing ovarian stimulation have a mild case of OHSS that can be managed with over-the-counter painkillers and a reduction in activity. In moderate OHSS, women develop or accumulate fluid within the abdominal cavity (ascites), and gastrointestinal symptoms may occur. These women are monitored closely, but generally do very well with simple outpatient management. The condition tends to resolve without intervention unless pregnancy occurs, in which case recovery may be delayed for several weeks. Up to 2% of women develop severe OHSS characterized by excessive weight gain, fluid accumulation in the abdomen and chest, electrolyte abnormalities, over-concentration of the blood (hemoconcentration), and, in rare cases, the development of blood clots (thromboembolism), kidney failure, or death. It may be medically necessary to drain fluid from the abdomen with a needle if breathing becomes difficult. Women with severe OHSS require hospitalization until the symptoms improve. If pregnancy occurs, OHSS can worsen. Occasionally, termination of pregnancy must be considered in the most severe cases.
- Cancer. Although initial reports suggested that women who use fertility drugs have an increased risk for ovarian cancer, numerous recent studies support the conclusion that fertility drugs are not linked to ovarian cancer. Nevertheless, there is still uncertainty whether a risk exists, and research continues to address this question. An annual gynecologic visit is recommended for all women with examination of the ovaries, regardless of prior use of ovulation medications.
- Risks related to the egg retrieval procedure. Laparoscopy carries the risks of any surgery that requires anesthesia. Removing eggs through an aspirating needle entails a slight risk of bleeding, infection, and damage to the bowel, bladder, or a blood vessel. This is true whether your doctor uses laparoscopy or ultrasound to guide the needle. Less than 1 patient in 1,000 will require major surgery to repair damage from complications of the egg retrieval procedure. In rare cases, infection may occur from the retrieval or embryo transfer.
- Multiple pregnancy. The chance of multiple pregnancy is increased in all assisted reproductivetechnologies when more than one embryo is transferred. Although some would consider twins a happy result, there are many problems associated with multiple births, and problems become progressively more severe and common with triplets and each additional fetus thereafter. Women carrying a multiple pregnancy may need to spend weeks or even months in bed or in the hospital in an attempt to delay preterm delivery. The risk of preterm delivery in multiple pregnancies is high, and babies may be born too early to survive. Premature babies require prolonged and intensive care and risk lifelong handicaps due to premature birth. Some couples may consider multifetal pregnancy reduction to decrease the risks due to multiple pregnancy, but this is likely to be a difficult decision. Data also suggest that IVF conceptions, even singletons, have a slightly increased risk of preterm delivery or low birth weight.
- First-trimester bleeding. First-trimester bleeding may signal a possible miscarriage or ectopic pregnancy. If bleeding or pain before 13 weeks occurs, a medical evaluation is needed to determine the cause. Some evidence suggests that early bleeding is more common in women who undergo in-vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) and is not associated with the same poor prognosis as it is in women who conceive spontaneously.
- Miscarriage may occur after ART, even after ultrasound identifies a pregnancy in the uterus. Miscarriage occurs after ultrasound in nearly 15% of women younger than age 35, in 25% at age 40, and in 35% at age 42 following ART procedures.
- There is approximately a 5% chance of ectopic pregnancy with ART.
- Birth defects. It is not clear whether the risk of birth defects is increased with IVF. Most studies do not show an increased risk, but several studies do. Research is ongoing to determine the amount, if any, of this risk. Furthermore, when intracytoplasmic sperm injection (ICSI) is used in cases of severe male factor infertility, a genetic cause of male infertility may be passed on to the offspring.
Assisted reproductive technologies (ART) involve significant physical, financial, and emotional commitments on the part of the couple. Psychological stress is common, and some couples describe the experience as an emotional roller coaster. The treatments are involved and costly. Patients have high expectations, yet failure is common in any given cycle. Couples may feel frustrated, angry,
isolated, and resentful. At times, frustration can lead to depression and feelings of low self-esteem, especially in the immediate period following a failed ART attempt. The support of friends and family members is very important at this time. Couples are encouraged to consider psychological counseling as an additional means of support and stress management. Many ART programs have a mental health professional on staff to help couples deal with the grief, tension, or anxieties associated with infertility and its treatment.
Assisted reproductive technology success rates
Success rates after assisted reproductive technology (ART) vary and depend on many factors. They can include the clinic or assisted reproductive technology center performing the ART procedure, the infertility diagnosis, and the age of the woman undergoing the procedure. The woman’s age is especially important. To find your chance of having a baby using assisted reproductive technology (ART), you can use the CDC’s 2021 Fertility Clinic Success Rates Report on Assisted Reproductive Technology (ART) Success Rates or the free IVF Success Estimator tool. The CDC’s 2021 Fertility Clinic Success Rates Report on ART Success Rates uses data from 453 reporting fertility clinics in the United States during 2021 using approximately 238,126 patients. This information is calculated based on the experiences of women and couples with similar characteristics. However, the IVF Success Estimator tool does have some limitations. The estimates are based on available data which may not be representative of your specific experience. For example, the IVF Success Estimator tool can generate estimates only for people ranging from age 20 to 50 years. If you are younger than 20 or older than 50 years of age and attempt to enter your true age, the tool will be unable to provide an estimate and you will be directed to enter a value between 20 and 50. You may choose to enter the value that is closest to your true age for an estimation. Available data to generate estimates are similarly limited to specific ranges for height and weight. Additionally, this the IVF Success Estimator tool does not provide medical advice, diagnosis, or treatment. You still need to speak with your doctor about your specific treatment plan and potential for success.
It is also important to understand the definitions of pregnancy rates and live birth rates. For example, a pregnancy rate of 40% does not mean that 40% of women took babies home. Pregnancy does not always result in live birth. A biochemical pregnancy is a pregnancy confirmed by blood or urine tests but not visible on ultrasound, because the pregnancy stops developing before it
is far enough along to be seen on ultrasound. A clinical pregnancy is one in which the pregnancy is seen with ultrasound, but stops developing sometime afterwards. Therefore, when comparing the “pregnancy” rates of different clinics, it is important to know which type of pregnancy is being compared. Most couples are more concerned with a clinic’s live birth rate, which is the probability of delivering a live baby per IVF cycle started. Pregnancy rates, and more importantly live birth rates, are influenced by a number of factors, especially the woman’s age.
According to the 2022 Society for Assisted Reproductive Technology live births rates per intended egg retrieval (all embryo transfers) by age group were as follows:
Women <35 years:
- Live births: 43.1%
- Singleton (percentage of live births): 95.7%
- Twins (percentage of live births): 4.2%
- Triplets (percentage of live births): 0%
Women 35-37 years:
- Live births: 31%
- Singleton (percentage of live births): 96%
- Twins (percentage of live births): 3.9%
- Triplets (percentage of live births): 0.1%
Women 38-40 years:
- Live births: 19%
- Singleton (percentage of live births): 95.7%
- Twins (percentage of live births): 4.3%
- Triplets (percentage of live births): 0%
Women 41-42 years:
- Live births: 9.4%
- Singleton (percentage of live births): 96.3%
- Twins (percentage of live births): 3.7%
- Triplets (percentage of live births): 0%
Women >42 years:
- Live births: 3.2%
- Singleton (percentage of live births): 96.9%
- Twins (percentage of live births): 3.1%
- Triplets (percentage of live births): 0%
Assisted reproductive technology cost
Costs for a Assisted Reproductive Technology (ART) vary from clinic to clinic. Including procedures, imaging, bloodwork, and medications, a single cycle can cost $15,000 to $30,000 or more. Note this is merely an approximation. Some people go through more than one IVF cycle, which can multiply treatment expenses. On average, the medication costs for a single IVF cycle can range from $3,000 to $6,000 per cycle. These are rough estimates, and the actual costs can differ based on individual circumstances, medication choices, and insurance coverage. Additional expenses may include loss of wages from time missed at work and expenses incurred due to travel and/or accommodations. In addition, the medications utilized in in-vitro fertilization (IVF) procedures (gonadotropins, hCG, GnRH analogs, or GnRH antagonists) are very expensive. All of these factors must be considered in determining the financial feasibility of participation in an in-vitro fertilization (IVF) program.
Do insurance plans cover infertility treatment?
The degree of services covered depends on where you live and the type of insurance plan you have. Many insurance companies do offer coverage for the various stages of the evaluation and treatment of infertility. Exactly what is covered often varies from insurer to insurer, and from policy to policy. Some insurers will only cover the examination and testing to determine the cause of infertility, while others will cover treatment and medications. The kind of treatment that is covered also varies. For example, some policies may cover intrauterine insemination (IUI) when used in conjunction with medication to boost ovulation, but may not cover in vitro fertilization (IVF) where as others may cover both. Fourteen states currently have laws that require insurers to either cover or offer to cover some form of infertility diagnosis and treatment. Those states are Arkansas, California, Connecticut, Hawaii, Illinois, Maryland, Massachusetts, Montana, New Jersey, New York, Ohio, Rhode Island, Texas and West Virginia. However, the laws vary greatly in their scope of what is and is not required to be covered. For more information about the specific laws for each of those states, please call your state’s Insurance Commissioner’s office or to learn about pending insurance legislation in your state, please contact your State Representatives.
It is also important to study your policy to determine if, and to what extent, you may be covered. Many fertility practices have financial counselors to help you through this, to answer your questions about your insurance benefits and to work with the insurer to determine what is covered.
Whether or not you live in a state with an infertility insurance law, you may want to consult with your employer’s director of human resources to determine the exact coverage your plan provides. Another good source of assistance is RESOLVE, an infertility patient advocacy and information organization.
When to end ART treatment?
Studies indicate that the chance for pregnancy in consecutive IVF cycles remains similar in up to 4 cycles. However, many other factors should be considered when determining the appropriate endpoint in therapy, including financial and psychological factors. Members of the IVF team can help couples decide when to stop treatment and discuss other options such as egg and/or sperm donation or adoption, if appropriate. Your doctor, support groups, and other couples undergoing infertility treatment can provide valuable support and guidance.
- Assisted Reproductive Technology. Fertility Clinic and National Summary Report 2021. https://www.cdc.gov/art/reports/2021/pdf/Report-ART-Fertility-Clinic-National-Summary-H.pdf[↩]
- American Society for Reproductive Medicine. (2012). Intrauterine insemination. http://www.fertilityanswers.com/wp-content/uploads/2016/04/intrauterine-insemination-iui.pdf[↩][↩]
- Infertility Workup for the Women’s Health Specialist: ACOG Committee Opinion, Number 781. Obstet Gynecol. 2019 Jun;133(6):e377-e384. doi: 10.1097/AOG.0000000000003271[↩]
- In vitro fertilization (IVF). https://www.mayoclinic.org/tests-procedures/in-vitro-fertilization/about/pac-20384716[↩][↩]
- American Society for Reproductive Medicine. (2015). Assisted reproductive technologies: A guide for patients. http://www.fertilityanswers.com/wp-content/uploads/2016/04/assisted-reproductive-technologies-booklet.pdf[↩]
- In-Vitro Fertilization (IVF). https://www.yashodahealthcare.com/blogs/in-vitro-fertilization-ivf/[↩]
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: [email protected]; Practice Committee of the Society for Assisted Reproductive Technology. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. 2017 Apr;107(4):901-903. https://doi.org/10.1016/j.fertnstert.2017.02.107[↩]
- American Society for Reproductive Medicine. (2012). Third-party reproduction (sperm, egg, and embryo donation and surrogacy): A guide for patients. https://www.reproductivefacts.org/globalassets/_rf/news-and-publications/bookletsfact-sheets/english-pdf/third-party_reproduction_booklet_web.pdf[↩][↩]
- 2021 National ART Summary. https://www.cdc.gov/art/reports/2021/summary.html[↩]