Contents
- Keratoconjunctivitis
- Table 1. Non-conjunctivitis causes of red eye
- Does a red or pink eye always mean infection?
- Can I catch keratoconjunctivitis?
- How long is keratoconjunctivitis contagious?
- Can Visine be used for keratoconjunctivitis?
- Can I use breast milk for keratoconjunctivitis?
- How do I clean my eyes with keratoconjunctivitis?
- Measles and conjunctivitis
- Keratoconjunctivitis causes
- Keratoconjunctivitis prevention
- Keratoconjunctivitis signs and symptoms
- Keratoconjunctivitis complications
- Keratoconjunctivitis diagnosis
- Keratoconjunctivitis treatment
- Keratoconjunctivitis sicca
- Allergic keratoconjunctivitis
- Allergic keratoconjunctivitis causes
- Allergic keratoconjunctivitis signs and symptoms
- Allergic keratoconjunctivitis complications
- Allergic keratoconjunctivitis diagnosis
- Allergic keratoconjunctivitis treatment
- Table 3. Allergic conjunctivitis treatment options
- Eye drops for allergic conjunctivitis
- Antihistamine eye drops
- Mast cell stabilizers
- Antihistamine and mast cell stabilizer eye drops
- Dual-Acting Antihistamine–Mast Cell Stabilizing Agents
- Leukotriene Receptor Antagonists
- Topical Vasoconstrictors (Decongestants)
- Combination eye drops including decongestant (Antihistamine-Vasoconstrictor Combinations)
- Other eye drops, to prevent allergy symptoms
- Oral antihistamines (tablets and syrups)
- Topical nonsteroidal anti-inflammatory drugs (NSAIDS)
- Corticosteroids
- Supratarsal steroids
- Topical Calcineurin inhibitors
- Biologicals
- Immunotherapy
- Surgery
- Allergic conjunctivitis home remedies
- Allergic conjunctivitis prognosis
- Vernal keratoconjunctivitis
- Vernal conjunctivitis causes
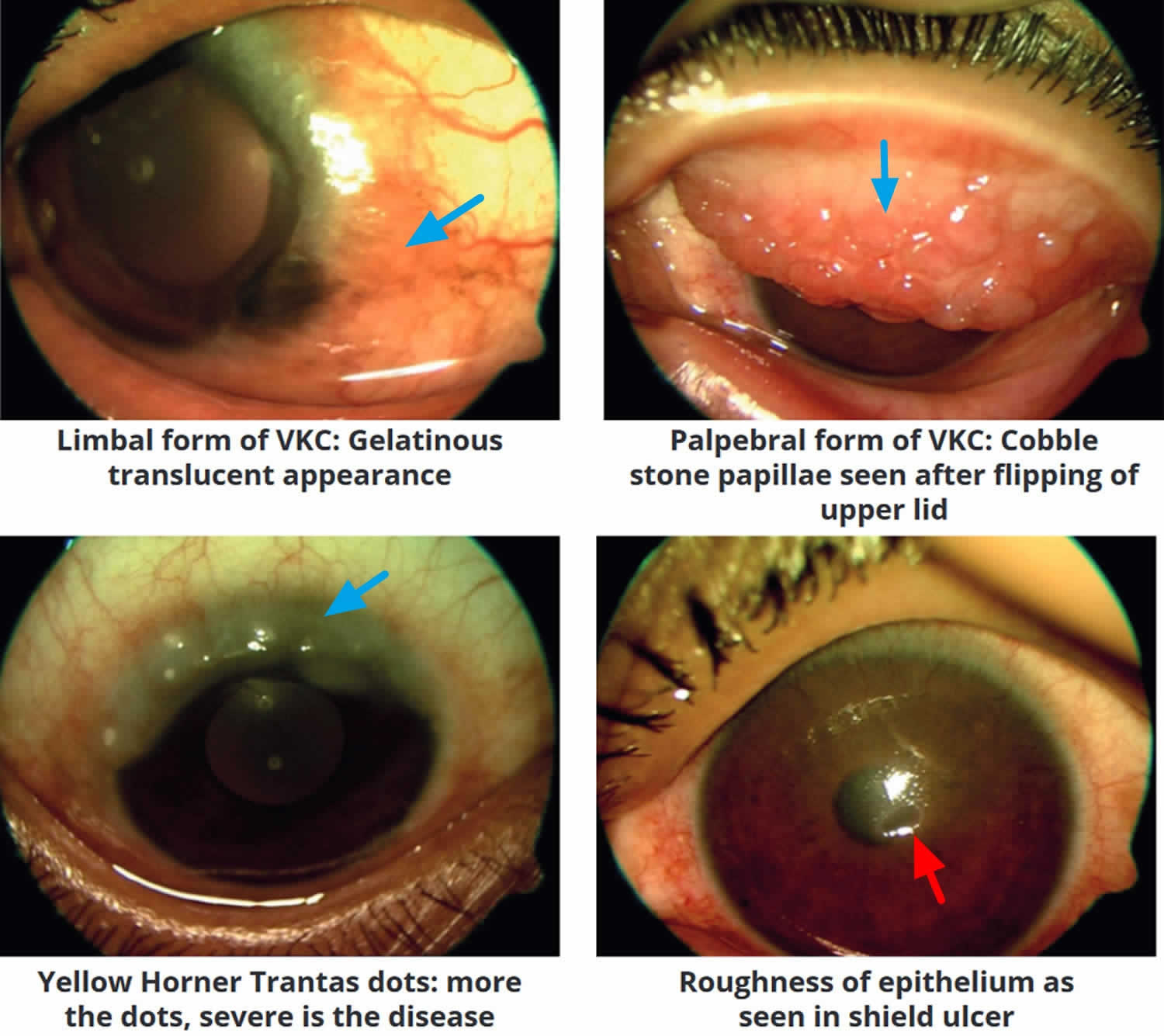
- Vernal conjunctivitis types
- Vernal conjunctivitis signs and symptoms
- Vernal conjunctivitis complications
- Vernal conjunctivitis differential diagnosis
- Vernal conjunctivitis treatment
- Vernal conjunctivitis prognosis
- Atopic keratoconjunctivitis
- Viral keratoconjunctivitis
- Herpes keratoconjunctivitis
- Herpetic keratoconjunctivitis cause
- Risk factors for developing herpetic keratoconjunctivitis
- Herpetic keratoconjunctivitis symptoms
- Herpetic keratoconjunctivitis complications
- Herpetic keratoconjunctivitis diagnosis
- Herpetic keratoconjunctivitis differential diagnosis
- Herpetic keratoconjunctivitis treatment
- Herpetic keratoconjunctivitis prognosis
- Bacterial keratoconjunctivitis
- Bacterial keratoconjunctivitis causes
- Bacterial keratoconjunctivitis signs and symptoms
- Bacterial keratoconjunctivitis complications
- Bacterial keratoconjunctivitis diagnosis
- Bacterial keratoconjunctivitis differential diagnosis
- Bacterial keratoconjunctivitis treatment
- Bacterial keratoconjunctivitis prognosis
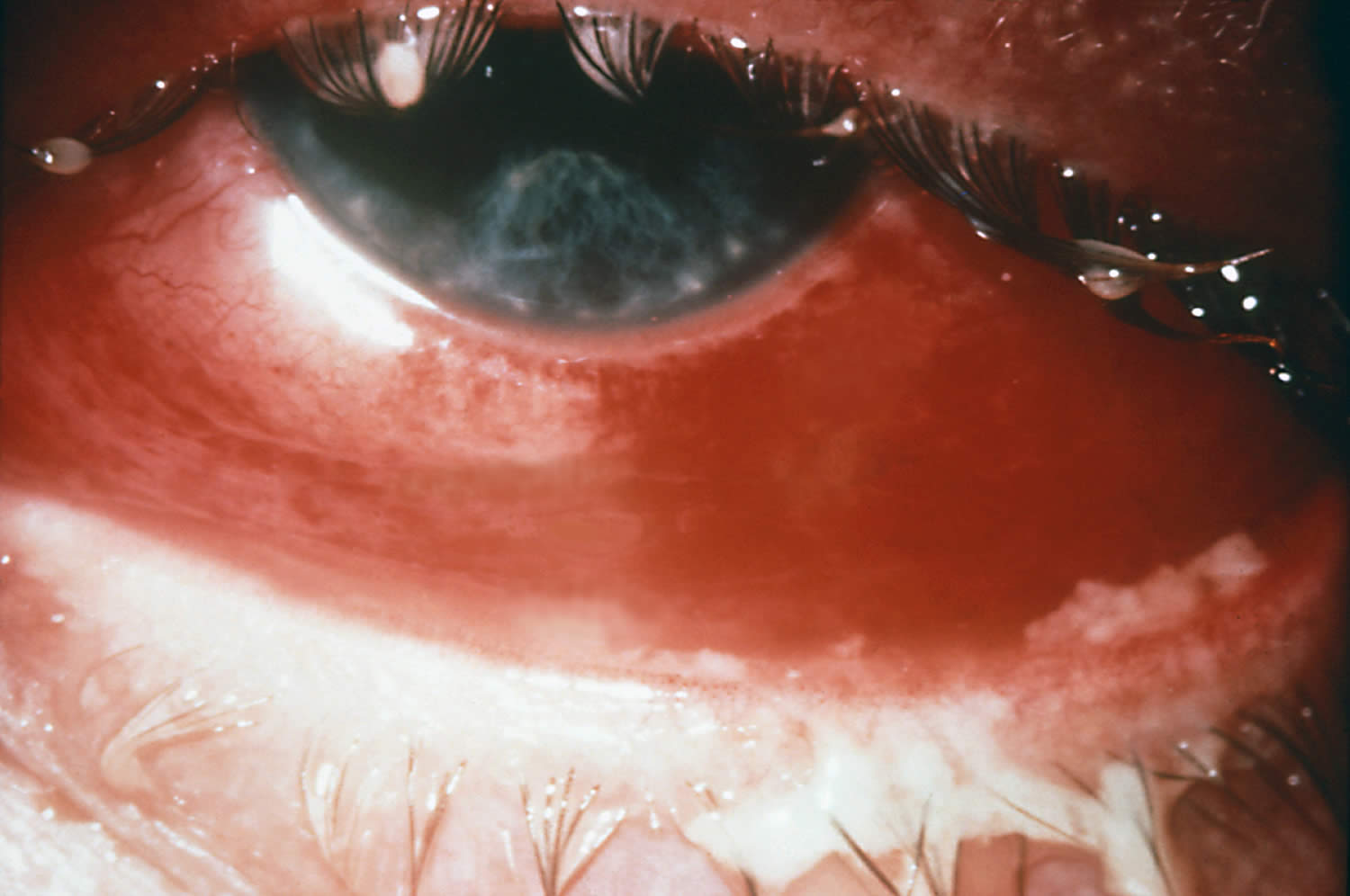
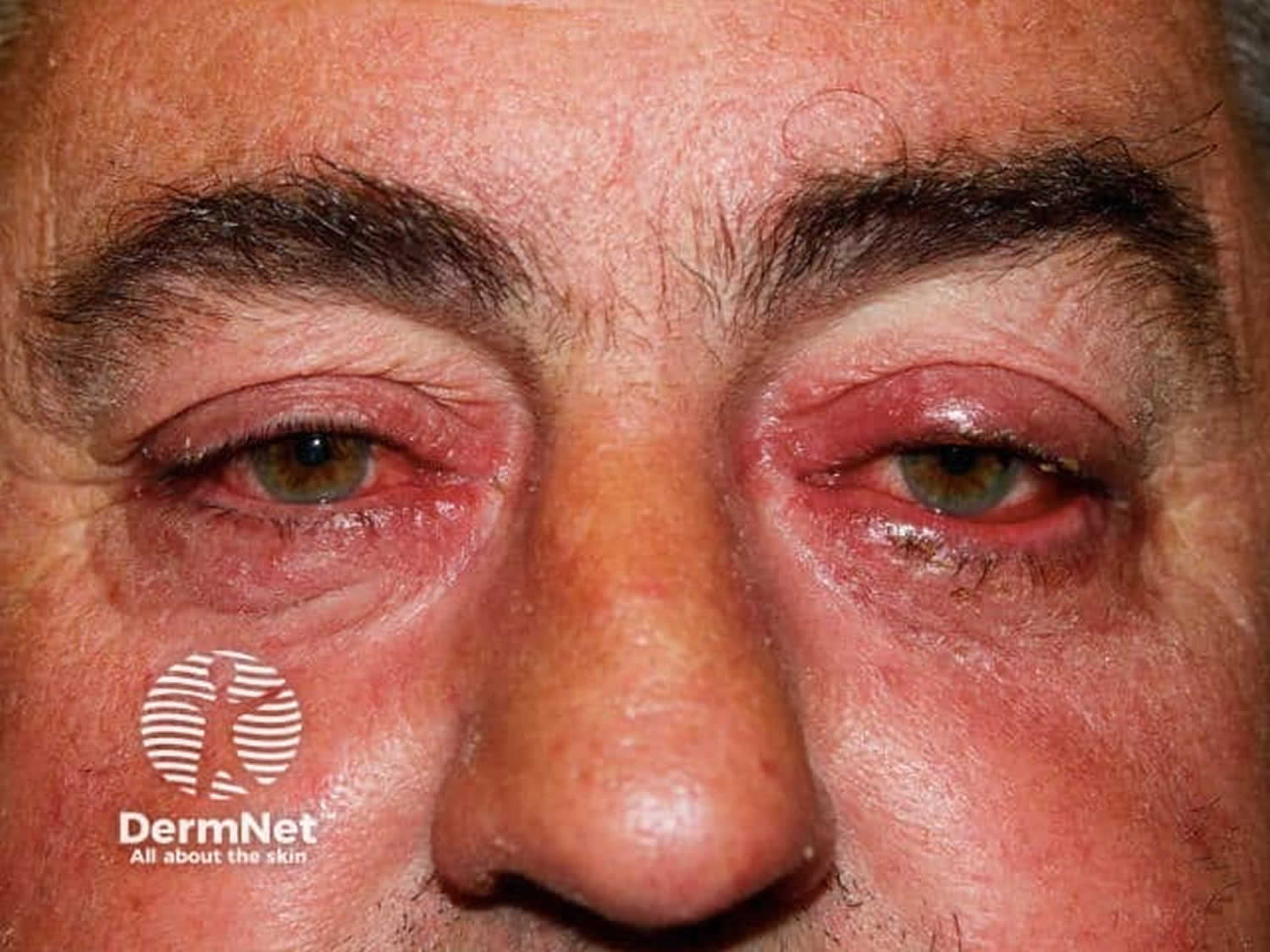
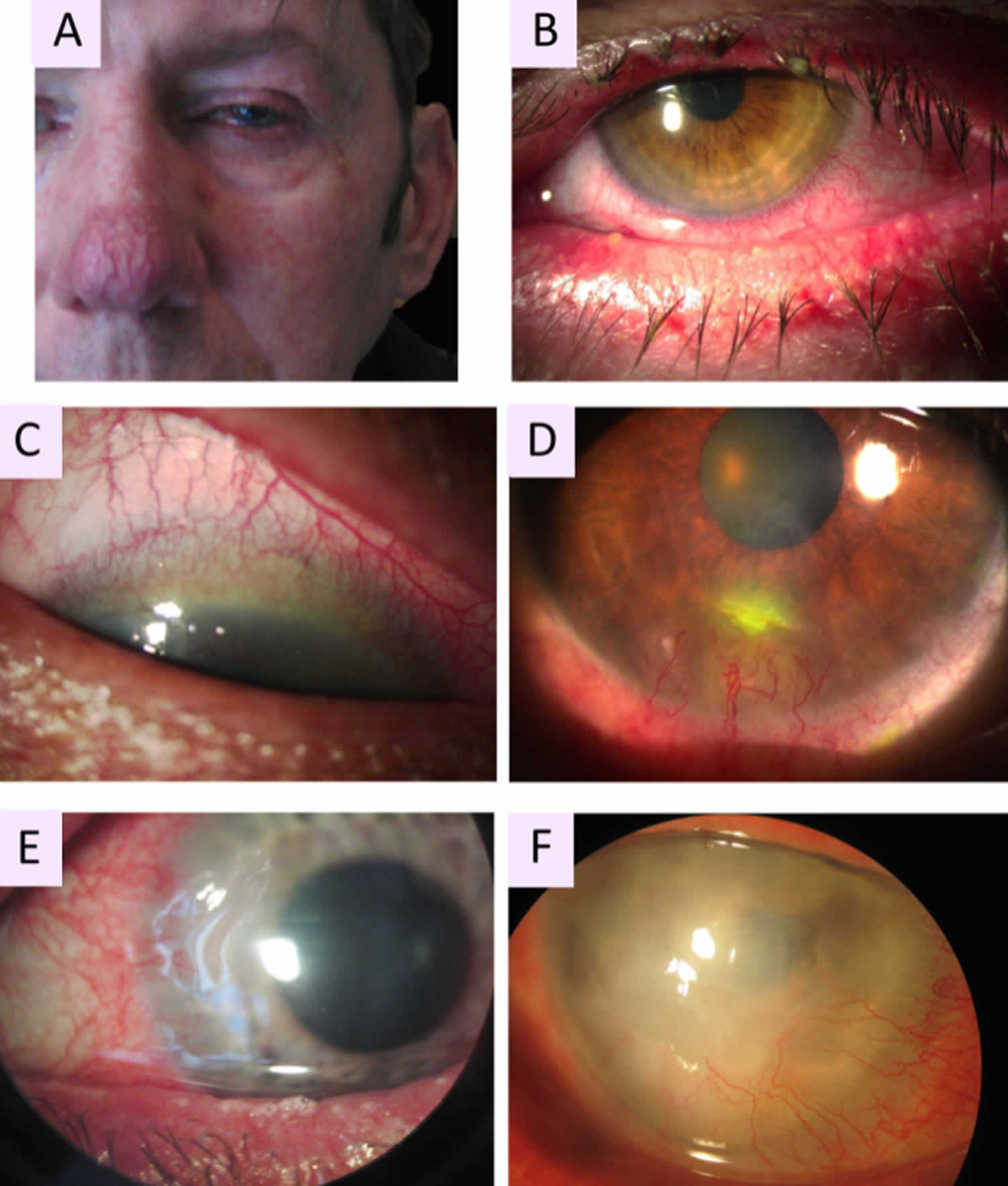
- Rosacea keratoconjunctivitis
Keratoconjunctivitis
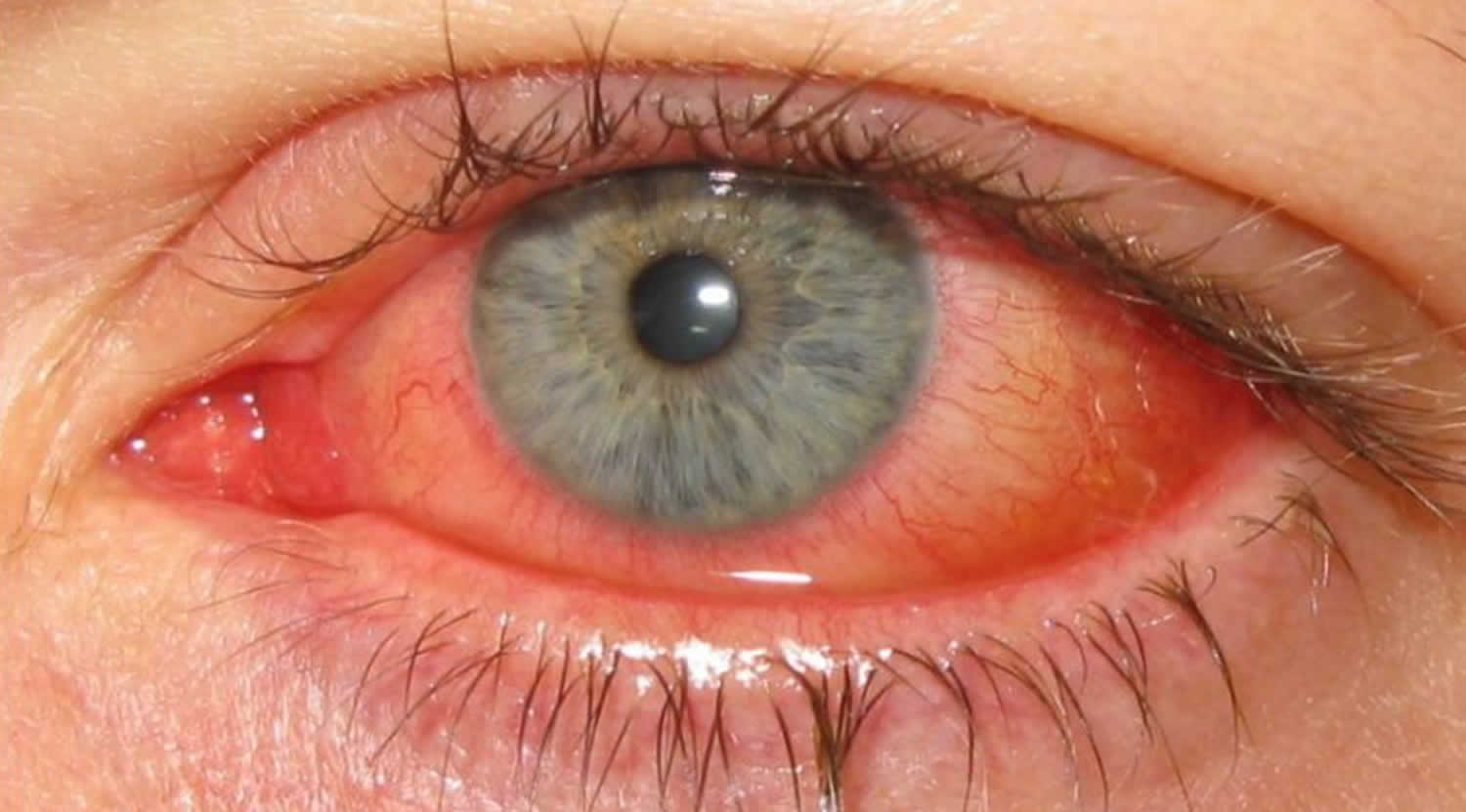
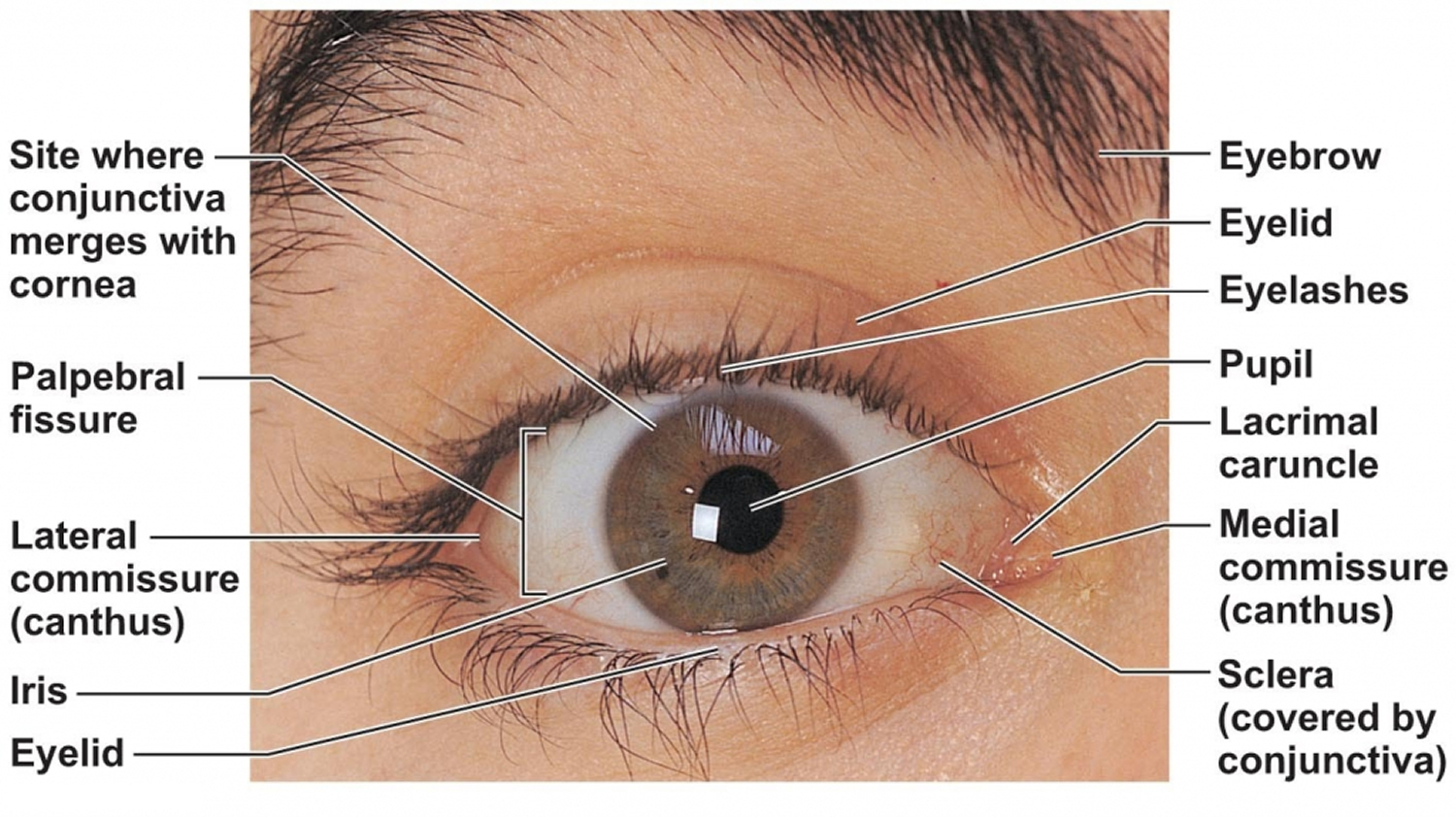
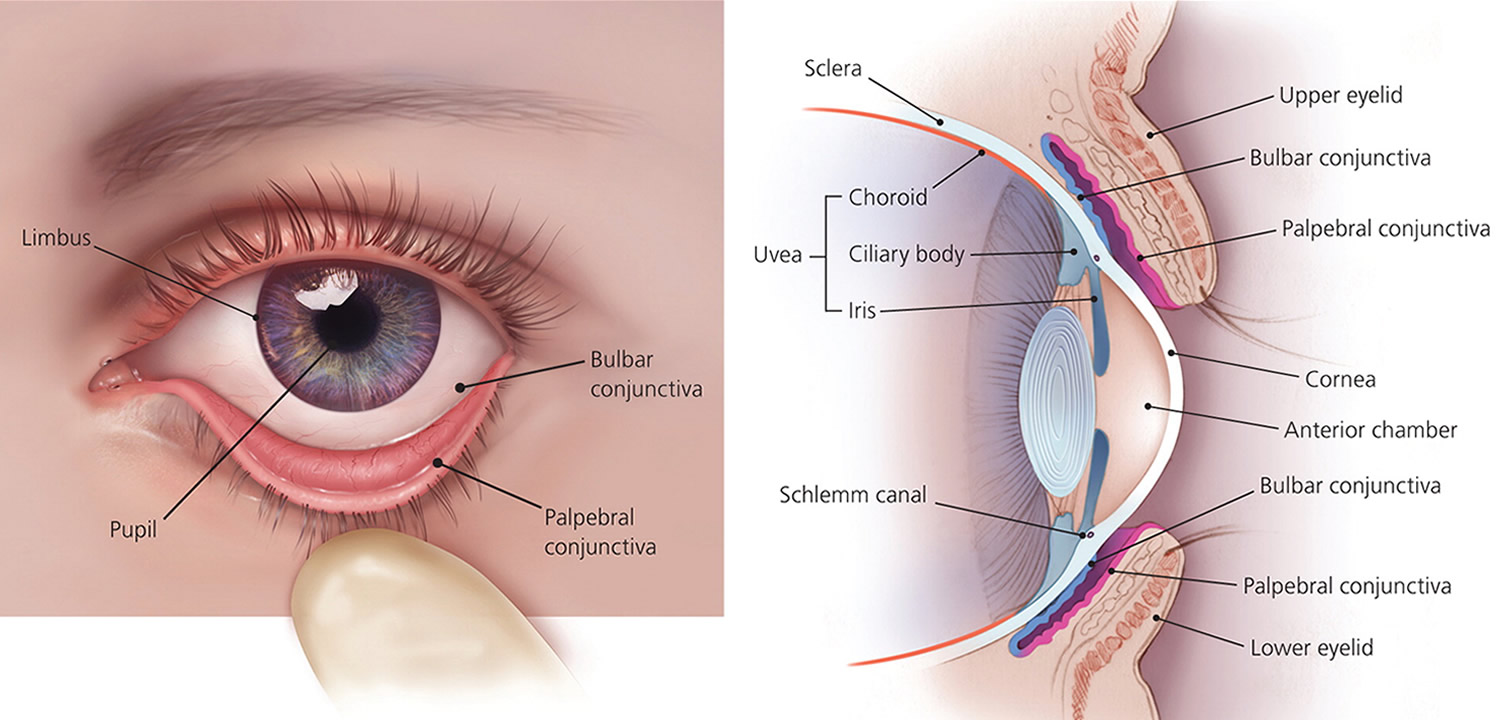
Keratoconjunctivitis also called pink eye, red eye or sticky eye is the inflammation or infection of the conjunctiva (conjunctivitis) that extends to the cornea (keratitis). The cornea is the clear, outermost layer of your eye (immediately in front to the anterior chamber, iris, and pupil) that allows light to enter your and helps focus it. The conjunctiva is the transparent membrane that lines inside your eyelid and covers the white part of your eyeball. The conjunctiva is a thin translucent mucous membrane that can be divided based on the location into palpebral conjunctiva (inside of the eyelids) and bulbar conjunctiva (begins at the edge of the cornea and covers the visible part of the sclera) (Figure 2). The conjunctiva contains nonkeratinizing, squamous epithelium and a thin, richly vascularized substantia propria containing lymphatic vessels and cells, such as lymphocytes, plasma cells, mast cells, and macrophages. The conjunctiva also has accessory lacrimal glands and goblet cells. When the small blood vessels in the conjunctiva become inflamed or infected, they’re more visible. This is what causes the whites of your eyes to appear reddish or pink. Conjunctivitis can be caused by bacterial or viral infection or an allergic reaction. “Pink eye” is most often associated with the bacterial infection or bacterial keratoconjunctivitis. Bacterial keratoconjunctivitis is more common in children, and viral keratoconjunctivitis is more common in adults. Keratoconjunctivitis can cause swelling, itching, burning, discharge, and redness. Though conjunctivitis can be irritating, it rarely affects your vision. Depending on what kind of keratoconjunctivitis you have and how bad it is, treatments can help ease the discomfort of conjunctivitis. Both viral and bacterial keratoconjunctivitis are very contagious and easily spread from one person to another. They are spread through direct or indirect contact with the liquid that drains from the eye of someone who’s infected. One or both eyes may be affected. Therefore, early diagnosis and treatment can help limit its spread. Bacterial and viral keratoconjunctivitis is a common cause of school absences and can spread quickly in schools. Viral keratoconjunctivitis usually gets better in a couple of weeks without treatment. However, you’ll need to see a doctor for bacterial keratoconjunctivitis to get treatment with antibiotic eye drops or ointment. Bacterial keratoconjunctivitis symptoms include yellow discharge, pus that causes the eyelids to stick together, and puffy eyelids. A viral eye infection does not lead to drainage or pus in and around your eye. Viral keratoconjunctivitis main symptom is eye redness. Both viral and bacterial keratoconjunctivitis can occur along with colds or symptoms of a respiratory infection, such as a sore throat. Wearing contact lenses that aren’t cleaned properly or aren’t your own can cause bacterial keratoconjunctivitis.
There are many causes of keratoconjunctivitis:
- Bacterial infection. Bacterial keratoconjunctivitis is the second most common cause and is responsible for the majority (50%-75%) of cases in children; it is observed more frequently from December through April 1.
- Viral infection. Viral keratoconjunctivitis is the most common cause of infectious keratoconjunctivitis both overall and in the adult population and is more prevalent in summer 2, 3, 4, 5, 6, 7, 8, 1.
- Allergies. Allergic keratoconjunctivitis is the most frequent cause of conjunctivitis, affecting 15% to 40% of the population and is observed more frequently in spring and summer 1, 9. Allergic keratoconjunctivitis may be seasonal, or triggered by specific allergens, for example, pollen or animal dander (skin cells that are shed by animals with hair, fur or feathers).
- Substances that cause irritation
- Contact lens products, eye drops, or eye ointments
- A chemical splash in the eye
- A foreign object in the eye
- In newborns, a blocked tear duct.
Keratoconjunctivitis can be divided into infectious and noninfectious causes. Viruses and bacteria are the most common infectious causes. Noninfectious keratoconjunctivitis includes allergies, irritation when the eyes are in contact with chemicals and cicatricial (scarring) keratoconjunctivitis, as well as keratoconjunctivitis secondary to immune-mediated diseases and neoplastic processes 10. Keratoconjunctivitis can also be classified into acute, hyperacute, and chronic according to the mode of onset and the severity of the clinical response 11. Furthermore, keratoconjunctivitis can be either primary or secondary to systemic diseases such as gonorrhea, chlamydia, graft-vs-host disease, and Reiter syndrome, in which case systemic treatment is warranted 10. The eye may look similar to you no matter what is causing the keratoconjunctivitis.
Check if you have keratoconjunctivitis
The main symptom of keratoconjunctivitis is red or pink eyes, often with itching, watering or discomfort.
Keratoconjunctivitis (pink eye) usually affects both eyes and makes them:
- Bloodshot
- Puffy eyes or swelling of the eyelids
- Burn or gritty feeling in one or both eyes
- Produce pus that sticks to lashes
- Itchy eyes
- Watery eyes
- Sensitive to light called photophobia.
If you have bacterial keratoconjunctivitis, you may also have yellow or green sticky discharge from the eyes. This can make your eyelids stick together, especially when you wake up from sleep.
If you have viral keratoconjunctivitis, one or both eyes might be affected, and the discharge is likely to be clear.
If you have allergic keratoconjunctivitis, both eyes are usually affected with a clear discharge. You might also have hay fever symptoms, such as an itchy nose, watery eyes and sneezing. Symptoms can be all year round or at certain times of the year (seasonal).
See your doctor or eye doctor (ophthalmologist) right away if:
- You’re in pain or are having trouble seeing
- You become sensitive to light
- Your symptoms have continued for a week or more, or are getting worse
- Your eye is producing a lot of pus or mucus
- You have any other symptoms of an infection, like fever, muscle ache or fatigue.
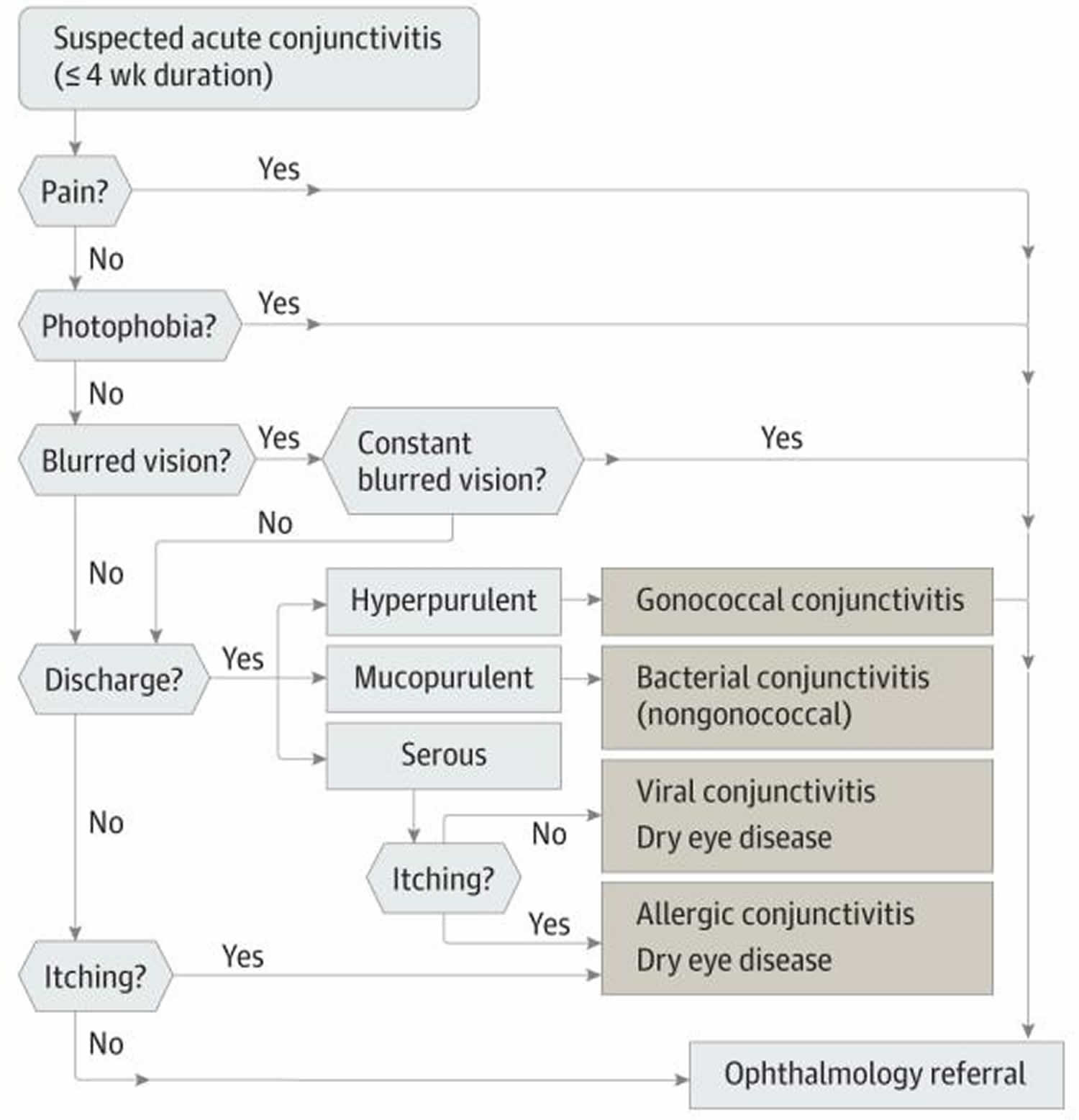
It is important to differentiate keratoconjunctivitis from other sight-threatening eye diseases that have similar clinical presentation and to make appropriate decisions about further testing, treatment, or referral. An algorithmic approach using a focused eye history along with a penlight eye examination may be helpful in diagnosis and treatment (Figure 3). Because keratoconjunctivitis and many other ocular diseases can present as “red eye”, the differential diagnosis of red eye and knowledge about the typical features of each disease in this category are important (see Table 1).
If keratoconjunctivitis is left untreated, severe symptoms and complications may occur including:
- Corneal scarring or ulceration: Persistent inflammation or infection can lead to damage to the cornea, potentially resulting in scarring or even ulceration.
- Infection spread: Untreated bacterial or viral keratoconjunctivitis can spread to other parts of your eye or to other people.
- Chronic conjunctivitis: Prolonged inflammation may result in long-lasting or recurring conjunctivitis, which can impact the quality of life.
- Vision loss and blindness: In extreme cases, untreated keratoconjunctivitis may lead to vision loss or blindness due to corneal damage or complications from severe infections.
Bacterial and viral conjunctivitis is highly contagious. Here’s how to avoid spreading it:
- Basic hygiene is enough to keep from spreading the infection to other people or your other eye.
- Change pillowcases and sheets every day.
- Use a fresh towel every day.
- Wash your hands often, especially after you touch your eyes.
- Don’t wear your contact lenses until your eyes are back to normal.
- Don’t share anything that touches your eyes.
- People who wear contact lenses need to stop wearing their contacts as soon as pink eye symptoms begin. If your symptoms don’t start to get better within 12 to 24 hours, make an appointment with your eye doctor to make sure you don’t have a more serious eye infection related to contact lens use.
Keratoconjunctivitis treatment depends on the cause. If the keratoconjunctivitis is caused by bacteria, antibiotic drops or ointment may be needed. It is important to use any prescription medication for the full number of days prescribed in order to prevent the infection from coming back and reduce antibiotic resistance in bacteria. In most cases, you won’t need antibiotic eye drops. Since keratoconjunctivitis is usually viral, antibiotics drops or ointments do not help viral, allergic or irritative conjunctivitis.
Viral keratoconjunctivitis, which is often seen with other symptoms like sore throat, runny nose and cough, does not respond to antibiotics and antibiotic medications are not needed. Viral keratoconjunctivitis often begins in one eye and then infects the other eye within a few days. Your symptoms should gradually clear on their own. This typically takes around 2 to 3 weeks. Antiviral medicines may be an option if your viral keratoconjunctivitis is caused by the herpes simplex virus (HSV).
Allergic keratoconjunctivitis is treated with antihistamine eye drops or allergy medicines by mouth and artificial tear drops. Some nasal sprays for hay fever are also helpful. Sometimes your doctor might suggest tests to help you find the allergic trigger.
Bacterial infections may require antibiotic eye drops or ointment. It’s important to keep applying the medicine for several days after your symptoms have improved.
Keratoconjunctivitis treatment is usually focused on symptom relief. Your doctor may recommend:
- Using artificial tears.
- Cleaning your eyelids with a wet cloth.
- Applying cold or warm compresses several times daily.
To help relieve your symptoms and prevent infection:
- Wash your hands thoroughly before touching your eyes.
- Wash your eye gently several times a day with clean cotton wool pad soaked in warm tap water.
- Use a new cotton wool pad for each eye, to prevent passing the infection into your other eye.
- Gently clean any eye discharge from your eye area. Always wipe from the corner of the eye (nearest the nose) outwards.
- If you wear contact lenses and have an infection, throw out your lenses. Wear glasses for at least a week after your symptoms have disappeared.
- Throw out any eye makeup or eyelash extensions used right before or during an eye infection.
If you wear contact lenses, you’ll be advised to stop wearing them until treatment is complete. Your doctor will likely recommend that you throw out soft contacts you’ve already worn. Disinfect hard lenses overnight before you reuse them. Ask your doctor if you should discard and replace your contact lens accessories, such as the lens case used before or during the illness. Also replace any eye makeup used before your illness.
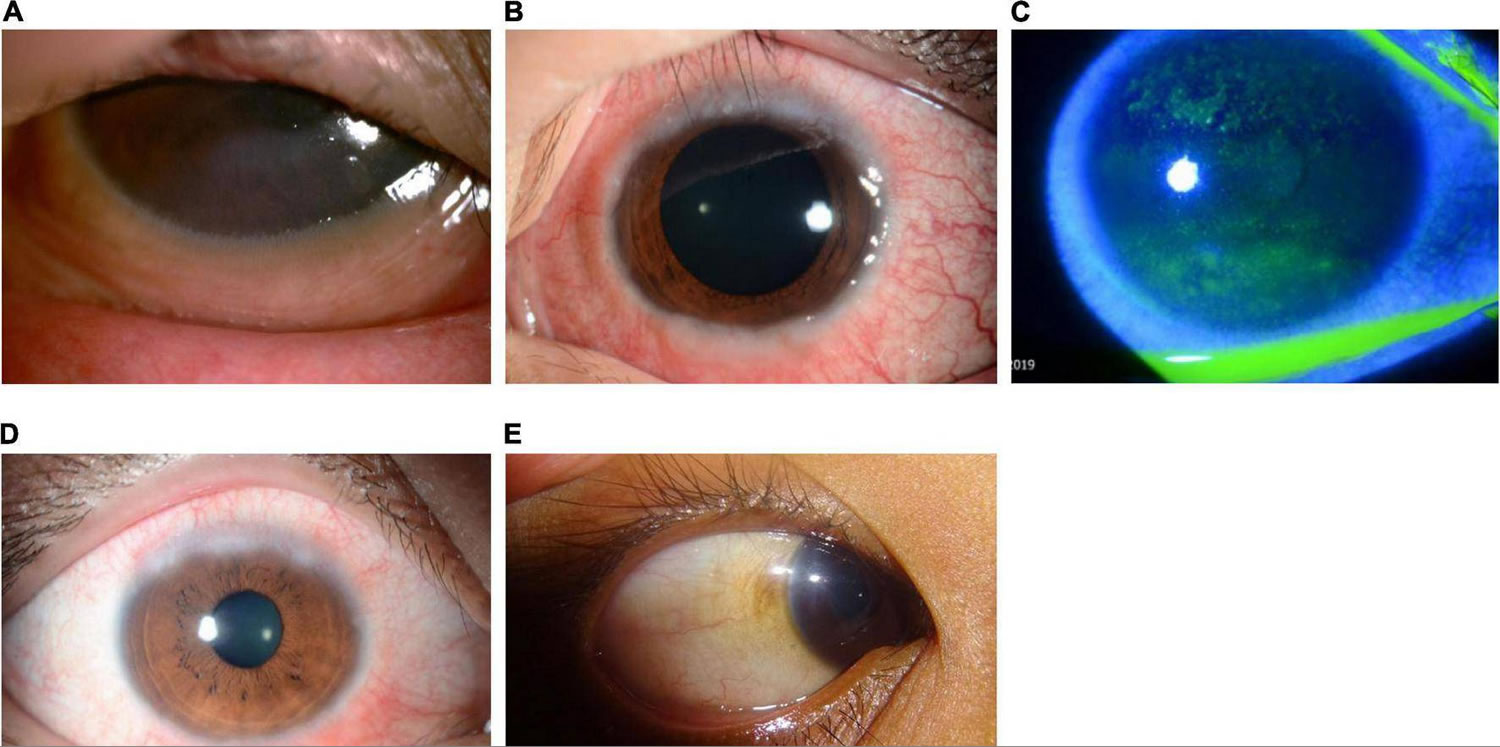
Figure 1. Human eye
Figure 2. Eye anatomy
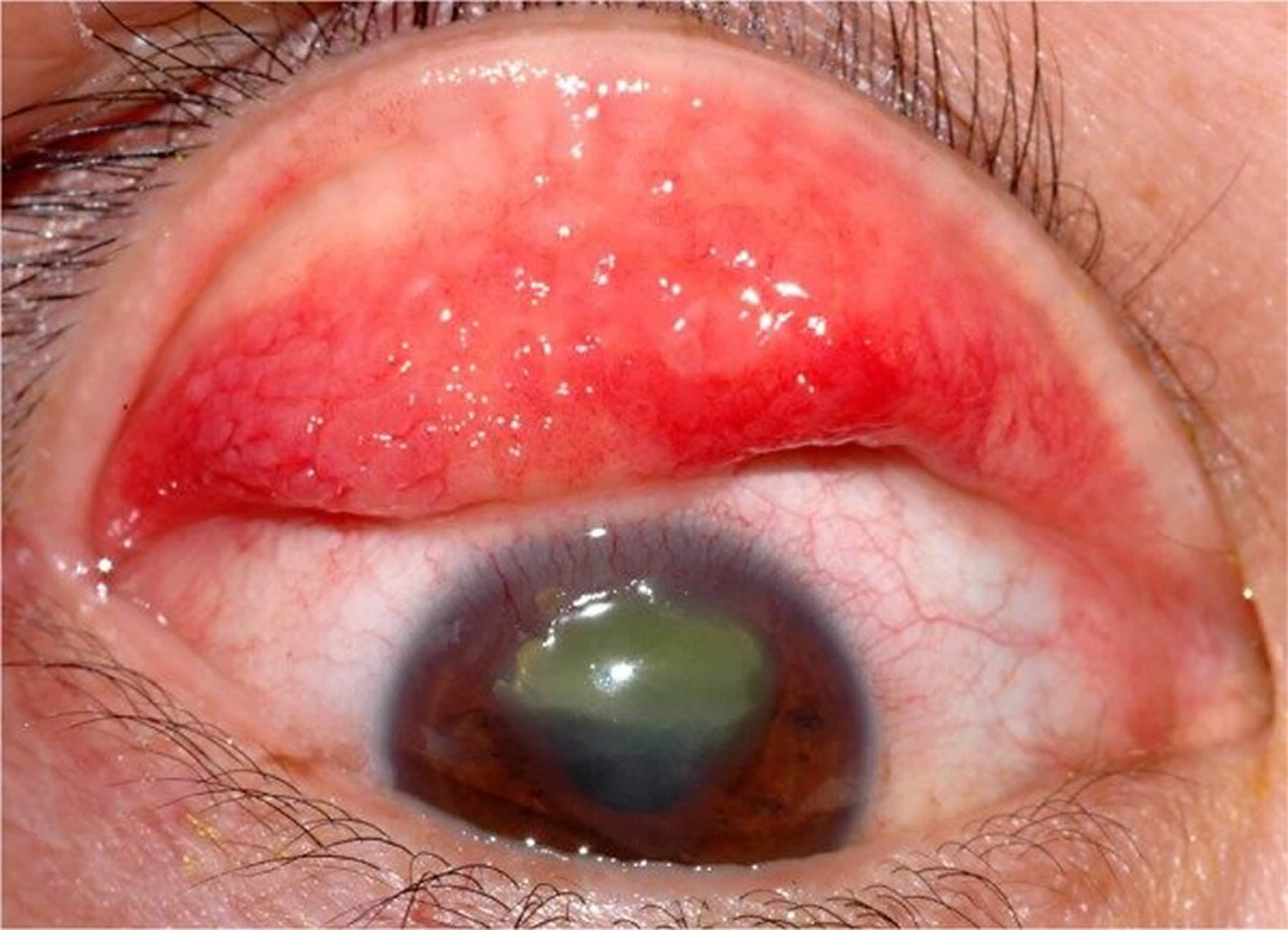
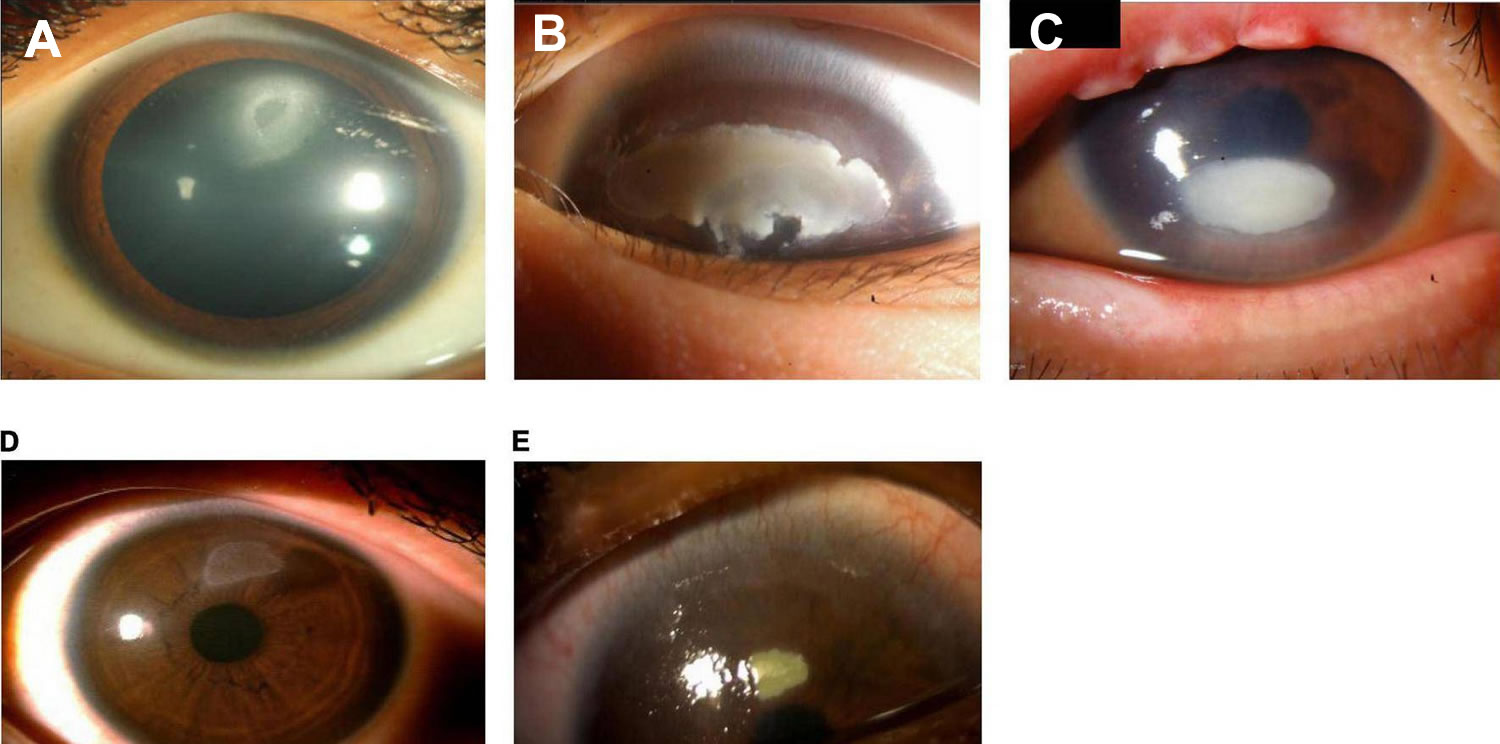
Figure 3. Keratoconjunctivitis
Figure 4. Acute keratoconjunctivitis diagnostic algorithm
[Source 12 ]Table 1. Non-conjunctivitis causes of red eye
| Differential Diagnosis | Symptoms | Penlight Examination Findings |
|---|---|---|
| Dry eye disease | Burning and foreign-body sensation. Symptoms are usually transient, worse with prolonged reading or watching television because of decreased blinking. Symptoms are worse in dry, cold, and windy environments because of increased evaporation. | Bilateral redness |
| Blepharitis | Similar to dry eyes | Redness greater at the margins of eyelids |
| Uveitis | Photophobia, pain, blurred vision. Symptoms are usually bilateral. | Decreased vision, poorly reacting pupils, constant eye pain radiating to temple and brow. Redness, severe photophobia, presence of inflammatory cells in the anterior chamber. |
| Angle closure glaucoma | Headaches, nausea, vomiting, ocular pain, decreased vision, light sensitivity, and seeing haloes around lights. Symptoms are usually unilateral. | Firm eye on palpation, ocular redness with limbal injection. Appearance of a hazy/steamy cornea, moderately dilated pupils that are unreactive to light. |
| Carotid cavernous fistula | Chronic red eye; may have a history of head trauma | Dilated tortuous vessels (corkscrew vessels), bruits on auscultation with a stethoscope |
| Endophthalmitis | Severe pain, photophobia, may have a history of eye surgery or ocular trauma | Redness, pus in the anterior chamber, and photophobia |
| Cellulitis | Pain, double vision, and fullness | Redness and swelling of lids, may have restriction of the eye movements, may have a history of preceding sinusitis (usually ethmoiditis) |
| Anterior segment tumors | Variable | Abnormal growth inside or on the surface of the eye |
| Scleritis | Decreased vision, moderate to severe pain | Redness, bluish sclera hue |
| Subconjunctival hemorrhage | May have foreign-body sensation and tearing or be asymptomatic | Blood under the conjunctival membrane |
Footnotes: There are some eye conditions which may look similar to keratoconjunctivitis.
[Sources 13, 14, 12 ]There are serious eye conditions that can cause eye redness. These conditions may cause eye pain, a feeling that something is stuck in your eye (foreign body sensation), blurred vision and light sensitivity. If you experience these symptoms, seek urgent care.
Make an appointment with your doctor if you notice any signs or symptoms you think might be pink eye. Pink eye can be highly contagious for as long as two weeks after signs and symptoms begin. Early diagnosis and treatment can protect people around you from getting pink eye too.
People who wear contact lenses need to stop wearing their contacts as soon as pink eye symptoms begin. If your symptoms don’t start to get better within 12 to 24 hours, make an appointment with your eye doctor to make sure you don’t have a more serious eye infection related to contact lens use.
See your doctor for advice if you have a pink eye that doesn’t start to improve after a few days.
Contact your doctor immediately or go to your nearest emergency room if:
- you have a painful pink eye or red eye
- you have other symptoms, including any changes in your vision like wavy lines or flashing, sensitivity to light (photophobia), a severe headache and feeling sick
- you’ve recently injured your eye, particularly if something has pierced it
- your baby has red eyes – get an urgent medical care if your baby is less than 28 days old
- you wear contact lenses and have conjunctivitis symptoms as well as spots on your eyelids – you might be allergic to the lenses
- your symptoms haven’t cleared up after 2 weeks
- sensitivity to light
- intense redness in one eye or both eyes
Does a red or pink eye always mean infection?
No. A pink or red eye may be a sign of another eye problem such as allergy, foreign body (something stuck in the eye), contact lens reaction, inflammation inside the eye, or glaucoma (high eye pressure).
Can I catch keratoconjunctivitis?
You can catch keratoconjunctivitis from droplets from the eyes, mouth and throat of an infective person. This can happen through touch, coughing or sneezing. You can also catch it from contact with objects that were contaminated with infectious eye secretions, such as towels, face washers and tissues.
Allergic conjunctivitis is caused by exposure to allergens such as:
- dust mites
- pollen
- animal dander (skin cells that are shed by animals with hair, fur or feathers)
- mould spores
- occasionally foods or food additives
Allergic conjunctivitis isn’t contagious so it can’t spread from person to person.
How long is keratoconjunctivitis contagious?
Keratoconjunctivitis (pink eye) generally remains contagious as long as you have tearing and matted eyes. Conjunctivitis (pink eye) is commonly caused by viruses or bacteria. Depending on the cause of your conjunctivitis, signs and symptoms usually improve within a few days to two weeks.
Viral and bacterial keratoconjunctivitis can spread very easily as easily as the common cold. If you have an infection in just one eye, be careful not to spread it to the other eye. And be careful not to spread the infection in public, either.
Good hygiene including washing your hands, avoiding close contact with others, and not sharing towels or pillowcases — is important. It may be okay to return to school or child care if your child does not have a fever, can practice good hygiene, and can avoid close contact with others.
Children who are not able to practice good hygiene or can’t avoid close contact with others should stay home until symptoms clear up.
Things to remember:
- You will be contagious as long as there is a discharge from your eye (usually 10-14 days after symptoms start).
- Do not attend work or school until discharge stops.
Check with your doctor if you have any questions about when your child can return to school or child care.
Can Visine be used for keratoconjunctivitis?
No! Whatever kind of keratoconjunctivitis you have, don’t use red-reducing eye drops, like Visine. These kinds of eye drops may be very uncomfortable if you have an infection. They also could make your symptoms worse.
Can I use breast milk for keratoconjunctivitis?
Breast milk could be more harmful than helpful for keratoconjunctivitis. One of the few studies on whether breast milk can fight infections found that it didn’t cure the most common causes of conjunctivitis and worse, breast milk can introduce new bacteria into the eye and cause serious infection. Eye infections in young children can be very serious even capable of causing blindness. Don’t delay seeing a doctor and don’t rely only on folk remedies.
There is lots of bad advice about conjunctivitis on the internet. Never put anything in your eye that isn’t approved by a doctor. Foods and herbal extracts are not sterile and can make eye conditions much worse. Bloggers who recommend breast milk for conjunctivitis say that substances in breast milk can cure infection and soothe inflammation. But there is no evidence that this helps.
How do I clean my eyes with keratoconjunctivitis?
Gentle cleaning with cotton balls soaked in warm water. Wipe from the inside corner of the eye to the outside corner, dispose of cotton ball, and repeat with a clean cotton ball as necessary. DO NOT try to clean inside the eyelids as this may cause damage to the conjunctiva.
Measles and conjunctivitis
Conjunctivitis can show up before a measles rash or at the same time. Ask these questions about whether conjunctivitis may be a sign of measles:
- Is there a reported outbreak of measles in the area?
- Has the child been vaccinated for measles? If so, then measles conjunctivitis is very unlikely.
- Are there other measles symptoms, like a red, blotchy rash or a high fever (above 104 degrees Farenheit/40 Celsius)? Note that other kinds of conjunctivitis can also cause fever, especially in children. So a mild fever, or fever by itself, isn’t necessarily a sign of measles.
- Is the child sensitive to regular, indoor light? Light-sensitivity is more likely to be a sign of measles-related conjunctivitis. Sensitivity to indoor light is always a sign of a serious eye condition, usually involving sight-threatening damage to the cornea. You should see an eye specialist (ophthalmologist), not just your family doctor or pediatrician.
If you think you or a loved one may have measles-related conjunctivitis, see an ophthalmologist right away and make sure they report it to local health authorities. In some cases, measles can damage the cornea, retina or optic nerve and result in vision loss or blindness.
Keratoconjunctivitis causes
Causes of keratoconjunctivitis include:
- Bacterial infection. Bacterial keratoconjunctivitis is the second most common cause and is responsible for the majority (50%-75%) of cases in children; it is observed more frequently from December through April.
- Viral infection. Viral keratoconjunctivitis is the most common cause of infectious conjunctivitis both overall and in the adult population and is more prevalent in summer.
- Allergies. Allergic keratoconjunctivitis is the most frequent cause, affecting 15% to 40% of the population and is observed more frequently in spring and summer.
- Substances that cause irritation
- Contact lens products, eye drops, or eye ointments
- A chemical splash in the eye
- A foreign object in the eye
- In newborns, a blocked tear duct.
Viral or bacterial keratoconjunctivitis is highly contagious and spread from person. You could develop viral or bacterial keratoconjunctivitis if you come into contact with:
- Discharge from the eyes, nose or throat of an infected person through touch, coughing or sneezing.
- Contaminated fingers or contact with contaminated surfaces or objects.
- Contaminated water while swimming.
Viral or bacterial keratoconjunctivitis can also be transmitted from an infected mother to her baby during vaginal delivery.
Allergic keratoconjunctivitis occurs more commonly among people who already have seasonal allergies. They develop it when they come into contact with a substance that triggers an allergic reaction in their eyes or because of a foreign body.
Risk factors for getting keratoconjunctivitis
Risk factors for keratoconjunctivitis include:
- Exposure to someone infected with the viral or bacterial form of conjunctivitis.
- Exposure to something you’re allergic to, for allergic conjunctivitis.
- Using contact lenses, especially extended-wear lenses.
Keratoconjunctivitis prevention
To prevent the spread of infective keratoconjunctivitis, practice good hygiene. For example:
- Don’t touch your eyes with your hands, unless you have just washed your hands.
- Wash your hands often with soap and warm water or alcohol-based hand sanitiser especially after touching their eyes or putting in eye drops. Hand washing is the best way to avoid getting conjunctivitis and avoid spreading it to others.
- Use a clean towel and washcloth daily.
- Don’t share towels or washcloths.
- Change your pillowcases every day.
- Throw away old eye cosmetics, such as mascara.
- Throw away tissues or cotton balls that have touched your eyes.
- Don’t share eye cosmetics or personal eye care items or eye drops with others.
Keep in mind that keratoconjunctivitis is no more contagious than the common cold. It’s okay to return to work, school or child care if you’re able to practice good hygiene and avoid close contact. However, if work, school or child care involves close contact with others it may be best to stay home until you or your child’s symptoms clear up.
Preventing keratoconjunctivitis in newborns
Newborns’ eyes are susceptible to bacteria present in the mother’s birth canal. These bacteria often cause no symptoms in the mother. In some cases, these bacteria can cause infants to develop a serious form of infective keratoconjunctivitis known as ophthalmia neonatorum, which needs immediate treatment to preserve sight. That’s why shortly after birth, an antibiotic ointment is applied to every newborn’s eyes. The ointment helps prevent eye infection.
Recommendations for contact lenses
If you wear contact lenses, wash your hands before putting in and taking out your lenses. Regularly cleaning and throwing out your lenses can also help keep infections away. Always store your
contact lenses in solutions recommended by contact lens guidelines. Never swim in a lake or hot tub with your contact lenses on. If you have been diagnosed with conjunctivitis, do NOT wear your contact lenses until you have healed, and then, start with a fresh pair of lenses.
Keratoconjunctivitis signs and symptoms
The most common symptoms of keratoconjunctivitis include:
- Redness in one or both eyes.
- Itchiness in one or both eyes.
- A gritty feeling in one or both eyes.
- A discharge in one or both eyes that forms a crust during the night that may prevent your eye or eyes from opening in the morning.
- Tearing.
- Sensitivity to light called photophobia.
Unequal pupil size (anisocoria) and photophobia are associated with serious eye conditions including anterior uveitis, keratitis, and scleritis 15.
Systemic diseases associated with conjunctivitis include skin and mucous membrane diseases (acne rosacea, ichthyosis, xeroderma pigmentosum), collagen vascular diseases (systemic lupus erythematosus, rheumatoid arthritis, Sjogren’s disease, granulomatosis with polyangiitis), and autoimmune disease (graft versus host disease, Steven-Johnson syndrome, and ocular cicatricial pemphigoid).
Keratoconjunctivitis complications
In both children and adults, keratoconjunctivitis can cause inflammation in the cornea called keratitis or corneal ulcer that can cause permanent eye damage and even blindness if they go too long without treatment. Prompt evaluation and treatment by your doctor or eye doctor can reduce your risk of complications. Most people who have keratoconjunctivitis recover within 2-5 days. Rarely, complications can occur.
Conjunctivitis complications can include:
- Trachoma
- Uveitis
- Corneal inflammation (keratitis) and cornea-conjunctiva inflammation (keratoconjunctivitis).
- More severe corneal diseases, especially corneal ulcers and recurrent corneal erosions.
Because of the risk of permanent damage, you shouldn’t ignore pink eye symptoms if they’re still getting worse after more than a few days.
See your doctor if you have:
- Eye pain.
- A feeling that something is stuck in your eye.
- Blurred vision.
- Light sensitivity.
Keratoconjunctivitis diagnosis
In most cases, your doctor can diagnose keratoconjunctivitis by asking about your recent health history and symptoms and examining your eyes. Rarely, your doctor may take a sample of the liquid that drains from your eye for laboratory analysis, called a culture.
A culture may be needed if your symptoms are severe or if your doctor suspects a high-risk cause, such as:
- A foreign body in your eye.
- A serious bacterial infection.
- A sexually transmitted infection.
Keratoconjunctivitis treatment
Keratoconjunctivitis treatment depends on what type of keratoconjunctivitis you have. Keratoconjunctivitis treatment is usually focused on symptom relief. Your doctor may recommend using artificial tears, cleaning your eyelids with a wet cloth, and applying cold or warm compresses several times daily.
If you have bacterial keratoconjunctivitis, you may need antibiotic ointment or drops. Put the drops in both eyes. Keep using the drops until 2 days after the discharge has gone.
If you have viral keratoconjunctivitis, you don’t need antibiotics. There is no specific treatment for viral keratoconjunctivitis, it generally improves on its own without treatment.
If you have allergic keratoconjunctivitis, the following things might help:
- avoiding anything that triggers the allergic reaction
- antihistamine eye drops or syrup — ask your doctor what antihistamines to take
- lubricating eye drops
- allergen immunotherapy for severe cases.
To reduce the symptoms of bacterial or viral keratoconjunctivitis you can:
- Take ibuprofen or another over-the-counter pain killer.
- Use over-the-counter lubricating eye drops (artificial tears).
- Put a warm, damp washcloth over your eyes for a few minutes. To make this warm compress:
- Soak a clean washcloth in warm water then wring it out so it’s not dripping.
- Lay the damp cloth over your eyes and leave it in place until it cools.
- Repeat this several times a day, or as often as is comfortable.
- Use a clean washcloth each time so you don’t spread the infection.
- Use a different washcloth for each eye if you have infectious conjunctivitis in both eyes.
If your eyelids are sticking together, a warm washcloth can loosen the dried mucus so you can open your eyes.
Use clean cotton wool (one piece for each eye). Boil water and then let it cool down before you:
- gently rub your eye lashes to clean off crusts
- hold a cold flannel on your eyes for a few minutes to cool them down
Stop wearing contact lenses. If you wear contact lenses, you’ll be advised to stop wearing them until treatment is complete. Your doctor will likely recommend that you throw out contact lens you’ve worn if your lenses are disposable. Use a new pair when you go back to wearing your contacts. Your old contacts are likely infected and could get you sick again if you wear them again.
Disinfect hard lenses overnight before you reuse them. Ask your doctor if you should discard and replace your contact lens accessories, such as the lens case used before or during the illness.
Stop wearing eye makeup. Throw out your old eye makeup and get new makeup once your eyes are healthy.
Viral conjunctivitis is like a common cold in the eye. There is no treatment for the virus and usually you just have to let it heal on its own. Viral keratoconjunctivitis should go away within a week or two without treatment. Since viral keratoconjunctivitis is caused by virus, antibiotics won’t help, and antibiotics may even cause harm by reducing their effectiveness in the future or causing a medication reaction.
Bacterial keratoconjunctivitis usually produces more mucus or pus than viral or allergic keratoconjunctivitis. Bacterial keratoconjunctivitis can be treated with antibiotics prescribed by a doctor.
Keratoconjunctivitis sicca
Keratoconjunctivitis sicca also called keratitis sicca or xerophthalmia is a general term for “dry eye syndrome” due to a breakdown in the natural oily layer outside of the tear film that coats the front of the eye and keeps tears from drying up too quickly 16, 17. Normally, the natural oily layer outside of the tear film is a stable homogenous layer that not only provides the cornea and conjunctiva a healthy buffer from damage were it constantly exposed to the air, but this interface between the tear film and the air is also responsible for a significant amount of the focusing power of the eye. When the tear film becomes unhealthy, it breaks down in different places on the cornea and conjunctiva, leading not only to symptoms of irritation, but also to unstable and intermittently changing vision. Keratoconjunctivitis sicca is sometimes caused or worsened by a condition called meibomianitis, which is inflammation of the oil-producing glands or meibomian glands along the edge of your eyelids where the eyelashes are found. There are approximately 100 small meibomian glands that run along the top and bottom of your eyelids. These glands produce an oily liquid that is an important part of the eye’s tears.
Keratoconjunctivitis sicca or dry eye syndrome commonly occurs in people who are otherwise healthy. Dry eye is a common eye condition and it is estimated to affetc 7.4% to 33.7% of the population 18, 19, 20, 21. Keratoconjunctivitis sicca is also more common with older age. This can occur due to hormonal changes that make your eyes produce fewer tears.
Other common causes of dry eyes or keratoconjunctivitis sicca include:
- Dry environment or workplace (wind, air conditioning)
- Sun exposure
- Smoking or second-hand smoke exposure
- Cold or allergy medicines
- Wearing contact lenses
Keratoconjunctivitis sicca can also be caused by:
- Heat or chemical burns
- Previous eye surgery
- Use of eye drops for other eye diseases
- An uncommon autoimmune disorder in which the meibomian glands that produce tears are destroyed (Sjögren syndrome). Sjögren’s syndrome is an autoimmune disease, where the body’s white blood cells attack healthy tissue and organs. With Sjögren’s syndrome, your immune system attacks the lacrimal and salivary glands that keep our eyes and mouth lubricated. This is why dry eyes are very common with Sjögren’s syndrome 22. Nine out of 10 people who have Sjögren syndrome are women between 40 and 60 years old. However, Sjögren syndrome can affect men and women of any age.
Stay away from dry environments and things that irritate your eyes to help prevent dry eye symptoms.
The first step in keratoconjunctivitis sicca treatment is artificial tears. These come as preserved (screw cap bottle) and unpreserved (twist open vial). Preserved tears are more convenient, but some people are sensitive to preservatives. There are many brands available without a prescription.
Start using the artificial tears at least 2 to 4 times per day. If your symptoms are not better after a couple of weeks of regular use:
- Increase use (up to every 2 hours).
- Change to unpreserved drops if you have been using the preserved type.
- Try a different brand.
- Talk to your doctor if you cannot find a brand that works for you.
Other treatments for keratoconjunctivitis sicca may include:
- Fish oil 2 to 3 times per day
- Glasses, goggles or contact lenses that keep moisture in the eyes
- Medicines such as cyclosporine (Restasis) or lifitegrast (Xiidra), corticosteroid eye drops, and oral tetracycline and doxycycline
- Tiny plugs placed in the tear drainage ducts to help moisture stay on the surface of the eye longer
Other helpful remedies include:
- DO NOT smoke.
- Avoid second-hand smoke, direct wind, and air conditioning.
- Use a humidifier, particularly in the winter.
- Limit allergy and cold medicines that may dry you out and worsen your symptoms.
- Purposefully blink more often. Rest your eyes once in a while.
- Clean eyelashes regularly and apply warm compresses.
Some dry eye symptoms are due to sleeping with the eyes slightly open. Lubricating ointments work best for this problem. You should use them only in small amounts since they can blur your vision. It is best to use them before sleep.
Surgery may be helpful if your dry eyes are caused by the eyelids being in an abnormal position.
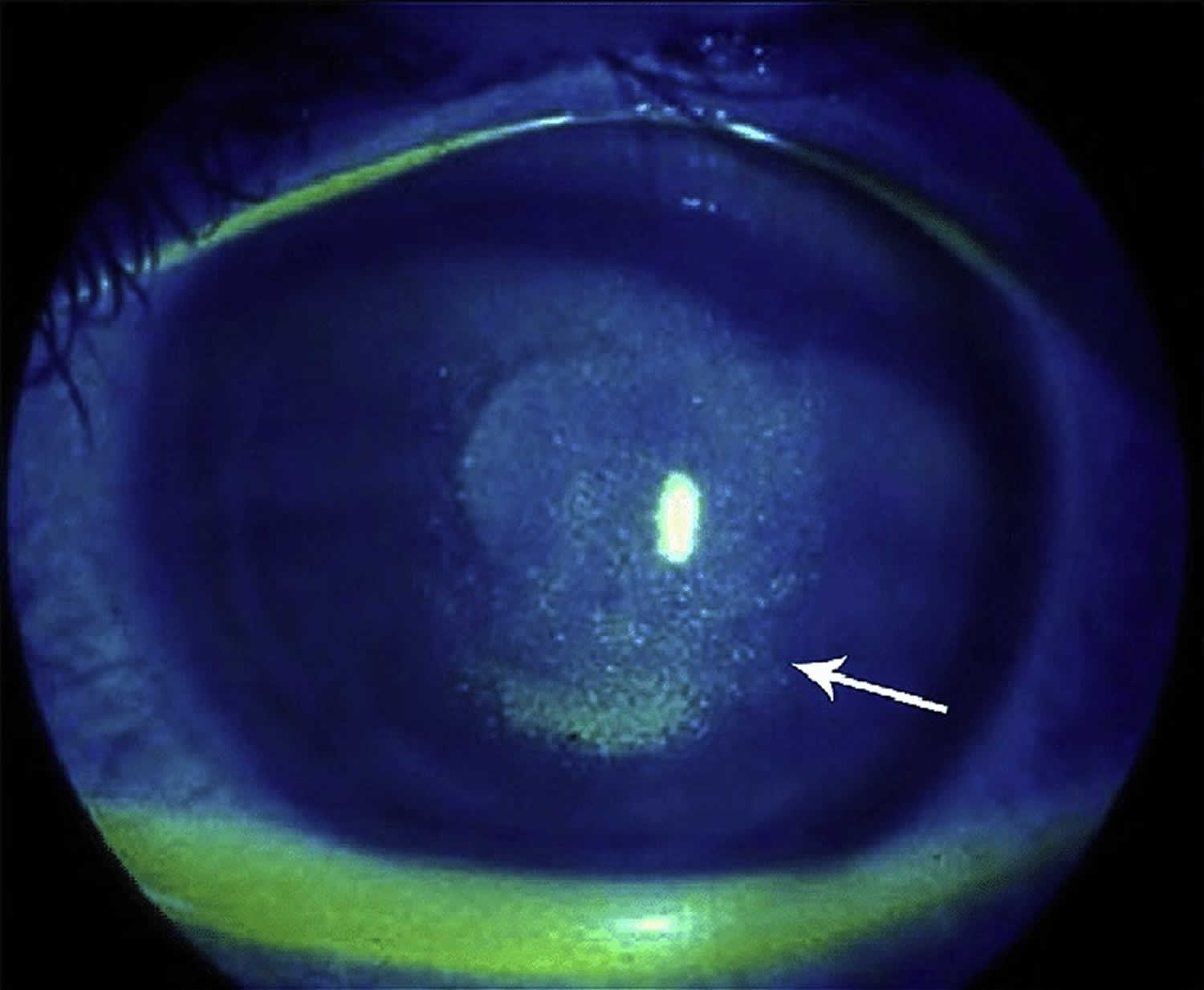
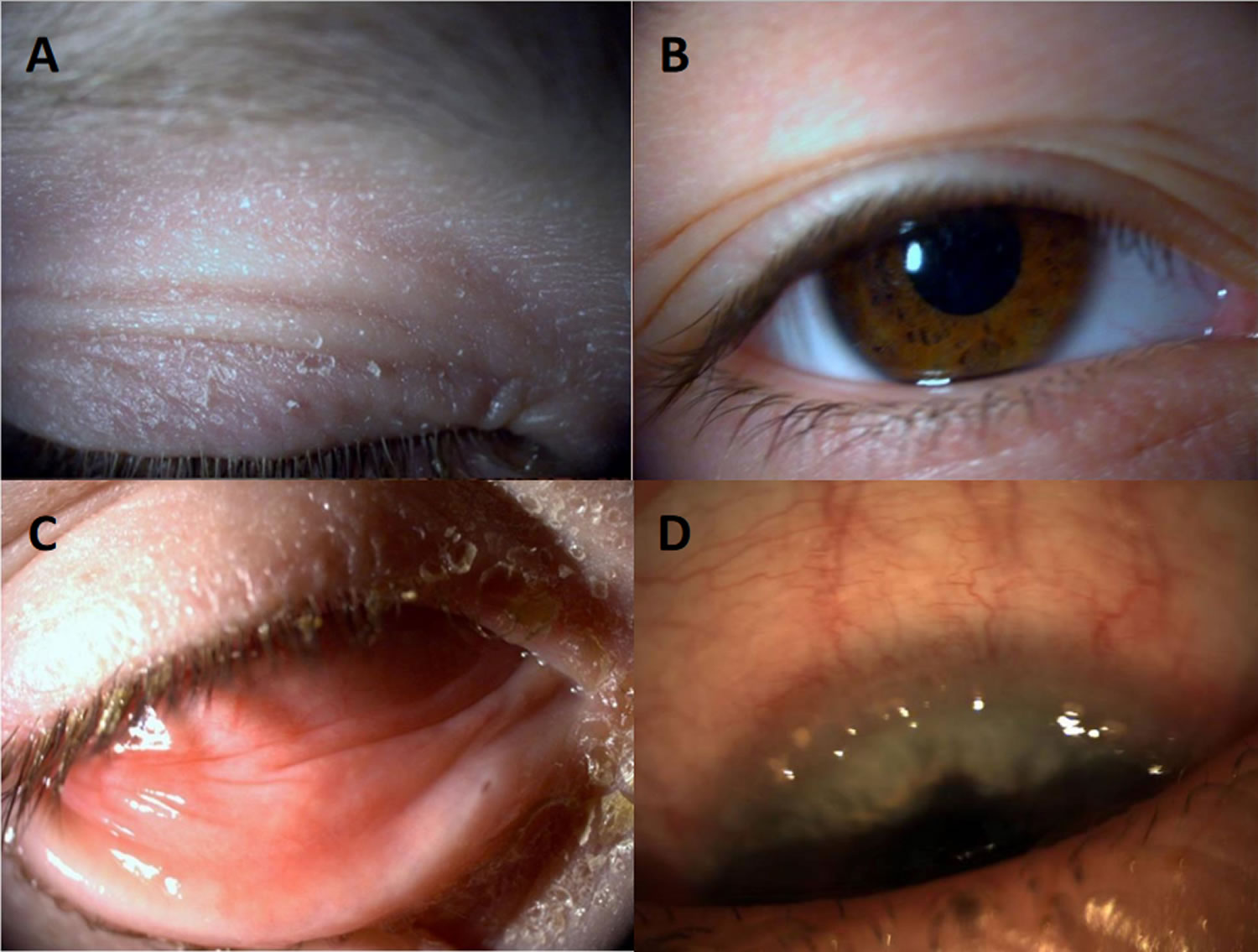
Figure 5. Keratoconjunctivitis sicca
Footnote: Keratoconjunctivitis sicca. Arrow points to punctate epithelial erosion resulting from corneal dessication.
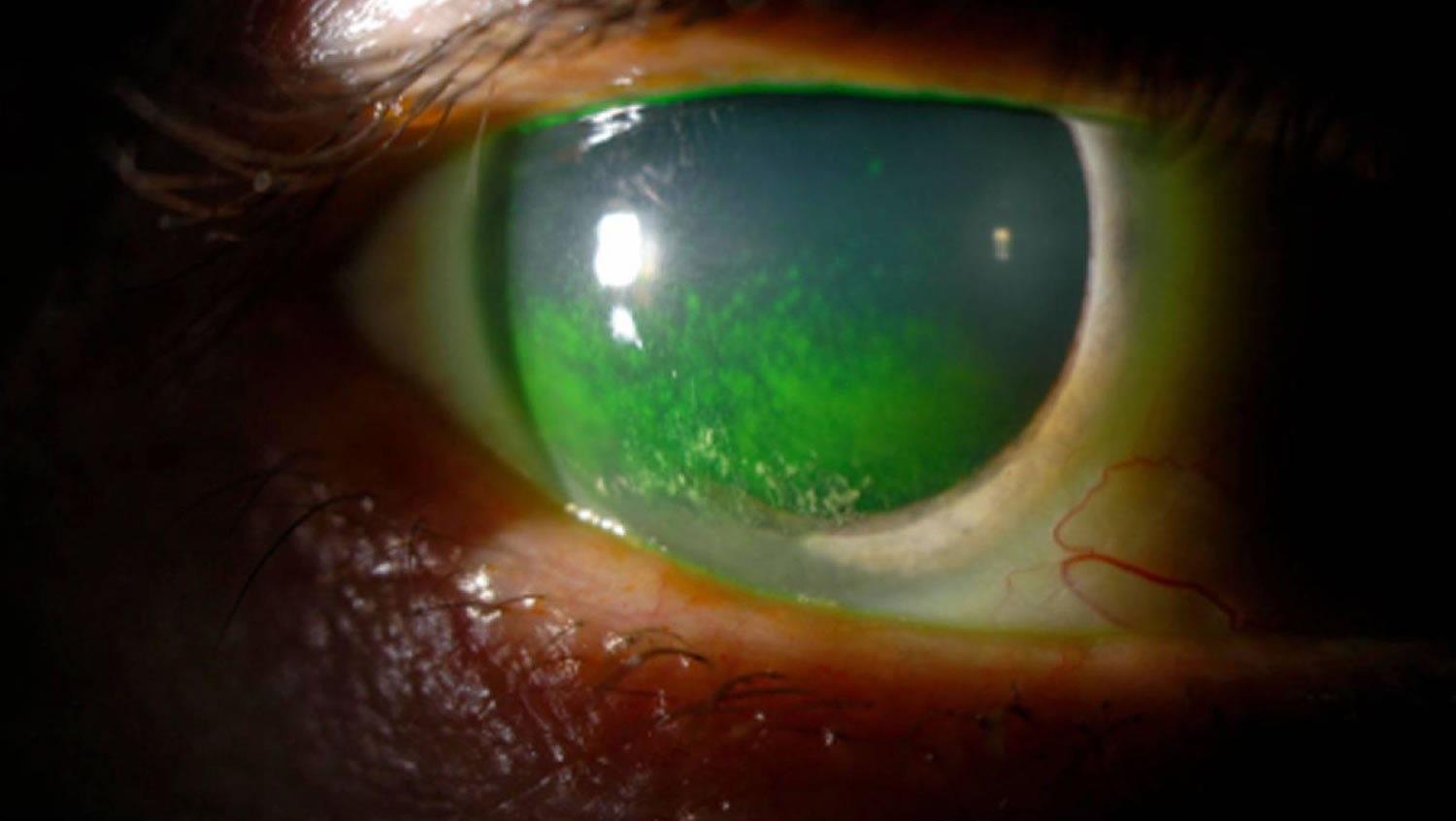
[Source 23 ]Figure 6. Keratoconjunctivitis sicca in a patient with Sjögren’s syndrome
[Source 24 ]Keratoconjunctivitis sicca causes
Keratoconjunctivitis sicca causes include 16:
- Allergies
- Aging associated with decreased hormones
- Pregnancy and associated hormonal changes
- Thyroid eye conditions
- Leukemia
- Lymphoma (cancer of the lymph system)
- Eyelid inflammation (blepharitis)
- Medication/supplement use including, but not limited to: psychiatric medicines, OTC cold medicines, anti-histamines, beta-blockers, pain relievers, sleeping pills, diuretics, hormonal replacement, and oral contraceptives
- Sjögren’s syndrome (dry mucus membranes throughout body)
- Other autoimmune disorders including Lupus and/or Rheumatoid Arthritis
- Chemical exposures / injuries to the eyes
- Previous eyelid, eye or facial surgery
- Laser vision correction
- Infrequent blinking, associated with staring at computer or video screens which is becoming a more frequent contributor and Parkinson’s disease
- Environmental (dusty, windy, hot/dry)
- Contact lens use
- Neurologic conditions including: stroke, Bell’s palsy, Parkinson’s, trigeminal nerve dysfunction
- Exposure keratitis, in which the eyelids do not close completely during sleep (i.e. lagophthalmos)
- Corneal ulcers and infections
- Post refractive surgery (LASIK or PRK)- while typically transient can become a chronic issue in some
- Inflammatory eye conditions, including conjunctivitis, uveitis or iritis
- Diabetes
- Infectious Keratitis, including Herpes Simplex and Herpes Zoster Keratitis
- Neurotrophic Keratitis
- Vitamin A deficiency (rare in US except in certain diseases such as Crohn’s disease).
Keratoconjunctivitis sicca symptoms
There are numerous different symptoms one can experience from keratoconjunctivitis sicca or dry eye syndrome, prominent amongst these symptoms is tearing 16. Naturally, a person with keratoconjunctivitis sicca may wonder why their eye(s) can be “dry” despite producing plenty of tears. This is because of the unhealthy tear film and the irritation that comes from it stimulates the brain to produce a wave or reflex of tears to help counteract the irritation. However, this reflex tearing is simply insufficient to correct the overall problem. For this reason, dry eye syndrome could more appropriately be termed “Tear Film Dysfunction”. Other symptoms of dry eye syndrome or tear film dysfunction include 16:
- Burning
- Stinging
- Itching
- Tearing
- Sandy or gritty feeling in the eye
- Scratchy or foreign-body sensation
- Discharge
- Frequent blinking
- Mattering or caking of the eyelashes (usually worse upon waking)
- Redness in the eye
- Blurry or fluctuating vision (made worse when reading, computer, watching television, driving, or playing video games)
- Sensitivity to light or photophobia
- Eye pain and/or headache
- Heavey eye lids
- Eye fatigue.
Keratoconjunctivitis sicca complications
Keratoconjunctivitis sicca complications range from mild to severe. Mild-to-moderate dry eye syndrome causes symptoms detailed above, including eye irritation or visual disturbances. More severe diseases can result in corneal complications, including infectious keratitis, ulceration, and scarring, which may cause subsequent loss of vision 25, 26. Although causation has not been established, several non eye complications exist with dry eye syndrome, including depression, sleep and mood disorders, dyslipidemia, and migraine headaches 27, 28.
Keratoconjunctivitis sicca diagnosis
Currently, there is NO “gold standard” diagnostic procedure for dry eye conditions 16. The debate on the most appropriate dry eye diagnostic tests for clinical practice is ongoing. Most people with dry eye syndrome or keratoconjunctivitis sicca have signs which are not even obvious on a general eye exam. An individual with dry eye syndrome or keratoconjunctivitis sicca may, in fact, have more than one cause acting simultaneously to produce his/her symptoms. This is actually the case for many persons who suffer from dry eye syndrome. For this reason, many persons who undergo casual examination and/or treatment attempts of dry eye syndrome without investigating for and treating all the possible causes can end up becoming frustrated, have persistent symptoms that can worsen, and may jump from doctor to doctor to seek relief.
A thorough history is essential in the workup of dry eye symptoms due to the frequent lack of correlation between symptoms and exam findings 29, 30. Examination should include evaluation of the face and eyelids, blinking patterns, eyelid margins, eyelashes, conjunctiva, cornea, and tear film.
Depending on the particular constellation of signs, symptoms, history and comorbidities, dry eye syndrome or keratoconjunctivitis sicca tests may include Schirmer’s tear test and blood tests to check for systemic disease may be warranted. The phenol red thread test is similar to the Schirmer’s tear test, except that red strips of special thread are used instead of paper strips. Numbing drops are not needed. The test takes 15 seconds.
People with dry eye syndrome or keratoconjunctivitis sicca may have these tests:
- Visual acuity measurement
- Slit lamp exam
- Diagnostic staining of the cornea and tear film
- Measurement of tear film break-up time (TBUT)
- Measurement of rate of tear production (Schirmer’s tear test or phenol red thread test)
- Measurement of concentration of tears (osmolality)
Schirmer’s tear test (Schirmer test)
A Schirmer’s tear test is a diagnostic tool that measures the amount of tears produced by your eyes to determine if they are producing enough to keep your eyes moist 31, 32. It’s often used to diagnose dry eye syndrome, but it can also be used to assess tear overproduction.
Here’s how Schirmer’s tear test is performed:
- The patient is given numbing eye drops to prevent your eyes from tearing due to irritation from the paper strips. Sometimes the test is done without numbing drops to test for other types of tear problems.
- The eye doctor will place the end of Schirmer strips or filter paper (Whatman filter paper #41) with dimensions 35 mm and 5 mm inside the lower eyelid of each eye 33
- The patient closes their eyes gently for 5 minutes. Closing the eyes tightly or rubbing the eyes during the test can cause abnormal test results.
- After 5 minutes, the eye doctor removes the paper strips and measures how much they became moistened.
- Do not rub the eyes for at least 30 minutes after the test. Leave contact lenses out for at least 2 hours after the test.
The amount of moisture on the paper strip indicates the level of tear production 31:
- Normal: More than 15 millimeters of moisture on the filter paper after 5 minutes (>10 mm). More than 15 mm of moisture is a sign of normal tear production. Both eyes normally release the same amount of tears
- Possible dry eyes: 10 to 15 millimeters (10 to 15 mm)
- Moderately dry eyes: 5 to 10 millimeters (5 to 10 mm)
- Extremely dry eyes: 0 to 5 millimeters (0 to 5 mm)
The Dry Eye Workshop (DEWS) proposes a Schirmer test cutoff value of 10 mm for 5 minutes as one of the criteria for diagnosing dry eye disease 34. The dry eye disease cutoff value varies in the literature, ranging from 15 mm originally reported by Schirmer, 10 mm by the Dry Eye Workshop (DEWS), 10 mm by Jones, and 5 mm by Sjogren 31. A test result of less than 5 mm indicates a pathologic dry eye, while more than 15 mm is considered normal 31.
Studies have shown that the Schirmer test has acceptable levels of intraexaminer reproducibility and interexaminer repeatability. Topical anesthesia does not seem to affect these parameters 35. Experts recommend that the same examiner conduct the testing during follow-up visits and repeated same-day measures if confirmation of results is needed.
Even though the Schirmer test has been available since 1903, several studies show that it does not properly identify a large group of people with dry eyes. It is most useful in the diagnosis of patients with severe aqueous deficiency, but is relatively insensitive for patients with mild dry eye 36. Newer and better tests are being developed. One test measures a molecule called lactoferrin. People with low tear production and dry eye have low levels of this molecule.
Another test measures tear osmolarity, or how concentrated the tears are. The higher the osmolarity, the more likely it is that you have dry eye.
Tear Osmolarity
Patients with dry eye disease have been found to have elevated tear film osmolarity (TFO) 37, 38. Tear hyperosmolarity can induce tear film instability by modifying the interaction between tear film lipids and proteins, damaging the epithelial cell membranes, triggering inflammation, and stimulating corneal nerves 39, 40.
Tear osmolarity can be determined easily in the office using the point of care TearLab Osmolarity System (TearLab, San Diego, CA), which measures the osmolarity of a 50 nanoliter (50 nL) tear sample. Normal values are considered to be 296±9.8 mOsm/L 37 41. Greater than 308 mOsm/L is considered to indicate at least mild dry eye and has been demonstrated to serve as an early indicator of ocular surface instability 42.
The test is performed by placing the tip of the handheld device at the lateral tear meniscus and then docking the sampler into the reader. The device contains a gold-plated microchip that measures electrical impedance in the sample and displays the osmolarity measurement within seconds.
Tear film osmolarity (TFO) testing is indicated for use in conjunction with other signs and symptoms. Combination of tear film osmolarity (TFO) with at least one other dry eye test will enhance the sensitivity and specificity 43, 44, 45. Schargus et al 42, however, did not find a significant correlation between tear film osmolarity (TFO) and MMP-9 levels or with any other clinical dry eye test.
Matrix metalloproteinase 9 (MMP-9)
Stressed epithelial cells on the eye surface can produce matrix metalloproteinases (MMP) 16. Matrix metalloproteinase 9 (MMP-9) has been shown to be elevated in the tears of patient with dry eye disease, and levels correlate with examination findings in patients with moderate to severe dry eye. The normal range of MMP-9 levels in human tears is 3 to 40 ng/mL 46. MMP-9 levels can be elevated in other inflammatory conditions, such as graft-versus-host disease, Stevens-Johnson syndrome, and following corneal surgery.
InflammaDry (Rapid Pathogen Screening Inc, Sarasota, FL) is a single use, noninvasive, disposable test that detects MMP-9 levels of 40 ng/mL or higher 16.
The InflammaDry test is performed prior to instillation of anesthetic eye drops by dabbing the sample collector at multiple sites along the palpebral conjunctiva. The lid can be released every 2 to 3 dabs to allow blinking. This should be repeated 6 to 8 times, after which the sampling fleece should rest against the conjunctiva for at least 5 seconds or until it is saturated with tears (indicated by a pink or glistening appearance). The sample collector is then snapped onto the test cassette and dipped into the buffer solution for activation. After 10 minutes, the test is read. One blue line and one red line indicate a positive test result, and the intensity of the red line is related to MMP-9 concentration. One blue line only indicates a negative test result.
The InflammaDry test was shown to have a sensitivity of 85% and specificity of 94% 47. In another study by Sambursky et al 48, the test was found to have a total positive and negative agreement of 81% (127/157) and 98% (78/80), respectively, with clinical assessment when Ocular Surface Disease Index (OSDI) was included in the definition of mild dry eye. When Ocular Surface Disease Index (OSDI) was excluded, the InflammaDry demonstrated a positive and negative agreement with clinical assessment of 86% (126/146) and 97% (88/91), respectively. Studies have also demonstrated that elevated MMP-9 levels correlate most with other dry eye tests in advanced disease and is likely a late sign that is rarely present in mild cases 42, 49.
Corneal sensation
Corneal hyperesthesia and/or reduced sensation may be present in severe and chronic dry eye disease 50. Sensory denervation may cause dry eye by reducing the afferent signaling of tear production, reducing the blink rate, and by altering trigeminal nerve influences on eye epithelial health. Decreased corneal sensation can also result from chronic dry eye.
Corneal sensation can be measured using a cotton tip applicator or more precisely with a Cochet-Bonnet esthesiometer 16.
Tear break up time (TBUT)
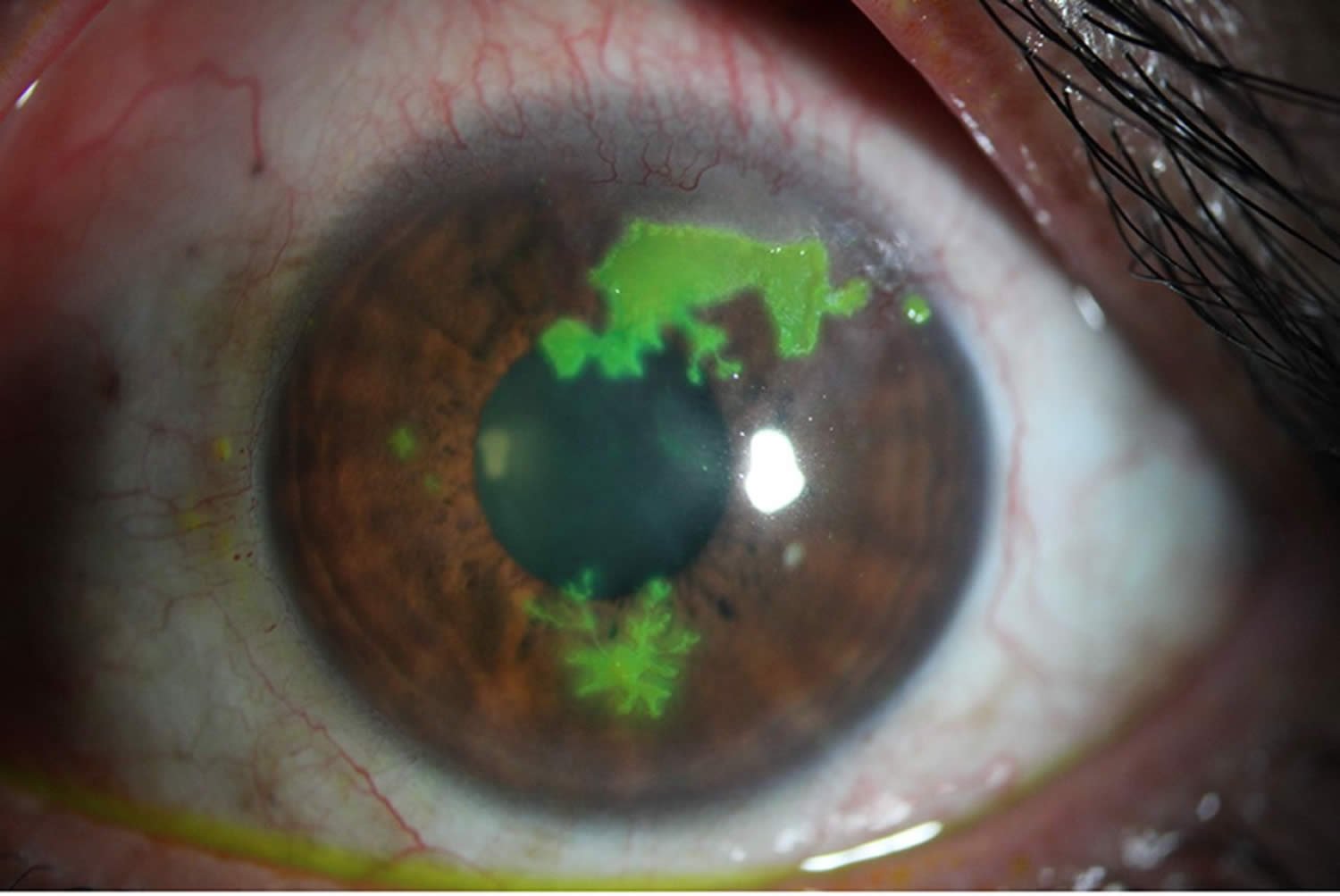
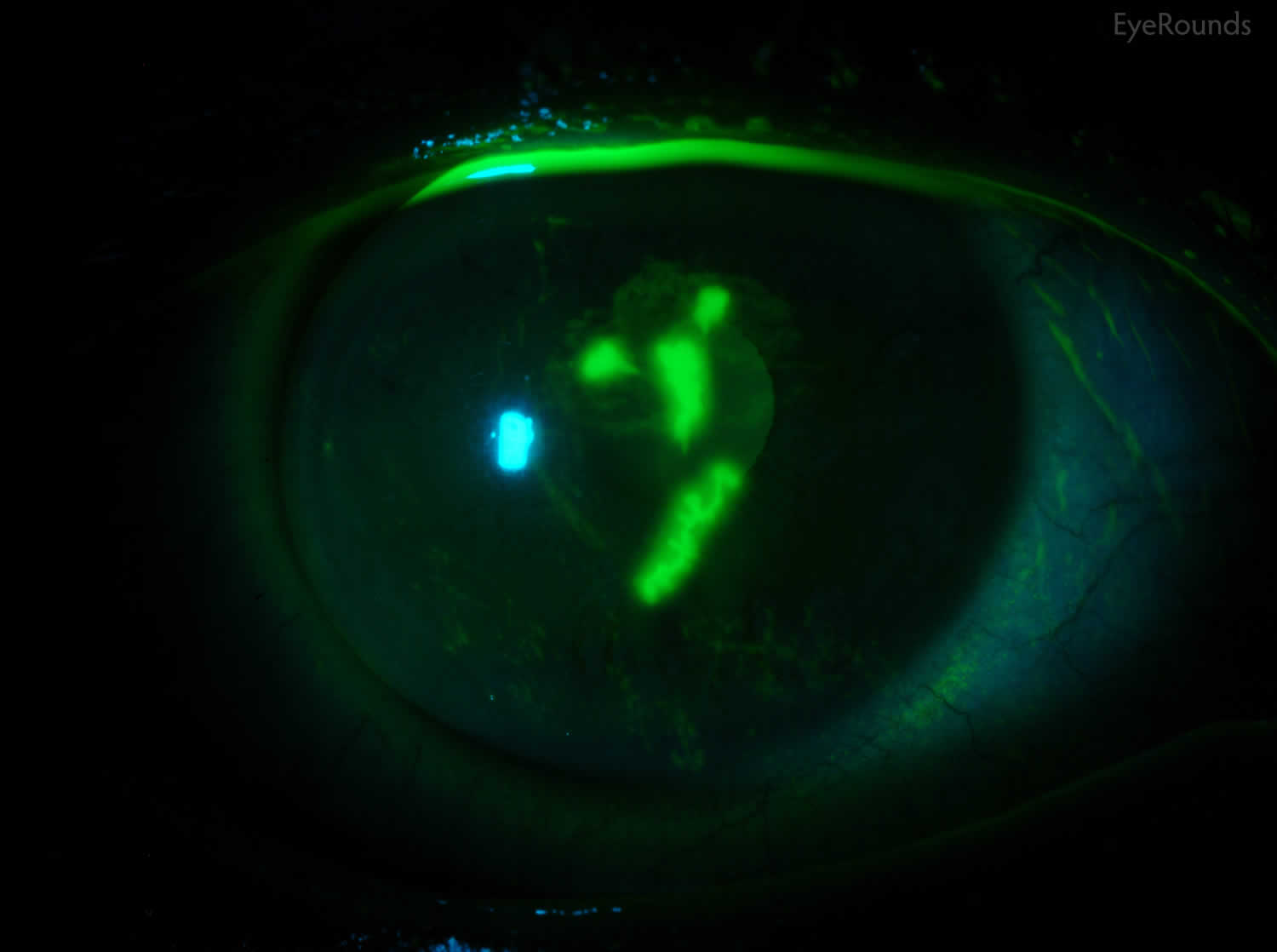
Tear break up time (TBUT) is an indication of tear film stability 16. The proper method of tear break up time (TBUT) testing is using a fluorescein-impregnated strip wet with non-preserved saline solution (benzalkonium chloride can increase tear break up speed) 16. The dye is distributed by blinking, and the patient is then asked to stare straight ahead without blinking. The tear film is observed under the cobalt blue light of a slit lamp, and the time between the last blink and the appearance of the first dry spot or hole in the tear film is measured and equal to the tear break up time (TBUT).
Tear break up time (TBUT) has been shown to be decreased in keratoconjunctivitis sicca, mucin deficiency, and Meibomian gland disease 16. Normal subjects show variability in tear break up time (TBUT), although 10 seconds is the typical cutoff between normal and abnormal results and has been found to be relatively specific in screening patients for tear film instability 51.
Delayed tear clearance
Following fluorescein placement, the persistence of fluorescein in the tear film at various time points can be determined. This may be more important to rule out nasolacrimal duct issues as a cause of tearing or epiphora.
Tear meniscus height (meniscometry)
The tear meniscus height can be used to estimate tear volume. A tear meniscus height less than 0.25 mm is suggestive of dry eye.
Tear film interferometry
Interferometry of the lipid layer of the tear film is a noninvasive method of grading tear film quality and estimating the thickness of the lipid layer, which have been shown to be abnormal in evaporative dry eye that is secondary to meibomian gland dysfunction. The LipiView interferometer (TearScience Inc, Morrisville, NC) is a commercially available tool that can measure lipid layer thickness.
Sjö test
Recently, additional autoantibodies were identified as diagnostic of Sjogrens syndrome. These include autoantibodies to salivary gland protein 1 (SP-1), carbonic anhydrase 6 (CA6), and parotid secretory protein (PSP). SP-1, CA6, and PSP were found in 45% of patients who met clinical criteria for Sjögren’s syndrome but tested negative for anti-Ro and anti-La. The novel autoantibodies may be present earlier in the disease course. In a study of patients with xerostomia and xerophthalmia for less than two years, 76% had autoantibodies to SP-1 or CA6 compared to 31% who had anti-Ro or anti-La antibodies 56. Currently in clinical practice, SP-1, CA-6, and PSP autoantibody levels can be determined using a commercially available blood test called Sjö (Bausch & Lomb), which also includes SS-A, SS-B, antinuclear antibody (ANA), and rheumatoid factor (RF) levels in its panel.
The test can be administered in the office using a simple finger stick with a lancet. Once a large drop of blood appears, the five dotted circles on the test card are filled. The sample is then allowed to air dry for 30 minutes, after which it can be sealed in a plastic envelope with a dessicating package. The sample along with the patient information is then mailed in. Test results are typically available within one week.
Ocular surface staining
Fluorescein sodium
Fluorescein dye is the most commonly used stain in ophthalmology. Areas in which the corneal or conjunctival surface epithelial cells are loose or desquamated will stain with fluorescein. Fluorescein dye should be instilled as described above. The degree of staining can be graded using various scales 51.
Rose Bengal
Rose Bengal is more sensitive for conjunctival staining, but also more difficult to visualize and less well tolerated compared to fluorescein. Rose Bengal stains devitalized epithelial cells that lack a healthy mucin coating. It is applied using a dye-impregnated paper strip.
Interpretation of staining is based on intensity and location using a grading scale described by van Bijsterveld 57. The nasal and temporal conjunctiva and the cornea are graded on a scale of 0-3 with a maximum possible score of 9.
In aqueous tear deficiency, the interpalpebral conjunctiva is the most common location for Rose Bengal staining 16. The severity of staining has been shown to correlate with the degree of aqueous deficiency, tear film instability, and reduced mucin production by conjunctival goblet and epithelial cells 51.
Lissamine green
Lissamine green has similar staining characteristics but is much better tolerated than Rose Bengal 16. Lissamine green is also available in dye-impregnated paper strips 16.
Keratoconjunctivitis sicca differential diagnosis
Many conditions may evoke symptoms similar to those caused by keratoconjunctivitis sicca 34. Some conditions may also be associated with or lead to keratoconjunctivitis sicca, such as allergic conjunctivitis, cicatricial conjunctivitis, filamentary keratitis, and neurotrophic keratitis 58. Identifying the underlying primary condition in these cases is key to reducing the progression of the disease and worsening of dry eye.
Differential diagnosis for keratoconjunctivitis sicca include 58:
- Conjunctivitis (allergic, viral, bacterial, parasitic/chlamydial)
- Anterior blepharitis
- Demodex blepharitis
- Cicatricial conjunctivitis (Stevens-Johnson Syndrome, mucous membrane pemphigoid)
- Bullous keratopathy
- Contact lens–related keratoconjunctivitis
- Eyelid malposition (entropion, ectropion) or abnormality (trichiasis) leading to ocular surface disease
- Keratitis (interstitial, filamentary, contact lens–related, neurotrophic)
Keratoconjunctivitis sicca treatment
Depending on the causes of keratoconjunctivitis sicca or your dry eyes, there are numerous treatments for dry eye syndrome or tear film dysfunction, but the more common treatment modalities include 16, 58, 59:
- Education about the condition
- Lid hygiene (warm compresses and eyelash and eyelid scrubs)
- Oral essential fatty acid supplements. Oral flaxseed oil or fish oil supplements 2000mg/day has also been found to be useful in alleviating symptoms and decreasing the frequency of topical agents anecdotally. However, the DREAM (Dry Eye Assessment and Management Study) Research Group concluded that patients with dry eye who received 3,000 mg of fish-derived n-3 fatty acids for 12 months had no significantly better outcomes than patients who received an olive oil placebo 60.
- Modification of the environment
- Eliminating direct high airflow or fans
- Reducing screen time
- Taking frequent screen breaks
- Using a humidifier
- Identification and elimination of offending topical and systemic agents
- Topical eye lubricants or artificial tears (preferably ones without a redness-reliever component in them)
- Longer acting agents such as artificial tear gel and ointments and LACRISERT® (hydroxypropyl cellulose ophthalmic insert)
- Tear conserving interventions such as punctal plugs or nighttime masks/goggles
- Prescription medicines such as Restasis (cyclosporine 0.05%) (increase tear-production), Cequa (cyclosporine 0.09%) (increase tear production) or Xiidra (lifitegrast 5%) (mechanism unknown-however is a small molecule integrin antagonist)
- Topical ophthalmic steroids are helpful in controlling the inflammatory aspect of the disease in short bursts.
- Oral antibiotics (macrolide or tetracycline) 61
- TearCare (Automated Meibomian Gland Heating/Manual Expression Procedure)
- LipiFlow (Automated Meibomian Gland Heating and Expression Procedure)
- Intense Pulsed Light (IPL) Therapy.
Additional treatment options include 58:
- Serum eye drops. Autologous serum tears, compounded artificial tears with mixed with the patient’s serum, can be particularly helpful in recalcitrant cases.
- Oral or topical secretagogues
- Therapeutic contact lenses
- Amniotic membrane grafting
- Surgical punctal occlusion
- Tarsorrhaphy.
Keratoconjunctivitis sicca prognosis
There are minimal published data describing the natural history of treated and untreated dry eye syndrome 62. Dry eye syndrome is often considered chronic, with periods of exacerbation due to intermittent contributing factors. Most people with dry eye syndrome who keep up with their regimen as prescribed by their eye doctor are able to have their symptoms controlled, allowing them to function either symptom-free or with minimal difficulty 16. Because of the nature of the causes of dry eye syndrome, most people do not get “cured” of their problem, but with regular maintenance can function as though they are cured 16. However, even the patient who is well-controlled on maintenance therapy can have break-through episodes and require a visit to their eye doctor, in addition to regularly scheduled visits.
Postsurgical dry eye such as following cataract surgery or refractive surgery often improves with time, possibly related to the regeneration of corneal nerves or reduction of ocular inflammation 63.
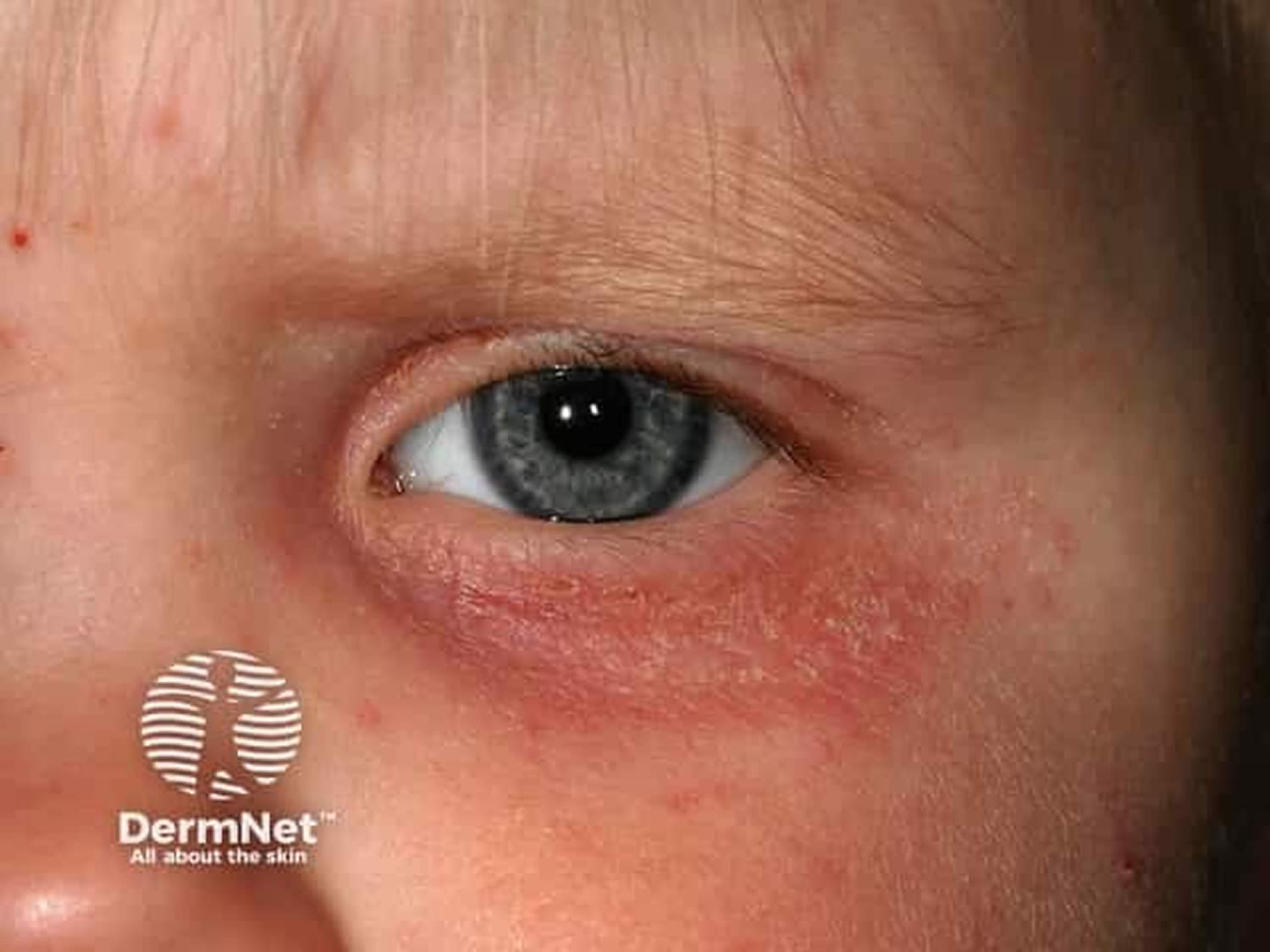
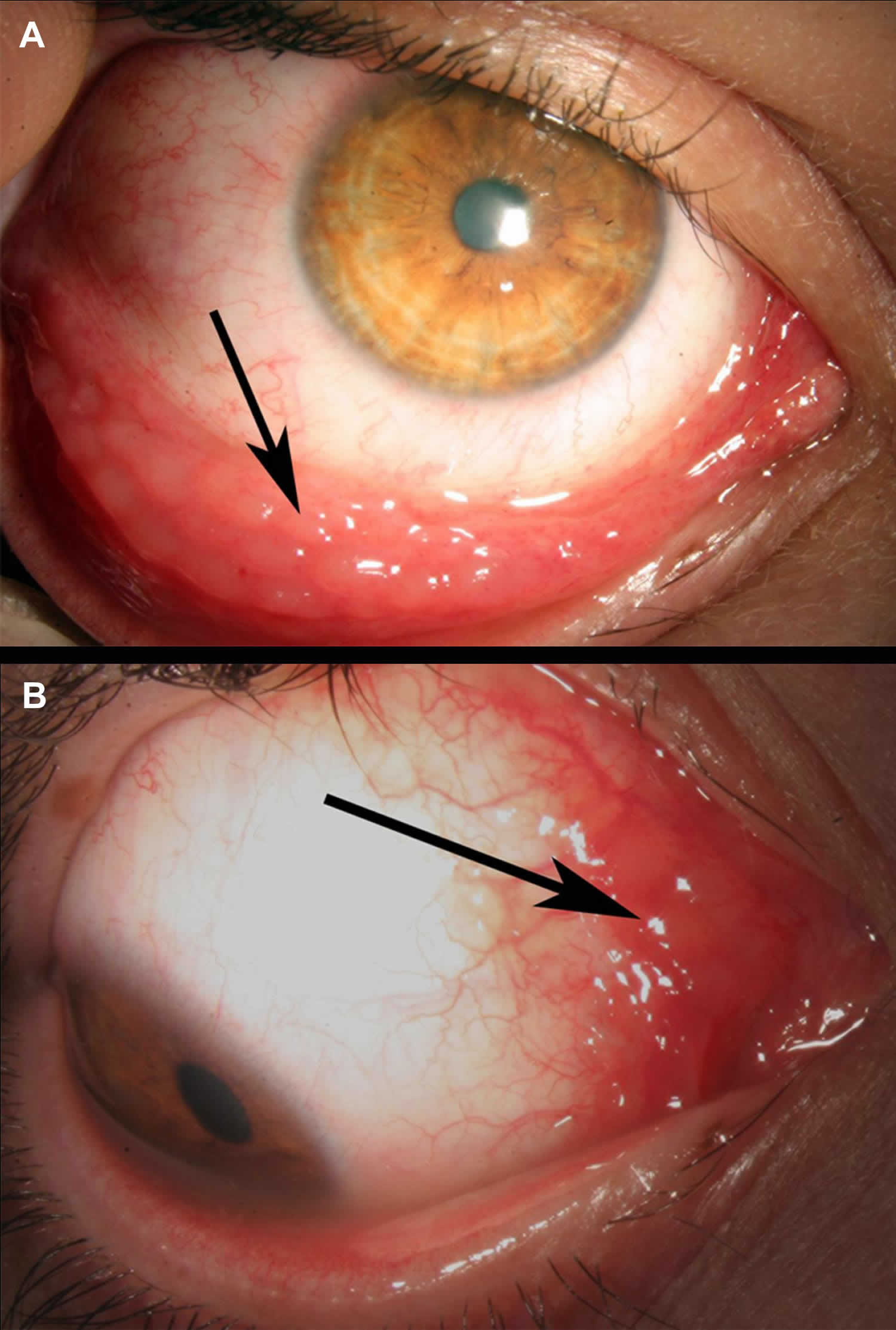
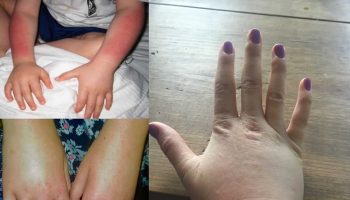
Allergic keratoconjunctivitis
Allergic keratoconjunctivitis also called allergic conjunctivitis is caused by an allergic reaction, with the majority of cases (90–95%) attributed to seasonal (certain times of the year) allergic conjunctivitis or perennial (all year round) allergic conjunctivitis 64, 65, 66, 67, 68, 69. Allergic conjunctivitis is an inclusive term that encompasses seasonal allergic conjunctivitis (SAC), perennial allergic conjunctivitis (PAC), vernal keratoconjunctivitis (VKC), and atopic keratocongiuntivitis (AKC). However, atopic keratocongiuntivitis (AKC) and vernal keratoconjunctivitis (VKC) have clinical and pathophysiological features quite different from seasonal allergic conjunctivitis (SAC) and perennial allergic conjunctivitis (PAC), in spite of some common markers of allergy 70, 66. Also contact lenses or ocular prosthesis associated giant papillary conjunctivitis (GPC) are often included in the group of eye allergy, however they should not be considered as real allergic diseases, but as chronic ocular micro-trauma related disorders, which need to be managed by ophthalmologists in association with contact lenses experts 66, 71.
Allergic conjunctivitis is a common conjunctivitis that is caused by immunoglobulin E (IgE) immune responses or immediate type 1 hypersensitivity immune reactions affecting more than 40% of the general population and is estimated to occur in up to 30% of children, either alone or in association with allergic rhinitis 72, 73, 74, 75, 76. Allergic conjunctivitis is a reaction of the outer lining of the eyeball (conjunctiva) to things in the environment to which a person is allergic (allergens). Dust, pollen, animal dander (skin cells that are shed by animals with hair, fur or feathers), and sometimes even medications can all be allergens. When your eyes are in contact with these allergens, the eyes get red, inflamed, watery, itchy or swollen eyelid 77, 78. Although these symptoms can look like the signs of an infection, allergic conjunctivitis is not an infection and is not contagious. However, these signs and symptoms can be sufficiently bothersome that people with allergic conjunctivitis often experience decreased work productivity, increased work or school absenteeism, limitation of everyday activities, and reduced quality of life 77.
Unlike conjunctivitis that is caused by bacterial or viral infection, allergic keratoconjunctivitis is not contagious, so it cannot be transferred from one person to another.
Allergic keratoconjunctivitis usually affect both eyes and are often accompanied with other signs of hay fever. Allergic keratoconjunctivitis signs can include an itchy, runny nose and sneezing or a history of other allergic conditions. The eyes are itchy (the hallmark of allergic eye disease) and watery 79, 80.
Allergic keratoconjunctivitis other symptoms can include:
- redness behind the eyelid, spreading up the white of the eye
- swelling of the eye/s making them appear puffy
- foreign body sensation
- excessive tears
- serous or mucous discharge
- conjunctival hyperemia (increased amount of blood in the vessels)
- tarsal papillary reaction
- a dislike of bright lights (photophobia).
Allergic keratoconjunctivitis symptoms may be:
- Perennial (all year round) due to constant exposure to dust mites, animal dander, indoor and outdoor mold spores and, in some cases, foods or food additives.
- Seasonal (certain times of the year) due to airborne allergens such as mold spores and pollen from grasses, trees, and weeds. The amount of airborne pollen varies from day to day and is dependent on the weather. People with pollen allergies often find their symptoms improve in wet weather and become worse on hot windy days or after thunderstorms.
If allergic keratoconjunctivitis is suspected, allergy testing can help identify the allergen responsible, or “trigger”.
Avoiding or minimizing exposure to known allergens is an important first step in managing allergic keratoconjunctivitis.
Allergic keratoconjunctivitis may be helped by treatments used in conditions such as hay fever e.g. antihistamines. Cool compresses and lubricating eye drops may soothe the eyes.
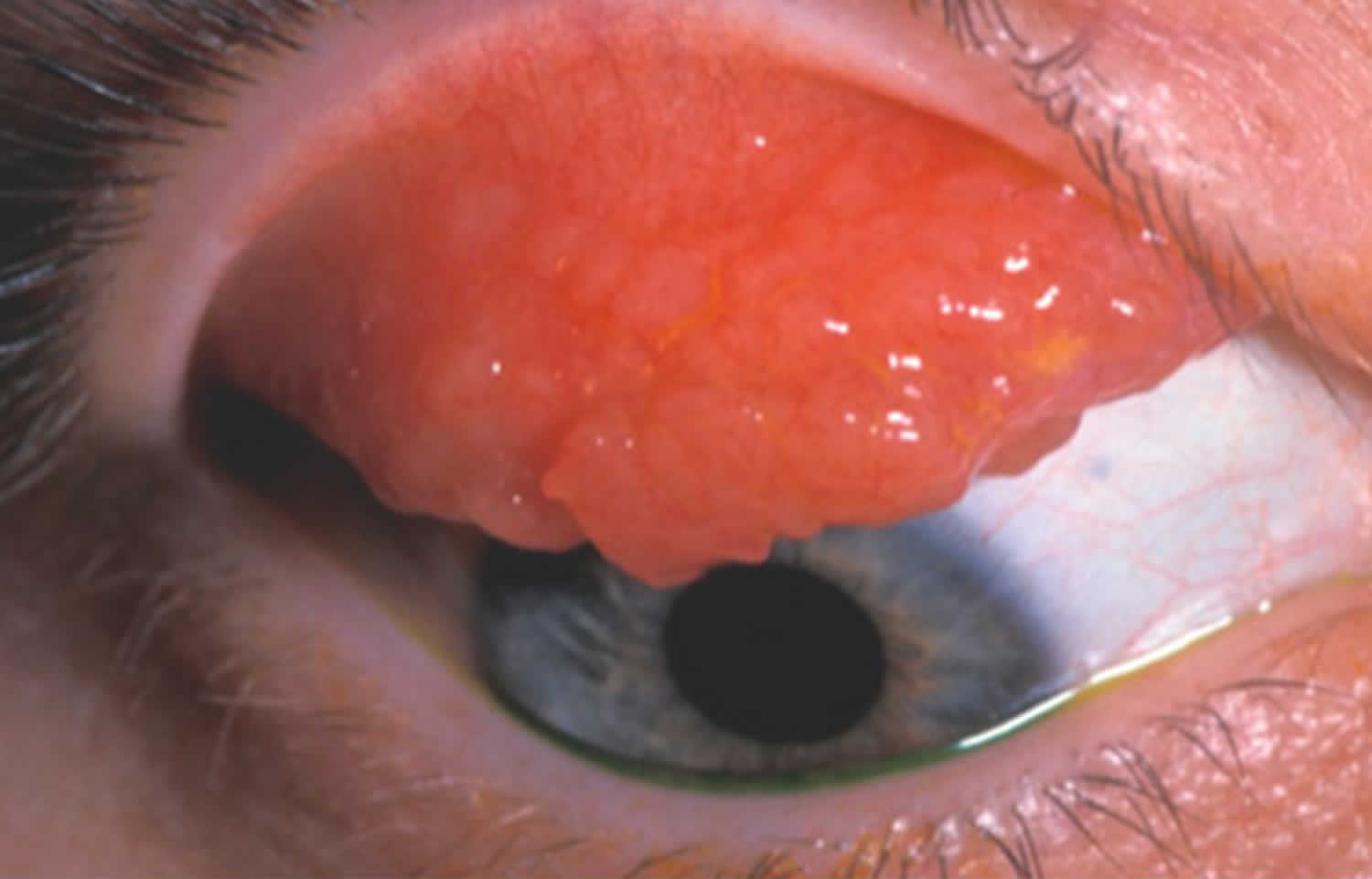
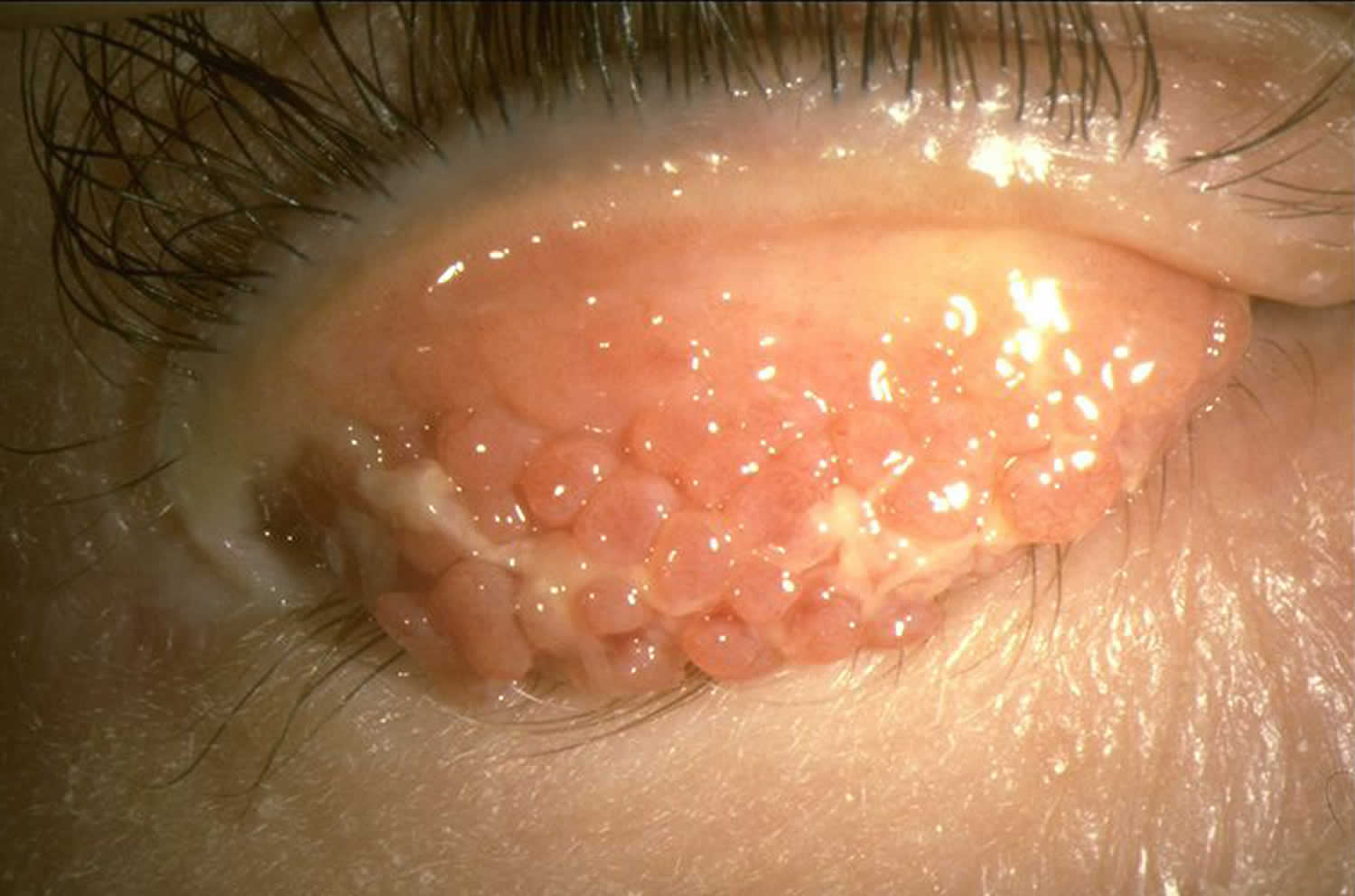
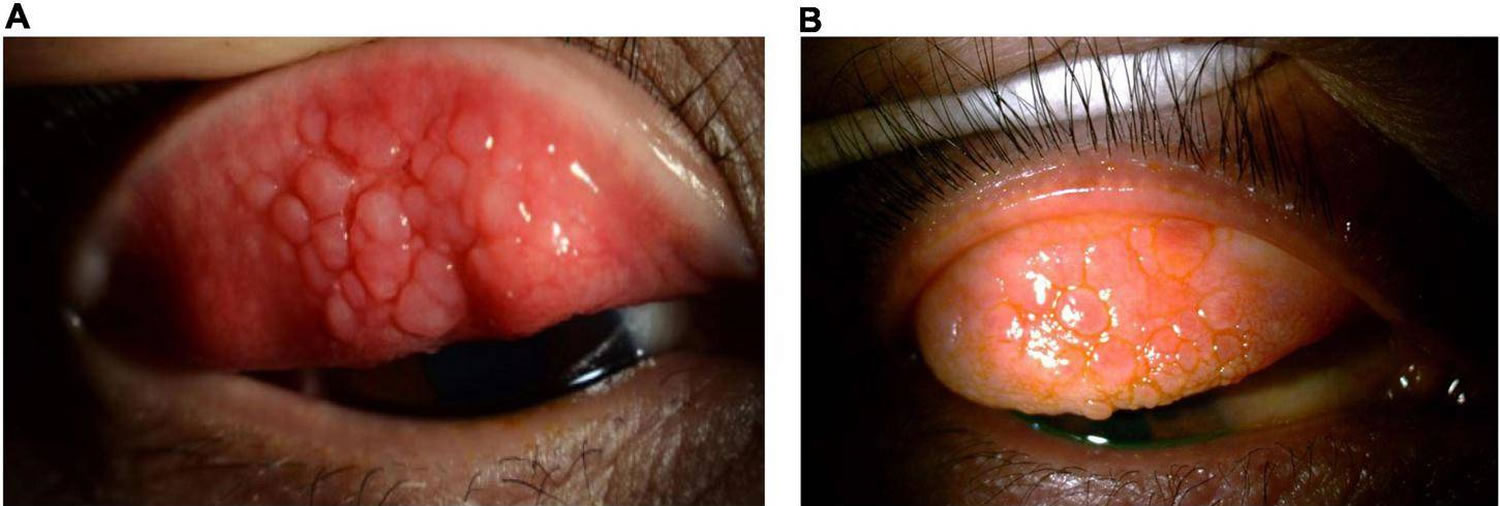
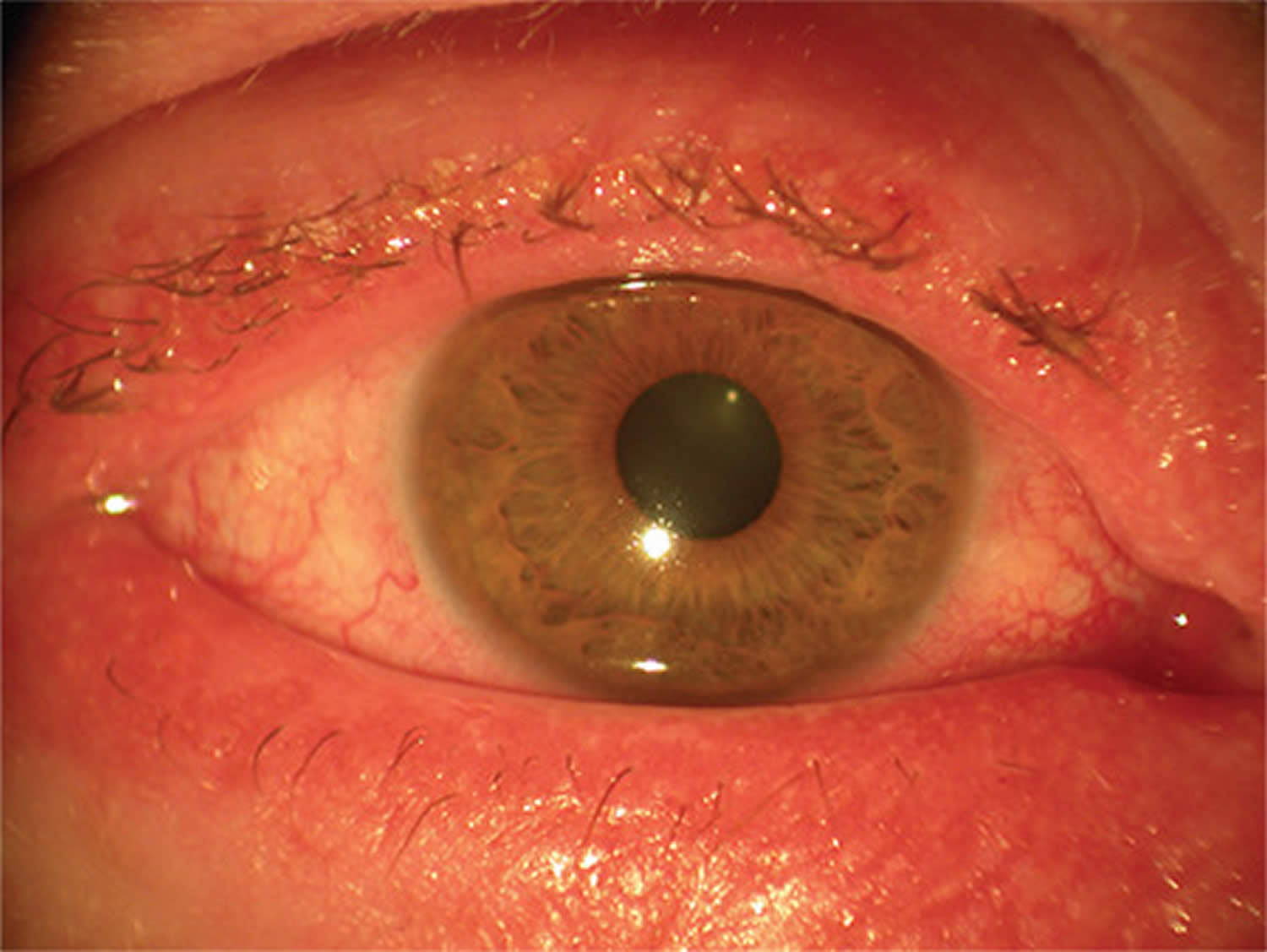
Figure 7. Allergic keratoconjunctivitis
Allergic keratoconjunctivitis causes
Allergic keratoconjunctivitis is caused by contact with something to which a person may be sensitive or allergic to (allergens), air pollution, atopy, pollen exposure, inflammation, and pet hair 64. Examples of common allergens to the conjunctival surface include tree/grass pollen, house dust mites, animal/pest dander, and mold spores 64, 82. Spring, summer and fall allergies tend to be caused by trees weed, grass, and flower pollen. Some people can have allergies all year round due to other household allergens, including dust, mold and animal dander/hair/fur. Some children may have an underlying medical problem making them more at risk for an allergic eye condition.
Allergic keratoconjunctivitis signs and symptoms
Allergic keratoconjunctivitis symptoms may vary from person to person. Allergic keratoconjunctivitis symptoms can be very mild or very severe. Itching is the most common symptom and eye allergy is unlikely to be present if itching is not present. Other symptoms may include stinging, tearing, and burning. The conjunctiva is usually pink and/or bloodshot. The white area immediately around the colored part of the eyes can also swell, causing tiny bumps visible on the surface of the eye. Eyelid skin can also be affected, becoming thick, swollen, itchy, or red. Children may frequently rub or roll their eyes if they have allergies. They may even tightly squeeze or blink frequently to help with the itchiness. Symptoms are often worse in the spring and/or summer months, but may stick around throughout the year.
Typical signs and symptoms of allergic keratoconjunctivitis include:
- Redness in both eyes.
- Itching and burning of both the eye and surrounding tissues.
- Watery discharge, often accompanied by acute discomfort in bright light (photophobia).
- Swollen eyelids which may become ‘heavy’ or ‘droopy’. In some severe cases, the eyelids are so swollen that they cannot completely open.
- Swollen conjunctiva which may look light purple and affect vision. Blurred vision or any change in the appearance of the cornea (clear part of the eye that covers the pupil) requires urgent referral to an eye specialist. Speak to your doctor or optometrist for a referral.
Allergic keratoconjunctivitis complications
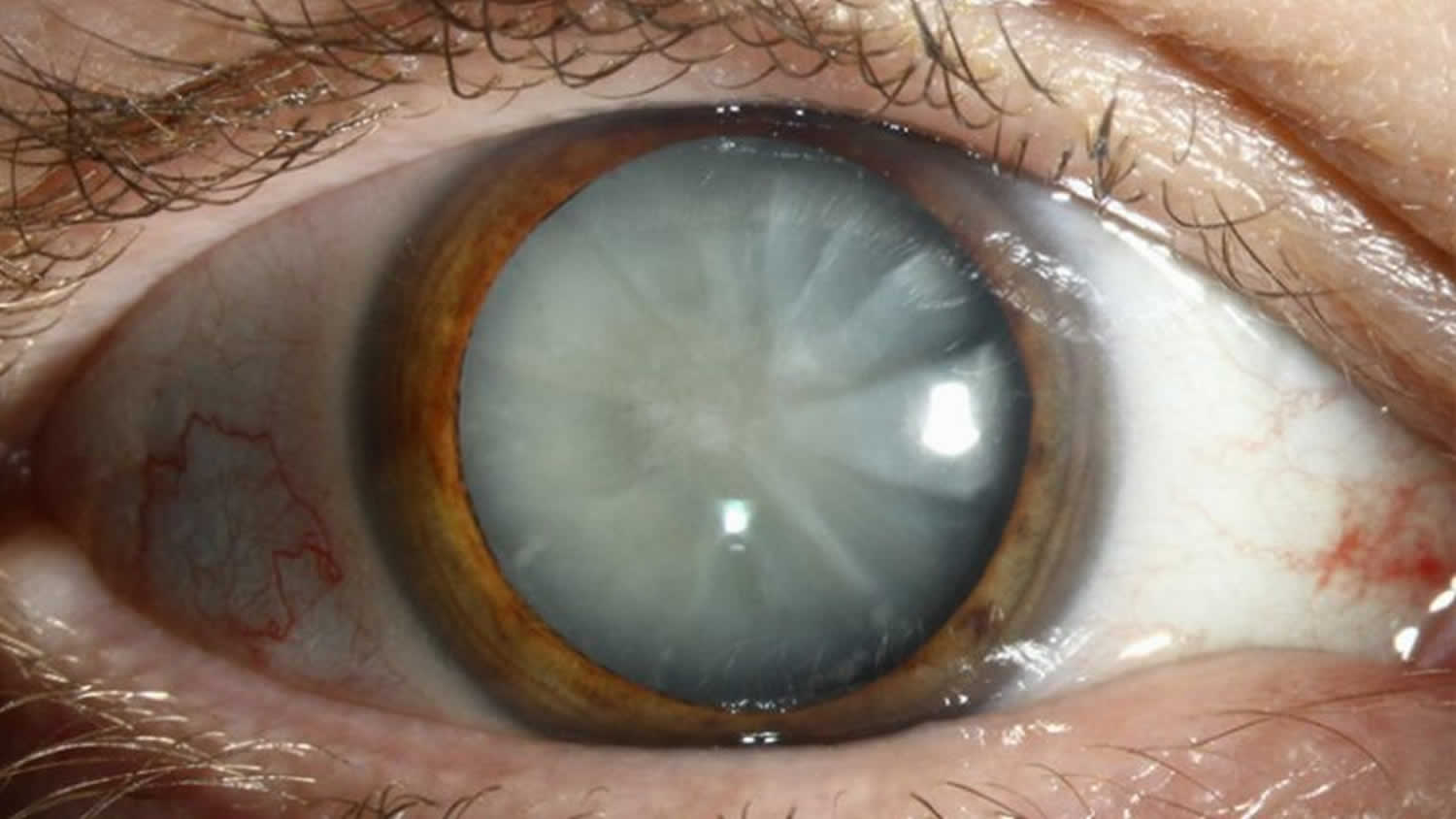
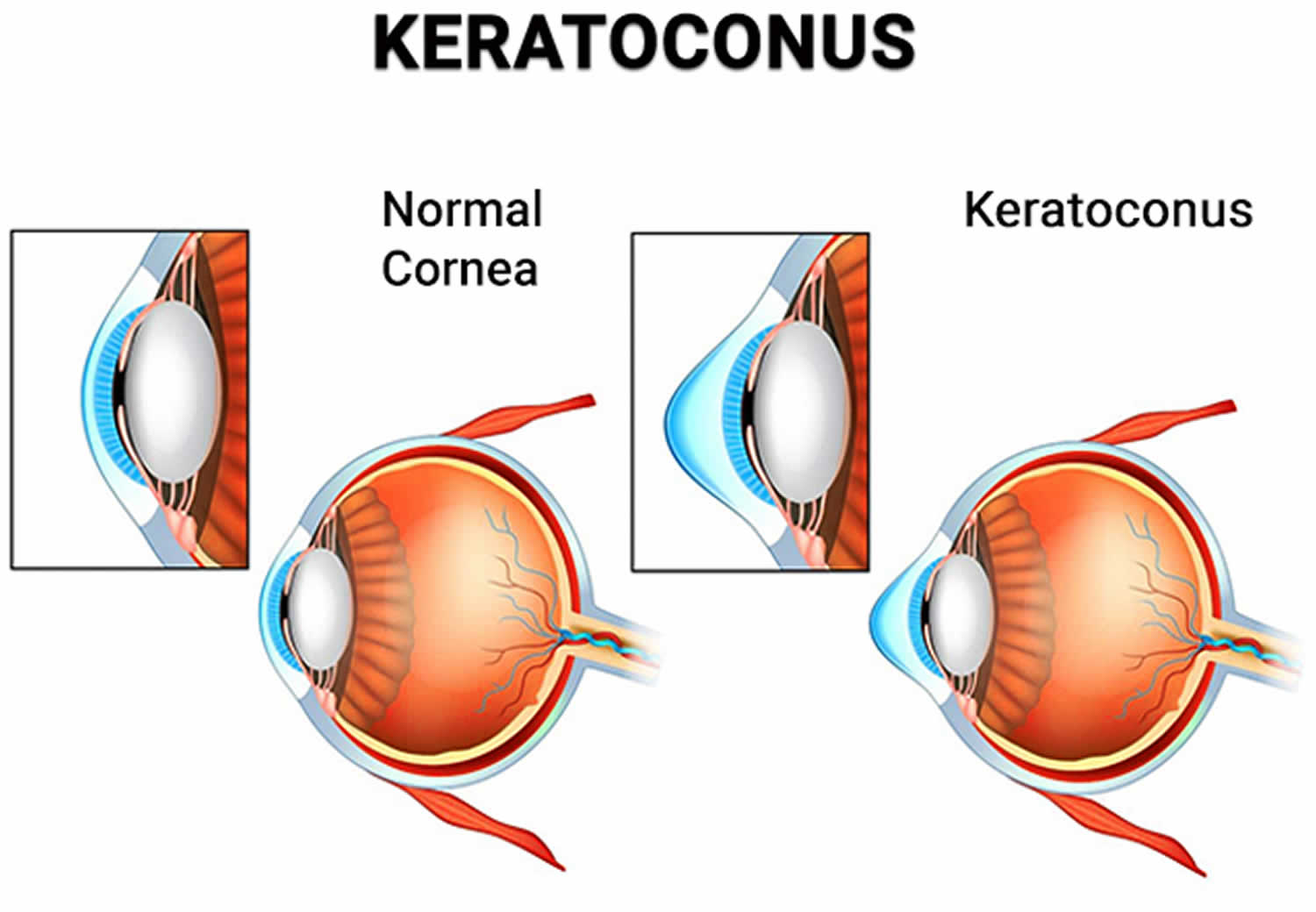
In most cases, allergic keratoconjunctivitis is not a severe health threat, but a common and often benign condition. Most often, allergic keratoconjunctivitis complications are because of poor compliance to treatment on the part of patient, or inadequate control of the disease when it presents in its severe form 83. Common complications include dry eye, infection and corneal scar 83. Chronicity of the untreated disease may lead to vision threatening problems like limbal stem cell deficiency (LSCD) and secondary keratoconus due to rubbing of the eyes 83.
As the treatment involves use of corticosteroids, steroid-induced raised intraocular pressure and cataract have been reported in these patients 84. Complications may lead to irreversible visual loss in some patients 84. Both keratoconus and limbal stem cell deficiency need timely surgical treatment to prevent visual malfunction 83.
Complications of allergic keratoconjunctivitis are rare, but when they do occur, they can be severe and may include 64:
- Scarring of the eye (severe cases).
- Progression to infective conjunctivitis, leading to the spread of infection to other areas of the body and potentially causing serious secondary infections.
- Conjunctival scarring
- Bacterial keratitis
- Corneal neovascularization
- Pannus
- Giant papillae
- Nonresolving epithelial defect
- Shield ulcer
- Steroid-induced glaucoma
- Dry eyes
- Keratoconus
- Herpetic keratitis
Allergic keratoconjunctivitis diagnosis
A diagnosis of allergic keratoconjunctivitis is made by history and examination. Although allergy testing may help pinpoint the specific allergens, it is usually not necessary since the types of allergens that usually cause conjunctivitis are very common, like grass, weed, and tree pollens. Eye drop treatments are the same no matter what allergen is causing the reaction.
If further investigation is needed, even if no identifiable allergens have been found, the second step is skin prick or patch tests 80. Patch tests are preferred in contact blepharoconjunctivitis, while skin prick tests are used in the other diseases 80. These tests are carried out with a standard battery of allergens and sometimes with others that are not normally tested but suspected as the cause of the allergy. If skin testing is indicated but not recommended (e.g., the patient is taking antihistaminic systemic medications), or if results are ambiguous (e.g., presence of dermatographism), or simply to complement the results of previous skin prick test, serum specific IgE measurements for the aeroallergens can be considered 85, 86.
In case of doubt after systemic allergy evaluation tests, a conjunctival allergen provocation test (CAPT) also known as conjunctival allergen challenge or ocular challenge test may be of use to identify the cause 86. In the conjunctival allergen provocation test (CAPT) an allergen is applied to the conjunctival mucosa to evaluate the patients’ immunoreactivity to the allergen. The conjunctival allergen provocation test (CAPT) is used to confirm which allergens the patient is sensitive to and has the same scientific background as other provocation tests used extensively in other mucosae such as nasal or digestive 86, 87. Non-specific or irritant challenges evaluate the hyperreactivity of the ocular mucosa, whilst direct mucosal challenges contain higher concentrations of the allergen encountered in environmental exposure and evaluate patients’ immunoreactivity to the allergen, following the guidelines for standard practice of the European Academy of Allergy and Clinical Immunology 86, 87, 88. A positive test will trigger the same signs and symptoms as those occurring when the allergen is encountered in real life, an IgE-mast cell-dependent immunoreactivity 89, 87. The conjunctival allergen provocation test (CAPT) is also useful to assess the relationship between symptoms and exposure in polysensitized patients and to assess response to therapy 85.
Allergic keratoconjunctivitis treatment
Allergen avoidance is the first line of treatment for allergic keratoconjunctivitis 90, 91, 92. In the case of pollen allergies, symptoms are often made worse by outdoor activities. Wearing glasses or goggles outdoors can limit contact with allergens. Minimization measures for house dust mites may include removing carpet, using dust mite covers for pillows and mattresses, and washing bedding in hot water are enough to reduce symptoms. Frequent washing of pillowcases and mattress covers as well as vacuuming the carpeting in your room help remove allergens from your surroundings. Regular washing your hair and face can help remove these allergens from the surface of your eyes, hair, and skin. Animal dander can be reduced by keeping animals outside, avoiding touching them or rubbing your eyes after exposure, and washing your hands/clothes after coming in contact 90, 91. Using artificial tear drops to rinse the eye and remove allergens from the eye can help with symptoms and help calm down the eye inflammation. These drops can provide even more relief when used cold (refrigerated) instead of at room temperature. Other non-pharmacological interventions include using cool compresses to decrease eye swelling and redness in irritated eyes. It is also important to avoid rubbing the eyes, as this can make allergic conjunctivitis worse.
If you have allergic keratoconjunctivitis, both prescription and over-the-counter allergy eye drops can treat allergic keratoconjunctivitis 12. These may include medicines that help control allergic reactions, such as antihistamines and mast cell stabilizers. Or your doctor may recommend medicines to help control inflammation, such as decongestants, steroids and anti-inflammatory drops. Nonprescription versions of these medicines also may be effective. Most of the easily available allergy eye drops work best when used daily for at least a few weeks, and it may take up to a week to get full symptom relief. Some eye drops can be used only on an as needed basis. Allergy eye drops may work better for some than pill or liquid medications as eye drops do not cause any drowsiness or changes in appetite. However, pill or liquid medications may be more helpful if allergies cause a lot of eyelid swelling or affect more than just the eyes. Please speak with your ophthalmologist if you have questions about allergy medications.
Given the different types of over-the-counter eye drops, sometimes what works well for one person may not work as well for another person. You may need to try different types of eye drops before you find one that works for you. If there are still allergic conjunctivitis symptoms even after trying different kinds of allergy eye drops, adding a short-term liquid or pill allergy medication by mouth may help relieve symptoms. Pills and liquid medications by mouth may also a good treatment for people who don’t do well eye drops, or who have other allergy symptoms like a runny nose.
In some cases, steroid eye drops may be needed along with allergy eye drops if the allergic reaction is very severe. However, steroid use needs to be monitored closely by your ophthalmologist and used only as directed to prevent serious eye problems. Use of steroid drops for a long time or at a large amount can cause serious vision problems, including glaucoma, cataracts and eye infections (keratitis). Only ophthalmologists (eye specialists) who can monitor for side effects should prescribe steroids for allergic conjunctivitis. Talk with your ophthalmologist if you have questions about steroid drops.
Topical medications (eye drops) treat the symptoms of allergic keratoconjunctivitis directly. Small drops of medication are delivered straight to the surface of the eye and are available in many different types.
- Antihistamine eye drops – effective but should not be used for longer than 6 weeks without medical advice.
- Antihistamine eye drops containing a vasoconstrictor (substances that cause the walls of blood vessels to narrow, or constrict) – minimize itch and remove redness by narrowing the swollen blood vessels in the eye. They should not be used for longer than 14 days without medical advice.
- Mast cell stabilizer eye drops – best used to prevent symptoms from occurring as they can take three to seven days to work. These can be used as long as necessary.
- Mast cell stabilizer eye drops with antihistamines – fast acting, effective and generally well tolerated.
- Steroid eye drops – effective in relieving symptoms quickly, but are associated with cataract formation, glaucoma and bacterial and viral infections of the cornea and conjunctiva. They should only be used under medical supervision as a short-term treatment and should never be used in the presence of herpes infections.
Antihistamine tablets or syrups help some people when it is difficult to avoid the allergen. Some side effects may include dryness of the eyes, nose, and mouth, and blurred vision. Antihistamines are usually contraindicated for people with glaucoma, advice should be sought from an eye specialist.
Allergen immunotherapy for specific allergens may benefit people with persistent, severe allergic conjunctivitis. However, relief of allergic conjunctivitis symptoms will not happen straight away.
Table 3. Allergic conjunctivitis treatment options
| Drug Class | Mechanism of Action | Common Examples | Eye Side Effects |
|---|---|---|---|
| Vasoconstrictors (Decongestants) | a-adrenergic agonists (mainly stimulation of a-1 receptors) | Phenylephrine, Brimonidine, Ephedrine, Naphazoline, Tetrahydrozoline | Rebound redness, conjunctivitis medicamentosa |
| Antihistamines | Competitive blockage of histamine receptors (all block H1 with some blocking H2, H3 and/or H4) | Levocabastine, Emedastine | Dryness, irritation |
| Mast Cell Stabilizers | Inhibit degranulation of mast cell and consequent histamine release | Sodium cromoglycate, Nedocromil sodium, Pemirolast, Lodoxamide | Stinging, Burning |
| Dual-Acting Agents | Inverse agonists of histamine receptors and prevent mast cell degranulation | Olopatadine, Ketotifen, Azelastine Epinastine, Alcaftadine | Burning, headache, dry eye |
| NSAIDS | Inhibits cyclooxygenase enzymes (COX-1 and COX-2) resulting in inhibition of prostaglandins | Ketorolac, Diclofenac Flurbiprofen | Stinging, burning, corneal melt |
| Corticosteroids | Inhibits phospholipase A resulting in the inhibition of prostaglandins and leukotriene synthesis | Dexamethasone, Prednisolone, Loteprednol, Fluorometholone, Rimexalone | Increased intraocular pressure, cataract formation, delayed wound healing |
| Immunomodulators | Cyclosporin A, Tacrolimus | Inhibiting production of IL-2 resulting in inhibition of T-cell activation | Burning, irritation |
Eye drops for allergic conjunctivitis
When you have an allergic reaction your body releases histamine from mast cells, which leads to hay fever. Antihistamines block this reaction. Antihistamines act via histamine receptor antagonism to block the inflammatory effects of histamine and relieve any associated signs and symptoms. Most antihistamines used in the treatment of eye allergy are histamine-1 (H1) receptor antagonists, although some agents have affinity for other subtypes. Histamine-2 (H2) antagonists have been shown to modulate both cell growth and migration. Animal model studies have shown that antihistamines may even reduce infiltration of eosinophils and thus reduce the clinical aspects of the late-phase reaction 93.
First-generation antihistamines are well tolerated and associated with a favorable long-term safety record, but are associated with instillation pain, short duration of action, and limited potency 94. First-generation antihistamines remain available in over-the-counter products, particularly in combination with vasoconstrictors (substances that cause the walls of blood vessels to narrow, or constrict). While newer antihistamines are also H1 antagonists, they have a longer duration of action (4–6 hours) and are better tolerated than their predecessors 93.
Topical antihistamines are widely available without a prescription. Antihistamines competitively block histamine receptors (e.g., H1 or H4) on nerve endings and blood vessels of the mucosal surface, thereby reducing itchiness and conjunctival redness 95. First-generation antihistamines were associated with a range of systemic side effects (e.g., sedation, dizziness, cognitive impairment, blurred vision) caused by anticholinergic actions and nonspecific binding to histamine H2 receptors in addition to drying of the ocular surface. Newer oral, intranasal, and topical ocular antihistamines demonstrate improved H1 receptor selectivity, with fewer adverse effects; however, eye side effects, such as burning and dryness, remain a concern. Topical antihistamines (e.g., levocabastine, emedastine difumarate) are useful for providing rapid relief of allergic conjunctivitis symptoms, but their duration of action is limited; most topical antihistamines require dosing four times.
Antihistamine eye drops
Antihistamines are competitive antagonists of histamine receptors that are present in the conjunctiva and eyelids. Once stimulated, histamine receptors lead to capillary dilation and increased vascular permeability, which leads to common allergic symptoms of itching and edema. Therefore, antihistamines work by preventing the binding of histamine to histamine-1 (H1) receptors and preventing the cascade of inflammatory events. In the eye, only H1 receptors are available 96. Examples include azelastine (Eyezep Eye Drops), levocabastine (Livostin Eye Drops, Zyrtec Levocabastine Eye Drops) and emedastine.
Mast cell stabilizers
Mast cell stabilizers work by preventing the degranulation of sensitized mast cells, thus stopping the release of histamine and other inflammatory mediators 90, 97. Since mast cell stabilizers act before the mast cell is degranulated, they rarely have an impact on the inflammatory mediators once they are already released 98, 97. In other words, mast cell stabilizers are not effective once the patient is symptomatic, and clinical trials have had a difficult time showing their efficacy 98. Since there are other quicker and more effective treatment agents available on the market, mast cell stabilizers are rarely used as monotherapy. The most common mast cell stabilizers used for allergic conjunctivitis are sodium lodoxamide 0.1% (Alomide), cromoglycate 2%, and nedocromil 2% 90, 92. Mast cell stabilizers can be used as a prophylactic measure to prevent mast cell degranulation for repeated exposures to the allergen 99.
Antihistamine and mast cell stabilizer eye drops
When you have an allergic reaction your body releases histamine from mast cells, which leads to hay fever. Mast cell stabilizer medicines help reduce this histamine release after allergen exposure and reduce allergic reactions and hayfever 100. Topical mast cell stabilizers act to prevent mast cell degranulation and subsequent release of proinflammatory molecules triggered by IgE binding to sensitized conjunctival mast cells. Topical mast cell stabilizers (e.g., cromolyn sodium, lodoxamide tromethamine, nedocromil sodium, pemirolast potassium) effectively prevent activation of the early phase response by preventing release of histamine, cytokines, and other inflammatory and chemotactic mediators. Preventing the early phase response blocks downstream inflammation events, including production of prostaglandins and leukotrienes, eosinophil infiltration, chemokine and adhesion molecule expression, and chronic mast cell activation that perpetuate the late-phase response in allergic conjunctivitis. Most mast cell stabilizers require administration four to six times daily; nedocromil sodium can be given twice daily. Because of the required loading time for maximal efficacy of mast cell stabilizers, these medications are most effective when treatment is initiated before symptoms manifest; their effectiveness is limited when allergic conjunctivitis cascades have been activated and mast cell degranulation and histamine release have already occurred.
Dual-Acting Antihistamine–Mast Cell Stabilizing Agents
Agents with dual antihistamine and mast cell stabilizing actions are more suitable for extant allergic conjunctivitis than single-action medications because they block binding of free histamine to receptors and inhibit further release of proinflammatory mediators from mast cells. This dual action rapidly alleviates multiple signs and symptoms of allergic conjunctivitis in the short term and blocks the feed-forward cycle of persistent inflammation caused by continuous mast cell activation in the long term to promote regression of allergic conjunctivitis. Antihistamine–mast cell stabilizing agents (e.g., olopatadine, alcaftadine, epinastine, bepotastine besilate) are currently considered first-line therapeutics for allergic conjunctivitis because they offer acute symptomatic relief and control inflammation, and can be used chronically without long-term safety concerns. Most dual-acting agents require twice-daily dosing. Olopatadine 0.2% and alcaftadine are indicated for once-daily dosing and maintain effectiveness through 16 hours after administration in conjunctival allergen challenge studies 101, 102, 103.
Olopatadine 0.1% (Pataday Twice Daily Relief) was the first topical anti-allergy medication that was approved for twice-daily usage 90. These agents are all preserved with a surfactant called benzalkonium chloride that may cause ocular surface toxicity 104. These are now considered the first line of treatment for allergic eye disease and are the most common ophthalmic agents recommended by eye care practitioners and allergists 90. These agents can be used to prevent mast cell degranulation and acutely following the onset of symptoms 105.
Compared to placebo, olopatadine has been found to improve symptoms of eyelid swelling, eye redness, chemosis (a condition where the conjunctiva, or the outer surface of the eye, swells due to fluid build-up), itch, and overall quality of life. Multiple randomized control trials have compared ketotifen and olopatadine. One meta-analysis found improvement in symptoms of itching after 14 days in favor of olopatadine 0.1% when compared to ketotifen 0.025% 106. Before 2020, olopatadine was only available as a prescription medication, and ketotifen, in the form of Zaditor or Alaway, was clinically commonly prescribed as the first line of relief as an OTC medication. Within the last 4 years, olopatadine became available OTC and has gained popularity to become clinically superior to ketotifen in terms of efficacy.
Leukotriene Receptor Antagonists
Leukotriene receptor antagonists (e.g., montelukast), which are currently available for oral dosing, prevent leukotrienes from binding to their conjunctival receptors to decrease inflammatory signaling and improve multiple ocular symptoms of allergic conjunctivitis. Leukotriene receptor antagonists have a slower onset of action, are less effective than topical antihistamines, and are not used as first-line therapy or monotherapy for allergic conjunctivitis 95.
Topical Vasoconstrictors (Decongestants)
Topical vasoconstrictors (decongestants) were the first ocular medication approved for the treatment of allergic conjunctivitis. Over-the-counter (OTC) topical vasoconstrictors are effective at temporarily decreasing conjunctival hyperemia by stimulating alpha-adrenergic receptors 98. Alpha-adrenergic agonists cause vasoconstriction of conjunctival blood vessels, resulting in decongestion and whitening of the eye 107, 98. However, the use of these agonists can lead to side effects such as rebound hyperemia and tachyphylaxis and, chronically, can lead to conjunctivitis medicamentosa 99, 98. Commonly used topical vasoconstrictors are oxymetazoline, naphazoline, tetrahydrozoline, and phenylephrine. These are best utilized as short-term solutions and should be avoided in narrow-angle glaucoma and cardiovascular issues 107, 98. These should not be recommended as a standalone treatment and used in combination with antihistamines for the treatment of allergic conjunctivitis 107, 108.
Combination eye drops including decongestant (Antihistamine-Vasoconstrictor Combinations)
Some eye drops contain an antihistamine such as pheniramine or antazoline to stop itching, and a vasoconstrictor (substances that cause the walls of blood vessels to narrow, or constrict) such as naphazoline to take away redness through stimulation of vascular alpha-adrenergic receptors e.g. naphazoline + antazoline (Antistine-Privine, Albalon-A), pheniramine + naphazoline (Visine Allergy with Antihistamine, Naphcon-A). Vasoconstrictors are commonly available in nonprescription combination formulations that contain an antihistamine (e.g., naphazoline-antazoline, naphazoline-pheniramine). These formulations exhibit a rapid onset of action and relieve redness and itchiness associated with allergic conjunctivitis. However, they are not recommended for long-term use because of reduced effectiveness over time and a potential rebound effect that can produce persistent red eye on discontinuation 100. As with topical antihistamines, combination antihistamine–vasoconstrictor formulations have a relatively short duration of action and are administered four times daily 78, 12. Limit use of combination eye drops to no more than 5 to 7 days to avoid a ‘rebound’ redness from overuse.
Other eye drops, to prevent allergy symptoms
These eye drops prevent allergic reactions in the eyes and need to be used 4 to 6 times per day, depending on the ingredient, for the entire time you are exposed to triggers, such as during spring e.g. cromoglycate (Cromolux Eye Drops, Opticrom), lodoxamide (Lomide Eye Drops 0.1%)
Oral antihistamines (tablets and syrups)
There are two types of oral antihistamines: newer, less sedating antihistamines, which do not typically cause drowsiness and older sedating antihistamines that cause drowsiness.
Oral antihistamines are good for treating hay fever symptoms, especially if you have a lot of different symptoms. You can also take oral antihistamines in advance if you know you are going to be exposed to allergens or triggers
Newer, less-sedating antihistamines
Newer antihistamines may rarely cause drowsiness; do not drive or operate machinery if you are affected e.g. cetirizine (Zilarex, Zyrtec), desloratadine (Aerius), fexofenadine (Fexotabs, Telfast), loratadine (Claratyne, Lorano).
Cetirizine and loratadine are available as syrups for children; check correct doses for different age groups. Cetirizine is more likely to cause drowsiness than other less sedating antihistamines
Older, sedating antihistamines
Older sedating antihistamines can cause drowsiness, sometimes the next day; it is important you do not drive or operate machinery e.g. chlorpheniramine + pseudoephedrine (Demazin 6 Hour Relief Tablets), dexchlorpheniramine (Polaramine), loratadine + pseudoephedrine (Claratyne-D with Decongestant Repetabs), promethazine (Phenergan, Sandoz Fenezal)
Older antihistamines are not available without a prescription for children under 2 years old. Do not drink alcohol with antihistamines that make you drowsy
If you have other medical conditions, such as glaucoma, epilepsy or prostate problems, or you take antidepressants, check with your doctor before taking these medicines
Topical nonsteroidal anti-inflammatory drugs (NSAIDS)
Topical nonsteroidal anti-inflammatory drugs (NSAIDS) act by blocking the cyclooxygenase enzymes (COX-1 and COX-2) within the cyclooxygenase pathway, resulting in the inhibition of inflammatory mediators such as prostaglandins and leukotrienes 98, 97. These drugs have proven efficacy against conjunctival hyperemia, pruritus, pain, and irritation 98. Topical NSAID agents commonly associated with the relief of ocular allergy symptoms include ketorolac, diclofenac, indomethacin, and flurbiprofen 98, 97. Although ketorolac has been approved for treating allergic conjunctivitis, studies have indicated that it is less effective compared to topical antihistamine agents 98, 97, 109. Moreover, these agents can cause burning and stinging sensations upon instillation, so long-term compliance is an issue. Thus, NSAIDs may be used for temporary relief of itching and hyperemia compared to no treatment; however, they do not aid with symptoms of mucous discharge, chemosis (a condition where the conjunctiva, or the outer surface of the eye, swells due to fluid build-up), and corneal damage, so alternative methods should be considered 98, 97, 109, 110.
Corticosteroids
Corticosteroids prevent production of multiple classes of late-phase response mediators, including prostaglandins, leukotrienes, histamine, and some cytokines. The numerous points of intervention in the inflammatory cascade make glucocorticoids an effective pharmacologic therapy for allergic conjunctivitis, but long-term topical use can lead to serious adverse effects, including increased intraocular pressure and corneal abnormalities 100. Long-term systemic corticosteroid use increases the risk of posterior subcapsular cataract formation 111. For this reason, patients at risk (e.g., those with glaucoma or diabetic retinopathy) or patients who receive higher doses or longer treatment courses of corticosteroids should be monitored by an ophthalmologist.
Corticosteroids (e.g., loteprednol etabonate, given four times daily) are generally not used as primary therapy for allergic conjunctivitis unless there is persistent or moderate-to-severe inflammation that the eye doctor does not feel will respond sufficiently to antihistamine–mast cell stabilizer medications alone 78. When corticosteroids are prescribed, they typically are used for short durations in the early stages of allergic conjunctivitis or during flare-ups until allergic conjunctivitis can be controlled with safer medications such as antihistamines, mast cell stabilizers, or dual-acting, single-molecule antihistamine–mast cell stabilizer agents 112. Topical corticosteroids are important in severe cases of allergic eye disease to break the cycle of inflammation and can be discontinued once the condition is under control. Most cases of seasonal allergic conjunctivitis or perennial allergic conjunctivitis do not often require corticosteroid intervention. For patients who require long-term use of corticosteroids, close observation by an ophthalmologist is warranted.
Supratarsal steroids
Supratarsal steroids are required in patients with the severe palpebral form of the disease who are unresponsive to topical steroids or are non-compliant. The conjunctiva is everted, and injection is given in the supratarsal conjunctiva. An injection of 0.1 ml betamethasone 4 mg/mL, dexamethasone 4 mg/mL, or triamcinolone can be provided 113.
Topical Calcineurin inhibitors
The topical calcineurin inhibitors cyclosporine A and tacrolimus are very effective in the treatment of giant papillary conjunctivitis (GPC), atopic keratocongiuntivitis (AKC), and vernal keratoconjunctivitis (VKC) and may serve as steroid-sparing agents when these forms of chronic allergic conjunctivitis become steroid-dependent 114, 86.
Cyclosporine A (CsA) is also used worldwide for the treatment of dry eye. Suspensions of cyclosporine A may be prepared in pharmacies, although some countries also have commercialized forms 115. The concentrations of cyclosporine A in the different ophthalmic formulations range between 0.01% and 2% and therefore the administration varies between 1 and 6 times per day 88. Tacrolimus may also be prepared as suspension by pharmacies and exists as an ointment for dermatological purposes in most countries at a concentration of 0.03-0.1%. Recent research shows that tacrolimus may have similar if not superior effectivity than cyclosporine A for the treatment of vernal keratoconjunctivitis (VKC). Moreover, dermatologic ointment containing tacrolimus is effective for the treatment of lid eczema in atopic keratocongiuntivitis (AKC) 114, 86. Topical treatment with calcineurin inhibitors has side effects such as stinging/burning sensation and the possibility of molluscum contagiosum virus, papillomavirus, or herpesvirus infection, although there is evidence from studies on dry eye syndrome that treatment with cyclosporine A can be topically administered long term and without systemic absorption 86, 88, 116, 115. At present, tacrolimus is generally administered topically in cases that do not respond to cyclosporine A.1 Finally, in very severe cases, allergic conjunctivitis such as VKC and atopic keratocongiuntivitis (AKC) may require systemic immunosuppression that is usually achieved with cyclosporine A, tacrolimus, or mycophenolate mofetil 114, 86, 117.
Biologicals
In theory, biological treatments could have superior results because they block the underlying inflammation pathways by bonding with specific biological molecules to decrease conjunctival inflammation 118. A few trials have reported the systemic use of the biologicals omalizumab, indicated for severe asthma, and dupilumab, indicated for atopic dermatitis, in vernal keratoconjunctivitis (VKC) and atopic keratocongiuntivitis (AKC). Omalizumab shows generally good results, though it has not yet been approved for allergic conjunctivitis, while dupilumab may increase the risk of blepharoconjunctivitis, which is tacrolimus-responsive in patients with severe atopic disease or previous atopic keratocongiuntivitis (AKC) 114, 119, 120, 121. Benralizumab, mepolizumab, and reslizumab, which are anti IL-5 biologic agents have not been studied in the context of allergic conjunctivitis 85.
Insunakinra (EBI-005) is the first inmunophilin synthesized for topical ophthalmologic use. It is an antagonist of the IL-1 receptor and binds to it, blocking the rest of the pathway. It has been documented to diminish ocular surface symptoms such as itching, inflammation, and discomfort 122.
Another molecule called liftitegrast (Shire Pharmaceuticals) has both activity as an antagonist of the IL-1 receptor and as antagonist of the lymphocyte functional antigen-1 and has proven effective for treatment of ocular surface symptoms 123.
Immunotherapy
The goal of immunotherapy is to diminish the symptoms and signs of rhinitis and conjunctivitis triggered by known allergens and to prevent their recurrence. Allergen-specific immunotherapy may be considered in cases of failure of first line treatments, or as a modifier of the natural course of the disease 114, 124, 125. Changes involve downregulation of Th2 response and upregulation of regulatory T-cells 85. It is carried out by administering gradually increasing amounts of the allergen to induce an immunological tolerance. According to the European Academy of Allergy and Clinical Immunology guidelines, it is indicated in patients with a documented IgE-mediated hypersensitivity to airborne agents, with severe forms of rhinoconjunctivitis that affect their quality of life in spite of allergen avoidance and pharmacotherapy 126, 127, 128. Immunotherapy can also be applied in children, but because it requires a strict regimen of desensitization, it may be difficult to treat children below 6 years of age 129, 125. There are commercial forms of many recognized allergens and the allergist determines the allergen to be prescribed based on previous hypersensitivity tests. Desensitization consists of two phases, an induction phase that lasts 5-8 months and a maintenance phase that last 3-5 years 125, 128. The standard method of administering the antigen has been subcutaneous injection, but recently other less invasive methods have been developed, such as sublingual or epicutaneous administration, with good results 116, 125. Adherence to sublingual is deemed better because it does not involve injections but has not been studied as exhaustively as subcutaneous injection; more randomized controlled trials are needed. Other forms of immunotherapy such as intralymphatic administration or edible vaccines are still being studied 116, 128.
In isolated allergic conjunctivitis (IgE- and non-IgE-mediated), allergen immunotherapy may be considered on the same premise as in rhinoconjunctivitis. However, there is less evidence of its beneficial effects and a few studies have documented an improvement of the clinical symptoms in vernal keratoconjunctivitis (VKC) but not in atopic keratocongiuntivitis (AKC) 116, 130, 117, 128.
Surgery
In very recalcitrant cases of atopic keratocongiuntivitis (AKC) and vernal keratoconjunctivitis (VKC), eye surgery may be needed. Papillae resection, in some cases with grafting of autologous conjunctiva, amniotic membrane or mucous membrane are effective in the treatment of severe forms of vernal keratoconjunctivitis (VKC) with corneal ulcers 127, 131, 132. Plaque resection may be necessary for subepithelial deposits in vernal keratoconjunctivitis (VKC) 133, 134. In atopic keratocongiuntivitis (AKC), surgery may be needed for eyelid and conjunctival scarring.
Atopic disease and atopic keratocongiuntivitis (AKC) can be complicated by subcapsular cataracts and/or severe ocular surface disease that may require complex surgery such as superficial keratectomy, limbal transplantation, or keratoprosthesis implantation 135.
Allergic conjunctivitis home remedies
If your conjunctivitis is caused by allergies, stopping the source of the allergy is important. Allergic conjunctivitis will continue as long as you’re in contact with whatever is causing it. Allergic conjunctivitis is not contagious. You can still go to work or school with allergic conjunctivitis and no one else will catch it.
To reduce your symptoms of allergic conjunctivitis you can:
- Take allergy medicine or use allergy eye drops.
- Put a cool, damp washcloth over your eyes for a few minutes.
- Use over-the-counter lubricating eye drops (artificial tears).
Allergic conjunctivitis prognosis
Most people with allergic keratoconjunctivitis the prognosis for allergic conjunctivitis is good 66, 64. However, visual impairment can occur related to certain types of severe eye allergies, too much eye rubbing, eye infections, corneal damage or steroid use. Additionally, the medications used to manage allergic conjunctivitis can be associated with the development of cataracts 64.
Vernal keratoconjunctivitis
Vernal conjunctivitis also called vernal keratoconjunctivitis is a severe type of seasonal allergic conjunctivitis that results in small, raised lumps on the inside of the upper eyelid called Horner Tranta dots and a stringy (ropey), mucus discharge (Figures 8, 9 and 10) 136, 137, 138. Large bumps or papillae on the conjunctiva are a classic sign of vernal conjunctivitis. Histopathology shows eosinophils in the conjunctival secretions. Vernal conjunctivitis is frequently associated with allergic rhinitis, atopic dermatitis, or asthma. Vernal conjunctivitis may be associated with a single allergen but more usually with multiple sensitivities. Vernal conjunctivitis is often seen in areas of the world where the weather is hot and dry than in cold climates, most commonly in Asia, Central and West Africa and South America 139, 140, 141, 142, 143, 138. Vernal conjunctivitis is also seen commonly in the Middle East, Japan, India, the Mediterranean area, North America, and Australia 144, 145. At least one study showed prevalence ranges from 4.0 to 11.1% prevalence of vernal keratoconjunctivitis in African countries schoolchildren 141, 146. Vernal conjunctivitis is thought to be relatively unusual in North America and Western Europe 147, 144. One European study demonstrated the prevalence of vernal conjunctivitis was between 1.2 to 10.6 per 10,000 and 0.8/10,000 with corneal complications has been reported 142. The increased incidence in hot regions is speculated to be secondary to a higher level of pollution by pollens and various other allergens 138.
Vernal conjunctivitis is a T-helper-2 (Th2) lymphocyte-driven disease characterized by infiltration of the conjunctiva by a number of inflammatory cell types, including eosinophils, mast cells, and T lymphocytes 148, 149. This differs from atopic keratoconjunctivitis, which has been shown to involve both Th1 and Th2 inflammatory cascades 150. Increased levels of tumor necrosis factor (TNF) alpha, histamine, tryptase, IgE, and IgG antibodies are observed on pathologic examination of tears 151. It is believed that the exaggerated immunoglobulin E (IgE) response observed with vernal conjunctivitis in response to common allergens may be a secondary event 149. Mast cells and basophils cause the immediate reaction through the release of histamine and the recruitment of inflammatory cells lymphocytes and eosinophils 136. This results in the release of a number of pro-inflammatory cytokines, including but not limited to interleukin (IL)-4, IL-5, and IL-13, as well as other toxic cell mediators such as eosinophil cationic protein, eosinophil-derived neurotoxin/eosinophil protein X (EDN/EPX) that result in corneal damage 149, 152. Release of these factors mediates the remodeling, eye inflammation, and itch that are commonly associated with vernal conjunctivitis.
Diagnosis and treatment of vernal conjunctivitis is a challenge for many ophthalmologists, since no precise diagnostic criteria have been established, the pathogenesis of the disease is unclear, and anti-allergic treatments are often ineffective in patients with moderate or severe disease 136.
Vernal conjunctivitis usually affects both eyes and is severe, occurring seasonally and mainly in children and more common in young boys than girls, but this difference becomes smaller as age increases 153. Often, patients with vernal conjunctivitis also have asthma or eczema. The majority of vernal conjunctivitis occurs in patients between the ages of 5 to 25 years of age with an age of onset between 10 to 12 years old; however there are reports of patients as young as 5-months-old 145, 154. Symptoms typically occur throughout the year, but are worse in spring (vernal means springtime in Latin) and summer time. 23% of patients may have a perennial (all year round) form of them disease and many may have recurrences outside of the springtime 145, 153. Symptoms can be so bad that children need to be treated with topical (eye drops) or systemic (oral tablets) steroids in addition to allergy eye drops such as topical cromolyn or antihistamine preparations. However, vernal conjunctivitis is more difficult to treat than other types of allergic conjunctivitis, and may need special immune based medications such as cyclosporine drops to control the eye inflammation and prevent other eye problems. Sleeping in an air-conditioned room, ice packs and cold compresses can help with symptom relief. Furthermore, without adequate treatment, the seasonal vernal conjunctivitis can evolve into a chronic perennial vernal conjunctivitis after a mean of 3 years from disease onset 149.
Most vernal conjunctivitis patients eventually do grow out of vernal conjunctivitis usually lasts between five to ten years and usually resolves after puberty or adolescence and is rarely seen after the age of thirty. In contrast, children diagnosed with atopic keratoconjunctivitis will suffer from signs and symptoms throughout their life, and may develop more severe complications, as the atopic keratoconjunctivitis is progressive 155.
Figure 8. Vernal conjunctivitis
Figure 9. Vernal keratoconjunctivitis
Footnote: Large cobblestone papillae. Upper tarsal giant papillae are typical of vernal conjunctivitis. These have characteristically flattened tops which sometimes demonstrate stain with fluorescein. Giant papillae can sometimes be seen near the limbus and, while relatively uncommon, symblepharon formation (an eye condition that causes the conjunctiva of the eye to stick to itself or the cornea) and conjunctival fibrosis (scar) can occur.
[Source 138 ]Figure 10. Horner–Trantas dots
Footnote: Peri-limbal Horner–Trantas dots are focal white dots consisting of degenerated epithelial cells and eosinophils and are indicative of vernal keratoconjunctivitis.
[Source 136 ]Figure 11. Vernal conjunctivitis treatment options
Footnotes: Treating vernal keratoconjunctivitis should entail a stepwise approach, identifying triggers, educating patients/caregivers on good ocular health, and addressing symptoms. In a new paradigm of management, the use of immunomodulators (e.g., topical cyclosporine A) should be considered early to tackle the inflammatory and chronic nature of vernal keratoconjunctivitis, with topical corticosteroids reserved as an add-on, short-pulse therapy for persistent disease, during acute exacerbations, or in patients with corneal involvement. Any use of corticosteroids requires tapering once symptoms have been controlled to avoid adverse events. For patients with an identified allergy, referral to an allergist is recommended for additional systemic treatment. In the rare patients who do not respond to medical treatment, surgery may be required.
*In the case of associated rhinitis, consider treatment according to Allergic Rhinitis and its Impact on Asthma (ARIA) protocol;
†No improvement is defined as no improvement in symptoms or changes in conjunctival, papillary or ocular surface clinical signs.
Abbreviations: CE = cationic emulsion; CsA = cyclosporine A; IgE = immunoglobulin E.
[Source 136. Adapted from Leonardi et al. 156]Vernal conjunctivitis causes
Vernal conjunctivitis is a severe type of seasonal allergic conjunctivitis that results in small, raised lumps on the inside of the upper eyelid called Horner Tranta dots and a stringy, mucus discharge 157, 158. A personal or family history of atopy is seen in a large proportion of vernal conjunctivitis patients 159. Atopy is a genetic tendency to develop allergic diseases, such as asthma, allergic rhinitis, and atopic dermatitis (eczema). Vernal conjunctivitis was originally thought to be due to a solely immunoglobulin E (IgE) mediate reaction via mast cell release and cell-mediated immune mechanisms 160. It has now been shown that IgE is not enough to cause the varied inflammatory response that is seen with vernal conjunctivitis 147. Activated eosinophils have also been implicated to play a significant role in the pathophysiology of vernal conjunctivitis and these can be shown consistently in conjunctival scrapings of vernal conjunctivitis patients; however mononuclear cells and neutrophils are also seen 160, 147. Additionally, the role of type 4 hypersensitivity mediated by CD4 T-helper-2 (Th2) lymphocytes with immunomodulators such as IL-4, IL-5, and bFGF has also been highlighted through a few studies 148, 149, 145, 147. This differs from atopic keratoconjunctivitis, which has been shown to involve both Th1 and Th2 inflammatory cascades 150. Immunomodulators like interleukins 4 (IL-4), IL-5, IL-13, and fibroblast growth factors have also been implicated. Increased levels of tumor necrosis factor (TNF) alpha, histamine, tryptase, IgE, and IgG antibodies are observed on pathologic examination of tears 151. There is also reported over-expression of cytokines and chemokines in the conjunctiva of these patients 161. Thought has been given to a possible endocrine predisposing factor as well as there is a decrease in symptoms and prevalence after puberty 160, 145.
Apart from personal allergy history, other predisposing factors include male gender, close contact with animals, and increased exposure to dust and sunlight 162.
A hereditary association or genetic link has been suggested, but no direct genetic associations have been made. Vernal conjunctivitis is seen more often in patients who have family history of atopy and might be positive in close to 49% of these cases. But no clear correlation with specific genetic loci has been identified 145.
Vernal conjunctivitis types
Vernal conjunctivitis or vernal keratoconjunctivitis is a subtype of allergic conjunctivitis 157. The episodes are often periodic and have seasonal recurrences. Seasonal exacerbations characterize vernal keratoconjunctivitis in the initial stages with a peak incidence during spring and summer. Over time, vernal conjunctivitis tends to become perennial (all year round).
Additional types of vernal conjunctivitis include perennial and seasonal rhinoconjunctivitis, atopic keratoconjunctivitis, and giant papillary conjunctivitis 137.
Vernal conjunctivitis is classified into three clinical subtypes based on the location of the papillae into palpebral (tarsal), limbal (bulbar), and mixed forms 148, 163, 136:
- Palpebral vernal conjunctivitis (tarsal vernal conjunctivitis). Palpebral vernal conjunctivitis is characterized by large, cobblestone-like papillae on the upper tarsal conjunctiva. These can differ in shape and size, but are usually defined as >1.0 mm in diameter 93, 148. There is a close association between the inflamed conjunctiva and the corneal epithelium, often leading to significant corneal disease.
- Limbal vernal conjunctivitis (bulbar vernal conjunctivitis). Limbal vernal conjunctivitis typically involves Horner–Trantas dots indicating lymphocytic and eosinophilic infiltration of the limbal conjunctiva 93, 148. Limbal vernal conjunctivitis typically affects the Black and Asian populations.
- Mixed vernal conjunctivitis. Mixed vernal conjunctivitis has features of both the palpebral (tarsal) and limbal (bulbar) subtypes in only one eye (as signs are often heterogeneous between eyes) 93.
According to the Management of Vernal Keratoconjunctivitis in Asia (MOVIA) Expert Working Group, the most common form of vernal conjunctivitis seen in clinics across Asia is the tarsal form; however, up to one-third of patients are assumed to have the mixed form 136. The limbal form of vernal conjunctivitis is considered less common in Asia (based on clinical experience) 136.
Figure 12. Vernal keratoconjunctivitis types
Footnote: Clinical forms of vernal keratoconjunctivitis: Limbal or bulbar, palpebral and mixed vernal keratoconjunctivitis.
[Source 67 ]Figure 13. Palpebral vernal conjunctivitis
Footnote: Palpebral vernal conjunctivitis also called tarsal vernal conjunctivitis is characterized by large, cobblestone-like papillae on the upper tarsal conjunctiva.
[Source 136 ]Figure 14. Limbal vernal conjunctivitis
Footnote: Limbal vernal conjunctivitis typically involves Horner–Trantas dots, indicating lymphocytic and eosinophilic infiltration of the limbal (bulbar) conjunctiva. The mixed form is characterized by the presence of both tarsal and limbal subtypes in only one eye.
[Source 136 ]Figure 15. Shield ulcer
Footnote: Shield ulcer formation. Shield ulcers usually form on the upper third of the cornea. Plaques can also form when inflammatory debris accumulates at the base of a shield ulcer.
[Source 136 ]Vernal conjunctivitis signs and symptoms
The most common eye symptoms of vernal conjunctivitis are initially severe itching, redness, and tearing. Other common symptoms include blurred vision, photophobia, foreign body sensation, burning, blinking or eye twitching (blepharospasm) and a characteristic ropey, stringy mucus, and/or serous discharge 93, 164, 160, 145. Other typical signs and symptoms include moderate-to-intense conjunctival hyperemia, mild-to-moderate chemosis, foreign-body sensation, and pain, all of which can be very intense upon awakening, causing what is called “the morning misery” 93. Vernal conjunctivitis typically affect both eyes but may also affect one eye only.
Clinical signs of vernal conjunctivitis include a papillary reaction of the upper tarsal conjunctiva and throughout the limbus. The signs of vernal conjunctivitis can be divided into conjunctival, limbal and corneal signs 138. Moreover, the clinical examination findings also vary dependent on the geographical location 165.
- Conjunctival signs include diffuse conjunctival injection and upper tarsal giant papillae. These are discrete >1mm in diameter that characteristically have flattened tops which sometimes demonstrate stain with fluorescein 160, 166. Additionally, these giant papillae can sometimes be seen near the limbus and, while relatively uncommon, symblepharon formation (an eye condition that causes the conjunctiva of the eye to stick to itself or the cornea) and conjunctival fibrosis can occur 167.
- Palpebral disease: In the early stages, there is conjunctival hyperemia and velvety papillary hypertrophy of the superior tarsal plate. In the intense disease, flat polygonal macropapillae, which are <1mm in size, are seen along with the whitish inflammatory disease. With further disease progression, these can form giant papillae that are>1mm in size and result from rupture of the dividing septa. Mucoid deposits can occur between giant papillae. The milder form of the pathology is characterized by minimal conjunctival congestion and decreased mucus production 168.
- Bulbar disease: The bulbar disease is also called limbal disease and is particularly more common and severe in tropical regions. This is characterized by congestion of the bulbar conjunctiva in the interpalpebral area. Gelatinous thickened papillae can form around the limbus and are associated with apically located whitish cellular collections known as Horner Tranta Spots 169.
- Limbal signs include thickening and opacification of the limbal conjunctiva as well as gelatinous appearing and sometime confluent limbal papillae. Peri-limbal Horner-Trantas dots are focal white limbal dots consisting of degenerated epithelial cells and eosinophils 160. Limbal disease can result in a limbal stem cell deficiency which can lead to pannus formation with corneal neovascularization 167.
- Corneal signs vary according to the severity of the disease process 160. Punctate epithelial erosions or keratitis can coalesce into macro-erosions of the epithelium 147.
- Plaques containing fibrin and mucous can accumulate into macro-erosions forming Shield ulcers (see Figure 15). Corneal neovascularization can ensue and resolution can leave a characteristic ring-like scar 166, 144.
- A waxing and waning gray-white lipid depositing in the peripheral, superficial stroma can occur and is known as pseudogerontoxon 160. Pseudogerontoxon is characterized by a paralimbal band of superficial scarring adjacent to the inflamed limbus resulting from recurrent limbal disease. It usually mimics arcus senalis.
- Vernal keratoconjunctivitis is also associated with corneal ectasias, particularly keratoconus, which results from persistent eye rubbing and occasionally superficial vascularization. Keratoconus has been shown to be more prominent in vernal conjunctivitis patients as well; possibly due to increased eye rubbing of chronically irritated patients 170.
- Associated bilateral herpes simplex keratitis has also been reported 171
- Lid signs. Blepharitis is often associated with patients with vernal keratoconjunctivitis. Some cases might be seen with eczema or maceration of the lid. Subconjunctival fibrosis and symblepharon can rarely develop due to the inflammatory complications 172.
Severe vernal conjunctivitis can result in sight-threatening complications 136. Eye surface damage as a result of repetitive eyelid trauma and vernal conjunctivitis-associated inflammatory activity can lead to corneal complications such as superficial punctate keratopathy, shield ulcers, corneal scarring, keratoconus, dry eyes, limbal stem-cell deficiency, and secondary infections 93, 139, 173, 174. Shield ulcers, which can be self-limiting or associated with bacterial keratitis, usually form on the upper third of the cornea, and can lead to loss of vision (see Figure 15) 148. Plaques can also form when inflammatory debris accumulates at the base of a shield ulcer 173 and can be particularly resistant to topical therapy or require surgical intervention 148. Limbal stem-cell deficiency can occur with longstanding inflammation 148. Keratoconus and irregular astigmatism can result from frequent eye rubbing in the pediatric population 175, 176, 177. In these patients, there is a fine balance between the benefits of corticosteroids and the risk for vision loss as a result of overtreatment. Patients with severe vernal conjunctivitis may also develop lid complications and acquire ptosis often with atopic dermatitis 136. The variable severity of these complications in each patient presents a challenge to ophthalmologists to not only manage acute episodes, but also to prevent reappearances.
Vernal conjunctivitis complications
The ocular complications reported among vernal keratoconjunctivitis patients include steroid-induced cataract, steroid-induced glaucoma, irregular astigmatism, keratoconus, acute hydrops, shield ulcer, central corneal scars, and limbal tissue hyperplasia 137.
Vernal conjunctivitis differential diagnosis
The close differentials of vernal keratoconjunctivitis include all types of allergic conjunctivitis, including seasonal allergic conjunctivitis, perennial allergic conjunctivitis, atopic keratoconjunctivitis (AKC) and giant papillary conjunctivitis 137. Most of these are IgE mediated responses except giant papillary conjunctivitis. None have gender predilection, except atopic keratoconjunctivitis (AKC) and vernal keratoconjunctivitis, which are more prevalent among males. History and physical examination often help in distinguishing between these clinical entities.
Atopic keratoconjunctivitis (AKC) typically has an older age of onset in 20-50 years of age, as opposed to onset prior to age 10 years with vernal conjunctivitis 137. Conjunctival involvement is classically on the upper tarsus in vernal conjunctivitis and on the lower tarsus in atopic keratoconjunctivitis. Additionally, atopic keratoconjunctivitis is typically more chronic in nature and more commonly results in scarring of the cornea and conjunctival cicatrization, whereas vernal conjunctivitis is typically more self-limiting and do not result in severe vision-threatening complications 66, 166. Atopic keratoconjunctivitis (AKC) is more commonly associated with asthma, rhinitis, dermatitis. Vernal keratoconjunctivitis is associated with increased goblet cells compared to decrease goblet cells in atopic keratoconjunctivitis.
Vernal conjunctivitis treatment
Management should follow a stepwise approach with active patient education 136:
- Address triggers and aggravating factors (e.g., environment and allergens).
- Maintain eye health, including frequent hand, face, and hair washing.
- Use of eye lubricants and cold compresses should always be considered.
- For the use of any eye drops on the eye surface, it is recommended to use preservative-free compounds, where possible, to minimize eye surface toxicity.
- Conventional topical anti-allergic drugs
- Dual-acting agents, antihistamines, and mast cell stabilizers are all effective for reducing signs and symptoms of mild or moderate vernal conjunctivitis.
- Dual-acting agents should be considered ahead of monotherapy with antihistamines or mast cell stabilizers.
- Cyclosporine A 0.1% cationic emulsion
- Topical cyclosporine A 0.1% cationic emulsion should be considered for patients with in moderate-to-severe or persistent vernal conjunctivitis.
- Patients should be instructed on how to apply cyclosporine A eye drops to minimize stinging or burning on instillation, such as using artificial tears prior to instillation.
- Topical corticosteroid eye drops
- In patients with only conjunctival involvement, topical corticosteroids should be reserved for use after loss of control or persistence of symptoms with immunomodulators such as cyclosporine A.
- Topical corticosteroids are effective for the management of acute exacerbations, or when the cornea is involved, and preferably only introduced in patients with more severe disease. In these individuals, corticosteroids should be used in combination with cyclosporine A to account for the fact that cyclosporine A may require ≥1 week to act.
- Because of an increased risk of adverse events/or vision loss with chronic use, topical corticosteroid eye drops should be used in short pulses alone or in combination with cyclosporine A under the supervision of an ophthalmologist and tapered rapidly.
- Tacrolimus
- In regions where available, tacrolimus should be reserved for patients with severe vernal conjunctivitis that is refractory to cyclosporine A.
- Tacrolimus can be considered as a treatment for moderate-to-severe vernal conjunctivitis in patients with allergy of the eyelid, but note this may be off-label.
- Vasoconstrictors
- Vasoconstrictors are not recommended for the treatment of vernal conjunctivitis. Topical vasoconstrictors can be effective at alleviating hyperemia but offer little to no relief from itchiness and have a short duration of action. If used to address hyperemia, vasoconstrictors should be used with caution and only for a short period (no longer than 5–7 days) due to adverse events and tachyphylaxis 178.
- Vasoconstrictors may cause several side effects including rebound redness, chronic follicular conjunctivitis, and tachyphylaxis. Vasoconstrictors are rarely used in the pediatric population. It is recommended that these agents should be avoided.
- Topical decongestants do not reduce the allergic response because they do not antagonize any of the mediators of allergic inflammation. Burning or stinging on instillation is a common adverse event, and prolonged use and/or discontinuation following longer-term use can lead to rebound hyperemia and conjunctivitis medicamentosa 179. These events are usually associated with topical combinations of vasoconstrictors and first-generation antihistamines, such as pheniramine and antazoline, which are available as over-the-counter products.
- Non-steroidal anti-inflammatory drugs (NSAIDs)
- Non-steroidal anti-inflammatory drugs (NSAIDs) are not recommended as they do not target the specific inflammatory mechanism associated with vernal conjunctivitis.
- Non-steroidal anti-inflammatory drugs (NSAIDs) used in the treatment of eye allergy typically inhibit cyclooxygenase (COX)-1 and COX-2 enzymes 93. Some have demonstrated a slight effect in the treatment of ocular allergy, by targeting itching, intercellular adhesion molecule-1 expression, and tear tryptase levels 93, 178. However, use of NSAIDs is not encouraged in vernal keratoconjunctivitis because of their local side effects, such as burning or stinging after application, increased risk of inducing keratitis on the ocular surface, and because they do not target the specific inflammatory mechanism associated with vernal keratoconjunctivitis.
- Oral antihistamines
- Second-generation systemic non-sedative antihistamines are preferred over older first-generation antihistamines.
- Allergen-specific immunotherapy
- Allergen-specific immunotherapy is only recommended when clearly defined systemic hypersensitivity to an identified allergen exists.
- Patients requiring allergen-specific immunotherapy should be referred to an allergist or specialist ophthalmologist.
- In selected patients with ocular complications or persisting symptoms following prior treatments
- Surgery, oral corticosteroids (short pulses) or corticosteroid lid injection, or systemic treatment with immunomodulators or biologics may be appropriate options for use by corneal specialists.
In patients with a history of vernal conjunctivitis, drug treatment should be planned early and started at the beginning of spring or continued all year, depending on allergen exposure and duration of symptoms 93.
Table 3. Overview of medications currently available for the management of vernal conjunctivitis
| Class | Drug | Standard dosing | Indication | Considerations |
|---|---|---|---|---|
| Topical antihistamines (second generation) | Antazoline Emedastine Levocabastine | QID | Relief of itching Relief of signs and symptoms | • Short duration of action • Frequently not enough alone to treat the entire disease |
| Mast cell stabilizers | DSCG Lodoxamide NAAGA Pemirolast potassium | BID to QID | Relief of signs and symptoms | • Long-term use • Slow onset of action • Prophylactic dosing • Frequently not enough alone to treat the entire disease |
| Dual-acting agents (antihistamine + mast cell stabilizers) | Alcaftadine Azelastine Bepotastine Epinastine Ketotifen Olopatadine | QD QD to BID BID BID BID to QID BID | Relief of itching Relief of signs and symptoms | • Side effects • Bitter taste (azelastine) • Frequently not enough alone to treat the entire disease |
| Immunomodulators (calcineurin inhibitors) | Cyclosporine A Tacrolimus | QD to QID QD | Treatment of severe vernal keratoconjunctivitis and AKC not responding to anti-allergic drugs | • Pharmacy-compounded preparations vary from center to center • Quality control and availability of pharmacy-compounded preparations are poor • Tacrolimus is largely used off-label in ocular allergy (tacrolimus is approved for vernal keratoconjunctivitis only in Japan) |
| Corticosteroids | Betamethasone Desonide Dexamethasone Fluorometholone Hydrocortisone Loteprednol Prednisolone Rimexolone | As required (up to 7 days) | Treatment of allergic inflammation Use in moderate-to-severe forms | • Risk for long-term adverse events • No mast cell stabilization • Potential for inappropriate patient use • Requires close monitoring |
| Vasoconstrictors (vasoconstrictor–antihistamine combinations) | Naphazoline/ pheniramine | BID to QID | Episodic itching and redness | • Rapid onset but short duration of action • Only addresses hyperemia • Associated with a range of side effects • Potential for inappropriate patient use |
Footnotes: * Availability and access to treatments may vary across clinics, hospitals, regions, and countries. Each treatment option should be considered in accordance with the level of evidence available at the time.
Abbreviations: AKC = atopic keratoconjunctivitis; BID = twice daily; DSCG = disodium cromoglycate; NAAGA = N-acetyl-aspartyl glutamic acid; QD = once daily; QID = four times daily; VKC = vernal keratoconjunctivitis.
[Source 136 ]Vernal conjunctivitis treatment options
Removal of any and all possible allergens as well as conservative management such as cool compresses and lid scrubs make up the first line of therapy 180. A topical antihistamine only may work in mild cases 180. Topical mast cell stabilizers (cromolyn sodium, nedocromil sodium, and lodoxamide) are typically used with topical antihistamines and have been shown to be effective in moderate presentations of vernal conjunctivitis 180. Mast-cell stabilizers have a loading period to reach their full therapeutic effect 147. Overall, topical antihistamines (instilled four times daily) appear to be safe and well tolerated in patients with vernal conjunctivitis 136. Alongside mast cell stabilizers and dual-acting agents, they are often the first-choice treatment and are effective in reducing the signs and symptoms of vernal conjunctivitis 178. Antihistamine drugs with eye activity are a good therapeutic option for patients with allergic conjunctivitis, including vernal conjunctivitis, since they can inhibit proinflammatory cytokine secretion from conjunctival epithelial cells 181.
Antihistamines
When you have an allergic reaction your body releases histamine from mast cells, which leads to hay fever. Antihistamines block this reaction. Antihistamines act via histamine receptor antagonism to block the inflammatory effects of histamine and relieve any associated signs and symptoms. Most antihistamines used in the treatment of eye allergy are histamine-1 (H1) receptor antagonists, although some agents have affinity for other subtypes. Histamine-2 (H2) antagonists have been shown to modulate both cell growth and migration. Animal model studies have shown that antihistamines may even reduce infiltration of eosinophils and thus reduce the clinical aspects of the late-phase reaction 93.
First-generation antihistamines are well tolerated and associated with a favorable long-term safety record, but are associated with instillation pain, short duration of action, and limited potency 94. First-generation antihistamines remain available in over-the-counter products, particularly in combination with vasoconstrictors (substances that cause the walls of blood vessels to narrow, or constrict). While newer antihistamines are also H1 antagonists, they have a longer duration of action (4–6 hours) and are better tolerated than their predecessors 93.
Topical antihistamines are widely available without a prescription. Antihistamines competitively block histamine receptors (e.g., H1 or H4) on nerve endings and blood vessels of the mucosal surface, thereby reducing itchiness and conjunctival redness 95. First-generation antihistamines were associated with a range of systemic side effects (e.g., sedation, dizziness, cognitive impairment, blurred vision) caused by anticholinergic actions and nonspecific binding to histamine H2 receptors in addition to drying of the ocular surface. Newer oral, intranasal, and topical ocular antihistamines demonstrate improved H1 receptor selectivity, with fewer adverse effects; however, eye side effects, such as burning and dryness, remain a concern. Topical antihistamines (e.g., levocabastine, emedastine difumarate) are useful for providing rapid relief of allergic conjunctivitis symptoms, but their duration of action is limited; most topical antihistamines require dosing four times.
Mast cell stabilizers
Alongside antihistamines and dual-acting agents, mast cell stabilizers (instilled two to four times daily) form the first line of treatment and are effective in reducing the signs and symptoms of vernal keratoconjunctivitis. Mast cell stabilizers have been shown to act on multiple cells involved in allergic inflammation, including eosinophils, neutrophils, macrophages, mast cells, and monocytes. For example, lodoxamide is known to have an effect on eosinophil activation (shown in studies by measuring tear eosinophil cationic protein before and after therapy), while N-acetyl-aspartyl glutamic acid (NAAGA) is known to inhibit leukotriene synthesis and release of histamine by mast cells 93. Overall, mast cell stabilizers are generally well tolerated especially preservative-free options; however, there are tolerability concerns with some agents, including burning and stinging sensations upon instillation, blurred vision, and an undesirable aftertaste. Data on long-term efficacy and safety of mast cell stabilizersare lacking 178.
Dual-Acting Antihistamine–Mast Cell Stabilizing Agents
Dual-acting agents are the combination of mast cell stabilizing properties and histamine receptor antagonism (instilled twice daily) has shown clear benefits in treating forms of eye allergy 136. This dual action rapidly alleviates multiple signs and symptoms of allergic conjunctivitis in the short term and blocks the feed-forward cycle of persistent inflammation caused by continuous mast cell activation in the long term to promote regression of allergic conjunctivitis. Antihistamine–mast cell stabilizing agents (e.g., olopatadine, alcaftadine, epinastine, bepotastine besilate) are currently considered first-line therapeutics for allergic conjunctivitis because they offer acute symptomatic relief and control inflammation, and can be used chronically without long-term safety concerns. Most dual-acting agents require twice-daily dosing. Olopatadine 0.2% and alcaftadine are indicated for once-daily dosing and maintain effectiveness through 16 hours after administration in conjunctival allergen challenge studies 101, 102, 103.
Dual-acting agents are well tolerated and are not associated with significant ocular drying effects 93, and should be preferred over monotherapy with antihistamines or mast cell stabilizers alone. A key benefit of dual-acting agents is the provision of rapid symptomatic relief by alleviating itching and redness 93. Olopatadine and ketotifen, for example, have shown to be effective in relieving itching, tearing, conjunctival hyperemia, mucus discharge, and photophobia 182.
If seasonal recurrence is known, it is suggested that mast-cell stabilization therapy be initiated prior to the season in which symptoms are encountered and continued throughout the season 147. Dual-action agents with both histamine-1 (H1) blocking mechanism and mast-cell stabilization have the benefits of working immediately and having long-term disease modifying effects. Topical corticosteroids are typically the most effective. High pulse dose with quick tapering and use of low-absorptions corticosteroids (fluoromethelone, loteprednol, remexolone, etc.) is preferred when using topical corticosteroid therapy 180. Oral corticosteroids can be considered in sight threatening conditions 160, 144. Supratarsal injection of local corticosteroid (triamcinolone or dexamethasone) into the upper tarsal papillae can sometimes offer short term relief as well 183.
Cyclosporine A
Long term immunomodulation (a process in which the immune system is modified or modulated in a beneficial way) with steroid sparing agents such as cyclosporine and tacrolimus is often needed. Topical calcineurin inhibitors (topical immunomodulators) are frequently prescribed for patients with vernal conjunctivitis 178. Topical calcineurin inhibitors are recommended in patients with acute-phase or persistent, moderate or severe vernal conjunctivitis that is not responding to anti-allergic drugs 178. Topical Cyclosporine A is effective in controlling ocular surface inflammation in vernal conjunctivitis and is thought to work by inhibiting Th2 proliferation and IL-2 production, and by reducing levels of immune cells and mediators acting on the ocular surface and conjunctiva 184. Different concentrations of cyclosporine A, ranging from 0.05 to 2.0%, are currently available in different countries with different clinical indications 185, 186. The 0.1% cationic emulsion (CE) formulation increases the bioavailability of cyclosporine A in the tear film compared with available oil-based (often used when cyclosporine A is pharmacy-compounded) or anionic emulsion cyclosporine A formulations 187. Clinical studies on the efficacy of topical cyclosporine A 0.1% cationic emulsion (CE) for treating vernal conjunctivitis have consistently shown a beneficial effect of cyclosporine A, with safety and efficacy demonstrated for up to 7 years and comparable with those seen in patients with dry eye disease 184, 188, 189, 190.
There is no consensus on the minimum effective concentration of cyclosporine A to treat vernal conjunctivitis, as this can depend on the emulsion vehicle 136. Topical cyclosporine A in concentrations of 0.05% to 2% has been shown to decrease inflammatory cytokines and the signs and symptoms of treated vernal conjunctivitis patients 191, 192. Currently, cyclosporine A 0.1% cationic emulsion (CE) is approved based on significant improvements in signs, symptoms, and quality of life in patients with severe vernal conjunctivitis, and is commercially available across Asia. It is recommended that cyclosporine A 0.1% CE should be initiated twice daily for patients with mild-to-moderate disease and four times daily for those with moderate-to-severe disease for up to 6 months. Other commercially available cyclosporine A formulations exist but are not approved for use in vernal conjunctivitis (as of December 2020) 136. In addition, cyclosporine A may be compounded in hospital-based pharmacies to provide variable concentrations and formulations; however, its quality may be compromised, and availability is limited to specialist centers 136.
The most commonly associated side effects with cyclosporine A is a stinging or burning sensation on instillation, and no new safety issues were identified in long-term follow-ups 136. Instillation pain can be minimized by following a standard technique for eye drop instillation, which includes using artificial tears prior to instillation (avoiding any potential washout effect of the subsequently administered cyclosporine A, by waiting at least 10 minutes between administrations), followed by one drop into the lower conjunctival sac of each eye in the morning, at noon, in the afternoon, and in the evening, approximately 4 hours apart 190. Patients should also be counseled to expect stinging and burning when the drops are applied, but that the sensation should taper off with regular use as prescribed.
After long cycles of treatment, cyclosporine A can allow control of symptoms without corticosteroids 136. There is a trend in the Asia-Pacific region, in using cyclosporine A as a first-line treatment for patients who present with moderate-to-severe or persistent vernal conjunctivitis 136. In patients presenting with conjunctival involvement only, cyclosporine A alone is recommended for treatment, while corticosteroids should only be prescribed (as short pulses) if there is an inadequate response to cyclosporine A 136. Otherwise, cyclosporine A can be used in combination with corticosteroids in patients with corneal complications (or limbitis) or acute exacerbations, in the understanding that cyclosporine A may take over a week to act due to its immunosuppressive mechanism of action 136.
Tacrolimus
Tacrolimus is a strong, non-steroidal immunosuppressant that binds to FK506-binding proteins in T lymphocytes and inhibits calcineurin activity 193. Tacrolimus has been investigated in a small randomized controlled trial in vernal keratoconjunctivitis, where it demonstrated improvements in symptoms of itching, foreign-body sensation, and photophobia, as well as in signs of limbal inflammatory activity and keratitis, with maintenance of disease control 194, 195. Tacrolimus may be administered as eye drops or an ointment. The use of tacrolimus (0.03%) ointment in ophthalmic and dermatologic form is currently off-label in Asia for patients with vernal keratoconjunctivitis. However, there are findings in published studies of small population sizes demonstrating effectiveness and tolerability 194, 195, 196, 197, 198, 199.
Tacrolimus 0.1% topically has also shown to improved signs and symptoms of vernal conjunctivitis, with one study showing improvement even in patients unresponsive to 0.1% topical cyclosporine A 200, 201. A study comparing the use of 0.1% tacrolimus ophthalmic ointment dosed twice a day versus 2% cyclosporine A eye drops four times a day for the treatment of vernal conjunctivitis, did not find a statistically significant difference in signs or symptoms or side effects between the two groups 202. Additionally, adult patients with vernal conjunctivitis may respond more favorably to topical cyclosporine A therapy 203. Oral anti-histamines are sometimes used, but there is no real evidence in their support 138.
Similar to cyclosporine A, tacrolimus may be associated with instillation pain 193. As a strong immunosuppressant, topical tacrolimus may be associated with an increased risk for corneal infections with prolonged use, and close monitoring of patients on long-term therapy is necessary 199. Topical tacrolimus (0.02–0.1%) should be reserved for patients with severe vernal keratoconjunctivitis involving allergy of the eyelid or whose disease is refractory to cyclosporine A 178.
Topical corticosteroid
Topical corticosteroid eye drops should be used as short, pulsed therapy to provide symptomatic relief in patients with more persistent vernal keratoconjunctivitis or acute exacerbations, or when the cornea is involved (e.g., patients with shield ulcers or hyperplasia in limbal vernal keratoconjunctivitis), and under an ophthalmologist’s supervision 178. Topical corticosteroids can be administered at a low or high dose, depending on the severity of vernal keratoconjunctivitis, and can be given up to four times daily with or without cyclosporine A. Corticosteroids act by suppressing the late-phase reaction in both experimental and clinical settings. Corticosteroids, in part, limit the inflammatory cascade by inhibiting phospholipase A2, and consequently act to prevent migration of leukocytes, release of hydrolytic enzymes, growth of fibroblasts, and lead to changes in vascular permeability 204.
Corticosteroids with low intraocular absorption (“soft steroids”), such as hydrocortisone, fluorometholone and loteprednol, may be preferred 93. Dosages are chosen based on the inflammatory state of the eye, with therapy prescribed in pulses of 3–5 days (1). Loteprednol is usually indicated for 7–8 days in the treatment of the acute phase 93. Prednisolone, dexamethasone, and betamethasone are sometimes considered only as second-line options, or as first-line treatment in more severe cases, due to their potential effect on intraocular pressure 93. Steroid–antibiotic combination eye drops are not recommended, since vernal keratoconjunctivitis is an allergic inflammation and not an infection 93.
Moderate-to-severe vernal keratoconjunctivitis may require repeated topical corticosteroid treatment to downregulate conjunctival inflammation 93. Persistent and severe symptoms, thick mucus discharge with moderate-to-severe corneal involvement, numerous and inflamed limbal infiltrates, and/or giant papillae may indicate a need for corticosteroids in addition to cyclosporine A use 93. However, use of corticosteroids as first-line therapy is not recommended in those with only conjunctival involvement 93.
Corticosteroids are not recommended for long-term use because of the increased risk for ocular adverse events, including increased intraocular pressure (IOP), glaucoma, cataracts, and susceptibility to infection 93. These adverse events depend, in part, on the structure of the steroid, the dose, and duration of treatment 205. Treatment with corticosteroids requires careful monitoring for the development of intraocular pressure (IOP), which should be promptly brought under control to prevent deterioration or loss of vision 206.
Supratarsal corticosteroid injections have demonstrated some value in treating adults with vernal keratoconjunctivitis but may not be an appropriate approach for children or for application by general ophthalmologists 206, 207. For more specialist ophthalmologists, this may be an option in children with refractory, severe, or challenging vernal keratoconjunctivitis.
Oral antihistamines or antileukotrienes
Systemic treatment with oral antihistamines or antileukotrienes can reduce the severity of flare-ups and generalized hyperreactivity 93. Oral antihistamines are good for treating hay fever symptoms, especially if you have a lot of different symptoms. You can also take oral antihistamines in advance if you know you are going to be exposed to allergens or triggers. Newer second-generation antihistamines are preferred over older first-generation antihistamines, in order to avoid the sedative and anticholinergic effects that are associated with first-generation agents 208.
Newer, less-sedating antihistamines
Newer antihistamines may rarely cause drowsiness; do not drive or operate machinery if you are affected e.g. cetirizine (Zilarex, Zyrtec), desloratadine (Aerius), fexofenadine (Fexotabs, Telfast), loratadine (Claratyne, Lorano).
Cetirizine and loratadine are available as syrups for children; check correct doses for different age groups. Cetirizine is more likely to cause drowsiness than other less sedating antihistamines.
Older, sedating antihistamines
Older sedating antihistamines can cause drowsiness, sometimes the next day; it is important you do not drive or operate machinery e.g. chlorpheniramine + pseudoephedrine (Demazin 6 Hour Relief Tablets), dexchlorpheniramine (Polaramine), loratadine + pseudoephedrine (Claratyne-D with Decongestant Repetabs), promethazine (Phenergan, Sandoz Fenezal)
Older antihistamines are not available without a prescription for children under 2 years old. Do not drink alcohol with antihistamines that make you drowsy.
If you have other medical conditions, such as glaucoma, epilepsy or prostate problems, or you take antidepressants, check with your doctor before taking these medicines.
Systemic immunotherapy
Systemic immunotherapy is also an option, especially for those patients with underlying systemic atopic disease such as atopic dermatitis and asthma. There are case reports of the successful use of omalizumab, an anti-IgE monoclonal antibody, in patients with vernal conjunctivitis recalcitrant to other treatment modalities, but this remains an off-label use 209, 210. Dupilumab is a human monoclonal antibody against interleukin-4 receptor alpha used in the treatment of atopic dermatitis. There is currently a multi-center, double-masked, randomized, and placebo-controlled, parallel-group study that is evaluating the safety and efficacy of dupilumab in the treatment of signs and symptoms of individuals with atopic keratoconjunctivitis (NCT04296864), and may also play a role in treating vernal disease in those patients older than 12 years 211. However, dupilumab has been found to itself induce atopic keratoconjunctivitis compared to placebo in treated patients, so its role as a primary treatment is debatable 138.
Allergen-specific immunotherapy
Allergen-specific immunotherapy is indicated only when a clearly defined systemic hypersensitivity to an identified allergen exists 178 and should be managed by an allergist or specialist ophthalmologist. The combination of clinical history with the results of a skin-prick test and specific serum IgE should be taken into consideration when the choice of immunotherapy is made 178. There are currently no robust studies of allergen-specific immunotherapy in vernal keratoconjunctivitis 178. A meta-analysis of several double-blind, randomized, placebo-controlled trials investigating sublingual immunotherapy for allergic conjunctivitis, reported a significant reduction in total ocular symptom scores and ocular signs (redness, itchiness, and tearing) vs. placebo in pollen-induced allergic conjunctivitis, but not in allergic conjunctivitis associated with house dust mites, in the pediatric population 212.
Surgical management
Case reports and ongoing clinical practice have indicated that, in rare instances, specialist surgical management may be beneficial in patients with vernal keratoconjunctivitis 136. Surgical approaches that focus on conjunctival cobblestone papillae, shield ulcers, corneal plaques, limbal insufficiency, and other ocular surface presentations that do not respond to medical treatment can be beneficial 213, 214, 215.
Surgical approaches can include excision of giant papillae, debridement of the corneal plaque to remove cytotoxic cells, and amniotic membrane transplantation 216. Surgical treatment, especially corneal plaque removal, may be appropriate for patients with ulcers of moderate-to-severe severity, because short-term re-epithelialization rates are higher and the number of complications is lower than that associated with medical therapy 216. Other reports suggest surgical treatment, such as giant papillae excision, may be appropriate in cases of corneal involvement and in the presence of coarse giant tarsal papillae resulting in ptosis (or mechanical pseudoptosis) 93, 217. Amniotic membrane transplantation following keratectomy has been described as a successful treatment for deep ulcers, in severe allergic patients with slight stromal thinning 218.
Patients with vernal keratoconjunctivitis who may benefit from surgical intervention should be referred to a corneal specialist first. Leonardi et al. 93 have suggested that cryotherapy and/or giant papillae excision papillae should otherwise be avoided because they only treat the complications of vernal keratoconjunctivitis and not the underlying disease, and may induce unnecessary scarring.
Vernal conjunctivitis prognosis
Generally vernal conjunctivitis is a rather benign and self-limiting disease that may resolve with age or spontaneously at puberty 160, 219, 220. Sometimes the debilitating nature of vernal conjunctivitis when it is active necessitates therapy to control symptoms. Complications typically arise from occasional corneal scarring and the unsupervised used of topical corticosteroids can result in cataracts, or glaucoma can lead to permanent visual impairment 160, 219. Rarely, complications in the form of keratoconus, shield ulcers, corneal scarring, and vascularisation may significantly affect visual acuity. Corneal ulcers are reported in close to 9.7% of patients 221, 222. In some patients (as many as 12% of cases) symptoms may persist beyond childhood, which in some cases may represent a conversion to an adult form of atopic keratoconjunctivitis 160. This persistence into adulthood has been shown to be as high as 12% 223.
Atopic keratoconjunctivitis
Atopic keratoconjunctivitis also called AKC is the most severe form of chronic allergic conjunctivitis or eye allergic reaction caused by immunoglobulin E (IgE) mediated mechanisms, which has been shown to involve both T-helper-1 (Th1) lymphocyte and T-helper-2 (Th2) lymphocyte inflammatory cascades, and that usually affects people with atopic disease, including atopic dermatitis, eczema, asthma, and food allergies and seasonal allergies, including a family history of allergies and hay fever 114, 116, 224, 225, 150, 86. Atopic keratoconjunctivitis is the chronic eye surface and eyelids (the conjunctiva and cornea) inflammation suffered by patients with atopic dermatitis (eczema). Atopic dermatitis also called atopic eczema, the most common inflammatory skin disease worldwide, presents as generalized skin dryness, itch, and rash 226. Atopic keratoconjunctivitis is the non-infectious inflammation of the conjunctiva (the transparent membrane that lines inside your eyelid and covers the white part of your eyeball) lining your eyelids (palpebral conjunctiva) which is red and swollen. The lower eyelid generally is affected more than the upper eyelid 225. This is a differentiating symptom from vernal keratoconjunctivitis (a severe type of seasonal allergic conjunctivitis) where the upper eyelid is most often affected. Atopy is a genetic condition where your immune system produces higher than normal antibodies in response to a given allergen. Allergic conjunctivitis is caused by contact with something to which a person may be sensitive or allergic to (allergens). However, 45% of the people with atopic keratoconjunctivitis do not display a hypersensitivity reaction to common allergens 133. Conjunctivitis is the inflammation or infection of the conjunctiva, which is the transparent membrane that lines inside your eyelid and covers the white part of your eyeball. The conjunctiva is a thin translucent mucous membrane that can be divided based on the location into palpebral conjunctiva (inside of the eyelids) and bulbar conjunctiva (begins at the edge of the cornea and covers the visible part of the sclera) (Figure 2). The conjunctiva contains nonkeratinizing, squamous epithelium and a thin, richly vascularized substantia propria containing lymphatic vessels and cells, such as lymphocytes, plasma cells, mast cells, and macrophages. The conjunctiva also has accessory lacrimal glands and goblet cells. When the small blood vessels in the conjunctiva become inflamed or infected, they’re more visible. This is what causes the whites of your eyes to appear reddish or pink. Atopic keratoconjunctivitis is a chronic eye surface inflammation with a relatively low expectation of resolution and can have low visual morbidity. If left untreated, atopic keratoconjunctivitis can progress to ulceration, scarring, cataract, keratoconus, and corneal vascularization (the process of growing blood vessels into a tissue).
Although atopic keratoconjunctivitis is a perennial (year round) disease, your symptoms may worsen in the winter. Unlike atopic dermatitis, which is generally seen early in childhood, atopic keratoconjunctivitis appears during late adolescence and early adulthood but may occur at all ages and there is a prevalence peak in patients between 20 and 50 years of age, with a personal or family history of atopic dermatitis or other allergic diseases such as eczema, asthma and/or urticaria 114, 227. The percentage of people with atopic dermatitis that develop atopic keratoconjunctivitis ranges from 25% to 42% 114. Men are more commonly affected than women with a ratio between 2 to 1 and 3 to 1 225. Approximately 5% of atopic keratoconjunctivitis patients have a history of childhood vernal keratoconjunctivitis (a severe type of seasonal allergic conjunctivitis) 64.
People with atopic keratoconjunctivitis usually show atopic dermatitis in the eyelids (see Figure 3A & 5). Eyelid dermatitis results in eyelid hyperpigmentation (panda eyes), swelling that causes horizontal eyelid creases (Dennie-Morgan lines), and absence of the lateral end of the eyebrows (Hertoghe’s sign) 114. More advanced chronic atopic keratoconjunctivitis may also cause keratinization of the eyelid margins, inflammation of the eyelid margins (blepharitis), the loss of eyelashes, and sometimes eyebrows (madarosis), thickening and fissuring of the skin (tylosis), eyelid deformities, and reactive droopy eyelids (ptosis) 114. People with atopic keratoconjunctivitis also show redness of the eyes, chemosis (a swelling of the conjunctiva), and tarsal papillae, typically in the inferior tarsal conjunctiva and sometimes even Horner-Trantas dots in the limbus (Figure 7), especially in more acute phases 114. Conjunctival scarring can lead to conjunctiva of the eyelid to stick to the conjunctiva of the eyeball (symblepharon) and shortening of the inferior conjunctival sac 114. The corneal involvement seems to be secondary to the conjunctival and palpebral involvement and may vary from superficial punctate keratitis to corneal ulcers, corneal scarring, and pannus 114.
People with atopic keratoconjunctivitis complain of severe itching most of the year that is usually more severe during the winter months and in colder climates. There is also discharge that tends to be more aqueous than in vernal keratoconjunctivitis (a severe type of seasonal allergic conjunctivitis), but it may also be mucous 114.
People with atopic keratoconjunctivitis may also complain of dry eyes that exacerbates itchiness and perpetuates conjunctival inflammation 114.
Chronic atopic keratoconjunctivitis leads to numerous complications: infections such as staphylococcal blepharoconjunctivitis and herpes simplex keratitis, cataracts (typically anterior subcapsular but also others), limbal stem-cell deficiency, keratoconus, glaucoma, retinal detachment, and corneoconjunctival tumors 114.
Atopic keratoconjunctivitis usually is diagnosed by clinical exam and a medical and family history, although a conjunctival biopsy may be helpful in distinguishing atopic keratoconjunctivitis from other conditions.
Atopic conjunctivitis treatment is complicated, often requiring input from multiple specialists such as cornea specialist, skin specialist (dermatologist) and doctor specializing in the diagnosis and treatment of allergies (allergist) to determine the most successful management.
The primary goal in managing allergic conjunctivitis, including vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC), is to avoid exposure to the allergen that triggers the allergic response 129.
If the allergen is known, steps can be taken to minimize exposure to it or, in some cases, get rid of it completely. For example, someone with an allergy to:
- House dust mites may find that minimisation measures such as removing carpet, using dust mite covers for pillows and mattresses, use of hypoallergenic bedding and washing bedding in hot water are enough to reduce symptoms.
- Animal dander may find the best option is to remove the animal or a pet from the house altogether, particularly if symptoms are severe.
- Use of air filtering devices, and dietary changes in cases of food sensitivity.
Mild to moderate symptoms of allergic conjunctivitis usually respond well to simple home treatments such as bathing eyes with cold water, ice packs and cold water compresses. Non-medicated eye drops can also help to lubricate the eye and gently flush allergens from the surface 86, 88, 228. More severe symptoms will usually require treatment with medication.
In some cases, eye patching may be required, and prevent complications that lead to vision loss. It is also important to balance this with limiting side effects of treatment.
- Cold compresses (mild cases): Cold compresses can be used as relief when you feel itchy and the urge to rub your eyes. Consistent eye itching and rubbing can cause thinning of the cornea, leading to keratoconus (corneal disease caused by the steepening and thinning of the cornea).
- Eye lubricants (mild cases): Artificial tear drops to soothe the eye surface, replenish the tear film and dilute the allergen.
- Antihistamine eye drops (mild cases): Antihistamine eye drops (e.g., Ketotifen ophthalmic such as Zaditor or Alaway, relieves itchiness) effective but should not be used for longer than 6 weeks without medical advice.
- Antihistamine tablets or syrups (mild cases): Antihistamine tablets or syrups help some people when it is difficult to avoid the allergen. Some side effects may include dryness of the eyes, nose, and mouth, and blurred vision. Antihistamines are usually contraindicated for people with glaucoma, advice should be sought from an eye specialist.
- Antihistamine eye drops containing a vasoconstrictor (mild cases): Minimize itch and remove redness by narrowing the swollen blood vessels in the eye. They should not be used for longer than 14 days without medical advice.
- Mast cell stabilizer eye drops (mild cases): Mast cell stabilizer eye drops (i.e., Olopatadine ophthalmic [Pataday, over the counter] or [Pazeo, prescription only], relieves itchiness, best used to prevent symptoms from occurring as they can take three to seven days to work. These can be used as long as necessary.
- Mast cell stabiliser eye drops with antihistamines (dual acting agents): These agents block histamine-1 (H1) receptors for acute therapy (antihistamine action) and inhibit mast cell degranulation for prophylaxis (mast cell stabilizer action). Compared with antihistamines and mast cell stabilizers, topical dual-activity agents are clinically superior in both symptom relief and tolerability 85. They are considered first-line therapies and are the most commonly prescribed treatment 85. They’re fast acting, effective and generally well tolerated. Dual acting agents include bepotastine, epinastine, azelastine, alcaftadine, and ketotifen, which are approved for itch treatment, and olopatadine, which is approved for all signs and symptoms of ocular hypersensitivity disorders 86. Olopatadine is safe, effective, and clinically superior to ketotifen, whilst some studies show that alcaftadine appears to be superior to olopatadine in reducing ocular itch 88, 116.
- Steroids: Steroid eye drops (moderate cases) or steroid oral medication (severe cases) effective in relieving symptoms quickly, but are associated with cataract formation, glaucoma and bacterial and viral infections of the cornea and conjunctiva. Steroid use needs to be monitored closely by your ophthalmologist and used only as directed to prevent serious eye problems and should never be used in the presence of herpes infections. Use of steroid drops for a long time or at a large amount can cause serious vision problems, including glaucoma, cataracts and eye infections (keratitis). Only ophthalmologists (eye specialists) who can monitor for side effects should prescribe steroids for allergic conjunctivitis. Talk with your ophthalmologist if you have questions about steroid drops.
- Immunomodulators such as Tacrolimus or Cyclosporine (moderate-severe cases): The topical calcineurin inhibitors cyclosporine A and tacrolimus are very effective in the treatment of atopic keratoconjunctivitis and may serve as steroid-sparing agents when these forms of chronic allergic conjunctivitis become steroid-dependent 114, 86. The concentrations of cyclosporine A in the different ophthalmic formulations range between 0.01% and 2% and therefore the administration varies between 1 and 6 times per day 88. Tacrolimus may also be prepared as suspension by pharmacies and exists as an ointment for dermatological purposes in most countries at a concentration of 0.03-0.1%. Recent research shows that tacrolimus may have similar if not superior effectivity than cyclosporine A for the treatment of vernal keratoconjunctivitis. Moreover, dermatologic ointment containing tacrolimus is effective for the treatment of lid eczema in atopic keratoconjunctivitis 114, 86. Topical treatment with calcineurin inhibitors has side effects such as stinging/burning sensation and the possibility of molluscum contagiosum virus, papillomavirus, or herpesvirus infection, although there is evidence from studies on dry eye syndrome that treatment with cyclosporine A can be topically administered long term and without systemic absorption 88, 86. 116, 115. At present, tacrolimus is generally administered topically in cases that do not respond to cyclosporine A 114. Finally, in very severe cases, allergic conjunctivitis such as vernal keratoconjunctivitis and atopic keratoconjunctivitis may require systemic immunosuppression that is usually achieved with cyclosporine A, tacrolimus, or mycophenolate mofetil 117, 114, 86.
- Allergen-specific immunotherapy. Allergen-specific immunotherapy is only recommended when clearly defined systemic hypersensitivity to an identified allergen exists. Patients requiring allergen-specific immunotherapy should be referred to an allergist or specialist ophthalmologist. Allergen immunotherapy for specific allergens may benefit people with persistent, severe allergic conjunctivitis. However, relief of allergic conjunctivitis symptoms will not happen straight away.
- Surgery, oral corticosteroids (short pulses) or corticosteroid lid injection, or systemic treatment with immunomodulators or biologics may be appropriate options for use by corneal specialists in selected patients with ocular complications or persisting symptoms following prior treatments. Insunakinra (EBI-005) is the first inmunophilin synthesized for topical ophthalmologic use. It is an antagonist of the IL-1 receptor and binds to it, blocking the rest of the pathway. It has been documented to diminish ocular surface symptoms such as itching, inflammation, and discomfort 122. Another molecule called liftitegrast (Shire Pharmaceuticals) has both activity as an antagonist of the IL-1 receptor and as antagonist of the lymphocyte functional antigen-1 and has proven effective for treatment of ocular surface symptoms 123. In very recalcitrant cases of vernal keratoconjunctivitis and atopic keratoconjunctivitis, eye surgery may be needed. Papillae resection, in some cases with grafting of autologous conjunctiva, amniotic membrane or mucous membrane are effective in the treatment of severe forms of vernal keratoconjunctivitis with corneal ulcers 127, 131, 132. In atopic keratoconjunctivitis, surgery may be needed for eyelid and conjunctival scarring. Atopic disease and atopic keratoconjunctivitis can be complicated by subcapsular cataracts and/or severe ocular surface disease that may require complex surgery such as superficial keratectomy, limbal transplantation, or keratoprosthesis implantation 135.
Usually combinations of oral and topical antihistamines and mast cell stabilizers usually are effective in controlling atopic keratoconjunctivitis symptoms. In more severe cases, there is potential for damage to the eye caused by scratching and rubbing. A eye doctor (ophthalmologist) may advise you to wear cotton gloves at night to prevent unintentional damage to your eye surface. Cold compresses and saline irrigation to lower the elevated tear pH also may be helpful. Only in severe cases should topical steroid therapy be considered and under the supervision of your eye doctor (ophthalmologist). Other steroid-sparing agents may be helpful, including cyclosporine or tacrolimus. Oral medications may be very helpful in some atopic conjunctivitis cases.
Figure 16. Atopic keratoconjunctivitis
Footnotes: Atopic keratoconjunctivitis. (A) Eczema of the eyelid; (B) Pseudoptosis and Dennie–Morgan double fold of the upper and lower lid; (C) Infiltration of the inferior conjunctiva and blepharitis; (D) Limbal infiltration with Horner–Trantas dots.
[Source 229 ]Figure 17. Atopic blepharokeratoconjunctivitis
[Source 80 ]Figure 18. Eyelid atopic dermatitis
Footnote: Atopic dermatitis of the eyelids in a child with characteristic lichenification (skin thickening, hyperpigmentation and exaggerated skin lines caused by sudden itching and excessive rubbing and scratching) and fine scaling 230.
[Source 226 ]Figure 19. Atopic dermatitis
[Source 231 ]What is atopy?
Atopy refers to genetic predisposition of experiencing an exaggerated immune response (hypersensitivity) to allergens via the overproduction of immunoglobulin E (IgE) 232. The term “atopy” is derived from the Greek word meaning “the state of being out of place” 232. Atopy typically consists of atopic dermatitis (skin), asthma (airways), and allergic rhinitis (nasal mucosa). Other atopic conditions include food allergy and drug allergy, allergic conjunctivitis, urticaria, angioedema, and anaphylaxis (a severe, life-threatening allergic reaction that requires immediate medical attention).
Individuals with atopy often have environmental allergies, allergic asthma, rhinitis, and atopic dermatitis or eczema. Less commonly, these individuals exhibit food allergies, urticaria, and nonhereditary angioedema.
While the specific cause of atopy is not well understood, a gene-environment interaction is thought to play an important role. Triggers that have been identified include viral respiratory infections, exercise, certain drugs, climatic factors, and psychological factors. Multiple genes with effects on immunoglobulin E (IgE) synthesis are also likely involved as well as changes in the skin microbiome potentially affecting skin barrier function.
The pathophysiology of atopy involves mast cell activation which leads to an inflammatory cascade of lipid mediators, cytokines, and histamine.
Atopic conjunctivitis causes
The exact cause of atopic keratoconjunctivitis is incompletely understood 64, 114, 224. Atopic keratoconjunctivitis is believed to arise from various factors, including allergen exposure, atopic dermatitis (present in more than 90% of cases), genetic predisposition and a combination of immune imbalance involving immediate type 1 hypersensitivity immune reactions (immunoglobulin E [IgE] mediated) and type 4 delayed hypersensitivity immune reactions to eye allergen exposure 233, 86. The tears of atopic keratoconjunctivitis patients will often contain high levels of immunoglobulin E (IgE) suggesting a type 1 hypersensitivity immune reaction 234, 64. However, 45% of the people with atopic keratoconjunctivitis do not display a hypersensitivity reaction to common allergens 133. Atopic keratoconjunctivitis tends to be more perennial (all year round), often worsening in the winter season, and individuals with atopic keratoconjunctivitis are sensitive to a wide range of airborne environmental pathogens 80. Recent studies suggest that microbes and especially colonization of the conjunctiva with Staphylococcus aureus may have a role in the disease 114.
The pathophysiology of atopic keratoconjunctivitis involves the release of cytokines by mast cells, eosinophils, T cells especially T-helper-1 (Th1) cells that produce chemotaxis and stimulate eosinophil production and conjunctival epithelial cells 235. Eosinophils initiate cytokine production, which heightens the inflammatory response 114. The release of allergic mediators leads to activation of T helper cells and chronic mast cell degranulation 236. Atopic keratoconjunctivitis patients have also been shown to have decreased corneal sensitivity and conjunctival goblet cell density compared to controls 237. The tears of patients who suffer from atopic keratoconjunctivitis have been shown to have elevated inflammatory cytokines and chemokines 238.
Conjunctival inflammation with eosinophil infiltrates in atopic keratoconjunctivitis, sometimes extending to the cornea and leading to corneal ulcer, scar formation and, ultimately, to vision loss 239, 240.
Risk factors for atopic keratoconjunctivitis
Atopic keratoconjunctivitis is a multifactorial disease with genetic and environmental risk factors 224. The strongest associations are genetic predisposition for poor skin barrier function and dysregulation of the immune system, asthma, allergic rhinitis, and environmental allergens 224. The presence of food allergies and food sensitivities in infancy and childhood is associated with a more severe form of atopic dermatitis 241. Allergy and sensitivity to pollen, pets and dust mites have also been associated with atopic keratoconjunctivitis 224.
Environmental factors such as climate, diet, urban living including pollution exposure, tobacco smoke, duration of breastfeeding during infancy, and obesity have been suggested as risk factors for atopic keratoconjunctivitis 241. It has also been proposed that antibiotic use may increase the risk of developing atopic disease via alterations to the gut microbiome 242.
Atopic conjunctivitis signs and symptoms
Atopic conjunctivitis signs and symptoms can vary greatly.
Atopic conjunctivitis usually affects both eyes and makes them:
- Bloodshot
- Puffy eyes or swelling of the eyelids
- Burn or gritty feeling in one or both eyes
- Itchy eyes
- Watery eyes
- Sensitive to light called photophobia.
The symptoms described above may not necessarily mean that you have atopic keratoconjunctivitis. If you experience one or more of these symptoms, contact your eye doctor (ophthalmologist) for a complete eye exam. Blurred vision or any change in the appearance of the cornea (clear part of the eye that covers the pupil) requires urgent referral to an eye specialist. Speak to your doctor or optometrist for a referral.
The main symptoms of atopic keratoconjunctivitis are usually perennial (all year round) and include bilateral itching of the conjunctiva, eyelids, and periorbital skin. Tearing, burning, photophobia, blurry vision and a foreign body sensation in the eyes are commonly encountered symptoms. Patients with atopic keratoconjunctivitis can have copious mucoid discharge, often described as rope-like 243.
In comparison to vernal keratoconjunctivitis, the symptoms of atopic keratoconjunctivitis are typically less severe but persistent. The skin changes in atopic keratoconjunctivitis are more pronounced, including eczema, redness, dryness, and scaly skin, often associated with thickening. Epidermal integrity is disrupted, leading to fissures, scratches (excoriation), cracks, and intense itching 238.
Other associated signs in atopic keratoconjunctivitis include chronic staphylococcal blepharitis, madarosis, keratinization of the lid margin, Hertoghe sign (absence of the lateral portion of eyebrows), and Dennie Morgan folds (skin folds on the lower eyelids due to regular itching) 64. Facial skin may have folds, ectropion, and epiphora. Patients can develop ptosis. In atopic keratoconjunctivitis, inferior palpebral conjunctival involvement is more common than superior involvement seen in vernal keratoconjunctivitis. The discharge in atopic keratoconjunctivitis is watery compared to the stringy mucoid discharge typically seen in vernal keratoconjunctivitis.
Hyperemia, inflammation, and chemosis may be present during active acute episodes 64. The papillae are smaller than in vernal keratoconjunctivitis, although marcopapillae can also develop. Diffuse conjunctival infiltration and scarring can give a whitish and featherless appearance. In cicatricial disease, symblepharon formation, shortening of the fornix, and caruncular keratinization may occur. Limbal papillae similar to those seen in vernal keratoconjunctivitis and Horner-Trantas dots can also arise in atopic keratoconjunctivitis 238.
In atopic keratoconjunctivitis, punctate epithelial erosions are commonly observed, particularly in the inferior part of the cornea, and these erosions can be significant 64. Persistent epithelial defects can occur, leading to cornea thinning, perforation, and descemetocele formation 64. Corneal plaque formation can also be observed. Another common finding feature in atopic keratoconjunctivitis is stromal scarring and peripheral vascularization, which tends to be more prevalent than vernal keratoconjunctivitis 64.
Atopic conjunctivitis complications
Untreated or refractory cases of atopic keratoconjunctivitis have a higher risk of developing sight threatening corneal complications such as bacterial and fungal keratitis, and occasionally, herpetic keratitis 244.
- Cataract: Patients with atopic keratoconjunctivitis may develop cataracts, particularly anterior or posterior subcapsular cataracts, secondary to long-term steroid therapy 245. Cataract is a condition that causes the cornea to become cloudy, resulting in blurred vision. These cataracts can be a side effect of using corticosteroid medications to manage the chronic inflammation associated with atopic keratoconjunctivitis 64. It is thought that 8-10% of people with a severe form of atopic dermatitis are at risk of developing cataract.
- Retina: Atopic keratoconjunctivitis has an increased risk of developing endophthalmitis due to a high lid margin and an increased concentration of Staphylococcus aureus in the lid margins 64. Additionally, atopic keratoconjunctivitis patients at high risk for cataract surgery may also face an increased risk of retinal detachment 246.
- Keratoconus: Keratoconus can be secondary to longterm rubbing of the eyes and herpes simplex keratitis in patients with atopic keratoconjunctivitis 247. The continuous eye rubbing associated with atopic keratoconjunctivitis can weaken and thin the corneal structure, predisposing the cornea to develop keratoconus, characterized by progressive bulging and thinning of the cornea. Additionally, herpes simplex keratitis, an infection caused by the herpes simplex virus, can contribute to corneal thinning and deformation, further increasing the risk of keratoconus development in atopic keratoconjunctivitis patients.
Corneal findings range from punctate epithelial erosions to ulcers (infectious or sterile), neovascularization, and lipid keratopathy. Herpes simplex keratitis is more common in patients with atopic keratoconjunctivitis and often more severe 243. Patients with atopic keratoconjunctivitis are also more likely to get bilateral herpetic keratitis, which is relatively unusual in the absence of atopy 248, 249. As with other eye allergic diseases, people with atopic keratoconjunctivitis are at increased risk of keratoconus due to chronic eye rubbing. Therefore, patients with atopic keratoconjunctivitis should be counseled to limit eye rubbing as much as possible and should be provided with treatment to reduce or eliminate itch. Due to common use of topical and oral corticosteroids, glaucoma is a potential complication of treatment. There are various case reports of conjunctival squamous cell carcinoma (SCC) in patients with atopic keratoconjunctivitis 250, 251.
Figure 20. Cataracts
[Source 252 ]Figure 21. Keratoconus
Footnote: Keratoconus is a degenerative eye disorder in which your cornea thins and stretches near the center, loses its shape and eventually bulges outward to form a cone shape. These physical changes come on gradually; in fact, they may start with just the loss of collagen in your eye. The misshapen cornea distorts your vision and requires immediate medical attention by a licensed ophthalmologist. A cone-shaped cornea may also cause sensitivity to light and doesn’t offer adequate protection for your eye.
[Source 253 ]Atopic conjunctivitis diagnosis
A diagnosis of atopic keratoconjunctivitis is made by history and examination. Examination findings are similar to those seen in simple allergic conjunctivitis. However, in atopic keratoconjunctivitis, there are additional chronic inflammatory changes to the ocular surface, such as corneal scarring and neovascularization. Changes to the eyelids, particularly the lower lid, and peri-orbital skin can range from mild atopy to lichenification (skin thickening, hyperpigmentation and exaggerated skin lines caused by sudden itching and excessive rubbing and scratching) 254.
Although allergy testing may help pinpoint the specific allergens, it is usually not necessary since the types of allergens that usually cause conjunctivitis are very common, like grass, weed, and tree pollens. Eye drop treatments are the same no matter what allergen is causing the reaction.
Physical examination
- Eyelids and periorbita: The eyelids and periorbital skin will almost always show evidence of eczematous dermatitis (erythematous, thickened dry skin with blistered patches). Eyelid findings include tylosis (thickening of the tarsal border of the eyelid) with crusting and scaling 229. Meibomian gland disease is very common in patients with AKC. Other lid findings often encountered are ectropion, trichiasis and madarosis.
- Conjunctiva: The conjunctiva is hyperemic and edematous, with prominent tarsal papillae (papillary conjunctivitis). Mucoid discharge is often present. Severe conjunctival disease can result in scarring and symblepharon. Horner-Trantas dots may or may not be present.
- Cornea: Corneal involvement is frequent and can range from punctate epithelial erosions to ulcers and even perforation. Peripheral vascularization and pannus are common 255.
- Anterior Segment: Anterior and posterior subcapsular cataracts are common.
Diagnostic testing
Diagnostic procedures and lab tests can aid in diagnosis and assessing severity of atopic keratoconjunctivitis. Many of these tests require equipment not readily available to most ophthalmologists.
Serum IgE and skin prick testing is non-specific, but can be helpful in establishing that a patient has atopic disease 256. IgE levels in tears have been shown to correlate with clinical severity of allergic eye disease 257.
Brush cytology, which involves taking scrapings from the tarsal conjunctiva, can quantify levels of inflammatory cells such as eosinophils and neutrophils. It has been show to correlate with the amount of corneal damage 258.
Confocal scanning laser microscopy has been described as a less invasive means of evaluating the ocular surface of atopic keratoconjunctivitis patients by identification of inflammatory cell densities 237. A significant correlation was found between the extent of inflammation as determined via brush cytology and that found by in vivo confocal microscopy 259.
Conjunctival biopsy is rarely used to assist in diagnosing atopic keratoconjunctivitis. Histology of atopic keratoconjunctivitis typically shows proliferation of goblet cells, invasion of eosinophils and mast cells into the epithelium as well as mononuclear cell infiltrate in the substantia propria 238. Conjunctival epithelium of atopic keratoconjunctivitis patients shows increased levels of T cells, T-helper cells, macrophages and dendritic cells 260.
Atopic keratoconjunctivitis differential diagnosis
Atopic keratoconjunctivitis differential diagnosis include 224:
- Vernal keratoconjunctivitis
- Seasonal allergic conjunctivitis
- Giant papillary conjunctivitis
- Phlyctenular keratoconjunctivitis. Phlyctenular keratoconjunctivitis is a nodular inflammation of the cornea or conjunctiva that results from a hypersensitivity reaction to a foreign antigen 261.
- Toxic conjunctivitis
- Viral conjunctivitis
- Infections conjunctivitis (especially trachoma)
- Adult blepharitis. Blepharitis refers to inflammatory disease processes of the eyelid(s). Blepharitis often is associated with systemic diseases, such as rosacea, atopy, and seborrheic dermatitis, as well as eye diseases, such as dry eye syndromes, chalazion, trichiasis, ectropion and entropion, infectious or other inflammatory conjunctivitis, and keratitis 262.
- Ocular rosacea.
Clinically, atopic keratoconjunctivitis is very similar to vernal keratoconjunctivitis (VKC), and there is controversy regarding classification of the two diseases. It has been proposed that vernal keratoconjunctivitis is a childhood form of atopic keratoconjunctivitis that evolves into atopic keratoconjunctivitis in adulthood. However, vernal keratoconjunctivitis can be present without signs of atopic disease. Vernal keratoconjunctivitis is generally regarded to end at puberty, and disease severity can fluctuate with seasons. Giant papillary reaction is more common in vernal keratoconjunctivitis. Atopic disease is typically a year-round condition (perennial). Though atopic keratoconjunctivitis peaks in adults, it can be present in childhood.
Atopic keratoconjunctivitis treatment
Consulting with cornea specialist, dermatologists and allergists can help determine the most successful management. The primary goal in managing allergic keratoconjunctivitis, including vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis, is to avoid exposure to the allergen that triggers the allergic response.
If the allergen is known, steps can be taken to minimize exposure to it or, in some cases, get rid of it completely. For example, someone with an allergy to:
- House dust mites may find that minimisation measures such as removing carpet, using dust mite covers for pillows and mattresses, use of hypoallergenic bedding and washing bedding in hot water are enough to reduce symptoms.
- Animal dander may find the best option is to remove the animal or a pet from the house altogether, particularly if symptoms are severe.
- Use of air filtering devices, and dietary changes in cases of food sensitivity.
Mild to moderate symptoms of allergic keratoconjunctivitis usually respond well to simple home treatments such as bathing eyes with cold water, ice packs and cold water compresses. Non-medicated eye drops can also help to lubricate the eye and gently flush allergens from the surface. More severe symptoms will usually require treatment with medication.
In some cases, eye patching may be required, and prevent complications that lead to vision loss. It is also important to balance this with limiting side effects of treatment.
Cold compresses, saline, and cold artificial tears or ointments are useful because they alleviate inflammation and discomfort and dilute the allergen, especially in acute allergic keratoconjunctivitis 86, 88, 228. Recent studies demonstrate the additive effect on the pharmacology of topical agents when combined with cold compresses and artificial tears 88. Other treatments such as eating probiotics like mandarin orange yogurt or antagonists of the prostaglandin D2 receptor 2 have shown promising results in clinical trials, decreasing the symptoms of patients with rhino-conjunctivitis 88, 228.
Maintaining good eyelid hygiene is also essential to prevent and treat staphylococcal blepharitis.
For patients with dry and fissured skin around the eyes, using a moisturizing cream such as E45 can help keep the skin hydrated and improve comfort.
A bandage contact lens may be recommended as part of the treatment plan for persistent epithelial defects. The bandage contact lens can protect the cornea and promote the healing of the corneal defect 263.
Medications for atopic keratoconjunctivitis
Topical therapy
Mild atopic keratoconjunctivitis can be treated similarly to other allergic eye disease, with emphasis placed on hand hygiene, cold compresses, and topical mast cells stabilizers (olopatadine 0.1% or lodoxamide 0.1%) and antihistamines (azelastine 0.05%) 224. While almost all patients will require more intensive treatment, these are vital to efficacious treatment. Corticosteroid drops and ointments are commonly employed in the treatment of atopic keratoconjunctivitis, especially to achieve control of exacerbations and breakthrough inflammation 224. Steroid therapy should be tapered as quickly as possible while avoiding rebound inflammation 224. Calcineurin inhibitors (cyclosporine and tacrolimus) are an effective steroid-sparing therapy 224. Calcineurin is an enzyme involved in the activation of T cells, a key player in atopic keratoconjunctivitis. Tacrolimus is available as a 0.03% and 0.1% topical ointment for dermatologic use (Protopic). It has been used off- label for treatment of ophthalmic diseases 264. Topical cyclosporine is available commercially in a 0.05% eye drop preparation (Restasis). Topical calcineurin inhibitors are safe and generally well tolerated, with stinging and eyelid skin maceration being the most common side effects 265. A recent meta-analysis demonstrated the efficacy of topical immunomodulators in treating atopic keratoconjunctivitis 266.
Oral Medications
Oral antihistamines are routinely used in atopic disease. Oral steroids and cyclosporine are generally reserved for severe/recalcitrant disease or for treatment of dermatologic disease and are often employed in conjunction with the patient’s dermatologist 224. Prolonged treatment with oral steroids is avoided due to unacceptable side effects. Systemic cyclosporine, given at dose of 5mg/kg per day has been shown helpful in inducing remission of severe atopic disease 267, 268. Once remission is obtained, frequency can be decreased to as little as every 5th day. Patients on systemic cyclosporine need regular monitoring of renal function, liver function, blood counts, and blood pressure.
Additional therapy
Zaouali et al 269 reported improvement of symptoms and clinical features of atopic keratoconjunctivitis with injection of supratarsal triamcinolone acetonide. Punctal occlusion can useful for concomitant dry eye.
Medical follow up
Patients with atopic keratoconjunctivitis require close, long- term follow up for development of vision threatening complications and medication side effects such as cataract formation and intraocular pressure elevation associated with steroid usage.
Surgery for atopic keratoconjunctivitis
Amniotic membrane transplantation has been shown to be very effective for persistent corneal epithelial defects 270.
Tectonic keratoplasty may be necessary for patients with severe corneal thinning or perforations. Penetrating keratoplasty (PK) may be indicated for corneal opacities or severe ulceration and thinning.
Patients with atopic keratoconjunctivitis are at increased risk of cataract formation (especially anterior and posterior subcapsular), independent of steroid exposure. Postoperative care and final visual outcomes are impacted by ongoing ocular surface disease.
Eyelid surgery may be necessary for correction of trichiasis, ectropion or entropion 243.
Surgical follow up
Penetrating keratoplasty in atopic keratoconjunctivitis patients is associated with higher rate of postoperative complications such as graft rejection, cataracts and glaucoma 270. Additionally, penetrating keratoplasty is not curative for atopic keratoconjunctivitis; these patients continue to have severe ocular surface disease that can affect viability of the corneal graft. Despite these complications, penetrating keratoplasty can provide significant improvements in visual acuity. One study looking at long term success of penetrating keratoplasty in atopic keratoconjunctivitis found a 50% failure rate in primary penetrating keratoplasty, with the primary causes of graft failure being rejection and stromal ulceration 243.
Atopic keratoconjunctivitis prognosis
Atopic keratoconjunctivitis (AKC) may result in decreased vision or blindness from corneal complications, such as chronic superficial punctate keratitis, persistent epithelial defects, corneal scarring or thinning, keratoconus, cataracts, and symblepharon formation 243.
Complications result from persistent surface keratopathy, corneal scarring or thinning, keratoconus, cataracts, and symblepharon formation. In addition, medical treatment with corticosteroids can further promote the development of cataracts, glaucoma, and secondary corneal infections.
Proper preventive measures, prompt effective treatment of exacerbations, and well-timed elective surgical intervention can reduce the incidence of poor vision and blindness 271.
Patients should be observed every few days or weeks until the ocular surface disease is stable 271. Moreover, when medically treating patients with steroids or immunosuppressants, a regular interval survey for drug-related side effects and complications is indicated 271.
Viral keratoconjunctivitis
Viral keratoconjunctivitis is caused by viruses and is very contagious. Virus is the most common cause of infectious keratoconjunctivitis in the adult population (80%) and is more prevalent in the summer 272, 273. Viral keratoconjunctivitis may involve one or both eyes, causing red itchy eyes with a ‘watery’ discharge, redness, blood vessel engorgement, pain, photophobia, and pseudomembranes 274. Viral keratoconjunctivitis is highly contagious and means you are likely to have come into contact with someone who already has conjunctivitis. Sometimes viral conjunctivitis is accompanied by cold or flu symptoms. Children are most susceptible to viral infections, and adults tend to get more bacterial infections.
Viral keratoconjunctivitis symptoms include:
- red, sore, watery or gritty eyes
- itchy and swollen eyes
- crusty eyelids.
Between 65% and 90% of cases of viral keratoconjunctivitis are caused by adenoviruses. The adenovirus is part of the Adenoviridae family that consists of a nonenveloped, double-stranded DNA virus. Frequently associated infections caused by the adenovirus include upper respiratory tract infections (URTI), eye infections, and diarrhea in children. Viral keratoconjunctivitis caused by adenoviruses produce two of the common clinical entities: pharyngoconjunctival fever, and epidemic keratoconjunctivitis 275.
- Pharyngoconjunctival fever is characterized by abrupt onset of high fever, pharyngitis, and bilateral conjunctivitis, and by periauricular lymph node enlargement.
- Epidemic keratoconjunctivitis is more severe and presents with watery discharge, hyperemia, chemosis, and ipsilateral periauricular lymphadenopathy. Lymphadenopathy (enlarged lymph glands) is observed in up to 50% of viral conjunctivitis cases and is more prevalent in viral conjunctivitis compared with bacterial conjunctivitis.
- Acute hemorrhagic conjunctivitis
- Acute follicular conjunctivitis
Viral keratoconjunctivitis secondary to adenoviruses is highly contagious, and the risk of transmission has been estimated to be 10-50% 275. Incubation and communicability are estimated to be 5-12 days and 10-14 days, respectively 275.
Viral keratoconjunctivitis secondary to herpes simplex virus (HSV) also known as herpes comprises 1.3 to 4.8% of all cases of acute conjunctivitis and is usually unilateral 276, 275. Primary herpes simplex virus type 1 (HSV-l) infection in humans occurs as a non-specific upper respiratory tract infection. Herpes simplex virus (HSV) spreads from infected skin and mucosal epithelium via sensory nerve axons to establish latent infection in associated sensory cranial nerve 5 (trigeminal nerve) and its ganglia. Latent infection of the trigeminal ganglion occurs in the absence of recognized primary infection, and reactivation of the virus may follow any of the three branches.
There is no specific treatment for most viral keratoconjunctivitis and it usually will get better on its own.
Viral keratoconjunctivitis causes
The most common cause of viral keratoconjunctivitis is adenoviruses. The adenovirus is part of the Adenoviridae family that consists of a nonenveloped, double-stranded DNA virus. Frequently associated infections caused by the adenovirus include upper respiratory tract infections, eye infections, and diarrhea in children.
Viral keratoconjunctivitis can be contracted by direct contact with the virus, airborne transmission, and reservoir such as swimming pools 277, 278.
Most cases of viral keratoconjunctivitis are highly contagious for 10 to 14 days. Washing hands and avoidance of eye contact are key to preventing transmission to others.
Up to 90% of viral keratoconjunctivitis cases are caused by adenoviruses 12. In children pharyngoconjunctival fever due to human adenovirus (HAdV) types 3, 4, and 7 results in acute follicular conjunctivitis with fever, sore throat (pharyngitis) and periauricular lymphadenopathy 274. Epidemic keratoconjunctivitis is the most severe eye infection caused by adenovirus and is classically associated with human adenovirus (HAdV) serotypes 8, 19, and 37 274. The cornea can be affected by the viral replication in the epithelium and anterior stroma leading to superficial punctate keratopathy and subepithelial infiltrates 279.. Monotherapy with povidone-iodine 2% have demonstrated the resolution of symptoms 280. Further combinations of povidone-iodine with corticosteroids are undergoing phase 3 randomized controlled studies 274. Visual symptoms caused by subepithelial infiltrates can be debilitating and the use of tacrolimus, 1% and 2% cyclosporine A eye drops have been shown to effective 281, 282, 283.
Herpetic keratoconjunctivitis
Herpes simplex virus (HSV) is estimated to be responsible for 1.3 to 4.8% of acute conjunctivitis cases 276. Herpes conjunctivitis is common in adults and children and associated with follicular conjunctivitis. Herpetic conjunctivitis treatment with topical antiviral agents is aimed at reducing virus shedding and the development of keratitis.
Varicella-zoster virus
Varicella-zoster virus, a virus that causes chickenpox or varicella, can cause conjunctivitis either by direct contact of the eye or skin lesions or inhalation of infected aerosolized particles, especially with the involvement of the first and second branches of the trigeminal nerve.
Acute Hemorrhagic Conjunctivitis (AHC)
Acute hemorrhagic conjunctivitis (AHC) is a very contagious form of viral conjunctivitis and symptoms include foreign body sensation, epiphora, eyelid edema, conjunctival chemosis, and subconjunctival hemorrhage 274. A small proportion of patients experience systemic symptoms of fever, fatigue, limb pain. Picornaviruses EV70 and coxsackievirus A24 variant (CA24v) are thought to be the responsible viruses 284. Transmission is primarily via hand to eye contact and fomites 285.
Figure 22. Acute hemorrhagic conjunctivitis
Footnotes: Acute hemorrhagic conjunctivitis caused by coxsackievirus A24 variant (CA24v) infection. (A) First day morning; (B) first day afternoon; (C) second day am; (D) second day pm; (E) third day am; (F) fourth day pm; (G) sixth day pm. The bilateral lid edema (*), vascular dilation, and conjunctival erythema were noted on the second day, and the inferior-nasal chemosis (arrow) was present on the third day post-acute hemorrhagic conjunctivitis. The clinical signs were less and resolved more quickly in the right (second infected) than the left (first affected) eye.
[Source 285 ]COVID-19 keratoconjunctivitis
COVID-19 has been reported to cause keratoconjunctivitis along with fever, cough, respiratory distress, and death 286. Retrospective and prospective studies show that 1% to 6% of patients display COVID-19 related conjunctivitis, with conjunctival swabs being positive in 2.5% of cases 287. Transmission through ocular tissues is incompletely understood. Ophthalmologists are at higher risk for COVID-19 infection due to the close proximity to patients, equipment intense clinics, direct contact with patients’ conjunctival mucosal surfaces, and high-volume clinics. Systemic COVID-19 is contracted through direct or airborne inhalation of respiratory droplets. Additional protective measures such as social distancing, wearing masks, reduced clinic volumes, and sterilization of surfaces should be practiced to reduce transmission risk.
Viral keratoconjunctivitis signs and symptoms
Viral keratoconjunctivitis can lead to one or more of these symptoms:
- eye irritation and redness
- excessive tearing from eyes
- discharge from the eyes
- swelling of the eyelids
- photophobia (unable to tolerate looking into light)
- often associated with an upper respiratory tract infection (cold).
Patients with viral keratoconjunctivitis present with sudden onset foreign body sensation, red eyes, itching, light sensitivity, burning, and watery discharge. Whereas with bacterial keratoconjunctivitis, patients present with all the above symptoms, but with pus (mucopurulent discharge) and sticky eyelids upon waking.
Visual acuity is usually at or near their baseline vision. The cornea can have subepithelial infiltrates that can decrease the vision and cause light sensitivity. The conjunctiva is injected (red) and can also be edematous. In some cases, a membrane or pseudomembrane can be appreciated in the tarsal conjunctiva. These are sheets of fibrin-rich exudates that are devoid of blood or lymphatic vessels. True membranes can lead to the development of subepithelial fibrosis and symblepharon, and also bleed heavily on removal 288. Follicles, small, dome-shaped nodules without a prominent central vessel, can be seen on the palpebral conjunctiva. The majority of viral keratoconjunctivitis patients will have follicles present, but the presence of papillae does not rule out a viral etiology 289.
Fever, malaise, fatigue, presence of enlarged lymph nodes (lymphadenopathy) and other constitutional signs help to differentiate viral keratoconjunctivitis from other causes. Unequal pupil size (anisocoria) and photophobia are associated with serious eye conditions including anterior uveitis, keratitis, and scleritis 15.
When primary eye herpes simplex virus (HSV) infection occurs, the patient typically manifests unilateral, thin, and watery discharge and sometimes vesicles may appear on the face or eyelids and vision may be affected. Corneal involvement may occur. With herpes zoster, there is a linear dermatomal pattern of vesicles. The conjunctiva is often red with a mucopurulent discharge. In a small percentage of patients, there is a history of external eye HSV infection that may lead to the diagnosis.
Keratoconjunctivitis caused by adenoviruses:
- Pharyngoconjunctival fever presents by abrupt onset of high fever, pharyngitis, subconjunctival hemorrhage, bilateral conjunctivitis, and by preauricular lymph node enlargement.
- Epidemic keratoconjunctivitis is more severe and presents with watery discharge, conjunctival membranes or pseudo membranes, hyperemia, chemosis, and ipsilateral preauricular lymphadenopathy. Can involve both epithelial and subepithelial corneal infiltrates.
- Can affect the cornea, look for subepithelial infiltrates.
- Lymphadenopathy is observed in up to 50% of viral keratoconjunctivitis cases and is more prevalent in viral keratoconjunctivitis compared with bacterial keratoconjunctivitis.
Herpes virus keratoconjunctivitis:
- The eyelids often are swollen with small bruise. Watery discharge and lymphadenopathy in front of the ear may be present. Usually unilateral.
- Skin or eyelid margin vesicles, or ulcers on the bulbar conjunctiva
- The cornea often demonstrates a punctate epitheliopathy. In severe cases, there can be a corneal epithelial defect (Dendritic epithelial keratitis). It typically begins in one eye and progresses to the fellow eye over a few days.
- It is important to note that herpes virus conjunctivitis does not form conjunctival membranes or pseudo membranes.
- Herpes zoster virus can involve ocular tissue, especially if the first and second branches of the trigeminal nerve are involved. Eyelids (45.8%) are the most common site of ocular involvement, followed by the conjunctiva. Corneal complication and uveitis may be present in 38.2% and 19.1% of cases, respectively. Severe forms include those presenting with the Hutchinson sign (vesicles at the tip of the nose, which has a high correlation with corneal involvement).
Viral keratoconjunctivitis complications
Viral keratoconjunctivitis complications may include 274:
- Punctate keratitis
- Bacterial superinfection
- Conjunctival scarring
- Corneal ulceration
- Chronic infection
Viral keratoconjunctivitis diagnosis
In most cases, your doctor can diagnose viral keratoconjunctivitis by asking about your recent health history and symptoms and examining your eyes. Palpation of the preauricular lymph nodes may reveal a reactive lymph node that is tender to the touch and will help differentiate viral keratoconjunctivitis versus bacterial keratoconjunctivitis.
Viral keratoconjunctivitis secondary to adenoviruses is highly contagious, and the risk of transmission has been estimated to be 10-50%. Patients commonly report recent contact with an individual with a red eye, or they may have a history of recent symptoms of an upper respiratory tract infection (URTI). Incubation and communicability are estimated to be 5-14 days for viral conjunctivitis secondary to adenoviruses.
Laboratory testing is typically not indicated unless the symptoms are not resolving and infection last longer than 4 weeks 274. Laboratory testing can be indicated in certain situations such as a suspected chlamydial infection in a newborn, an immune-compromised patient, excessive amounts of discharge, or suspected gonorrhea co-infection 274. In the office, doctors can run tests to positively identify adenovirus with a specificity and sensitivity of 89% and 94%, respectively. However, ophthalmologists usually can make the diagnosis clinically with confirmational additional testing 290.
Regarding adenovirus testing, the gold standard has traditionally been cell culture because once the virus is isolated, the diagnosis is definitive and the characterization of the virus can be undertaken 291. Disadvantages include cost and increased time associated with cell culture-based testing. The mainstay of viral conjunctivitis testing in the developed world is the detection of viral DNA by polymerase chain reaction (PCR). PCR for adenovirus has been shown to be 93% sensitive and 97.3% specific from conjunctival swabs, with similar values, found for HSV diagnosis 291. A recent development has been the creation of a rapid detection testing platform for adenovirus, the AdenoPlus assay (Rapid Pathogen Screening Inc., Sarasota, Florida, USA). This is a swift office-based test designed to detect 53 serotypes of adenovirus and provide a result within 10 minutes 292. Studies have demonstrated high specificity values of 92% to 98% in detecting adenoviral conjunctivitis 293, 294, 295. However, the test has lower sensitivity reported compared with PCR analysis 293, 294, 295. Rapid detection testing can be more useful in the future once viral conjunctivitis treatments reach the clinical setting 296.
Viral keratoconjunctivitis treatment
Viral keratoconjunctivitis treatment is aimed at symptomatic relief and not to eradicate the self-limiting viral infection 274. The resolution of viral keratoconjunctivitis can take up to 3 weeks. Treatment includes using artificial tears for lubrication four times a day or up to ten times a day with preservative-free tears. Cool compresses with a wet washcloth to the periocular area may provide symptomatic relief. Preventing the spread of infection to the other eye or other people requires the patient to practice good hand hygiene with frequent washing, avoidance of sharing towels or linens, and avoiding touching their eyes. A person is thought to shed the virus while their eyes are red and tearing.
If a membrane or pseudomembrane is present, it can be peeled at the slit lamp to improve patient comfort and prevent any scar formation from occurring 274. These membranes can either be peeled with a jeweler forceps or a cotton swab soaked with topical anesthetic 274. Topical steroids can help with the resolution of symptoms. However, they can also cause the shedding of the virus to last longer. Patients should be informed that they are highly contagious and should refrain from work or school until their symptoms resolve. While using steroids, they may still shed the virus without the visual symptoms that would indicate that they have an infection. Steroids should be reserved for patients with decreased vision due to their subepithelial infiltrates or severe conjunctival injection causing more the expected discomfort 297, 298.
The use of povidone-iodine, a non-specific disinfectant, is a promising new treatment for adenoviral keratoconjunctivitis 292. This is an inexpensive and widely available antiseptic solution which is used as part of the aseptic preparation for ocular surgery. It is able to kill extracellular organisms but has no effect intracellularly. It does not induce drug resistance because its mechanism of action is not immunologically dependent. A single dose of 2.5% povidone-iodine in infants with adenoviral conjunctivitis resulted in a reduction in symptom severity and reduced recovery time without significant side effects 299.
Topical corticosteroids alone are contraindicated in epithelial herpes simplex keratitis and are associated with prolonged viral shedding and infection 296. When used in combination with an anti-infective, corticosteroids have demonstrated good tolerability and efficacy in treating the inflammatory and infectious components of conjunctivitis 300, 301.
Viral keratoconjunctivitis prognosis
The majority of cases of virus keratoconjunctivitis resolve on their own 274. In rare cases, chronic infection may occur. Most cases resolve within 14-30 days. In some patients, photophobia, diminished vision and glare may be a problem 274.
Herpes keratoconjunctivitis
There are 2 common types of herpes virus – herpes simplex virus 1 (HSV-1), also called oral herpes, and herpes simplex virus 2 (HSV-2), which is genital herpes. These two forms of the herpes virus can spread to other parts of the body and cause lesions or sores. Herpes simplex virus 1 (HSV-1) is the most common and primarily infects the face, causing the familiar “cold sore” or “fever blister”. Herpes simplex virus 2 (HSV-2) is the sexually transmitted form of herpes, infecting the genitals. While both HSV-1 and HSV-2 can spread to the eye and cause infection, herpes simplex virus 1 (HSV-1) is by far the most frequent cause of eye infections leading to epithelial keratitis, viral keratitis, herpes keratitis, or herpetic keratoconjunctivitis. Herpes simplex virus type 1 (HSV-1) is the leading cause of corneal blindness in the developed world 302, 303. Herpes simplex virus 1 (HSV-1) infection can be transferred to the eye by touching an active lesion and then your eye. It is rare to transfer herpes simplex virus 2 (HSV-2) to the eye.
Globally, the incidence of HSV keratitis is 1.5 million yearly, including 40,000 new cases that result in severe visual impairment 304. In the US, approximately 500,000 people are afflicted with eye HSV infection 304.
Herpes virus keratoconjunctivitis:
- The eyelids often are swollen with small bruise. Watery discharge and lymphadenopathy in front of the ear may be present. Usually unilateral.
- Skin or eyelid margin vesicles, or ulcers on the bulbar conjunctiva
- The cornea often demonstrates a punctate epitheliopathy. In severe cases, there can be a corneal epithelial defect (Dendritic epithelial keratitis). It typically begins in one eye and progresses to the fellow eye over a few days.
- It is important to note that herpes virus conjunctivitis does not form conjunctival membranes or pseudo membranes.
- Herpes zoster virus can involve ocular tissue, especially if the first and second branches of the trigeminal nerve are involved. Eyelids (45.8%) are the most common site of ocular involvement, followed by the conjunctiva. Corneal complication and uveitis may be present in 38.2% and 19.1% of cases, respectively. Severe forms include those presenting with the Hutchinson sign (vesicles at the tip of the nose, which has a high correlation with corneal involvement).
Left untreated, herpes keratitis can severely damage your eye. It is very important to consult an eye specialist (ophthalmologist) before beginning any treatment, because some medications or eye drops may actually make the infection worse. Topical corticosteroids should be avoided because they potentiate the virus and may cause harm.
Mild infection is usually treated with topical and oral antiviral medication (acyclovir or valacyclovir or famciclovir for 10 to 14 days and/or topical antiviral medications), as well as antibiotic eye drops or ointments 305. Topical ganciclovir 0.15% can be utilized and is approved by the FDA for the treatment of acute herpetic keratitis since 2009. Topical ganciclovir 0.15% is typically dosed five times a day until the cornea ulcer heals, and then three times a day for another week 306. Topical therapy with trifluridine 1% eight to nine times a day can also be prescribed, but care must be taken to ensure antiviral drops are discontinued within 10-14 days due to significant corneal toxicity 306. Topical acyclovir is not approved by the FDA, but used commonly outside the United States for the treatment of HSV epithelial keratitis 306. Topical steroids are contraindicated in the presence of active epithelial disease, although cycloplegia drops and topical antibiotics may be added 307. Your ophthalmologist may also gently scrape the affected area of the cornea to remove the diseased cells.
Herpes keratitis (infectious or inflammatory) can recur. To decrease the risk of recurrence, antiviral pills may be used for years. According to the landmark Herpetic Eye Disease Study (HEDS) published in 1991, long-term prevention with oral antivirals decreases the risk of recurrent HSV keratitis 308. Specifically, long-term acyclovir therapy (400mg twice a day for 1 year) decreased the risk of epithelial and stromal HSV disease by almost half 309.
Figure 23. Herpes simplex keratitis
Footnotes: Slit-lamp photograph showing three phases of lesions: epithelial dots (9 o’clock), dendritic pattern (6 o’clock) and a geographic epithelial keratitis (12–2 o’clock), suggesting herpes simplex virus (HSV) epithelial keratitis.
[Source 310 ]Figure 24. Herpes simplex keratitis
Footnote: Slit lamp photo demonstrating classic epithelial dendrites in a patient with herpes simplex virus (HSV) keratitis after fluorescein staining.
[Source 311 ]Figure 25. Necrotizing herpes simplex keratitis
Footnote: Necrotizing herpes simplex keratitis associated with stromal thinning and overlying epithelial defect in right eye.
[Source 303 ]Herpetic keratoconjunctivitis cause
Herpes simplex virus 1 (HSV-1) or “oral herpes” is very contagious and is commonly transmitted by skin contact with someone who has the virus. Almost everyone, about 90 percent (9 out of 10) of the population, is exposed to HSV-1, usually during childhood. After the original infection, herpes simplex virus 1 (HSV-1) lies in a dormant state, living in nerve cells of the skin or eye. However, studies have shown that both herpes simplex virus 1 (HSV-1), also called oral herpes, and herpes simplex virus 2 (HSV-2) also called genital herpes may affect either location, and mixed infections have been reported 307.
Herpes simplex virus (HSV) spreads from infected skin and mucosal epithelium via sensory nerve axons to establish latent infection in associated sensory cranial nerve 5 (trigeminal nerve) and its ganglia. Latent infection of the trigeminal ganglion occurs in the absence of recognized primary infection, and reactivation of the virus may follow any of the three branches.
Reactivation can be triggered in a number of ways, including:
- stress
- sun exposure or other UV light exposure (such as tanning beds)
- fever
- trauma to the body (such as injury or surgery)
- menstruation
- certain medications
Once herpes simplex is present in your eye, it typically infects the eyelids, conjunctiva and cornea. It may also infect the inside of the eye; however, this is much less common.
Risk factors for developing herpetic keratoconjunctivitis
Risk factors for development of primary herpes simplex virus (HSV) infection involve direct contact with infected secretions or lesions 302.
Risk factors for reactivation of herpes simplex virus (HSV) infection have been theorized to include 312, 307:
- Sunlight
- Fever
- Trauma
- Heat
- Menstruation
- Stress
- Trigeminal nerve manipulation
- Infectious disease and immunocompromised states.
- COVID-19 infection 313
- COVID-19 vaccination 314
Herpetic keratoconjunctivitis symptoms
The symptoms of herpes keratitis may include:
- eye pain
- red eye
- facial rash
- blurred vision
- tearing
- eye discharge
- sensitivity to light or photophobia
- the eyelids often are swollen with bruises. Watery discharge and preauricular lymphadenopathy may be present. Usually unilateral.
- skin or eyelid margin vesicles, or ulcers on the bulbar conjunctiva
- the cornea often demonstrates a punctate epitheliopathy. In severe cases, there can be a corneal epithelial defect (Dendritic epithelial keratitis). It typically begins in one eye and progresses to the fellow eye over a few days.
- It is important to note that herpes virus conjunctivitis does not form conjunctival membranes or pseudo membranes.
The sign of HSV keratitis is the presence of multiple small branching epithelial dendrites on the surface of the cornea. Although often times it first presents as a coarse, punctuate epithelial keratitis, which may be mistaken for a viral keratitis. The HSV dendrite possesses terminal bulbs that distinguish it from the herpes zoster pseudodendrite and follows the nerve pattern of the cornea.
When primary eye herpes simplex virus (HSV) infection occurs, the patient typically presents with unilateral (one eye) or more rarely bilateral, blurred vision, photophobia, pain, redness, and/or thin, and watery discharge and sometimes accompanying vesicular eyelid lesions. Eye pain is often a hallmark of HSV epithelial keratitis, as it tends to be the most painful form of HSV keratitis 307. In a small percentage of patients, there is a history of external eye herpes simplex virus (HSV) infection that may lead to the diagnosis.
Bilateral eye involvement are more common in patients with atopy due to proposed immune dysregulation increasing susceptibility to viral infections 315. The incidence of bilateral eye involvement range from 1.3% to 12% 303. Herpes simplex virus (HSV) can affect all layers of the cornea, and may be accompanied by a blepharoconjunctivitis, which may result in lesions of the eyelids and a follicular conjunctivitis. Characteristically, herpes simplex virus (HSV) epithelial keratitis presents with classic dendritic lesions with terminal bulbs. Recurrent activations within the sensory ganglion can result in cornea scarring, necrosis, and decreased corneal sensation (neurotrophic cornea), all of which can be vision threatening.
If the herpes simplex virus (HSV) infection is superficial, involving only the cornea’s outer layer called the epithelium, it will usually heal without scarring. However, if the herpes simplex virus (HSV) involves the deeper layers of cornea which can happen after some time, the infection may lead to scarring of the cornea, loss of vision and sometimes even blindness.
Worldwide, approximately one million people suffer from HSV epithelial keratitis yearly 304. Several large scale studies have demonstrated that epithelial keratitis is the most common form of HSV eye involvement 316, 317.
Herpetic keratoconjunctivitis complications
Corneal complications of herpetic eye disease range from epitheliopathy to frank neurotrophic or metaherpetic ulcers 306. Neurotrophic corneas, defined as corneas with decreased sensation due to corneal nerve changes after HSV infection, can cause a wide array of issues ranging from severe dry eyes to corneal perforation from a non- healing neurotrophic ulcer.
Herpetic keratoconjunctivitis diagnosis
The diagnosis of HSV is often made clinically, however, laboratory tests are available to confirm the diagnosis in more ambiguous and difficult cases and in all cases of neonatal herpetic infections. Key aspects to inquire about in the history of patients with suspected HSV eye infection include past infections (history of recurrent “red eye”, particularly unilateral), underlying systemic diseases, immunosuppression or immunocompromised state, history of eyelid lesions, history of oral and genital ulcers, and recent infections and vaccinations.
The clinical diagnosis of HSV may be suggested by the presence of the multiple arborizing dendritic epithelial ulcers with terminal bulbs. The bed of the ulcer stains with fluorescein, while the swollen corneal epithelium at the edge of the ulcer typically stains with rose bengal. Several dendrites may also coalesce to form a geographic epithelial ulcer 318. In addition, there may be mild conjunctival injection, ciliary flush, mild stromal edema and subepithelial white blood cell infiltration. Following resolution of the primary infection, a “ghost dendrite” may be visible just beneath the prior area of epithelial ulceration.
Physical examination
In addition to a standard biomicroscopy, special attention should be paid to the presence of a preauricular lymph node, vesicular lesions on the lids or adnexa, bulbar follicles, decreased corneal sensation, and most notably the presence of epithelial dendrites on the cornea.
For primary HSV keratitis, it is more common for patients to present with a blepharoconjunctivitis than with cornea involvement. Primary keratitis typically occurs in childhood and is spread by direct contact 307 Diffuse punctate keratopathy or corneal microcysts may be visible 319.
Recurrent HSV keratitis can also present as epithelial keratitis 306. The earliest sign of epithelial disease include raised clear vesicles that later coalesce to form the classic dendritic lesion. The dendritic ulcer, the dichotomous branching cornea lesion, can be stained with fluorescein and is the hallmark of HSV epithelial keratitis. These epithelial lesions represent active replicating virus. As the dendritic ulcer may evolve into a geographic ulceration of the cornea, especially in patients with compromised immunity, atopy, or on topical steroids.
Diagnostic procedures
Serologic testing may be performed but is usually not helpful in recurrent disease as most adults are laterally infected with HSV. Thus, serum antibody testing is typically of limited use. However, conjunctival scrapings, impression cytology specimens, and scrapings from vesicular lesions on the skin may be tested by cytology, culture, or polymerase chain reaction (PCR) for the presence of HSV 320. Corneal scrapings of HSV keratitis prepared with Giemsa stain may reveal the presence of intranuclear viral inclusion bodies 320. Multinucleated giant cells may also be found. Fluorescent antibody (FAB) testing involving impression cytology using nitrocellulose membrane or a cornea smear. A Tzanck smear can reveal multinucleated giant cells and intranuclear eosinophilic inclusion bodies.
Herpetic keratoconjunctivitis differential diagnosis
The differential diagnoses of HSV keratoconjunctivitis include herpes zoster ophthalmicus and other types of viral keratitis (adenovirus, cytomegalovirus, Epstein- Barr virus), neurotrophic keratopathy, epithelial regeneration line, iatrogenic (topical drops such as antivirals), acanthamoeba keratitis, soft contact lens overwear, microbial keratitis, staphylococcal marginal keratitis, and Thygeson’s superficial punctuate keratitis 307.
Herpetic keratoconjunctivitis treatment
It is very important to consult an ophthalmologist before beginning any treatment, because some medications or eye drops may actually make the infection worse. Topical corticosteroids should be avoided because they potentiate the virus and may cause harm. Treatment of herpes keratitis depends on its severity 306. Mild infection is usually treated with topical and oral antiviral medication (acyclovir or valacyclovir or famciclovir for 10 to 14 days and/or topical antiviral medications), as well as antibiotic eye drops or ointments 305. Topical ganciclovir 0.15% can be utilized and is approved by the FDA for the treatment of acute herpetic keratitis since 2009. Topical ganciclovir 0.15% is typically dosed five times a day until the cornea ulcer heals, and then three times a day for another week 306. Topical therapy with trifluridine 1% eight to nine times a day can also be prescribed, but care must be taken to ensure antiviral drops are discontinued within 10-14 days due to significant corneal toxicity 306. Topical acyclovir is not approved by the FDA, but used commonly outside the United States for the treatment of HSV epithelial keratitis 306. Topical steroids are contraindicated in the presence of active epithelial disease, although cycloplegia drops and topical antibiotics may be added 307. Your ophthalmologist may also gently scrape the affected area of the cornea to remove the diseased cells.
Amniotic membrane may promote epithelial healing and reduce scar formation in conjunction with antivirals 321. According to the landmark Herpetic Eye Disease Study (HEDS) published in 1991, long- term prevention with oral antivirals decreases the risk of recurrent HSV keratitis 308. Specifically, long-term acyclovir therapy (400mg twice a day for 1 year) decreased the risk of epithelial and stromal HSV disease by almost half 309.
In case of severe scarring and vision loss, a corneal transplant may be required.
There is no complete cure for herpes; once the virus is in the body, you cannot get rid of it. However, if you develop herpes keratitis, there are some things you can do to help prevent recurring outbreaks:
- If you have an active cold sore or blister, avoid touching your eyes.
- When herpes eye infections happen often, your doctor might place you on a preventative antiviral medication that you take by mouth.
- Steroids can increase the herpes virus in the body. You should not use steroid eye drops unless you are taking an anti-viral medicine as well.
- Stop wearing contact lenses if you keep getting infections.
- See an ophthalmologist immediately if symptoms of ocular herpes return.
Medical follow up
The patient should be closely monitored and if no response to treatment occurs after 1 week of therapy, the possibility of resistance to antiviral therapy, antiviral toxicity, neurotrophic disease, poor compliance with medication, and/or an alternative diagnosis should be considered 306.
Surgery
If there is visually significant stromal scarring, a penetrating keratoplasty may be performed once the disease is inactive 306. Depending on the location and size of the scar, a lamellar keratoplasty may also be used to clear the visual axis. Of note, in eyes that are unable to sustain a clear graft, a Boston keratoprosthesis may be a viable option.
Surgical follow up
Follow-up should be performed as standard of practice for penetrating keratoplasty 322. Special attention should be paid to signs of recurrence of herpetic disease. Oral antiviral therapy may improve the rate of graft survival by decreasing the number of recurrences.
Herpetic keratoconjunctivitis prognosis
Herpetic keratoconjunctivitis prognosis is usually good if treated early, but greatly varies depending on severity and number of recurrences of the disease.
Bacterial keratoconjunctivitis
Bacterial keratoconjunctivitis is caused by bacteria and is very contagious. The most common bacteria are Staphylococcus species, Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis 323, 324. Bacterial keratoconjunctivitis presents with conjunctival injection, mucopurulent discharge, and crusty eyelids. The diagnosis is usually clinical. The condition is often self-limiting, but there is good evidence that antibiotics improve remission rates. Most of the current evidence suggests that the choice of topical antibiotics and the treatment regimen do not significantly affect the rate of recovery from infection. Failure to recognize and treat bacterial keratoconjunctivitis may lead to complications, such as keratitis or anterior uveitis. Bacterial keratoconjunctivitis is commonly associated with hyperacute (<24 hours) onset of severe and very rapid progressing conjunctivitis. Bacteria rank second among the most common causes of infectious keratoconjunctivitis. Bacterial keratoconjunctivitis almost always affects both eyes, although it may start in just one eye. Symptoms include gritty feeling like sand in your eye and a thick yellow discharge (pus) that crust over your eyelashes, especially after sleep. With bacterial keratoconjunctivitis, you have sore, red eyes with a lot of sticky pus in the eye. Some bacterial infections, however, may cause little or no discharge. Sometimes the bacteria that cause pink eye are the same that cause strep throat.
Bacterial keratoconjunctivitis has an incubation period and communicability of 1 to 7 days and is commonly caused by 325:
- Neisseria gonorrhoeae
- Neisseria meningitidis
Bacterial keratoconjunctivitis with acute or subacute onset (hours-days) of moderate to severe severity are commonly caused by 325:
- Haemophilus influenzae biotype III (previously known as Haemophilus aegyptius)
- Haemophilus influenzae
- Streptococcus pneumoniae
- Streptococcus viridans
- Staphylococcus aureus
Chronic bacterial keratoconjunctivitis is used to describe any keratoconjunctivitis lasting more than 4 weeks. It has an incubation period of 2 to 7 days 275.
Clinically distinguishing bacterial keratoconjunctivitis from other keratoconjunctivitis is essential as it can help direct treatments and potentially curb unnecessary empiric antibiotic administration. Traditionally, a purulent or mucopurulent discharge has been associated with the diagnosis of bacterial keratoconjunctivitis while watery discharge has been more consistent with viral or allergic keratoconjunctivitis 12. A 2003 study contested this assertion based on a lack of evidence to support discharge characteristics correlating with the cause of keratoconjunctivitis 12, 326. A later study by the same authors concluded that three findings were significantly predictive of bacterial keratoconjunctivitis, including glued eyes, lack of itching, and no previous history of keratoconjunctivitis 12, 326. A 2006 prospective study of children with conjunctivitis described five clinical history and physical examination variables associated with bacterial culture-positive infections. These included a history of gluey or sticky eyelids in the morning, examination findings of mucoid or purulent discharge, eyelids or eyelashes crusting or gluing on examination, absence of burning sensation, and lack of watery discharge 327. The inconsistencies of these findings concerning discharge as a predictor of bacterial infection might be attributable to the 2003 survey focusing on history, while the 2006 study included in-office physical examination findings. These differences reinforce the idea of variability in the presentation of bacterial keratoconjunctivitis.
Gonococcal infection of the eye, when it does occur, typically presents as keratoconjunctivitis (Figure 13). Gonococcal conjunctivitis in adults most often results from the accidental transfer of microorganisms from one part of the body to another (autoinoculation) in persons with genital gonococcal infection. Persons with gonococcal conjunctivitis may initially develop mild, nonpurulent conjunctivitis, that, if untreated, typically progresses to marked conjunctival redness, copious purulent discharge, and conjunctival edema 328. Less often, ulcerative keratitis develops. Untreated gonococcal conjunctivitis can cause complications that may include corneal perforation, endophthalmitis (an infection of the tissues or fluids inside the eyeball) and blindness.
Bacterial keratoconjunctivitis may need antibiotic ointment or drops prescribed by a doctor. Treatment should be applied to both eyes, even if only one eye appears to be infected. Always treat the unaffected eye first. The findings of 2023 Cochrane review suggest that the use of topical antibiotics is associated with a modestly improved chance of resolution in comparison to the use of placebo 329. Since no evidence of serious side effects was reported, use of antibiotics may therefore be considered to achieve better clinical and microbiologic efficacy than placebo.
Figure 26. Gonococcal conjunctivitis
Footnote: Severe case of gonococcal conjunctivitis. Note the purulent material on the upper and lower lids.
[Source 330 ]Figure 27. Chlamydial conjunctivitis
Footnotes: 22-year-old man presenting with a long-standing (>4 months) history of redness in his right eye accompanied by scant mucopurulent discharge was treated by several courses of topically administered antibiotics. Owing to the poor response to therapy, he was referred to our ocular inflammatory clinic. External examination of the right eye revealed prominent conjunctival follicles almost exclusively located at the inferior fornix of the conjunctiva (A) and prominent conjunctival bulbar follicles at the semilunar fold (B). The rest of the examination was unremarkable except for a symptom of mild burning on urination for the past 3 months. Nucleic acid amplification testing (NAAT) on a conjunctival swab confirmed the presence of Chlamydia trachomatis in both samples and excluded the presence of Neisseria gonorrhoeae. The patient was prescribed a 1-week course of oral doxycycline 100 mg twice a day (because topical antibiotics are ineffective). Symptoms and signs resolved completely within 1-month thereafter.
[Source 331 ]Bacterial keratoconjunctivitis causes
Bacterial keratoconjunctivitis is inflammation and infection of the conjunctiva and cornea caused by direct contact with infected secretions.
The most common bacteria that cause bacterial keratoconjunctivitis are 324:
- Staphylococcus, the same family of bacteria that cause staph infections.
- Streptococcus, the bacteria family that causes strep throat and pneumococcal disease.
- Haemophilus influenzae, a bacteria family best known for causing meningitis in young children.
- Sexually transmitted infections (STIs), like Chlamydia trachomatis (chlamydia), Neisseria gonorrhoeae (gonorrhea) and Treponema pallidum (syphilis). When these infections pass from birthing parent to child during birth, it can lead to neonatal conjunctivitis, a condition that can cause permanent eye damage and blindness.
Bacterial pathogens in adults are more often staphylococcal species with Haemophilus influenzae and Streptococcus pneumoniae responsible for a smaller percentage of cases 332. Staphylococcus aureus is more commonly found in adults and the elderly but is also present in pediatric cases of bacterial conjunctivitis 327. There has also been an increase in the frequency of conjunctivitis secondary to methicillin-resistant Staphylococcus aureus (MRSA) 332. Contact lens wearers are more susceptible to gram-negative infections 327. Pseudomonas aeruginosa is more likely to be the isolate from critically ill, hospitalized patients 327. Neonates can be affected by the vertical, oculogenital transmission of Neisseria gonorrhoeae and Chlamydia trachomatis resulting in acute bacterial conjunctivitis 333. Neisseria gonorrhoeae and Chlamydia trachomatis organisms can also cause a hyperacute infection in sexually active adolescents and adults 333.
The most common causative organism of bacterial keratoconjunctivitis in children is Haemophilus influenzae, followed by Streptococcus pneumoniae and Moraxella catarrhalis 334, 335, 336.
Bacterial keratoconjunctivitis can be quite contagious. The most common ways to get the contagious bacterial keratoconjunctivitis include:
- Direct contact with an infected person’s bodily fluids, usually through hand-to-eye contact.
- Contact with contaminated fingers, fomites or oculo-genital contact with someone infected. Young, sexually active adults below the age of 25 years, have a high risk, especially if they do not use condoms on sexual encounters.
- Spread of the infection from bacteria living in the person’s own nose and sinuses.
- Not cleaning contact lenses properly. Using poorly fitting contact lenses or decorative contacts are risks as well.
- Compromised tear production or drainage
- Disruption of the natural epithelial barrier
- Abnormality of adnexal structures
- Trauma
- Immunosuppressed status.
Children are the people most likely to get pink eye from bacteria or viruses. This is because they are in close contact with so many others in school or day care centers. Also, they don’t practice good hygiene.
In children, bacterial keratoconjunctivitis is often caused by Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis.
The most common pathogens for bacterial keratoconjunctivitis in adults are Staphylococcal species, followed by Streptococcus pneumoniae and Haemophilus influenzae.
The course of bacterial keratoconjunctivitis usually lasts 7-10 days.
- Hyperacute bacterial keratoconjunctivitis is often caused by Neisseria gonorrhoeae. When the infection does not respond to standard antibiotic therapy in sexually active patients, Chlamydia trachomatis should be suspected.
- Chronic bacterial keratoconjunctivitis is commonly caused Staphylococcus aureus, Moraxella lacunata, and enteric bacteria.
Bacterial keratoconjunctivitis signs and symptoms
Bacterial keratoconjunctivitis symptoms may include:
- the feeling that something is in your eye, or a gritty sensation in your eye
- red eyes
- burning eyes
- itchy eyes
- painful eyes (this is usually with the bacterial form)
- watery eyes
- puffy eyelids
- blurry or hazy vision
- sensitivity to light (photophobia)
- lots of mucus, pus, or thick yellow discharge from your eye. There can be so much that your eyelashes stick together.
Bacterial keratoconjunctivitis complications
Complications from bacterial keratoconjunctivitis are uncommon; however, severe infections can result in keratitis, corneal ulceration and perforation, and blindness 12, 327, 335.
Bacterial keratoconjunctivitis diagnosis
In most cases, your doctor can diagnose bacterial keratoconjunctivitis by asking about your recent health history and symptoms and examining your eyes.
Classic physical examination findings of bacterial keratoconjunctivitis are conjunctival erythema and purulent discharge 333. A complete eye examination should include assessment of visual acuity and corneal involvement 12, 337. Although slit lamps are beneficial to a comprehensive eye assessment, they are not routinely available in primary care offices 12. An otoscopic examination is warranted if ear symptoms are reported in children to diagnose concurrent acute otitis media 335.
Rarely, your doctor may take a swab to test for sample of the liquid that drains from your eye for laboratory analysis, called a culture 324. A culture may be needed if your symptoms are severe or if your doctor suspects a high-risk cause, such as:
- A foreign body in your eye.
- A serious bacterial infection.
- A sexually transmitted infection (STI).
Conjunctival cultures are also the recommended in cases where newborn conjunctivitis (ophthalmia neonatorum) is suspected, or where copious purulent discharge makes the diagnosis of gonococcal or chlamydial infection more likely 333, 12.
Your doctor can use the results to guide your treatment.
In the following days or weeks, your doctor may recommend that you have a follow-up visit with an eye care specialist to check on how your eye is healing and adjust your treatment if necessary.
Bacterial keratoconjunctivitis differential diagnosis
The differential diagnosis for bacterial keratoconjunctivitis includes viral and allergic keratoconjunctivitis 327. Clear discharge and itching are more characteristic of allergies and viral infections 12, 327. Trauma can also present with similar symptoms to keratoconjunctivitis of bacterial origin. Keratitis and iridocyclitis should be ruled out as corneal infections, and iris inflammation can lead to significant morbidity 327.
Bacterial keratoconjunctivitis treatment
Bacterial keratoconjunctivitis can resolve without treatment; however, your doctor may prescribe antibiotic eye drops, depending on how severe your symptoms are and with the consideration of benefits of treatment, the natural course of the disease if left untreated, antibiotic resistance, and the philosophy of antibiotic stewardship 324. And distinguishing bacterial keratoconjunctivitis from other causes of keratoconjunctivitis can be difficult, and doctors often err on the side of empiric antibiotic therapy 337. And antibiotic eye drops or ointments may speed up your recovery.
Studies have indicated that around 50 percent of pediatric infectious keratoconjunctivitis presentations are attributed to bacteria, while physicians prescribe antibiotics in up to 80 to 95 percent of these cases 337, 338. Ophthalmologists use antibiotic therapy in a smaller percentage of cases than general practitioners 337, 338. Treatment with topical antibiotics has demonstrated a decrease in symptoms, improved resolution times, decreased transmission, and hastened return to school or work 12, 335, 336, 337.
Keratoconjunctivitis usually goes away on its own within 1 to 2 weeks. If your symptoms last longer than that, you should see your ophthalmologist. He or she can make sure you don’t have a more serious eye problem.
The natural course of untreated bacterial keratoconjunctivitis is the resolution of the infection within one week 327. Another consideration is the continued resistance patterns of the eye bacterial pathogens 335. With an understanding of the described management variables, uncomplicated bacterial keratoconjunctivitis can be treated empirically with topical antibiotics, or managed expectantly without antibiotics 12. In complicated cases involving patients with immunocompromise, contact lens use, and suspected gonococcal or chlamydial infections, antibiotic therapy should be provided 12. If the decision is made to initiate empiric treatment, the antibiotic chosen should be broad-spectrum and include coverage of gram-positive and gram-negative ocular bacteria 335. Topical aminoglycosides, polymyxin B combination drugs, macrolides, and fluoroquinolones are the most commonly prescribed ophthalmic agents 339, 335, 12. The duration of treatment is generally five to seven days 335.
Recently, data has shown recorded emerging resistance to most classes of these drugs 335. Topical erythromycin has been a therapeutic choice for years; however, microbial resistance and unsatisfactory coverage for Haemophilus influenzae have limited its usefulness. Topical polymyxin B/trimethoprim and several fluoroquinolones effectively manage most cases of acute bacterial conjunctivitis 333, 335. Newer fluoroquinolones have the least documented resistance; however, they are costly 335. They should be considerations in areas of increased local antibiotic resistance 333, 339. Bacterial conjunctivitis secondary to gonococcal or chlamydial infections requires systemic treatment 12. Oral antibiotics are also indicated in cases of bacterial conjunctivitis with concurrent acute otitis media 335. Ophthalmia neonatorum secondary to Chlamydia trachomatis requires oral or intravenous erythromycin in addition to topical erythromycin for 14 days 333, 340, 341.When gonorrhea is the cause of the newborn infection, hospital admission, a single dose of intravenous or intramuscular ceftriaxone, and eye irrigation are the indicated therapy until resolution of the infection 12.
Follow up for acute bacterial keratoconjunctivitis should be encouraged if there is no improvement in symptoms after one to two days 327.
Gonococcal conjunctivitis treatment
In the only published study of the treatment of gonococcal conjunctivitis in adults, all 12 study participants responded to a single 1 gram intramuscular injection of ceftriaxone 342. Based on this study, the recommended treatment for gonococcal conjunctivitis is a single ceftriaxone 1 gram intramuscular dose 343, 344. In addition, a one-time lavage of the infected eye with saline should be considered 343, 344.
Chlamydial conjunctivitis treatment
The recommended treatment regimen for the baby with chlamydial conjunctivitis is a 14-day course of oral erythromycin base or erythromycin ethylsuccinate 50 mg/kg/day orally divided into 4 doses daily for 14 days 345. Treatment with oral erythromycin or oral azithromycin in infants during the first 6 weeks of life has been associated with an increased risk of infantile hypertrophic pyloric stenosis (IHPS) 346, 347, 348, 349. Therefore, infants treated with either of these antibiotics should be followed for infantile hypertrophic pyloric stenosis signs and symptoms 348, 345. The use of topical erythromycin alone is not effective for baby with chlamydial conjunctivitis and it is not recommended for use in combination with oral antibiotics 345.
Data on oral azithromycin for the treatment of neonatal chlamydial infection are limited, but a small study suggested a short 3-day course of azithromycin may be effective 350. The use of a recommended or alternative therapy has an efficacy of only approximately 80% and some infants require treatment with a second course of antibiotics.
Bacterial keratoconjunctivitis prognosis
The prognosis for uncomplicated bacterial keratoconjunctivitis is good with complete resolution and rare adverse events with both antibiotic treatment and expectant management strategies 12, 335.
Rosacea keratoconjunctivitis
According to its clinical presentation, rosacea has been classified by the National Rosacea Society Expert Committee into the 4 subtypes 361:
- Erythematotelangiectatic rosacea. Erythrotelangiectatic rosacea is characterized by facial redness, often with telangiectasias present.
- Papulopustular rosacea. Papulopustular rosacea presents with facial redness and a variable number of erythematous papules and pustules
- Phymatous rosacea. Phymatous rosacea involves the thickening of the skin and hyperplasia of sebaceous glands, often in the nasal region (rhinophyma).
- Ocular (eye) rosacea. Sometimes rosacea affects the eyes, eyelids and the front of the eye. This is called ocular rosacea. Ocular rosacea is characterized by inflammation of the eye surface tissues often presents with blepharitis, conjunctivitis, rosacea-associated keratitis and chalazion due to tear film instability, eye irritation, red eyes and eye dryness 362, 363.
Rosacea can also occur with other skin conditions.
Rosacea affects about 10% of the population with a greater risk in sun-sensitive fair skin individuals 364. Rosacea can also develop in Asian and African populations 365. According to the National Rosacea Society estimates, 16 million Americans suffer from acne rosacea, and 58%–72% of rosacea patients develop eye involvement or ocular rosacea. In a 1993 to 2010 US National Ambulatory Medical Care Survey analysis of the racial distribution of patients with rosacea, it was found that of all patients diagnosed with rosacea, 3.9% were Hispanic, 2.3% were Asian or Pacific Islander, and 2.0% were Black 366. It is important to note that the lower prevalence of rosacea in individuals with skin of color may be partly attributed to underdiagnosis due to the difficulty observing redness and other rosacea features in darker skin 367.
Ocular rosacea or eye rosacea affects adult males and females equally, and generally starts after the age of 30 years with one study reporting an average age at presentation of 56 years. Ocular rosacea or eye rosacea is uncommon in children.
Ocular rosacea or eye rosacea usually occurs in patients with existing rosacea but it can be the first sign of the disease. Ocular rosacea or eye rosacea tends to occur in patients with facial flushing.
People with eye rosacea frequently develop evaporative dry eye with burning and foreign body sensation. The hallmarks of ocular rosacea or eye rosacea include bilateral chronic blepharitis, meibomian gland dysfunction, and chronic scarring 354. There are subsequent tear film instability and debris, tearing, discomfort, photophobia, keratitis, and blurred vision. Some patients also develop recurrent chalazia secondary to meibomian gland dysfunction 368. People with eye rosacea may complain of burning and foreign body sensation 369. Eye rosacea in the most severe cases if left untreated can lead to peripheral corneal ulcers, corneal edemas, corneal perforations, corneal scarring and corneal neovascularization and lead to visual loss 360, 370, 371.
Figure 28. Rosacea keratoconjunctivitis
Figure 29. Skin and eye rosacea
Footnotes: (A) Patient with phymatous rosacea–associated rhinophyma, blepharophyma, nasal and facial erythema with telangiectasia. (B) Blepharophyma with thickened lid edges, lid margin telangiectasia, meibomian gland dysfunction. (C) Corneal neovascularization of eye rosacea growing from the superior limbus with a crescent pattern forming a vascular pannus. (D) Catarrhal corneal infiltrate caused by rosacea. (E) Typical peripheral ulcerative keratitis of rosacea, corresponding to sterile corneal melting of a crescentic area with newly formed stromal vessels. (F) Advanced stage of ocular rosacea with white corneal infiltrates and whole corneal neovascularization, including the visual axis.
[Source 353 ]Rosacea keratoconjunctivitis causes
The exact cause of ocular rosacea is unknown 351, 372. However, immunological factors, microorganisms on the skin surface, genetics, environmental factors, and neurovascular dysregulation and reactive blood vessels are involved 373, (Paiva-Santos AC, Gonçalves T, Peixoto D, Pires PC, Velsankar K, Jha NK, Chavda VP, Mohammad IS, Cefali LC, Mazzola PG, Mascarenhas-Melo F, Veiga F. Rosacea Topical Treatment and Care: From Traditional to New Drug Delivery Systems. Mol Pharm. 2023 Aug 7;20(8):3804-3828. doi: 10.1021/acs.molpharmaceut.3c00324)), 372.
- Demodex mites, normal inhabitants of eyelash follicles, may stimulate inflammation in ocular rosacea and anterior blepharitis. Though it is not clear if Demodex mites is a cause or consequence of rosacea 374.
- Bacteria may play a role, as ocular rosacea improves with antibiotics. One theory is that bacterial lipases release toxic free fatty acids and glycerides from lipids secreted by meibominan glands.
- The pro-inflammatory cytokine interleukin 1-alpha (IL-1α) increases matrix metalloproteinase-9 (MMP-9) in the tear fluid. Upregulation of MMP-9 damages ocular tissues.
Ultraviolet (UV) exposure is a trigger for rosacea 375. A genetic predisposition is supported by a higher incidence of disease in patients with a family history of rosacea. Furthermore, specific human leucocyte antigen (HLA) loci have been identified in patients with rosacea 376.
Obstruction of meibomian glands changes tear film composition leading to:
- Reduced tear film lipid layer
- Tear film instability
- Tear hyperosmolarity.
Studies show that certain foods and activities can cause rosacea to flare up. Rosacea triggers you might try to avoid include:
- Being outside in the heat, sun, wind, or cold
- Doing very active sports, such as running
- Drinking alcohol
- Eating spicy foods
- Drinking hot coffee or tea
- Feeling stressed or upset.
Rosacea keratoconjunctivitis signs and symptoms
Ocular rosacea mostly affect the eyelids, conjunctiva and cornea. Rarely, it can involve the iris and sclera.
Rosacea keratoconjunctivitis signs and symptoms can include:
- Red, burning or watering eyes
- Dry eyes
- Itching in your eyes
- Feeling like something is stuck in your eye or foreign body sensation.
- Eye sensitive to light (photophobia)
- Redness and swelling on your eyelids and at the base of eyelashes
- Clogging of the oily glands of your eyelids
- Corneal ulcers
- Chalazion or stye.
Eyelid and conjunctiva
- Anterior blepharitis involves the lid margin and lash line. Signs include 356:
- Swelling and thickening of lid margin
- Redness and dilated blood vessels
- Scaly debris at the base of the eyelashes
- Loss of eyelashes (madarosis) or misdirected eyelashes.
- Posterior blepharitis involves obstruction of the ducts and loss of the Meibomian glands 377. This leads to 356:
- Conjunctival hyperaemia (dilated conjunctival vessels)
- Cloudy secretions
- Papillary and follicular reactions in the tarsal plate
- Conjunctival scarring
- Chalazions (granulomatous inflammatory lesions around meibomian glands)
- Hordeolum externum (stye is an eyelash infection with Staphyloccocus aureus)
Cornea
- An inflamed cornea (keratitis) is a rare but serious ocular complication of rosacea and can threaten vision 356, 373.
- Keratitis may affect one or both eyes.
- The thinned cornea becomes inflamed with superficial punctate erosions, increased blood vessels and opacities.
- Keratitis due to ocular rosacea tends to begin at one edge or the bottom of the eye and then spread to affect lower half to two-thirds of the cornea.
- Recurrent attacks lead to corneal thinning, increased corneal opacity and vessel invasion.
- Severe ocular disease rarely can result in descemetocoele (deep ulcer) and corneal perforation.
- Corneal disease can include pannus formation (a layer of vascular fibrous tissue) and phlyctenules (allergic corneal nodules).
Iris and sclera
- Iritis: inflamed iris
- Episcleritis: inflammation of the layer between conjunctiva and cornea
- Scleritis: inflammation of the white of the eye
Iritis, episcleritis and scleritis are rare in ocular rosacea. They cause a painful, watery red eye and may affect vision.
Some people have rosacea affecting their skin but no symptoms of eye rosacea. Others have eye rosacea but no skin symptoms. You can also have both forms of rosacea. Women are more likely than men to have rosacea.
Rosacea keratoconjunctivitis diagnosis
The diagnosis of rosacea has been established clinically based on observation and interpretation of skin and eye signs. Eye rosacea may be suspected in a patient with skin rosacea that has eyelid or eye disease. While many patients show both ocular and skin signs, some may only show eye signs, which can make diagnosis more difficult in the absence of skin rosacea 378, 379, 380.
Blepharitis can also be due to seborrheic dermatitis, a scaly skin condition, and inflammatory papules on and around the eyelids may be due to periorificial dermatitis.
Recently, the National Rosacea Society Expert Committee proposed both major and minor features that would classify rosacea 381. Diagnostic features are a persistent centrofacial erythema associated with periodic intensification by potential trigger factors and phymatous changes. Major features: flushing/transient centrofacial erythema, inflammatory papules and pustules, telangiectasia, and ocular manifestations (lid margin telangiectasia, blepharitis, and keratitis/conjunctivitis/sclerokeratitis). Minor features: burning sensation of the skin, stinging sensation of the skin, edema, and dry sensation of the skin 382. Ocular manifestations may appear in the absence of other diagnostic phenotypes 381.
Rosacea keratoconjunctivitis differential diagnosis
Rosacea keratoconjunctivitis differential diagnosis include:
- Systemic lupus erythematosus (SLE)
- Herpes simplex keratitis
- Seborrheic or contact dermatitis
- Cellulitis
- Morbihan Syndrome (often associated with Rosacea)
- Atopic dermatitis
- Blepharoconjunctivitis associated with other causes
Rosacea keratoconjunctivitis treatment
While there is currently no cure for rosacea, several treatment options are available focusing on symptom management 352. Rosacea keratoconjunctivitis treatment includes lifestyle modification (avoidance of triggers), warm compresses, eyelid scrubs, and digital eye massage 382, 369, 383. Medical management varies depending on severity. To relieve the dry eye associated with eye rosacea, artificial tears and lubricating agents are used 382. Systemic, subantimicrobial tetracyclines (doxycycline, minocycline) and macrolides (azithromycin) are thought to decrease inflammation associated with rosacea and are often first-line agents for patients with moderate disease 382, 384, 385. While antibiotics may present a great treatment option for patients with ocular rosacea, side effects should be considered (vaginal/oral candidiasis, gastrointestinal distress, photosensitivity, teratogenicity, Stevens-Johnson syndrome, and allergies). At the lower doses used for eye rosacea management, it is postulated that oral antibiotics have a favorable impact on matrix metalloproteinases (MPP), interleukins, nitric oxide, activated B-lymphocytes, and collagen abnormalities that have been associated with rosacea 382. Topical metronidazole has a grade A evidence rating for the treatment of mild to moderate inflammatory rosacea, with a small study showing an improvement in rosacea-related eyelid health 382, 369.
Immunosuppressive agents, such as cyclosporine, have also been used to reduce chronic eyelid and corneal inflammation 382, 369. Although the medical literature reports mixed results, interventional office treatments include Lipiflow/Intense Pulse Light therapy, meibomian gland expression, and punctal occlusion may be considered 382, 386, 387. Intense Pulsed Light therapy in particular was demonstrated to increase the outflow of the meibomian glands thereby reducing bacterial counts seen in meibum stasis. It also causes the downregulation of pro-inflammatory mediators and stimulate alteration in the microcirculation thereby decreasing periocular inflammation and erythema 388. Treatment of chalazia may require excision. Severe ophthalmic disease may require ocular surface reconstruction (amniotic membrane placement, conjunctival flap, tissue adhesives), and penetrating keratoplasty for corneal perforation 382
Future interventions may include novel therapies for various cytokines, chemokines, and angiogenesis factors. Chemodenervation (i.e. Botox) has recently been trialed for rosacea management, hypothesized to inhibit vasodilatory mechanisms 389.
Conservative management
- Lid hygiene: dilute baby shampoo, dilute bicarbonate solution or proprietary preparation can be applied with a cotton bud to clean eyelid margins and remove scale along the lash line.
- Warm compresses to closed eyelids for five to ten minutes daily can improve flow of meibomian gland secretions, chalazion and hordeolum.
- Artificial tears (eye lubricants) reduce symptoms due to dry eye.
- Avoid wearing contact lenses if they irritate the inflamed eye.
Topical antibiotics
Anterior blepharitis can be successfully treated with various topical antiseptics and antibiotics including:
- Erythromycin
- Metronidazole
- Azithromycin
- Bacitracin
- Polymyxin B
- Fusidic acid
Topical anti-inflammatory agents
- Topical nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen may be helpful.
- Topical steroids are used short-term to treat marked lid inflammation or rosacea keratitis. Long-term use of topical steroids should be avoided, as they can lead to glaucoma and cataracts.
- Topical ciclosporin is typically used in ocular rosacea that has not responded to topical steroids.
Topical adrenergic receptor agonists
Oxymetazoline and brimonidine are alpha-1 and alpha-2 adrenergic receptor agonists, respectively. When applied topically, both compounds relieve the facial erythema observed in rosacea by exerting a local vasoconstriction effect. This is achieved through binding to the adrenergic receptors of vascular smooth muscle, stimulating contraction 390. Unlike the other treatments discussed, oxymetazoline and brimonidine only provide temporary symptoms relief, effective for up to 12 hours.
Topical adrenergic receptor antagonists
Carvedilol and propranolol are nonselective beta-adrenergic receptor antagonists. Unlike alpha adrenergic receptors, beta receptor activation promotes vasodilation. By inhibiting beta-adrenergic receptors on vascular smooth muscle, vasoconstriction is achieved. Studies have shown propranolol to be effective in relieving facial flushing, while carvedilol has demonstrated effectiveness in reducing redness 391, 392. Carvedilol is also able to reduce ROS production and scavenge free ROS, which may further contribute to its effectiveness in treating rosacea 393.
Ivermectin
Ivermectin is a semisynthetic antiparasitic drug derived from avermectin that has demonstrated effectiveness in treating papules and pustules 394. The effectiveness of ivermectin in treating rosacea is believed to involve both its antiparasitic and anti-inflammatory properties. Ivermectin eliminates parasites including Demodex folliculorum (a microscopic mite that lives in human hair follicles, usually on the face, and eats dead skin cells) by binding to gamma-aminobutyric acid (GABA) receptors in motor synapses, causing paralysis 395. In a group of 20 patients treated with topical ivermectin 1.0% once-daily, the density of Demodex folliculorum (a microscopic mite that lives in human hair follicles, usually on the face, and eats dead skin cells) mites were reduced by week 6 396. In an experimental study on human epidermal keratinocytes, ivermectin was found to reduce expression of CAMP and KLK5 397. Ivermectin has also been shown to decrease production of IL-1β and TNF-α 398.
Oral antibiotics
The following oral antibiotics are used for ocular rosacea:
- Tetracyclines such as doxycycline. Of the tetracycline antibiotics, the first-generation tetracycline and the second-generation doxycycline are commonly prescribed for treating rosacea. These tetracyclines are typically prescribed at a sub-antimicrobial dose, relying on its anti-inflammatory and antioxidative properties to alleviate rosacea symptoms. Subantimicrobial-dose doxycycline, modified-release 40 to 100 mg daily. In one experimental study, doxycycline was shown to directly inhibit MMP activity and expression, suppressing KLK5 activation and subsequent LL-37 production 399. Doxycycline also reduces levels of IL-1β, IL-8, and TNF-α 400. In addition, doxycycline has demonstrated effectiveness in scavenging neutrophil derived reactive oxygen species (ROS) 401.
- Macrolides such as erythromycin. Macrolides have been shown to reduce production of IL-1β, IL-8, and TNF-α by neutrophils and reduce reactive oxygen species (ROS) levels in rosacea patient skin 402.
They reduce bacteria, improve tear film stability and normalise meibonian gland secretions.
Oral antibiotics are generally continued for 6–12 weeks, and then slowly tapered over the course of one to two months. Further courses of oral antibiotics can be used for disease flare-ups.
Styes failing to clear with topical antibiotic are treated with oral anti-staphylococcal antibiotics such as flucloxacillin.
Oral retinoids
Retinoids are a class of compounds derived from vitamin A commonly used for regulating skin turnover. Oral isoretinoin can be used in low dose to treat ocular rosacea but with caution because its adverse effects include increased infections, dry eye, and other ocular effects. Topical retinoids, such as the third-generation adapalene, have demonstrated effectiveness in treating papules and pustules 403. The first-generation isotretinoin when taken orally has shown efficacy in treating severe recalcitrant rosacea 404.
Omega-3 fatty acid supplementation
Oral omega-3 fatty acid supplementation has been reported to be beneficial for some patients with dry eyes.
Surgery
Surgery may be required to repair corneal opacification or perforation due to rosacea keratitis. The procedure is called keratoplasty.
Hordeola that fail to improve with warm compresses and antibiotic therapy may be excised.
Rosacea keratoconjunctivitis prognosis
Rosacea is not a life-threatening disease, and the overall prognosis of rosacea is good 372. However, rosacea can lead to depression and anxiety. If left untreated, patients can develop permanent scarring and persistent red skin. In addition, eye complications could be a complication of untreated eye rosacea. Recent studies elaborated on the possible correlation of rosacea with neurologic, cardiovascular, endocrine, and gastrointestinal comorbidities. Consideration of these comorbidities in patients with rosacea may be warranted, though currently no evidence-based recommendations for screening have been established 405, 406.
- Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86(1):5–17. doi: 10.1111/j.1600-0420.2007.01006.x[↩][↩][↩]
- Hørven I. Acute conjunctivitis: a comparison of fusidic acid viscous eye drops and chloramphenicol. Acta Ophthalmol (Copenh) 1993;71(2):165–168. doi: 10.1111/j.1755-3768.1993.tb04983.x[↩]
- Stenson S, Newman R, Fedukowicz H. Laboratory studies in acute conjunctivitis. Arch Ophthalmol. 1982;100(8):1275–1277. doi: 10.1001/archopht.1982.01030040253009[↩]
- Rönnerstam R, Persson K, Hansson H, Renmarker K. Prevalence of chlamydial eye infection in patients attending an eye clinic, a VD clinic, and in healthy persons. Br J Ophthalmol. 1985;69(5):385–388. doi: 10.1136/bjo.69.5.385[↩]
- Harding SP, Mallinson H, Smith JL, Clearkin LG. Adult follicular conjunctivitis and neonatal ophthalmia in a Liverpool eye hospital, 1980-1984. Eye (Lond) 1987;1(pt 4):512–521. doi: 10.1038/eye.1987.77[↩]
- Uchio E, Takeuchi S, Itoh N, et al. Clinical and epidemiological features of acute follicular conjunctivitis with special reference to that caused by herpes simplex virus type 1. Br J Ophthalmol. 2000;84(9):968–972. doi: 10.1136/bjo.84.9.968[↩]
- Woodland RM, Darougar S, Thaker U, et al. Causes of conjunctivitis and keratoconjunctivitis in Karachi, Pakistan. Trans R Soc Trop Med Hyg. 1992;86(3):317–320. doi: 10.1016/0035-9203(92)90328-a[↩]
- Fitch CP, Rapoza PA, Owens S, et al. Epidemiology and diagnosis of acute conjunctivitis at an inner-city hospital. Ophthalmology. 1989;96(8):1215–1220. doi: 10.1016/s0161-6420(89)32749-7[↩]
- Bielory BP, O’Brien TP, Bielory L. Management of seasonal allergic conjunctivitis: guide to therapy. Acta Ophthalmol. 2012;90(5):399–407. doi: 10.1111/j.1755-3768.2011.02272.x[↩]
- American Academy of Ophthalmology. Cornea/External Disease Panel . Preferred Practice Pattern Guidelines: Conjunctivitis-Limited Revision. American Academy of Ophthalmology; San Francisco, CA: 2011.[↩][↩]
- Mannis MJ, Plotnik RD. Bacterial conjunctivitis. In: Tasman W, Jaeger EA, editors. Duanes Ophthalmology on CD-ROM. Lippincott Williams & Wilkins; 2006.[↩]
- Azari AA, Barney NP. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013 Oct 23;310(16):1721-9. doi: 10.1001/jama.2013.280318. Erratum in: JAMA. 2014 Jan 1;311(1):95. Dosage error in article text.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Leibowitz HM. The red eye. N Engl J Med. 2000;343(5):345–351. doi: 10.1056/NEJM200008033430507[↩]
- Cronau H, Kankanala RR, Mauger T. Diagnosis and management of red eye in primary care. Am Fam Physician. 2010 Jan 15;81(2):137-44. https://www.aafp.org/pubs/afp/issues/2010/0115/p137.html[↩]
- Narayana S, McGee S. Bedside Diagnosis of the ‘Red Eye’: A Systematic Review. Am J Med. 2015 Nov;128(11):1220-1224.e1. doi: 10.1016/j.amjmed.2015.06.026[↩][↩]
- Dry Eye Syndrome. https://eyewiki.org/Dry_Eye_Syndrome[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Dry eye syndrome. https://medlineplus.gov/ency/article/000426.htm[↩]
- Alshamrani AA, Almousa AS, Almulhim AA, Alafaleq AA, Alosaimi MB, Alqahtani AM, Almulhem AM, Alshamrani MA, Alhallafi AH, Alqahtani IZ, Alshehri AA. Prevalence and Risk Factors of Dry Eye Symptoms in a Saudi Arabian Population. Middle East Afr J Ophthalmol. 2017 Apr-Jun;24(2):67-73. doi: 10.4103/meajo.MEAJO_281_16[↩]
- Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan: The Shihpai Eye Study. Ophthalmology. 2003;110:1096–101. doi: 10.1016/S0161-6420(03)00262-8[↩]
- Lee AJ, Lee J, Saw SM, Gazzard G, Koh D, Widjaja D, et al. Prevalence and risk factors associated with dry eye symptoms: A population based study in Indonesia. Br J Ophthalmol. 2002;86:1347–51. doi: 10.1136/bjo.86.12.1347[↩]
- McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998;105:1114–9. doi: 10.1016/S0161-6420(98)96016-X[↩]
- What Is Sjögren’s Syndrome? https://www.aao.org/eye-health/diseases/what-is-sjogren-syndrome[↩]
- Jeganathan VS, Wirth A, MacManus MP. Ocular risks from orbital and periorbital radiation therapy: a critical review. Int J Radiat Oncol Biol Phys. 2011 Mar 1;79(3):650-9. doi: 10.1016/j.ijrobp.2010.09.056[↩]
- Simpson RG, Moshirfar M, Edmonds JN, Christiansen SM, Behunin N. Laser in situ keratomileusis in patients with collagen vascular disease: a review of the literature. Clin Ophthalmol. 2012;6:1827-37. doi: 10.2147/OPTH.S36690[↩]
- Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011 Jan;36(1):1-7. doi: 10.3109/02713683.2010.519850[↩]
- Ayaki M, Kawashima M, Negishi K, Tsubota K. High prevalence of sleep and mood disorders in dry eye patients: survey of 1,000 eye clinic visitors. Neuropsychiatr Dis Treat. 2015 Mar 31;11:889-94. doi: 10.2147/NDT.S81515[↩]
- Aldaas KM, Ismail OM, Hakim J, Van Buren ED, Lin FC, Hardin JS, Meyer JJ. Association of Dry Eye Disease With Dyslipidemia and Statin Use. Am J Ophthalmol. 2020 Oct;218:54-58. doi: 10.1016/j.ajo.2020.05.007[↩]
- Ismail OM, Poole ZB, Bierly SL, Van Buren ED, Lin FC, Meyer JJ, Davis RM. Association Between Dry Eye Disease and Migraine Headaches in a Large Population-Based Study. JAMA Ophthalmol. 2019 May 1;137(5):532-536. doi: 10.1001/jamaophthalmol.2019.0170[↩]
- The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):93-107. doi: 10.1016/s1542-0124(12)70082-4[↩]
- Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004 Nov;23(8):762-70. doi: 10.1097/01.ico.0000133997.07144.9e[↩]
- Brott NR, Zeppieri M, Ronquillo Y. Schirmer Test. [Updated 2024 Feb 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559159[↩][↩][↩][↩]
- Schirmer test. https://medlineplus.gov/ency/article/003501.htm[↩]
- Negrel AD, Lemasson JM. Papier filtre Whatman-41 et prélèvement des sécrétions du cul-de-sac conjonctival [Whatman-41 filter paper and sample of the secretions of the conjunctival cul-de-sac]. Rev Int Trach Pathol Ocul Trop Subtrop. 1979;56(3-4):69-72. French.[↩]
- Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S, Pult H, Sullivan BD, Tomlinson A, Tong L, Villani E, Yoon KC, Jones L, Craig JP. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017 Jul;15(3):539-574. doi: 10.1016/j.jtos.2017.05.001[↩][↩]
- Lee JH, Hyun PM. The reproducibility of the Schirmer test. Korean J Ophthalmol. 1988 Jun;2(1):5-8. doi: 10.3341/kjo.1988.2.1.5[↩]
- Krachmer. (2013). Cornea, 3rd Edition. Elsevier.[↩]
- Versura P, Profazio V, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010 Jul;35(7):553-64. doi: 10.3109/02713683.2010.484557[↩][↩]
- Lemp MA, Bron AJ, Baudouin C, Benítez Del Castillo JM, Geffen D, Tauber J, Foulks GN, Pepose JS, Sullivan BD. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011 May;151(5):792-798.e1. doi: 10.1016/j.ajo.2010.10.032[↩]
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):75-92. doi: 10.1016/s1542-0124(12)70081-2[↩]
- Baudouin C. The pathology of dry eye. Surv Ophthalmol. 2001 Mar;45 Suppl 2:S211-20. doi: 10.1016/s0039-6257(00)00200-9[↩]
- TearLab® Osmolarity System. https://www.labtician.com/therapeutics/product/tearlab/[↩]
- Schargus M, Ivanova S, Kakkassery V, Dick HB, Joachim S. Correlation of Tear Film Osmolarity and 2 Different MMP-9 Tests With Common Dry Eye Tests in a Cohort of Non-Dry Eye Patients. Cornea. 2015 Jul;34(7):739-44. doi: 10.1097/ICO.0000000000000449[↩][↩][↩]
- Sullivan BD, Crews LA, Sönmez B, de la Paz MF, Comert E, Charoenrook V, de Araujo AL, Pepose JS, Berg MS, Kosheleff VP, Lemp MA. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012 Sep;31(9):1000-8. doi: 10.1097/ICO.0b013e318242fd60[↩]
- Szalai E, Berta A, Szekanecz Z, Szûcs G, Módis L Jr. Evaluation of tear osmolarity in non-Sjögren and Sjögren syndrome dry eye patients with the TearLab system. Cornea. 2012 Aug;31(8):867-71. doi: 10.1097/ICO.0b013e3182532047[↩]
- Baudouin C, Aragona P, Messmer EM, Tomlinson A, Calonge M, Boboridis KG, Akova YA, Geerling G, Labetoulle M, Rolando M. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf. 2013 Oct;11(4):246-58. doi: 10.1016/j.jtos.2013.07.003[↩]
- Sambursky R, O’Brien TP. MMP-9 and the perioperative management of LASIK surgery. Curr Opin Ophthalmol. 2011 Jul;22(4):294-303. doi: 10.1097/ICU.0b013e32834787bb[↩]
- Sambursky R, Davitt WF 3rd, Latkany R, Tauber S, Starr C, Friedberg M, Dirks MS, McDonald M. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013 Jan;131(1):24-8. doi: 10.1001/jamaophthalmol.2013.561. Erratum in: JAMA Ophthalmol. 2013 Mar 1;131(3):364.[↩]
- Sambursky R, Davitt WF 3rd, Friedberg M, Tauber S. Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease. Cornea. 2014 Aug;33(8):812-8. doi: 10.1097/ICO.0000000000000175[↩]
- Chotikavanich S, de Paiva CS, Li de Q, Chen JJ, Bian F, Farley WJ, Pflugfelder SC. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009 Jul;50(7):3203-9. doi: 10.1167/iovs.08-2476[↩]
- De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004 Jan;137(1):109-15. doi: 10.1016/s0002-9394(03)00897-3[↩]
- Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):108-52. doi: 10.1016/s1542-0124(12)70083-6[↩][↩][↩]
- Carsons SE, Patel BC. Sjogren Syndrome. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431049[↩][↩][↩]
- De Vita S, Gandolfo S, Zandonella Callegher S, Zabotti A, Quartuccio L. The evaluation of disease activity in Sjögren’s syndrome based on the degree of MALT involvement: glandular swelling and cryoglobulinaemia compared to ESSDAI in a cohort study. Clin Exp Rheumatol. 2018 May-Jun;36 Suppl 112(3):150-156.[↩]
- Martel A, Coiffier G, Bleuzen A, Goasguen J, de Bandt M, Deligny C, Magnant J, Ferreira N, Diot E, Perdriger A, Maillot F. What is the best salivary gland ultrasonography scoring methods for the diagnosis of primary or secondary Sjögren’s syndromes? Joint Bone Spine. 2019 Mar;86(2):211-217. doi: 10.1016/j.jbspin.2018.06.014[↩]
- Baer AN, Walitt B. Update on Sjögren Syndrome and Other Causes of Sicca in Older Adults. Rheum Dis Clin North Am. 2018 Aug;44(3):419-436. doi: 10.1016/j.rdc.2018.03.002[↩]
- Shen L, Suresh L, Lindemann M, Xuan J, Kowal P, Malyavantham K, Ambrus JL Jr. Novel autoantibodies in Sjogren’s syndrome. Clin Immunol. 2012 Dec;145(3):251-5. doi: 10.1016/j.clim.2012.09.013[↩]
- van Bijsterveld OP. Diagnostic tests in the Sicca syndrome. Arch Ophthalmol. 1969 Jul;82(1):10-4. doi: 10.1001/archopht.1969.00990020012003[↩]
- Golden MI, Meyer JJ, Zeppieri M, et al. Dry Eye Syndrome. [Updated 2024 Feb 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470411[↩][↩][↩][↩]
- Morthen MK, Magno MS, Utheim TP, Snieder H, Hammond CJ, Vehof J. The physical and mental burden of dry eye disease: A large population-based study investigating the relationship with health-related quality of life and its determinants. Ocul Surf. 2021 Jul;21:107-117. doi: 10.1016/j.jtos.2021.05.006[↩]
- Dry Eye Assessment and Management Study Research Group; Asbell PA, Maguire MG, Pistilli M, Ying GS, Szczotka-Flynn LB, Hardten DR, Lin MC, Shtein RM. n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N Engl J Med. 2018 May 3;378(18):1681-1690. doi: 10.1056/NEJMoa1709691[↩]
- Mohamed HB, Abd El-Hamid BN, Fathalla D, Fouad EA. Current trends in pharmaceutical treatment of dry eye disease: A review. Eur J Pharm Sci. 2022 Aug 1;175:106206. doi: 10.1016/j.ejps.2022.106206[↩]
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, Viso E, Vitale S, Jones L. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017 Jul;15(3):334-365. doi: 10.1016/j.jtos.2017.05.003[↩]
- Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf. 2014 Jan;12(1):32-45. doi: 10.1016/j.jtos.2013.09.001[↩]
- Baab S, Le PH, Gurnani B, et al. Allergic Conjunctivitis. [Updated 2024 Jan 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448118[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Allergic conjunctivitis: Update on pathophysiology and prospects for future treatment. Ono, Santa Jeremy et al. Journal of Allergy and Clinical Immunology, Volume 115, Issue 1, 118 – 122. https://www.jacionline.org/article/S0091-6749(04)03032-5/fulltext[↩]
- La Rosa M, Lionetti E, Reibaldi M, Russo A, Longo A, Leonardi S, Tomarchio S, Avitabile T, Reibaldi A. Allergic conjunctivitis: a comprehensive review of the literature. Ital J Pediatr. 2013 Mar 14;39:18. doi: 10.1186/1824-7288-39-18[↩][↩][↩][↩][↩]
- Rathi, V.M., & Murthy, S.I. (2017). Allergic conjunctivitis. Community Eye Health, 30, S7 – S10. https://pdfs.semanticscholar.org/d820/987003b832dc90f868f531c9616a4444ac4c.pdf[↩][↩]
- Butrus S, Portela R. Ocular allergy: diagnosis and treatment. Ophthalmol Clin North Am. 2005 Dec;18(4):485-92, v. doi: 10.1016/j.ohc.2005.07.007[↩]
- Gomes PJ. Trends in prevalence and treatment of ocular allergy. Curr Opin Allergy Clin Immunol. 2014 Oct;14(5):451-6. doi: 10.1097/ACI.0000000000000100[↩]
- Bielory L, Frohman L. Allergic and immunologic disorders of the eye. J Allergy Clin Immunol. 1992;86:1–20. doi: 10.1016/s0091-6749(05)80033-8[↩]
- Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin Exp Immunol. 2008;153:17–21. doi: 10.1111/j.1365-2249.2008.03716.x[↩]
- Tariq F. Allergic Conjunctivitis: Review of Current Types, Treatments, and Trends. Life (Basel). 2024 May 21;14(6):650. doi: 10.3390/life14060650[↩][↩]
- Leonardi A, Castegnaro A, Valerio AL, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. 2015 Oct;15(5):482-8. doi: 10.1097/ACI.0000000000000204[↩]
- What is allergic conjunctivitis? https://aapos.org/glossary/allergic-conjunctivitis[↩]
- Carr W, Schaeffer J, Donnenfeld E. Treating Allergic Conjunctivitis: A Once-daily Medication that Provides 24-hour Symptom Relief. Allergy & Rhinology. 2016;7(2). doi:10.2500/ar.2016.7.0158[↩]
- Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988-1994. J Allergy Clin Immunol. 2010 Oct;126(4):778-783.e6. doi: 10.1016/j.jaci.2010.06.050[↩]
- Chigbu D.I. The pathophysiology of ocular allergy: A review. Cont Lens Anterior Eye 32: 3–15; quiz 43–44, 2009.[↩][↩]
- Bielory L., Meltzer E.O., Nichols K.K.et al. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 34: 408–420, 2013.[↩][↩][↩]
- Palmares J, Delgado L, Cidade M, Quadrado MJ, Filipe HP; Season Study Group. Allergic conjunctivitis: a national cross-sectional study of clinical characteristics and quality of life. Eur J Ophthalmol. 2010;20:257–264. doi: 10.1177/112067211002000201[↩]
- Villegas BV, Benitez-Del-Castillo JM. Current Knowledge in Allergic Conjunctivitis. Turk J Ophthalmol. 2021 Feb 25;51(1):45-54. doi: 10.4274/tjo.galenos.2020.11456[↩][↩][↩][↩][↩]
- Allergic Conjunctivitis. https://www.mrverity.com/patient-information/images/dry-and-irritable-eyes/allergic-conjunctivitis/[↩]
- Wise S.K., Lin S.Y., Toskala E., Orlandi R.R., Akdis C.A., Alt J.A., Azar A., Baroody F.M., Bachert C., Canonica G.W., et al. International consensus statement on allergy and rhinology: Allergic rhinitis—2023. Int. Forum. Allergy Rhinol. 2023;13:293–859. doi: 10.1002/alr.23090[↩]
- Rathi VM, Murthy SI. Allergic conjunctivitis. Community Eye Health. 2017;30(99):S7-S10. https://pmc.ncbi.nlm.nih.gov/articles/PMC5968423/[↩][↩][↩][↩]
- Saboo US, Jain M, Reddy JC, Sangwan VS. Demographic and clinical profile of vernal keratoconjunctivitis at a tertiary eye care center in India. Indian J Ophthalmol. 2013 Sep;61(9):486-9. doi: 10.4103/0301-4738.119431[↩][↩]
- Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin Immunol. 2020;16:5. doi: 10.1186/s13223-020-0403-9[↩][↩][↩][↩][↩][↩]
- Fauquert JL, Jedrzejczak-Czechowicz M, Rondon C, Calder V, Silva D, Kvenshagen BK, Callebaut I, Allegri P, Santos N, Doan S, Perez Formigo D, Chiambaretta F, Delgado L, Leonardi A Interest Group on Ocular Allergy (IGOA) from the European Academy of Allergy and Clinical Immunology. Conjunctival allergen provocation test : guidelines for daily practice. Allergy. 2016;72:43–54. doi: 10.1111/all.12986[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Pepper AN, Ledford DK. Nasal and ocular challenges. J Allergy Clin Immunol. 2018;141:1570–1577. doi: 10.1016/j.jaci.2017.11.066[↩][↩][↩]
- Leonardi A, Bogacka E, Fauquert JL, Kowalski ML, Groblewska A, Jedrzejczak-Czechowicz M, Doan S, Marmouz F, Demoly P, Delgado L. Ocular allergy: recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy. 2012;67:1327–1337. doi: 10.1111/all.12009[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Bilkhu PS, Wolffsohn JS, Naroo SA, Robertson L, Kennedy R. Effectiveness of nonpharmacologic treatments for acute seasonal allergic conjunctivitis. Ophthalmology. 2014;121:72–78. doi: 10.1016/j.ophtha.2013.08.007[↩]
- Bielory L. Ocular allergy overview. Immunol. Allergy Clin. N. Am. 2008;28:1–23. doi: 10.1016/j.iac.2007.12.011[↩][↩][↩][↩][↩][↩]
- Chan V.F., Yong A.C., Azuara-Blanco A., Gordon I., Safi S., Lingham G., Evans J., Keel S. A Systematic Review of Clinical Practice Guidelines for Infectious and Non-infectious Conjunctivitis. Ophthalmic Epidemiol. 2022;29:473–482. doi: 10.1080/09286586.2021.1971262[↩][↩]
- Palmares J., Delgado L., Cidade M., Quadrado M.J., Filipe H.P. Allergic conjunctivitis: A national cross-sectional study of clinical characteristics and quality of life. Eur. J. Ophthalmol. 2010;20:257–264. doi: 10.1177/112067211002000201[↩][↩]
- Leonardi A. Management of vernal keratoconjunctivitis. Ophthalmol Ther. (2013) 2:73–88. 10.1007/s40123-013-0019-y[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Yanni JM, Sharif NA, Gamache DA, Miller ST, Weimer LK, Spellman JM. A current appreciation of sites for pharmacological intervention in allergic conjunctivitis: effects of new topical ocular drugs. Acta Ophthalmol Scand Suppl. (1999) 228:33–7. 10.1111/j.1600-0420.1999.tb01171.x[↩][↩]
- Gane J, Buckley R. Leukotriene receptor antagonists in allergic eye disease: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2013 Jan;1(1):65-74. doi: 10.1016/j.jaip.2012.07.001[↩][↩][↩]
- Simons F.E., Simons K.J. Histamine and H1-antihistamines: Celebrating a century of progress. J. Allergy Clin. Immunol. 2011;128:1139–1150.e4. doi: 10.1016/j.jaci.2011.09.005[↩]
- Sanchez-Hernandez M.C., Campo P., Benitez del Castillo J.M., Merayo-Lloves J., Montero J., Valero A., Antón E., Rondon C., Matheu V., Fernández-Parra B., et al. Consensus document on allergic conjunctivitis (DECA) J. Investig. Allergol. Clin. Immunol. 2015;25:94–106.[↩][↩][↩][↩][↩][↩]
- Abelson M.B., Shetty S., Korchak M., Butrus S.I., Smith L.M. Advances in pharmacotherapy for allergic conjunctivitis. Expert. Opin. Pharmacother. 2015;16:1219–1231. doi: 10.1517/14656566.2015.1040760[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- La Rosa M., Lionetti E., Reibaldi M., Russo A., Longo A., Leonardi S., Tomarchio S., Avitabile T., Reibaldi A. Allergic conjunctivitis: A comprehensive review of the literature. Ital. J. Pediatr. 2013;39:18. doi: 10.1186/1824-7288-39-18[↩][↩]
- Bielory B.P., O’Brien T.P., and Bielory L. Management of seasonal allergic conjunctivitis: Guide to therapy. Acta Ophthalmol 90: 399–407, 2012.[↩][↩][↩]
- Abelson M.B., and Gomes P.J. Olopatadine 0.2% ophthalmic solution: The first ophthalmic antiallergy agent with once-daily dosing. Expert Opin Drug Metab Toxicol 4: 453–461, 2008.[↩][↩]
- Greiner JV, Edwards-Swanson K, Ingerman A. Evaluation of alcaftadine 0.25% ophthalmic solution in acute allergic conjunctivitis at 15 minutes and 16 hours after instillation versus placebo and olopatadine 0.1%. Clin Ophthalmol. 2011 Jan 13;5:87-93. doi: 10.2147/OPTH.S15379[↩][↩]
- Ackerman S, D’Ambrosio F Jr, Greiner JV, Villanueva L, Ciolino JB, Hollander DA. A multicenter evaluation of the efficacy and duration of action of alcaftadine 0.25% and olopatadine 0.2% in the conjunctival allergen challenge model. J Asthma Allergy. 2013 Apr 8;6:43-52. doi: 10.2147/JAA.S38671[↩][↩]
- Kari O., Saari K.M. Updates in the treatment of ocular allergies. J. Asthma Allergy. 2010;3:149–158. doi: 10.2147/JAA.S13705[↩]
- Berger W., Gomes P.J., Beck M., Kimura S., Westbrook T., Storms W., Galant S. Effects of adjuvant therapy with 0.1% olopatadine hydrochloride ophthalmic solution on quality of life in patients with allergic rhinitis using systemic or nasal therapy. Ann. Allergy Asthma Immunol. 2005;95:361–371. doi: 10.1016/S1081-1206(10)61155-6[↩]
- Kam K.W., Chen L.J., Wat N., Young A.L. Topical Olopatadine in the Treatment of Allergic Conjunctivitis: A Systematic Review and Meta-analysis. Ocul. Immunol. Inflamm. 2017;25:663–677. doi: 10.3109/09273948.2016.1158282[↩]
- Dordal M.T., Lluch-Bernal M., Sánchez M.C., Rondón C., Navarro A., Montoro J., Matheu V., Ibáñez M.D., Fernández-Parra B., Dávila I. Allergen-specific nasal provocation testing: Review by the rhinoconjunctivitis committee of the Spanish Society of Allergy and Clinical Immunology. J. Investig. Allergol. Clin. Immunol. 2011;21:1–12. quiz 12.[↩][↩][↩]
- Dupuis P., Prokopich C.L., Hynes A., Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin. Immunol. 2020;16:5. doi: 10.1186/s13223-020-0403-9[↩]
- Mishra G.P., Tamboli V., Jwala J.K., Mitra A. Recent patents and emerging therapeutics in the treatment of allergic conjunctivitis. Recent Pat. Inflamm. Allergy Drug Discov. 2011;5:26–36. doi: 10.2174/187221311794474883[↩][↩]
- Swamy B.N., Chilov M., McClellan K., Petsoglou C. Topical non-steroidal anti-inflammatory drugs in allergic conjunctivitis: Meta-analysis of randomized trial data. Ophthalmic Epidemiol. 2007;14:311–319. doi: 10.1080/09286580701299411[↩]
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000 Dec;11(6):478-83. doi: 10.1097/00055735-200012000-00016[↩]
- O’Brien TP. Allergic conjunctivitis: an update on diagnosis and management. Curr Opin Allergy Clin Immunol. 2013 Oct;13(5):543-9. doi: 10.1097/ACI.0b013e328364ec3a[↩]
- Singh S, Pal V, Dhull CS. Supratarsal injection of corticosteroids in the treatment of refractory vernal keratoconjunctivitis. Indian J Ophthalmol. 2001 Dec;49(4):241-5.[↩]
- Patel N, Venkateswaran N, Wang Z, Galor A. Ocular involvement in atopic disease: a review. Curr Opin Ophthalmol. 2018;29:576–581. doi: 10.1097/ICU.0000000000000532[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Hoy SM. Ciclosporin Ophthalmic Emulsion 0.1%: A Review in Severe Dry Eye Disease. Drugs. 2017;77:1909–1916. doi: 10.1007/s40265-017-0834-x[↩][↩][↩]
- Bielory L, Schoenberg D. Emerging therapeutics for ocular surface disease. Curr Allergy Asthma Rep. 2019;28:19–16. doi: 10.1007/s11882-019-0844-8[↩][↩][↩][↩][↩][↩][↩]
- Bielory L, Schoenberg D. Ocular allergy: update on clinical trials. Curr Opin Allergy Clin Immunol. 2010;19:495–502. doi: 10.1097/ACI.0000000000000564[↩][↩][↩]
- De Bosscher K, Haegeman G, Elewaut D. Targeting inflammation using selective glucocorticoid receptor modulators. Curr Opin Pharmacol. 2010;10:497–504. doi: 10.1016/j.coph.2010.04.007[↩]
- Bozkurt MK, Tülek B, Bozkurt B, Akyürek N, Öz M, Kiyici A. Comparison of the efficacy of prednisolone, montelukast, and omalizumab in an experimental allergic rhinitis model. Turk J Med Sci. 2014;44:439–447. doi: 10.3906/sag-1212-23[↩]
- van der Schaft J, Thijs JL, de Bruin-Weller MS, Balak DMW. Dupilumab after the 2017 approval for the treatment of atopic dermatitis: what’s new and what’s next? Curr Opin Allergy Clin Immunol. 2019;19:341–349. doi: 10.1097/ACI.0000000000000551[↩]
- Nahum Y, Mimouni M, Livny E, Bahar I, Hodak E, Leshem YA. Dupilumabinduced ocular surface disease (DIOSD) in patients with atopic dermatitis: clinical presentation, risk factors for development and outcomes of treatment with tacrolimus ointment. Br J Ophthalmol. 2020;104:776–779. doi: 10.1136/bjophthalmol-2019-315010[↩]
- Amparo F, Dastjerdi MH, Okanobo A, Ferrari G, Smaga L, Hamrah P, Jurkunas U, Schaumberg DA, Dana R. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA Ophthalmol. 2013;131:715–723. doi: 10.1001/jamaophthalmol.2013.195[↩][↩]
- Tauber J, Karpecki P, Latkany R, Luchs J, Martel J, Sall K, Raychaudhuri A, Smith V, Semba CP; OPUS-2 Investigators. Lifitegrast Ophthalmic Solution 5.0% versus Placebo for Treatment of Dry Eye Disease: Results of the Randomized Phase III OPUS-2 Study. Ophthalmology. 2015;122:2423–2431. doi: 10.1016/j.ophtha.2015.08.001[↩][↩]
- Moote W, Kim H, Ellis AK. Allergen-specific immunotherapy. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):53. doi: 10.1186/s13223-018-0282-5[↩]
- Calderon MA, Penagos M, Sheikh A, Canonica GW, Durham S. Sublingual immunotherapy for treating allergic conjunctivitis. Cochrane Database Syst Rev. 2011;7:CD007685. doi: 10.1002/14651858.CD007685.pub2[↩][↩][↩][↩]
- Roberts G, Pfaar O, Akdis CA, et al. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy. 2018;73:765–798. doi: 10.1111/all.13317[↩]
- Iyer G, Agarwal S, Srinivasan B. Outcomes and Rationale of Excision and Mucous Membrane Grafting in Palpebral Vernal Keratoconjunctivitis. Cornea. 2018;37:172–176. doi: 10.1097/ICO.0000000000001421[↩][↩][↩]
- Bielory BP, Shah SP, O’Brien TP, Perez VL, Bielory L. Emerging therapeutics for ocular surface disease. Curr Opin Allergy Clin Immunol. 2016;16:477–486. doi: 10.1097/ACI.0000000000000309[↩][↩][↩][↩]
- Berger WE, Granet DB, Kabat AG. Diagnosis and management of allergic conjunctivitis in pediatric patients. Allergy Asthma Proc. 2017;38:16–27. doi: 10.2500/aap.2017.38.4003[↩][↩]
- Addis H, Jeng BH. Vernal Keratoconjunctivitis. Clinical Ophthalmol. 2018;12:119–123. doi: 10.2147/OPTH.S129552[↩]
- Nishiwaki-Dantas MC, Dantas PE, Pezzutti S, Finzi S. Surgical resection of giant papillae and autologous conjunctival graft in patients with severe vernal keratoconjunctivitis and giant papillae. Ophthalmic Plast Reconstr Surg. 2000;16:438–442. doi: 10.1097/00002341-200011000-00007[↩][↩]
- Guo P, Kheirkhah A, Zhou WW, Qin L, Shen XL. Surgical resection and amniotic membrane transplantation for treatment of refractory giant papillae in vernal keratoconjunctivitis. Cornea. 2013;32:816–820. doi: 10.1097/ICO.0b013e31826a1e53[↩][↩]
- La Rosa M, Lionetti E, Reibaldi M, Russo A, Longo A, Leonardi S, Tomarchio S, Avitabile T, Reibaldi A. Allergic conjunctivitis: a comprehensive review of the literature. Ital J Pediatr. 2013;39:18. doi: 10.1186/1824-7288-39-18[↩][↩][↩]
- Irkeç MT, Orhan M, Erdener U. Role of tear inflammatory mediators in contact lens-associated giant papillary conjunctivitis in soft contact lens wearers. Ocul Immunol Inflamm. 1999;7:35–38. doi: 10.1076/ocii.7.1.35.8107[↩]
- Jabbehdari S, Starnes TW, Kurji KH, Eslani M, Cortina MS, Holland EJ, Djalilian AR. Management of advanced ocular surface disease in patients with severe atopic keratoconjunctivitis. Ocul Surf. 2019;17:303–309. doi: 10.1016/j.jtos.2018.12.002[↩][↩]
- Mehta JS, Chen WL, Cheng ACK, Cung LX, Dualan IJ, Kekunnaya R, Khaliddin N, Kim TI, Lam DK, Leo SW, Manurung F, Tesavibul N, Bremond-Gignac D. Diagnosis, Management, and Treatment of Vernal Keratoconjunctivitis in Asia: Recommendations From the Management of Vernal Keratoconjunctivitis in Asia Expert Working Group. Front Med (Lausanne). 2022 Aug 1;9:882240. doi: 10.3389/fmed.2022.882240[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kaur K, Gurnani B. Vernal Keratoconjunctivitis. [Updated 2023 Jun 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK576433[↩][↩][↩][↩][↩]
- Vernal Keratoconjunctivitis. https://eyewiki.org/Vernal_Keratoconjunctivitis[↩][↩][↩][↩][↩][↩][↩]
- Katelaris CH. Ocular allergy in the Asia Pacific region. Asia Pac Allergy. (2011) 1:108–14. 10.5415/apallergy.2011.1.3.108[↩][↩]
- Zicari AM, Capata G, Nebbioso M, De Castro G, Midulla F, Leonardi L, et al. Vernal keratoconjunctivitis: an update focused on clinical grading system. Ital J Pediatr. (2019) 45:64. 10.1186/s13052-019-0656-4[↩]
- Miyazaki D, Fukagawa K, Okamoto S, Fukushima A, Uchio E, Ebihara N, et al. Epidemiological aspects of allergic conjunctivitis. Allergol Int. (2020) 69:487–95. 10.1016/j.alit.2020.06.004[↩][↩]
- Bremond-Gignac D, Donadieu J, Leonardi A, Pouliquen P, Doan S, Chiambarretta F, et al. Prevalence of vernal keratoconjunctivitis: a rare disease? Br J Ophthalmol. (2008) 92:1097–102. 10.1136/bjo.2007.117812[↩][↩]
- Leonardi A. Management of vernal keratoconjunctivitis. Ophthalmol Ther. 2013 Dec;2(2):73-88. doi: 10.1007/s40123-013-0019-y[↩]
- De Smedt, S., G. Wildner, and P. Kestelyn, Vernal keratoconjunctivitis: an update. Br J Ophthalmol, 2013. 97(1): p. 9-14.[↩][↩][↩][↩]
- Kumar, S., Vernal keratoconjunctivitis: a major review. Acta Ophthalmologica, 2009. 87(2): p. 133-147.[↩][↩][↩][↩][↩][↩][↩]
- Smedt, S.D., et al., Vernal keratoconjunctivitis in school children in Rwanda and its association with socio-economic status: a population-based survey. Am J Trop Med Hyg, 2011. 85(4): p. 711-7.[↩]
- La Rosa, M., et al., Allergic conjunctivitis: a comprehensive review of the literature. Ital J Pediatr, 2013. 39: p. 18.[↩][↩][↩][↩][↩][↩][↩]
- Addis H, Jeng BH. Vernal keratoconjunctivitis. Clin Ophthalmol. (2018) 12:119–23. 10.2147/OPTH.S129552[↩][↩][↩][↩][↩][↩][↩][↩]
- Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye. (2004) 18:345–51. 10.1038/sj.eye.6700675[↩][↩][↩][↩][↩]
- Baudouin C, Liang H, Bremond-Gignac D, Hamard P, Hreiche R, Creuzot-Garcher C, et al. CCR4 and CCR5 expression in conjunctival specimens as differential markers of T(H)1/T(H)2 in ocular surface disorders. J Allergy Clin Immunol. (2005) 116:614–9. 10.1016/j.jaci.2005.05.033[↩][↩][↩]
- Mishra GP, Tamboli V, Jwala J, Mitra AK. Recent patents and emerging therapeutics in the treatment of allergic conjunctivitis. Recent Pat Inflamm Allergy Drug Discov. (2011) 5:26–36. 10.2174/187221311794474883[↩][↩]
- Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res. (2002) 21:319–39. 10.1016/S1350-9462(02)00006-X[↩]
- Bonini, S., et al., Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology, 2000. 107(6): p. 1157-63.[↩][↩]
- Ukponmwan, C.U., Vernal keratoconjunctivitis in Nigerians: 109 consecutive cases. Trop Doct, 2003. 33(4): p. 242-5.[↩]
- Bremond-Gignac D. Atopic and vernal keratoconjunctivitis: differences and similarities. Acta Ophthalmologica. (2016) 94:S256. 10.1111/j.1755-3768.2016.0005[↩]
- Leonardi A, Silva D, Perez Formigo D, Bozkurt B, Sharma V, Allegri P, Rondon C, Calder V, Ryan D, Kowalski ML, Delgado L, Doan S, Fauquert JL. Management of ocular allergy. Allergy. 2019 Sep;74(9):1611-1630. doi: 10.1111/all.13786[↩]
- Vichyanond P, Kosrirukvongs P. Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep. 2013 Jun;13(3):308-14. doi: 10.1007/s11882-013-0345-0[↩][↩]
- Oray M, Toker E. Tear cytokine levels in vernal keratoconjunctivitis: the effect of topical 0.05% cyclosporine a therapy. Cornea. 2013 Aug;32(8):1149-54. doi: 10.1097/ICO.0b013e31828ffdf8[↩]
- Zicari, A.M., et al., Vernal keratoconjunctivitis: atopy and autoimmunity. Eur Rev Med Pharmacol Sci, 2013. 17(10): p. 1419-23.[↩]
- Buckley, R.J., Vernal keratoconjunctivitis. Int Ophthalmol Clin, 1988. 28(4): p. 303-8.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res. 2002 May;21(3):319-39. doi: 10.1016/s1350-9462(02)00006-x[↩]
- Alemayehu AM, Yibekal BT, Fekadu SA. Prevalence of vernal keratoconjunctivitis and its associated factors among children in Gambella town, southwest Ethiopia, June 2018. PLoS One. 2019 Apr 18;14(4):e0215528. doi: 10.1371/journal.pone.0215528[↩]
- Barot RK, Shitole SC, Bhagat N, Patil D, Sawant P, Patil K. Therapeutic effect of 0.1% Tacrolimus Eye Ointment in Allergic Ocular Diseases. J Clin Diagn Res. 2016 Jun;10(6):NC05-9. doi: 10.7860/JCDR/2016/17847.7978[↩]
- Sacchetti M, Baiardini I, Lambiase A, Aronni S, Fassio O, Gramiccioni C, et al. Development and testing of the quality of life in children with vernal keratoconjunctivitis questionnaire. Am J Ophthalmol. (2007) 144:557–63. 10.1016/j.ajo.2007.06.028[↩]
- Brindisi G, Cinicola B, Anania C, De Castro G, Nebbioso M, Miraglia Del Giudice M, Licari A, Caffarelli C, De Filippo M, Cardinale F, Duse M, Zicari AM. Vernal keratoconjunctivitis: state of art and update on treatment. Acta Biomed. 2021 Nov 29;92(S7):e2021517. doi: 10.23750/abm.v92iS7.12419[↩]
- Krachmer, Jay H., Mark J. Mannis, and Edward J. Holland. Cornea. 2nd ed. Philadelphia: Elsevier/Mosby, 2005. Print. p667-674.[↩][↩][↩]
- Bonini, S., et al., Vernal keratoconjunctivitis. Eye (Lond), 2004. 18(4): p. 345-51.[↩][↩]
- Lai B, Phan K, Lewis N, Shumack S. A rare case of vernal keratoconjunctivitis in a patient with atopic dermatitis treated with tralokinumab. J Eur Acad Dermatol Venereol. 2022 May;36(5):e343-e345. doi: 10.1111/jdv.17824[↩]
- Zengarini C, Roda M, Schiavi C, Bruni F, Bardazzi F, Bellusci C, Raone B. Successful treatment of severe recalcitrant vernal keratoconjunctivitis and atopic dermatitis associated with elevated IgE levels with omalizumab. Clin Exp Dermatol. 2022 Mar;47(3):604-606. doi: 10.1111/ced.15000[↩]
- Cameron, J.A., A.A. Al-Rajhi, and I.A. Badr, Corneal ectasia in vernal keratoconjunctivitis. Ophthalmology, 1989. 96(11): p. 1615-23.[↩]
- Jeng BH, Whitcher JP, Margolis TP. Pseudogerontoxon. Clin Exp Ophthalmol. 2004 Aug;32(4):433-4. doi: 10.1111/j.1442-9071.2004.00849.x[↩]
- Turgut B, Kurt J, Catak O, Demir T. Phthriasis palpebrarum mimicking lid eczema and blepharitis. J Ophthalmol. 2009;2009:803951. doi: 10.1155/2009/803951[↩]
- Cameron JA. Shield ulcers and plaques of the cornea in vernal keratoconjunctivitis. Ophthalmology. (1995) 102:985–93. 10.1016/S0161-6420(95)30925-6[↩][↩]
- Reddy JC, Basu S, Saboo U, Murthy SI, Vaddavalli PK, Sangwan VS. Management, clinical outcomes, and complications of shield ulcers in vernal keratoconjunctivitis. Am J Ophthalmol. (2013) 155:550–9. 10.1016/j.ajo.2012.09.014[↩]
- Bonini S, Bonini S, Lambiase A, Marchi S, Pasqualetti P, Zuccaro O, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology. (2000) 107:1157–63. 10.1016/s0161-6420(00)00092-0[↩]
- Tabbara KF. Ocular complications of vernal keratoconjunctivitis. Can J Ophthalmol. (1999) 34:88–92.[↩]
- Sen P, Jain S, Mohan A, Shah C, Sen A, Jain E. Pattern of steroid misuse in vernal keratoconjunctivitis resulting in steroid induced glaucoma and visual disability in Indian rural population: an important public health problem in pediatric age group. Indian J Ophthalmol. (2019) 67:1650–5. 10.4103/ijo.IJO_2143_18[↩]
- Leonardi A, Silva D, Perez Formigo D, Bozkurt B, Sharma V, Allegri P, et al. Management of ocular allergy. Allergy. (2019) 74:1611–30. 10.1111/all.13786[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Spector SL, Raizman MB. Conjunctivitis medicamentosa. J Allergy Clin Immunol. 1994 Jul;94(1):134-6. doi: 10.1016/0091-6749(94)90081-7[↩]
- Leonardi, A., Management of vernal keratoconjunctivitis. Ophthalmol Ther, 2013. 2(2): p. 73-88.[↩][↩][↩][↩]
- Yanni JM, Weimer LK, Sharif NA, Xu SX, Gamache DA, Spellman JM. Inhibition of histamine-induced human conjunctival epithelial cell responses by ocular allergy drugs. Arch Ophthalmol. (1999) 117:643–7. 10.1001/archopht.117.5.643[↩]
- Hida WT, Nogueira DC, Schaefer A, Dantas PEC, Dantas MCN. Comparação entre o uso tópico do fumarato de cetotifeno 0,025% e do cloridrato de olopatadina 0,1% no tratamento da ceratoconjuntivite primaveril [comparative study between 0.025% ketotifen fumarate and 0.1% olopatadine hydrochloride in the treatment of vernal keratoconjunctivitis]. Arq Bras Oftalmol. (2006) 69:851–6. 10.1590/S0004-27492006000600013[↩]
- Holsclaw, D.S.; Whitcher, J.P.; Wong, I.G.; Margolis, T.P. Supratarsal injection of corticosteroid in the treatment of refractory vernal keratoconjunctivitis. Am. J. Ophthalmol. 1996, 121, 243–249.[↩]
- Gokhale NS. Systematic approach to managing vernal keratoconjunctivitis in clinical practice: severity grading system and a treatment algorithm. Indian J Ophthalmol. (2016) 64:145–8. 10.4103/0301-4738.179727[↩][↩]
- Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. (2017) 117:14–28. 10.1016/j.ejpb.2017.03.006[↩]
- Nebbioso M, Alisi L, Giovannetti F, Armentano M, Lambiase A. Eye drop emulsion containing 0.1% cyclosporin (1 mg/mL) for the treatment of severe vernal keratoconjunctivitis: an evidence-based review and place in therapy. Clin Ophthalmol. (2019) 13:1147–55. 10.2147/OPTH.S181811[↩]
- Lallemand F, Daull P, Benita S, Buggage R, Garrigue JS. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. J Drug Dev. (2012) 2012:6044204. 10.1155/2012/604204[↩]
- Wan KH, Chen LJ, Rong SS, Pang CP, Young AL. Topical cyclosporine in the treatment of allergic conjunctivitis: a meta-analysis. Ophthalmology. (2013) 120:2197–203. 10.1016/j.ophtha.2013.03.044[↩]
- Bremond-Gignac D, Doan S, Amrane M, Ismail D, Montero J, Németh K, et al. Twelve-month results of cyclosporine a cationic emulsion in a randomized study in patients with pediatric vernal keratoconjunctivitis. Am J Ophthalmol. (2020) 212:116–26. 10.1016/j.ajo.2019.11.020[↩]
- Leonardi A, Doan S, Amrane M, Ismail D, Montero J, Németh J, et al. A randomized, controlled trial of cyclosporine a cationic emulsion in pediatric vernal keratoconjunctivitis: the VEKTIS study. Ophthalmology. (2019) 126:671–81. 10.1016/j.ophtha.2018.12.027[↩][↩]
- Oray, M. and E. Toker, Tear cytokine levels in vernal keratoconjunctivitis: the effect of topical 0.05% cyclosporine a therapy. Cornea, 2013. 32(8): p. 1149-54.[↩]
- Vichyanond, P. and P. Kosrirukvongs, Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep, 2013. 13(3): p. 308-14.[↩]
- Al-Amri AM, Mirza AG, Al-Hakami AM. Tacrolimus ointment for treatment of vernal keratoconjunctivitis. Middle East Afr J Ophthalmol. (2016) 23:135–8. 10.4103/0974-9233.164616[↩][↩]
- Müller EG, Santos MSD, Freitas D, Gomes JAP, Belfort R., Jr Tacrolimus eye drops as monotherapy for vernal keratoconjunctivitis: a randomized controlled trial. Arq Bras Oftalmol. (2017) 80:154–8. 10.5935/0004-2749.20170038[↩][↩]
- Müller GG, José NK, de Castro RS, de Holanda EC. Long-term use of topical tacrolimus ointment: a safe and effective option for the treatment of vernal keratoconjunctivitis. Arq Bras Oftalmol. (2019) 82:119–23. 10.5935/0004-2749.20190026[↩][↩]
- Ohashi Y, Ebihara N, Fujishima H, Fukushima A, Kumagai N, Nakagawa Y, et al. A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther. (2010) 26:165–74. 10.1089/jop.2009.0087[↩]
- Chatterjee S, Agrawal D. Tacrolimus in corticosteroid-refractory vernal keratoconjunctivitis. Cornea. (2016) 35:1444–8. 10.1097/ICO.0000000000000918[↩]
- Liu FY, Liu HY, Chu HS, Chen WL, Hu FR, Wang IJ. Dermatologic tacrolimus ointment on the eyelids for steroid-refractory vernal keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol. (2019) 257:967–74. 10.1007/s00417-019-04287-1[↩]
- Fukushima A, Ohashi Y, Ebihara N, Uchio E, Okamoto S, Kumagai N, et al. Therapeutic effects of 0.1% tacrolimus eye drops for refractory allergic ocular diseases with proliferative lesion or corneal involvement. Br J Ophthalmol. (2014) 98:1023–7.[↩][↩]
- Barot, RK et al., Therapeutic effect of 0.1% Tacrolimus Eye Ointment in Allergic Ocular Diseases. J Clin Diagn Res. 2016 Jun;10(6):NC05-9.[↩]
- Wan Q et al., Therapeutic effect of 0.1% Tacrolimus Eye Drops in the Tarsal Form of Vernal Keratoconjunctivitis. Ophthalmic Res. 2018;59(3):126-134.[↩]
- Labcharoenwongs P, Jirapongsananuruk O, Visitsunthorn N, Kosrirukvongs P, Saengin P, Vichyanond P. A double-masked comparison of 0.1% tacrolimus ointment and 2% cyclosporine eye drops in the treatment of vernal keratoconjunctivitis in children. Asian Pac J Allergy Immunol. 2012 Sep;30(3):177-84. https://apjai-journal.org/wp-content/uploads/2017/12/2AdoublemaskedcomparisonVol30No3September2012P177.pdf[↩]
- Leonardi, A., et al., Vernal keratoconjunctivitis-like disease in adults. Am J Ophthalmol, 2013. 155(5): p. 796-803.[↩]
- Niederkorn JY, Dana MR. Immune System and the Eye Ocular Therapeutics. Amsterdam: Elsevier; (2008). p. 199–237. 10.1016/b978-012370585-3.50012-1[↩]
- McGhee CNJ, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. (2002) 25:33–55.[↩]
- Holsclaw DS, Whitcher JP, Wong IG, Margolis TP. Supratarsal injection of corticosteroid in the treatment of refractory vernal keratoconjunctivitis. Am J Ophthalmol. (1996) 121:243–9.[↩][↩]
- Zaouali S, Kahloun R, Attia S, Jelliti B, Trigui M, Yahia SB, et al. Supratarsal injection of triamcinolone acetonide and childhood allergic keratoconjunctivitis. Int Ophthalmol. (2012) 32:99–106. 10.1007/s10792-011-9421-4[↩]
- Bielory L, Lien KW, Bigelsen S. Efficacy and tolerability of newer antihistamines in the treatment of allergic conjunctivitis. Drugs. (2005) 65:215–28.[↩]
- de Klerk, T.A., et al., Severe vernal keratoconjunctivitis successfully treated with subcutaneous omalizumab. J aapos, 2013. 17(3): p. 305-6.[↩]
- Doan, S., Amat, F., Gabison, E. et al. Omalizumab in Severe Refractory Vernal Keratoconjunctivitis in Children: Case Series and Review of the Literature. Ophthalmol Ther 6, 2017, 195–206.[↩]
- Evaluation of Dupilumab in Patients With Atopic Keratoconjunctivitis (AKC). https://clinicaltrials.gov/study/NCT04296864[↩]
- Calderon MA, Penagos M, Sheikh A, Canonica GW, Durham SR. Sublingual immunotherapy for allergic conjunctivitis: cochrane systematic review and meta-analysis. Clin Exp Allergy. (2011) 41:1263–72.[↩]
- Lin HY, Yeh PT, Shiao CS, Hu FR. Surgical management and immunohistochemical study of corneal plaques in vernal keratoconjunctivitis. J Formos Med Assoc. (2013) 112:569–73. 10.1016/j.jfma.2012.07.017[↩]
- Pelegrin L, Gris O, Adán A, Plazas A. Superficial keratectomy and amniotic membrane patch in the treatment of corneal plaque of vernal keratoconjunctivitis. Eur J Ophthalmol. (2008) 18:131–3. 10.1177/112067210801800123[↩]
- Solomon A, Zamir E, Levartovsky S, Frucht-Pery J. Surgical management of corneal plaques in vernal keratoconjunctivitis: a clinicopathologic study. Cornea. (2004) 23:608–12. 10.1097/01.ico.0000121710.58571.c4[↩]
- Stock RA, Lazzari SL, Martins IP, Bonamingo EL. Surgical debridement of corneal shield ulcers in pediatric patients: two case reports and a review of the literature. J Med Case Rep. (2020) 14:70. 10.1186/s13256-020-02407-8[↩][↩]
- AlHaran DH. Management of vernal keratoconjunctivitis in children in Saudi Arabia. Oman J Ophthalmol. (2020) 13:3–12.[↩]
- Tanaka M, Dogru M, Takano Y, Miyake-Kashima M, Asano-Kato N, Fukagawa K, et al. Quantitative evaluation of the early changes in ocular surface inflammation following MMC-aided papillary resection in severe allergic patients with corneal complications. Cornea. (2006) 25:281–5. 10.1097/01.ico.0000183533.14899.8d[↩]
- Kumar S. Vernal keratoconjunctivitis: a major review. Acta Ophthalmol. 2009 Mar;87(2):133-47. doi: 10.1111/j.1755-3768.2008.01347.x[↩][↩]
- De Smedt S, Wildner G, Kestelyn P. Vernal keratoconjunctivitis: an update. Br J Ophthalmol. 2013 Jan;97(1):9-14. doi: 10.1136/bjophthalmol-2011-301376[↩]
- NEUMANN E, GUTMANN MJ, BLUMENKRANTZ N, MICHAELSON IC. A review of four hundred cases of vernal conjunctivitis. Am J Ophthalmol. 1959 Feb;47(2):166-72. doi: 10.1016/s0002-9394(14)76417-7[↩]
- Cameron JA. Shield ulcers and plaques of the cornea in vernal keratoconjunctivitis. Ophthalmology. 1995 Jun;102(6):985-93. doi: 10.1016/s0161-6420(95)30925-6[↩]
- Saboo, U.S., et al., Demographic and clinical profile of vernal keratoconjunctivitis at a tertiary eye care center in India. Indian J Ophthalmol, 2013. 61(9): p. 486-9.[↩]
- Atopic Keratoconjunctivitis. https://eyewiki.org/Atopic_Keratoconjunctivitis[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Atopic Keratoconjunctivitis (AKC). https://www.umkelloggeye.org/conditions-treatments/atopic-keratoconjunctivitis[↩][↩][↩]
- Atopic dermatitis. https://dermnetnz.org/topics/atopic-dermatitis[↩][↩]
- Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res. 2002;21:319–339. doi: 10.1016/s1350-9462(02)00006-x[↩]
- O’Brien TP. Allergic conjunctivitis: an update on diagnosis and management. Curr Opin Allergy Clin Immunol. 2013;13:543–549. doi: 10.1097/ACI.0b013e328364ec3a[↩][↩][↩]
- Brémond-Gignac D, Nischal KK, Mortemousque B, Gajdosova E, Granet DB, Chiambaretta F. Atopic Keratoconjunctivitis in Children: Clinical Features and Diagnosis. Ophthalmology. 2016 Feb;123(2):435-437. doi: 10.1016/j.ophtha.2015.07.012[↩][↩]
- Aboobacker S, Harris BW, Limaiem F. Lichenification. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537332[↩]
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016 Mar 12;387(10023):1109-1122. doi: 10.1016/S0140-6736(15)00149-X[↩]
- Atopy. https://dermnetnz.org/topics/atopy[↩][↩]
- Gupta J, Johansson E, Bernstein JA, Chakraborty R, Khurana Hershey GK, Rothenberg ME, Mersha TB. Resolving the etiology of atopic disorders by using genetic analysis of racial ancestry. J Allergy Clin Immunol. 2016 Sep;138(3):676-699. doi: 10.1016/j.jaci.2016.02.045[↩]
- Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010 Feb;125(2 Suppl 2):S73-80. doi: 10.1016/j.jaci.2009.11.017[↩]
- Enríquez-de-Salamanca A, Calder V, Gao J, Galatowicz G, García-Vázquez C, Fernández I, Stern ME, Diebold Y, Calonge M. Cytokine responses by conjunctival epithelial cells: an in vitro model of ocular inflammation. Cytokine. 2008 Oct;44(1):160-7. doi: 10.1016/j.cyto.2008.07.007[↩]
- Metz DP, Hingorani M, Calder VL, Buckley RJ, Lightman SL. T-cell cytokines in chronic allergic eye disease. J Allergy Clin Immunol. 1997 Dec;100(6 Pt 1):817-24. doi: 10.1016/s0091-6749(97)70279-3[↩]
- Hu Y, Adan ES, Matsumoto Y, Dogru M, Fukagawa K, Takano Y, Tsubota K, Fujishima H. Conjunctival in vivo confocal scanning laser microscopy in patients with atopic keratoconjunctivitis. Mol Vis. 2007 Aug 10;13:1379-89.[↩][↩]
- Chen JJ, Applebaum DS, Sun GS, Pflugfelder SC. Atopic keratoconjunctivitis: A review. J Am Acad Dermatol. 2014 Mar;70(3):569-75. doi: 10.1016/j.jaad.2013.10.036[↩][↩][↩][↩]
- Chen JJ, Applebaum DS, Sun GS, Pflugfelder SC. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569–575. doi: 10.1016/j.jaad.2013.10.036[↩]
- Takamura E, et al. Japanese guideline for allergic conjunctival diseases. Allergol Int. 2011;60:191–203. doi: 10.2332/allergolint.11-RAI-0335[↩]
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66 Suppl 1:8-16. doi: 10.1159/000370220[↩][↩]
- Dom S, Droste JH, Sariachvili MA, Hagendorens MM, Oostveen E, Bridts CH, Stevens WJ, Wieringa MH, Weyler JJ. Pre- and post-natal exposure to antibiotics and the development of eczema, recurrent wheezing and atopic sensitization in children up to the age of 4 years. Clin Exp Allergy. 2010 Sep;40(9):1378-87. doi: 10.1111/j.1365-2222.2010.03538.x[↩]
- Power WJ, Tugal-Tutkun I, Foster CS. Long-term follow-up of patients with atopic keratoconjunctivitis. Ophthalmology. 1998 Apr;105(4):637-42. doi: 10.1016/S0161-6420(98)94017-9[↩][↩][↩][↩][↩]
- Vaidyanathan U, Hopping GC, Liu HY, Somani AN, Ronquillo YC, Hoopes PC, Moshirfar M. Persistent Corneal Epithelial Defects: A Review Article. Med Hypothesis Discov Innov Ophthalmol. 2019 Fall;8(3):163-176.[↩]
- Liu A, Manche EE. Bilateral posterior subcapsular cataracts associated with long-term intranasal steroid use. J Cataract Refract Surg. 2011 Aug;37(8):1555-8. doi: 10.1016/j.jcrs.2011.05.020[↩]
- Wang T, Moinuddin O, Abuzaitoun R, Hwang M, Besirli C, Wubben TJ, Zacks DN. Retinal Detachment After Endophthalmitis: Risk Factors and Outcomes. Clin Ophthalmol. 2021 Apr 13;15:1529-1537. doi: 10.2147/OPTH.S302757[↩]
- Hanet MS, Zimpfer A, Lepper S, Seitz B. Keratoconus-like tomographic changes in a case of recurrent interstitial keratitis. J Ophthalmic Inflamm Infect. 2018 Mar 7;8(1):4. doi: 10.1186/s12348-018-0146-7[↩]
- Garrity JA, Liesegang TJ. Ocular complications of atopic dermatitis. Can J Ophthalmol. 1984 Feb;19(1):21-4.[↩]
- Easty D, Entwistle C, Funk A, Witcher J. Herpes simplex keratitis and keratoconus in the atopic patient. A clinical and immunological study. Trans Ophthalmol Soc U K (1962). 1975 Jul;95(2):267-76.[↩]
- Kallen C, Reinhard T, Schilgen G, Cartsburg O, Böcking A, Auw-Hädrich C, Sundmacher R. Atopische KeratokonjunktivitisWahrscheinlich ein Risikofaktor für die Entstehung von Bindehautkarzinomen [Atopic keratoconjunctivitis: probably a risk factor for the development of conjuntival carcinoma]. Ophthalmologe. 2003 Oct;100(10):808-14. German. doi: 10.1007/s00347-003-0809-z[↩]
- Heinz C, Fanihagh F, Steuhl KP. Squamous cell carcinoma of the conjunctiva in patients with atopic eczema. Cornea. 2003 Mar;22(2):135-7. doi: 10.1097/00003226-200303000-00011[↩]
- Different Types of Cataracts. https://insighteye.com.au/different-types-cataracts/[↩]
- Keratoconus. https://www.vrmny.com/conditions/keratoconus/[↩]
- Azari AA, Arabi A. Conjunctivitis: A Systematic Review. J Ophthalmic Vis Res. 2020 Aug 6;15(3):372-395. doi: 10.18502/jovr.v15i3.7456[↩]
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010 Oct;10(5):478-85. doi: 10.1097/ACI.0b013e32833e16e4[↩]
- Correale CE, Walker C, Murphy L, Craig TJ. Atopic dermatitis: a review of diagnosis and treatment. Am Fam Physician. 1999 Sep 15;60(4):1191-8, 1209-10. https://www.aafp.org/pubs/afp/issues/1999/0915/p1191.html[↩]
- Inada N, Shoji J, Kato H, Kiely S, Mulyanto, Sawa M. Clinical evaluation of total IgE in tears of patients with allergic conjunctivitis disease using a novel application of the immunochromatography method. Allergol Int. 2009 Dec;58(4):585-9. doi: 10.2332/allergolint.09-OA-0101[↩]
- Takano Y, Fukagawa K, Dogru M, Asano-Kato N, Tsubota K, Fujishima H. Inflammatory cells in brush cytology samples correlate with the severity of corneal lesions in atopic keratoconjunctivitis. Br J Ophthalmol. 2004 Dec;88(12):1504-5. doi: 10.1136/bjo.2004.047167[↩]
- Wakamatsu TH, Tanaka M, Satake Y, Dogru M, Fukagawa K, Igarashi A, Fujishima H. Eosinophil cationic protein as a marker for assessing the efficacy of tacrolimus ophthalmic solution in the treatment of atopic keratoconjunctivitis. Mol Vis. 2011 Apr 13;17:932-8. https://pmc.ncbi.nlm.nih.gov/articles/PMC3084241/[↩]
- Foster CS, Rice BA, Dutt JE. Immunopathology of atopic keratoconjunctivitis. Ophthalmology. 1991 Aug;98(8):1190-6. doi: 10.1016/s0161-6420(91)32154-7[↩]
- Phlyctenular Keratoconjunctivitis. https://eyewiki.org/Phlyctenular_Keratoconjunctivitis[↩]
- Gaddie IB, Donnenfeld ED, Karpecki P, Vollmer P, Berdy GJ, Peterson JD, Simmons B, Edell ARP, Whitson WE, Ciolino JB, Baba SN, Holdbrook M, Trevejo J, Meyer J, Yeu E; Saturn-2 Study Group. Lotilaner Ophthalmic Solution 0.25% for Demodex Blepharitis: Randomized, Vehicle-Controlled, Multicenter, Phase 3 Trial (Saturn-2). Ophthalmology. 2023 Oct;130(10):1015-1023. doi: 10.1016/j.ophtha.2023.05.030[↩]
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016 Aug 8;8:71-78. doi: 10.2147/OPTO.S84795[↩]
- Al-Amri AM. Long-term follow-up of tacrolimus ointment for treatment of atopic keratoconjunctivitis. Am J Ophthalmol. 2014 Feb;157(2):280-6. doi: 10.1016/j.ajo.2013.10.006[↩]
- González-López JJ, López-Alcalde J, Morcillo Laiz R, Fernández Buenaga R, Rebolleda Fernández G. Topical cyclosporine for atopic keratoconjunctivitis. Cochrane Database Syst Rev. 2012 Sep 12;(9):CD009078. doi: 10.1002/14651858.CD009078.pub2[↩]
- Erdinest N, Noyman DBE, London N, Lavy I, Levinger N, Landau D, Solomon A, Morad Y, Naroo SA. Applications of topical immunomodulators enhance clinical signs of vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC): a meta-analysis. Int Ophthalmol. 2024 Mar 24;44(1):157. doi: 10.1007/s10792-024-03097-7[↩]
- Munro CS, Levell NJ, Shuster S, Friedmann PS. Maintenance treatment with cyclosporin in atopic eczema. Br J Dermatol. 1994 Mar;130(3):376-80. doi: 10.1111/j.1365-2133.1994.tb02936.x[↩]
- Salek MS, Finlay AY, Luscombe DK, Allen BR, Berth-Jones J, Camp RD, Graham-Brown RA, Khan GK, Marks R, Motley RJ, et al. Cyclosporin greatly improves the quality of life of adults with severe atopic dermatitis. A randomized, double-blind, placebo-controlled trial. Br J Dermatol. 1993 Oct;129(4):422-30. doi: 10.1111/j.1365-2133.1993.tb03170.x[↩]
- Zaouali S, Kahloun R, Attia S, Jelliti B, Trigui M, Yahia SB, Messaoud R, Khairallah M. Supratarsal injection of triamcinolone acetonide and childhood allergic keratoconjunctivitis. Int Ophthalmol. 2012 Apr;32(2):99-106. doi: 10.1007/s10792-011-9421-4[↩]
- Koçluk Y, Yalniz-Akkaya Z, Burcu A, Örnek F. Atopic keratoconjunctivitis: long-term results of medical treatment and penetrating keratoplasty. Arq Bras Oftalmol. 2016 Nov-Dec;79(6):376-379. doi: 10.5935/0004-2749.20160107[↩][↩]
- Atopic Keratoconjunctivitis (AKC). https://emedicine.medscape.com/article/1194480-overview[↩][↩][↩]
- Keen M, Thompson M. Treatment of Acute Conjunctivitis in the United States and Evidence of Antibiotic Overuse: Isolated Issue or a Systematic Problem? Ophthalmology. 2017 Aug;124(8):1096-1098. doi: 10.1016/j.ophtha.2017.05.029[↩]
- Udeh BL, Schneider JE, Ohsfeldt RL. Cost effectiveness of a point-of-care test for adenoviral conjunctivitis. Am J Med Sci. 2008 Sep;336(3):254-64. doi: 10.1097/MAJ.0b013e3181637417[↩]
- Solano D, Fu L, Czyz CN. Viral Conjunctivitis. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470271[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Conjunctivitis. https://eyewiki.org/Conjunctivitis[↩][↩][↩][↩][↩][↩]
- Sheikh A, Hurwitz B, van Schayck CP, McLean S, Nurmatov U. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2012 Sep 12;(9):CD001211. doi: 10.1002/14651858.CD001211.pub3. Update in: Cochrane Database Syst Rev. 2023 Mar 13;3:CD001211. doi: 10.1002/14651858.CD001211.pub4[↩][↩][↩]
- Li J, Lu X, Jiang B, Du Y, Yang Y, Qian H, Liu B, Lin C, Jia L, Chen L, Wang Q. Adenovirus-associated acute conjunctivitis in Beijing, China, 2011-2013. BMC Infect Dis. 2018 Mar 20;18(1):135. doi: 10.1186/s12879-018-3014-z[↩]
- Sow AS, Kane H, Ka AM, Hanne FT, Ndiaye JMM, Diagne JP, Nguer M, Sow S, Saheli Y, Sy EHM, De Meideros Quenum ME, Ndoye Roth PA, Ba EA, Ndiaye PA. Expérience sénégalaise des conjonctivites virales aiguës [Senegalese experience with acute viral conjunctivitis]. J Fr Ophtalmol. 2017 Apr;40(4):297-302. French. doi: 10.1016/j.jfo.2016.12.008[↩]
- Chigbu DI, Labib BA. Pathogenesis and management of adenoviral keratoconjunctivitis. Infect Drug Resist. 2018 Jul 17;11:981-993. doi: 10.2147/IDR.S162669[↩]
- Trinavarat A, Atchaneeyasakul LO. Treatment of epidemic keratoconjunctivitis with 2% povidone-iodine: a pilot study. J Ocul Pharmacol Ther. 2012 Feb;28(1):53-8. doi: 10.1089/jop.2011.0082[↩]
- Levinger E, Slomovic A, Sansanayudh W, Bahar I, Slomovic AR. Topical treatment with 1% cyclosporine for subepithelial infiltrates secondary to adenoviral keratoconjunctivitis. Cornea. 2010 Jun;29(6):638-40. doi: 10.1097/ICO.0b013e3181c33034[↩]
- Berisa Prado S, Riestra Ayora AC, Lisa Fernández C, Chacón Rodríguez M, Merayo-Lloves J, Alfonso Sánchez JF. Topical Tacrolimus for Corneal Subepithelial Infiltrates Secondary to Adenoviral Keratoconjunctivitis. Cornea. 2017 Sep;36(9):1102-1105. doi: 10.1097/ICO.0000000000001279[↩]
- Arici C, Mergen B. Late-term topical tacrolimus for subepithelial infiltrates resistant to topical steroids and ciclosporin secondary to adenoviral keratoconjunctivitis. Br J Ophthalmol. 2021 May;105(5):614-618. doi: 10.1136/bjophthalmol-2020-316196[↩]
- Zhang L, Zhao N, Huang X, Jin X, Geng X, Chan TC, Liu S. Molecular epidemiology of acute hemorrhagic conjunctivitis caused by coxsackie A type 24 variant in China, 2004-2014. Sci Rep. 2017 Mar 23;7:45202. doi: 10.1038/srep45202[↩]
- Langford MP, Anders EA, Burch MA. Acute hemorrhagic conjunctivitis: anti-coxsackievirus A24 variant secretory immunoglobulin A in acute and convalescent tear. Clin Ophthalmol. 2015 Sep 10;9:1665-73. doi: 10.2147/OPTH.S85358[↩][↩]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Feb 15;395(10223):496. doi: 10.1016/S0140-6736(20)30252-X[↩]
- Danesh-Meyer HV, McGhee CNJ. Implications of COVID-19 for Ophthalmologists. Am J Ophthalmol. 2021 Mar;223:108-118. doi: 10.1016/j.ajo.2020.09.027[↩]
- Chintakuntlawar AV, Chodosh J. Cellular and tissue architecture of conjunctival membranes in epidemic keratoconjunctivitis. Ocul Immunol Inflamm. 2010 Oct;18(5):341-5. doi: 10.3109/09273948.2010.498658[↩]
- Marinos E, Cabrera-Aguas M, Watson SL. Viral conjunctivitis: a retrospective study in an Australian hospital. Cont Lens Anterior Eye. 2019 Dec;42(6):679-684. doi: 10.1016/j.clae.2019.07.001[↩]
- Sethuraman U, Kamat D. The red eye: evaluation and management. Clin Pediatr (Phila). 2009 Jul;48(6):588-600. doi: 10.1177/0009922809333094[↩]
- Elnifro EM, Cooper RJ, Klapper PE, Yeo AC, Tullo AB. Multiplex polymerase chain reaction for diagnosis of viral and chlamydial keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2000 Jun;41(7):1818-22.[↩][↩]
- Kaufman HE. Adenovirus advances: new diagnostic and therapeutic options. Curr Opin Ophthalmol. 2011 Jul;22(4):290-3. doi: 10.1097/ICU.0b013e3283477cb5[↩][↩]
- Kam KY, Ong HS, Bunce C, Ogunbowale L, Verma S. Sensitivity and specificity of the AdenoPlus point-of-care system in detecting adenovirus in conjunctivitis patients at an ophthalmic emergency department: a diagnostic accuracy study. Br J Ophthalmol. 2015 Sep;99(9):1186-9. doi: 10.1136/bjophthalmol-2014-306508[↩][↩]
- Sambursky R, Trattler W, Tauber S, Starr C, Friedberg M, Boland T, McDonald M, DellaVecchia M, Luchs J. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013 Jan;131(1):17-22. doi: 10.1001/2013.jamaophthalmol.513. Erratum in: JAMA Ophthalmol. 2013 Mar;131(3):320.[↩][↩]
- Holtz KK, Townsend KR, Furst JW, Myers JF, Binnicker MJ, Quigg SM, Maxson JA, Espy MJ. An Assessment of the AdenoPlus Point-of-Care Test for Diagnosing Adenoviral Conjunctivitis and Its Effect on Antibiotic Stewardship. Mayo Clin Proc Innov Qual Outcomes. 2017 Jul 25;1(2):170-175. doi: 10.1016/j.mayocpiqo.2017.06.001[↩][↩]
- Jhanji V, Chan TC, Li EY, Agarwal K, Vajpayee RB. Adenoviral keratoconjunctivitis. Surv Ophthalmol. 2015 Sep-Oct;60(5):435-43. doi: 10.1016/j.survophthal.2015.04.001[↩][↩]
- Usher P, Keefe J, Crock C, Chan E. Appropriate prescribing for viral conjunctivitis. Aust Fam Physician. 2014 Nov;43(11):748-9.[↩]
- Shiota H, Ohno S, Aoki K, Azumi A, Ishiko H, Inoue Y, Usui N, Uchio E, Kaneko H, Kumakura S, Tagawa Y, Tanifuji Y, Nakagawa H, Hinokuma R, Yamazaki S, Yokoi N. [Guideline for the nosocomial infections of adenovirus conjunctivitis]. Nippon Ganka Gakkai Zasshi. 2009 Jan;113(1):25-46. Japanese.[↩]
- Özen Tunay Z, Ozdemir O, Petricli IS. Povidone iodine in the treatment of adenoviral conjunctivitis in infants. Cutan Ocul Toxicol. 2015 Mar;34(1):12-5. doi: 10.3109/15569527.2014.888077[↩]
- Kovalyuk N, Kaiserman I, Mimouni M, Cohen O, Levartovsky S, Sherbany H, Mandelboim M. Treatment of adenoviral keratoconjunctivitis with a combination of povidone-iodine 1.0% and dexamethasone 0.1% drops: a clinical prospective controlled randomized study. Acta Ophthalmol. 2017 Dec;95(8):e686-e692. doi: 10.1111/aos.13416[↩]
- Faraldi F, Papa V, Rasà D, Santoro D, Russo S. Netilmicin/dexamethasone fixed combination in the treatment of conjunctival inflammation. Clin Ophthalmol. 2013;7:1239-44. doi: 10.2147/OPTH.S44455[↩]
- Lobo AM, Agelidis AM, Shukla D. Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf. 2019 Jan;17(1):40-49. doi: 10.1016/j.jtos.2018.10.002[↩][↩]
- Agarwal R, Maharana PK, Titiyal JS, Sharma N. Bilateral herpes simplex keratitis: lactation a trigger for recurrence! BMJ Case Rep. 2019 Mar 9;12(3):e223713. doi: 10.1136/bcr-2017-223713[↩][↩][↩]
- Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012 Sep;57(5):448-62. doi: 10.1016/j.survophthal.2012.01.005[↩][↩][↩]
- Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2015 Jan 9;1(1):CD002898. doi: 10.1002/14651858.CD002898.pub5[↩][↩]
- Herpes Simplex Epithelial Keratitis. https://eyewiki.org/Herpes_Simplex_Epithelial_Keratitis[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Roozbahani M, Hammersmith KM. Management of herpes simplex virus epithelial keratitis. Curr Opin Ophthalmol. 2018 Jul;29(4):360-364. doi: 10.1097/ICU.0000000000000483[↩][↩][↩][↩][↩][↩][↩]
- Austin A, Lietman T, Rose-Nussbaumer J. Update on the Management of Infectious Keratitis. Ophthalmology. 2017 Nov;124(11):1678-1689. doi: 10.1016/j.ophtha.2017.05.012[↩][↩]
- Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med. 1998 Jul 30;339(5):300-6. doi: 10.1056/NEJM199807303390503[↩][↩]
- Prakash G, Avadhani K, Srivastava D. The three faces of herpes simplex epithelial keratitis: a steroid-induced situation. BMJ Case Rep. 2015 Apr 2;2015:bcr2014209197. doi: 10.1136/bcr-2014-209197[↩]
- Herpes Simplex Keratitis. https://eyerounds.org/cases/160-HSV.htm[↩]
- Arshad S, Petsoglou C, Lee T, Al-Tamimi A, Carnt NA. 20 years since the Herpetic Eye Disease Study: Lessons, developments and applications to clinical practice. Clin Exp Optom. 2021 Apr;104(3):396-405. doi: 10.1080/08164622.2021.1877531[↩]
- Majtanova N, Kriskova P, Keri P, Fellner Z, Majtan J, Kolar P. Herpes Simplex Keratitis in Patients with SARS-CoV-2 Infection: A Series of Five Cases. Medicina (Kaunas). 2021 Apr 24;57(5):412. doi: 10.3390/medicina57050412[↩]
- Alkwikbi H, Alenazi M, Alanazi W, Alruwaili S. Herpetic Keratitis and Corneal Endothelitis Following COVID-19 Vaccination: A Case Series. Cureus. 2022 Jan 5;14(1):e20967. doi: 10.7759/cureus.20967[↩]
- Borkar DS, Gonzales JA, Tham VM, Esterberg E, Vinoya AC, Parker JV, Uchida A, Acharya NR. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014 Mar;132(3):326-31. doi: 10.1001/jamaophthalmol.2013.6277[↩]
- Predictors of recurrent herpes simplex virus keratitis. Herpetic Eye Disease Study Group. Cornea. 2001 Mar;20(2):123-8. doi: 10.1097/00003226-200103000-00001[↩]
- Young RC, Hodge DO, Liesegang TJ, Baratz KH. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976-2007: the effect of oral antiviral prophylaxis. Arch Ophthalmol. 2010 Sep;128(9):1178-83. doi: 10.1001/archophthalmol.2010.187[↩]
- White ML, Chodosh J. Herpes Simplex Virus Keratitis: A Treatment Guideline. AAO Compendium of Evidence-Based Eye Care; 2014. Available at: http:// www.aao.org/clinical-statement/herpes-simplex-virus-keratitis-treatment- guideline[↩]
- Tabery HM. Epithelial changes in early primary herpes simplex virus keratitis. Photomicrographic observations in a case of human infection. Acta Ophthalmol Scand. 2000 Dec;78(6):706-9. doi: 10.1034/j.1600-0420.2000.078006706.x[↩]
- Subhan S, Jose RJ, Duggirala A, Hari R, Krishna P, Reddy S, Sharma S. Diagnosis of herpes simplex virus-1 keratitis: comparison of Giemsa stain, immunofluorescence assay and polymerase chain reaction. Curr Eye Res. 2004 Aug-Sep;29(2-3):209-13. doi: 10.1080/02713680490504911[↩][↩]
- Kalogeropoulos D, Geka A, Malamos K, Kanari M, Kalogeropoulos C. New Therapeutic Perceptions in a Patient with Complicated Herpes Simplex Virus 1 Keratitis: A Case Report and Review of the Literature. Am J Case Rep. 2017 Dec 27;18:1382-1389. doi: 10.12659/ajcr.906506[↩]
- Reed JW, Joyner SJ, Knauer WJ 3rd. Penetrating keratoplasty for herpes zoster keratopathy. Am J Ophthalmol. 1989 Mar 15;107(3):257-61. doi: 10.1016/0002-9394(89)90309-7[↩]
- Hutnik C, Mohammad-Shahi MH. Bacterial conjunctivitis. Clin Ophthalmol. 2010 Dec 6;4:1451-7. doi: 10.2147/OPTH.S10162[↩]
- Pippin MM, Le JK. Bacterial Conjunctivitis. [Updated 2023 Aug 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546683[↩][↩][↩][↩]
- Basic and Clinical Science Course 2019-2020: External Disease and Cornea. San Francisco, CA: American Academy of Ophthalmology; 2019.[↩][↩]
- Sheikh A, Hurwitz B. Topical antibiotics for acute bacterial conjunctivitis: Cochrane systematic review and meta-analysis update. Br J Gen Pract. 2005 Dec;55(521):962-4. https://pmc.ncbi.nlm.nih.gov/articles/PMC1570513/[↩][↩]
- Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008 Feb;86(1):5-17. doi: 10.1111/j.1600-0420.2007.01006.x[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Wan WL, Farkas GC, May WN, Robin JB. The clinical characteristics and course of adult gonococcal conjunctivitis. Am J Ophthalmol. 1986 Nov 15;102(5):575-83. doi: 10.1016/0002-9394(86)90527-1[↩]
- Chen YY, Liu SH, Nurmatov U, van Schayck OC, Kuo IC. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2023 Mar 13;3(3):CD001211. doi: 10.1002/14651858.CD001211.pub4[↩]
- Centers for Disease Control and Prevention Public Health Image Library. CDC, 1977.[↩]
- Rothschild PR, Brezin AP, Dupin N. A 22-year-old man with chronic red eye and dysuria. BMJ Case Rep. 2014 Mar 20;2014:bcr2013202113. doi: 10.1136/bcr-2013-202113[↩]
- Azari AA, Barney NP. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013 Oct 23;310(16):1721-9. doi: 10.1001/jama.2013.280318. Erratum in: JAMA. 2014 Jan 1;311(1):95. Dosage error in article text[↩][↩]
- Beal C, Giordano B. Clinical Evaluation of Red Eyes in Pediatric Patients. J Pediatr Health Care. 2016 Sep-Oct;30(5):506-14. doi: 10.1016/j.pedhc.2016.02.001[↩][↩][↩][↩][↩][↩][↩]
- Leung AKC, Hon KL, Wong AHC, Wong AS. Bacterial Conjunctivitis in Childhood: Etiology, Clinical Manifestations, Diagnosis, and Management. Recent Pat Inflamm Allergy Drug Discov. 2018;12(2):120-127. doi: 10.2174/1872213X12666180129165718[↩]
- Pichichero ME. Bacterial conjunctivitis in children: antibacterial treatment options in an era of increasing drug resistance. Clin Pediatr (Phila). 2011 Jan;50(1):7-13. doi: 10.1177/0009922810379045[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Epling J. Bacterial conjunctivitis. BMJ Clin Evid. 2012 Feb 20;2012:0704. https://pmc.ncbi.nlm.nih.gov/articles/PMC3635545/[↩][↩]
- Patel PB, Diaz MC, Bennett JE, Attia MW. Clinical features of bacterial conjunctivitis in children. Acad Emerg Med. 2007 Jan;14(1):1-5. doi: 10.1197/j.aem.2006.08.006[↩][↩][↩][↩][↩]
- Rietveld RP, ter Riet G, Bindels PJ, Sloos JH, van Weert HC. Predicting bacterial cause in infectious conjunctivitis: cohort study on informativeness of combinations of signs and symptoms. BMJ. 2004 Jul 24;329(7459):206-10. doi: 10.1136/bmj.38128.631319.AE[↩][↩]
- Chen FV, Chang TC, Cavuoto KM. Patient demographic and microbiology trends in bacterial conjunctivitis in children. J AAPOS. 2018 Feb;22(1):66-67. doi: 10.1016/j.jaapos.2017.08.008[↩][↩]
- Zloto O, Gharaibeh A, Mezer E, Stankovic B, Isenberg S, Wygnanski-Jaffe T. Ophthalmia neonatorum treatment and prophylaxis: IPOSC global study. Graefes Arch Clin Exp Ophthalmol. 2016 Mar;254(3):577-82. doi: 10.1007/s00417-016-3274-5[↩]
- Matejcek A, Goldman RD. Treatment and prevention of ophthalmia neonatorum. Can Fam Physician. 2013 Nov;59(11):1187-90. https://pmc.ncbi.nlm.nih.gov/articles/PMC3828094/[↩]
- Haimovici R, Roussel TJ. Treatment of gonococcal conjunctivitis with single-dose intramuscular ceftriaxone. Am J Ophthalmol. 1989 May 15;107(5):511-4. doi: 10.1016/0002-9394(89)90495-9[↩]
- CDC’s Sexually Transmitted Infections (STI) Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/default.htm[↩][↩]
- Gonococcal Infections Among Adolescents and Adults. https://www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm[↩][↩]
- Chlamydial Infections. https://www.cdc.gov/std/treatment-guidelines/chlamydia.htm[↩][↩][↩]
- Centers for Disease Control and Prevention (CDC). Hypertrophic pyloric stenosis in infants following pertussis prophylaxis with erythromycin–Knoxville, Tennessee, 1999. MMWR Morb Mortal Wkly Rep. 1999 Dec 17;48(49):1117-20.[↩]
- Honein MA, Paulozzi LJ, Himelright IM, Lee B, Cragan JD, Patterson L, Correa A, Hall S, Erickson JD. Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromcyin: a case review and cohort study. Lancet. 1999 Dec 18-25;354(9196):2101-5. doi: 10.1016/s0140-6736(99)10073-4. Erratum in: Lancet 2000 Feb 26;355(9205):758.[↩]
- Eberly MD, Eide MB, Thompson JL, Nylund CM. Azithromycin in early infancy and pyloric stenosis. Pediatrics. 2015 Mar;135(3):483-8. doi: 10.1542/peds.2014-2026[↩][↩]
- Smith C, Egunsola O, Choonara I, Kotecha S, Jacqz-Aigrain E, Sammons H. Use and safety of azithromycin in neonates: a systematic review. BMJ Open. 2015 Dec 9;5(12):e008194. doi: 10.1136/bmjopen-2015-008194[↩]
- Zikic A, Schünemann H, Wi T, Lincetto O, Broutet N, Santesso N. Treatment of Neonatal Chlamydial Conjunctivitis: A Systematic Review and Meta-analysis. J Pediatric Infect Dis Soc. 2018 Aug 17;7(3):e107-e115. doi: 10.1093/jpids/piy060[↩]
- Paiva-Santos AC, Gonçalves T, Peixoto D, Pires PC, Velsankar K, Jha NK, Chavda VP, Mohammad IS, Cefali LC, Mazzola PG, Mascarenhas-Melo F, Veiga F. Rosacea Topical Treatment and Care: From Traditional to New Drug Delivery Systems. Mol Pharm. 2023 Aug 7;20(8):3804-3828. doi: 10.1021/acs.molpharmaceut.3c00324[↩][↩]
- Geng RSQ, Bourkas AN, Mufti A, Sibbald RG. Rosacea: Pathogenesis and Therapeutic Correlates. J Cutan Med Surg. 2024 Mar-Apr;28(2):178-189. doi: 10.1177/12034754241229365[↩][↩]
- Rodrigues-Braz D, Zhao M, Yesilirmak N, Aractingi S, Behar-Cohen F, Bourges JL. Cutaneous and ocular rosacea: Common and specific physiopathogenic mechanisms and study models. Mol Vis. 2021 May 13;27:323-353. https://pmc.ncbi.nlm.nih.gov/articles/PMC8131178/[↩][↩]
- Ocular Rosacea. https://eyewiki.org/Ocular_Rosacea[↩][↩]
- Rosacea. https://dermnetnz.org/topics/rosacea[↩]
- Ocular rosacea. https://dermnetnz.org/topics/ocular-rosacea[↩][↩][↩][↩][↩]
- Ocular Rosacea. https://www.aao.org/eye-health/diseases/ocular-rosacea-facts[↩]
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: A review of recent findings. J Am Acad Dermatol. 2013;69(Supplement 1):S15–26. doi: 10.1016/j.jaad.2013.04.045[↩]
- Steinhoff M, Schmelz M, Schauber J. Facial Erythema of Rosacea – Aetiology, Different Pathophysiologies and Treatment Options. Acta Derm Venereol. 2016;96:579–86. doi: 10.2340/00015555-2335[↩]
- Gallo RL, Granstein RD, Kang S, Mannis M, Steinhoff M, Tan J, Thiboutot D. Standard classification and pathophysiology of rosacea: The 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148–55. doi: 10.1016/j.jaad.2017.08.037[↩][↩]
- Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, Powell F. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584–7. doi: 10.1067/mjd.2002.120625[↩]
- Patiño-Ramírez BE, Rodríguez-García A, Díaz JC, Perfecto Ávalos Y. Alteraciones de la superficie ocular en pacientes con rosácea ocular. Rev Mex Oftalmol. 2012;86:86–96.[↩]
- Vieira AC, Mannis MJ. Ocular rosacea: common and commonly missed. J Am Acad Dermatol. 2013;69(Suppl 1):S36–41. doi: 10.1016/j.jaad.2013.04.042[↩]
- Gold LM, Draelos ZD. New and Emerging Treatments for Rosacea. Am J Clin Dermatol. 2015;16:457–61. doi: 10.1007/s40257-015-0156-2[↩]
- Alexis AF, Callender VD, Baldwin HE, Desai SR, Rendon MI, Taylor SC. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: Review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722–1729.e7. doi: 10.1016/j.jaad.2018.08.049[↩]
- Al-Dabagh A, Davis SA, McMichael AJ, Feldman SR. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014 Oct 15;20(10):13030/qt1mv9r0ss[↩]
- Alexis AF, Callender VD, Baldwin HE, Desai SR, Rendon MI, Taylor SC. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80(6):1722-1729.e7. doi: 10.1016/j.jaad.2018.08.049[↩]
- Awais M, Anwar MI, Iftikhar R, Iqbal Z, Shehzad N, Akbar B. Rosacea – the ophthalmic perspective. Cutan Ocul Toxicol. 2015;34(2):161-6. doi: 10.3109/15569527.2014.930749[↩]
- Oge’ LK, Muncie HL, Phillips-Savoy AR. Rosacea: Diagnosis and Treatment. Am Fam Physician. 2015 Aug 1;92(3):187-96. https://www.aafp.org/pubs/afp/issues/2015/0801/p187.html[↩][↩][↩][↩]
- Rainer BM, Kang S, Chien AL. Rosacea: Epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:e1361574. doi: 10.1080/19381980.2017.1361574[↩]
- López-Valverde G, Garcia-Martin E, Larrosa-Povés JM, Polo-Llorens V, Pablo-Júlvez LE. Therapeutical Management for Ocular Rosacea. Case Rep Ophthalmol. 2016;7:237–42. doi: 10.1159/000446104[↩]
- Farshchian M, Daveluy S. Rosacea. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557574[↩][↩][↩]
- Rosacea: Pathogenesis, clinical features, and diagnosis. https://www.uptodate.com/contents/rosacea-pathogenesis-clinical-features-and-diagnosis[↩][↩]
- van Zuuren EJ. Rosacea. N Engl J Med. 2017 Nov 2;377(18):1754-1764. doi: 10.1056/NEJMcp1506630[↩]
- Ahn CS, Huang WW. Rosacea Pathogenesis. Dermatol Clin. 2018 Apr;36(2):81-86. doi: 10.1016/j.det.2017.11.001[↩]
- Rainer BM, Kang S, Chien AL. Rosacea: Epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017 Oct 4;9(1):e1361574. doi: 10.1080/19381980.2017.1361574[↩]
- Adult Blepharitis. https://emedicine.medscape.com/article/1211763-overview[↩]
- Oltz M, Check J. Rosacea and its ocular manifestations. Optometry. 2011;82:92–103. doi: 10.1016/j.optm.2010.01.015[↩]
- Alvarenga LS, Mannis MJ. Ocular Rosacea. Ocul Surf. 2005;3:41–58. doi: 10.1016/s1542-0124(12)70121-0[↩]
- Rodriguez-Garcia A. Ocular Rosacea: Recent Advances in Pathogenesis and Therapy. In 2015. p. 175–199.[↩]
- Gallo RL, Granstein RD, Kang S, Mannis M, Steinhoff M, Tan J, Thiboutot D. Standard classification and pathophysiology of rosacea: The 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018 Jan;78(1):148-155. doi: 10.1016/j.jaad.2017.08.037[↩][↩]
- Wladis EJ, Adam AP. Treatment of ocular rosacea. Surv Ophthalmol. 2018 May-Jun;63(3):340-346. doi: 10.1016/j.survophthal.2017.07.005[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Layton AM. Pharmacologic treatments for rosacea. Clin Dermatol. 2017 Mar – Apr;35(2):207-212. doi: 10.1016/j.clindermatol.2016.10.016[↩]
- Wladis EJ, Bradley EA, Bilyk JR, Yen MT, Mawn LA. Oral Antibiotics for Meibomian Gland-Related Ocular Surface Disease: A Report by the American Academy of Ophthalmology. Ophthalmology. 2016 Mar;123(3):492-6. doi: 10.1016/j.ophtha.2015.10.062[↩]
- Webster GF, Durrani K, Suchecki J. Ocular rosacea, psoriasis, and lichen planus. Clin Dermatol. 2016 Mar-Apr;34(2):146-50. doi: 10.1016/j.clindermatol.2015.11.014[↩]
- Wladis EJ, Aakalu VK, Foster JA, Freitag SK, Sobel RK, Tao JP, Yen MT. Intense Pulsed Light for Meibomian Gland Disease: A Report by the American Academy of Ophthalmology. Ophthalmology. 2020 Sep;127(9):1227-1233. doi: 10.1016/j.ophtha.2020.03.009[↩]
- Dell SJ, Gaster RN, Barbarino SC, Cunningham DN. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin Ophthalmol. 2017 May 2;11:817-827. doi: 10.2147/OPTH.S130706[↩]
- Bunya V, March de Ribot F, Barmettler A, Yen MT, Nguyen Barkat C. American Academy of Opthalmology Eye Wiki. Intense pulsed light (IPL) therapy. 2023.[↩]
- Scala J, Vojvodic A, Vojvodic P, Vlaskovic-Jovicevic T, Peric-Hajzler Z, Matovic D, Dimitrijevic S, Vojvodic J, Sijan G, Stepic N, Wollina U, Tirant M, Thuong NV, Fioranelli M, Lotti T. Botulin Toxin Use in Rosacea and Facial Flushing Treatment. Open Access Maced J Med Sci. 2019 Aug 30;7(18):2985-2987. doi: 10.3889/oamjms.2019.784[↩]
- Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part II. Topical and systemic therapies in the treatment of rosacea. J Am Acad Dermatol. 2015;72(5):761-770. doi: 10.1016/j.jaad.2014.08.027[↩]
- Hsu CC, Lee JY. Carvedilol for the treatment of refractory facial flushing and persistent erythema of rosacea. Arch Dermatol. 2011;147(11):1258-1260. doi: 10.1001/archdermatol.2011.204[↩]
- Craige H, Cohen JB. Symptomatic treatment of idiopathic and rosacea-associated cutaneous flushing with propranolol. J Am Acad Dermatol. 2005;53(5):881-884. doi: 10.1016/j.jaad.2005.07.021[↩]
- Dandona P, Ghanim H, Brooks DP. Antioxidant activity of carvedilol in cardiovascular disease. J Hypertens. 2007;25(4):731-741. doi: 10.1097/HJH.0b013e3280127948[↩]
- Stein L, Kircik L, Fowler J, Tan J, Draelos Z, Fleischer A, Appell M, Steinhoff M, Lynde C, Liu H, Jacovella J. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014 Mar;13(3):316-23. https://jddonline.com/articles/efficacy-and-safety-of-ivermectin-1-cream-in-treatment-of-papulopustular-rosacea-results-of-two-rand-S1545961614P0316X/[↩]
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44(12):981-988. doi: 10.1111/j.1365-4632.2004.02253.x[↩]
- Schaller M, Gonser L, Belge K, et al. Dual anti-inflammatory and anti-parasitic action of topical ivermectin 1% in papulopustular rosacea. J Eur Acad Dermatol Venereol. 2017;31(11):1907-1911. doi: 10.1111/jdv.14437[↩]
- de Menonville ST, Rosignoli C, Soares E, et al. Topical treatment of rosacea with ivermectin inhibits gene expression of cathelicidin innate immune mediators, LL-37 and KLK5, in reconstructed and ex vivo skin models. Dermatol Ther (Heidelb). 2017;7(2):213-225. doi: 10.1007/s13555-017-0176-3[↩]
- Zhang X, Song Y, Ci X, et al. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm Res. 2008;57(11):524-529. doi: 10.1007/s00011-008-8007-8[↩]
- Kanada KN, Nakatsuji T, Gallo RL. Doxycycline indirectly inhibits proteolytic activation of tryptic kallikrein-related peptidases and activation of cathelicidin. J Invest Dermatol. 2012;132(5):1435-1442. doi: 10.1038/jid.2012.14[↩]
- Stechmiller J, Cowan L, Schultz G. The role of doxycycline as a matrix metalloproteinase inhibitor for the treatment of chronic wounds. Biol Res Nurs. 2010;11(4):336-344. doi: 10.1177/1099800409346333[↩]
- Griffin MO, Fricovsky E, Ceballos G, Villarreal F. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am J Physiol Cell Physiol. 2010;299(3):C539-C548. doi: 10.1152/ajpcell.00047.2010[↩]
- Alzolibani AA, Zedan K. Macrolides in chronic inflammatory skin disorders. Mediators Inflamm. 2012;2012:159354. doi: 10.1155/2012/159354[↩]
- Altinyazar HC, Koca R, Tekin NS, Esturk E, Adapalene vs. metronidazole gel for the treatment of rosacea. Int J Dermatol. 2005;44(3):252-255. doi: 10.1111/j.1365-4632.2004.02130.x[↩]
- Park H, Del Rosso JQ. Use of oral isotretinoin in the management of rosacea. J Clin Aesthet Dermatol. 2011 Sep;4(9):54-61. https://pmc.ncbi.nlm.nih.gov/articles/PMC3175801/[↩]
- Holmes AD, Spoendlin J, Chien AL, Baldwin H, Chang ALS. Evidence-based update on rosacea comorbidities and their common physiologic pathways. J Am Acad Dermatol. 2018 Jan;78(1):156-166. doi: 10.1016/j.jaad.2017.07.055[↩]
- Haber R, El Gemayel M. Comorbidities in rosacea: A systematic review and update. J Am Acad Dermatol. 2018 Apr;78(4):786-792.e8. doi: 10.1016/j.jaad.2017.09.016[↩]